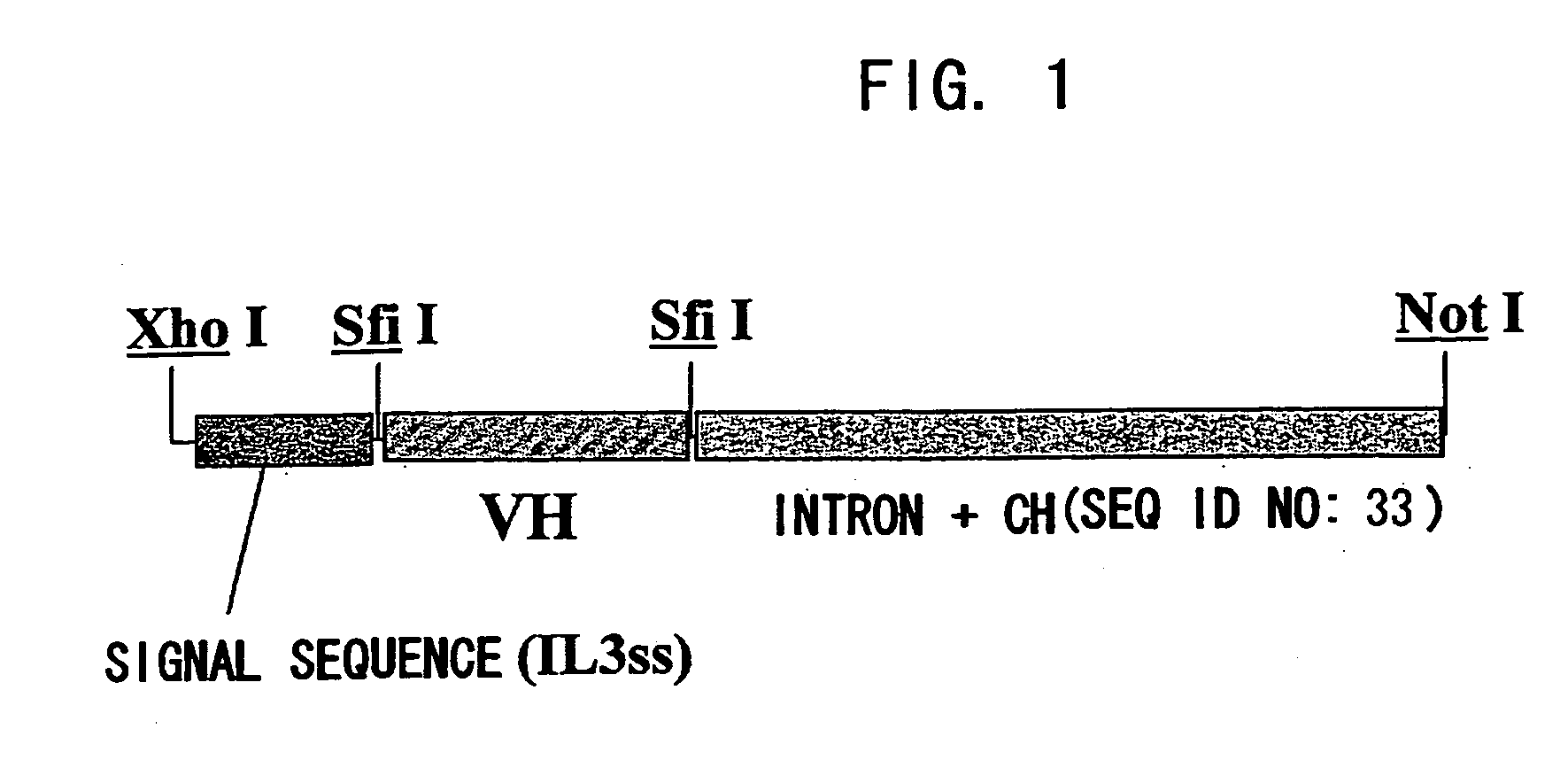
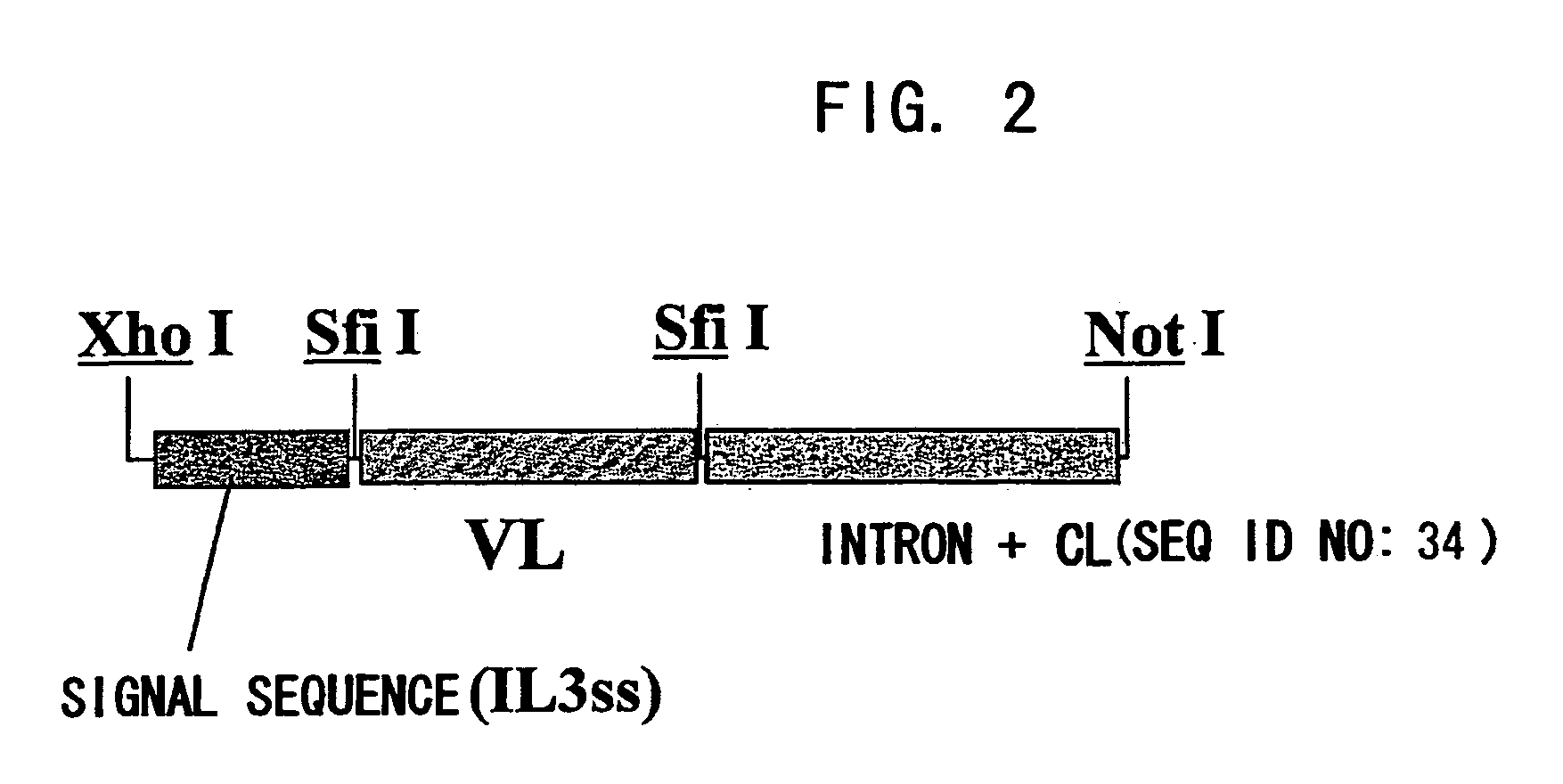
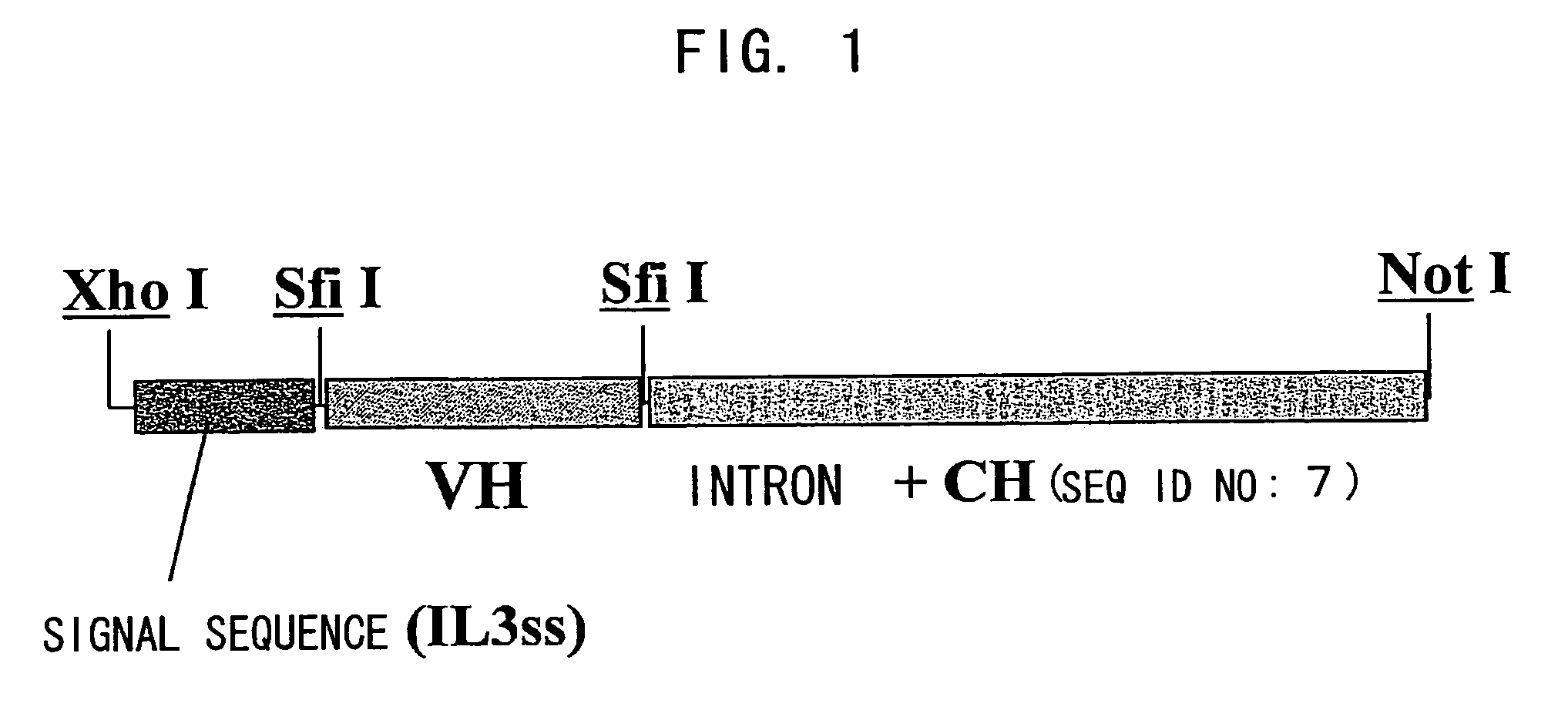
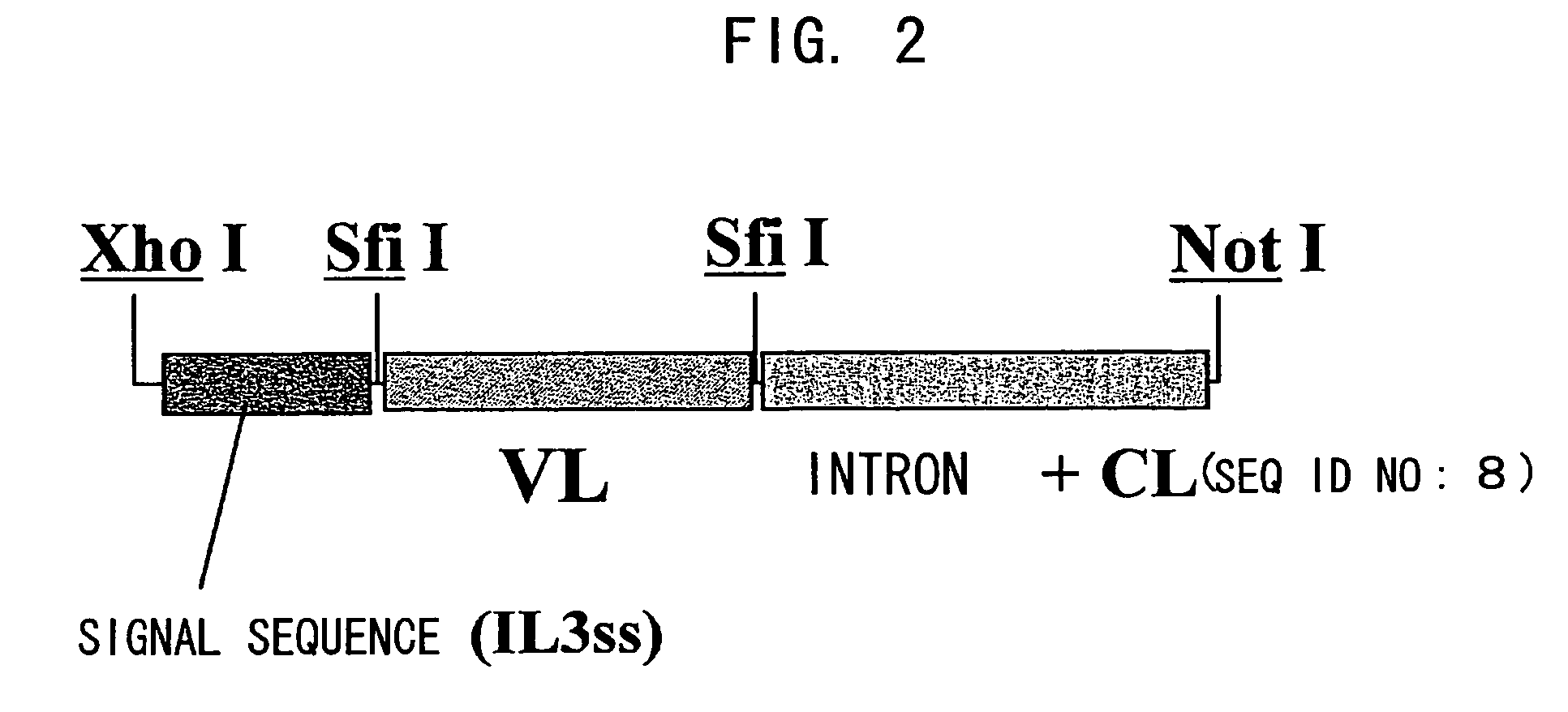
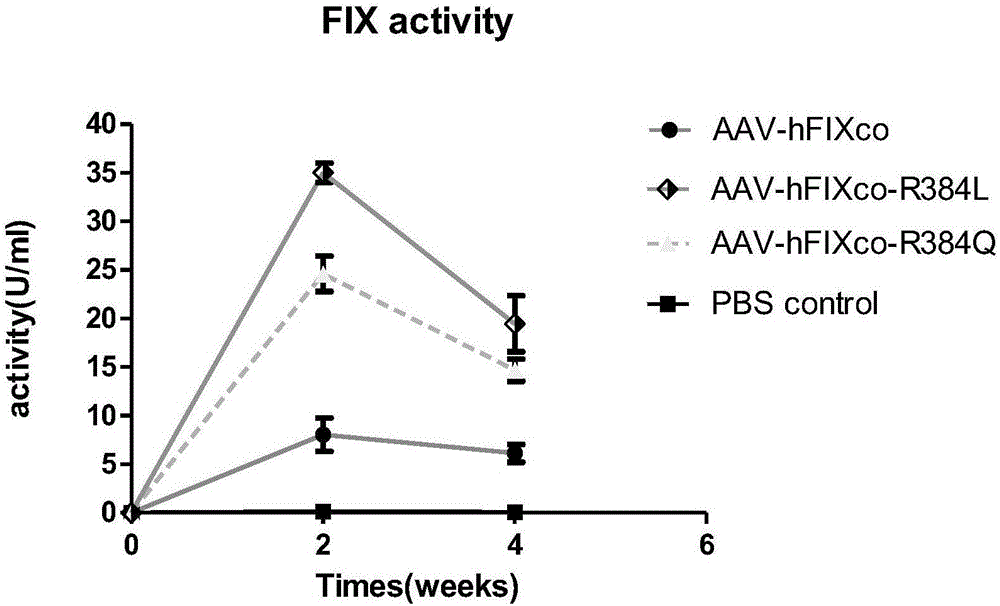
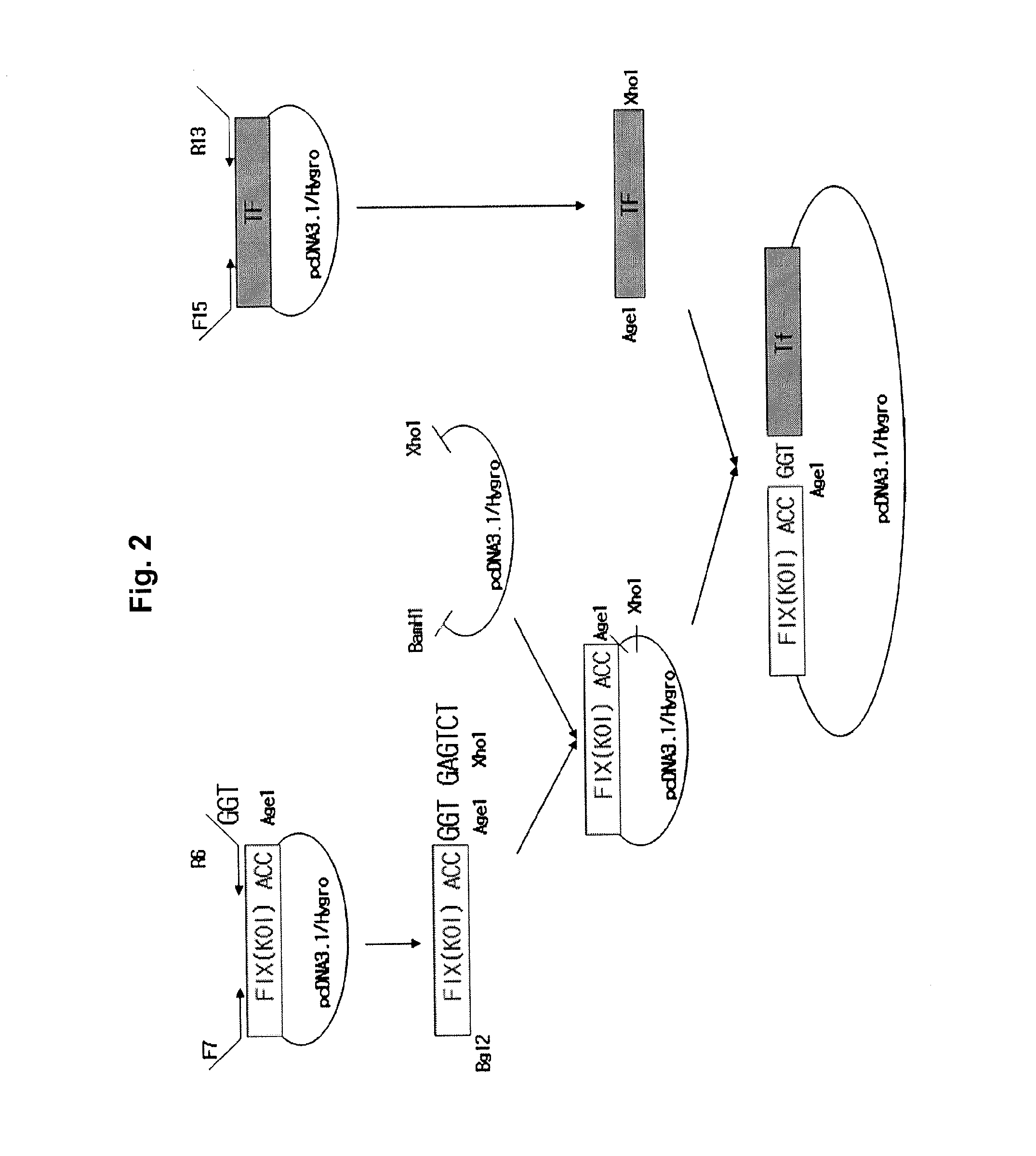
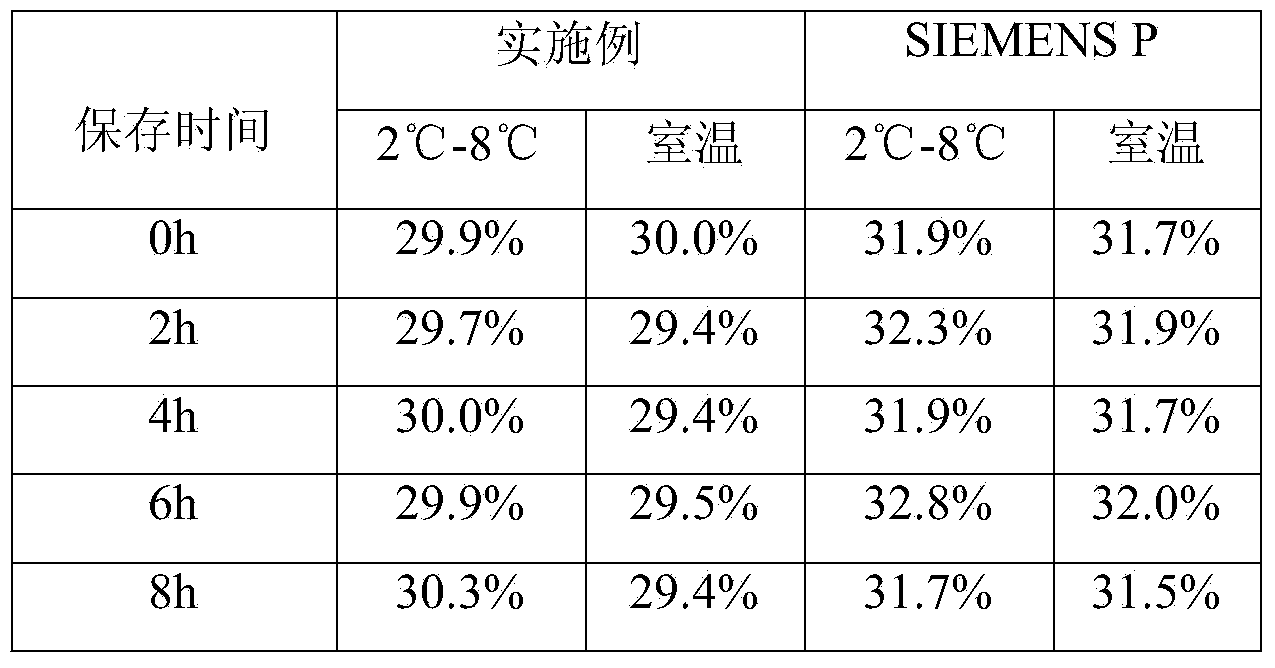
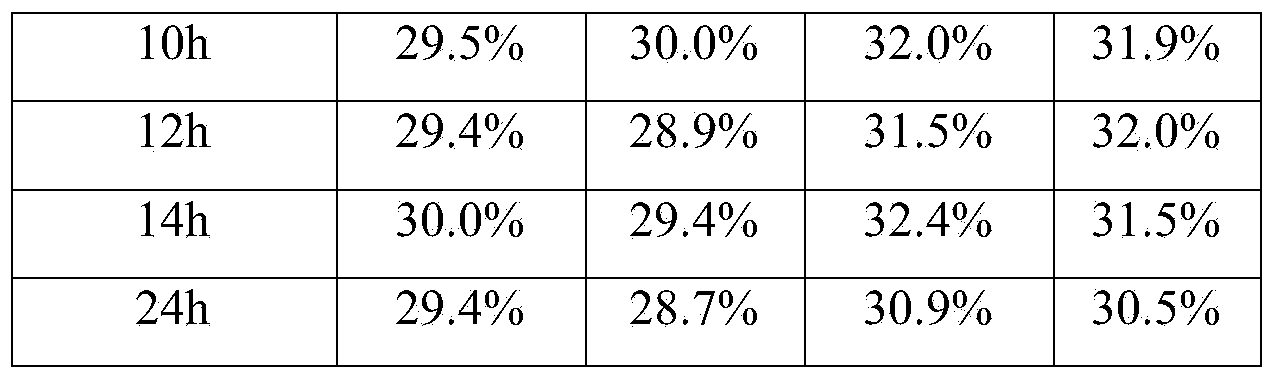
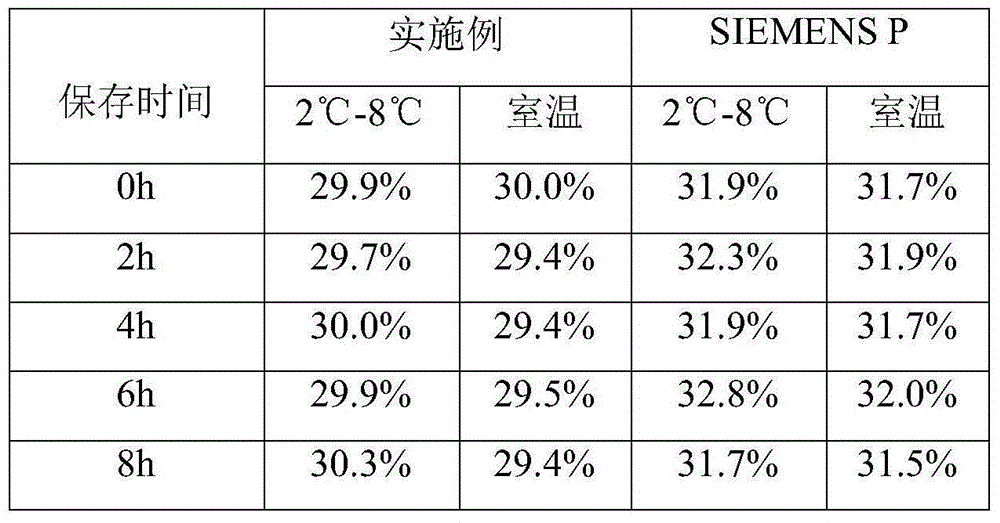
