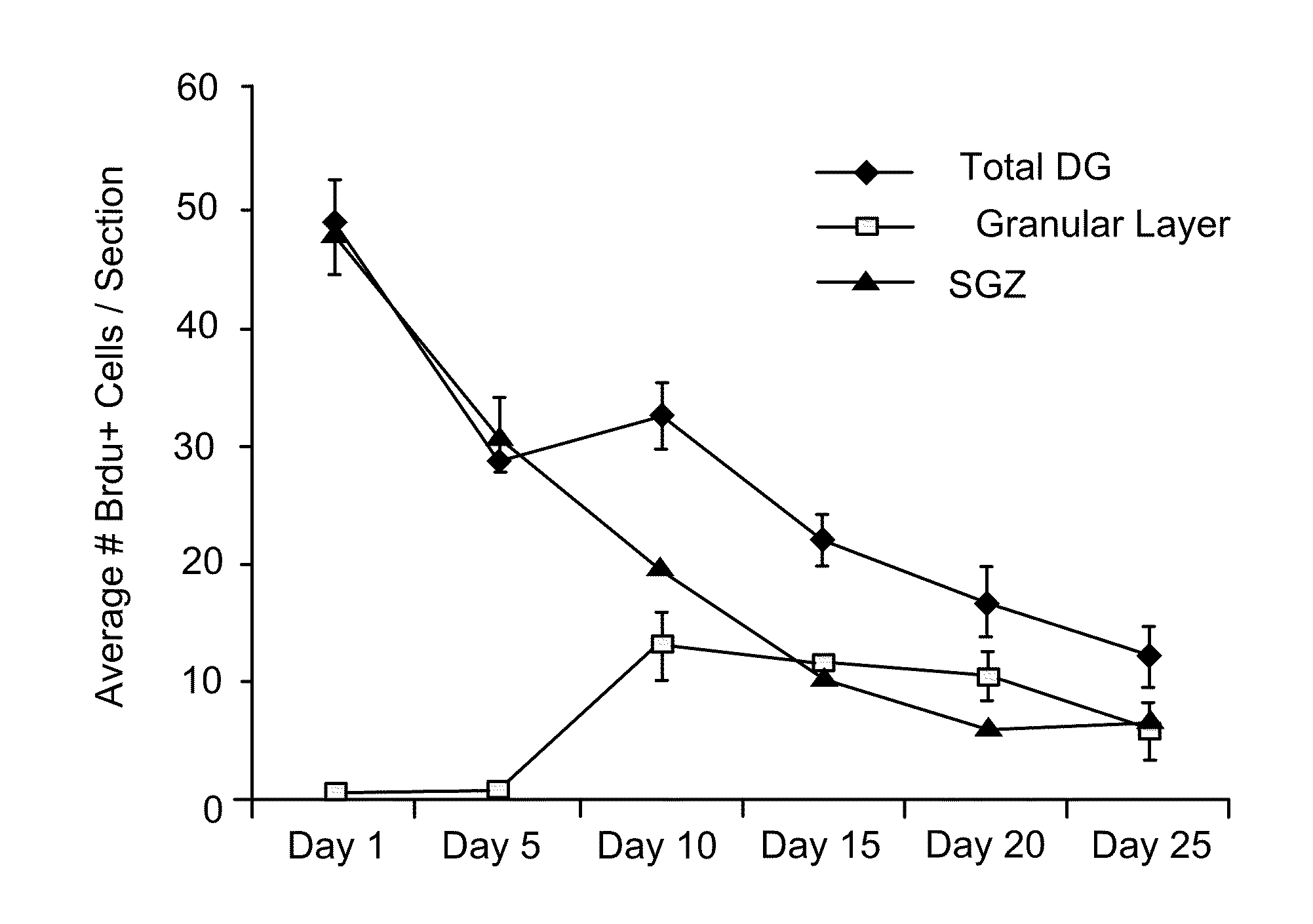
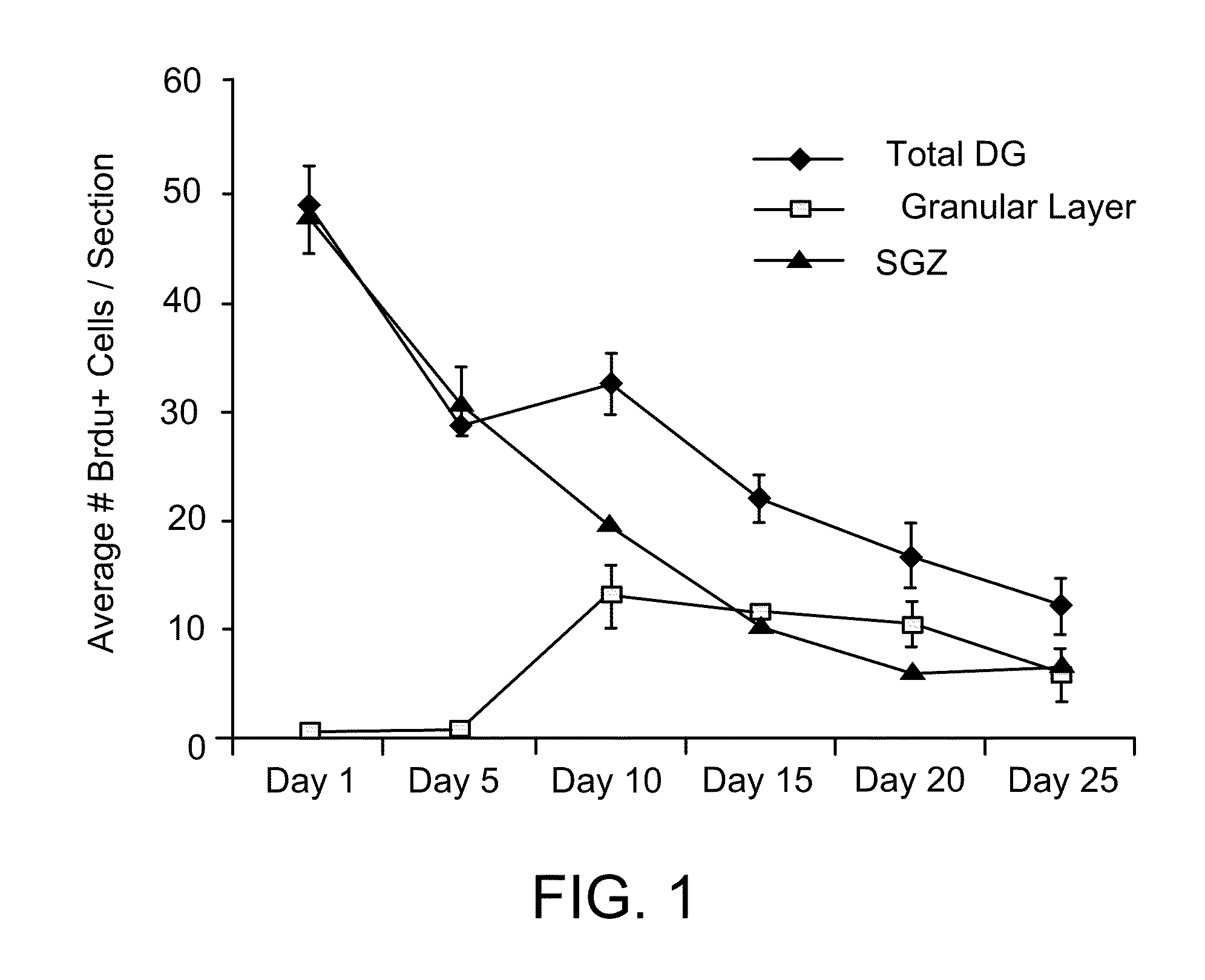
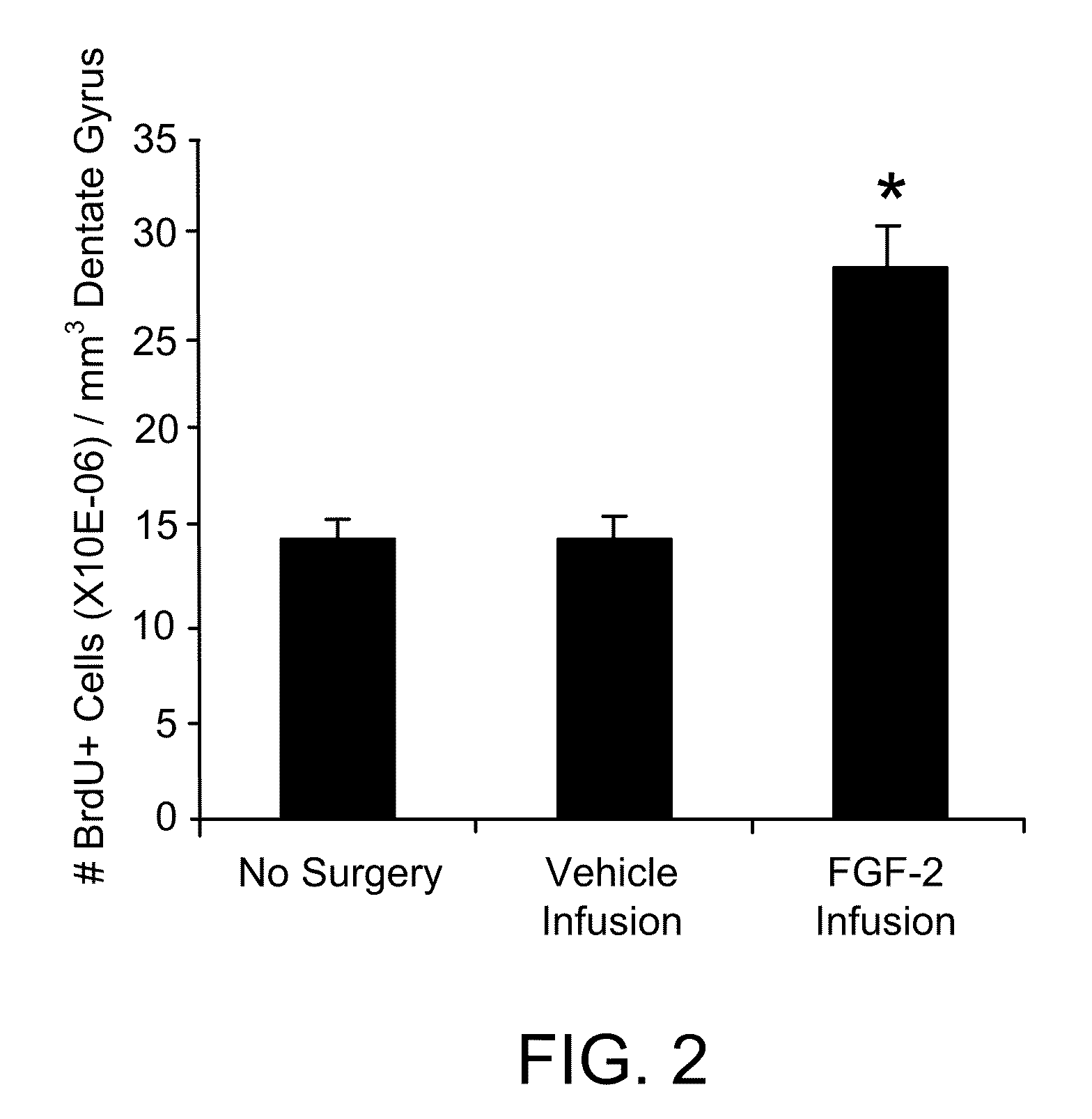
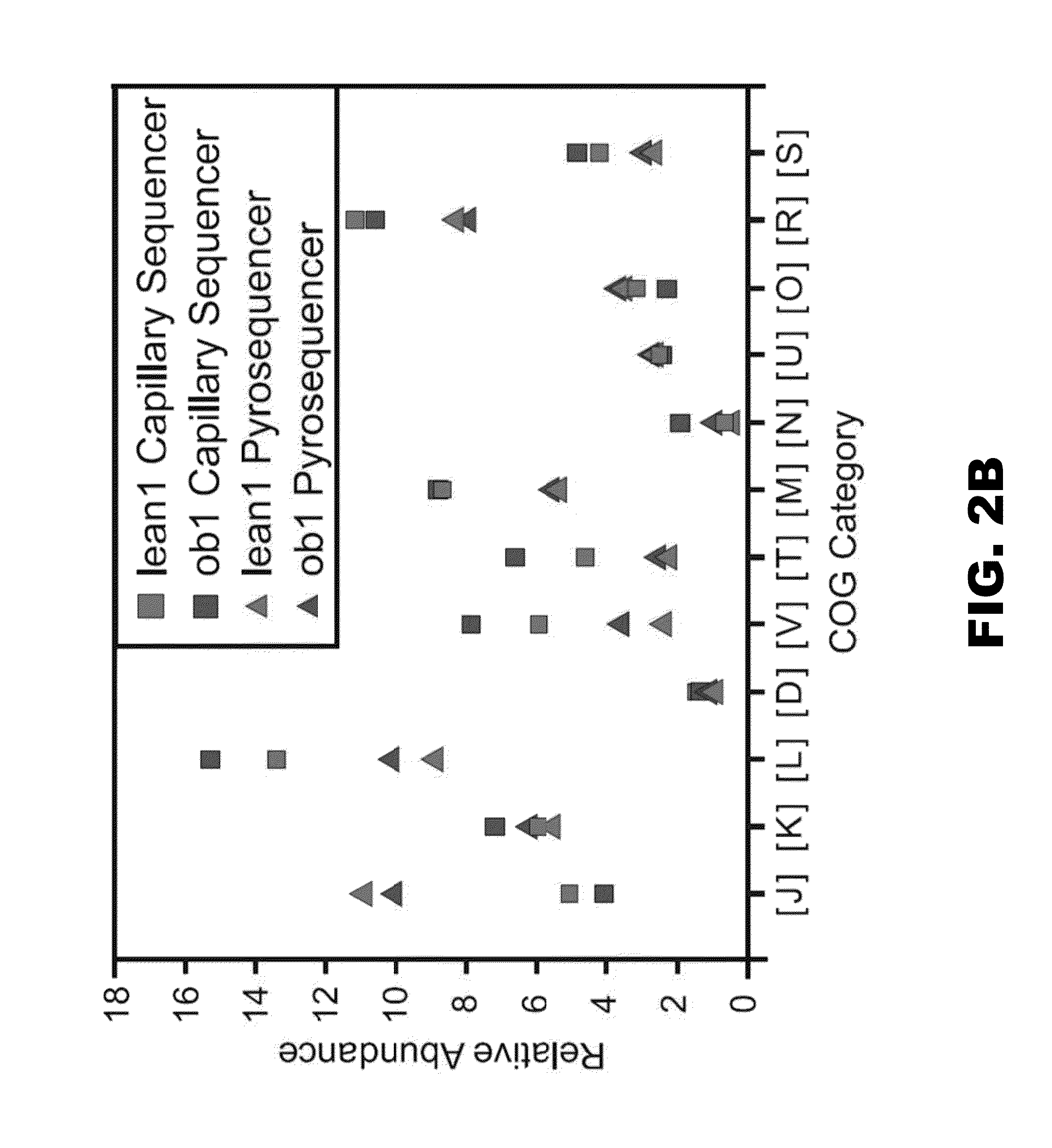
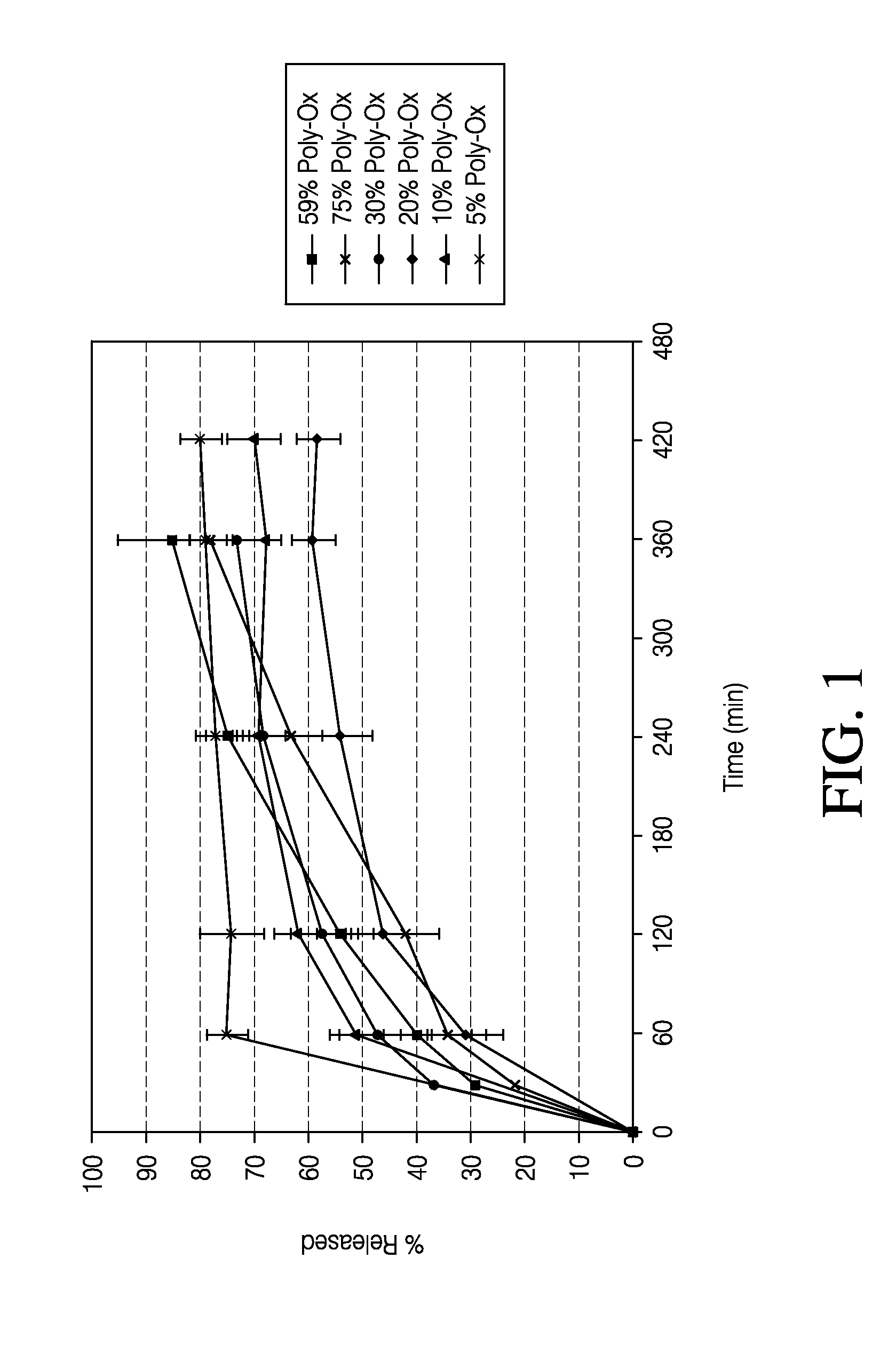
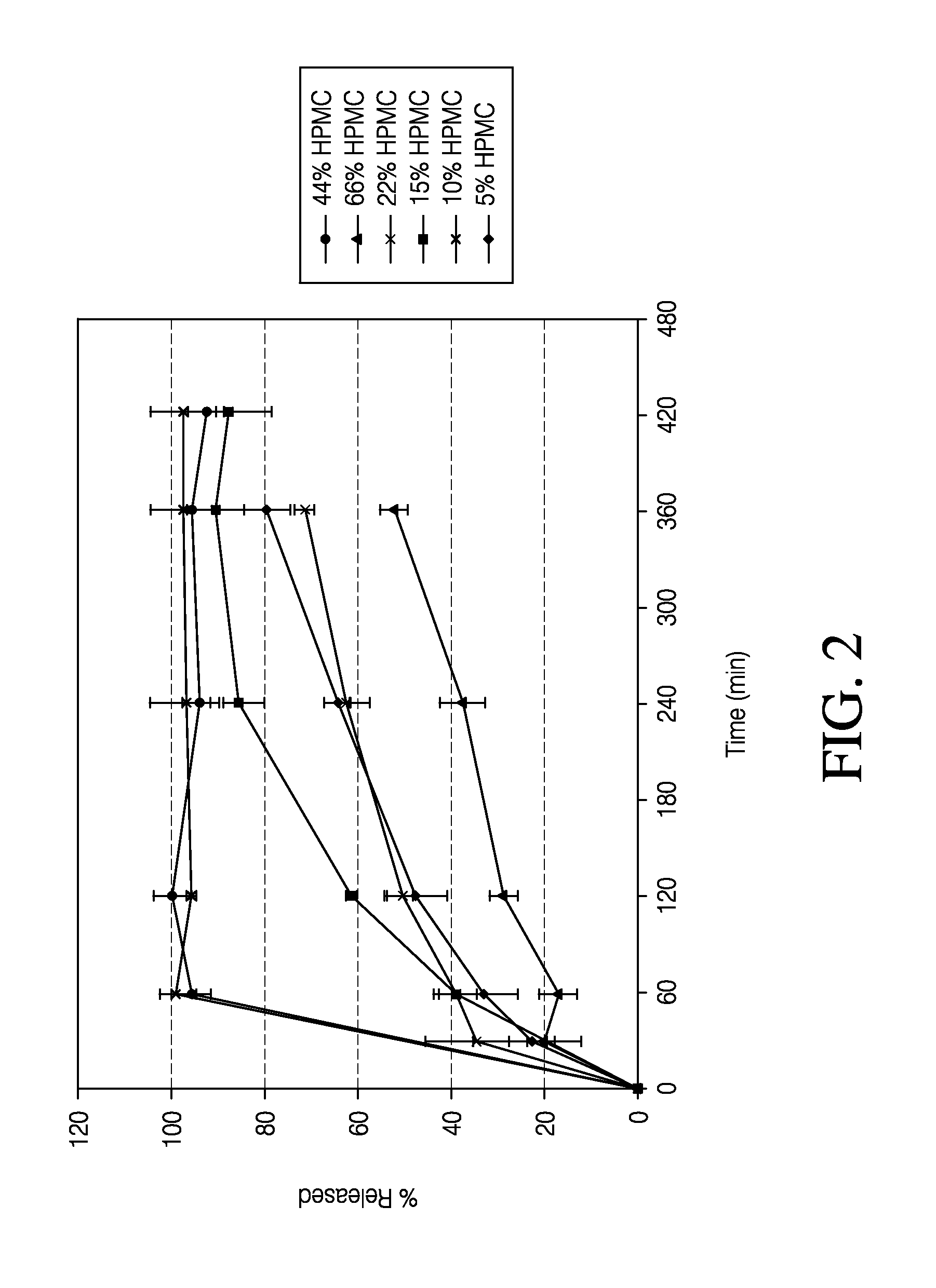
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2016 results about "Weight loss" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
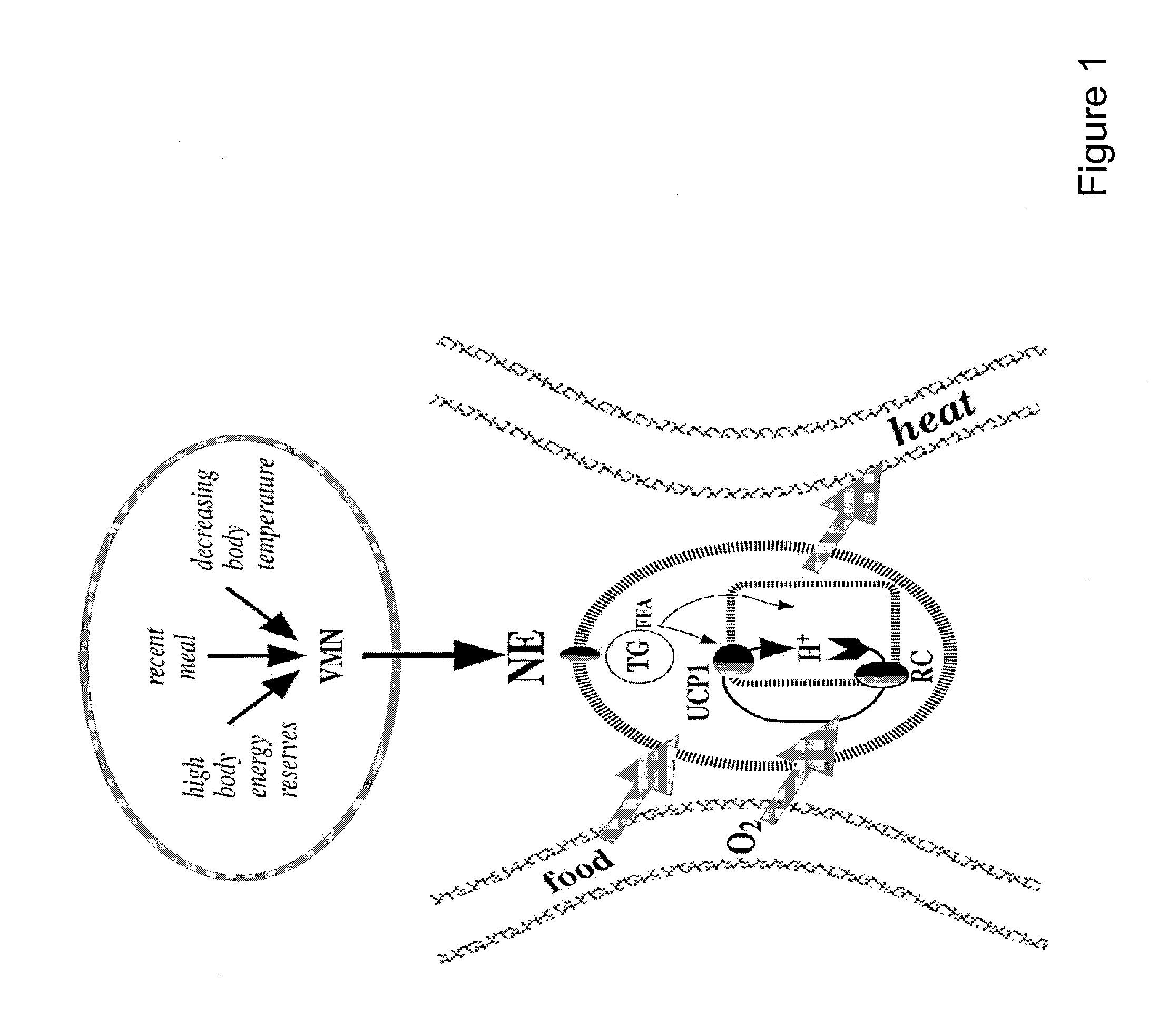
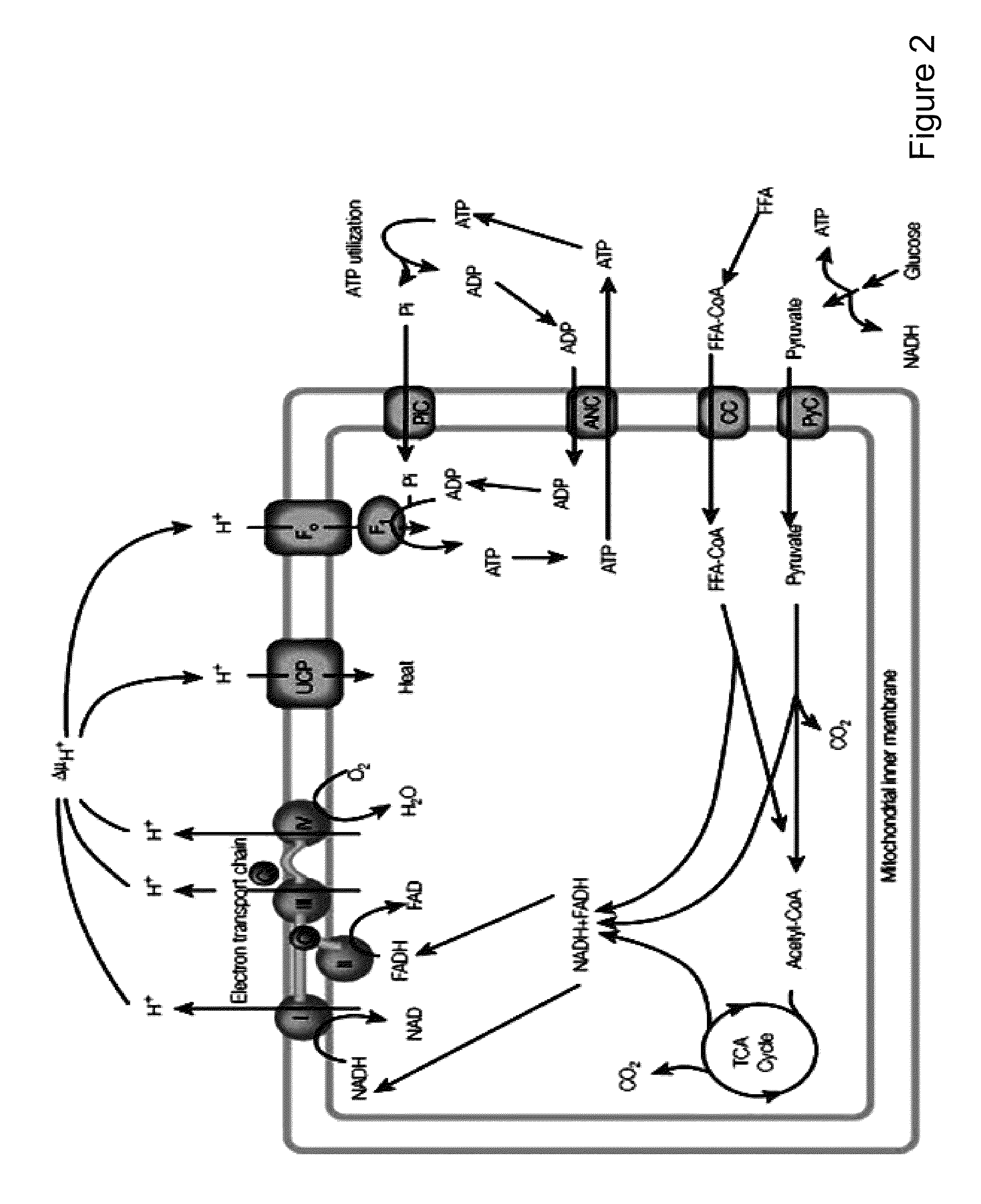
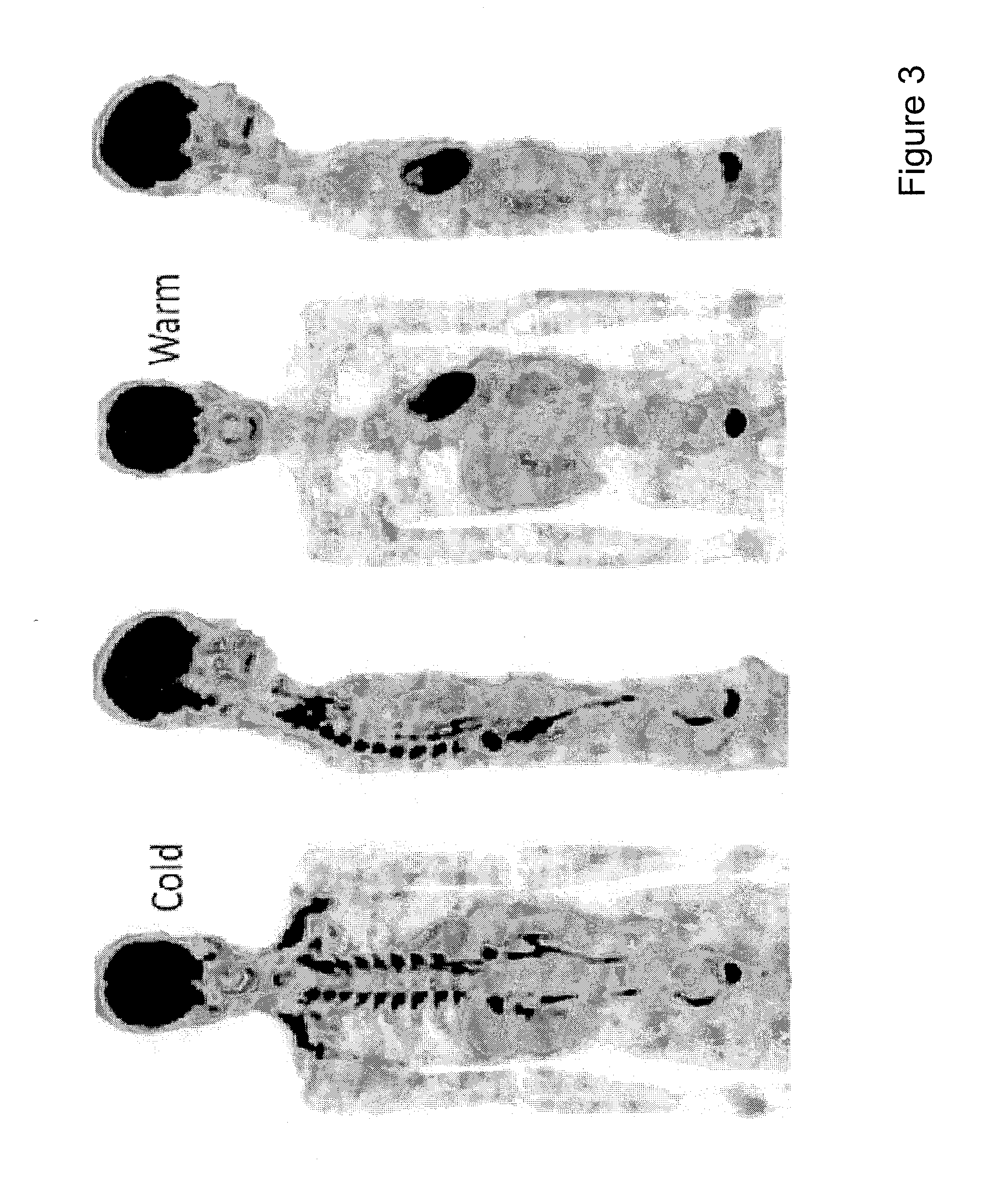
Decrease in body weight without trying.
