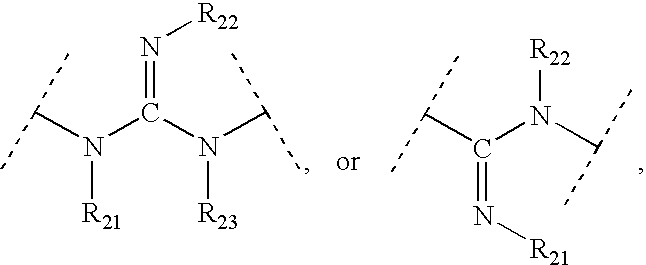
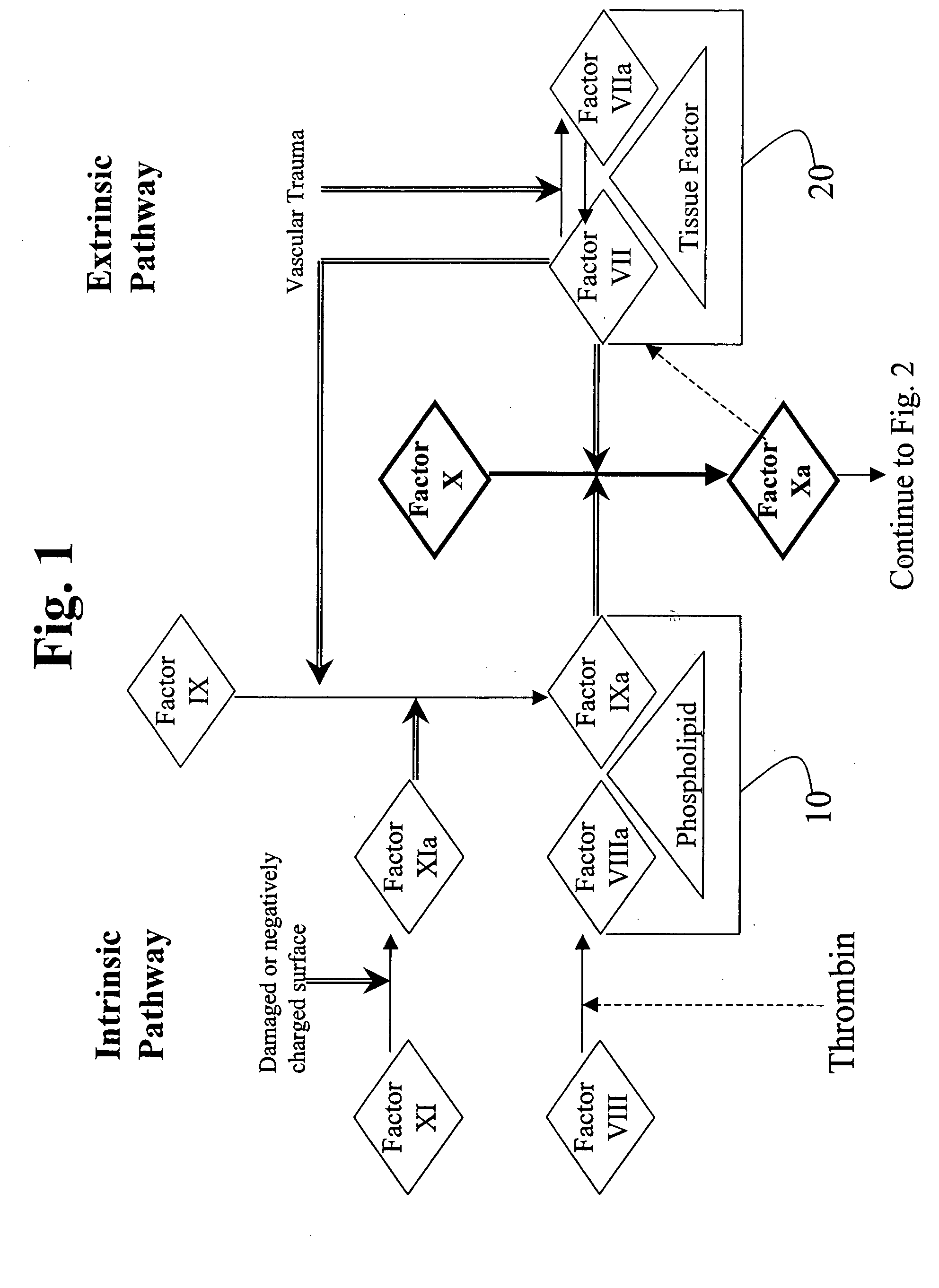
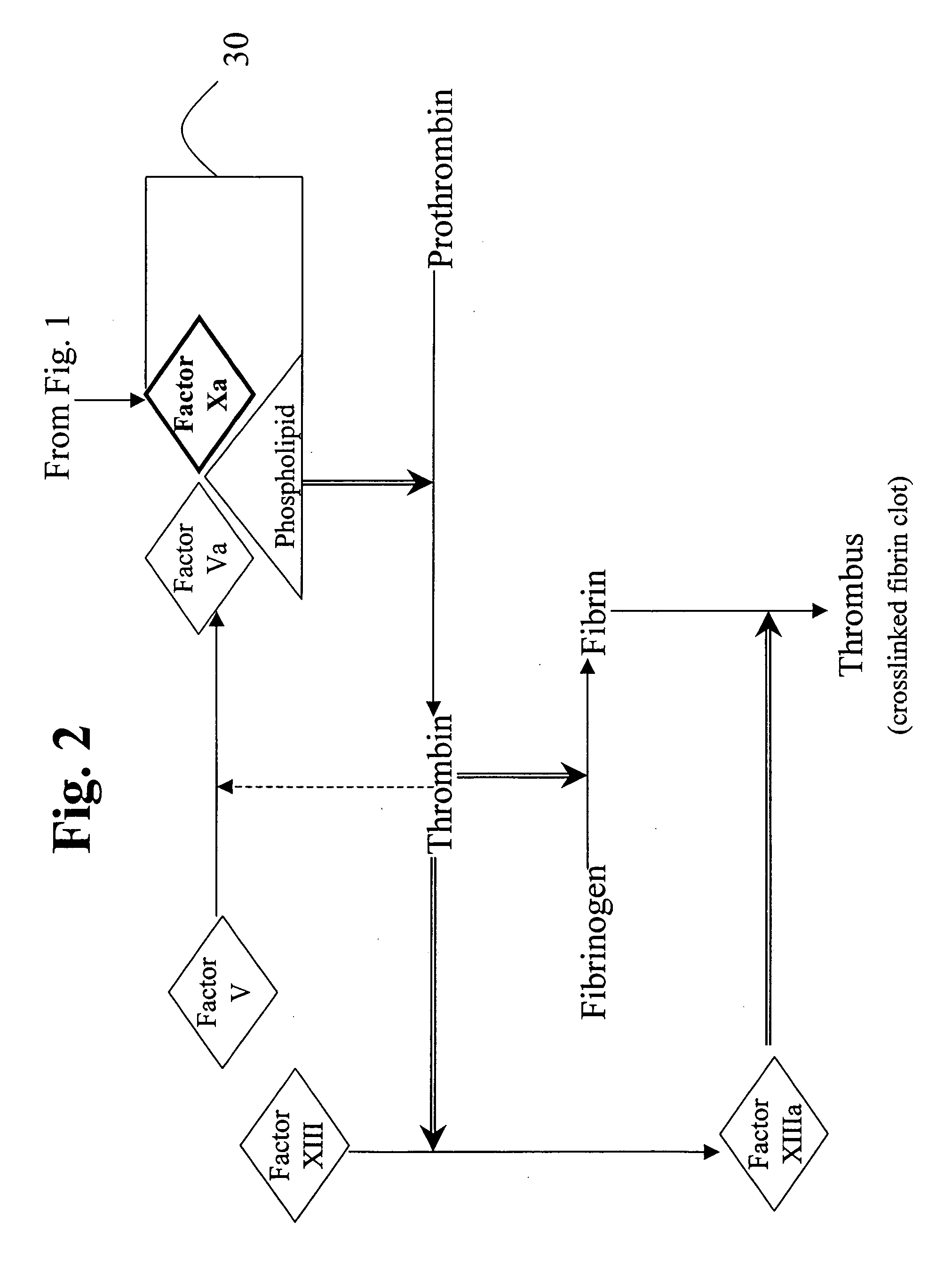
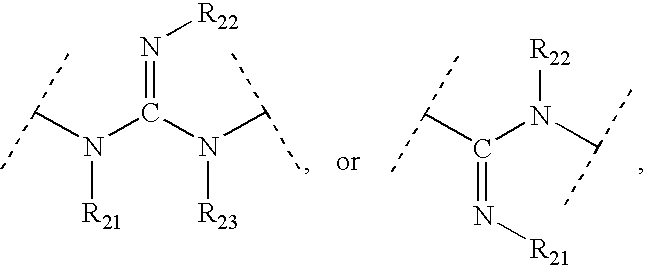
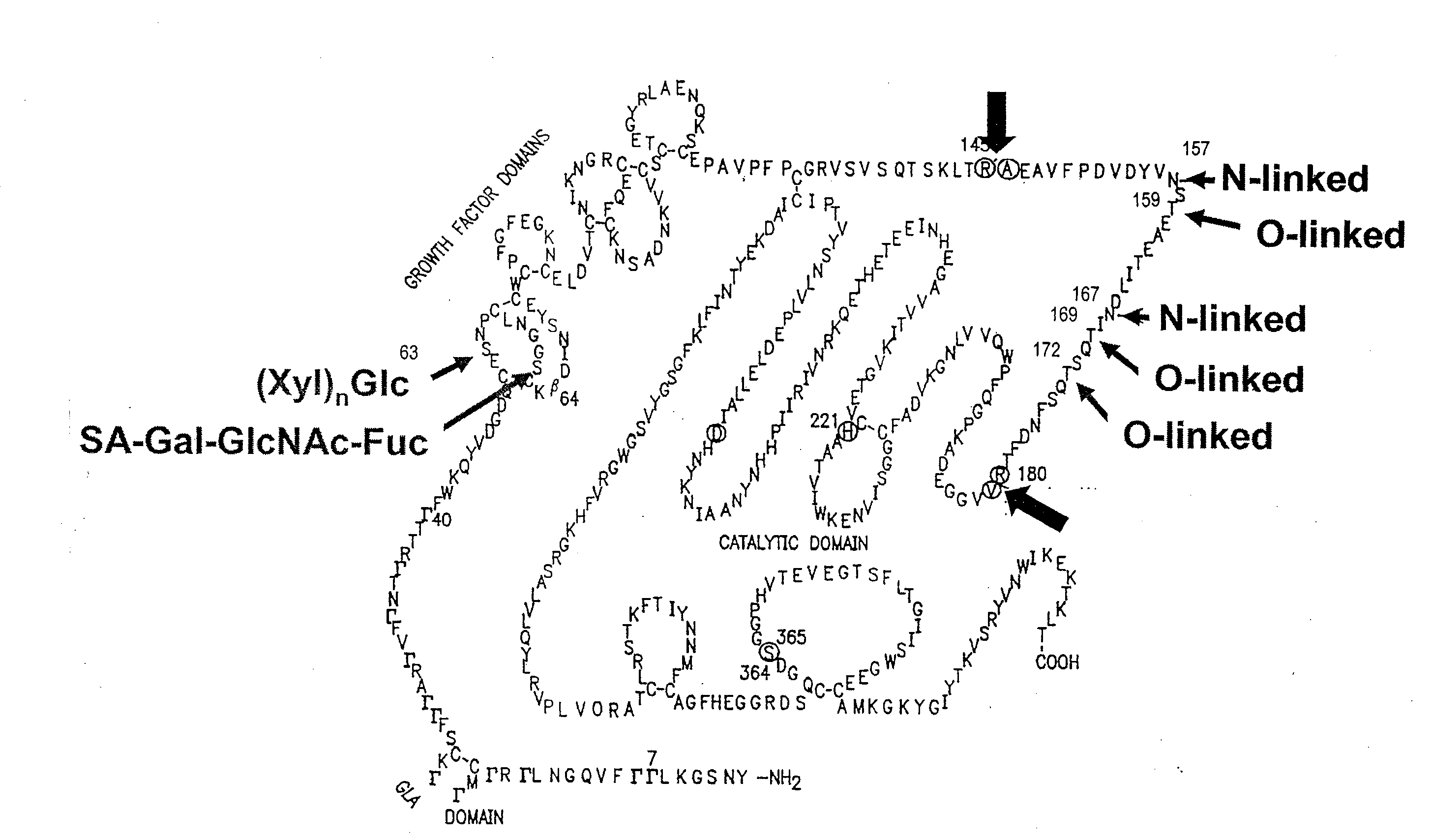
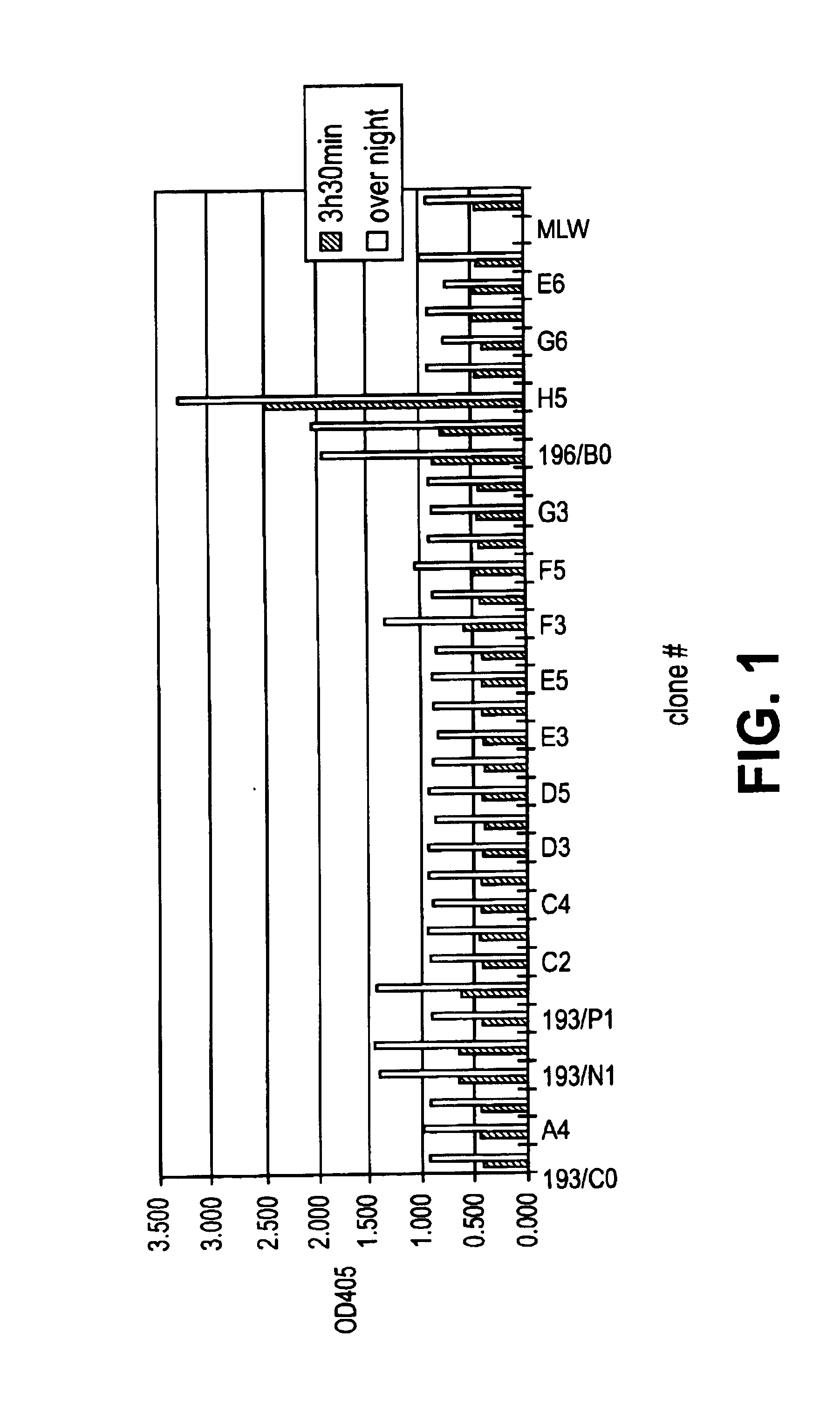
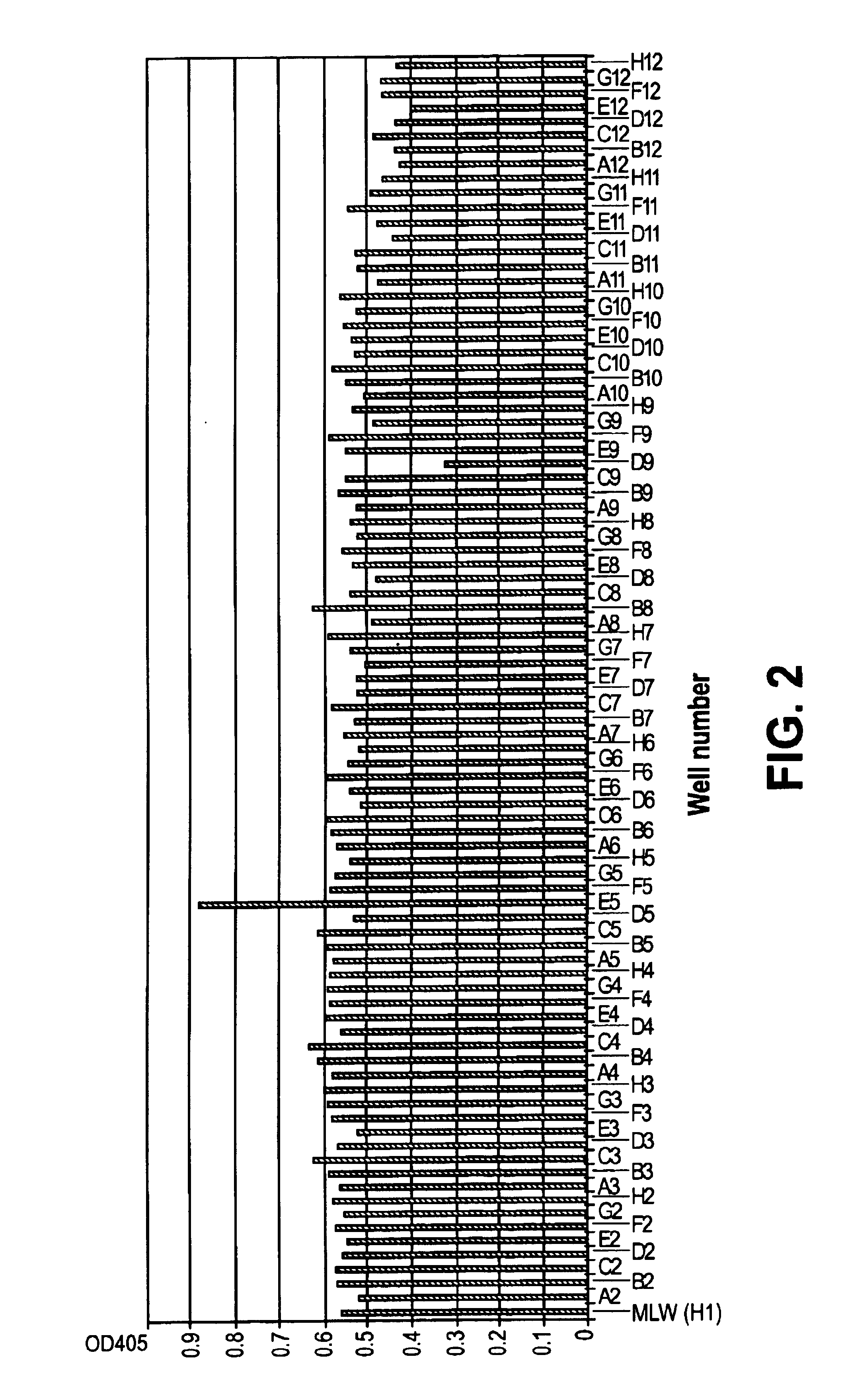
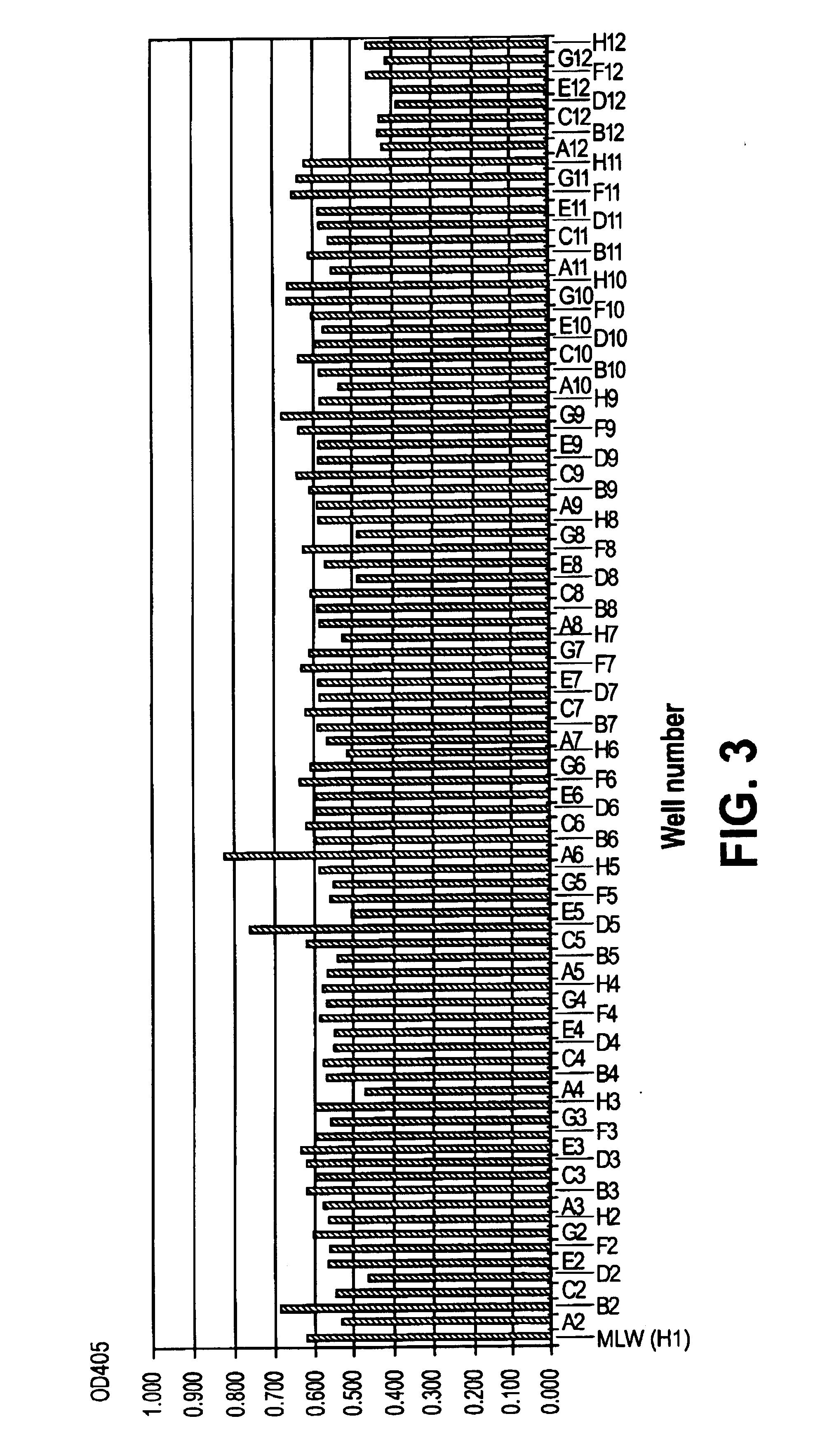
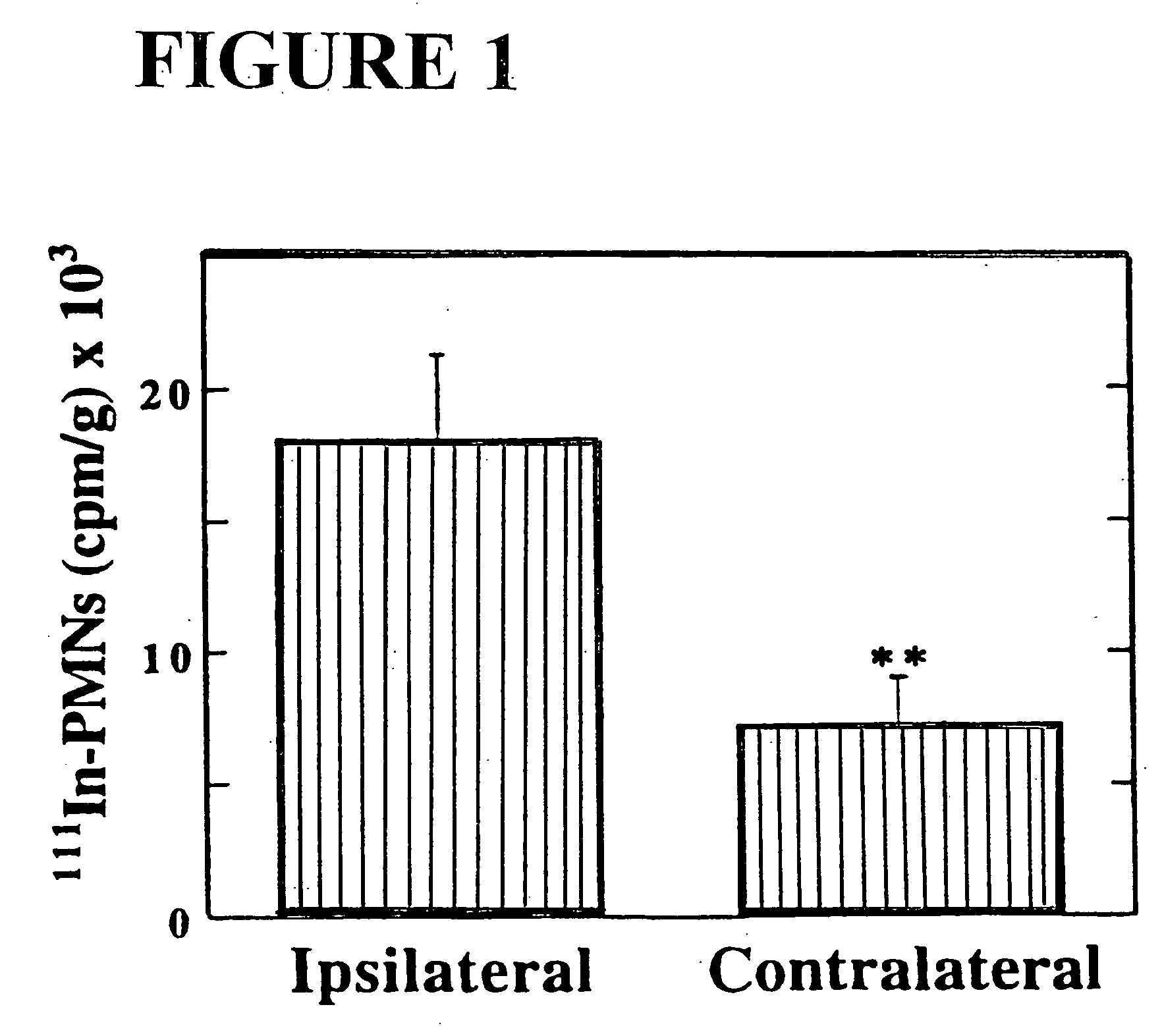
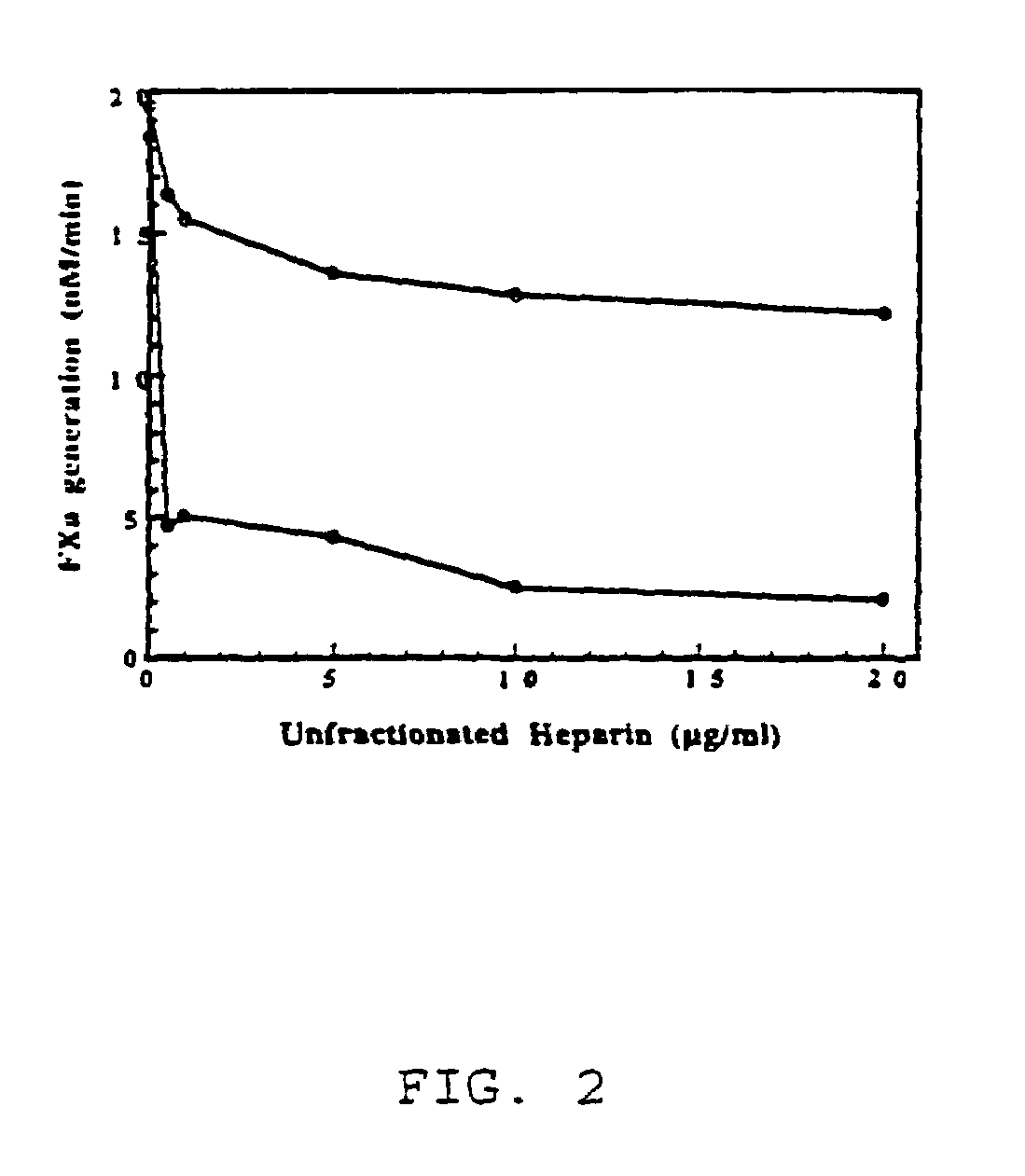
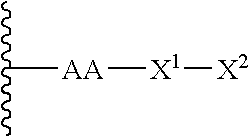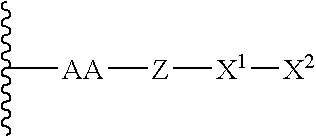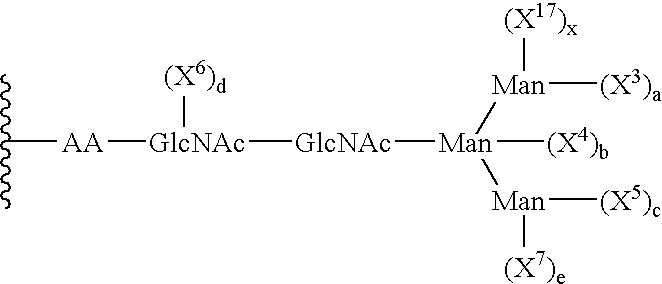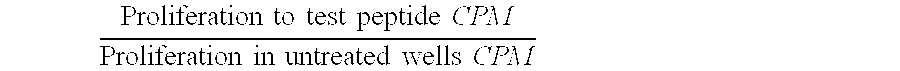Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
157 results about "Factor IX" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to prevent or control bleeding in people with little or no factor IX (due to hemophilia B, Christmas disease).
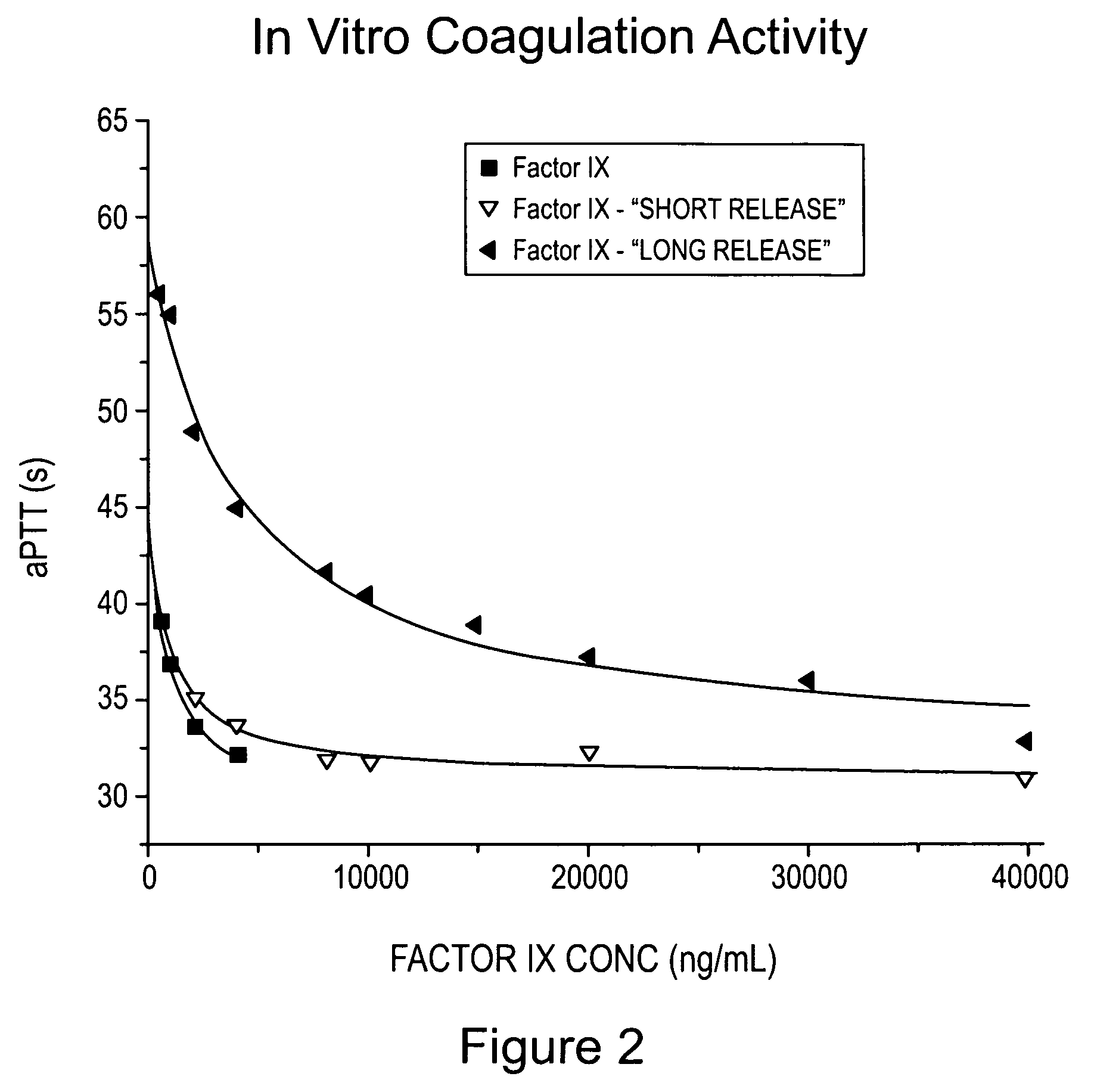
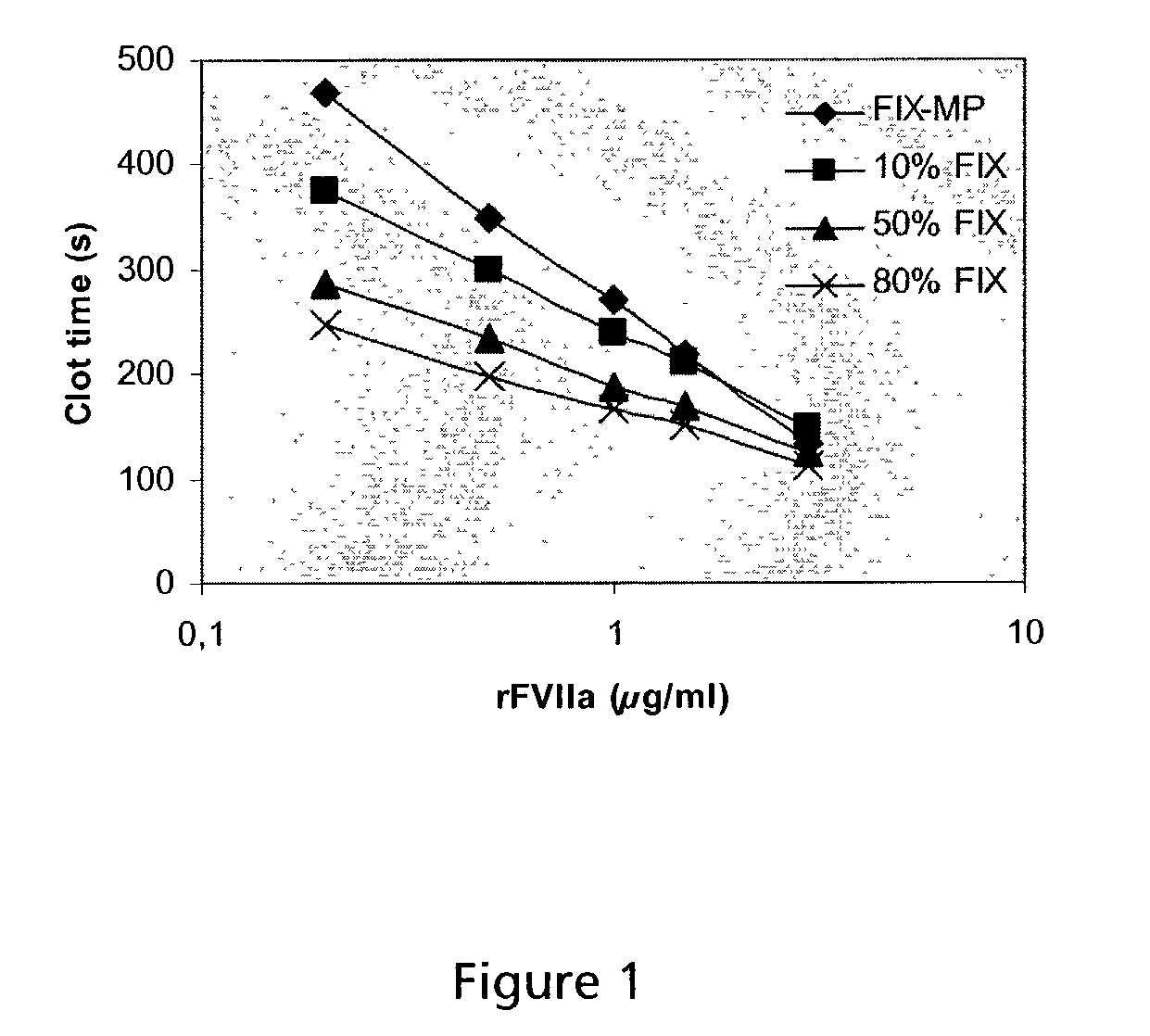
Formulations for factor IX
