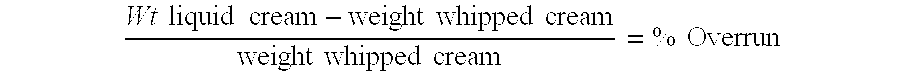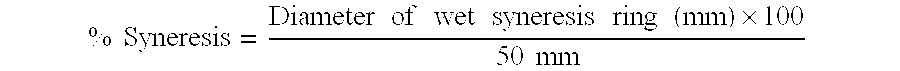Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1490 results about "Sterilization process" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
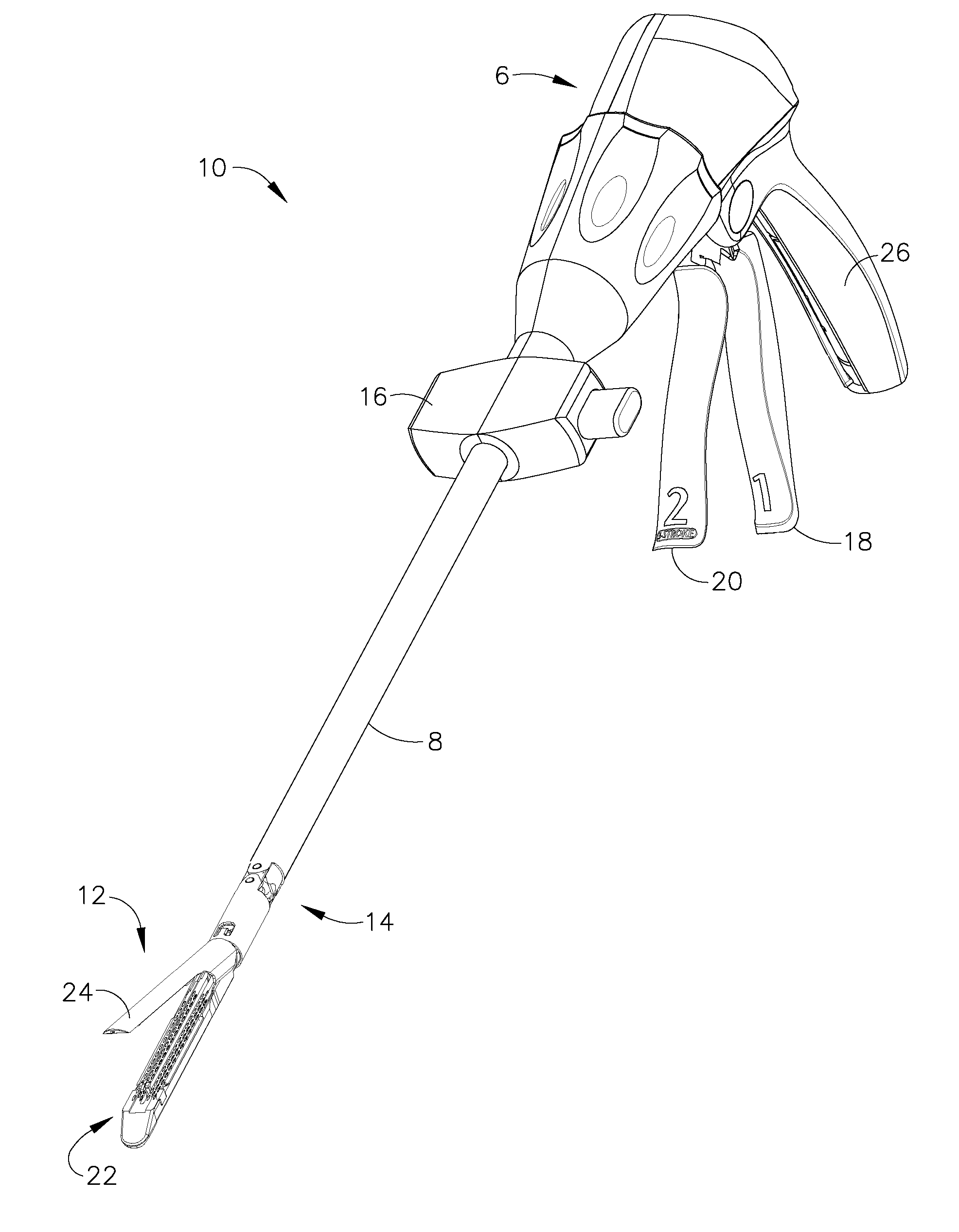
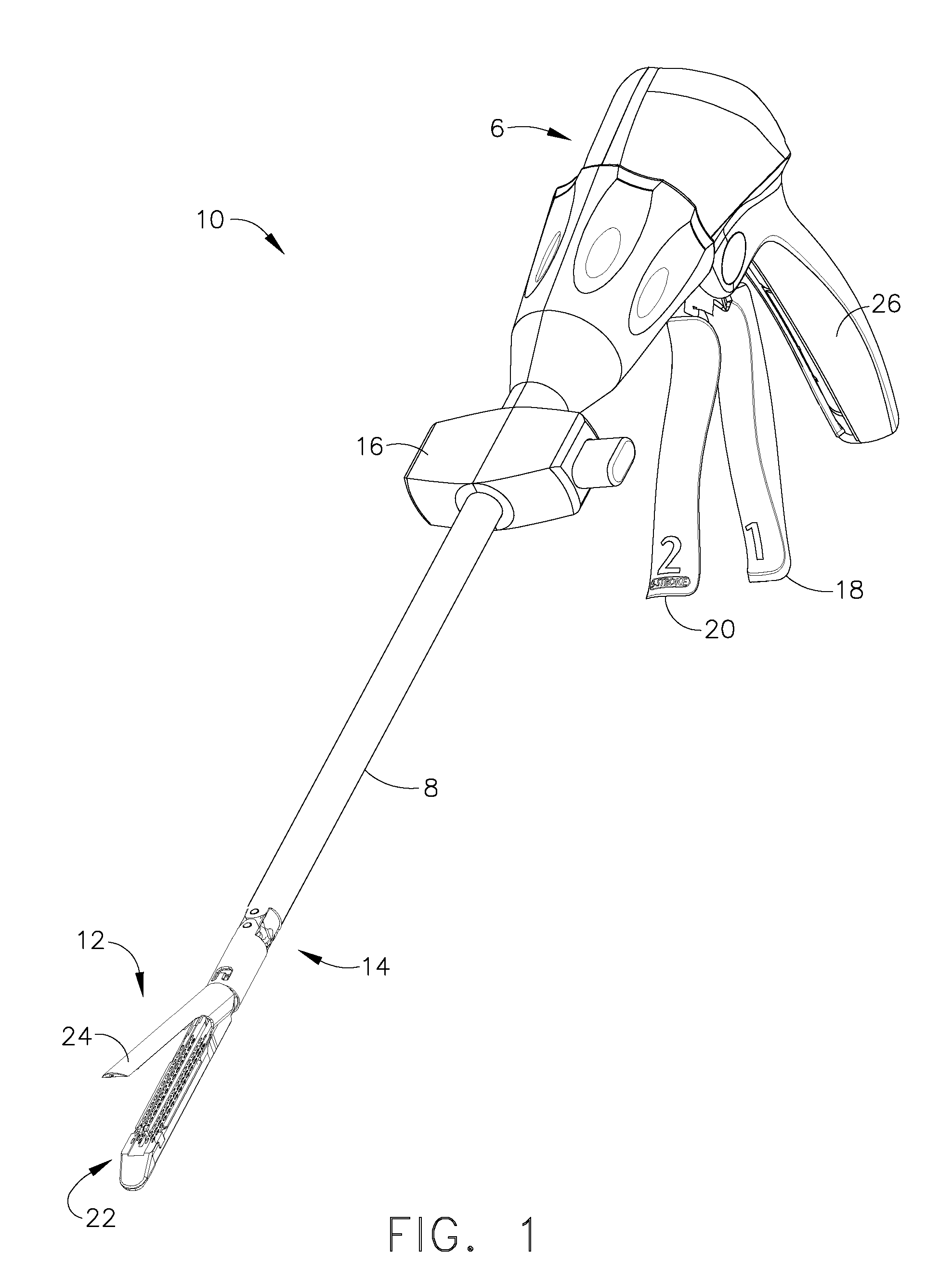
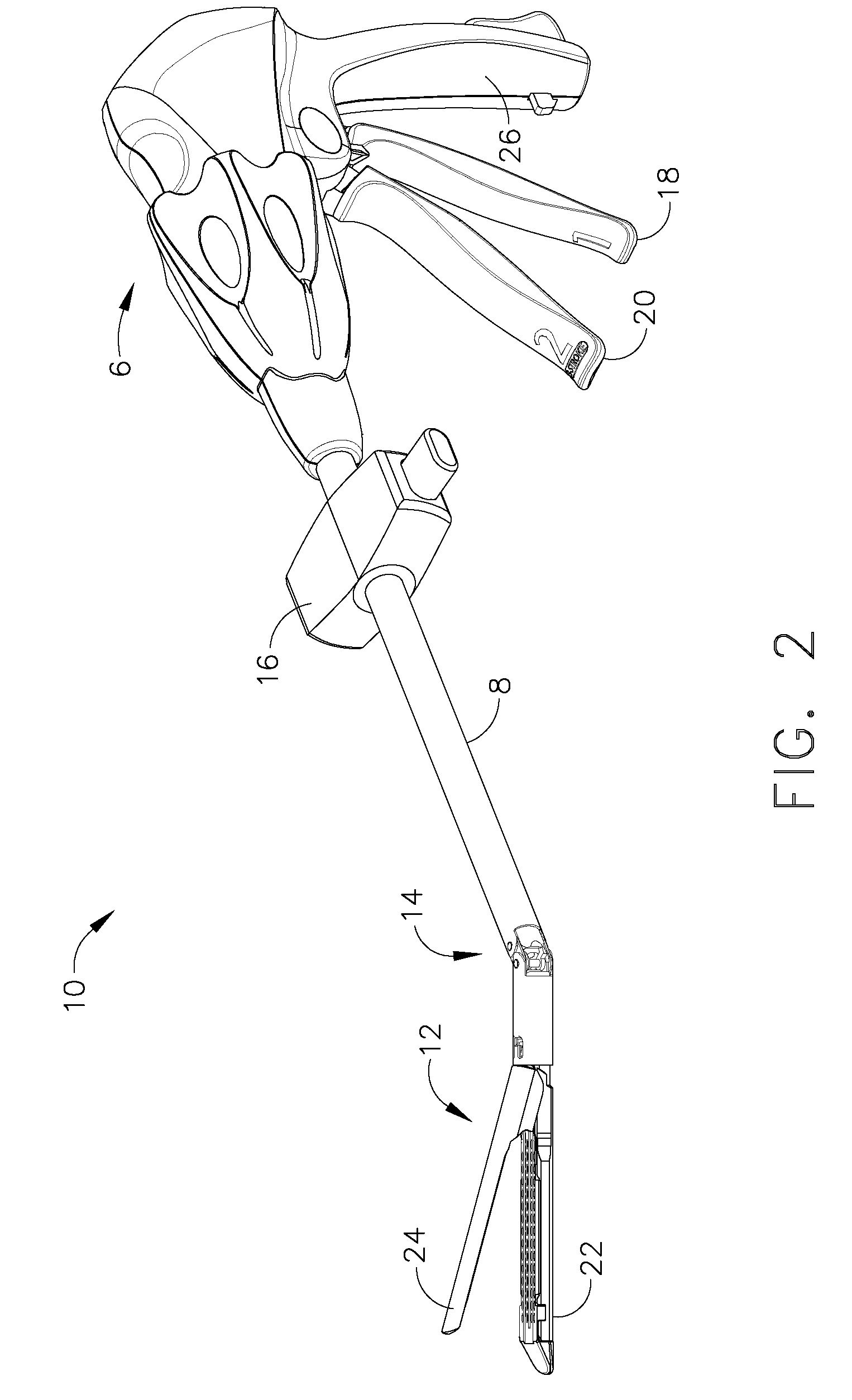
A sterilization process is a combination of the sterilizer (including the sterilizer agent), the process, the load, the loading pattern and the wrapping (system).
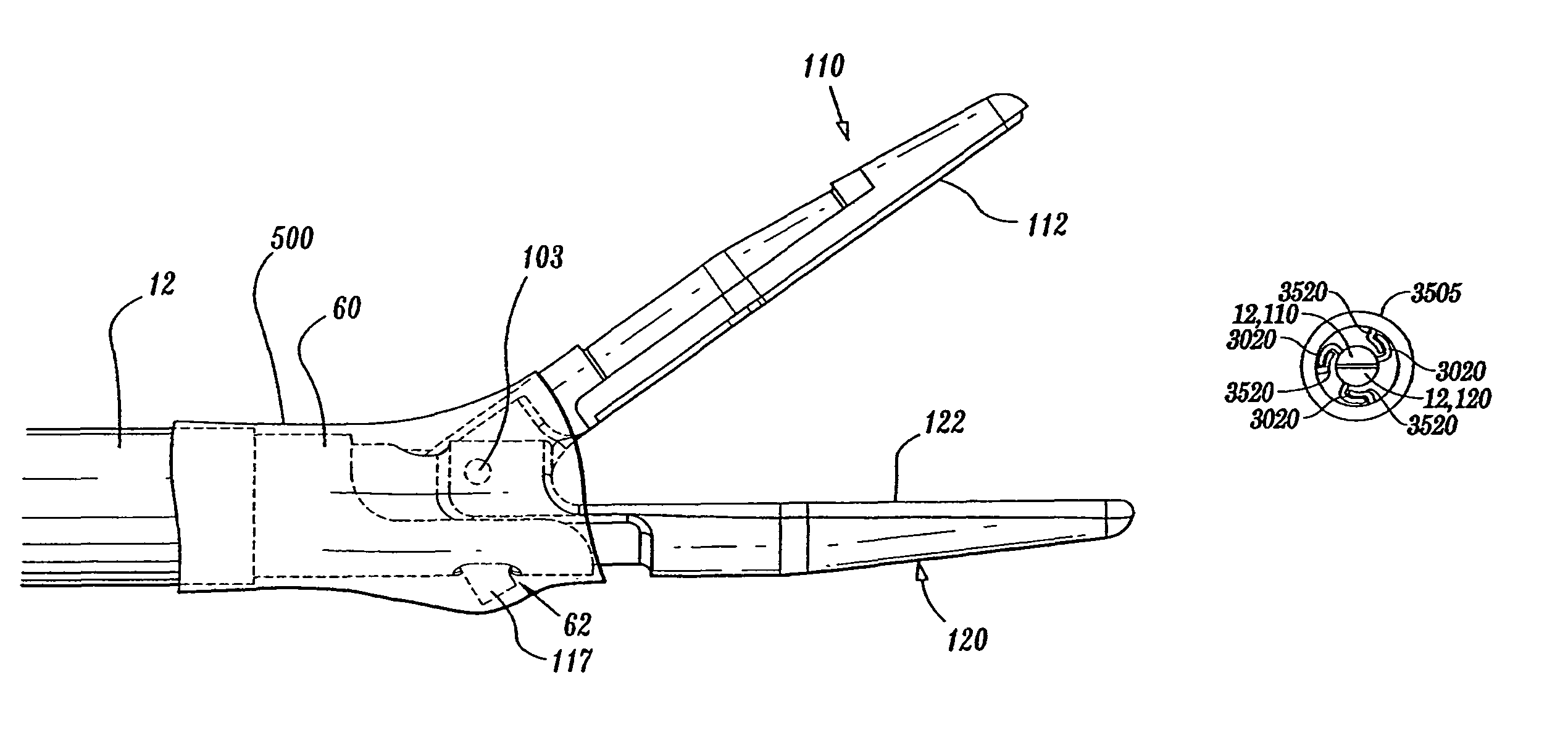
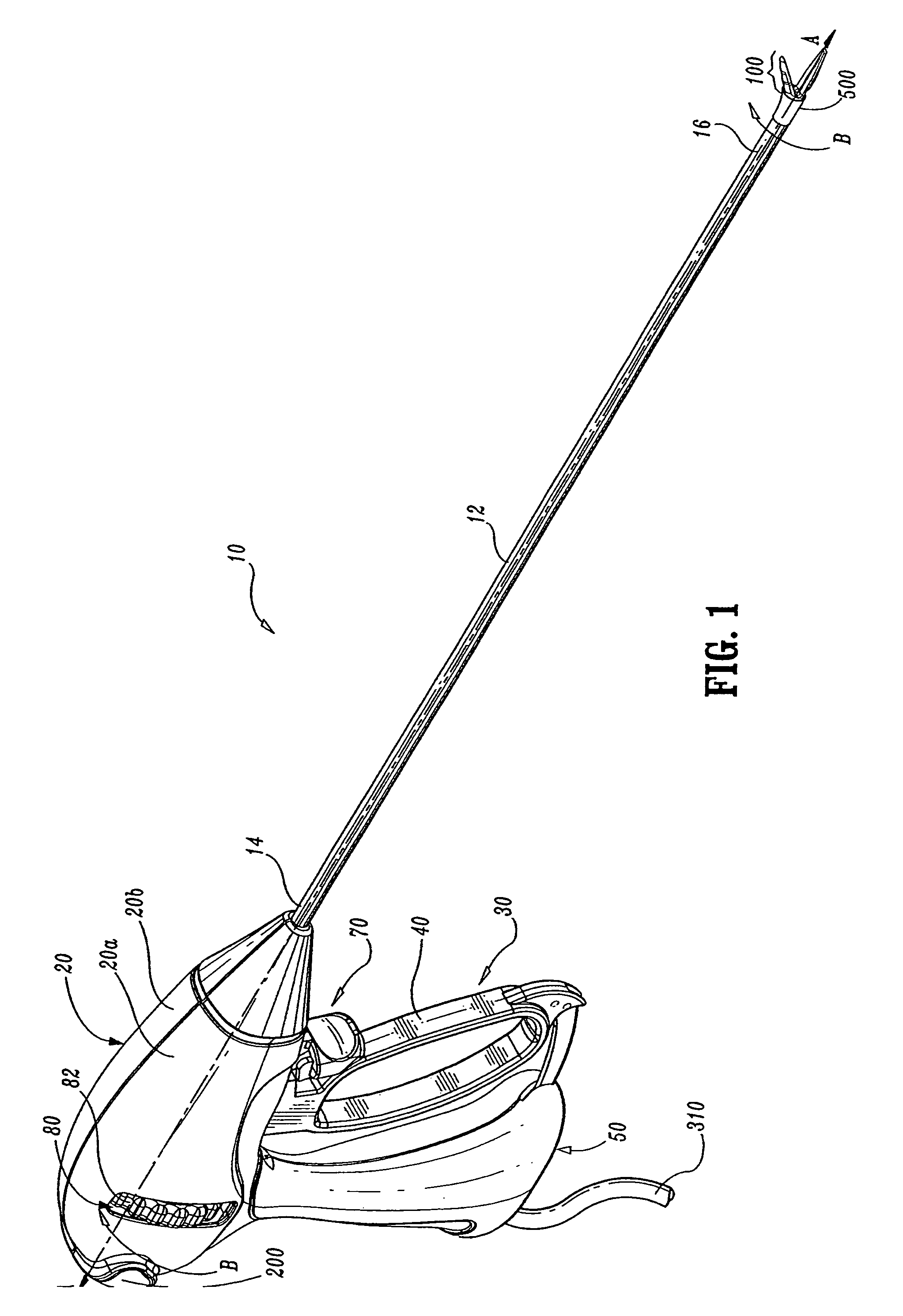
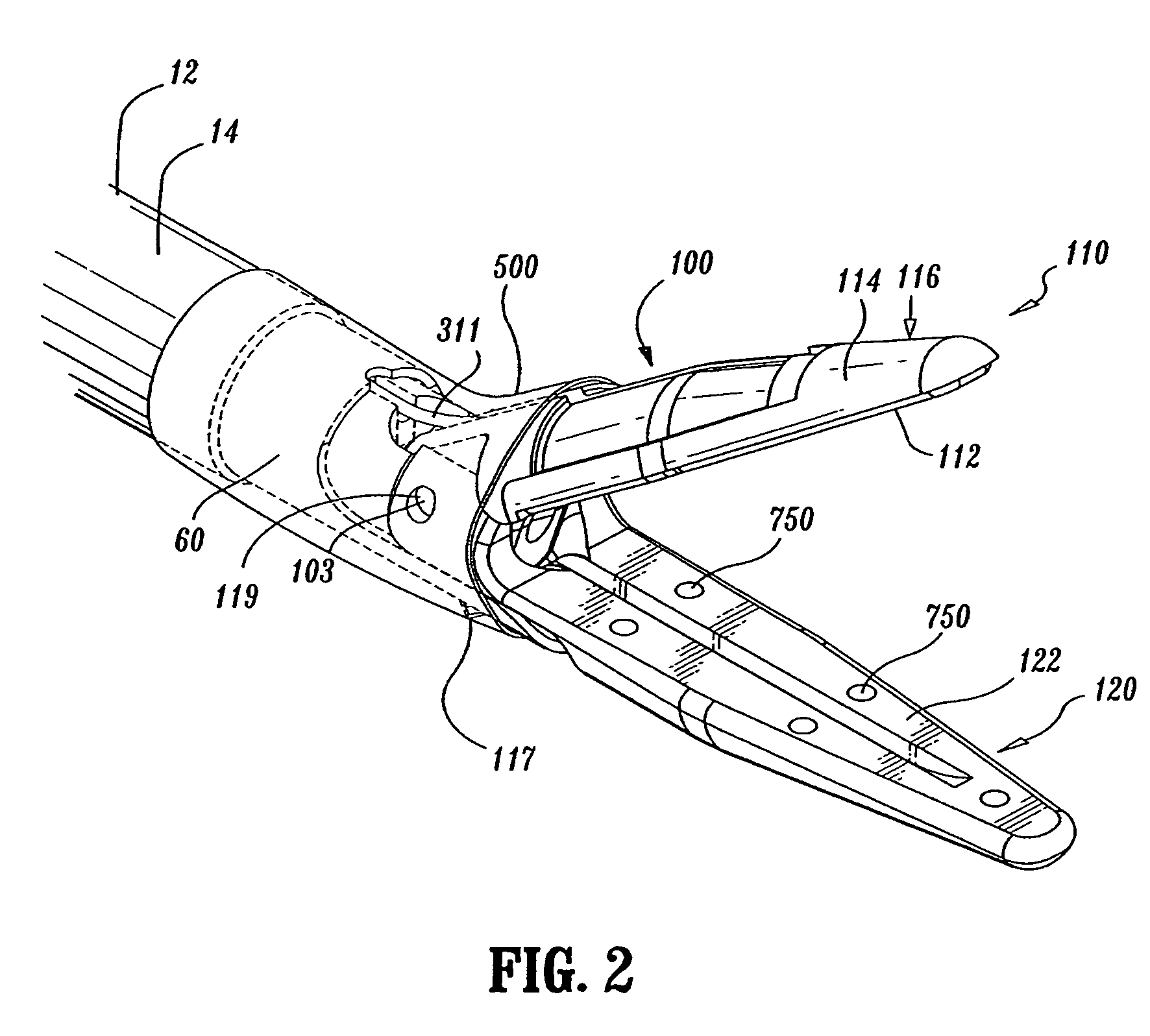
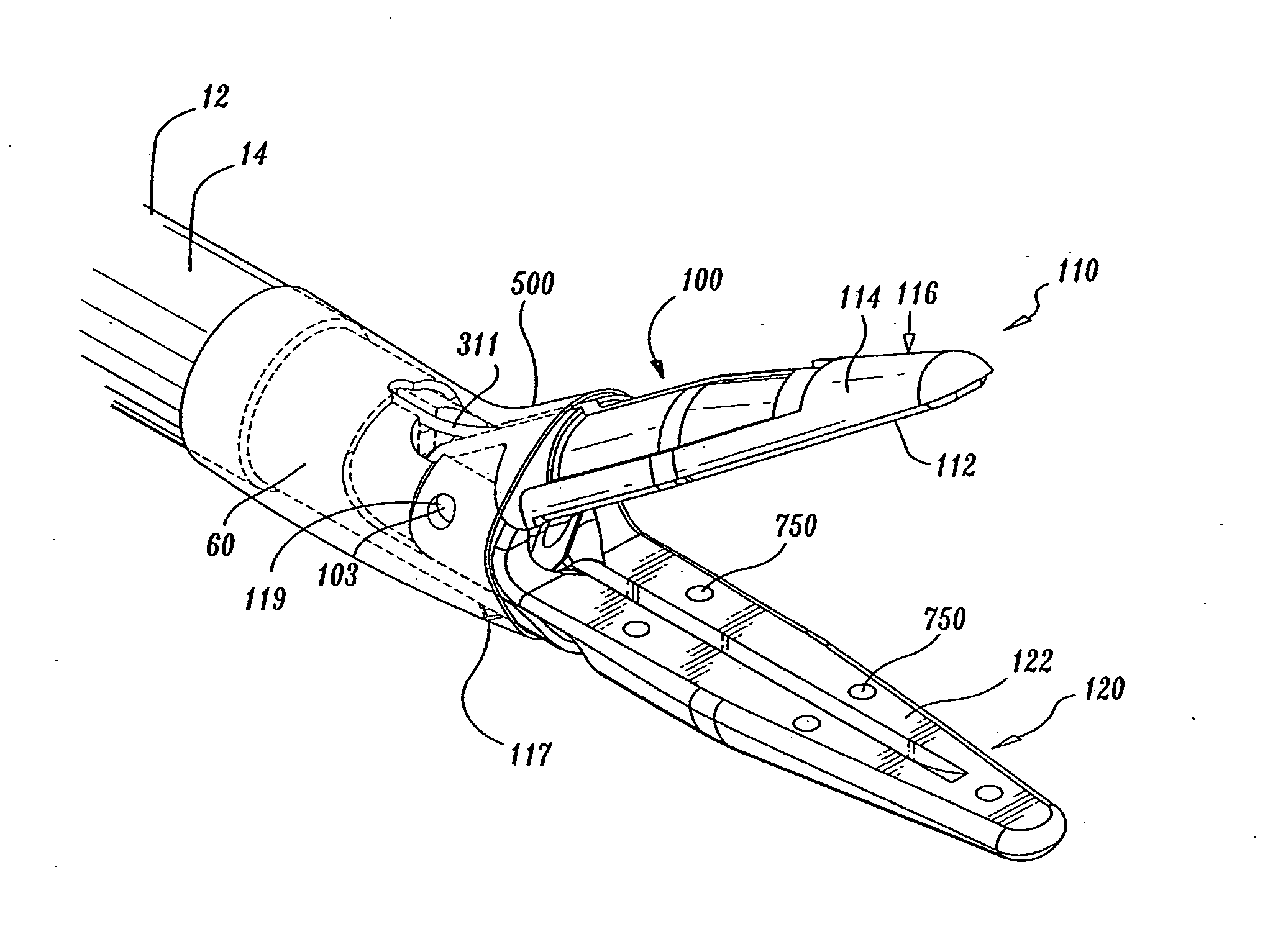
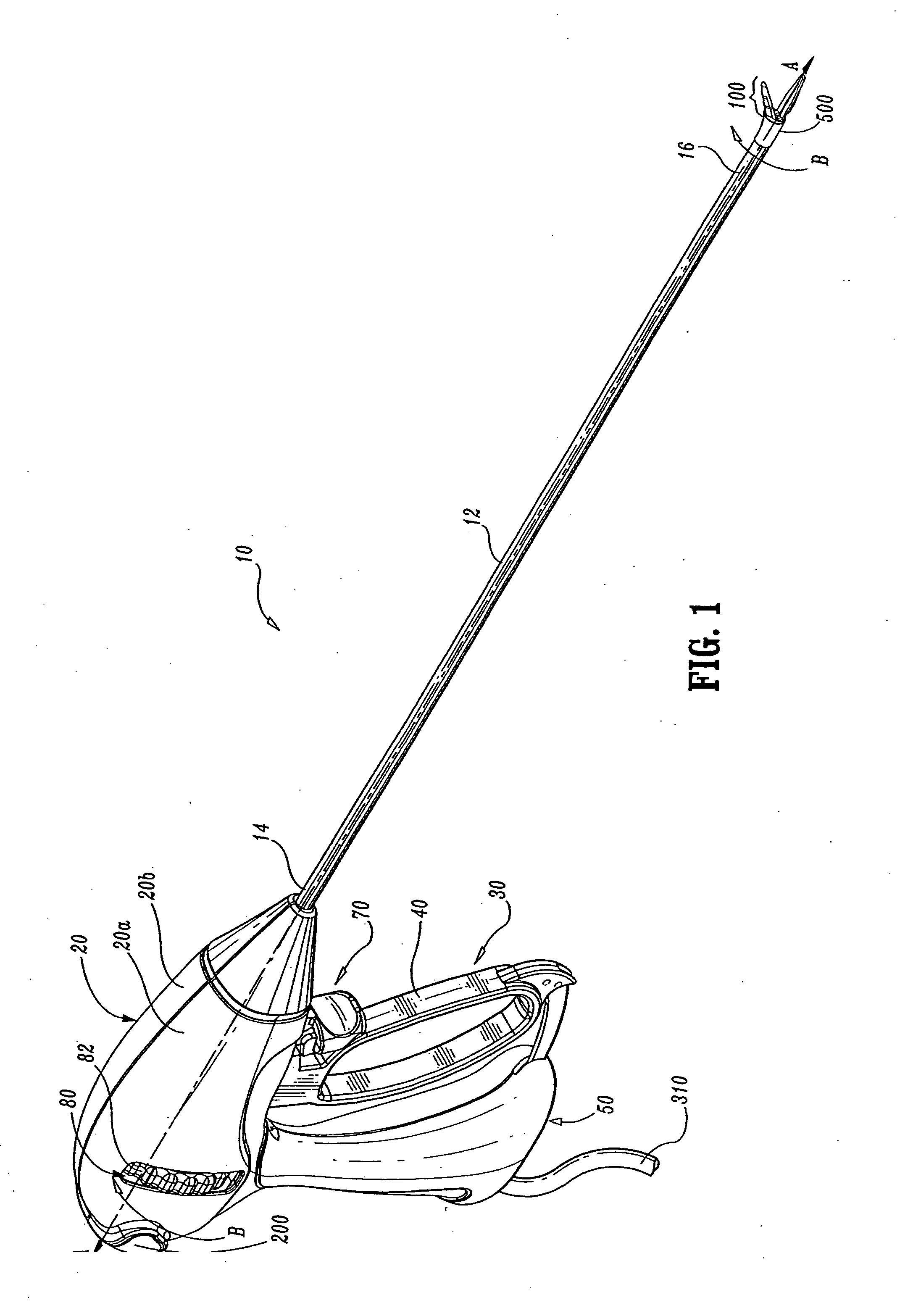
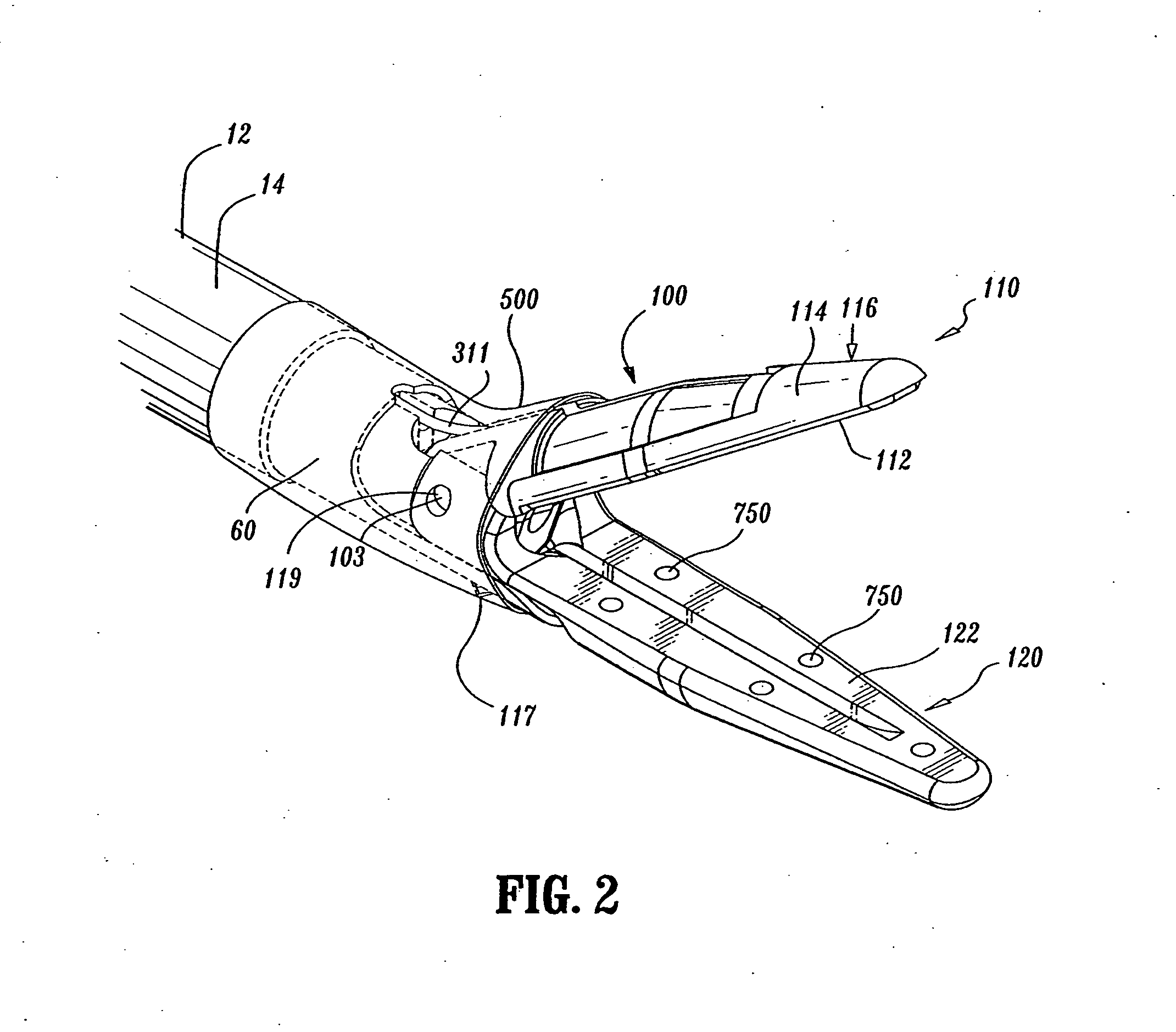
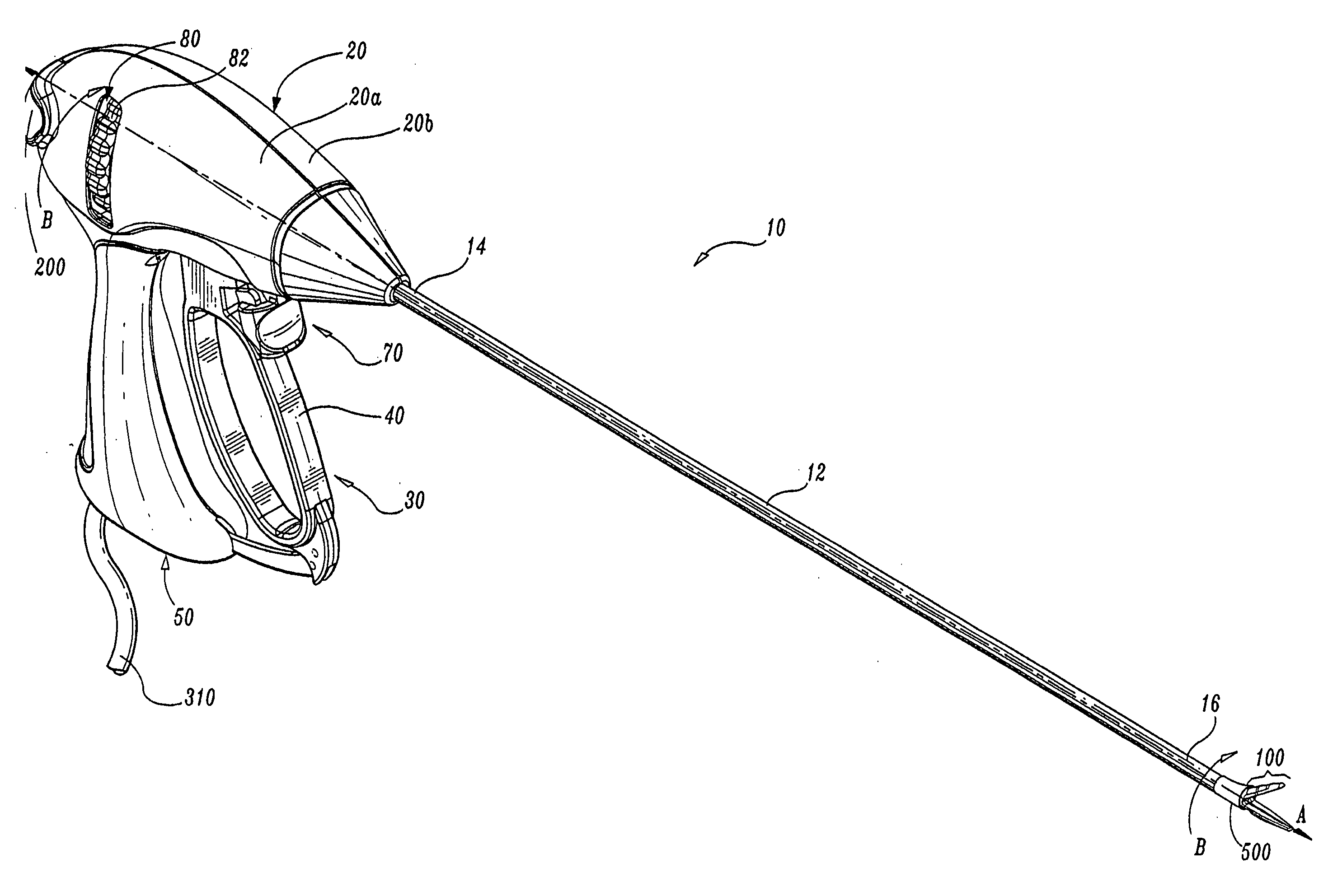
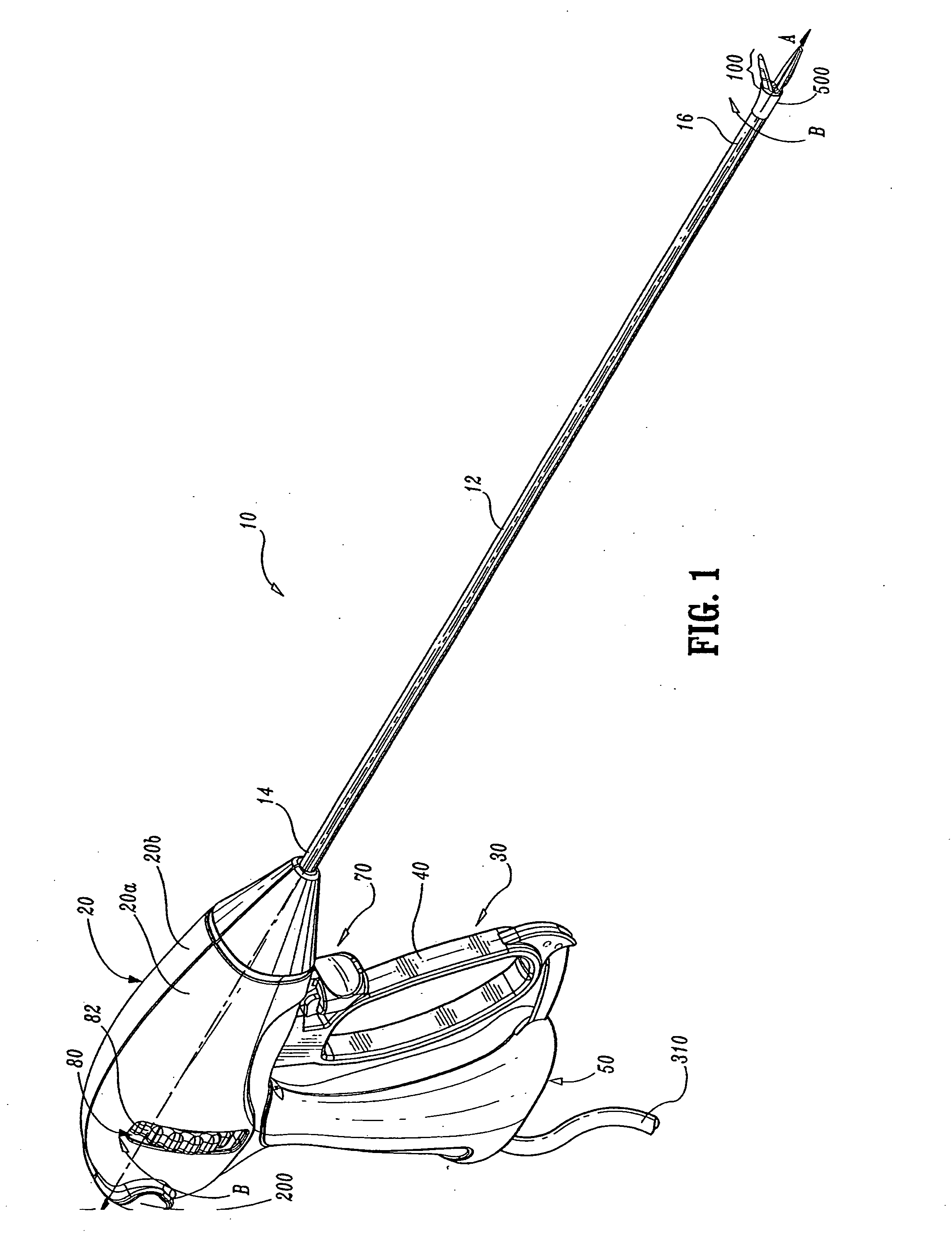
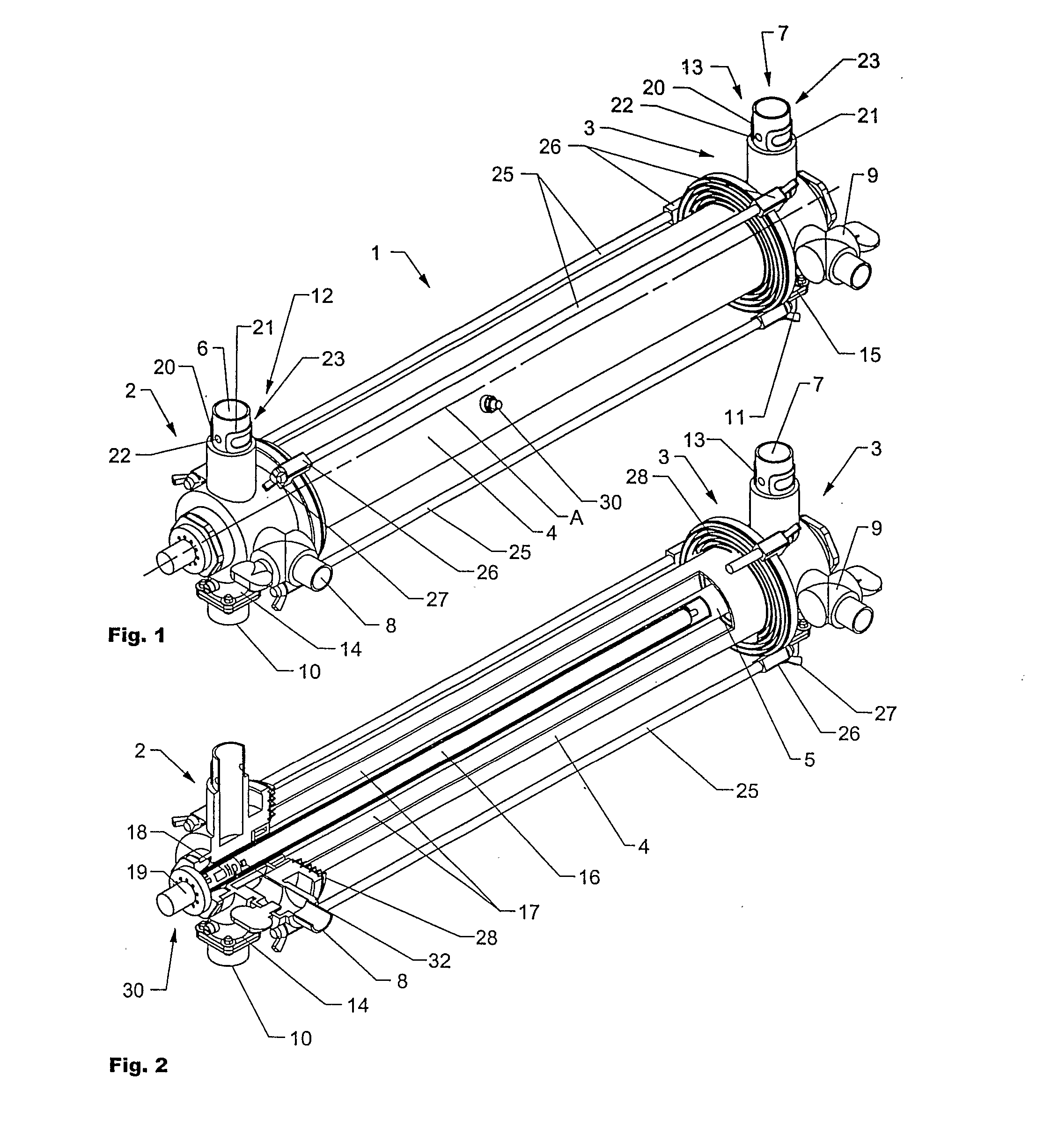
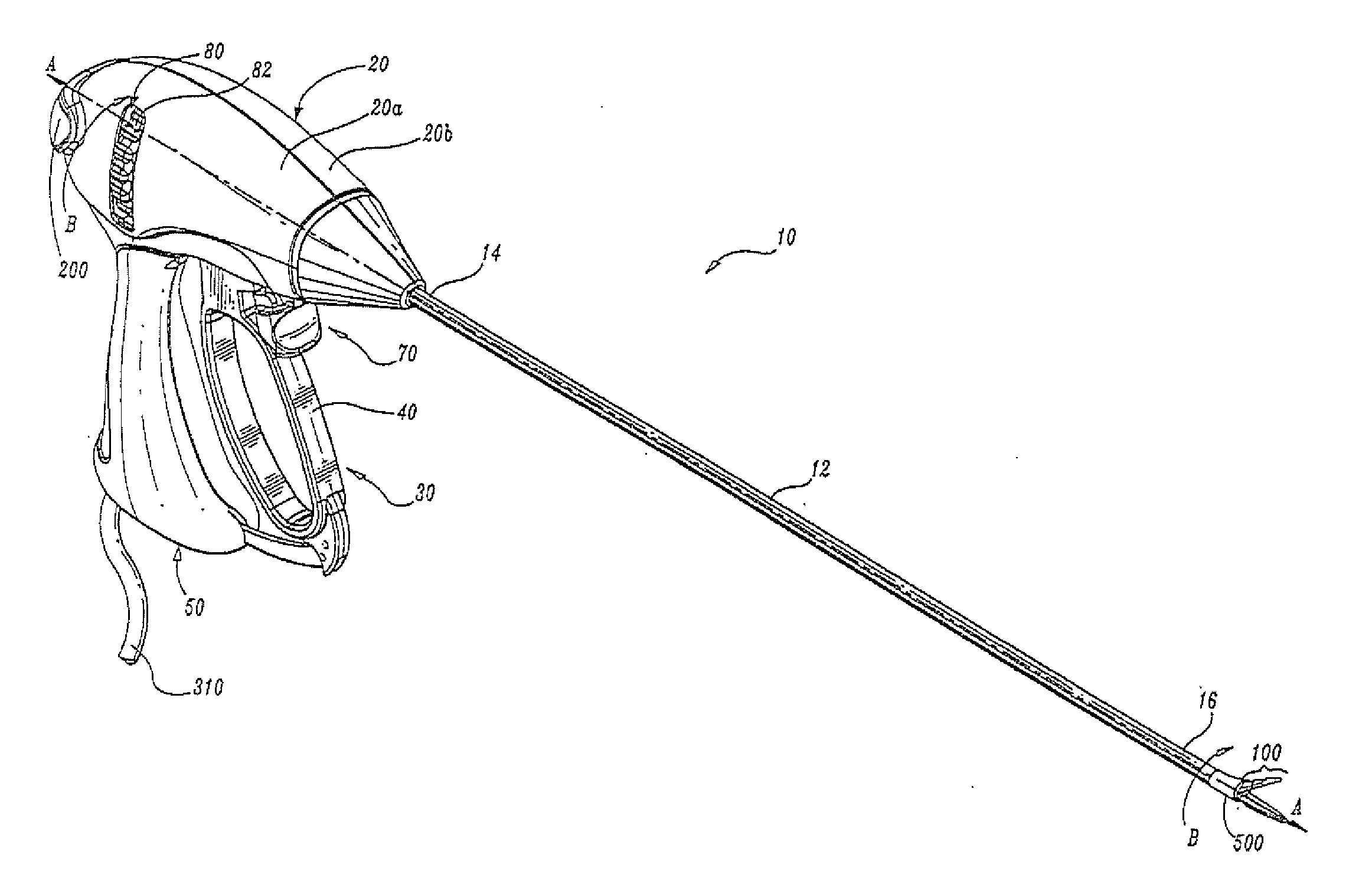
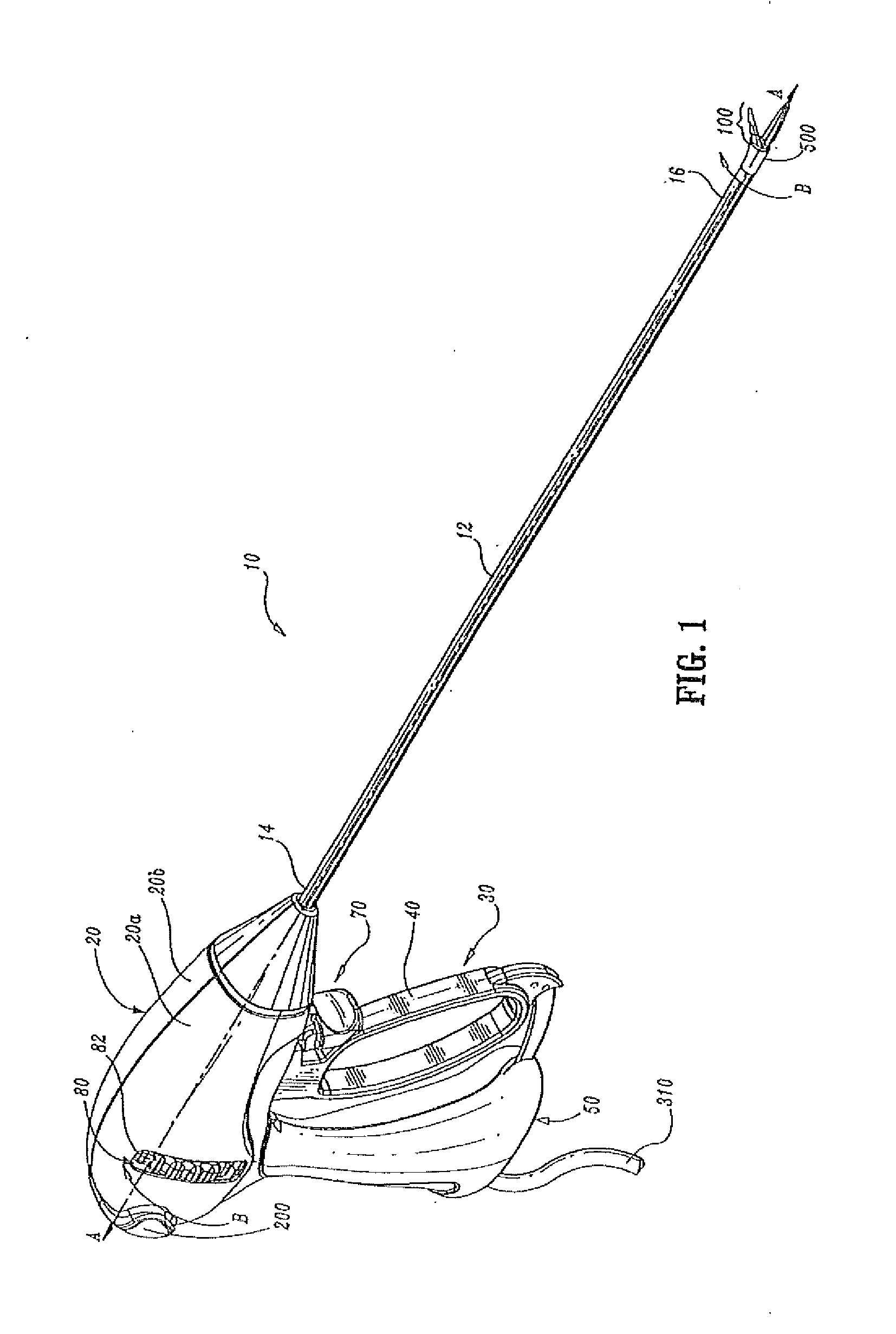
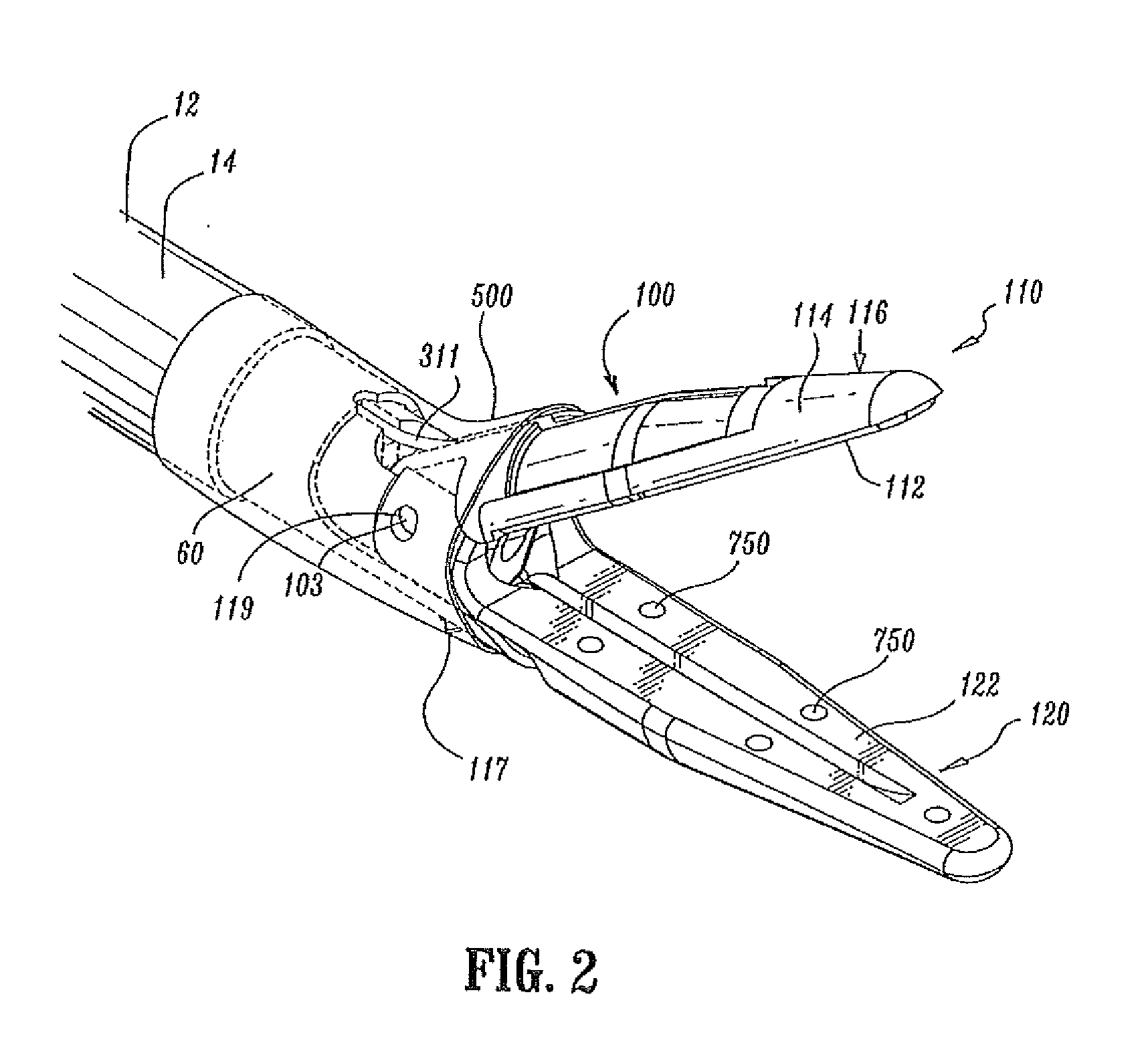
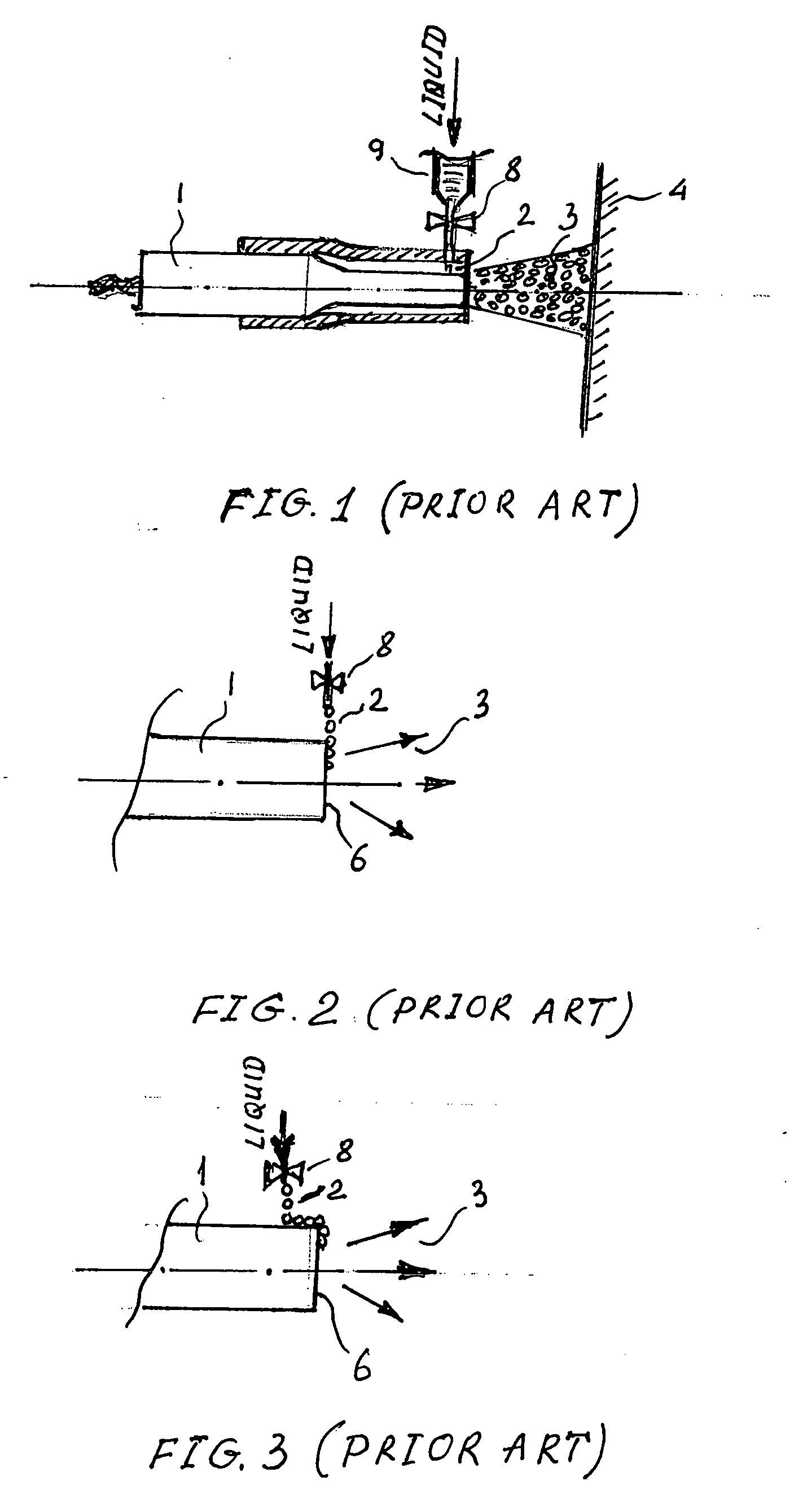
Insulating boot for electrosurgical forceps
ActiveUS7879035B2Reduces the potential for strayDiagnosticsSurgical instrument detailsEthylene oxideEngineering
