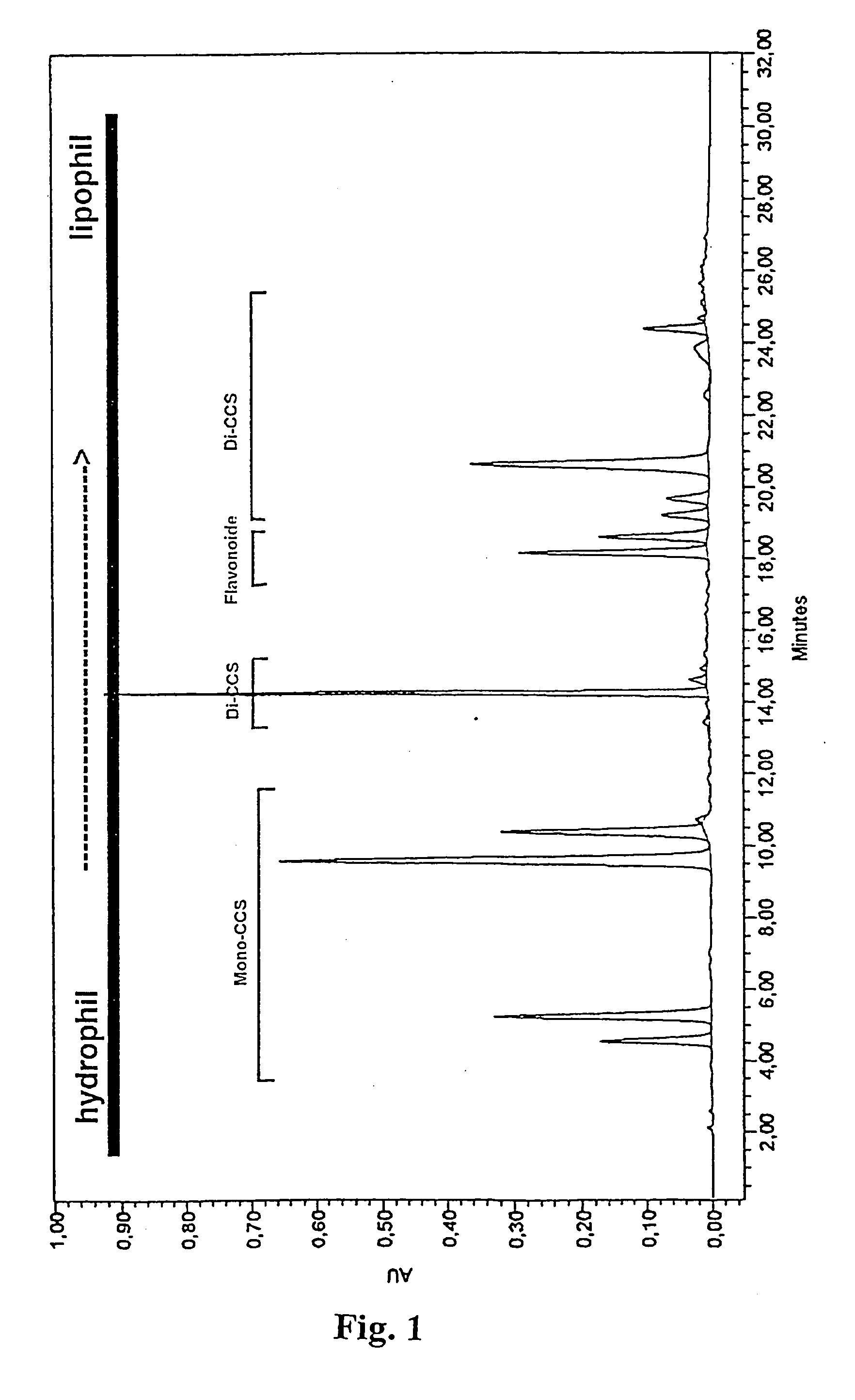
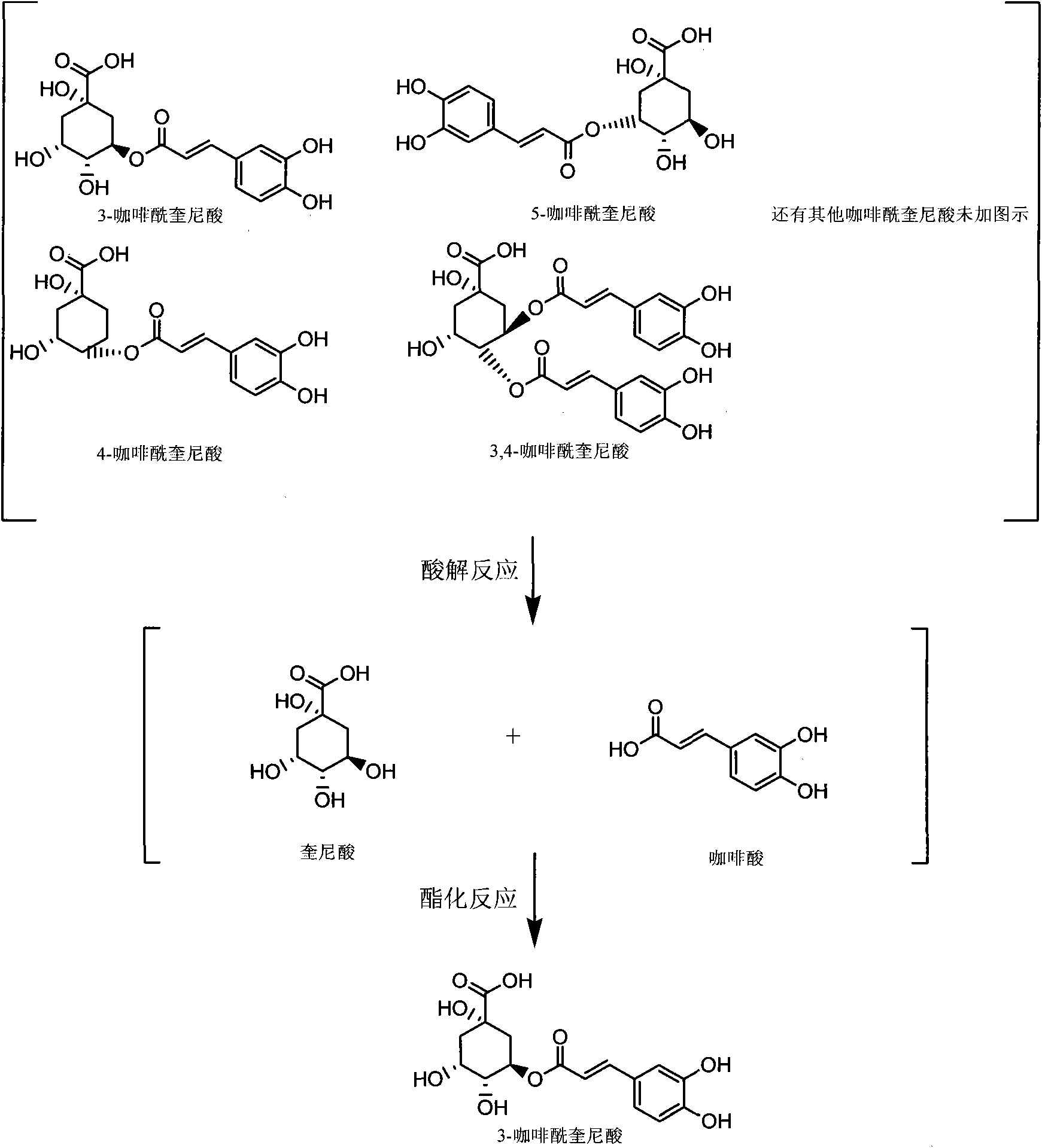
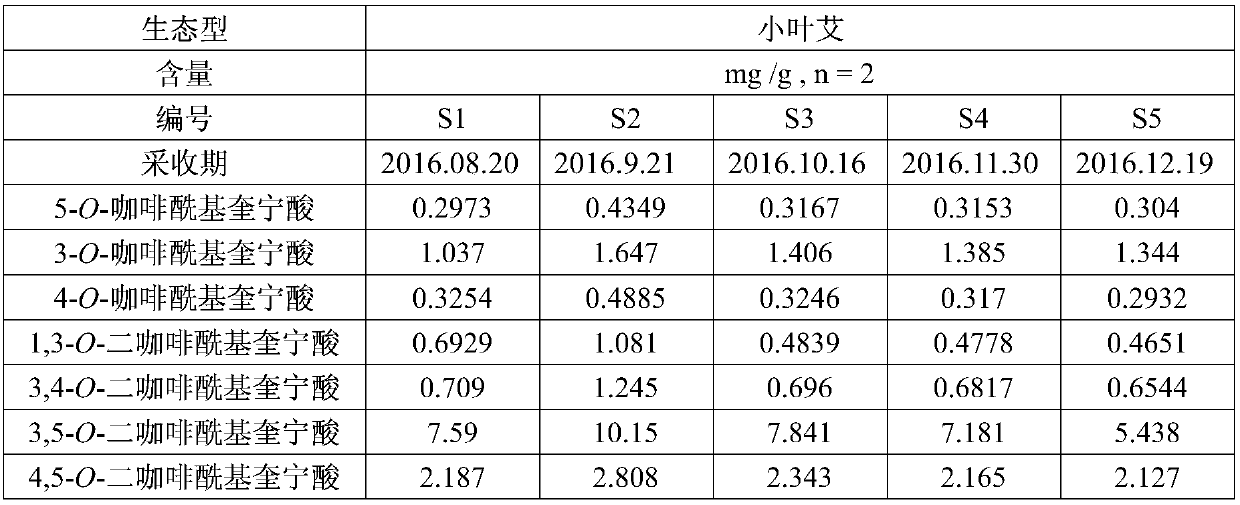
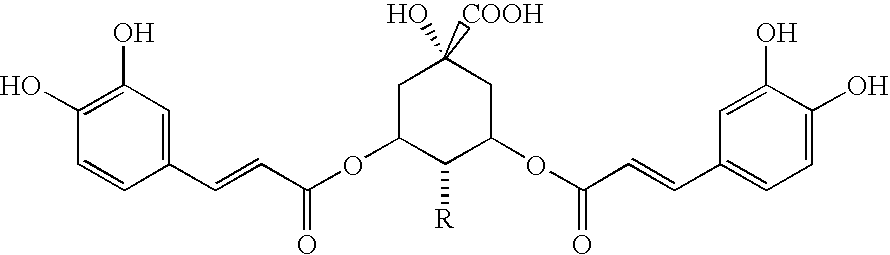
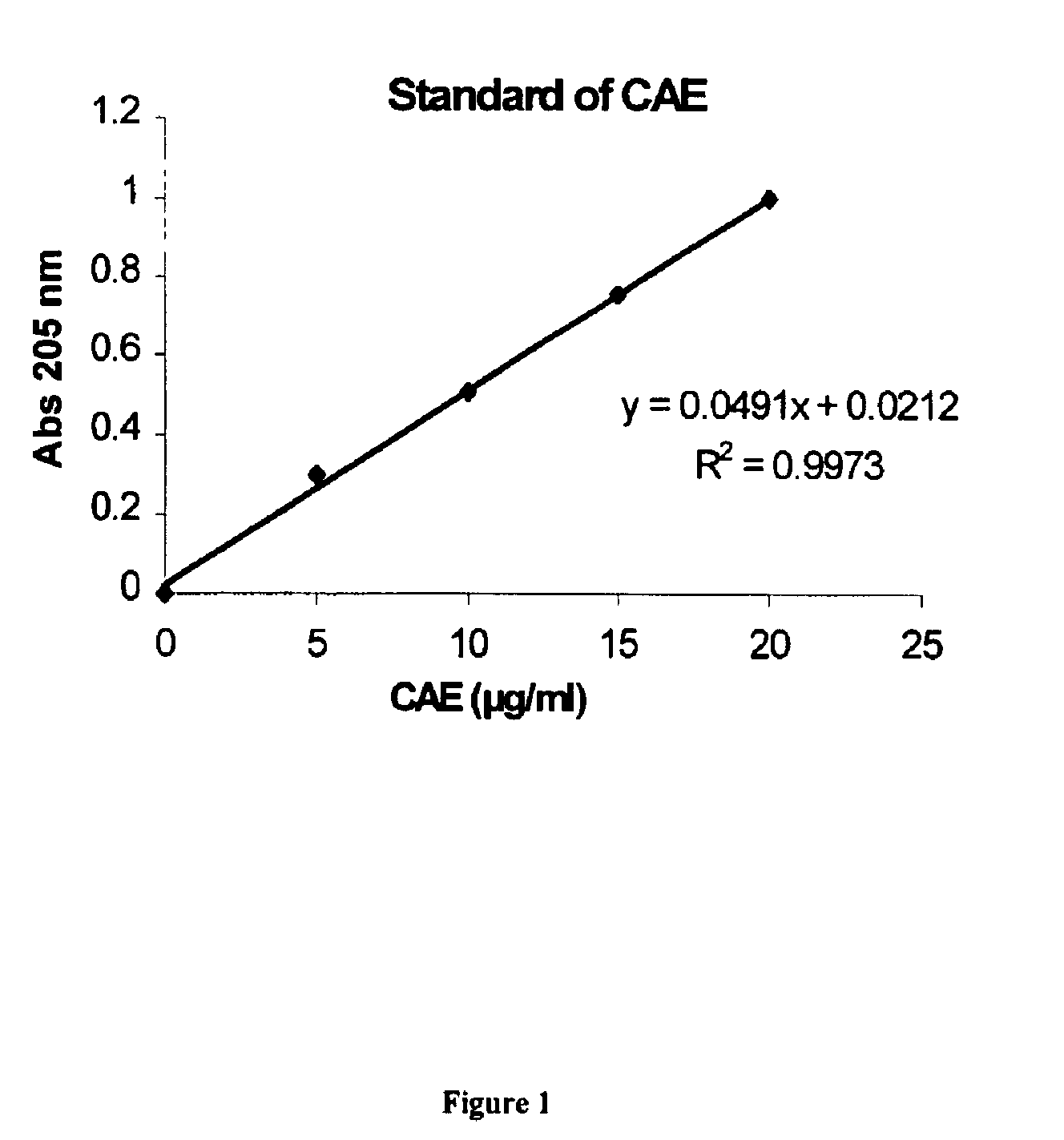
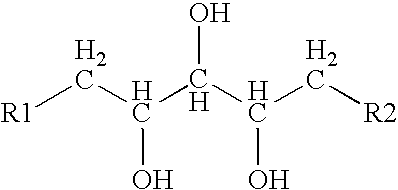
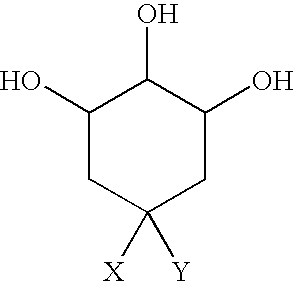
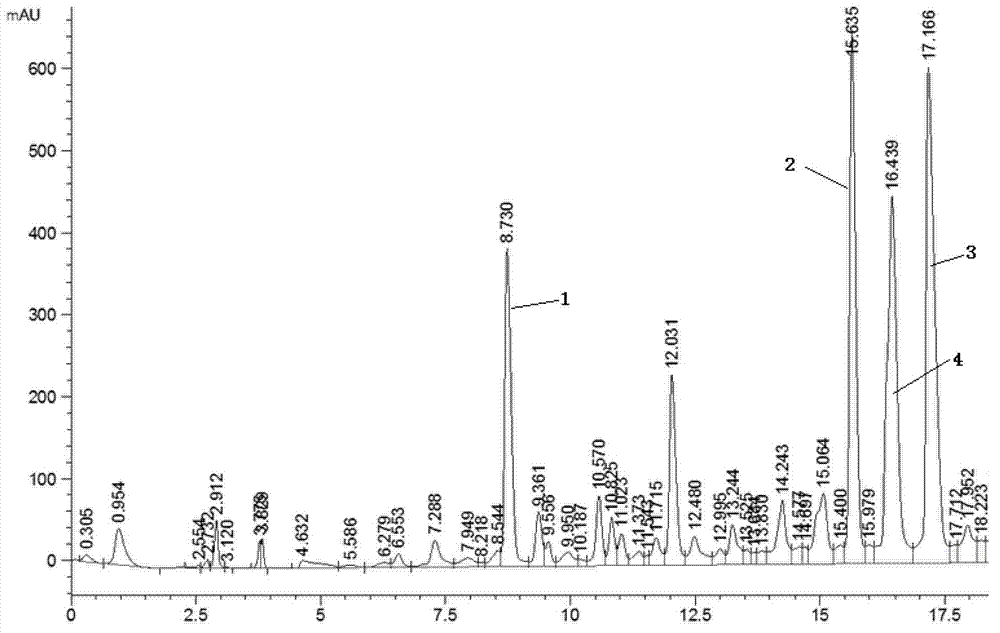
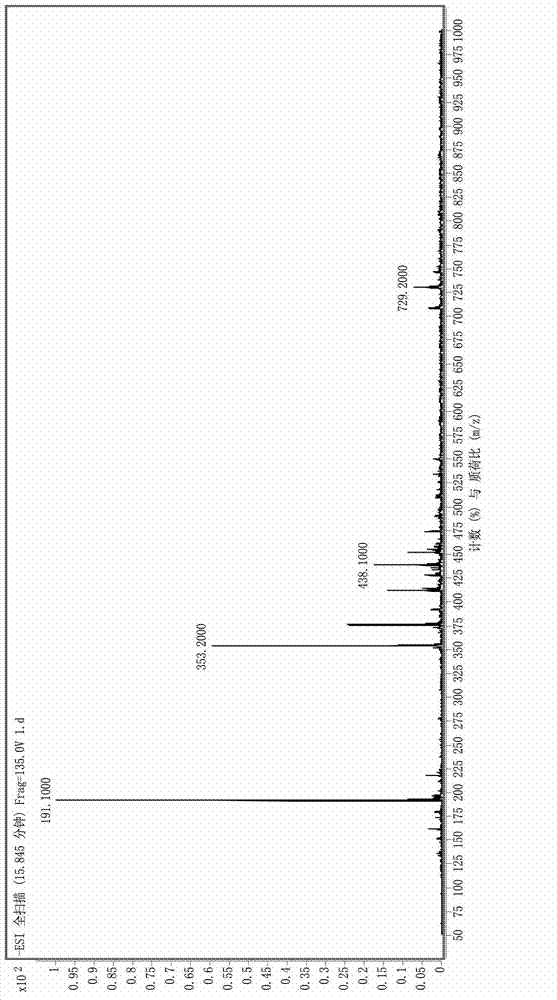
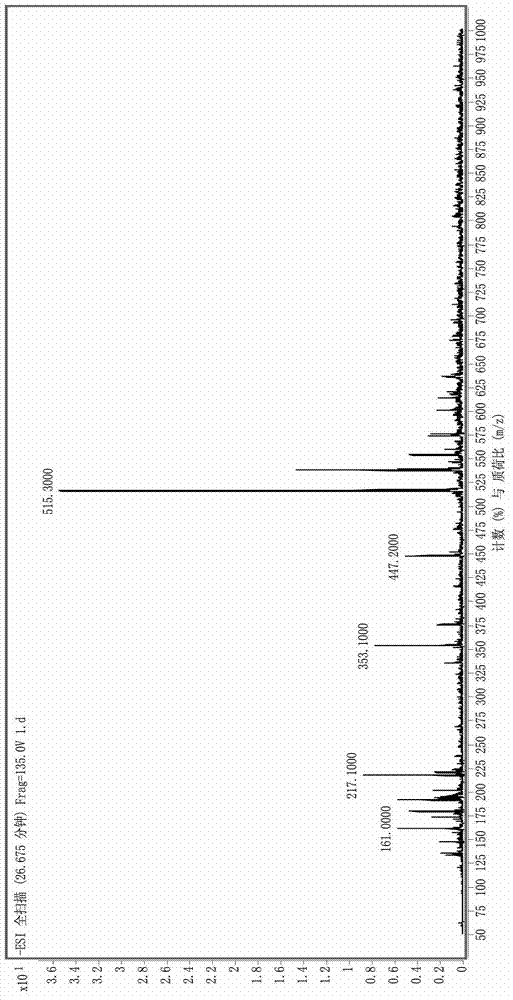
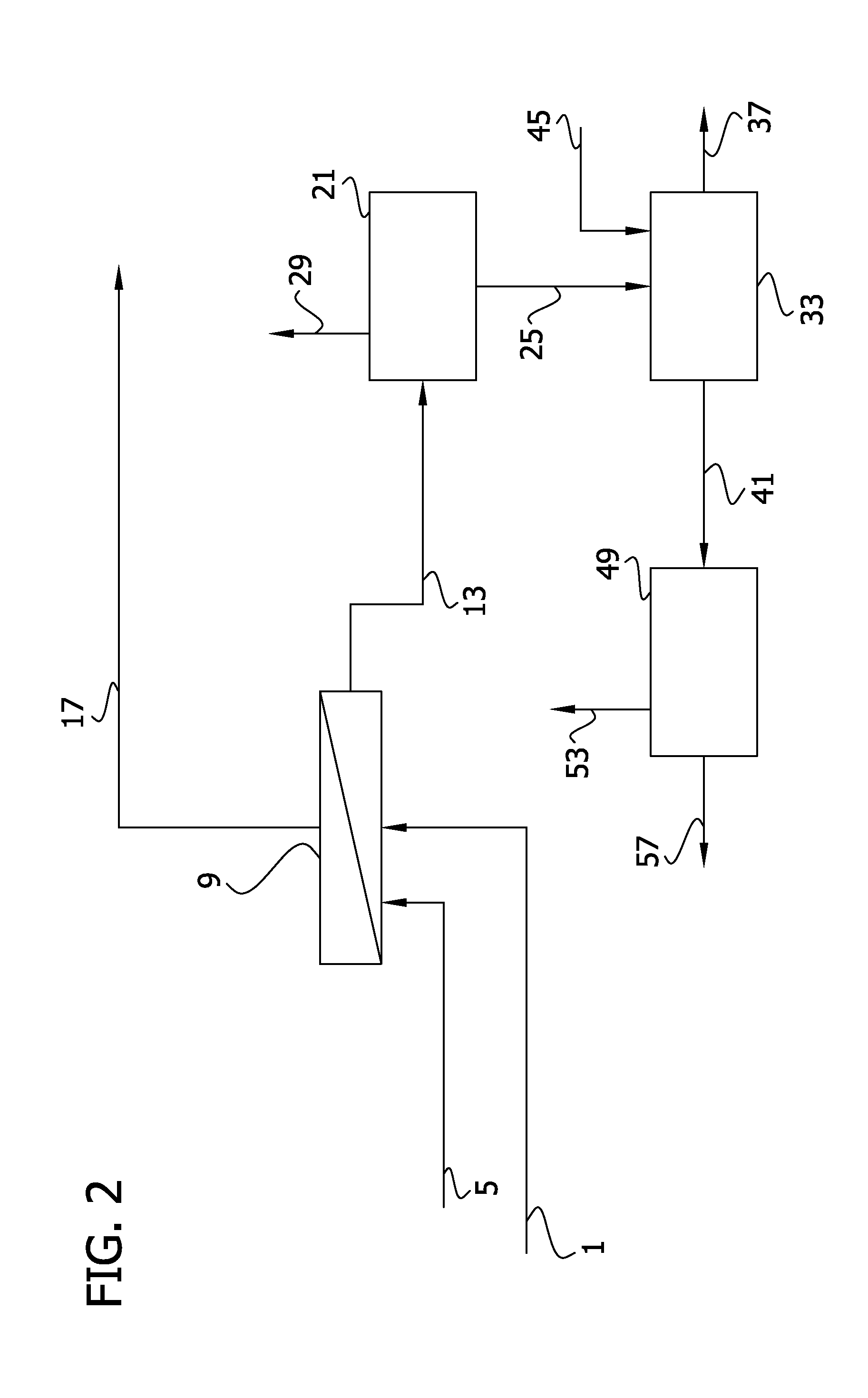
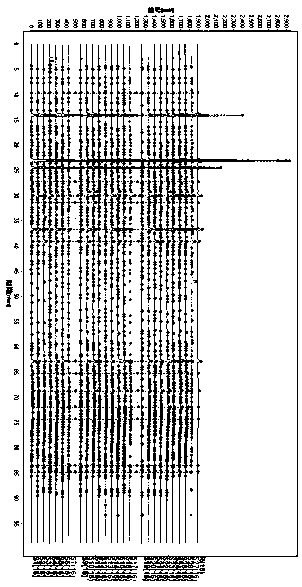
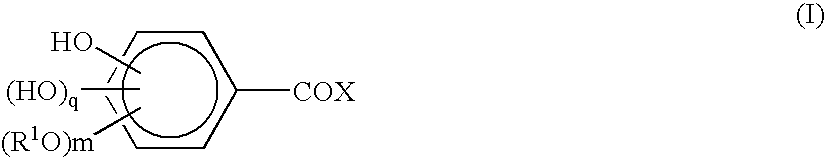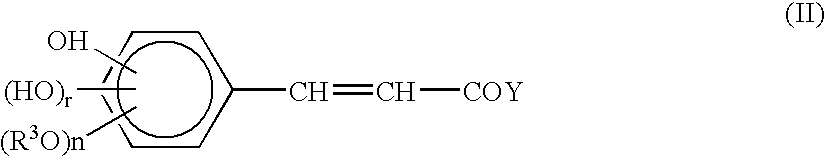Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
178 results about "Quinic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
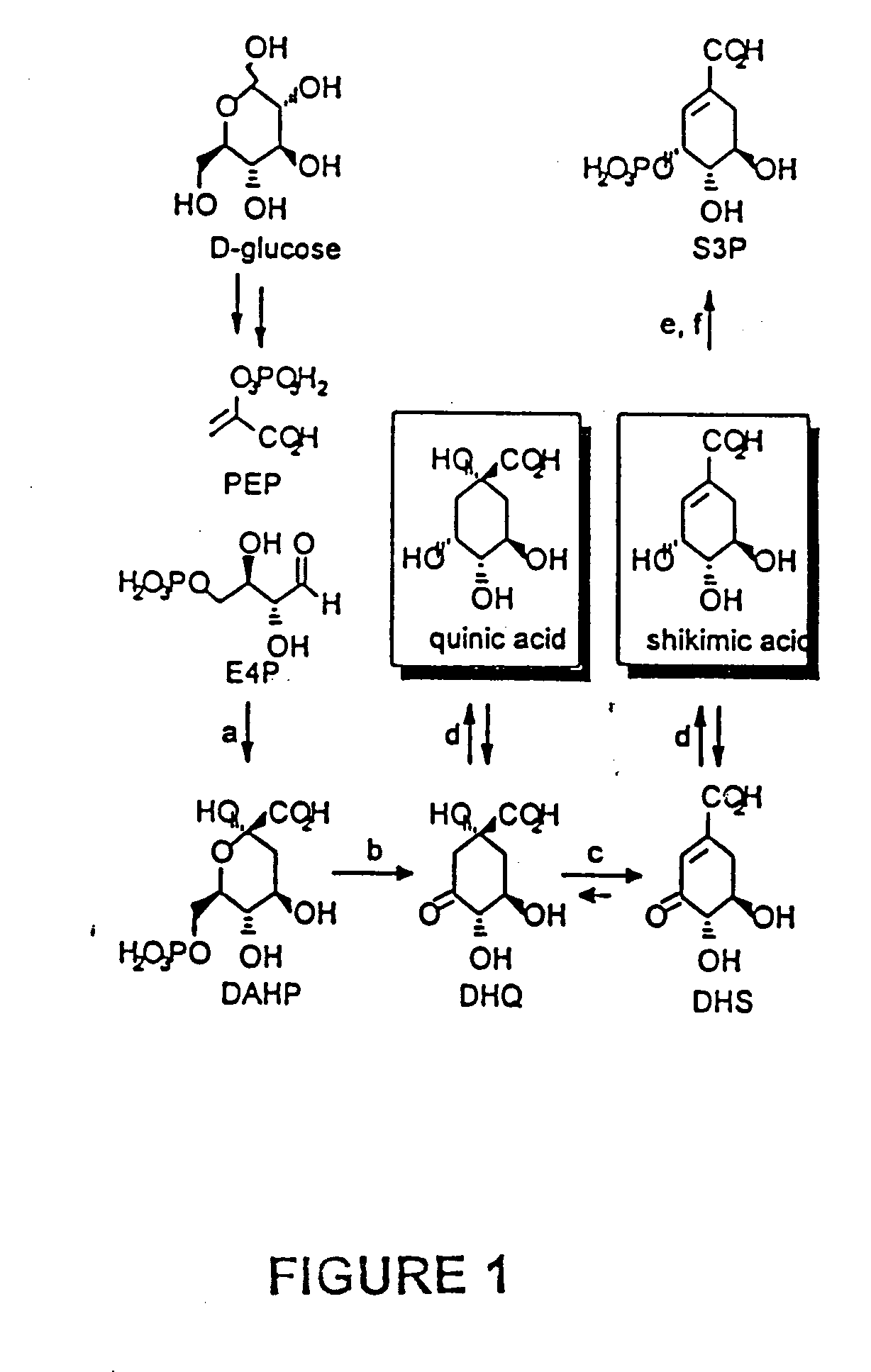
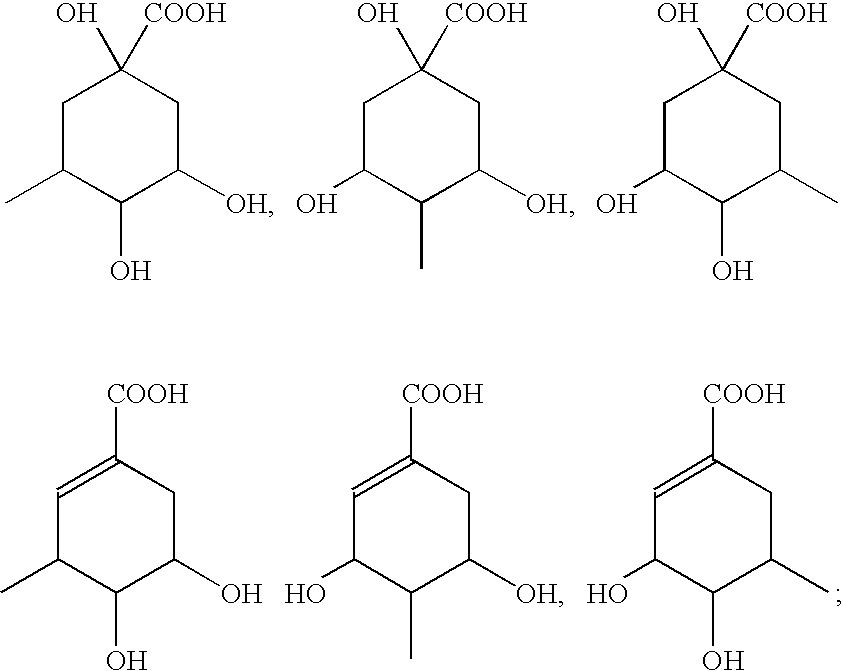
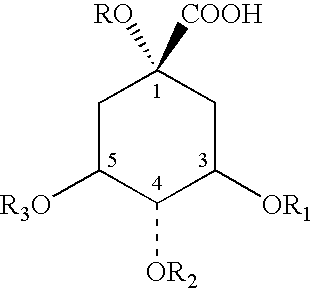
Quinic acid is a cyclitol, a cyclic polyol, and a cyclohexanecarboxylic acid. It is a colorless solid that can be extracted from plant sources. Quinic acid is implicated in the perceived acidity of coffee.
Biocatalytic synthesis of quinic acid and conversion to hydroquinone
A bioengineered synthesis scheme for the production of quinic acid from a carbon source is provided. Methods of producing quinic acid from a carbon source based on the synthesis scheme as well as conversion of quinic acid to hydroquinone are also provided.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
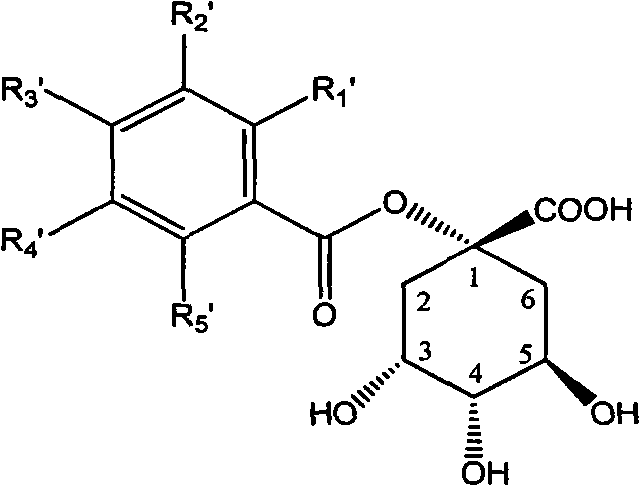
1-oxygen-substituted benzene formyl quinic acid pharmaceutical use of inhibiting hepatitis b virus
