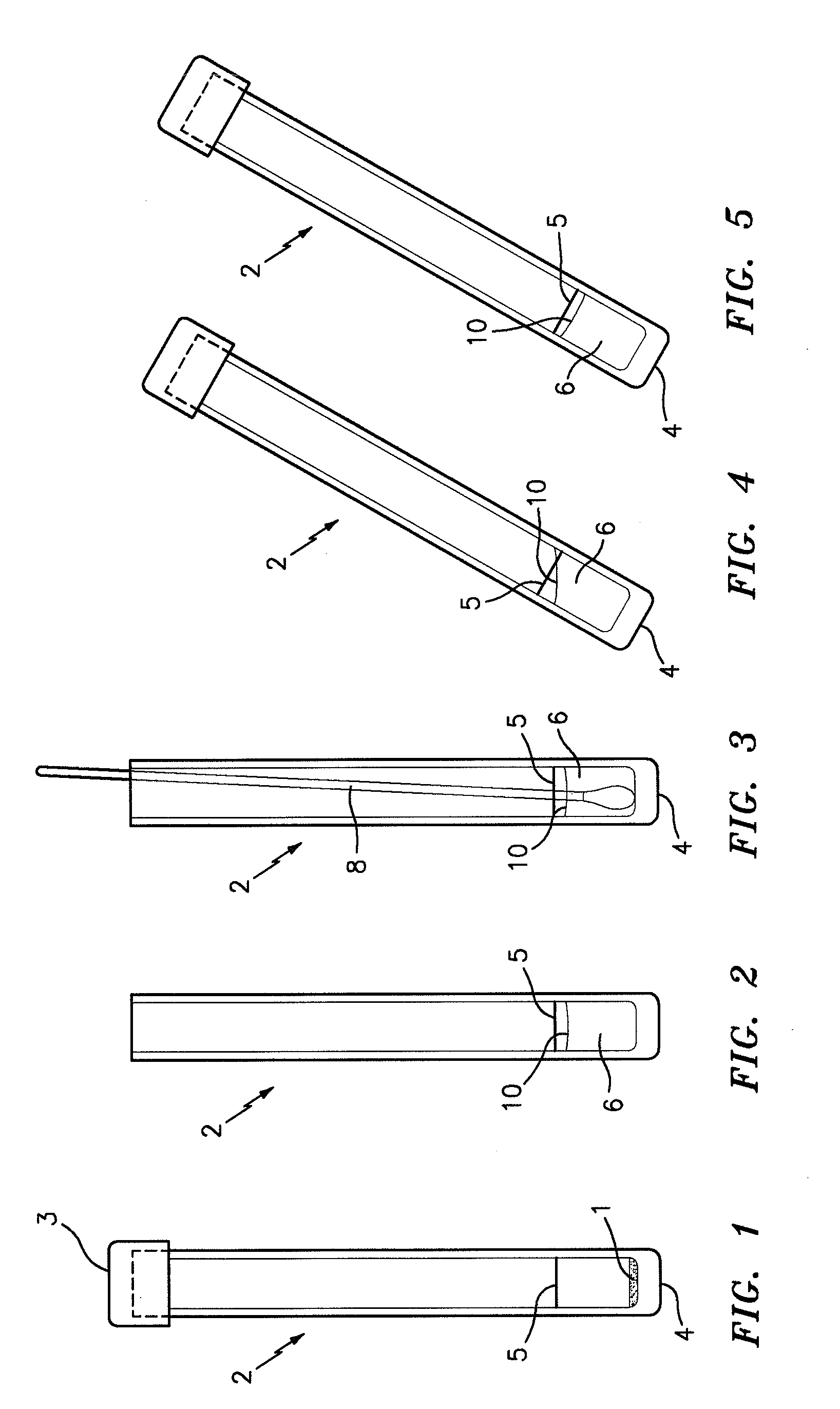
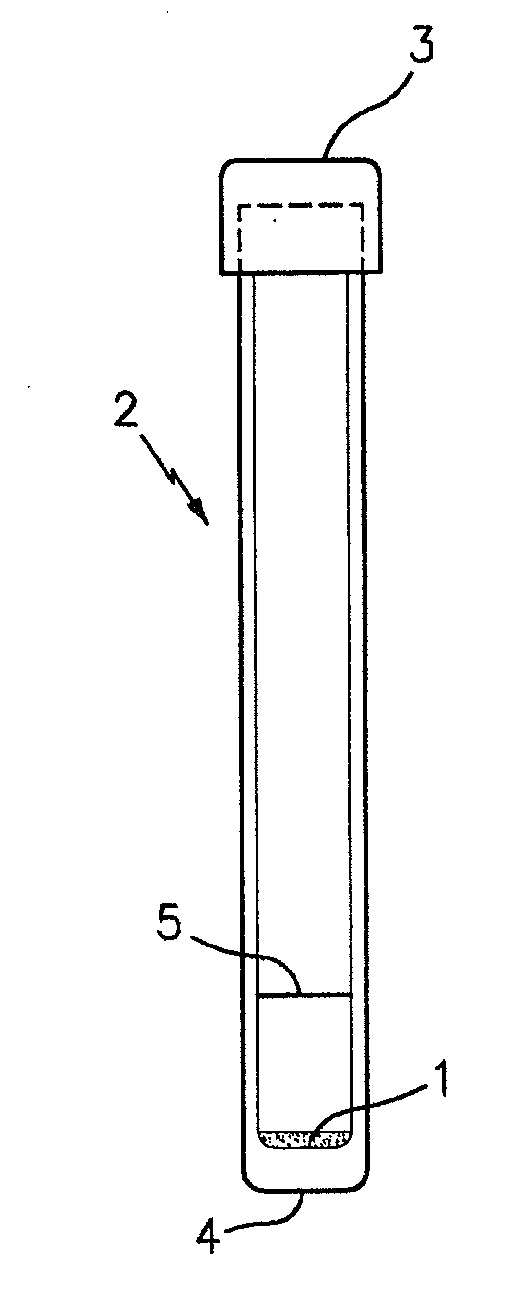
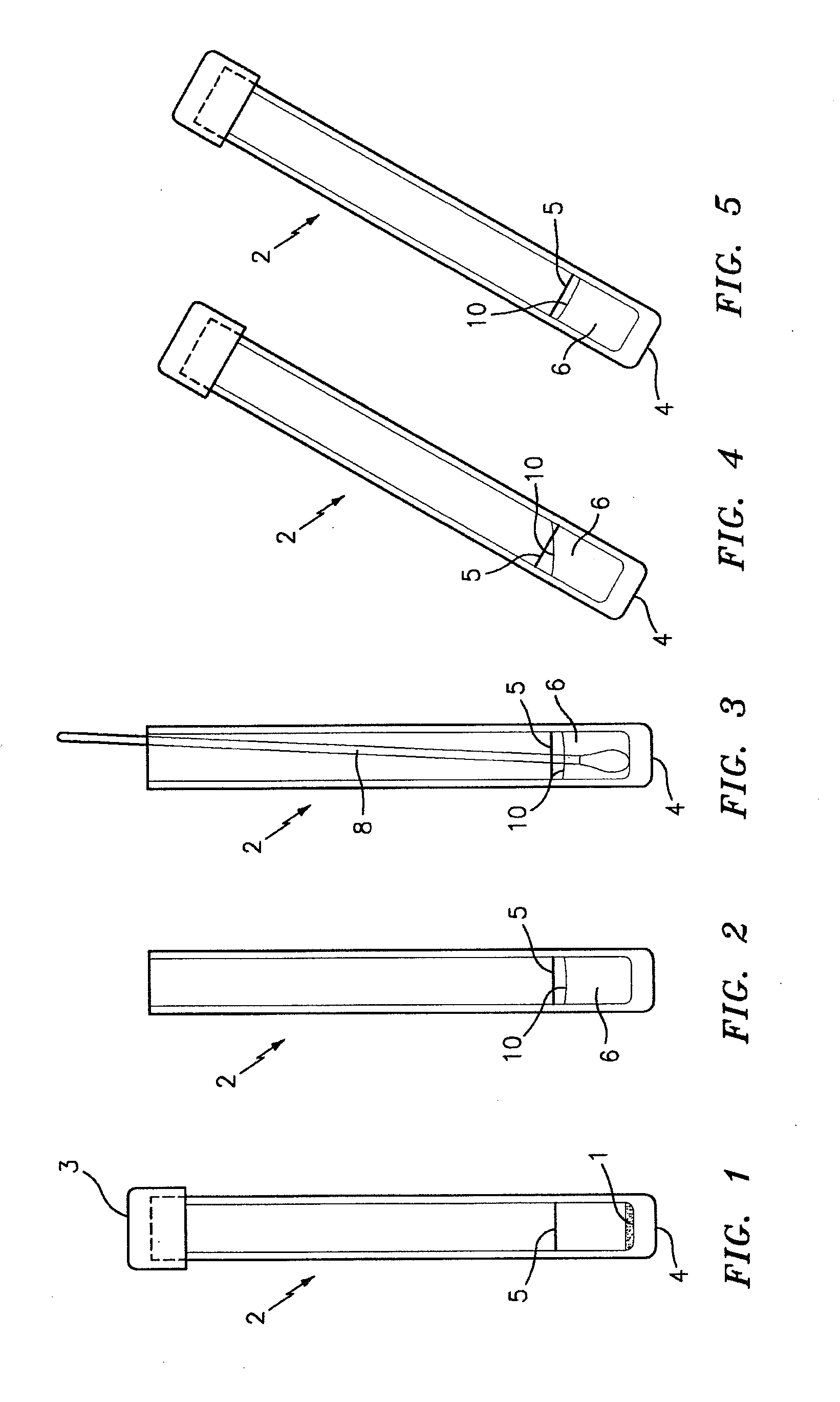
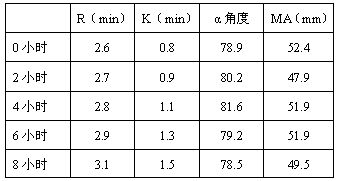
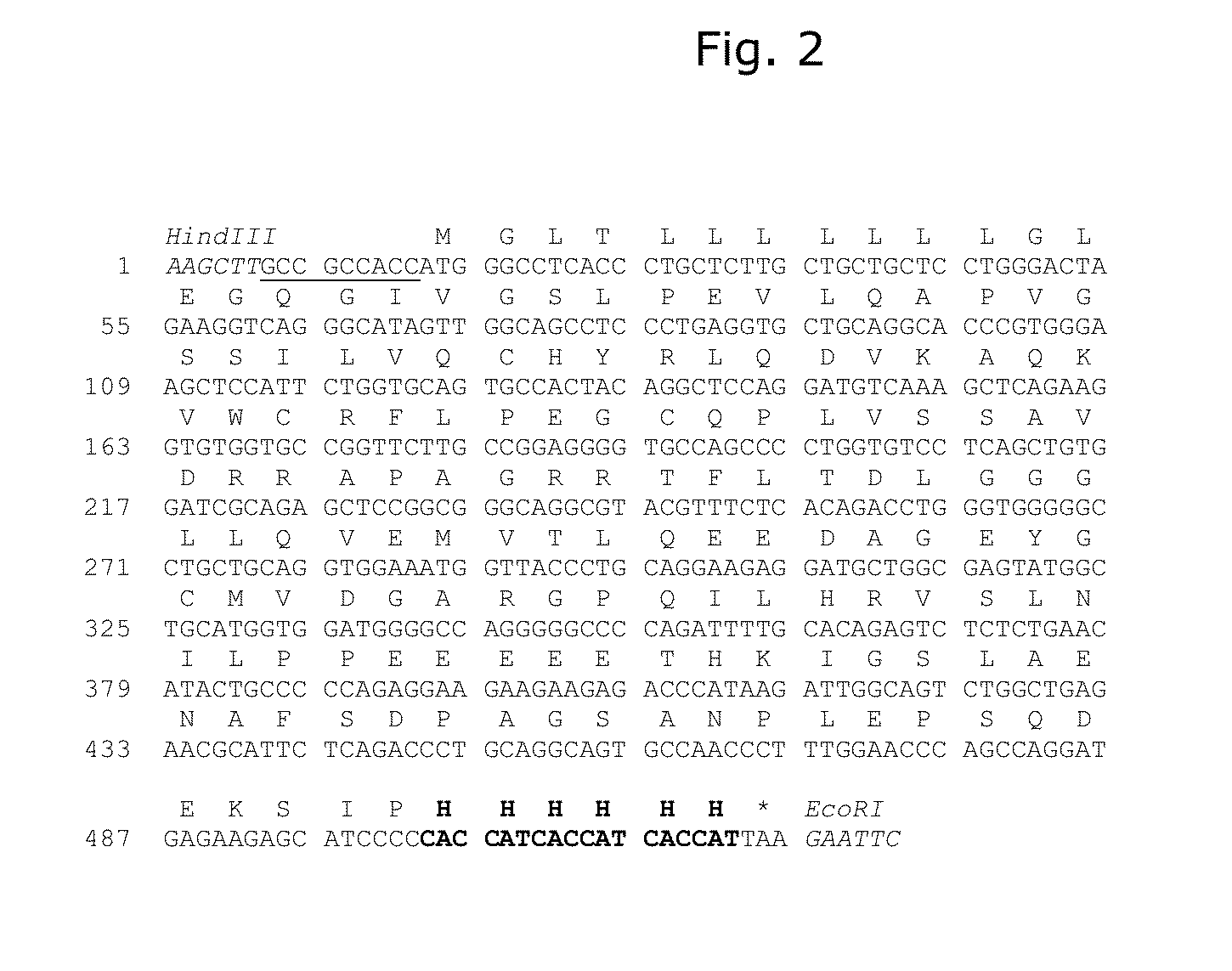
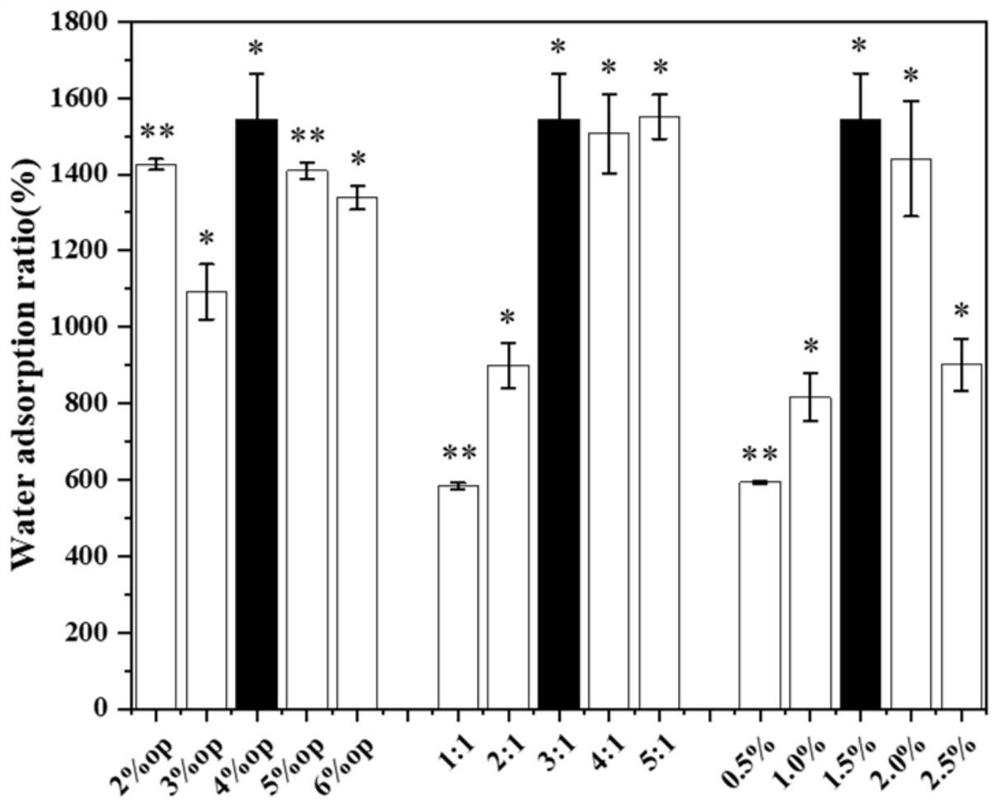
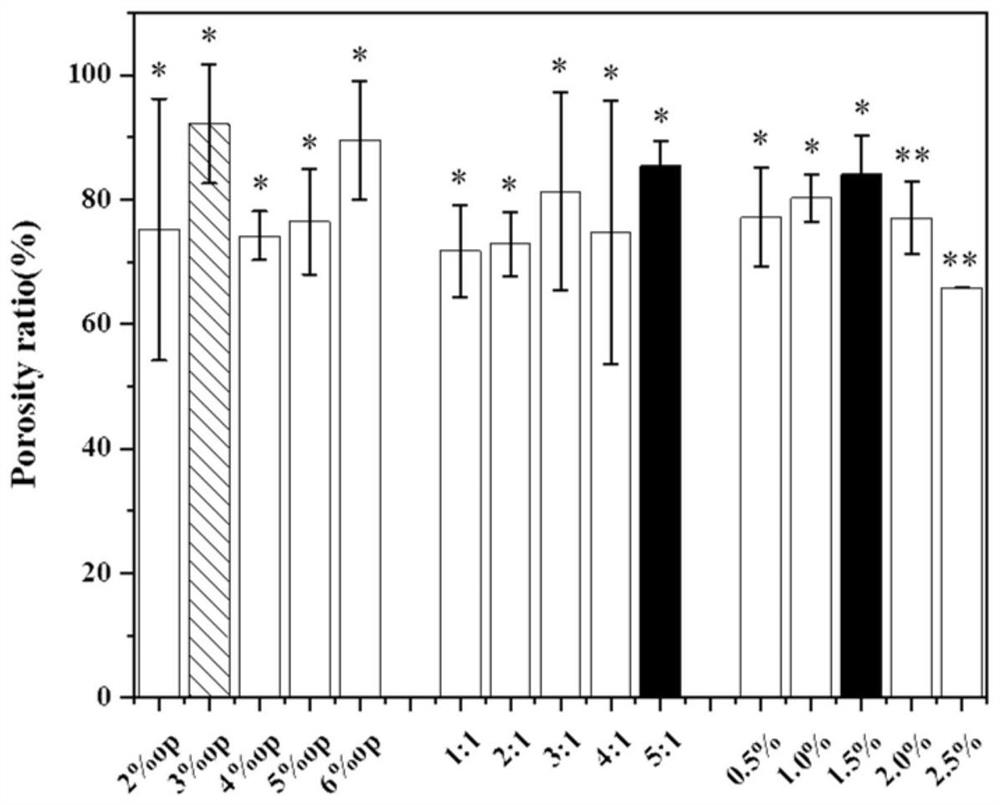
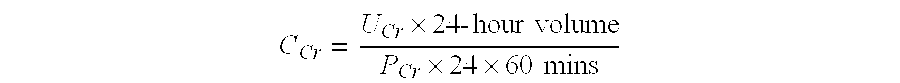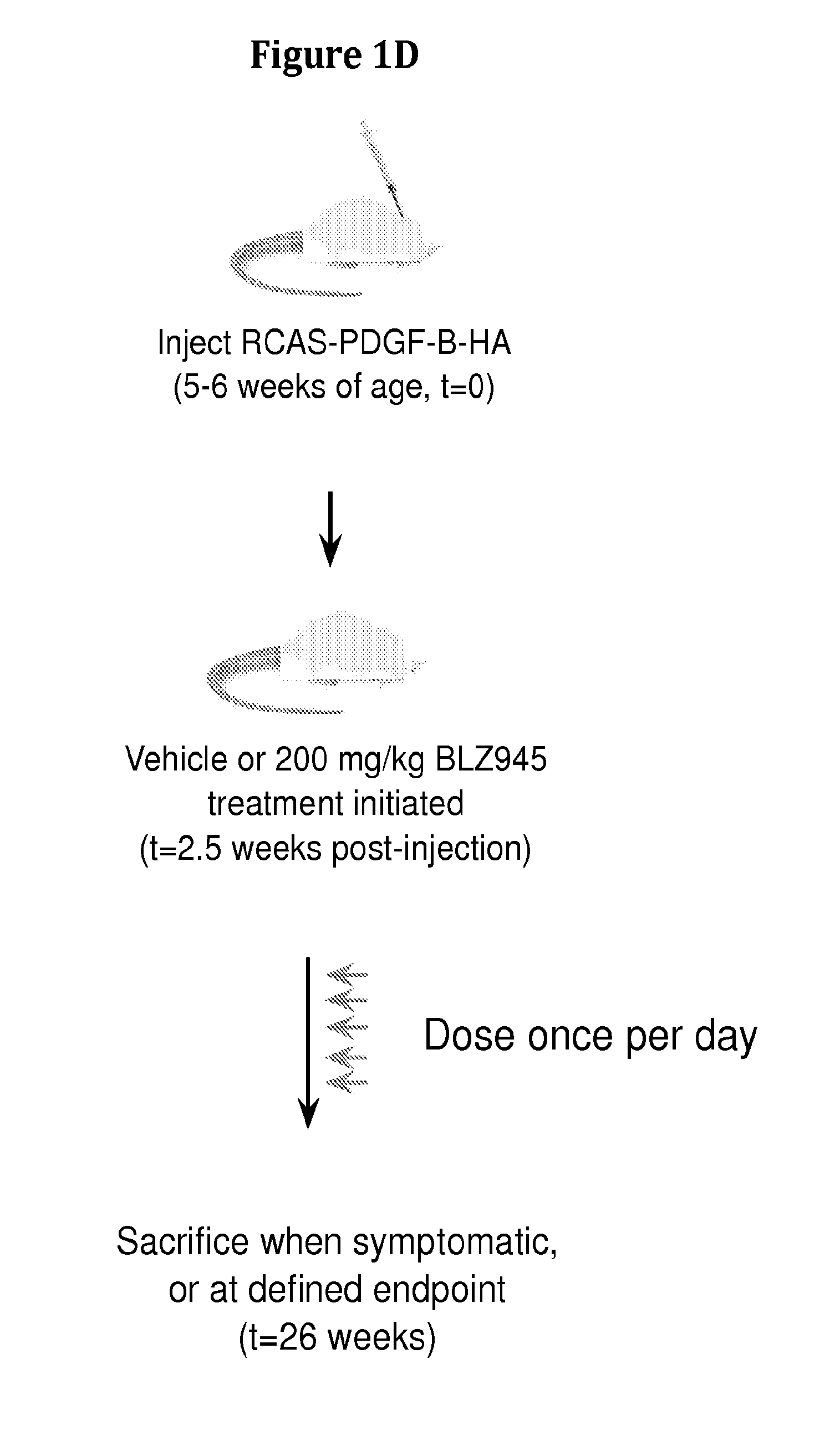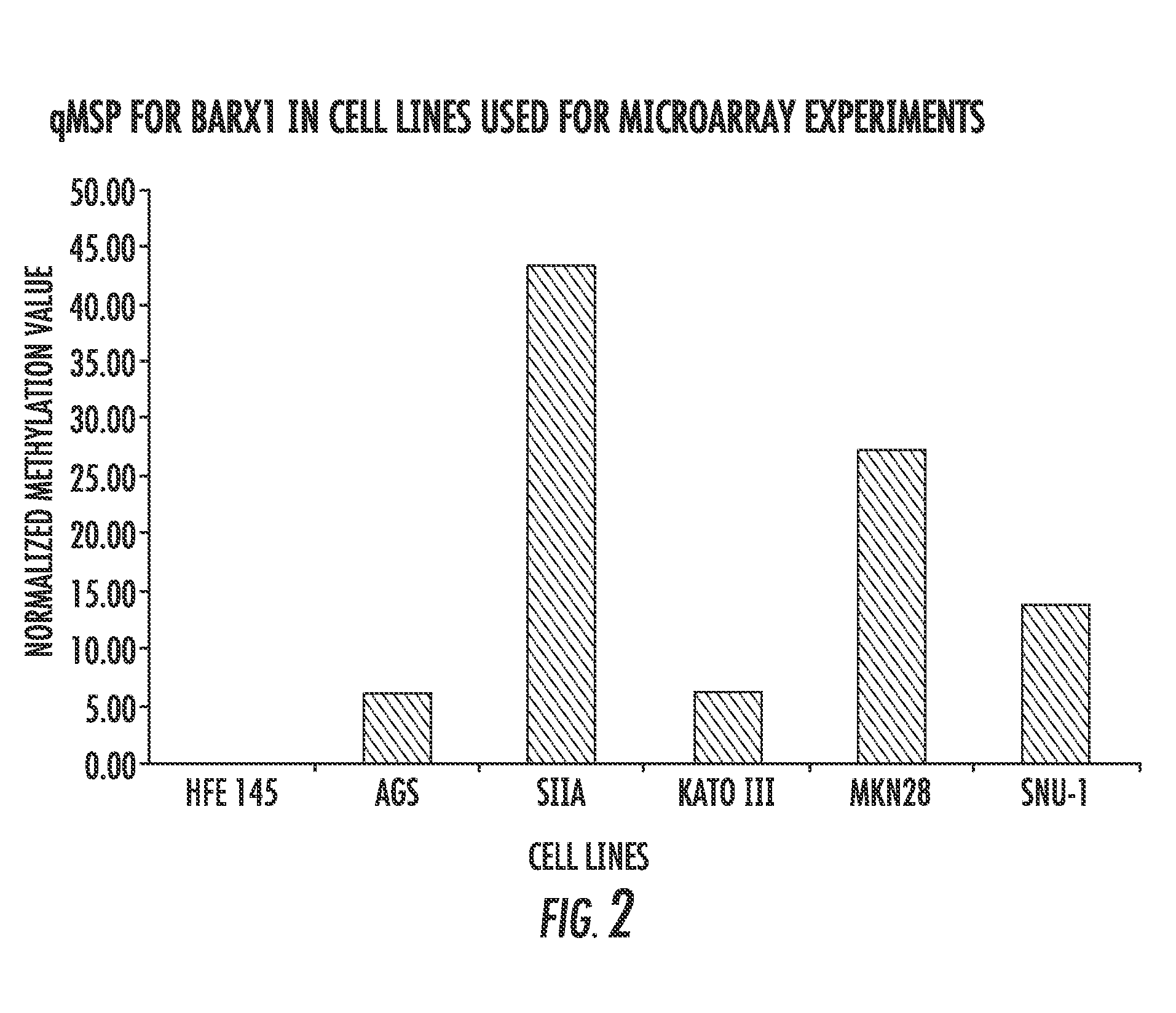Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
66 results about "Clotting factors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
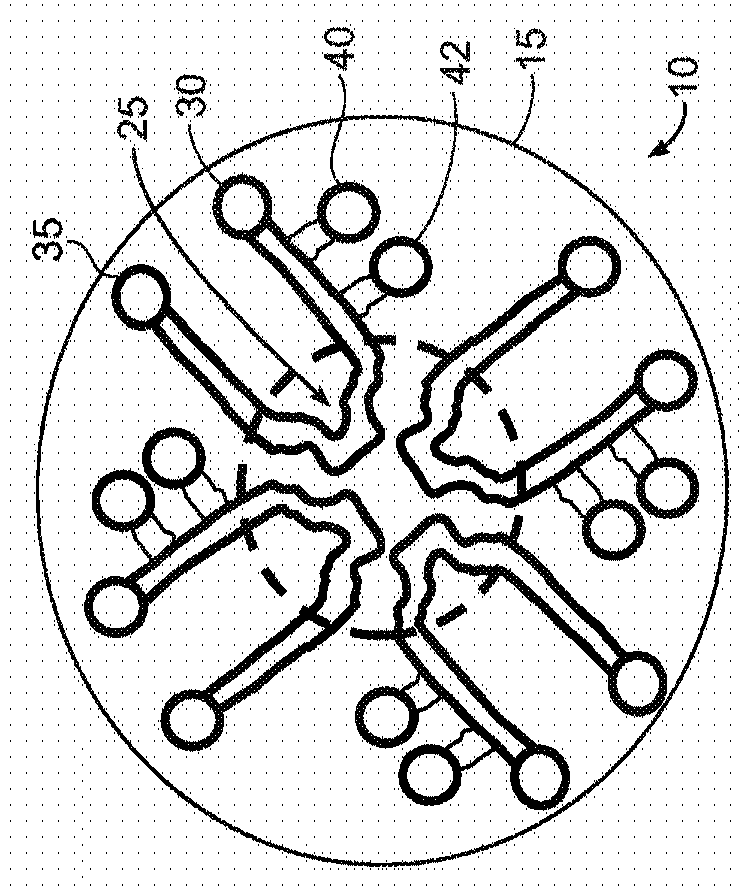
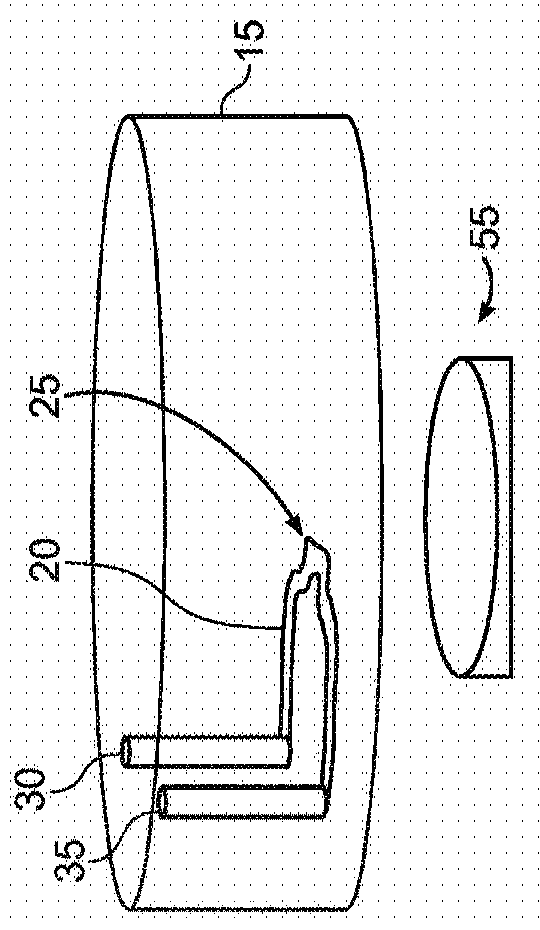
Microneedle device for extraction and sensing of bodily fluids
InactiveUS7344499B1Simple wayMinimal and no damageAdditive manufacturing apparatusMicroneedlesMetaboliteIrritation
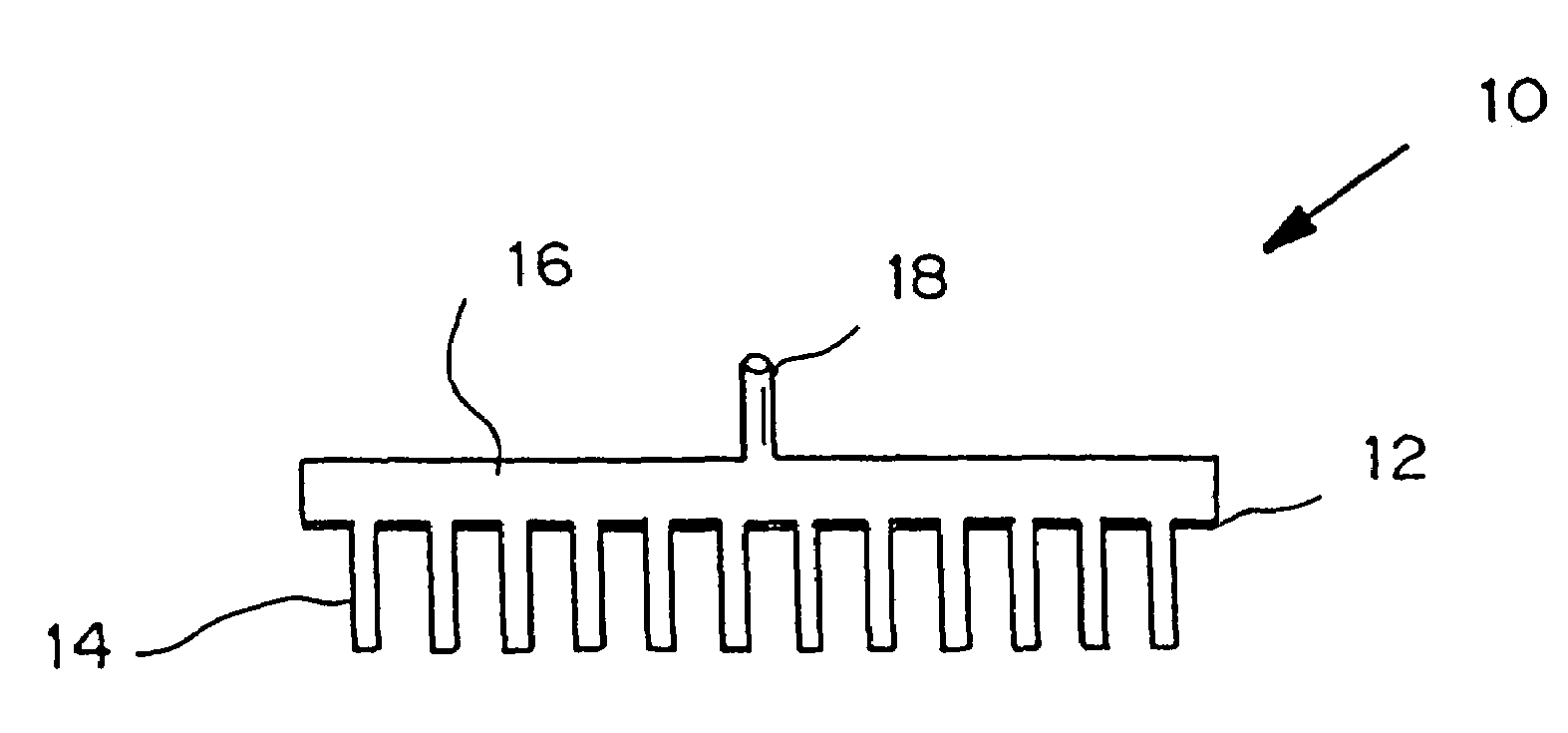
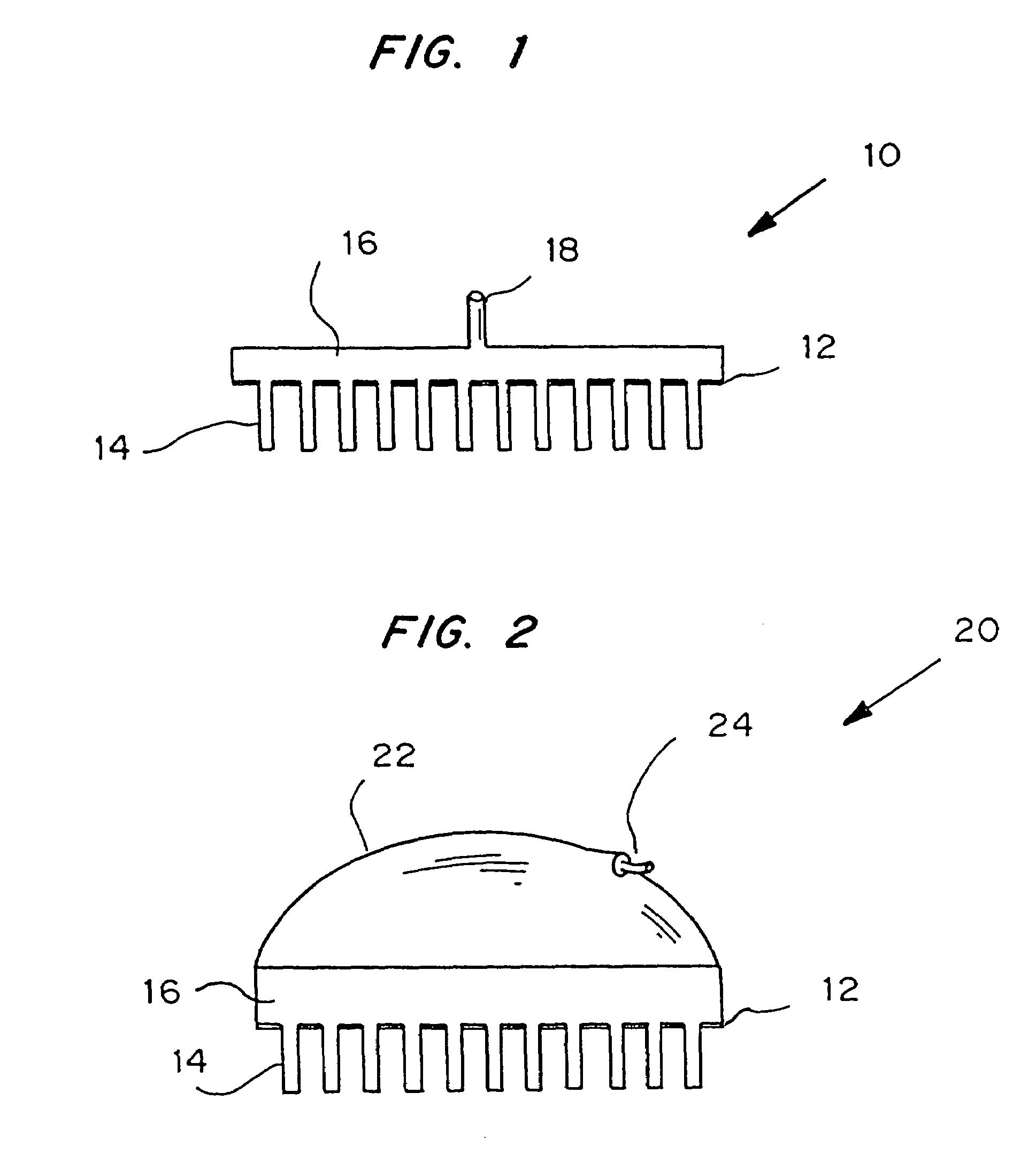
Microneedle devices are provided for controlled sampling of biological fluids in a minimally-invasive, painless, and convenient manner. The microneedle devices permit in vivo sensing or withdrawal of biological fluids from the body, particularly from or through the skin or other tissue barriers, with minimal or no damage, pain, or irritation to the tissue. The microneedle device includes one or more microneedles, preferably in a three-dimensional array, a substrate to which the microneedles are connected, and at least one collection chamber and / or sensor in communication with the microneedles. Preferred embodiments further include a means for inducing biological fluid to be drawn through the microneedles and into the collection chamber for analysis. In a preferred embodiment, this induction is accomplished by use of a pressure gradient, which can be created for example by selectively increasing the interior volume of the collection chamber, which includes an elastic or movable portion engaged to a rigid base. Preferred biological fluids for withdrawal and / or sensing include blood, lymph, interstitial fluid, and intracellular fluid. Examples of analytes in the biological fluid to be measured include glucose, cholesterol, bilirubin, creatine, metabolic enzymes, hemoglobin, heparin, clotting factors, uric acid, carcinoembryonic antigen or other tumor antigens, reproductive hormones, oxygen, pH, alcohol, tobacco metabolites, and illegal drugs.
