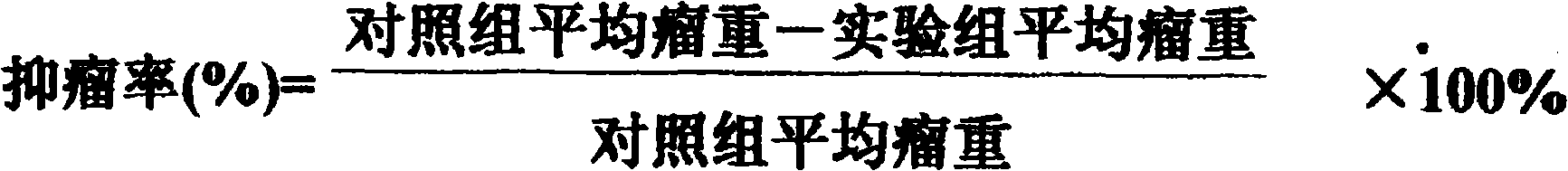
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
201 results about "Agkistrodon acutus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Plaster mainly for curing cervical vertebra and lumbar vertebra disease
InactiveCN101085304ANo allergic reactionHeavy metal active ingredientsAnthropod material medical ingredientsDiseaseSalvia miltiorrhiza
The invention discloses a medicinal plaster for treating neck and lumbar spine diseases which comprises the following raw materials (by weight portion): Chinese angelica root 45-55, Ligusticum wallichii 45-55, notopterygium root 45-55, giant knotweed rhizome 45-55, pubescent angelica root 45-55, radix salvia miltiorrhiza 45-55, red peony root 45-55, Clematis chinensis 45-55, large-leaf gentian root 45-55, saffron 25-35, Achyranthes bidentatae 25-35, spatholobus stem 25-35, eucommia bark 25-35, drynaria 25-35 and agkistrodon acutus 1 pieces.
Owner:付先进
Medicine for treating rheumatism and rheumatoid disease
InactiveCN101085223AGood treatment effectAnthropod material medical ingredientsAntipyreticMedicinal herbsRheumatism
The invention discloses a medicament for treating rheumatism and rheumatoid disease, which is prepared from 50 kinds of Chinese medicinal herbs including pubescent angelica root, Clematis chinensis, Sichuan aconite root, Ligusticum wallichii, achyranthes and cyathula root, zanthoxylum piperitum, rhizoma dioscoreae, prepared aconite root, notopterygium root, white-stiff silkworm, safflower, gastrodia tuber, myrrh, frankincense, drynaria, tiger bone, earthworm, agkistrodon acutus, hooked uncaria, large-leaf gentian root and cinnamon twig through steps of drying the raw materials herbs, disintegrating into 120 mesh fines, sieving, mixing proportionally, carrying out germicidal treatment, making powders, watered pills, honeyed pills or capsules.
Owner:于永
Plaster for treating rheumatism bone disease
InactiveCN101450184AQuick resultsShort course of treatmentHeavy metal active ingredientsHydroxy compound active ingredientsCentipedeMyrrh
The invention relates to a plaster for treating rheumatism which is prepared from 40 traditional Chinese medicinal materials including syngnathus, pubescent angelica, angelica, agkistrodonbungarus minimus, peach kernel, safflower, phellodendron, radix sileris, catnip, asarum, pinellia ternate, yazao, erhua, forsythia, pangolin, nux vomica, rhizoma gastrodiae, panax notoginseng, papaya, hyssop, cortex eucommiae, cassia twig, olibanum, myrrh, angelica, coptis, notopterygium, fritillaria, sophora, whole worm, centipede, dragon's blood, radix auckladiae, realgar, musk, clove, borneol, astragalus, sesame oil and guangdan. The plaster has special curative effect for treating intractable rheumatism, and has advantages of quick result, short treatment period, high cure rate and low cost.
Owner:陈振山
Incubation and cultivation methods of agkistrodon acutus
ActiveCN103004687AImprove hatching survival rateSnake grows fastAnimal husbandryAgkistrodon acutusIncubation temperature
The invention relates to incubation and cultivation methods of agkistrodon acutus. An incubation method comprises the following steps of: egg selection and incubation, wherein the incubation comprises the following technical points of: a, controlling the incubation temperature to be 26-30 DEG C; b, controlling the relative incubation humidity to be 88-96 percent; c, incubating for about 23 days; and d, checking eggs timely, and processing mucid eggs: checking the eggs through lighting once a day to see whether mildew or fertilization occurs or not, and processing timely so as to avoid affecting other eggs. A cultivation method comprises the following steps of: feeding of the eggs, overwinter cultivation of the of agkistrodon acutus, and feeding and management of the of agkistrodon acutus. The incubation and cultivation methods of the agkistrodon acutus have high snakelet incubation survival rate, and fast agkistrodon acutus growth, and are suitable for large-scale artificial breeding.
Owner:YONGZHOU CITY STRANGE SNAKE SCI & TECH IND
Method utilizing fermentation bed pool to raise agkistrodon acutus
The invention discloses a method utilizing a fermentation bed pool to raise agkistrodon acutus. The method includes the steps of placing the agkistrodon acutus into the fermentation bed pool, opening sliding doors every three to seven days from the next week, using new fermentation bed padding to cover excrement on the surface of fermentation bed padding, spraying EM bacterial liquid on the surface of the fermentation bed padding after 10-15 days when the snake is placed, and carrying out harrow-turning treatment on the fermentation bed padding after 15-30 days when the snake is placed. The fermentation bed pool is low in cost and simple to manufacture. The snake pool is simple in structure and reasonable in design. A snake house is complete in configuration, and environment is convenient to control. The mode utilizing the fermentation bed pool to raise the agkistrodon acutus facilitates health environment control, prevents harmful germs from breeding, and lowers risks of daily operation and cultivation.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Zhuifengtouguhuoluo pills(capsule) and its prepn process
InactiveCN101015650AEasy to makeRemissionAnthropod material medical ingredientsAntipyreticMedicineAgkistrodon acutus
The invention relates to a Chinese medicinal capsule for treating chronic infectious arthritis and rigid rachitis and process for preparation, wherein the raw materials include agkistrodon acutus, black-tail snake, black ants, ginseng, asaryl, scorpion, earthworm, large-leaf gentian root, seed of nuxvomica, dried rehmannia root, eucommia bark and epimeddium.
