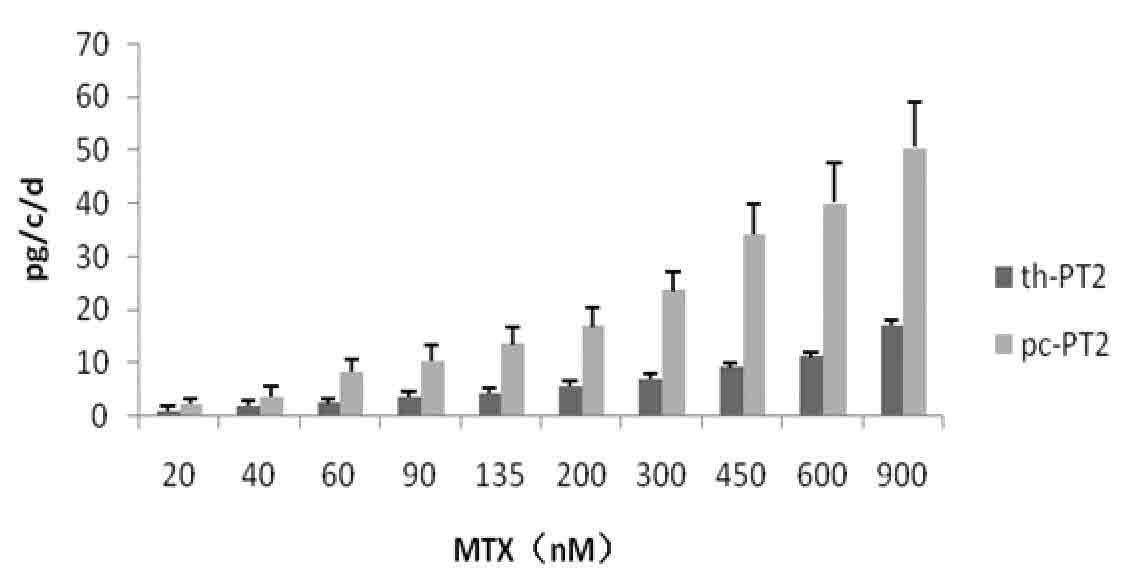
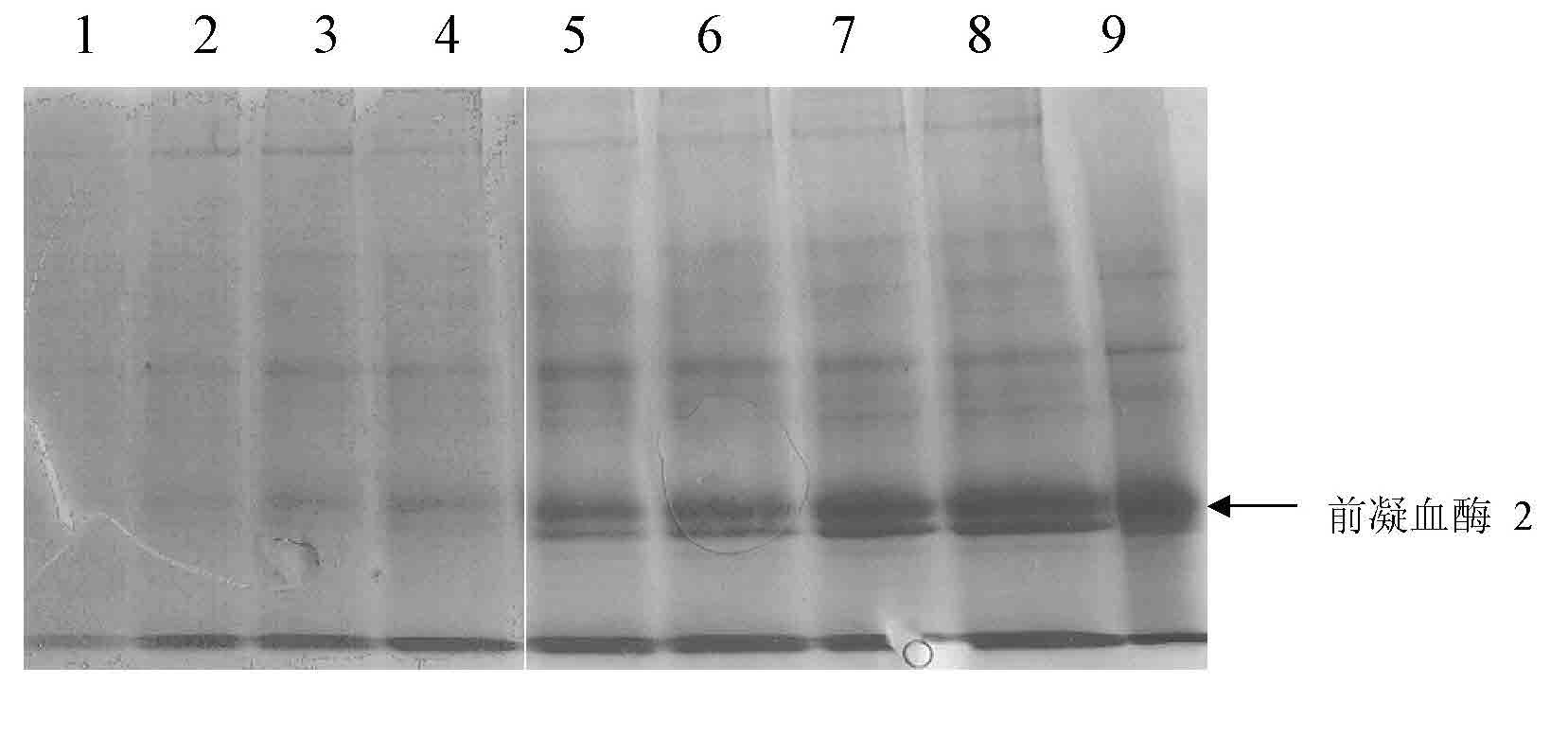
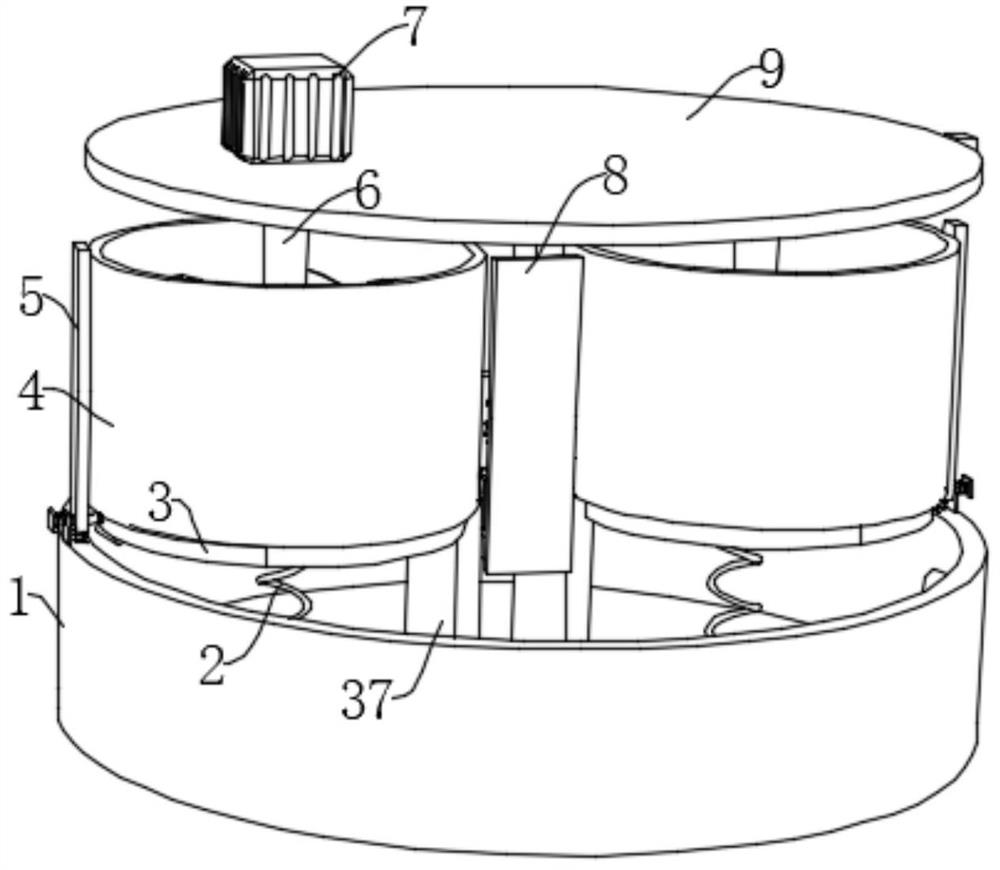
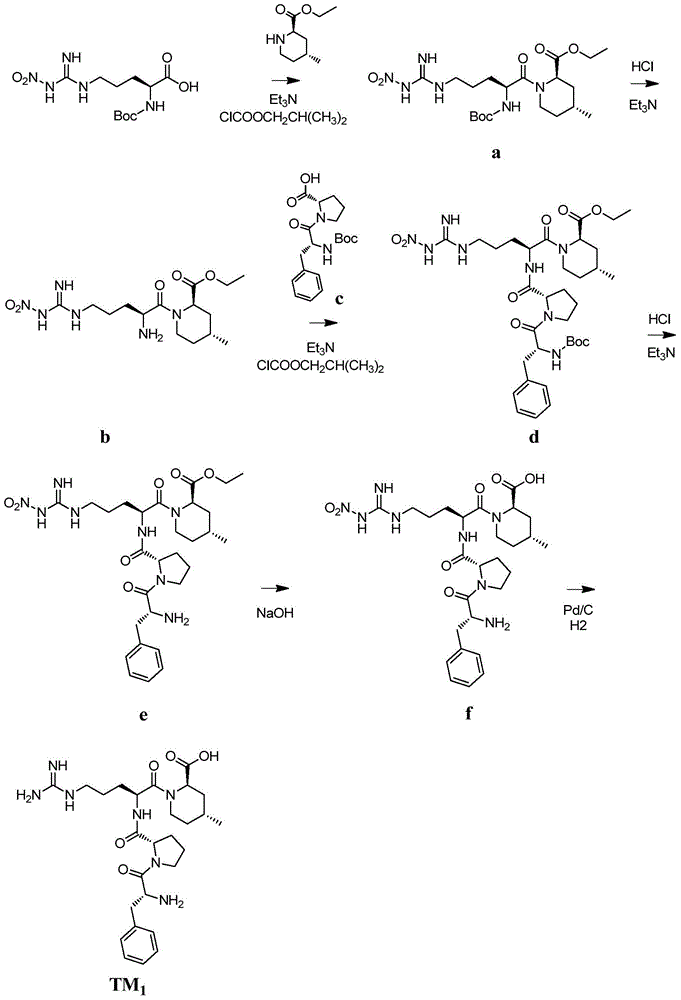
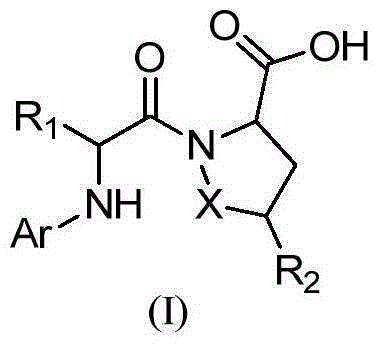
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Human Thrombin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human Thrombin. Source: Human. Background : Thrombin is the final protease in the blood coagulation cascade and serves both pro- and anticoagulant functions through the cleavage of several targets.
