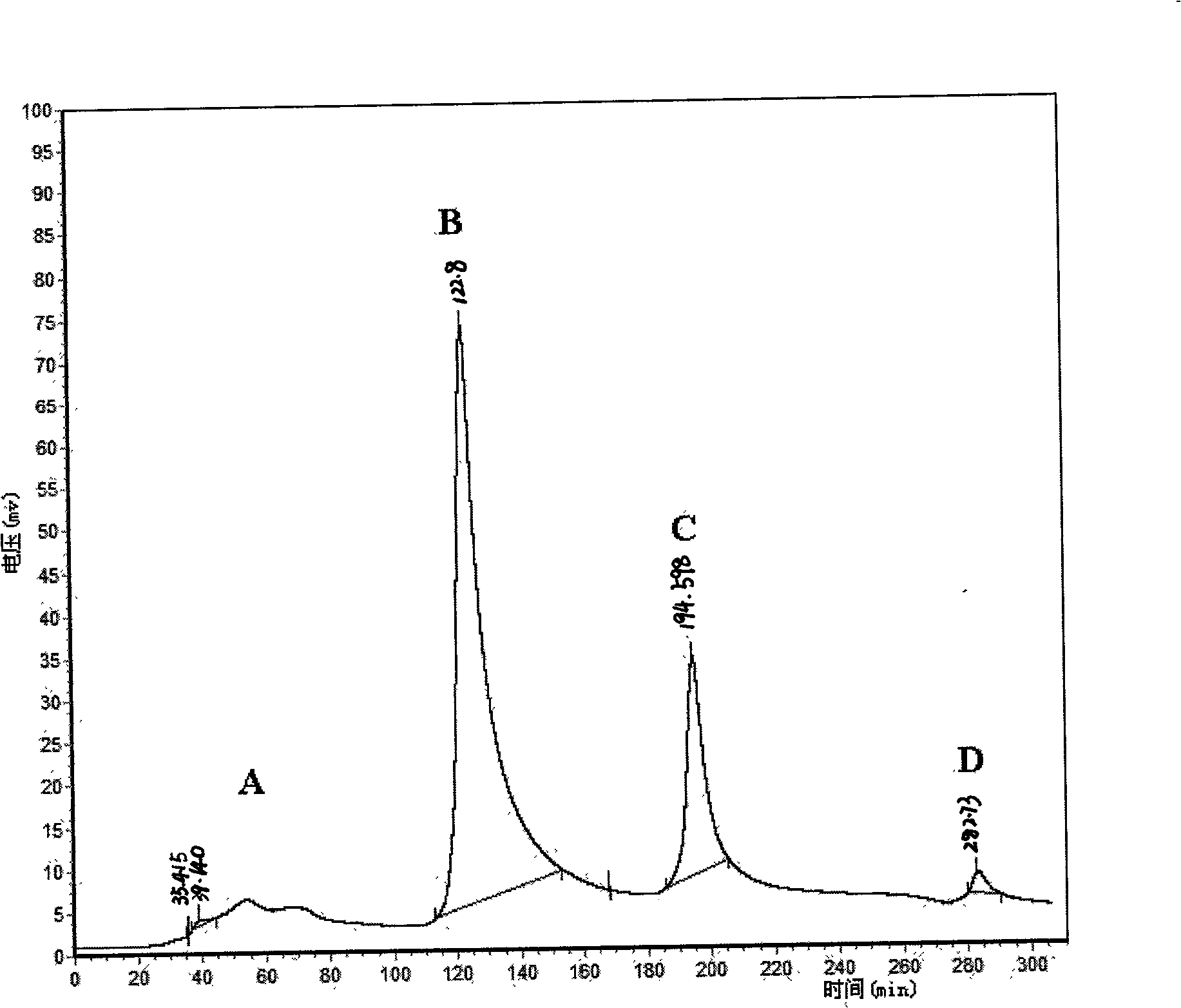
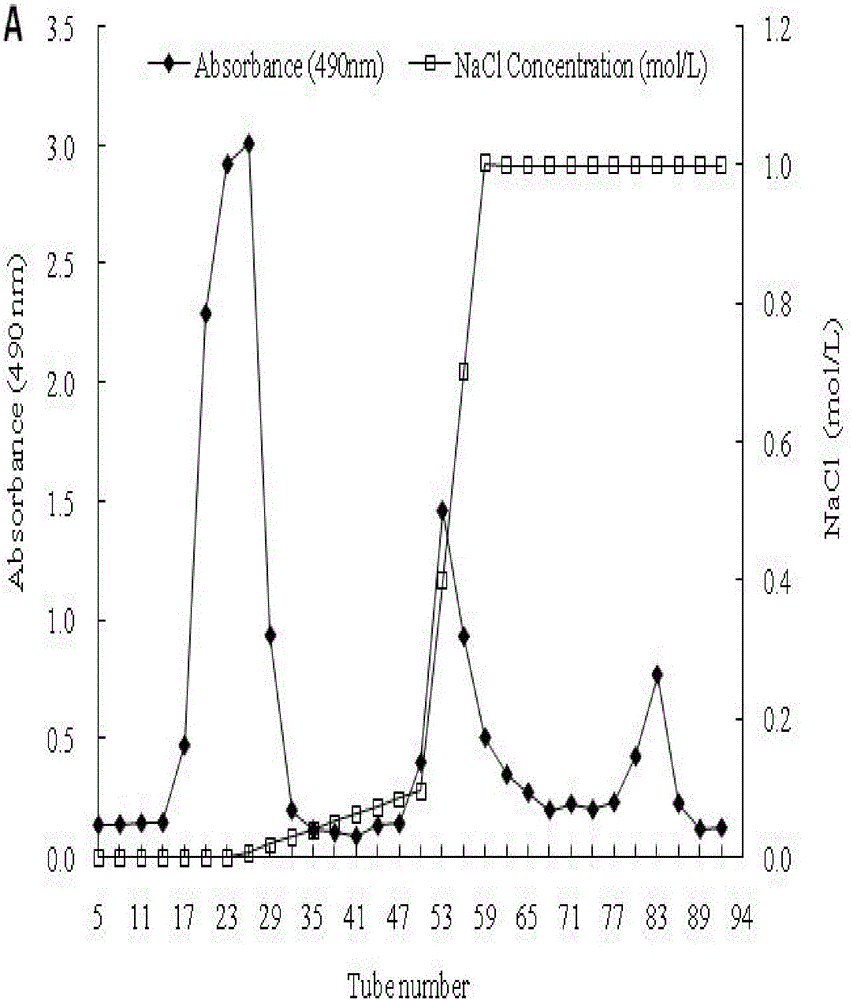
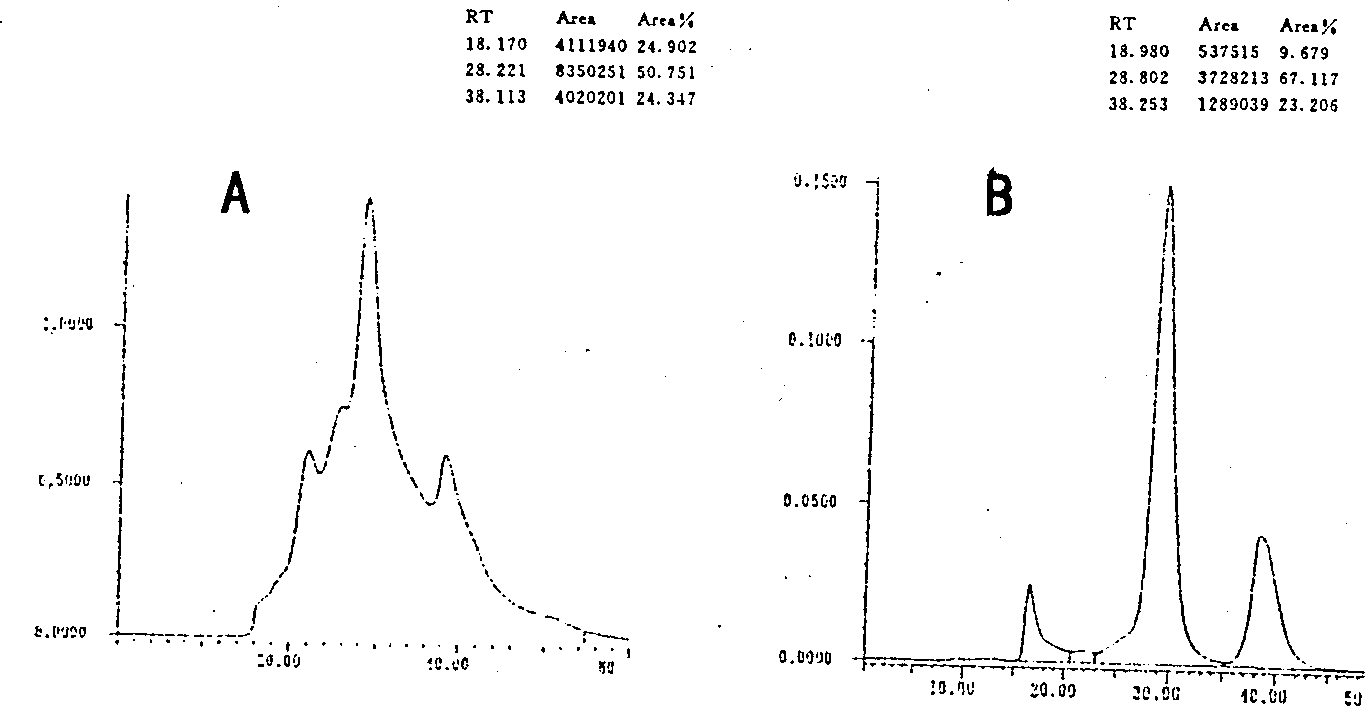
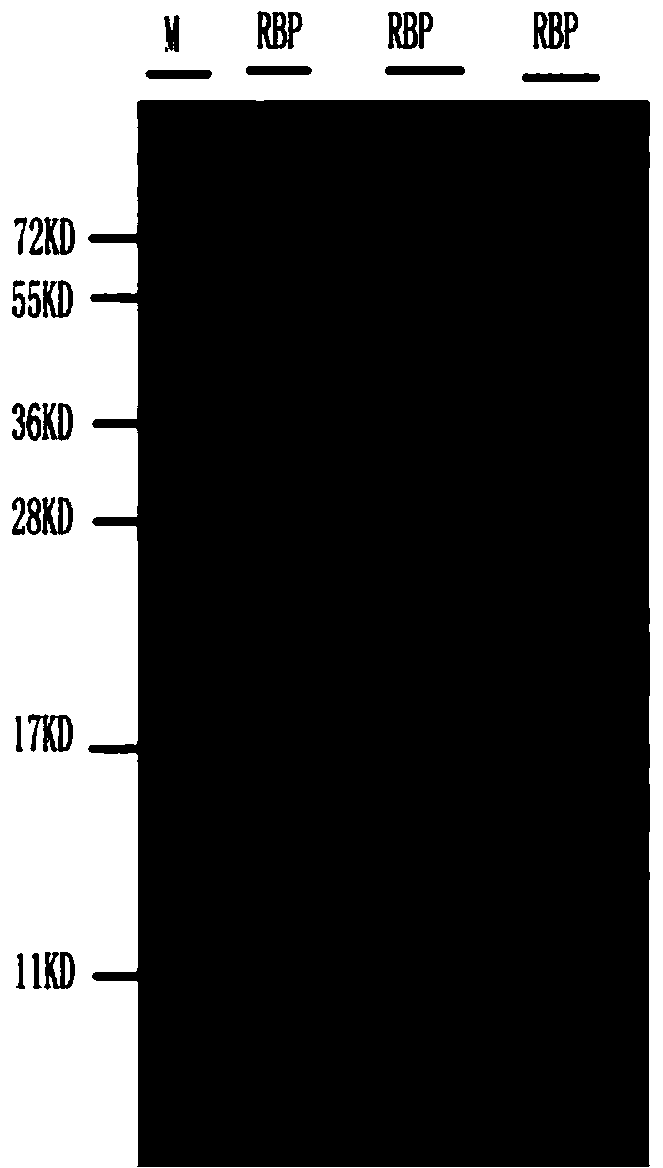
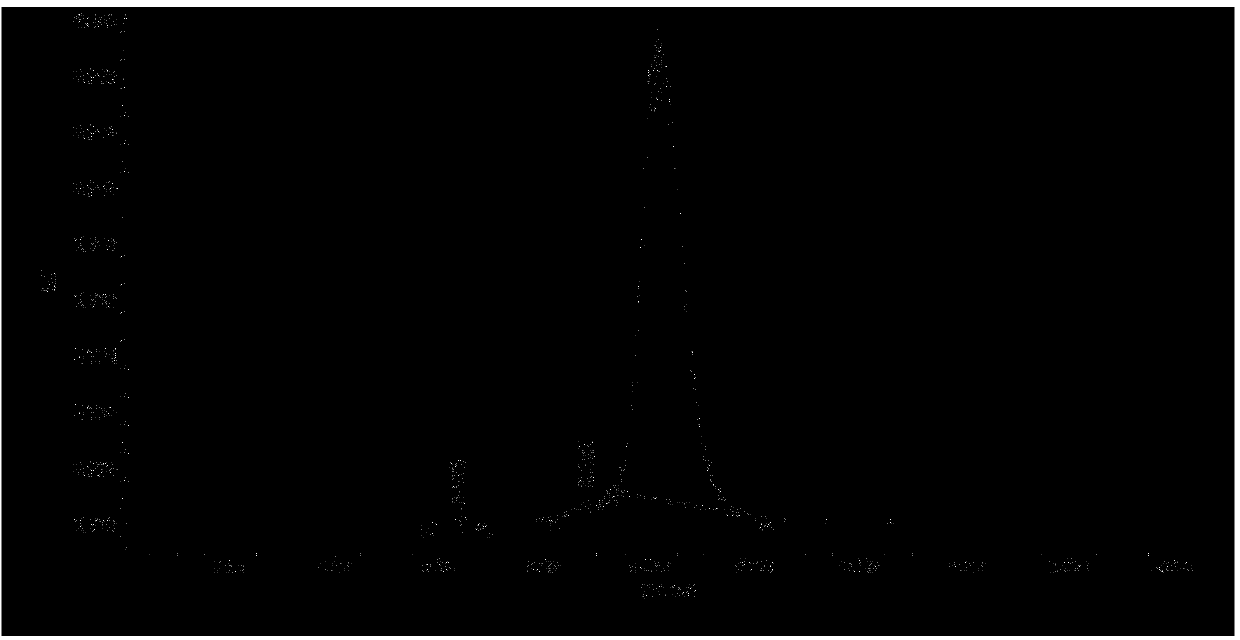
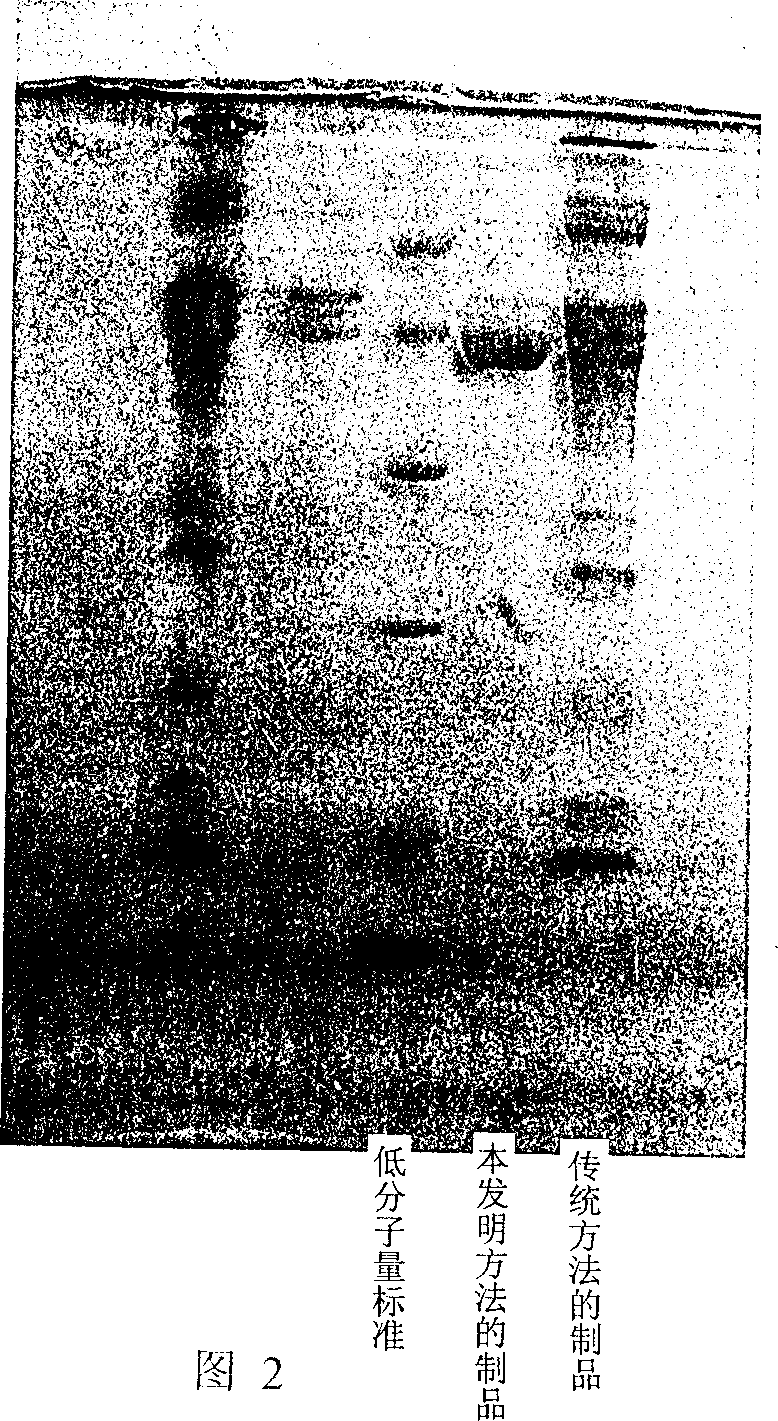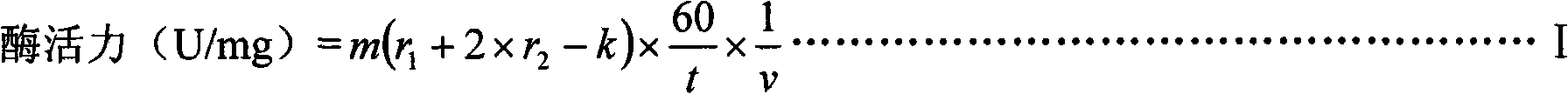Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
95 results about "DEAE-Sepharose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
DEAE-Sepharose is a tradename for the anion-exchange reactive group, diethylaminoethanol (DEAE) covalently linked to Sepharose (a polysaccharide polymer).
Phellinus linteus mycelia active glucoprotein and use thereof and preparation
InactiveCN101297821AHigh yieldReduce manufacturing costAntibacterial agentsFungiArginineAdditive ingredient
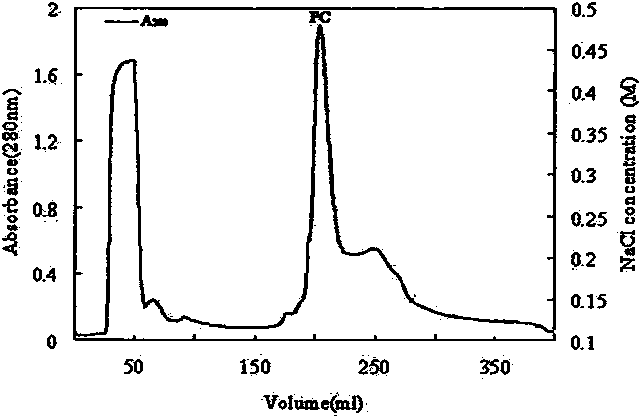
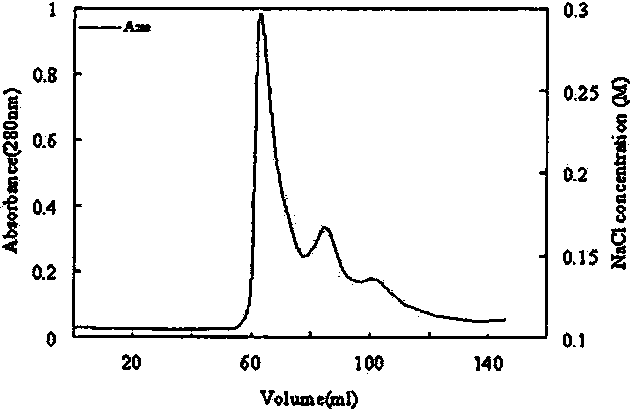
The invention discloses a submerged fermentation phellinus linteus mycelium glycoprotein, a usage and a separation extraction preparation method thereof, the complex is the complex of heteropolysaccharide and protein, wherein, the content of the heteropolysaccharide is 15 to 20 percent, and the heteropolysaccharide is composed of three monosaccharides of glucose monosaccharide, xylose and mannose; the content of the protein is 80 to 85 percent, and the protein is composed of 18 amino acids of aspartic acid, glutamic acid, arginine and so on; and the weight-average molecular weight is 20 to 40KD. The glycoprotein complex uses bran extract liquid as a main ingredient for preparing a culture medium, the phellinus linteus mycelium is produced by the submerged fermentation of the phellinus linteus bacterial strain liquid, the homogenization, the cold-water extraction, the centrifugalization, the collection of supernatant liquid, the precipitation of ammonium sulfate, the dialysis and the DEAE-Sepharose Fast Flow column chromatography are carried out for system separating and purifying the glycoprotein complex. The anti-bacterial glycoprotein is used for preparing the anti-bacterial dugs which have inhibitory effects on escherichia coil and staphylococcus aureus. At the same time, the glycoprotein complex can be used for the separation and the purification of the glycoprotein from the mycelium obtained by the submerged fermentation of various medical and edible fungi.