Owner:尹政德
Medicinal composition for treating dermatosis, preparation process and use thereof
InactiveCN1923244AAchieve recoveryAchieve the purpose of recoveryAmphibian material medical ingredientsPowder deliveryDiseasePeppermints
Disclosed is a pharmaceutical composition for treating skin diseases which comprises the following components (by weight ratio): ginseng 20-40 parts, astragalus root 30-80 parts, capsule of weeping forsythia 5-20 parts, ledebouriella root 10-40 parts, notopterygium root 5-30 parts, levisticum 5-50 parts, flavescent sophora root 10-50 parts, phellodendron bark 5-20 parts, broom cypress fruit 10-30 parts, cnidium fruit 10-30 parts, centipede, cassia seed 5-20 parts, hornet nest 10-30 parts, licorice root 5-30 parts, herba schizonepetae 5-20 parts, peppermint 5-20 parts, antelope's horn 10-80 parts, cocklebur fruit 10-50 parts, black-tail snake 10-80 parts, dried human placemta 10-30 parts, agkistrodon acutus 10-50 parts, coralbean bark 5-30 parts, secretio bufonis 10-30 parts, ophicalcite 10-50 parts, lead monoxide 10-50 parts, large-leaf gentian root 10-80 parts, and musk 5-20 parts.
Owner:王积善
Traditional Chinese medicine preparation for treating liver cancer and preparation method thereof
InactiveCN102078569AEnhance immune functionProlong lifeAmphibian material medical ingredientsAnthropod material medical ingredientsBarbed Skullcap HerbToad Venom
The invention provides a traditional Chinese medicine preparation for treating liver cancer, comprising the following raw medicines in parts by weight: 10-20 parts of acanthopanax, 10-20 parts of oldenlandia diffusa, 10-20 parts of radix astragali, 10-20 parts of cambogia, 10-20 parts of glossy privet fruit, 10-20 parts of white atractylodes rhizome, 10-20 parts of radix polygoni multiflori preparata, 10-20 parts of oriental wormwood, 10-20 parts of turmeric, 10-20 parts of selfheal, 10-20 parts of umbellate pore fungus, 10-20 parts of magnolia vine fruit, 10-20 parts of curcuma zedoary, 10-20 parts of tuckahoe, 10-20 parts of eclipta, 10-20 parts of root of red-rooted salvia, 10-20 parts of white peony root, 10-20 parts of barbed skullcap herb, 10-20 parts of baical skullcap root, 10-20 parts of epimedium, 1-10 parts of toad venom and 1-10 parts of scorpion. The traditional Chinese medicine preparation for treating liver cancer has the beneficial effects of prolonging the life time of inoperable patients, playing an adjuvant treating effect on patients treated mainly by operation, radiation treatment and chemo-treatment, and having certain curative effects on liver cancer-specific backgrounds of liver diseases such as hepatitis and liver cirrhosis.
Owner:李秀举
Chinese patent medicine for treating rheumatoid arthritis
InactiveCN102247497AEnsure balanceUnblock circulatory disordersAnthropod material medical ingredientsAntipyreticEphedra sinicaRheumatoid arteritis
The invention provides a Chinese patent medicine for treating rheumatoid arthritis, which is characterized in that the medicine comprises the following components by weight: 8-12 parts of ephedra sinica, 8-12 parts of Chinese mugwort leaves, 12-18 parts of zaocys dhumnades, 12-18 parts of agkistrodon acutus, 8-12 parts of frankincense, 8-12 parts of angelica dahurica, 45-55 parts of clematis roots, 8-12 parts of rhizoma homalomena, 8-12 parts of root-bark of Chinese hydrangeavine, 27-33 parts of common clubmoss herb, 27-33 parts of millettia dielsiana, 12-18 parts of notopterygium incisum, 12-18 parts of pubescent angelica, 12-18 parts of achyranthes, 9-15 parts of angelica, 9-15 parts of menispermaceae, 12-18 parts of cassia twig, 9-15 parts of pawpaw, 12-18 parts of kudzu roots, 12-18 parts of pangolin, 45-55 parts of ants, 18-22 parts of radix astragali, 12-18 parts of bighead atractylodes rhizome, 75-85 parts of fructus eucalypti robustae, 12-18 parts of cherokee roses, and 12-18 parts of licorice. The Chinese patent medicine of the present invention has the efficacy of dispelling wind and dissipating binds, freeing channels and promoting qi, activating blood and dissolving stasis, dispersing blood stasis and detumescence; the plurality of traditional Chinese medicine components can complement each other, dredge circulatory disturbance, and repair damaged joints. The invention can regulate body endocrine, and maintain internal hormone balance. The invention has significant curative effect, and has no toxic or side effect.
