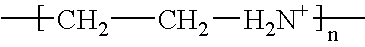Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
9909 results about "Surface coating" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
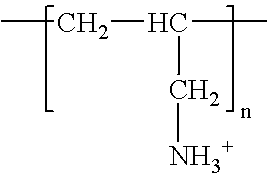
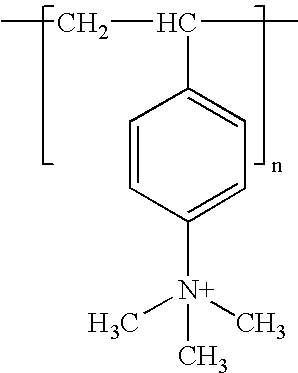
Surface coating, any mixture of film-forming materials plus pigments, solvents, and other additives, which, when applied to a surface and cured or dried, yields a thin film that is functional and often decorative. Surface coatings include paints, drying oils and varnishes, synthetic clear coatings,...
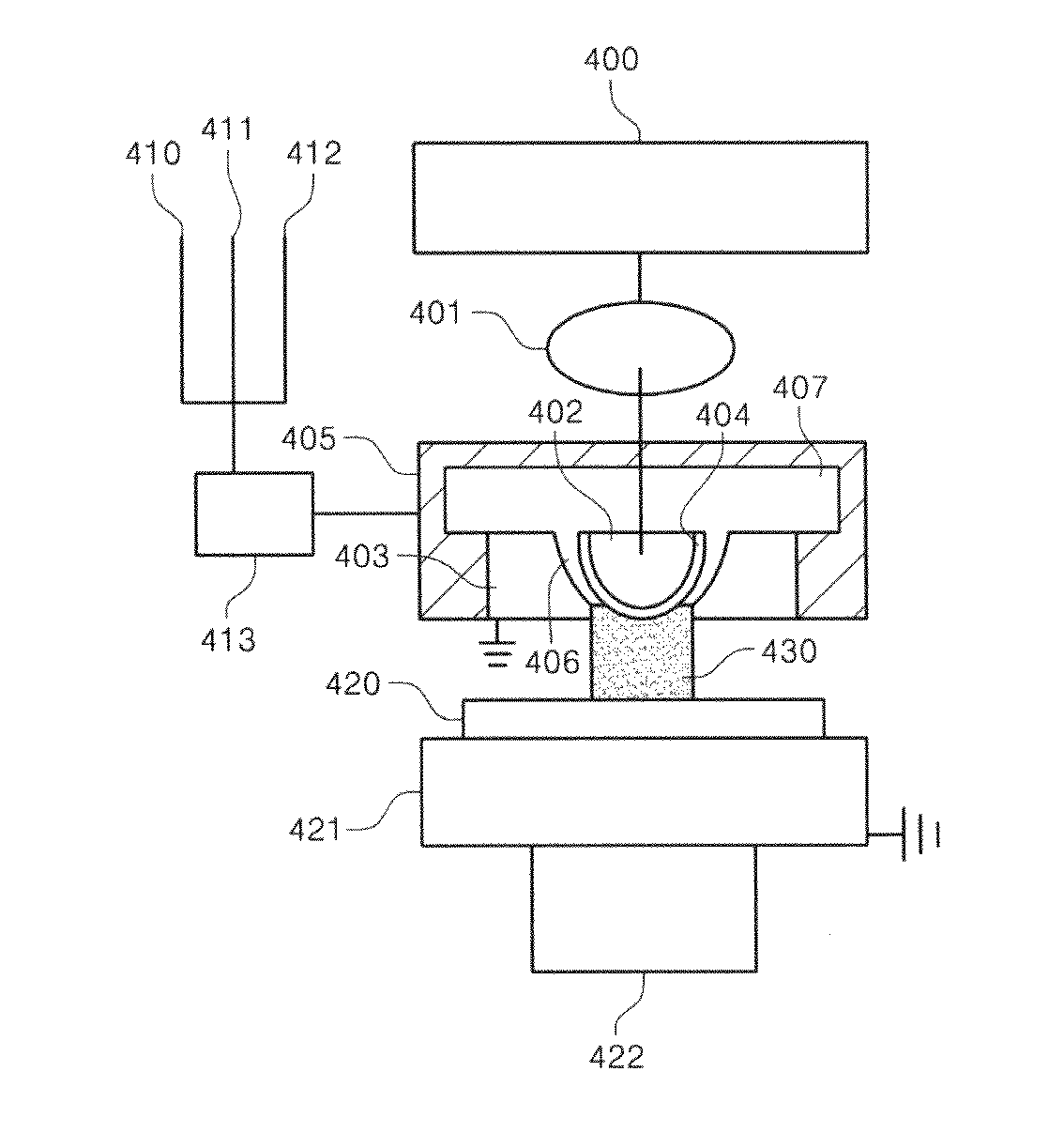
Electrosurgical working end with replaceable cartridges
InactiveUS7041102B2Reduce conductancePrevent any substantial dehydrationSurgical instruments for heatingCoatingsEngineeringMedical device
An electrosurgical medical device and method for creating thermal welds in engaged tissue that carries the electrosurgical components in a disposable cartridge. The instrument also can carry a blade member in a disposable cartridge, thus making the instrument system inexpensive to use. In another embodiment, the electrical leads of the cartridge have a surface coating of a thermochromic material to provide the physician with a visual indicator of operational parameters when applying energy to tissue.
Owner:ETHICON ENDO SURGERY INC
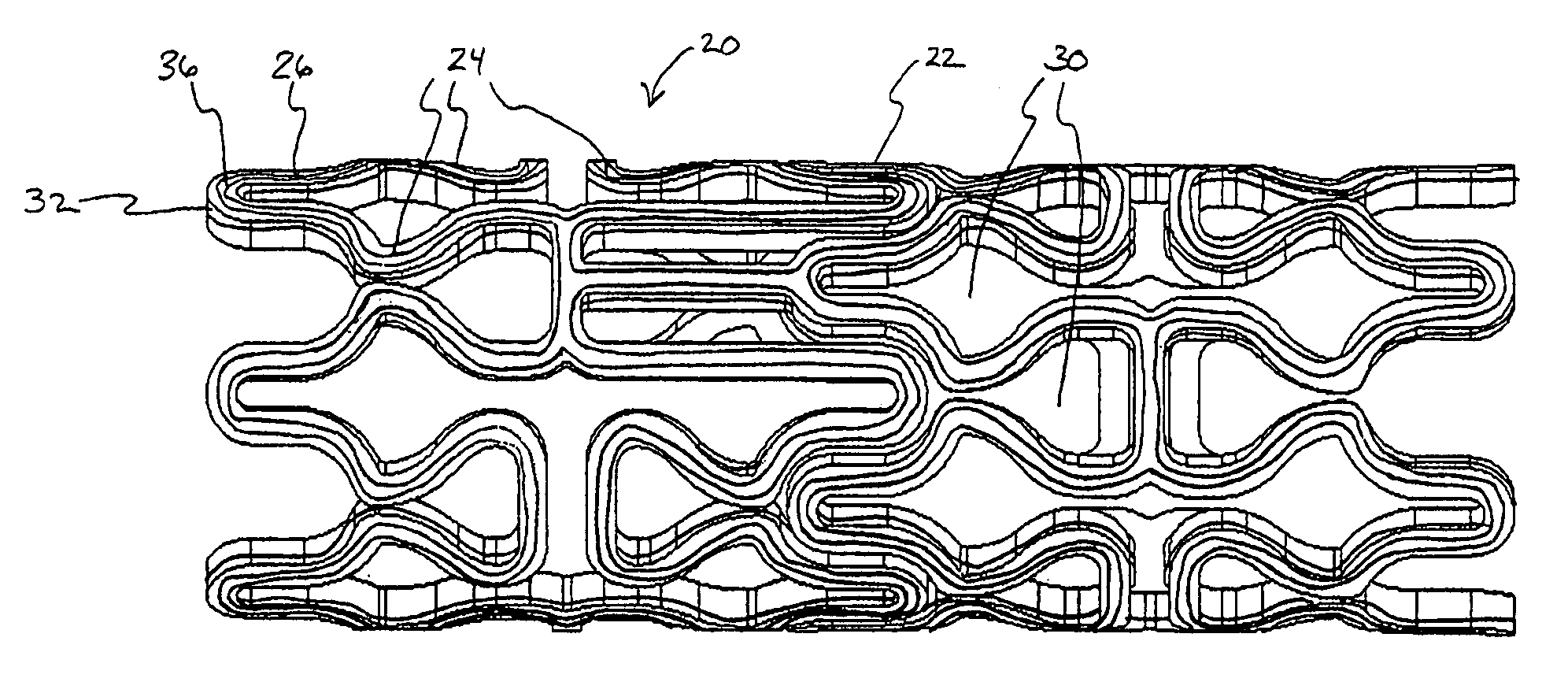
Absorbent articles comprising nanoparticles
Owner:NANO MET ZERO
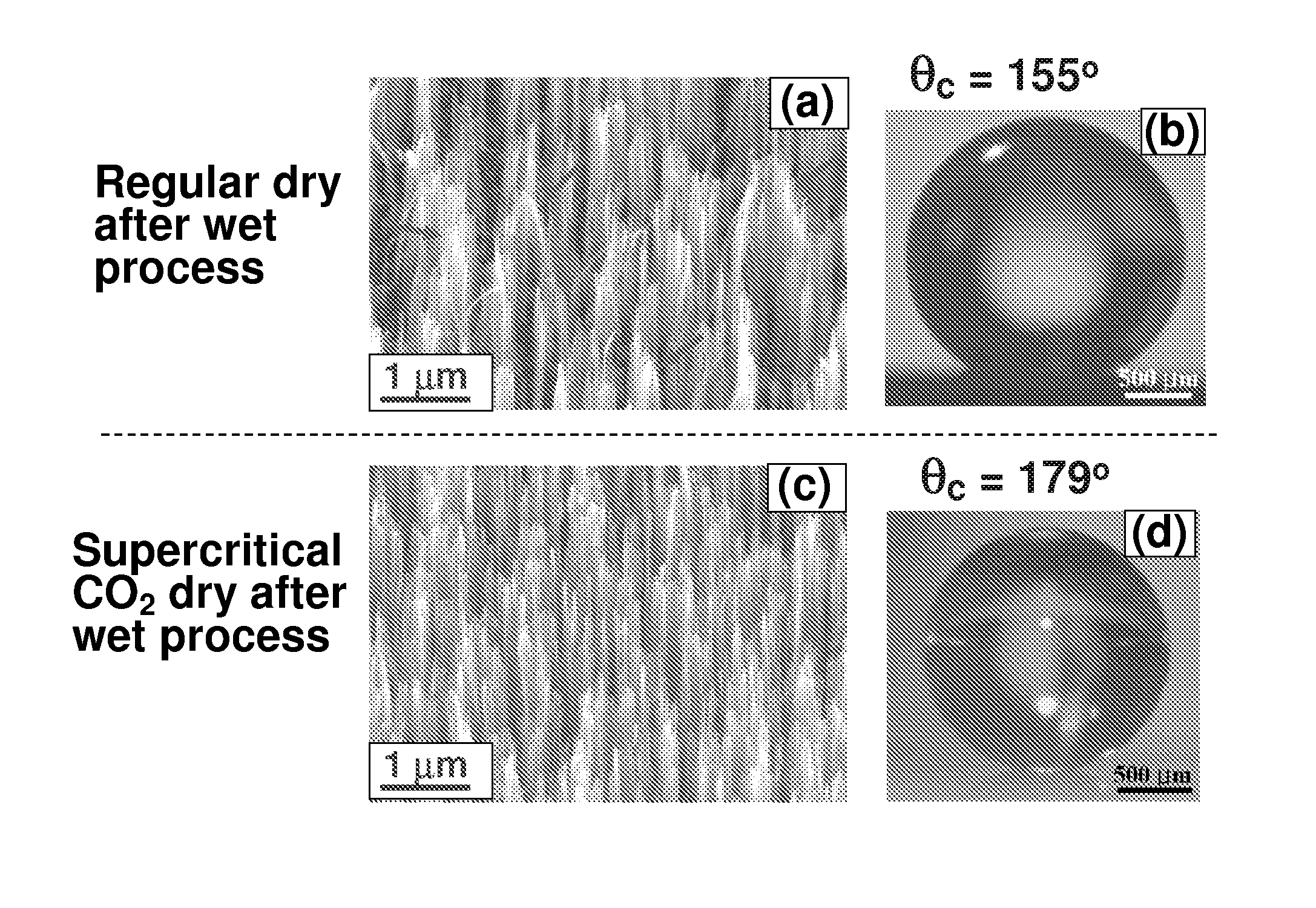
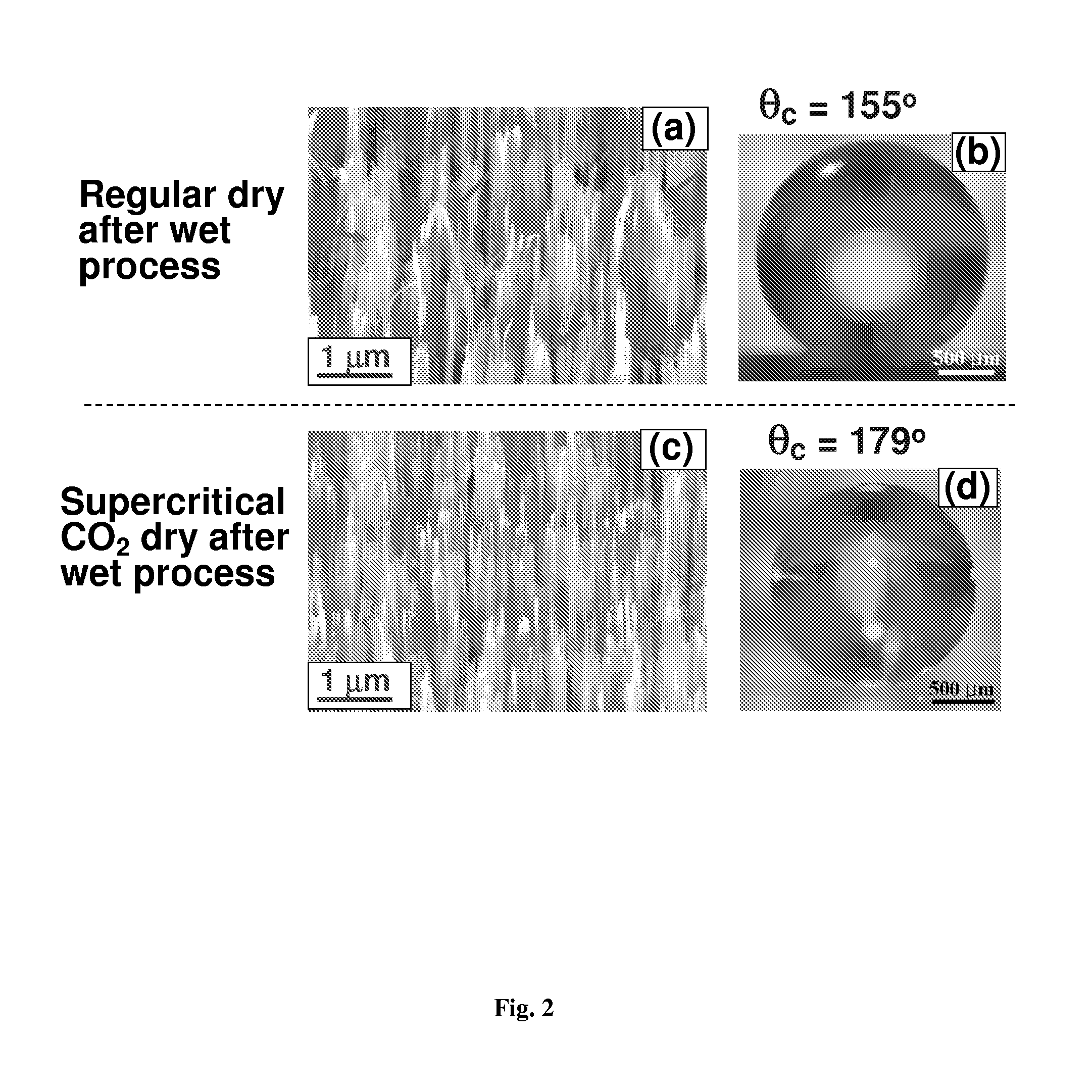
Surface coating method for hydrophobic and superhydrophobic treatment in atmospheric pressure plasma
The present invention relates to a method of coating fluorocarbon or hydrocarbon on the surface of a workpiece using atmospheric pressure plasma. More particularly, the present invention relates to a method of coating hydrocarbon or fluorocarbon on the surface of a workpiece using plasma generated under atmospheric pressure such that the workpiece can have a hydrophobic or super-hydrophobic surface.The method of coating a surface of a workpiece with fluorocarbon to be hydrophobic or super-hydrophobic according to the present invention comprises the steps of generating first atmospheric pressure glow plasma by supplying a reaction gas into a discharge space formed between a first electrode and a second electrode, the reaction gas containing hydrogen gas, fluorocarbon gas and inert gas, the first and second electrodes being connected to an RF power supply of an atmospheric pressure plasma generator; and approaching the workpiece to the first electrode downstream of a reaction gas flow passing through the discharge space, such that the plasma created in the discharge space is transferred into a space between the first electrode and the workpiece to generate a second atmospheric pressure glow plasma therein, whereby a fluorocarbon coating layer can be formed on the surface of the workpiece.
Owner:KANG BANG KWON
Method of preparing nano-structured surface coatings and coated articles
InactiveUS7892606B2Improve adhesionMore readily manufacturableMaterial nanotechnologyNanostructure manufactureCross-linkNano structuring
Owner:DSM IP ASSETS BV
LED-based luminaire
InactiveUS20070041220A1Maximize lighting effectivenessLow costCoupling device connectionsPlanar light sourcesEffect lightEngineering
An LED-based luminaire includes a driver configured to convert line voltage into a desired power configuration. Elongate fasteners attach one or more LED-based lighting modules to a mount member and also to energized poles of the power driver. The fasteners communicate electrical energy from the power driver to the lighting module. In one embodiment, the mount member functions as a heat sink, and it includes a bumpy surface coating having a texture with sufficient feature heights to enhance heat transfer between the heat sink and the surrounding environment.
