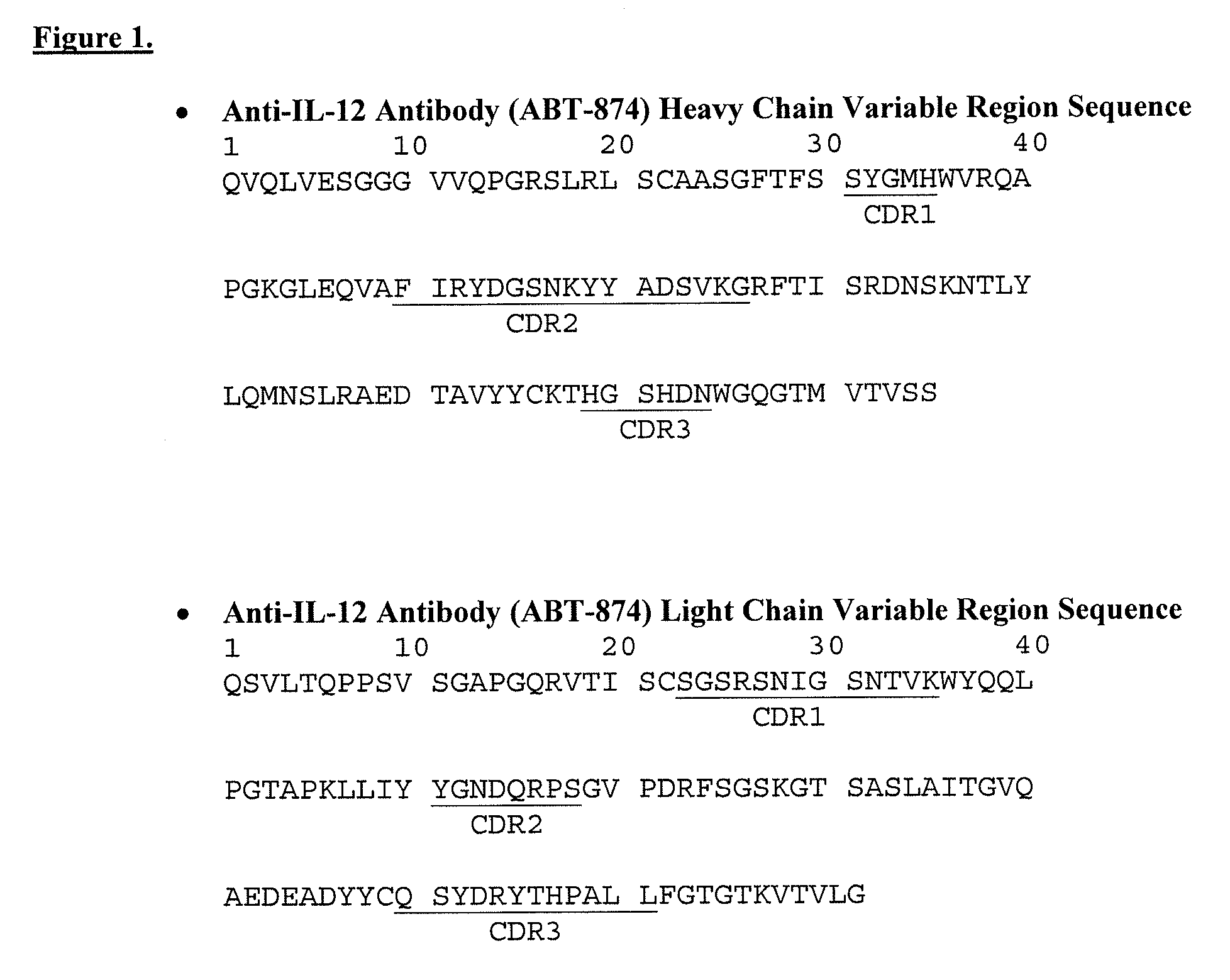
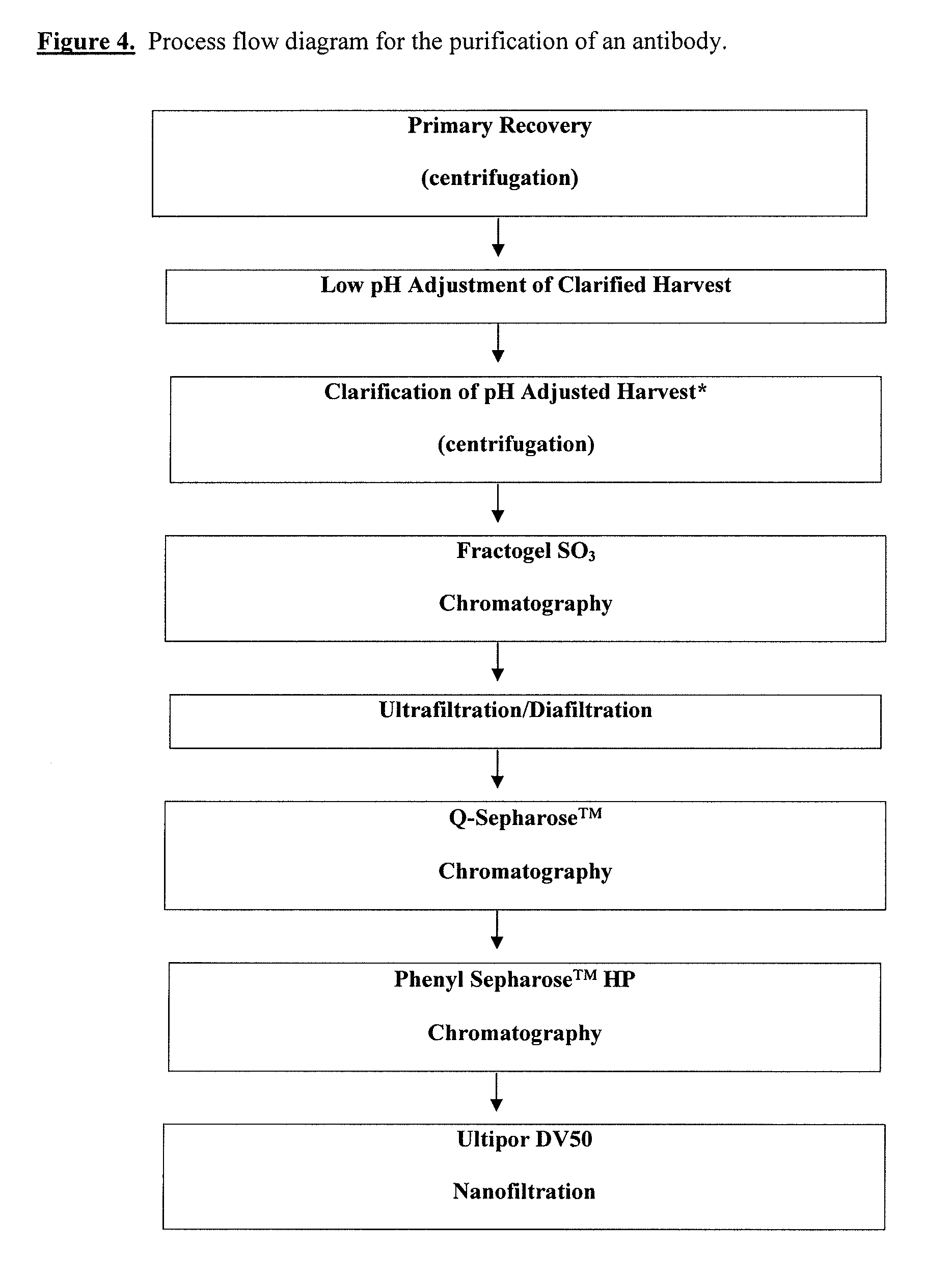
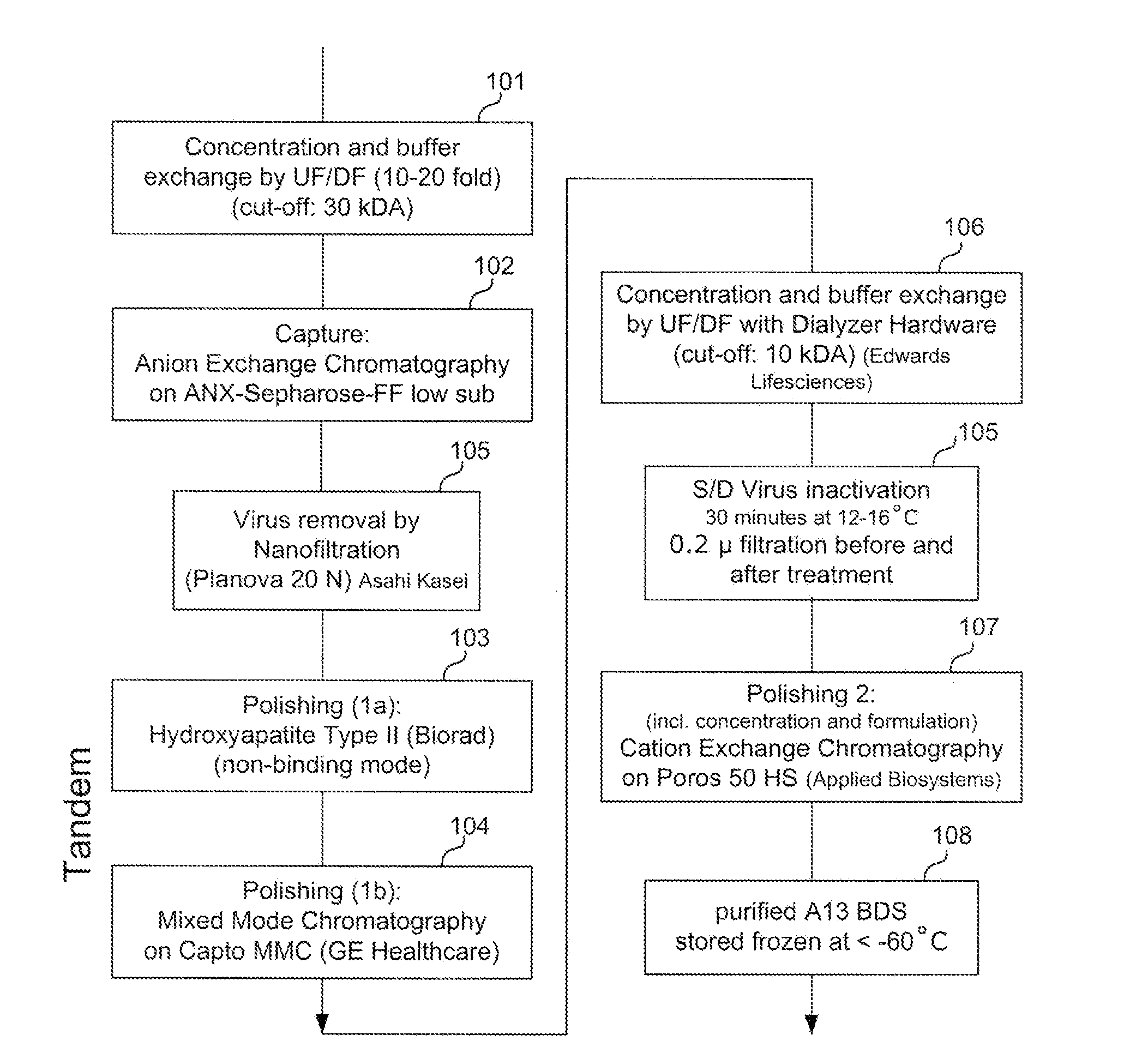
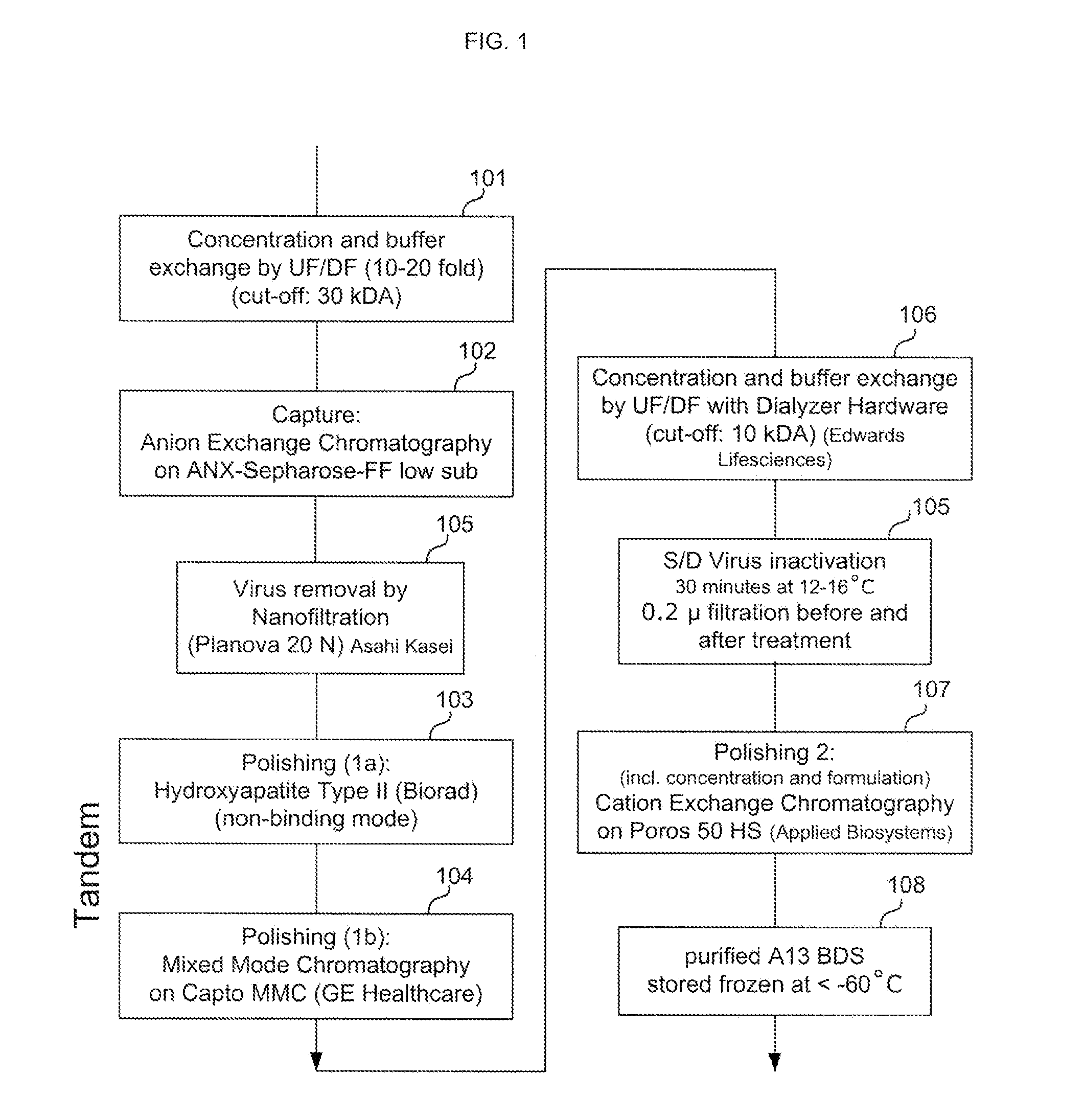
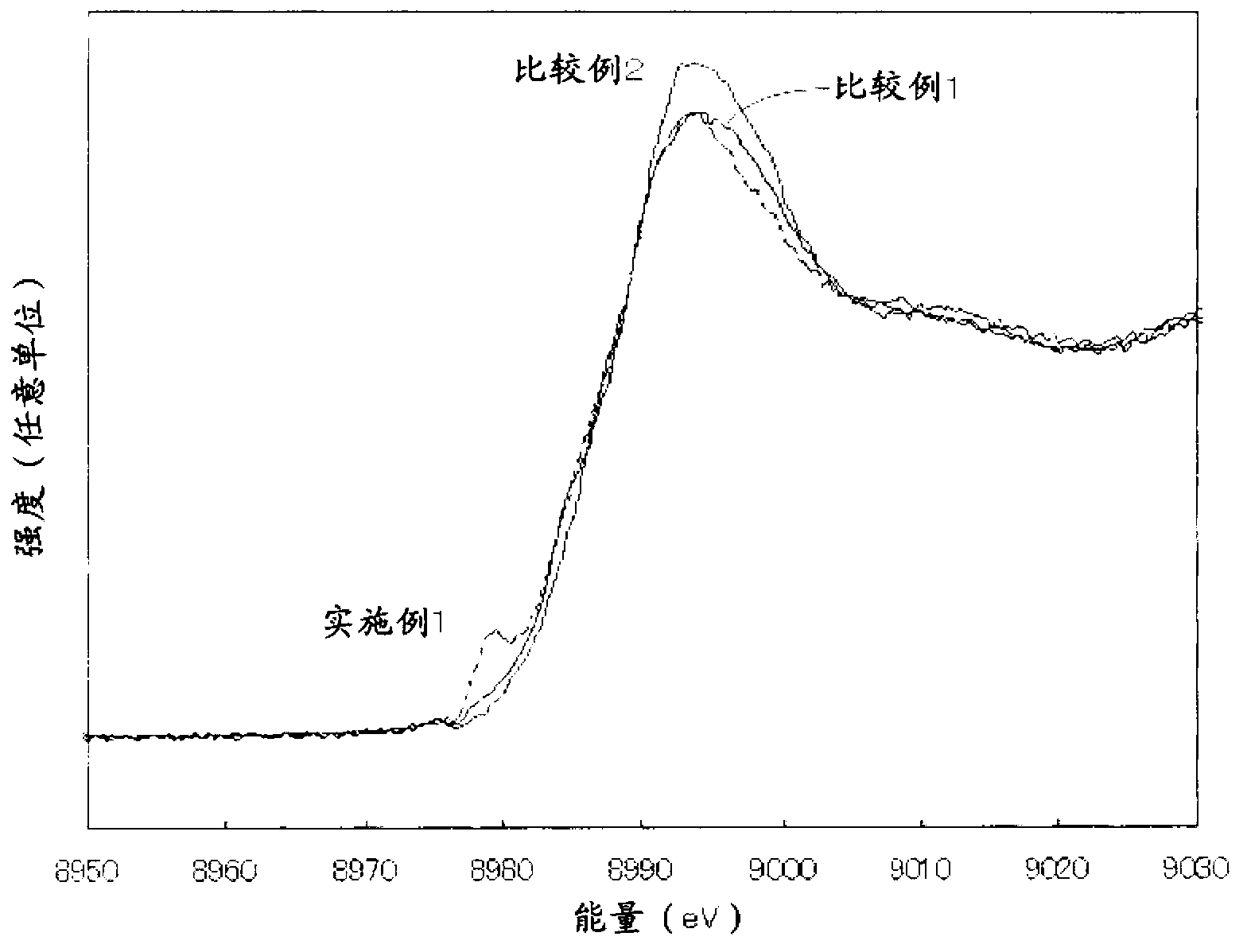
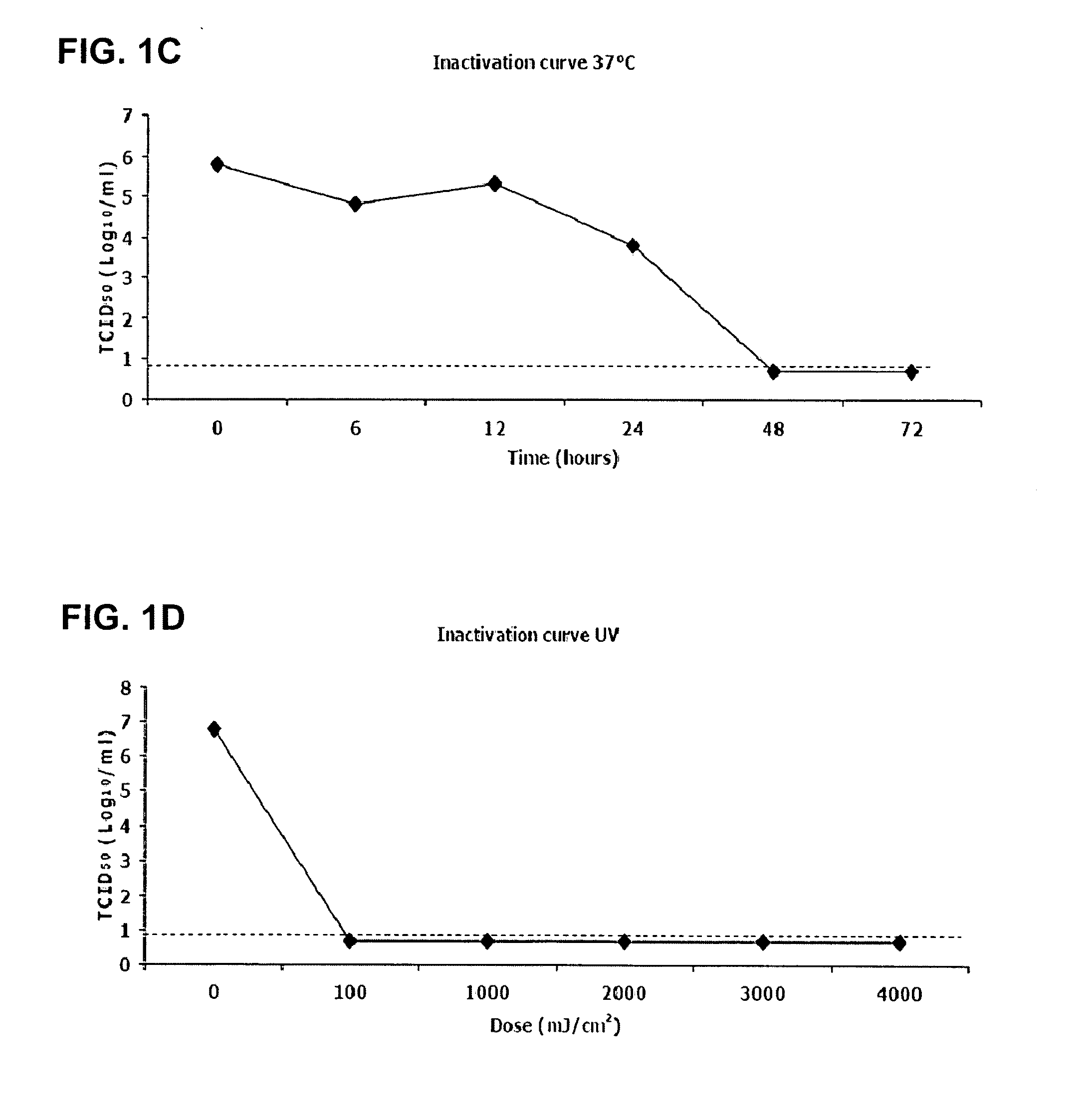