Composite fat granule and preparation method thereof
ActiveCN102058905APromote proliferationPrevent necrotic liquefactionProsthesisHuman ThrombinAnimal science
The invention discloses a composite fat granule and a preparation method thereof. The composite fat granule comprises the components in the following proportions: 2ml of platelet-rich plasma, 1ml of human thrombin solution, (2+ / -0.5)*107 fat adipose-derived stem cells and 4ml of fat granules. Through the composite fat granule, the survival rate of transplanted fat can be improved, the long-term absorption rate is reduced, and situations, such as the formation of ectopic fibrotic nodules and the like can not occur.
Owner:成都世联康健生物科技有限公司
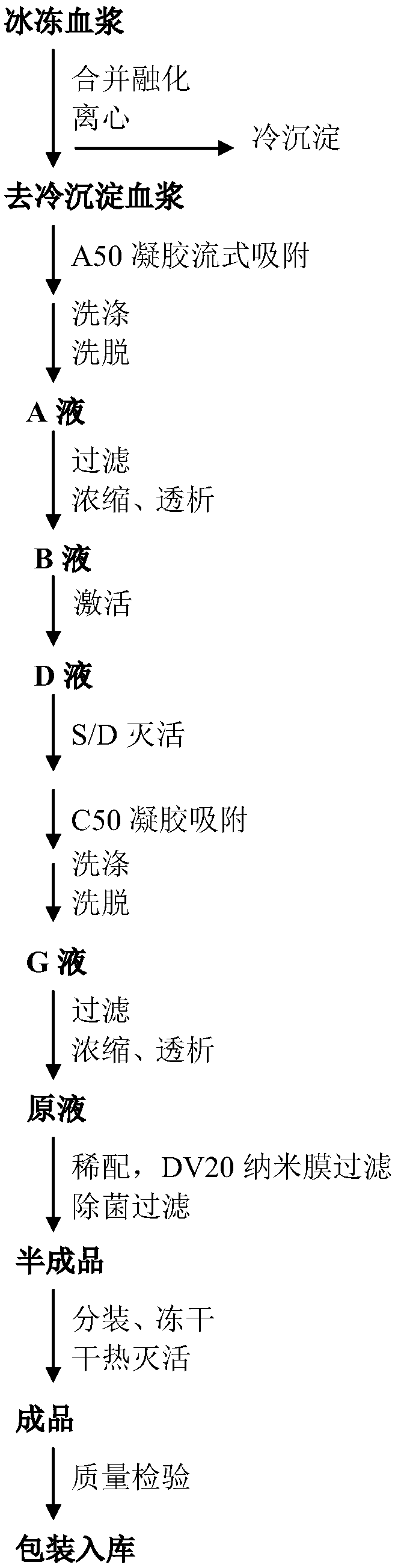
Method for preparing human thrombin from cold-removing glue plasma
InactiveCN105039295ASignificant technological progressHigh yieldPeptidasesFreeze-dryingUltrafiltration
The invention discloses a method for preparing human thrombin from cold-removing glue plasma. Firstly, anion resins are used to absorb prothrombin in the cold-removing glue plasma, then elution and filtering are conducted, and a prothrombin solution is obtained; secondly, the prothrombin solution is activated through a CaCL2 solution, and a thrombin solution is obtained; thirdly, S / D viral inactivation is conducted on the thrombin solution; fourthly, chromatography is conducted through cation columns, and a purified thrombin solution is obtained; fifthly, ultrafiltration, dialysis and concentrate are conducted on the thrombin solution, and the thrombin solution with the titer meeting the product specification requirement is obtained; sixthly, a filter element of 20 nm in size is used to conduct virus removal filtering; seventhly, sterilization filtering is conducted through a filter element of 0.22 microns in size, and then subpackage is conducted according to the required specifications; eighthly, freeze-drying is conducted, and dry heat viral inactivation is conducted on freeze-drying powder, and a human thrombin product is obtained. According to the method, the thrombin is purified through the one-step cation columns, operation is simple, thrombin specific activity is high, and safety of clinic use of the product is guaranteed through a three-step virus elimination mode.
Owner:上海洲跃生物科技有限公司
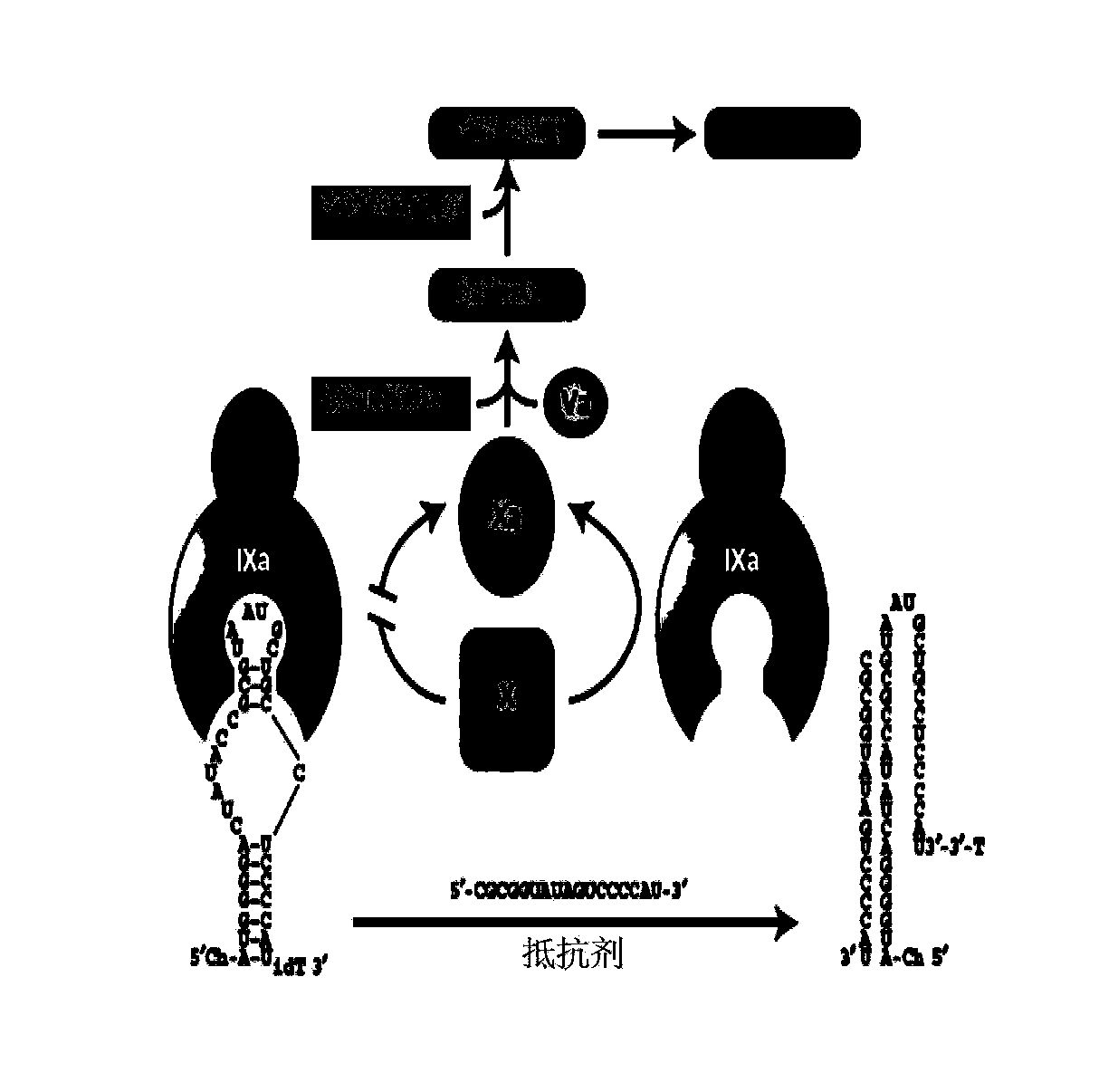
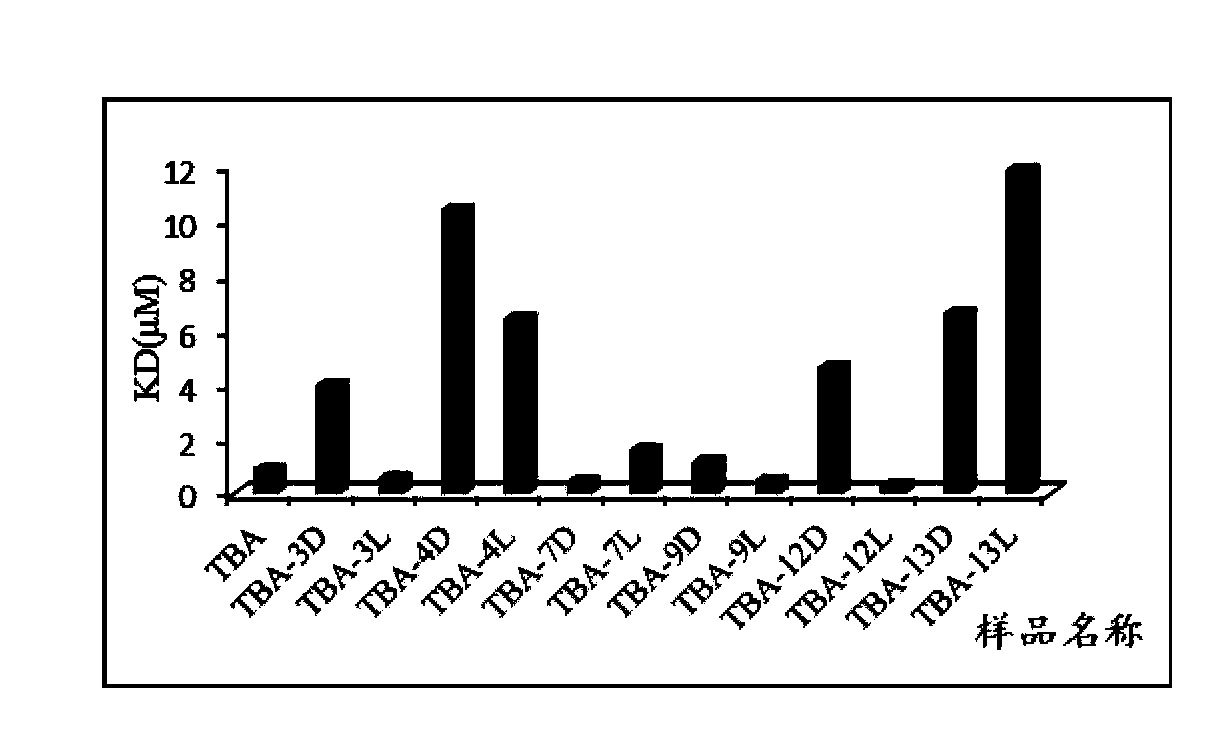
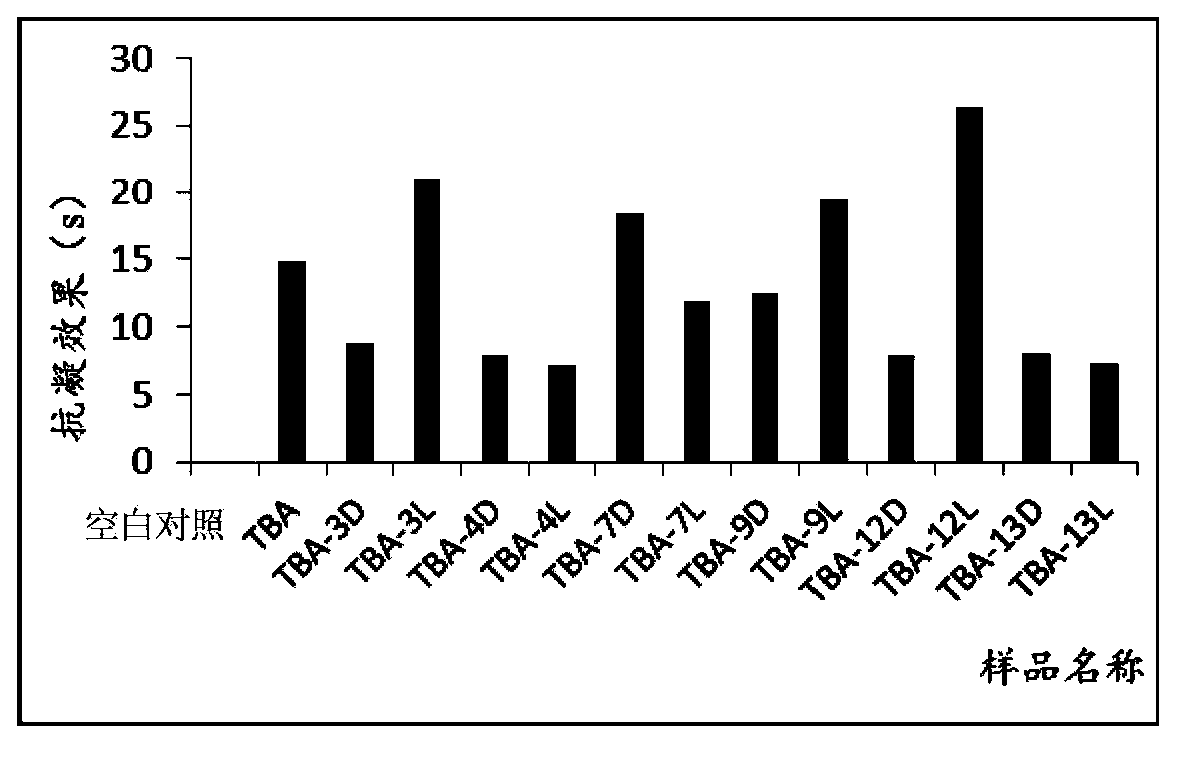
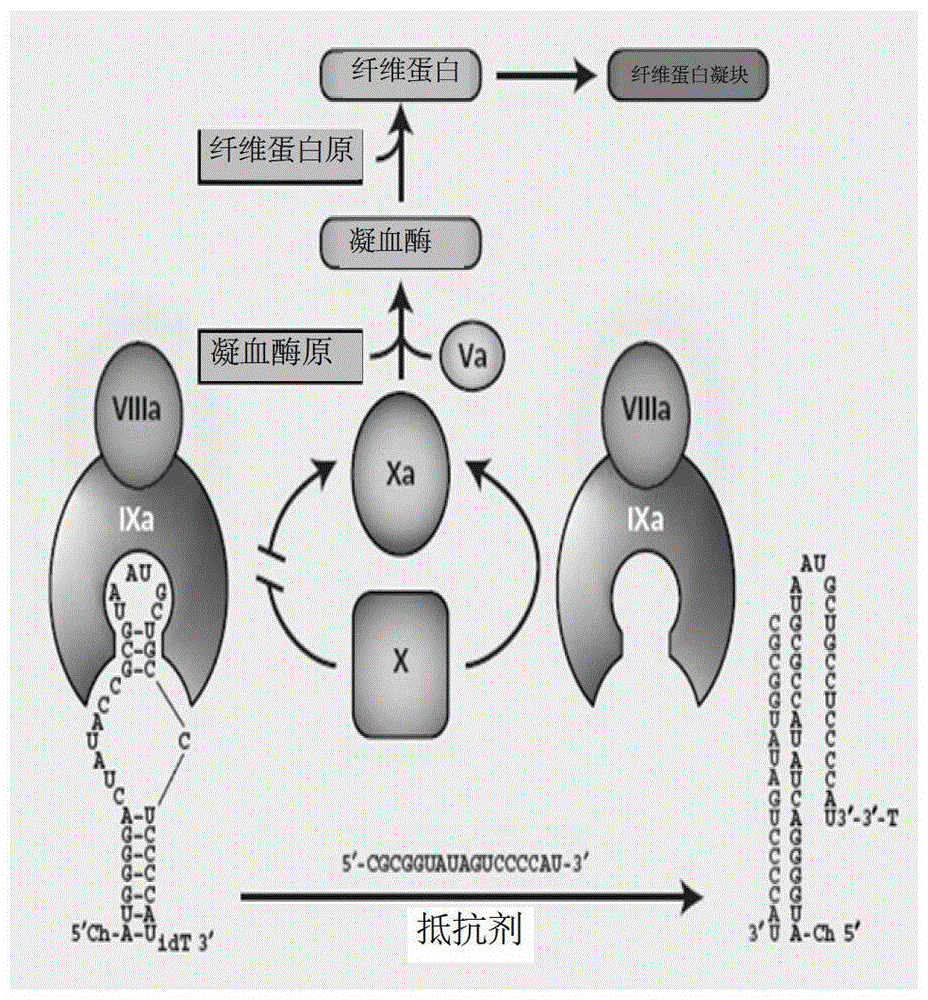
