Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
285 results about "Chimera Protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
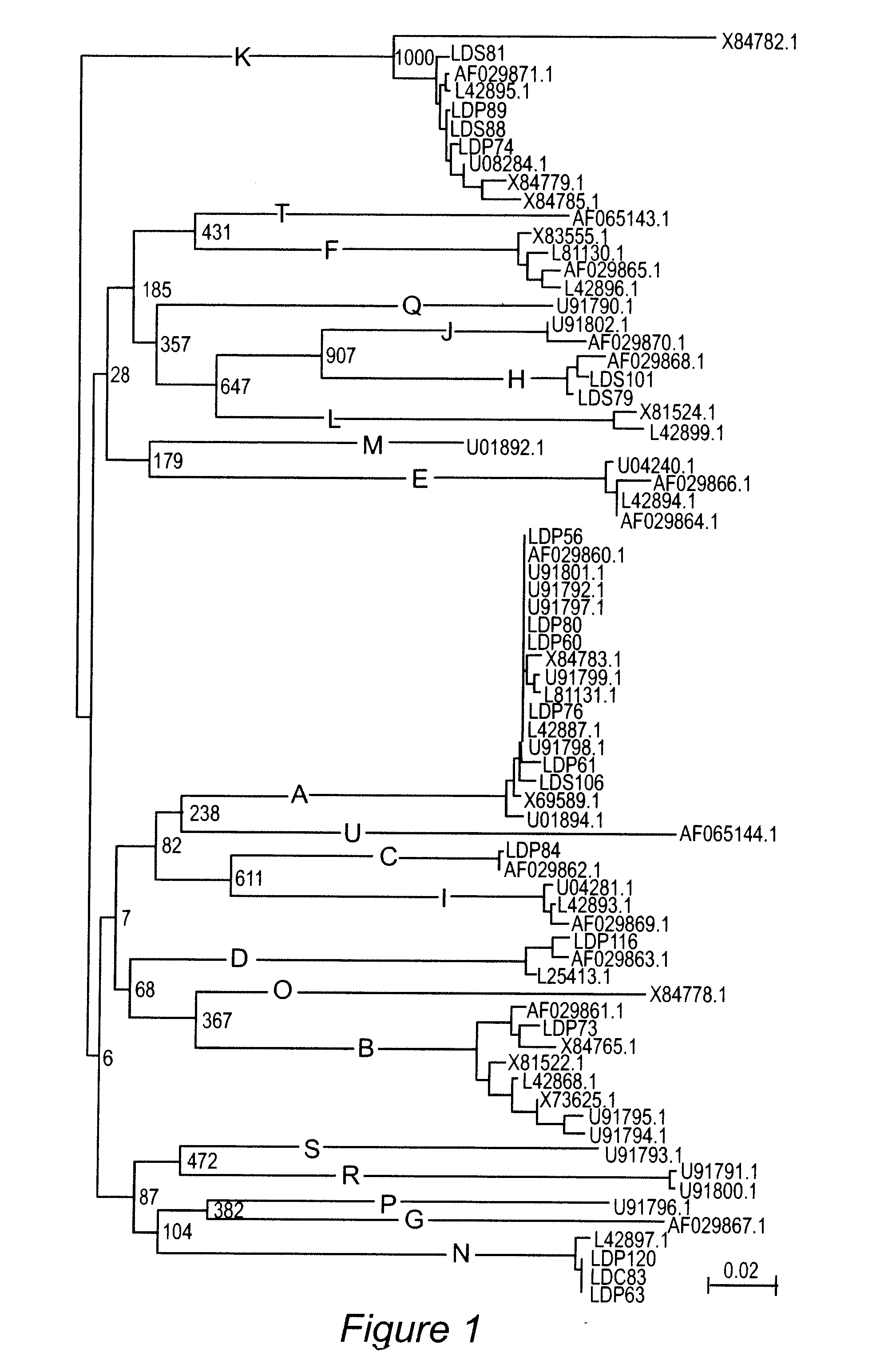
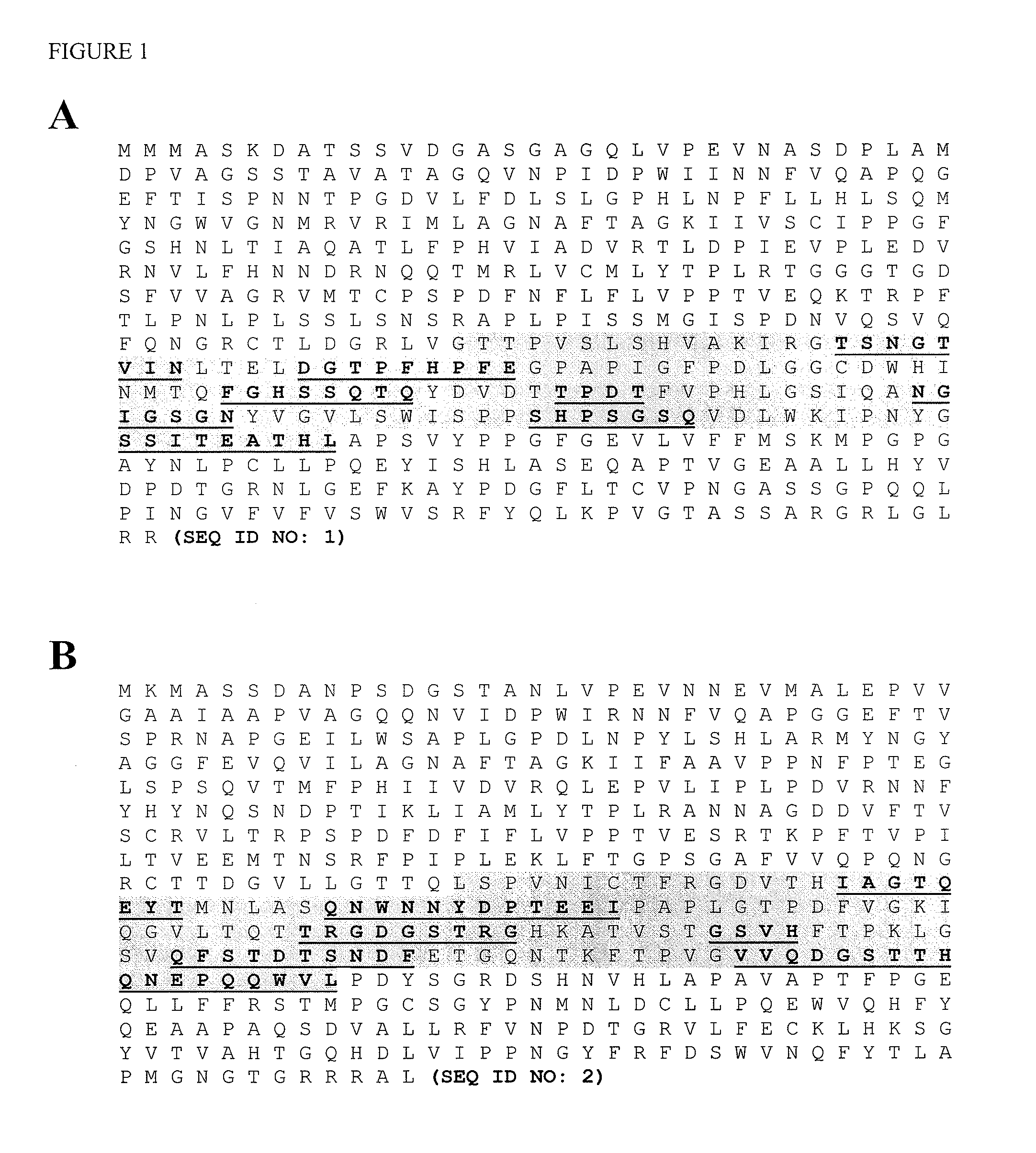
Chimera (protein) Fusion proteins or chimeric (\kī-ˈmir-ik) proteins (literally, made of parts from different sources) are proteins created through the joining of two or more genes that originally coded for separate proteins.
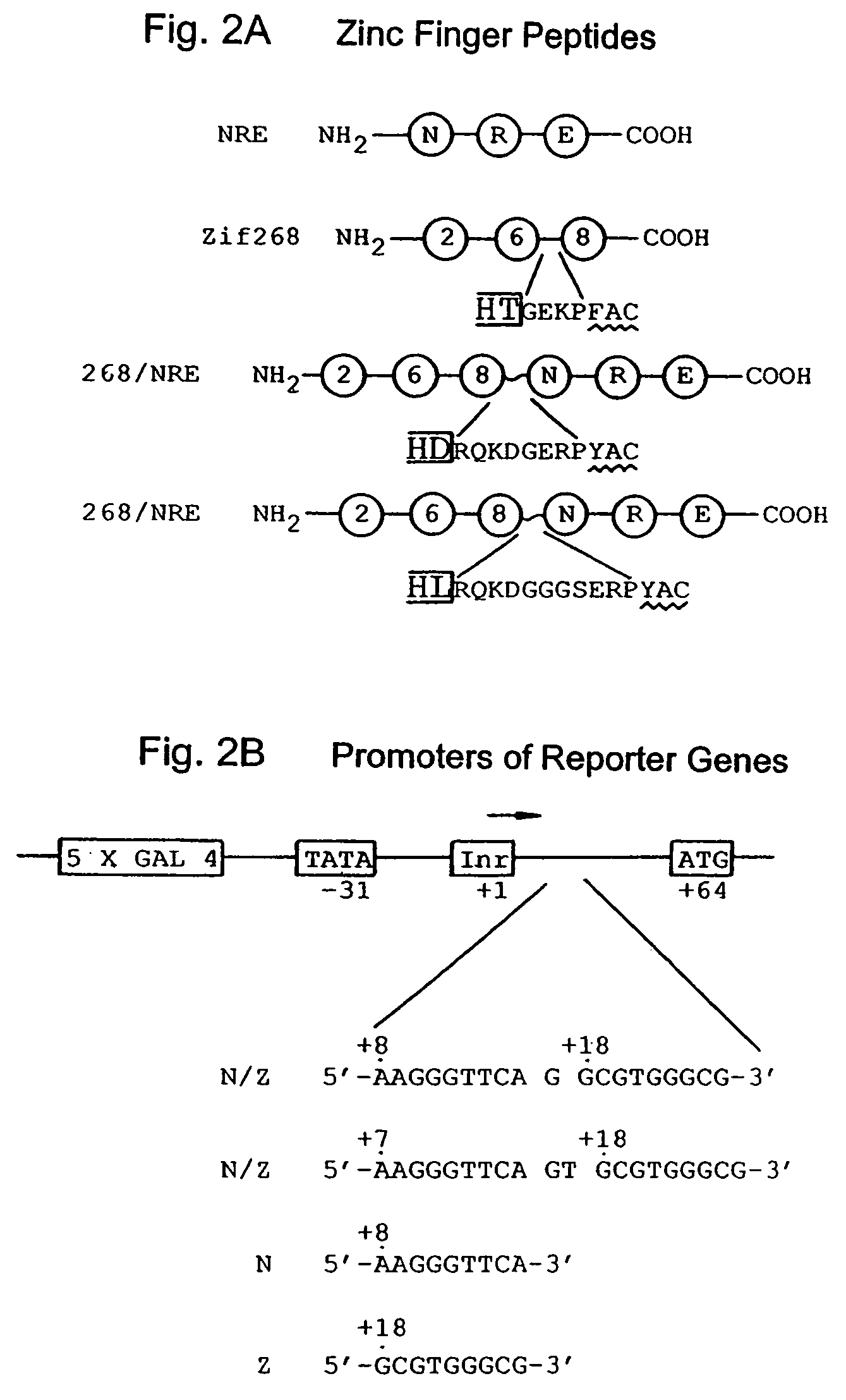
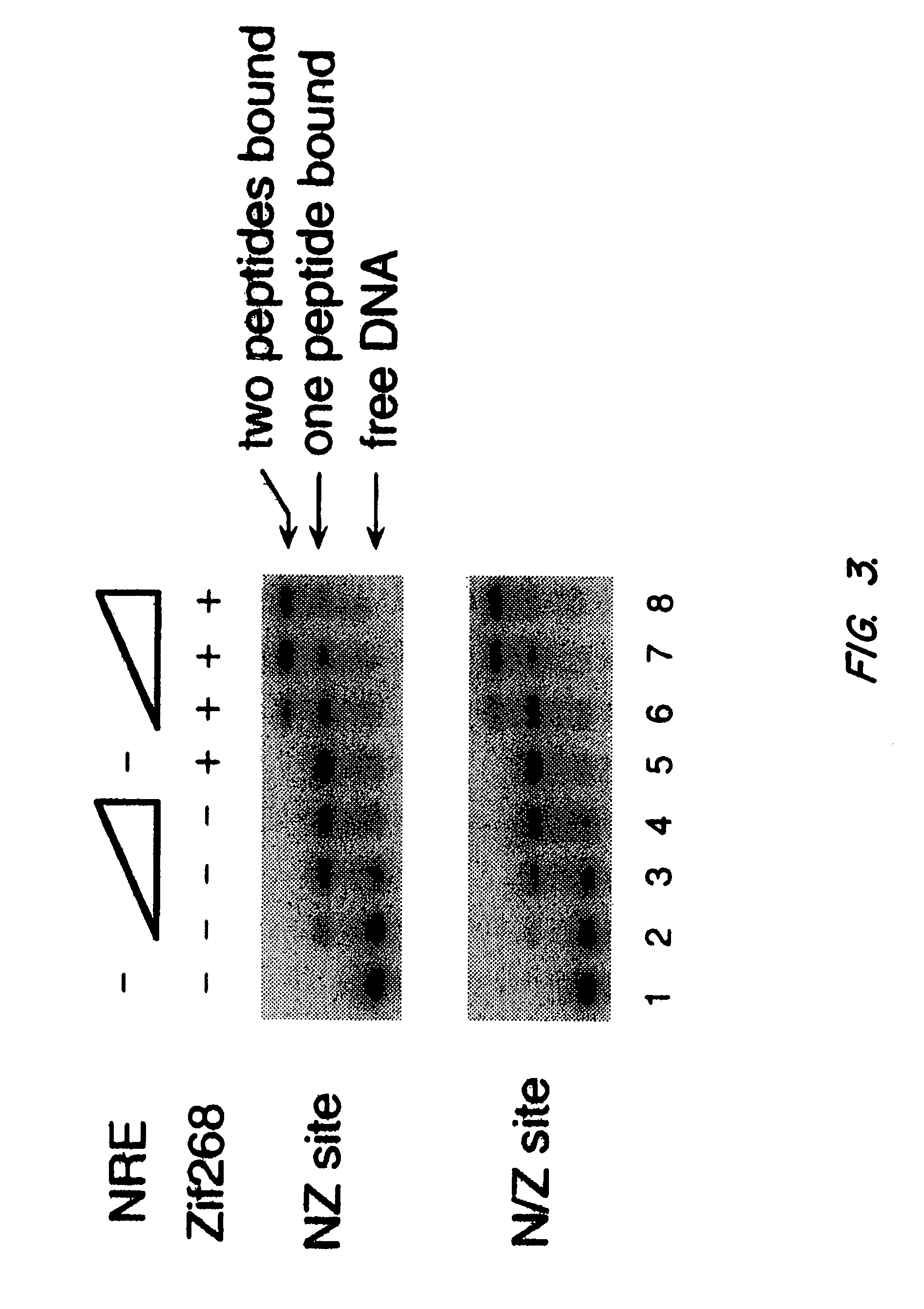
Nucleic acid encoding poly-zinc finger proteins with improved linkers
InactiveUS7153949B2Enhanced affinity and specificityImprove the level ofPeptide/protein ingredientsAntibody mimetics/scaffoldsDNA-binding domainNucleotide
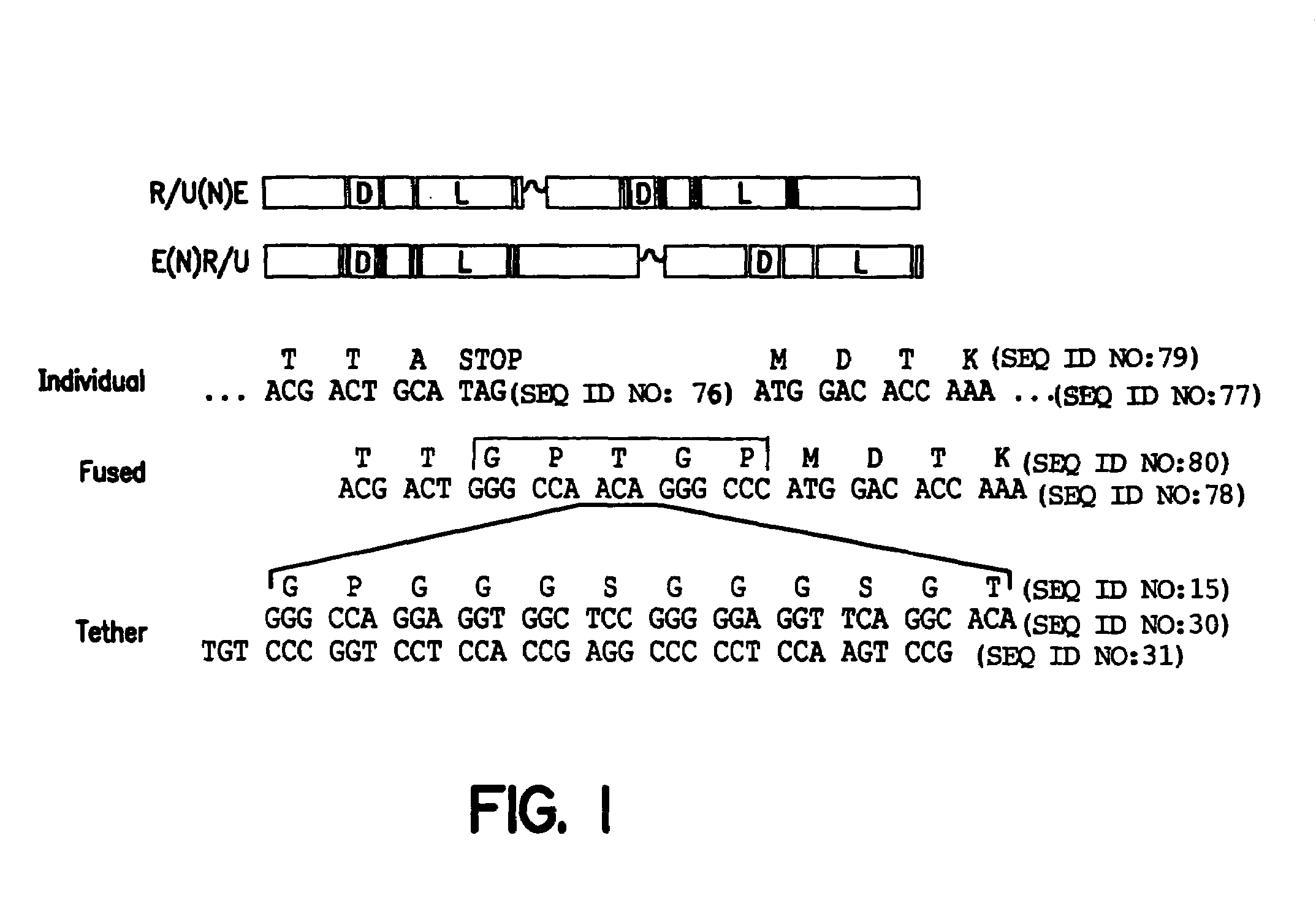
Polynucleotides encoding chimeric proteins, and methods for their production and use are disclosed. The chimeric proteins comprise a flexible linker between two zinc finger DNA-binding domains, wherein the linker contains eight or more amino acids between the second conserved histidine residue of the carboxy-terminal zinc finger of the first domain and the first conserved cysteine residue of the amino-terminal zinc finger of the second domain.