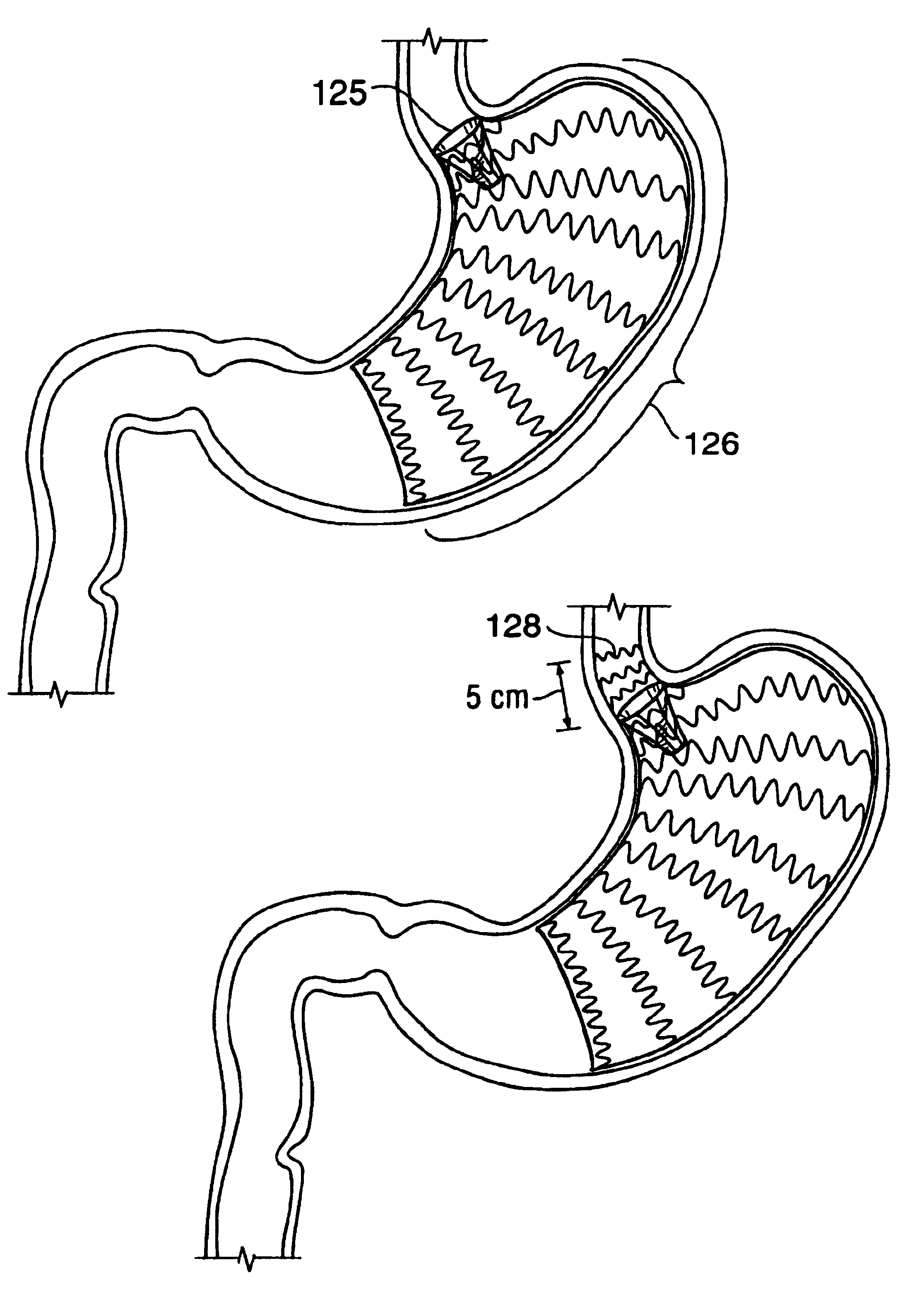
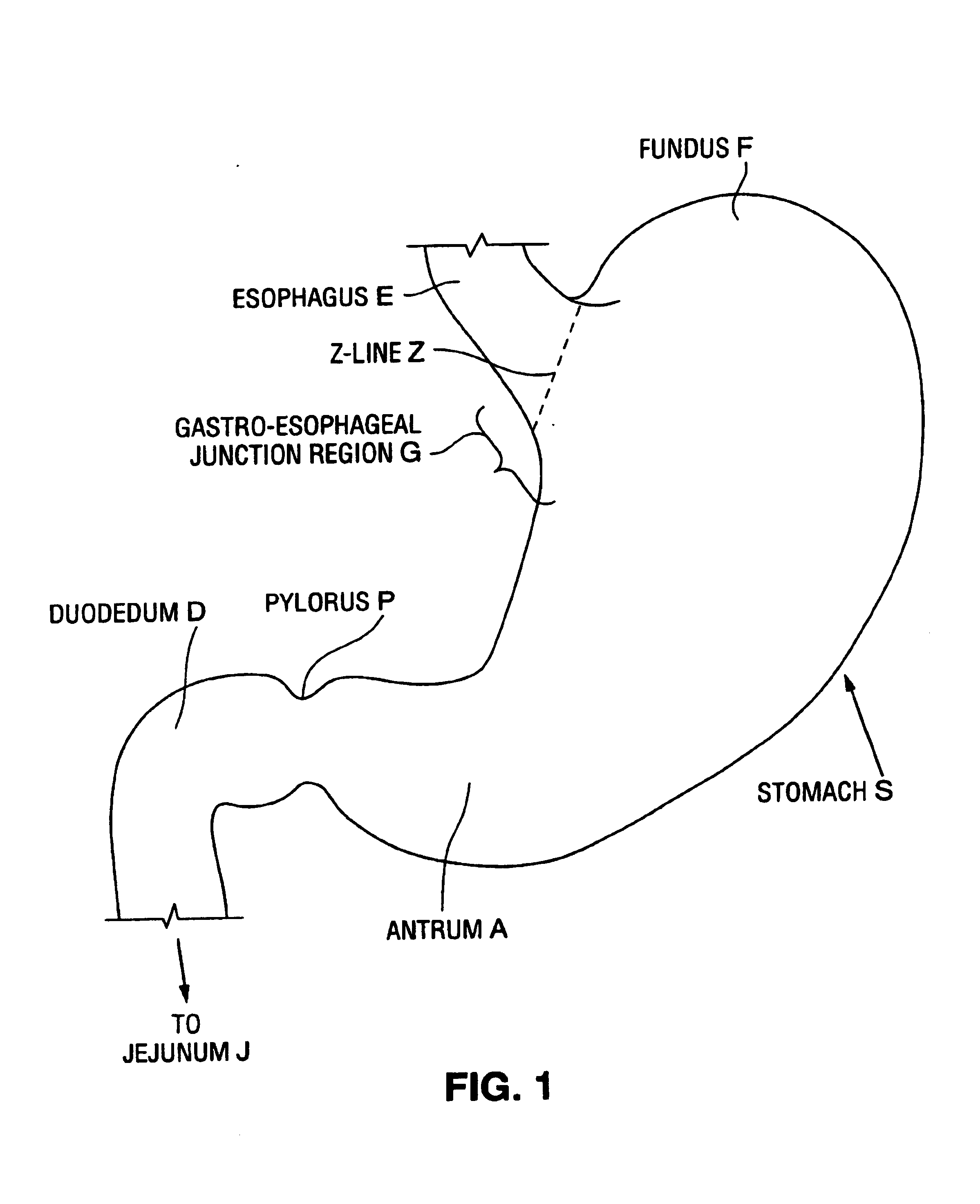
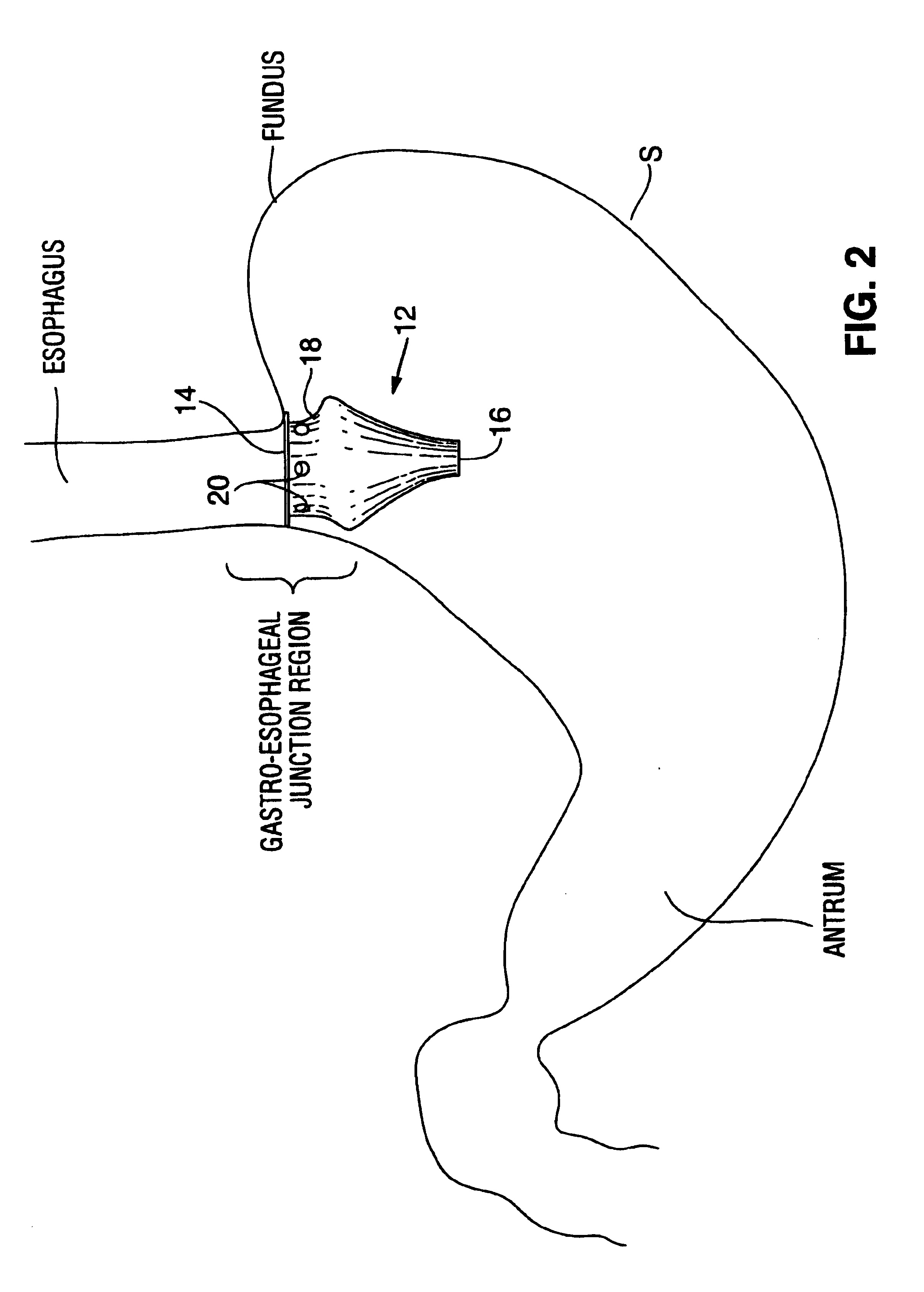
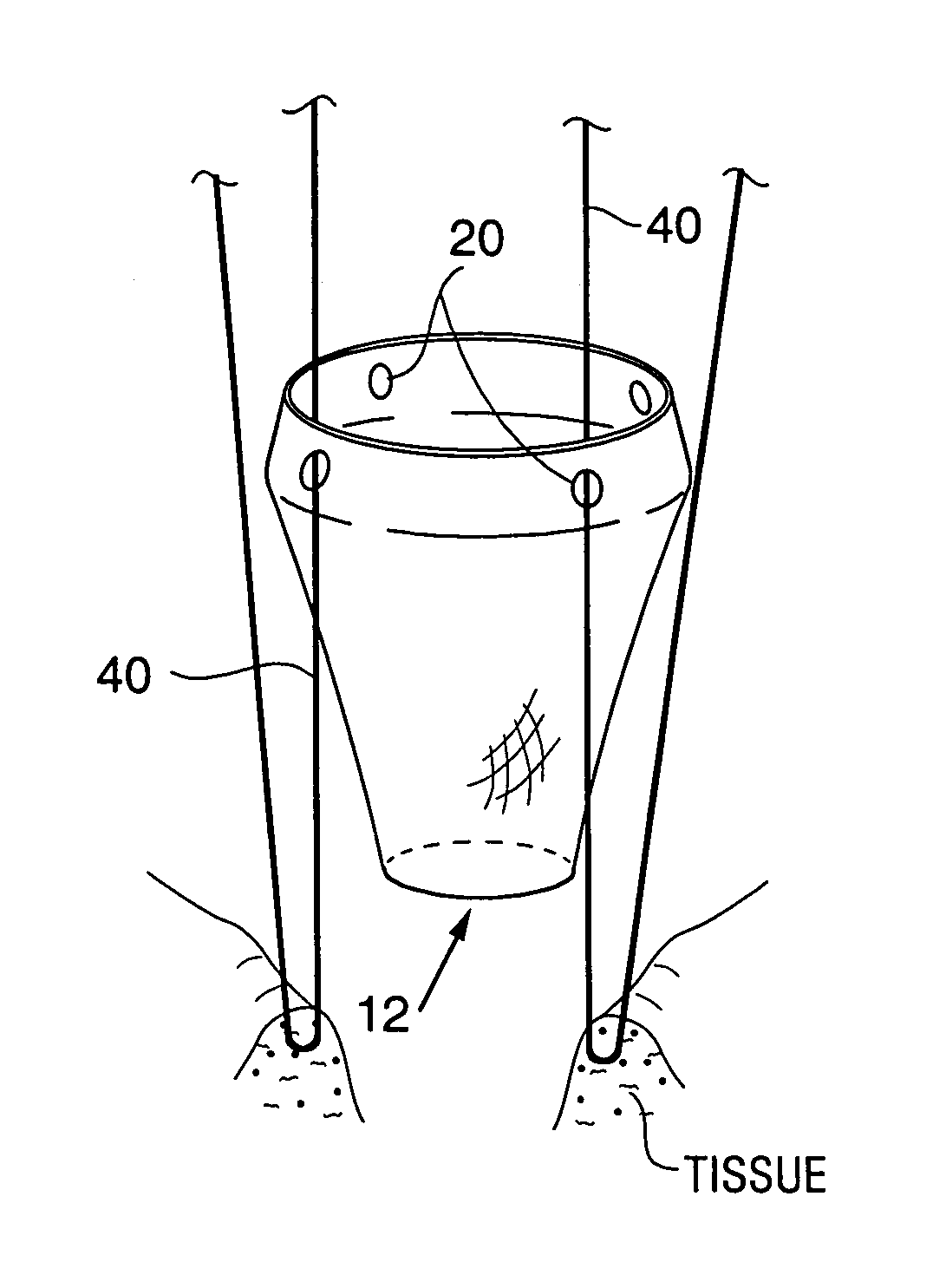
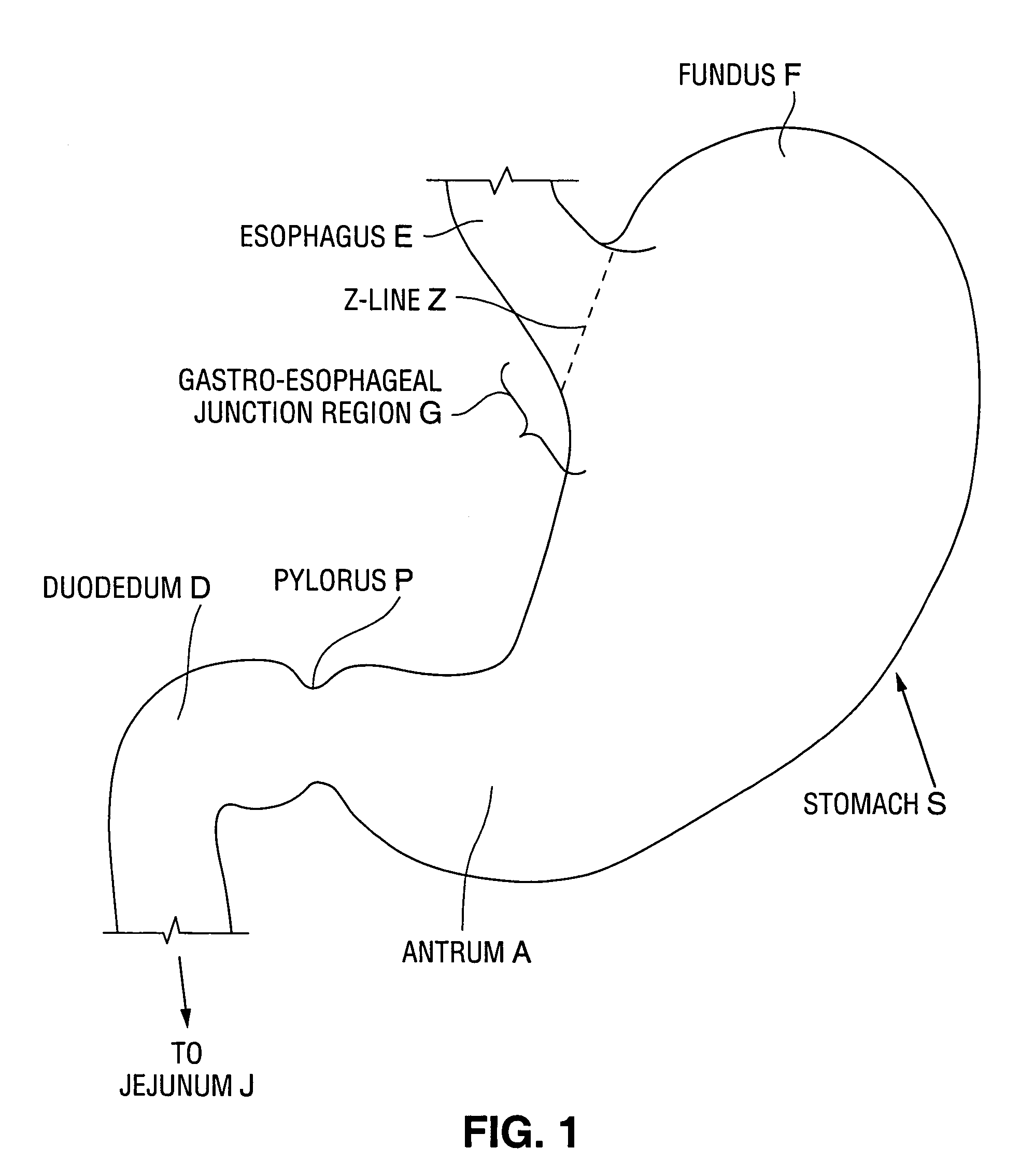
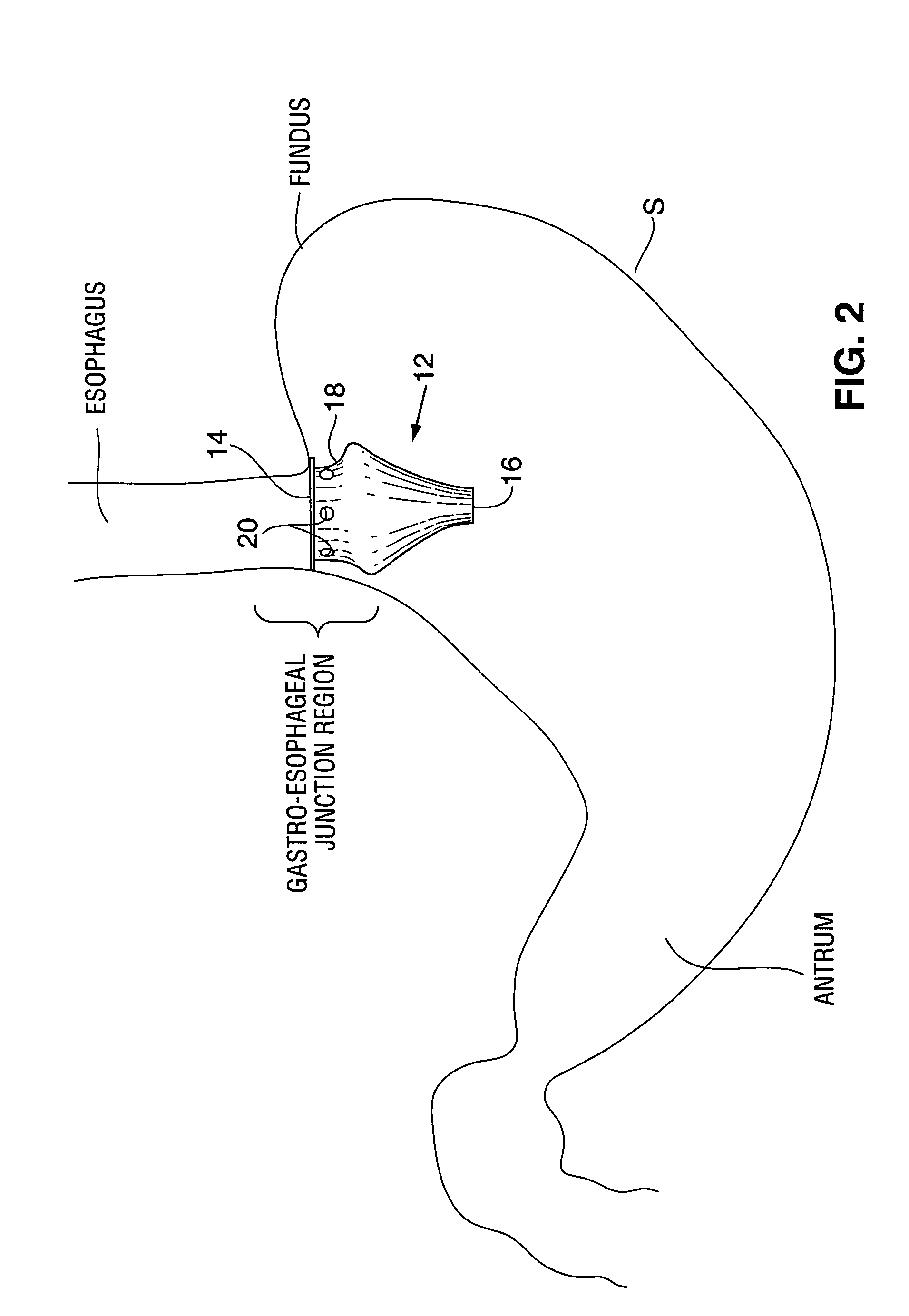
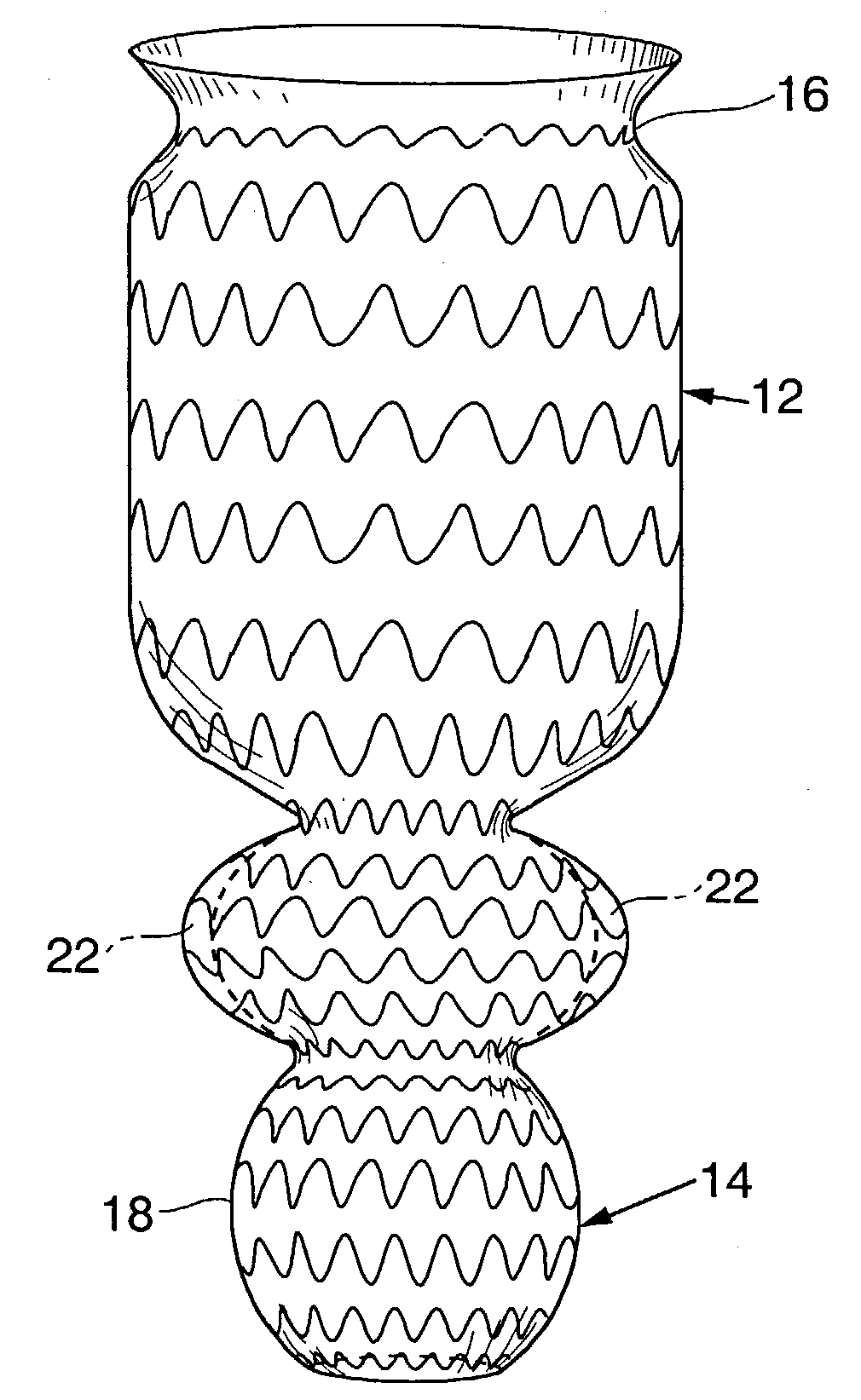
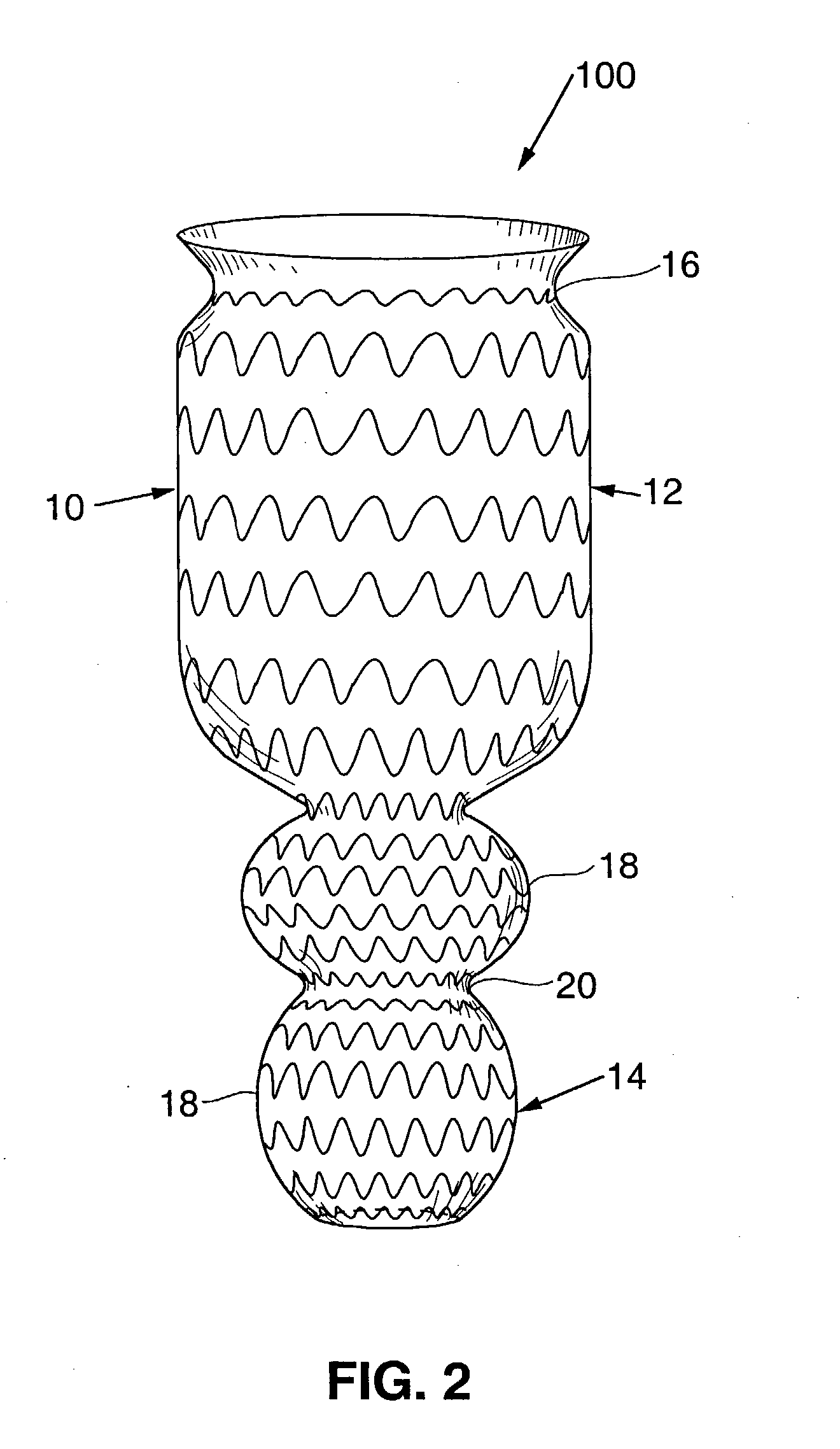
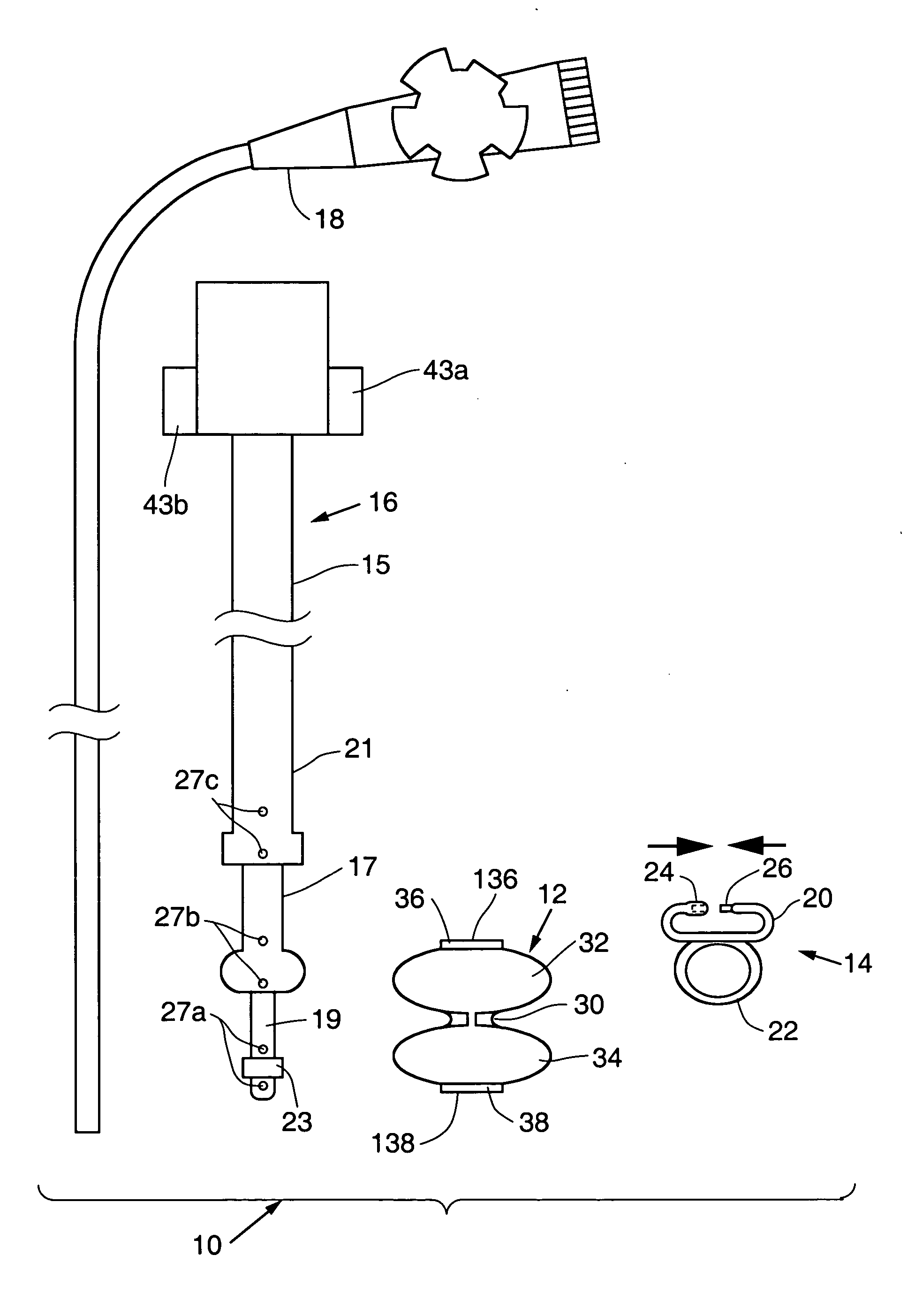
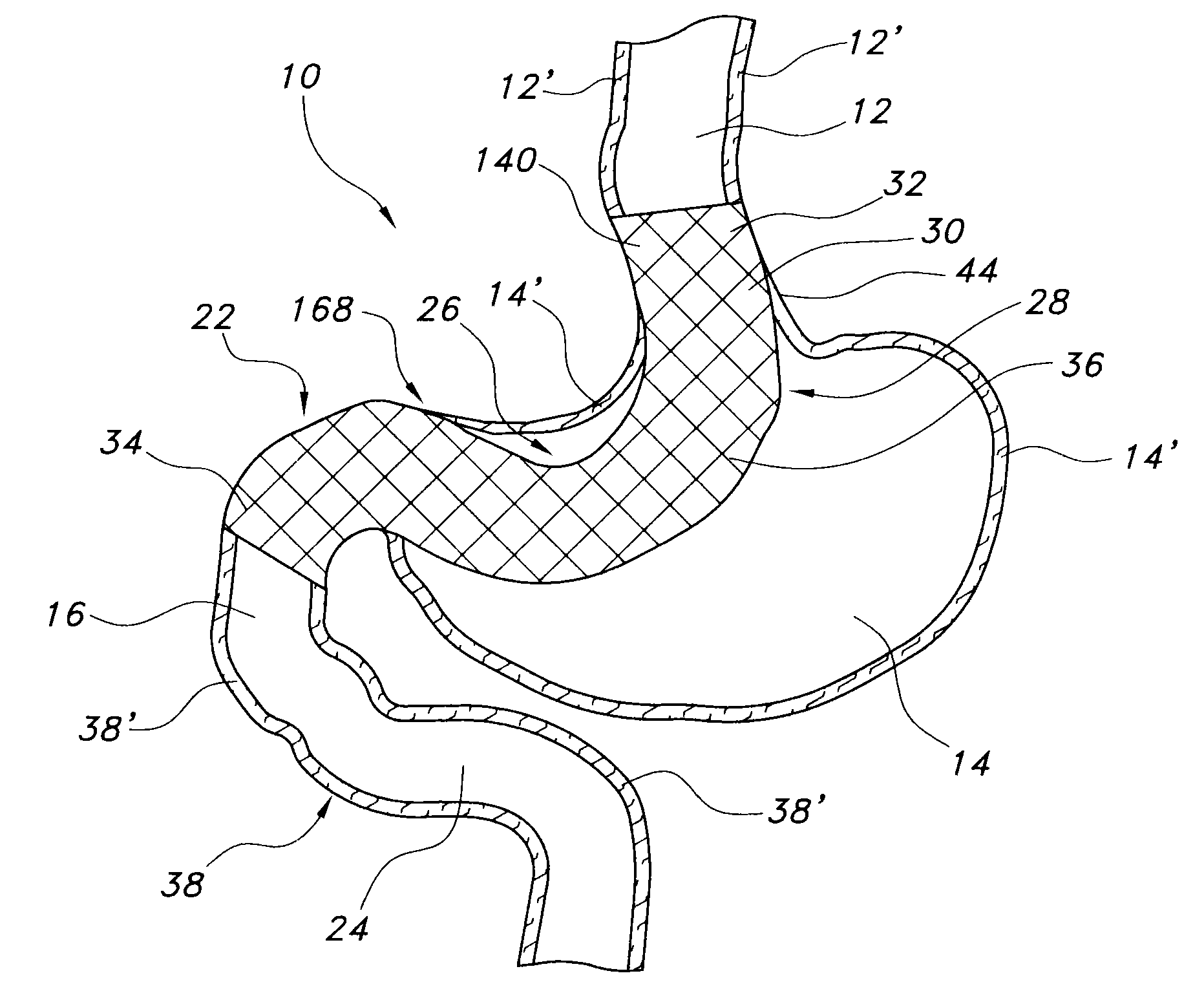
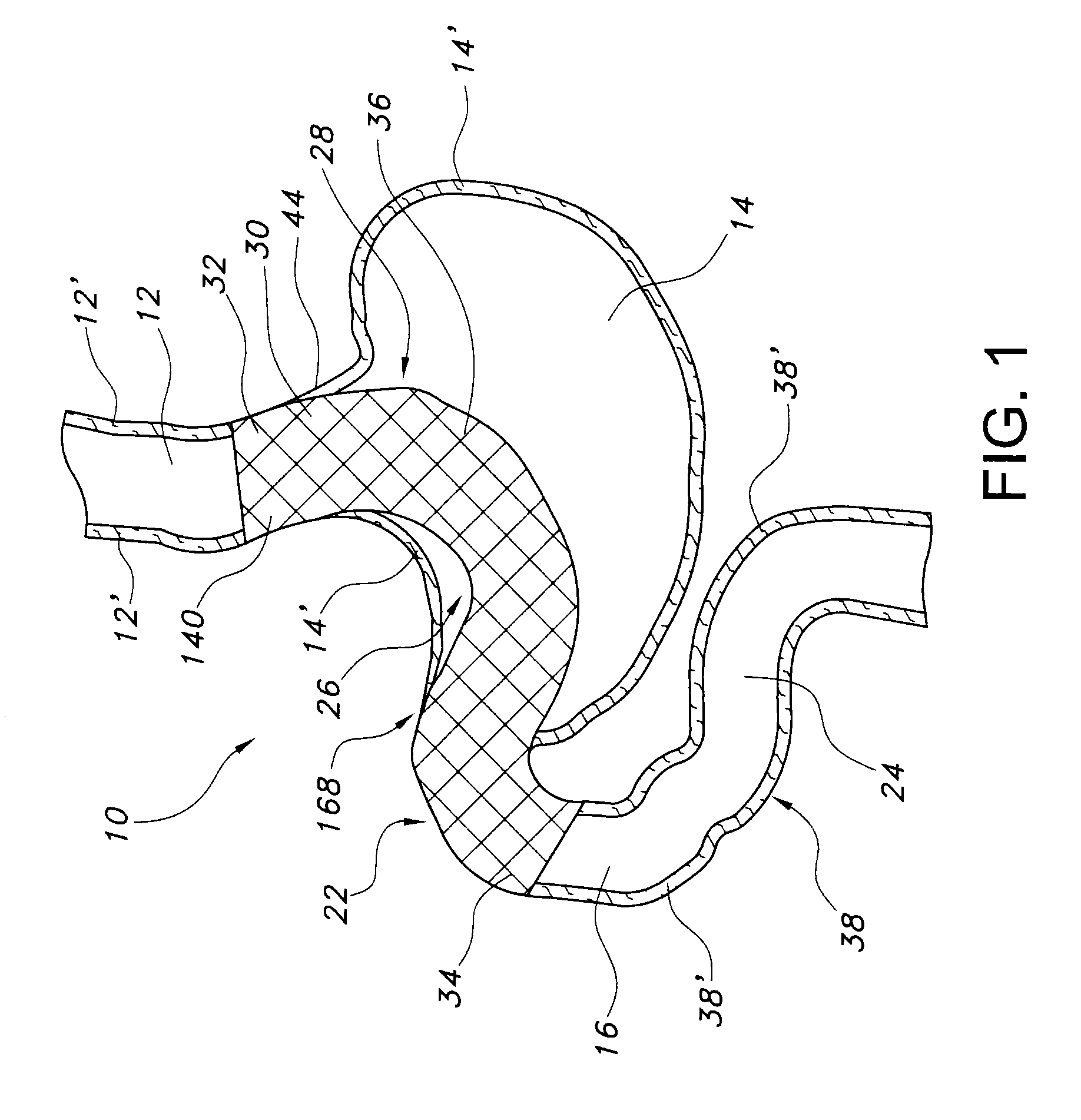
