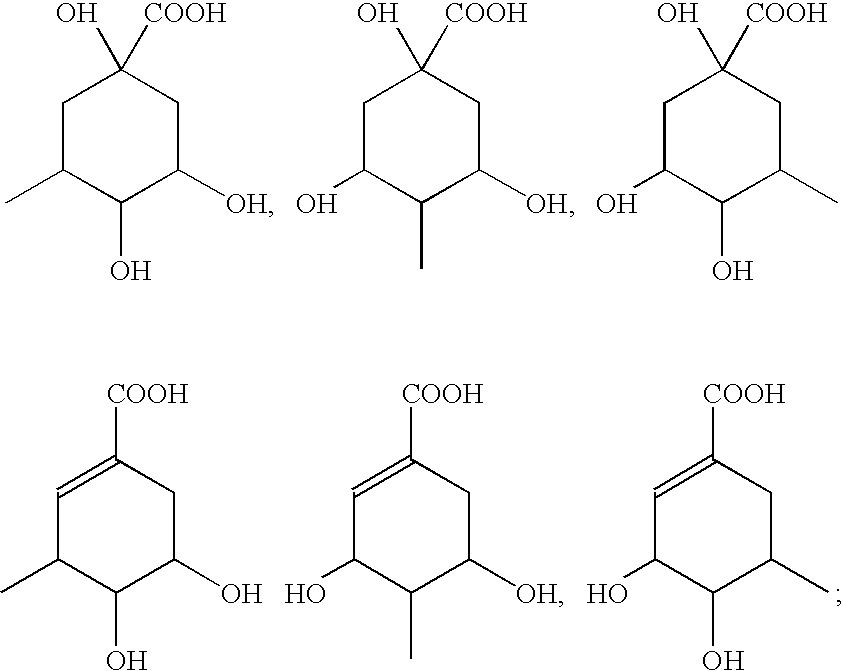
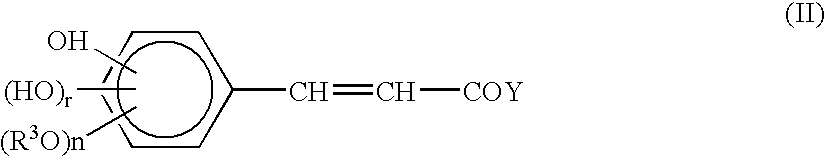Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1187 results about "Immune protection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immune Protect with Paractin contains a combination of patented ingredients that have been clinically shown to boost immune function, increasing the body's natural ability to combat challenges.
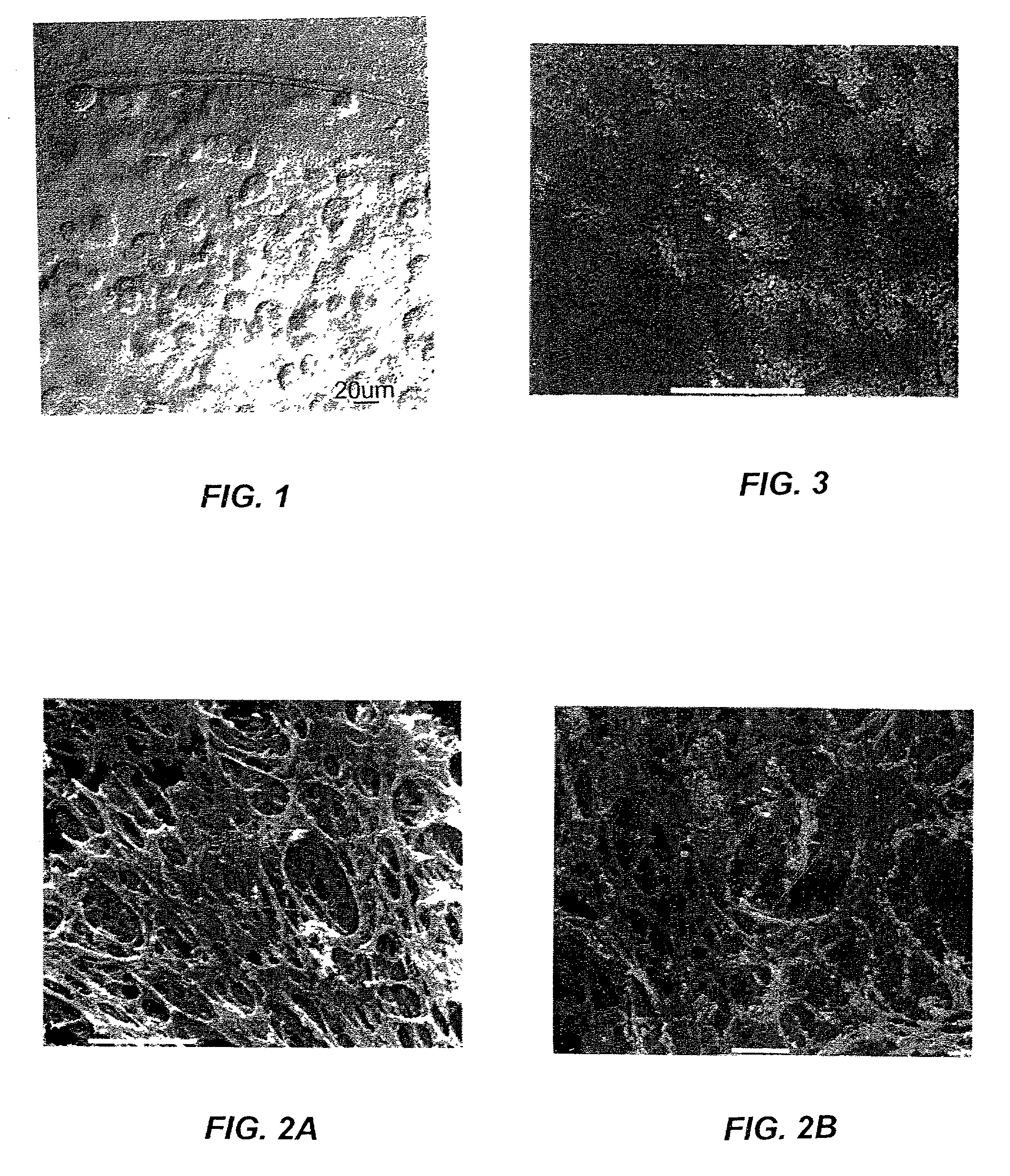
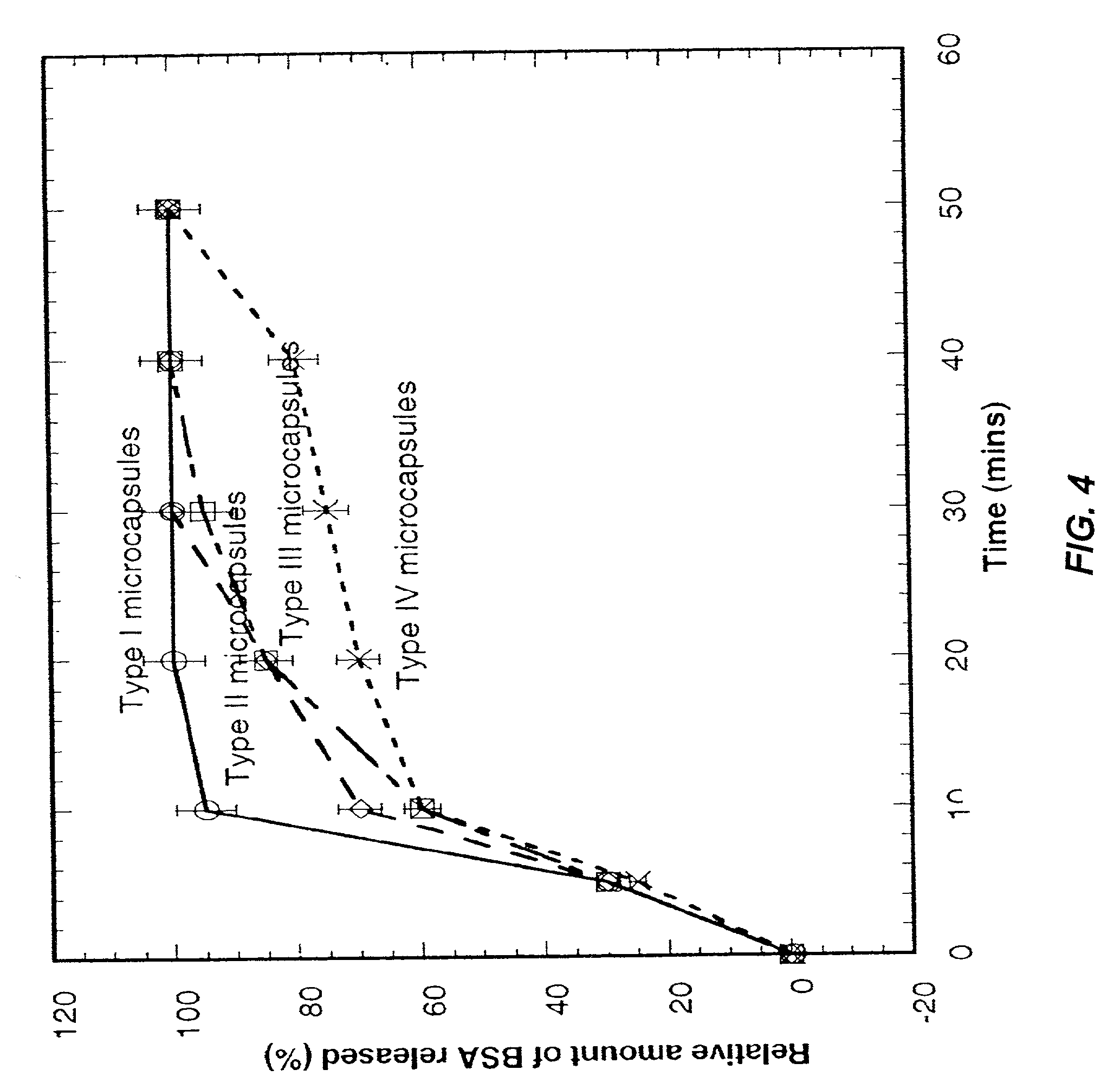
Multi-layer cell encapsulation for tissue engineering
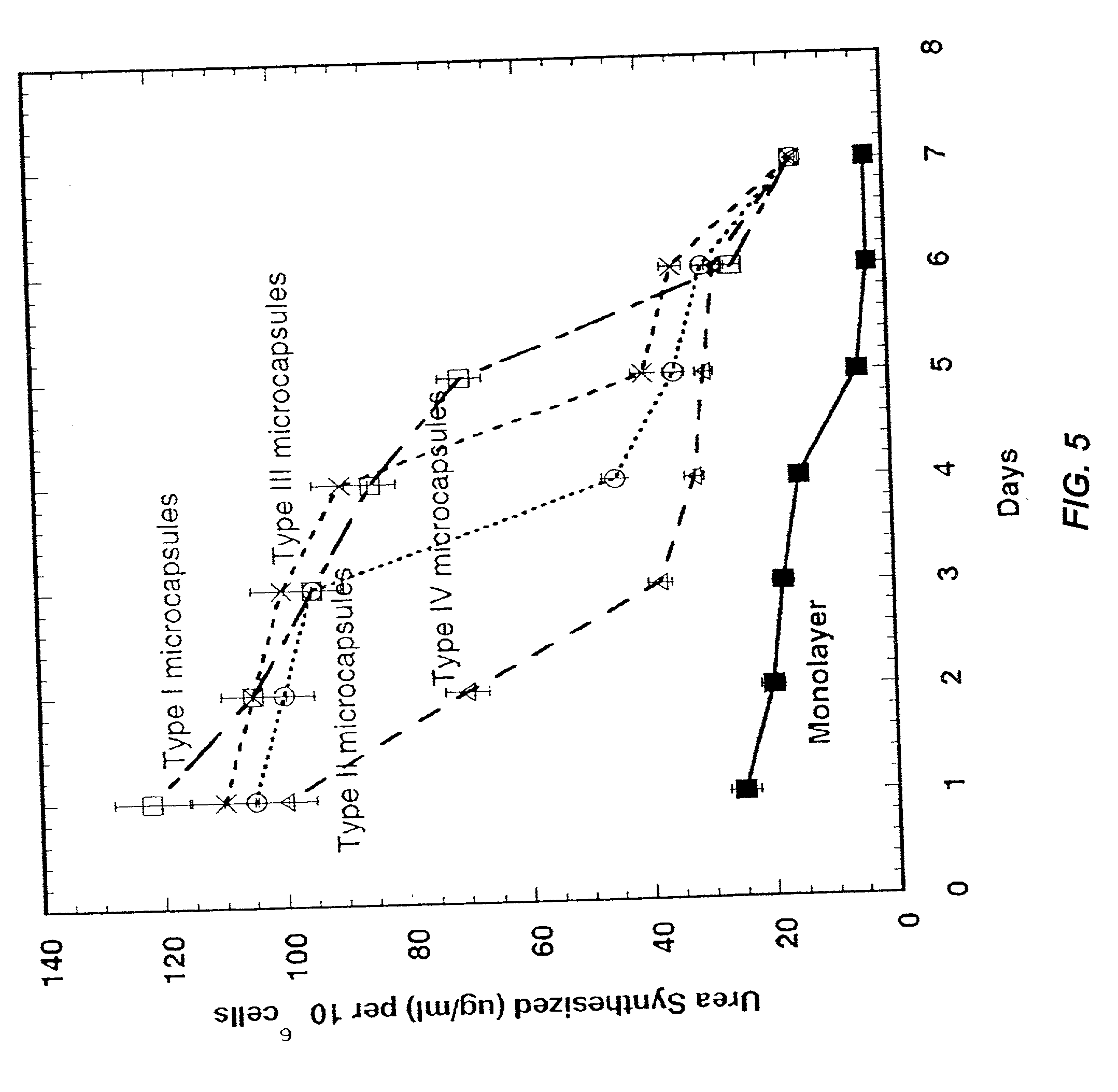
A multi-layered microcapsule has an inner extracellular matrix and an outer shell. The inner extracellular matrix includes a first inner layer of biopolymer and a second intermediate layer of polymer that provides partial immune-protection and holds the first layer in place. The outer shell can form an exoskeleton to provide mechanical stability. Each of the individual layers can be varied to optimize mechanical stability, cell function, and immuno-protection.
