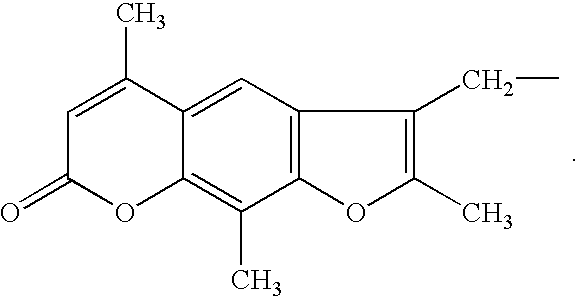
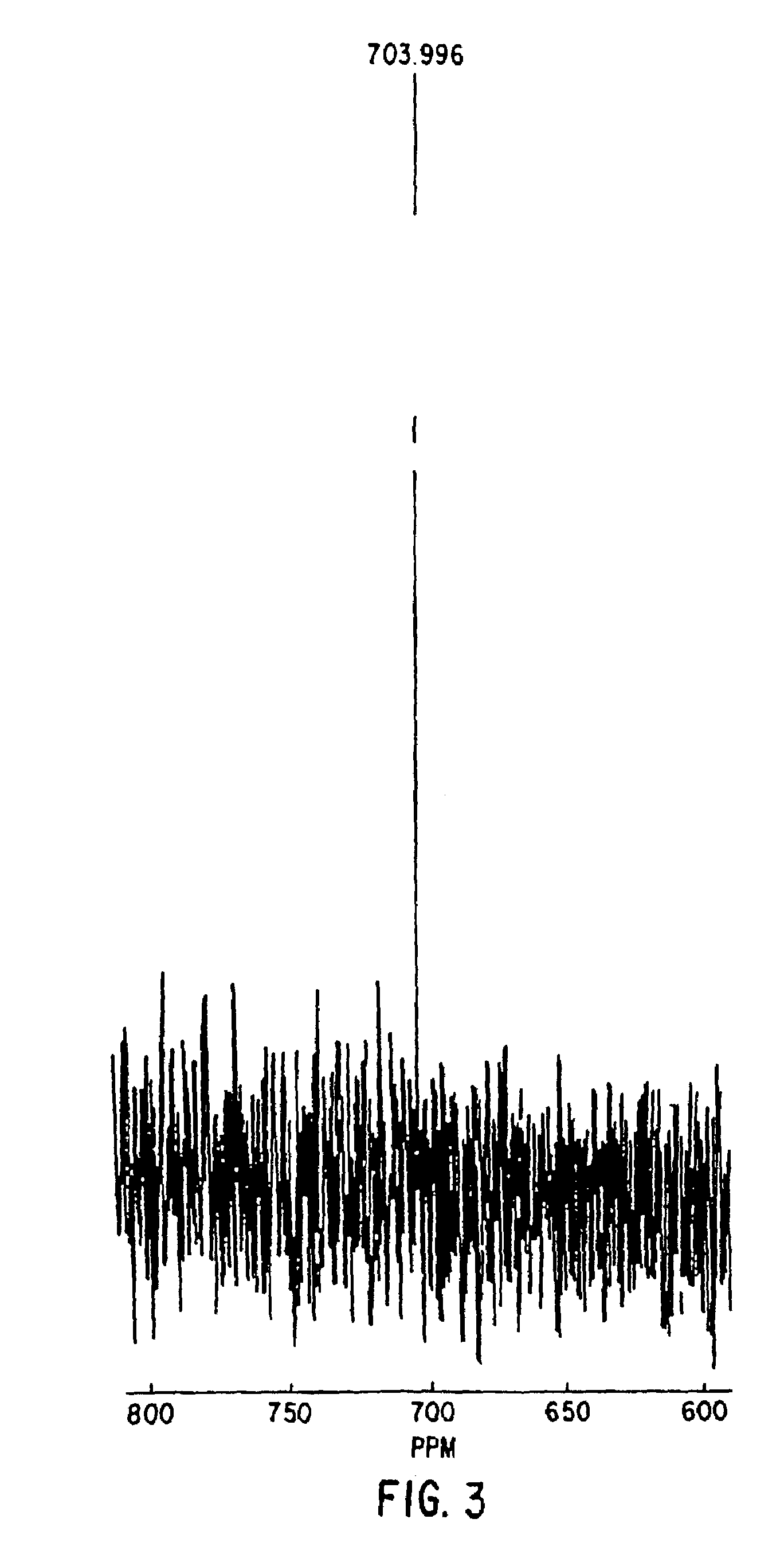
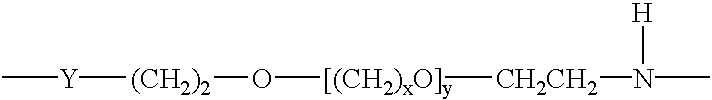Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
515results about "Haemoglobins/myoglobins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Kits for amplifying and detecting nucleic acid sequences, including a probe
InactiveUS6040166AStrong specificityReduce manpowerNanotechSequential/parallel process reactionsOligonucleotide primersNucleic acid sequencing
The present invention is directed to a process for amplifying any target nucleic acid sequence contained in a nucleic acid or mixture thereof using a thermostable enzyme. The process comprises treating separate complementary strands of the nucleic acid with a molar excess of two oligonucleotide primers, extending the primers with a thermostable enzyme to form complementary primer extension products which act as templates for synthesizing the desired nucleic acid sequence, and detecting the sequence so amplified. The steps of the reaction can be repeated as often as desired and involve temperature cycling to effect hybridization, promotion of activity of the enzyme, and denaturation of the hybrids formed.
Owner:ROCHE MOLECULAR SYST INC
Method and device for electrochemical immunoassay of multiple analytes
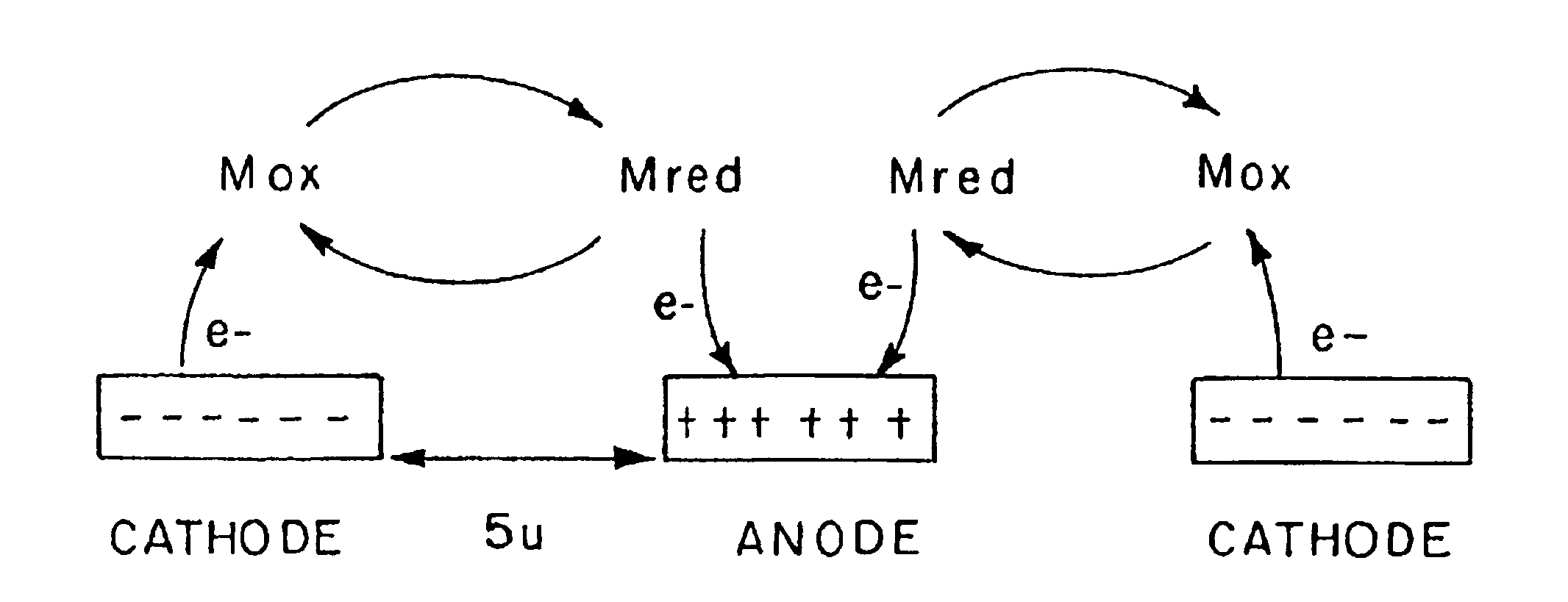
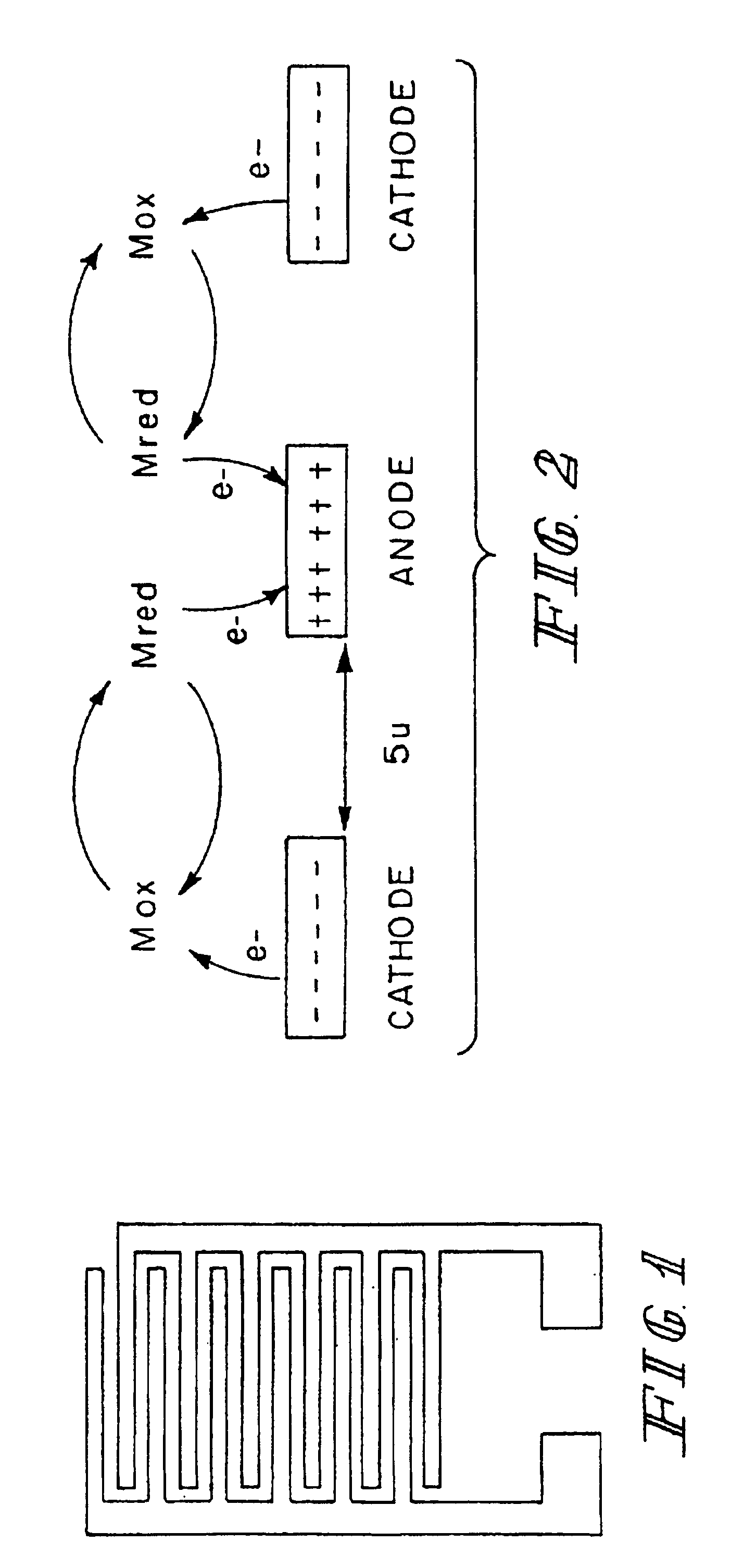
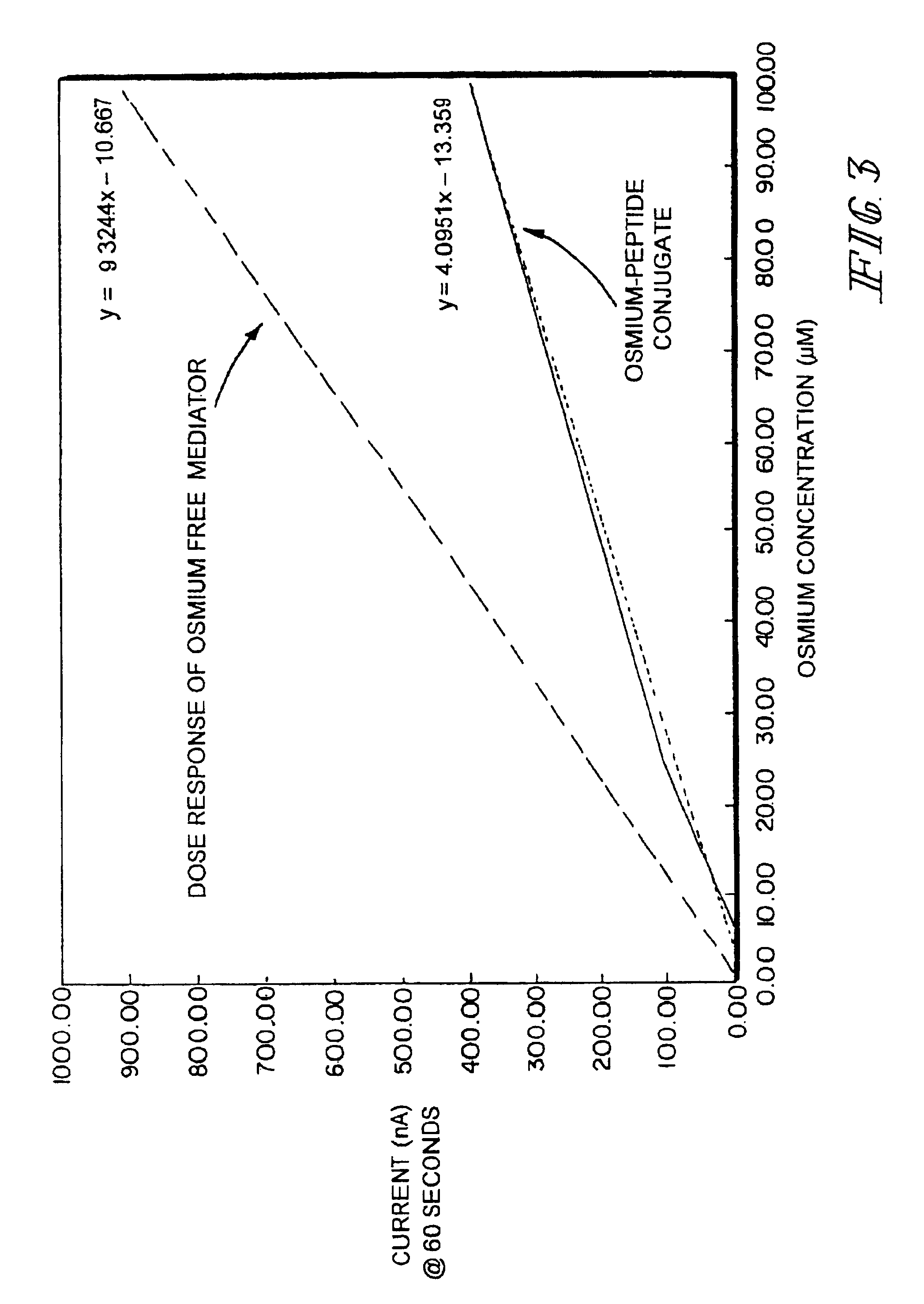
InactiveUSRE40198E1High measurement accuracyImprove accuracyImmobilised enzymesBioreactor/fermenter combinationsAnalyteRedox
Owner:ROCHE DIABETES CARE INC
Kits for amplifying and detecting nucleic acid sequences
InactiveUS6514736B1Strong specificityReduce manpowerNanotechSequential/parallel process reactionsOligonucleotide primersNucleic acid sequencing
The present invention is directed to a process for amplifying any target nucleic acid sequence contained in a nucleic acid or mixture thereof using a thermostable enzyme. The process comprises treating separate complementary strands of the nucleic acid with a molar excess of two oligonucleotide primers, extending the primers with a thermostable enzyme to form complementary primer extension products which act as templates for synthesizing the desired nucleic acid sequence, and detecting the sequence so amplified. The steps of the reaction can be repeated as often as desired and involve temperature cycling to effect hybridization, promotion of activity of the enzyme, and denaturation of the hybrids formed.
Owner:ROCHE MOLECULAR SYST INC
Method for the preparation of a heat stable oxygen carrier-containing pharmaceutical composition
ActiveUS7932356B1Increase oxygenationHigh sensitivityPeptide/protein ingredientsMammal material medical ingredientsArteriolar VasoconstrictionCross-link
A highly purified and heat stable cross-linked nonpolymeric tetrameric hemoglobin suitable for use in mammals without causing renal injury and vasoconstriction is provided. A high temperature and short time (HTST) heat processing step is performed to remove undesired dimeric form of hemoglobin, uncross-linked tetrameric hemoglobin, and plasma protein impurities effectively. Addition of N-acetyl cysteine after heat treatment and optionally before heat treatment maintains a low level of met-hemoglobin. The heat stable cross-linked tetrameric hemoglobin can improve and prolong oxygenation in normal and hypoxic tissue. In another aspect, the product is used in the treatment of various types of cancer such as leukemia, colorectal cancer, lung cancer, breast cancer, liver cancer, nasopharyngeal carcinoma and esophageal cancer. Another application is heart preservation in situations where there is a lack of oxygen supply in vivo, such as in heart transplant or oxygen-deprived heart.
