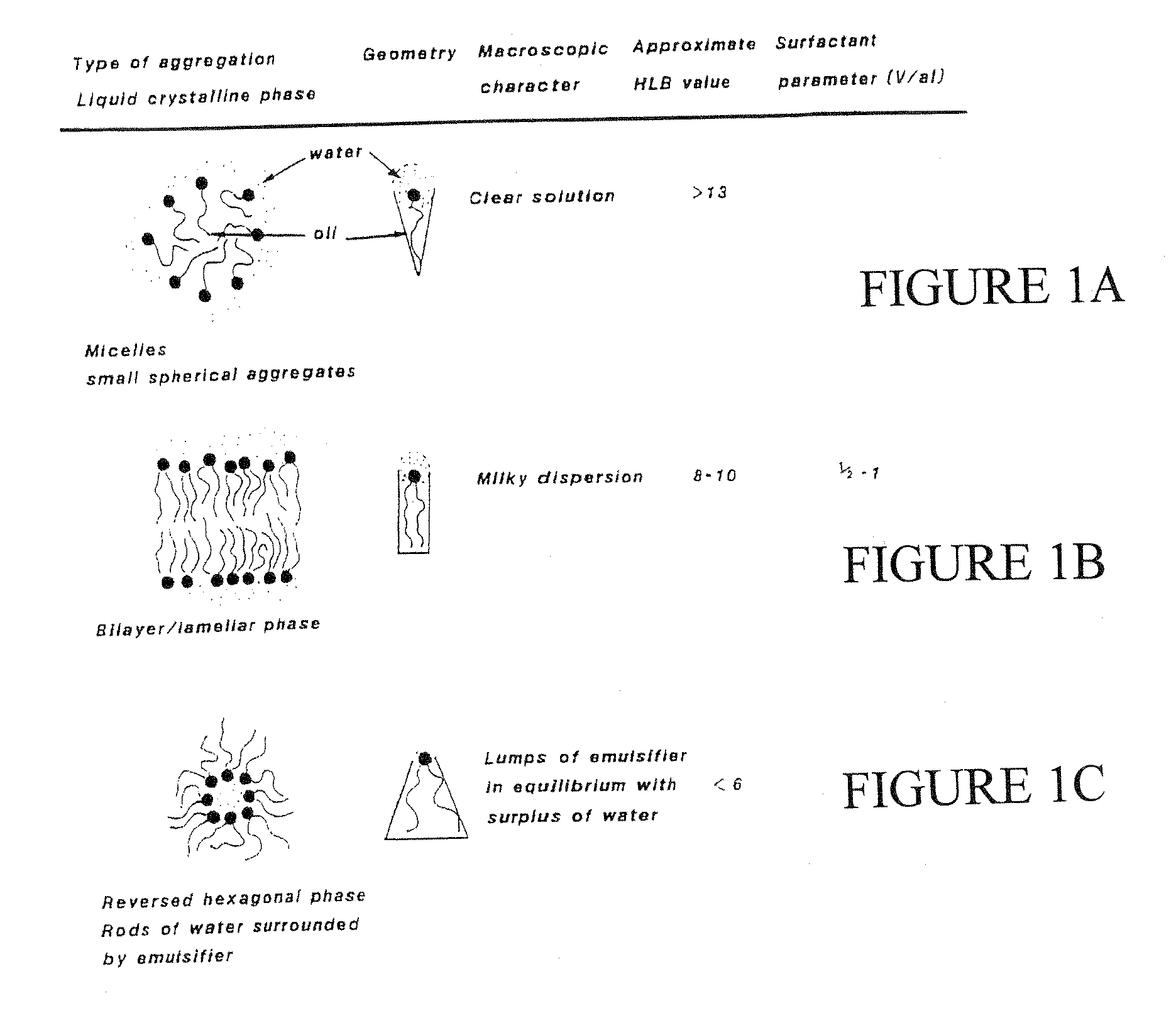
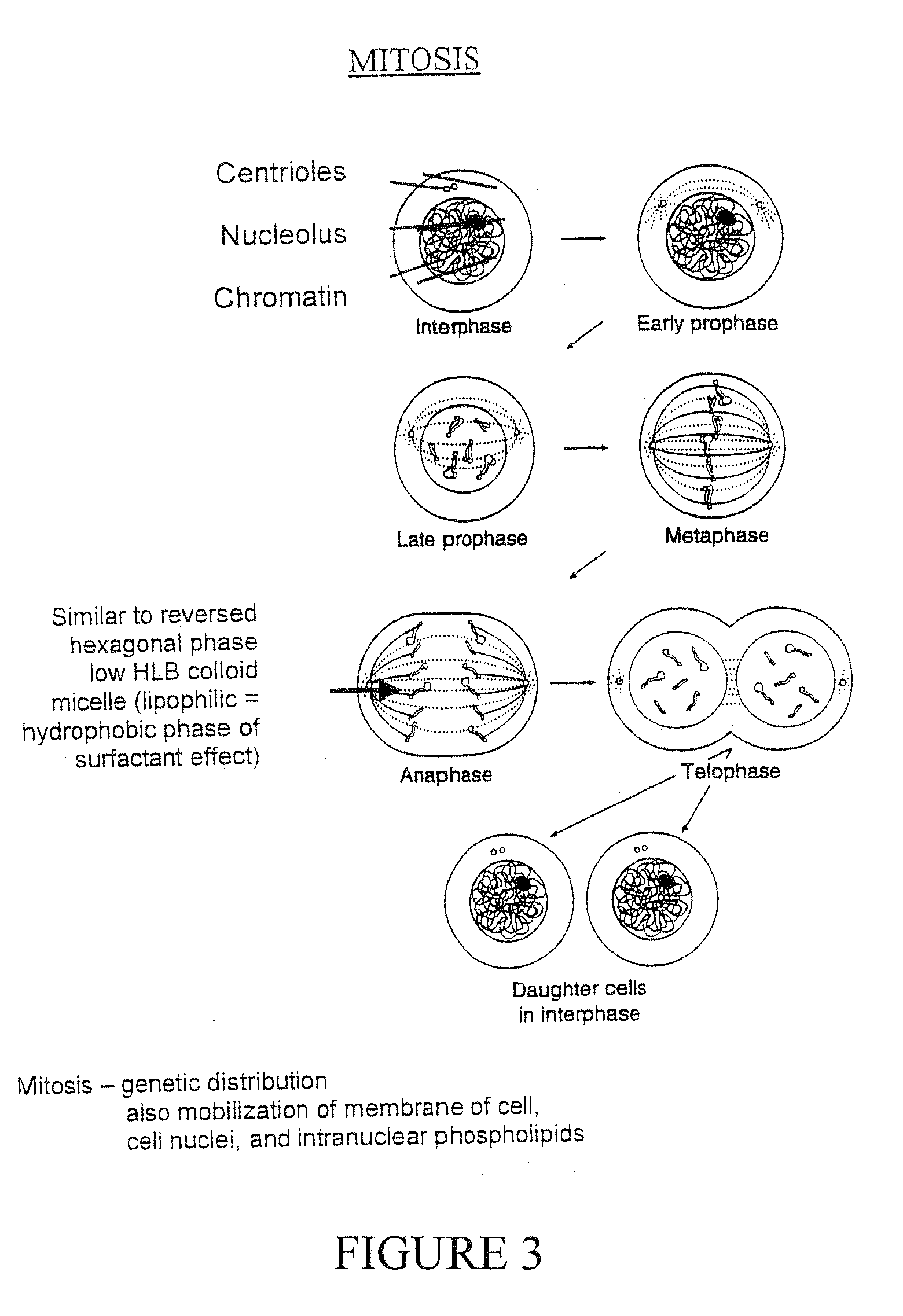
Pluripotent therapeutic compositions and uses thereof
a therapeutic composition and a technology of a composition, applied in the direction of bacteria material medical ingredients, dispersed delivery, peptide/protein ingredients, etc., can solve the problem that no active treatment is offered that can save the patient, and achieve the effect of reducing reducing the dosage of medication, and avoiding the risk of allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Example of Multi-Center Study
[0406]Within three to six weeks of initiation of this treatment using the compositions of the invention approaching 95% of Crohn's disease (CD) and pediatric Crohn's disease (PCD) patients are being studied in double-blind, placebo-controlled multi-center including 35 radiographically tagged inflammatory neutrophile permeability studies as well as the open study are able to discontinue the immune suppression therapy.
[0407]Remarkably, the discontinuance is not associated with relapse for a period of at least six months. More than 95% are maintained in the disease-free state for as long as one year. Growth arrest and puberty suppression are overcome within six weeks in more than 20 patients with a predictable efficacy of over 95% in 150 patients (⅓ of 450 being studied, (PCD cases treated exceed 200 patients). Controlled in-vitro tissue culture studies of biopsied Crohn's tissue evidences significant (approaching more than 95%) reduction in inflammatory me...
example 2
