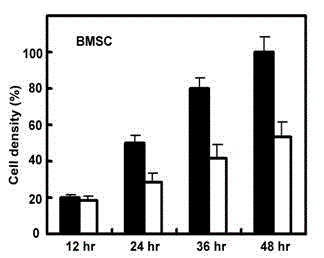
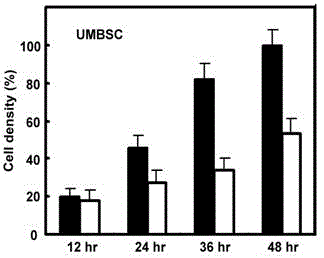
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1492results about "Cell culture media" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Modulation of stem and progenitor cell differentiation, assays, and uses thereof
InactiveUS20030235909A1Modulate their differentiationIncrease speedOrganic active ingredientsSenses disorderAssayPlacenta
The present invention relates to methods of modulating mammalian stem cell and progenitor cell differentiation. The methods of the invention can be employed to regulate and control the differentiation and maturation of mammalian, particularly human stem cells along specific cell and tissue lineages. The methods of the invention relate to the use of certain small organic molecules to modulate the differentiation of stem or progenitor cell populations along specific cell and tissue lineages, and in particular, to the differentiation of embryonic-like stem cells originating from a postpartum placenta or for the differentiation of early progenitor cells to a granulocytic lineage. Finally, the invention relates to the use of such differentiated stem or progenitor cells in transplantation and other medical treatments.
Owner:SIGNAL PHARMA LLC +2
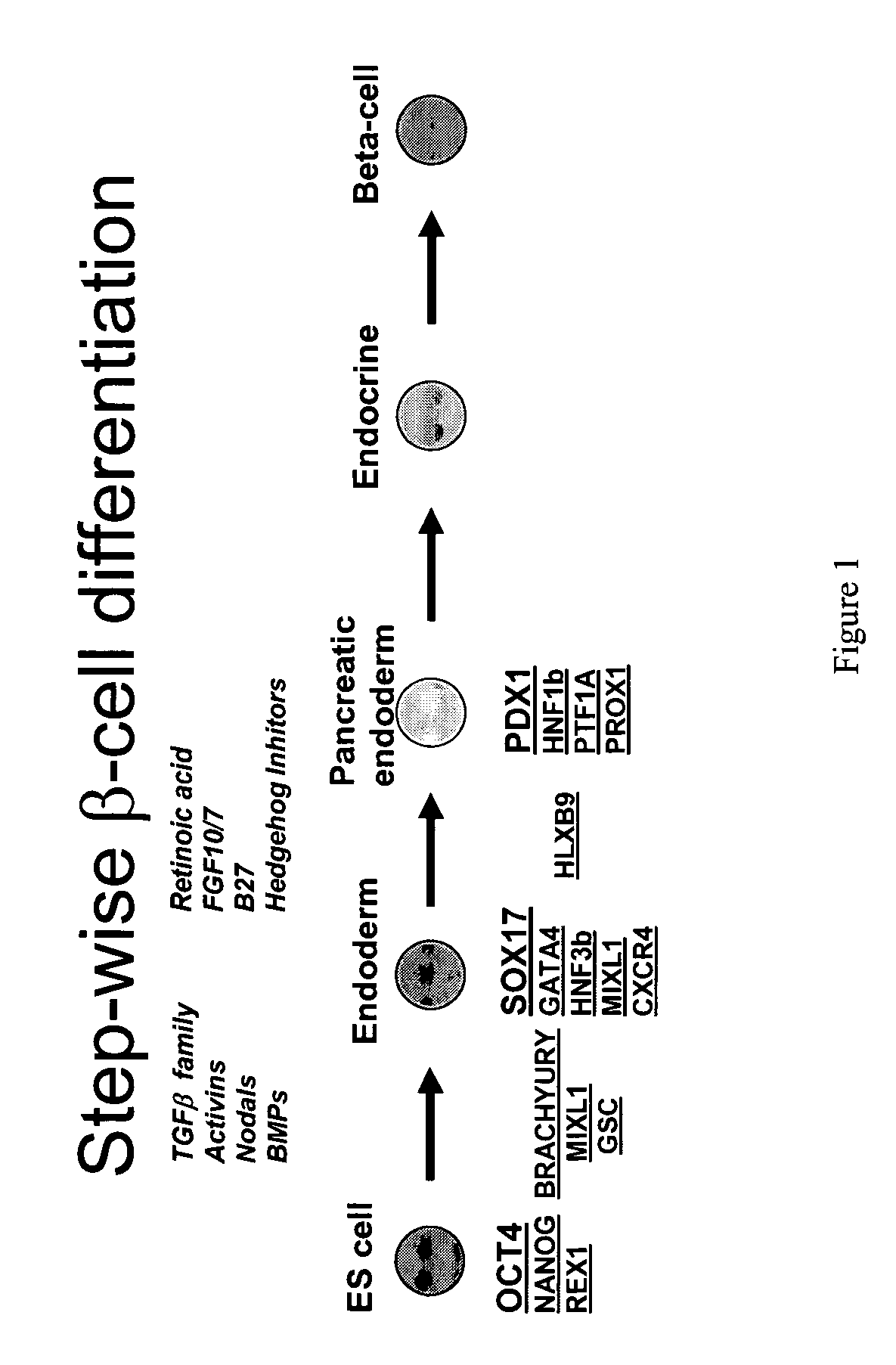
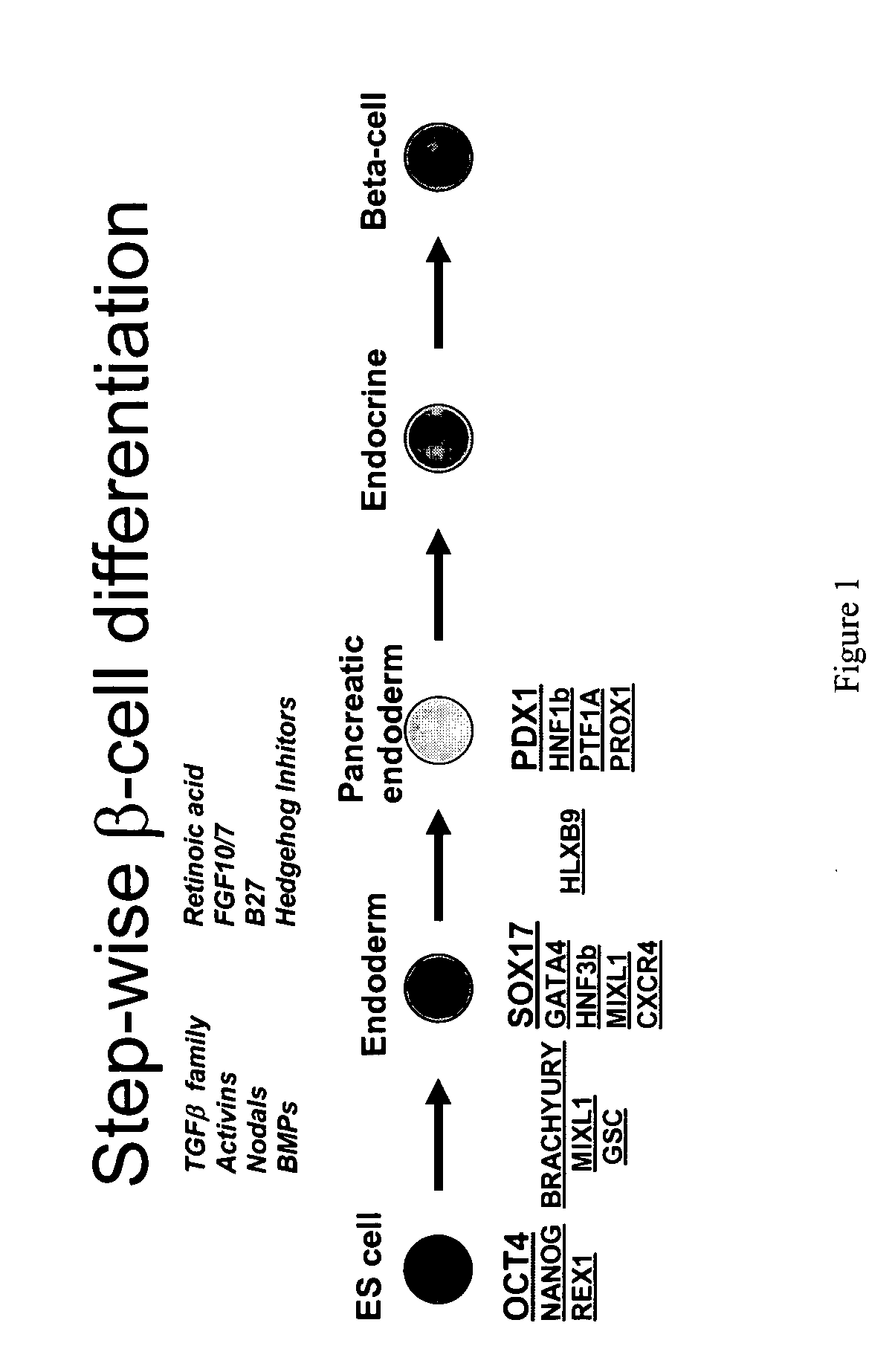
PDX1 expressing endoderm
InactiveUS20050266554A1Increase differentiationIncrease productionGastrointestinal cellsDiagnosticsGerm layerCell type
Disclosed herein are cell cultures comprising PDX1-positive endoderm cells and methods of producing the same. Also disclosed herein are cell populations comprising substantially purified PDX1-positive endoderm cells as well as methods for enriching, isolating and purifying PDX1-positive endoderm cells from other cell types. Methods of identifying differentiation factors capable of promoting the differentiation of endoderm cells, such as PDX1-positive foregut endoderm cells and PDX1-negative definitive endoderm cells, are also disclosed.
Owner:CYTHERA
Method for expansion of stem cells
InactiveUS20060182724A1Increase oxygen contentAvoid clotsBiocideCosmetic preparationsCell culture mediaCell growth
A method of increasing the growth of stem cells by mixing the stem cells with a growth medium that has been conditioned by an incubation with placental tissue. The method increases the expansion of the stem cell population.
Owner:RIORDAN NEIL H
Modulation of stem and progenitor cell differentiation, assays, and uses thereof
InactiveUS7498171B2Increase speedWell formedOrganic active ingredientsSenses disorderProgenitorAssay
The present invention relates to methods of modulating mammalian stem cell and progenitor cell differentiation. The methods of the invention can be employed to regulate and control the differentiation and maturation of mammalian, particularly human stem cells along specific cell and tissue lineages. The methods of the invention relate to the use of certain small organic molecules to modulate the differentiation of stem or progenitor cell populations along specific cell and tissue lineages, and in particular, to the differentiation of embryonic-like stem cells originating from a postpartum placenta or for the differentiation of early progenitor cells to a granulocytic lineage. Finally, the invention relates to the use of such differentiated stem or progenitor cells in transplantation and other medical treatments.
Owner:SIGNAL PHARMA LLC +2
Oligopeptide-free cell culture media
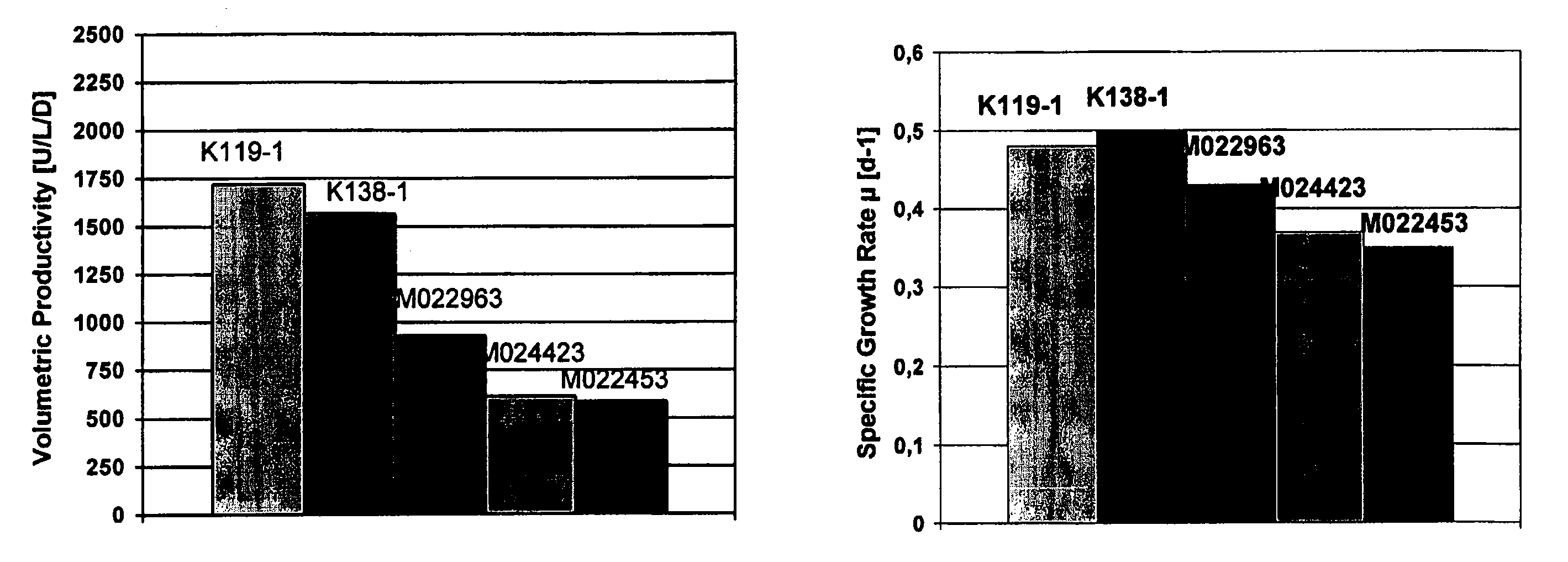
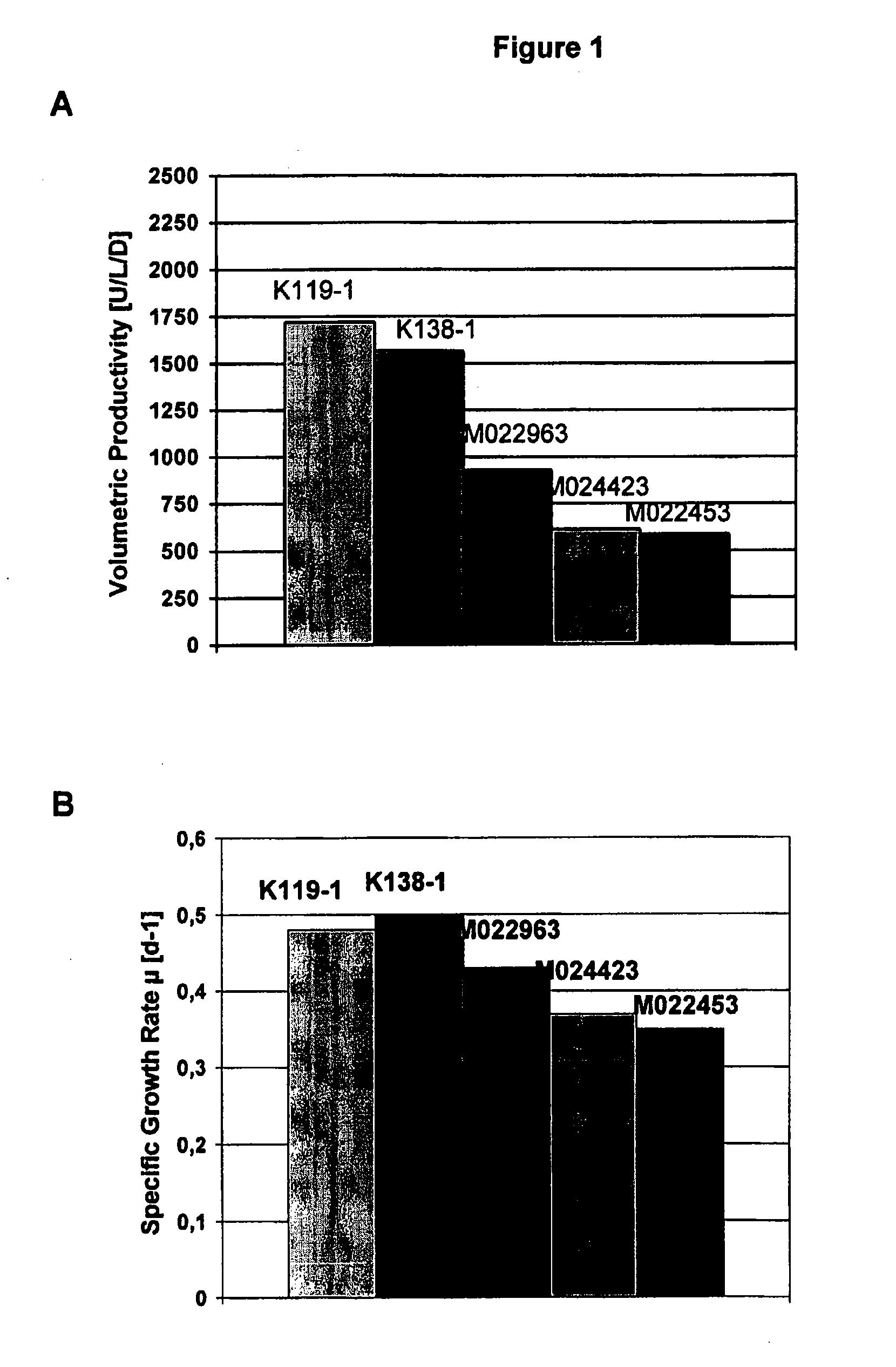
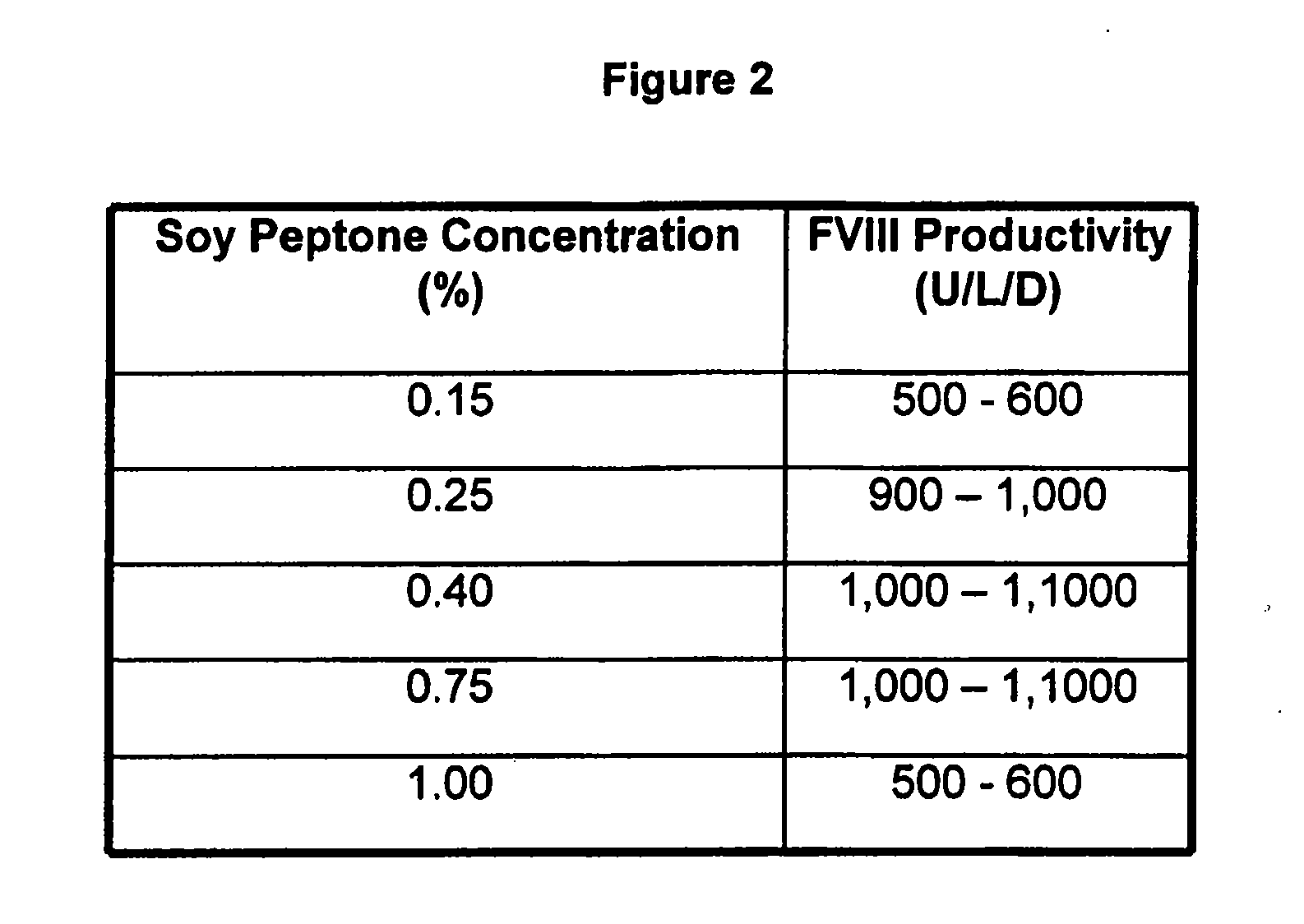
InactiveUS20070212770A1Efficient expression of recombinantEfficient productionFactor VIIBacteriaCulture cellCell culture media
The present invention relates to oligopeptide-free cell culture media comprising at least 0.5 mg / L of a polyamine and to methods for cultivating cells in said oligopeptide-free cell culture media comprising at least 0.5 mg / L of a polyamine. The invention also relates to methods for expressing at least one protein in a medium comprising at least 0.5 mg / L of a polyamine and to methods for producing at least one virus in a medium comprising at least 0.5 mg / L of a polyamine.