Owner:BILLION KING INT
Reduced side-effect hemoglobin compositions
The invention relates to novel hemoglobin compositions, particularly novel recombinant mutant hemoglobin compositions, which eliminate or substantially reduce 1) the creation of heart lesions, 2) gastrointestinal discomfort, 3) pressor effects, and 4) endotoxin hypersensitivity associated with the administration of extracellular hemoglobin compositions in various therapeutic applications. Applications described include treatments for anemia, head injury, hemorrhage or hypovolemia, ischemia, cachexia, sickle cell crisis and stroke; enhancing cancer treatments; stimulating hematopoiesis; improving repair of physically damaged tissues; alleviating cardiogenic shock; and shock resuscitation.
Owner:BAXTER INT INC +1
Hemoglobin mutants with increased soluble expression and/or reduced nitric oxide scavenging
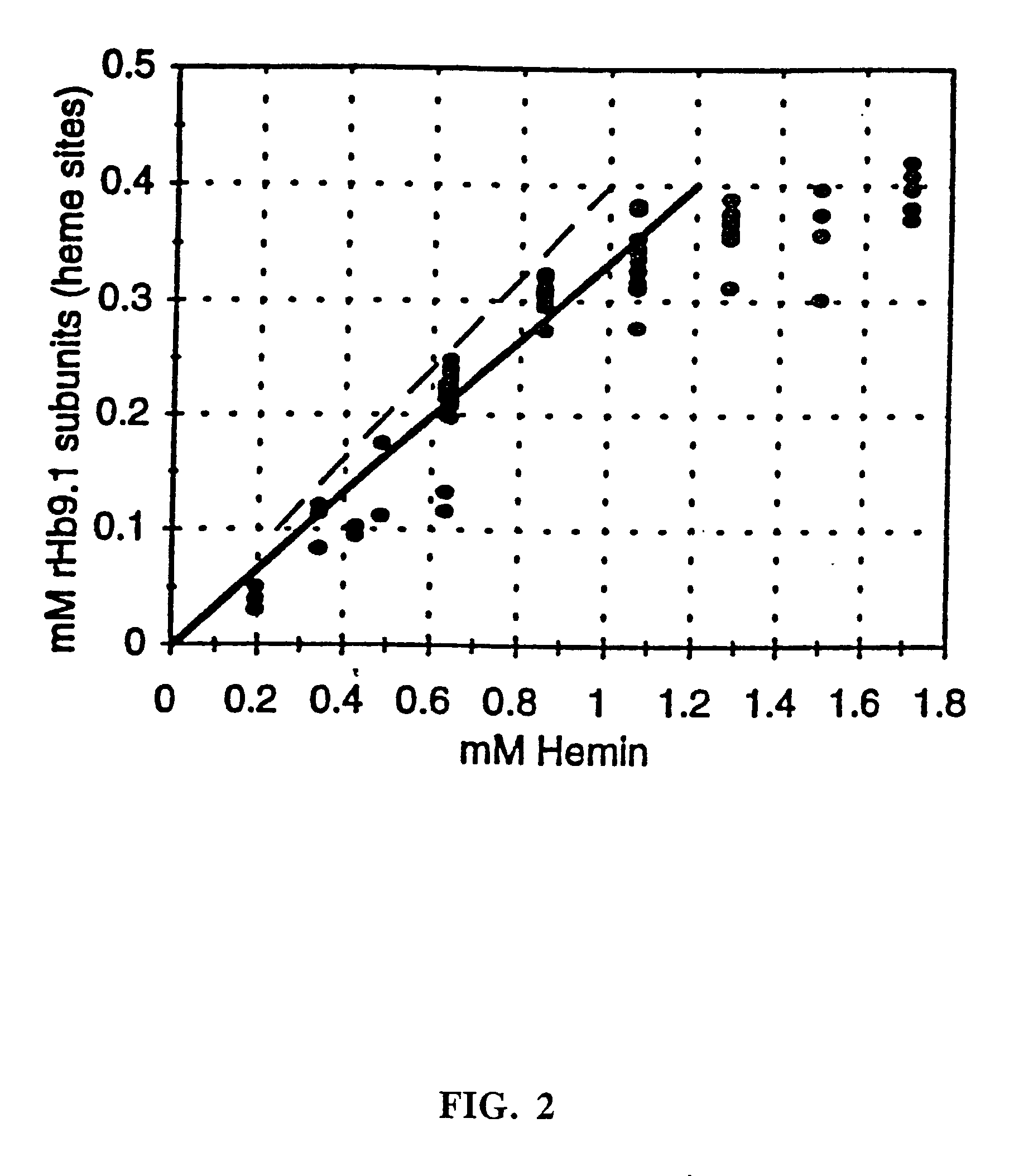
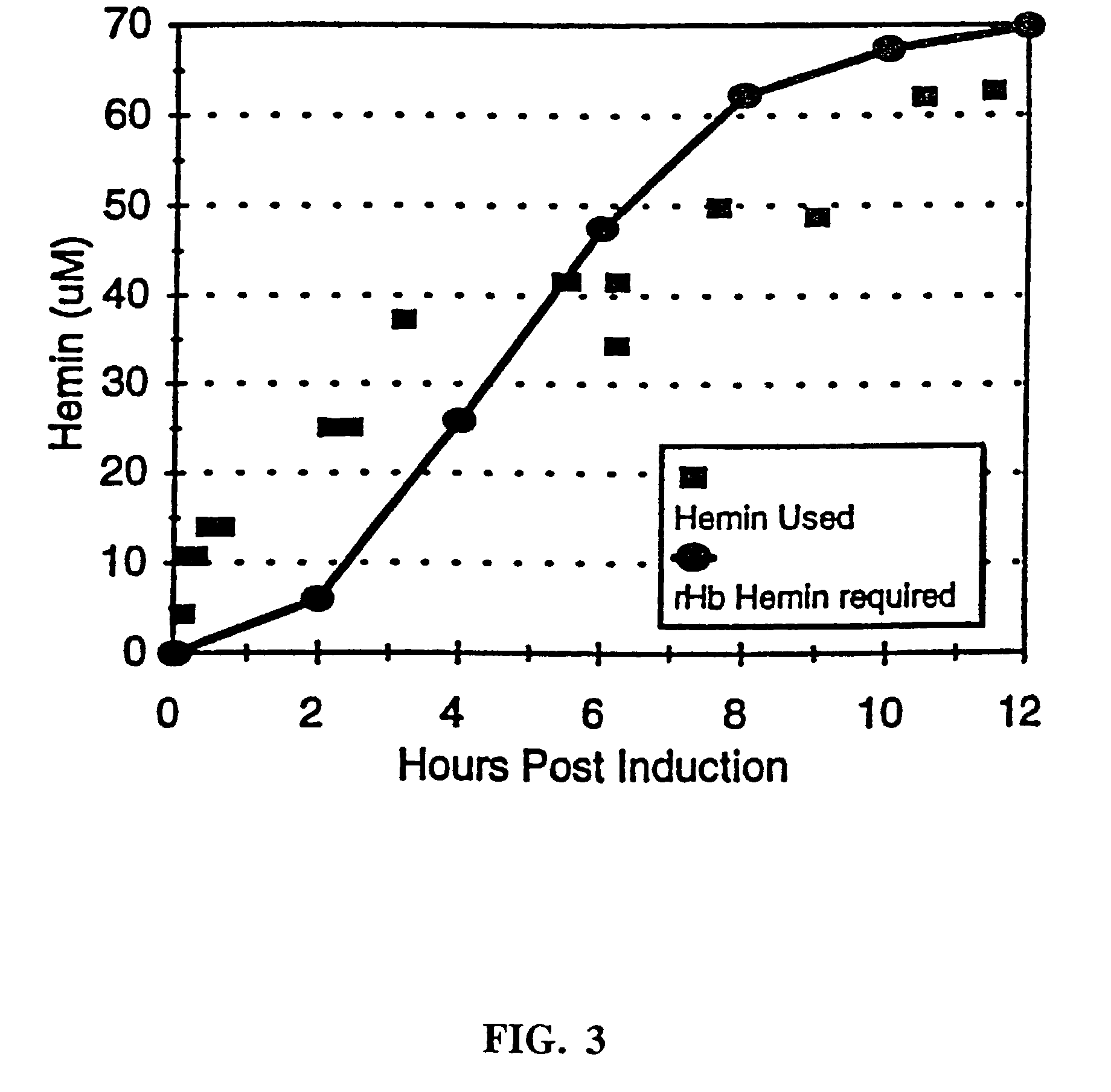
The invention relates to novel recombinant hemoglobins having reduced nitric oxide scavenging and / or increased high soluble expression. The invention further relates to methods of increasing the soluble expression of recombinant hemoglobin by adding exogenous hemin in molar excess of the heme binding sites of recombinant hemoglobin.
Owner:BAXTER BIOTECH TECHNOLOGY SARL +1
Therapeutic retroviral vectors for gene therapy
InactiveUS7901671B2Maintaining therapeutic levelImprove the level ofBiocidePeptide/protein ingredientsHematopoietic cellRed blood cell
Owner:MASSACHUSETTS INST OF TECH
Pharmaceutical Composition Comprising An Immunoglobulin FC Region as a Carrier
Disclosed is a novel use of an immunoglobulin Fc fragment, and more particularly, a pharmaceutical composition comprising an immunoglobulin Fc fragment as a carrier. The pharmaceutical composition comprising an immunoglobulin Fc fragment as a carrier remarkably extends the serum half-life of a drug while maintaining the in vivo activity of the drug at relatively high levels. Also, when the drug is a polypeptide drug, the pharmaceutical composition has less risk of inducing immune responses compared to a fusion protein of the immunoglobulin Fc fragment and a target protein, and is thus useful for developing long-acting formulations of various polypeptide drugs.
