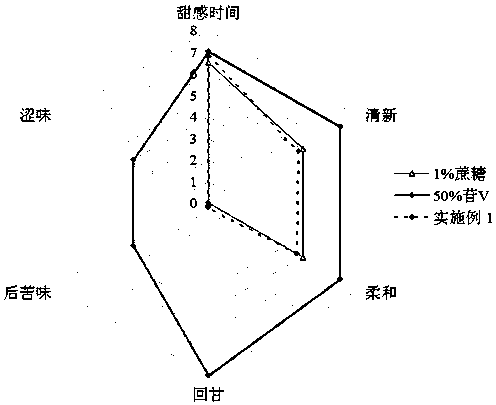
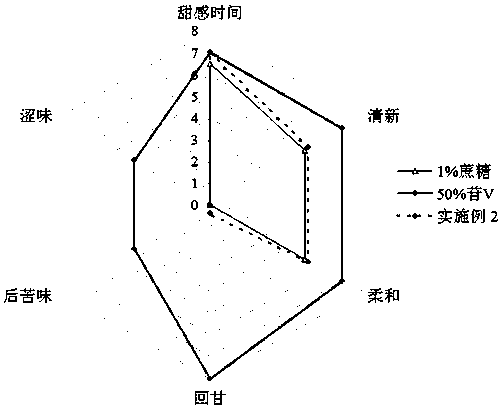
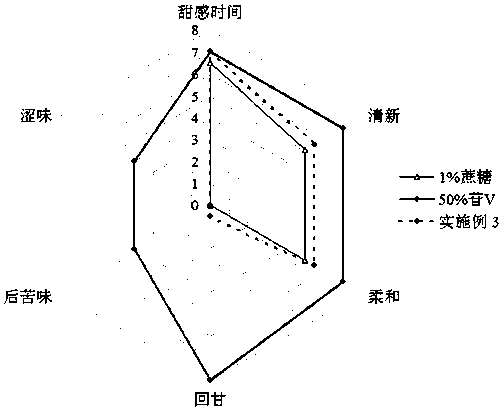
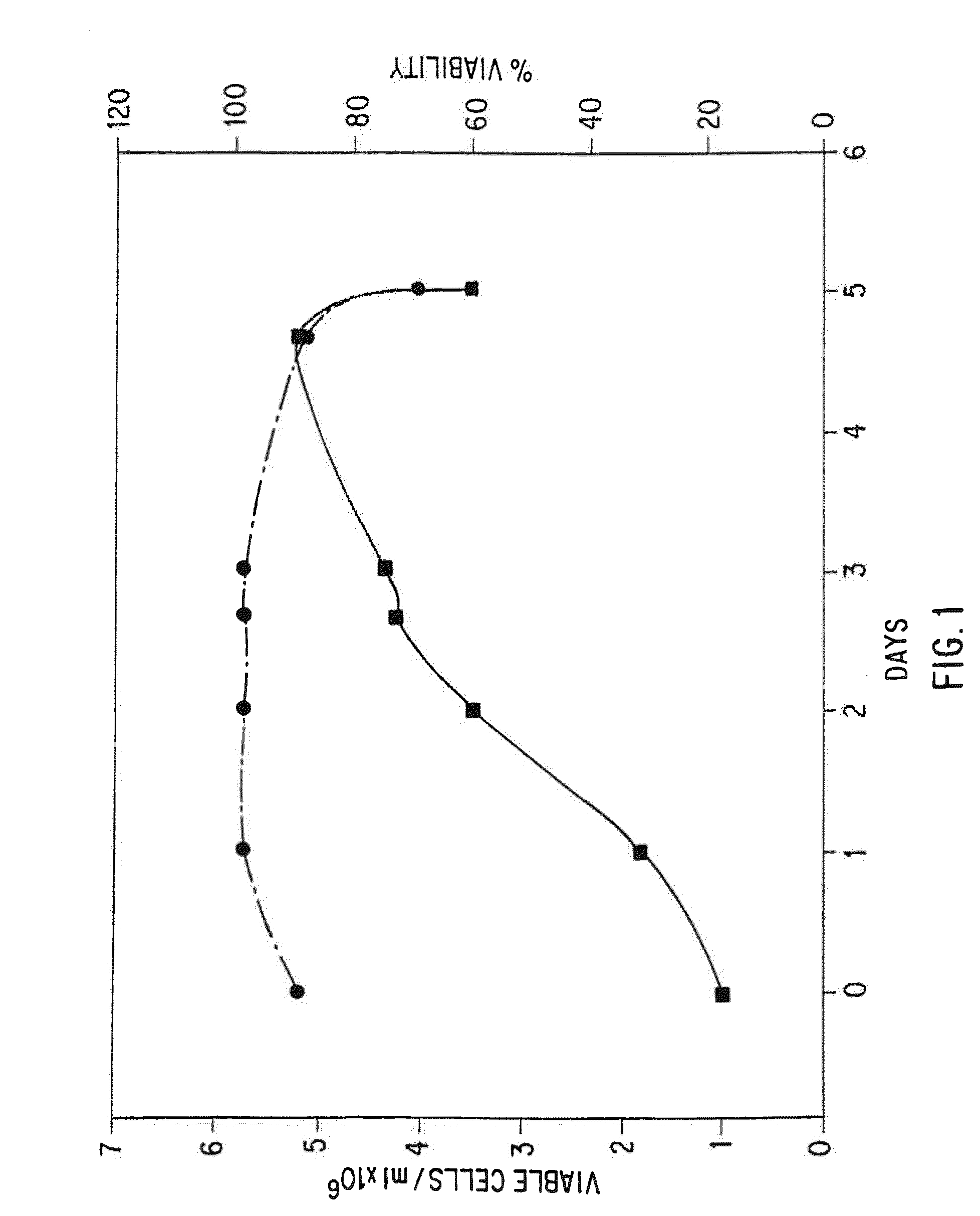
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
134 results about "Protein free" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
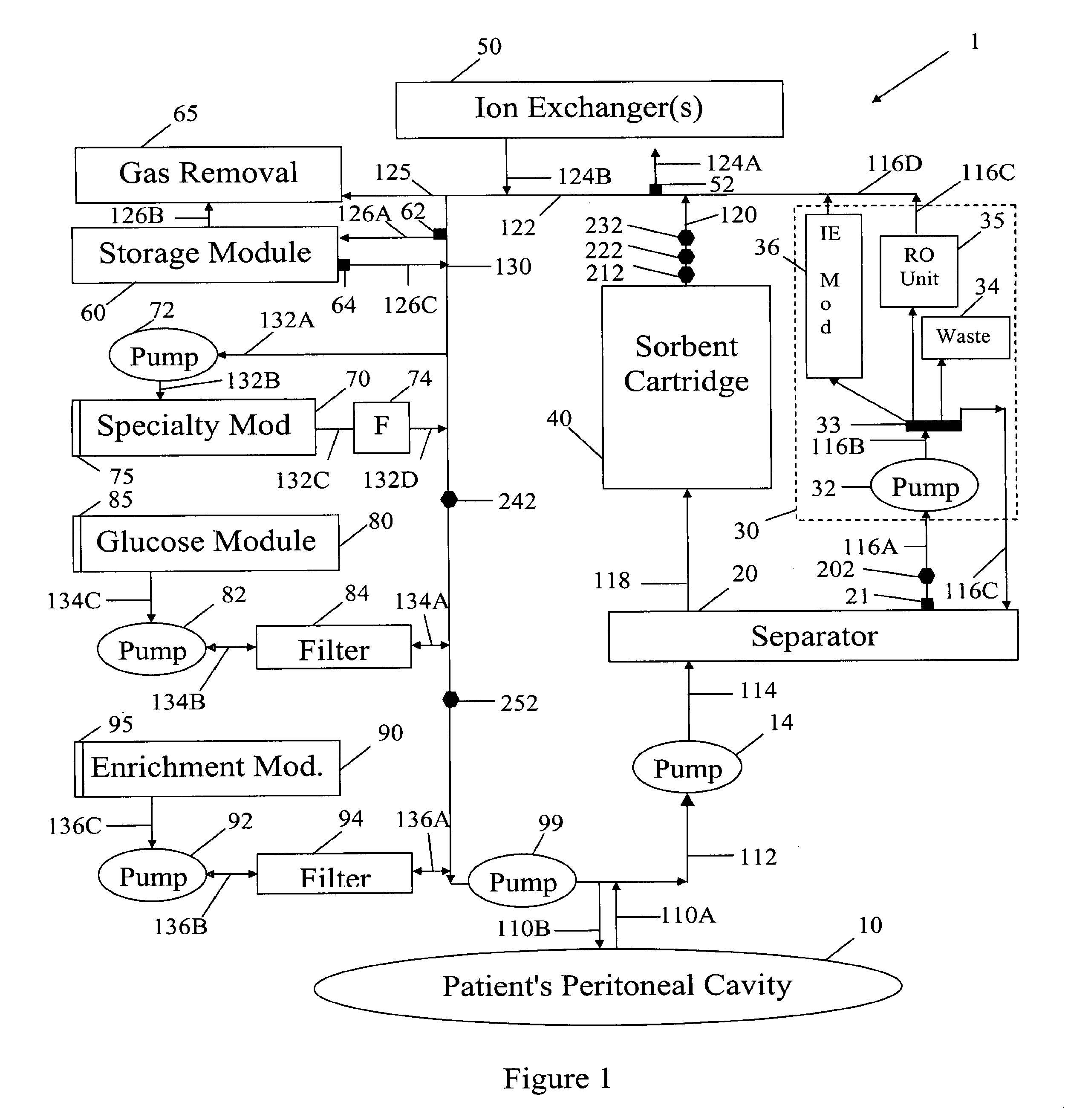
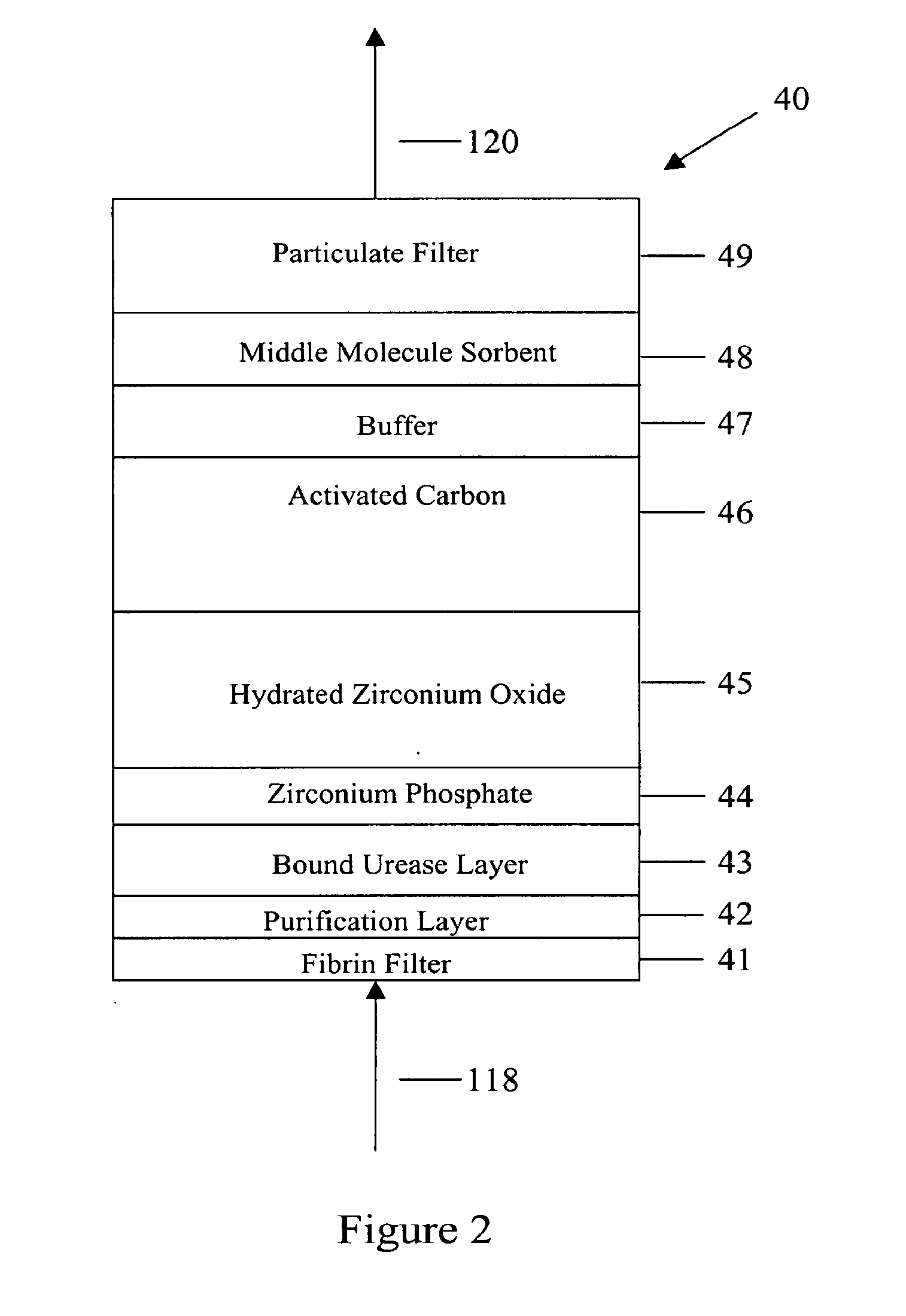
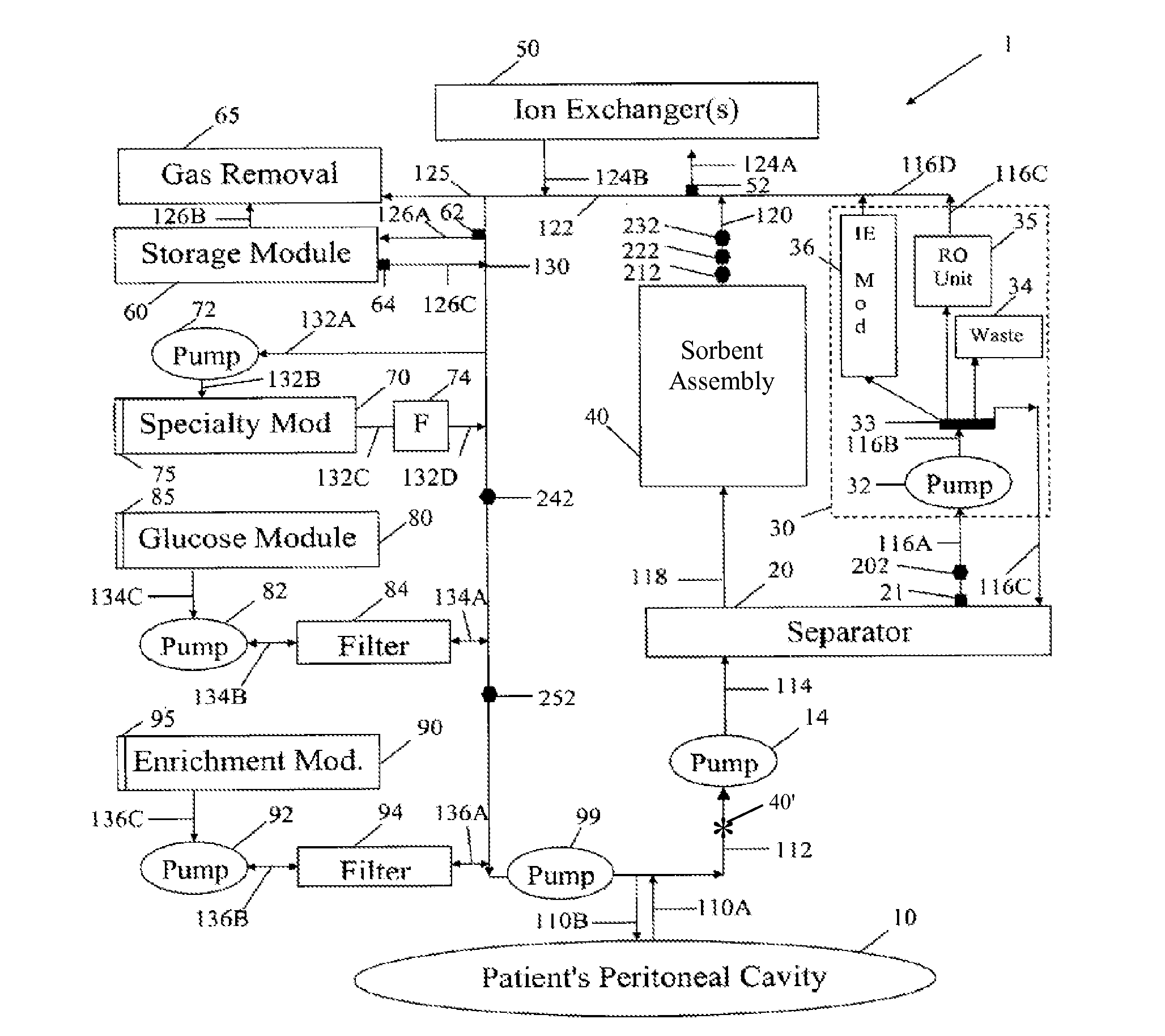
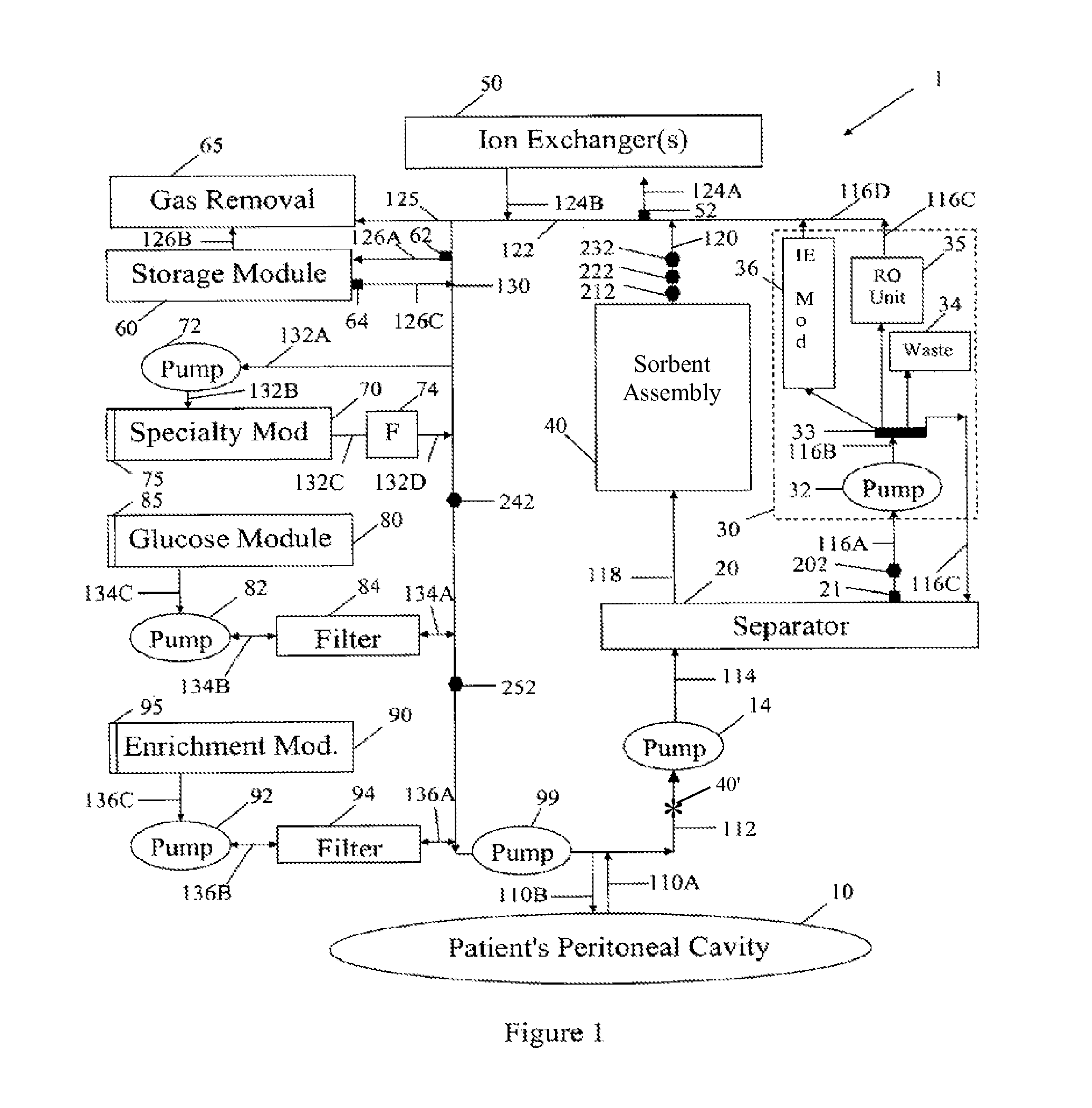
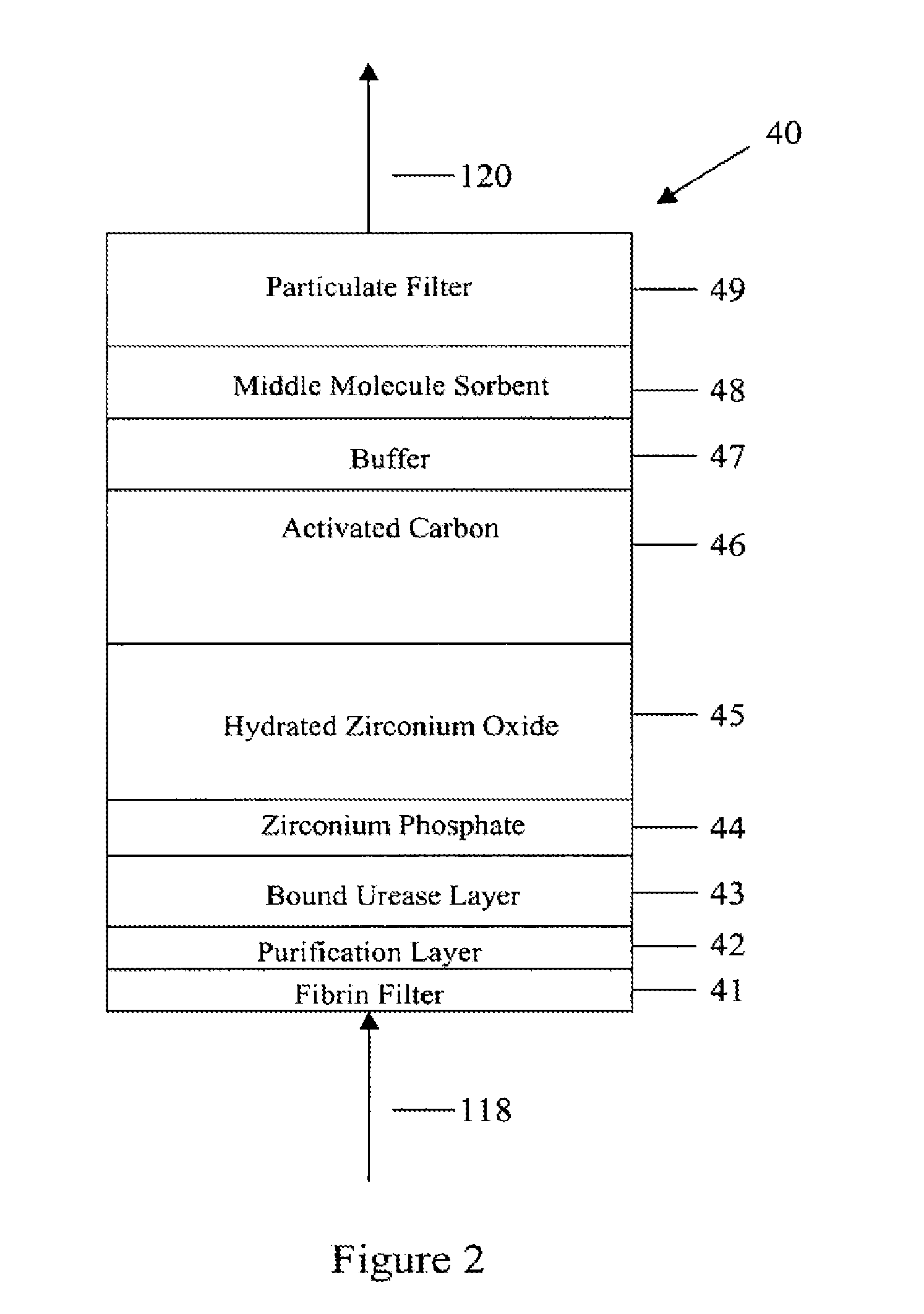
Peritoneal dialysis methods and apparatus
ActiveUS20070179431A1Increase percentageImprove wear resistancePeritoneal dialysisProtein compositionPeritoneal fluid
