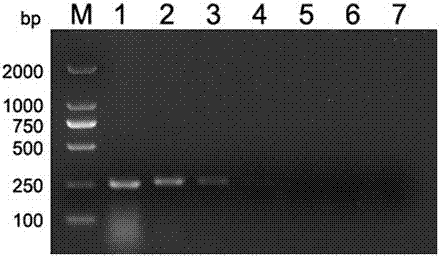
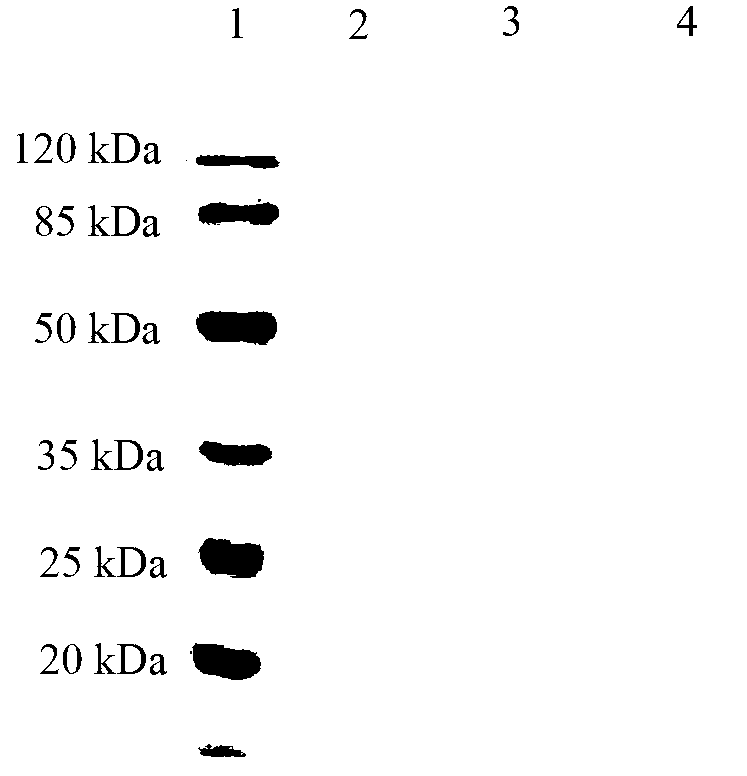
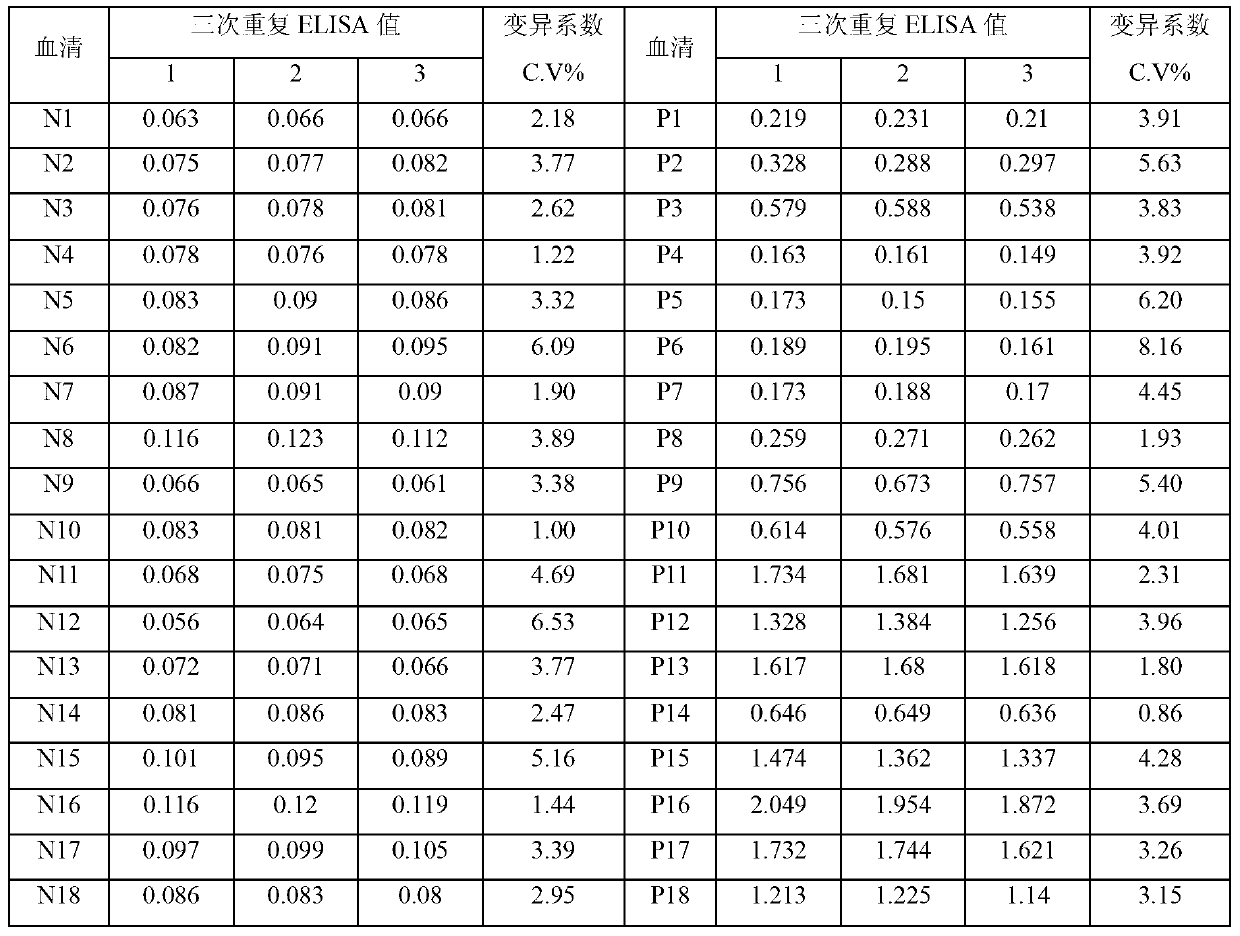
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
634 results about "Epidemiologic survey" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Epidemiological Survey. a medical survey conducted in an epidemic focus to discover the source of an infection, the means by which the causative agent was transmitted, and the circumstances that gave rise to the disease. The findings are used to devise ways of preventing the disease from spreading.
Methods for rapid detection and identification of biogents in epidemiological and forensic investigations
InactiveUS20070048735A1Microbiological testing/measurementBiological testingGenotypingEpidemiologic survey
The present invention provides methods for rapid forensic investigations by identification of bioagents associated with biowarfare and acts of terrorism or crime. The methods are also useful for epidemiological investigations by genotyping of bioagents.