Owner:GEORGIA TECH RES CORP +1
Methods and compositions for immunotherapy and detection of inflammatory and immune-dysregulatory disease, infectious disease, pathologic angiogenesis and cancer
InactiveUS20060140936A1Antibacterial agentsOrganic active ingredientsDendritic cellAutoimmune condition
Methods and compositions for immunotherapy of inflammatory and immune-dysregulatory diseases, using multispecific antagonists that target at least two different markers are disclosed. The different targets include (i) proinflammatory effectors of the innate immune system, (ii) coagulation factors, and (iii) targets specifically associated with an inflammatory or immune-dysregulatory disorder, with a pathologic angiogenesis or cancer, or with an infectious disease, wherein the targets included in group (iii) are neither a proinflammatory effector of the immune system nor a coagulation factor. When the multispecific antagonist reacts specifically with a target associated with an inflammatory or immune-dysregulatory disorder, with a pathologic angiogenesis or cancer, or with an infectious disease, it also binds specifically with at least one proinflammatory effector of the immune system or at least one coagulation factor. Thus, the multispecific antagonist contains at least one binding specificity related to the diseased cell or condition being treated and at least one specificity to a component of the immune system, such as a receptor or antigen of B cells, T cells, neutrophils, monocytes and macrophages, and dendritic cells, a modulator of coagulation, or a proinflammatory cytokine. The multispecific antagonists are used in the treatment of various diseases that are generated or exacerbated by, or otherwise involve, proinflammatory effectors of the innate immune system or coagulation factors. Such diseases more particularly include acute and chronic inflammatory disorders, autoimmune diseases, giant cell arteritis, septicemia and septic shock, coagulopathies (including diffuse intravascular coagulation), neuropathies, graft versus host disease, infectious diseases, acute respiratory distress syndrome, granulomatous diseases, transplant rejection, asthma, cachexia, myocardial ischemia, and atherosclerosis. Other diseases also responsive to these therapies include cancers and conditions with pathological angiogenesis.
Owner:IMMUNOMEDICS INC
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20120190044A1Eliminate needBioreactor/fermenter combinationsBiological substance pretreatmentsGlycoproteinMetalloproteinase inhibitor
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a plurality of assays, one or more of which is configured to detect a kidney injury marker selected from the group consisting of metalloproteinase inhibitor 2, soluble oxidized low-density lipoprotein receptor 1, interleukin-2, von Willebrand factor, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor receptor superfamily member 11B, neutrophil elastase, interleukin-1 beta, heart-type fatty acid-binding protein, beta-2-glycoprotein 1, soluble CD40 ligand, coagulation factor VII, C—C motif chemokine 2, IgM, CA 19-9, IL-10, TNF-α, and myoglobin as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Kit for measuring the thrombin generation in a sample of a patient's blood or plasma
InactiveUS20050221414A1Simple and efficient and fast and reproducible assayConvenient typeMicrobiological testing/measurementBiological material analysisTissue factorThrombin activity
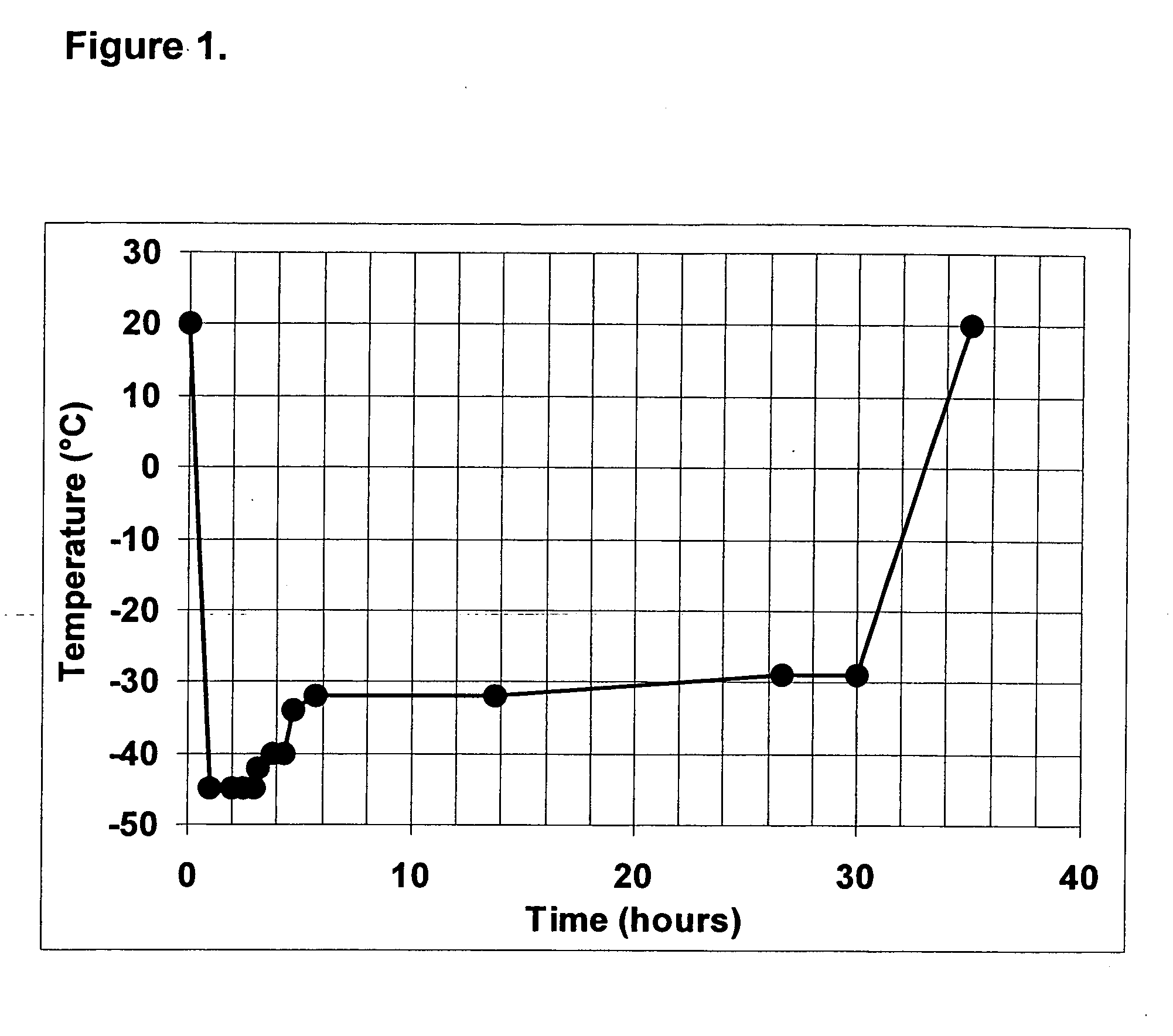
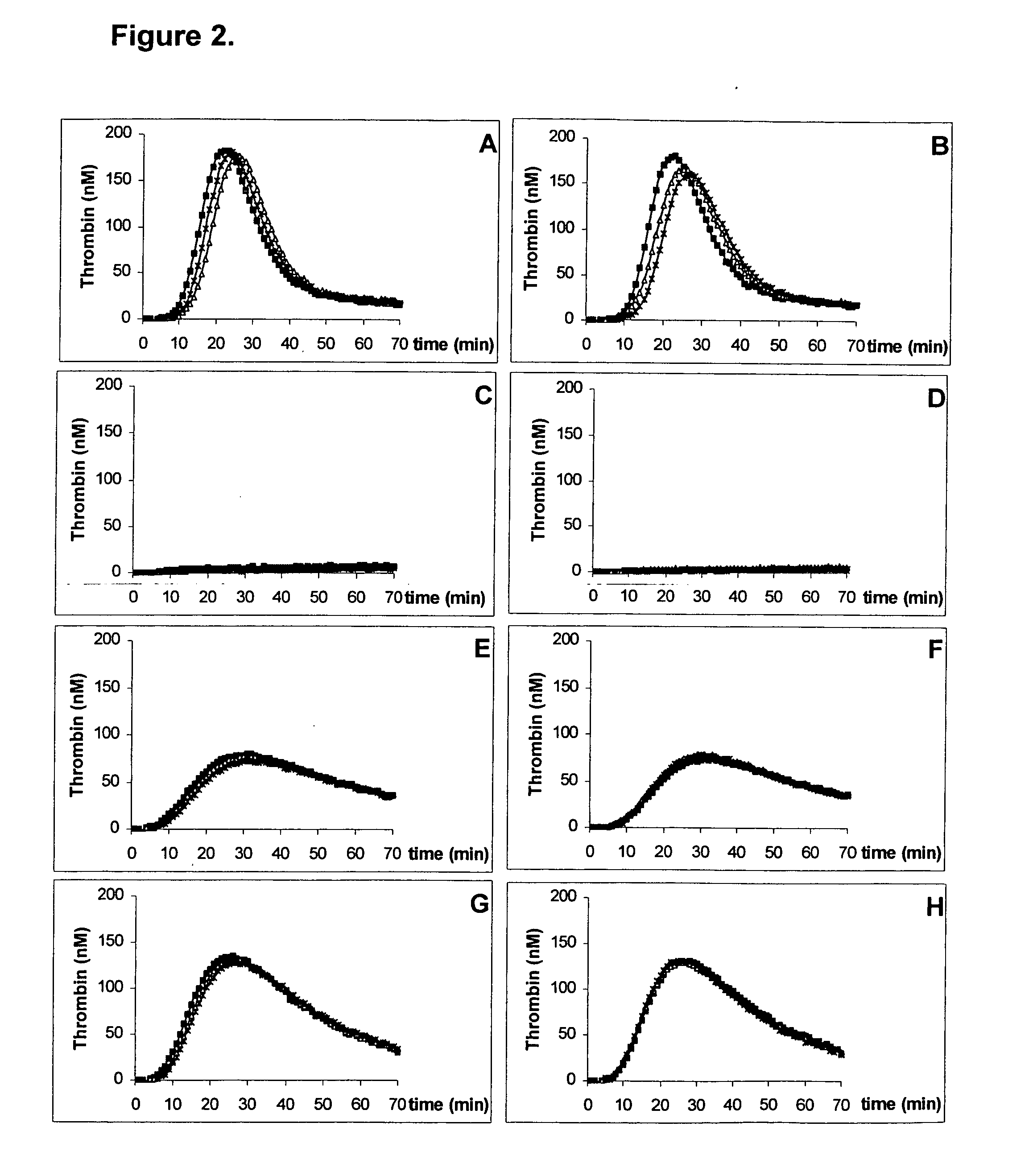
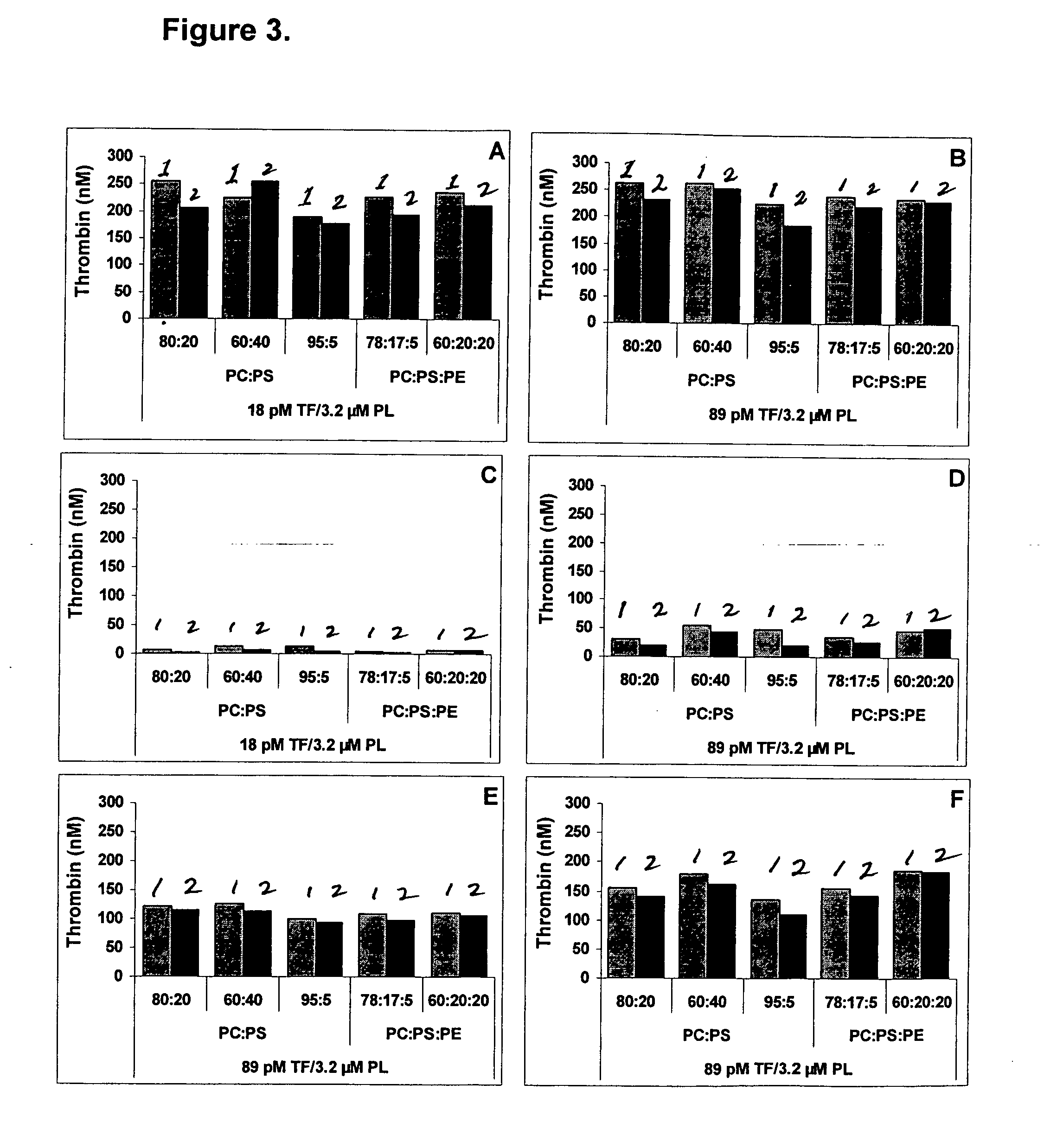
The invention provides a kit for measuring the thrombin generation in a sample of a patient's blood or plasma, or in a sample of clotting factors. The kit contains lyophilized tissue factor / phospholipid-complex and a lyophilized mixture containing a thrombin-substrate and CaCl2. The invention also provides processes for preparing the reagents for the kit. The kit can be used in a method for measuring the thrombin generation in a sample, wherein it is possible to detect changes in thrombin generation kinetics, for example after administration of inhibitor bypassing agents to a patient who has developed inhibitors to an exogenous clotting factor such as Factor VIII.