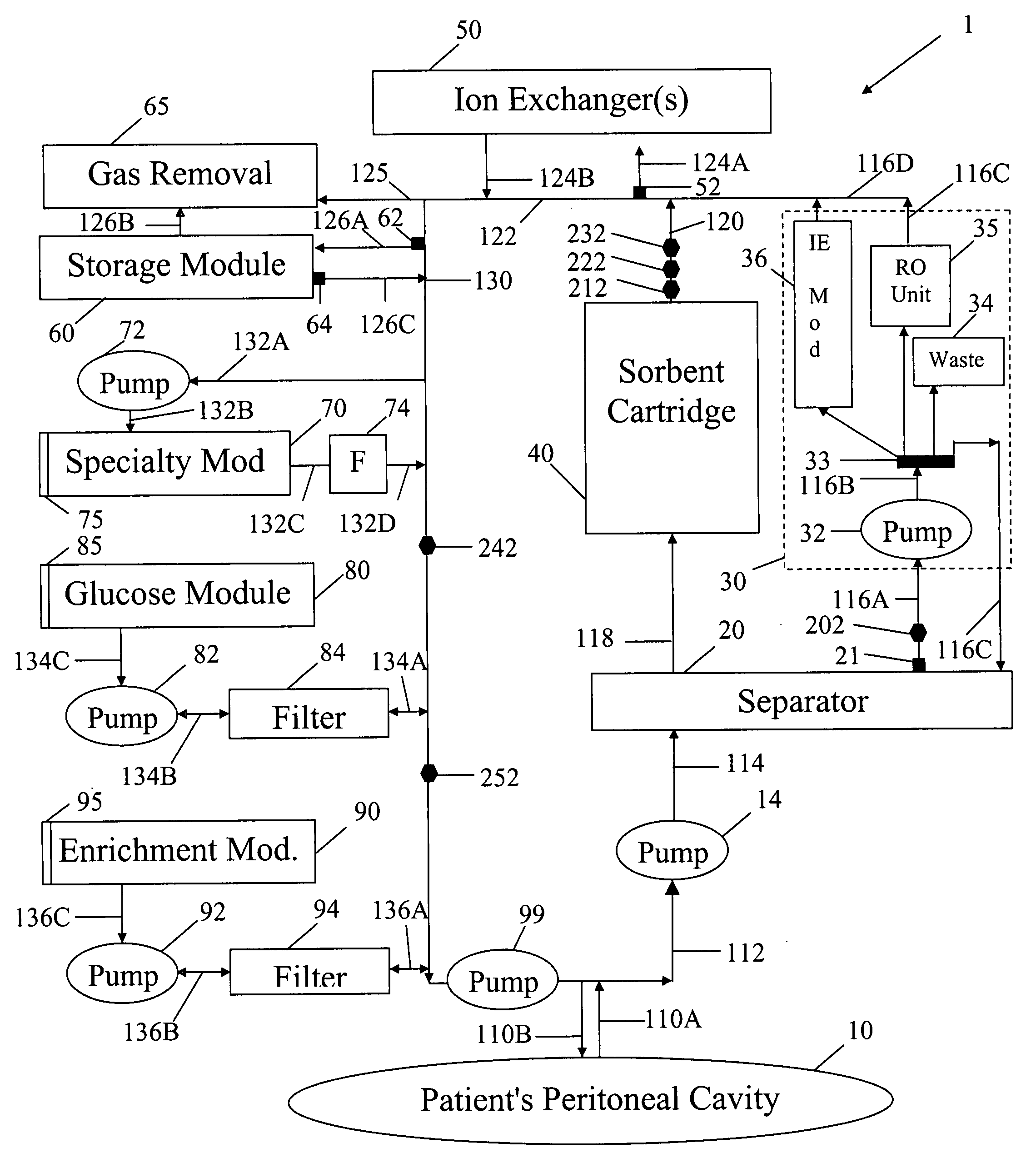
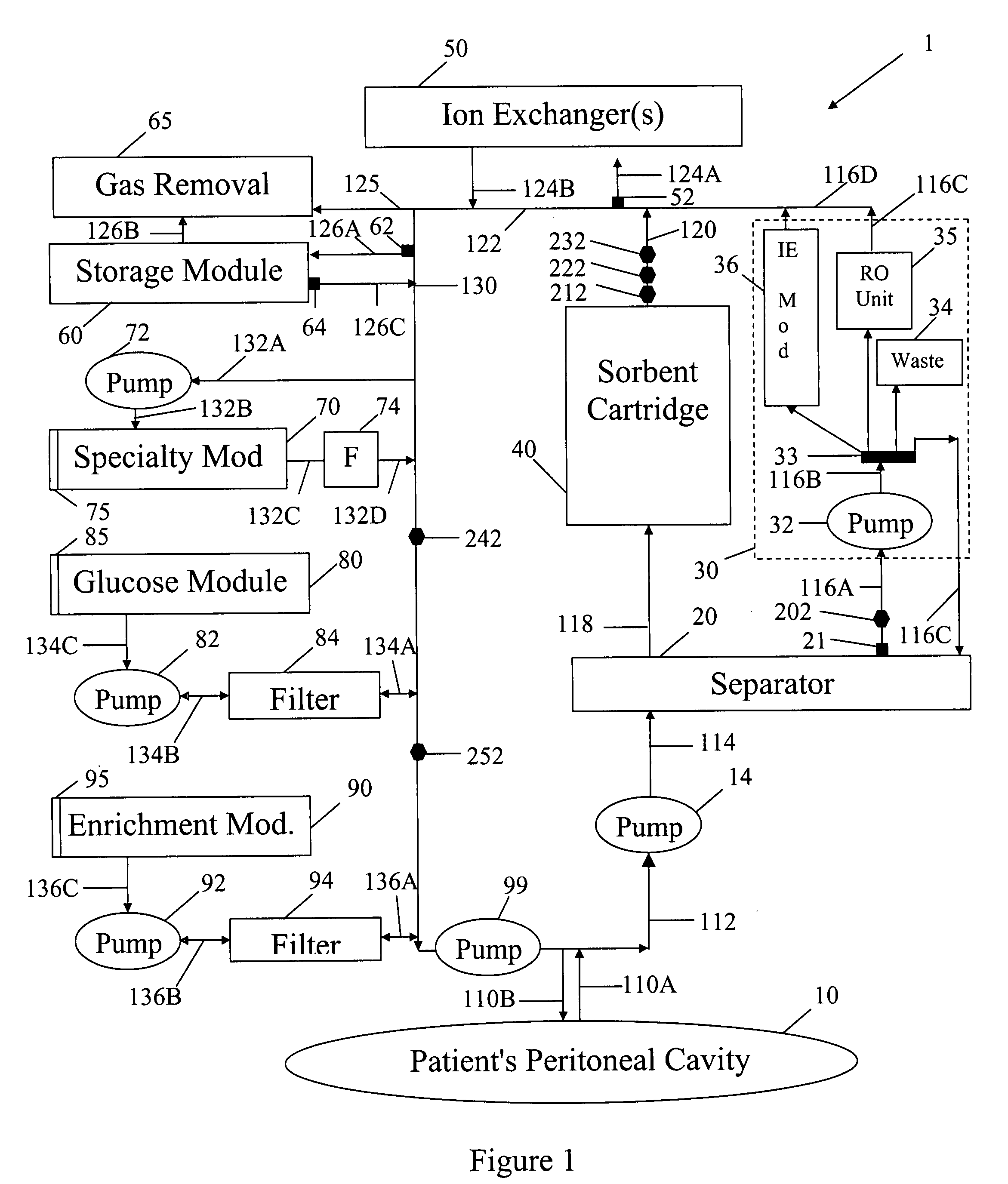
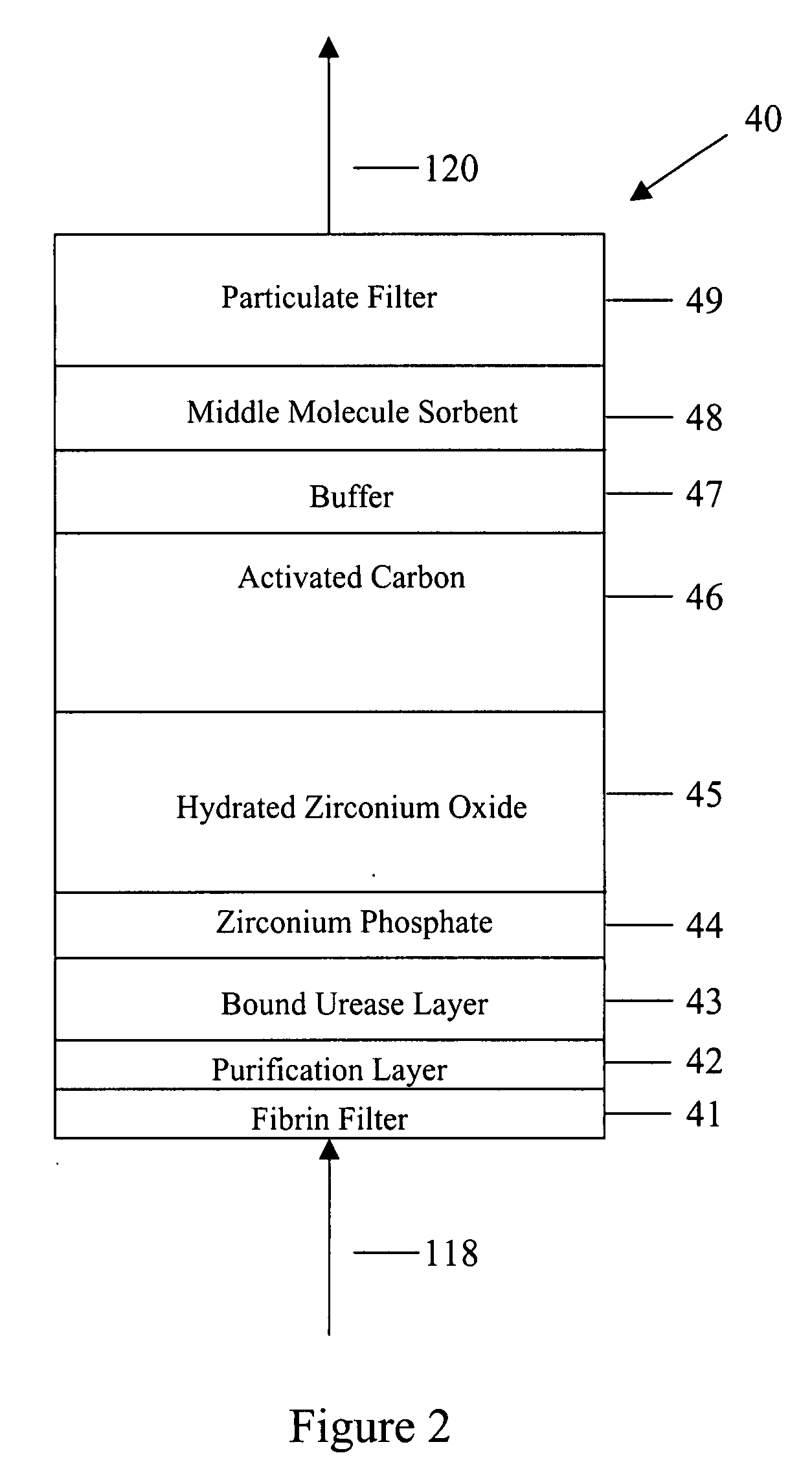
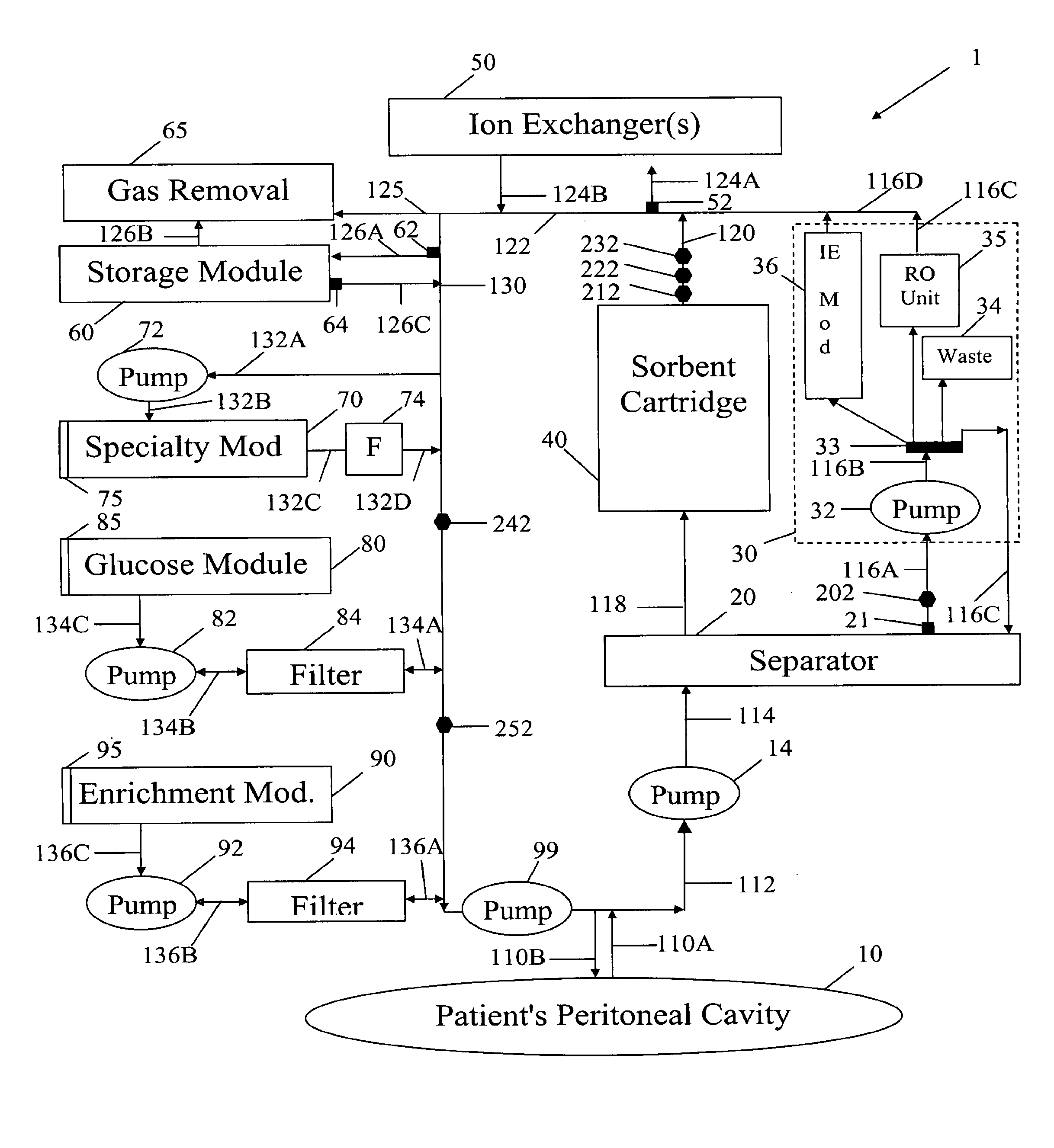
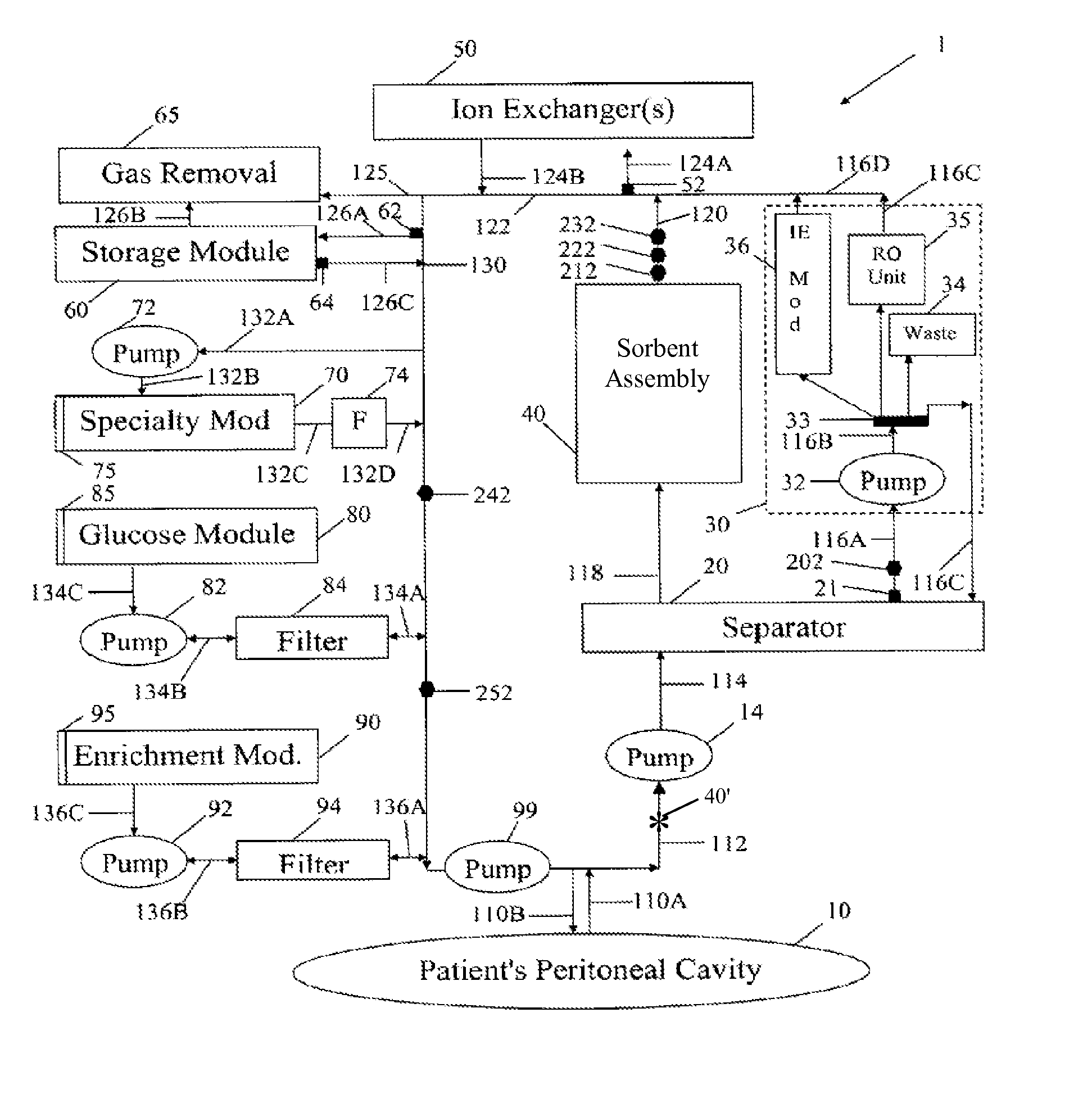
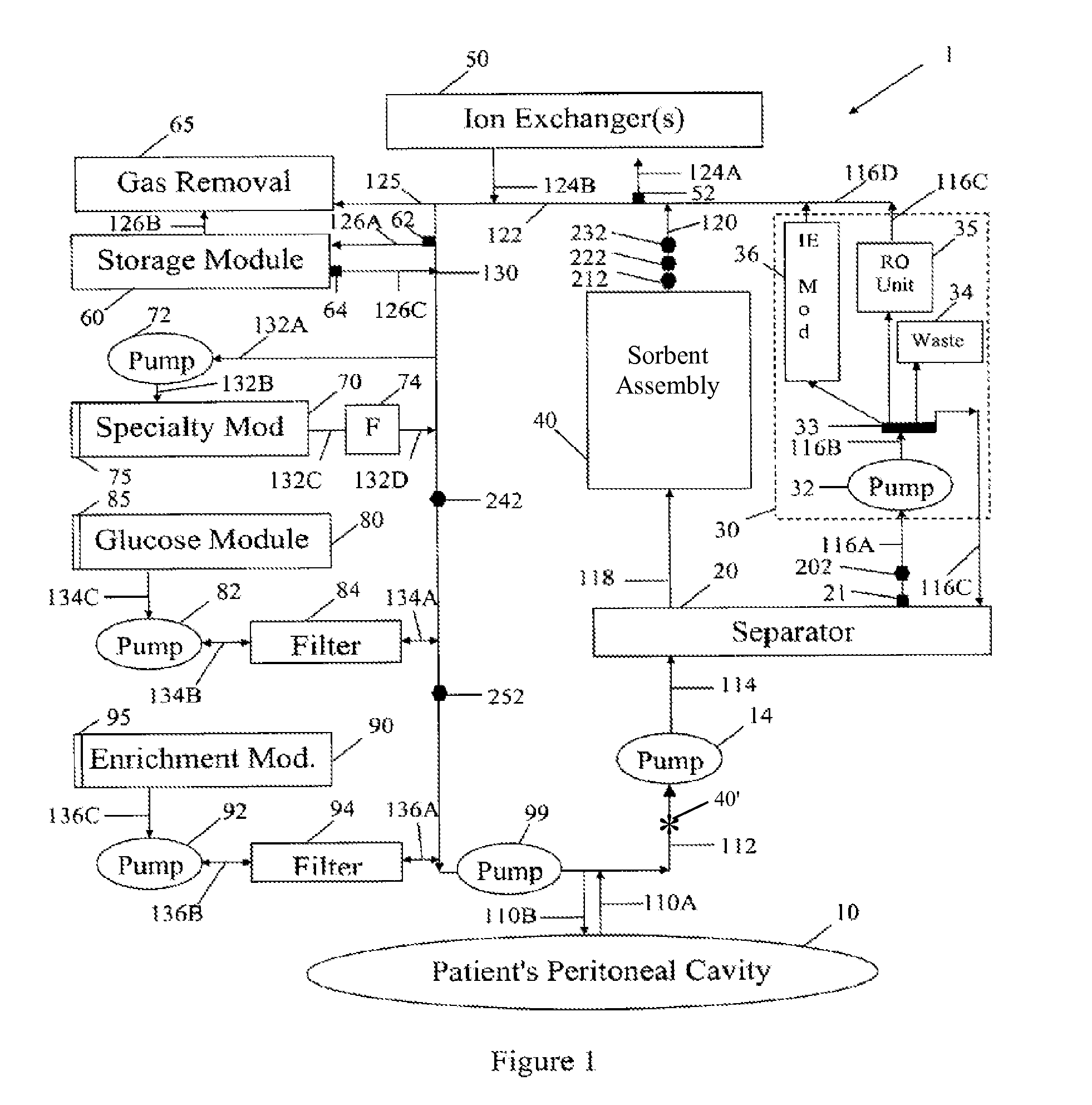
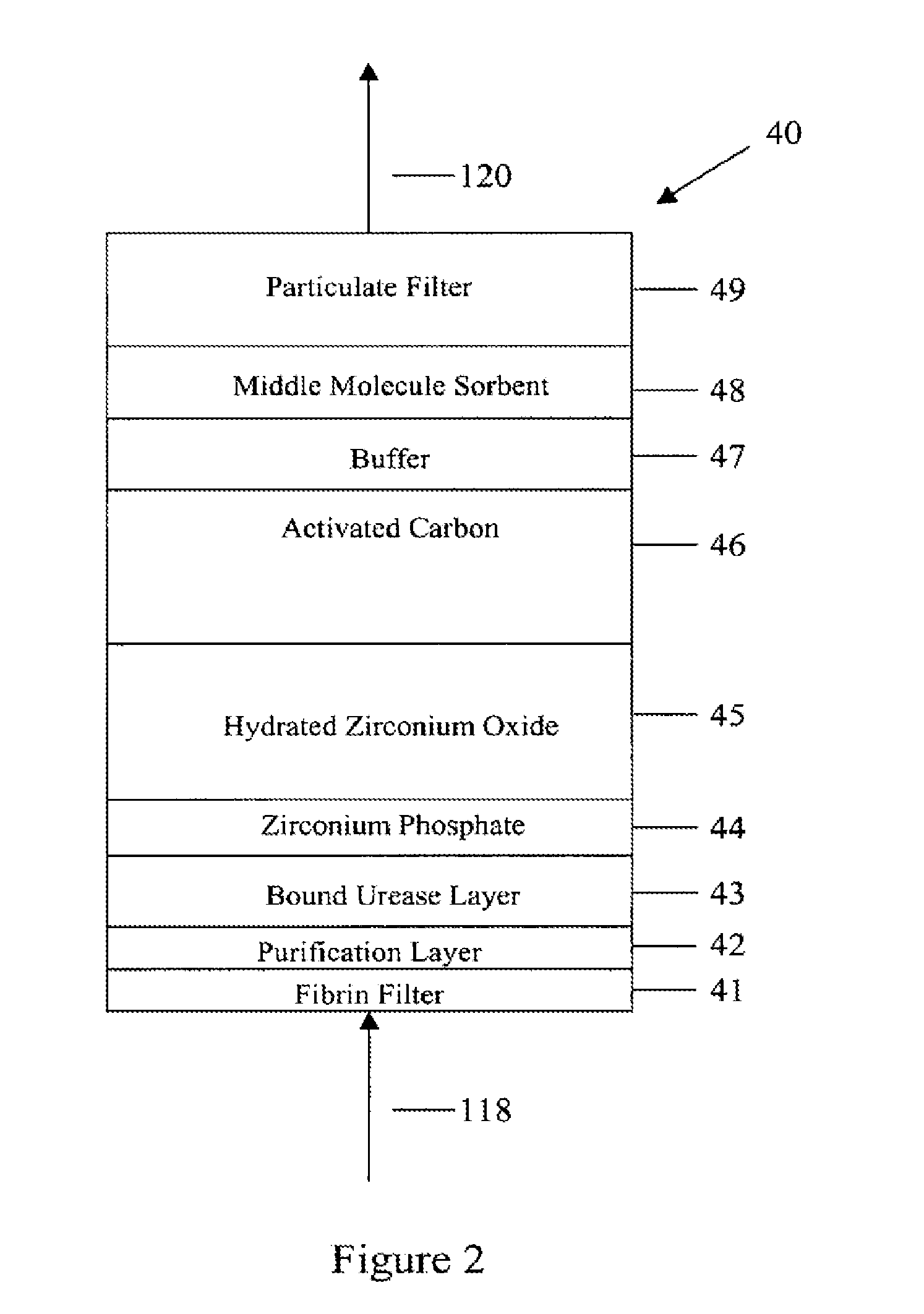
A peritoneal-based (“bloodless”) artificial kidney processes peritoneal fluid without need for additional fluids (“waterless”). Fluid is separated into a protein-rich stream and a protein-free stream. The protein-rich stream is regenerated using a sorbent assembly, and its protein composition can be modified by removal of selected protein(s) (“dialysate-pheresis”). It is then reconstituted with additives and returned into the peritoneal cavity, thereby reducing protein-loss and providing oncotic-pressure for ultrafiltration. The protein-free stream is used to produce free water, and an alkaline or acid fluid for optimization of the composition of the regenerated stream. The unused protein-free stream can be used to “reverse flush” the separator to maintain its patency and the excess discarded for fluid-balance regulation. Compared to prior art, immobilization of urease allows more protein rich fluid to be regenerated and re-circulated into the peritoneal cavity for toxin removal and allows practicable development of portable and wearable artificial kidneys.