Owner:IBIS BIOSCI
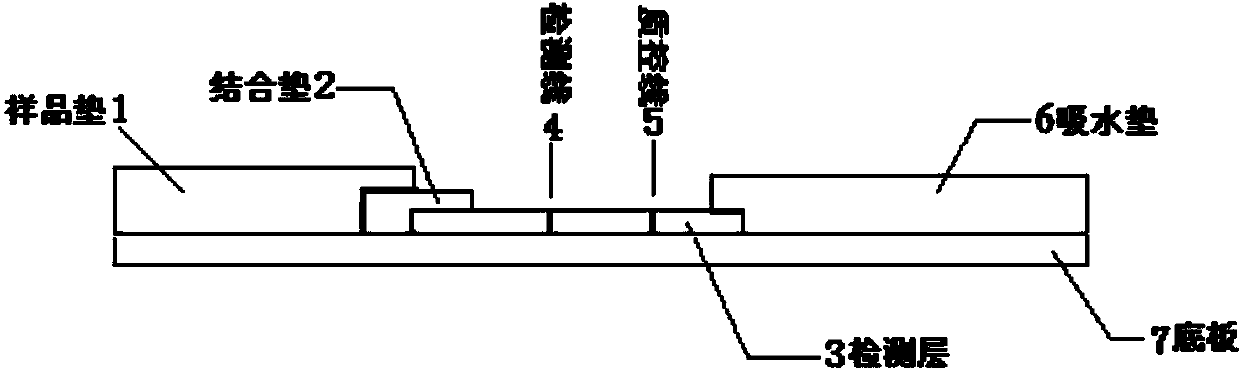
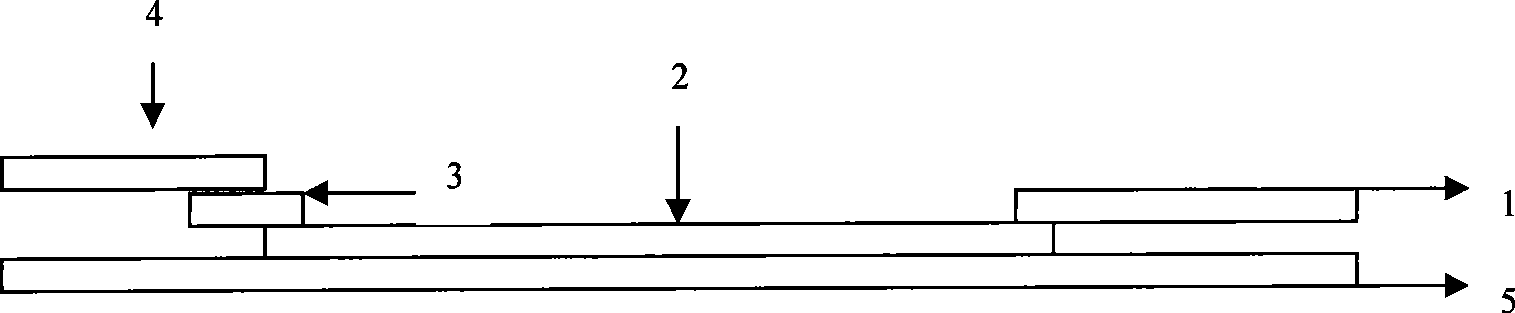
Human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and preparation method and application thereof
The invention provides a human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and a preparation method and application thereof. The assay kit comprises a detection card and a silver-stained sensitivity-enhanced pad, wherein the detection card is composed of a bottom plate, a sample pad, an absorbent pad, a conjugate pad and a detection layer; the conjugate pad is coated with a colloidal gold-marked polyclonal antibody mixture of colloidal gold marked rabbit anti-human mycoplasma pneumoniae P1 protein and P30 protein; the detection layer is composed of a solid phase nitrocellulose membrane with a detection line and a quality control line; the detection layer is bonded on the bottom plate, the conjugate pad and the absorbent pad are partially overlapped with the detection layer respectively and are bonded with the detection layer and the bottom plate respectively; the sample pad and the conjugate pad are partially overlapped to be bonded with the conjugate pad and the bottom plate respectively; and the silver-stained sensitivity-enhanced pad consists of a AgNO3 pad and a restoring pad. The human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit can effectively improve the detection sensitivity of the human mycoplasma pneumoniae, has the strong specificity and has the high application value in the aspects of clinical diagnosis of human mycoplasma pneumoniae, etiology identification, epidemiological investigation and the like.
Owner:HUBEI UNIV OF TECH +1
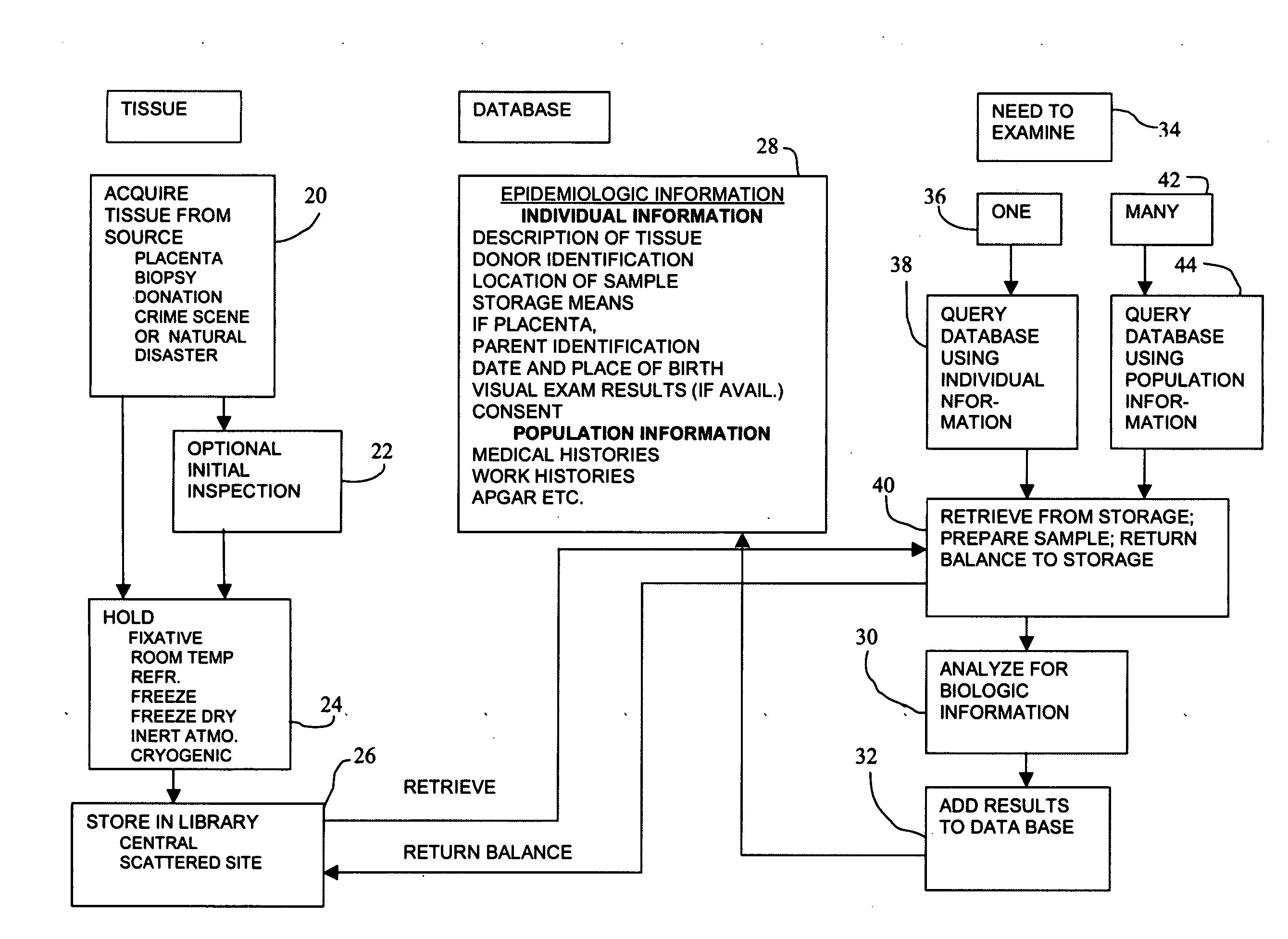
Method of making and using a library of biological information
InactiveUS20080027353A1Lower initial costLow costPreparing sample for investigationSurgeryPhysician attendingEpidemiologic survey
Biologic information is obtained concerning a member of a population by obtaining a tissue sample from the member, storing the sample without embedding it in an embedding medium, retrieving from storage the sample associated with the member and thereafter analyzing it for biologic information. The tissue sample may be all or part of the member's placenta. Storage may be in a fixative such as formalin or a formalin substitute. Storage may also be by other means of preserving such as freezing or the like. When a tissue sample from more than one member is collected, a library is created that may be used for a variety of purposes, including reducing the incidence of medical malpractice claims, identification of members such as paternity testing or suspect identification. The library may also be used for pharmaceutical development and epidemiological surveys and research. Each sample may have associated with it certain epidemiologic information such as the donor's identity and medical history, residence, place of employment and the like. If the sample is of placental tissue similar information concerning the member's parents may also be recorded, as well as the hospital where the delivery occurred, the attending physician's name and the like. If and when a sample is retrieved and analyzed for biologic information, that information may also be associated with the sample so that it is available for future researchers.
Owner:KLIMAN HARVEY J
Rapid parallel nucleic acid detection method and system based on micro-fluidic chip
ActiveCN104630373AMeet automatic sampling requirementsUniform heating temperature fieldBioreactor/fermenter combinationsSequential/parallel process reactionsFluorescenceDisplay device
The invention relates to a rapid parallel nucleic acid detection method and system based on a micro-fluidic chip. The nucleic acid detection system comprises a micro-fluidic chip, a motor, exciting light, a double-focal-plane imaging lens set, a detector, a signal acquisition processor and a display; the micro-fluidic chip comprises at least one reaction channel; a heating film is arranged at the periphery of the micro-fluidic chip; a submillimeter air layer is maintained between the micro-fluidic chip and the heating film; and the micro-fluidic chip is irradiated by adopting the exciting light, so that a nucleic acid sample generates fluorescence under excitation of the exciting light, the fluorescence is gathered on the detector by virtue of the double-focal-plane imaging lens set so as to generate an analog signal, the detector transmits the generated analog signal to the signal acquisition processor so as to generate a real-time fluorescence detection signal, and the real-time fluorescence detection signal is displayed by the display. The method and the system disclosed by the invention can be applied to the fields of clinical pathogenic bacterium molecular diagnosis, food inspection and quarantine, food poisoning pathogenic bacterium detection, bacteriology classification and epidemiological investigation and have huge economical and social benefits.
