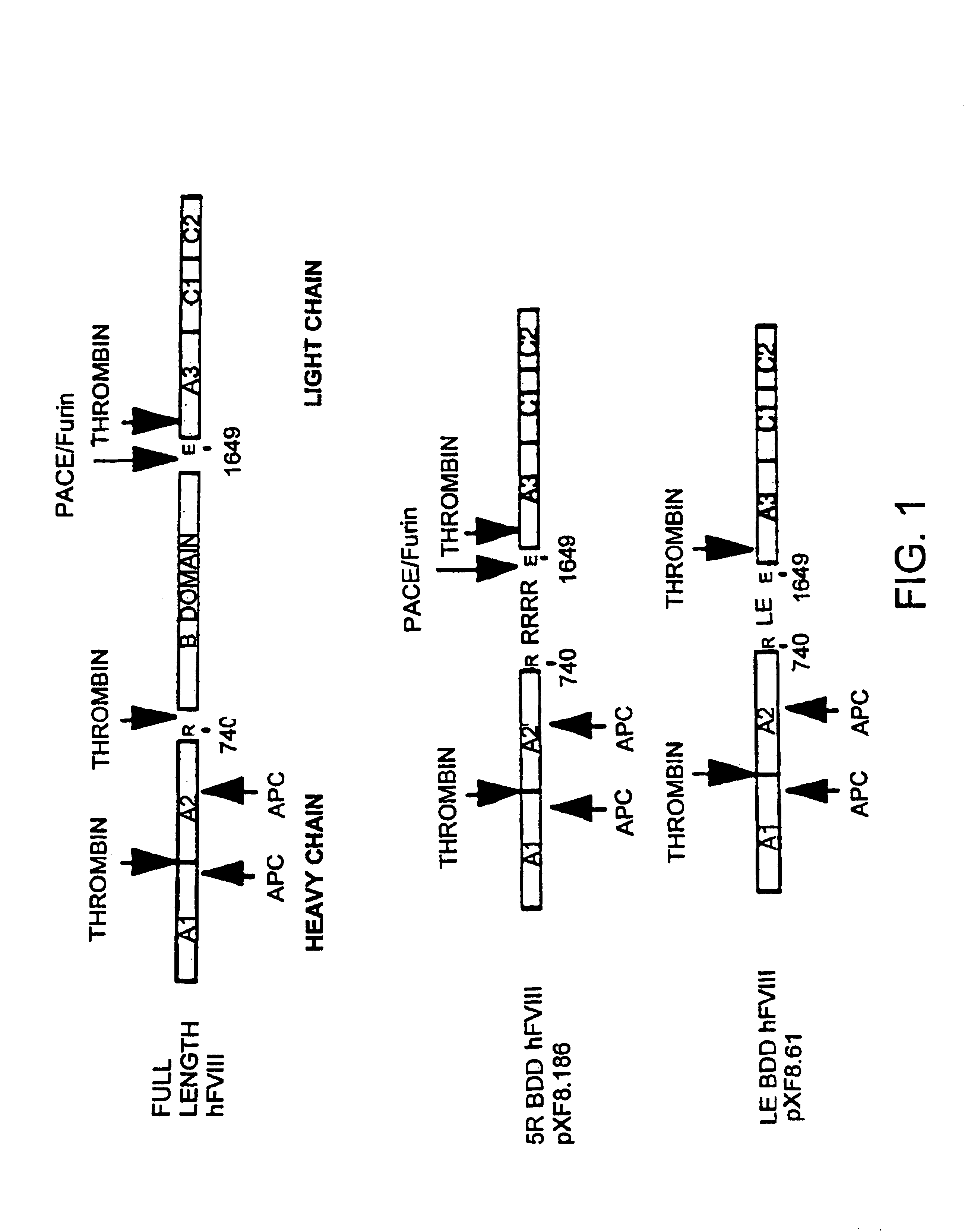
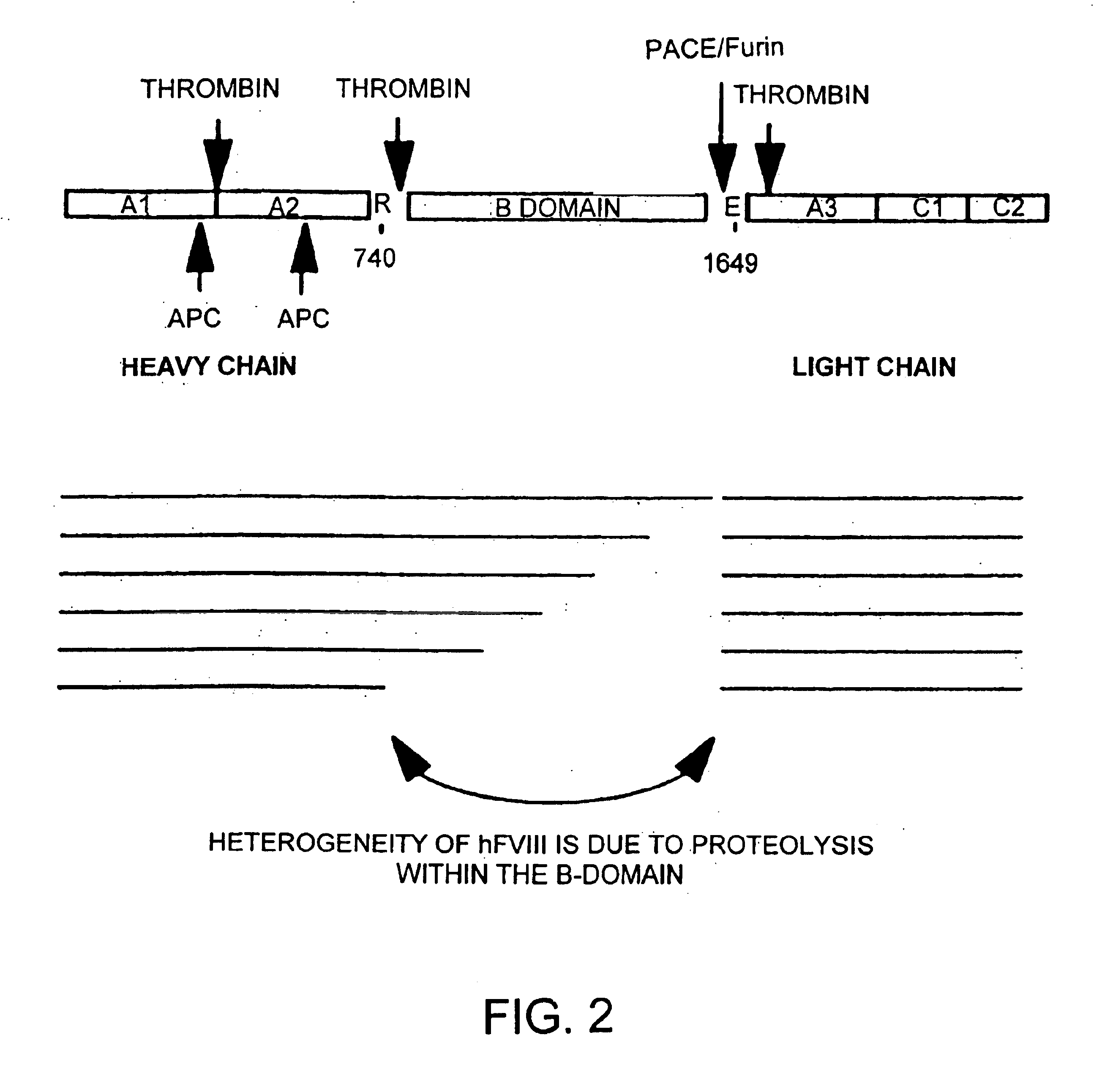
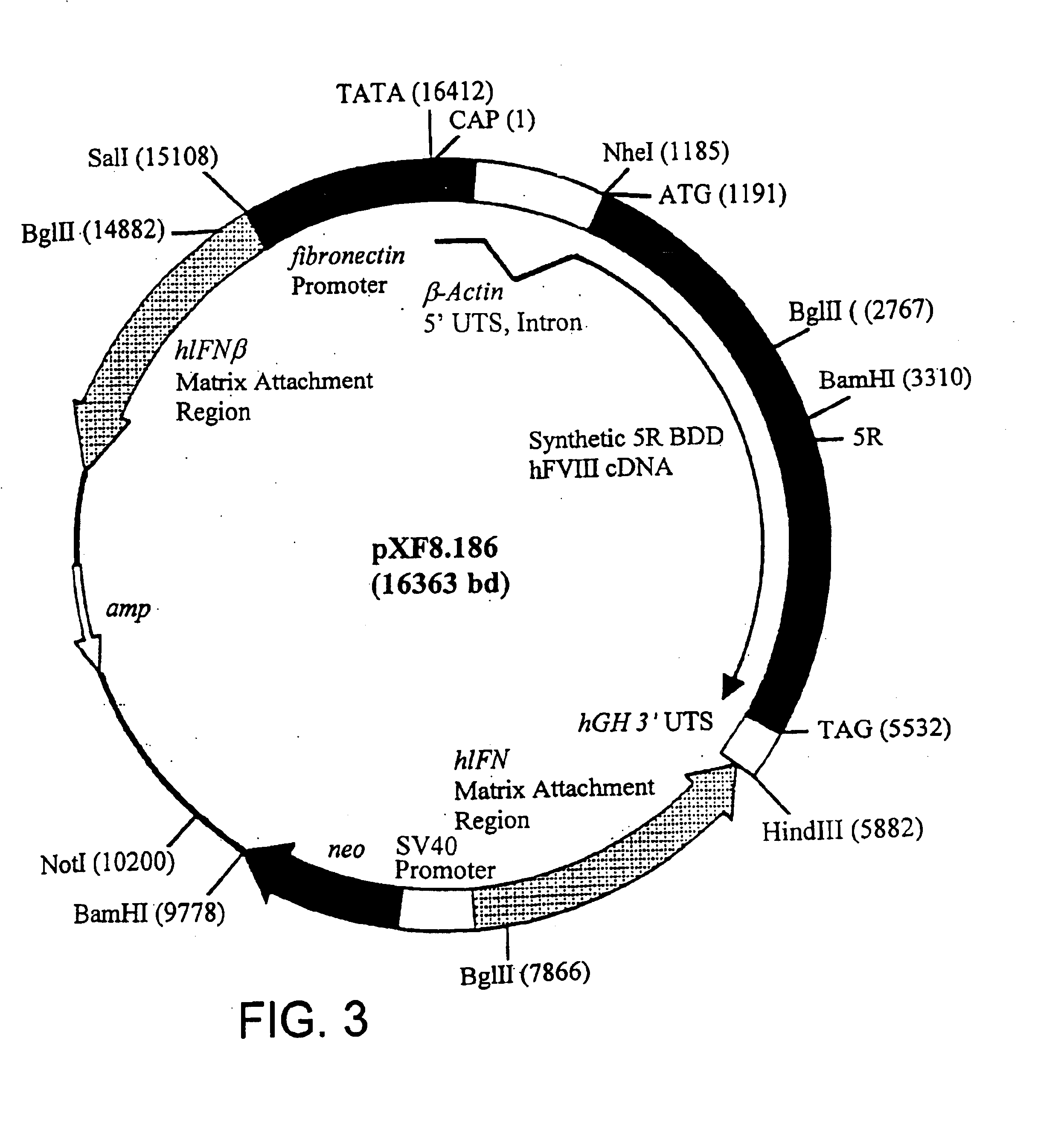
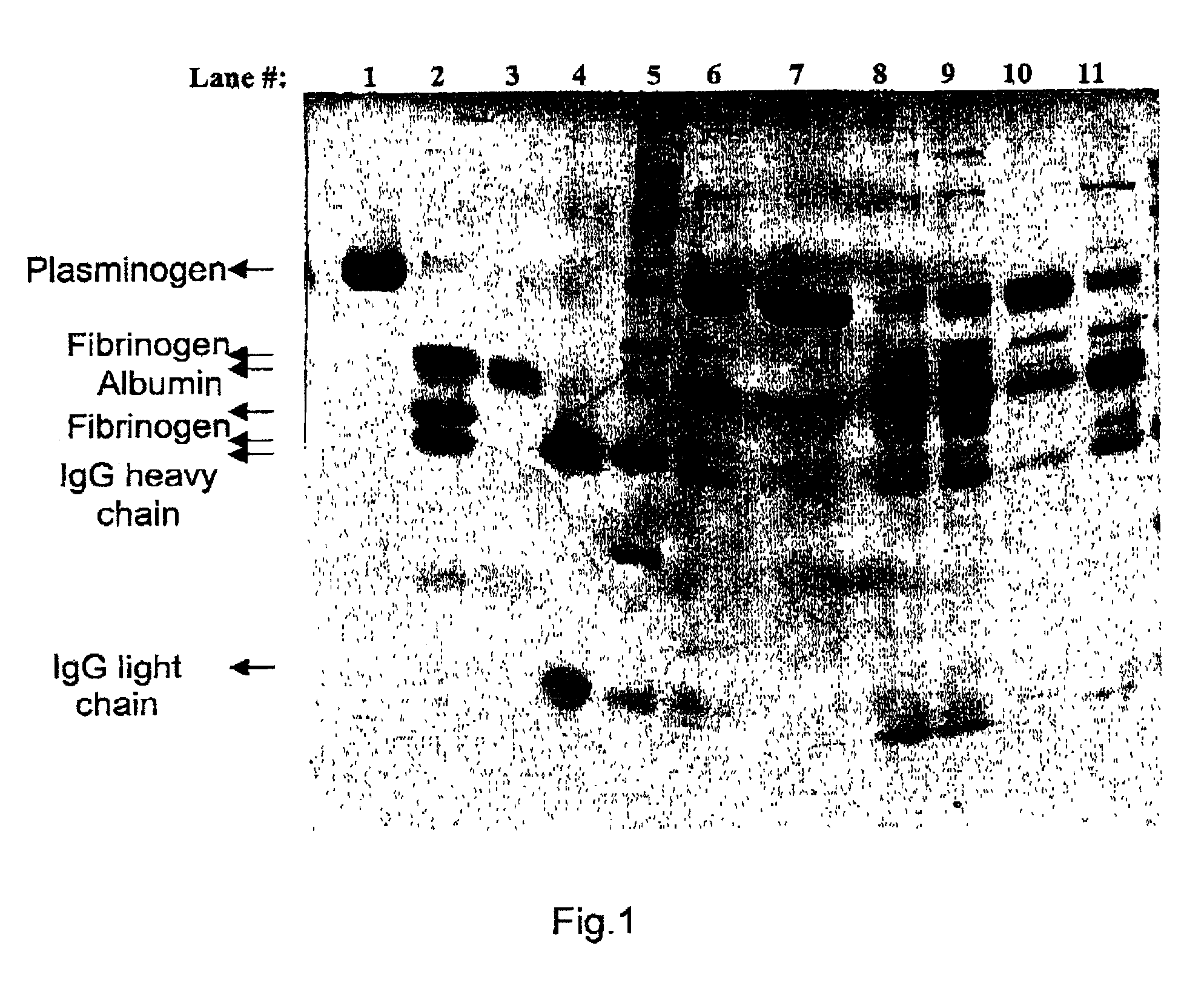
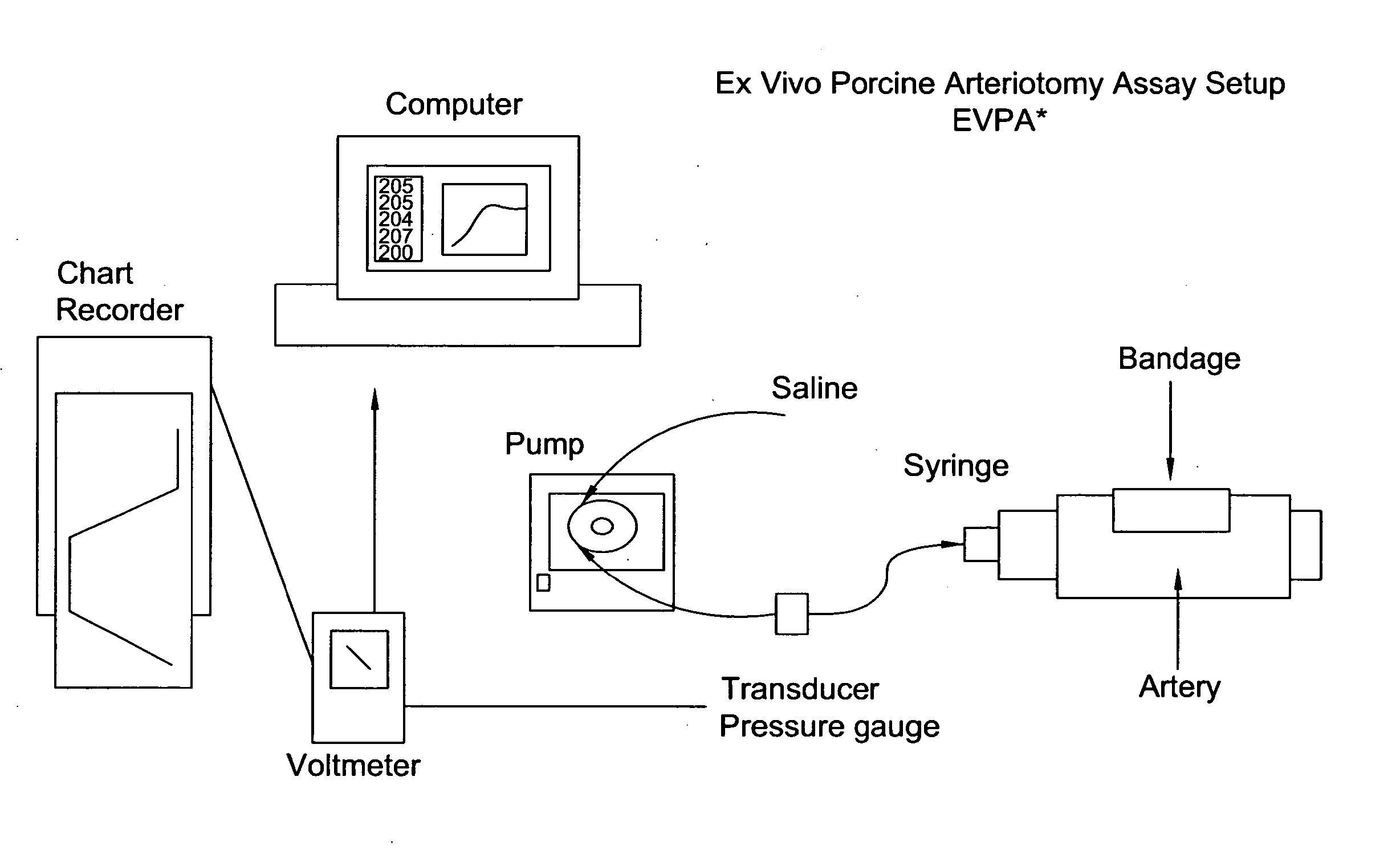
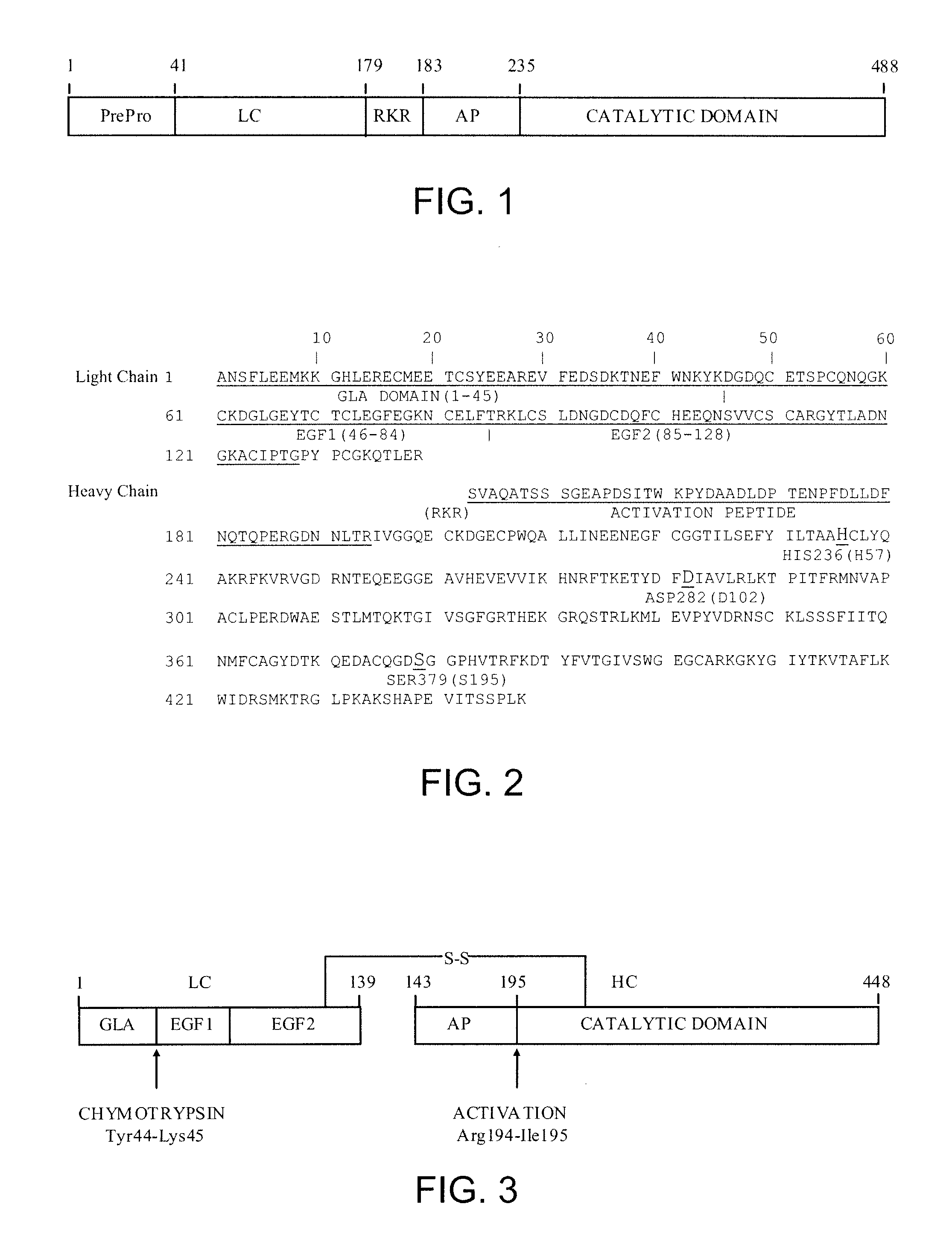
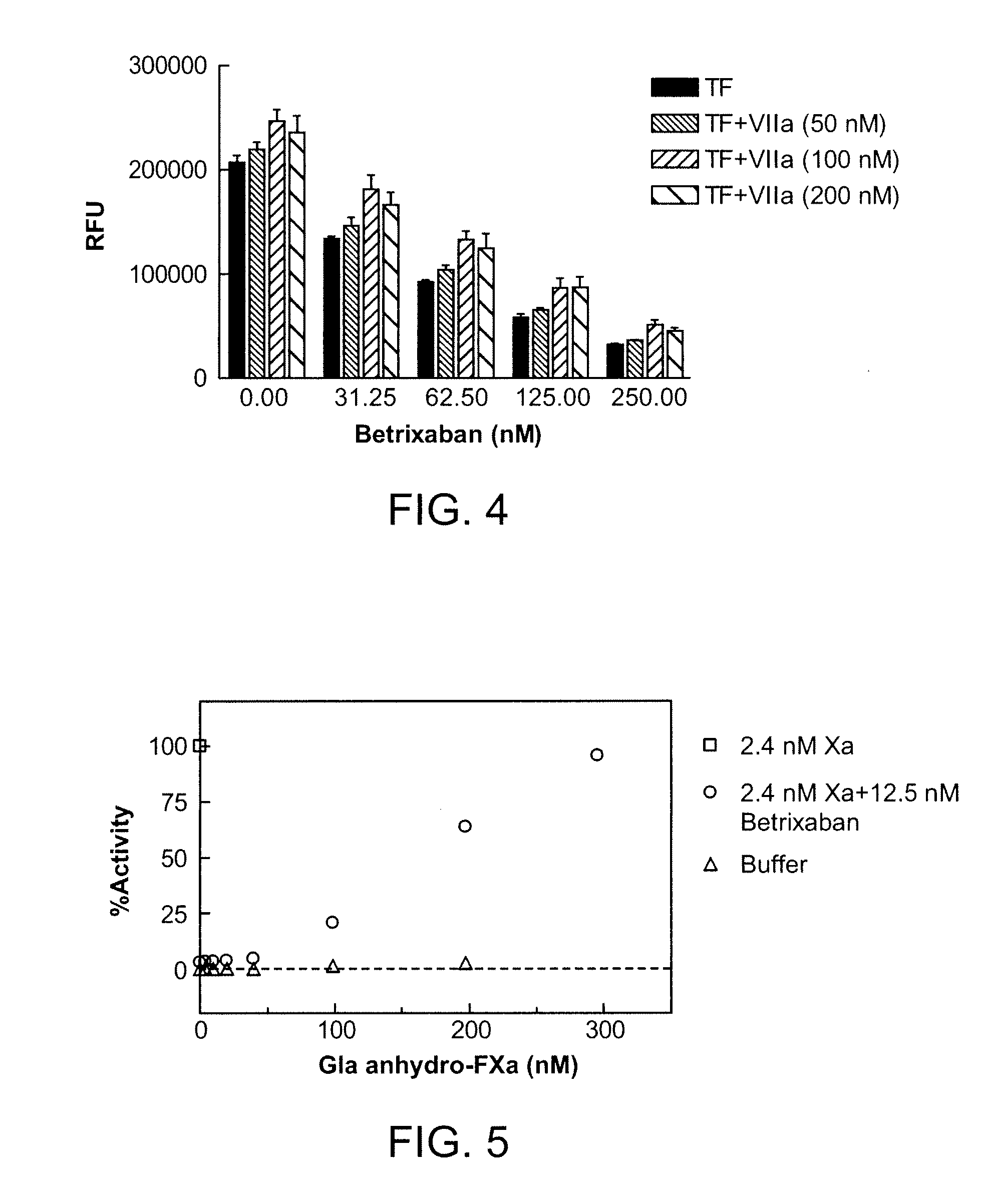
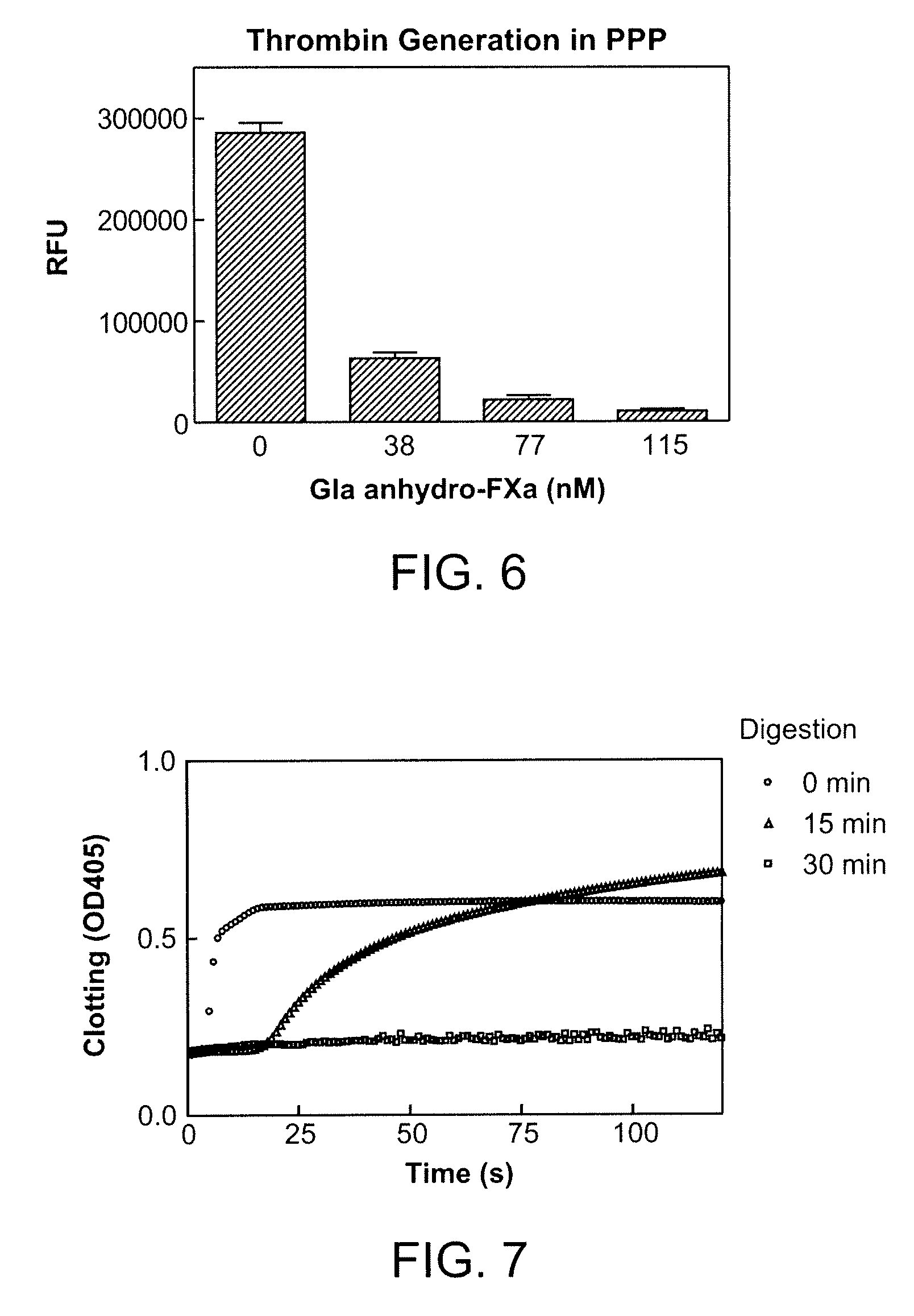
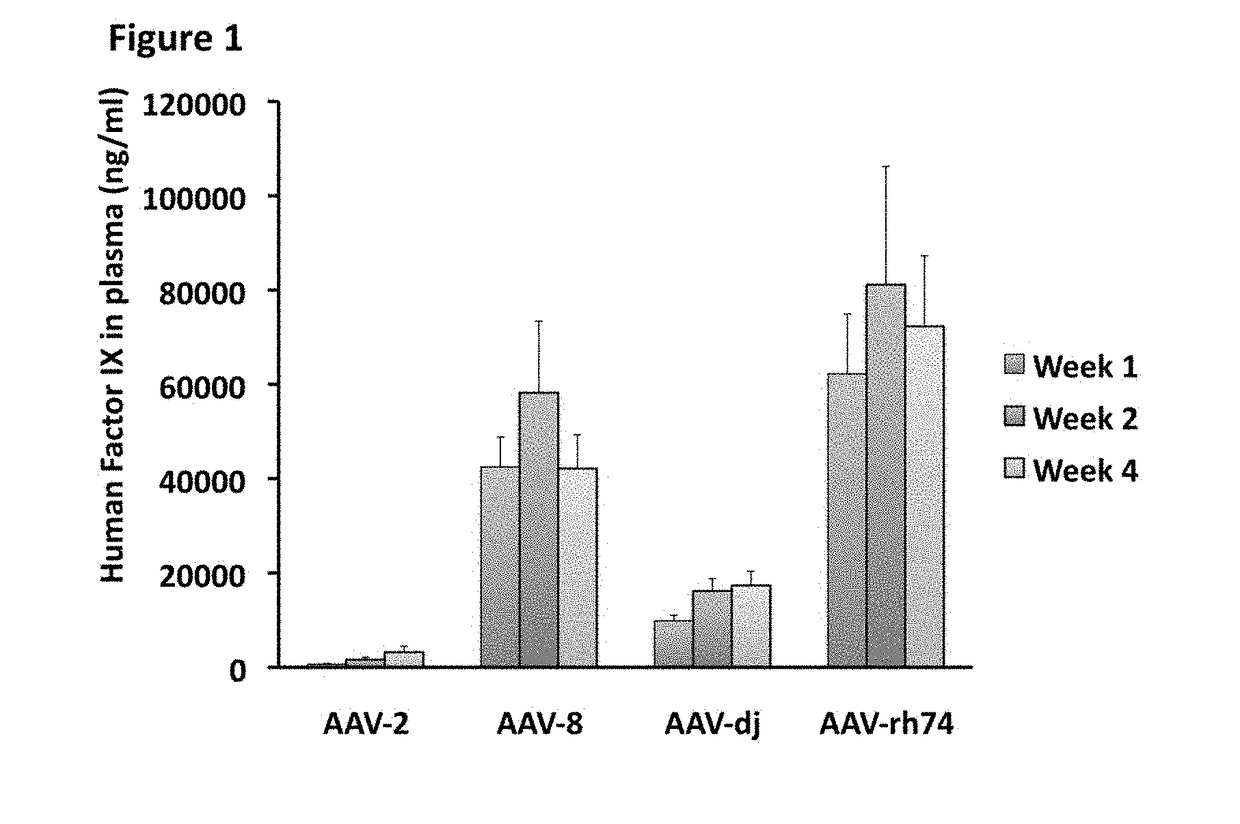
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4573results about "Peptidases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
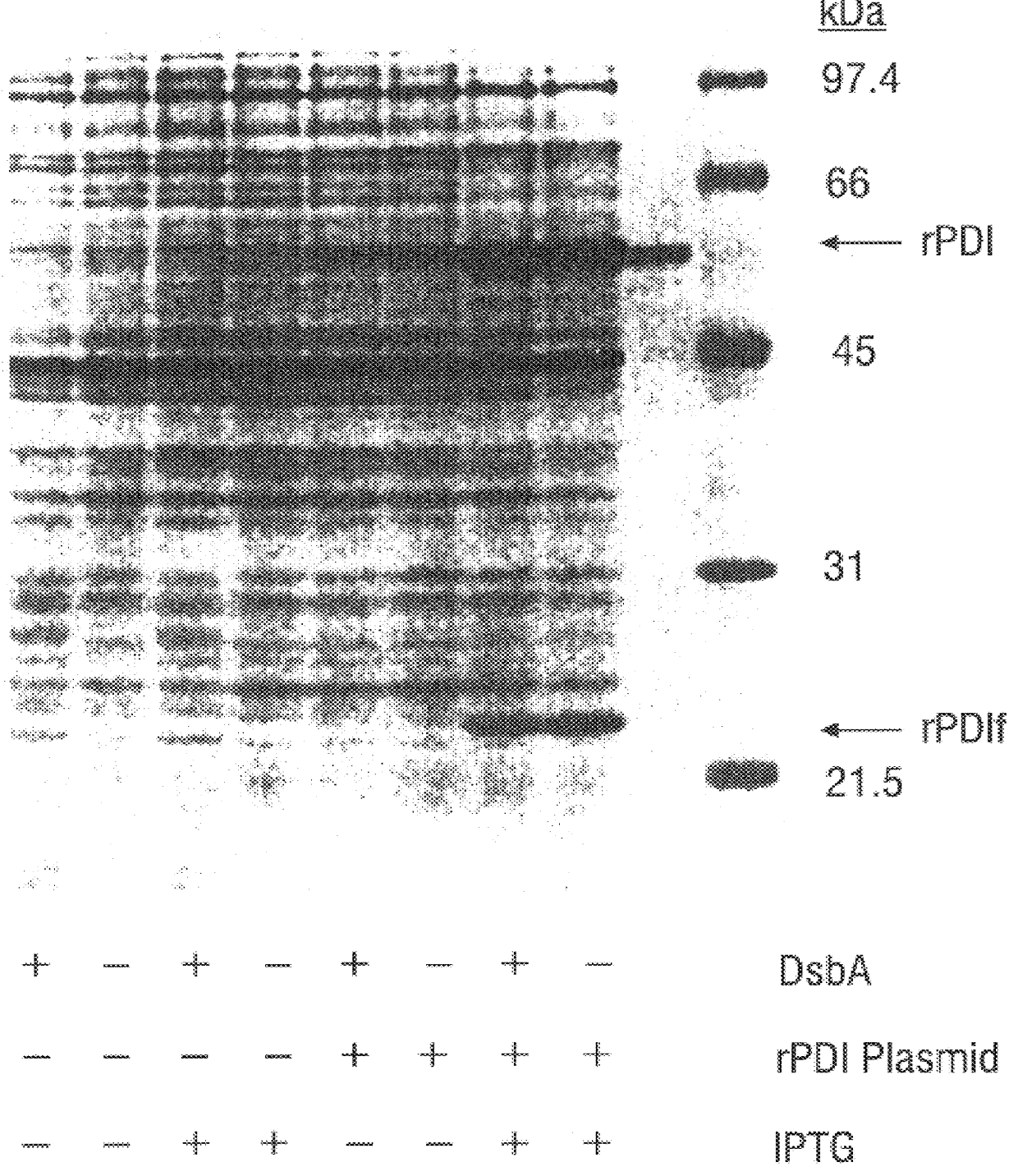
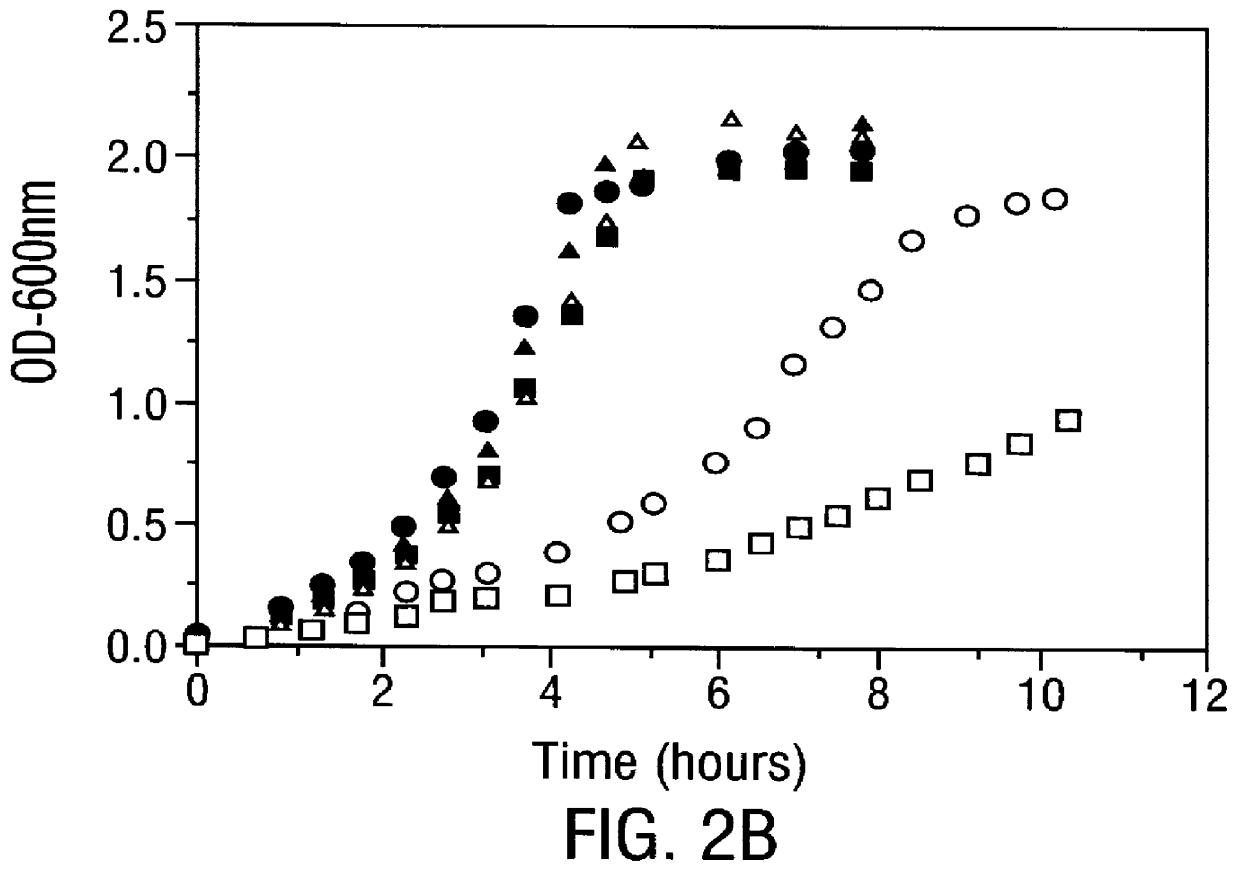
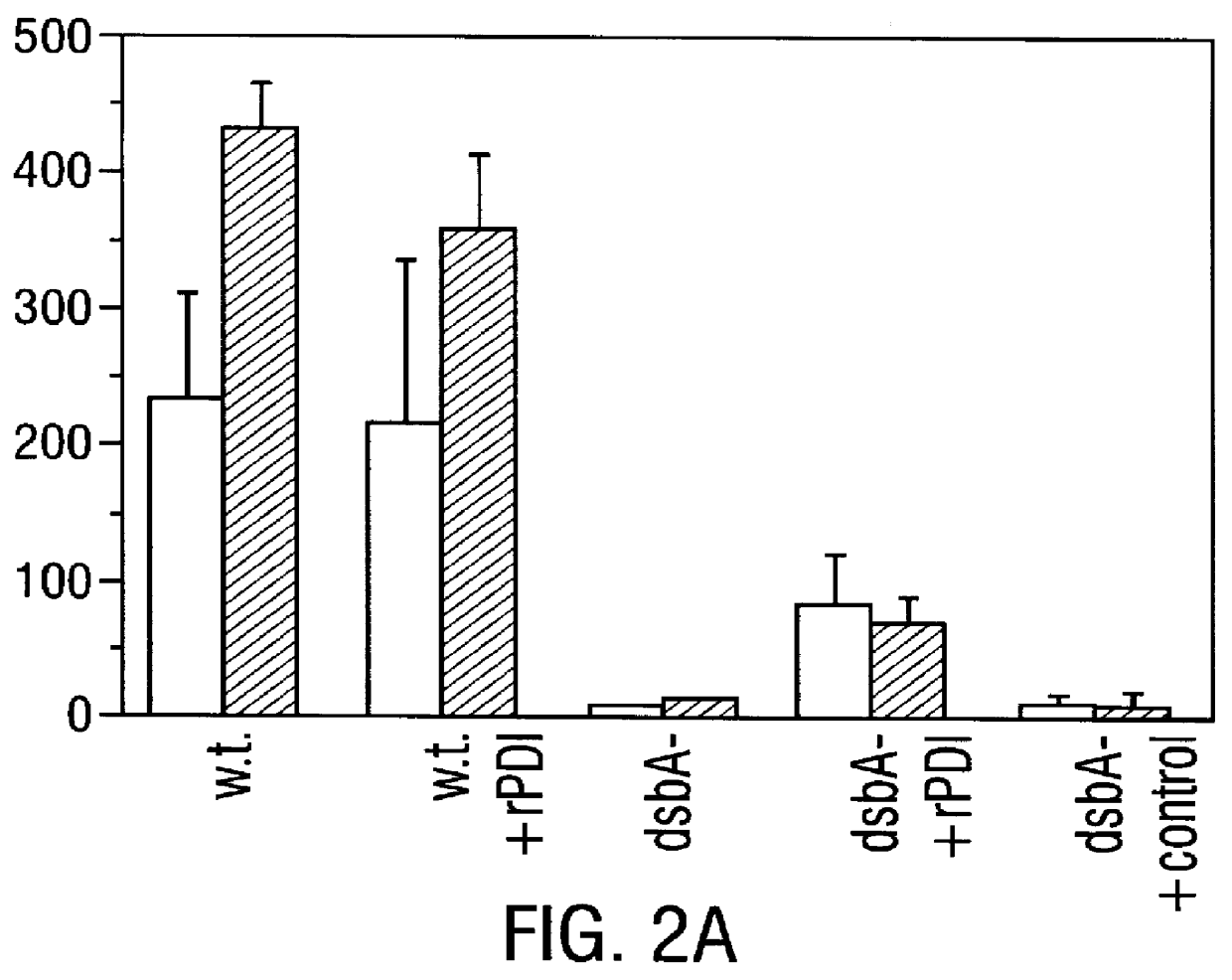
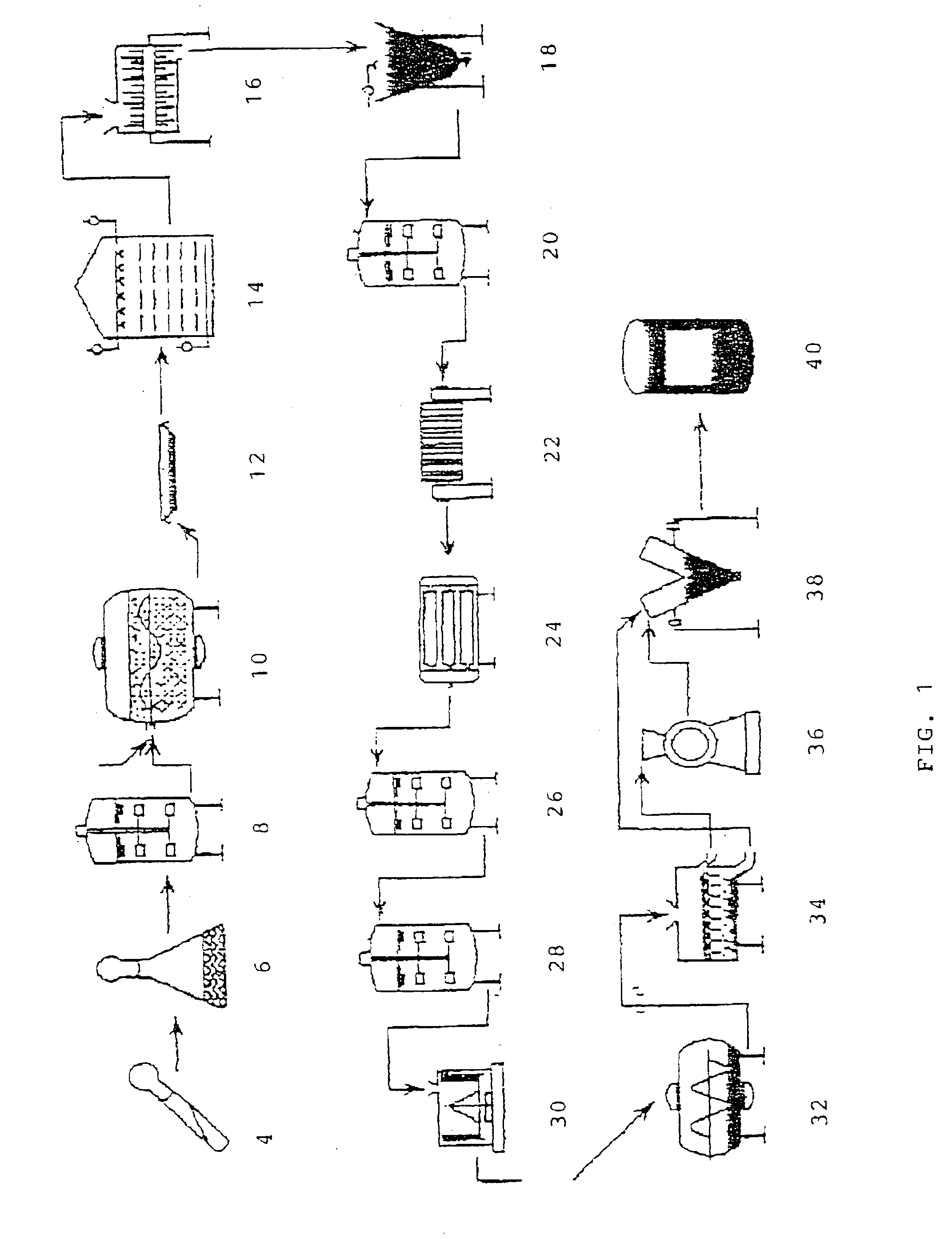
Methods for producing soluble, biologically-active disulfide-bond containing eukaryotic proteins in bacterial cells
InactiveUS6027888AEfficient productionFold preciselyPeptide/protein ingredientsMicroorganismsDisulfide bondingZymogen
Disclosed are methods of producing eukaryotic disulfide bond-containing polypeptides in bacterial hosts, and compositions resulting therefrom. Co-expression of a eukaryotic foldase and a disulfide bond-containing polypeptide in a bacterial host cell is demonstrated. In particular