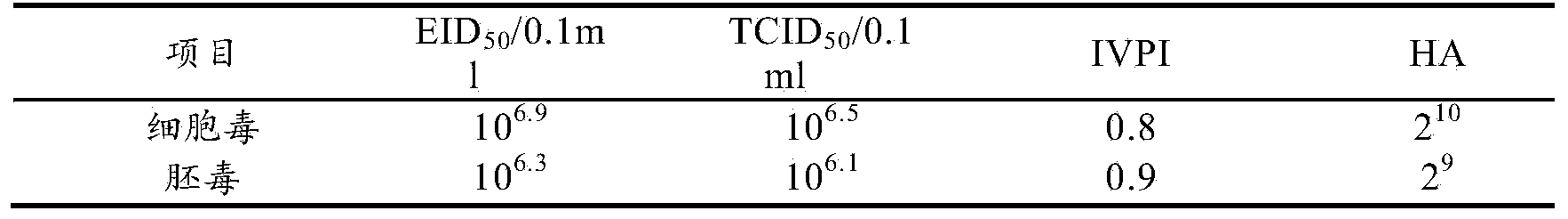
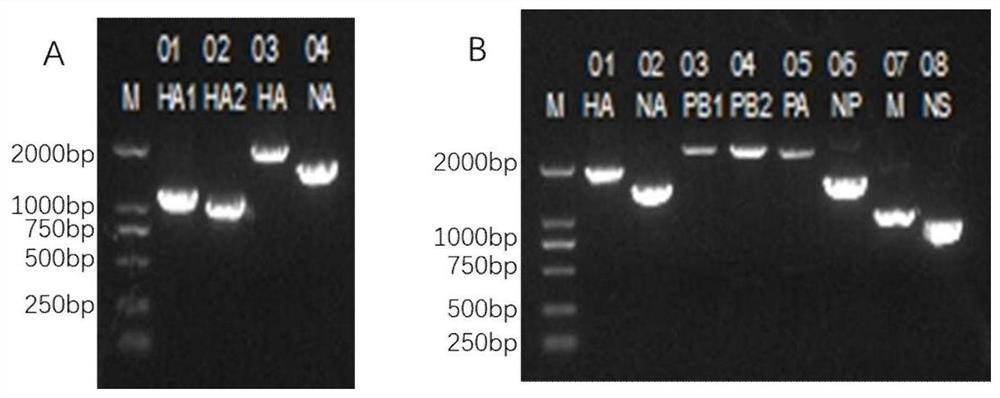
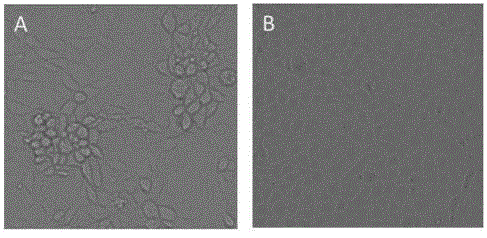
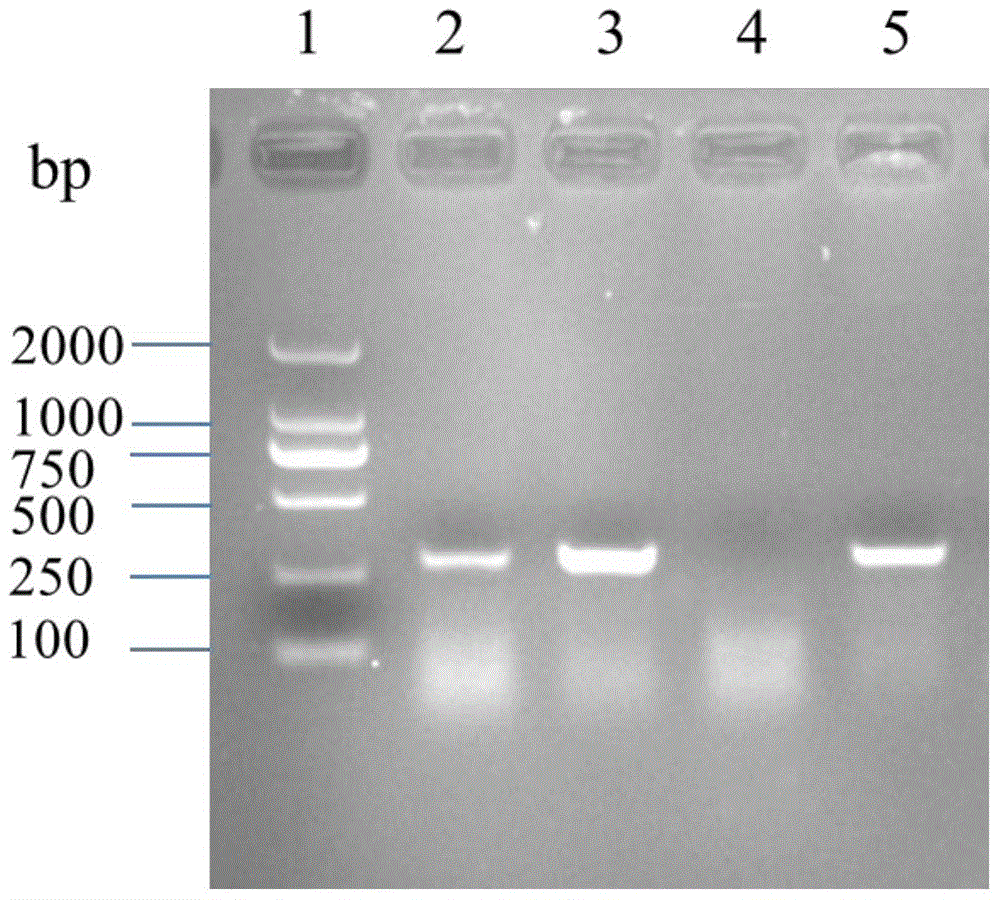
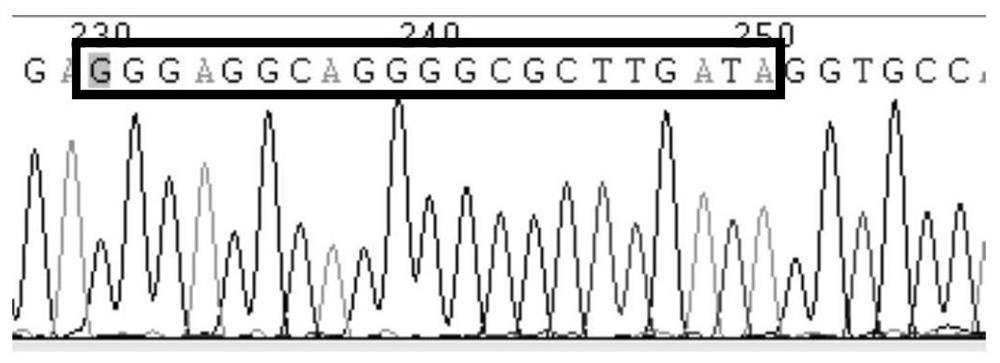
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Low pathogenic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Low Pathogenic Avian Influenza (LPAI) is classified as a low risk disease, and therefore organizations such as the Organization International des Epizooties (OIE), an international body that classifies and regulates animal disease, does not require it to be reported.
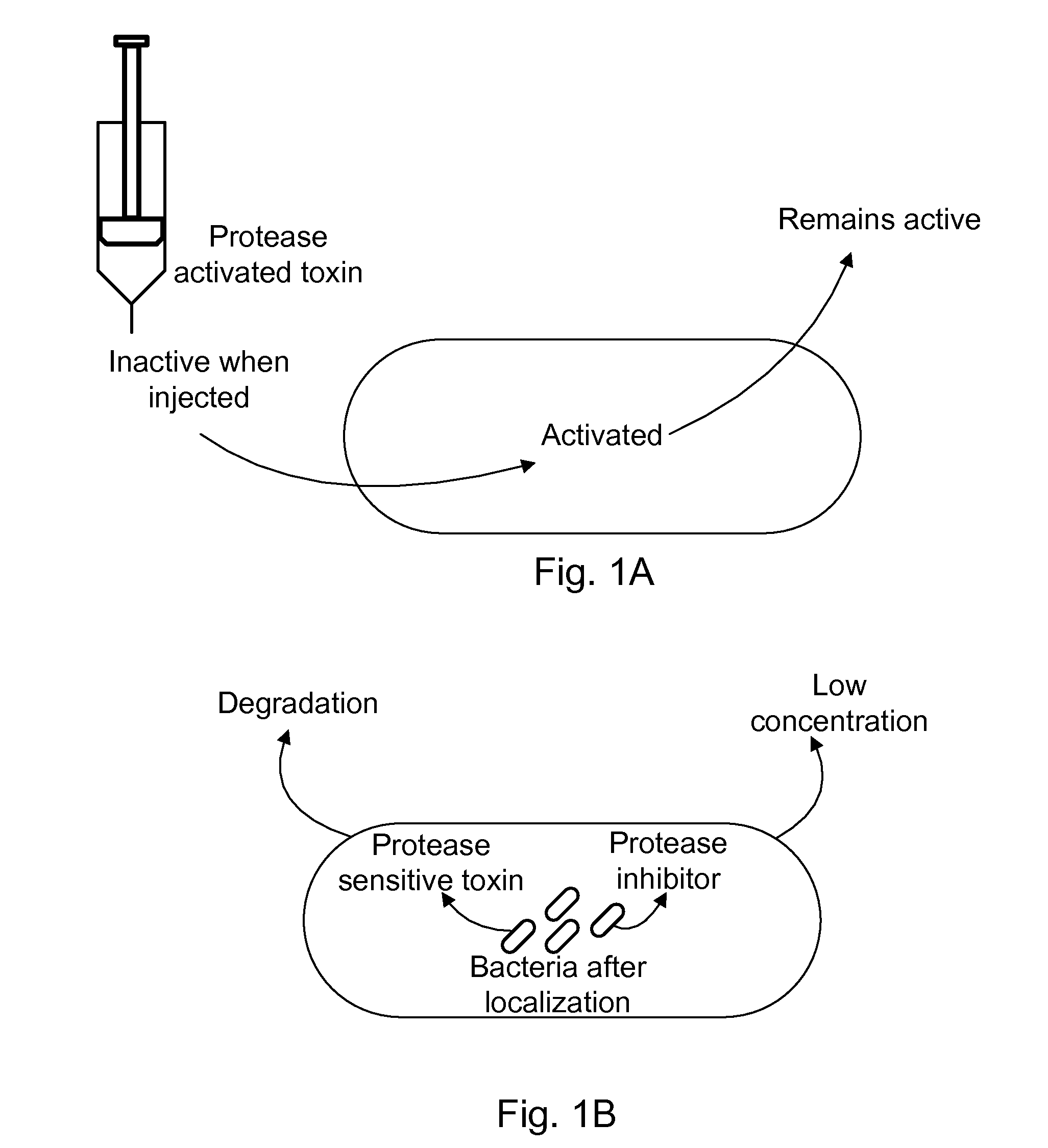
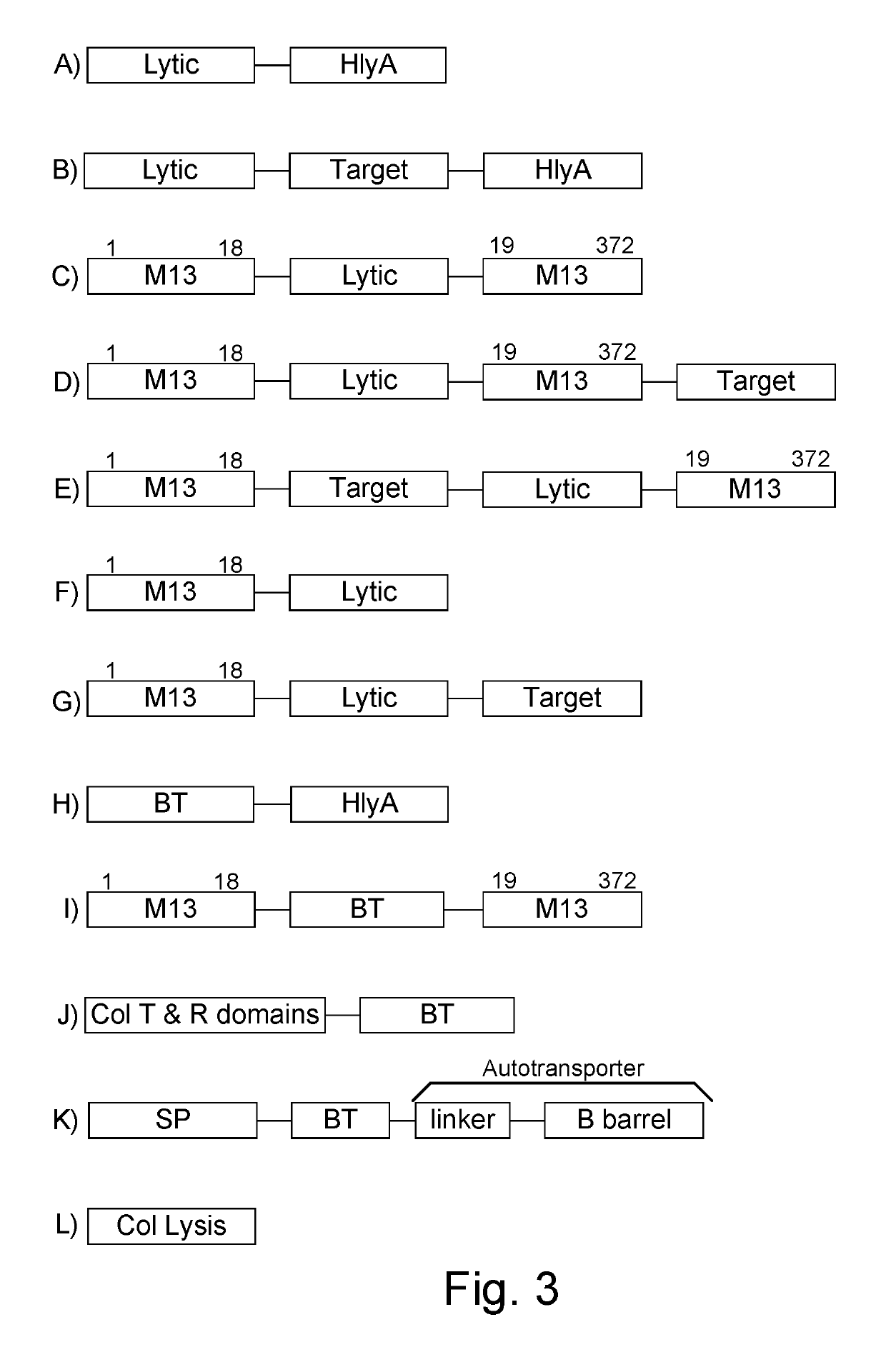
Protease inhibitor: protease sensitivity expression system composition and methods improving the therapeutic activity and specificity of proteins delivered by bacteria
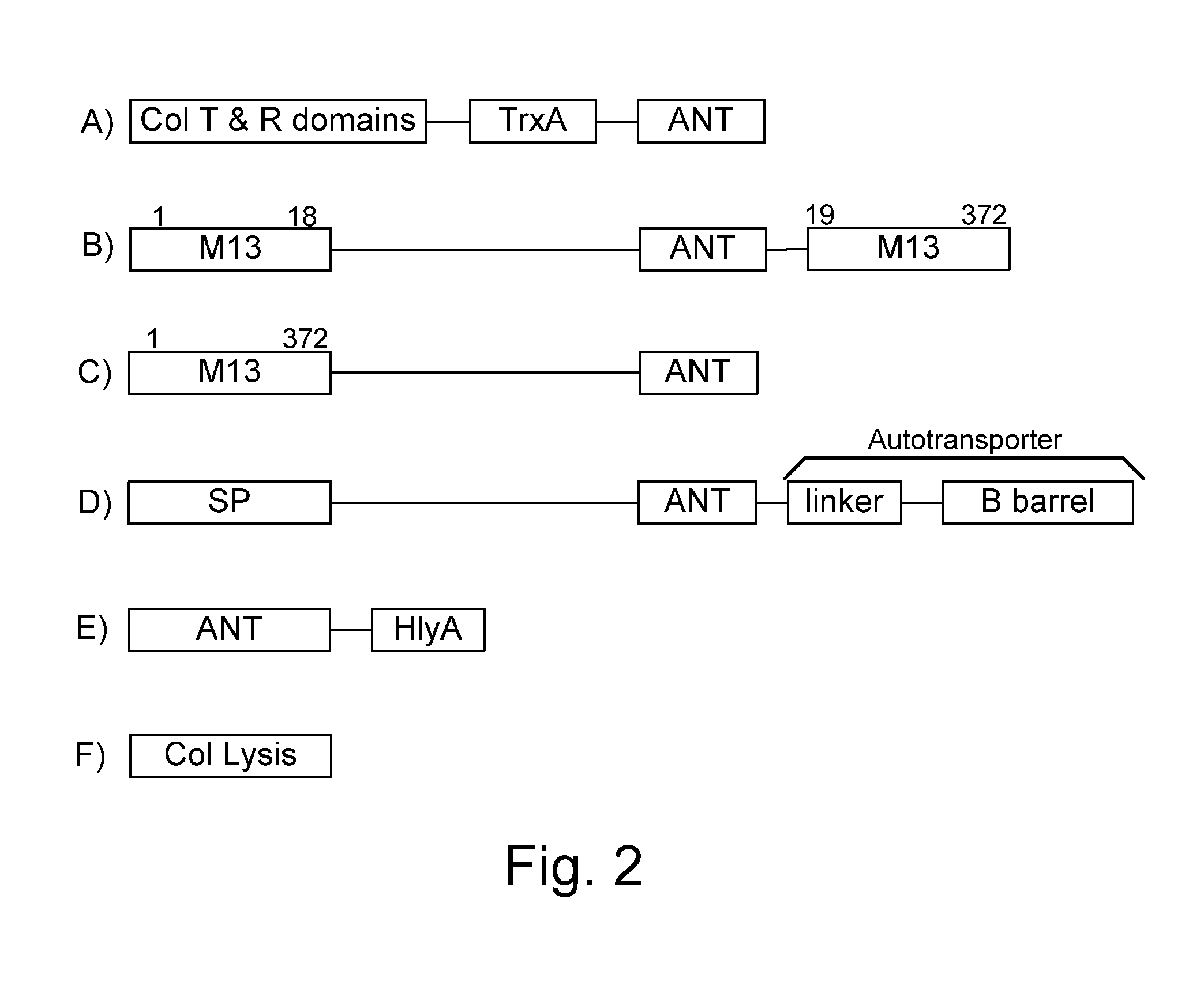
Bacteria which co-express protease inhibitors and protease sensitive therapeutic agents, which are surface displayed, secreted and / or released and result in their localized production and maintenance within a target tissue and inactivation outside of the target tissue, thereby increasing therapeutic activity and reducing the systemic toxicity. The bacteria may be attenuated, non-pathogenic, low pathogenic or a probiotic. Protease sensitivity may be further accomplished by engineering protease degradation sites within the therapeutic agents, further enhancing the inactivation outside of the target tissue while retaining activity within the target tissue through co-expression of a protease inhibitor. Novel chimeric proteins secreted by bacteria, including chimeric toxins targeted to neoplastic cells, tumor matrix cells and cells of the immune system, and combination therapies of these protease inhibitor:chimeric toxin-expressing bacteria together with small-molecule and biologic agents are also described. Non-conjugative bacteria limiting exchange of genetic material, and antibody resistant bacteria are also provided.