Owner:MASSACHUSETTS INST OF TECH
Methods for chemically synthesizing immunoglobulin chimeric proteins
ActiveUS7381408B2Prolong lifeEasy to purifyHydrolasesPeptide/protein ingredientsChimera ProteinBiochemistry
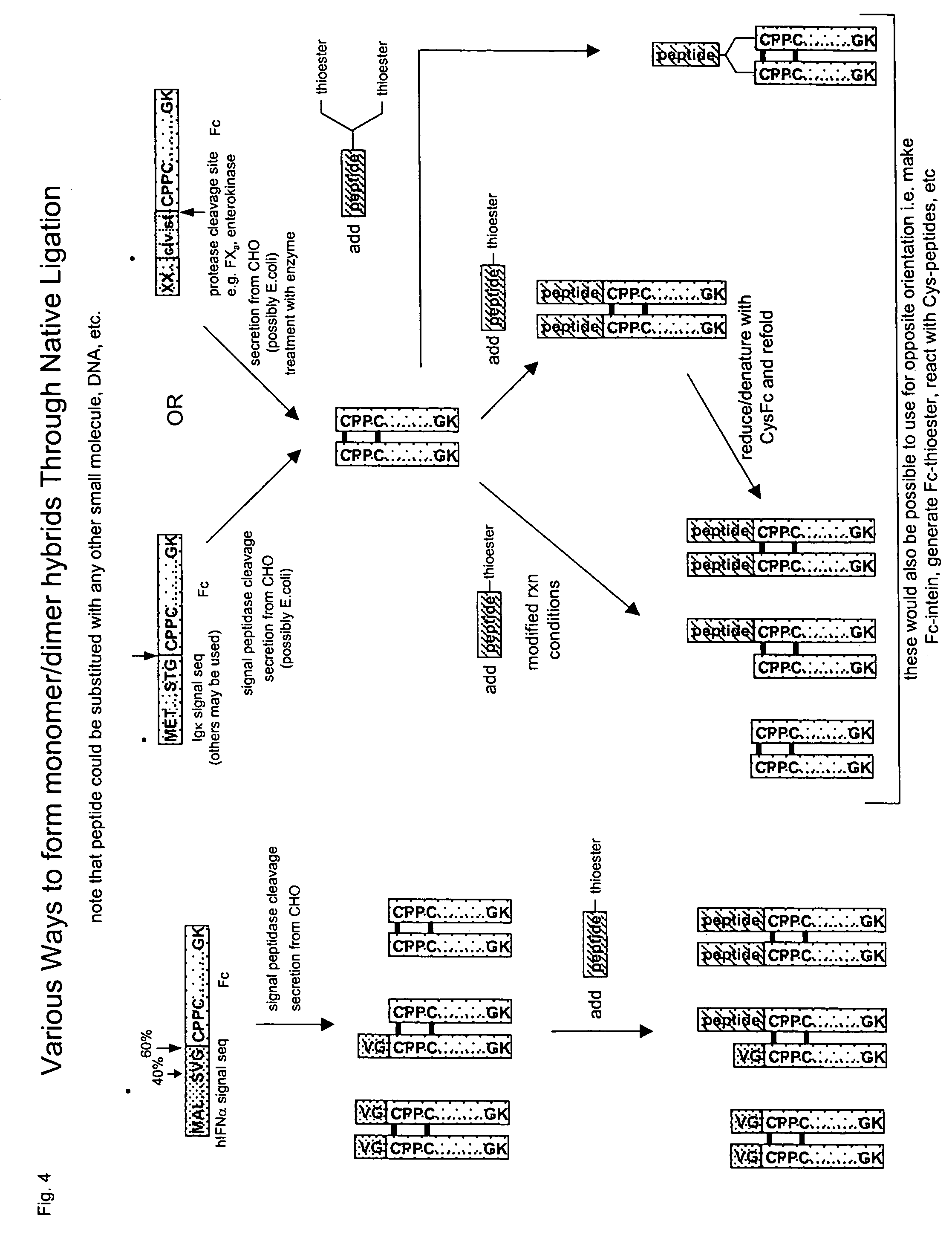
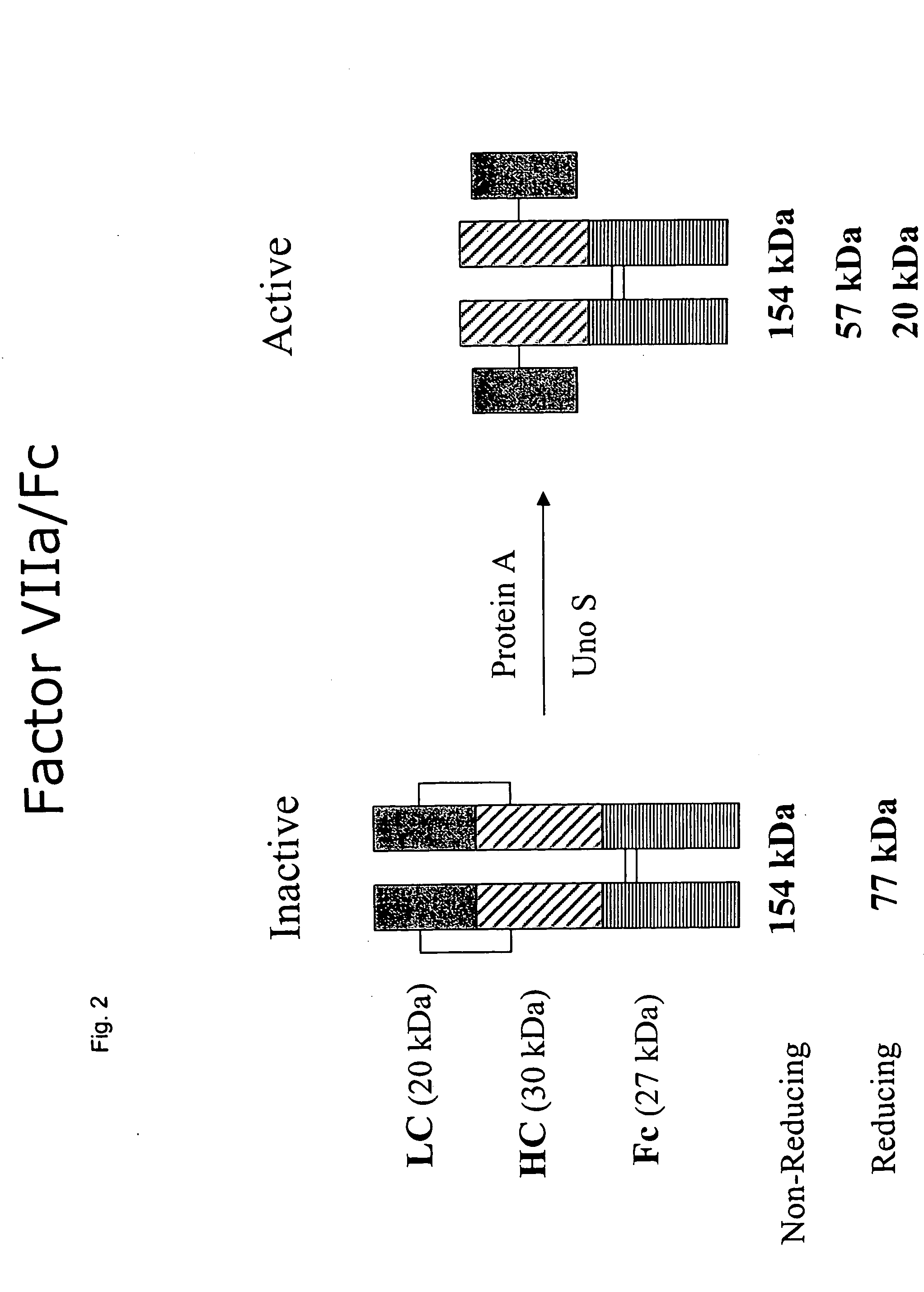
The invention provides methods of chemically synthesizing chimeric proteins comprising at least a portion of an immunoglobulin constant region and a biologically active molecule.
Owner:BIOVERATIV THERAPEUTICS INC
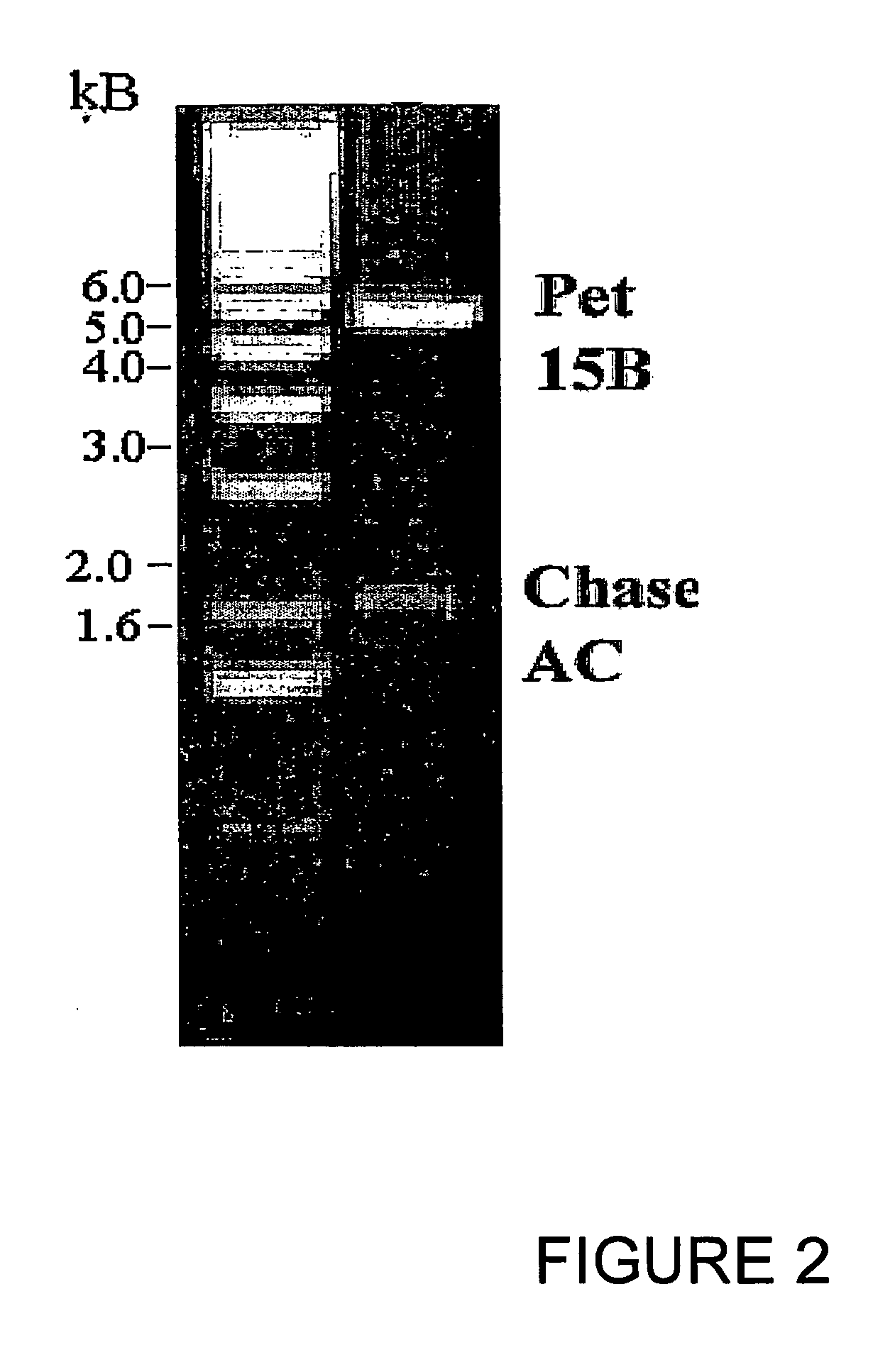
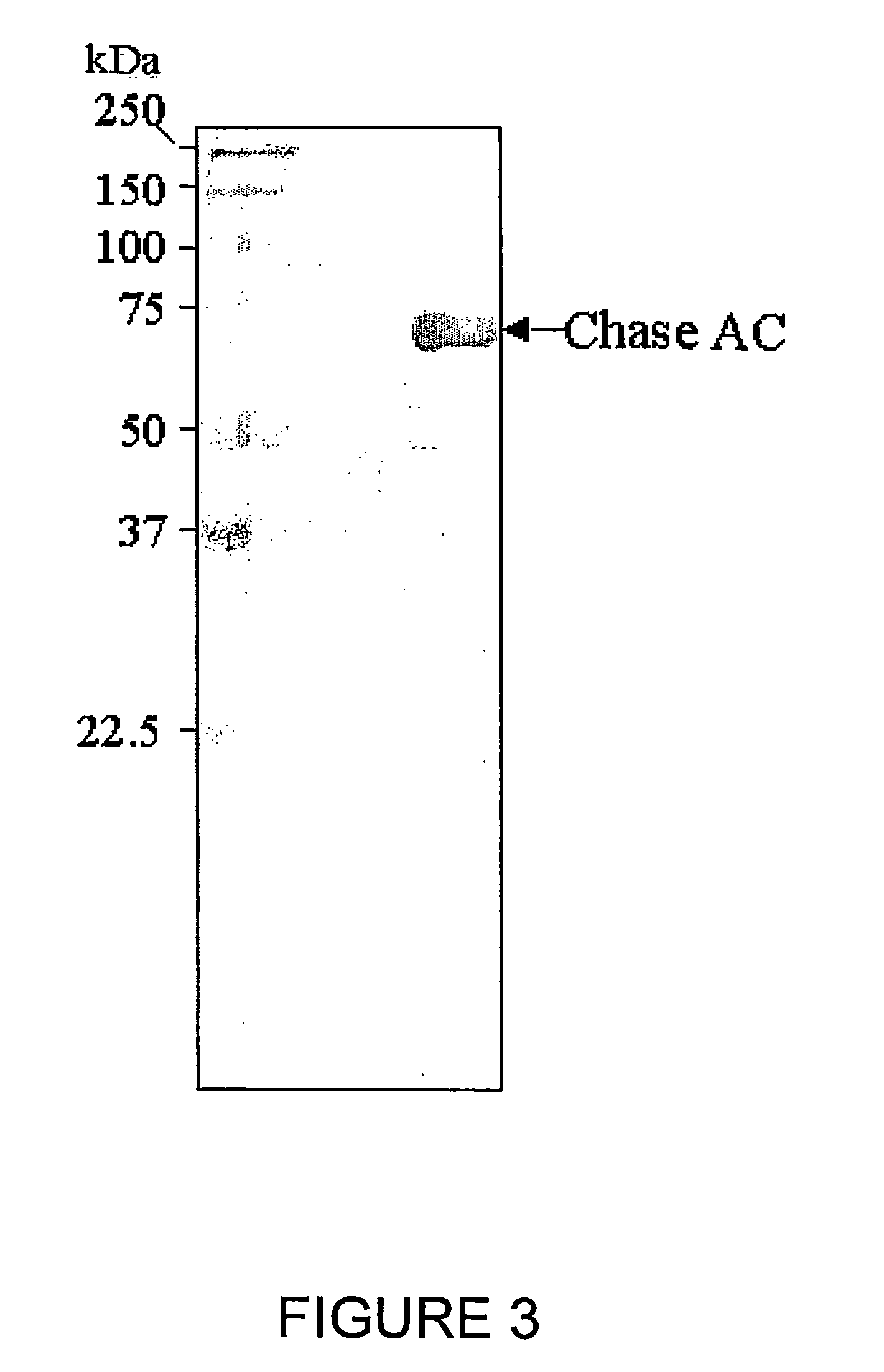
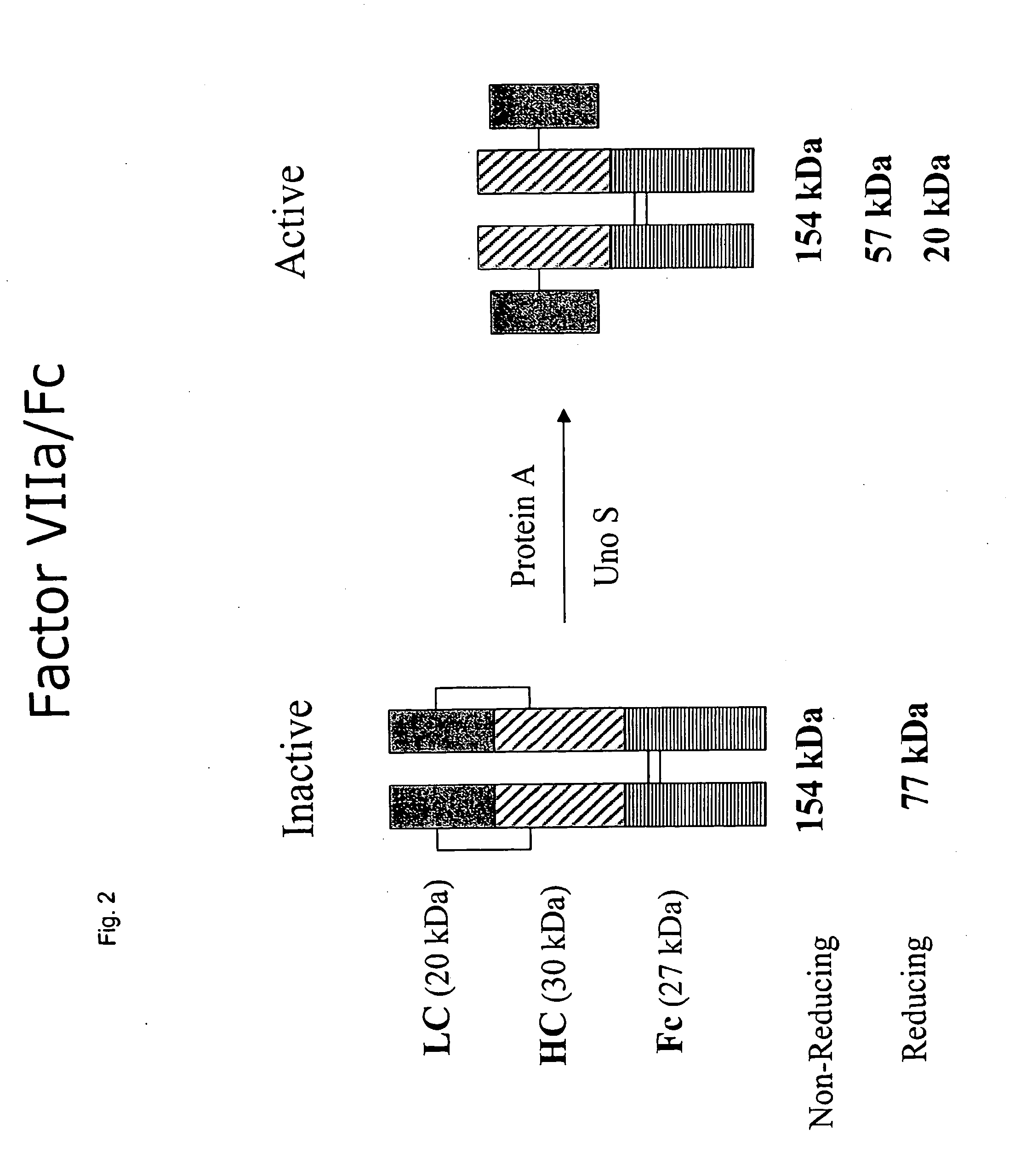
Clotting factor-Fc chimeric proteins to treat hemophilia
InactiveUS20050147618A1Peptide/protein ingredientsAntibody mimetics/scaffoldsHemostatic DisordersChimera Protein
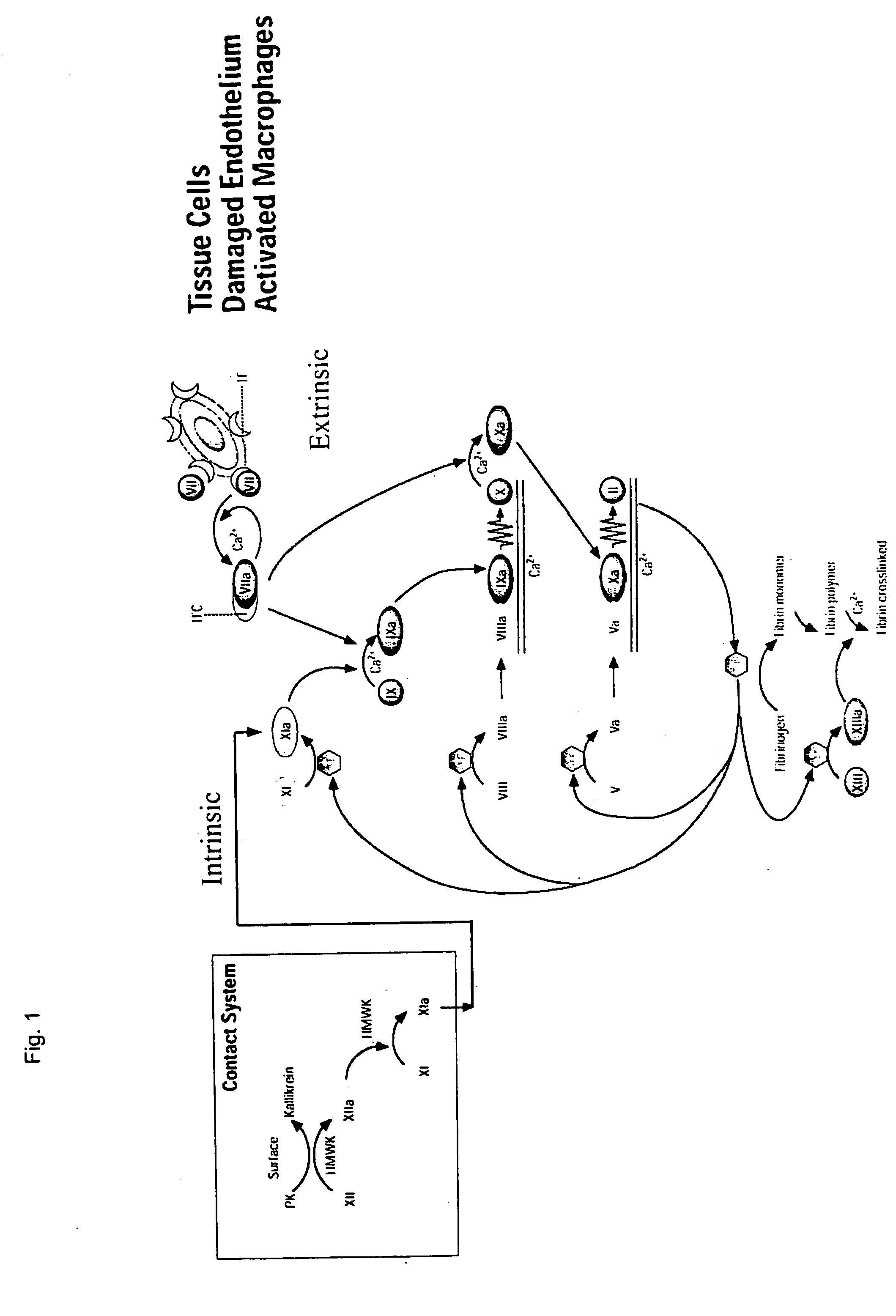
The invention relates to a chimeric protein comprising at least one clotting factor and at least a portion of an immunoglobulin constant region. The invention relates to a method of treating a hemostatic disorder comprising administering a therapeutically effective amount of a chimeric protein wherein the chimeric protein comprises at least one clotting factor and at least a portion of an immunoglobulin constant region.
