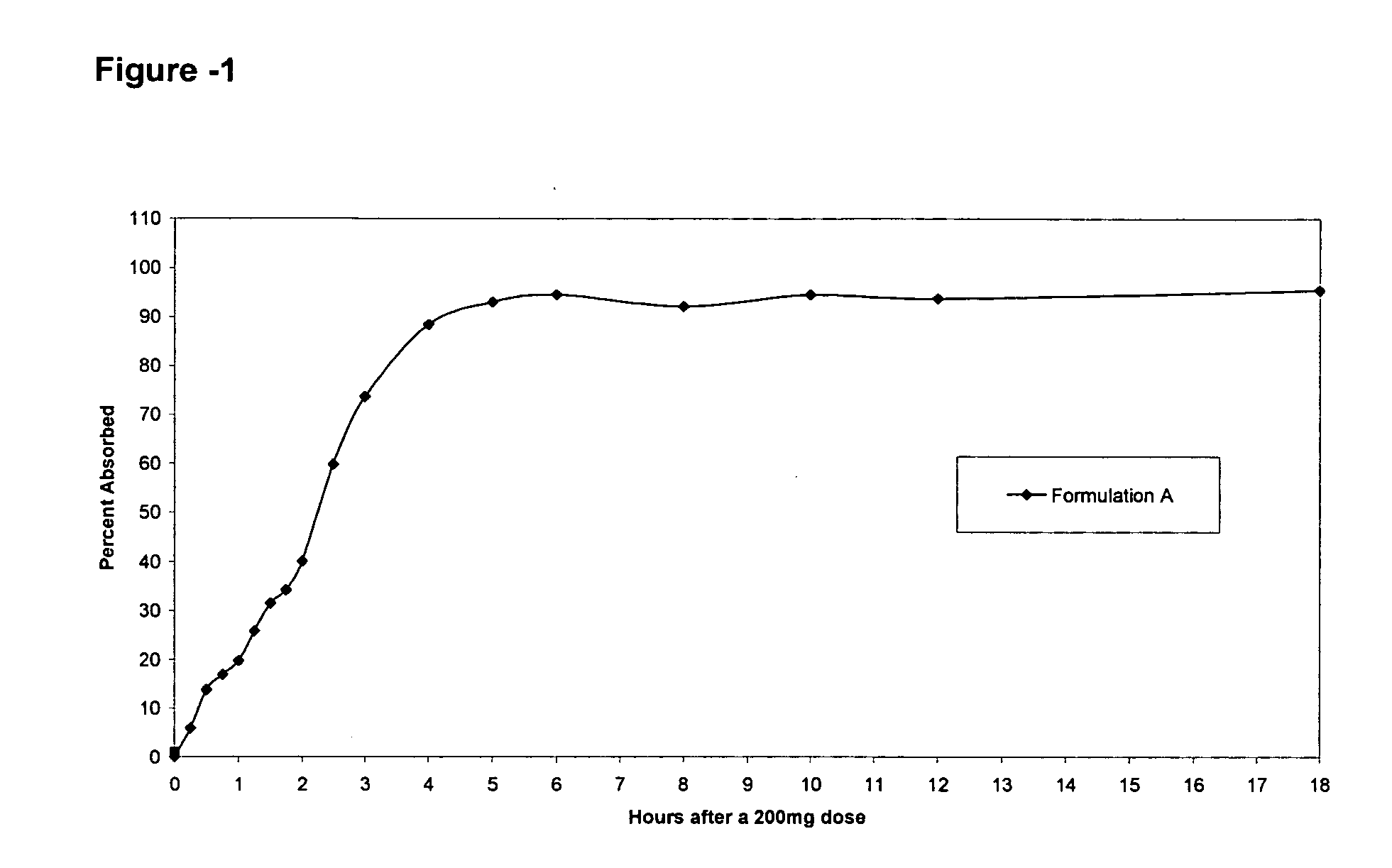
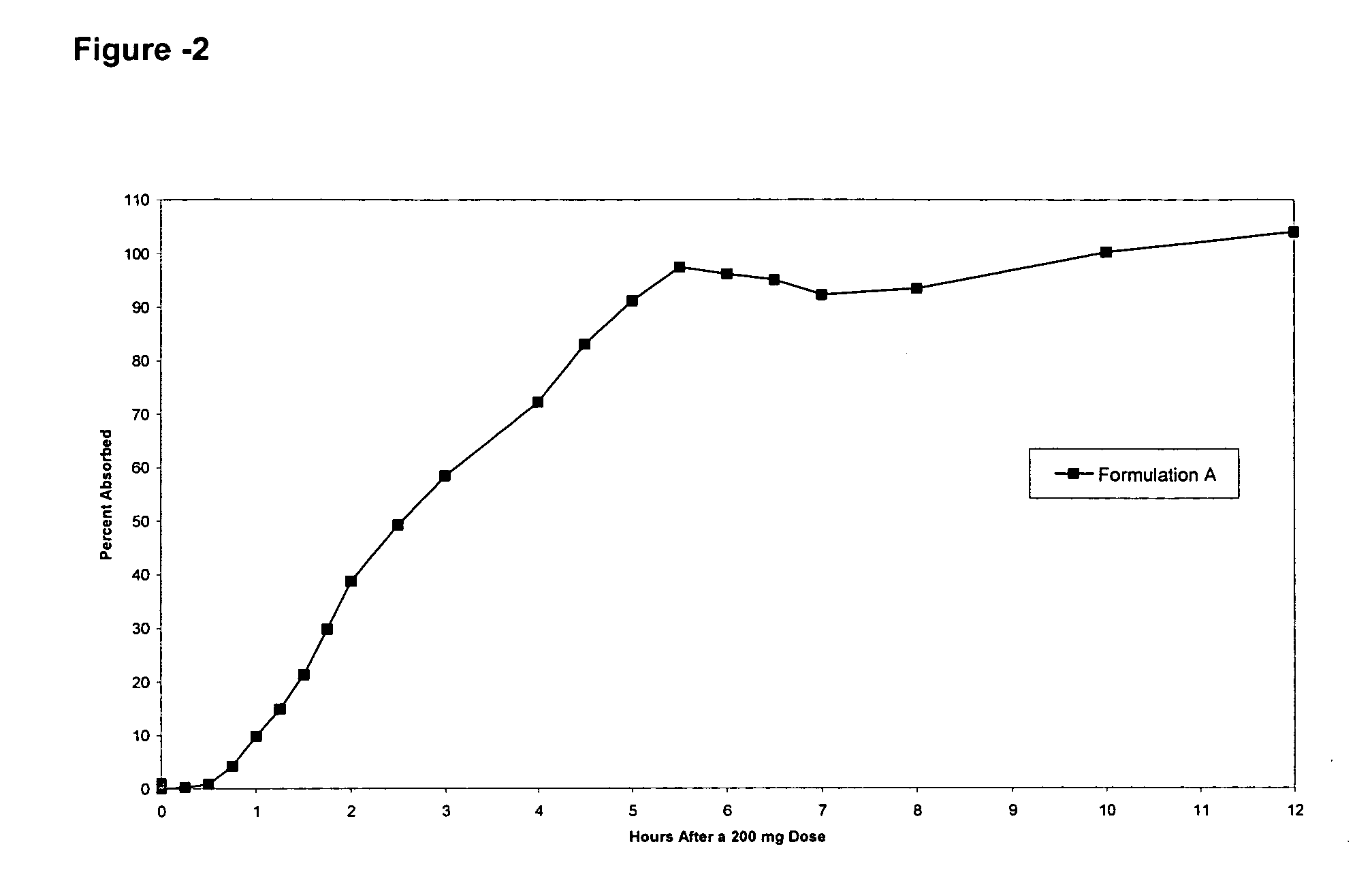
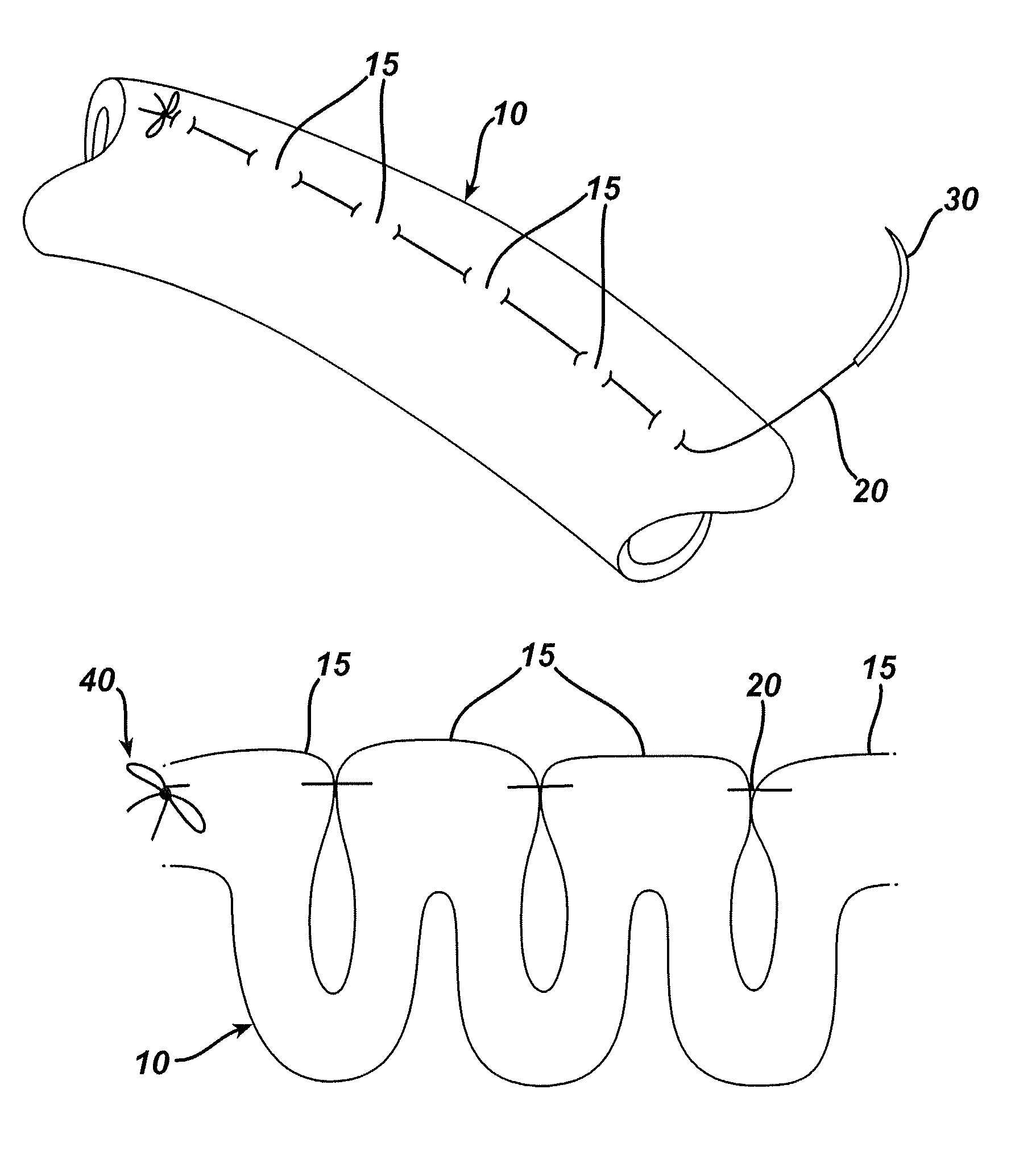
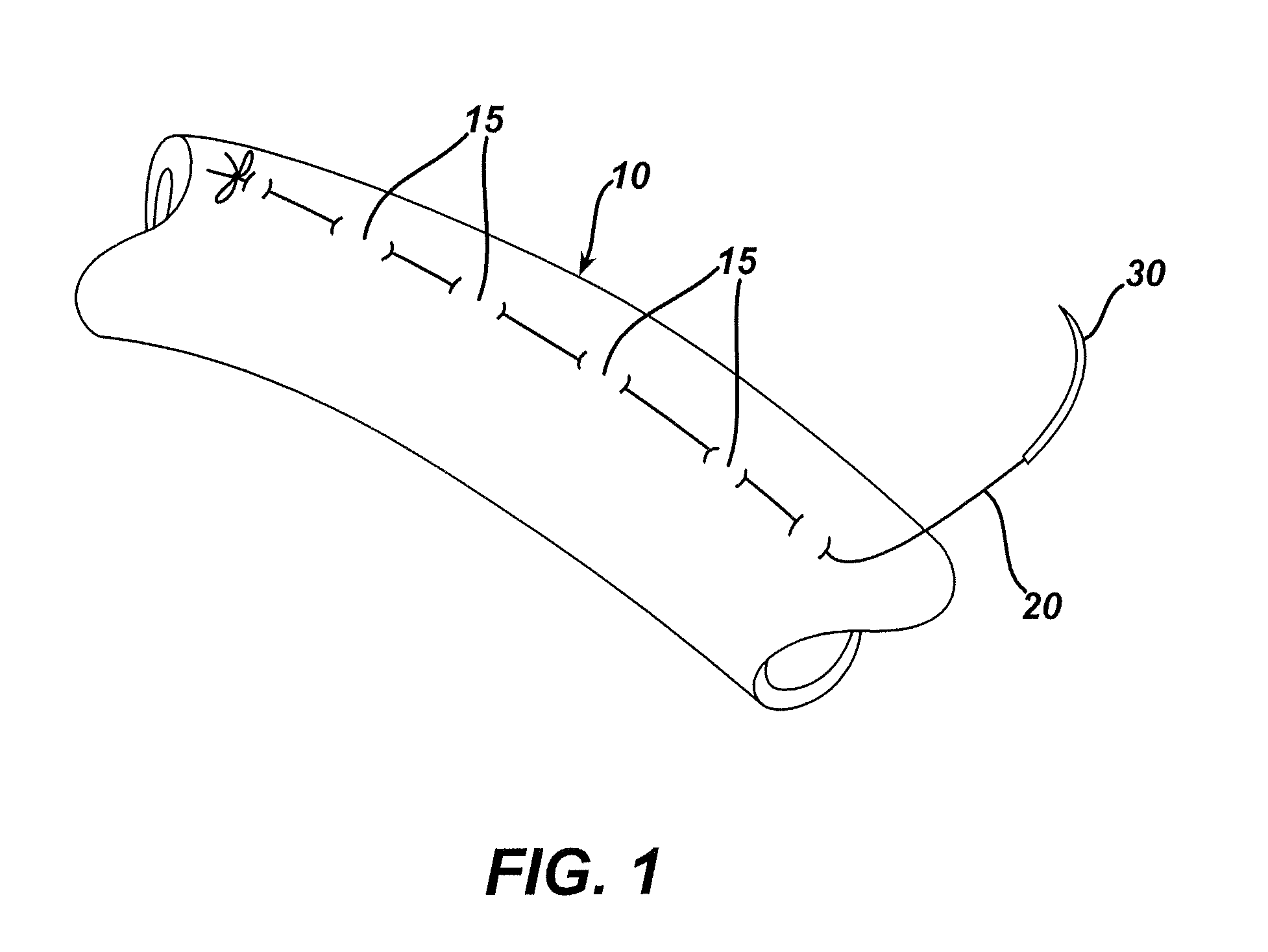
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
112 results about "Ileum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
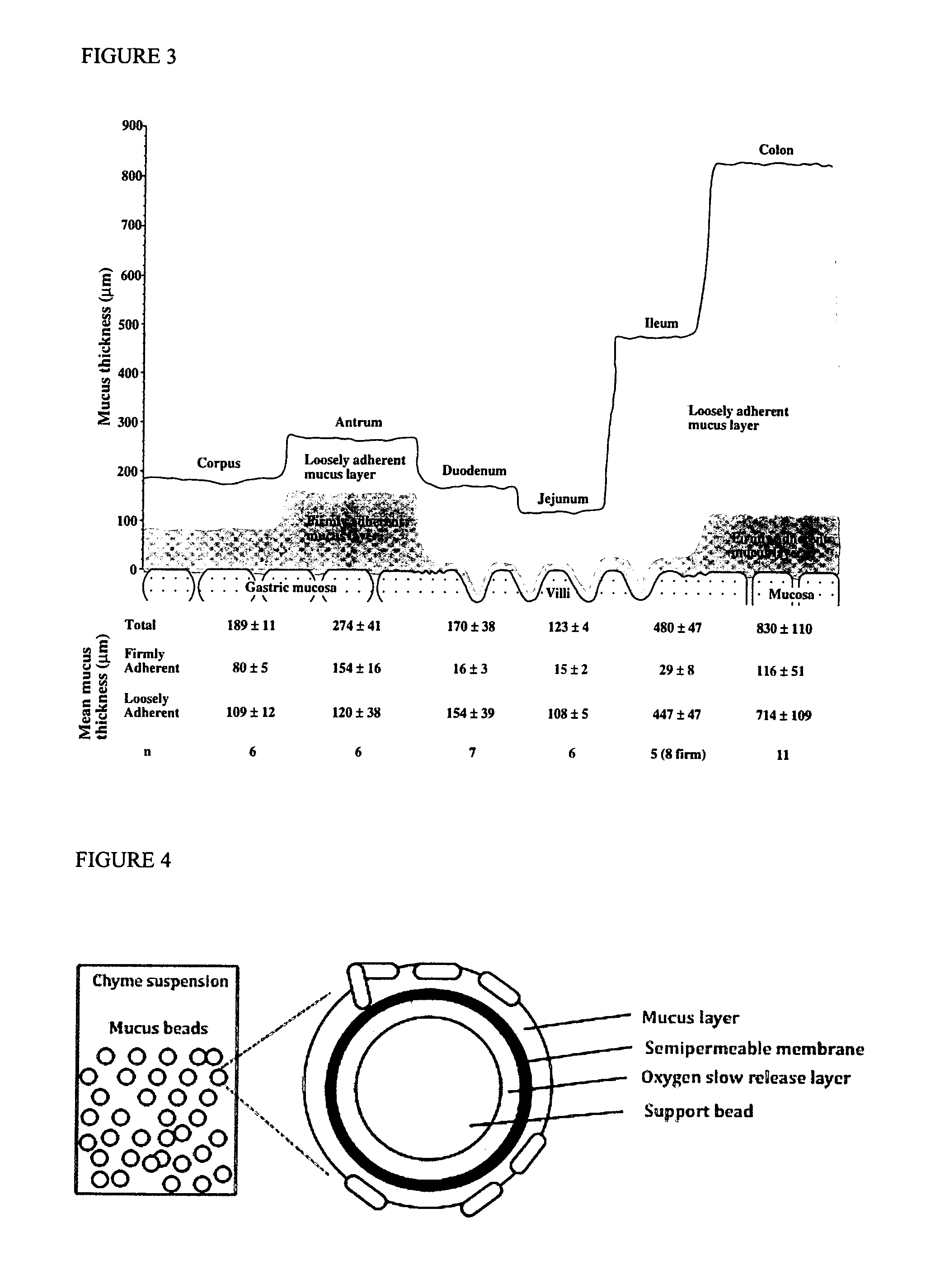
The ileum /ˈɪliəm/ is the final section of the small intestine in most higher vertebrates, including mammals, reptiles, and birds. In fish, the divisions of the small intestine are not as clear and the terms posterior intestine or distal intestine may be used instead of ileum. Its main function is to absorb vitamin B₁₂, bile salts, and whatever products of digestion were not absorbed by the jejunum.
