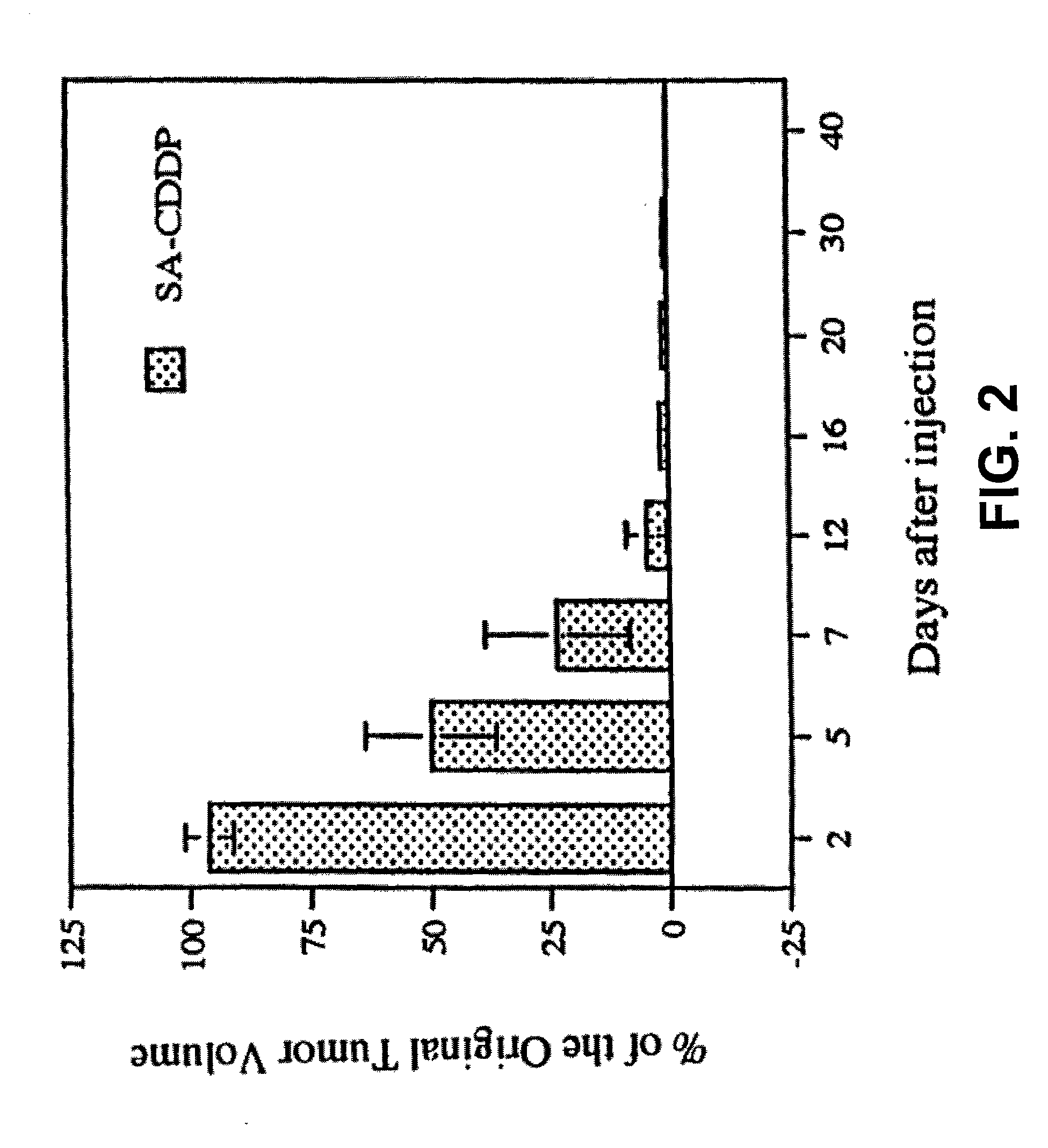
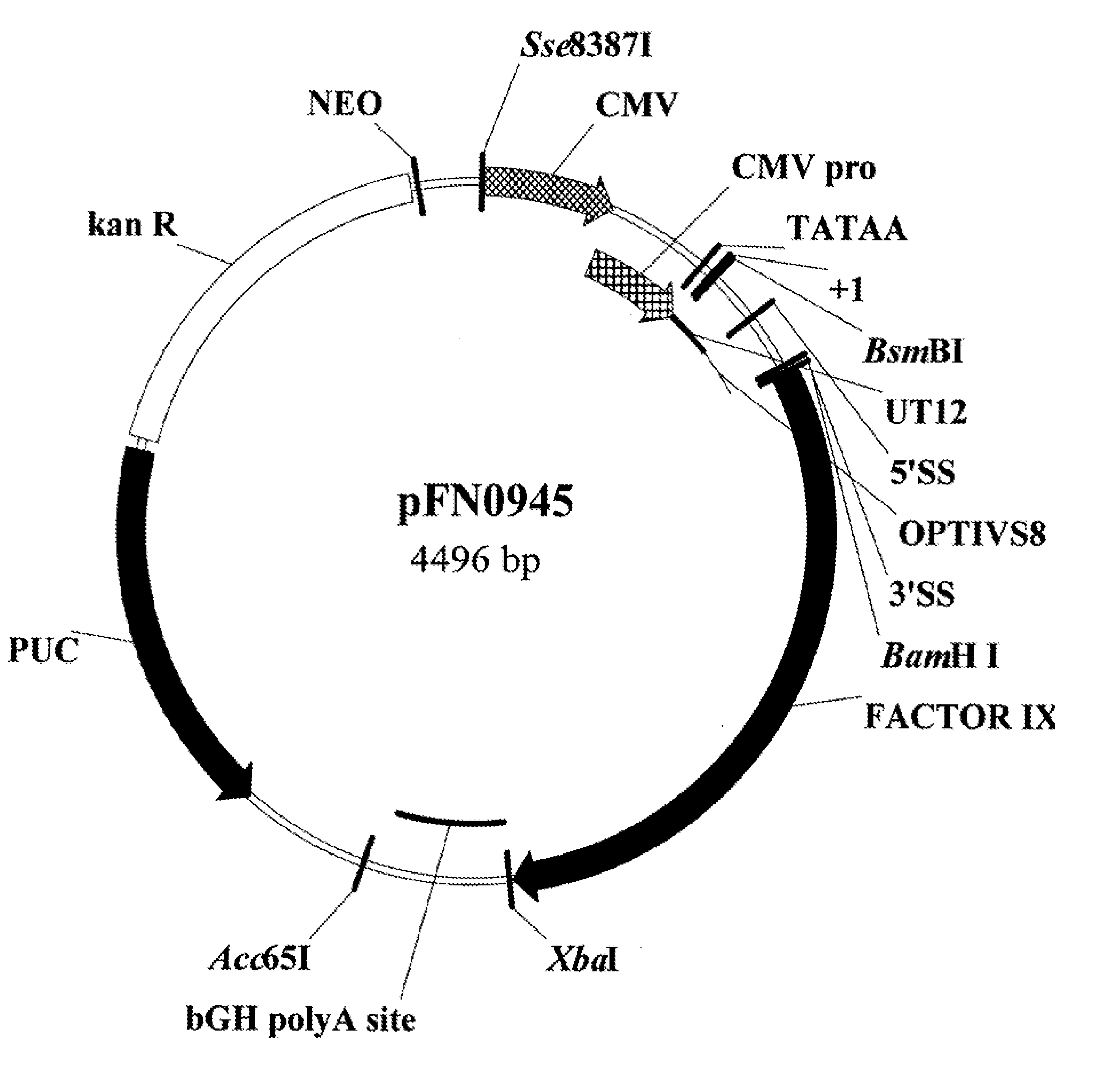
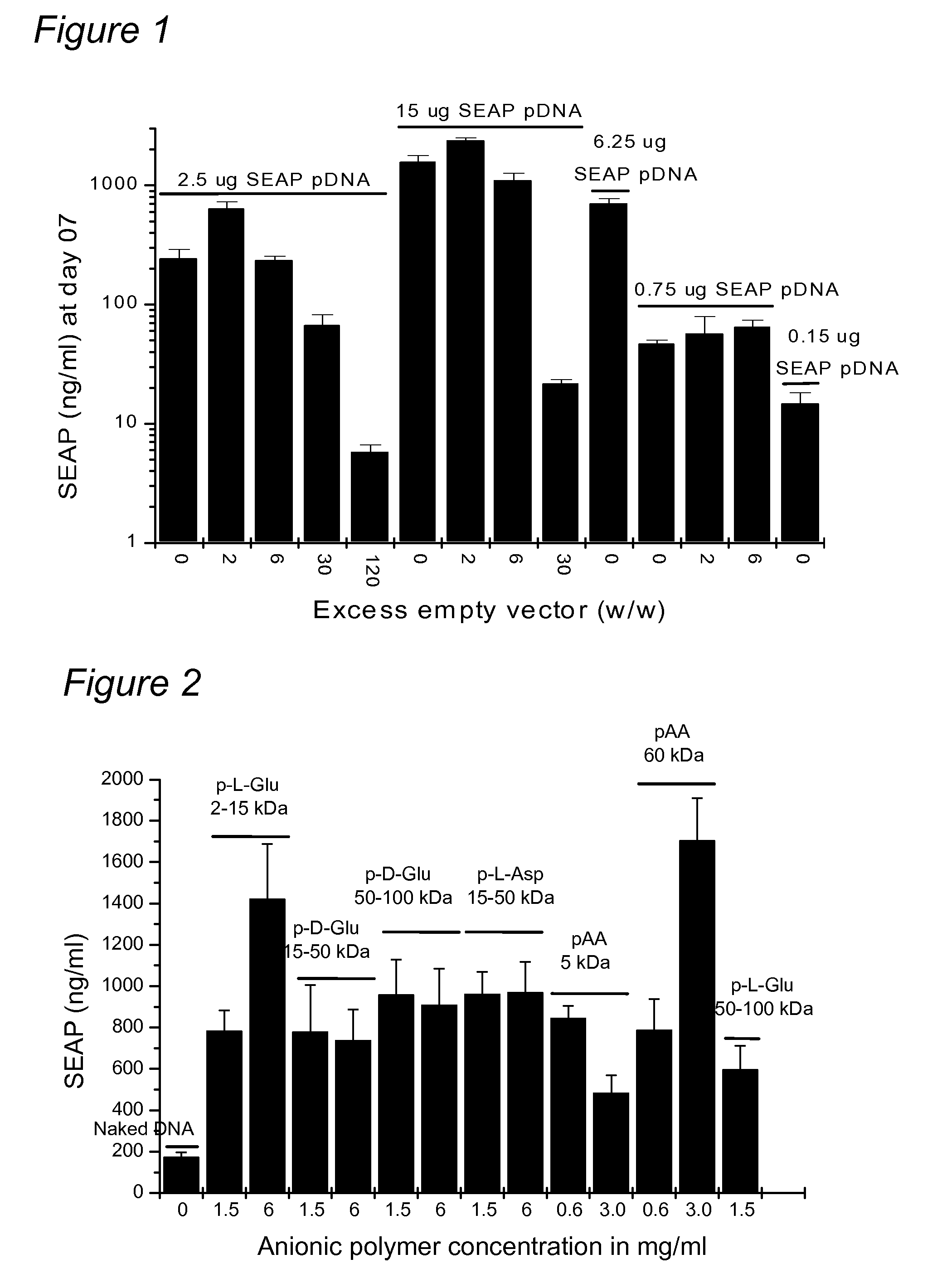
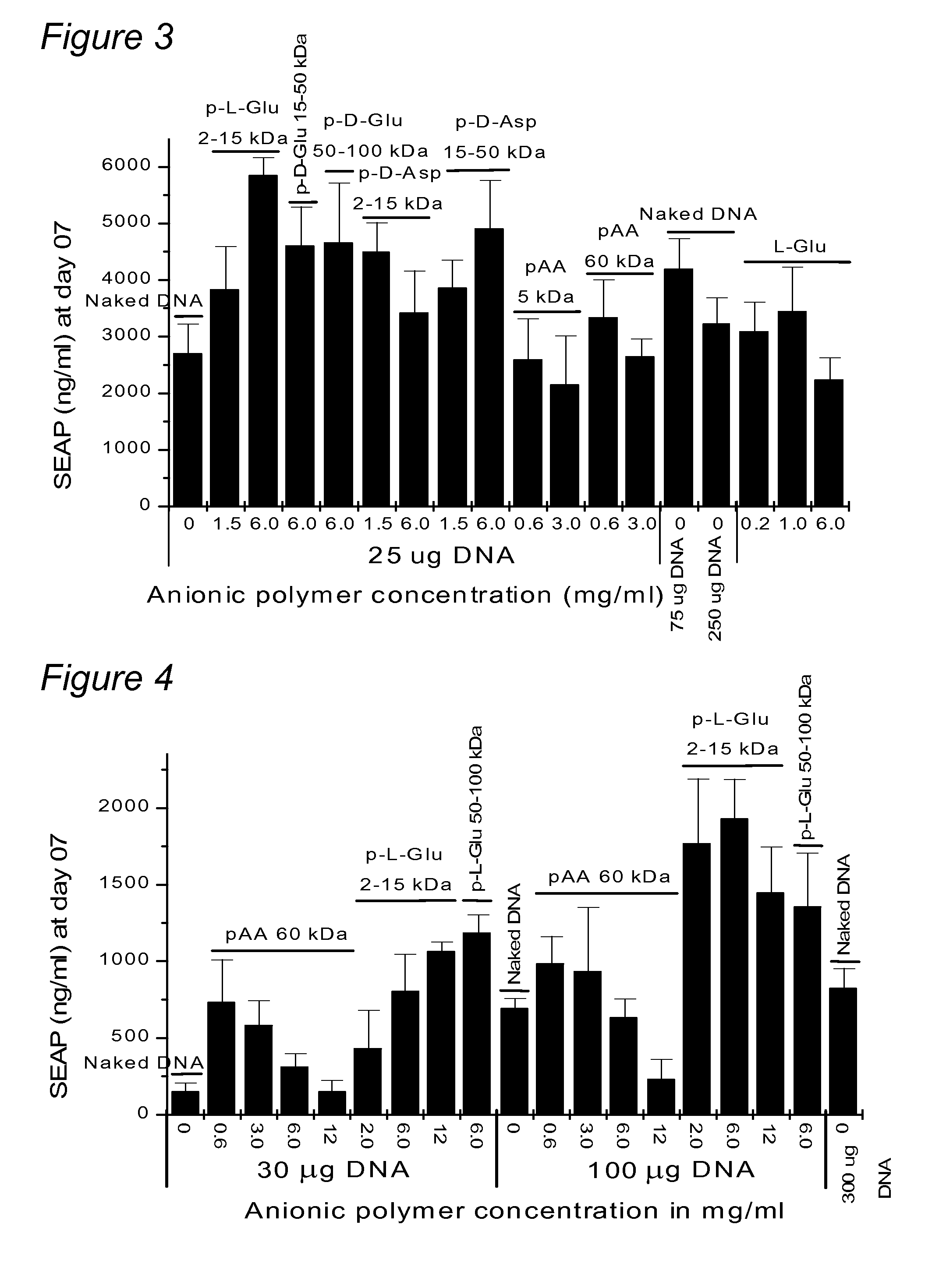
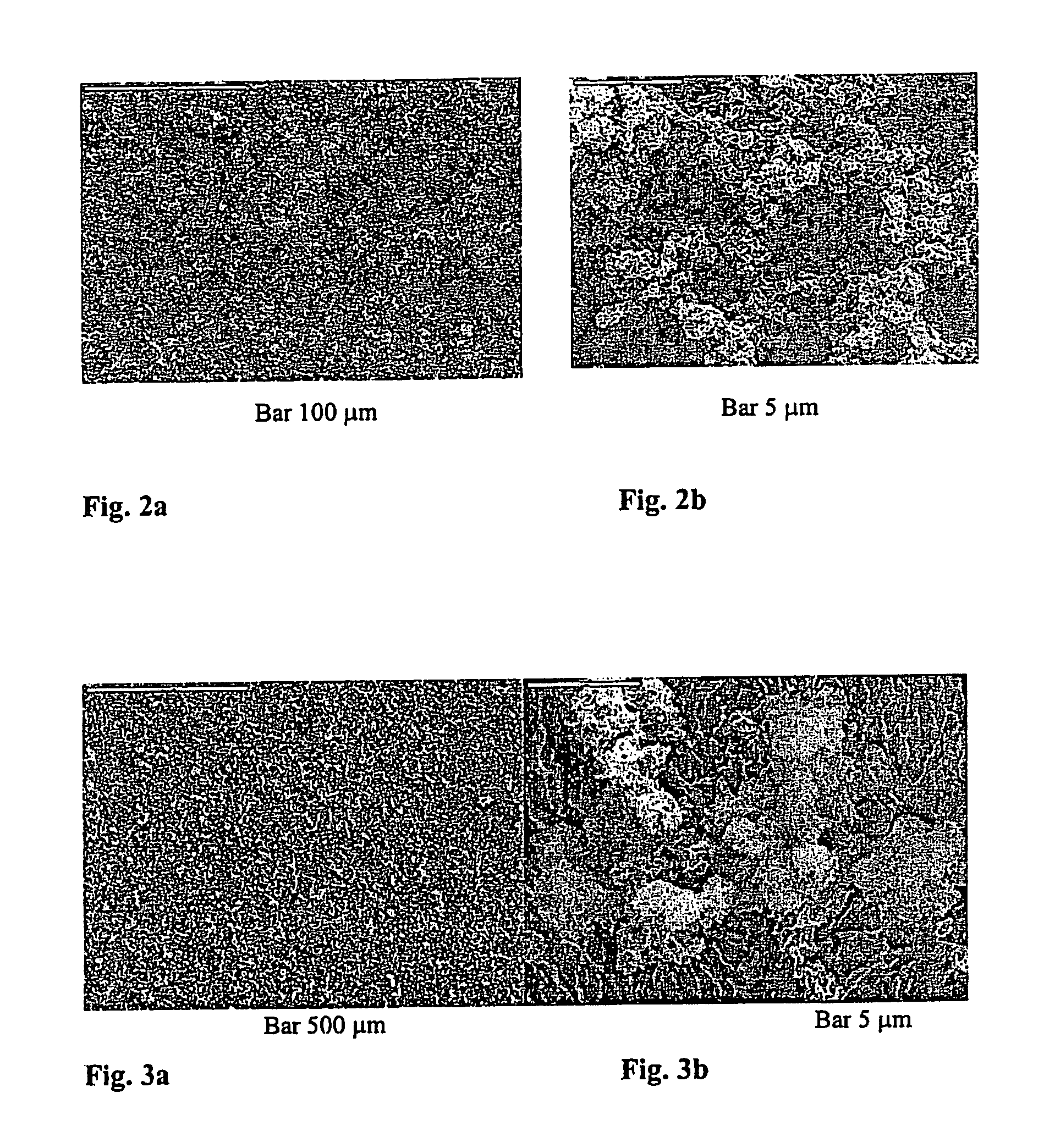
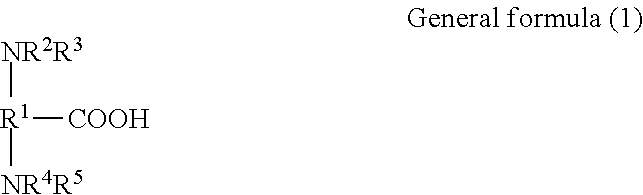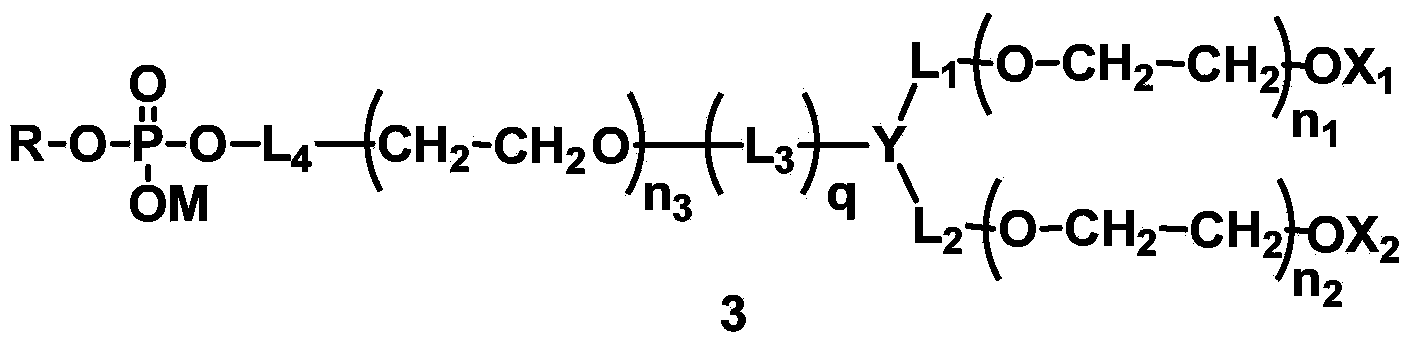Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
692 results about "Polyamino acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polyamino Acids. Polyamino acids (PAA) have properties that mimic proteins, making them ideal for both drug delivery and the delivery of nucleic acids both in vitro and in vivo. These properties include increasing solubility and stability of drug attachments, drug encapsulation, drug targeting, bypassing multidrug resistance (MDR) factors,...
