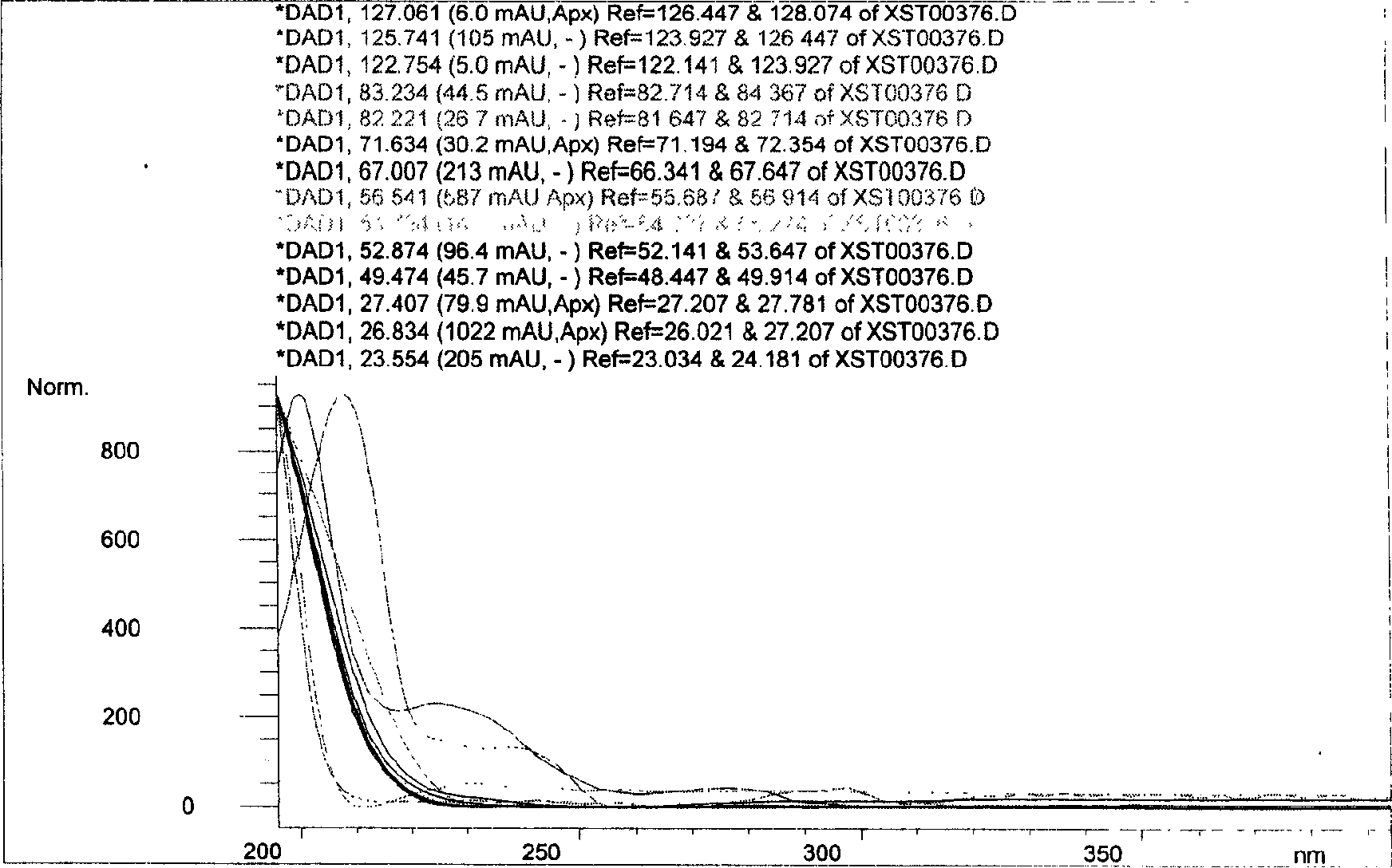
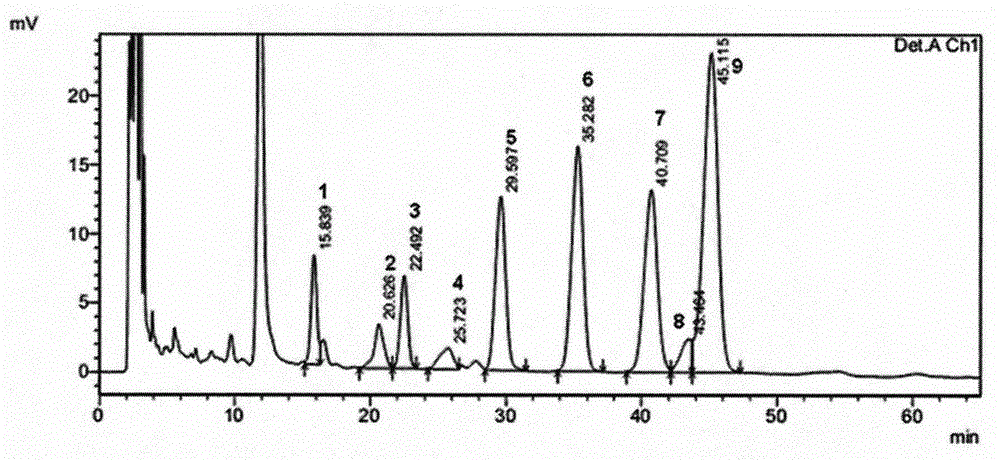
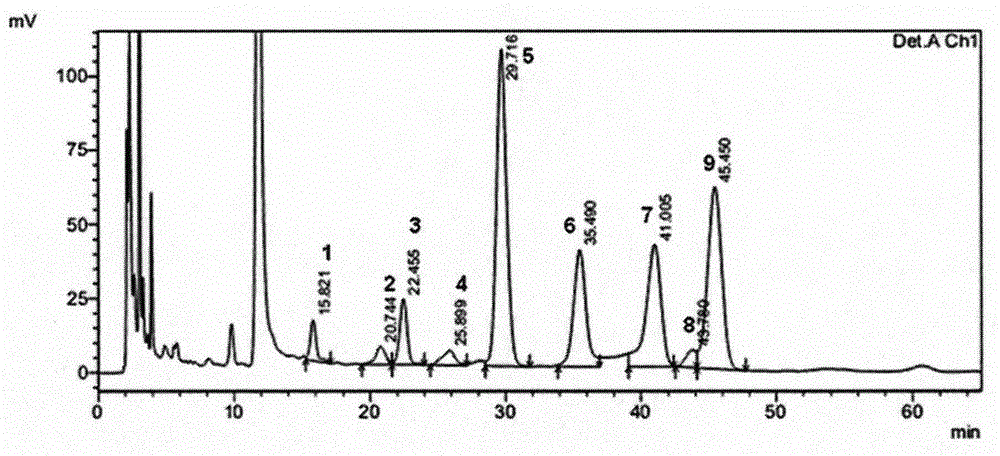
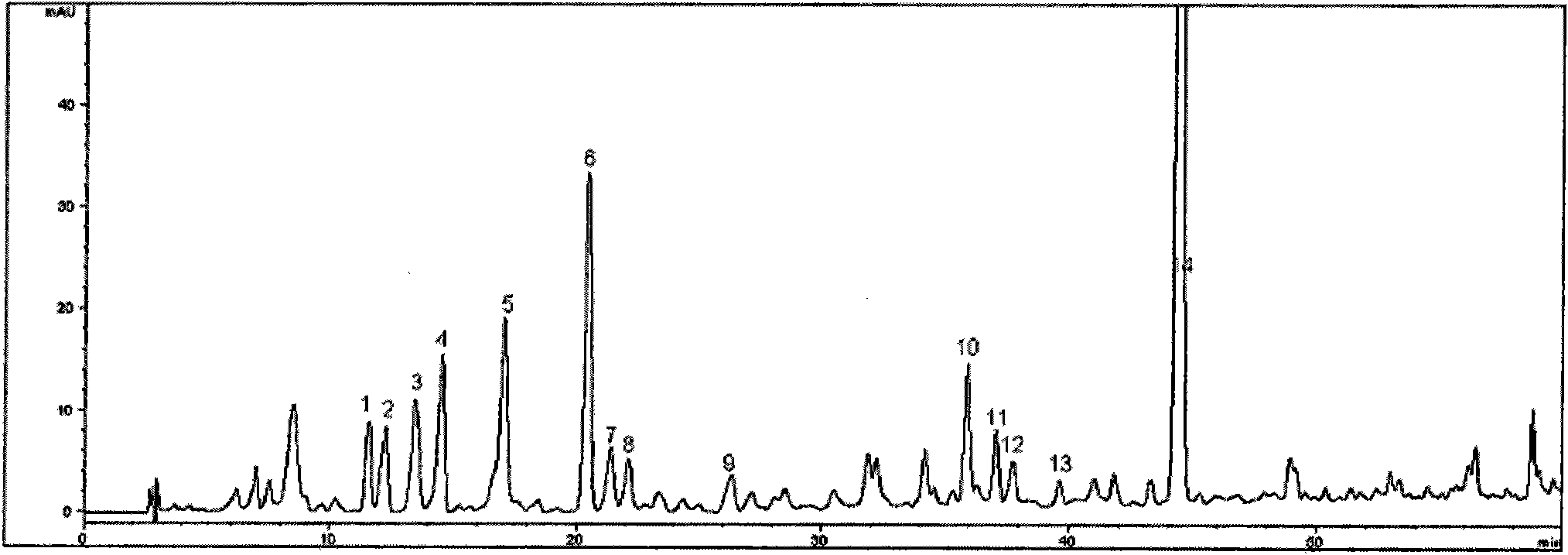
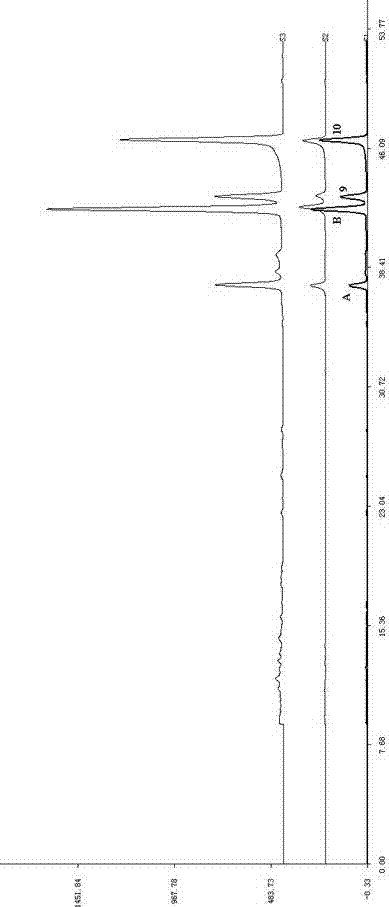
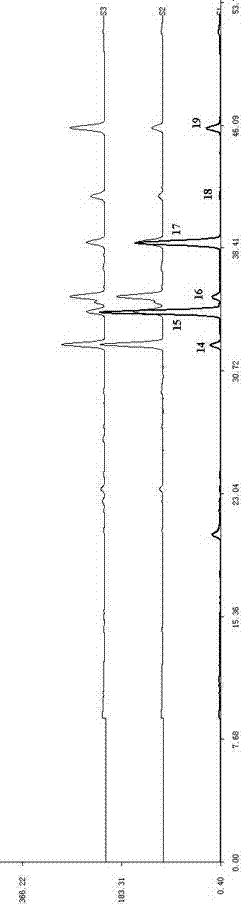