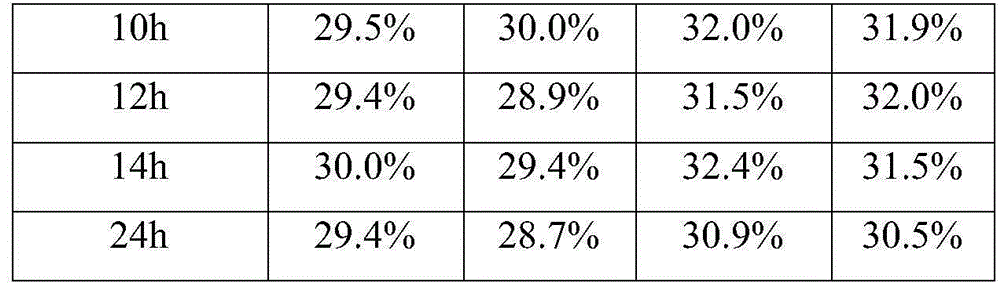
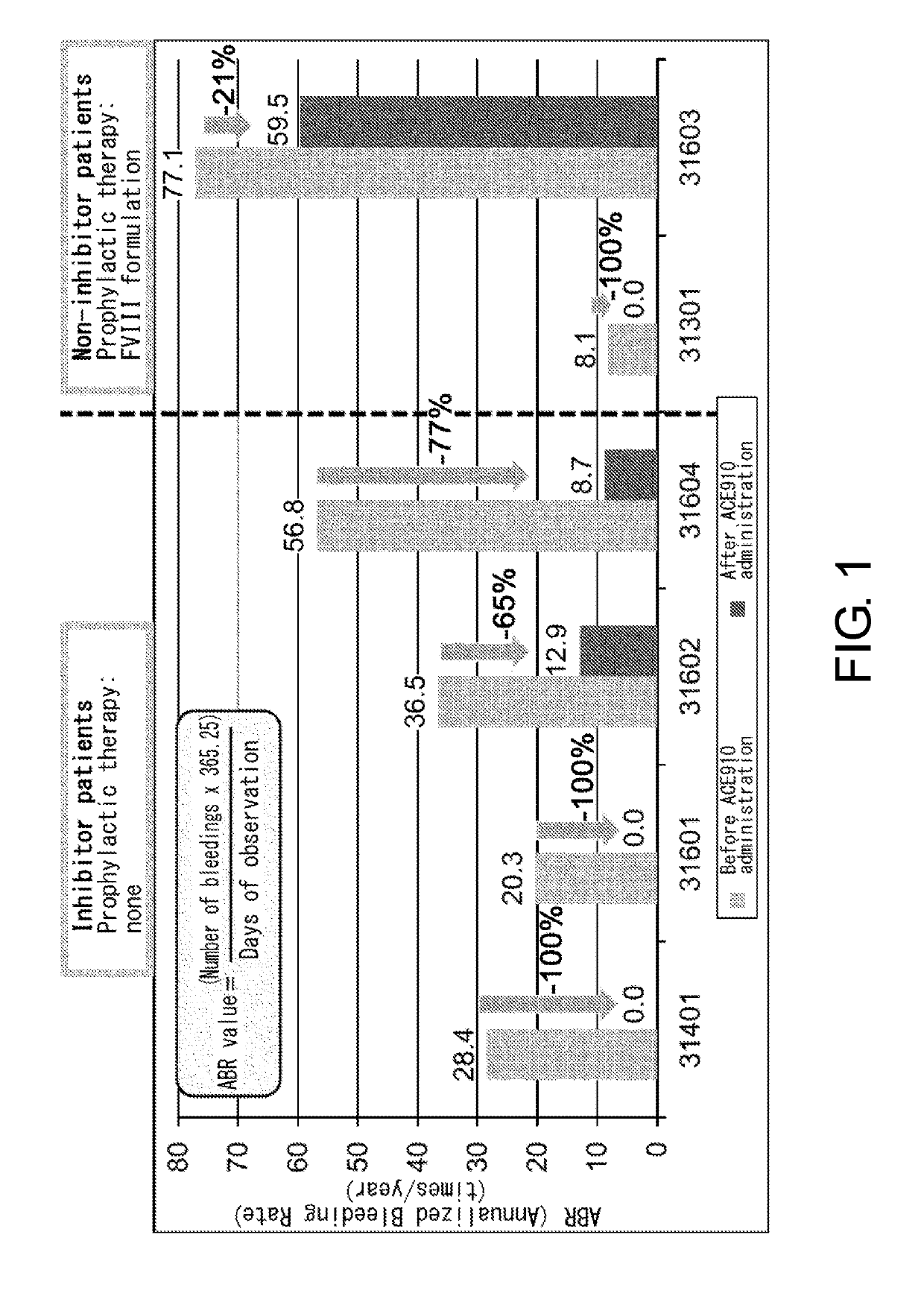
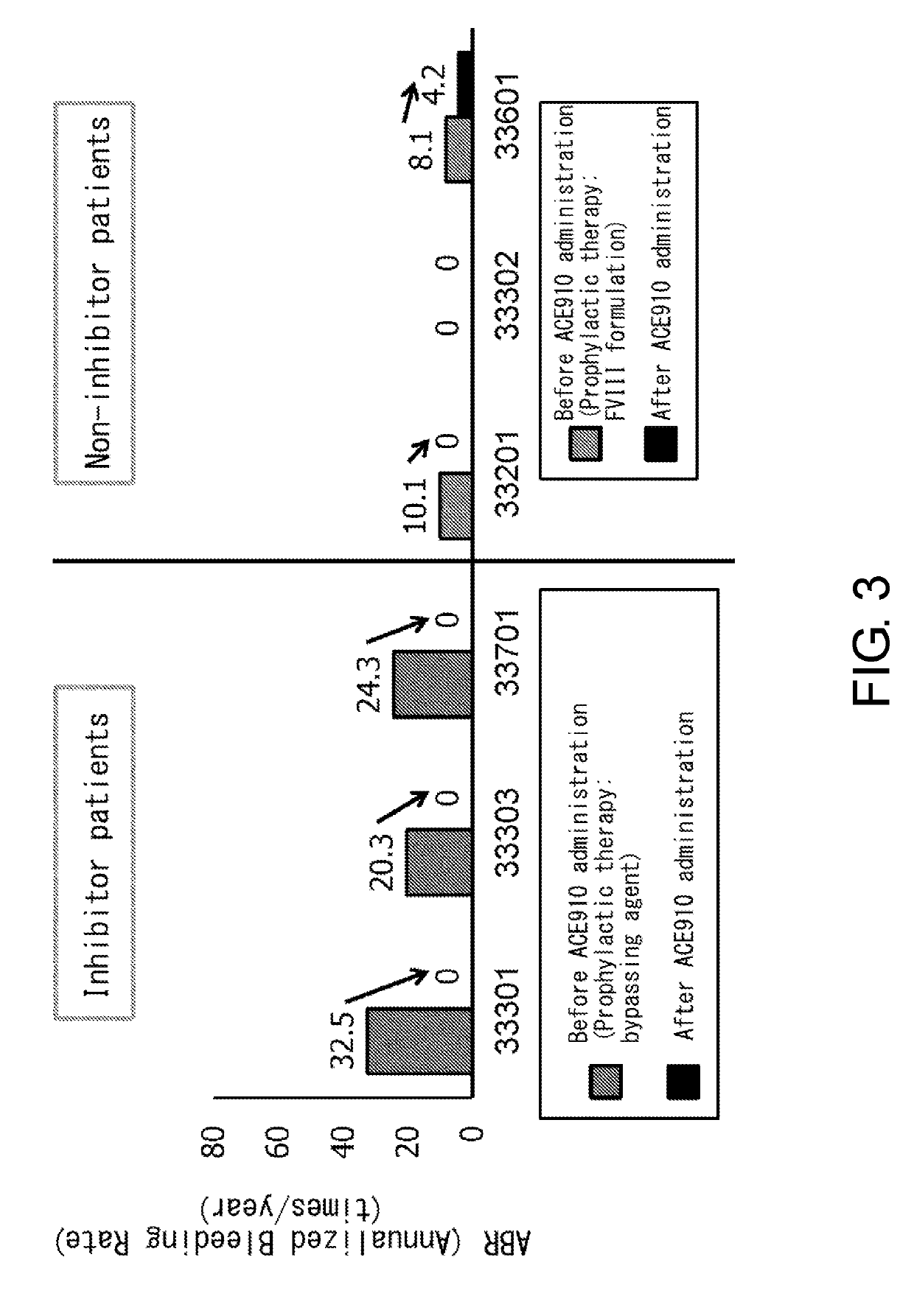
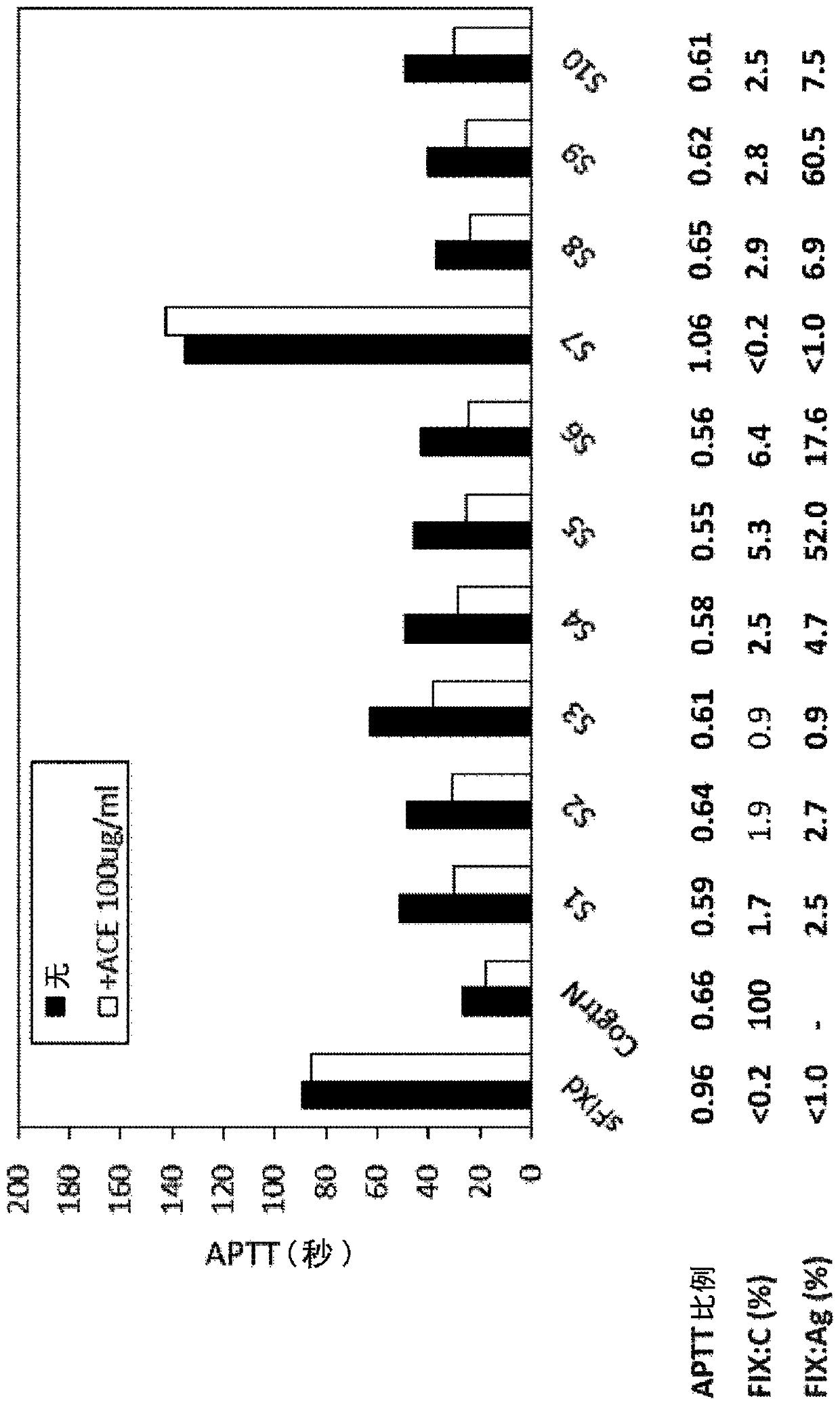
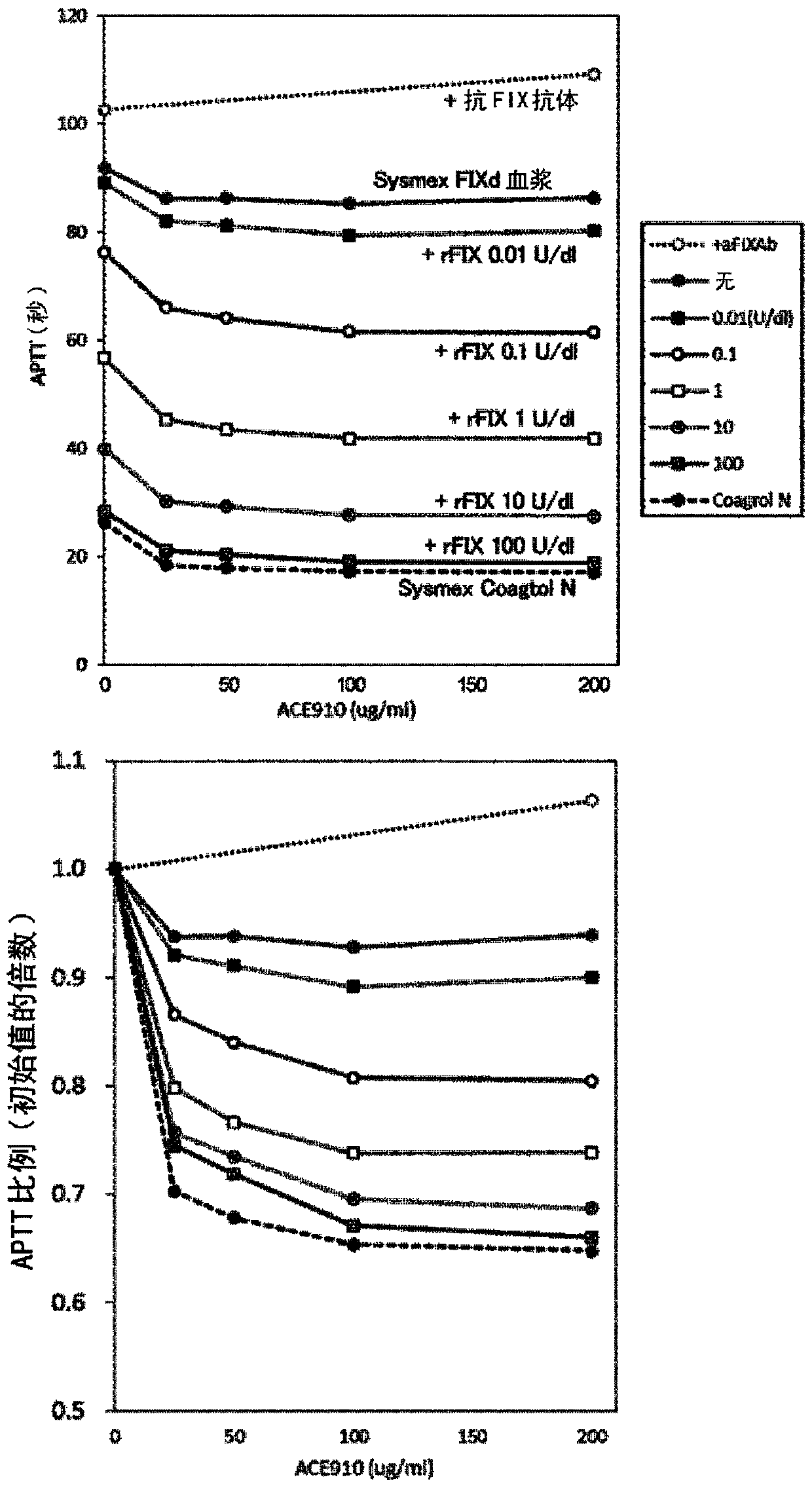
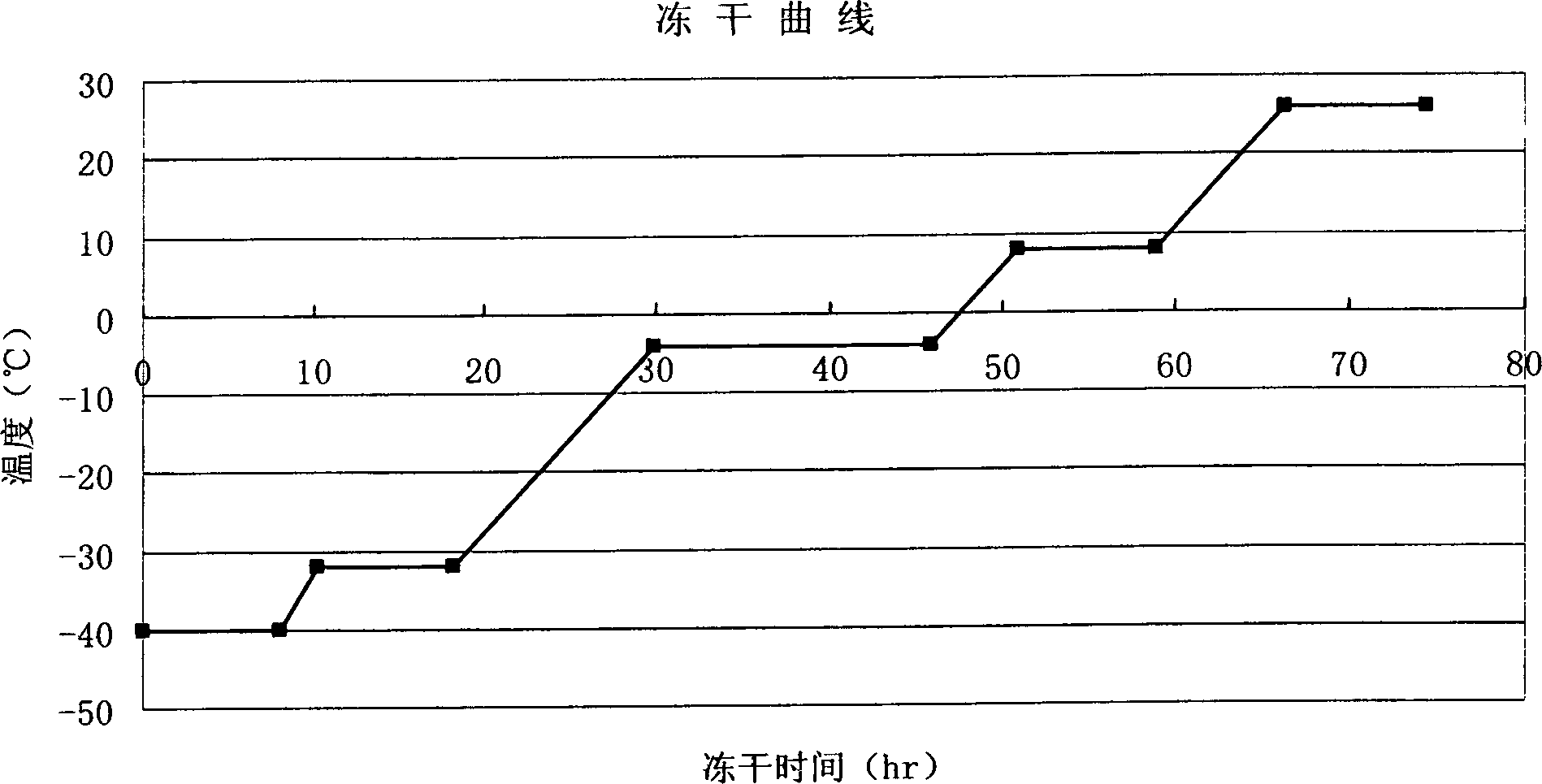
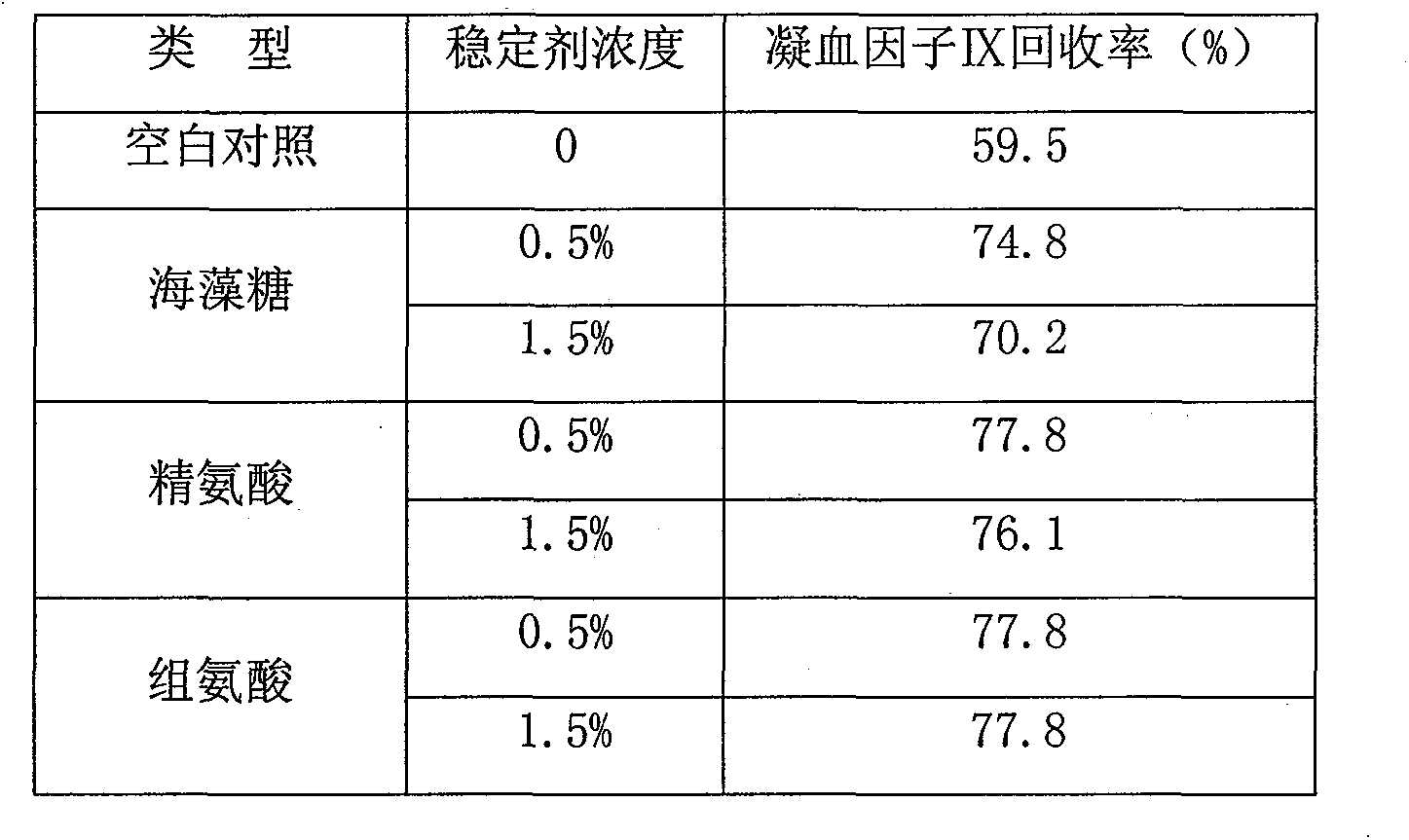