Owner:BAXTER INT INC +1
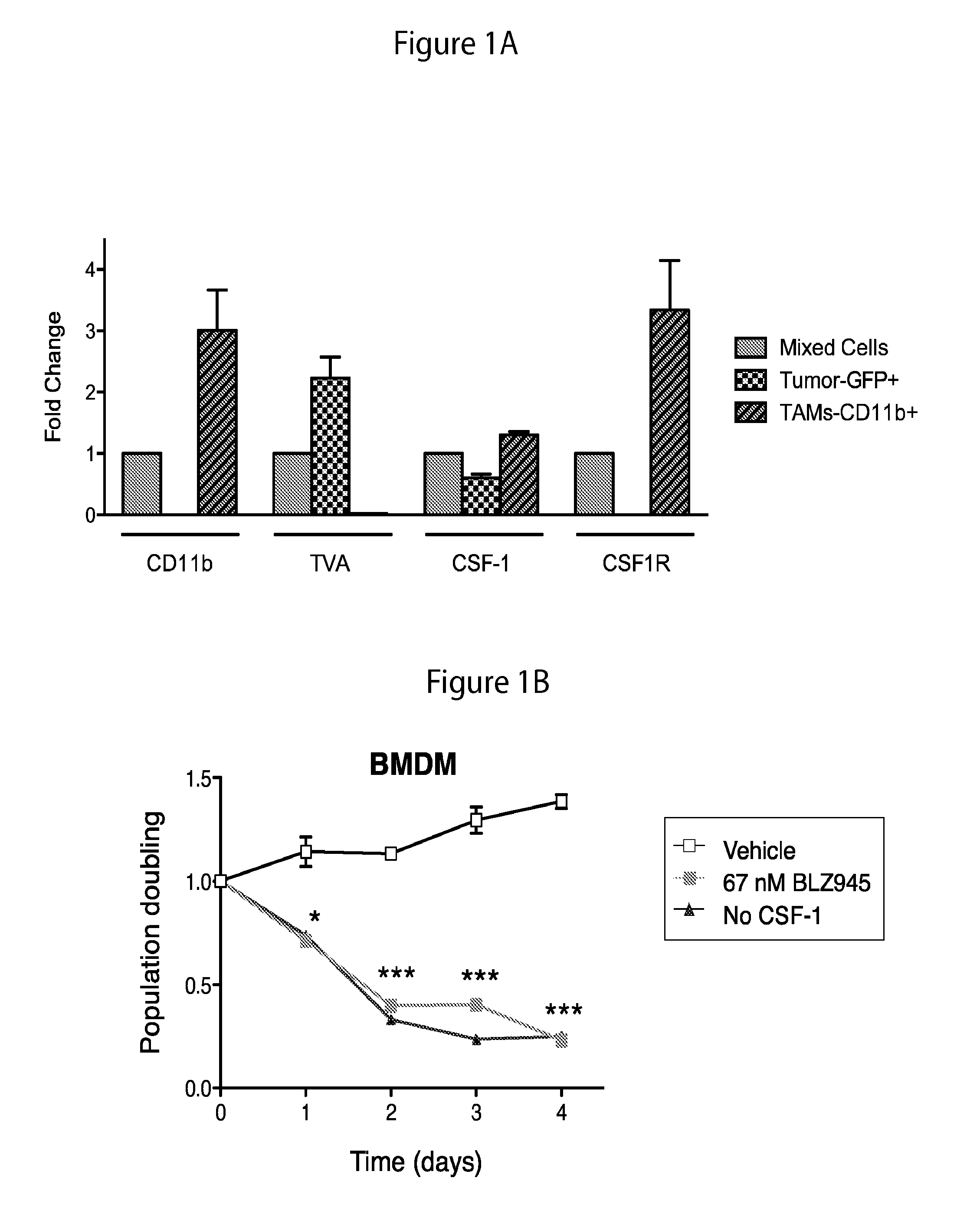
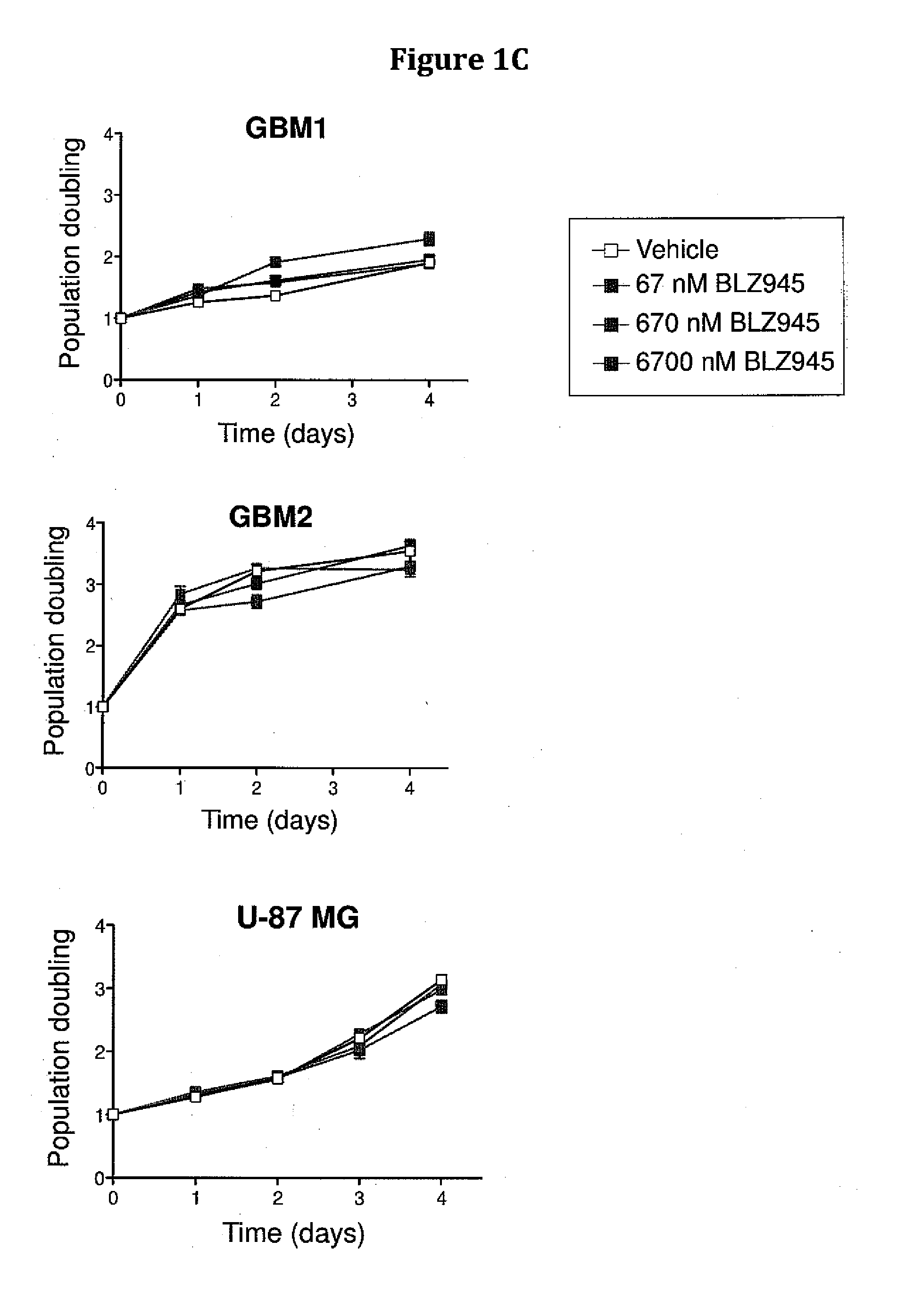
Inhibition of colony stimulating factor-1 receptor signaling for the treatment of brain cancer
InactiveUS20150119267A1Compound screeningApoptosis detectionAfter treatmentColony-stimulating factor
The present invention provides a method of screening brain tumor patients for treatment with inhibitor of CSF-1R, based on differential gene expression including adrenomeduUin (ADM), arginase 1 (ARG1), clotting factor F13A1, mannose receptor C type 1 (MRC1 / CD206), and protease inhibitor SERPINB2 after treatment with the inhibitor. Based on the same differential gene expression profile, the present invention also provides a method of screening a compound to treat brain cancer.
Owner:SLOAN KETTERING INST FOR CANCER RES
Methods and compositions for immunotherapy and detection of inflammatory and immune-dysregulatory disease, infectious disease, pathologic angiogenesis and cancer
InactiveUS20080108794A1Antibacterial agentsOrganic active ingredientsDendritic cellAutoimmune condition
Owner:IMMUNOMEDICS INC
Methods and compositions for immunotherapy and detection of inflammatory and immune-dysregulatory disease, infectious disease, pathologic angiogenesis and cancer
InactiveUS20080241141A1Antibacterial agentsOrganic active ingredientsDendritic cellAutoimmune condition
Methods and compositions for immunotherapy of inflammatory and immune-dysregulatory diseases, using multispecific antagonists that target at least two different markers are disclosed. The different targets include (i) proinflammatory effectors of the innate immune system, (ii) coagulation factors, and (iii) targets specifically associated with an inflammatory or immune-dysregulatory disorder, with a pathologic angiogenesis or cancer, or with an infectious disease, wherein the targets included in group (iii) are neither a proinflammatory effector of the immune system nor a coagulation factor. When the multispecific antagonist reacts specifically with a target associated with an inflammatory or immune-dysregulatory disorder, with a pathologic angiogenesis or cancer, or with an infectious disease, it also binds specifically with at least one proinflammatory effector of the immune system or at least one coagulation factor. Thus, the multispecific antagonist contains at least one binding specificity related to the diseased cell or condition being treated and at least one specificity to a component of the immune system, such as a receptor or antigen of B cells, T cells, neutrophils, monocytes and macrophages, and dendritic cells, a modulator of coagulation, or a proinflammatory cytokine. The multispecific antagonists are used in the treatment of various diseases that are generated or exacerbated by, or otherwise involve, proinflammatory effectors of the innate immune system or coagulation factors. Such diseases more particularly include acute and chronic inflammatory disorders, autoimmune diseases, giant cell arteritis, septicemia and septic shock, coagulopathies (including diffuse intravascular coagulation), neuropathies, graft versus host disease, infectious diseases, acute respiratory distress syndrome, granulomatous diseases, transplant rejection, asthma, cachexia, myocardial ischemia, and atherosclerosis. Other diseases also responsive to these therapies include cancers and conditions with pathological angiogenesis.
Owner:IMMUNOMEDICS INC
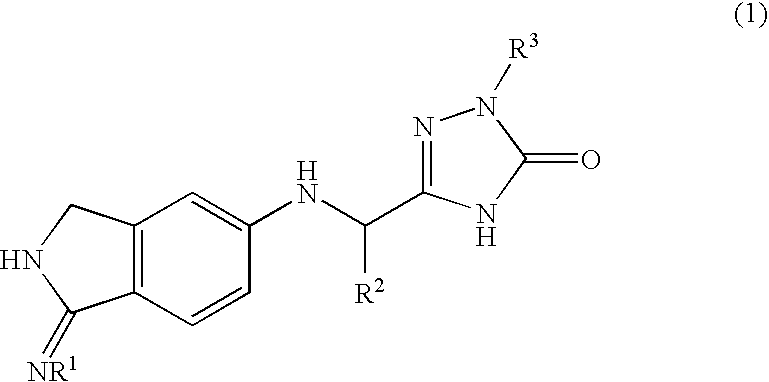
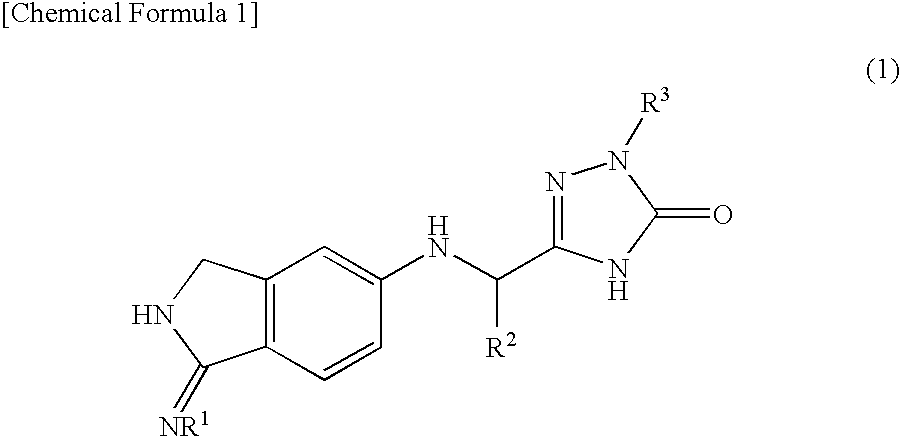
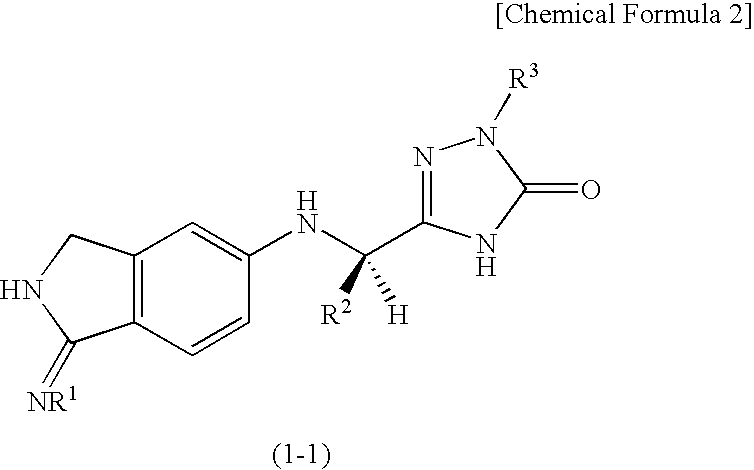
2,3-dihydro-iminoisoindole derivatives
InactiveUS20090270433A1Inhibition effectGood anticoagulant effectBiocideOrganic chemistryArylDisease
A compound represented by the following general formula (1), or a salt thereof has serine protease inhibiting activity, and particularly excellent inhibiting activity against clotting factor VIIa. This compound or a salt thereof is useful as therapeutic and / or prophylactic agents for diseases associated with thrombus formation.[wherein R1 represents hydrogen, R2 represents optionally substituted phenyl, etc., R3 represents optionally substituted C6-10 aryl, etc.]
Owner:EISIA R&D MANAGEMENT CO LTD
Modified coagulation factors with prolonged in vivo half-life
InactiveUS8754194B2Prolong half-life in vivoPromote recoveryFactor VIIPeptide/protein ingredientsHalf-lifeADAMTS Proteins
The present invention relates to nucleic acid sequences coding for modified coagulation factors, preferably coagulation factor VIII, and their derivatives; recombinant expression vectors containing such nucleic acid sequences; host cells transformed with such recombinant expression vectors; and recombinant polypeptides and derivatives coded for by said nucleic acid sequences, whereby said recombinant polypeptides and derivatives have biological activities and prolonged in vivo half-lives compared to the unmodified wild-type proteins. The invention also relates to corresponding sequences that result in improved in vitro stability. The present invention further relates to processes for the manufacture of such recombinant proteins and their derivatives. The invention also relates to a transfer vector for use in human gene therapy, which comprises such nucleic acid sequences.
Owner:CSL BEHRING GMBH
Method and medium for detecting the presence or absence of pathogenic staphylococci in a first generation test sample
InactiveUS8268579B2Facilitate the processBacteriaMicrobiological testing/measurementMethicillin resistant StaphylococcusLesion swab
A presence / absence test for Staphylococcus aureus (S. aureus) involves placing a first generation test sample in a solution that will clot in the presence of S. aureus. The solution contains components that will selectively grow S. aureus and also contains clotting factors that will react with S. aureus, if S. aureus is present in the sample, to clot the solution. Examples of specimen samples that can be tested include nasal swabs and lesion swabs, among others. The test can also be modified to detect the presence or absence of methicillin resistant S. Aureus (MRSA).
Owner:PILOTS POINT LLC
Method and medium for detecting the presence or absence of staphylococcus aureus in a test sample
InactiveUS20110269163A1Facilitates handling and packaging and storingFavorable source of coagulase substrateBacteriaMicrobiological testing/measurementLesion swabStaphylococcus cohnii
A presence / absence test for Staphylococcus aureus (S. aureus) involves placing a first generation test sample in a solution that will clot in the presence of S. aureus. The solution contains components that will selectively grow S. aureus and also contains clotting factors that will react with S. aureus, if S. aureus is present in the sample, to clot the solution. Examples of specimen samples that can be tested include nasal swabs and lesion swabs, among others. The test can also be modified to detect the presence or absence of methicillin resistant S. Aureus (MRSA).