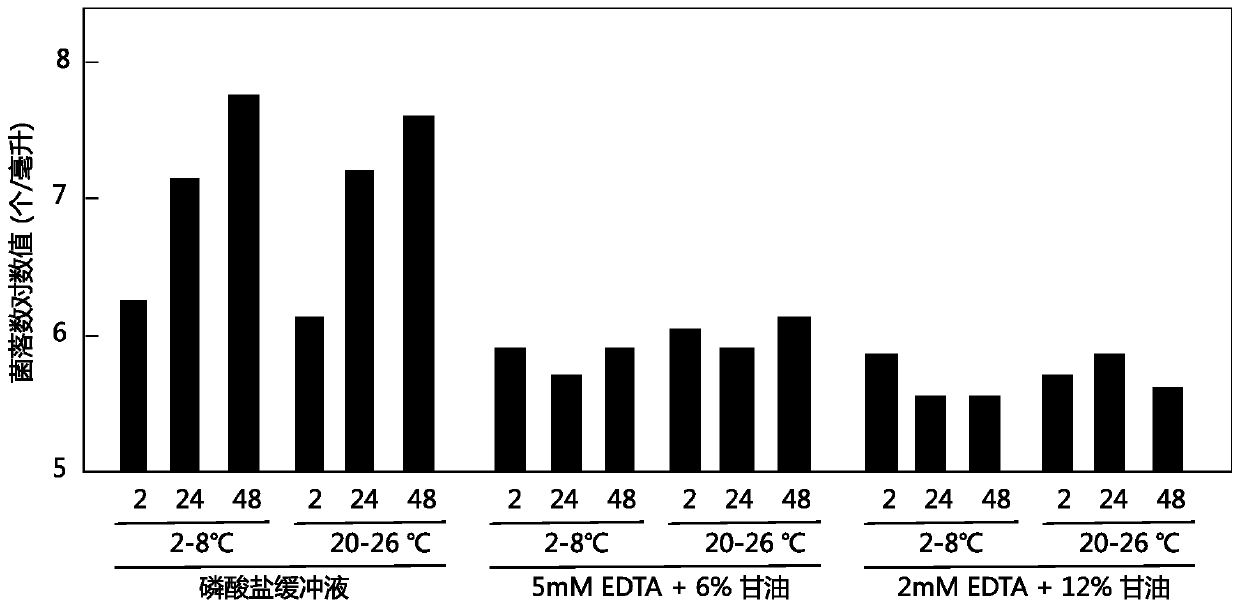
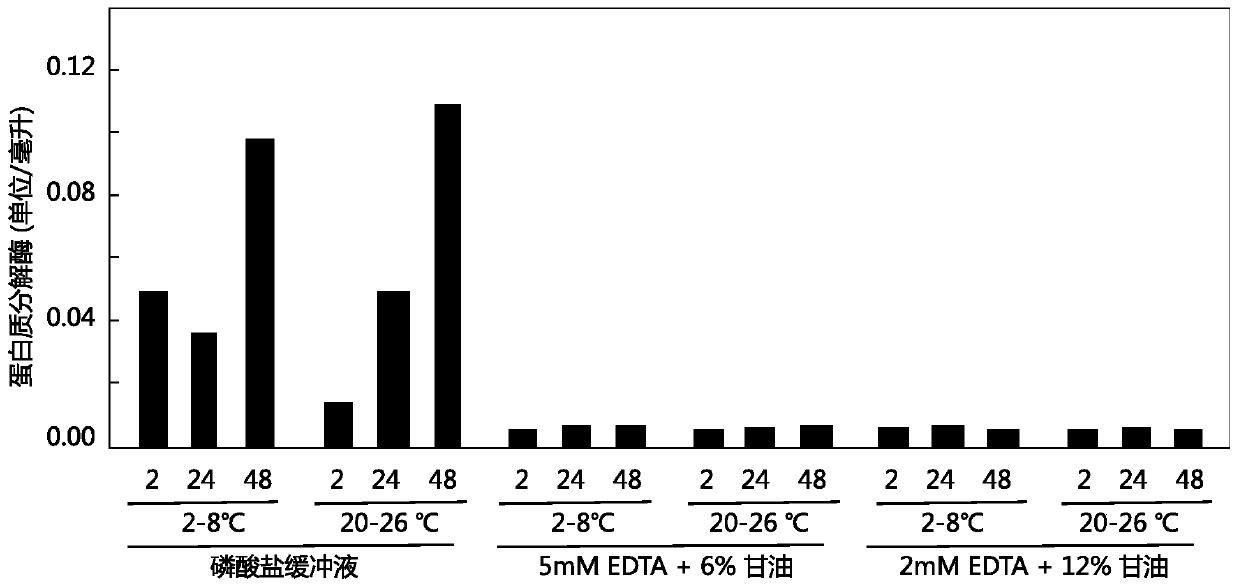
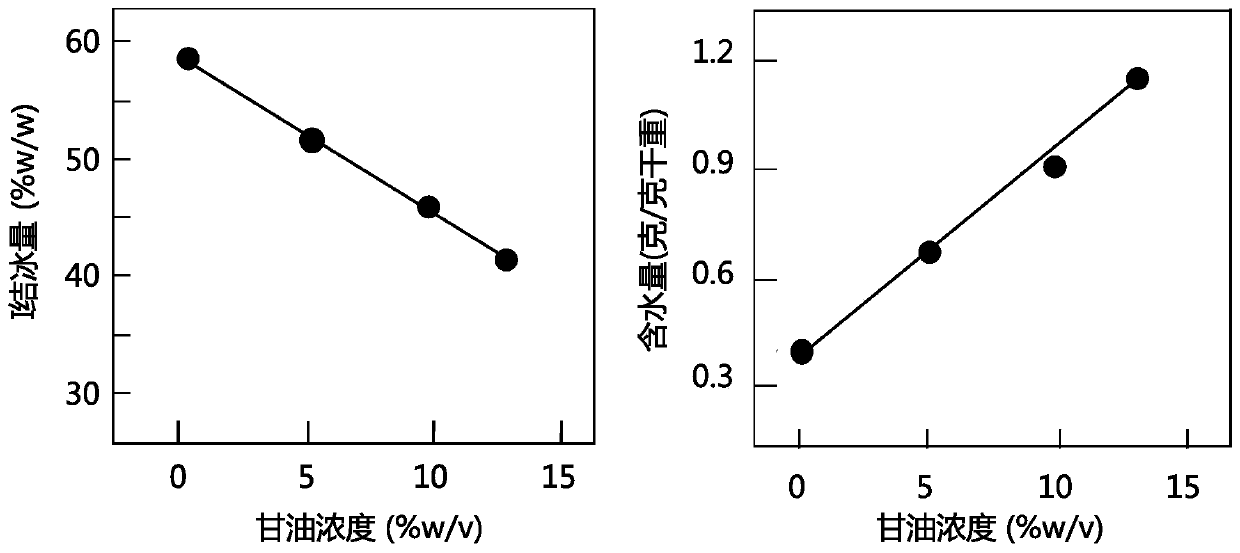
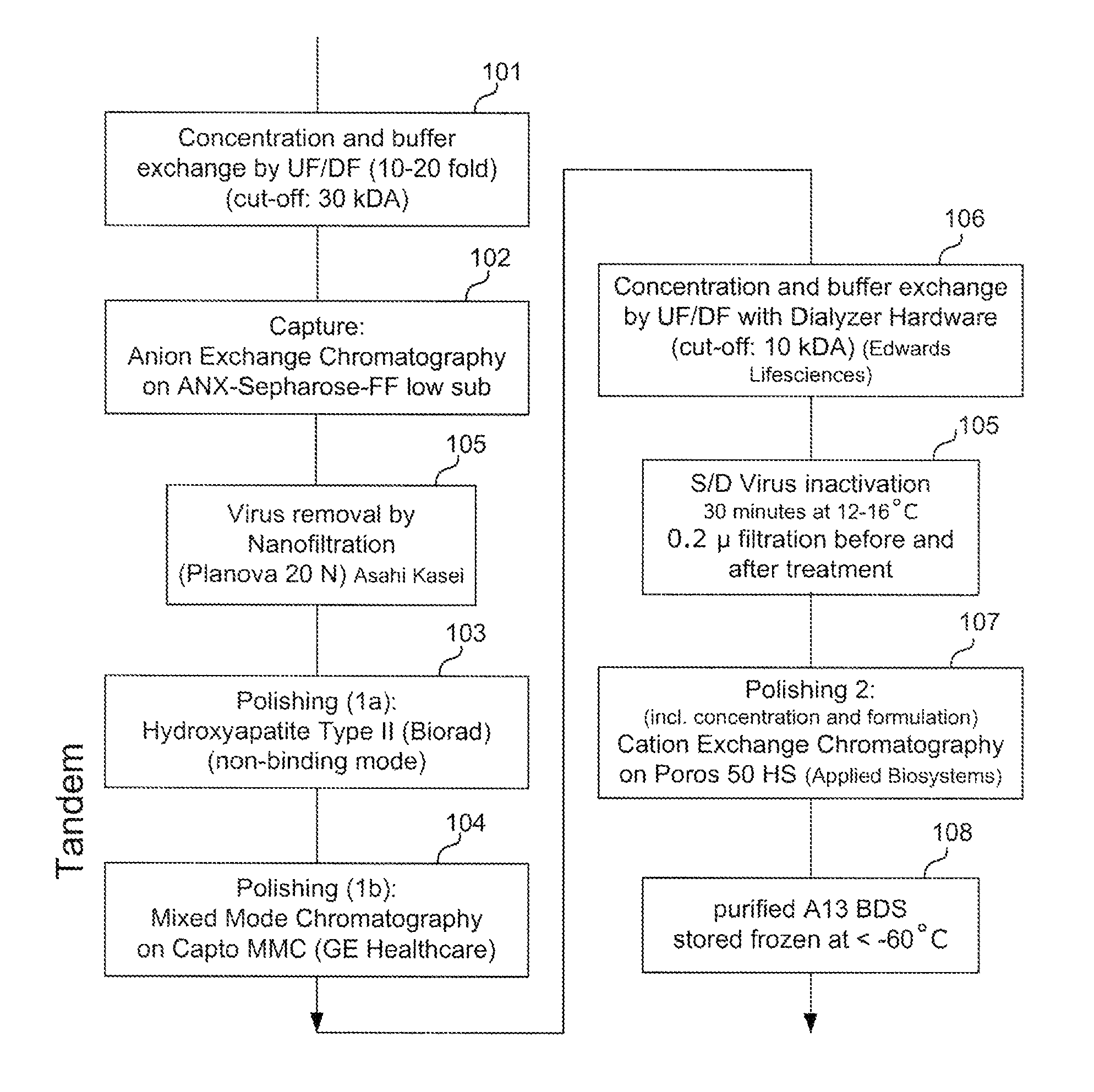
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
199 results about "Viral Inactivation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viral inactivation is different from viral removal because, in the former process, the surface chemistry of the virus is altered and in many cases the (now non-infective) viral particles remain in the final product.