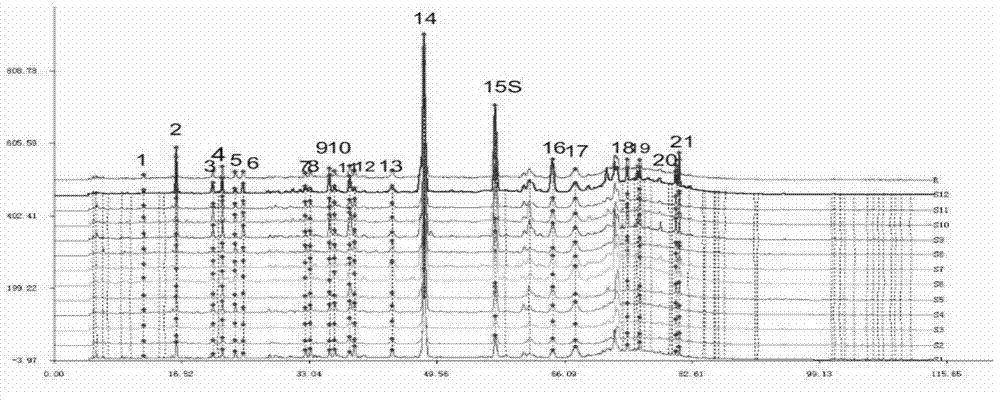
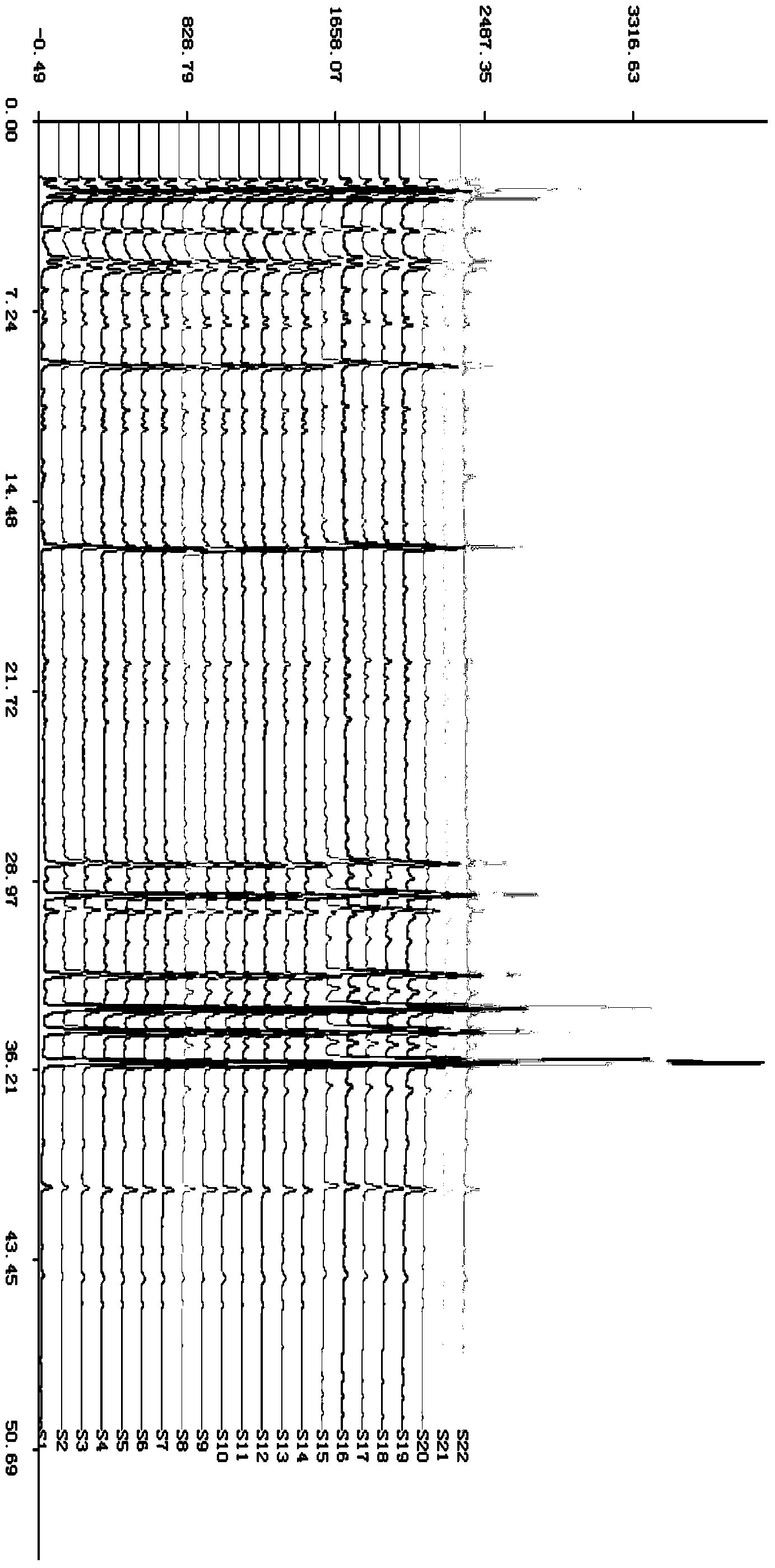
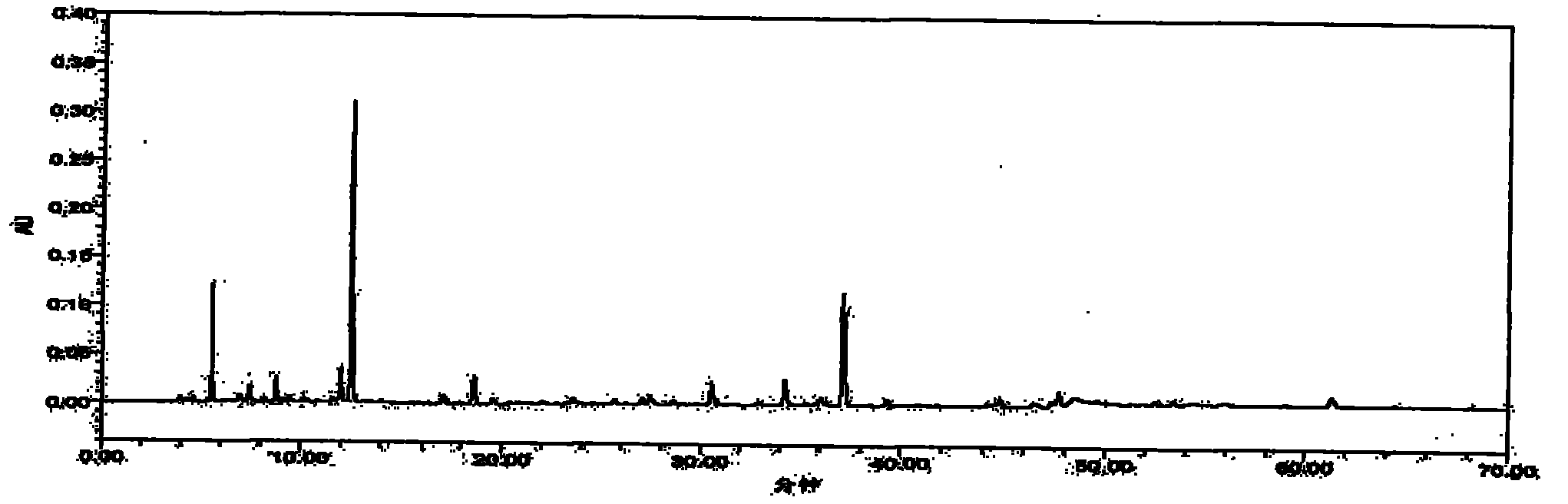
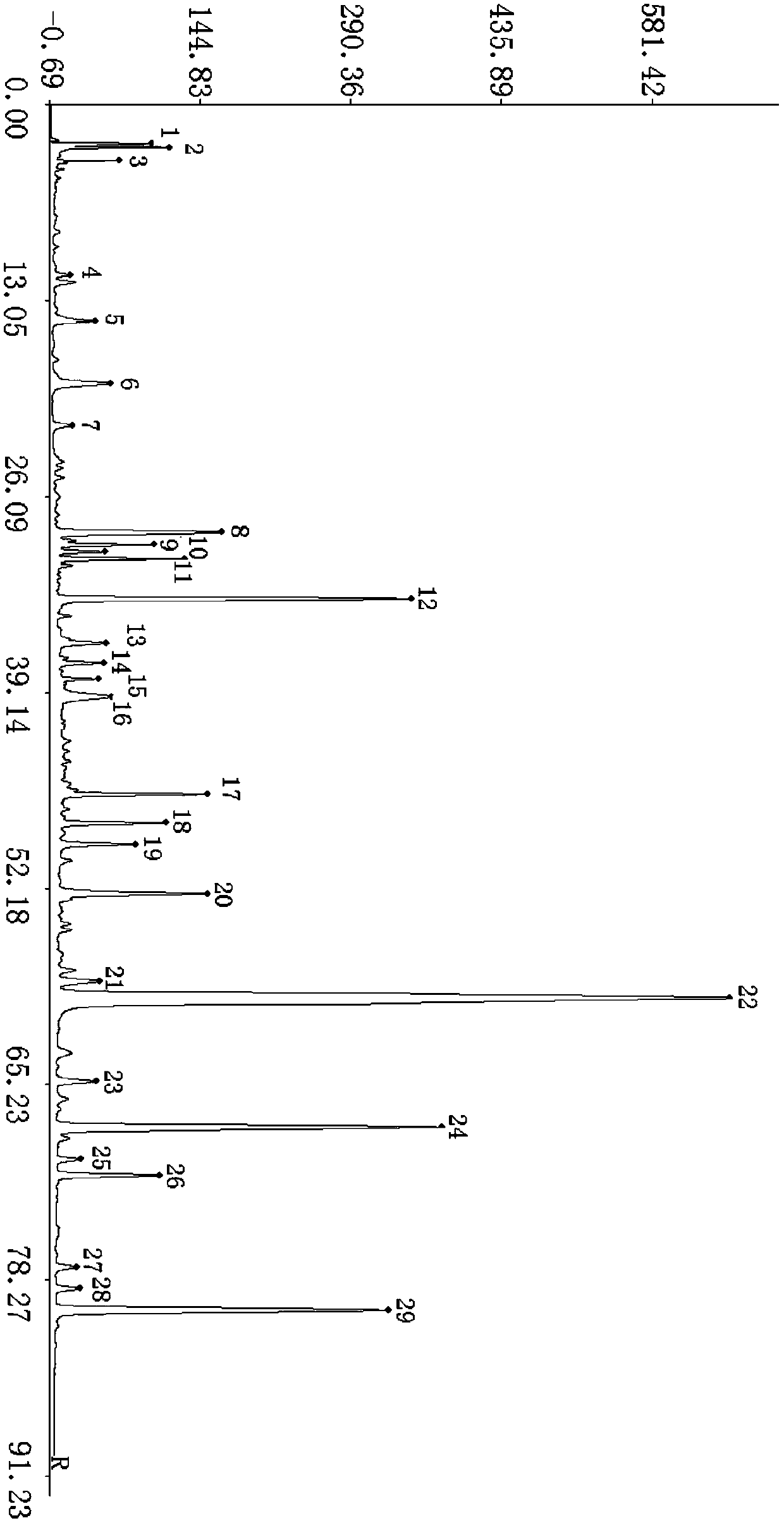
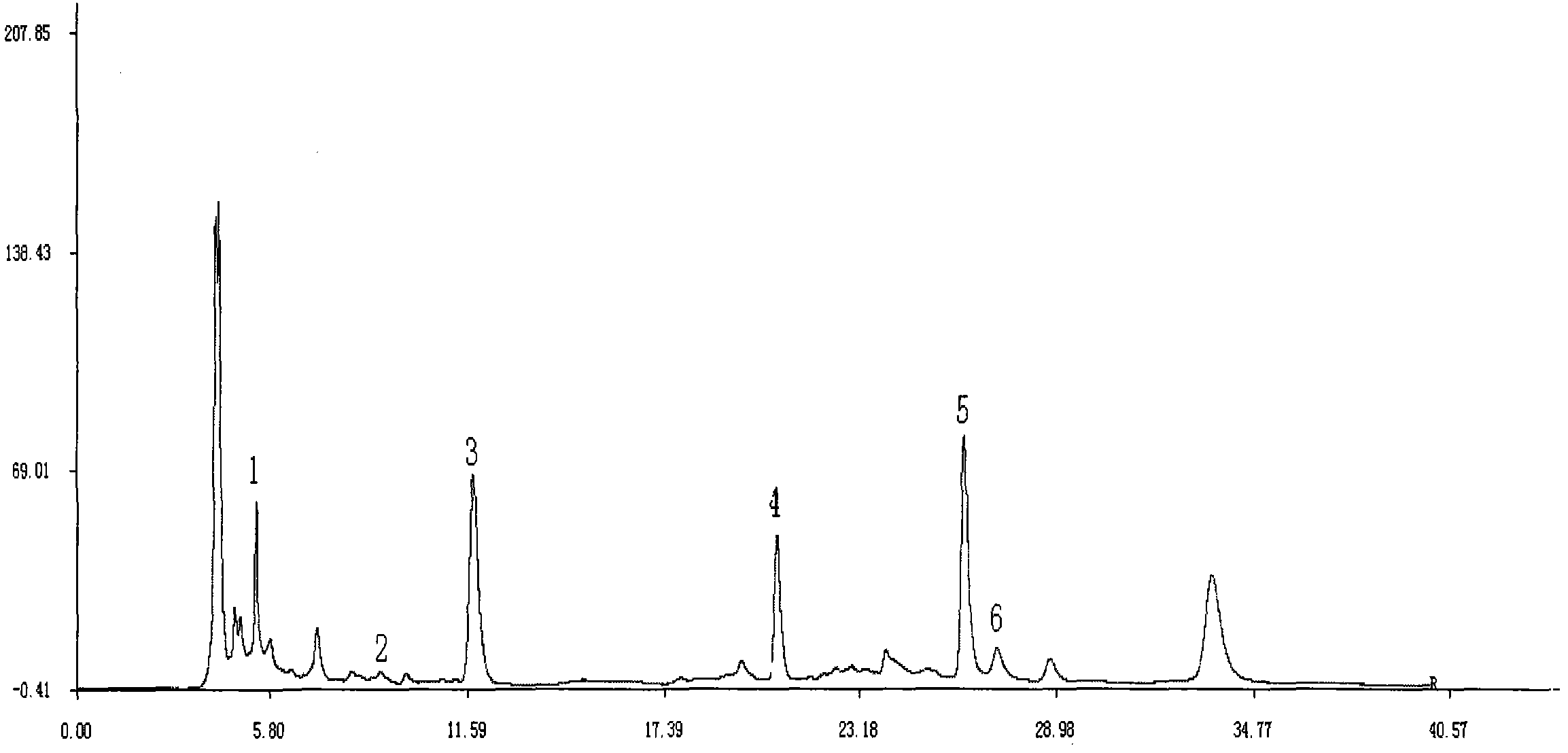
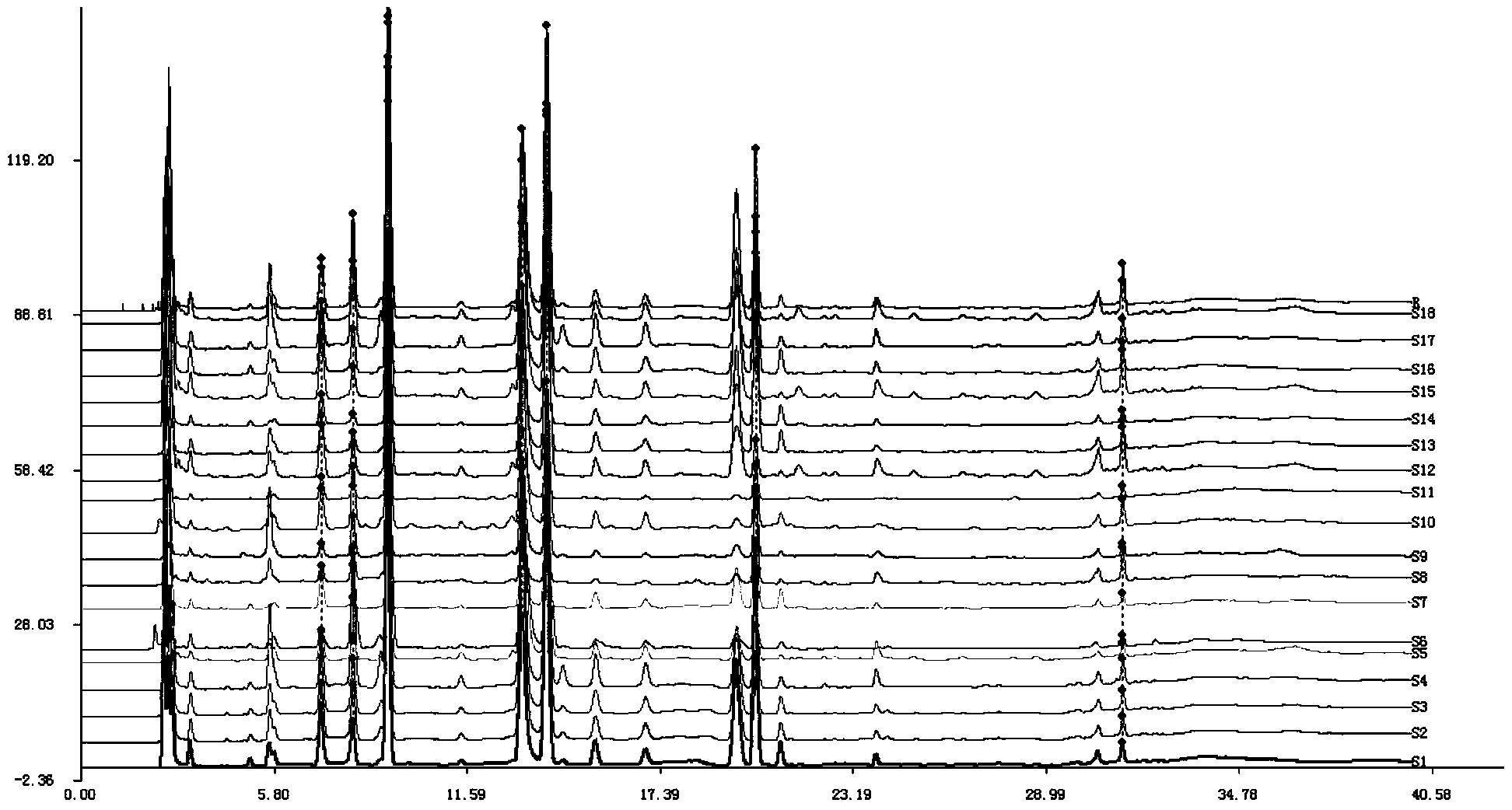