Owner:BERMUDES DAVID GORDON
Immunization and/or treatment of parasites and infectious agents by live bacteria
ActiveUS8771669B1Reducing eliminatingReducing or eliminating the targeted parasite, infectious diseaseVirusesBacteriaLytic peptideHuntingtons chorea
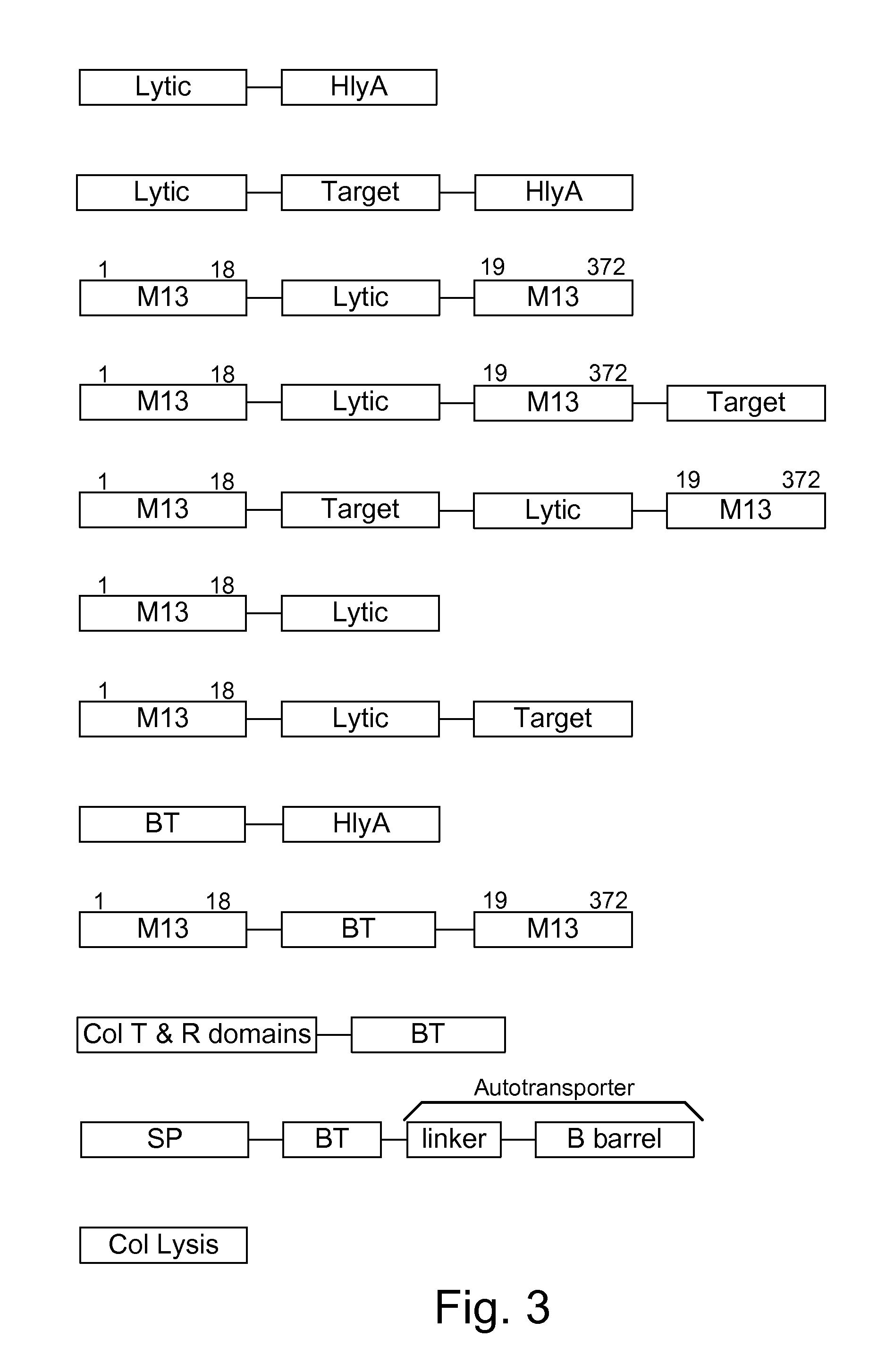
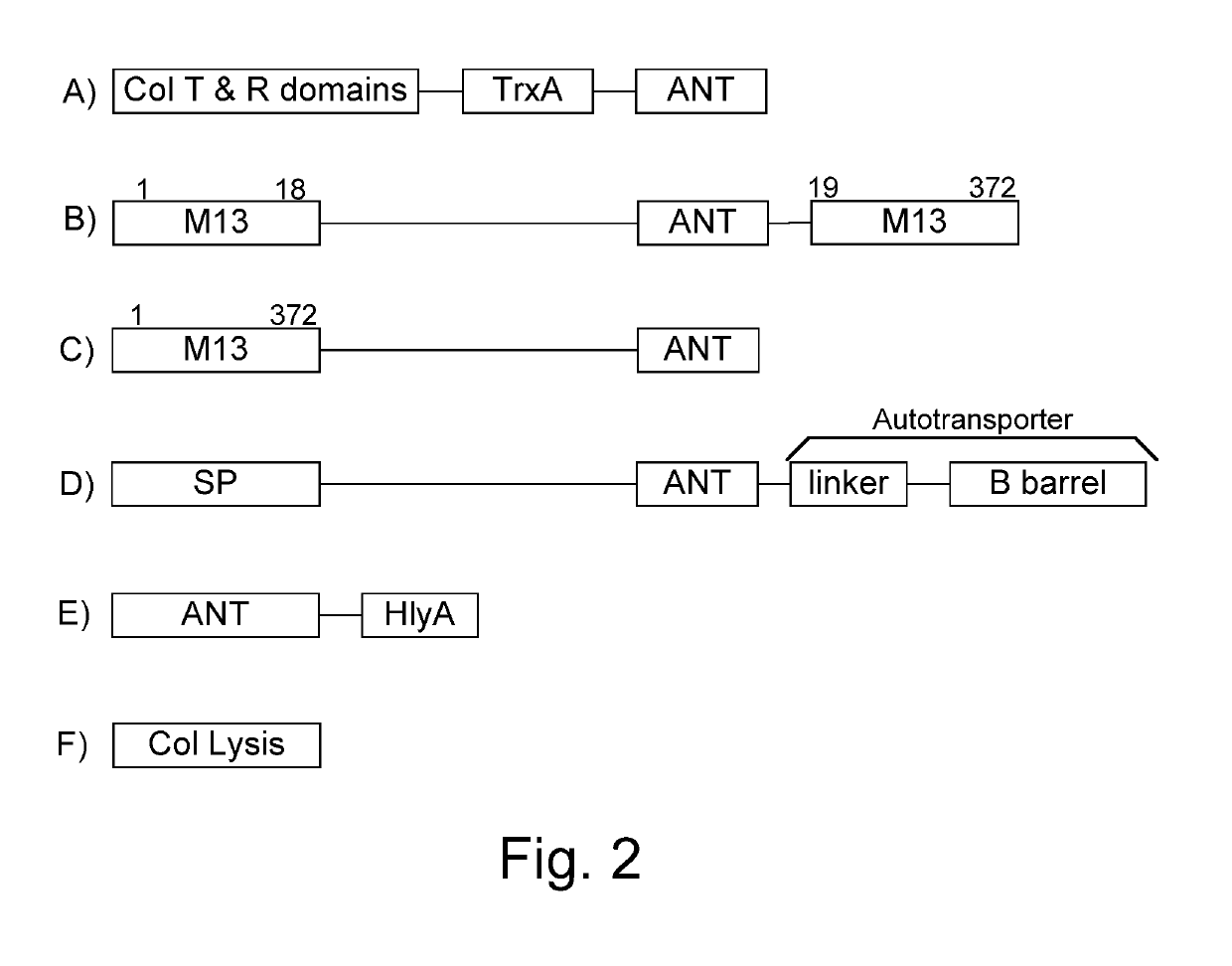
Chimeric proteins are expressed, secreted or released by a bacterium to immunize against or treat a parasite, infectious disease or malignancy. The delivery vector may also be attenuated, non-pathogenic, low pathogenic, or a probiotic bacterium. The chimeric proteins include chimeras of, e.g., phage coat and / or colicin proteins, bacterial toxins and / or enzymes, autotransporter peptides, lytic peptides, multimerization domains, and / or membrane transducing (ferry) peptides. The active portion of the immunogenic chimeric proteins can include antigens against a wide range of parasites and infectious agents, cancers, Alzheimer's and Huntington's diseases, and have enhanced activity when secreted or released by the bacteria, and / or have direct anti-parasite or infectious agent activity. The activity of the secreted proteins is further increased by co-expression of a protease inhibitor that prevents degradation of the effector peptides. Addition of an antibody binding or antibody-degrading protein further prevents the premature elimination of the vector and enhances the immune response.
Owner:BERMUDES DAVID G DR
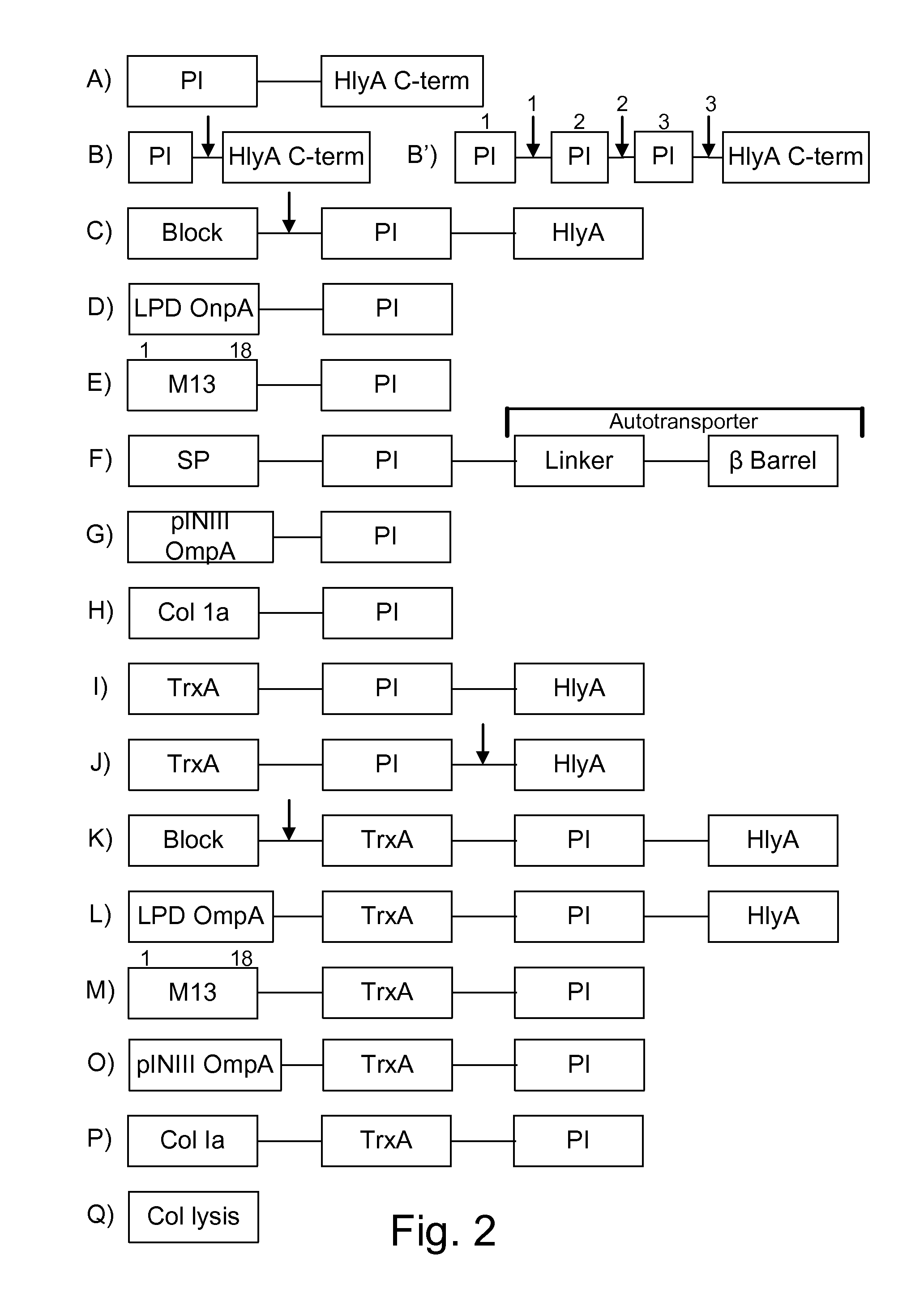
Protease inhibitor: protease sensitivity expression system composition and methods improving the therapeutic activity and specificity of proteins delivered by bacteria
ActiveUS9068187B1Direct cytotoxicDirect inhibitoryBiocidePeptide/protein ingredientsBacteroidesSurface display
Bacteria which co-express protease inhibitors and protease sensitive therapeutic agents, which are surface displayed, secreted and / or released and result in their localized production and maintenance within a target tissue and inactivation outside of the target tissue, thereby increasing therapeutic activity and reducing the systemic toxicity. The bacteria may be attenuated, non-pathogenic, low pathogenic or a probiotic. Protease sensitivity may be further accomplished by engineering protease degradation sites within the therapeutic agents, further enhancing the inactivation outside of the target tissue while retaining activity within the target tissue through co-expression of a protease inhibitor. Novel chimeric proteins secreted by bacteria, including chimeric toxins targeted to neoplastic cells, tumor matrix cells and cells of the immune system, and combination therapies of these protease inhibitor:chimeric toxin-expressing bacteria together with small-molecule and biologic agents are also described. Non-conjugative bacteria limiting exchange of genetic material, and antibody resistant bacteria are also provided.