The invention relates to 1-o-substituted benzoyl quinic acid compounds having the formula of (I) and anti-hepatitis B virus activity and pharmaceutical salts thereof. The invention also relates to a preparation method of the quinic acid compounds and intermediate compounds of the quinic acid compounds. The invention also relates to a pharmaceutical application of the quinic acid compounds, and pharmaceutical compositions containing the same. The compounds (I) and intermediate compounds thereof, and pharmaceutical salts thereof inhibit hepatitis B virus DNA (HBVDNA) replication and reduces hepatits B virus surface antigen (HBsAg) expression. Thus, the 1-o-substituted benzoyl quinic acid compounds and intermediate compounds thereof have prospect pharmaceutical application in the preparation of a drug for treating correlated hepatitis B virus infectious disease.
Owner:WENZHOU MEDICAL UNIV
Artichoke leaf extracts
InactiveUS20040234674A1Organic active ingredientsCosmetic preparationsAdditive ingredientCaffeoylquinic acid
Owner:DIVAPHARMA CHUR
Photoprotector and/or photoimmunoprotector compositions of the skin and their uses
InactiveUS20070025933A1Prevent and minimise damaging effectPrevent and minimise reactionCosmetic preparationsBiocideBenzoic acidPhototherapy unit
The composition comprises of a component A selected from a hydroxylated derivative of benzoic acid or of cinamic acid, their esters, amides or salts, a glycoside of a hexose, and their mixtures; and a component B selected from quinic acid, shikimic