Owner:HANMI SCI CO LTD
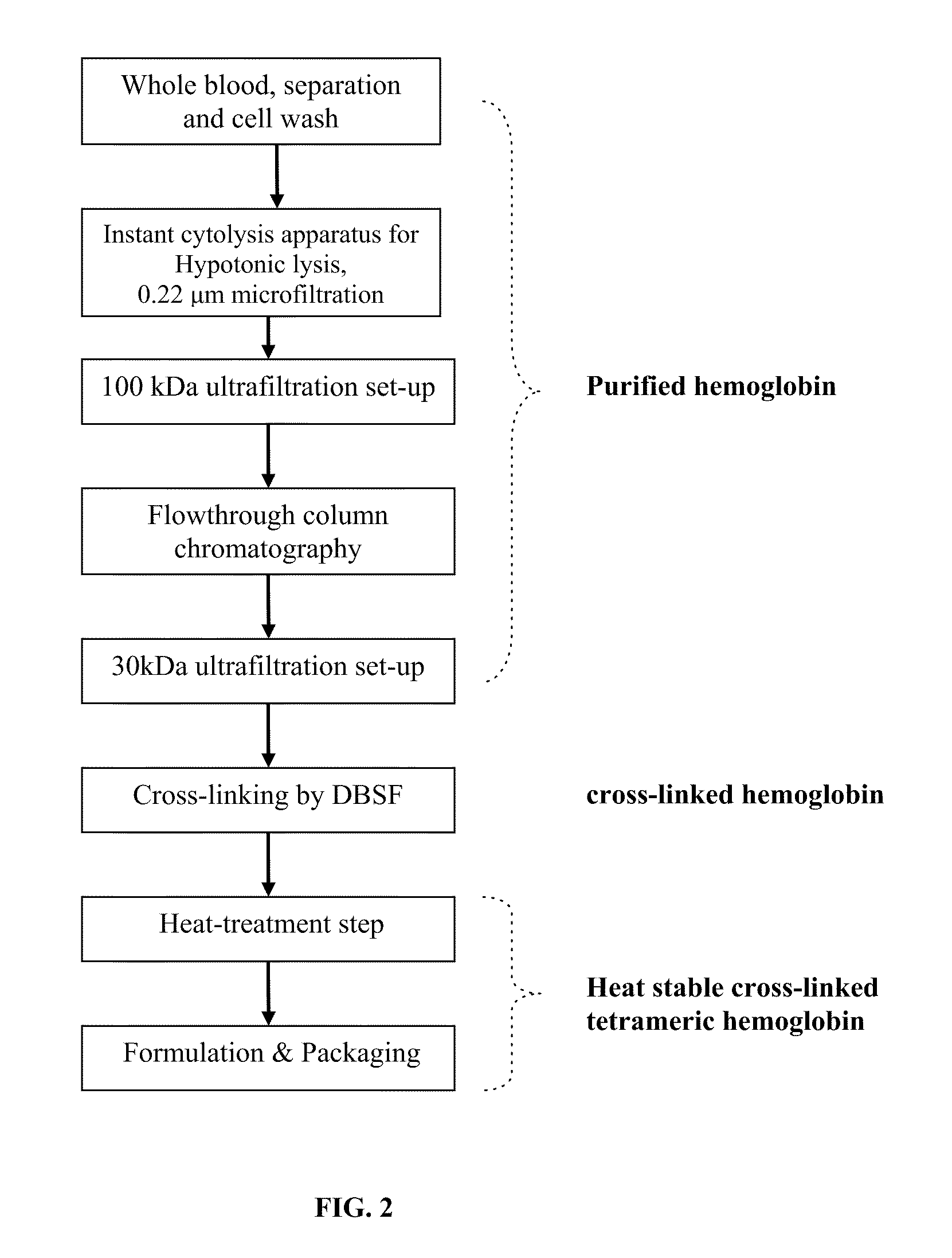
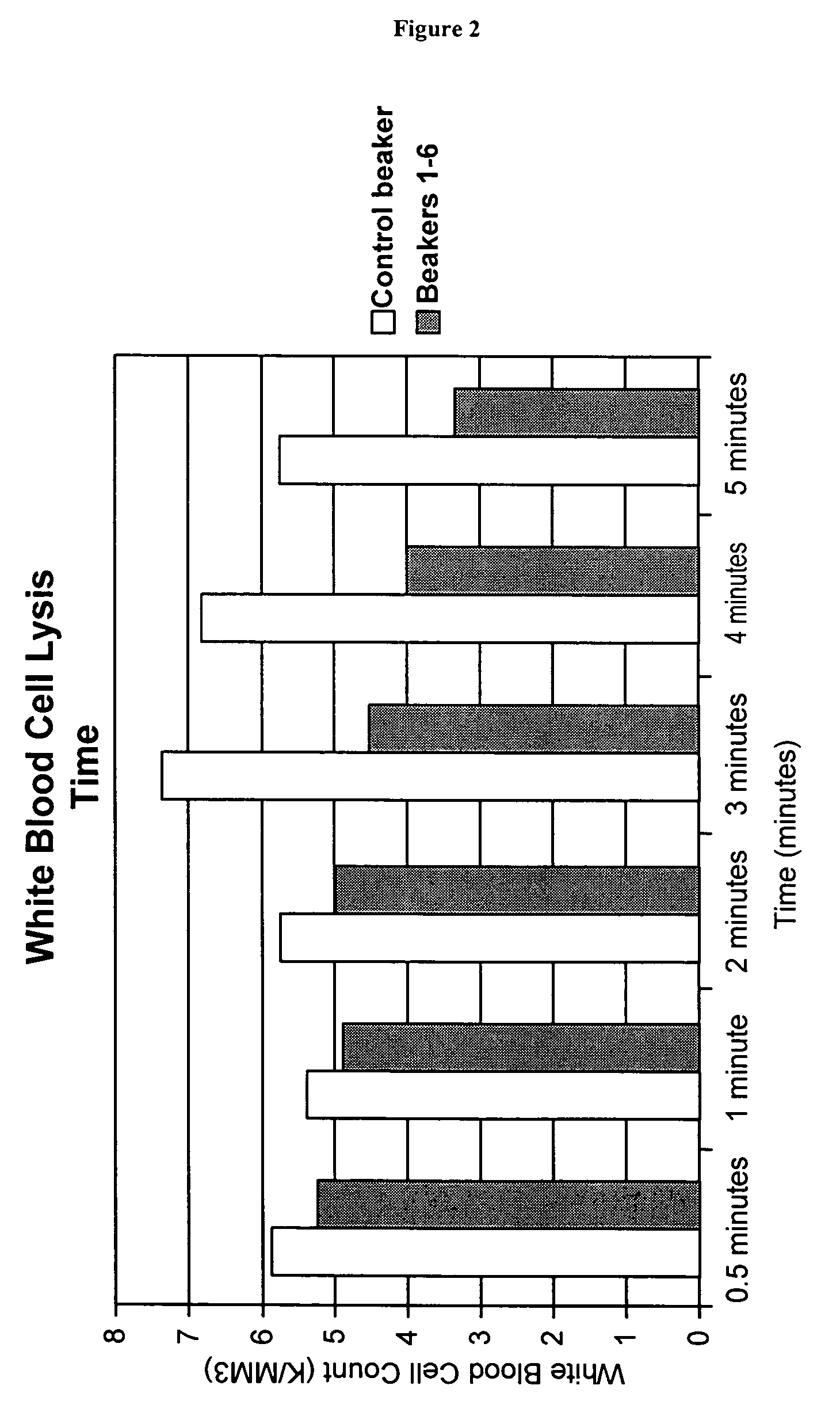
Method for the preparation of a high-temperature stable oxygen-carrier-containing pharmaceutical composition and the use thereof
ActiveUS7989593B1Increase oxygenationHigh sensitivityPeptide/protein ingredientsMammal material medical ingredientsArteriolar VasoconstrictionWhite blood cell
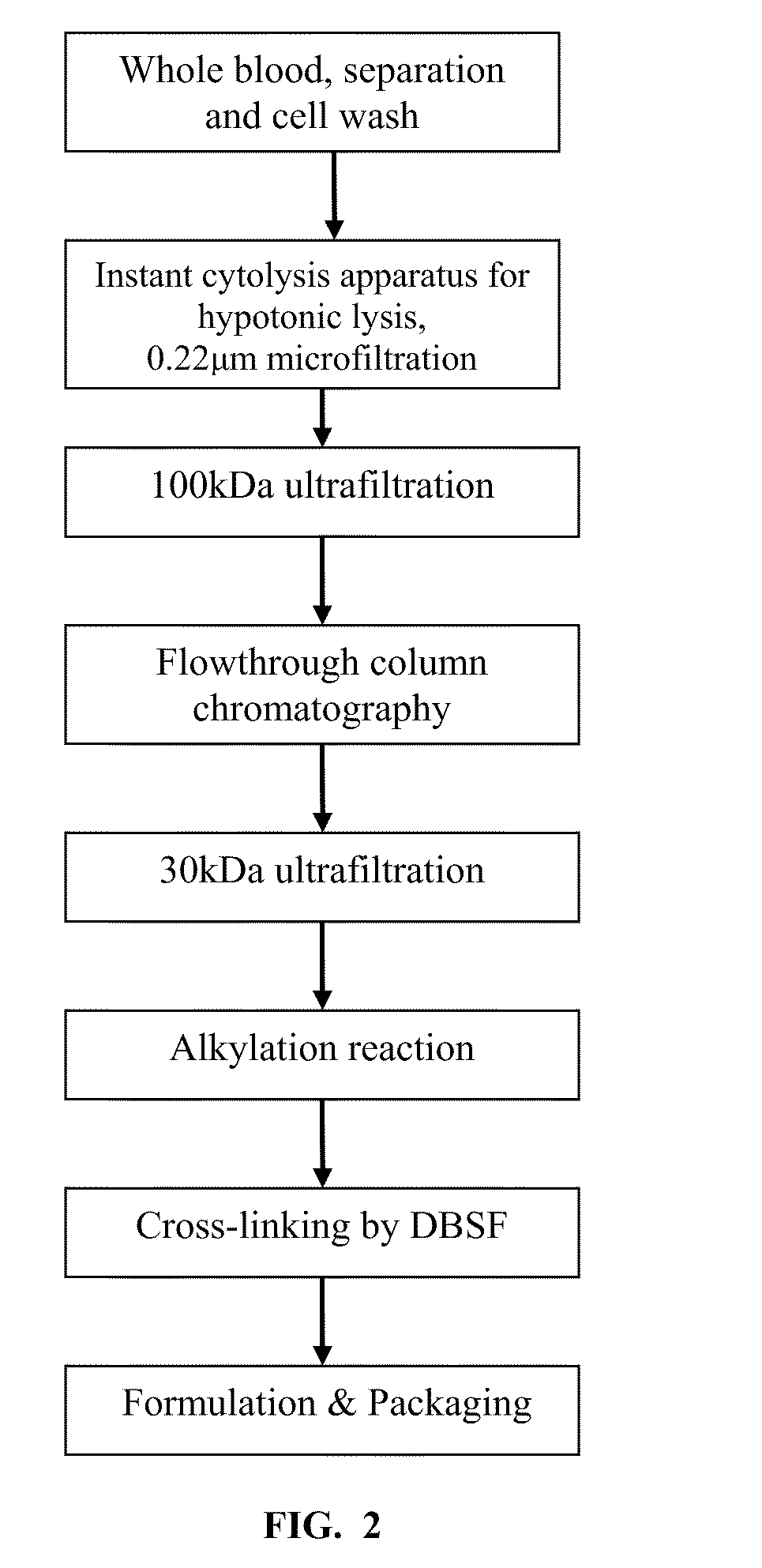
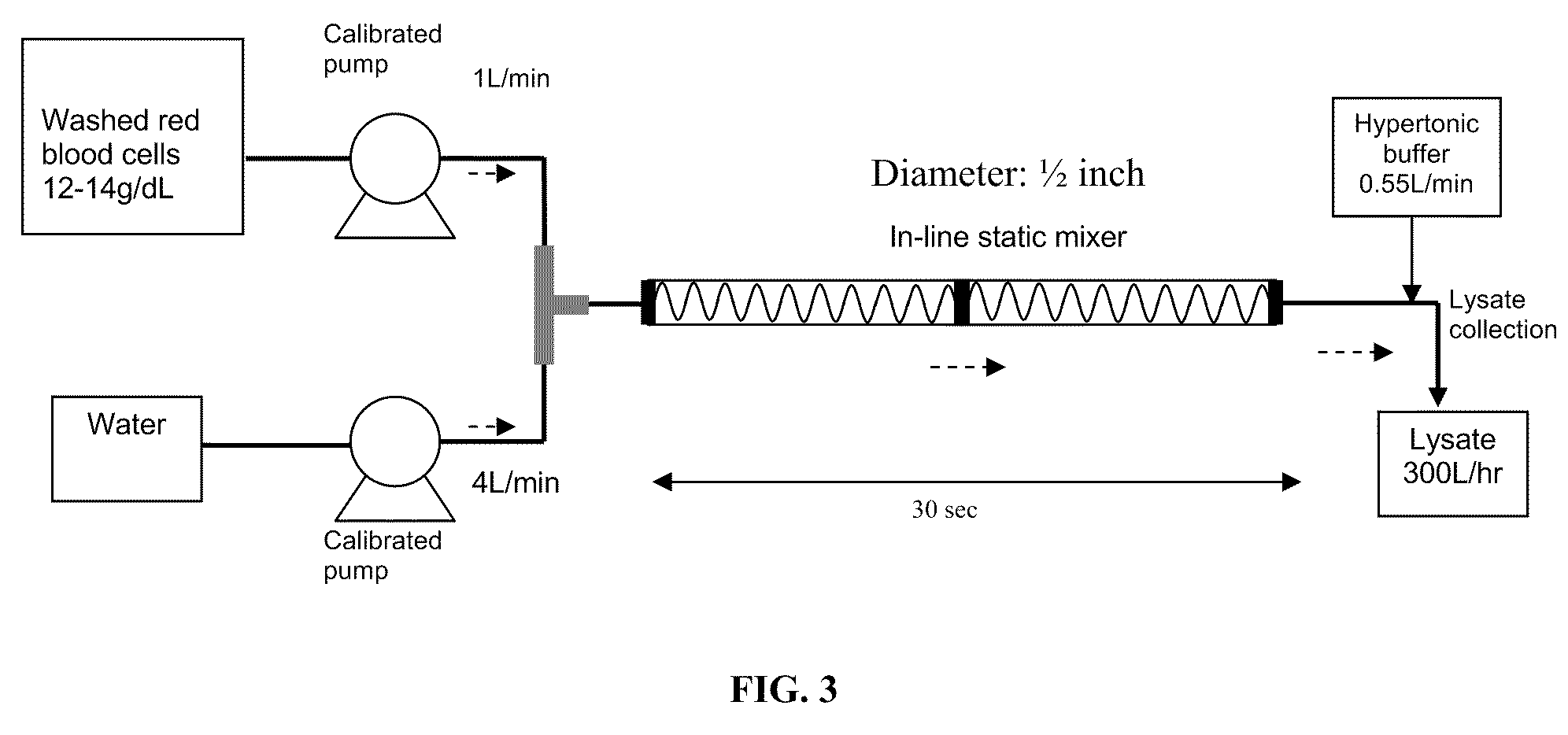
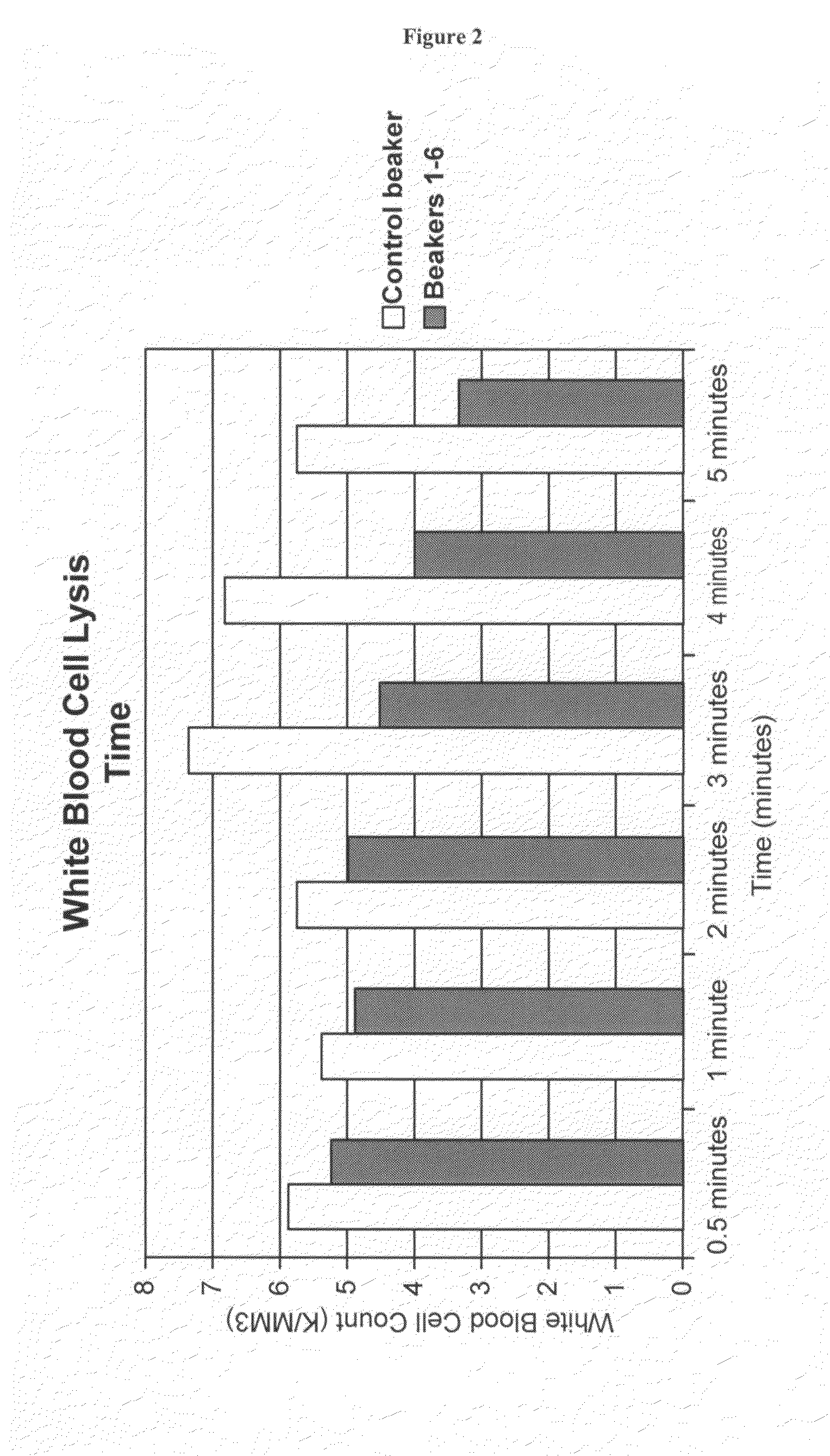
A high temperature-stable and highly purified cross-linked (optionally ≧70% β-β linked) tetrameric hemoglobin with high efficiency of oxygen delivery suitable for use in mammals without causing renal injury and vasoconstriction is provided. The dimeric form of hemoglobin is degenerated and purification processes are performed on red blood cells from whole blood. Controlled hypotonic lysis in an instant cytolysis apparatus prevents lysis of white blood cells. Nucleic acids from white blood cells and phospholipids impurities are not detected. Blocking of reactive sulfhydryl groups by a sulfhydryl reagent is performed in an oxygenated environment. Flowthrough column chromatography removes different plasma protein impurities. N-acetyl cysteine is added to the cross-linked tetrameric hemoglobin to maintain a low level of met-hemoglobin. The stabilized hemoglobin is preserved in an infusion bag with aluminum overwrap to prevent formation of inactive met-hemoglobin from oxygen intrusion. The product finds use in tissue oxygenation and cancer treatment.
Owner:BILLION KING INT
Modified hemoglobin and methods of making same
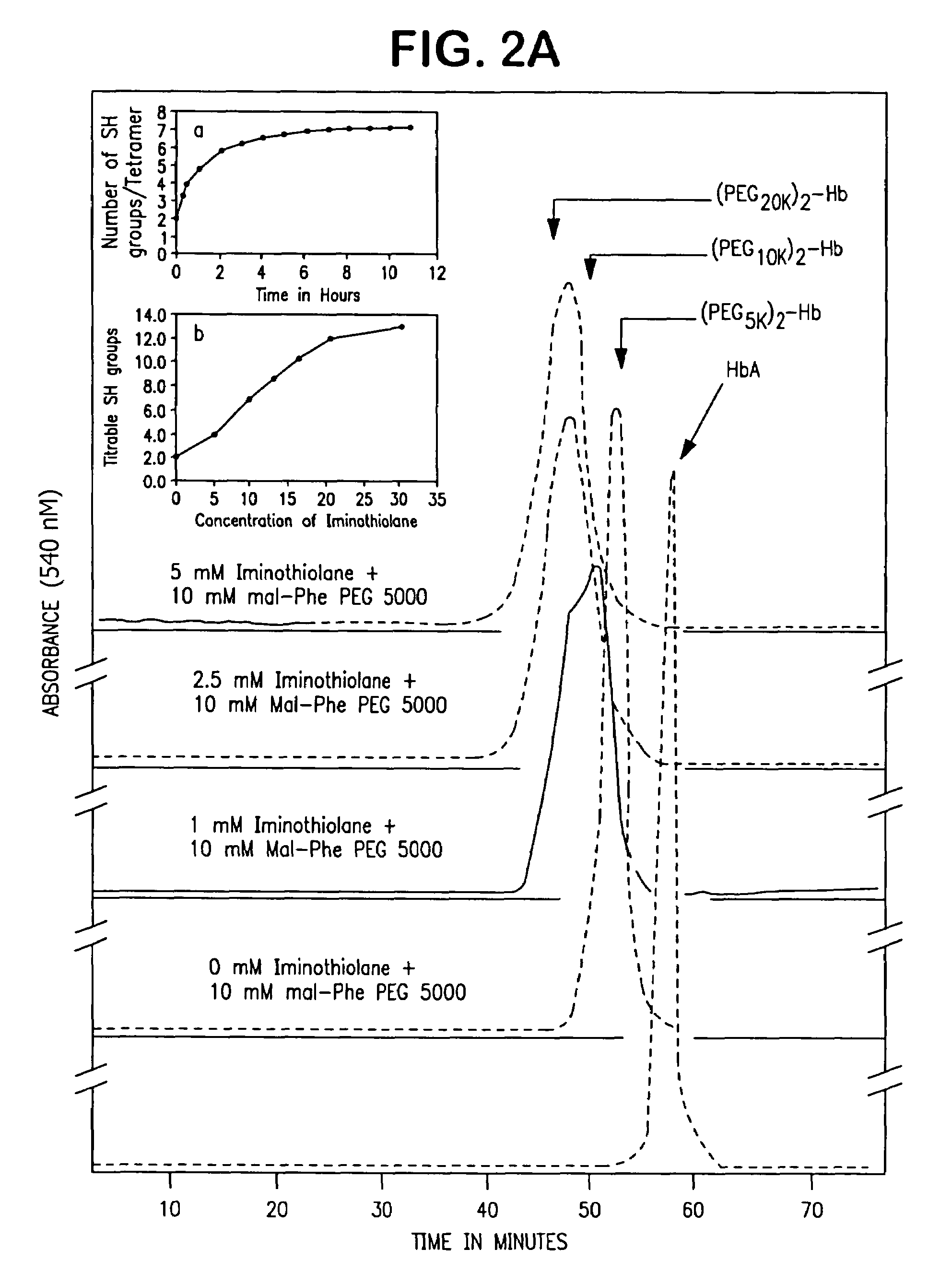
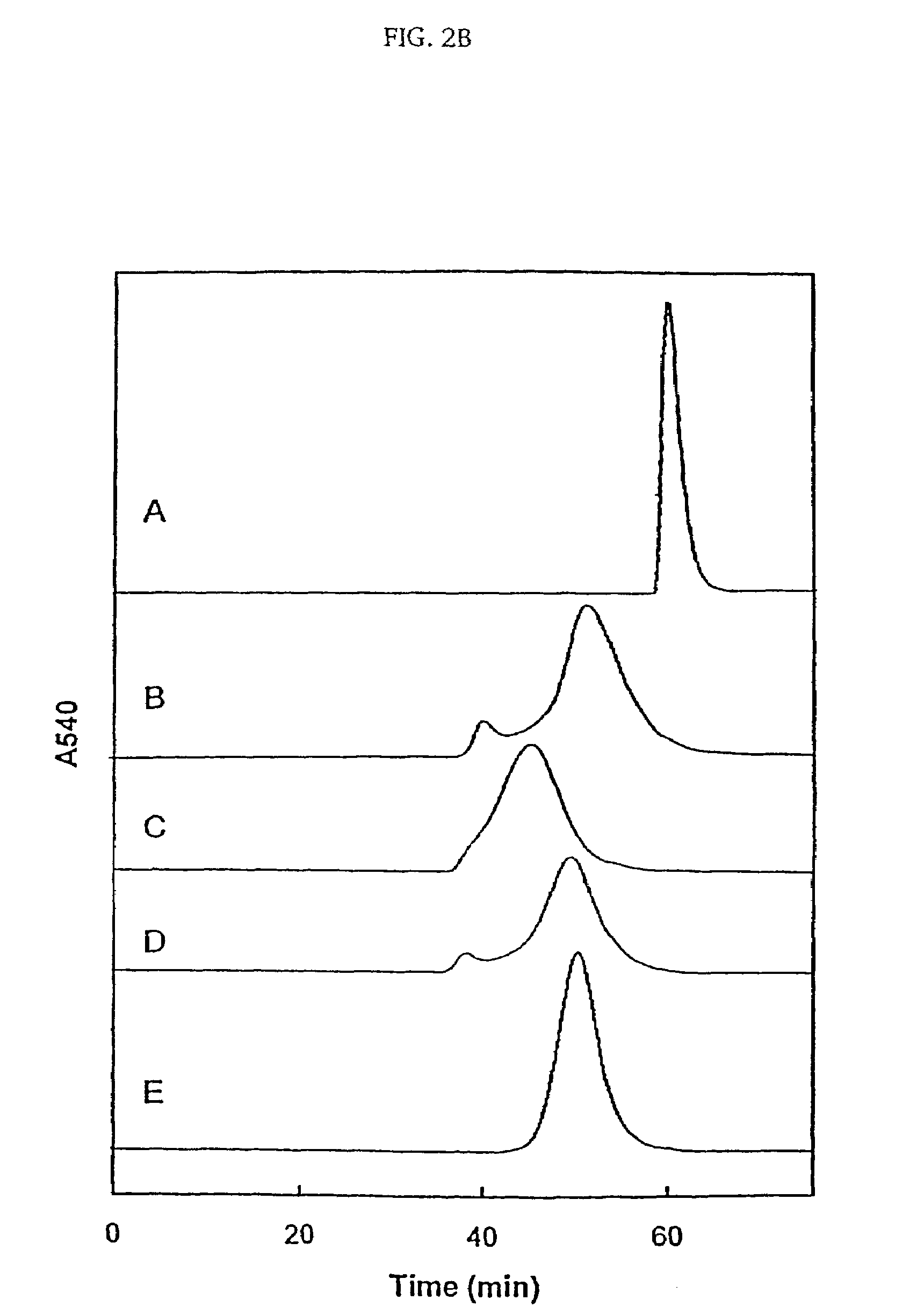
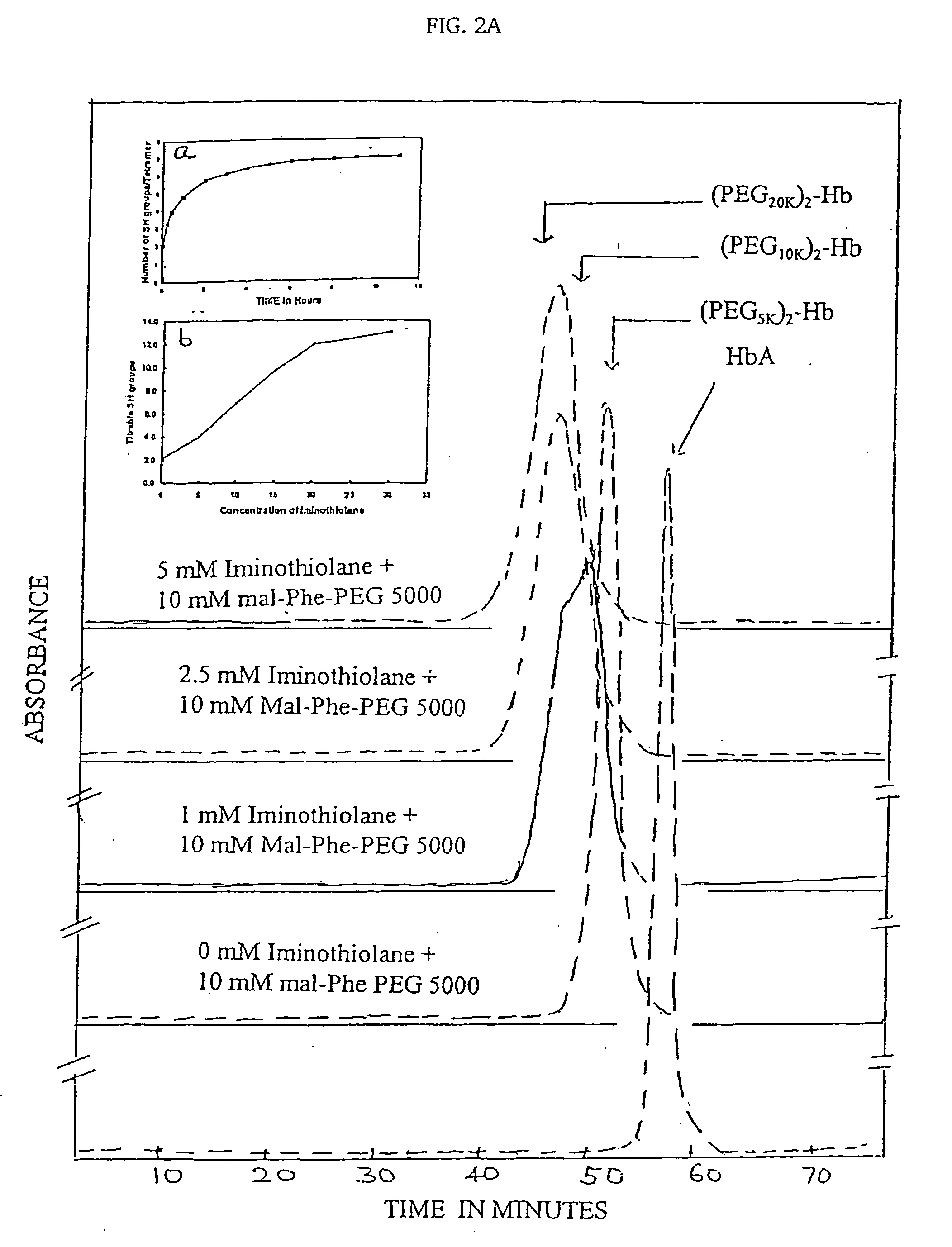
InactiveUS7501499B2Increase volumeHigh viscosityPeptide/protein ingredientsHaemoglobins/myoglobinsSulfurHemeprotein
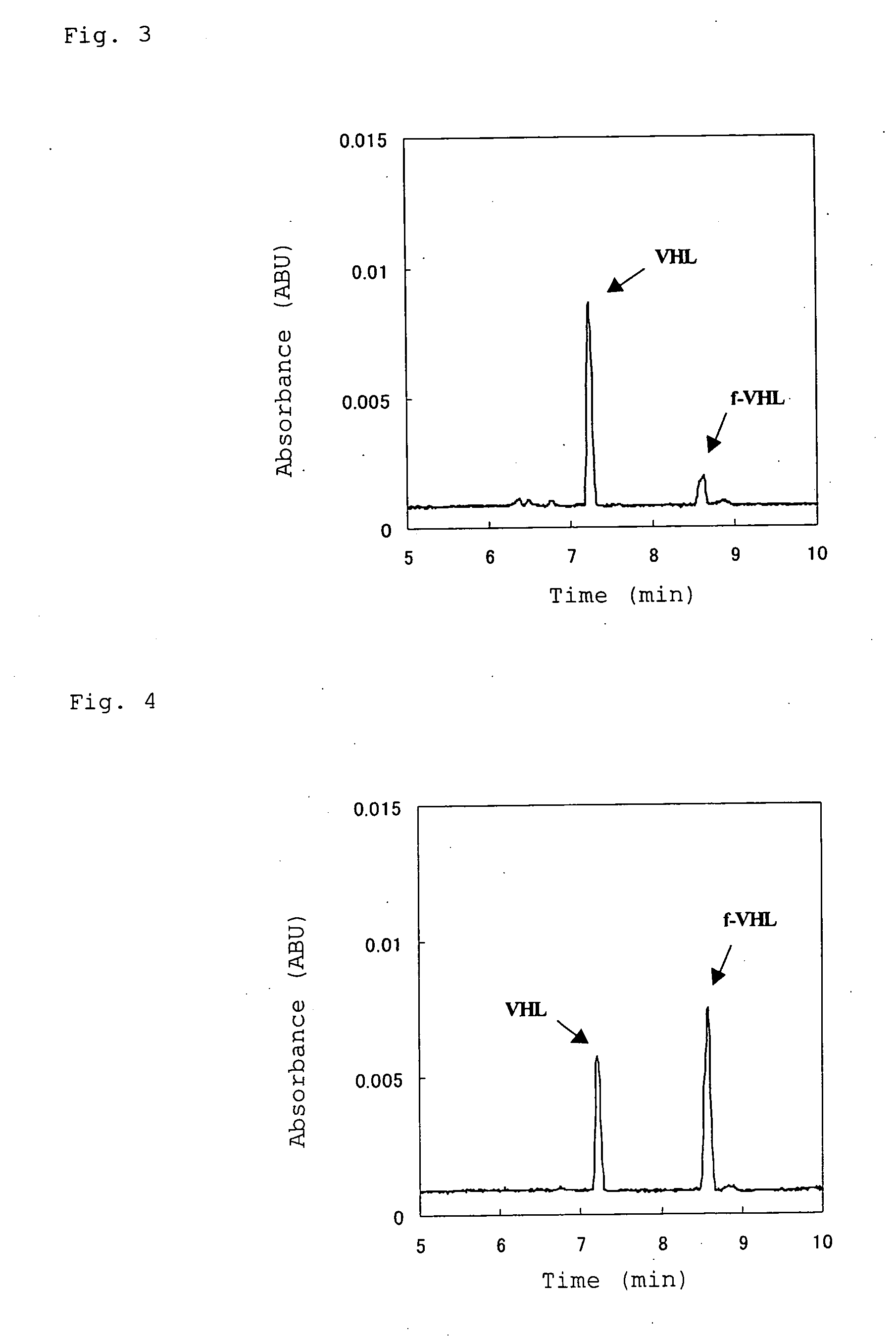