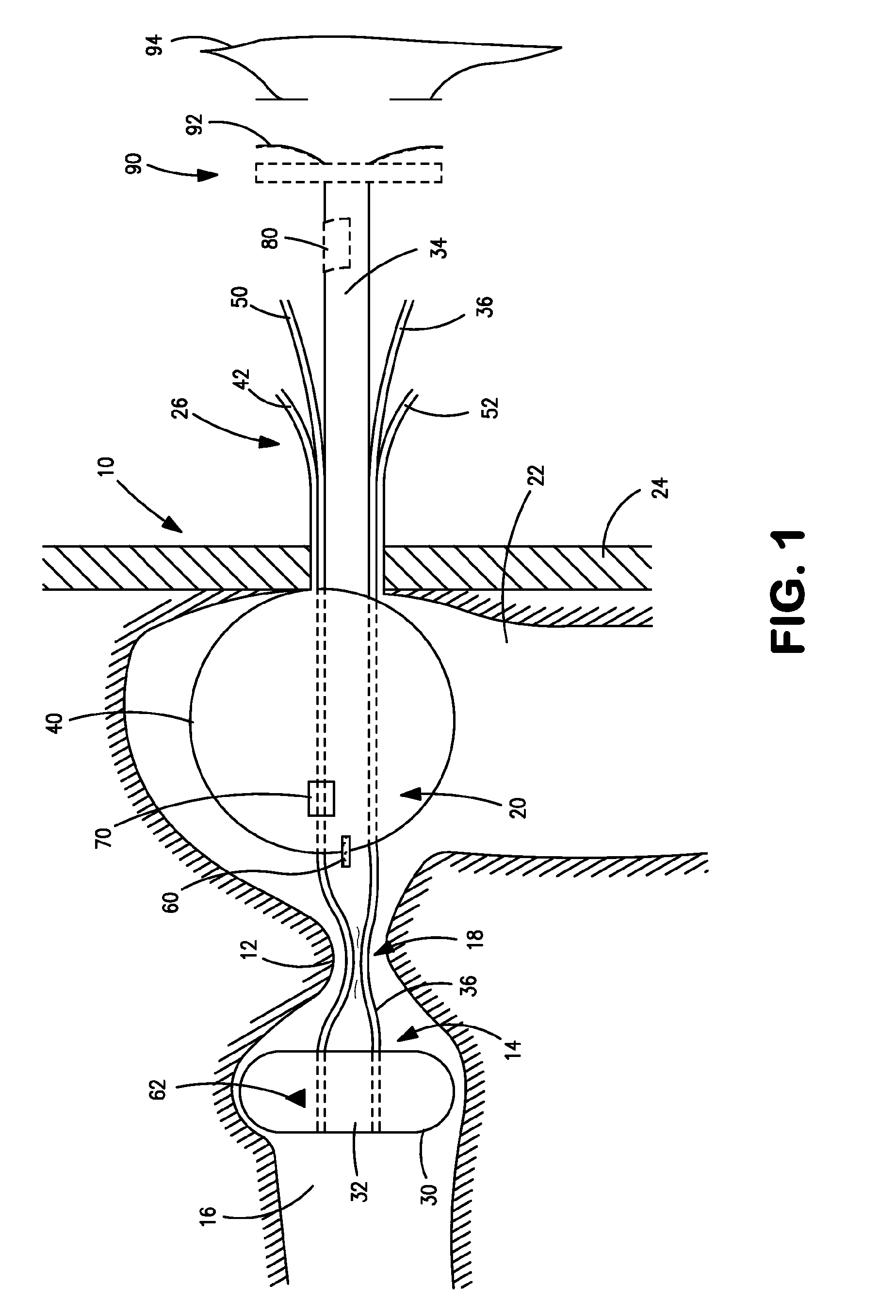
Distender device and method for treatment of obesity and metabolic and other diseases
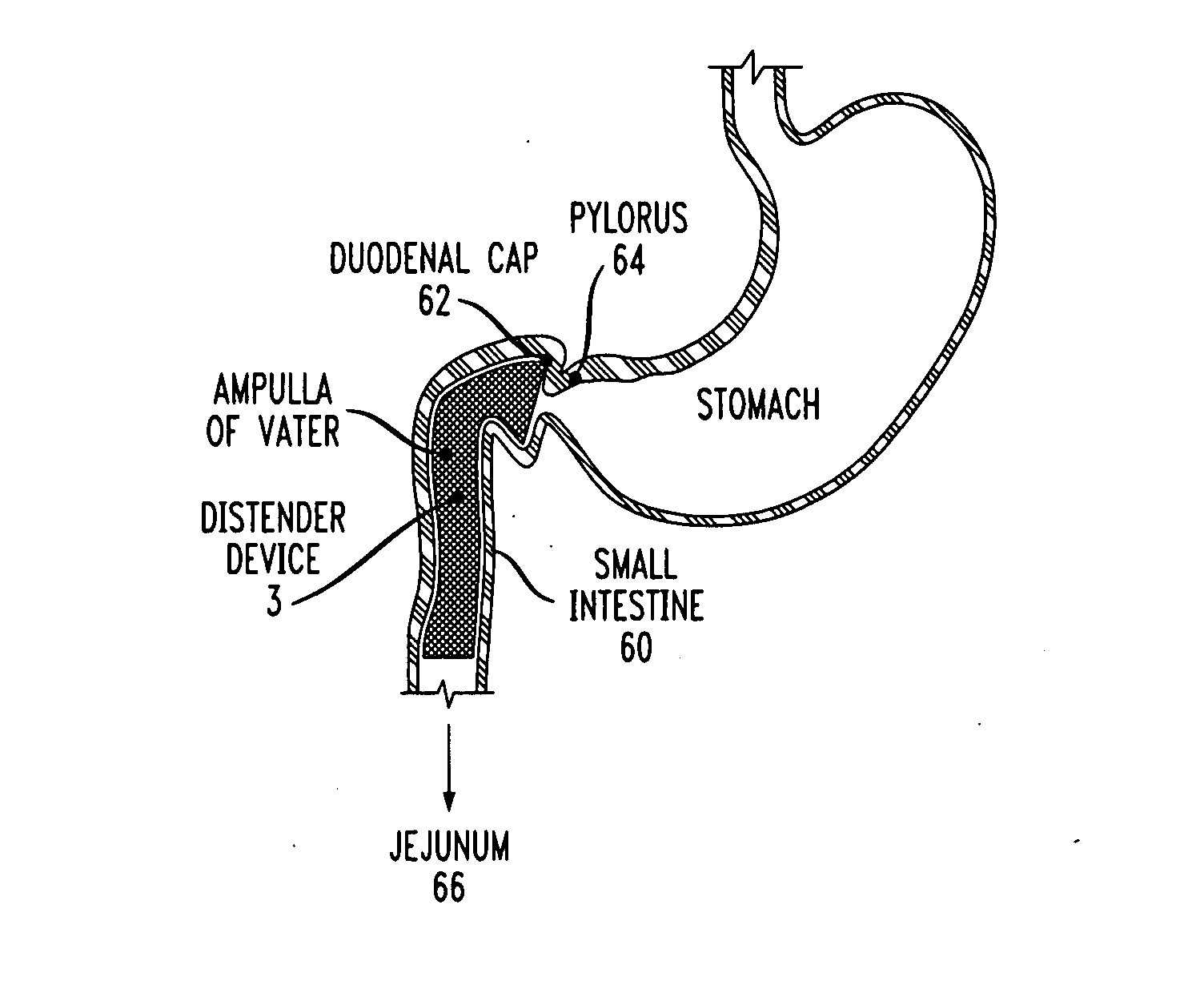
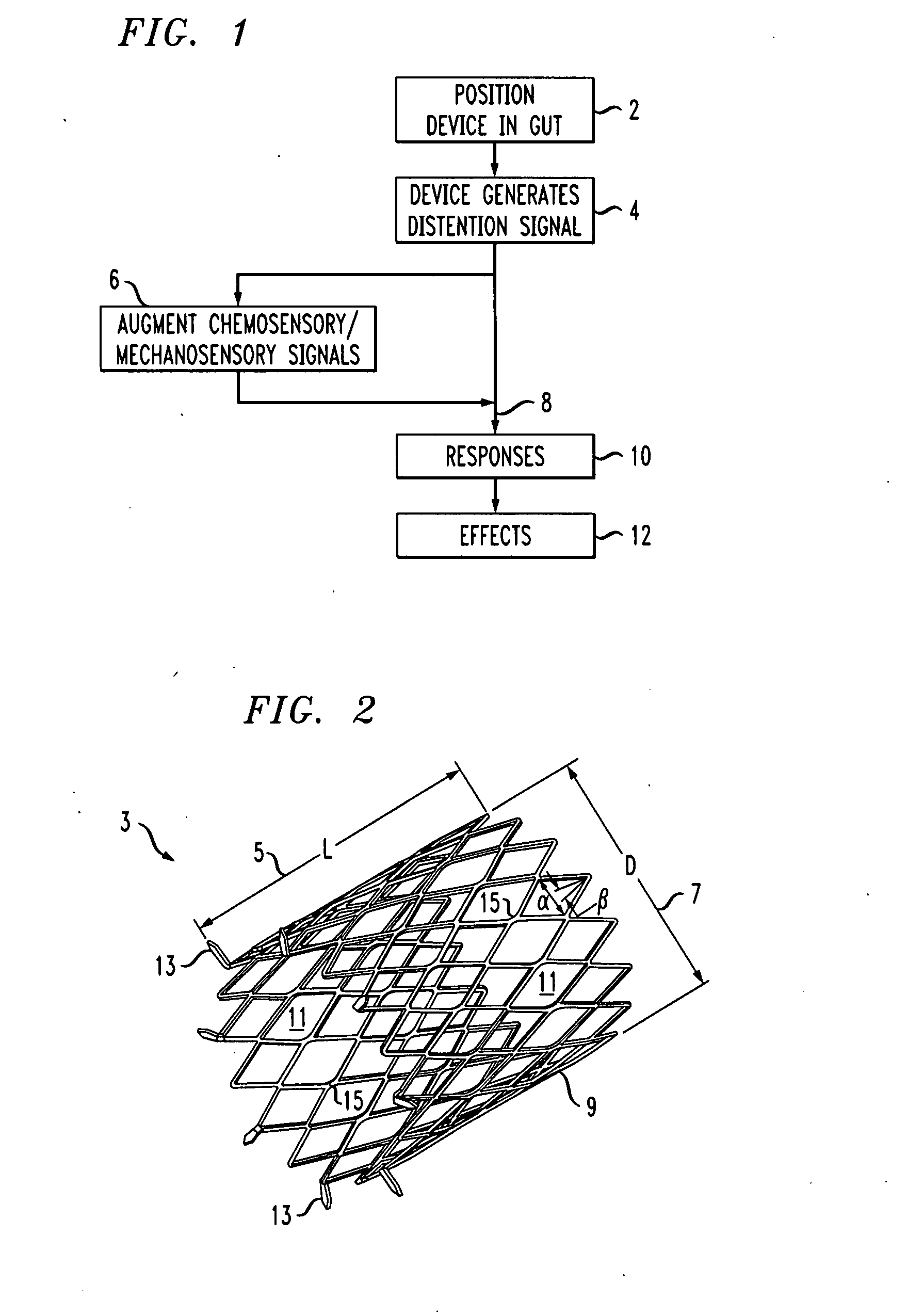
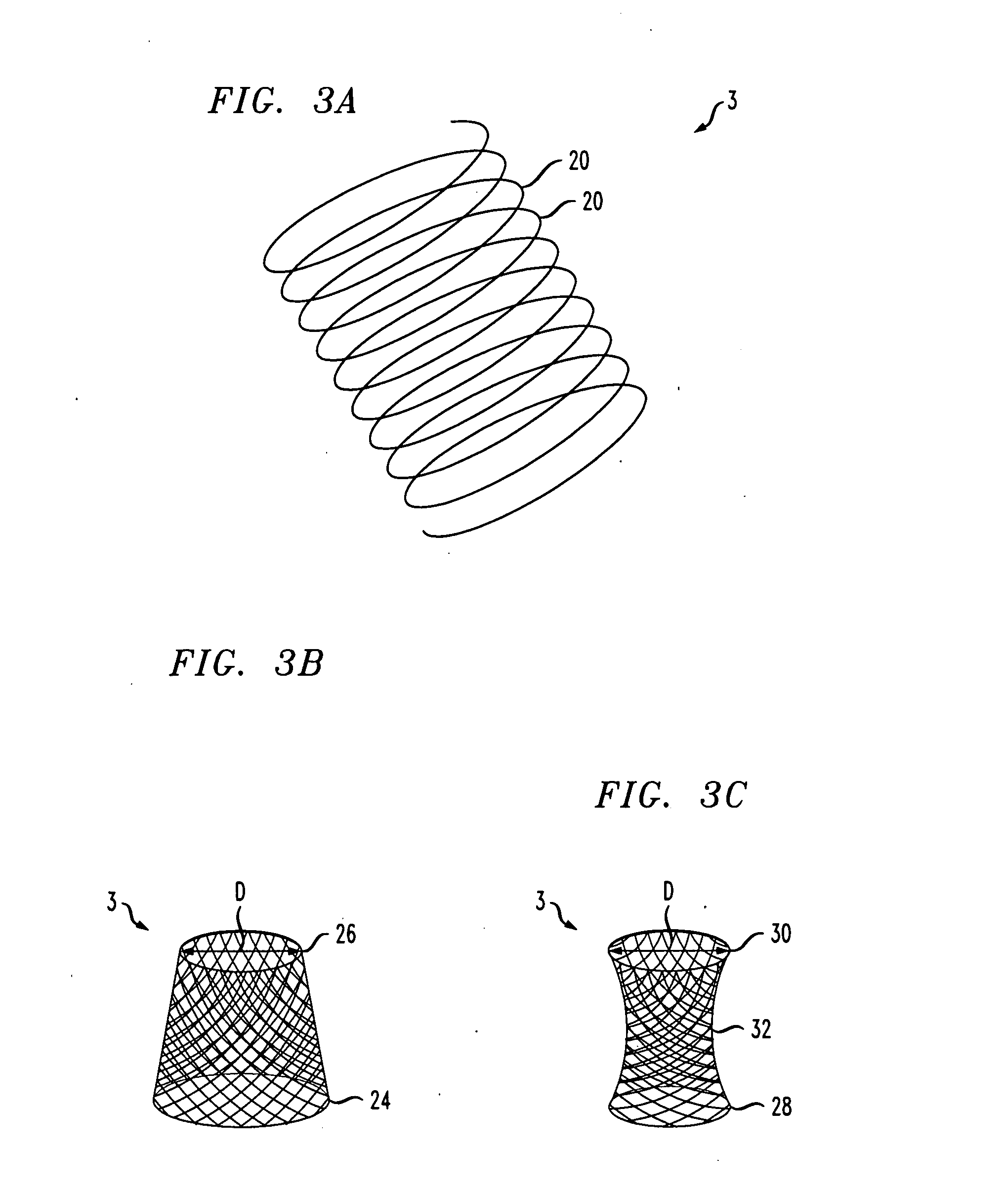
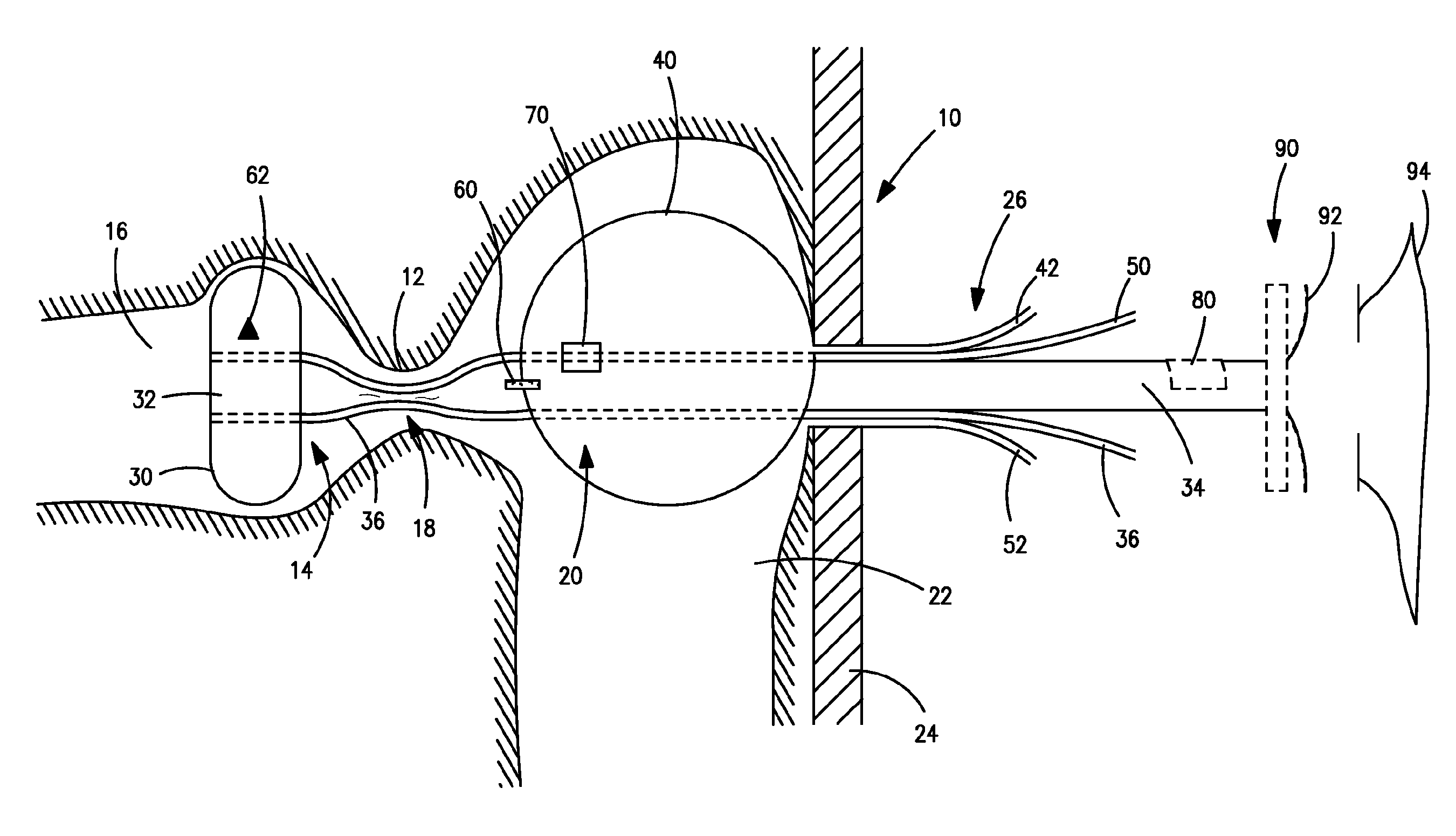
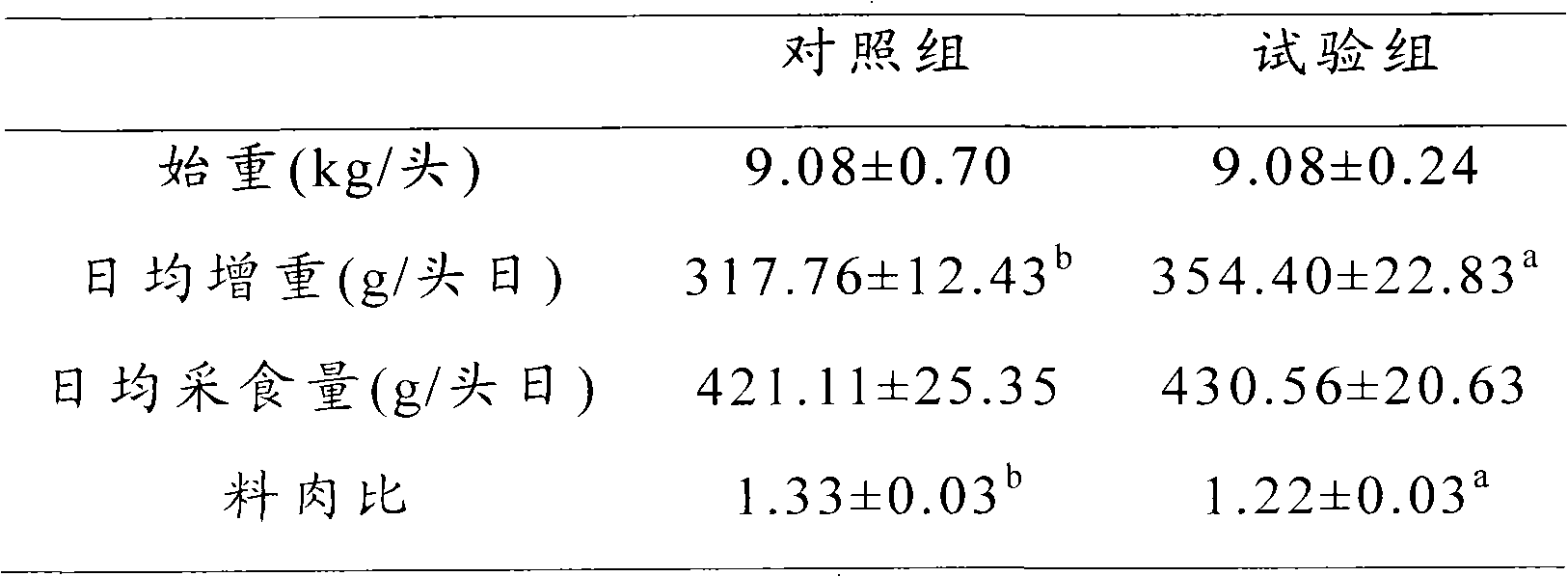
A gastrointestinal implant device is positioned in a patient's small intestine or rectum and produces an outward force that itself produces a distension signal which is a therapeutically useful neural or humoral signal that evokes satiogenic or weight loss effects by itself. The device may advantageously be placed in the duodenum adjacent the pylorus or in the jejunum, ileum or rectum. The distension signals may amplify chemosensory or mechanosensory signals such as enteroendocrine secretions within the patient. The device may be a mesh and include a low material density that allows for unrestricted chyme absorption within the small intestine and unrestricted chyme flow through the gastrointestinal system. A method includes inserting the device into the patient then either retrieving the device after treatment is complete or allowing a device formed of a biodegradable material to degrade in time after treatment is complete.
