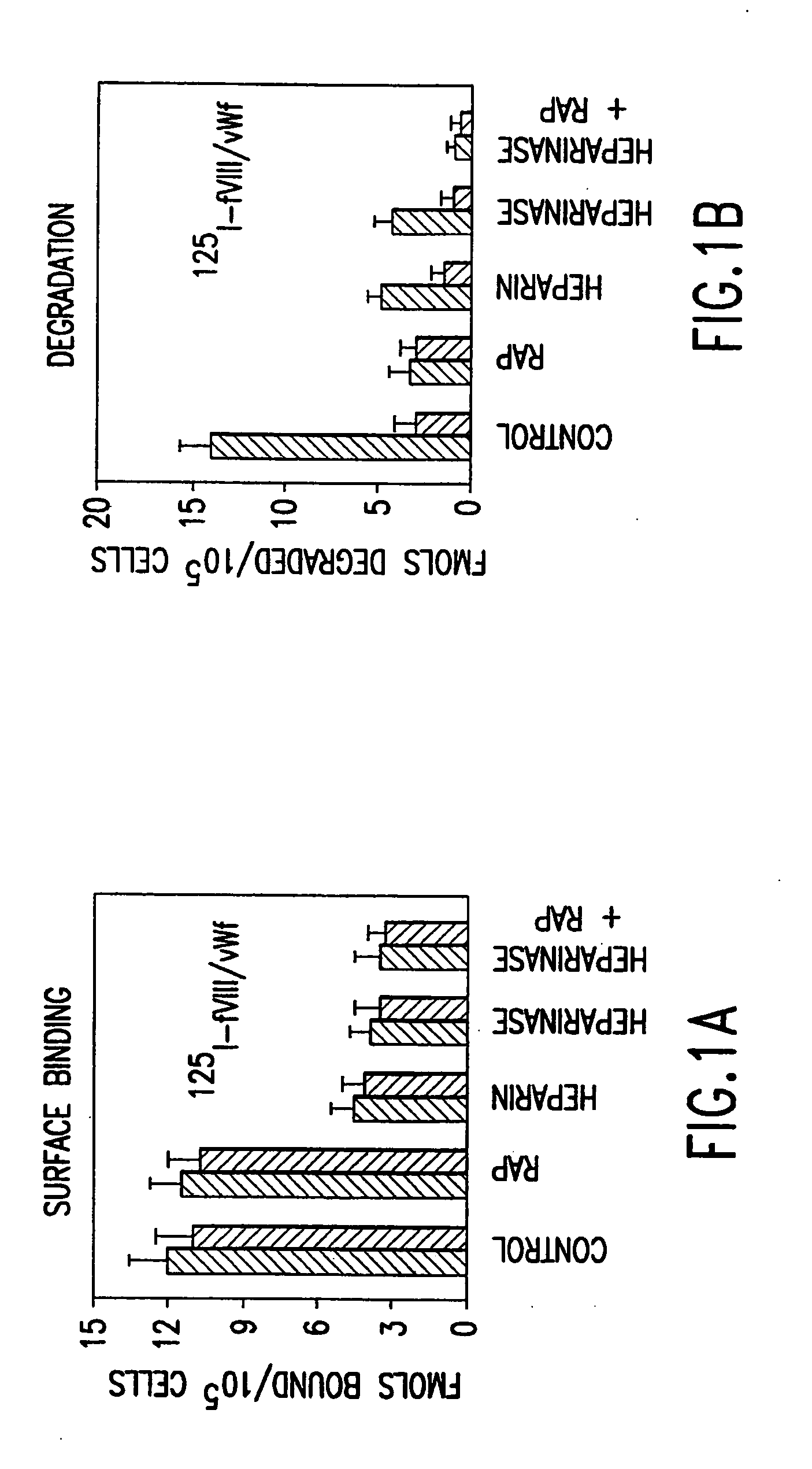
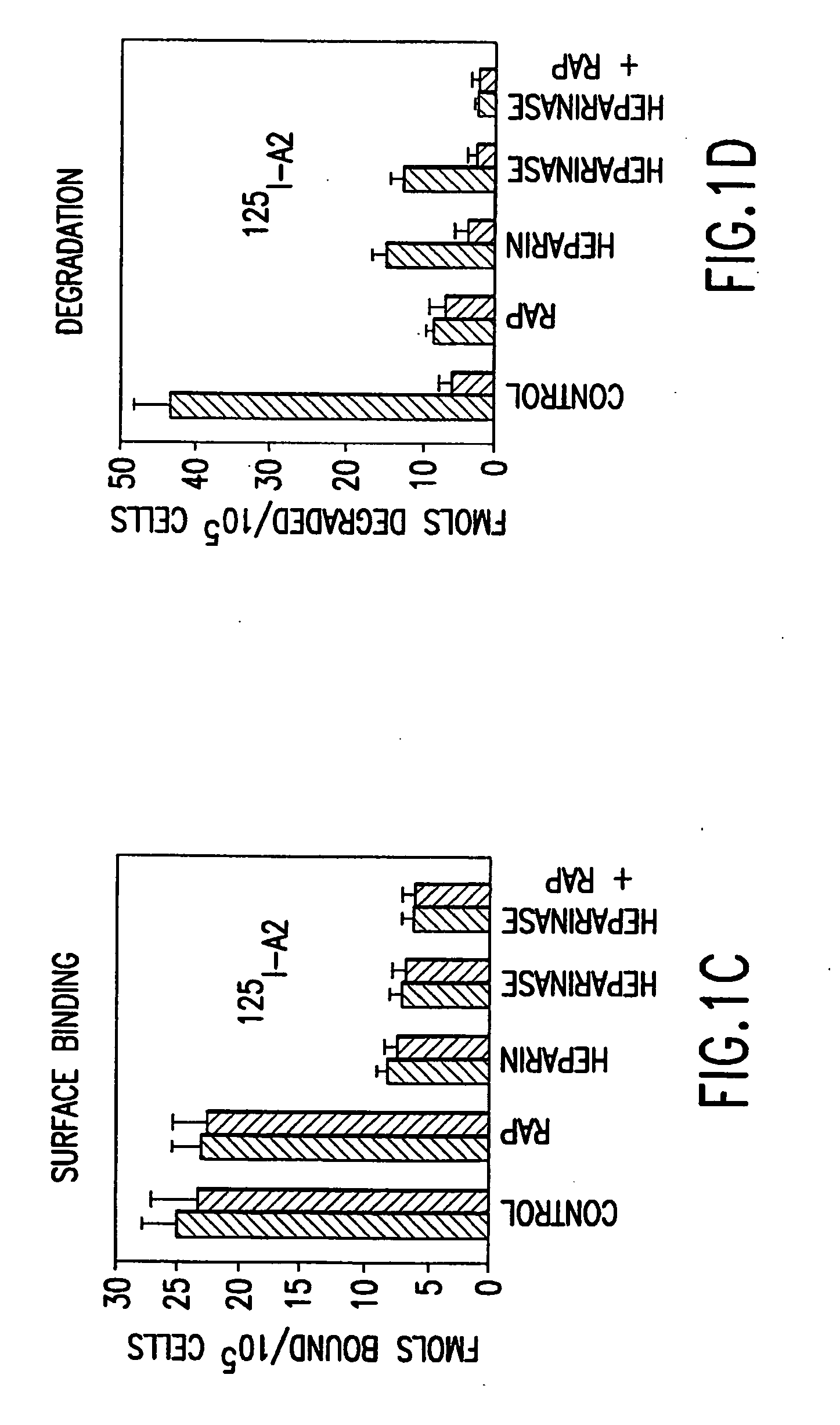
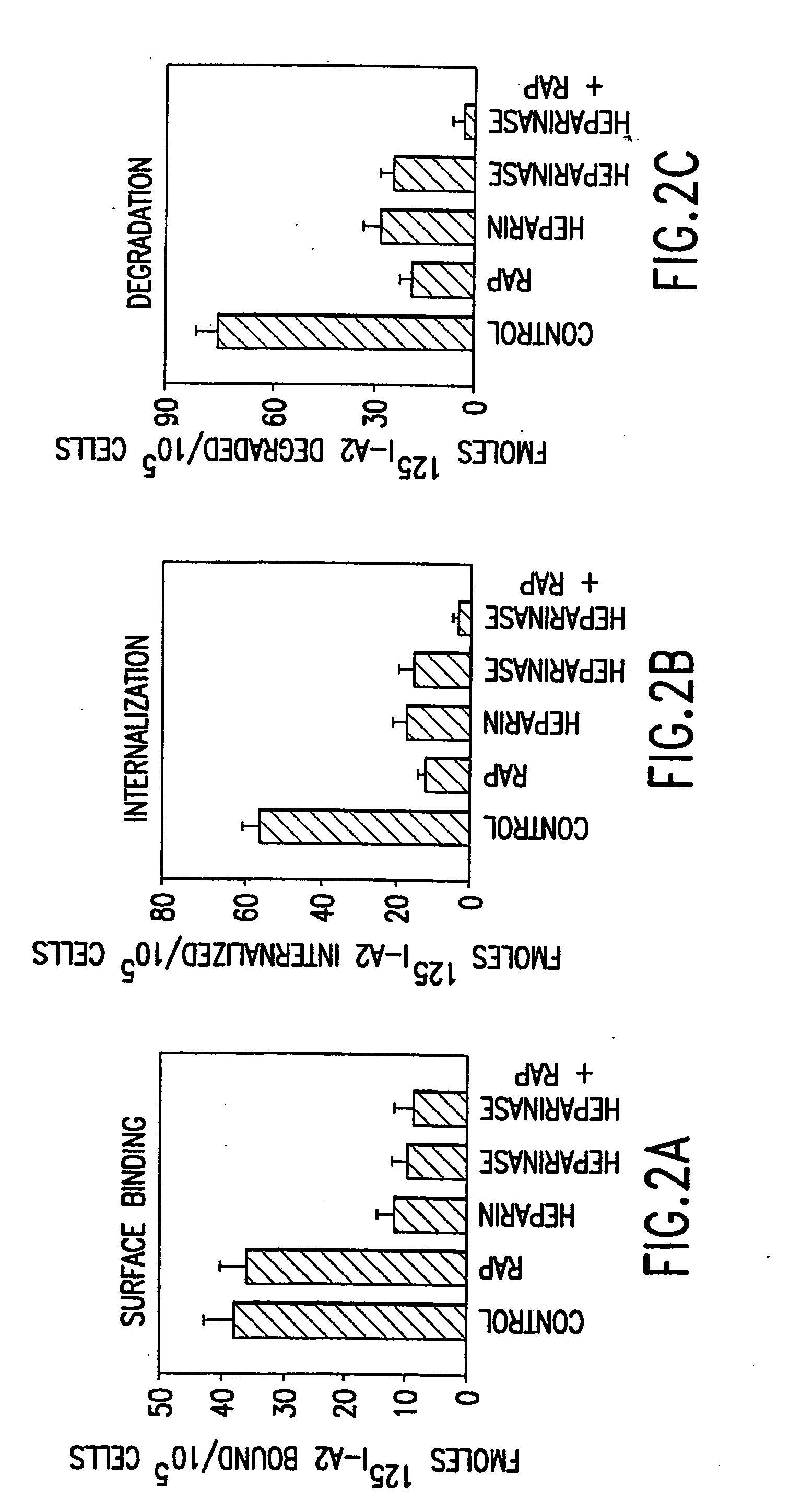
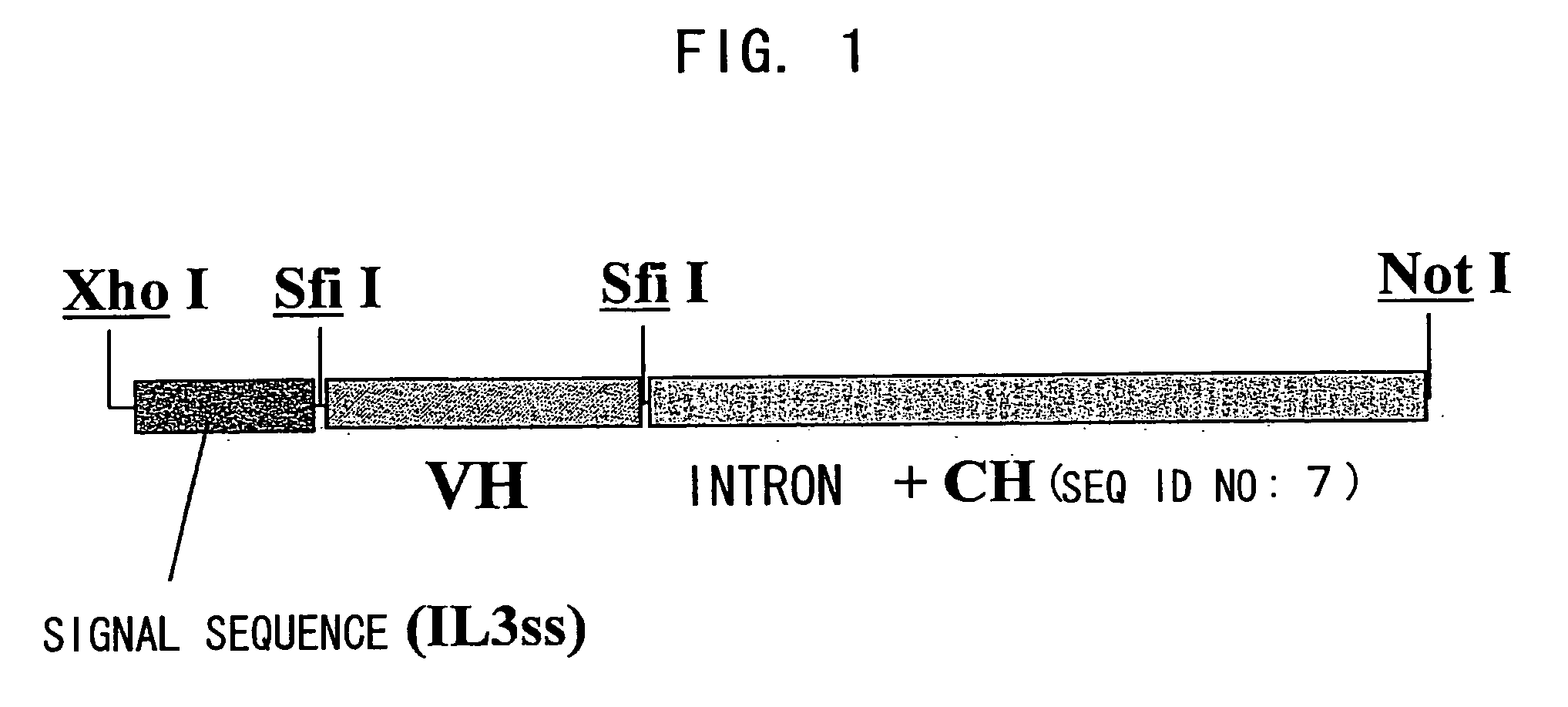
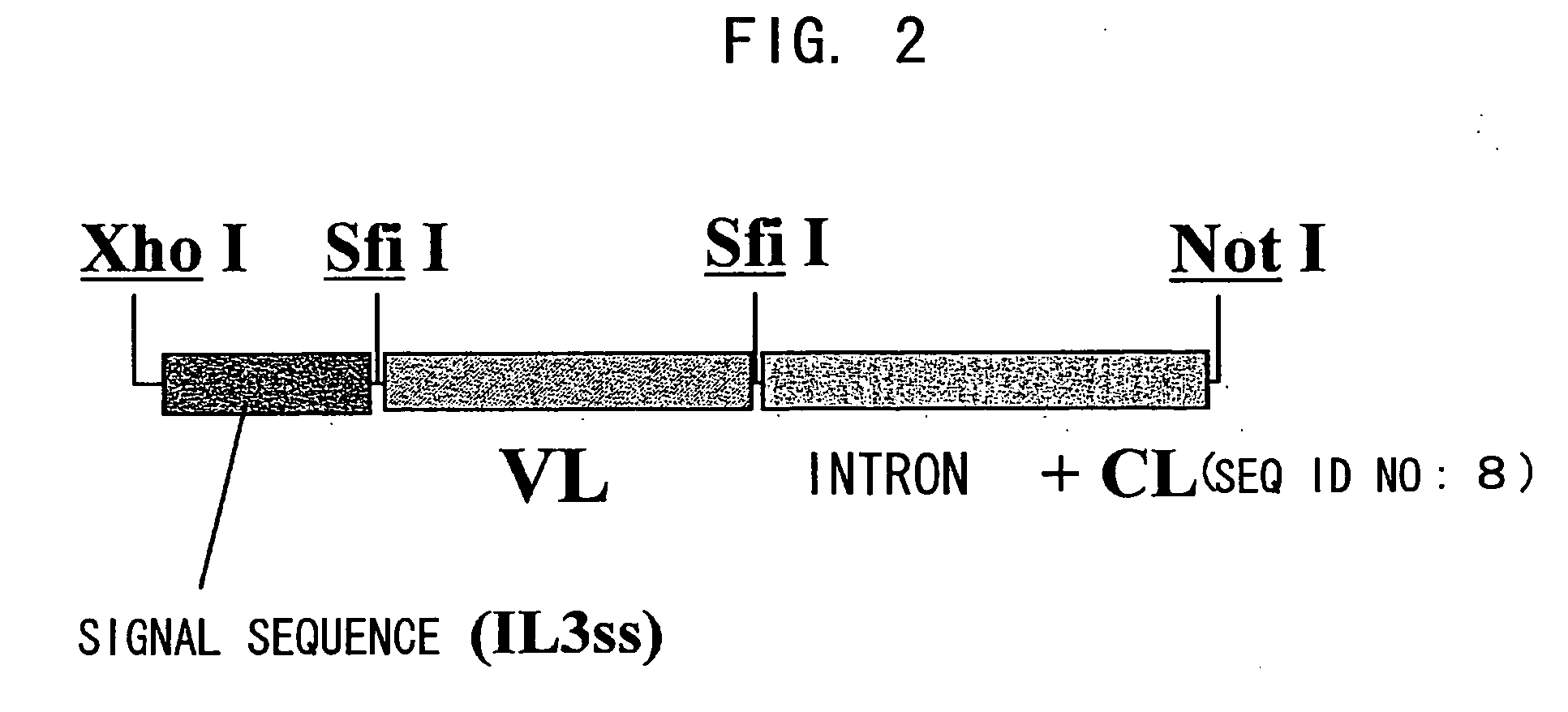
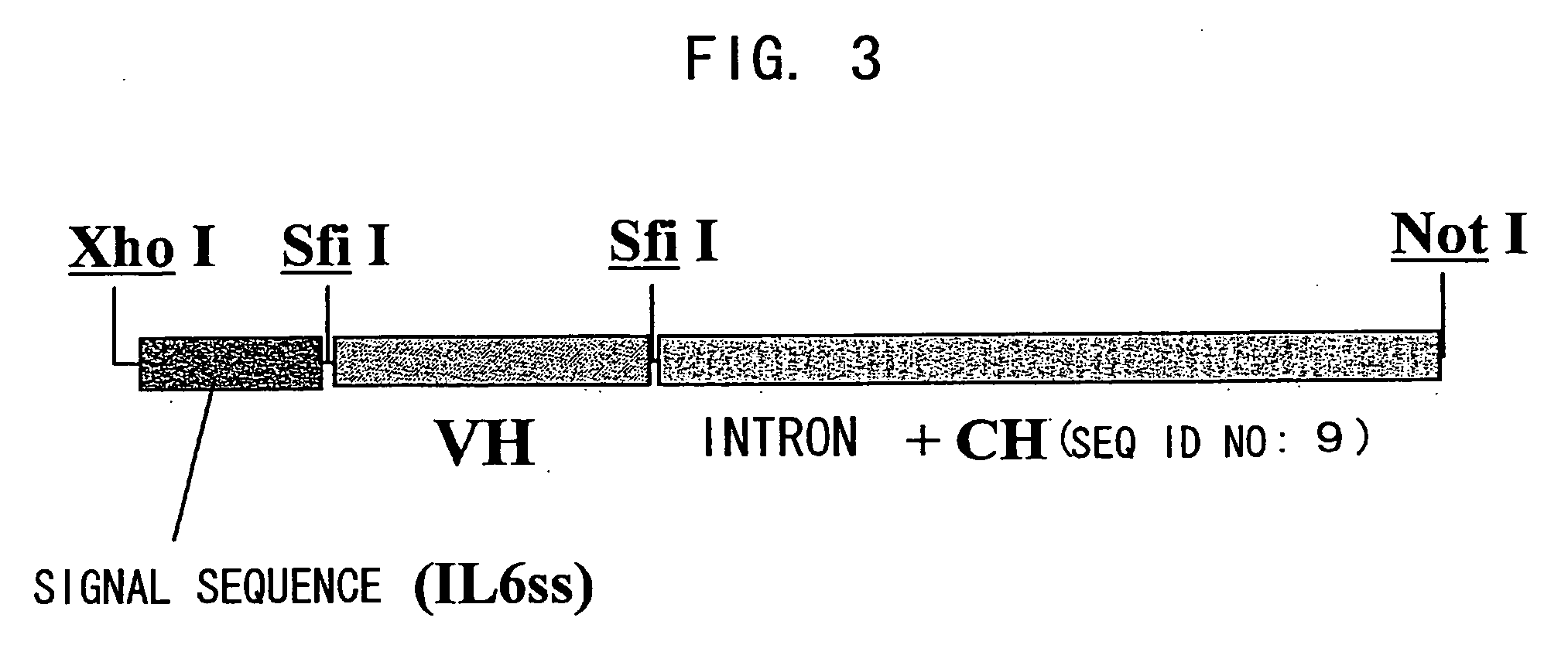
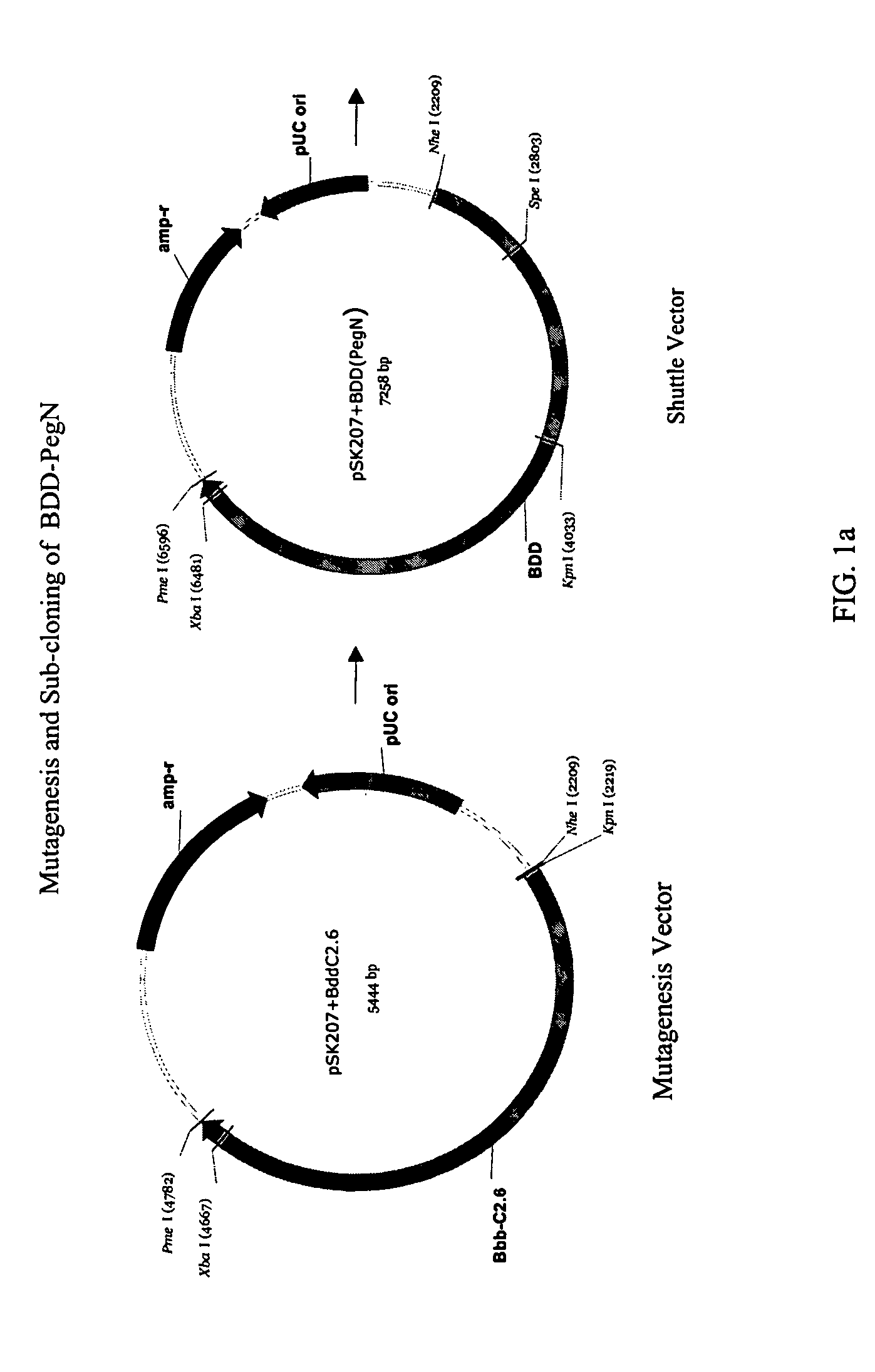
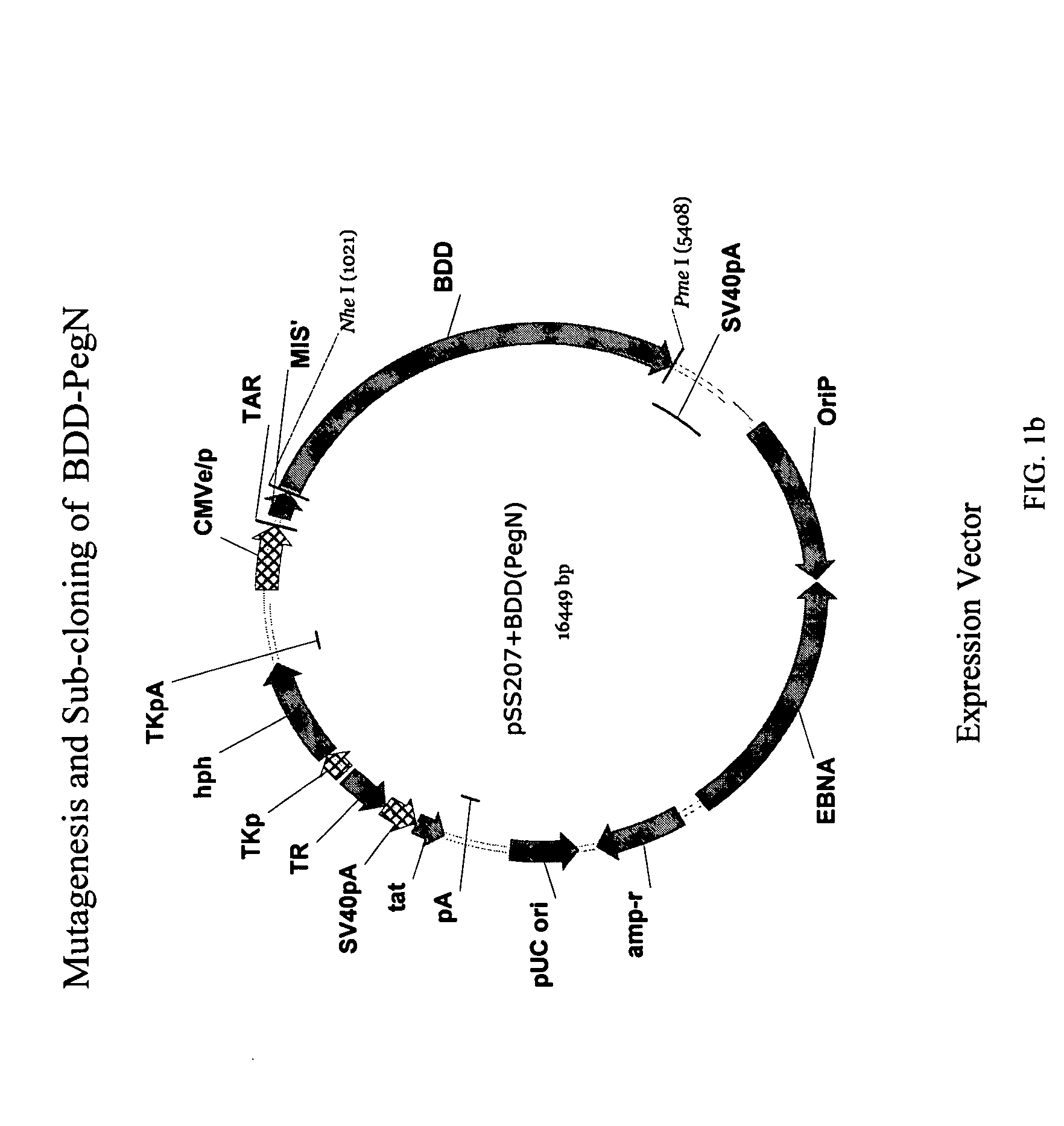
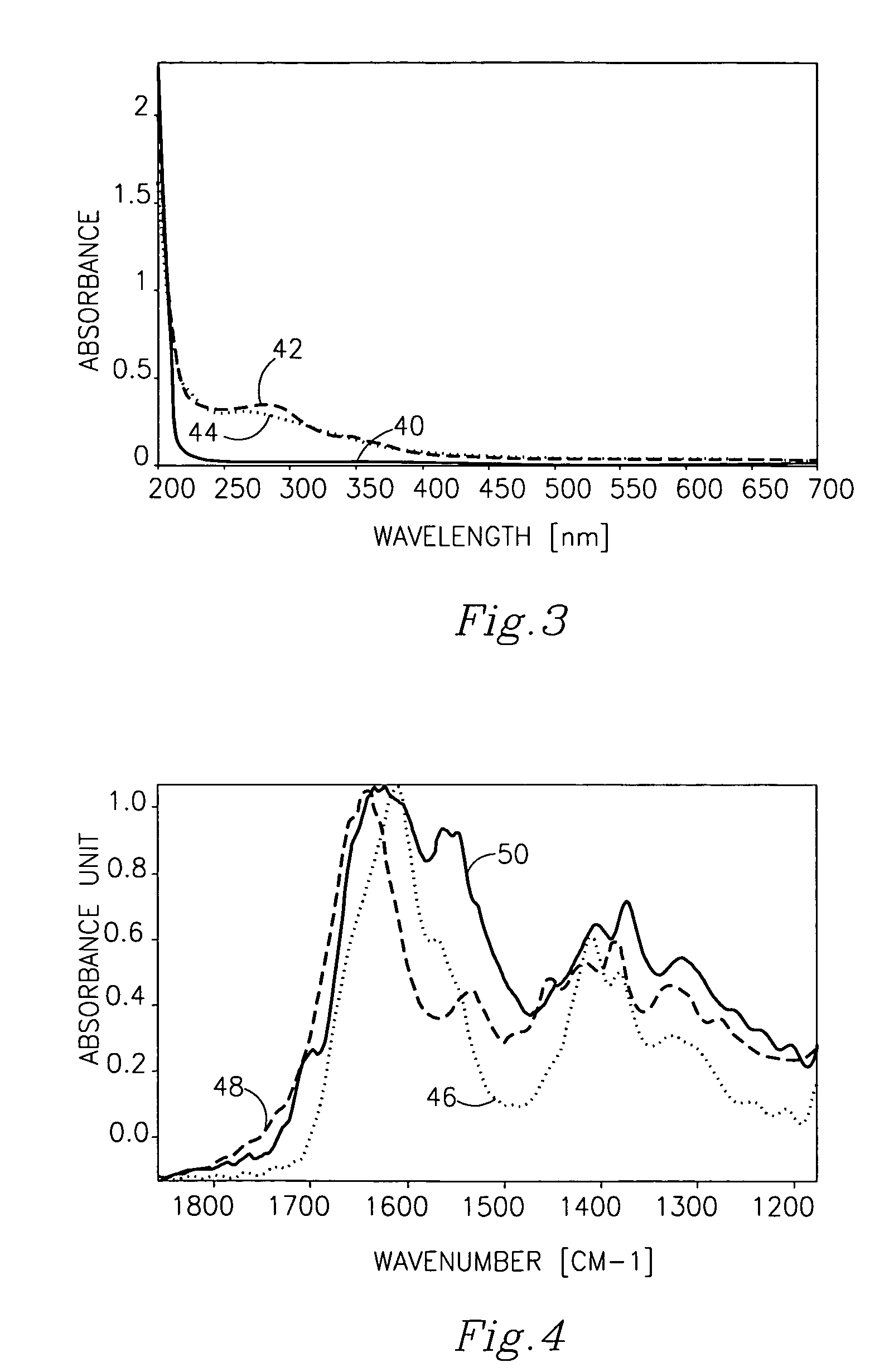
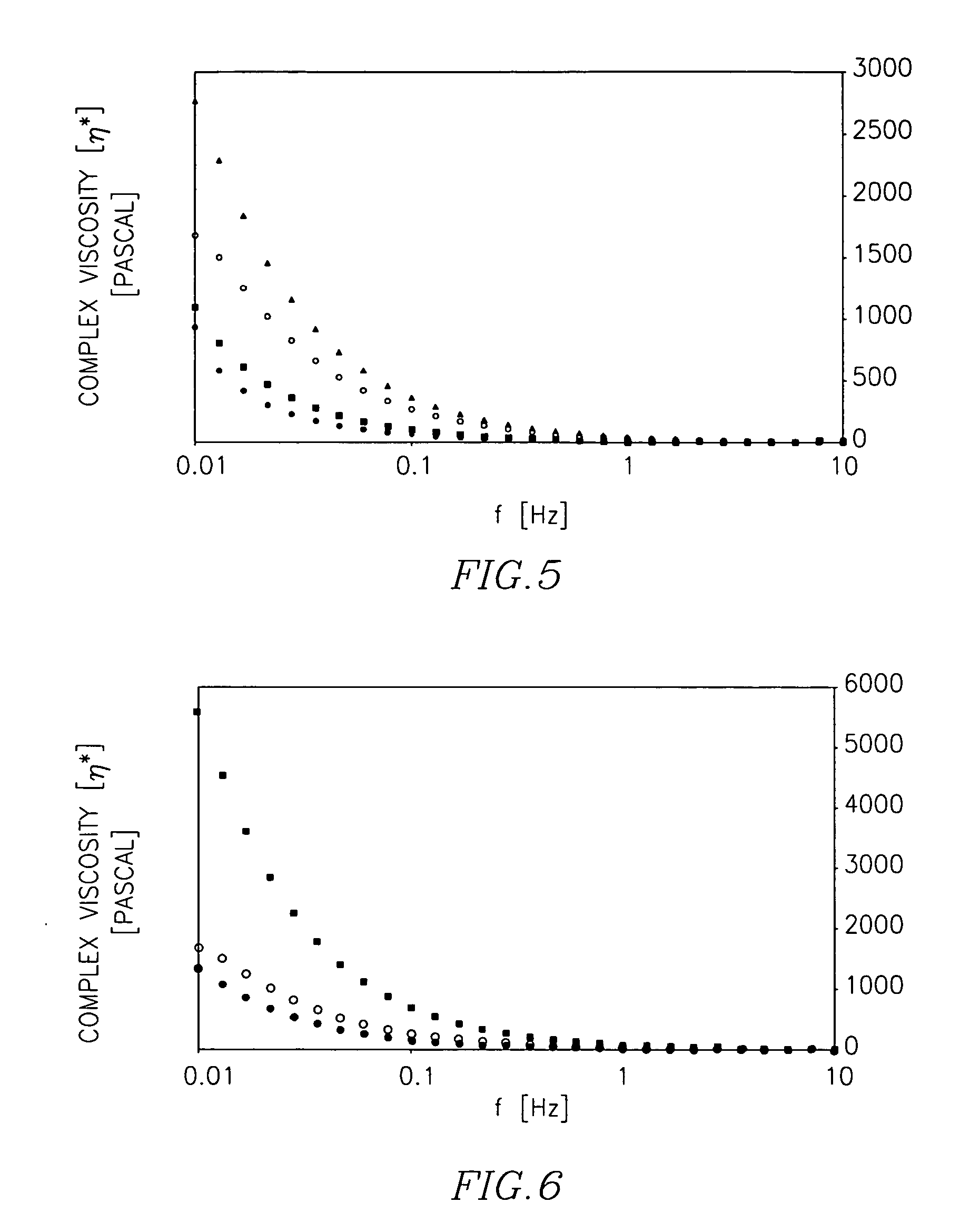
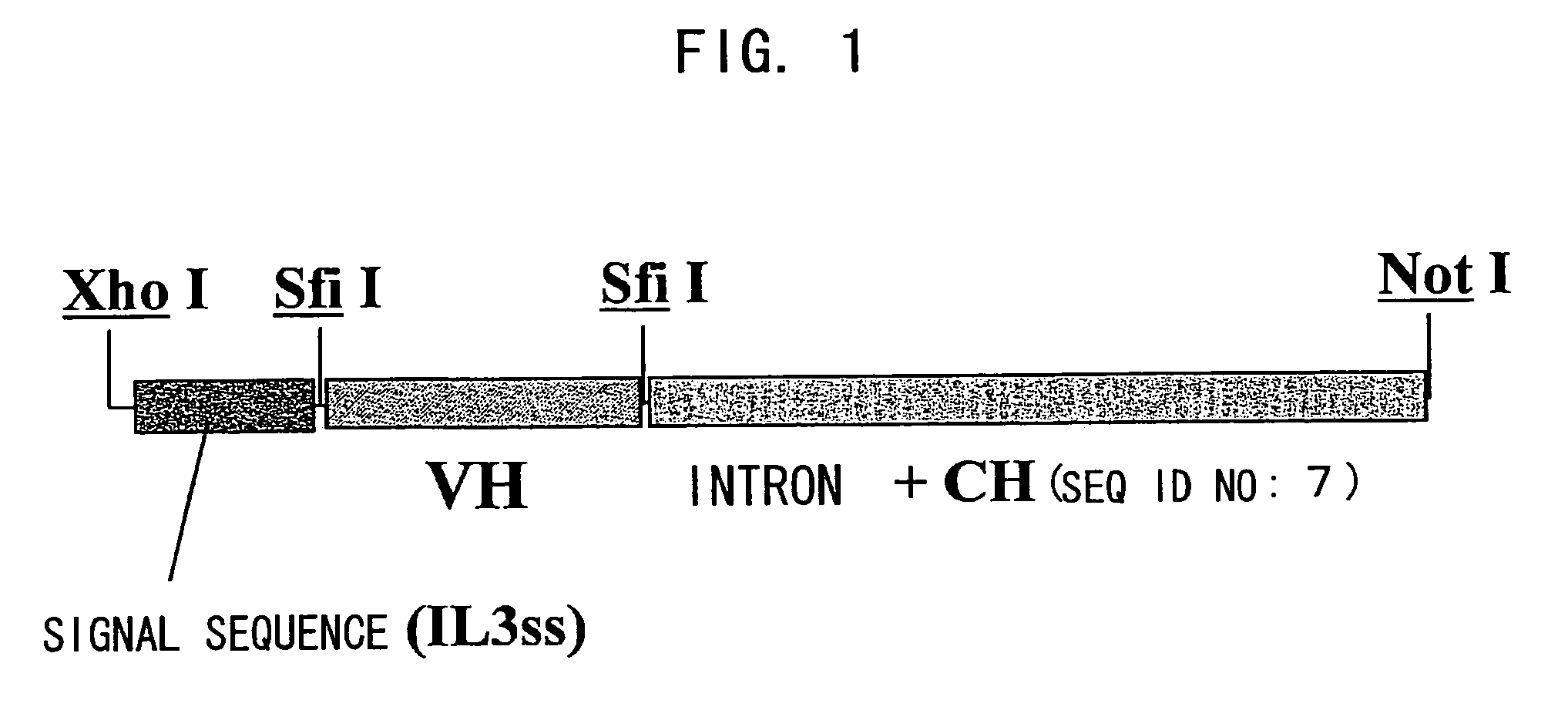
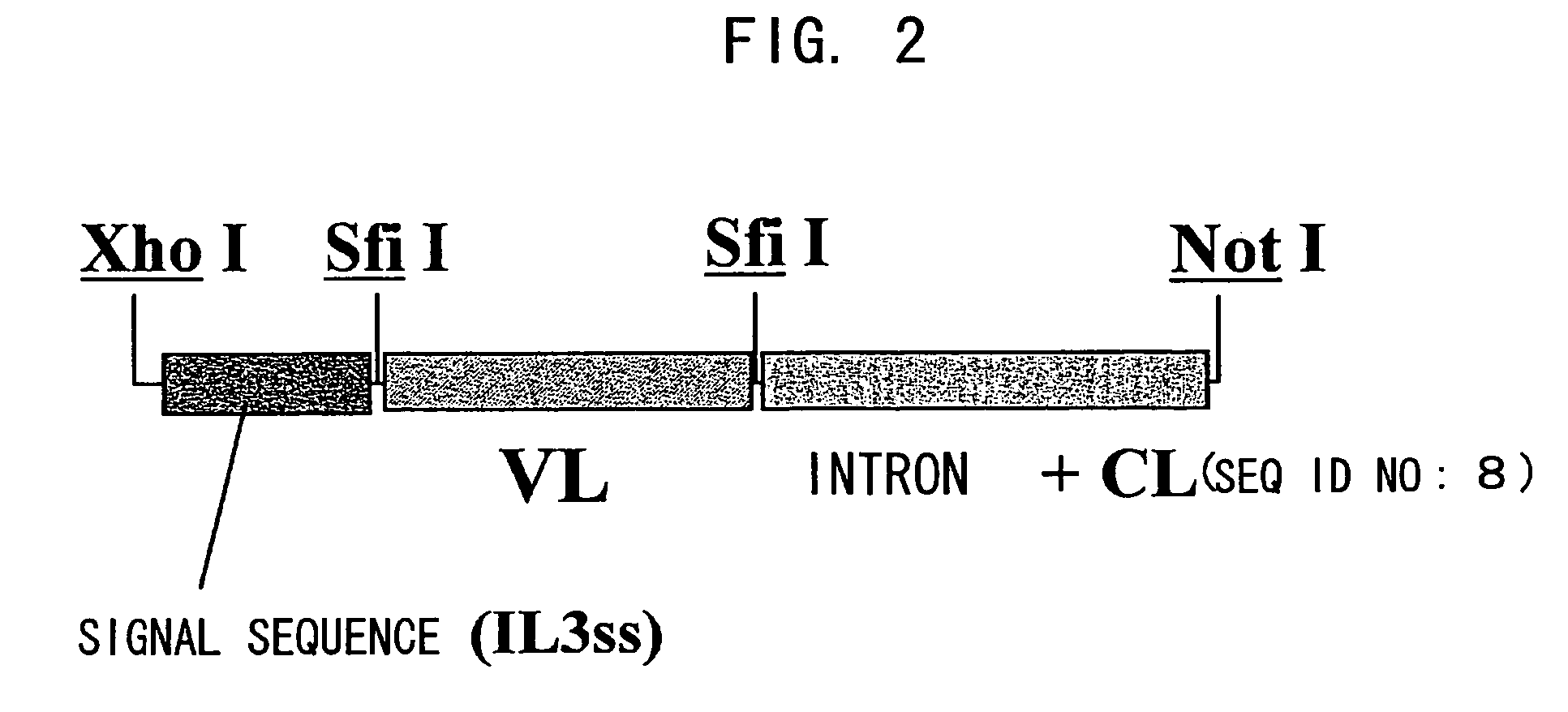
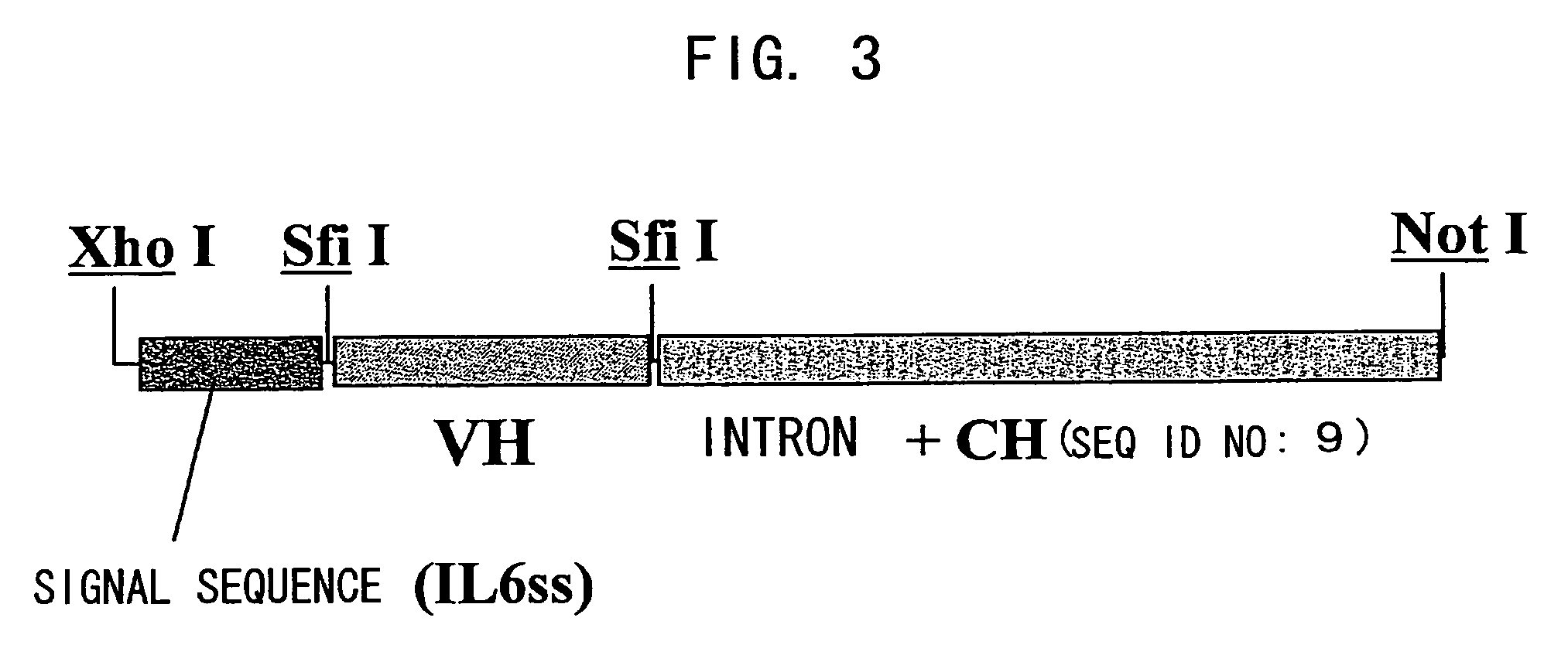
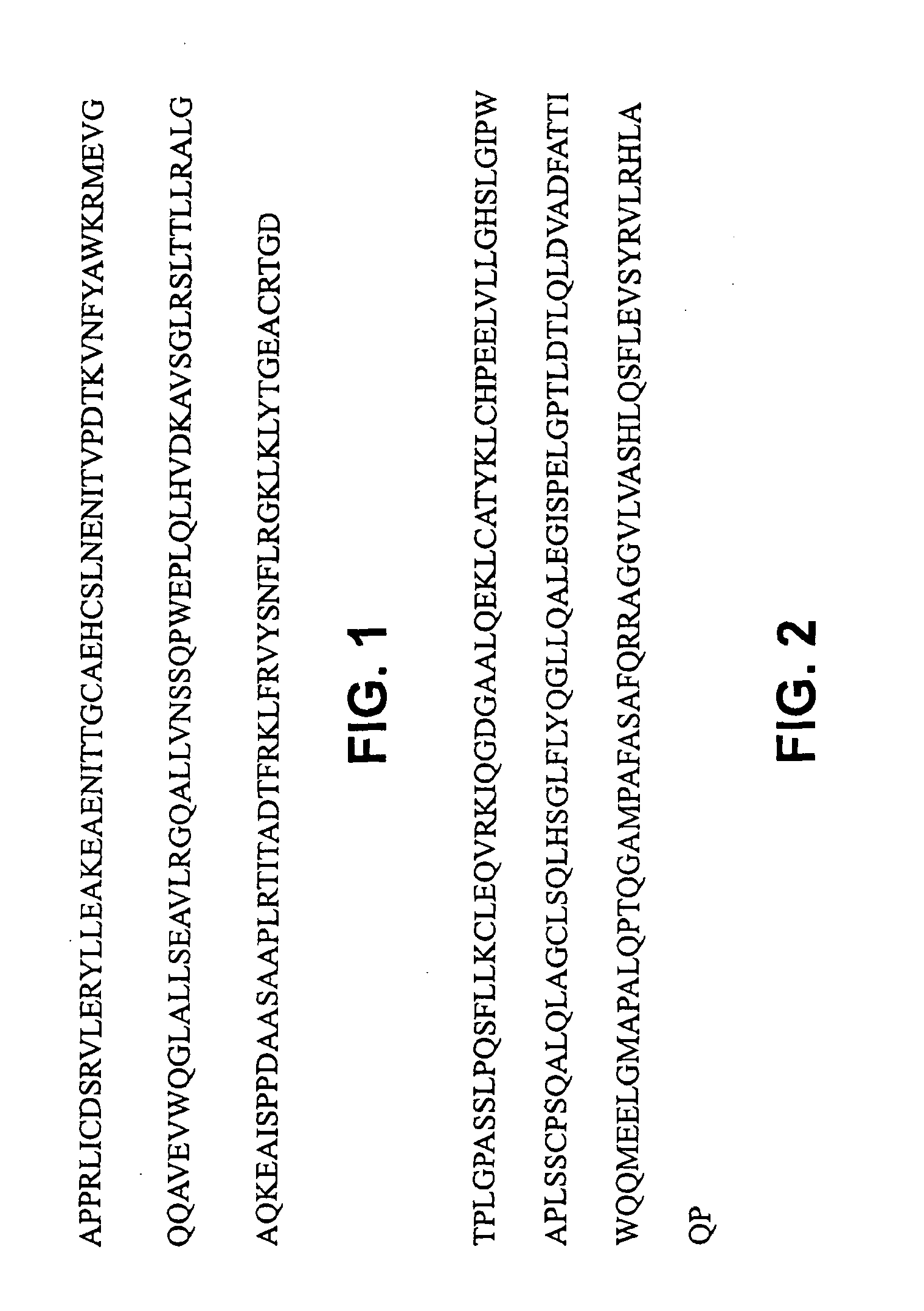
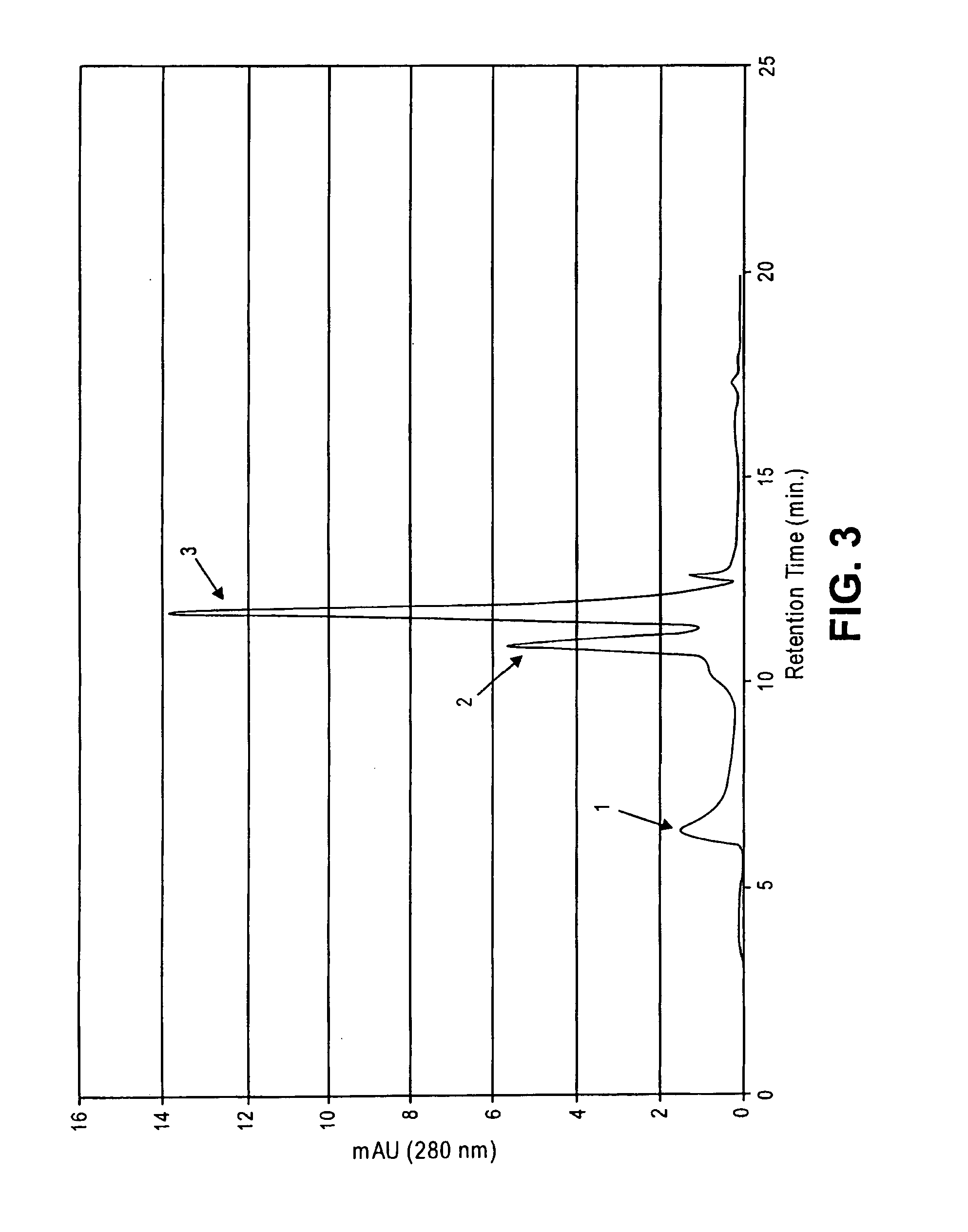
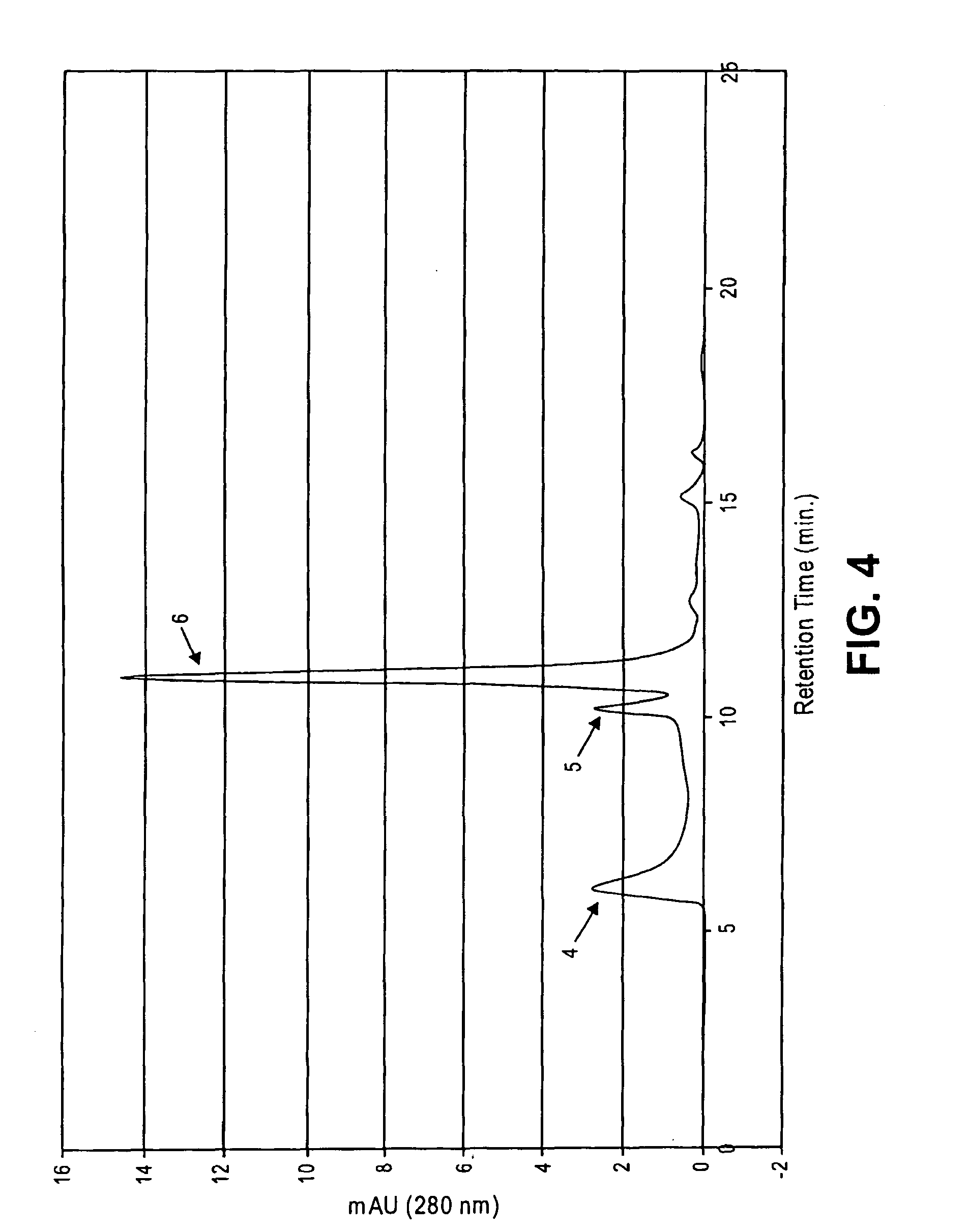
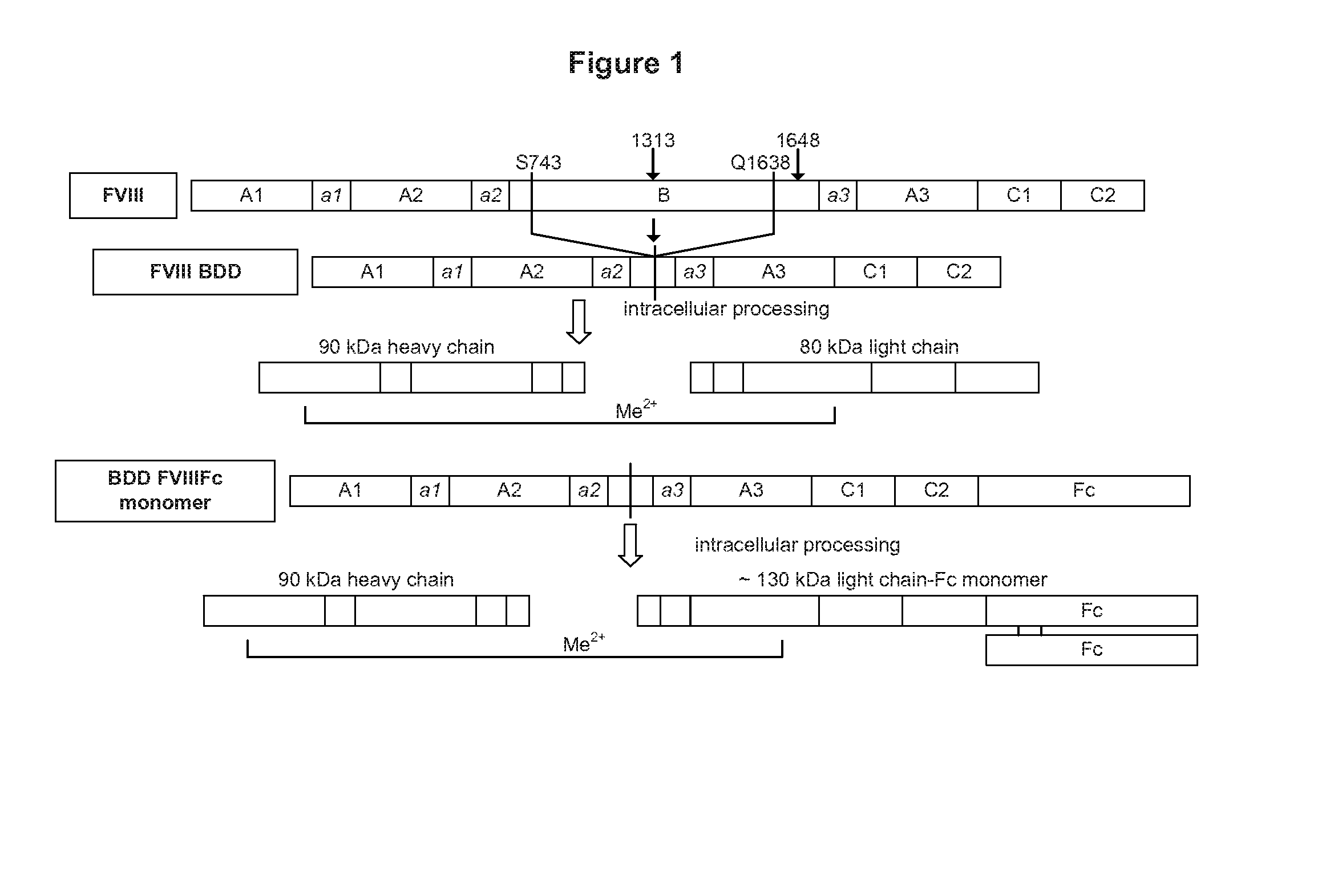