Owner:BAXTER HEALTHCARE SA +1
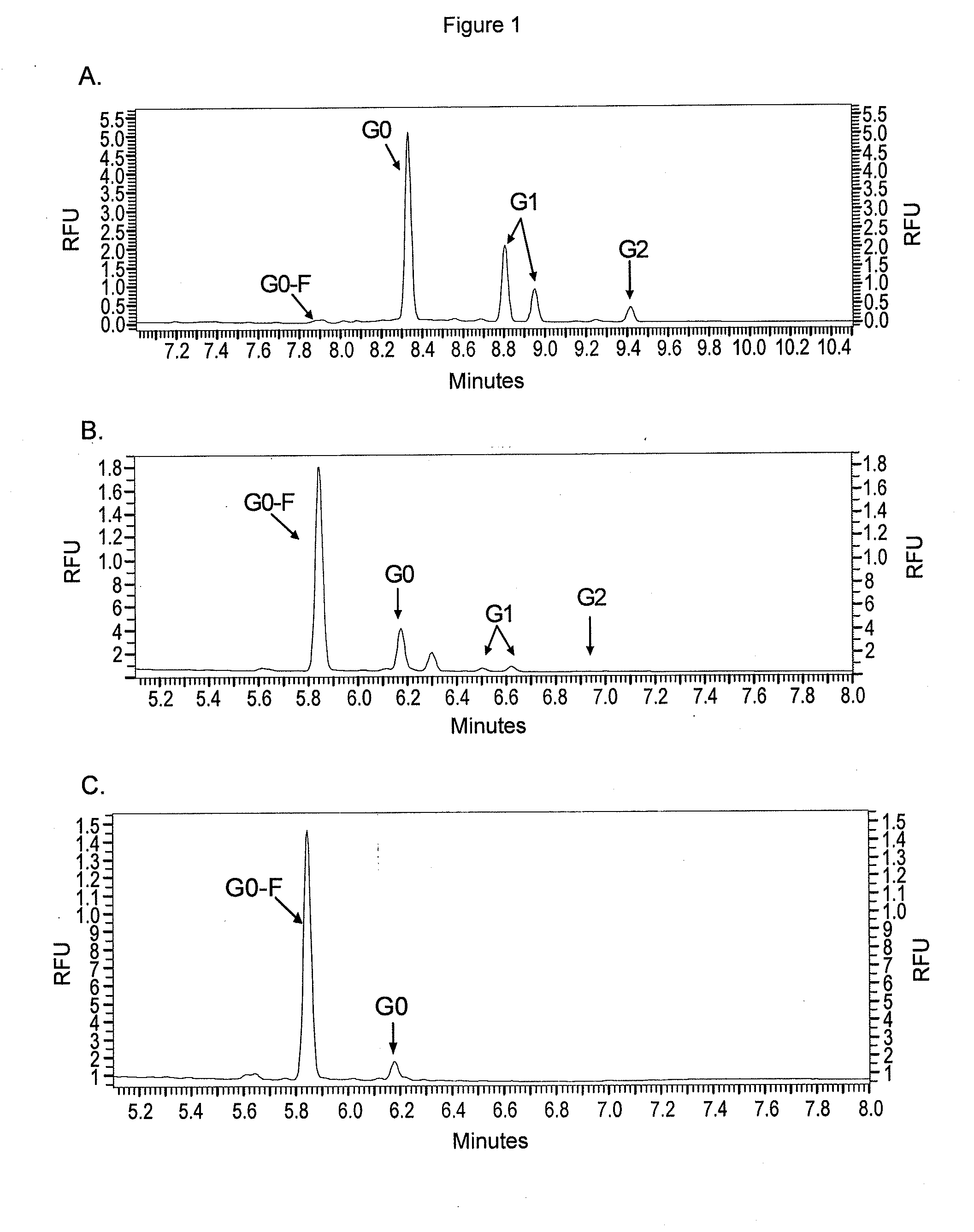
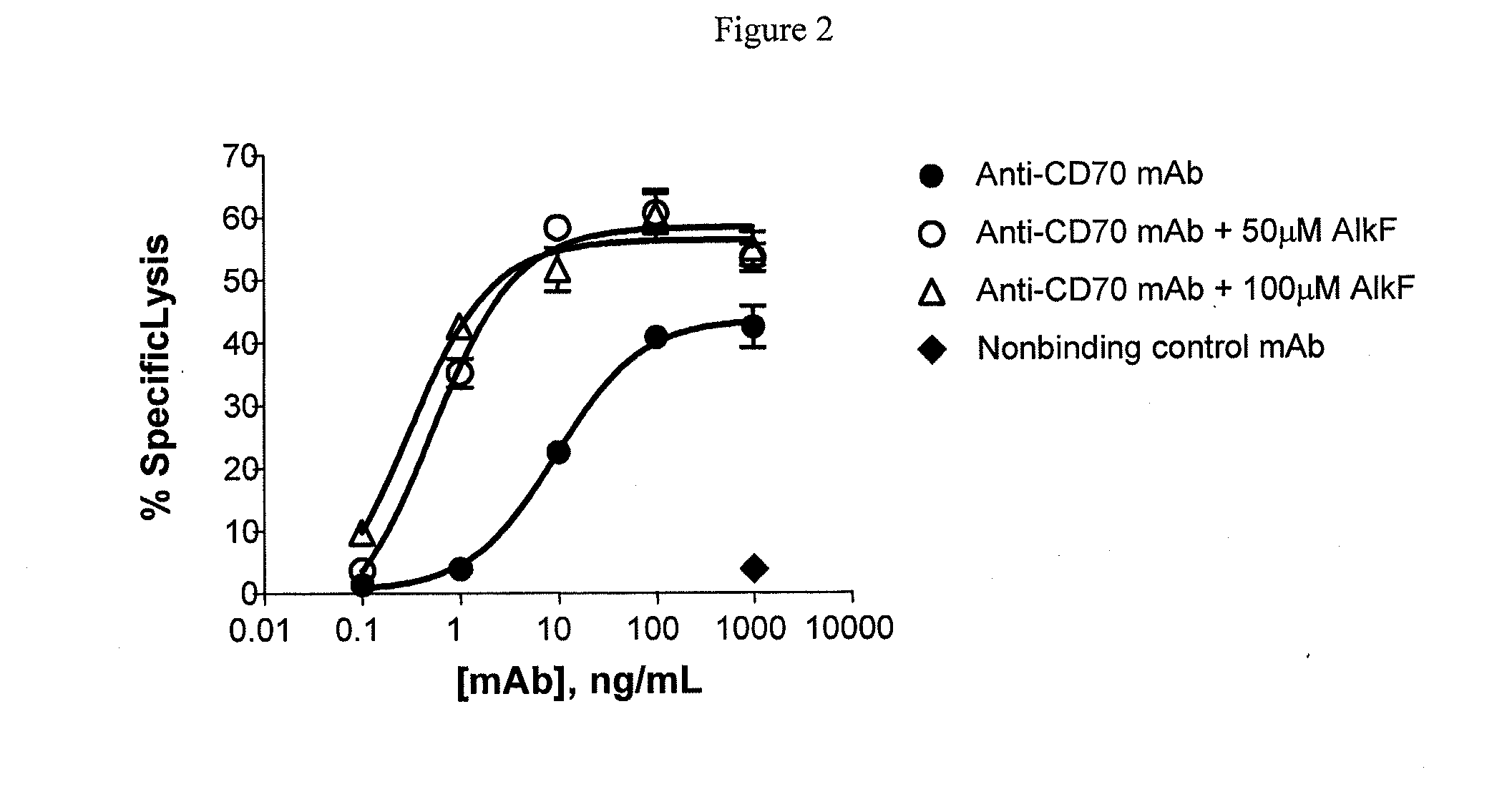
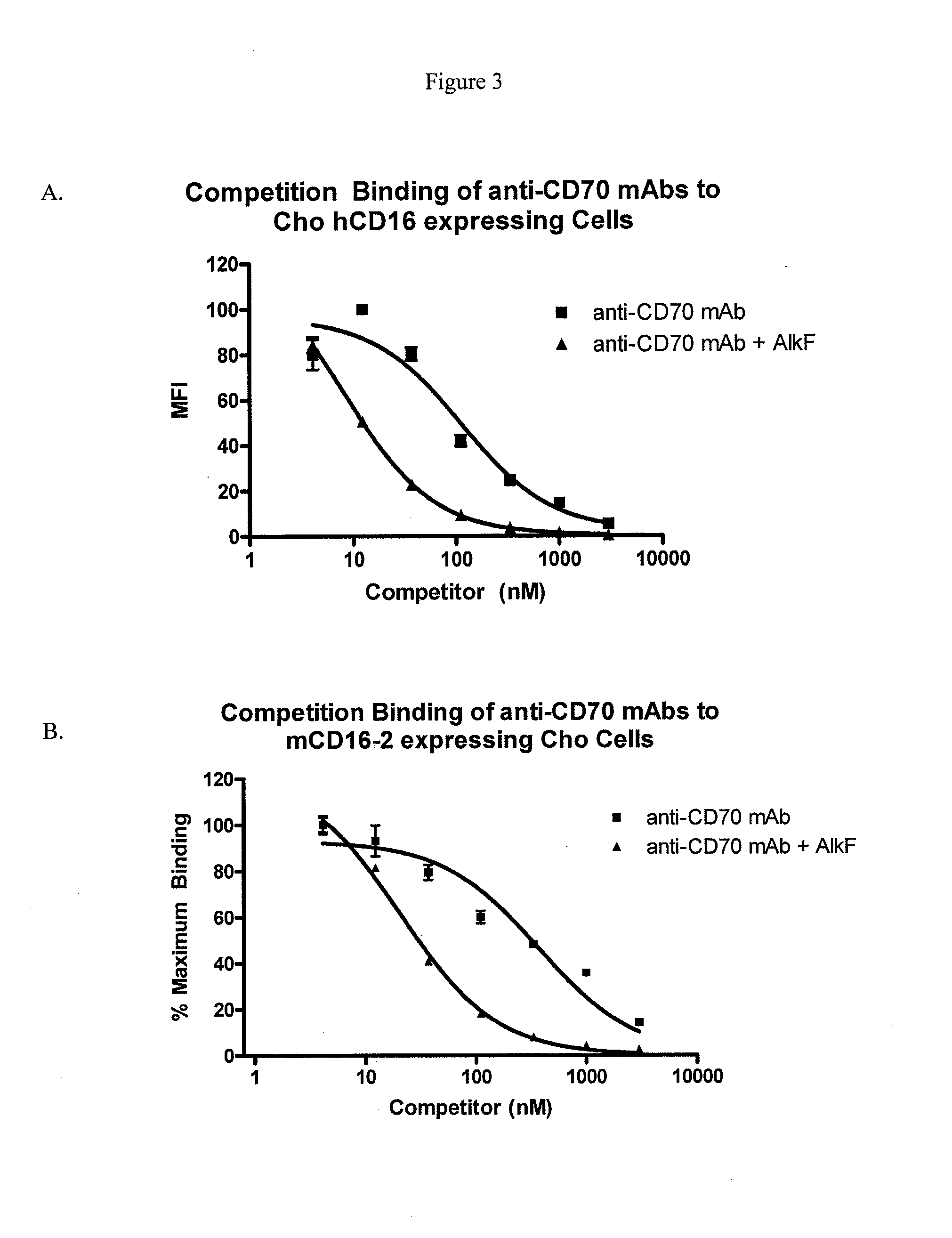
Methods and compositions for making antibodies and antibody derivatives with reduced core fucosylation
ActiveUS20090317869A1Inhibit and reduce core fucosylationEsterified saccharide compoundsSugar derivativesFucosylationAntibody
The invention provides methods and compositions for preparing antibodies and antibody derivatives with reduced core fucosylation.
Owner:SEAGEN INC
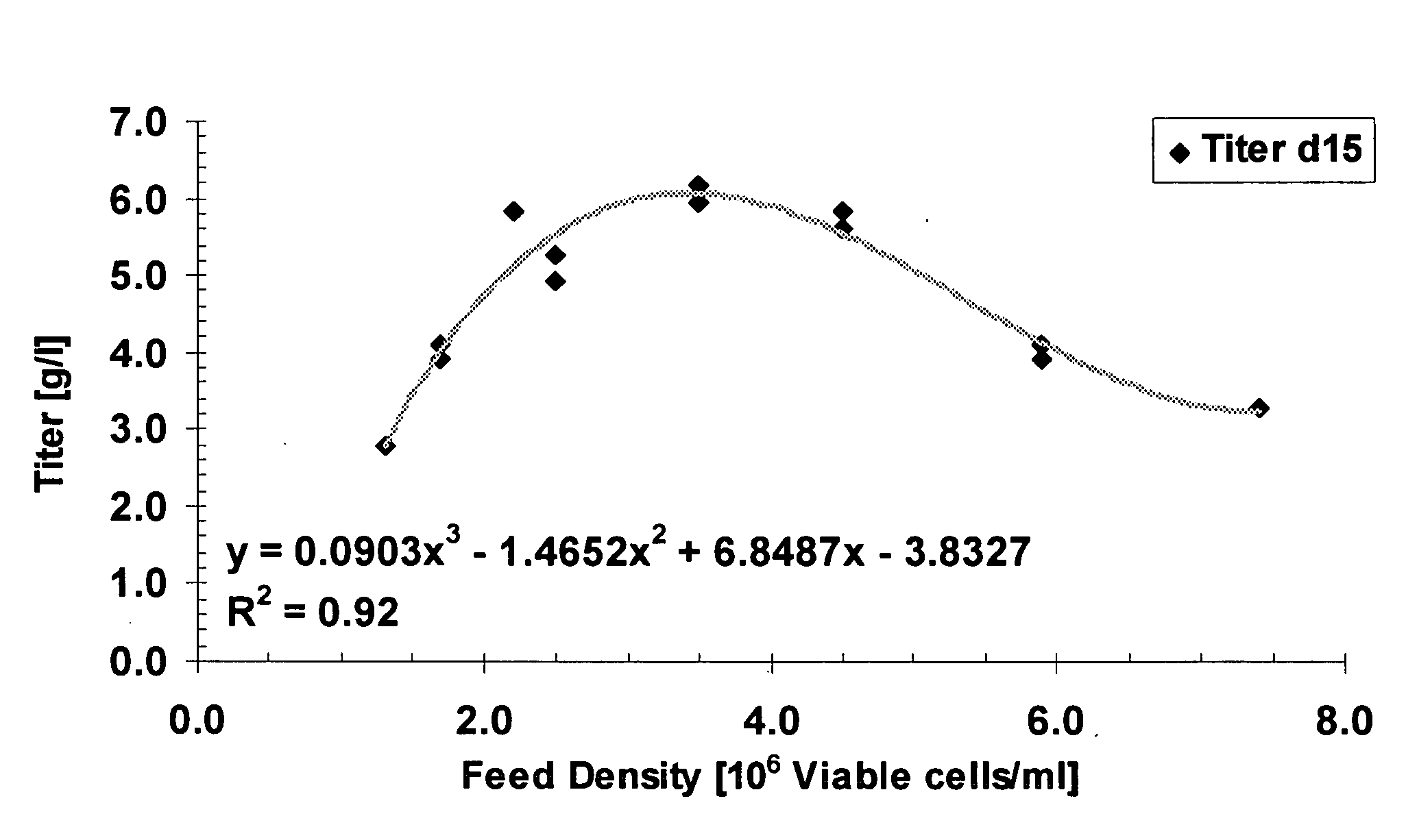
Cell culture improvements
ActiveUS20080227136A1Improve productivityImproved cell culture longevityGenetically modified cellsMicrobiological testing/measurementCell culture mediaImproved method
The invention describes improved methods and compositions for producing a recombinant protein, e.g., an antibody, in mammalian cell culture. In addition, the invention provides improved cell culture media, including improved production media, feed solutions, and combination feeds, which may be used to improve protein productivity in mammalian cell culture.
Owner:ABBVIE INC
Methods for identifying factors for differentiating definitive endoderm
Disclosed herein are methods of identifying one or more differentiation factors that are useful for differentiating cells in a cell population comprising definitive endoderm cells into cells which are capable of forming tissues and / or organs that are derived from the gut tube.