acid, their alkaline metal or alkaline earth salts, their methyl esters, and mixtures of the same. This composition is suitable for protecting the skin against ultraviolet radiation coming from the sun or artificial sources, such as those used in phototherapy units and in sun tanning rooms. For application in the field of dermatology and nutrition, and, in particular, in the photoprotection of the skin and mucosa, photo-ageing and photocarcinogenesis, including protection of the immune system associated with the skin.
Owner:IND FARM CANTABRIA
Bottled beverage
InactiveUS20070092624A1Great tasteAdd flavorPre-extraction tea treatmentTea extractionHigh concentrationAdditive ingredient
A packaged beverage of pH 2 to 6 with a green tea extract mixed therein, comprising the following ingredients (A) to (E) (A) from 0.01 to 1.0 wt % of non-polymer catechins, (B) quinic acid or a salt thereof, (C) from 0.0001 to 15 wt % of a sweetener, (D) from 0.001 to 0.5 wt % of sodium ions, and (E) from 0.001 to 0.2 wt % of potassium ions, wherein a content weight ratio [(B) / (A)] of said quinic acid or salt thereof (B) to said non-polymer catechins (A) is from 0.0001 to 0.5. The packaged beverage contains catechins at high concentration, is reduced in bitterness and astringency and is suited for long-term drinking, is excellent in the stability of bitterness and astringency and also in the feeling when it is swallowed, and further, remains stable in color tone even when filled in a clear container and stored at high temperatures.