Owner:BERMUDES DAVID GORDON
Immunization and/or treatment of parasites and infectious agents by live bacteria
ActiveUS9486513B1Reducing or eliminating the targeted parasite, infectious diseaseSsRNA viruses negative-sensePeptide/protein ingredientsHuntingtons choreaAntiparasitic
Chimeric proteins are expressed, secreted or released by a bacterium to immunize against or treat a parasite, infectious disease or malignancy. The delivery vector may also be attenuated, non-pathogenic, low pathogenic, or a probiotic bacterium. The chimeric proteins include chimeras of, e.g., phage coat and / or colicin proteins, bacterial toxins and / or enzymes, autotransporter peptides, lytic peptides, multimerization domains, and / or membrane transducing (ferry) peptides. The active portion of the immunogenic chimeric proteins can include antigens against a wide range of parasites and infectious agents, cancers, Alzheimer's and Huntington's diseases, and have enhanced activity when secreted or released by the bacteria, and / or have direct anti-parasite or infectious agent activity. The activity of the secreted proteins is further increased by co-expression of a protease inhibitor that prevents degradation of the effector peptides. Addition of an antibody binding or antibody-degrading protein further prevents the premature elimination of the vector and enhances the immune response.
Owner:BERMUDES DAVID GORDON
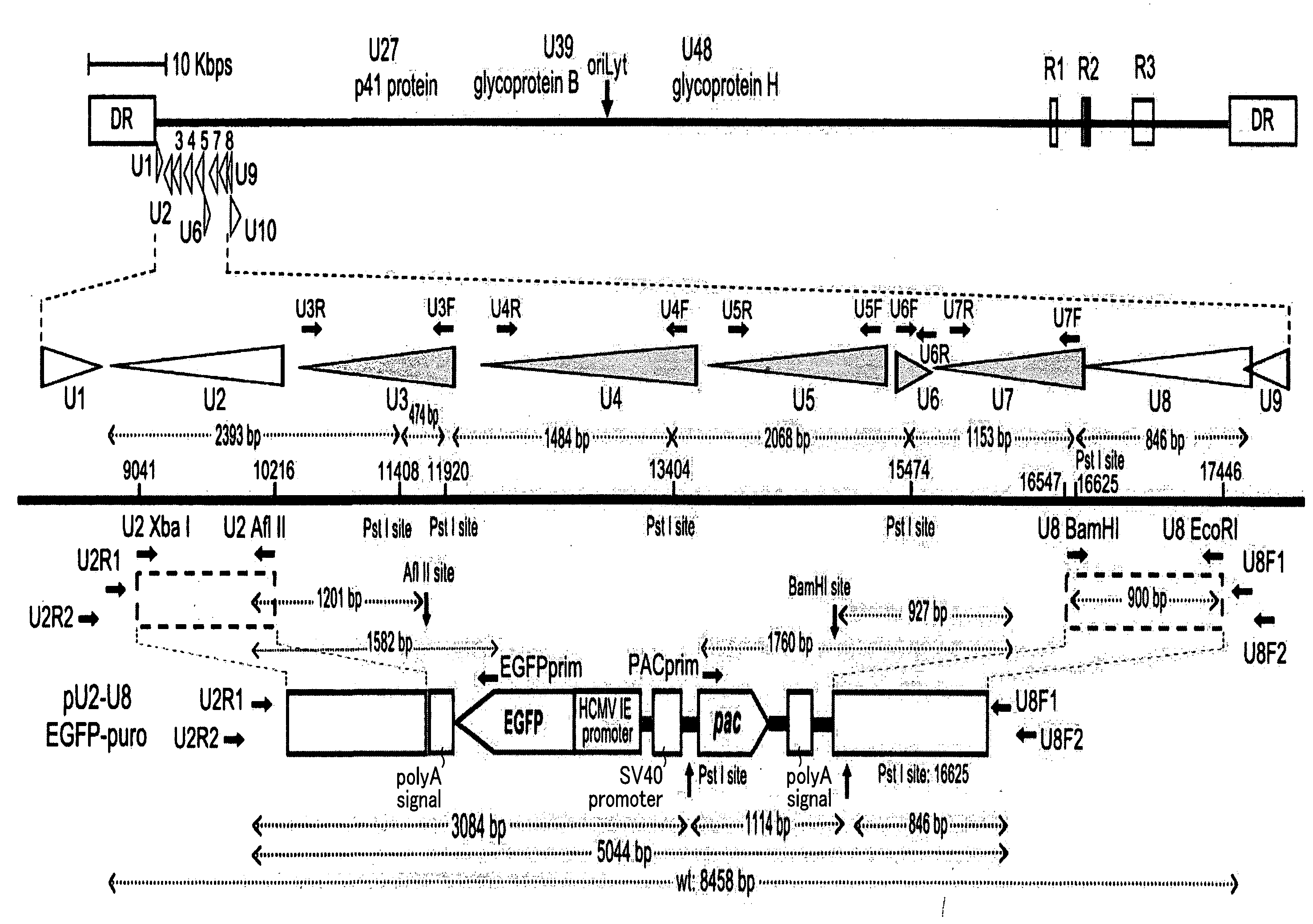
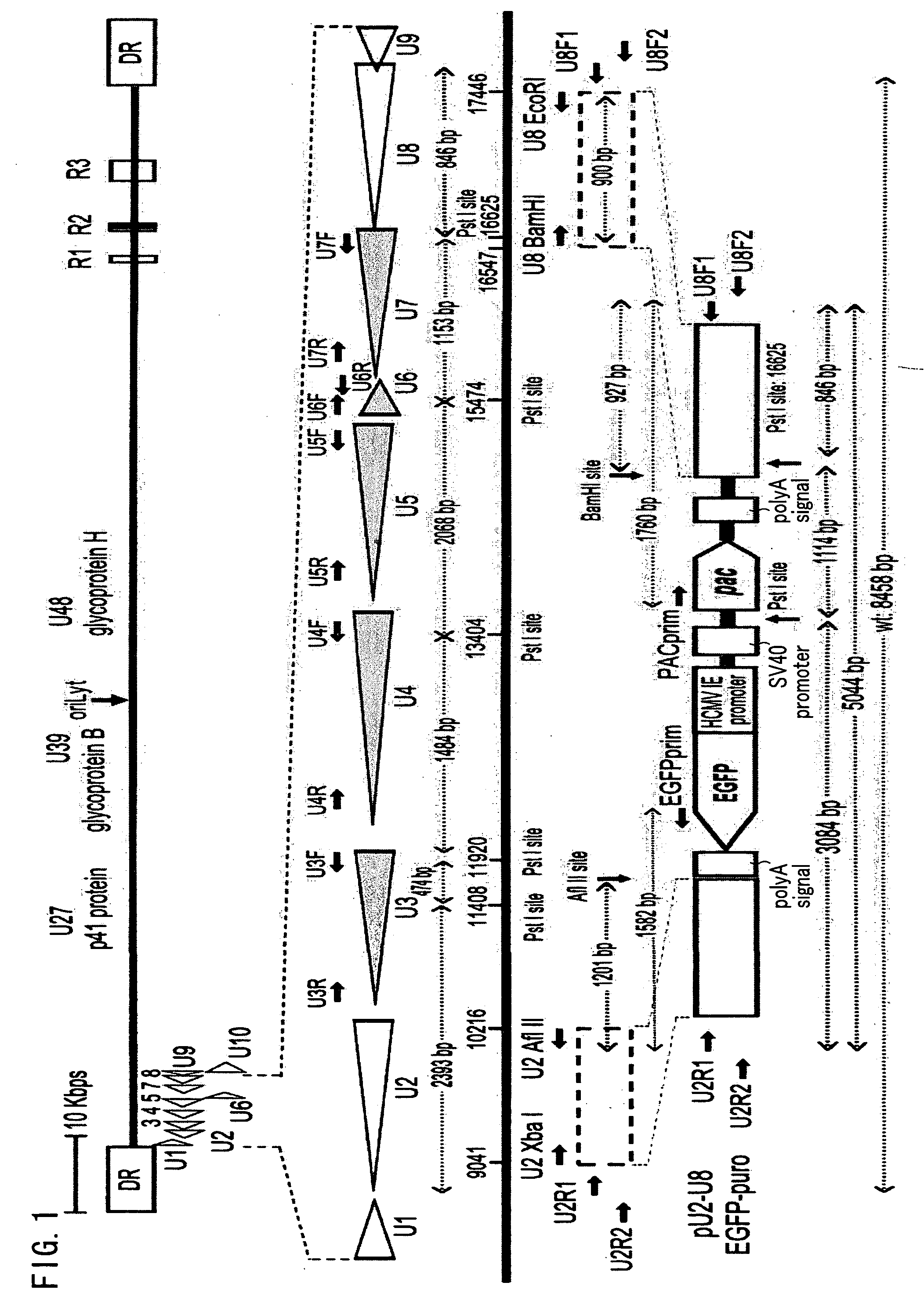
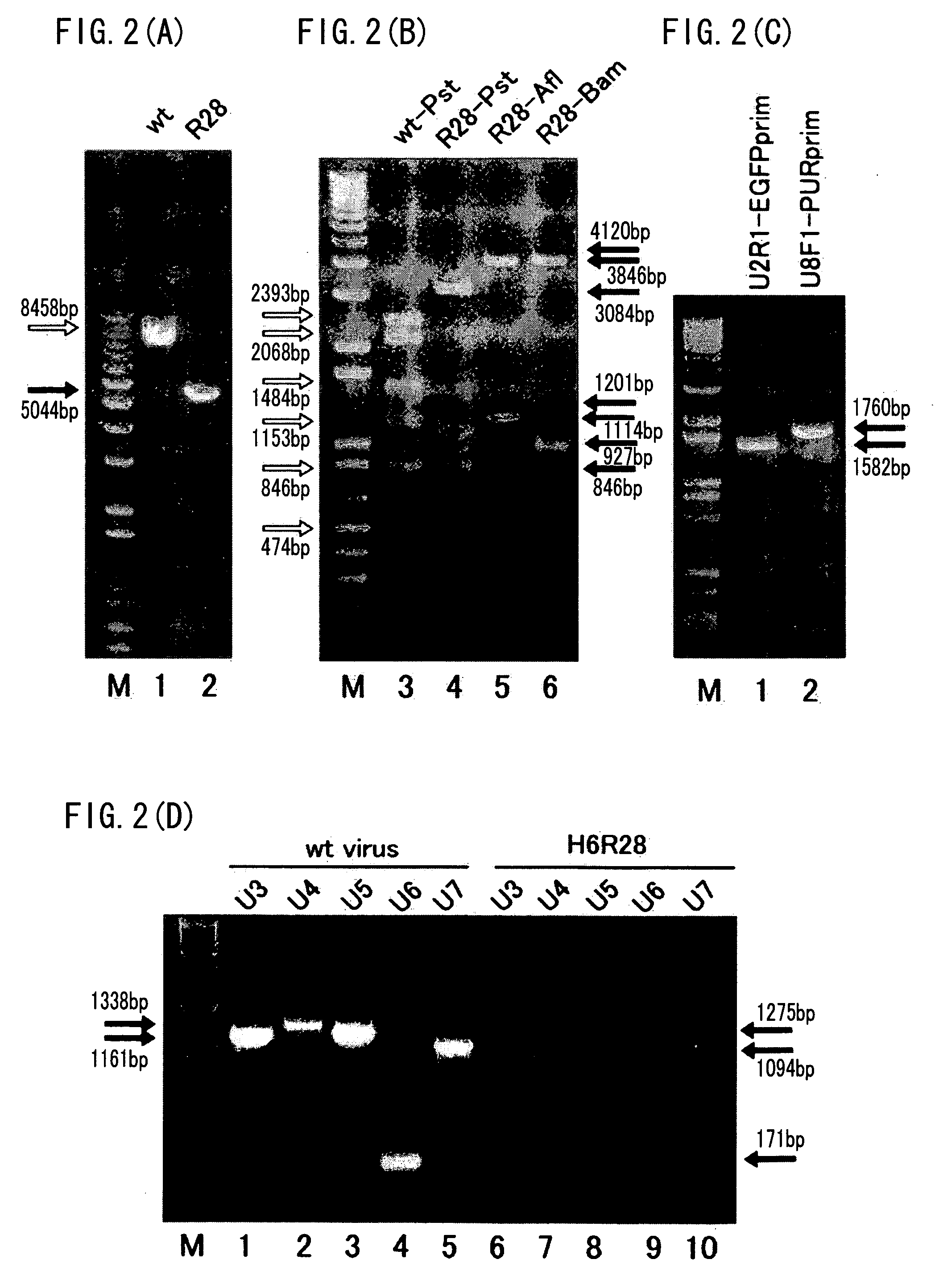
Recombinant virus vector originating in HHV-6 or HHV-7, method of producing the same, method of transforming host cell using the same, host cell transformed thereby and gene therapy method using the same
It is intended to provide a virus vector by which an exogenous nucleotide sequence can be inserted and easily transferred into a mammalian host cell and a gene encoded by the exogenous nucleotide sequence can be expressed in the host cell, and which has a low risk of pathogenicity and is appropriately usable in gene therapy of mammals. Namely, a recombinant vector originating in HHV-6 which has an exogenous nucleotide sequence in a portion corresponding to at least one region selected from the group consisting of U2, U3, U4, U5, U6, U7, U8, U24, and U25 regions of HHV-6; or a recombinant vector originating in HHV-7 which has an exogenous nucleotide sequence in a portion corresponding to at least one region selected from the group consisting of U2, U3, U4, U7, U8, U24, U24a, and U25 regions of HHV-7.