Viral inactivation during purification of antibodies cross reference to related applications
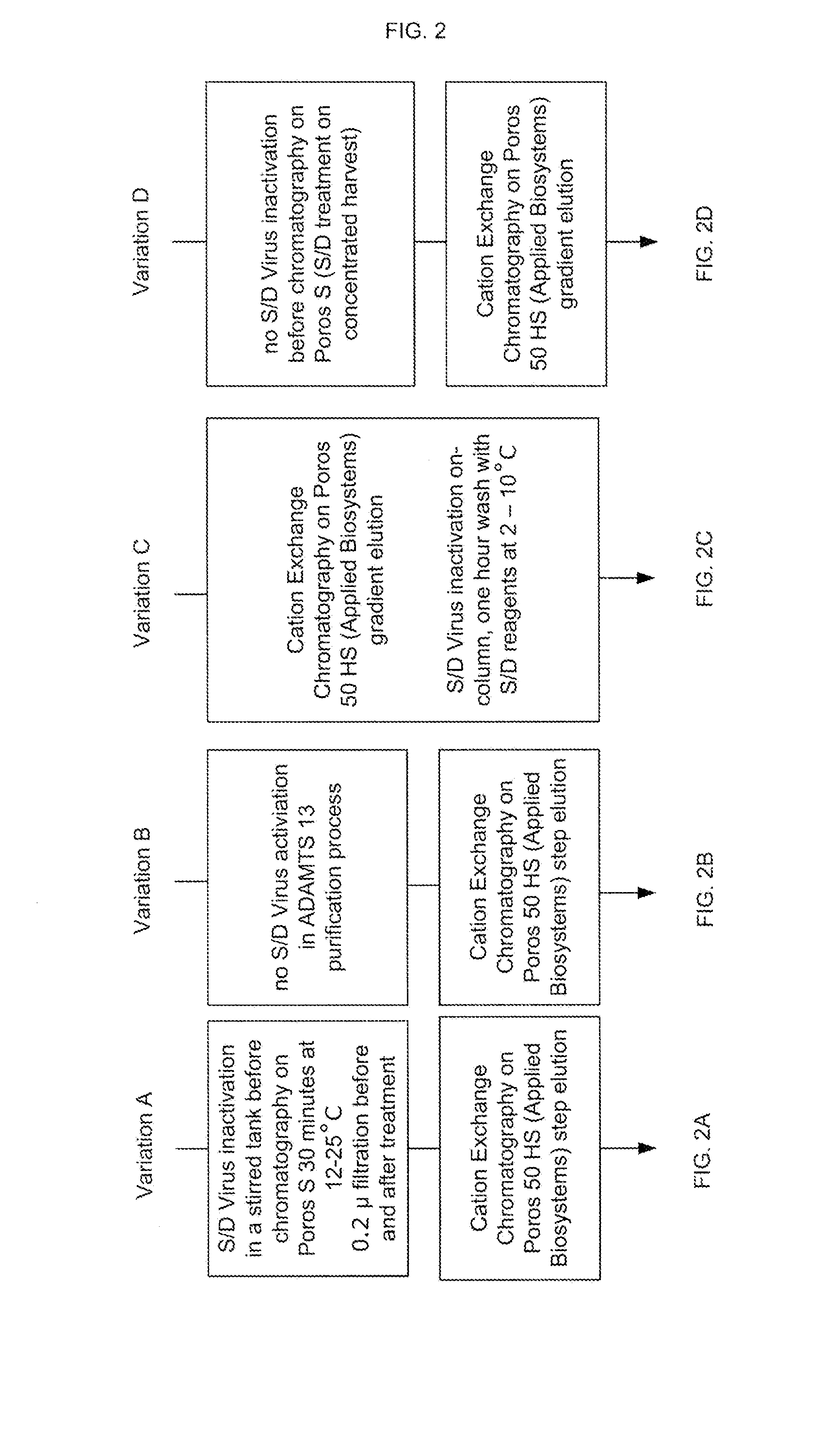
ActiveUS20100136025A1Easy to eliminateEnhanced interactionNervous disorderAntipyreticIon exchangeViral Inactivation
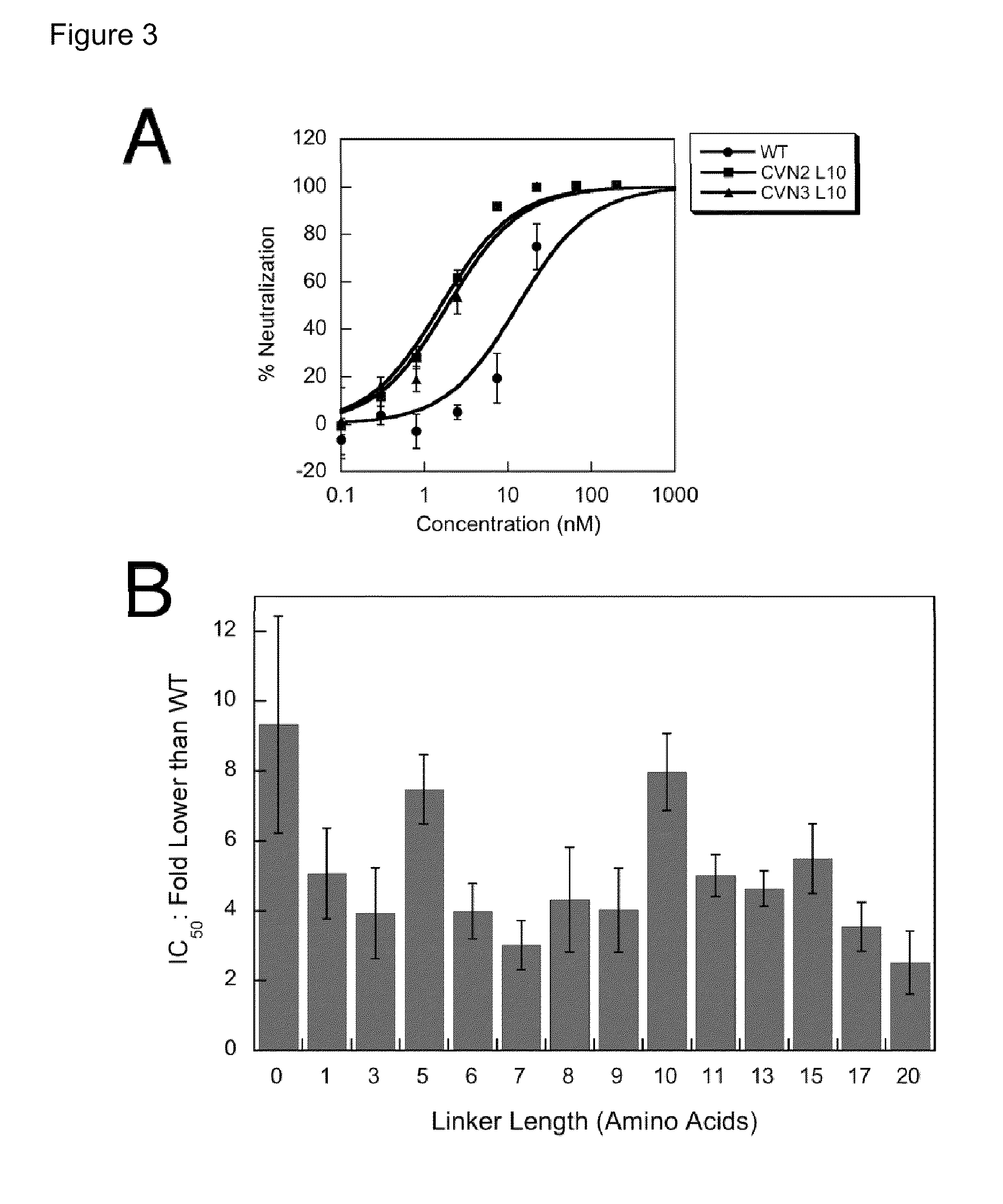
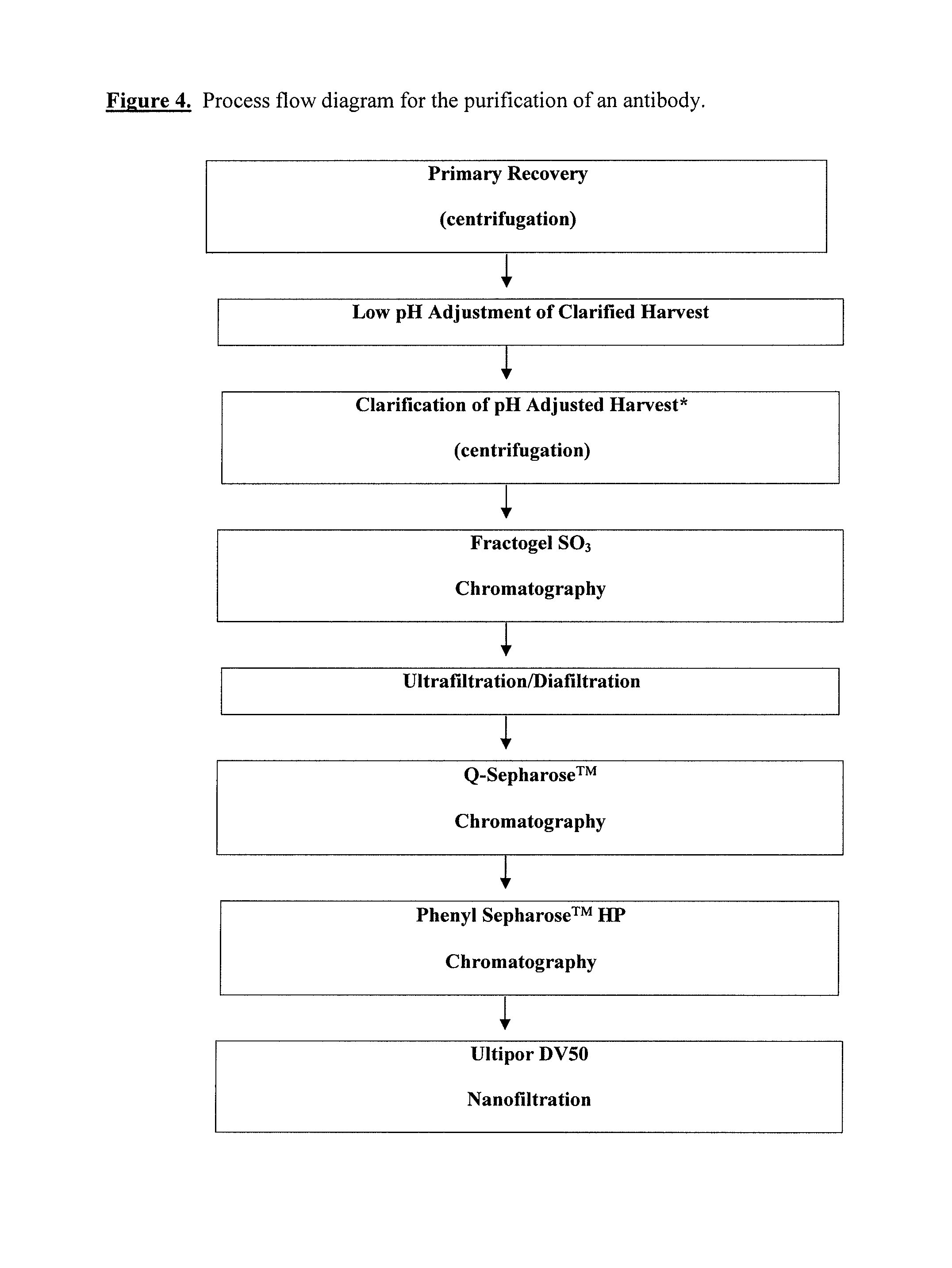
Described herein are methods for isolating and purifying antibodies from a sample matrix. One aspect of the present disclosure is directed to viral reduction / inactivation of samples generated in the various steps of antibody purification. In a particular aspect, methods herein employ an acidification step followed by one or more chromatography steps. The chromatography steps can include one or more of the following chromatographic procedures: ion exchange chromatography, affinity chromatography, and hydrophobic interaction chromatography.
Owner:ABBVIE INC
Cellular and viral inactivation
The invention provides compositions of inactivated viruses, bacteria, fungi, parasites and tumor cells that can be used as vaccines. Methods for making such inactivated viruses, bacteria, fungi, parasites and tumor cells are also provided.
Owner:UNITED STATES OF AMERICA
EB Matrix Production from Fetal Tissues and its Use for Tissue Repair
InactiveUS20020146393A1Reducing biological propertyPreserve strengthBiocideHepatocytesCross-linkTissue repair
<heading lvl="0">Abstract of Disclosure< / heading> A method of forming and preserving a bioremodelable, biopolymer scaffold material by subjecting animal tissue, particularly fetal or neo-natal tissue, to chemical and mechanical processing. The process includes, but is not limited t, harvesting the tissue, optionally extracting growth and differentiation factors from the tissue, inactivating infective agents of the tissue, mechanically expressig undesirable components frm the tissue, delipidizing the tissue, washing the tissue, optionally drying the tissue, and optionally cross-linking the tissue not necessarily in the order described. The resulting product, EBM, is characterized by its microbial, fungal, viral and prion inactivated state. EBM is storng, bioremodelable, drapable and does not undergo calicification. EBM supplants previous inventions because of its unique method of preparation and broad applicability in tissue reengineering
Owner:TEI BIOSCI
Methods of Purifying Recombinant Adamts13 and Other Proteins and Compositions Thereof