Owner:PILOTS POINT LLC
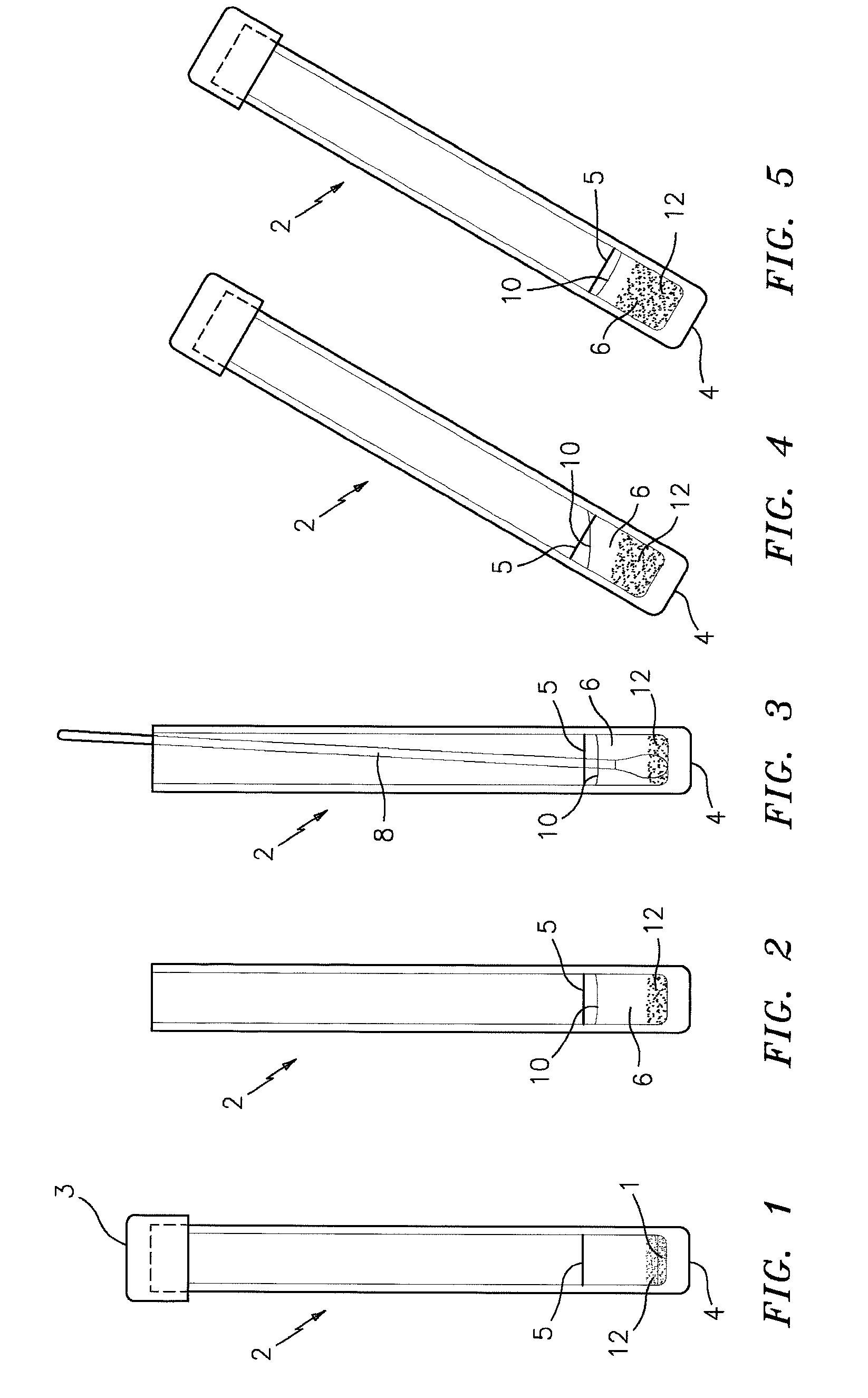
Clotting factor preparations for delivery into tissue of the intestinal tract using a swallowable drug delivery device
ActiveUS10603275B2Improved long-term tolerance to and efficacyProcess controlPeptide/protein ingredientsCatheterIntestinal wallsTherapeutic effect
Owner:RANI THERAPEUTICS
Transgenic Non-Human Animals Expressing Human Blood Clotting Factors and Uses Thereof
Owner:BAXALTA GMBH
Method for detecting the presence or absence of pathogenic Staphylococci in a test sample, including test mixture with micro particles
InactiveUS8546103B2Favorable source of coagulase substrateImprove performance consistencyMicrobiological testing/measurementStaphylococcus cohniiFirst generation
Owner:PILOTS POINT LLC
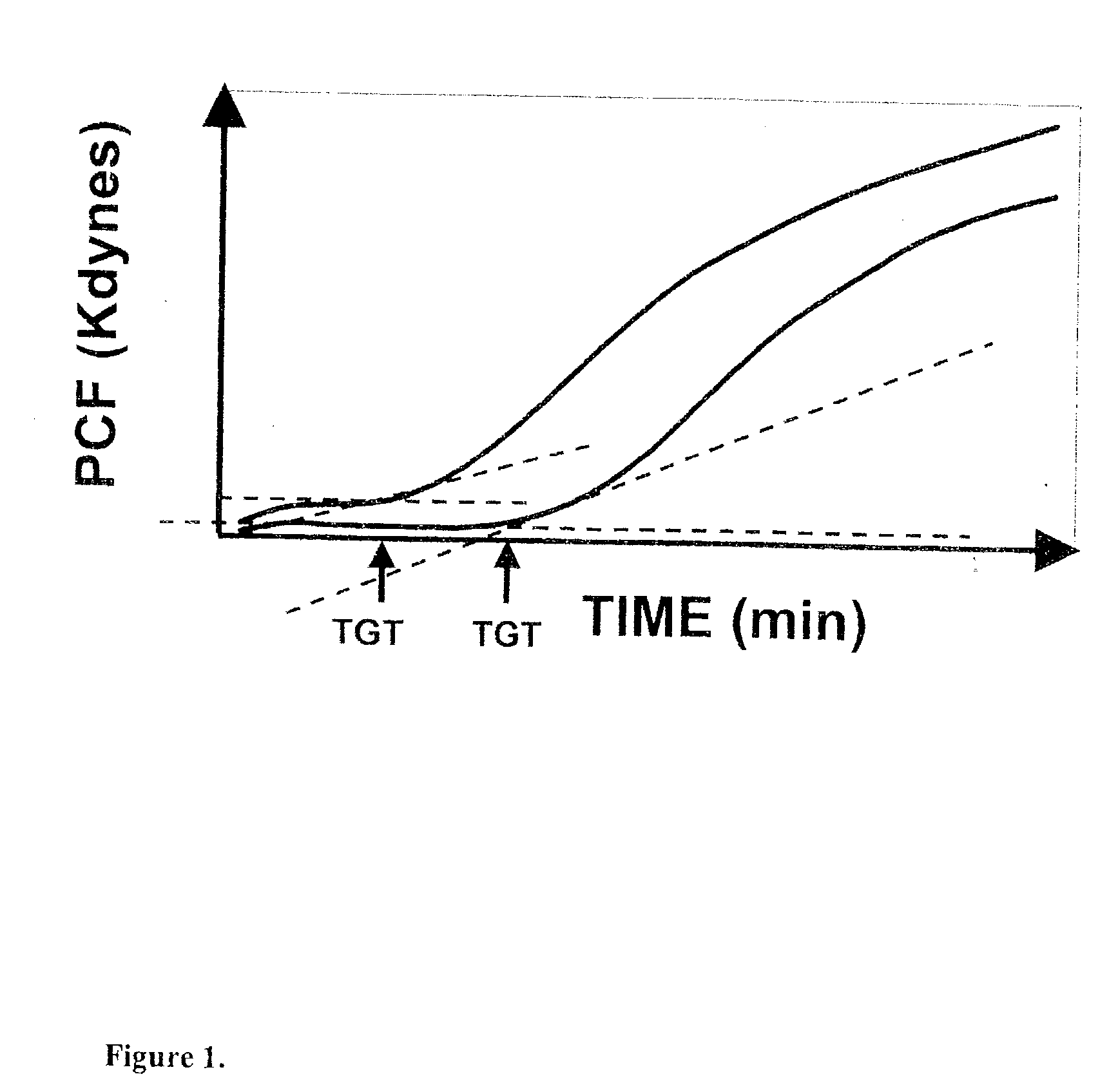
Onset of force development as a marker of thrombin generation
InactiveUS20030199428A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseCoronary artery disease
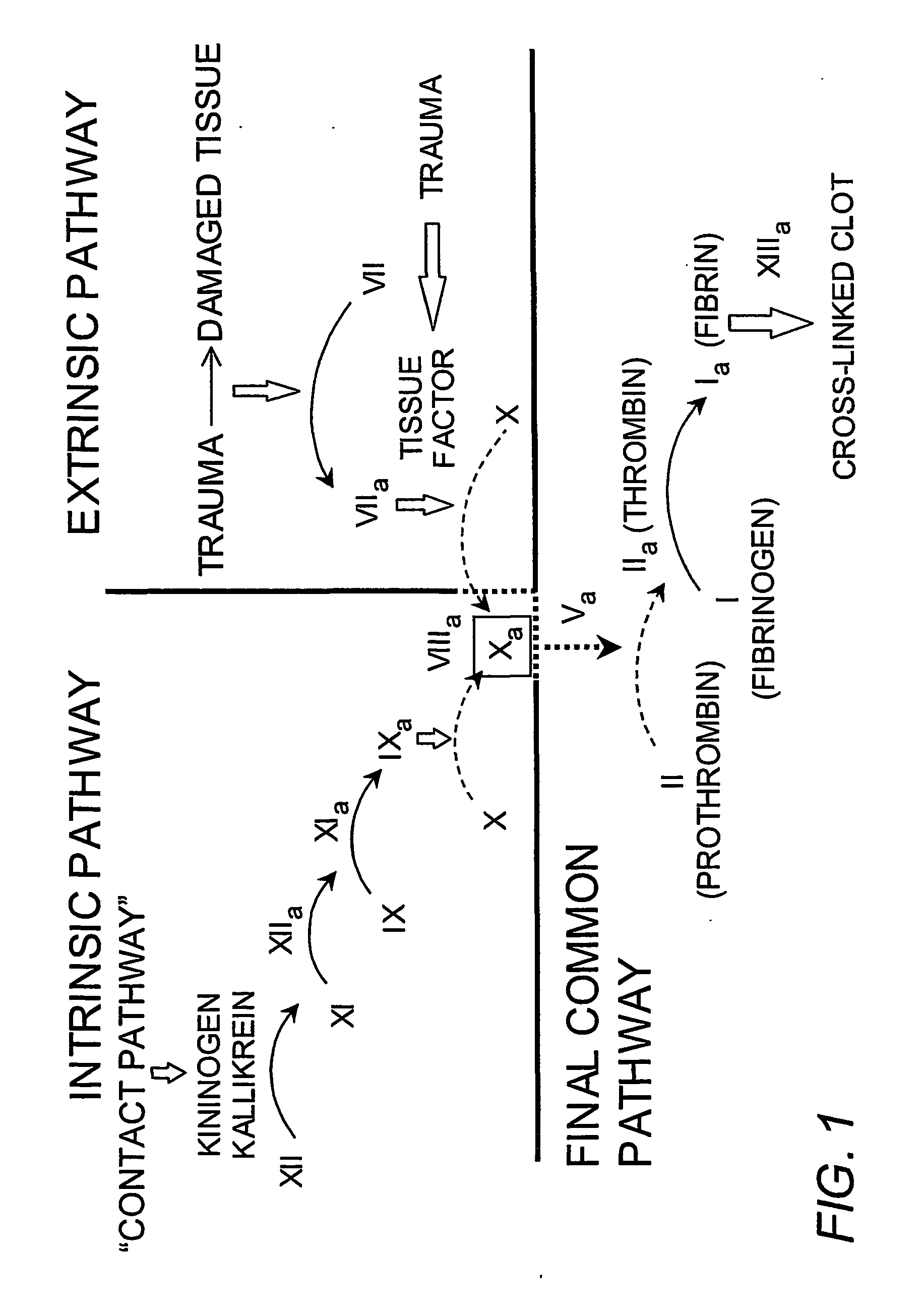
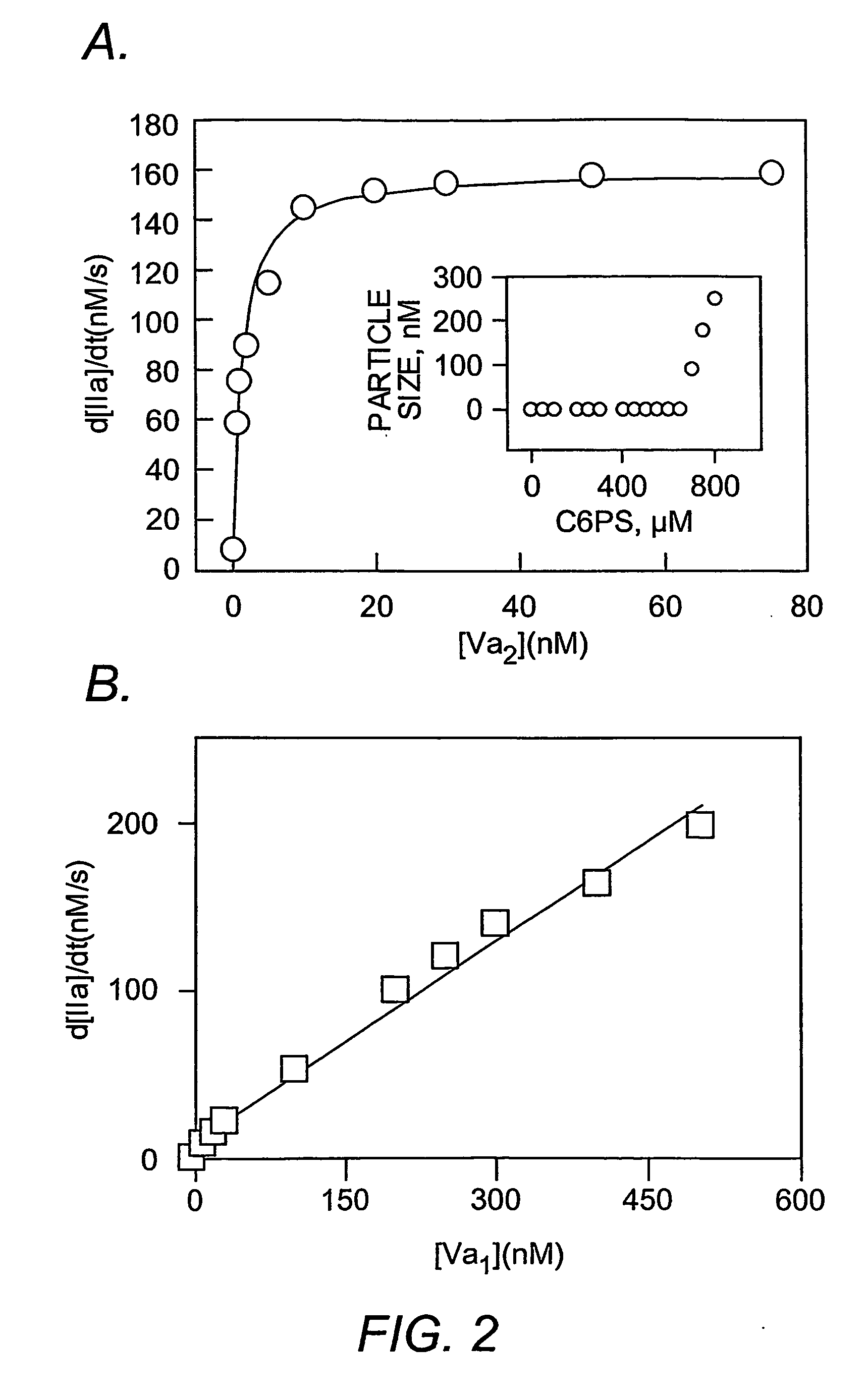
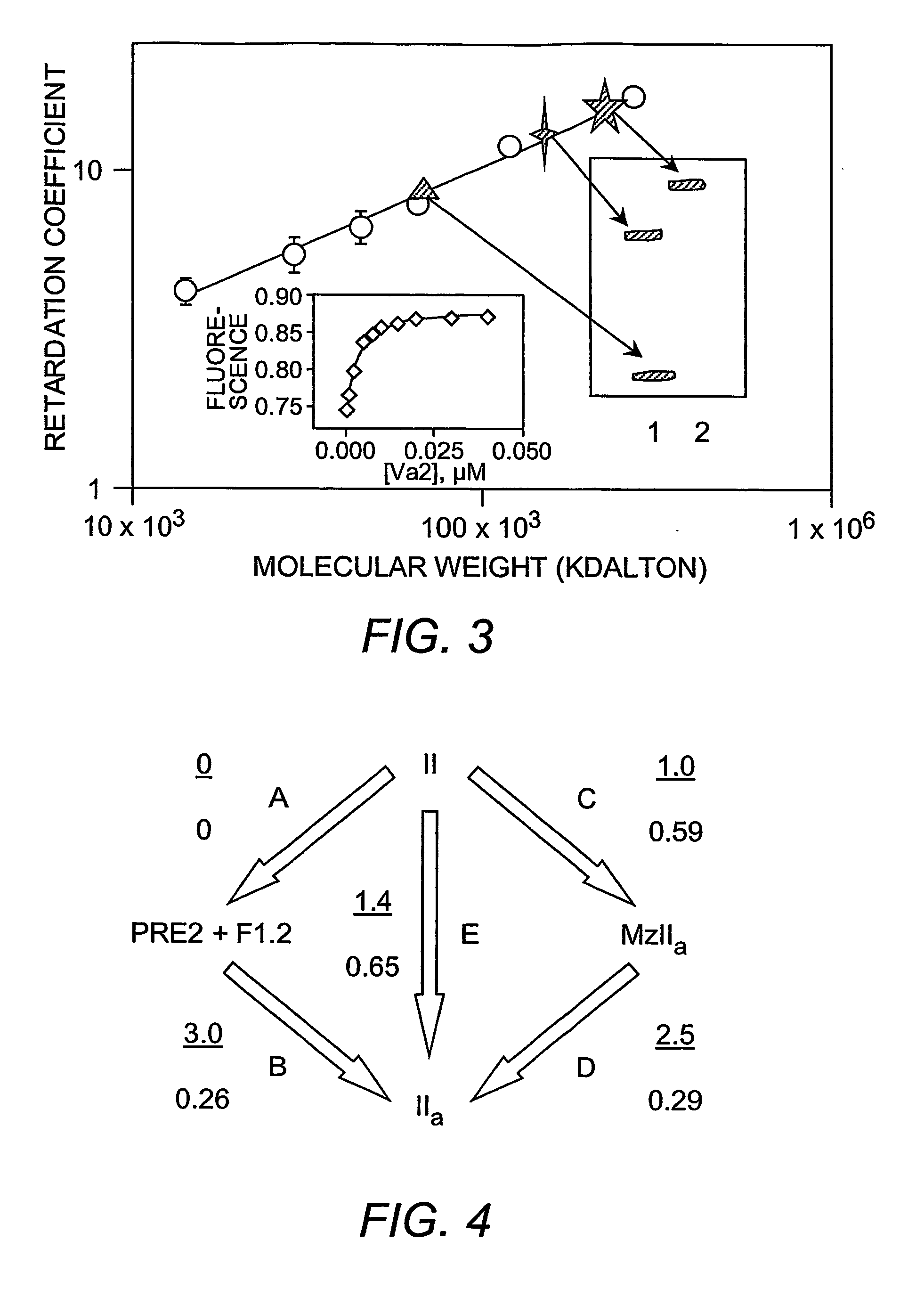
Platelet contractile force (PCF) is used as a surrogate marker of thrombin generation. PCF generation occurs concomitant with the burst of prothrombin fragment F 1+2 release. The time between assay start and PCF onset is identified as the thrombin generation time (TGT), and is used in assessing risk of bleeding, in diagnosing various disorders, and in monitoring the effects of pharmaceutical and other treatments. TGT is prolonged in clotting factor deficiencies and in the presence of direct and indirect thrombin inhibitors. TGT shortens to normal with clotting factor replacement and shortens with administration of rVIIa. TGT is short in thrombophilic states such as coronary artery disease, diabetes and thromboangiitis obliterans and prolongs toward normal with oral and intravenous anticoagulants.