Owner:AGENCY FOR SCI TECH & RES +1
Porcine circovirus 2 type inactivated vaccine
InactiveCN101240264ASimple processEasy to operateViral antigen ingredientsMicroorganism based processesAdjuvantVaccine Production
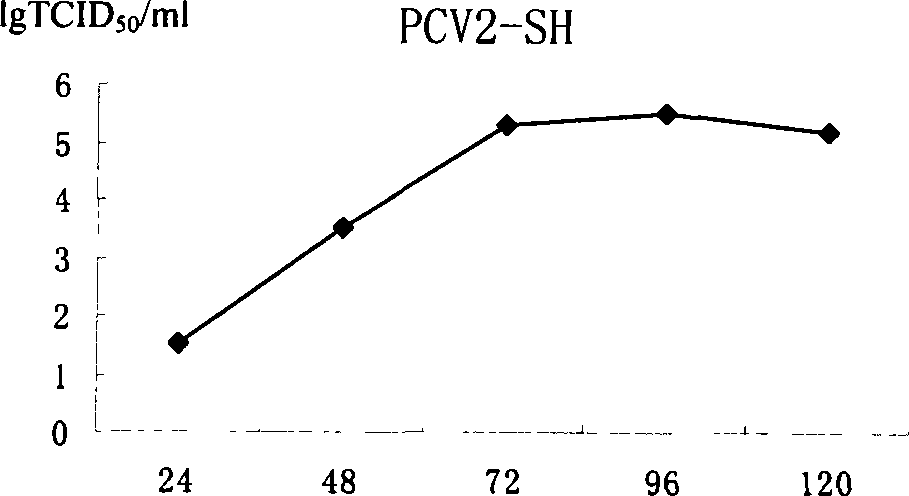
The pig circular ring virus 2 type (PVC2) inactivated vaccine (SH individual plant) of the invention belongs to biotechnology field. The pig circular ring virus 2 type poisonous individual plant SH belongs to circular ring virus section circular ring virus genus which has been preserved in Wuhan institute of virology, Chinese academy of sciences. The shanghai separated individual plant SH of purified PCV2 virus is obtained by gathering raw material from hogpen which happened bad weaning piglet multisystem exhaustion failure syndrome in Shanghai in 2002 year, separating, appraising and purifying virus. The PCV2-SH plant is proliferated in mass in PK-15 cell, inactivated through methyl aldehyde and emulsified with liquid paraffine adjuvant to prepare conventional liquid paraffin(e) adjuvant immunomodulators for vaccines. The laboratory has trial-manufactured five lots vaccines successfully which are good safety and also can induce pig bring immune protection effect, made out a draft rules for vaccines production and testing. The inactivated vaccine proved by every aspects experiment has met state biological products standard completely.
Owner:NANJING AGRICULTURAL UNIVERSITY
Recombinant SARS-CoV-2 vaccine using human replication-defective adenovirus as vector
ActiveCN111218459AReduce loadSimple manufacturing methodSsRNA viruses positive-senseViral antigen ingredientsProtective antigenCoronavirus vaccination
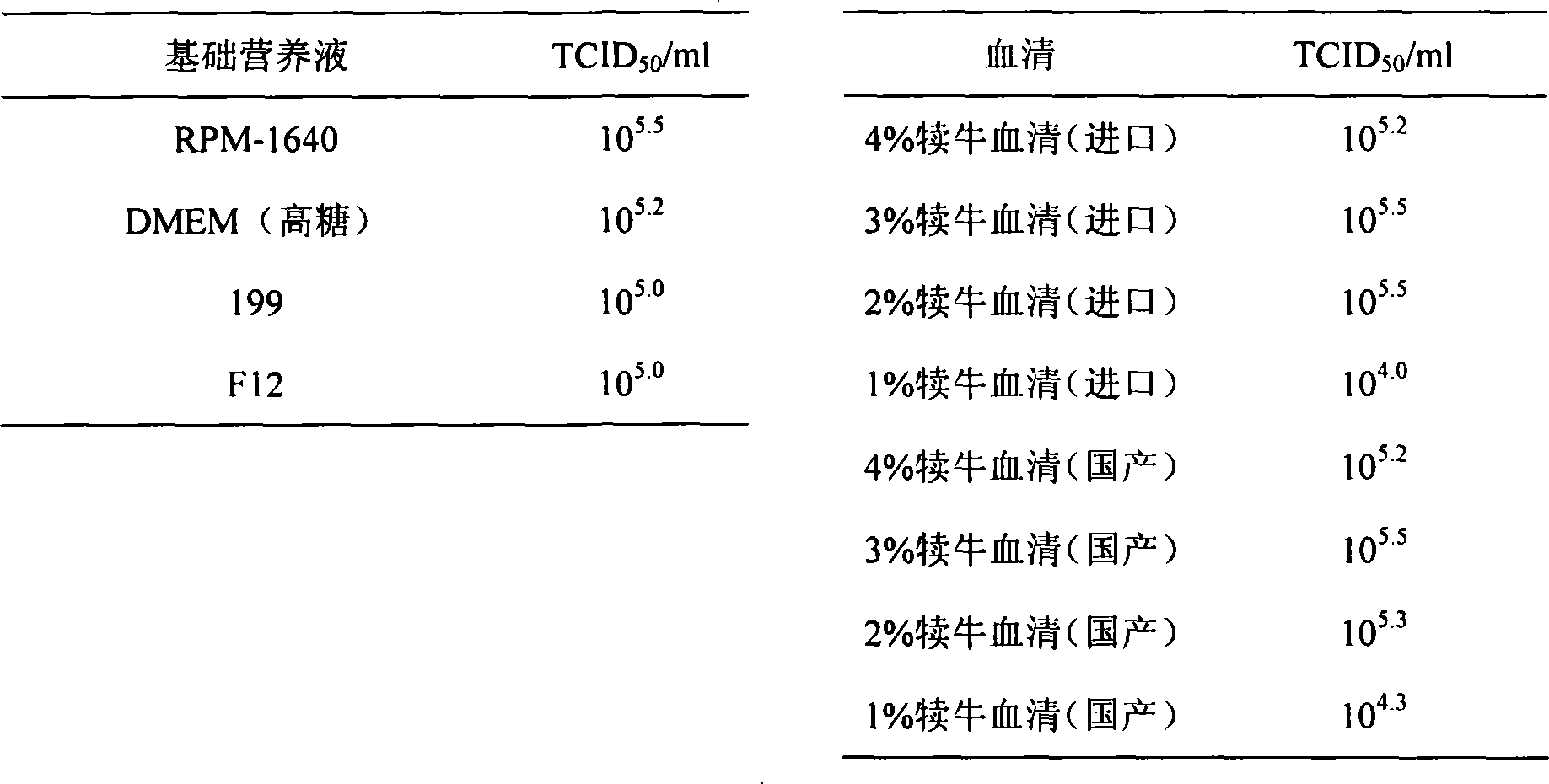
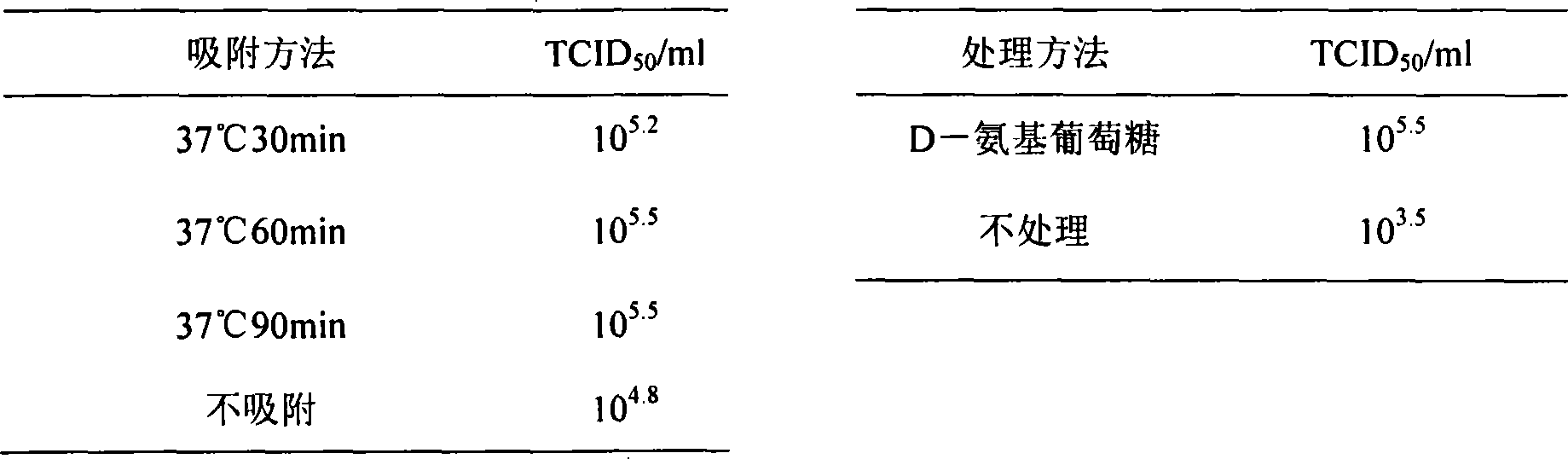
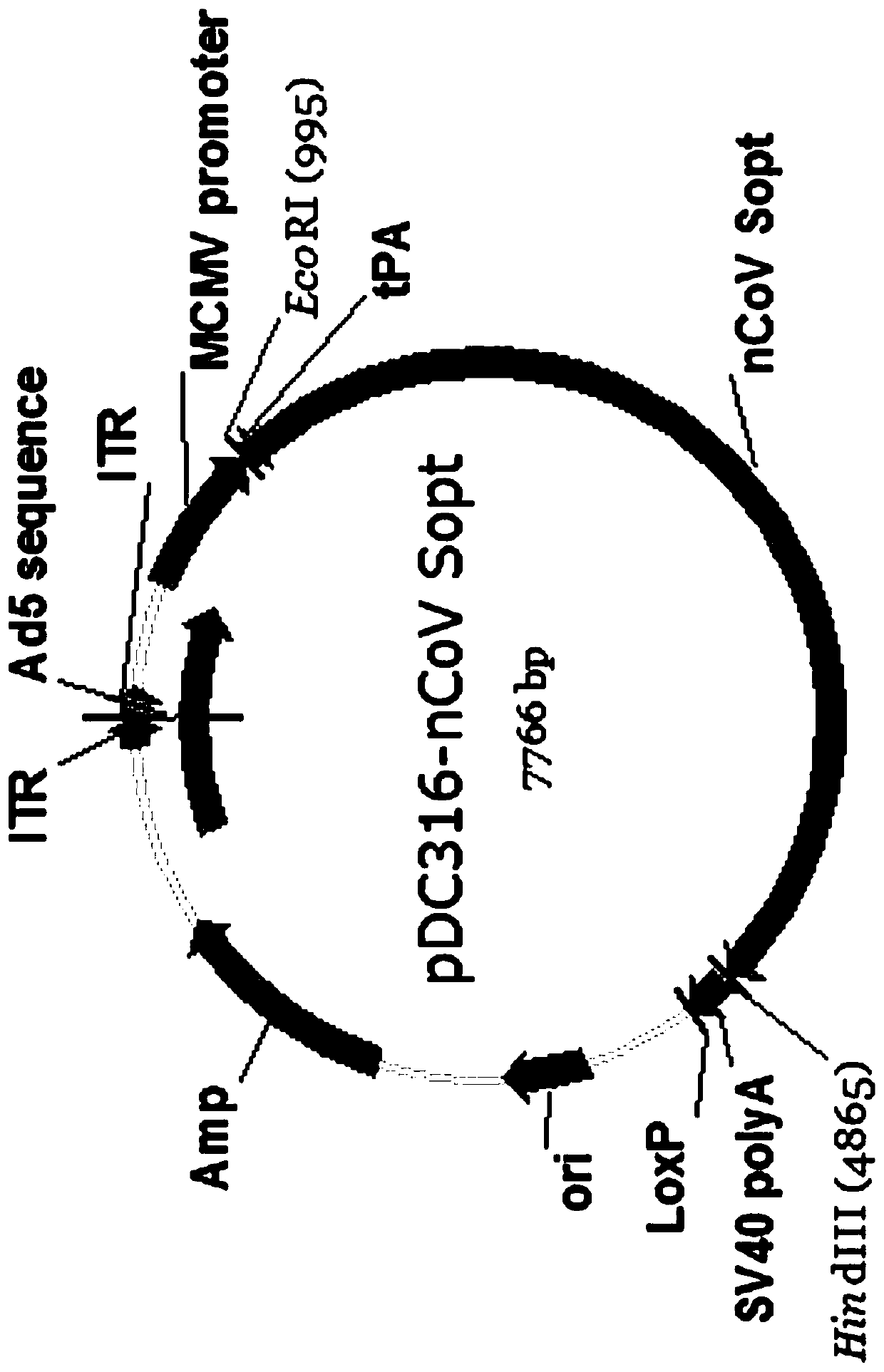
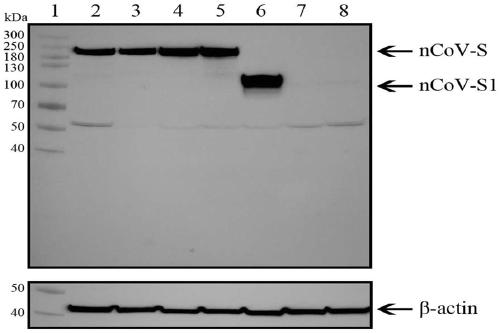
The invention provides a SARS-CoV-2 vaccine using human type-5 replication-defective adenovirus as a vector. The vaccine uses E1 and E3 to be combined with replication-defective human type-5 adenovirus as the vector and HEK293 cells integrating adenovirus E1 gene as a packaging cell line, and a protective antigen gene carried is the 2019 SARS-CoV-2 S protein gene (Ad5-nCoV) which is subjected to optimization design. After the S protein gene is optimized, the expression level in transfected cells is increased significantly. The vaccine has good immunogenicity in mouse and guinea pig models, andcan induce a body to produce a strong cellular and humoral immune response in a short time. Studies on the protective effect of hACE2 transgenic mice show that after 14 days of single immunization ofAd5-nCoV, the viral load in lung tissue can be significantly reduced, and it is indicated that the vaccine has a good immunoprotective effect on the 2019 SARS-CoV-2. In addition, the vaccine is quick, simple and convenient to prepare, and can be mass-produced in a short period of time to respond to sudden outbreaks.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
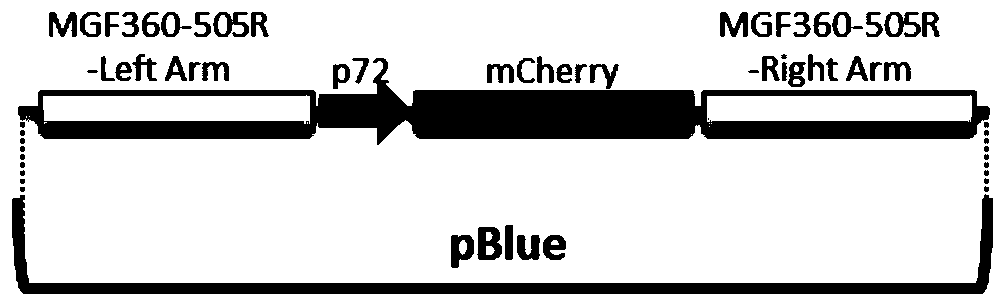
Gene deletion attenuated African swine fever virus and application thereof as vaccine
ActiveCN110093324AGood immune protectionFull Poison Attack ProtectionViral antigen ingredientsVirus peptidesAfrican swine feverGenetic engineering
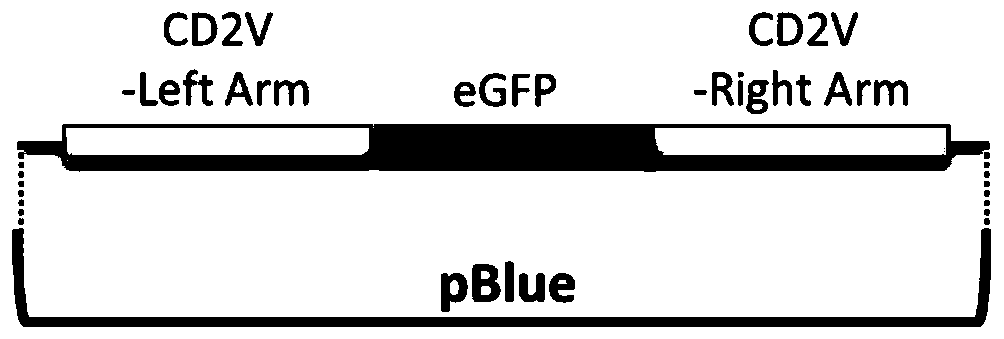
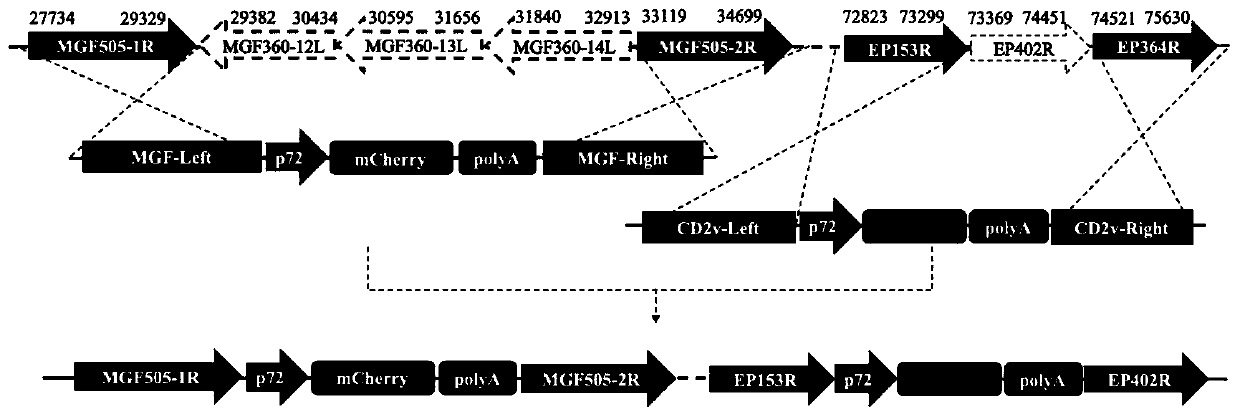
The invention relates to a gene deletion attenuated African swine fever virus as a vaccine and the vaccine, and a construction method thereof. An African swine fever Chinese epidemic strain Pig / CN / HLJ / 2018 is adopted, a virulence gene of the African swine fever virus is deleted by a genetic engineering technology, and the gene deletion virus of MGF360-505R deletion and joint deletion of CD2V and MGF360-505R is obtained. Experiments show that the two virus strains can provide 100% immune protection against the African swine fever Chinese epidemic virulent strains, can be used as vaccines for safe and effective prevention and control of African swine fever in China, and have great social value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