ActiveUS20110081700A1Reduce formationExtension of timeInactivation/attenuationBiomass after-treatmentHydroxylapatiteAnion-exchange chromatography
Provided herein are methods for purifying recombinant A Disintegrin-like and Metallopeptidase with Thrombospondin Type 1 Motif 13 (ADAMTS13) protein from a sample. The method comprises enriching for ADAMTS13 protein by chromatographically contacting the sample with hydroxyapatite under conditions that allow ADAMTS13 protein to appear in the eluate or supernatant from the hydroxylapatite. The methods may further comprise tandem chromatography with a mixed mode cation exchange / hydrophobic interaction resin that binds ADAMTS13 protein. Additional optional steps involve ultrafiltration / diafiltration, anion exchange chromatography, cation exchange chromatography, and viral inactivation. Also provided herein are methods for inactivating virus contaminants in protein samples, where the protein is immobilized on a support. Also provided herein are compositions of ADAMTS13 prepared according to said methods.
Owner:TAKEDA PHARMA CO LTD
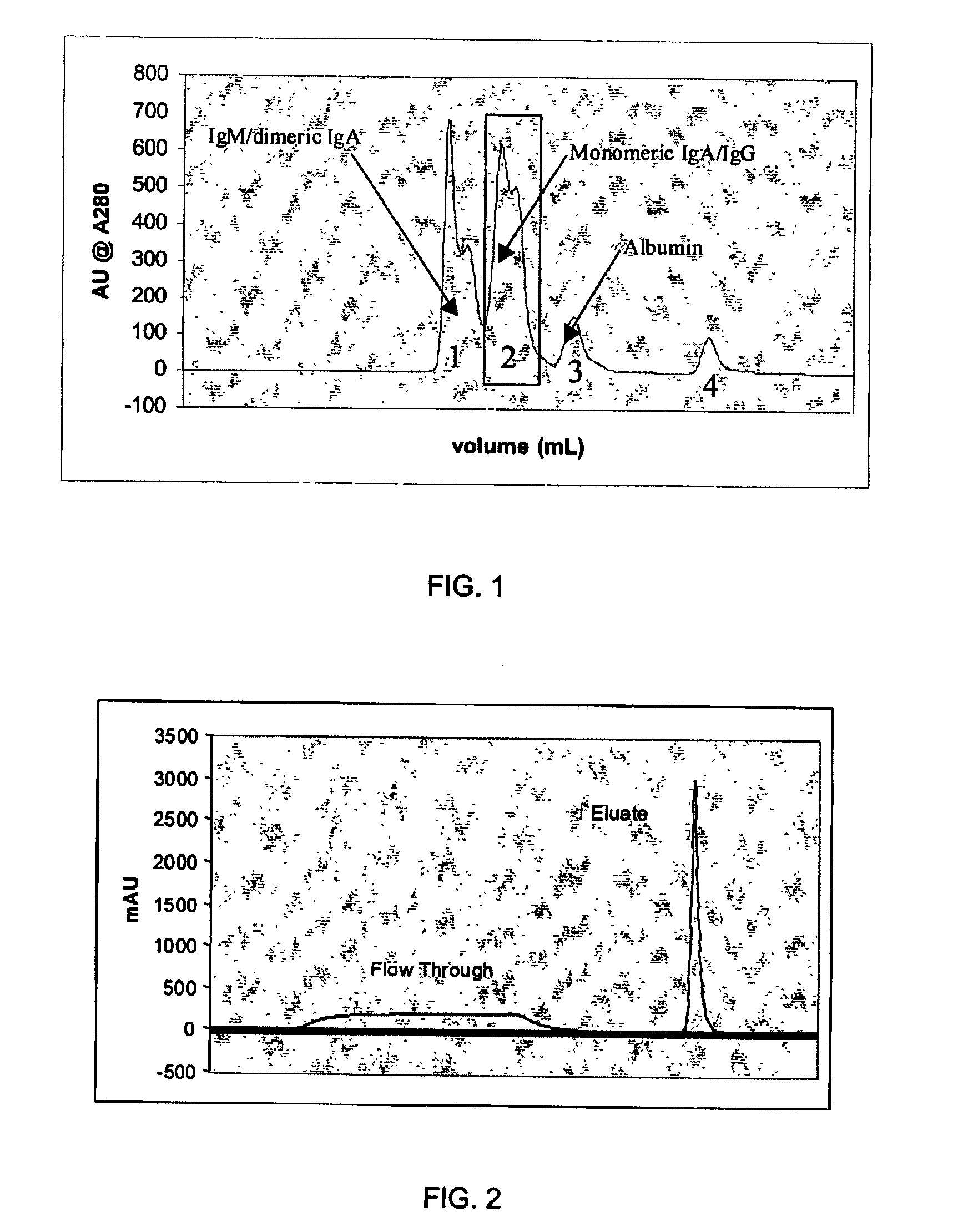
Chromatographic method for high yield purification and viral inactivation of antibodies
InactiveUS6955917B2Minimizes post virus treatment manipulationYield maximizationPeptide/protein ingredientsSerum immunoglobulinsLipid formationLow ionic strength
An improved process for the purification of antibodies from human plasma or other sources is disclosed. The process involves suspension of the antibodies at pH 3.8 to 4.5 followed by addition of caprylic acid and a pH shift to pH 5.0 to 5.2. A precipitate of contaminating proteins, lipids and caprylate forms and is removed, while the majority of the antibodies remain in solution. Sodium caprylate is again added to a final concentration of not less than about 15 mM. This solution is incubated for 1 hour at 25° C. to effect viral inactivation. A precipitate (mainly caprylate) is removed and the clear solution is diluted with purified water to reduce ionic strength. Anion exchange chromatography using two different resins is utilized to obtain an exceptionally pure IgG with subclass distribution similar to the starting distribution. The method maximizes yield and produces a gamma globulin with greater than 99% purity. The resin columns used to obtain a high yield of IgG retain IgM and IgA. IgA and IgM may be eluted from these resins in high yield and purity.