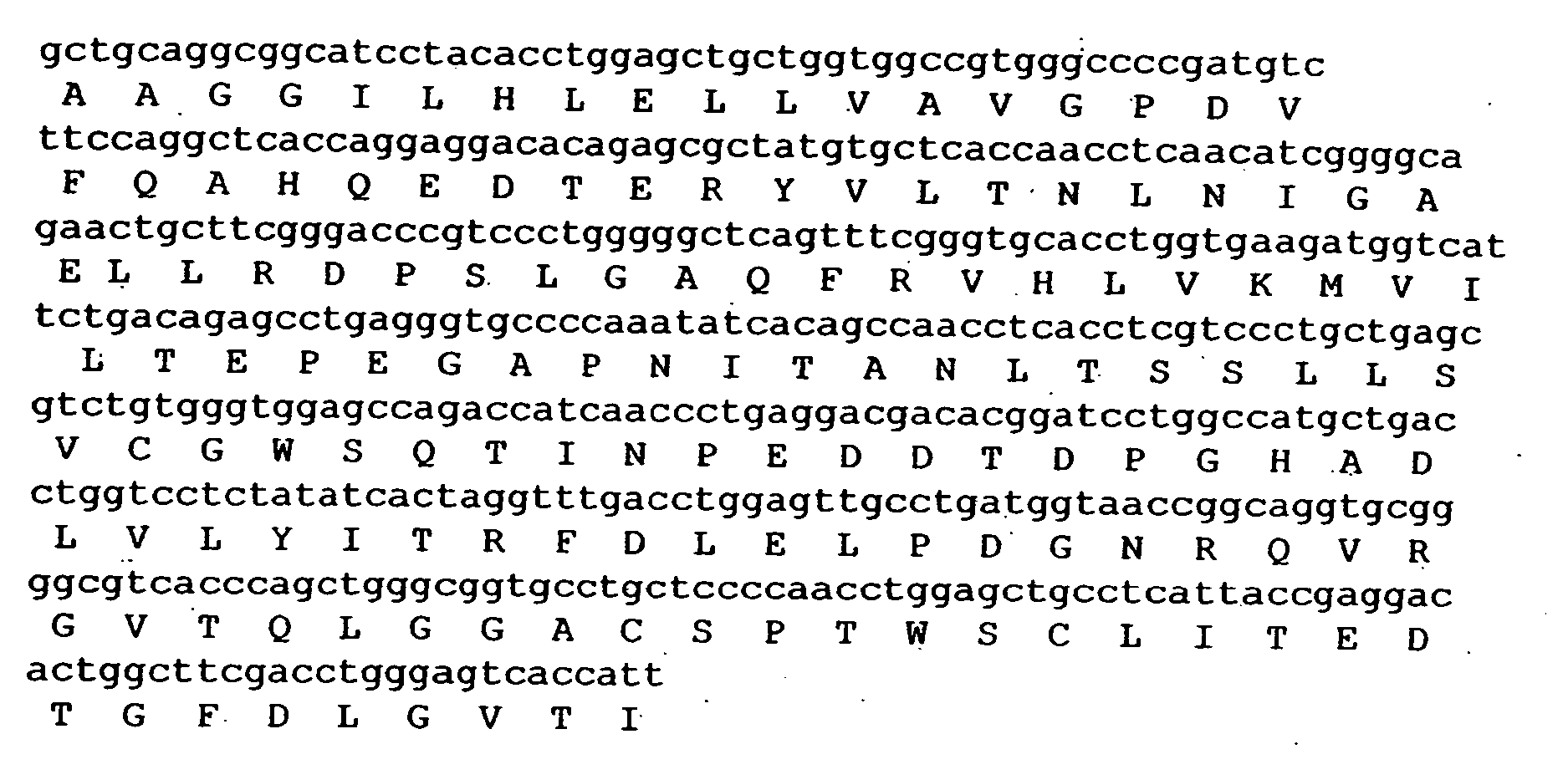Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
779results about "Factor VII" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
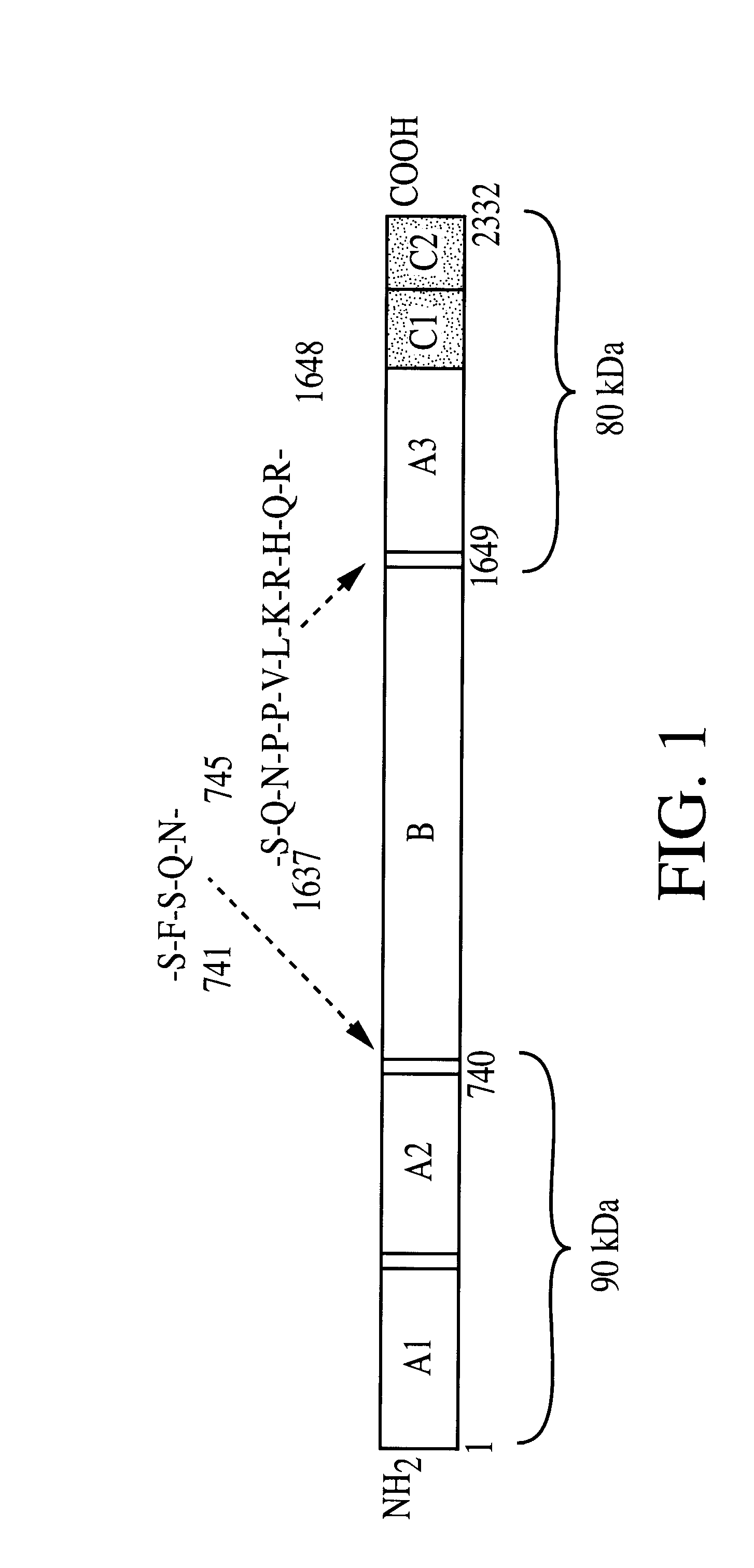
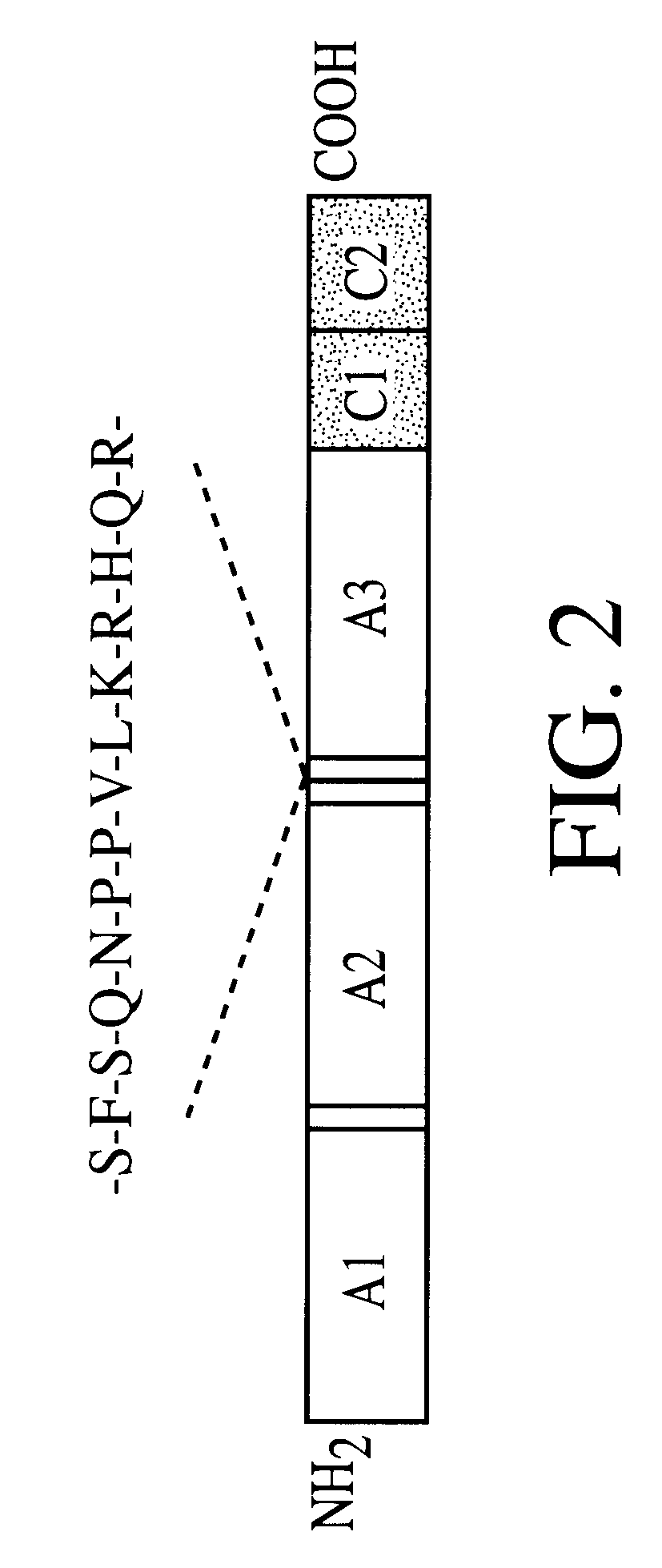
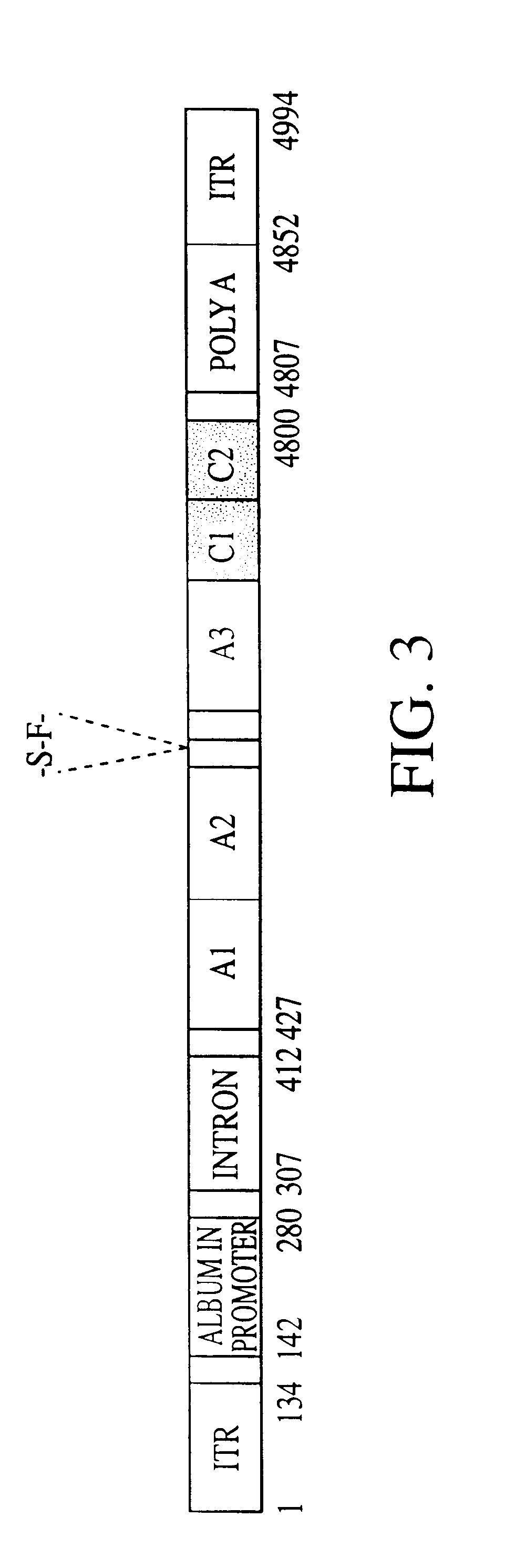
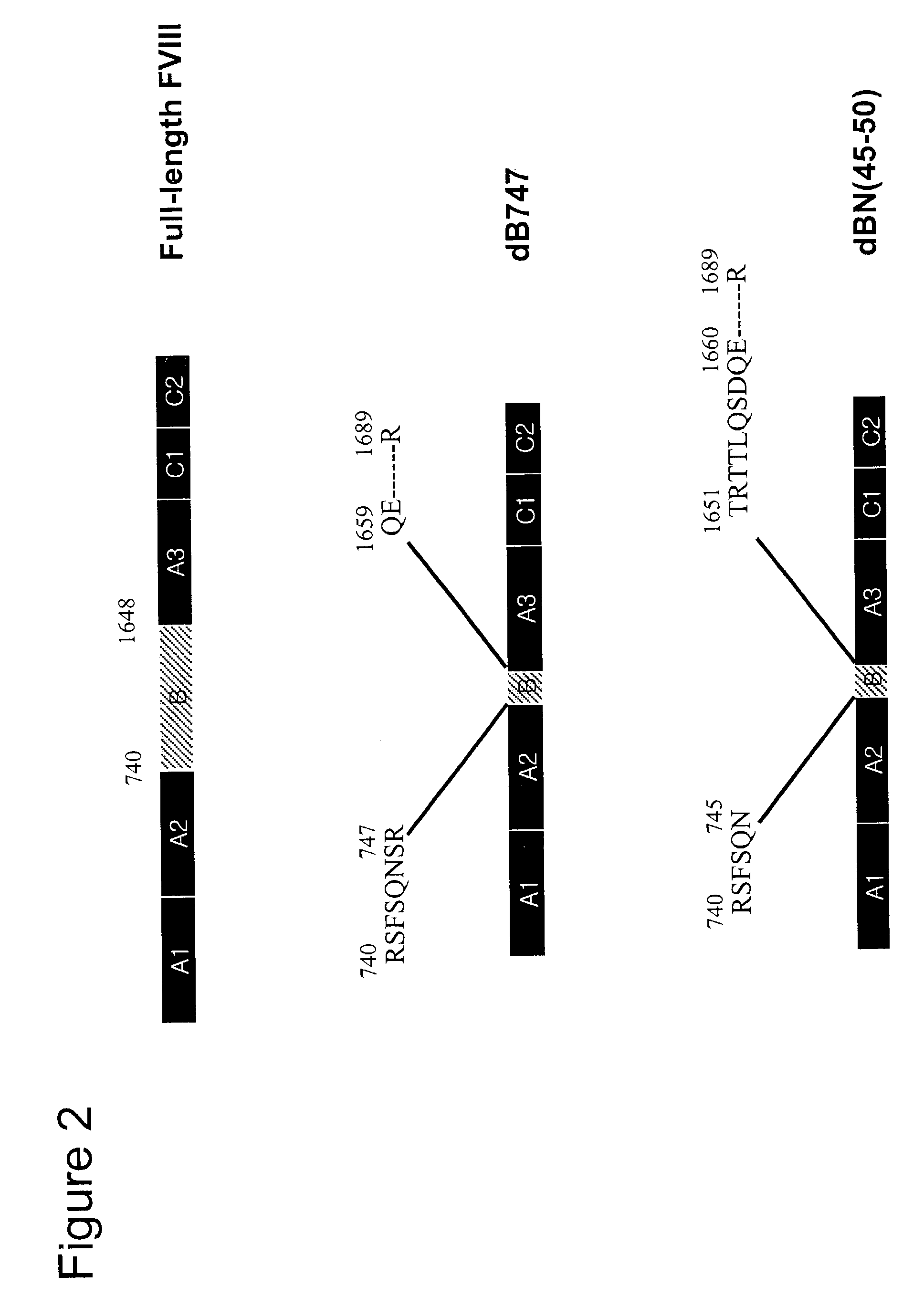
Adeno-associated virus vectors for expression of factor VIII by target cells
InactiveUS6200560B1Easily transfectedConvenient platformBiocideFactor VIIHigh level expressionHuman cell
The present invention provides improved viral vectors useful for the expression of genes at high levels in human cells. In particular, the present invention provides recombinant