Owner:VIACYTE INC
Animal protein-free media for cultivation of cells
InactiveUS20080009040A1Efficient expressionGuaranteed efficient growthMicroorganismsCulture processHydrolysateCell culture media
The present invention relates to animal protein-free cell culture media comprising polyamines and a plant- and / or yeast-derived hydrolysate. The invention also relates to animal protein-free culturing processes, wherein cells can be cultivated, propagated and passaged without adding supplementary animal proteins in the culture medium. These processes are useful in cultivating cells, such as recombinant cells or cells infected with a virus, and for producing biological products by cell culture processes.
Owner:BAXTER INT INC +1
Integration of sample storage and sample management for life science
InactiveUS20060099567A1Reduce degradationImmobilised enzymesMaterial nanotechnologyEnzymeDry storage
Compositions and methods are disclosed for automated storing, tracking, retrieving and analyzing biological samples, including dry storage at ambient temperatures of nucleic acids, proteins (including enzymes), and cells using a dissolvable dry storage matrix that permits recovery of biologically active materials. RFID-tagged biological sample storage devices featuring dissolvable or dissociable matrices are described for use as supports of biological samples, which matrices can be dried and subsequently rehydrated for sample recovery. Also disclosed are computer-implemented systems and methods for managing sample data.
Owner:BIOMATRICA INC
Methods for identifying factors for differentiating definitive endoderm
Disclosed herein are methods of identifying one or more differentiation factors that are useful for differentiating cells in a cell population comprising definitive endoderm cells into cells which are capable of forming tissues and / or organs that are derived from the gut tube.
Owner:VIACYTE INC
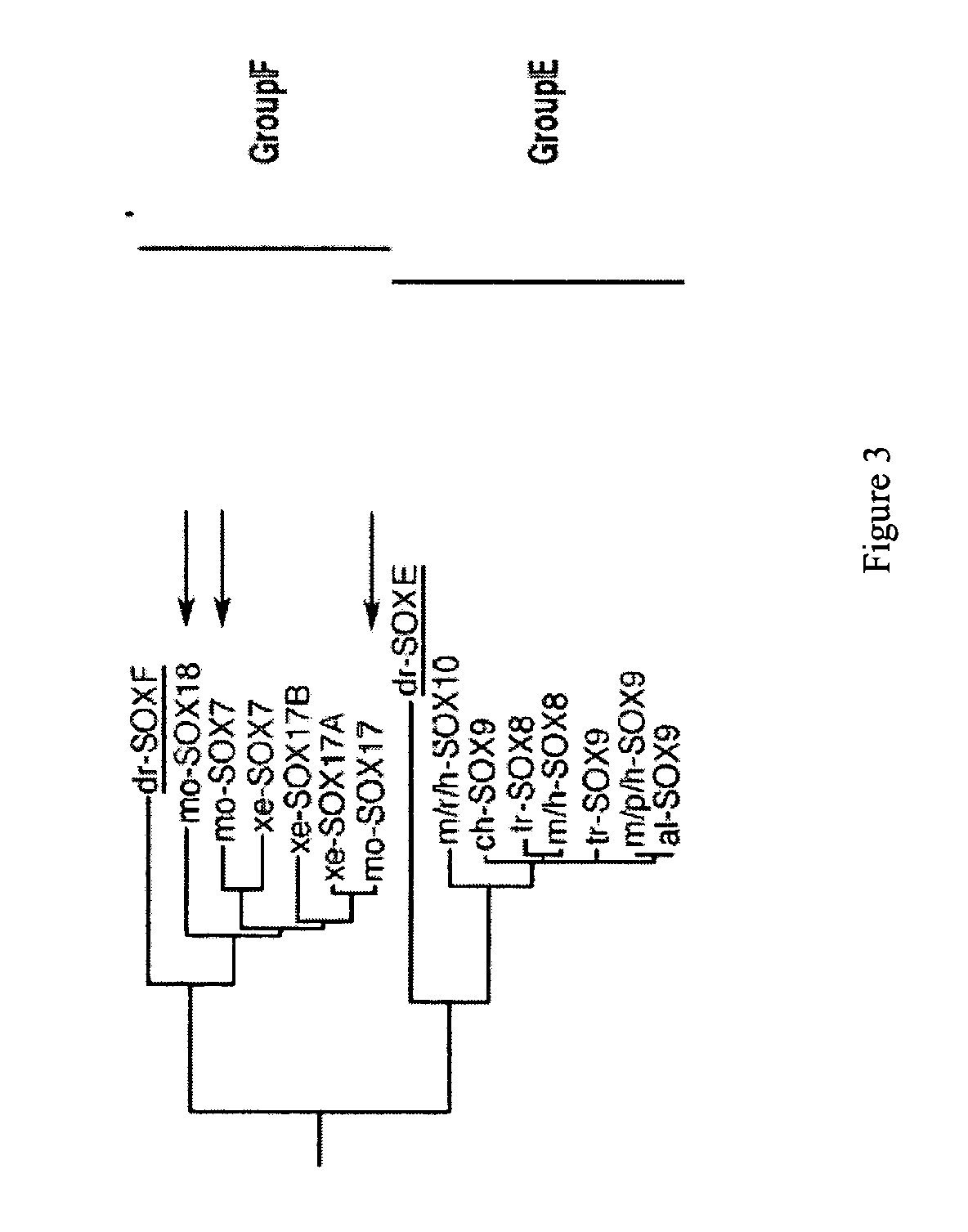
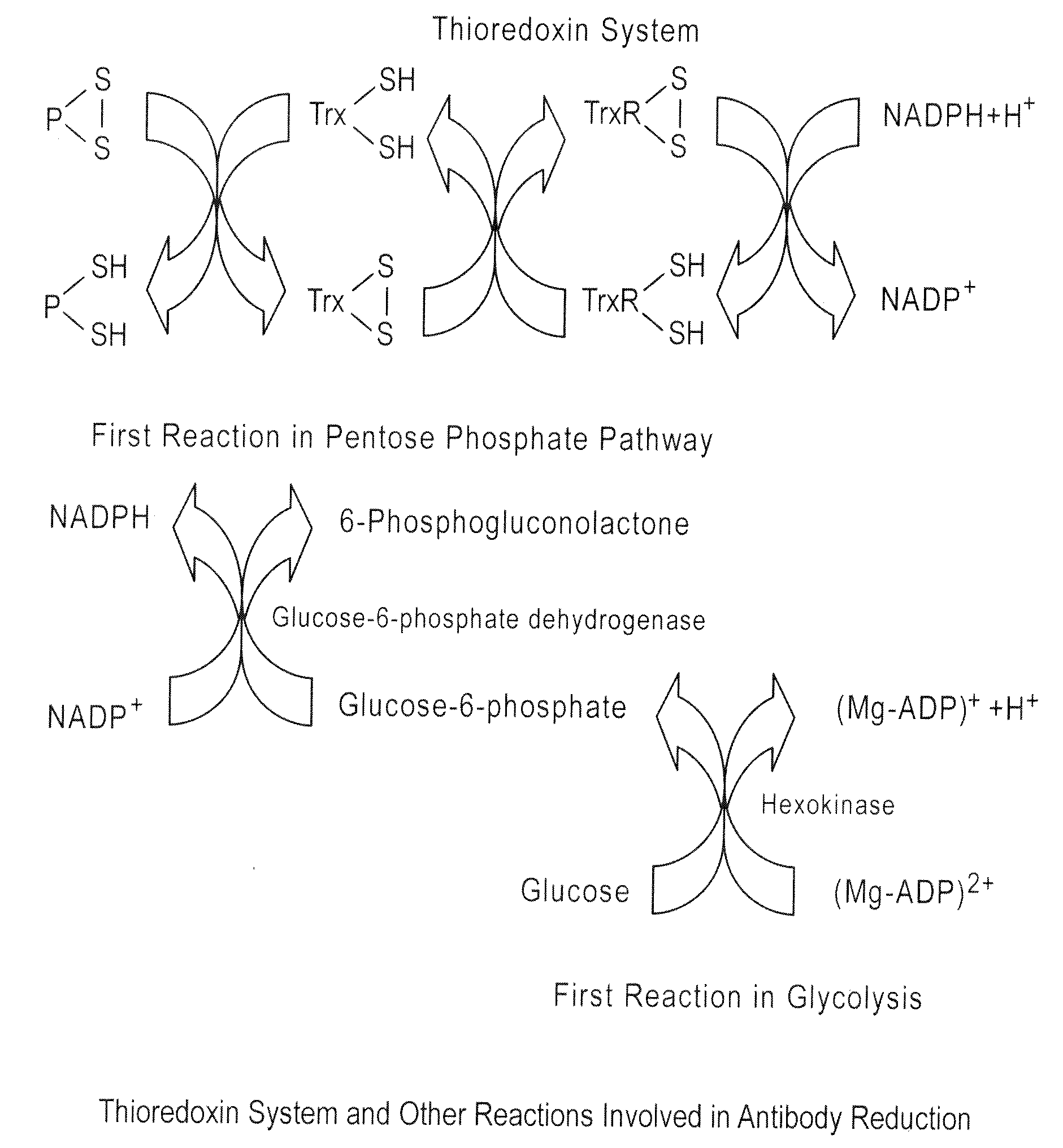
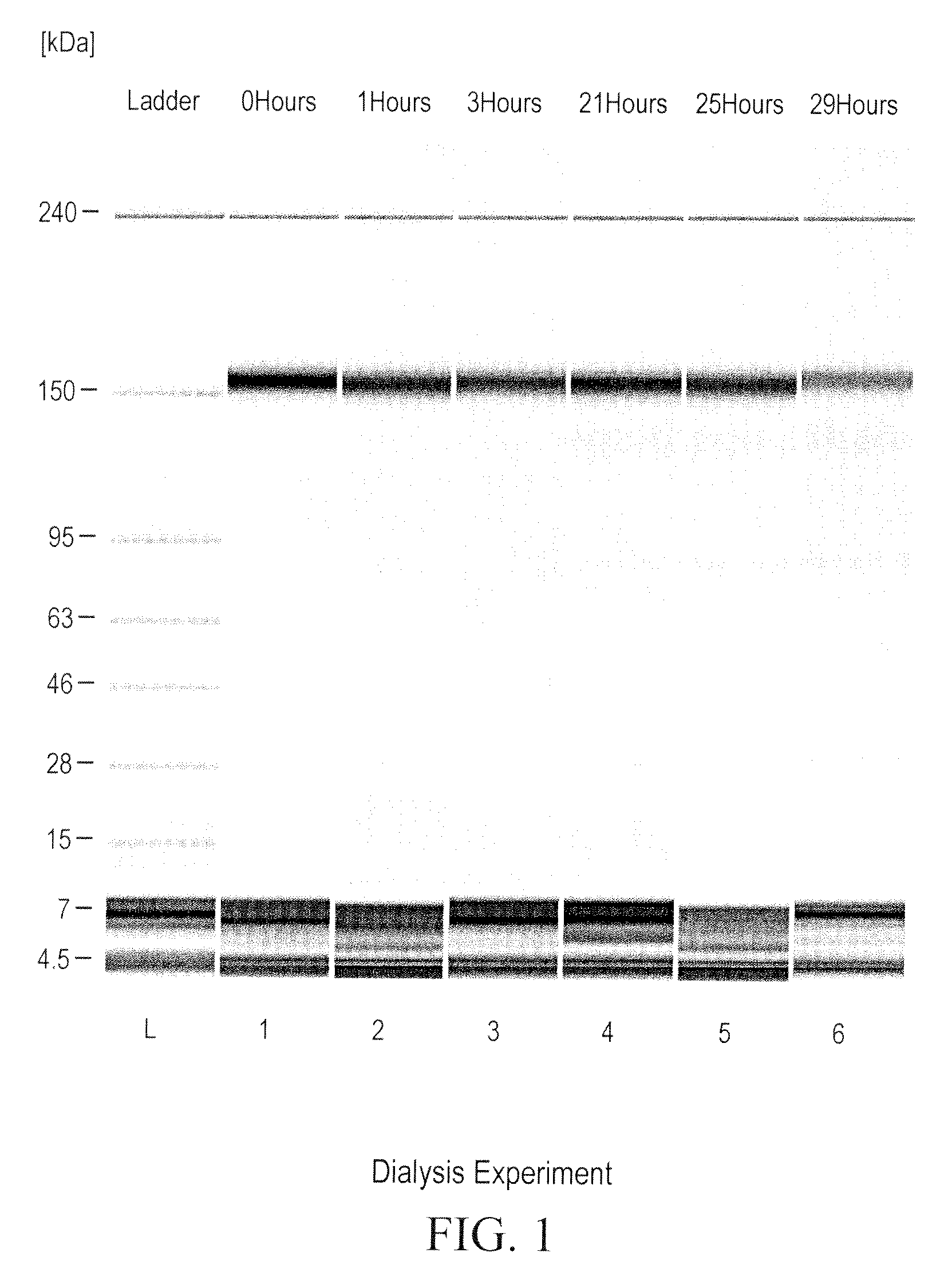
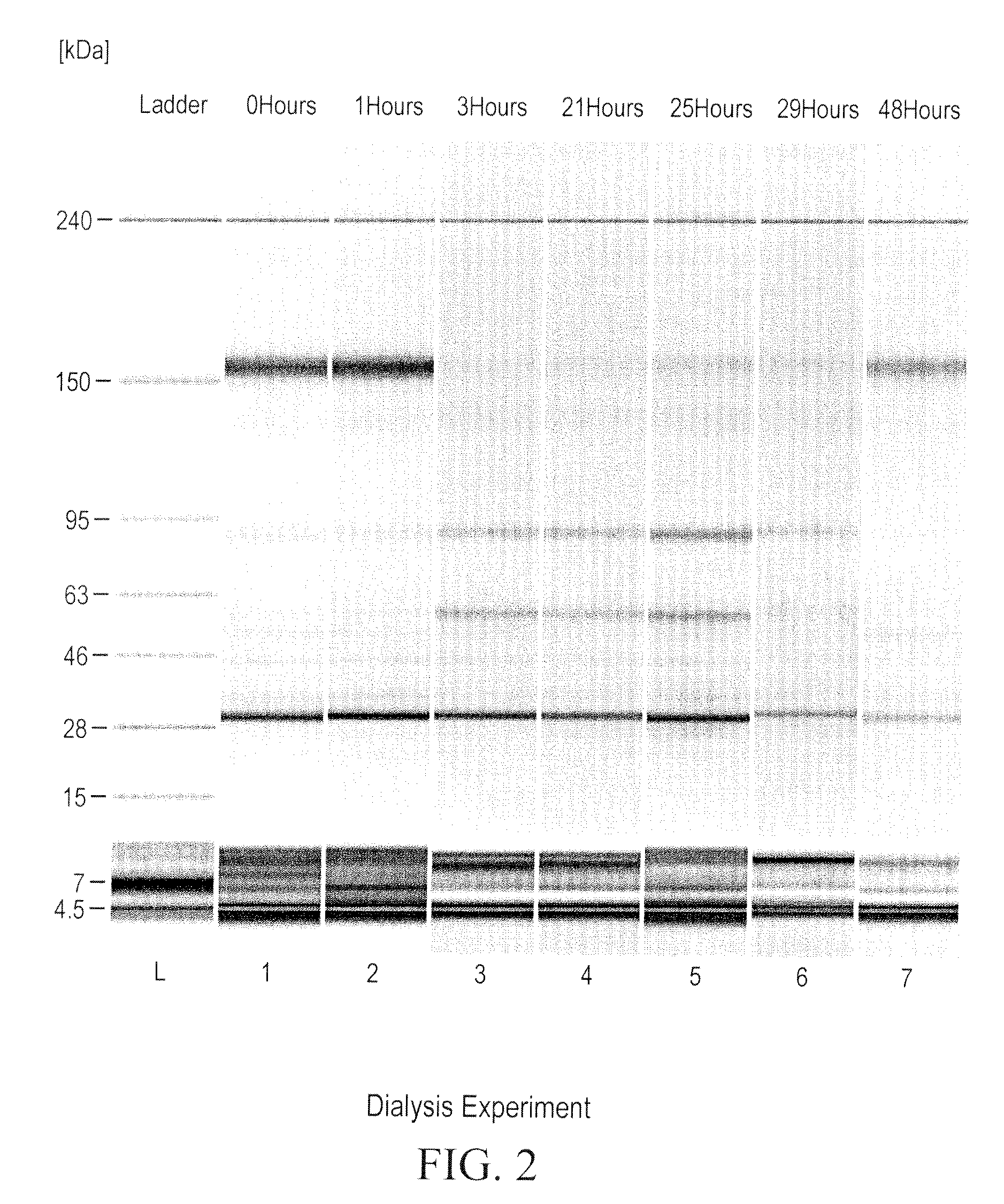
Prevention of disulfide bond reduction during recombinant production of polypeptides
The invention concerns methods and means for preventing the reduction of disulfide bonds during the recombinant production of disulfide-containing polypeptides. In particular, the invention concerns the prevention of disulfide bond reduction during harvesting of disulfide-containing polypeptides, including antibodies, from recombinant host cell cultures.