Either an endoscopic or open bipolar forceps includes a flexible, generally tubular insulating boot for insulating patient tissue, while not impeding motion of the jaw members. The jaw members are movable from an open to a closed position and the jaw members are connected to a source of electrosurgical energy such that the jaw members are capable of conducting energy through tissue held therebetween to effect a tissue seal. A knife assembly may be included that allows a user to selectively divide tissue upon actuation thereof. The insulating boot may be made from a viscoelastic, elastomeric or flexible material suitable for use with a sterilization process including ethylene oxide. An interior portion of the insulating boot may have at least one mechanically interfacing surface that interfaces with a mechanically interfacing surface formed between the shaft and a jaw member or with a mechanically interfacing surface disposed or formed on the shaft or a jaw member.
Owner:COVIDIEN AG
Insulating boot for electrosurgical forceps
ActiveUS20070078458A1Reduces the potential for strayDiagnosticsSurgical instruments for heatingElastomerEngineering
Either an endoscopic or open bipolar forceps includes a flexible, generally tubular insulating boot for insulating patient tissue, while not impeding motion of the jaw members. The jaw members are movable from an open to a closed position and the jaw members are connected to a source of electrosurgical energy such that the jaw members are capable of conducting energy through tissue held therebetween to effect a tissue seal. A knife assembly may be included that allows a user to selectively divide tissue upon actuation thereof. The insulating boot may be made from a viscoelastic, elastomeric or flexible material suitable for use with a sterilization process including ethylene oxide.
Owner:COVIDIEN AG
Insulating boot for electrosurgical forceps
ActiveUS20070106295A1Reduces the potential for strayDiagnosticsSurgical instruments for heatingBipolar forcepsEndoscope
Either an endoscopic or open bipolar forceps includes a flexible, generally tubular insulating boot for insulating patient tissue, while not impeding motion of the jaw members. The jaw members are movable from an open to a closed position and the jaw members are connected to a source of electrosurgical energy such that the jaw members are capable of conducting energy through tissue held therebetween to effect a tissue seal. A knife assembly may be included that allows a user to selectively divide tissue upon actuation thereof. The insulating boot may be made from a viscoelastic, elastomeric or flexible material suitable for use with a sterilization process including ethylene oxide. An interior portion of the insulating boot may have at least one mechanically interfacing surface that interfaces with a mechanically interfacing surface formed between the shaft and a jaw member or with a mechanically interfacing surface disposed or formed on the shaft or a jaw member.