Owner:BAYER HEALTHCARE LLC
Engineered lectins for viral inactivation
ActiveUS20090297516A1Easy to neutralizeGood curative effectAntibacterial agentsBiocideHalf-lifeComplement-dependent cytotoxicity
Engineered lectins and methods of using such reagents for both preventing and treating a broad array of viral infections are provided. The lectins of the invention are engineered in two ways, first through the enhancement of the natural mode of action of lectins against viruses through linked multimerization, and second through the creation of a new class of reagents, hereinafter referred to as a “lectibody” or “lectibodies”, that engage host immune function in addition to simply binding glycosylated viral proteins via the combination of a lectin and the Fc region of an antibody in order to drive Fc-mediated effector functions including ADCC (antibody-dependent cell-mediated cytotoxicity), increased half-life, complement-dependent cytotoxicity (CDC), and antibody-dependent cell-mediated phagocytosis (ADCP) in response to a lectin-mediated carbohydrate-binding event.
Owner:CALIFORNIA INST OF TECH
Methods and Compositions for Simultaneously Isolating Hemoglobin from Red Blood Cells and Inactivating Viruses
InactiveUS20080138790A1Facilitate viral inactivationFacilitating red blood cell lysisHaemoglobins/myoglobinsCentrifugal force sediment separationLysisRed blood cell
The present invention relates to methods arid compositions for isolating hemoglobin from red blood cells. Such methods and compositions also facilitate viral inactivation in a manner that allows recovery of biologically active hemoglobin. More particularly, this method relates to the use of solvents and detergents that are capable of facilitating red blood cell lysis to release hemoglobin (and solubilizing red blood cell membranes), while simultaneously inactivating viruses.
Owner:SANGART INC
Method for preparing human body or animal accellular tissues
ActiveCN103191466AReasonable formulaReduce procurement costsTissue cultureAbsorbent padsHuman bodyCell-Extracellular Matrix
The invention discloses a method for preparing human body or animal accellular tissues. According to the method, the decellularized tissue obtained by treating human or animal tissues, such as skin, pleuroperitoneal membrane, esophagus mucous membrane, small intestine mucous membrane, pericardium, vessel, nervus, cardiac valves, bone, cartilage, muscle tendon, and the like through processes of inactivation of virus, derosination, decellularization, and the like is an acellular three-dimensional network structure tissue, and extracellular matrix components are kept. The tissue prepared by the method can be used as an implantable repair material for a human body and also can be used as a support material of tissue engineering cell culture. The skin, the pleuroperitoneal membrane, the esophagus mucous membrane, the small intestine mucous membrane, the pericardium, and the like also can be used as a wound covering material.
Owner:上海优越生物医学科技有限公司
Decellularization cornea preparation method
The invention relates to a decellularization cornea preparation method, which adopts steps of corneal epithelium layer removing, ultraviolet cross-linking, viral inactivation, decellularization treatment, gradient dehydration, radiation protection and sterilization. According to the present invention, the prepared decellularization cornea has characteristics of complete antigen removing, no excitation of host acquired immunity reaction, good biocompatibility, low damage on nature corneal stroma, maintaining of structure characteristics of the nature corneal, and maintaining of effective components of the nature corneal so as to provide physical and chemical properties similar to the nature corneal; and after the prepared decellularization cornea is transplanted, animal experiment results show that characteristics of transparent transplanted cornea, no scar formation, no melting generation and no neovascularization are provided, the transplanted cornea and the recipient bed are completely integrated after transplanting a month, the transplanted cornea is subjected to complete epithelization after three months, corneal stroma cells migrate toward the decellularization cornea graft so as to prove that the tissue just takes place slow reconstruction, and the transplanted cornea after 6 months does not show significant difference in histology and appearance detection compared with the nature cornea.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
Method for stabilizing a cryoprecipitate of plasmatic proteins for being subjected to a viral inactivation thermal treatment
ActiveUS20060247426A1Shorten the timeImprove protectionPowder deliveryPeptide/protein ingredientsCryoprecipitateMedicine
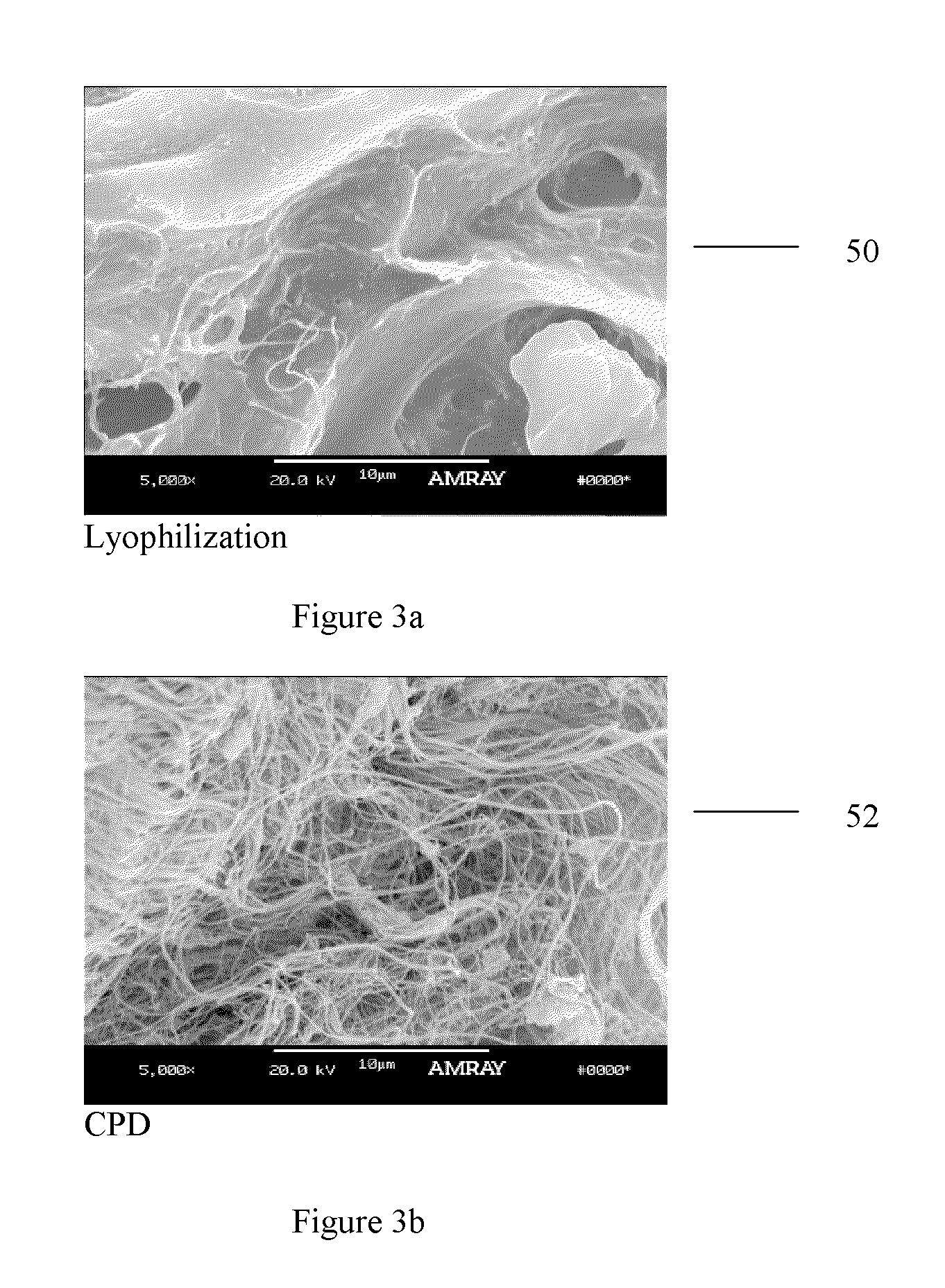
The invention relates to a method for obtaining cryoprecipitatable proteins, comprising a viral inactivation step by thermally treating a lyophilisate of these proteins, comprising, before rendering the proteins in the form of a lyophilisate, an initial addition step, to these proteins, of a stabilizing and solubilizing formulation containing a mixture consisting of arginine, at least one hydrophobic amino acid and of tribasic sodium citrate. The invention also relates to a concentrate consisting of at least one cryoprecipitable protein containing the stabilizing and solubilizing formulation introduced according to the method and being suited for therapeutic use.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Viral inactivation during purification of antibodies cross reference to related applications
ActiveUS9109010B2Easy to disassembleReduce or inactivate pH-sensitive virusesNervous disorderAntipyreticIon exchangeViral Inactivation
Described herein are methods for isolating and purifying antibodies from a sample matrix. One aspect of the present disclosure is directed to viral reduction / inactivation of samples generated in the various steps of antibody purification. In a particular aspect, methods herein employ an acidification step followed by one or more chromatography steps. The chromatography steps can include one or more of the following chromatographic procedures: ion exchange chromatography, affinity chromatography, and hydrophobic interaction chromatography.