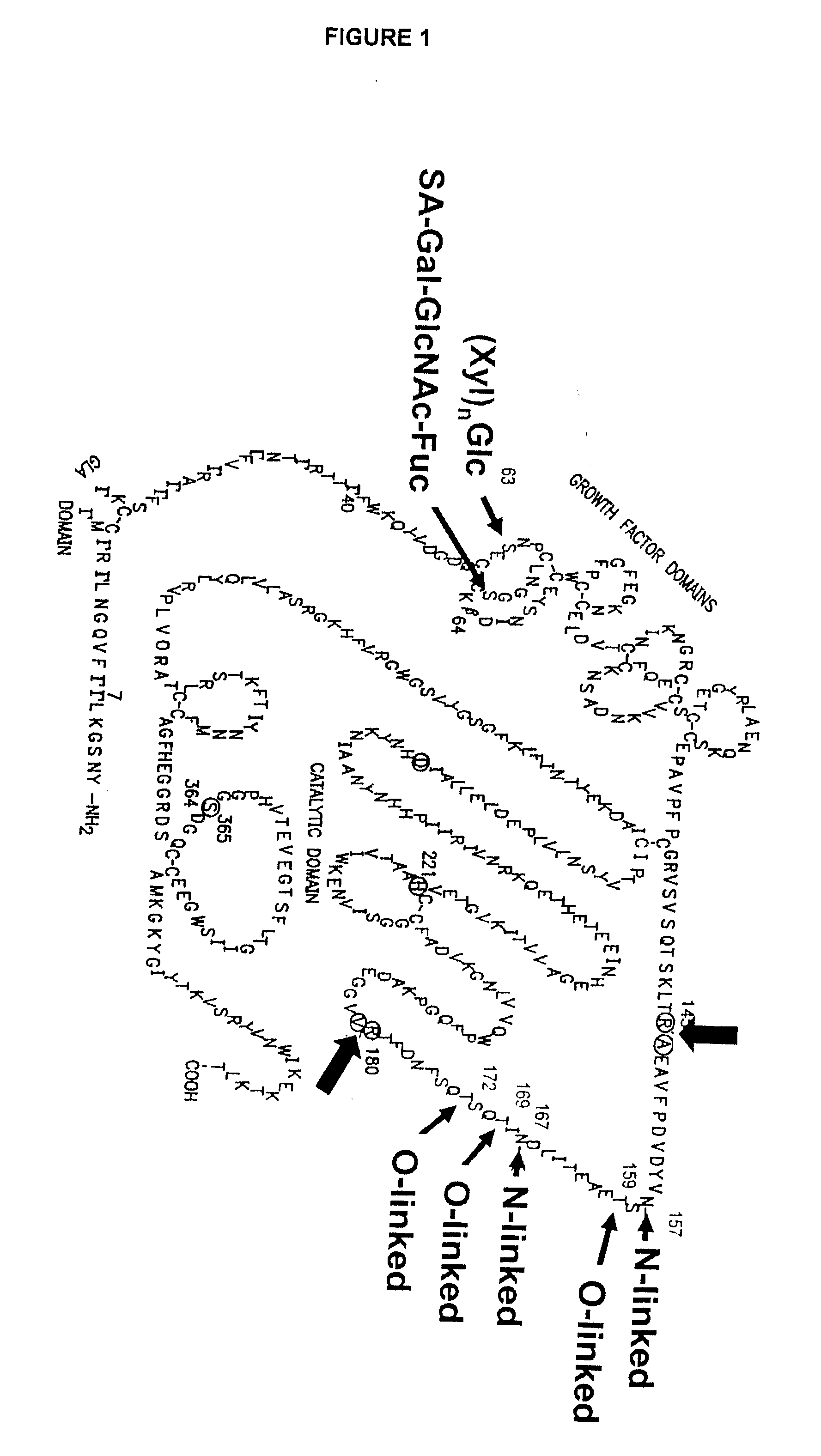
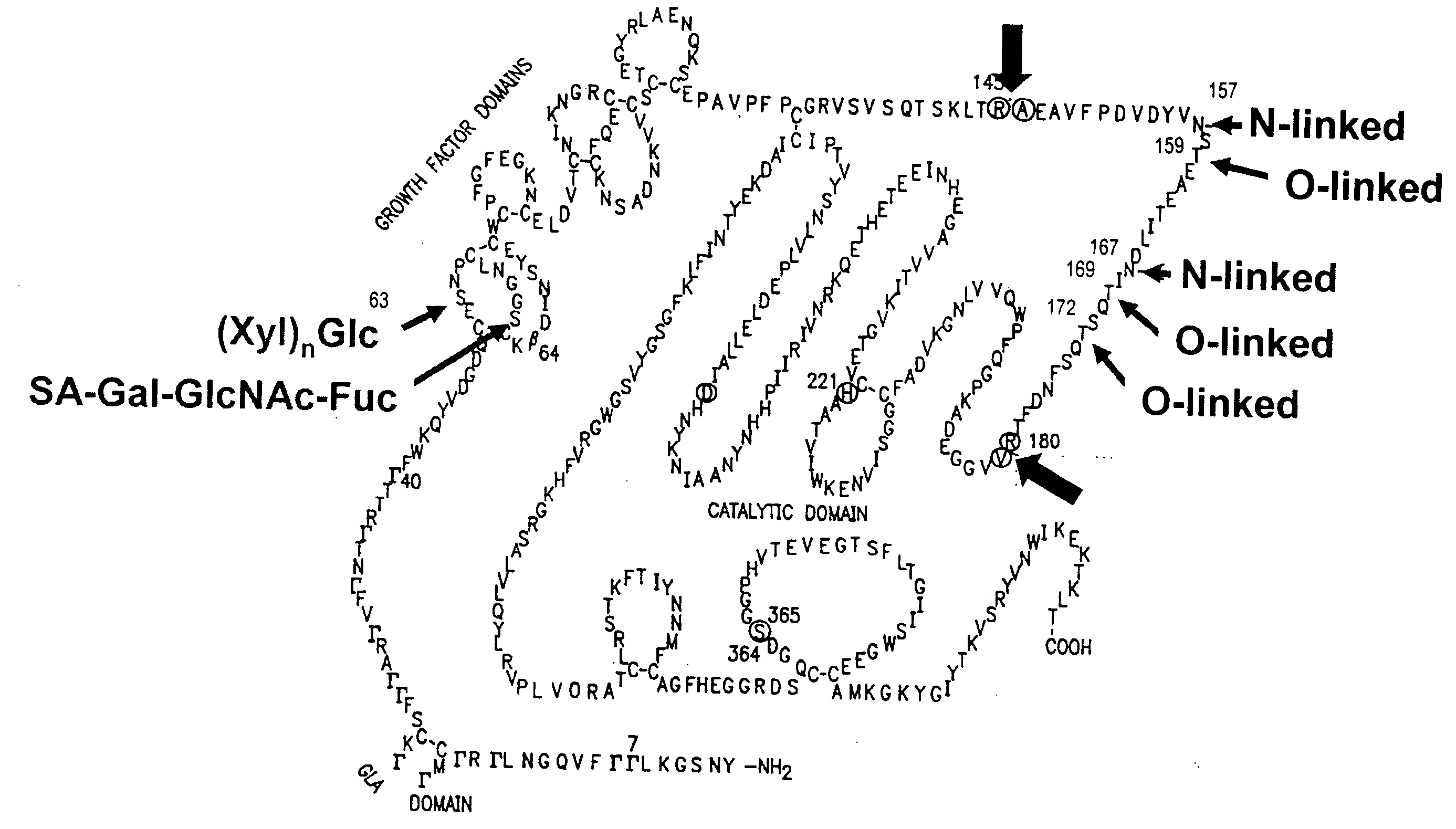
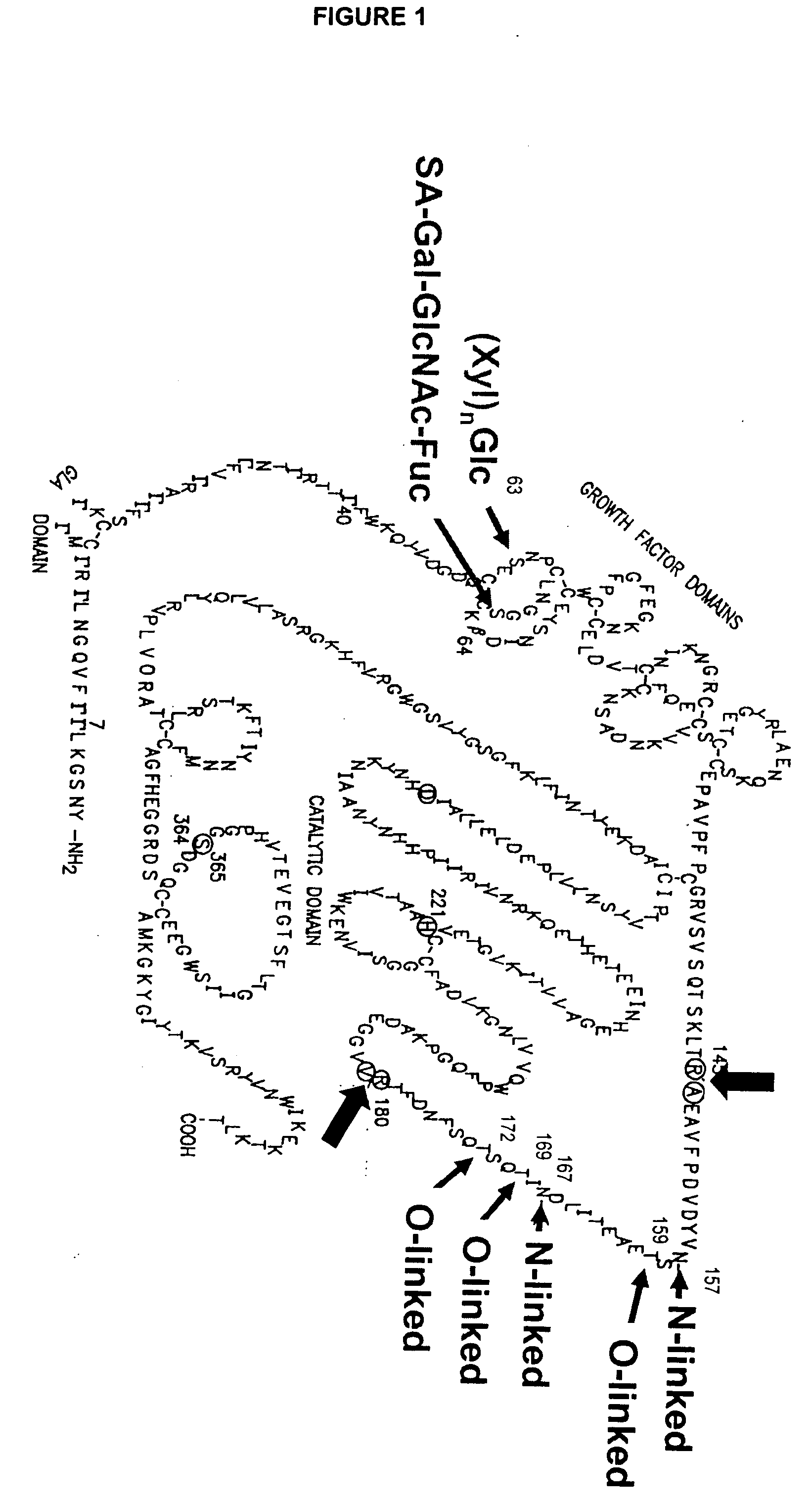
InactiveUS6372716B1Pharmaceutical delivery mechanismSaccharide peptide ingredientsFactor iiFactor IX
Owner:GENETICS INST INC
Modified polynucleotides for the production of factor ix
InactiveUS20130245103A1Organic active ingredientsPeptide/protein ingredientsNucleotidePolynucleotide
Provided are formulations, compositions and methods for delivering biological moieties such as modified nucleic acids into cells to modulate protein expression. Such compositions and methods include the delivery of biological moieties, and are useful for production of proteins.
Owner:MODERNATX INC
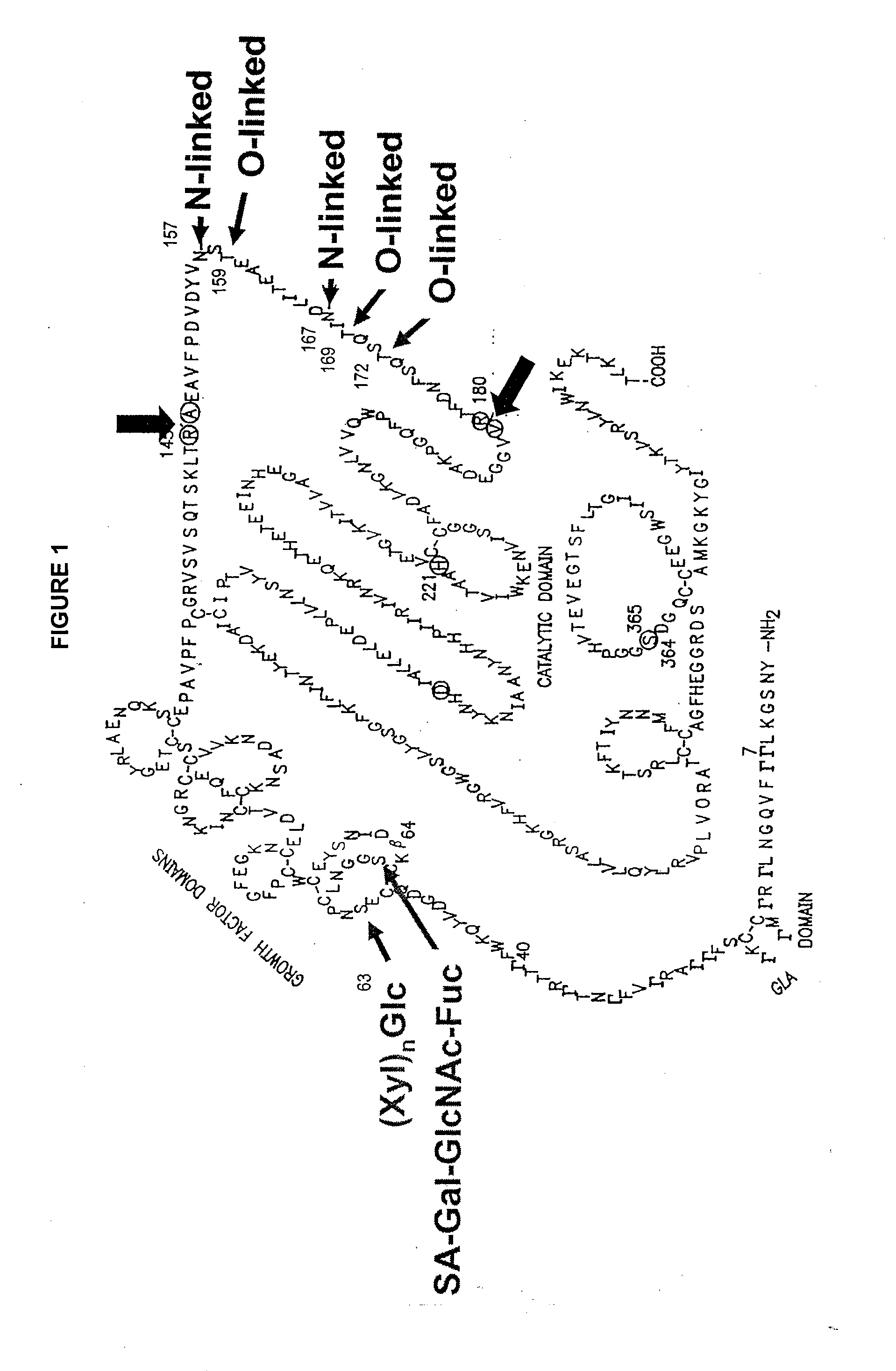
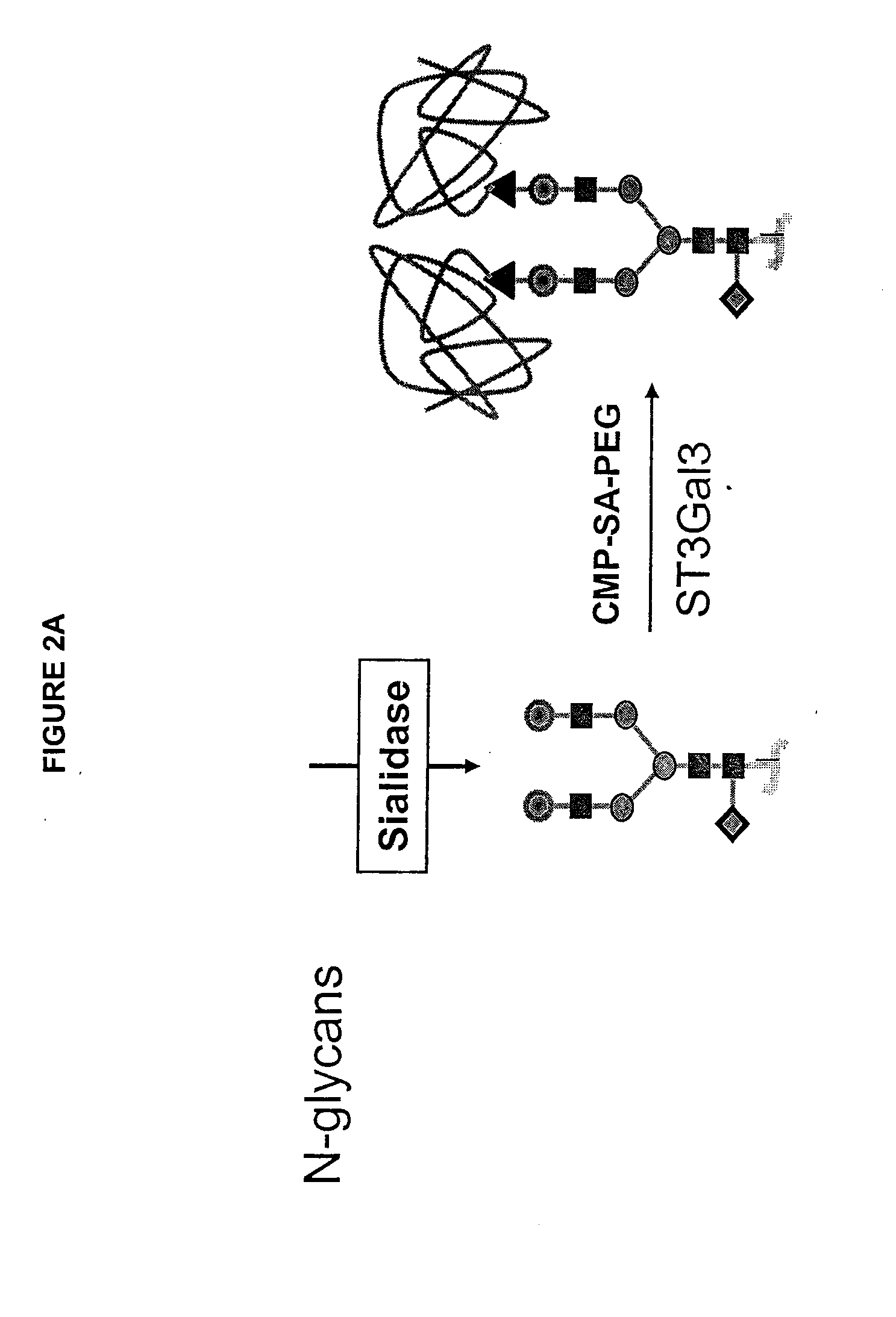
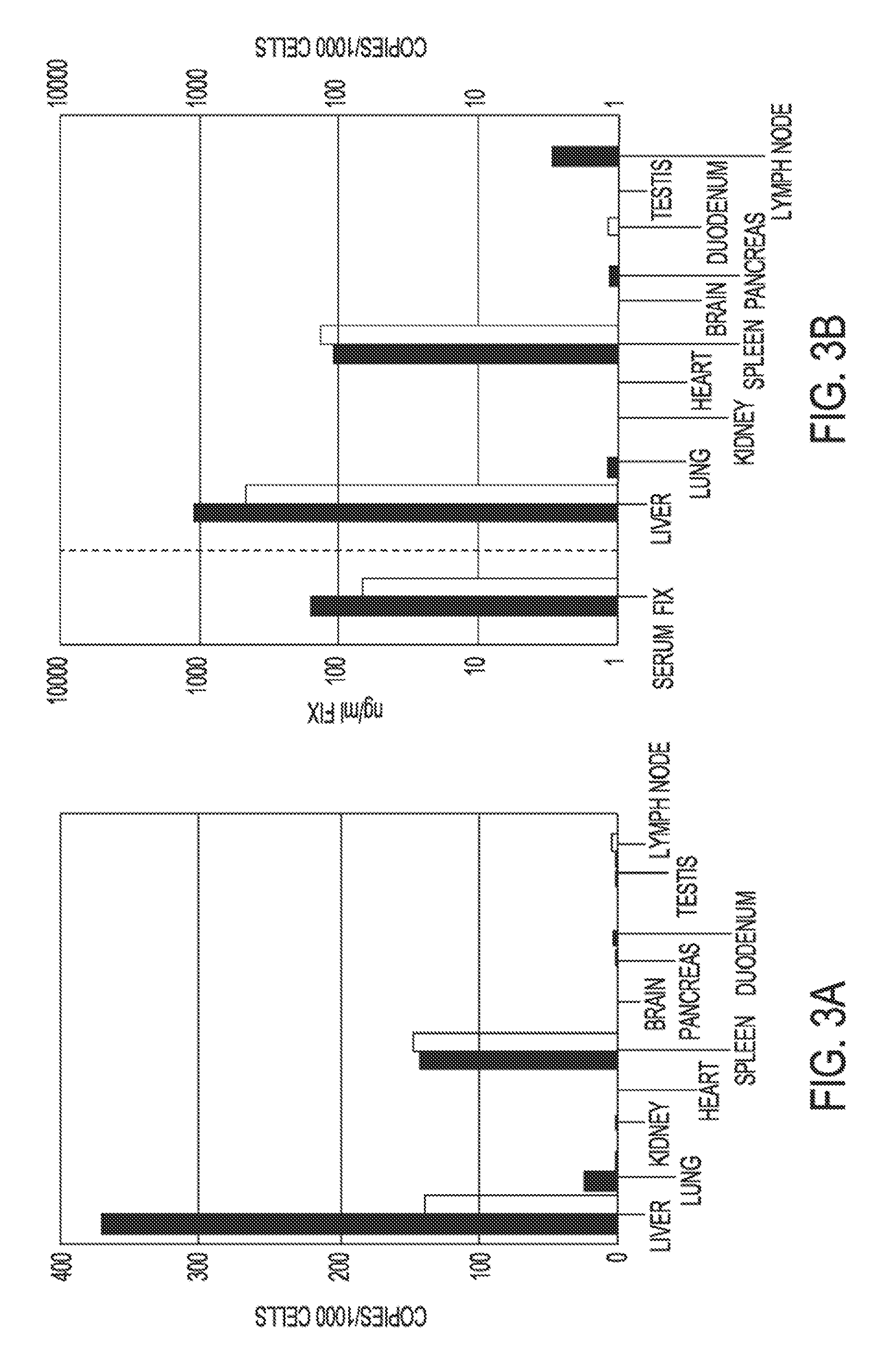
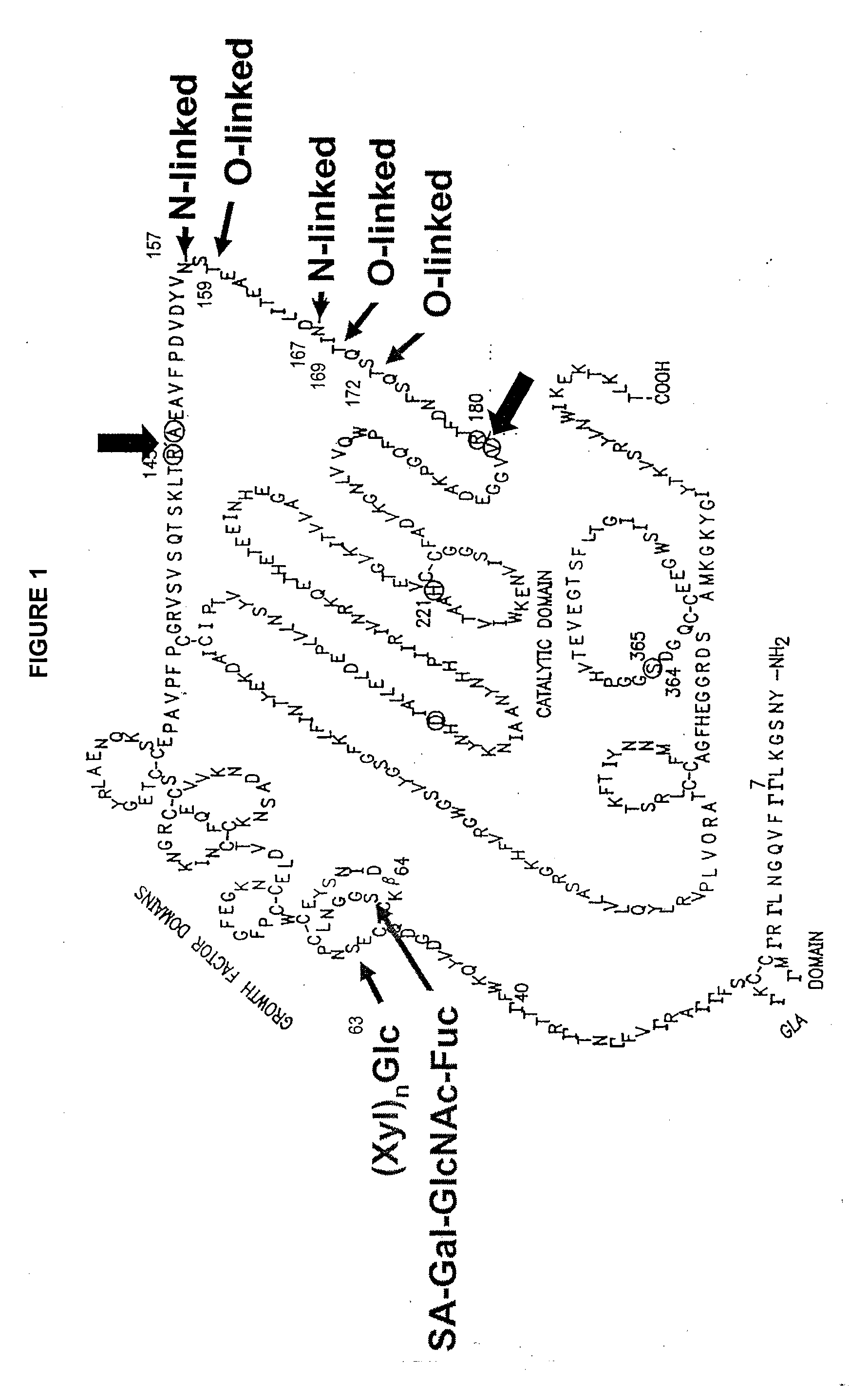
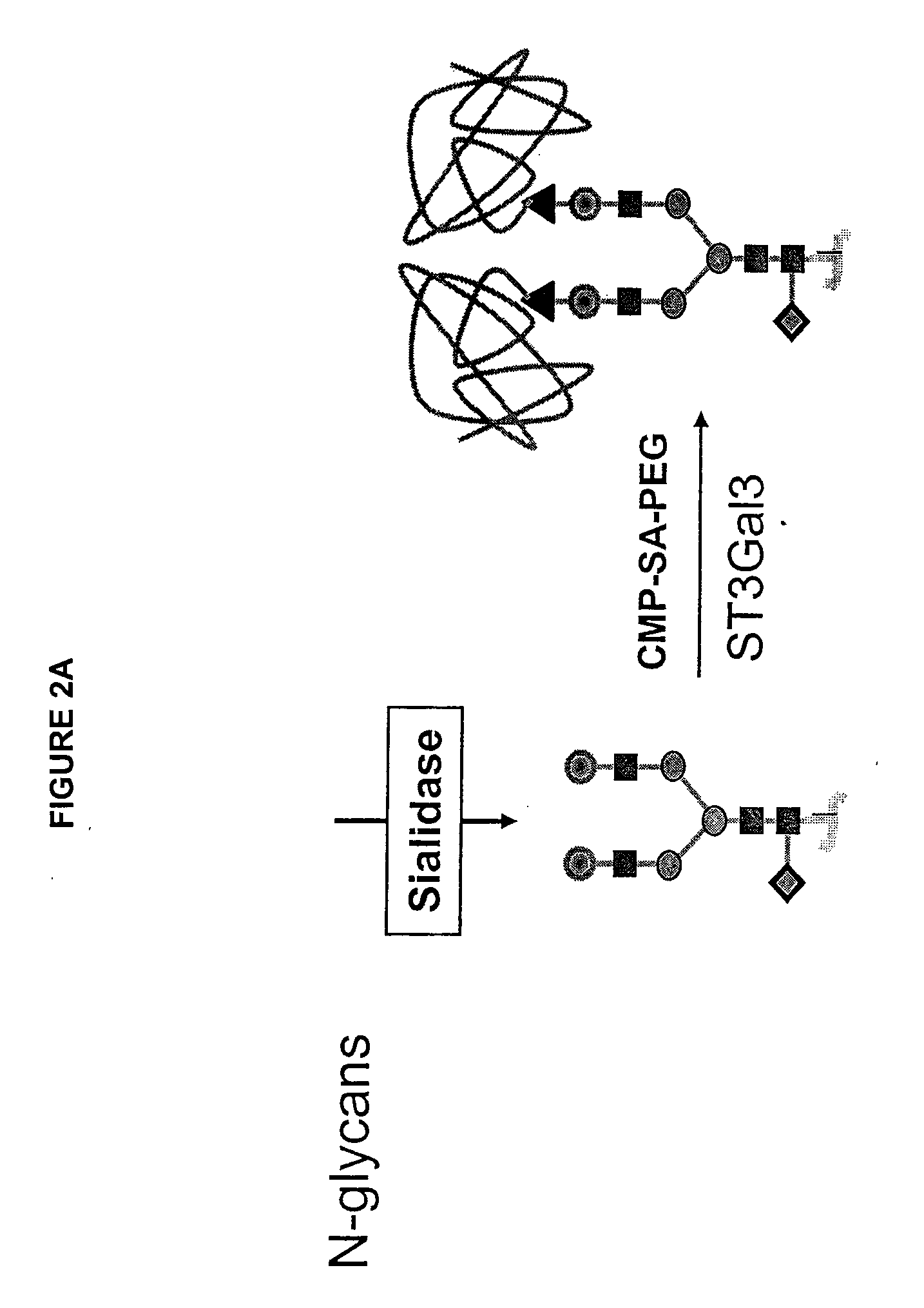