Owner:VIRUS IKAGAKU KENKYUSHO
Real-time fluorescent RCR molecular detection kit for leptosphaeria maculans and detection method thereof
InactiveCN101928779AQuick checkSensitive detectionMicrobiological testing/measurementPositive controlFluorescence
The invention relates to a real-time fluorescent RCR molecular detection kit for leptosphaeria maculans, which contains a general hybrid reagent for gene expression, ddH2O, a probe and primer mixture and a positive control. The probe and primer mixture contains 5mumol / L of LMpro probe, 18mumol / L of LMf upstream primer and 18mumol / L of LMr downstream primer; and the positive control is 100ng / muL leptosphaeria maculans DNA solution. The kit can directly detect the sick rape seed or plant residues, has the advantages of quickness, sensitivity, accuracy and good repeatability, particularly can be used for detecting a sample with low pathogenic bacteria content, is particularly suitable for quickly detecting the leptosphaeria maculans in seeds, plants and sick residues of cruciferous crops such as imported and exported rape seeds and the like, and has good application prospect.
Owner:ANIMAL AND PLANT & FOOD DETECTION CENTER JIANGSU ENTRY EXIT INSPECTION AND QUARANTINE BUREAU
H9N2 subtype avian influenza virus strain as well as inactivated vaccine and application thereof
The invention discloses an H9N2 subtype avian influenza virus strain as well as an inactivated vaccine and application thereof, and belongs to the field of separation and application of avian influenza virus strains. The H9N2 subtype avian influenza virus strain J / ZY strain is separated from a suspected H9N2 avian influenza pathogenetic chicken flock, and the microbial collection number of the strain is CGMCC No. 8853. The invention further discloses a preparation method for the H9N2 subtype avian influenza inactivated vaccine, and the preparation method comprises the following steps: culturing viruses to obtain a virus supernate; adding an inactivating agent to inactivate the virus supernate; mixing adjuvants and the virus supernate uniformly, and emulsifying to obtain the inactivated vaccine. Immune protection experiments prove that the separated H9N2 subtype avian influenza virus strain J / ZY strain serving as a vaccine strain has good immunogenicity and a relatively high antibody level can be produced after an animal is immunized by the strain.
Owner:JILIN ZHENGYE BIOLOGICAL PROD
Immunization and/or treatment of parasites and infectious agents by live bacteria
ActiveUS10364435B1Reducing or eliminating the targeted parasite, infectious diseaseSsRNA viruses negative-sensePeptide/protein ingredientsBacteroidesLytic peptide
Chimeric proteins are expressed, secreted or released by a bacterium to immunize against or treat a parasite, infectious disease or malignancy. The delivery vector may also be attenuated, non-pathogenic, low pathogenic, or a probiotic bacterium. The chimeric proteins include chimeras of, e.g., phage coat and / or colicin proteins, bacterial toxins and / or enzymes, autotransporter peptides, lytic peptides, multimerization domains, and / or membrane transducing (ferry) peptides. The active portion of the immunogenic chimeric proteins can include antigens against a wide range of parasites and infectious agents, cancers, Alzheimer's and Huntington's diseases, and have enhanced activity when secreted or released by the bacteria, and / or have direct anti-parasite or infectious agent activity. The activity of the secreted proteins is further increased by co-expression of a protease inhibitor that prevents degradation of the effector peptides. Addition of an antibody binding or antibody-degrading protein further prevents the premature elimination of the vector and enhances the immune response.
Owner:BERMUDES DAVID GORDON
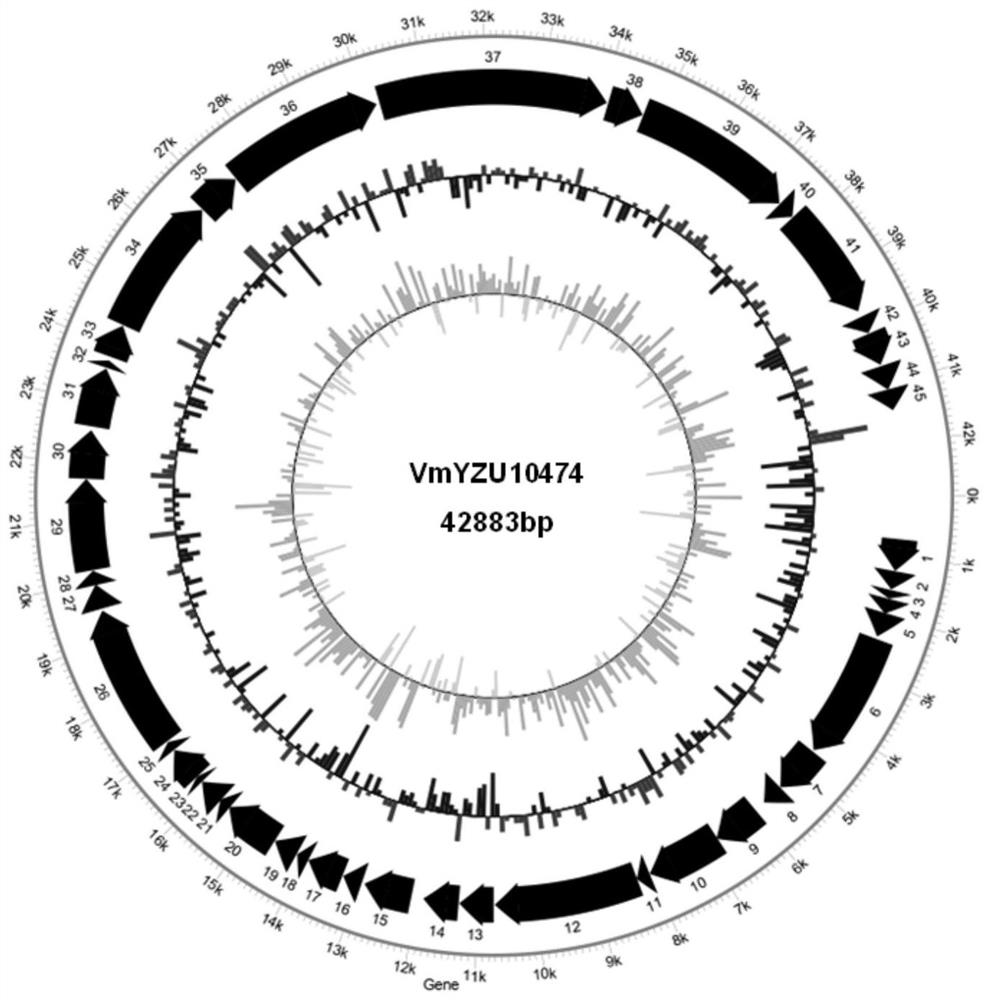
Pathogenic vibrio phage VmYZU10474 and application thereof
ActiveCN112063593AEfficient crackingUnique shapeBiocideMicroorganism based processesBiotechnologyDisease
The invention discloses a pathogenic vibrio phage VmYZU10474 and application thereof. The phage is identified to have unique genome structure characteristics, belongs to a novel phage, and has been preserved in China Center for Type Culture Collection on August 12, 2020 with the preservation number of CCTCC NO: M 2020417. The phage capable of efficiently cracking vibrio is obtained, can inhibit pathogenic vibrio in various matrixes, such as mimicus vibrio, vibrio parahaemolyticus, vibrio alginolyticus and vibrio fluvialis, and can be applied to preparation of bacteriostatic agents for controlling pathogenic vibrio pollution in aquatic products and environments thereof. The prepared bacteriostatic agents can effectively control growth of pathogenic vibrio in culture media, fish juice and aquatic product samples, and meanwhile can effectively inhibit pathogenic vibrio pellicle formation on solids such as equipment and vessels; and in addition, the pathogenic vibrio phage VmYZU10474 is simple to prepare and convenient to use, and the risk that pathogenic vibrio is spread and causes food-borne diseases can be reduced.
Owner:YANGZHOU UNIV
Chimeric protein toxins for expression by therapeutic bacteria
ActiveUS20170051260A1Improve the display effectFacilitated releasePeptide/protein ingredientsAntibody mimetics/scaffoldsBacteroidesSurface display
Bacteria with tumor-targeting capability express, surface displayed, secreted and / or released modified chimeric therapeutic proteins with enhanced therapeutic activity against a neoplastic tissue including solid tumors, lymphomas and leukemias. The bacteria may be attenuated, non-pathogenic, low pathogenic or a probiotic. The chimeric proteins may be protease sensitive and may optionally be further accompanied by co-expression of a secreted protease inhibitor as a separate molecule or as a fusion.
Owner:BERMUDES DAVID DR
Triple RT-PCR primer composition for identification of H4 subtype, H6 subtype and H9 subtype AIV, and kit and use thereof
PendingCN106939357AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesDisease monitoringLow pathogenic
The invention discloses a triple RT-PCR primer composition for rapid simultaneous identification of H4 subtype and / or H6 subtype and / or H9 subtype AIV. The triple RT-PCR primer composition comprises 3 pairs of specific amplification primers. The specific amplification primers comprise a primer pair of H4-F and H4-R, a primer pair of H6-F and H6-R and a primer pair of H9-F and H9-R. The invention also discloses a kit comprising the triple RT-PCR primer composition, a use of the triple RT-PCR primer composition in rapid simultaneous identification of H4 subtype and / or H6 subtype and / or H9 subtype AIV and a method for rapid simultaneous identification of H4 subtype and / or H6 subtype and / or H9 subtype AIV through the triple RT-PCR primer composition. The triple RT-PCR primer composition has the characteristics of simple operation, short detection time, strong specificity and high accuracy, provides an effective diagnostic detection scheme for low pathogenic AIV inspection and quarantine and disease monitoring and control and has an important meaning for prevention, control and monitoring of low pathogenic AIV.