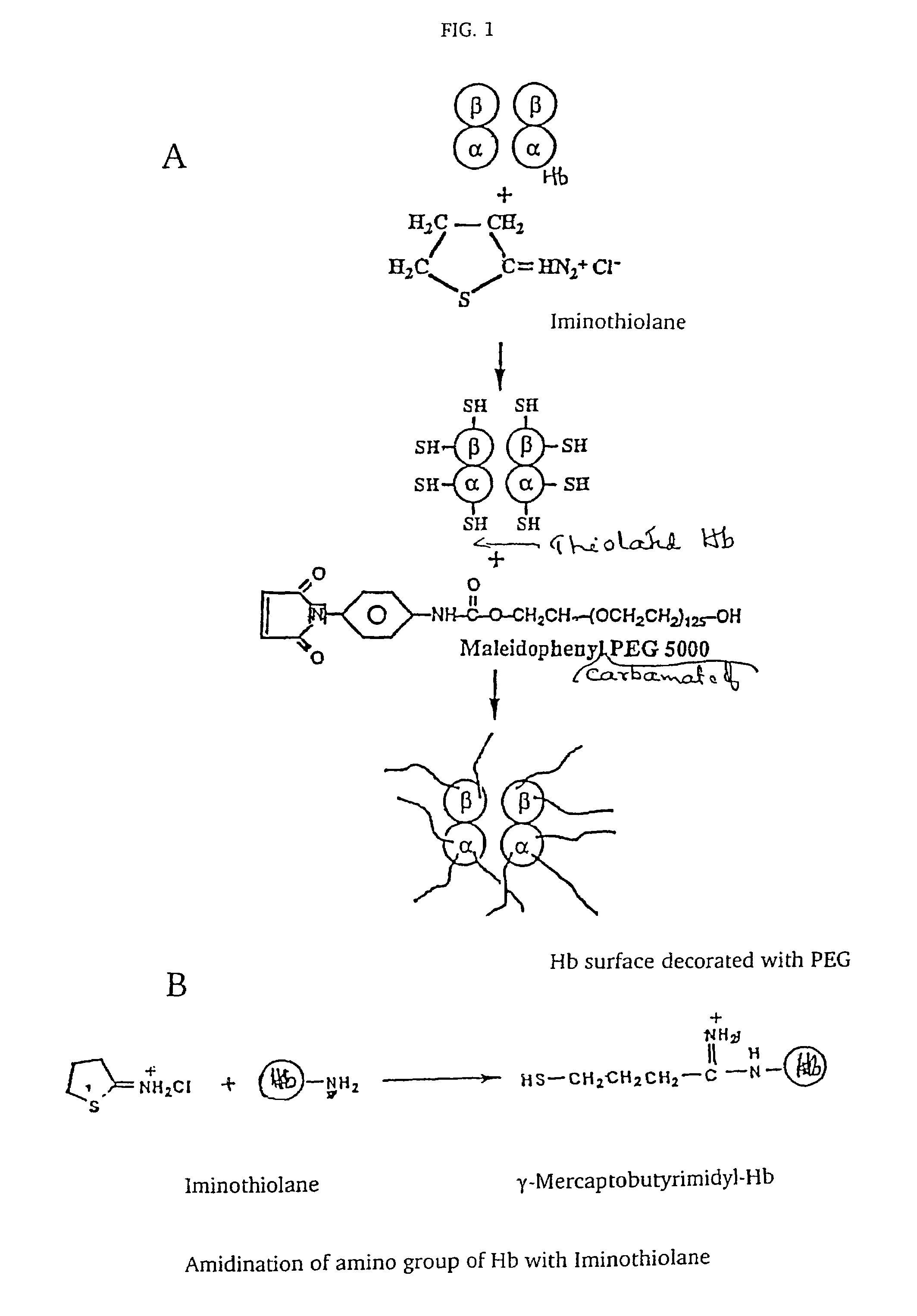
The present invention provides a hemoglobin molecule (Hb) having six ± one PEG chains, wherein two of said PEG chains are bound to Cys-93 (β) of Hb, and the remaining PEG chains are bound to thiol groups introduced on ε-NH2 of Hb. The present invention also provides a process for preparing a modified hemoglobin molecule (Hb), comprising the steps of: (a) reacting Hb with 8-15 fold excess of iminothiolane to form thiolated Hb; and (b) reacting the thiolated Hb with 16-30 fold excess of PEG functionalized with a maleimide moiety, to form the modified Hb.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Methods and Compositions for Simultaneously Isolating Hemoglobin from Red Blood Cells and Inactivating Viruses
InactiveUS20080138790A1Facilitate viral inactivationFacilitating red blood cell lysisHaemoglobins/myoglobinsCentrifugal force sediment separationLysisRed blood cell
The present invention relates to methods arid compositions for isolating hemoglobin from red blood cells. Such methods and compositions also facilitate viral inactivation in a manner that allows recovery of biologically active hemoglobin. More particularly, this method relates to the use of solvents and detergents that are capable of facilitating red blood cell lysis to release hemoglobin (and solubilizing red blood cell membranes), while simultaneously inactivating viruses.
Owner:SANGART INC
Stabilized hemoglobin solutions
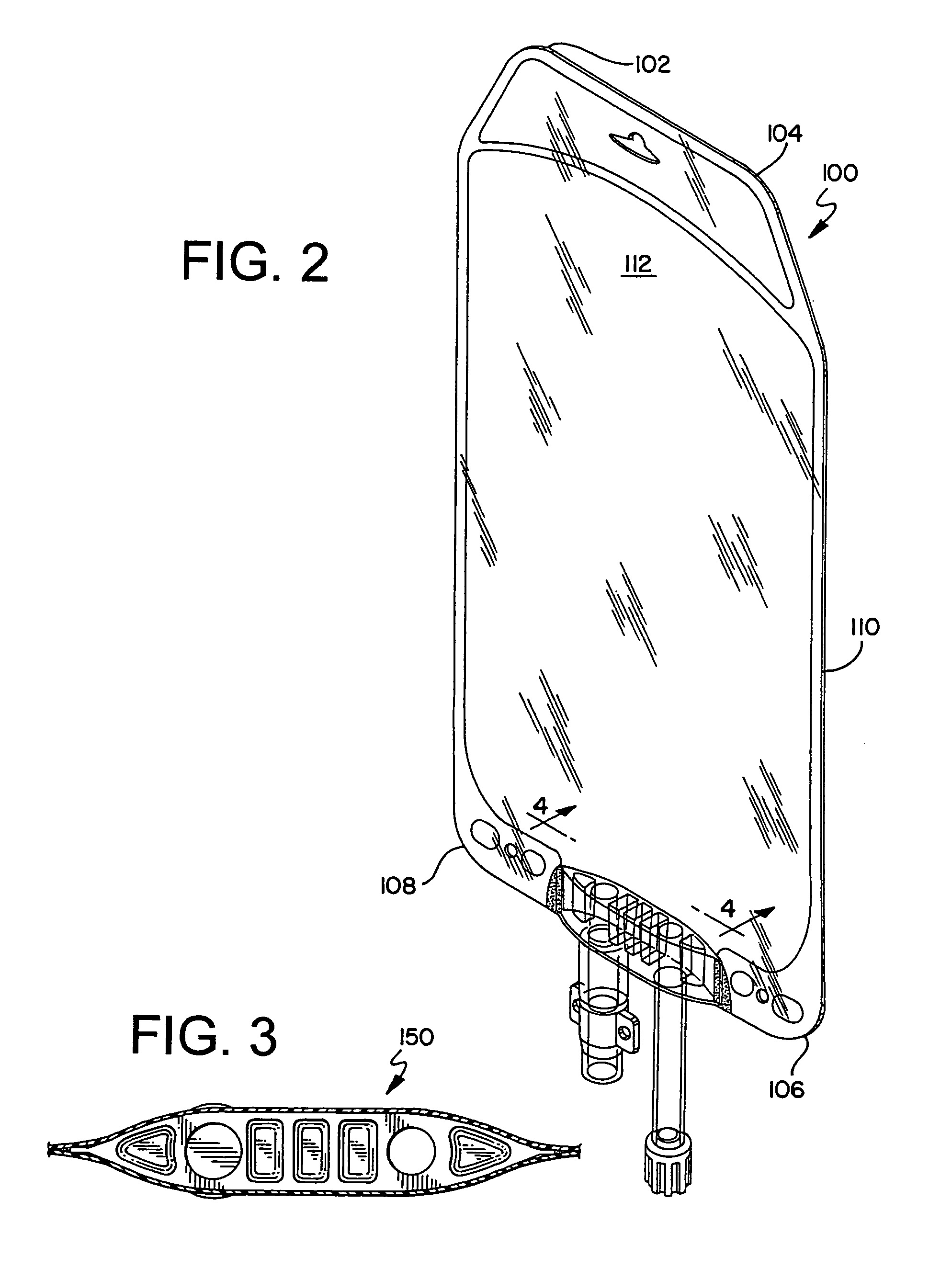
A hemoglobin solution packaged in a flexible oxygen-impermeable container system. The container system includes a multi-layer film having at least a product contact layer, an oxygen and moisture barrier layer and an exterior layer. The flexible container system further includes an interface port for filling the flexible container with the hemoglobin solution and delivering the hemoglobin solution. The hemoglobin solution comprises a substantially stroma and tetramer free, cross linked, pyridoxylated hemoglobin solution including preservatives such as ascorbic acid, glycine and dextrose.