Owner:GENENTECH INC
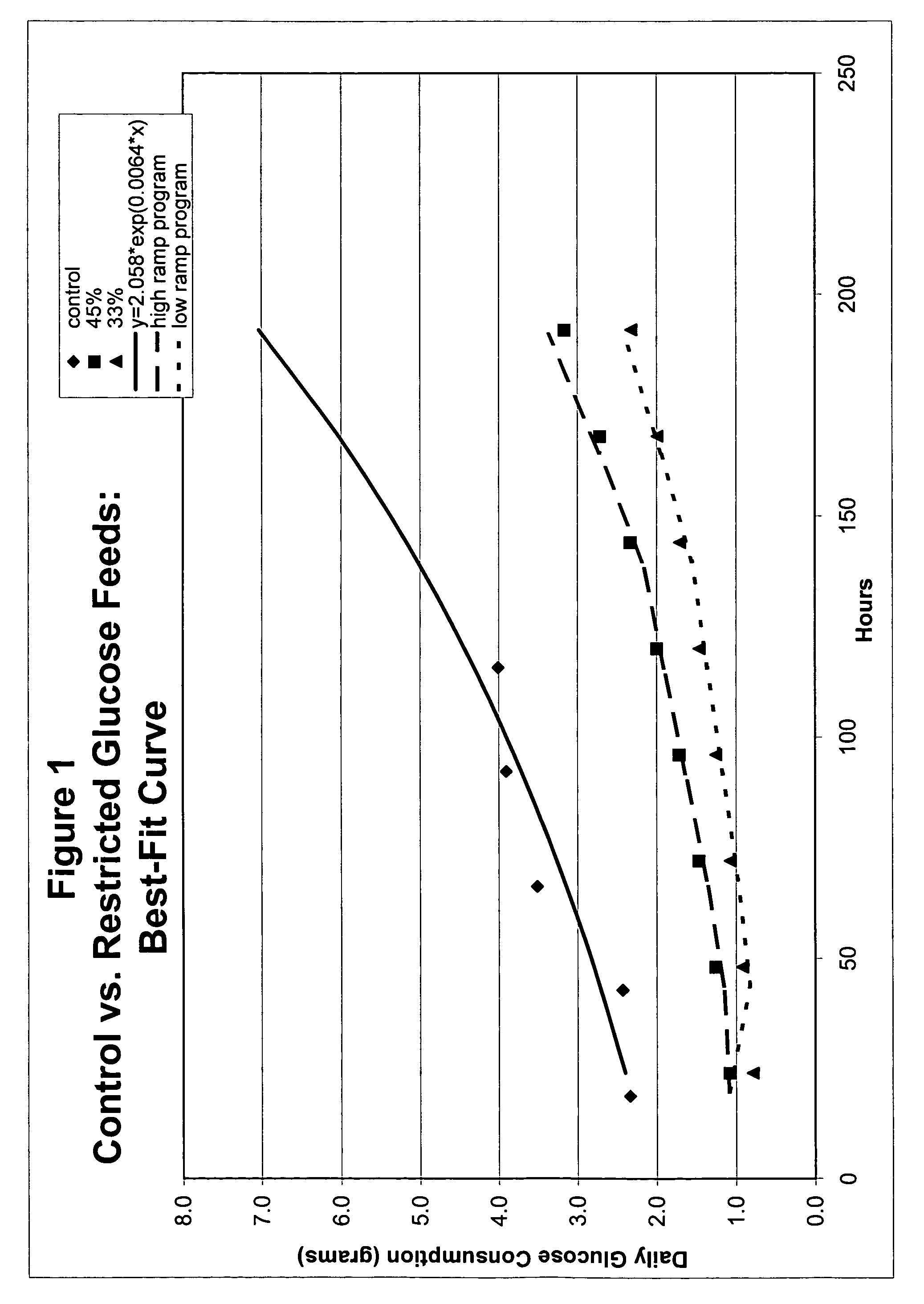
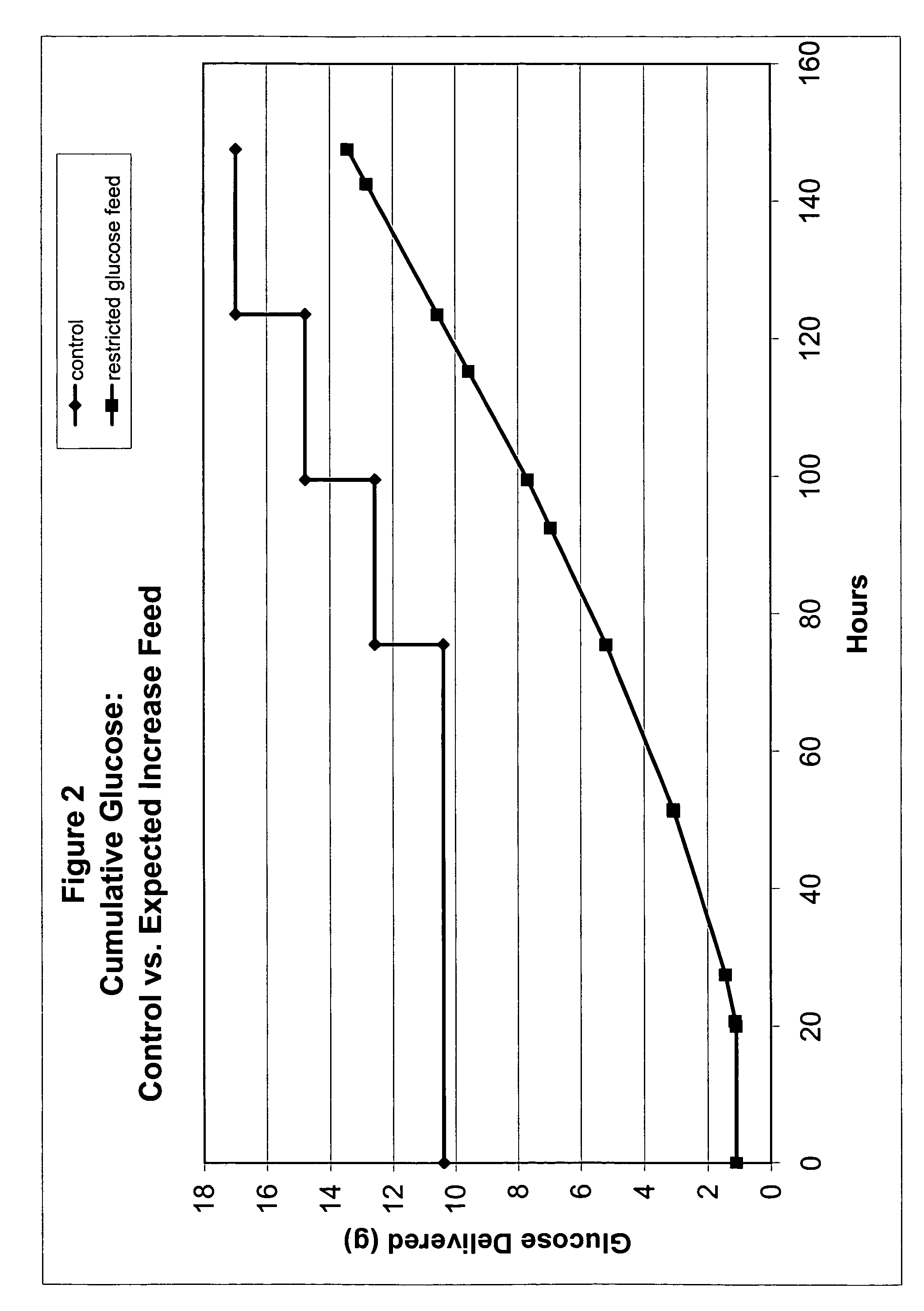
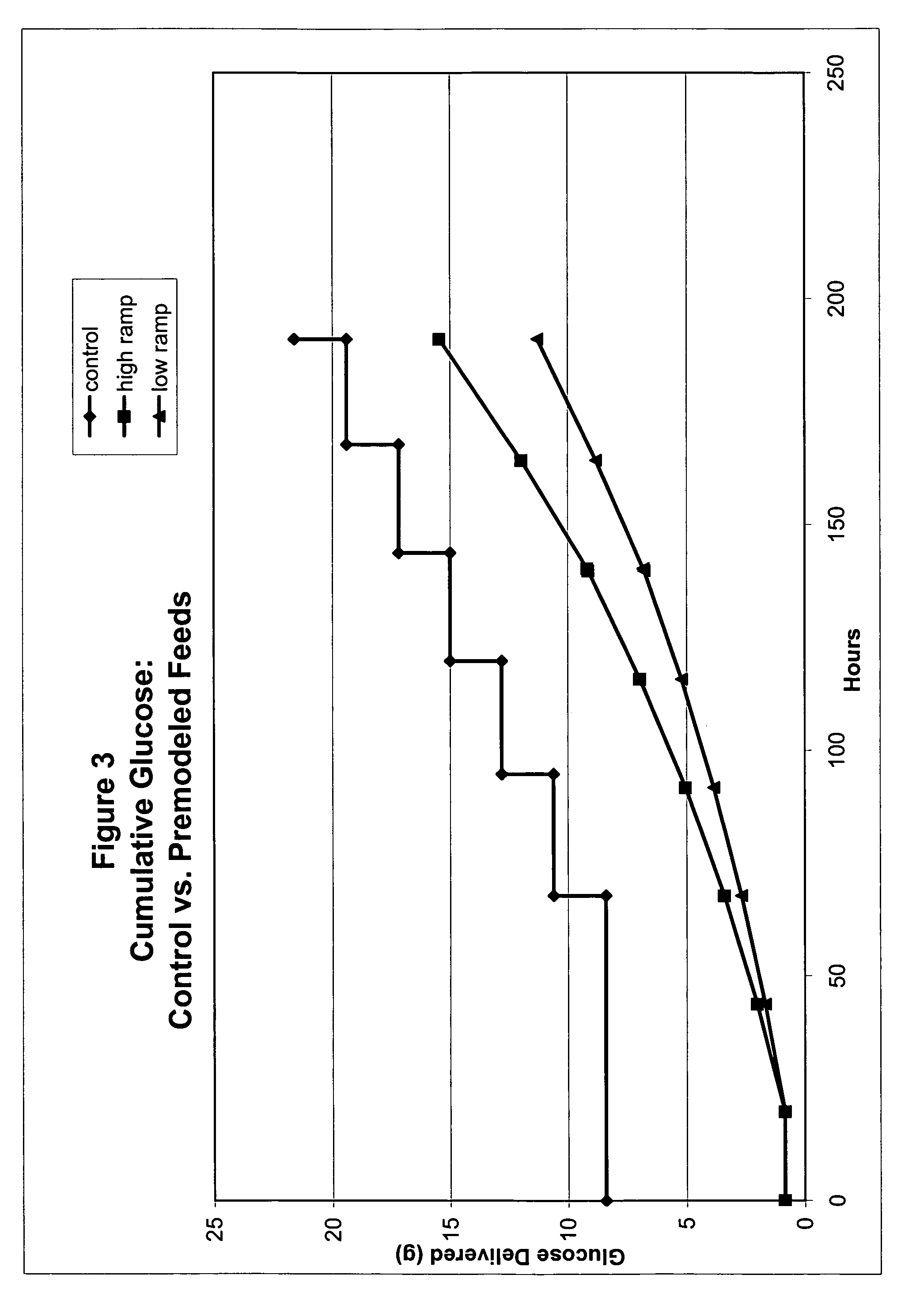
Restricted glucose feed for animal cell culture
ActiveUS7429491B2Promote productionFlexible controlCulture processCell culture mediaBiotechnologyBiochemistry
Methods of improving protein production in animal cell cultures are provided. Cell culture methods are presented wherein glucose is fed in a restricted manner to cell culture; this restricted feeding of glucose to the cell culture results in lactate production being controlled to a low level. The restricted feeding of glucose in a fed-batch process is not accomplished through a constant-rate feeding of glucose, and the restricted feeding need not depend on sampling. Instead, restricted feeding of glucose to the culture is accomplished through feeding of glucose to the culture at a rate that is a function of an expected or a premodeled rate of glucose consumption by the animal cells when exposed to medium containing a high level of glucose. Because lactate production is controlled to low levels, recombinant protein production is increased.
Owner:WYETH LLC
Stem cell culture medium and application thereof and stem cell cultivation method
ActiveCN103060264AHigh speedRapid expansionCulture processCell culture mediaMesenchymeCell culture media
The invention discloses a stem cell culture medium, an application of the stem cell culture medium and a stem cell cultivation method. Blood serum does not exist in the stem cell culture medium. The stem cell culture medium comprises amino acid, vitamin, salt, lipoid, cytokines and egg white polypeptide. The stem cell culture medium is suitable for fast cultivating stem cells which are tissue sources of human and mammal, and comprises but not is limited by fat mesenchyme stem cells, mesenchymal stem cells and umbilical cord blood stem cells. The culture medium enables increasing speed of the cells to be improved by 3-5 times, and differential potentials of the cells can not be affected. Compared with an ordinary stem cell culture medium, stem cells from different sources can be fast expanded, passage number is prolonged, and proficiency properties of the stem cells can be well kept.
Owner:苏州博棠再生医学科技有限公司
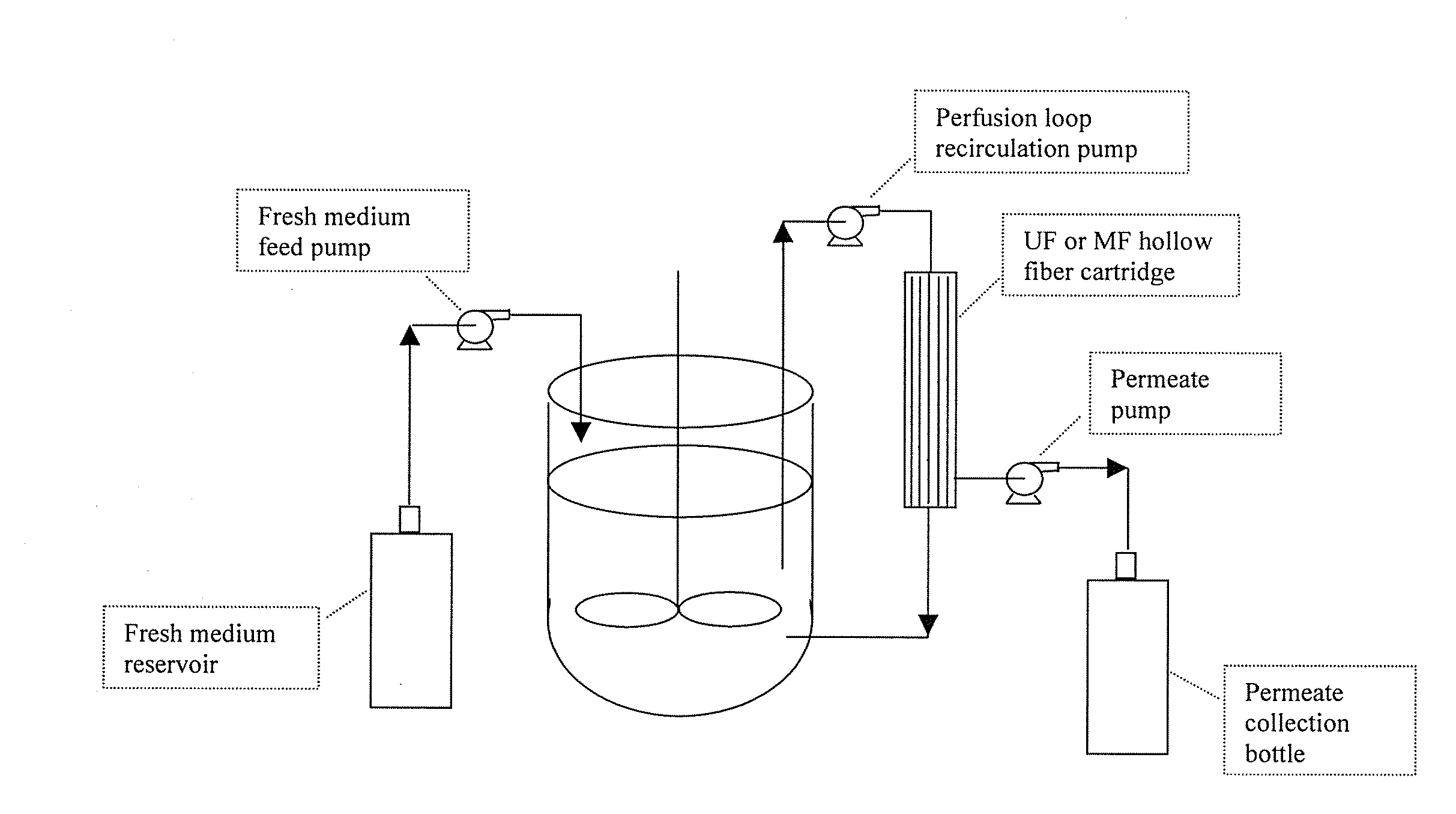
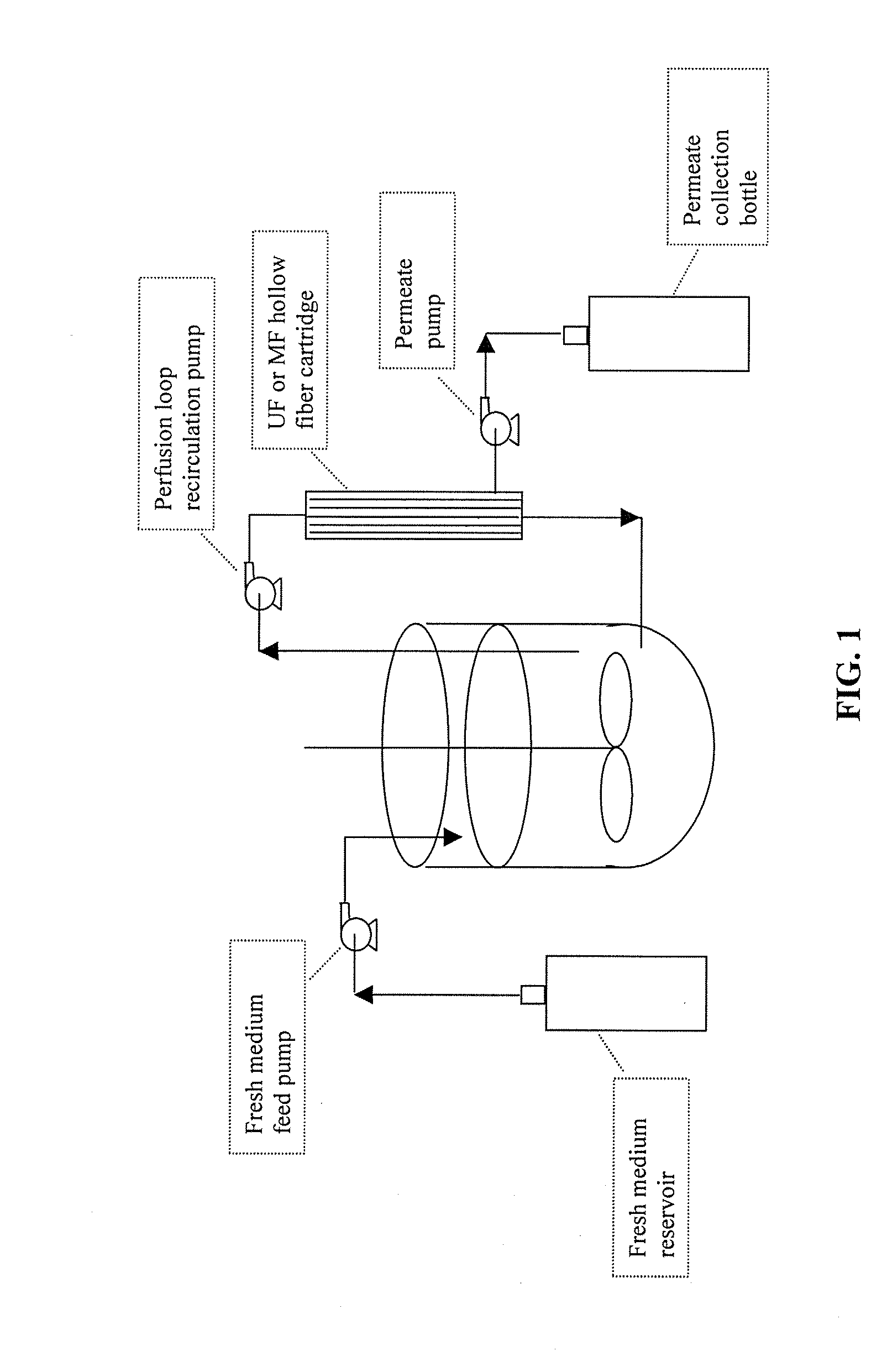
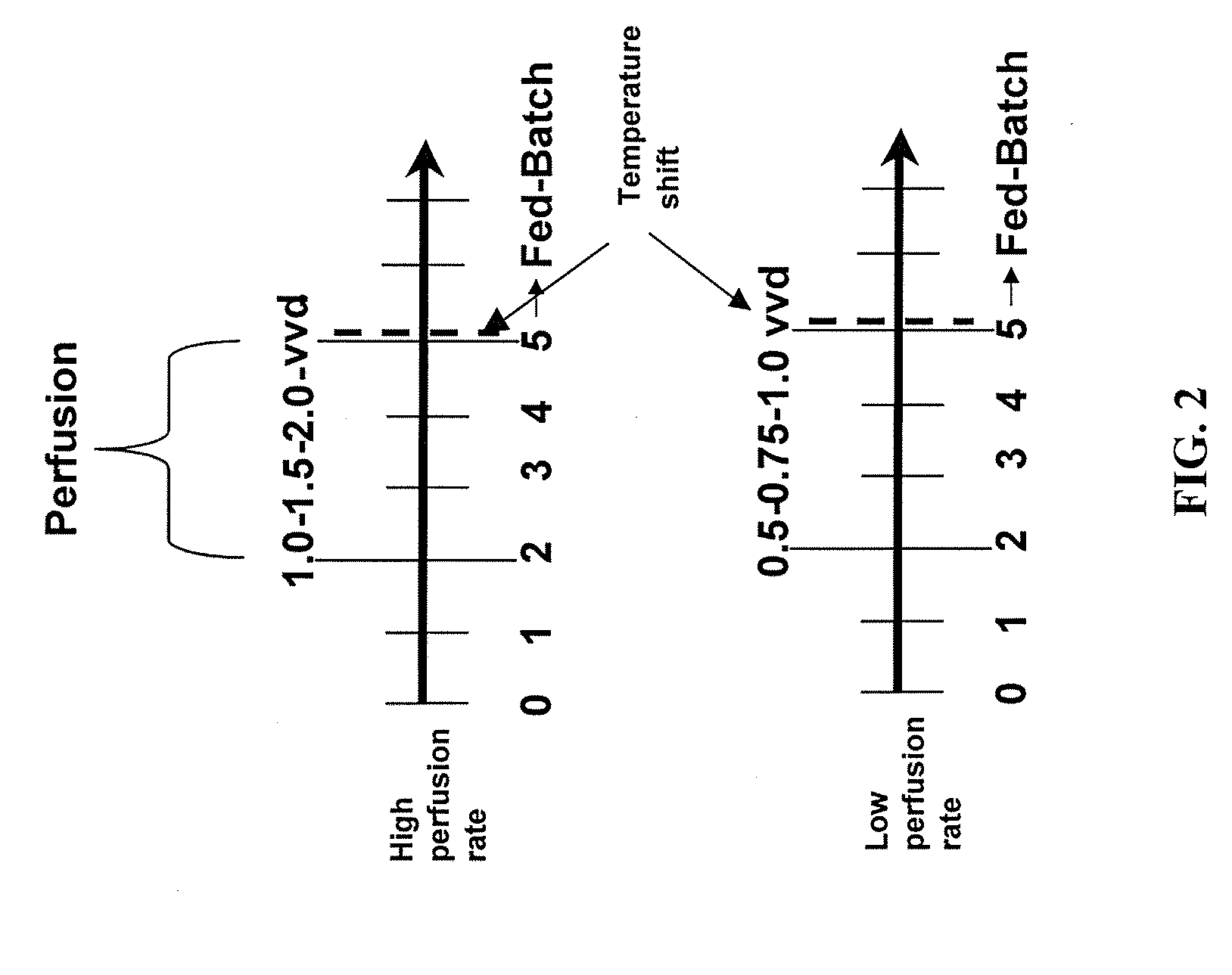
Use of perfusion to enhance production of fed-batch cell culture in bioreactors
InactiveUS20090042253A1Bioreactor/fermenter combinationsBiological substance pretreatmentsFiltrationFeed pump
The invention relates to methods of improving protein production, e.g., large-scale commercial protein production, e.g., antibody production, utilizing a modified fed-batch cell culture method comprising a cell growth phase and a polypeptide production phase. The modified fed-batch cell culture method combines both cell culture perfusion and fed-batch methods to achieve higher titers of polypeptide products. Because the modified fed-batch cell culture method of the invention produces higher polypeptide product titers than fed-batch culture alone, it will substantially improve commercial-scale protein production. The invention also relates to a perfusion bioreactor apparatus comprising a fresh medium reservoir connected to a bioreactor by a feed pump, a recirculation loop connected to the bioreactor, wherein the recirculation loop comprises a filtration device, e.g., ultrafiltration or microfiltration, and a permeate pump connecting the filtration device to a permeate collection container.