Owner:DIAMOND CREEK CAPITAL LLC
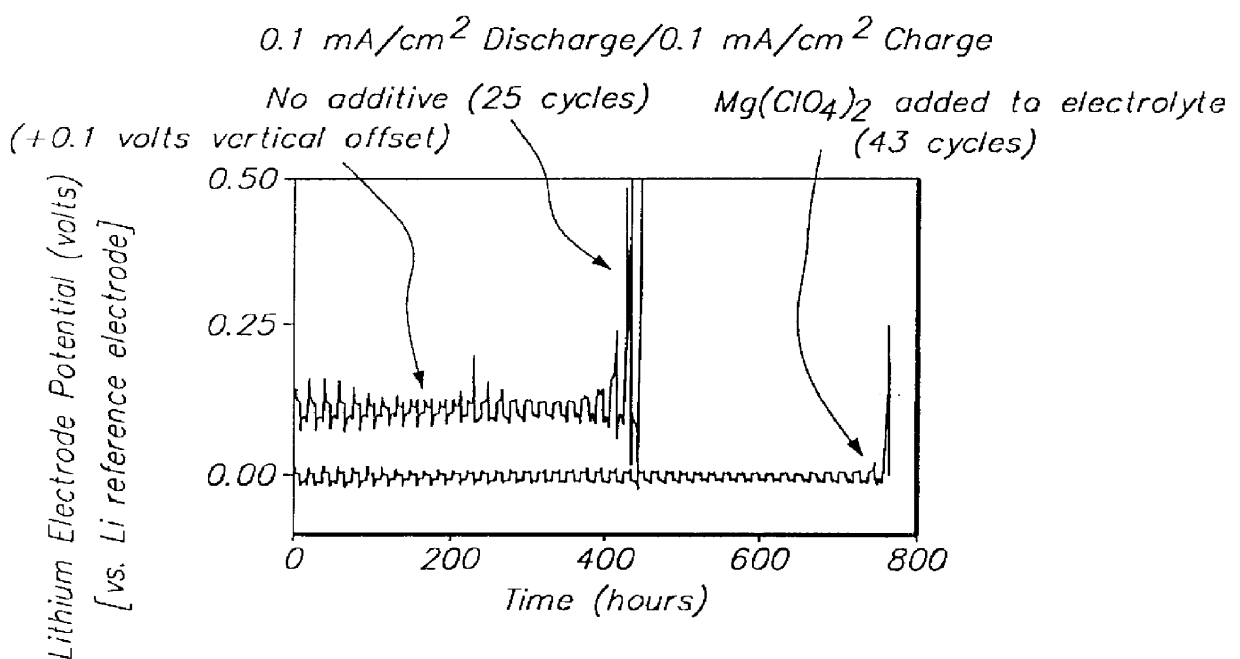
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6017651AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
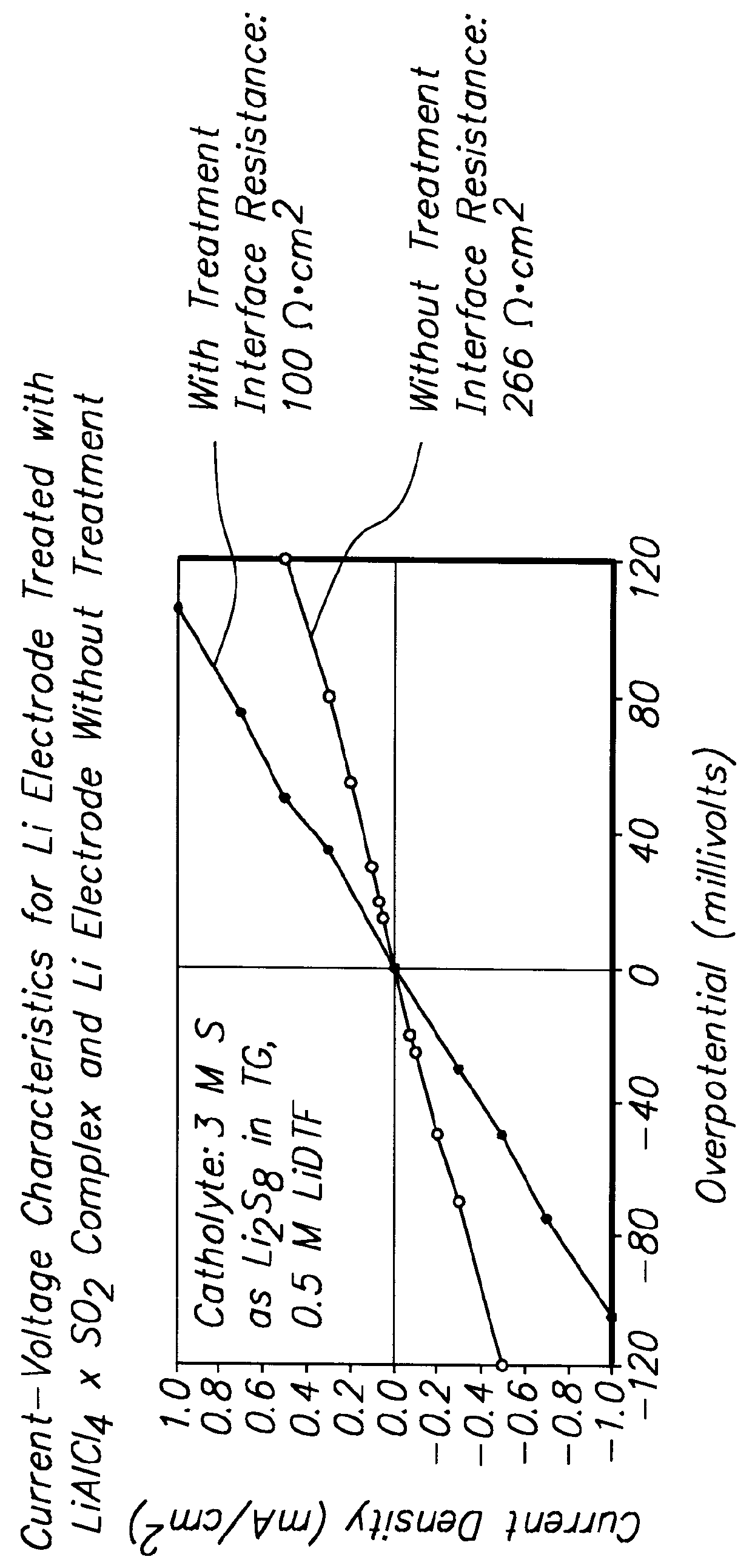
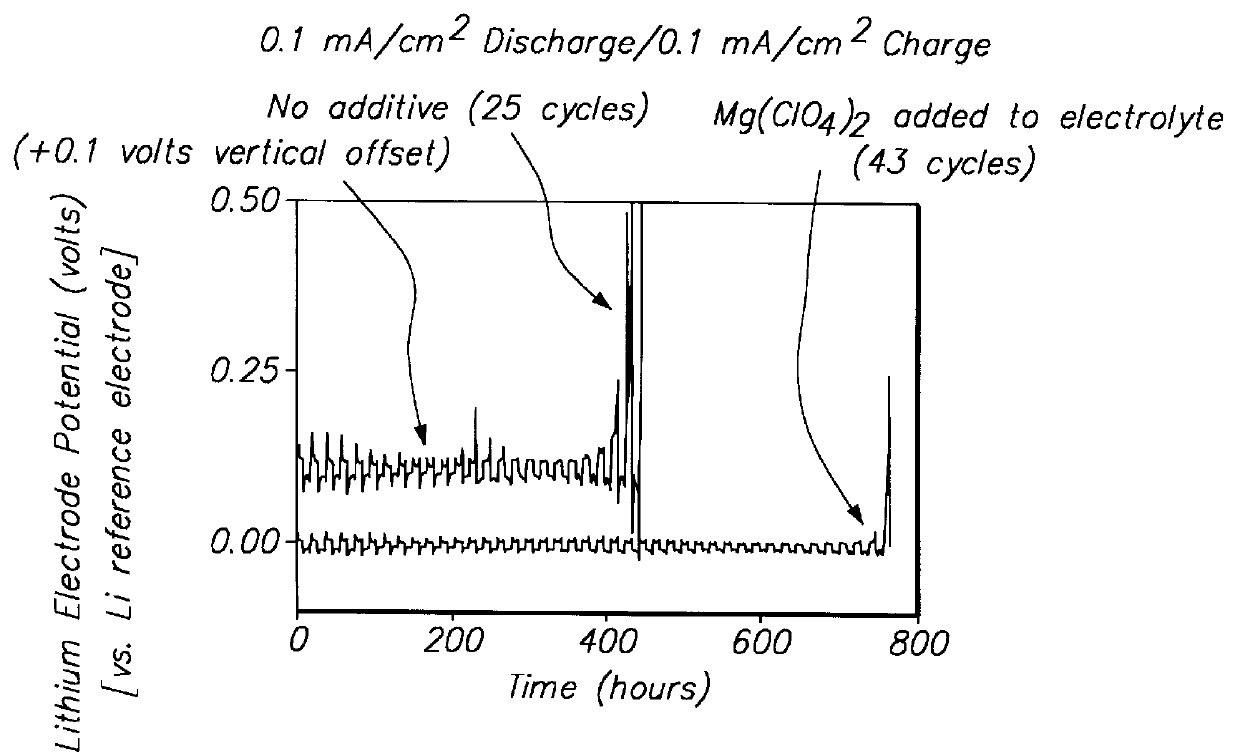
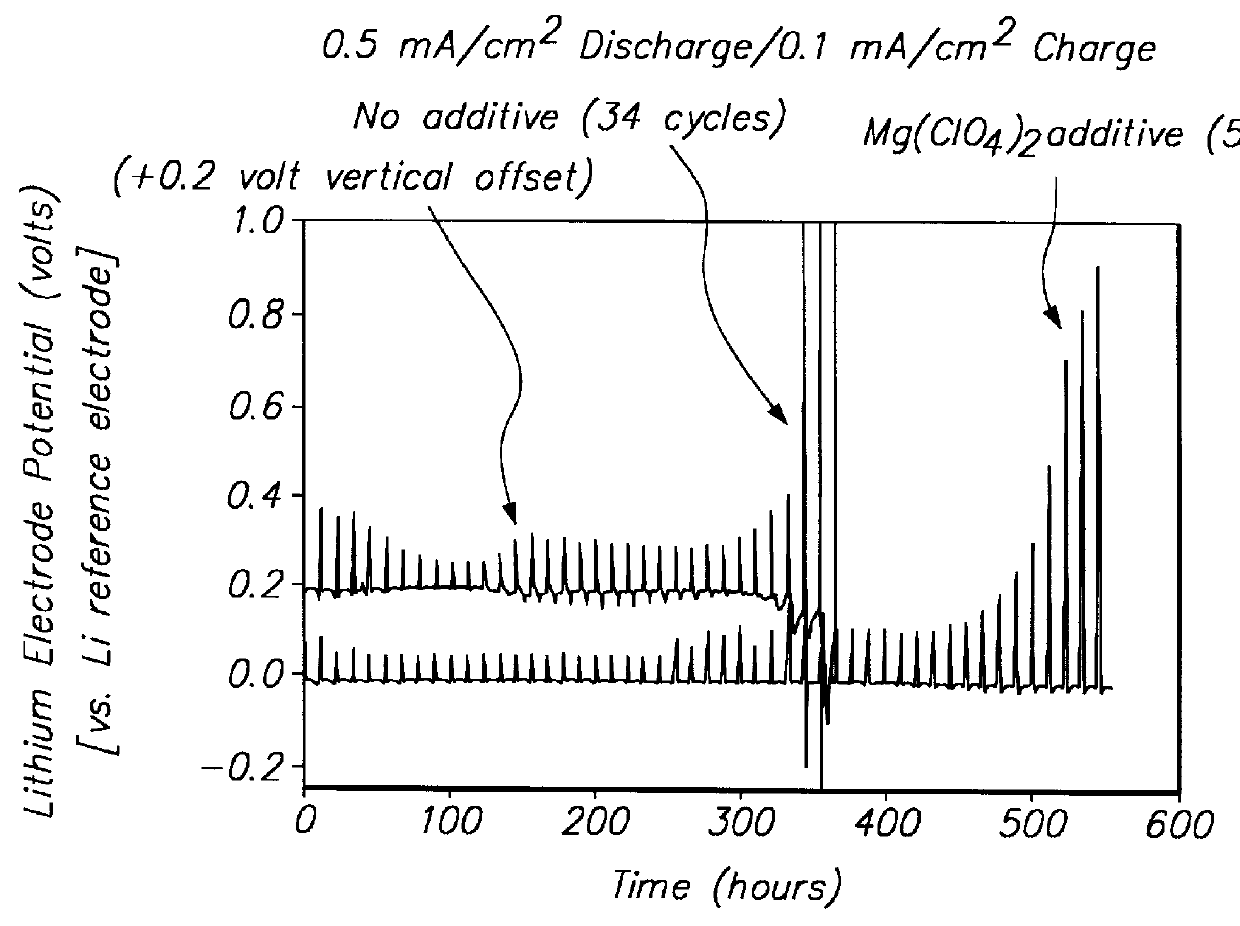
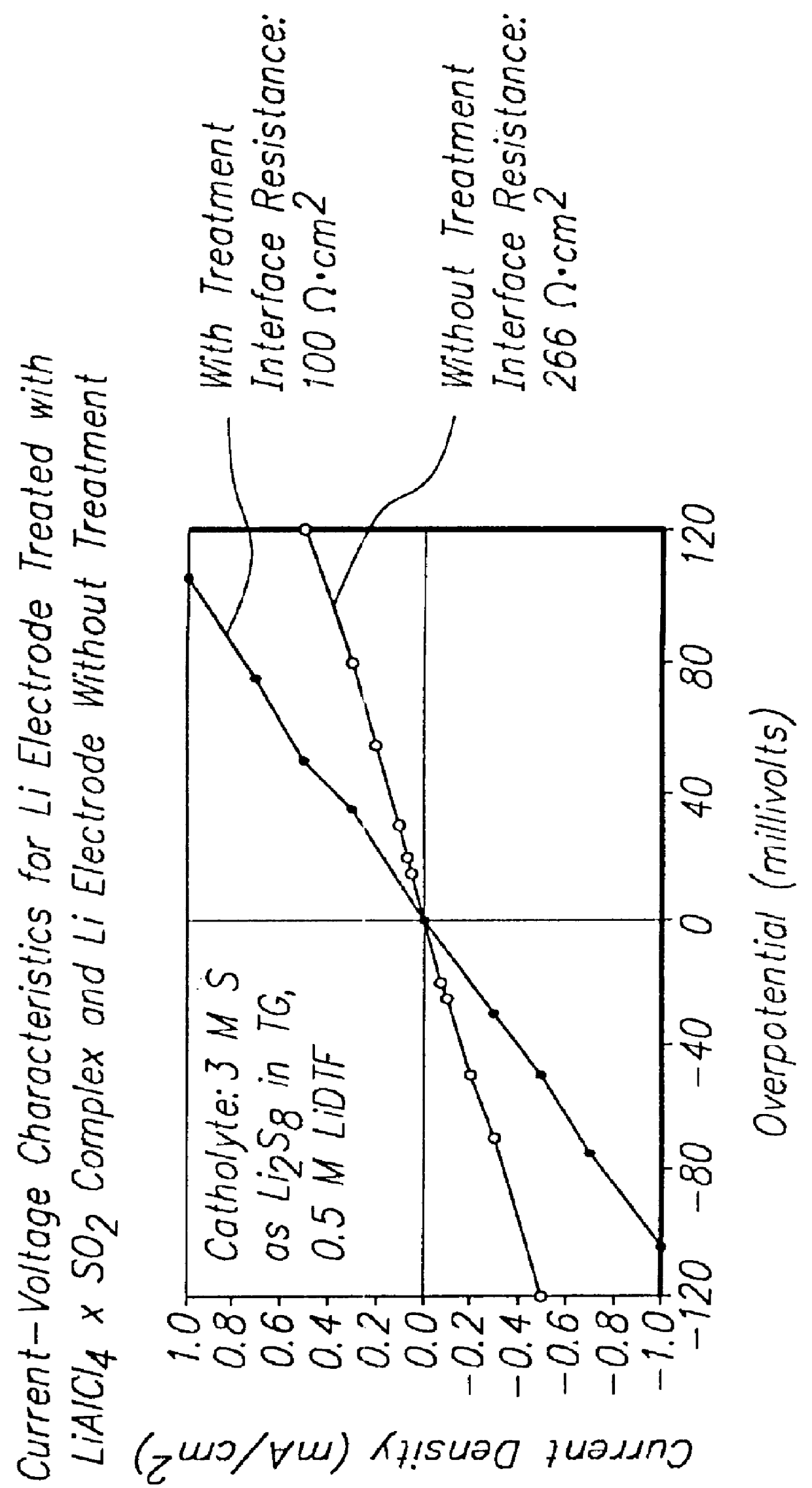
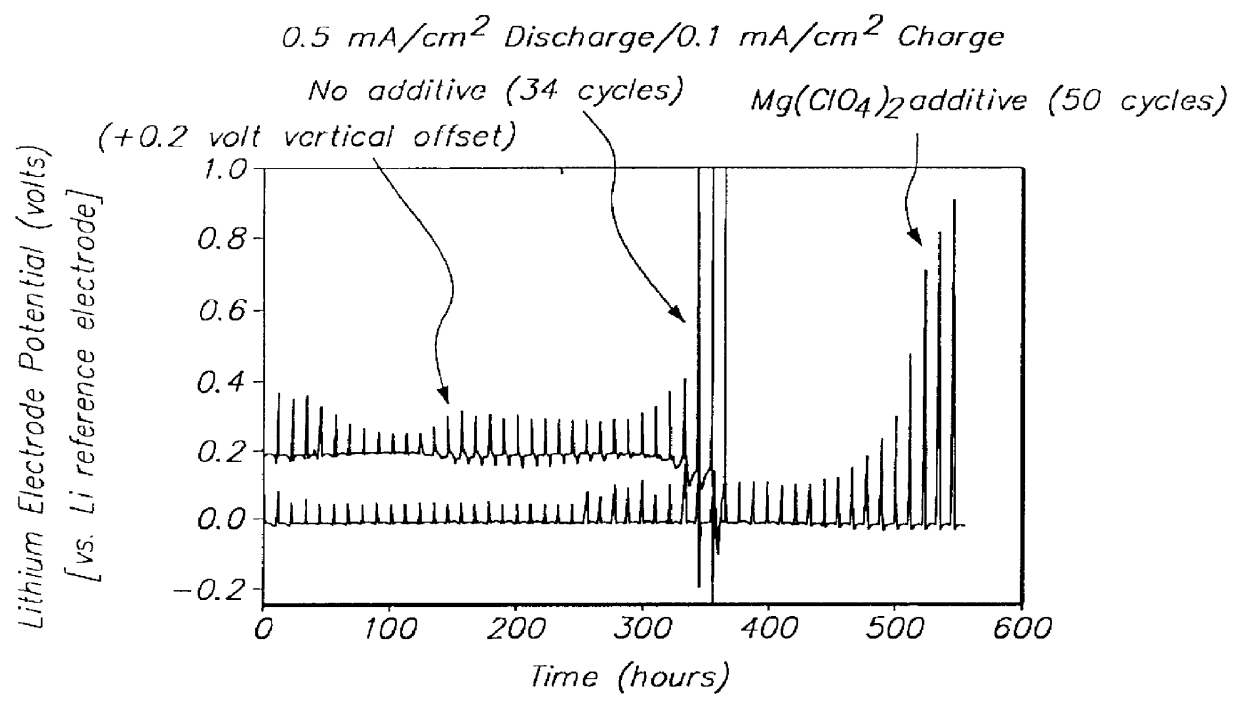
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Poly(beta-amino alcohols), their preparation, and uses thereof
ActiveUS20130302401A1Organic active ingredientsPeptide/protein ingredientsChemical structureFibrosis
A new class of poly(beta-amino alcohols) (PBAAs) has been prepared using combinatorial polymerization. The inventive PBAAs may be used in biotechnology and biomedical applications as coatings (such as coatings of films or multilayer films for medical devices or implants), additives, materials, excipients, non-biofouling agents, micropatterning agents, and cellular encapsulation agents. When used as surface coatings, these PBAAs elicited different levels of inflammation, both in vitro and in vivo, depending on their chemical structures. The large chemical diversity of this class of materials allowed us to identify polymer coatings that inhibit macrophage activation in vitro. Furthermore, these coatings reduce the recruitment of inflammatory cells, and reduce fibrosis, following the subcutaneous implantation of carboxylated polystyrene microparticles. These polymers may be used to form polyelectrolyte complex capsules for cell encapsulation. The invention may also have many other biological applications such as antimicrobial coatings, DNA or siRNA delivery, and stem cell tissue engineering.
Owner:MASSACHUSETTS INST OF TECH
Ballistic fabric laminates
InactiveUS6846758B2Improved ballistic protectionImprove protectionSynthetic resin layered productsPersonal protection gearElastomerYarn
Woven fabric laminates having superior resistance to penetration by ballistic projectiles, assemblies thereof, and the method by which they are made. In one embodiment, among others, a laminate of the invention is comprised of a fabric woven from a high strength, high modulus yarn, a surface coating of a low modulus elastomer and a plastic film bonded to its elastomer-coated surface.