Owner:KAO CORP
Pharmaceutical composition and application thereof in preparing medicine for treating multidrug resistance of tumors
ActiveCN108159038AGood reversal of drug resistanceAvoid drug resistanceOrganic active ingredientsImmunoglobulinsPD-L1 inhibitorChlorogenic acid
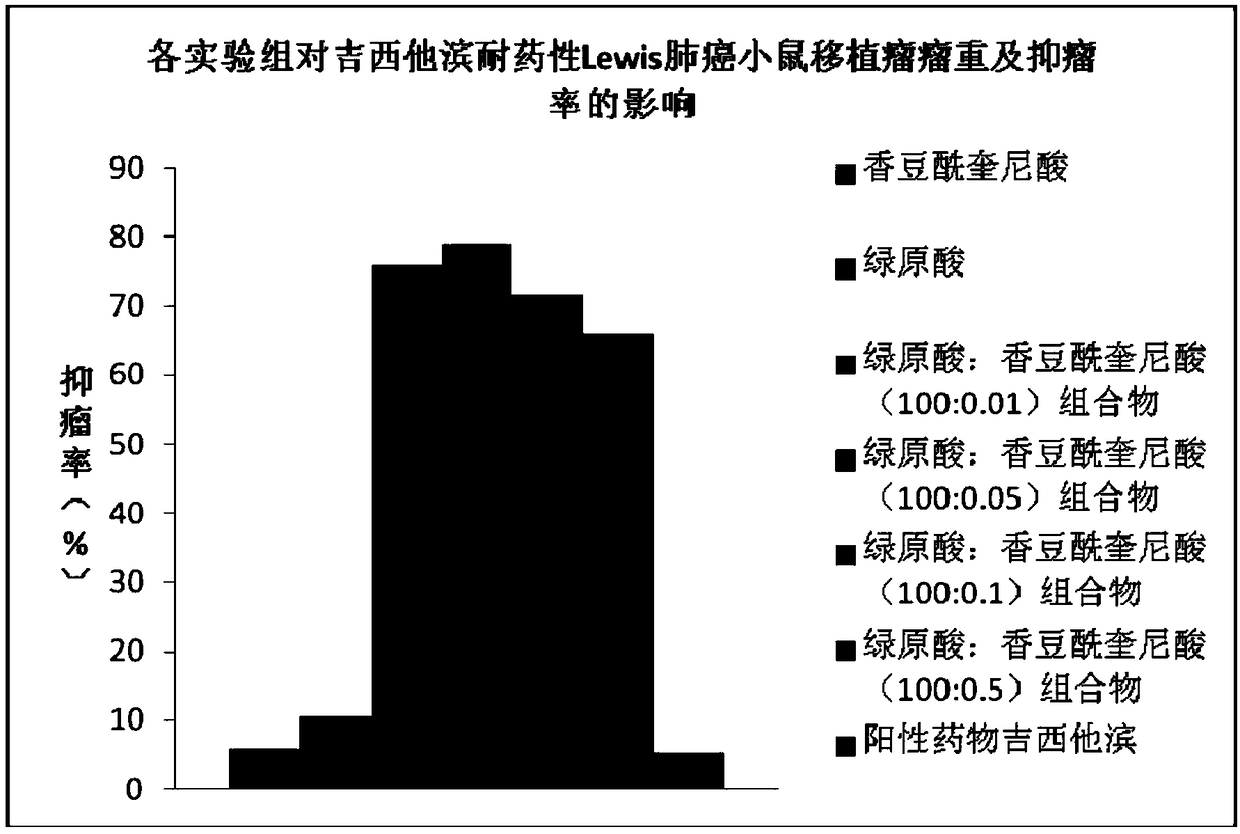
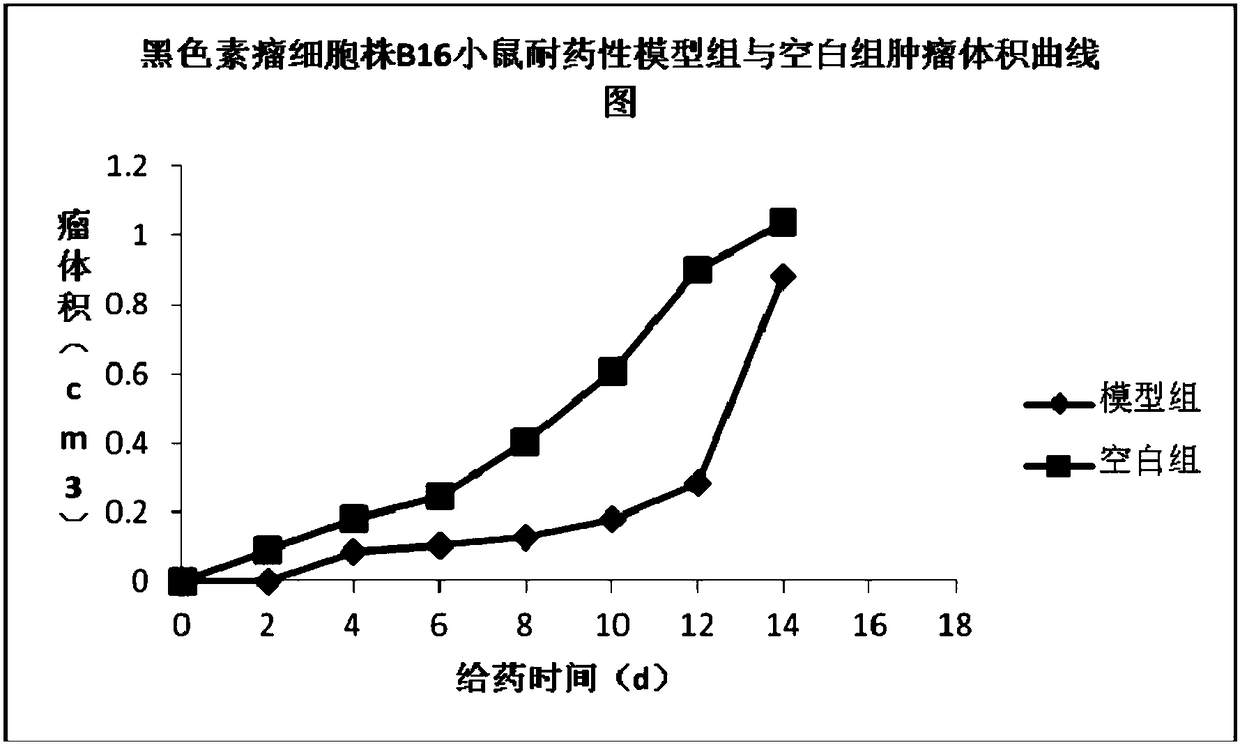
The invention discloses a pharmaceutical composition and an application thereof in preparing a medicine for treating multidrug resistance of tumors and belongs to the field of biomedicines. The pharmaceutical composition is prepared from chlorogenic acid and tonka-bean quinic acid and can be used for preparing a reversal agent and a PD-1 / PD-L1 inhibitor for the multidrug resistance of the tumors.The pharmaceutical composition and the application disclosed by the invention have the beneficial effects that by combined use of the chlorogenic acid and the tonka-bean quinic acid, the synergistic interaction can be exerted, the drug-resistance reversal action on the tumor cell strain generating multidrug resistance for chemotherapeutic drugs and immunotherapeutic drugs is good, the expression of PD-1 / PD-L1 of tissues of transplanted tumors of mice with drug-resistant B16 melanoma and drug-resistant Lewis lung cancer can be effectively suppressed, and a role in proliferating CD4+T and CD8+Tcells of the mice with the drug-resistant B16 melanoma and the drug-resistant Lewis lung cancer can be effectively played.
Owner:SICHUAN JIUZHANG BIO TECH CO LTD
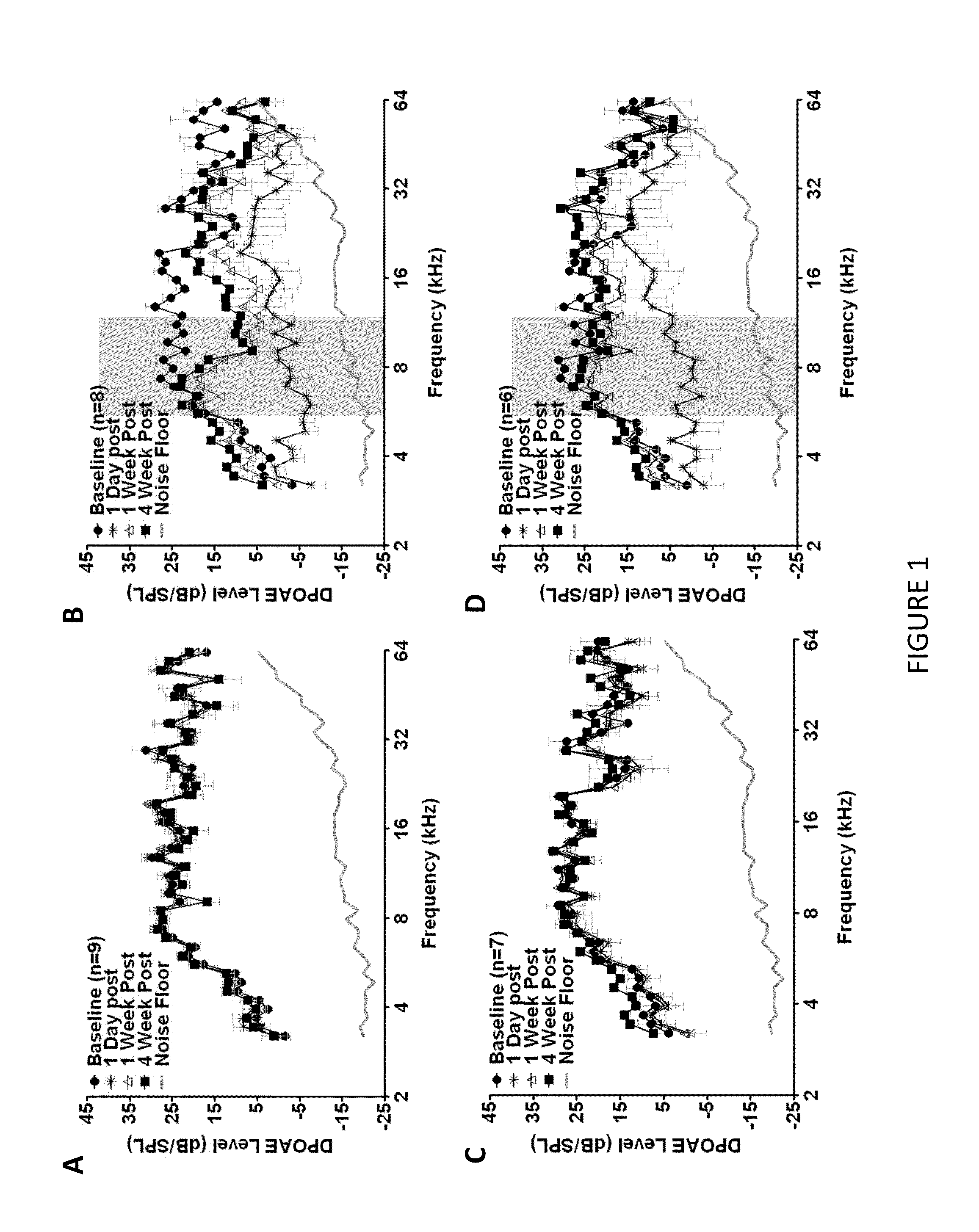
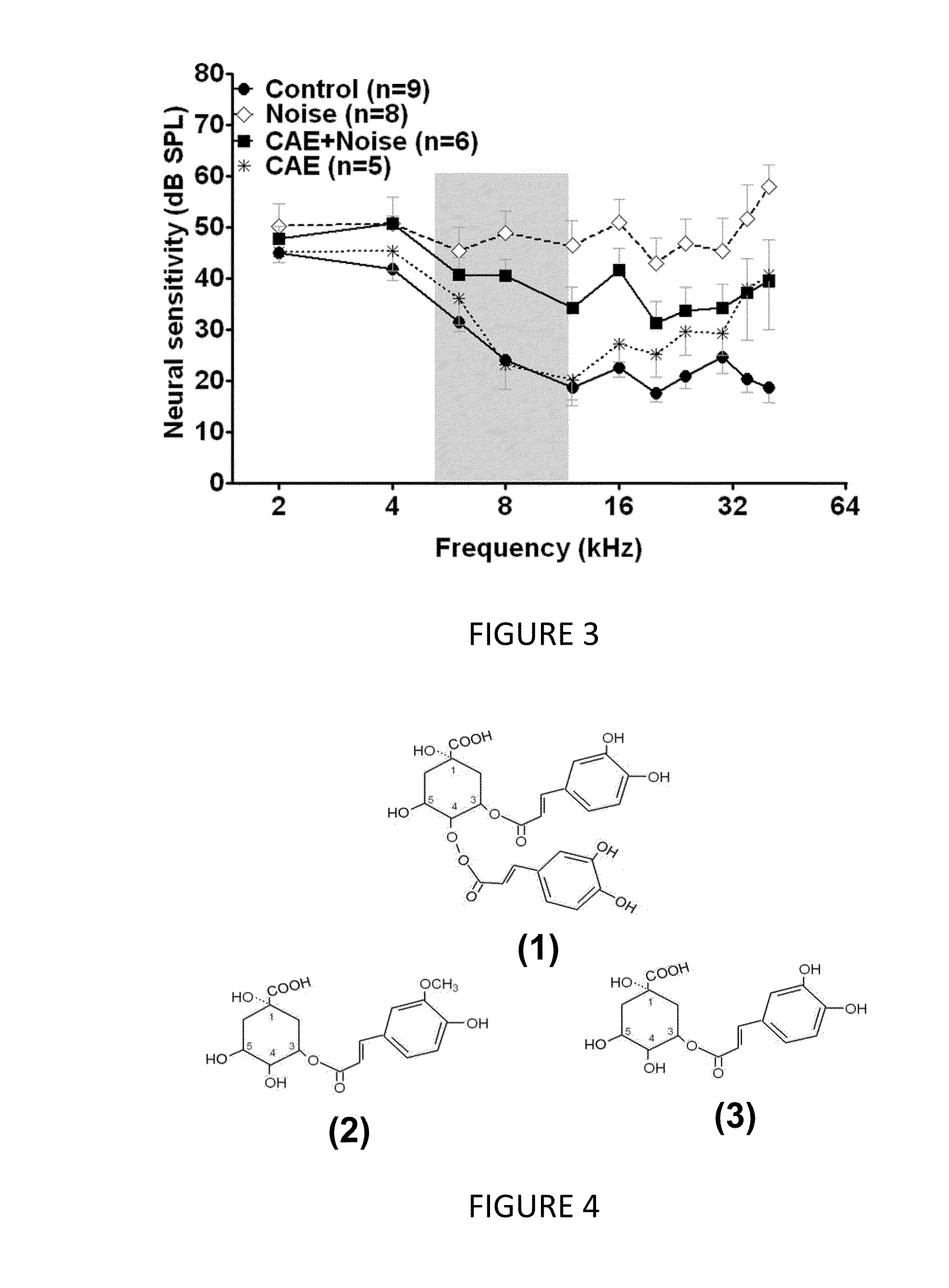
Methods and compositions for preventing and treating auditory dysfunctions
The invention provides methods for treating auditory impairments in a subject in need of treatment comprising administering to said subject an effective amount of a composition comprising, as an active agent, one or more of a carboxy alkyl ester, a quinic acid derivative, a caffeic acid derivative, a ferulic acid derivative, or a quinic acid lactone or derivative thereof or pharmaceutically acceptable salt thereof and an acceptable carrier or excipient, so as to treat auditory impairments in the subject.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Packaged coffee drink
ActiveUS20090035421A1Prevent precipitationReady-for-oven doughsFruit and vegetables preservationHigh concentrationAdditive ingredient
To provide a packaged coffee beverage, which contains chlorogenic acids at high concentration, has good flavor and taste, and is suppressed in the occurrence of sediment during long-term storage. A packaged coffee beverage subjected to heat sterilization treatment, the beverage comprising (A) monocaffeoylquinic acid, (B) feruloylquinic acid and (C) dicaffeoylquinic acid, wherein (a) a total content of the ingredients (A), (B) and (C) contained in dissolved states in the beverage is from 0.14 to 4% by weight based on the beverage, and the beverage comprises (b) 80% by weight or more of water, (c) magnesium and sodium at a Mg / Na weight ratio of from 0.04 to 1, (d) a coffee extract obtained from roasted coffee beans having an L value of form 16 to 25, and (e) from 0.0024 to 0.0122% by weight of brown color in terms of Food Yellow No. 4.
