Agkistrodon acutus anticoagulant factor XI polypeptide and application thereof
A technology with antithrombotic activity, AVCSQEAMTGPCRAVMPRWYFDMYKKKCIRFIYGGCGGNRNNFESEEYCMAVCKKMI, which is applied in the application field of antithrombotic drugs, can solve bleeding and other problems, and achieve the effect of prolonging the time of carotid artery thrombosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
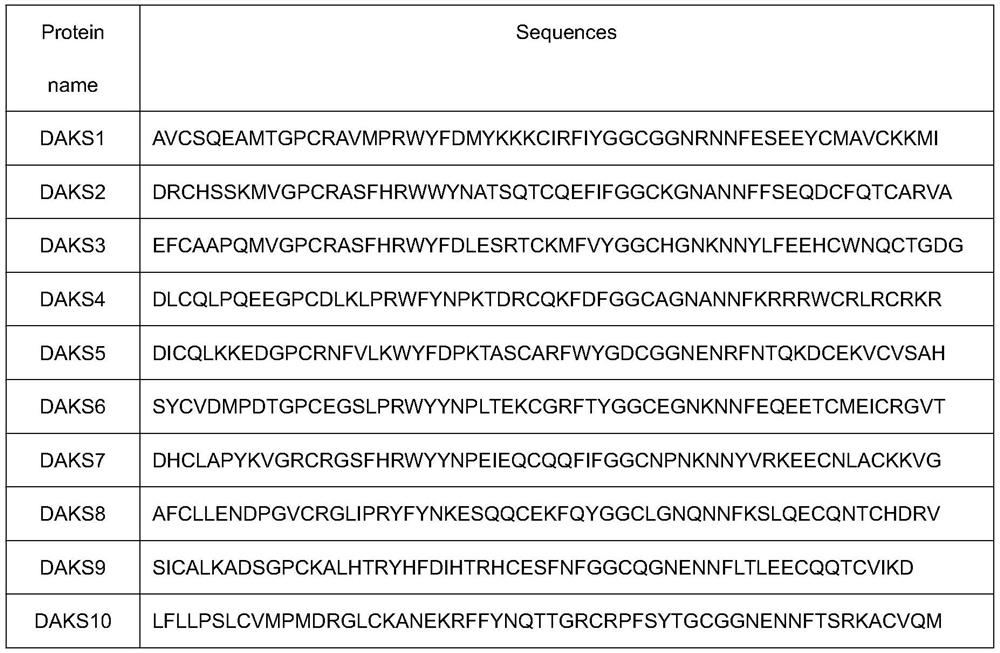
[0029] Example 1: Discovery of DAKS series polypeptides
[0030] The venom glands of the five-step snake were taken, and the mRNA was enriched with magnetic beads, fragmented, and the first and second strands of cDNA were synthesized with random primers, and purified using the QIAQuickPCR kit. The target fragment was recovered by agarose gel electrophoresis, amplified by PCR, and library construction was completed. Sequencing was performed using an Illumina Hiseq 4000. Trinity (version 2.4.0) was used for de novo analysis of transcriptome without parameters. Blast annotation and Pfam analysis, searched with "Kunitz" as the keyword to get 10 sequences, named DAKS 1-DAKS10. The sequence information is as follows:
[0031]
[0032]
Embodiment 2
[0033] Example 2: Inhibitory effect of DAKS series polypeptides on blood coagulation factor XI
[0034] Construction of recombinant plasmid pPIC9k / DAKS: DAKS sequence was cloned into pPIC9k using seamless cloning (cloning site NotI), linearized with SacI and electroporated into Pichia pastoris GS115, positive colonies were identified by PCR and sequencing, and positive colonies were picked Colony into the growth medium BMGY, 28.5°C, shake culture at 220r / min, when the OD600 of the bacterial solution is around 4-6, centrifuge at 4000r / min for 5min, transfer the bacteria into the expression medium BMMY, add a final concentration of 1 % methanol induction, centrifuged after 72h to collect the supernatant, purified to obtain the DAKS target protein. To test the inhibitory activity of DAKS series peptides on blood coagulation factor XIa, pipette 100 μL FXIa (1 nM) and 50 μL DAKS (1-10) and mix evenly in a 96-well plate, incubate at 37°C for 1 hour, add 50 μL of substrate (FXIa chro...