embodiments, the methods have been used to produce mammalian pancreatic trypsin inhibitor and tissue plasminogen activator (tPA) in soluble, biologically-active forms, which are isolatable from the bacterial periplasm. Also disclosed are expression systems, recombinant vectors, and transformed host cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
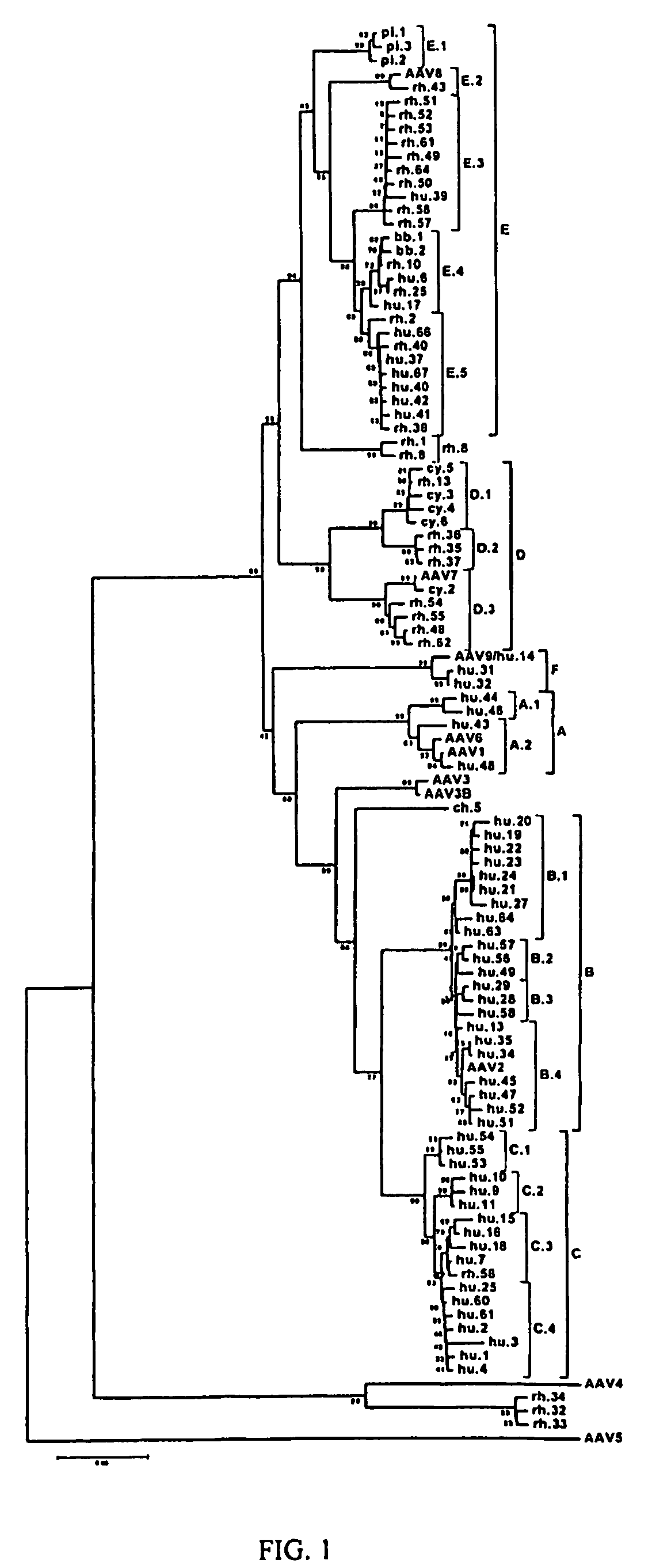
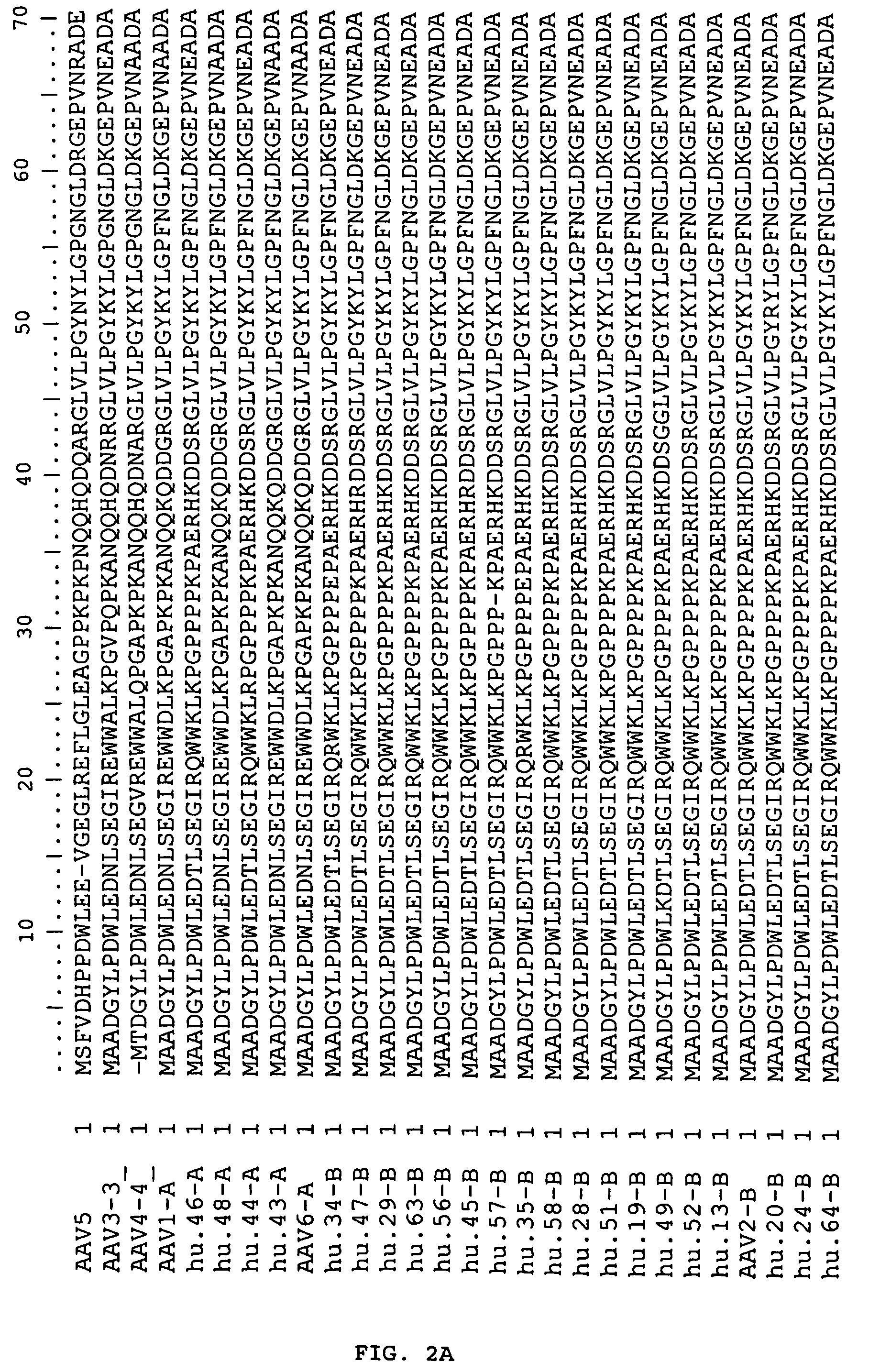
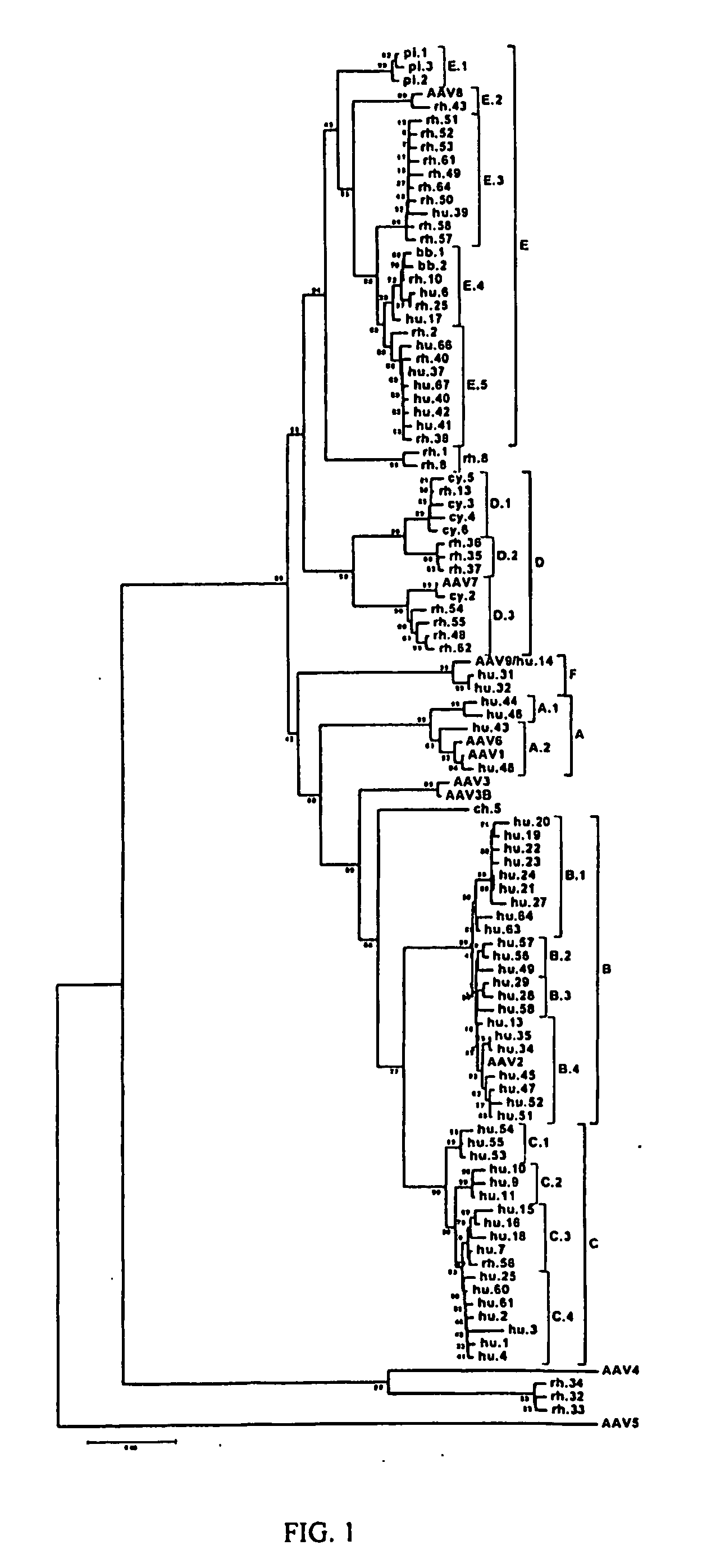
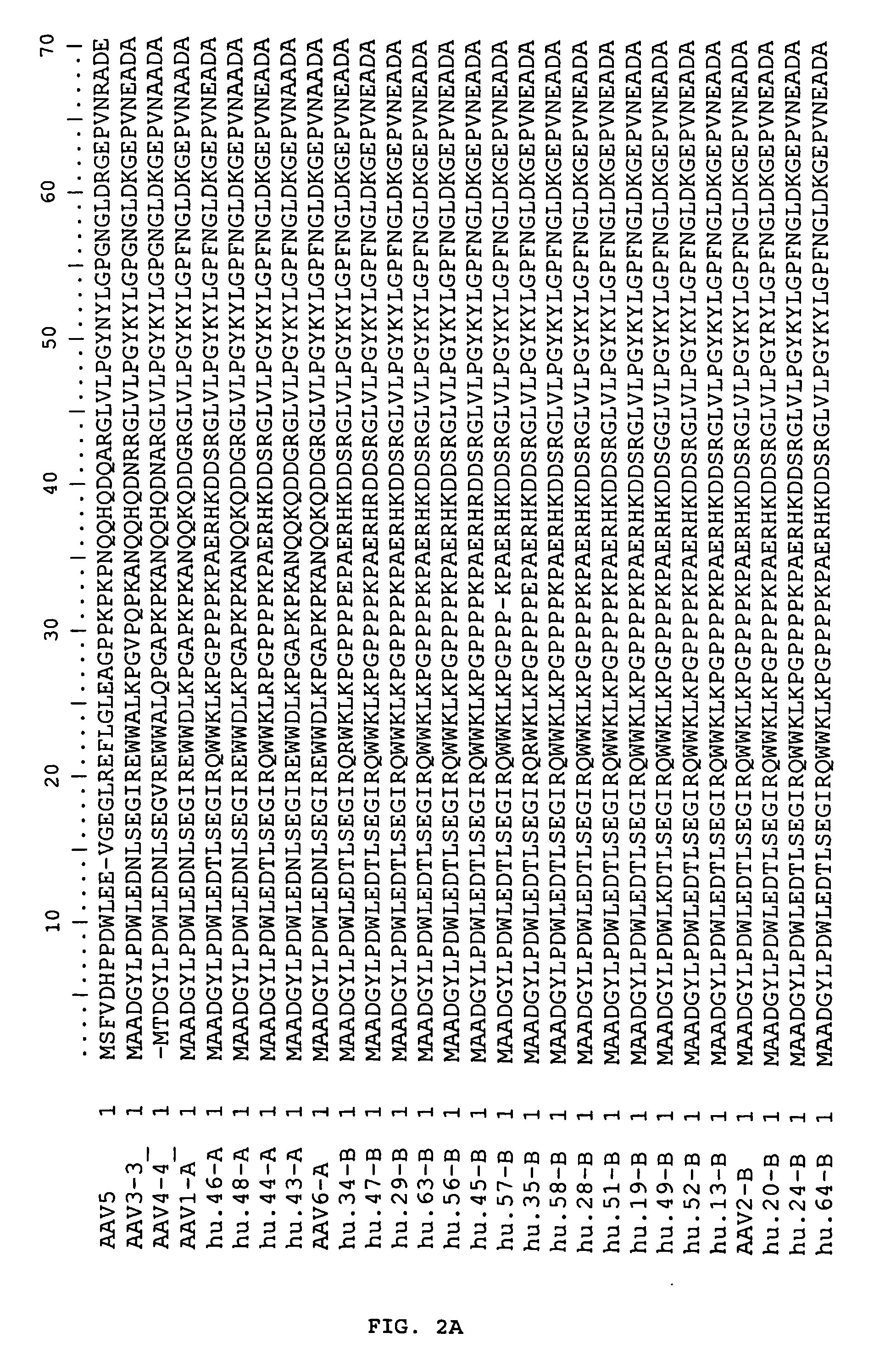
Adeno-associated virus (AAV) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Adeno-associated virus (aav) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
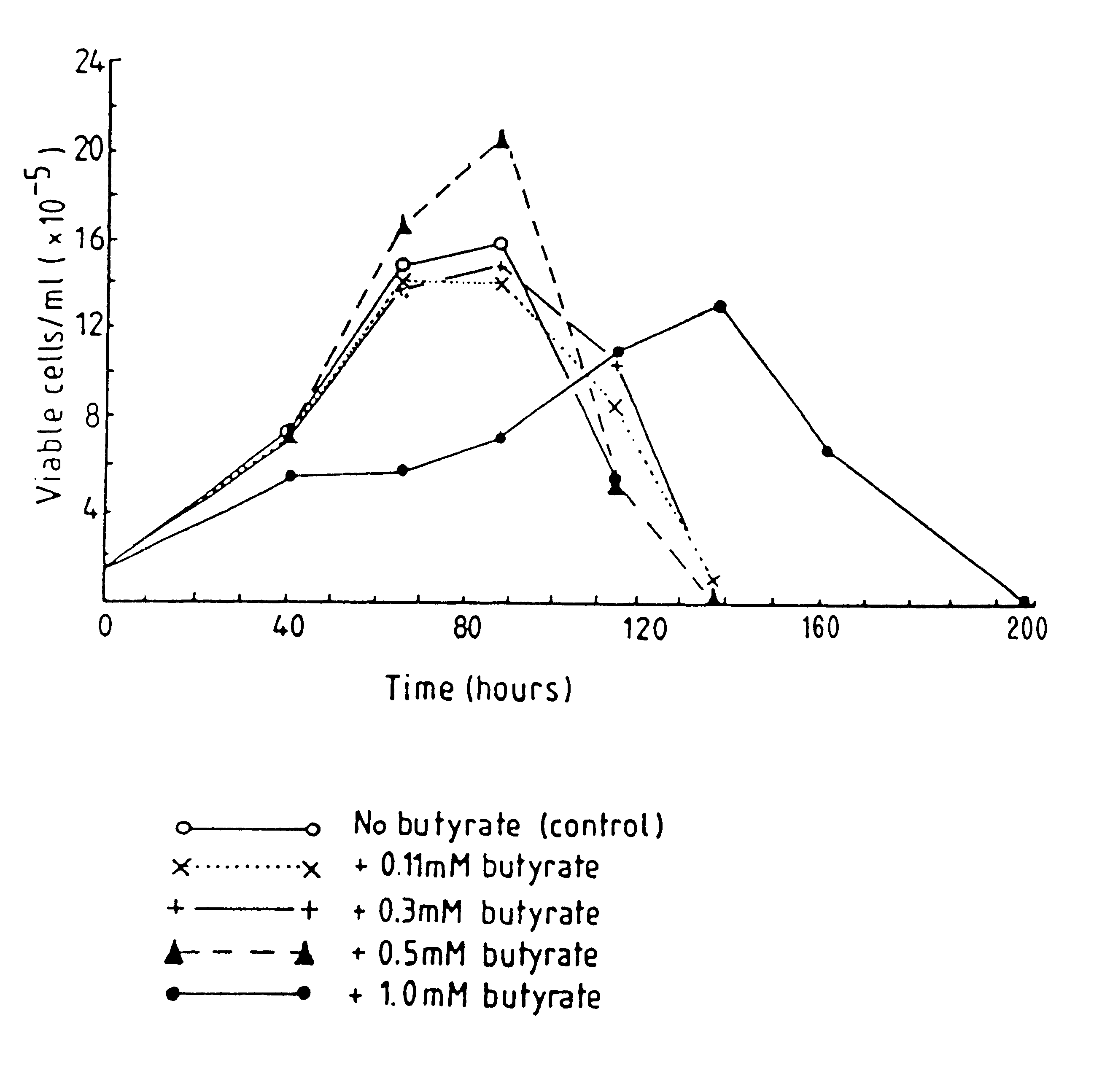
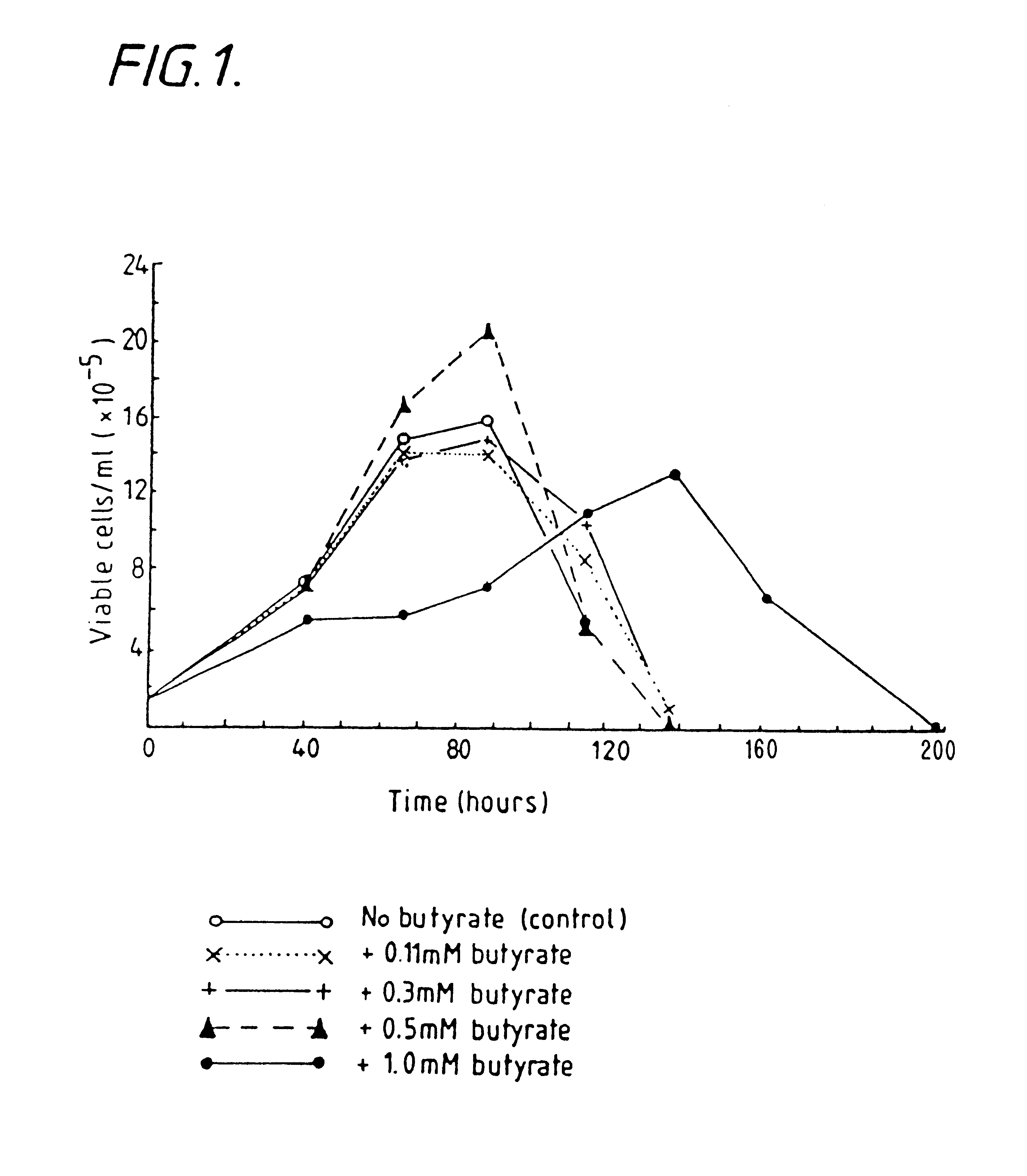
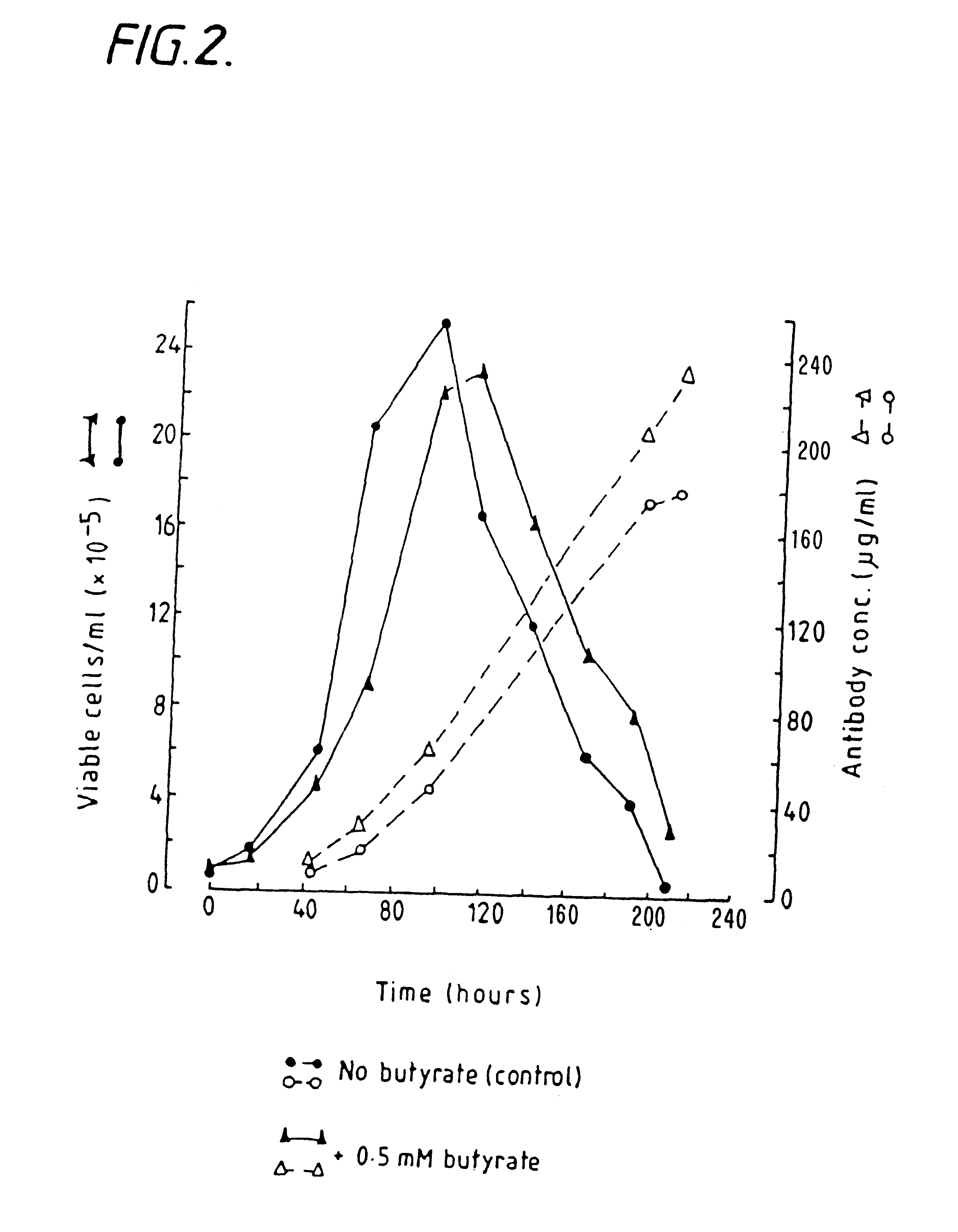
Production of proteins by cell culture
InactiveUS6413746B1High protein yieldReduce cell viabilityImmunoglobulins against blood group antigensPeptide/protein ingredients3D cell cultureBiochemistry
Methods for obtaining a protein by culture of hybridoma cells, wherein said protein is an immunoglobulin, are disclosed. The methods involve culturing animal hybridoma cells in continuous presence of an alkanoic acid or salt thereof, which enhances protein production, wherein said alkanoic acid or salt thereof is present at 2 concentration range of 0.1 mM to 200 mM.
Owner:LONZA LTD
Method for preparing thrombin for use in a biological glue
InactiveUS6472162B1Derive fast acting, stable autologous thrombinSimple preparatory procedureBioreactor/fermenter combinationsBiological substance pretreatmentsTissue sealantDonors plasma
A sterile method for preparing stable thrombin component from a single donor's plasma in which the thrombin component and the clotting and adhesive proteins component are harvested simultaneously from the same donor plasma in less than one hour. The combined components provide an improved biological hemostatic agent and tissue sealant by virtue of its freedom from the risk of contaminating viruses or bacteria from allogenic human or bovine blood sources. The thrombin provides polymerization of the clotting and adhesive proteins in less than five seconds, and is sufficiently stable to provide that fast clotting over a six hour period. Further, the clotting times can be predictably lengthened by diluting the thrombin with saline.