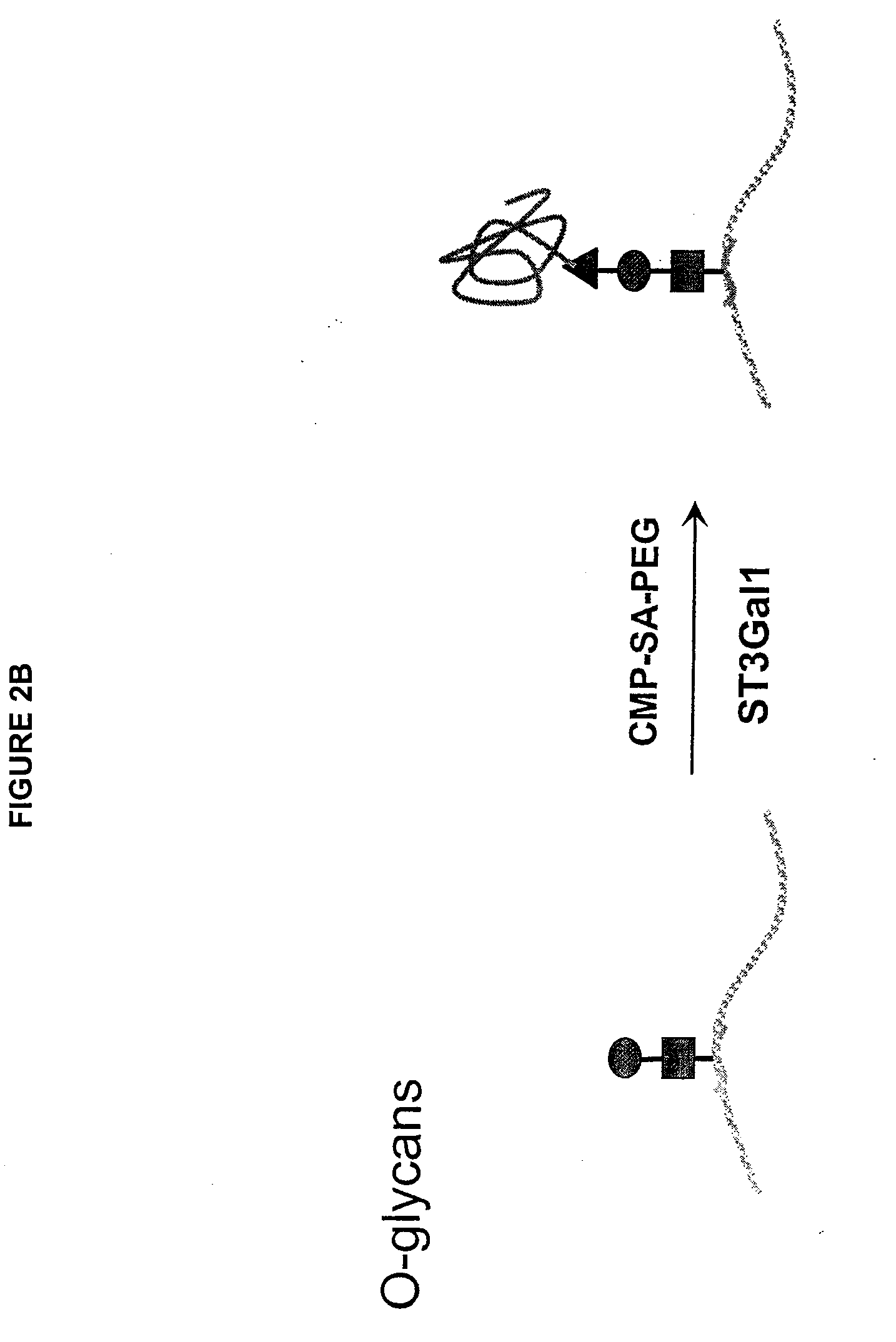
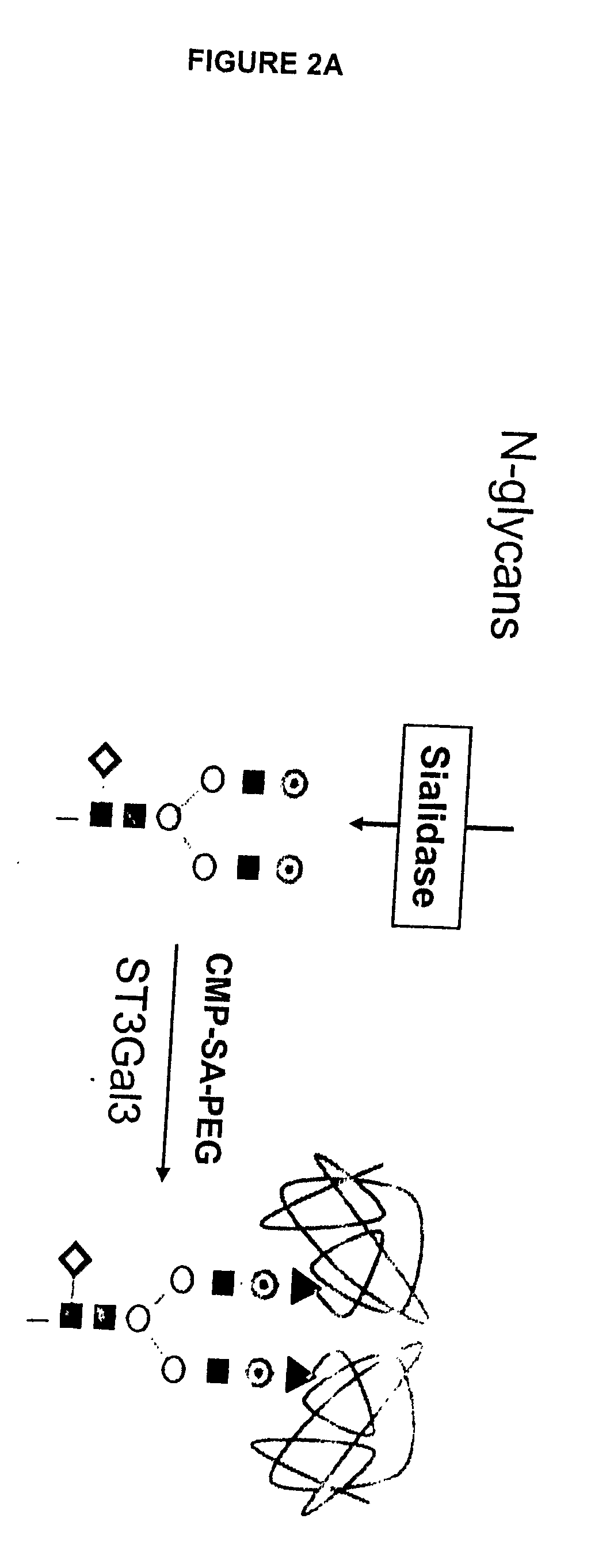
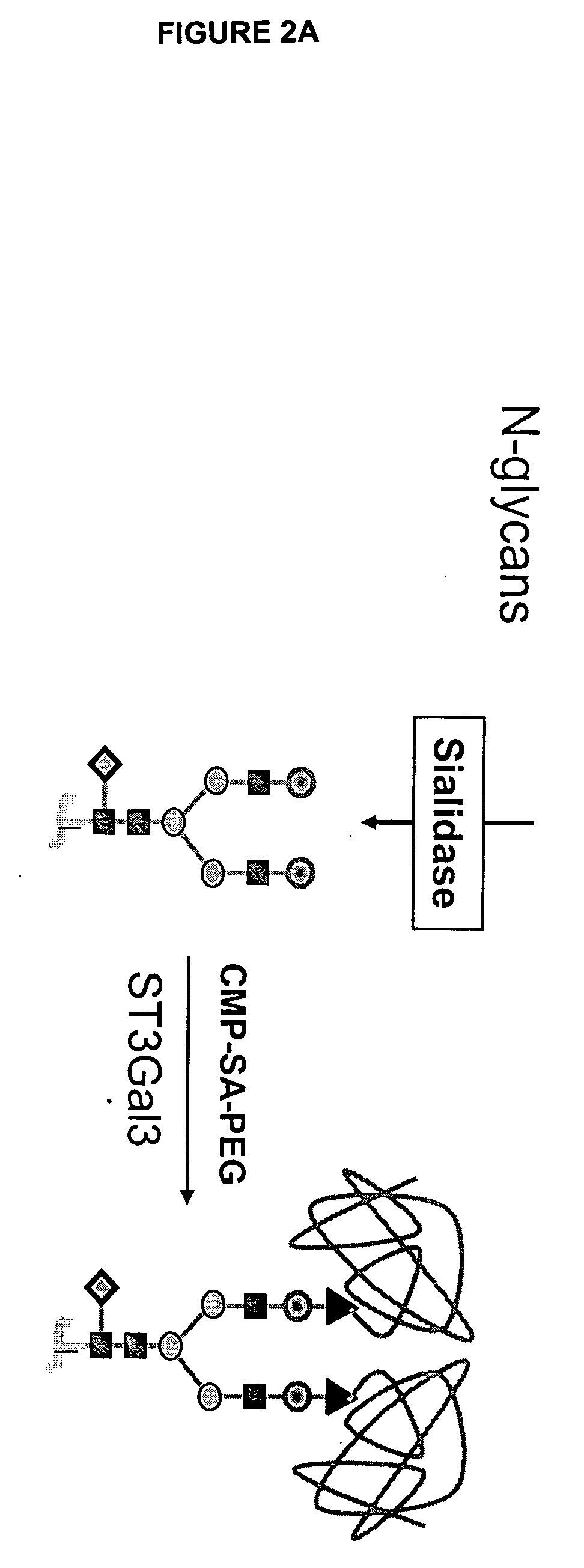
Glycopegylated factor IX
InactiveUS20060040856A1Improved pharmacokinetic propertiesRetain pharmacological activitySaccharide peptide ingredientsMammal material medical ingredientsGlycoPEGylated factor VIIaPharmaceutical formulation
The present invention provides conjugates between Factor IX and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NEOSE TECH
Aryl and heteroaryl compounds, compositions, and methods of use
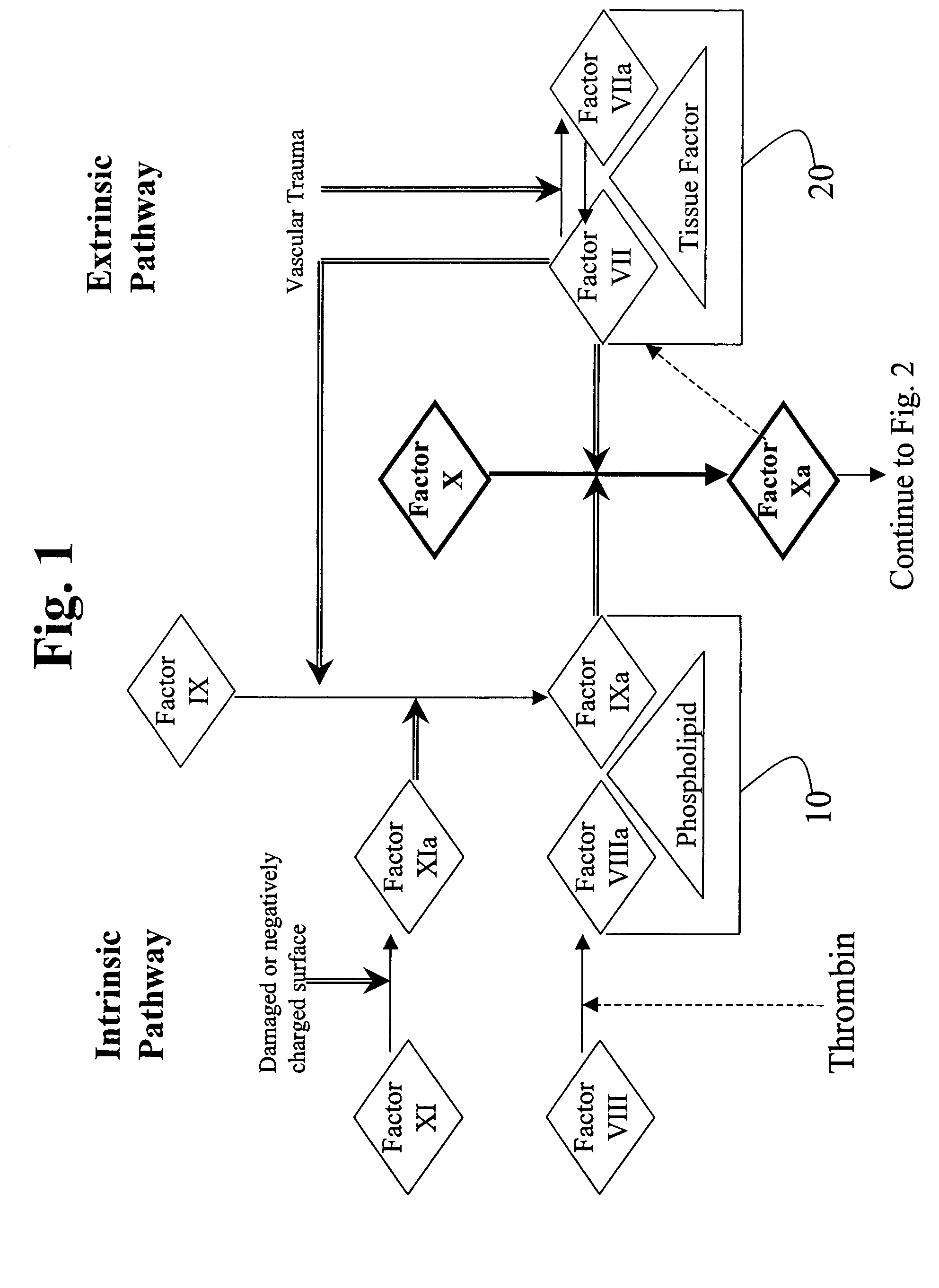
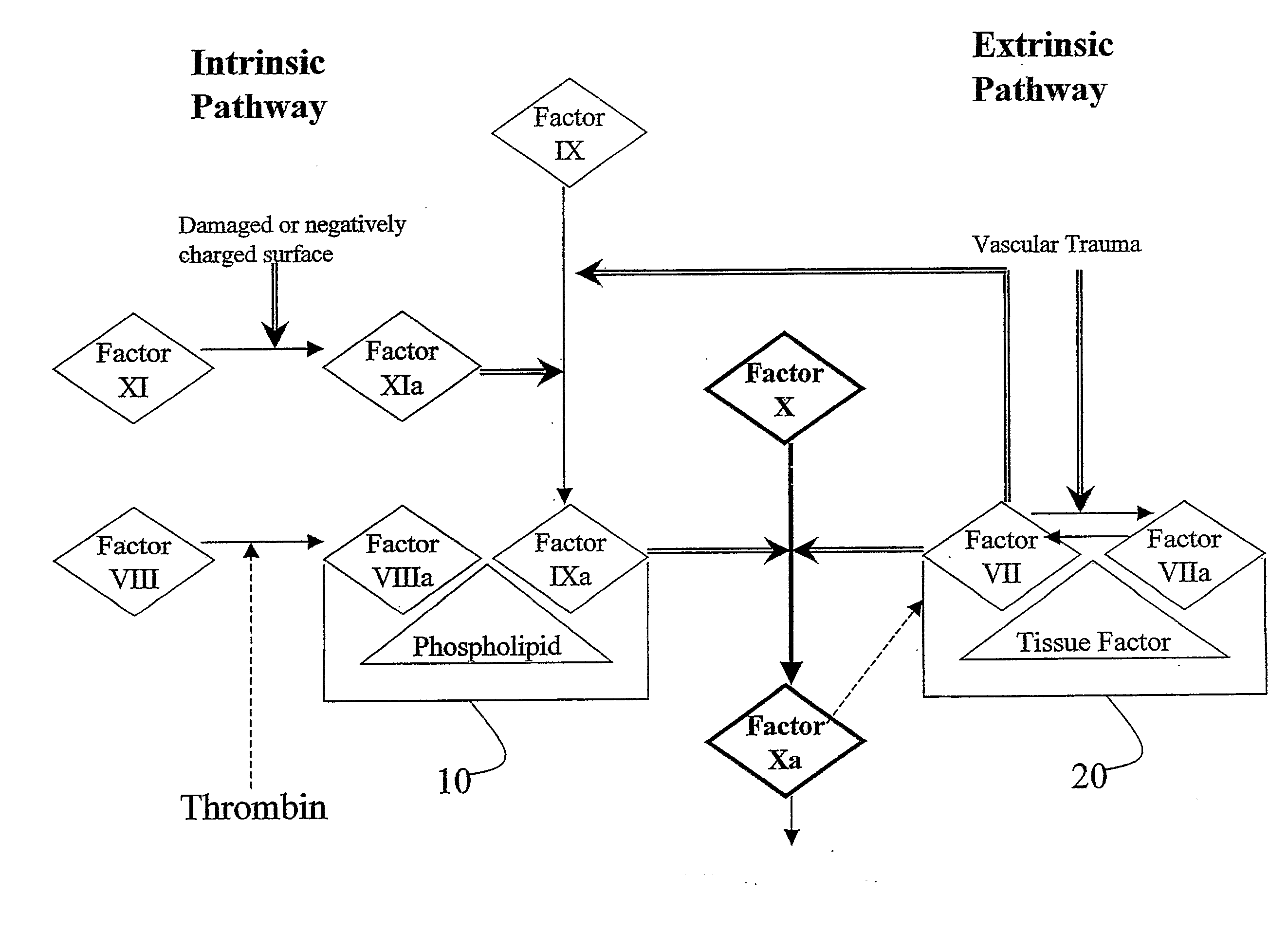
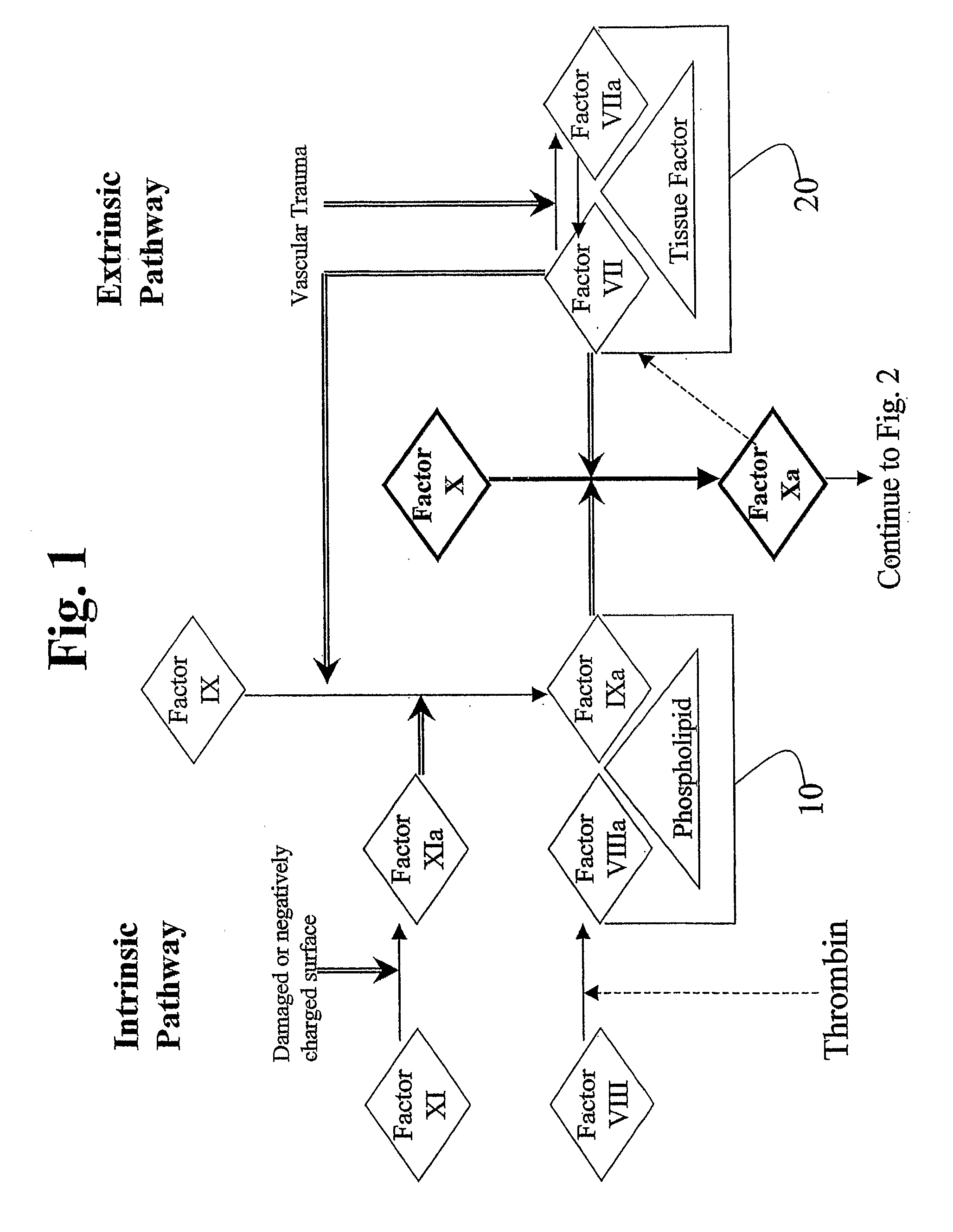
This invention provides aryl and heteroaryl compounds, methods of their preparation, pharmaceutical compositions comprising the compounds, and their use in treating human or animal disorders. The compounds of the invention may be useful as antagonists, or partial antagonist