Owner:JIANGSU UNIV
Preparation method of cordyceps militaris active polysaccharide
InactiveCN105085704AHigh in polysaccharidesLight colorOrganic active ingredientsAntineoplastic agentsCordycepsDEAE-Sepharose
The invention relates to cordyceps militaris active polysaccharide prepared by a method comprising the following steps: performing ultrafine grinding on cordyceps militaris sporophores, then extracting with boiling water, and collecting supernatant fluid; and concentrating the supernatant fluid, then adding ethanol for deposition, passing a deposited part through a DEAE-sepharose column, desalting a part of 0-0.5N, and then performing freeze-drying. The invention also relates to an application of a cordyceps militaris extract in preparation of an anti-tumor activity product.
Owner:上海瑞丰农业科技有限公司 +2
Preparation method of high-purity phycocyanin
InactiveCN103992402AWide variety of sourcesSimple extraction processPeptide preparation methodsDepsipeptidesPhosphateDEAE-Sepharose
The invention discloses a preparation method of high-purity phycocyanin, and is characterized in that the preparation method comprises the following steps: (1) taking a fresh spirulina powder, fully mixing with a phosphate buffer solution evenly, repeatedly freezing and thawing for 7-10 times to break and remove cell walls, centrifuging to remove spirulina mud, and thus obtaining a supernatant; (2) adopting a two-step precipitation method with a 20%-30% ammonium sulfate and a 40%-60% ammonium sulfate to obtain a phycocyanin crude extract; (3) after dialyzing the crude extract, loading the sample onto a weak anion exchange column DEAE Sepharose FF, carrying out gradient elution after ion exchange, collecting an outflow component with A620 / A280 of more than 3; and (4) dialyzing the collected sample, then loading the sample onto a strong anion exchange column SOURCE30Q, carrying out gradient elution after ion exchange, collecting an outflow component with A620 / A280 of more than 4, again carrying out one-time ammonium sulfate precipitation concentration, and thus obtaining the high-purity phycocyanin having the purity of more than 4.5. The extraction purification method is simplified in process, wide in source of the raw material spirulina, simple in required equipment, and high in purity of the product, and has quite high application value.
Owner:CHINA PHARM UNIV
Purification of recommbined human urokinase zymogen
ActiveCN1680550AEasy to fillLarge amount of processingPeptide preparation methodsPeptidasesZymogenUrokinase Plasminogen Activator
The invention is about a method to purify the recombined human urokinase by the chromatography. The invention relates to recovery the gene engineering production from the mammal cell culture using the Streamline-SP, Sephacryl S-200, Sepharose Fast Flow and DEAE-Sepharose Fast Flow method. The recombined human urokinase can meet the SFDA standard and the percent recovery is above 70%, the purity is above 99% by using the method.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
Method for preparing Fuzhuan tea polysaccharide with intestinal tract benefiting function
InactiveCN106188323AHyperpigmentationGood decolorization effectFood ingredient functionsFood extractionAlcoholDEAE-Sepharose
The invention relates to a method for preparing Fuzhuan tea polysaccharide with an intestinal tract benefiting function. The Fuzhuan tea polysaccharide is obtained through decoloration, drying, smashing, hot water extraction, ethanol precipitation, redissolving, DEAE Sepharose Fast Flow resin decoloration, dialysis, concentration and freeze drying of 80% ethyl alcohol. In-vitro anaerobic fermentation experiments prove that the Fuzhuan tea polysaccharide has the intestinal tract benefiting function. The method has the advantages that utilization rate is high, decoloration effect is good, the process is mild and does not damage the activity of tea polysaccharide, and a new approach is provided for developing and utilizing Fuzhuan tea polysaccharide.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for extracting and preparing low molecular fucoidan from Ascophyllum mackaii
InactiveCN103788219AIncrease contentImproves and preserves active ingredientsAscophyllumDEAE-Sepharose
The invention relates to the alga chemical field, and concretely relates to a method for extracting and preparing low molecular fucoidan from Ascophyllum mackaii. The method is characterized in that Ascophyllum mackaii is immersed and extracted by employing dilute hydrochloric acid and water, a soak solution is used for purifying an extract through a foaming machine, an upper layer floater and a lower layer sediment are respectively collected, and polysaccharide in the Ascophyllum mackaii is fully extracted and collected by using a filtering collection and a cell disruption extraction method. An ultrafiltration technology is used for removing salinity and the graded products in 20000 Dalton can be obtained. The fucoidan crude product is obtained by an ethanol precipitation method. Purification and classification of an anion exchange resin chromatographic column of DEAE-Sepharose F.F. can be carried out. Next classification and concentration of an 8-14KD dialysis bag are carried out, and cryoconcentration and freeze drying are carried out to obtain the fucoidan product.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Method for extracting and purifying neutral pseudo-ginseng polysaccharide, research and application for pharmacological activity for promoting cell proliferation
ActiveCN107286265APromotes significant proliferationStrong value-added effectOrganic active ingredientsCosmetic preparationsOsteoblastPeriodontal ligament stem cells
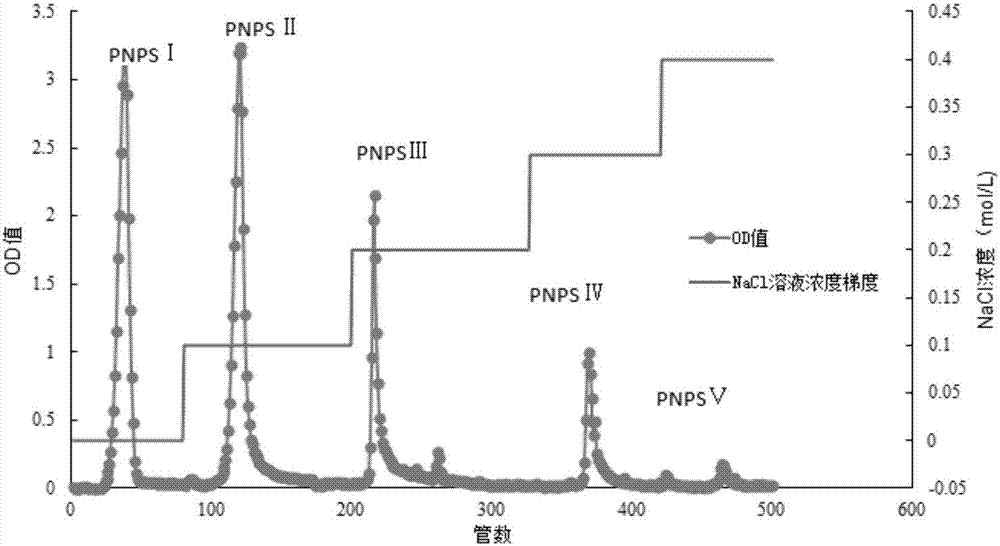
The invention relates to a method for extracting and purifying neutral pseudo-ginseng polysaccharide, a research and an application for pharmacological activity for promoting cell proliferation. The pseudo-ginseng waste residue of the notoginsenoside extracted in the production process is taken as a raw material, a water extract and alcohol precipitation method is adopted for acquiring crude pseudo-ginseng polysaccharide and the DEAE Sepharose Fast Flow anion exchange chromatography is adopted for purifying the crude pseudo-ginseng polysaccharide so as to acquire five components: neutral pseudo-ginseng polysaccharide PNPS I and acidic pseudo-ginseng polysaccharide PNPS II, PNPS III, PNPS IV and PNPS V five samples. The research on the influence of the pseudo-ginseng polysaccharide on human periodontal ligament stem cell, mice osteoblast and human skin epidermis cell in vitro proliferation proves that the neutral pseudo-ginseng polysaccharide PNPS I is effective in boosting the periodontal ligament stem cell, mice osteoblast and human skin epidermis cell in vitro proliferation and the neutral pseudo-ginseng polysaccharide PNPS I can be used as an important drug intermediate for developing a new drug and a natural raw material for a functional healthcare food and also can be used as an active raw material of the household chemicals.
