Preparation method of blood coagulation factor IX quality control product
A technology of blood coagulation factors and quality control products, which is applied in the field of preparation of clinical blood coagulation test preparations, can solve the problems of single level, high product price, and inability to meet the needs of multiple activity levels, and achieve improved ability, good stability, and reduced The effect of testing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] In conjunction with embodiment, further set forth the present invention:
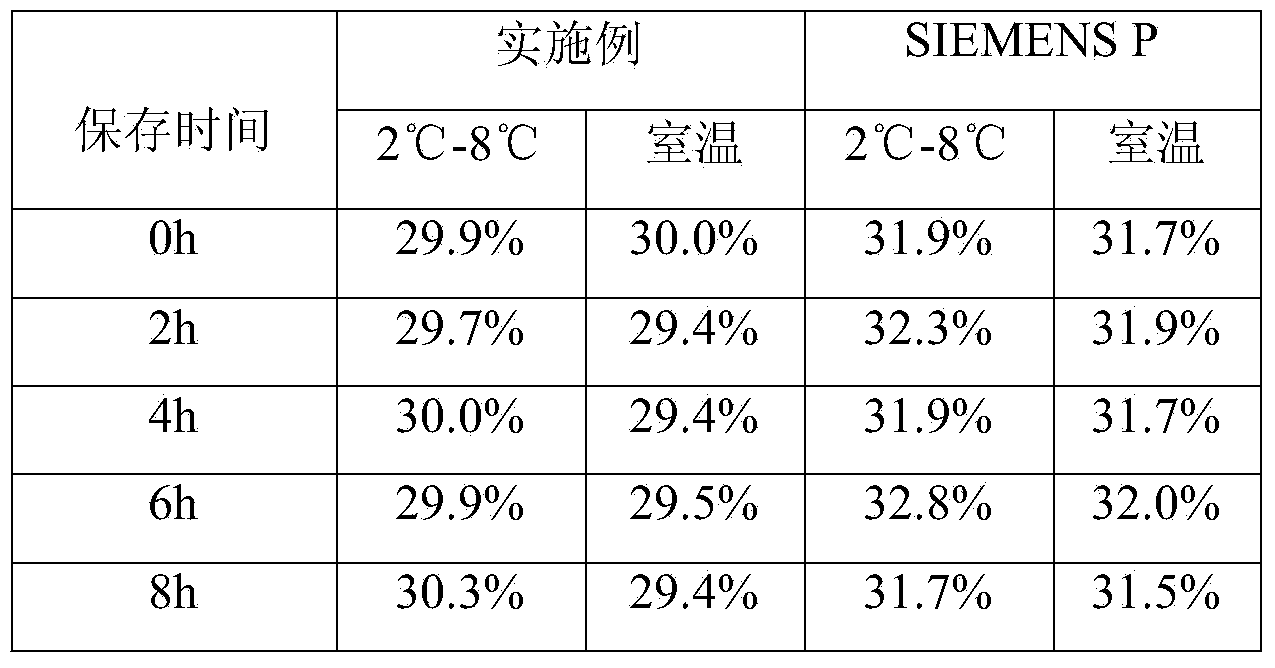
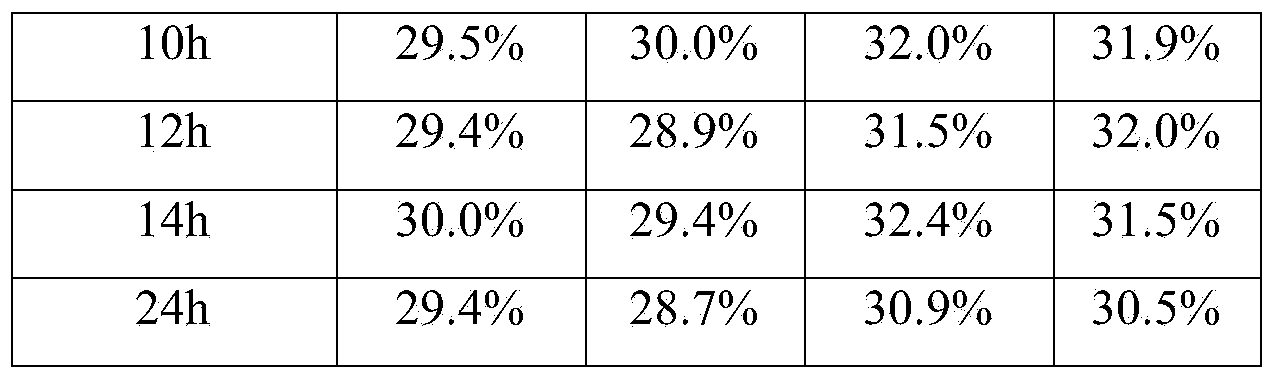
[0016] The embodiment takes the preparation of a blood coagulation factor IX quality control product with an activity of 30% as an example.
[0017] (1) Add and mix the blood collected from human veins and anticoagulant at a volume ratio of 9:1, centrifuge at 2°C to 8°C for 15 minutes, and the relative centrifugal force is 2500g, separate and absorb the upper layer of plasma, and separate the plasma from several people Mixing, that is, multiple servings of mixed plasma; the anticoagulant is trisodium citrate solution, and its concentration is 0.109mol / L to 0.129mol / L.
[0018] (2) The immunoaffinity chromatography column is pre-balanced with a buffer at 10°C to 18°C, and the buffer includes 0.02mol / L Tris, 0.15mol / L NaCl, 8mmol / L MgCl 2 , pH7.25.
[0019] (3) Add MgCl to raw plasma 2 After that, the MgCl in raw plasma 2 The concentration is 4.5-5.5mmol / L. Put on the immunoaffinity chromatogr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com