Owner:CAPITALBIO CORP +2
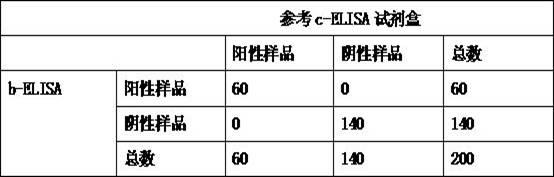
Kit for detecting antibody against Peste des petits ruminants virus b-ELISA and preparation method thereof
InactiveCN102419369AReduce economic costsLow costMaterial analysisViral antibodyEpidemiologic survey
The invention relates to the technical field of biology, particularly the field of viral antibody detection. A kit for detecting the antibody against Peste des petits ruminants virus b-ELISA comprises the following ingredients which are arranged respectively: Peste des petits ruminants nucleoprotein antigen, Peste des petits ruminants monoclonal antibody, diluent, strong positive serum, weak positive serum, negative serum, HRP sheep anti-mouse secondary antibody, 20 times the concentration of washing liquid, substrate liquid, stopping solution and enzyme-linked immunosorbent plate. The optimum proportion of each ingredient in the kit is determined by experiments. The kit can be used for rapid diagnosis and detection of animal Peste des petits ruminants virus antibody, especially for the antibody detection of a lot of samples in the epidemiological survey of Peste des petits ruminants. The detection method of Peste des petits ruminants virus b-ELISA has different detection principle and experiment operating procedures and the like from those of a c-ELISA detection method in a BIRAD laboratory. The Peste des petits ruminants nucleoprotein antigen and Peste des petits ruminants monoclonal antibody in the kit are self-developed. The detection sensitivity, singularity and other indexes of the kit are the same with those of the c-ELISA detection method in the internationally recognized BIRAD laboratory.
Owner:CHECKOUT & QUARANTINE TECH CENT YUNNAN ENTRY &EXIT CHECKOUT & QUARANTINE BUR +1
Oligonucleotide probe kit for detecting common intestine trac kpathogenic bacteria and its use
InactiveCN1683565AQuick checkAccurate detectionMicrobiological testing/measurementAgainst vector-borne diseasesAntigenBio engineering
The present invention belongs to the field of microbe detecting technology. The oligonucleotide probe for detecting common intestinal tract pathogenic bacteria is designed on 16S rRNA and 23S rRNA of bacteria, ipaH of dysentery bacillus giant plasmid, VipR of Salmonella typhi and other gene sequence, has length of 25-50 bp, and relatively high sensitivity and specificity. The oligonucleotide probe is suitable for detection based on nucleic acid hybridization principle, especially detection based on gene chip principle. Under certain use condition, it can detect Listeria, parahemolutic vibrio, Campylobacter, etc. It may be used in many aspects, such as disease diagnosis, environment detection, food poisoning detection, etc.
Owner:RADIOLOGY INST ACAD OF MILITARY MEDICINE SCI PLA
Oligonucleotide primer for detecting common pathogenic bacteria by adopting fluorescent quantitation PCR (Rich Client Platform) technology, method thereof for detecting common pathogenic bacteria and application thereof
InactiveCN101928773AEfficient and wideWide range of applicationsMicrobiological testing/measurementAgainst vector-borne diseasesBiotechnologyFood poisoning
The invention discloses an oligonucleotide primer for detecting common pathogenic bacteria by adopting a fluorescent quantitation PCR (Rich Client Platform) technology, a method thereof for detecting common pathogenic bacteria and the application thereof. The method comprises the following steps of: providing 10 pairs of specific oligonucleotide primer sequences at annealing temperature of 50-60 DEG C without differing 5 DEG C; and simultaneously, quickly, accurately and effectively identifying and quantificationally detecting various pathogenic bacteria at the same time. A detection range comprises bacillus cereus, enterobacter sakazakii, vibrio parahaemolyticus, enterohemorrhagic escherichia coli O157, salmonella, Listeria monocytogenes, Shigella, campylobacter jejuni, pseudomonas aeruginosa, klebsiella pneumoniae, and the like. The invention also can be used for the fields of disease diagnosis, environmental monitoring, water-quality and food supervision and detection, food poisoning pathogenicbacteria detection, bacteriological classification, epidemiological investigation, biological agent detection, and the like, is convenient, quick, accurate and effective and has wide application range.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Primer, method and kit for detecting animal clonorchiasis sinensis specificity
InactiveCN101586161AQuick checkEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationSequence databaseUltraviolet lights
The present invention discloses a primer, a method and a kit for detecting the animal clonorchiasis sinensis specificity, a nucleotide sequence of an upstream primer of the primer is represented by SEQ ID NO:1, a nucleotide sequence of a downstream primer is represented by SEQ ID NO:2. the present invention implements an PCR amplification to a detectingformwork DNA by the primer, an amplifying outcome yield is processed by an agarose gel electrophoresis and observed under an ultraviolet light, if it is a positive result, there will be a specificity amplifying band, otherwise there will not be a band. According to an ITS zone sequence database OF THE animal clonorchiasis sinensis, the invention designs the primer, builds a rapid, special and sensitive PCR method, and it is capable of authenticating the animal clonorchiasis sinensis accurately. The operation of the kit of the invention is simple and programmable, the method specificity is strong, the sensibility is high, the result judgement is objective, and the invention is capable of being used for diagnosing the animal clonorchiasis sinensis and inquiring epidemiology.
Owner:SOUTH CHINA AGRI UNIV
Enzyme-linked immunosorbent assay (ELISA) kit for duck hepatitis virus type-I serum antibody, test method and application thereof
InactiveCN101839917AStrong specificityIncreased sensitivityDepsipeptidesFermentationAntigenDuck hepatitis A virus
The invention relates to an enzyme-linked immunosorbent assay (ELISA) kit for duck hepatitis virus type-I serum antibody and relates to a test method and application of the kit. The kit comprises an enzyme label plate coated by the recombinant VP1 (virus protein) protein, a rabbit anti-duck IgY antibody marked by horseradish peroxidase, a TMB substrate colour reagent, a positive serum, a negative serum and a kit specification. In the invention, by adopting the polymerase chain reaction, the VP1 genes are amplified from the DHV-1genome and the VP1 gene-containing recombinant expression plasmid pET32a-VP1 is constructed; the plasmid is transferred to host cells BL21 (DE3), and the in-vitro expression VP1 protein is purified by a nickel column and then used as the antigen; the enzyme-linked immunosorbent assay kit is established; the positive serum is the standard positive serum of duck hepatitis virus type-I and the negative control is the standard negative serum of duck. The test kit has the advantages of strong specificity, high sensitivity, simple operation, easy large-scale popularization and application, very important application value in diagnosis of duck hepatitis virus type-I, survey of epidemiology and immunization survey and the like.