Owner:云南多糖生物科技有限公司
Method for preparing human immunoglobulin for intravenous injection
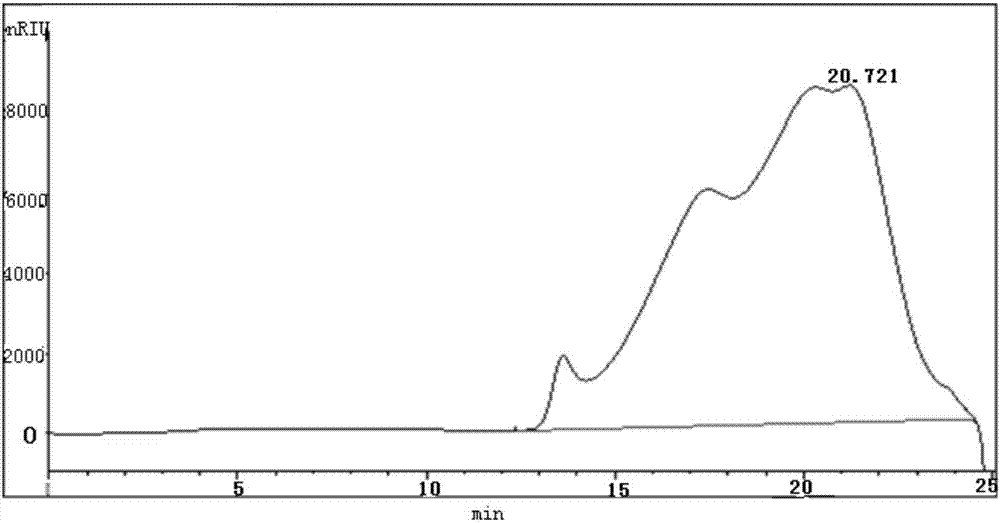
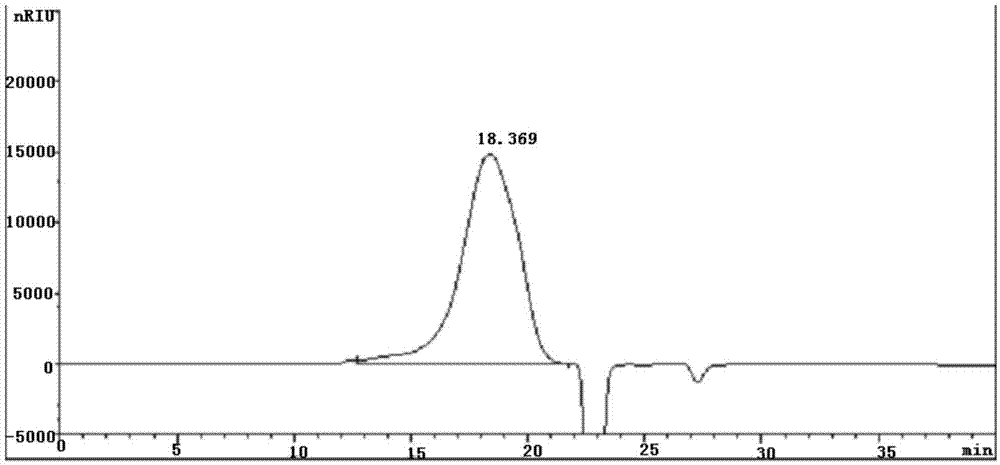
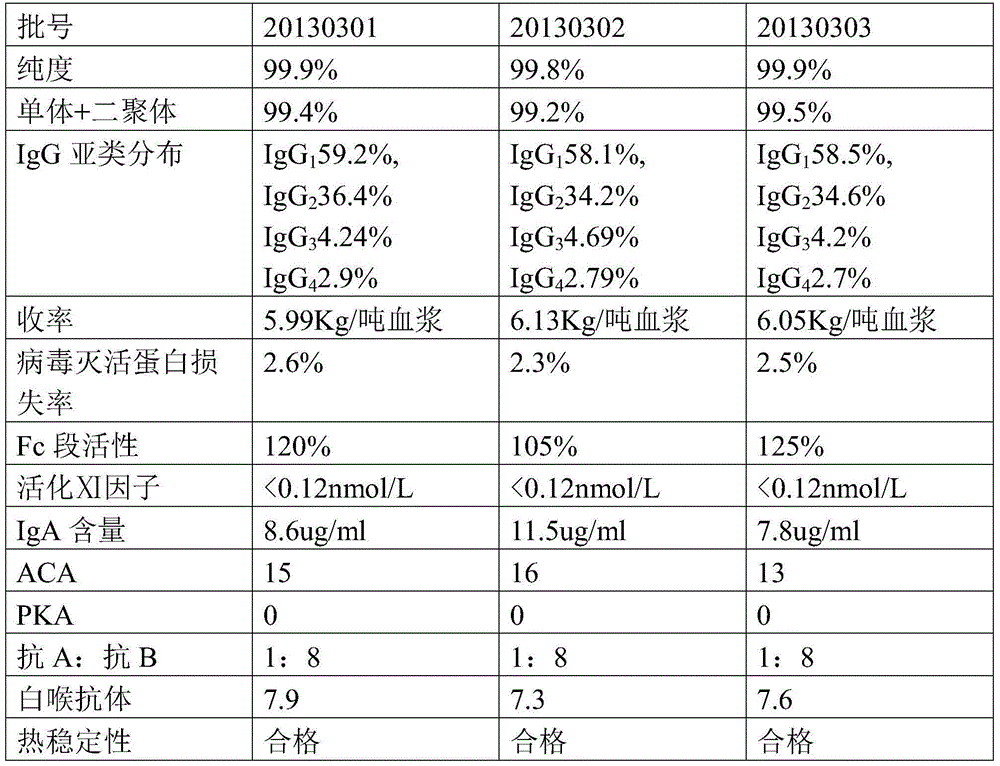
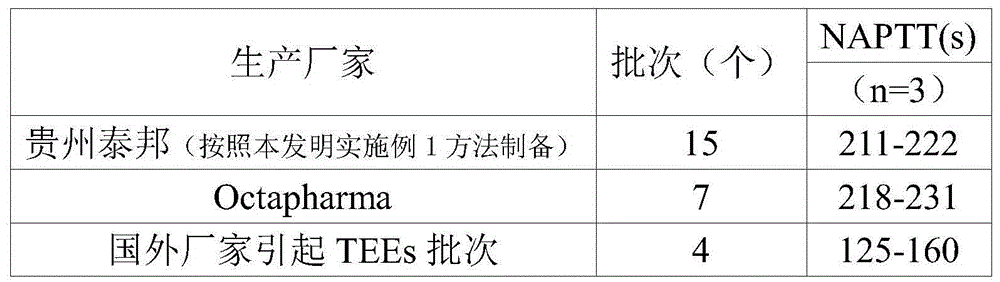
ActiveCN104402993AHigh purityImprove qualitySerum immunoglobulinsPeptide preparation methodsSide effectFiltration
The invention provides a method for preparing human immunoglobulin for intravenous injection. The method comprises the following steps of a, dissolving: taking a Cohn method F II precipitation; dissolving the precipitation in water for injection; performing clarifying filtration; adjusting the pH value to 6.60+ / -0.1; uniformly stirring; adjusting the electrical conductivity to 1.40+ / -0.05ms / cm; adjusting the temperature to 0-5DEG C, and uniformly stirring; b, performing chromatography: purifying by using a chromatographic column of DEAE Sepharose Fast Flow, and collecting human immunoglobulin component liquid; c, performing ultrafiltration: performing ultrafiltration and dialysis on flow penetrating liquid in the step b; when the osmotic pressure is lower than 30mOs mol / kg, concentrating the treated liquid until the protein concentration is 4.0 percent+ / -1 percent (w / v) to obtain concentrated solution; d, inactivating virus: adding sorbitol in the concentrated solution until the concentration is (33+ / -1) percent (w / v), adjusting the pH value to 5.00+ / -0.1; keeping the temperature 60+ / -0.5DEG C constant for 10 hours, cooling and performing clarifying filtration; e, performing ultrafiltration and dialysis and preparing to obtain the human immunoglobulin. The human immunoglobulin prepared by the method disclosed by the invention is high in purity; an Fc segment is high in biological activity and good in quality; IgA and other impurity factors are in low in content; the method has the advantages of few side effects, high yield and good market application prospect.
Owner:GUIZHOU TAIBANG BIOLOGICAL PROD
Technological process of continuously perfused culture of hybrid tumor and CHO cell to prepare antibody
InactiveCN1339606AEliminate pollutionLow costFused cellsFermentationAbnormal tissue growthDEAE-Sepharose
The technological process includes inoculation of cell to carrier in fixed bed; continuous perfused culture; detection; regulation and analysis of cell metabolism, growth rate and product synthesis balance; and product recovering and purification. The regulation and analysis of cell metabolism, growth rate and product synthesis balance includes the analysis of glucose, lactic acid, pH and other indexes, adding corresponding amino acid before the cell decline phase, decreasing glucose and blood serum concentration, etc. to realize high density and high yield culture. The product recovering andpurification inclues utilizing Streamline SP medium to recover antibody in supernatant, utilizing DEAE-Sepharose FF anionic exchanger to purify product, freeze drying.
Owner:陈志南
Extraction and purification method and application of fermented astragalus polysaccharide
InactiveCN104277133AEliminates the decolorization stepReduce distractionsOrganic active ingredientsDigestive systemAstragalus polysaccharideAlcohol
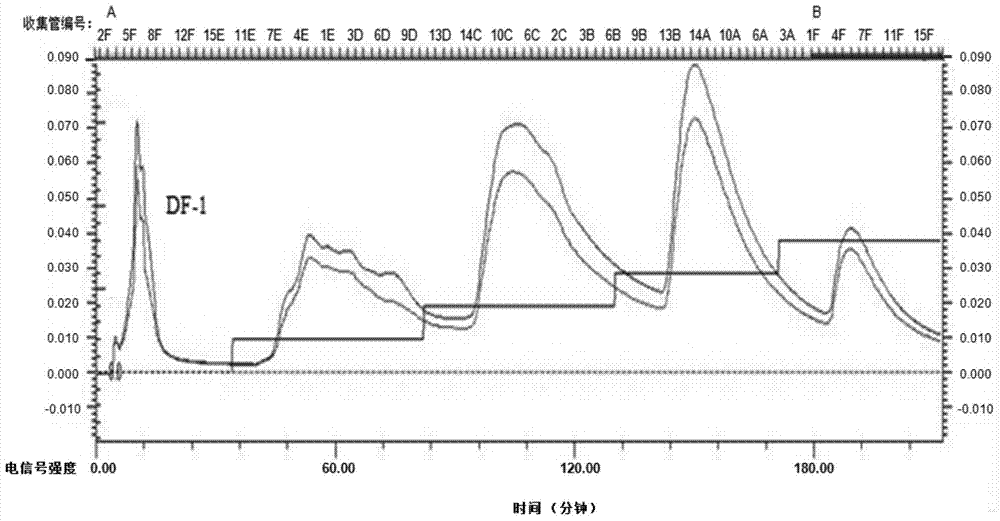
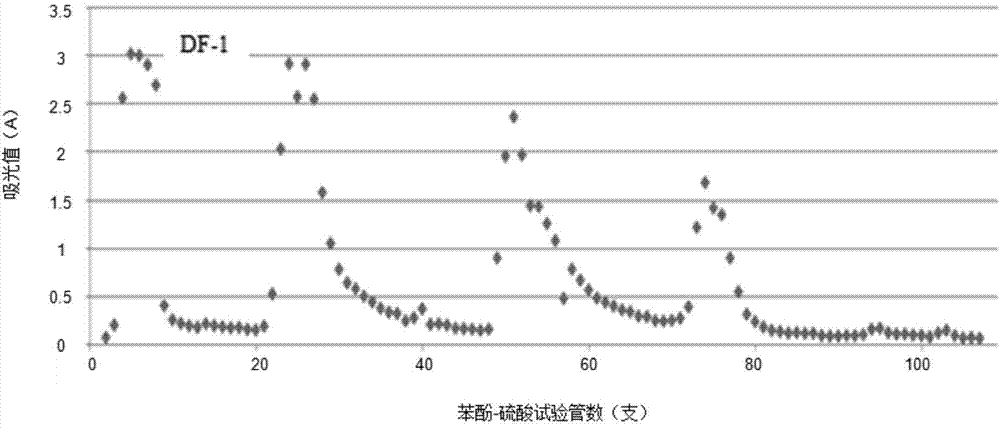
The invention provides an extraction and purification method and application of a fermented astragalus polysaccharide. The extraction and purification method of the fermented astragalus polysaccharide comprises the following steps of (1) carrying out warm immersion extraction on fermented astragalus, concentrating an extracting solution by using a membrane intercepting ultrafilter, removing protein through a Sevag method, and carrying out dialysis, alcohol precipitation and washing to obtain a crude fermented astragalus polysaccharide; (2) purifying the fermented astragalus polysaccharide by adopting DEAE Sepharose FastFlow, eluting by using ultrapure water, concentrating and freeze-drying an eluant to obtain a neutral polysaccharide DF-1, eluting by using gradient NaCl, and concentrating, dialyzing, concentrating and freeze-drying the eluant to obtain a weakly acidic polysaccharide; (3) purifying the neutral polysaccharide DF-1 obtained from the step (2) by adopting a Sephacryl S-400 High Reslution preparation chromatographic column, eluting by using ultrapure water, concentrating and freeze-drying the eluant to obtain neutral polysaccharides SF-1 and SF-2. Compared with a crude drug APS, the FAPS (Fermented Astragalus Polysaccharide) extracted by applying the extraction and purification method provided by the invention is outstandingly increased in yield and content.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Method for fast separating and purifying C-phycocyanin and isophycocyanin from blue algae
InactiveCN101240010AReduce manufacturing costHigh yieldPeptide preparation methodsDEAE-SepharoseLow ionic strength
A rapid separation and purification process of C-phycocyanin and allophycocyanin from blue algae, which pertains to separation and purification of phycocyanin technical field. The invention extracts C-phycocyanin and allophycocyanin by freeze dissolving and intensified swelling with low ions, finally carries out primary purification after precipitation with ammonia sulfate, elutes buffer liquid with constant ionic strength and pH gradient by DEAE Sepharose Fast Flow ionexchange chromatography, so that C-phycocyanin and allophycocyanin with high purity is obtained by one step. The process is easy in operation, time and energy saving, with little requirements to apparatus, high in yield. The process also dramatically lower preparation cost of CPC and APC, thus lays a foundation for CPC and APC application in ultrasensitive detection in biomedical.