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
474 results about "Hplc fingerprint" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
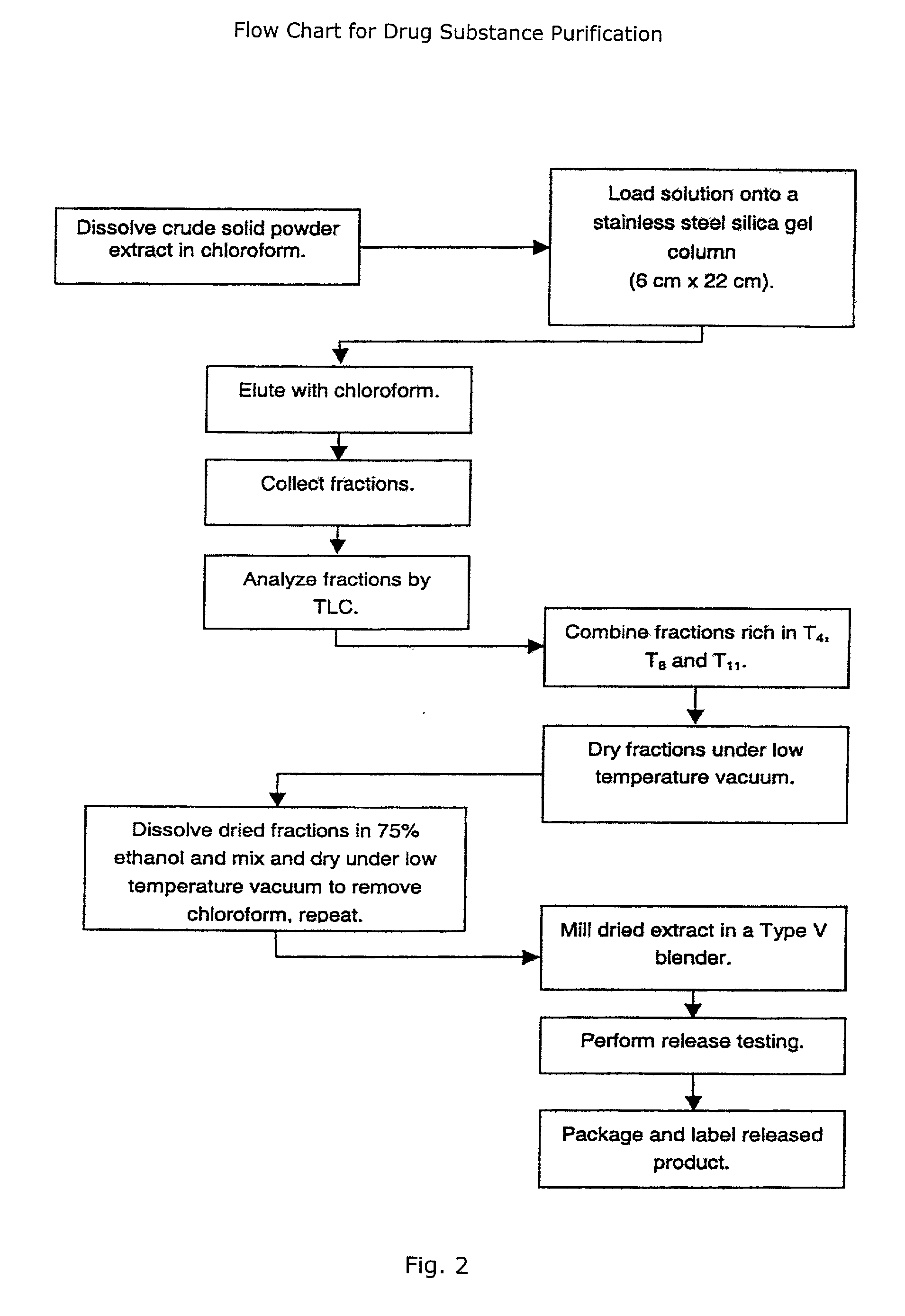
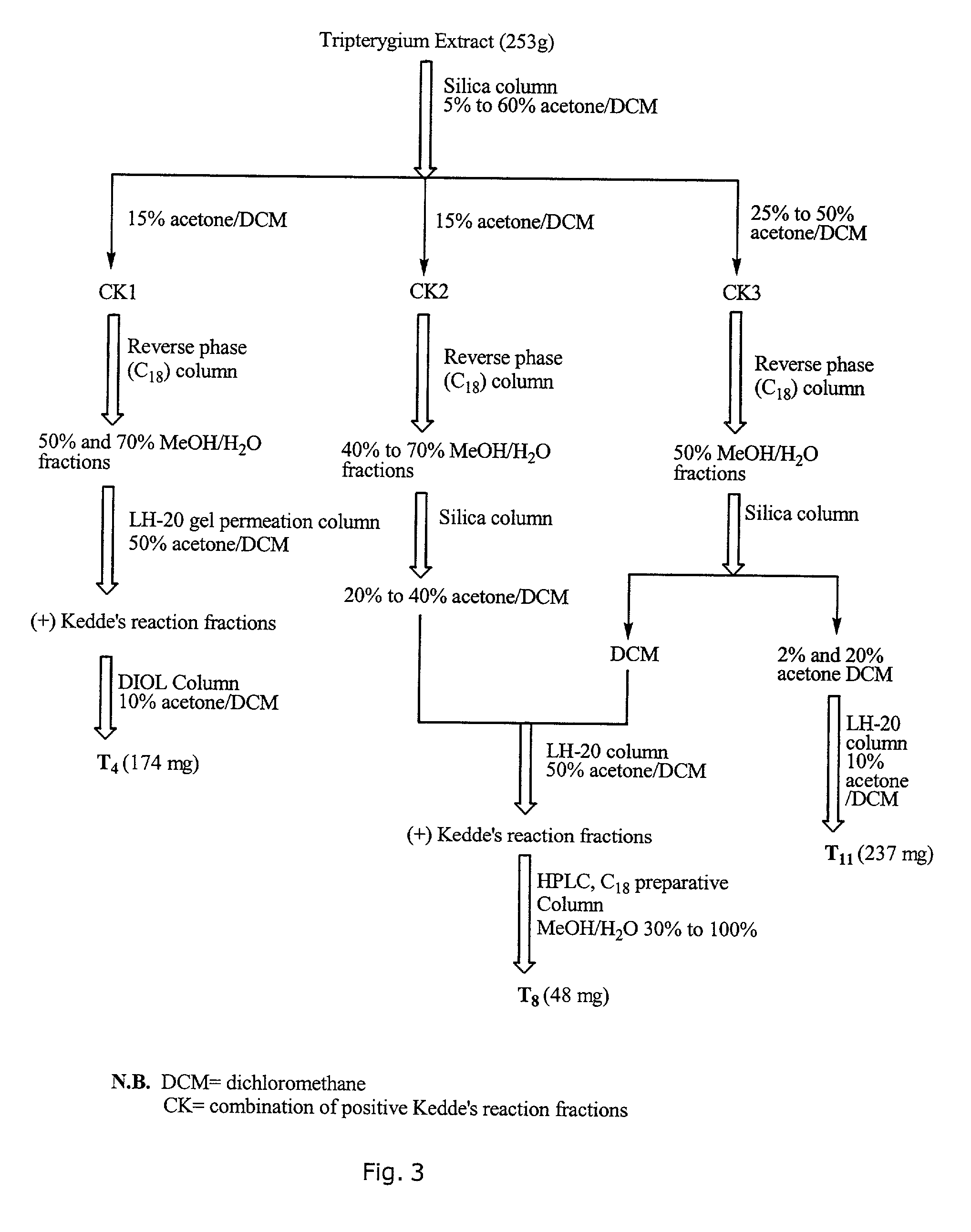
Novel botanical extract of Tripterygium Wilfordii Hook F.