Owner:RGT UNIV OF CALIFORNIA +1
Preparation of recombinant factor VIII in a protein free medium
InactiveUS6171825B1Eliminate and at least greatly reduce riskImprove productivityFactor VIICulture processFactor iiManganese
Recombinant Factor VIII can be produced in relatively large quantities on a continuous basis from mammalian cells in the absence of any animal-derived proteins such as albumin by culturing the cells in a protein free medium supplemented with polyol copolymers, preferably in the presence of trace metals such as copper. In very preferred embodiments, the medium includes a polyglycol known as Pluronic F-68, copper sulfate, ferrous sulfate / EDTA complex, and salts of trace metals such as manganese, molybdenum, silicon, lithium and chromium. With an alternative medium which included trace copper ions alone (without polyol copolymers) we were also able to enhance the productivity of Factor VIII in recombinant cells such as BHK cells that are genetically engineered to express Factor VIII.
Owner:BAYER HEALTHCARE LLC +1
Peritoneal Dialysis Methods and Apparatus
ActiveUS20100217181A1Increase percentageImprove wear resistancePeritoneal dialysisProtein compositionPeritoneal fluid
A peritoneal-based (“bloodless”) artificial kidney processes peritoneal fluid without need for additional fluids (“waterless”). Fluid is separated into a protein-rich stream and a protein-free stream. The protein-rich stream is regenerated using a sorbent assembly, and its protein composition can be modified by removal of selected protein(s) (“dialysate-pheresis”). It is then reconstituted with additives and returned into the peritoneal cavity, thereby reducing protein-loss and providing oncotic-pressure for ultrafiltration. The protein-free stream is used to produce free water, and an alkaline or acid fluid for optimization of the composition of the regenerated stream. The unused protein-free stream can be used to “reverse flush” the separator to maintain its patency and the excess discarded for fluid-balance regulation. Compared to prior art, immobilization of urease allows more protein rich fluid to be regenerated and re-circulated into the peritoneal cavity for toxin removal and allows practicable development of portable and wearable artificial kidneys.
Owner:RGT UNIV OF CALIFORNIA +1
Non-protein foaming compositions and methods of making the same
ActiveUS20060040034A1High densityHigh gas contentMilk preparationFrozen sweetsProtein freeProduct gas
A protein-free soluble foaming composition is provided which contains carbohydrate particles having a plurality of voids containing entrapped pressurized gas. The composition may include a surfactant and may be contained in a food product such as a beverage mix or an instant food. In addition, a method is provided for manufacturing the foaming composition in which the protein-free soluble foaming particles are heated and an external pressure exceeding atmospheric pressure is applied to the protein-free soluble foaming particles. The soluble foaming particles are cooled and the external gas pressure is released resulting in pressurized gas remaining in internal voids of the foaming composition.
Owner:INTERCONTINENTAL GREAT BRANDS +1
Microencapsulated Oil Product and Method of Making Same
ActiveUS20090004333A1Increase loadAvoid excessive shear and damageDough treatmentConfectionerySolid shellProtein free
A microencapsulated product comprises a core, a first shell comprising a protein and being substantially carbohydrate-free, and a second shell comprising a carbohydrate and being substantially protein-free. The double shell structure provides a strong shell that makes the microcapsule suitable for use in food products. The core can be a lipid, and in particular a structured lipid with nutritional benefits, such that the nutritional benefits can be passed on to the consumer. The microcapsules of the present invention can be used in making foods products, beverage products, and mixes for making such food and beverage products.
Owner:BUNGE OILS INC
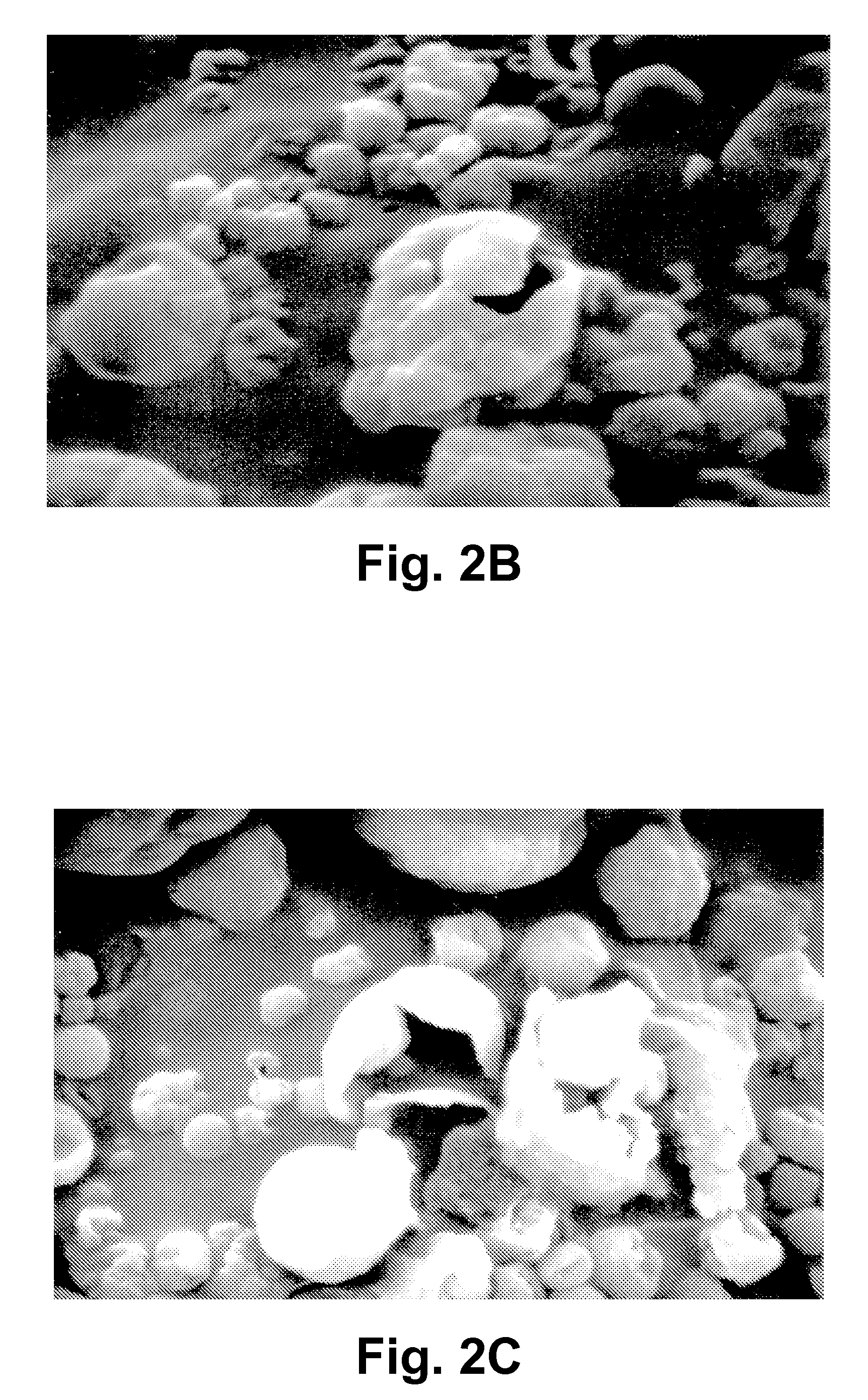
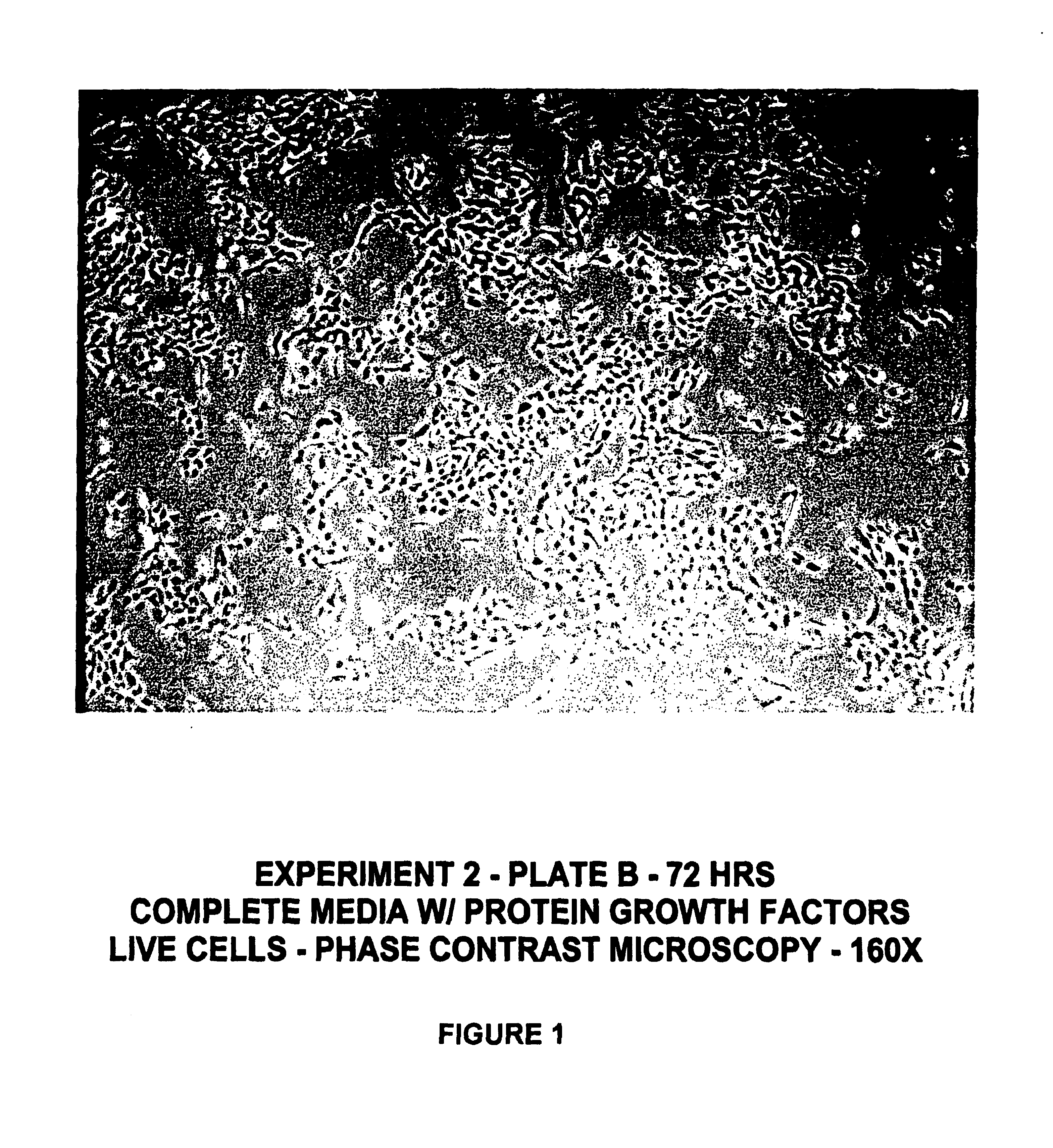
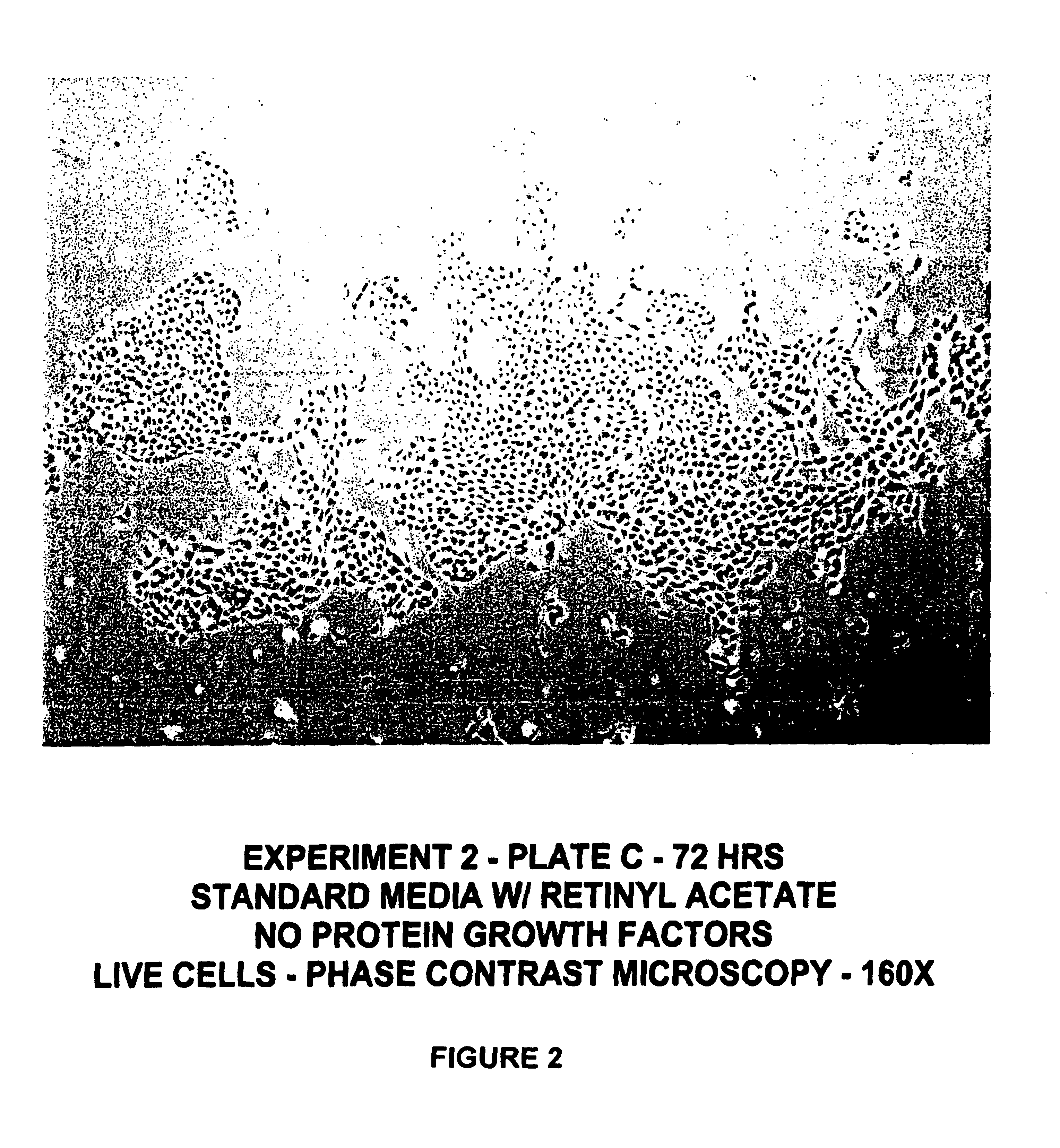
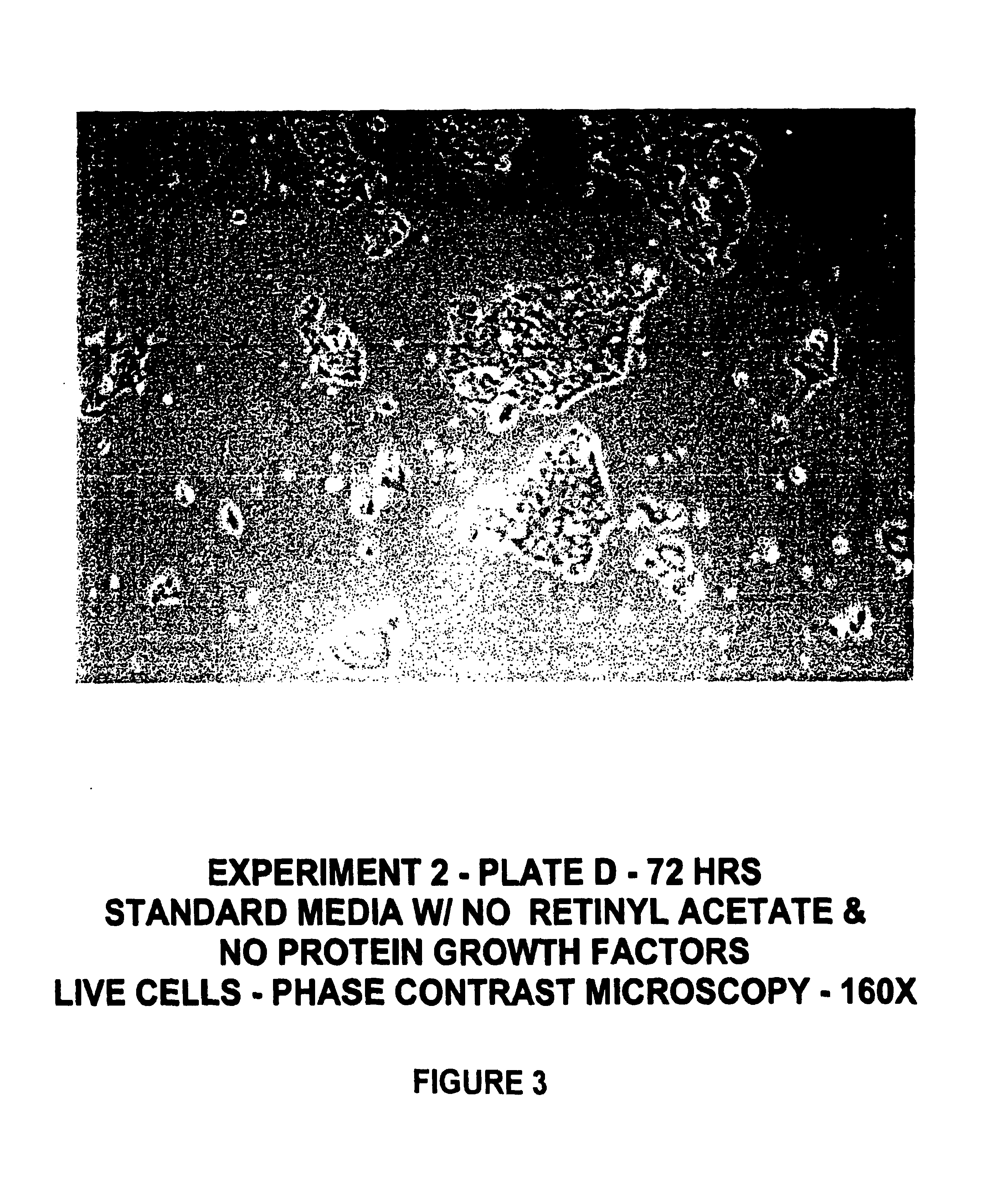
Protein-free defined media for the growth of normal human keratinocytes
Improvements are made to a novel media that replace the requirement for all protein growth factors by the addition to the medium of physiological concentrations of retinyl acetate. The media are serum-free, companion cell or feeder layer-free and organotypic, matrix free solutions for the cultivation of clonally competent basal keratinocytes. The media and methods are useful in the production of epidermal epithelial tissue that is suitable for skin grafting.
Owner:BIOPLAST MEDICAL
Soluble recombinant botulinum toxin proteins