adeno-associated vectors (AAV) suitable for gene therapy. These vectors are capable of delivering nucleic acid containing constructs which result in the production of full-length therapeutic levels of biologically active Factor VIII in the recipient individual in vivo. The present invention also provides pharmaceutical compositions comprising such AAV vectors, as well as methods for making and using these constructs.
Owner:GENZYME CORP
Factor VIII polypeptide
ActiveUS7041635B2Stable and efficiently expressed formFull coagulation activityFactor VIIPeptide/protein ingredientsThrombin activityAmino acid
Owner:SK BIOSCI CO LTD
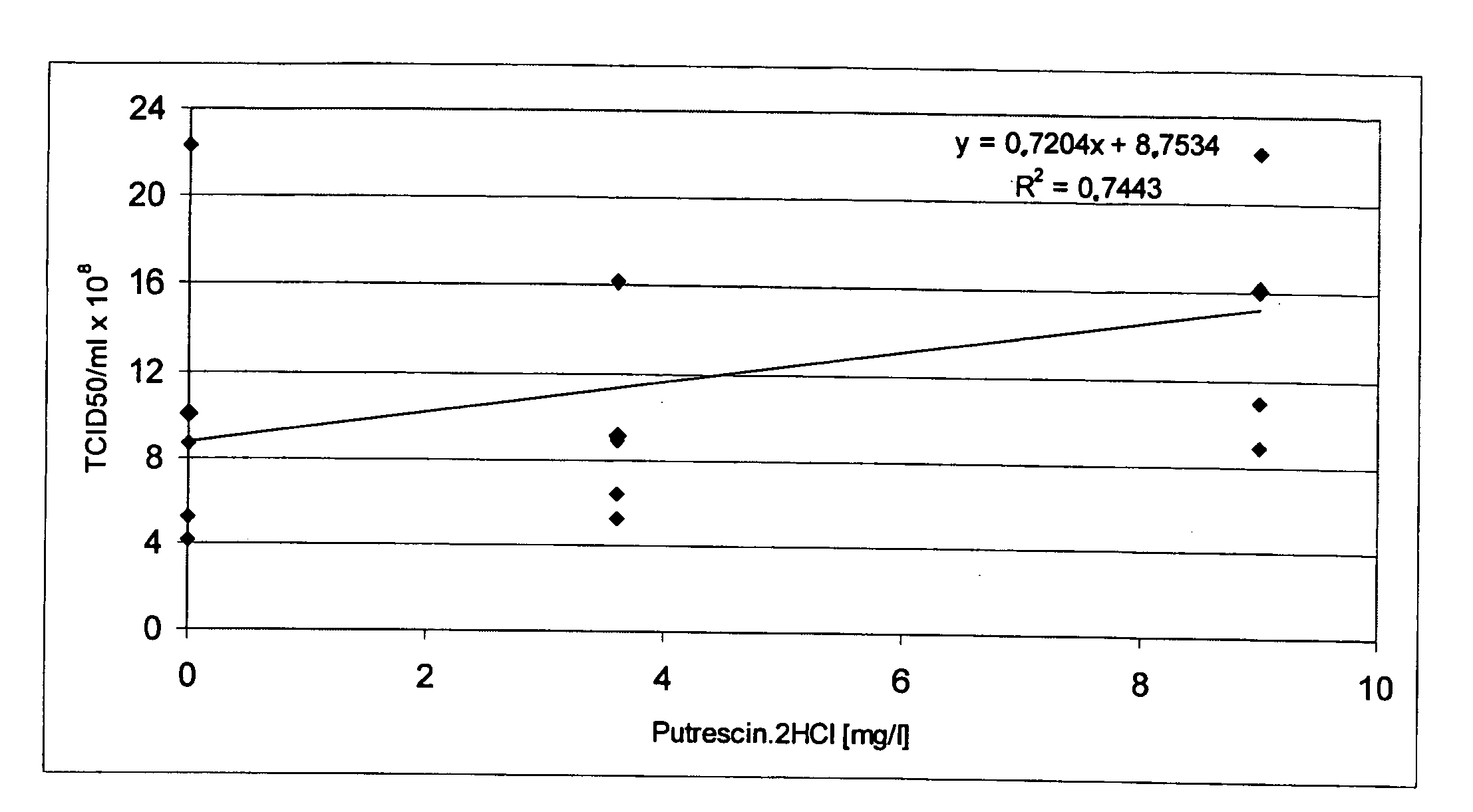
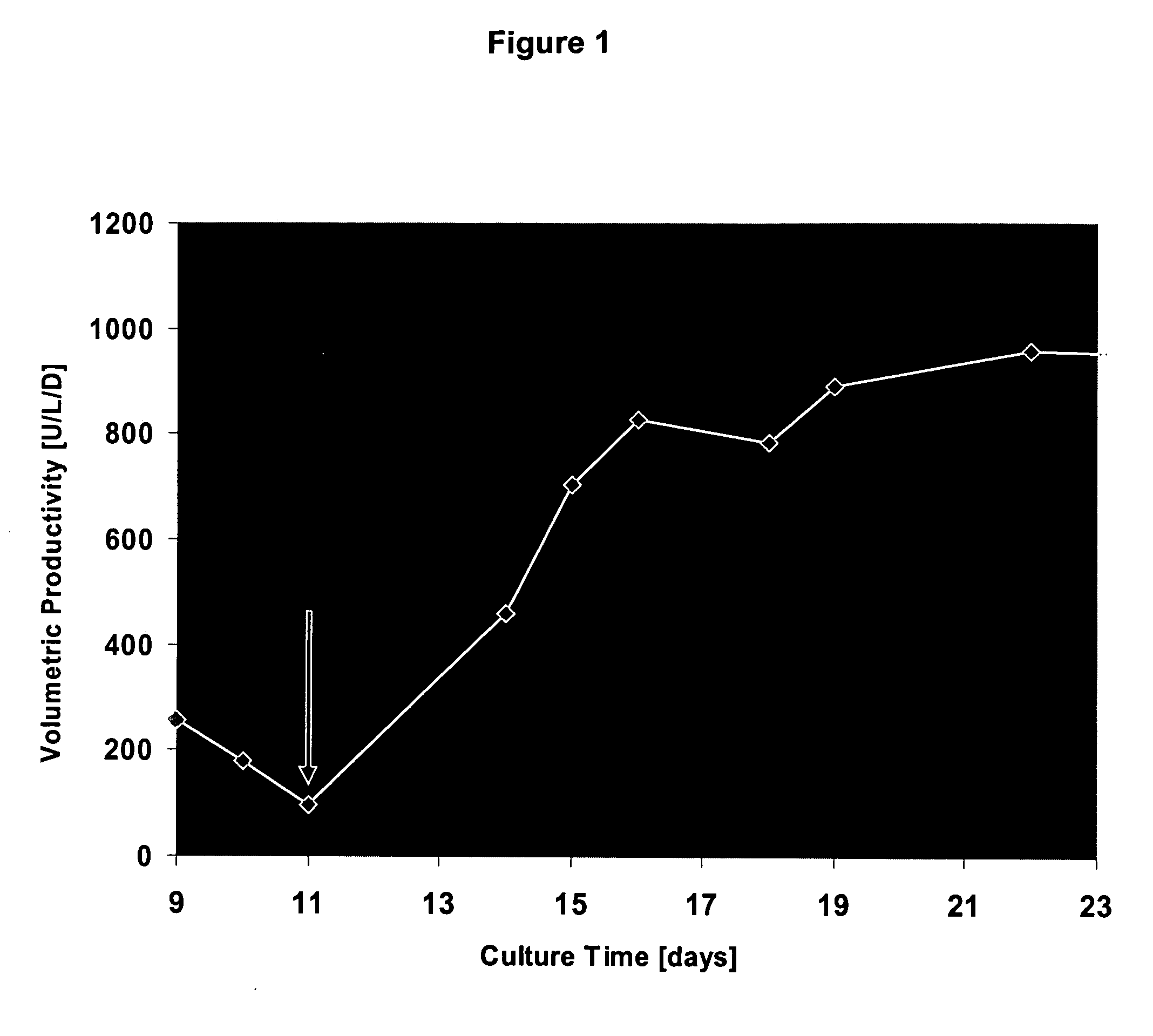