Owner:WYETH LLC
Fed-batch cell culture methods using non-animal-based hydrolysates
ActiveUS8093045B2Improve productivityHigh potencyGenetically modified cellsMicrobiological testing/measurementHydrolysateCell culture media
The invention describes improved methods and compositions for producing a recombinant protein, e.g., an antibody, in mammalian cell culture. In addition, the invention provides improved cell culture media, including improved production media, feed solutions, and combination feeds, which may be used to improve protein productivity in mammalian cell culture.
Owner:ABBVIE INC
Production of polypeptides
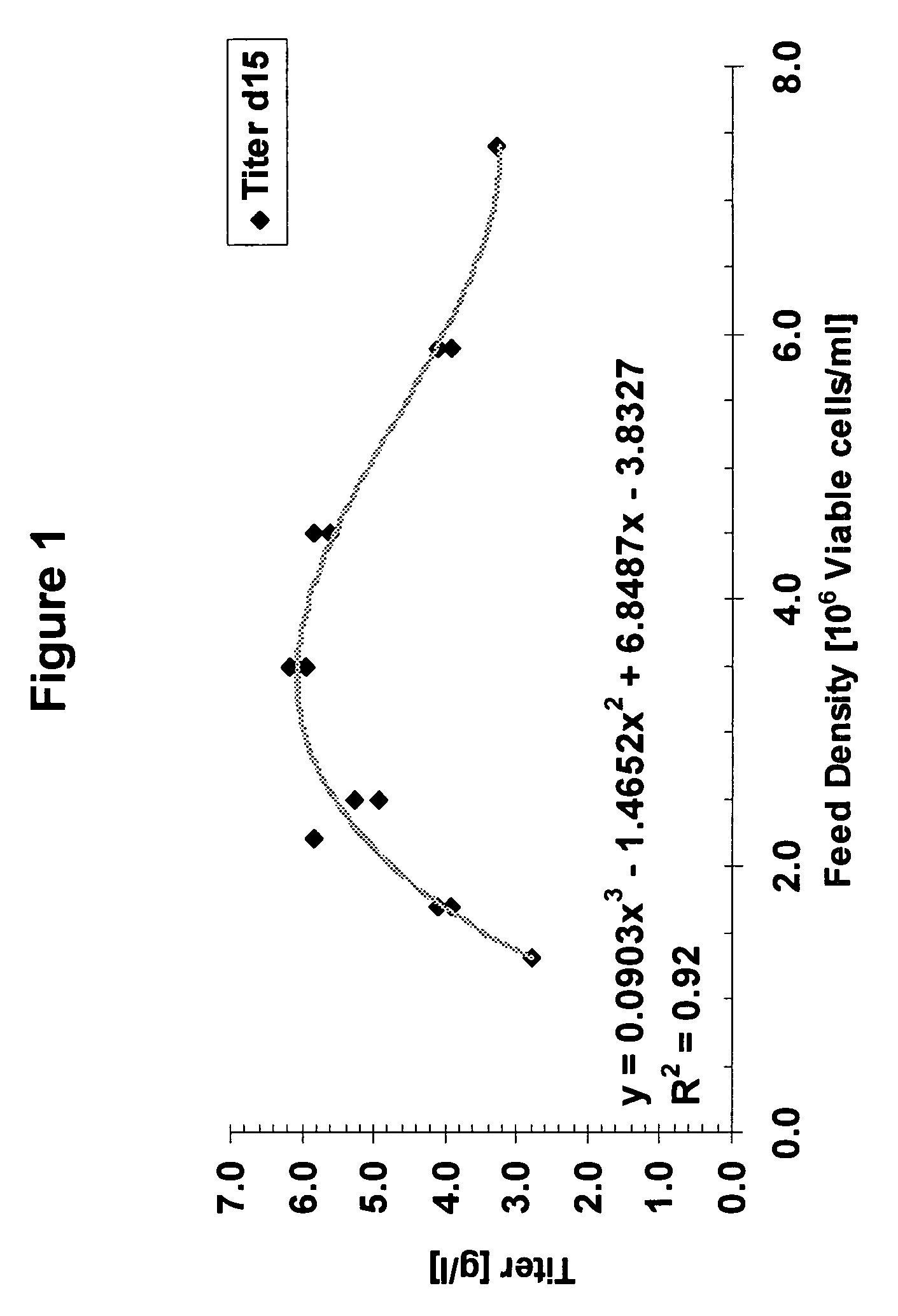
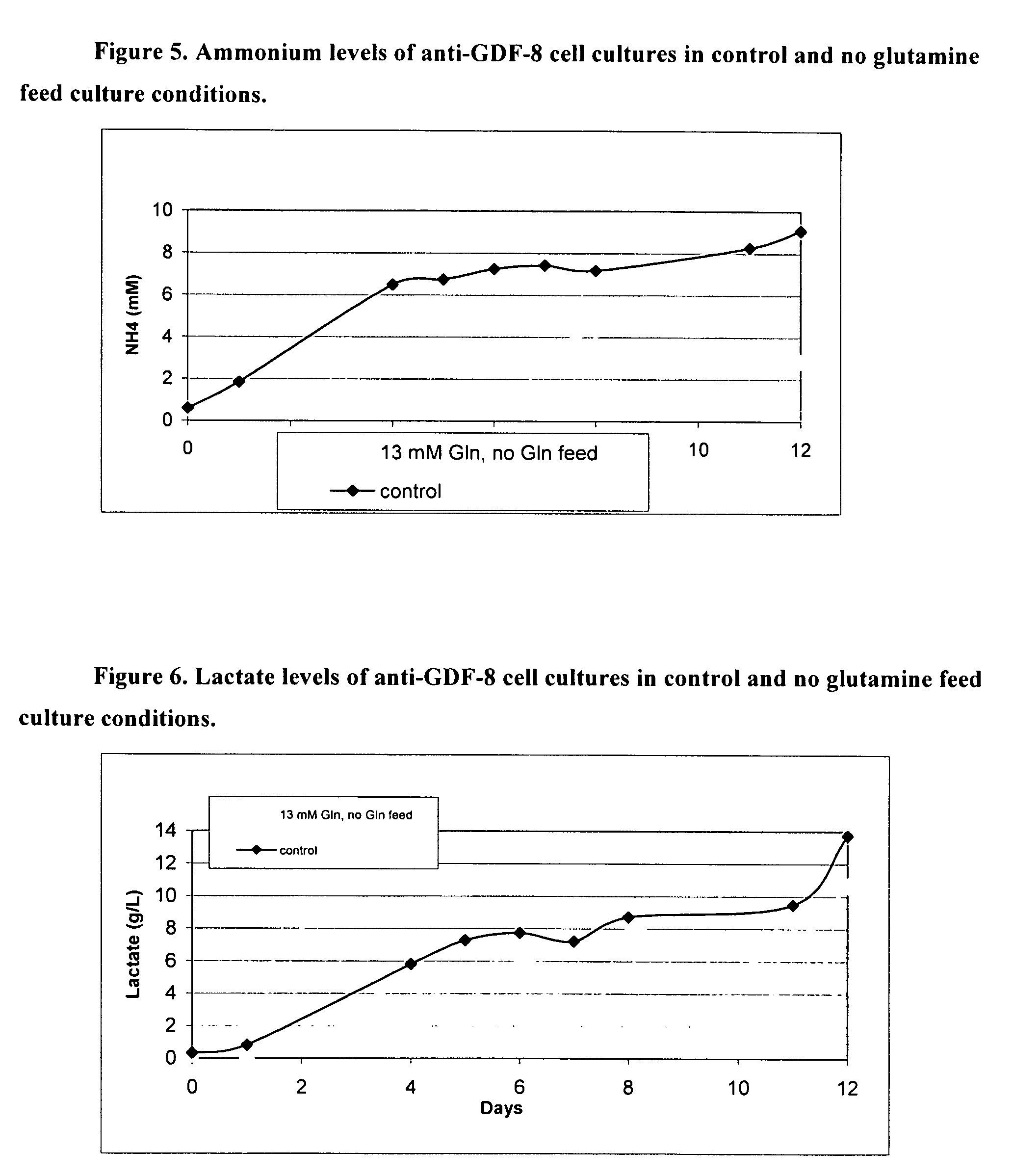
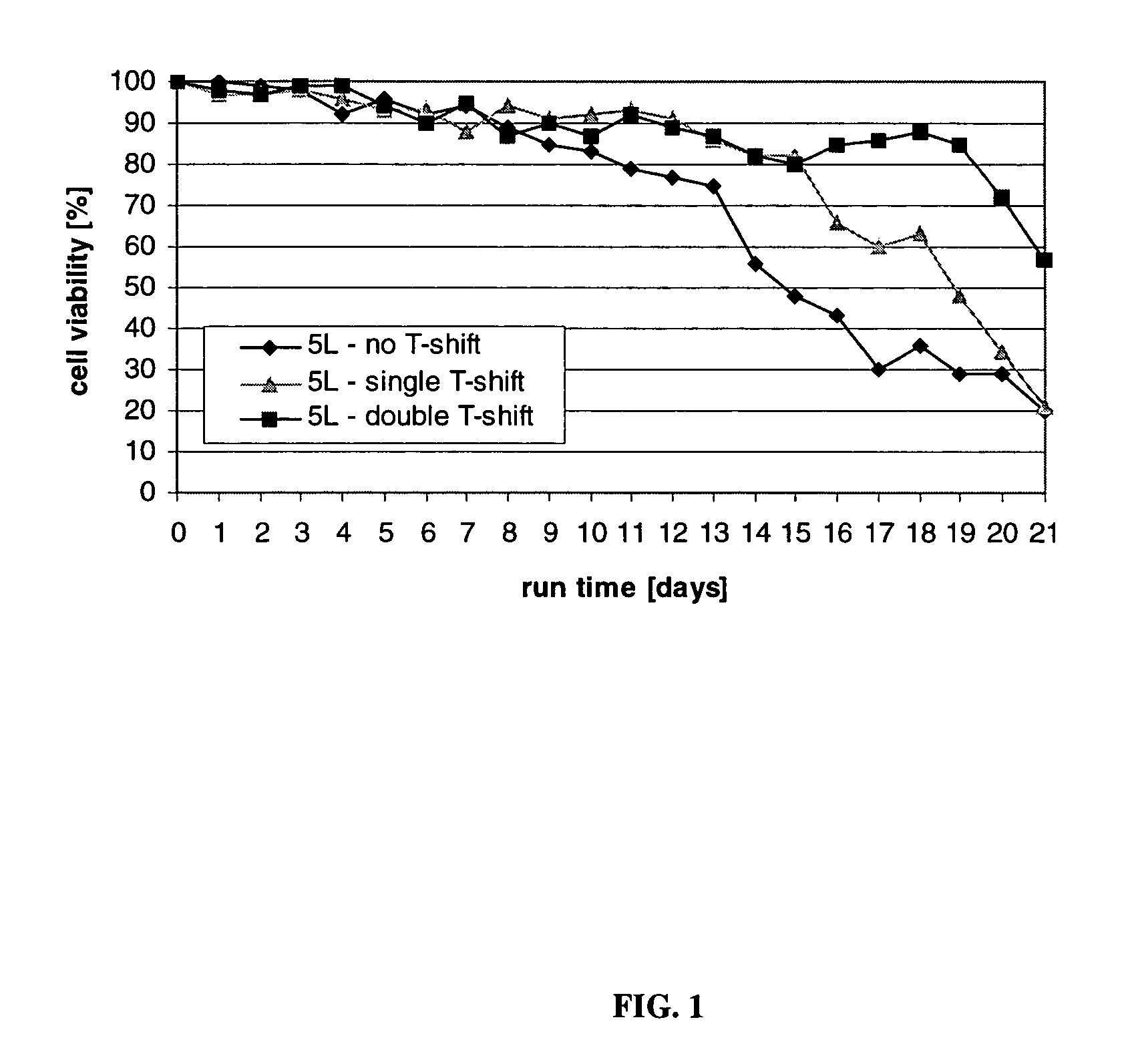
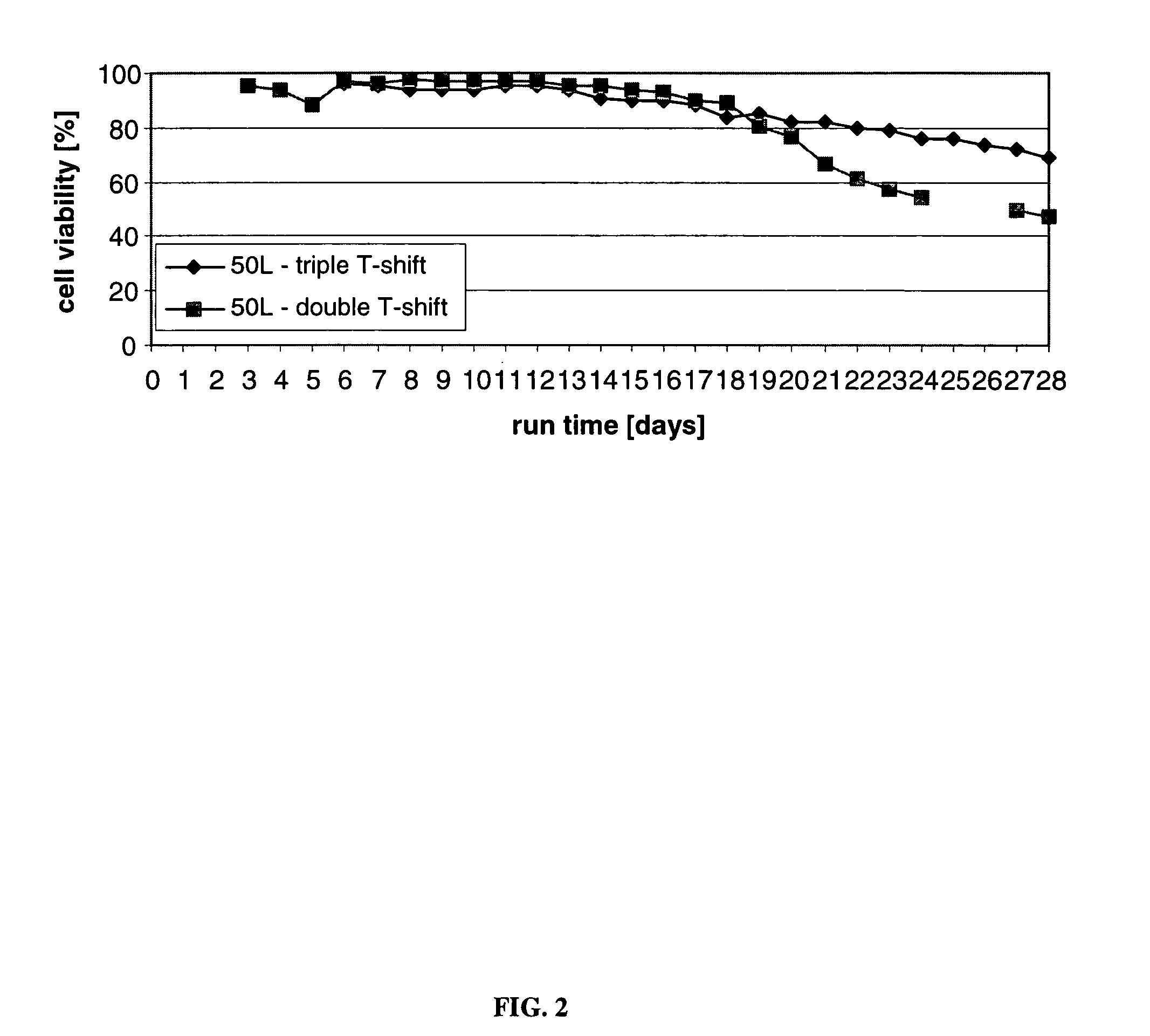
An improved system for large scale production of proteins and / or polypeptides in cell culture, particularly in media characterized by one or more of: i) a cumulative amino acid concentration greater than about 70 mM; ii) a molar cumulative glutamine to cumulative asparagine ratio of less than about 2; iii) a molar cumulative glutamine to cumulative total amino acid ratio of less than about 0.2; iv) a molar cumulative inorganic ion to cumulative total amino acid ratio between about 0.4 to 1; or v) a combined cumulative glutamine and cumulative asparagine concentration between about 16 and 36 mM, is provided. The use of such a system allows high levels of protein production and lessens accumulation of certain undesirable factors such as ammonium and / or lactate. Additionally, culture methods including a temperature shift, typically including a decrease in temperature when the culture has reached about 20-80% of it maximal cell density, are provided. Alternatively or additionally, the present invention provides methods such that, after reaching a peak, lactate and / or ammonium levels in the culture decrease over time.