Owner:ASAHI KASEI MEDICAL CO LTD
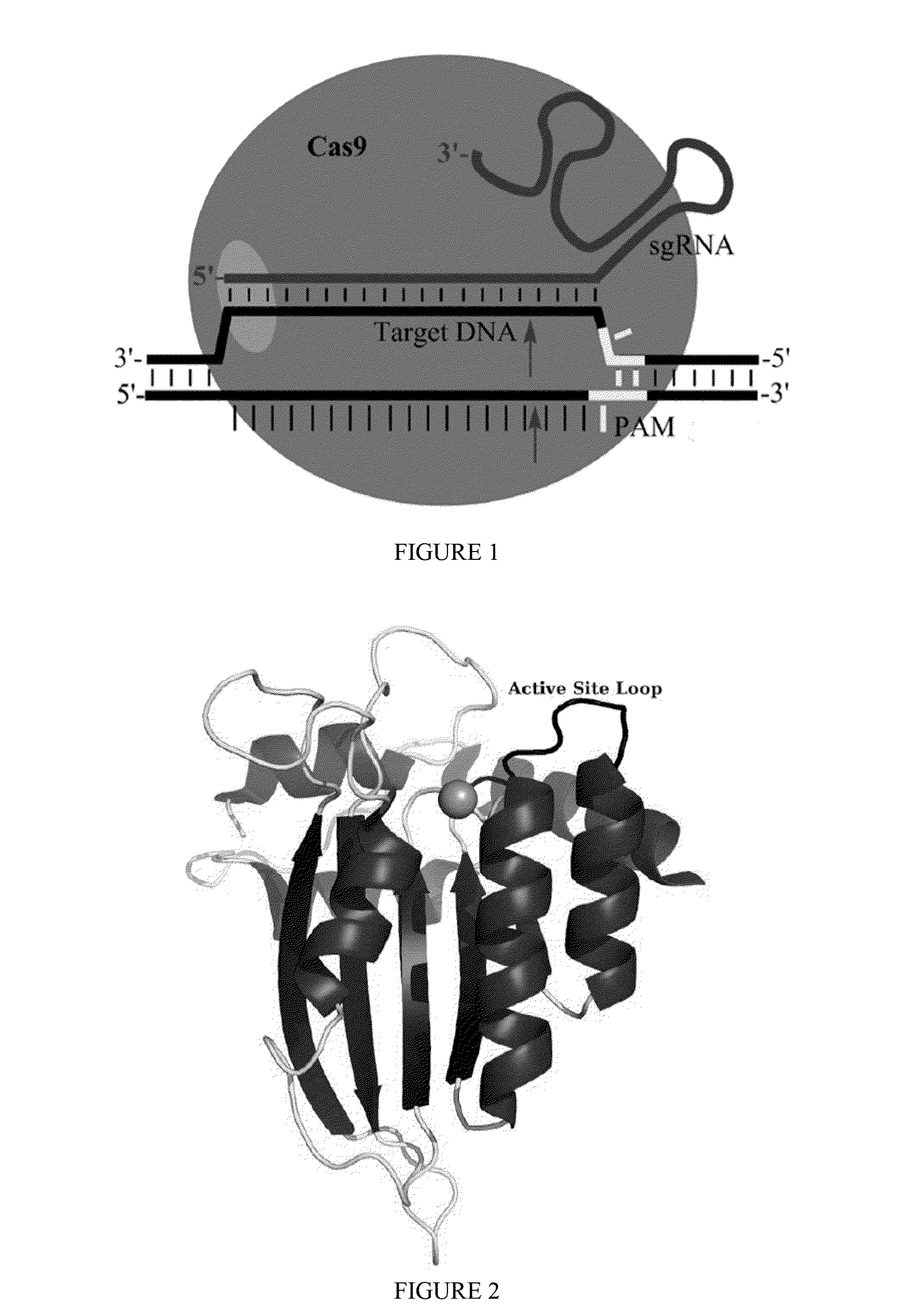
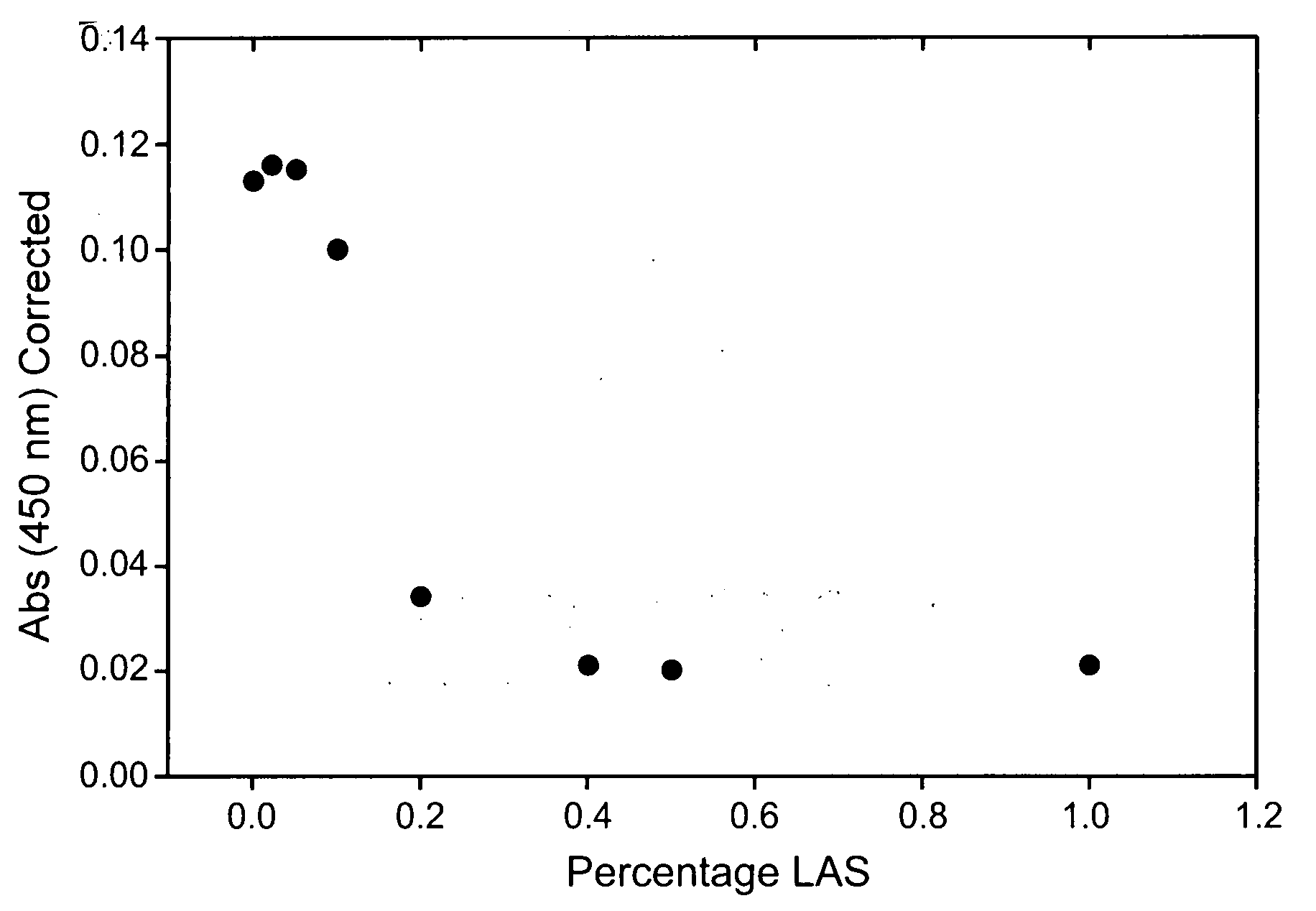
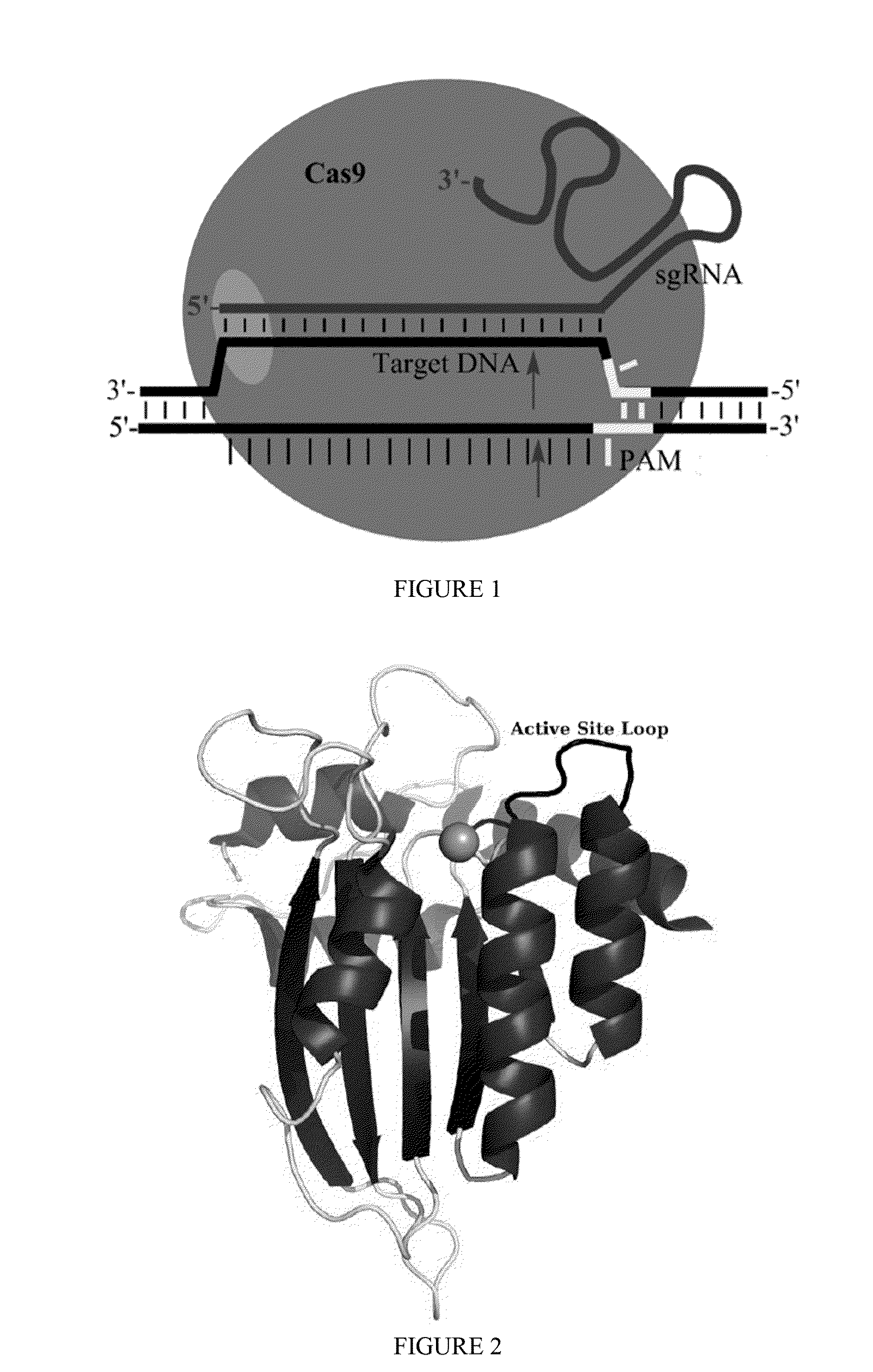
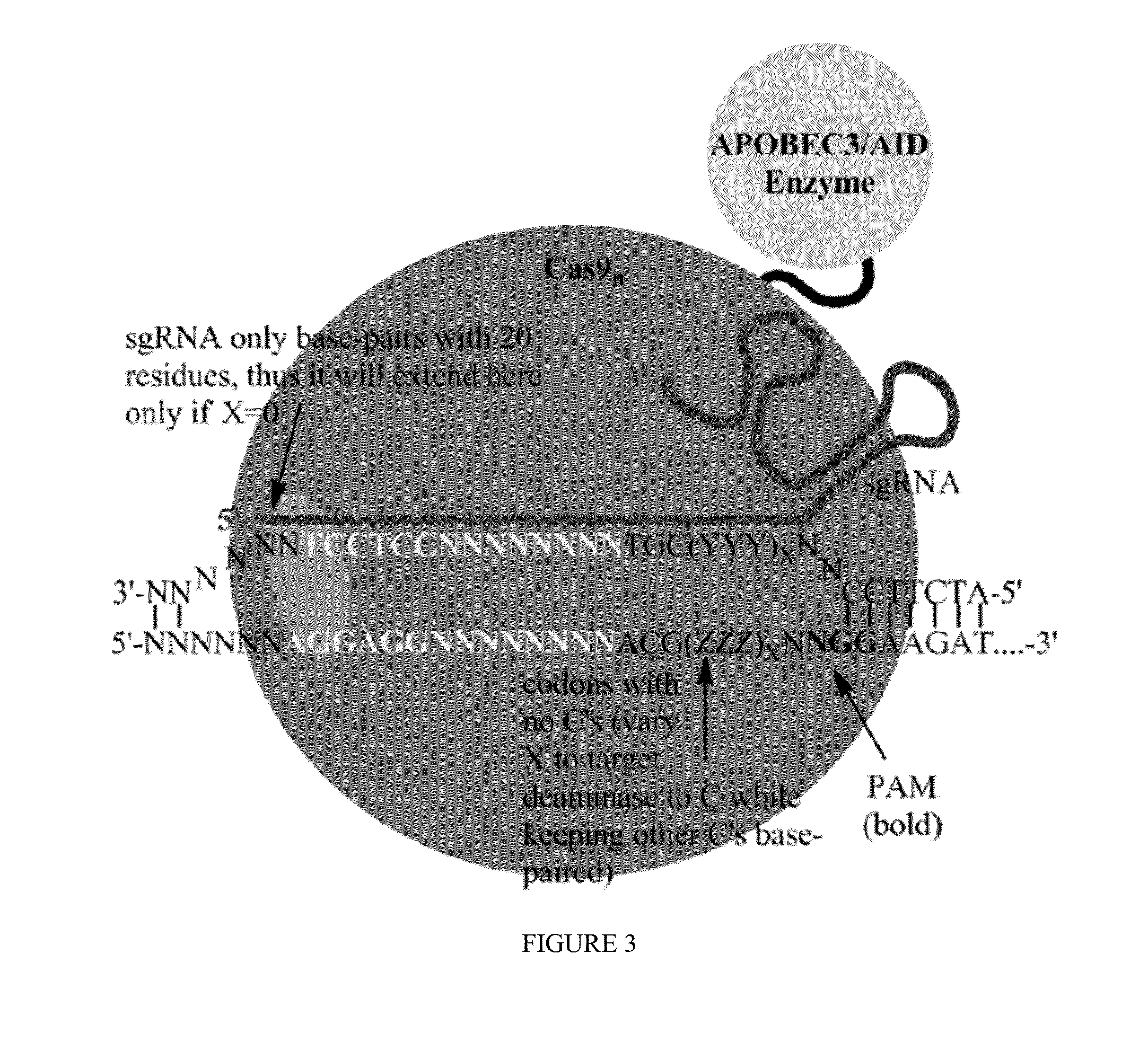
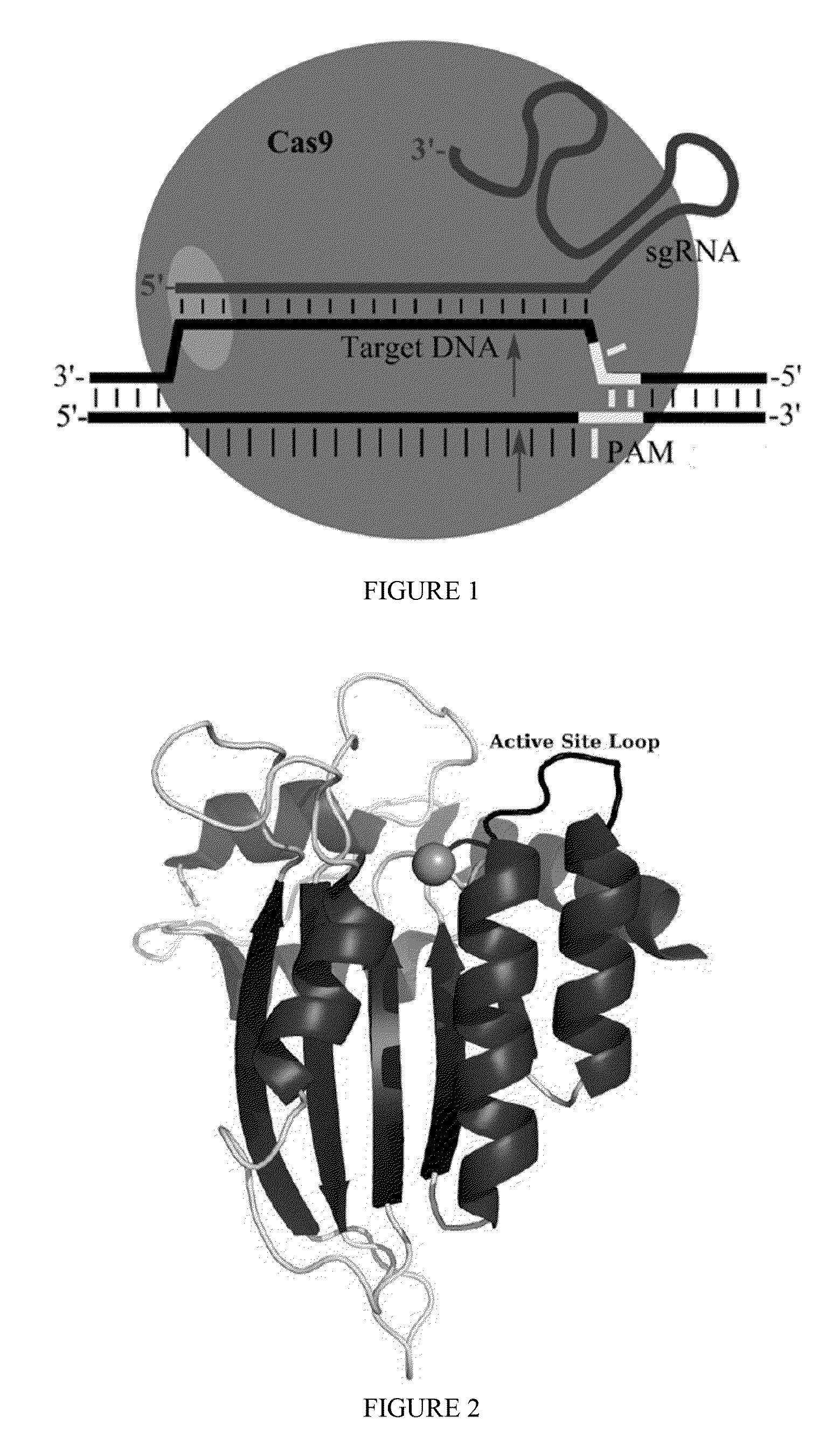
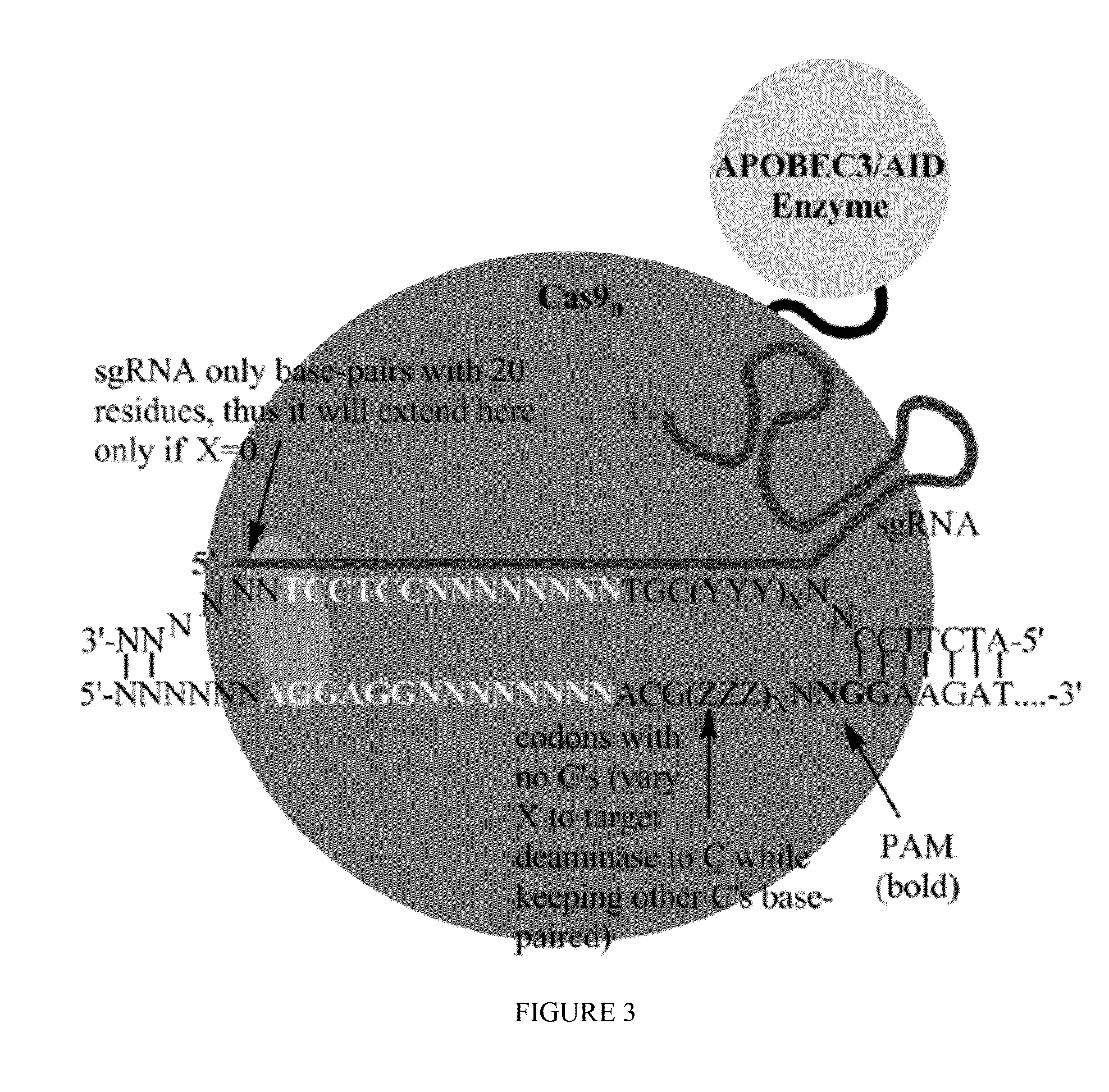
Fusions of cas9 domains and nucleic acid-editing domains
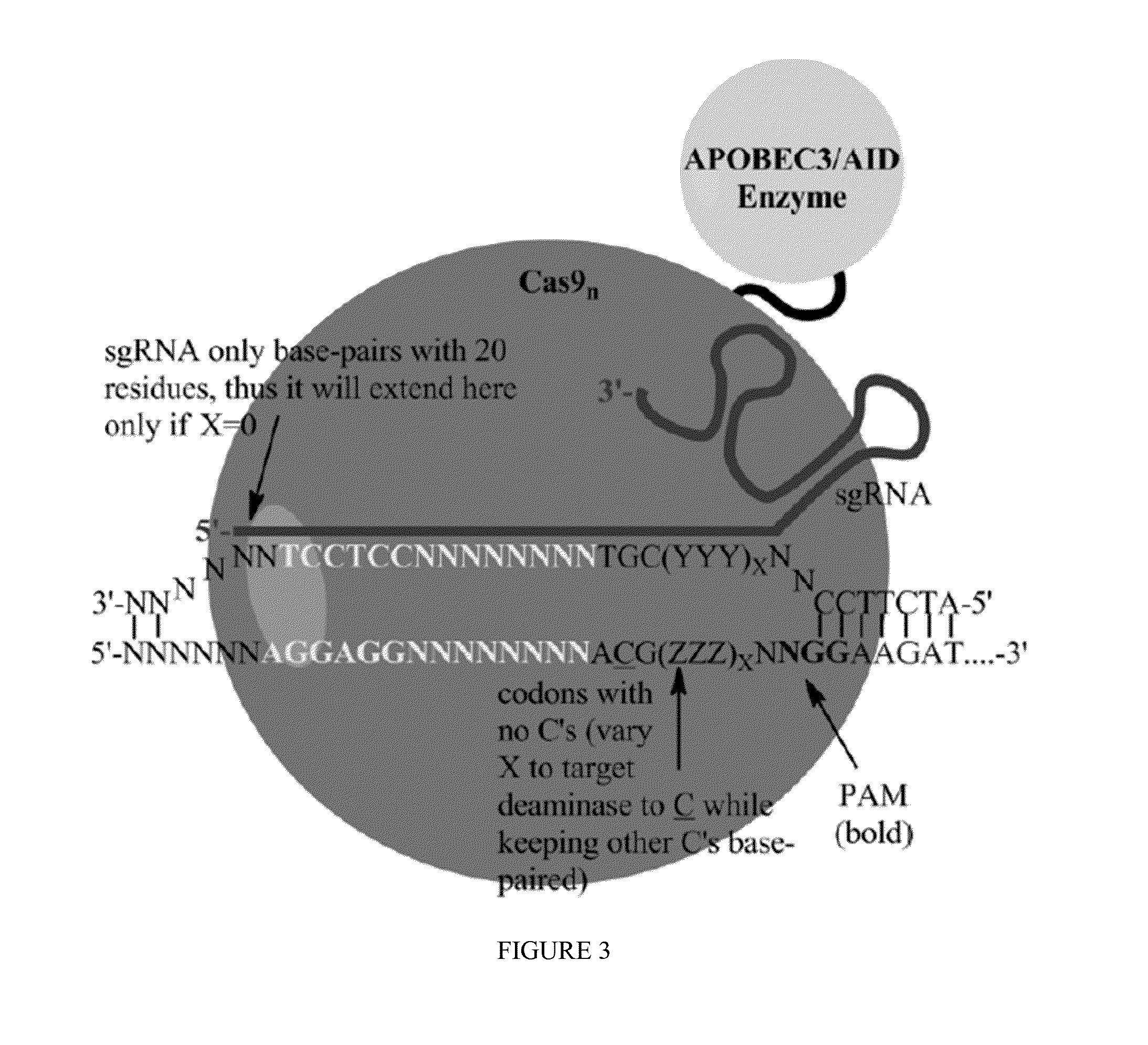
Some aspects of this disclosure provide strategies, systems, reagents, methods, and kits that are useful for the targeted editing of nucleic acids, including editing a single site within the genome of a cell or subject, e.g., within the human genome. In some embodiments, fusion proteins of Cas9 and nucleic acid editing enzymes or enzyme domains, e.g., deaminase domains, are provided. In some embodiments, methods for targeted nucleic acid editing are provided. In some embodiments, reagents and kits for the generation of targeted nucleic acid editing proteins, e.g., fusion proteins of Cas9 and nucleic acid editing enzymes or domains, are provided.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Topical Pharmaceutical Foam Composition
InactiveUS20070154402A1Reduced intensity of colorReduce odor intensityAntibacterial agentsBiocideAlcohol freeActive agent
A stable topical alcohol-free aerosol foam containing one or more keratolytic agents is provided. The foam-forming formulation is an emulsion which contains an HFA propellant and one or more keratolytic agents. The emulsion has an oil phase and an aqueous, i.e. water-containing, phase. The active agent(s) may be present in either phase of the emulsion or dispersed in the emulsion. The oil phase may consist at least in part of the HFA propellant. Either or both of the oil phase and the aqueous phase may contain one or more surfactants, emulsifiers, emulsion stabilizers, buffers, and / or other excipients. The foam is stable on the skin, for example, for at least 5 minutes at body temperature, preferably at least 20 minutes at body temperature, and disappears into the skin upon rubbing or after prolonged standing. In one embodiment, the formulation contains an HFA propellant which does not contain additional co-solvents or co-propellants. The formulations demonstrate reduced intensity of the odor and / or color associated with the keratolytic agent(s) as compared to conventional formulations containing keratolytic agents.
Owner:PRECISION DERMATOLOGY
Use and production of storage-stable neutral metalloprotease
InactiveUS20080293610A1Good storage stabilityImprove performanceBacteriaFermentationBiologyGenus Bacillus
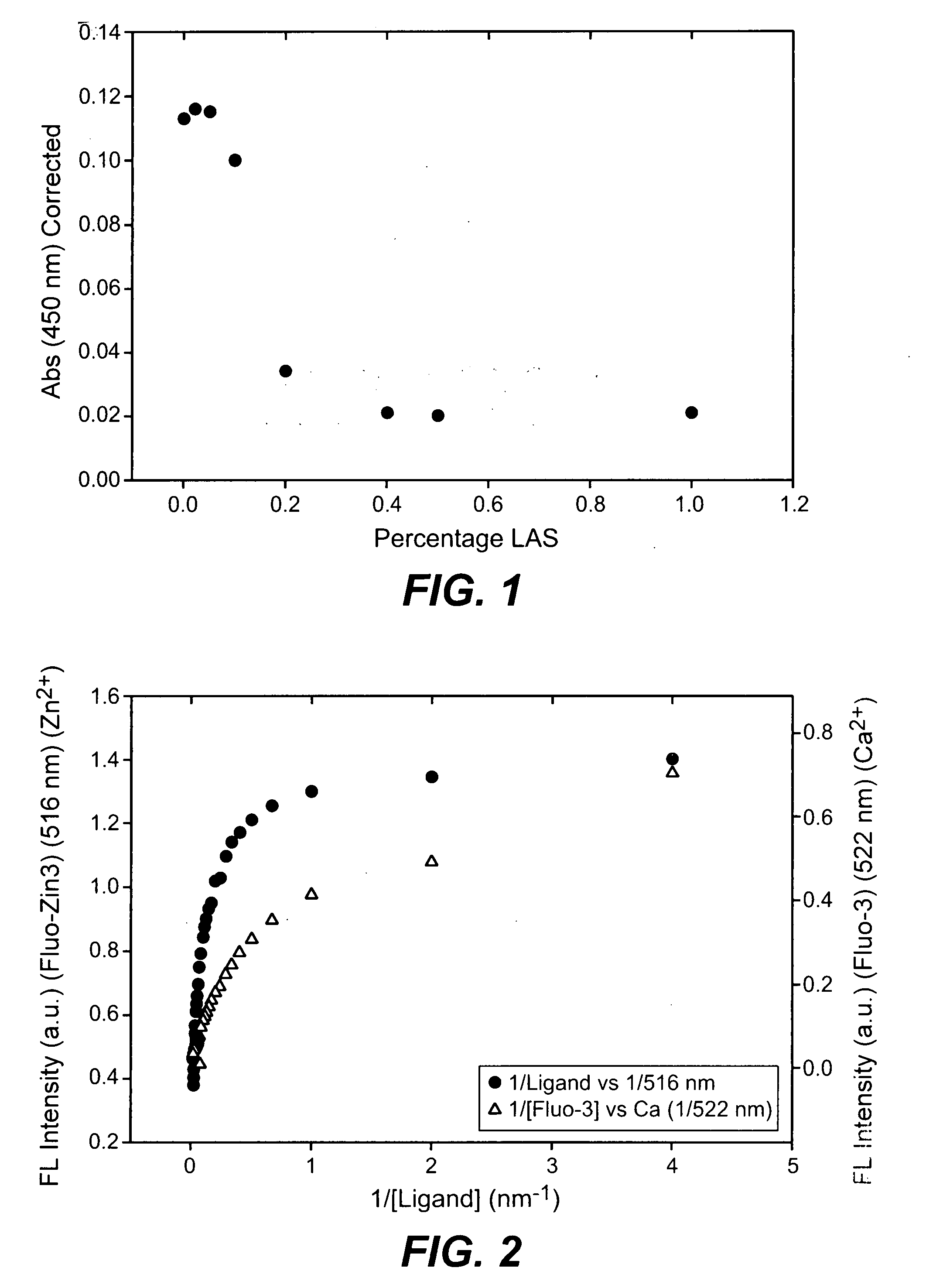
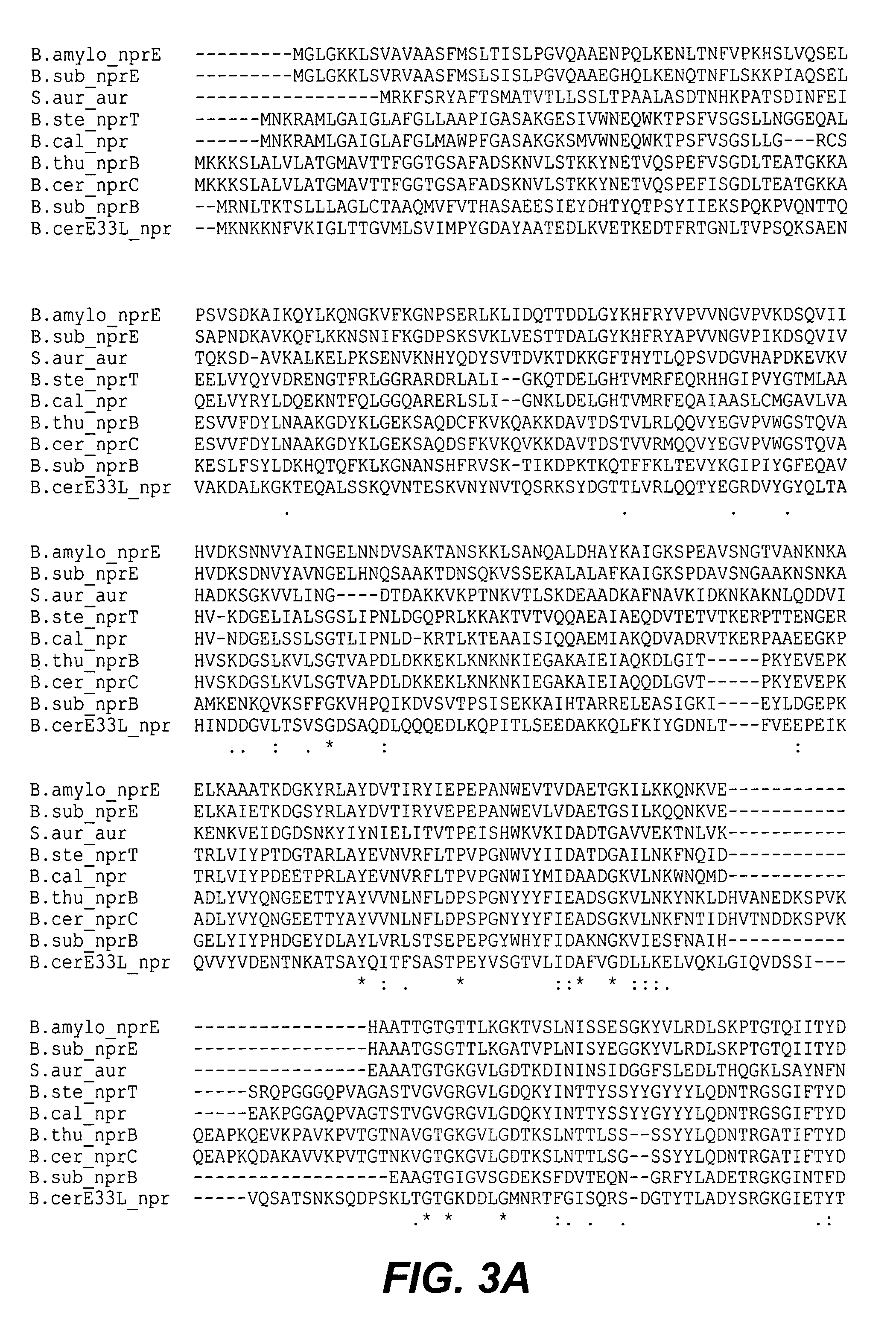
The present invention provides methods and compositions comprising at least one neutral metalloprotease enzyme that has improved storage stability. In some embodiments, the neutral metalloprotease finds use in cleaning and other applications. In some particularly preferred embodiments, the present invention provides methods and compositions comprising neutral metalloprotease(s) obtained from Bacillus sp. In some more particularly preferred embodiments, the neutral metalloprotease is obtained from B. amyloliquefaciens. In still further preferred embodiments, the neutral metalloprotease is a variant of the B. amyloliquefaciens neutral metalloprotease. In yet additional embodiments, the neutral metalloprotease is a homolog of the B. amyloliquefaciens neutral metalloprotease. The present invention finds particular use in applications including, but not limited to cleaning, bleaching and disinfecting.