Owner:COVIDIEN AG
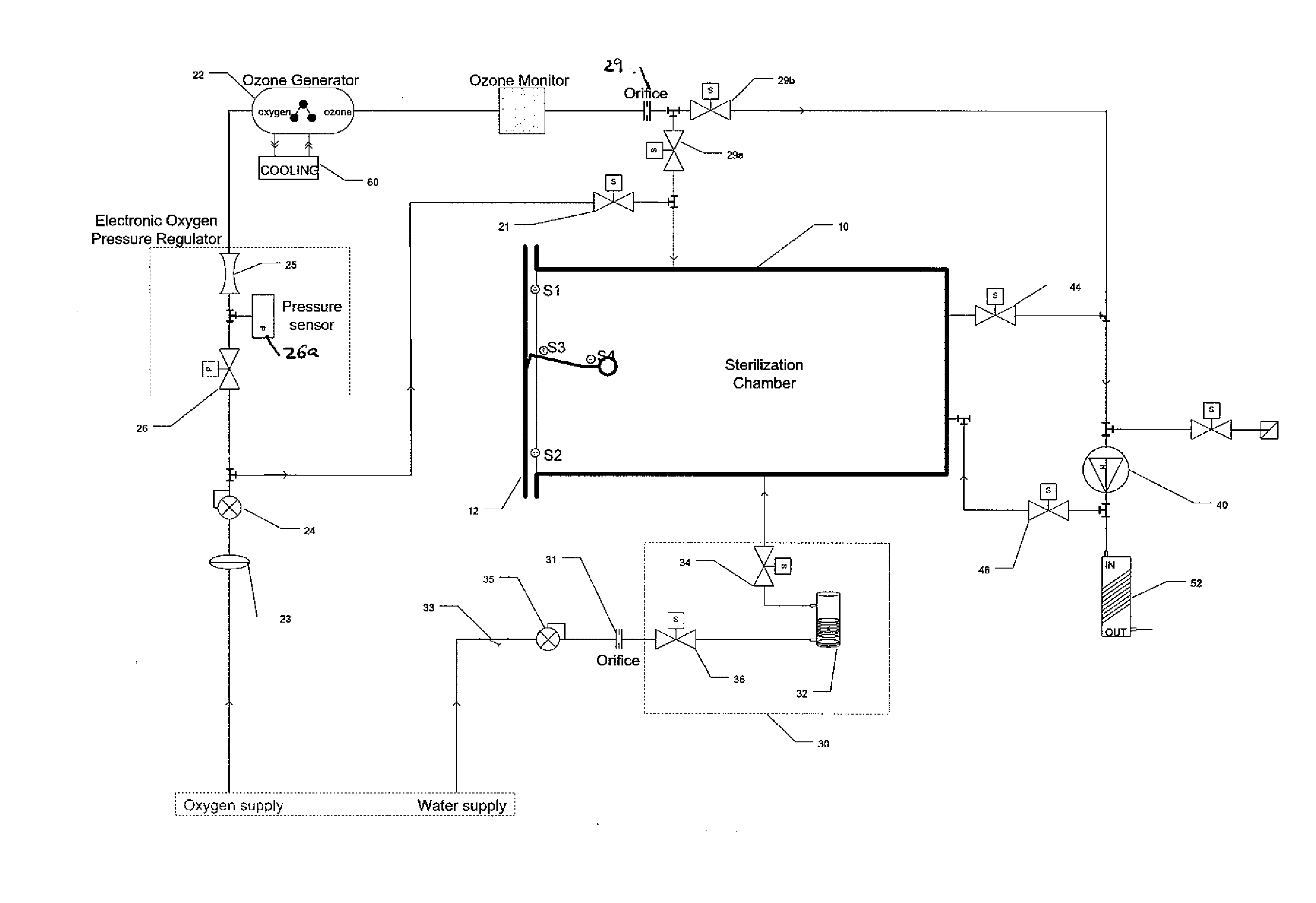
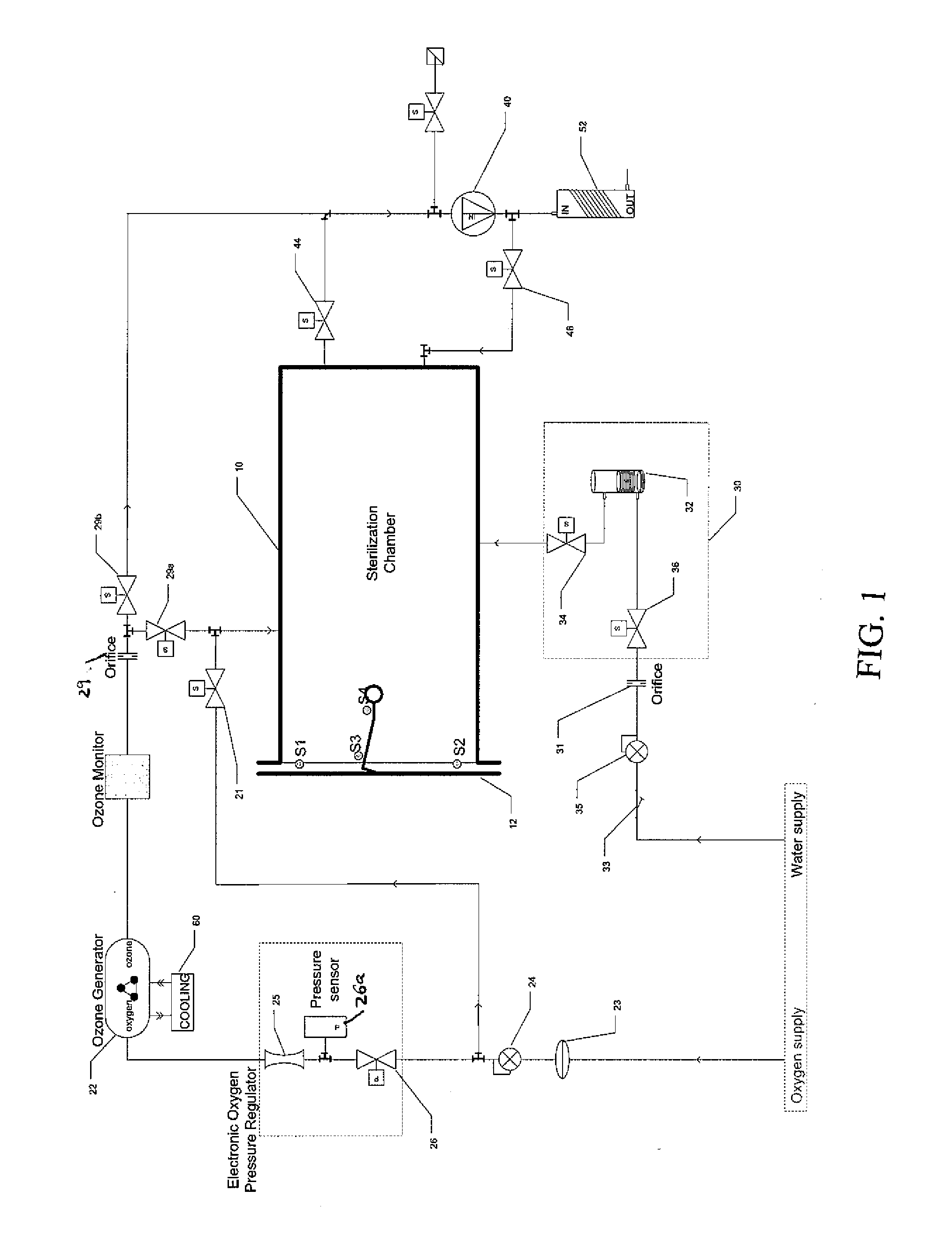
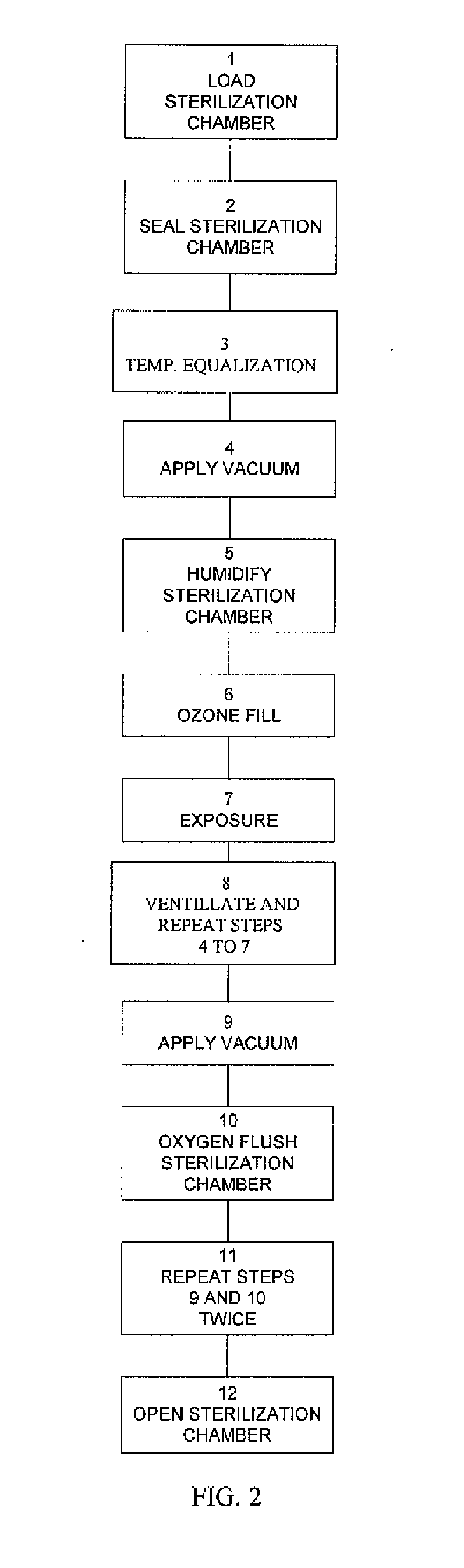
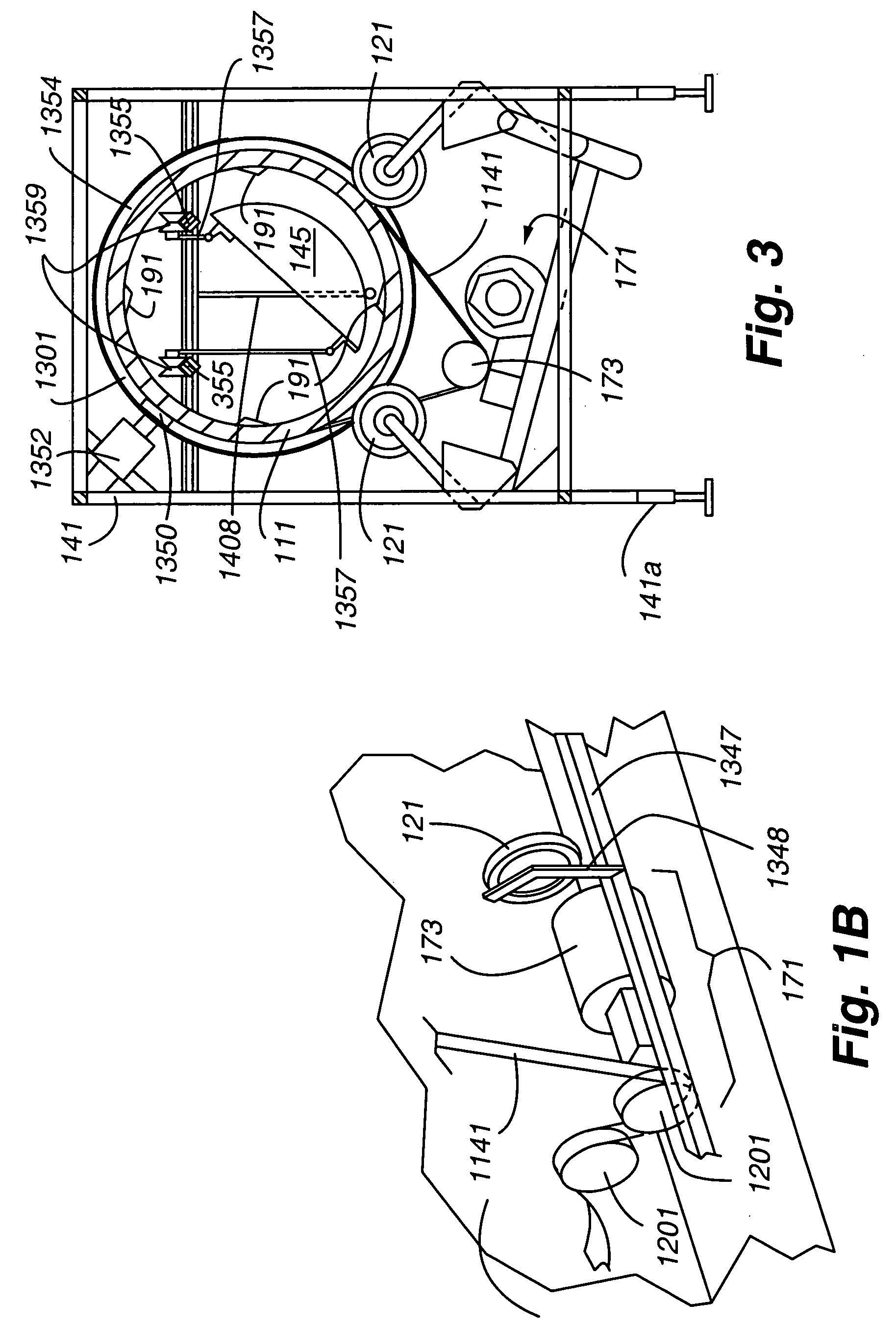
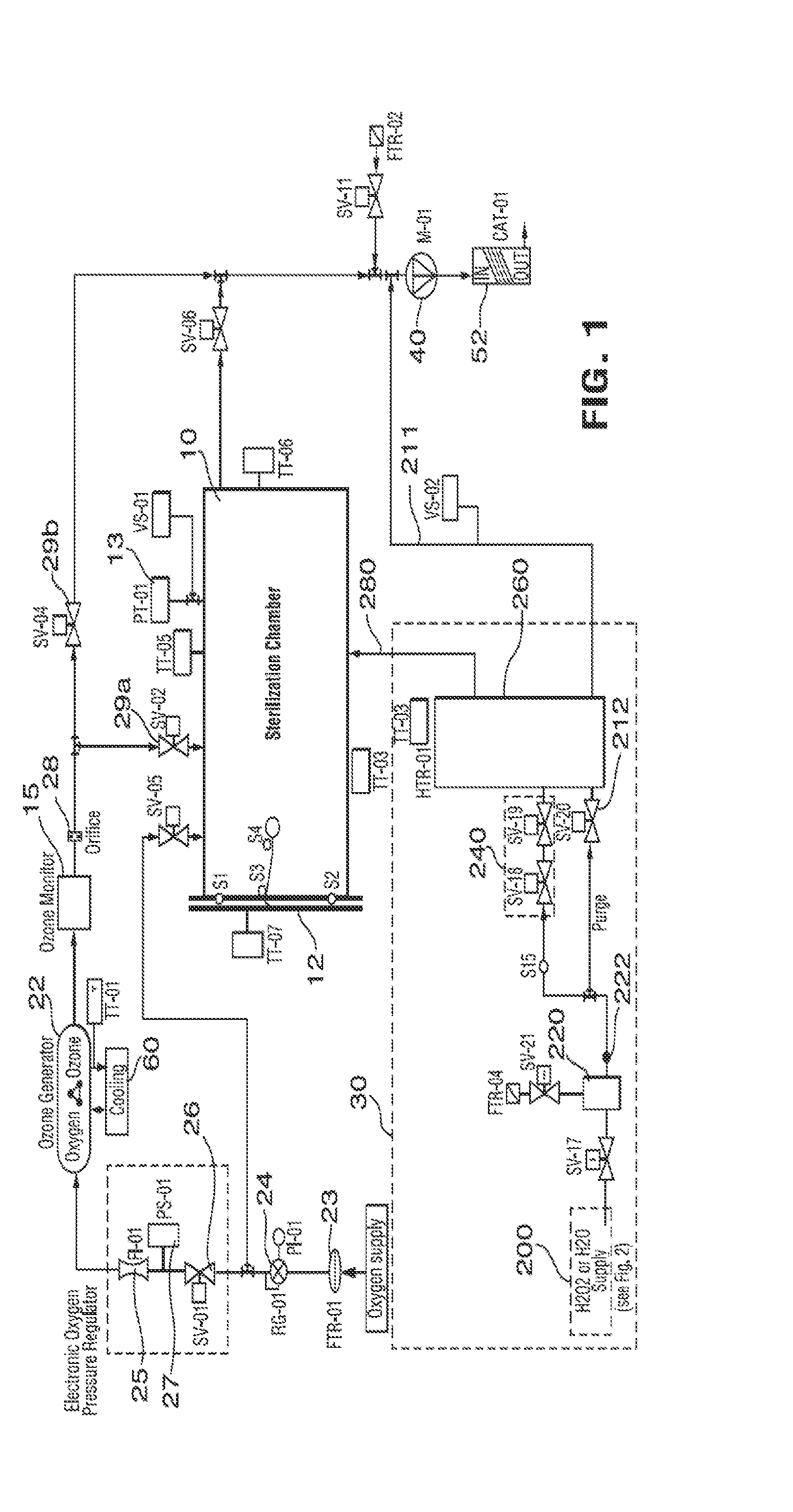
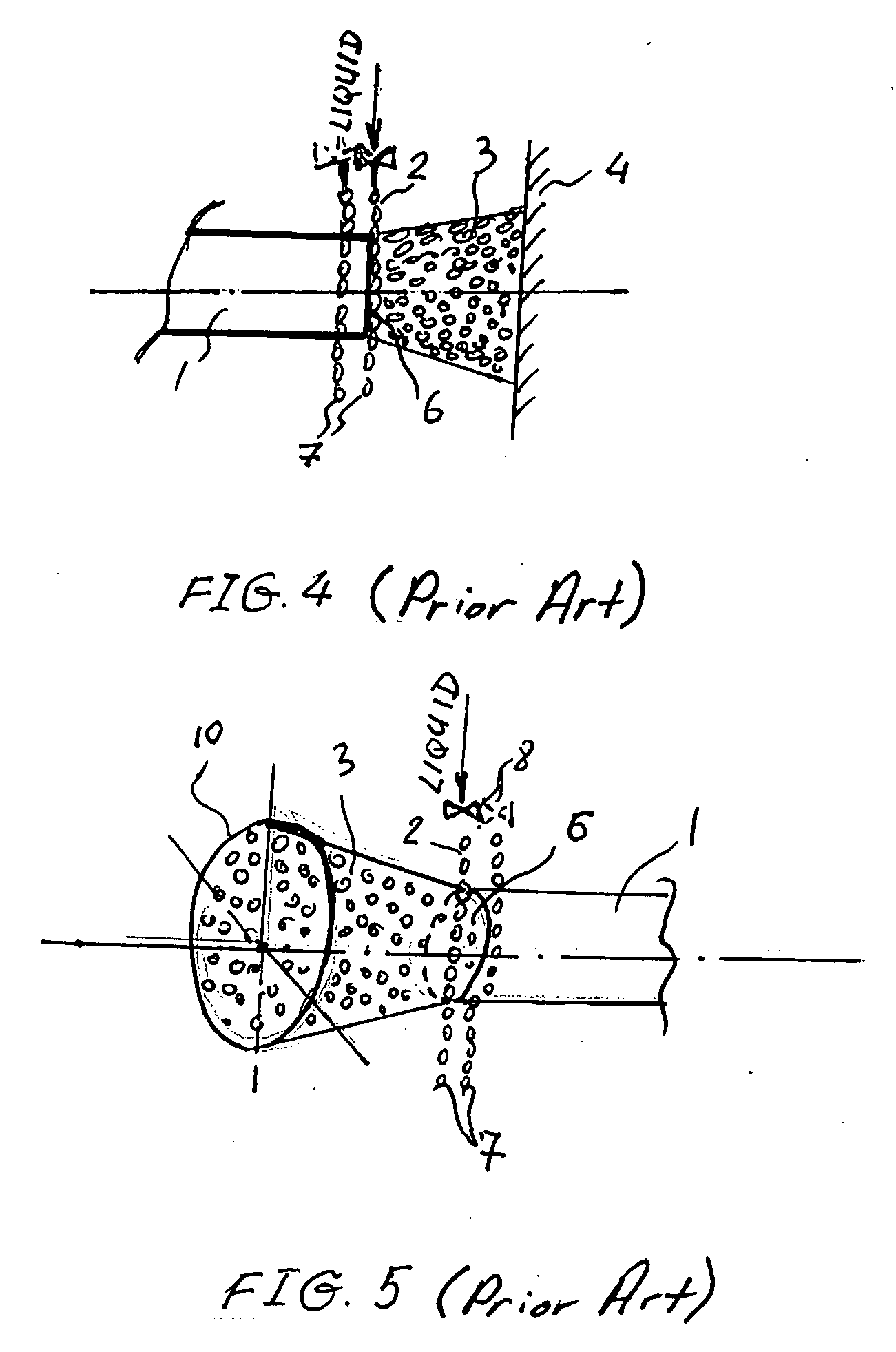
Method and apparatus for ozone sterilization
InactiveUS20070258855A1Avoid condensationPrevent water condensationElectrolysis componentsExhaust apparatusVacuum pressureWater vapor
The present invention provides a method and apparatus for sterilizing articles using an ozone-containing gas, where condensation of water from the sterilization atmosphere during the sterilization process is substantially prevented. The inventive sterilization method includes providing a sterilization chamber and placing an article into the sterilization chamber. The sterilization chamber is sealed prior to equalizing the temperature of the article and the atmosphere in the sterilization chamber. A vacuum is applied to achieve a preselected vacuum pressure in the sterilization chamber. Once the vacuum pressure is set, water vapour is supplied to the sterilization chamber. Ozone-containing gas is then supplied to the sterilization chamber and the sterilization chamber remains sealed for a preselected treatment period, where the sterilization chamber remains sealed throughout the whole process. Finally, vacuum in the sterilization chamber is released.
Owner:STRYKER CORP
Sterilizable surgical instrument
ActiveUS20100193569A1Avoid damageReduce the possibilitySuture equipmentsStapling toolsElectronic componentHydrogen Peroxide Sterilization
A surgical instrument including a first portion and a second portion, wherein the second portion can be sterilized separately from the first portion. The first portion can comprise an anvil, a staple cartridge channel and / or staple cartridge, and a movable cutting member. The second portion can comprise electronic components configured to control the surgical instrument and / or record data collected during the use of the surgical instrument. The first portion can be sterilized using a gamma radiation sterilization process while the second portion can be sterilized using a different sterilization process, such as steam, ethylene oxide, ozone, and / or hydrogen peroxide sterilization processes, for example. As a result, the first and second portions can be sterilized separately and delivered in two separate containers. The second portion can be stored within a sealed bag and can include an electrical terminal which can penetrate the bag and communicate with the first portion.
Owner:CILAG GMBH INT
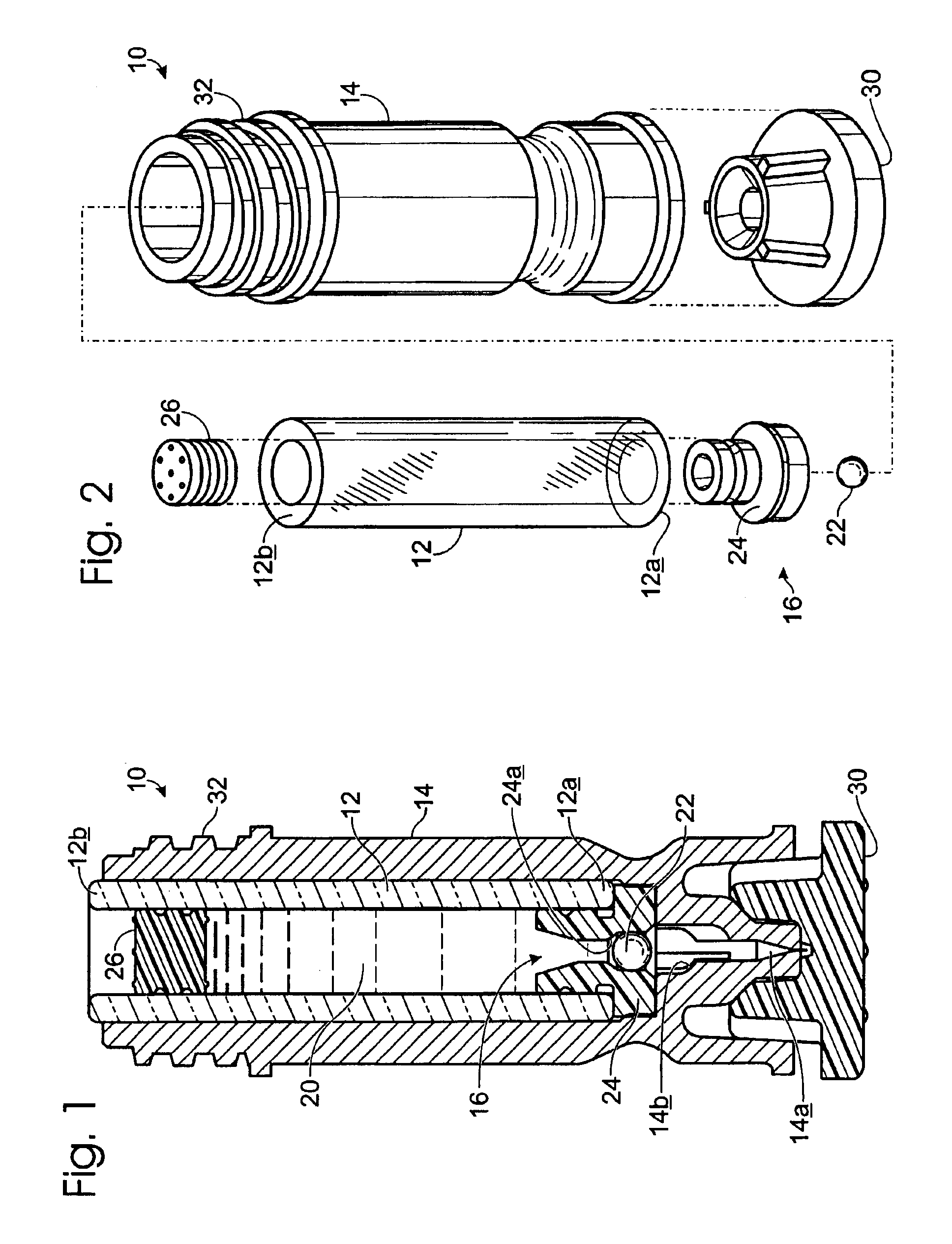
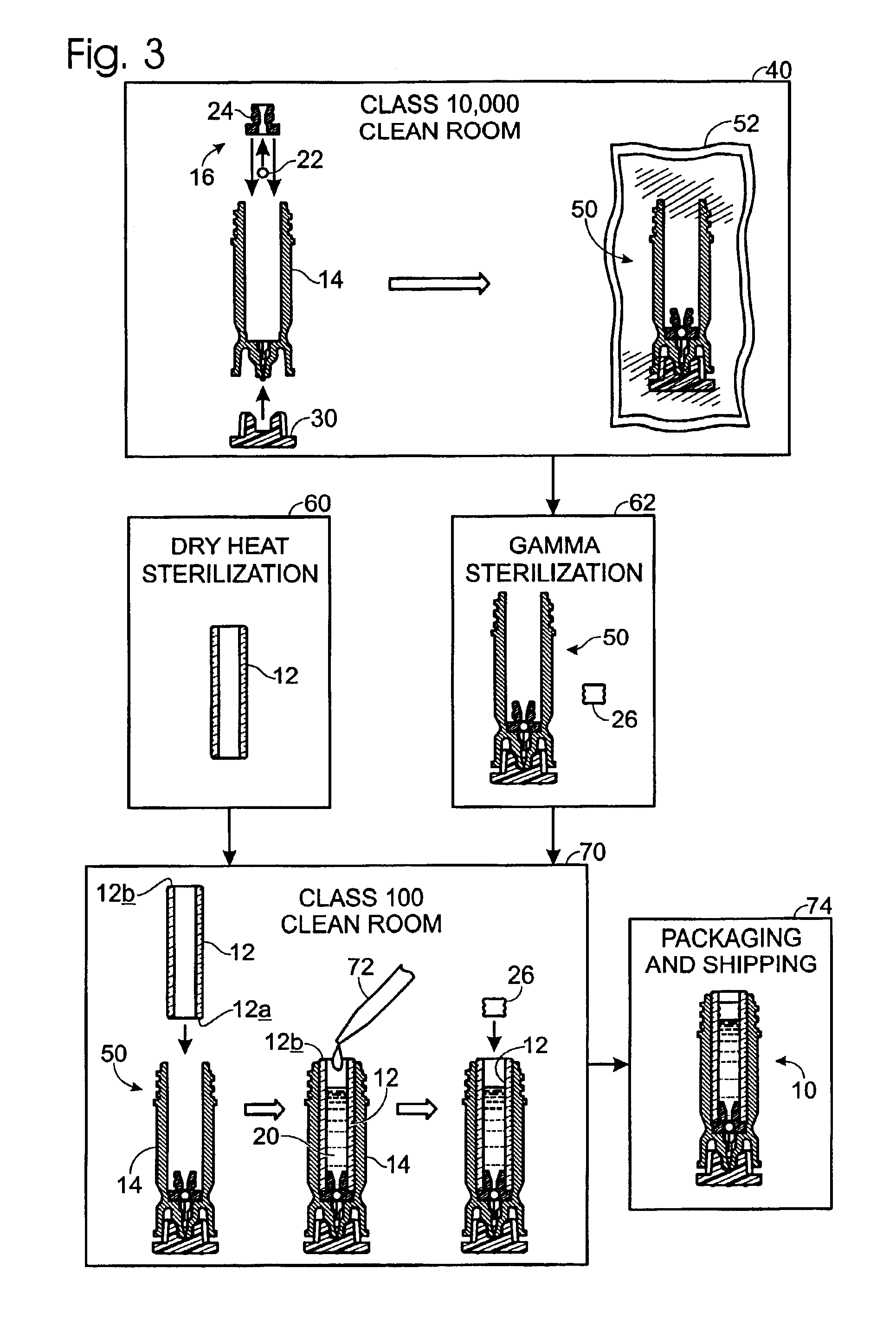
Drug cartridge assembly and method of manufacture
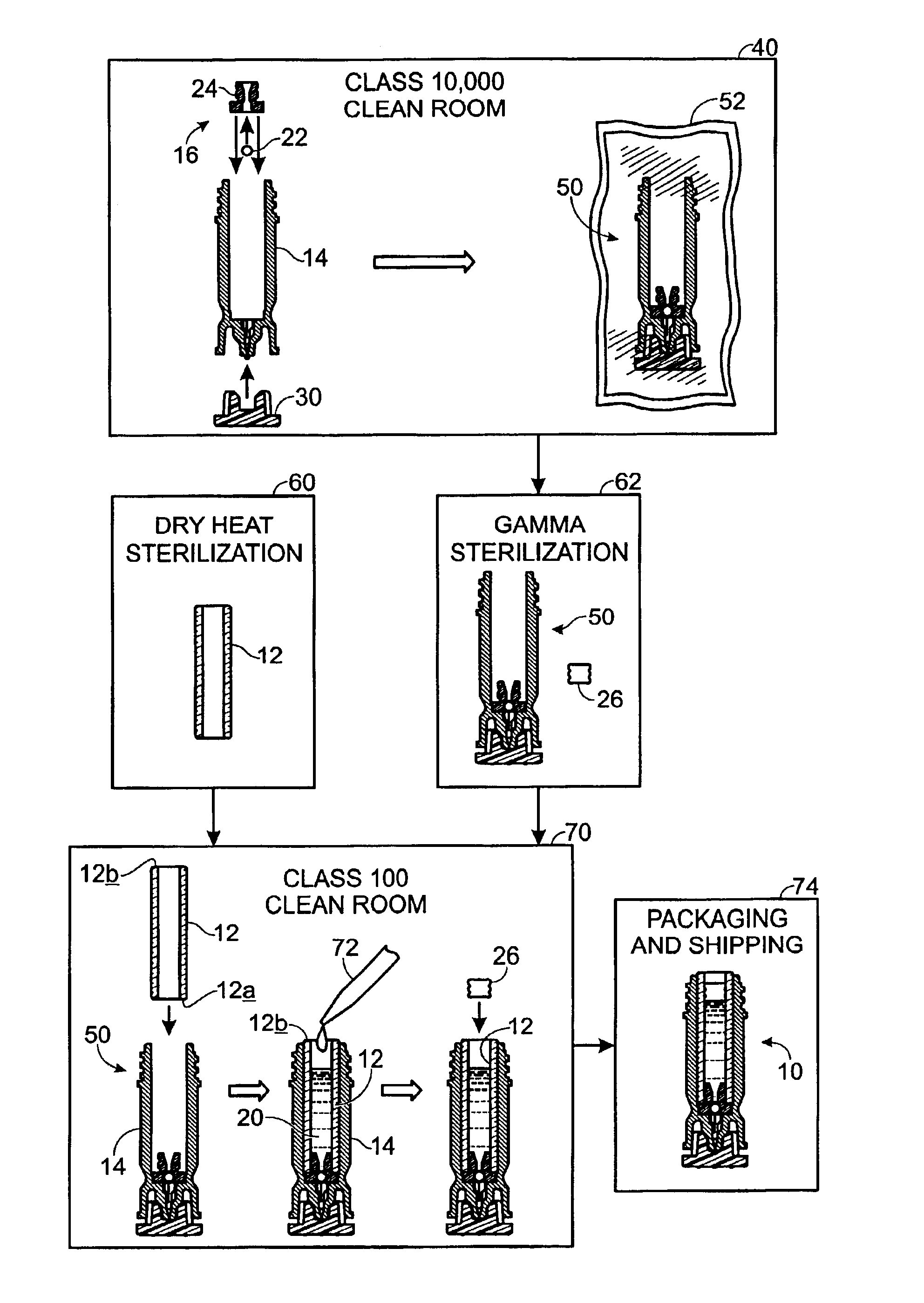
A method of manufacture for a drug cartridge assembly. The method includes providing a drug cartridge, providing a nozzle sub-assembly, and sterilizing the drug cartridge and nozzle sub-assembly. The method further includes assembling the drug cartridge and nozzle sub-assembly together in a configuration that enables ejection of liquid out of the drug cartridge through the nozzle sub-assembly. The method further includes filling the drug cartridge with a liquid, such as an injectable drug. The method may include separate sterilization of the drug cartridge and nozzle sub-assembly, using different sterilization processes. Portions of the method may be performed prior to sterilization within a first cleanroom, with subsequent steps being performed in a second cleanroom having a substantially lower particulate-per-volume rating than the first cleanroom.