of factor IX and / or factor XI and thus, may be used to inhibit the intrinsic pathway of blood coagulation. The compounds may be useful in a variety of applications including the management, treatment and / or control of diseases caused in part by the intrinsic clotting pathway utilizing factor IX and / or XI.
Owner:TRANSTECH PHARMA INC
Aryl and heteroaryl compounds, compositions, methods of use
This invention provides aryl and heteroaryl compounds, methods of their preparation, pharmaceutical compositions comprising the compounds, and their use in treating human or animal disorders. The compounds of the invention may be useful as antagonists, or partial antagonist of factor IX and / or factor XI and thus, may be used to inhibit the intrinsic pathway of blood coagulation. The compounds may be useful in a variety of applications including the management, treatment and / or control of diseases caused in part by the intrinsic clotting pathway utilizing factor IX and / or XI.
Owner:TRANSTECH PHARMA
Glycopegylated factor ix
InactiveUS20090081188A1Improved pharmacokinetic propertiesRetain pharmacological activityPeptide/protein ingredientsEnzyme stabilisationDiseasePharmaceutical formulation
The present invention provides conjugates between Factor IX and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Aryl and Heteroaryl Compounds, Compositions, Methods of Use
This invention provides aryl and heteroaryl compounds of formula (X). The compounds of the invention may be useful as antagonists, or partial antagonist of factor IX and / or factor XI and thus, may be used to inhibit the intrinsic pathway of blood coagulation. Formula (X) wherein R102 is selected from the group consisting of —C(O)OH, —C(O)OCH3, —C(O)O-t-butyl, —C(O)NH—OCH2-phenyl, C(O)NHOH, and —C(O)NHSO2CH3; and wherein R101, R103, R104 and Y are as defined herein.