Owner:KAO CORP
Taste improver for high intensity sweetener
InactiveUS20120156351A1Food ingredient as taste affecting agentFood preparationGreen Tea PolyphenolsSucrose
A taste improver which is capable of effectively suppressing unpleasant bitter taste, harsh taste, and astringent taste peculiar to high intensity sweeteners without changing original flavor and taste of food and of reproducing taste intensity and a flavor profile by using the high intensity sweeteners, which are equal to those attained by using sugars such as sucrose.It is possible to improve the unpleasant aftertastes of a high intensity sweetener and to reproduce taste intensity and a flavor profile which are equal to those attained by using sugars such as sucrose by adding the taste improver for high intensity sweetener, characterized by containing (A), (B), and (C), and further (D) and / or (E), to a beverage or food containing the high intensity sweetener:(A) spilanthol, or an extract or essential oil of a plant containing spilanthol;(B) quinic acid or a composition containing quinic acid;(C) vanilla polyphenol or a composition containing vanilla polyphenol;(D) green tea polyphenol or a composition containing green tea polyphenol; and(E) rosaceous plant polyphenol or a composition containing rosaceous plant polyphenol.
Owner:OGAWA & CO LTD
Taste improver for high intensity sweetener
ActiveCN102481004ASuppress bitternessFood ingredient as taste affecting agentFood preparationSucroseRosaceae
Owner:OGAWA & CO LTD
Beverage having a concentrated or purified tea extract
InactiveUS7029718B2Great tasteIncrease concentrationTea extractionAcidic food ingredientsAdditive ingredientSweetness
Provided is a beverage having a concentrated or purified tea extract incorporated therein and containing the following ingredients (A) and (B): (A) non-polymer catechins from 0.092 to 0.5 wt. %, (B) quinic acid at a weight ratio of [(B) / (A)] falling within a range of from 0.01 to 0.1. The beverage of the present invention is suited for daily drinking, because it does not generate an uncomfortable aftertaste which will otherwise remain after bitterness or astringency peculiar to catechins is alleviated by a sweetener or the like.
Owner:KAO CORP
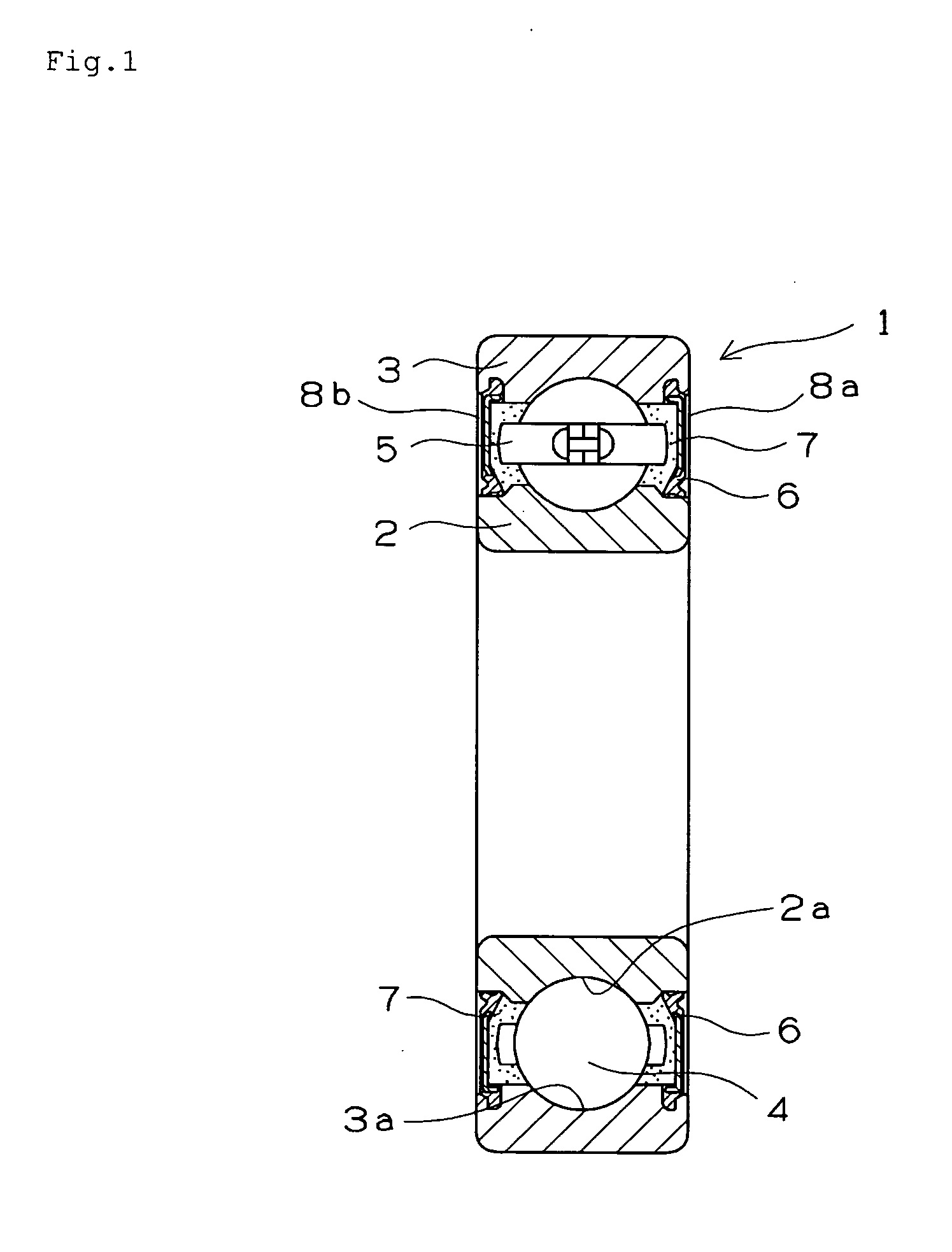
Grease composition, grease-packed bearing, universal joint for propeller shaft, lubricating oil composition, and oil-impregnated sintered bearing
InactiveUS20110306429A1Prevent hydrogen brittleness-caused peculiar peelingImprove resistance to oxidative deteriorationClutchesRoller bearingsGallic acid esterChlorogenic acid
The present invention provides a grease composition or a lubricating oil composition which is capable of effectively preventing hydrogen brittleness-caused peeling from occurring on a rolling surface of a rolling bearing, is excellent in durability in a high temperature and speed operation, and can be used for a long time. A grease-packed bearing (1) has an inner ring (2), an outer ring (3), and a plurality of rolling elements (4). A sealing member (6) for sealing a grease composition (7) is provided at openings (8a) and (8b) disposed at both axial ends of the inner ring (2) and the outer ring (3). The grease composition (7) includes a base grease composed of a base oil and a thickener and an additive added to the base grease. The additive contains at least one compound selected from among plant-derived polyphenolic compounds and compounds formed by decomposition thereof. The above-described compounds include tannin, gallic acid, ellagic acid, chlorogenic acid, caffeic acid, curcumin, quercetin, and quinic acid.
Owner:NTN CORP
Method for processing stevia rebaudiana dark green tea
The invention relates to a method for producing dark green tea from stevia rebaudiana as a raw material. The method comprises the following steps of: selecting stevia rebaudiana tender shoots or fresh leaves or dried leaves as the raw material; after bacterial inoculation, performing pile fermentation at the temperature ranging from 15 to 35 DEG C for 1-10 d; performing roasting drying after the pile fermentation; and pressing to obtain the stevia rebaudiana dark green tea product. The stevia rebaudiana dark green tea obtained by the method provided by the invention has the effects of reducing blood pressure, resisting diabetes, resisting virus and the like as the stevia rebaudiana leaves contain bioactive substances such as stevioside, caffeoyl quinic acid, and flavone.