Owner:HENAN UNIV OF SCI & TECH
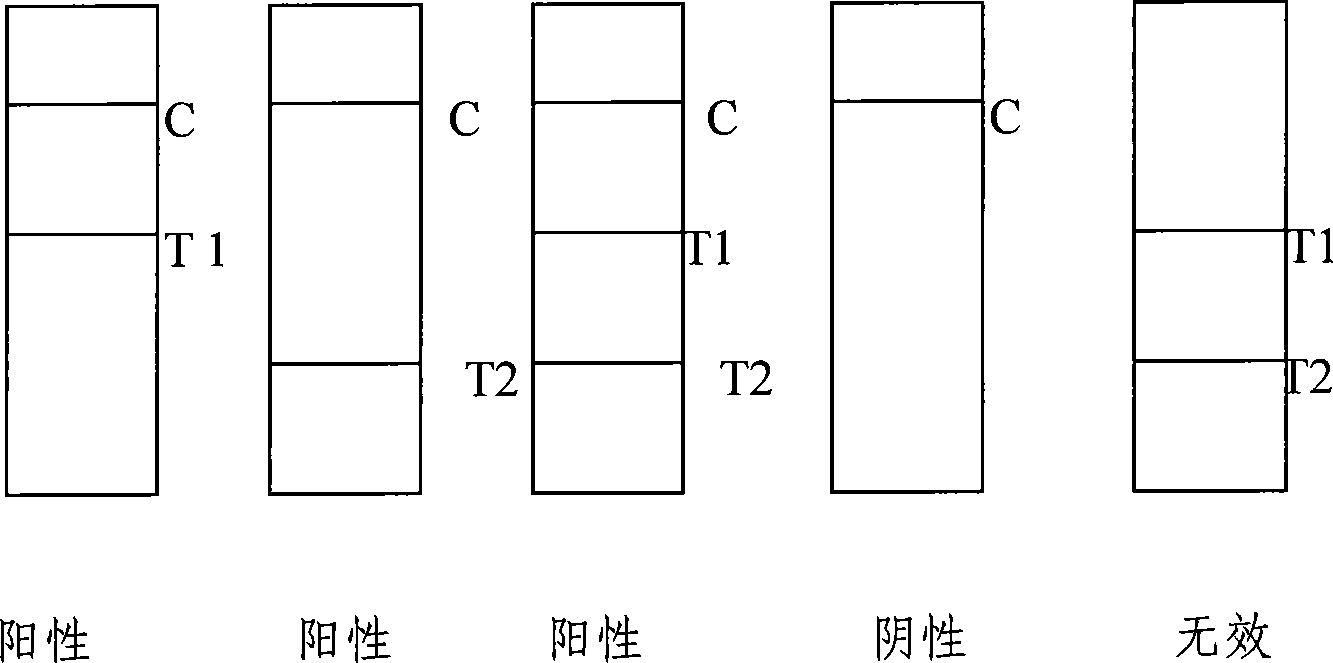
Colloidal gold method detection test strip and reagent kit for IgM and IgG antibodies of mycoplasma pneumoniae and preparation method of reagent kit
The invention discloses a colloidal gold method detection test strip and a reagent kit for IgM and IgG antibodies of mycoplasma pneumonia (MP) and a preparation method of the reagent kit. The test strip determines the IgM antibody and the IgG antibody by using a principle of an immunocapture method; the specific IgM and IgG antibodies of the MP can be detected jointly by one operation; the operation process is simplified; and the reagent kit is simple, convenient, rapid and accurate in detection, is particularly applicable to primary screening and epidemiological survey, and has an auxiliary diagnosis effect on early and interim mycoplasma pneumoniae infection.
Owner:JIANGSU KEYGEN BIOTECH CORP LTD
Food-originated pathogenic bactenium quick detection gene chip and its application
InactiveCN1536090AMicrobiological testing/measurementAgainst vector-borne diseasesFood poisoningBeta-hemolytic streptococcus
The present invention provides a gene chip for quickly detecting pathogens from food source, discloses the preparation method of said gene chip and provides 26 oligonucleotide probe sequences for detection. Said gene chip can quickly, accurately and high-effectively detect and identify the class of the pathogens in food, and its detection range includes staphylococcus aureus, Shiga's bacillus, salmonella, colibacillus 0157, bacillus proteus, mononuclear hyperplastic listerella, enterocolitis yersinia, aeruginous pseudomonads, vibrio parahaemolyticus, vibrio cholerae, bacillus cereus, beta hemolytic streptococcus, coconut fermentation pseudomonads, boticin, vibrio jejuni and bacillus perfringens, etc.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Primer system for detecting gene SNPs (single nucleotide polymorphisms) related to hereditary hearing loss and application of primer system
InactiveCN103276065AHigh detection sensitivityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationNucleotideQuantitative epidemiology
The invention discloses a primer system which is used for simultaneously detecting 10 gene SNPs (single nucleotide polymorphisms) related to hereditary hearing loss. Based on a product prepared by using the primer system, the 10 gene SNPs related to the related to hereditary hearing loss can be simultaneously detected. The product can be used for detecting the gene types of 10 gene SNPs of a testee, and the detection results can be used as a reference to clinical diagnosis and also can be used in the fields, such as epidemiological investigation, prenatal screening and neonatal screening. According to the primer system, 10 gene SNPs on different genes can be simultaneously detected in one reaction system; and therefore compared with technologies, such as sequencing, real-time fluorogenic quantitative PCR (polymerase chain reaction), the primer system is lower in cost and simple and convenient to operate, and the accuracy and sensitivity are improved.
Owner:向华
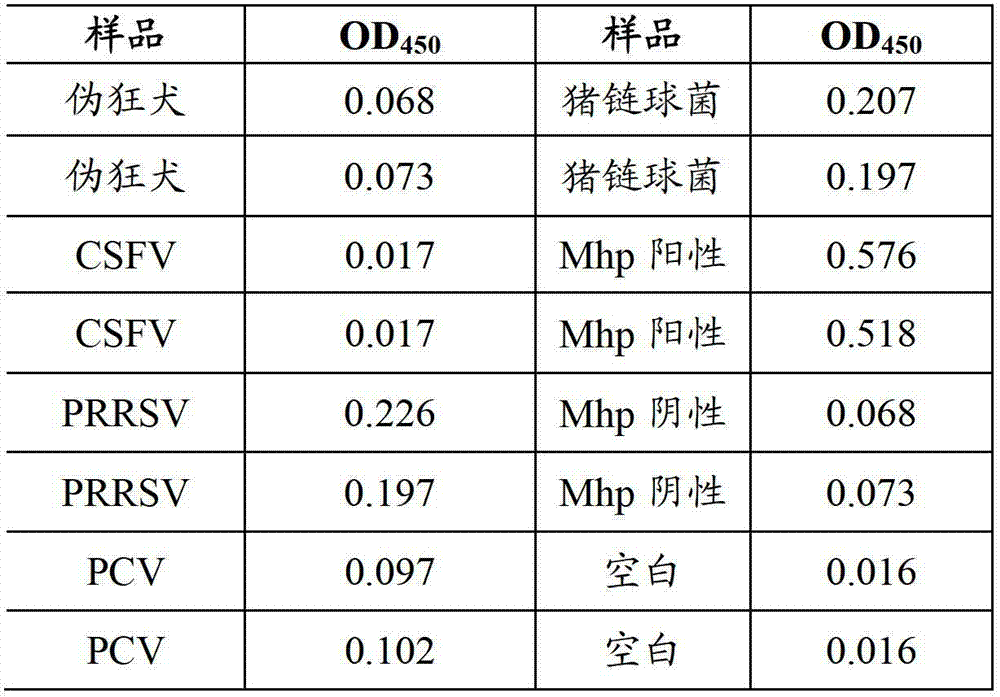
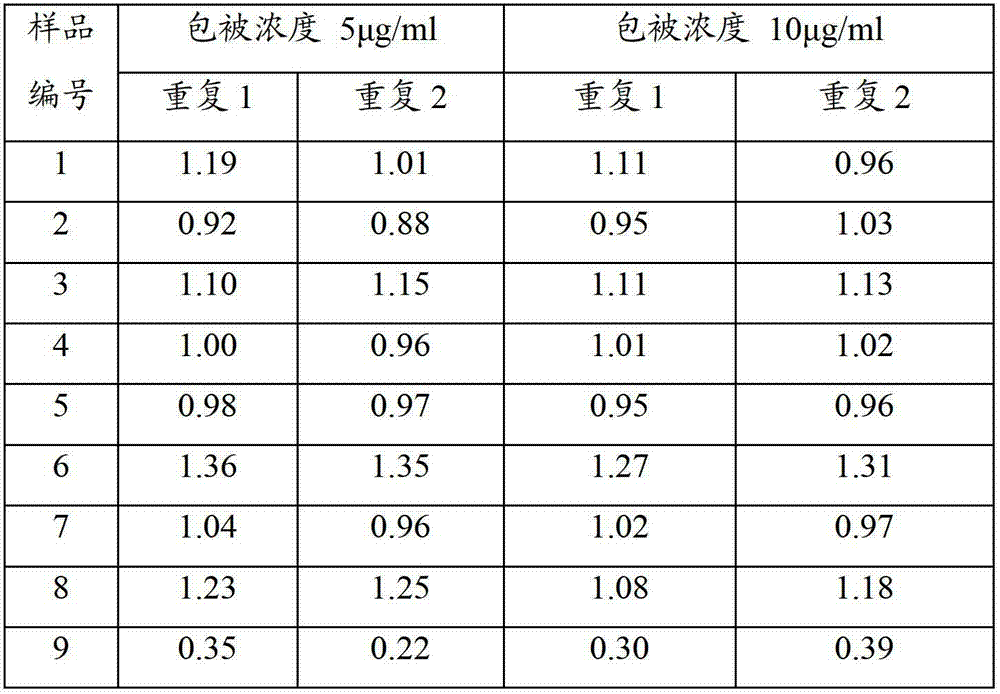
Mycoplasma hyopneumoniae indirect ELISA antibody detection kit and application