The present invention includes recombinant proteins derived from Clostridium botulinum toxins. In particular, soluble recombinant Clostridium botulinum type A, type B and type E toxin proteins are provided. Methods which allow for the isolation of recombinant proteins free of significant endotoxin contamination are provided. The soluble, endotoxin-free recombinant proteins are used as immunogens for the production of vaccines and antitoxins. These vaccines and antitoxins are useful in the treatment of humans and other animals at risk of intoxication with clostridial toxin.
Owner:ALLERGAN INC
Protein-Free Gamete and Embryo Handling and Culture Media Products
ActiveUS20090226879A1Need can be overcomeConvenient researchCulture processArtificial cell constructsAssisted fertilizationEmbryo
Owner:ALI BIN M ABDULLAH JAFFAR
Peritoneal dialysis methods and apparatus
ActiveUS8876753B2Improve wear resistanceImprove aesthetic qualityPeritoneal dialysisMedical syringesPeritoneal fluidProtein composition
A peritoneal-based artificial kidney processes peritoneal fluid without need for additional fluids. Spent dialysate is separated into a protein-rich stream and a protein-free stream. The protein-rich stream is regenerated using a sorbent assembly, and its protein composition can be modified by removal of selected protein(s). Alternatively, the spent dialysate is first processed in a sorbent assembly and then separated into the protein-rich and protein-free streams. Immobilization of urease allows more protein rich fluid to be regenerated and re-circulated into the peritoneal cavity for toxin removal and allows practicable development of portable and wearable artificial kidneys.
Owner:RGT UNIV OF CALIFORNIA +1
Non-protein foaming compositions and methods of making the same
ActiveUS7534461B2Improve the immunityAllergenicity reductionMilk preparationFrozen sweetsProduct gasAtmospheric pressure
A protein-free soluble foaming composition is provided which contains carbohydrate particles having a plurality of voids containing entrapped pressurized gas. The composition may include a surfactant and may be contained in a food product such as a beverage mix or an instant food. In addition, a method is provided for manufacturing the foaming composition in which the protein-free soluble foaming particles are heated and an external pressure exceeding atmospheric pressure is applied to the protein-free soluble foaming particles. The soluble foaming particles are cooled and the external gas pressure is released resulting in pressurized gas remaining in internal voids of the foaming composition.
Owner:INTERCONTINENTAL GREAT BRANDS LLC +1
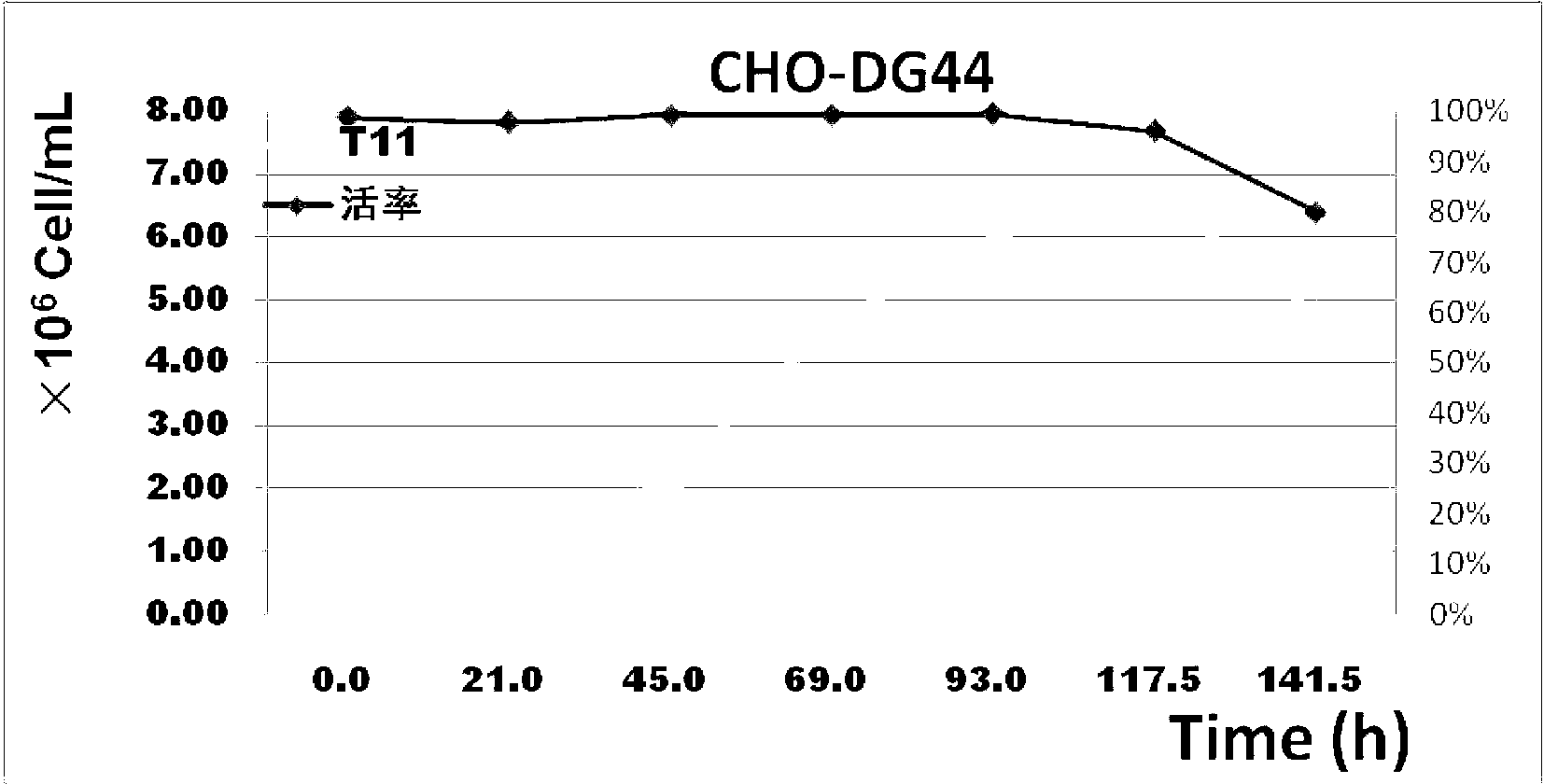
Serum-free and protein-free all-chemical-component-definition culture medium for supporting CHO high-density suspension growth
InactiveCN102876626AEasy to prepareEasy to storeVertebrate cellsArtificial cell constructsCulture mediumsAmino acid
The invention discloses a serum-free and protein-free all-chemical-component-definition culture medium for supporting the CHO high-density suspension growth. The serum-free culture medium contains amino acids, inorganic salts, trace elements, vitamins, carbohydrates and other organic molecules. The serum-free culture medium has the advantages of no containment of any extracted or recombinant proteins, polypeptides or hydrolyzates, short cell growth time, high density, low metabolic refuse accumulation, good cell vitality maintenance, realization of the protein expression higher than that of products of same kind, and low cost because of all chemical component definition.
Owner:NINGBO PD ACRO BIO SYST
Protein-free creamers, stabilizing systems, and process of making same
InactiveUS20090142469A1Pleasant mouth-feelSufficient whitening capacityFood preservationTeaEmulsionRefrigeration
Protein-free creamer compositions and stabilizing systems contained therein. The creamer composition includes an emulsifying component of at least two low molecular weight emulsifiers in relative amounts sufficient to provide a stabilized emulsion, a cellulose component including a blend of microcrystalline cellulose and carboxymethylcellulose in an amount sufficient to maintain homogeneity of the composition; and a carageenan gum component present in an amount sufficient to maintain homogeneity of the composition. The creamer composition can be in the form of a shelf stable aseptic liquid creamer that is stable for at least about 9 months, an extended-shelf life (ESL) liquid creamer that is stable for at least about four months at refrigeration, or a powder that is stable for at least 24 months at ambient conditions. The creamer composition provides sufficient whitening capacity and a pleasant mouth feel without discernable feathering and without discernable fat separation when added to liquid beverages.