Owner:HONEYWELL INT INC
Surface coating for chamber components used in plasma systems
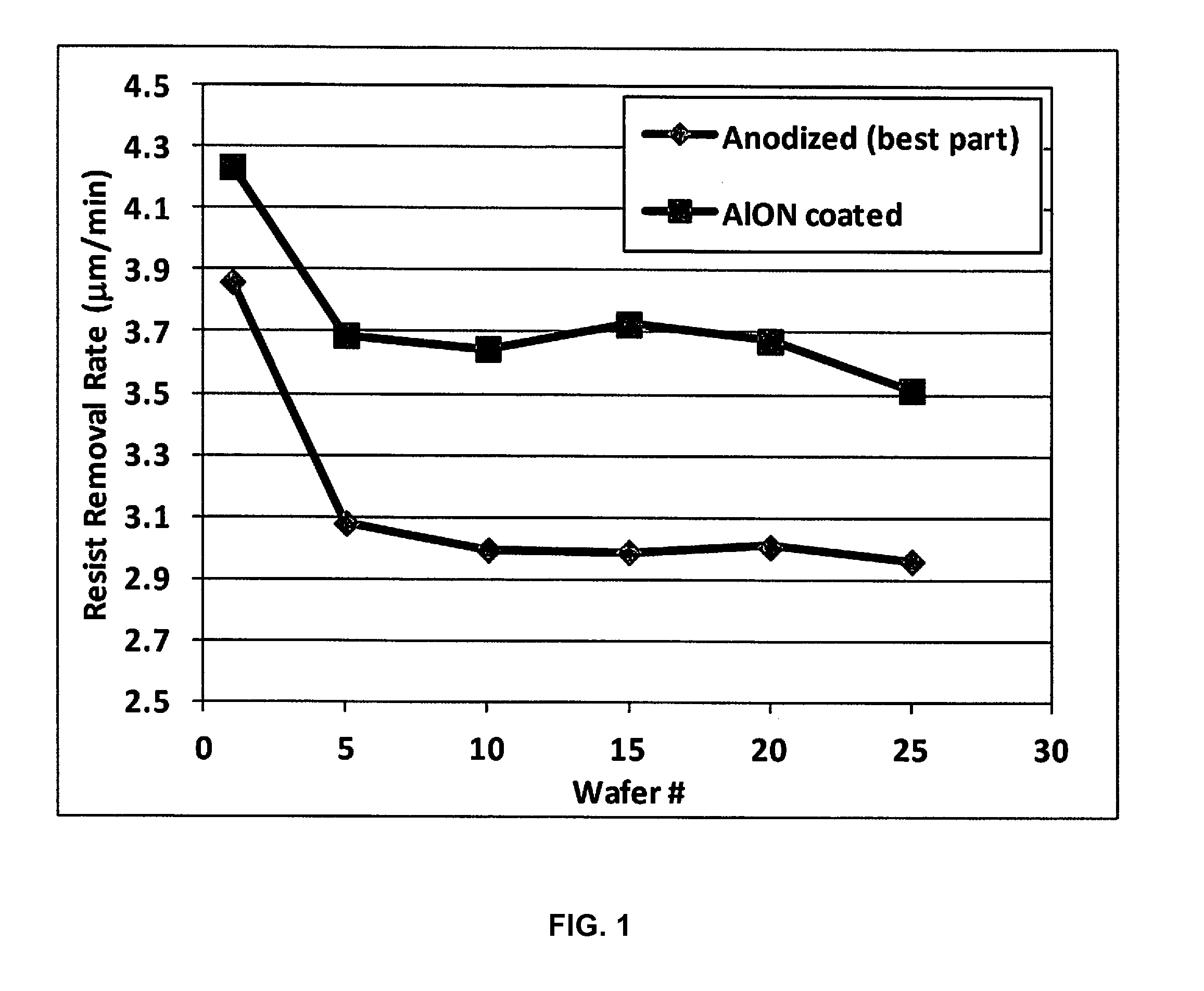
ActiveUS20170032942A1Low plasma surface recombination rateHigh trafficElectric discharge tubesVacuum evaporation coatingBlood plasmaPlasma chamber
Disclosed herein are surface coatings for plasma components that have the benefit of being robust against chemical and plasma physical attack in aggressive (e.g., fluorine-based) plasma environments. The coatings also provide low plasma surface recombination rates for active oxygen, nitrogen, fluorine, and hydrogen species when compared with other known surface treatments. The coatings can be applied to any plasma system component not requiring etching or plasma cleaning including but not limited to materials like quartz, aluminum, or anodized aluminum. Additionally, the efficiency of the system is increased by applying a non-reactive coating to system components thereby increasing the flow of excited plasma species to the plasma chamber of the system.
Owner:ENTEGRIS INC
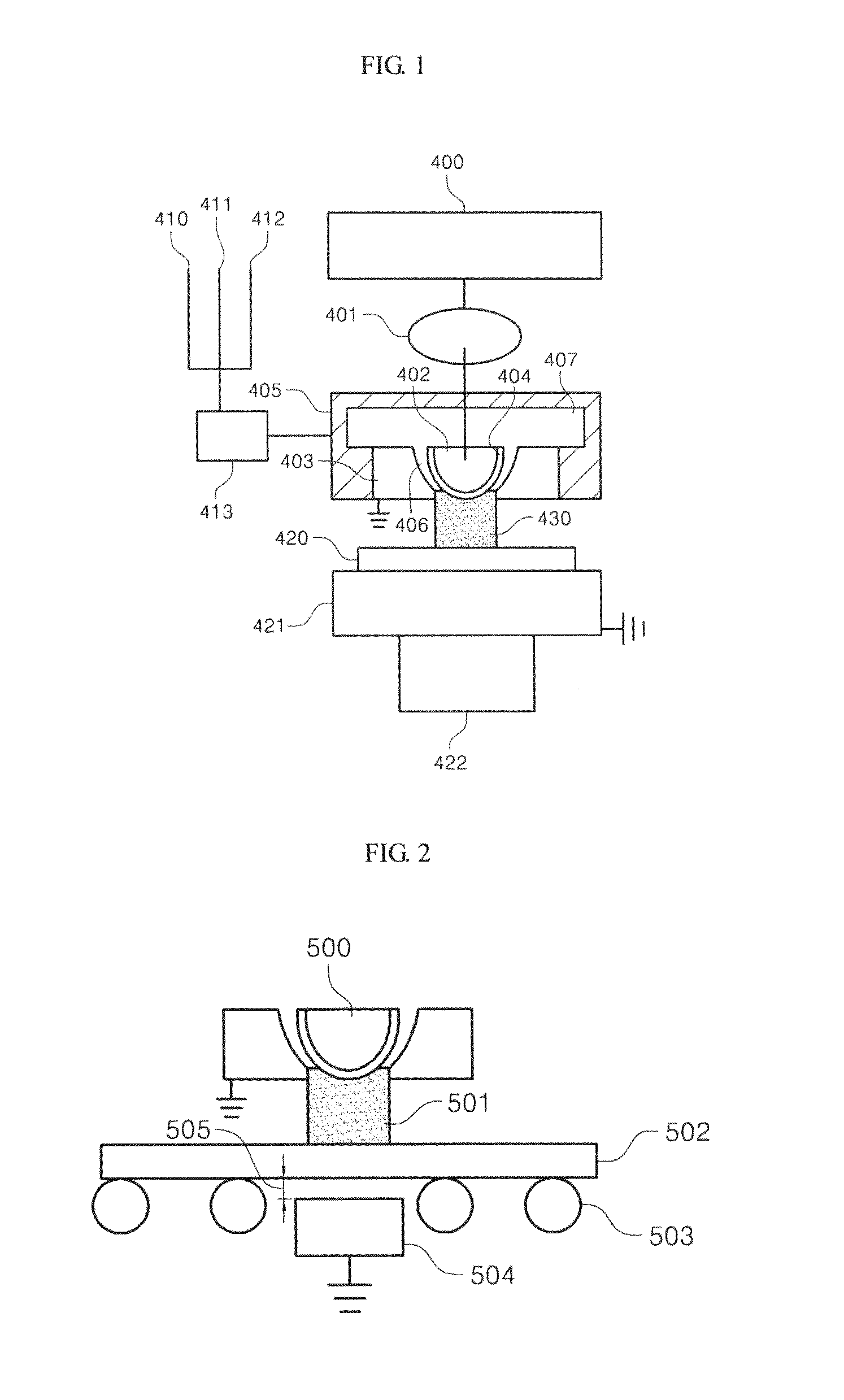
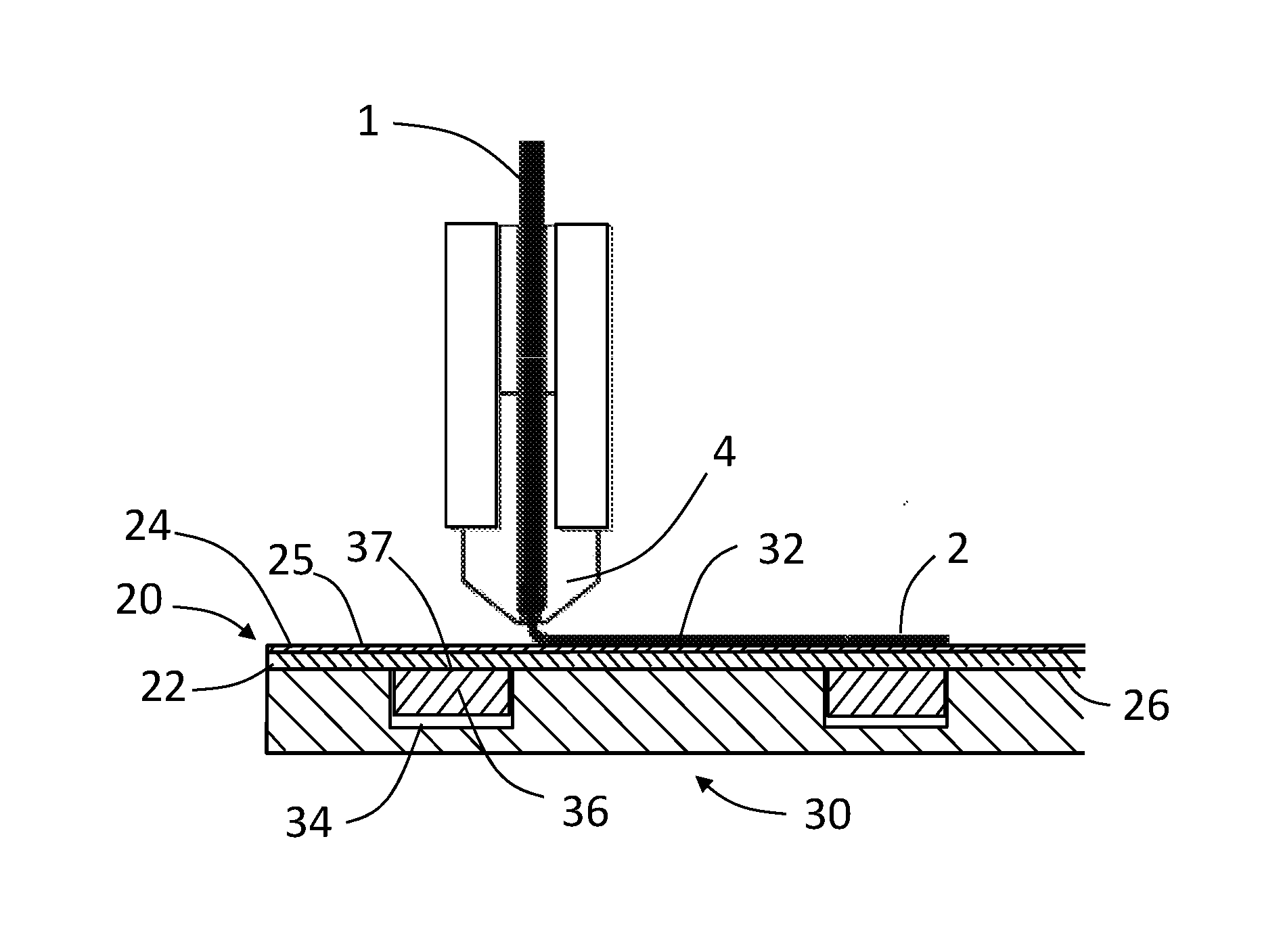
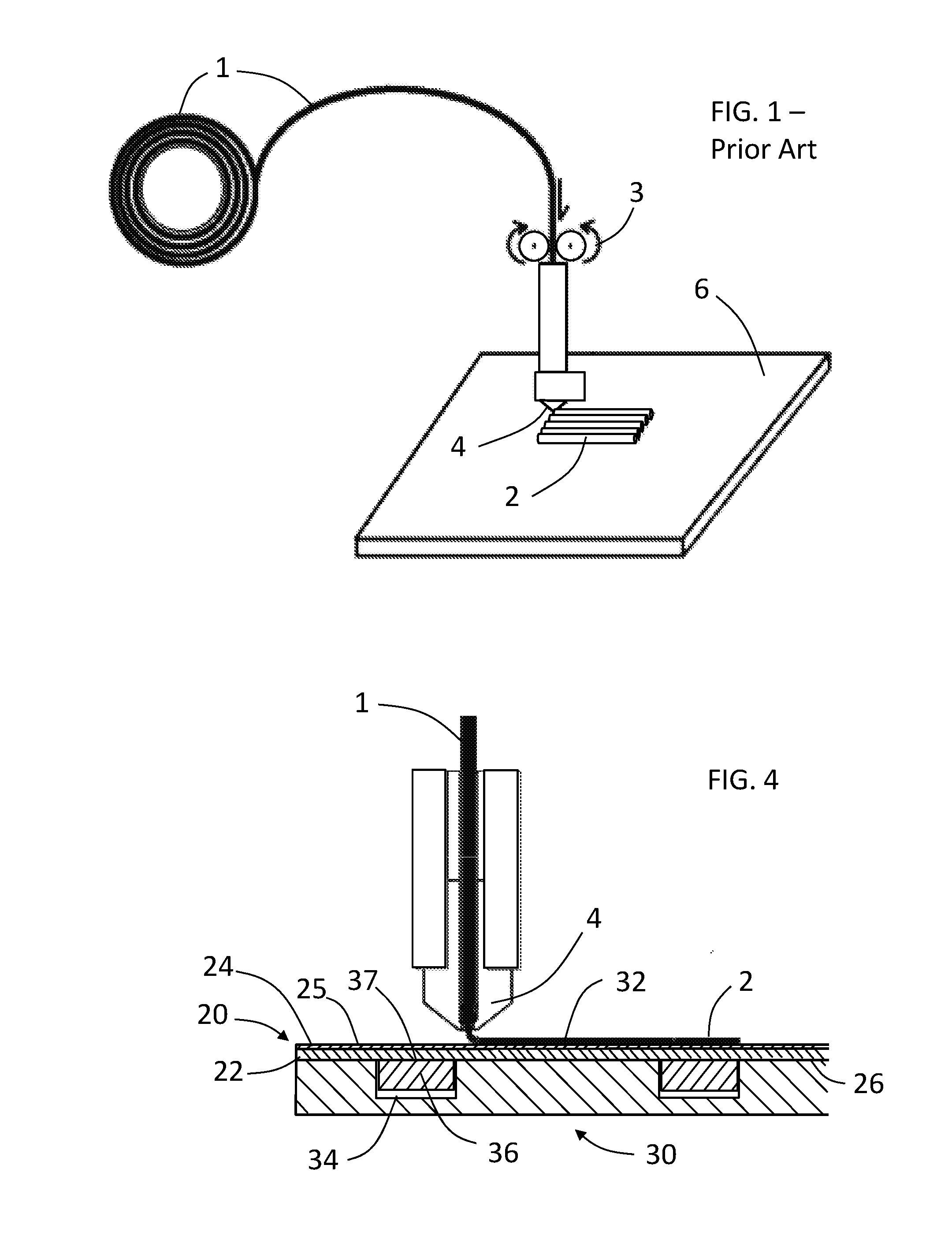
Process of and apparratus for three-dimensional printing
InactiveUS20060054039A1Low adhesion characteristicAvoid mechanical deformationManufacturing platforms/substratesPattern printingTemperature controlEngineering
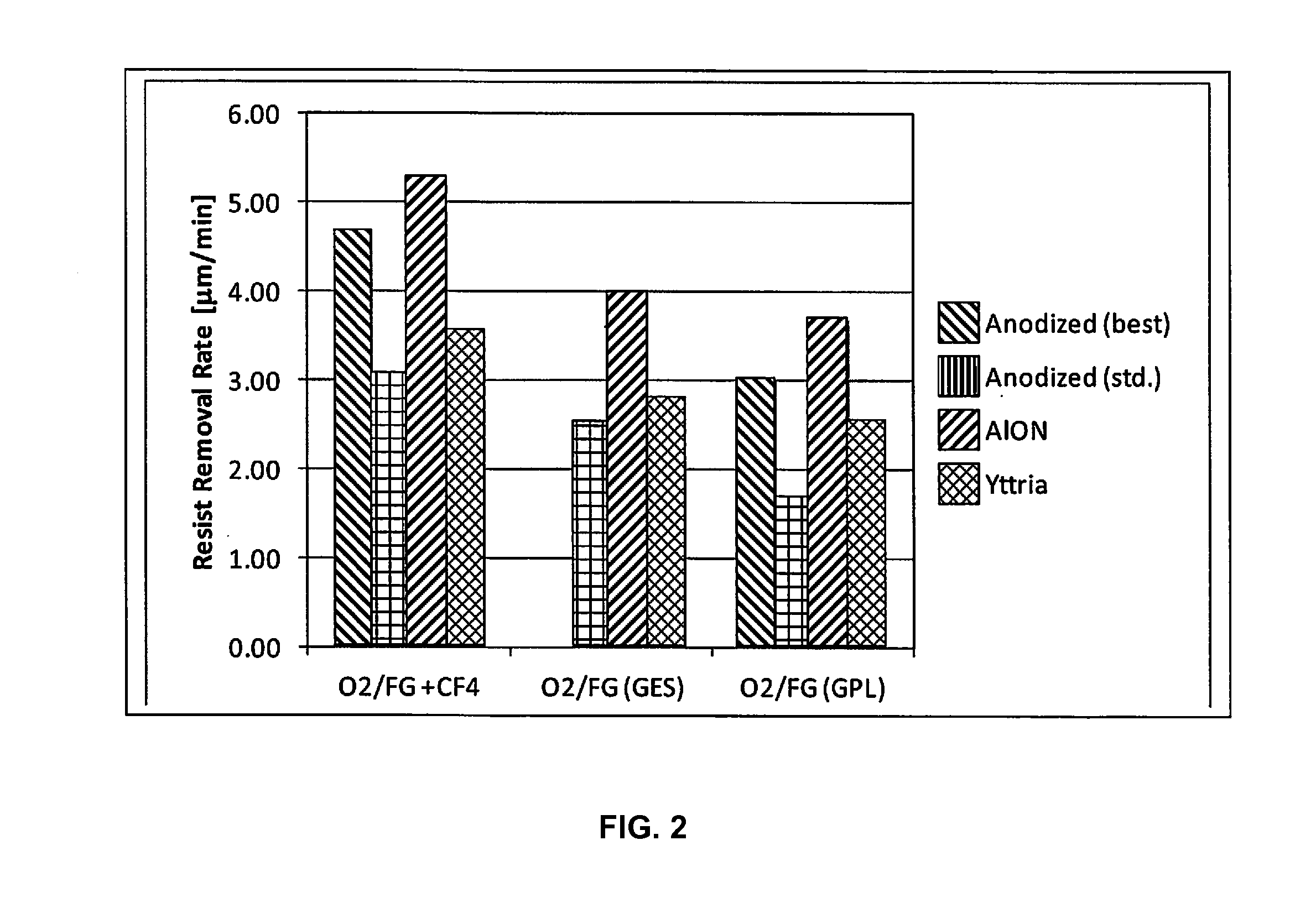
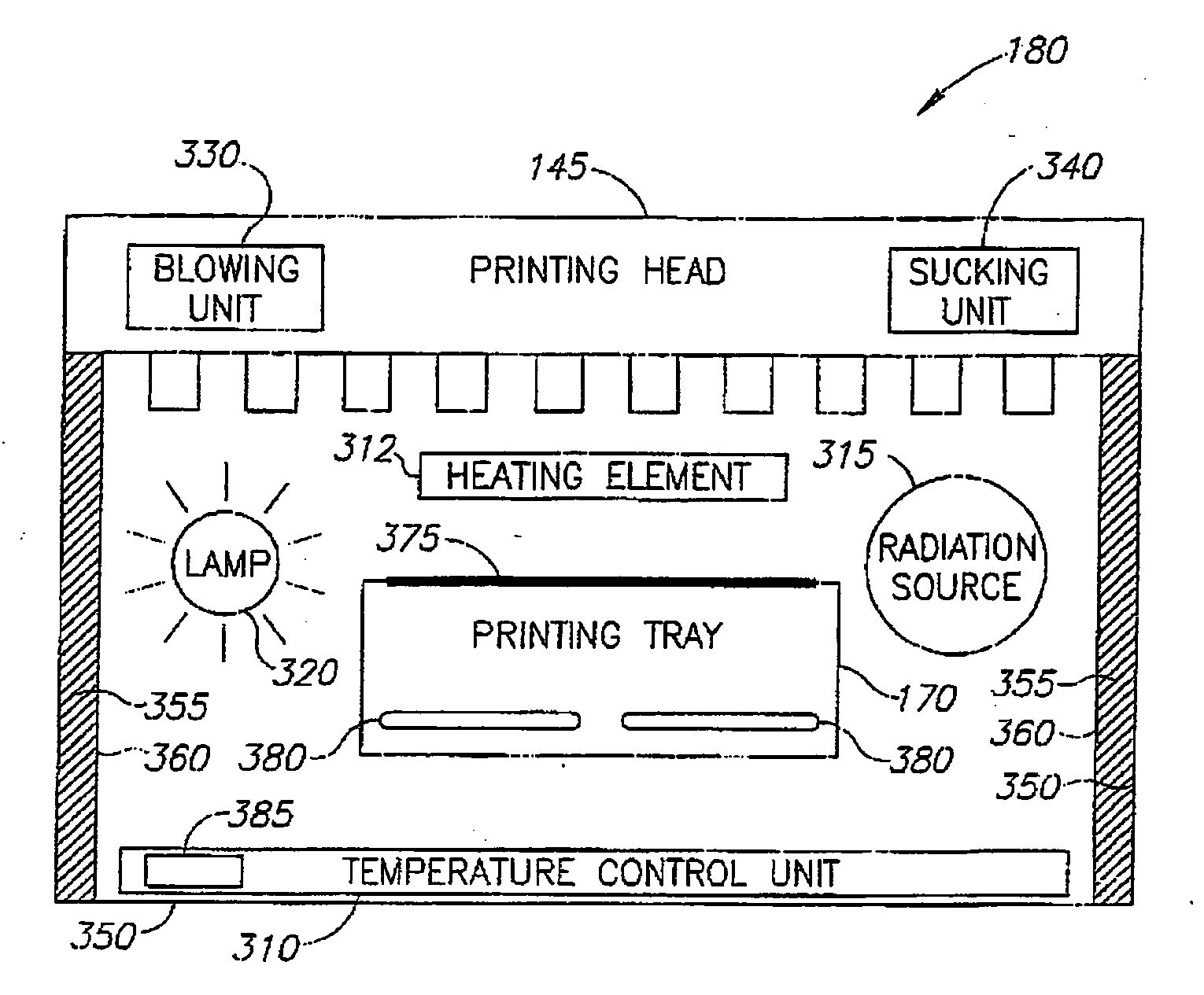