Owner:ADVANCED NEUROMODULATION SYST INC
Temporary ostomy appliance
ActiveUS20110040231A1Low inflation pressureReduce potential riskSurgeryDilatorsCatheterAbdominal wall
A temporary ostomy appliance is disclosed, including a catheter for extending through the abdominal wall into the intestine. The catheter may be a transcecal catheter for extending through the cecal valve into the ileum. A portion of a catheter that extends through the cecal valve is made collapsible when the catheter is empty. The collapsed portion expands to permit passage of effluent. A balloon carried on the catheter is preformed with a shape and size in order to permit inflation without elastic stretching of the balloon wall material. A filament is provided for permitting a portion of the catheter to be fastened to internal body tissue by surgical sutures or staples. In order to release the fastening without further surgery, the filament is withdrawn by pulling on a proximal portion outside the body.
Owner:CONVATEC TECH INC
Compositions and methods for inducing satiety and treating non-insulin dependent diabetes mellitus, prediabetic symptoms, insulin resistance and related disease states and conditions
ActiveUS20110268795A1Increase muscle massReduce fatBiocidePeptide/protein ingredientsDiseaseInsulin dependent diabetes
The invention provides methods of treatment that induce satiety in a subject for a period of at least around twenty-four hours by once-daily administration to the subject of a controlled release dosage form, wherein the dosage form is administered while the subject is in the fasted state and at a time of around six to around nine hours prior to the subject's next intended meal, and wherein the dosage form comprises a controlled release composition, which comprises an enterically-coated, ileum hormone-stimulating amount of a nutritional substance and releases the majority of the nutritional substance in vivo upon reaching the subject's ileum. The invention also provides a diagnostic tool for probing the health and disease state of the ileal hormones, excess or deficiencies. The invention provides a safe vehicle for targeted deliveries of chemical, pharmaceuticals, natural substances and nutrition to the ileum. The present invention also provides a method for treating noninsulin dependent diabetes mellitus, pre-diabetic symptoms, and insulin resistance, as well as a number of disease states and conditions including gastrointestinal disorders as otherwise described herein.
Owner:SAPIENZA RES LLC +1
Methods and Devices For The Rerouting Of Chyme To Induct Intestinal Brake
Methods and devices reroute chyme to induce intestinal brake in order to improve the effectiveness of bariatric surgical procedures and to improve comorbidity resolution. A bowel is manipulated to provide a shortened path for chyme to travel to the ileum. These methods and devices of rerouting chyme to induce intestinal brake may comprise one or more of a surgical procedure, an implanted device, or a combination of an implant with an improved surgical procedure.
Owner:ETHICON ENDO SURGERY INC
Controlled release compositions comprising nimesulide
InactiveUS20070128276A1Extended stayDecreased gastrointestinal motilityBiocideNervous disorderActive agentArthritis