Composite gel microparticles as active principle carriers
The invention relates to vectors for delivering medicinal, nutritional, plant-protection or cosmetic active principles, these delivery particles being of small, controllable and adjustable particle size, which protect the active principle, and being biocompatible, biodegradable, non-immunogenic, stable and free of solvent. The particles do not denature the active principle and allow the active principle to be released. The microparticles of the invention are of a cohesive structure made of a physicochemically stable and integral composite gel which includes an oil such as coconut oil, an aqueous phase and a linear, non-crosslinked copolyamino acid of Leu / Glu type (random or diblock). The microparticles have a controllable and adjustable size of between 0.05 and 500 mum.
Owner:FLAMEL TECHNOLOGIES
Nucleic acid formulations for gene delivery and methods of use
InactiveUS7173116B2Improve topical bioavailabilityHigh expressionPowder deliveryPeptide/protein ingredientsPentagalacturonic acidPolyamino acid
A nucleic acid formulation for use in gene delivery comprising a nucleic acid and an anionic polymer is disclosed. Examples of the anionic polymer includes aniionic amino acid polymer or poly-amino acid (such as poly-L-glutamic acid, poly-D-glutamic acid, poly-L-aspartic acid, poly-D-aspartic acid), poly-acrylic acid, polynucleotides, poly galacturonic acid, and poly vinyl sulfate.
Owner:INOVIO PHARMA
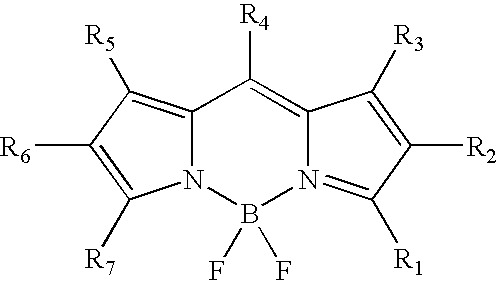
Labeling of immobilized proteins using dipyrrometheneboron difluoride dyes
InactiveUS6972326B2Hybrid immunoglobulinsChemiluminescene/bioluminescenceFluorescenceImmobilized protein
The invention describes methods for labeling or detecting of immobilized poly(amino acids), including peptides, polypeptides and proteins, on membranes and other solid supports, using fluorescent dipyrrometheneboron difluoride dyes. Such immobilized poly(amino acids) are labeled or detected on blots or on arrays of poly(amino acids), or are attached to immobilized aptamers.
Owner:MOLECULAR PROBES
Polyethylene glycol/polycation block copolymers
ActiveUS20070059271A1High gene transfer effectivityPowder deliveryOther foreign material introduction processesSide chainPolyethylene glycol
The invention provides block copolymers formed of poly(ethylene glycol) segments and poly(amino acid derivative) segments having side chains of at least one kind of specific amine residue. The invention also provides polyion complexes of such copolymers with polynucleotides and the like. These block copolymers are useful as carriers for in vivo delivery of active substances such as DNA.
Owner:THE UNIV OF TOKYO
Modified-release microparticles based on amphiphilic copolymer and on active principles(s) and pharmaceutical formulations comprising them
The present invention relates to novel microparticles formed of amphiphilic polyamino acids which transport active principle(s), AP(s), in particular protein and peptide active principle(s), and to novel modified-release pharmaceutical formulations comprising said AP microparticles. The aim of the invention is to develop novel microparticles, charged with AP, obtained by aggregation of nanoparticles of amphiphilic polyamino acids and having improved properties, in particular in the dry solid form, with regard to their ability to be dispersed and, concerning the reconstituted suspension, its stability and its ability to be easily handled and injected. The invention relates firstly to microparticles of amphiphilic polyamino acid (PO) comprising at least one AP (associated noncovalently) which spontaneously form a colloidal suspension of nanoparticles in water, at pH 7.0, under isotonic conditions; which microparticles a. are obtained by atomization of a solution or colloidal suspension of PO comprising at least one AP, b. have a size of between 0.5 and 100 microns, c. and are dispersible in colloidal suspension. The invention also relates to the process for the preparation of these microparticles, to a liquid formulation comprising a suspension of these PO / AP microparticles, to a reconstitution process and kit for this formulation and to a dry form of this formulation.