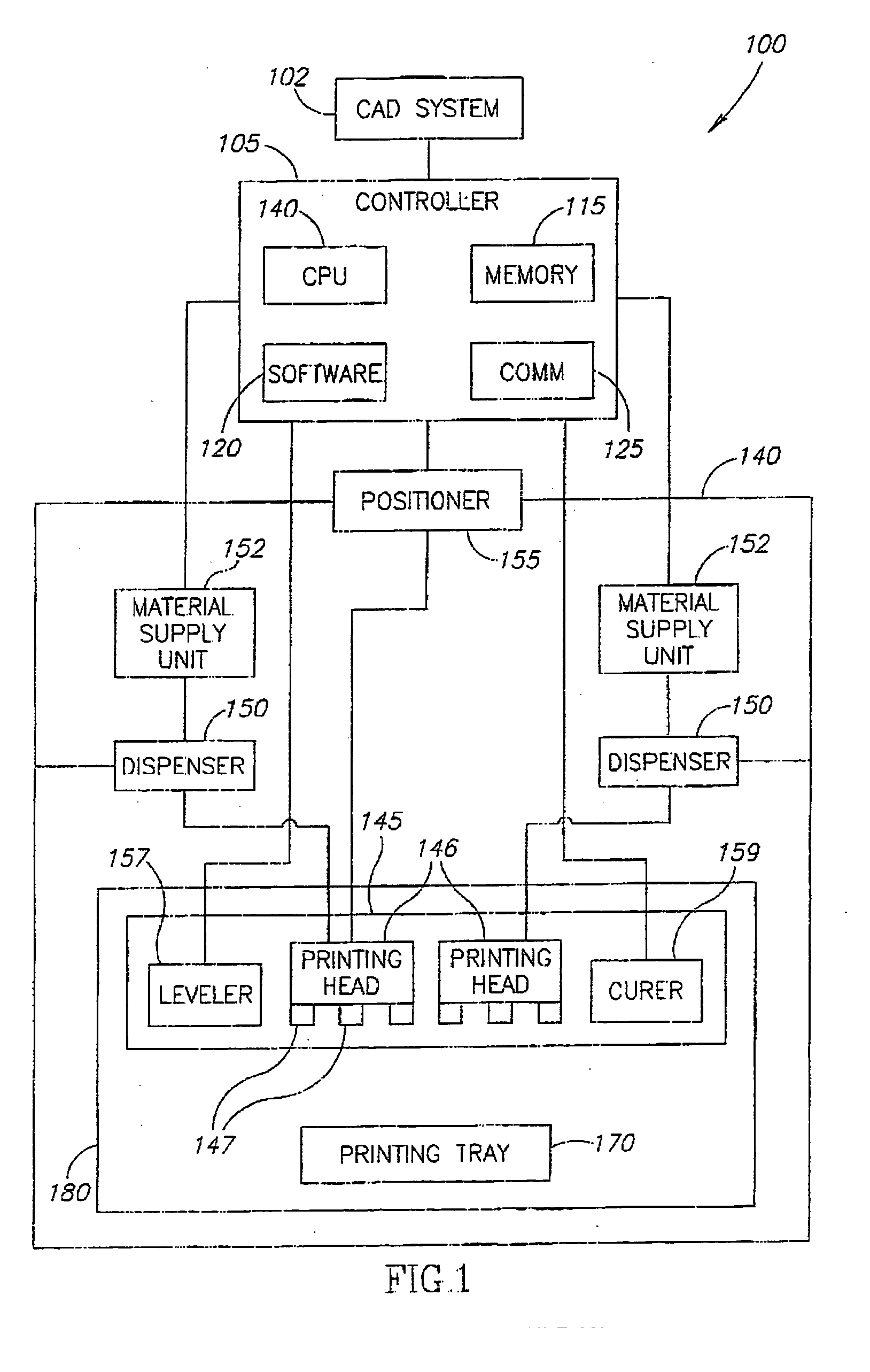
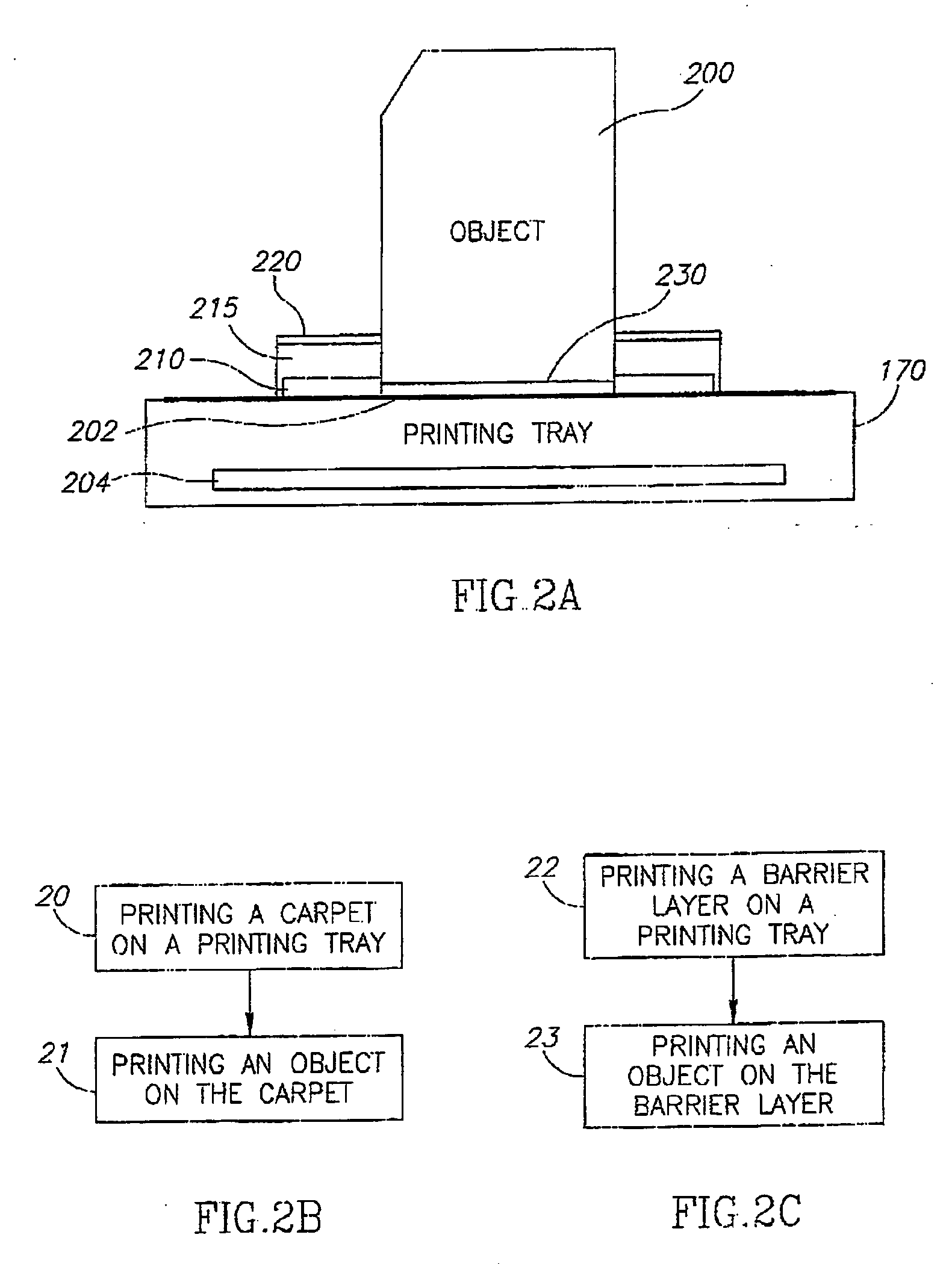
A process of, and apparatus for, three-dimensional printing of an object (200) from modeling material (220) including a printing tray (170) having a surface coating (202), a carpet (210), a support material (215), a support pedestal (210), a barrier layer (230) and a temperature control unit (204).
Owner:OBJET GEOMETRIES
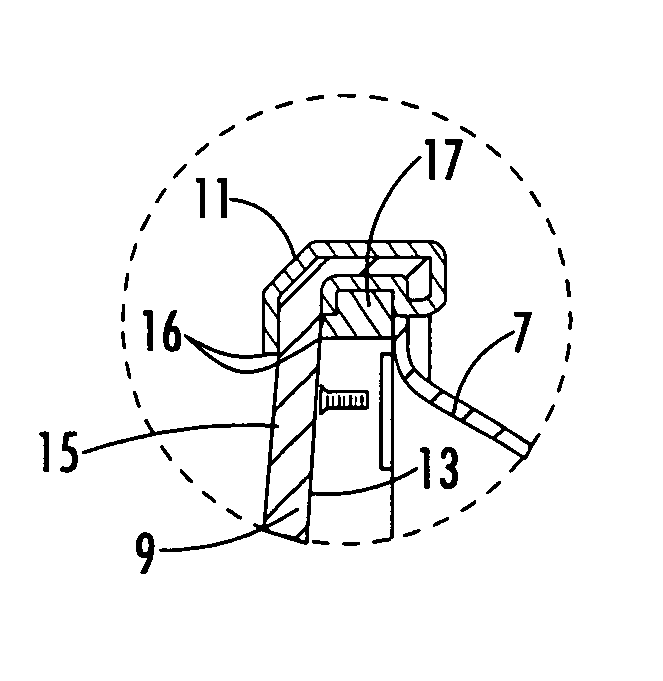
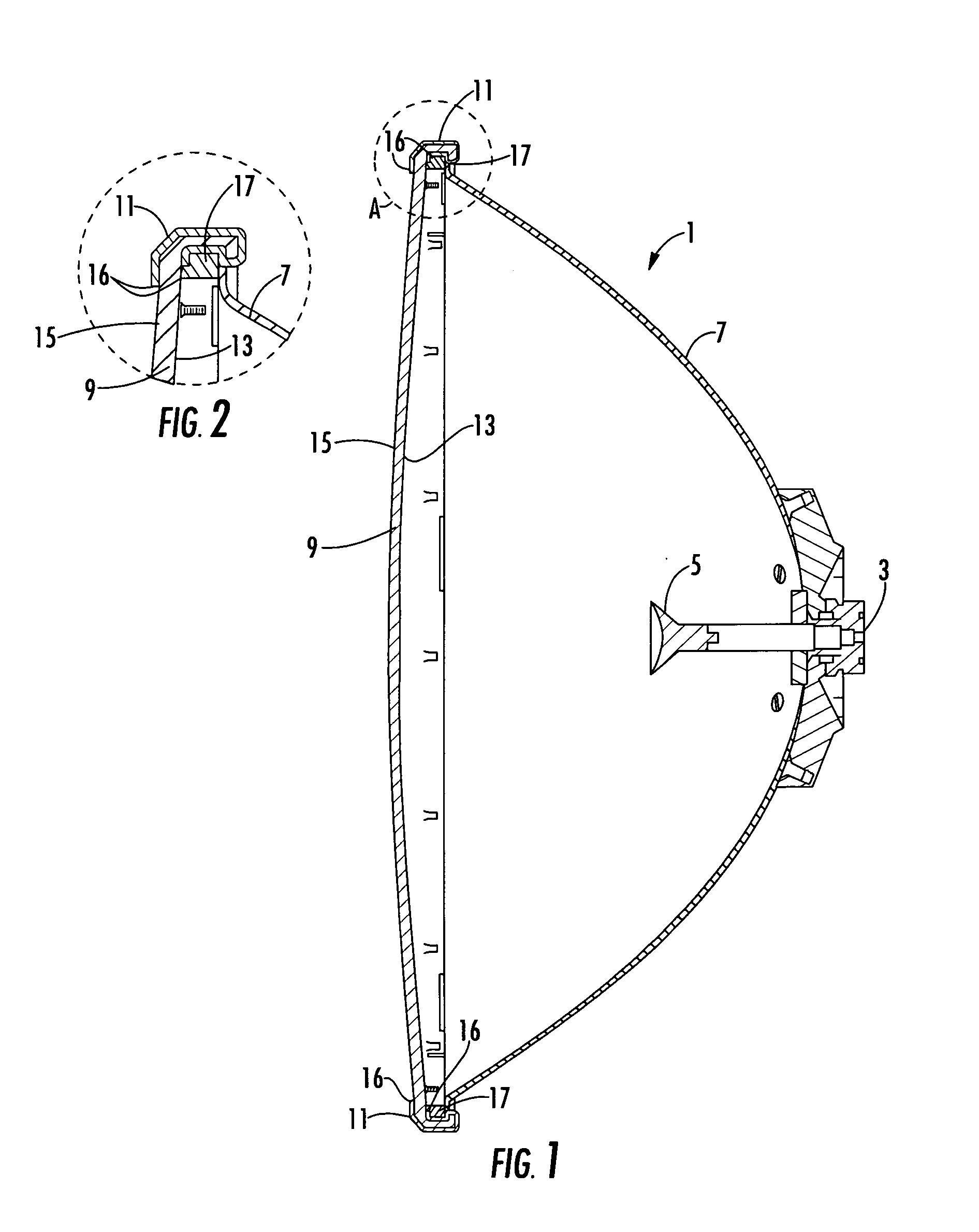
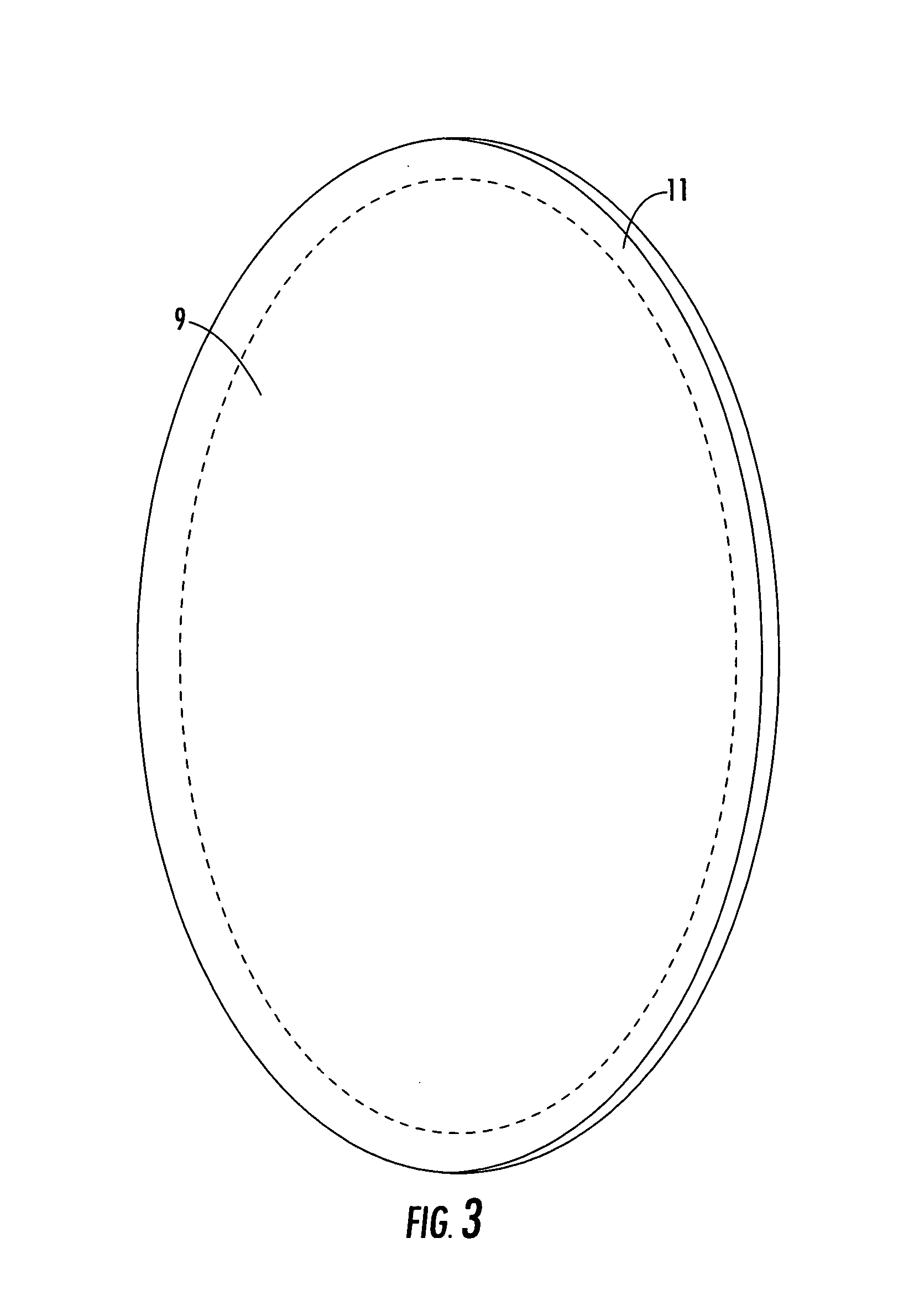
Reflector antenna radome with backlobe suppressor ring and method of manufacturing
ActiveUS7138958B2Reduce wind loadsCost efficientRadiating element housingsCollapsible/retractable loop antennasMetallic foilSuppressor
A radome adapted to reduce backlobes of an associated reflector antenna via application of a conductive ring with an inward facing edge about the periphery of the radome. The conductive ring may be applied extending around the radome periphery to an inside and or outside surface of the radome. The conductive ring may be formed upon the radome by metalising, electrodaging, over molding or the like. Further, the conductive ring may be a metal, metallic foil, conductive foam or the like which is coupled to the radome. An absorber in the form of a ring or a surface coating applied to the radome and or the distal end of the reflector may also be added between the radome and the reflector.