Owner:BIOJECT
Process of reducing fouling during heat processing of foods and beverages
InactiveUS20060240159A1Reduce dirtFood preservationPackaging protectionMethyl cellulosePasteurization
A pasteurization or sterilization process reduces fouling of a food or beverage composition containing protein during the heat treatment. An antifouling agent is added to the food or beverage composition that is selected from hydroxypropylcellulose (HPC) with a hydroxypropyl molar substitution of greater than 3.0 and a weight average molecular weight (Mw) as measured by SEC of greater than 350,000 Dalton, methylhydroxypropylcellulose (MHPC) with a methoxyl content of greater than 17% and a hydroxypropyl content of greater than 3%, methylcellulose (MC) with a methoxyl content greater than 17% and a viscosity in water at ambient temperatures and a concentration of 2% of greater than 1,000 cps, or mixtures thereof, This food or beverage composition is then heated in a first heat exchanger at a temperature between 50 and 100° C. for a time of from about 2 seconds to 30 minutes for pasteurization or it is further heated to sterilization temperatures before being packaged out or further processed. The improvements of this process is that the heat exchangers are fouled at least 10% by weight less or run-time increased at least 10% as compared to when heat-treating a similar food or beverage composition without the antifouling agent.
Owner:HERCULES INC
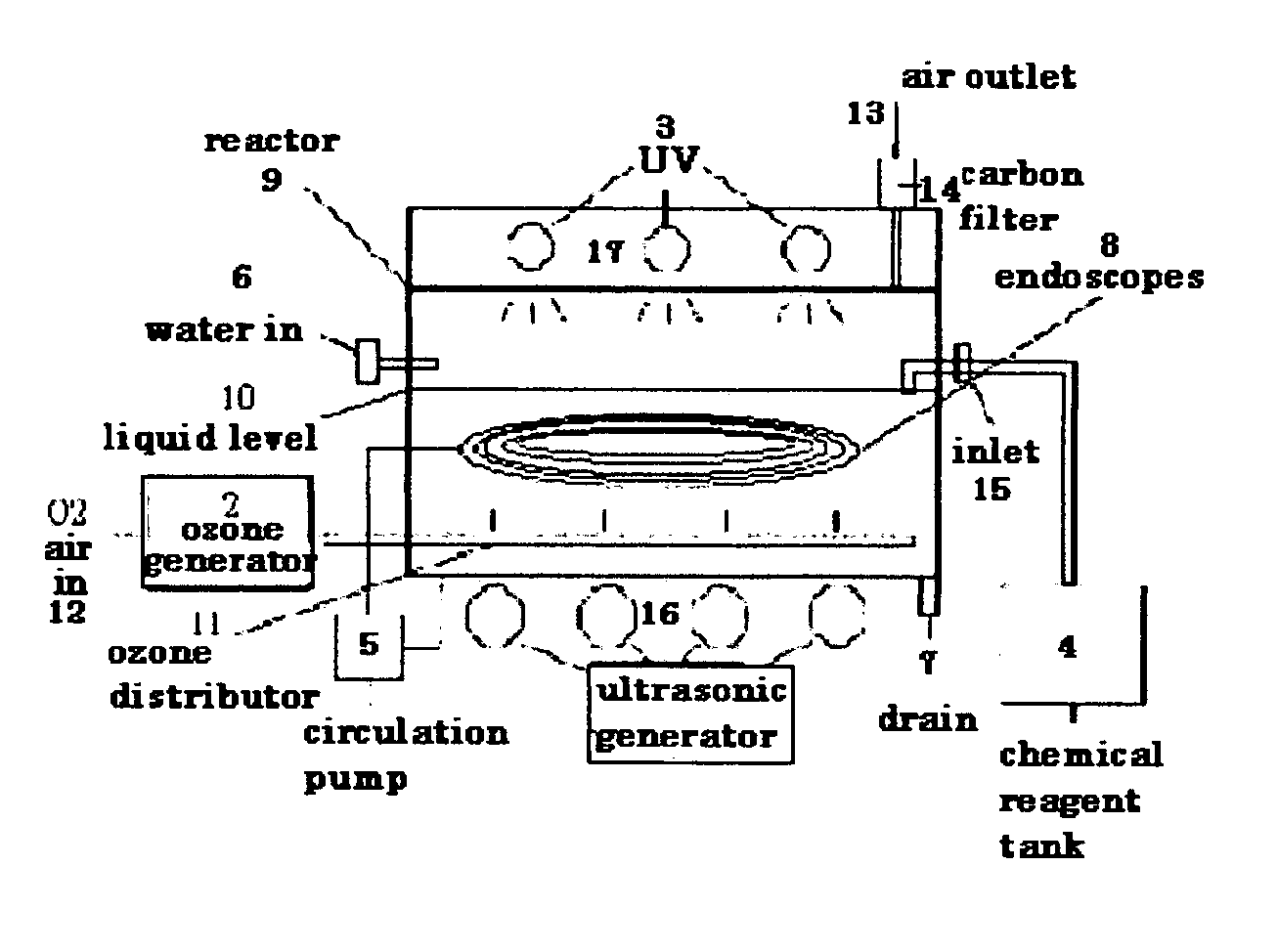
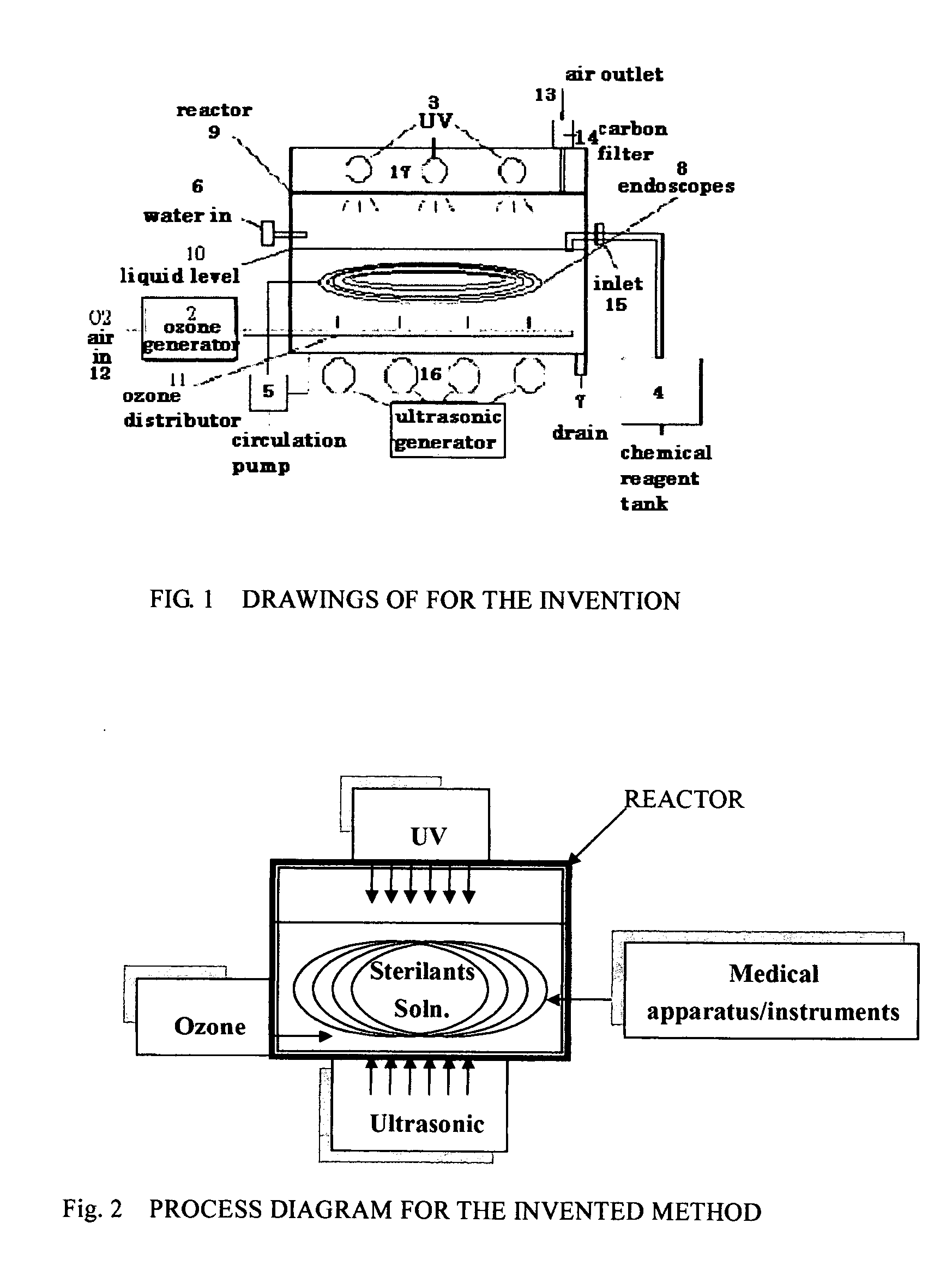
Low temperature sterilization and disinfections method and apparatus for medical apparatus and instruments
A method and apparatus for the low temperature sterilization and disinfections to be applied to medical apparatus / instruments. This invention perfects the use of ultrasonic, UV, ozone in a liquid bath with at least one chemical reagent which can be either sterilant or disinfectant solution for reprocessing many classes of medical equipment / instruments especially those which do not withstand high temperature sterilization process and require sterilization or high level disinfections. The invented method can effectively remove and destroy contaminates, particularly living organisms, bacterial spores, virus, and organics from used or contaminated medical apparatus / instruments surface, inner hollow lumen tube, joints and crevices. Having greatest immediate application for the cleaning, disinfecting and sterilizing all in one simple process for reprocessing used or contaminated medical apparatus / instruments after treating patients.
Owner:DING LAMBERT LISHING
Food product surface sterilization apparatus and method
InactiveUS20040052702A1Increase temperatureReduce food product temperature changeFood preservationIndirect heat exchangersUnsafe conditionUltraviolet
An apparatus is disclosed for the micro-organism surface sterilization of foods using, a "germicidal" such as light waves (e.g., ultraviolet), and in some cases in combination with (or replaced by) one or more of sound waves and ozone. The surface sterilizer apparatus may include a plurality of germicidal (e.g., ultraviolet) emitters for surface sterilization of foods that are, e.g., rotated in a drum or rotated via a screw auger. Assemblies of emitters for the germicidal may be constructed to be watertight (i.e., withstand a high pressure, heated water spray), and movable relative to the drum or screw conveyor for easy cleaning and maintenance. The apparatus may also include a controller (e.g., programmable logic controller) for controlling the sterilization process so that the apparatus does not endanger personnel nearby, and so that the food is properly sterilized. The controller may vary the amount of germicidal used, the rate that food traverses the apparatus, the inclination of the apparatus, and terminate sterilization processing when an unsafe condition is detected. The apparatus may be used in-line with other food processing equipment for the real-time sterilization of food. The controller may also communicate with food processing components upstream of the apparatus for controlling the flow of food to the surface sterilizer
Owner:C & S EQUIP COMPANY
Sterilization method and apparatus
ActiveUS20110076192A1Reduce disadvantagesReduce humiditySamplingSupporting meansDecompositionWater vapor
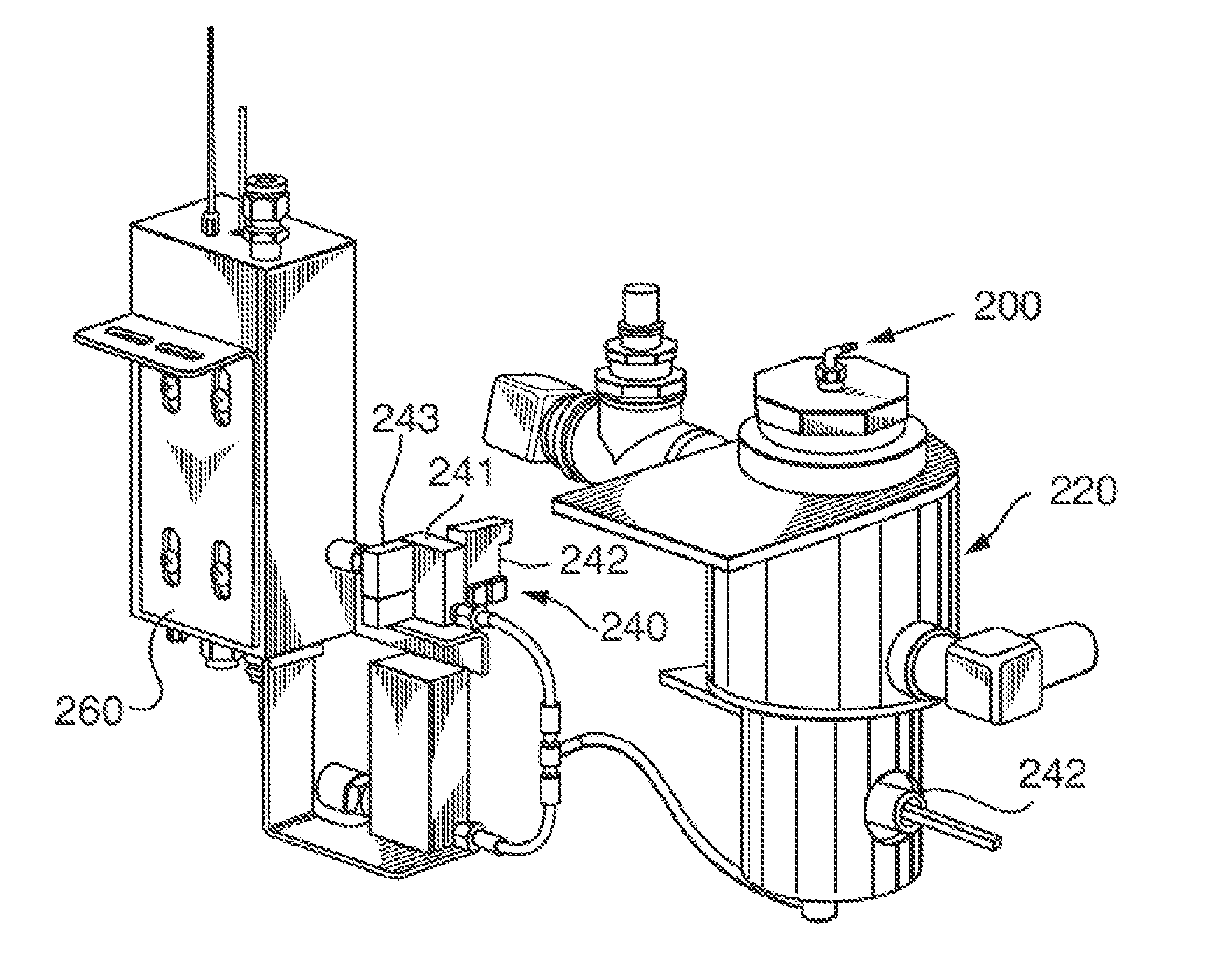
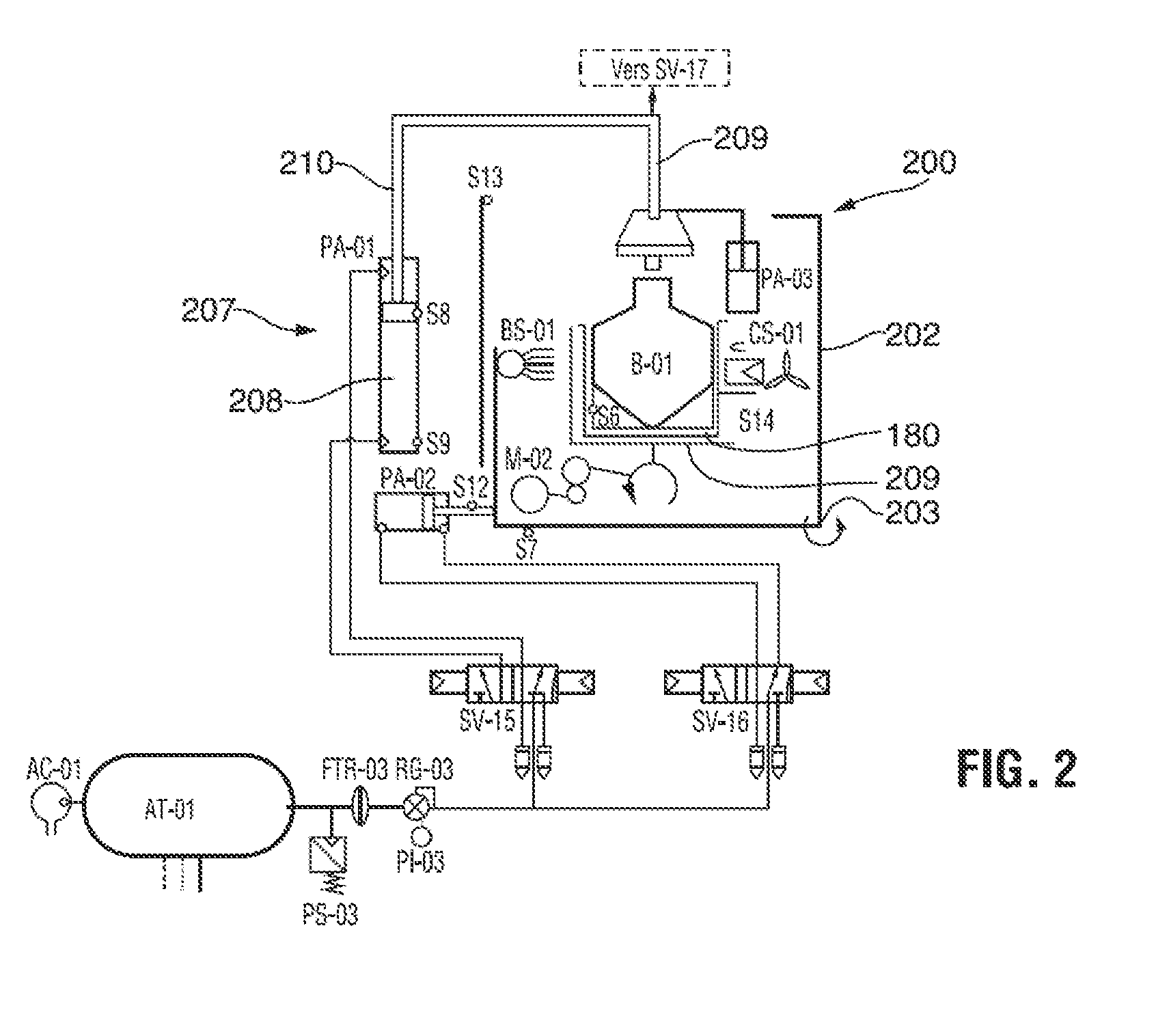
A method of sterilizing an article by sequentially exposing the article to hydrogen peroxide and ozone is disclosed. The article is exposed under vacuum first to an evaporated aqueous solution of hydrogen peroxide and subsequently to an ozone containing gas. The exposure is carried out without reducing the water vapor content of the sterilization atmosphere, the water vapor content being derived from the aqueous solvent of the hydrogen peroxide solution and from the decomposition of the hydrogen peroxide into water and oxygen. The complete sterilization process is carried out while the chamber remains sealed and without removal of any component of the sterilization atmosphere. For this purpose, the chamber is initially evacuated to a first vacuum pressure sufficient to cause evaporation of the aqueous hydrogen peroxide at the temperature of the chamber atmosphere. The chamber is then sealed for the remainder of the sterilization process and during all sterilant injection cycles. Keeping the chamber sealed and maintaining the hydrogen peroxide and its decomposition products in the chamber for the subsequent ozone sterilization step results in a synergistic increase in the sterilization efficiency and allows for the use of much lower sterilant amounts and sterilization cycle times than would be expected from using hydrogen peroxide and ozone in combination.