Owner:HEMOGLOBIN OXYGEN THERAPEUTICS
Modified hemoglobin and methods of making same
InactiveUS20060135753A1Enhanced molecular volumeHigh viscosityPeptide/protein ingredientsHaemoglobins/myoglobinsSulfurHemeprotein
The present invention provides a hemoglobin molecule (Hb) having six±one PEG chains, wherein two of said PEG chains are bound to Cys-93 (β) of Hb, and the remaining PEG chains are bound to thiol groups introduced on ε-NH2 of Hb. The present invention also provides a process for preparing a modified hemoglobin molecule (Hb), comprising the steps of: (a) reacting Hb with 8-15 fold excess of iminothiolane to form thiolated Hb; and (b) reacting the thiolated Hb with 16-30 fold excess of PEG functionalized with a maleimide moiety, to form the modified Hb.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
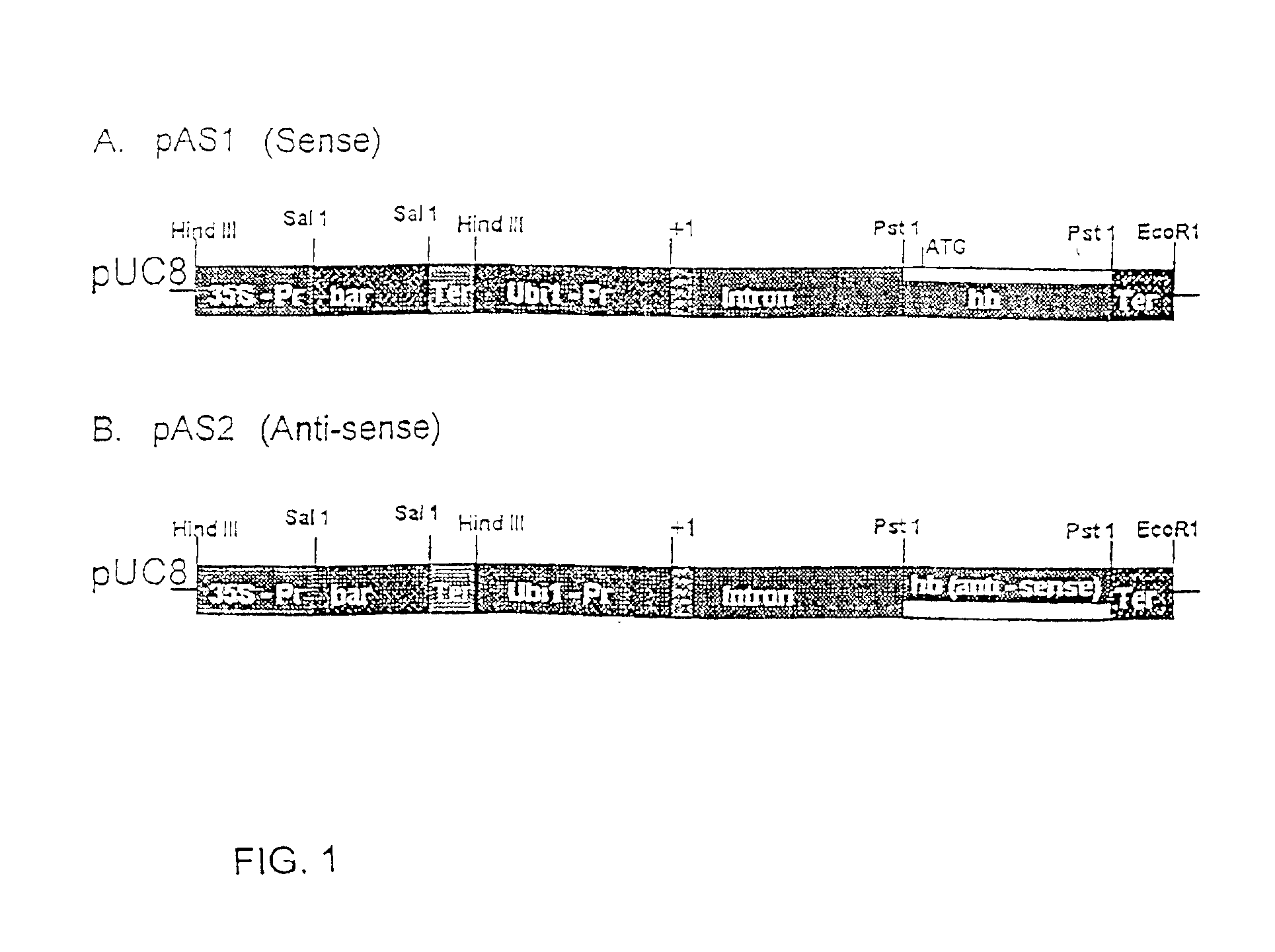
Nonsymbiotic plant hemoglobins to maintain cell energy status
InactiveUS6936749B1Decrease in levelLower Level RequirementsSugar derivativesBacteriaSubstrate-level phosphorylationProgenitor
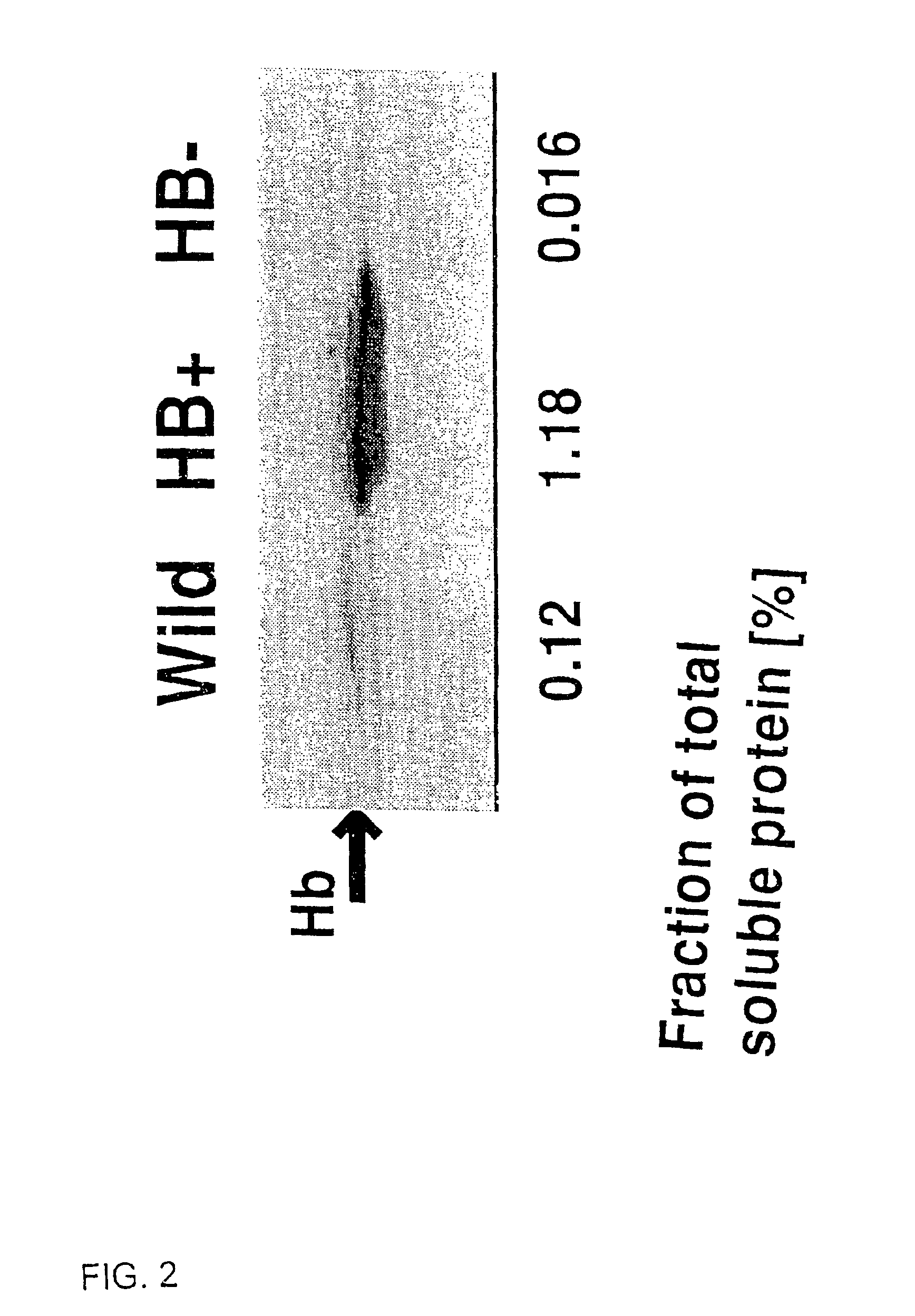
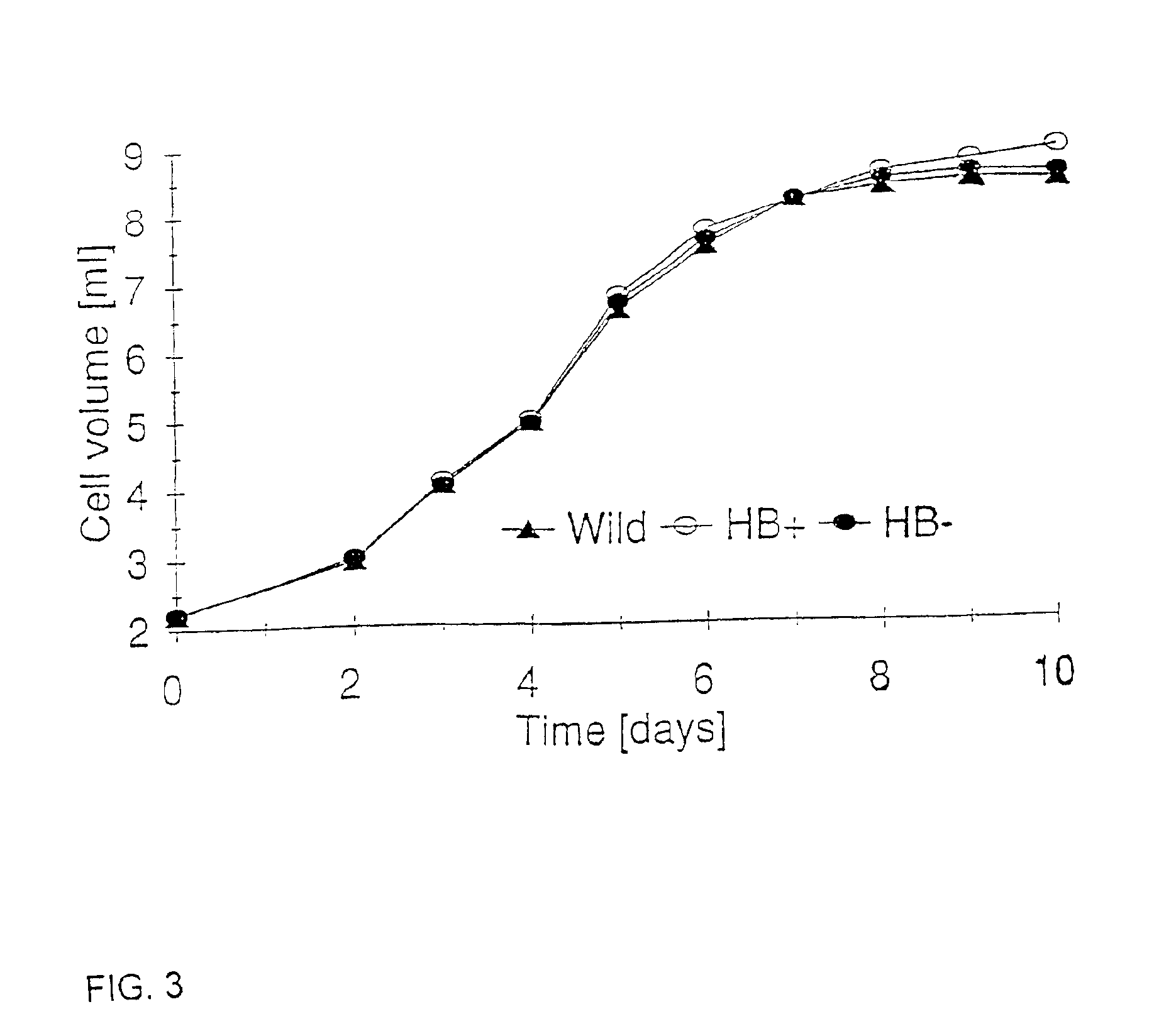
Nonsymbiotic hemoglobins are broadly present across evolution; however, the function of these proteins is unknown. Cultured maize cells have been transformed to constitutively express a barley hemoglobin gene in either the sense (HB+) or antisense (HB−) orientation. Hemoglobin protein in the transformed cell lines was correspondingly higher or lower than in wild type cells under normal atmospheric conditions. Limiting oxygen availability, by placing the cells in a nitrogen atmosphere for 12 hours, had little effect on the energy status of cells constitutively expressing hemoglobin, but had a pronounced effect on both wild type and HB− cells, where ATP levels declined by 27% and 61% respectively. Energy charge was relatively unaffected by the treatment in HB+ and wild type cells, but was reduced from 0.91 to 0.73 in HB− cells suggesting that the latter were incapable of maintaining their energy status under the low oxygen regime. Similar results were observed with P. aeruginosa cells transformed with an Hb expression vector. It is suggested that nonsymbiotic hemoglobins act to maintain the energy status of cells in low oxygen environments and that they accomplish this effect by promoting glycolytic flux through NADH oxidation, resulting in increased substrate level phosphorylation. Nonsymbiotic hemoglobins are likely ancestors of an early form of hemoglobin that sequestered oxygen in low oxygen environments, providing a source of oxygen to oxidize NADH to provide ATP for cell growth and development. This in turn suggests that cells containing increased levels of Hb protein will survive longer under low oxygen tension or high energy demand.