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Blood coagulation factor IX" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
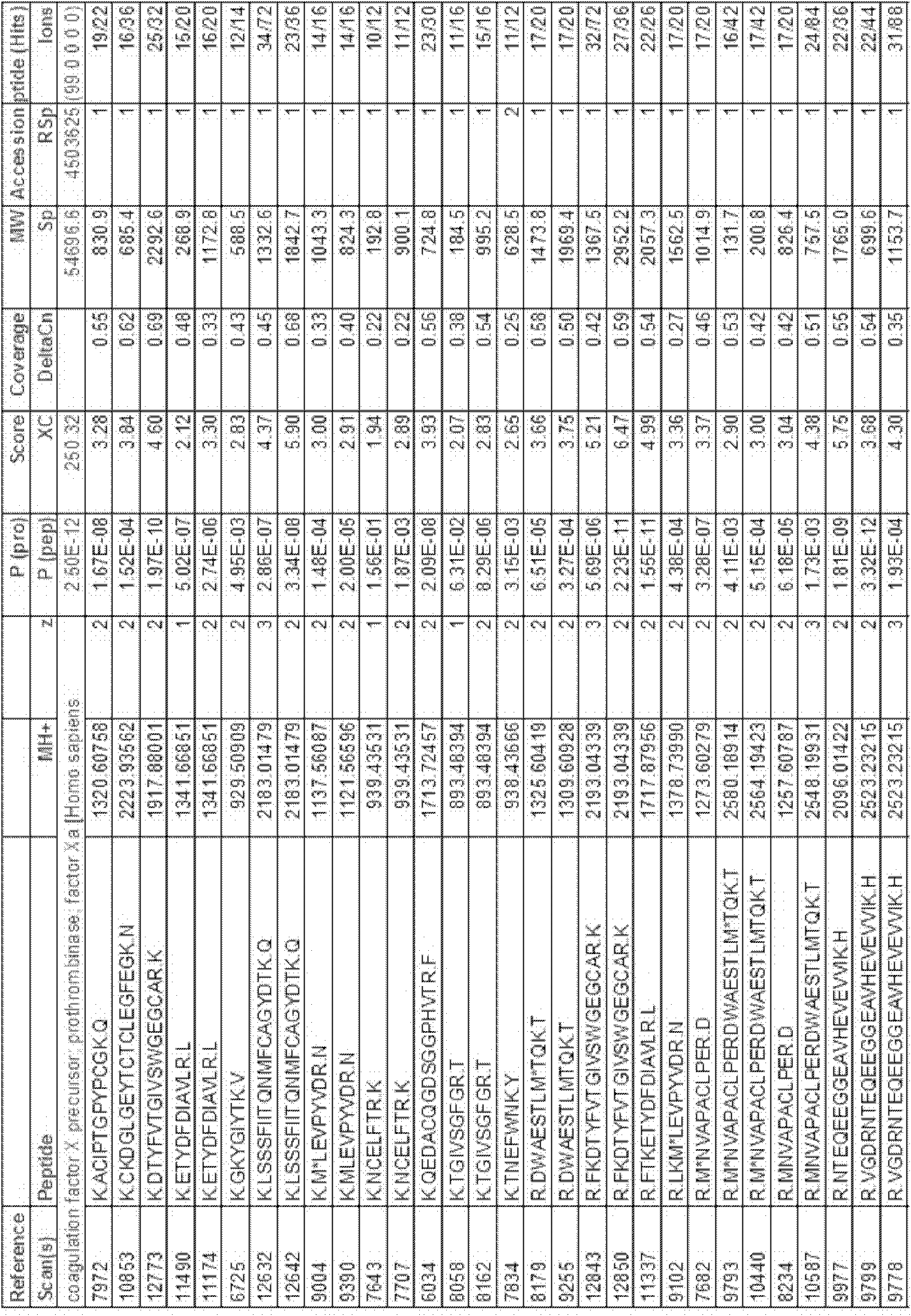
Coagulation factor IX, also known as clotting factor IX, is a natural protein found in the human body. This protein is produced by the liver and is one of several different proteins that help the body to form blood clots.