Owner:TRANSTECH PHARMA INC
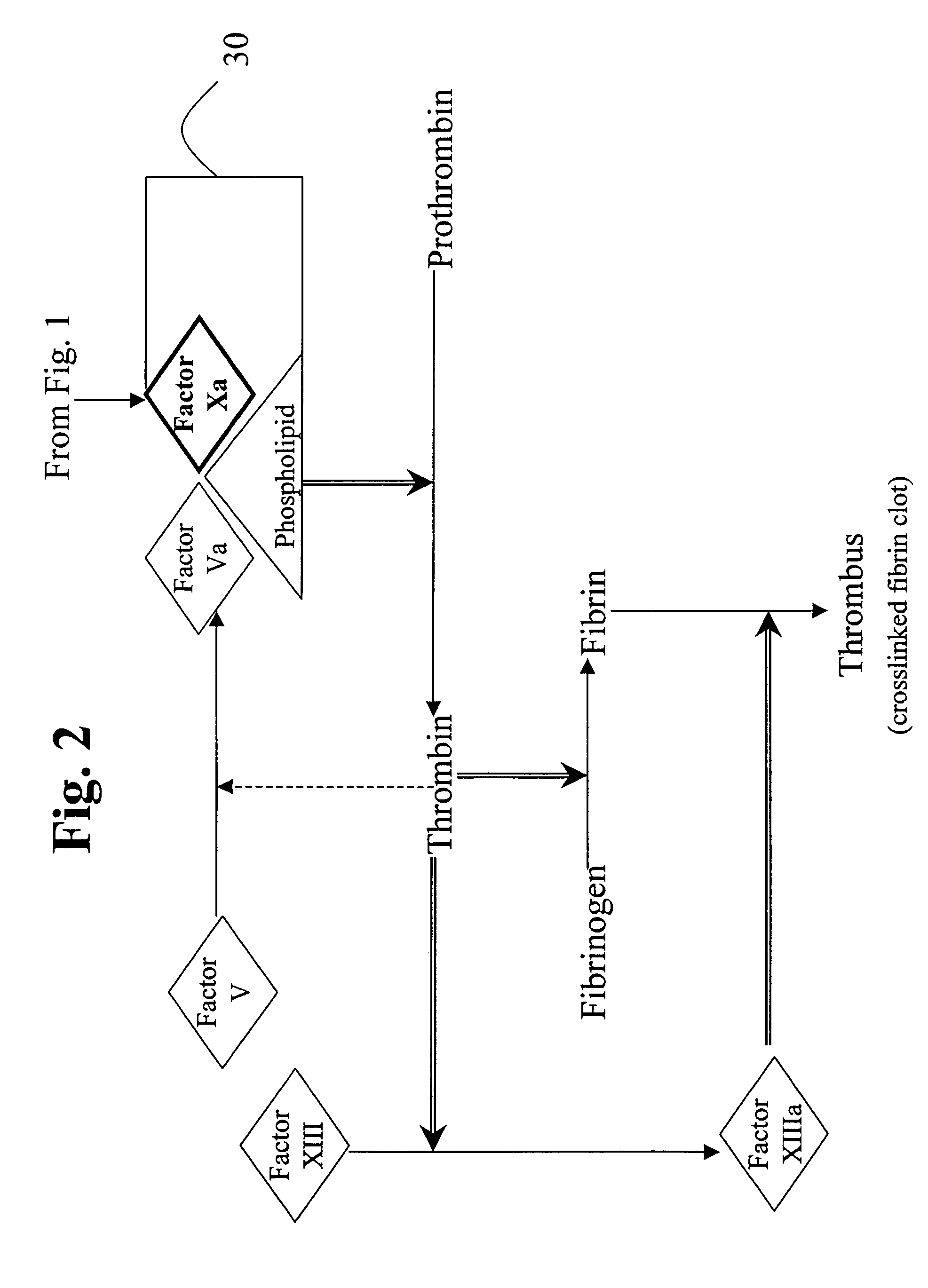
Compositions and methods for treating coagulation related disorders
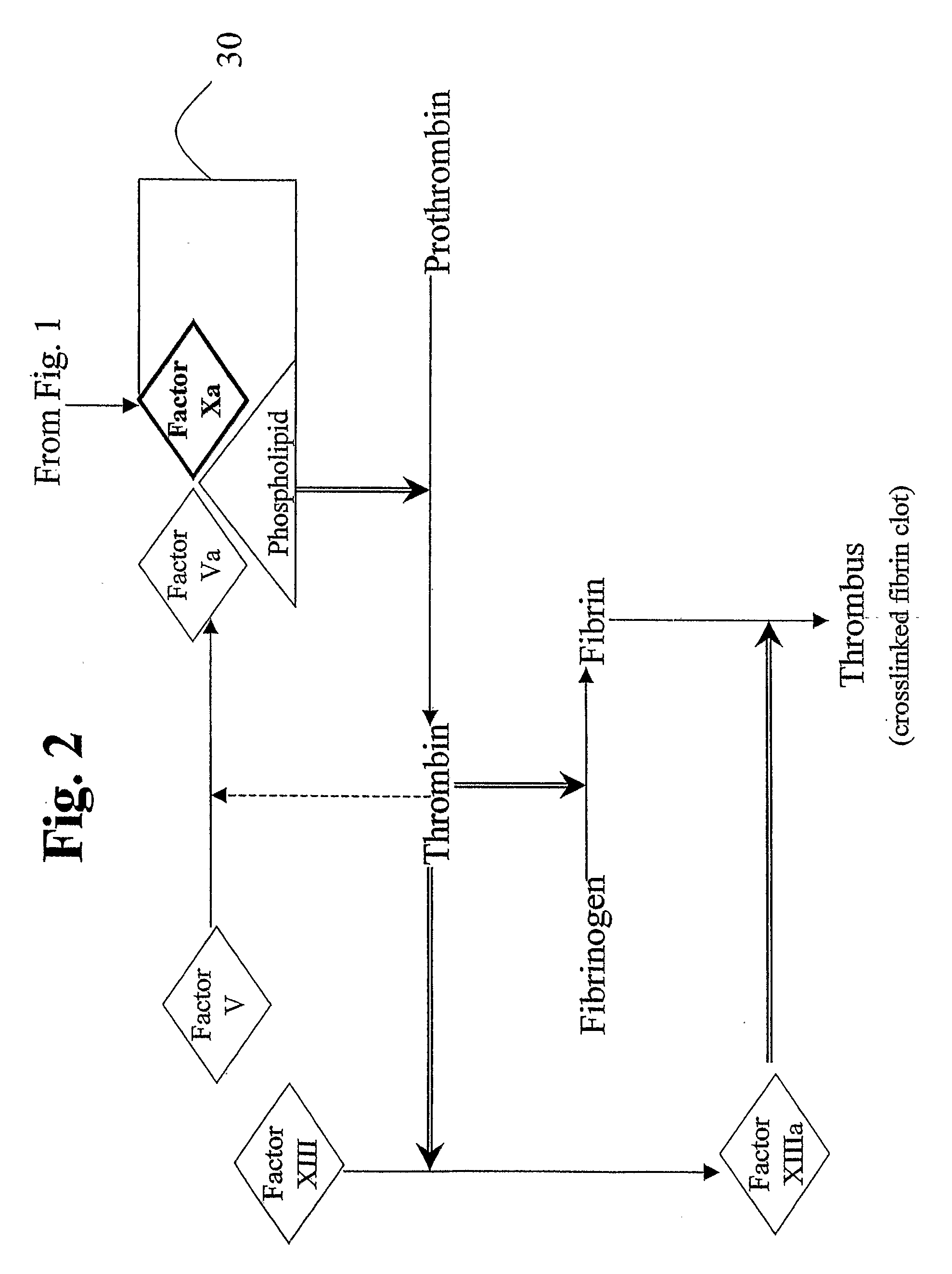
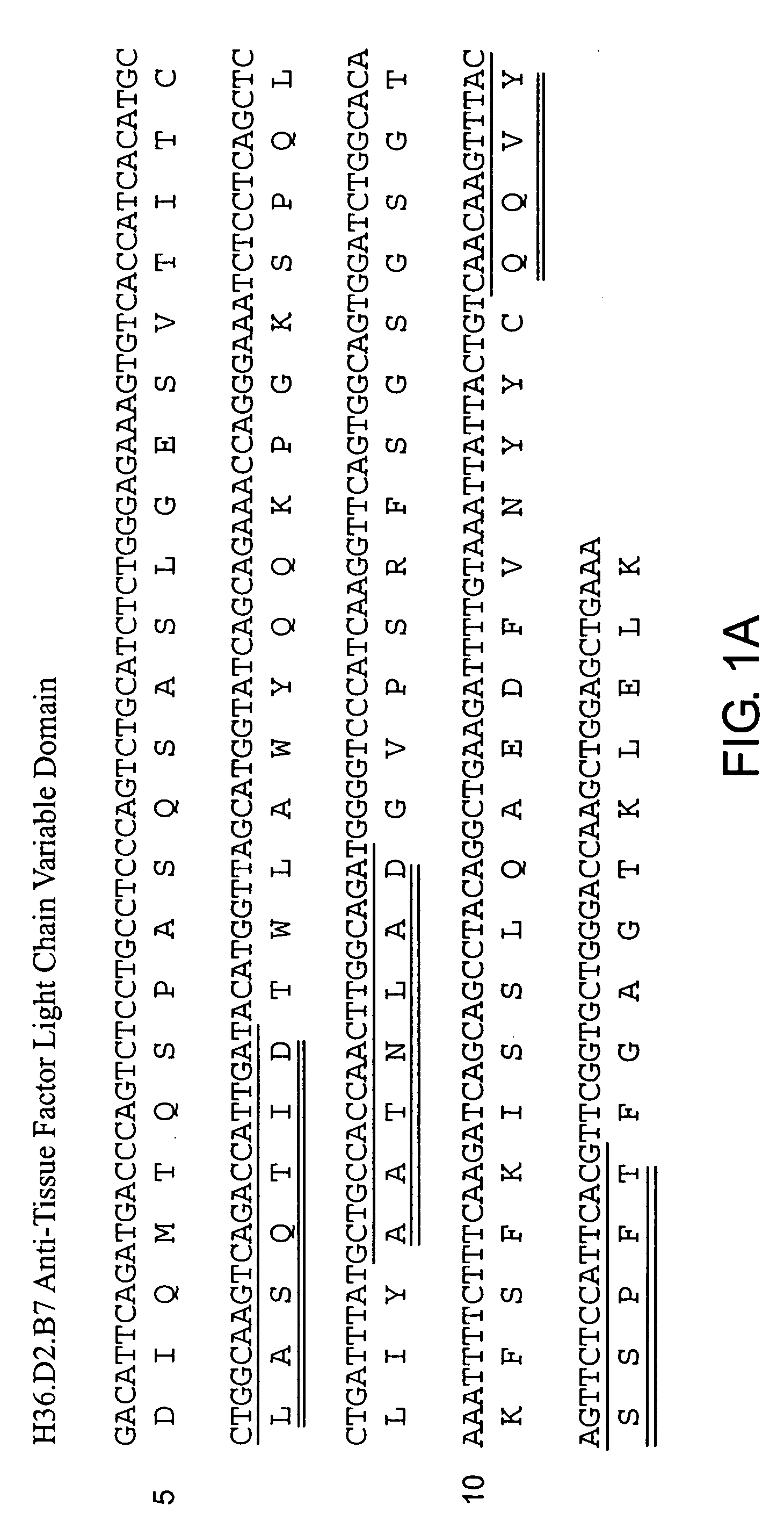
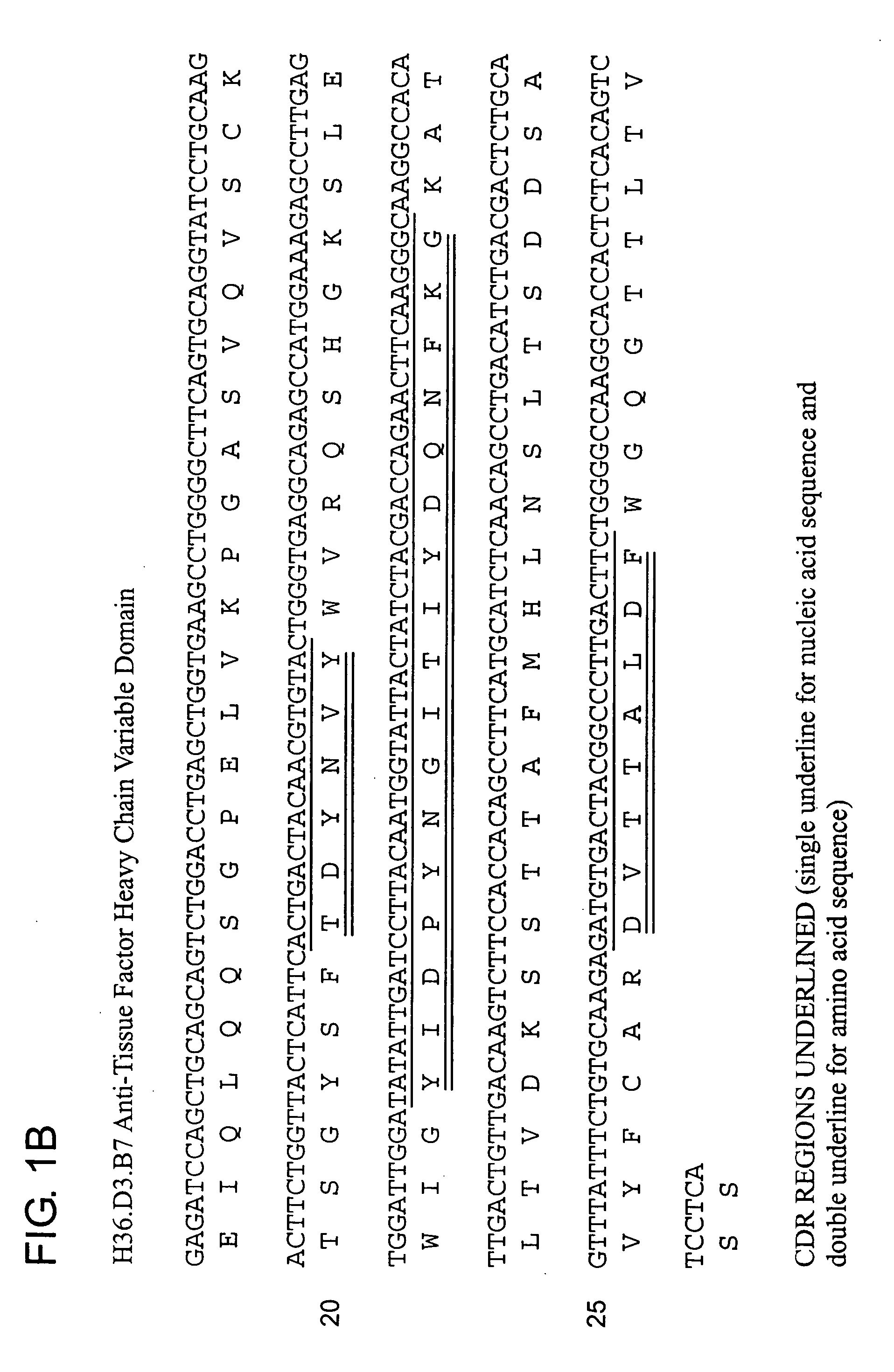
InactiveUS20060159675A1Initiate and prolong such disorderRelieve symptomsImmunoglobulins against blood coagulation factorsAntibacterial agentsDiseaseTissue factor
Disclosed are methods for preventing or treating sepsis, a sepsis-related condition or an inflammatory disease in a mammal. In one embodiment, the method includes administering to the mammal a therapeutically effective amount of at least one humanized antibody, chimeric antibody, or fragment thereof that binds specifically to tissue factor (TF) to form a complex in which factor X or factor IX binding to the complex is inhibited and the administration is sufficient to prevent or treat the sepsis in the mammal. The invention has a wide spectrum of useful applications including treating sepsis, disorders related to sepsis, and inflammatory diseases such as arthritis.
Owner:GENENTECH INC
Factor IX Polypeptides and Methods of Use Thereof
Owner:BIOVERATIV THERAPEUTICS INC
Aryl and heteroaryl compounds, compositions, and methods of use
This invention provides aryl and heteroaryl compounds, methods of their preparation, pharmaceutical compositions comprising the compounds, and their use in treating human or animal disorders. The compounds of the invention may be antagonists, or partial antagonist of factor IX and / or factor XI and thus, may be useful for inhibiting the intrinsic pathway of blood coagulation. The compounds may be useful in a variety of applications including the management, treatment and / or control of diseases caused in part by the intrinsic clotting pathway.
Owner:VTV THERAPEUTICS LLC
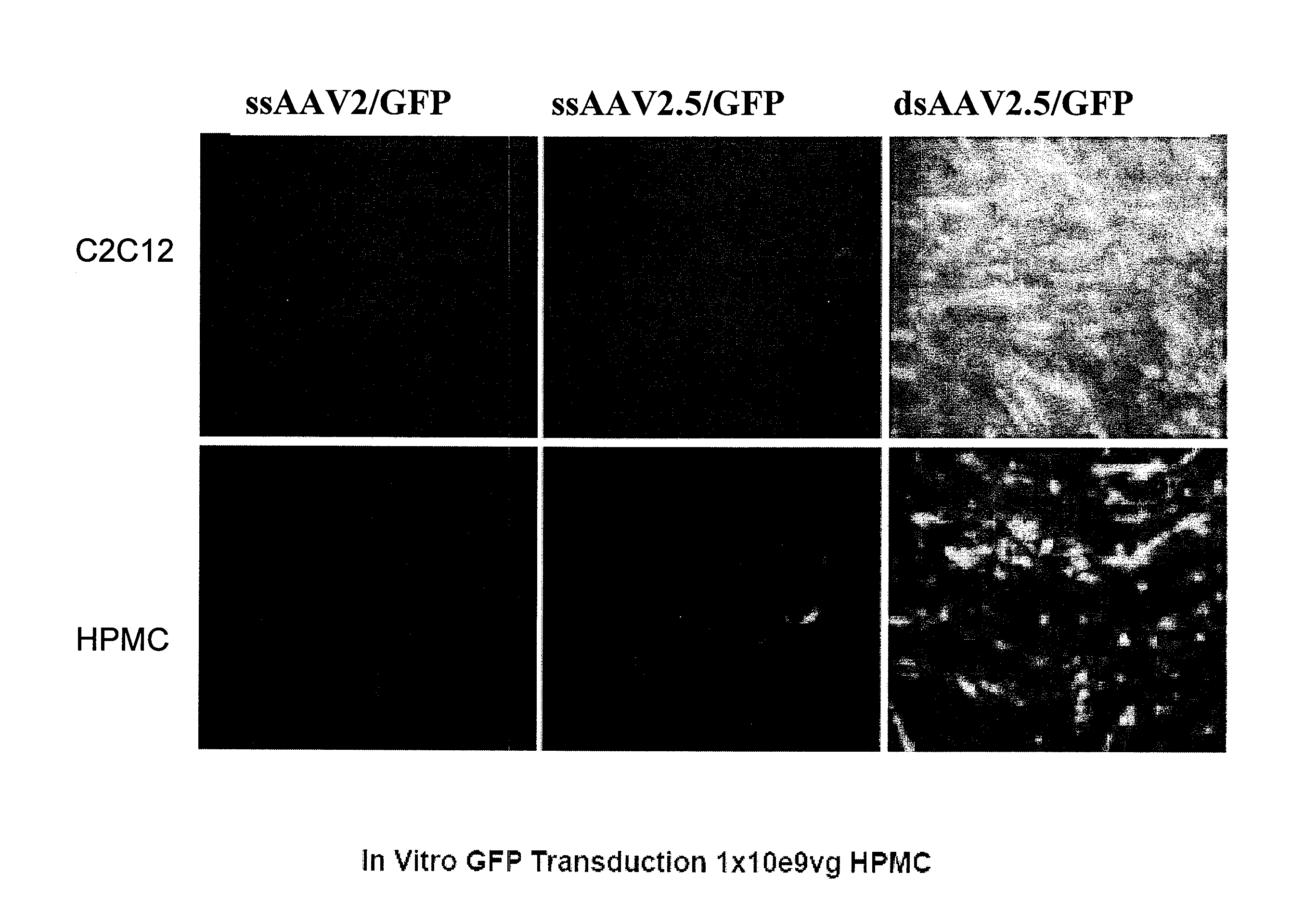
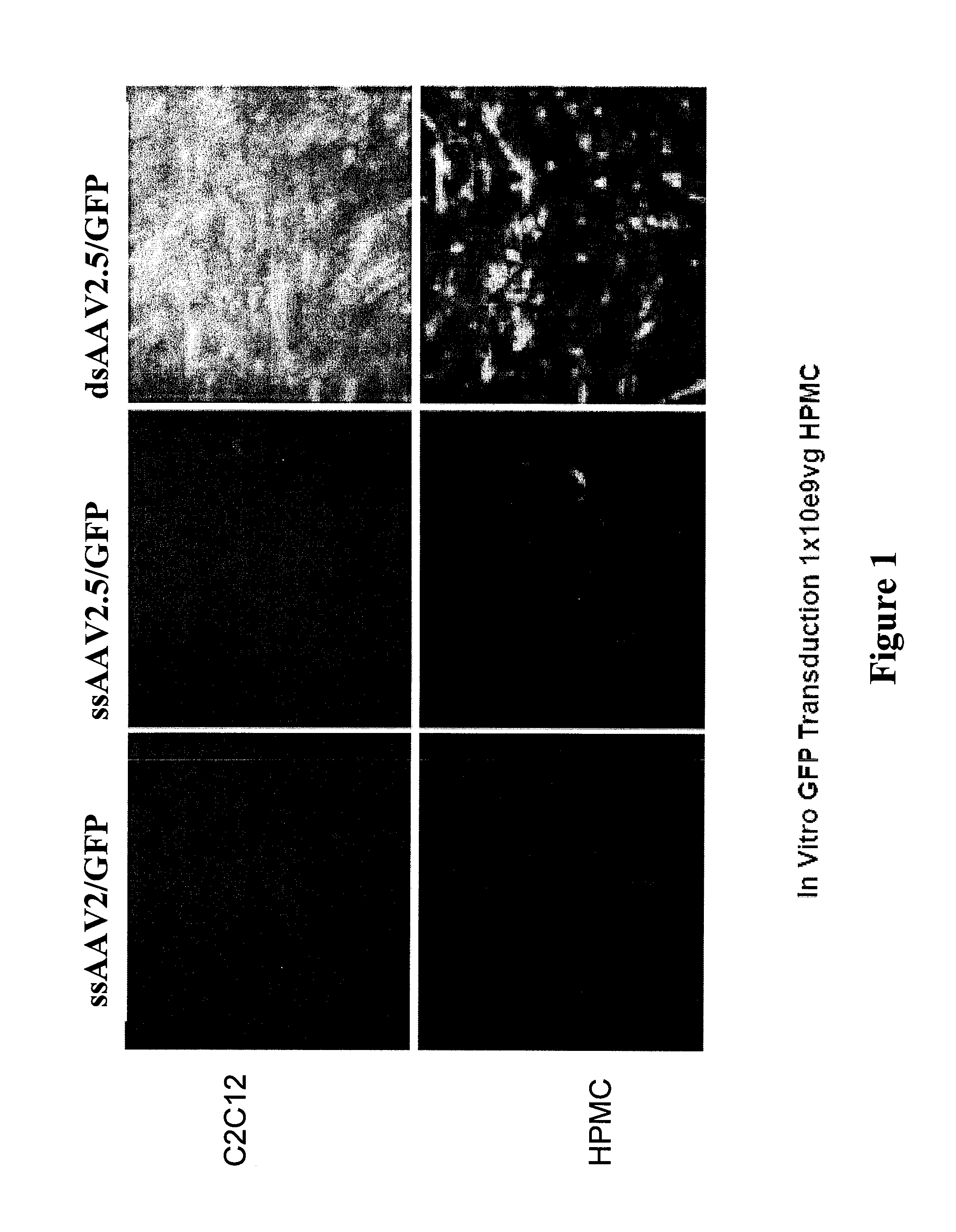
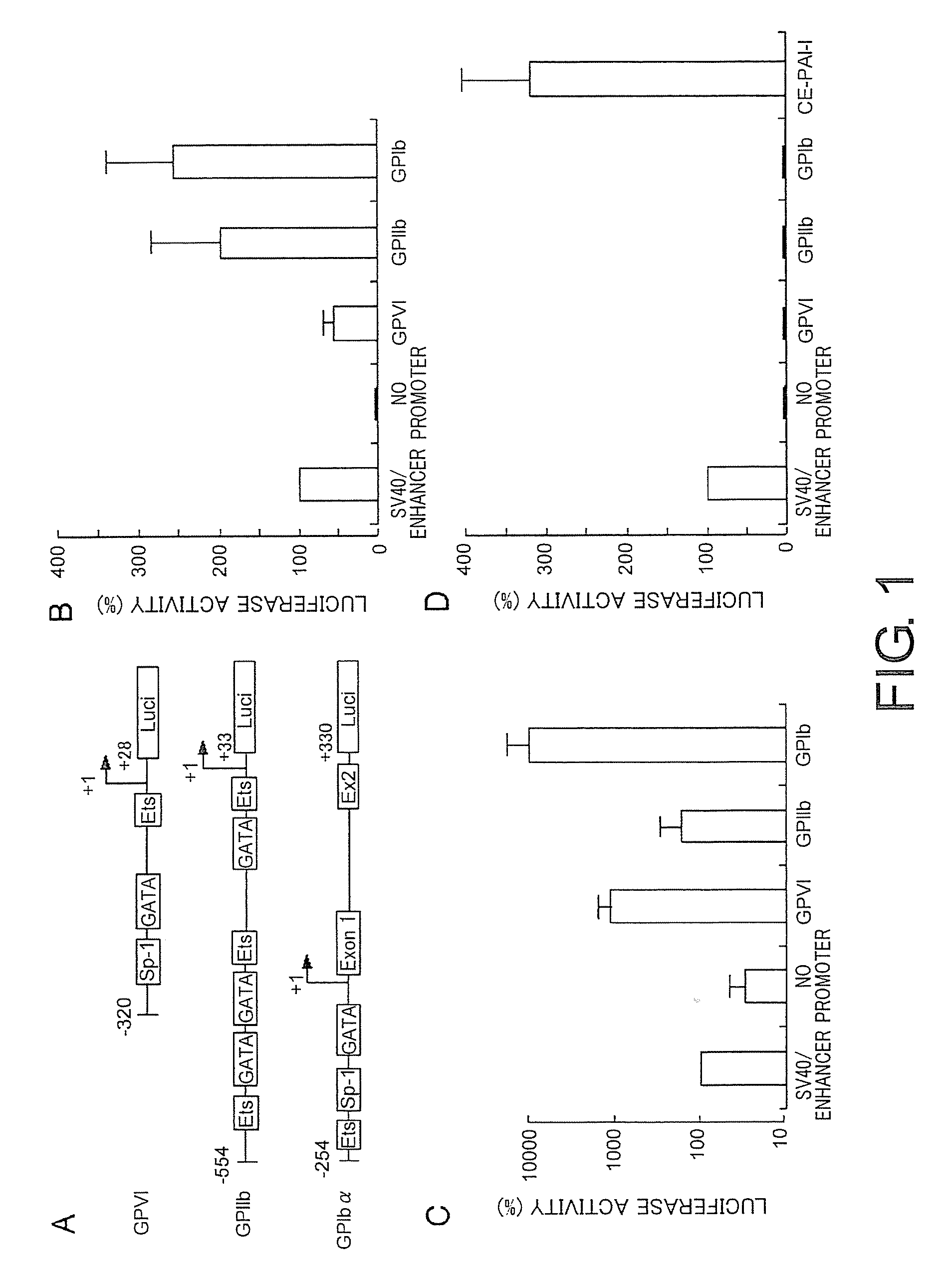
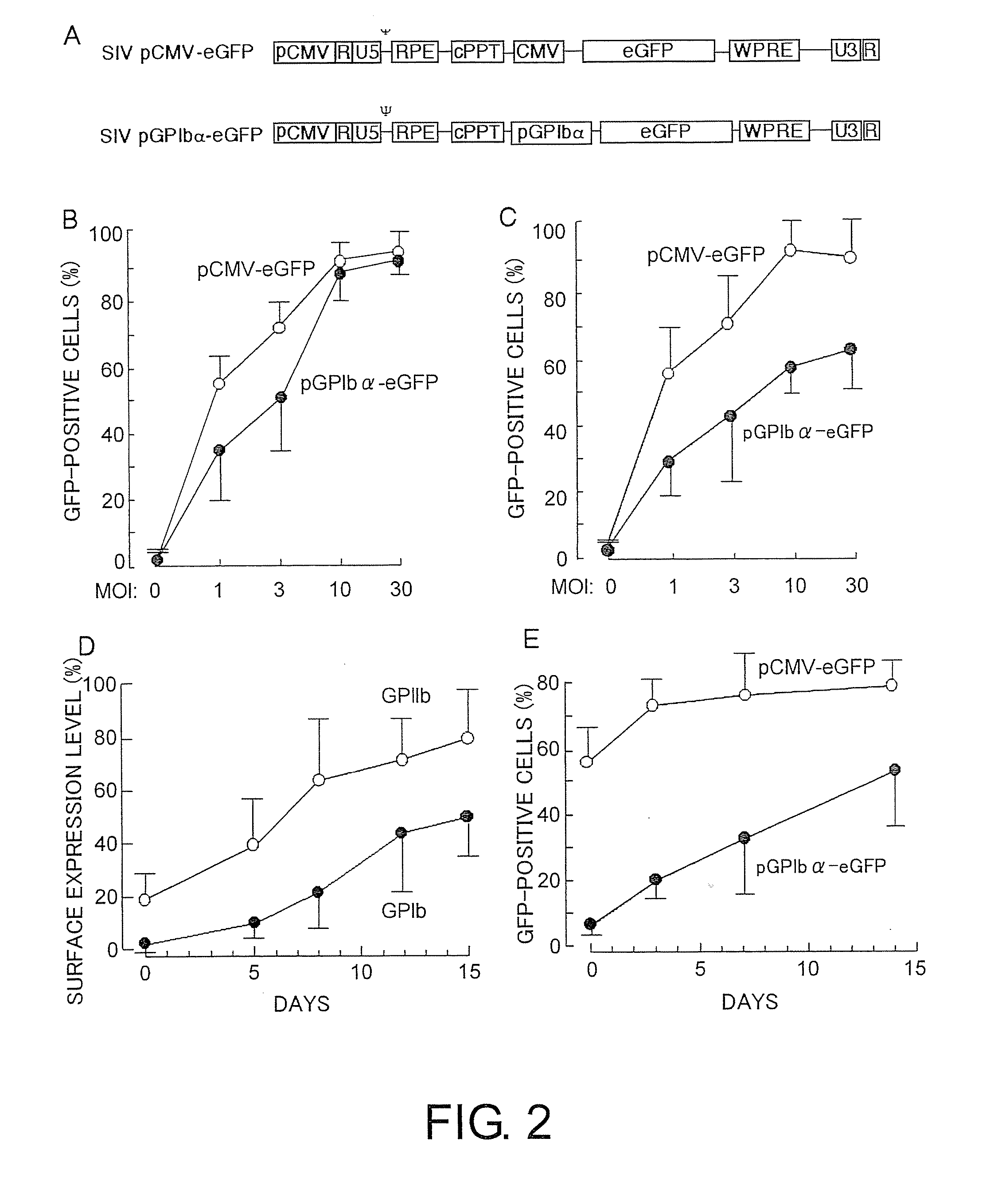
Lentiviral vectors featuring liver specific transcriptional enhancer and methods of using same
Recombinant lentiviruses and transfer vectors for transgene delivery as well as methods for gene therapy using such vectors are disclosed. The invention provides a third generation lentiviral packaging system and a set of vectors for producing recombinant lentiviruses, as well as novel tissue specific enhancer and promoter elements useful for optimizing liver specific transgene delivery. The transgene is preferably a blood clotting factor such as human factor IX (hFIX) or human factor VIII (hFVIII) and can be used for treatment of hemophilia.
Owner:MILTENYI BIOTEC B V & CO KG
Glycopegylated factor ix
ActiveUS20100081791A1Promote recoveryPeptide/protein ingredientsMammal material medical ingredientsSugar moietyPharmaceutical formulation