Owner:FLAMEL TECHNOLOGIES
Immobilized and activity-stabilized complexes of LHRH antagonists and processes for their preparation
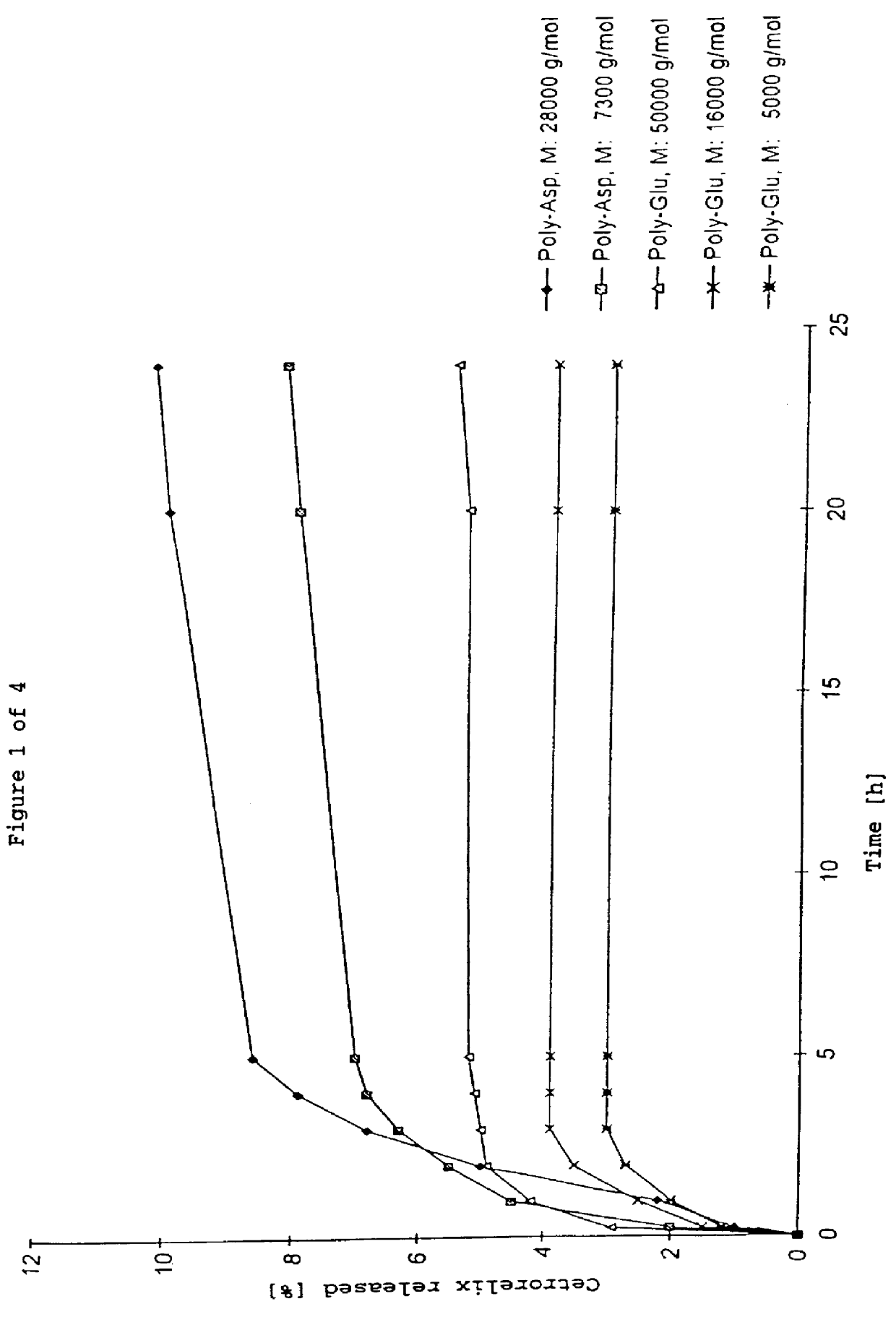
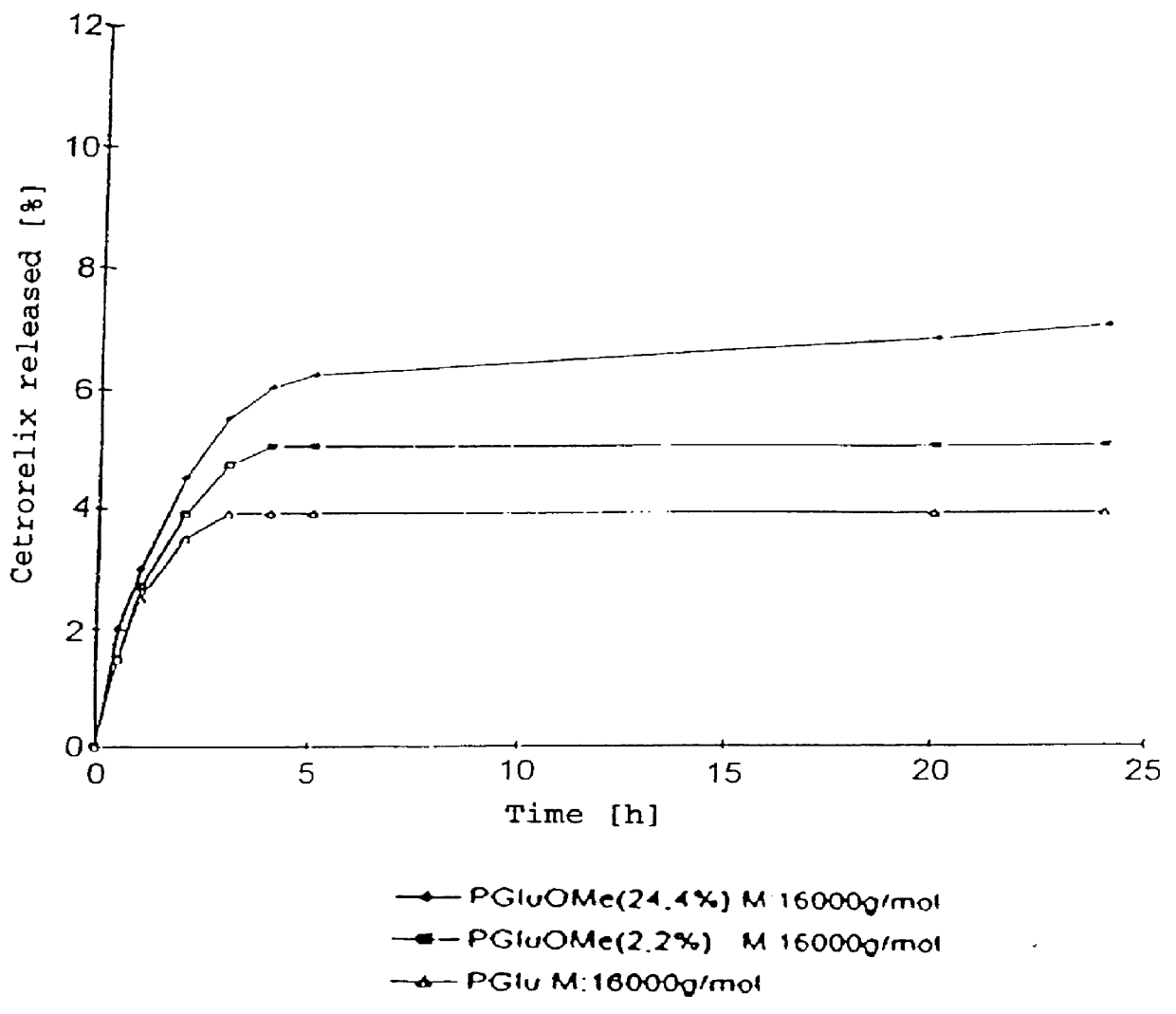
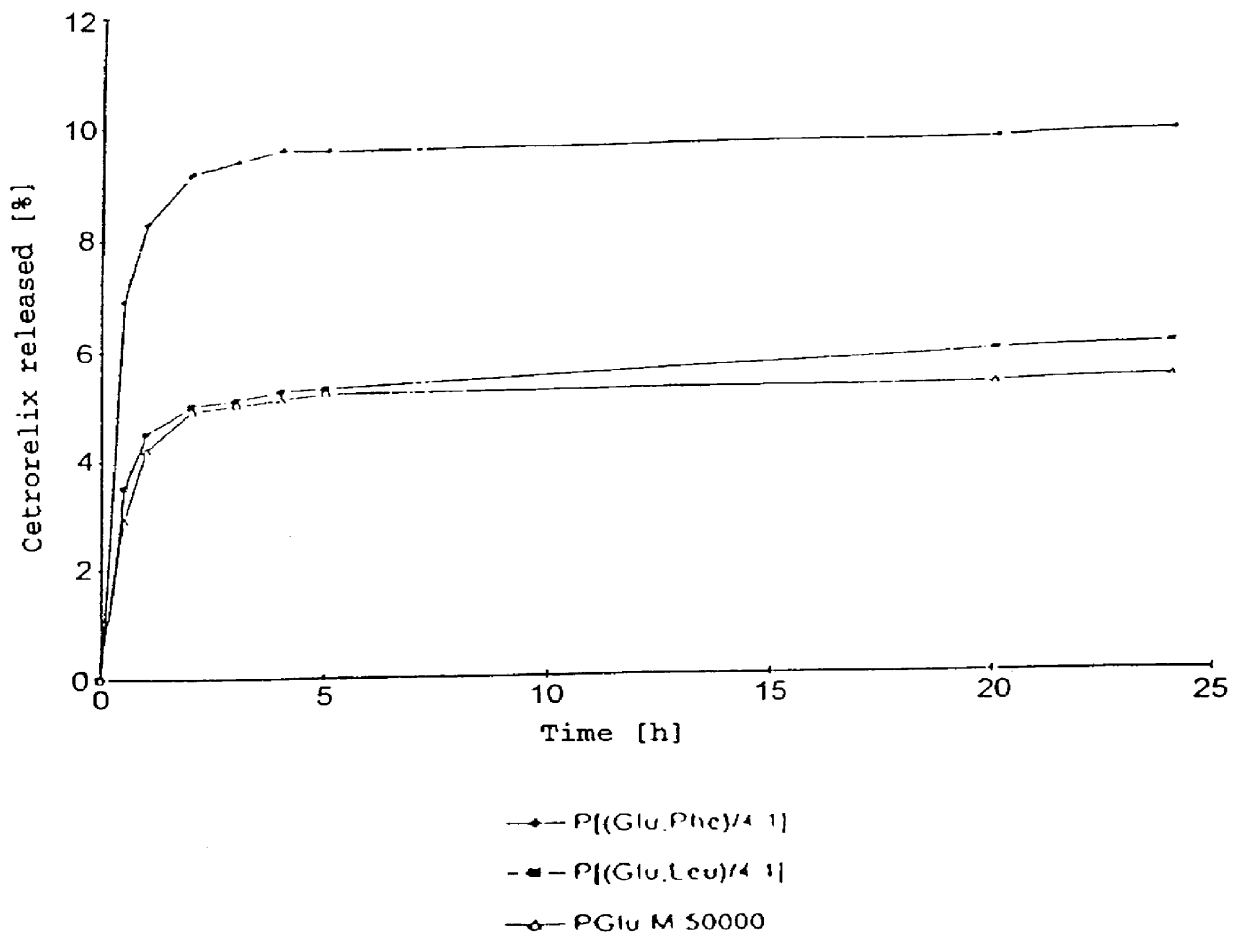
In this invention, a release-delaying system is to be developed for LHRH antagonists, in particular for cetrorelix, which allows the active compound to be released in a controlled manner over several weeks by complexation with suitable biophilic carriers. The acidic polyamino acids polyglutamic acid and polyaspartic acid were selected for complexation with cetrorelix. The cetrorelix polyamino acid complexes are prepared from aqueous solutions by combination of the solutions and precipitation of the complexes, which are subsequently centrifuged off and dried over P2O5 in vacuo. If complexes having a defined composition are to be obtained, lyophilization proves to be a suitable method. The cetrorelix-carboxylic acid complexes were also prepared from the aqueous solutions. In the random liberation system, the acidic polyamino acids poly-Glu and poly-Asp showed good release-delaying properties as a function of the hydrophobicity and the molecular mass of the polyamino acid. In animal experiments, it was possible to confirm the activity of the cetrorelix-polyamino acid complexes as a depot system in principle. It is thus possible by complexation of cetrorelix with polyamino acids to achieve testosterone suppression in male rats over 600 hours. The release of active compound here can be controlled by the nature and the molecular mass of the polymers.
Owner:ZENTARIS GMBH
Detergent mixture
InactiveUS20040048766A1Good cleaning and foaming propertyProcess economyCationic surface-active compoundsOrganic detergent compounding agentsActive agentSugar amine
A process for making a surfactant composition involving: (a) providing a starting mixture containing: (i) an aqueous alkali solution; (ii) at least one amino acid and / or a salt thereof; (iii) a fatty acid chloride; (iv) an acylatable surfactant precursor selected from the group consisting of a protein hydrolyzate, a polyamino acid, an aminosulfonic acid, an amino sugar, a nonionic surfactant, and mixtures thereof; and (v) up to about 15% by weight, based on the weight of the starting mixture, of a polyol component; (b) providing a stirring mechanism; and (c) reacting (ii) and (iii), with stirring, to form the surfactant composition.
Owner:COGNIS DEUT GMBH & CO KG
Transfer ink jet recording method
InactiveUS20120127250A1Satisfactory transferabilitySatisfactory wet scratch resistance of a final imageInksInk transfer from master sheetPolymerizationPolyamino acid
Provided is a transfer ink jet recording method, which provides satisfactory transferability and satisfactory wet scratch resistance of a final image even at high printing duty. The transfer ink jet recording method includes using an aggregation liquid, in which the aggregation liquid includes a polymer compound including one of a polyamino acid and a polyamino acid salt each having an amino group, each of which is obtained by polymerization of an amino acid compound represented by the general formula (1).
Owner:CANON KK
Oral formulation for delivery of poorly absorbed drugs
InactiveUS20060088592A1Promote absorptionReduce inactivationHeavy metal active ingredientsMultiple-port networksSucrosePolyvinyl alcohol
A composition for oral delivery of a poorly absorbed drug is disclosed. The composition includes the drug, an enhancer for increasing absorption of the drug through the intestinal mucosa, a promoter, which alone does not increase absorption of the drug through the intestinal mucosa, but which further increases the absorption of the drug in the presence of the enhancer, and optionally a protector for protecting the drug from physical or chemical decomposition or inactivation in the gastrointestinal tract. Illustrative enhancers include sucrose fatty acid esters, and illustrative promoters include aminosugars and amino acid derivatives, such as poly(amino acids). Illustrative protectors include methylcellulose, poly(vinyl alcohol), and poly(vinyl pyrrolidone).