Owner:DANISCO US INC +1
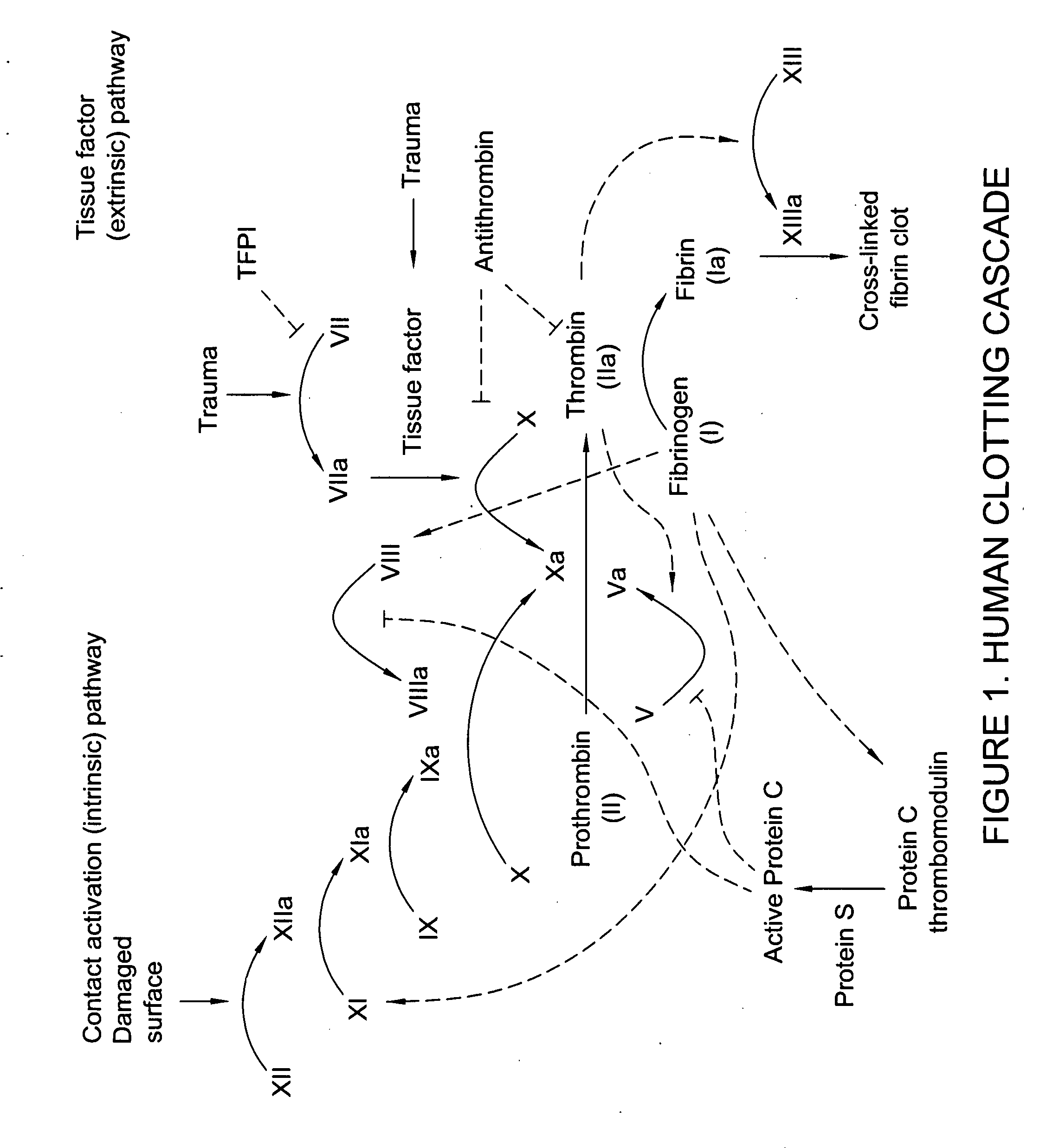
Clotting factor-Fc chimeric proteins to treat hemophilia
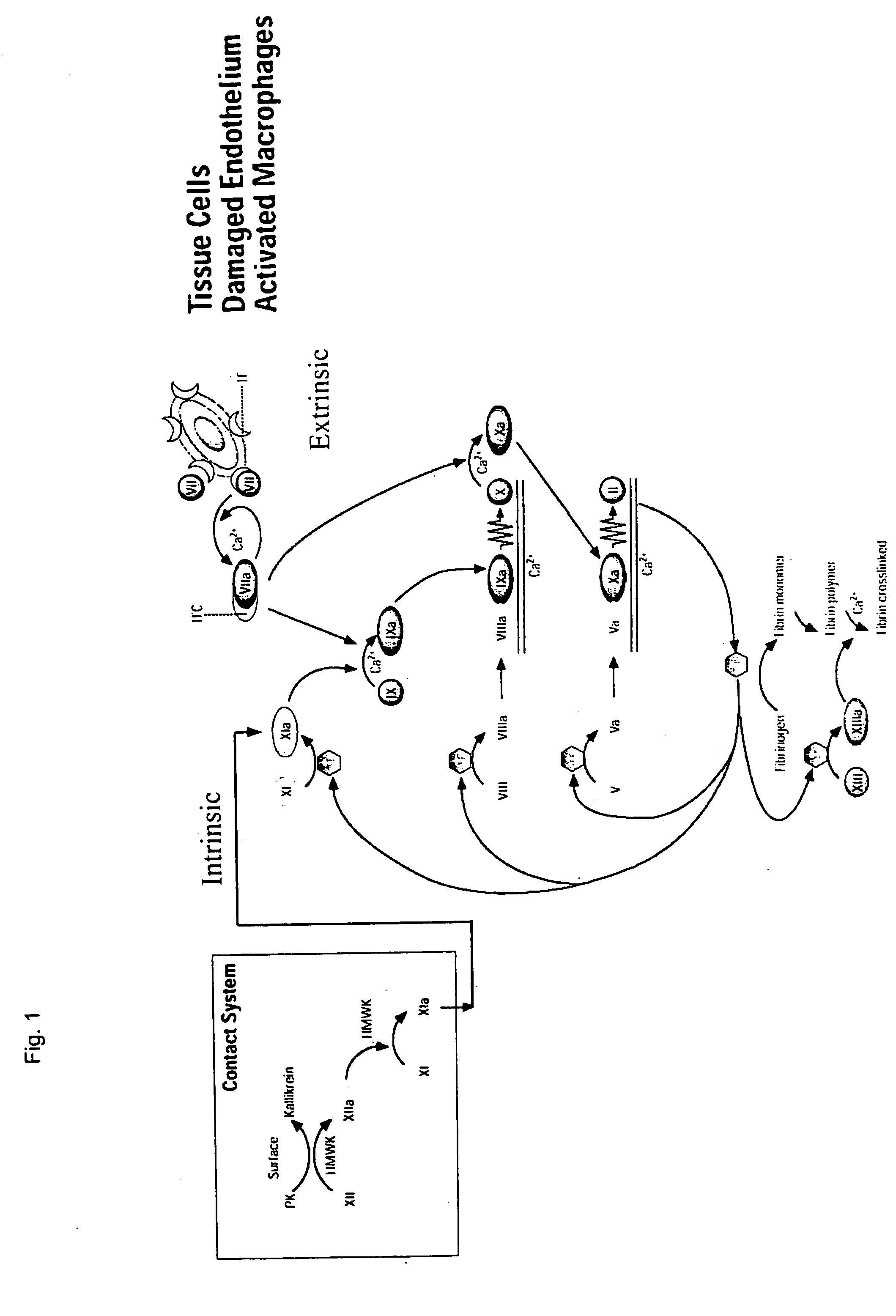
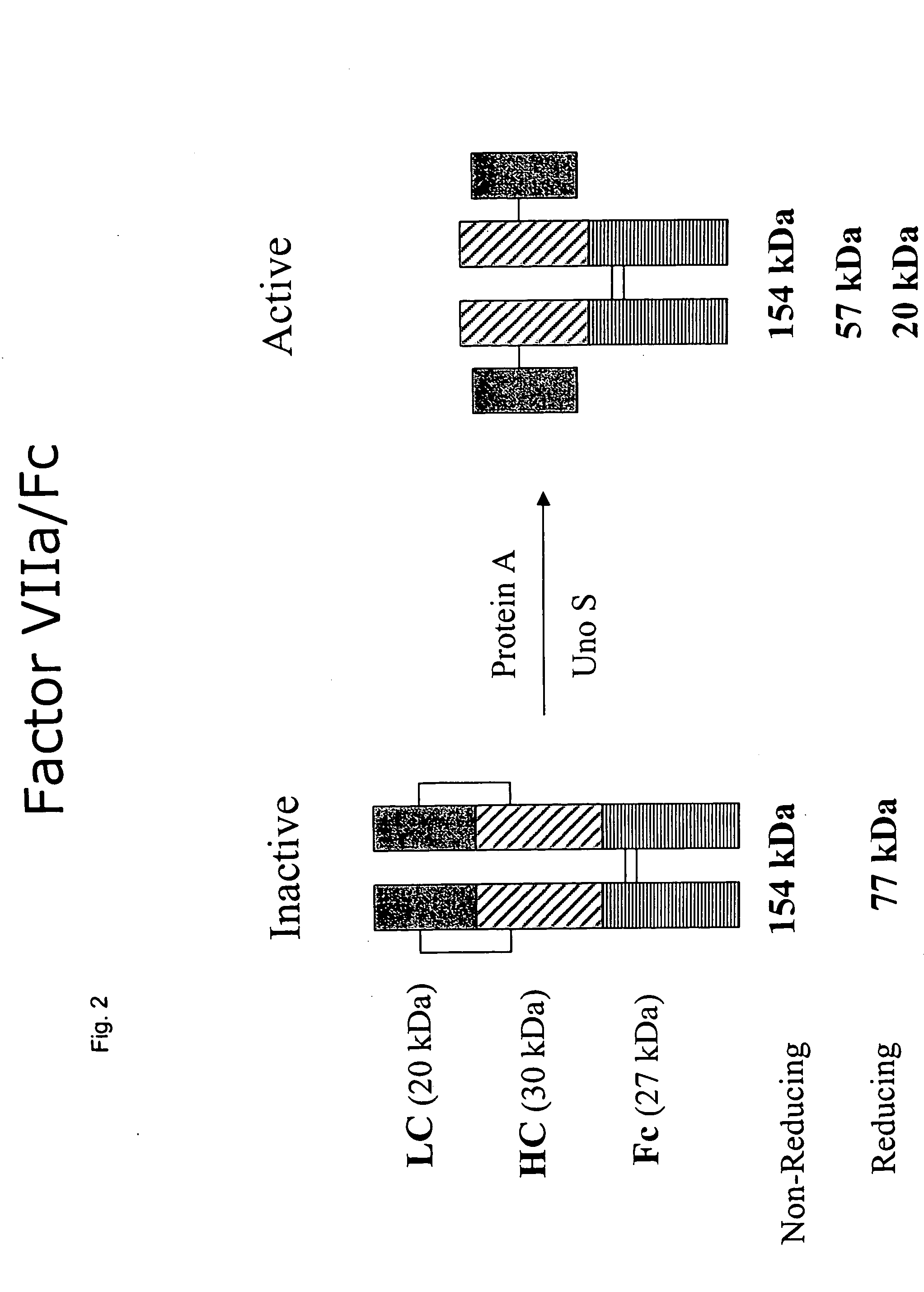
InactiveUS20050147618A1Peptide/protein ingredientsAntibody mimetics/scaffoldsHemostatic DisordersChimera Protein
The invention relates to a chimeric protein comprising at least one clotting factor and at least a portion of an immunoglobulin constant region. The invention relates to a method of treating a hemostatic disorder comprising administering a therapeutically effective amount of a chimeric protein wherein the chimeric protein comprises at least one clotting factor and at least a portion of an immunoglobulin constant region.
Owner:BIOVERATIV THERAPEUTICS INC
Optimized messenger RNA
InactiveUS6924365B1Precise functionEliminates issues concerning patient complianceFactor VIIPeptide/protein ingredientsMessenger RNANucleic acid sequence
The present invention is directed to a synthetic nucleic acid sequence which encodes a protein wherein at least one non-common codon or less-common codon is replaced by a common codon. The synthetic nucleic acid sequence can include a continuous stretch of at least 90 codons all of which are common codons.
Owner:SHIRE HUMAN GENETIC THERAPIES INC
Removal of plasmin(ogen) from protein solutions
A method for specifically removing or isolating plasmin(ogen) or plasmin in presence of fibrinogen from a mixture containing plasmin(ogen) or plasmin by contacting the mixture with a rigid amino acid wherein the amino group of the amino acid and the carboxylic group of the amino acid are about 6–8 Angstroms, preferably about 7 Angstroms apart and the rigid amino acid is covalently bound to the support via the amino group of the amino acid.
Owner:OMRIX BIOPHARM
Enzyme delivery systems and methods of preparation and use
ActiveUS20100260857A1Improve stabilityEnhanced administration propertyPowder deliveryNervous disorderDiseaseCystic fibrosis lungs
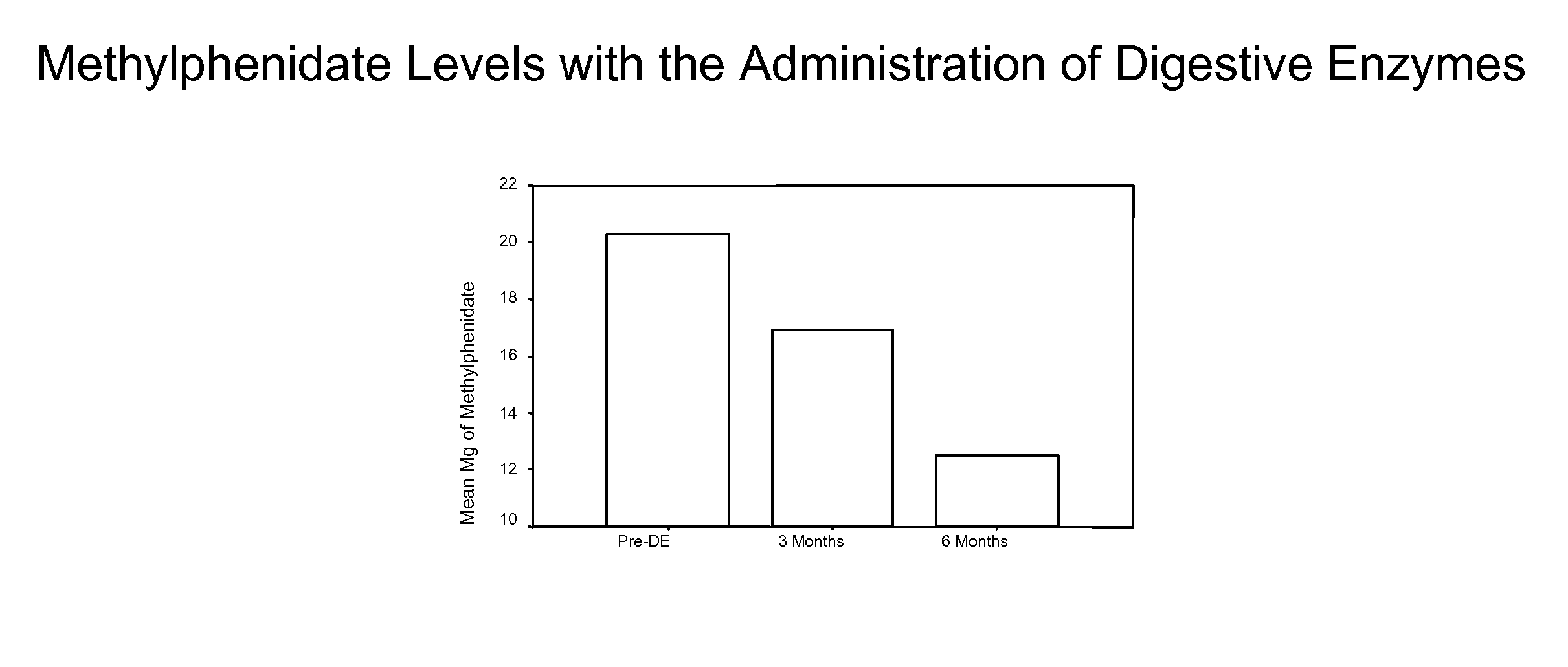
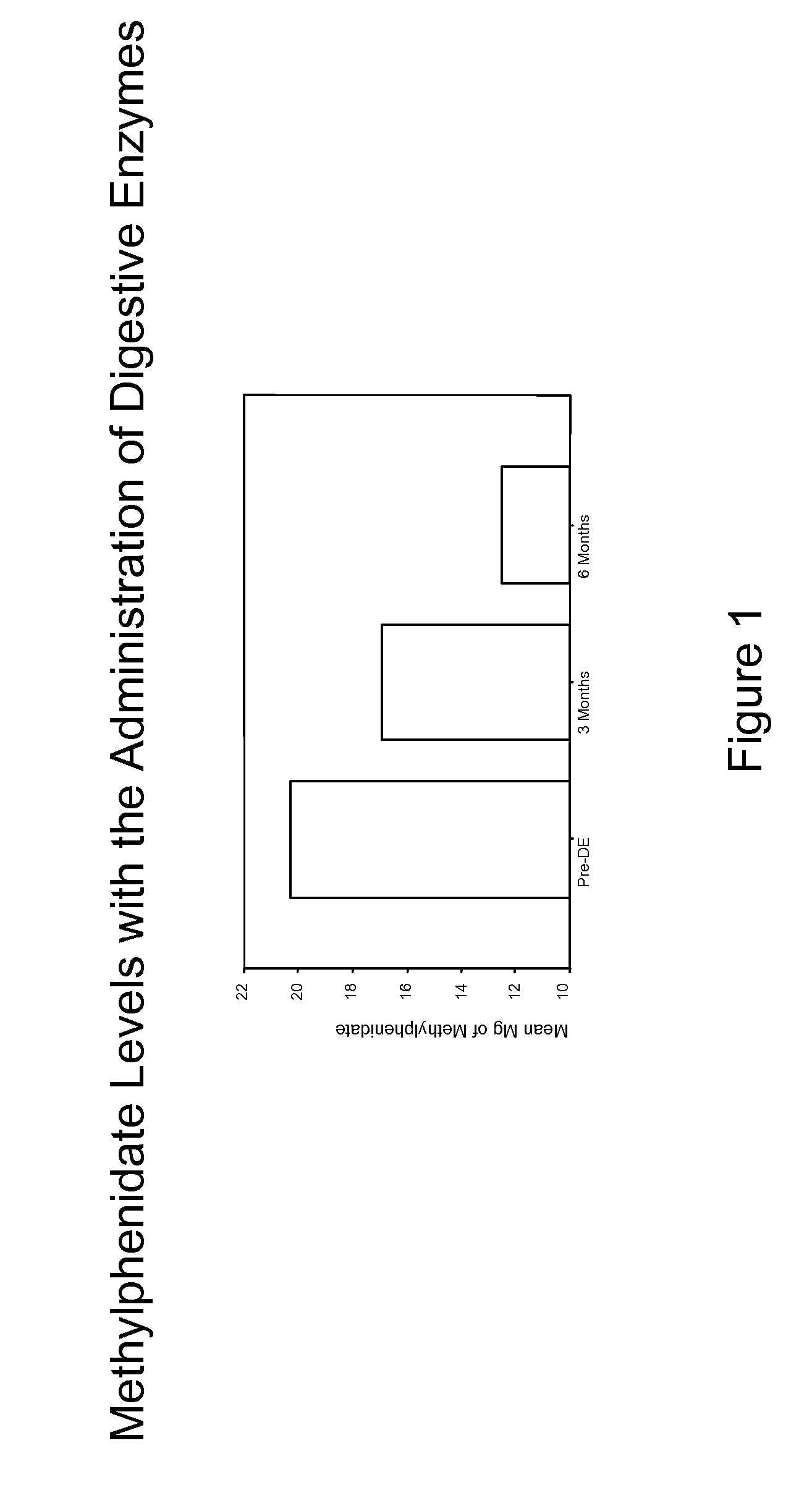
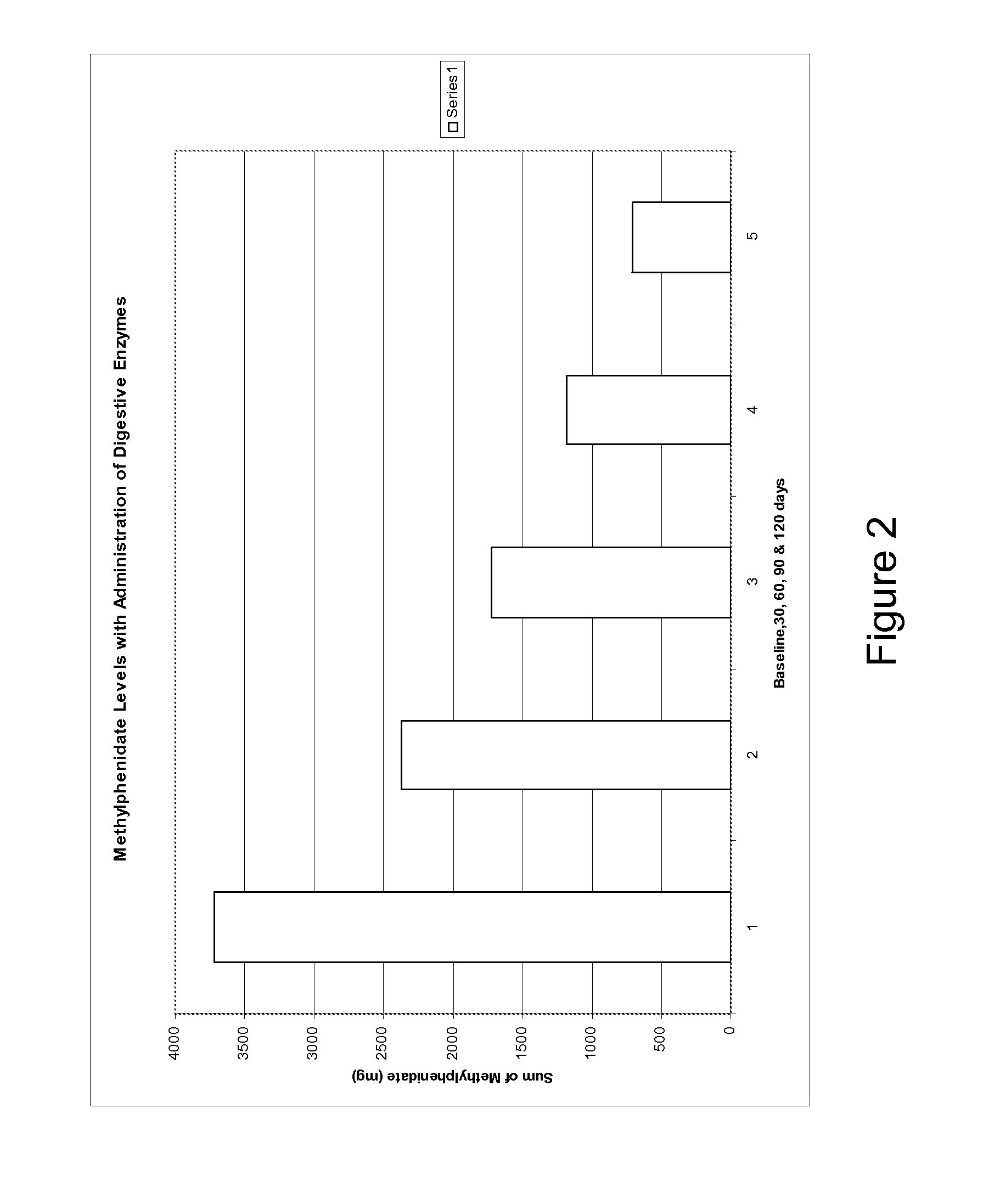
This invention relates to coated digestive enzyme preparations and enzyme delivery systems and pharmaceutical compositions comprising the preparations. This invention further relates to methods of preparation and use of the systems, pharmaceutical compositions and preparations to treat persons having ADD, ADHD, autism, cystic fibrosis and other behavioral and neurological disorders.