Owner:ABBVIE INC
Method of treating bone tissue
In one embodiment, the method comprises providing tissue, preparing the tissue, and treating the tissue to improve remodeling characteristics of the tissue. The tissue may be, for example, cortical bone. Treating the tissue to improve remodeling characteristics may comprise heating the tissue, treating the tissue with a chemical, or other. Heating the tissue may be done in the absence of oxygen and may comprise heating the tissue in a vacuum, heating the tissue in an inert atmosphere, heating the tissue in a reducing atmosphere, coating the tissue with a protective coating and heating the tissue, or other. Further embodiments comprise treating the tissue in supercritical fluids, for example, to dry or virally inactivate the tissue.
Owner:WARSAW ORTHOPEDIC INC
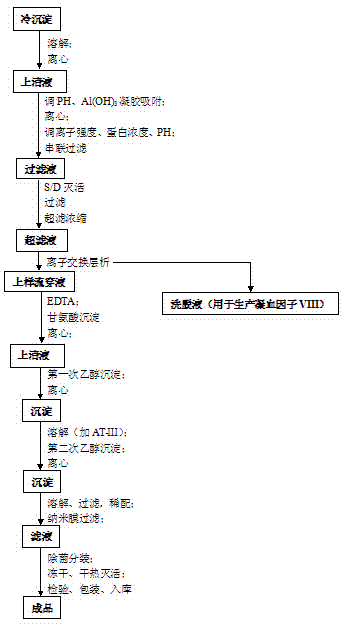
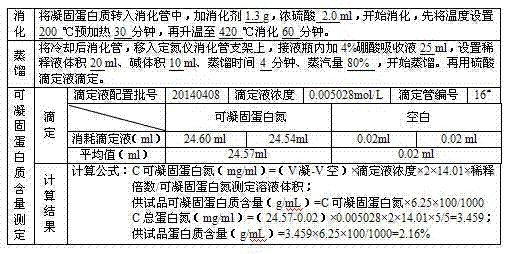
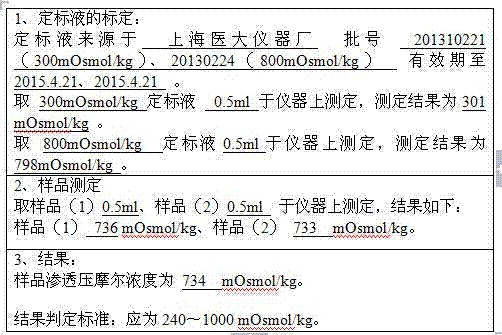
Preparation process for extracting human fibrinogens from waste for extracting cryoprecipitated blood coagulation factor VIII
ActiveCN104231072AHigh purityShort reconstitution timeFactor VIIFibrinogenBlood coagulation factor VIIIEthanol precipitation
The invention discloses a preparation process for extracting human fibrinogens from waste for extracting cryoprecipitated blood coagulation factor VIII. The preparation process is characterized by comprising the steps of cryoprecipitation dissolution, 2% aluminium hydroxide gel absorption, ion strength adjustment, series connection filtering, S / D viral inactivation, ion-exchange chromatography, EDTA Ca2+ removal, glycine precipitation, primary low-temperature ethanol precipitation, AT-III thrombin inhibition, secondary low-temperature ethanol precipitation, nanofilm filtering and dry-heat inactivation. For ensuring the safety, a nanofilm ia added to filter virus except S / D and dry-heat inactivation. By means of an added AT-III inactivated thrombin and EDTA Ca2+ removal process, fibrinogen in the production process is effectively prevented from being activated into fibrous protein. The glycine precipitation is utilized to remove fibrous protein monomers and polymers in products so as obtain high-purity human fibrinogen. The preparation products are safe and reliable, redissolution time is short, the clinic first-aid demand is met, and meanwhile the preparation process has important significance on indirect saving of scarce plasma resources.
Owner:华润博雅生物制药集团股份有限公司
Sanitization of chromatographic media
InactiveUS20050006307A1Allow useIon-exchange process apparatusSemi-permeable membranesViral InactivationChaotropic agent
A method of sanitizing chromatographic media is provided. The method includes contacting the media with an acidic chaotropic agent, at low temperature and low pH. The method provides pathogen removal and / or inactivation, including viral inactivation in particular embodiments.
Owner:BAYER HEALTHCARE LLC
Virus sample preservation solution as well as preparation method and application thereof
ActiveCN111718908AAchieve preservationWide applicabilitySsRNA viruses positive-senseMicrobiological testing/measurementViral InactivationBiological organism
The invention provides a virus sample preservation solution as well as a preparation method and application thereof, and relates to the technical field of biology. The virus sample preservation solution provided by the invention is prepared from a buffer compound with specific concentration, a chaotropic agent, a detergent and a chelating agent. Through the mutual matching of the specific components with the specific concentration, the virus sample preservation solution provided by the invention has the advantages of safety, stability and high efficiency; viruses can be inactivated within 10 minutes; the virus sample nucleic acid can be stably preserved. In addition, the preservation on the virus sample can be realized without ultra-low temperature environment; and the virus sample preservation solution can be applicable to the virus sample collection and transportation in clinic and field environment.
Owner:HANGZHOU BIOER TECH CO LTD
Coxsackie virus A6 strain (WF057R) and applications thereof
InactiveCN106947745AProtectiveGood repeatabilityOrganic active ingredientsSsRNA viruses positive-senseTGE VACCINEViral Inactivation
The invention discloses a Coxsackie virus A6 strain (WF057R) and applications thereof. The preservation number of the Coxsackie virus A6 strain (WF057R) in China General Microbiological Culture Collection Center (CGMCC) is CGMCC No.13393. A vaccine and CVA6 antiserum prepared from WF057R can treat and prevent diseases caused by CVA6. Furthermore, the maternal-transferred antibody of WF057R also has a protective effect on newborn mice. WF057R can also be used to establish a stable Coxsackie virus A6 infected animal model with good repeatability, and the animal model can be applied to drug antivirus treatment and immune protective effect evaluation of viral inactivation vaccines.
Owner:TAISHAN MEDICAL UNIV
Methods for viral inactivation using eco-friendly detergents
ActiveUS20160333046A1Maintain product qualityIncreased FragmentationPeptide/protein ingredientsInactivation/attenuationViral Inactivation
The invention provides for methods of inactivating virus in product feedstream in a manufacturing process of a therapeutic polypeptide using an environmentally compatible detergent. The environmentally compatible detergent does not adversely affect product quality while effectively inactivating virus in the feedstream.
Owner:GENENTECH INC
Method for preparing human blood coagulation factors IX and VII subcutaneously from cold-glue-removed blood plasma
InactiveCN105330736AIncrease profitSimple processPeptide preparation methodsBlood coagulation/fibrinolysis factorsUltrafiltrationEngineering
The invention discloses a method for preparing human blood coagulation factors IX and VII subcutaneously from cold-glue-removed blood plasma. The method comprises the following steps: 1, removing cold glue from blood plasma; 2, conducting strong anion-exchange column chromatography the first time; 3, conducting PEG sedimentation for removing impure protein; 4, conducting S / D viral inactivation; 5, conducting strong anion-exchange column chromatography the second time, and obtaining FVII eluent and FIX eluent; 6, conducting weak anion-exchange column chromatography, and concentration for purifying blood coagulation FVII; 7, conducting heparin affinity column chromatography for purifying blood coagulation FIX; 8, conducting ultrafiltration; 9, adding a stabilizing agent, and conducting adjustment; 10, conducting virus-removal filtration through nanofilms; 11, conducting sterilization, filtration and subpackage; 12, conducting freeze-drying; 13, conducting dry-hot viral inactivation. According to the method, PEG sedimentation is adopted for removing the impure protein, the target of preparing high-purity FVII and FIX simultaneously is achieved through combination of an ion-exchange column chromatography technology and an affinity chromatography technology, the process flow is simple, the production cycle is short, a product is subjected to three steps of virus eradicating measures, and use safety is high.
Owner:上海洲跃生物科技有限公司
Method for preparing human thrombin from cold-removing glue plasma
InactiveCN105039295ASignificant technological progressHigh yieldPeptidasesFreeze-dryingUltrafiltration
The invention discloses a method for preparing human thrombin from cold-removing glue plasma. Firstly, anion resins are used to absorb prothrombin in the cold-removing glue plasma, then elution and filtering are conducted, and a prothrombin solution is obtained; secondly, the prothrombin solution is activated through a CaCL2 solution, and a thrombin solution is obtained; thirdly, S / D viral inactivation is conducted on the thrombin solution; fourthly, chromatography is conducted through cation columns, and a purified thrombin solution is obtained; fifthly, ultrafiltration, dialysis and concentrate are conducted on the thrombin solution, and the thrombin solution with the titer meeting the product specification requirement is obtained; sixthly, a filter element of 20 nm in size is used to conduct virus removal filtering; seventhly, sterilization filtering is conducted through a filter element of 0.22 microns in size, and then subpackage is conducted according to the required specifications; eighthly, freeze-drying is conducted, and dry heat viral inactivation is conducted on freeze-drying powder, and a human thrombin product is obtained. According to the method, the thrombin is purified through the one-step cation columns, operation is simple, thrombin specific activity is high, and safety of clinic use of the product is guaranteed through a three-step virus elimination mode.
Owner:上海洲跃生物科技有限公司
Titanium oxide photocatalyst having copper compounds supported thereon, and method for producing same
ActiveCN103228358AImprove photocatalytic activityPigmenting treatmentBiocideCopperViral Inactivation
A copper compound-carried titanium oxide photocatalyst which is excellent in a photocatalytic activity and a viral inactivation property and a production process for the same can be provided by a copper compound-carried titanium oxide photocatalyst comprising titanium oxide in which a content of rutile type titanium oxide is 50 % by mole or more and a monovalent copper compound and a divalent copper compound which are carried on a surface of the titanium oxide described above and a production process for a copper compound-carried titanium oxide photocatalyst, comprising a step of carrying a monovalent copper compound and a divalent copper compound on a surface of titanium oxide in which a content of rutile type titanium oxide is 50 % by mole or more.