Owner:STRYKER CORP
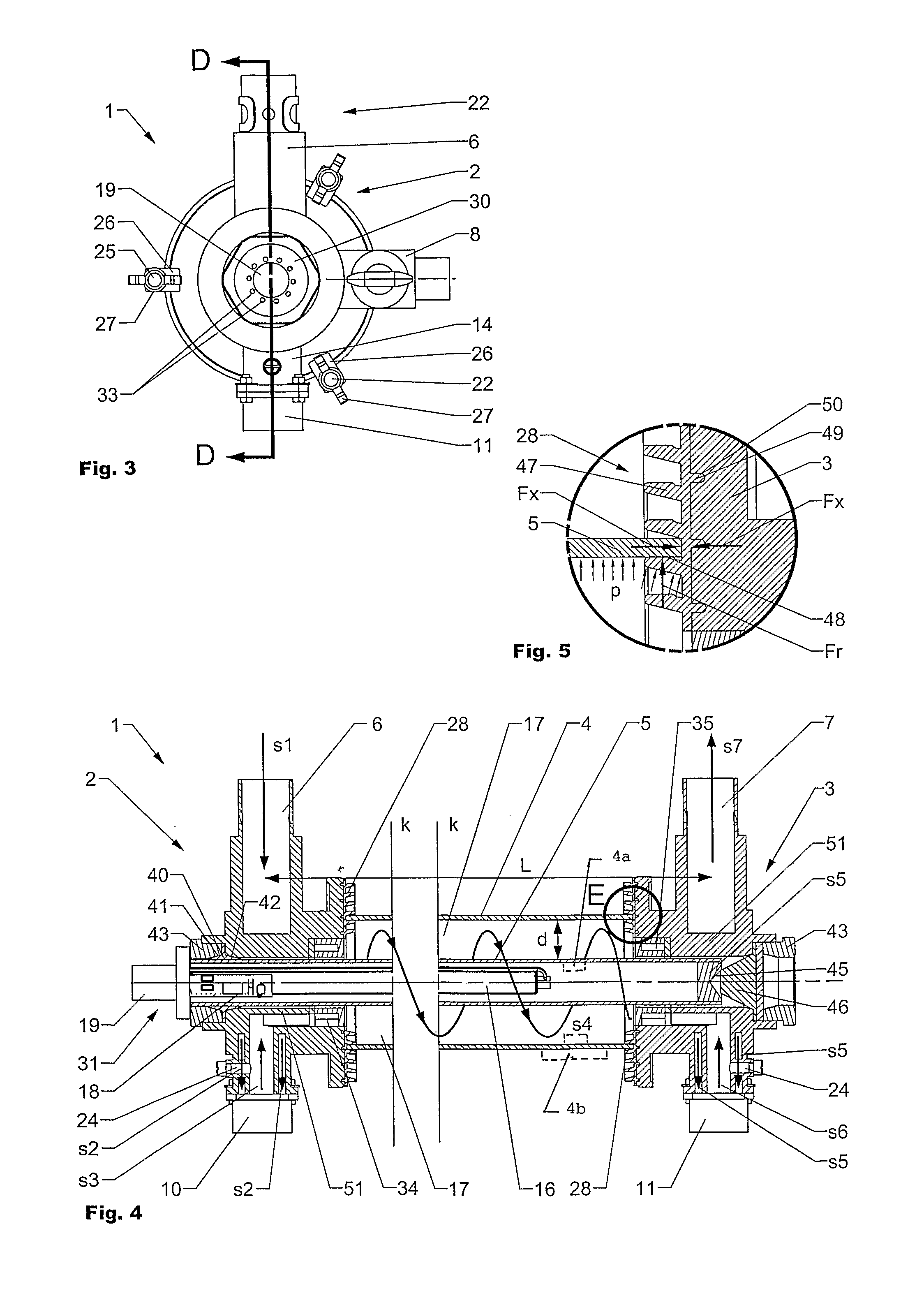
Sterilizing Device And A Method For Sterilizing Of Fluids
InactiveUS20080095661A1Low costReduce operating costsSamplingWater/sewage treatment by irradiationUltraviolet lightsControl cell
The invention is directed to an improved sterilizing device (1) for sterilizing of a fluid by UV-radiation. The sterilizing device (1) has a modular setup with at least one flange (2, 3), an inner and an outer tube (4, 5) and comprises at least one UV-lamp (16) for emitting UV-radiation. A lamp tag (32) attached to or incorporated in the at least one UV-lamp (16) comprises information regarding the UV-lamp (16). The lamp tag (32) is interconnected with a lamp sensor unit (18) and / or a control unit (19) and may comprise sensors to control the sterilizing process.
Owner:KOHLER CO
Sterilization container capable of providing an indication regarding whether or not surgical instruments sterilized in the container were properly sterilized
A sterilization container for sterilizing at least one surgical instrument. The container includes at least one sensor for measuring an environmental characteristic of the container during the sterilization of the instrument. The measure of the environmental characteristic is supplied to a processor. The processor compares the measurement of the container environment to a validated measurement for the sterilization process. If the measured environmental characteristic is at least equal to the validated sterilization process measurement, the processor presents an indication that the surgical instruments was properly sterilized.
Owner:STRYKER CORP
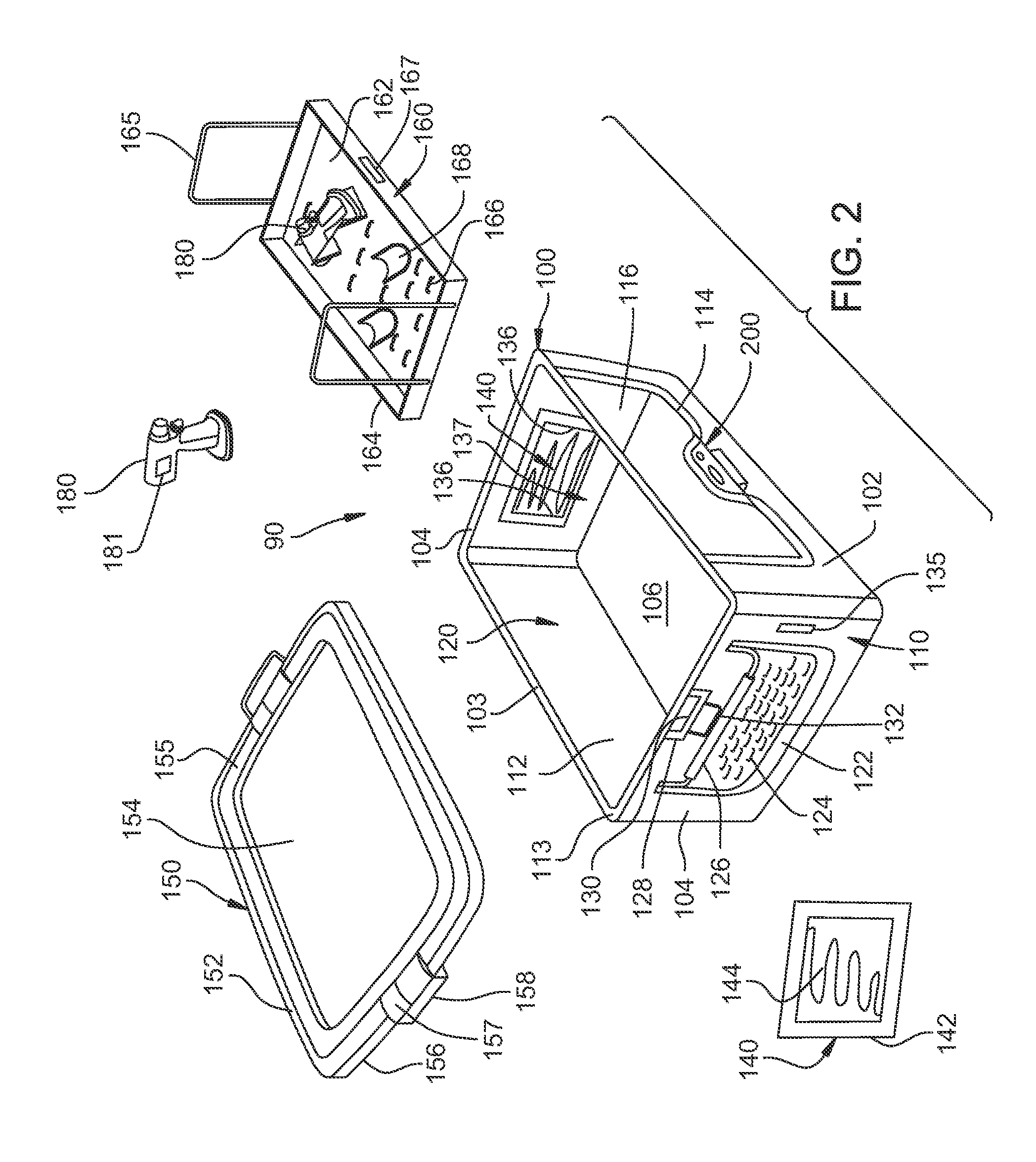
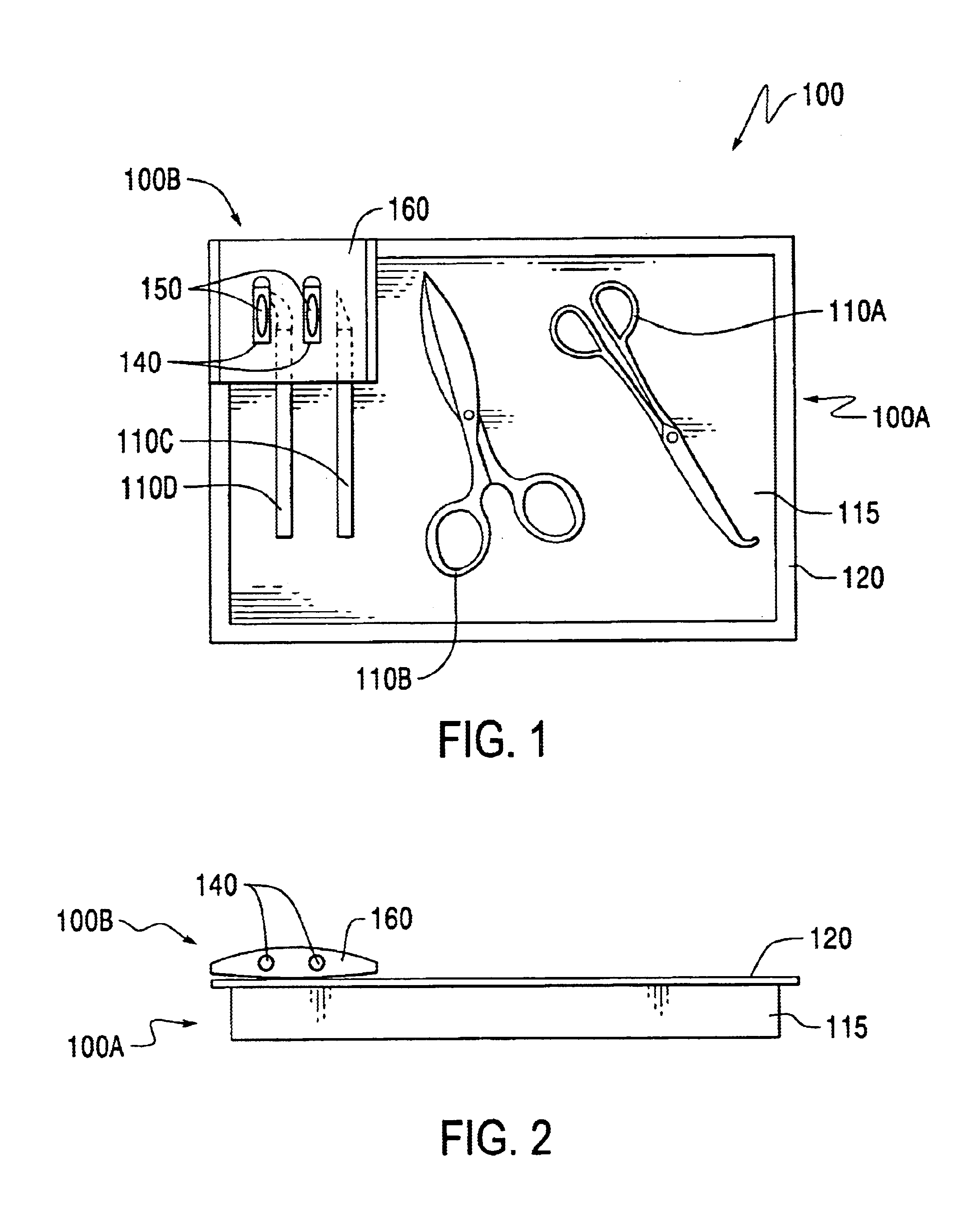
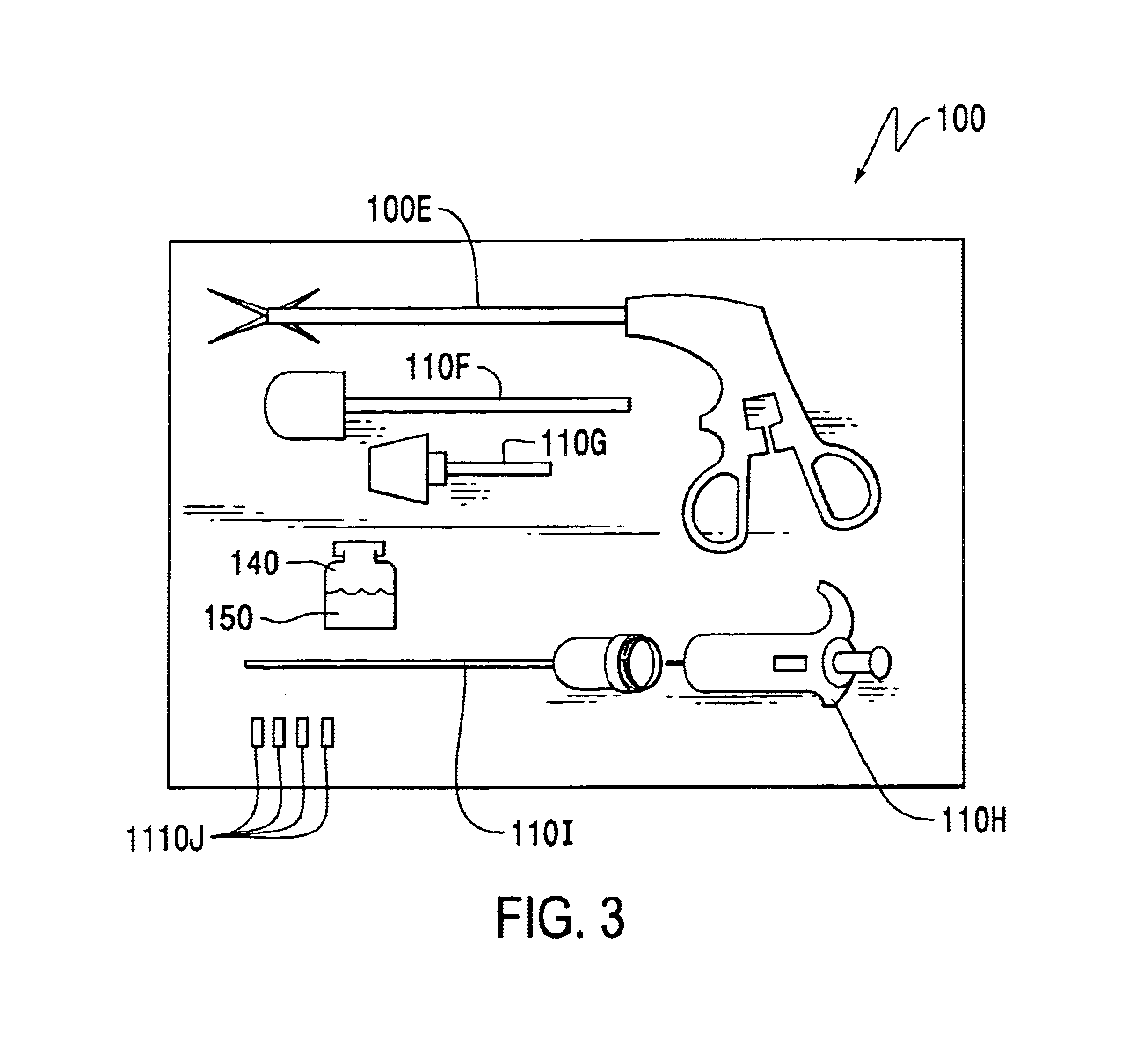
Method of sterilizing a medical procedure kit containing a medical adhesive
InactiveUS6837027B2Avoid degradationInhibition of polymerizationSurgical furnitureDiagnosticsSurgical operationSurgical department
A medical procedure kit incorporates one or more surgical tools necessary to perform at least part of a medical procedure, along with a container having a quantity of a medical adhesive. Medical adhesives are useful as an alternate or an adjunct to surgical sutures and / or staples in wound closure, as well as for covering and protecting surface wounds such as lacerations, abrasions, burns, stomatitis, sores, minor cuts and scrapes, and other wounds. As such, the inclusion of such an adhesive into a composite medical procedure kit can be of great assistance to a medical doctor or surgeon by providing in single kit form, tools necessary to complete many medical procedures, without having to use multiple procedure kits. The adhesive may be independently sterilized and wrapped from the other surgical tools of the resultant kit, and then associated into kit form, or assembled and sterilized together as a unitary kit. When the adhesive is incompatible with sterilizing procedures of the other components of the kit, the adhesive may be pre-sterilized and protected by a sterilization barrier that shields the adhesive from exposure to the sterilization process used to sterilize other tools of the procedure kit. The adhesive is preferably a 1,1 -disubstituted ethylene monomer, such as an alpha-cyanoacrylate.
Owner:CLOSURE MEDICAL
Automated biological indicator incubator
InactiveUS20060263258A1Analysis using chemical indicatorsHeating or cooling apparatusCommunication interfaceTemperature control
A system and method of automated incubating and reading of biological indicators is disclosed. In one embodiment, the automated biological indicator incubator is configured to control the temperature of incubation test wells to a desired temperature range suitable for use with a biological indicator, control the incubation period, and detect a change in the biological indicator colored media providing an indication of growth or lack of growth. In other embodiments, the automated biological indicator incubator is self-calibrating, and provides a communication interface to an external device, such as a computer. The communication interface is suitable for use to collect and analyze data associated with the biological indicator during the incubator period, and for making a determination of growth or lack of growth and success of the sterilization process as well as a permanent document of the monitoring of the sterilization process.
Owner:HARRIS MATTHEW +3
Insulating Boot for Electrosurgical Forceps
ActiveUS20110106079A1Reduces the potential for strayDiagnosticsSurgical instruments for heatingEthylene oxideEngineering
Owner:COVIDIEN AG
Endoscope suitable for autoclave sterilization
An endoscope suitable for autoclave sterilization includes an insertion section having an objective lens section disposed at the extreme end thereof for focusing a subject image and an eyepiece section located on the base end side of the inserting section and including at least an eyepiece lens. an image guide fiber is disposed in the bundle for transmitting the subject image in an observation section focused by the objective lens section. An eyepiece lens unit is disposed in the eyepiece section, the eyepiece lens unit causing the eyepiece lens to confront the base end structure whose hermetic seal level is higher than the watertight seal level of the shell of the endoscope. A focus position changing means is disposed to the eyepiece lens unit to change the focus position of the eyepiece lens. The eyepiece section is so constructed and / or assembled that the autoclave sterilization process does not cause water vapor or the like to adversely affect the operability of the eyepiece section.