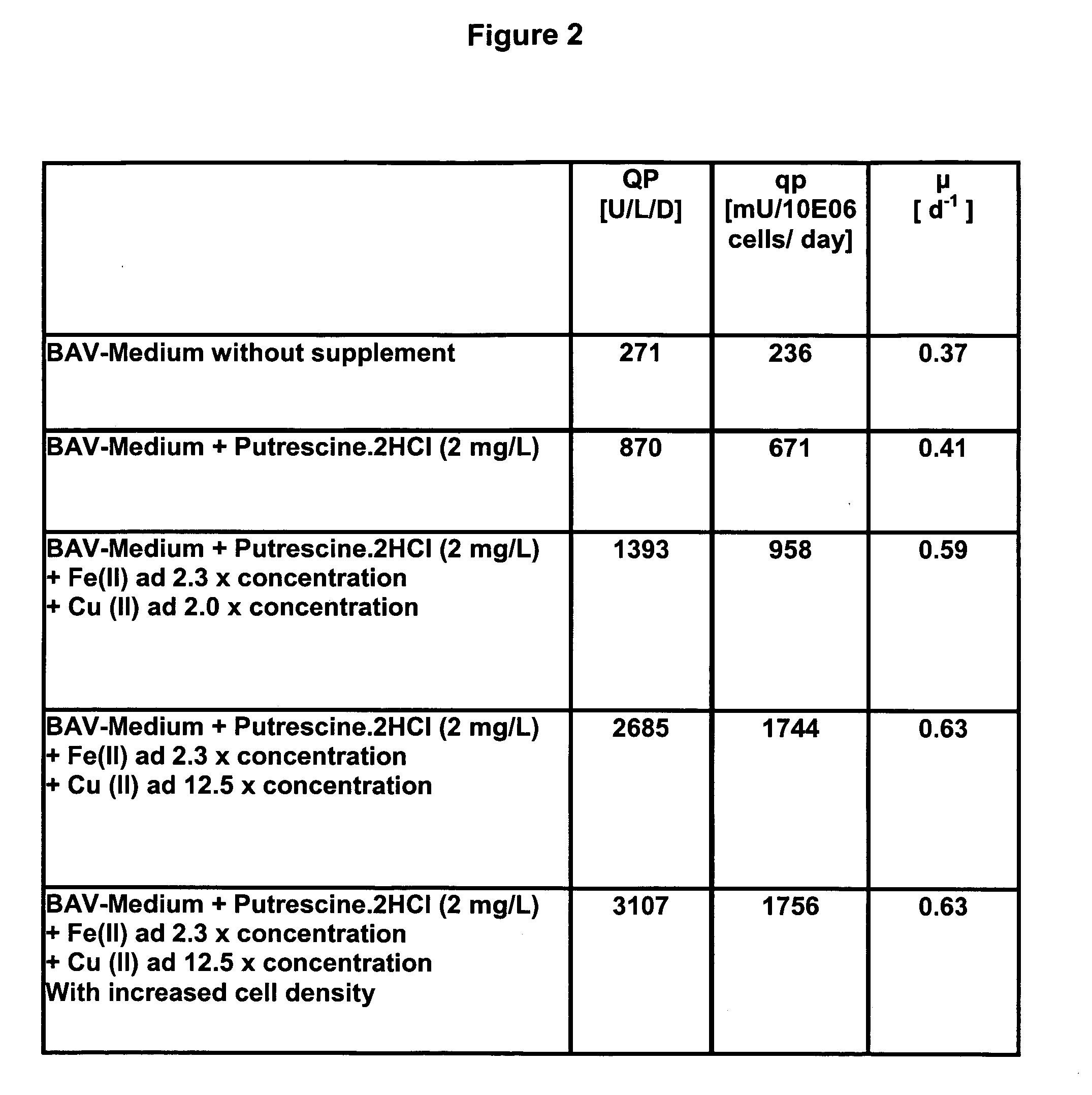
Oligopeptide-free cell culture media
InactiveUS20070212770A1Efficient expression of recombinantEfficient productionFactor VIIBacteriaCulture cellCell culture media
The present invention relates to oligopeptide-free cell culture media comprising at least 0.5 mg / L of a polyamine and to methods for cultivating cells in said oligopeptide-free cell culture media comprising at least 0.5 mg / L of a polyamine. The invention also relates to methods for expressing at least one protein in a medium comprising at least 0.5 mg / L of a polyamine and to methods for producing at least one virus in a medium comprising at least 0.5 mg / L of a polyamine.