Owner:HEMODYNE
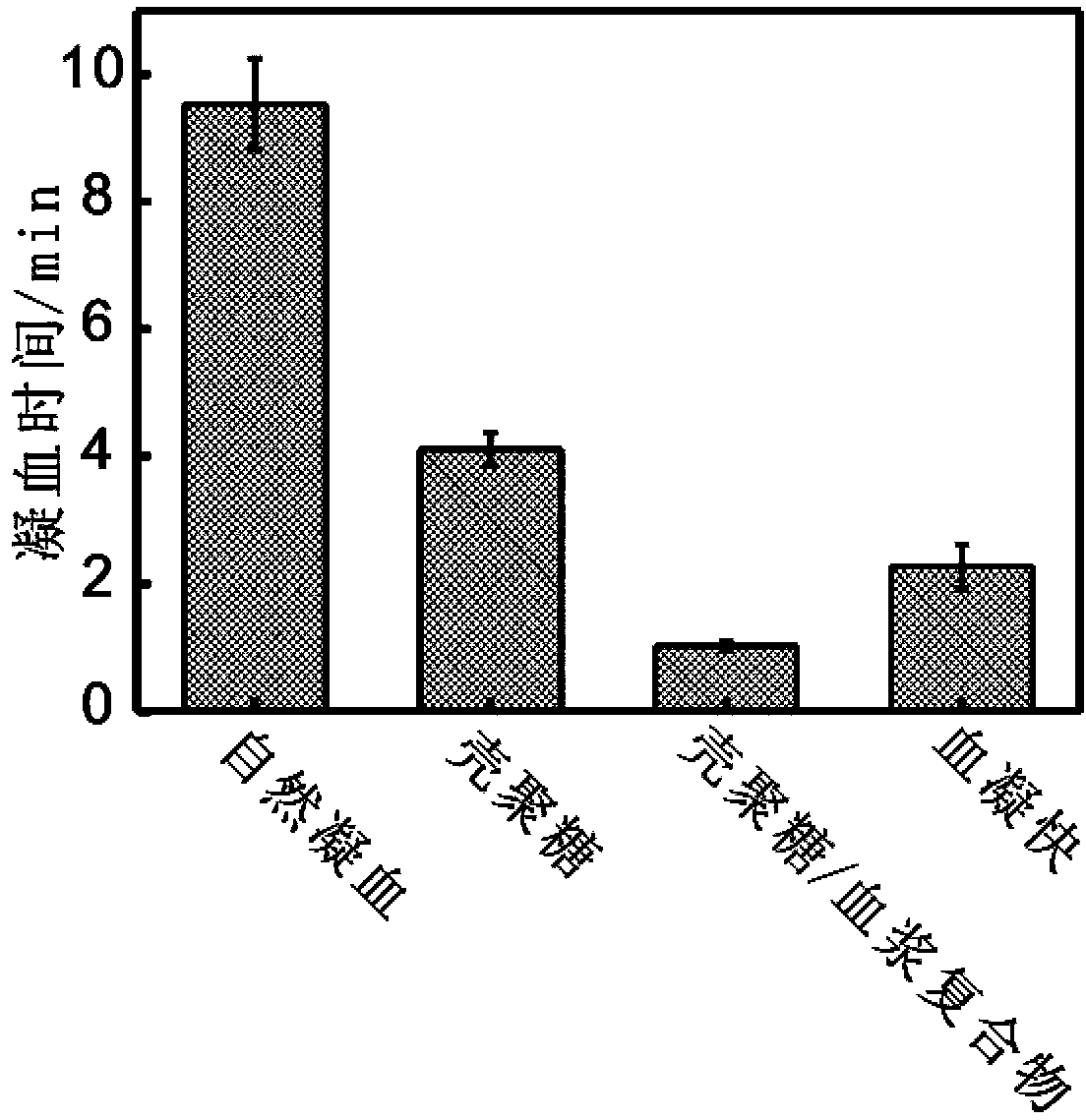
Insoluble polysaccharide compound with hemostatic function and preparation method thereof
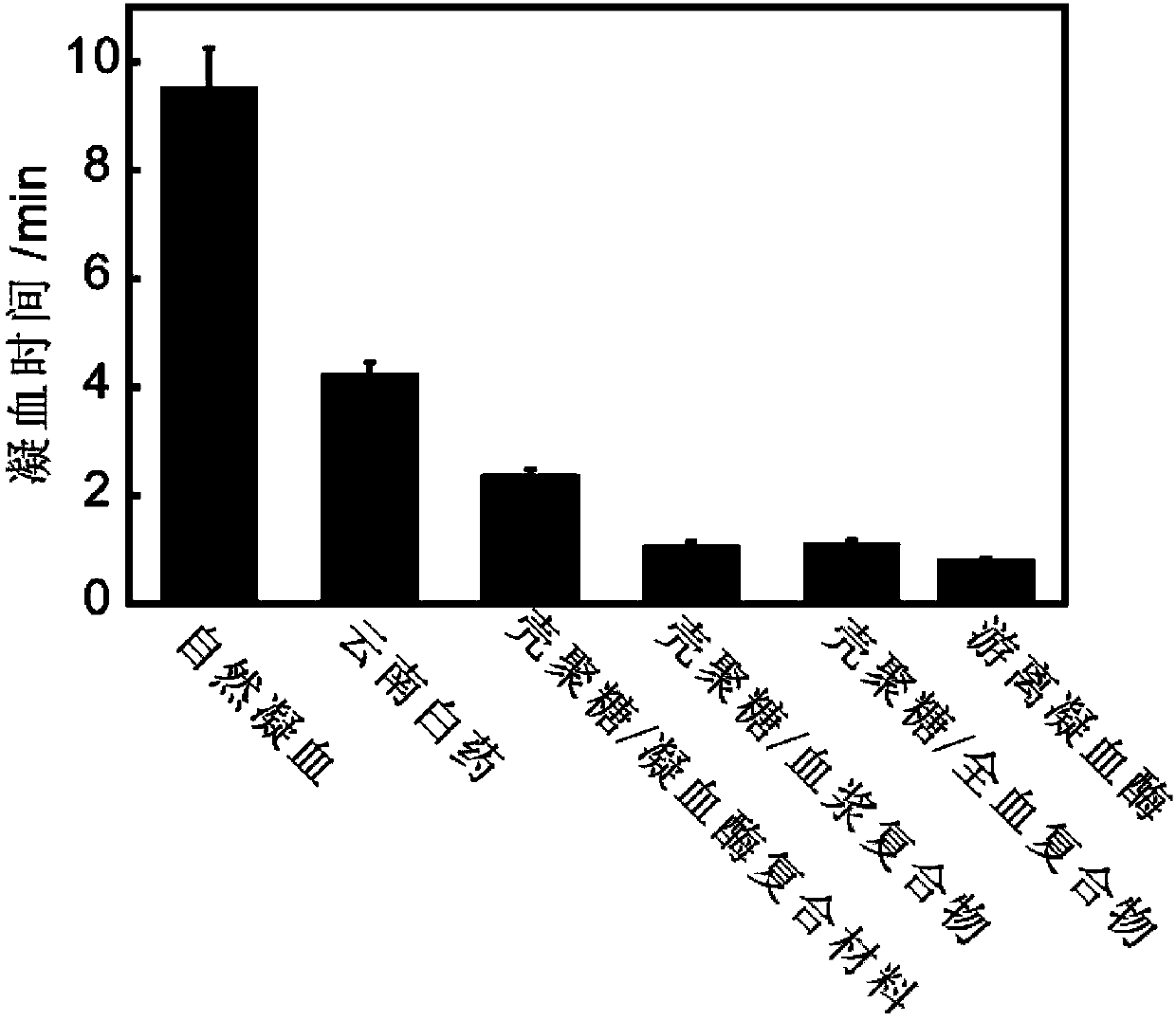
ActiveCN104208096AHigh coagulation efficiencyEvenly dispersedAerosol deliveryOintment deliveryAntibiotic YBlood plasma
The invention provides an insoluble polysaccharide compound with a hemostatic function. The compound includes insoluble polysaccharide and a blood component. The blood component is whole blood or plasma, and the insoluble polysaccharide is macromolecular polysaccharide insoluble in whole blood or plasma. According to the invention, by mixing the blood component with the insoluble polysaccharide, the blood component undergoes clotting reaction evenly and dispersedly on the surface of the insoluble polysaccharide, so that clotting factors generated in the clotting process can be adsorbed by the insoluble polysaccharide surface, and high clotting activity can be maintained, thereby obtaining a composite hemostatic material with an excellent clotting effect. The preparation method of the insoluble polysaccharide compound provided by the invention is simple, and is convenient and safe to use, thus being widely applicable to trauma and surgical hemostasis. The insoluble polysaccharide compound can be combined with other medical materials and medical instruments so as to be prepared into band aids, infusion bands, self-adhesive dressings, tablets and capsule core materials, operation packages and first-aid packets, etc. At the same time, the adsorption performance and other characteristics of the insoluble polysaccharide compound are utilized to adsorb antibiotics, painkillers and the like thereinto, thus achieving better curative effect.
Owner:ZHEJIANG UNIV
Improved-type human coagulation factor FVII-Fc fusion protein as well as preparation method and application thereof
ActiveCN103397009AIncrease productionEfficient and convenient purificationPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
The invention discloses a recombined human coagulation factor FVII-Fc fusion protein as well as a preparation method and an application thereof. The fusion protein sequentially comprises human FVII, a flexible peptide junction and an IgG2Fc variant from an N terminal to a C terminal, wherein the Fv variant has no splitting property and shows tiny Fc-mediated adverse side effect. The fusion protein has the biological activity similar to or higher than that of the human FVII and greatly prolonged plasma half-life, thus the pharmacodynamics and the drug effect are improved.
Owner:上海日耘生物医药有限公司
Nucleic acid molecules and uses thereof for non-viral gene therapy
PendingUS20200069817A1Organic active ingredientsFactor VIITherapeutic proteinInverted Terminal Repeat
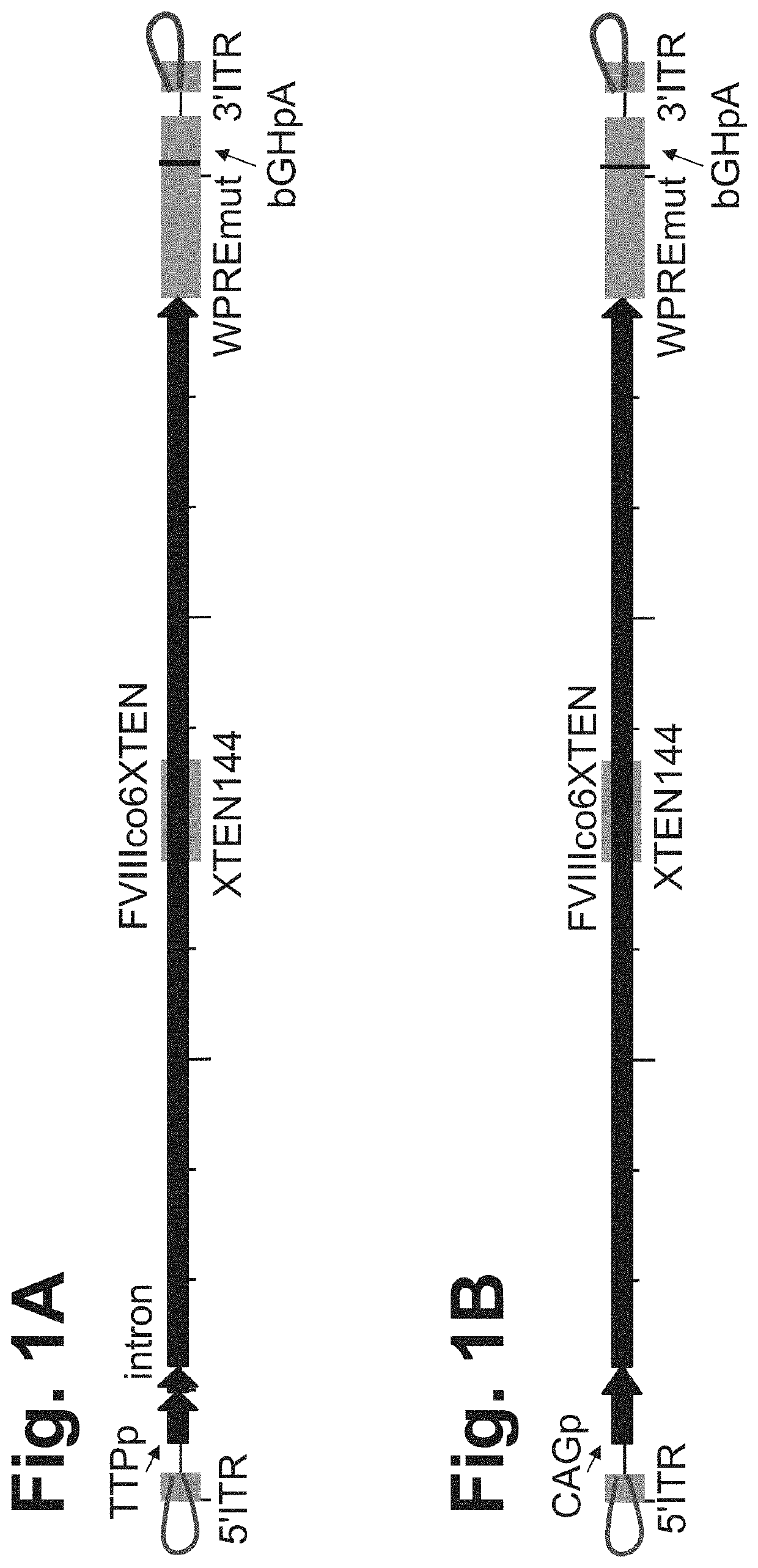
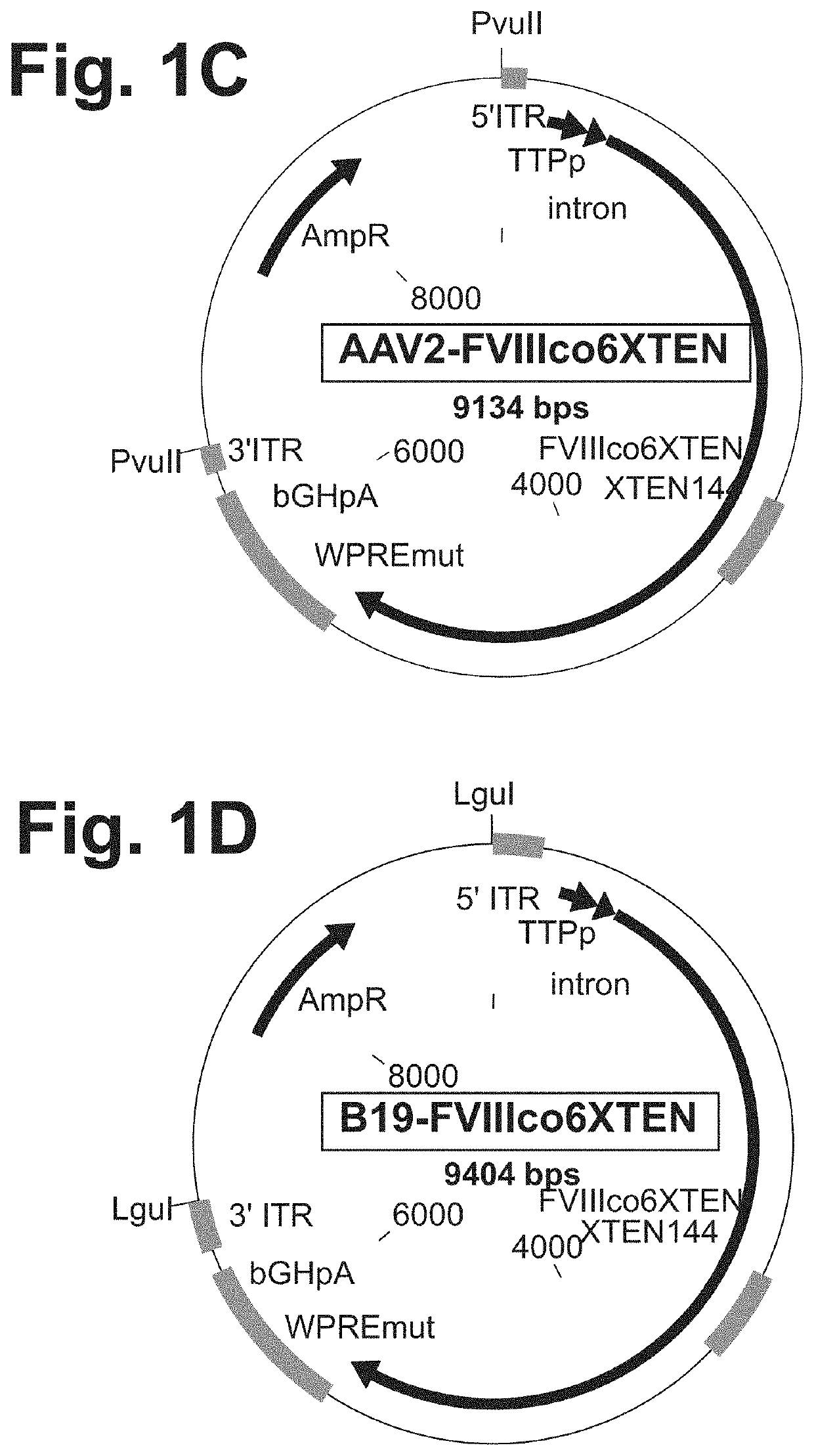
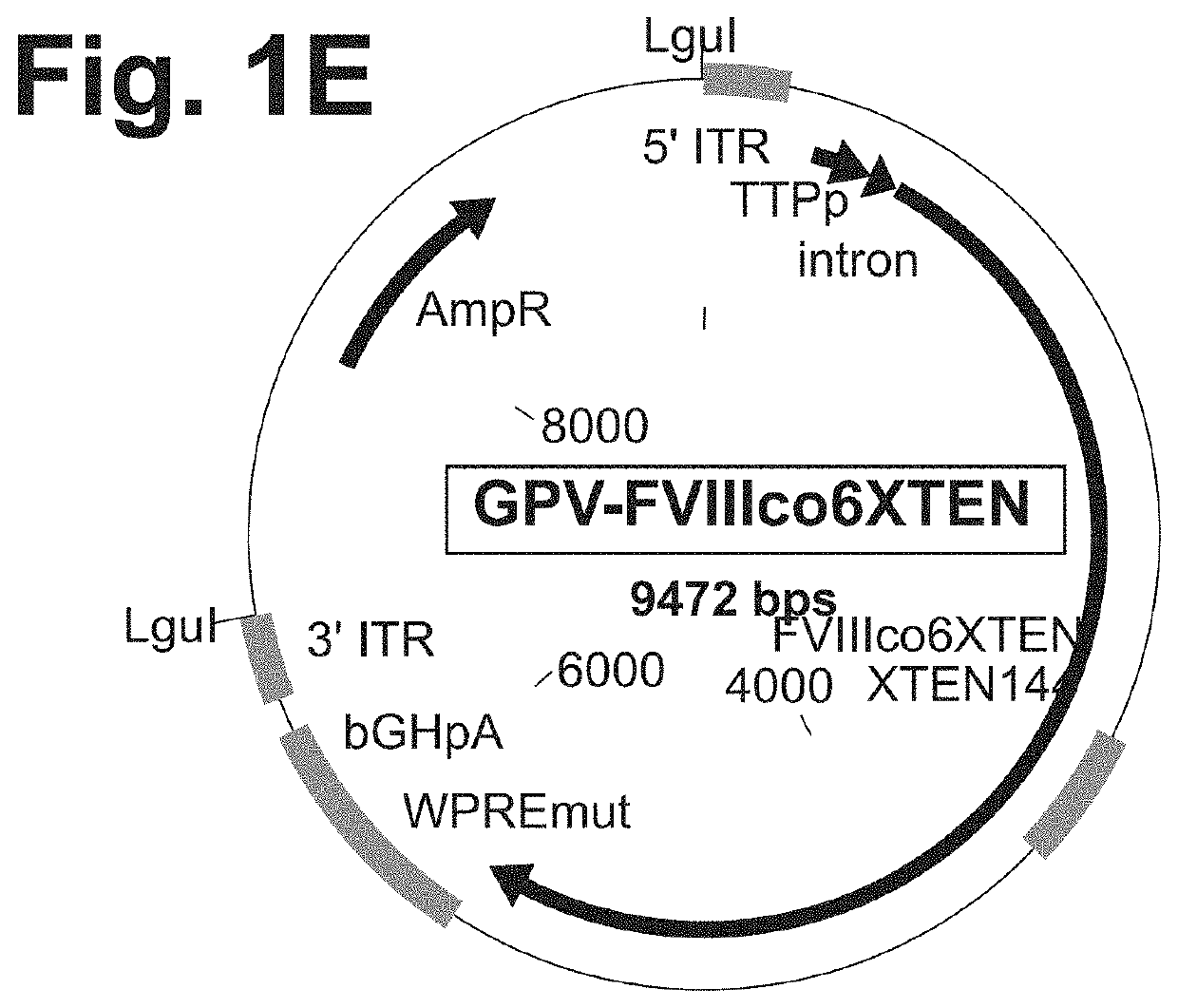
The present disclosure provides nucleic acid molecules comprising a first inverted terminal repeat (ITR), a second ITR, and a genetic cassette encoding a target sequence. In some embodiments, the target sequence encodes a miRNA and / or a therapeutic protein. In certain embodiments, the therapeutic protein comprises a clotting factor, a growth factor, a hormone, a cytokine, an antibody, a fragment thereof, and a combination thereof. In some embodiments, the first ITR and / or the second ITR is an ITR of a non-adeno-associated virus (AAV). The present disclosure also provides methods of treating a metabolic disorder of the liver in a subject comprising administering to the subject the nucleic acid molecule or a polypeptide encoded thereby.