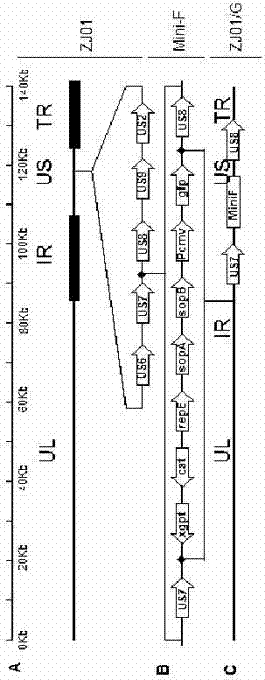
Porcine pseudorabies virus (PRV) variant PRV-ZJ01 and application thereof
ActiveCN103627678AImprove securityImproving immunogenicityMicroorganism based processesAntiviralsRabiesEngineering
The invention relates to the technical field of porcine pseudorabies viruses (PRVs) and in particular relates to a porcine PRV variant PRV-ZJ01 with collection number of CGMCCNo.8170 and an application of the porcine PRV variant PRV-ZJ01 in preparation of vaccines. The porcine PRV variant PRV-ZJ01 has the beneficial effects that a water-soluble inactivated vaccine is prepared by adopting a PRV-ZJ01 variant virus solution and is subjected to a swine immune protection test with live vaccines of Bartha-K61, Bucharest and HB-98 strains and the results show that the inactivated vaccine of the ZJ01 strain has relatively high safety and has the immune protection efficiency obviously higher than that of immunity groups of the live vaccines of the Bartha-K61, Bucharest and HB-98 strains, and the live vaccines of the Bartha-K61, Bucharest and HB-98 strains can not provide full protection for the ZJ01 very virulent strain; the inactivated vaccine of ZJ01 has relatively good immune protection effects on the PRV variant and the traditional strains; infected with 10<6.0>TCID50 (Tissue culture infectious dose 50) / ml nasal drops of the PRV-ZJ01 variant, all the 85-day-old non-immune swine can become ill and die; results prove that the virulence of the virus strain is obviously enhanced, the antigenicity is varied and the virus strain has relatively good immunogenicity after being inactivated and can be used for research and development of the vaccine of the virus strain and the diagnostic methods.
Owner:NANJING AGRICULTURAL UNIVERSITY
Immunoprotective influenza antigen and its use in vaccination
The present invention relates to an influenza antigen, comprising a fusion product of at least the extracellular part of a conserved influenza membrane protein or a functional fragment thereof and a presenting carrier, which may be a presenting (poly)peptide or a non-peptidic structure, such as glycans, peptide mimetics, synthetic polymers. The invention further relates to a vaccine against influenza, comprising at least an antigen of the invention, optionally in the presence of one or more excipients. The invention also relates to use of the antigen, a method for preparing the antigen and acceptor cells expressing the antigen.
Owner:VLAAMS INTERUNIVIR INST VOORS BIOTECH
Pluripotent therapeutic compositions and uses thereof
InactiveUS20090274660A1Reduce riskReduce significant riskBiocideOrganic active ingredientsSelf-healingAnticarcinogen
Synthetic Stem Cell-like Tissue Healing and Regeneration Medication with Anti-inflammatory, Protein Synthesis, Enzyme Deficiency Activation and Genetic Therapy, and Anti-cancer Agent derived from a series of inventions that include these products of Biomolecular Engineering, Drug Discovery from a Biologic Periodic Table of Applied Biochemistry and Biophysics. Tissue has a self healing effect promoting tissue healing and tissue regeneration. Not only does it maintain good health but also it has been observed that the patient's blood is withdrawn from the patient and applied to the ulcer has healing qualities. Cartilage placed in a wound promotes and accelerates wound healing. The anabolic biochemical and biophysical equivalent of tissue has been found in these embodiments to have the same pharmacologic qualities, when devoid of genetic DNA mismatch and other catabolic factors including the catabolic effects of microorganism overgrowth that lacks pro-biotic qualities. The healing efficacy of these tissue components gives us further appreciation of the protective action of human tissue over and above and other than the immune protective system or perhaps an integral component part of the immune system.