Owner:CHENGDU LYUDI TECH
Refined polyvalent anti-snake poison lyophilized blood serum and using method
ActiveCN101347617ATimely treatmentEffective treatmentAntinoxious agentsAntibody ingredientsAntigen Binding FragmentSnake venom
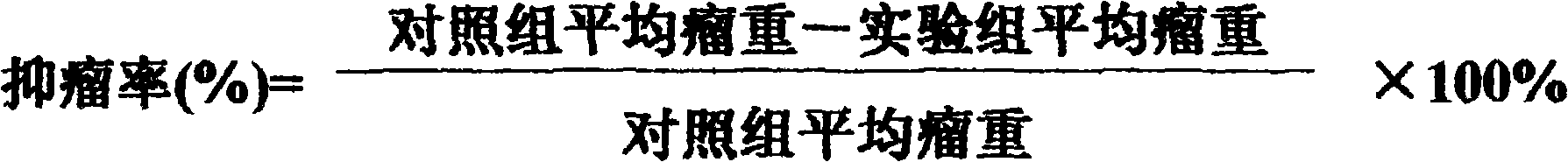
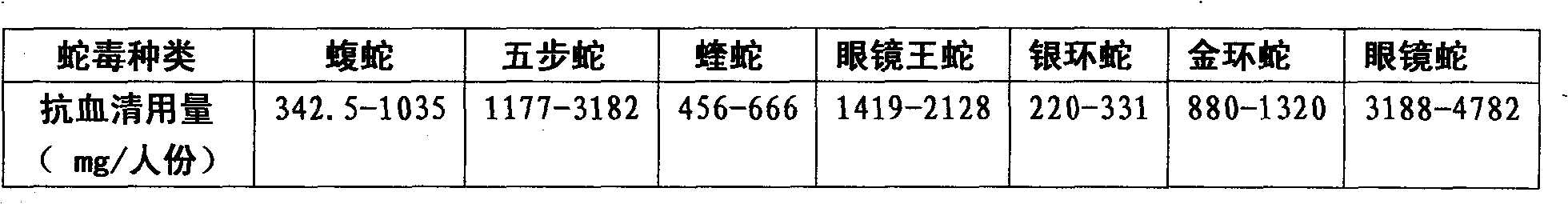
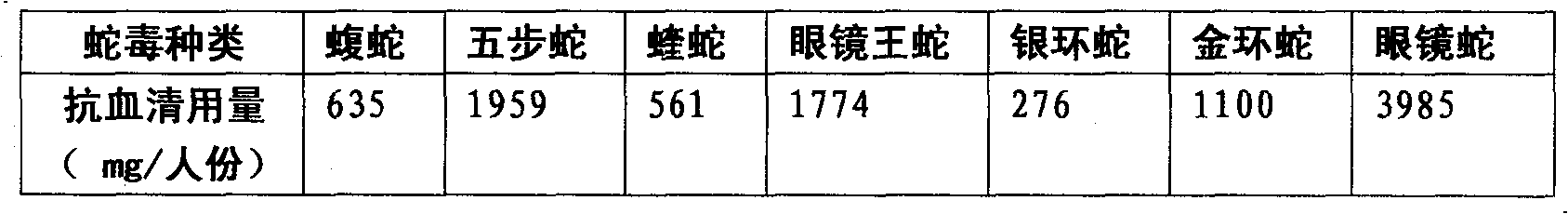
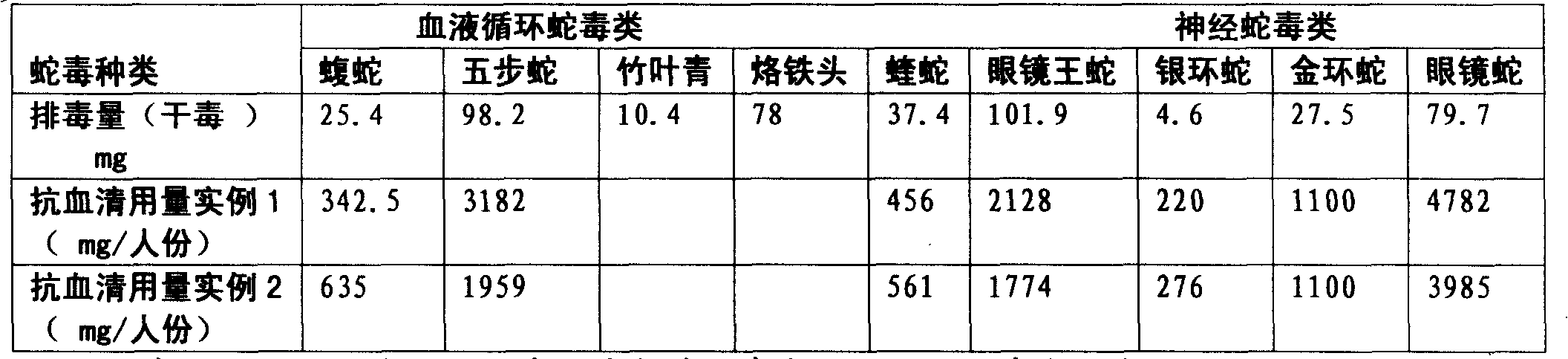
Purified multivalent and lyophilized antivenin and a use method belong to antibody immunity serums that aim at more than two antigens. The invention aims at preparing the multivalent antivenin by a plurality of lyophilized antivenins and the use method. The invention is characterized in that: the antigen-binding fragment F(ab)2 has 60 to 80 percent of lyophilized antiserum, the unit is mg per person, 342.5 to 1035 of vipers, 1177 to 3182 of agkistrodon acutus, 456 to 666 of adders, 1419 to 2128 of king cobras, 220 to 331 of coral snakes, 880 to 1320 of gold banded kraits, 3188 to 4782 of cobras are combined; the optimized combination is as follows: 635 of vipers, 1959 of agkistrodon acutus, 561 of adders, 1774 of king cobras, 276 of coral snakes, 1100 of gold banded kraits and 3985 of cobras. Blood circulation snake venom antiserum and nerves snake venom antiserum are respectively filled and measured. The use method is as follows: the antivenin that is diluted by sodium chloride and an anti-allergic agent, such as chlorphenamine maleate, are injected into muscle or a vein. The antivenin is prepared by only seven antivenins, and can effectively and in time treat injuries caused by various poisonous snakes in our country when the antiserum is directly injected in condition of the undetermined variety of poisonous snakes, and the invention reaches the advanced world level of the antivenin.