Owner:NESTEC SA
Peritoneal Dialysis Methods and Apparatus
ActiveUS20120271227A1Overcome deficienciesImprove wear resistancePeritoneal dialysisPeritoneal fluidProtein composition
A peritoneal-based artificial kidney processes peritoneal fluid without need for additional fluids. Spent dialysate is separated into a protein-rich stream and a protein-free stream. The protein-rich stream is regenerated using a sorbent assembly, and its protein composition can be modified by removal of selected protein(s). Alternatively, the spent dialysate is first processed in a sorbent assembly and then separated into the protein-rich and protein-free streams. Immobilization of urease allows more protein rich fluid to be regenerated and re-circulated into the peritoneal cavity for toxin removal and allows practicable development of portable and wearable artificial kidneys.
Owner:RGT UNIV OF CALIFORNIA +1
Universal, glycosylation enhancer, completely chemically defined medium formulation
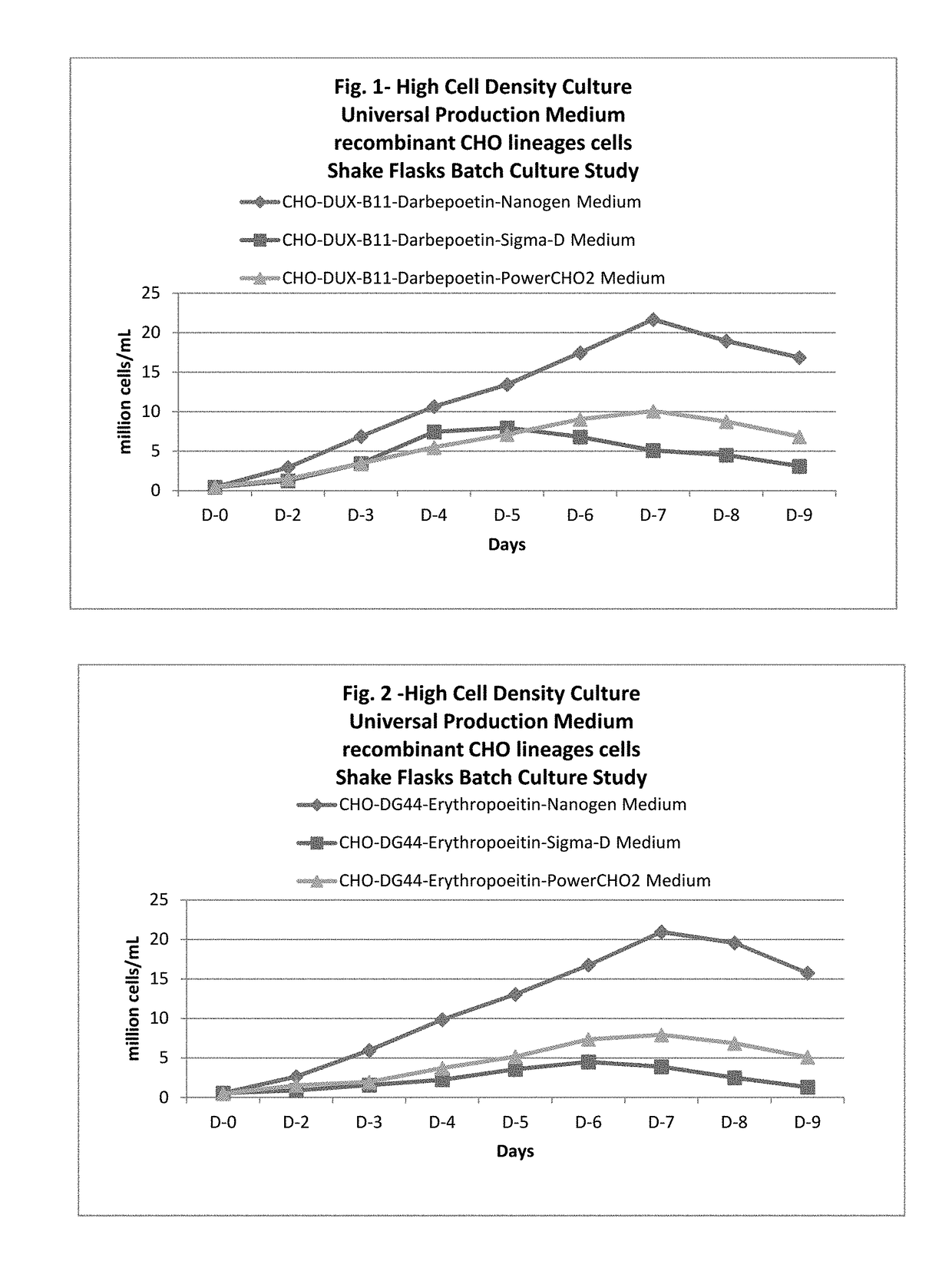
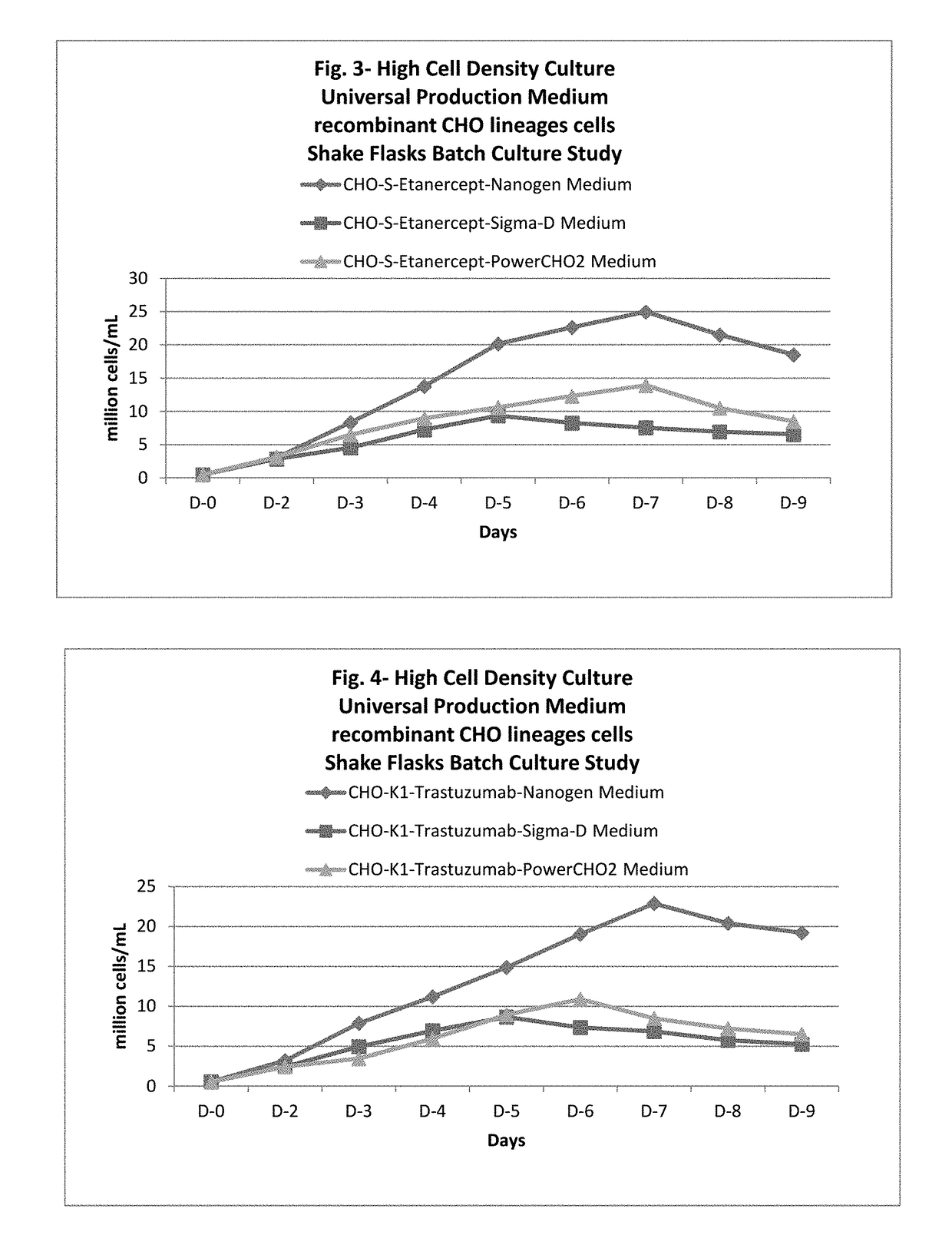
ActiveUS20170218328A1Therapy is also rapidCulture processCell culture mediaSodium bicarbonateHydrolysate
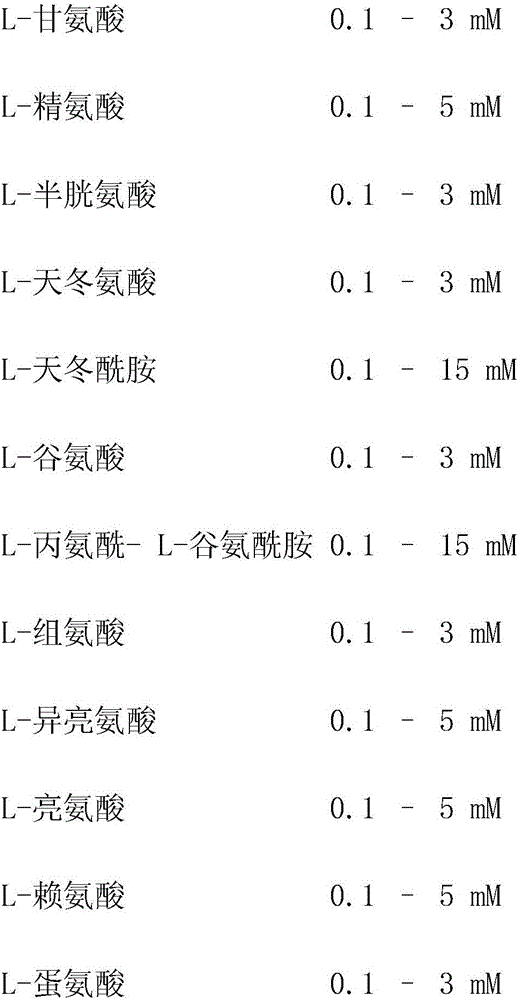
In one embodiment, the present application discloses a cell culture medium for culturing cell lines suitable for producing a therapeutic protein, comprising an amino acid selected from a group consisting of L-arginine, L-asparagine, L-proline, L leucine and L hydroxyproline and a mixture thereof; a vitamin selected from a group consisting of ascorbic acid Mg2+ salt, biotin, pyridoxine HCL, folic acid, riboflavin and D-calcium pantothenate, and a mixture thereof; an element selected from a group consisting of ammonium meta vanadate, sodium meta vanadate, germanium dioxide, barium acetate, aluminum chloride, rubidium chloride, cadmium chloride, ammonium molybedate, stannous chloride, cobalt chloride, chromium sulfate, silver nitrate, sodium metasilicate, zinc sulfate, manganese sulfate H2O, manganous chloride, ferric nitrate 9H2O, ferrous sulfate 7H2O, ferric ammonium citrate, magnesium chloride anhydrous, and magnesium sulfate anhydrous, and a mixture thereof; a nucleoside selected from a group consisting of uridine and cystidine; a sugar selected from a group consisting of galactose, mannose and N-Acetyl-D-Mannosamine; and a triple buffering system comprising sodium carbonate, sodium bicarbonate and HEPES; wherein the cell culture medium is animal component-free, plant component-free, serum-free, growth factors-free, recombinant protein-free, lipid-free, steroid-free, and free of plant or animal hydrolysates and / or extracts.
Owner:NANOGEN PHARMA BIOTECH CO LTD
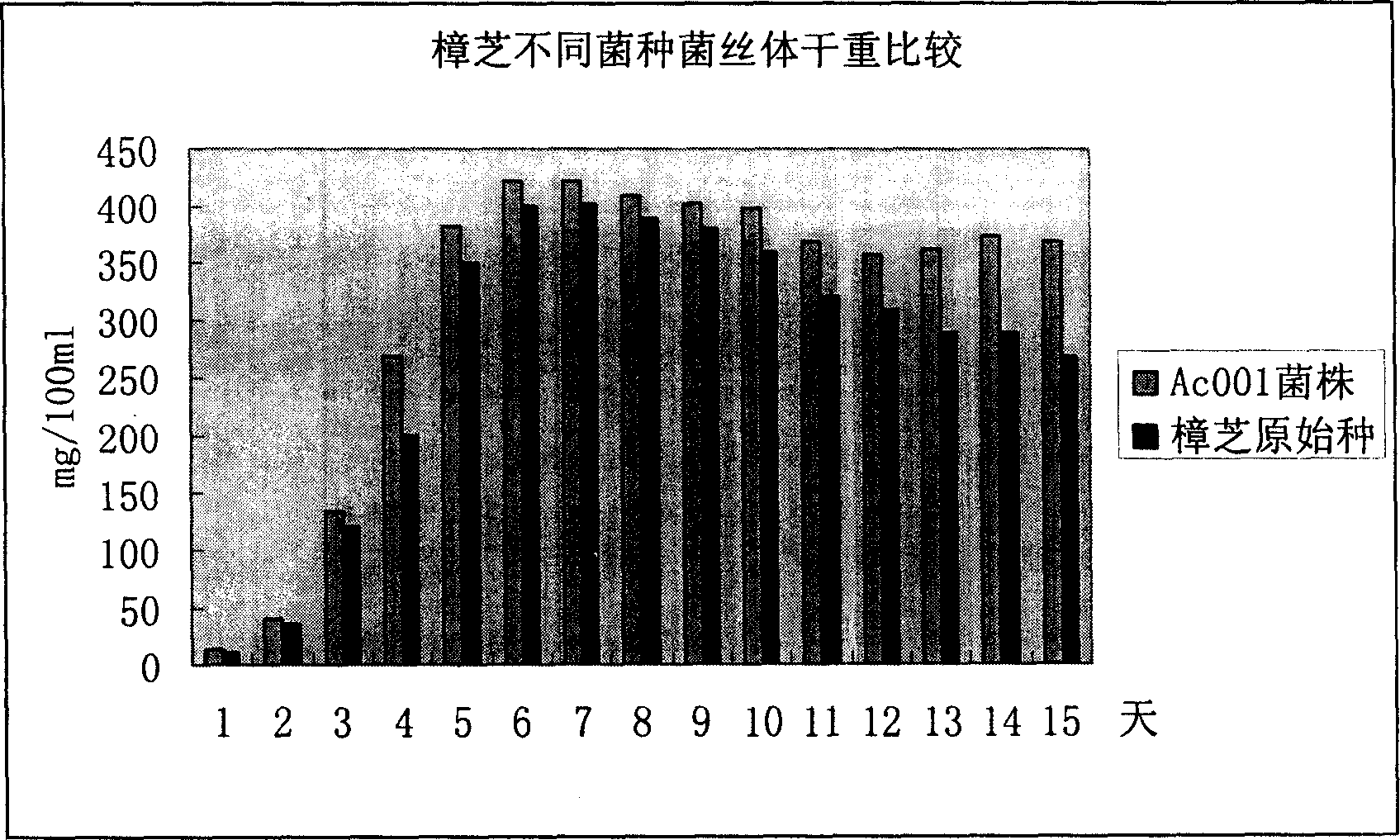
Liver cancer resistant Antrodia camphorata and preparation method thererof
The invention discloses an Antrodia camphorata capsule for resisting hepatic carcinoma, which comprises the following constituents (by weight portions): Antrodia camphorate mycelium fermentation extract 20-100, protein-free maize starch or medicinal starch gum 200-480. The Antrodia camphorate mycelium fermentation extract is abstracted by ethanol and dried. The Antrodia camphorata bacterial strain Ac001 was preserved in the China General Microbiological Culture Collection Center with a docket number of CGMCC No.1460. The Antrodia camphorate mycelium fermentation extract in the hepatic carcinoma resisting Antrodia camphorate capsule has appreciable actions in inhibiting hepatitis B virus HbsAg, e antigen HbeAg and HBV DNA secretion and resisting cancers especially liver cancer.