Owner:THE UNIV OF TOKYO
Method for preparing human body or animal accellular tissues
ActiveCN103191466BReasonable formulaReduce procurement costsTissue cultureAbsorbent padsHuman bodyCell-Extracellular Matrix
The invention discloses a method for preparing human body or animal accellular tissues. According to the method, the decellularized tissue obtained by treating human or animal tissues, such as skin, pleuroperitoneal membrane, esophagus mucous membrane, small intestine mucous membrane, pericardium, vessel, nervus, cardiac valves, bone, cartilage, muscle tendon, and the like through processes of inactivation of virus, derosination, decellularization, and the like is an acellular three-dimensional network structure tissue, and extracellular matrix components are kept. The tissue prepared by the method can be used as an implantable repair material for a human body and also can be used as a support material of tissue engineering cell culture. The skin, the pleuroperitoneal membrane, the esophagus mucous membrane, the small intestine mucous membrane, the pericardium, and the like also can be used as a wound covering material.
Owner:上海优越生物医学科技有限公司
Natural food preservative substance formula and preparation
InactiveCN101263924AStrong sterilization functionStrong antibacterial functionClimate change adaptationFood preservationSide effectPreservative
The invention relates to food anticorrosion technical field, in particular to a natural food anticorrosion preservative prescription and the preparation method. The invention is characterized in that the prescription comprises pure bamboo vinegar of 1 to 5% total acid and chitosan of 80 to 99% deacetylation degree (weight percentage); pure bamboo vinegar: 92 to 99%, chitosan: 1 to 8%; the vinegar and chitosan of the proportion is added into the reator and is mixed well; the mixed solution in the reator is agitated in the normal temperature or the raised 100 DEG C; the agitating speed is 100 to 200 time / m; the preservative is formed after 5 to 30m dissolution. The natural food anticorrosion preservative has the advantages that no toxic side effect exist; the application scope is wide; the cost is low; the preservative can make sterilization, bacteriostasis and viral inactivation.
Owner:叶礼奎
Animal bladder decellularizing matrix as well as preparation method and application thereof
ActiveCN107397978AEnsure Structural IntegrityReduce the risk of transmissionTissue regenerationProsthesisAntigenFreeze thawing
The invention provides a preparation method of an animal bladder decellularizing matrix. The preparation method comprises the steps of extracting a strata submucosum of an animal bladder, and carrying out virus inactivation, degreasing and decellularization, wherein the decellularization step sequentially comprises a repeated freeze thawing step and a decellularization solution decellularization step, the freezing temperature in the freeze thawing step is (-70)-(-90) DEG C, and the freezing time is 5-15 hours. The invention further provides the animal bladder decellularizing matrix prepared by virtue of the preparation method and the application of the animal bladder decellularizing matrix. According to the preparation method, the bladder decellularizing matrix is prepared by combining the repeated freeze thawing with the decellularization solution decellularization, elastic fibers and a collagen net structure of an extracellular matrix are furthest preserved, and meanwhile, antigens are thoroughly removed. The animal bladder decellularizing matrix can be applied to the repairing fields of bladders, urethrae and ureters.
Owner:BEIJING NATON TECH GRP CO LTD +1
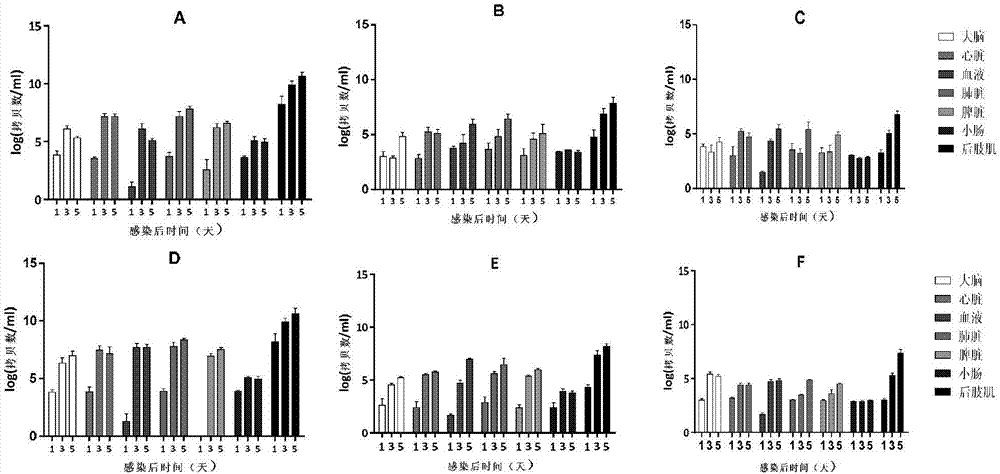
Viral inactivation process
ActiveUS20110020406A1Reduce viremiaSsRNA viruses positive-senseViral antigen ingredientsFamily ArteriviridaePorcine reproductive and respiratory syndrome virus
The invention relates generally to the field of virology. More particularly, the present invention relates to methods for determining the effect of a viral inactivation procedure on the antigenicity of the inactivated virus. In particular for a virus that is a member of the family Arteriviridae or Coronaviridae or Asfarviridae, in particular for Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). The invention further provides methods to determine the antigenicity of an inactivated virus as well as methods to screen for anti-viral compounds using any one of the aforementioned methods Methods of using the inactivated and immunogenic virus thus obtained, in particular in the manufacture of a vaccine are also provided by the present invention.
Owner:UNIV GENT
Placenta tissue matrix material and preparation method thereof
The invention discloses a placenta tissue matrix material. A placenta extracellular matrix is prepared by taking placenta tissue as a raw material through the technologies of tissue preservation, tissue treatment, sterilization, virus inactivation and the like, the placenta extracellular matrix retains a natural matrix structure and basic biological characteristics, and the extracellular matrix particularly retains receptors of various active factors. A preparation method comprises the following steps: tissue pretreatment; sterilization and virus inactivation; tissue DNA removal; decellularization; and preparation of various forms such as gel, sponge or powder from the acellular matrix according to specific clinical requirements. The acellular matrix disclosed by the invention is a specialscaffold material with an active factor receptor, can actively attract active ingredients in a living body into the material, is beneficial to accumulation of cell active ingredients, supports cell migration, adhesion, proliferation and differentiation, and can accelerate damaged tissue repair and local tissue reconstruction.
Owner:银丰低温医学科技有限公司 +2
Method for stabilizing a cryoprecipitate of plasmatic proteins for being subjected to a viral inactivation thermal treatment
ActiveUS7727743B2Improve protectionImprove solubilityPowder deliveryPeptide/protein ingredientsProtein compositionCryoprecipitate
The invention relates to a method for obtaining cryoprecipitatable proteins, comprising a viral inactivation step by thermally treating a lyophilisate of these proteins, comprising, before rendering the proteins in the form of a lyophilisate, an initial addition step, to these proteins, of a stabilizing and solubilizing formulation containing a mixture consisting of arginine, at least one hydrophobic amino acid and of tribasic sodium citrate. The invention also relates to a concentrate consisting of at least one cryoprecipitable protein containing the stabilizing and solubilizing formulation introduced according to the method and being suited for therapeutic use.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Methods of purifying recombinant ADAMTS13 and other proteins and compositions thereof
ActiveUS8945895B2Reduce conductivityPeptide/protein ingredientsInactivation/attenuationHydroxylapatiteAnion-exchange chromatography
Provided herein are methods for purifying recombinant A Disintegrin-like and Metallopeptidase with Thrombospondin Type 1 Motif 13 (ADAMTS13) protein from a sample. The method comprises enriching for ADAMTS13 protein by chromatographically contacting the sample with hydroxyapatite under conditions that allow ADAMTS13 protein to appear in the eluate or supernatant from the hydroxylapatite. The methods may further comprise tandem chromatography with a mixed mode cation exchange / hydrophobic interaction resin that binds ADAMTS13 protein. Additional optional steps involve ultrafiltration / diafiltration, anion exchange chromatography, cation exchange chromatography, and viral inactivation. Also provided herein are methods for inactivating virus contaminants in protein samples, where the protein is immobilized on a support. Also provided herein are compositions of ADAMTS13 prepared according to said methods.
Owner:TAKEDA PHARMA CO LTD
Viral inactivation using ozone
InactiveUS20050051497A1Maximize gas-fluid mass transferSmooth connectionWater treatment parameter controlMedical devicesLipid formationBiological integrity
A method to inactivate viruses in a biological fluid and a non-fluid target to produce a non-infectious biological fluid or non-fluid target. The method involves subjecting an amount of a fluid or a target containing a virus including lipid-enveloped viruses, to an amount of ozone delivered by an ozone delivery system. The method may provide for maintaining the biological integrity of the biological fluid or the non-fluid target. All gas contacting surfaces of the system, including one or more gas-fluid contact devices are made from ozone-inert construction.
Owner:LIPIDVIRO TECH
Rabbit haemorrhagic disease tissue inactivated vaccine and preparation method thereof
InactiveCN102755644AEasy to solveEasy to controlViral antigen ingredientsInactivation/attenuationAntigenDisease
The invention relates to a rabbit haemorrhagic disease tissue inactivated vaccine and a preparation method thereof. The rabbit haemorrhagic disease tissue inactivated vaccine comprises a rabbit haemorrhagic disease tissue virus liquid and a propolis adjuvant, wherein every liter of virus extracting solution contains 5-15mg of propolis. The preparation method mainly comprises the following steps of: treating pathological materials of a disease and obtaining a virus liquid; inactivating viruses; preparing a vaccine; inspecting the safety and immune efficiency of the vaccine; and performing split charging on the vaccine. A vaccine pathogen is passed and proliferated after separation, and comprehensive antigen components are used for comprehensively inducing inoculated animal groups on multiple aspects, so that good effects of preventing and controlling diseases can be achieved. The adopted propolis adjuvant is a good immunopotentiator as well as an irritant, has broad spectrum biologic activity, can be used for causing specific immune response and starting a nonspecific defense mechanism, and has the remarkable effects of improving immune antibody generation and increasing the leucocyte phagocytosis.
Owner:河南后羿生物工程股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com