Owner:LIAOCHENG UNIV
Application of corynebacterium diphtheroid as immune adjuvant in poultry oil emulsion inactivated vaccine
InactiveCN107684621AReduce pollutionReduce lossPharmaceutical non-active ingredientsEmulsion deliveryBiological propertyOil emulsion
The invention discloses application of corynebacterium diphtheroid as an immune adjuvant in a poultry oil emulsion inactivated vaccine. The corynebacterium diphtheroid refers to the corynebacterium similar to corynebacterium diphtheriae in respect of morphological and biological characteristics, namely the low-pathogenic or non-pathogenic corynebacterium except the high-pathogenic corynebacteriumdiphtheriae. The corynebacterium diphtheroid active adjuvant disclosed by the invention has high immunity stimulation activity and can be used for non-specifically stimulating the immune functions ofT and B lymphocytes so as to promote secretion of a plurality of cytokines; by adding the immune adjuvant into the poultry oil emulsion inactivated vaccine, the vaccine induced generation of animal antibody can be obviously promoted.
Owner:SOUTH CHINA AGRI UNIV +1
Method of biochip for simultaneous testing avian influenza infection of human and fowls
A biochip for detecting avain fluenza virus of both human and fowls is prepared as providing activated chip substrate, providing various fluenza virus subset sections to be sample-set, providing quality control protein, carrying out arrangement pattern design of point measurement, making sample-sets of quality control protein and various avain fluenza virus subset sections according to prepared pattern for detecting out antibodies generated by various subsets simultaneously.
Owner:深圳市赛尔生物技术有限公司
Chimeric virus RvHBJX of porcine reproductive and respiratory syndrome and application of chimeric virus
ActiveCN104593336AImprove the efficacy of immune protectionGood immune protectionViral antigen ingredientsMicroorganism based processesPathogenicityStructural protein
The invention provides a chimeric virus RvHBJX of porcine reproductive and respiratory syndrome and an application of the chimeric virus and belongs to the technical field of genetic engineering in virus. The chimeric virus RvHBJX of porcine reproductive and respiratory syndrome provided by the invention is a chimeric virus which comprises a JXwn06 structural protein coding region of a high pathogenicity PRRSV strain by taking a low pathogenicity PRRSV strain HB-1 / 3.9 as a framework. After the chimeric virus is continuously passed for 80 times in vitro through an MARC-145 cell, the hereditary property of the virus is stable. The adaptability of the virus on a host cell is gradually enhanced along increase of the passage numbers. After the chimeric virus is passed for 60 times (P60) or over 60 times, the animal body is relatively good in safety, and the inoculated virus can provide 100% immune protection for high pathogenicity PRRSV strain and can be used as a vaccine candidate for the porcine reproductive and respiratory syndrome. The chimeric virus for preventing and treating porcine reproductive and respiratory syndrome has a good application prospect.
Owner:CHINA AGRI UNIV
Recombinant virus vector originating in HHV-6 or HHV-7, method of producing the same, method of transforming host cell using the same, host cell transformed thereby and gene therapy method using the same
It is intended to provide a virus vector by which an exogenous nucleotide sequence can be inserted and easily transferred into a mammalian host cell and a gene encoded by the exogenous nucleotide sequence can be expressed in the host cell, and which has a low risk of pathogenicity and is appropriately usable in gene therapy of mammals. Namely, a recombinant vector originating in HHV-6 which has an exogenous nucleotide sequence in a portion corresponding to at least one region selected from the group consisting of U2, U3, U4, U5, U6, U7, U8, U24, and U25 regions of HHV-6; or a recombinant vector originating in HHV-7 which has an exogenous nucleotide sequence in a portion corresponding to at least one region selected from the group consisting of U2, U3, U4, U7, U8, U24, U24a, and U25 regions of HHV-7.
Owner:VIRUS IKAGAKU KENKYUSHO
Method of saving chimeric viral strain and chimeric attenuated strain with A/B type chimeric HA plasmids
InactiveCN109913496ASolve the problem that is prone to virulence reversionImprove securityInactivation/attenuationMicroorganism based processesAttenuated strainChimeric RNA
The invention disclose a method of saving a chimeric viral strain and chimeric attenuated strain with A / B type chimeric HA plasmids. The method comprises: subjecting an HA sequence of H5 subtype influenza 2.3.4.4 lineage to alkaline cleavage site modification such that a highly pathogenic virus is converted into a lowly pathogenic virus; synthesizing an HA gene of the H5 subtype influenza 2.3.4.4lineage; designing and synthesizing an amplification primer to construct A / B type chimeric HA plasmids by using the synthesized HA gene and constructed pBD-Ya1688-HA transflective plasmids as templates; cloning the HA gene of H5 subtype influenza virus directionally to the pBD-Ya1688-HA transflective plasmids to form A / B type chimeric HA plasmids by means of seamless cloning; saving the chimeric viral strain and chimeric attenuated strain with A / B type chimeric HA plasmids through the A / B type chimeric HA plasmids. The problem is solved that an influenza virus vaccine easily causes virulent recovery.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
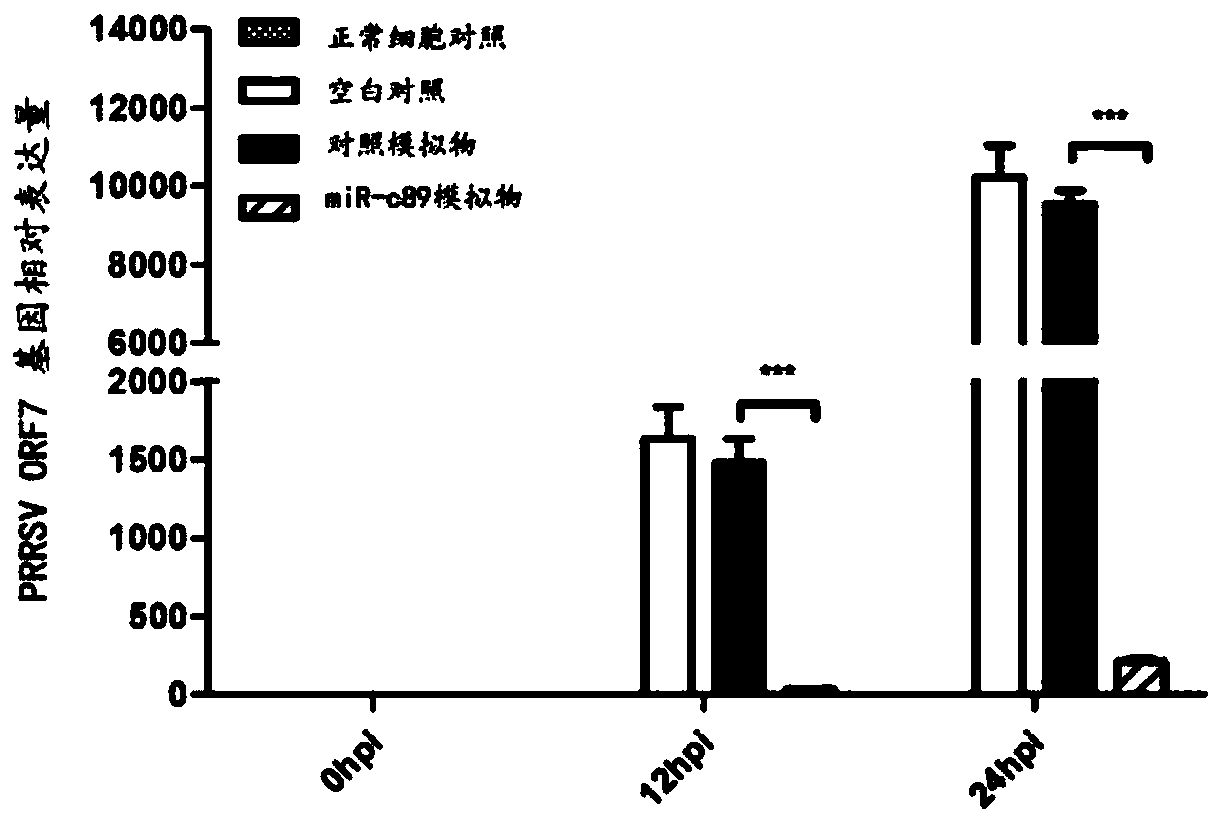
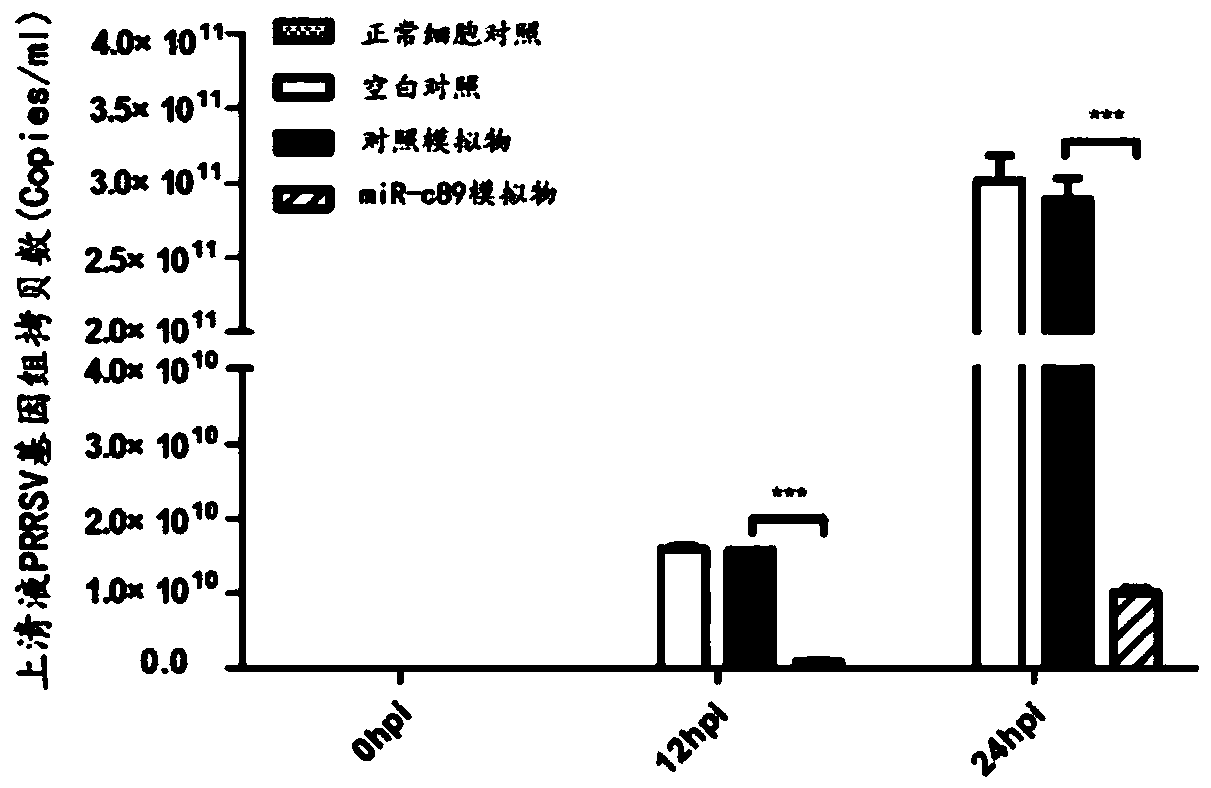
Swine-origin miR-c89 capable of resisting PRRSV (Porcine reproductive and respiratory syndrome virus) infection, and application thereof
ActiveCN109679952AInhibition of replicationPrevent proliferationOrganic active ingredientsAntiviralsWestern blotPorcine reproductive and respiratory syndrome virus
The invention discloses a swine-origin miR-c89 capable of resisting PRRSV (Porcine reproductive and respiratory syndrome virus) infection. Through RT-qPCR (Real Time-quantitative Polymerase Chain Reaction), TCID50 (Tissue Culture Infectious Dose 50) and western blot methods, miR-c89 derived from swine coding is proved to obviously inhibit the replication and the proliferation of high pathogenicityand low pathogenicity PRRSV for the first time, the micoRNA is hopefully developed into a novel medicine capable of preventing and curing porcine reproductive and respiratory syndrome and used for researching the gene-modified pig capable of resisting the porcine reproductive and respiratory syndrome, and a foundation is laid for the prevention and control of the PRRSV.