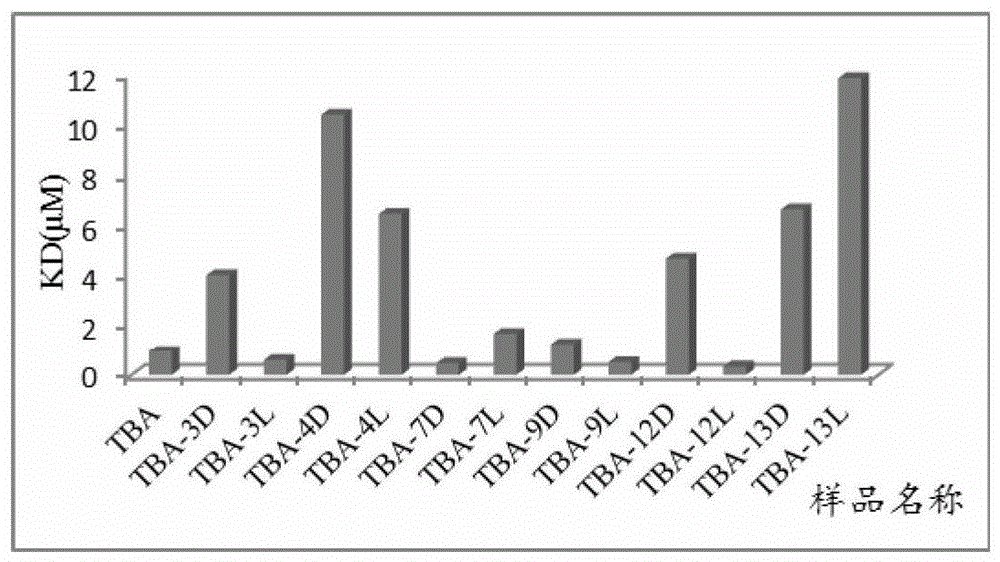
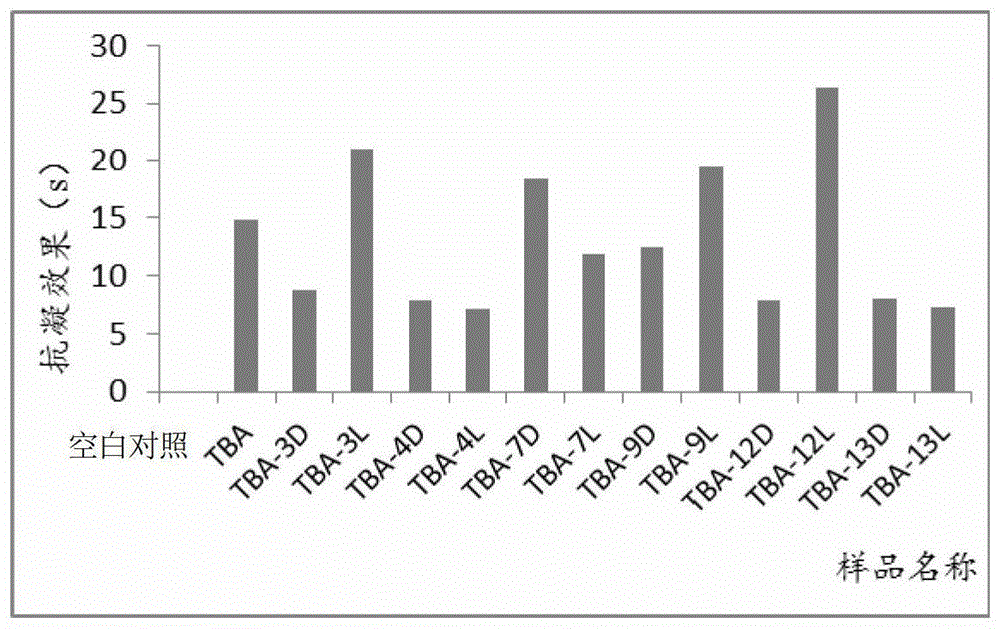
Isothymidine-modified thrombin nucleic-acid aptamer, and preparation method and application thereof
ActiveCN104278035AStable physical and chemical propertiesHigh synthesis efficiencyGenetic material ingredientsGene therapyAptamerHuman Thrombin
The invention discloses an isothymidine-modified thrombin nucleic-acid aptamer, and a preparation method and application thereof, and belongs to the field of biomedicine. By doping isothymidine shown as a chemical formula I and / or II into synthesized thrombin nucleic-acid aptamer, the isothymidine-modified thrombin nucleic-acid aptamer is obtained. Experiments prove that the human thrombin nucleic-acid aptamer modified by employing the method is capable of relatively well recognizing thrombin, is relatively strong in bonding force and has relatively strong anticoagulation effect, and therefore the isothymidine-modified thrombin nucleic-acid aptamer is hopeful to be prepare a high-efficiency high-selectivity anticoagulation medicine with low toxic and side effects.
Owner:PEKING UNIV
Preparation process of lyophilized human thrombin
The invention discloses a preparation process of lyophilized human thrombin. The preparation process is characterized by comprising the following steps: preparing cooled precipitated blood plasma intoa human prothrombin, activating the human prothrombin to obtain a human thrombin crude solution, preparing human thrombin from the human thrombin crude solution, wherein the activation conditions ofthe human prothrombin are that: 0.3mol / L of calcium chloride solution is added in an amount of one tenth of an original human prothrombin solution, so that the calcium chloride final concentration is0.025 to 0.03mol / L, then soybean lecithin or egg yolk lecithin is added, so that calcium chloride with the concentration of 0.1 to 0.2 percent is taken as an activating agent; slowly stirring the solution, and activating at the temperature of 20 to 25 DEG C for 1 to 2 hours to obtain a human thrombin solution. The prepared human thrombin is 400 to 700IU / ml in titer, is over 1,000IU / mg proteins inspecific activity, and is over 20IU / ml in yield. The preparation process has the advantages of good purifying effect, high yield and easiness in operation, and the situations of poor activation effect, low coversion rate, low yield, over long activation time, turbidity after re-dissolving, lowering of the titer after long-time storage in the prothrombin are avoided effectively.