Bispecific antibody substituting for functional proteins
ActiveUS20070041978A1Enhances enzymatic reactionImmunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIBlood Coagulation Factor X
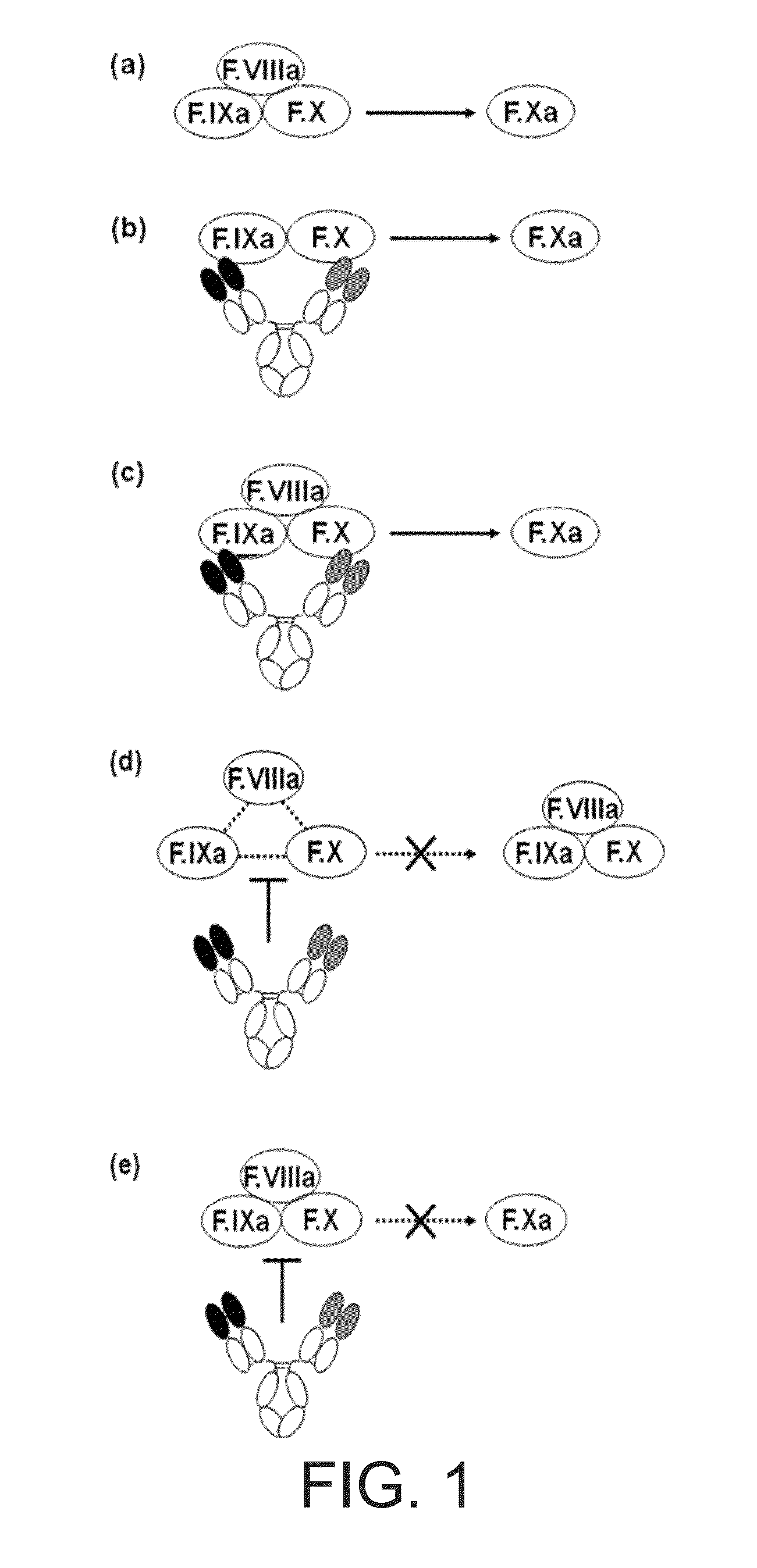
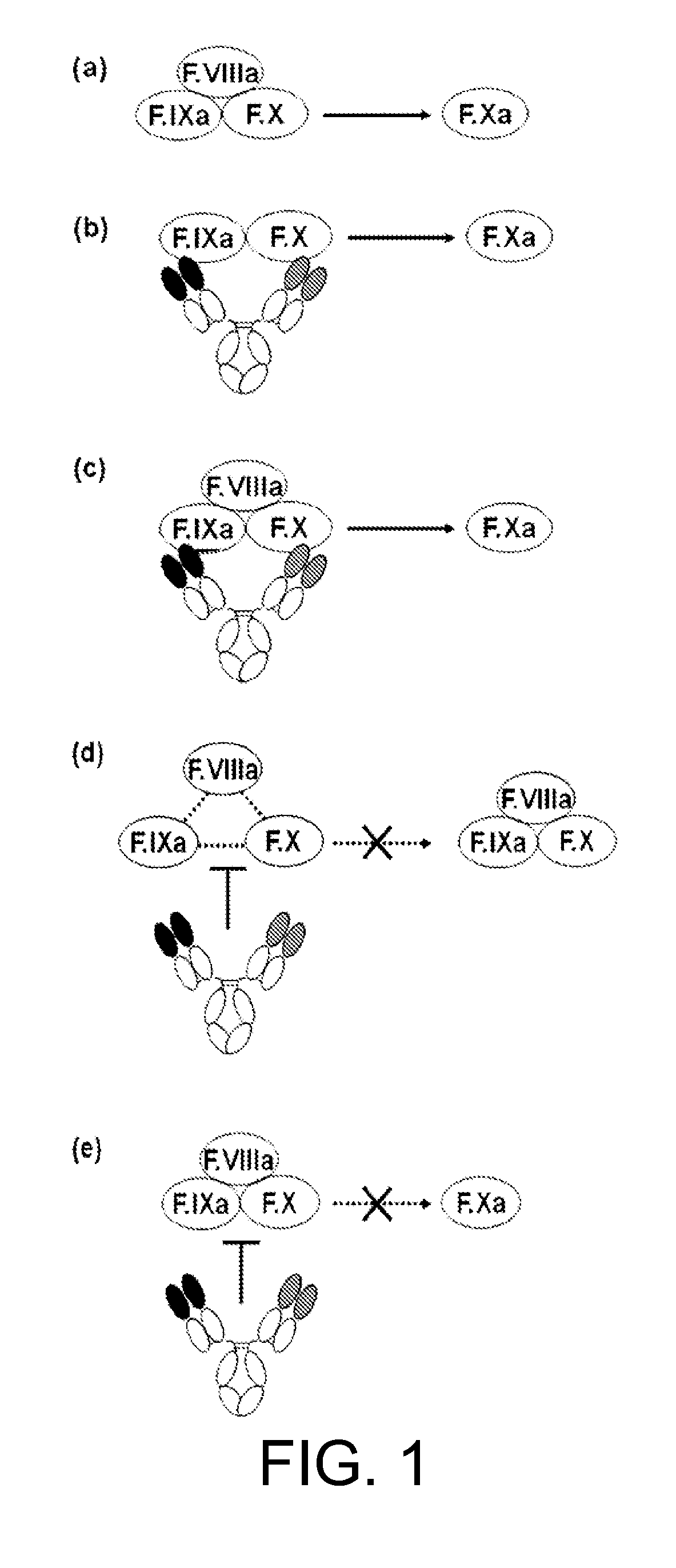
The present inventors succeeded in constructing bispecific antibodies, which bind to both the blood coagulation factor IX / activated blood coagulation factor IX and blood coagulation factor X, and functionally substitute for blood coagulation factor VIII / activated blood coagulation factor VIII which enhances the enzymatic reaction.
Owner:CHUGAI PHARMA CO LTD
Double Specific Antibodies Substituting For Functional Proteins
InactiveUS20080075712A1Reduced activityOutstanding sustainabilityImmunoglobulins against blood coagulation factorsNervous disorderBlood coagulation factor VIIIBlood Coagulation Factor X
The present inventors succeeded in separating bispecific antibodies that functionally substitute for ligands of type I interferon receptors comprising two types of molecules: AR1 chain and AR2 chain. Furthermore, the present inventors succeeded in producing bispecific antibodies that substitute for the enzyme reaction-accelerating function of blood coagulation factor VIII / activated blood coagulation factor VIII, which bind to both blood coagulation factor IX / activated blood coagulation factor IX and blood coagulation factor X.
Owner:CHUGAI PHARMA CO LTD
Bispecific antibody substituting for functional proteins
ActiveUS8062635B2Enhances enzymatic reactionImmunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIBlood Coagulation Factor X
The present inventors succeeded in constructing bispecific antibodies, which bind to both the blood coagulation factor IX / activated blood coagulation factor IX and blood coagulation factor X, and functionally substitute for blood coagulation factor VIII / activated blood coagulation factor VIII which enhances the enzymatic reaction.
Owner:CHUGAI PHARMA CO LTD
Multi-specific antigen-binding molecule having alternative function to function of blood coagulation factor viii
ActiveUS20130330345A1High activityLow F.Xase inhibitory actionImmunoglobulins against blood coagulation factorsAnimal cellsBlood coagulation factor VIIIAntigen
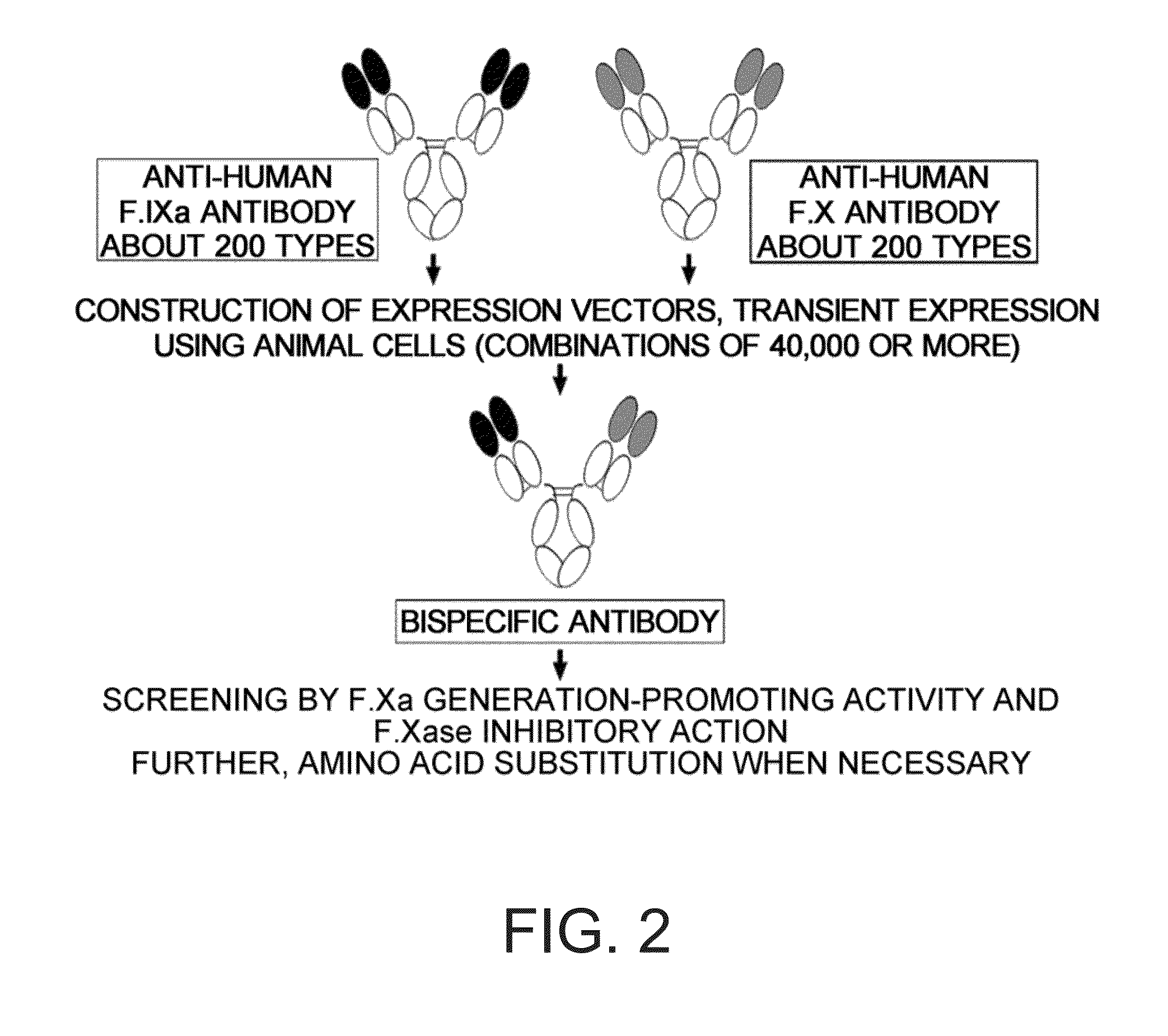
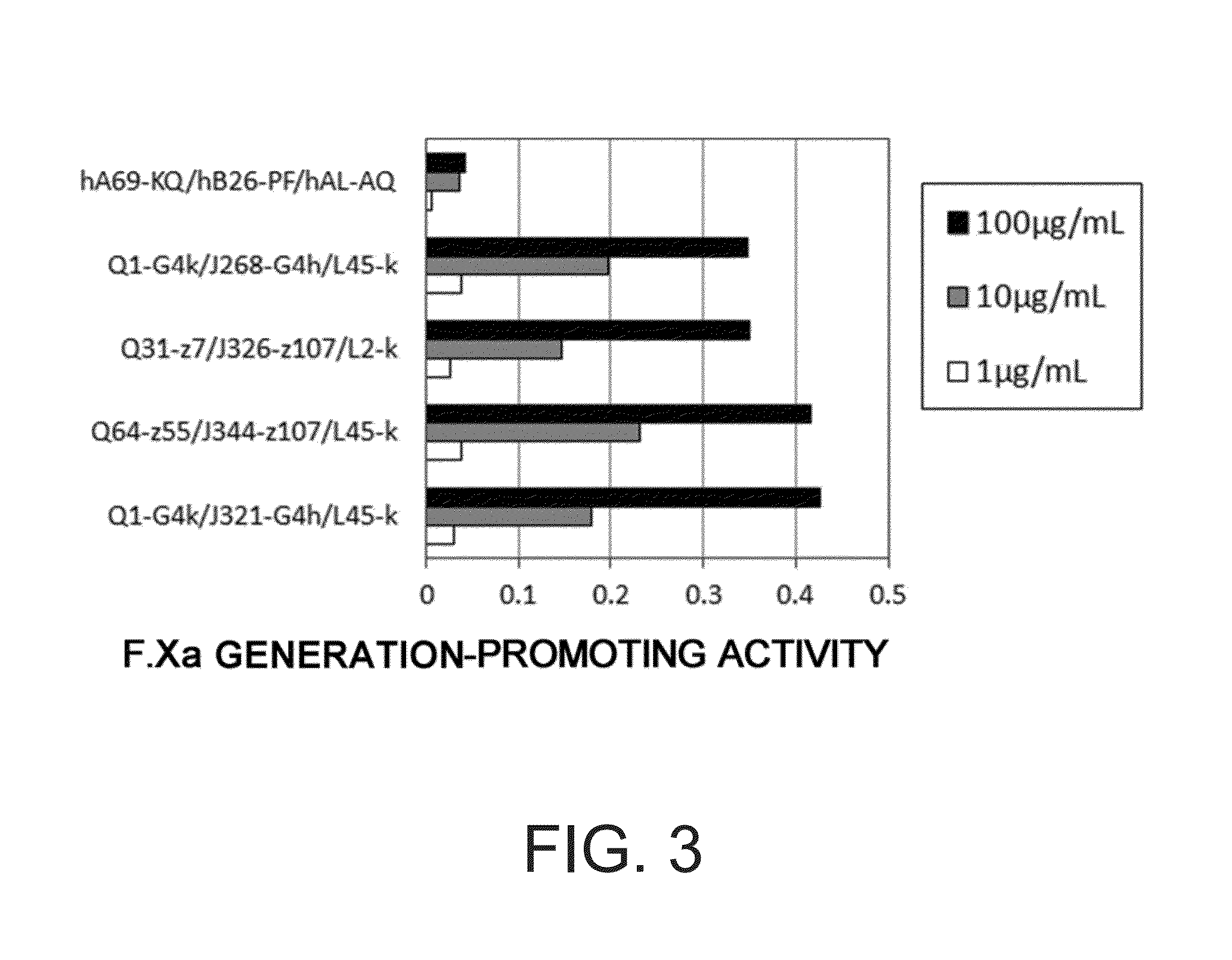
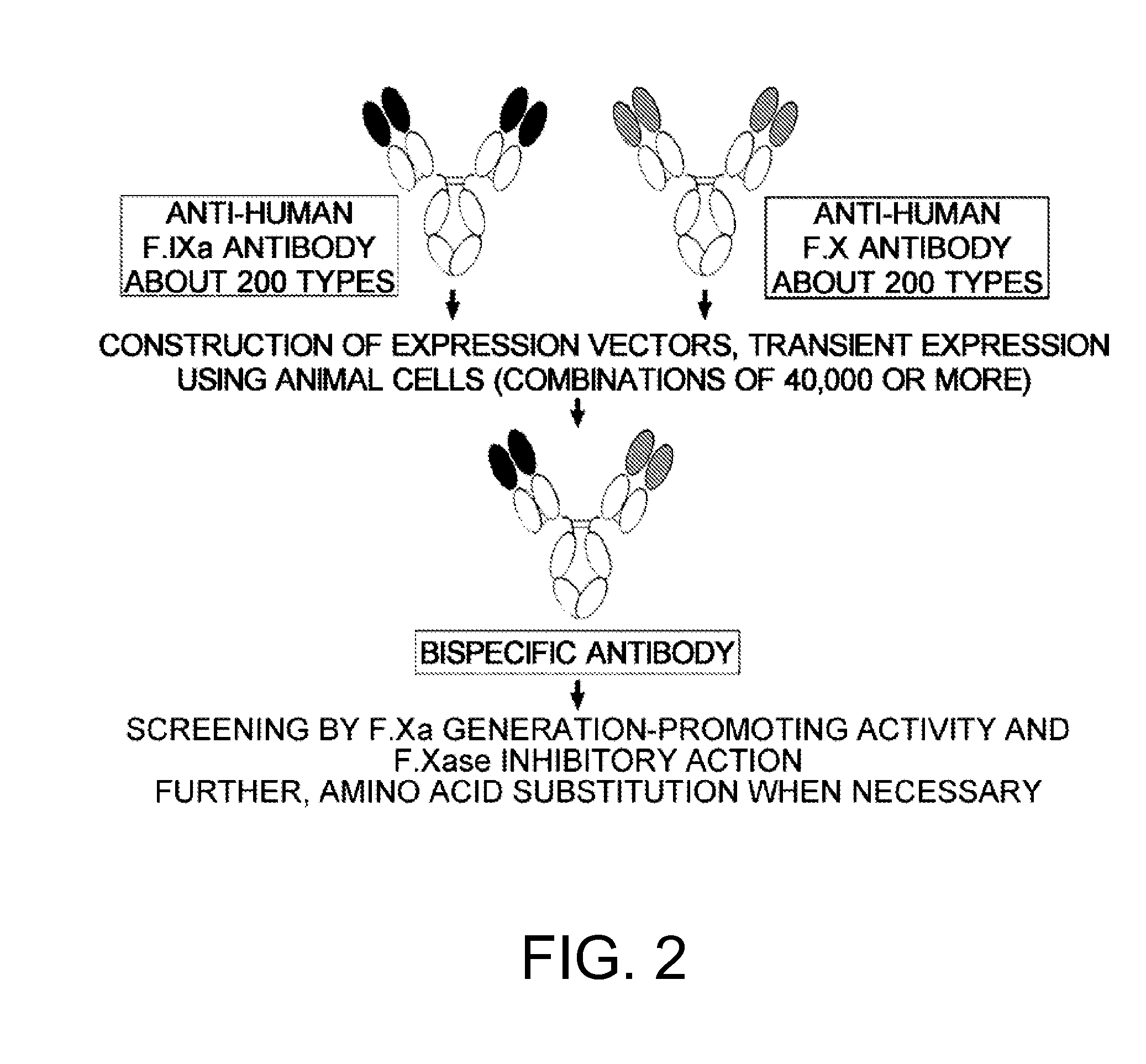
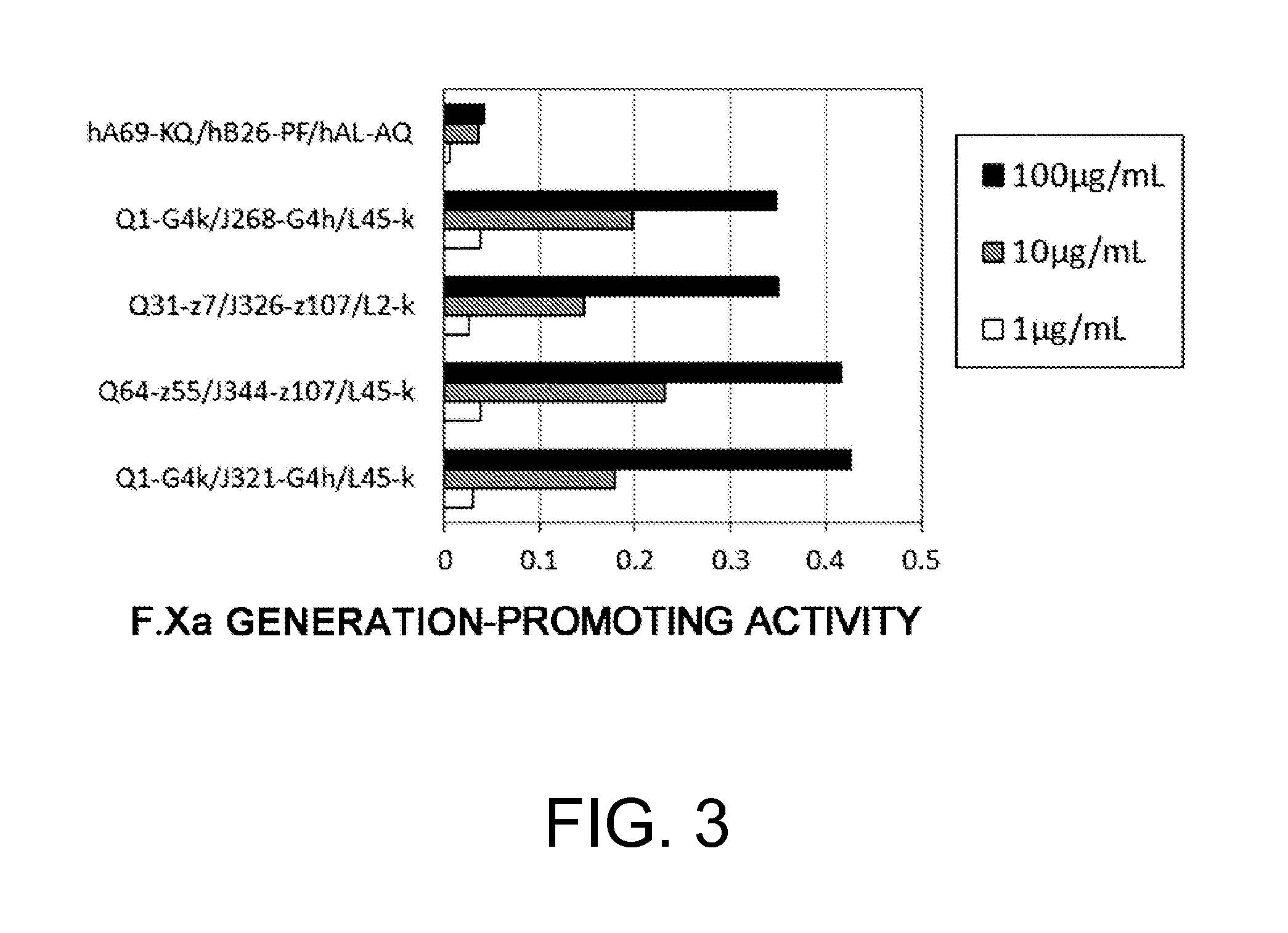
Various bispecific antibodies that specifically bind to both blood coagulation factor IX / activated blood coagulation factor IX and blood coagulation factor X and functionally substitute for the cofactor function of blood coagulation factor VIII, that is, the function to promote activation of blood coagulation factor X by activated blood coagulation factor IX, were produced. From these antibodies, multispecific antigen-binding molecules having a high activity of functionally substituting for blood coagulation factor VIII were successfully discovered.