Owner:SHANDONG UNIV
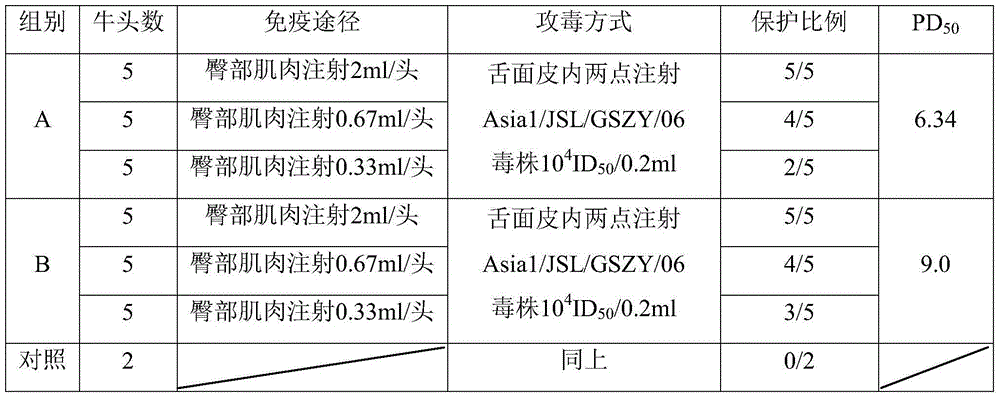
Preparation method of foot-and-mouth disease vaccines
InactiveCN104826097AImprove throughputNo undesired filter effectsAntiviralsAntibody medical ingredientsFiberAdjuvant
The invention provides a preparation method of foot-and-mouth disease vaccines. The method comprises the following steps: (1) filtering a cell culture fluid of viruses by using a deep filter device at a flow velocity of 300-500 L / m<2> / hr; (2) carrying out ultrafiltration and concentration on antigen liquid by using a hollow fiber column or membrane envelope; (3) enabling a virus antigen obtained by using the purification and concentration method to pass through DEAE-Sepharose FF and Sephawse6FF two-step chromatographic columns; (4) carrying out inactivation by using BEI, and blocking by using sodium thiosulfate; and (5) carrying out emulsification by using a 206 adjuvant. Due to vaccines prepared by using the method, immune animals can achieve a good immune effect, less adverse reaction and high safety.
Owner:北京必威安泰科技有限公司 +1
Preparing method for pig thrombin freeze-dried powder
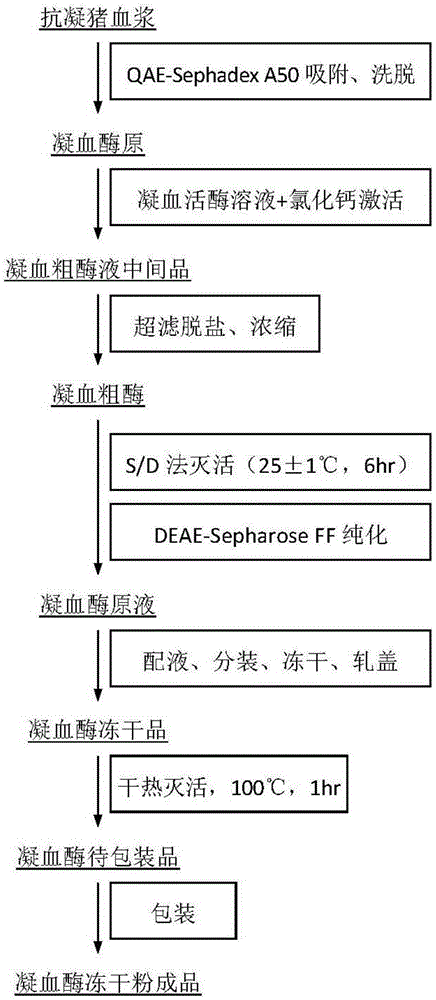
ActiveCN105250226AEffective inactivationImprove securityPowder deliveryPeptide/protein ingredientsFreeze-dryingDEAE-Sepharose
The invention discloses a preparing method for pig thrombin freeze-dried powder. The preparing method comprises the following steps that anticoagulant pig plasma and gel are mixed and stirred for adsorption and pass through a column for elution, and a prothrombin solution is obtained; normal saline is added to rabbit brain powder, then the prothrombin solution and CaCl2 are added for zymogen activation, a crude thrombin enzyme solution is obtained, subjected to ultrafiltration for desalination and concentrated, viral inactivation is carried out, an obtained crude enzyme solution passes through DEAE-Sepharose Fast Flow chromatographic column, elution is carried out, target peaks are collected, and pig thrombin is obtained; the pig thrombin is added with mannitol or dextran 40, filtering, sterilizing, subpackaging, freeze drying and vacuum tamponing are carried out, an aluminum plastic combined cover is rolled, and a pig thrombin freeze-dried product is obtained; the freeze-dried product is subjected to dry heat treatment at 100 DEG C for secondary virus inactivation, and the pig thrombin freeze-dried powder is obtained after packaging. The specific activity of pig thrombin in the product is not lower than 130 U / mg, the whole process is simple in step and easy to implement, the product safety is improved, and the preparing method is suitable for industrial production.
Owner:WUHAN HITECK BIOLOGICAL PHARMA
Preparation method for mung bean protein with effect of reducing blood fat
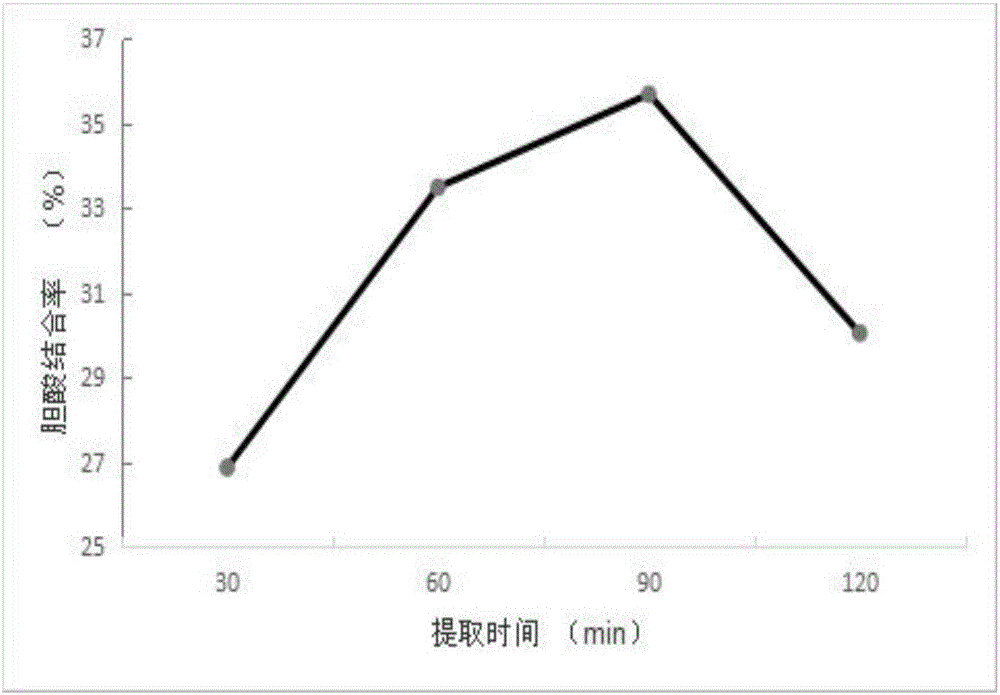
ActiveCN105924498ALow priceSimple extraction processMetabolism disorderPeptide preparation methodsCholic acidDEAE-Sepharose
The invention discloses a preparation method for mung bean protein with an effect of reducing blood fat. The preparation method comprises the following steps of: 1) taking clean mung bean, crushing and sieving, soaking for removing grease, drying, extracting a phosphate buffer, centrifugally collecting supernatant and salting out, thereby acquiring a mung bean protein extract; 2) performing anion exchange chromatography on the mung bean protein extract acquired in the step 1) and respectively collecting eluting peak of 280nm detection line; and 3) respectively performing in vitro cholic acid binding rate determination on the eluting peak acquired in the step 2) and collecting the target peak at the highest binding rate, thereby acquiring the target protein. The mung bean protein with the effect can be prepared according to the preparation method provided by the invention; the purified protein acquired after eluting and purifying DEAE-Sepharose Fast Flow is stronger in effect of reducing blood fat; the method has the advantages of functions of screening and purifying mung bean, simple rough protein extracting operation and convenience in wide-range popularization.
Owner:INST OF CROP SCI CHINESE ACAD OF AGRI SCI
Purification method of white gourd prolease
The invention relates to a purification method of wax gourd proteinase. The wax gourd is grinded, filtered and centrifuged; the supernatant is precipitated by ammonium sulfate with various saturation to obtain protein precipitation which is dissolved by TS buffer; through dialysis and DEAE-Sepharose FF ion exchange column gradient elution, 0.2mol / L NaCl eluate is collected for concentration and Superdex 75 gel filtration chromatography; the purified wax gourd proteinase with 65KD to 67KD molecular weight. The proteinase has significant casein hydrolysis activity; the best pH is 6.2 to 6.6 and the best temperature is 68 degree to 72 degree; the invention has good thermal stability; the proteinase still preserves 90 percent activity at 80 degree for 100 minutes. The invention can be applied in areas such as food processing, textile processing and medical healthcare etc.