A novel botanical extract from the plant Tripterygium Wilfordii Hook. F. containing bioactive components triptriolide, tripdiolide, tripchloride and tiptolide for treating inflammatory and autoimmune diseases is prepared and the bioactive components are quantified. A HPLC fingerprinting technology is also developed to produce a characteristic fingerprint for the botanical extract.
Owner:NOVEMED GROUP
Method for screening traditional Chinese medicine effect related ingredients and model building method
ActiveCN102879486ASimple methodReliable methodComponent separationHplc fingerprintAdditive ingredient
The invention discloses a method for screening traditional Chinese medicine effect related ingredients and a model building method. The method comprises the steps as follows: taking the researched traditional Chinese medicines as objects for preparing multiple sets of sample solutions containing chemical components with different concentrations; selecting part of the chemical components for HPLC (High Performance Liquid Chromatography) analysis, and investigating linear relations between the peak areas and the concentrations; selecting the multiple sets of sample solutions in the linear range, and determining the respective HPLC fingerprint spectrums and the medicine effect indexes of the sample solutions; and obtaining relations between the peak areas and the medicine effect indexes of the ingredients by adopting a linear regression analysis method in the linear relation range, and confirming the actions of the medicine effect related ingredients on the medicine effect indexes according to the positive value and the negative value of the standard regression coefficients and / or the t check results of the ingredients so as to screen out the traditional Chinese medicine effect related ingredients. The method is simple, convenient, easy to implement, reliable and good in commonality, can embody the characteristics of comprehensive actions of the multiple ingredients on the medicine effect indexes, is suitable for screening the traditional Chinese medicine effect related ingredients, and provides a technical data support for discovering and screening traditional Chinese medicine active ingredients.
Owner:SHANDONG UNIV
Quality control method of liuwei wuling tablets
InactiveCN105758962AReduce wear and tearShort analysis timeComponent separationBiological testingMedicinal herbsHplc fingerprint
The invention relates to the technical field of compound traditional Chinese preparations, and in particular relates to a quality control method of liuwei wuling tablets. The quality control method is characterized in that 20 different proportion groups are formed by randomly sampling six medicinal materials of the liuwei wuling tablets; a corresponding HPLC fingerprint spectrum is established, and in-vitro anti-hepatic fibrosis activity is measured; and an SPSS software is utilized to analyze, so that a pectrum-effect relationship of in-vitro anti-hepatic fibrosis effect of the liuwei wuling tablets is established. The relatively perfect quality control method is established on the basis of pharmacodynamic substances of the liuwei wuling tablets. Compared with a conventional single-index content measuring method, the quality control method is more comprehensive, scientific and standard, and has the advantages that the content of multiple components is simultaneously measured, so that not only the analysis time is shortened, but also the loss of a solvent is saved.
Owner:山东世博金都药业有限公司
HPLC fingerprint spectrum measurement method for standard double-harmonizing decoction
ActiveCN106501434AGuaranteed stabilityGuarantee the safety of useComponent separationHplc fingerprintData matching
The invention provides a HPLC fingerprint spectrum measurement method for standard double-harmonizing decoction, belonging to the technical field of traditional Chinese medicine. The method comprises the following steps: (1) preparing a test sample solution; (2) preparing a mixed reference solution; (3) preparing a single medicine negative reference solution; (4) precisely absorbing the test sample solution, the mixed reference solution and the single medicine negative reference solution respectively and injecting into a high performance liquid chromatograph and measuring to obtain the liquid chromatogram of the test sample solution, the mixed reference solution and the single medicine negative reference solution; and (5) performing data matching on the liquid chromatogram of the test sample solution, the mixed reference solution and the single medicine negative reference solution by use of the traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system formulated by the Chinese committee of pharmacopeia to obtain a standard fingerprint spectrum. According to the invention, the quality of standard granules of double-harmonizing decoction is effectively controlled, the curative effect of the drug is guaranteed, and more formal quality control is realized on the classical famous prescription.
Owner:天津同仁堂集团股份有限公司
Method for constructing Compound Xueshuantong preparation HPLC fingerprint pattern and method standard fingerprint pattern thereof
ActiveCN1670529AMonitor qualityMonitor stabilityTesting dairy productsBiological testingSalvia miltiorrhizaHplc fingerprint
This invention relates to compound thrombus-relaxing agent fingerprint spectrum recreation method by use of natural plants medicinal materials, which comprises the following parts: test solution process; comparing liquid process; measuring with effective liquid chromatograph measurement with chromatograph relative keeping time and peak area as one; computing the test items relative keeping time and relative peak area to get the HPLC fingerprint chromatograph. The required HPLC standard fingerprint chromatograph to notoginseng chromatograph has five common peaks; the one to astragalus root has two common peaks; chromatograph to radix salvia miltiorrhiza has two common peaks; one to scrophularia root has two common peaks; the one to radix salvia miltiorrhiza under 270 nm has three common peaks; the one to scrophularia root has common peak.
Owner:SUN YAT SEN UNIV +1
Construction method of wolfberry fruit polysaccharide multi-element fingerprint spectrum and wolfberry fruit polysaccharide standard fingerprint spectrum
ActiveCN104458985ASimple and fast operationEasy to operateComponent separationHplc fingerprintLighting spectrum
The invention provides a construction method of a wolfberry fruit polysaccharide multi-element fingerprint spectrum and a wolfberry fruit polysaccharide standard fingerprint spectrum, belonging to the technical field of traditional Chinese medicine and functional food raw materials and a fingerprint spectrum of the product. The construction method comprises the following steps: pretreating wolfberry fruits, extracting wolfberry fruit polysaccharide, determining a UV standard fingerprint spectrum, determining an HPSEC standard fingerprint spectrum, analyzing an IR light spectrum fingerprint and determining the standard fingerprint spectrum, completely hydrolyzing, deriving a hydrolyzed product, and analyzing a PCD-HPLC fingerprint and determining the standard fingerprint spectrum. The pretreatment, extraction of the wolfberry fruit polysaccharide and construction method of the multi-element fingerprint spectrum of 16 types of wolfberry fruits in different production areas are the same as above and can be operated simultaneously and respectively. The construction method of the wolfberry fruit polysaccharide multi-element fingerprint spectrum has the advantages of simplicity in operation, stability, flexibility, high precision, good repeatability and the like; and the quality condition and production area resources of the wolfberry fruit polysaccharides can be grasped from a whole characteristic appearance of a chromatogram and a novel scientific method is provided for wolfberry fruit quality control and true and false identification.
Owner:CHINA PHARM UNIV
Method for authenticating dendrobium officinale by using HPLC fingerprint
InactiveCN102370891ASignificant advantagesSignificant useSenses disorderComponent separationHplc fingerprintOrganic solvent
The present invention provides a method for authenticating dendrobium officinale by using a HPLC fingerprint. The method comprises the following steps: (1) establishing a HPLC fingerprint of dendrobium officinale: a, precisely weighing powder of a dendrobium officinale medicinal material, adding water or a monohydric alcohol to carry out extraction and concentration, extracting by an organic solvent, carrying out treatments of recovering, concentrating, volume metering, filtering to obtain a tested sample solution; b, adopting a high performance liquid chromatography method to analyze the tested sample solution to obtain the fingerprint of the dendrobium officinale medicinal material; (2) establishing a standard fingerprint of the dendrobium officinale: carrying out analysis and comparison for 20 batches of the dendrobium officinale medicinal materials to obtain the standard fingerprint of the dendrobium officinale and the similarity of the dendrobium officinale medicinal material, wherein the similarity is more than 0.8; (3) adopting the same steps to detect the fingerprint of the dendrobium officinale counterfeit / substitute, carrying out comparison for the fingerprint of the dendrobium officinale counterfeit / substitute and the standard fingerprint of the dendrobium officinale, calculating the similarity, wherein the similarity is less than 0.6. According to the present invention, the standard fingerprint and the similarity can be adopted to effectively monitor the authenticity of the dendrobium officinale and the medicinal material quality.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Medicinal material of polygonum capilalum, extractive, and quality control method
InactiveCN101091749AReflect qualityQuality improvementComponent separationUrinary disorderHplc fingerprintMedicinal herbs
The present invention provides a Chinese medicinal material polygonum capitatum. Said invention provides its HPLC fingerprint chromatogram map, it contains 13 characteristic peaks. Its chromatographic condition is as flows: chromatographic column: Hypersil ODS chromatographic column (4.6mmX150mm, 5micrometers); column temperature: 25deg.C; mobile phase: acetonitrile (A)-0.4% phosphoric acid (B) solution, binary gradient elution, weight percentage is 0-30%: 100-70%; flowing rate: 0.8ml.min-1; detection wavelength: 310nm; and sample size: 20 microliters. Besides, said invention also provides its extract and its quality control method.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Fingerprint of Polygonum multiflorum and establishment method and application thereof
InactiveCN102114083AGood reproducibilityQuality improvementComponent separationDigestive systemHplc fingerprintPolygonum limbatum
The invention discloses a quality control method of Polygonum multiflorum through the fingerprint thereof. The fingerprint is HPLC (high-performance liquid chromatography) fingerprint obtained through HPLC analysis of Polygonum multiflorum. The fingerprint can be used for completely monitoring the quality of Polygonum multiflorum to ensure the stable and uniform quality of Polygonum multiflorum and related pharmaceutical preparations containing Polygonum multiflorum. The invention further discloses the establishment method and the application of the fingerprint of Polygonum multiflorum.