Owner:UNIVERSITY OF MANITOBA
Nitrosated and nitrosylated heme proteins
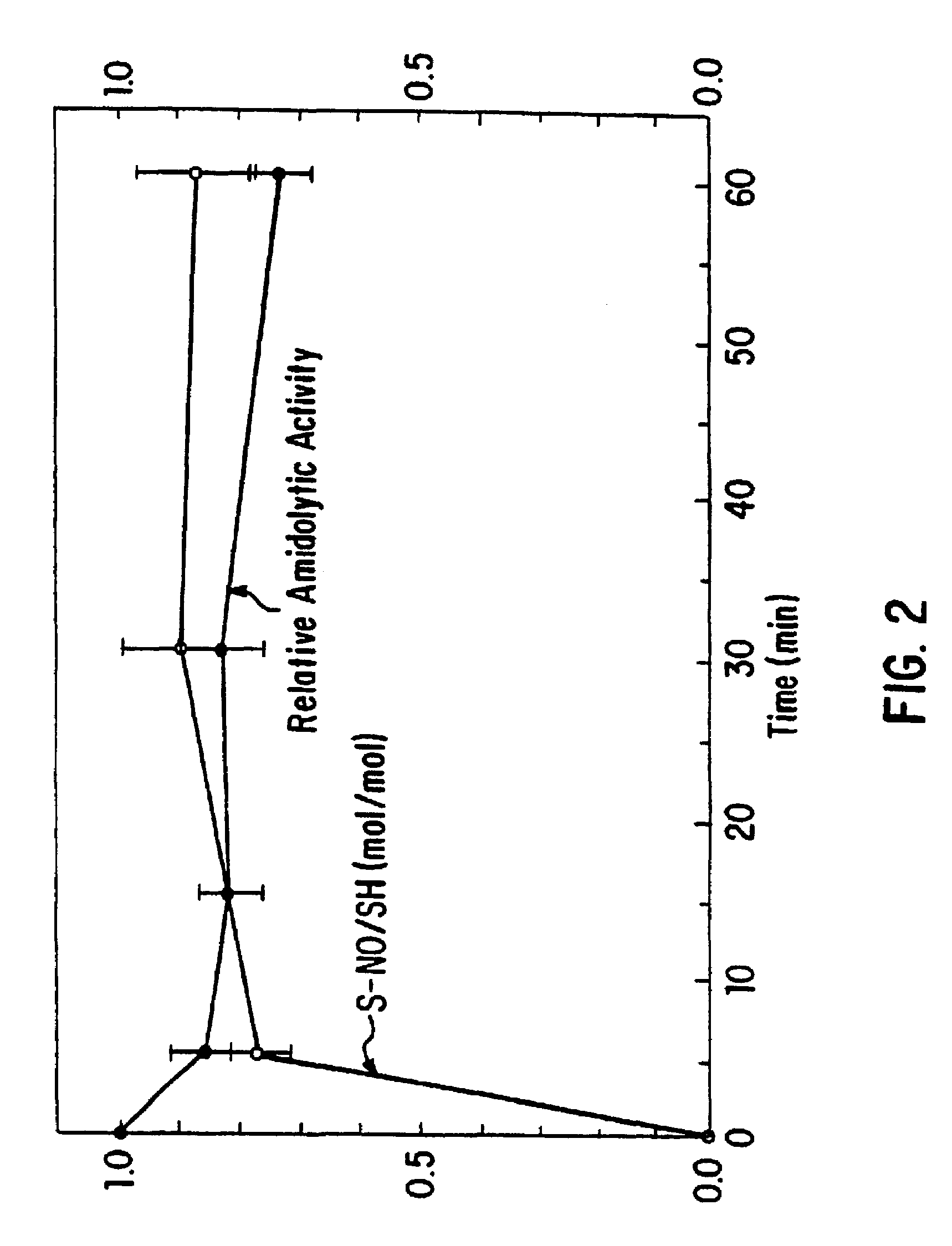
Nitrosylation of proteins and amino acid groups enables selective regulation of protein function, and also endows the proteins and amino acids with additional smooth muscle relaxant and platelet inhibitory capabilities. Thus, the invention relates to novel compounds achieved by nitrosylation of protein thiols. Such compounds include: S-nitroso-heme proteins, S-nitroso-t-PA, S-nitroso-cathepsin; S-nitroso-lipoproteins; and S-nitroso-immunoglobulins. The invention also relates to therapeutic use if S-nitroso-protein compounds for regulating protein function, cellular metabolism and effecting vasodilation, platelet inhibition, relaxation of non-vascular smooth muscle, and increasing blood oxygen transport by hemoglobin and myoglobin. The compounds are also used to deliver nitric oxide in its most bioactive form in order to achieve the effects described above, or for in vitro nitrosylation of molecules present in the body. The invention also relates to the nitrosylation of oxygen, carbon and nitrogen moieties present on proteins and amino acids, and the use thereof to achieve the above physiological effects.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Heme-binding photoactive polypeptides and methods of use thereof
InactiveUS20110243849A1Useful applicationMammal material medical ingredientsAnalysis by subjecting material to chemical reactionHeme bindingChemistry
Owner:RGT UNIV OF CALIFORNIA +1
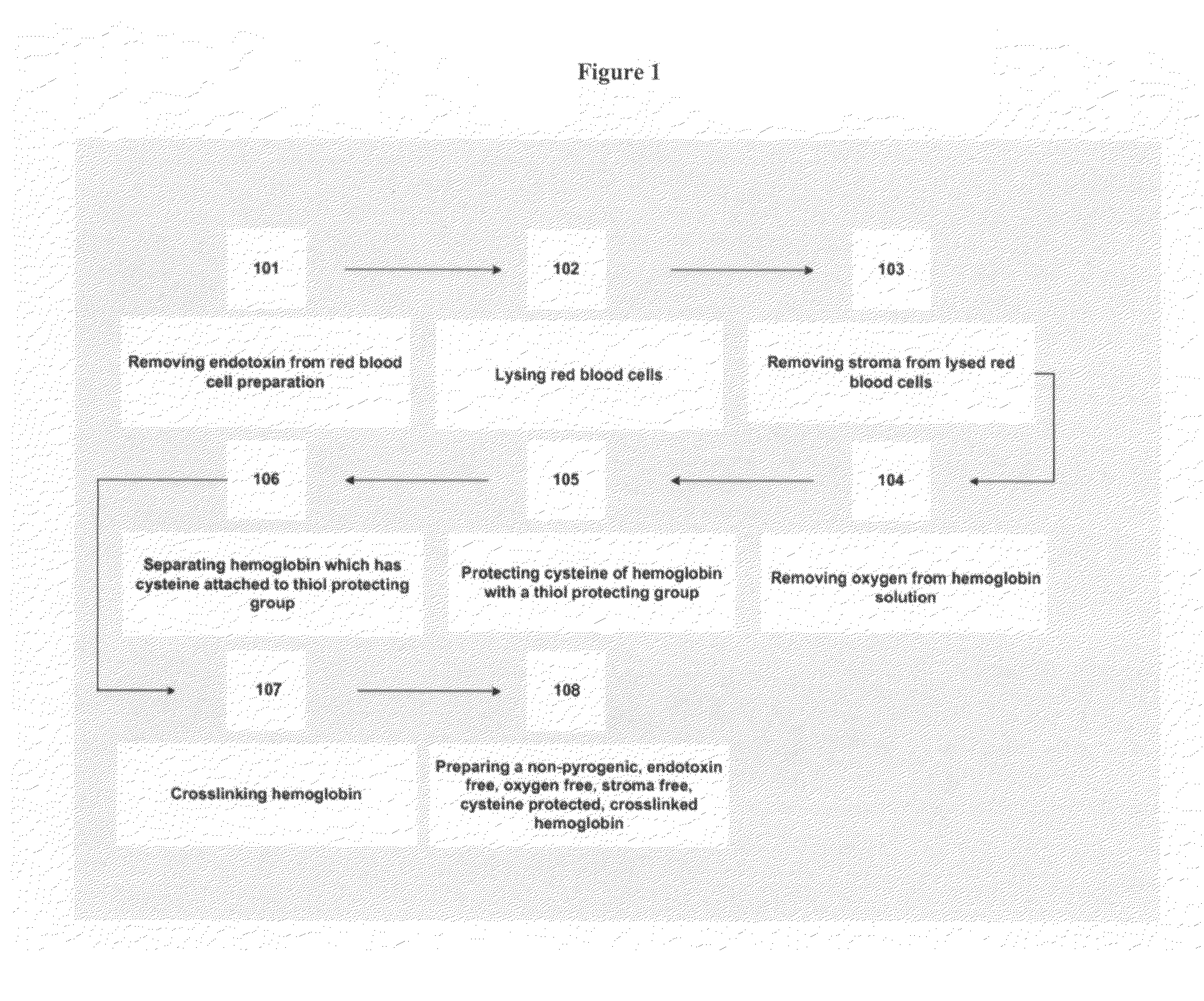
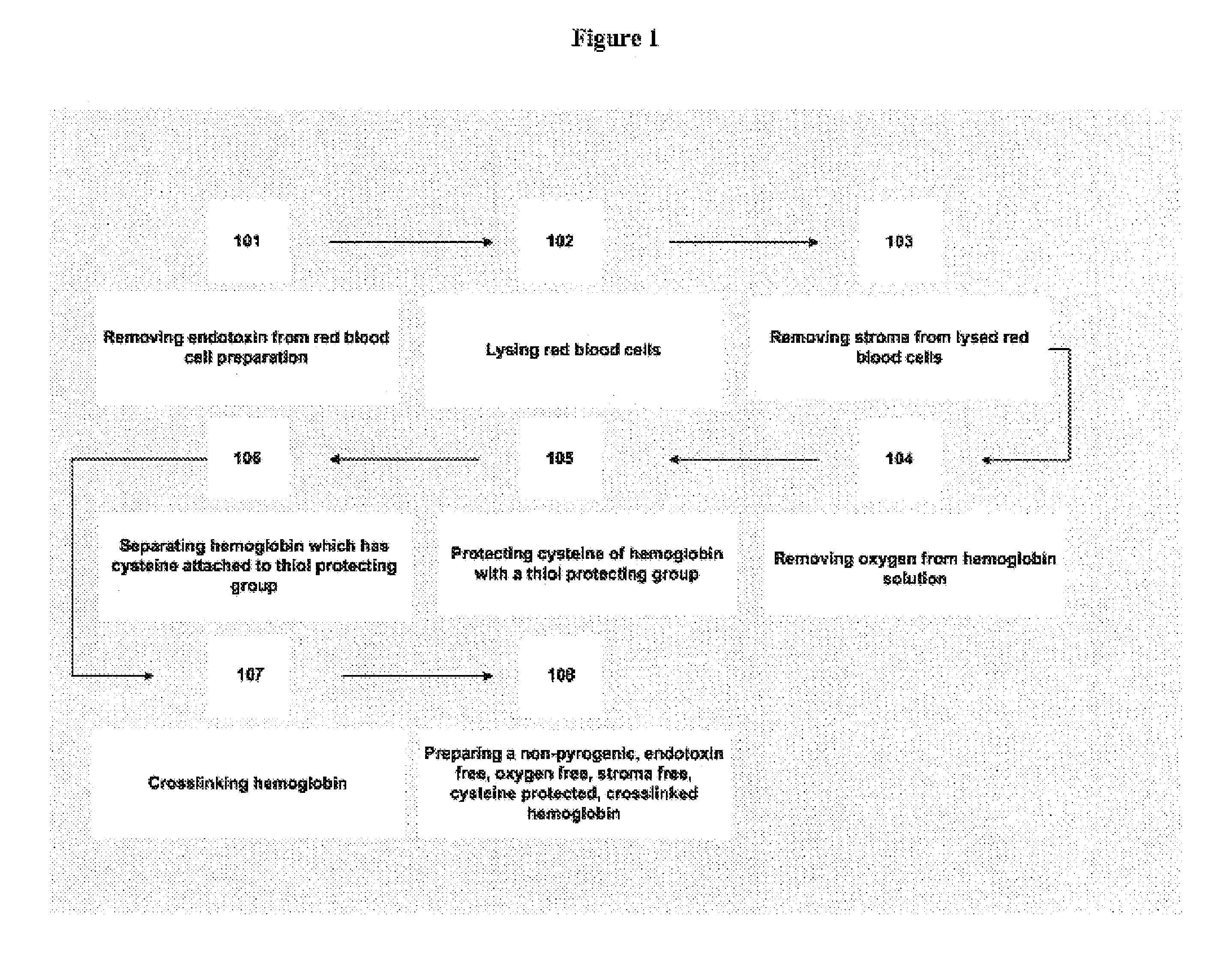
Non-pyrogenic, endotoxin-free, stroma-free tetrameric hemoglobin
InactiveUS6894150B1Improve oxygen carrying capacityPeptide/protein ingredientsHaemoglobins/myoglobinsMammalBlood substitute
A non-pyrogenic, endotoxin-free, stroma-free, blood substitute capable of being used in humans or other mammals to provide oxygen to tissue and to deliver carbon dioxide to the lung and a process for its preparation are described.
Owner:IKOR +1
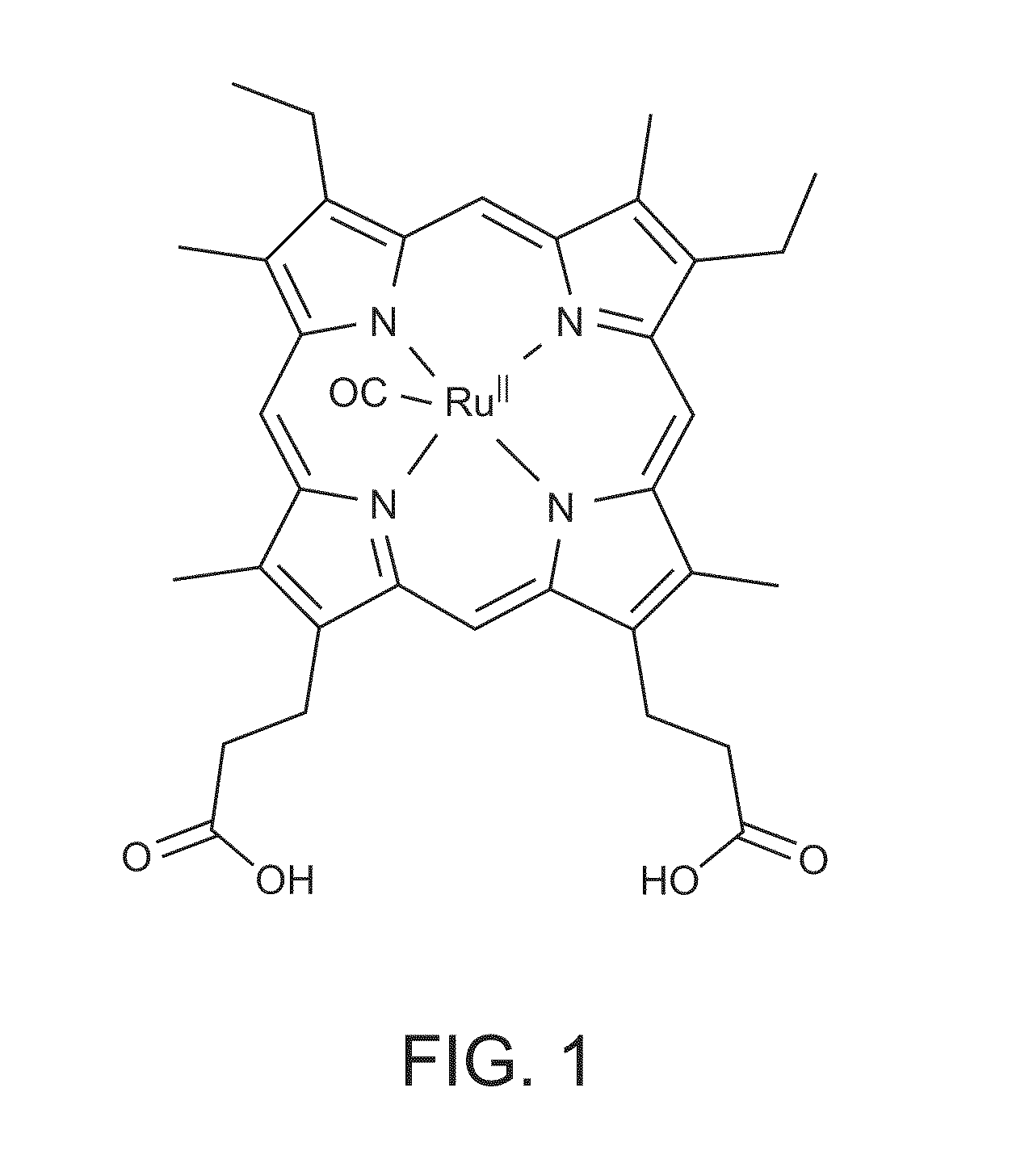
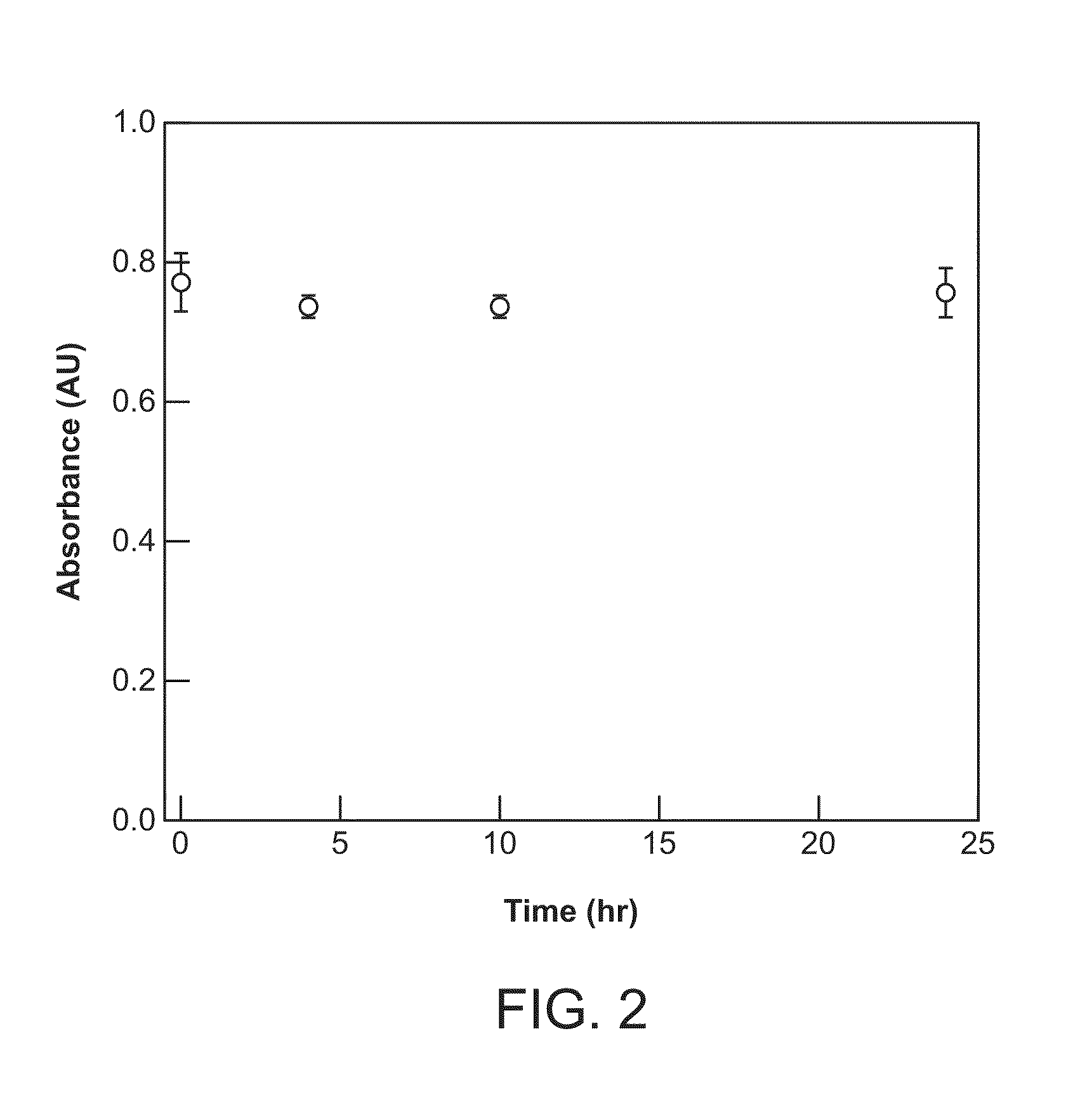
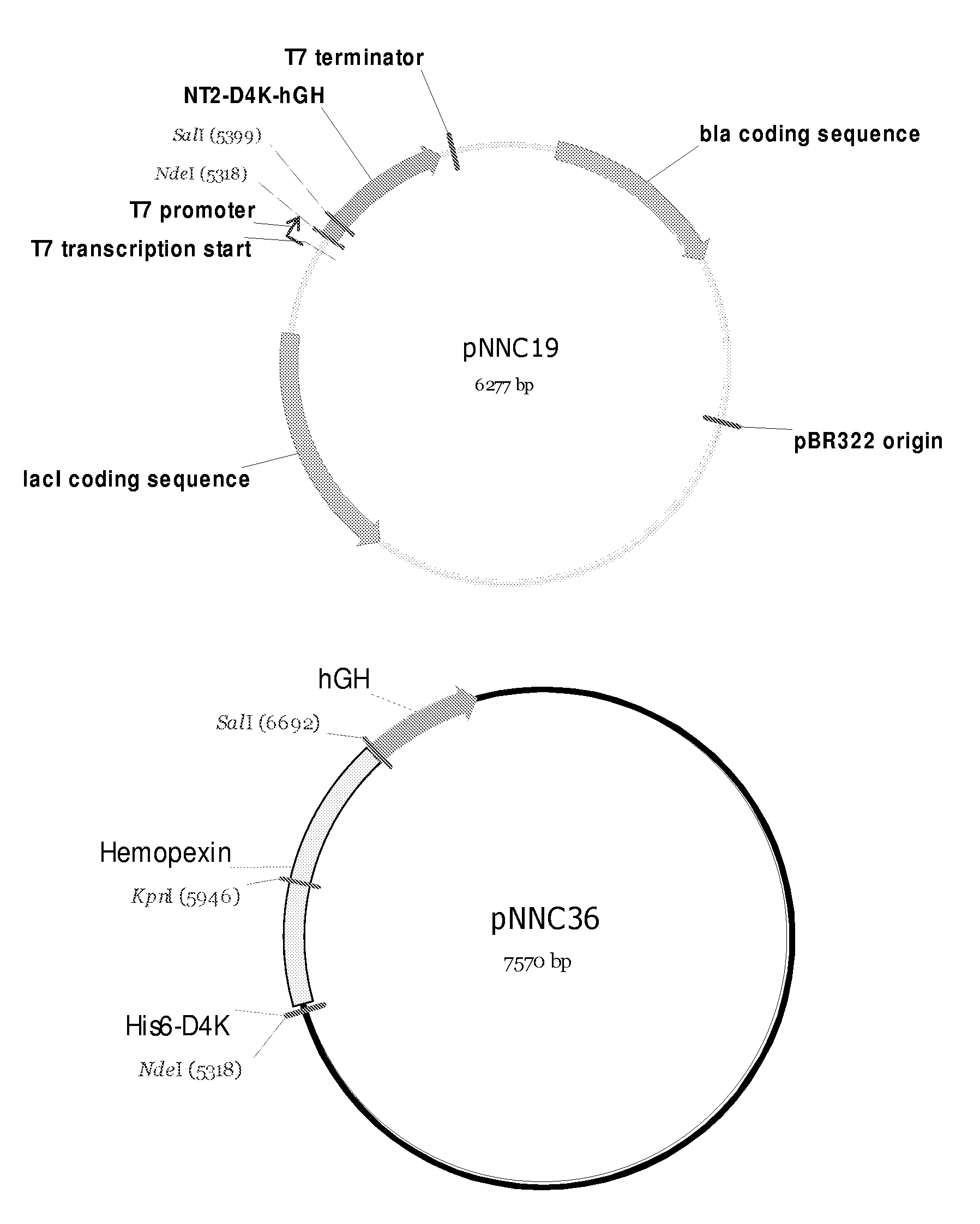
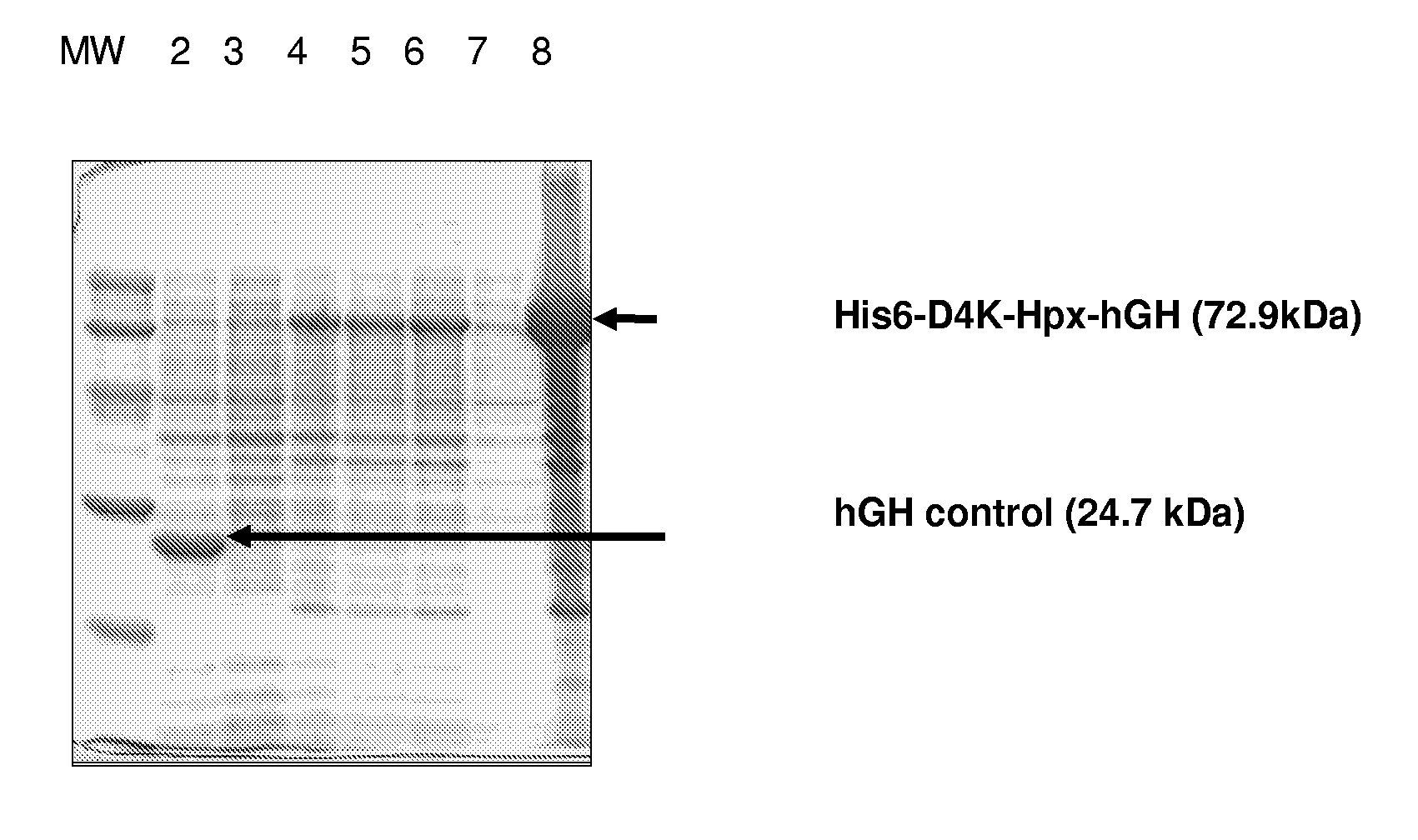
Hemopexin fusion proteins
Fusion proteins comprising a first protein fused to hemopexin are provided, said fusion proteins exhibit an increased circulation time.