Owner:华润博雅生物制药集团股份有限公司
Preparation method of freeze-dried human thrombin
ActiveCN106474461AHigh yieldImprove qualityPowder deliveryPeptide/protein ingredientsSp sephadexUltrafiltration
The invention relates to a preparation method of freeze-dried human thrombin. The preparation method comprises the following steps: (1) taking a Cohn component III as the raw material, and preparing human prothrombin by an isoelectric precipitation method; (2) using calcium chloride to activate prothrombin prepared in the step (1) to obtain a coarse product of thrombin; (3) subjecting the coarse product to S / D virus inactivation and SP-Sephadex C-50 resin chromatographic purification to obtain refined thrombin; and (4) subjecting the refined thrombin to ultrafiltration and concentration, adding an stabilizing agent into the refined thrombin, removing virus and filtering the refined thrombin by a nano membrane, sterilizing and filtering the refined thrombin, freeze-drying the refined thrombin, and carrying out a dry heating treatment to obtain the target product. Compared with the prior art, the preparation steps are concise, the product yield is high, and the production quality is good.
Owner:SHANGHAI RAAS BLOOD PRODUCTS CO LTD +1
Method for production of recombinant human thrombin
Owner:MEDIMMUNE LTD
Preparation method of human thrombin
The invention relates to the field of blood products and particularly relates to a preparation method of human thrombin. The invention provides a preparation method of human thrombin, which comprises the following steps: 1) performing redissolution and virus inactivation of a component III obtained by Cohn fraction of human plasma; and purifying the obtained liquid through anion exchange resin to obtain prothrombin complex eluent; 2) adding calcium ions into the eluent obtained in the step 1), and incubating until the prothrombin is effectively activated; 3) separating and purifying the liquid obtained in the step 2) through cation exchange resin; and 4) performing nano membrane filtration of the liquid obtained in the step 3) to obtain a finished product of human thrombin. In the preparation method provided by the invention, by adopting the thrombin product of the technology market, the human thrombin has high vigor, high yield and good long-term stability, meets the requirements on safety and effectiveness of clinical application and is of relatively obvious economic value and practical significance.