[0411]Congenital biliary atresia (CBA) is a rare fatal (prior to treatment with this invention) disease without liver transplant, with a U.S. incidence of only 300 cases per year (approximately 1 per 1 million of U.S. population) with symptoms occurring at onset of infancy that include: poor appetite, poor food intake, extreme jaundice, (21 mgm percent bilirubin, with biliary obstruction further confirmed by an abnormal dye excretion study. stools were gray, acholic, lacking normal stool bilirubin color), lassitude, weight loss, failure to thrive, abnormal liver function tests, abnormal liver ultrasound, and abnormal biopsy.
[0412]Therapy with the compositions of the invention in a CBA patient using components No. 1 and No. 2 re-established organ function, stimulated tissue healing and tissue protein synthesis concurrent with significant anti-inflammatory activity, clearance of all abnormal liver function, and averted the required for a liver transplant.
[0413]Components No. 1 and No....
example 3
Crohn's Disease, (CD) Case Report, Part (a)
[0415]A 71 year old female patient with more than 3 decades of Crohn's disease whose symptoms included diarrhea, constipation, severe bouts of abdominal pain and fever, generalized aching, extreme fatigue, nausea, food and dairy intolerance, increased sedimentation rate, recently had a flare up of the Crohn's disease. Response from 4 mgm of corticosteroid, once daily was unsatisfactory. Corticosteroid dosing was then increased to 4 times daily for acute flare ups.
[0416]The patient received a composition comprising 5 to 25 grams of L-amino acids and glycine, lecithin (phospholipid-PC), and extracellular matrix components comprising collagen, proteoglycan aggregate complex of cartilage and chondrotin sulfate (shark cartilage 740 mg. per capsule, 4 capsules twice daily). Symptoms of severe abdominal pain and diarrhea, and the flare-up were cleared within 24 hours. The improvement continued over the next few weeks, and the patient responded to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com