Owner:CHUGAI PHARMA CO LTD
Multi-specific antigen-binding molecules and uses thereof
InactiveUS20140037632A1High activityHigh stability in bloodImmunoglobulins against blood coagulation factorsAntibody ingredientsBlood coagulation factor VIIIAntigen
Various bispecific antibodies that specifically bind to both blood coagulation factor IX / activated blood coagulation factor IX and blood coagulation factor X and functionally substitute for the cofactor function of blood coagulation factor VIII, that is, the function to promote activation of blood coagulation factor X by activated blood coagulation factor IX, were produced. From these antibodies, multispecific antigen-binding molecules having a high activity of functionally substituting for blood coagulation factor VIII were successfully discovered.
Owner:CHUGAI PHARMA CO LTD
FIX-Mutant Proteins for Hemophilia B Treatment
InactiveUS20080214462A1Improved clot activityHigh activityPeptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsDisease
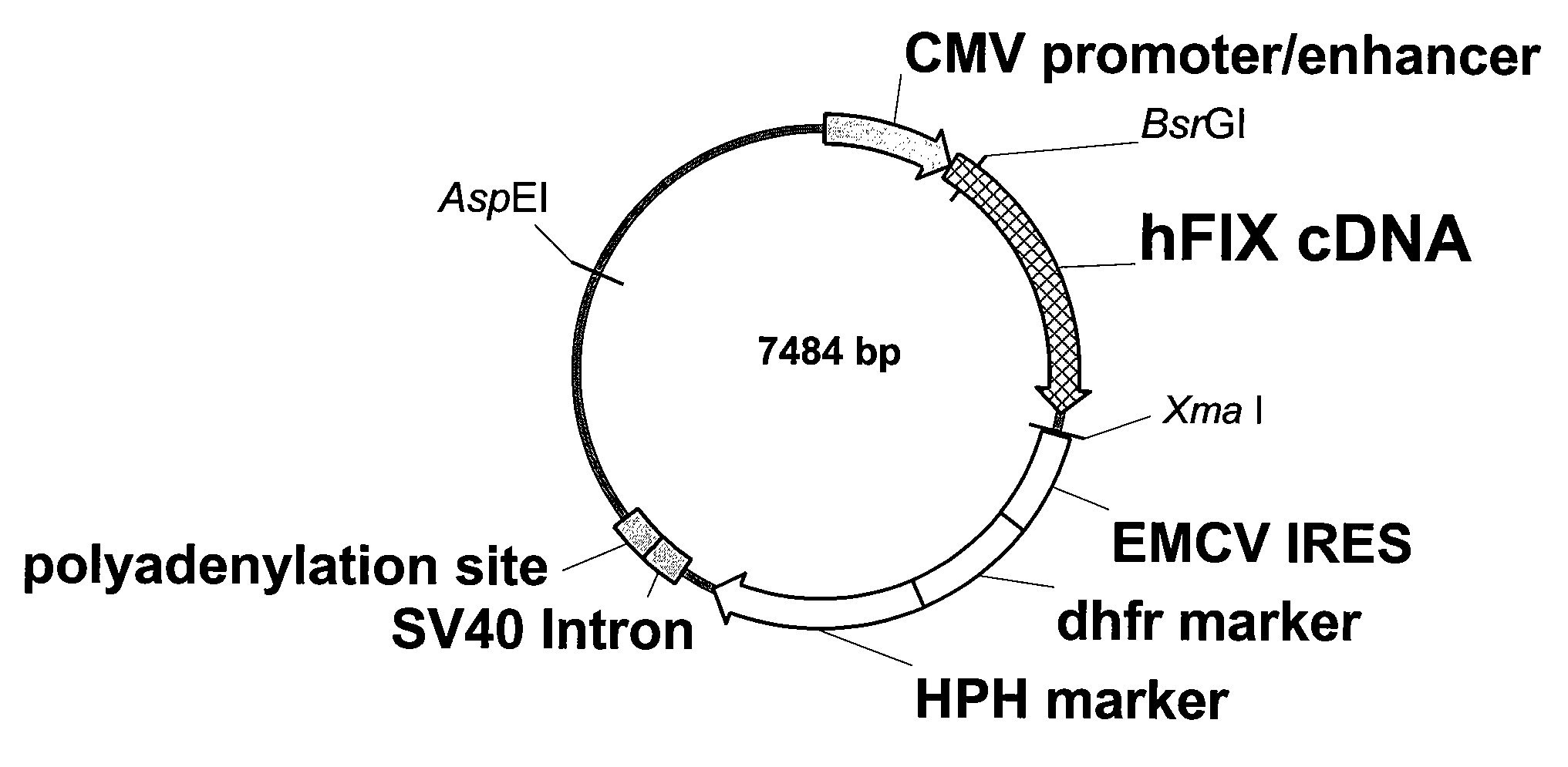
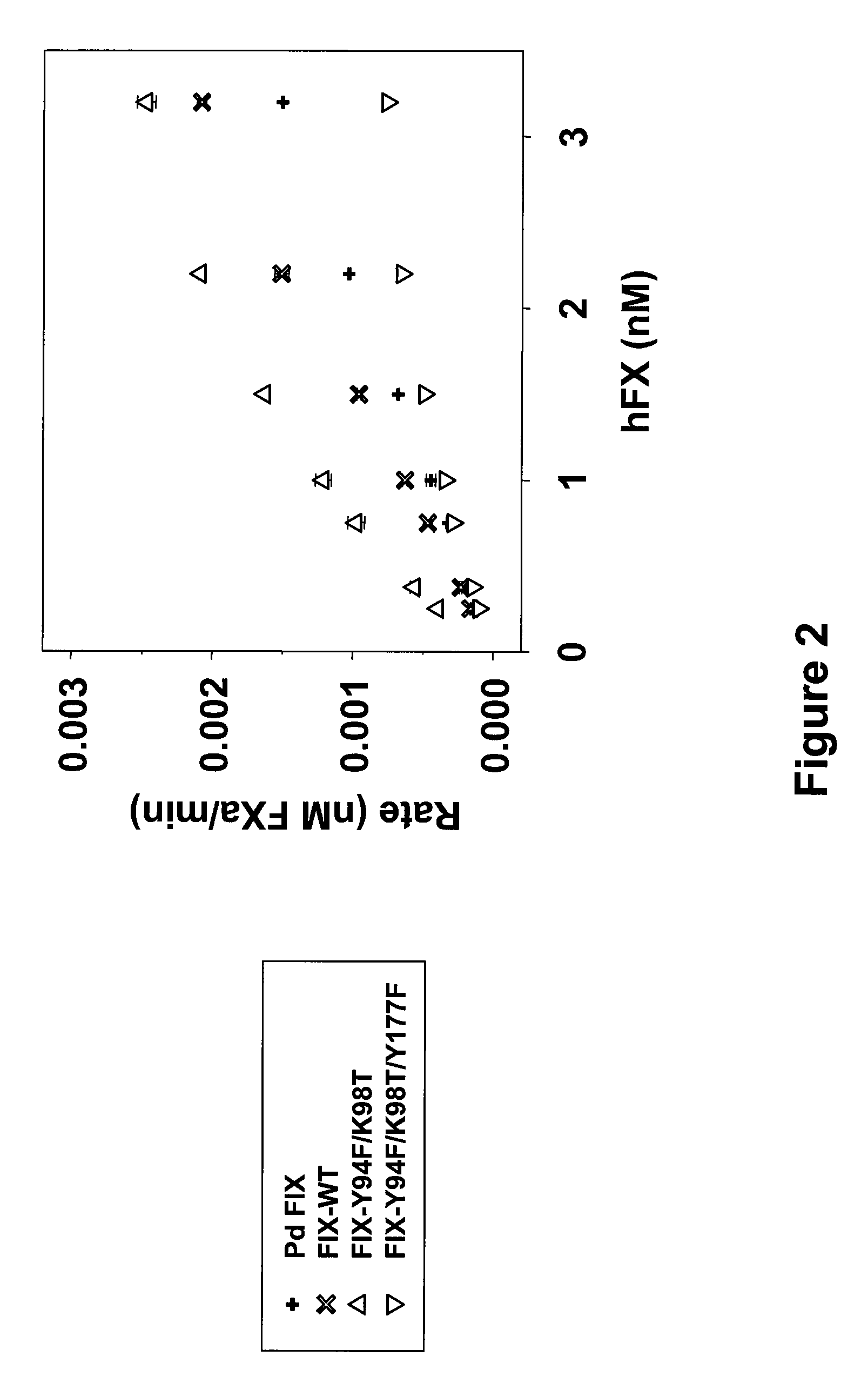
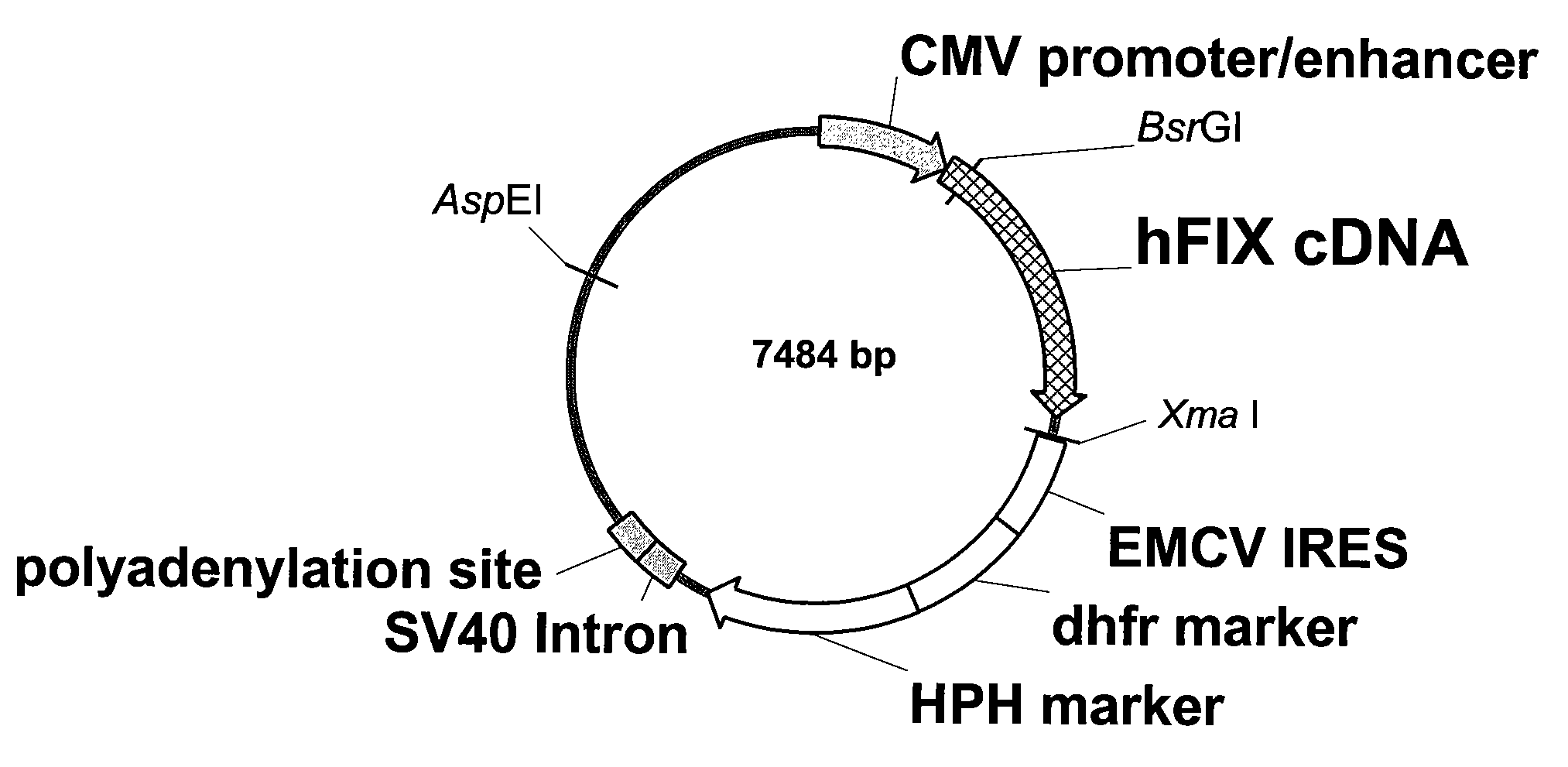
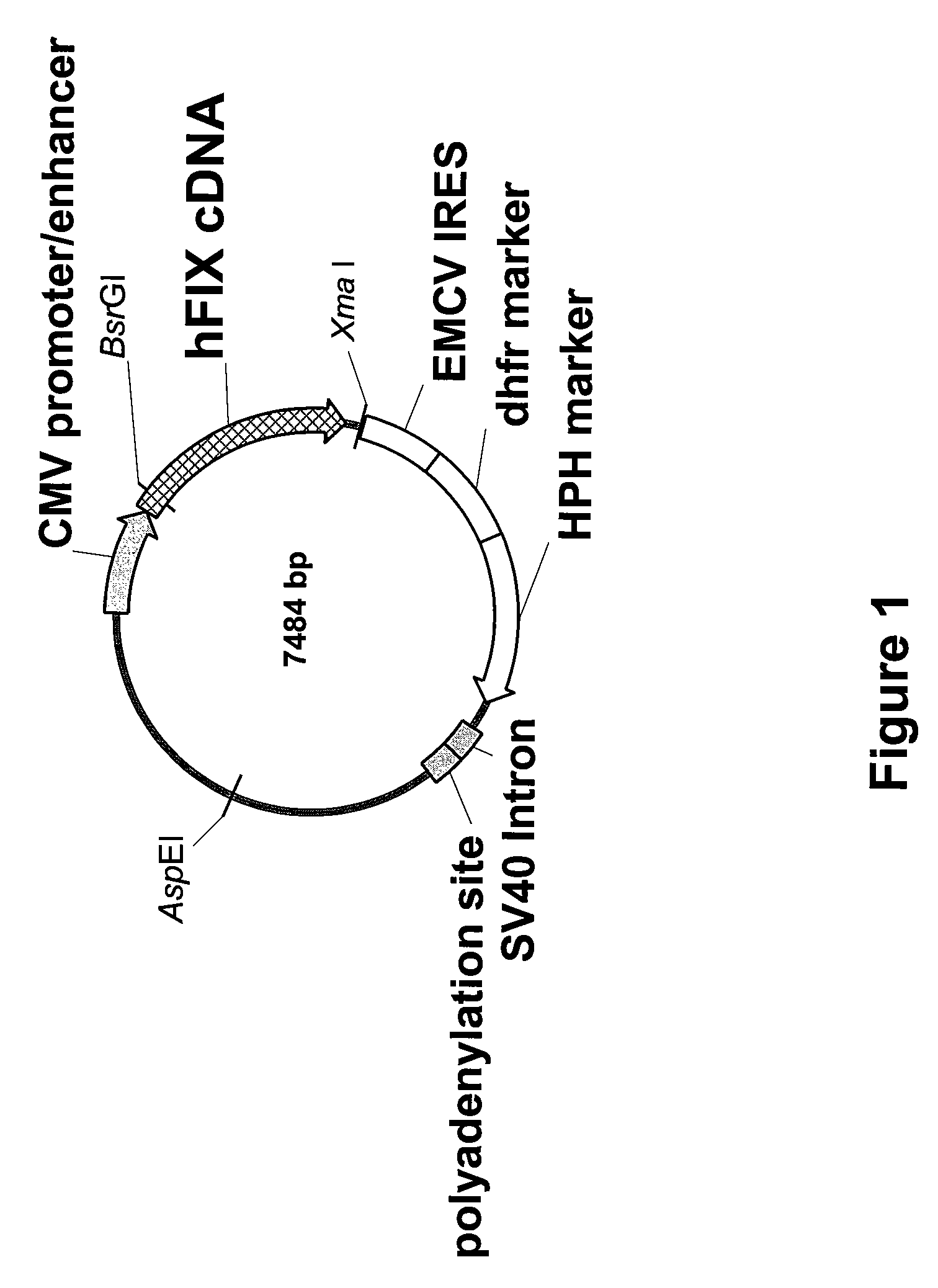
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having improved FIX clotting activity. Three full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assays in FIX-depleted plasma. Two mutant proteins had increased specific FIX activity. Furthermore, a pre-activated FIX protein had an increased activity in FIX-depleted plasma. Therefore these FIX mutants can be used for the treatment of FIX associated bleeding disorders.