Owner:NANJING NORMAL UNIVERSITY
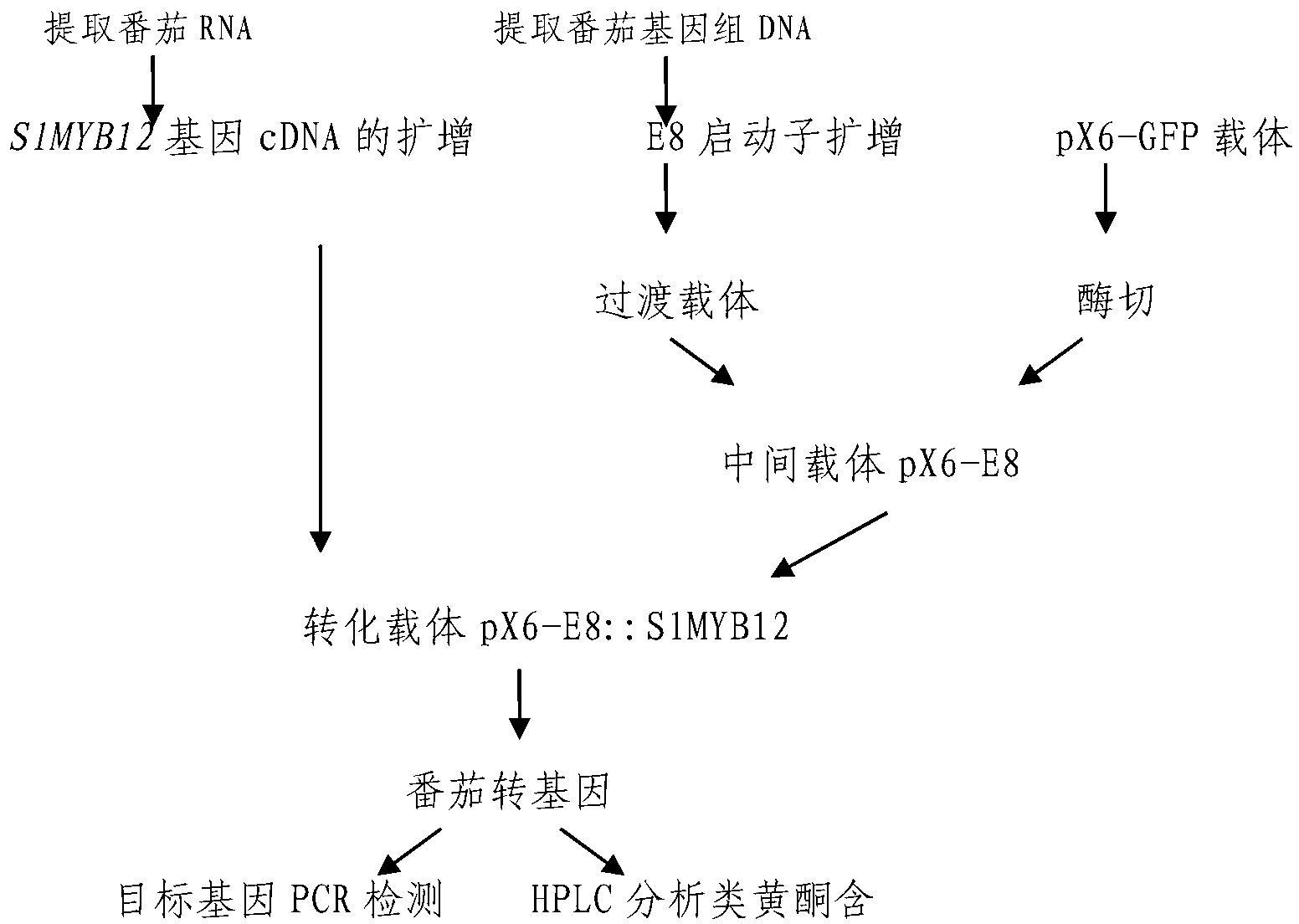
Gene segment for regulating synthesis of solanaceae flavonoids and caffeoyl quinic acid
InactiveCN102796746AImplement industrializationGenetic engineeringFermentationNaringenin chalconeCaffeoylquinic acid
The invention relates to the technical field of plant genetic engineering, and provides a gene segment DNA capable of assigning capacities to solanaceae accumulated flavonoids such as rutin, kaempferol rutin and naringenin chalcone, and caffeoyl quinic acid, wherein the gene segment has a gene sequence shown in SEQ ID NO:1, and is obtained from tomato, and comprises a control gene S1MYB12 for controlling the synthesis of solanaceae flavonoids and caffeoyl quinic acid. The inventor provides a specific method of separation cloning, function verifying and applying of the gene segment. A tomato endogenous gene S1MYB12 is driven to be specifically expressed in the tomato fruit by using a tomato fruit specificity expression promoter E8, thus the contents of the flavonoids and the caffeoylquinic acid in the tomato fruit are greatly increased, and the function of the tomato endogenous gene S1MYB12 of regulating the synthesis of the flavonoids and the caffeoyl quinic acid is verified.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Method for preparing and partially-synthesizing chlorogenic acid from processing residual liquid of aqua lonicerae foliae
InactiveCN101941908ASolve the problem of low extraction yieldRealize comprehensive utilizationOrganic compound preparationCarboxylic acid esters preparationOrganic solventSeparation technology
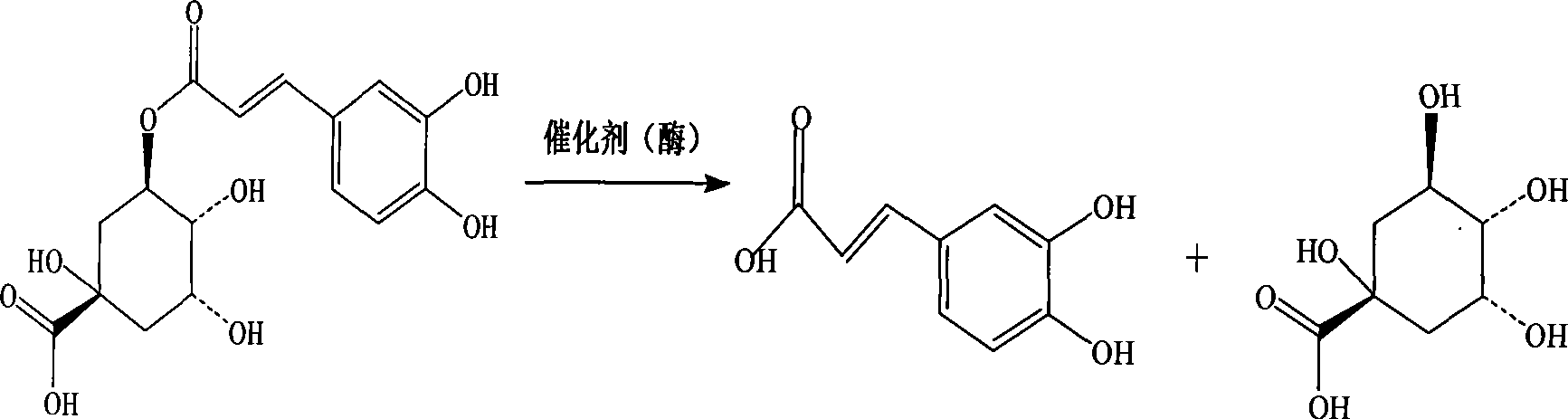
The invention discloses a method for preparing and partially-synthesizing chlorogenic acid from the processing residual liquid of aqua lonicerae foliae, which belongs to a method for manufacturing chemical substances or medicines and solves the problem of the existing chlorogenic acid extraction and preparation technology that a large amount of organic solvent is used and causes environmental pollution, or the production is difficult to expand as a relatively complex separation technology is used, or the extraction efficiency is low, the utilization rate of raw materials is relatively low and so on. The method comprises a deposition step, a separation step by using macroporous absorption resin, an acid hydrolysis step, a separation step by using ion exchange column, a semi-synthesis step and a crystallization step. The invention realizes comprehensive utilization of resources by adopting the processing residual liquid of aqua lonicerae foliae as a raw material, and can make full use of the caffeoylquinic acid substances during the extraction, hydrate the compounds into caffeic acid and quinic acid, then synthesize the chlorogenic acid and finally obtain chlorogenic acid with the purity over 99% through the crystallization step. The process of the invention is simple and can be easily expanded to industrial production while greatly improving the utilization rate of the raw material.
Owner:WUHAN JUHUI GREEN IND DEV
Composition containing dicaffeoyl quinic acid and its preparing method
InactiveCN1615838AOrganic active ingredientsPharmaceutical delivery mechanismDispersed mediaCaffeoylquinic acid
The present invention discloses composition containing dicaffeoyl quinic acid and its preparation process. The composition is suspension, gel or solution with or without dispersing medium. The composition is easy to take, acceptable, and especially suitable for long term taking and for children, aged people and people with dysphagia. The present invention also provides the preparation process of dicaffeoyl quinic acid solution.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Emulsion containing dicaffeoyl quinic acid and its preparing method
InactiveCN1615837AImprove stabilityEasy to transportOrganic active ingredientsDigestive systemIntramuscular injectionOrganic solvent
The present invention discloses emulsion containing dicaffeoyl quinic acid and its preparation process. The emulsion has high stability and small particle size, and may be used in intravenous injection, intramuscular injection or oral taking. The emulsion may be obtained directly via dispersing the organic solution of dicaffeoyl quinic acid and emulsifier directly into water phase component.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
HPLC (High Performance Liquid Chromatography) method for simultaneously determining contents of seven components in blumea balsamifera
InactiveCN107561196AImprove detection efficiencySimple methodComponent separationHplc methodContent determination
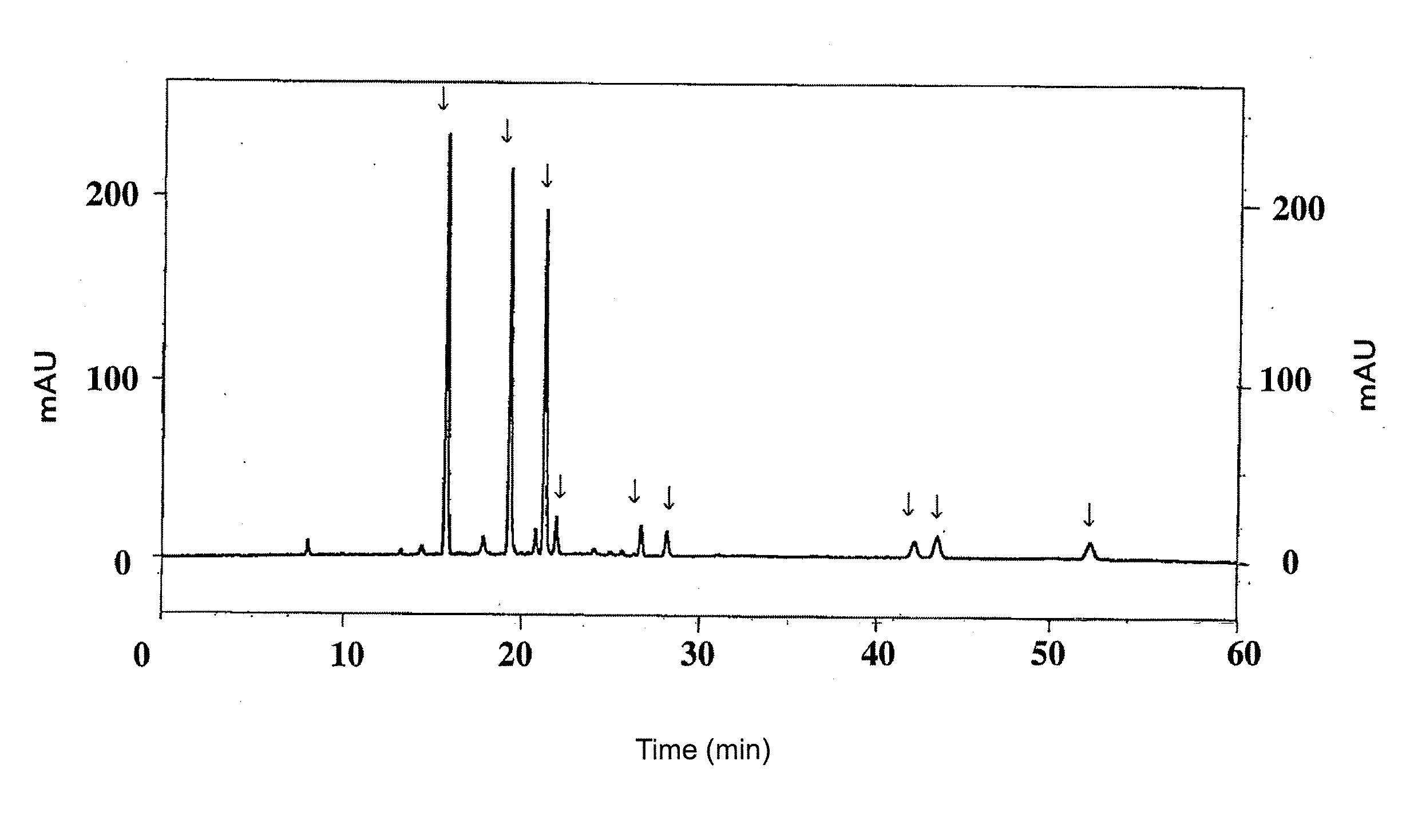
The invention discloses an HPLC (High Performance Liquid Chromatography) method for simultaneously determining the contents of seven components in blumea balsamifera. Seven caffeoyl quinic acid components determined by the method are 5-O-caffeoyl quinic acid, 3-O-caffeoyl quinic acid, 4-O-caffeoyl quinic acid, 1,3-O-dicaffeoyl quinic acid, 3,4-O-dicaffeoyl quinic acid, 3,5-O-dicaffeoyl quinic acidand 4,5-O-dicaffeoyl quinic acid. The HPLC method comprises the following determining steps of (1) preparation of a standard solution; (2) preparation of a tested solution; (3) establishment of content determination standard curves of seven caffeoyl quinic acid components; (4) determination of the contents of seven caffeoyl quinic acid components in a sample. According to the HPLC method disclosed by the invention, the operation is simple and convenient, the precision degree, the repeatability and the stability are good, the accuracy is high, the contents of seven caffeoyl quinic acid components in the blumea balsamifera can be simultaneously determined, and higher practical value is obtained.
Owner:GUIZHOU MEDICAL UNIV
Pharmaceutical compositions from ethnobotanicals
This invention relates to the field of drug discovery. Specifically, it describes a method (“Inverted Drug Screening” or “IDS™”) of identifying therapeutics from ethnobotanical (EB) preparations by repeatedly fractionating and testing fractions from EB sources. One aspect of the invention relates to quinic acid derivatives (e.g., derivatives of 3,5-dicaffeoyl quinic acid) for the treatment of respiratory syncytial virus (RSV) infection.