Owner:PROCARRIER
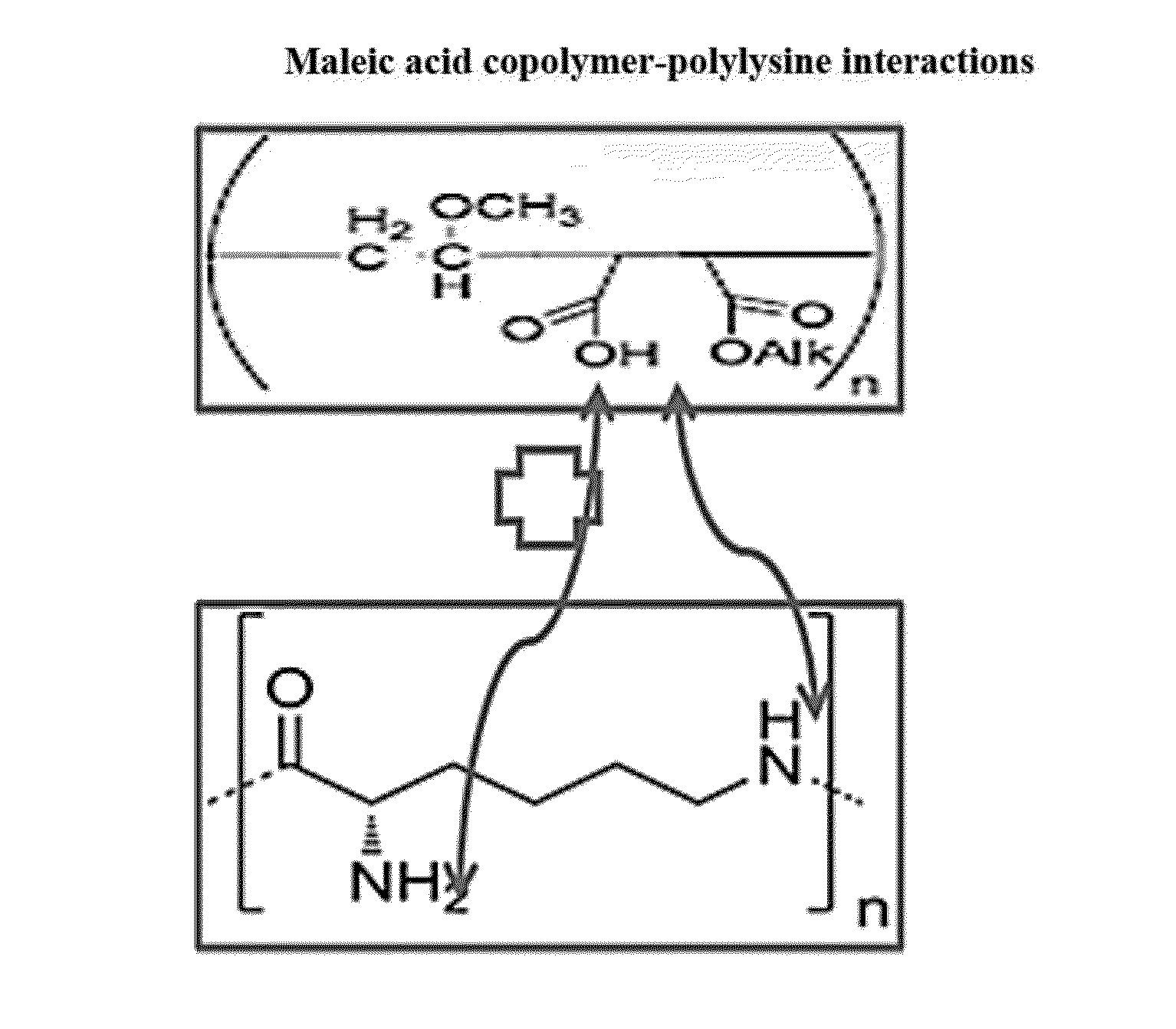
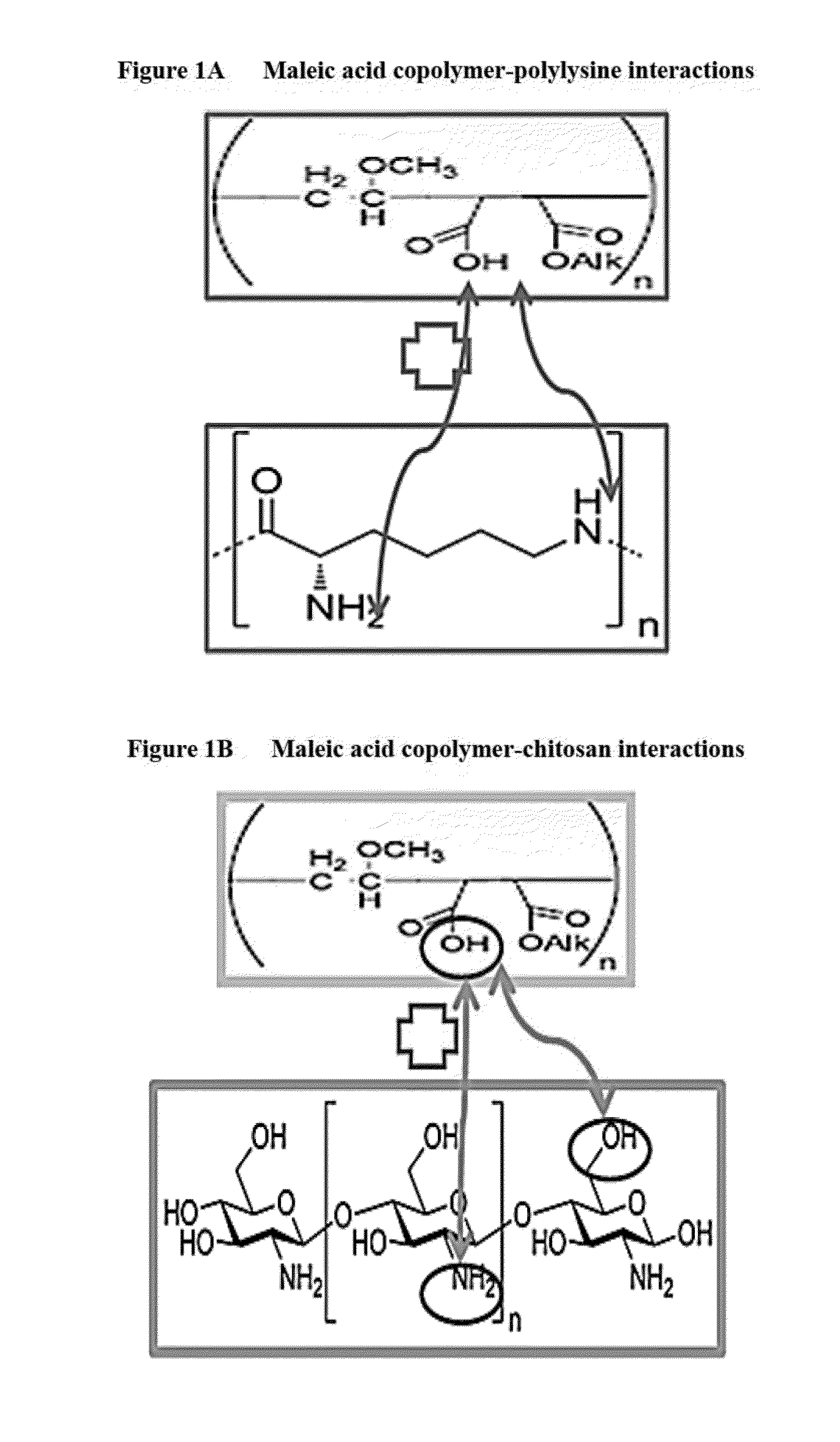
Hair cosmetic and styling compositions based on maleic acid copolymers and polyamines
Disclosed are cosmetic compositions which include hair cosmetic or styling compositions, comprising: a maleic acid copolymer; a polyamine selected from polyamino acids and aminated polysaccharides; a neutralizer; and water, wherein the polyamine is present in an amount less than 0.1% by weight based on the total weight of the composition, and methods of making and using the compositions to impart or maintain hair style.
Owner:LOREAL SA
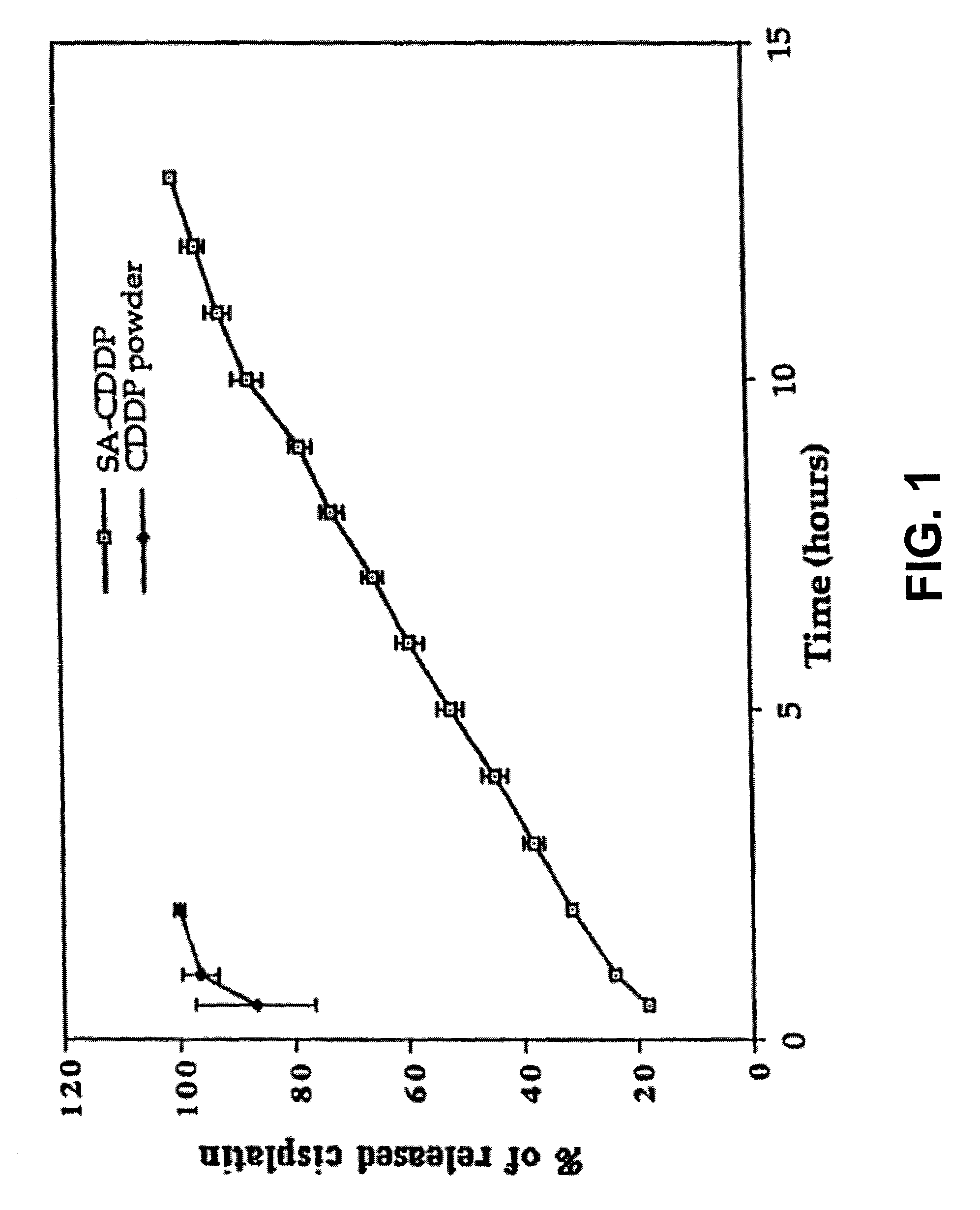
Local regional chemotherapy and radiotherapy using in situ hydrogel
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Nucleic Acid Formulations for Gene Delivery and Methods of Use
InactiveUS20070213287A1Improve topical bioavailabilityHigh expressionOrganic active ingredientsPeptide/protein ingredientsGene deliveryD-glutamic acid
A nucleic acid formulation for use in gene delivery comprising a nucleic acid and an anionic polymer is disclosed. Examples of the anionic polymer includes anionic amino acid polymer or poly-amino acid (such as poly-L-glutamic acid, poly-D-glutamic acid, poly-L-aspartic acid, poly-D-aspartic acid), poly-acrylic acid, polynucleotides, poly galacturonic acid, and poly vinyl sulfate.