Owner:NOVO NORDISK AS
Preserving a hemoglobin blood substitute with a transparent overwrap
InactiveUS20060160724A1High purityExtended shelf lifeWrappersPeptide/protein ingredientsMedicineBlood substitute
The invention relates to a method for preserving the stability of a hemoglobin blood substitute comprising maintaining the hemoglobin blood substitute in an atmosphere substantially free of oxygen. The method for preserving the deoxygenated hemoglobin blood substitute comprises maintaining the deoxygenated blood substitute in an oxygen barrier film overwrap package, wherein at least one face of the overwrap package comprises a transparent laminate material and wherein at least one other face of the overwrap package comprises a foil laminate material. The preserved deoxygenated hemoglobin blood substitute comprises a deoxygenated hemoglobin blood substitute and an oxygen barrier film overwrap package wherein at least one face of the overwrap package comprises a transparent laminate material and wherein at least one other face of the overwrap package comprises a foil laminate material.
Owner:OPK BIOTECH
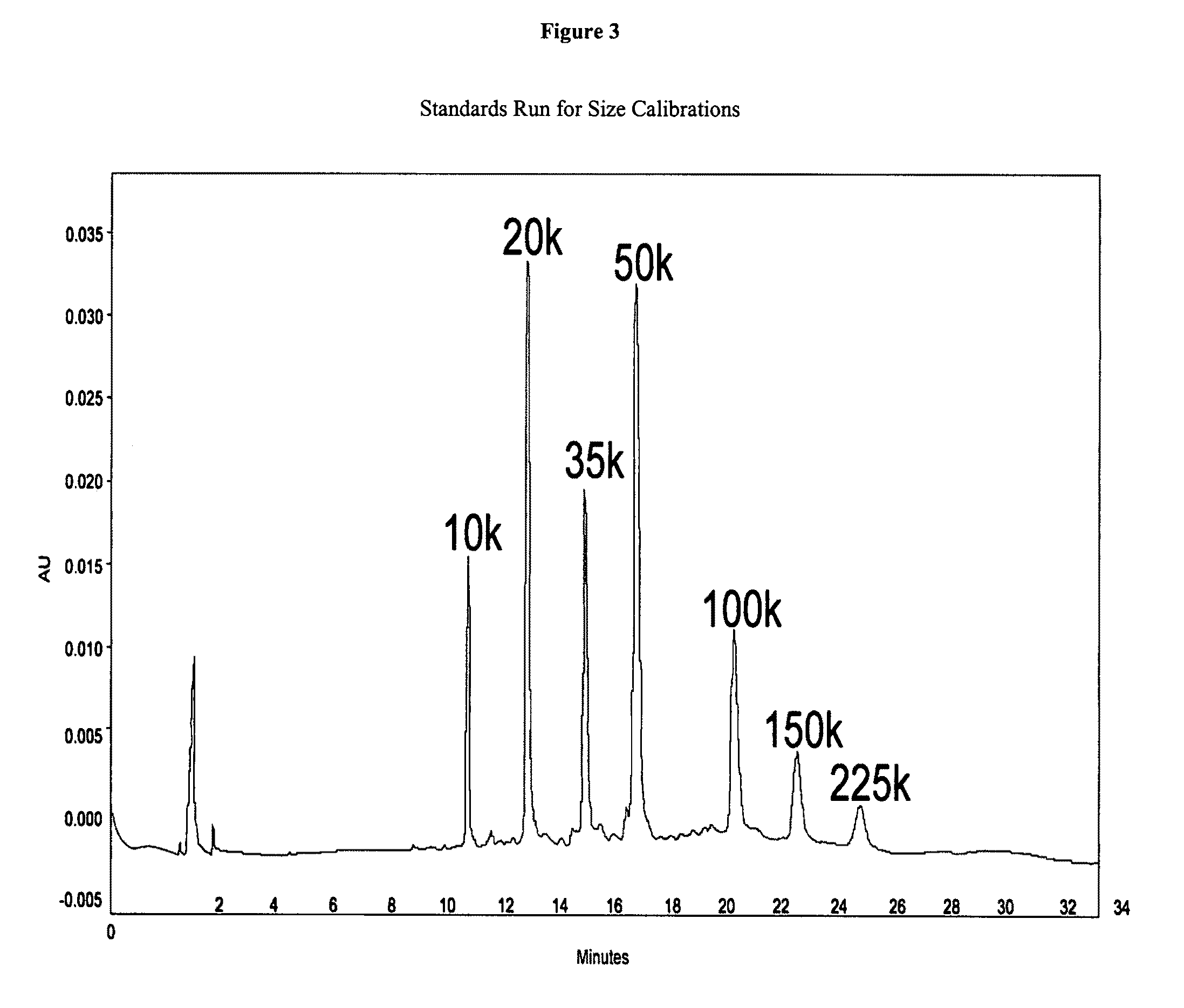
Ultra pure hemoglobin solutions and blood-substitutes
InactiveUS6506725B1Avoid a lot of problemsPeptide/protein ingredientsHaemoglobins/myoglobinsChemistryChromatographic separation
A blood substitute and plasma expander comprising a cross-linked, substantially endotoxin-free hemoglobin solution and process for preparing same. The process comprises fractionating whole blood, separating out a stromal-free, sterile hemoglobulin solution, chromatographically separating endotoxins from said hemoglobin solution and crosslinking the resulting endotoxin-free hemoglobin solution.
Owner:OPK BIOTECH
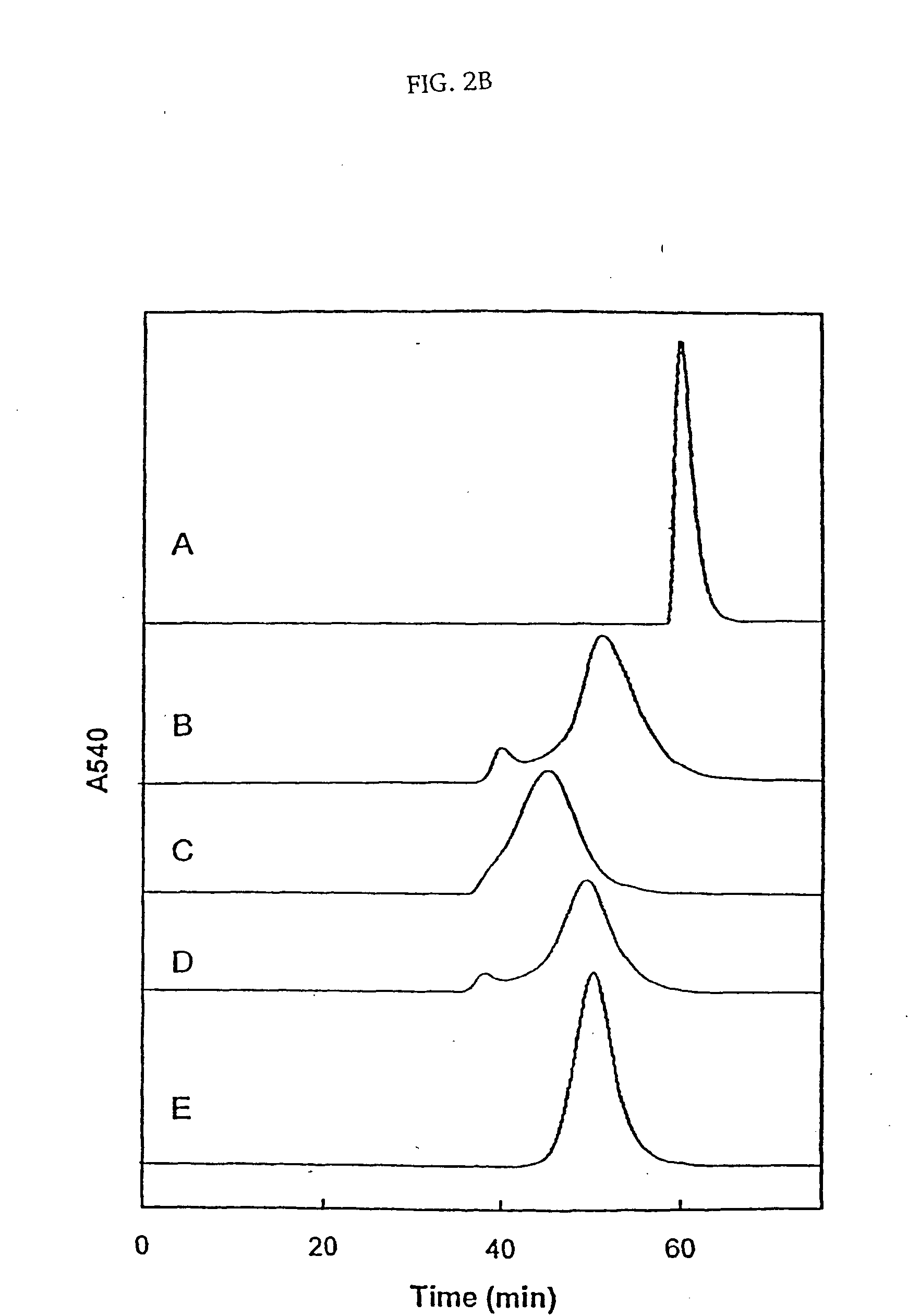
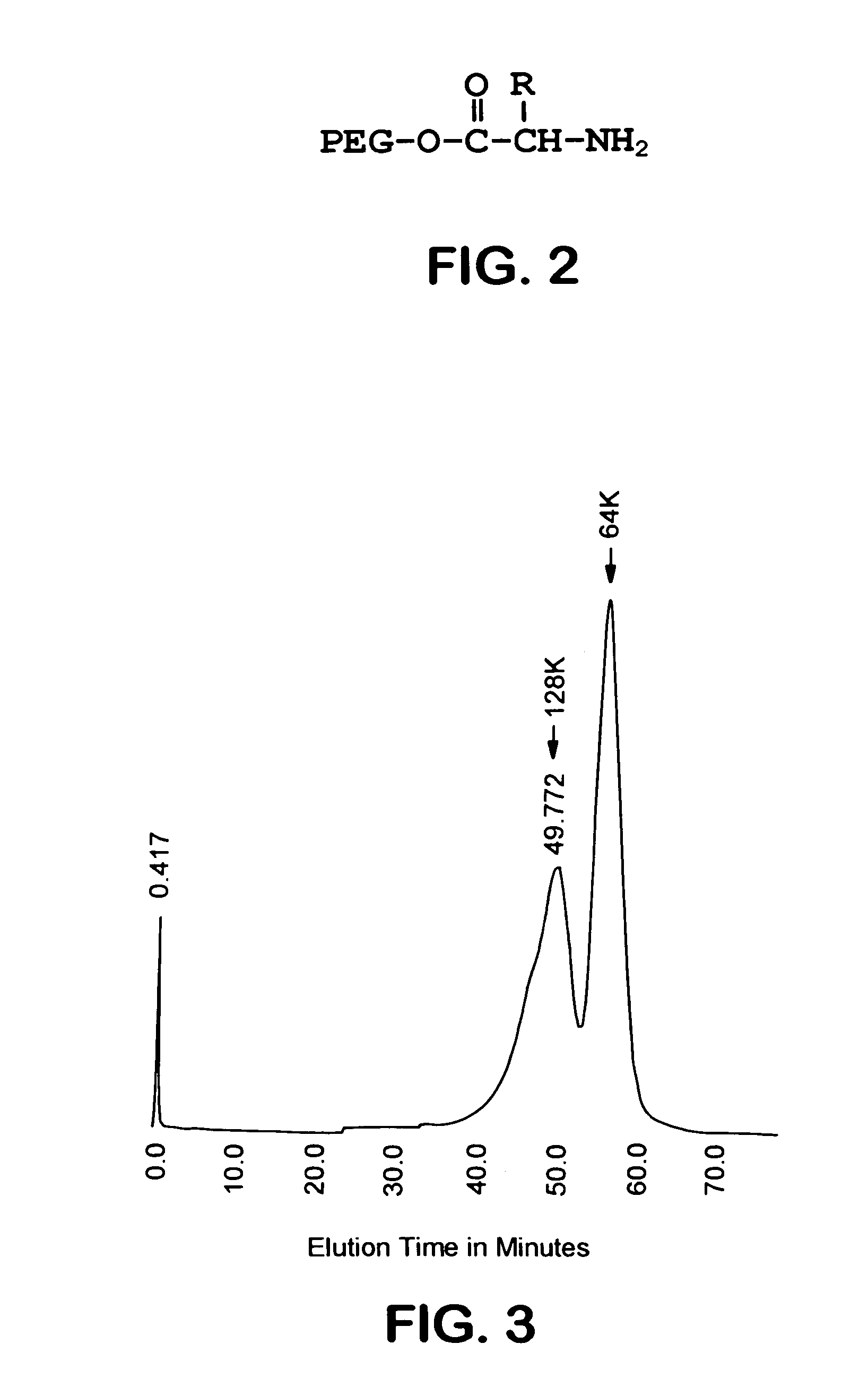
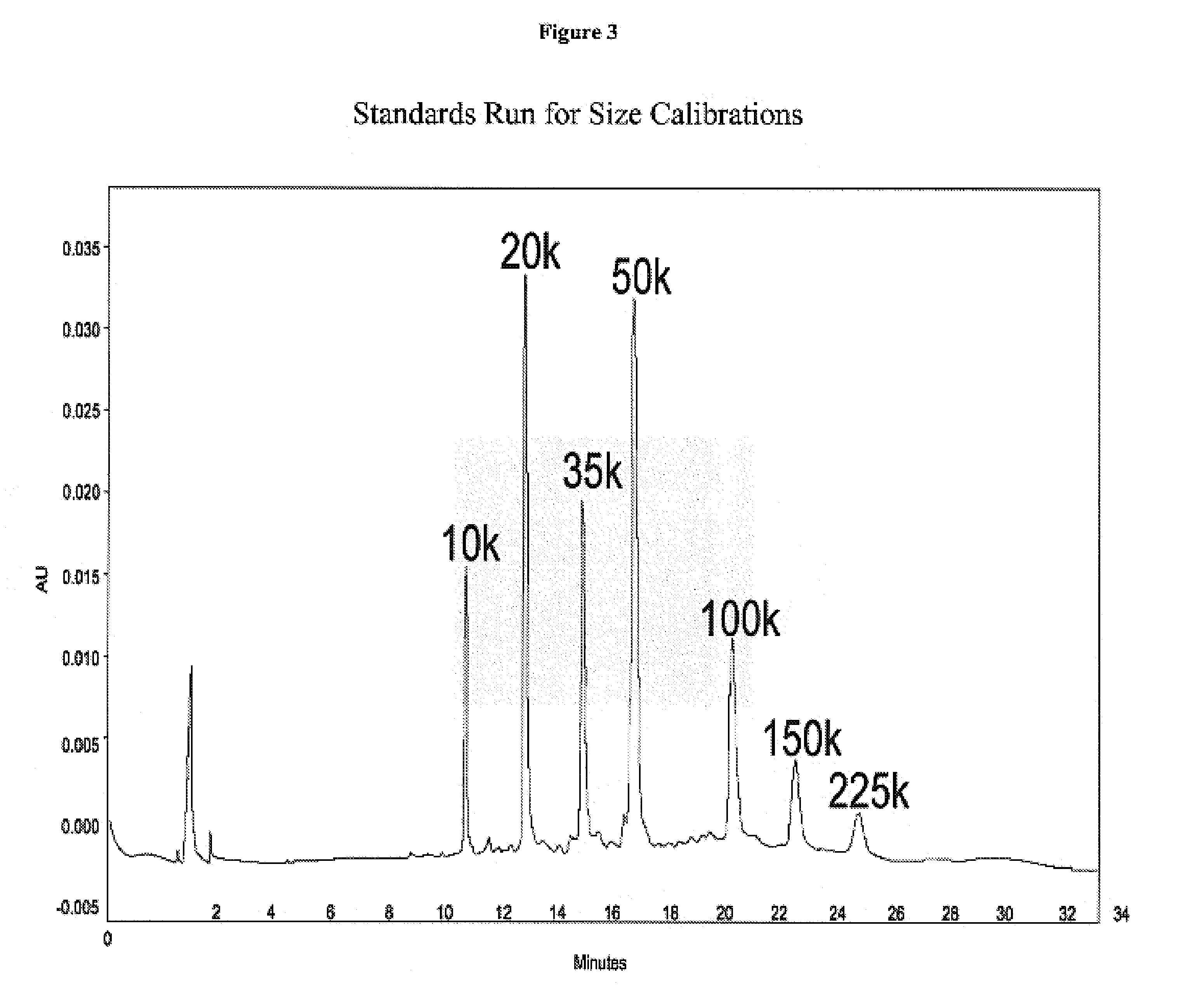
Size enhanced hemoglobins: surface decoration and crosslinking of the protein with polyoxy alkylene glycols
Novel modified hemoglobins comprising polyalkylene glycols and novel methods for making those hemoglobins are provided. One group of modified hemoglobins comprise polyalkylene glycols bonded to the hemoglobin with an amide linkage at Glu-43(β). Additional polyalkylene glycols can also be bonded to the Glu-22(β) and / or the Asp-47(β). These hemoglobins are made by a novel amidation procedure. A second group of modified hemoglobins comprise a polyalkylene glycol covalently bonded to the hemoglobin at the α-amino of a Val-1(β) or a Val-1(α). Additional polyalkylene glycols can optionally be covalently bonded to a limited number of ε-amino groups. This second group of hemoglobins is made using a novel reductive alkylation procedure. A third group of modified hemoglobins comprise a polyalkylene glycol bonded to a thiol group of the hemoglobin through a phenylsuccinimido linkage, wherein no polyalkylene glycol is bonded to a Cys-93(β). This third group of modified hemoglobins is made by an improvement in a hemoglobin-polyalkylene linkage procedure utilizing thiolation-mediated maleimide chemistry.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Nitric oxide-blocked cross-linked tetrameric hemoglobin
InactiveUS7504377B2Increase capacityImprove abilitiesBiocidePeptide/protein ingredientsCross-linkThiol
The present invention includes compositions containing carboxamidomethylated cross-linked hemoglobin where the cysteine moiety of the hemoglobin includes a thiol protecting group and where the hemoglobin has a reduced ability to bind with nitric oxide. Preferably, the hemoglobin is deoxygenated, endotoxin free, and stroma free. The present invention also includes method of preparation, process of preparation and the method of use including supplementing blood volume in mammals and treating disorders in mammals where oxygen delivery agents are of benefit.