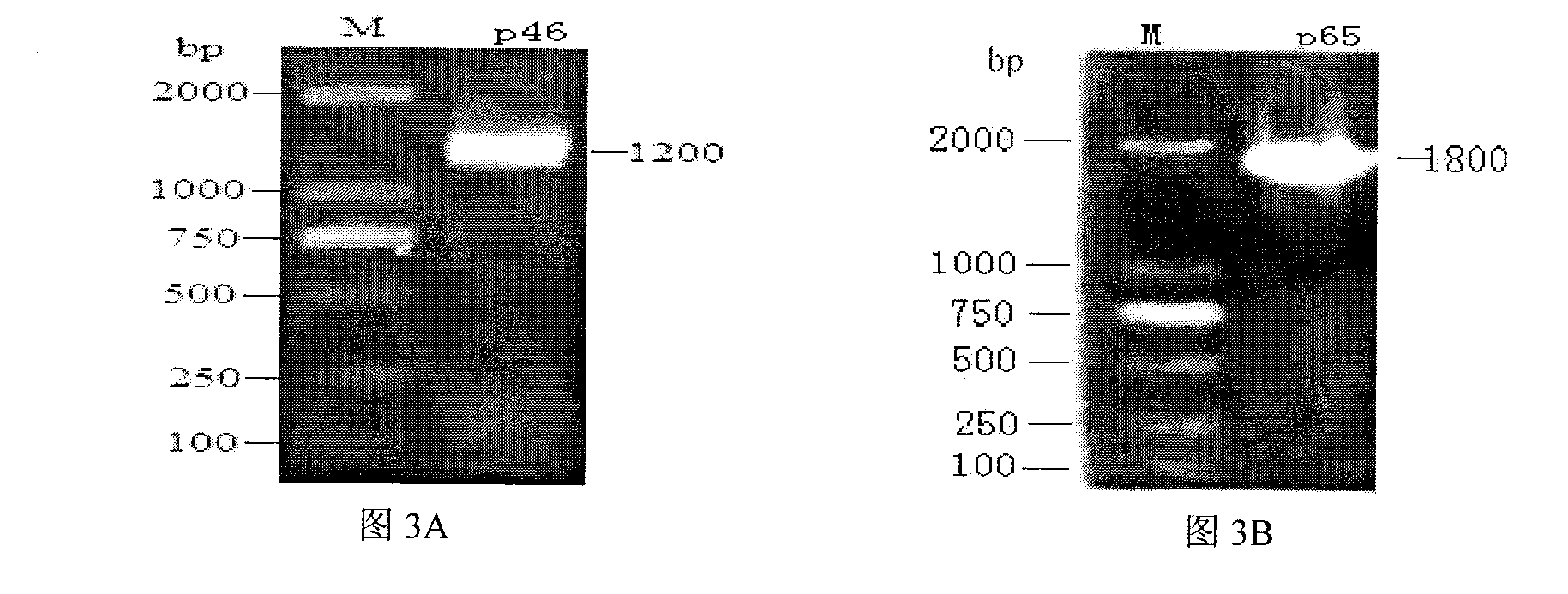
ActiveCN103018442AStrong specificityHigh sensitivityMaterial analysisEscherichia coliPurification methods
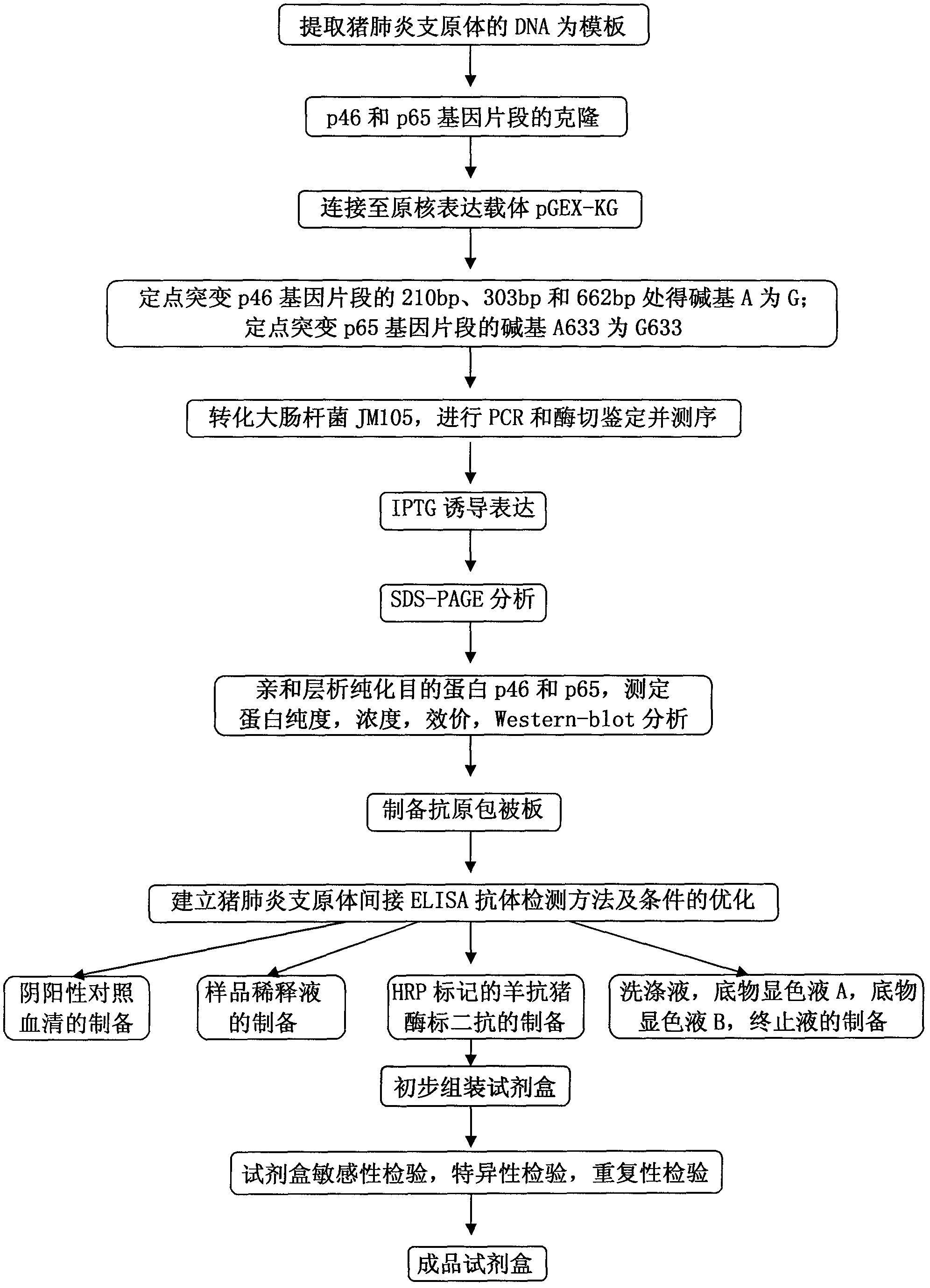
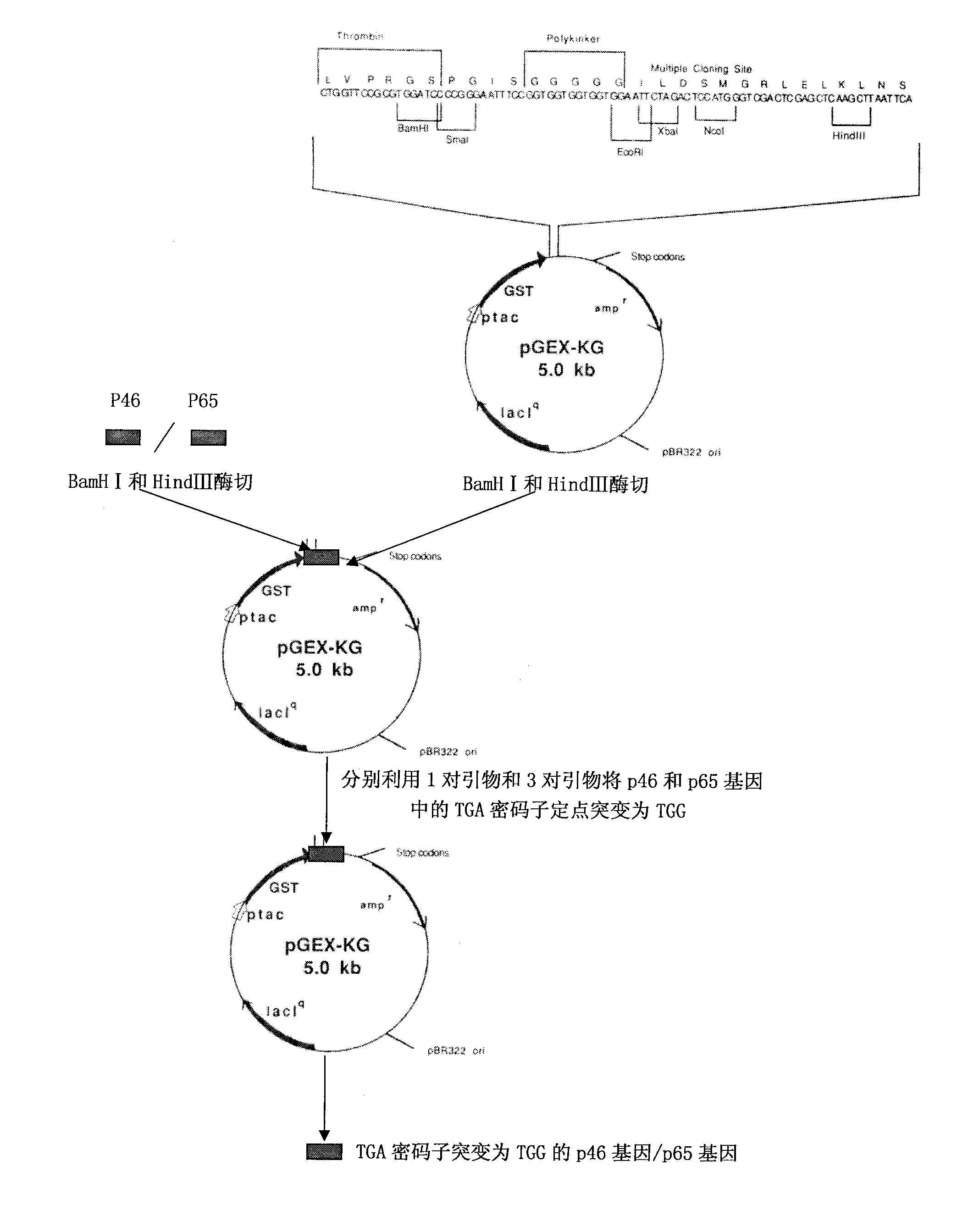
The invention belongs to the technical field of animal virology and animal infectious diseases detection, and concretely relates to a Mycoplasma hyopneumoniae indirect ELISA antibody detection kit and an application. According to the invention, two strains of recombinant E. coli pGEX-KG-46 and pGEX-KG-65 expressing M. hyopneumoniae p46 protein and p65 protein are obtained through a gene engineering recombinant technology. The kit provided by the invention comprises an ELISA plate using expression proteins of mutant P46 gene and P65 gene of the Mycoplasma hyopneumoniae membrane proteins as common antigen coating, and other core reagents. The invention discloses clone of the P46 gene and the P65 gene of the Mycoplasma hyopneumoniae membrane proteins, site-directed mutagenesis, and expression and purification methods of the p46 protein and the p65 protein, and further discloses a Mycoplasma hyopneumoniae indirect ELISA antibody detection method. The indirect ELISA antibody detection kit provided by the invention can be used for a large-scale clinical testing and an epidemiological investigation of the Mycoplasma hyopneumoniae antibody, and has a wide market prospect.
Owner:HUAZHONG AGRI UNIV +1
Vibrio parahemolyticus dual-real-time fluorescence PCR (Polymerase Chain Reaction) detecting primer, probe, detecting kit and detecting method
InactiveCN102146469AEasy to detectQuick checkMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceQuarantine
The invention discloses a vibrio parahemolyticus dual-real-time fluorescence PCR (Polymerase Chain Reaction) detecting primer, a probe, a detecting kit and a detecting method. The primer, the probe and the reaction condition are optimized and designed by using a dual-real-time fluorescence PCR method and adopting a toxR gene and a tdh gene of vibrio parahemolyticus as target genes, so that the toxic gene is detected when specific detection is carried out on the vibrio parahemolyticus in an amplification reaction, the toxR gene is used for specifically detecting the vibrio parahemolyticus, andthe tdh gene is used for detecting whether a toxic gene is carried or not. The invention has the advantages of rapid detection, reliable result, high sensitivity and high specificity, can finish the process from sample preparation to detecting result issuing in 8 to 10 hours, is free from the inference of false positive gender, cross contamination, and the like, provides a favorable tool for carrying out epidemiological survey of vibrio parahemolyticus, is suitable for inspection and quarantine of foods and marine products and can enable an inspection and quarantine bureau, a disease prevention and control center and a quality supervision department to carry out simple, rapid and accurate detection on the sample.
Owner:许龙岩 +1
Quadruple fluorescent PCR (Polymerase Chain Reaction) detection kit for common chicken food-borne bacteria and application method thereof
InactiveCN103436626AQuick checkHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesDiseaseBacteroides
The invention discloses a quadruple fluorescent PCR (Polymerase Chain Reaction) detection kit for common chicken food-borne bacteria and an application method thereof. The kit contains a fluorescent PCR solution and standards of Clostridium perfringens, Salmonella enteritidis, Escherichia coli O157: H7 and Campylobacter jejuni, wherein the fluorescent PCR solution contains Taq DNA (deoxyribonucleic acid) polymerase, a PCR buffer solution, Mg<2+>, dNTPs (deoxy-ribonucleoside triphosphates), four pairs of bacterial specific primers and four kinds of corresponding fluorescent probes. According to the detection kit and the application method thereof, four kinds of chicken-carried main food-borne pathogenic bacteria, namely Clostridium perfringens, Salmonella enteritidis, Escherichia coli O157: H7 and Campylobacter jejuni, can be rapidly and accurately detected by using quadruple fluorescent PCR, and the detection kit can be applied to the assay of bacteria, the diagnosis of diseases and epidemiological investigation.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Gold and silver combined inspection test paper for feline and canidae animals important pestilence pathogen and its production
A colloidal gold-silver united test paper used for detecting etiology of important disease on Felidae and Canidae animal is prepared mix-enveloping mouse source RV, CDV, RPV and CPV single-anti being labeled with colloidal gold on glass cellulose membrane to form combination pad; enveloping IgG of purified dog-resisting RV, CDV, FPV and CPV on R, D, F and P positions of detection line at nitric acid cellulose membrane and enveloping rabbit resisting mouse IgG on C position of quality control line at nitric acid cellulose membrane to from detection membrane.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Fluorescent quantitation PCR (polymerase chain reaction) detection kit for four conventional herpesvirus hominises
InactiveCN103820573AMicrobiological testing/measurementMicroorganism based processesFluoProbesHerpesvirus hominis
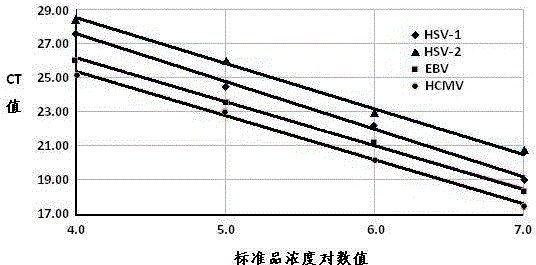
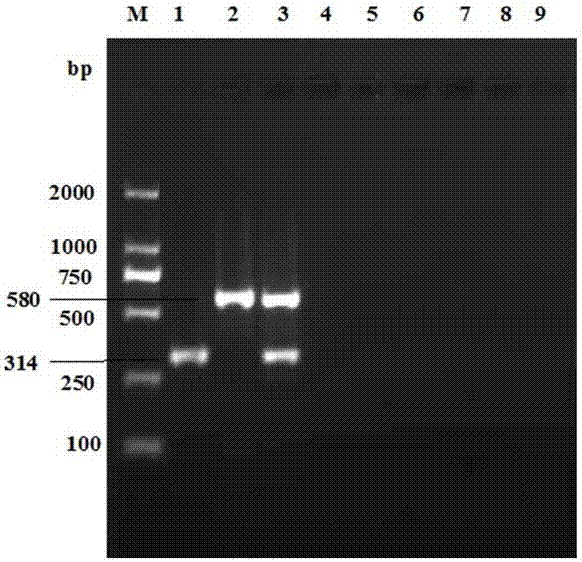
The invention provides a real-time fluorescent quantitation PCR (polymerase chain reaction) kit for simultaneously detecting conventional herpesvirus hominises HSV (herpes simplex virus)-1, HSV-2, EBV and HCMV (human cytomegalovinis). The kit consists of quantitation PCR reaction liquid, quantitation reaction liquid, an HSV-1 standard substance, an HSV-2 standard substance, an EBV standard substance, an HCMV standard substance, a negative reference substance, an HSV-1 positive reference substance, an HSV-2 positive reference substance, an EBV positive reference substance, an HCMV positive reference substance, a specification and a kit body. The kit disclosed by the invention adopts a real-time fluorescent quantitation PCR technology and two specific fluorescent probes in the same reaction system; the detection range covers the four most conventional herpesvirus hominises, so that the four herpesvirus hominises in a sample can be simultaneously subjected to parting detection; real-time and accurate quantitation can be realized; the need for early, quick and accurate diagnosis can be met; a powerful basis is supplied to epidemiological investigation and immediate formulation of a targeted treating scheme.