Owner:BIOVERATIV THERAPEUTICS INC
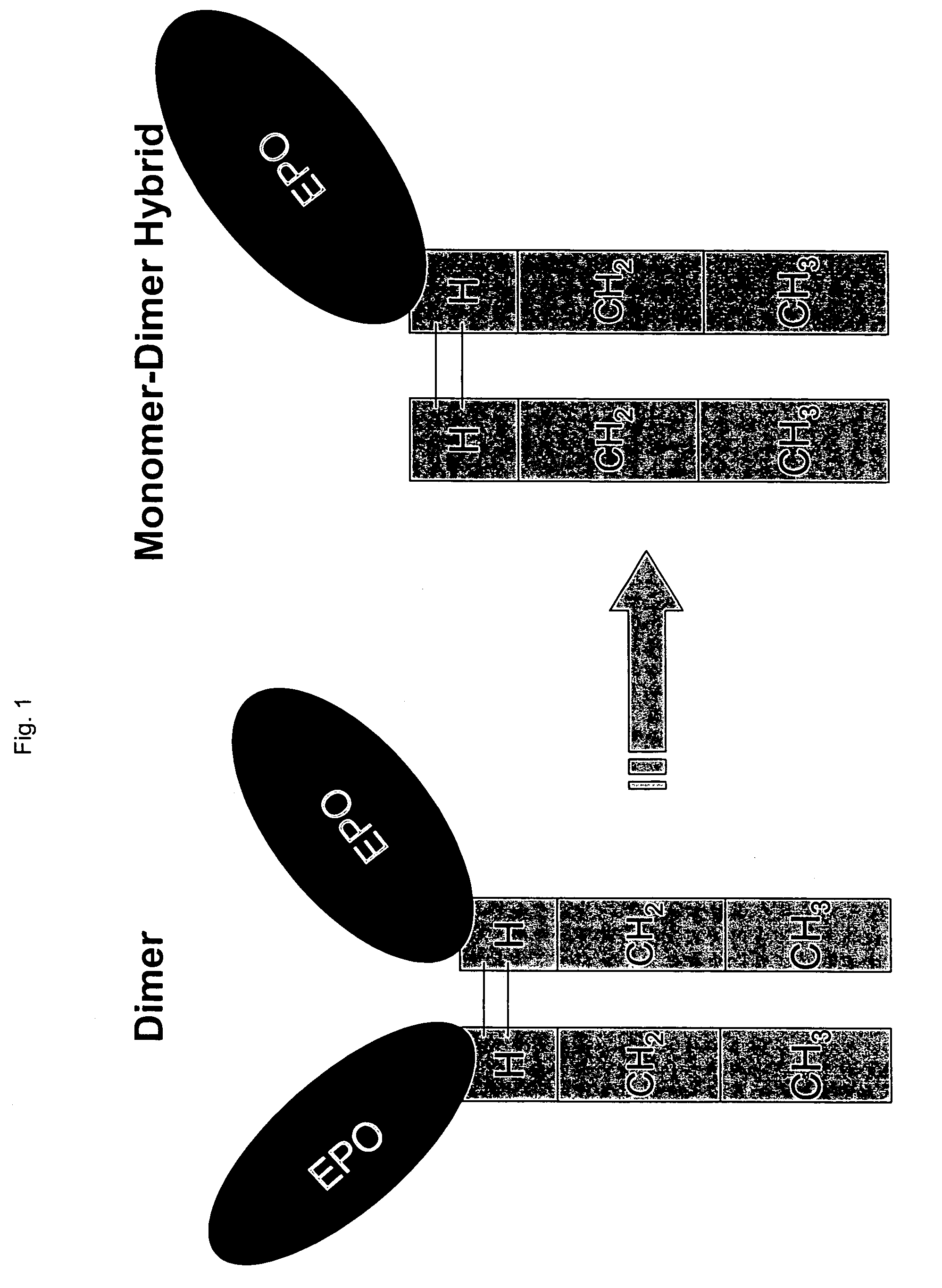
Hormone receptor functional dimers and methods of their use
InactiveUS7057015B1Enhance possibility of producingIncrease flexibilityFusion with DNA-binding domainSugar derivativesADAMTS ProteinsProtein Unit
The invention provides chimeric proteins having at least two functional protein units, each containing the dimerization domain of a member of the steroid / thyroid hormone nuclear receptor superfamily. The chimeric proteins can fold under crystallization conditions to form functional entities. The functional entities optionally contain a novel flexible peptide linker of variable lengths between at least two of the protein units. In a preferred embodiment, the linker is designed to be increased in increments of 12 amino acids each to aid in preparation of variant chimeric proteins. The DNA binding characteristics of the invention functional entities differ from those of wild-type complexes formed between “monomeric” receptors and their binding partners. Some functional entities, e.g. dimers expressed as fusion proteins, transactivate responsive promoters in a manner similar to wild-type complexes, while others do not promote transactivation and function instead essentially as constitutive repressors. The invention further provides nucleotide sequences encoding the invention chimeric proteins, cells containing such nucleotide sequences, and methods for using the invention chimeric proteins to modulate expression of one or more exogenous genes in a subject organism. In addition, isolated protein crystals suitable for x-ray diffraction analysis and methods for obtaining putative ligands for the invention chimeric proteins are provided.
Owner:SALK INST FOR BIOLOGICAL STUDIES
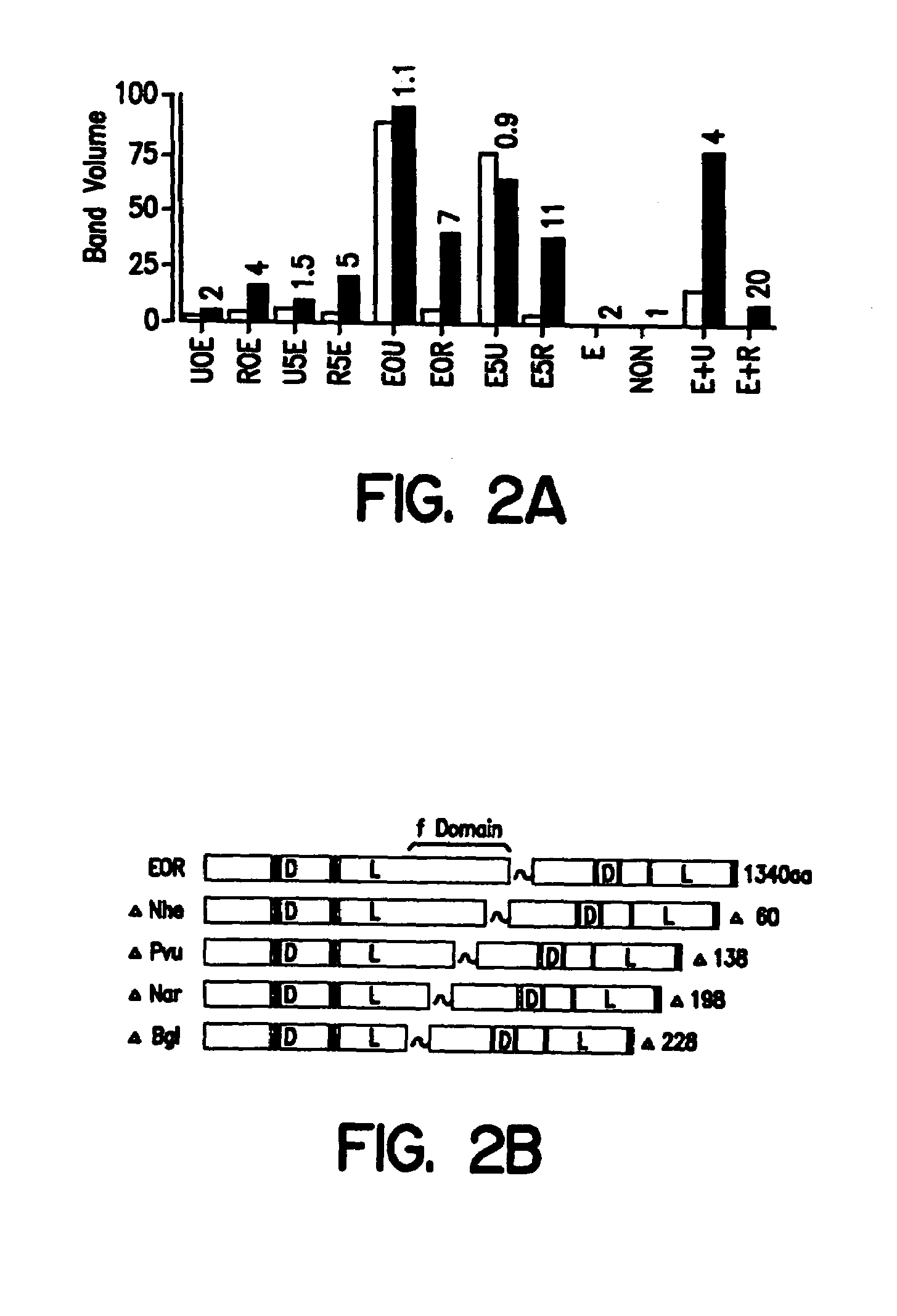
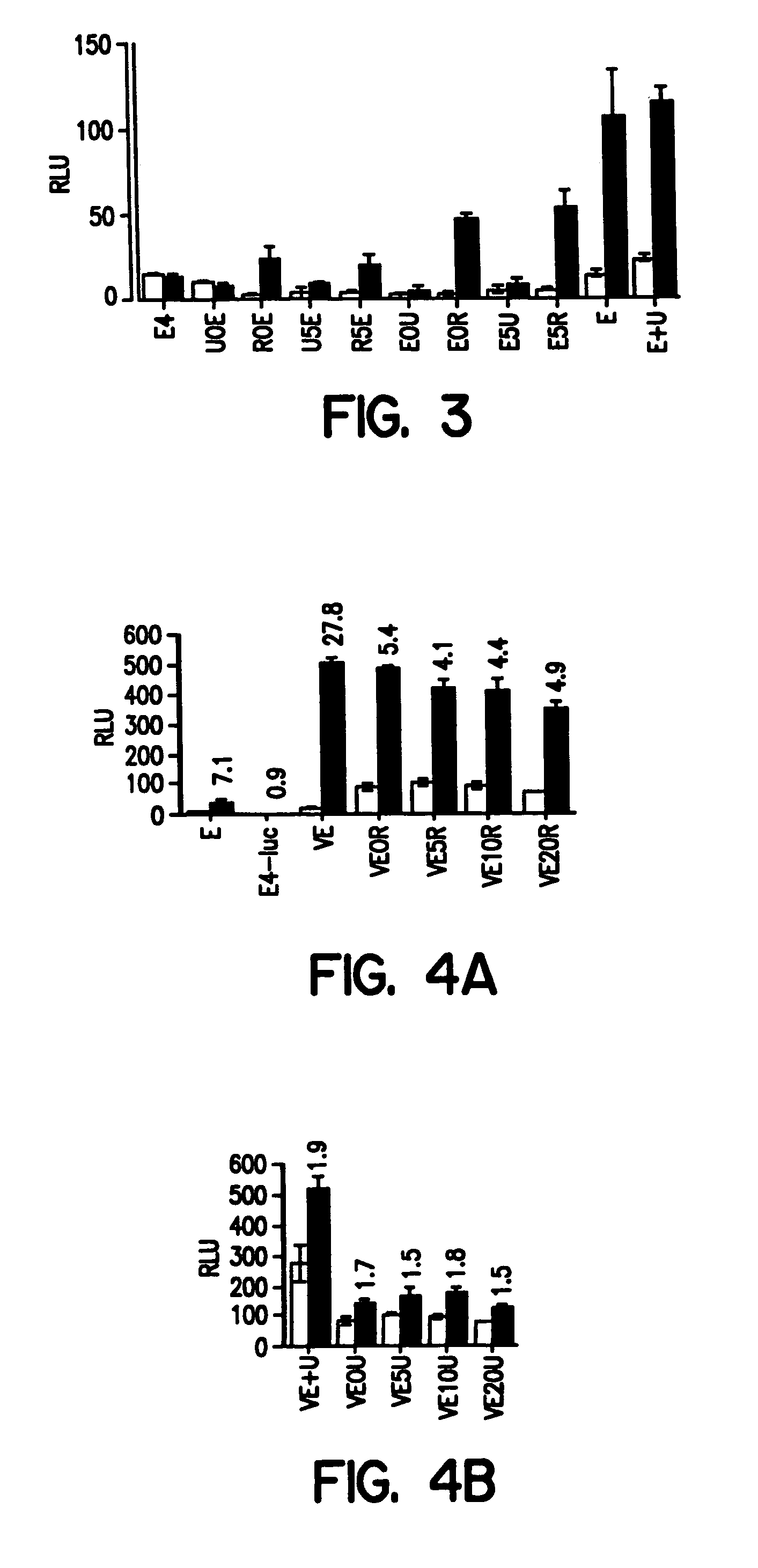
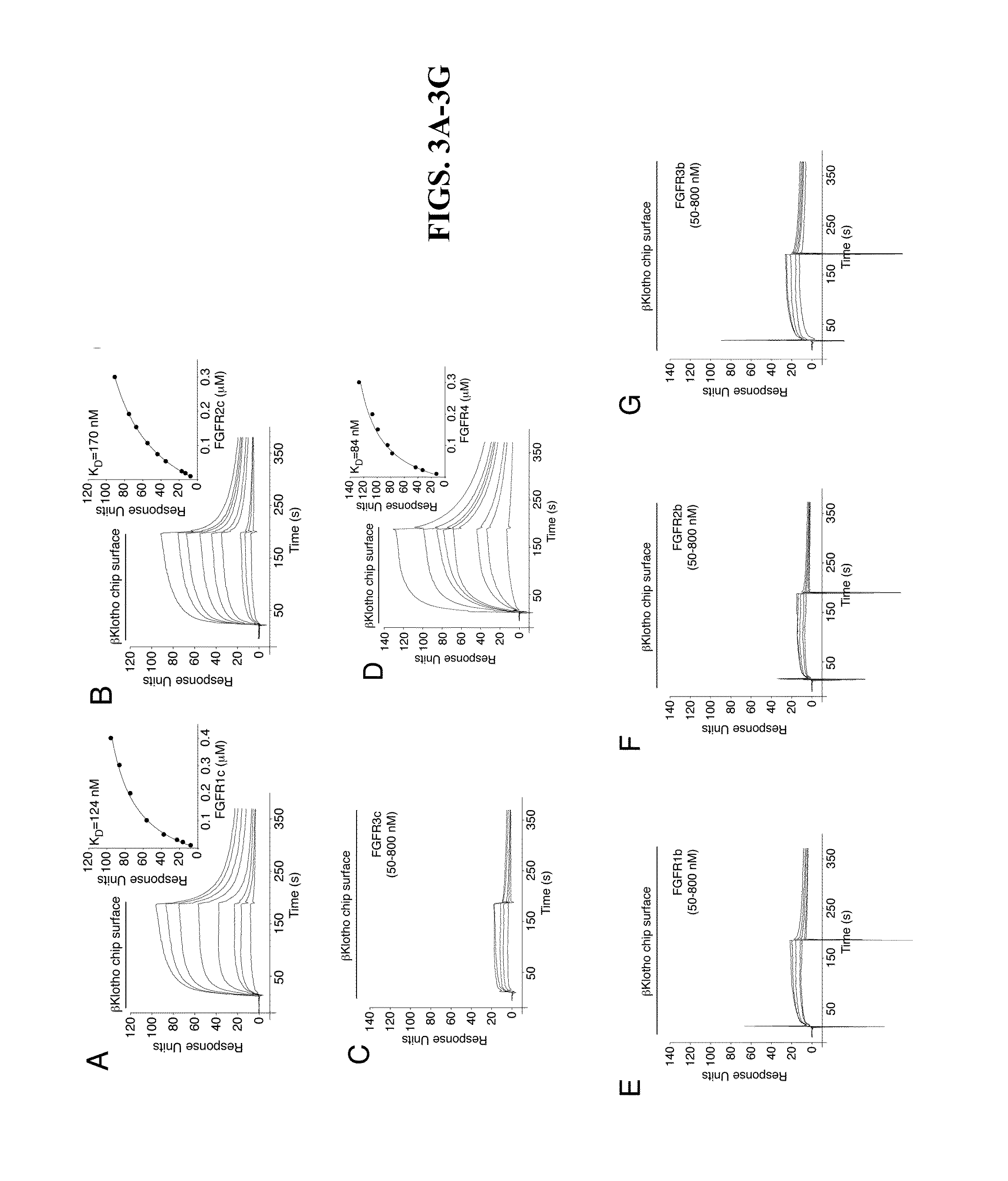
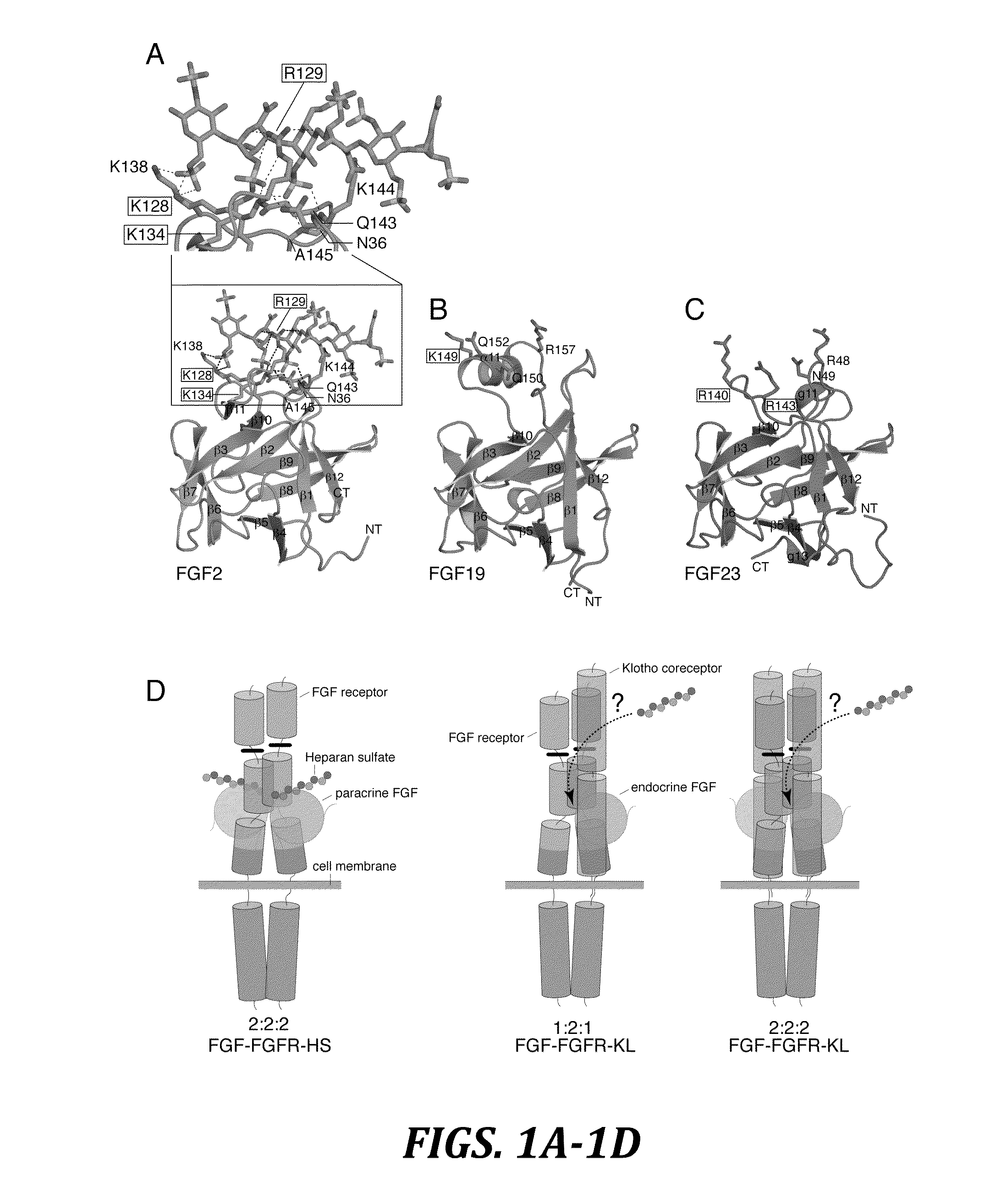
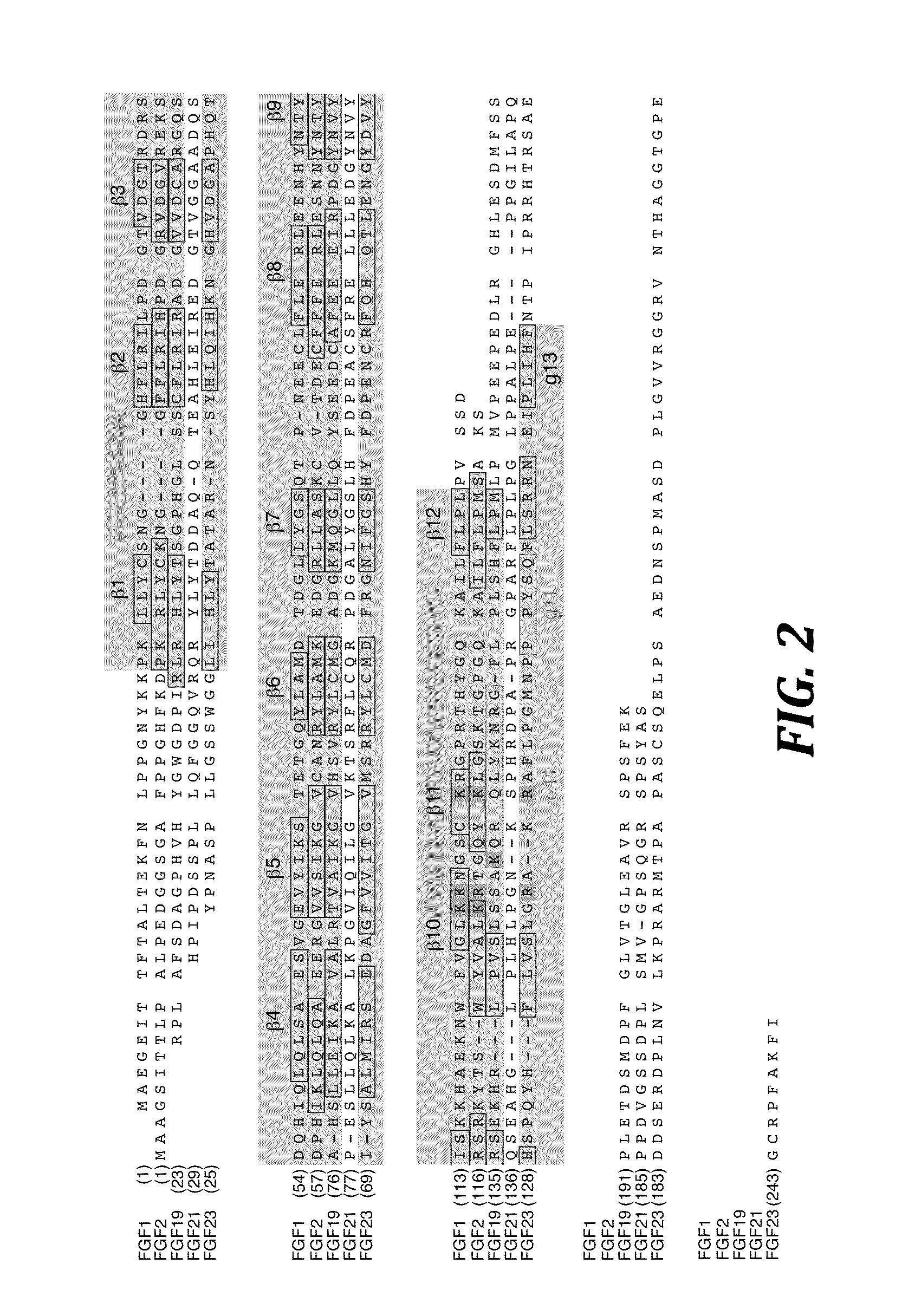
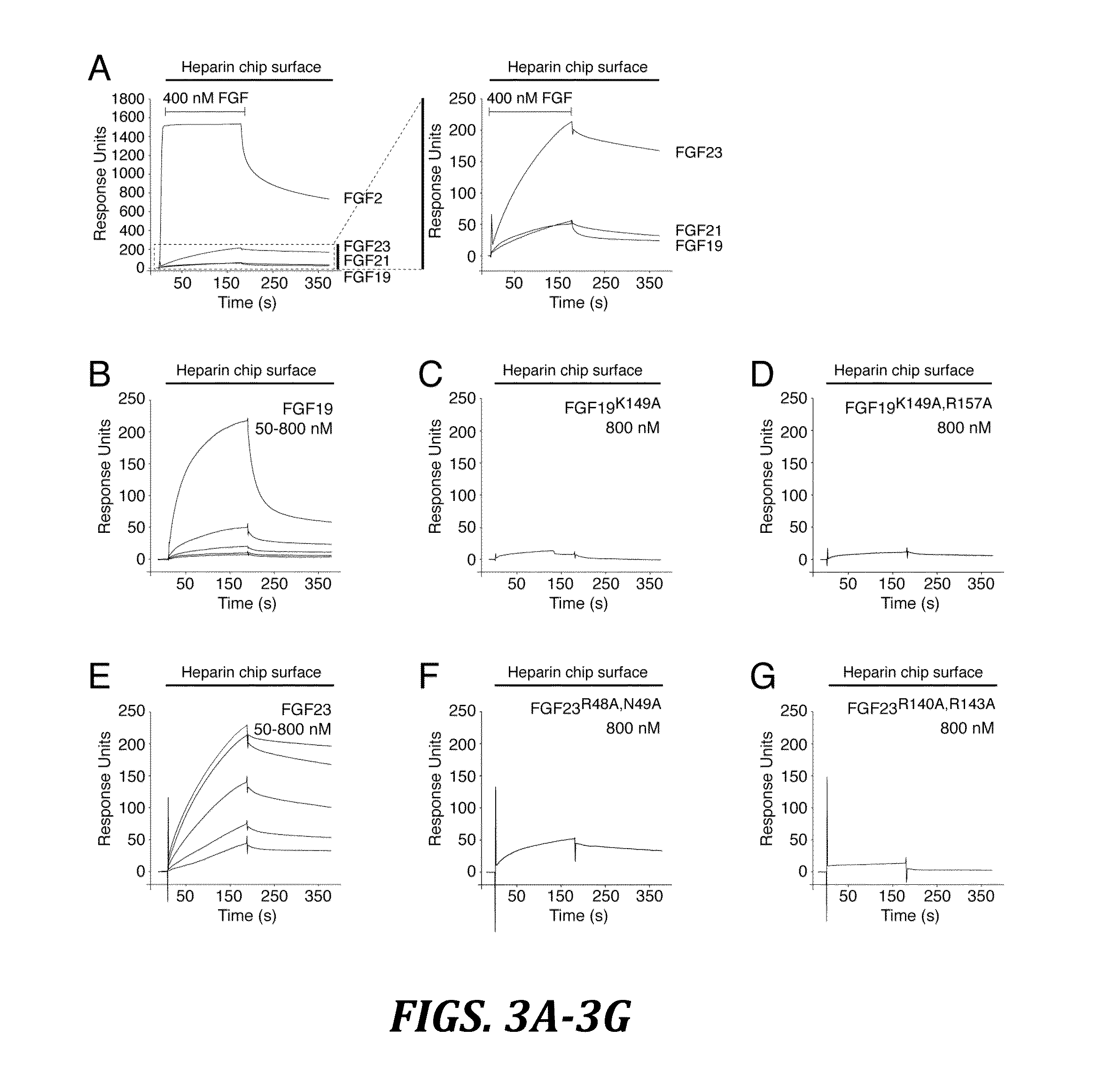
Chimeric fgf21 proteins with enhanced binding affinity for beta-klotho for the treatment of type ii diabetes, obesity, and related metabolic disorders
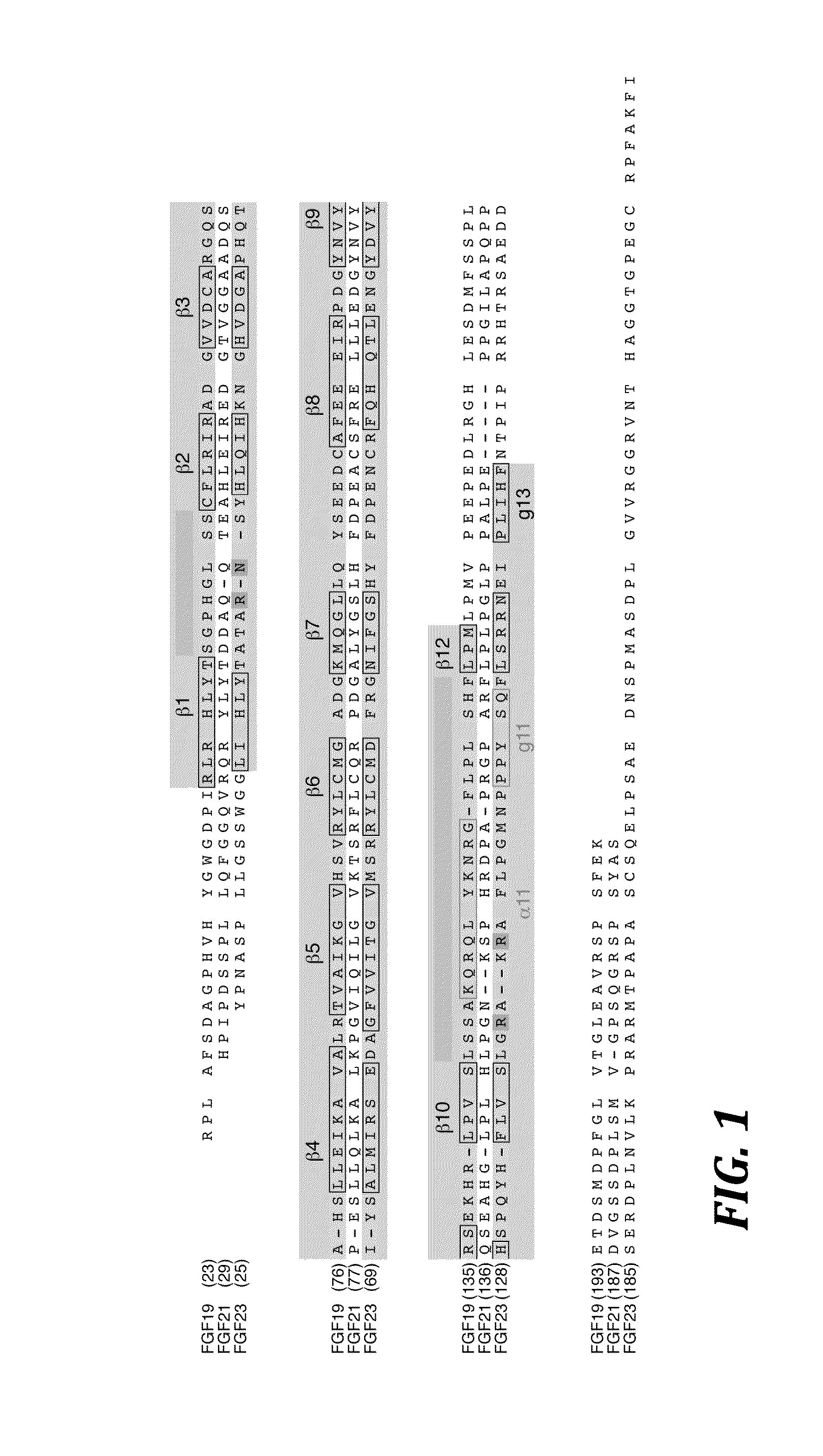
The present invention relates to chimeric proteins that include an N-terminus coupled to a C-terminus, where the N-terminus includes an N-terminal portion of fibroblast growth factor 21 (“FGF21”) and the C-terminus includes a C-terminal portion of fibroblast growth factor 19 (“FGF19”). The present invention also relates to pharmaceutical compositions including chimeric proteins according to the present invention, as well as methods for treating a subject suffering from diabetes, obesity, or metabolic syndrome, methods of treating a subject in need of increased FGF21-βKlotho-FGF receptor complex formation, methods of causing increased FGF21 receptor agonist-βKlotho-FGF receptor complex formation, and methods of screening for compounds with enhanced binding affinity for the βKlotho-FGF receptor complex involving the use of chimeric proteins of the present invention.