Owner:KANGMEI PHARMA
Fingerprint pattern quality control method for cordyceps sinensis bacterium powder raw material in herbs medicaments for strengthening the body resistance and activating blood and dissolving stasis
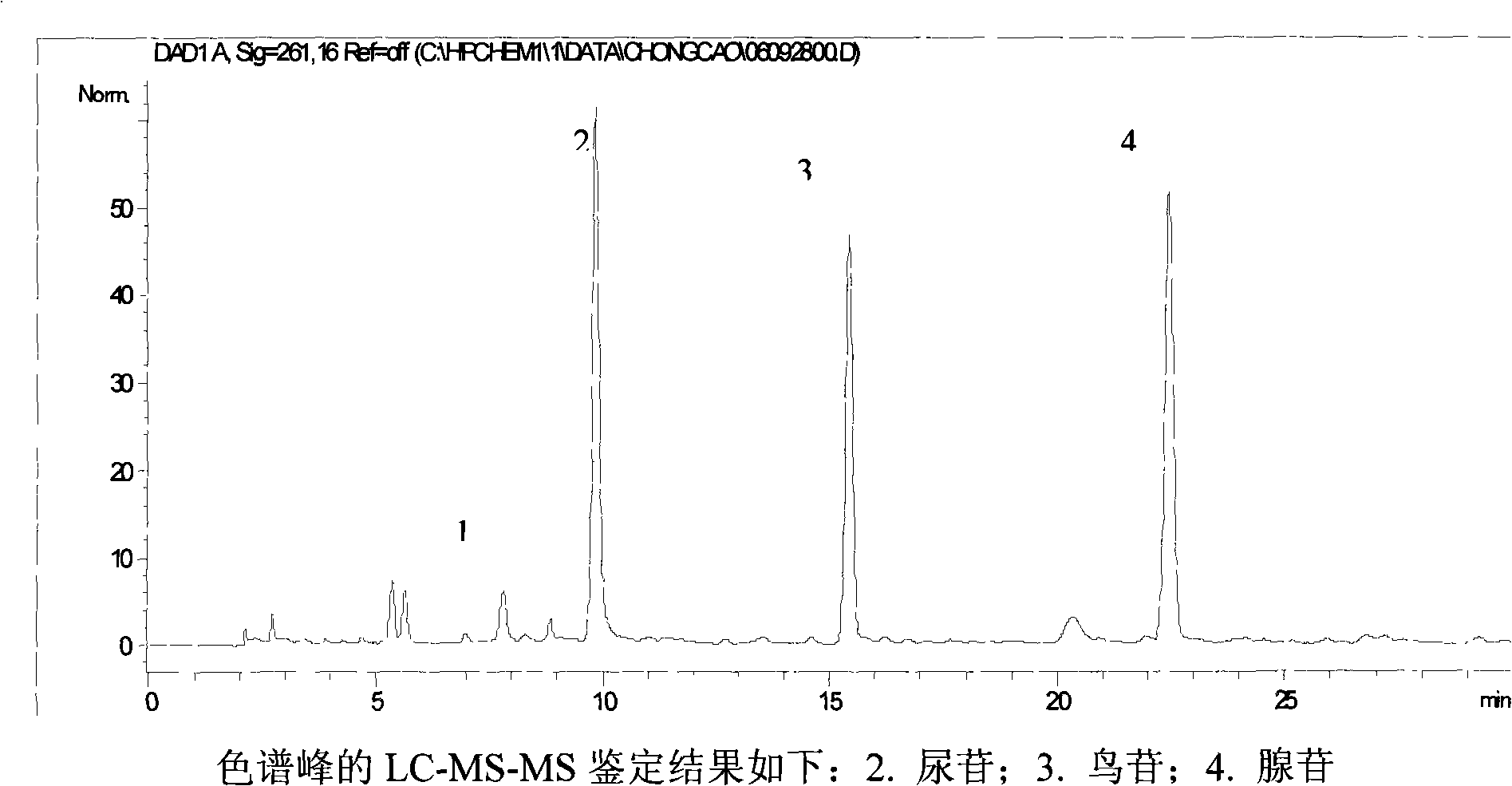
ActiveCN101293002AGuarantee normal implementationHigh sensitivityFungiComponent separationHplc fingerprintRetention time
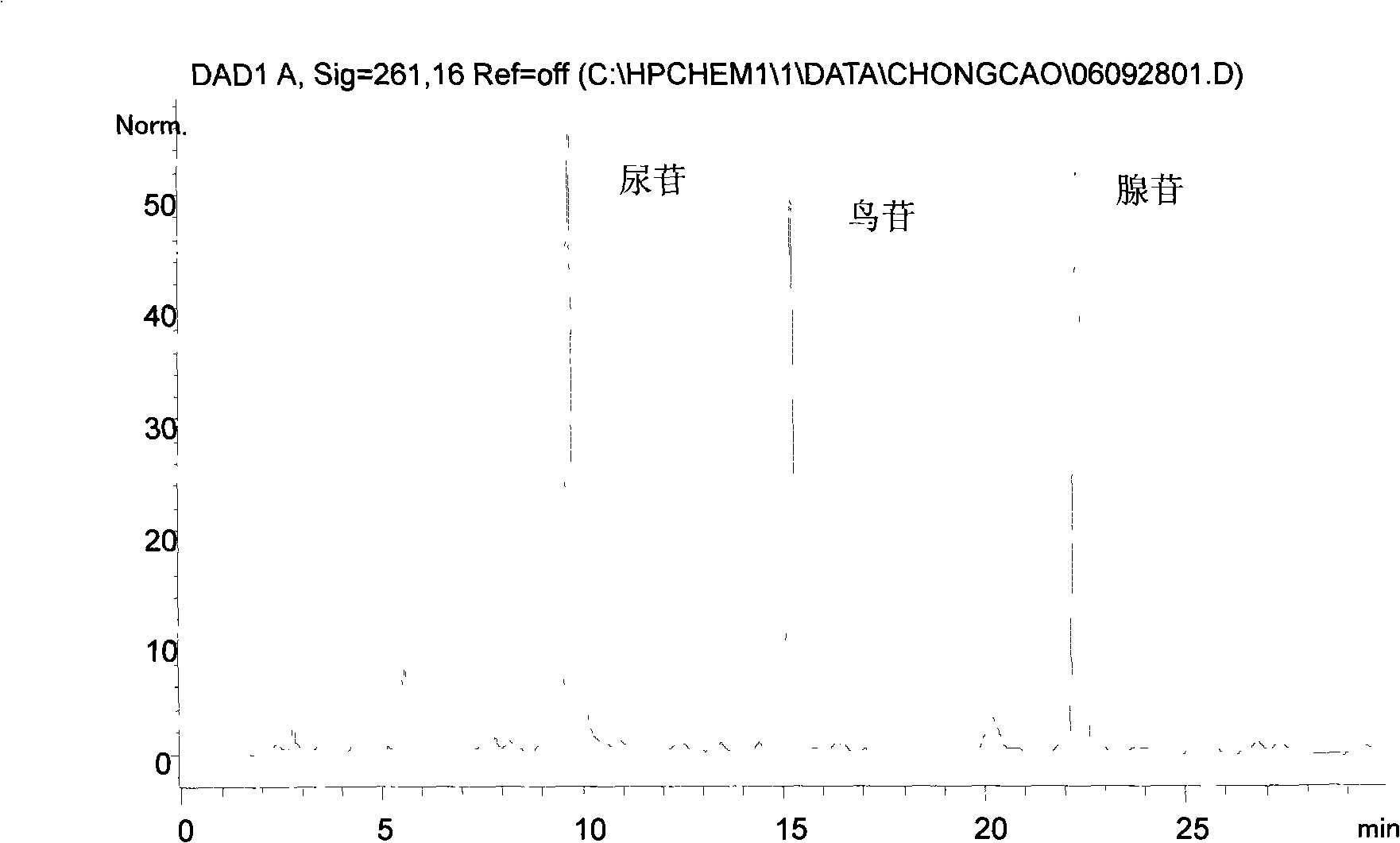
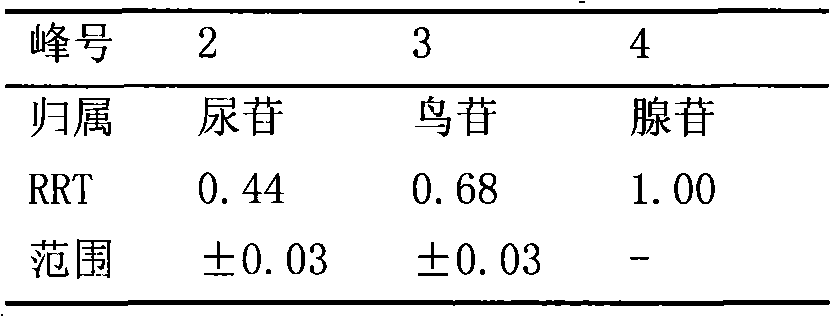
The invention relates to a control method of the fingerprint spectrum quality of cordyceps sinensis powder raw material in botanical drug for strengthening vital qi and removing blood stasis, comprising the steps that: (1) cordyceps sinensis powder is extracted: 0.100g of cordyceps sinensis powder is taken, purified water is added, the ultrasonic extraction, the filtration and the sample injection are carried out; (2) the gradient elution with mobile phase is carried out: octadecyl silane bonded silica gel is taken as a filler, water and acetonitrile are taken as mobile phase to carry out the gradient elution for 0 to 30min and 0 to 7 percent B; (3) a standard fingerprint spectrum is established: the HPLC standard fingerprint spectrum of the cordyceps sinensis powder is determined, and 3 characteristic peaks are selected; (4) the quality control of the fingerprint spectrum is carried out: the relative retention time of No.2 peak uridine, No.3 peak guanosine and No.4 peak adenosine are 0.44 plus or minus 0.03, 0.68 plus or minus 0.03 and 1.00 respectively; the HPLC fingerprint spectrum of the sample is compared with the contrast fingerprint spectrum. The similarity calculated by the 5 common peaks is not less than 0.9, (5) the preparation of the cordyceps sinensis powder raw material is carried out; the control method has good repetitivity and can fully reflect the basic characteristics of nucleoside ingredients of the cordyceps sinensis powder.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV
Construction method of HPLC fingerprint and DNA fingerprint of Saussurea Involucrata
InactiveCN101418348AQuality improvementTotal authenticityComponent separationMicrobiological testing/measurementHplc fingerprintHomoplantaginin
The invention relates to an HPLC fingerprint spectrum of Saussurea involucrate and a method for establishing a DNA fingerprint spectrum of the Saussurea involucrate. The invention is characterized in that the HPLC fingerprint spectrum comprises fingerprint spectrums of two extraction positions with different polarities of ethyl acetate and n-butyl alcohol, and the fingerprint spectrum of the extraction position of the ethyl acetate has 20 common peaks, wherein 7 peaks refer to scopoletin, rutoside, isoquercitrin, xylostein, quercetrin, homoplantaginin and hispidulin respectively; the fingerprint spectrum of the extraction position of the n-butyl alcohol has 11 common peaks, wherein 3 peaks refer to bergapten, scopoletin and rutoside; and the DNA fingerprint spectrum consists of an RAPD molecular fingerprint spectrum and an ISSR molecular fingerprint spectrum. The quality and the reality and falsity of the Saussurea involucrate can be universally and effectively detected in the aspects of chemistry and biology by combination of HPLC fingerprint and DNA fingerprint.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Identification method of propolis medicinal materials and preliminary classification method of propolis medicinal material growing district
ActiveCN101327224AFully reflectFully reflectedAnthropod material medical ingredientsComponent separationMedicinal herbsHplc fingerprint
The invention relates to a propolis (as a medicinal material) distinguishing method and a preliminary classification method of propolis production areas. First test sample solution is prepared, then control solution is prepared. The measurement method is as follows: 10mul of the reference solution and 10mul of the test sample solution are respectively and accurately extracted and then injected into a high performance liquid chromatograph; the test is performed according to the high performance liquid chromatograph; with the control solution as the reference, the chromatogram is measured and recorded; then a propolis HPLC finger-print map is established. The invention has the advantages of convenient method, stability, high accuracy, good reproduction and easiness in mastering, and also has the advantages that the trueness of the propolis can be mastered from the whole characteristic appearance of the chromatogram, and the propolis distinguishing method can be used as one of the indexes for controlling the propolis quality and distinguishing the trueness of the propolis. Meanwhile the propolis from different production areas also undergoes preliminary classification.
Owner:广州市宝生园股份有限公司 +1
HPLC (High performance liquid chromatography) detection method for distinguishing different cultivars of uncaria
ActiveCN103869010AEasy to identifyEfficient separationComponent separationHplc fingerprintMedicinal herbs
The invention belongs to the field of analysis of traditional Chinese medicinal materials, and particularly discloses an HPLC (High performance liquid chromatography) method for distinguishing different cultivars of uncaria. All chromatographic peaks are separated effectively when the uncaria traditional Chinese medicinal materials are subjected to HPLC detection by optimizing each experimental parameter; the phenomena of mutual interference of the chromatographic peaks, trailing and the like are reduced; mutual interference of the chromatographic peaks of different ingredients in different cultivars of uncaria in HPLC detection is basically avoided, so as to provide basis for authenticating different cultivars of uncaria and constructing a uncaria HPLC fingerprint spectrum.
Owner:GUANGDONG PHARMA UNIV
Preparation methods of medicine combination and HPLC fingerprint establishment method thereof
ActiveCN110455965AEasy to takeGuaranteed active substanceComponent separationHplc fingerprintChinese cinnamon
The application relates to the field of traditional Chinese medicine, and particularly to preparation methods of a medicine combination and an HPLC fingerprint establishment method of thereof. 15-25 parts of astragalus, 8-12 parts of ginseng, 3-8 parts of glycyrrhiza, 1-3 parts of cinnamon and 1-3 parts of ginger are mixed, then extraction is carried out 2-4 times, and then extracts are combined,and are concentrated into an extract with relative density of 1.05-1.20g / cm<3> of at 60-80 DEG C. The methods can ensure that the finally obtained medicine combination extract contains valid substances of all raw materials of the scutellaria, the ginseng, the glycyrrhiza, the cinnamon and the ginger, ensure full utilization of all the valid substances, and thus improve quality of the medicine combination. The HPLC fingerprint establishment method can be used to judge the valid substances in the prepared medicine combination, and thus identify the quality of the medicine combination.