Owner:IMMUNOPATH PROFILE INC A CORP OF PA
Four-gene-deletion weak-toxin strain for African swine fever viruses and application of four-gene-deletion weak-toxin strain
InactiveCN110551695AEasy to solveViral antigen ingredientsMicrobiological testing/measurementAfrican swine feverToxin
The invention discloses a four-gene-deletion weak-toxin strain for African swine fever viruses. The weak-toxin strain is the four-gene-deletion weak-toxin strain for an African swine fever virus SY18separation strain, and the following gene function protein is deleted: CD2v gene coding products and three multigene family genes( MGF360-12L, MGF360-13L and MGF360-14L ) coding products. The invention further discloses an application of the weak-toxin strain of the African swine fever viruses to preparation of vaccines for preventing or treating African swine fever. The weak-toxin strain of the African swine fever viruses can provide complete immunoprotection effect on attack of ASFV parent toxin strains, is high in safety, and is suitable for being used as vaccine candidate strains for preventing the African swine fever.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Infant formula for optimal growth, gastrointestinal protection and immunological protection of infants
InactiveUS20150237902A1Better nutritional balanceImprove toleranceSugar food ingredientsVitamin food ingredientsOptimal growthImmune protection
An infant formula specially designed for covering nutrition necessities of infants between 0 and 36 months of life is described, which reduces the intolerance problems related to the consumption of infant formulas currently found in the art.
Owner:NUCITEC DE C V
gE- and gI-deleted porcine pseudorabies virus variant strain and use thereof
The invention relates to the technical field of porcine pseudorabies viruses and especially relates to a gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G and a use thereof. The gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G has the accession number of CGMCC No.7957. The invention discloses the use of the gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G in vaccine preparation. After the New Zealand big white rabbit is inoculated with the 106.0TCID50 recombinant viruses, clinical symptoms such as pruritus are not caused. An oil-in-water inactivated vaccine prepared from the gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G is injected into a piglet and after four weeks, the BELISA antibody is produced but the gE antibody does not exist, and the immunization protection efficiency is 100%. After immunization on sows, the piglets produced by the sows get immunization protection and the efficiency of PRV variant virus and traditional virus immunization protection is 100%. It is proved that the ZJ011G recombinant virus has good immunogenicity and can be used for vaccine preparation.
Owner:JIANGSU NANNONG HI TECH
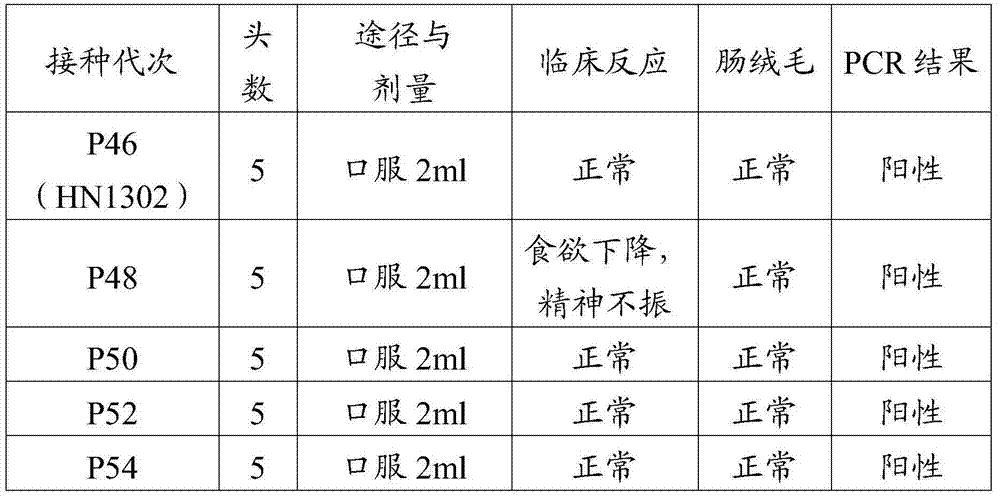
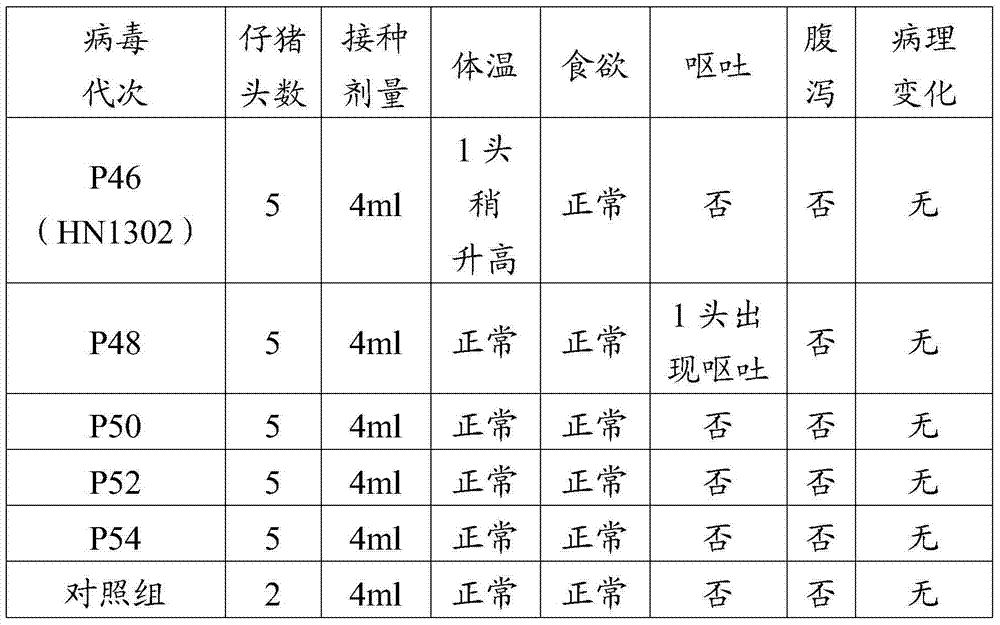
Porcine epizootic diarrhea virus strain, attenuated vaccine strain thereof and application thereof
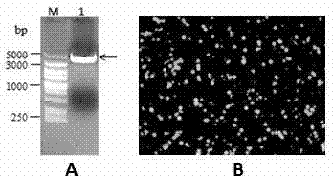
The invention discloses a porcine epizootic diarrhea virus strain (HN1301) and an attenuated vaccine strain (HN1302) attenuated by passage of the porcine epizootic diarrhea virus. The attenuated vaccine strain of the porcine epizootic diarrhea virus is assigned the accession number CCTCC-V201342. The attenuated vaccine strain (HN1302) is good in safety, is strong in immune protective capability and is high in immune efficacy.
Owner:PU LIKE BIO ENG
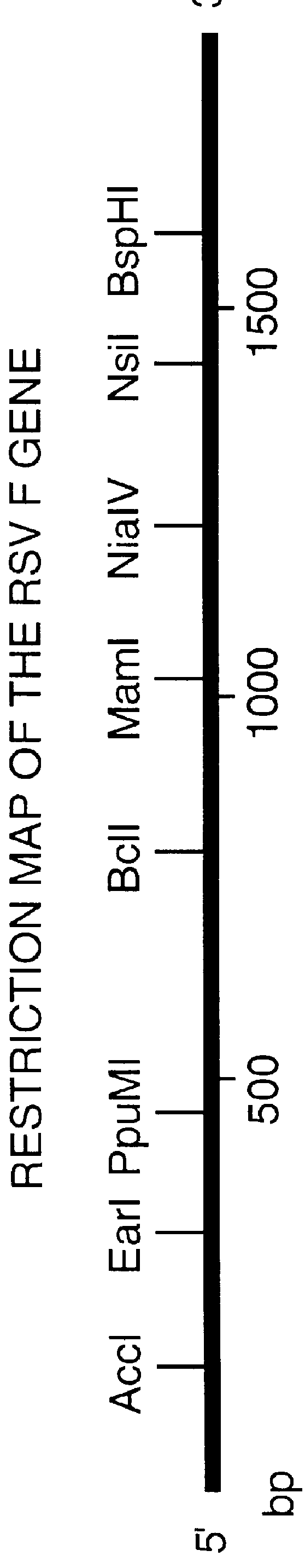
Nucleic acid respiratory syncytial virus vaccines
InactiveUS6083925AGood immune protectionImprove expression levelSsRNA viruses negative-senseGenetic material ingredientsHeterologousF protein
Non-replicating vectors containing a nucleotide sequence coding for an F protein of respiratory syncytial virus (RSV) and a promoter for such sequence, preferably a cytomegalovirus promoter, are described for in vivo immunization. The nucleotide sequence encloding the RSV F protein may lack a sequence encoding the homologous signal peptide but possessing a heterologous signal peptide enhancing RSV F protein expression. Such non-replicating vectors, including plasmids, also may contain a further nucleotide sequence located adjacent to the RSV F protein encoding sequence to enhance the immunoprotective ability of the RSV F protein when expressed in vivo. Such non-replicating vectors may be used to immunize a host against disease caused by infection with RSV, including a human host, by administration thereto, and may be formulated as immunogenic compositions with pharmaceutically-acceptable carriers for such purpose. Such vectors also may be used to produce antibodies for detection of RSV infection in a sample.
Owner:CONNAUGHT LAB
A type foot-and-mouth disease recombinant vaccine strains and preparation method and application thereof
ActiveCN103266091AMultiple phenotype improvements and enhancedPhenotype Improvement and EnhancementMicroorganism based processesAntiviralsAntigenDisease
The invention relates to A type foot-and-mouth disease recombinant vaccine strains prepared by using a reverse genetic manipulation technology and a preparation method and application of the strains. One strain is an A type foot-and-mouth disease recombinant vaccine strain with high titer, antigen matching property and immune protection rate, and the other strain is an A type foot-and-mouth disease recombinant non-pathogenic vaccine strain with high titer, antigen matching property and immune protection rate and without pathogenicity for a host; an antigen nucleotide sequence of each of the vaccine strains is shown as SEQ ID NO: 1; eukaryotic plasmids of viruses can be saved by using a reverse genetic manipulation system; after pigs and cattle are immunized by using the inactivated vaccines prepared from the prepared recombinant vaccine strains, the bodies can be effectively stimulated to produce immune response, and an immune protection effect is provided for the bodies of the pigs and the cattle; through a 10,000-times cattle median infectious dose (BID50) challenge assay of A type AISA topological strains, the immune protection rate reaches 100 percent, and the median protective dose (PD50) is 10.81 to 13.59; and the recombinant vaccine strains can be applied to prevention and control of A type foot-and-mouth disease viruses of China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for producing swine fever live vaccine with cell line
ActiveCN101181637AGuaranteed to be pureEnsure safetyAntiviralsAntibody medical ingredientsQuality controlSeedling
The invention discloses a method for producing a live swine fever vaccine by using a cell line. The present invention comprises the following technical steps: (1) selecting a cell line as the cells for making seedlings; (2) subculture and cultivation of cells for making seedlings; (3) breeding of cytotoxic species; (4) breeding of venom for making seedlings; 5) Mixing seedlings, subpackaging and freeze-drying. The invention has the advantages of simple and stable production process, easy operation, high virus content, small difference between batches, easy quality control, and can significantly improve the yield and quality of vaccines. The live swine fever vaccine produced by the invention has good safety and high immune efficacy, and has complete immune protection against the virulent attack of swine fever.
Owner:CHINA INST OF VETERINARY DRUG CONTROL +1
H9 subtype avian influenza virus isolate and vaccine composition prepared thereby
ActiveCN103789272AGood effectImprove immune efficiencyMicroorganism based processesAntiviralsHemagglutininAvian influenza virus
The invention discloses an avian influenza virus isolate belonging to an H9 subtype. An amino acid sequence of an HA1 structural domain of hemagglutinin has the following characteristic sites: 69-bit P, 180-bit A, 221-bit N and 236-bit R; the vaccine composition prepared by the H9 subtype avian influenza virus isolate with the following characteristic sites has good immune efficiency, and is superior to the vaccine prepared by the strain in the prior art in effect, cross protection can be provided for a popular wild strain, significant cross immunogen features are displayed, and the avian influenza virus isolate has a good application prospect in the aspect of preventing and treating poultry cross immune protection.
Owner:PU LIKE BIO ENG +1
Attenuated Live Vaccine for Prevention of Porcine Reproductive and Respiratory Syndrome
ActiveUS20120189655A1Organic active ingredientsSsRNA viruses positive-senseBiologyAttenuated Live Vaccine
The present disclosure provides an attenuated live vaccine strain and the formulations thereof, for preventing pigs from infection of porcine reproductive and respiratory syndrome (PRRS). The preparation methods for the vaccines and the formulations are also provided. The attenuated live vaccine strain provided herein offers significant immunological protection to pigs against PRRS. The vaccine formulations of the present disclosure also have advantages in long shelf lives as well as good stability during storage.