Owner:COMMSCOPE TECH LLC
Topographic coatings and coating methods for medical devices
InactiveUS20050228477A1Easy to operateImprove adhesionStentsPretreated surfacesInsertion stentElution
Medical devices having topographic coatings are provided. The topographic coatings have regions of high and low elevation and may be composed of polymers, metals, ceramics, proteins and other biocompatible materials. Such topographic coatings facilitate the deposition, elution, and protection of therapeutic agents on the medical device, manipulation of the medical device, and other purposes. In particularly preferred embodiments, the medical device comprises a stent for vascular implantation.
Owner:XTENT INC
Process for manufacturing photovoltaic cells
InactiveUS20050268963A1Semiconductor/solid-state device manufacturingPhotovoltaic energy generationBack surface fieldEngineering
A process for making a photovoltaic cell using a semiconductor wafer doped with a first dopant, the wafer comprising a front surface and a back surface, the process comprising the steps of forming a first layer on the front surface of the wafer, the first layer comprising a second dopant of a conductivity type opposite the first dopant; depositing a surface coating on the front surface over the first layer; forming grooves in the front surface after depositing the surface coating thereon; adding a second dopant to the grooves; depositing a doping material on the back surface; treating the wafer having the doping material deposited thereon to form a back surface field, masking at least a portion of the back surface of the wafer after the treating; and adding a conductive material to the grooves to form an electrical contact.
Owner:BP CORP NORTH AMERICA INC
Biomedical devices having improved surface characteristics
InactiveUS7297725B2Reduce complexityHigh oxygen permeabilityOptical articlesCoatingsSpray coatingOxygen
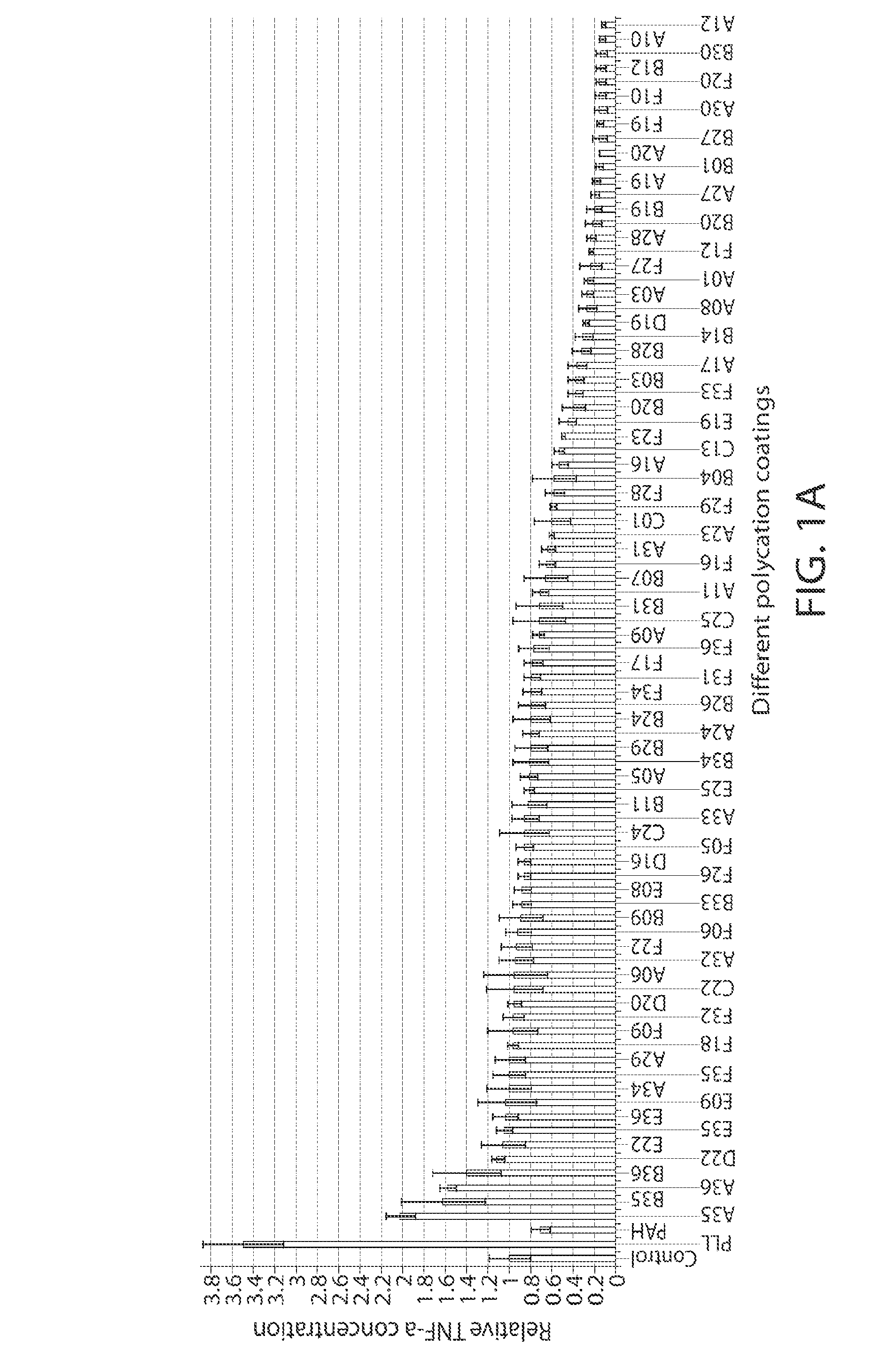
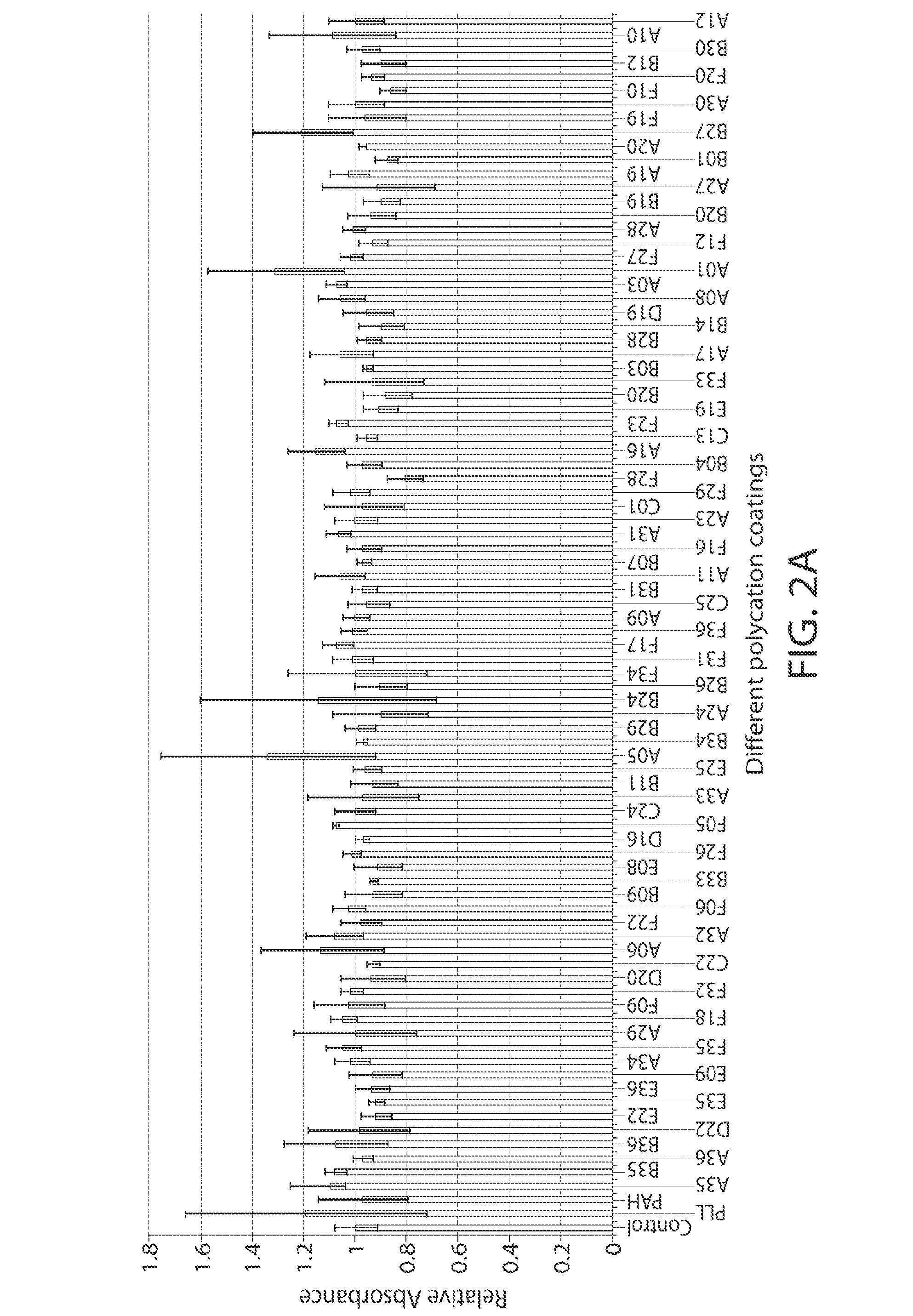
Biomedical devices, such as ophthalmic lenses, and methods of making such devices having a surface coating including at least one polyionic layer. A preferred method involves spray coating a polycationic material onto a core lens, rinsing and drying the lens, followed by spray coating a polyanionic material, rinsing and drying. The coating process may be applied a plurality of times to achieve a multi-layer coating on the lens surface. A particularly preferred embodiment is a contact lens comprising a highly oxygen permeable hydrophobic core coated with a 5 to 20 bilayers of hydrophilic polyionic materials.
Owner:NOVARTIS AG
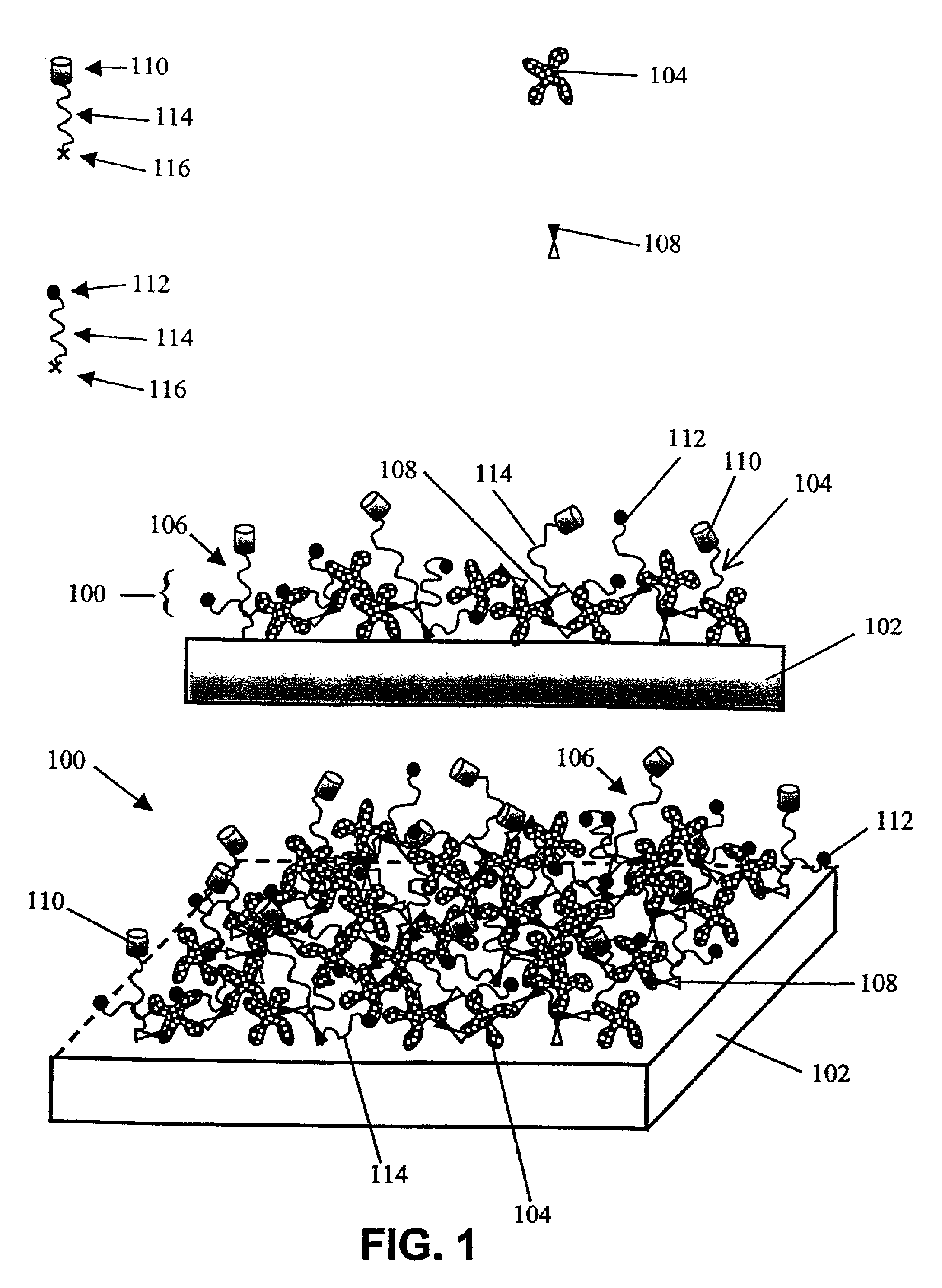
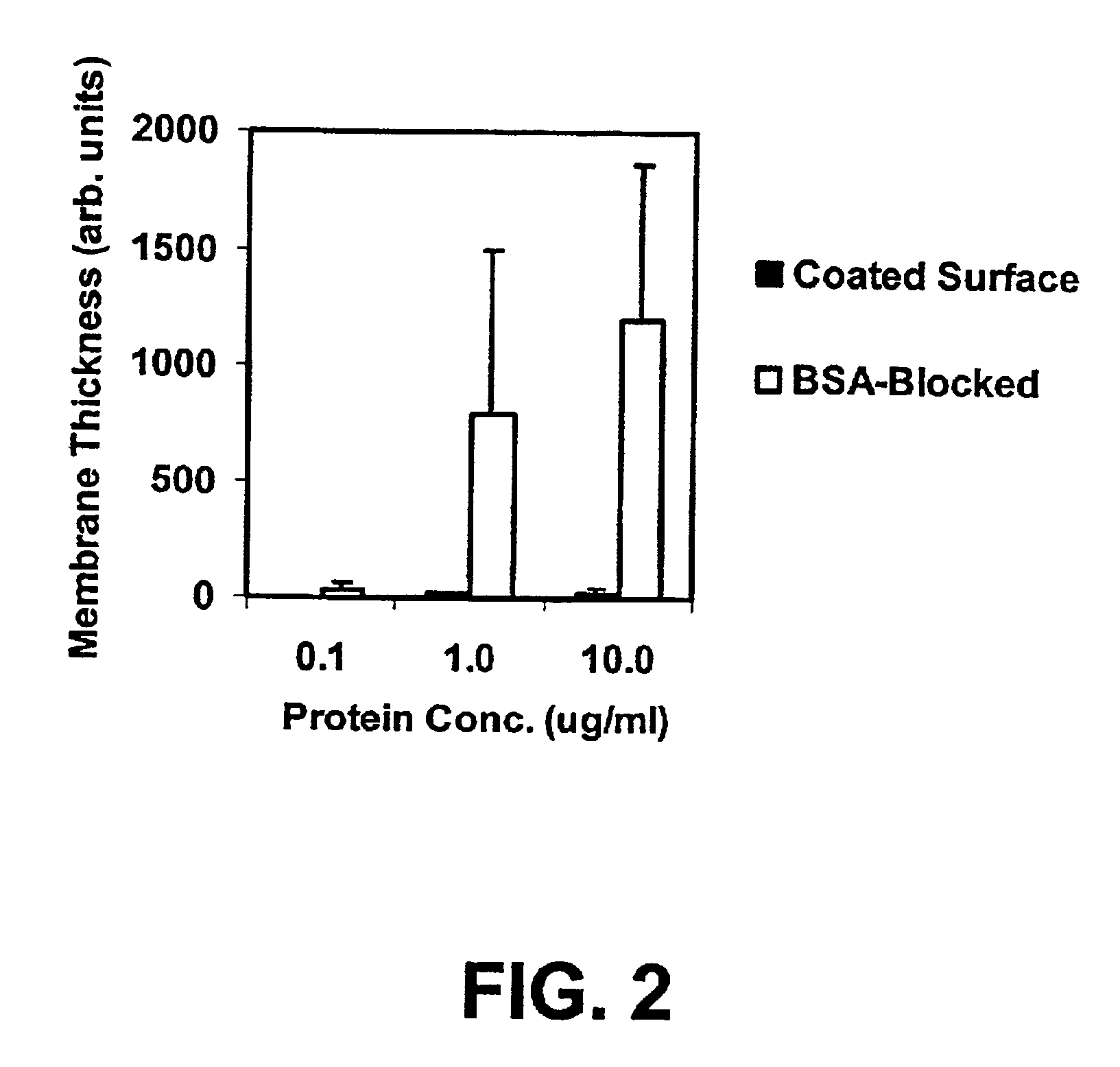
Functional surface coating
InactiveUS6844028B2Inhibit non-specific bindingImprove propertiesMicrobiological testing/measurementPreparing sample for investigationProtein targetBound property
Compositions and methods of preparing functional thin films or surface coatings with low non-specific binding are described. The thin films contain specified functional groups and non-specific binding repellant components. The thin films are either covalently bound to or passively adsorbed to various solid substrates. The specified functional group provides specified activity for the thin film modified solid surfaces and non-specific binding repellant components significantly reduce the non-specific binding to the thin film modified solid surfaces. Non-specific binding repellant components do not affect specified functional group's activity in the thin films. In these methods, specified functional groups are anchored to the solid substrates through a spacer. Surface coatings are also described having both non-specific protein binding properties combined with functional groups for specific binding activity thereby providing surface coating that specifically recognize target proteins but limit binding to non-specific protein.