Owner:惠静璇
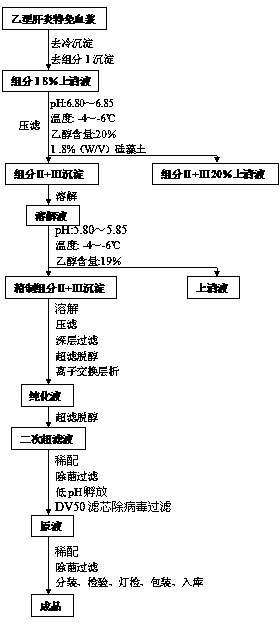
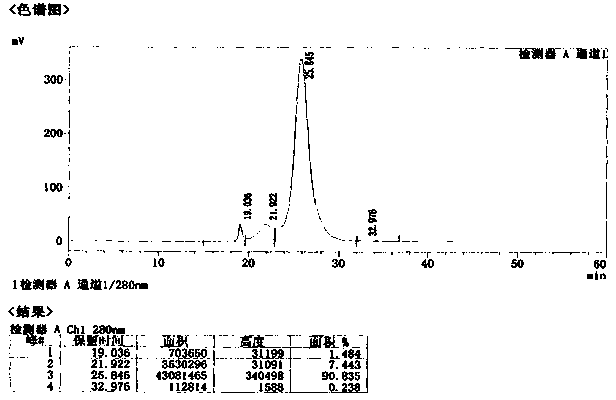
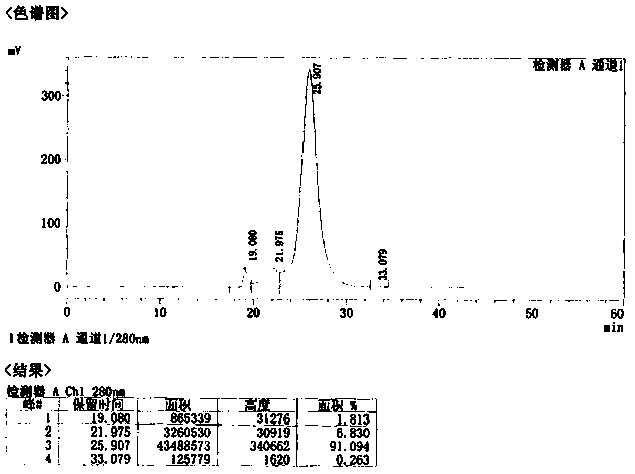
Preparation process of human hepatitis B immunoglobulin
ActiveCN104231075AEfficient removalImprove securityImmunoglobulins against virusesPeptide preparation methodsDEAE-SepharoseUltrafiltration
The invention discloses a preparation process of human hepatitis B immunoglobulin. The preparation process comprises the following steps: adopting plasma higher than the standard, and separating by a low-temperature ethanol method and a filter press technique to obtain sediment of components II and III; dissolving and carrying out dissolution and filter pressing to obtain refined sediment of components II and III; dissolving, adjusting reaction parameters, carrying out filter pressing, depth filtration, ultrafiltration and dialysis, and adjusting protein fluid parameters; carrying out column chromatography purification by using DEAE Sepharose Fast Flow weak anion exchange gel; carrying out ultrafiltration, and adjusting the pH to 4.05-4.15; adding maltose as a protecting agent; carrying out inactivation of low-pH inoculated viruses and virus removal by a DV20 filter element, carrying out secondary ultrafiltration, and controlling the maltose residue to be not higher than 2g / L; and then adding glycine as a protecting agent and the like, so as to prepare the specific human hepatitis B immunoglobulin of which the purity is over 99.0% and the valence is not lower than 100 IU / ml, wherein the total content of IgG monomer and dimer in the specific human hepatitis B immunoglobulin is over 98.0%. Compared with a traditional low-temperature ethanol method, the preparation process can improve the yield of human hepatitis B immunoglobulin by 7.5%-10%.
Owner:华润博雅生物制药集团股份有限公司
Preparation method of human serum albumin
ActiveCN102816230AImprove utilizationReduce demandSerum albuminPeptide preparation methodsSerum protein albuminDEAE-Sepharose
The invention relates to a preparation method of a human serum albumin. The human serum albumin is extracted and purified from a waste component IV precipitate separated from human plasma, and thus the comprehensive utilization of plasma can be improved. The preparation method comprises the following steps of: 1, dissolving the component IV precipitate and then press-filtering and separating to obtain a filtrate A; 2, adding polyethylene glycol while stirring the filtrate A, and press-filtering and separating to obtain a filtrate B; 3, adjusting the pH of the filtrate B, controlling the temperature of the filtrate B, and press-filtering and separating to obtain a filtrate C; 4, adding the polyethylene glycol while stirring the filtrate C to obtain a reaction solution C, press-filtering and separating to obtain a precipitate; 5, dissolving the precipitate, carrying out DEAE Sepharose fast flow weak anion exchange chromatography, and ultrafiltering; and 6, adding sodium caprylate after the concentration of protein in an ultra-filtrate is diluted, adjusting the pH to 6.8 to 7.0, sterilizing and filtering, and carrying out pasteurized inactivation to obtain a human serum albumin finished product.
Owner:TONROL BIOLOGICAL PHARM CO LTD
Extracellular antiseptic protein for Bacillus subtilis and its separation and purification method
InactiveCN1974597APreserve natural qualitiesBroad spectrum of biological functionsHydrolasesMicroorganism based processesDEAE-SepharoseRNA - Ribonucleic acid
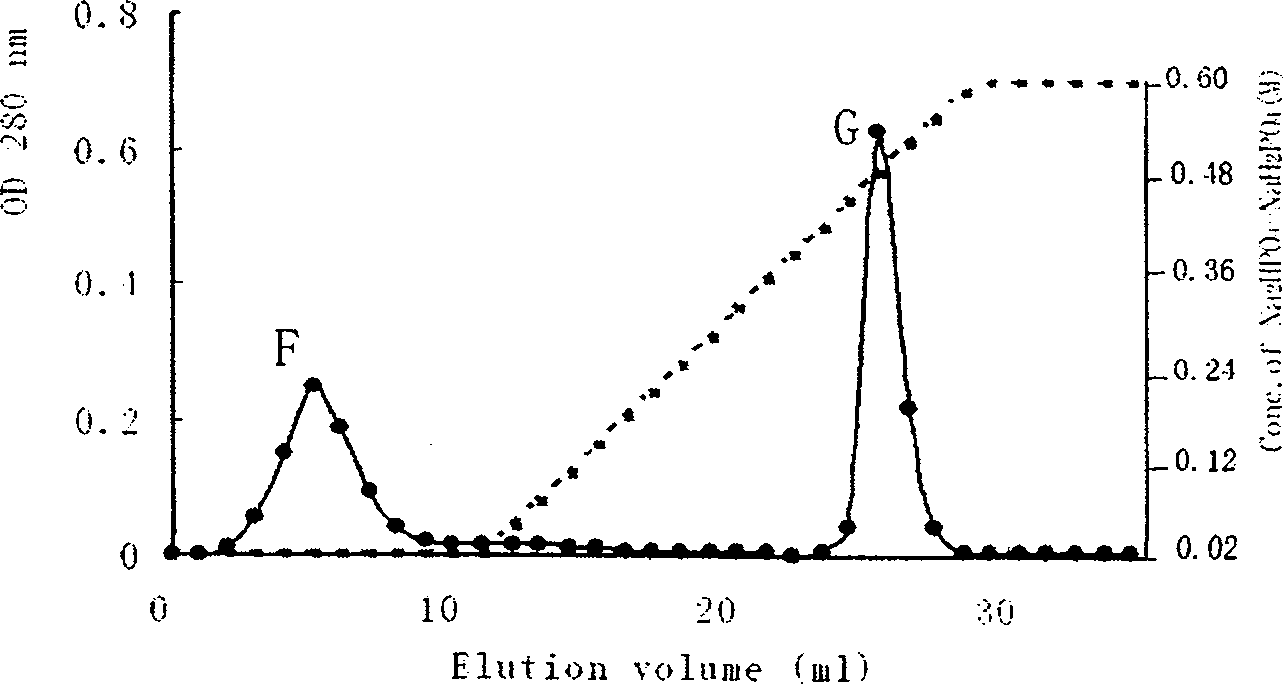
The present invention relates to one kind of extracellular antiseptic protein of Bacillus subtilis and its separation and purification process. The extracellular antiseptic protein has molecular weight of 41.9 kD, 26 mass-to-charge ratio peaks, and 5 peptide amino acid sequences including VTIVDEKGR, FSFSDVHNR, VYIADSTNFK, ELPISENLASVNMR and EAEWAYMITGK. Its separation and purification process includes the following steps: separating coarse protein from Bacillus subtilis Bs-916 via DEAE Sepharose F.F. separation, separating its B peak drips via Phenyl Sepharose 6 F.F. separation, separating the E peak drips via with hydroxyapatite column and obtaining the G peak drips as the purified extracellular antiseptic protein. The extracellular antiseptic protein has broad spectrum antiseptic activity, ribonuclease activity and agglutinin activity.
Owner:JIANGSU ACAD OF AGRI SCI
Cuttlebone polysaccharide CPS-1 and its preparation and use
The invention discloses a cuttle-bone polysaccharide CPS-1 and preparing method and usage in the medical domain, which is characterized by the following: boiling cuttle-bone powder to get raffinate; depositing raffinate; dialyzing; cooling; drying; getting cuttle-bone polysaccharide crude product; separating and purifying by DEAE Sepharose F.F ion exchange column, Sephacryl S-300 molecule coagulation column and Sepharose CL-6B coagulation column; getting cuttle-bone polysaccharide CPS-1. The invention can treat ulcerative colitis, which can be used for preparing the medicine of treating ulcerative colitis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for extracting purified high ferro myoglobins from cardiac muscle
InactiveCN1800202AHigh purityHigh economic valueHaemoglobins/myoglobinsPeptide preparation methodsFiltrationDEAE-Sepharose
The invention relates to a protein extracting purify craft which is a method for extracting and purifying ferrihemoglobin. It dose first treatment to the fresh animal's myocardium, obtains the vegetable protein course extracting matter by ejection; the course matter uses sulfuric acid diammonium salt out and the dialyzing treatment with trapping molecular weight 14000 to obtain the dialysis example; the dialysis example uses DEAE-Sepharose Fast Flow anion exchange color printing to collect the brunneus eluent; it collects the eluent and uses Sephacryl S-200HR gel filtration chromatography to collect the brunneus eluent which is corresponding to the clean peek to obtain the ferrihemoglobin.