Owner:BAXTER INT INC +1
FVIII-Independent FIX-Mutant Proteins for Hemophilia A Treatment
ActiveUS20080214461A1Peptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsMutated protein
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having factor VIII (FVIII) independent factor X (FX) activation potential. Five full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assay in FVIII-depleted plasma as well as in FVIII-inhibited patient plasma. In FVIII-depleted plasma functional activity of the FIX mutants was calculated as increased FVIII equivalent activity. The mutant proteins had increased FVIII equivalent activity. In FVIII-inhibited patient plasma the FEIBA equivalent activity was calculated for analysis of FVIII independent FX activation potential. The proteins had also increased FEIBA equivalent activity. Furthermore, the pre-activated FIX proteins had an increased activity in FIX-depleted plasma containing FVIII inhibitors. Therefore these FIX mutants are alternatives as bypassing agents for treatment of FVIII inhibitor patients.
Owner:TAKEDA PHARMA CO LTD
Monoclonal antibody of blood coagulation factor IX
ActiveCN103396494AImmunoglobulins against blood coagulation factorsPeptide preparation methodsDiseaseHeavy chain
The invention discloses a monoclonal antibody of a blood coagulation factor IX. The antibody comprises a light chain and a heavy chain, wherein the variable region amino acid sequence of the light chain is represented by SEQ ID NO.3, and the variable region amino acid sequence of the heavy chain is represented by SEQ ID NO.4. The invention also discloses a preparation method of the antibody. The antibody can specifically identify the blood coagulation factor IX. The invention further discloses a method for purifying the blood coagulation factor IX from blood plasma through utilizing the antibody. The blood coagulation factor IX can be conveniently and efficiently purified from the blood plasma through utilizing the antibody, so the infection risks of the diseases comprising hepatitis A, the hepatitis B, AIDS and the like caused by the patient blood transfusion supplementation of the blood coagulation factor IX are reduced.
Owner:浙江耶大生物医药有限公司
Preparation and application of high-activity blood coagulation factor IX mutant, recombinant protein and fusion protein
PendingCN106497949AGood prospects for alternative treatmentsPeptide/protein ingredientsFermentationSerum igeCoagulation factor IX activity
The invention relates to preparation and application of high-activity blood coagulation factor IX mutant, recombinant protein and fusion protein. The nucleotide sequence of the mutant is as shown in SEQ ID No. 1.
Owner:四川至善唯新生物科技有限公司
Fusion protein having factor ix activity
Disclosed is a fusion protein comprising blood coagulation factor IX (FIX) and transferrin. The fusion protein exhibits improved specific FIX activity, as compared to native FIX, and can be useful in the treatment of FIX deficiency-associated diseases.
Owner:TIUMBIO CO LTD
Preparation method of blood coagulation factor IX quality control product
InactiveCN104181313AEasy to detectImprove uniformityPreparing sample for investigationBiological testingFreeze-dryingBlood plasma
The invention relates to a preparation method of a clinical blood coagulation inspection preparation and particularly relates to a preparation method of a blood coagulation factor IX quality control product. The preparation method comprises the following steps: carrying out affinity chromatography on the mixed blood plasma of multiple persons by using an anti-human blood coagulation factor IX monoclonal antibody immunoaffinity chromatography column, removing a blood coagulation factor IX in the mixed blood plasma of multiple persons to obtain a blood plasma in shortage of the blood coagulation factor IX; mixing the mixed blood plasma of multiple persons with the blood plasma in shortage of the blood coagulation factor IX according to a certain proportion to prepare a blood coagulation factor IX quality control product with the content of the blood coagulation factor IX at different concentration levels, adding a freeze-drying protective additive, carrying out sub-packaging, and carrying out freeze drying so as to obtain the blood coagulation factor IX quality control product. According to the blood coagulation factor IX quality control product prepared by the preparation method, the uniformity, the stability and the stability of freeze-dried aquatic product subjected to re-melting are good, and the quality control product can replace an imported product to be used for quality control on detection of blood coagulation factor IX, so that the reduction of the detection cost is facilitated and the capability of detecting the blood coagulation factor IX in China can be promoted.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
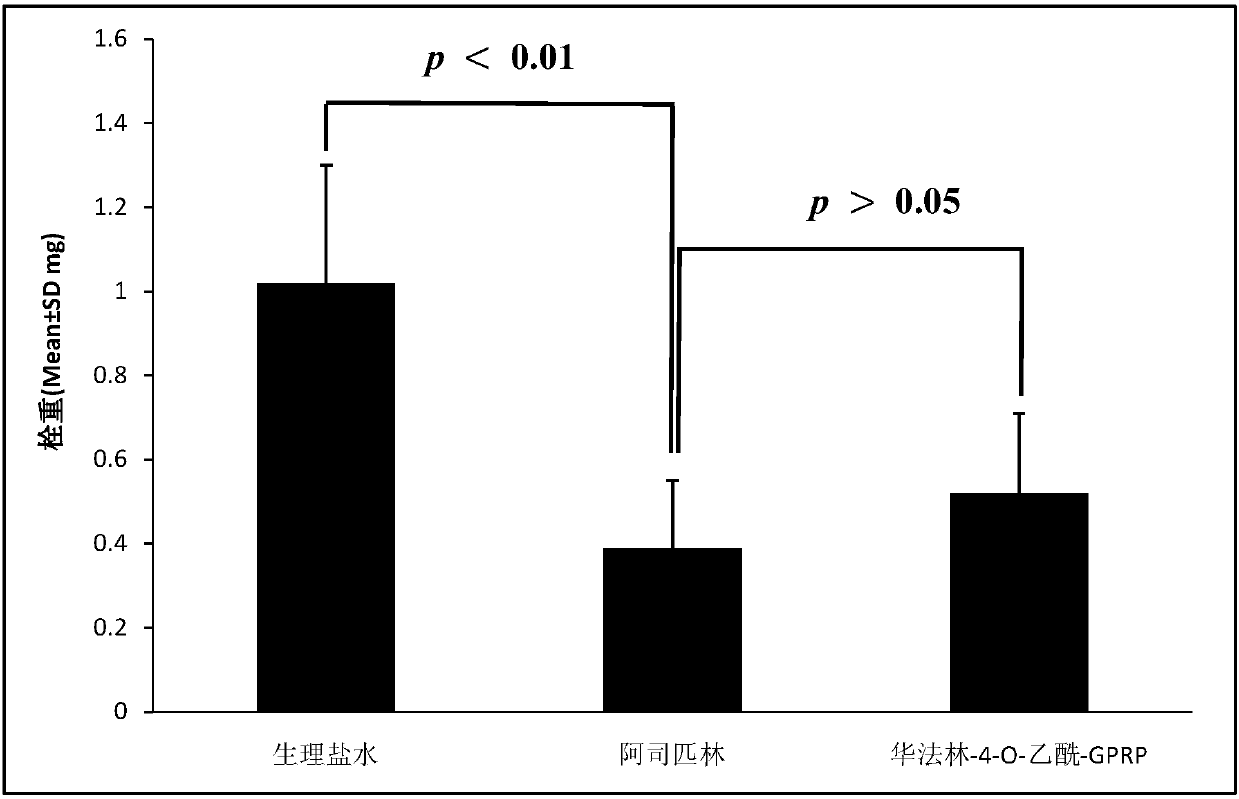
Warfarin-4-O-acetyl-GPRP and synthesis, activity and application thereof
ActiveCN107686506AReduce the level of coagulation factor IXTetrapeptide ingredientsPeptide preparation methodsWarfarinMedicine
The invention discloses warfarin-4-O-acetyl-GPRP, discloses a preparation method thereof, discloses phlebothrombosis resisting activity thereof, discloses ferric trichloride induced mouse artery thrombosis resisting activity thereof, discloses in-vivo blood coagulation factor IX content reducing activity thereof and discloses the advantage thereof no warfarin sample bleeding risk. Therefore, the invention discloses application thereof in preparing phlebothrombosis resisting medicine, application thereof in preparing artery thrombosis resisting medicine and application thereof in preparing blood coagulation factor IX antagonists.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
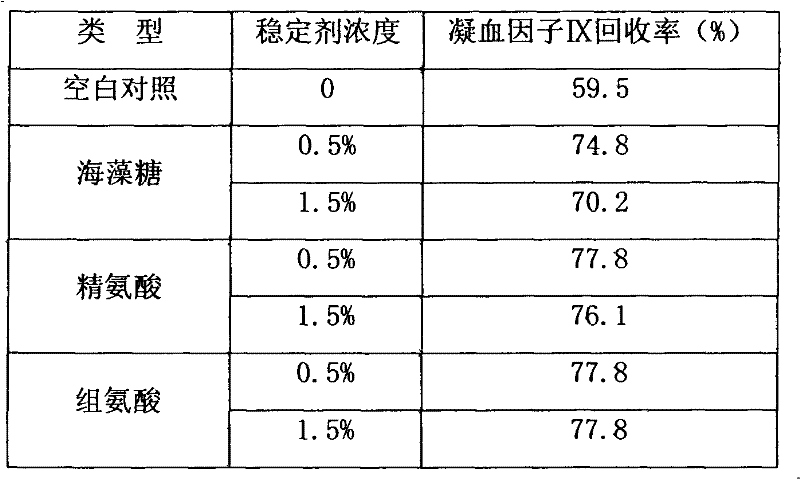
High-purity prothrombin complex product freeze-drying stabilizer
InactiveCN102441172AImprove protectionPeptide/protein ingredientsPharmaceutical non-active ingredientsZymogenArginine
The invention relates to the field of medicinal biotechnology, and in particular to a high-purity prothrombin complex product stabilizer capable of effectively preventing the inactivation of a blood coagulation factor IX with a main effect and blood coagulation factors II, VII and X in a product in a freeze-drying process, wherein the specific activity of each of four blood coagulation factors II, VII, IX and X is greater than or equal to 3.5IU / mg protein. The stabilizer provided by the invention contains arginine or / and trehalose or / and histidine or / and glycine. The tests show that so long as the high-purity prothrombin complex contains 0.1-10% of arginine or / and 0.1-10% of trehalose or / and 0.1-10% of histidine, the activity of the blood coagulation factor IX and the activity of the blood coagulation factors II, VII and X can be effectively protected in the freeze-drying process. Thus, the stabilizer provided by the invention can be applied to the freeze-drying process of the high-purity prothrombin complex.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +3
Method for efficiently extracting and purifying blood coagulation factor IX and blood coagulation factor X
InactiveCN102146134AHigh affinityImprove stabilityPeptide preparation methodsBlood coagulation/fibrinolysis factorsBlood Coagulation Factor XBlood plasma
The invention provides a method for efficiently extracting and purifying a blood coagulation factor IX and a blood coagulation factor X. The method provided by the invention comprises the following steps: collecting blood, treating, and collecting blood plasma; and extracting an IX crude extract and an X crude extract from the blood plasma, and purifying. The method is characterized in that the purifying step is to use FIX / FX-bp-Sepharose 4B to purify the blood coagulation factor IX crude extract and the blood coagulation factor X crude extract respectively through the affinity chromatography; and the affinity ligand FIX / FX-bp is selected from ACF I, ACF II or AHP. The purified blood coagulation factor IX can be directly used as a medicament and the purified blood coagulation factor X canbe directly used as a reagent.
Owner:UNIV OF SCI & TECH OF CHINA
Genetically-modified non-human animal and application thereof
The invention relates to a genetically-modified non-human animal and application thereof, in particular to a transgenic mouse model and application thereof in the aspect of screening of a targeted medicine. According to the transgenic mouse model and application thereof in the aspect of screening of the targeted medicine, by targetedly knocking out and replacing a mouse protein C gene by means ofa human protein C gene expression cassette, a humanized protein C knock-in mouse is generated. The mouse has fertility, and can hybridize with another mouse disease model (such as a mouse in the deficiency of a blood coagulation factor VIII or a blood coagulation factor IX) to produce a humanized protein C mouse disease model (such as a humanized protein C factor VIII-deficient mouse model or a humanized protein C factor IX-deficient mouse model). The mouse model can be used for studying functions in vivo of human protein C and human activated protein C (APC). The mouse model is the first mouse model used for testing a therapeutic candidate medicine, which targets the human protein C or the APC, in vivo, and has very high economic value and scientific research value.
Owner:SHANGHAI RAAS BLOOD PRODUCTS CO LTD
Prepn. of blood coagulation factor IX compound
InactiveCN1336178AHigh recovery rateReduce contentPeptide/protein ingredientsMammal material medical ingredientsPhosphateDEAE-Sepharose
The present invention relates to the preparation of plasma thromboplastin antecedent IX complex phosphate buffer solution or citrate buffer solution with 4-7 milli mole concertration and pH 5.5-6.5 is used to balance the gel used for adsorption, with fresh frozen human plasmia being added. DEAE-sepharose Fast Flow is used to proceed ion exchange adsorption separation, then same buffer solution with pH 5.5-7.0 is used to wash adsorption gel. further same buffer solution with pH 6-8 is used to elute adsorption gel, collect plasma thromboplastin antecedent IX complex containing factor II, VII, IX and X.
Owner:四川高维系统工程技术有限公司 +2
Method for detecting anticoagulant capacity of human prothrombin complex
InactiveCN104459165AQuantitative determination of comprehensive anticoagulant capacitySimple and fast operationMaterial analysis by observing effect on chemical indicatorBiological testingWavelengthChemistry
The invention discloses a method for detecting anticoagulant capacity of a human prothrombin complex. The method comprises the following steps: (1) taking the human prothrombin complex, diluting until a blood coagulation factor IX is 0.8-1.3IU / ml, adding a human prothrombin solution with the isovolumetric concentration being 4-6IU / ml, mixing evenly, and standing at room temperature for 1-2 minutes; (2) adding a thrombin chromogenic substrate solution which is isovolumetric to the human prothrombin solution, mixing evenly, and incubating at 22-28 DEG C for 4-6 minutes, wherein the concentration of the chromogenic substrate solution is 0.5-0.7mg / ml; (3) determining a light absorption value at the wavelength of 405nm, and determining once 30-50 seconds for 5-8 times in all; and (4) drawing the time changing trend along with the light absorption value by taking the determined light absorption value as a longitudinal coordinate (y) and time (s) as a cross coordinate (x), building a linear equation y=kx+a, wherein k is slope, and calculating the value of 1 / k. The method for detecting the anticoagulant capacity of the human prothrombin complex is simple and convenient to operate and good in repeatability; the comprehensive anticoagulant ability of the PCCs product can be quantitatively detected; and the safety of the PCCs product can be evaluated.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI
Protective agent in process for performing dry heat virus inactivation on high-purity prothrombin complex concentrate products
InactiveCN102416171AHigh activity yieldPeptide/protein ingredientsPharmaceutical non-active ingredientsProthrombin complex concentrateZymogen
The invention relates to the field of medicinal biotechnologies, and discloses a protective agent which can effectively prevent a mainly acting blood coagulation factor IX and other blood coagulation factors II, VII and X in high-purity prothrombin complex concentrate products (the specific activities of the blood coagulation factors II, VII, IX, and X are more than and equal to 3.5IU / mg protein) from being inactivated in the process of performing dry heat virus inactivation at the temperature of 100 DEG C for 30 minutes. The protective agent is trehalose or / and histidine and also contains two or three of common glycine, sodium citrate and NaCl. Experiments prove that if only high-purity prothrombin complex concentrate products contain 0.1 to 8 percent of trehalose or / and 0.1 to 8 percent of histidine, the activities of the blood coagulation factors IX, II, VII and X can be effectively protected in the process of performing dry heat virus inactivation at the temperature of 100 DEG C for 30 minutes. Therefore, the invention can be used as the protective agent in the process for performing dry heat virus inactivation on the high-purity prothrombin complex concentrate products.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +3
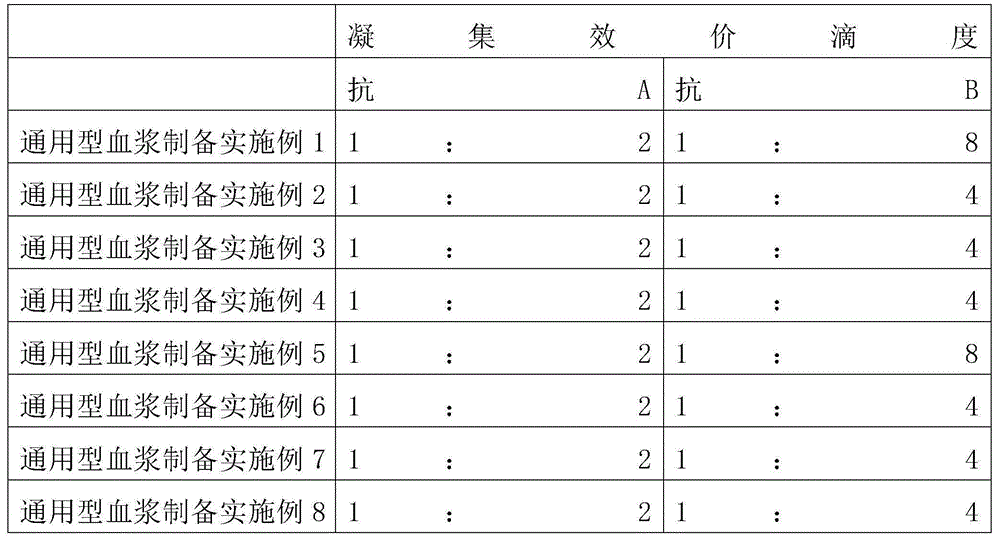
Human plasma with multiple functions and preparation method thereof
InactiveCN105832768ASave transfusion timeIncreased flexibility of usePeptide/protein ingredientsMammal material medical ingredientsPatient needBlood Coagulation Factor X
The invention provides a human plasma with multiple functions and a preparation method thereof, including general-purpose plasma and blood component additives; the general-purpose plasma is general-type fresh frozen plasma or general-purpose freeze-dried plasma; the blood component additive is selected from albumin, C Globulins, platelets, fibrinogen, factor II, factor V, factor VII, factor VIII, factor IX, factor X, factor XI, factor XII, factor XIII, and prothrombin complex any one or more of them. The advantages are: for patients who need to supplement plasma and at least one blood component additive at the same time, only the plasma including the corresponding blood component additive needs to be transfused, thereby increasing the scope and flexibility of use, saving the patient's blood transfusion time, and saving Blood transfusion equipment.
Owner:杜祖英
Fusion protein having factor IX activity
Disclosed is a fusion protein comprising blood coagulation factor IX (FIX) and transferrin. The fusion protein exhibits improved specific FIX activity, as compared to native FIX, and can be useful in the treatment of FIX deficiency-associated diseases.