Owner:PFIZER IRELAND PHARM CORP
Non-radical photochemical crosslinked hydrogel material preparation method, product and application
ActiveCN105131315APrecise and controllable time and spaceEasy to operateOrganic active ingredientsCosmetic preparationsPolymer scienceHydroxylamine
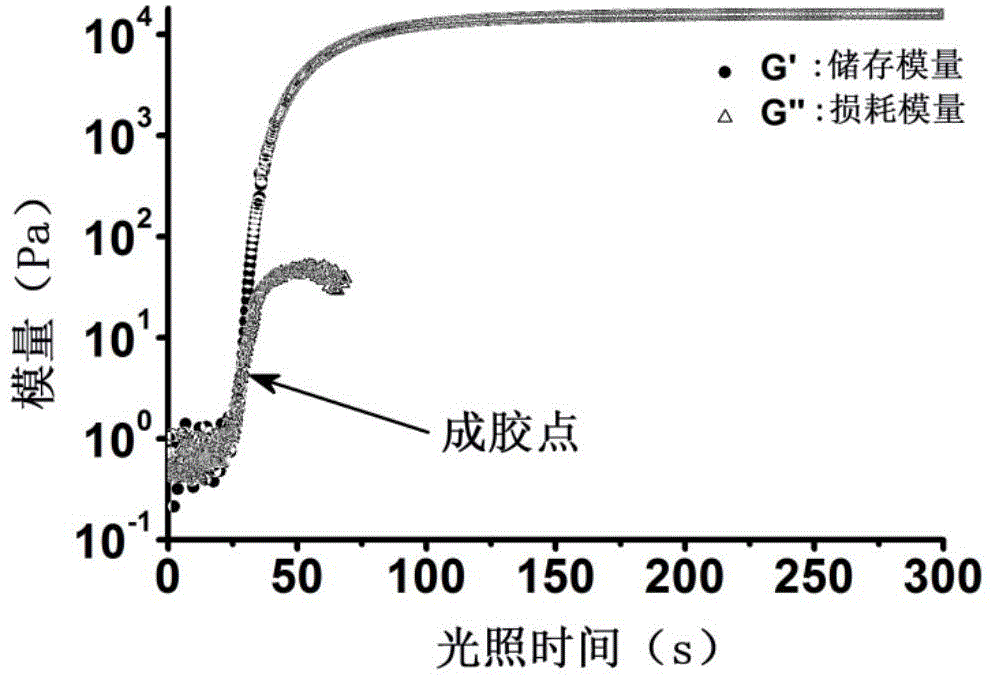
The present invention provides a non-radical photo-crosslinked hydrogel preparation method, comprising the following steps: a component A is dissolved in a biocompatible medium to obtain a solution A, component B-hydrazide, hydroxylamine or primary amine high molecular derivative is dissolved in a biocompatible medium to obtain a solution B; the solution A and the solution B are evenly mixed to obtain a hydrogel precursor solution; under illumination, aldehyde group produced by light excitation of o-nitrobenzyl in the component A of the hydrogel precursor solution is crosslinked with hydrazone, hydroxylamine or primary amine group in the component B in the form of respective formation of oxime and Schiff base to produce the hydrogel. The present invention also provides a kit for the hydrogel preparation, and application of the hydrogel in tissue repair, beauty and as a cell, protein or drug carrier. The tissue surface light-situ gel can be achieved by the hydrogel, in particular, wound surface in-situ thin glue formation can be achieved, and the hydrogel is especially suitable for clinical wound surface tissue repair and isolation.
Owner:上海戴云化工科技有限公司 +2
Production of glycoproteins using manganese
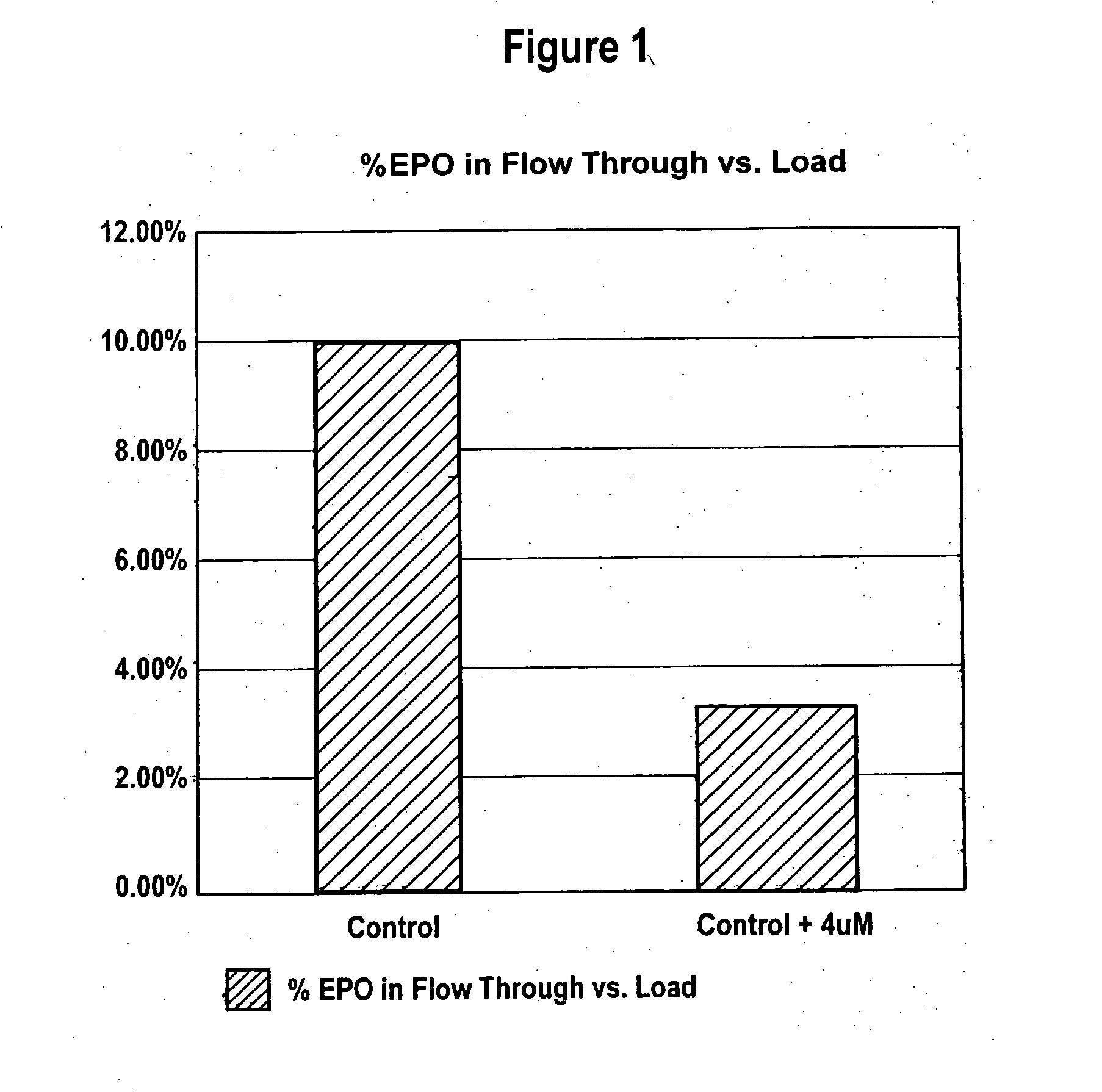
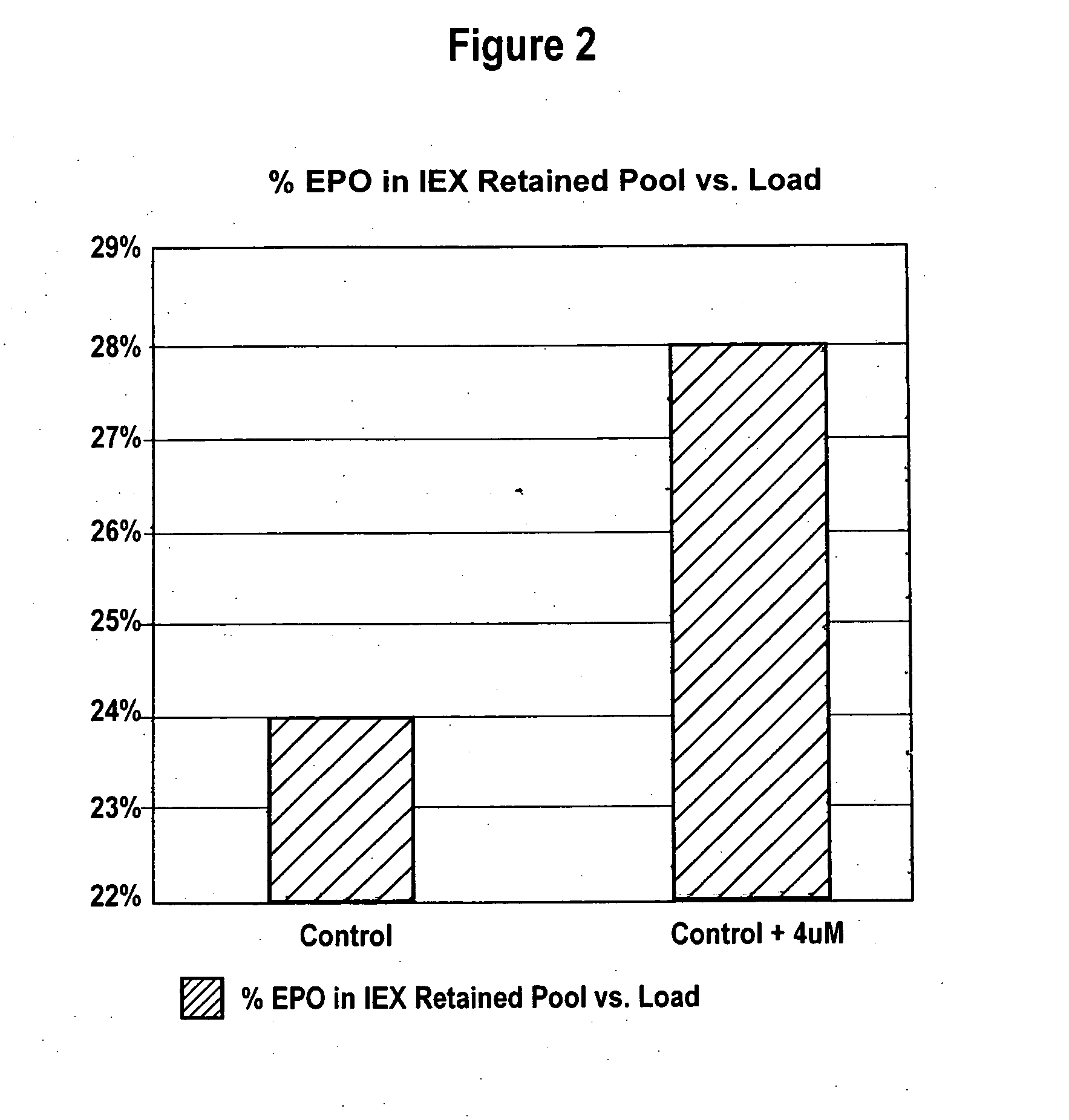
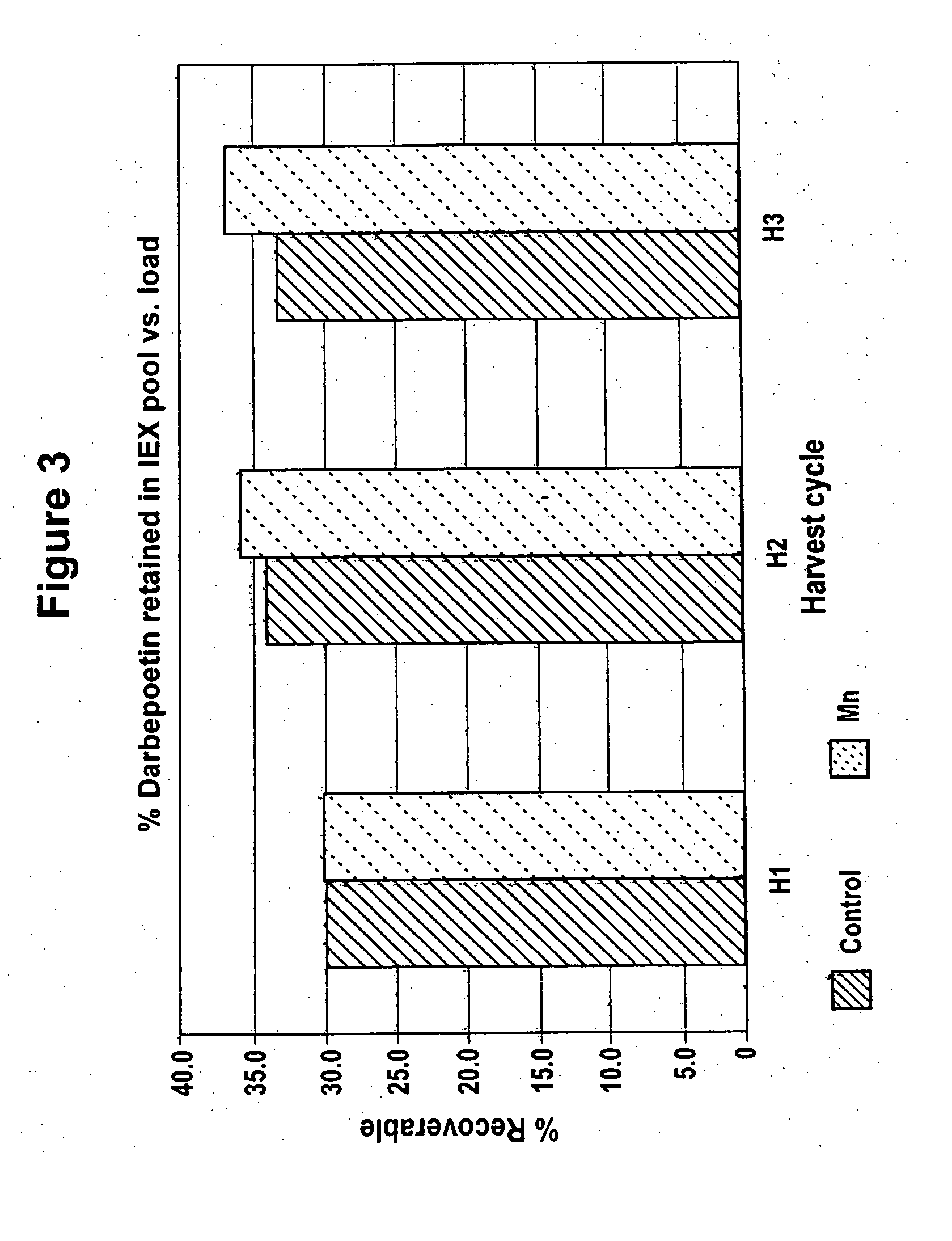
ActiveUS20070161084A1Increase sialylationProduced in advanceImmunoglobulinsFermentationSialic acid aldolaseBiotechnology
Culture media comprising manganese and methods of culturing cells to improve sialylation and glycosylation of glycoproteins are provided.
Owner:AMGEN INC
Preparation and use of plant embryo explants for transformation
ActiveUS20080280361A1Bioreactor/fermenter combinationsBiological substance pretreatmentsEmbryoBiology
The present invention relates to excision of explant material comprising meristematic tissue from seeds, and storage of such material prior to subsequent use in plant tissue culture and genetic transformation. Methods for tissue preparation, storage, and transformation are disclosed, as is transformable meristem tissue produced by such methods, and apparati for tissue preparation.
Owner:MONSANTO TECH LLC
Methods and products for transfection
The present invention relates in part to methods for producing tissue-specific cells from patient samples, and to tissue-specific cells produced using these methods. Methods for reprogramming cells using RNA are disclosed. Therapeutics comprising cells produced using these methods are also disclosed.
Owner:FACTOR BIOSCI
Production of polypeptides
ActiveUS20060121568A1Cell receptors/surface-antigens/surface-determinantsGenetically modified cellsTotal amino acidsInorganic ions
An improved system for large scale production of proteins and / or polypeptides in cell culture, particularly in media characterized by one or more of: i) a cumulative amino acid concentration greater than about 70 mM; ii) a molar cumulative glutamine to cumulative asparagine ratio of less than about 2; iii) a molar cumulative glutamine to cumulative total amino acid ratio of less than about 0.2; iv) a molar cumulative inorganic ion to cumulative total amino acid ratio between about 0.4 to 1; or v) a combined cumulative glutamine and cumulative asparagine concentration between about 16 and 36 mM, is provided. The use of such a system allows high levels of protein production and lessens accumulation of certain undesirable factors such as ammonium and / or lactate. Additionally, culture methods including a temperature shift, typically including a decrease in temperature when the culture has reached about 20-80% of it maximal cell density, are provided. Alternatively or additionally, the present invention provides methods such that, after reaching a peak, lactate and / or ammonium levels in the culture decrease over time.
Owner:PFIZER IRELAND PHARM CORP
Glycolysis-inhibiting substances in cell culture
ActiveUS8192951B2Reduce accumulationHigh levelGenetically modified cellsCulture processMetabolic wastePharmaceutical drug
An improved system for large scale production of proteins and / or polypeptides in cell culture is provided. In accordance with the present invention, cells expressing the protein or polypeptide of interest are grown in media that comprise a glycolysis-inhibiting substance. Additionally and / or alternatively, cells expressing the protein or polypeptide of interest are grown in media in which glutamine is limited. The use of such a system allows high levels of protein or polypeptide production and lessens accumulation of undesirable metabolic waste products such as lactate. Proteins and polypeptides expressed in accordance with the present invention may be advantageously used in the preparation of pharmaceutical, immunogenic, agricultural or other commercial compositions.