Owner:OLYMPUS CORP
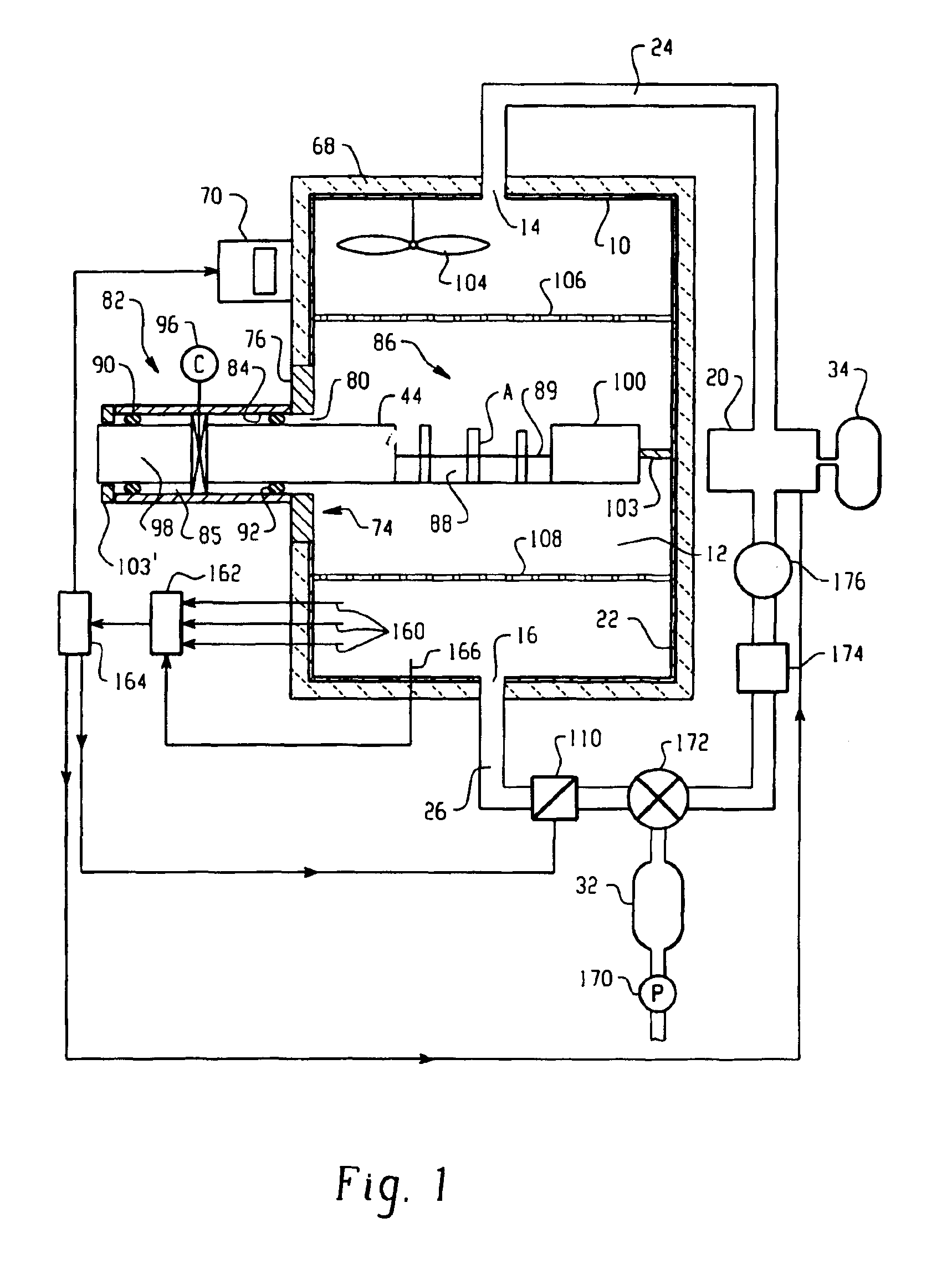
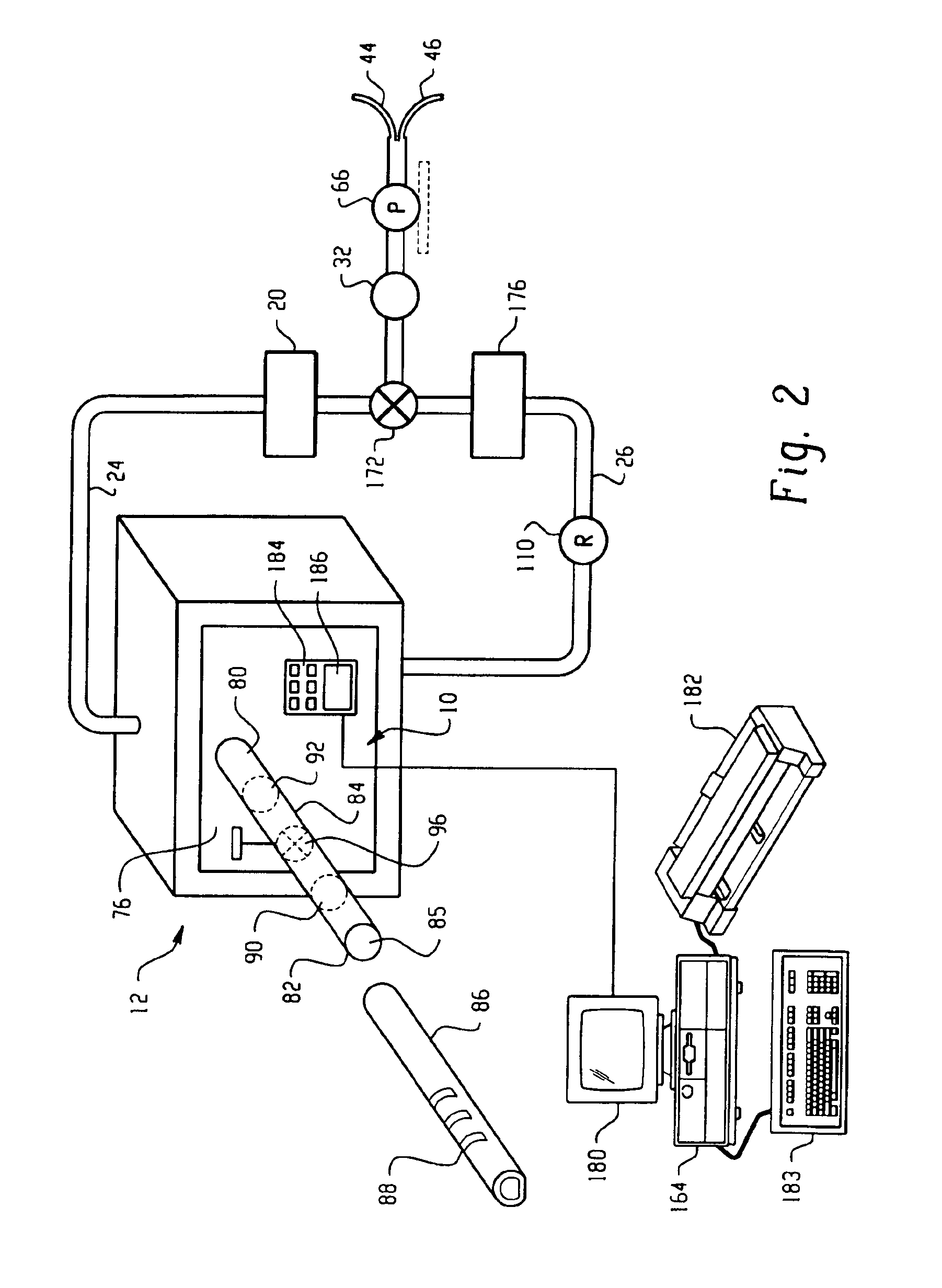
Method and apparatus for validation of sterilization process
InactiveUS20050135965A1Analysis using chemical indicatorsMaterial analysis using wave/particle radiationDosimeterEngineering
An apparatus, system and method for verifying the achievement of a desired sterility assurance level (SAL) for components manipulated within a low-energy electron beam sterilization chamber. The components are preferably pre-sterilized and connected together in an assembly fashion which creates and maintains the sterility of the connection by subjecting the components to low-energy (less than 300 KeV) electron beam radiation. The verification is completed by measuring the sterilization dose delivered to a sensor, also known as a dosimeter, positioned within the sterilization process to simulate the components.
Owner:BAXTER INT INC
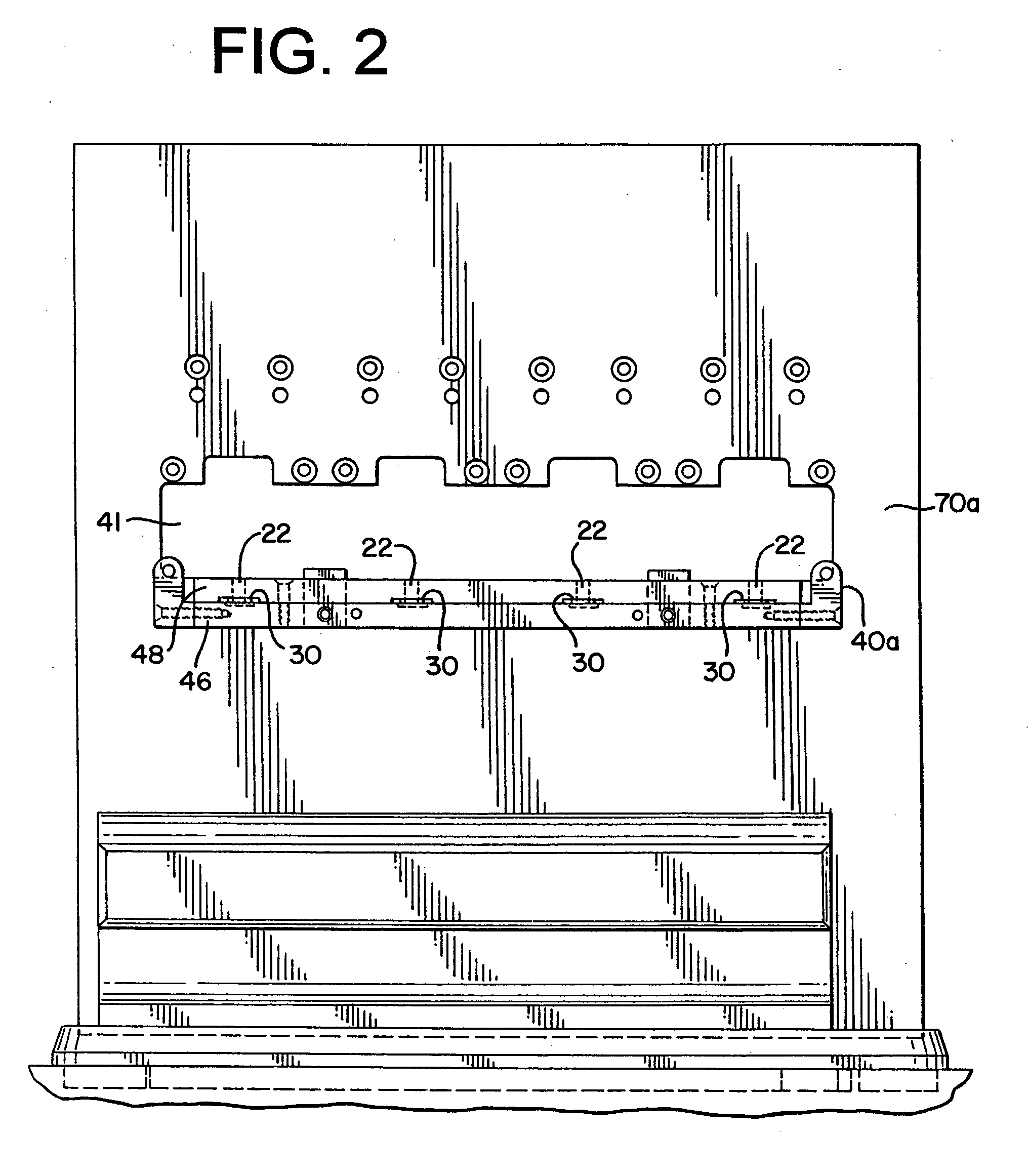
Absorbent instrument trayliner for sterilization process and method of sterilizing surgical instruments
InactiveUS6440375B1Potential damageSterilizationSurgical furnitureDispensing apparatusDry weightEthylene oxide
The present invention is directed to a super-absorbent trayliner for use in a sterilization process and, more particularly, to a super-absorbent trayliner for cushioning surgical instruments and providing an advantageous moisture absorption functionality during and after completion of a sterilization process. The super-absorbent trayliner functions advantageously with steam or ethylene oxide gas as the sterilization agent. The super-absorbent trayliner is fabricated from a material having a desired level of moisture absorption, e.g., on the order of at least about thirty percent (30%) by dry weight, and is preferably fabricated from a hydrophilic polymeric foam material, e.g., a hydrophilic polyurethane foam. The disclosed super-absorbent trayliner may be advantageously utilized in sterilizing surgical instruments such that potential residual moisture is eliminated from the surface of the sterilized surgical instruments.
Owner:GENERAL HOSPITAL SUPPLY CORP
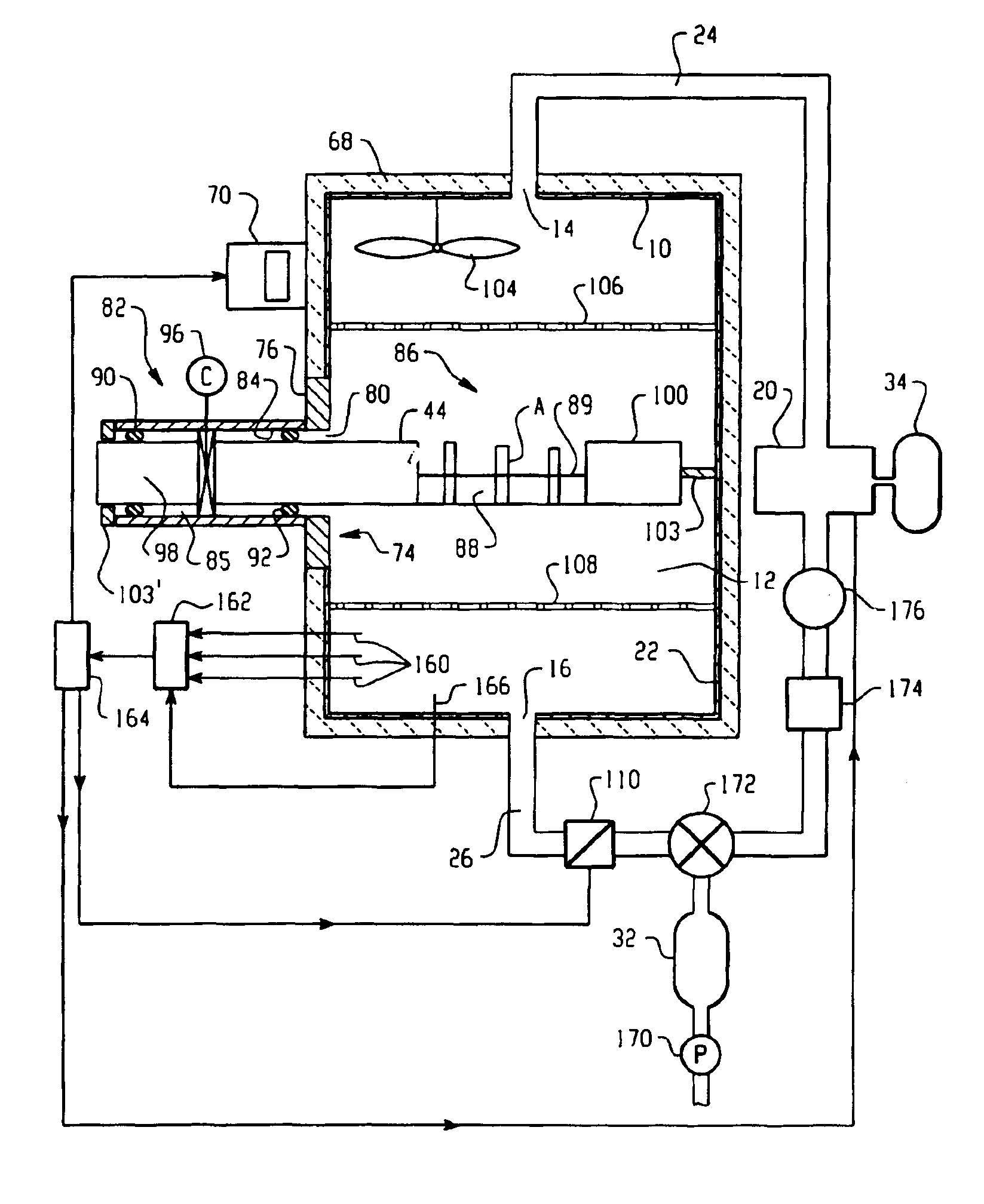
Vapor phase decontamination process biological indicator evaluator resistomer (BIER) vessel
InactiveUS6936434B2Evenly distributedApparent advantageBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringVapor phase
A BIER vessel evaluates biological indicators for sterilization processes. By flowing gaseous sterilant, such as vaporized hydrogen peroxide, through a chamber (12) before, during, and after introducing the indicators, the indicators are instantaneously exposed to preselected steady state conditions, allowing accurate and reproducible evaluation of the indicator response. A door (32) to an opening (30) in the chamber opens for introducing the indicators to the chamber without appreciably disturbing the steady state conditions therein. After a preselected time, the biological indicators are removed and evaluated for remaining biological activity.
Owner:AMERICAN STERILIZER CO
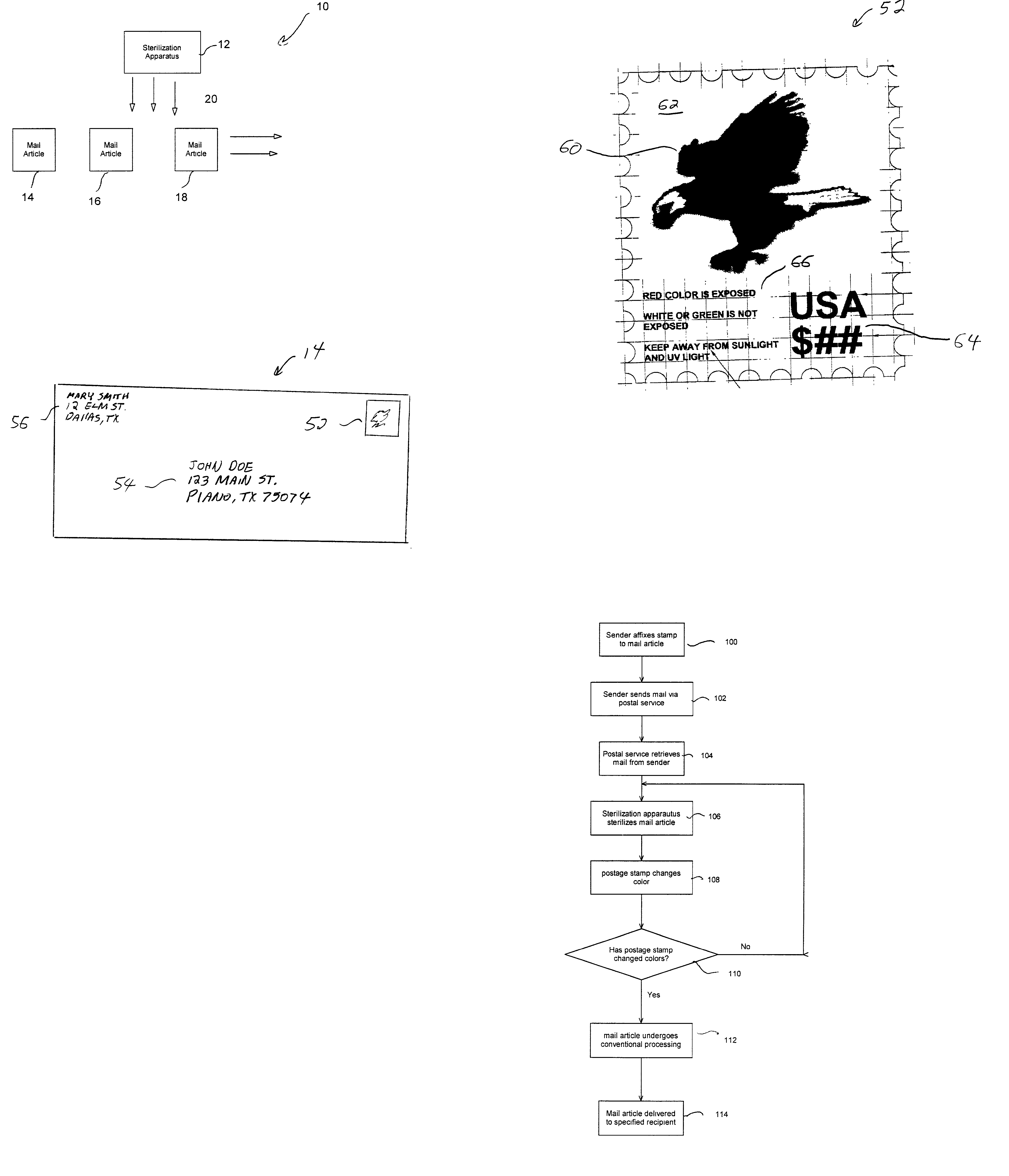
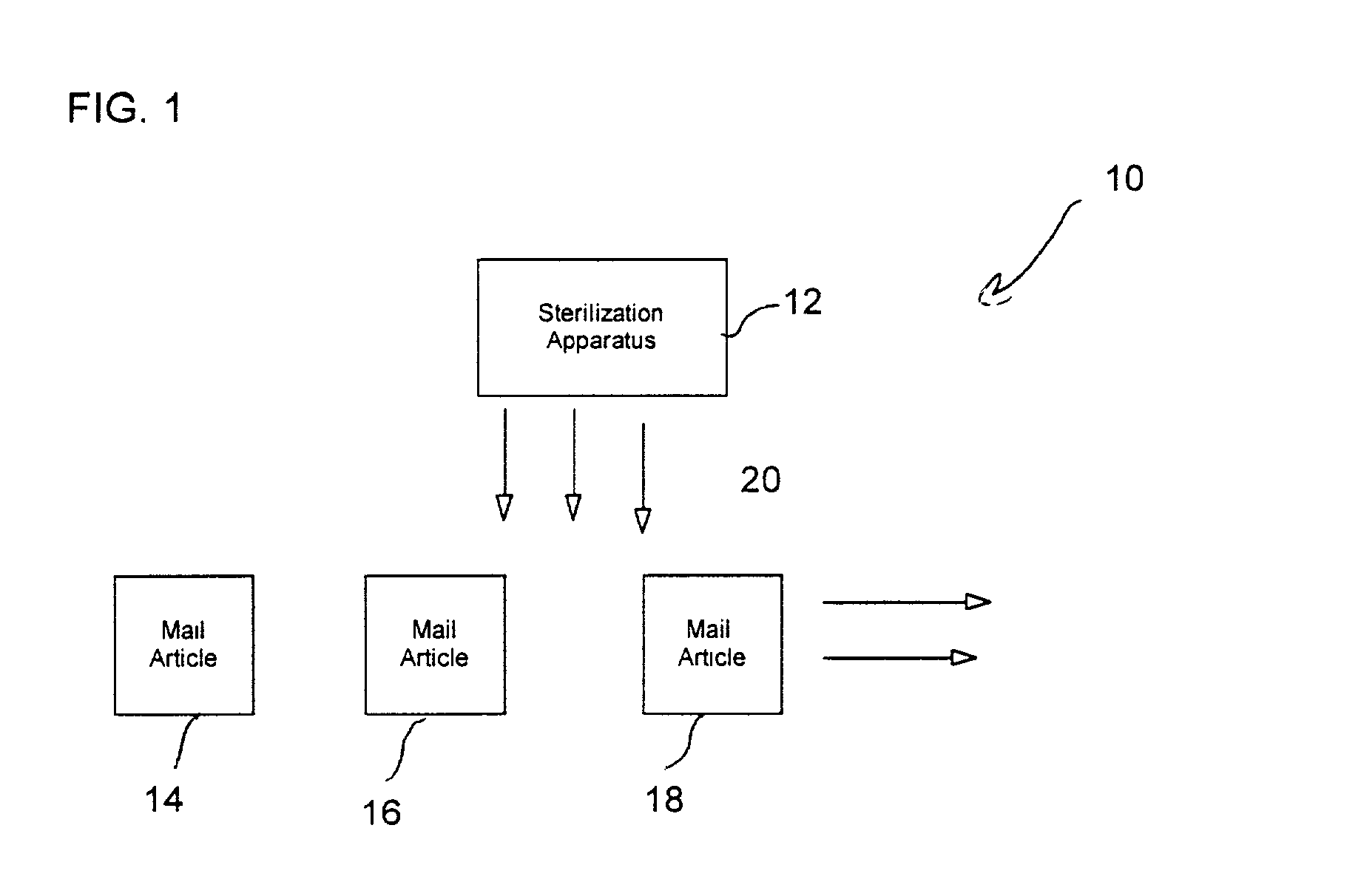
Postage stamp indicating completion of sterilization process
InactiveUS20030138345A1Withdrawing sample devicesPreparing sample for investigationSuccessful completionPostal service
A postage stamp providing an indication of successful completion of a sterilization process. The postage stamp is affixed to a mail article. The mail article is then collected by the postal service for processing. The mail article is subjected to sterilization by a sterilization apparatus to sterilize the mail article. Upon being successfully sterilized, the postage stamp turns color to indicate completion of the sterilization process.
Owner:SCHWABE NIKOLAUS Z
Integrated washing and sterilization process
A method for cleaning and sterilizing a medical device comprises the steps of placing the device into a container, contacting the device with a cleaning solution, contacting the device with a liquid sterilant and lowering pressure in the container to vaporize the liquid sterilant in the container, thereby simultaneously completing sterilization of the device and drying the device. The washing and sterilization processes can proceed simultaneously.