Owner:NEW YORK UNIV
Chimeric proteins and methods for using the same
InactiveUS7569663B2Enhanced advantageImprove propertiesCompounds screening/testingPeptide/protein ingredientsTrans signalingDisease
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Chimeric activators: quantitatively designed protein therapeutics and uses thereof
ActiveUS20110274658A1Reduced cell-activating propertyAvoid and reduce unwanted side effectObesity gene productsPeptide/protein ingredientsChimerin ProteinsApoptosis
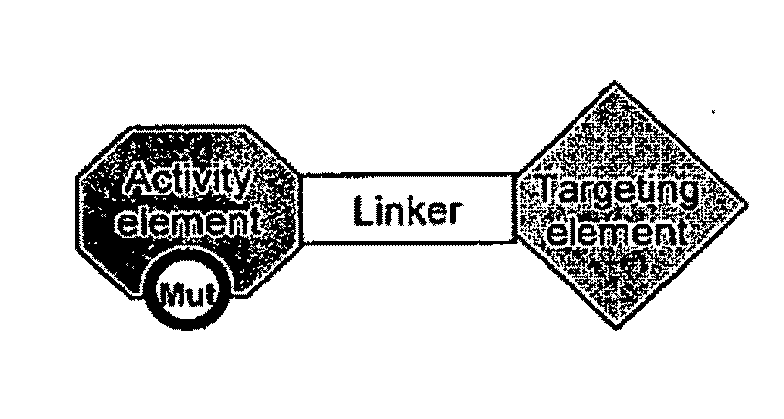
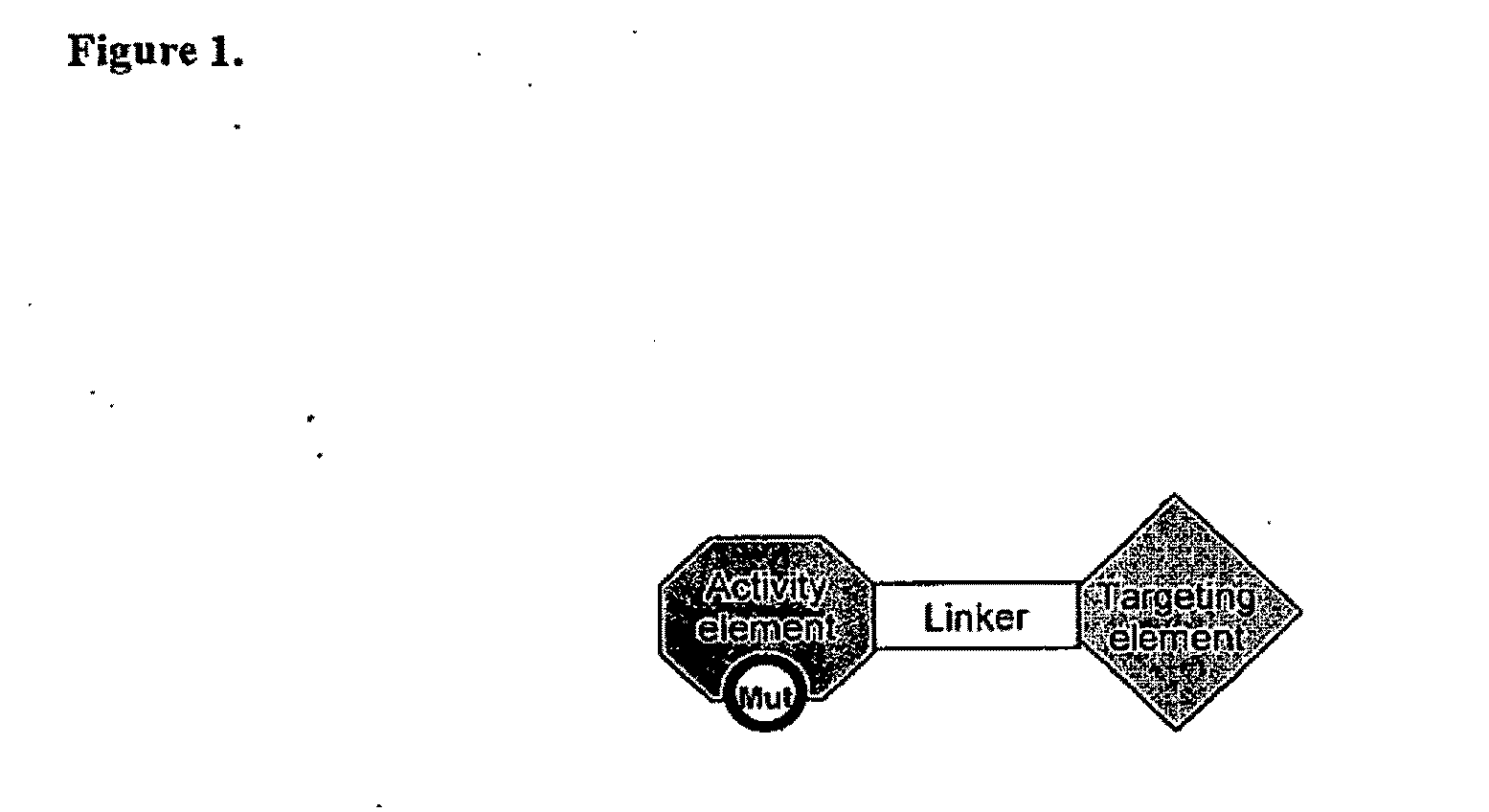
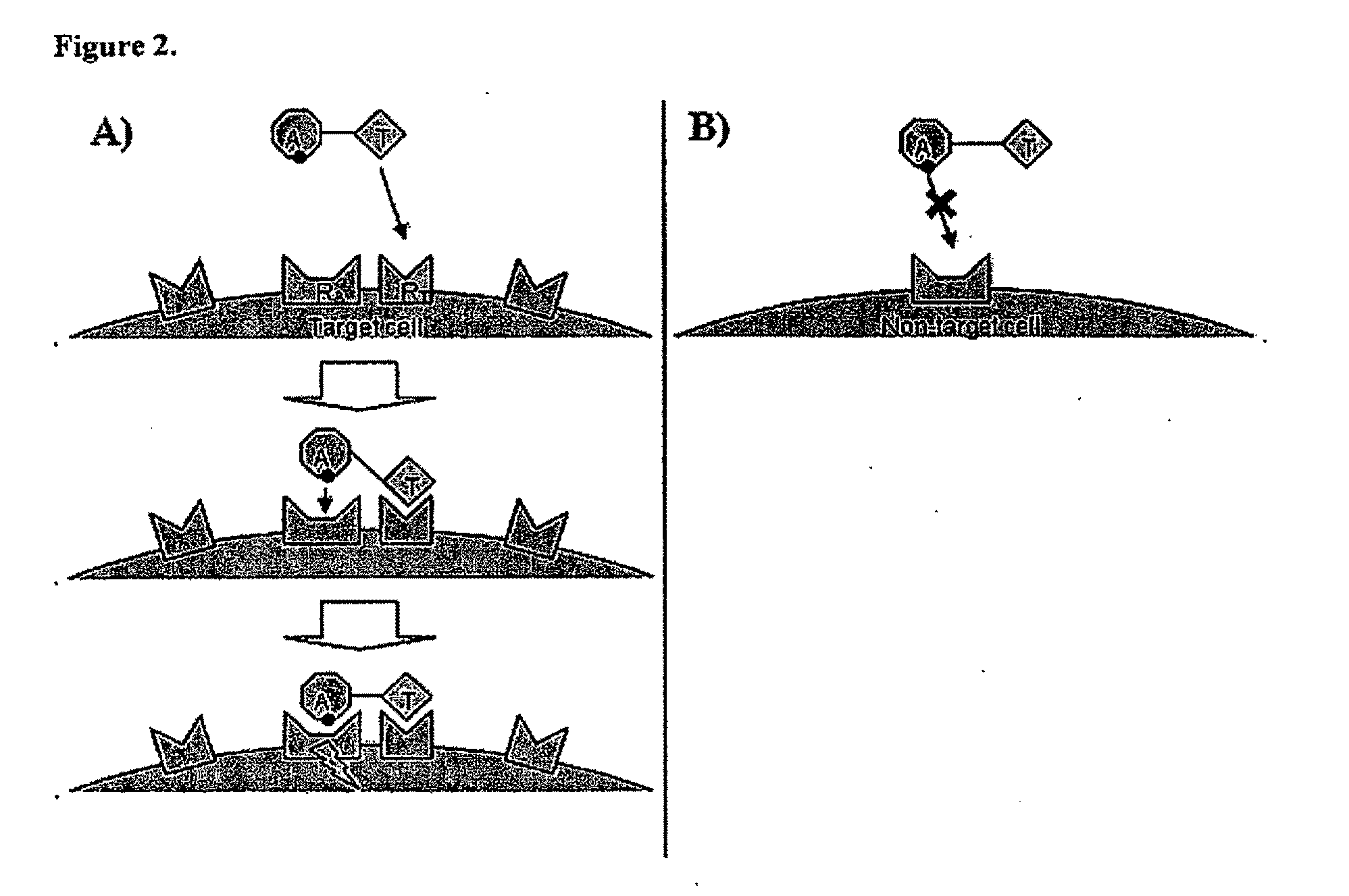
Aspects of the invention provide methods for harnessing the potential of proteins that occur naturally (e.g., in humans) and that have serious but finite toxicity. Aspects of the invention relate to a quantitative systems-biological and structural approach to design a class Mof chimeric proteins that avoid the toxicity of protein drugs while retaining their desired activities. In particular, chimeric proteins containing a variant form of a natural protein fused to a targeting moiety may be administered to a subject to target a signal (e.g., induction of apoptosis) to particular cells without having a generalized toxic effect
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Chimeric fibroblast growth factor 23 proteins and methods of use
ActiveUS20140243260A1Peptide/protein ingredientsAntibody mimetics/scaffoldsChimera ProteinFibroblast growth factor 23
The present invention relates to an isolated chimeric protein. The isolated chimeric protein includes an N-terminus coupled to a C-terminus, where the N-terminus includes an N-terminal portion from a fibroblast growth factor (“FGF”) 23 molecule and the C-terminus includes a C-terminal portion from an FGF19 molecule. The present invention also relates to a pharmaceutical composition including an isolated chimeric protein and a pharmaceutically acceptable carrier. The isolated chimeric protein includes an N-terminus coupled to a C-terminus, where the N-terminus includes an N-terminal portion from a fibroblast growth factor (“FGF”) 23 molecule and the C-terminus includes a C-terminal portion from an FGF19 molecule, and a pharmaceutically-acceptable carrier. Yet another aspect of the present invention relates to a method for treating a subject suffering from a disorder. This method includes selecting a subject suffering from the disorder and administering to the subject a therapeutically effective amount of a chimeric protein according to the present invention.