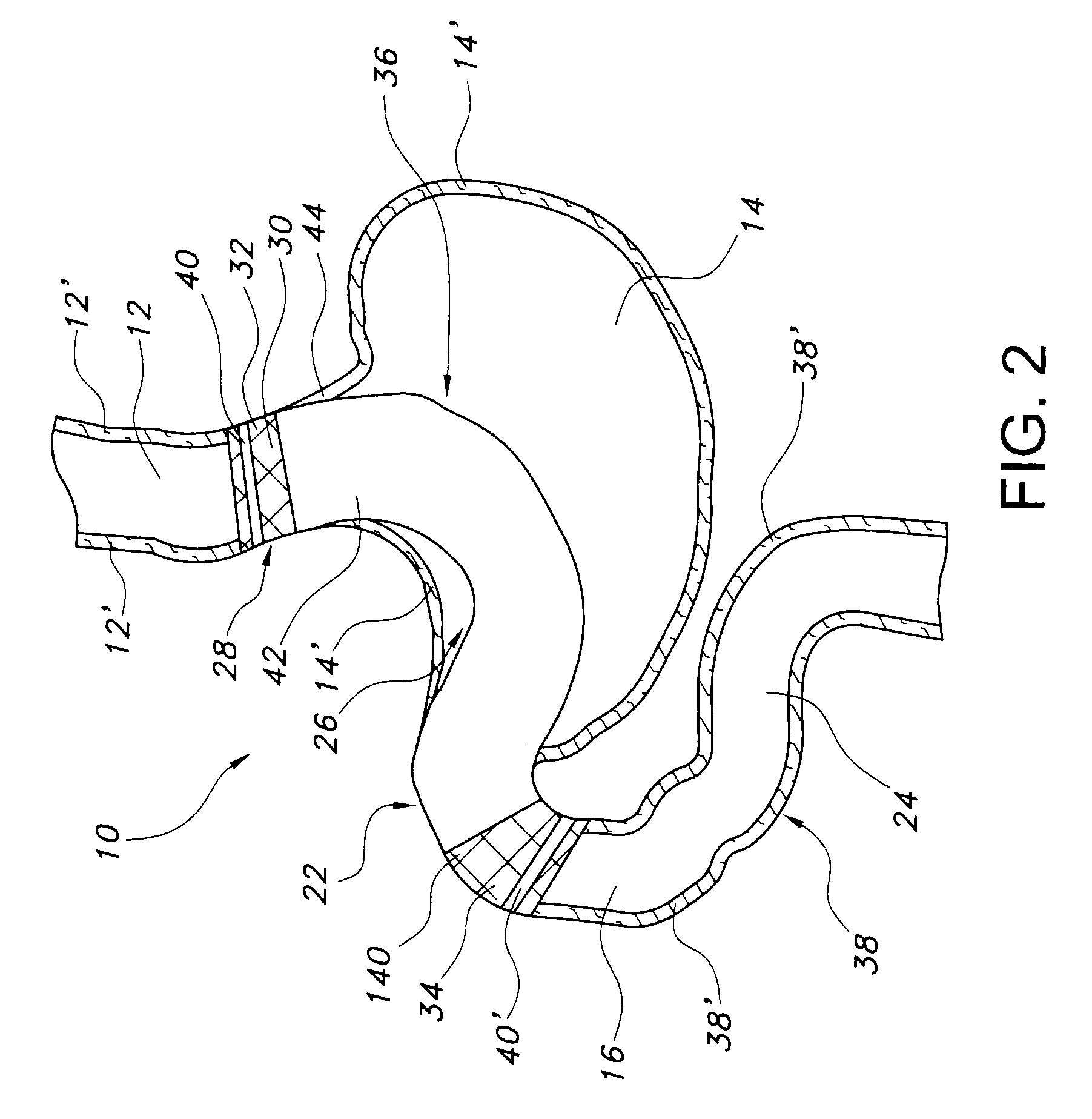
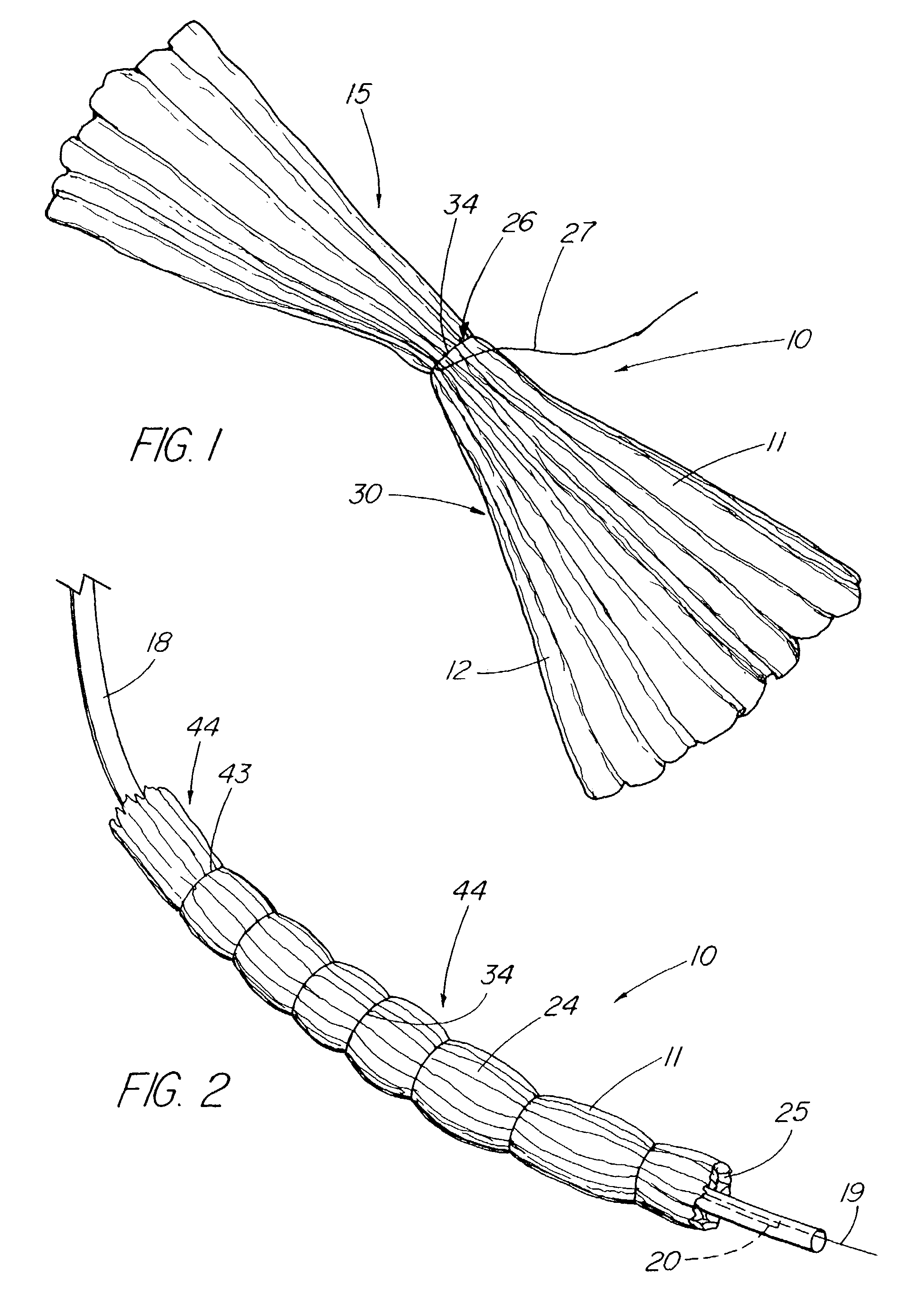
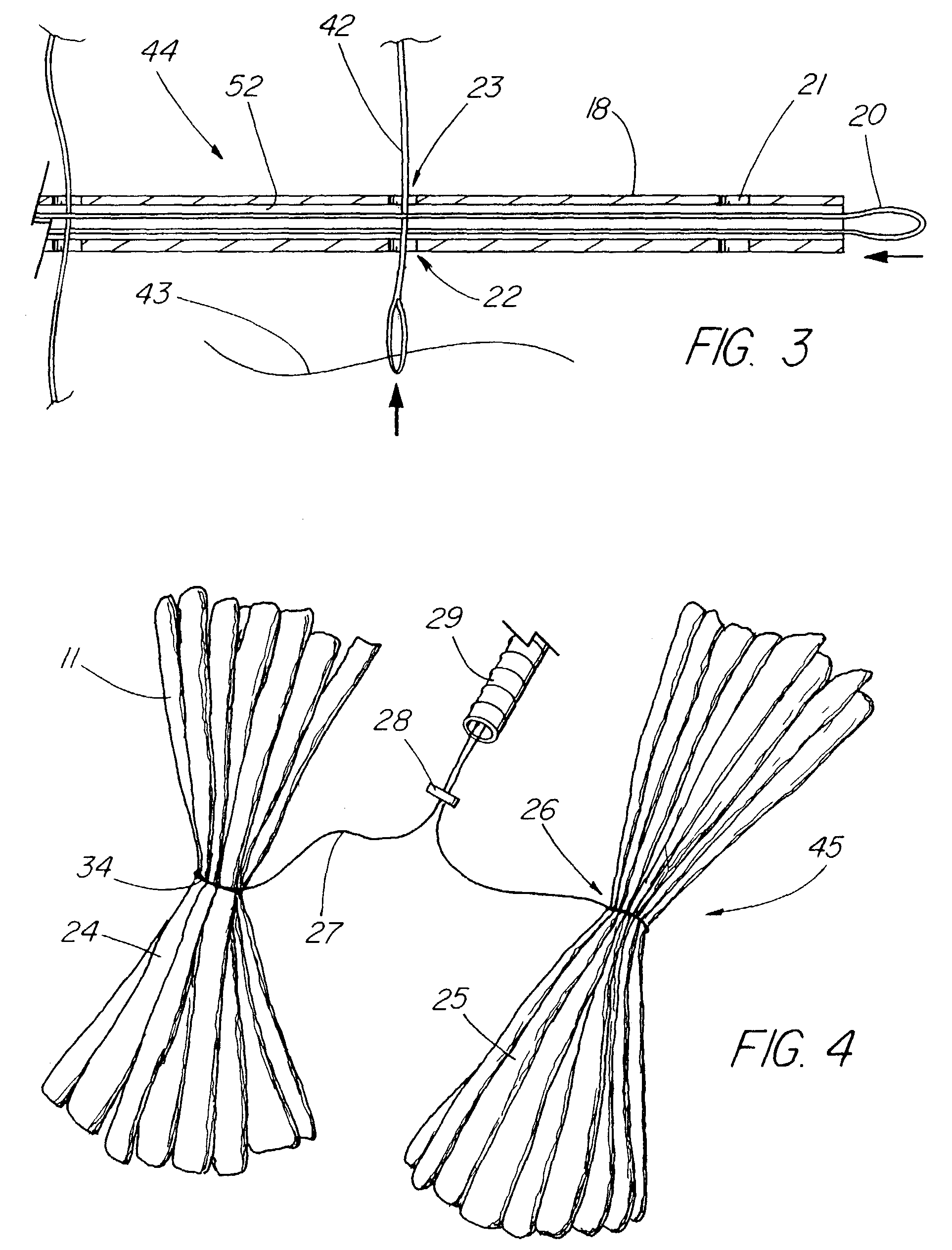
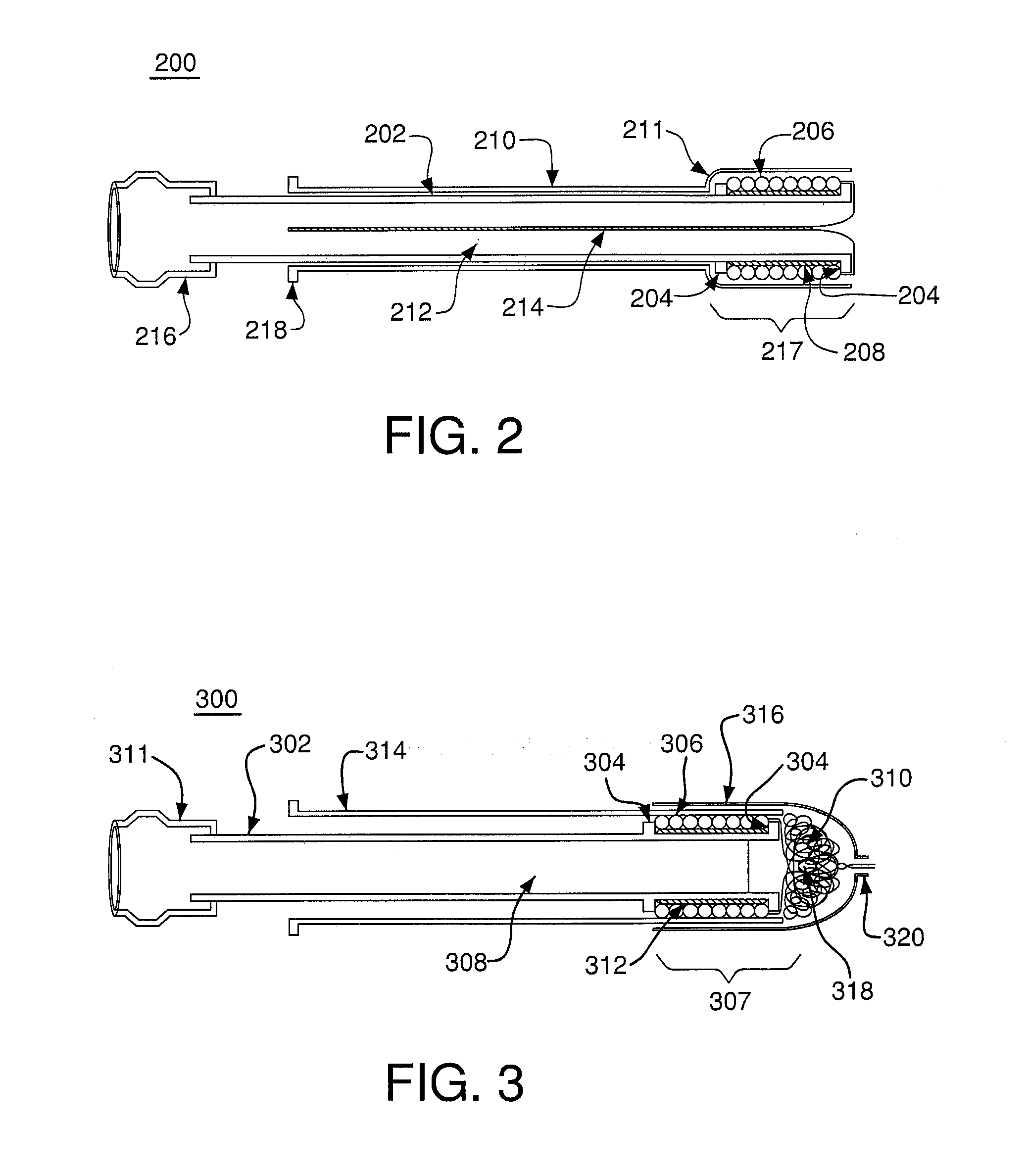
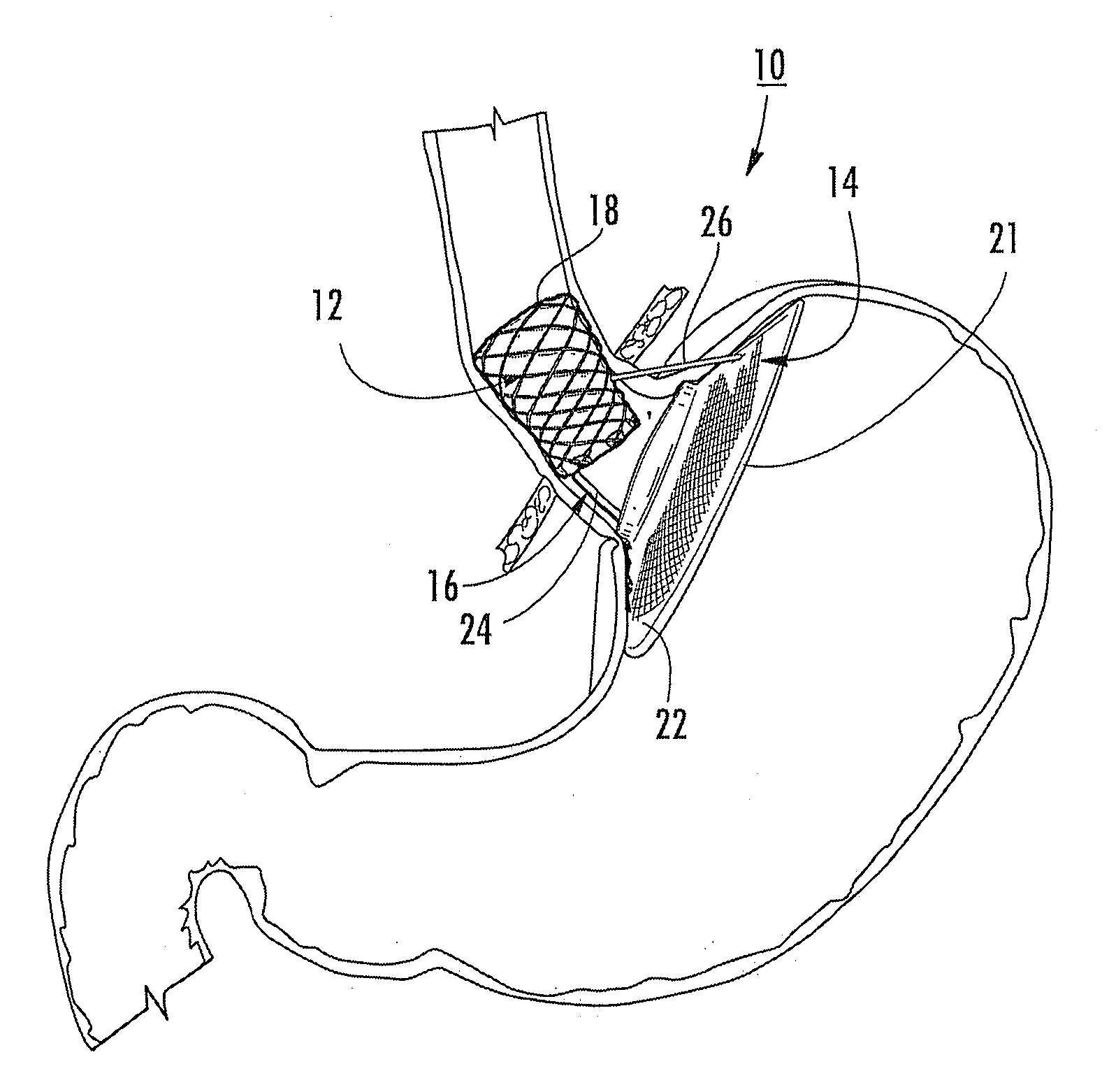
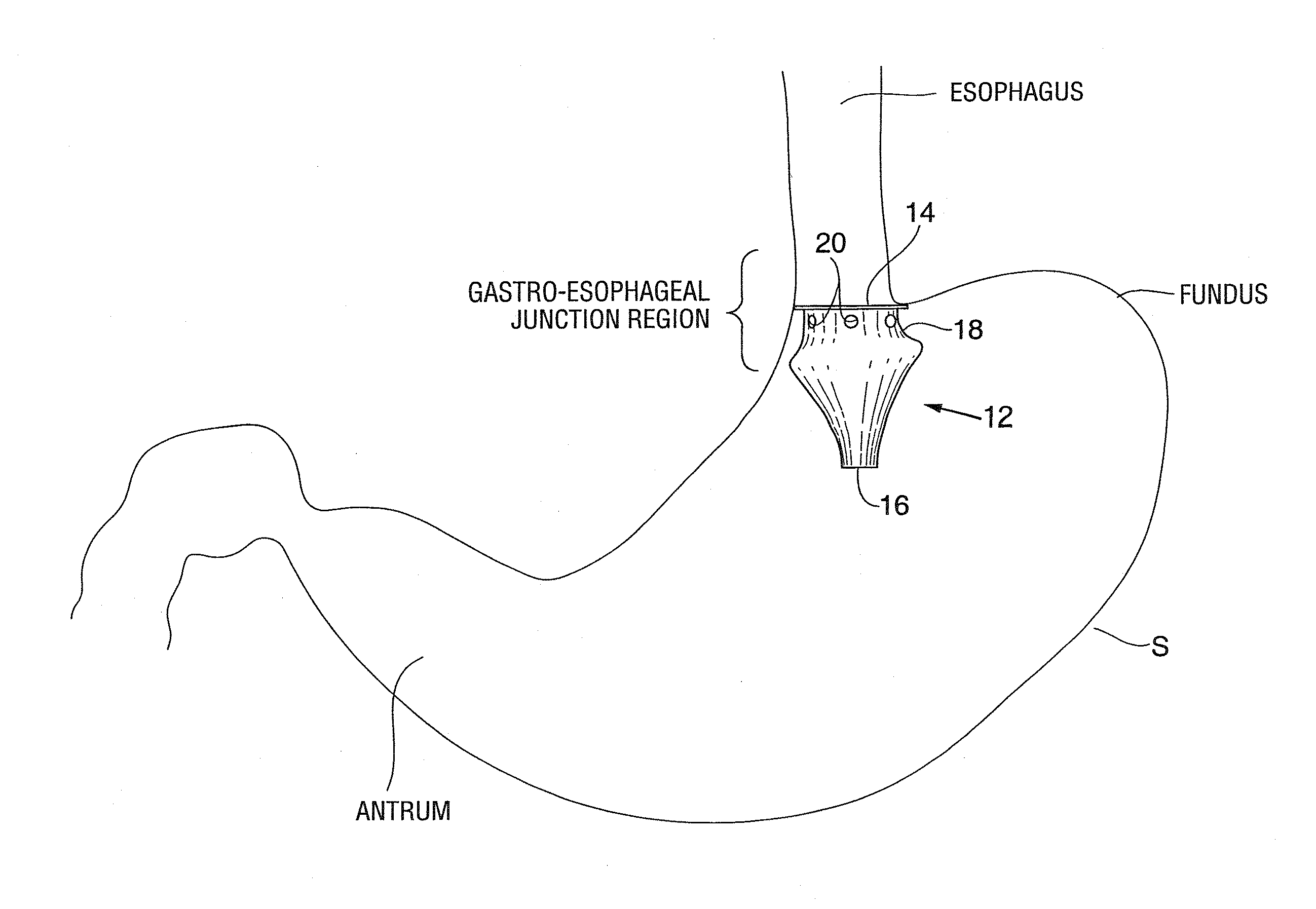
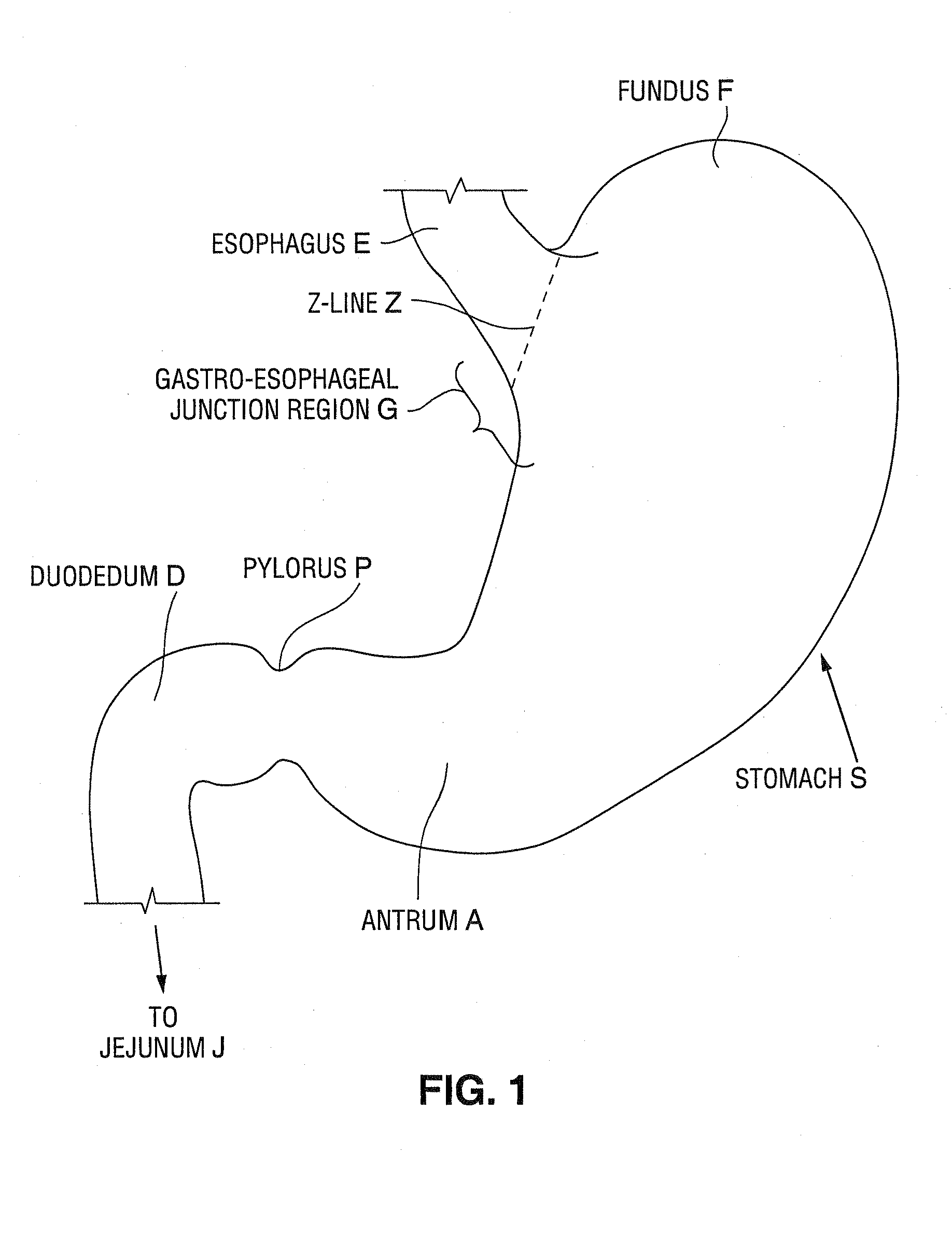
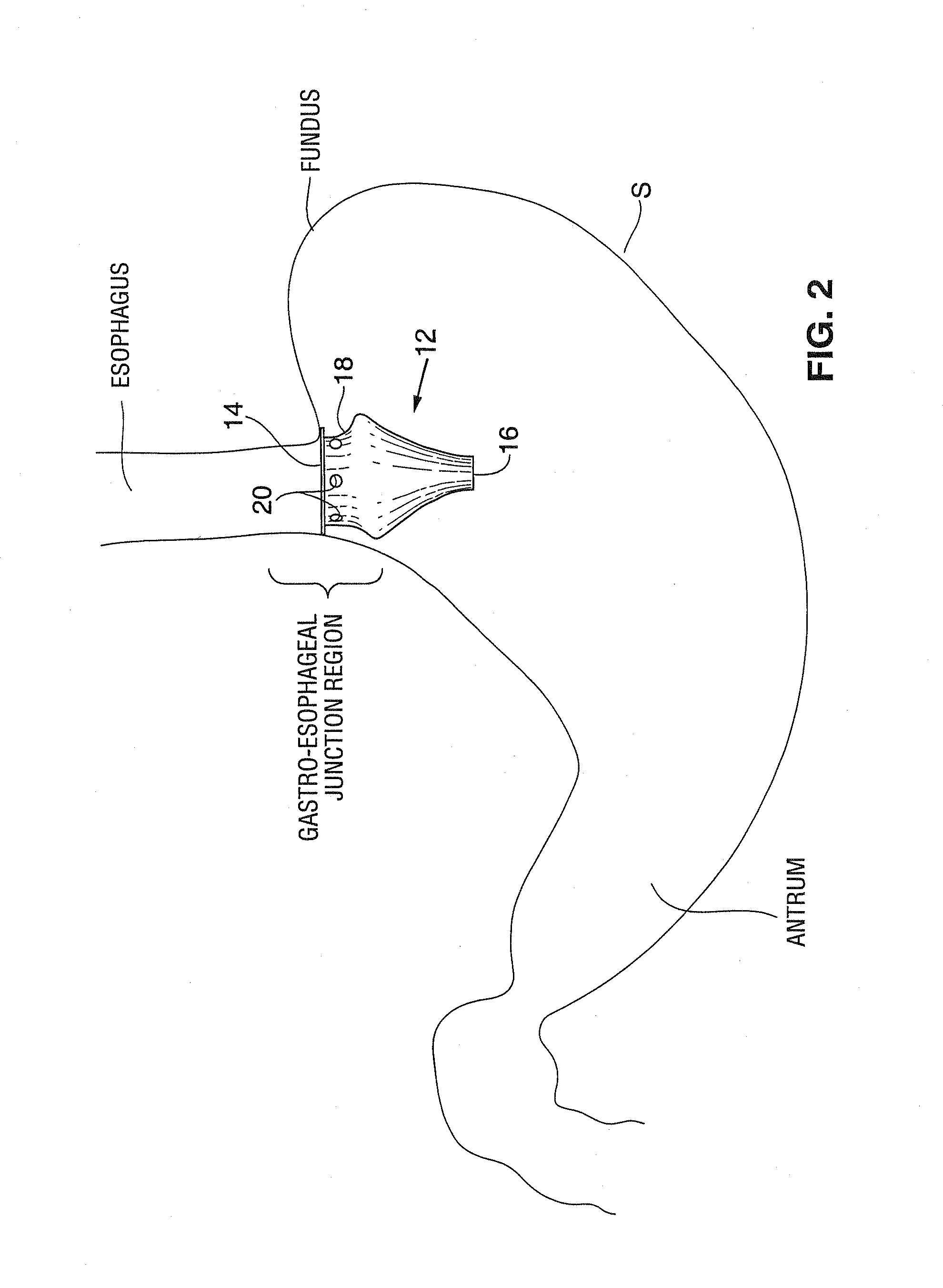
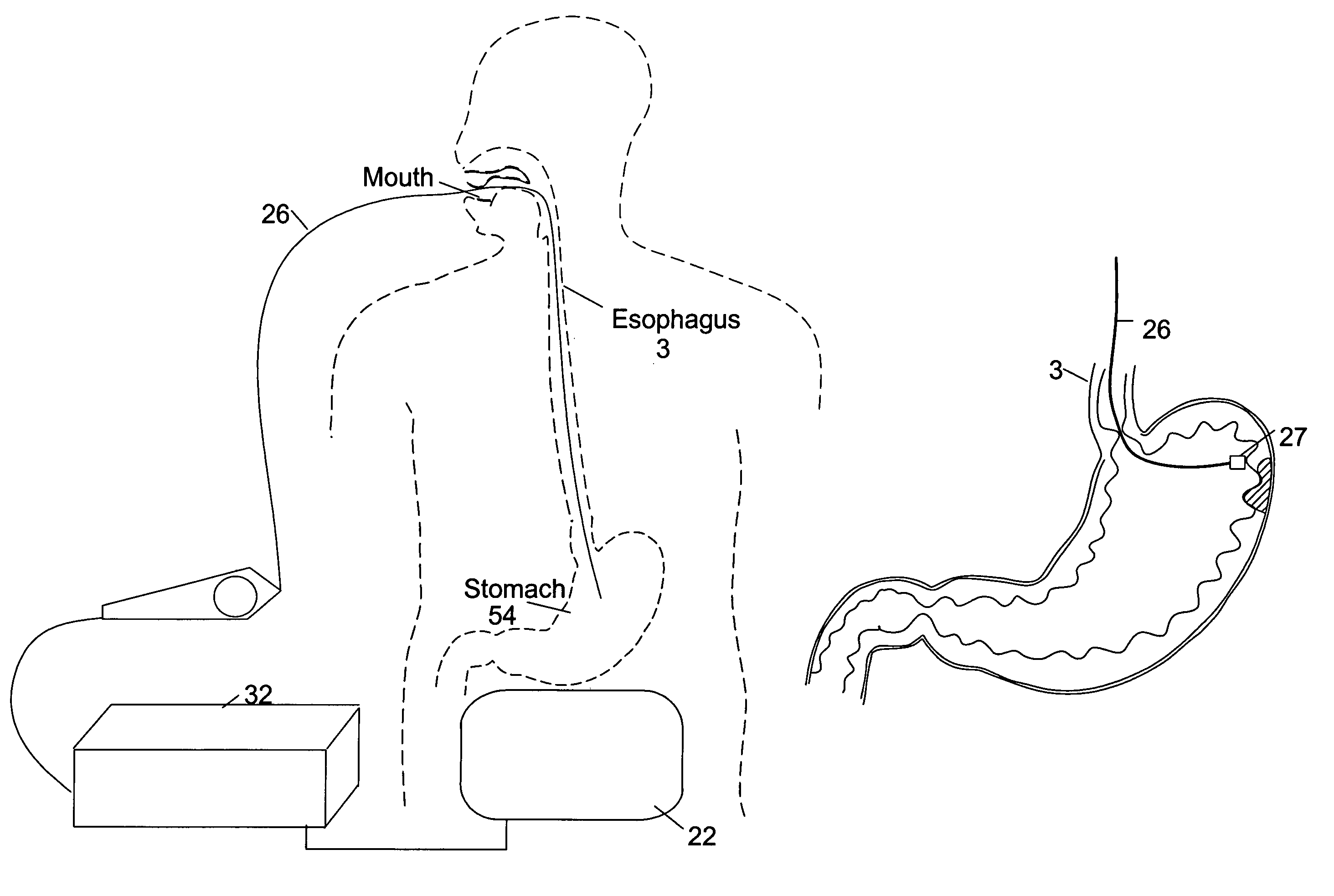
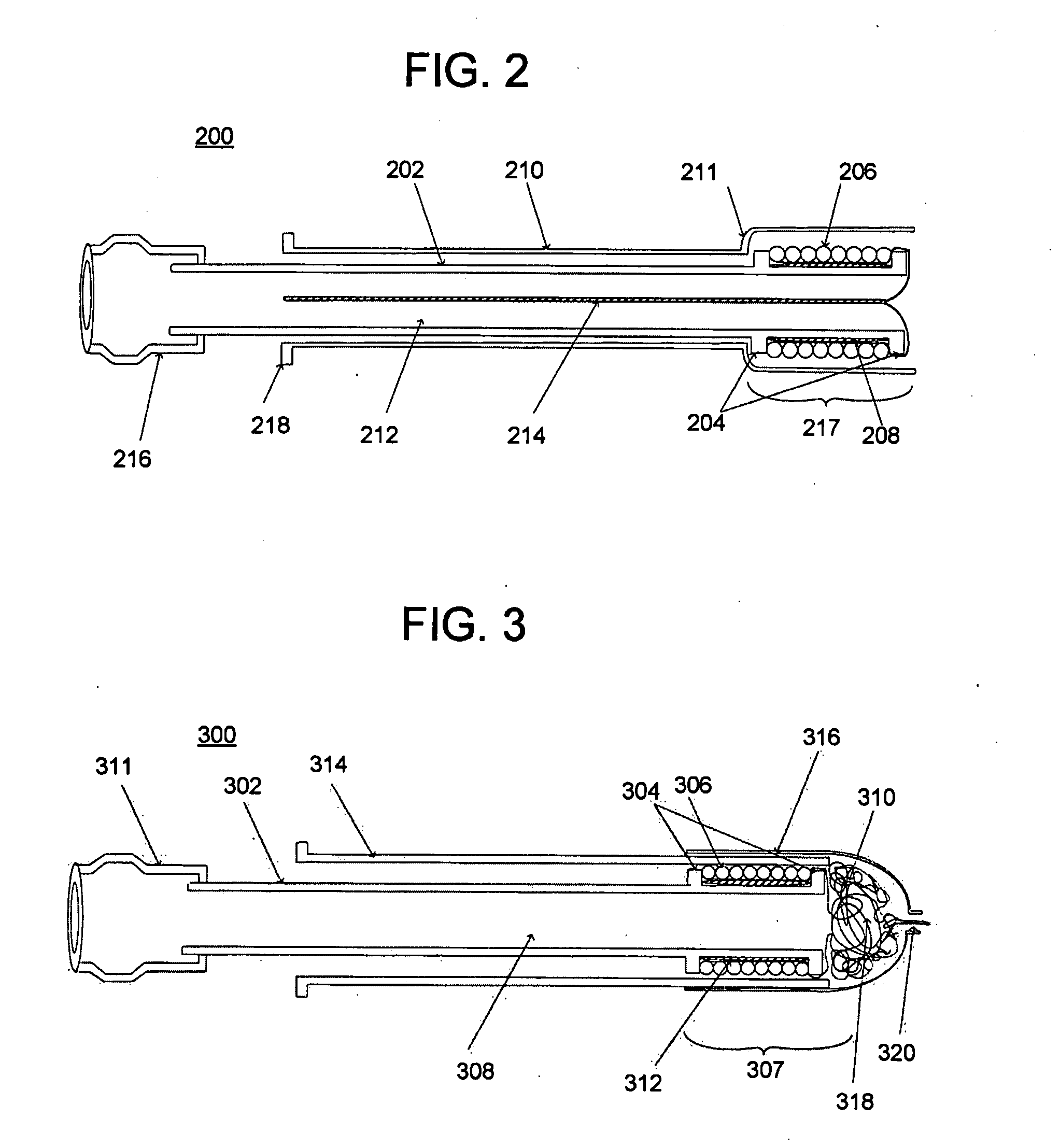
Satiation devices and methods