Owner:BAXTER HEALTHCARE SA +1
Protein complexes having Factor VIII:C activity and production thereof
InactiveUS6060447AImprove stabilityHigh yieldPeptide/protein ingredientsMammal material medical ingredientsFactor iiADAMTS Proteins
Recombinant protein complexes having human Factor VIII:C activity are expressed in a eukaryotic host cell by transforming the host cell with first and second expression cassettes encoding a first polypeptide substantially homologous to human Factor VIII:C A domain and a second polypeptide substantially homologous to human Factor VIII:C C domain, respectively. In the present invention, the first polypeptide may be extended having at its C-terminal a human Factor VIII:C B domain N-terminal peptide, a polypeptide spacer of 3-40 amino acids, and a human Factor VIII:C B domain C-terminal peptide. Expression of the second polypeptide is improved by employing an .alpha..sub.1 -antitrypsin signal sequence.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Preparation of recombinant factor VIII in a protein free medium
InactiveUS6171825B1Eliminate and at least greatly reduce riskImprove productivityFactor VIICulture processFactor iiManganese
Recombinant Factor VIII can be produced in relatively large quantities on a continuous basis from mammalian cells in the absence of any animal-derived proteins such as albumin by culturing the cells in a protein free medium supplemented with polyol copolymers, preferably in the presence of trace metals such as copper. In very preferred embodiments, the medium includes a polyglycol known as Pluronic F-68, copper sulfate, ferrous sulfate / EDTA complex, and salts of trace metals such as manganese, molybdenum, silicon, lithium and chromium. With an alternative medium which included trace copper ions alone (without polyol copolymers) we were also able to enhance the productivity of Factor VIII in recombinant cells such as BHK cells that are genetically engineered to express Factor VIII.
Owner:BAYER HEALTHCARE LLC +1
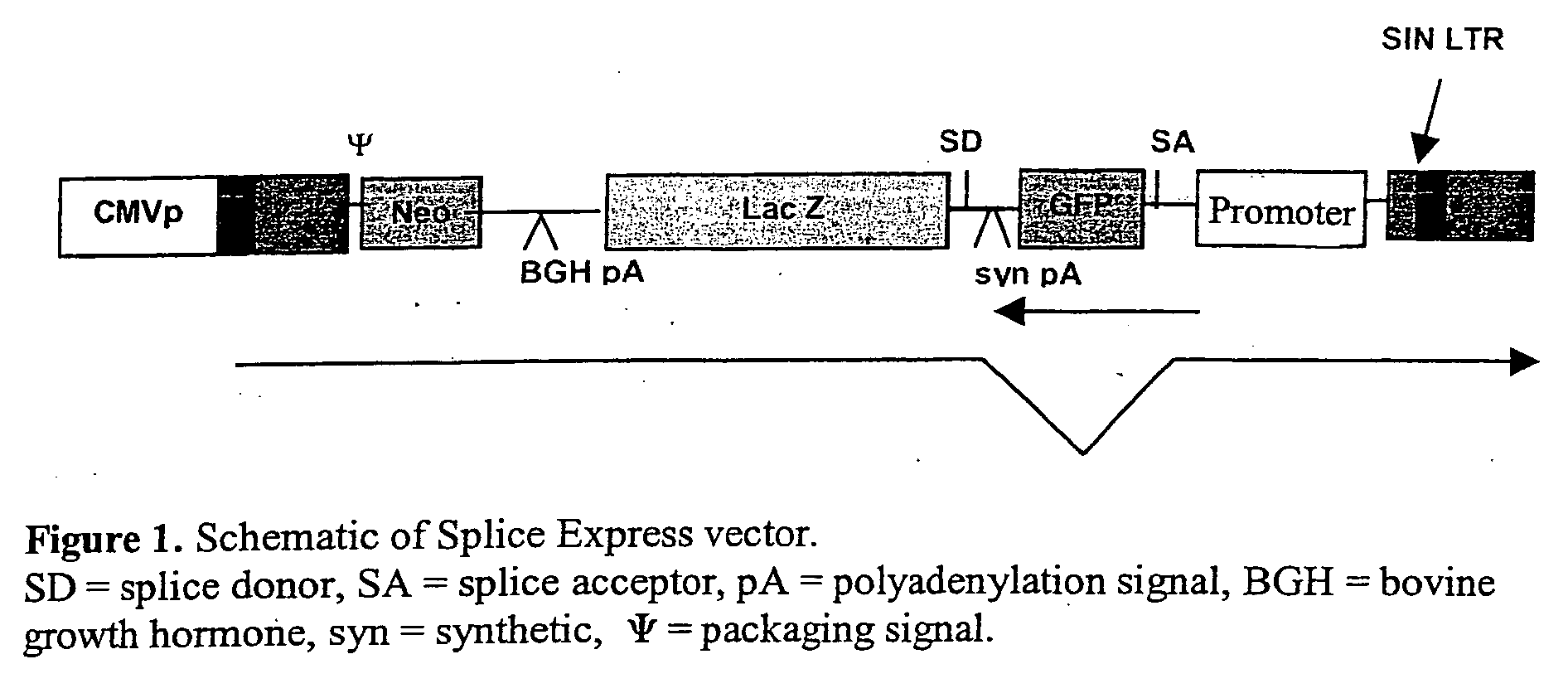
Vectors
InactiveUS20060281180A1Stable long-term expressionEfficient expressionAntibacterial agentsFactor VIIGeneticsViral vector
Provided is a lentiviral vector capable of delivering a nucleotide of interest (NOI) to a desired target site and wherein the NOI encodes the Factor VIII and the Factor VIII is expressed following delivery of the NOI to the desired target site.
Owner:OXFORD BIOMEDICA (UK) LTD
Optimized messenger RNA
InactiveUS6924365B1Precise functionEliminates issues concerning patient complianceFactor VIIPeptide/protein ingredientsMessenger RNANucleic acid sequence
The present invention is directed to a synthetic nucleic acid sequence which encodes a protein wherein at least one non-common codon or less-common codon is replaced by a common codon. The synthetic nucleic acid sequence can include a continuous stretch of at least 90 codons all of which are common codons.
Owner:SHIRE HUMAN GENETIC THERAPIES INC
Method for Purifying Antibodies
ActiveUS20130171095A1Increased serum half-lifeMinimize the possibilityFactor VIIPeptide/protein ingredientsIsoelectric pointAntibody
The invention relates generally to compositions and methods for purifying the desired species from a mixture of desired heterodimer and contaminating homodimer immunoglobulin variants by modifying the isoelectric point(s) of the individual chains.
Owner:XENCOR
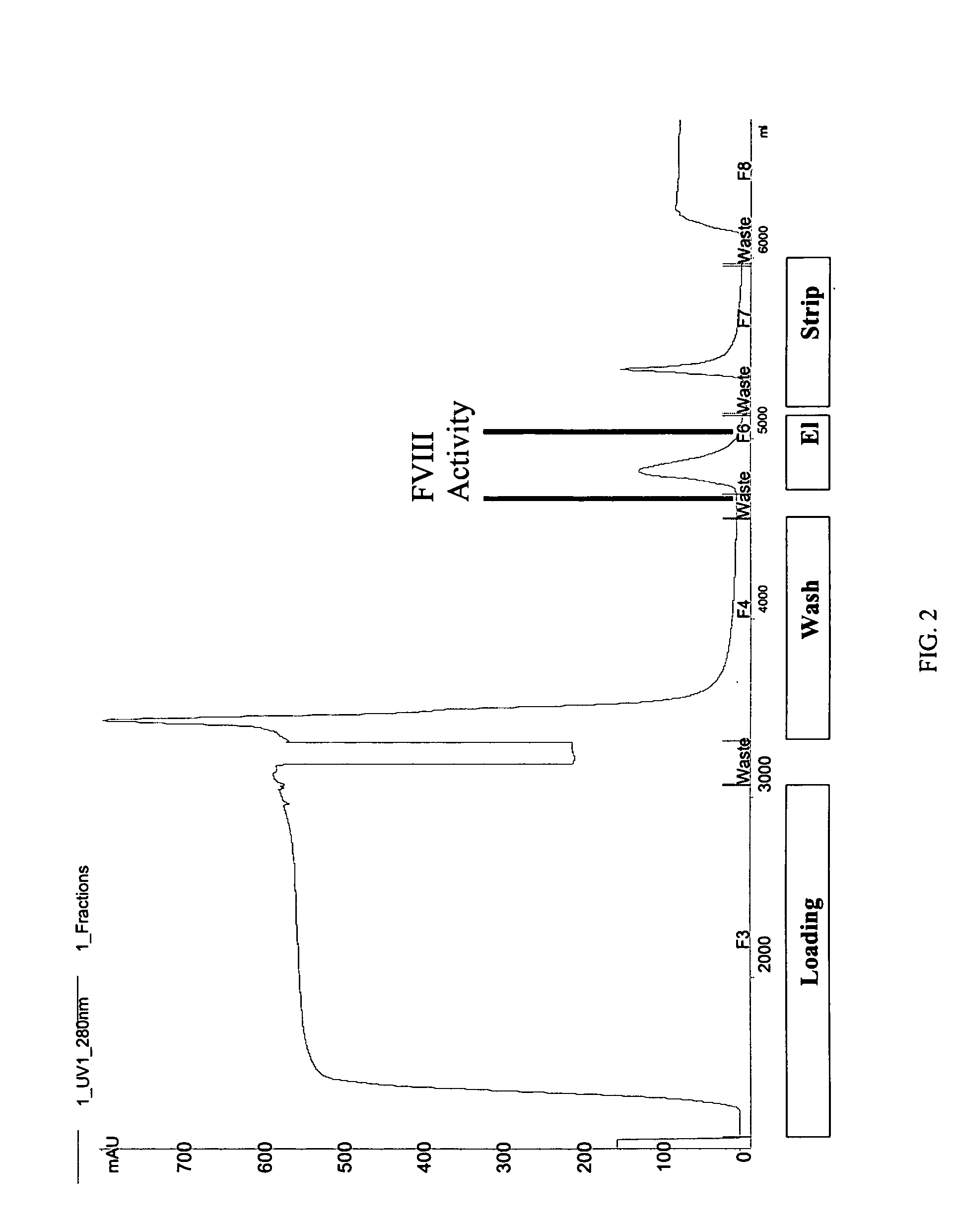
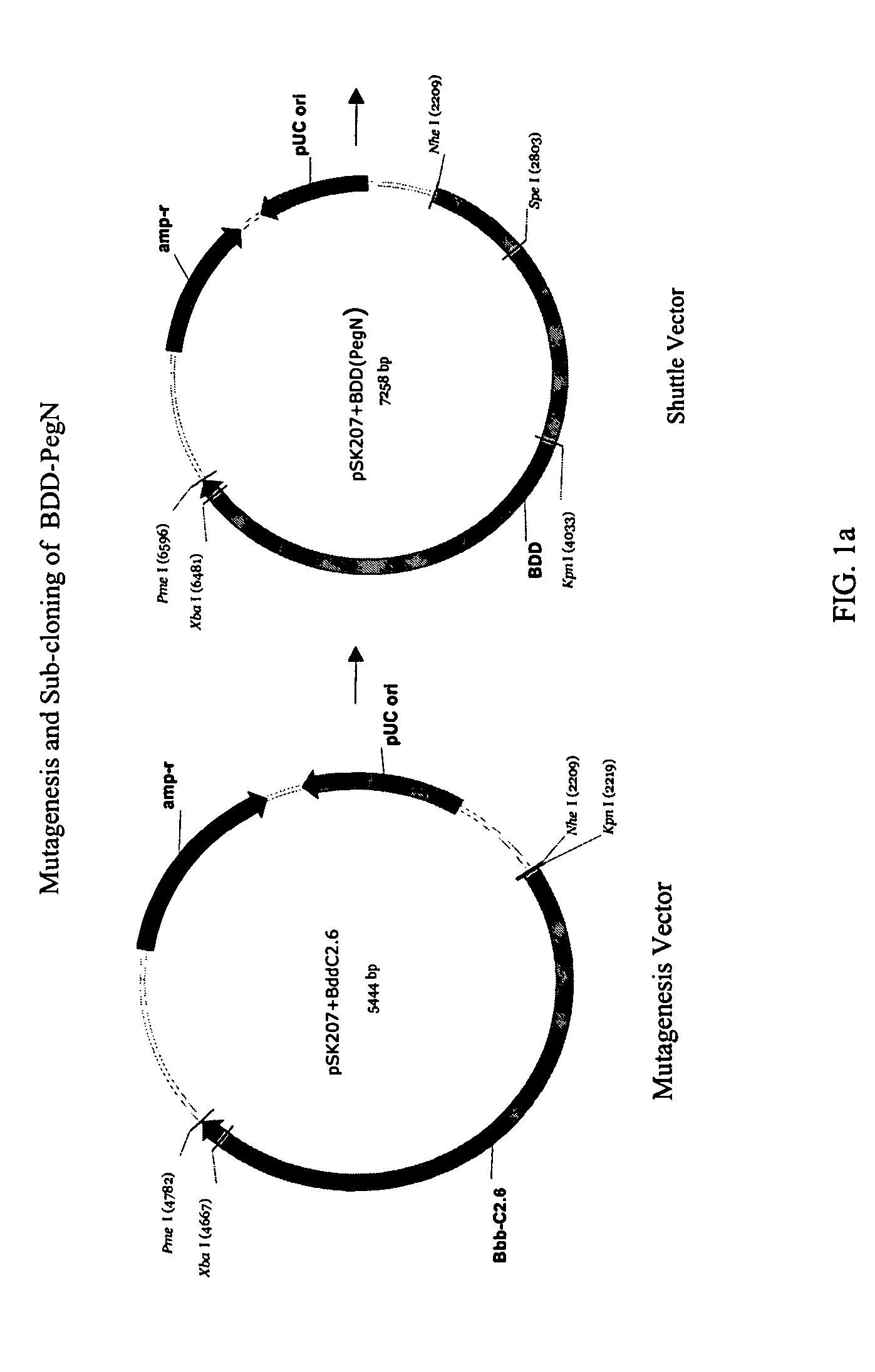
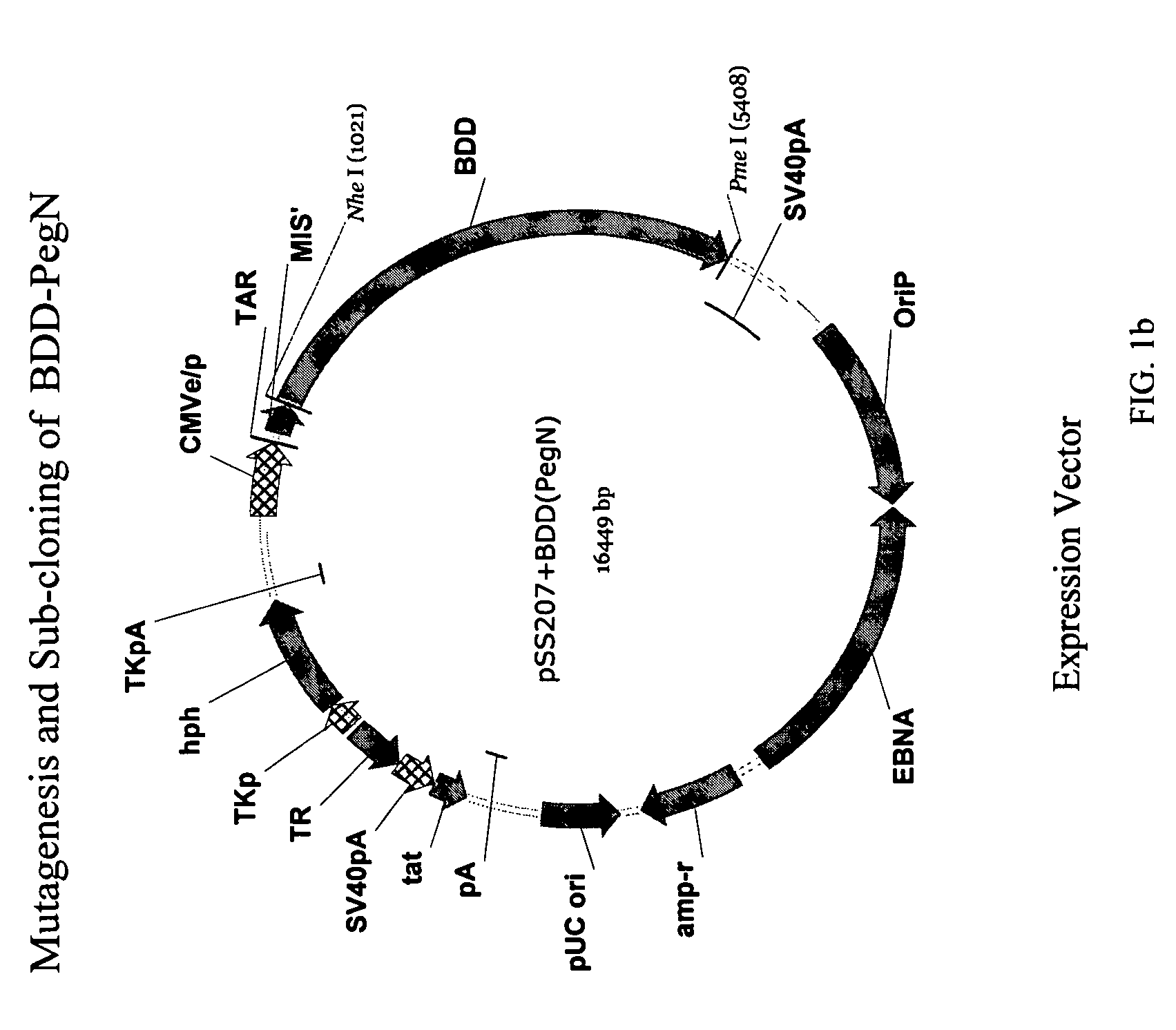
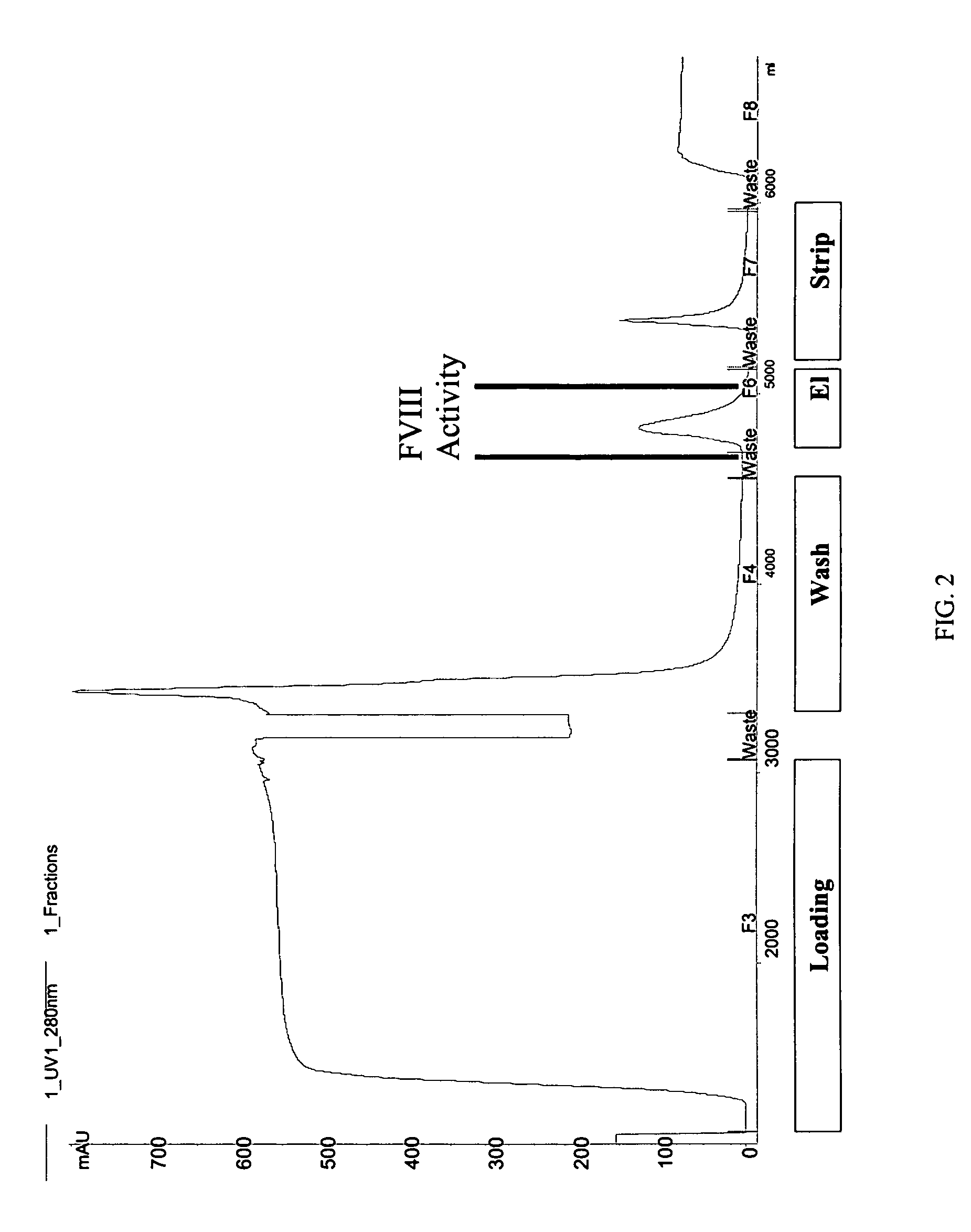
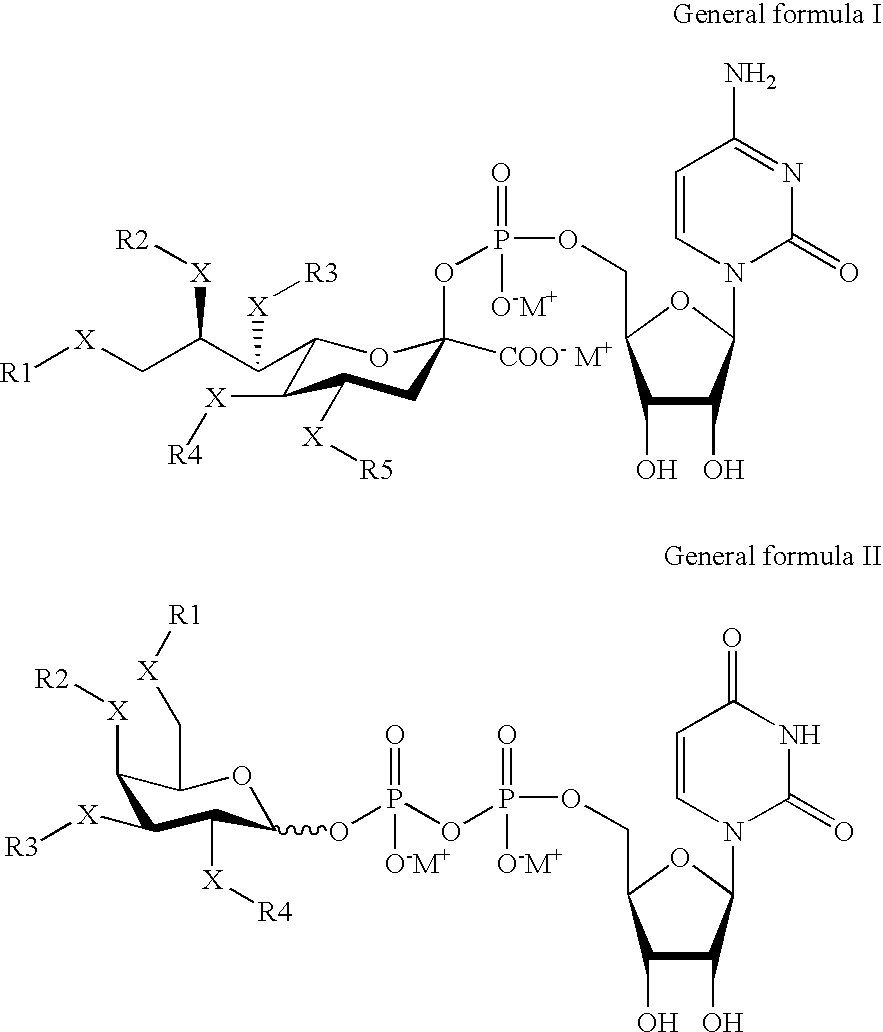
Pegylated factor VIII
The invention is a proteinaceous construct comprising a Factor VIII molecule having at least a portion of the B domain intact, which is conjugated to a water-soluble polymer such as polyethylene glycol having a molecular weight of greater than 10,000 Daltons. The construct has a biological activity of at least 80% of the biological activity of native Factor VIII, and the in vivo half-life of the construct is increased by at least 1.5 fold as compared to the in vivo half-life of native factor FVIII.
Owner:TAKEDA PHARMA CO LTD
Factor VIII compositions and methods