Owner:ZHEJIANG UNIV
Dual PCR detection probe and kit for duck adenovirus 2 and duck adenovirus A
InactiveCN107058634AEquivalent sensitivitySimplify operating proceduresMicrobiological testing/measurementDNA/RNA fragmentationDuplex pcrMicrobiology
The invention provides a dual PCR detection probe and kit for duck adenovirus 2 and duck adenovirus A, and belongs to the field of epizootiology. Two pairs of primers which are shown in SEQ ID NO.1-4 are adopted, a dual PCR detection method through which differential diagnosis can be conducted on duck adenovirus 2 and duck adenovirus A in a duck flock is built, a basis is laid for developing an epidemiological survey of adenovirus infection types and scientific prevention and control of relevant diseases in the duck flock, and thus the dual PCR detection probe and kit for duck adenovirus 2 and duck adenovirus A have very important study significance.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
PCR (Polymerase Chain Reaction) primer for detecting and identifying porcine circovirus 3 (PCV3) and detection method and detection kit
InactiveCN107012259ASimple and efficient operationStrong specificityMicrobiological testing/measurementMicroorganism based processesConserved sequenceMicrobiology
The invention discloses a PCR (Polymerase Chain Reaction) primer for detecting and identifying porcine circovirus 3 (PCV3) and a detection method and a detection kit. A pair of PCR primers for detecting the PCV3 is designed by performing preference comparison with genomic sequences of the PCV3 according to a conserved sequence on a cap protein of the PCV3. Sequences of the forward and reverse primers PCV3-F / R of the PCR are sequentially shown as SEQ ID NO.1 to SEQ ID NO.2. The invention further provides a PCR detection method and kit for the PCV3. The primer and the detection method disclosed by the invention are capable of rapidly, conveniently and efficiently detecting and identifying the PCV3, the specificity is high, the sensitivity is high, and the detection method is simple and high-efficiency in operation, is convenient for clinical detection and convenient for developing epidemiological investigation and has great application prospects.
Owner:SOUTH CHINA AGRI UNIV
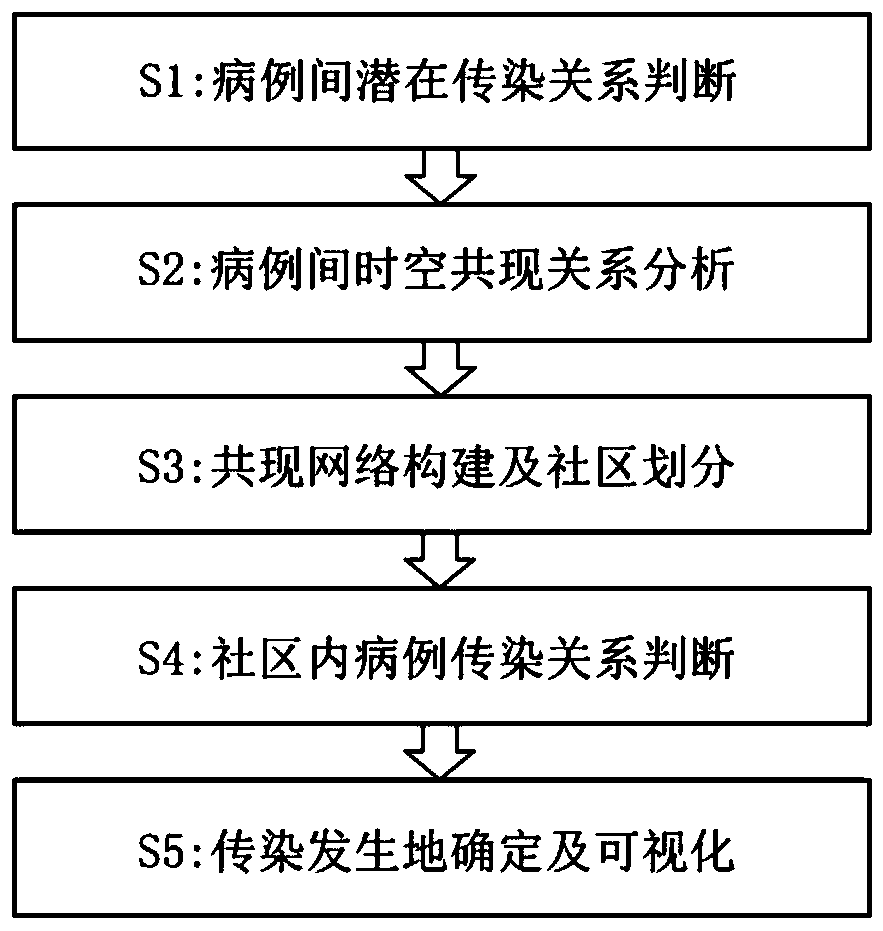
Respiratory infectious disease infection tree reconstruction method based on mobile phone signaling data
ActiveCN111540476AQuick fixAchieve traceabilityEpidemiological alert systemsCharacter and pattern recognitionDiseaseEpidemiologic survey
The invention discloses a respiratory infectious disease infection tree reconstruction method based on mobile phone signaling data. The method comprises the following steps: S1, judging a potential infection relationship between cases; S2, analyzing a space-time co-occurrence relationship between cases; S3, performing co-occurrence network construction and community division; S4, judging a case infection relationship in the community; and S5, determining and visualizing an infection place. According to the invention, rapid judgment of a case infection relationship and determination and visualization of an infection position can be realized during outbreak of respiratory infectious diseases, and the limitations that the traditional epidemiological investigation is time-consuming and labor-consuming and the result is not clear and visual enough are overcome; the method can quickly reconstruct infection tree between cases, determine the spatial position of infection, and display the spatial position on a map in a visual manner, and is beneficial to mastering the disease propagation process in time, improving the virus traceability and blocking the propagation efficiency.
Owner:INST OF GEOGRAPHICAL SCI & NATURAL RESOURCE RES CAS
Multi-antigen ELISA (Enzyme Linked Immunosorbent Assay) kit for detecting African swine fever virus antibody
The invention provides a multi-antigen enzyme linked immunosorbent assay (ELISA) kit for detecting an African swine fever virus (ASFV) antibody, belonging to the field of a biotechnology and diagnosis and research of animal-borne diseases. The multi-antigen enzyme linked immunosorbent assay kit comprises expression and purification of three types of ASFV recombined antigens, preparation of positive and negative control blood serum of an ASFV antibody, optimal envelope antigen combination and concentration determination, optimization of multi-antigen ELISA (MA-ELISA (Microalbumin-Enzyme Linked Immunosorbent Assay)) reaction parameters, determination of an ASFV antibody negative blood serum critical value, MA-ELISA detection artificial infection and determination of sensitivity, specificity and repeatability of field blood serum samples. By detecting and testifying a large quantity of known blood serum samples, the sensitivity, the specificity and the repeatability of detecting the ASFV antibody by the MA-ELISA are obviously higher than those of an ELISA method recommended by World Organization for Animal Health and oversea similar kits; and the multi-antigen enzyme linked immunosorbent assay kit can be used for ASFV serological diagnosis, epidemiological investigation and live pig import and export quarantine inspection.