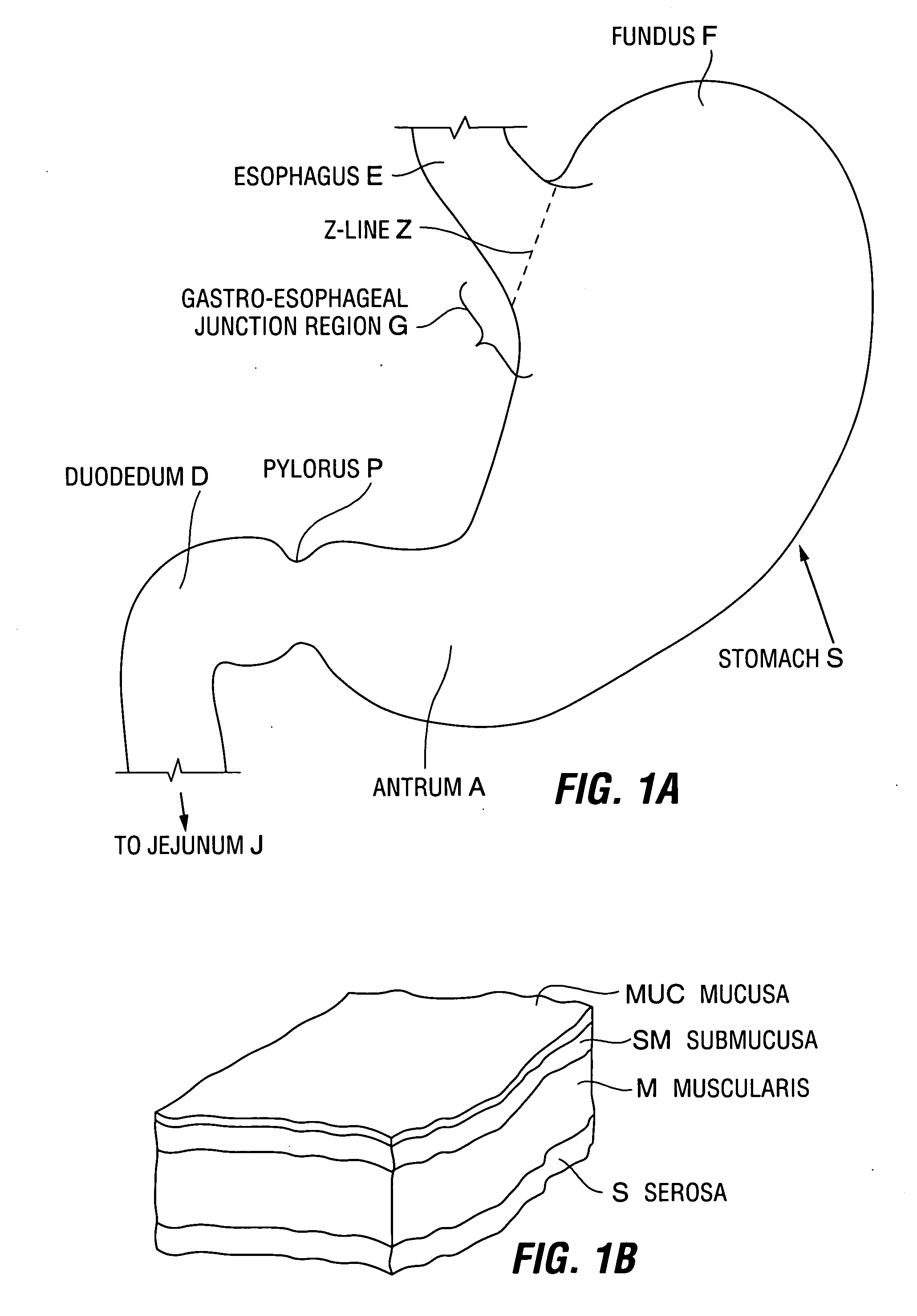
A device for inducing weight loss in a patient includes a tubular prosthesis positionable at the gastro-esophageal junction region, preferably below the z-line. In a method for inducing weight loss, the prosthesis is placed such that an opening at its proximal end receives masticated food from the esophagus, and such that the masticated food passes through the pouch and into the stomach via an opening in its distal end.