The present invention provides conjugates between Factor IX and PEG moieties. The conjugates are linked via a glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. Conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Recombinant human factor ix and use thereof
ActiveUS20080167219A1Easy to adaptPeptide/protein ingredientsGenetic material ingredientsWild typeFactor ii
The present invention aims at converting factor IX into a molecule with enhanced activity which provides an alternative for replacement therapy and gene therapy for hemophilia B. Using recombinant techniques, factor IX with replacement at positions 86, 277, and 338 exhibits better clotting activity than recombinant wild type factor IX.
Owner:LIN SHU WHA
Factor IX/factor IXa activating antibodies and antibody derivatives
InactiveUS7033590B1High activityRapid blood coagulationImmunoglobulins against blood coagulation factorsImmunoglobulins against blood group antigensActivated factor IXFactor ii
Owner:BAXALTA INC
Methods for treating ischemic disorders using carbon monoxide
InactiveUS20050048133A1Avoid accumulationBiocidePeptide/protein ingredientsGynecologySufficient time
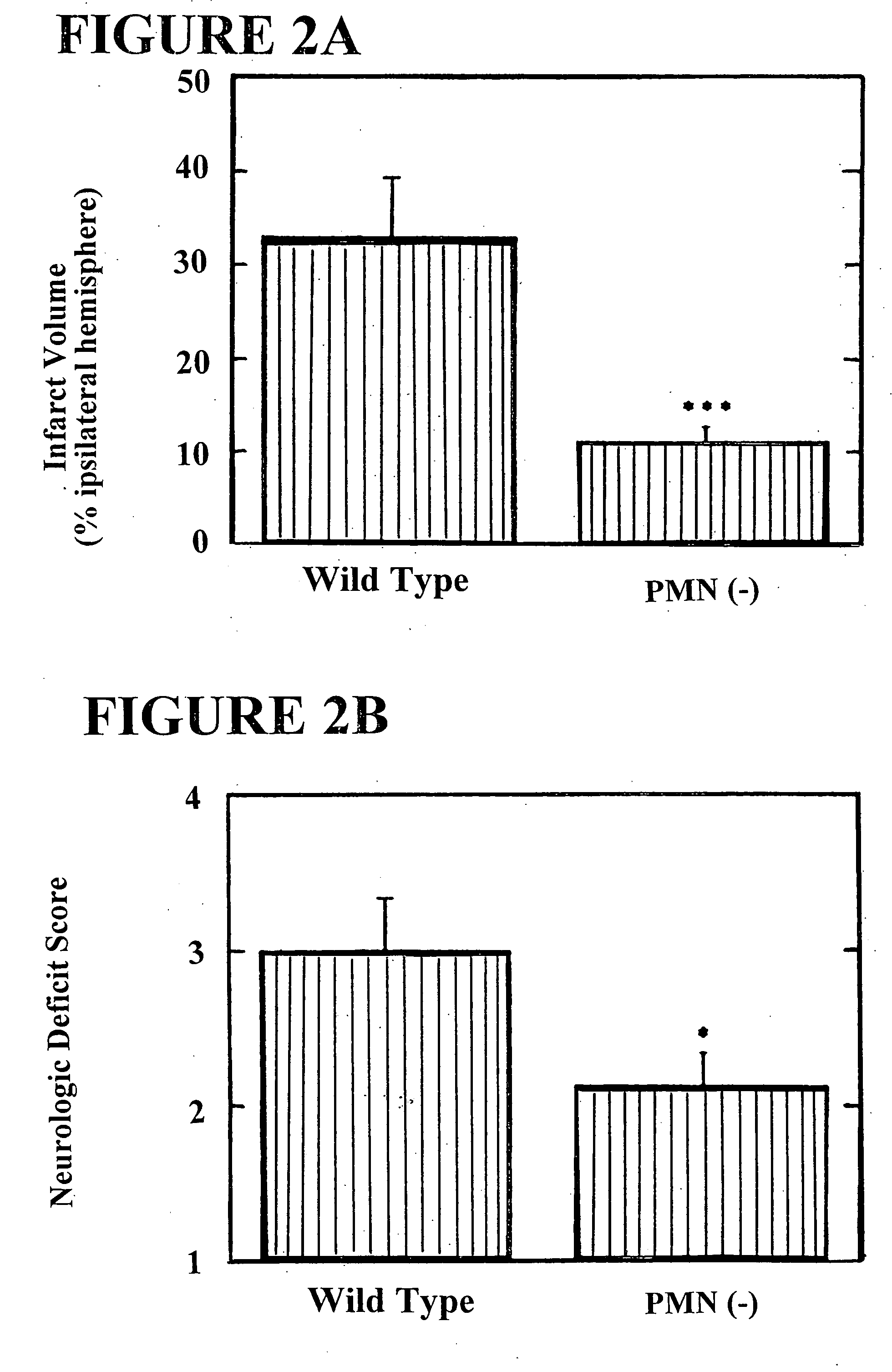
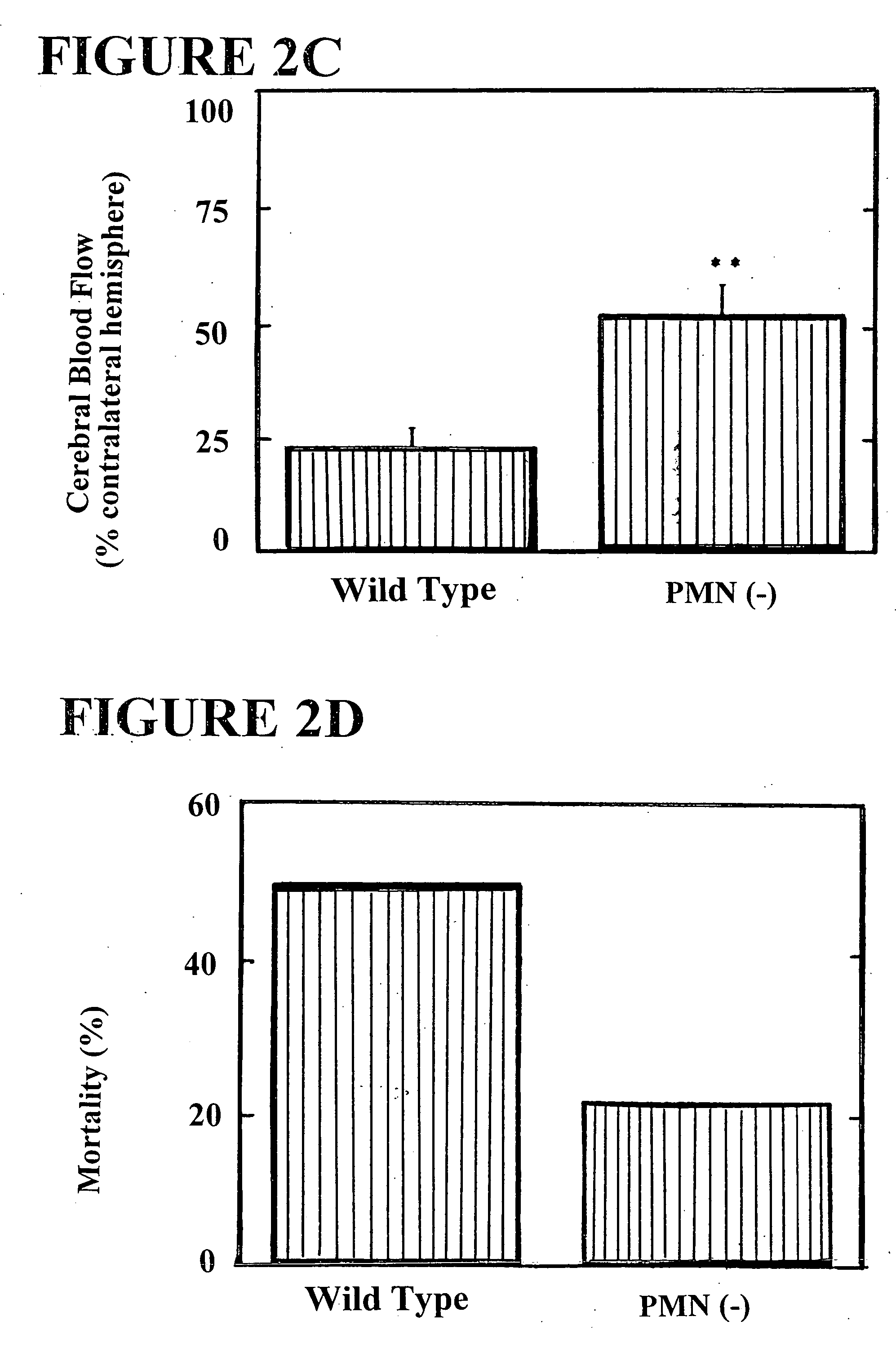
The present invention provides for a method for treating an ischemic disorder in a subject which comprises administering to the subject a pharmaceutically acceptable form of a selectin antagonist in a sufficient amount over a sufficient time period to prevent white blood cell accumulation so as to treat the ischemic disorder in the subject. The invention further provides a method for treating an ischemic disorder in a subject which comprises administering to the subject carbon monoxide gas in a sufficient amount over a sufficient period of time thereby treating the ischemic disorder in the subject. The invention further provides a method for treating an ischemic disorder in a subject which comprises administering to the subject a pharmaceutically acceptable form of inactivated Factor IX in a sufficient amount over a sufficient period of time to inhibit coagulation so as to treat the ischemic disorder in the subject.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Combined use of factor VII polypeptides and factor IX polypeptides
InactiveUS20030203845A1Effective treatmentPeptide/protein ingredientsPharmaceutical drugBleeding episodes
The invention concerns a pharmaceutical preparation comprising a factor VII or factor VII-related polypeptide and a factor IX or factor IX-related polypeptide. The invention also concerns use of a factor VII or factor VII-related polypeptide and a factor IX or factor IX-related polypeptide for manufacture of a medicament for pharmaceutical use as well as methods for prevention or treatment of bleeding episodes in subjects.