A controlled release composition comprising nimesulide as an active agent formulated as a gastroretentive system, preferably as a solid oral dosage form is provided, wherein the residence time of the active agent is increased in the stomach, duodenum, jejunum or ileum. The present invention also provides process of preparing such dosage form and methods of using such dosage form compositions. The dosage form compositions are preferably administered once-a-day or twice-a-day and are particularly very useful in the prophylaxis or treatment of NSAID indicated disorder(s) such as acute painful conditions like post-operative trauma, pain associated with cancer, sports injuries, migraine headache and the like, or chronic diseases such as arthritis and the like.
Owner:PANACEA BIOTEC
Methods and devices for the rerouting of chyme to induce intestinal brake
Methods and devices reroute chyme to induce intestinal brake in order to improve the effectiveness of bariatric surgical procedures and to improve comorbidity resolution. A bowel is manipulated to provide a shortened path for chyme to travel to the ileum. These methods and devices of rerouting chyme to induce intestinal brake may include one or more of a surgical procedure, an implanted device, or a combination of an implant with an improved surgical procedure.
Owner:ETHICON ENDO SURGERY INC
Formulations for oral delivery of adsorbents in the gut
ActiveUS20130052269A1Eliminate and reduce side effectEliminating and reducing side effectAntibacterial agentsBiocideIntestinal structureDisease
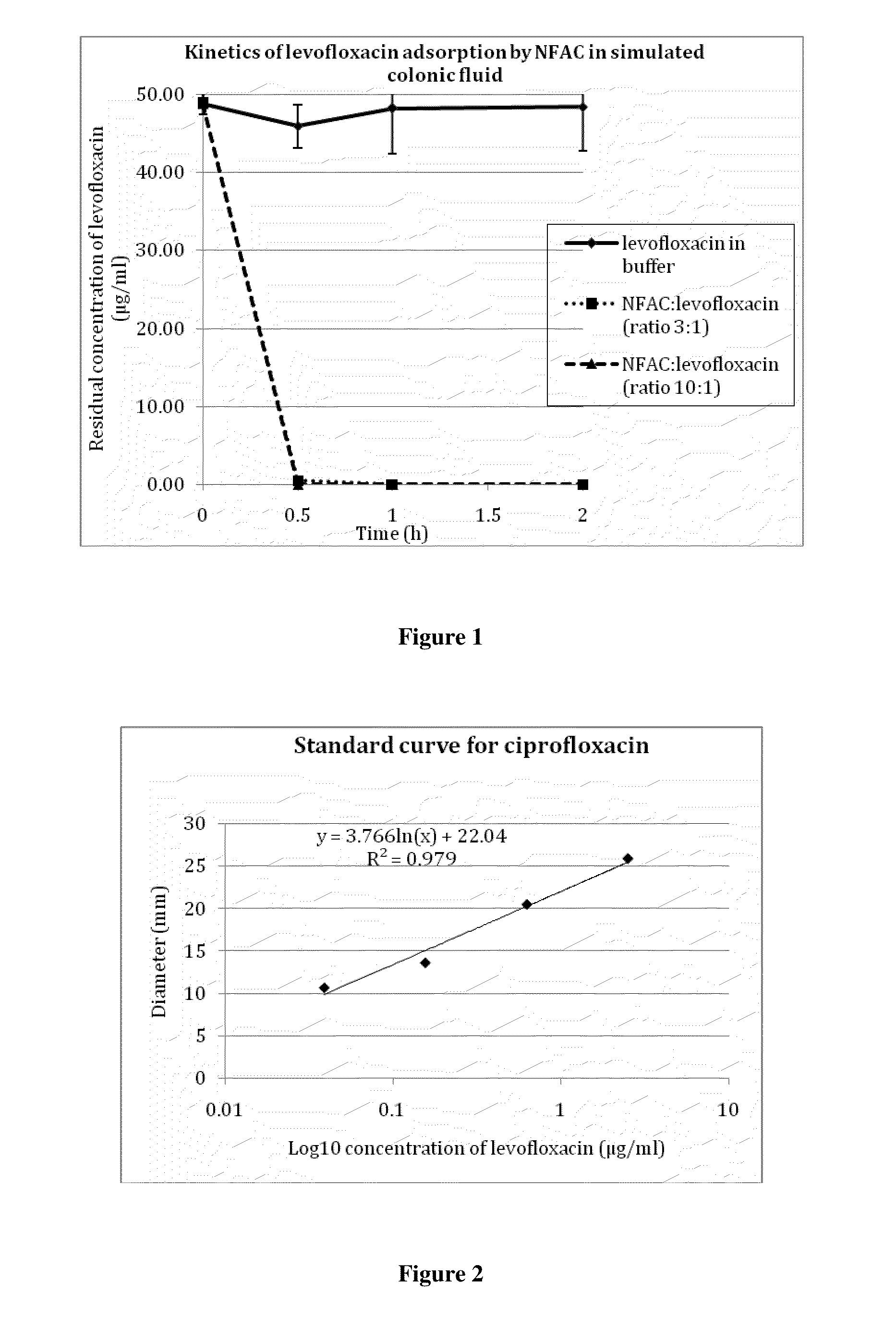
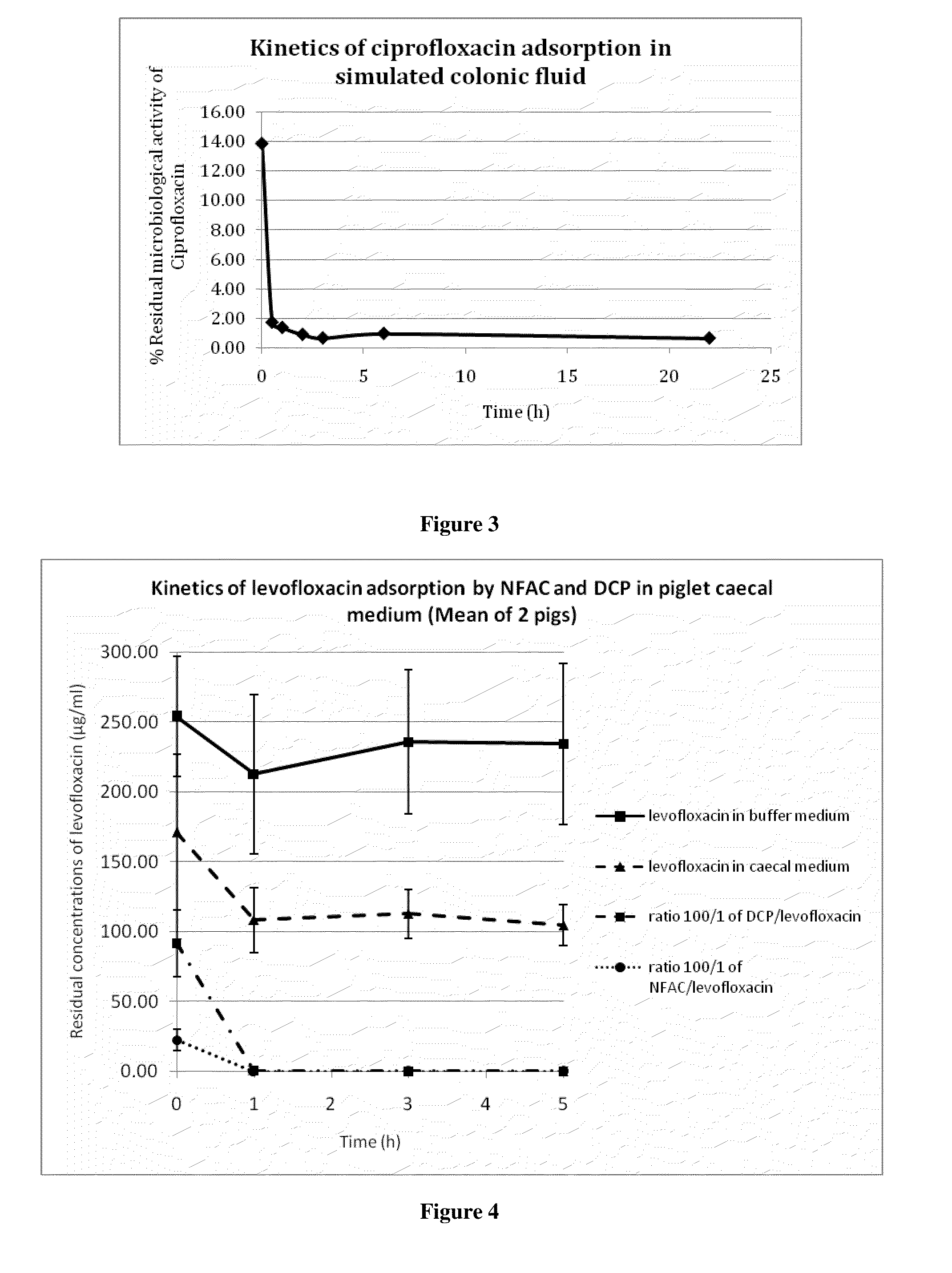
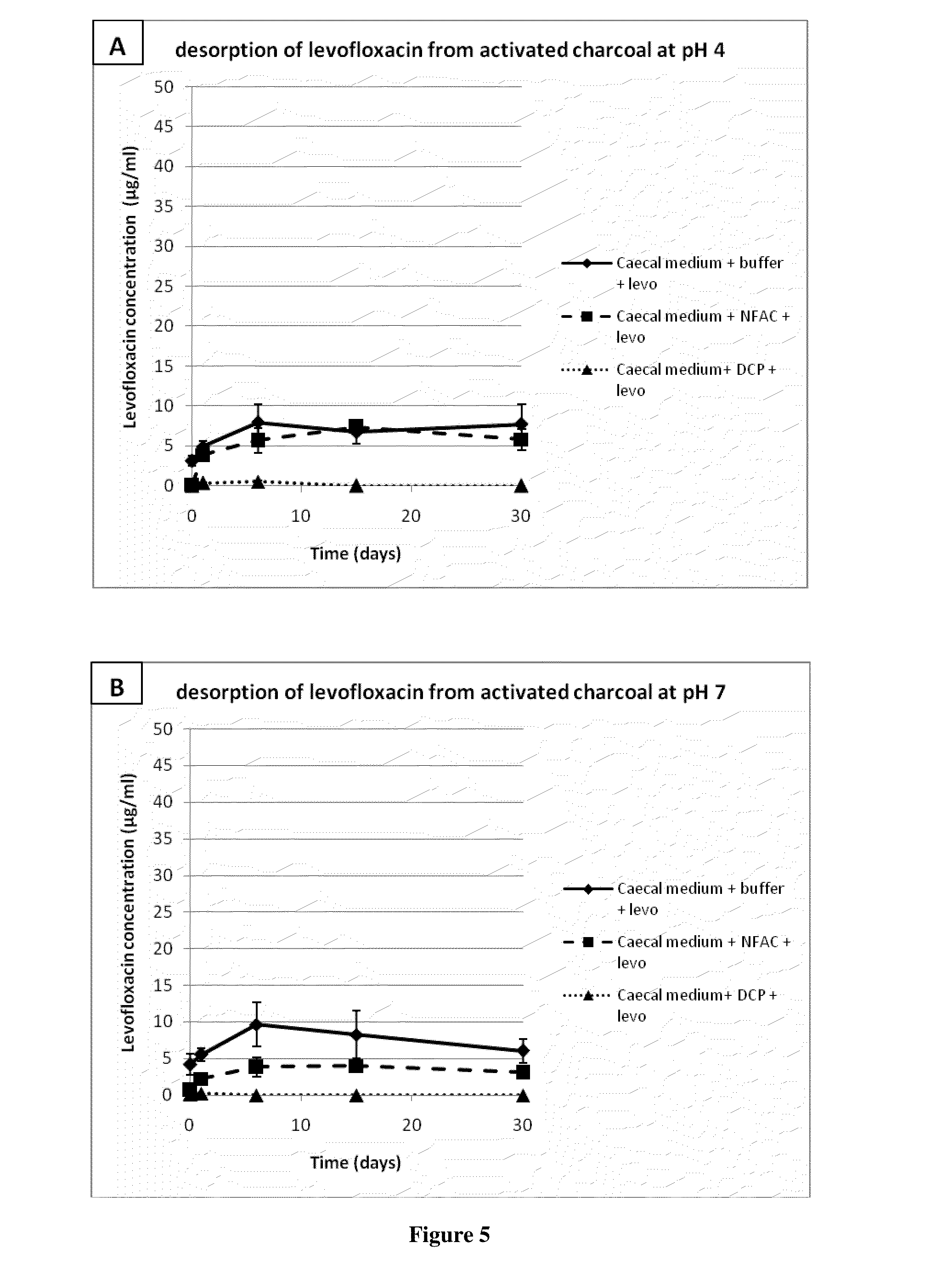
The invention relates to a formulation for the delayed and controlled delivery of an adsorbent into the lower intestine of mammals. The formulation includes a carrageenan and an adsorbent, such as activated charcoal. The invention further relates to uses of this formulation, in particular to pharmaceutical uses. In one embodiment, the formulation is used to eliminate or reduce the side effects in the intestine, in particular in the colon, of pharmaceutical agents that are administered as a treatment for a disorder, but that have side effects when they reach the late ileum, the caecum or the colon.
Owner:DA VOLTERRA
Pharmaceutical formulation with enhanced solubility for the delivery of corticosteroids
InactiveUS20080081070A1Improve solubilityPrecision releaseOrganic active ingredientsBiocidePorositySolubility
Formulations have been developed to improve the solubility of corticosteroids such as fluticasone proprionate in a composition designed to achieve localized release of the drug in the small intestine and / or colon. In one embodiment, solid dispersions of fluticasone are prepared wherein the drug is blended with or coated onto a highly water soluble substrate such as nonpareil (sugar beads) then coated with a layer of polymer soluble in small intestinal fluid, then coated with an enteric coating. The inner polymer layer controls release of the drug, and the enteric coating, a pH sensitive polymer that is broken down in the ileum and colon, controls localized release of drug at various sites within the gastrointestinal tract. The multilayer pharmaceutical composition can be in the form of pellets, tablets compressed from pellets or pellets packed into capsules. The release profile of the drug can be manipulated by (1) altering size or shape (i.e., surface area) and solubility of the inert substrate; (2) the ratio of drug to polymer, the polymer composition and solubility, the porosity of the polymer; (3) the drug form (i.e., free base or salt, or which salt); and the thickness and / or surface area of the drug / polymer and / or enteric coating. In a preferred embodiment, the composition is administered orally. This may also be packaged to provide for an escalating or tapering dosage.