Owner:BOSTON SCI SCIMED INC
Gut microbiome as a biomarker and therapeutic target for treating obesity or an obesity related disorder
InactiveUS20100172874A1Decreasing energy harvestingGood for weight lossBiocideMetabolism disorderDiseaseMicroorganism
The present invention relates to the gut microbiome as a biomarker and therapeutic target for energy harvesting, weight loss or gain, and / or obesity in a subject. In particular, the invention provides methods of altering and monitoring the relative abundance of Bacteroides and Firmicutes in the gut microbiome of a subject.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Satiation devices and methods
A device for inducing weight loss in a patient includes a tubular prosthesis positionable at the gastro-esophageal junction region, preferably below the z-line. In a method for inducing weight loss, the prosthesis is placed such that an opening at its proximal end receives masticated food from the esophagus, and such that the masticated food passes through the pouch and into the stomach via an opening in its distal end.
Owner:BOSTON SCI SCIMED INC
Satiation devices and methods
A device for inducing weight loss in a patient includes a tubular prosthesis self-expandable from a collapsed position in which the prosthesis has a first diameter to an expanded position in which the prosthesis has a second, larger, diameter. In a method for inducing weight loss, the prosthesis is placed in the collapsed position and inserted into a stomach of a patient. The prosthesis is allowed to self-expand from the collapsed position to the expanded position and into contact with the walls of the stomach, where it induces feelings of satiety and / or inhibits modulation of satiety-controlling factors such as Ghrelin.
Owner:BOSTON SCI SCIMED INC
Methods of activating a melanocortin-4 receptor pathway in obese subjects
ActiveUS8476227B2Induce weight lossOrganic active ingredientsPeptide/protein ingredientsDefined ProcedureMC4 Receptor
Methods and therapeutics are provided for treating metabolic disorders by activation of melanocortin signaling pathways. Generally, the methods and therapeutics can induce activation of melanocortin receptor signaling to increase energy expenditure and induce weight loss. In one embodiment, a method for performing a diagnostic procedure can be chosen, energy expenditure then assess in light of the diagnostic procedure and a definitive procedure(s) can be selected dependent on the outcome of the energy assessment. In another embodiment, a diagnostic procedure can be chosen to activate melanocortin receptor pathways, energy expenditure can be assessed and a definitive procedure(s) can be chosen that selectively and optimally activate melanocortin receptor pathways.
Owner:ETHICON ENDO SURGERY INC +1
Restrictive and/or obstructive implant system for inducing weight loss
The present application describes an implant system useable for positioning an implant device such as a device useful for restricting passage of ingested food into the stomach. In one embodiment, the disclosed system includes a plurality of anchors that may be coupled to tissue within the stomach, or to a tissue tunnel formed by plicating stomach wall tissue. The anchor includes a loop. During use, the implant device is inserted through the loop and expanded such that it retains its position within the loop until removed. Instruments for implanting and explanting the implant device are also described.
Owner:BOSTON SCI SCIMED INC
Electrical system for weight loss and laparoscopic implanation thereof
InactiveUS6564101B1Avoid necessityEasy to deployInternal electrodesExternal electrodesElectricityCardiac pacemaker electrode
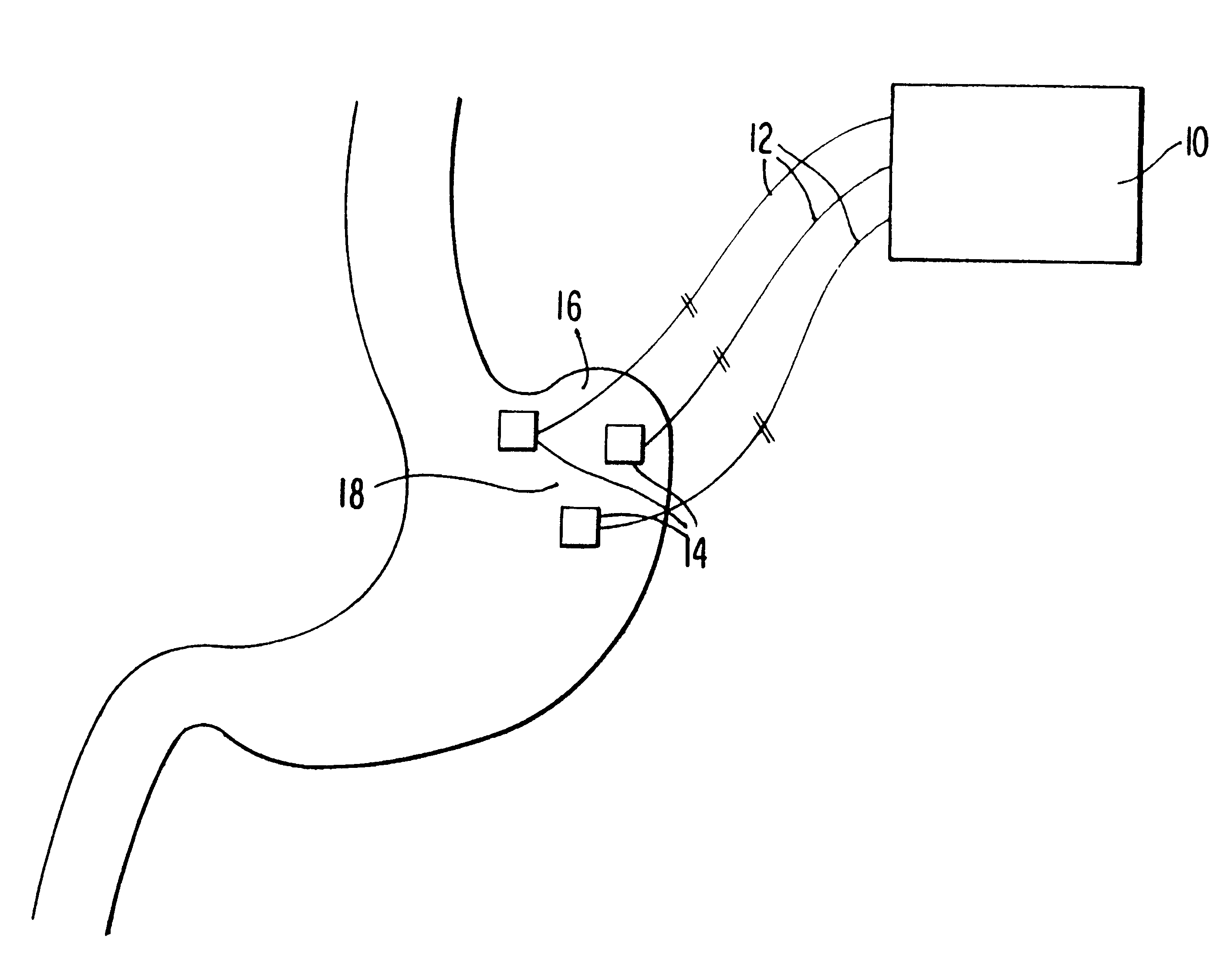
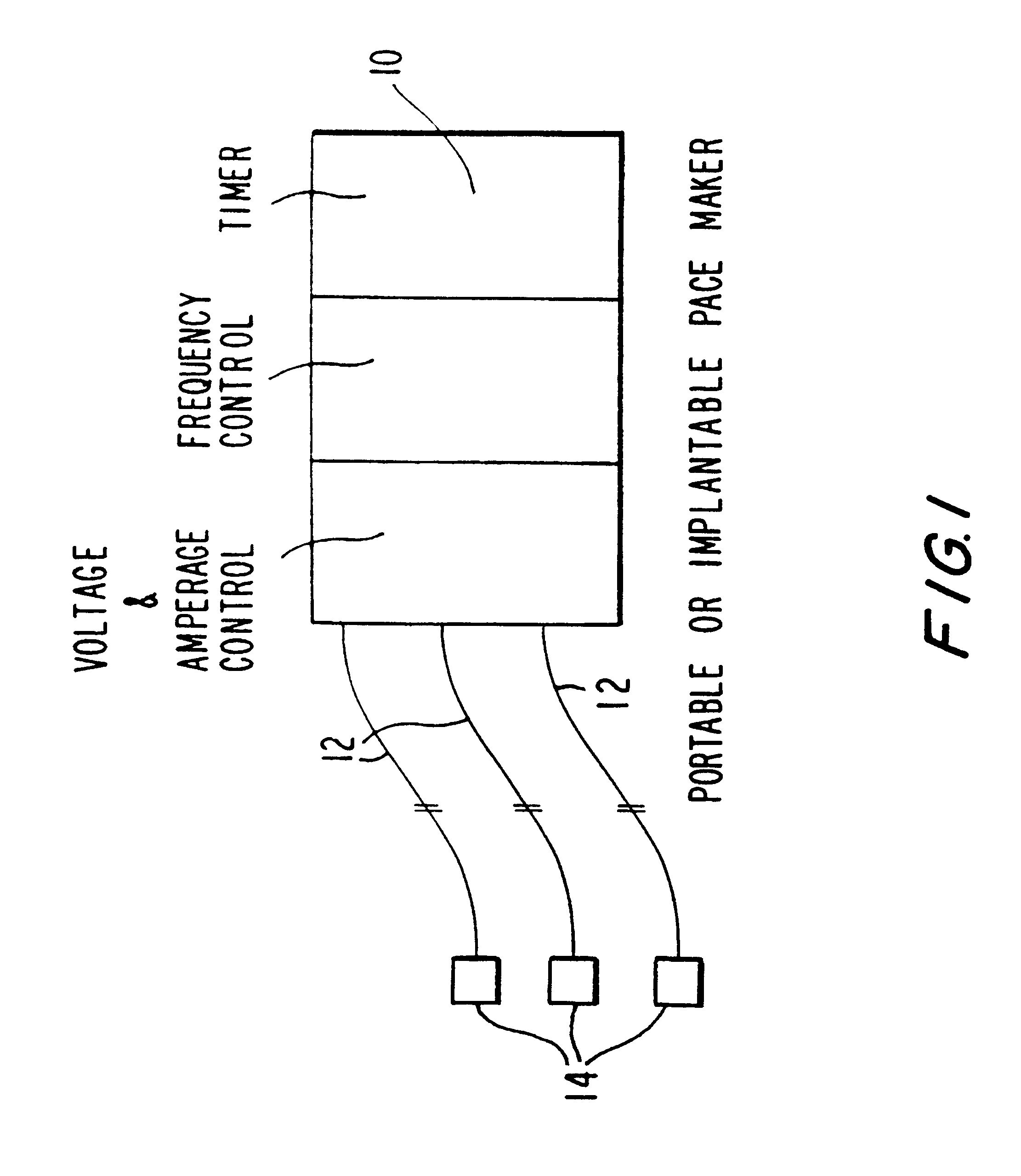
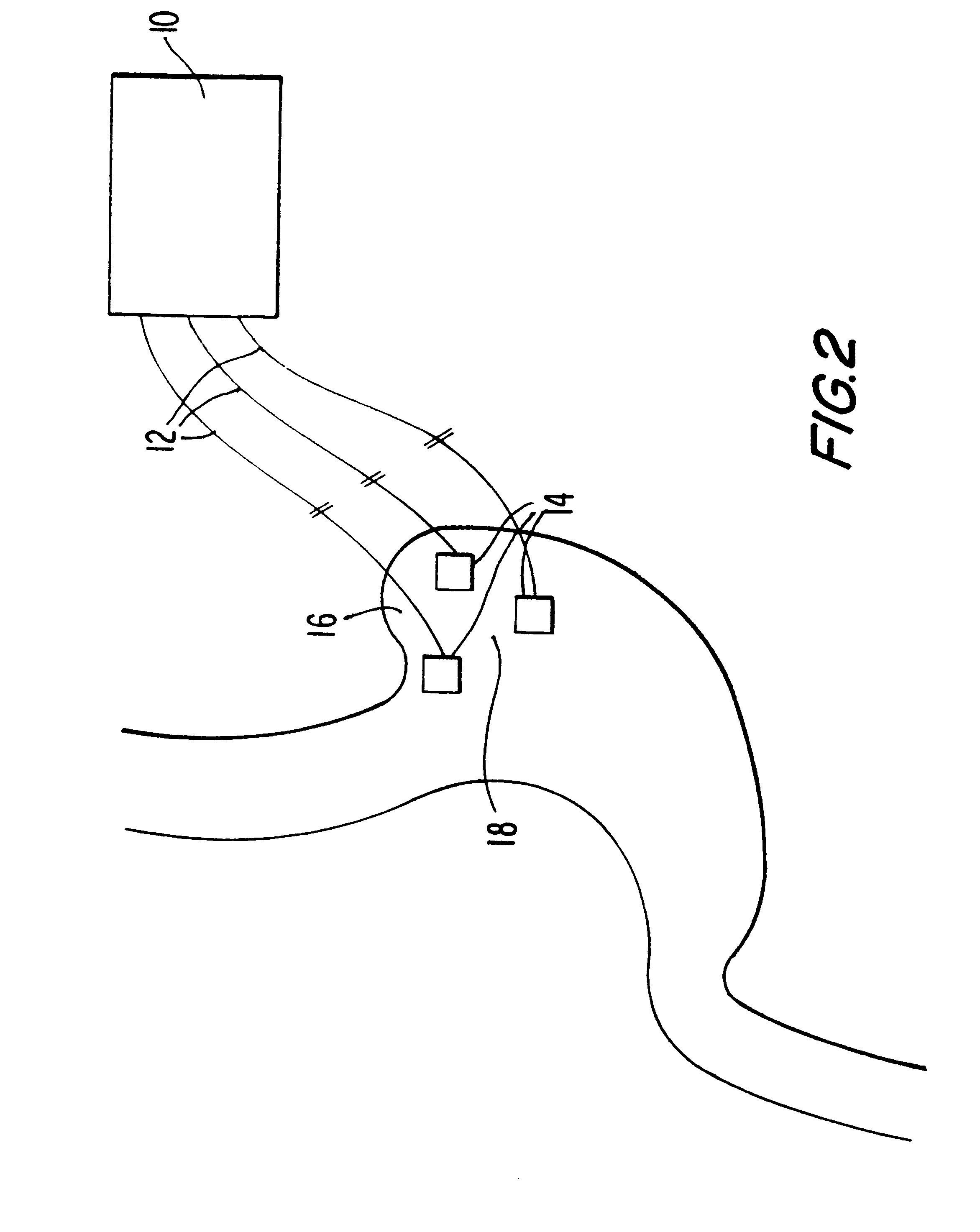
An electrical device utilized to control the body weight of a medically overweight human being comprises of at least two electrical leads for implanting on the fundus of the stomach. An electrical generator / controller (pacemaker) generates and regulates the frequency and degree of electrical stimulation. The device can be used surgically, laproscopically, and / or endoscopically.
Owner:ZIKRIA BASHIR A
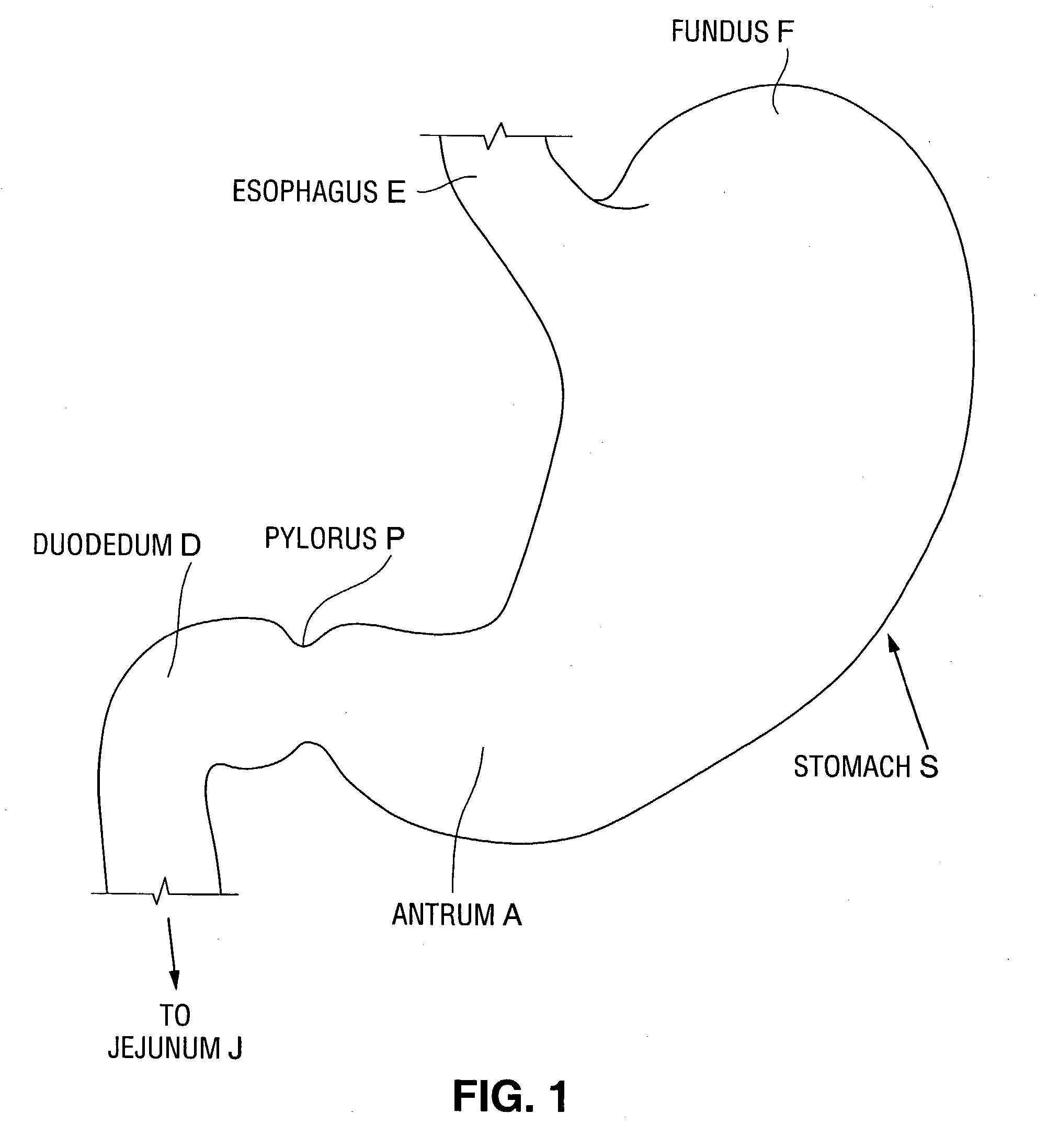
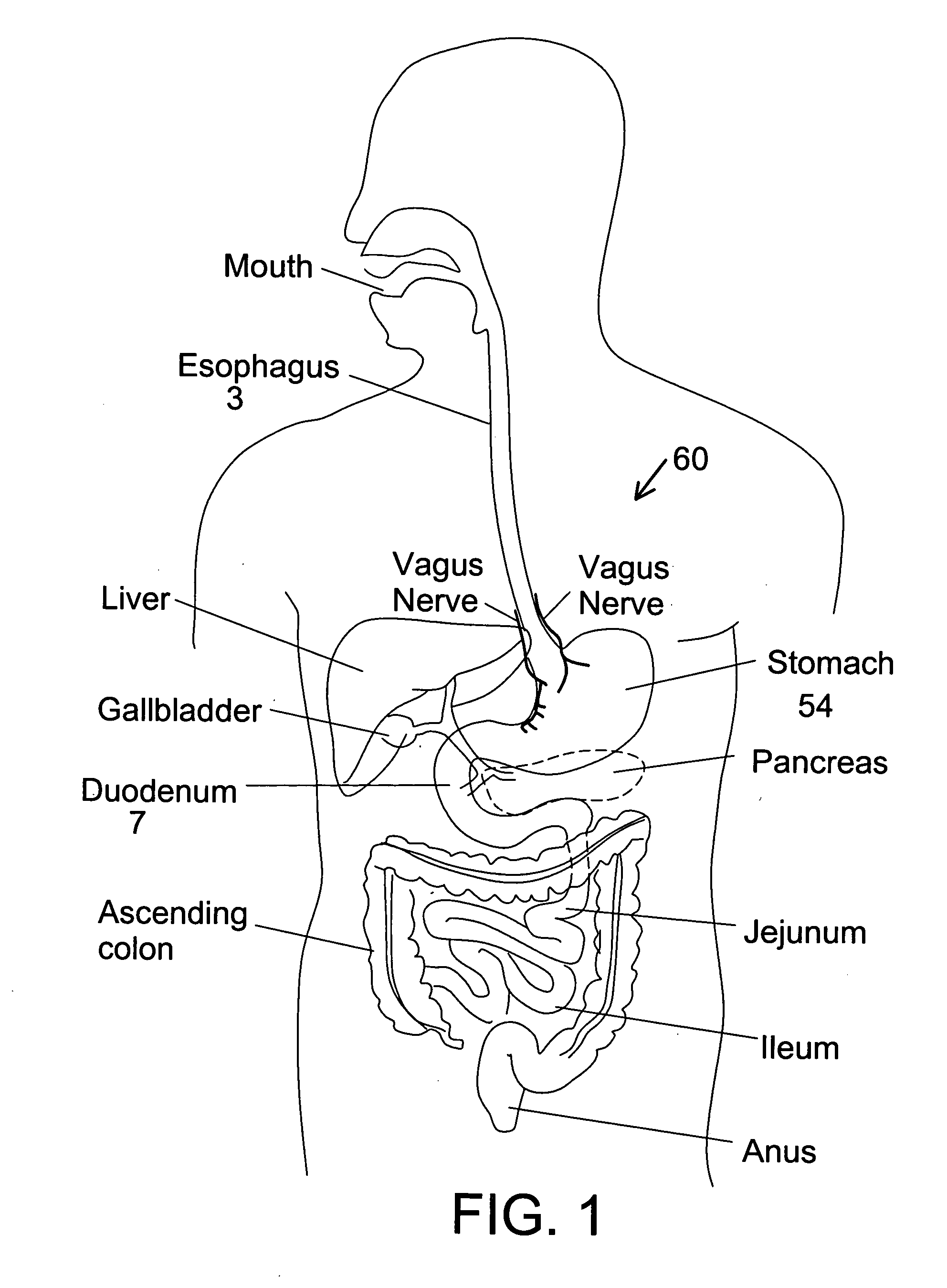
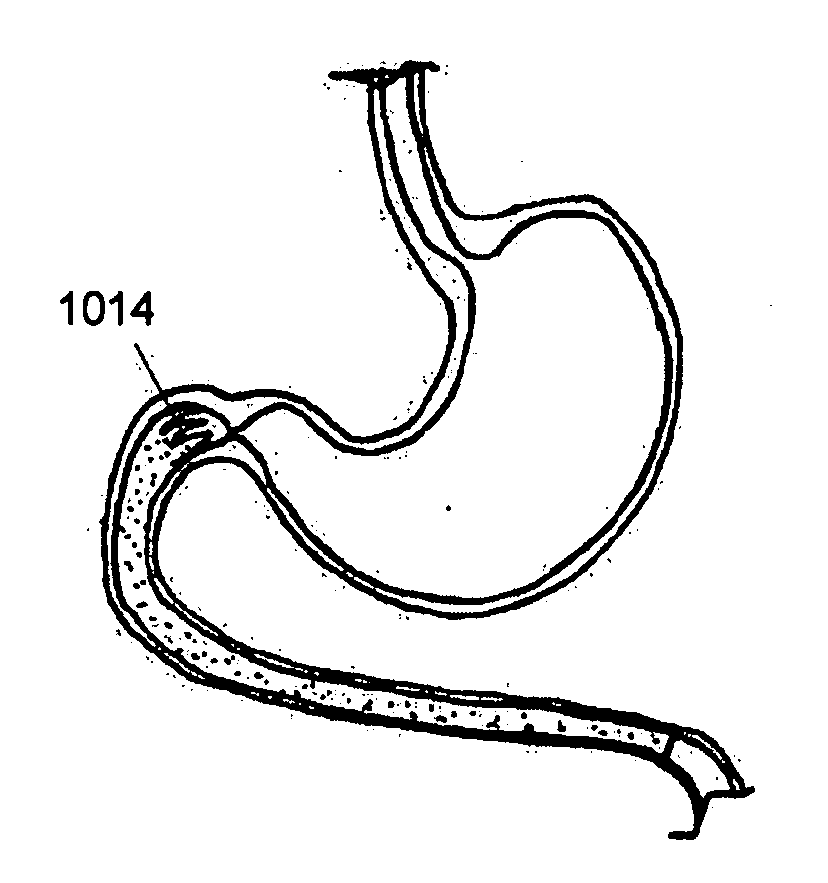
Stomach bypass for the treatment of obesity
ActiveUS20110087146A1Reduce and eliminate digestionEliminate digestionStentsOesophagiDigestionStomach bypass
The present application provides devices and methods for inducing weight loss. In particular, the present application provides devices which are secured in the stomach or external to the stomach to reduce digestion and / or absorption of food in accordance with the methods of the invention
Owner:BOSTON SCI SCIMED INC
Intragastric device for treating obesity
An apparatus and method comprising at least one intragastric member or artificial bezoar made of a digestive-resistant or substantially indigestible material that is introduced into a gastric lumen of a mammal for the treatment of obesity. The intragastric member or artificial bezoar is typically at inserted into the gastric lumen in a partially compacted configuration, whereby it is then manipulated into, or allowed to assume, a second expanded configuration sufficiently large to remain within the reservoir of the stomach during normal activities and not be passed through the pylorus into the intestines. In animals, the present invention has been found to be effective in achieving weight loss over a several month period, while being easy to place and retrieve.