Owner:TIUMBIO CO LTD
Single-domain antibody specific to coagulation factor IX (FIX)
ActiveCN110343181AImmunoglobulins against blood coagulation factorsPreparing sample for investigationSingle-domain antibodyMedical biology
The invention relates to the field of medical biology, discloses a single-domain antibody specific to a coagulation factor IX (FIX), particularly discloses a FIX binding molecule derived from the single-domain antibody, and especially discloses application in FIX detection and FIX-poor plasma preparation.
Owner:SUZHOU ALPHAMAB
Methods for treating a disease that develops or progresses as a result of decrease or loss of activity of blood coagulation factor viii and/or activated blood coagulation factor viii
InactiveUS20170253663A1EffectiveImmunoglobulins against blood coagulation factorsHybrid immunoglobulinsBlood coagulation factor VIIIAntigen
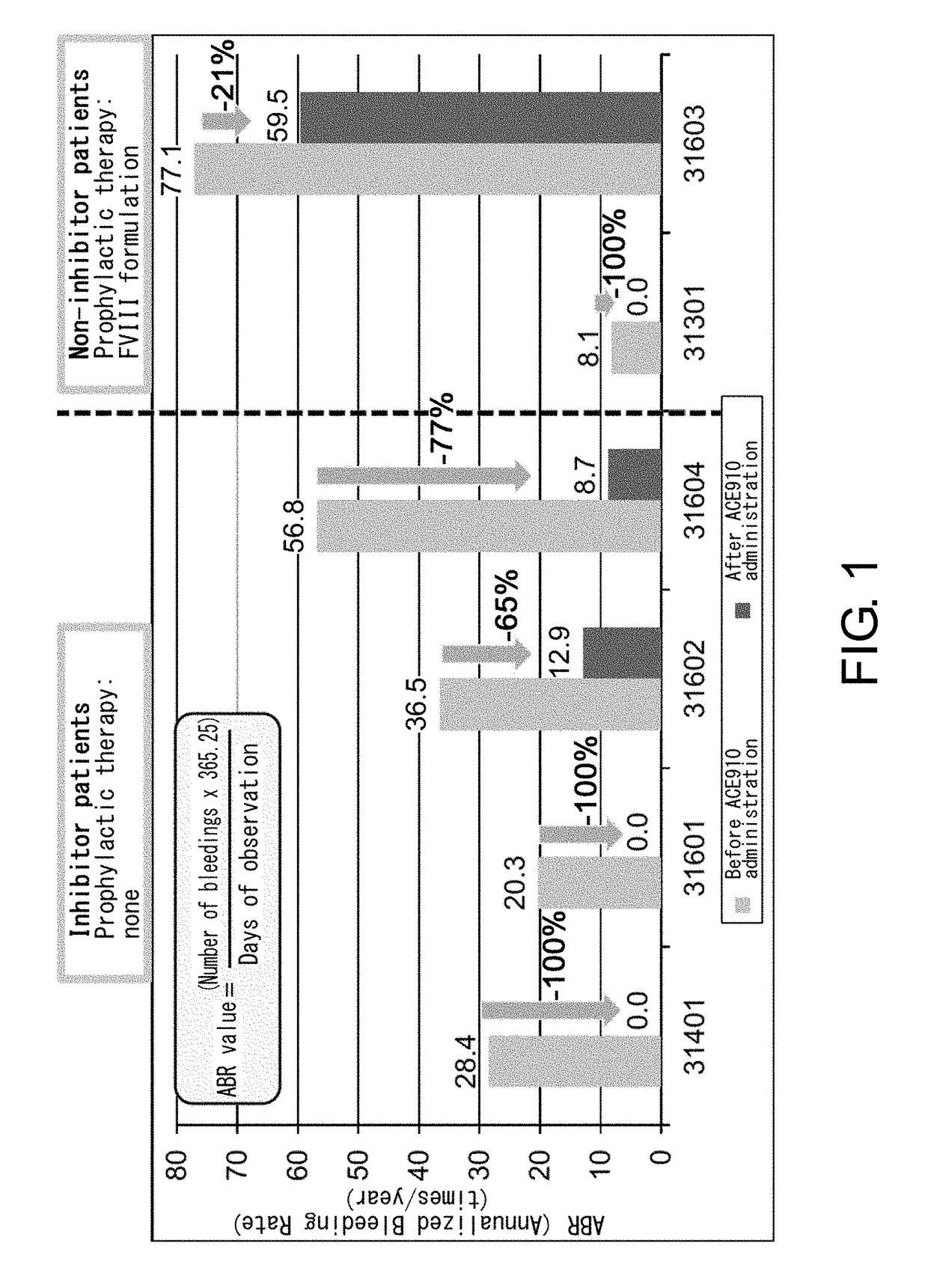
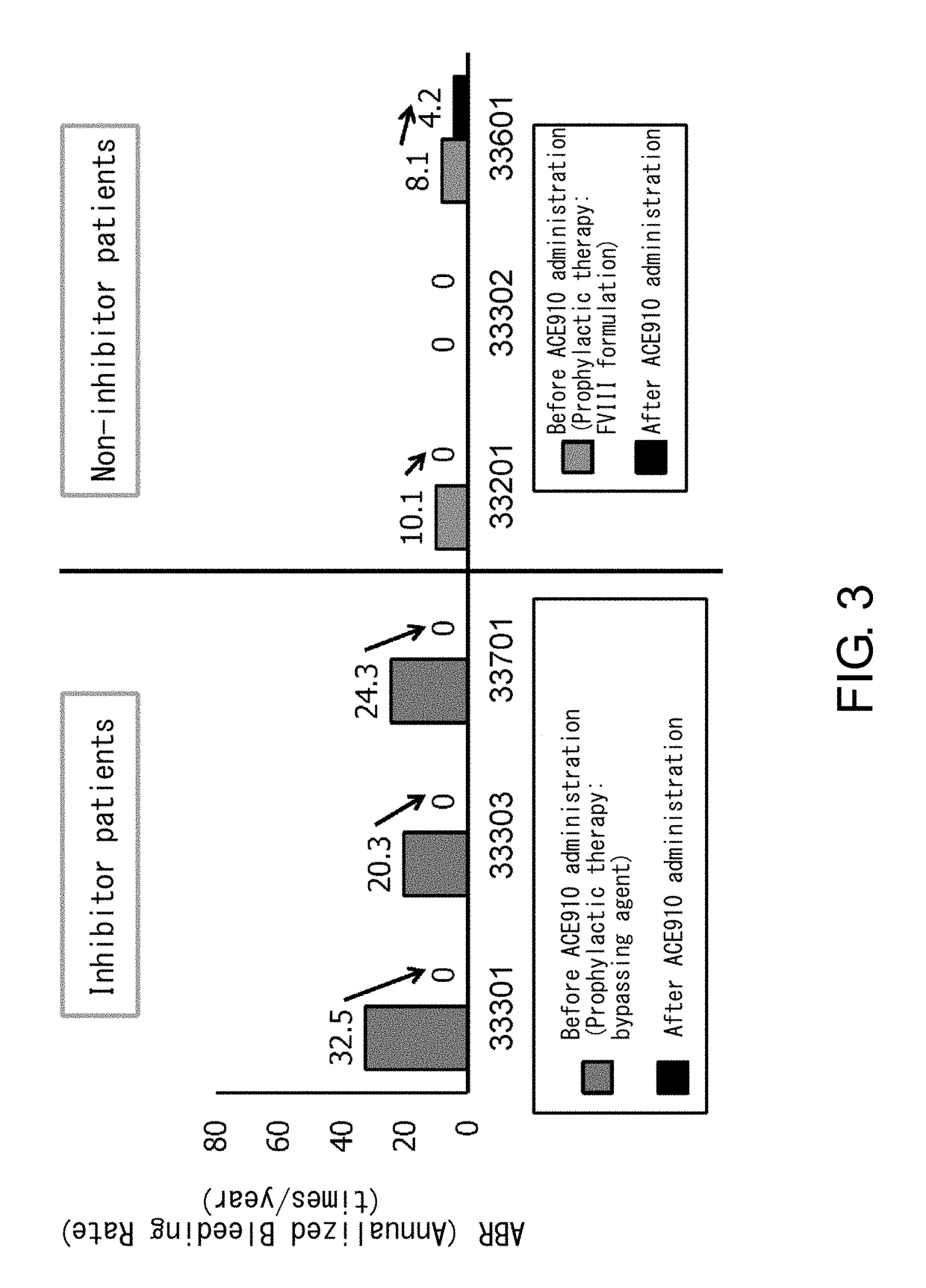
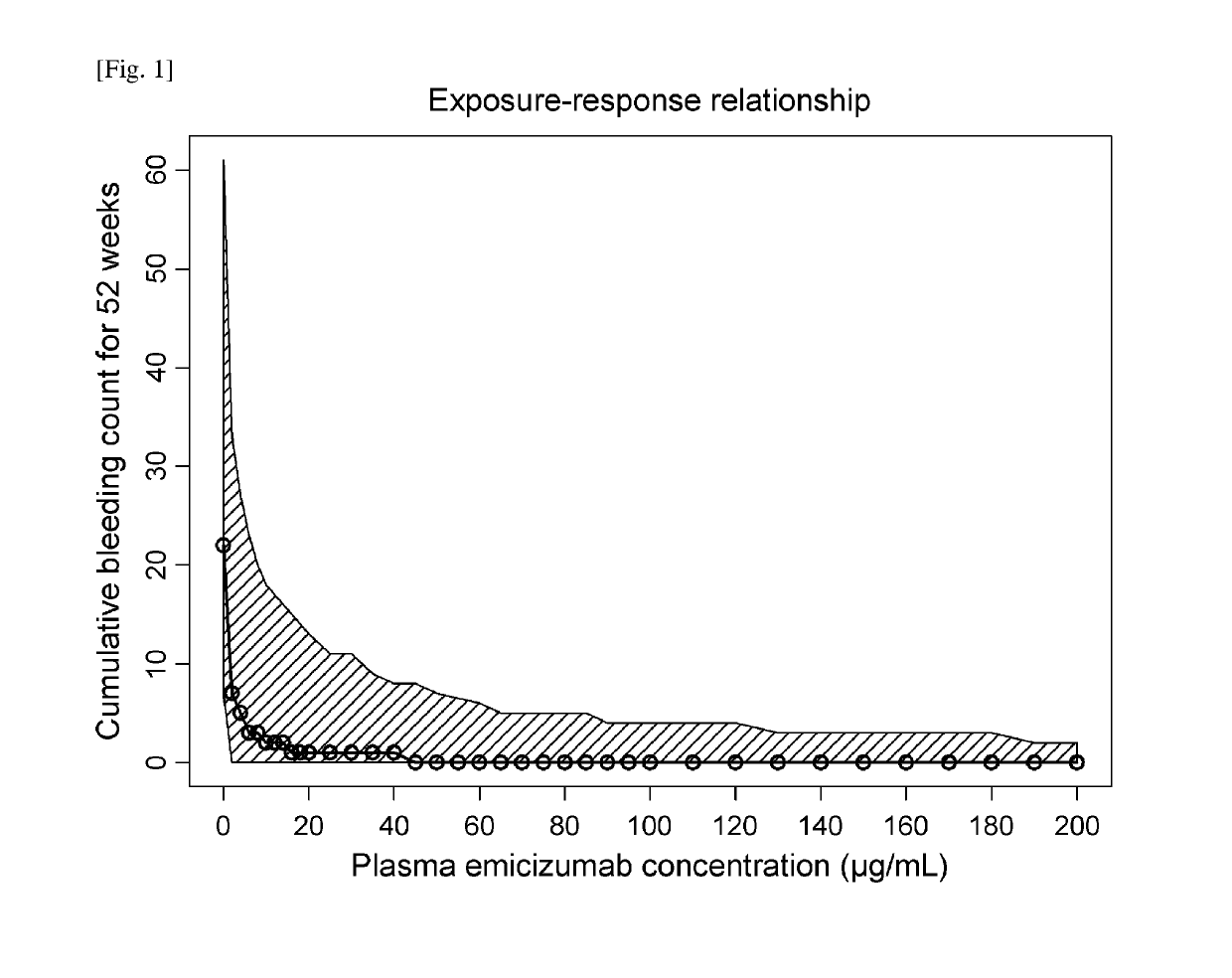
The inventors discovered that by administering a pharmaceutical composition comprising a bispecific antigen-binding molecule that recognizes blood coagulation factor IX and / or activated blood coagulation factor IX and blood coagulation factor X and / or activated blood coagulation factor X according to a given dosage regimen, diseases that develop and / or progress due to a decrease or deficiency in the activity of blood coagulation factor VIII and / or activated blood coagulation factor VIII can be prevented and / or treated more effectively.
Owner:CHUGAI PHARMA CO LTD
Cell migration regulator
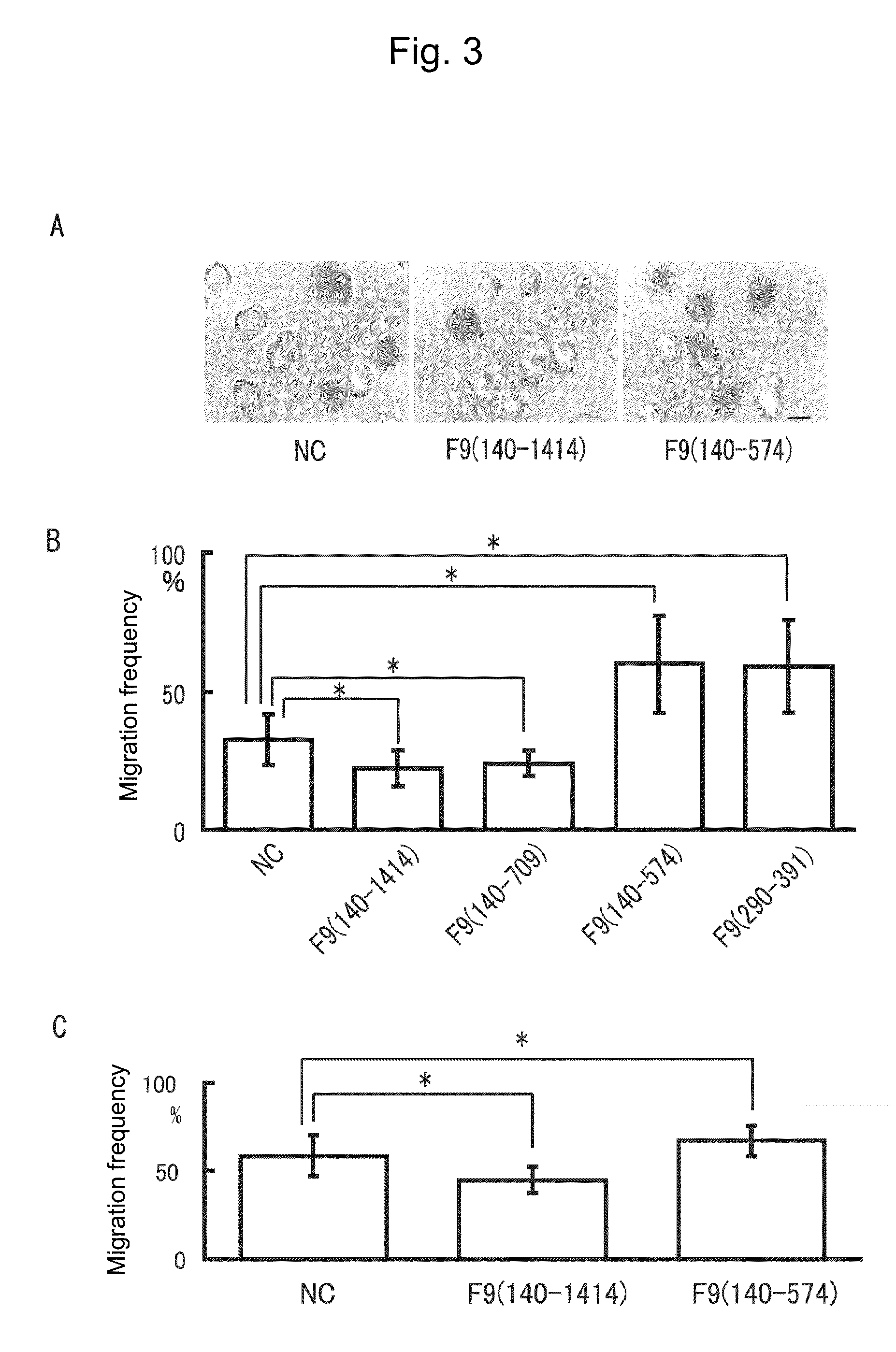
InactiveUS9371523B2Promote disseminationMaintaining an epithelial morphology of cellsPeptide/protein ingredientsTissue cultureCell migrationBiology
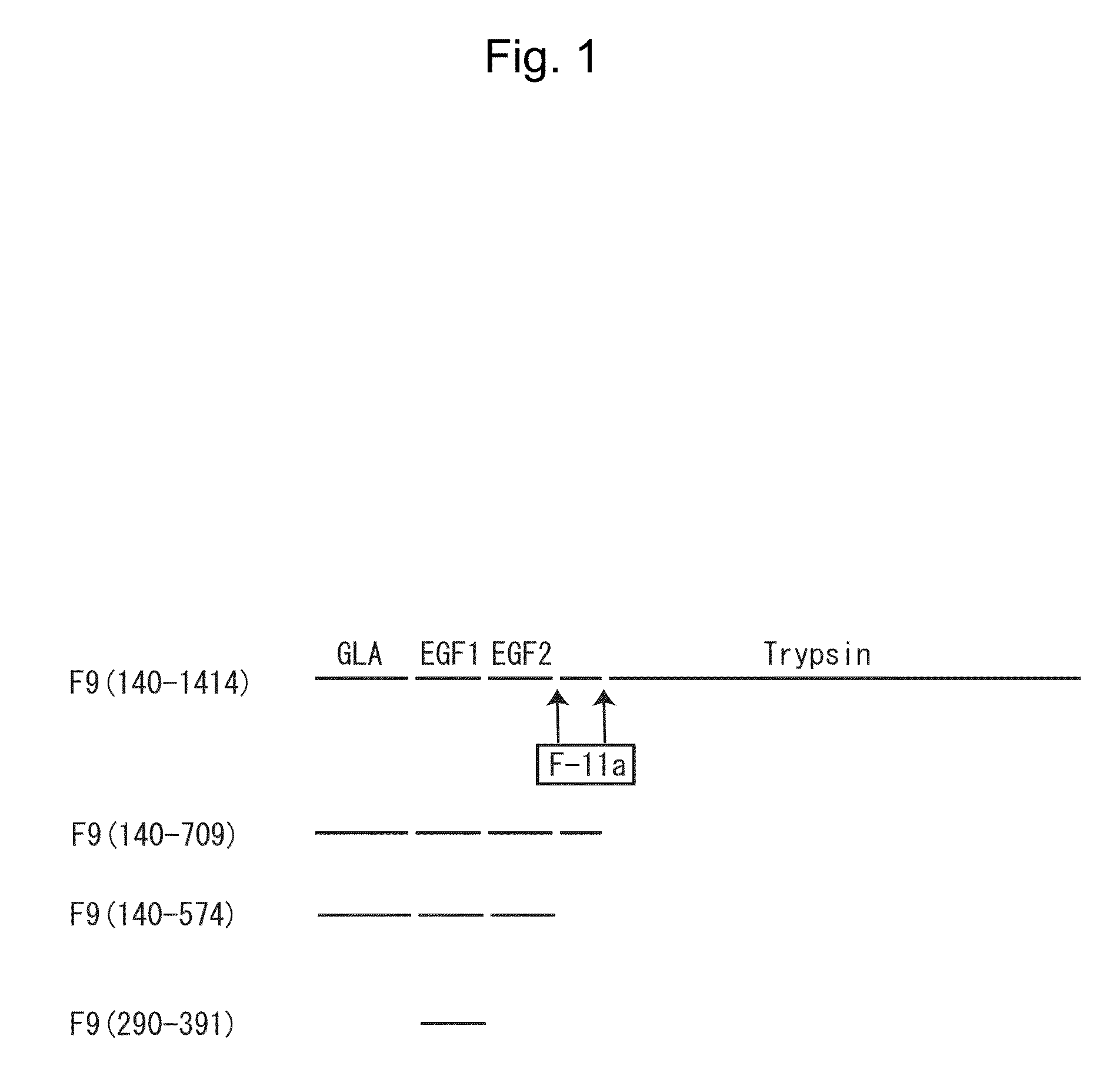
The present invention provides a cell migration regulator capable of promoting or inhibiting cell migration, a method for regulating cell migration, and a pharmaceutical composition comprising such a regulator, etc. The cell migration regulator of the present invention comprises a peptide, a derivative thereof, or a salt of the peptide or the derivative, wherein the peptide comprises the full-length blood coagulation factor IX, a segment derived from the full-length blood coagulation factor IX by removal of the trypsin domain, the light chain of blood coagulation factor IX, or the EGF1 domain of blood coagulation factor IX, or the EGF3 domain of the endothelial cell locus-1 protein.