Owner:NANJING NORMAL UNIVERSITY
Ahylysantinfarctase 36KD single-stranded haemocoagulase and its preparing method
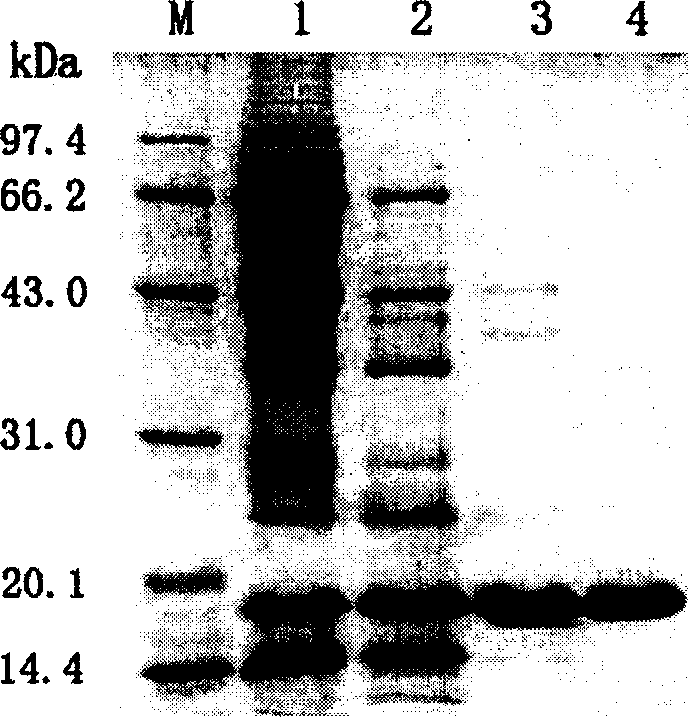
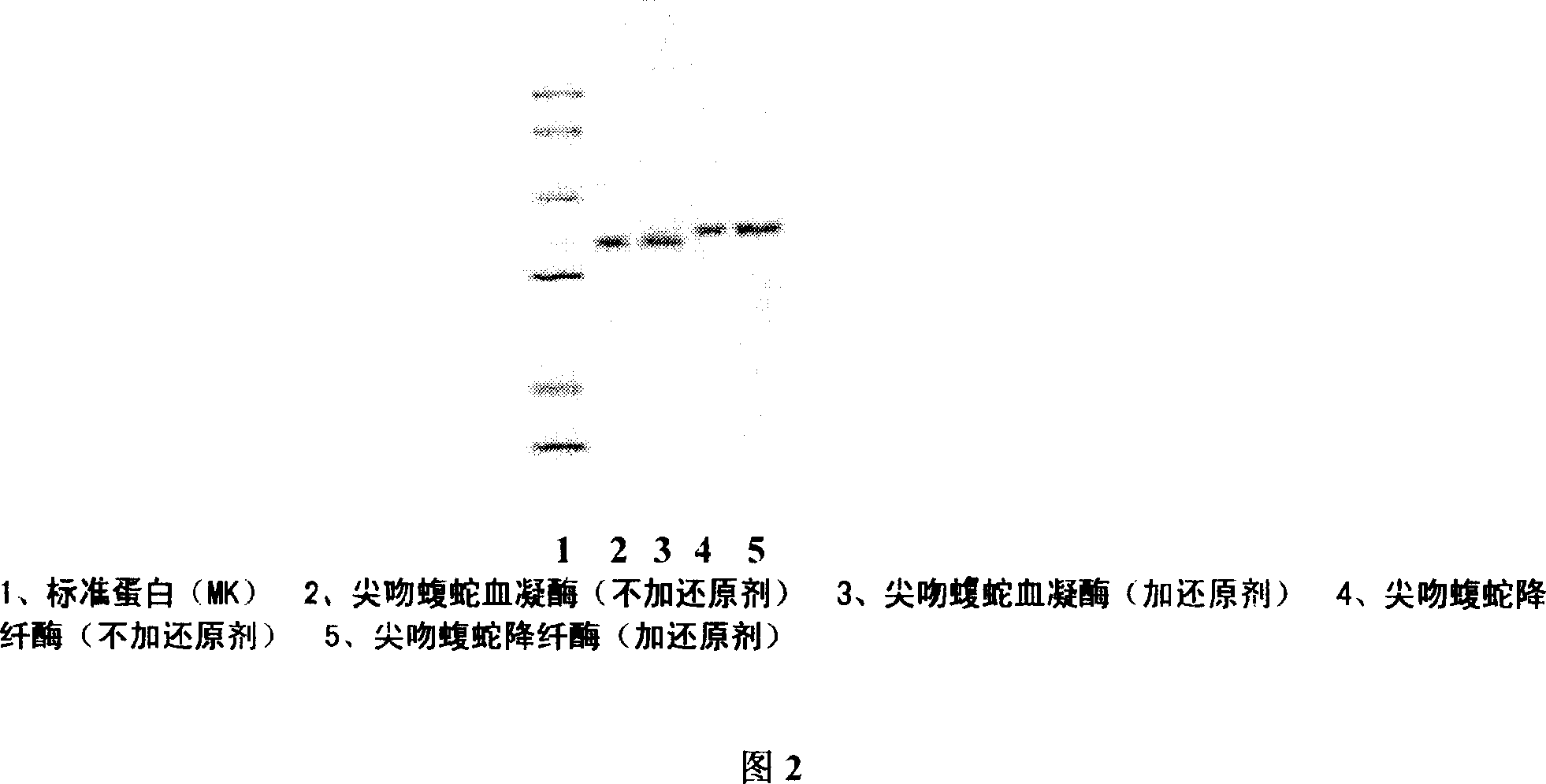
The present invention is Agkistrodon acutus venon 36KD single-stranded hemocoagulase and its preparation process, and aims at providing single-stranded Agkistrodon acutus venon hemocoagulase with high potency and low toxicity. SDS-PAGE electrophoresis shows that the hemocoagulase has molecular weight of 36KD+ / -2KD, single color zone, and at least 90 % homogeneity with the N end sequence of the single-stranded amino acid shown in SEQ ID No. 1. The preparation process includes dissolving Agkistrodon acutus venon in Tris-HCl buffering solution (pH8.0) and dialysis; adding the supernatant to Metal Sepharose F.F affinity chromatographic column and collecting the penetrating peak component; adding the penetrating peak component to DEAE-Sepharose F.F ion exchange column and collecting active peak component; separating in Superdex 75 column and Sephacryl S-100 column; desalting in Sephadex G-25 column; sterilizing and freeze drying.
Owner:沈居仁 +2
Extracellular antiseptic protein for Bacillus subtilis and its separation and purification method
InactiveCN100463919CPreserve natural qualitiesBroad spectrum of biological functionsHydrolasesMicroorganism based processesPurification methodsDEAE-Sepharose
The present invention relates to one kind of extracellular antiseptic protein of Bacillus subtilis and its separation and purification process. The extracellular antiseptic protein has molecular weight of 41.9 kD, 26 mass-to-charge ratio peaks, and 5 peptide amino acid sequences including VTIVDEKGR, FSFSDVHNR, VYIADSTNFK, ELPISENLASVNMR and EAEWAYMITGK. Its separation and purification process includes the following steps: separating coarse protein from Bacillus subtilis Bs-916 via DEAE Sepharose F.F. separation, separating its B peak drips via Phenyl Sepharose 6 F.F. separation, separating the E peak drips via with hydroxyapatite column and obtaining the G peak drips as the purified extracellular antiseptic protein. The extracellular antiseptic protein has broad spectrum antiseptic activity, ribonuclease activity and agglutinin activity.
Owner:JIANGSU ACAD OF AGRI SCI
Prepn. of blood coagulation factor IX compound
InactiveCN1336178AHigh recovery rateReduce contentPeptide/protein ingredientsMammal material medical ingredientsPhosphateDEAE-Sepharose
The present invention relates to the preparation of plasma thromboplastin antecedent IX complex phosphate buffer solution or citrate buffer solution with 4-7 milli mole concertration and pH 5.5-6.5 is used to balance the gel used for adsorption, with fresh frozen human plasmia being added. DEAE-sepharose Fast Flow is used to proceed ion exchange adsorption separation, then same buffer solution with pH 5.5-7.0 is used to wash adsorption gel. further same buffer solution with pH 6-8 is used to elute adsorption gel, collect plasma thromboplastin antecedent IX complex containing factor II, VII, IX and X.
Owner:四川高维系统工程技术有限公司 +2
Process for purifying human retinol binding protein and preparation process of polyclonal antibody thereof
ActiveCN104193817APreserve immunogenicitySerum immunoglobulinsImmunoglobulins against animals/humansSeparation technologyDEAE-Sepharose
The invention discloses a process for purifying a human retinol binding protein and a preparation process of a polyclonal antibody thereof. According to the invention, by using a separation technology by multiple chromatographic columns such as a DEAE-Sepharose Fast Flow (DEAE-S) ion exchange column, a molecular sieve (Superdex 75, Sephacryls-200) and a hydrophobic column Phenyl SepharoseTM High Performance (PHSP), the human retinol binding protein is successfully purified from urine of a patient suffering from renal injury, and the immunogenicity of the RBP protein is remained to the greatest degree. The obtained human RBP antigen is used for immunizing animals, thus obtaining a polyclonal antibody of the human retinol binding protein. The obtained polyclonal antibody of the human retinol binding protein can be applied to an immunonephelometry kit.
Owner:桂林英美特生物技术有限公司
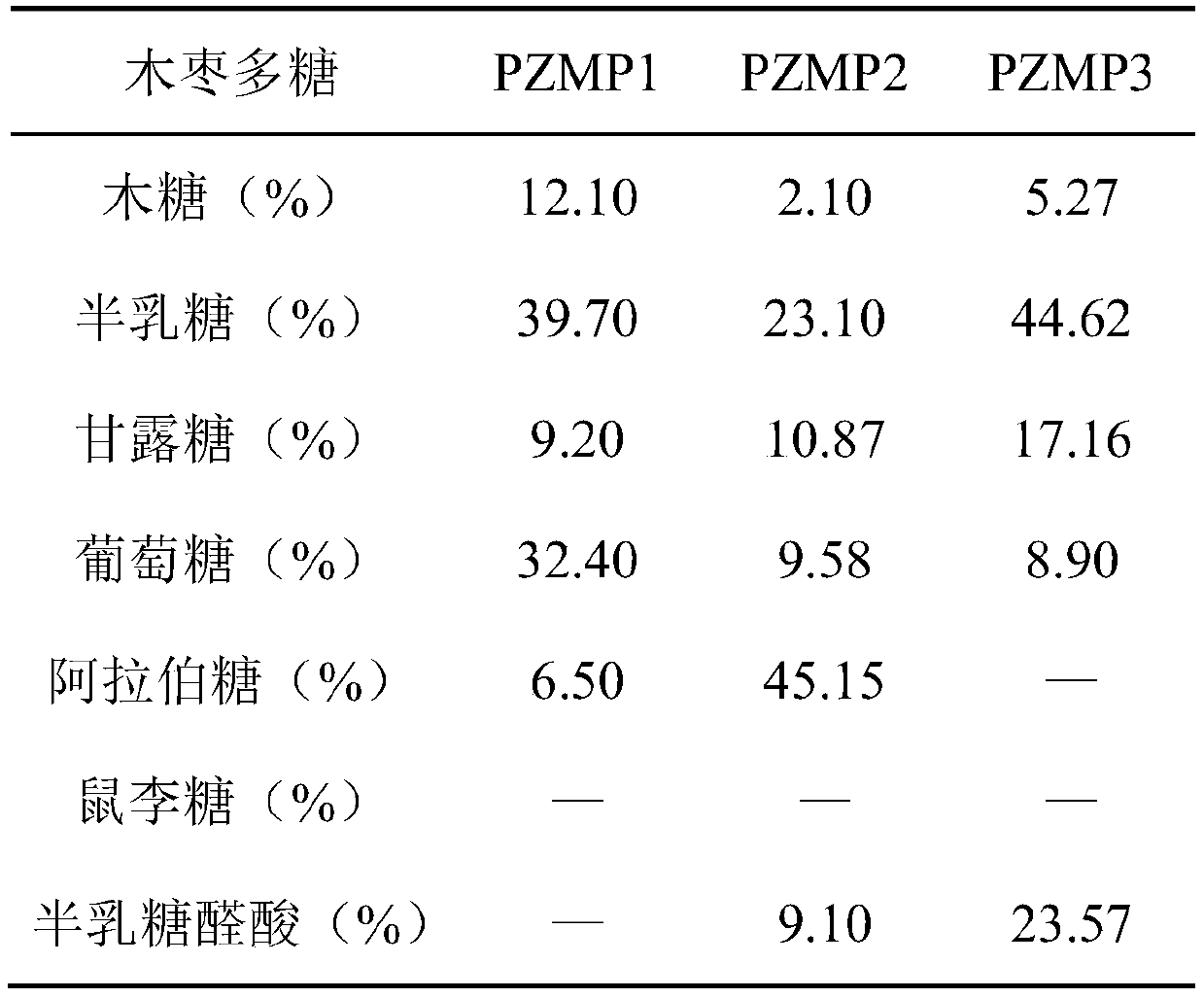
Separation and purification method of jujube polysaccharide with antioxidant effect
ActiveCN110606900APromote sustainable developmentHigh purityFood ingredient functionsPolysaccharide/gum food ingredientsPurification methodsDEAE-Sepharose
The invention provides a separation and purification method of jujube polysaccharide with an antioxidant effect. The method comprises the following steps: separating and purifying the jujuba polysaccharide; the method for separating and purifying the jujube polysaccharide comprises the following steps: separating and purifying jujube polysaccharide; enabling the jujuba polysaccharide to pass through a DEAE Sepharose Fast Flow anion exchange column, sequentially carrying out gradient elution by using a PBS solution and a NaCl-PBS solution for separation, and purifying the jujuba polysaccharideby using a Sepharol S-300 gel column. According to the preparation method disclosed by the invention, three jujube antioxidant active polysaccharides, PZMP1, PZMP2 and PZMP3, can be obtained through one-time separation and purification; the three jujube antioxidant active polysaccharides obtained through separation and purification are high in purity, the purity can reach 90% or above, the yield is high, the jujube crude polysaccharides serve as the base number, the yield of PZMP1 is 2.95%, the yield of PZMP2 is 14.15%, and the yield of PZMP3 is 14.61%. In addition, the three kinds of jujube antioxidant active polysaccharides obtained through separation and purification have high oxidation resistance, raw materials are provided for antioxidant functional food, and sustainable development of jujubes is promoted.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Preparation method of chitosanase
InactiveCN108531418ALow costSimplify separation and purification stepsBacteriaMicroorganism based processesDEAE-SepharoseFreeze-drying
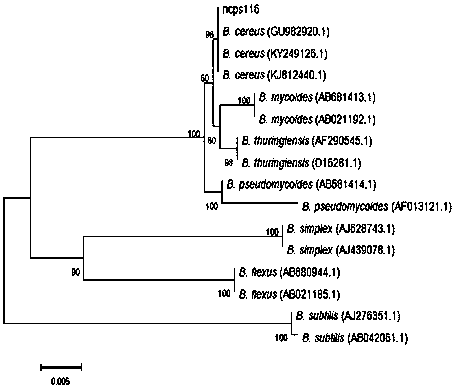
The invention discloses a preparation method of cihtosanase. Bacillid cereus 116 is used as starting strain, the extracellular chitosanase is prepared by optimizing a culture condition by virtue of liquid fermentation, the enzymatic activity of the chitosanase in the fermented solution can reach 40 to 80 U / mL, the fermented solution is frozen and centrifuged and precipitated level by level by virtue of ammonium sulfate, and the solid chitosanase is obtained by virtue of DEAE-Sepharose Fast Flow ion-exchange column chromatography, concentration and freeze drying.