Owner:CUREMARK
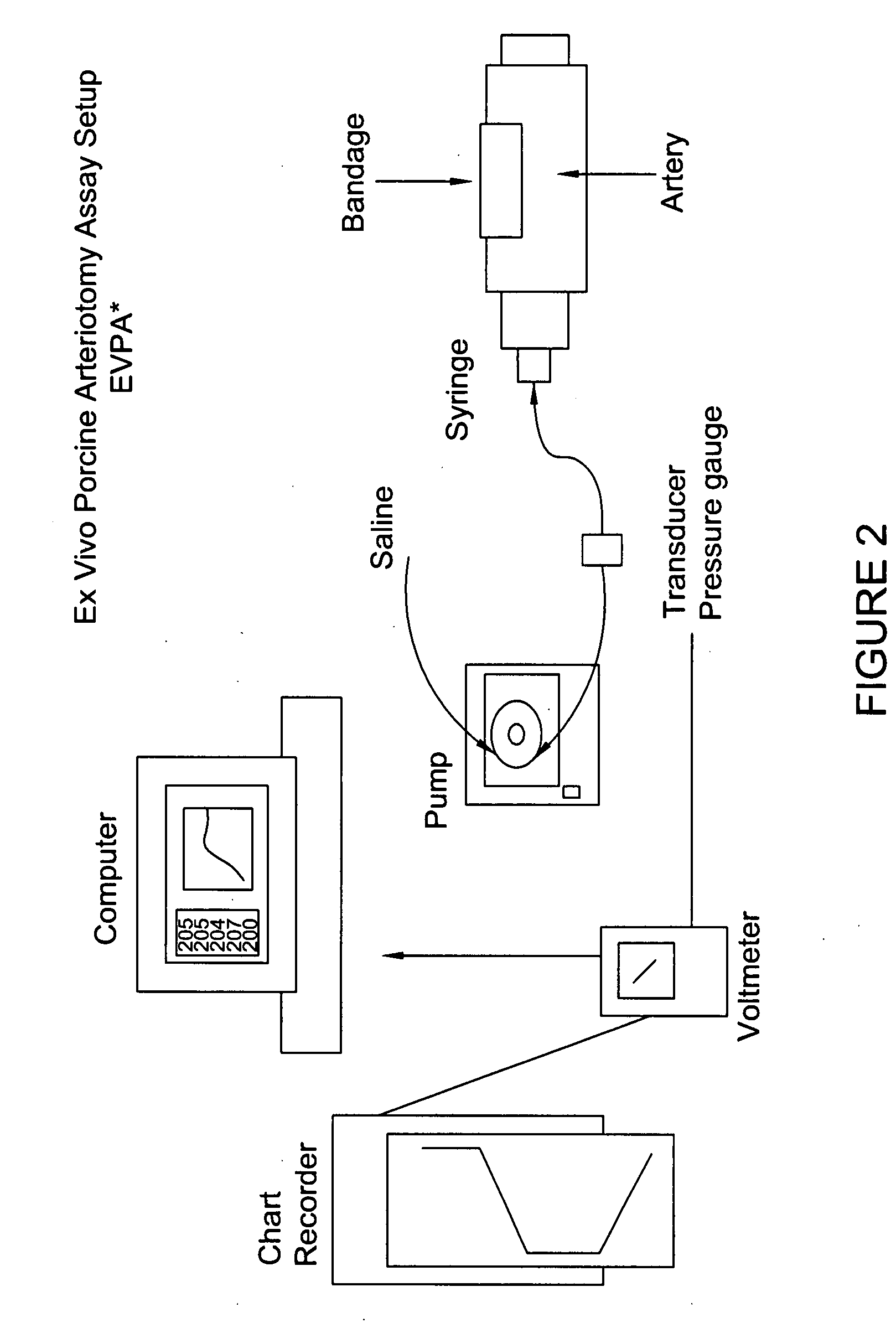
Processes for the production of solid dressings for treating wounded tissue
ActiveUS20080031934A1Reduce the temperatureAvoid componentsNon-adhesive dressingsPeptide/protein ingredientsFibrinogenBiomedical engineering
Disclosed are processes for preparing solid dressings for treated wounded tissue in mammalian patients, such as a human, comprising a haemostatic layer consisting essentially of a fibrinogen component and a fibrinogen activator. Also disclosed are methods for treating wounded tissue using these dressings and frozen and liquid compositions useful for preparing the haemostatic layer(s) of these dressings or for treating wounded tissue in a mammal.
Owner:HDG
Anti-cancer vaccines
The present provides tumor-associated HLA-restricted antigens, and in particular HLA-A2 restricted antigens, as vaccines for treating or preventing cancers in a patient. In specific aspects, neutrophil elastase peptides other than PR1, cyclin E1 peptides, cyclin D peptides, or cyclin E2 peptides are provided. Such peptides can be used to elicit specific CTLs that preferentially attack tumor cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
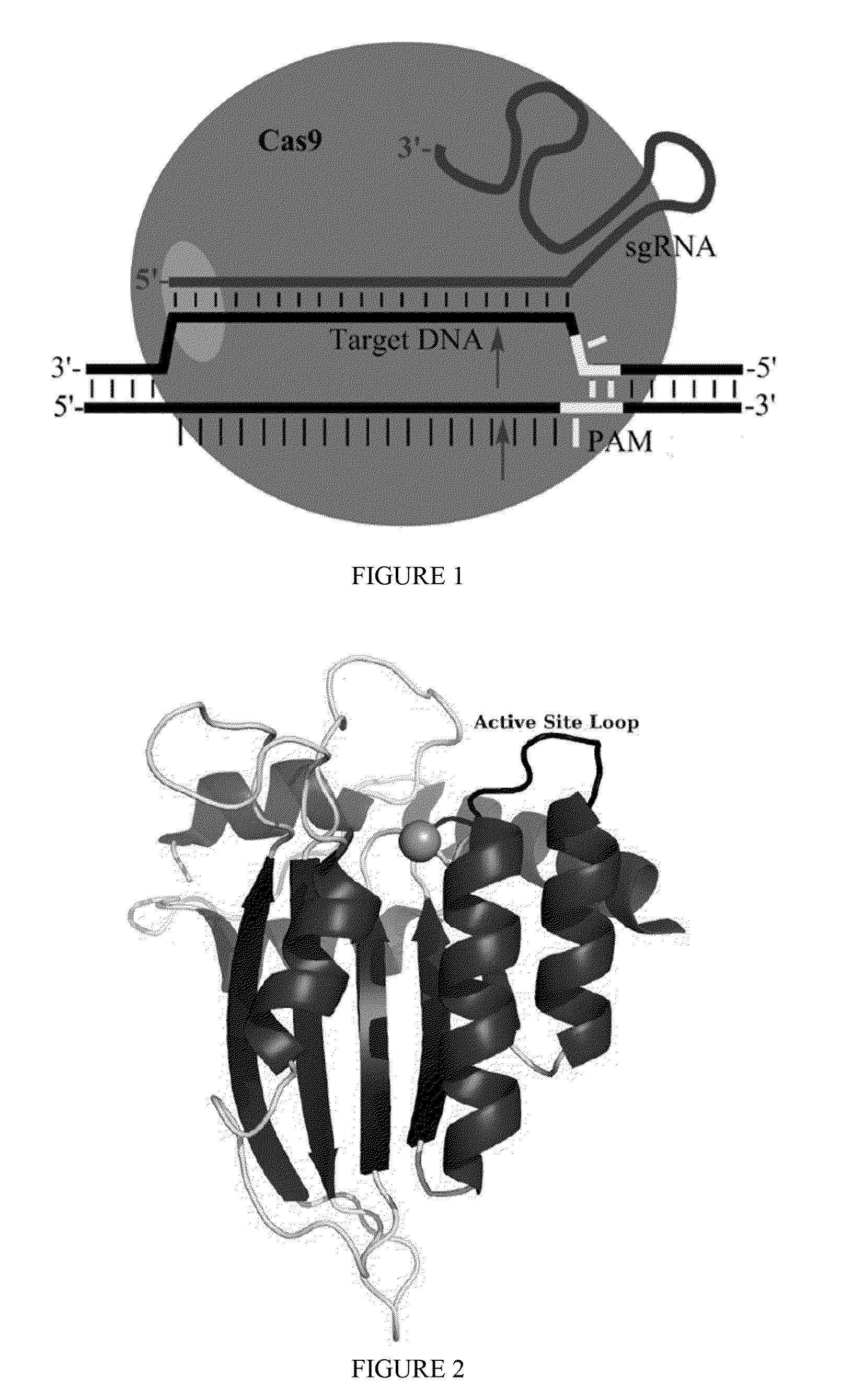
Methods for correcting caspase-9 point mutations
Some aspects of this disclosure provide strategies, systems, reagents, methods, and kits that are useful for the targeted editing of nucleic acids, including editing a nucleic acid encoding a mutant Caspase-9 protein to correct a point mutation associated with a disease or disorder, e.g., with neuroblastoma. The methods provided are useful for correcting a Caspase-9 point mutation within the genome of a cell or subject, e.g., within the human genome. In some embodiments, fusion proteins of Cas9 and nucleic acid editing enzymes or enzyme domains, e.g., deaminase domains, are provided. In some embodiments, reagents and kits for the generation of targeted nucleic acid editing proteins, e.g., fusion proteins of Cas9 and nucleic acid editing enzymes or domains, are provided.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
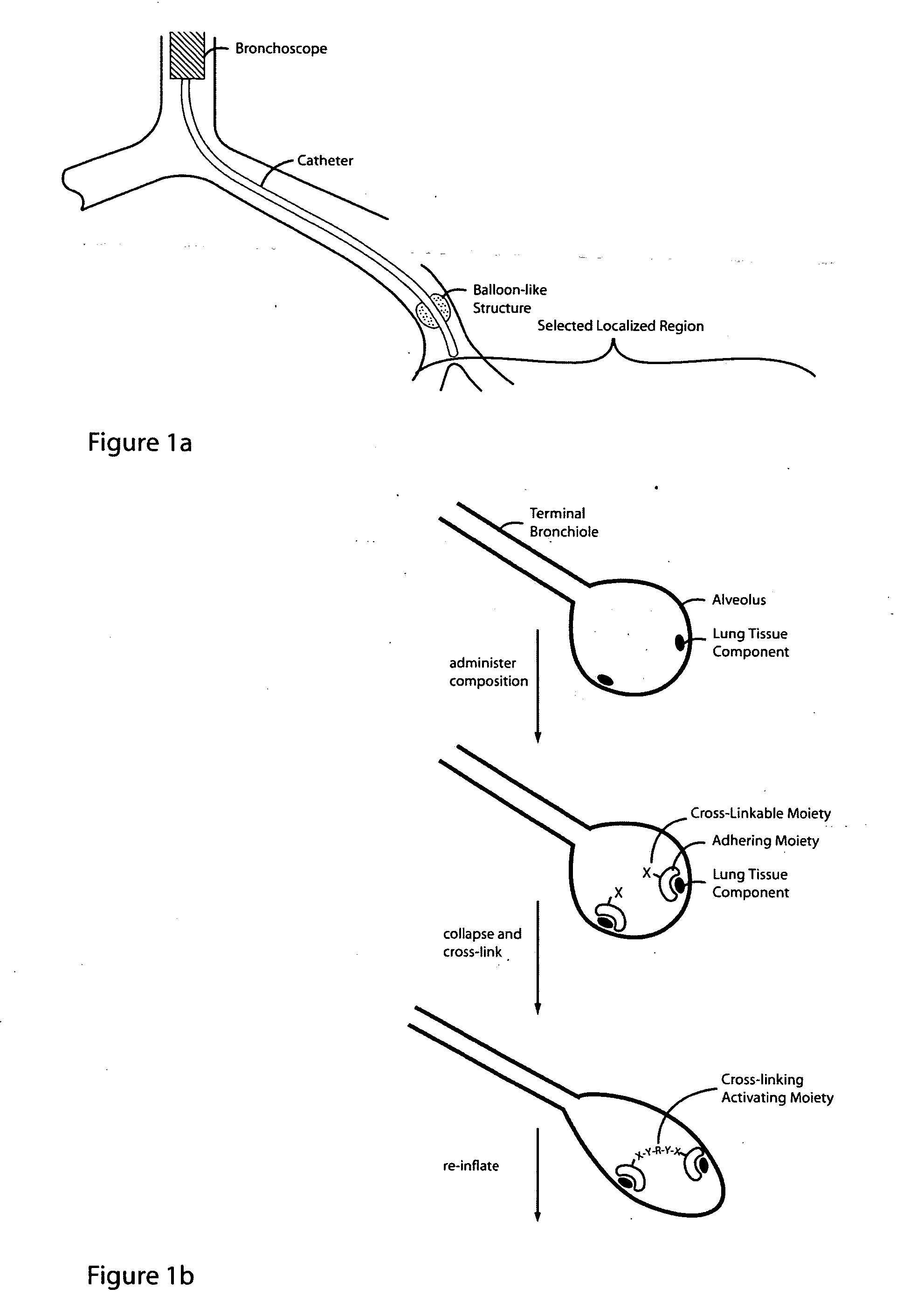
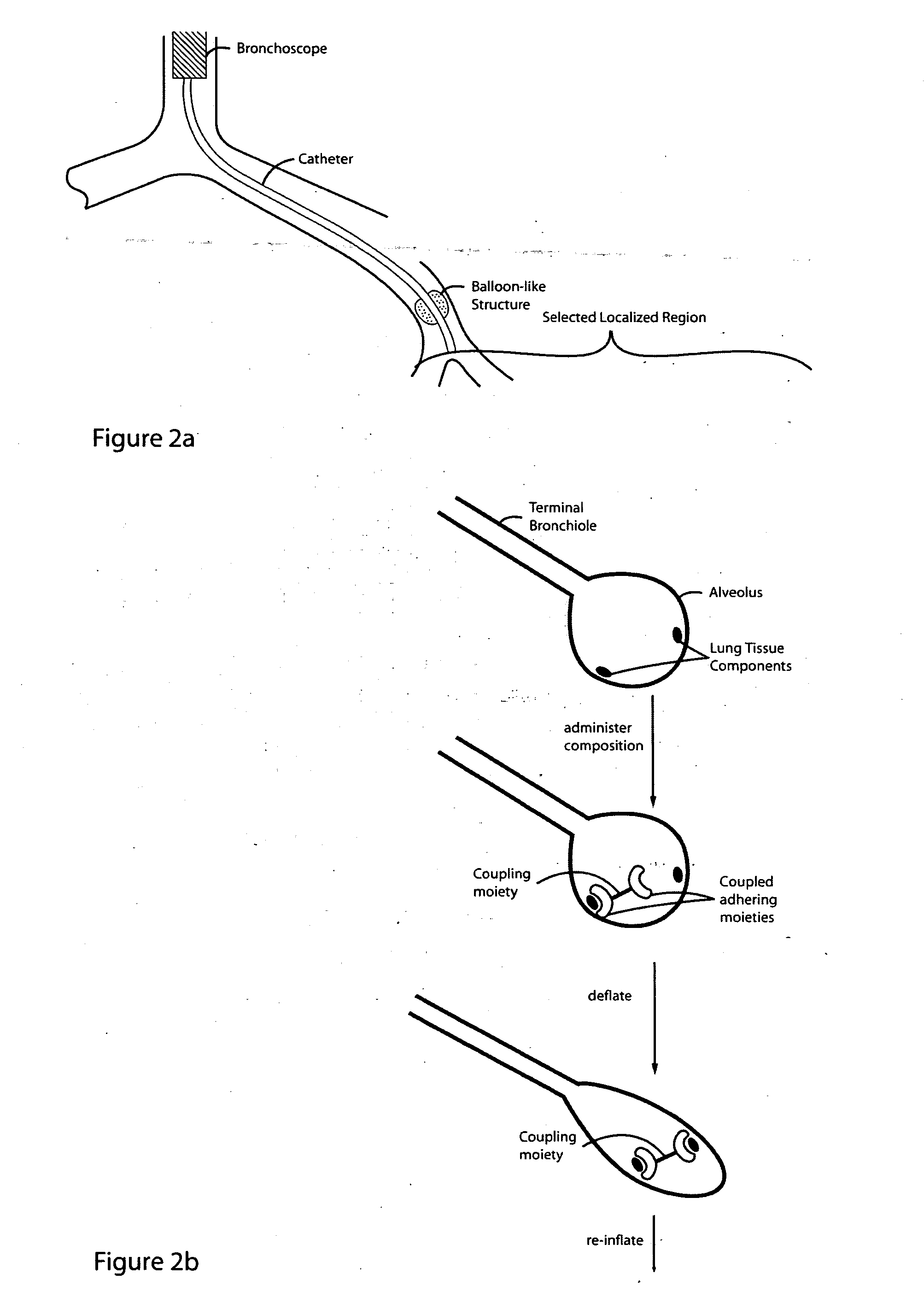
Lung volume reduction using glue composition
InactiveUS20050281802A1Reducing lung volumeSurgical adhesivesBacteria material medical ingredientsLung volumesLung tissue
The present invention relates to methods and compositions for sealing localized regions of damaged lung tissue to reduce overall lung volume. The glue compositions provide a glue featuring an adhering moiety coupled to one or more other moieties including, for example, a cross-linkable moiety and / or one other adhering moiety. The methods and compositions of the invention find use, for example, in treating pulmonary conditions, such as emphysema.
Owner:EKOS CORP
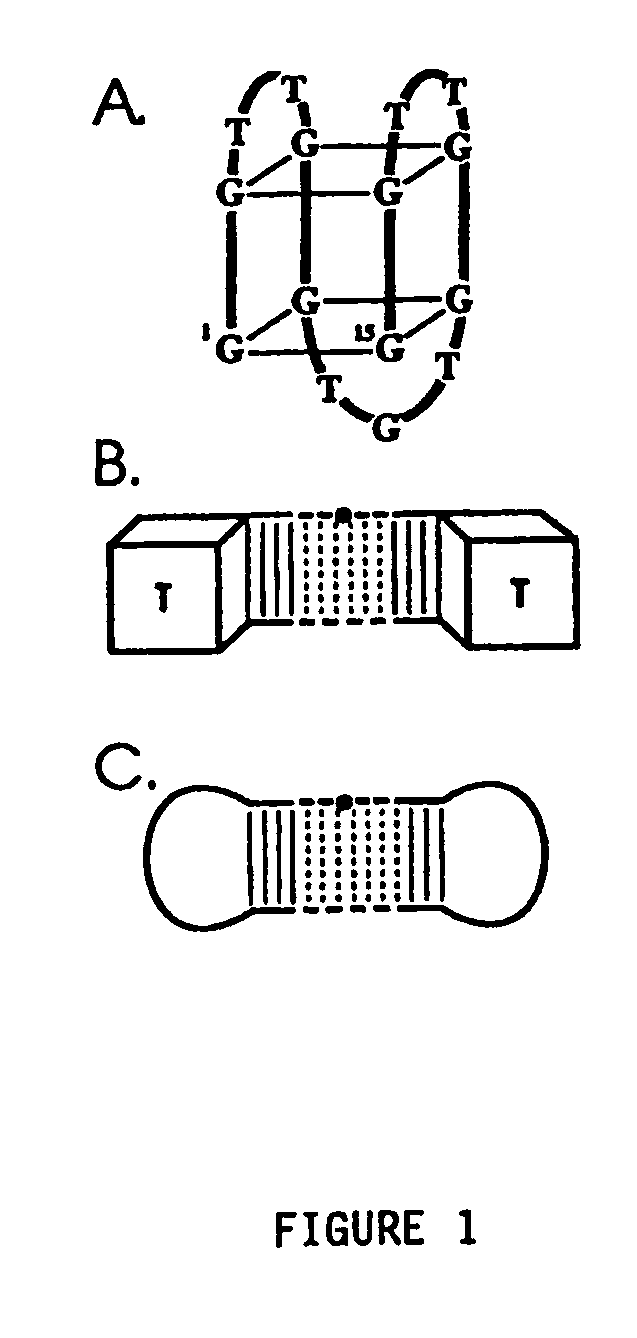
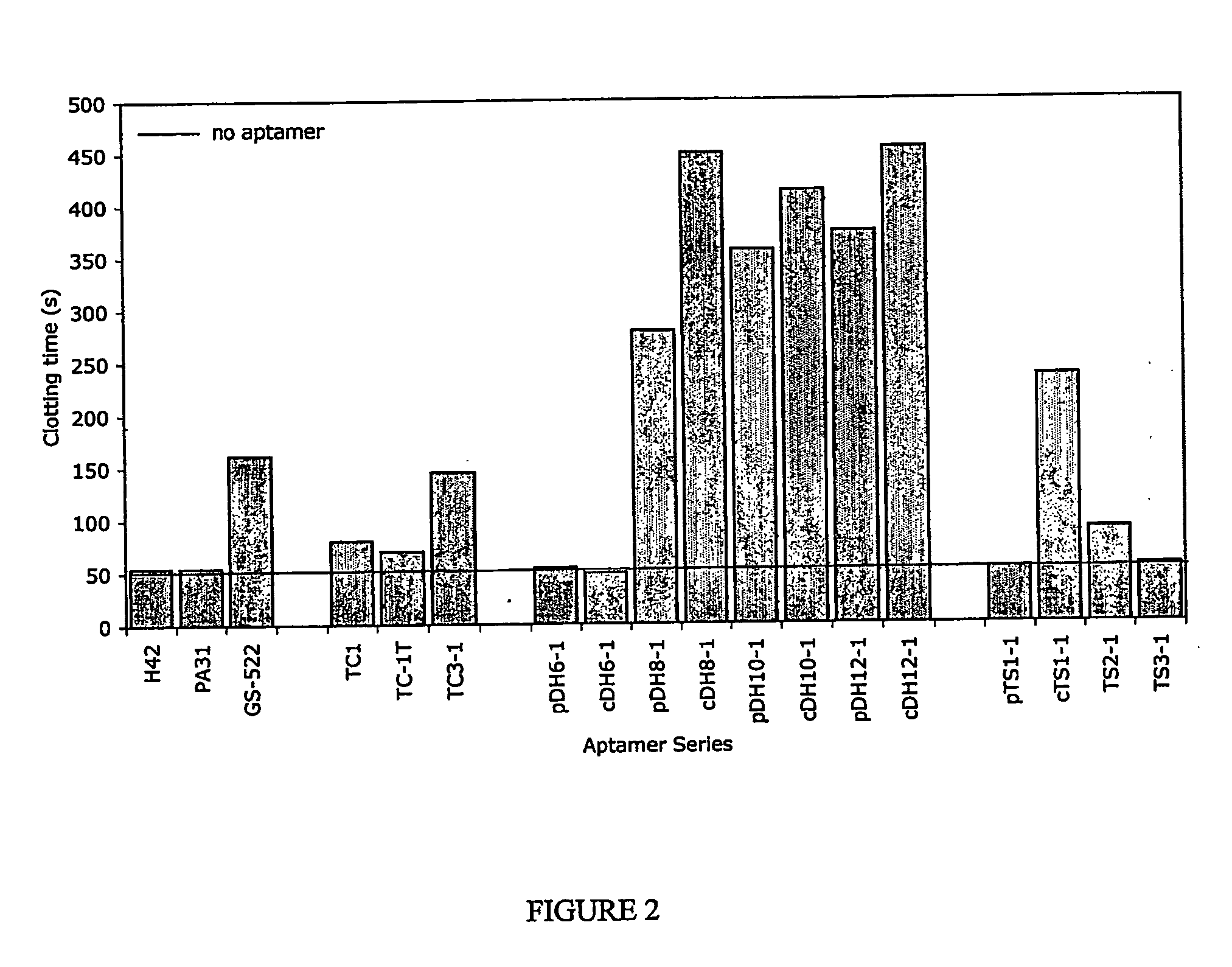
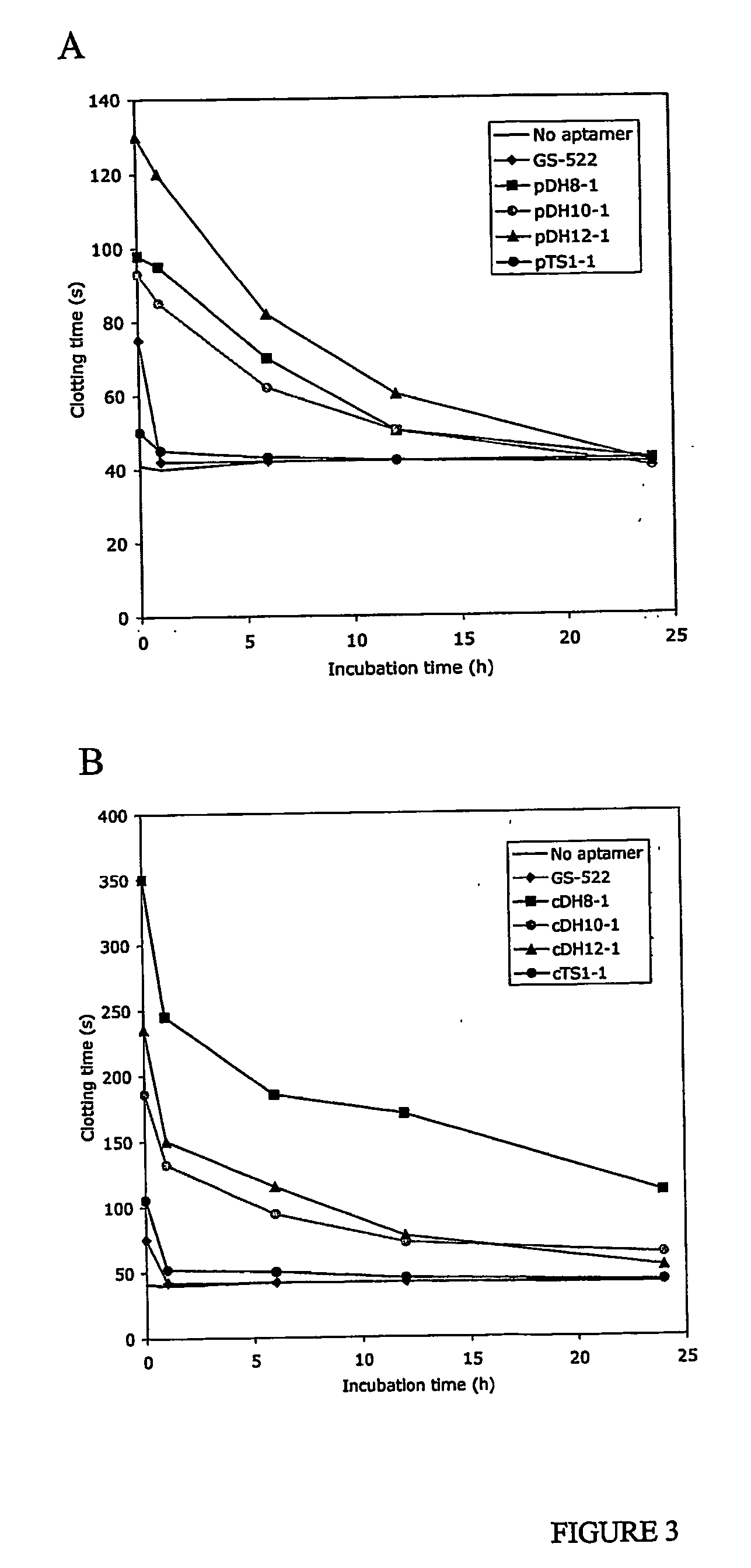
Aptamers and antiaptamers
InactiveUS20050176940A1Preventing and reducing coagulation of bloodOrganic active ingredientsSugar derivativesNucleotideThrombin activity
The present invention relates to: An aptamer comprising a circular oligonucleotide defining one to four target binding regions; An aptamer comprising an oligonucleotide defining two, three or four thrombin binding quadruplex regions separated by at least partially duplex regions, wherein the quadruplex regions comprise a GGTMGGXGGTTGG sequence wherein M represents A or T and X represents a sequence of two to five nucleotides and / or nucleotide analogues; An aptamer represented by formula (I): 5′D1, wQxD1D2yQzD2,3′—the variables are as defined in the specification; and Aptamers selected from specific sequences.