Owner:AURIGA LAB +1
Corn bean pulp type feed complex enzyme additive and application thereof
ActiveCN102132770APromote digestionLower feed to meat ratioAnimal feeding stuffAccessory food factorsFiberDigestion
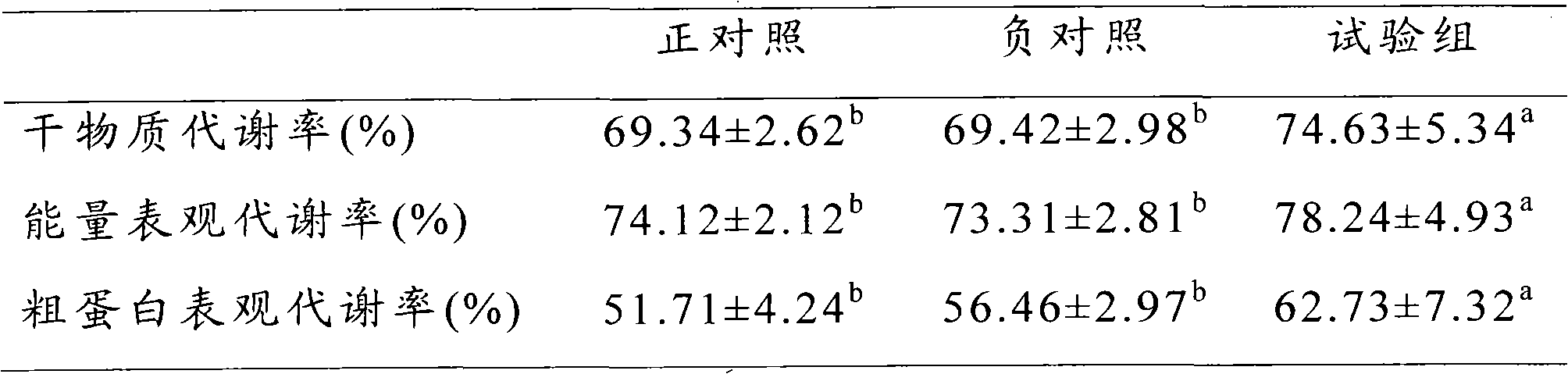
The invention provides a corn bean pulp type feed complex enzyme additive and an application thereof. The complex enzyme additive comprises the components based on weight percentage: 10%-15% of xylanase, 8%-15% of cellulase, 3%-8% of beta-seminase, 5-15% of alpha-galactosidase, 5%-10% of acid protease, 10%-20% of moderate temperature amylase, 5%-10% of glucoamylase and 7%-54% of carrier. The complex enzyme additive can decompose the nutrient s in the corn bean pulp, a mass of anti-nutritional factors which are hard to digest by animals such as the crude fiber and the like, so that energy in the corn bean pulp can be abundantly explored, the digestion capability of the animals to the corn bean pulp type feed can be improved, and the material-meat ratio can be reduced; the digestibility of the nutrient substance and the appearance metabolic energy can be improved, and the microorganism quantity of the ileum can be reduced; the corn bean pulp type feed complex enzyme additive has the advantages of being high-efficiency, specific, small in additive amount and the like compared with the similar additive; and after the complex enzyme additive is used, the growth of the animals can be promoted, and the feed converting efficiency can be improved.
Owner:菏泽海鼎饲料科技有限公司
Administration of serine protease inhibitors to the stomach
ActiveUS9504736B2The way is simple and fastNervous disorderPeptide/protein ingredientsMulti organHigh doses
The inventors have unexpectedly discovered that shock and / or potential multi-organ failure due to shock can be effectively treated by administration of liquid high-dose protease inhibitor formulations to a location upstream of where pancreatic proteases are introduced into the gastrointestinal tract. Most preferably, administration is directly to the stomach, for example, via nasogastric tube under a protocol effective to treat shock by such administration without the need of providing significant quantities of the protease inhibitor to the jejunum and / or ileum.
Owner:LEADING BIOSCI +1
3D culture, passage, cryopreservation, recovery and identification method for in-vitro organoids based on small intestines of different segments of mice
InactiveCN108728399ASignificant advantagesSignificant progressGastrointestinal cellsDead animal preservationIntestinal structureImmunofluorescent labeling
The invention discloses a 3D culture, passage, cryopreservation, recovery and identification method for in-vitro organoids based on small intestines of different segments of mice. The method comprisesthe following steps: (1) separating recesses of duodenum, jejunum and ileum segments of mice; (2) performing 3D culture on the recesses of the duodenum, jejunum and ileum segments of mice, and forming organoids; (3) performing passage on organoids of small intestines of mice; (4) performing cryopreservation on the organoids of small intestines of mice; (5) performing recovery on the organoids ofsmall intestines of mice; (6) preparing frozen sections of the organoids of small intestines of mice; and (7) performing immunofluorescence labeling and identification on the frozen sections of the organoids of small intestines of mice. Compared with the prior art, the method disclosed by the invention optimizes a recess extraction manner, an in-vitro culture system and a culture medium based on the recesses of the small intestines containing stem cells, and comprises the steps of complete culture, passage, cryopreservation, recovery and identification. The small intestine organoids obtained by the method are capable of performing repeated passage, and the passage organoids have character stability.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Empty targeting capsule and preparation method thereof
ActiveCN104055750ALow costEnter the positioning accuratelyPharmaceutical non-active ingredientsCapsule deliveryCarrageenanSide effect
The invention discloses an empty targeting capsule. The empty targeting capsule comprises chitosan, pectin, carrageenan, resistant starch, plasticizer and water repellent agents, wherein sorbitol, glycerol, propylene glycol, mannitol and polyethylene glycol are selected and used as the plasticizer, and polyvinyl alcohol and silicon dioxide are selected and used as the water repellent agents. A preparation method includes the first step of preparing pectin-chitosan polymers, the second step of mixing and dissolving raw materials, and the third step of forming the empty targeting capsule through the conventional mold dipping method. The duodenal targeting capsule, the ileum targeting capsule and the colon targeting capsule can be prepared according to different components and different methods as required; protonated amidogen in the chitosan and ionized carboxyl in the pectin are combined into secondary valence bonds through electrostatic attraction, and polyelectrolyte coordination complexes are formed; the prepared duodenal targeting capsule, the prepared ileum targeting capsule and the prepared colon targeting capsule are accurate in positioning and good in drug release effect and have the advantages of being small in dosage, high in curative effect and low in toxic and side effect.
Owner:王荣辉 +1
Administration of serine protease inhibitors to the stomach
ActiveUS20130310325A1The way is simple and fastEffective treatmentBiocideNervous disorderMulti organNose
The inventors have unexpectedly discovered that shock and / or potential multi-organ failure due to shock can be effectively treated by administration of liquid high-dose protease inhibitor formulations to a location upstream of where pancreatic proteases are introduced into the gastrointestinal tract. Most preferably, administration is directly to the stomach, for example, via nasogastric tube under a protocol effective to treat shock by such administration without the need of providing significant quantities of the protease inhibitor to the jejunum and / or ileum.