Owner:ETHICON INC
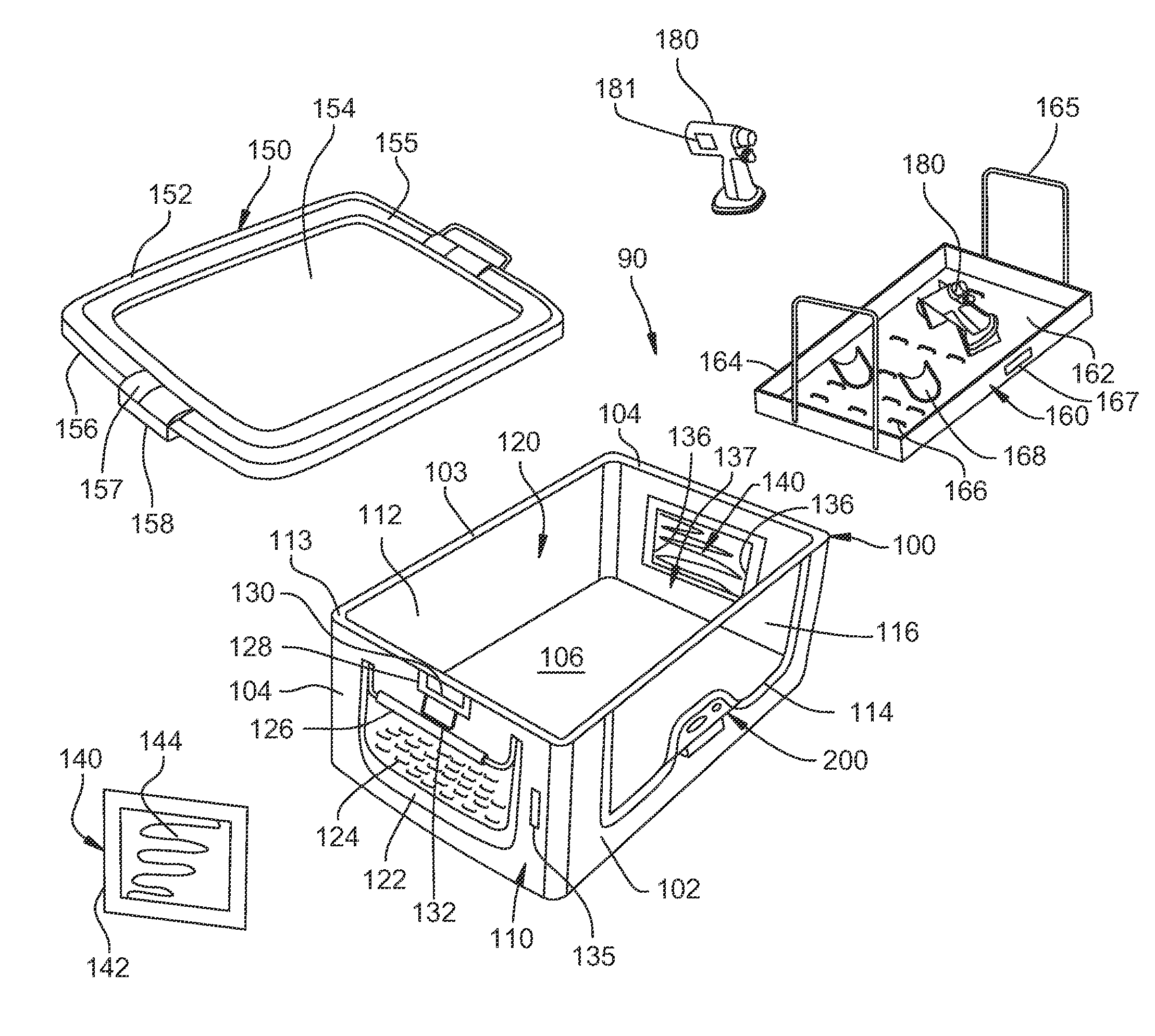
Sealed medical device enclosure
ActiveUS7694814B1Prevent illegal useRemoval and insertion can be facilitatedUltrasonic/sonic/infrasonic diagnosticsSurgical furnitureProtection sexEngineering
Systems and methods providing an enclosure for protecting portions of a medical device are shown. Embodiments individually protect multiple portions of a medical device during sterilization. Protective enclosures of embodiments provide a minimized size adapted to provide protection to portions of a medical device susceptible to damage by a sterilization process. Enclosures of embodiments provide protection by preventing unauthorized use of the medical device being protected.
Owner:FUJIFILM SONOSITE
Supercritical carbonic anhydride wall-breaking method for melissa powder
ActiveCN101268815ARemove the effect of active ingredientsImprove permeabilityFood shapingFood preparationExhaust valveAdditive ingredient
The invention discloses a method using supercritical carbon dioxide to perform bee pollen broken-face. Bee pollen is put in an extraction plant of supercritical carbon dioxide, to react for a certain time in supercritical carbon dioxide liquid at the proper pressure and temperature, a vent valve is opened to rapidly empty carbon dioxide, so that a pollen cell expands acutely due to larger pressure difference of the inside and the outside, thereby cracking. The method has the advantages that the operation is simple and convenient, the cost is low, the whole process is performed below 50 DEG C, the broken-face time does not exceed 30 minutes, the influence of the product by thermotropy is reduced, so that the active ingredient is preserved completely, at the same time, bacillus can be killed, the unification of the broken-face and the sterilization processes is realized, the broken-face ratio of the pollen cell is high, but the percentage of damage is low, and the inclusion still exists in a cracked pollen wall under the dry condition, thereby favoring the preservation of the nutritious content.
Owner:陕西康泰莱生物医药工程有限公司
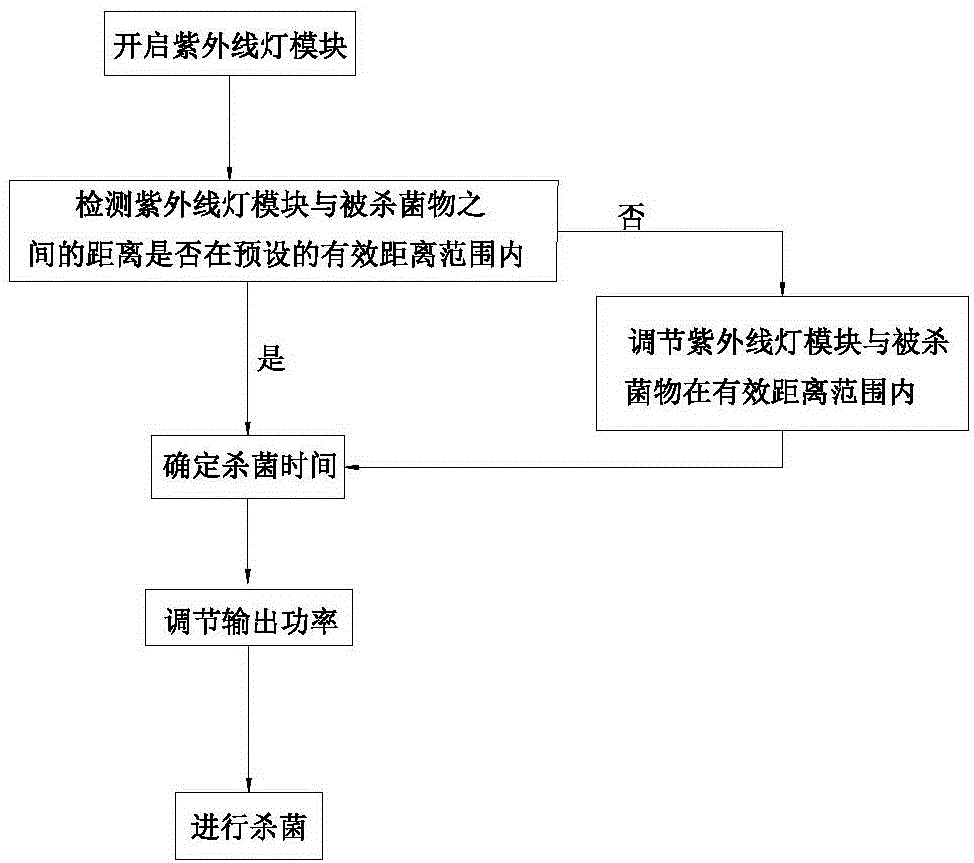
Mobile terminal with ultraviolet sterilization function and controlling method thereof
ActiveCN105159159AImprove securityEffective sterilizationProgramme control in sequence/logic controllersRadiationComputer moduleUltraviolet
The invention discloses a mobile terminal with an ultraviolet sterilization function and a controlling method thereof. The mobile terminal comprises a mobile terminal body, an ultraviolet lamp module and a controller. According to the controlling method, when the ultraviolet lamp module is turned on, the controller of the mobile terminal determines whether the distance between the ultraviolet lamp module and a sterilized object is within a preset effective distance range, if not, the distance between the ultraviolet lamp module and the sterilized object is adjusted to be within the preset effective distance range, and when the distance between the ultraviolet lamp module and the sterilized object is within the preset effective distance range, the output power of the ultraviolet lamp module is adjusted according to the distance between the ultraviolet lamp module and the sterilized object. The method provided by the invention solves the problems that the distance between an ultraviolet sterilization lamp and the sterilized object cannot be adjusted in an existing sterilization process, invalid sterilization occurs in the sterilization process of the ultraviolet sterilization lamp, and the safety is not ensured.
Owner:HONGLI ZHIHUI GRP CO LTD
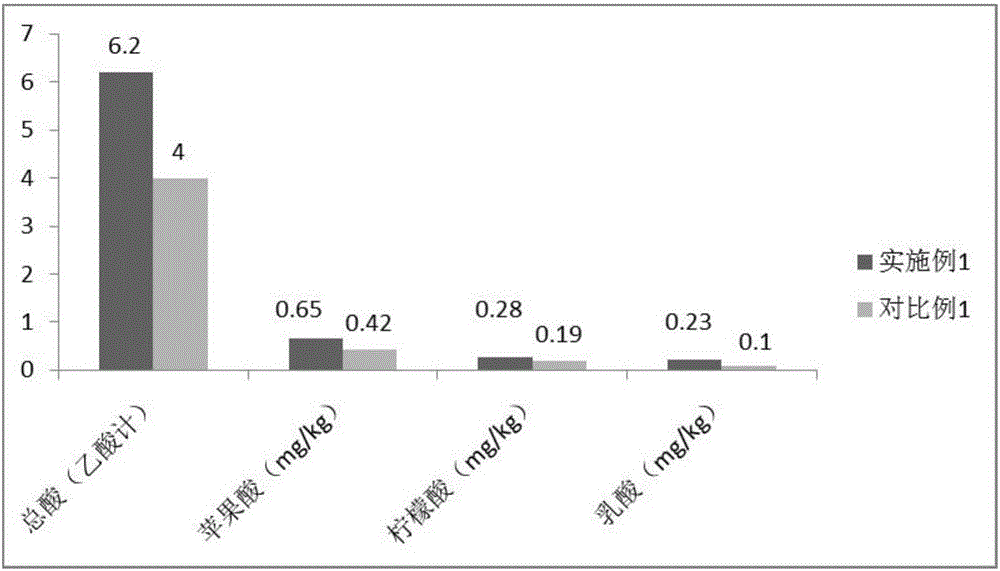
Preparation method for plant/fruit/vegetable enzymes
InactiveCN106343547AExtract comprehensiveShorten the production cycleFood ingredient functionsFlavorEnzymatic hydrolysis
The invention discloses a preparation method for plant / fruit / vegetable enzymes. The method mainly comprises the following steps: (1) preparing plant / fruit / vegetable juice; (2) carrying out enzymatic hydrolysis; (3) carrying out first-time fermentation; (4) carrying out second-time fermentation; (5) carrying out dipping. According to the preparation method provided by the invention, the fermentation cycle is short, and fermented raw materials can be decomposed thoroughly, so that the content of substances related to flavor and taste in the plant / fruit / vegetable enzymes is increased, the content of nutrients is higher, and the absorption is easier; through repeated sterilization processes, the stability of the plant / fruit / vegetable enzymes is guaranteed.
Owner:NINGBO XINUOYA MARINE BIOTECH CO LTD
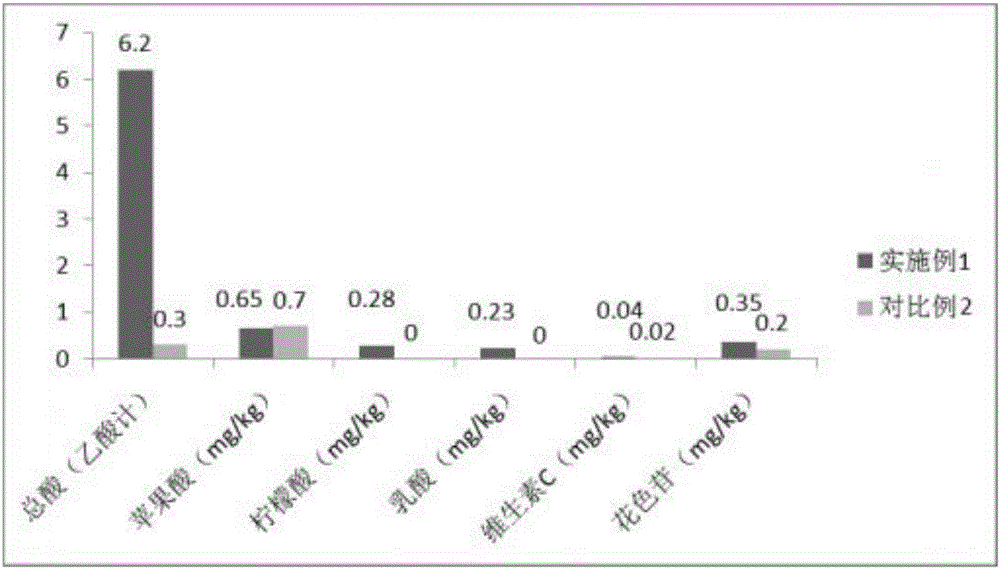
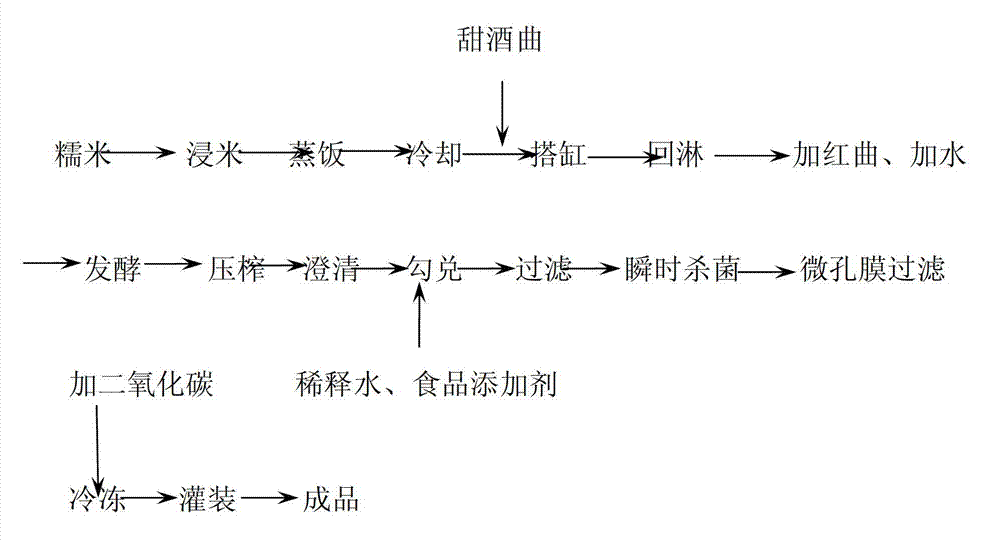
Method for preparing low-degree distilled red kojic rice liquor
The invention provides a method for preparing low-degree distilled red kojic rice liquor. The liquor is prepared by selecting materials, immersing and washing rice, cooking, cooling, filling a jar, culturing grains, fermenting, squeezing and settling, preparing, filtering, instantly sterilizing, crystallizing and adding carbon dioxide, filling and a series of processes, wherein the step of selecting materials is to select milled round grain glutinous rice in the present year as a brewing raw material, and the rice in the freshness detection is required to be grass green; monascus with high-diastatic power and high tone is selected to be combined with sweet distiller yeast with high sugar yield and rich ester yield to brew blending base liquor, so that the problems of light taste and single flavor after the product is diluted can be solved; the material-water ratio in thick mash fermentation is 1:0.5, so that the sugar, acid and alcohol contents in the fermenting process are in a controllable state; and due to the application of 30-second instant sterilization process at 90 to 92 DEG C and low-temperature freezing process, the fresh and strong taste of the low-degree distilled red kojic rice liquor prepared by the invention can be effectively guaranteed.
Owner:ZHEJIANG HONGSHILIANG GRP JIGONGJIA WINERY CO LTD
Ultrasound medical stent coating method and device
InactiveUS20070031611A1Improve adhesionImprove propertiesSurgeryPharmaceutical containersMedicineInsertion stent
An ultrasound apparatus and technique produces precise and uniform coatings on various substrates such as stents or other medical devices. The apparatus and technique increases adhesiveness of the surface of the stent or other medical device. In addition, the coating, drying, sterilization processes take place concurrently. The apparatuses generate and deliver targeted, gentle, and highly controllable dispensation of continuous liquid spray. The ultrasound coating apparatuses and techniques provide an instant on-off coating process with no atmospheric therpeutic agent contamination, no “webbing,” no “stringing” or other surface coating anomalies. Furthermore, the technology reduces wastage of expensive pharmaceuticals or other expensive coating materials.
Owner:BABAEV EILAZ P
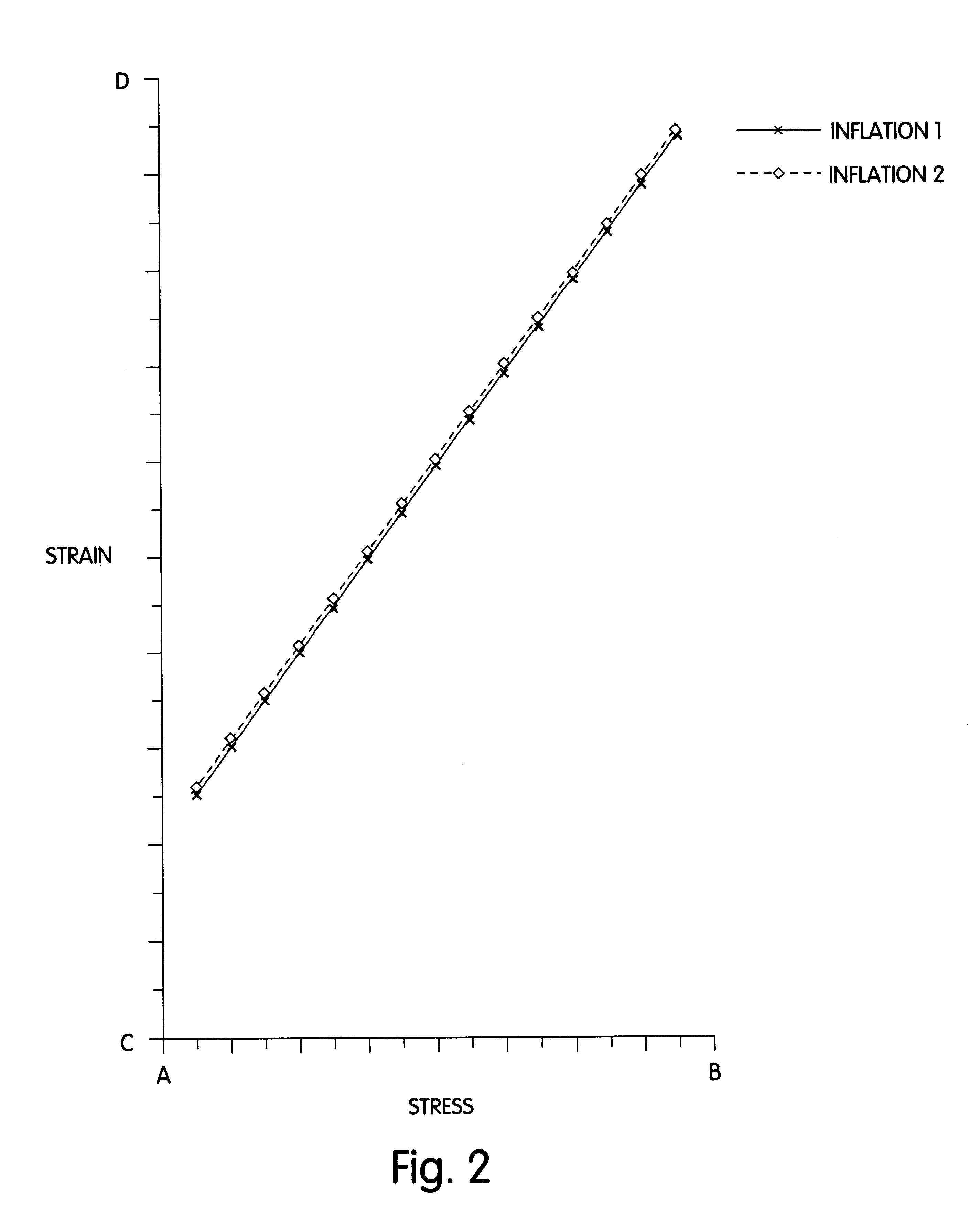
Distensible dilatation balloon with elastic stress
Balloons and balloon catheters with a superior overall combination of distensibility, elastic stress response and strength. The improved properties of the balloons result from the method or process used to form the balloons, as well as the polymeric materials used in said balloon forming process. Additionally, the enhanced combination of properties of the balloons will not be adversely affected by the novel sterilization process contemplated by this invention.
Owner:MEDTRONIC AVE
Tea making technology using light microwaves
InactiveCN102754707AShorten the production cycleReduce lossesPre-extraction tea treatmentMicroorganismMicrowave applications
The invention discloses a tea making technology using light microwaves. The technology comprises the following steps of: sunning with light waves; putting fresh tea leaves in light wave equipment; turning on a power supply, wherein the wavelength of the light waves is 250-800nm, the power is 400-3,000W, and the illumination time is 1-10min; removing green with light microwaves, drying and sterilizing; extracting aroma with light waves; and performing tea screening, check and vacuum packing to obtain a tea product. The light microwaves are applied to the tea processing; and due to the non-thermal effect of the light microwave electric field in the green removing and drying processes, the processing time is greatly shortened, and the tea quality is maintained. Meanwhile, the light microwaves have a sterilization function and can effectively kill microorganisms in tea. Through the tea making technology using light microwaves, the fresh leaves can be collected and processed on the site, and the processes of sunning, green removing, drying, aroma extraction and sterilization are finished in one step, thus the efficiency is high, the operation is simple and the production cost is effectively reduced. Through the invention, the prepared tea has high and stable quality.
Owner:FUZHOU HUAINANZI BIOLOGICAL TECH
Tissue culture and rapid propagation method of acer palmatum
InactiveCN101849506AImprove survival ratePrevent browningCultivating equipmentsPlant tissue cultureSucroseThidiazuron
The invention relates to a tissue culture and rapid propagation method of acer palmatum. The method comprises the following steps of: after a series of sterilization processes, carrying out isolated culture on acer palmatum stems by adopting NN69 as a basic culture medium; and establishing a rapid propagation system of the acer palmatum by utilizing hormone regulation and control to successfully culture an integrated rooting regeneration plant with a transplant survival rate of about 90 percent, wherein a starting culture medium for inoculating a primary generation comprises the NN69, 0.1mg / L of NAA (Naphthylacetic Acid), 0.3mg / L of TDZ (Thidiazuron) and 30g / L of sucrose with a pH value of 5.8; an enrichment culture medium comprises the NN69, 30g / L of sucrose, 0.1mg / L of NAA and 0.8mg / L of KT-30; and a rooting culture medium comprises 1 / 2 NN69, 0.2mg / L of NAA, 20g / L of sucrose and 0.2g / L of active carbon. The method overcomes the defects of long conventional seedling culturing period of the acer palmatum, low cuttage propagation coefficient, and the like and realizes the industrial production of acer palmatum nursery stocks.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com