Owner:WU HUA
Photoprotector and/or photoimmunoprotector compositions of the skin and their uses
InactiveUS20070025933A1Prevent and minimise damaging effectPrevent and minimise reactionCosmetic preparationsBiocideBenzoic acidPhototherapy unit
The composition comprises of a component A selected from a hydroxylated derivative of benzoic acid or of cinamic acid, their esters, amides or salts, a glycoside of a hexose, and their mixtures; and a component B selected from quinic acid, shikimic acid, their alkaline metal or alkaline earth salts, their methyl esters, and mixtures of the same. This composition is suitable for protecting the skin against ultraviolet radiation coming from the sun or artificial sources, such as those used in phototherapy units and in sun tanning rooms. For application in the field of dermatology and nutrition, and, in particular, in the photoprotection of the skin and mucosa, photo-ageing and photocarcinogenesis, including protection of the immune system associated with the skin.
Owner:IND FARM CANTABRIA
Porcine epidemic diarrhea virus stain and application thereof
ActiveCN103725651AImprove securityGood immune protectionMicroorganism based processesAntiviralsEpidemic diarrheaMicroorganism
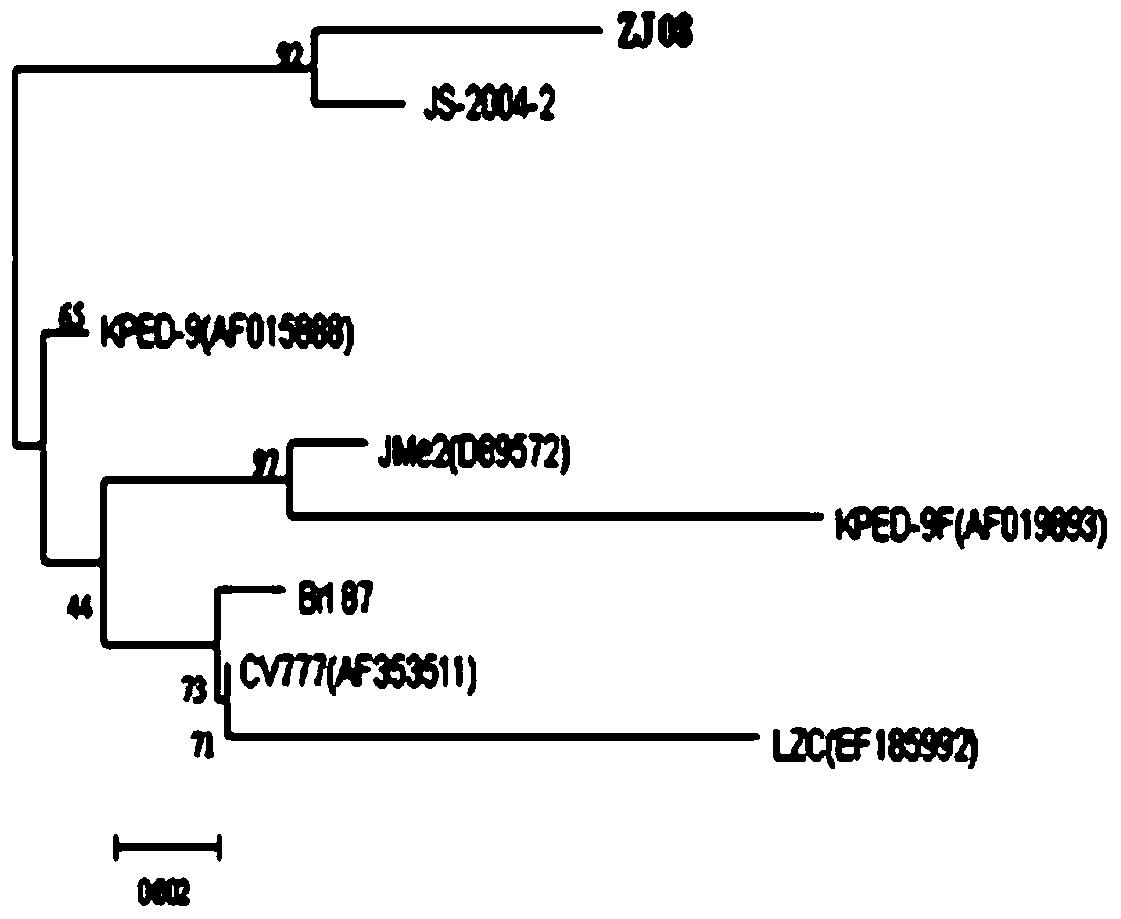
The invention discloses a porcine epidemic diarrhea virus low-virulent stain ZJ08 and application thereof. The microbial collection number of the low-virulent stain is CGMCC No.7806. The PEDV low-virulent stain has high safety, and is safe for pregnant sows, baby pigs and pigs in all ages. The active protection ratio of the low-virulent stain for 3-day baby pigs reaches 100%, and the passive protective ratio reaches higher than 94.7%, which indicates that the low-virulent stain has favorable immunoprotection effect on porcine epidemic diarrhea.
Owner:兆丰华生物科技(南京)有限公司 +3
Genetic engineering marked attenuated vaccine strain of porcine reproductive and respiratory syndrome virus and application thereof
ActiveCN102250843AMeet the differential diagnosisEasy to solveViral antigen ingredientsAntiviralsNucleotideGenetic engineering
The invention discloses a genetic engineering marked attenuated vaccine strain of a porcine reproductive and respiratory syndrome virus (PRRSV). The attenuated vaccine strain comprises a genomic nucleic acid of a porcine reproductive and respiratory syndrome virus attenuated vaccine strain HuN4-F112; the HuN4-F112 genome includes a mutation in a genetic region for coding an Nsp2 protein, and the mutation is as follows: a nucleotide sequence for coding a Newcastle disease virus NP protein is inserted to a lacking region of a nucleotide sequence for coding 480-532-site amino acid of the Nsp2 protein; or the nucleotide sequence for coding the Newcastle disease virus NP protein is inserted to the lacking region of a nucleotide sequence for coding 508-532-site amino acid of the Nsp2 protein. The invention also discloses an application of the genetic engineering marked attenuated vaccine strain. The genetic engineering marked attenuated vaccine strain of the porcine reproductive and respiratory syndrome virus provided by the invention not only can provide completely safe immune protection to resist high-pathogenicity PRRSV after the porcine is immunized, but also can effectively distinguish the immunized porcine of the porcine reproductive and respiratory syndrome vaccine with the naturally infected porcine of the field virus.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
MntC recombinant protein of staphylococcus aureus and preparation method and application thereof