Owner:劲牌持正堂药业有限公司
Establishment method of curcuma aromatica medicine fingerprint map and the fingerprint map thereof
The invention discloses an establishment method of a curcuma aromatica medicine fingerprint map and the fingerprint map thereof, and particularly, provides establishment of a HPLC fingerprint map for controlling the quality of curcuma aromatica medicine. The method includes the steps of: 1) preparation of a reference substance solution: preparing the reference substance solutions of curdione, curcumadiol, procurcumenol; 2) preparation of a sample solution: extracting the curcuma aromatica medicine and filtering an extract liquid; 3) HPLC detection to obtain the fingerprint map: performing HPLC detection with octadecylsilane chemically bonded silica as a filler and an acetonitrile-tetrahydrofuran-0.1% phosphoric acid water system as a mobile phase in a chromatographic column in a gradient elution manner, wherein column temperature is 30 DEG C, ultraviolet detection wavelength is 230-250 nm and time is 60-65 min; and 4) similarity evaluation: introducing the obtained fingerprint map into "fingerprint similarity evaluation system of traditional Chinese medicine chromatography" (2004A version) similarity software for performing similarity evaluation. The method is simple, quick and accurate, has good repeatability, and provides powerful guarantee for comprehensive and effective control on quality of the curcuma aromatica medicine.
Owner:GUILIN EIGHT PLUS ONE PHARMA CO LTD
Establishment of HPLC (High Performance Liquid Chromatography) fingerprint spectrum of rabdosia lophanthide medicinal materials and fingerprint spectrum of of rabdosia lophanthide medicinal materials
ActiveCN103776926AMonitor qualityRealize base source identificationComponent separationHplc fingerprintPhosphoric acid
The invention discloses establishment of an HPLC (High Performance Liquid Chromatography) fingerprint spectrum of rabdosia lophanthide medicinal materials and a fingerprint spectrum of the rabdosia lophanthide medicinal materials, relates to a quality control method of traditional Chinese medicine medicinal materials, and particularly relates to a method for detecting an extracting solution of the rabdosia lophanthide medicinal materials by establishing the HPLC fingerprint spectrum. The method comprises the following steps: preparing a comparison product solution; selecting an extracting solvent and an extracting method; preparing a sample solution; inspecting chromatographic conditions, namely carrying out gradient elution by taking octadecyl bonded silica gel as filler of a chromatographic column, wherein a gradient elution solution is composed of acetonitrile-phosphoric acid with a flowing phase being 0.1%-0.3%, the column temperature is 25-40 DEG C, the ultraviolet detection wavelength is 230-270nm and the time is 100-120min; determining the HPLC fingerprint spectrums of the different types of rabdosia lophanthide medicinal materials; and marking a characteristic peak and selecting a reference peak. By adopting the method disclosed by the invention, the dispute of using rabdosia lophanthide as a medicine base source is solved. Methods including common peak comparison, clustering analysis, characteristic peak analysis and the like of the fingerprint spectrum are comprehensively utilized and are used for identifying the three types of HPLC fingerprint spectrums, so as to realize the base source identification of the rabdosia lophanthide medicinal materials, divide production area classification and provide evidences on quality control and accurate clinical utilization of the rabdosia lophanthide medicinal materials.
Owner:DONGGUAN MATHEMATICAL ENG ACAD OF CHINESE MEDICINE GUANGZHOU UNIV OF CHINESE MEDICINE
Method for establishing infantile stagnation-removing and cough-relieving oral solution-HPLC (High Performance Liquid Chromatography) fingerprint spectrum and infantile stagnation-removing and cough-relieving oral solution-HPLC fingerprint spectrum
ActiveCN103954719AAccurate massQuality is evaluated comprehensively and accuratelyComponent separationHplc fingerprintRetention time
The invention discloses a method for establishing an infantile stagnation-removing and cough-relieving oral solution-HPLC (High Performance Liquid Chromatography) fingerprint spectrum. The method comprises the following steps: preparing a tested product solution; determining an HPLC chromatographic condition; making an HPLC standard fingerprint spectrum. The invention also discloses the standard infantile stagnation-removing and cough-relieving oral solution-HPLC fingerprint spectrum obtained by adopting the method. The standard infantile stagnation-removing and cough-relieving oral solution-HPLC fingerprint spectrum has 19 common peaks, the relative retention times tR of the 19 common peaks are sequentially as follows: 0.066, 0.077, 0.088, 0.124, 0.157, 0.166, 0.192, 0.220, 0.229, 0.275, 0.481, 0.837, 0.872, 0.890, 0.962, 1.000, 1.026, 1.061 and 1.203. The method disclosed by the invention has the advantages of easiness and convenience for operation, high stability and good repeatability; the obtained spectrum has the multiple characteristic peaks, can be used for comprehensively evaluating the quality of an infantile stagnation-removing cough-relieving oral solution through the comparison of the common peaks of the standard fingerprint spectrum, is favorable to stabilizing the quality of products and ensuring the safety and the effectiveness of clinical medications.
Owner:LUNAN PHARMA GROUP CORPORATION
Processing method of honey-fried liquorice
ActiveCN101780137AQuality improvementTo make up for the disadvantages of subjective observation and control of the degree of processingAntipyreticDigestive systemHplc fingerprintEngineering
The invention discloses a processing method of honey-fried liquorice, which can effectively overcome the weaknesses that the quality is unstable for the frying time is subjectively controlled when the prepared liquorice pieces are made into the honey-fried liquorice pieces, and comprises the following steps that: prepared liquorice pieces are obtained to be arranged inside refined honey with water to be mixed and moistened until the refined honey is adequately absorbed by the liquorice, the liquorice pieces are arranged inside a frying container to be fried for a given time, when the surface reaches a given temperature, the fried liquorice pieces are taken out to be cooled to the room temperature, and after the scraping is sieved, the fried liquorice pieces are sealed. The method is simple, saves time and is easy to operate. The prepared honey-fried liquorice pieces have uniform color and brightness. The HPLC fingerprint of the honey-fried liquorice pieces which are made with the traditional processing method and the processing method of the invention are compared, the peak area of main chromatographic peak is proved to be larger than that of the liquorice piece which is made with the traditional processing method. The benefit of the honey-fried liquorice pieces which are prepared with the processing method is proved by the pharmacodynatics to be better than that of the honey-fried liquorice pieces which are prepared with the traditional processing method.
Owner:山东百味堂中药饮片有限公司
Method for detection, identification and content determination of folia eriobotryae or drug containing folia eriobotryae raw material
The invention provides a method for the detection of folia eriobotryae or drugs containing folia eriobotryae raw material. The detection method adopting HPLC (high performance liquid chromatography) fingerprint for detection includes the steps of: (1) preparing a test solution; (2) preparing a reference solution by taking chlorogenic acid, neochlorogenic acid and cryptochlorogenic acid; (3) conducting precise drawing of the reference solution and the test solution into a liquid chromatograph and then performing elution with 0.4% of phosphoric acid-acetonitrile as a mobile phase. The quality detection method of the folia eriobotryae or the drugs containing the folia eriobotryae raw material has the advantages of being capable of performing content determination and feature mapping and saving inspection costs, and being capable of distinguishing between folia eriobotryae (medicinal materials, decoction pieces or formula granules) and honeyed folia eriobotryae (medicine, decoction piecesor formula granules) and conducting quality control of the whole process of folia eriobotryae or honeyed folia eriobotryae production.
Owner:SICHUAN NEO GREEN PHARMA TECH DEV
Fingerprint detection method of cyclomastopathy eliminating pill preparation
ActiveCN103983704AOptimizationEasy to operateComponent separationHplc fingerprintFritillaria thunbergii
The invention discloses an HPLC fingerprint detection method of a cyclomastopathy eliminating pill preparation. The method comprises the following steps: preparing a test substance solution, preparing a reference substance solution, and determining the correlation between all bulk drugs and a finished product; and determining by using a high performance liquid chromatograph, calculating the relative retention time and the relative peak area of the test substance with the retention time and the peak area of a reference peak as 1 to obtain the HPLC fingerprint of the cyclomastopathy eliminating pill preparation. The HPLC fingerprint of athe cyclomastopathy eliminating pill preparation obtained through the method shows the numbers of characteristic peaks of radix bupleuri, Rhizoma Cyperi, Rheum officinale, Ligusticum wallichii, Rhizoma Curcumae, Chinese angelica, white peony root, Fritillaria thunbergii Miq., Vaccaria segetalis and green tangerine peel below 254nm are 4, 4, 17, 7, 5, 7, 11, 4, 6 and 11 respectively. Experiment verification results show that the fingerprint detection method has the advantages of high sensitivity and precision, good stability and repeatability, high similarity, objective, comprehensive and accurate evaluation of the quality of the cyclomastopathy eliminating pill preparation, and importance for guaranteeing of the clinic drug effect.
Owner:雷允上药业集团有限公司
Nuclear magnetic resonance quality control method for ginseng and astragalus strengthening injection
ActiveCN103884731ASimple methodImprove stabilityAnalysis using nuclear magnetic resonanceHplc fingerprintAdditive ingredient
The invention provides a method of controlling the quality of amino acids, organic acids and sugars in a ginseng and astragalus strengthening injection by combining a proton nuclear magnetic resonance fingerprint with the content determination of a plurality of ingredients. A quality control method aiming at the amino acids, the organic acids and sugar compounds in the ginseng and astragalus strengthening injection is built by adopting a qualitative and quantitative nuclear magnetic resonance fingerprint technique, and can be combined with a high-performance liquid chromatography (HPLC) fingerprint in industrial production. Thus, total quantity control on the ginseng and astragalus strengthening injection product is achieved.