Owner:NORTHWEST A & F UNIV
Method and device for identifying pathogenic influence of concurrent bacteria infection against poultry influenza virus infection
InactiveCN1442485AHighly pathogenicAchieve infectionMicrobiological testing/measurementTissue/virus culture apparatusBacteroidesBird flu
A method for determining the pathogenic affection of concurrent bacterial infection to avian influenza virus includes such steps as inoculating the bacterium able to secrete subtilisinoid and / or the bacterium able to secrete tryptase and the low-pathogenicity avian influenza virus in culture medium of CEF in a particular mode, culturing, and observing if CPE presents. Its cell culture equipment is composed of cell culture bottle and bacteria culture chamber. Its advantages are simple method, short time, high correctness and low cost.
Owner:SUN YAT SEN UNIV
Lactobacillus plantarum and microecological preparation, preparation method and application thereof
ActiveCN111635873AEnhance immune functionImprove thymus indexAntibacterial agentsBacteriaBiotechnologyDisease
The invention provides lactobacillus plantarum and a microecological preparation, a preparation method and application thereof. The lactobacillus plantarum is named as Lactobacillus Plantarum BLCC2-0125, and is preserved in the China Center for Type Culture Collection in Wuhan City on April 24th, 2020, and the preservation number is CCTCC NO: M 2020078. The lactobacillus plantarum can improve thebody immunity, has a prevention effect on low-pathogenicity avian influenza, and can prevent and treat mycoplasma gallisepticum disease.
Owner:山东宝来利来生物工程股份有限公司
Reagent and method for highly pathogenic H7 avian influenza virus detection and application of reagent
ActiveCN106834540AReduce economic lossProtect health and safetyMicrobiological testing/measurementMicroorganism based processesAgricultural sciencePathogenicity
The invention discloses a reagent and method for highly pathogenic H7 avian influenza virus detection and application of the reagent. The reagent for highly pathogenic H7 avian influenza virus detection in the present application comprises a primer pair and / or a probe. The primer pair and / or the probe is designed for a specific recognition sequence of highly pathogenic H7 avian influenza virus. The specific recognition sequence is a sequence shown in Seq ID No. 1, or a reverse complementary sequence of the sequence shown in Seq ID No. 1, or a synonymous mutation sequence of the sequence shown in Seq ID No. 1 or the reverse complementary sequence of the sequence shown in Seq ID No. 1, or a sequence substituted with 1-4 bases. The reagent in the present application can effectively distinguish the highly pathogenic H7 avian influenza virus from low pathogenic H7 avian influenza virus, thereby providing technical support and guidance for timely taking effective prevention and control measures, and reducing economic losses in livestock and poultry industry due to a failure in distinguishing between high pathogenicity and low pathogenicity of the H7 avian influenza virus.
Owner:SOUTH CHINA AGRI UNIV +1
Artificial recombinant H5N8 influenza virus and preparation method and application thereof
ActiveCN113913395AReduce manufacturing costQuality improvementSsRNA viruses negative-senseVirus peptidesHemagglutininVaccine Production
The invention discloses an artificial recombinant H5N8 influenza virus and a preparation method and application thereof. The artificial recombinant H5N8 influenza virus has a highly pathogenic avian influenza virus A / swan / Shanxi / 4-1 / 2020 (H5N8) surface antigen Hemagglutinin (HA) gene and a Neuraminidase (NA) gene, but an HA cleavage site has a typical low pathogenic avian influenza virus molecular characteristic (-RETR-), and has six internal genes of PB2, PB1, PA, NP, M and NS of an influenza virus chick embryo high-titer adaptive strain A / PR / 8 / 34(H1N1), the strain number is H5-Re14, and the preservation number is CCTCC NO: V202160. The artificial recombinant H5N8 influenza virus has good chick embryo adaptability, and the virus growth titer can be increased by 9 times or above compared with that of a wild virus; and the artificial recombinant H5N8 influenza virus is used as a vaccine strain for producing a vaccine, the production cost of the vaccine can be greatly reduced, and the quality of the vaccine is improved.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Low-pathogenicity porcine reproductive and respiratory syndrome virus, and vaccine and application thereof
ActiveCN105779396AStrong immune responseViral antigen ingredientsMicroorganism based processesMicroorganismPathogenicity
The invention relates to a low-pathogenicity porcine reproductive and respiratory syndrome virus (PRRSV), and a vaccine and an application thereof. The PRRSV is separated from porcine lung tissues by using Marc-145 cells, the above separated strain can proliferate on the Marc-145 cells and make the Marc-145 cells have typical cytopathy, the Marc-145 is detected to obtain the tilter of the separated strain of 10<7.0> TCID50 / mL, and the strain is preserved in China General Microbiological Culture Collection Center on Oct. 22, 2015 with the preservation number of CGMCC No.9819; and leaned piglets artificially inoculated with the new virus strain only have weak thermal response, have no obvious clinic symptoms, and normally grow without untoward effects. The new strain artificially inoculated to pigs can induce the pigs to have good immune response I order to generate high level PRRSV antibodies.
Owner:兆丰华生物科技(南京)有限公司北京生物医药科技中心 +3
Carrier pigeon Newcastle disease virus genetic engineering modified attenuated strain as well as preparation method and application thereof
PendingCN114774373AWeak toxicityImprove securitySsRNA viruses negative-senseViral antigen ingredientsF proteinTGE VACCINE
The invention discloses a carrier pigeon Newcastle disease virus genetic engineering modified attenuated strain as well as a preparation method and application of the carrier pigeon Newcastle disease virus genetic engineering modified attenuated strain. The method specifically comprises the following steps: carrying out site-directed mutagenesis on amino acids of three cleavage sites of F protein of a carrier pigeon Newcastle disease virus strain (PNDV-YQ strain) by using a reverse genetic manipulation technology, constructing and obtaining a full-length cDNA plasmid pOK-YQ of a low-toxicity gene in a segmented cloning manner, respectively cloning ORF genes of NP, P and L of the PNDV-YQ strain to an eukaryotic expression vector pCIneo, and carrying out expression by using a recombinant vector pCIneo, according to the invention, helper plasmids pCI-NP, pCI-P and pCI-L are constructed and obtained. After a BSR-T7 cell is co-transfected by the full-length plasmid and a helper plasmid, a recombinant attenuated virus, namely the pigeon Newcastle disease virus (PNDV-aYQ strain), is rescued. The strain of virus is a low-pathogenicity or non-pathogenicity strain, and has good proliferation characteristics and stable hereditary characteristics. Compared with the existing newcastle disease vaccine, the inactivated vaccine prepared from the virus strain has the advantages of low side reaction, early immune production period and high protection rate.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES +1