Owner:COOK MEDICAL TECH LLC
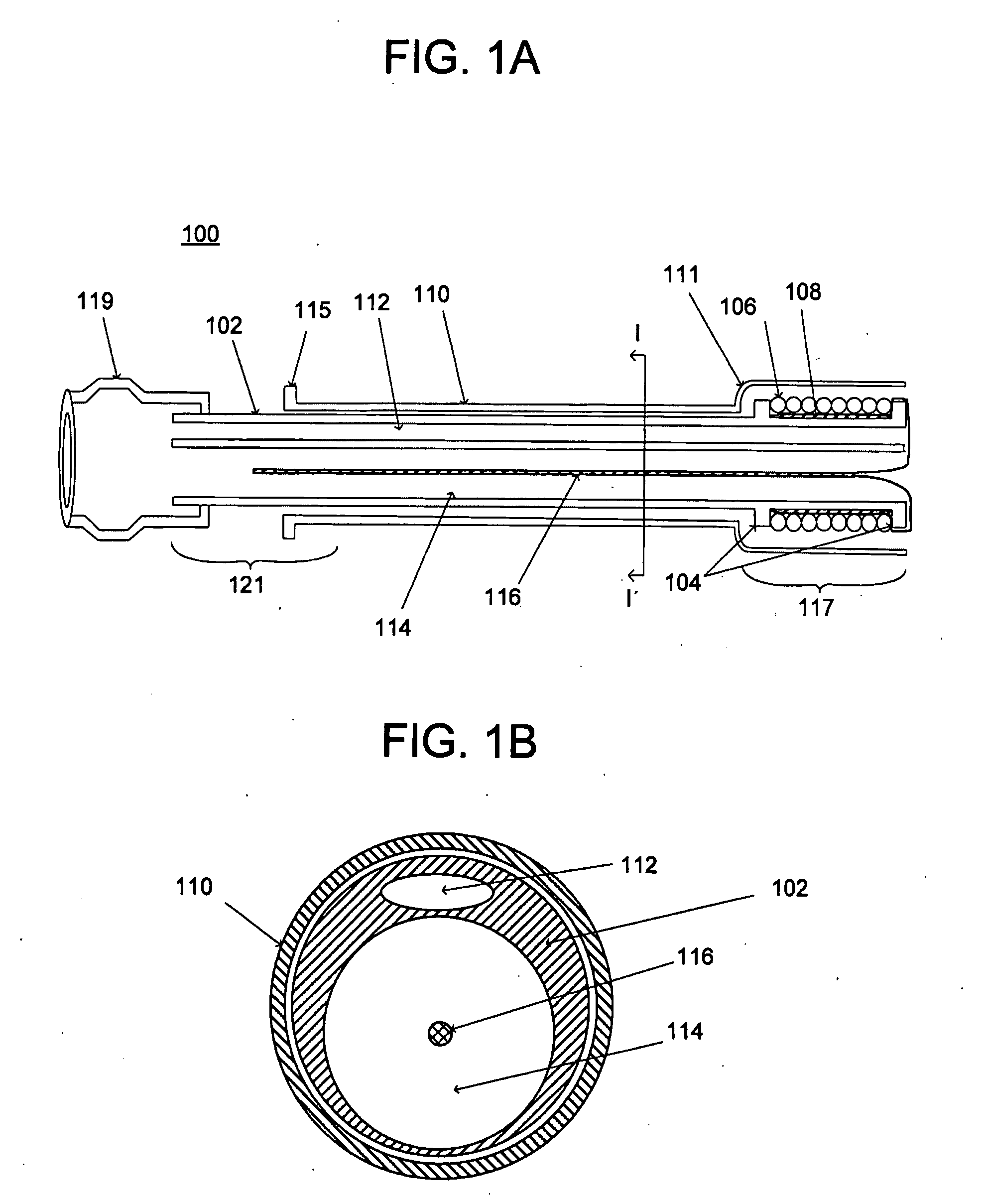
Medical apparatus and method of making the same
InactiveUS20080255678A1Minimize complicationsLess invasiveEar treatmentSurgeryMedical deviceEndoscope
The invention relates to a medical apparatus including a device used in the treatment of weight loss, obesity and potentially other associated health problems, e.g., type II diabetes. The device is used to impede absorption of nutrients within the gastrointestinal tract, i.e., bypassing a portion of the gastrointestinal tract. The medical apparatus enables implantation of the device using minimally invasive techniques, such a transesophageal approach under visualization. The device may be implanted via a working channel of a medical scope, e.g., an endoscope or in combination with a medical scope.
Owner:WL GORE & ASSOC INC
Solid form
InactiveUS20080286344A1Strong enoughAvoids and reduces processing and product drawbackPowder deliveryNervous disorderFilling materialsVolumetric Mass Density
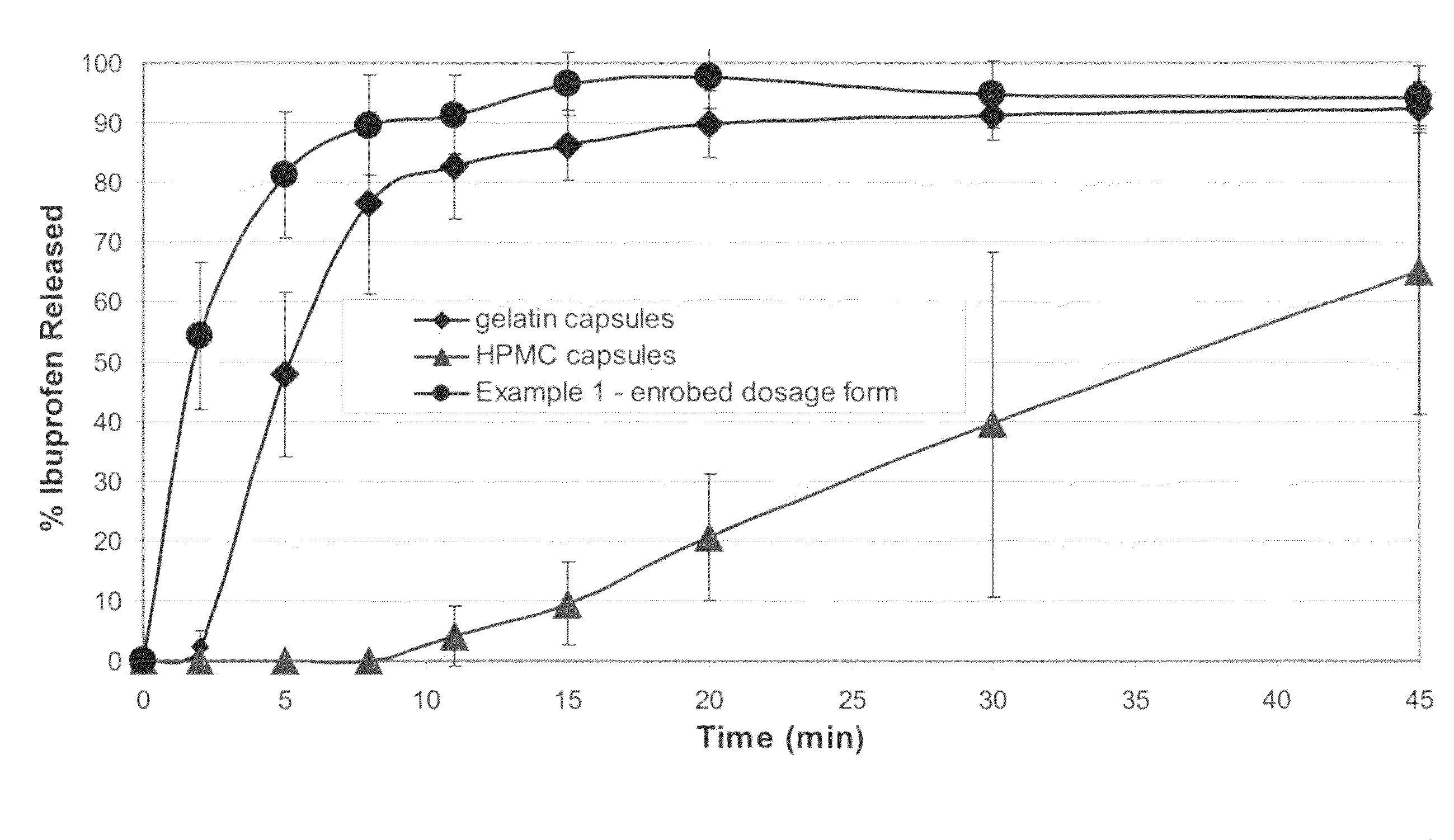
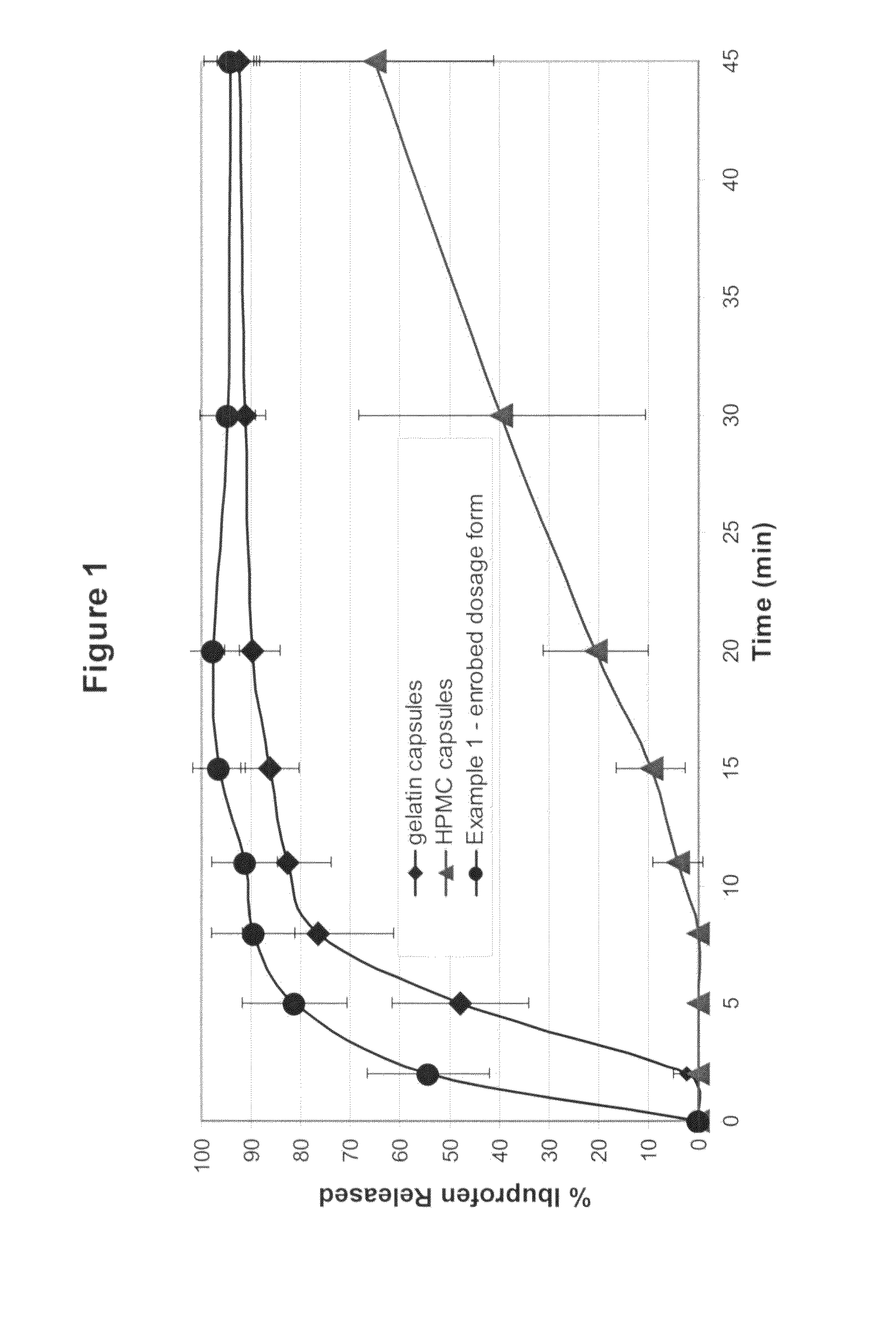
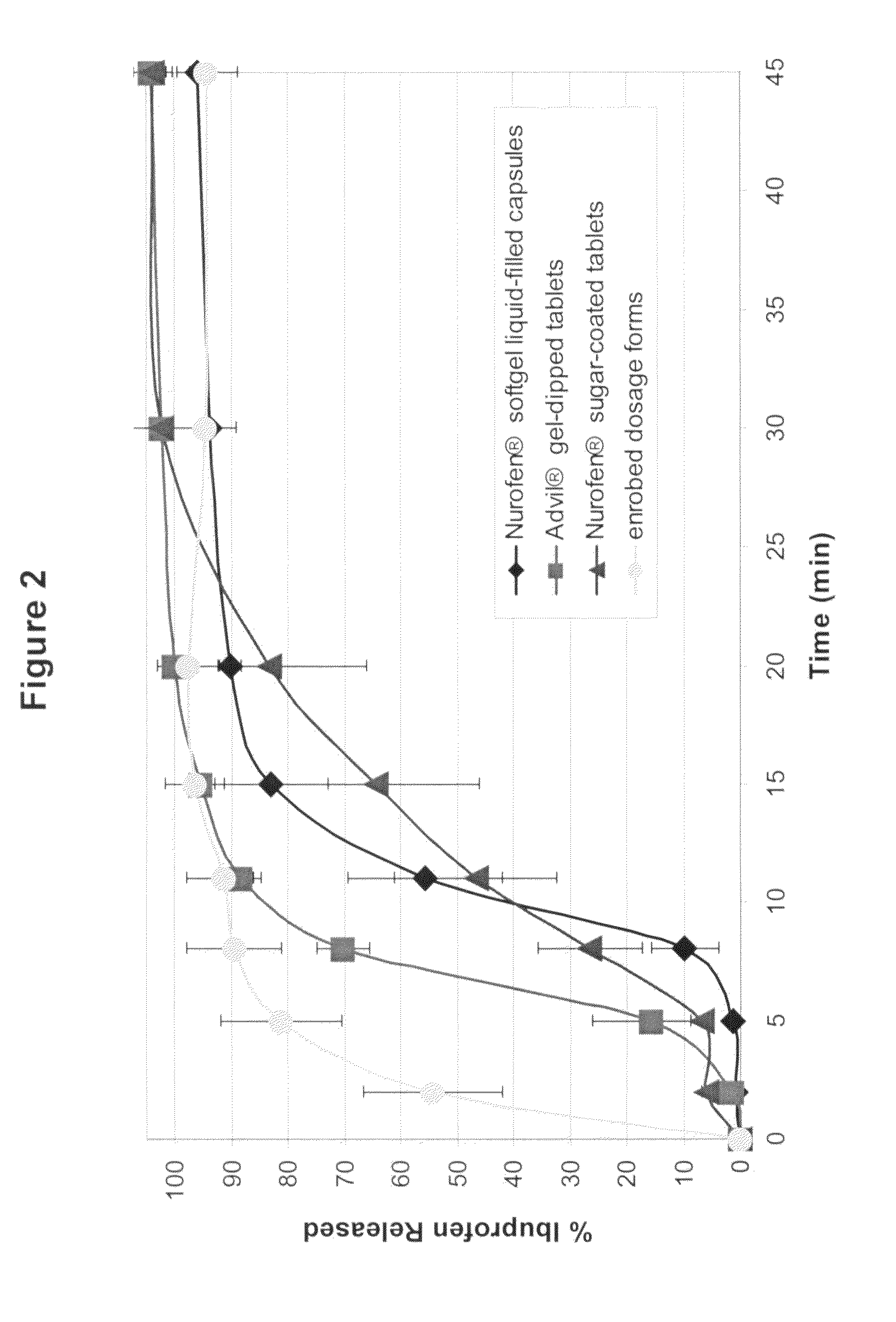
A solid form comprising at least one film enrobing a compacted fill material wherein:i) the compacted fill material comprises at least one active material;ii) the solid form shows a weight loss that is less than 1% during a 30 minutes USP friability test United States Pharmacopeia (USP) 29 Test Number 1216 (page 3046);iii) the compacted fill material has a density of at least 0.5 g / ml based on the total solid volume of the solid form and a tensile strength of less than 0.9 MPa; andiv) the compacted fill material is present in the solid form in at least a first zone and a second zone and the active material is present in at least one of the zones.
Owner:FMC CORP
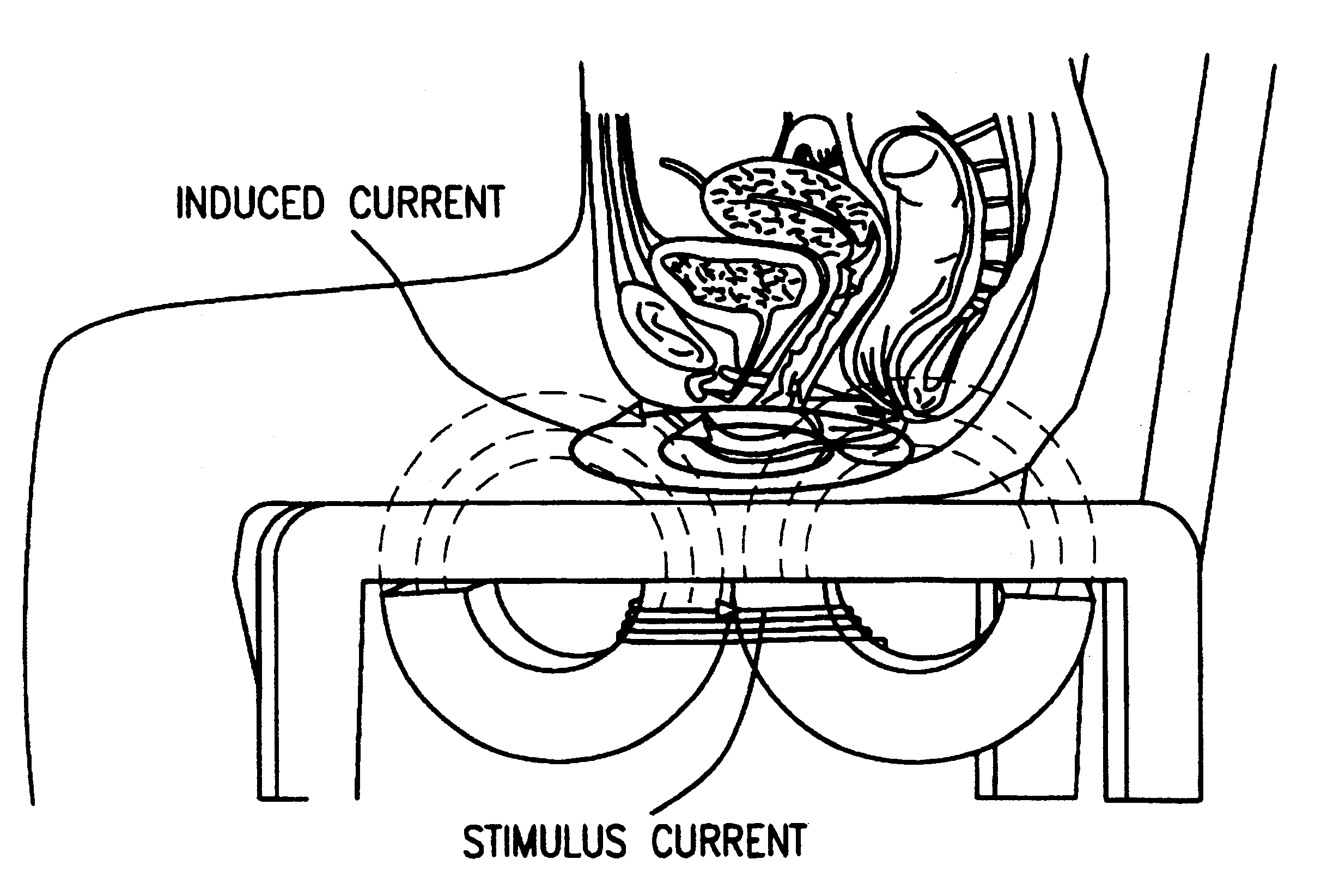
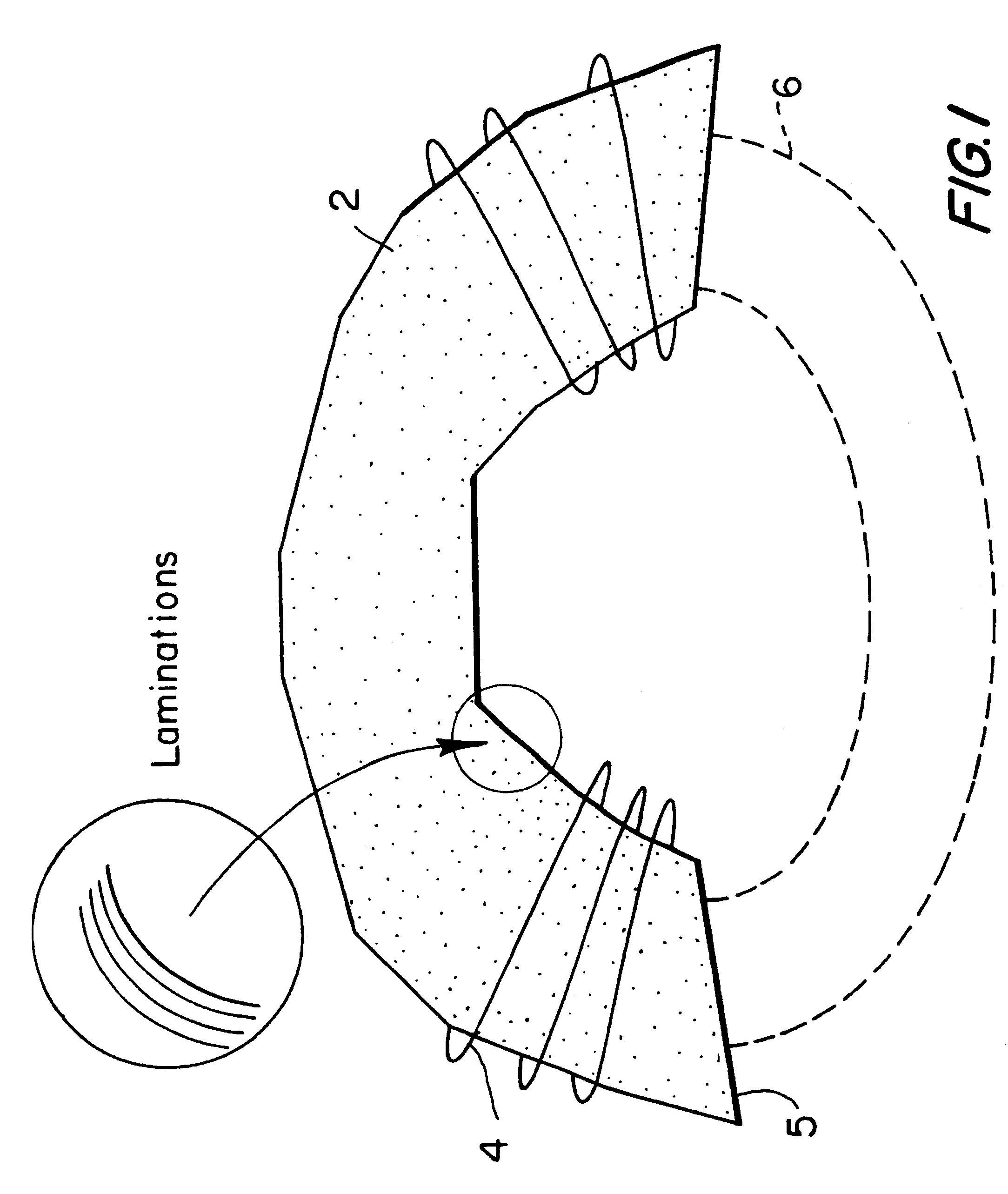
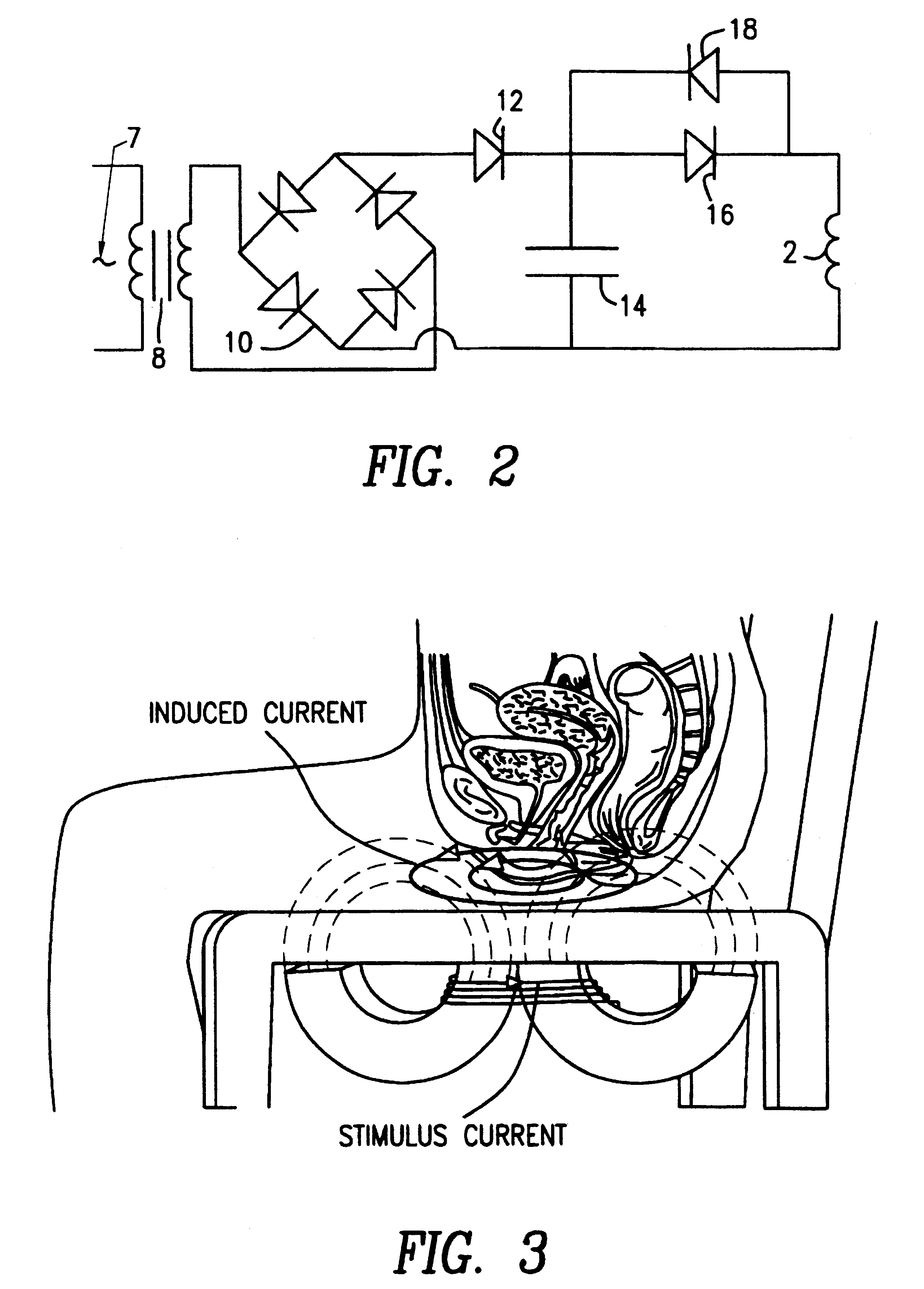
Magnetic nerve stimulation seat device
A magnetic nerve stimulator system is comprised of a core constructed from a material having a high field saturation with a coil winding. A thyrister capacitive discharge circuit pulses the device. A rapidly changing magnetic field is guided by the core, preferably vanadium permendur. For task specific excitation of various nerve groups, specially constructed cores allow for excitation of nerves at deeper levels with higher efficiency than is possible with air-core stimulators. Among the applications possible with this invention are treatment of incontinence, rehabilitation of large muscle groups in the leg and arm, and excitation of abdominal wall muscle groups to aid in weight loss and metabolic rate increase. A C-shape is employed for focussing the stimulation as desired.