Owner:INOVIO PHARMA
3D printing modified polyamino acid material and preparation method thereof
Owner:INST OF CHEM CHINESE ACAD OF SCI
Preparation method of polyamino acid and polyamino acid nano-hydrogel
ActiveCN102167817ASimple stepsGood biocompatibilityPharmaceutical non-active ingredientsCross-linkControlled release
The invention provides a preparation method of polyamino acid, which comprises the following steps: dissolving a terminal-aminated hydrophilic polymer, L-cystine-N-endo-carboxylic acid anhydride and amino acid-N-endo-carboxylic acid anhydride in an organic solvent, stirring and reacting, thus obtaining the polyamino acid. The invention further provides polyamino acid nano-hydrogel which comprises the polyamino acid prepared by the method adopting the technical scheme, and water. The polyamino acid which can form the nano-hydrogel can be prepared by only one step, and the step is simple, convenient and fast. The obtained polyamino acid comprises a hydrophilic polymer and a cross-linked polyamino acid, the nano-hydrogel can be formed by dissolving the polyamino acid in the water, and the nano-hydrogel can be used as carrier materials for medicament transmission, and medicament control and release. The terminal-aminated hydrophilic polymer, the L-cystine-N-endo-carboxylic acid anhydrideand the amino acid-N-endo-carboxylic acid anhydride are used as raw materials, and the nano-hydrogel formed by the obtained polyamino acid has good biocompatibility and biodegradability.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Novel solid preparation containing block copolymer and anthracycline anticancer agent and process for producing the same
There has been required a clinically applicable solid preparation for injection which contains a block copolymer composed of a hydrophilic polymer structure moiety and a hydrophobic polyamino acid structure moiety and an anthracycline anticancer agent. It is intended to provide a clinically applicable solid preparation for injection which contains a block copolymer composed of a hydrophilic polymer structure moiety and a hydrophobic polyamino acid structure moiety, an anthracycline anticancer agent, a saccharide and a base.
Owner:NIPPON KAYAKU CO LTD +2
Biological compatibility surface coating of implantation type medical instruments and coating method thereof
The invention discloses a biological compatibility surface coater and self-assembling coating method of planted medical apparatus, which is characterized by the following: the coater is prepared by self-assembling coating method layer by layer, which accelerates esoderma and resists thrombus constituted by two or more macromolecular materials with certain positive and negative load; the positive load material is chitose, chitin, polylysine and so on; the negative load material is disebrin, hyaluronic acid, alginic acid, polyamino acid and so on; the crosslinker and biological active factor or drug can be added to accelerate the growth of the cell; the coater satisfies the request of biologically medical transplantation.
Owner:FUDAN UNIV +1
Fruit and vegetable fresh-keeping agent as well as application thereof
ActiveCN103548994AReduce breathing intensityReduced ethylene emissionsFruit and vegetables preservationLoss rateChitin formation
The invention relates to a fruit and vegetable fresh-keeping agent as well as application thereof, belongs to the field of food storage, and aims at providing a fruit and vegetable fresh-keeping agent. Active ingredients of the fruit and vegetable fresh-keeping agent comprise the following components in parts by weight: 1-4 parts of chitin as well as derivative thereof and 0.1-1 part of polyamino acid, wherein chitin as well as derivative thereof and polyamino acid are both hydrosoluble. The fresh-keeping agent provided by the invention can prolong the storage period of fruits and vegetables, can reduce the rotting rate and the loss rate, can improve the quality of fruits and vegetables, and increase the economic benefit, meanwhile has the advantages of low cost, simple formula, convenience in operation and the like, additionally is safe and reliable in component, and is harmless to human bodies.
Owner:CHENGDU NEWSUN CROPSCI
Non-phosphorus reverse osmosis membrane scale inhibitor and preparation method thereof
ActiveCN101948189AAvoid depositionImprove compatibilityScale removal and water softeningReverse osmosisRoom temperature
The invention discloses a non-phosphorus reverse osmosis membrane scale inhibitor and a preparation method thereof. The non-phosphorus reverse osmosis membrane scale inhibitor comprises the following components: 5-20% of carboxylic acid polymer, 6-36% of sulfonic acid copolymer, 5-15% of polyamino acid, 0.1-20% of Dimethyldioctadecylammonium bromide and the balance deionized water. The preparation method is as follows: adding the above components in a container at room temperature, and stirring to form a uniform and transparent solution. The non-phosphorus reverse osmosis membrane scale inhibitor provided by the invention is suitable for pretreatment of surface water, can efficiently prevent inorganic scales and colloid scales from depositing on a reverse osmosis membrane, ensures the pH value to be 2-7 in the use process, has no toxicity and good compatibility with a common flocculant, can not cause any damage on the membrane compared with acid.
Owner:北京蓝星清洗有限公司
Topical cosmetic formulations for regulating and improving the moisture content of the skin
InactiveUS20060275238A1Improve skinImproved mucosa compatibilityCosmetic preparationsToilet preparationsTopical treatmentBULK ACTIVE INGREDIENT