InactiveUS20050100990A1Reduce gapExtended half-lifeFactor VIIPeptide/protein ingredientsHalf-lifeNucleotide
The present invention provides methods of increasing the half-life and / or specific activity of factor VIII. More specifically, the invention provides methods of increasing the half-life and / or specific activity of factor VIII by substituting one or more amino acids in the A2 domain. It further provides methods for producing such factor VIII mutants. The invention also provides polynucleotides encoding the mutant factor VIII, and methods of treating hemophilia using the polypeptides and polynucleotides of the invention.
Owner:UNIV OF MARYLAND
Bispecific antibody substituting for functional proteins
ActiveUS20070041978A1Enhances enzymatic reactionImmunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIBlood Coagulation Factor X
The present inventors succeeded in constructing bispecific antibodies, which bind to both the blood coagulation factor IX / activated blood coagulation factor IX and blood coagulation factor X, and functionally substitute for blood coagulation factor VIII / activated blood coagulation factor VIII which enhances the enzymatic reaction.
Owner:CHUGAI PHARMA CO LTD
Site-directed modification of FVIII
ActiveUS20060115876A1Improve featuresImproved pharmacokinetic propertiesFactor VIIPeptide/protein ingredientsPolyethylene glycolMutant protein
This invention relates to Factor VIII muteins that are covalently bound, at a predefined site that is not an N-terminal amine, to one or more biocompatible polymers such as polyethylene glycol. The mutein conjugates retain FVIII procoagulant activity and have improved pharmacokinetic properties.
Owner:BAYER HEALTHCARE LLC
Site-directed modification of FVIII
ActiveUS7632921B2Improve featuresImproved pharmacokinetic propertiesFactor VIIPeptide/protein ingredientsFactor VIII deficiencyPolyethylene glycol
Owner:BAYER HEALTHCARE LLC
Method for stabilizing biomolecules in liquid formulations
InactiveUS20020110524A1Improve stabilityOrganic active ingredientsFactor VIIActive proteinPharmaceutical medicine
The invention is directed to a stable formulation of a biologically active protein useful for aerosol delivery to the respiratory tract of a patient in need of treatment comprising: (a) a carrier liquid comprising from about 10% to from about 100% V / V water and from about 0% to from about 90% V / V of an organic liquid; (b) a biologically effective amount of a protein suspended or dissolved in a carrier liquid; and (c) a stabilizing effective amount of a derivatized carbohydrate stabilizing agent suspended or dissolved in said carrier liquid. The stable formulations of the invention may optionally contain about 0.1% to about 5.0% W / V of a pharmaceutically acceptable excipient.
Owner:BATTELLE MEMORIAL INST
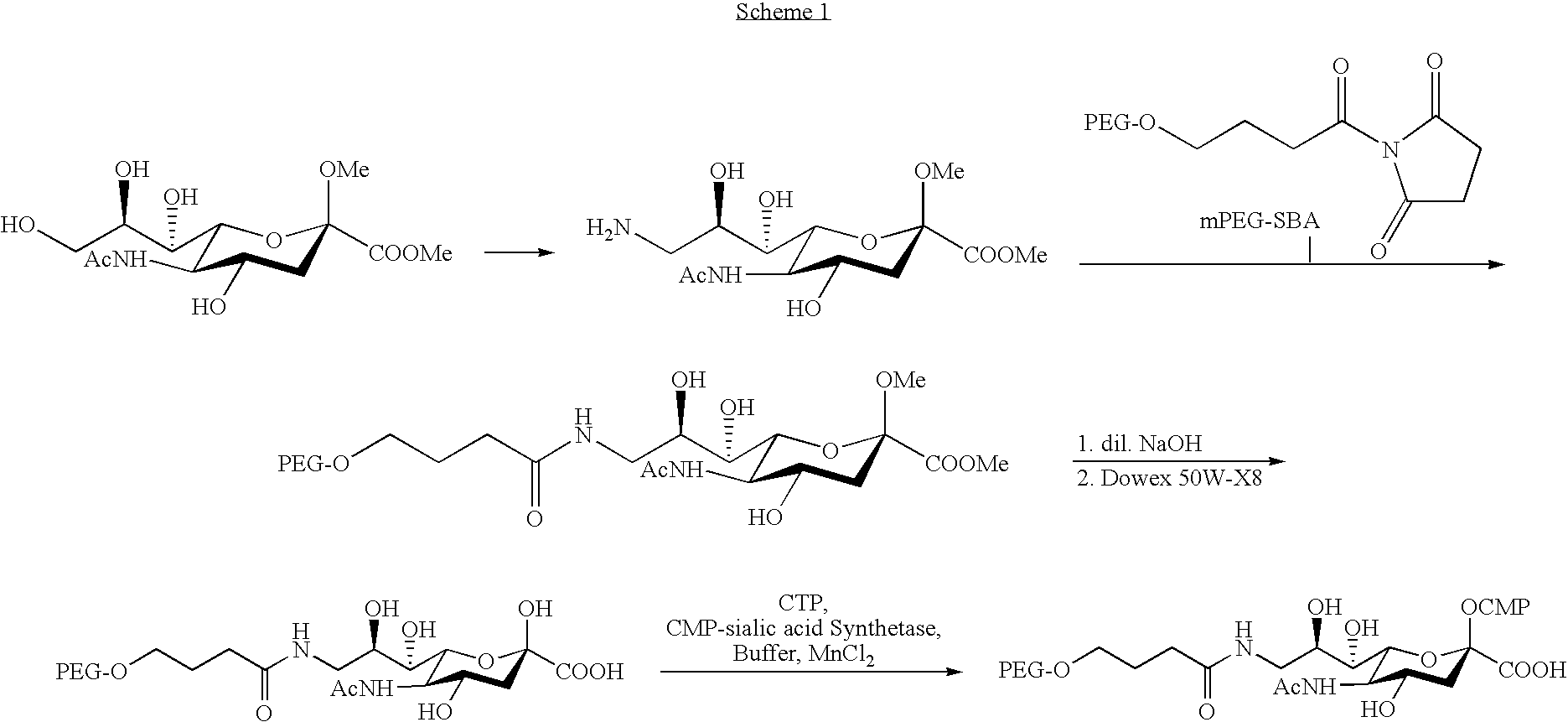
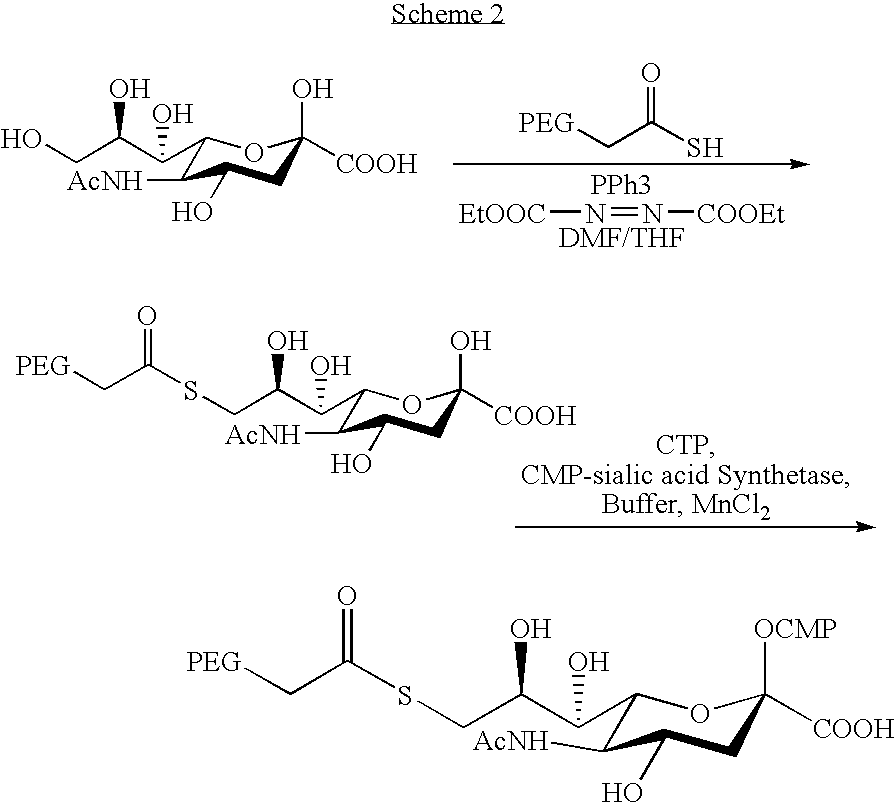
Pegylated factor VII glycoforms
InactiveUS20050113565A1Improve functional propertiesFactor VIIPeptide/protein ingredientsFactor VIIOligosaccharide
The invention concerns a preparation comprising a plurality of Factor VII polypeptides or Factor VII-related polypeptides, wherein the polypeptides comprise asparagine-linked and / or serine-linked oligosaccharide chains, and wherein at least one oligosaccharide group is covalently attached to at least one polymeric group.
Owner:KLAUSEN NIELS +3
Cross-linked polysacharide and protein matrices and methods for their preparation
Methods for preparing cross-linked polysaccharide matrices by cross-linking one or more amino group containing polysaccharides or amino-functionalized polysaccharides with reducing sugars and / or reducing sugar derivatives. The resulting matrices may include polysaccharide matrices and composite cross-linked matrices including polysaccharides cross-linked with proteins and / or polypeptides. Additives and / or cells may also be included in or embedded within the matrices. Various different solvent systems and reducing sugar cross-linkers for performing the cross-linking are described. The resulting matrices exhibit various different physical, chemical and biological properties.
