Styptic powder
A technology of hemostatic powder and calcium polyglutamate, applied in the field of hemostatic powder, can solve problems such as poor batch stability and difficult quality control, and achieve the effects of controllable structure, good biocompatibility, and easy quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
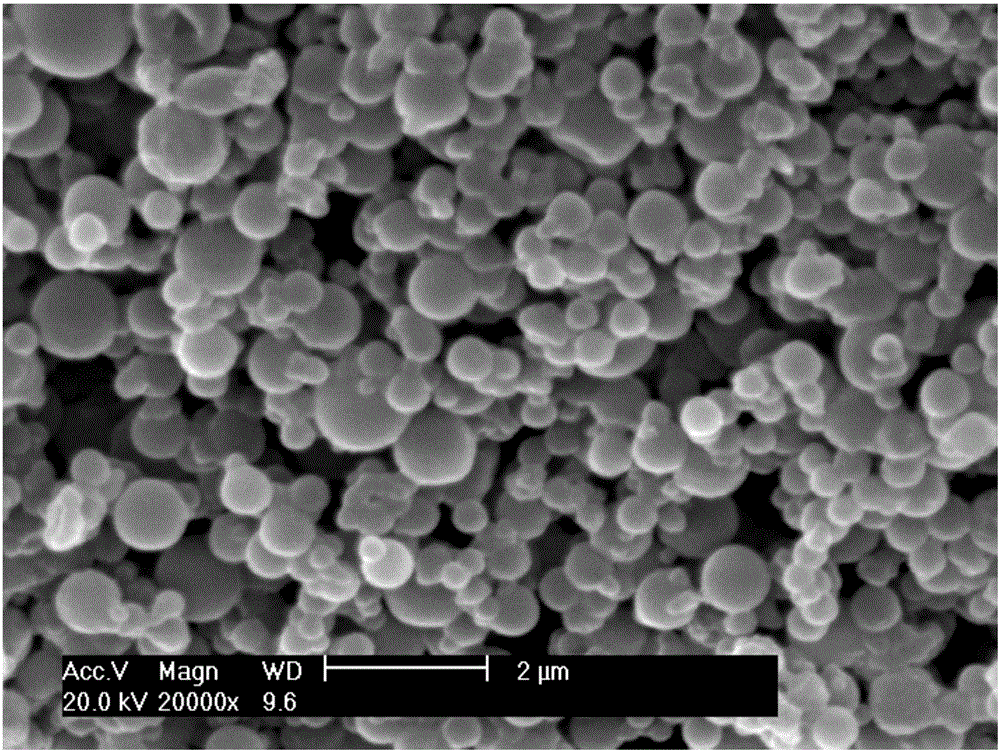
[0026] Prepare 100 mL of an aqueous solution containing 0.2 g calcium polyglutamate and 0.2 g adipic dihydrazide as the water phase; use n-heptane as the oil phase medium, add emulsifier / oil phase = 1 / 100 (w / w) After the emulsifier Tween80 is mixed evenly, the water-oil two-phase mixture is emulsified evenly according to the water / oil ratio=1:4 (v / v). Add 4 mL of aqueous solution containing 0.24 g EDAC and 1 mL of 0.1 mol / L hydrochloric acid in sequence, stir for 6 hours, add 400 mL of water, centrifuge, wash thoroughly with isopropanol, and dry in vacuum to obtain adipic acid dihydrazide cross-linked polyglutamic acid Calcium hemostatic powder, its scanning electron microscope photo is as follows figure 1 shown.
Embodiment 2
[0028] Prepare 100 mL of an aqueous solution containing 0.4 g of calcium polyglutamate and 0.2 g of adipate dihydrazide as the water phase; use cyclohexane as the oil phase medium, and add emulsifier / oil phase = 1 / 100 (w / w) After the emulsifier Tween80 is mixed evenly, the water-oil two-phase mixture is emulsified evenly according to the water / oil ratio=1:4 (v / v). Add 4 mL of aqueous solution containing 0.24 g EDAC and 0.1 mL of 0.1 mol / L hydrochloric acid in sequence, stir for 24 hours, add 200 mL of isopropanol, centrifuge, wash thoroughly with isopropanol, and dry in vacuo to obtain adipic acid dihydrazide cross-linked polymer Calcium glutamate hemostatic powder.
Embodiment 3
[0030] Hemostatic performance evaluation test of cross-linked polycalcium glutamate-based hemostatic powder. Establish rat femoral artery hemorrhage model, respectively evaluate and compare cross-linked polyglutamate calcium-based hemostatic powder 1 (prepared in Example 1), cross-linked polyglutamate calcium-based hemostatic powder 2 (prepared in embodiment 2) and Yunnan Baiyao (control group) hemostatic effect. Select 48 SD rats (body weight: 200 ± 20 g), half male and half female, and randomly divided into 4 groups, 12 rats in each group, half male and half male. The 4 groups are respectively: cross-linked calcium polyglutamate-based hemostatic powder 1 (prepared in Example 1), cross-linked polyglutamate calcium-based hemostatic powder 2 (prepared in Example 2), Yunnan Baiyao (control group) and gauze (blank group). The hemostatic powder of the experimental group and the control group was added at a dose of 0.1 g. All animals were anesthetized by intraperitoneal injectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com