Owner:NIHON UNIVERSITY
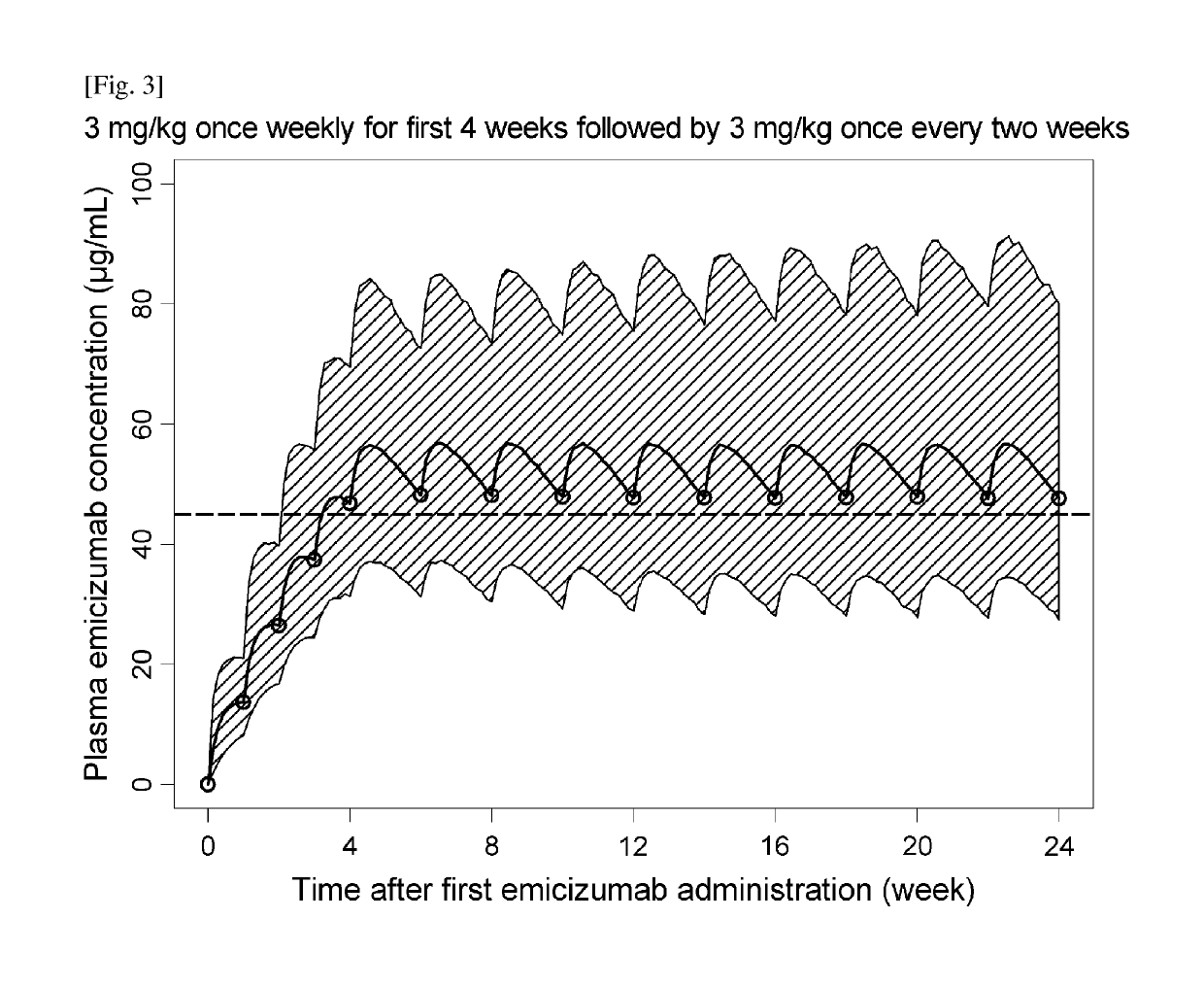
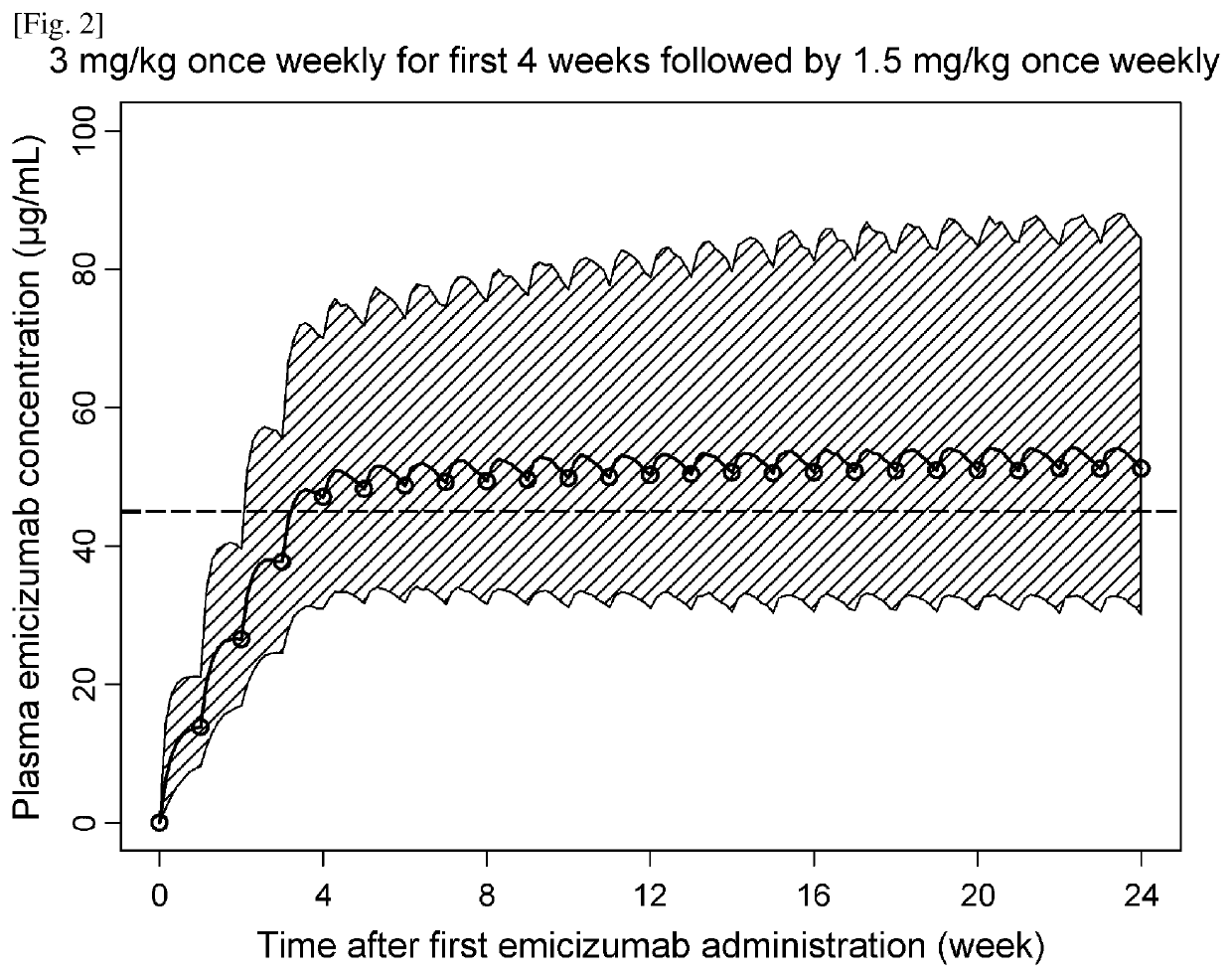
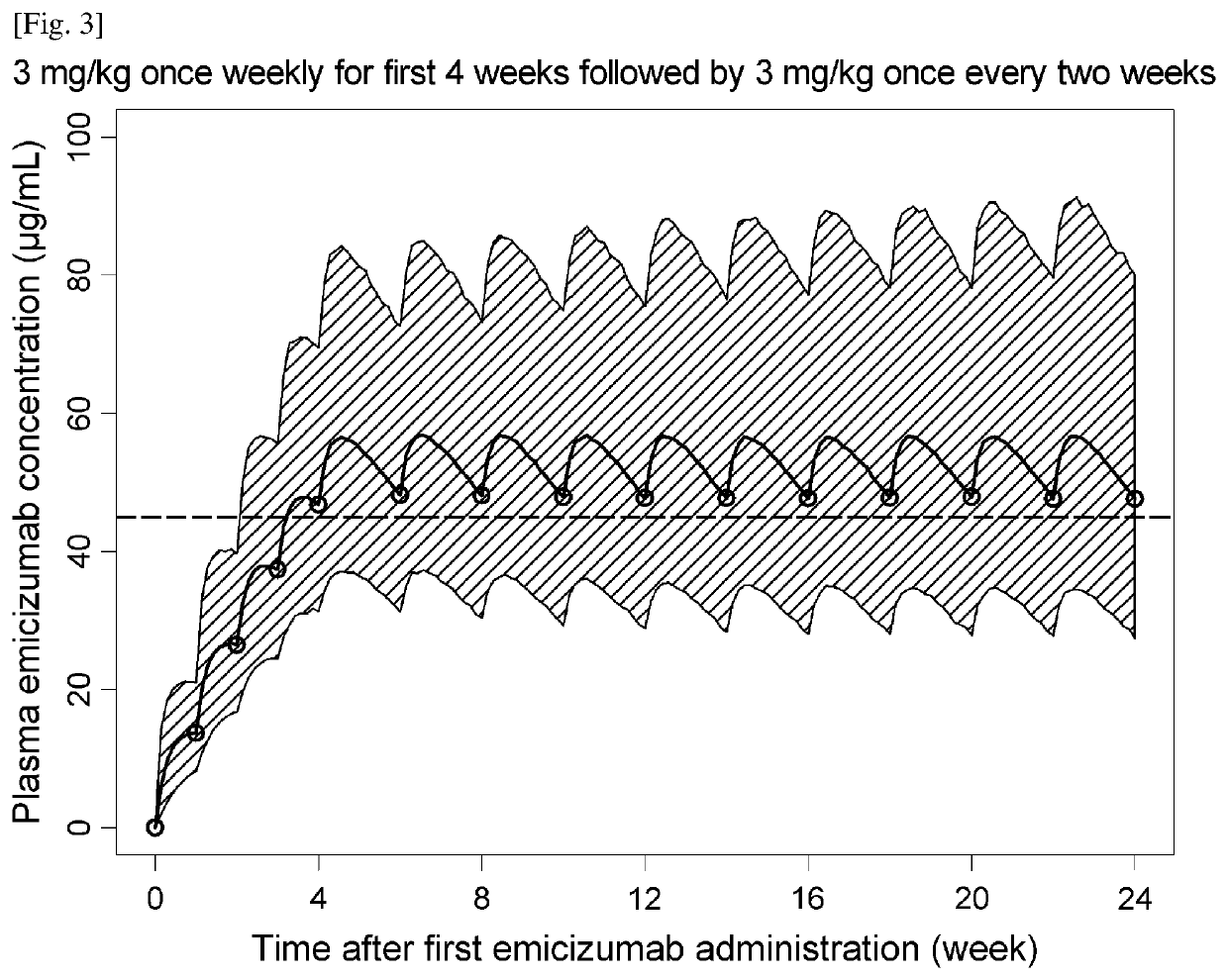
Methods of using a bispecific antibody that recognizes coagulation factor ix and/or activated coagulation factor ix and coagulation factor x and/or activated coagulation factor x
ActiveUS20190194352A1Reduce morbidityImmunoglobulins against blood coagulation factorsAntibody ingredientsRegimenBlood Coagulation Factor X
An objective of the present invention is to provide an effective pharmaceutical composition or a dosage regimen for preventing and / or treating bleeding, a disease accompanying bleeding, or a disease caused by bleeding. The inventors discovered that by administering a pharmaceutical composition comprising a bispecific antigen-binding molecule that recognizes (a) blood coagulation factor IX and / or activated blood coagulation factor IX and (b) blood coagulation factor X and / or activated blood coagulation factor X according to a given dosage regimen, bleeding, a disease accompanying bleeding, or a disease caused by bleeding can be prevented and / or treated more effectively.
Owner:F HOFFMANN LA ROCHE & CO AG +1
Methods of using a bispecific antibody that recognizes coagulation factor IX and/or activated coagulation factor IX and coagulation factor X and/or activated coagulation factor X
ActiveUS11352438B2Reduce morbidityImmunoglobulins against blood coagulation factorsAntibody ingredientsDosing regimenAntigen
An objective of the present invention is to provide an effective pharmaceutical composition or a dosage regimen for preventing and / or treating bleeding, a disease accompanying bleeding, or a disease caused by bleeding. The inventors discovered that by administering a pharmaceutical composition comprising a bispecific antigen-binding molecule that recognizes (a) blood coagulation factor IX and / or activated blood coagulation factor IX and (b) blood coagulation factor X and / or activated blood coagulation factor X according to a given dosage regimen, bleeding, a disease accompanying bleeding, or a disease caused by bleeding can be prevented and / or treated more effectively.
Owner:F HOFFMANN LA ROCHE & CO AG +1
Coagulation factor ⅸ quality control product preparation method
InactiveCN104181313BEasy to detectImprove uniformityPreparing sample for investigationBiological testingFreeze-dryingBlood plasma
The invention relates to a preparation method of a clinical blood coagulation inspection preparation and particularly relates to a preparation method of a blood coagulation factor IX quality control product. The preparation method comprises the following steps: carrying out affinity chromatography on the mixed blood plasma of multiple persons by using an anti-human blood coagulation factor IX monoclonal antibody immunoaffinity chromatography column, removing a blood coagulation factor IX in the mixed blood plasma of multiple persons to obtain a blood plasma in shortage of the blood coagulation factor IX; mixing the mixed blood plasma of multiple persons with the blood plasma in shortage of the blood coagulation factor IX according to a certain proportion to prepare a blood coagulation factor IX quality control product with the content of the blood coagulation factor IX at different concentration levels, adding a freeze-drying protective additive, carrying out sub-packaging, and carrying out freeze drying so as to obtain the blood coagulation factor IX quality control product. According to the blood coagulation factor IX quality control product prepared by the preparation method, the uniformity, the stability and the stability of freeze-dried aquatic product subjected to re-melting are good, and the quality control product can replace an imported product to be used for quality control on detection of blood coagulation factor IX, so that the reduction of the detection cost is facilitated and the capability of detecting the blood coagulation factor IX in China can be promoted.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
Pharmaceutical composition for use in prevention and/or treatment of disease that develops or progresses as a result of decrease or loss of activity of blood coagulation factor viii and/or activated blood coagulation factor viii
InactiveUS20190309090A1Immunoglobulins against blood coagulation factorsHybrid immunoglobulinsBlood coagulation factor VIIIDosing regimen
The inventors discovered that by administering a pharmaceutical composition comprising a bispecific antigen-binding molecule that recognizes blood coagulation factor IX and / or activated blood coagulation factor IX and blood coagulation factor X and / or activated blood coagulation factor X according to a given dosage regimen, diseases that develop and / or progress due to a decrease or deficiency in the activity of blood coagulation factor VIII and / or activated blood coagulation factor VIII can be prevented and / or treated more effectively.
Owner:CHUGAI PHARMA CO LTD
Improved FIX fusion protein, conjugate and application of fusion protein and conjugate
The present invention relates to a blood coagulation factor IX fusion protein having an extended circulating half-life, a conjugate containing the fusion protein, a pharmaceutical composition containing the fusion protein and the conjugate and an application of the fusion protein, the conjugate and the composition in the treatment of hemorrhagic diseases (such as hemophilia B).
Owner:江苏晟斯生物制药有限公司
Medicinal composition usable for preventing and/or treating blood coagulation factor ix abnormality, comprising multispecific antigen binding molecule replacing function of blood coagulation factor VIII
PendingCN110461358AImmunoglobulins against blood coagulation factorsHybrid immunoglobulinsBlood coagulation factor VIIIVon willebrand
The present inventors verified the coagulation promoting effect of multispecific antigen binding molecules, said molecules replacing the function of FVIII, using blood and plasma collected from patients with FIX abnormality. As a result, it is clarified that a multispecific antigen binding molecule replacing the function of FVIII is not only usable in a method for preventing and / or treating bleeding in hemophilia A, acquired hemophilia A, von Willebrand's disease and hemophilia C caused by the dysfunction of FVIII but also usable in a method for preventing and / or treating bleeding in FIX abnormality owing to the coagulation promoting activity thereof. It is also clarified that the effect of a FIX preparation can be enhanced by the combined use of the FIX preparation with the multispecificantigen binding molecule replacing the function of FVIII and this combined use appears promising as a combination therapy showing a stable hemostatic effect.
Owner:NARA MEDICAL UNIVERSITY +1
High-purity prothrombin complex product freeze-drying stabilizer
InactiveCN102441172BImprove protectionPeptide/protein ingredientsPharmaceutical non-active ingredientsZymogenFreeze-drying
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com