Owner:LAIYANG AGRI COLLEGE
Haemophilus outer membrane protein
InactiveUS6013514AImprove the protective effectHigh protection levelAntibacterial agentsBacteriaSerum igeHaemophilus
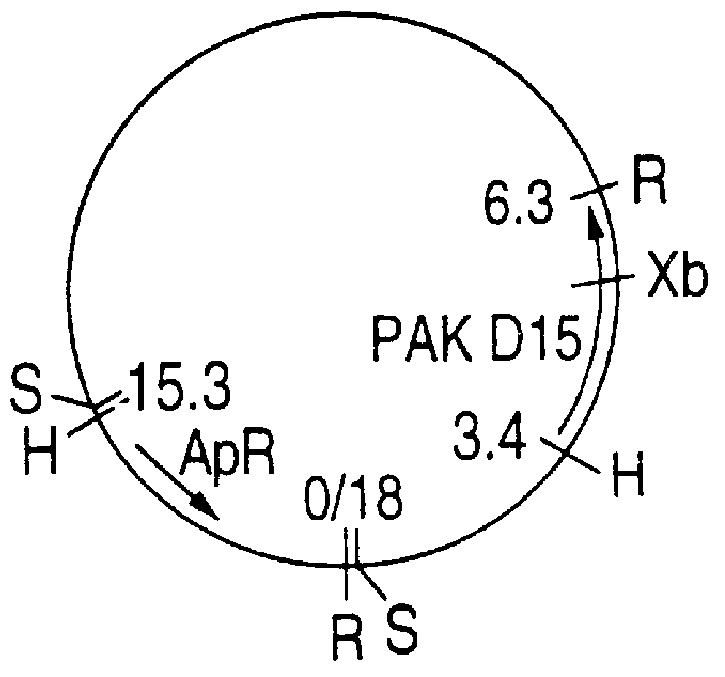
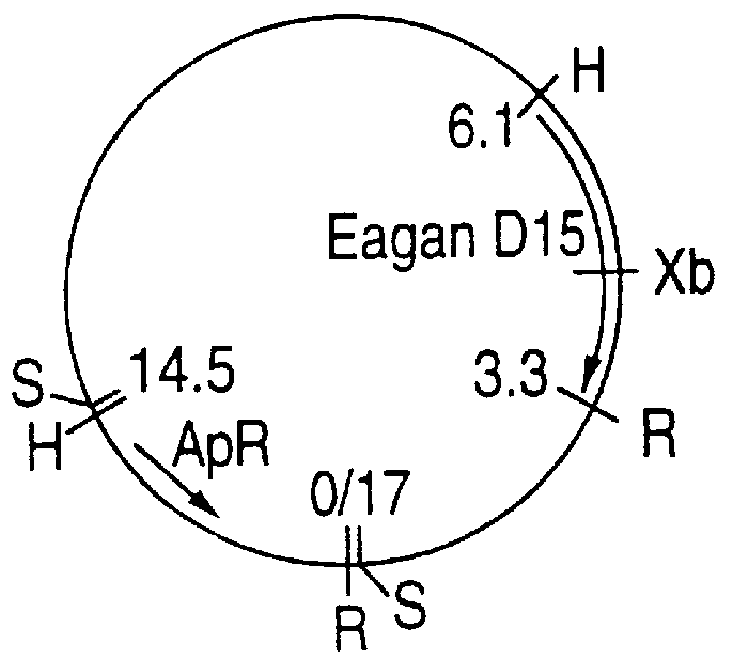
PCT No. PCT / CA93 / 00501 Sec. 371 Date Sep. 12, 1995 Sec. 102(e) Date Sep. 12, 1995 PCT Filed Nov. 23, 1993 PCT Pub. No. WO94 / 12641 PCT Pub. Date Jun. 9, 1994Purified and isolated nucleic acid from specific strains of Haemophilus influenzae is provided which encodes at least a portion of the D15 outer membrane protein of Haemophilus. The nucleic acid is used to produce peptides, polypeptides and proteins free of contaminant associated with Haemophilus for purposes of diagnosis and medical treatment. Furthermore, the nucleic acid may be used in the diagnosis of Haemophilus infection. Antisera obtained following immunization with the nucleic acid D15 outer membrane protein or peptides also may be used for the purpose of diagnosis and medical treatment.
Owner:COONNAUGHT LAB
Serum-free protein-free cell culture medium
ActiveCN106635953AHigh expressionReduce manufacturing costAnimal cellsCulture processBiotechnologyVaccine Production
The invention relates to the technical field of biology, specifically discloses a serum-free protein-free cell culture medium, and aims to provide a serum-free protein-free cell culture medium capable of supporting the growth of high density cells. The culture medium comprises amino acids, vitamins, inorganic salts, trace elements, carbohydrates, other chemical compounds, polyamine (or derivatives of polyamine), and hydrolysate. The manufacturing cost is low. The stability is high, the live cell density and cell survival rate are extremely high, and the culture medium is suitable for culture cells for recombinant protein and vaccine production.
Owner:昆明润什生物科技有限公司
Protein free formula
ActiveUS20100172876A1Avoid developmentReduce severityBiocidePeptide/protein ingredientsIntact proteinFeces
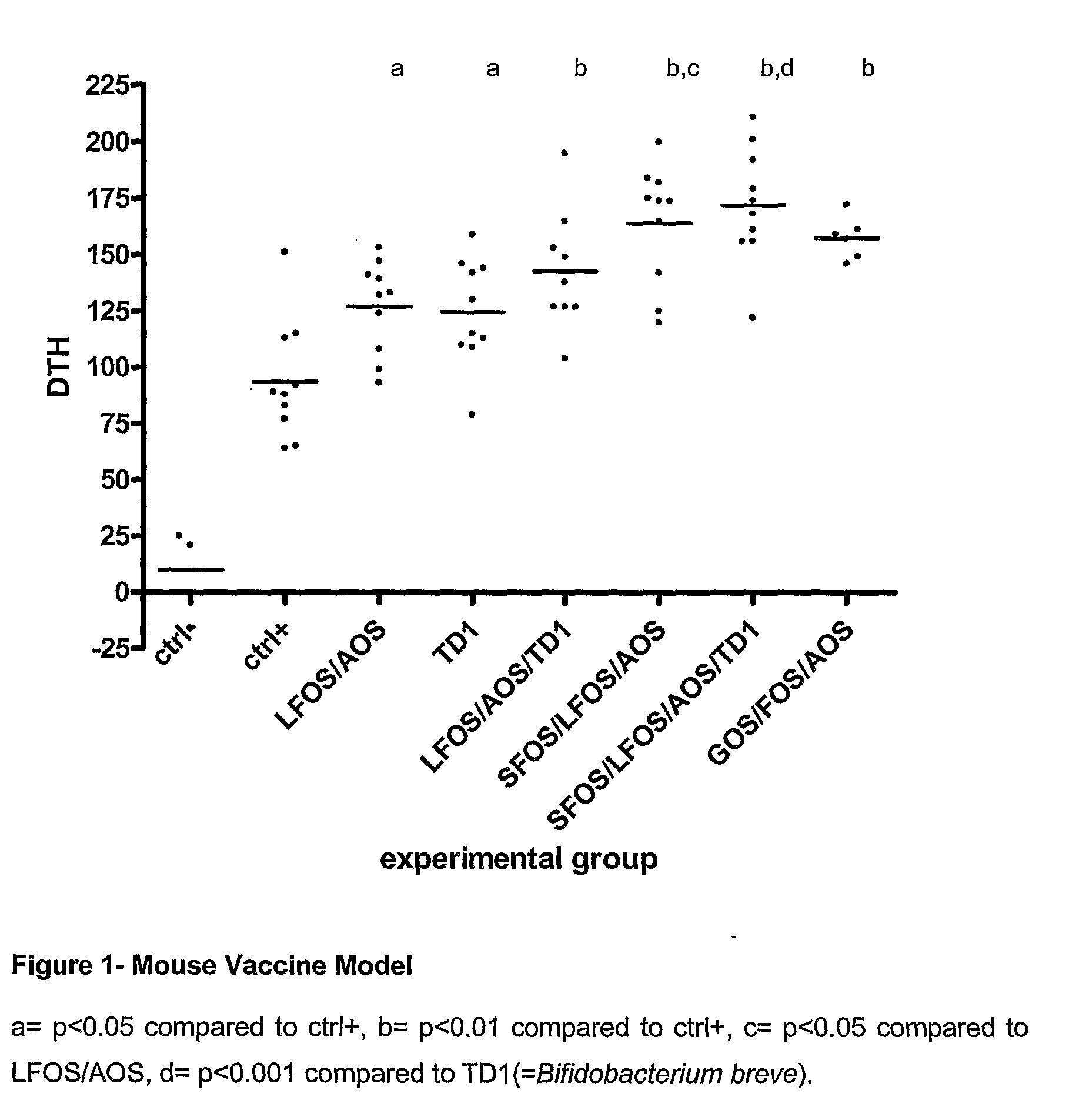
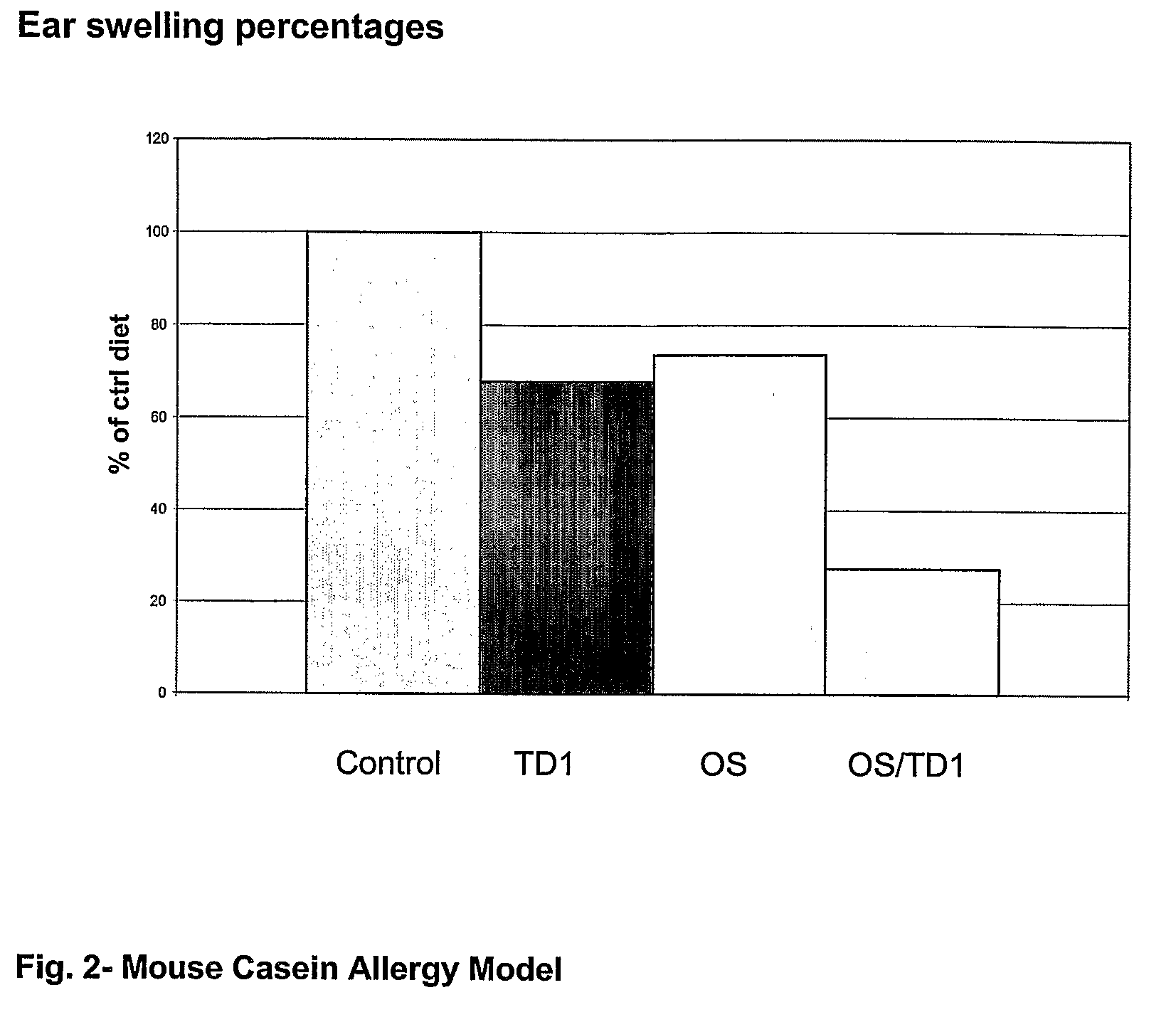
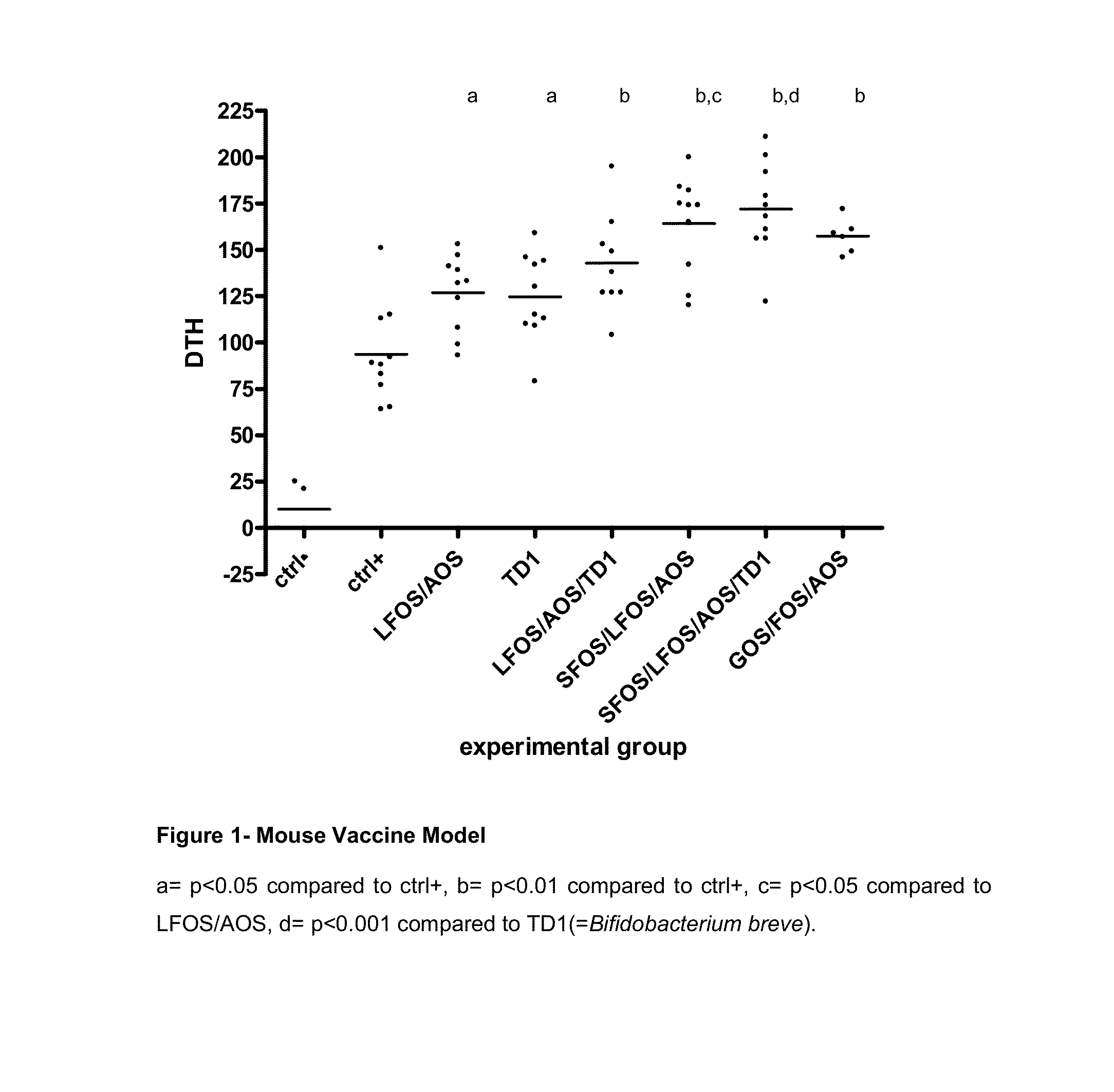
The invention relates to the use of composition comprising free amino acids as a sole source of protein, a fatty acid source comprising long chain polyunsaturated fatty acids, a carbohydrate source comprising digestible and indigestible carbohydrates, and milk protein free Bifidobacteria for treating a person suffering from (a) colic, congestion, runny nose, wheezing, vomiting, diarrhoea, bloody stools, mucus in stools, rash, eczema, gastroesophageal reflux, eosinophilic esophagitis or asthma; (b) cow's milk allergy and / or food protein intolerance; and / or (c) infections, wherein the indigestible carbohydrate is selected from a milk protein free source and the total composition is essentially free of intact proteins.