Owner:WYETH LLC
Methods for growing mammalian cells in vitro
InactiveUS7390660B2Increase cell densityEnhance cell viabilityCulture processCell culture mediaMammalGlucose polymers
Owner:F HOFFMANN LA ROCHE & CO AG
Media for culturing stem cells
Well-defined, xeno-free culture media which comprise a TGF-beta isoform or the chimera formed between IL6 and the soluble IL6 receptor (IL6RIL6), which are capable of maintaining stem cells, and particularly, human embryonic stem cells, in an undifferentiated state are provided. Also provided are cell cultures comprising the culture media and the stem cells and methods of expanding and deriving embryonic stem cells in such well-defined, xeno-free culture media. In addition, the present invention provides methods of differentiating ESCs or EBs formed therefrom for the generation of lineage specific cells.
Owner:TECHNION RES & DEV FOUND LTD
Culture media for stem cells
PendingUS20140243227A1Slow proliferationIncrease surface areaBioreactor/fermenter combinationsBiological substance pretreatmentsStem cell cultureBiology
Culture media and methods for expanding and differentiating populations of stem cells and for obtaining organoids. Expanded cell populations and organoids obtainable by methods of the invention and their use in drug screening, toxicity assays and regenerative medicine.
Owner:KONINK NEDERLANDSE AKADE VAN WETENSCHAPPEN
Polypeptide production in animal cell culture
InactiveUS20050272124A1Promote cell growthHigh protein yieldGenetically modified cellsCulture processBiotechnologyGrowth phase
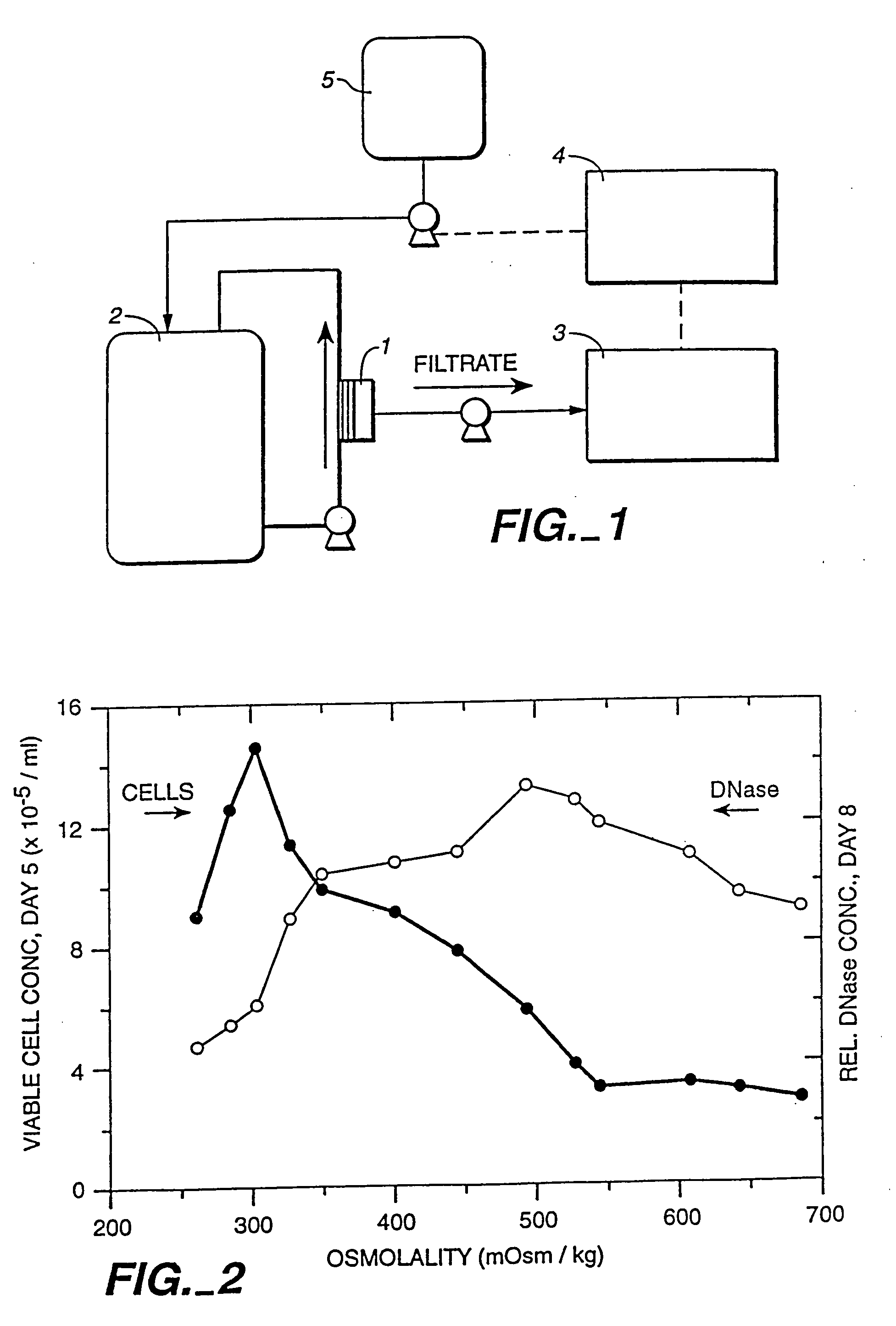
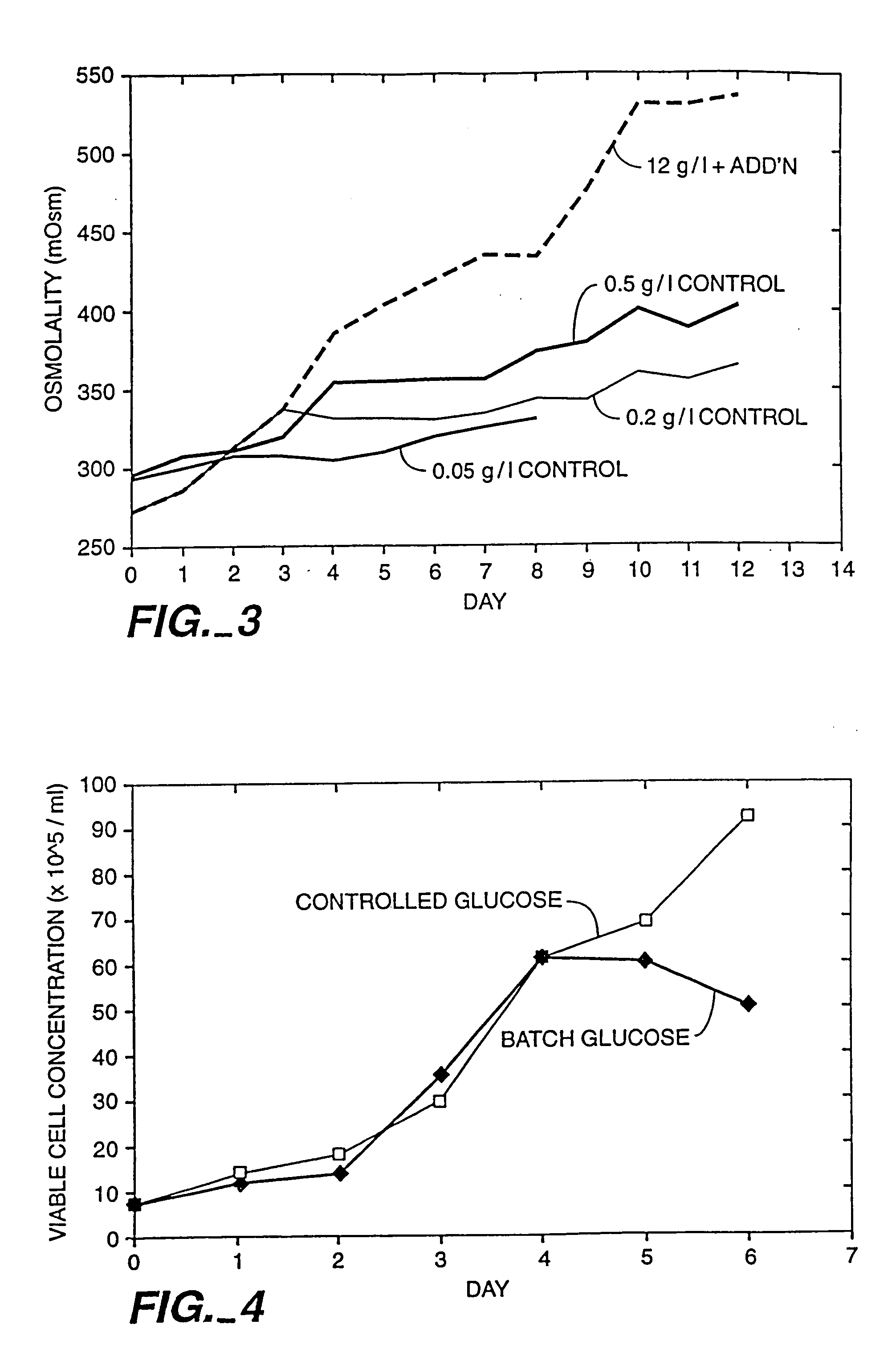
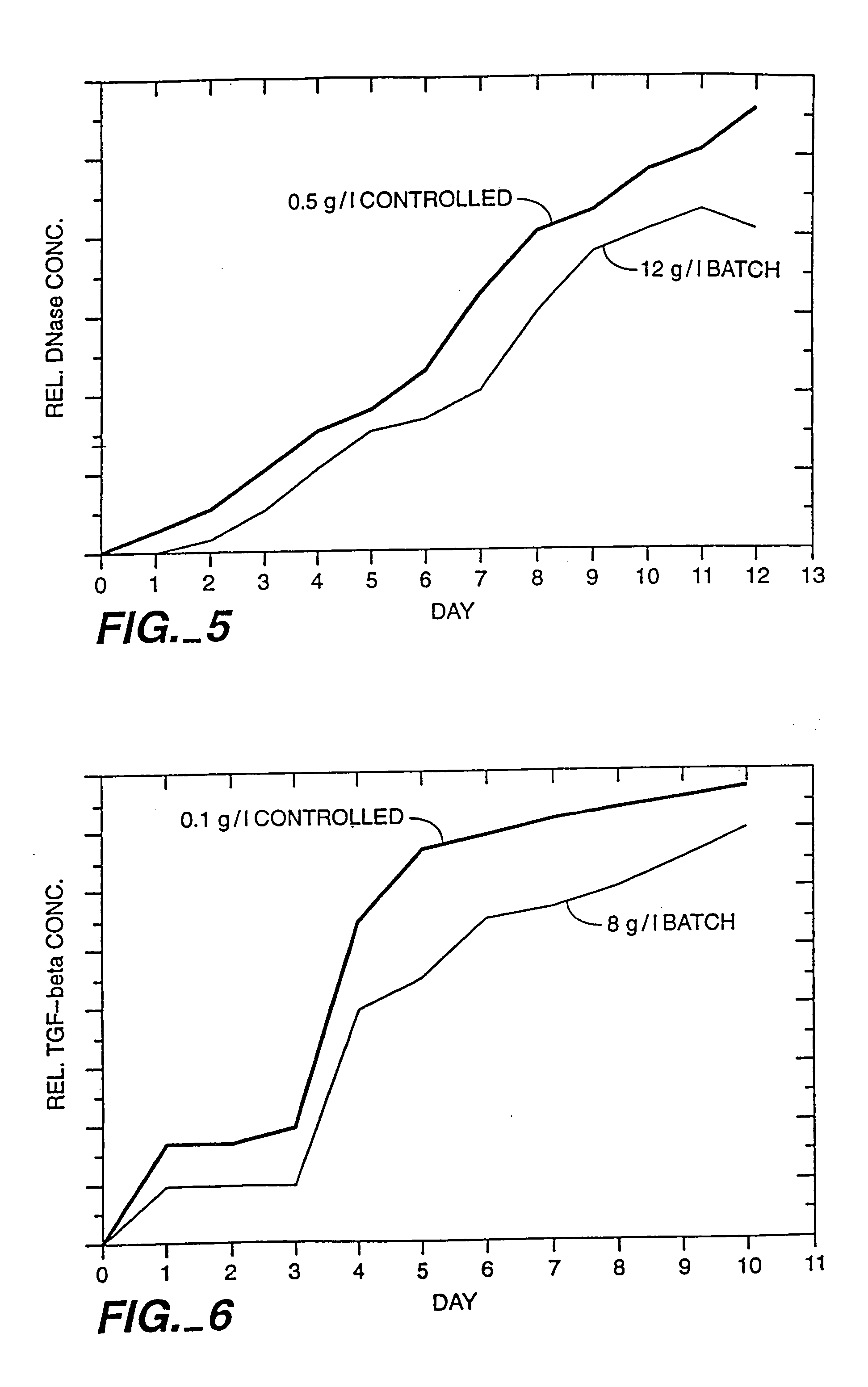
A method of producing a polypeptide in fed batch cell culture is provided which involves an initial cell growth phase and a distinct production phase. In the initial growth stage, animal cells having nucleic acid encoding the polypeptide are cultured at a starting osmolality of about 280-330 mOsm in the presence of a concentration of glucose controlled throughout the culturing to be within a range between about 0.01 and 1 g / L. This is followed by a production phase, where the cultured animal cells of the growth phase are inoculated at a cell seed density of at least 1.0×106 cells / mL and the cells are cultured at a starting osmolarity of about 400-600 mOsm in the presence of a concentration of glucose controlled throughout the culturing to be within a range between about 0.01 and 1 g / L. Preferably, the glutamine concentration in the cell culture medium is simultaneously controlled in order to curtail production of lactic acid and ammonia which result from unnecessarily high glutamine concentrations. During the growth phase, production of potentially detrimental metabolic waste products, such as lactic acid, is controlled thereby curtailing the increase of osmolality due to accumulation and neutralization of waste products. Thus, the cell growth can be improved. In the production phase, the cell culture conditions are modified in order to arrest or reduce cell growth and thereby direct nutrient utilization toward production, as opposed to cell growth. Overall, it is intended that the method results in an improvement in specific productivity, reduction in production run times and / or an increase in final product concentration.