Owner:BIOVERATIV THERAPEUTICS INC
Macrocyclic factor VIIa inhibitors useful as anticoagulants
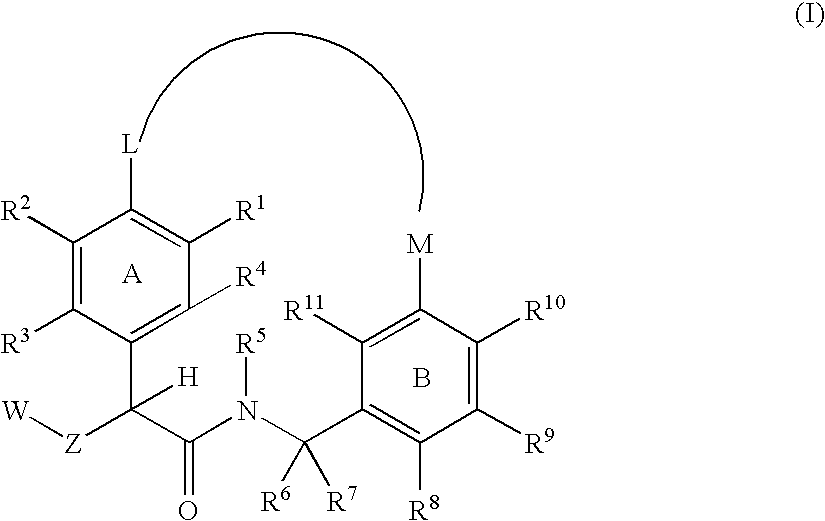
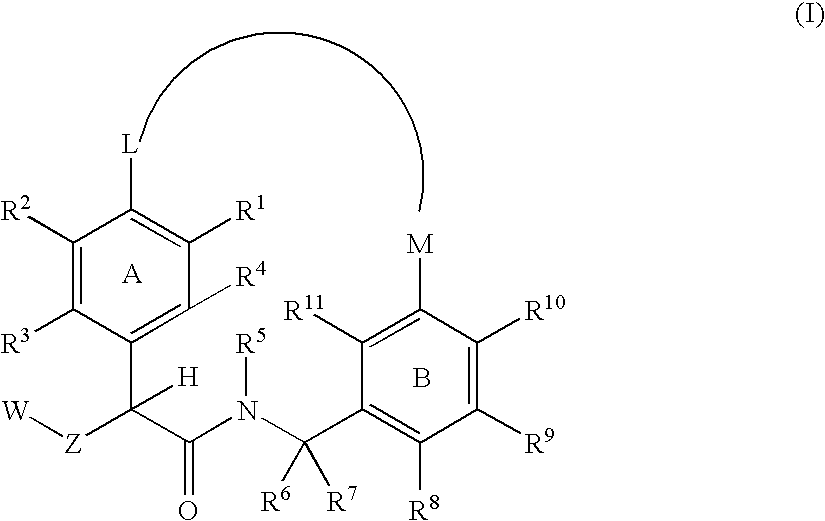
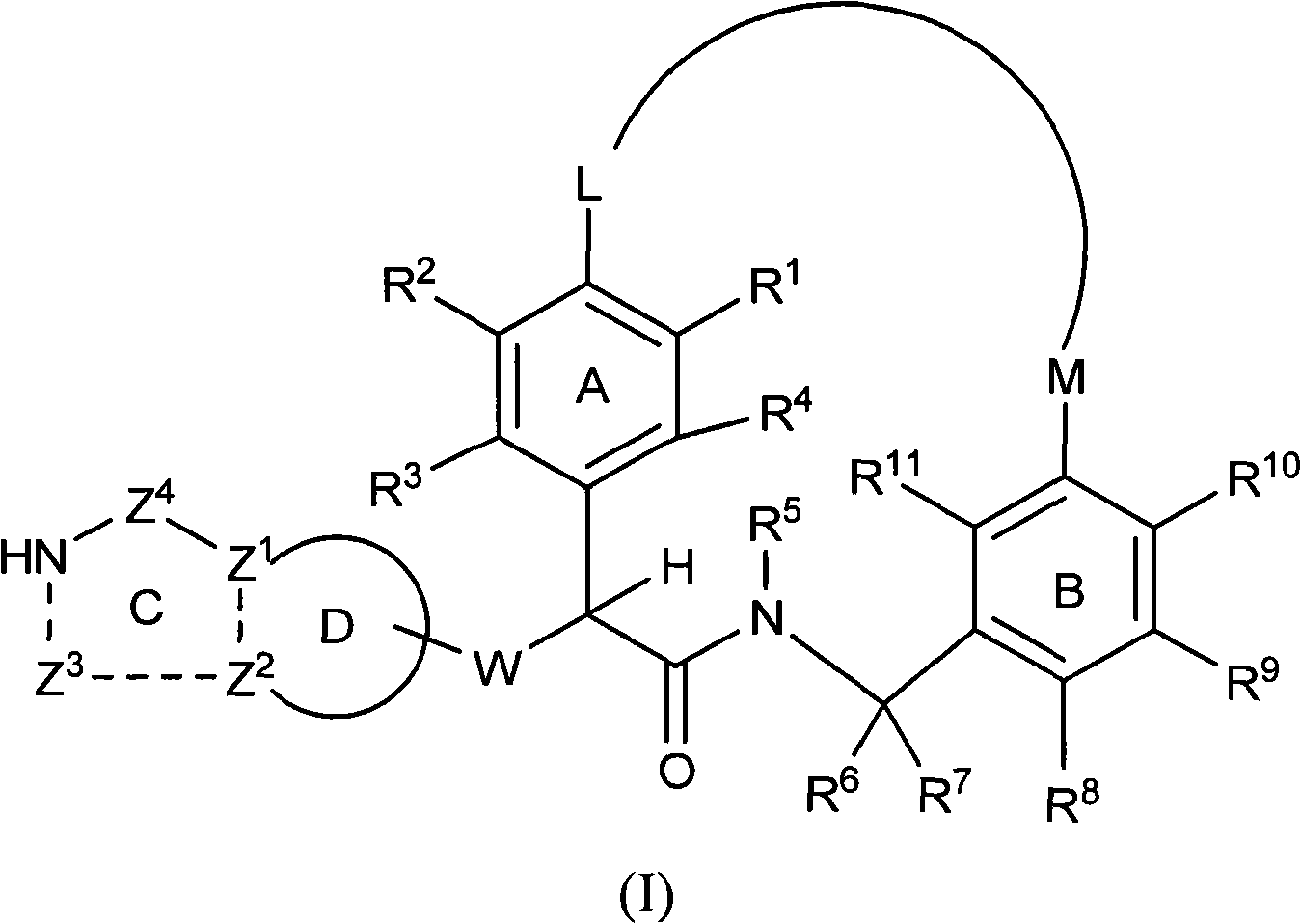
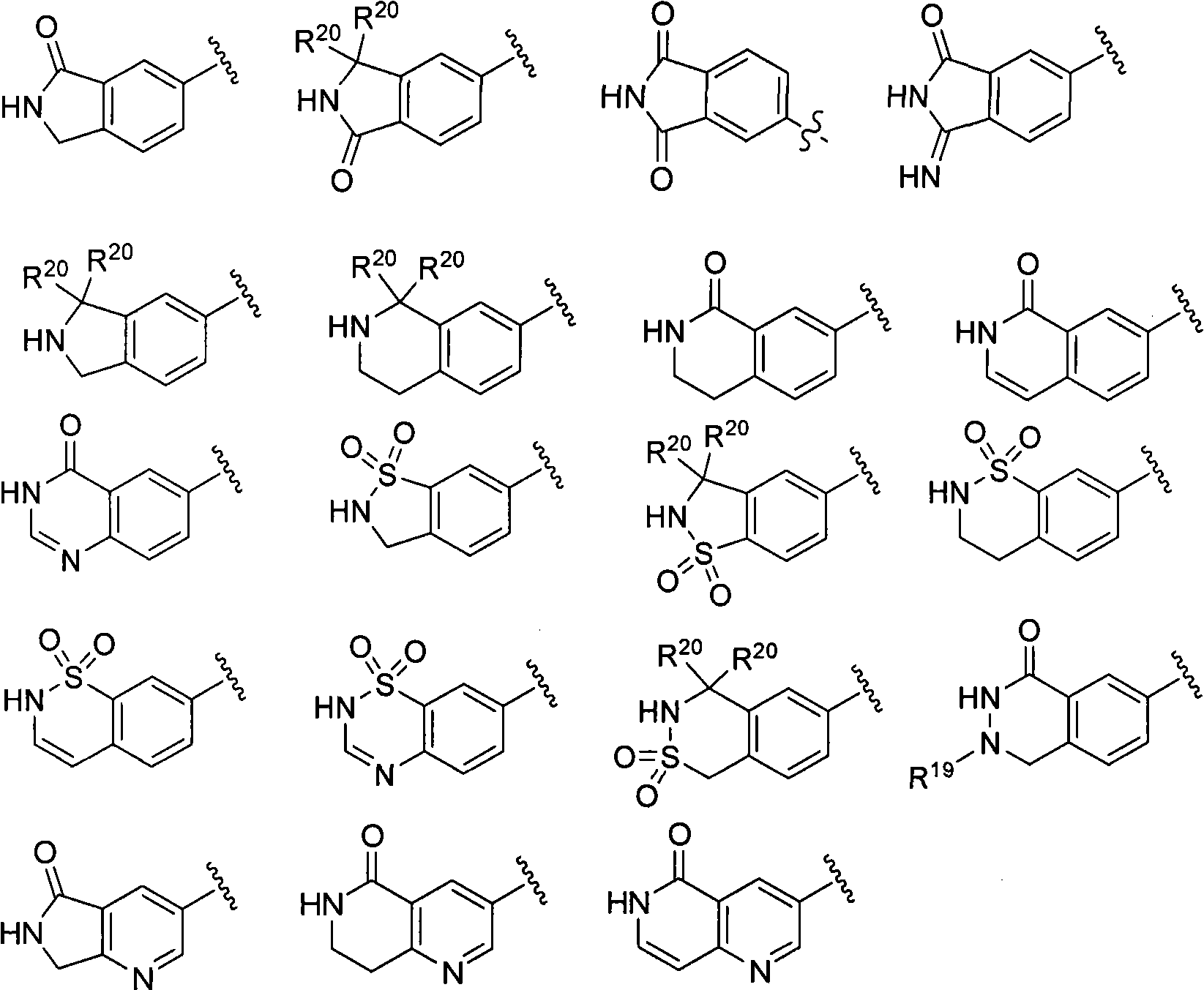
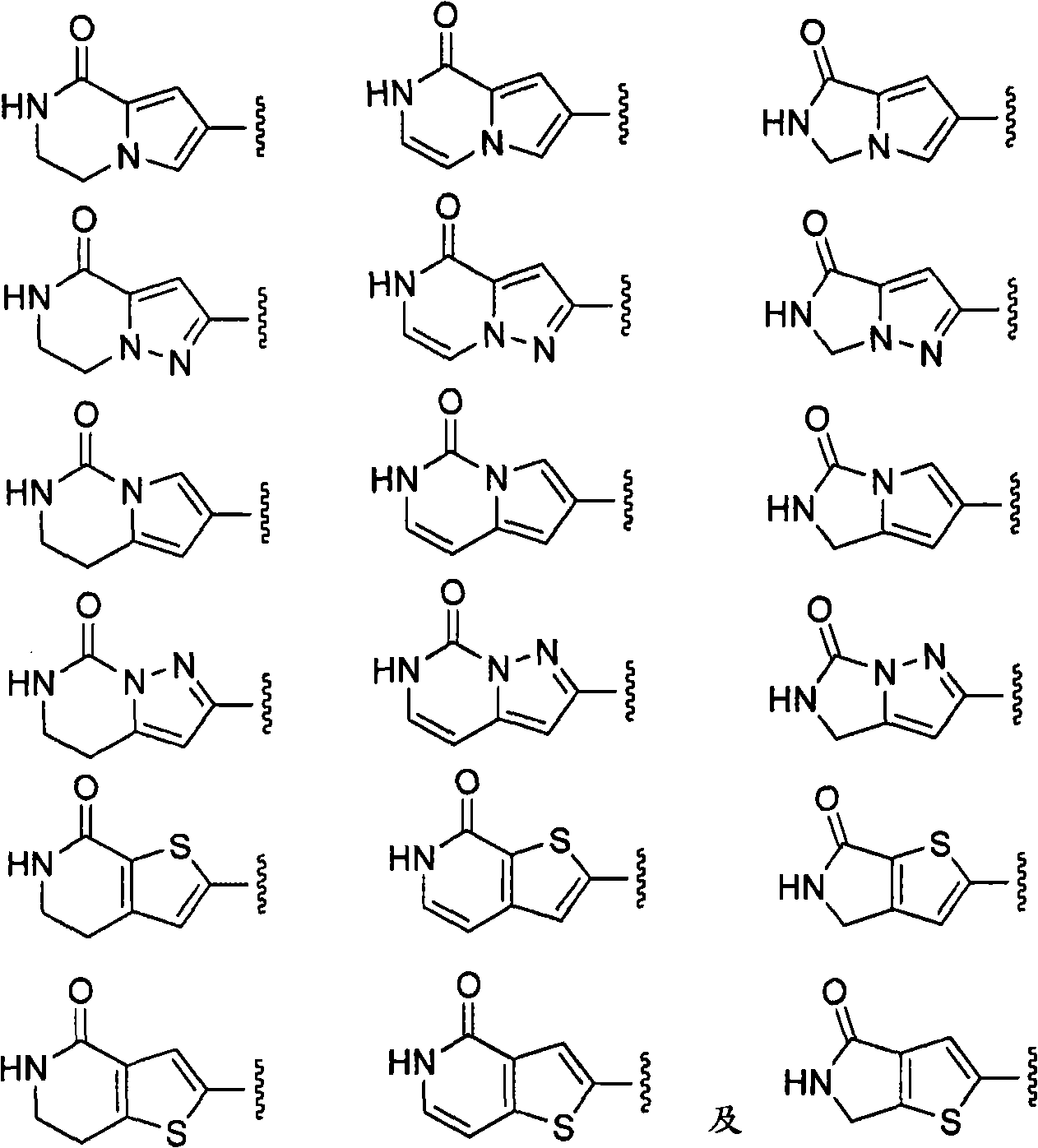
The present invention relates generally to novel macrocycles of Formula (I):or stereoisomers, tautomers, pharmaceutically acceptable salts, solvates, or prodrugs thereof, wherein the variables A, B, L, M, W, Z, R1, R2, R3, R4, R5, R6, R7, R8, R9, and R10 are as defined herein. These compounds are selective inhibitors of the serine protease coagulation factor VIIa which can be used as medicaments.
Owner:BRISTOL MYERS SQUIBB CO
Thromboelastography instrument quality control product and preparation method thereof
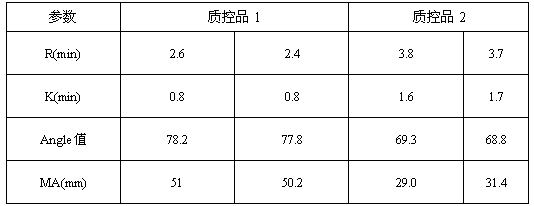
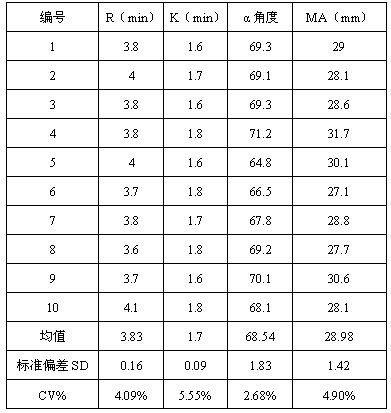
ActiveCN107588998AReduce use costSimple preparation stepsPreparing sample for investigationBiological testingBiochemical engineeringQuality control
The invention discloses a quality control product which comprises a quality control product 1 and a quality control product 2, wherein the quality control product 1 comprises the following components:2-6 parts by weight of factor-rich goat plasma, 2-5 parts by weight of sheep serum and 1-4 parts by weight of fibrinogen mother liquor; the quality control product 2 comprises the following components: 1 to 4 parts by weight of factor-deficient sheep plasma, 5 to 10 parts by weight of sheep serum and 0. 3 to 2 parts by weight of the fibrinogen mother liquor, the fibrinogen mother liquor is prepared by dissolving fibrinogen in the sheep serum, wherein the mass percentage of the fibrinogen is 10%; clotting factor extraction is not needed in the preparation process of the thromboelastography instrument quality control product, preparation steps are simplified, operation is easy, cost is low, results are accurate, and stability is good; test results of the thromboelastography instrument quality control product basically meet that of a quality control product produced by already listed US Haemoscope company, the thromboelastography instrument quality control product can replace the qualitycontrol product produced by the already listed US Haemoscope company, and customer use cost is reduced.