Owner:SHANGHAI RAAS BLOOD PRODUCTS CO LTD
Preparation method of hemostatic material based on fibrin gel
InactiveCN107080857APromote formationAccelerates blood clotting processSurgical adhesivesPharmaceutical delivery mechanismFiberFibrin glue
The invention discloses a preparation method of a hemostatic material based on fibrin gel. The preparation method comprises the following steps: A. dissolution of bovine fibrinogen: at the temperature of 30-50 DEG C, bovine fibrinogen is dissolved by the use of a 0.9% NaCl solution so as to obtain a bovine fibrinogen solution with the concentration of 5-30 mg / Ml; B. dissolution of human thrombin: at the temperature of 30-50 DEG C, human thrombin is dissolved by the use of 330-660 mol / L of a CaCl2 solution so as to obtain a human thrombin solution with the concentration of 3-7 * 104 unit / L; and C. synthesis of gel: at the temperature of 30-50 DEG C, the fibrinogen solution obtained in the Step A and the thrombin solution obtained in the Step B are mixed according to the volume ratio of 1:1-5 so as to obtain mixed liquor; and the mixed liquor is dropped on gauze and then reacts on a shaker of 30-50 DEG C for 4-12 h. The hemostatic material prepared by the method has a good hemostatic effect, has good biocompatibility, can promote formation of granulation tissues and epithelial tissues and speed up healing and repairing of skin tissues, is beneficial to observation of wounds and healing situation, and is convenient for diagnosis and treatment of wounds.
Owner:SOUTHWEST JIAOTONG UNIV
High expression and production method of recombinant human thrombin in animal cell
The invention provides a high expression and production method of recombinant human thrombin in animal cells. Specifically, the invention provides a fusion gene and a fusion protein coded by the same. The gene contains signal peptide sequence of human protein C and Gla structural domain deleted human prothrombin gene sequence, and can enhance expression level of human prothrombin-2. The invention also discloses a method for employing a recombinant expression vector containing the above nucleotide sequence to express and produce human prothrombin in animal cells. The invention also discloses a method for producing recombinant human thrombin by purification and activation. The recombinant human thrombin produced by the invention has biological activity satisfying treatment of correlative clinic diseases.
Owner:SUZHOU ZELGEN BIOPHARML
Preparation method for producing fibrin gel and platelect gel using human thromblase
InactiveCN1507924ADoesn't exclude sexual issuesSurgical adhesivesMammal material medical ingredientsHuman ThrombinFibrin glue
The present invention relates to a method for producing fibrin colloid and preparing platelet gel by using human thrombosin, in particular it utilizes ingle blood donor or self-body thrombin to produce fibrin colloid and platelet gel with bio-compatibility and bio-degradability. Said invented preparation method can reduce viral infection and antigenic hazard, and can raise application of fibrin colloid in the fields of medicine and cell culture.
Owner:新扬行实业有限公司
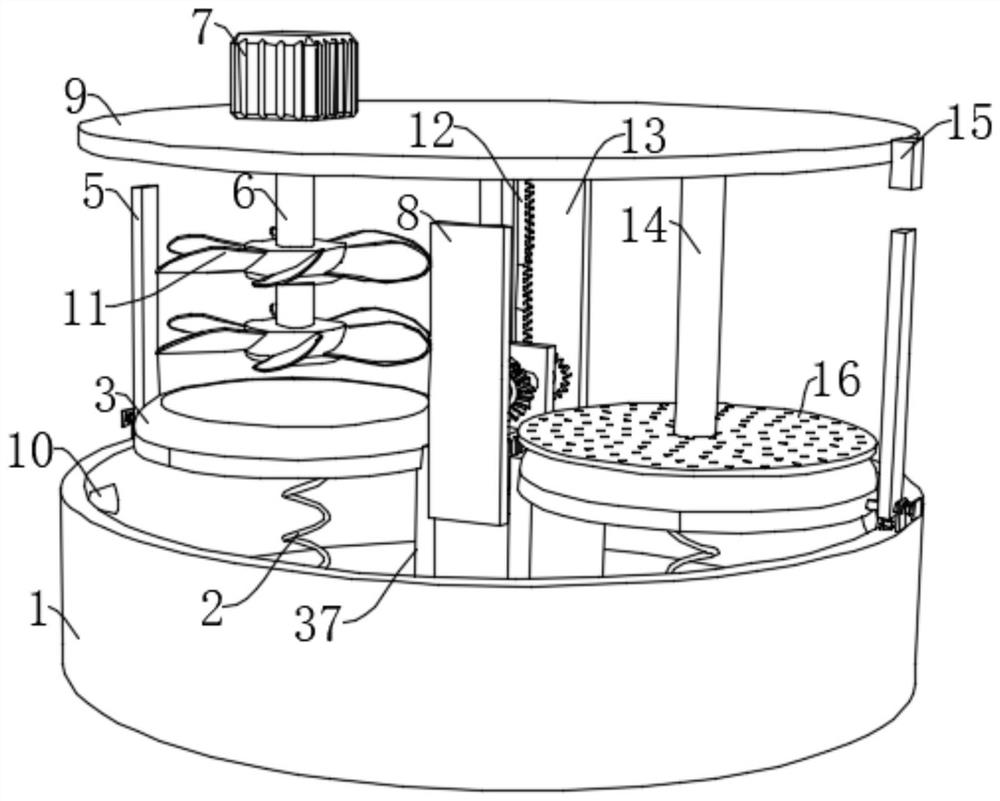
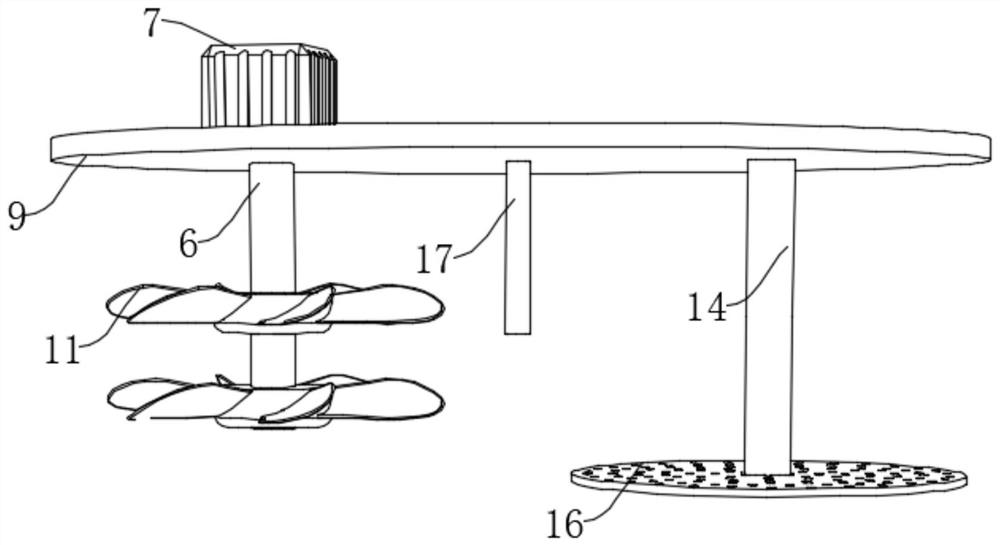
Freeze-dried human thrombin preparation device for blood product manufacturing
PendingCN114717087AImprove efficiencyEasy to useBioreactor/fermenter combinationsBiological substance pretreatmentsHuman ThrombinWhole blood product
The invention belongs to the field of freeze-dried human thrombin, particularly relates to a freeze-dried human thrombin preparation device for manufacturing blood products, and aims to solve the problems that existing filter pressing and stirring need to be carried out in different devices, materials subjected to filter pressing are difficult to take out completely, a large amount of time is wasted in the material transferring process, and the working efficiency is reduced. According to the technical scheme, the device comprises a collecting box, the inner wall of the bottom of the collecting box is fixedly connected with two symmetrically-arranged fixing plates, the tops of the fixing plates are fixedly connected with fixing cylinders, one sides of the fixing plates are slidably connected with circular table plates, and the circular table plates extend into the fixing cylinders. According to the device, the function of stirring and filter pressing can be achieved at the same time, the working efficiency is greatly improved, meanwhile, precipitates obtained after filter pressing can be easily taken out, the lifting and filter pressing functions can be completed only through one servo motor, and the device cost is reduced.
Owner:华润博雅生物制药集团股份有限公司
Method for the manufacturing of di-chain proteins for use in humans
InactiveUS20140377248A1The method is accurate and reliableHigh purityBacteriaSugar derivativesHuman ThrombinThrombin activity
This invention relates to a novel method for producing di-chain proteins for use in humans from single-chain precursors, including di-chain clostridial neurotoxins. The method comprises the step of expressing a nucleic acid sequence encoding a single-chain precursor comprising a thrombin-cleavage site and the step of cleaving the single-chain precursor with a human factor Xa or a human thrombin, particularly a human thrombin drug product authorized for human therapeutic use. The invention further relates to novel di-chain clostridial neurotoxins and nucleic acid sequences encoding such novel di-chain clostridial neurotoxins.
Owner:MERZ PHARMA GMBH & CO KGAA
Method for the manufacturing of di-chain proteins for use in humans
InactiveUS20170204391A1Peptide/protein ingredientsFusion with protease siteHuman ThrombinThrombin activity
This invention relates to a novel method for producing di-chain proteins for use in humans from single-chain precursors, including di-chain clostridial neurotoxins. The method comprises the step of expressing a nucleic acid sequence encoding a single-chain precursor comprising a thrombin-cleavage site and the step of cleaving the single-chain precursor with a human factor Xa or a human thrombin, particularly a human thrombin drug product authorized for human therapeutic use. The invention further relates to novel di-chain clostridial neurotoxins and nucleic acid sequences encoding such novel di-chain clostridial neurotoxins.