Owner:NEW YORK UNIV
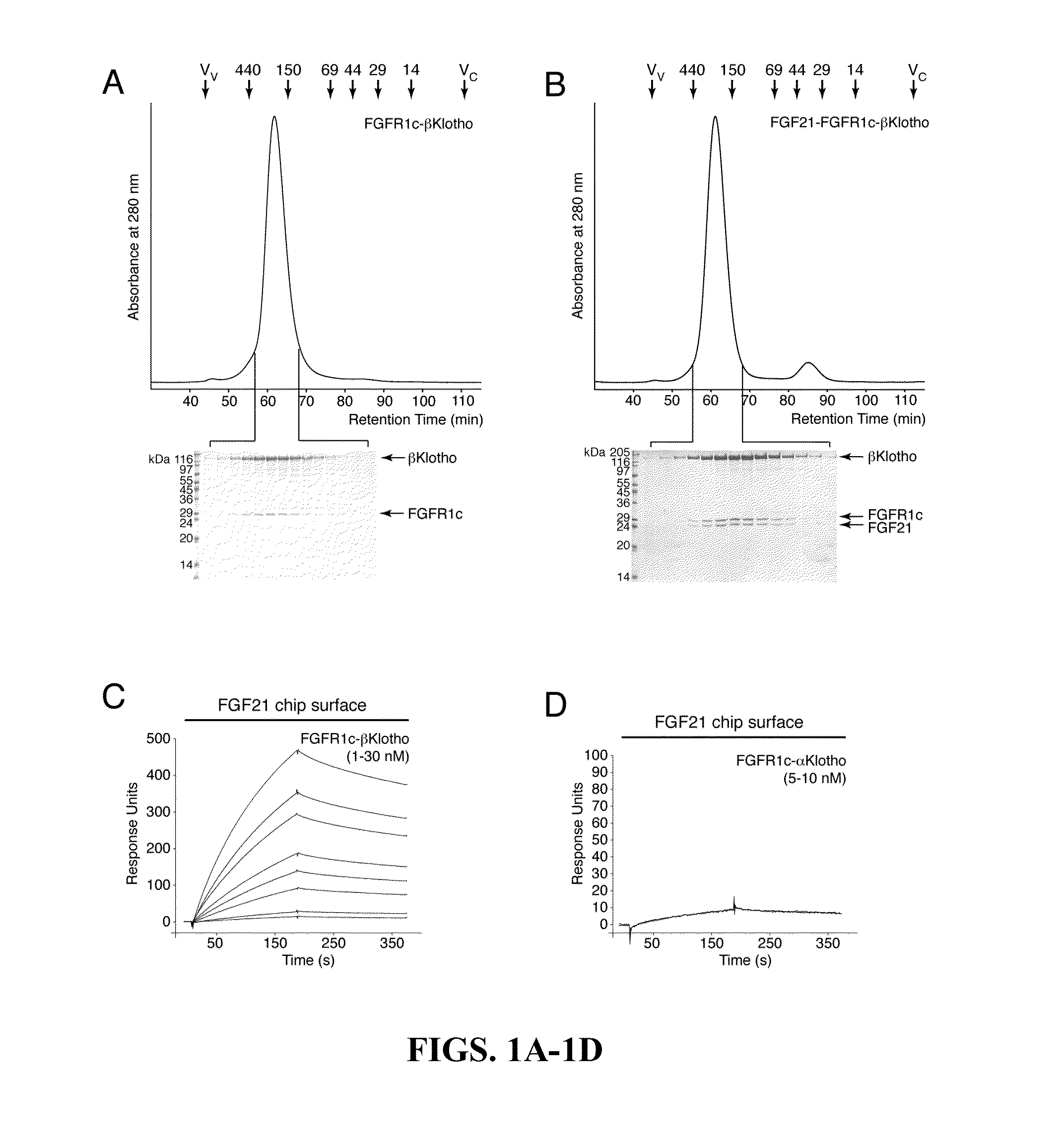
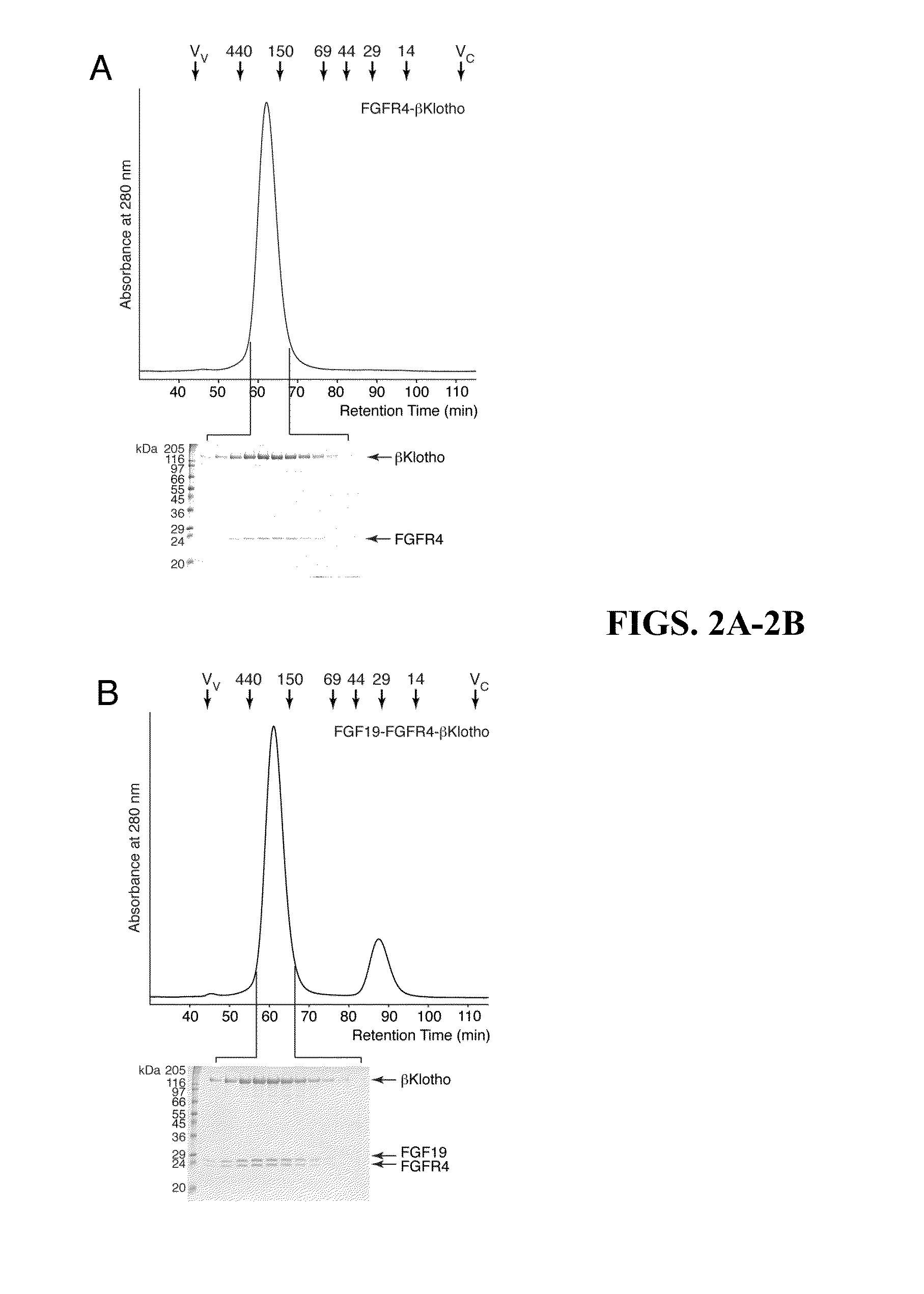
Chimeric fibroblast growth factor 19 proteins and methods of use
ActiveUS20130331317A1Enhanced endocrine activityLow affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsKlothoHeparin-DHE
The present invention relates to a chimeric protein that includes an N-terminus coupled to a C-terminus, where the N-terminus includes a portion of a paracrine fibroblast growth factor (“FGF”) and the C-terminus includes a C-terminal portion of an FGF19 molecule. The portion of the paracrine FGF is modified to decrease binding affinity for heparin and / or heparan sulfate compared to the portion without the modification. The present invention also relates to pharmaceutical compositions including chimeric proteins according to the present invention, methods for treating a subject suffering from diabetes, obesity, or metabolic syndrome, and methods of screening for compounds with enhanced binding affinity for the βKlotho-FGF receptor complex involving the use of chimeric proteins of the present invention.
Owner:SALK INST FOR BIOLOGICAL STUDIES +2
Novel chimeric proteins and methods for using the same
InactiveUS20030216546A1Compounds screening/testingPeptide/protein ingredientsDiseaseProtein insertion
Novel chimeric proteins are disclosed. The proteins comprise at least two portions. The first portion binds to a first cell and decreases the cell's ability to send a trans signal to a second cell; the second portion sends its own trans signal to the second cell. Methods for making and using these proteins in the treatment of cancer, viral infections, autoimmune and alloimmune diseases are also disclosed, as are pharmaceutical formulations comprising the novel chimeric proteins and genes. Either the proteins themselves or a genetic sequence encoding the protein can be administered. Other methods are also disclosed in which two molecular components result in decrement of a first trans signal from a first cell and the conferring of a second trans signal to a second cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Mutant ctla4 gene transfected t cell and composition including same for anticancer immunotherapy
ActiveUS20140242049A1Enhance luciferase activityMaximize the effectBiocideAntibody mimetics/scaffoldsSide effectCancer cell
A transformed T-cell for T-cell therapy, and a composition including the same for anticancer immunotherapy. More particularly, the transformed T-cell is characterized by the transfection of a gene for coding a chimera protein. The T-cell, to which the gene for coding the chimera protein is transected, may improve the therapeutic effects induced by immune tolerance of cancer cells, and furthermore maximize anti-cancer effects by activating signal transduction to induce the activation of T-cells. Also, the disclosure allows treatments that minimize side effects such as the development of autoimmune diseases due to systematic T-cell activation.
Owner:NAT CANCER CENT
Immunization and/or treatment of parasites and infectious agents by live bacteria
ActiveUS8771669B1Reducing eliminatingReducing or eliminating the targeted parasite, infectious diseaseVirusesBacteriaLytic peptideHuntingtons chorea
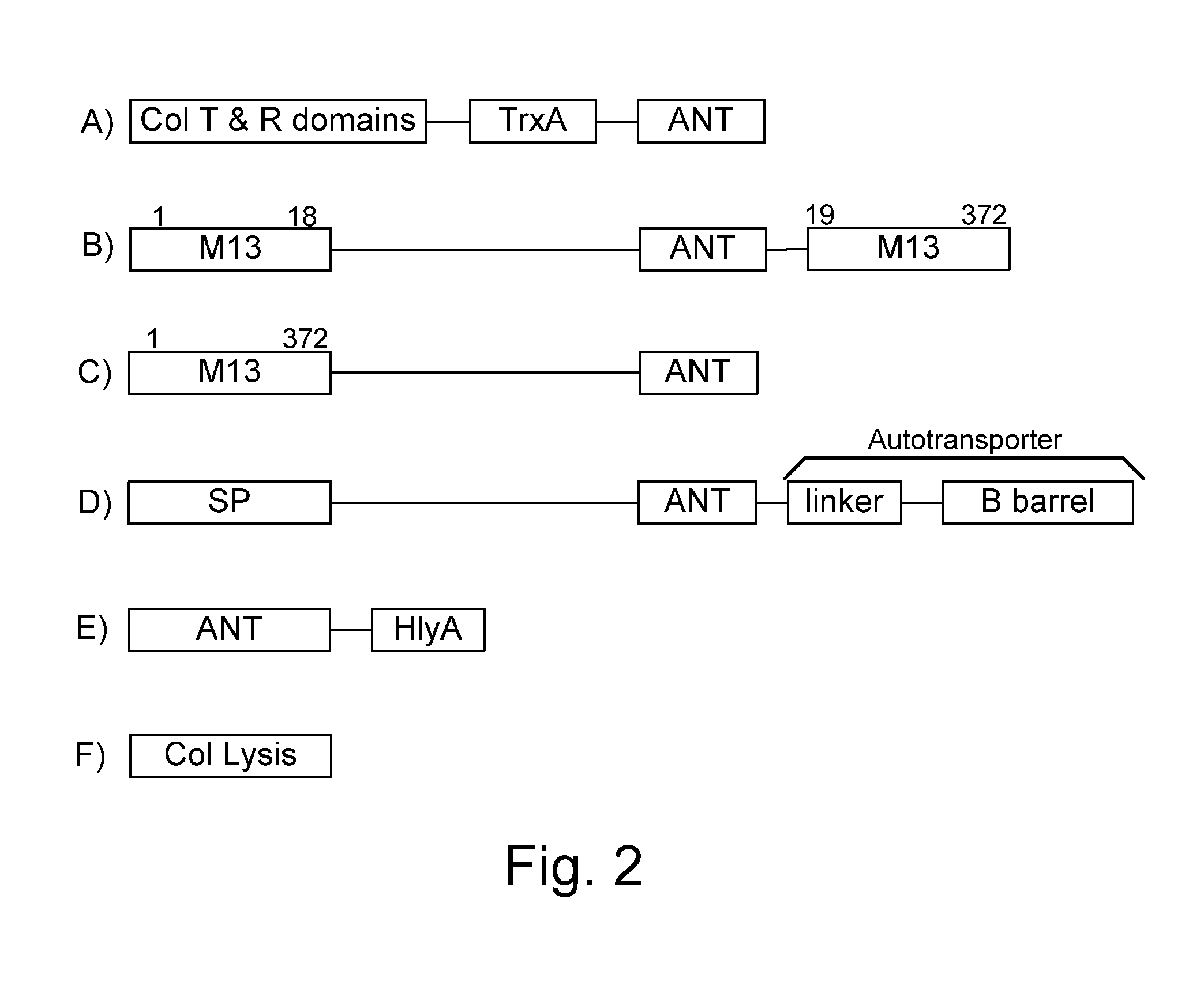
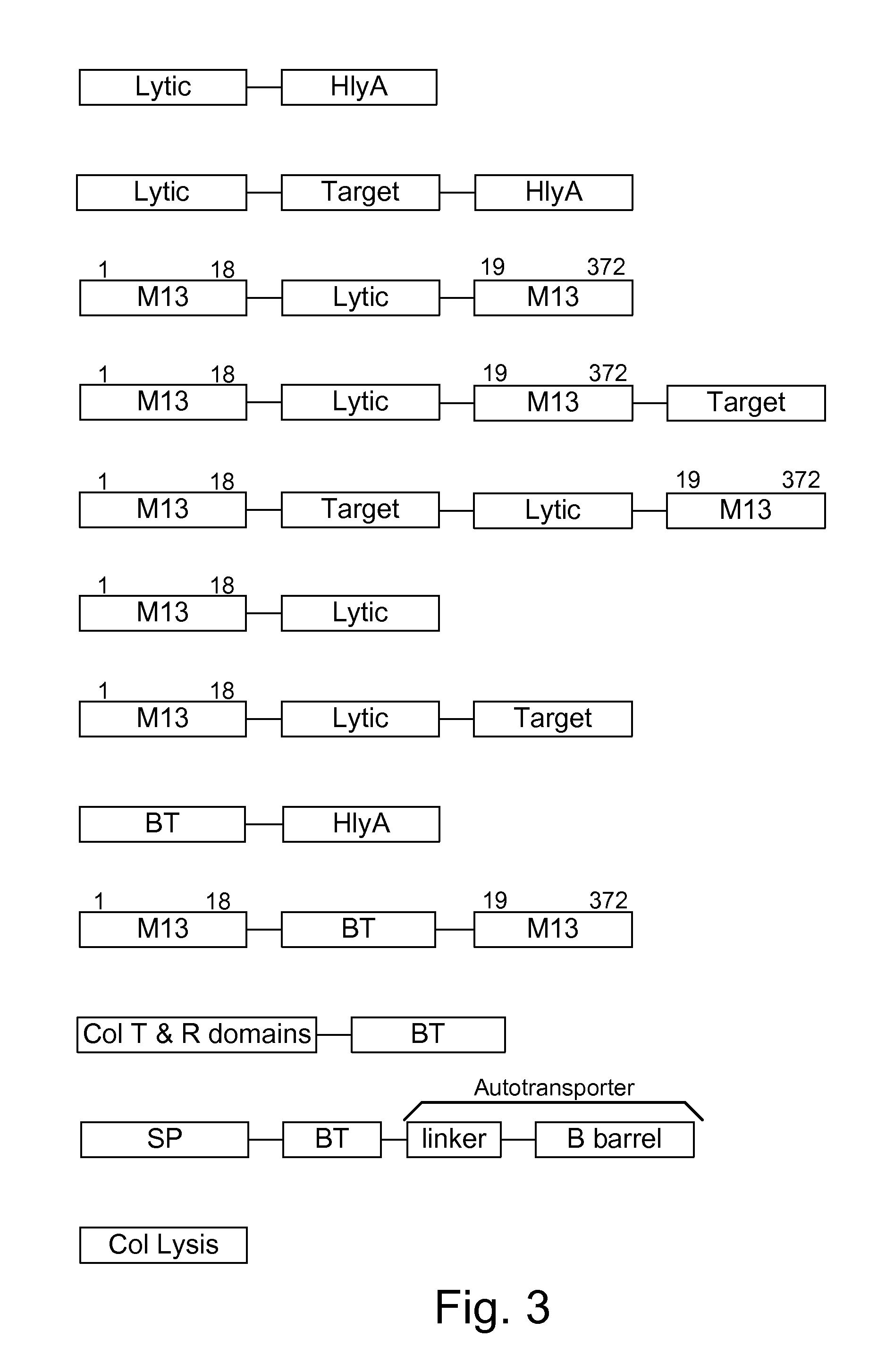
Chimeric proteins are expressed, secreted or released by a bacterium to immunize against or treat a parasite, infectious disease or malignancy. The delivery vector may also be attenuated, non-pathogenic, low pathogenic, or a probiotic bacterium. The chimeric proteins include chimeras of, e.g., phage coat and / or colicin proteins, bacterial toxins and / or enzymes, autotransporter peptides, lytic peptides, multimerization domains, and / or membrane transducing (ferry) peptides. The active portion of the immunogenic chimeric proteins can include antigens against a wide range of parasites and infectious agents, cancers, Alzheimer's and Huntington's diseases, and have enhanced activity when secreted or released by the bacteria, and / or have direct anti-parasite or infectious agent activity. The activity of the secreted proteins is further increased by co-expression of a protease inhibitor that prevents degradation of the effector peptides. Addition of an antibody binding or antibody-degrading protein further prevents the premature elimination of the vector and enhances the immune response.