ActiveCN103694323AHigh expressionEasy to separate and purifyAntibacterial agentsBacteriaPurification methodsStaphylococcus aureus
The invention belongs to the field of biotechnology, and relates to a MntC recombinant protein of staphylococcus aureus (SA), a carrier comprising the recombinant protein, a host, a composition or a kit, application, preparation, fermentation and purification method of the protein. The MntC recombinant protein prepared by the method has strong immunogenicity, is safe and non-toxic, and is proved by animal tests to be able to effectively stimulate an organism to generate high efficient humoral immune response and good immune protection.
Owner:CHENGDU OLYMVAX BIOPHARM +1
A-type kreotoxin receptor combination region Hc, coding protein and application thereof
The present invention discloses A type botulin receptor binding domain Hc gene and its coding protein and uses. The gene is one of the following nucleotides sequence 1) SEQ ID NO:1 DNA sequence in sequence list, 2) SEQ ID NO:2 DNA sequence in sequence list, 3) nucleotides sequence of above 90% homology with SEQ ID NO:1 DNA sequence and capable of stimulating body to produce immune protection to A type botulin,4) nucleotides sequence capable of hybridizing with SEQ ID NO:1 DNA sequence under strict condition. The present A type botulin receptor binding domain Hc gene and its coding protein will play an important role in A type botulin vaccine.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Recombinant adenovirus of porcine reproductive and respirator syndrome virus and porcine Circovirus, and vaccine
InactiveCN1800375AHas the copy featureStable potencyViruses/bacteriophagesAntibody medical ingredientsEscherichia coliCircovirus
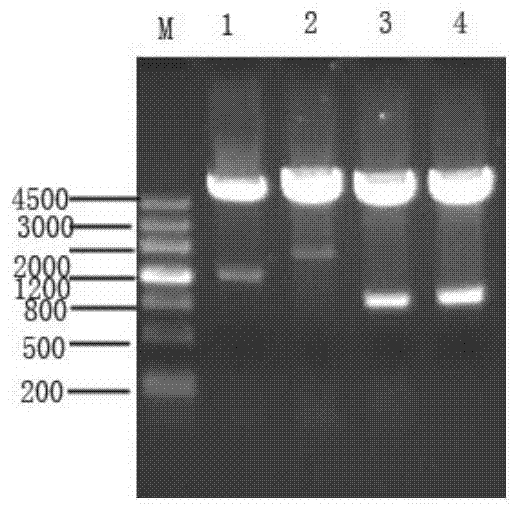
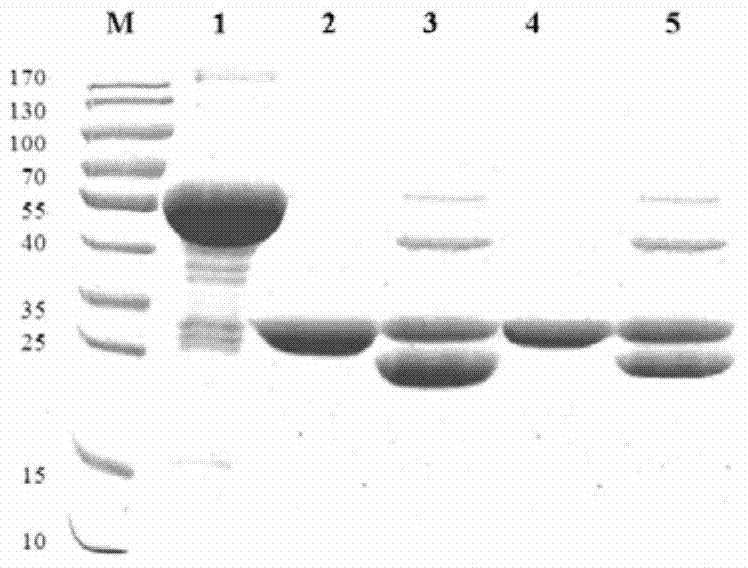
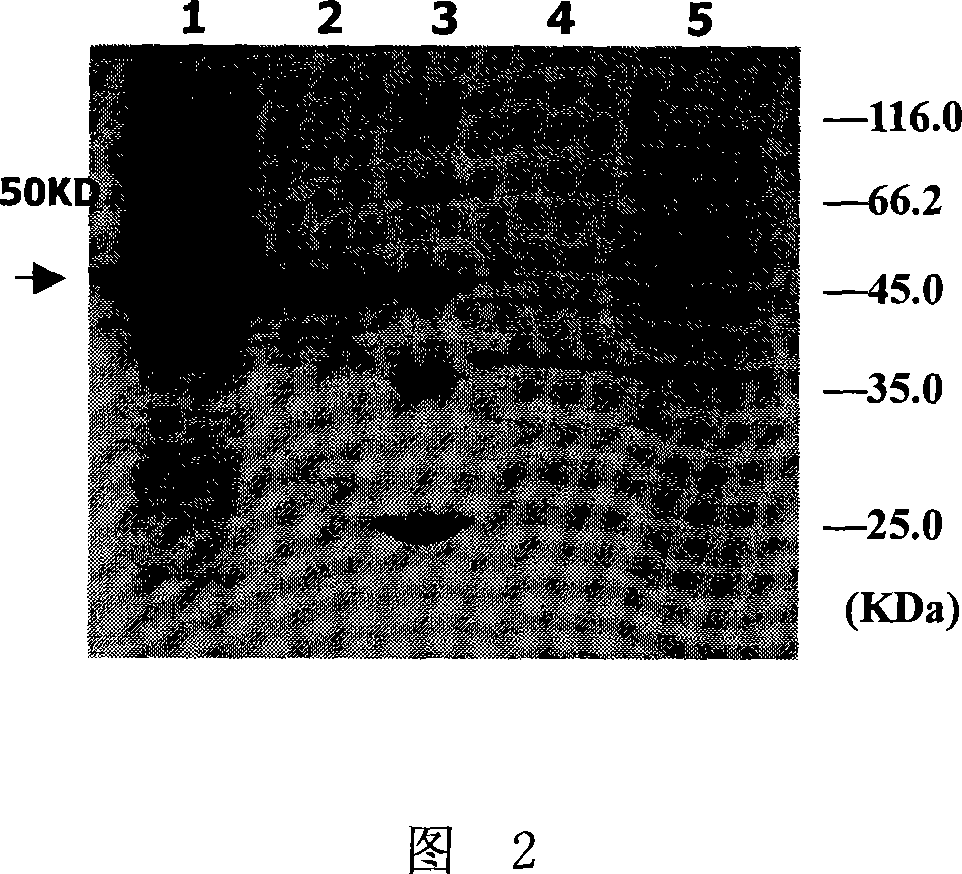
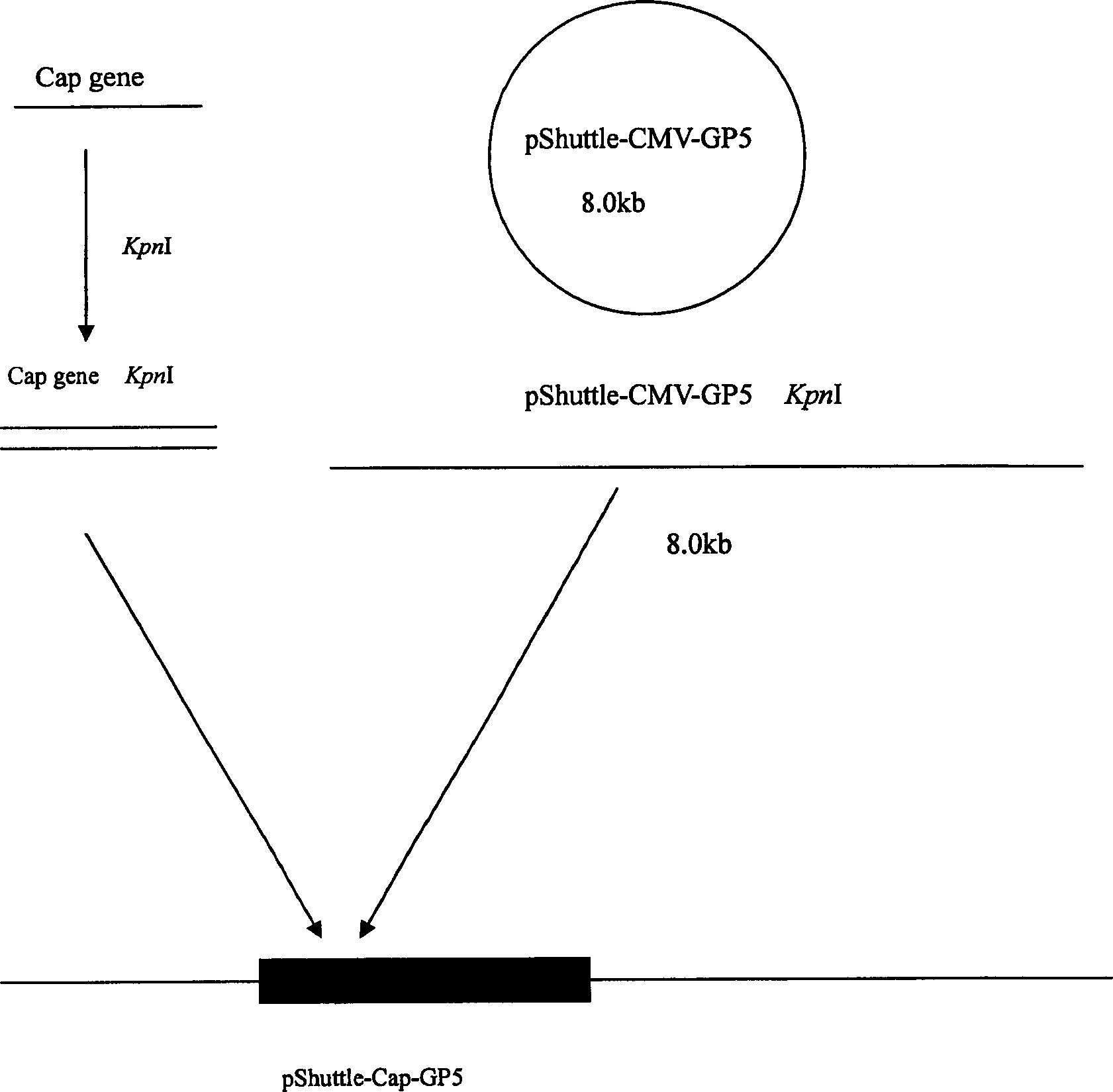
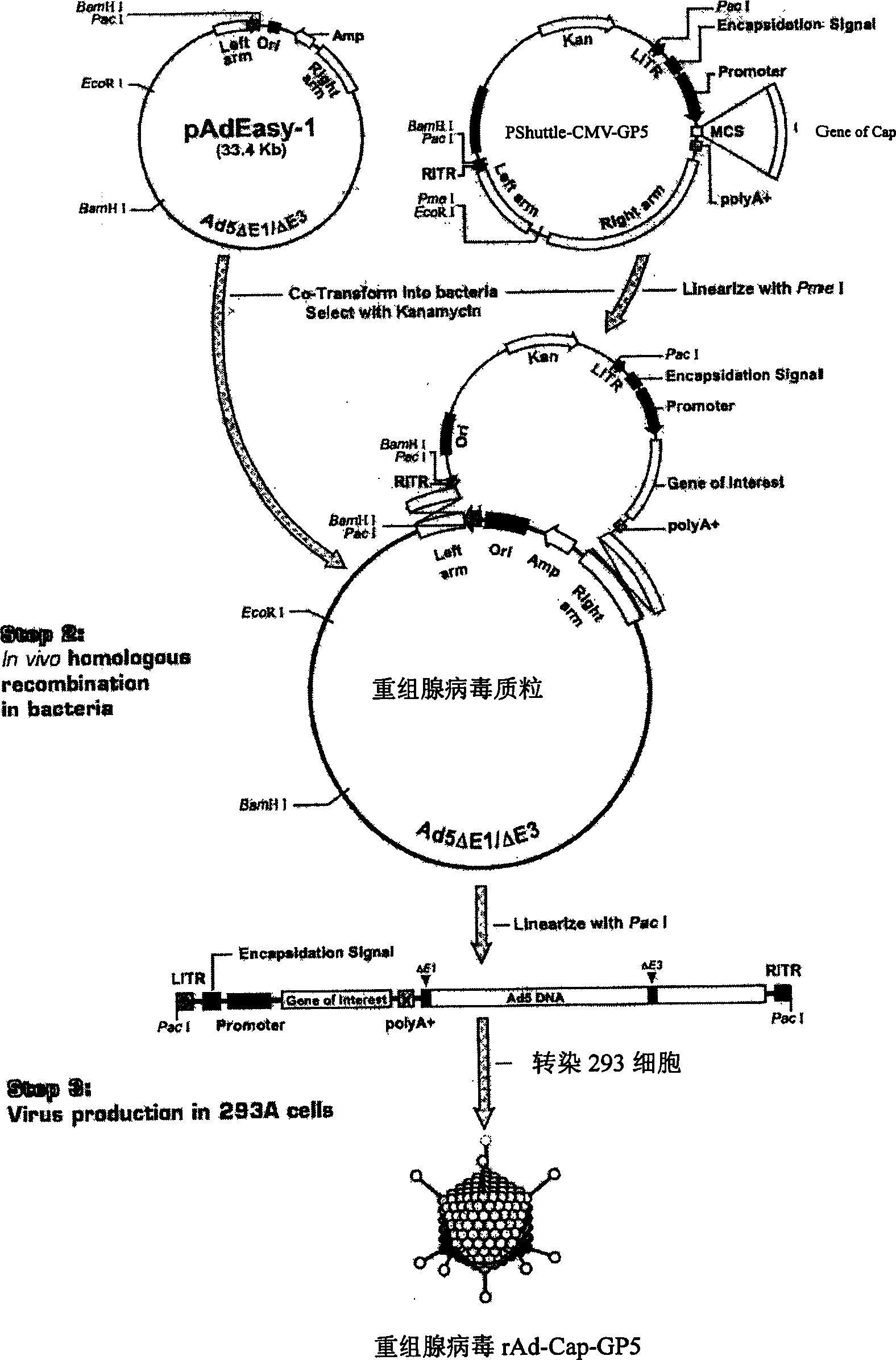
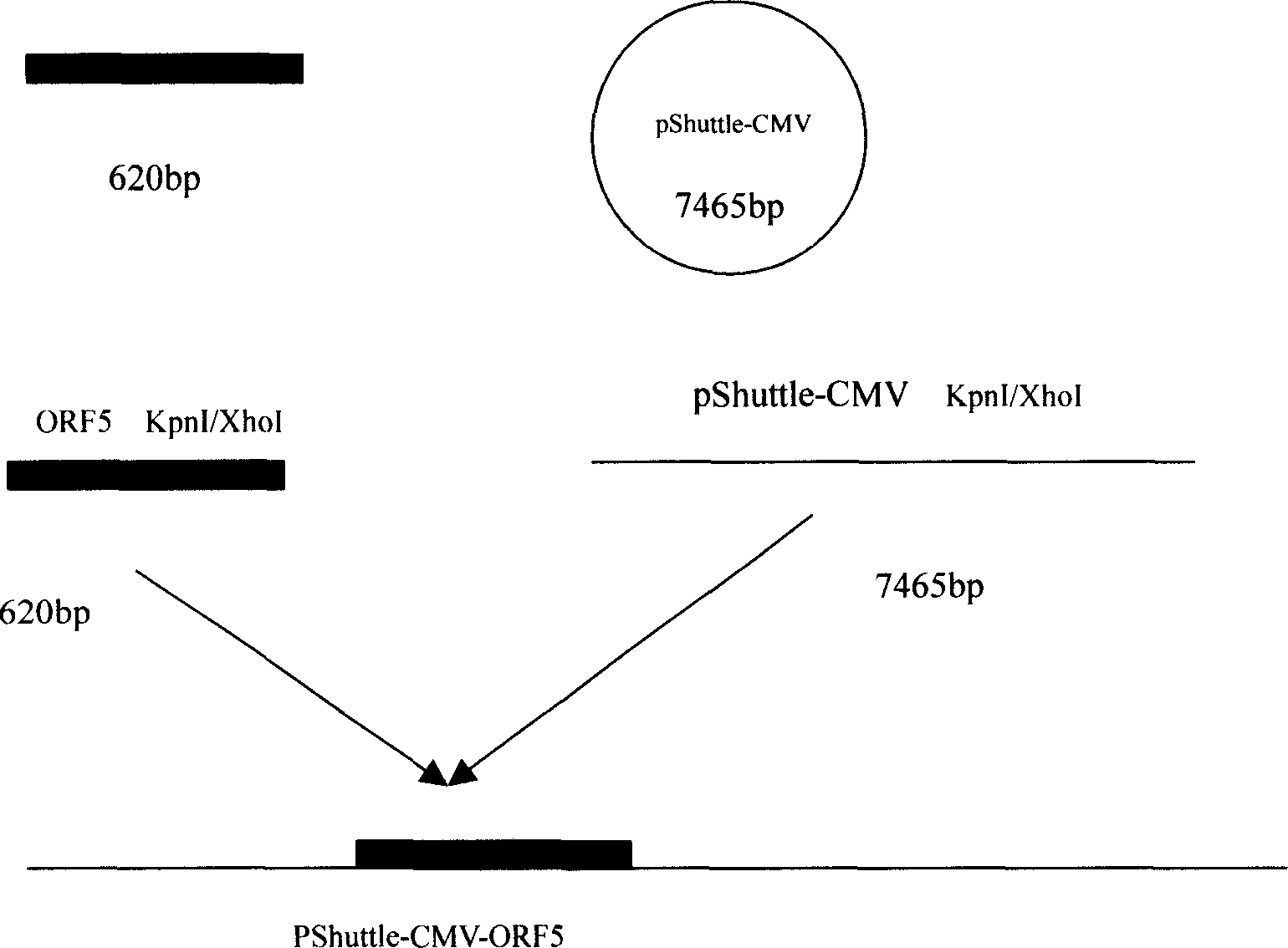
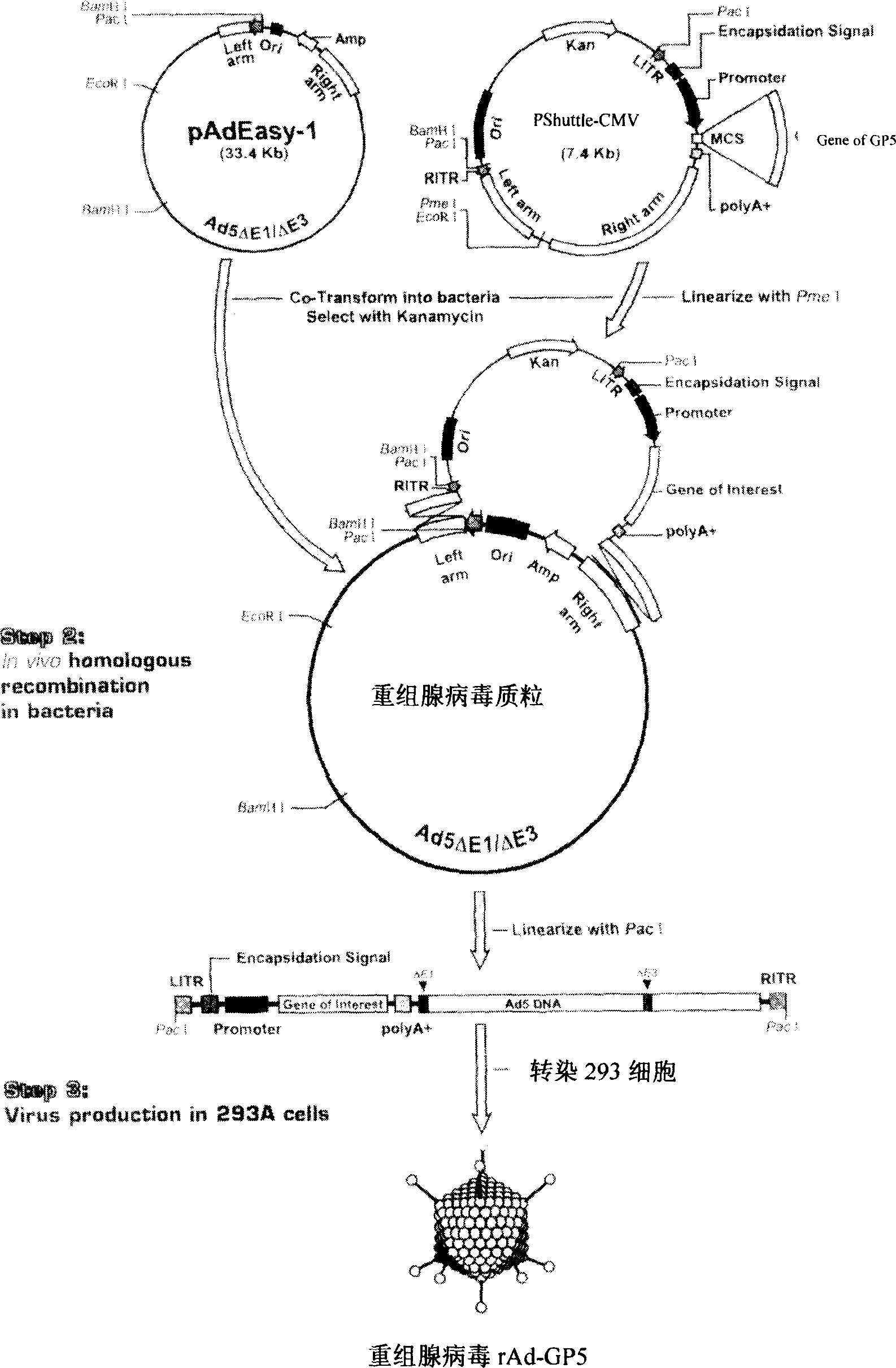
The invention relates to a pig breeding and breathing complex virus (PPRSV) and pig 2-type loop virus (PCV 2) series gene recombination adenovirus and vaccine in the field of high and new biotechnology. It uses PCR technology to clone the Cap protein gene into the plasmid carrier pShuttle-CMV-GP5 by open type reading rule and transforms tobacillus coli BJ5183strain with cage carrier pAdEasy-1 to capture the recombination plasmid; it uses recombination plasmid HEK293-A cell to capture recombination adenovirus and purifies it to express the recombination adenovirus rAd-Cap-GP5 of PRRSV GP5protein and PCV-2 Cap protein.
Owner:NANJING AGRICULTURAL UNIVERSITY
Seneca valley virus strain and application thereof
ActiveCN109182278AImproving immunogenicityDistant relationshipSsRNA viruses positive-senseViral antigen ingredientsDiseaseVaccine Immunogenicity
The invention discloses a Seneca valley virus strain and an application thereof, wherein the Seneca valley virus SVV-HeNXX / swine / 2017 is deposited in China Center For Type Culture Collection with theserial number of CCTCCNO: V201767. The Seneca valley virus SVV-HeNXX / swine / 2017 has good immunogenicity. It can induce immune animals to produce higher level of immune protection and can be used for preparing vaccine against Seneca Valley Virus Disease. The vaccine has high safety and no detoxification after immunization, and can induce animals to produce higher level of antibody quickly, so as toachieve high-efficient prevention of Porcine Seneca Valley Virus Disease, and has good popularization and application value.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Immune protective antigen of haemophilus parasuis