Owner:IKOR +1
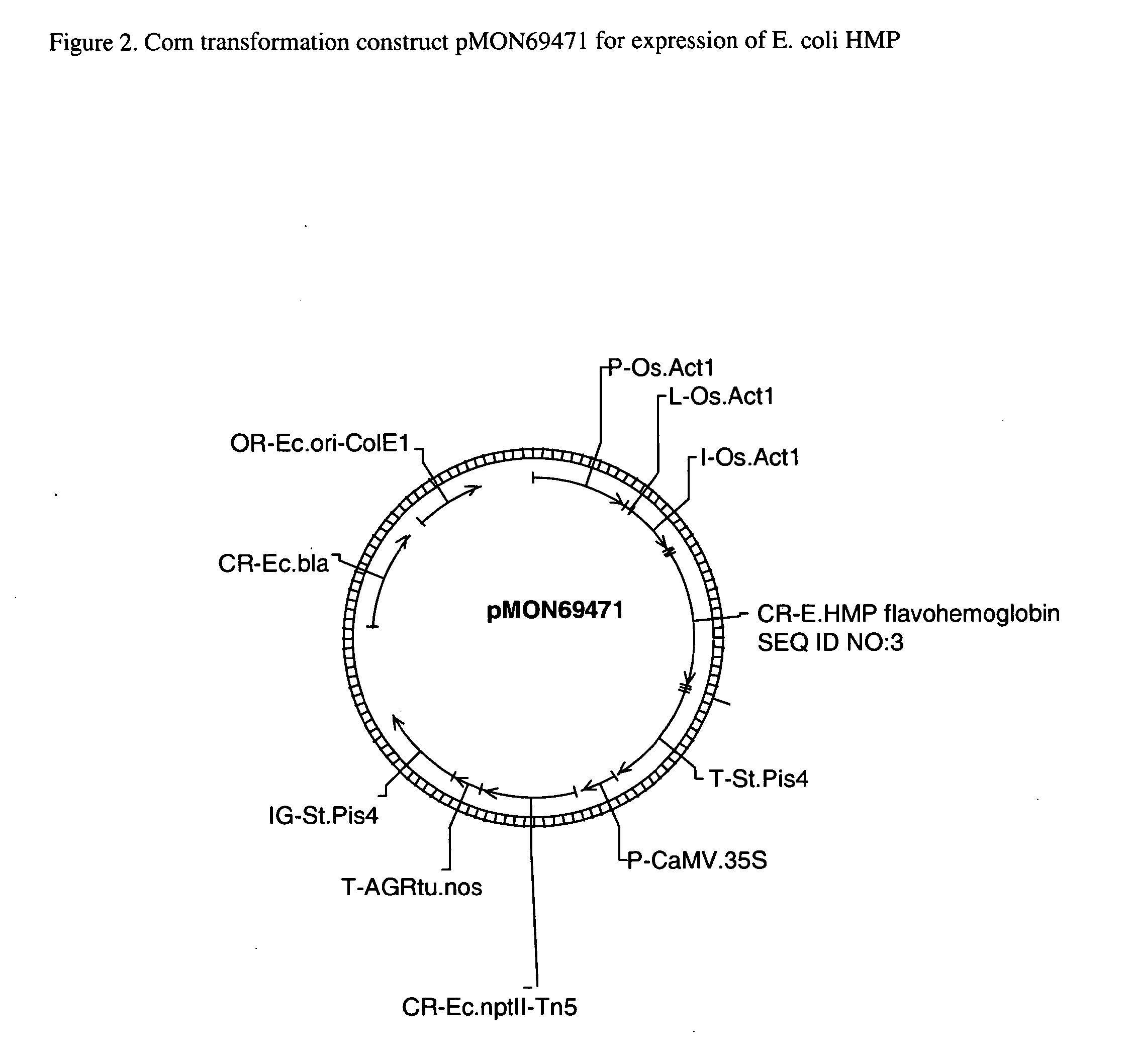
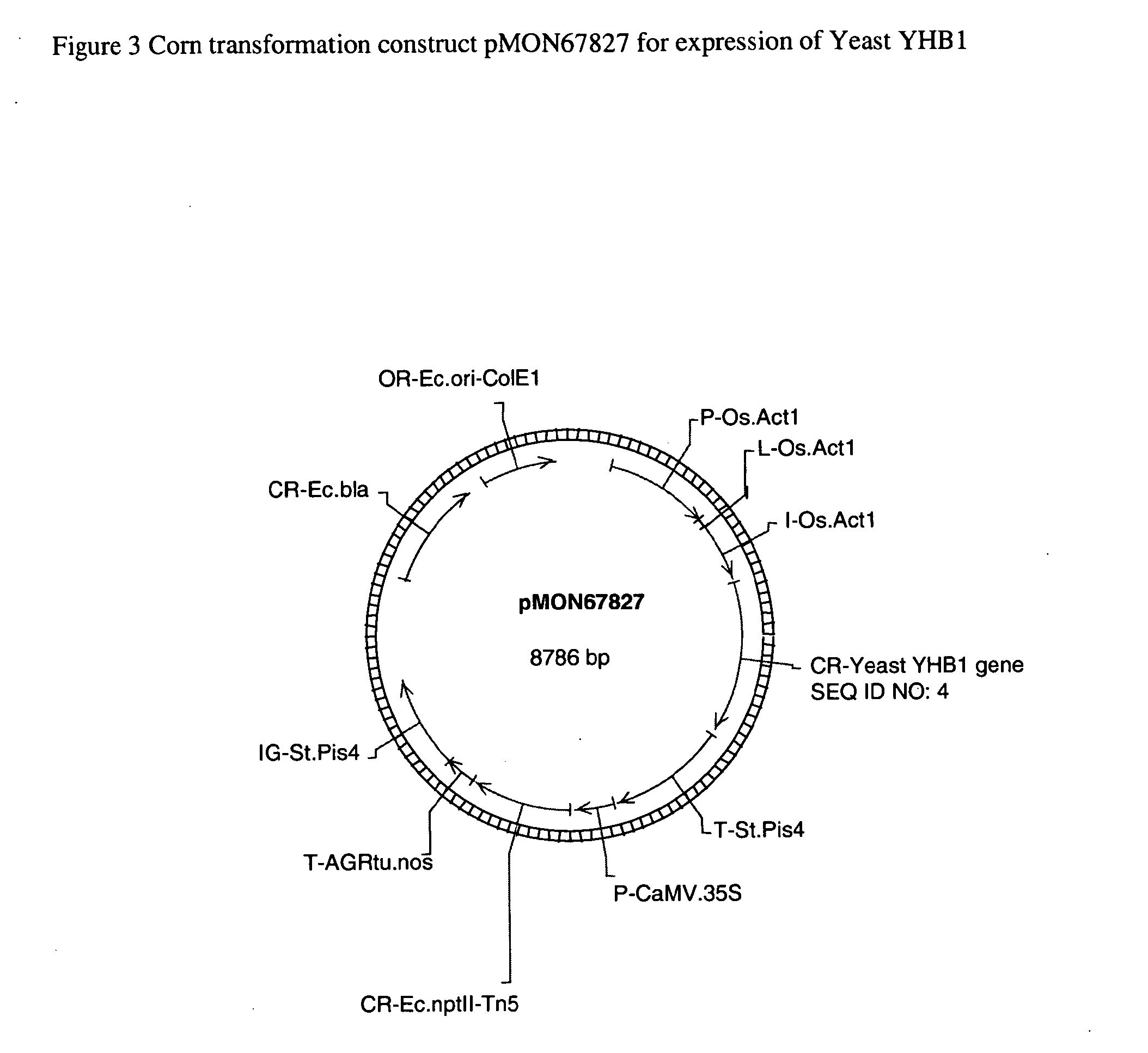
Plants containing a heterologous flavohemoglobin gene and methods of use thereof
InactiveUS20150275224A1Improved agronomic traitHigh speedSugar derivativesHaemoglobins/myoglobinsNitrogenNitric oxide
Plant nitrogen use efficiency in corn has been improved by transformation with a flavohemoglobin gene. Plants comprising a flavohemoglobin gene have decreased nitric oxide (NO) levels, increased biomass accumulation under a sufficient nitrogen growth condition, and increased chlorophyll content under a limiting nitrogen growth condition. Additionally, these transformed plants evidence higher levels of yield.
Owner:MONSANTO TECH LLC
Therapeutic retroviral vectors for gene therapy
ActiveUS20060057725A1Maintaining therapeutic levelImprove the level ofPeptide/protein ingredientsGenetic material ingredientsHemoglobinopathyRed blood cell
Retroviral gene therapy vectors that are optimized for erythroid specific expression and treatment of hemoglobinopathic conditions are disclosed.
Owner:MASSACHUSETTS INST OF TECH
Carboxymethylated cross-linked tetrameric hemoglobin
InactiveUS7494974B2Improve oxygen carrying capacityBiocidePeptide/protein ingredientsCross-linkThiol
The present invention includes compositions containing carboxymethylated cross-linked hemoglobin where the cysteine moiety of the hemoglobin includes thiol protecting group and where the hemoglobin is incapable of binding with the nitric oxide. Preferably, the hemoglobin is deoxygenated, endotoxin free, and stroma free. The present invention also includes method of preparation, process of preparation and the method of supplementing blood in mammals.
Owner:IKOR
Protein expression strains
InactiveUS6379924B1Reduced activityReduction in translatable UBC mRNAFungiHaemoglobins/myoglobinsActive proteinPlasmid
The use of a means to vary Ubc4p or Ubc5p activity in a fungal cell to control the copy number of a plasmid in the cell. The level of Ubc4p or Ubc5p activity may be reduced / abolished (for example by gene deletion, mutagenesis to provide a less active protein, production of antisense mRNA or production of competitive peptides) to raise the copy number and increase yield of a protein encoded by the plasmid.
Owner:ALBUMEDIX AS
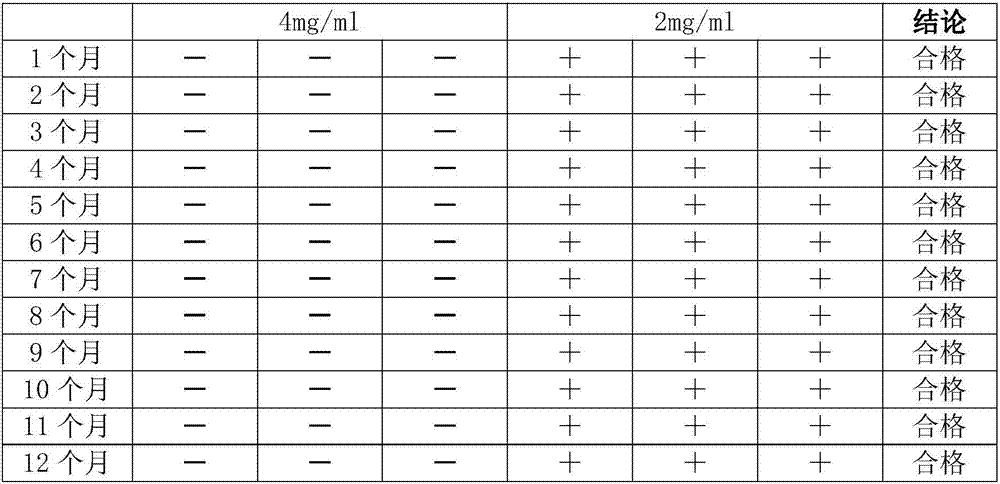
Preparation method of hemoglobin quality control product
ActiveCN107266563AHigh activityMonitor performanceHaemoglobins/myoglobinsPeptide preparation methodsMaterials preparationHigh volume manufacturing
The invention discloses a preparation method of a hemoglobin quality control product. The preparation method comprises following steps: 1, material preparation; 2, washing and centrifugation; 3, obtaining of an erythrocyte solution; 4, extraction of hemoglobin; 5, dialysis; and 6, sub-packaging of freeze-dried powder. According to the preparation method, hemoglobin obtained via dialysis is added into a diluent containing a freeze-drying protective agent and a filling agent for dilution to a set quality control product concentration; after sub-packaging, low temperature pre-freezing and freeze-drying are carried out so as to obtain freeze-dried powder. In the preparation process, the activity of hemoglobin is protected effectively; the form of freeze-dried powder is adopted for storage, so that the stability is high, and the performance of the reagent can be monitored in clinical applications effectively. The preparation method is low in cost; operation is simple; large scale production can be realized; and long term storage can be realized.
Owner:SICHUAN ORIENTER BIOLOGICAL TECH
Ferritin fusion proteins for use in vaccines and other applications
An isolated ferritin fusion protein is provided in which ferritin is fused with a protein or peptide capable of being fused to ferritin without interfering with the polymeric self-assembly of the resulting fusion protein, and the protein may be of the endocapsid form when fused at the C terminus or an exocapsid form when fused at the N terminus. These fusion proteins may self-assemble into a variety of useful higher polymeric forms, e.g., capsid or other polymeric aggregate, and they are advantageous in that they are useful in a variety of applications, including human and veterinary vaccines and therapeutics, blood substitutes, image contrast agents, metal chelating agents, gelling agents, protein purification platforms, and therapeutic receptor-binding proteins.
Owner:NEW CENTURY PHARMA INC
Nucleic acid structure and expression carrier for enhancing recombinant protein production and mass-production of recombinant protein
InactiveCN1712534AImprove propertiesProtection from decompositionHaemoglobins/myoglobinsAntibody mimetics/scaffoldsNucleic acid structureGene product
Nucleic acid construction and expression carrier for improving recombinant protein production and production of recombinant protein are disclosed. The process is carried out by cloning first nucleic acid sequence of coded thioredoxin and second nucleic acid sequence of coded ferrohemoglobin into recombinant host cell, reinforcing the recombinant host cell production and helping to release intracellular stress.
Owner:FENG CHIA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com