Owner:INST OF AGRO FOOD SCI & TECH SHANDONG ACAD OF AGRI SCI
Chorionic gonadotrophin purification method
PendingCN105968185ASimple purification processEasy to purifyDepsipeptidesPeptide preparation methodsAnimal scienceDEAE-Sepharose
The invention relates to a chorionic gonadotrophin purification method which adopts three times of chromatography for purification, wherein the three times of chromatography includes affinity chromatography, hydrophobic chromatography and ion-exchange chromatography, and finally, a high-purity chorionic gonadotrophin solid is obtained. The filler of the affinity chromatography is capto blue (high sub), the filler of the hydrophobic chromatography is Phenyl Sepharose 6 Fast Flow (high sub), and the filler of the ion-exchange chromatography is DEAE Sepharose Fast Flow. According to the chorionic gonadotrophin purification method, the purification process is optimized, the purification flows are simplified, the yield is obviously increased, the titer is higher than that of the existing process, the floor area of the equipment is reduced, and the energy consumption of a workshop is reduced. In the invention, the titer of preprocessed chorionic gonadotrophin is about 200iu / mg, and the titer of chorionic gonadotrophin processed by three times of chromatography can reach 10000iu / mg, so that the titer is obviously increased.
Owner:宁波人健药业集团股份有限公司
Enzymolysis-modified sargassum horneri polysaccharide and application thereof
The invention discloses an enzymolysis-modified sargassum horneri polysaccharide and an application thereof. A preparation method of the enzymolysis-modified sargassum horneri polysaccharide comprises steps as follows: (1) performing microwave extraction on a sargassum horneri polysaccharide to obtain a coarse polysaccharide extract; (2) performing grading alcohol precipitation: performing precipitation on the coarse polysaccharide extract with the final ethanol volume fraction of 30-40% and 55-65% successively to obtain an SHP60 coarse polysaccharide component; (3) performing decolorization and deproteinization on the SHP60 coarse polysaccharide component to obtain an SHPA polysaccharide; (4) performing enzymolysis on the SHPA polysaccharide with a dextranase to obtain an enzymatic product; (5) performing ion column chromatography on the enzymatic product with a DEAE-Sepharose Fast Flow ion column chromatography system, and concentrating and filtering an eluent to obtain a filtrate; (6) performing gel column chromatography on the filtrate obtained in Step (5) with a Sephadex G-25 gel chromatography system, collecting a salt-free polysaccharide part, and performing concentration and freeze-drying to obtain the enzymolysis-modified sargassum horneri polysaccharide. The enzymolysis-modified sargassum horneri polysaccharide can be applied to preparation of an antioxidant, a moisture absorbent and a moisturizer.
Owner:ZHEJIANG UNIV OF TECH
Agkistrodonacutus thrombin preparation method and uses
The invention discloses a preparation method for Hemocoagulase of Agkistrodon acutus peculiar to China, which comprises dissolving, centrifuging at low temperature, dialyzing, DEAE-Sepharose FF chromatography, and Sephadex G25 chromatography. The product comprises A and B subunits with molecular weight as 15.0KD and 14.2KD respectively, and the amino acid sequences of first to twenty-five sites are DCSSGWSSYEGHCYKVFKQSKTWTA and DCPSDWSSYECHCYKPDEPKTWEDA respectively. This product can be used to haemostasis in clinic.
Owner:BEIJING KONRUNS PHARM CO LTD
Method for extracting CEL I nuclease in celery
InactiveCN101538561ANo significant difference in purityNo significant difference in activityHydrolasesSulfite saltFiltration
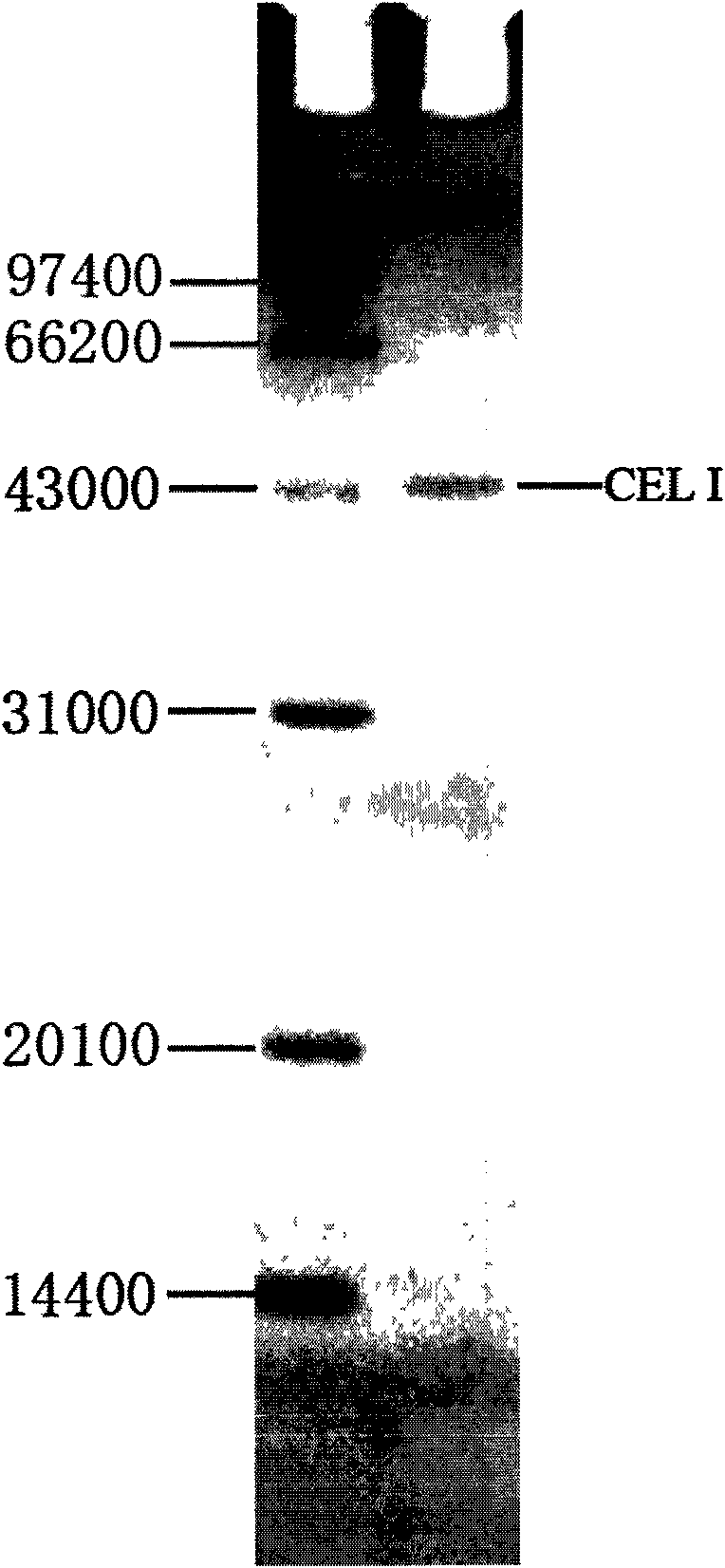
The invention relates to a method for extracting CEL I nuclease in celery and discards measures adopting a plurality of high-speed or ultra-speed centrifugation and a plurality of gel filtration and ion exchange chromatography in the prior art. The method comprises the following steps: adding a small amount of sodium sulfite to extract so as to reduce and clear colored substances in the extract; adopting a thermal denaturation physical method to rapidly clear a great amount of foreign protein with poor temperature toleration; utilizing the characteristic that CEL I is the combination of glycoprotein and activated concanavalin to combine CEL I nuclease and concanavalin so as to remove the foreign protein and further purify the CEL I; and finally, selecting DEAE-Sepharose FF as a suitable medium for the CEL I by once chromatography purification. Accordingly, the extraction method replaces the time-consuming and expensive measures adopting a plurality of high-speed even ultra-speed refrigerated centrifugation, a plurality of gel filtration and ion exchange chromatography, and the like and achieves the aims that the CEL I nuclease is rapidly extracted from the celery and purified.
Owner:GUANGZHOU UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com