Owner:BERMUDES DAVID G DR
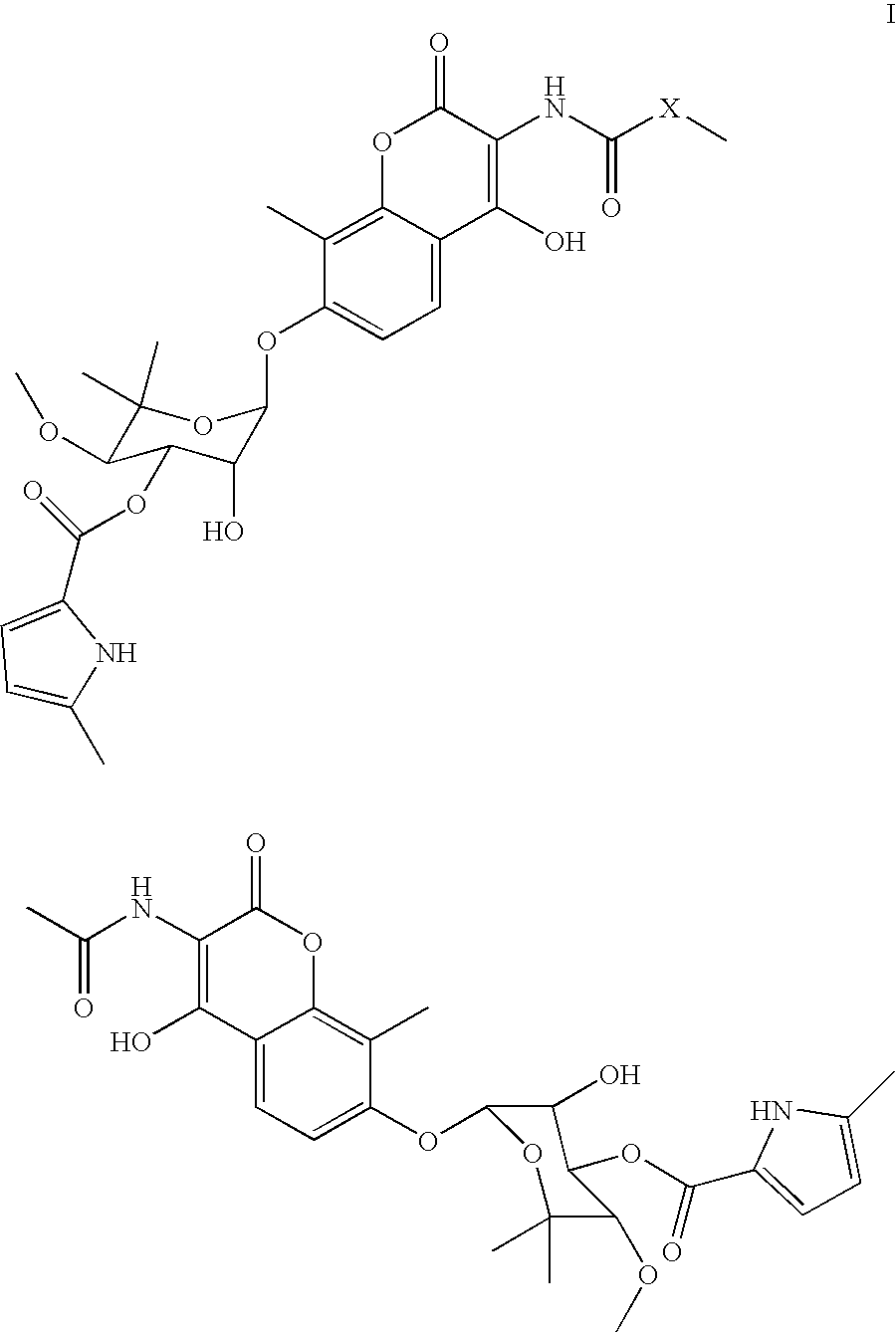
Coumermycin analogs as chemical dimerizers of chimeric proteins
Coumermycin analogs of general formula I: wherein X, a linking group, is selected from the group consisting of alkyl, aryl, diaryl, substituted alkyl, substituted aryl, alkyl with hereroatoms in the chain, heteroaryl, cyclic and bicyclic alkyl, and a combination of alkyl, aryl and heteroaryl substituents. The compounds are suitable for use as chemical dimerizers of chimeric proteins.
Owner:MERCK & CO INC
Transcription regulatory gene and peptide
ActiveUS20050183169A1Simple structurePotent capacity for repressing transcription of genePeptide/protein ingredientsImmunoglobulinsTranscription Regulation GeneMetabolic enzymes
This invention relates to a peptide or protein capable of converting a transcription factor into a transcriptional repressor, a gene encoding such peptide or protein, a chimeric protein in which the aforementioned peptide or protein is fused to a transcription factor, a chimeric gene in which the gene encoding a peptide or protein is fused to a gene encoding a transcription factor, a recombinant vector having such chimeric gene, and a transformant comprising such recombinant vector. The peptide of the invention that is capable of converting a transcription factor into a transcriptional repressor is very short. Thus, it can be very easily synthesized, and it can effectively and selectively repress the transcription of a specific gene. Accordingly, such gene is applicable to and useful in a wide variety of fields, such as repression of the expression of cancerous genes and regulation of the expression of genes encoding pigment-metabolic enzymes.
Owner:GREENSOGNA +1
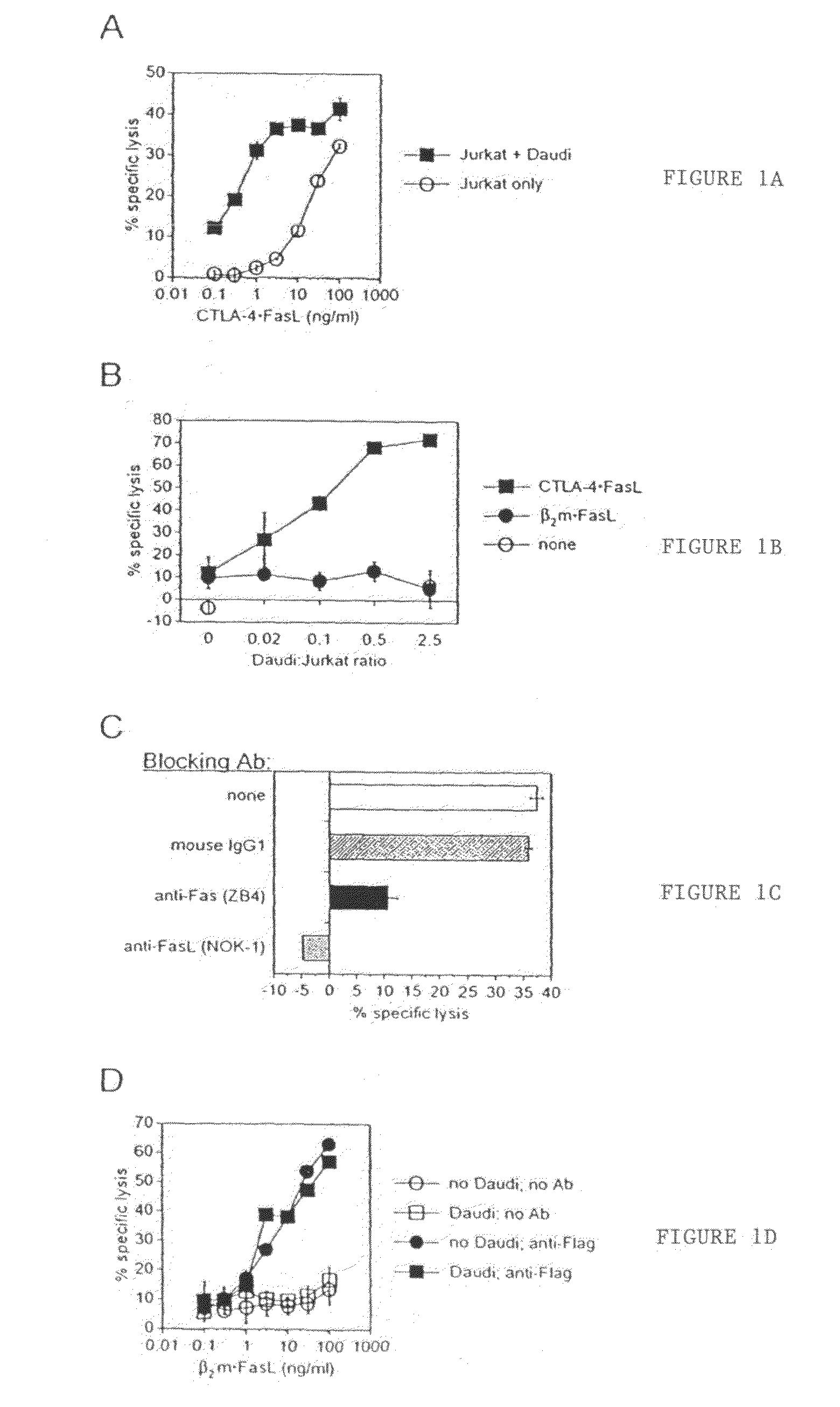
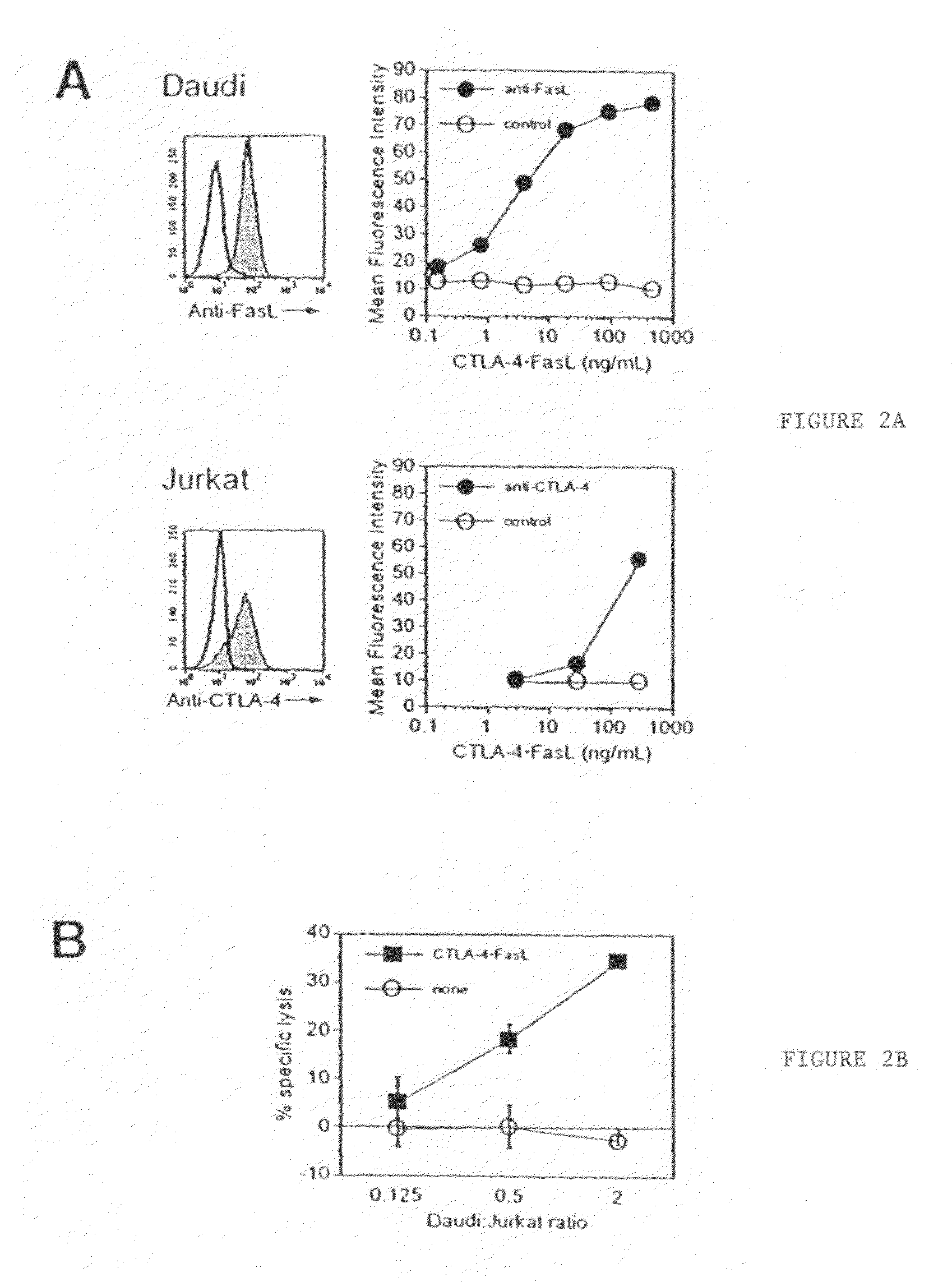
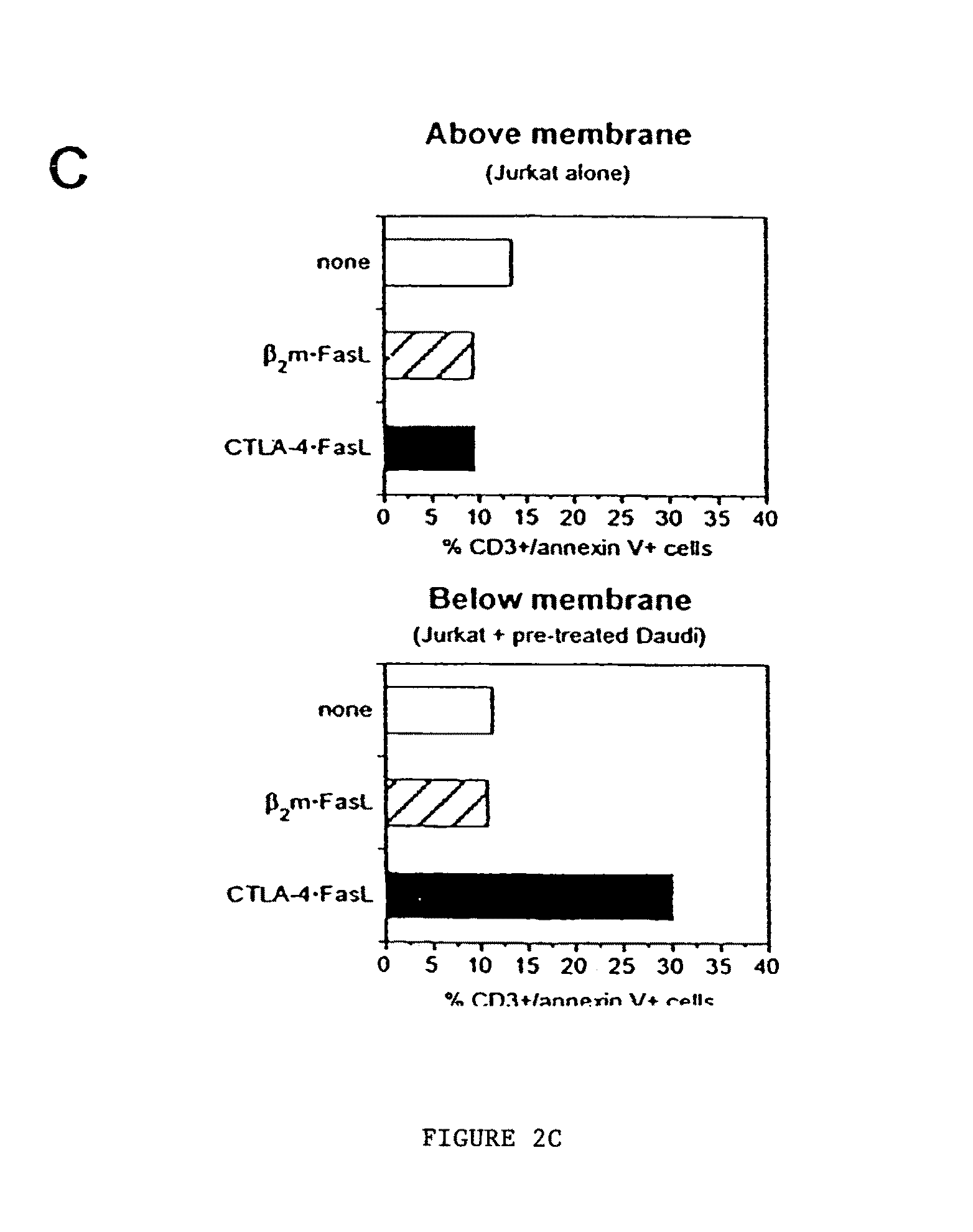
Chimeric proteins with cell-targeting specificity and apoptosis-inducing activities