Owner:浙江健博生物科技股份有限公司
Externally-coating Chinese medicine in combination with needle warming moxibustion for dispelling cold and pathogenic dampness
InactiveCN101214327AHarmonize qi and bloodSymptoms disappearDevices for heating/cooling reflex pointsAnthropod material medical ingredientsCentipedeSalvia miltiorrhiza
The present invention relates to a Chinese medicine preparation, in particular to an external Chinese medicine combined with warming moxibustion for dispelling wind chill and pathogenic dampness. The present invention is made from the crude drugs with the following weight portions: 4 to 6g of saffron, radix notoginseng and liquorice respectively, 8 to 14g of angelica, chuanxikong rhizme, motherwort herb, white peony, prepared rehmannia rhizome, corktree bark, rhizoma atractylodis, largehead atractylode, notopterygium root , pubescent angelica root, cortex eucommiae, radix saposhinikoviae, grassleaf sweetflag rhizome and acanthopanax root respectively, 13 to 17g of danshen, 1 to 3g of Buthus martensi kirsch and agkistrodon acutus and 1 to 2g of centipede; all the crude drugs are added with 500ml water and cooked into paste. The medicine, as an external Chinese medicine combined with warming moxibustion, is coated on the acupoints of human body in acupuncture, and utilizes the physical stimulus of moxibustion hyperthermia and the pharmacological action of warmly dredging channels and collaterals of Chinese mugwort leaf, as well as the characteristics of ''qi and blood will circulate in warmness but coagulate in coldness''. The present invention has immediate effects of warmly dredging channels and collaterals, harmonizing qi and blood, activating the collaterals, dispelling wind and eliminating dampness, and dispelling cold and relieving pain through acupoint administration combined with the warm effect of warming moxibustion.
Owner:陈铁雷
A kind of preparation method of snake skin active peptide
The invention relates to a preparation method of snake skin active peptide, in particular to snakes such as black snake, pit viper, red snake, coral snake, cobra, king snake, five step snake, pine snake, tiger tail snake, Chinese water snake, etc. The fresh snake skin is used as the raw material, after cleaning → mincing → refining → adjusting the material concentration → adjusting the material temperature → adding protease enzymatic hydrolysis → centrifugal filtration → deenzyme → nanofiltration desalination concentration → ultrafiltration purification → freeze drying and other technological processes , to obtain snake skin active peptide. More than 90% of the molecular weight of the product is distributed between 200-5000Dalton, of which 200-2000Dalton (2-10 peptide) accounts for more than 75%, and it also contains docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) It has good water solubility, excellent moisture absorption and moisturizing function, good acid and heat stability. It can be used in the fields of medicine and health food production.
Owner:唐作安
A Chinese traditional medicinal composition for treatment of rheumatism and its preparation method
A Chinese traditional medicine composition used in treatment of rheumatism, comprising cortex phellodendri chinsis, rhizoma atractylodis, radix et rhizoma clematidis, caulis spatholobi, nidus vespae, zaocys, bungarus parvus, agkistrodon, flos carthami, eupolyphaga seu steleophaga, myrrha, olibanum, radix angelicae pubescentis, scorpio, scolopendra, pheretima, rhizoma et radix notopterygii, ramulus cinnamomi, rhizoma curcumae longae, etc, used in the treatment of rheumatism in clinic. In addition, the invention discloses the preparation method of the Chinese traditional medicine composition.
Owner:温继臣
Medicinal use of agkistrodon acutus thrombase for treating hemorrhagic disease
The invention refers to a ThrombinLikeEnzyme, called Haemocoagulase Acutus, in brief Halase, separated and purified from Agkistrodon acutus midew. It also refers to the usage of the drug of curing haemorrhagic disease made by using Halase. Halase is a glycoprotein composed of double sub-groups, connected by bisulfur bond. On N end, determining result of 15 amino acid sequences as follows: A sub-group: DCPSDWSSYEGHCYK; Bsub-group: DCSSGWSS- YEGHCYK. Molecular weight: 29076.1Da, equipotential point 5.78, and containing 1.89%-1.95% neutral hexose. Take Halase and excipient dextrose to add in injection water to dissolve, filter and sterilize, make split charging, and freeze-dry to obtain white injecting Halase powder injection. Halase is a new haemostatic drug.
Owner:GUANGZHOU RUIKANG BIOLOGICAL PHARMA
Pharmaceutical composition for adjuvant therapy of cancers and preparation method thereof
InactiveCN103127443APromote absorptionIncrease profitAnthropod material medical ingredientsAntineoplastic agentsRadix Astragali seu HedysariEfficacy
The invention relates to a pharmaceutical composition for adjuvant therapy of cancers and a preparation method thereof. The pharmaceutical composition of the invention comprises the following traditional Chinese medicine raw material herbs: radix astragali, gen-seng, radix codonopsis, bighead atractylodes rhizome, poria cocos, sculellaria barbata, oldenlandia diffusa, glaucescent fissistigma roots, scorpion, centipede, radices trichosanthis, rhizoma sparganii, curcuma zedoary, licorice, and the like, has the efficacy of breaking blood, removing blood stasis, softening hardness and dissipating binds, is applicable to adjuvant therapy of various cancers, and has significant curative effect after years of clinical application.
Owner:周昌明
Traditional Chinese medicine composition for drug rehabilitation
InactiveCN101011476AAchieving withdrawal treatmentHas analgesiaAmphibian material medical ingredientsNervous disorderWithdrawal syndromeLicorice roots
The invention discloses a Chinese medicinal composition for drug rehabilitation which comprises the following constituents (by weight portions): corydalis tuber 150-300 parts, root of Dahurain angelica 50-130 parts, red peony root 20-100 parts, black slice of aconite 20-100 parts, rhizome of Sichuan lovage 20-100 parts, herba patriniae 30-100 parts, Chinese angelica root 50-100 parts, rheum officinale 30-100 parts, licorice root 30-100 parts, cow-bezoar 1-10 parts, secretio bufonis 0.1-2.5 parts, borneol 0.5-1.0 part, agkistrodon acutus 1-4 parts, buthus martensi kirsch 1-10 parts, deer placenta 5-30 parts, fine Chinese caterpillar fungus 20-100 parts, American ginseng 15-40 parts.