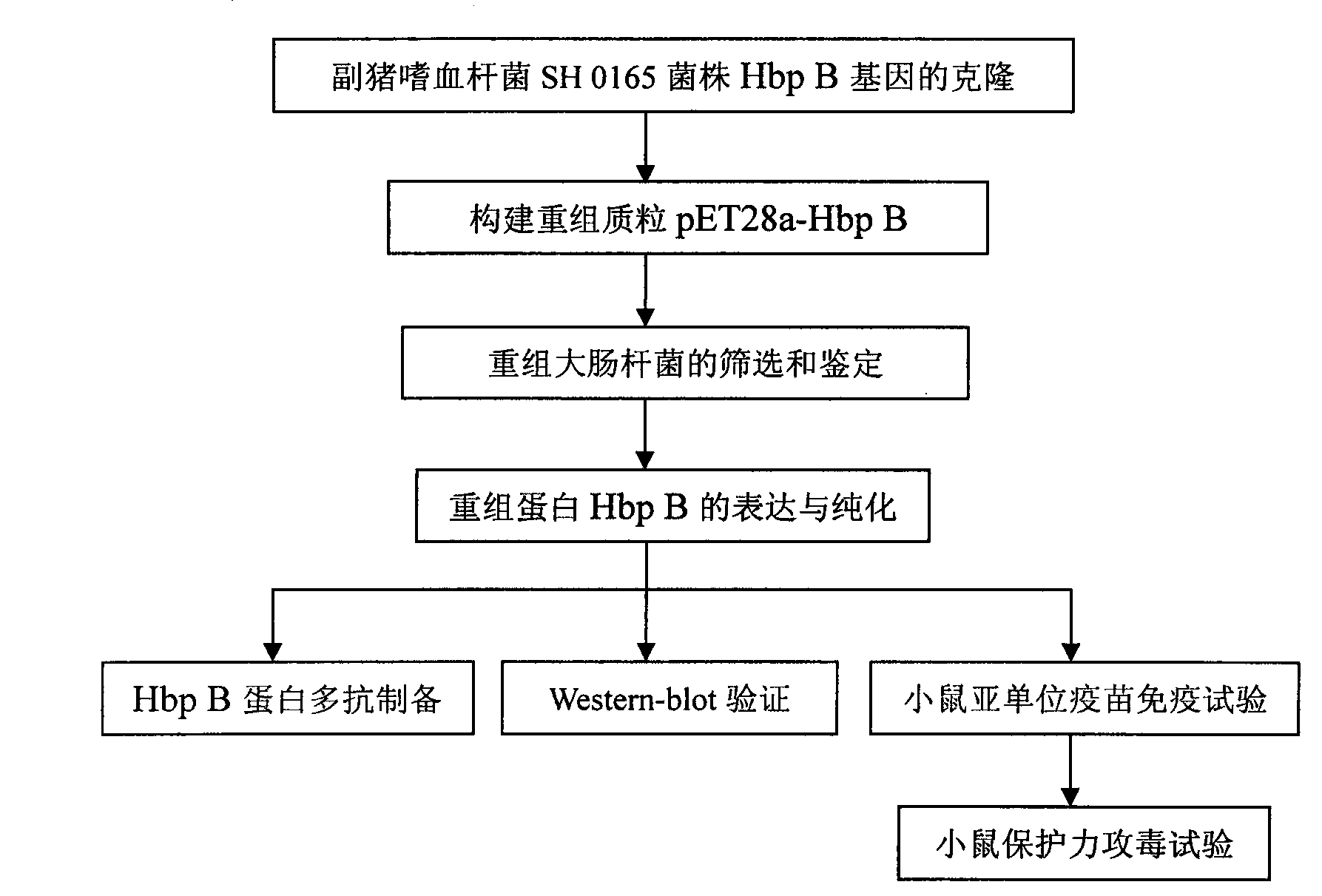
ActiveCN102864157AGood protective antigen proteinAntibacterial agentsBacteriaProtective antigenEscherichia coli
The invention belongs to the technical field of animal-borne disease subunit vaccine preparation and relates to preparation and application of the immune protective antigen of the haemophilus parasuis. Outer membrane protein Hbp B genes of the haemophilus parasuis are cloned, a nucleotide sequence is indicated as SEQID NO:1, and the sequence of gene code is indicated as SEQ ID NO:2. Recombination Escherichia coli BL21 / Pet-28a-Hbp B (preservation number is CCTCC NO:M2011228) is built and comprises genes in a sequence table SEQ ID NO:1. Antigen protein of the haemophilus parasuis is obtained and expressed through gene transformation Escherichia coli in the SEQ ID NO:1. The invention further discloses a preparation method and application of the recombination Escherichia coli. The haemophilus parasuis subunit vaccine has good safety, and an immune protection effect reaches 83%.
Owner:HUAZHONG AGRI UNIV +1
Asia1 type foot-and-mouth disease recombinant virus and preparation method and application thereof
The invention relates to an Asia1 type foot-and-mouth disease recombinant virus without pathogenicity for a host and a preparation method and application thereof. A saving system is efficient eukaryotic plasmids which are constructed by gene engineering and can express exact foot-and-mouth disease virus genome RNA (Ribonucleic Acid), and therefore the foot-and-mouth disease recombinant virus can be constructed and prepared; vaccine strains with high titer and good antigen matching property can be prepared by using the plasmids, can be prepared into live vaccines or inactivated vaccines and can effectively stimulate bodies to produce immune response after being used for immunizing pigs and cattle, provide an immune protective effect on the pigs and the cattle and effectively protect GV and GII prevalent strains, the immune protection rate can reach 100 percent, and the median protective dose (PD50) is 6.34 to 13.59; and the recombinant virus has the advantages of high titer, high antigen matching property with the prevalent strains, wide antigen spectrum and high immune protection rate, does not have pathogenicity for pig and cattle hosts, does not form toxemia or expel toxin, and can be applied to prevention and control of Asia1 type foot-and-mouth disease viruses of China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Pig breeding and respiratory syndrome recombined adenovirus and vaccine
InactiveCN1554766ALittle changeChange propertiesGenetic material ingredientsInactivation/attenuationEscherichia coliBiotechnology
The present invention relates to pig reproduction and respiratory syndrome virus recombined adenovirus and vaccine, and belongs to the field of biological high-tech. Through RT-PCR process to proliferate whole PRRSV GP5 sequence, cloning the gene sequence to the shuttle vector pShuttle-CMV of adenovirus carrier system, cotransforming colibacillus BJ5183 strain together with the skeleton vector of adenovirus carrier system to obtain recombinant plasmid, transfecting HEK293-A cell to obtain recombinant adenovirus and plaque purification, and RT-PCR and indirect immunofluorescence technique inspection, the recombinant adenovirus rAd-GP5 expressing PRRSV GP5 protein is constituted. The recombinant adenovirus can set ahead the expression of PRRSV GP5 protein and raise the expression amount to simulate the immune protecting reaction of body effectively.