Owner:ACCELERATED MEDICAL DIAGNOSTICS INC
3D Print Bed Having Permanent Coating
PendingUS20170036403A1Improve adhesionSufficient level of adhesionManufacturing platforms/substratesPretreated surfacesEpoxyWater based
A coated print bed for a 3D printer having a permanent print-surface coating permanently secured to a print bed substrate plate, having a smooth, planar surface that provides an adhesive interface layer between a first layer of an applied plastic print material and the coated print bed. The coating contains a matrix-forming compound, such as a solvent- or water-based epoxy resin, an adhesive material, and optionally a filler. The user can print a series of print object directly onto the permanent print surface coating of the coated print bed, without having to refresh or refurbish the print surface, such as by applying to the print bed surface a temporary coating such as painter's tape, or a liquid adhesive.
Owner:EZ PRINT
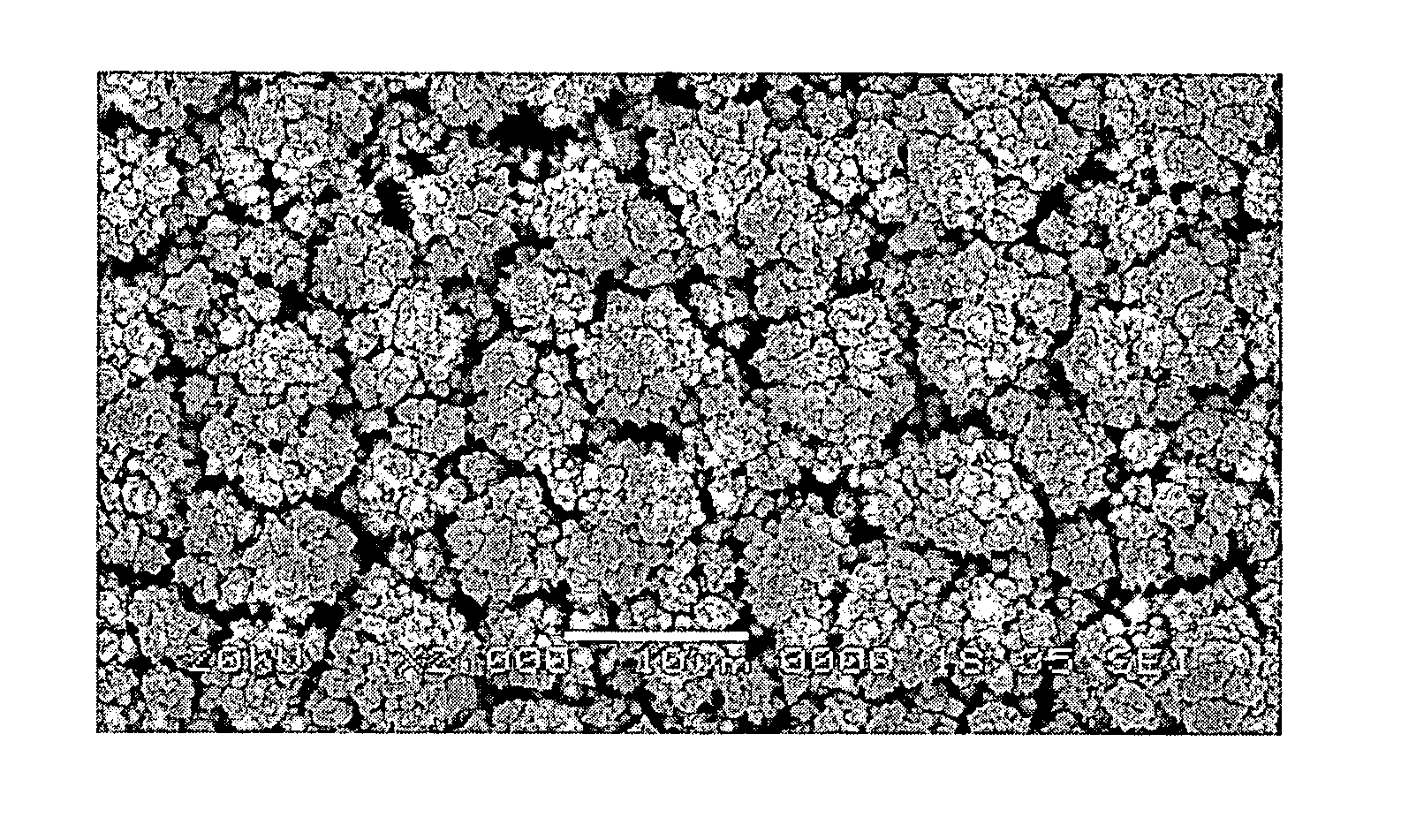
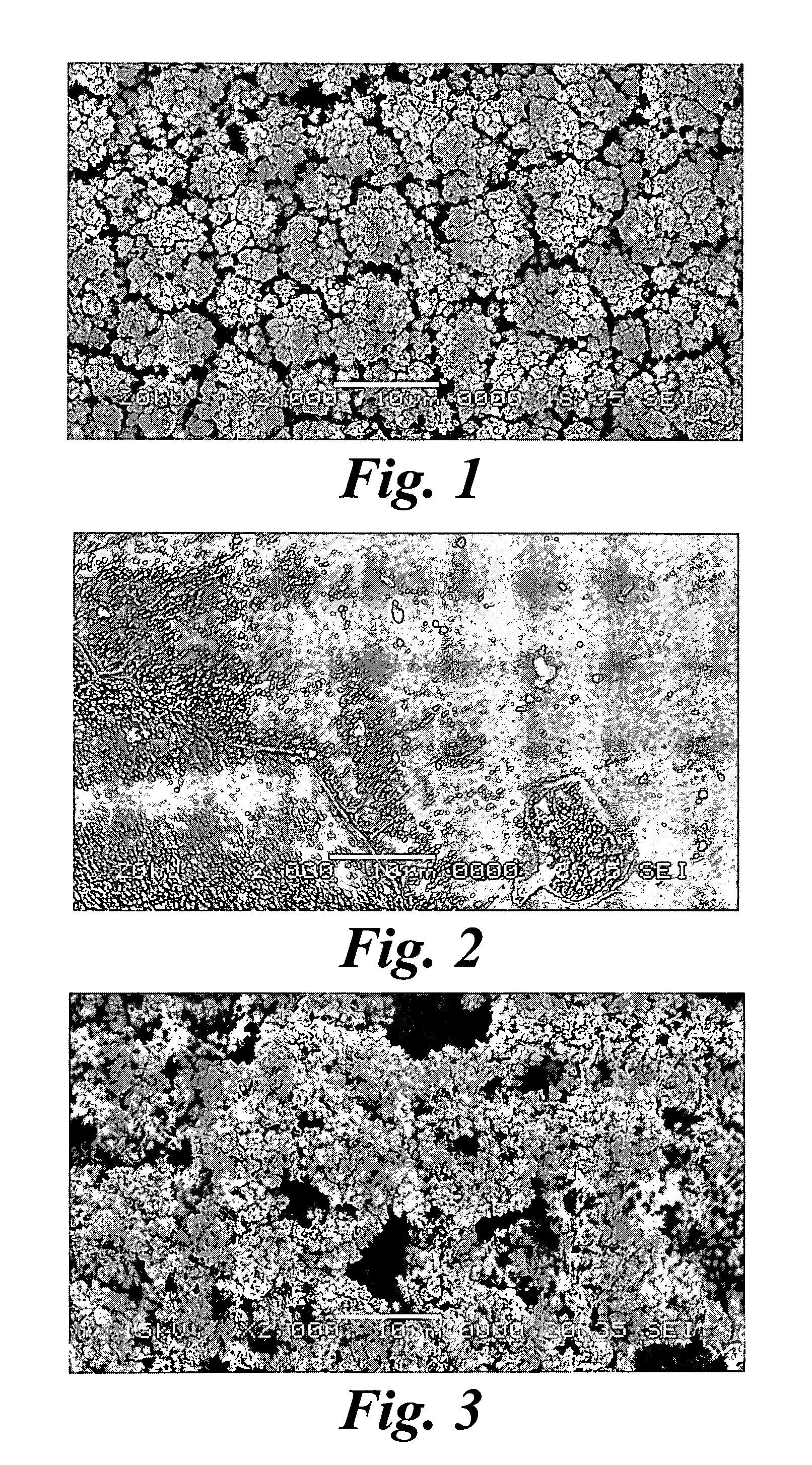
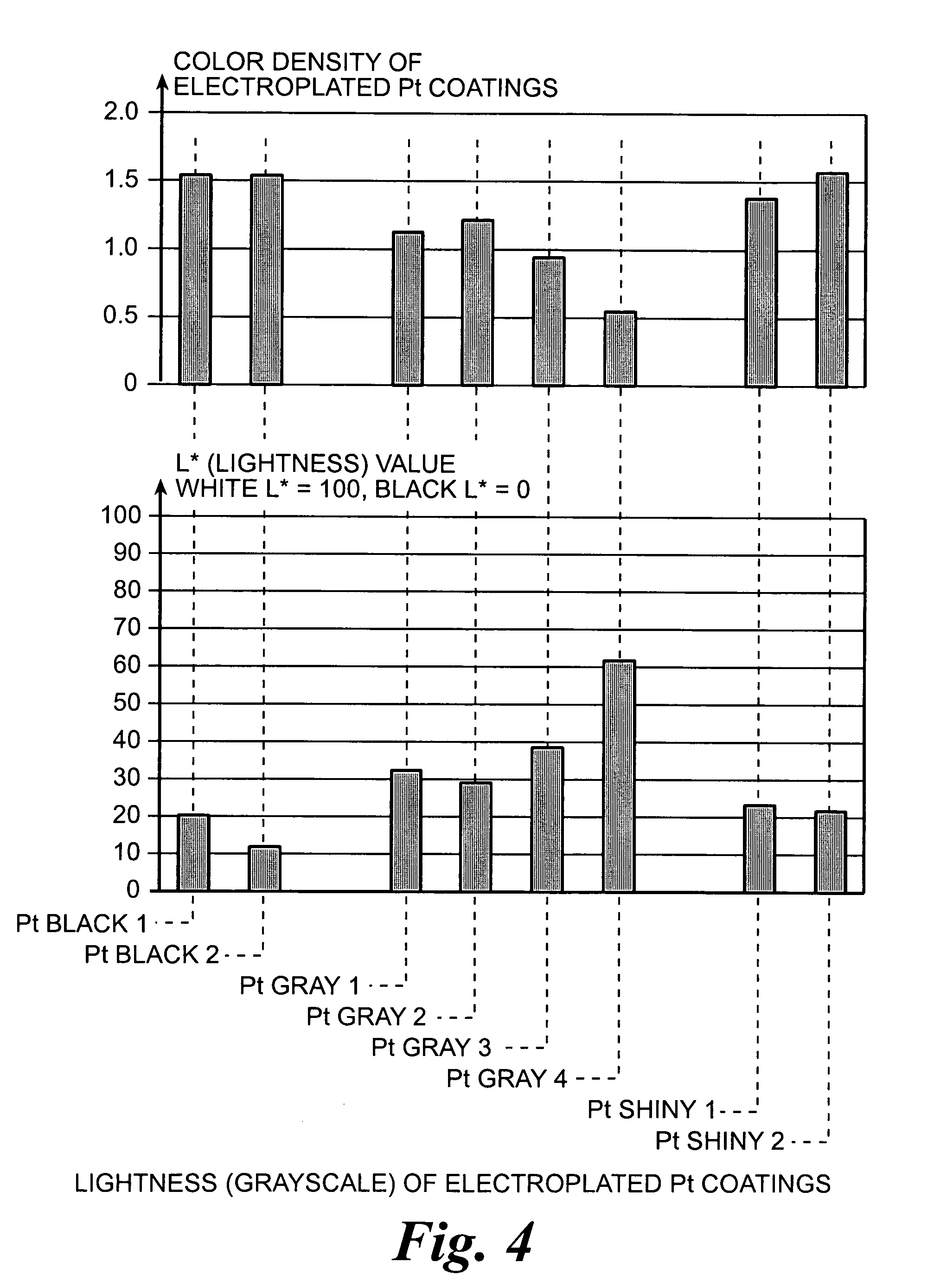
Platinum electrode and method for manufacturing the same
InactiveUS6974533B2Increase surface areaSufficient physical and structural strengthCellsHead electrodesMetallurgyPt element
An improved electrode and method for manufacturing the improved electrode wherein the electrode having a fractal surface coating of platinum [which the present inventor refers to as “platinum gray”] with a increase in surface area of at least 5 times when compared to shiny platinum of the same geometry and also having improved resistance to physical stress when compared to platinum black having the same surface area. The process of electroplating the surface coating of platinum gray comprising plating at a moderate rate, i.e., at a rate that is faster than the rate necessary to produce shiny platinum and that is less than the rate necessary to produce platinum black.
Owner:CORTIGENT INC
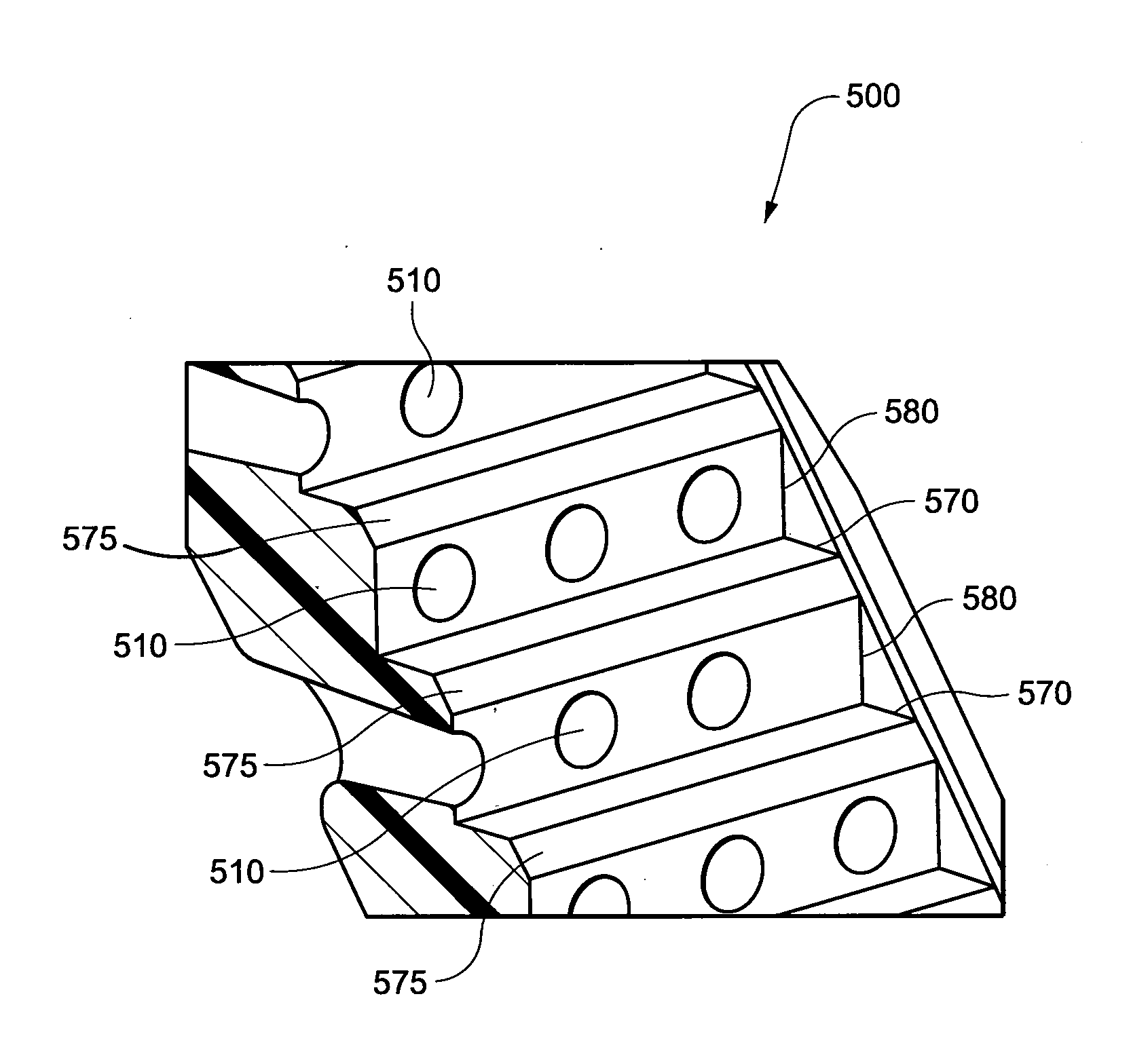
Patient interface systems
ActiveUS20120132209A1Easy to useEasy to appreciateControlling ratio of multiple fluid flowsBreathing masksElastomerEngineering
A headgear for use with a patient interface for delivering a flow of breathable gas to a patient includes at least a strap (741) adapted to position the patient interface in sealing engagement with the patient's airways. The strap is constructed from an elastomer and a first side of the strap includes a first region (215) on a portion of its surface that is textured to reduce friction with objects contacting the strap. A textured surface coating for a portion of an elastomer strap included in a headgear system is adapted to contact the skin of a patient, when in use, and the coating has a Ra value greater than zero. A vent (500) for use with a patient interface for delivering a flow of breathable gas to a patient includes a plurality of rises (580) and runs (570) in a stepped arrangement; and a plurality of holes (510) in the stepped arrangement for the venting of gas.