Owner:GENENTECH INC
Methods for increasing polypeptide production
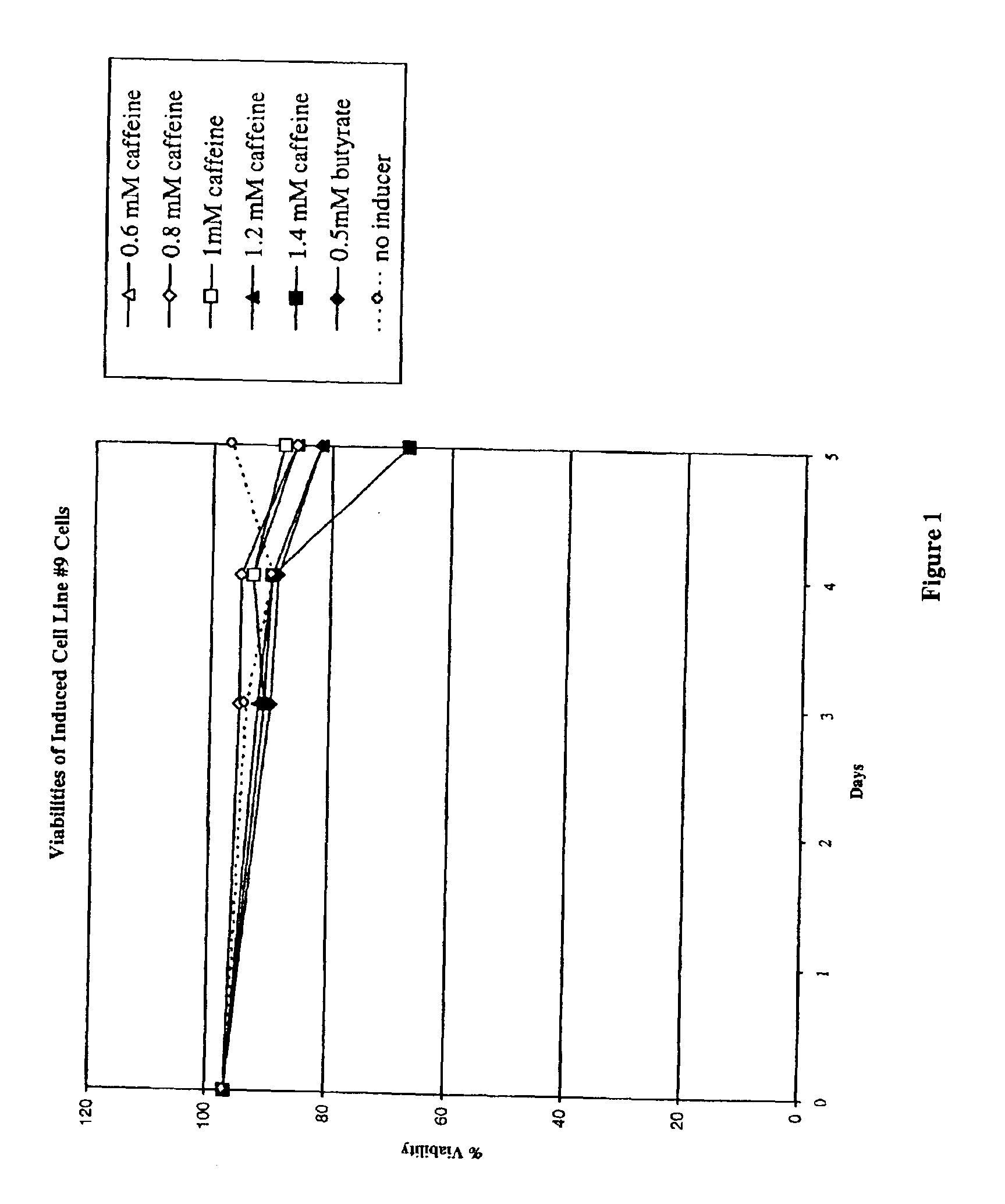
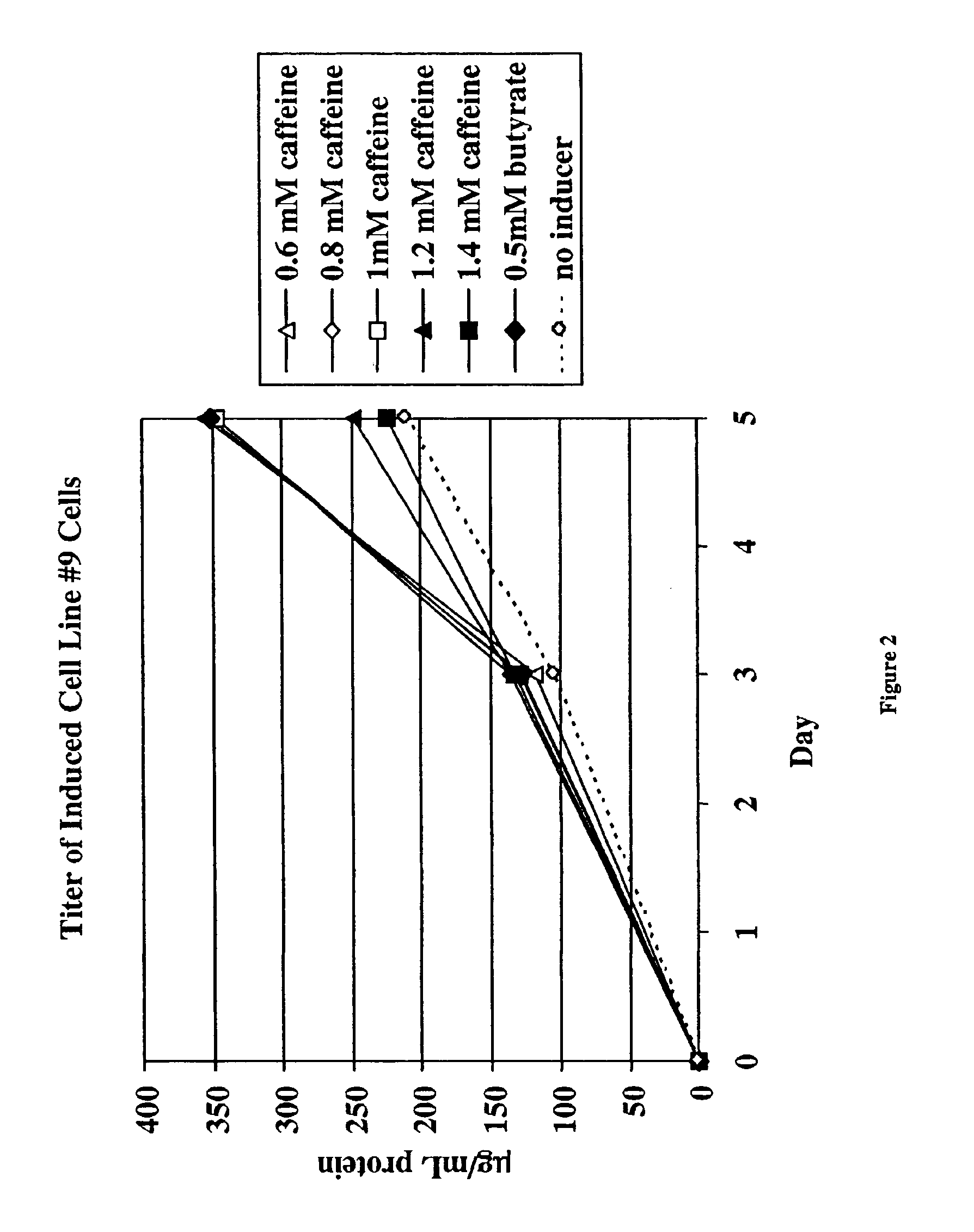
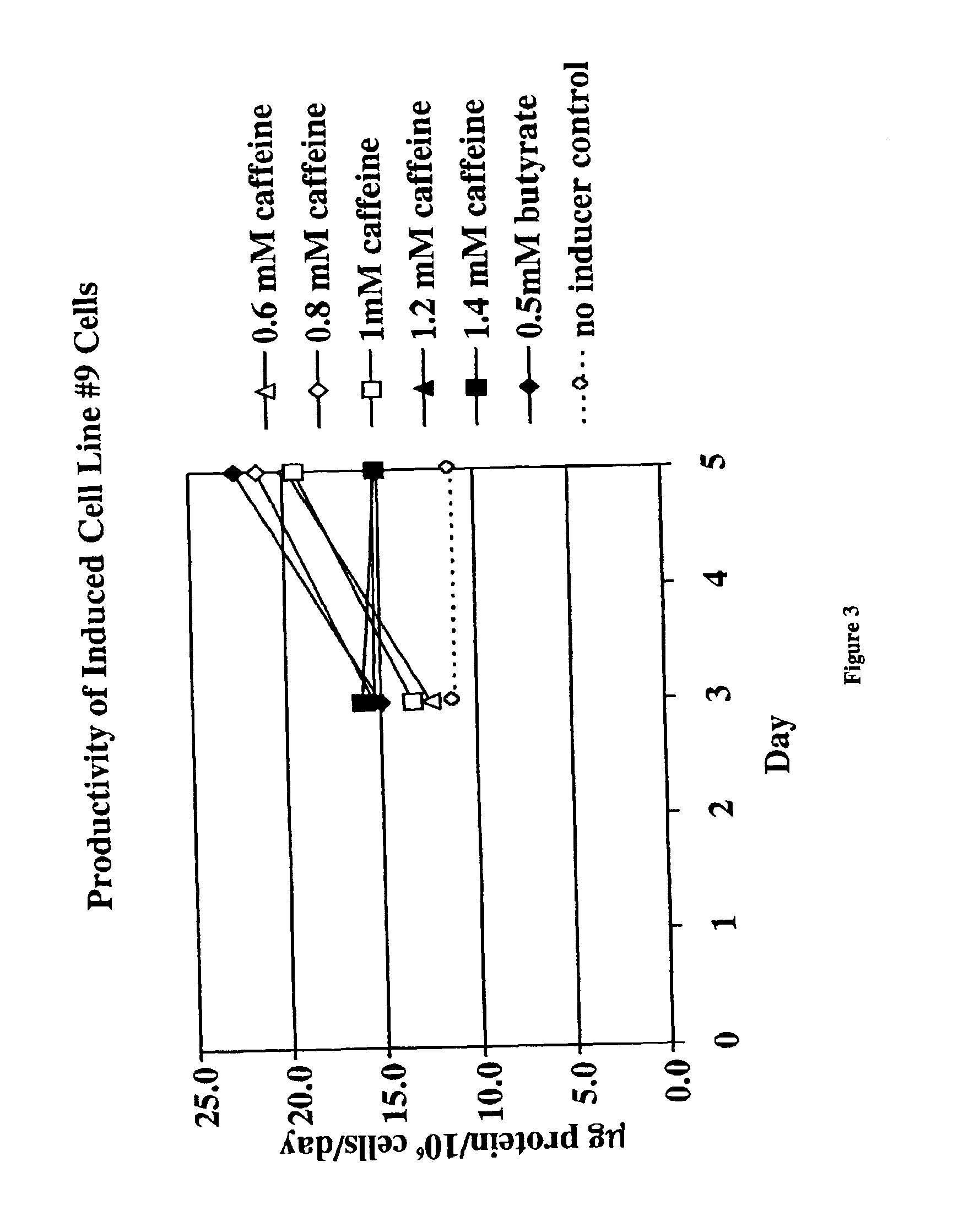
InactiveUS6872549B2Increase polypeptide expressionInduce productionAntibody mimetics/scaffoldsImmunoglobulinsXanthineInducer
The invention provides methods of increasing the production of polypeptides, optionally recombinant polypeptides, from mammalian cells using xanthine derivatives or hybrid polar compounds and cultures containing the same. Combinations of inducers including a hybrid polar compound and / or a xanthine derivative and / or an alkanoic acid can also be used, optionally at temperatures less than 37° C.
Owner:IMMUNEX CORP
Mammalian cell culture processes for protein production
ActiveUS7541164B2Enhance cell viabilityPeptide/protein ingredientsImmunoglobulinsGrowth phaseTwo temperature
Owner:BRISTOL MYERS SQUIBB CO
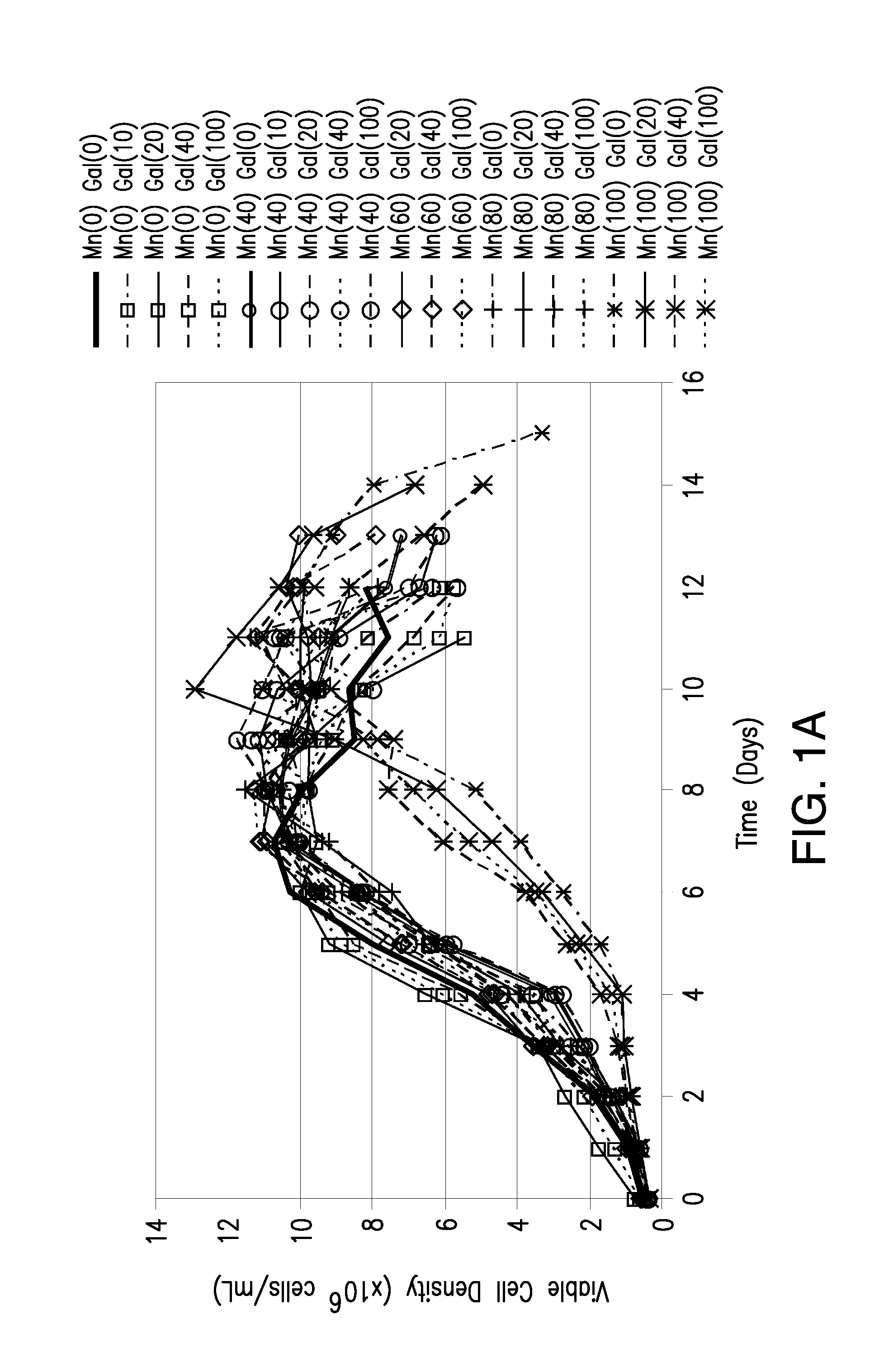
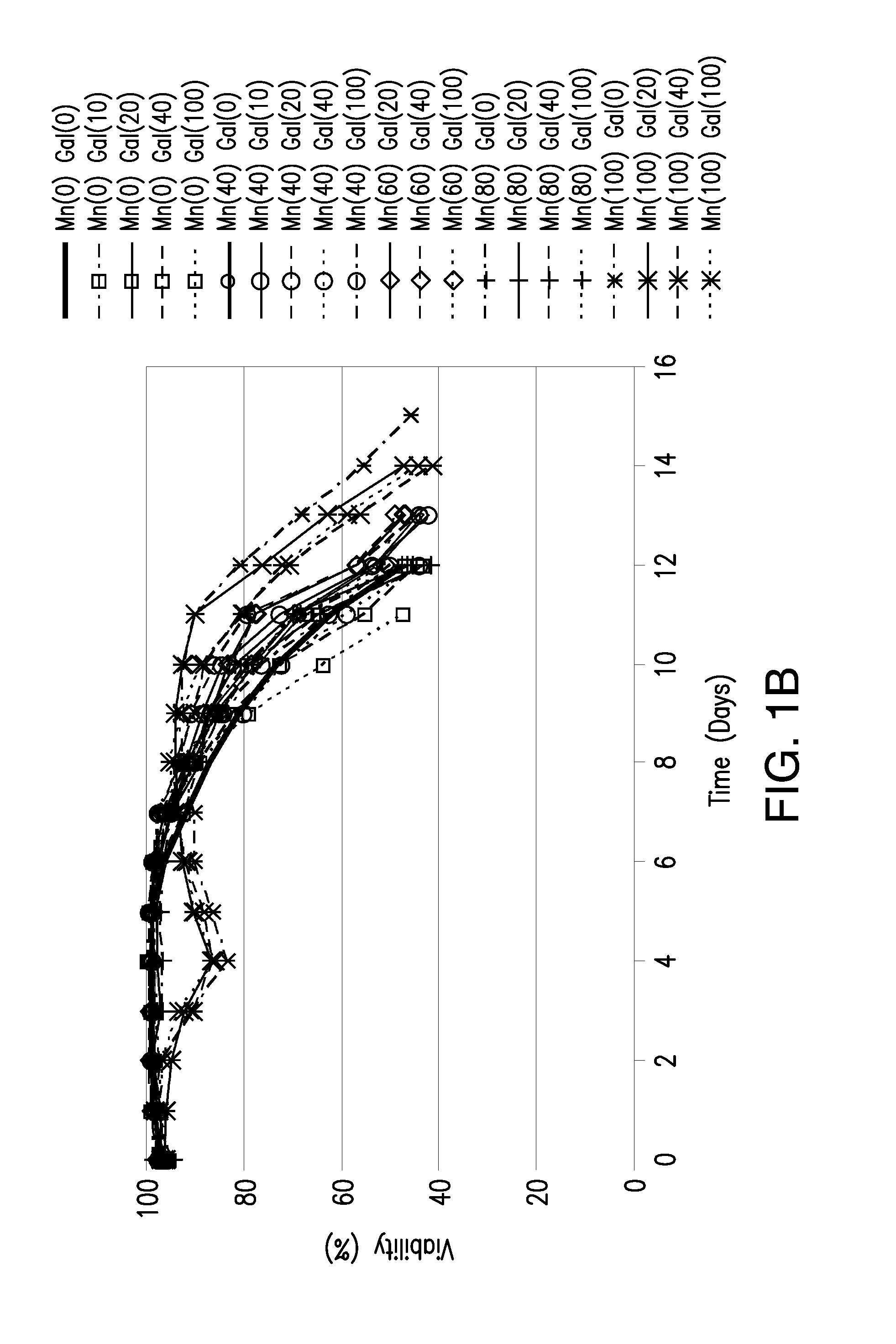
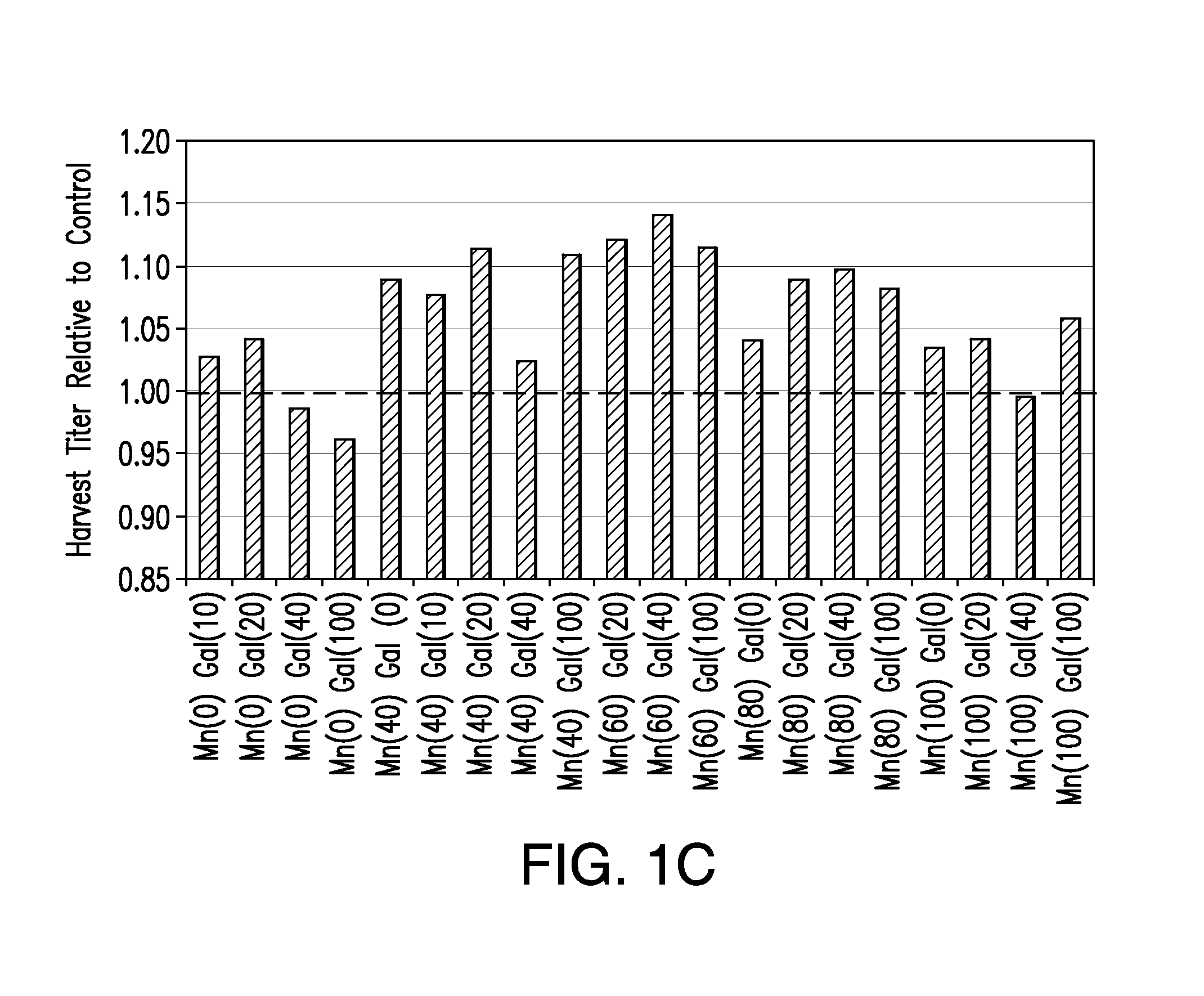
Methods for controlling the galactosylation profile of recombinantly-expressed proteins
The present invention relates to methods for modulating the glycosylation profile of recombinantly-expressed proteins. In particular, the present invention relates to methods of controlling the galactosylation profile of recombinantly-expressed proteins by supplementing production medium, e.g., a hydrolysate-based or a chemically defined medium, with manganese and / or D-galactose.
Owner:ABBVIE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com