Owner:TIANJIN DEVAL AREA QIANTUO BIOSCI
Medicine for external application for curing hyperosteogeny and disc herniation and method of preparing the same
InactiveCN101249179AGood curative effectShort course of treatmentSkeletal disorderMammal material medical ingredientsSide effectAdditive ingredient
The invention discloses an external medicine for treating hyperosteogeny and disc herniation and a preparation method. The external medicine has the raw ingredients and weight parts that: pangolin is 10 to 20, agkistrodon acutus is 10 to 20, saffron is 5 to 15, panax notoginseng is 10 to 20, radix cyathulae is 5 to 15, kudzu roots are 15 to 30, herba lycopodti is 10 to 20, olibanum is 10 to 15, dog bones are 20 to 30, eucommia bark is 10 to 20, wild celery is 5 to 10, and musk is 0.2 to 0.5. The invention has the preparation method that: the raw ingredient medicines are mixed and crushed into powders which are mixed into a paste by adding with rice vinegar; after the paste is evaporated for 15 to 20 minutes under water insulation, the rice vinegar is bedashed in; the evaporation is continued for 5 to 10 minutes; or the raw ingredients are crushed into powders which are mixed evenly and stir-fried until done, and then the powders is made into a plaster for bonding application through mixing with vinegar or water. The external medicine and the preparation method have the advantages of good curative effect, short course of treatment, hard relapse, no toxic side effects, and low treatment cost by apply the external medicine to act the external application on patients.
Owner:李武平
Compound plaster of dahurian angelica root and its preparation method
InactiveCN1602944AAchieve the purpose of healingDefinite curative effectNervous disorderAnthropod material medical ingredientsConvulsionSide effect
Compound prescription Bai Zhi paste and the preparation method involves a kind of medicine to treat the facial nerve paralysis, belongs to the Chinese native medicine domain.The invention provides a kind of curative effect paste, its raw material is composed by golden scorpion, system typhoneum giganteum, south system star, centipede, agkistrodon acutus, elevated gastrodia, dead silkworms, Sichuan, borneol and sesame oil.The paste producted in high temperature cooling oil, no destroyed medicine effective component, curative effect accurate to treat the facial nerve inflammation, the facial nerve lacking in vigilance, facial nerve convulsion, effect is satisfied, treatment course is short, does not have poisonous side effect, the use is convenient, does not need to heat and fire roast, is advantageous for the treatment at the spot.
Owner:邓从斌
Refined polyvalent anti-snake poison lyophilized blood serum and using method
ActiveCN101347617BTimely treatmentEffective treatmentAntinoxious agentsAntibody ingredientsAntigen Binding FragmentNaja
Purified multivalent and lyophilized antivenin and a use method belong to antibody immunity serums that aim at more than two antigens. The invention aims at preparing the multivalent antivenin by a plurality of lyophilized antivenins and the use method. The invention is characterized in that: the antigen-binding fragment F(ab)2 has 60 to 80 percent of lyophilized antiserum, the unit is mg per person, 342.5 to 1035 of vipers, 1177 to 3182 of agkistrodon acutus, 456 to 666 of adders, 1419 to 2128 of king cobras, 220 to 331 of coral snakes, 880 to 1320 of gold banded kraits, 3188 to 4782 of cobras are combined; the optimized combination is as follows: 635 of vipers, 1959 of agkistrodon acutus, 561 of adders, 1774 of king cobras, 276 of coral snakes, 1100 of gold banded kraits and 3985 of cobras. Blood circulation snake venom antiserum and nerves snake venom antiserum are respectively filled and measured. The use method is as follows: the antivenin that is diluted by sodium chloride and an anti-allergic agent, such as chlorphenamine maleate, are injected into muscle or a vein. The antivenin is prepared by only seven antivenins, and can effectively and in time treat injuries caused by various poisonous snakes in our country when the antiserum is directly injected in condition of the undetermined variety of poisonous snakes, and the invention reaches the advanced world level of the antivenin.
Owner:浙江健博生物科技股份有限公司
A kind of preparation method of snake meat oligopeptide
The invention discloses a method for preparing oligopeptide of snake meat, which comprises the following steps of: selecting a healthy and live adult snake from zaocys dhumnade, hagworm, gloydius saxatilis, dinodon rufozonatum, cobra, coral snakes, agkistrodon acutus, elaphe schrenckii, elaphe anomala, enhydris chinensis, and the like, slaughtering, removing the head, tail, skin and viscera of the snake, cleaning a carcass part with clear water, and performing processes such as grinding, jordaning, enzymolysis, filtration, enzyme deactivating, salt and arsenic removal, nanofiltration and concentration, spray drying and the like to obtain dry oligopeptide powder of the snake meat. The obtained product has the molecular weight of over 85 percent and the molecular weight distribution of 200-5,000Dalt; and bioactive substances have the effects of resisting tumors, coagulation, oxidation and fatigue, improving immunity, preventing heart cerebrovascular disease, preventing and treating rheumatic arthritis and the like.
Owner:唐作安
Vein relaxing plaster for curing cervical vertebra and lumbar vertebra disease
InactiveCN101085305APain reliefPromote circulationHeavy metal active ingredientsAntipyreticDiseaseMedicinal herbs
The invention discloses a Chinese medicament for treating neck and lumbar spine diseases which is prepared from the following medicinal herbs (by weight ratio): Clematis chinensis 50g, frankincense 40g, myrrh 40g, kansui root 30g, buthus martensi kirsch 30g, earthworm 30g, dried body of ground beetle 60g, Sichuan aconite root 40g, wild aconkite root 40g, curcuma longa 60g, notopterygium root 40g, pubescent angelica root 40g, ledebouriella root 40g, agkistrodon acutus 50g, centipede 5 pieces, peach kernel 30g, safflower 30g, grassleaved sweetflag rhizome 40g, borneol 15g, musk 5g and white pepper 30g. And the preparing process comprises the steps of charging 2500g of sesame oil into pot, charging the Chinese medicinal herbs, frying and deslagging, charging yellow lead 1000g and stirring, cooling down to 60 Deg C, charging cold water, releasing fire-toxin, filtering to obtain the plaster.