Owner:MERZ PHARMA GMBH & CO KGAA
Binding proteins to the human thrombin receptor, par4
PendingCN111670198AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman ThrombinReceptor
Owner:MONASH UNIV
Affinity chromatographic process of separating and purifying plasmin
InactiveCN101067131AHigh recovery rateHigh purityIon-exchange process apparatusIon-exchanger regenerationCross-linkIon
The present invention discloses affinity chromatographic process of separating and purifying plasmin. The process includes preparing affinity chromatographic medium and affinity chromatography. The preparation of affinity chromatographic medium includes the steps of: dissolving human fibrinogen in sterilized water at 35-40 deg.c, agarose in barbital sodium-HCl buffer solution, sterilizing and cooling; adding human thrombin and dropping into vinyl tetrachloride at 2-10 deg.c to form spherical grain; washing with ion-free water and maintaining at 35-40 deg.c for 1-2 hr; and adding glutaraldehyde to cross link. The affinity chromatography includes the steps of balancing the chromatographic column with the buffer liquid at 2-10 deg.c, adding the sample solution, rinsing with the buffer liquid and final eluting with the eluting solution. The present invention has high plasmin recovering rate and high plasmin purity.
Owner:SOUTH CHINA UNIV OF TECH
Multi-target compound with anticoagulant and antiplatelet activity, preparation method and application
ActiveCN108137653BDirect specific antithrombin functionAchieve anticoagulant and antithrombotic effectPeptide-nucleic acidsPeptide/protein ingredientsHuman ThrombinBinding site
Provided is an anticoagulant and platelet GPⅡb / Ⅲa receptor multi-target compound, its general formula is as follows: A-L-B-L'-C, wherein A and B are binding sites for thrombin, and C is For the platelet GPⅡb / Ⅲa receptor binding site, L is the first linking group, and L' is the second linking group. Preparation methods and uses of the above compounds are also provided. The compound has in vitro human thrombin inhibitory activity, in vitro platelet GPIIb / IIIa receptor inhibitory activity, in vitro / in vivo antiplatelet aggregation, in vivo anticoagulant and antithrombotic effects.
Owner:SHAANXI MICOT TECH LTD
Application of human thrombin-sensitive protein-1 in preparation of kit for predicting chemotherapy effect of intrahepatic cholangiocarcinoma
The invention relates to the technical field of medical biological detection, and concretely relates to application of human thrombin-sensitive protein-1 in preparation of a kit for predicting the chemotherapy effect of intrahepatic cholangiocarcinoma. The application has the advantages that the protein chip is used for screening out the protein with expression difference in tissues of patients of an intrahepatic cholangiocarcinoma (ICC) chemotherapy drug-resistant group and an ICC chemotherapy sensitive group in the middle and advanced stages, the protein can be detected in serum, and it is found that the thrombin-sensitive protein-1 has the most obvious difference in two groups of patients and has a very high expression level in sensitive group patients. The invention provides the application of human thrombin-sensitive protein-1 as a serum marker for determining whether chemotherapy of an intrahepatic cholangiocarcinoma patient is sensitive or not. The expression level of the human thrombin-sensitive protein-1 is closely related to survival prognosis of the patient. The application provides a new clinical means for serological diagnosis.
Owner:上海柏运医疗器材有限公司
Autologous or homologous coagulant produced from anticoagulated whole blood
A method for the preparation of a stable autologous or homologous coagulant from whole blood is disclosed. The direct precipitation of anticoagulated whole blood obviates the need for a plasma isolation step with unexpected results. The autologous or homologous coagulant produced by the method of the present invention demonstrated clotting times equivalent to commercially available bovine thrombin and human thrombin preparations, with improved kinetics of growth factor release from activated platelets over preparations of bovine thrombin.
Owner:HARVEST TECH
Binding proteins to the human thrombin receptor, par4
PendingUS20210179707A1Big impactHigh sensitivityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman ThrombinReceptor
Owner:MONASH UNIV
A method for activating and preparing human thrombin from human coagulation factor ⅸ chromatography waste liquid
ActiveCN104560925BDoes not affect productionDoes not affect medicationPeptidasesFreeze-dryingDry heat
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Composite fat granule and preparation method thereof
ActiveCN102058905BPromote proliferationPrevent necrotic liquefactionProsthesisHuman ThrombinAnimal science
The invention discloses a composite fat granule and a preparation method thereof. The composite fat granule comprises the components in the following proportions: 2ml of platelet-rich plasma, 1ml of human thrombin solution, (2+ / -0.5)*107 fat adipose-derived stem cells and 4ml of fat granules. Through the composite fat granule, the survival rate of transplanted fat can be improved, the long-term absorption rate is reduced, and situations, such as the formation of ectopic fibrotic nodules and the like can not occur.
Owner:成都世联康健生物科技有限公司
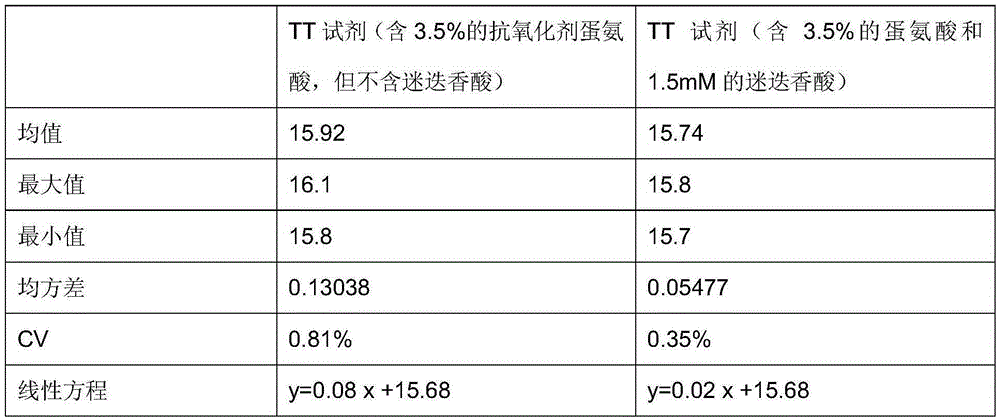
Rosmarinic acid-containing thromboplastin time measuring reagent
ActiveCN105424946AStrong chelation abilityPlay a role in chelationBiological testingFreeze-dryingBovine serum albumin
The invention relates to a rosmarinic acid-containing thromboplastin time measuring reagent. The rosmarinic acid-containing thromboplastin time measuring reagent is composed of a Tris-HCl buffer containing 25 U / ml of human thrombin, 2% of bovine serum albumin, 1.5 mM of rosmarinic acid, 0.05% of sodium azide, 3.5% of methionine, 1.5% of PEG, 3% of mannitol, 2.5% of maltose and 10 mM of calcium chloride, wherein percentage is mass-volume ratio, and a pH value is 7.4. A preparation method of the reagent comprises the following steps: preparing the buffer with the pH value being 7.4 and Tris concentration being 50 mM, adding BSA with final concentration being 2%, 1.5mM of rosmarinic acid with final concentration being 2%, triazacyclononane with final concentration being 0.05%, methionine with final concentration being 3.5%, PEG with final concentration being 1.5%, mannitol with final concentration being 3%, maltose with final concentration being 2.5%, and 10mM of calcium chloride in the buffer, wherein, besides calcium chloride, the unit of the materials is mass-volume fraction; filtering a mixed liquor with a 0.22-micrometer filter membrane, and adding a human thrombin freeze-drying preparation in a filtrate so that the concentration of human thrombin in a final filtrate is 25 U / ml, and obtaining a TT reagent. According to the invention, rosmarinic acid is taken as a stabilizing agent, so that antioxidation performance and stabilization performance of the thromboplastin time measuring reagent can be obviously increased.
Owner:QINGDAO GUGAO BIOTECH CO LTD
Method for preparing human thrombin by activating human coagulation factor IX chromatography waste liquor
ActiveCN104560925ADoes not affect productionDoes not affect medicationPeptidasesHuman ThrombinVirus inactivation
The invention discloses a method for preparing human thrombin by activating human coagulation factor IX chromatography waste liquor. Human thrombin is prepared from a raw material which is waste liquor produced when a human coagulation factor IX is prepared by a chromatographic technology. The method specifically comprises the following steps: (1) obtaining a PCC (Prothrombin Concentration Complex) crude product from human blood plasma; (2) performing S / D inactivation; (3) obtaining a human coagulation factor IX crude product and chromatography waste liquor by chromatography; (4) activating the chromatography waste liquor; (5) purifying thrombin; (6) performing freeze-drying and dry-heat inactivation. According to the method, the F IX chromatography waste liquor is used as the raw material, and influence on the purification of F IX is prevented; the addition of any animal-derived substance in a waste liquor activation process is avoided; the method comprises two virus inactivation steps, so that high clinical safety is achieved.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
A kind of isothymidine modified thrombin nucleic acid aptamer and its preparation method and application
ActiveCN104278035BStable physical and chemical propertiesHigh synthesis efficiencyGenetic material ingredientsBlood disorderHuman ThrombinAptamer
The invention discloses an isothymidine-modified thrombin nucleic-acid aptamer, and a preparation method and application thereof, and belongs to the field of biomedicine. By doping isothymidine shown as a chemical formula I and / or II into synthesized thrombin nucleic-acid aptamer, the isothymidine-modified thrombin nucleic-acid aptamer is obtained. Experiments prove that the human thrombin nucleic-acid aptamer modified by employing the method is capable of relatively well recognizing thrombin, is relatively strong in bonding force and has relatively strong anticoagulation effect, and therefore the isothymidine-modified thrombin nucleic-acid aptamer is hopeful to be prepare a high-efficiency high-selectivity anticoagulation medicine with low toxic and side effects.