Owner:UNISEARCH LTD
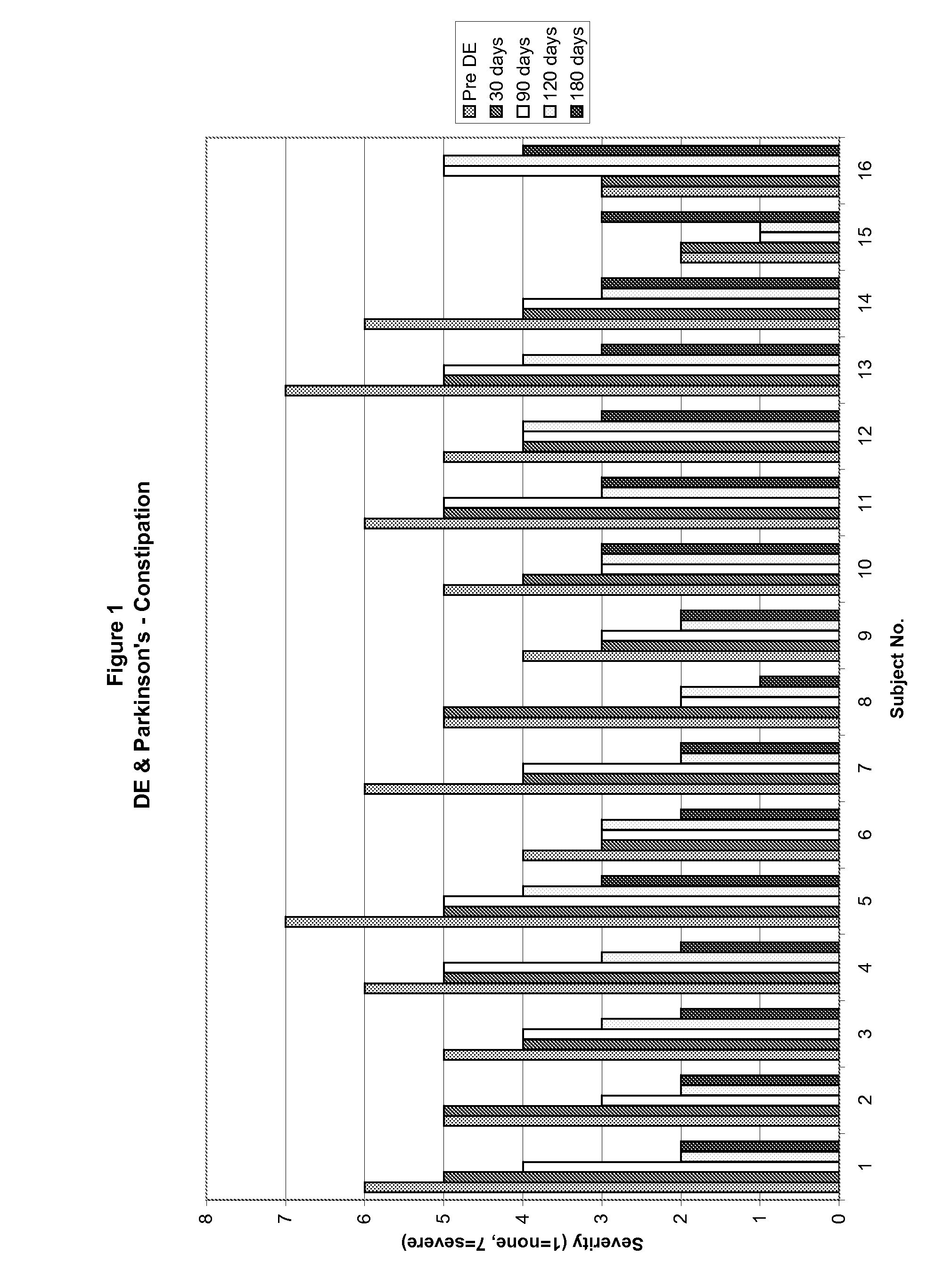
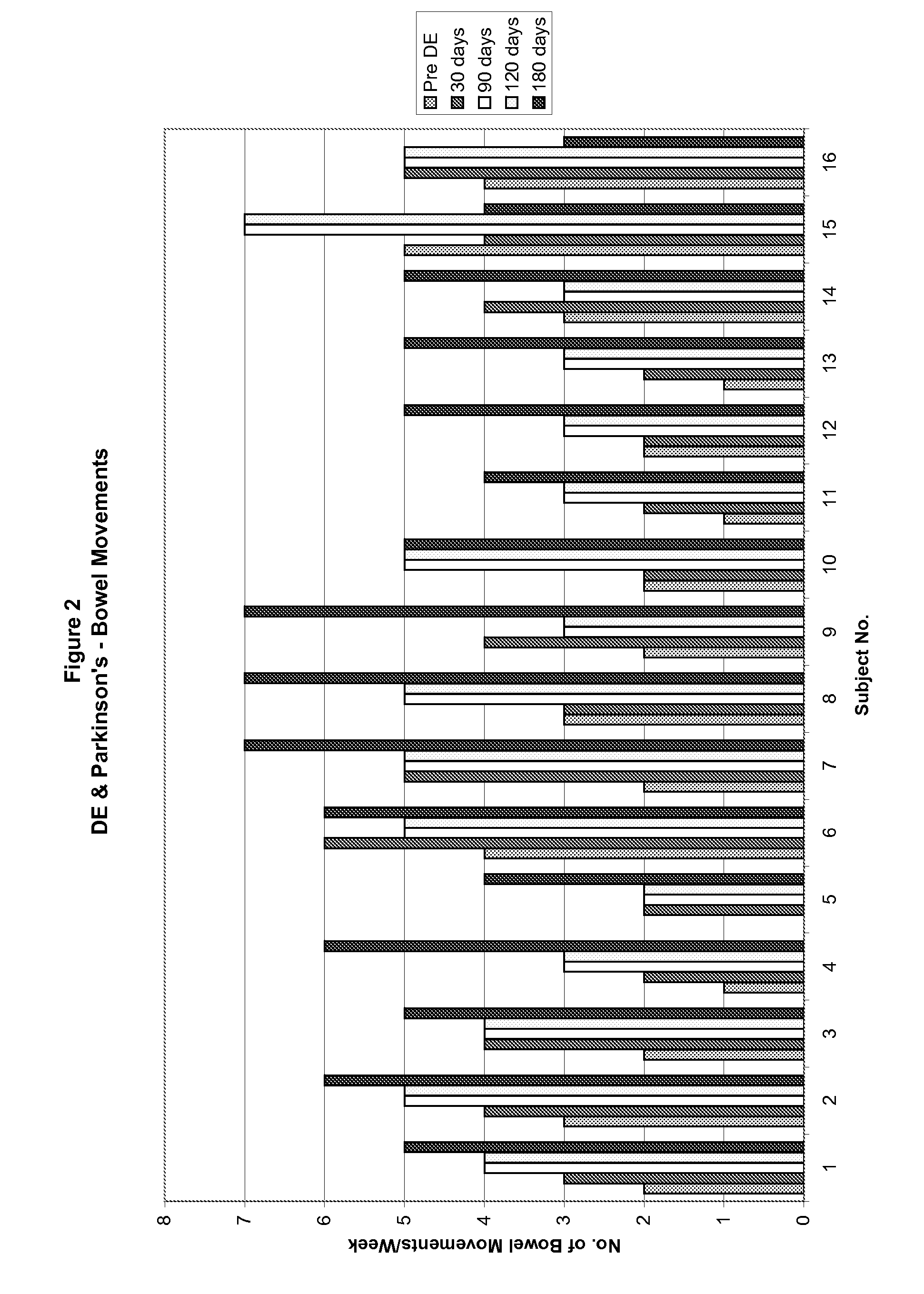
Method of treating and diagnosing parkinsons disease and related dysautonomic disorders
InactiveUS20070053895A1Symptoms improvedNervous disorderPeptide/protein ingredientsAutonomic bladder dysfunctionPsychiatry
A method for treating a Parkinson's patient with digestive / pancreatic enzymes involves administering an effective amount of digestive / pancreatic enzymes to an individual having the disorder in order to improve a symptom of the disorder. In addition, a method is provided for determining whether an individual has, or may develop, Parkinson's disease or related dysautonomic disorders and for determining whether an individual will benefit from the administration of pancreatic / digestive enzymes to treat the dysautonomic disorder.
Owner:CUREMARK
Pharmaceutical preparations for attention deficit disorder, attention deficit hyperactivity disorder and other associated disorders
InactiveUS20070116695A1Well formedFormulation stabilityBiocidePeptide/protein ingredientsChymotrypsinAttention deficits
A pharmaceutical preparation for the treatment of attention deficit disorders combines a therapeutically effective amount of digestive enzymes, such as chymotrypsin, and medication used to treat attention deficit disorders, such as Ritalin®, Concert®, Adderall® and Strattera®. The preparation may be in the form of a tablet, capsule or time released formula in order to reduce the amount of pills per dosage. The pharmaceutical preparation ameliorates the symptoms of the attention deficit disorder. The preparation has a stabilizing matrix containing a solidified microcrystalline cellulose which captures and protects therapeutically effective amounts of digestive enzyme particles within the stabilizing matrix.
Owner:CUREMARK
Novel pharmaceutical preparation for preeclampsia, eclampsia, and toxemia, and their related symptoms and related disorders of pregnancy
A therapeutic agent for the treatment of toxemia, preeclampsia and eclampsia and the method for preparing the therapeutic agents is disclosed. The therapeutic agent is a stable pharmaceutical preparation containing, but not limited to, digestive / pancreatic enzymes. The therapeutic agent may be manufactured by a variety of encapsulation technologies. Delivery of the therapeutic agent may be made orally, through injection, by adherence of a medicated patch or other method. Further, a method of using of a biomarker, the presence of chymotrypsin in the maternal GI tract to determine the likelihood of developing preeclampsia, pregnancy induced hypertension, and eclampsia / toxemia is disclosed.
Owner:CUREMARK
Methods for correcting alpha-antitrypsin point mutations
InactiveUS20150166984A1Nervous disorderFusion with DNA-binding domainHuman DNA sequencingObstructive Pulmonary Diseases
Some aspects of this disclosure provide strategies, systems, reagents, methods, and kits that are useful for the targeted editing of nucleic acids, including editing a nucleic acid encoding a mutant α-antitrypsin protein to correct a point mutation associated with a disease or disorder, e.g., with chronic obstructive pulmonary disease (COPD) disease. The methods provided are useful for correcting an α-antitrypsin point mutation within the genome of a cell or subject, e.g., within the human genome. In some embodiments, fusion proteins of Cas9 and nucleic acid editing enzymes or enzyme domains, e.g., deaminase domains, are provided. In some embodiments, reagents and kits for the generation of targeted nucleic acid editing proteins, e.g., fusion proteins of Cas9 and nucleic acid editing enzymes or domains, are provided.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Methods for correcting presenilin point mutations
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Method and compositions for detecting botulinum neurotoxin
ActiveUS20060134722A1Antibacterial agentsNervous disorderSurface plasmon resonance imagingFluorophore
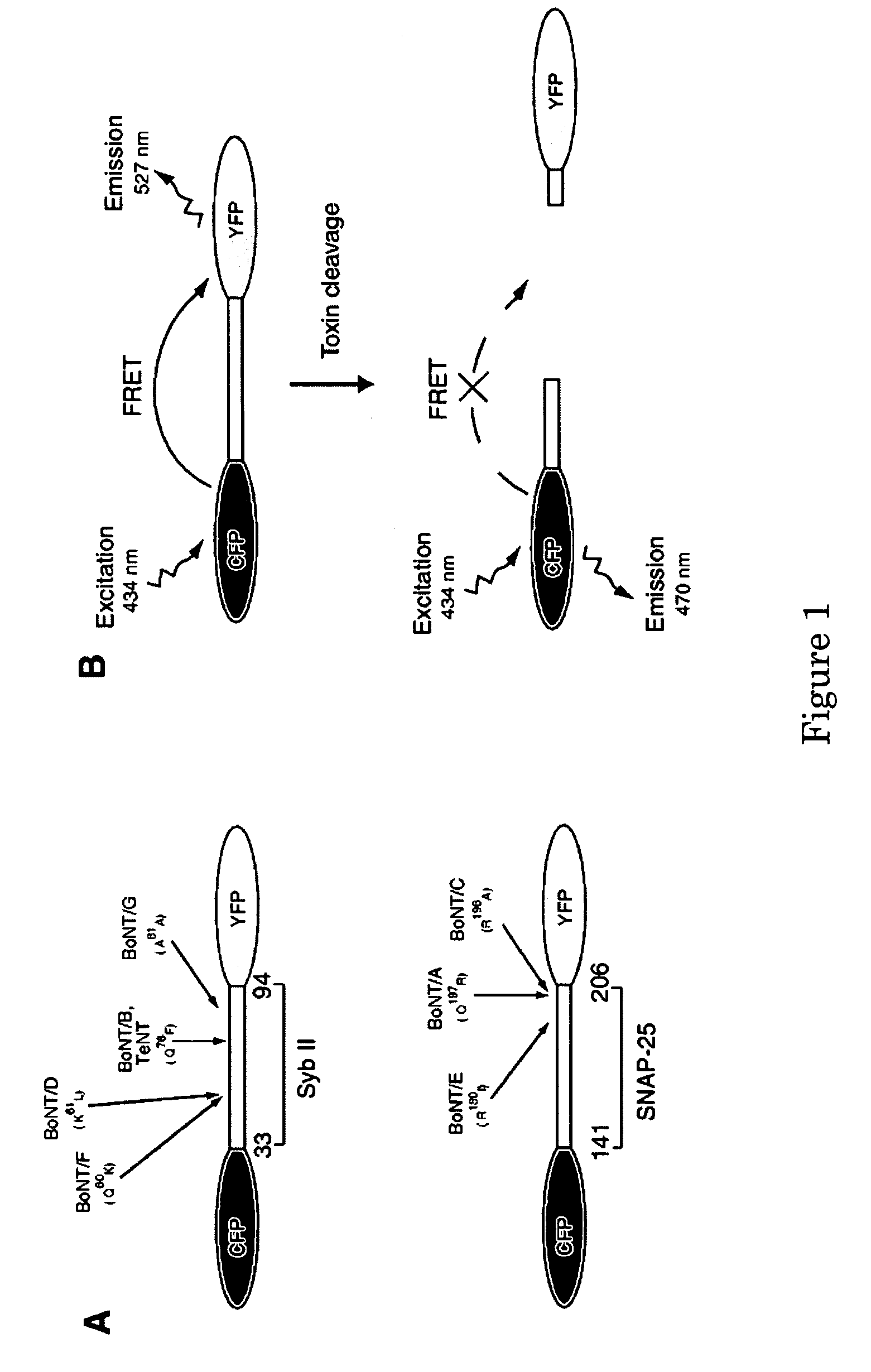
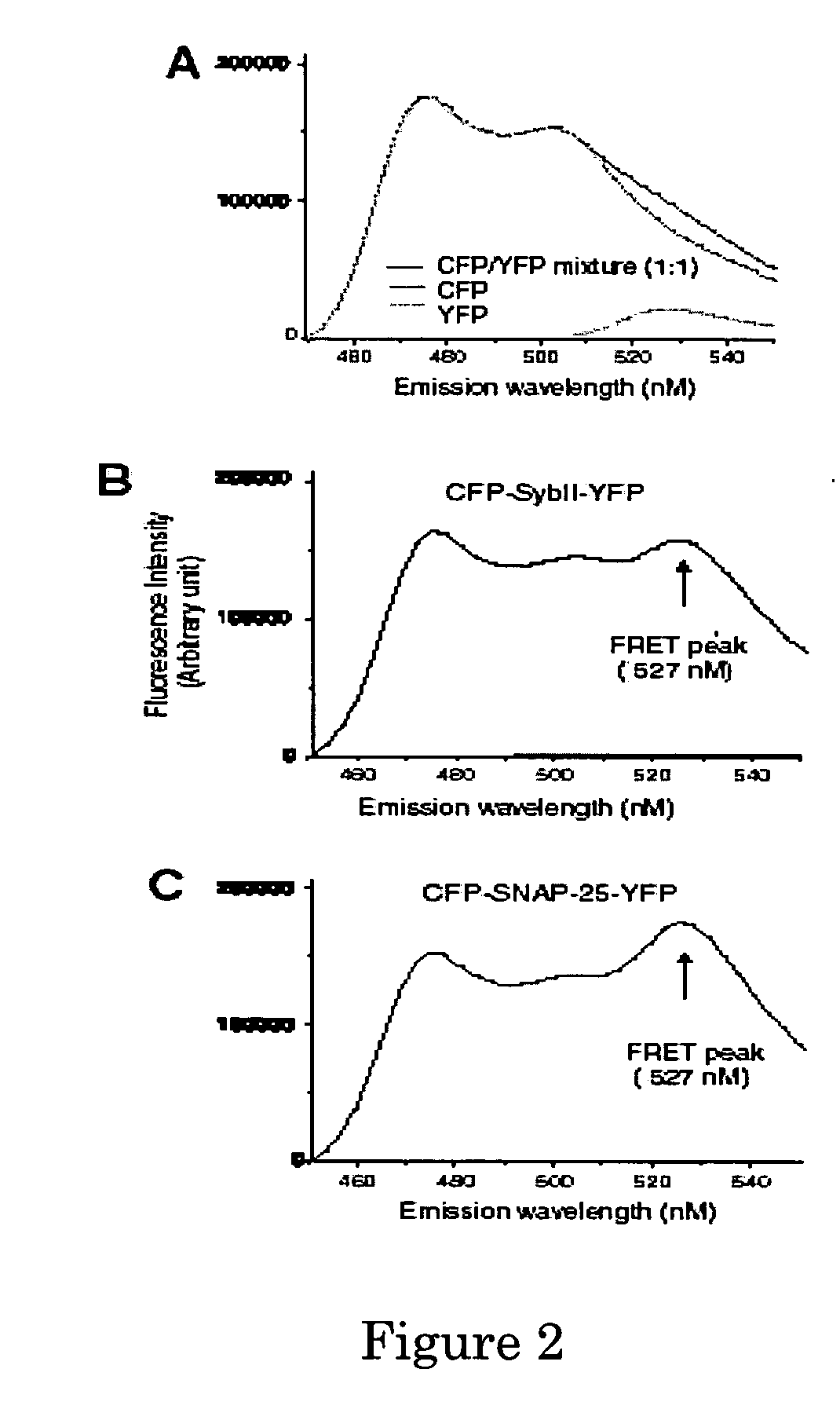
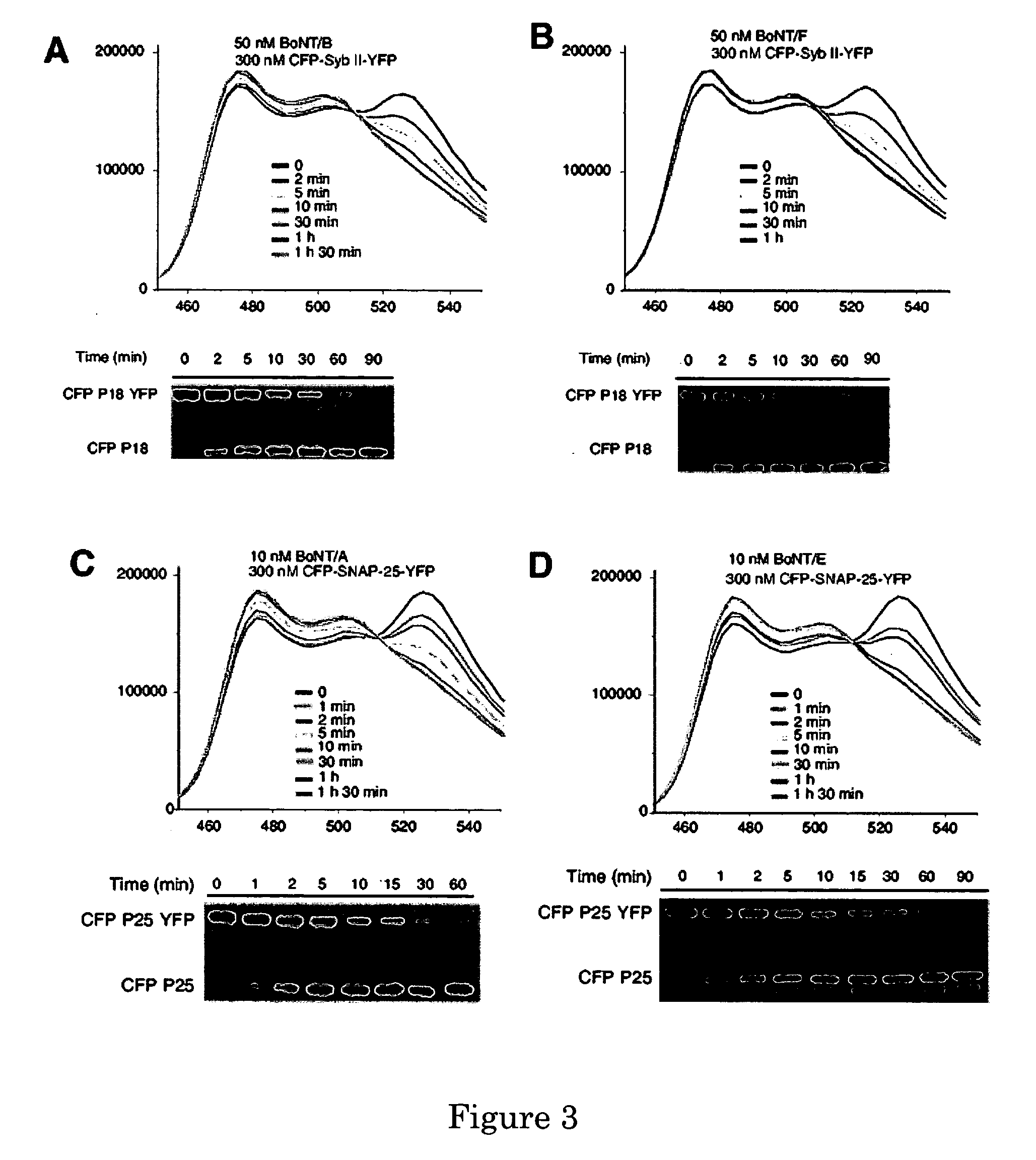
A molecular construct capable of fluorescent resonance energy transfer (FRET), comprising a linker peptide, a donor fluorophore moiety and an acceptor fluorophore moiety, wherein the linker peptide is a substrate of a botulinum neurotoxin selected from the group consisting of synaptobrevin, syntaxin and SNAP-25, or a fragment thereof capable being cleaved by the botulinum neurotoxin, and separates the donor and acceptor fluorophores by a distance of not more than 10 nm, and wherein emission spectrum of the donor fluorophore moiety overlaps with the excitation spectrum of the acceptor fluorophore moiety; or wherein the emission spectra of the fluorophores are detectably different. Also provided are isolated nucleic acid expressing the construct, kits comprising said construct and cell lines comprising said nucleic acid. Further provided are methods of detecting a BoNT using the above described construct via FRET, and methods for detecting a BoNT using surface plasmon resonance imaging.