Owner:LI MIN PHARM FAB OF LIVZON PHARM GRP +1
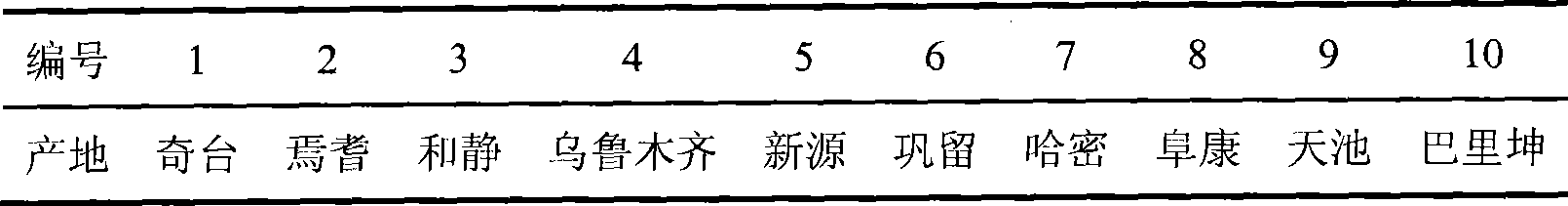
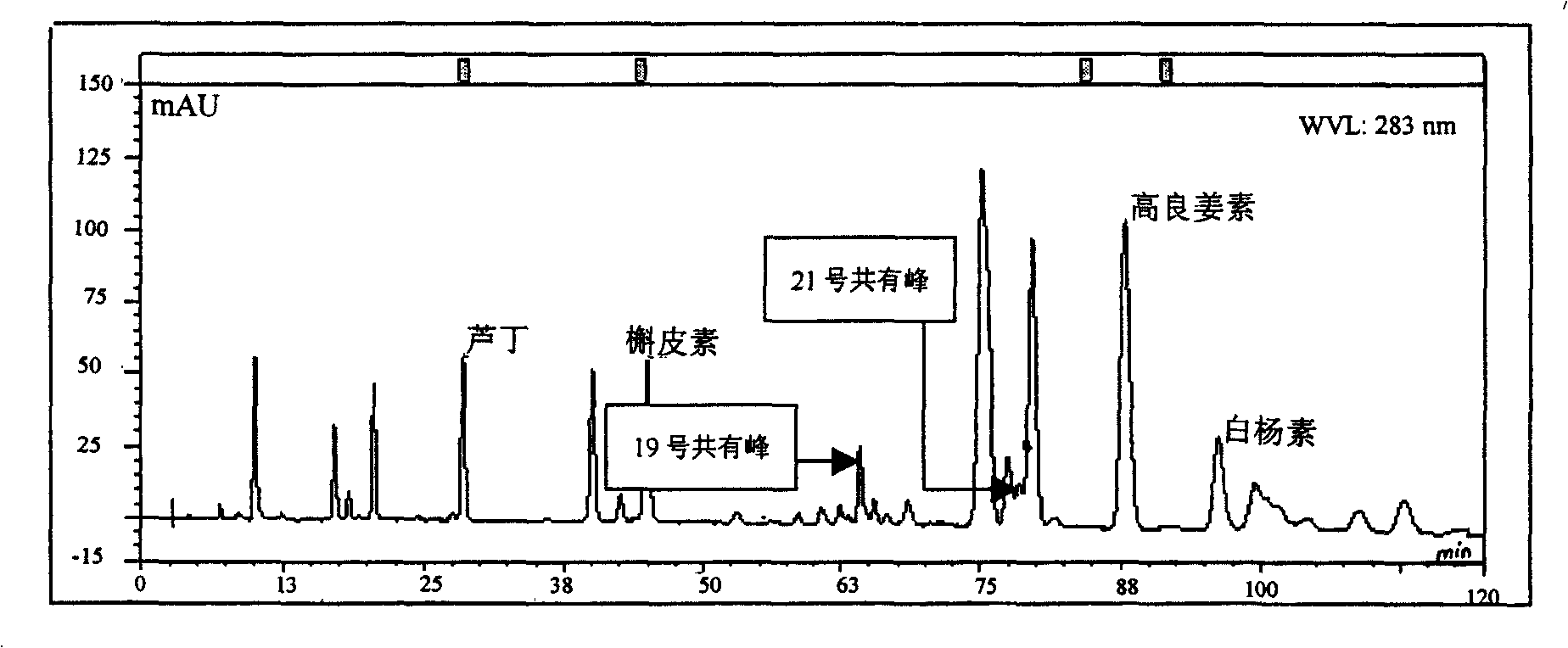
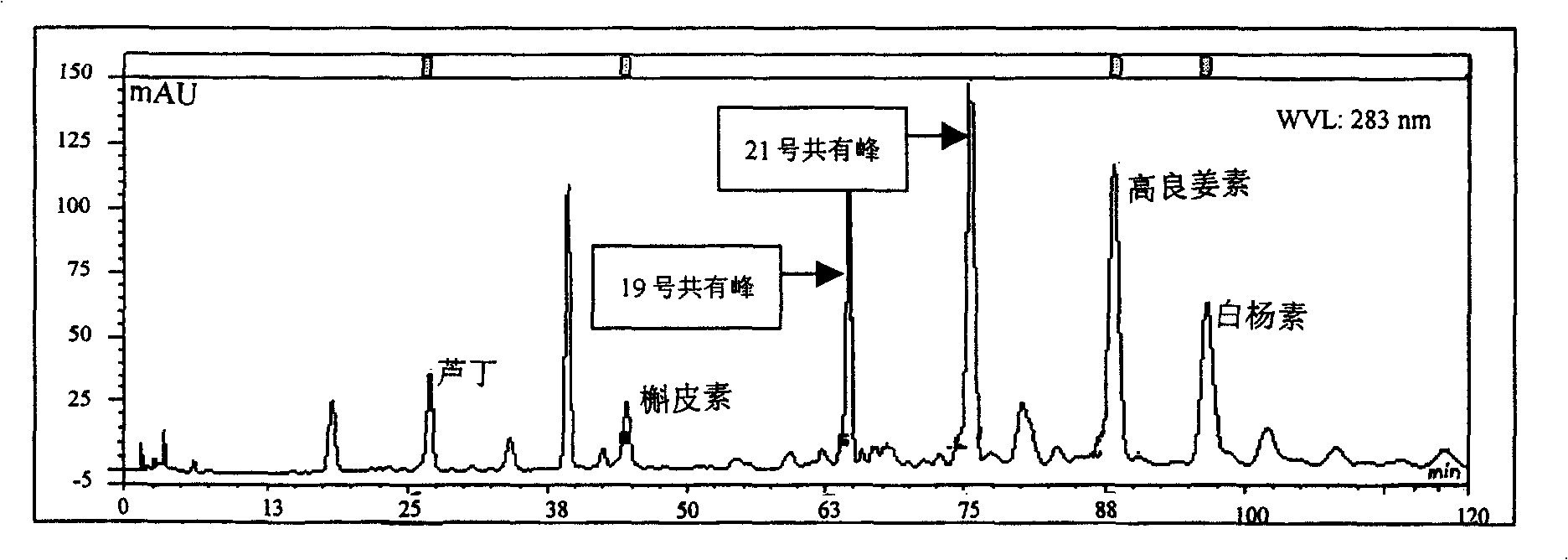
Medlar HPLC fingerprint establishment method and its standard fingerprint
InactiveCN101216466ASignificant advantagesSignificant useComponent separationTesting medicinal preparationsHplc fingerprintComputer science
The invention provides a method for establishing an HPLC fingerprint spectrum of Fructus Lycii and a standard fingerprint spectrum thereof. The method comprises the following steps of: preparing a test solution, preparing a reference solution, selecting chromatographic conditions, and carrying out HPLC determination to obtain the fingerprint spectrum of Fructus Lycii. The standard fingerprint spectrum of Fructus Lycii can be obtained by comparing HPLC fingerprint spectra of 21 batches of Fructus Lycii materials and determining the common fingerprint characteristics. The method has the advantages of simple operation, good reproducibility, a plurality of characteristic peaks, accurate and reliable result, etc. The standard fingerprint spectrum can be used for effectively monitoring the quality of Fructus Lycii.
Owner:BIOCHEM ENG COLLEGE OF BEIJING UNION UNIV
Detection method for atractylodes macrocephala koidz medicinal materials
InactiveCN102680631ATrue reflection of qualityEnsure medication safetyComponent separationHplc fingerprintMedicine
The invention discloses a detection method for atractylodes macrocephala koidz medicinal materials. The method adopts HPLC (high performance liquid chromatography) fingerprint spectrum for detection and includes the operation steps of: (1) preparing sample solution; (2) preparing reference solution; and (3) respectively and precisely absorbing the sample solution and the reference solution to inject into a liquid chromatograph so as to elute by taking an acetonitrile-water system as a flowing phase and detect at wavelengths of 248+ / -5nm. After the atractylodes macrocephala koidz medicinal materials are decocted with water, and then the HPLC fingerprint spectrum is applied for detection, so that quality of the atractylodes macrocephala koidz medicinal materials can be reflected more truly. Moreover, chromatographic conditions such as the flowing phase are selected specifically, so that chromatogram baselines are stable and convenient to integrate, resolution of characteristic peaks is good, and similarity among different medicinal materials is high. The detection method for the atractylodes macrocephala koidz medicinal materials can be effectively used for quality control of atractylodes macrocephala koidz and provides a guarantee of medication security of the atractylodes macrocephala koidz medicinal materials.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Use of cereour bacillus in preparing thrombus treating medicine
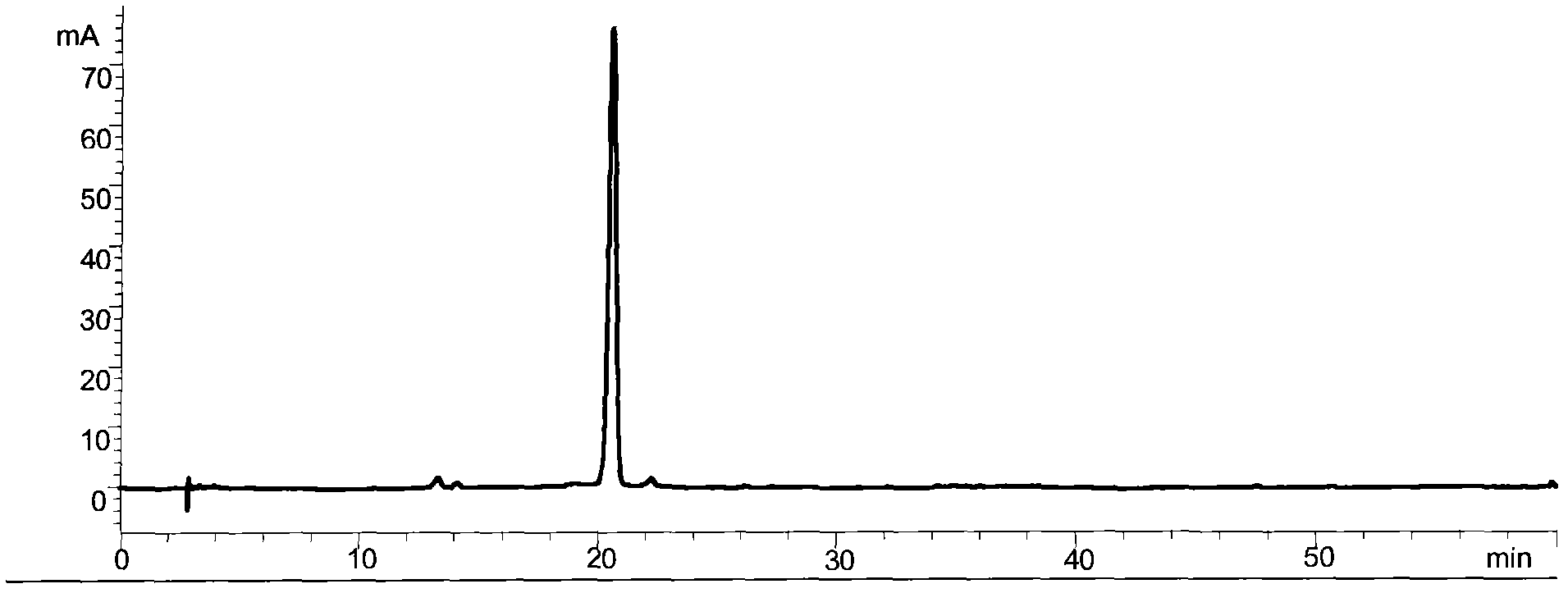
InactiveCN1857722AProlong formation timeObvious antithrombotic effectBacteriaPeptide/protein ingredientsHplc fingerprintDisease
The present invention relates to a kind of streptokinase, and is especially one kind of Bacillus cereus produced fibrinolysin, or ceryl kinase. The ceryl kinase has HPLC fingerprint with peak within 7-9 min in the conditions of chromatographic column Waters Protein -PakTM 60 7.8*300 mm, mobile phase 0.2mol / L NaH2PO4-CH3OH(95:5), flow rate 1ml / min and detecting wavelength 220 nm. The present invention also provides the preparation process of the ceryl kinase, and the use of the Bacillus cereus and the ceryl kinase in preparing thrombus treating medicine. The ceryl kinase of the present invention, the rat carotid artery thrombosis experiment shows, possesses obvious thrombosis resisting effect, so that the present invention provides one new option for treatment of thrombus diseases.
Owner:CHENGDU DIAO JIU HONG PHARMACEUTICAL FACTORY
Method for building quality control chromatography fingerprint maps of traditional Chinese medicine herbal tea and herbal medicine beverage products
The invention discloses a method for building quality control chromatography fingerprint maps of traditional Chinese medicine herbal tea and herbal medicine beverage products. The method comprises the steps of: (1) preprocessing a sample and processing the sample by one method selected from following methods: (1) a freeing, drying-extracting method, (2) a thermal evaporating, drying-extracting method, and (3) a solid-phase extraction method; (2) conducting HPLC (high performance liquid chromatography) fingerprint map analysis. According to the method, the quality control chromatography fingerprint maps of the traditional Chinese medicine herbal tea and herbal medicine beverage products built by the method disclosed by the invention can be used for carrying out macroscopic and fine constituent quality control on the product, and the blank that the Chinese herbal medicine beverage such as the traditional Chinese medicine herbal tea and herbal medicine herbal tea only has requirements on raw material and auxiliary material, sensory indexes and health indexes in quality control, but has no macroscopic quality control indexes of related phytochemical constituents at present is filled.
Owner:HUNAN NORMAL UNIVERSITY
Finger-print spectrum construction method and quality detection method of chrysanthemum cell-disruption decoction pieces
ActiveCN104833749AQuality improvementControl and identify counterfeit goodsComponent separationHplc fingerprintActive component
The invention relates to a finger-print spectrum mutual mode construction method and a quality detection method of chrysanthemum cell-disruption decoction pieces. In the method, with isochlorogenic acid A as a contrast peak, mutual mode standard spectrums and determination indexes are established through HPLC analysis to not less than 10 batches of the samples under following chromatographic conditions: column temperature: 35 DEG C; wavelength: 348 nm; mobile phase: an acetonitrile-0.5% phosphoric acid solution; elution gradient: 0-8 min-24 min-50 min-75 min; and acetonitrile change: 14%-18%-18%-25%-45%. The spectrums of the samples to be detected under the same chromatographic condition are compared with the mutual mode standard spectrums to detect the quality of the samples to be detected. The invention firstly discloses the HPLC finger-print spectrum and the quality detection method aiming to the chrysanthemum cell-disruption decoction pieces. The spectrums comprehensively contain the spectrum information of main active components of the chrysanthemum cell-disruption decoction pieces. The method is strong in specificity, is quick and accurate in detection, and can effectively control the total quality of medicines, cell-disruption powders and cell-disruption decoction pieces.