InactiveUS20020090374A1Peptide/protein ingredientsAntibody mimetics/scaffoldsApoptosisInterleukin II
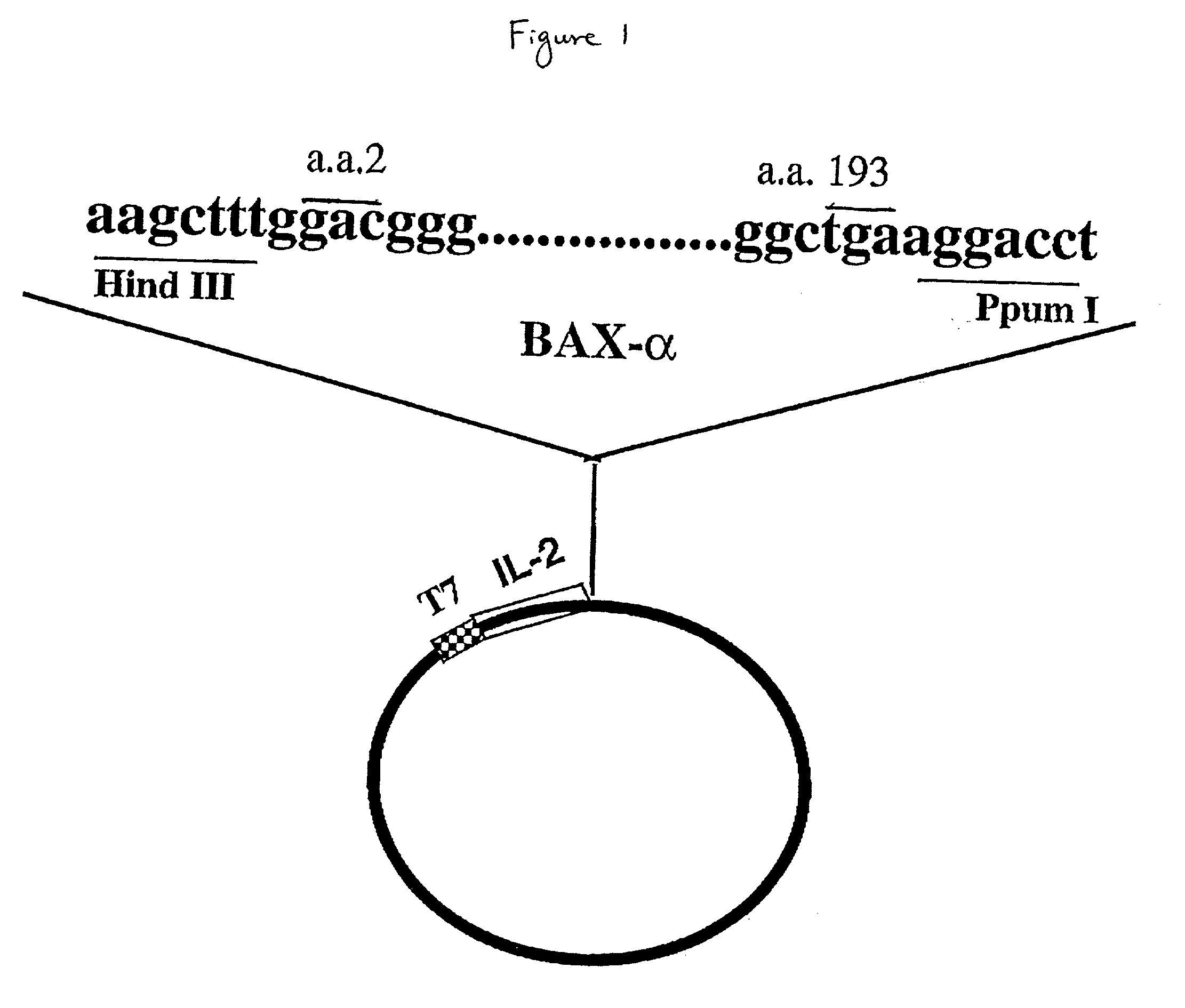
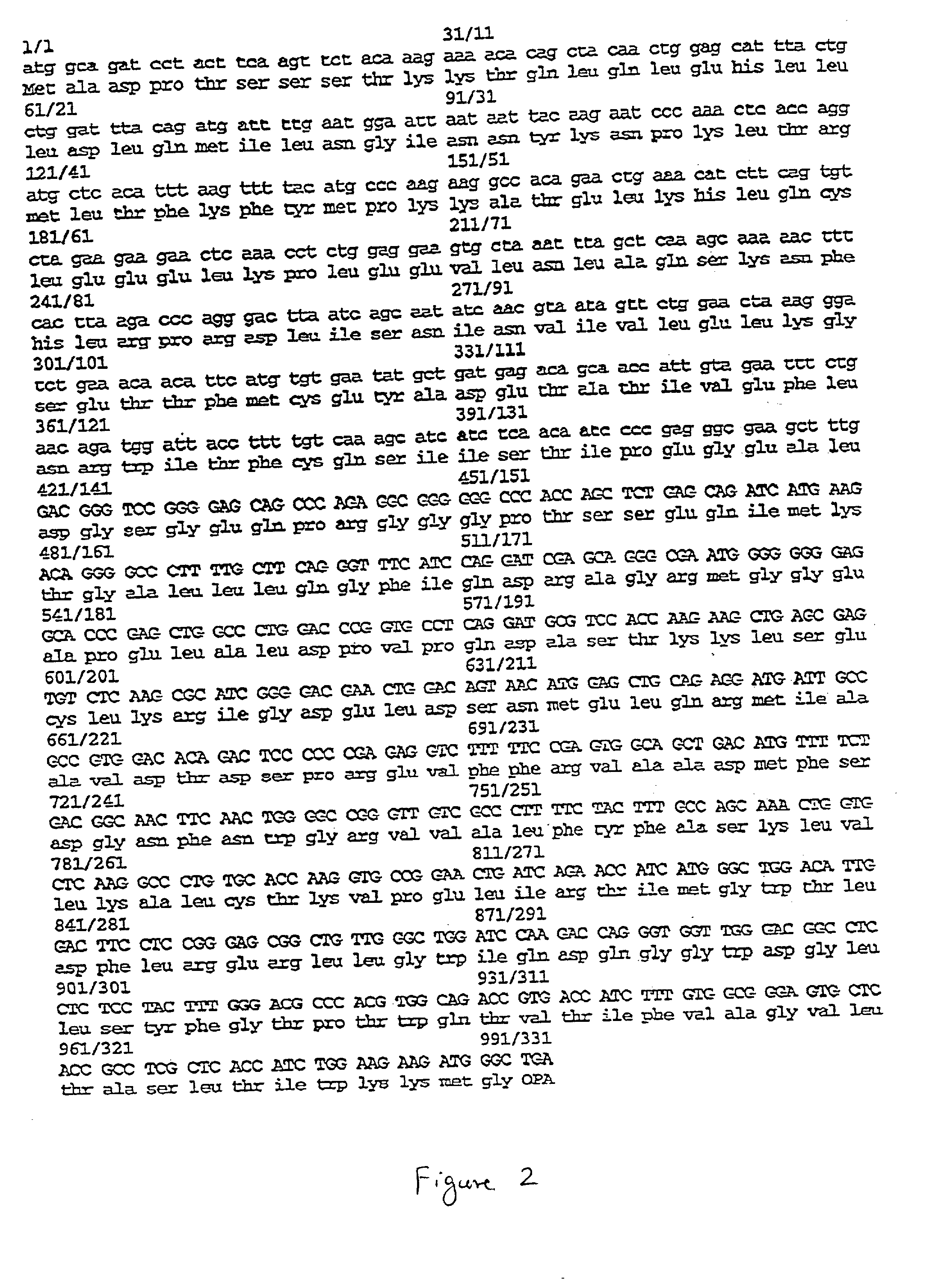
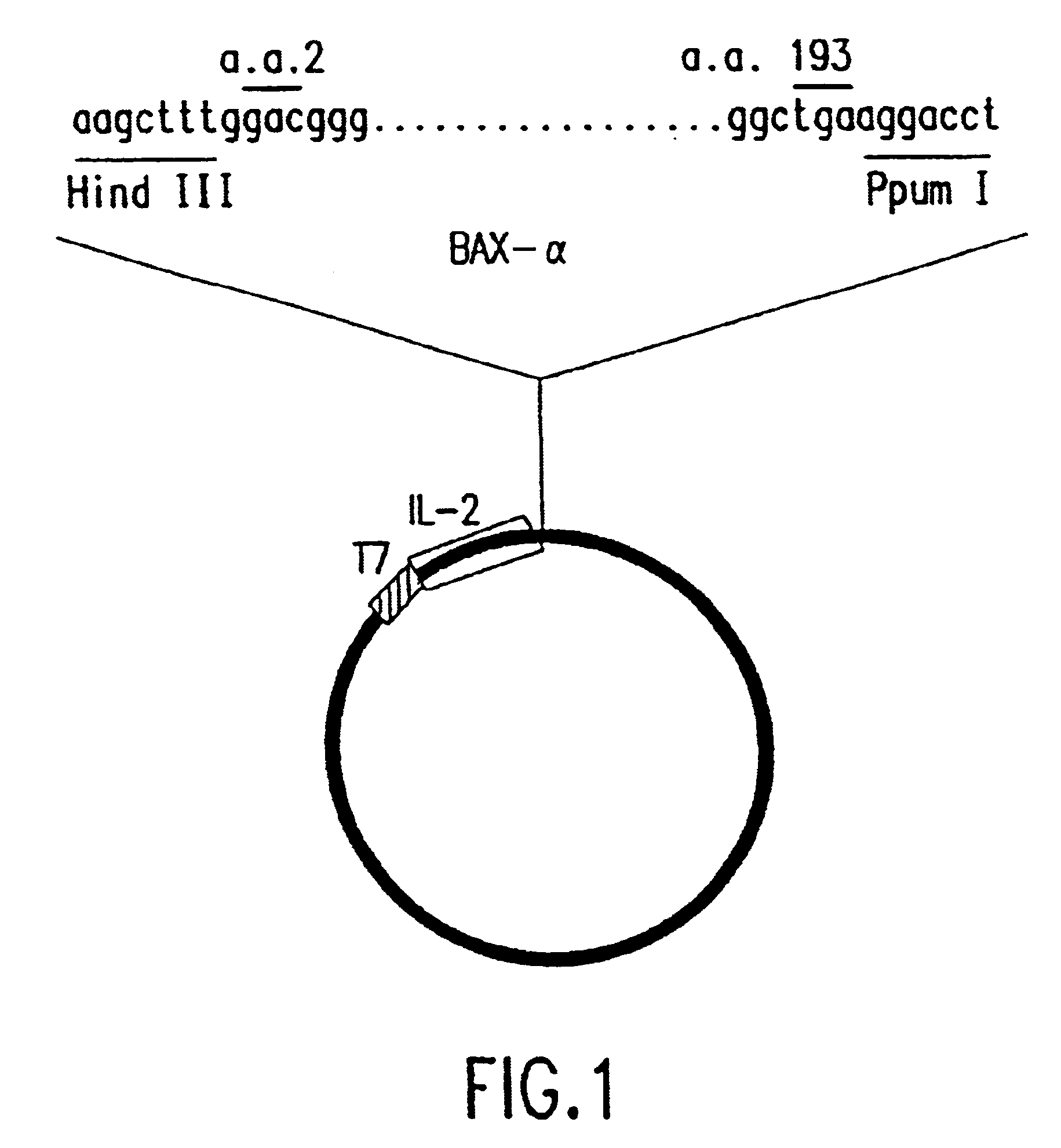
The present invention relates to chimeric proteins with cell-targeting specificity and apoptosis-inducing activities. In particular, the invention is illustrated by a recombinant chimeric protein between human interleukin-2 (IL2) and Bax. The chimeric protein specifically targets IL2 receptor (IL2R)-expressing cells and induces cell-specific apoptosis.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Gene and peptide for transcriptional repressor
Owner:GREENSOGNA +1
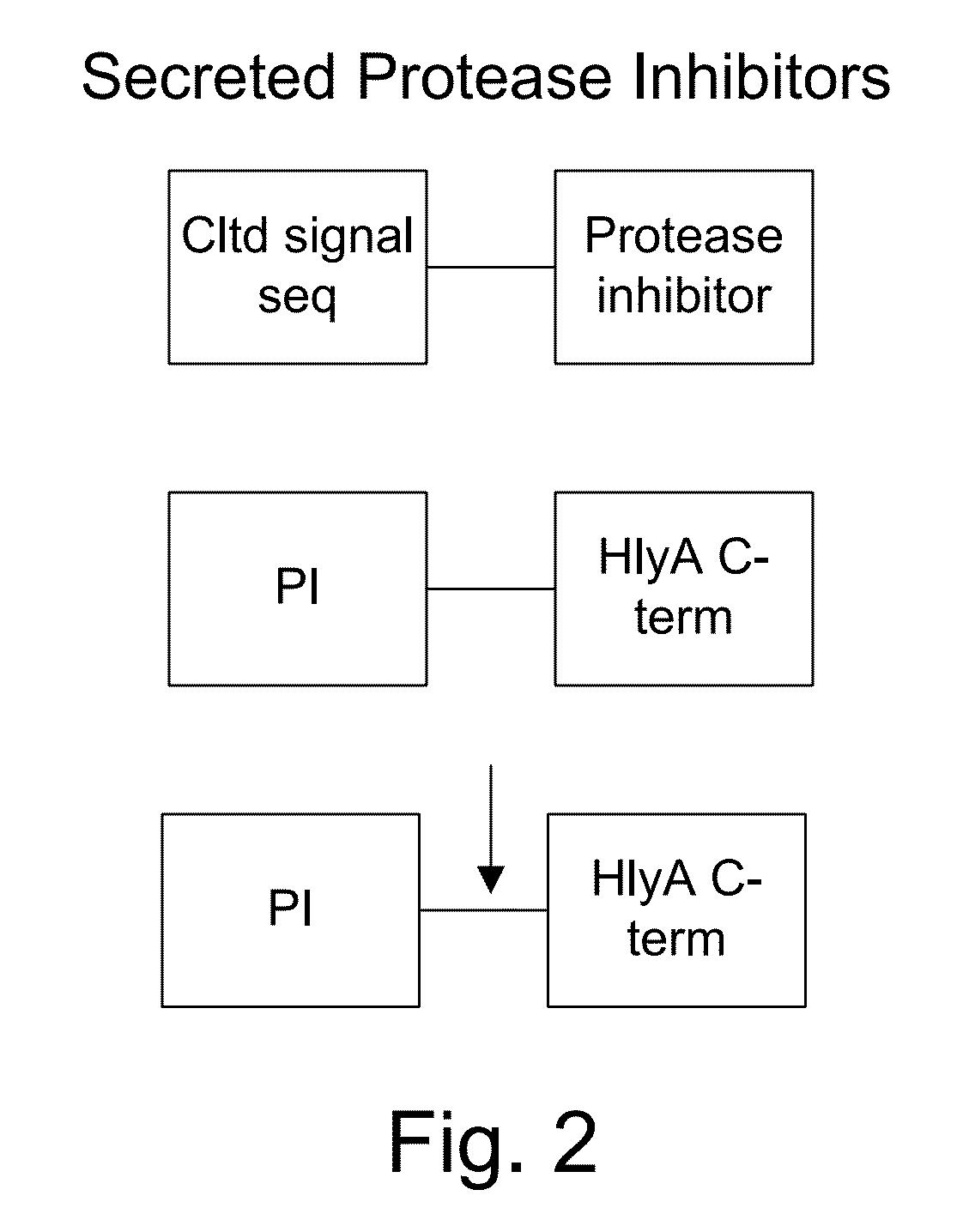
Protease inhibitor: protease sensitivity expression system and method improving the therapeutic activity and specificity of proteins and phage and phagemids delivered by bacteria
The present invention uses co-expression of protease inhibitors and protease sensitive therapeutic agents that results in their localized production within the target tissue and inactivation outside of the target tissue, thereby increasing therapeutic activity and reducing the systemic toxicity. Inactivation is also accomplished by engineering protease degradation sites within the therapeutic construct for proteases, preferably those that are under-expressed within the target tissue yet present in non-target tissues within the body, resulting in therapeutic activity within the target tissue and inactivation outside of the target tissue. Novel chimeric proteins secreted by bacteria are also described. The chimeric proteins include chimeric toxins targeted to neoplastic cells and cells of the immune system. Novel combination therapies of these protease inhibitor:chimeric toxin-expressing bacteria together with small-molecule and biologic agents are also described. Non-conjugative bacteria capable of delivering phage / phagemids expression cassettes for DNA and RNA-based therapeutics are also described.