Owner:NOVO NORDISK AS
Glycopegylated Factor Ix
InactiveUS20080255026A1Promote recoveryPeptide/protein ingredientsPeptide sourcesPharmaceutical formulationSugar moiety
The present invention provides conjugates between Factor Ix and PEG moieties. The conjugates are linked via a glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. Conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Glycopegylated Factor Ix
InactiveUS20080318850A1Improved pharmacokinetic propertiesReliable productPeptide/protein ingredientsMammal material medical ingredientsDiseasePharmaceutical formulation
The present invention provides conjugates between Factor IX and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
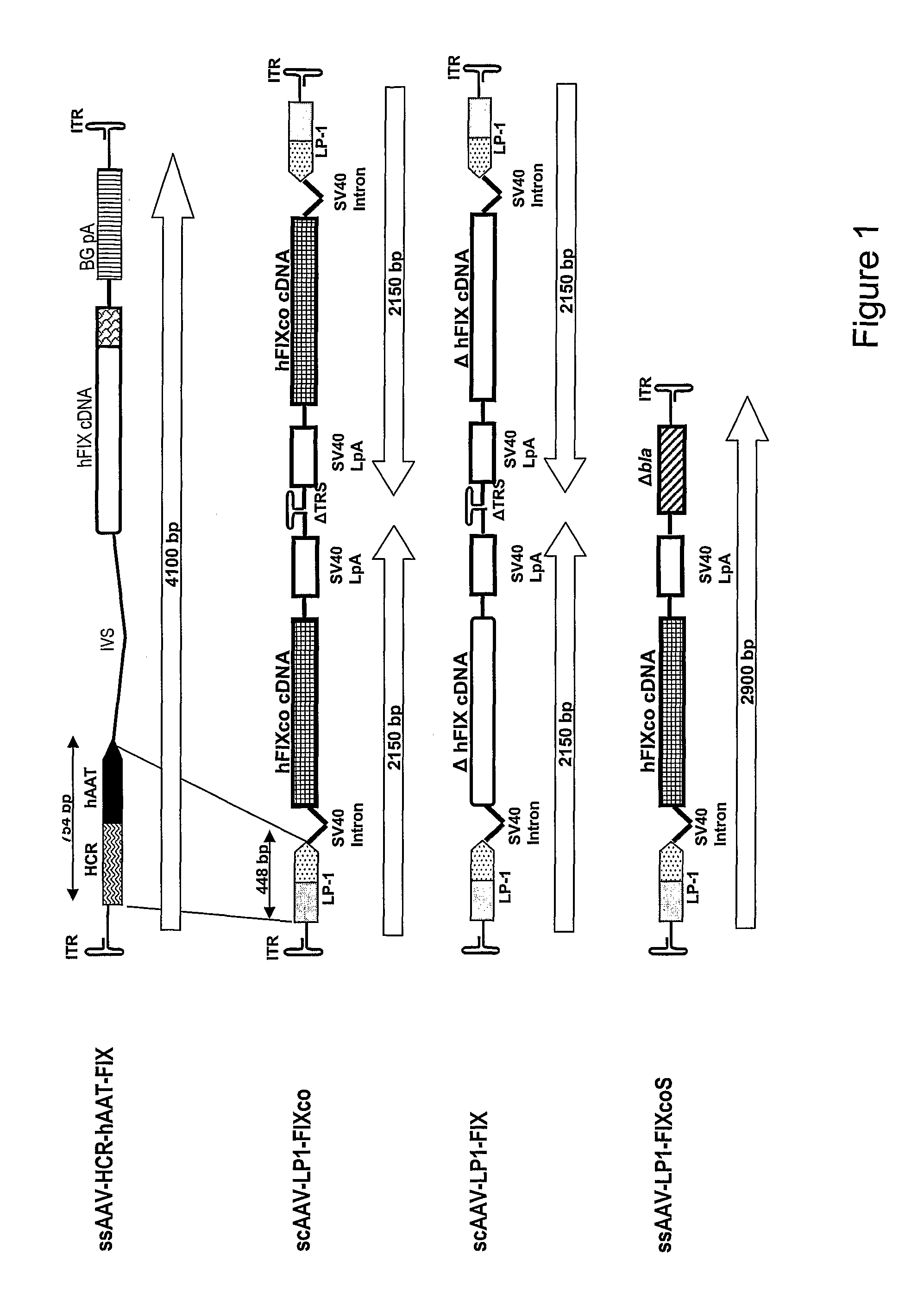
Expression of factor IX in gene therapy vectors
Two mechanisms are provided for improving the expression of Factor IX in gene therapy vectors. The first is the use of a specific Factor IX polynucleotide coding sequence designed for optimal expression. The second is the use of transcriptional regulatory regions minimized in size so that they can be used to express Factor IX, as well as any other gene of interest, in a size-constrained environment such as in a self complementary gene therapy vector system.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Modified factor viii and factor ix genes and vectors for gene therapy
The present invention relates to a modified and optimized Factor VIII or Factor IX nucleic acid for inclusion in a chimeric virus vector. Use of such vector can be used for treatment of hemophilia.
Owner:ASKLEPIOS BIOPHARMACEUTICAL INC
Factor IXa for the treatment of bleeding disorders
Owner:BAXALTA GMBH
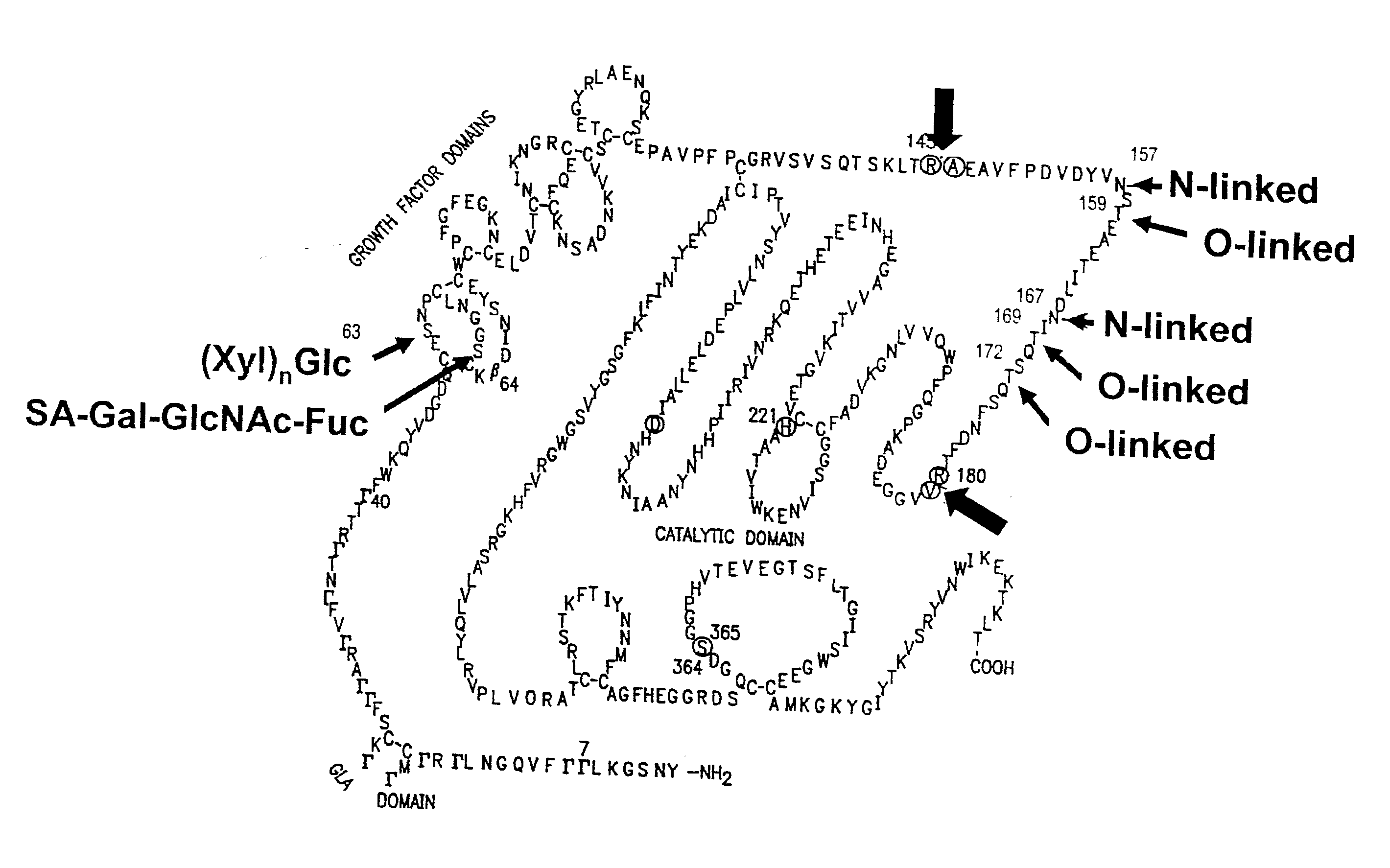
Modified factor ix
InactiveUS20040254106A1Modify characteristicMagnitude is largeFactor VIIPeptide/protein ingredientsEpitopeIn vivo
The invention in particular relates to the modification of human factor IX to result in factor IX proteins that are substantially non-immunogenic or less immunogenic than any non-modified counterpart when used in vivo. The invention relates, furthermore, to T-cell epitope sequences deriving from human factor IX, which are immunogenic.
Owner:MERCK PATENT GMBH
Mutant human factor IX with an increased resistance to inhibition by heparin
InactiveUS7125841B2Improve the immunityShorten clotting timePeptide/protein ingredientsMammal material medical ingredientsFactor iiBiology
The present invention is related to a novel composition of matter and methods of using the same. More particularly, the invention describes mutant human factor IX which has an increased resistance to inhibition by heparin. Methods of making and using this composition for the therapeutic intervention of hemophilia are disclosed.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Recombinant blood clotting factors
The present invention relates to an improved method for the production of recombinant human blood clotting factors, in particular of factor VIII and factor IX. An immortalized human cell line can be used to stably express viral transcription activator proteins and carrying a vector having a promoter functionally linked to a DNA sequence coding for a blood coagulating factor, provided that said promoter is not a viral promoter which is stimulated by said viral transcription activator proteins. The invention further relates to an immortalized human cell line carrying said vector, factor VIII muteins particularly suitable for the above production method; pharmaceutical compositions comprising such factor VIII muteins, and the use of such factor VIII muteins for preparing a medicament for treating hemophilia.
Owner:OCTAPHARMA +1
Therapeutic method for blood coagulation disorder
InactiveUS20090148425A1Ensure adequate treatmentImprove abilitiesBiocidePeptide/protein ingredientsFactor iiPlatelet
The present invention provides agents for treating blood coagulation abnormalities, which contain as an active ingredient a lentiviral vector carrying a blood coagulation factor gene operably linked to a promoter which induces platelet-specific expression. Agents for treating hemophilia A or hemophilia B are provided by application of the gene encoding Factor VIII or Factor IX. Blood coagulation abnormalities can be treated by gene therapy by infecting hematopoietic stem cells or such with the therapeutic agents of the present invention.
Owner:DNAVEC CORP
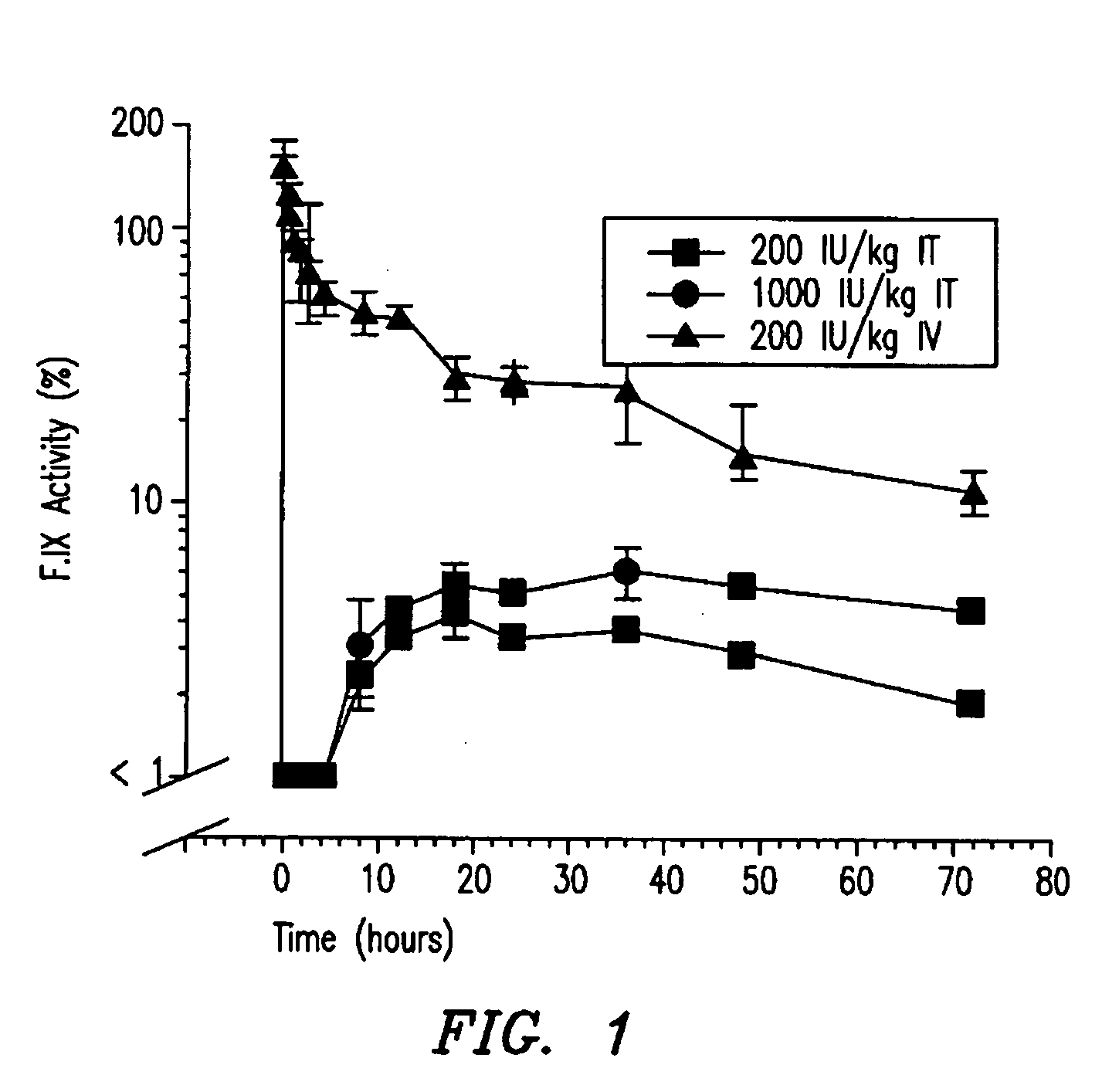
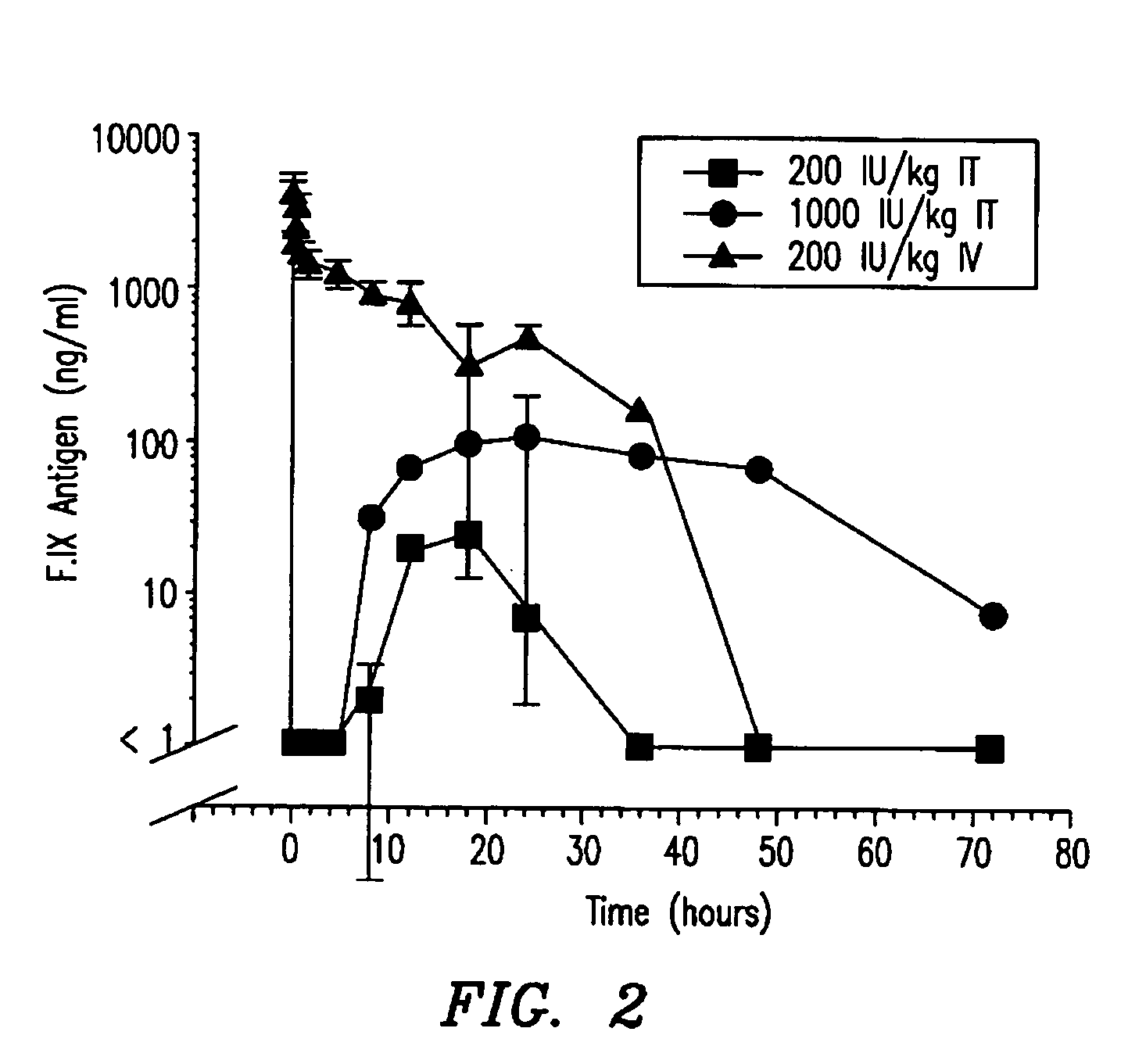
Hemophilia treatment by inhalation of coagulation factors
Hemophilia treatment by the inhalation of coagulation factors. Dry powder Factor IX is aerosolized to a mass median aerodynamic diameter of 4 μm or less, with at least 90% monomer content, at least 80% activity level, and 10% water or less. The aerosol is slowly, and deeply inhaled into the lung, and followed by a maximal exhale.
Owner:WYETH LLC +1
Modified factor ix, and compositions, methods and uses for gene transfer to cells, organs, and tissues
ActiveUS20160375110A1Reduced duration severity frequencyReduce dosagePeptide/protein ingredientsBlood disorderFactor iiGene transfer
The invention relates to modified Factor IX coding sequence, expression cassette, vectors such as viral (e.g., lenti- or adeno-associated viral) vectors, and gene transfer methods and uses. In particular, to target Factor IX nucleic acid to cells, tissues or organs for expression (transcription) of Factor IX.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com