Owner:DATUM BIOTECH LTD
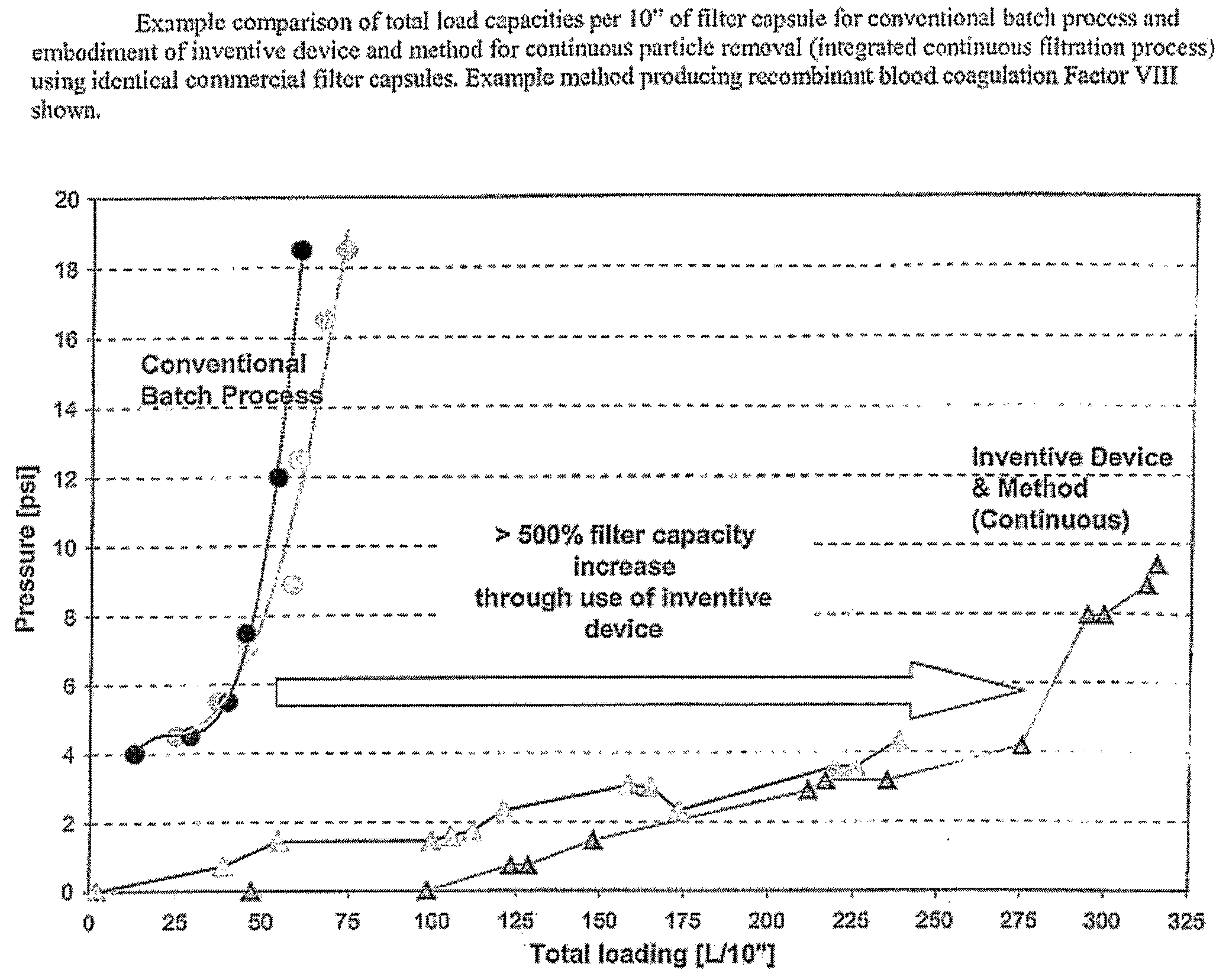
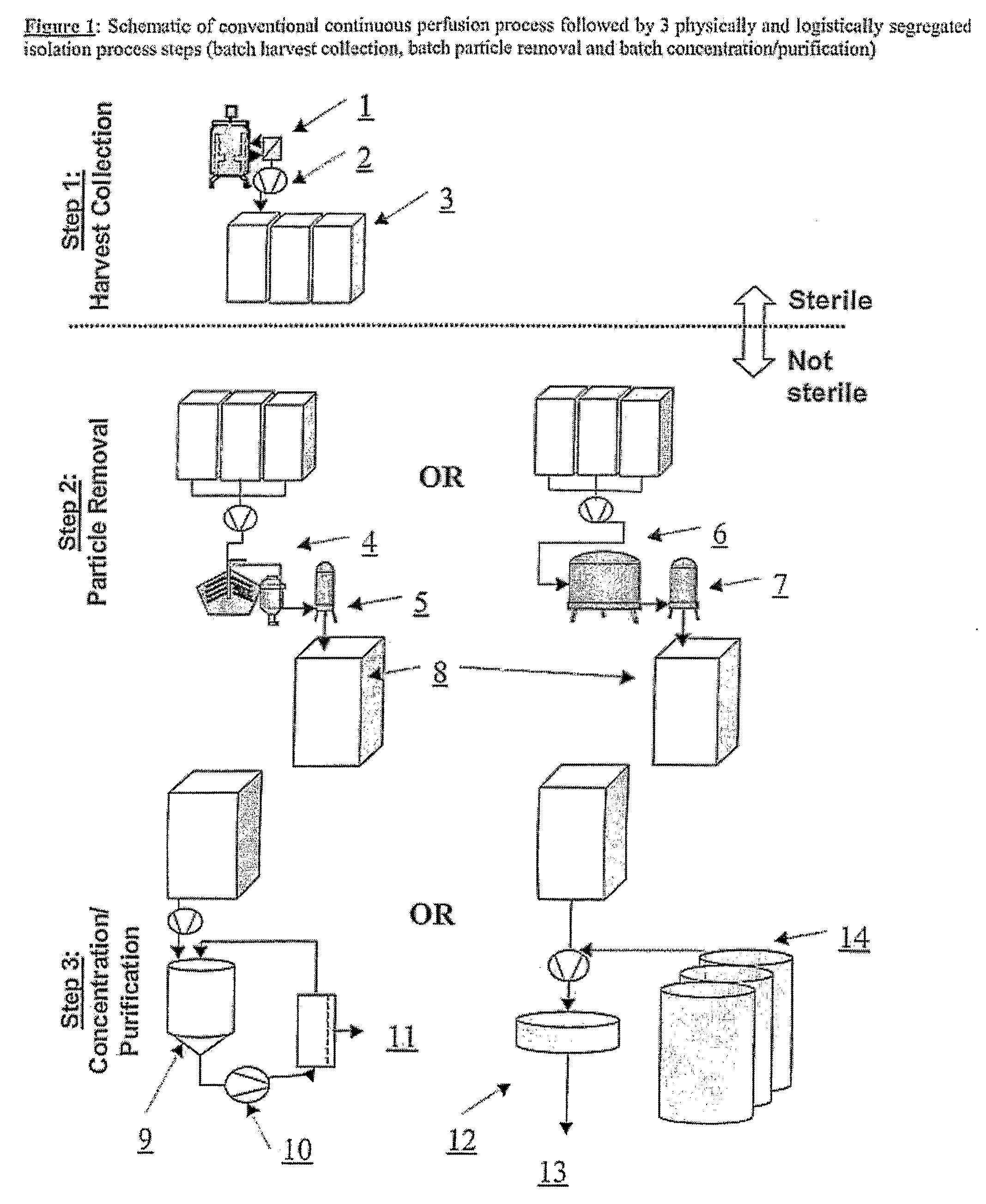
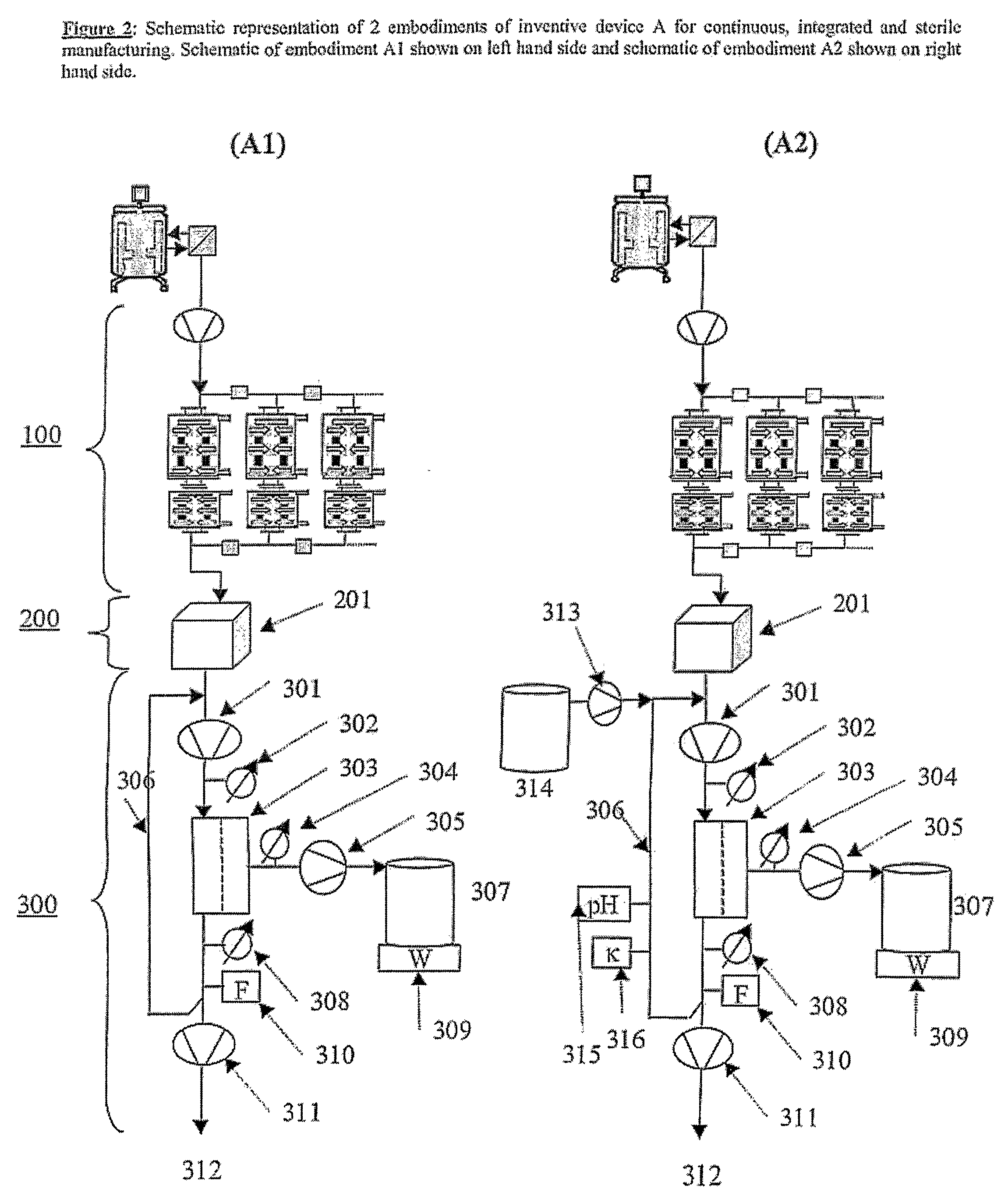
Devices and Methods for Integrated Continuous Manufacturing of Biological Molecules
ActiveUS20080269468A1Bioreactor/fermenter combinationsFactor VIIContinuous perfusionChemical physics
The present invention relates to a process and apparatus for purifying a molecule of interest from a heterogeneous clarified fluid mixture. The apparatus of the invention generally comprises a continuous perfusion fermentation system, a continuous particle removal system integrated with the perfusion fermentation system; and a continuous purification system integrated with the particle removal system, which is maintained under sterile conditions. The process comprises filtering a heterogeneous clarified fluid mixture by continuous ultrafiltration at a specific flow rate below the transition point of the molecule of interest in the pressure-dependent region of the flux versus TMP curve, wherein the specific flow rate is maintained substantially constant throughout the continuous ultrafiltration.
Owner:BAYER HEALTHCARE LLC
Modified proteins
InactiveUS20100056428A1Prolonged Circulatory Half-LifeReduce in quantityFactor VIIPeptide/protein ingredientsGlycoprotein iOrganic chemistry
Method of conjugating glycoproteins by means of chemical modification is provided as well as new modified glycoproteins.
Owner:NOVO NORDISK AS
Bispecific antibody substituting for functional proteins
ActiveUS8062635B2Enhances enzymatic reactionImmunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIBlood Coagulation Factor X
The present inventors succeeded in constructing bispecific antibodies, which bind to both the blood coagulation factor IX / activated blood coagulation factor IX and blood coagulation factor X, and functionally substitute for blood coagulation factor VIII / activated blood coagulation factor VIII which enhances the enzymatic reaction.
Owner:CHUGAI PHARMA CO LTD
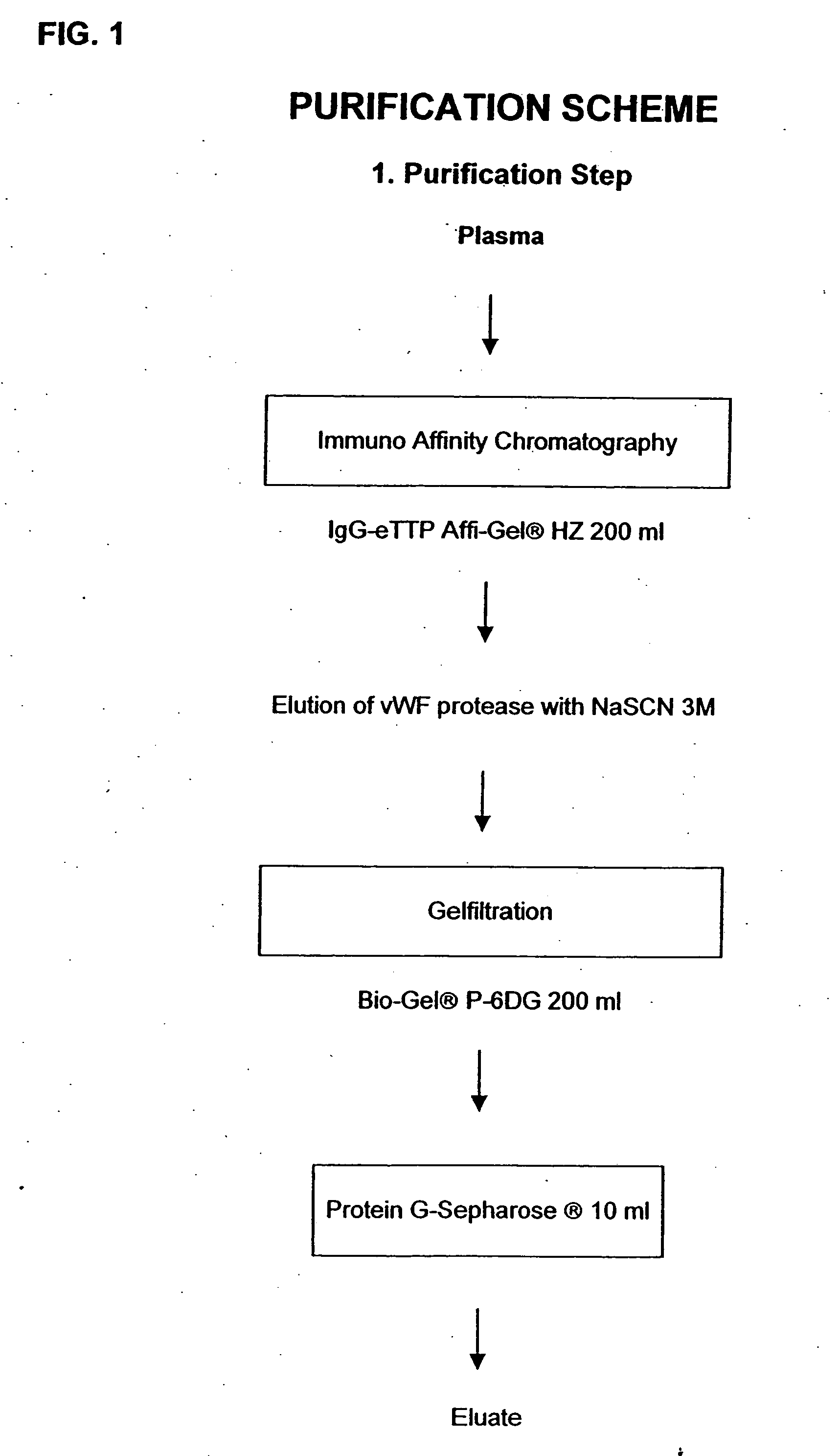
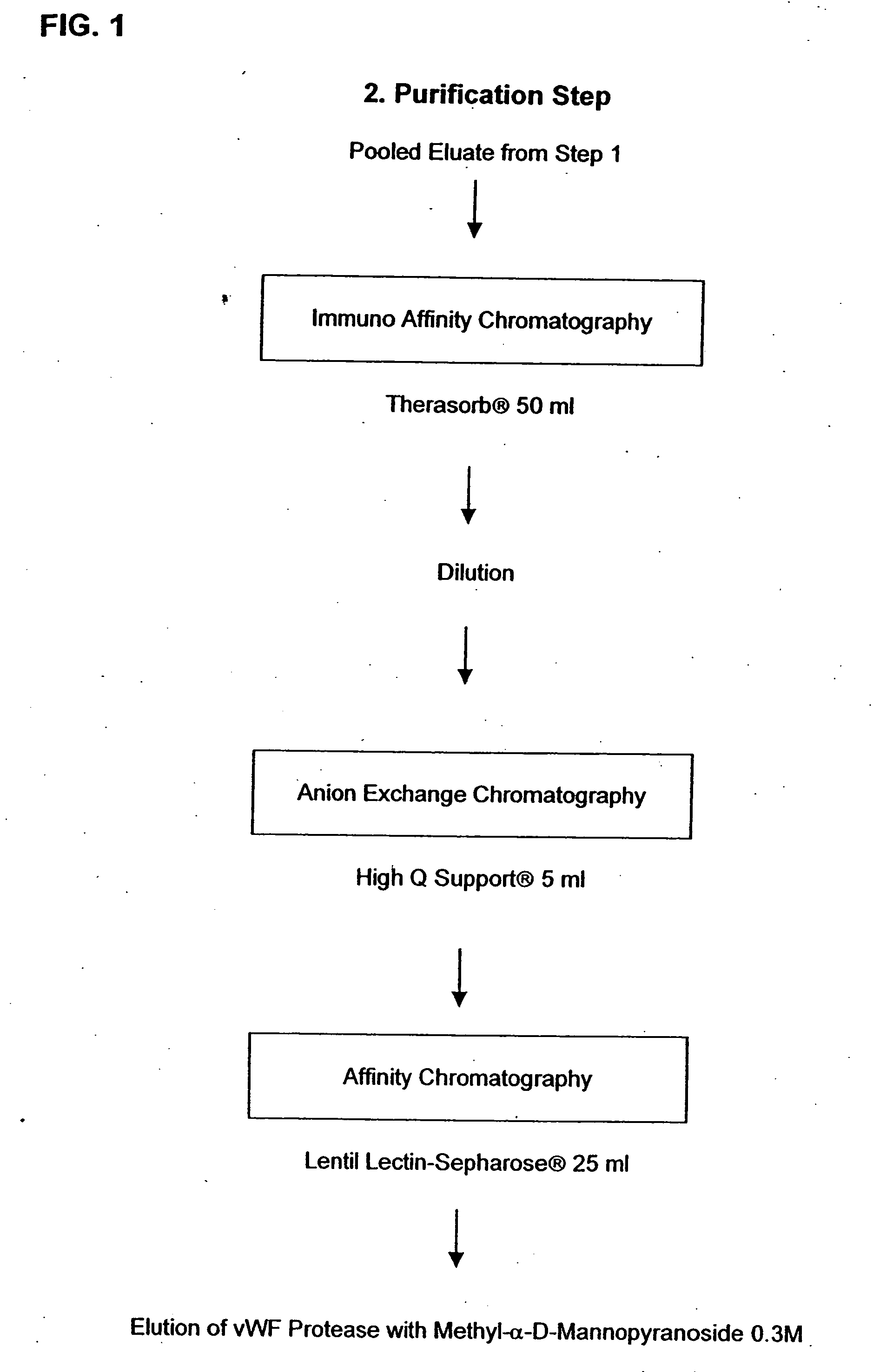
Composition exhibiting a von Willebrand factor (vWF) protease activity comprising a polypeptide chain with the amino acid sequence AAGGILHLELLV
InactiveUS20050266528A1Immunoglobulins against blood coagulation factorsFactor VIIFactor VIII vWFProteinase activity
Owner:BAXALTA INC
Lentiviral vectors featuring liver specific transcriptional enhancer and methods of using same
Recombinant lentiviruses and transfer vectors for transgene delivery as well as methods for gene therapy using such vectors are disclosed. The invention provides a third generation lentiviral packaging system and a set of vectors for producing recombinant lentiviruses, as well as novel tissue specific enhancer and promoter elements useful for optimizing liver specific transgene delivery. The transgene is preferably a blood clotting factor such as human factor IX (hFIX) or human factor VIII (hFVIII) and can be used for treatment of hemophilia.
Owner:MILTENYI BIOTEC B V & CO KG
Optimized messenger RNA
InactiveUS20080076174A1Broaden applicationEliminates issue concerning patient complianceFactor VIISugar derivativesMessenger RNAComputational biology
The present invention is directed to a synthetic nucleic acid sequence which encodes a protein wherein at least one non-common codon or less-common codon is replaced by a common codon. The synthetic nucleic acid sequence can include a continuous stretch of at least 90 codons all of which are common codons.
Owner:SHIRE HUMAN GENETIC THERAPIES INC
Acryloyloxyethylphosphorylcholine Containing Polymer Conjugates and Their Preparation
InactiveUS20100166700A1Prevents and reduces efficiencyExtended half-lifeFactor VIINervous disorderPolymer sciencePharmaceutical drug
The present invention relates to polymeric reagents and conjugates thereof, methods for synthesizing the polymeric reagents and conjugates, pharmaceutical compositions comprising the conjugates and methods of using the polymer conjugates including therapeutic methods where conjugates are administered to patients.