Owner:于洪文
Primer combination for identifying three medicinal snakes and application thereof
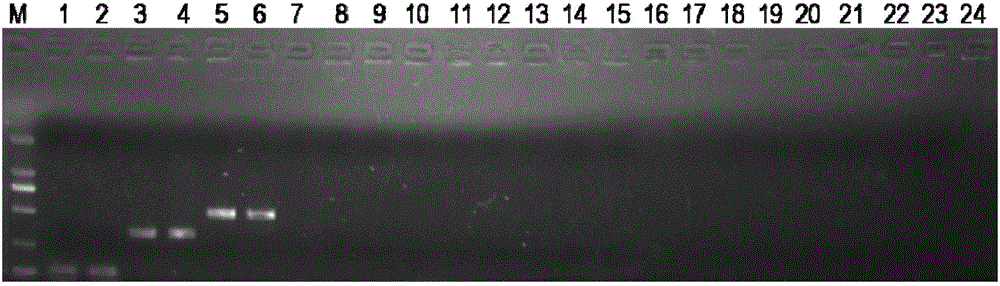
ActiveCN106636397AMicrobiological testing/measurementDNA/RNA fragmentationPit viperAgkistrodon acutus
The invention discloses a primer combination for identifying three medicinal snakes and an application thereof. The primer combination disclosed by the invention is composed of six single stranded DNA molecules as shown by the sequences 1-6. The invention further sets forth the application of the primer combination for identifying a long-nosed pit viper, a zaocys dhumnade and a little multibanded krait. The invention further discloses a method for identifying long-nosed pit viper, zaocys dhumnade and little multibanded krait. According to the invention, the existence of the medicinal snakes (long-nosed pit viper, zaocys dhumnade and little multibanded krait) can be simultaneously identified by carrying out PCR reaction once and the existence of specific medicinal snake species can be detected according to the size of the gel electrophoresis stripe. Meanwhile, the primer combination disclosed by the invention also can be used for identifying the mixed sample of long-nosed pit viper, zaocys dhumnade and little multibanded krait.
Owner:华润三九现代中药制药有限公司 +2
Medicament for treating rheumatism and rheumatoid disease, and preparing method thereof
InactiveCN101530470AReduce joint painReasonable medicationAnthropod material medical ingredientsAntipyreticCentipedePainful joints
The invention discloses a medicament for treating heumatism and rheumatoid disease which is prepared by 11 kinds raw materials as follows: white-dappled snake, whole worm, ginger worm, jiazhu, centipede, notopterygium root, radix angelicae pubescentis, frankincense, myrrh and pheretima aspergillum. The medicament is reasonable and scientific which can reduce arthrosis ache and eliminate swelling of the intractable rheumatism and rheumatoid patient obviously by a treating principle of expelling wind and cold, activating blood and dissolving stasis and has obvious treating effect. Normal rheumatism patient can be cured in one treating period by taking the medicament in clinic, severe patient can be cured in two-five treating periods. The medicament also has certain effect to hyperosteogeny, apoplexy sequelae and trauma sore.
Owner:冯大海
Pharmaceutical formulation for treating burn and scald
InactiveCN102885875ACutting costsShort burn timeReptile material medical ingredientsDermatological disorderSide effectTreatment effect
The invention relates to a pharmaceutical formulation for treating burn and scald in the pharmaceutical formulation field, in particular to a pharmaceutical formulation for treating burn and scald, which is short in treatment time, low in cost and excellent in treatment effect. The pharmaceutical formulation is formed by mixing cobra fat, agkistrodon acutus fat, zaocys dhumnade fat and pearl tea oil together according to a certain extracting and purifying manner and in a certain ratio. The pharmaceutical formulation is quite short in the time of treating burn and scald and generally enables the wound to be healed in a week; besides, the formulation is simple and very low in cost, thereby saving unnecessary expenditure for patients; and moreover, the pharmaceutical formulation has the treatment effect of leaving no scar and has no any other side-effect.
Owner:廖光辉
Tendon-relaxing pain-relieving Chinese herbal powder
InactiveCN1840100AEffective treatmentPromote circulationPowder deliveryAnthropod material medical ingredientsMedicinal herbsGastrodia
The invention relates to a Chinese medicinal powder for treating waist and leg pains, which is prepared form 28 kinds of Chinese medicinal herbs including cornus officinalis, epimeddium, east Asian tree fern rhizome, dipsacus root, drynaria, eucommia bark, Loranthus mulberry mistletoe, Clematis chinensis, large-leaf gentian root, pawpaw, cortex acanthopanacis, agkistrodon acutus, Kusnezoff monkshood root, homalomena rhizoma, achyranthes and cyathula root, frankincense, pubescent angelica root, notopterygium root, buthus martensi karsch, Chinese angelica root, gastrodia tuber, white atractylodes rhizome, atractylodes rhizome, root of red rooted saliva, dried body of ground beetle, prepared rhizome of rehmannia and mulberry twigs.