Cosmetic and / or dermatological formulations for the topical treatment of the skin for regulating and improving the moisture content of the skin, comprising as active ingredient combination a) at least one anionic polyamino acid matrix, b) at least one polysaccharide, and optionally c) one or more osmoprotectants for topical application are provided.
Owner:EVONIK GOLDSCHMIDT GMBH
Method for improving the cleaning action of a detergent or cleaning agent
InactiveUS20110201536A1Easy to cleanOrganic detergent compounding agentsAnionic surface-active compoundsBiotechnologyMetabolite
Owner:HENKEL KGAA
Organic-inorganic nanocomposite coatings for implant materials and methods of preparation thereof
The present invention provides inorganic-organic nanocomposite coatings for implant materials and methods for the production thereof. The coatings consist of a sequentially adsorbed polyelectrolyte film (SAPF) intergrown with calcium phosphate crystals. The substrate is selected from glass, polymer, metal or metal alloys. The SAPFs consist of successions of positively and negatively charged monolayers, comprising biocompatible polyelectrolytes, preferably polyaminoacids. The calcium phosphate crystals may comprise octacalcium phosphate, calcium deficient apatites, carbonate apatites, hydroxyapatite, or mixtures thereof, with particle sizes 50 nm to 2 μm. The inorganic phase is grown “in situ” within the polyelectrolyte organic matrix.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Methods of increasing lean tissue mass using OB protein compositions
InactiveUS7208577B2Quality improvementLower Level RequirementsObesity gene productsPeptide/protein ingredientsTreatment useOb Protein
The present invention provides methods of creating and using OB protein compositions with an antibody constant region or portion thereof fused to an OB protein. The fusion protein is created by attaching the polyamino acids to the OB protein moiety. The fusion proteins can then be used for various therapeutic uses.
Owner:AMGEN INC
Spherical amino acid adsorbent and its preparation method
ActiveCN1476908ALow cost of treatmentImprove adsorption capacityHaemofiltrationSurgeryDiseaseSide effect
The present invention relates to a ring amino acid adsorbing agent and its preparation method. It uses natural macromolecule as carrier, its grain size is 0.45-0.9 mm, it is formed from resin which has amino acid ligand with fixed effective dose after activation of epoxy chloropropane, in which amino acid content is 12.5-39.7 micro mol / ml resin, and the described ligand is: amino acid, polyamino acid, polypeptide or protein. It can be directly used for blood perfusion method to cure the diseases of rheumatoid arthritis, systemic lupus erythematosus, myasthenia gravis, endotoxemia and infective shock, etc. The animal tests show that adsorbing agent has no toxic side effect and heat source. It is a good medical blood-cleaning adsorption material.
Owner:JAFRON BIOMEDICAL
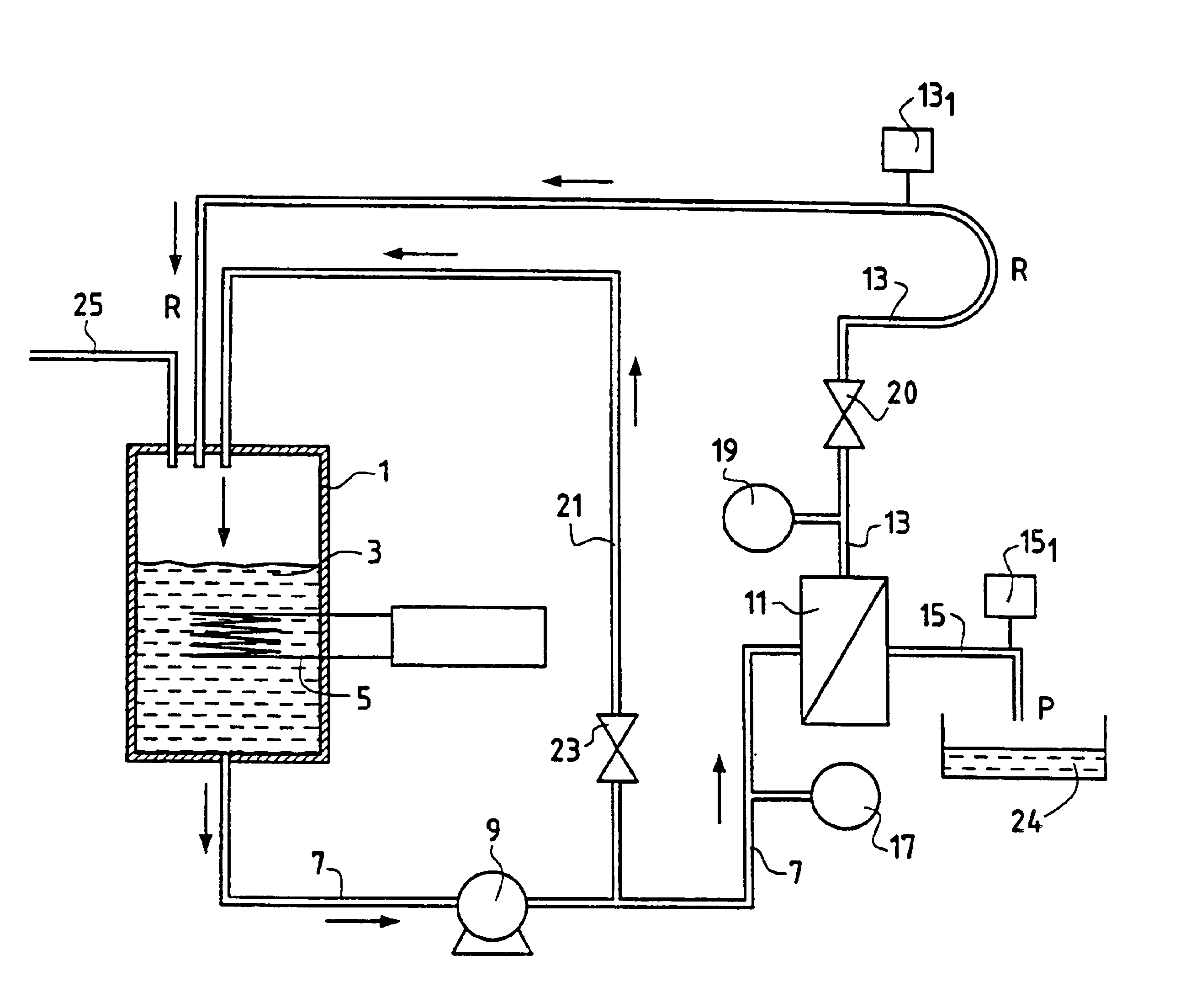
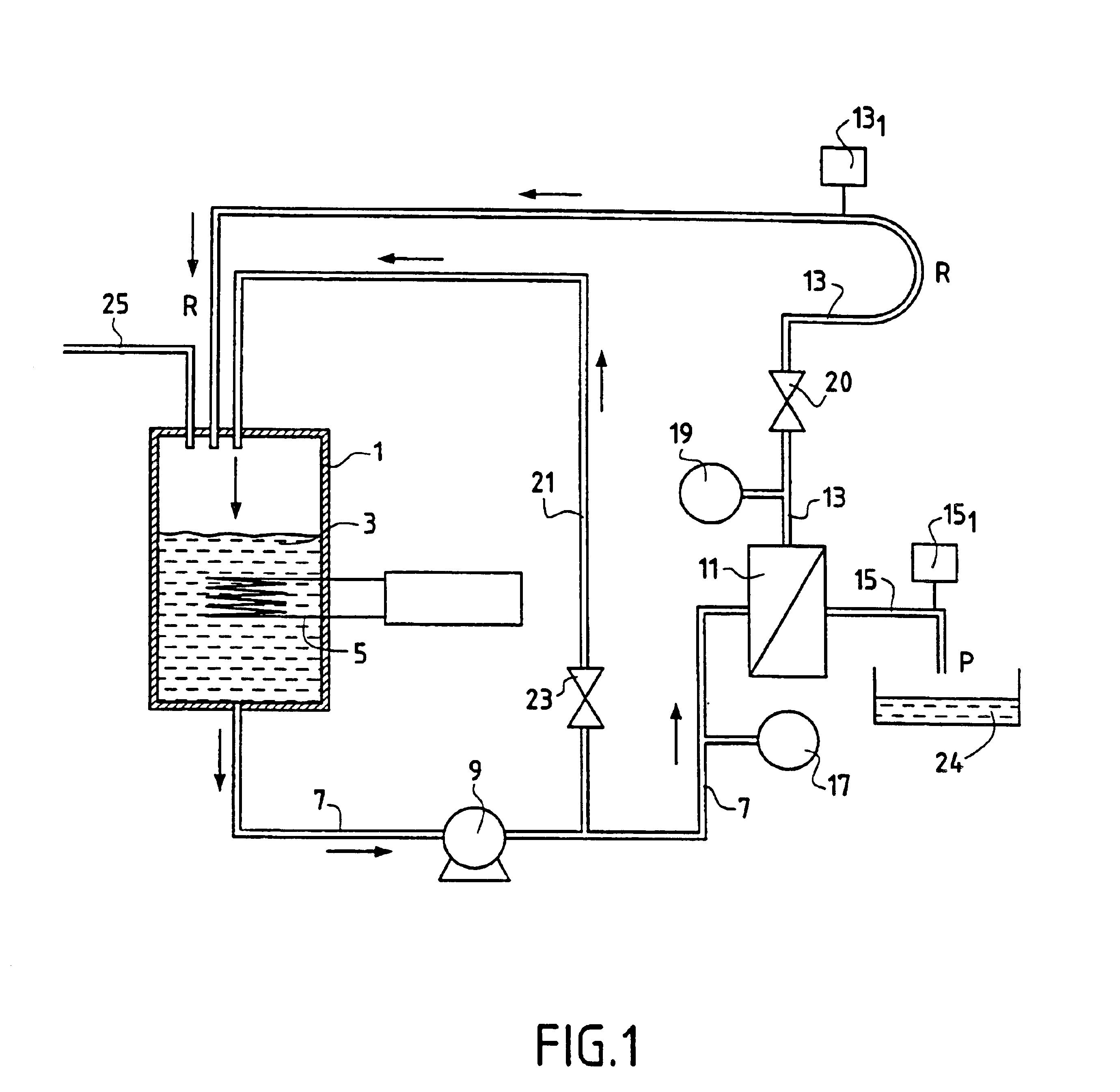
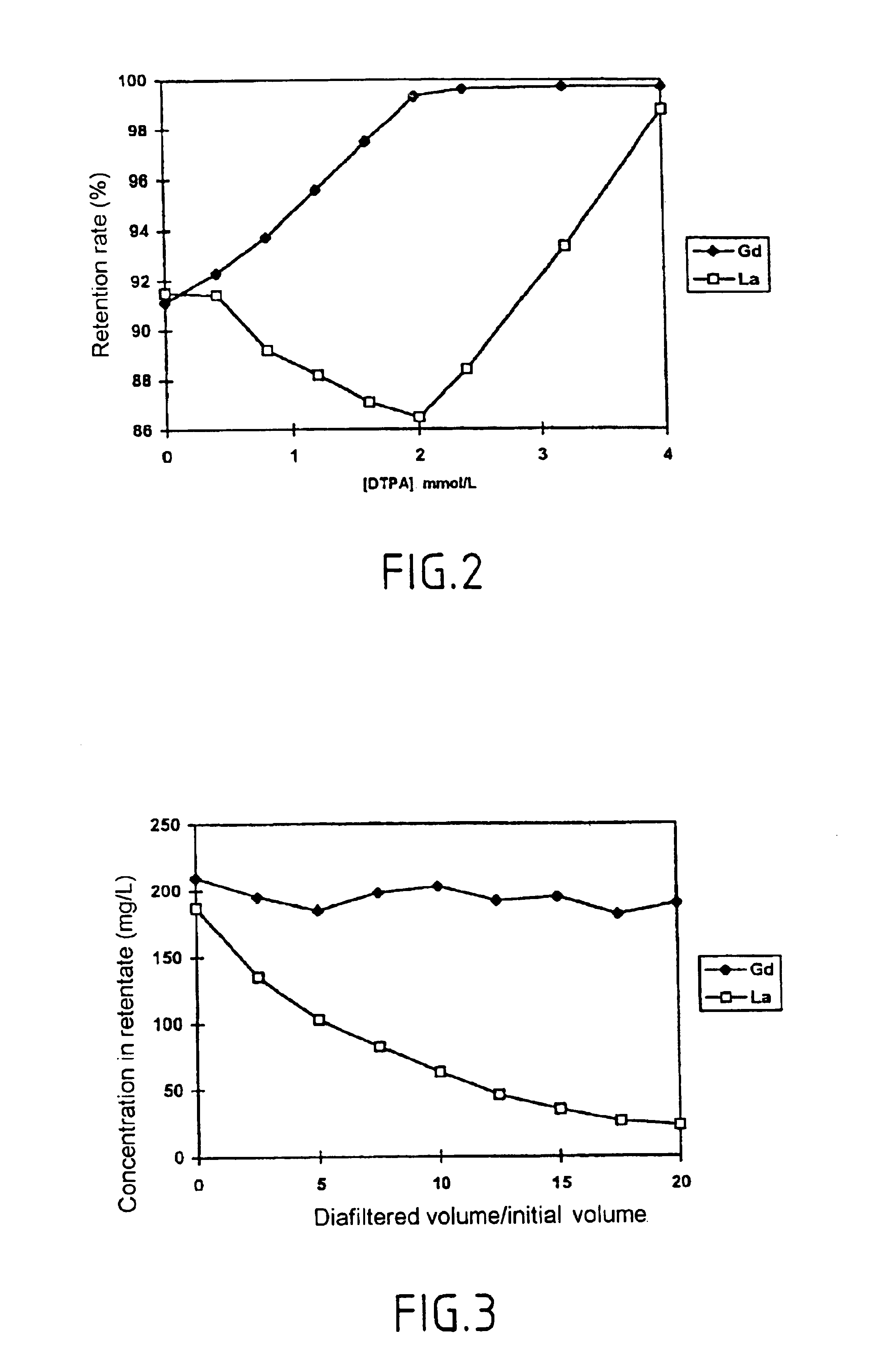
Method for separating in an aqueous medium lanthanides and/or actinides by combined complexing-nanofiltration, and novel complexing agents therefor
InactiveUS6843917B1Easy to implementEasily degradableMembranesOrganic chemistryWaste processingLanthanide
The invention relates to the separation of lanthanides and actinides by nanofiltration complexation. The object of the invention is to satisfy the existing need for a simple, efficient and economical technique for separating lanthanides and actinides. This object is achieved by a process consisting of using ligands of the polyamino acid type, such as EDTA or DTPA, for complexing lanthanides and / or actinides before separating them by nanofiltration. The invention further relates to novel polyamino acid ligands incorporating ligand structures additional to EDTA and DTPA. Application to the production of rare earths or nuclear waste processing, especially to recycling operations carried out on spent nuclear fuels is also discussed.
Owner:UNIV CLAUDE BERNARD LYON 1 +1
Dispersion of polyamino acids in a continuous lipid phase
InactiveUS20080152675A1Extend posting timeImprove protein stabilityBiocidePharmaceutical non-active ingredientsLipid formationWater in oil emulsion
The invention relates to injectable pharmaceutical compositions for the prolonged release of at least one active principle, comprising at least one active principle in an aqueous phase of amphiphilic polymer, said aqueous phase being in the form of a dispersion in a continuous lipid phase. The composition is in the form of a water-in-oil emulsion comprising:a pharmaceutically acceptable, continuous lipid phase,an aqueous disperse phase containing at least one amphiphilic polymer and at least one active principle not covalently bonded to said amphiphilic polymer, andat least one pharmaceutically acceptable surfactant.
Owner:FLAMEL TECHNOLOGIES
Mildewproof waterproof coating composition
ActiveCN101298533AStrong scrub resistanceHigh tensile strengthAntifouling/underwater paintsRosin coatingsPolymer scienceEmulsion
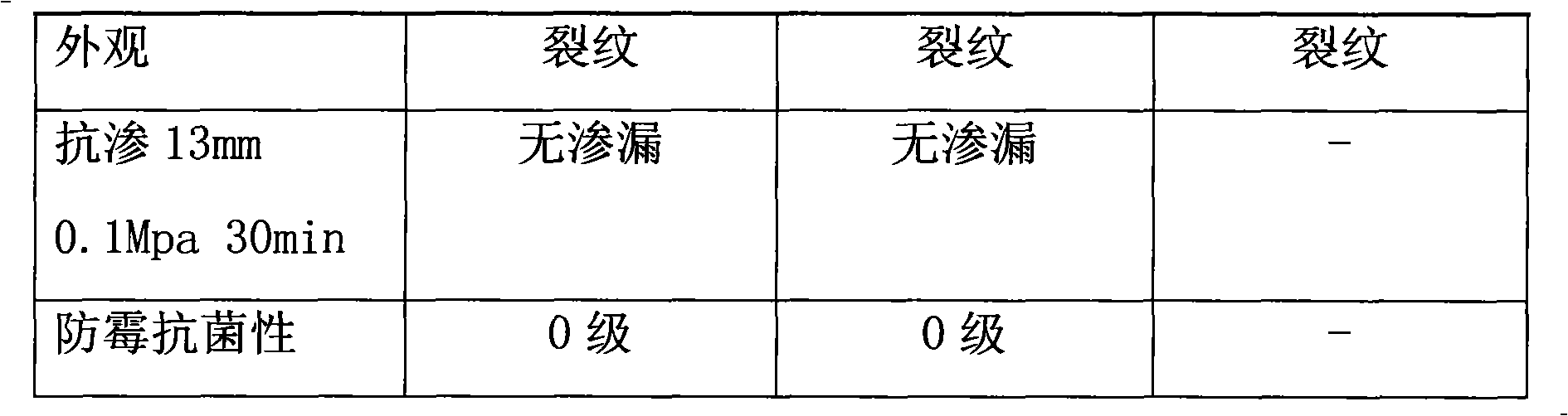
The invention relates to an anti-leakage, anti-seepage and anti-mildew acrylic ester waterproof coating compound used for buildings, which mainly consists of acrylic ester emulsion, polyamino acid emulsion, rosin resin, anti-mildew agent and filler. A coating film formed after the coating is used is characterized by high tensile strength and high elongation at break, which can make up cracks already existing and in developing on the wall and can be directly scrubbed, with strong scrubbing resistance. The coatings of the invention can be coated on moist building surfaces and can not go mouldy, thus being applicable to cement, metal, wood and paper surfaces and playing the role of waterproof, anti-seepage and anti-mildew.