Owner:SHS INTERNATIONAL
Antibiotic-free homologous protein-free creep feed for weaned piglets
The invention discloses an antibiotic-free homologous protein-free creep feed for weaned piglets, comprising corn, puffed corn, flour, puffed soybean, Hamlet protein, yeast nucleotide, super steamed fish meal, cheese plus cheese, sucrose, glucose, whey powder, milk powder, whey protein concentrate, calcium monohydrogen phosphate, calcium formate, edible salt, lysine, methionine, tryptophan, threonine, a compound acidifier, coated zinc oxide, coated plant essential oil, coated sodium butyrate, alkaloids, a vitamin complex, trace element premix and a compound enzyme preparation. The Hamlet protein, yeast nucleotide and whey protein concentrate are used herein to replace plasma protein powder and intestine membrane protein; attracting property, palatability and high digestive utilization areguaranteed for the antibiotic-free homologous protein-free creep feed for weaned piglets; piglets can gain smooth transition from breast milk to feed; the comprehensive utilization of the compound acidifier, coated sodium butyrate, coated zinc oxide, coated plant essential oil and alkaloids helps improve immunity in piglets and control diarrhea in the piglets.
Owner:CONTI FEED CHINA
Method for preparing bee pollen polysaccharide through combining enzymolytic wall-breaking with hot-water ultrasonic extracting
The invention discloses a method for preparing bee pollen polysaccharide through combining enzymolytic wall-breaking and hot-water ultrasonic extracting. The method comprises the following steps: drying, purifying, grinding and screening bee pollen; carrying out enzymolytic wall-breaking; carrying out heating and ultrasonic extracting; dehydrating; deproteinizing with perchloric acid; carrying out (diethylamino) ethyl column chromatography and elution; separating acid polysaccharide precipitation and neutral polysaccharide precipitation with cetyl trimethyl ammonium bromide (CTAB); and dissolving the polysaccharide precipitations, then dehydrating, vacuum-freezing, and drying to obtain the high-purity impurity-free bee pollen polysaccharide. By the method, the polysaccharide extraction ratio is improved by 66.03%, and the polysaccharide yield reaches 18.54mg / g which is improved by 2.02 times compared with that of the extraction method of the prior art; and the obtained bee pollen polysaccharide liquid is negative relatively to the ninhydrin reaction, no protein residual is left, and the obtained bee pollen polysaccharide has no impurities and high purity.
Owner:TONGLU XINGYUAN HEALTH PROD
Method for extracting desoxyribonucleic acid from formalin fixed and paraffin embedded tissues
ActiveCN102146112AHarm reductionNo bodily harmSugar derivativesSugar derivatives preparationAbsorption columnSalt free
The invention relates to a method for extracting desoxyribonucleic acid from formalin fixed and paraffin embedded tissues, and belongs to the technical field of nucleic acid application in biology. The method comprises the following steps of: preparing special lysis solution, adding the lysis solution into paraffin section tissues, boiling at high temperature for 30 minutes, and centrifuging to take supernate; adding absolute ethanol for uniform mixing, and adding the mixed solution into a silicon membrane absorption column for centrifuging; rinsing by using protein-free liquid and salt-free rinsing liquid; and eluting by using elution buffer. In the method, a toxic reagent of dimethylbenzene is not used for dewaxing, and harm to bodies of experimenters is avoided; and precious protease Kis not needed to perform long-time incubation enzymolysis, the operation is simple and quick, the extracted desoxyribonucleic acid in genome is high in quality and stability, and the cost and time can be saved to the greatest degree. The extracted product is subjected to polymerase chain reaction (PCR) detection, long segments with about 750pb can be obtained through amplification, and the work in the aspects such as scientific research, biomedicine and the like is greatly facilitated.
Owner:TIANGEN BIOTECH BEIJING
Protein free formula
ActiveUS8691213B2Avoid developmentReduce severityBiocidePeptide/protein ingredientsSerum rashIntact protein
The invention relates to the use of composition comprising free amino acids as a sole source of protein, a fatty acid source comprising long chain polyunsaturated fatty acids, a carbohydrate source comprising digestible and indigestible carbohydrates, and milk protein free Bifidobacteria for treating a person suffering from (a) colic, congestion, runny nose, wheezing, vomiting, diarrhea, bloody stools, mucus in stools, rash, eczema, gastroesophageal reflux, eosinophilic esophagitis or asthma; (b) cow's milk allergy and / or food protein intolerance; and / or (c) infections, wherein the indigestible carbohydrate is selected from a milk protein free source and the total composition is essentially free of intact proteins.
Owner:SHS INTERNATIONAL
Method for decreasing bitterness and improving taste of protein-free and hydrolyzed infant formulas
InactiveUS20090117256A1Reduce bitternessGreat tasteFood ingredient as taste affecting agentFood preparationMedicineAdditive ingredient
The present invention relates to a method for decreasing the bitterness or improving the taste of a protein-free or hydrolyzed infant formula. The method comprises intermixing ingredients of the formula and adjusting the pH of the formula to between about 6.5 and about 7.2.
Owner:MEAD JOHNSON NUTRITION
Serum-free protein-free feed culture medium as well as preparation method and application thereof
ActiveCN107460159AReduce the risk of contaminationRich varietyCulture processArtificial cell constructsPollutionCulture mediums
The invention relates to a serum-free protein-free high-efficiency feed culture medium as well as a preparation method and application thereof. The feed culture medium comprises amino acids, inorganic salts, trace elements, vitamins, carbohydrates and other organic matters. Moreover, the culture medium does not contain any glutamine. Since the feed culture medium contains serum-free protein-free animal-source-free components, the viral pollution risk can be greatly reduced, and downstream purification is facilitated. Meanwhile, since the culture medium is full in types of formula components and balanced in proportion, the culture medium can be applicable to high-density culture and high-protein expression of multiple CHO cell strains.
Owner:上海多宁生物科技股份有限公司
Protein-free momordica grosvenori pure-flavor concentrated juice and production method thereof
The invention relates to a protein-free momordica grosvenori pure-flavor concentrated juice and a production method thereof. The production method comprises the following steps: (1) after washing thefresh momordica grosvenori, squeezing the momordica grosvenori, extracting the pomace with ice water, and merging the juice and an ice water extract; (2) adjusting the pH value to alkaline, adding a protease preparation, performing enzymatic hydrolysis, heating and boiling the material, cooling the material, and performing centrifugation; (3) passing the material through a cation exchange resin column, and collecting an effluent; (4) passing the material through an anion exchange resin column, collecting the effluent, and concentrating the product. The concentrated juice obtained by the methodhas a protein content of less than 0.1%, a mass content of mogroside V as high as 4.53%, and a yield of up to 98%; The obtained concentrated juice is light yellow transparent and thick liquid, the smell is fragrant, the taste is sweet and saturated, no odor is generated, the sugar content is as high as 68 brix, and the sweetness is close to that of sucrose; The production method has the advantages of strong operability, safety, environmental protection and no pollution, and is suitable for industrial production.
Owner:HUNAN HUACHENG BIOTECH
Low protein and protein-free extended shelf life (ESL) and shelf-stable aseptic liquid creamers and process of making thereof
InactiveUS20110293800A1Sufficient effectGood physical and chemical stabilityFood ingredient as chelating agentAcidic food ingredientsVegetable oilCarrageenan
The invention provides low protein and protein-free liquid creamer compositions, and processes for making them. The liquid creamer composition includes an emulsifying component comprising a combination of at least two low molecular weight emulsifiers; a hydrocolloid system comprising microcrystalline cellulose (MCC) / carboxymethylcellulose (CMC) / carrageenan; a chelating system comprising at least one chelating agent of an organic or inorganic acid or organic or inorganic acid salt; a buffer system comprising at least one buffering agent; and a whitening agent in an amount sufficient to provide additional whitening to an aqueous media to which the creamer is added. The composition has a vegetable oil content of about 0.1% to about 33% by weight of the composition and a protein content of no more than 3% by weight of the composition. The composition is in the form of an aseptic liquid creamer that is shelf-stable for at least nine months, and provides high whitening capacity and a pleasant mouth-feel with no discernable feathering or fat separation when added to aqueous media of beverages at different pHs, hardnesses and temperatures.
Owner:NESTEC SA
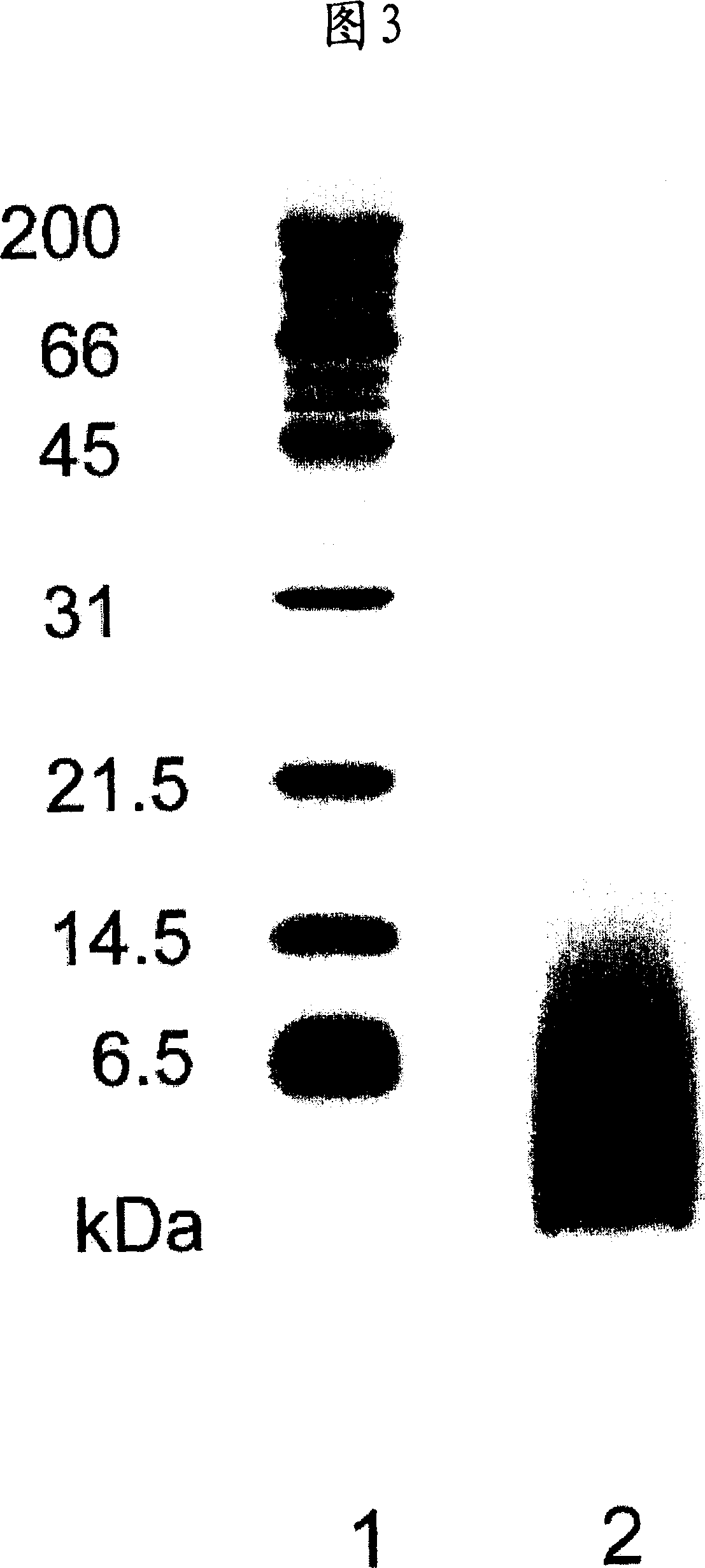
Protein-free natural rubber latex, process for producing the same and use thereof
A natural rubber latex which is substantially free from proteins specified respectively by bands at 14, 31 and 45 kDa in the SDS-PAGE method; and a process for producing the natural rubber latex as described above which comprises saponifying a natural rubber latex with an alkali hydroxide in the presence of a surfactant. Because of substantially being free from the above proteins causative of the expression of type I allergy, the above-described natural rubber latex is appropriately usable in producing various products such as catheters, rubber gloves, condoms and foamed articles.
Owner:SUMITOMO RUBBER IND LTD
Serum-free mammalian cell culture medium, and uses thereof
InactiveUS20090280532A1Improve expression levelIncrease productionBiocideInorganic active ingredientsMammalCell culture media
Owner:LIFE TECH CORP
CHO (Chinese hamster ovary) cell serum-free protein-free culture medium and preparation method thereof
InactiveCN106190950AGrow fastClear chemical compositionCulture processArtificial cell constructsMinor elementHydrolysate
The invention relates to preparation of a cell culture medium, and particularly provides preparation of a novel serum-free protein-free defined-chemical-component cell culture medium. The culture medium contains multiple amino acids, vitamins, inorganic salts, minor elements, carbohydrates and other supplementary factors. The culture medium is free of any extract or hydrolysate. The culture medium is free of any animal-derived component. The culture medium can well support in-vitro suspension culture of CHO (Chinese hamster ovary) cells, and satisfies the demands for CHO cell culture. The culture medium contains defined chemical components, and is low in cost.
Owner:BEIJING SL PHARMA +2
Amino acid formula powder and preparing method thereof
InactiveCN107041546AAddress Nutritional IssuesVitamin food ingredientsInorganic compound food ingredientsVegetable oilGalactooligosaccharide
The objective of the invention is to provide amino acid formula powder and preparing method thereof. Effective components of the amino acid formula powder are prepared from, by mass, 15-35% of refined vegetable oil, 30-50% of maltodextrin, 12-20% of compound amino acids, 0.07-0.5% of docosahexoenoic acid (DHA), 6-14% of medium-chain fatty acids, 0.1-1.8% of arachidonic acid (ARA), 0.01-0.1% of vitamins, 1.2-2.4% of mineral compounds, 1-5% of fructooligosaccharide (Fos), 1-10% of galacto-oligosaccharides (Gos), 0.03-1.0% of taurine, 0.05-0.5% of L-carnitine and 0.038-0.240% of choline. Adoption of protein-free formula powder is of great significance for prevention and treatment of infant protein allergy. The amino acids, the vitamins, polysaccharides, and the like are adopted as supplements to form the novel amino acid formula powder, thus overcoming the nutrition issue of protein-allergic infants. The amino acid formula powder is suitable for protein-allergic infants. The objective is realized.
Owner:浙江科露宝食品有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com