Owner:NANJING AGRICULTURAL UNIVERSITY
H9N2 avian influenza virus vaccine strain and application of H9N2 avian influenza virus vaccine strain in immune protection
The present invention relates to the field of animal virology, and provides a recombinant chicken-origin H9N2 avian influenza virus vaccine strain and a method for isolation, identification and purification of the strain. The invention further relates to a research of biological characteristics of the strain, especially to a research of characteristics of the strain adopted as the vaccine strain,and an evaluation of immune effects of the strain on SPF chickens. The preservation number of the strain is CCTCCNO:V201030. According to the present invention, the antigen variation conditions of the virus strain and other isolated virus strains are represented from the molecular level; after the virus strain is prepared into the vaccine, the prepared vaccine is adopted to immunize the 4 week old SPF chickens, with the protection effect analysis of the homologous H9 influenza wild virus strain and the heterologous H9 influenza wild virus strain, the results show that the influenza virus strain can be adopted as the spare vaccine strain of H9 subtype avian influenza. With the present invention, the spare vaccine strain is provided for prevention of the avian influenza outbreak by using the vaccine, the molecular biology technology program is provided for screen of the avian influenza virus vaccine strain, the molecular biology background is provided for study of the mechanism of animal infection by the avian influenza, and the important public health significance is provided.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
High-prolificacy porcine circovirus type-2 strain and application thereof
ActiveCN102787100AImprove reproductive performanceGood immune protectionViral antigen ingredientsMicroorganism based processesNucleotideMicrobiology
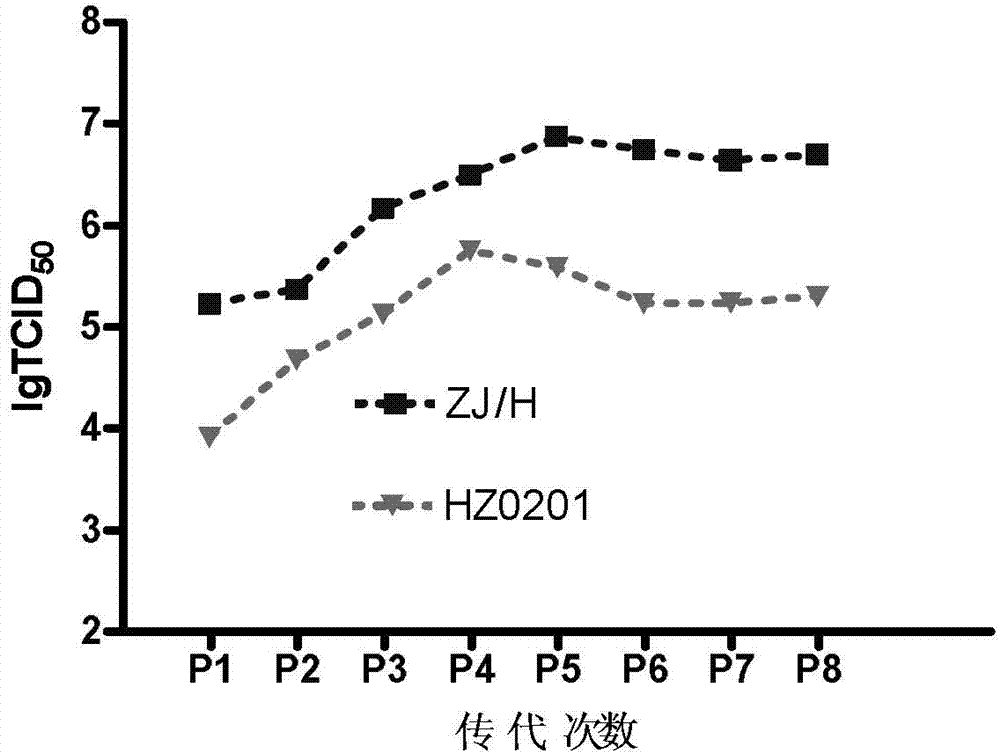
The invention discloses a high-prolificacy porcine circovirus type-2 strain and application thereof. The porcine circovirus type-2 strain is named as PCV2-ZJ / H strain, the preservation number of the strain is CGMCC No.6391, the gene sequence of the strain is shown as SEQ ID NO:1, and nucleotide at a 1376 locus in the nucleotide sequence of a genome of the strain is absent. The porcine circovirus type-2 strain has high prolificacy, the virus titer (TCID50) in PK-15 cells reaches 107.6 / mL and is more than 10 times higher than that (106.3 / mL) of a parent virus, and a vaccine prepared with the strain has high immune protection capacity.
Owner:ZHEJIANG UNIV
Pneumococcus polysaccharide protein coupling vaccine and its preparing method
The present invention is pneumococus polysaccharide protein coupling vaccine comprising covalently connected pneumococus capsule polysaccharide and recombinant pneumolysin without hemolytic activity modification and its preparation process. The vaccine has pneumolysin without hemolytic activity as protein carrier, no need of eliminating hemolysis toxicity of pneumolysin with formalin and ensured safety, and may be used for infant below 2 yeas old to prevent tympanitis. Owing to the pneumolysin as the self protein, the vaccine has no probable immune interference reaction and strengthened immune protecting effect. The vaccine has cross immunizing protection effect on various kinds of serum type pneumococus and raised immune memory response to pneumococus infection.
Owner:EYE & ENT HOSPITAL SHANGHAI MEDICAL SCHOOL FUDAN UNIV
Vaccine composition
InactiveUS20060216307A1UpregulationReducing lipid A toxicityAntibacterial agentsSenses disorderProtective antigenBacteroides
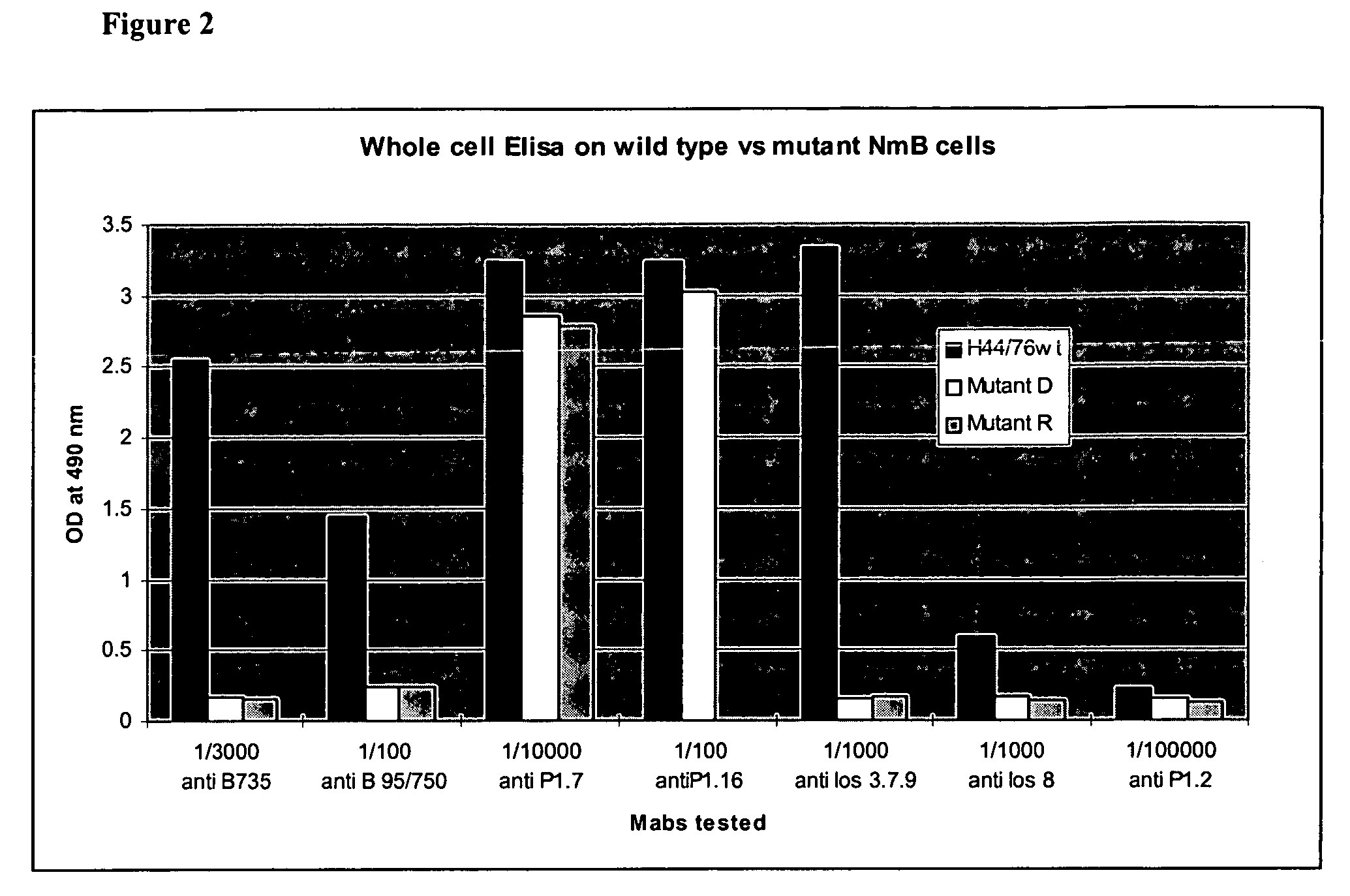
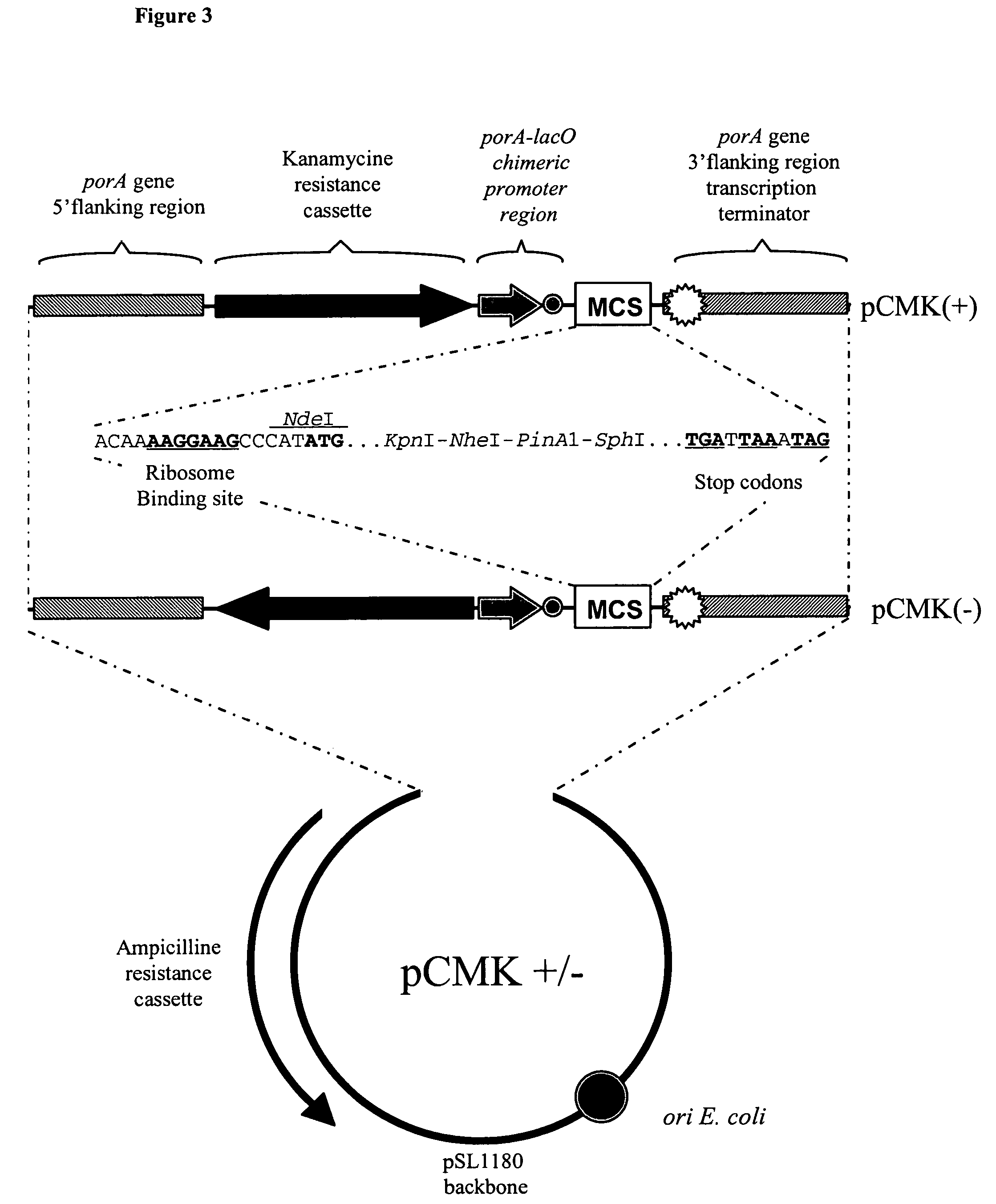
The present invention relates to an immuno-protective and non-toxic Gram-negative bleb vaccine suitable for paediatric use. Examples of the Gram-negative strains from which the blebs are made are N. meningitidis, M. catarrhalis and H. influenzae. The blebs of the invention are improved by one or more genetic changes to the chromosome of the bacterium, including up-regulation of protective antigens, down-regulation of immunodominant non-protective antigens, and detoxification of the Lipid A moiety of LPS.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com