Owner:YANGZHOU UNIV
Primers for triple PCR of three types of sheep pathogenic mycoplasmas and detection method
InactiveCN106434919AOvercome limitationsQuick checkMicrobiological testing/measurementDNA/RNA fragmentationEpidemiologic surveyMycoplasma pneumonia
The invention provides primers for triple PCR of three types of sheep pathogenic mycoplasmas and a detection method. The three pairs of special primers MO, MCC and MCCP are synthesized respectively according to the designs of sheep mycoplasma pneumoniae, a goat mycoplasma mycoide subspecies and a goat mycoplasma pneumonia subspecies, and a triple PCR optimum reaction system and reaction conditions for the three types of sheep pathogenic mycoplasmas are provided. The simultaneous pathogenic detection of the sheep mycoplasma pneumoniae, the goat mycoplasma mycoide subspecies and the goat mycoplasma pneumonia subspecies can be rapidly carried out without cloning, sequencing and sequence comparison, and the detection method has the advantages of being rapid, accurate, strong in specificity, good in repeatability and the like, suitable for rapid detection of the sheep mycoplasma pneumoniae, the goat mycoplasma mycoide subspecies and the goat mycoplasma pneumonia subspecies and large-scale epidemiological investigation and has great economic and social benefits.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Colloidal gold immunochromatographic test paper detecting African swine fever virus antigen, preparation method and application thereof
The invention relates to colloidal gold immunochromatographic test paper detecting African swine fever virus antigen, a preparation method and application thereof, belonging to virus disease diagnosistechnologies and the field of animal quarantine. The test paper comprises a PVC bottom board, wherein a sample pad, a colloidal gold pad, a nitrocellulose membrane and a water absorption pad are fixed on the PVC bottom board in sequence, the colloidal gold pad is internally provided with one epitope of monoclonal antibody Mab1 of an anti-ASFV p30 antigen marked by colloidal gold, the surface of the nitrocellulose membrane is provided with a detection line and a quality control line, the detection line is designed for another epitope of monoclonal antibody Mab2 for ASFVp30 antigen, and the quality control line is a goat anti-mouse IgG antibody. The colloidal gold immunochromatographic test paper has the advantages of being convenient, rapid, sensitive and specific, can detect ASFV infectedcells directly, and is suitable for ASFV infected diagnosis, epidemiologic investigation and international trade quarantine for swine.
Owner:ROHI BIOTECH
Primer system for detecting gene SNP related to genetic deafness, and use thereof
ActiveCN103352073AHigh detection sensitivityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationAccuracy improvementFluorescence
The present invention discloses a primer system for concurrently detecting 10 gene polymorphism sites related to genetic deafness. Based on the product prepared by the primer system, 10 gene polymorphism sites related to genetic deafness can be concurrently detected. Use of the product, genotypes of 10 gene polymorphism sites of a subject are detected, and the detection results can be used for assisted clinical diagnosis, and can further be used for epidemiological investigation, and can also be used for prenatal screening, neonate screening and other fields. With the present invention, 10 gene polymorphism sites on different genes can be concurrently detected in a reaction system, such that advantages of low cost, convenient operation, accuracy improvement and sensitivity improvement are provided compared with sequencing, real-time fluorescence quantitative PCR and other technologies.
Owner:BIOYONG TECH
ELISA detection method and reagent kit for bluetongue viral antigen
InactiveCN101403759AImprove featuresIncreased sensitivityBiological testingSerum igeMonoclonal antibody
The invention discloses an ELISA detection method and a kit of blue-tongue virus antigen. The detection method uses the monoclonal antibody of anti-blue-tongue virus VP7 protein as dual-anti sandwich ELISA detection method of a coating and enzyme-marked antibody; and the kit comprises the monoclonal antibody of the anti-blue-tongue virus VP7 protein of the coating and enzyme-marked antibody. The kit and the detection method can be used for detecting the blue-tongue virus antigen, have higher specificity and sensitiveness, can be used in large-scale forserological detection, is suitable for epidemiological investigation and has wide application prospect.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Preparation method for African swine fever virus antibody detection colloidal gold immunochromatography test paper strip
InactiveCN103293306AEasy to manufactureShorten detection timeMaterial analysisAfrican swine fever virus AntibodyTrue positive rate
The invention relates to a preparation method for an African swine fever virus (ASFV) antibody detection colloidal gold immunochromatography test paper strip, belonging to the field of bioengineering and animal epidemic disease diagnosis. The preparation method comprises the following steps of: expressing an ASFV recombinant antigen p54, purifying, preparing an antibody, preparing a colloidal gold immunochromatography test paper strip, and detecting the sensitivity and specificity of the ASFV antibody. Compared with the other conventional serological methods, the colloidal gold immunochromatography test paper strip prepared by the method has the advantages of convenience, quickness, sensitivity, specificity and the like, can be used for obtaining a definite diagnosis result within 5 minutes and directly detecting a suspicious swine serum (or anticoagulation) virus antibody, and is particularly suitable for field ASFV serological diagnosis, epidemiological survey and swine international trade quarantine and inspection.
Owner:YANGZHOU UNIV
Test paper strip for detecting clostridium difficile toxin A and toxin B colloidal gold, method for making same and applications
ActiveCN101363867ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisCelluloseClostridium difficile infections
The invention provides a test strip for rapid detection of clostridium difficile toxins A and B. A monoclonal antibody or polyclonal antibody of the clostridium difficile toxin A, a monoclonal antibody or polyclonal antibody of the clostridium difficile toxin B, and a quality control double-antibody IgG coat a nitrate cellulose film (NC film), and a membrane chromatography double antibody sandwich method is adopted to detect the clostridium difficile toxins A and B in a specimen in combination with a monoclonal antibody of colloidal gold labeled clostridium difficile toxins A and B. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base course, site detection and epidemiological investigation, and has auxiliary effect on the diagnosis of clostridium difficile toxin infection.
Owner:辽宁迪浩生物科技有限公司
Deer epidemic hemorrhage competitive enzyme-linked immunosorbent assay kit and preparation method and use thereof
InactiveCN101672849AStrong specificityIncreased sensitivityMaterial analysisSerum igeEpizootic haemorrhagic disease virus
The invention relates to a deer epidemic hemorrhage competitive enzyme-linked immunosorbent assay kit and a preparation method and a user thereof, the kit comprises an EHDV antigen-coated ELISA plate,a monoclonal antibody IgG-HRP enzyme conjugate, positive serum, negative serum, 20 times of concentrated washing liquid, 10 times of concentrated dilution buffer, a substrate I, a substrate II, a substrate III and stop solution. The preparation method comprises the following steps: a, preparing a deer epidemic hemorrhage virus ELISA-coated antigen, and detecting the safety of the deer epidemic hemorrhage virus ELISA-coated antigen; b, preparing a monoclonal antibody of deer epidemic hemorrhage virus and carrying out preparation and identification on the antibody-horseradish peroxidase (HRP) enzyme conjugate; c, preparing the positive serum and the negative serum; d, preparing the deer epidemic hemorrhage virus antigen-coated ELISA plate; e, preparing the 20 times of the concentrated washing liquid and the 10 times of the concentrated dilution buffer, and preparing the substrate I, the substrate II, the substrate III and the stop solution; and f, assembling the kit. The kit is used fordiagnosis, quarantine, detection and epidemiological investigation of deer epidemic hemorrhage and is characterized by strong specificity, high sensitivity and the like.
Owner:花群义
Mycoplasma hyopneumoniae antibody detection kit and manufacture method thereof
ActiveCN102928585AIncrease binding rateHigh detection sensitivityMaterial analysisSorbentImmunologic Surveillance
The invention belongs to the detection field of biotechnology and particularly relates to a mycoplasma hyopneumoniae antibody detection kit and a manufacture method thereof. A solid carrier is wrapped by a link-coupled mycoplasma hyopneumoniae peculiar polypeptide antigen, and the manufactured mycoplasma hyopneumoniae peculiar polypeptide antigen enzyme-linked immuno sorbent assay (ELISA) detection kit is used for clinical detection of mycoplasma hyopneumoniae infection, epidemiological investigation and immunologic surveillance. The non-protein polymer directional link-coupled mycoplasma hyopneumoniae peculiar polypeptide antigen serves as the wrapping solution, the combination efficiency of polypeptide and a target antibody is effectively improved, accordingly, detection sensitivity is remarkably improved, non-singular background value reading is remarkably reduced simultaneously, the specificity is high, and the detection kit has the advantages of being easy to operate, fast in diagnosis, economic and convenient in large-scale detection and the like. The manufacture method is convenient to popularize and has wide application prospect. When the kit is used for detecting samples, the using amount of the wrapping antigen can be reduced to 10ng / ml, so that cost is reduced, and popularization and using are facilitated.
Owner:GUANGDONG HAID ANIMAL HUSBANDRY & VETERINARY RES INST +1
Colloidal gold method detection test strip and reagent kit for IgG antibody of respiratory disease and preparation method of reagent kit
InactiveCN102928589AEasy to detectQuick checkMaterial analysisBovine respiratory diseasePrimary screening
The invention discloses a colloidal gold method detection test strip and a reagent kit for an IgG antibody of a respiratory disease and a preparation method of the reagent kit. The test strip determines the IgG antibody by using a principle of an immunocapture method; respiratory herpes viruses, adenoviruses, influenzaviruses and an IgG antibody of mycoplasma pneumoniae can be detected jointly by one operation; the operation process is simplified; the test strip is simple, convenient, rapid and accurate in detection, suitable for mass detection and applicable to primary screening and epidemiological survey; and a result is distinct and easy to distinguish.
Owner:JIANGSU KEYGEN BIOTECH CORP LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com