Owner:WISCONSIN ALUMNI RES FOUND
Antidotes for factor xa inhibitors and methods of using the same
ActiveUS20090098119A1Reduce and remove intrinsic procoagulantReduce and remove and anticoagulant activityOrganic active ingredientsHydrolasesMedicineAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Variant AAV and compositions, methods and uses for gene transfer to cells, organs and tissues
ActiveUS9840719B2Reduce the possibilityCost of treatmentVectorsPeptide/protein ingredientsTherapeutic proteinNucleic acid sequencing
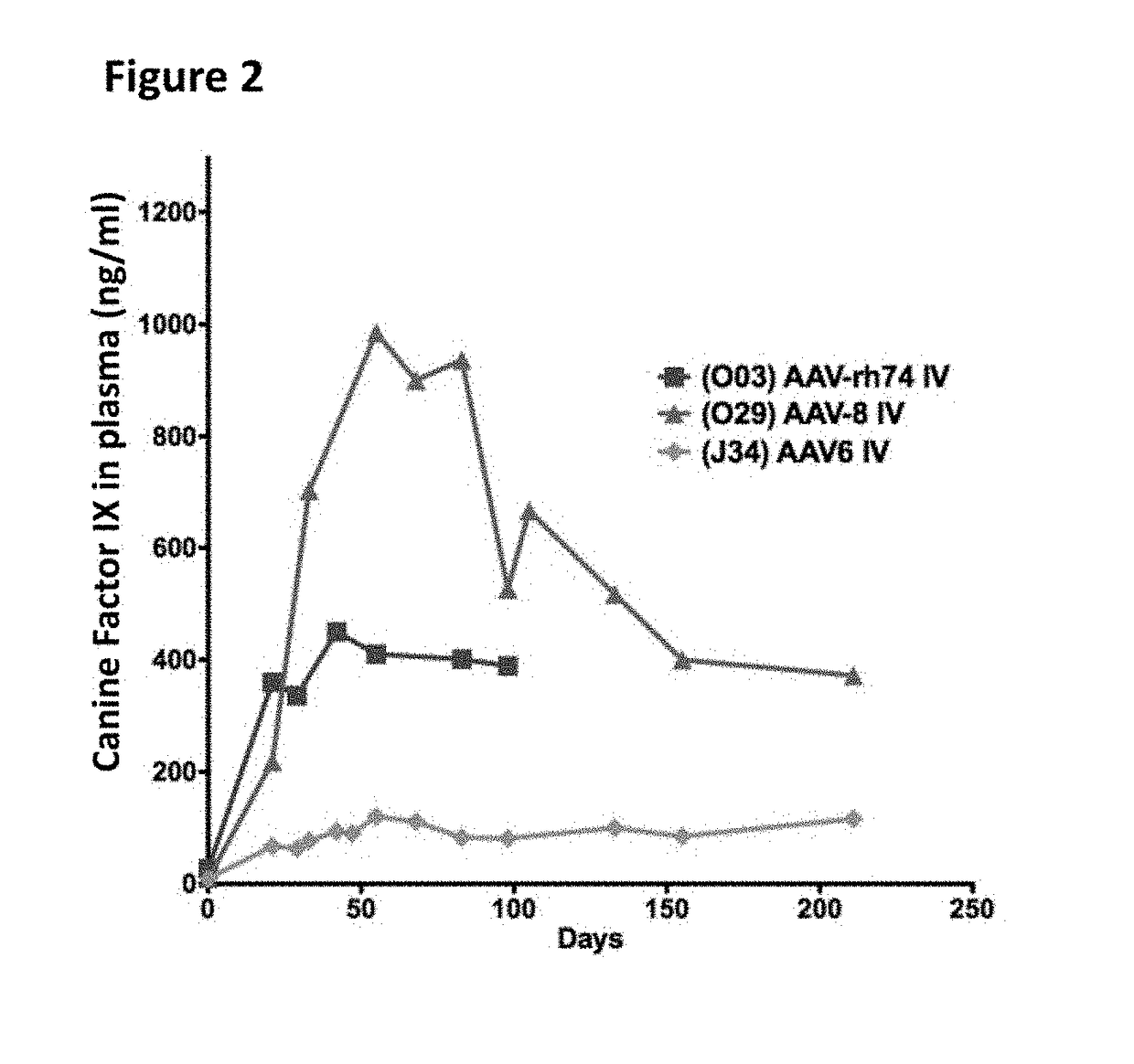
The invention relates to adeno-associated virus (AAV) serotype AAV-Rh74 and related AAV vectors, and AAV-Rh74 and related AAV vector mediated gene transfer methods and uses. In particular, AAV-Rh74 and related AAV vectors target polynucleotides to cells, tissues or organs for expression (transcription) of genes encoding therapeutic proteins and peptides, and polynucleotides that function as or are transcribed into inhibitory nucleic acid sequences.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Long-acting coagulation factors and methods of producing same
ActiveUS20130243747A1Prevent coagulationPreventing hemophiliaBacteriaPeptide/protein ingredientsNucleotidePolynucleotide
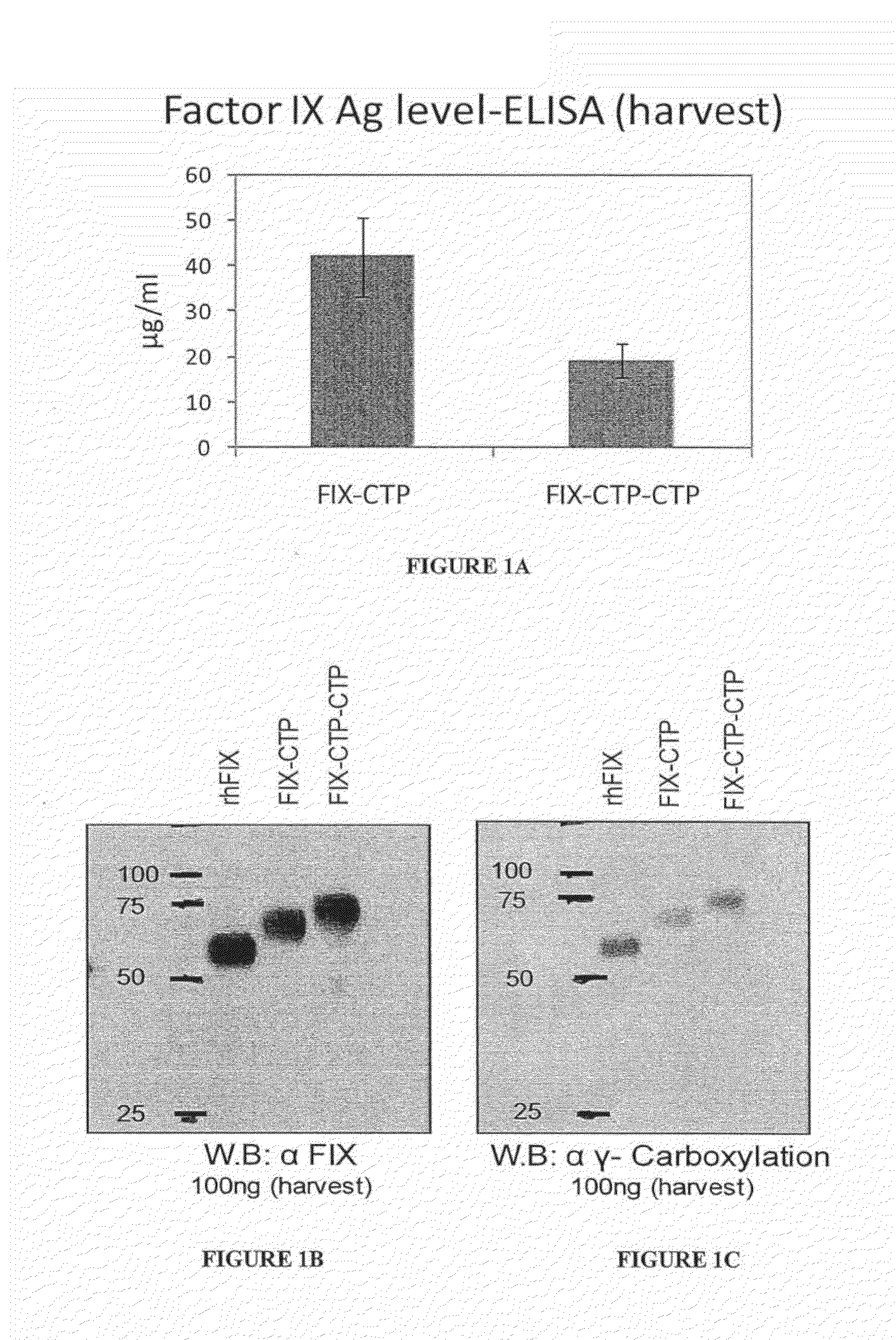
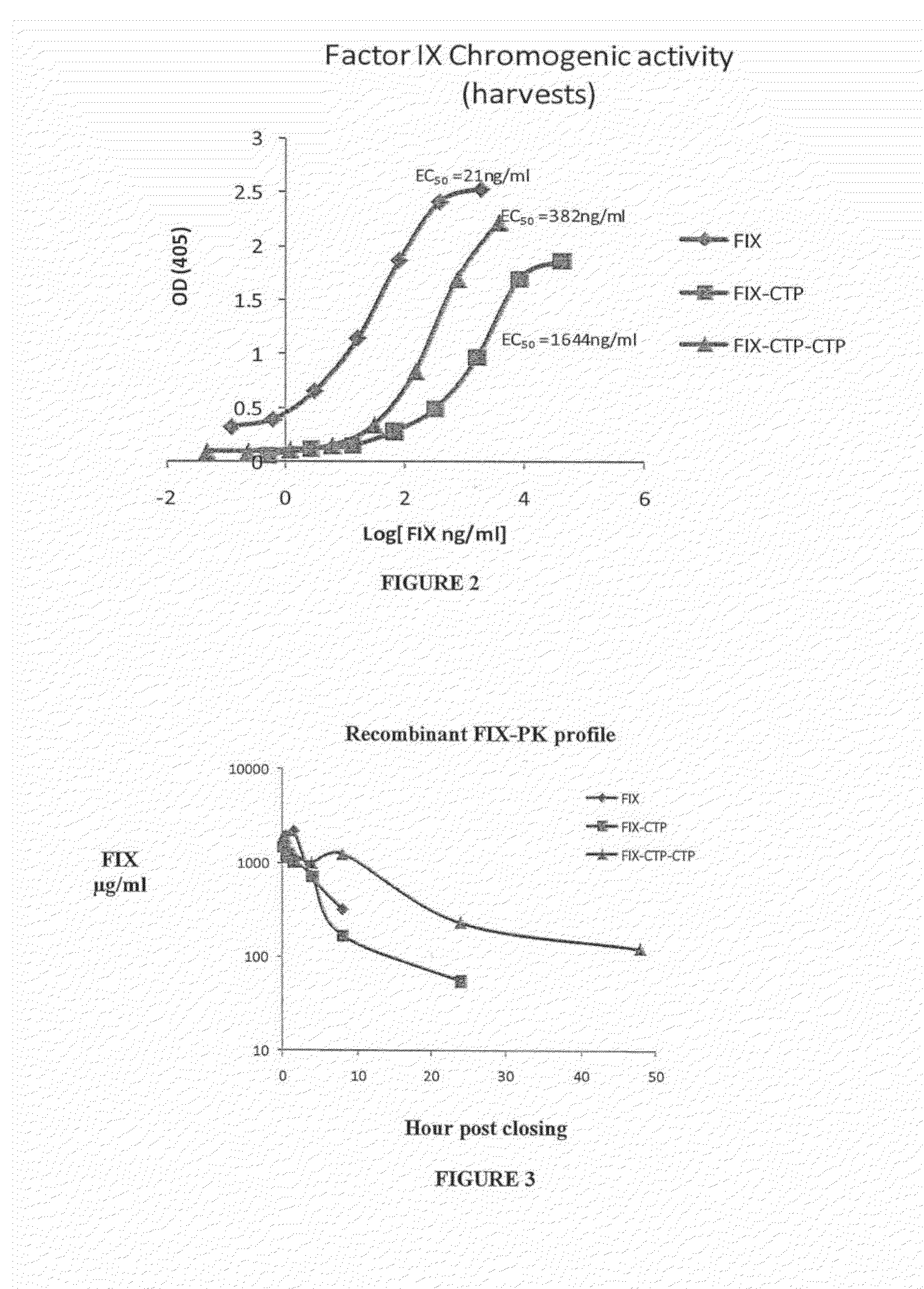
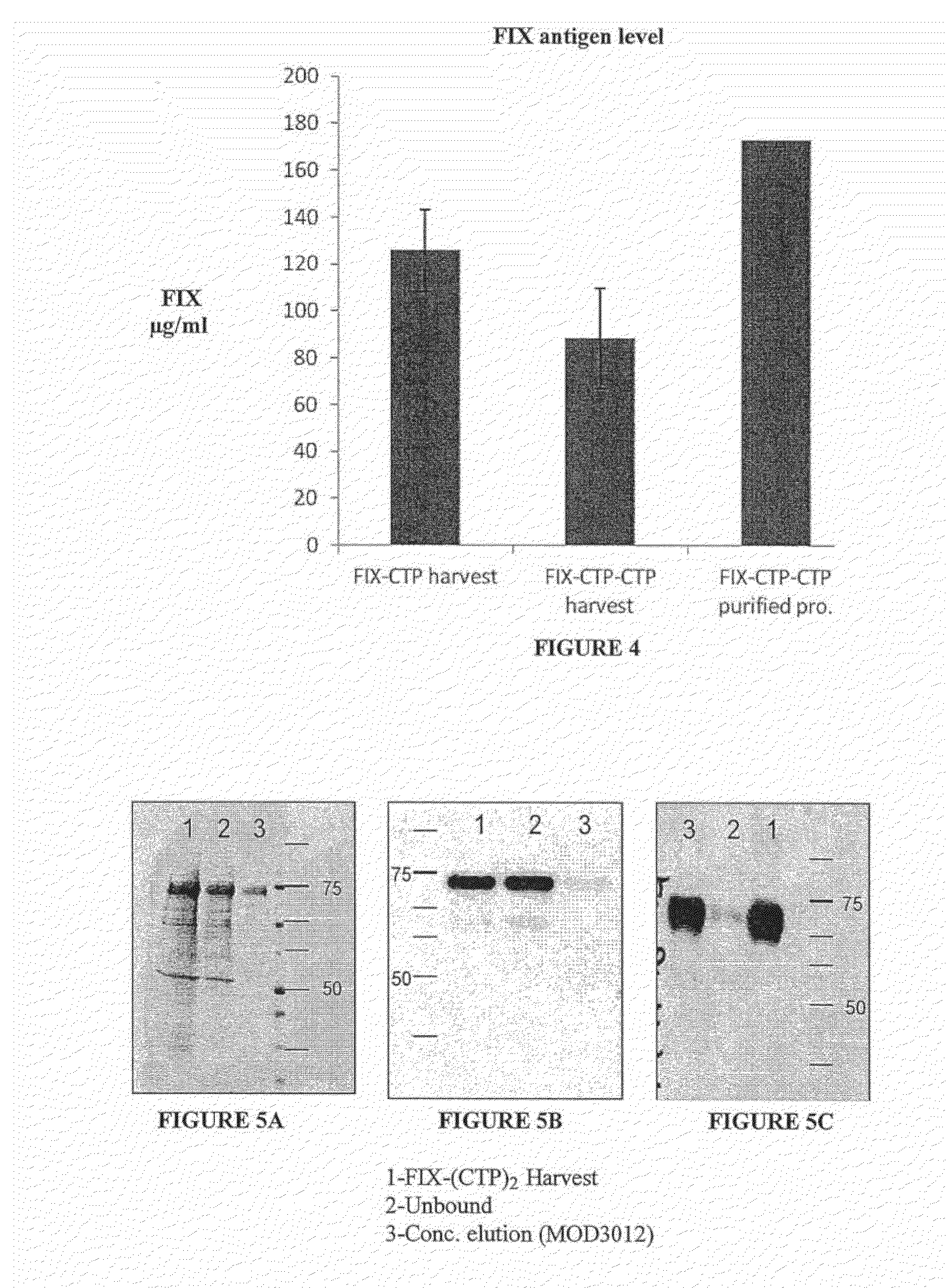
Polypeptides comprising at least one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to the carboxy terminus but not to the amino terminus of a coagulation factor and polynucleotides encoding the same are disclosed. Pharmaceutical compositions comprising the polypeptides and polynucleotides of the invention and methods of using and producing same are also disclosed.
Owner:OPKO BIOLOGICS
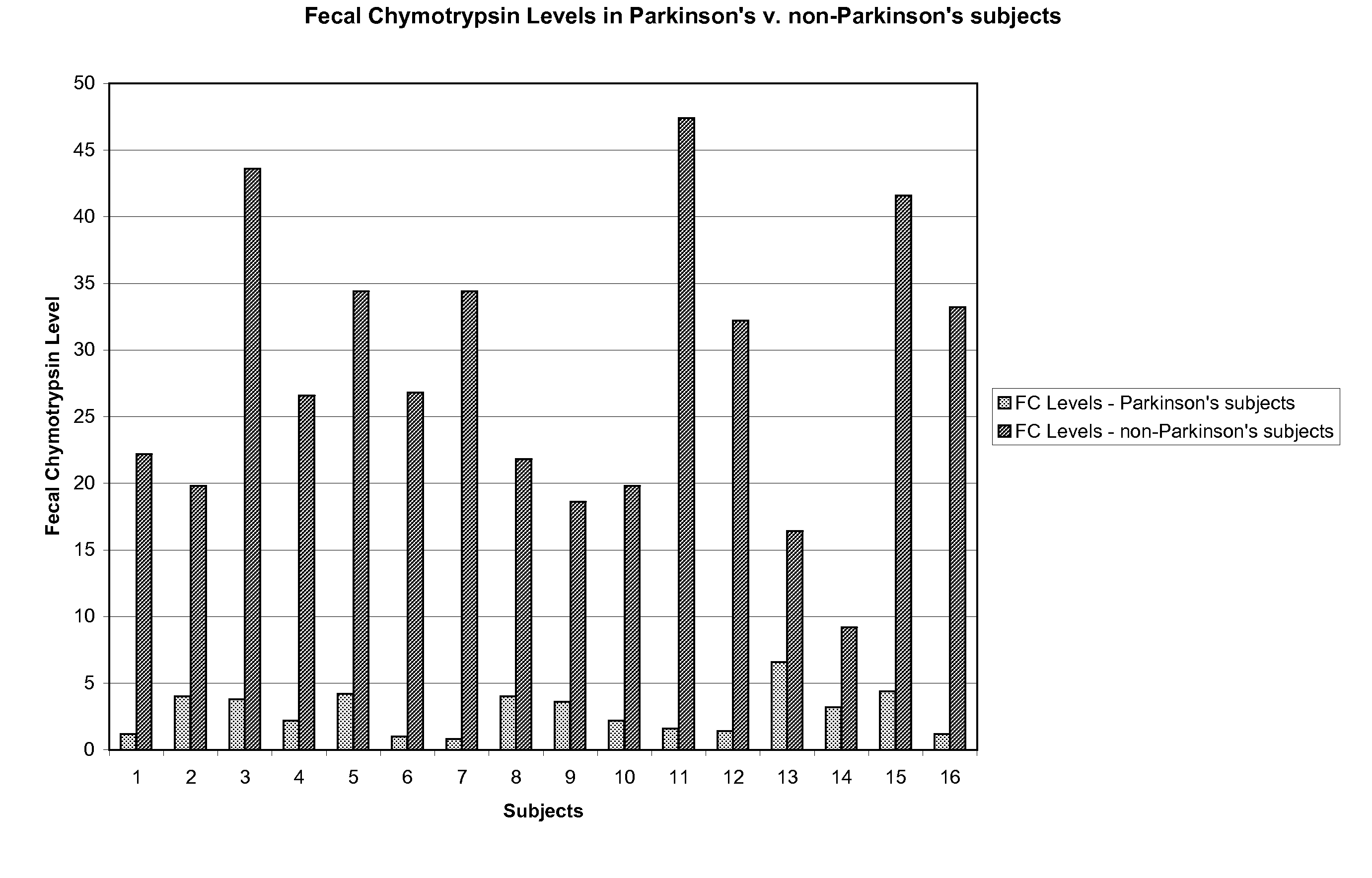
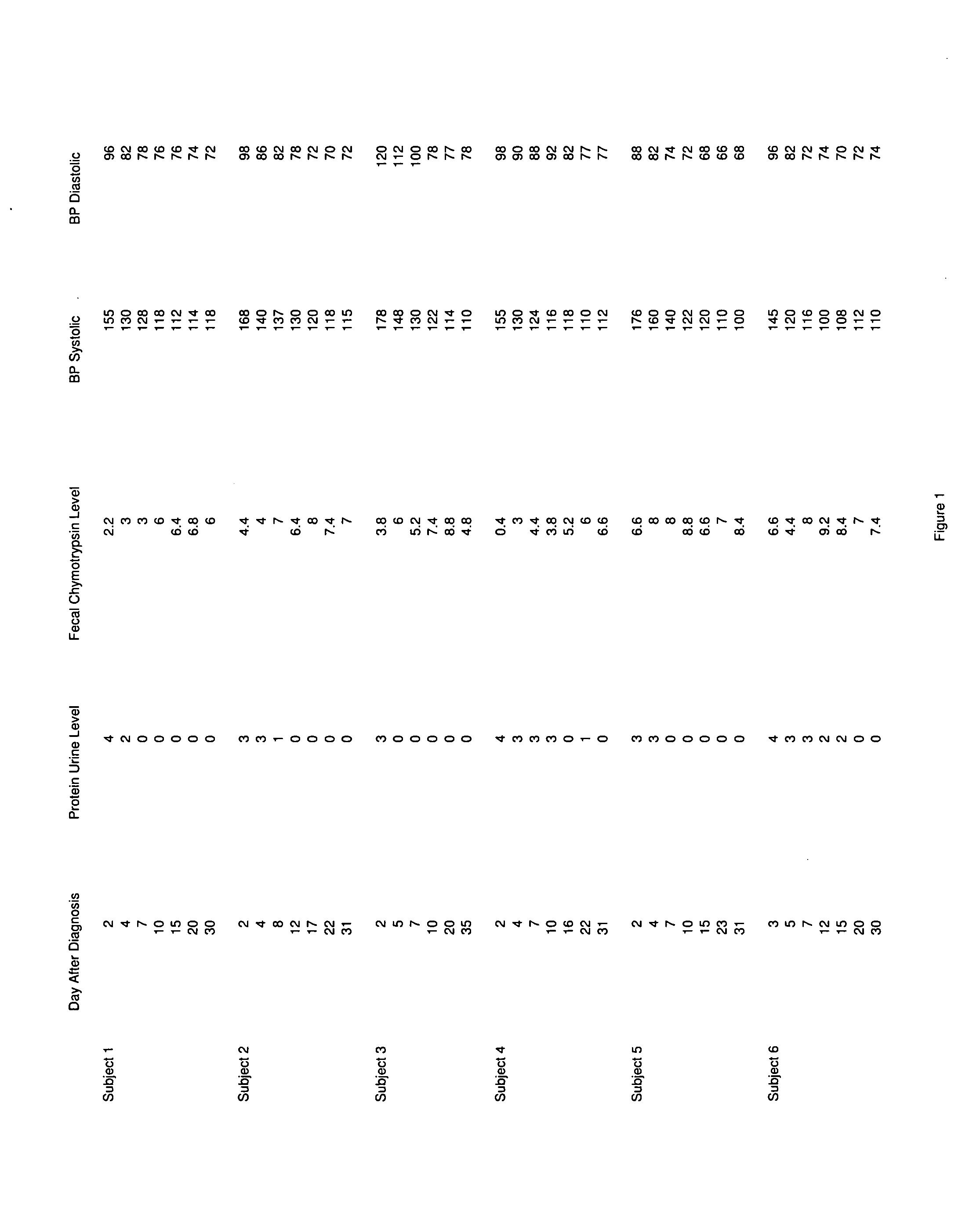
Methods and compositions for the treatment of symptoms of prion diseases
InactiveUS20100092447A1Peptide/protein ingredientsMicrobiological testing/measurementDiseaseFaecal chymotrypsin
A therapeutic composition for the treatment of the symptoms of prion diseases and the method for preparing the therapeutic agents is disclosed. The therapeutic composition is a stable pharmaceutical composition comprising one or more digestive and / or pancreatic enzymes. The therapeutic composition may be manufactured by a variety of encapsulation technologies. Delivery of the therapeutic composition may be made orally, through injection, by adherence of a medicated patch or other method. Further, a method of using fecal chymotrypsin level as a biomarker for the presence of a prion disease, or the likelihood of an individual to develop a prion disease is disclosed.
Owner:CUREMARK
Compositions and methods relating to reduction of symptoms of autism
InactiveUS6899876B2Relieve symptomsMany symptomPeptide/protein ingredientsOxidoreductasesNormorphineMedicine
Methods and compositions that can reduce the symptoms of autism in a human patient comprising administering a physiologically effective amount of one or both of a purified casomorphin inhibitor selected from the group consisting of a casomorphinase and a casomorphin ligand, and a physiologically effective amount of a purified gluteomorphin inhibitor selected from the group consisting of a gluteomorphinase and a gluteomorphin ligand, to a human patient in sufficient quantities to reduce the effects of the autism. In some embodiments, the compositions and methods further comprise a physiologically effective amount of an enkephalin inhibitor, preferably an enkephalinase, and a physiologically effective amount of an endorphin inhibitor, preferably an endorphinase.
Owner:PROTHERA
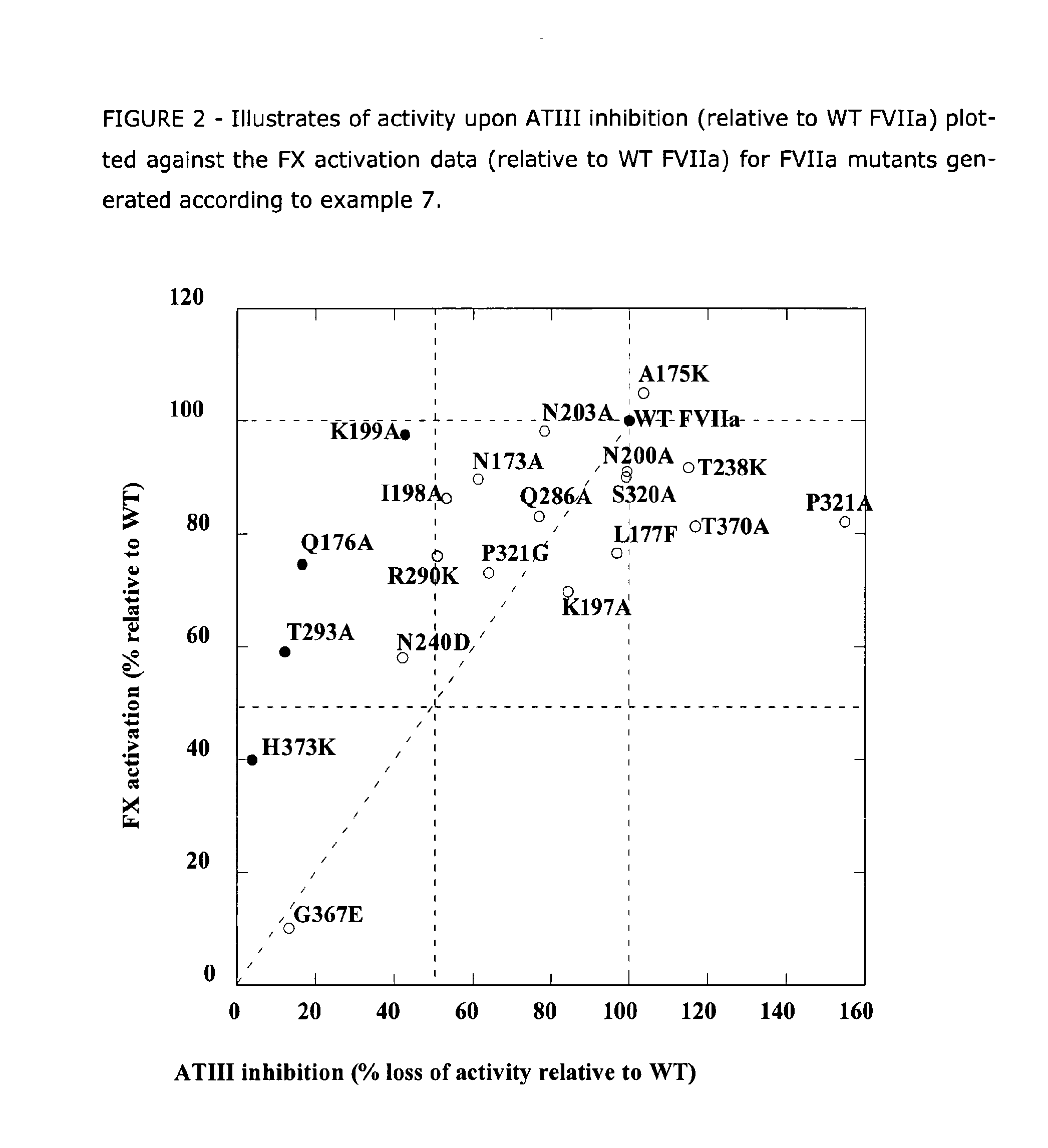
Human Coagulation Factor VII Polypeptides
InactiveUS20090055942A1Prolong half-life in vivoPeptide/protein ingredientsFermentationFactor VIIClotting factor
The present invention relates to novel human coagulation Factor Vila variants having substitutions of one or more amino acids at a position selected from the group consisting of position 172, 173, 175, 176, 177, 196, 197, 198, 199, 200, 203, 235, 237, 238, 239, 240, 286, 287, 288, 289, 290, 291, 292, 293, 294, 295, 297, 299, 319, 320, 321, 327, 341, 363, 364, 365, 366, 367, 370, 373 corresponding to amino acid positions of SEQ ID NO:1 and wherein said Factor VII polypeptide exhibits increased resistance to inactivation by an endogenous inhibitor of said FVII polypeptide relative to wild-type human FVIIa.
Owner:NOVO NORDISK AS
Nucleic acids encoding plasminogen fragments
Nucleic acid sequences encoding kringle region fragments of plasminogen. Ribonucleic and deoxyribonucleic acid sequences that encode for kringle region fragments are useful for gene therapy or recombinant expression for the treatment of angiogenesis-related diseases, specifically angiogenesis-dependent cancer.
Owner:CHILDRENS MEDICAL CENT CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com