Fluorescent RT-PCR primer, probe and method for detecting highly pathogenic H7N9 avian influenza virus
PendingCN110564892AProtect production securityEnsure public health safetyMicrobiological testing/measurementDNA/RNA fragmentationAvian influenza virusInfectious bursitis
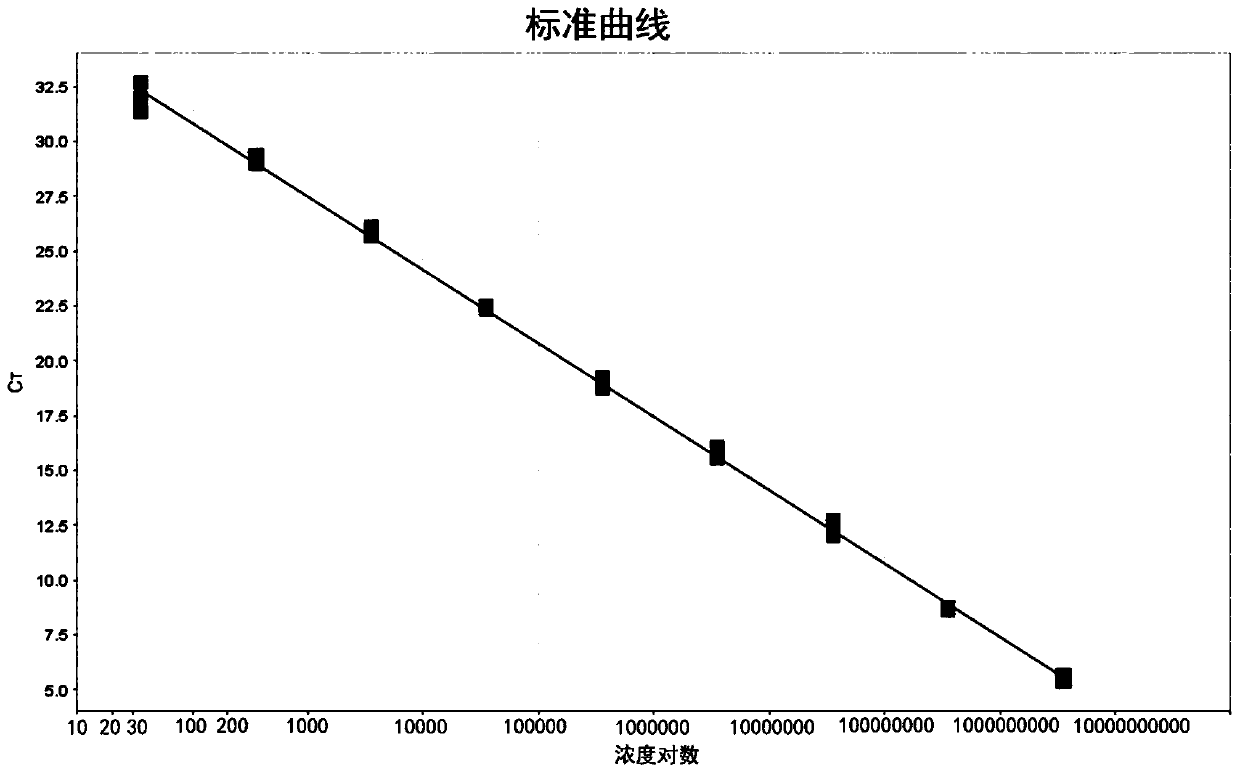
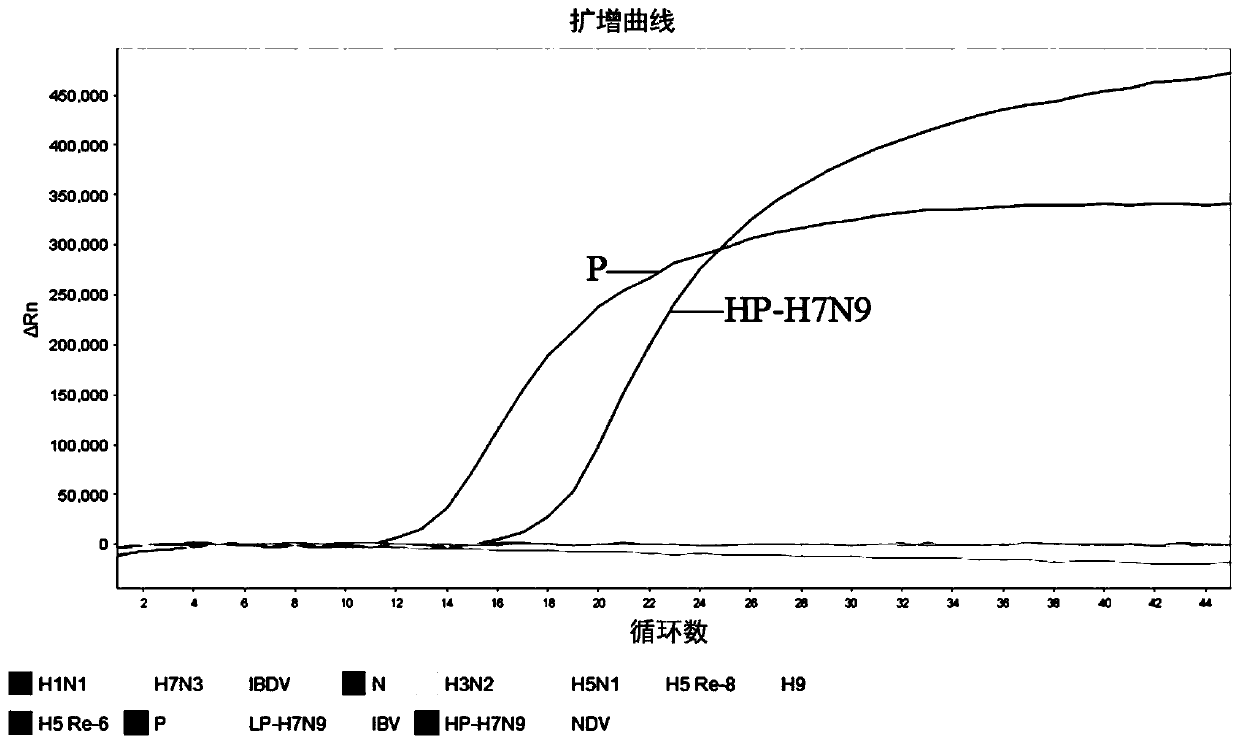
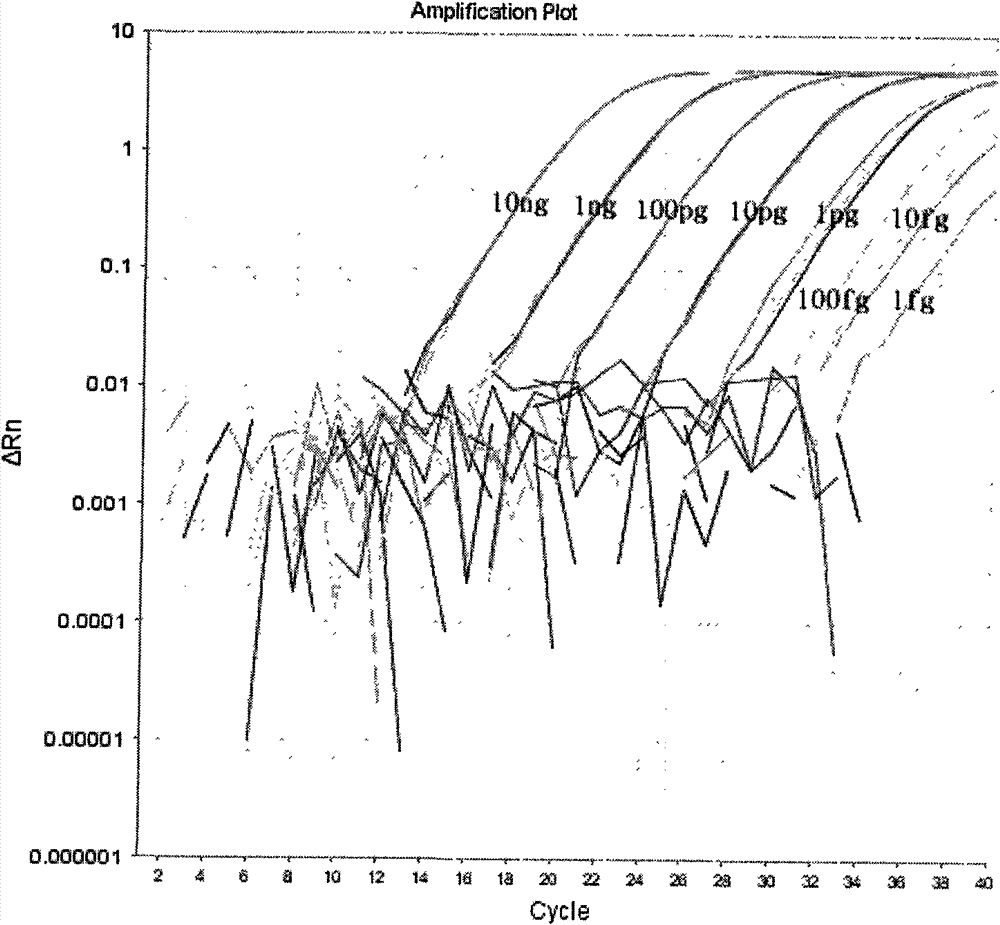
The invention discloses a fluorescent RT-PCR primer, probe and method for detecting a highly pathogenic H7N9 avian influenza virus (HP-H7N9). The established HP-H7N9 fluorescent RT-PCR detection method can specifically detect the HP-H7N9, and no cross reactions appear with a low pathogenicity H7N9 influenza virus (LP-H7N9), H7N3 influenza virus, H3N2 influenza virus, H5N1 avian influenza virus, H5subtype avian influenza virus (Re-6 vaccine strain), H5 subtype avian influenza virus (Re-8 vaccine strain), H9 subtype avian influenza virus, influenza A (H1N1) virus (H1N1), infectious bursal disease virus (IBDV), chicken infectious bronchitis virus (IBV), newcastle disease virus (NDV) and the like; positive plasmids with the lowest concentration of 7.09 copies / [mu]L can be detected; the variable coefficient (CV) value of a repeatability test is between 1.97% and 4.89%, the method is indicated to have good repeatability. The established HP-H7N9 fluorescent RT-PCR method has the advantages of specificity, sensitivity, rapidness and good repeatability, and is a good method for rapidly detecting HP-H7N9.
Owner:GONGBEI CUSTOMS TECH CENT
Primer and kit for distinguishing and detecting low-pathogenicity and high-pathogenicity babesia motasi
ActiveCN104561343AAvoid pollutionHigh sensitivityMicrobiological testing/measurementMicroorganism based processesPathogenicityBioinformatics
The invention discloses a primer and a detecting kit for distinguishing and detecting low-pathogenicity and high-pathogenicity babesia motasi. The primers in the primer sequence and the kit disclosed by the invention are respectively primers in SEQ ID No.1, SEQ ID No.2, SEQ ID No.4 and SEQ ID No.5, and probe primers SEQ ID No.3 and SEQ ID No.6. Through using the primer and the kit disclosed by the invention for detection, the advantages of high sensitivity, high speed, high specificity and the like are achieved, and the primer and the kit disclosed by the invention can be widely applied to the qualitative and quantitative detection of pathogens.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Real-time fluorescent RCR molecular detection kit for leptosphaeria maculans and detection method thereof
InactiveCN101928779BQuick checkSensitive detectionMicrobiological testing/measurementPositive controlFluorescence
Owner:ANIMAL AND PLANT & FOOD DETECTION CENTER JIANGSU ENTRY EXIT INSPECTION AND QUARANTINE BUREAU
Artificially recombined h5n8 influenza virus and its preparation method and application
ActiveCN113913395BReduce manufacturing costQuality improvementSsRNA viruses negative-senseVirus peptidesHemagglutininVaccine Production
The invention discloses an artificially recombined H5N8 influenza virus, a preparation method and application thereof. An artificial recombinant H5N8 influenza virus of the present invention has highly pathogenic avian influenza virus A / swan / Shanxi / 4‑1 / 2020 (H5N8) surface antigen hemagglutinin (HA) and neuraminidase (NA) genes , but the HA cleavage site shows the molecular characteristics of typical low pathogenic avian influenza virus (‑RETR‑), and has the PB2, PB1, PA, There are 6 internal genes including NP, M and NS, the strain number is H5‑Re14, and the deposit number is CCTCC NO: V202160. It has good chicken embryo adaptability, and the virus growth titer can be increased by more than 9 times compared with wild virus; using this as a vaccine strain to produce vaccines can greatly reduce vaccine production costs and improve vaccine quality.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Low pathogenic porcine reproductive and respiratory syndrome virus, vaccine and its application
ActiveCN105779396BNo adverse effects on growthStrong immune responseViral antigen ingredientsMicroorganism based processesPorcine lungPig reproduction
The invention relates to a low-pathogenicity porcine reproductive and respiratory syndrome virus (PRRSV), and a vaccine and an application thereof. The PRRSV is separated from porcine lung tissues by using Marc-145 cells, the above separated strain can proliferate on the Marc-145 cells and make the Marc-145 cells have typical cytopathy, the Marc-145 is detected to obtain the tilter of the separated strain of 10<7.0> TCID50 / mL, and the strain is preserved in China General Microbiological Culture Collection Center on Oct. 22, 2015 with the preservation number of CGMCC No.9819; and leaned piglets artificially inoculated with the new virus strain only have weak thermal response, have no obvious clinic symptoms, and normally grow without untoward effects. The new strain artificially inoculated to pigs can induce the pigs to have good immune response I order to generate high level PRRSV antibodies.
Owner:兆丰华生物科技(南京)有限公司北京生物医药科技中心 +3
Reagents, methods and applications for detection of highly pathogenic h7 avian influenza virus
ActiveCN106834540BReduce economic lossProtect health and safetyMicrobiological testing/measurementMicroorganism based processesPathogenicityViral test
Owner:SOUTH CHINA AGRI UNIV +1
Method and device for identifying pathogenic influence of concurrent bacteria infection against poultry influenza virus infection
InactiveCN1442486AHigh pathogenicityEasy to operateMicrobiological testing/measurementTissue/virus culture apparatusBacteroidesPathogenicity
A method for determining the affection of concurrent bacterial infection to virus infection pathogenicity includes such steps as inoculating the bacteria and low-pathogenicity virus in cell culturingmedium, culturing, and observing in cell lesion (CPE) presents. Its culture equipment is composed of cell culture bottle and bacteria culture chamber. Its advantages are simple process, short time, high currectness and low cost.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com