Owner:ZYMETX
Method for improving active ingredients of honeysuckle flowers
ActiveCN107616992AIngredient content increase valueIdeal ingredient contentAntinoxious agentsPlant ingredientsMedicineWavelength
The invention relates to a method for improving the active ingredients of honeysuckle flowers, in particular to a method for improving the active ingredients of honeysuckle flower by a photoenzymaticinduction technology. The method comprises the following sequential steps of (1) taking the honeysuckle flowers; (2) performing -20 DEG C freezing; (3) performing alternate illumination by UV-A being320 to 400nm, UV-B with the wave length being 280 to 320nm and UV-C with the wave length being 200 to 280nm. The method has the advantages that the contents of caffeoyl quinic acid and iridoid glycoside of the active ingredients of the honeysuckle flowers can be obviously increased; the ultraviolet treatment time is greatly shortened; the environment-friendly effect is achieved; meanwhile, small experiments prove that the method is applicable to large-scale industrial production.
Owner:山东省医学科学院
Method of preparation and composition of a water soluble extract of the bioactive component of the plant species uncaria for enhancing immune, anti-inflammatory, anti-tumor and DNA repair processes of warm blooded animals
InactiveUS20050176825A1Inhibit productionIncrease heightBiocideHydroxy compound active ingredientsDiseaseFluorescence
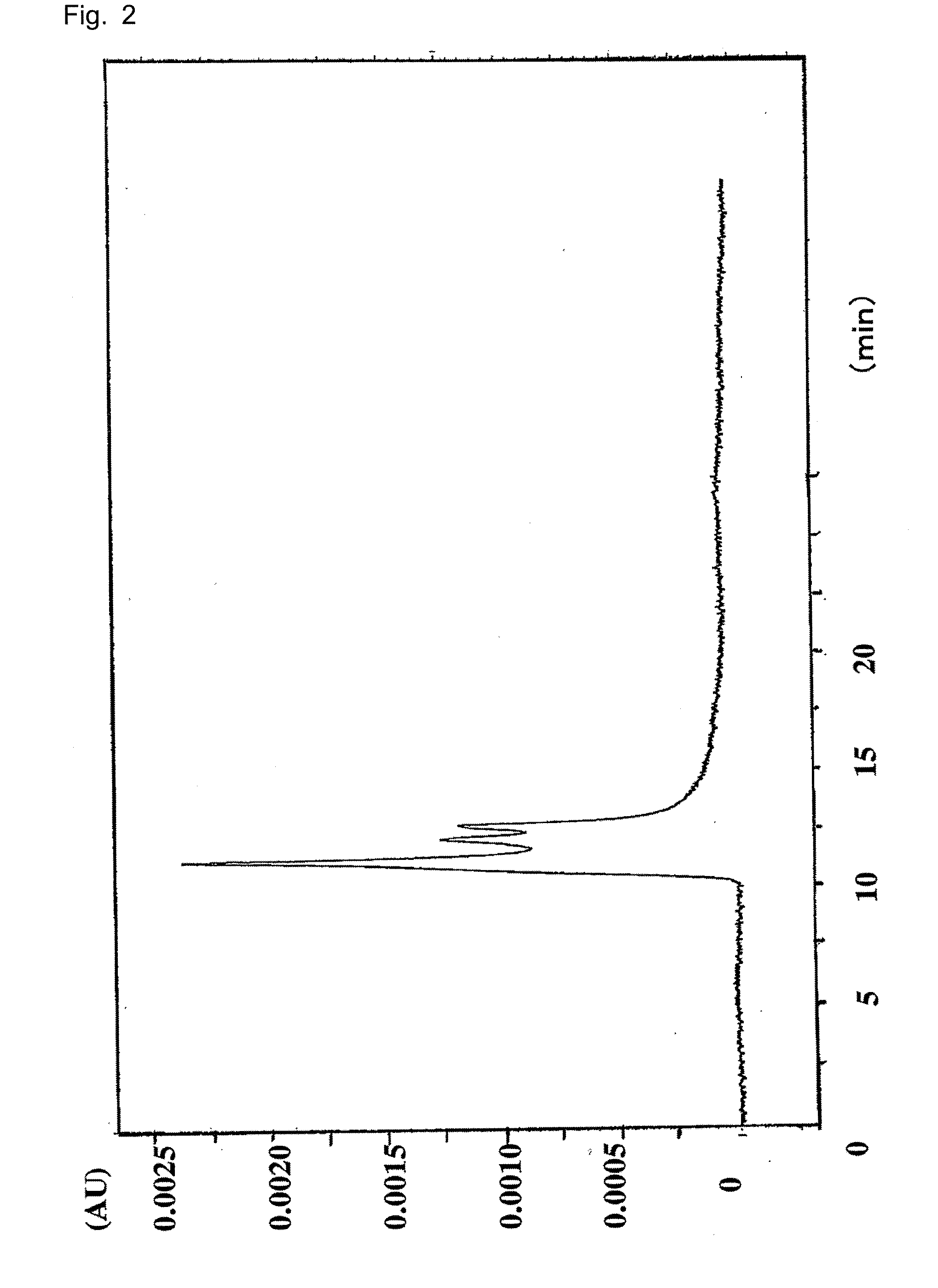
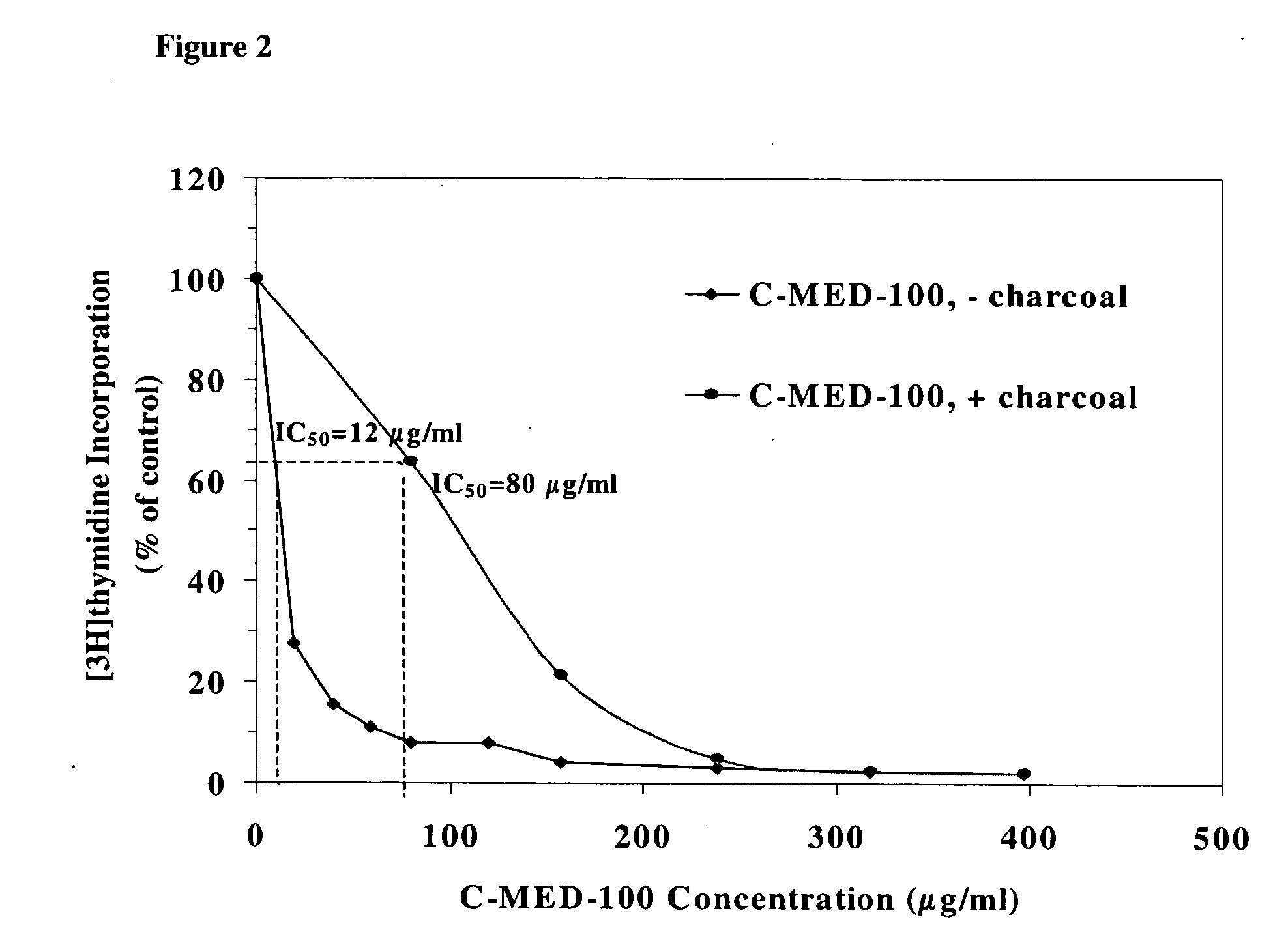
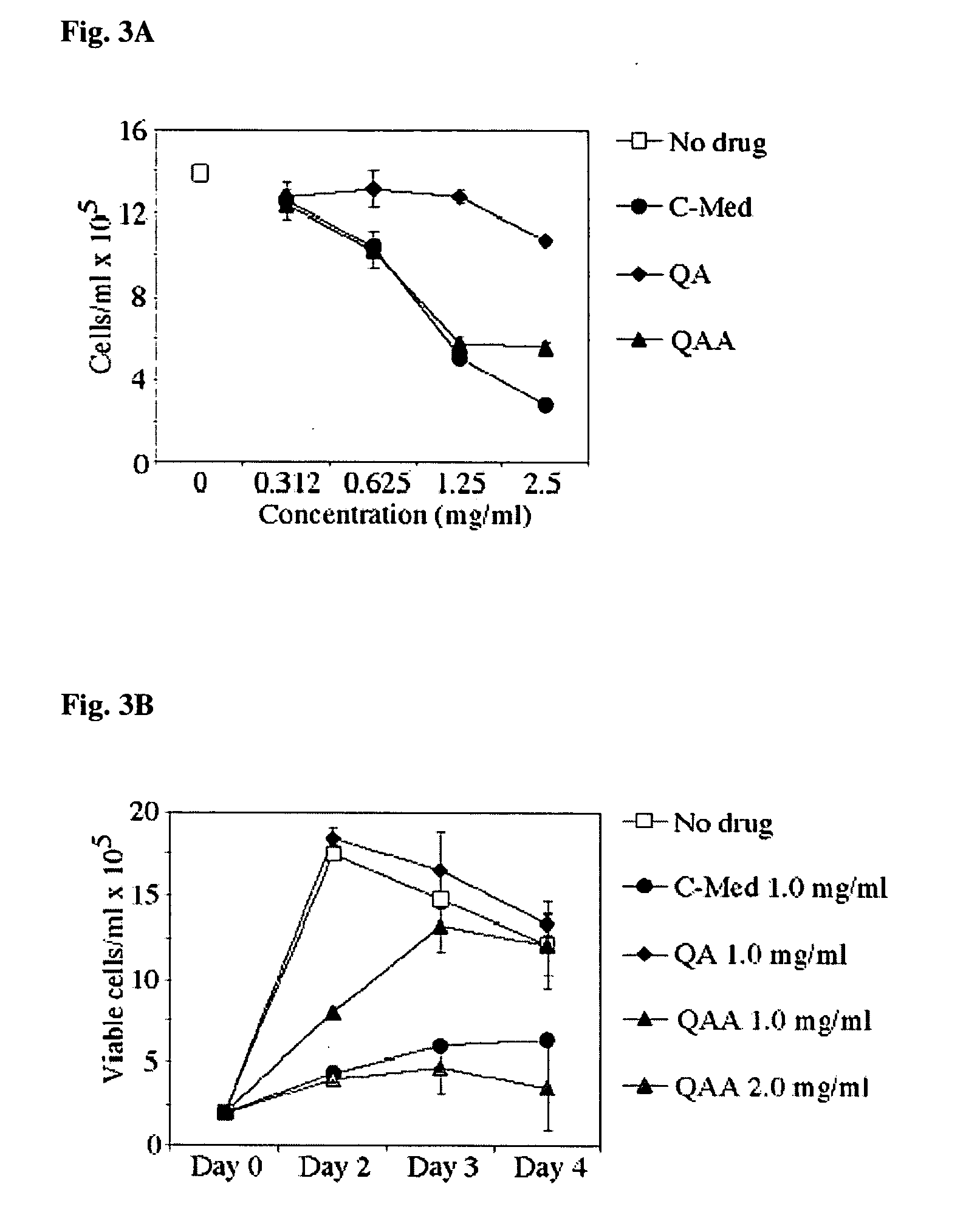
A method for isolating the bioactive component of the water-soluble extract of Uncaria tomentosa known as C-MED-100®, comprising (i) precipitating the spray drying carrier from C-MED-100®; (ii) using the resulting C-MED-100® to obtain a spotting mixture for thin layer chromatography (TLC); (iii) spotting the C-MED-100® spotting mixture on pre-run TLC plates and eluting the plates to obtain the fluorescing band with Rf=0.2-0.3; (iv) scraping off the Rf=0.2-0.3 band, eluting it in ammonia and freeze drying the eluted band to form a powder; and (v) extracting the powder with methanol to remove solubilized silica gel, concentrating the methanol solution and crystalizing the concentrated solution to obtain the bioactive component. The isolated bioactive component in vitro is a quinic acid analog, preferably quinic acid lactone. By contrast, the isolated bioactive component in vivo is quinic acid, whether as free acid or as a quinic acid salt, including quinic acid ammonium salt. A pharmaceutical composition comprising a pharmaceutically effective amount of the bioactive component and a nontoxic inert carrier or diluent. The bioactive component may be used to enhance immune competency, treat disorders associated with the immune system, inhibit the inflammatory response, treat disorders associated with the inflammatory response, enhance the anti-tumor response, and treat disorders associated with the response to tumor formation and growth, all in mammals.