Owner:张国柱
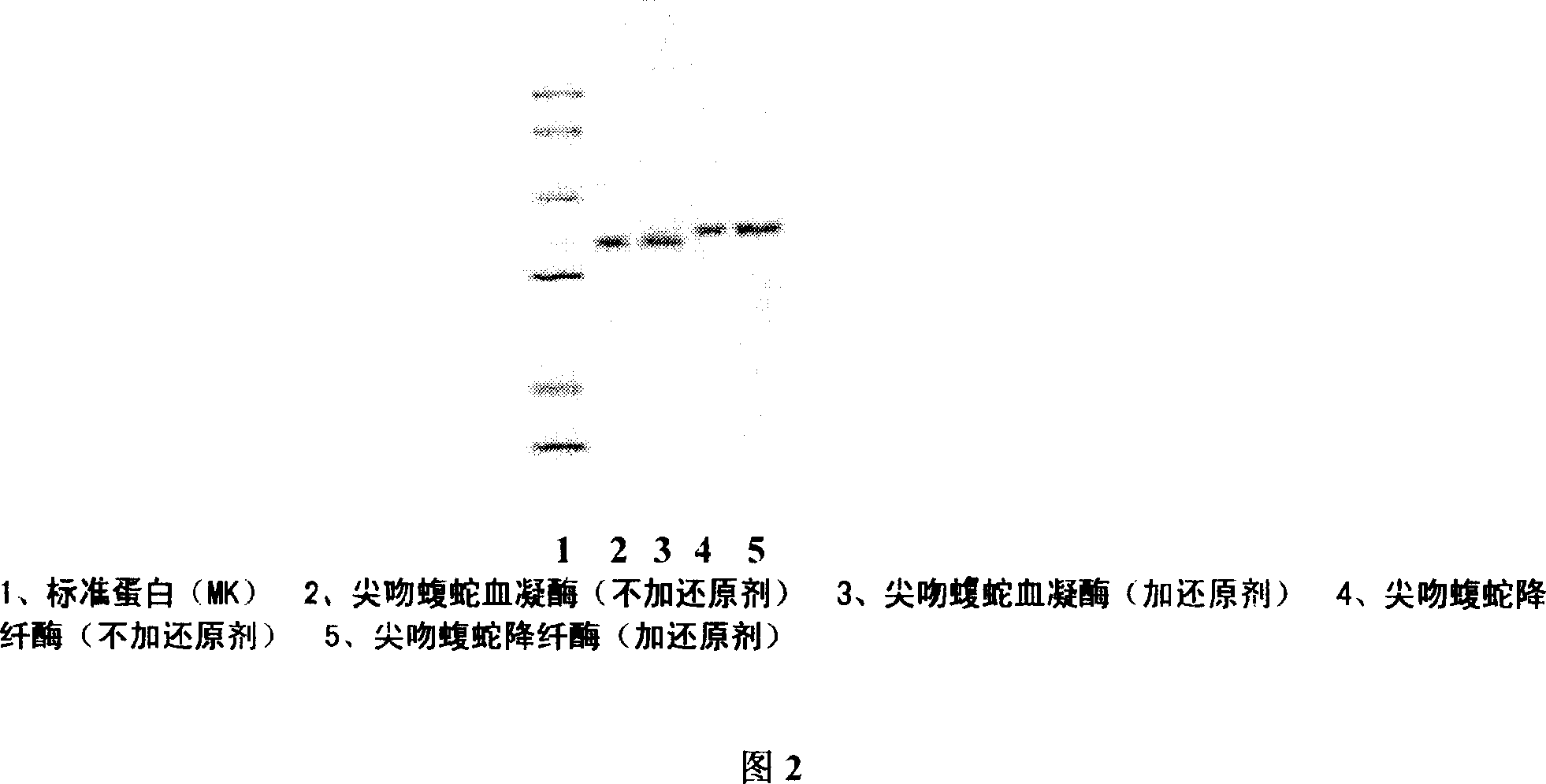
Ahylysantinfarctase 36KD single-stranded haemocoagulase and its preparing method
The present invention is Agkistrodon acutus venon 36KD single-stranded hemocoagulase and its preparation process, and aims at providing single-stranded Agkistrodon acutus venon hemocoagulase with high potency and low toxicity. SDS-PAGE electrophoresis shows that the hemocoagulase has molecular weight of 36KD+ / -2KD, single color zone, and at least 90 % homogeneity with the N end sequence of the single-stranded amino acid shown in SEQ ID No. 1. The preparation process includes dissolving Agkistrodon acutus venon in Tris-HCl buffering solution (pH8.0) and dialysis; adding the supernatant to Metal Sepharose F.F affinity chromatographic column and collecting the penetrating peak component; adding the penetrating peak component to DEAE-Sepharose F.F ion exchange column and collecting active peak component; separating in Superdex 75 column and Sephacryl S-100 column; desalting in Sephadex G-25 column; sterilizing and freeze drying.
Owner:沈居仁 +2
Bone disease treatment plaster and making method thereof
InactiveCN106692814ANo side effectsReduce viscosityHeavy metal active ingredientsNervous disorderCentipedeSea urchin
The invention discloses a bone disease treatment plaster. The bone disease treatment plaster comprises Smilax hypoglauca Benth, Hedyotis diffusa, Zanthoxylum nitidum, Cynanchum amplexicaule (Sieb. et Zucc.) Hemsl., Herba Patriniae, Pseudobulbus Cremastrae seu Pleiones, Cornu Bubali, Cordyceps sinensis, barbed skullcap herb, fresh dendrobium, Polygonum multiflorum, geckos, Santalum album L., Dioscorea nipponica Makino, rheum officinale, toad venom, geckos, American ginseng, Salvia miltiorrhiza, Adenophora stricta, Radix Scrophulariae, Sophora flavescens, Radix Pseudostellariae, Corn Cervi Pantotrichum, sea horses, starfishes, sea urchins, Folium Cordylines Fruticosae, Rhizoma Paridis, Rhodiola rosea, Crocus sativus, Radix Astragali, Human Placenta, pangolins, long-noded pit viper, Agkistrodon acutus, Zaocys Dhumnades, Changbai Mountain Mythic Fungus, dragon's blood, panax notoginseng, black maca, Cistanche deserticola, Radix Morindae officinalis, polygonatum rhizome, Semen plantaginis, semen cuscutae, centipedes, lumbricus, musk, rhizoma gastrodiae and whole scorpions. The bone disease treatment plaster aims at macroscopic regulation, and realizes integral conditioning and treatment through regulating the self tissue ability of a complex and open huge system and depending on the self rehabilitation ability of patients.
Owner:李立宏
External used medicine for treating tumor
The invention relates to an external used medicine prepared based on traditional Chinese medicines for treating tumor, wherein powder product is prepared by taking gecko, nidus vespae, cynanchum paniculatum, astragalus mongholicus, codonopsis pilosula, rhizoma atractylodis alba, hairyvein agrimony, semen coicis, safflower carthamus, amethyst, manyleaf paris rhizome, honey-fried licorice root, toad skin, stiff silkworm, pillis ophidiae, zaocys dhumnade, agkistrodon, rhizoma corydalis, angelica sinensis, cortex albiziae, Flos Albiziae, scorpio, centipede, lumbricus, polygala tenuifolia, amber, chlorite schist, leech, gadfly, ground beeltle, manis pentadactyla, and oldenlandia diffusa, which are finely selected and cleaned with clear water, mixing uniformly, crushing to 60-80 meshes medicine powders, and sterilizing. The medicine has functions in combating poison with poison, dispelling wind and removing obstruction in the meridians, clearing away heat and toxic materials, promoting blood circulation to remove blood stasis, smoothing the nerves, combating poison, removing stasis, tonifying Qi and strengthening healthy energy, can inhibit and kill tumor cells effectively, and has significant treating effect to tumors without toxic and side effects.