Owner:RESMED LTD
Ablation catheter with contoured openings in insulated electrodes
InactiveUS20070005053A1Minimize changesSurgical instruments for heatingEdge effectsBiomedical engineering
An array of ring electrodes or a wire electrode is mounted about the outside surface of the distal end of the ablation catheter. Substantially all of the outer surface of each ring or wire electrode is covered by an electrically insulating coating. The insulating surface coating on each ring defines a contoured opening in the insulating surface coating that exposes the conductive band or wire beneath. An array of contoured openings are formed along a wire electrode. The insulating coating mitigates potential edge effects that create hot spots and can result in unwanted tissue damage during an ablation procedure.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
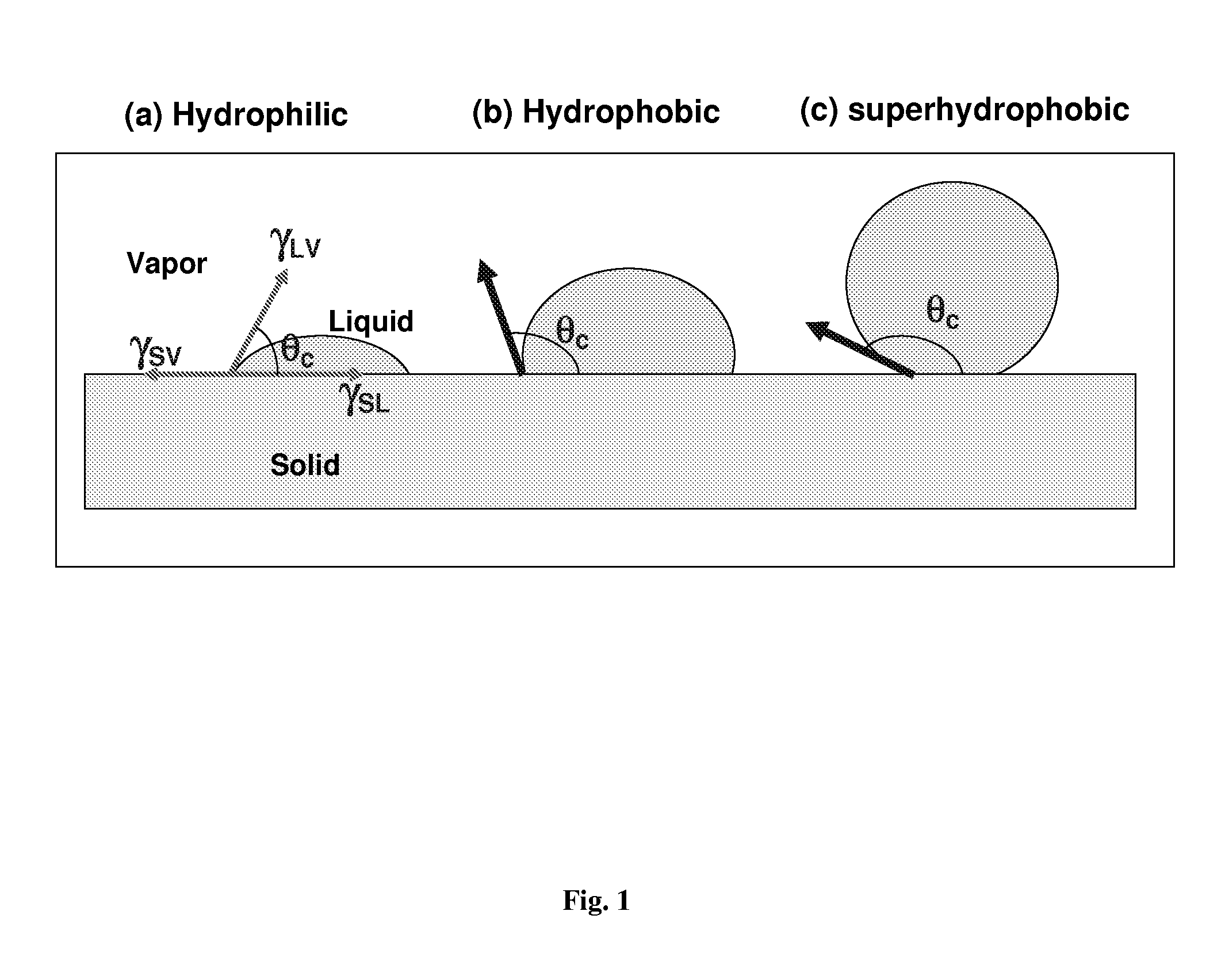
Self-cleaning lotus effect surfaces having antimicrobial properties
A self-cleaning or lotus-effect surface that has antimicrobial properties, commercial products comprising such a surface, and uses thereof. A process for the production of an antimicrobial self-cleaning or lotus-effect surface in which one or more antimicrobial polymer(s) is secured to a surface-coating system for securing structure-formers to generate a self-cleaning surface. This method lastingly binds antimicrobial polymers to the self-cleaning surface. Commercial products comprising an antimicrobial self-cleaning or lotus-effect surface.
Owner:CREAVIS GES FUER TECH
Appliance and method for surface treatment of a board shaped material and floorboard
InactiveUS20080000417A1Improve accuracyCovering/liningsSpecial ornamental structuresMaterials scienceSurface coating
A device for coating surface portions of a board material with a liquid material. The device includes a wheel which transfers the coating material and compressed air which positions the coating material. A method for surface coating and a floorboard with a finished surface portion.
Owner:VÄLINGE INNOVATION AB
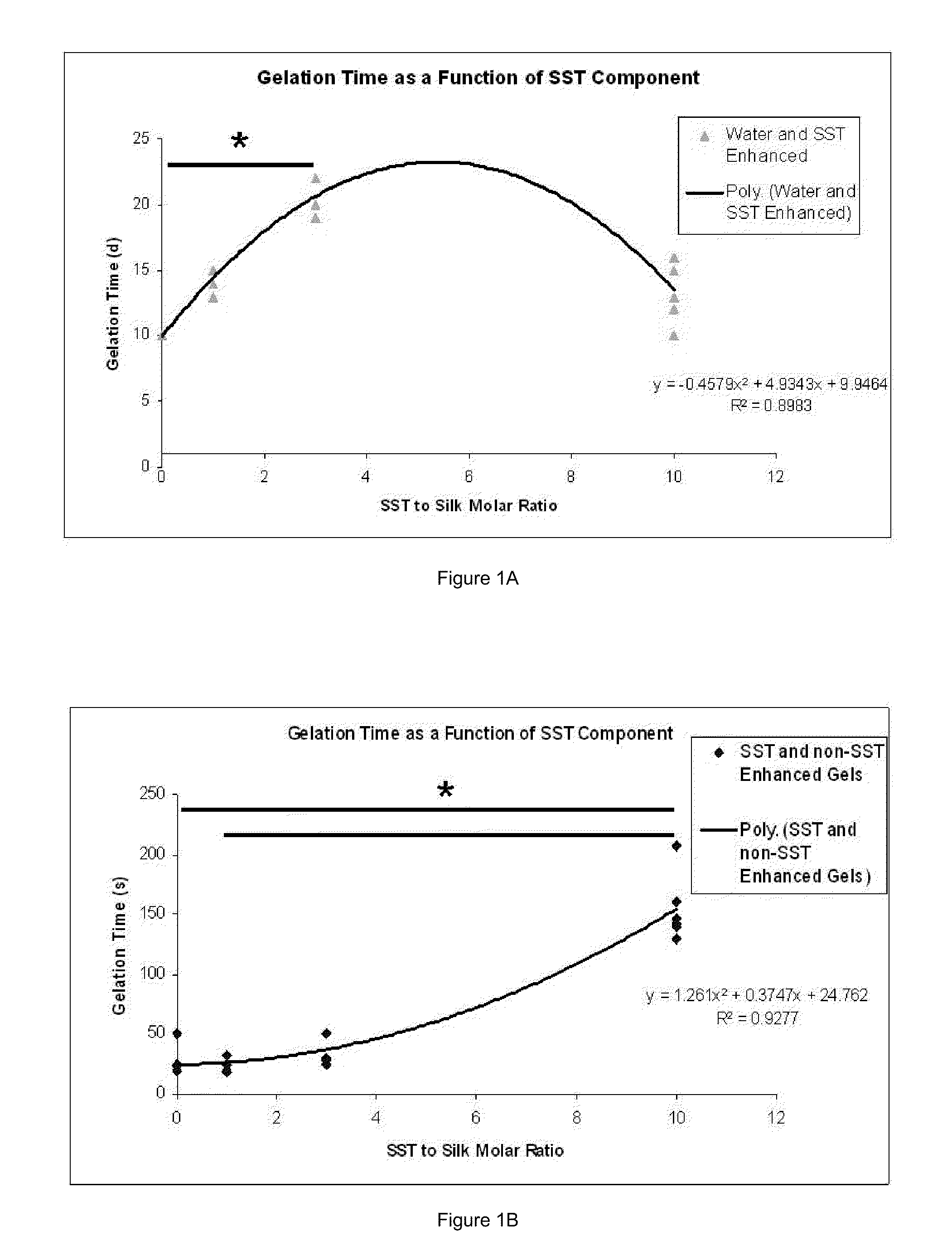
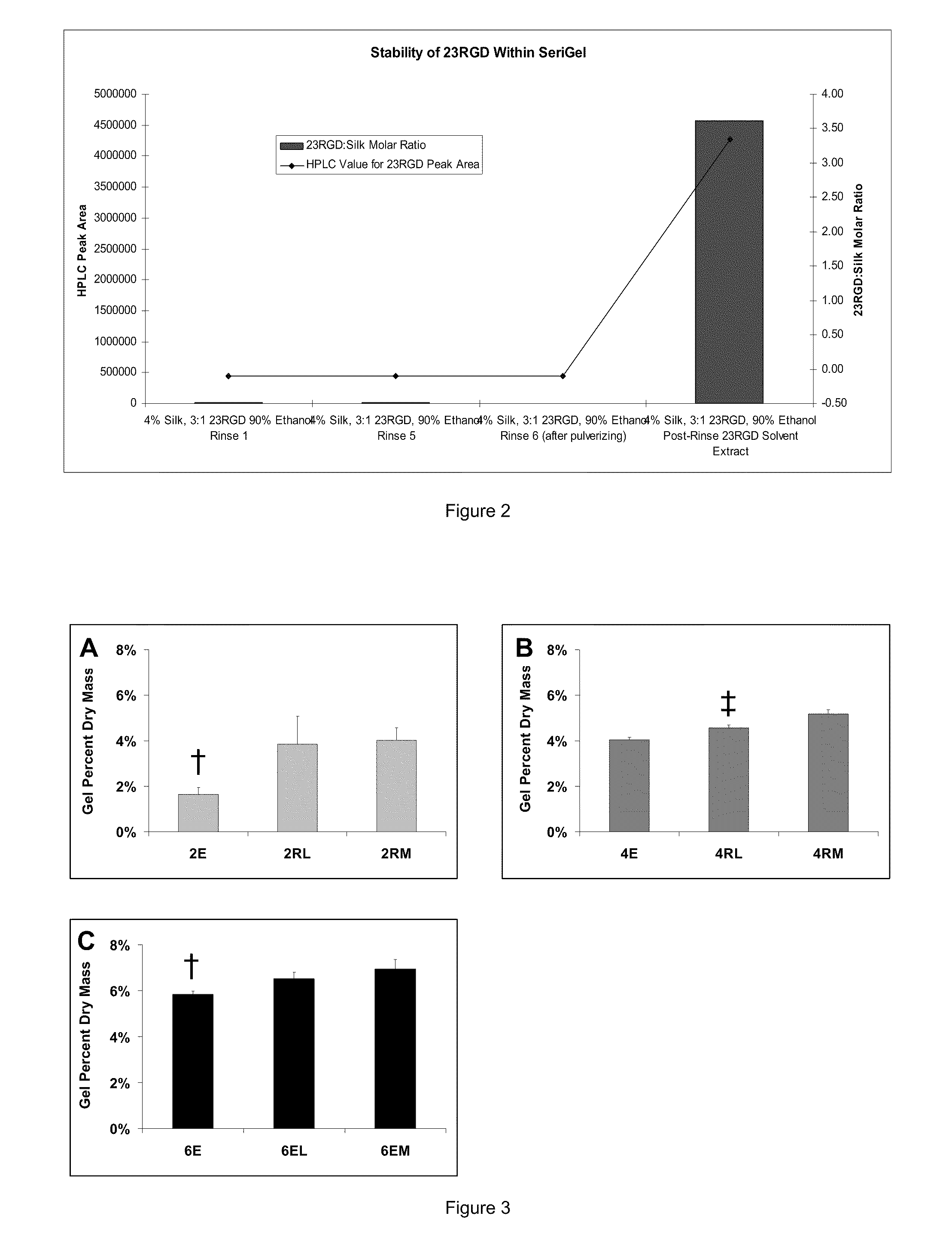
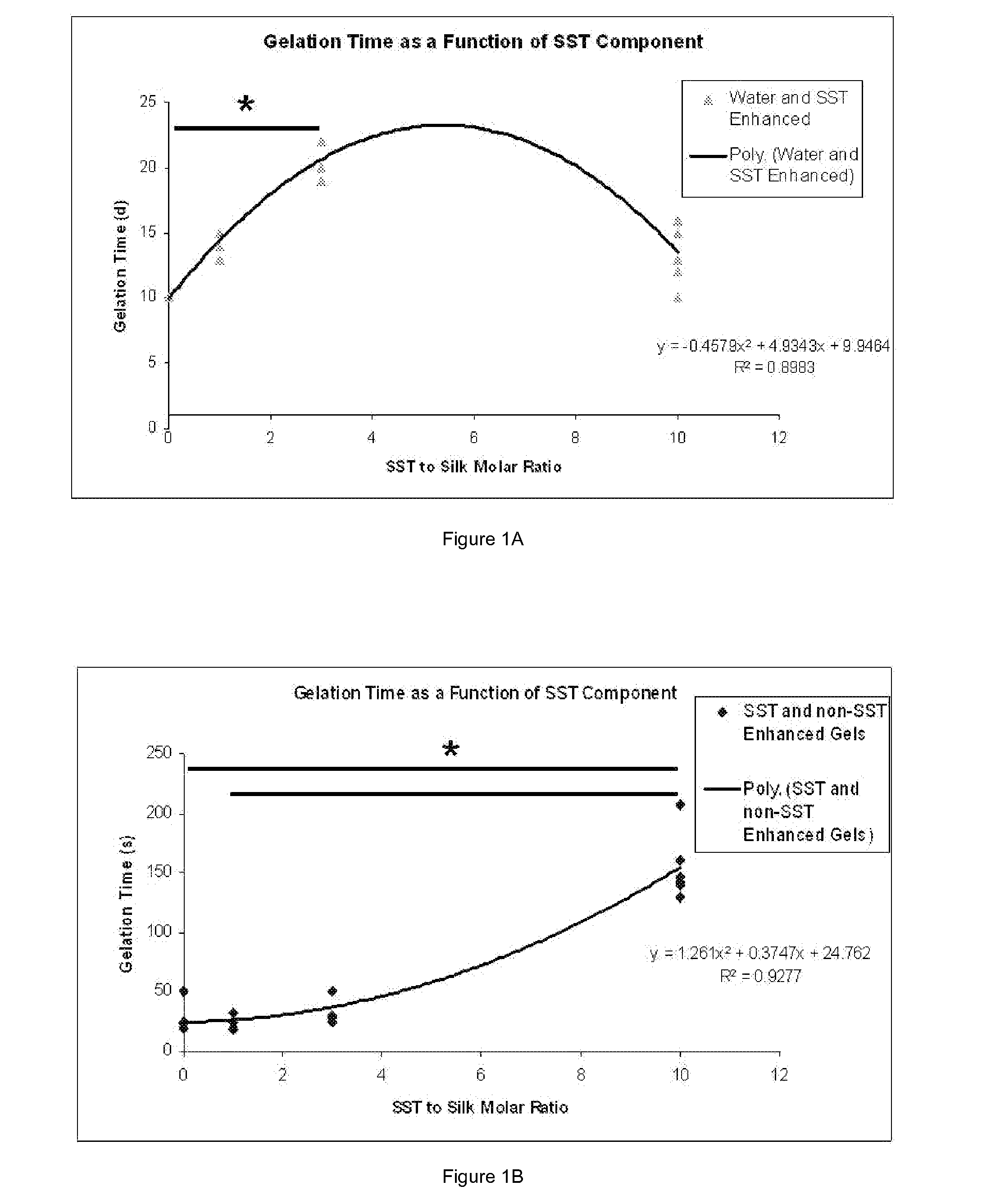
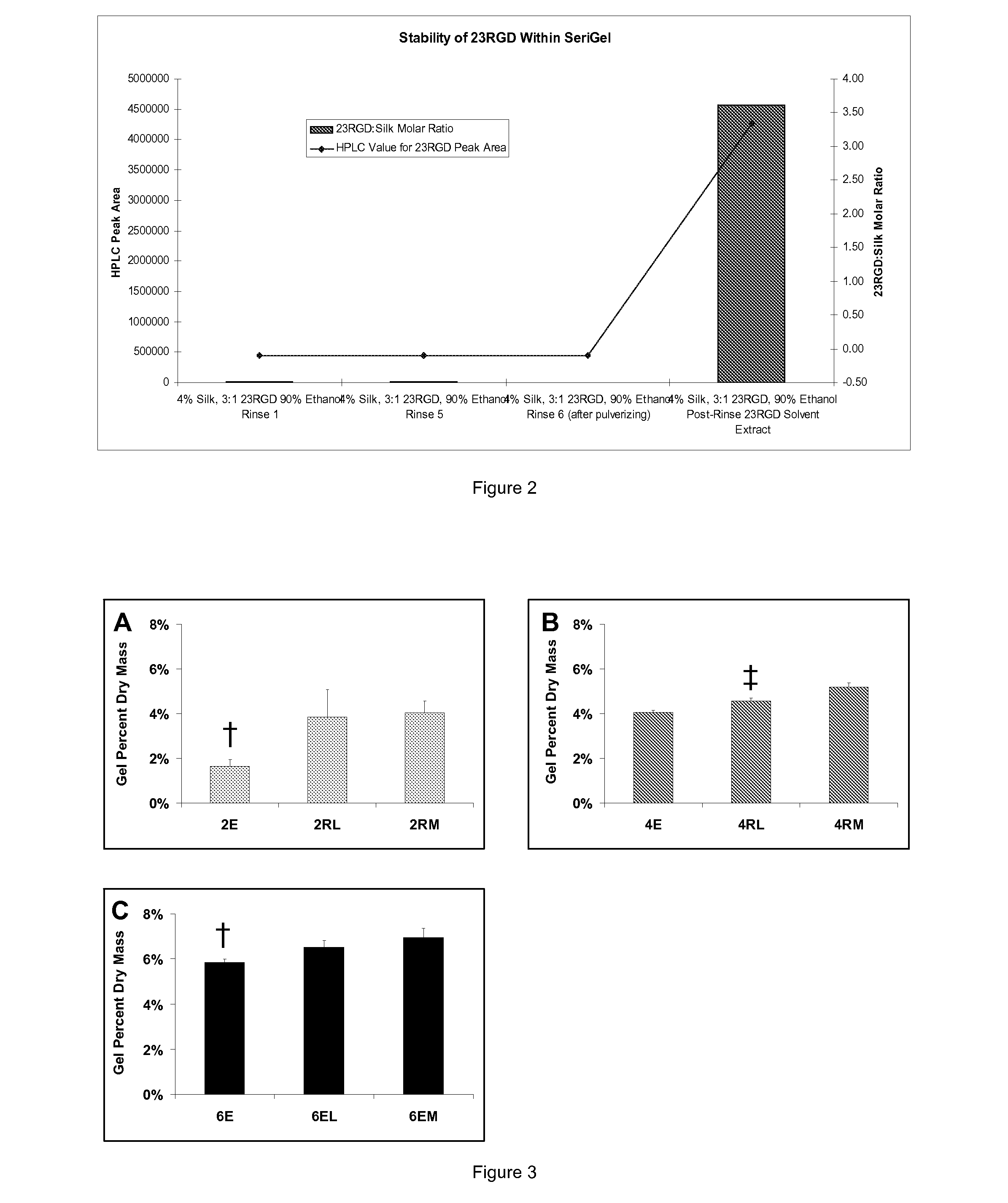
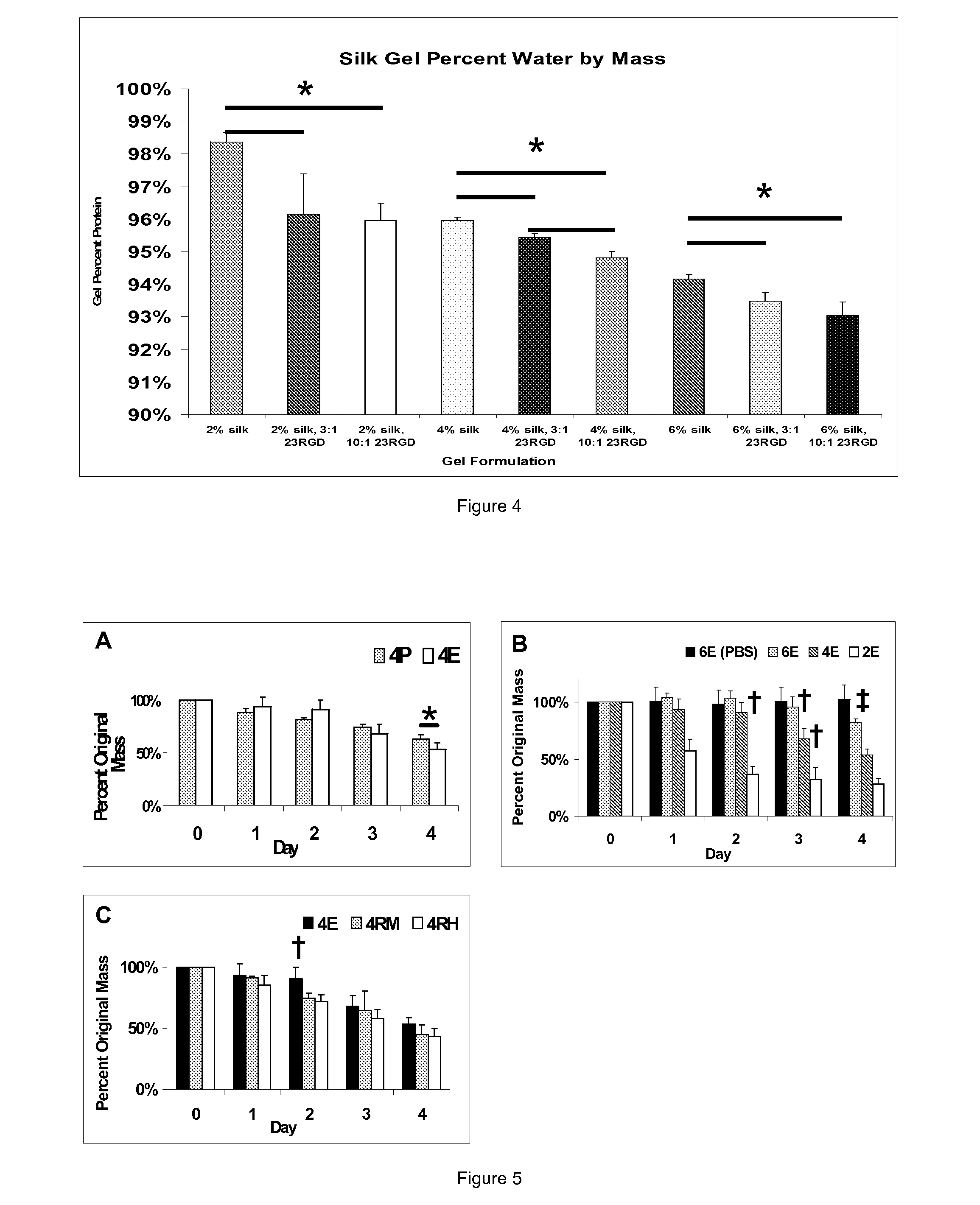
Silk Fibroin Hydrogels and Uses Thereof
InactiveUS20110008406A1Increase profitGood biocompatibilityBiocideCosmetic preparationsDiseaseFibronectin binding
The present specification provides for methods for purifying fibroins, purified fibroins, methods of conjugating biological and synthetic molecules to fibroins, fibroins conjugated to such molecules, methods of making fibroin hydrogels, fibroin hydrogels and fibroin hydrogel formulations useful for a variety of medical uses, including, without limitation uses as bulking agents, tissue space fillers, templates for tissue reconstruction or regeneration, cell culture scaffolds for tissue engineering and for disease models, surface coating to improve medical device function, or drug delivery devices.