Owner:NANJING CONSTR ENG GRP
Multi-effect soil conditioner and preparation method thereof
ActiveCN102925162AImprove water absorption and retention capacityStrong resistance to external physical pressureAgriculture tools and machinesOrganic fertilisersWater holdingFluoride
The invention discloses a multi-effect soil conditioner and a preparation method of the multi-effect soil conditioner. The multi-effect soil conditioner comprises the raw materials, by weight, 600-740 parts of organic solid waste, 150-210 parts of flyash and 110-190 parts of polyamino acid superabsorbent. The 600-740 parts of the organic solid waste, the 150-210 parts of the flyash and the 110-190 parts of the polyamino acid superabsorbent are mixed sufficiently and dried to granulate. The soil conditioner has various advantages of being water-holding and fertilizer-saving, and increasing plant soil nutrient and improving soil physico-chemical property and the like. The soil conditioner can be applied to various qualitative soils and play a crucial role in restoring special soils such as high level of fluoride soil and clayey soil, and also play a crucial role in wind prevention and sand fixation.
Owner:NANJING UNIV OF TECH
Corn lodging resistant production gain conditioning agent and its preparation and application
InactiveCN101011063AImprove lodging resistanceNo side effectsBiocidePlant growth regulatorsGrowth plantOrganic acid
The invention relates to a corn lodging resistant production-increase adjusting agent, comprising plant growth adjusting agent, polyamino acid, organic acid, and microelements. The inventive production comprises that using solvent to dissolve the organic acid, and dissolve the plant growth adjusting agent, microelements and polyamino acid, making volume constant. The inventive adjusting agent can be diluted to be sprayed on the leaves of corn, to optimize the shape and yield of corn, to improve the anti-lodge ability of corn and improve the production.
Owner:INST OF CROP SCI CHINESE ACAD OF AGRI SCI
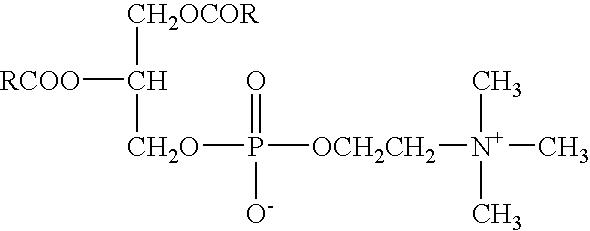
Phospholipid derivatives for branching polyethylene glycol, and lipid membrane structural body composed of same
ActiveCN103881084APromote formationLong metabolic half-lifeOrganic active ingredientsPharmaceutical non-active ingredientsHydrogen atomPolyethylene glycol
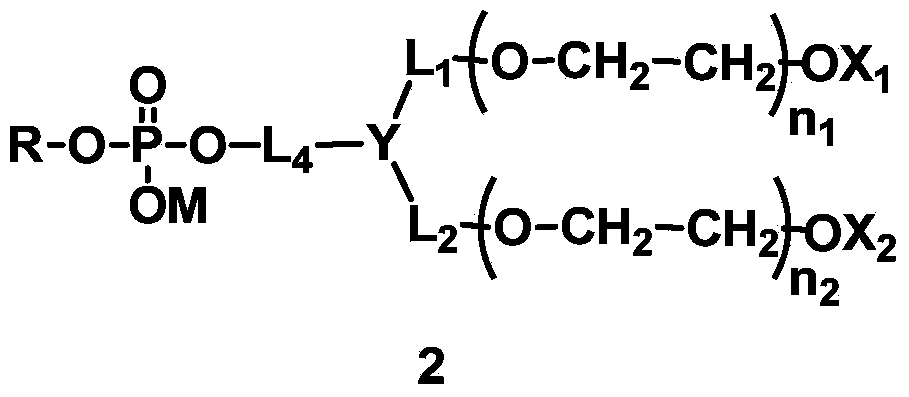
The invention discloses phospholipid derivatives for branching polyethylene glycol, and a lipid membrane structural body composed of the same. The general formula of the phospholipid derivatives is as shown in the specification, wherein X1 and X2 are hydroxyl with 1-20 carbon atoms respectively and independently; n1 and n2 are integers ranging from 1 to 1000 respectively and independently, n3 is an integer ranging from 0 to 1000, and n1+n2+n3 is not less than 2 and not greater than 2000; Y is a branching centre with 1-20 carbon atoms, and connected with L1, L2 and L3 by a covalent bond; L1, L2 and L3 are linking groups for Y and polyethylene glycol units; q is 0 or 1; when n3=0, q is 0 and Y is not the polyamino acid residue of 2-10 amino acid residues; L4 is a linking group; M is hydrogen atom or cation; R is the residue of hydrophobic lipids. The lipid membrane structural body composed of the phospholipid derivatives for branching polyethylene glycol is used for wrapping a medicine and capable of effectively prolonging the cycle time of the medicine in a body.
Owner:XIAMEN SINOPEG BIOTECH
Aliphatic polyester-polyamino acids copolymer with biological function and its synthesis method
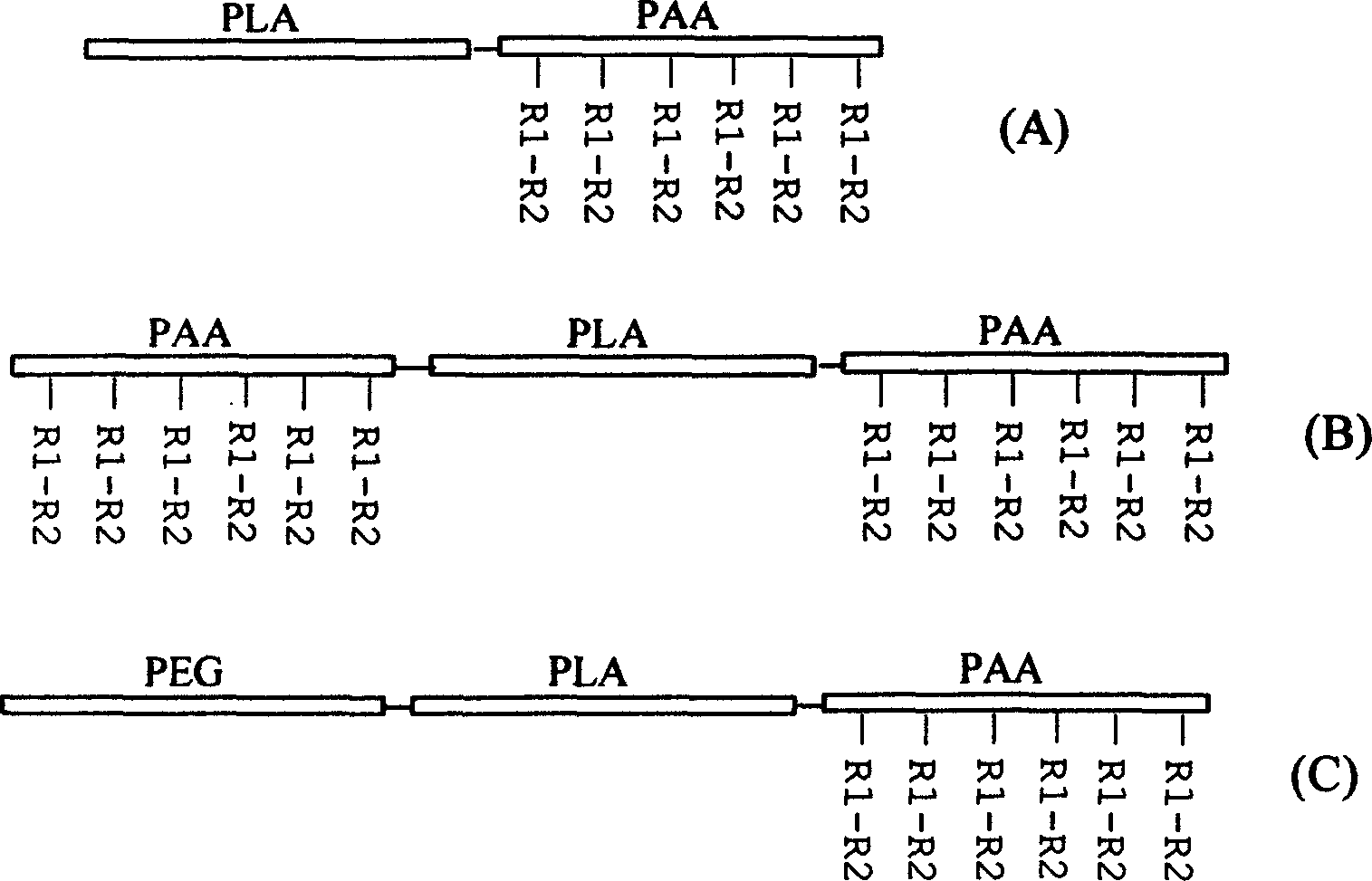
InactiveCN1800238AImprove hydrophilicityGood biocompatibilityOrganic active ingredientsPharmaceutical non-active ingredientsPolyesterSynthesis methods
The invention relates to a fatty group polyester-polyamino acid copolymer with biology function and its synthesis method in the field of macromole biological medicine material technique. The block copolymer comprises: the two block copolymer (A) of fatty group polyester -polyamino acid with biology function and three block copolymer (B), and polyoxyethylene-fatty group polyester-polyamino acid three block copolymer (C). The synthesis method comprises: synthesizing the block copolymer of the carboxylic which is protected by benzyl, doing catalytic reduction to the palladium wood coal or hydrolyzing hydrogen bromide to generate the corresponding polymer with free carboxyl, then using NHS (N- hydroxyl succinimide-sauba) to active the flank carboxyl of the polyamino acid section, reacting it with short peptide, sugar or drug with RGD to obtain the fatty group polyester-polyamino acid copolymer with biology function.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com