Owner:ZHONGSHAN ZHONGZHI PHARMA GRP
Method for establishing HPLC fingerprint of Yinzhihuang granule, and standard fingerprint obtained therethrough
The invention discloses a method for establishing the HPLC fingerprint of a Yinzhihuang granule. The method comprises the following steps: preparing a sample solution, determining HPLC chromatographicconditions, and making an HPLC standard fingerprint. The invention also discloses a the HPLC standard fingerprint of the Yinzhihuang granule, obtained through the method. The fingerprint has twenty-nine common peaks, and the relative retention times tR of the common peaks sequentially respectively are 0.079, 0.087, 0.113, 0.341, 0.436, 0.563, 0.648, 0.866, 0.891, 0.905, 0.919, 1.000, 1.089, 1.129, 1.161, 1.197, 1.395, 1.453, 1.496, 1.597, 1.776, 1.808, 1.979, 2.071, 2.136, 2.168, 2.353, 2.396 and 2.440. The method has the advantages of simplicity in operation, high stability, good reproducibility, a large quantity of characteristic peaks in the obtained fingerprint, realization of comprehensive evaluation of the quantity of the Yinzhihuang granule through comparing the common peaks in thestandard fingerprint, facilitation of the stabilization of the product quality, and ensuring the clinical medication safety and effectiveness.
Owner:LUNAN PHARMA GROUP CORPORATION
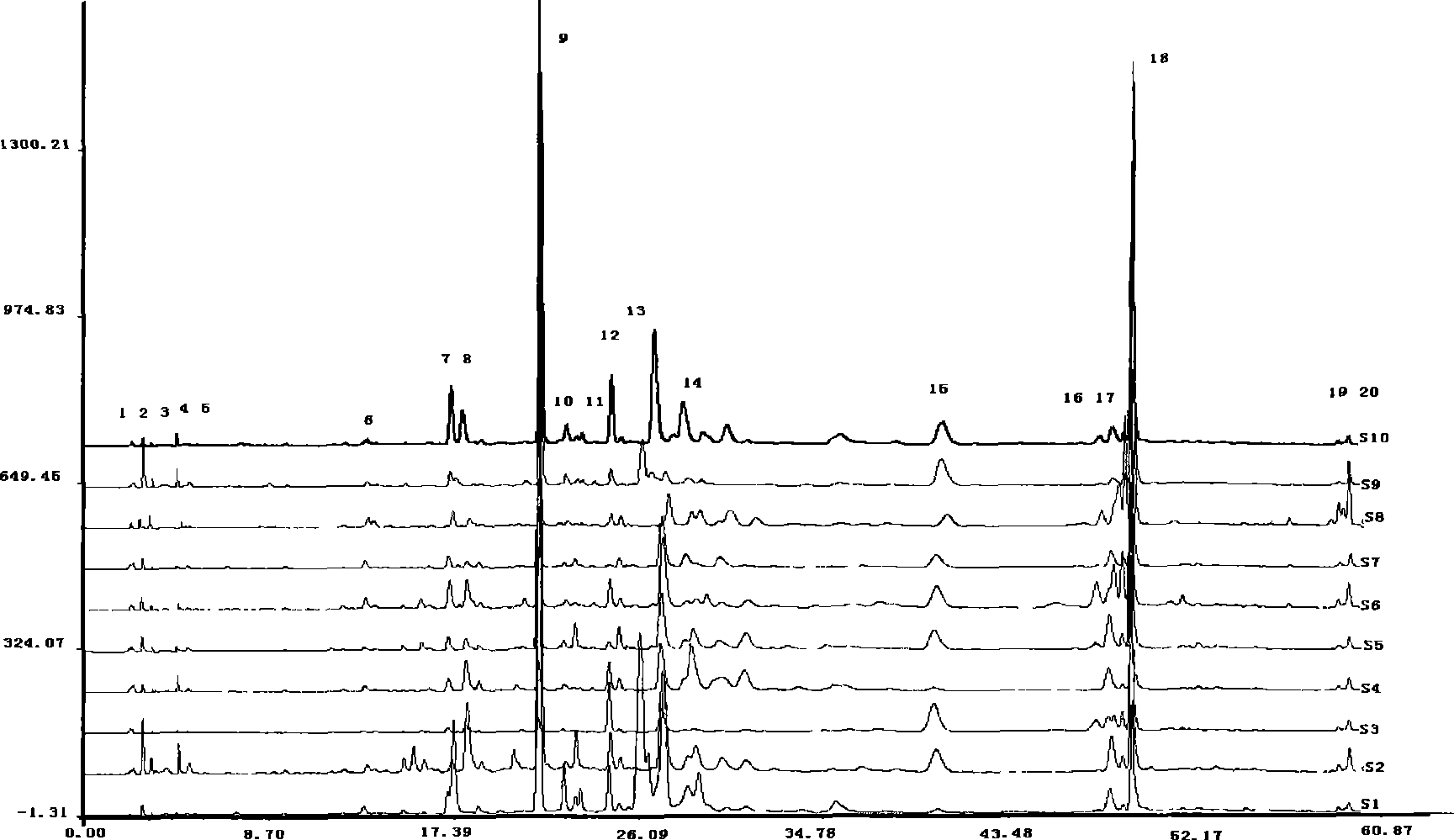
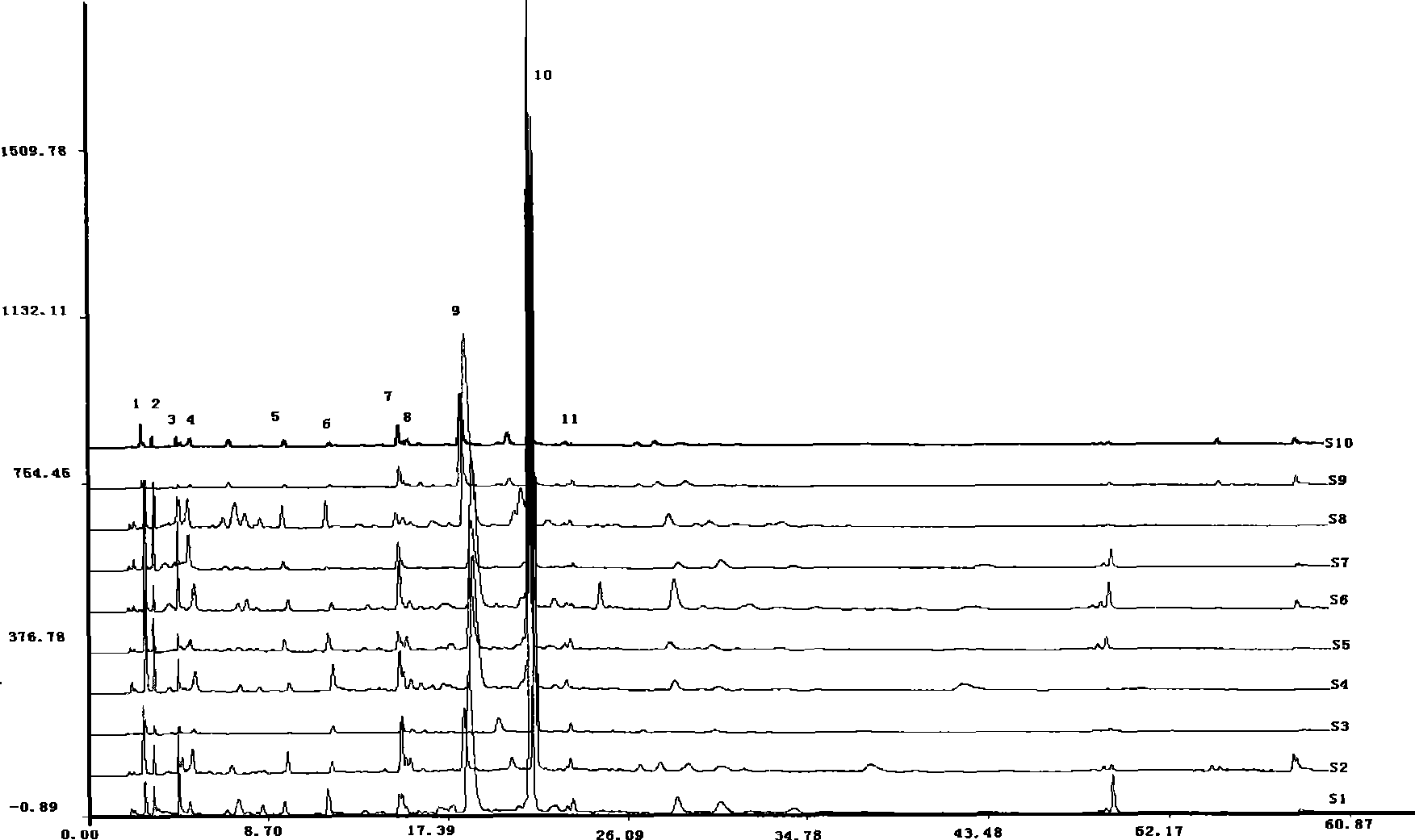
Establishing method for cordyceps fungus fingerprints and standard fingerprints
ActiveCN103484522AAvoid one-sidednessReduce counterfeitingComponent separationMicrobiological testing/measurementHplc fingerprintCordyceps
The invention relates to an establishing method for HPLC fingerprints of cordyceps fungus nucleoside components in different product forms, and standard fingerprints. The employed chromatographic column is Agilent Zorbax Extend C18 (250 mm*4.6 mm, 5 mu m), the column temperature is 25 DEG C, the detection wavelength is 260 nm, the mobile phase is a methanol-water system, and a gradient elution mode is employed for obtaining of the HPLC fingerprints of nucleoside components of cordyceps stromata, mycelia and encarpia. Through comparison of the HPLC fingerprints of the stromata, mycelia and encarpia of the six cordyceps fungus, common fingerprint characteristics of the three-form cordycepses are respectively determined, and the standard fingerprints are obtained, and qualitative determination and quantitive determination are performed on common fingerprint peaks. The method of the invention has the advantages of simplicity, stability, high precision and good repeatability, and helps to accurately reflect quality state of cordyceps fungus from the integrated chromatographic characteristics of different-form cordycepses. The common fingerprint characteristics can be taken as one of the indexes for cordyceps quality control and discrimination.
Owner:OCEAN UNIV OF CHINA
Detection method forqualified pinellia tubers
ActiveCN103969361AImprove accuracyThe detection method is simpleComponent separationHplc fingerprintPinellia
The invention discloses a detection method for qualified pinellia tubers. According to the method, determination is performed through HPLC (high performance liquid chromatography) fingerprints and the method comprises the operation steps as follows: (1) taking a to-be-detected sample, crushing the sample, performing extraction with water or methanol serving as a solvent, and preparing a test solution; (2) injecting the test solution into a high performance liquid chromatograph to be determined. Chromatographic conditions are as follows: the detection wavelength is in a range of 254-265 nm; a chromatographic column takes octadecylsilane as filler; and a mobile phase is eluted by a methanol-water system. The detection method for the qualified pinellia tubers is high in precision, good and accurate in reproducibility, convenient to operate, low in cost and broad in market application prospect.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Tibet gadol medicinal material HPLC finger print construction method and standard finger print thereof
InactiveCN1547009AMonitor qualityQuality improvementComponent separationHplc fingerprintPhosphoric acid
The invention refers to a constructing method for HPLC fingerprint diagram of Rhodiola tibetica (Hook.f.et Thomas) Fu medicinal materials and the standard fingerprint diagram. At first, takes the Rhodiola tibetica (Hook.f.et Thomas) Fu powder, adds in alcohol, carries on microwave extraction, the lead acetate liquid is added into the extraction liquid and generates deposit, it is centrifugated, the acquired supernate is used as testing liquid; then, carries on high efficiency liquid phase color spectrum analysis: absorbs the testing sample liquid, the color spectrum pole is hypersil ODS (5m, 2504.0mm), the flow phase is 0.05% phosphoric acid buffer solution-methyl alcohol, the invention uses ladder eluting method; the testing wavelength: 360nm; acquires the fingerprint diagram of HPLC, confirms the fingerprint character, acquires the standard fingerprint diagram of the medicine. The HPLC fingerprint character can monitor the quality of Rhodiola tibetica (Hook.f.et Thomas) Fu, ensures it is stable, even, and controllable.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com