Owner:PEKING UNIV
Anticoagulant compound, preparation method and application of anticoagulant compound and drug composition containing anticoagulant compound
InactiveCN104418934AEasy to synthesizeGood human thrombin inhibitory effectDipeptide ingredientsTetrapeptide ingredientsHuman ThrombinAnticoagulant
The invention relates to a compound shown in a general formula (I) in the specification and a stereoisomer or physiologically acceptable salts thereof and also relates to a preparation method and application of the compound. Besides, the invention also relates to a drug composition containing the compound and an application of the drug composition. The compound which is provided by the invention and can serve as a thrombin inhibitor has good in-vitro human thrombin inhibiting effects and does not have obvious toxicity. Besides, the compound provided by the invention has a novel structure and good biological activity, is simple and convenient to synthesize and is a thrombin inhibitor with a good application prospect.
Owner:北京赛而生物药业有限公司
Multi-target compound with anticoagulation and antiplatelet activity as well as preparation method and application of multi-target compound
ActiveCN113773369ADirect specific antithrombin functionAchieve anticoagulant and antithrombotic effectPeptide-nucleic acidsPeptide/protein ingredientsBinding siteAnti platelet
The invention relates to a general formula and physiologically acceptable salts thereof: A-L-B-L'-C, wherein A and B are binding sites with thrombin, C is a binding site with a platelet GPIIb / IIIa receptor, L is a first linking group, and L' is a second linking group. The invention also relates to a preparation method and application of the compound. The invention provides an anticoagulant and antiplatelet GPIIb / IIIa receptor multi-target compound, which has good in-vitro human thrombin inhibition activity, in-vitro platelet GPIIb / IIIa receptor inhibition effect, in-vitro / in-vivo anti-platelet aggregation, in-vivo anticoagulant and antithrombotic effects; and the multi-target compound can be clinically used for preventing and treating peripheral artery thrombosis and arteriovenous bypass thrombosis, treating thrombosis in acute coronary syndrome (ACS) or percutaneous coronary intervention (PCI), and preventing and treating cerebral arterial thrombosis.
Owner:SHAANXI MICOT TECH LTD
Multi-target compound with anticoagulation and antiplatelet activity as well as preparation method and application of multi-target compound
ActiveCN113201049ADirect specific antithrombin functionAchieve anticoagulant and antithrombotic effectPeptide-nucleic acidsPeptide/protein ingredientsBinding siteAnti platelet
The invention relates to a general formula and physiologically acceptable salts thereof: A-L-B-L'-C, wherein A and B are binding sites with thrombin, C is a binding site with a platelet GPIIb / IIIa receptor, L is a first linking group, and L' is a second linking group. The invention also relates to a preparation method and application of the compound. The invention provides an anticoagulant and antiplatelet GPIIb / IIIa receptor multi-target compound, which has good in-vitro human thrombin inhibition activity, in-vitro platelet GPIIb / IIIa receptor inhibition effect, in-vitro / in-vivo anti-platelet aggregation, in-vivo anticoagulant and antithrombotic effects, and can be used for preparing antithrombotic drugs. The traditional Chinese medicine composition can be clinically used for preventing and treating peripheral artery thrombosis and arteriovenous bypass thrombosis, treating thrombosis in acute coronary syndrome (ACS) or percutaneous coronary intervention (PCI), and preventing and treating cerebral arterial thrombosis.