Owner:冯宇伟
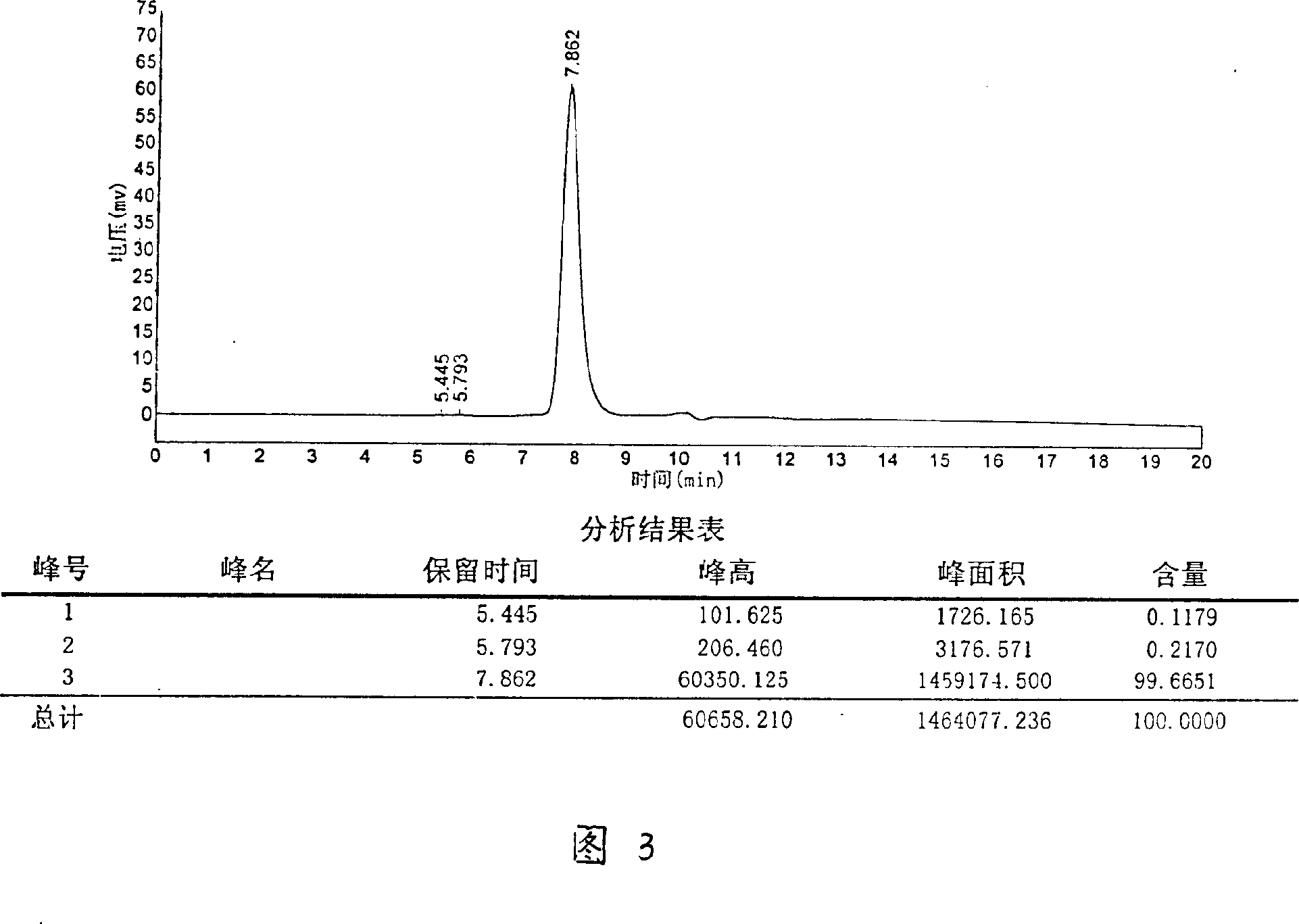
Angiogenesis inhibitant, purification method and medicinal composition therewith
ActiveCN102372770ASuppress generationGood effectPeptide/protein ingredientsPharmaceutical delivery mechanismLymphatic SpreadPurification methods
The invention discloses an angiogenesis inhibitant, which has an amino acid sequence selected from SEQ ID NO.1 and SEQ ID NO.2 and a molecular weight of 25000-35000 Dalton measured by a non-reducing SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) method. The purification method comprises the steps of: first dissolving agkistrodon acutus venom in a buffer solution, conducting centrifugation so as to take supernate; subjecting the obtained supernate to anion exchange chromatography so as to obtain an angiogenesis inhibitant crude solution; then coupling a monoclonal antibody secreted by a hybridoma cell strain 1B9 (CCTCC C200970) with an affinity chromatography vector so as to prepare an immune affinity chromatography column; finally purifying the crude solution with the obtained immune affinity chromatography column, thus obtaining the angiogenesis inhibitant. The angiogenesis inhibitant of the invention can be prepared into powder injections for injection, and has an anti-angiogenesis activity, especially angiogenesis related to tumor growth, and also has an obvious effect in tumor resistance and tumor metastasis resistance.
Owner:ZHAOKE GUANGZHOU OPTHALMIC DRUG
External application medicine for treating hypertension
InactiveCN101129805AEasy to useLittle side effectsAnthropod material medical ingredientsHydroxy compound active ingredientsGround beetleAdemetionine
The invention relates to a medicament for external application for treating hypertension, which comprises the following active raw materials (by weight portion): notoginseng 4-10 parts, selfheal 0-6 parts, coptis root 4-10 parts, cicada skin 4-10 parts, centipede 9-20 parts, root of red rooted saliva 0-30 parts, cassia seed 0-20 parts, hooked uncaria 0-20 parts, kudzuvine root 10-20 parts, gastrodia elata 0-30 parts, dried body of ground beetle 4-10 parts, earthworm 4-10 parts, agkistrodon acutus 15-45 parts, borneol 2-6 parts, kansui root 3-5 parts, rhizoma corydalis 4-10 parts, asarum herb 5-8 parts and white mustard seed 4-10 parts. The medicament is prepared through mixing the raw materials and grinding into medicinal powder.
Owner:马瑞珍
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com