Owner:MAGIC RACE
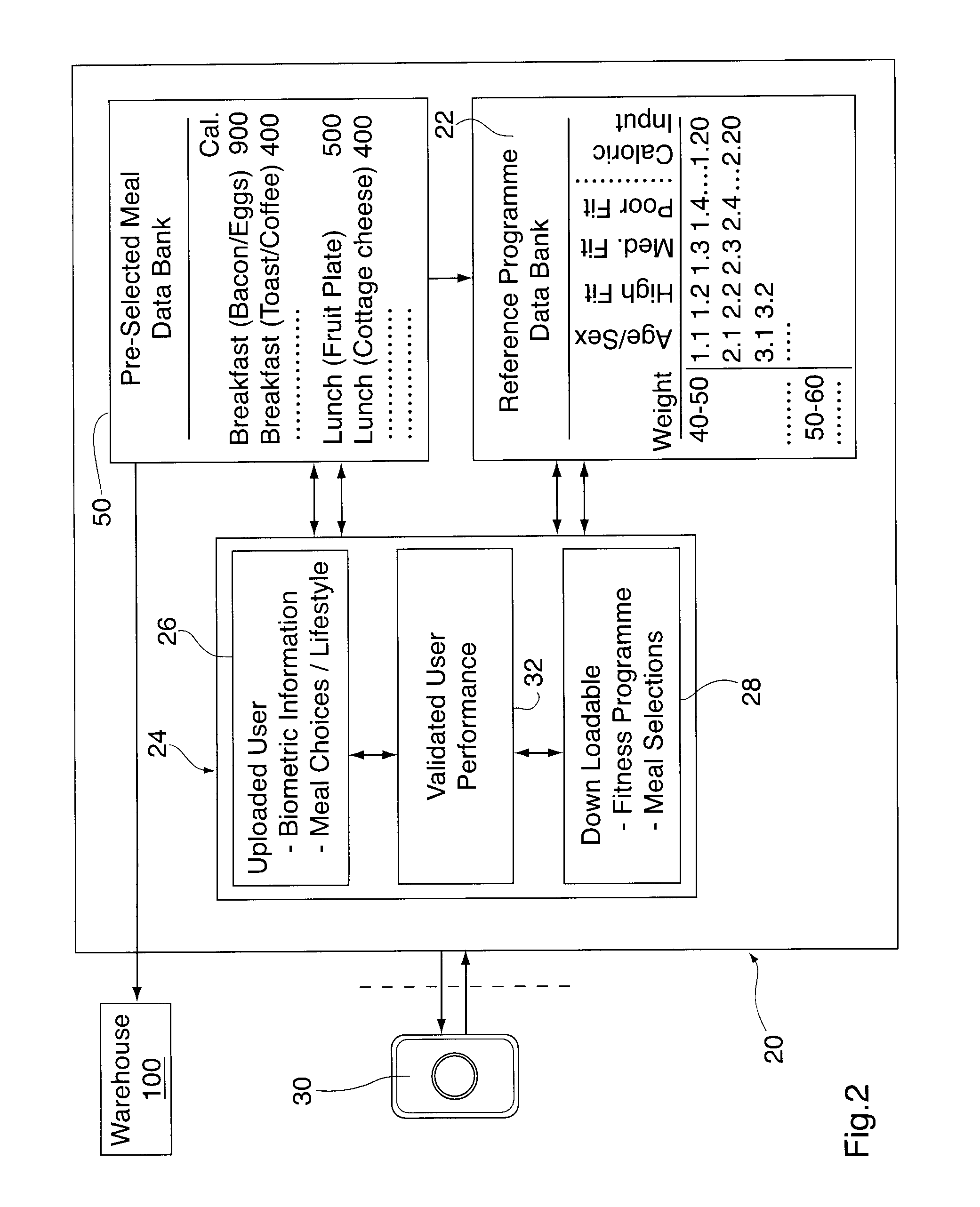
Mobile fitness and personal caloric management system
InactiveUS20110087137A1Continuous monitoringPhysical therapies and activitiesCosmonautic condition simulationsJob descriptionAccelerometer
A user's personal biometric information such as age, sex, weight, height as well as the user's lifestyle information, such as daily caloric input, job description, smoker status and physical fitness, is uploaded onto a host computer. Target heart rate, energy and / or caloric consumption levels related to desired fitness and weight loss goals for a particular individual are then selected having regard to fitness levels for an individual of comparable age, and consuming similar calories are then downloaded to a caloric monitoring unit. The caloric monitoring unit is provided for measuring the user's heart rate and dynamic energy and / or caloric expenditure over one to four weeks. The caloric monitoring unit includes a heart rate monitor, a unit accelerometer, a global positioning system (GPS), and an audio and / or video output. The audio / video output is operable to provide information and / or motivational prompts to the user in the event the heart rate, energy expenditure and / or caloric expenditure falls below or exceeds pre-selected target expenditures over a particular time segment of the selected time period having regard to the calories which are consumed. A display provides a continuously updated visual indication of whether or not the use has achieved the pre-selected optimum caloric burn or energy expenditure for that particular time segment. An internal calendar / clock, a processor and / or memory in the caloric monitoring unit compares measured heart rate and energy expenditures for multiple time segments against target levels stored as the user-specific fitness programme tailored to achieve the desired weight loss. The comparison is then used to generate compliance output data to either the user and / or a nutritionist.
Owner:SALIENT IMAGING
Phytochemicals for promoting weight loss
New dietary supplement compositions are disclosed that comprise the phytochemical Diindolylmethane (DIM), as well as its precursor, Indole-3-carbinol (I3C), and cogener, 2-(Indol-3-ylmethyl)-3,3' diindolylmethane (LTR-1), dietary supplement acceptable carriers and / or excipients. The use of these dietary supplement compositions facilitate weight loss as part of a nutritional system targeting release and metabolism of stored fat.
Owner:BIORESPONSE
Solid form
InactiveUS20080014228A1Reduce deliveryImprove robustnessCosmetic preparationsNervous disorderFilling materialsImmediate release
An enrobed solid form comprising a film enrobing a compacted fill material having at least one active material, the solid form shows a weight loss that is less than 1% during a 30 minutes USP Friability Test, the fill material having a density of at least 0.5 g / ml based on the total solid volume of the solid form and a tensile strength less than 0.9 MPa, and the at least one active material within the solid form has an immediate release profile. The solid form is useful in effective delivery of high dose levels of active material.
Owner:FMC CORP
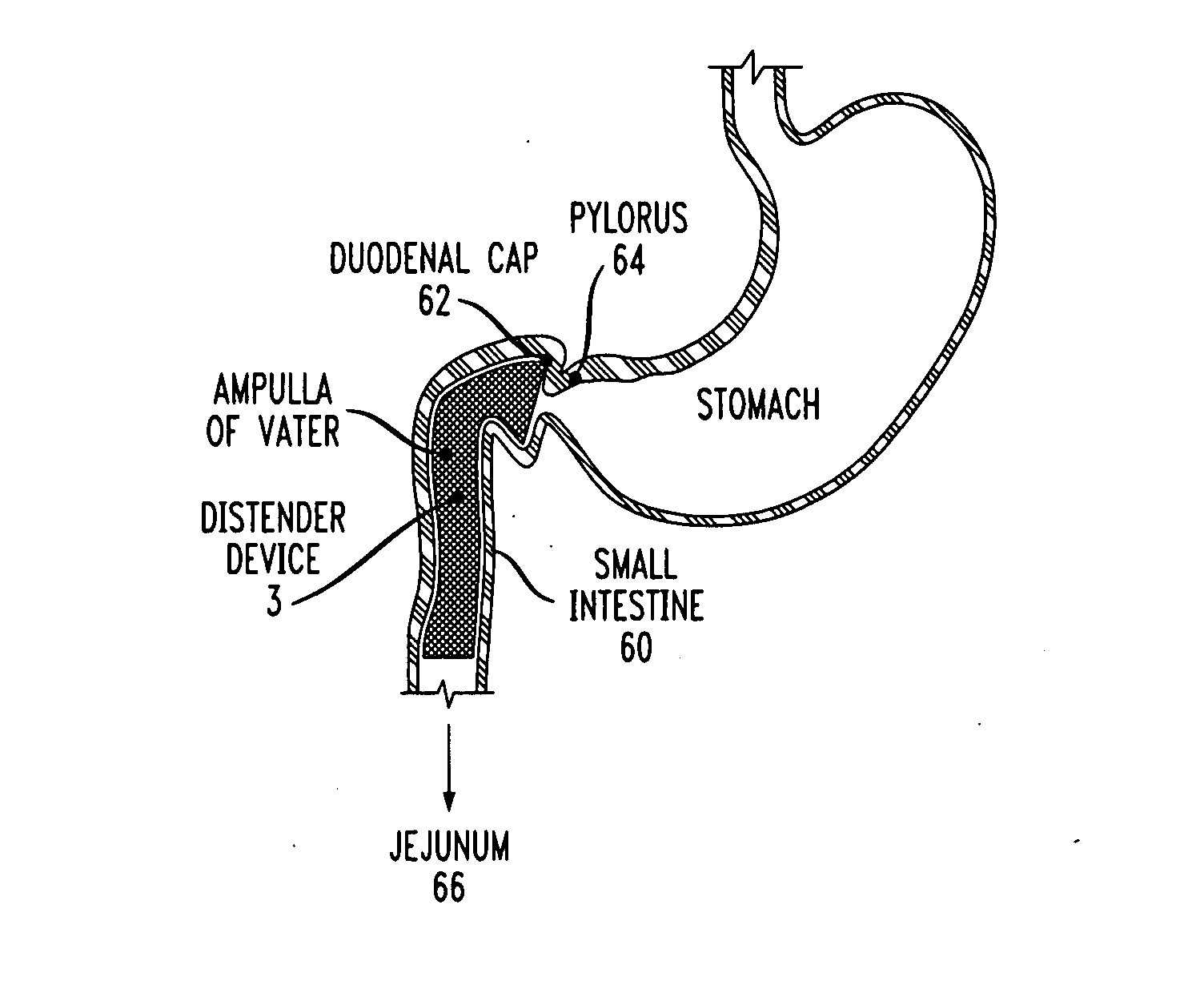
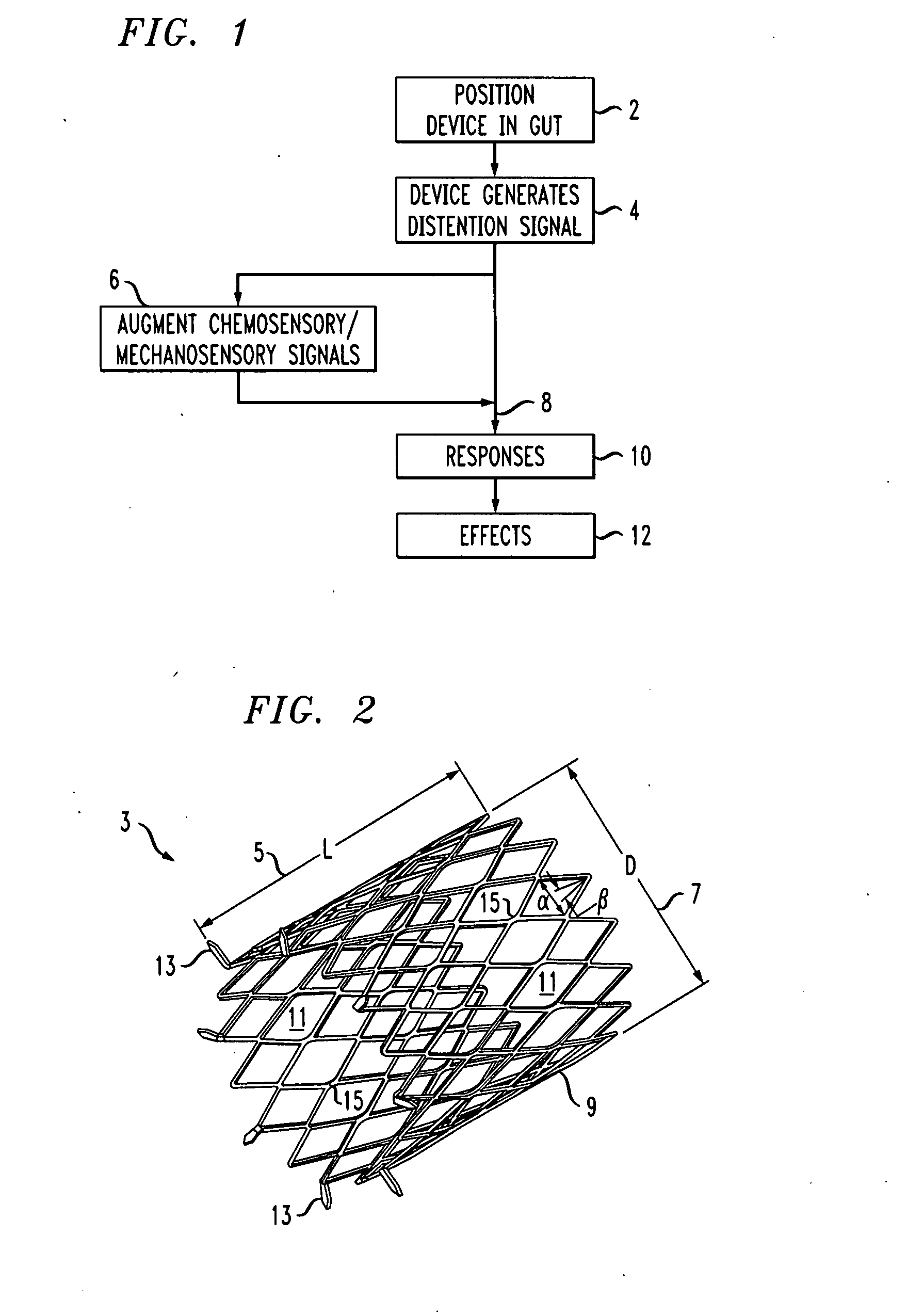
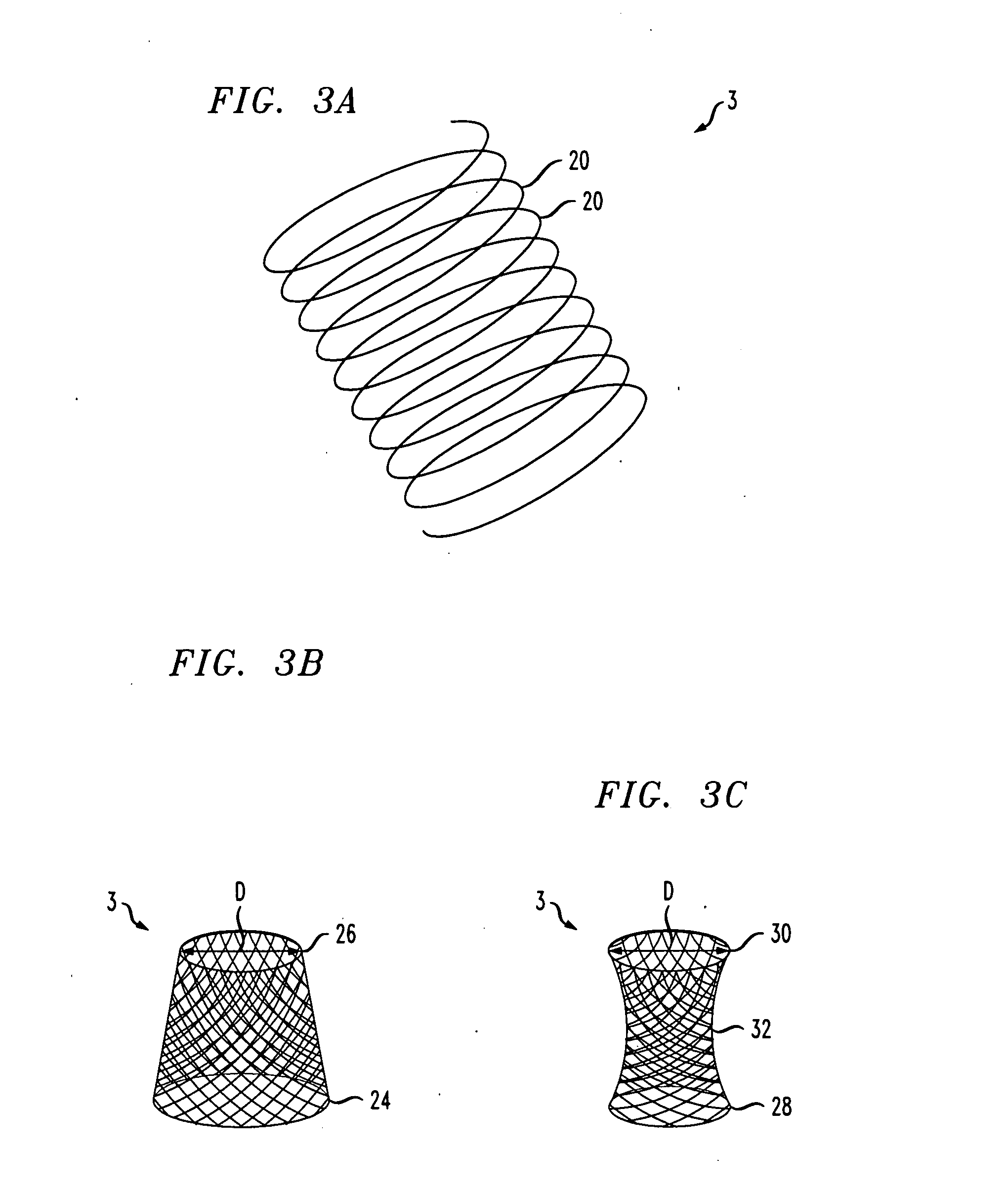
Distender device and method for treatment of obesity and metabolic and other diseases
A gastrointestinal implant device is positioned in a patient's small intestine or rectum and produces an outward force that itself produces a distension signal which is a therapeutically useful neural or humoral signal that evokes satiogenic or weight loss effects by itself. The device may advantageously be placed in the duodenum adjacent the pylorus or in the jejunum, ileum or rectum. The distension signals may amplify chemosensory or mechanosensory signals such as enteroendocrine secretions within the patient. The device may be a mesh and include a low material density that allows for unrestricted chyme absorption within the small intestine and unrestricted chyme flow through the gastrointestinal system. A method includes inserting the device into the patient then either retrieving the device after treatment is complete or allowing a device formed of a biodegradable material to degrade in time after treatment is complete.
Owner:ADVANCED NEUROMODULATION SYST INC
Strawberry-flavor peony compound beverage and preparation method thereof
InactiveCN103960721AThe proportion is scientific and reasonableEliminate toxins from the bodyNatural extract food ingredientsFood preparationAdditive ingredientNutrient content
The invention relates to the field of beverages compounded by fruits and vegetables as well as flowering tea and preparation methods thereof, and provides a strawberry-flavor peony compound beverage and a preparation method thereof. The compound beverage is formed mainly by strawberries, white gourds, mung beans and a mixed extracting solution of peonies and greenish lily flowers in proportion, wherein the mixed extracting solution of peonies and greenish lily flowers is obtained in the manner that an ethanol solution with the concentration of 90 percent is adopted to be added with tartaric acid used as an extracting agent for extraction; the preparation method comprises the following steps: strawberries and white gourds are respectively juiced for standby use, then the juiced strawberries and white gourds are mixed with ground mung bean milk and the mixed extracting solution of peonies and greenish lily flowers, then high fructose corn syrup, citric acid and potassium sorbate are added into the mixture, and homogenizing, degassing, sterilization and filling are performed, so that finished products are obtained. The compound beverage provided by the invention is complete in nutrition and delicious in taste, greatly reserves respective nutritional ingredients, and has the health functions of preventing hypertension and arteriosclerosis and weight loss.
Owner:LUOYANG CHUNKUI AGRI DEV
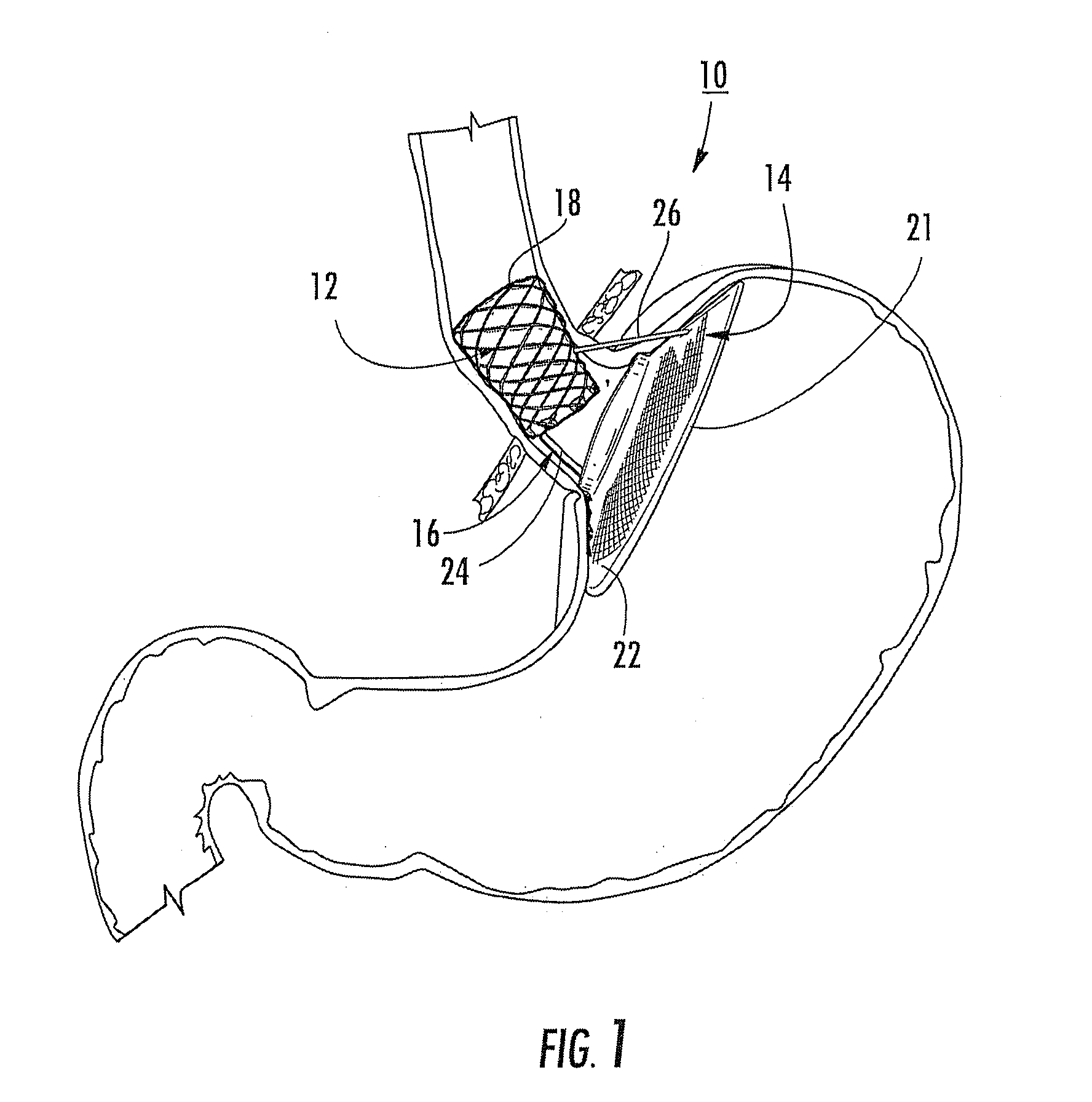
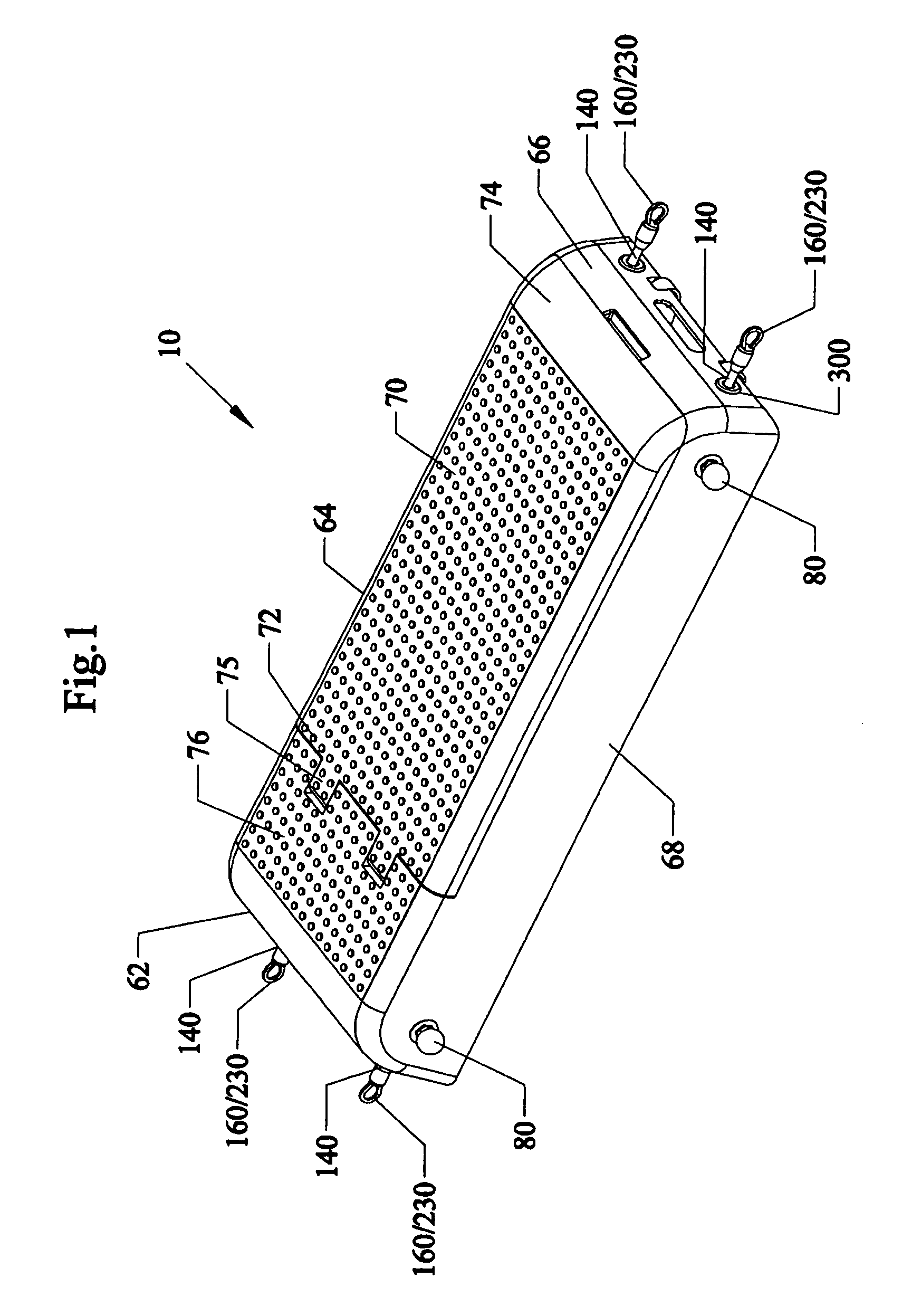
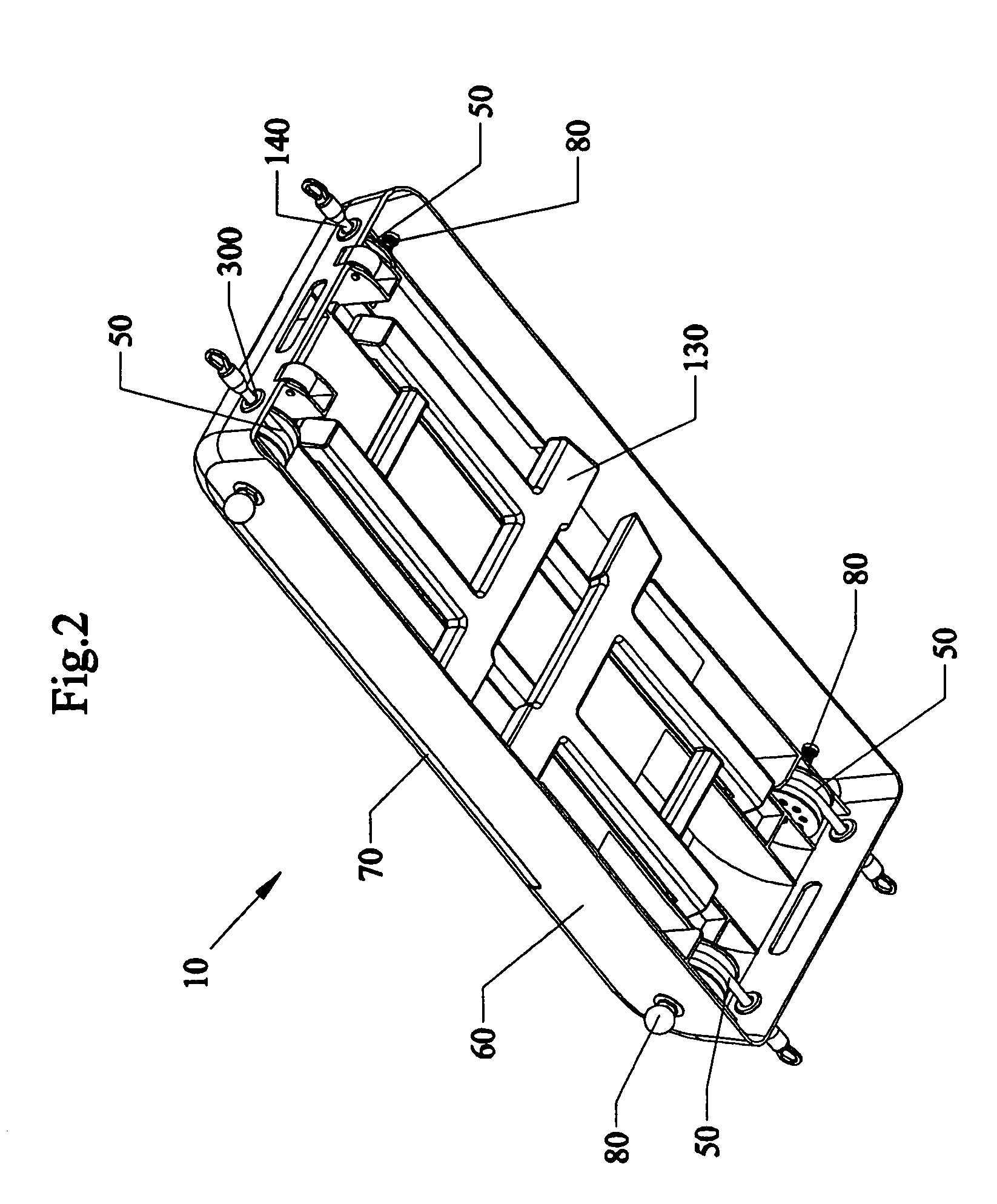
Bariatric device and method
ActiveUS20100030017A1Effective and invasive mannerEffective and minimally invasiveSuture equipmentsDilatorsCardiac surfaceHeart Part
A bariatric device and method of causing weight loss in a recipient includes providing a bariatric device having an esophageal member, a cardiac member and a connector connected with the esophageal member and the cardiac member. The esophageal member has an esophageal surface that is configured to generally conform to the shape and size of a portion of the esophagus. The cardiac member has a cardiac surface that is configured to generally conform to the shape and size of a portion of the cardiac portion of the stomach. The esophageal surface is positioned at the esophagus. The cardiac surface is positioned at the cardiac portion of the stomach. The bariatric device stimulates receptors in order to influence a neurohormonal mechanism in the recipient.