Owner:KODIAK SCI
Factor viii chimeric and hybrid polypeptides, and methods of use thereof
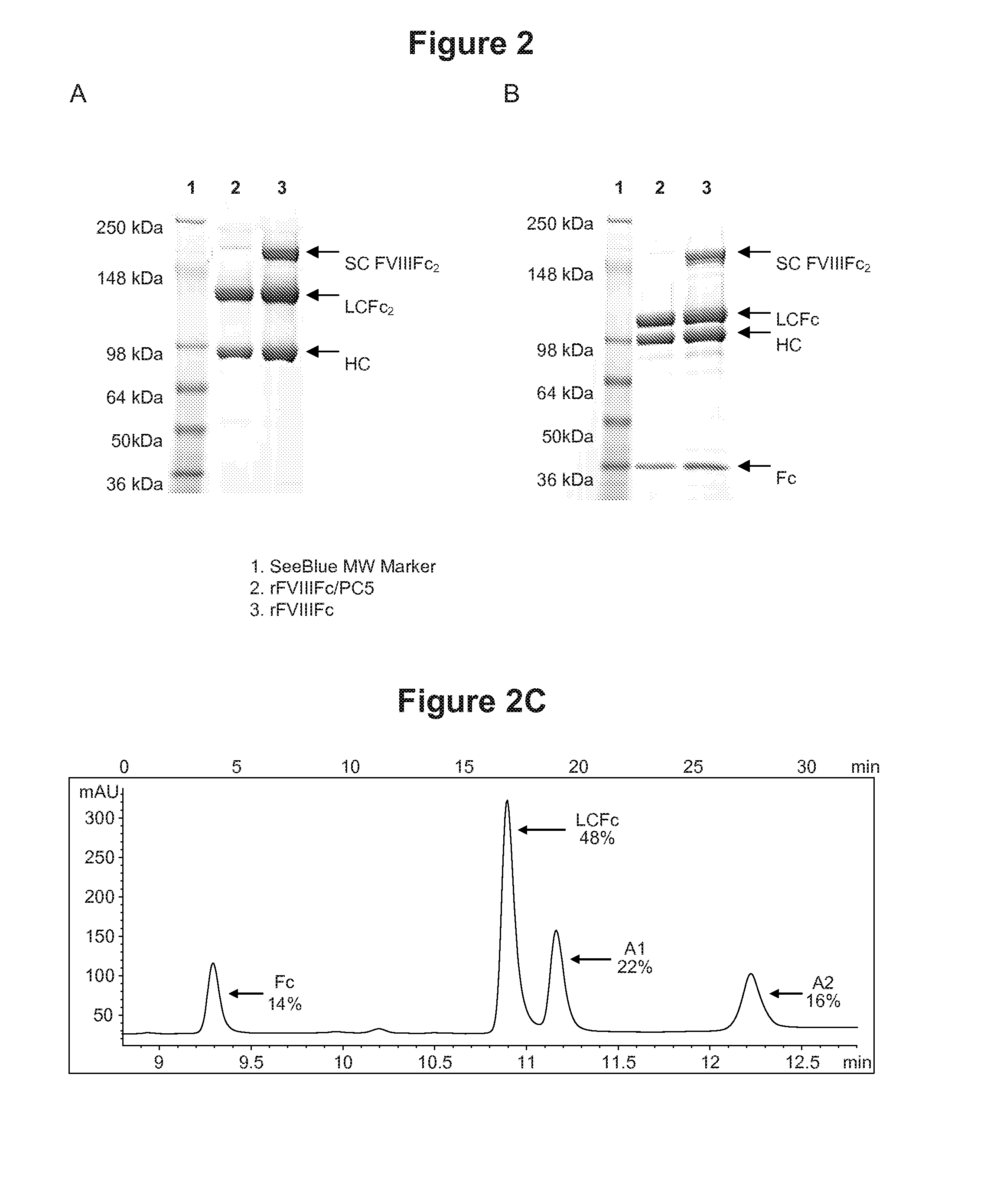
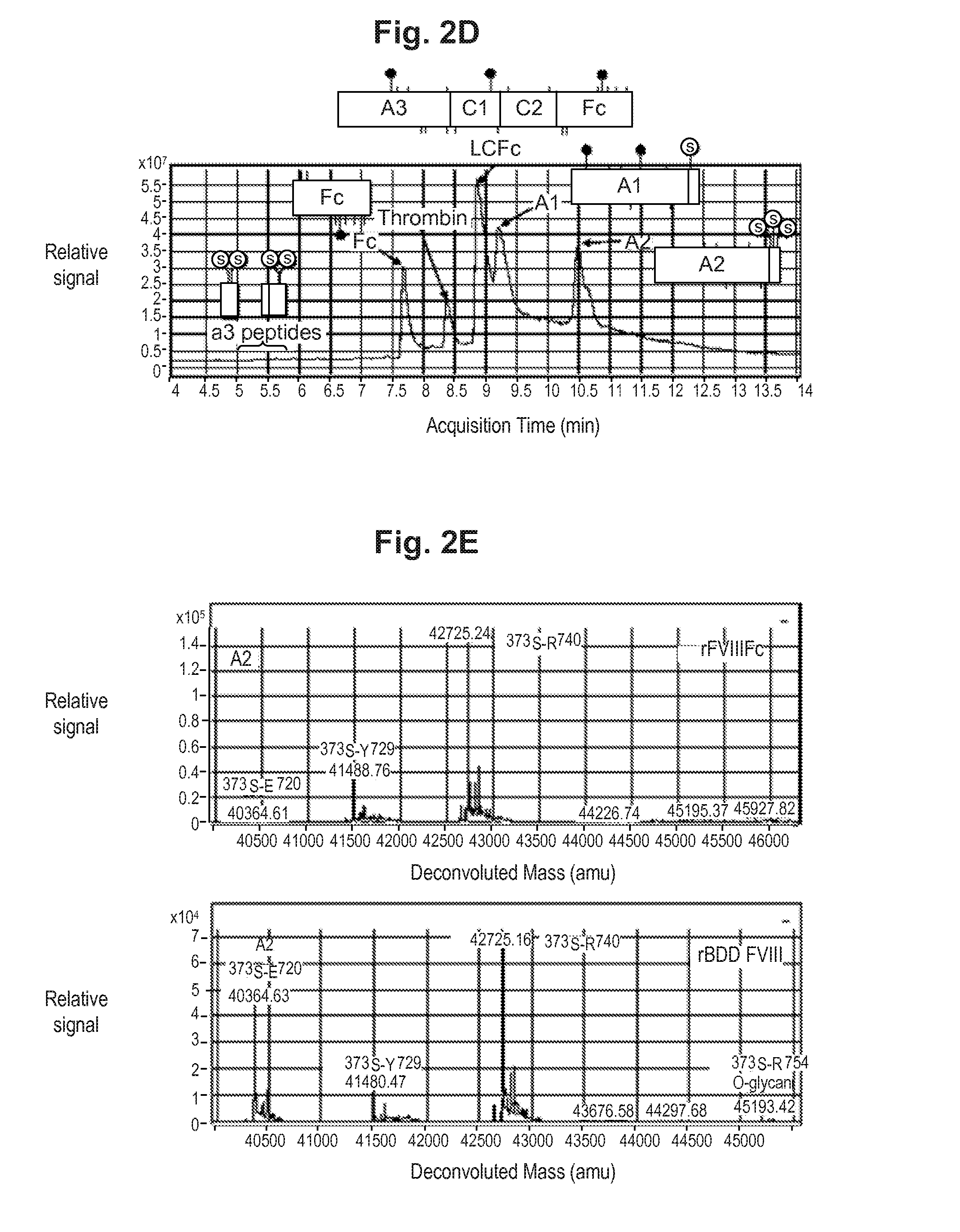
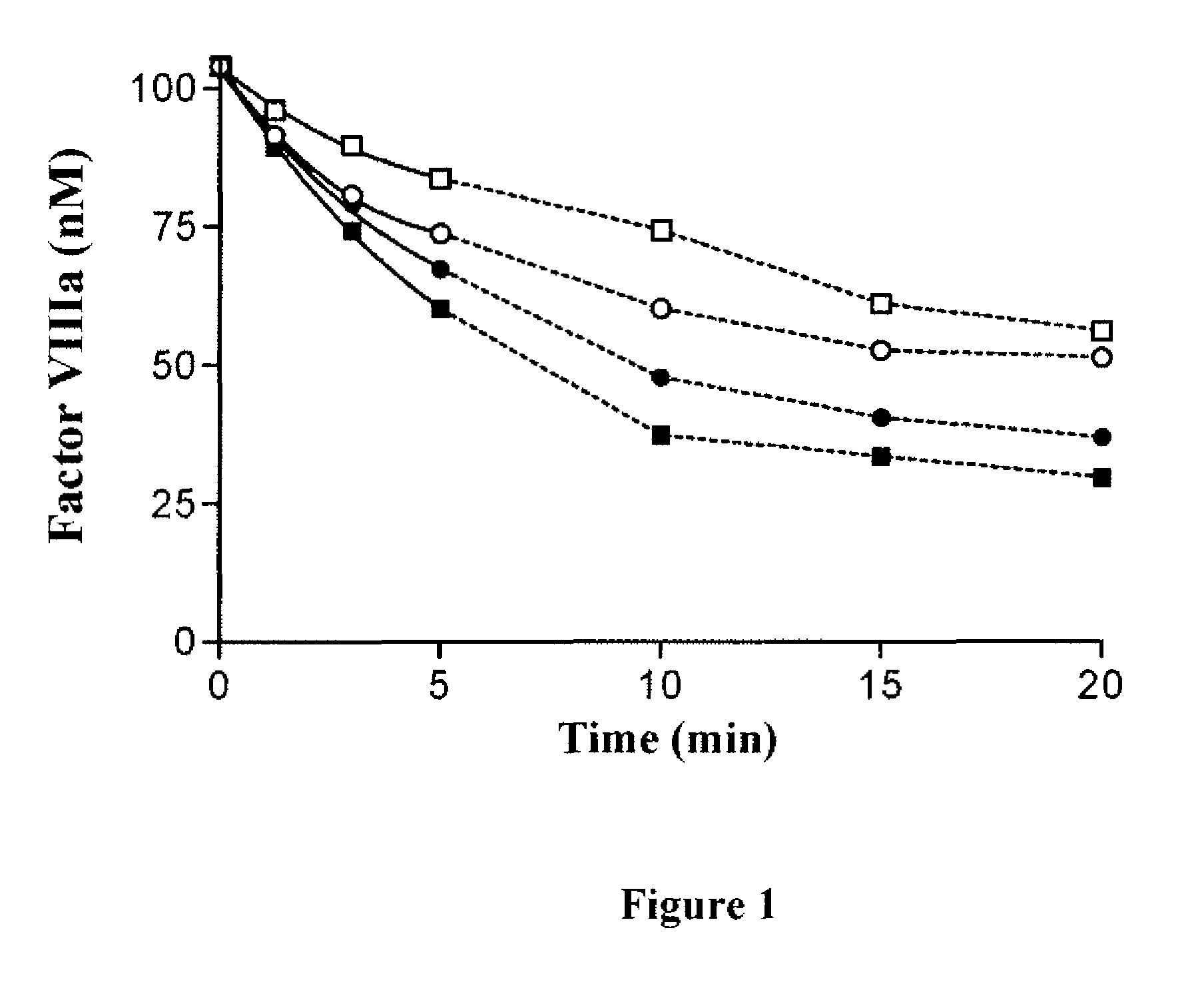
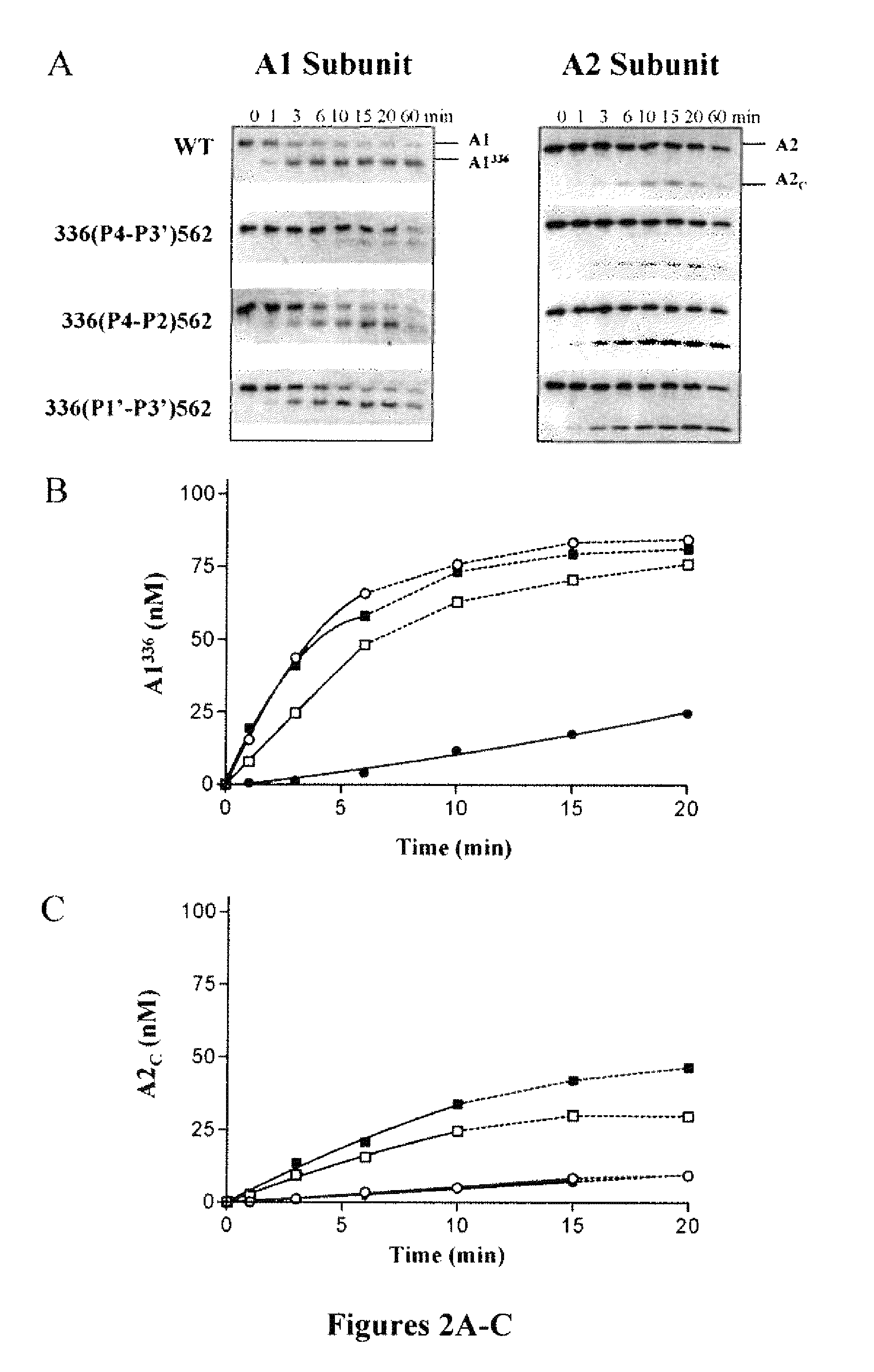
ActiveUS20140294821A1Evaluating efficacyEvaluate FVIIIFc consumptionFactor VIIPeptide/protein ingredientsNucleotidePolynucleotide
The present invention provides methods of administering Factor VIII (processed FVIII, single chain FVIII, or a combination thereof); methods of administering chimeric and hybrid polypeptides comprising Factor VIII; chimeric and hybrid polypeptides comprising Factor VIII; polynucleotides encoding such chimeric and hybrid polypeptides; cells comprising such polynucleotides; and methods of producing such chimeric and hybrid polypeptides using such cells
Owner:BIOVERATIV THERAPEUTICS INC
Recombinant factor VIII having reduced inactivation by activated protein C
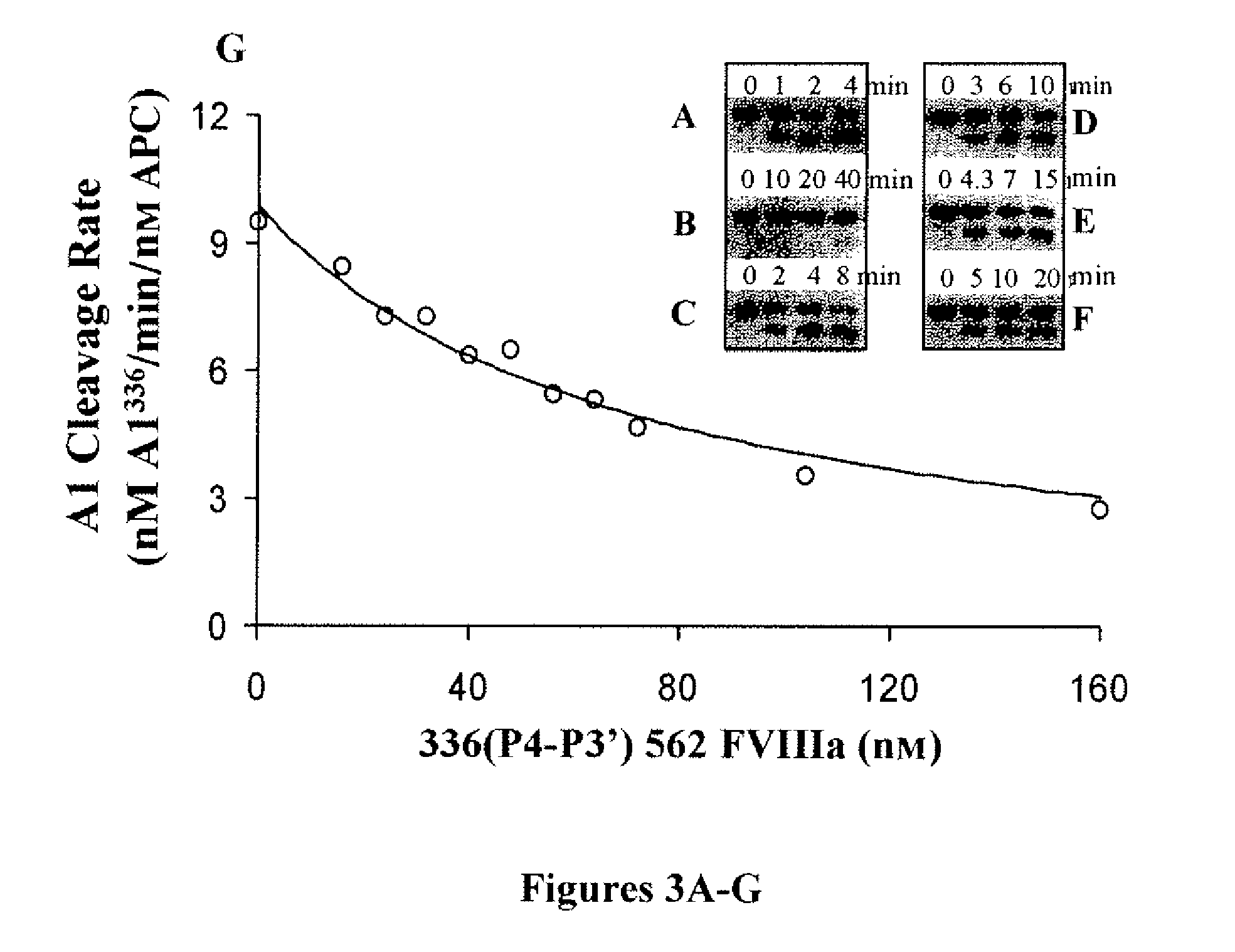
ActiveUS8183345B2High catalytic efficiencyPromote localizationFactor VIIBacteriaProtein activationClotting disorders
The present invention relates to a recombinant factor VIII that is characterized by one or more mutations within a region surrounding an activated protein C cleavage site, which one or more mutations result in a reduced rate of inactivation by activated protein C. Isolated nucleic acid molecules, recombinant expression vectors, and host cells suitable for expression of the recombinant factor VIII are also disclosed. The recombinant factor VIII can be used for the treatment of clotting disorders, such as hemophilia A.
Owner:UNIVERSITY OF ROCHESTER
von Willebrand factor (vWF)-containing preparation, process for preparing vWF-containing preparations, and use of such preparations
A high-purity von Willebrand factor preparation, a process for making it, and use of the preparation and compositions containing it for the treatment of disorders are disclosed.
Owner:GE HEALTHCARE BIOPROCESS R&D +1
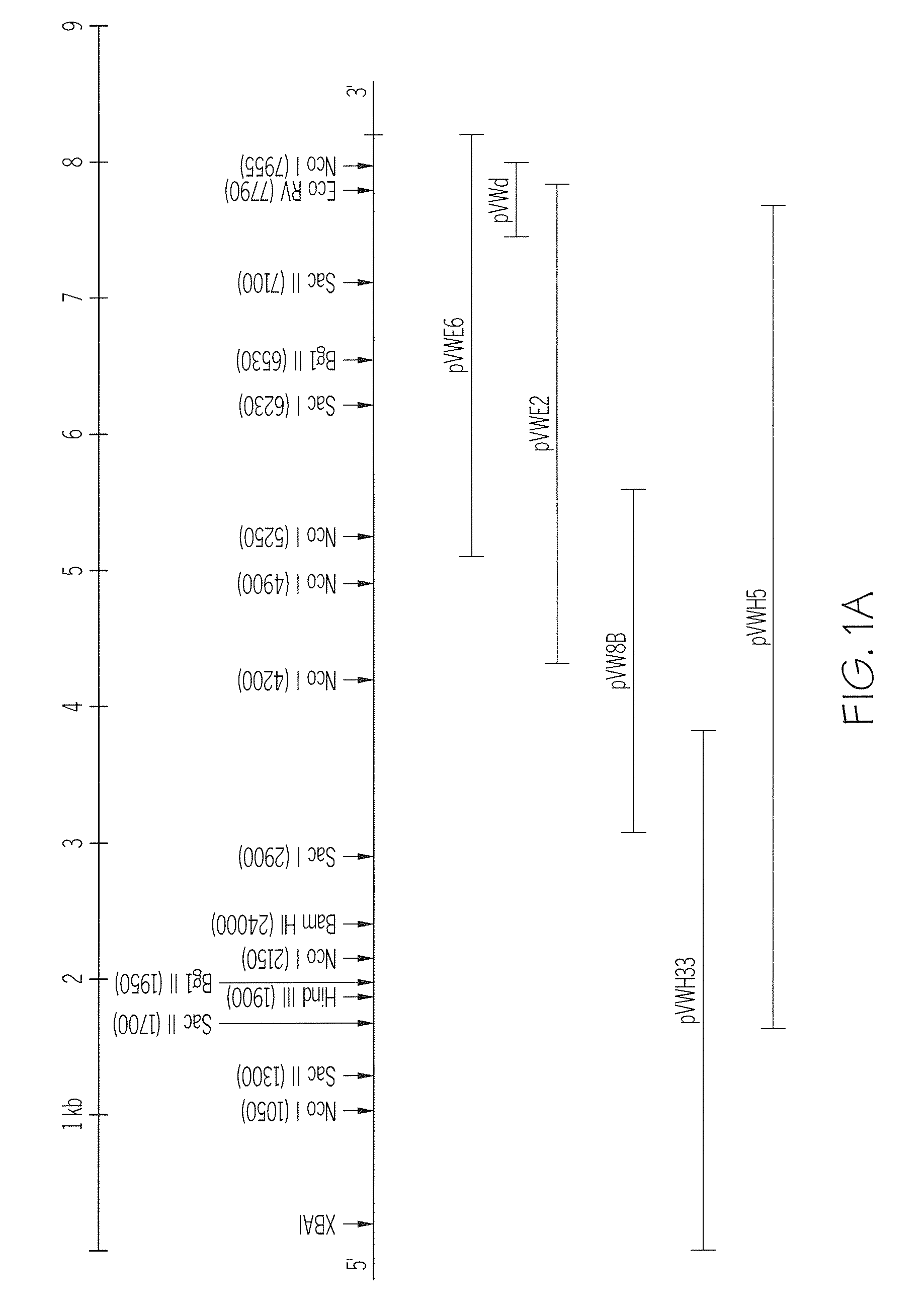
DNA encoding Von Willebrand Factor (VWF) and methods and cells for producing VFW, and VFW produced by the DNA, methods and cells
Von Willebrand's Factor (VWF) is produced using an expression vector that includes: 1) a DNA sequence encoding a functional VWF protein; and 2) regulatory DNA capable of effecting expression of that DNA sequence in a host cell transformed with the vector. Restriction fragment length polymorphisms (RFLP's) associated with the VWF gene are identified and used in a probe for determining the source of a VWF gene in a DNA sample. The gene for VWF is localized to the short arm of human chromosome 12 (12p).
Owner:CHILDRENS MEDICAL CENT CORP
Haemostatic composition comprising hyaluronic acid
The present invention relates to a haemostatic composition comprising a biologically absorbable material and hyaluronic acid or a derivative thereof, methods of producing such compositions and the use of these compositions. In particular the method of producing said haemostatic composition comprises treating it with dry heat at a temperature between 110-200° C.
Owner:FERROSAN MEDICAL DEVICES
Stabilization of aqueous compositions of proteins with displacement buffers
InactiveUS20100028372A1Improve stabilityImproves pH stabilityFactor VIIPeptide/protein ingredientsAnalytical chemistryProtein stability
An aqueous composition having increased protein stability is obtained by: a. determining a pH at which the protein has stability at the desired temperature; b. adding to the composition at least one displacement buffer wherein the displacement buffer has a pKa that is at least 1 unit greater or less than the pH of step (a); and c. adjusting the pH of the composition to the pH of step (a); wherein the aqueous composition does not comprise a conventional buffer at a concentration greater than about 2 mM and wherein the conventional buffer has a pKa that is within 1 unit of the pH of step (a).
Owner:ARECOR LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com