Owner:BERMUDES DAVID GORDON
Adeno-associated-virus rep sequences, vectors and viruses
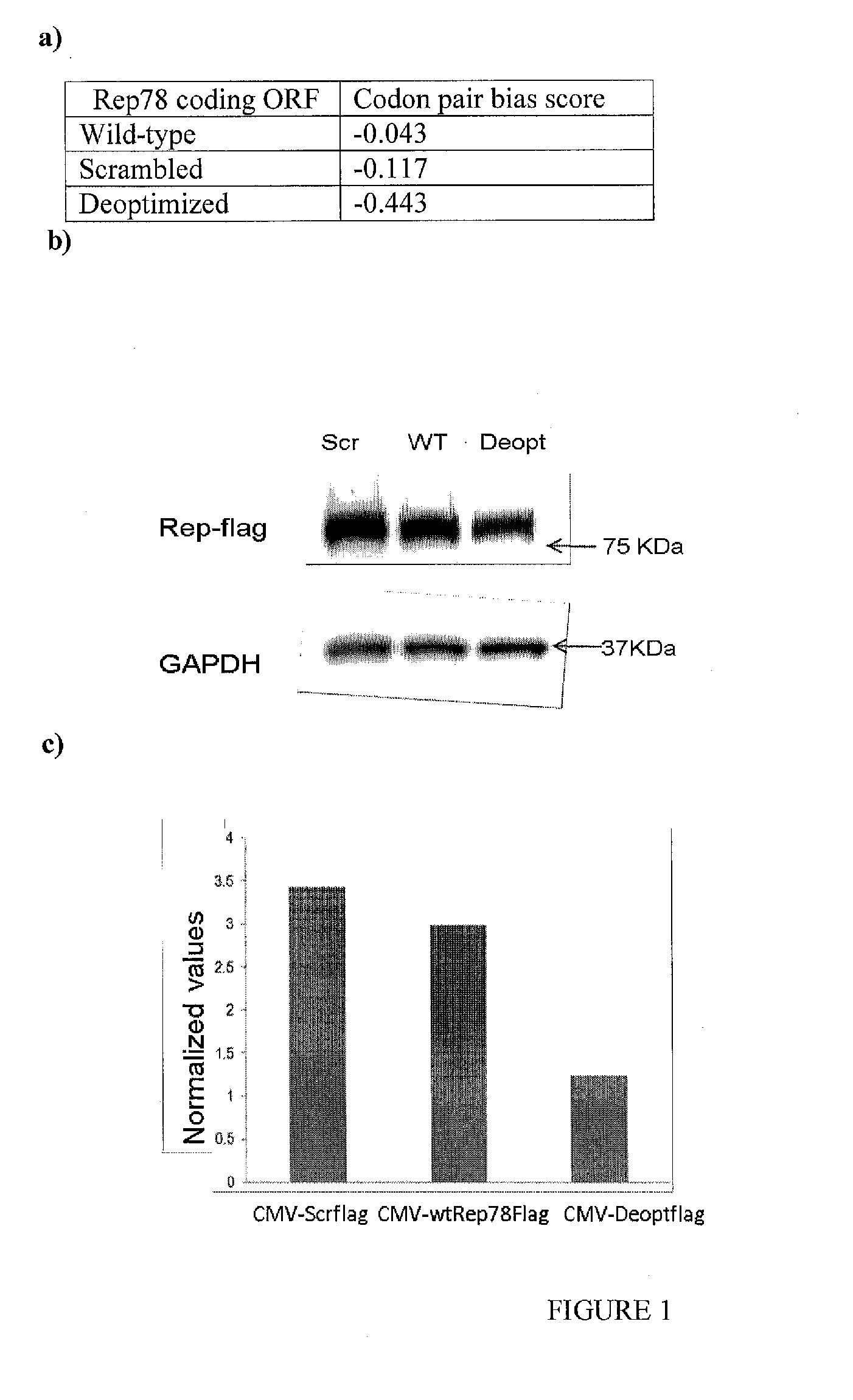
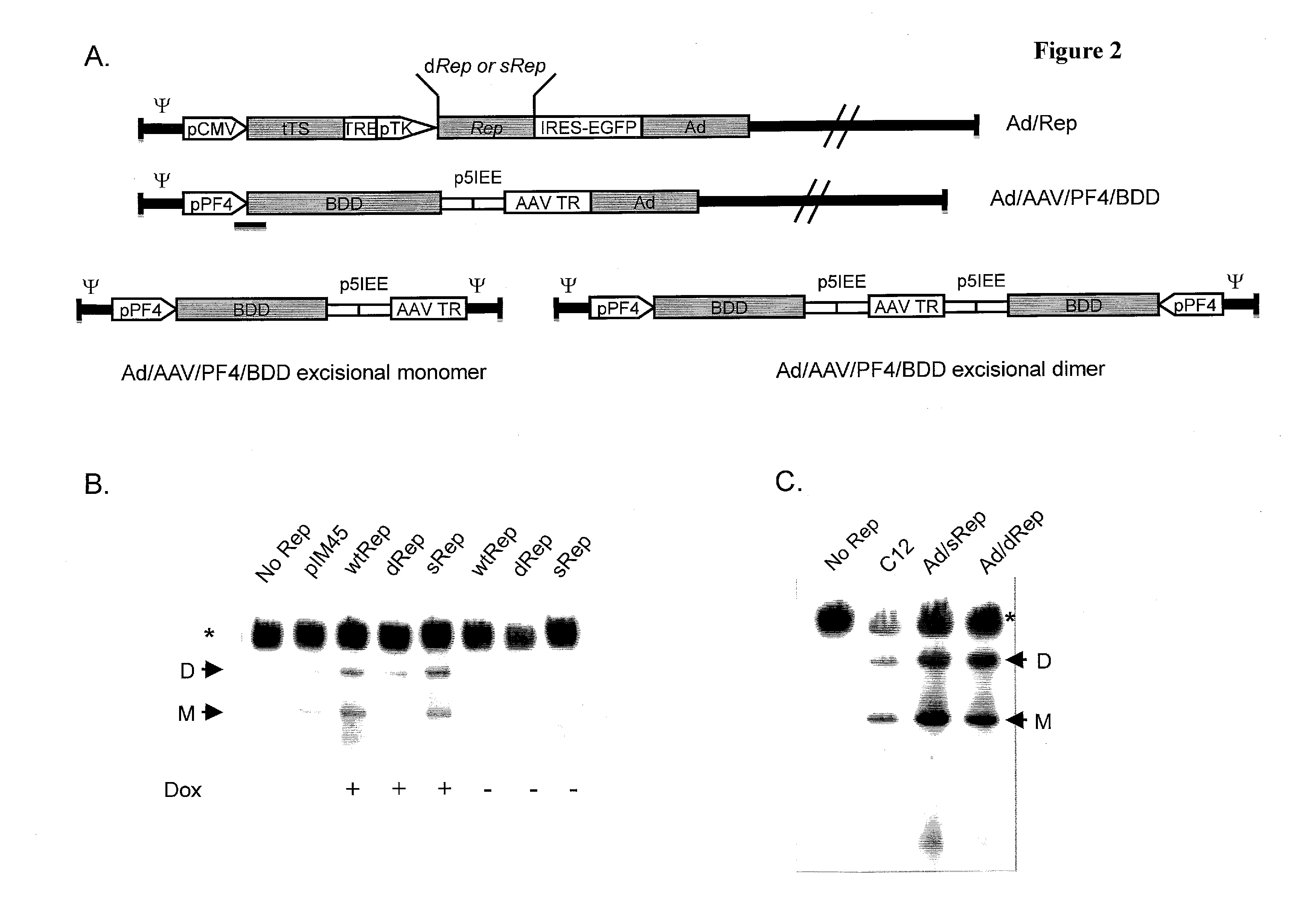
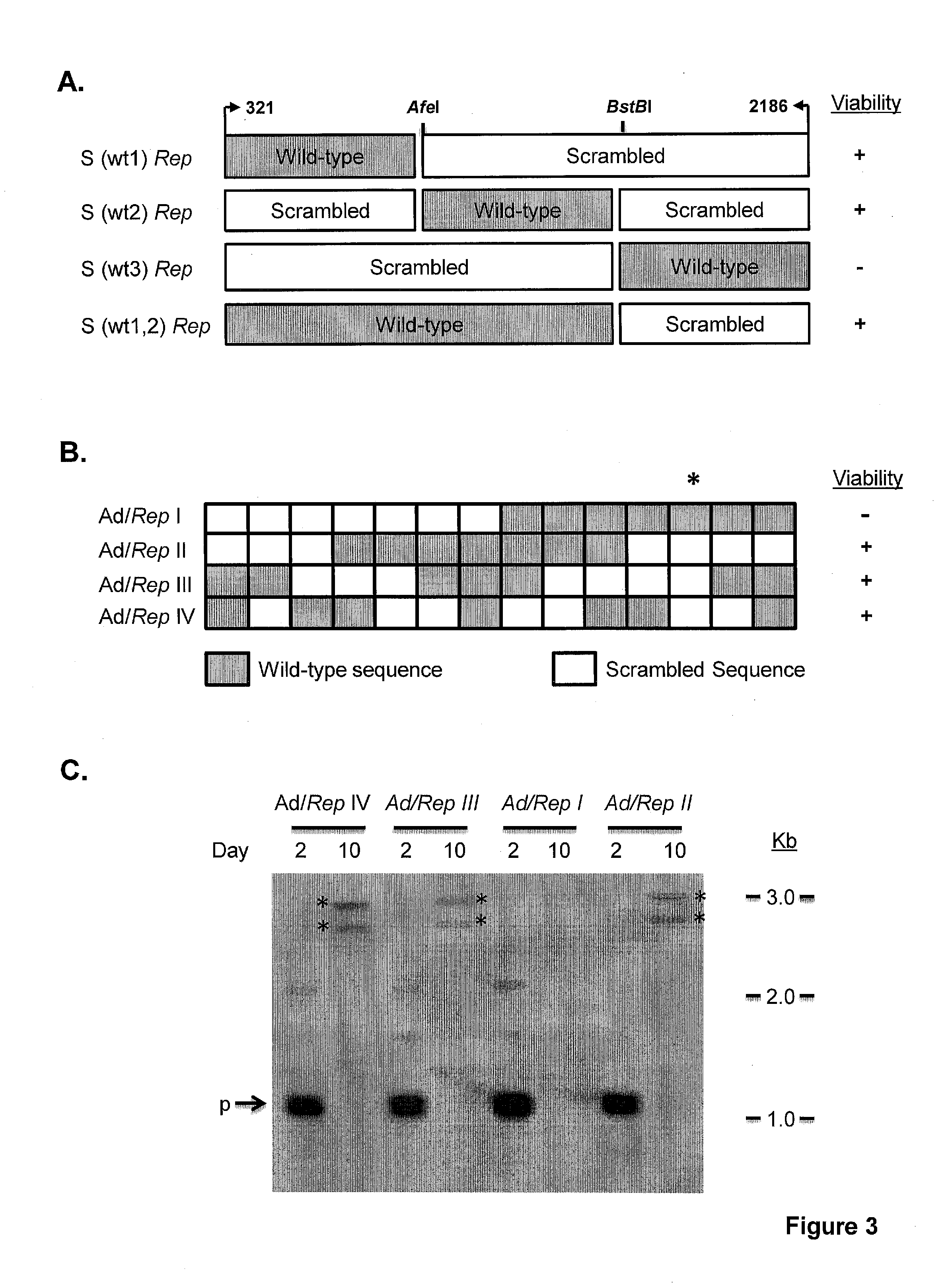
The invention provides adeno-associated virus (AAV) replication (Rep) sequences. In one embodiment, the invention provides nucleotide sequences encoding a chimeric protein, wherein the encoded chimeric protein contains a wild type AAV Rep inhibitory amino acid sequence, and wherein the nucleotide sequences contain a scrambled and / or deoptimized polynucleotide sequence encoding the wild type AAV Rep inhibitory amino acid sequence. The invention provides vectors, cells, and viruses containing the invention's sequences. Also provided are methods for detecting portions of the AAV Rep inhibitory amino acid sequence, which reduce replication and / or infection and / or productive infection by viruses. The invention's compositions and methods are useful for site-specific integration and / or expression of heterologous sequences by recombinant adeno-associated virus (rAAV) vectors and by rAAV virus particles, such as hybrid viruses (e.g., Ad-AAV) comprising such vectors. The invention's compositions and methods find application in, for example, gene therapy and / or vaccines.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Chimeric protein
InactiveUS20060153827A1Avoid developmentInhibit growthHydrolasesPeptide/protein ingredientsNeural cellChimera Protein
A chimeric protein is disclosed for promoting repair and regeneration of neurons damaged by disease or physical injury wherein the chimeric protein is a combination of a first polypeptide possessing matrix modification activity and a second polypeptide possessing regenerating activity for neural cells.
Owner:ACORDA THERAPEUTICS INC
Fc chimeric proteins with anti-HIV drugs
InactiveUS20050281829A1Improve stabilityStable HIV chimeric proteinsPeptide/protein ingredientsAntibody mimetics/scaffoldsAntiviral drugImmunoglobulin Fc Fragments
The invention relates to anti viral agents comprised of viral fusion inhibitors and at least a portion of an immunoglobulin constant region. The invention further relates to anti viral agents comprised HIV viral fusion inhibitors and an Fc fragment of an immunoglobulin. The invention also relates to methods of treating a viral infection, including HIV infection.
Owner:HEHIR CRISTINA +5
CLOTTING FACTOR-Fc CHIMERIC PROTEINS TO TREAT HEMOPHILIA
ActiveUS20110182896A1Reduce riskEffective treatmentPeptide/protein ingredientsHydrolasesHemostatic DisordersImmunoglobulin IgE
Owner:BIOVERATIV THERAPEUTICS INC
Chimeric proteins with cell-targeting specificity and apoptosis-inducing activities
The present invention relates to chimeric proteins with cell-targeting specificity and apoptosis-inducing activities. In particular, the invention is illustrated by a recombinant chimeric protein between human interleukin-2 (IL2) and Bax. The chimeric protein specifically targets IL2 receptor (IL2R)-expressing cells and induces cell-specific apoptosis.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Genetically engineered clostridial genes, proteins encoded by the engineered genes, and uses thereof
ActiveUS20100196421A1Optimization propertiesOptimized therapeuticAntibacterial agentsSenses disorderEngineered geneticGenetically engineered
The present invention relates to an isolated Clostridial neurotoxin propeptide having a light chain region, a heavy chain region, where the light and heavy chain regions are linked by a disulfide bond, and an intermediate region connecting the light and heavy chain regions. An isolated nucleic acid molecule encoding a Clostridial neurotoxin propeptide is also disclosed. Also disclosed is an isolated, physiologically active Clostridial neurotoxin produced by cleaving a Clostridial neurotoxin propeptide, a vaccine or antidote thereof, and methods of immunizing against or treating for toxic effects of Clostridial neurotoxins. Methods of expressing recombinant physiologically active Clostridial neurotoxins are also disclosed. Also disclosed is a chimeric protein having a heavy chain region of a Clostridial neurotoxin and a protein with therapeutic functionality. A treatment method is also disclosed.
Owner:NEW YORK UNIV
Novel Chimeric Proteins and Methods for Using The Same
InactiveUS20130243697A1Enhanced advantageImprove propertiesCompounds screening/testingPeptide/protein ingredientsTrans signalingDisease
Novel chimeric proteins are disclosed. The proteins comprise at least two portions. The first portion binds to a first cell and decreases the cell's ability to send a trans signal to a second cell; the second portion sends its own trans signal to the second cell. Methods for making and using these proteins in the treatment of cancer, viral infections, autoimmune and alloimmune diseases are also disclosed, as are pharmaceutical formulations comprising the novel chimeric proteins and genes. Either the proteins themselves or a genetic sequence encoding the protein can be administered. Other methods are also disclosed in which two molecular components result in decrement of a first trans signal from a first cell and the conferring of a second trans signal to a second cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Polyvalent Chimeric OspC Vaccinogen and Diagnostic Antigen
A chimeric polyvalent recombinant protein for use as a vaccine and diagnostic for Lyme disease is provided. The chimeric protein comprises epitopes of the loop 5 region and / or the alpha helix 5 region of outer surface protein C (OspC) types. The OspC types may be associated with mammalian Borrelia infections.
Owner:VIRGINIA COMMONWEALTH UNIV
Chimeric MHC protein and oligomer thereof for specific targeting
InactiveUS20050074848A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsCoiled coil proteinOligomer
An oligomeric Major Histocompatibility Complex (MHC) complex comprising at least two chimeric proteins, said chimeric proteins comprising a first section derived from an MHC peptide chain or a functional part thereof and a second section comprising an oligomerising domain derived from an oligomer-forming coiled-coil protein, said complex further comprising attaching means for selectively attaching said MHC complex to a target cell, wherein formation of the oligomeric MHC complex occurs by oligomerisation at the oligomerising domain of the chimeric proteins, and wherein at least two of the first sections are derived from the same MHC peptide chain. In one embodiment, the complex further includes peptide bound to the MHC portions of the complex in the groove formed by the MHC α1 and α2 domains for class I complexes or the MHC α1 and β1 domains for class II complexes. Related processes for amplifying T-cells, chimeric proteins, vectors, recombinant expression cassettes, pharmaceutical compositions and processes for making pharmaceutical compositions are also disclosed.
Owner:PROIMMUNE
Targeted heterologous antigen presentation on calicivirus virus-like particles
Owner:TAKEDA VACCINES INC
Uses of labeled hsp90 inhibitors
The disclosure provides evidence that the abundance of this particular “oncogenic HSP90” species, which is not dictated by HSP90 expression alone, predicts for sensitivity to HSP90 inhibition therapy, and thus is a biomarker for HSP90 therapy. The disclosure also provides evidence that identifying and measuring the abundance of this oncogenic HSP90 species in tumors predicts of response to HSP90 therapy. “Oncogenic HSP90” is defined herein as the HSP90 fraction that represents a cell stress specific form of chaperone complex, that is expanded and constitutively maintained in the tumor cell context, and that may execute functions necessary to maintain the malignant phenotype. Such roles are not only to regulate the folding of overexpressed (i.e. HER2), mutated (i.e. mB-Raf) or chimeric proteins (i.e. Bcr-Abl), but also to facilitate scaffolding and complex formation of molecules involved in aberrantly activated signaling complexes (i.e. STATS, BCL6).
Owner:SLOAN KETTERING INST FOR CANCER RES +1
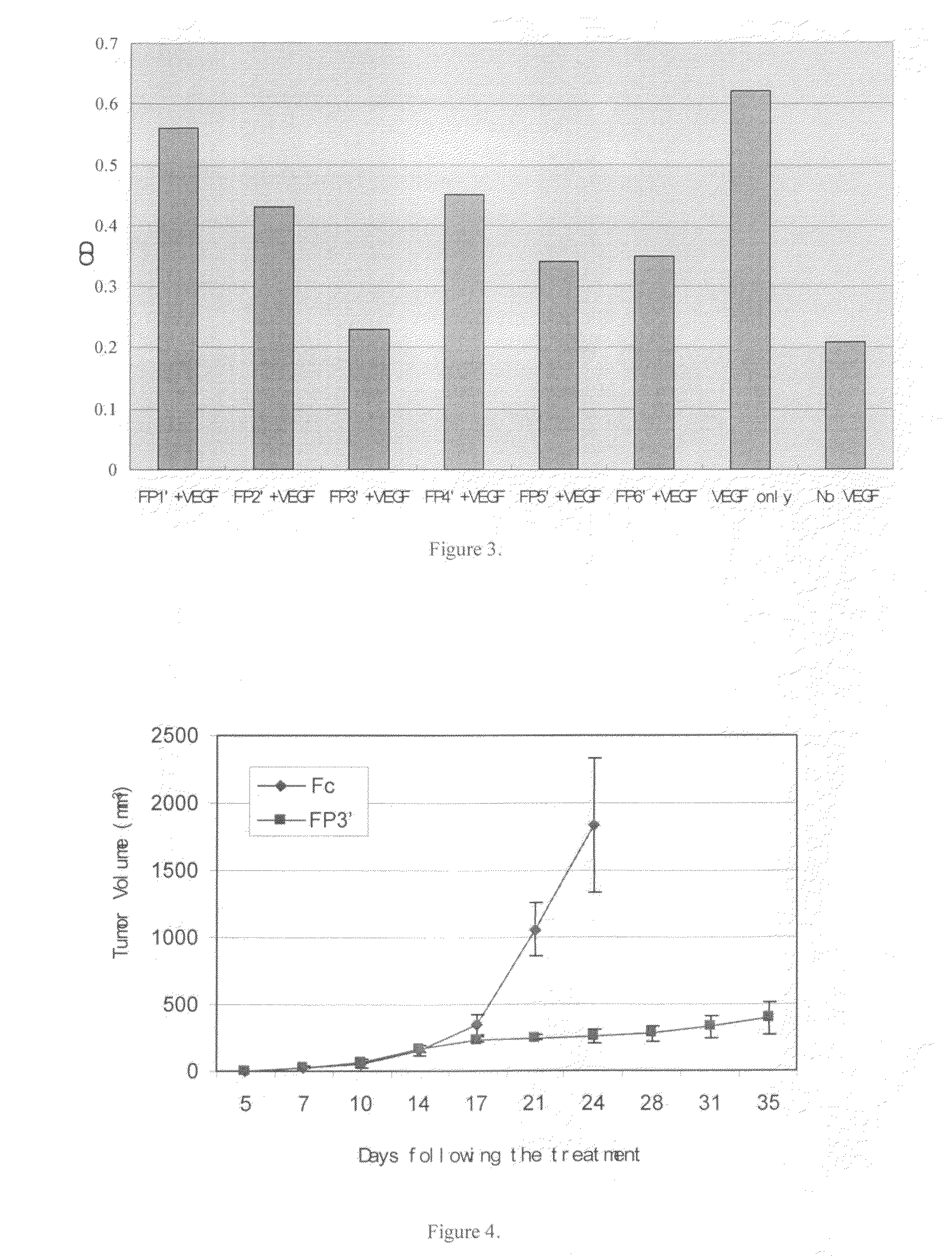
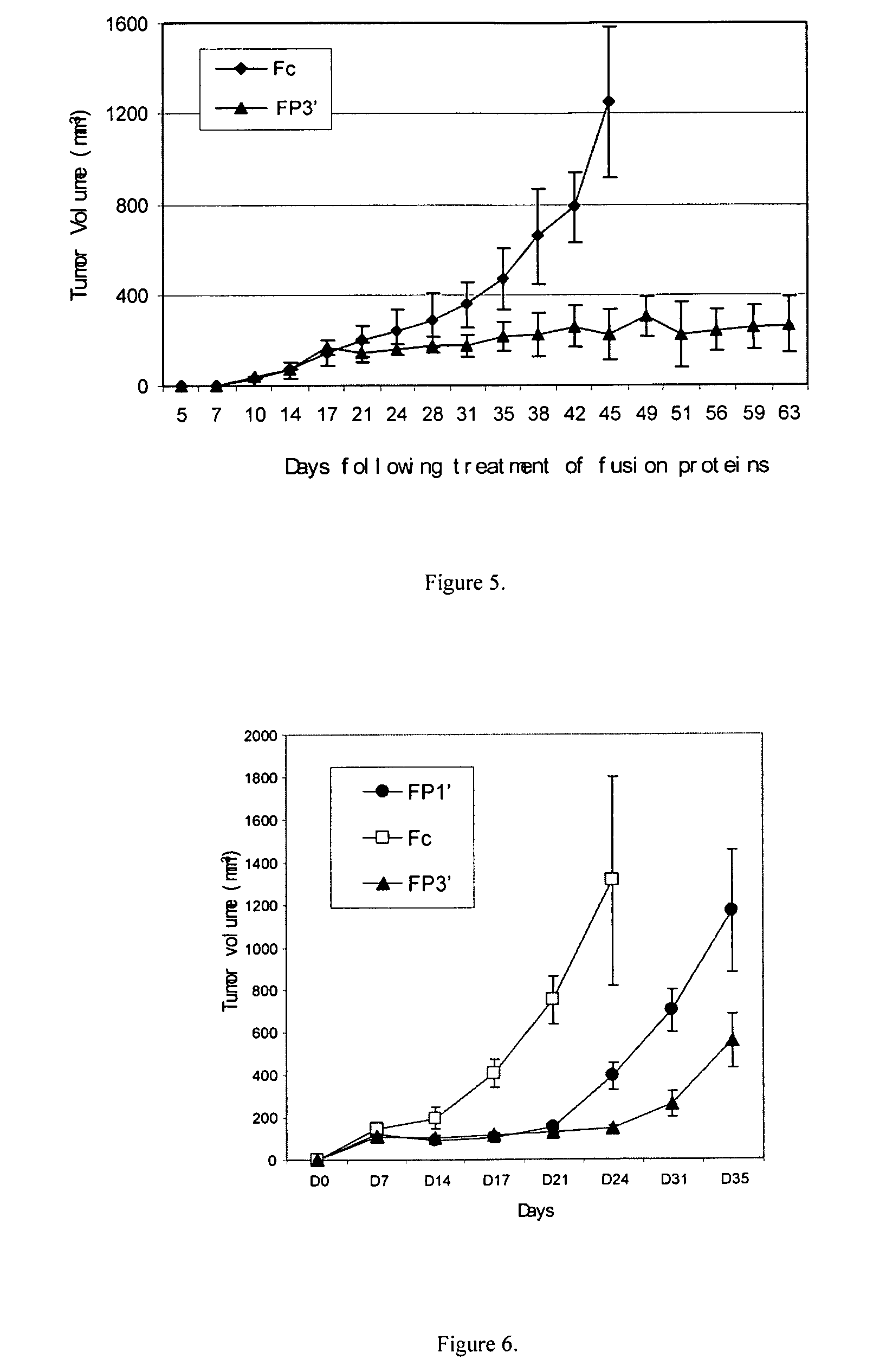
Angiogenesis-inhibiting chimeric protein and the use
ActiveUS7750138B2Increase binding siteHigh affinitySenses disorderPeptide/protein ingredientsAngiogenesis growth factorChimera Protein
Owner:CHENGDU KANGHONG BIOTECH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com