Owner:BFKW
Pro-neurogenic compounds
This technology relates generally to compounds and methods for stimulating neurogenesis (e.g., post-natal neurogenesis, including post-natal hippocampal and hypothalamic neurogenesis) and / or protecting neuronal cell from cell death. Various compounds are disclosed herein. In vivo activity tests suggest that these compounds may have therapeutic benefits in neuropsychiatric and / or neurodegenerative diseases such as schizophrenia, major depression, bipolar disorder, normal aging, epilepsy, traumatic brain injury, post-traumatic stress disorder, Parkinson's disease, Alzheimer's disease, Down syndrome, spinocerebellar ataxia, amyotrophic lateral sclerosis, Huntington's disease, stroke, radiation therapy, chronic stress, abuse of a neuro-active drug, retinal degeneration, spinal cord injury, peripheral nerve injury, physiological weight loss associated with various conditions, as well as cognitive decline associated with normal aging, chemotherapy, and the like.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Satiation devices and methods
A device for inducing weight loss in a patient includes a tubular prosthesis positionable at the gastro-esophageal junction region, preferably below the z-line. In a method for inducing weight loss, the prosthesis is placed such that an opening at its proximal end receives masticated food from the esophagus, and such that the masticated food passes through the pouch and into the stomach via an opening in its distal end.
Owner:BAROSENSE
Gut microbiome as a biomarker and therapeutic target for treating obesity or an obesity related disorder
Owner:WASHINGTON UNIV IN SAINT LOUIS
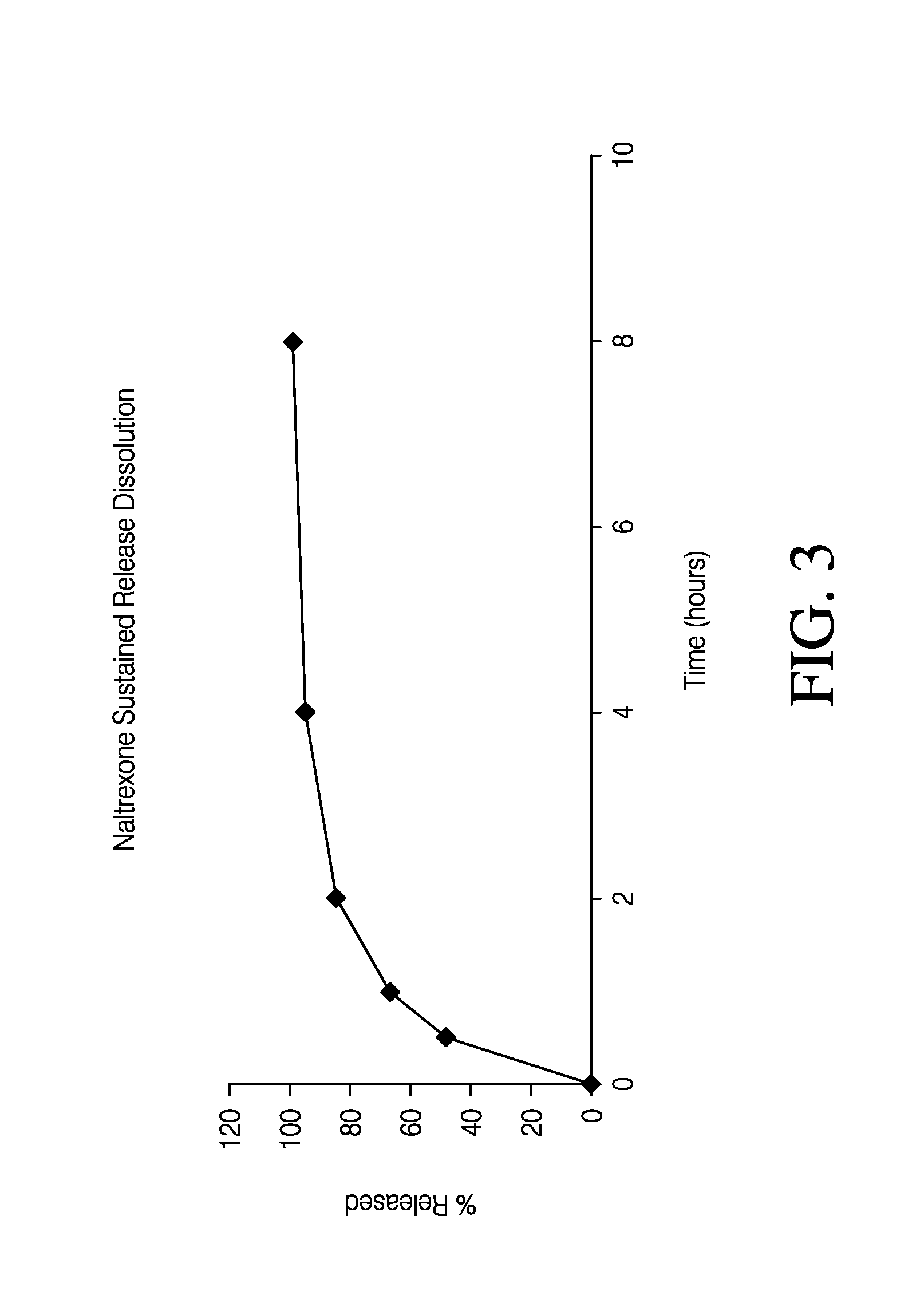
Sustained release formulation of naltrexone
ActiveUS20070281021A1Cause weight lossAvoid weight gainBiocideKetone active ingredientsSide effectCompound (substance)
A sustained-release oral dosage form of naltrexone or a pharmaceutically acceptable salt thereof is provided. The oral dosage form may be administered with another compound. Administration of the oral dosage form may reduce a side effect, which may be a side effect at least partially attributable to a weight-loss treatment. The oral dosage form may be administered to treat a weight-loss condition.
Owner:OREXIGEN THERAPEUTICS INC
Method and system for gastric ablation and gastric pacing to provide therapy for obesity, motility disorders, or to induce weight loss
InactiveUS20050240239A1Avoid side effectsProviding therapyUltrasound therapyInternal electrodesCardiac pacemaker electrodeMotility
Method and system to provide therapy for obesity, gastric motility, or to induce weight loss comprises ablating the gastric tissue around the “pacemaker” region of the stomach, and electrically pacing the stomach with a pulse generator / stimulator to control the electrical activity of the gastric muscle. The ablation to the gastric tissue may be from the epigastric side, or may be from inside the stomach. The ablation may be performed utilizing any one of: radiofrequency catheter ablation; radiofrequency catheter ablation using an irrigated tip catheter; microwave ablation; cryoablation; high intensity focused ultrasound (HIFU) ablation; and laser ablation. The ablation of the “pacemaker” region of the stomach may be partial or complete. A gastric pulse generator / stimulator is implanted to provide electrical pulses to the stomach. The function of the gastric stimulator after complete ablation of the pacemaker region, is to provide a basic electrical rhythm (BER) to regulate and control electrical activity of the stomach. Alternatively, if partial ablation is performed the function of the gastric pulse generator / stimulator is to enhance the residual basic electrical rhythm (BER), or to interfere with the residual basic electrical rhythm (BER).
Owner:BOVEJA BIRINDER R +1
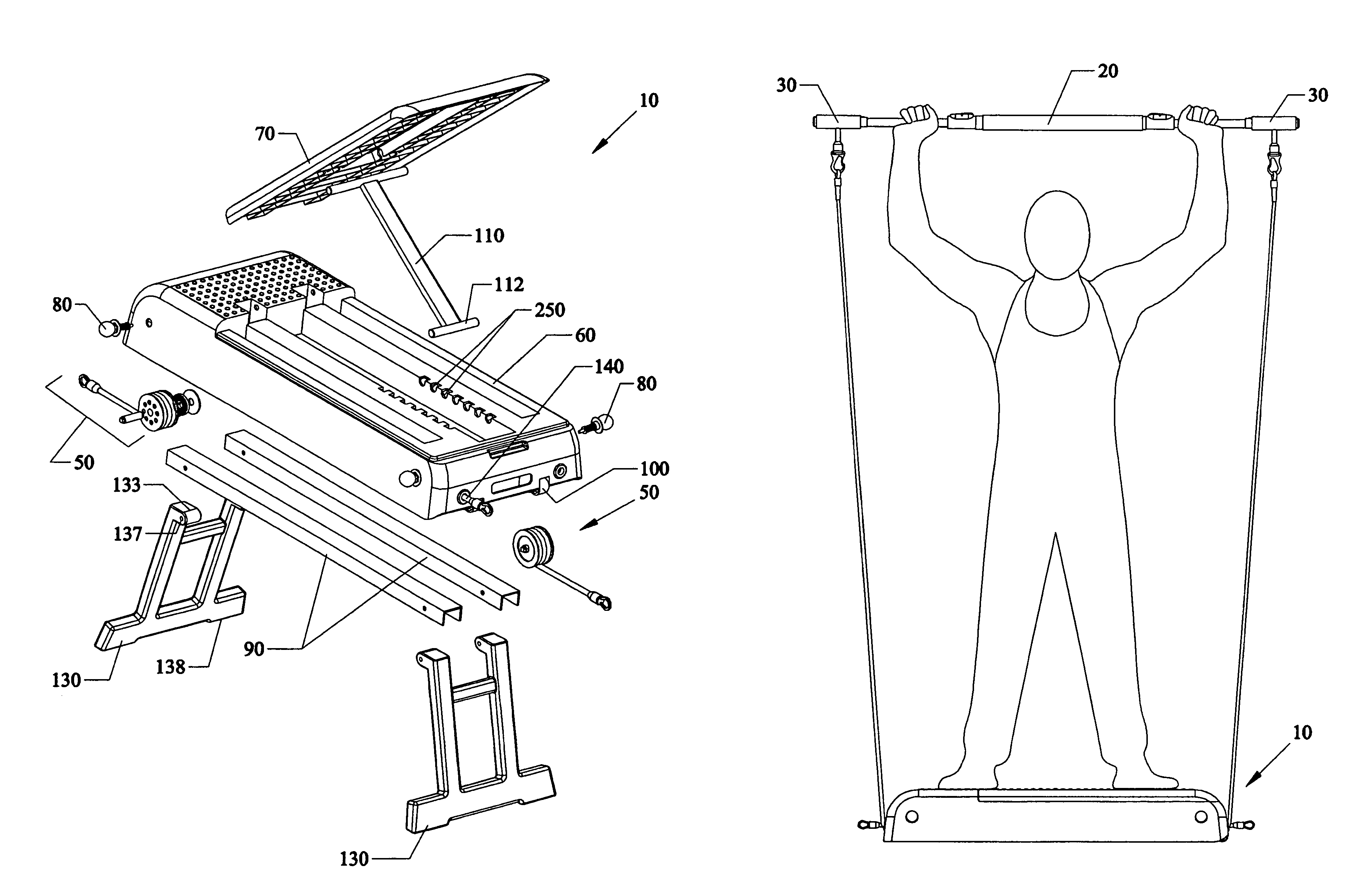
Portable convertible multifunction exercise apparatus and method
InactiveUS7591763B1Low costGuaranteed rapid successionResilient force resistorsStiltsWhole bodyMuscle group
Portable, convertible and multifunction exercise apparatus, devices, systems and methods of using that allows individuals to accomplish their fitness, health conditioning, weight loss and rehabilitation goals with a single platform having selectively length adjustable resistance bands. Rotatable spring biased wheels can lock the bands to different lengths, where the longer the length the less resistance, and the shorter the length the more the resistance. The platform can transform from a stepper into a bench to an incline seat with foldable legs. Contained within the platform storage unit are several bars handles and leg attachments. An exercise bar having a rotatable midportion can have handle grip ends removably attachable to the bands and / or the bar so that a variety of additional exercises can be performed allowing the user to exercise all muscle groups for a total body workout.
Owner:ZHANG CHAO
Medical apparatus and method of making the same
The invention relates to a medical apparatus including a device used in the treatment of weight loss, obesity and potentially other associated health problems, e.g., type II diabetes. The device is used to impede absorption of nutrients within the gastrointestinal tract, i.e., bypassing a portion of the gastrointestinal tract. The medical apparatus enables implantation of the device using minimally invasive techniques, such a transesophageal approach under visualization. The device may be implanted via a working channel of a medical scope, e.g., an endoscope or in combination with a medical scope.
Owner:WL GORE & ASSOC INC
Injectable hollow tissue filler
The present invention comprises a plurality of injectable hollow particulate fillers suspended in a biocompatible fluid carrier to significantly improve the clumping resistance and injectability of the composition. The hollow particulate fillers have a lower effective density and are able to suspend in the carrier without precipitation. The loss of skin volume as a result of aging, diseases, weight loss, and injury can lead to uneven skin surface (e.g. wrinkle, etc.). The uneven skin can be repaired by injecting appropriate amount of hollow fillers underneath the skin. Some cases of urinary incontinence occur when the resistance to urine flow has decreased excessively. Continence is restored by injecting the present invention to the urethra tissue to increase resistance to urine outflow. Similarly, the present invention allows for the control of gastric fluid reflux by submucosal injections of the fillers to the esophageal-gastric and gastric-pyloric junction. For patients with vesicoureteral reflux, it can be treated by injection of the present invention into patients' ureteral tissue. This invention can also be used to repair defective or inadequately functioning muscles of the anal sphincter by administering an effective amount of injectable hollow fillers into the defect or anal sinuses.
Owner:CHU JACK FA DE
Chemotherapy treatment
InactiveUS20030040478A1Improve welfareImprove survivalBiocideOrganic active ingredientsRegimenApoptosis
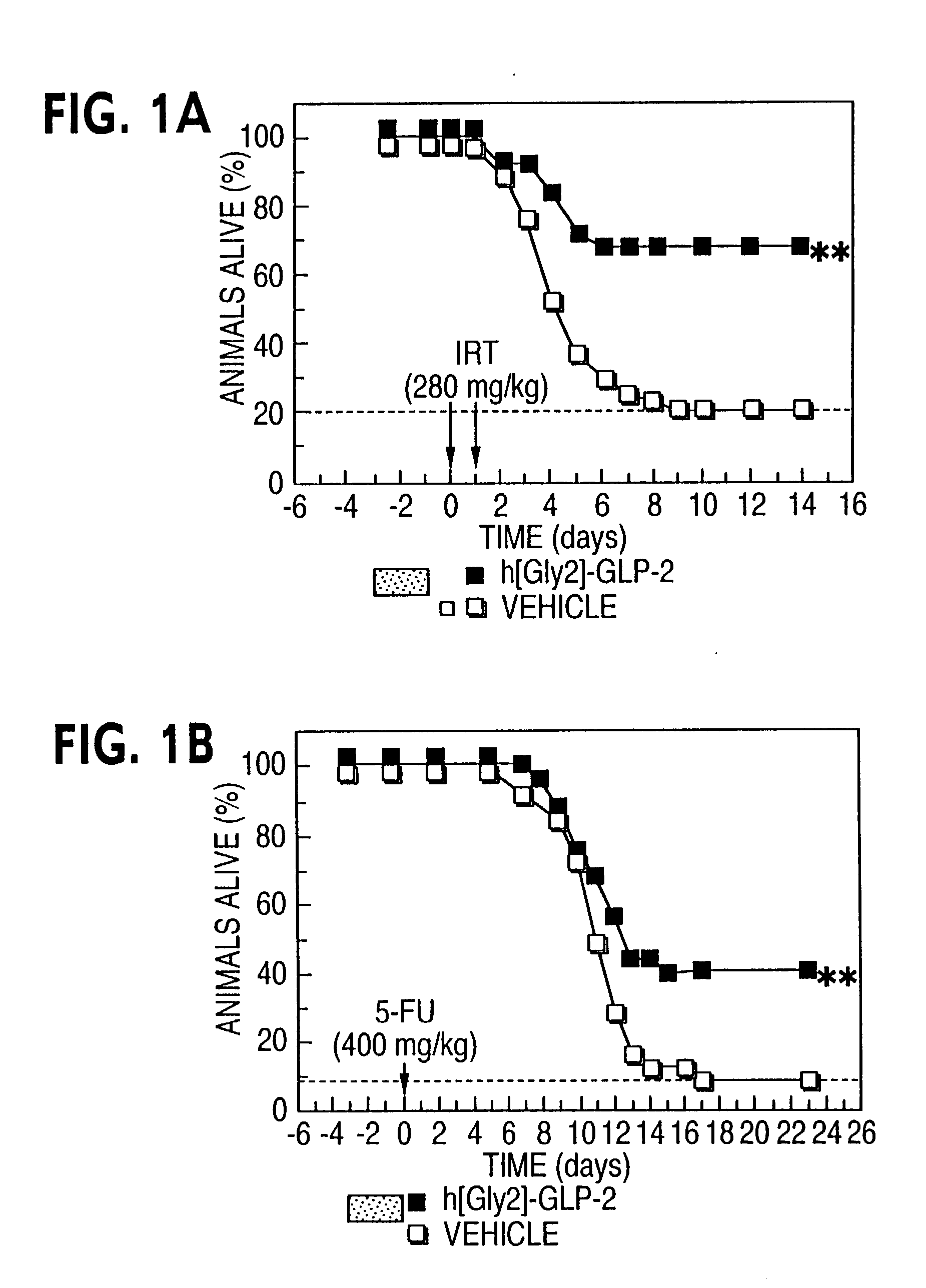
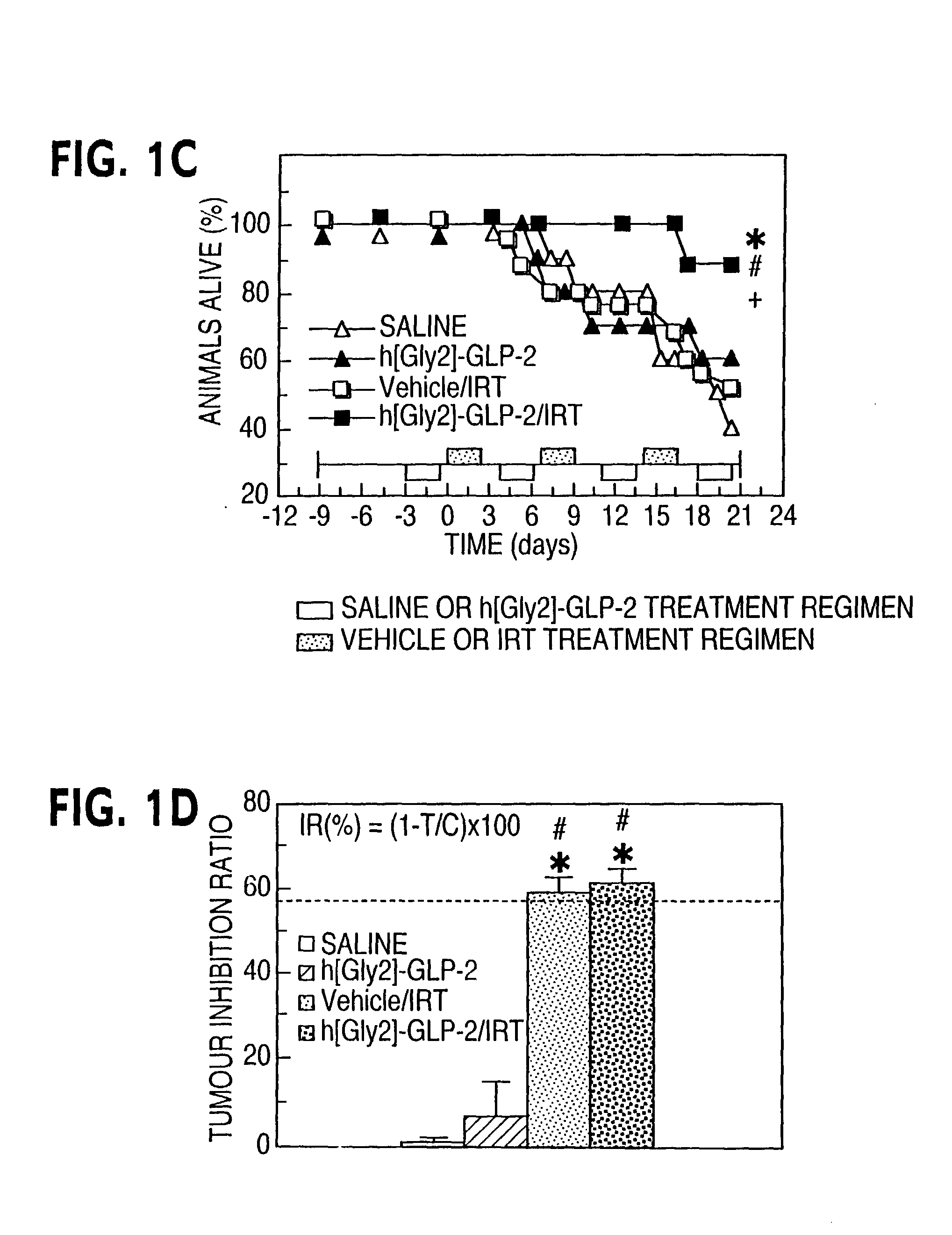
This invention provides a treatment regimen that is effective in inhibiting chemotherapy-induced apoptosis and promoting cell survival. The invention also relates to a treatment regimen that confers resistance to caspase activation, thereby inhibiting caspase-mediated, proteolytic cleavage of functional cellular enzymes. Specifically, subjects undergoing chemotherapy are first exposed to a pretreatment regimen. Under this regimen, a GLP-2 receptor activator, such as h[GLY2]-GLP2, is administered each day for a predetermined beneficial period, e.g., three consecutive days. Approximately about 1 week following pretreatment, the subjects are exposed to an appropriate chemotherapy treatment regimen. Pretreatment with a GLP-2 receptor activator followed by administration of chemotherapeutic agents improves cell survival, reduces bacteremia, attenuates epithelial injury, and inhibits cellular apoptosis. Moreover, it does not impair the effectiveness of chemotherapy nor result in weight loss. The anti-apoptotic effects of GLP-2 may be useful in the reduction of cytoxicity and bacterial infection induced by chemotherapeutic agents.
Owner:1149336 ONTARIO
Ultra-high fiber supplement and method of reducing weight cardiovascular risks and ingested toxins.
Owner:SMALL GIANT
Compositions for affecting weight loss
Disclosed are compositions for affecting weight loss comprising a first compound and a second compound, where the first compound is an antidiabetic and the second compound is a anticonvulsant. Also disclosed are methods of affecting weight loss, increasing energy expenditure, increasing satiety in an individual, or suppressing the appetite of an individual, comprising identifying an individual in need thereof and treating that individual with an antidiabetic and an anticonvulsant.
Owner:KRISHNAN RANGA +1
Modulation of fiaf and the gastrointestinal microbiota as a means to control energy storage in a subject
Owner:WASHINGTON UNIV IN SAINT LOUIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com