Owner:上海原科实业发展有限公司
Coagulation Factor-Targeting to TLT-1 on Activated Platelets
InactiveUS20160263230A1Peptide/protein ingredientsAntibody mimetics/scaffoldsCoagulation proteinPolynucleotide
Owner:NOVO NORDISK HEALTH CARE AG
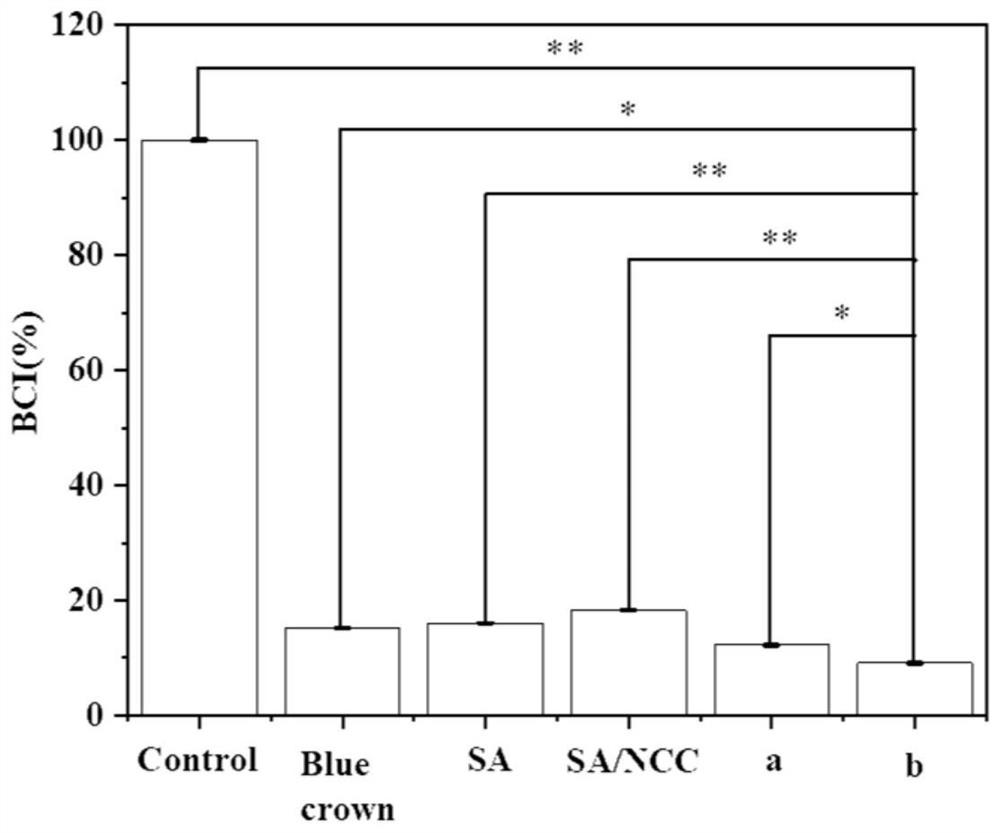
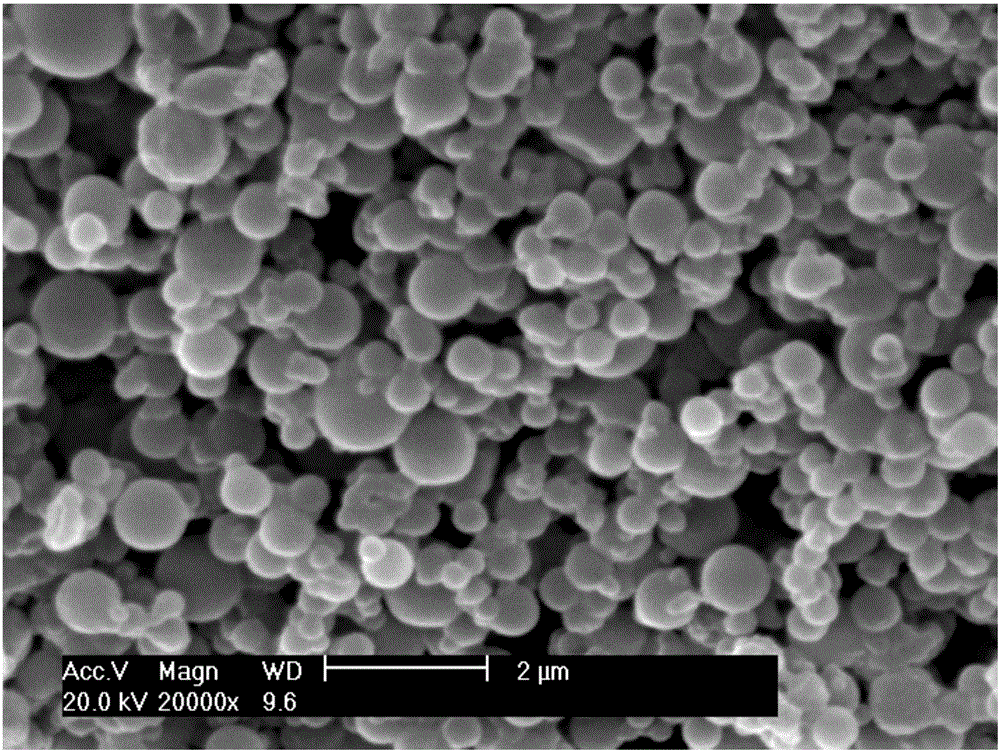
Antibacterial hemostatic porous microsphere based on sodium alginate and nanocrystalline cellulose
ActiveCN113908328AEasy to prepareMaterials are readily availableSurgical adhesivesPharmaceutical delivery mechanismCelluloseRed blood cell
The invention provides preparation of a antibacterial hemostatic porous microsphere with good biocompatibility, nontoxicity and biodegradability. The sodium alginate / cellulose nanocrystalline porous microsphere is prepared by using an inverse emulsion method. The antibacterial hemostatic porous microsphere of sodium alginate and cellulose nanocrystalline has simple preparation method, available materials, and good safety coefficient. The sodium alginate / nanocrystalline cellulose loaded epsilon-polylysine porous microsphere prepared by the method of the invention is applied in wound hemostasis to quickly absorb blood, reduce blood flow and promote local aggregation of red blood cells, blood coagulation factors and platelets at a wound, thereby promoting hemostasis. In addition, the antibacterial hemostatic porous microsphere of the present invention can prevent wound infection and promote tissue healing due to antibacterial performance.
Owner:ZHEJIANG OCEAN UNIV
Styptic powder
InactiveCN104998293AGood bioadhesionGood hemostatic abilityAbsorbent padsBandagesMicrosphereBiocompatibility Testing
The invention aims to provide a crosslinking poly glutamic acid calcium microsphere. The adopted base material poly glutamic acid is a polyamino acid material obtained through microorganism fermentation synthesis, controllable in structure, biodegradable, excellent in biocompatibility, simple in product forming technology and easy to control in quality; in addition, the hemostasis process of the crosslinking poly glutamic acid calcium microsphere is not affected by normal clotting mechanism and can still play the stypticity for patients using an anticoagulant and with low blood platelets and complete blood cells; release of Ca <2+> in the material can accelerate activation of intrinsic coagulation factors to realize the auxiliary stypticity; moreover, poly glutamic acid has better biological adhesiveness, facilitates control of continuous errhysis of tissues, and is higher in hemostasis capacity.
Owner:青岛卫辽医用生物材料有限公司
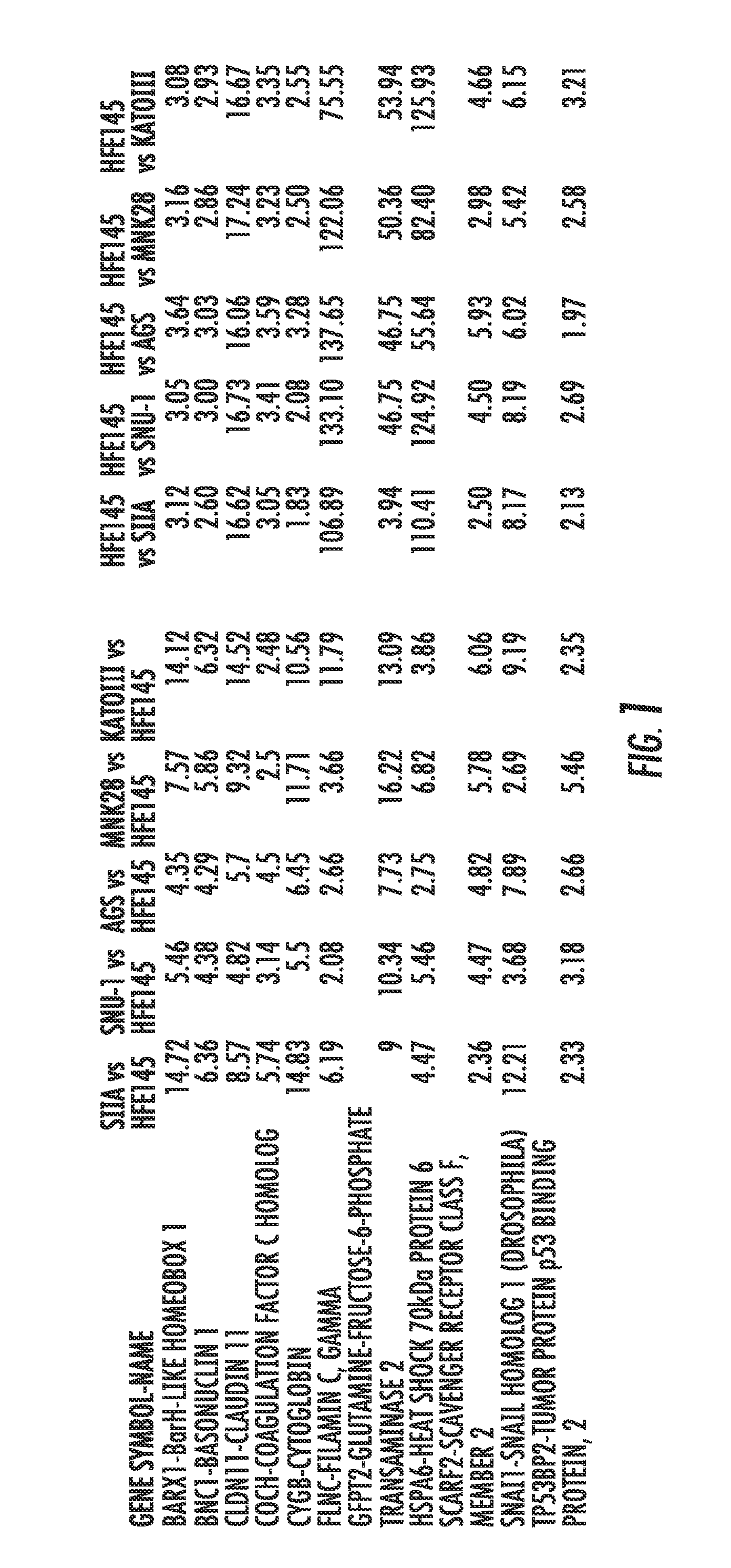
Methods and compositions for diagnosing and treating gastric cancer
ActiveUS20150292029A1Therapeutic utilityElevated methylation levelNucleotide librariesMicrobiological testing/measurementGlutamineCancer research
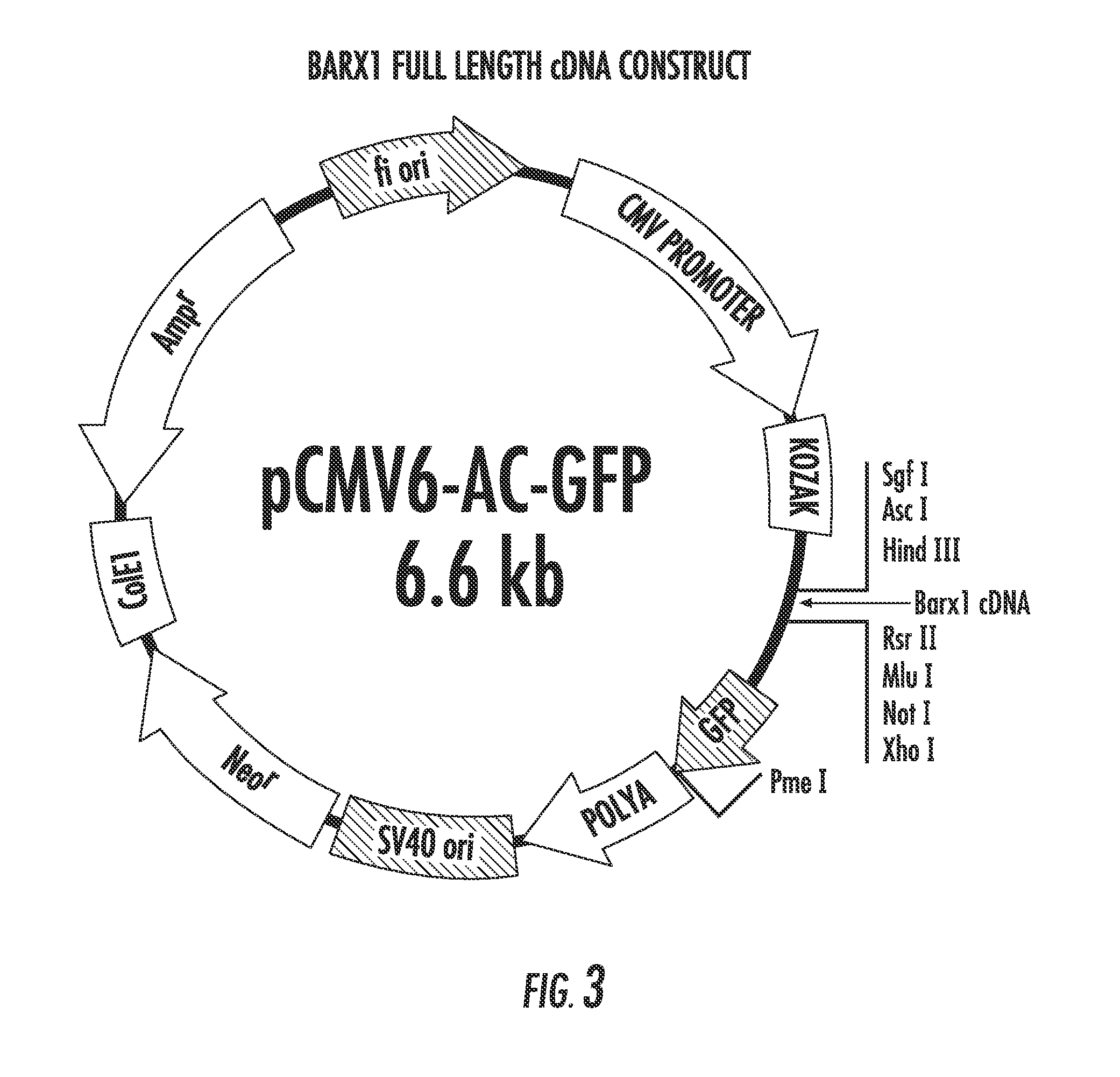
The present invention relates to the field of gastric cancer. More specifically, the present invention provides methods and compositions for diagnosing and treating gastric cancer. In a specific embodiment, a method for diagnosing gastric cancer or a likelihood thereof in a patient comprises the steps of (a) providing a sample from the patient; (b) measuring the methylation levels of one or more biomarkers in the sample collected from the patient; and (c) predicting gastric cancer in the patient if the biomarkers are hypermethylated. In a more specific embodiment, the one or more biomarkers comprises tight junction protein claudin-1 1 (CLD-N11), the transcription factor BarH-like homeobox (BARX1), basonuclin1 (BNC1), Coagulation factor C homolog (COCH), filamin C gamma (FLNC), cytoglobin B (CYGB), glutamine-fructose-6-phospahe-transaminase-2 (GFPT2), heat-shock-70 kDa protein-6 (HSPA6), snail homolog 1(SNAL1), skin calmodulin-related factor (SCARF), and tumor protein p53 binding protein 2 (TP53BP2).
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Soluble phospholipids for use in clotting factor assays
InactiveUS20070037235A1Reduce and eliminate interferenceReduce abundanceMicrobiological testing/measurementBiological testingPhospholipidClotting factor
The present invention provides a soluble phospholipid reagent and assays of clotting activity using the same. The methods of the invention can be used to carry out any clotting assay or other assay of clotting activity that traditionally relies on platelet membranes or synthetic membrane preparation by substituting therefor the soluble phospholipids of the invention. Assay compositions and kits comprising the soluble phospholipids of the invention are also provided.
Owner:NORTH CAROLINA AT CHAPEL HILL UNIVERSTIY OF THE +1
Macrocyclic factor VIIa inhibitors useful as anticoagulants
The present invention relates generally to novel macrocycles of Formula (I) or stereoisomers, tautomers, pharmaceutically acceptable salts, solvates, or prodrugs thereof, wherein the variables A, B, C, D, L, M, W, Z1, Z2, Z3, Z4, R1, R2, R3, R4, R5, R6, R7, R8, R9, and R10 are as defined herein. These compounds are selective inhibitors of factor VIIa which can be used as medicaments.
Owner:BRISTOL MYERS SQUIBB CO
Submicron particles to decrease transfusion
A submicron protein sphere and method to intravenously treat a patient requiring blood component transfusion. The submicron protein spheres have a size ranging from 1.0 micron to less than 0.1 micron and a molecular weight ranging from 780 billion Daltons to less than 0.8 billion Daltons. The protein spheres have no biologically active molecules added or bound to the protein spheres prior to administering to the patient. The protein used to construct the spheres can be human serum albumin from natural sources or recombinant DNA-derived serum albumin, or other proteins such as gelatin or synthetic polypeptides. However, the protein spheres can bind the various clotting factors including fibrinogen after the spheres have entered the blood stream, binding the necessary additional biologically active molecules supplied in vivo from the patient's own blood, and possibly in vitro.
Owner:PTLNV SERIES 8 LLC
Methods and devices for detection of anticoagulants in plasma and whole blood
PendingCN111094990AUnderstanding PathophysiologyPeptide/protein ingredientsLaboratory glasswaresAnticoagulant AgentAnti coagulation
Methods and devices for evaluating coagulation are described, including methods and devices for detecting an anticoagulant agent or a coagulation abnormality. In various embodiments, the methods and devices of the invention measure the coagulation of a sample in response to a gradient of one or more coagulation factors. These responses can be evaluated to accurately profile the coagulation impairments of the sample, including the presence of anticoagulant medication. In various embodiments, the invention provides the point-of-care or bedside testing with a convenient, microfluidic device thatcan be used by the minimally trained personnel.
Owner:MASSACHUSETTS INST OF TECH +1
Lipid nanodiscs and nanorods as modulators of clotting factor function in vivo
InactiveUS20160367677A1High expressionStable and firmPeptide/protein ingredientsPharmaceutical non-active ingredientsLipid formationFactor X
The present invention includes composition and methods of using a lipid nanodisk or nanotube composition comprising a lipid composition of phosphatidylserine and galactosylceramide and a membrane-bound Factor VIII protein, a membrane-bound Factor IX protein, a membrane-bound Factor VIII-Factor IX protein complex, or a membrane-bound Factor V-Factor X protein complex in or about the lipid nanodisks or nanotubes.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Agkistrodon acutus anticoagulant factor XI polypeptide and application thereof
ActiveCN113773377AStrong inhibitory activityProlonged thromboplastin timePeptide/protein ingredientsProtease inhibitorsAntithrombotic AgentAgkistrodon acutus
The invention belongs to the field of polypeptides in biochemistry, and relates to an agkistrodon acutus anticoagulant factor XI polypeptide and application thereof. According to the polypeptide, a Kunitz type polypeptide sequence is screened from an agkistrodon acutus snake venom transcriptome database, the Kunitz type polypeptide sequence is constructed and expressed to obtain the polypeptide with antithrombotic activity, and the polypeptide can be applied to preparation of antithrombotic drugs.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com