Owner:ALLERGAN INC
Silk Fibroin Hydrogels and Uses Thereof
InactiveUS20110008437A1Increase profitGood biocompatibilityPowder deliveryCosmetic preparationsDiseaseFibronectin binding
The present specification provides for methods for purifying fibroins, purified fibroins, methods of conjugating biological and synthetic molecules to fibroins, fibroins conjugated to such molecules, methods of making fibroin hydrogels, fibroin hydrogels and fibroin hydrogel formulations useful for a variety of medical uses, including, without limitation uses as bulking agents, tissue space fillers, templates for tissue reconstruction or regeneration, cell culture scaffolds for tissue engineering and for disease models, surface coating to improve medical device function, or drug delivery devices.
Owner:ALLERGAN INC
Process for producing detachable dirt- and water-repellent surface coatings
ActiveUS7083828B2Good removal effectSimple wayFouling preventionSolid waste managementWaxSurface structure
Process for producing detachable dirt- and water-repellent surface coatings on articles, wherein during the coating process, hydrophobic particles are applied to the surface of the articles, thus generating a surface structure with elevations on that surface of the articles that has dirt- and water-repellent properties, which comprises suspending the hydrophobic particles in a solution of a silicone wax in a highly volatile siloxane, and applying this suspension to at least one surface of an article, and then removing the highly volatile siloxane.
Owner:EVONIK DEGUSSA GMBH
Nanostructured superhydrophobic, superoleophobic and/or superomniphobic coatings, methods for fabrication, and applications thereof
Systems, techniques and applications for nanoscale coating structures and materials that are superhydrophobic with a water contact angle greater than about 140° or 160° and / or superoleophobic with an oil contact angle greater than about 140° or 160°. The nanostructured coatings can include Si or metallic, ceramic or polymeric nanowires that may have a re-entrant or mushroom-like tip geometry. The nanowired coatings can be used in various self-cleaning applications ranging from glass windows for high-rise buildings and non-wash automobiles to pipeline inner surface coatings and surface coatings for biomedical implants.
Owner:RGT UNIV OF CALIFORNIA
Screw thread placement in a porous medical device
Methods are provided for manufacturing an internal thread in a porous orthopedic implant, such as an orthopedic anchor. In one embodiment, the internal thread is formed in the orthopedic implant by bonding a pre-formed, internally threaded component to the orthopedic implant. In another embodiment, the internal thread is formed in the orthopedic implant by bonding a solid plug to the orthopedic implant or forming a surface coating on the orthopedic implant and then machining the solid plug or surface coating.
Owner:ZIMMER INC
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6165644AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Enhanced surfaces, coatings, and related methods
Disclosed are methods for incorporating additives, such as chemically active particles, into the surfaces of articles or into coatings disposed atop articles. The disclosed methods are also applicable to conventional molding techniques, and can be performed in batch or continuous fashion.
Owner:TPK HLDG
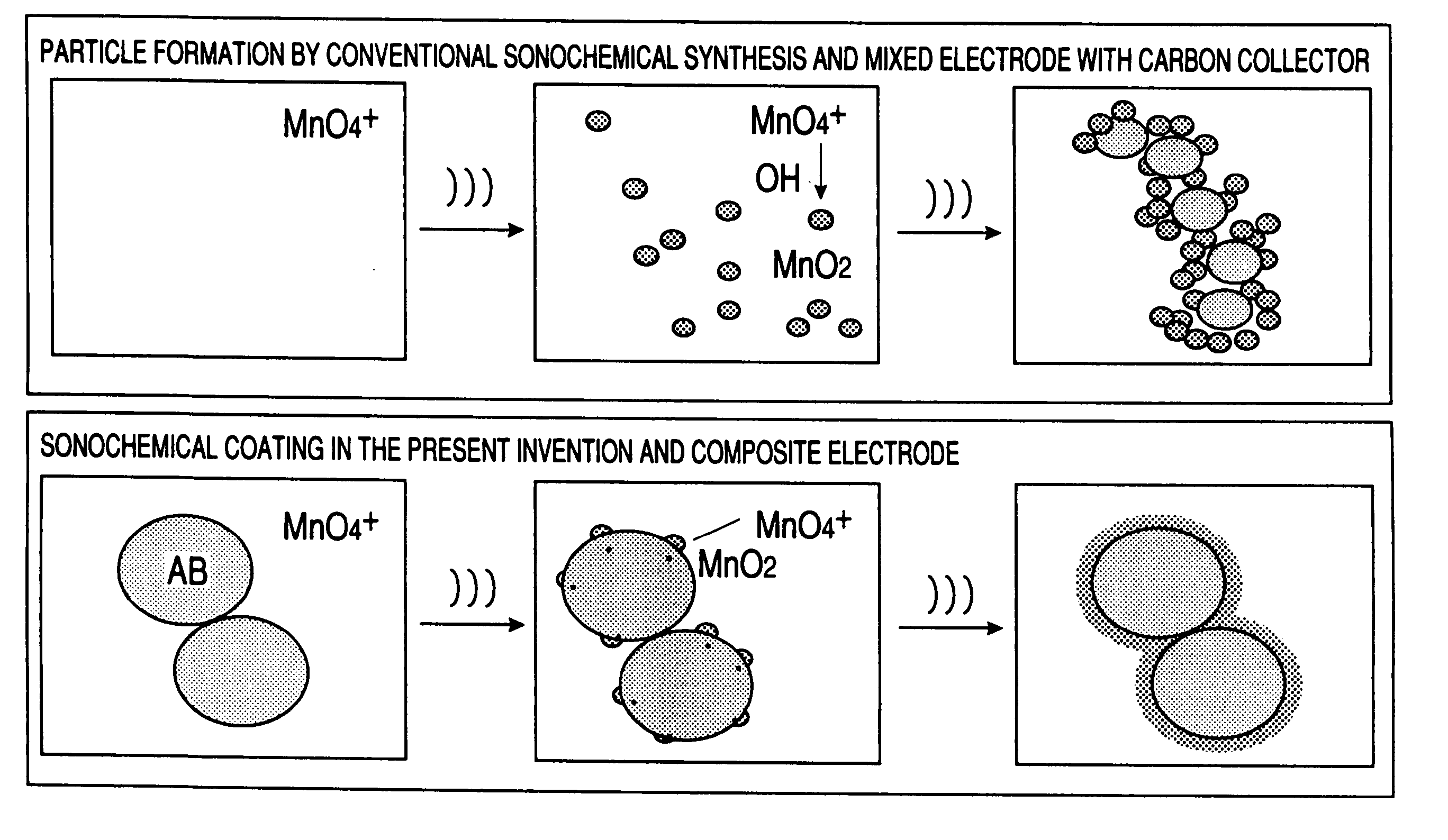
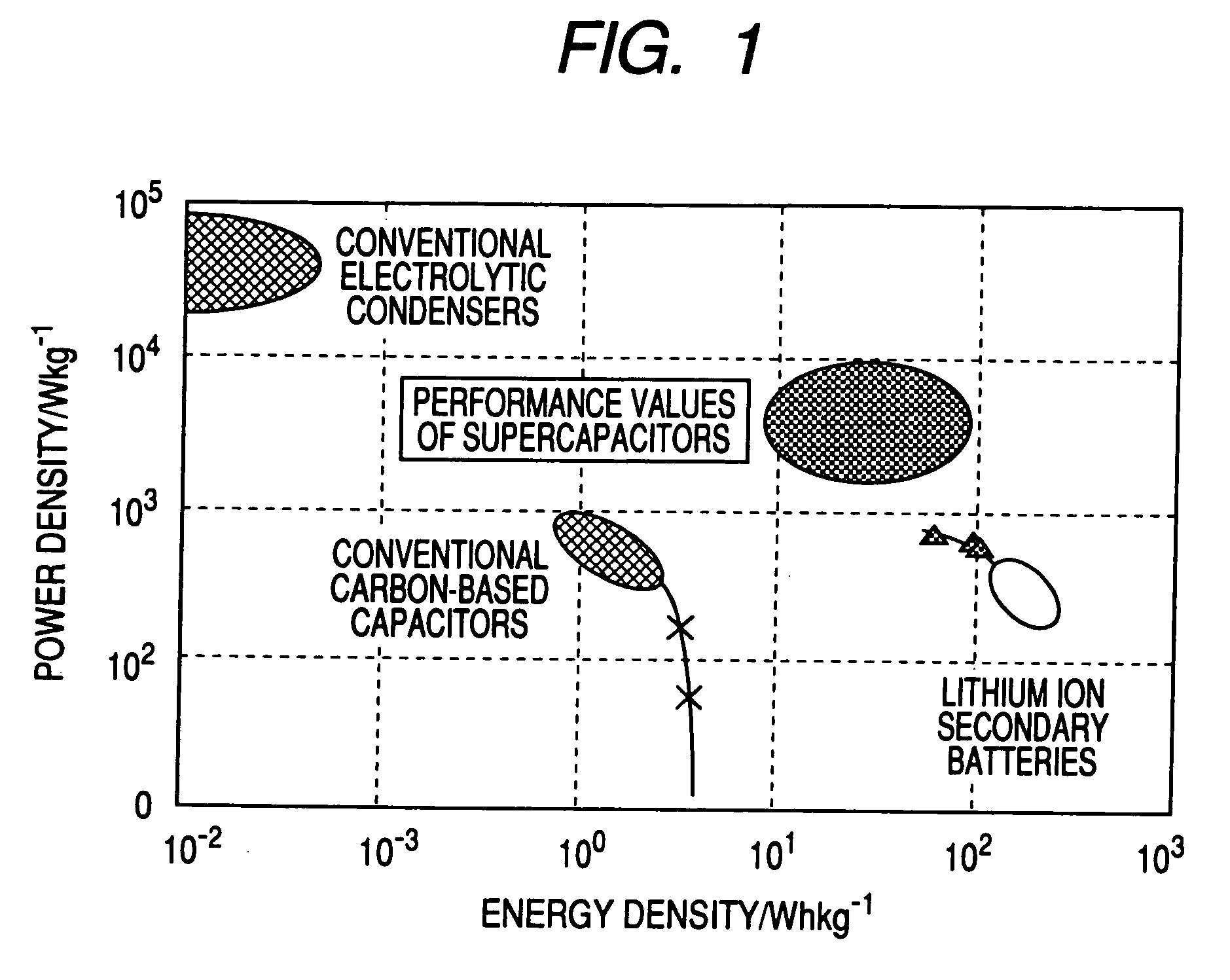
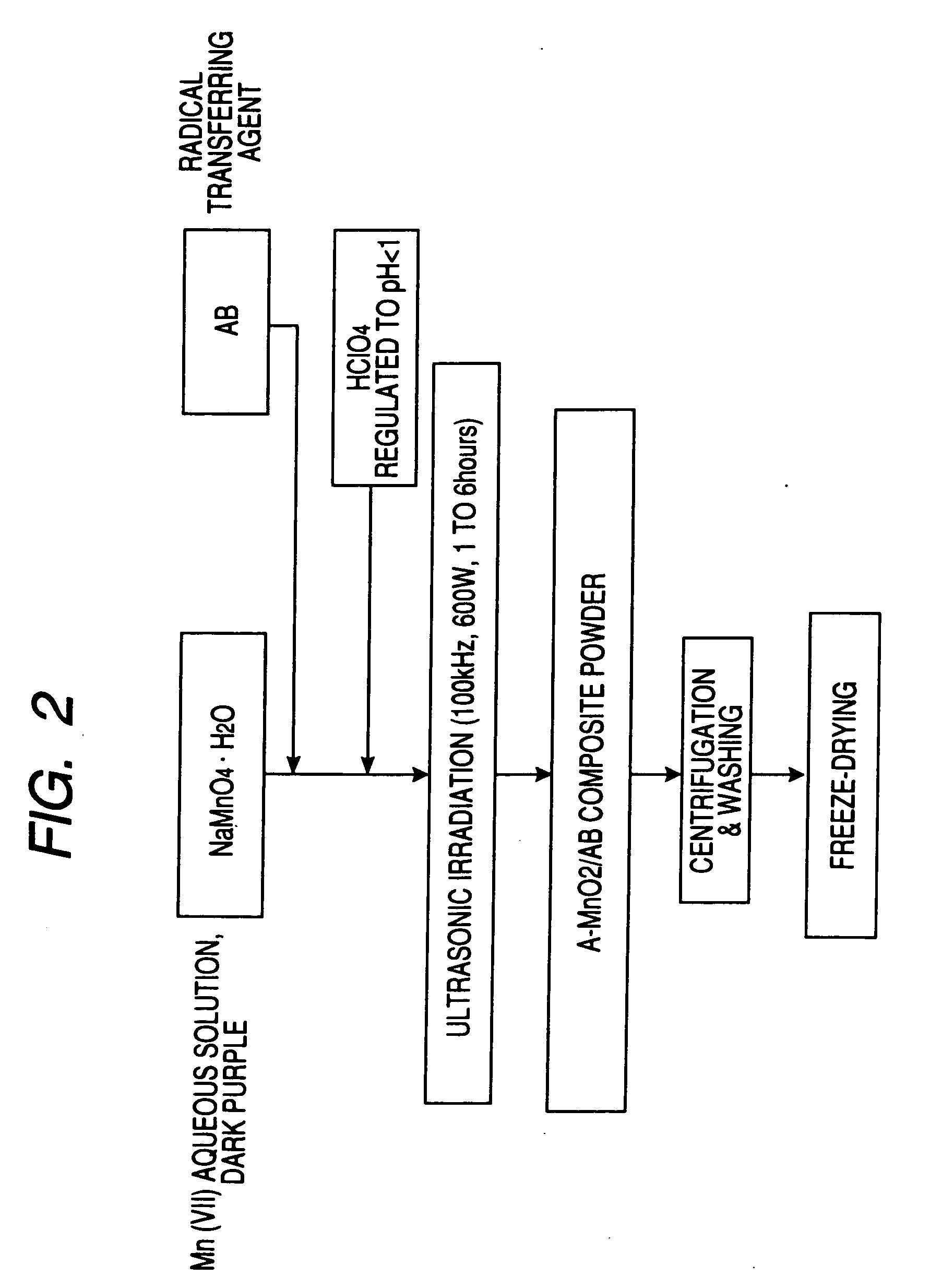
Carbon fine powder coated with metal oxide, metal nitride or metal carbide, process for producing the sdame, and supercapacitor and secondary battery carbon fine powder
InactiveUS20060154071A1Increase surface areaPigmenting treatmentDouble layer capacitorsCapacitanceElectrical resistance and conductance
A carbon fine powder is obtained by uniformly coating the surface of a carbon fine powder having a large specific surface area with a uniform thin film layer of a metal oxide, metal nitride or metal carbide as an electrode active substance material through induction of a sonochemical reaction. It is found that the obtained carbon fine powder has a low electrical resistance and shows a rapid faradaic process (pseudo-capacitance) of the surface coating layer. The coated carbon fine powder, a process for producing the same, and a supercapacitor and a secondary battery using the carbon fine powder are disclosed.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Thermoplastic fluoropolymer-coated medical devices
A medical device provided with at least a partial surface coating of a thermoplastic copolymer of tetrafluoroethylene and perfluoroalkylvinylether that is free of cross-linking monomers and curing agents. The fluoropolymer coating is preferably an amorphous thermoplastic, is highly inert and biocompatible, has elastomeric characteristics that provide desirable mechanical properties such as good flexibility and durability. These characteristics allow the coating to be considered “functionally transparent” because it withstands mechanical deformations required for the assembly, deployment, expansion, and placement of medical devices, without any adverse effect on the mechanical and biological functionality of the coated device. Further, its inertness, derived from the perfluorocarbon structure, contributes to its functionally transparent nature. The coating can be provided with various liquid or solid additives, can be loaded with large quantities of additives including a wide range of therapeutic agents, and has excellent drug elution characteristics when elutable additives are used. The desirable mechanical characteristics are surprising given the absence of cross-linking monomers and curing agents that would otherwise render such materials inadequately biocompatible. The perfluoroalkylvinylether may be perfluoromethylvinylether, perfluoroethylvinylether or perfluoropropylvinylether.
Owner:WL GORE & ASSOC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com