Owner:SHAANXI MICOT TECH LTD
Method for the manufacturing of di-chain proteins for use in humans
This invention relates to a novel method for producing di-chain proteins for use in humans from single-chain precursors, including di-chain clostridial neurotoxins. The method comprises the step of expressing a nucleic acid sequence encoding a single-chain precursor comprising a thrombin-cleavage site and the step of cleaving the single-chain precursor with a human factor Xa or a human thrombin, particularly a human thrombin drug product authorized for human therapeutic use. The invention further relates to novel di-chain clostridial neurotoxins and nucleic acid sequences encoding such novel di-chain clostridial neurotoxins.
Owner:MERZ PHARMA GMBH & CO KGAA
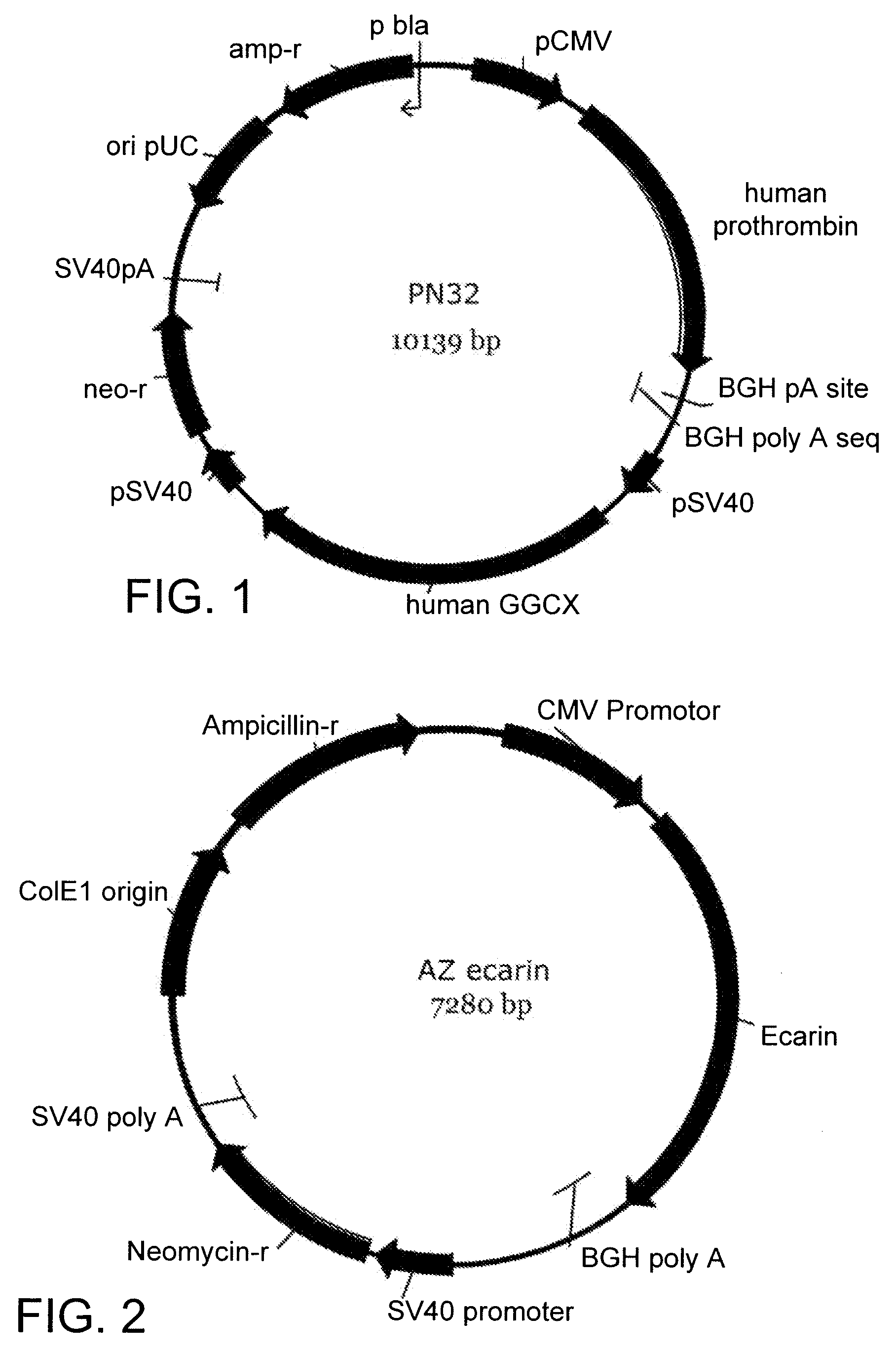
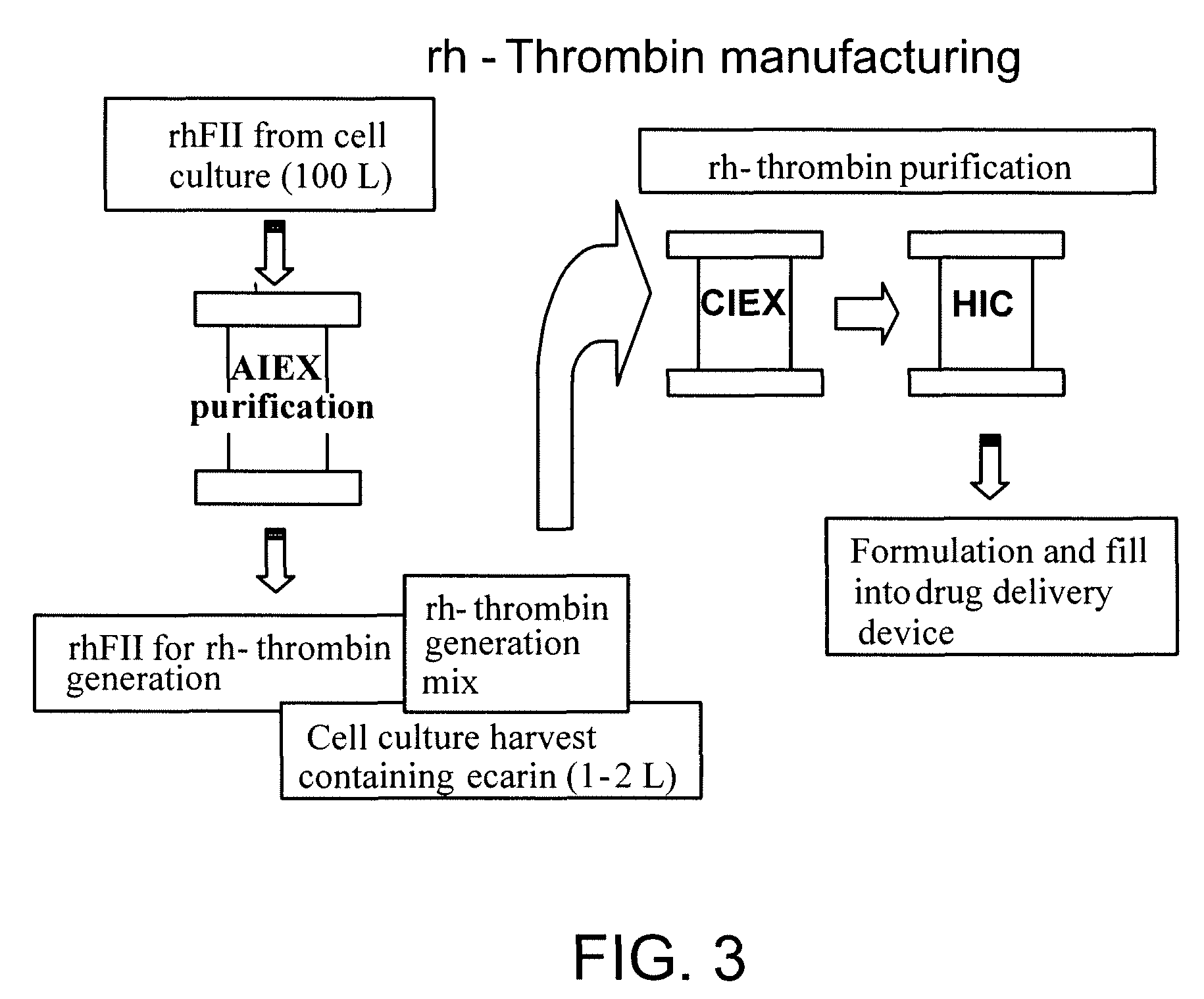
Process for producing human thrombin by gene modification technique
InactiveUS7635577B2Safe and economical processHigh expressionSugar derivativesMicrobiological testing/measurementZymogenHuman Thrombin
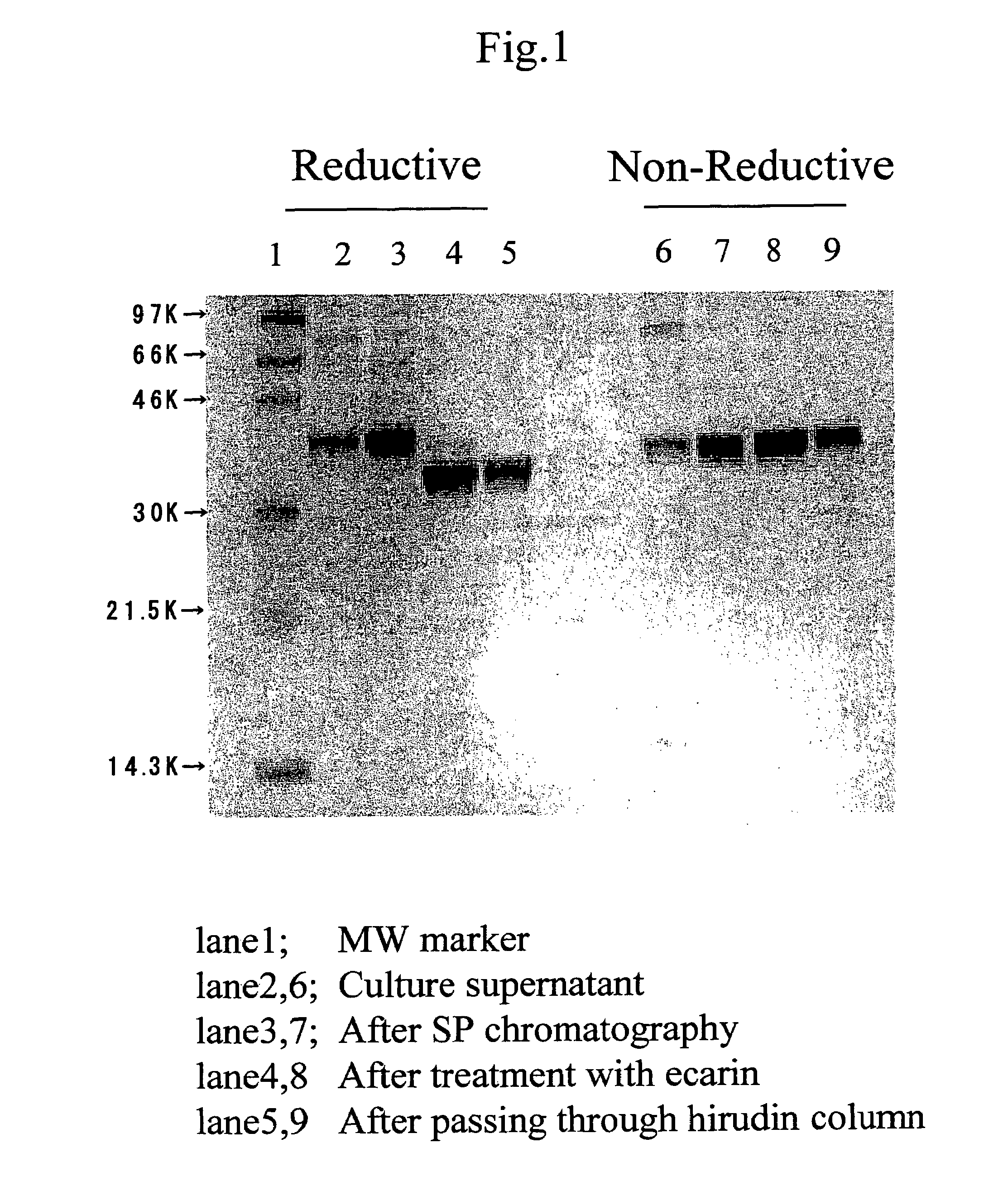
A genetic recombinant human thrombin is provided. Human thrombin is efficiently prepared by the genetic engineering technique comprising the steps: (1) culturing a transfectant animal cell transfected with an expression vector in which a gene encoding human prethrombin is incorporated to the downstream of a promoter so as to produce and accumulate prethrombin in culture supernatant and recovering the produced human prethrombin; (2) treating a solution containing human prethrombin recovered in step (1) with ecarin so as to convert human prethrombin into human thrombin; and (3) purifying the solution obtained after the above activation process to obtain purified human thrombin. The present invention allows for provision of human thrombin in a large scale in a safe and economical manner due to exclusion of blood-derived components.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Multi-target compound with anticoagulation and antiplatelet activity as well as preparation method and application of multi-target compound
ActiveCN113201048ADirect specific antithrombin functionAchieve anticoagulant and antithrombotic effectPeptide-nucleic acidsPeptide/protein ingredientsBinding siteAnti platelet
The invention relates to a general formula and physiologically acceptable salts thereof: A-L-B-L'-C, wherein A and B are binding sites with thrombin, C is a binding site with a platelet GPIIb / IIIa receptor, L is a first linking group, and L' is a second linking group. The invention also relates to a preparation method and application of the compound. The invention provides an anticoagulant and antiplatelet GPIIb / IIIa receptor multi-target compound, which has good in-vitro human thrombin inhibition activity, in-vitro platelet GPIIb / IIIa receptor inhibition effect, in-vitro / in-vivo anti-platelet aggregation, in-vivo anticoagulant and antithrombotic effects, and can be used for preparing antithrombotic drugs. The traditional Chinese medicine composition can be clinically used for preventing and treating peripheral artery thrombosis and arteriovenous bypass thrombosis, treating thrombosis in acute coronary syndrome (ACS) or percutaneous coronary intervention (PCI), and preventing and treating cerebral arterial thrombosis.
Owner:SHAANXI MICOT TECH LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com