Owner:OPTIGENEX INC
Aspergillus niger strain for high yield of chlorogenic acid hydrolase and use thereof
InactiveCN101392224AFast growthImprove conversion rateFungiHydrolasesChlorogenic acidScreening method
The invention relates to an Aspergillus niger with a high yield of chlorgenic acid hydrolase screened from plant soil rich of chlorgenic acid, which is nominated as Aspergillus niger LN-1 by classification. The collection unit of the strain is the Center of General Microorganisms, China Committee for Culture Collection of Microorganisms, the collection date is July 14, 2008, and the collection registration number is CGMCC No.2589. The invention further mainly relates to a screening method of the strain, an enzyme production method and the application thereof for the preparation of caffeic acid and quinic acid. The Aspergillus niger LN-1 screened by the method is an aerobic fungus that is easy to be cultivated, produces a plurality of complex enzymes and has the advantages of rapid growth speed, strong reproduction, high-efficiency enzyme production, safeness, high efficiency, the production of chlorgenic acid hydrolase that has activity of orientatedly catalyzing and hydrolyzing the chlorgenic acid so as to compose the caffeic acid and the quinic acid, and high transformation efficiency.
Owner:NANJING UNIV OF TECH
Methods and compositions for preventing and treating auditory dysfunctions
The invention provides methods for treating auditory impairments in a subject in need of treatment comprising administering to said subject an effective amount of a composition comprising, as an active agent, one or more of a carboxy alkyl ester, a quinic acid derivative, a caffeic acid derivative, a ferulic acid derivative, or a quinic acid lactone or derivative thereof or pharmaceutically acceptable salt thereof and an acceptable carrier or excipient, so as to treat auditory impairments in the subject.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Taste-improving agent for potassium salt or potassium salt-containing food or drink
InactiveUS20130224361A1Increase heightEnhancing salt tasteAlcoholic beverage preparationFood ingredient as taste affecting agentSalt substituteFlavor
The invention improves the unpleasant taste characteristic of potassium salts without affecting the original flavor of foods or drinks and increases the value of potassium salt as a salt substitute, in order to provide means and a salt composition for reducing sodium in foods and drinks.By adding one or more of the following: quinic acid or a quinic acid-containing composition, spilanthol or a spilanthol-containing plant extract or plant essential oil, or an Allium plant extract, to a potassium salt or a potassium salt-containing food or drink, the unpleasant tastes such as acrid taste and metallic taste characteristic of potassium salts are improved while the salt flavor and taste are augmented.
Owner:MIYAZAWA TOSHIO +3
Method of preparation and composition of a water soluble extract of the bioactive component of the plant species uncaria for enhancing immune, Anti-inflammatory, Anti-tumor and DNA repair processes of warm blooded animals
InactiveUS6964784B2Enhance the immune competency of a mammalInhibit productionBiocideHydroxy compound active ingredientsMammalFreeze-drying
A method for isolating the bioactive component of the water-soluble extract of Uncaria tomentosa known as C-MED-100®, comprising (i) precipitating the spray drying carrier from C-MED-100®; (ii) using the resulting C-MED-100® to obtain a spotting mixture for thin layer chromatography (TLC); (iii) spotting the C-MED-100® spotting mixture on pre-run TLC plates and eluting the plates to obtain the fluorescing band with Rf=0.2-0.3; (iv) scraping off the Rf=0.2-0.3 band, eluting it in ammonia and freeze drying the eluted band to form a powder; and (v) extracting the powder with methanol to remove solubilized silica gel, concentrating the methanol solution and crystalizing the concentrated solution to obtain the bioactive component. The isolated bioactive component is a quinic acid analog, preferably quinic acid lactone. A pharmaceutical composition comprising a pharmaceutically effective amount of the bioactive component and a nontoxic inert carrier or diluent. The bioactive component may be used to enhance immune competency, treat disorders associated with the immune system, inhibit the inflammatory response, treat disorders associated with the inflammatory response, enhance the anti-tumor response, and treat disorders associated with the response to tumor formation and growth, all in mammals.
Owner:OPTIGENEX INC +1
Beverage Packed in Sealed Container
An object of the present invention is to provide a beverage packed in a sealed container, which can suppress occurrence of precipitation for a long period and retain high flavor. The above-described problem can be overcome by containing (1) theanine ranging from 100 mg to 1000 mg and (2) quinic acid ranging from 30 mg to 300 mg per 500 ml beverage.
Owner:TAIYO KAGAKU CO LTD
Use of artemisia annua and artemisia annua industrial-extraction residues as raw materials for caffeoylquinic acid preparation
InactiveCN102816065ALow costReduce manufacturing costCarboxylic acid esters separation/purificationBiotechnologyEconomic benefits
The invention discloses a novel use of artemisia annua and provides a novel raw material source for caffeoylquinic acid preparation. The use provided by the invention has the advantages that limitation of artemisia annua development and utilization are overcome; comprehensive and effective utilization of an artemisia annua resource is realized; and artemisia annua industrial-extraction residues are changed into things of value so that large economic benefits, social benefits and environmental benefits are obtained.
Owner:NANJING NORMAL UNIVERSITY
Processes for producing and recovering shikimic acid
ActiveUS20100298599A1FermentationCarboxylic compound separation/purificationShikimic acidCarboxylic acid
The present invention generally relates to processes for producing and recovering cyclitolcarboxylic acids such as shikimic acid and quinic acid. In particular, the present invention is directed to processes for producing shikimic acid that comprise contacting glyphosate and an organism that has the common aromatic biosynthetic pathway. The present invention is also directed to recovery of the shikimic acid product from aqueous process streams utilizing membrane separation techniques
Owner:MONSANTO TECH LLC
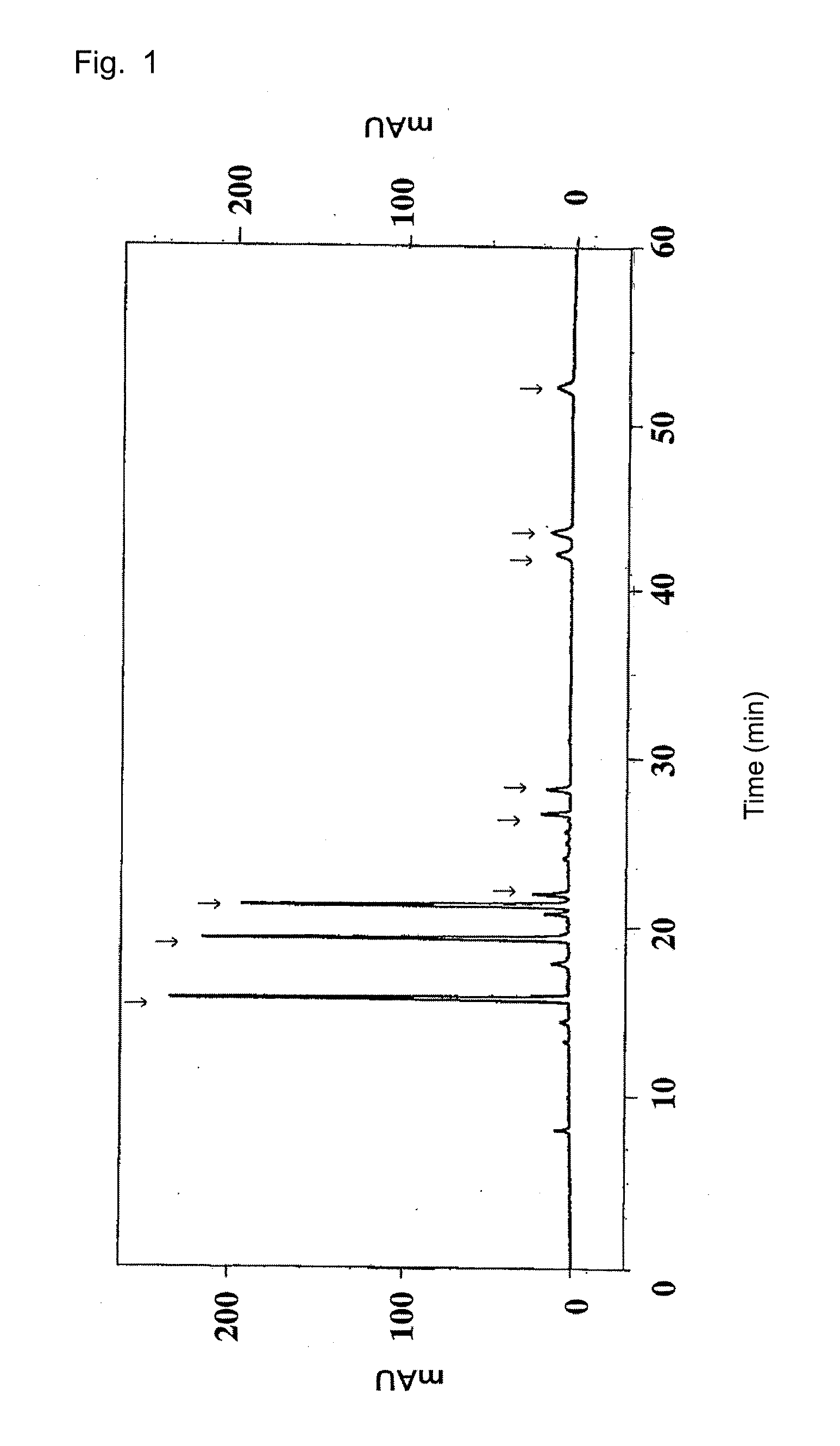
Miao medicine Periploca forrestii Schltr. HPLC fingerprint study and multi-component content determination method
The invention discloses a Miao medicine Periploca forrestii Schltr. HPLC fingerprint study and multi-component content determination method. An HPLC method is used to establish fingerprints of Periploca forrestii Schltr. of different producing areas, contents of four kinds of caffeoyl quinic acid-type chemical constituents therein are determined, and a basis is provided for Periploca forrestii Schltr. medicinal material quality evaluation; a chromatographic column is Xtimate C18 (4.6mm x 250mm, 5[mu]m); 0.1% phosphoric acid aqueous solution (A)-acetonitrile (B) is used as mobile phases, and gradient elution is carried out; a flow rate is 1 mL / min; a column temperature is 30 DEG C; injection volume is 10[mu]L, and detection wavelength is 330nm; and a similarity evaluation system for chromatographic fingerprint of TCM (version 2012) is used for similarity analysis on samples, contents of four kinds of caffeoyl quinic acid-type chemical constituents can be determined simultaneously through the Periploca forrestii Schltr. HPLC fingerprints and content determination, the four kinds of constituents of the different producing areas have large differences, the content of each constituent has a certain relationship with a producing area, and a foundation is laid for later Miao medicine Periploca forrestii Schltr. quality standard study.
Owner:贵州中医药大学
Method for extracting shikimic acid and quinic acid from ginkgo leaves
ActiveCN111233658AIncrease production capacityAchieve regenerationBulk chemical productionCarboxylic compound separation/purificationBiotechnologyQuinamic acid
The invention belongs to the field of organic acid extraction and separation, and relates to a method for extracting shikimic acid and quinic acid from ginkgo leaves. The problems of long consumed time, large wastewater treatment capacity, environmental pollution, complicated operation and the like in the existing method are solved; the chemical properties of each component in the ginkgo leaves are compared; a carbon dioxide supercritical extraction-extraction-membrane filtration-electrodialysis-crystallization method is combined for use; on the premise of commercial value, various active ingredients, including chlorophyll, ginkgetin, bilobalide, gingko polysaccharide and the like, in the ginkgo leaves are developed to the maximum extent, and finally, shikimic acid and quinic acid productsare obtained through separation, and the content of the shikimic acid and the content of the quinic acid are respectively greater than 98%.
Owner:SHAANXI JIAHE PHYTOCHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com