Embodiment 3
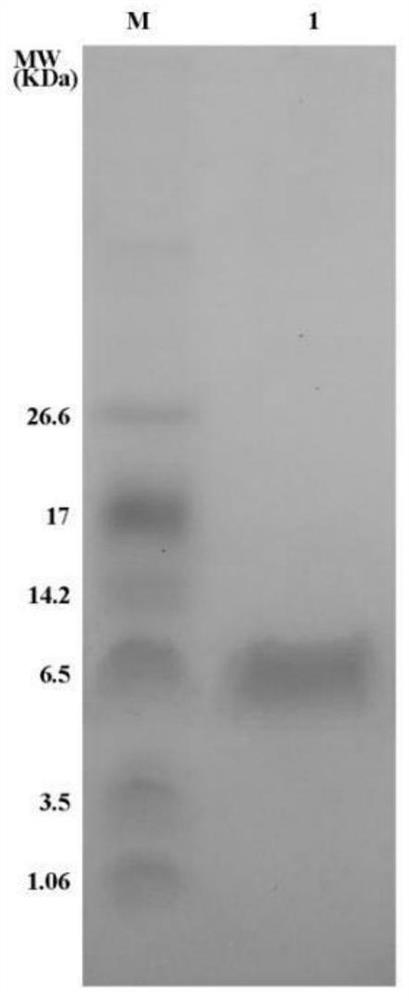
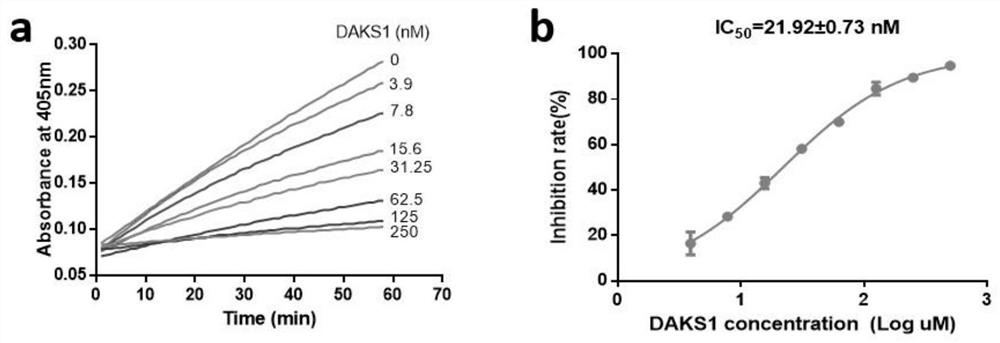
[0040] Example 3: Inhibitory effect of polypeptide DAKS1 provided by the present invention on blood coagulation factor XI
[0041] The polypeptide DAKS1 described in this example is obtained by recombinant expression in Pichia pastoris and nickel column affinity chromatography. The inhibitory effect of the recombinant polypeptide on blood coagulation factor XI was detected by a chromogenic substrate method.
[0042] Pipette 100 μL of FXIa (1 nM) and 50 μL of DAKS1 (0-500 nM) into a 96-well plate and mix evenly, incubate at 37°C for 1 hour, add 50 μL of substrate (FXIa chromogenic substrate is S2366, the final concentration is 0.25 mM, and continue at 405 nm wavelength Detect for 1 hour, scan once every minute. Negative control: 50 μL TBS-BSA buffer + 100 μL HFXIa + 50 μL substrate, blank control: 50 μL sample + 100 μL TBS-BSA buffer + 50 μL substrate (all wells are set as duplicate wells). Draw the absorbance value -Reaction time curve, the slope of the curve is the speed V o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com