Owner:LEADING BIOSCI +1
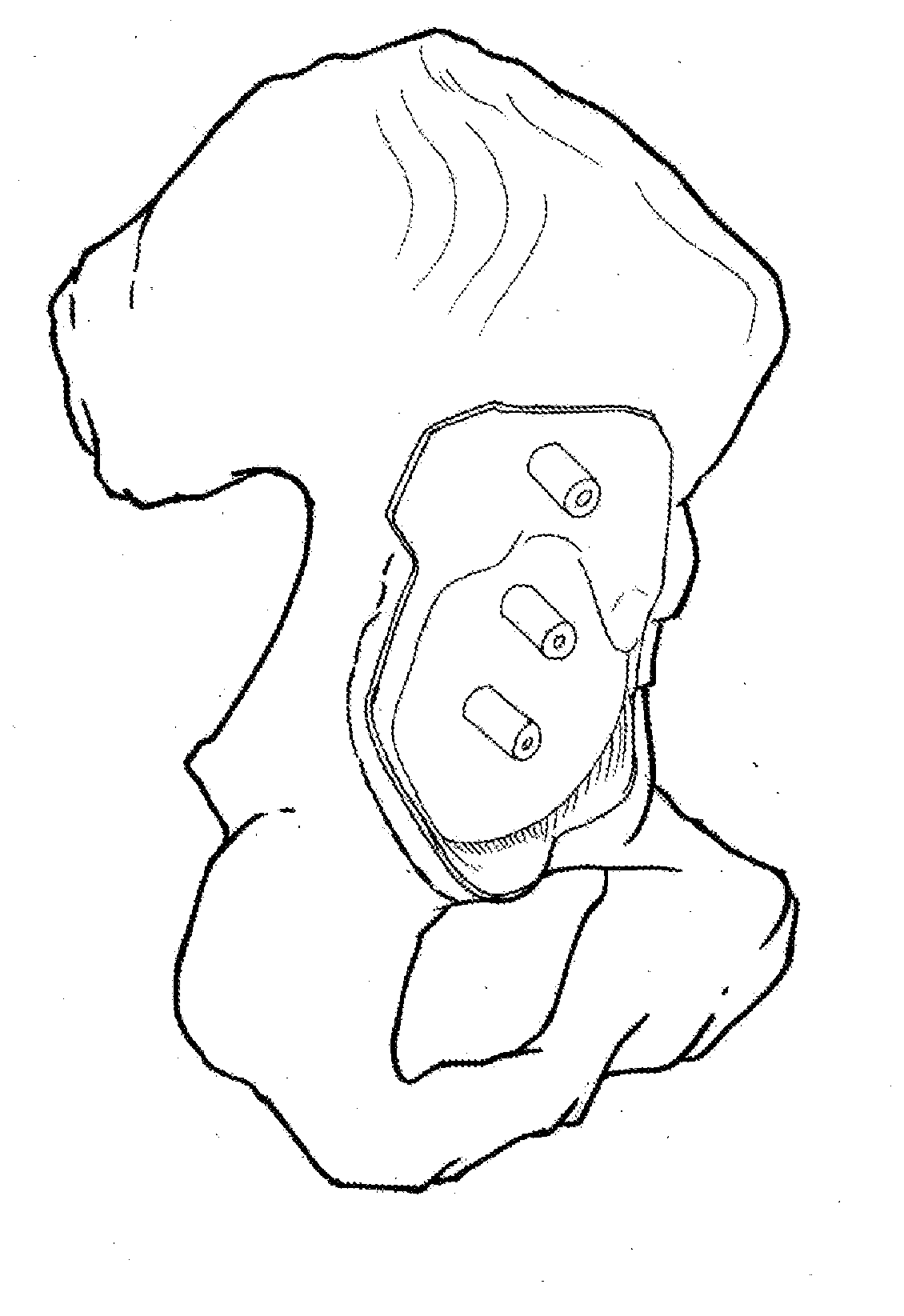
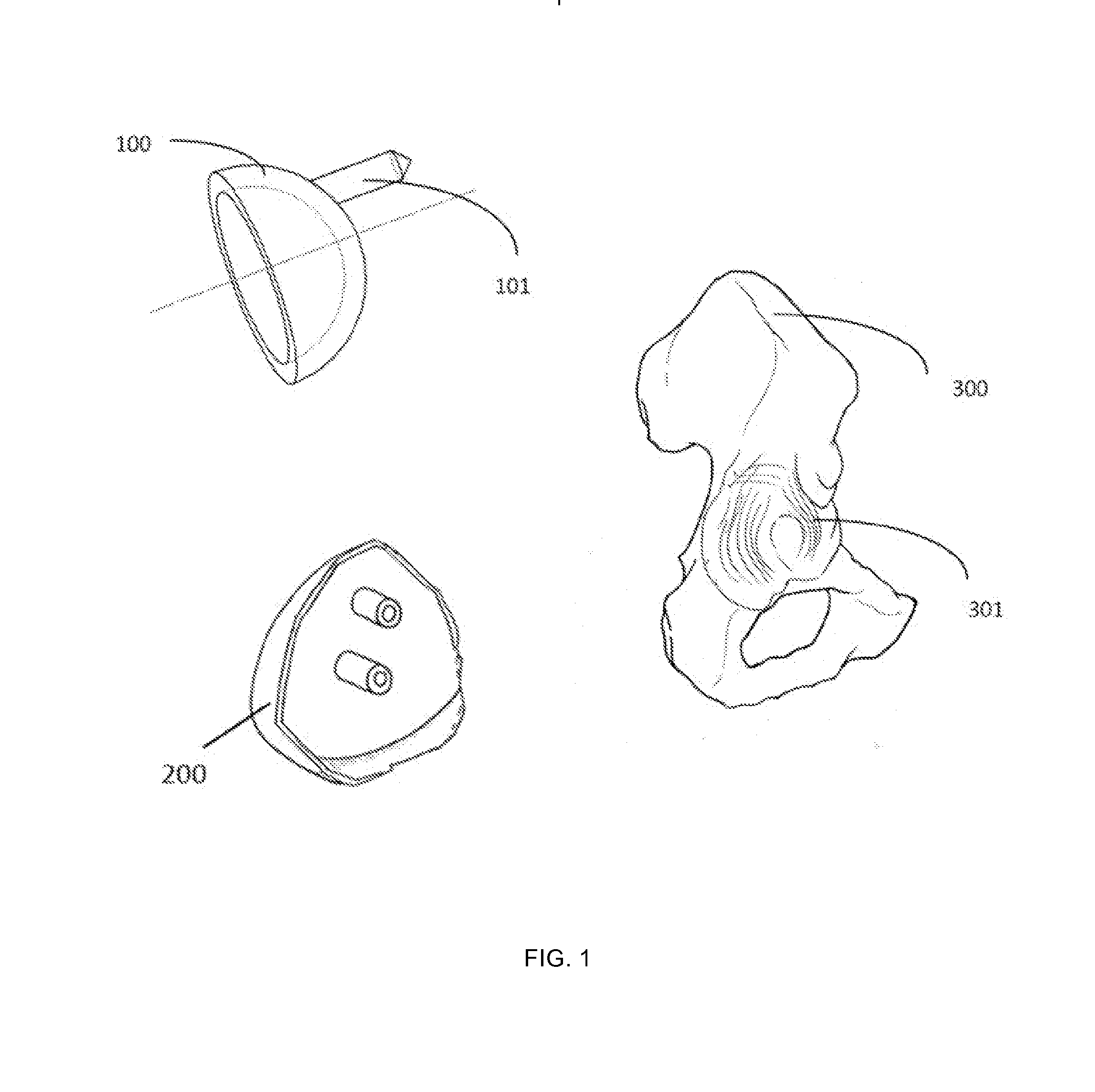
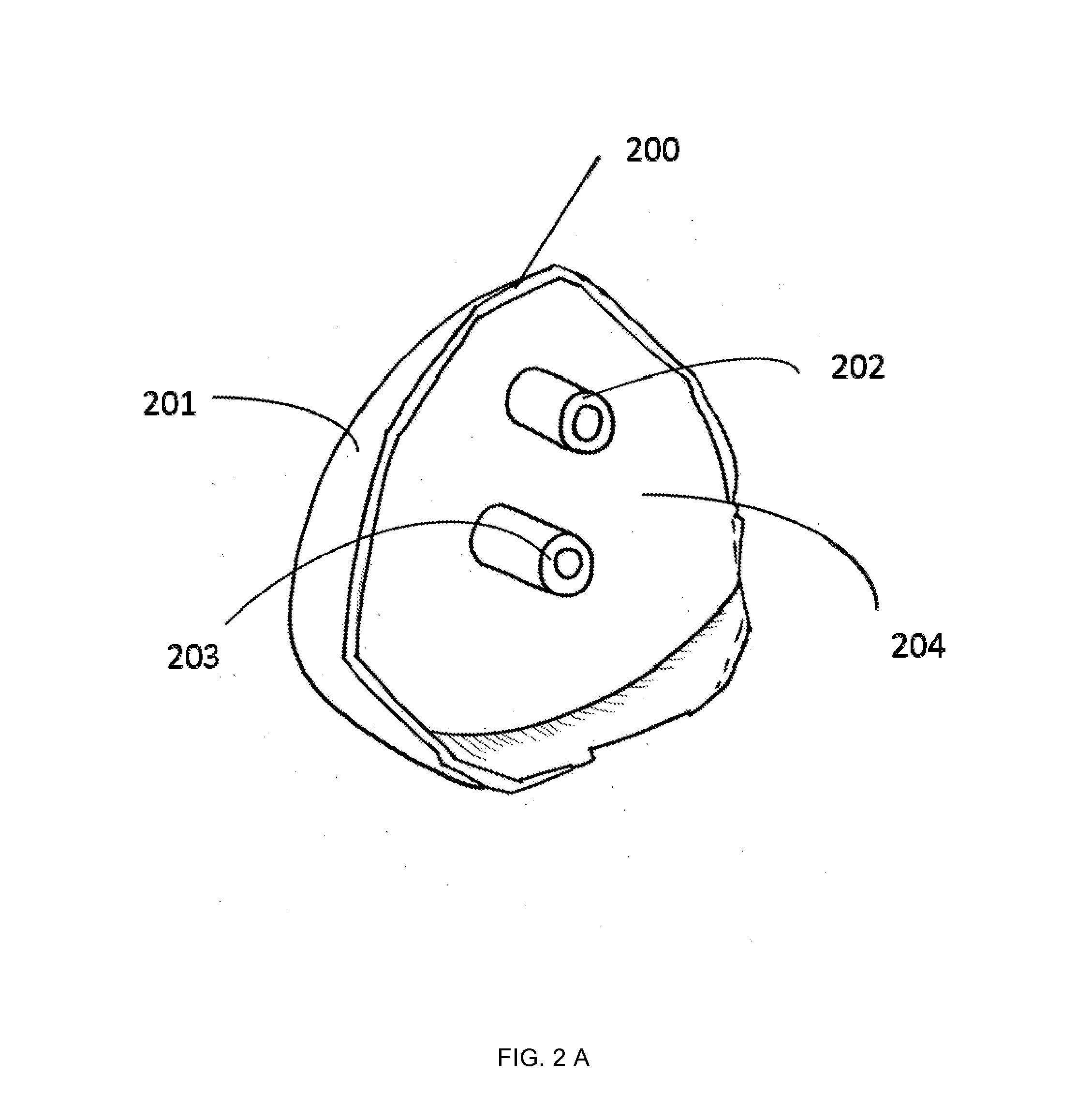
Patient-specific guidance system and acetabular component with offset stems for hip arthroplasty
InactiveUS20140316416A1Accurate placementReduce failureJoint implantsNon-surgical orthopedic devicesAcetabular componentGuidance system
A method provide a patient-specific guidance system developed from 3D bone models, which are generated based on the patient's 2D image data. The surgical guide that is generated from this 3D data has an exterior surface that conforms to the interior surface of the acetabulum of an individual patient. Once this custom guide is placed into the patient's acetabulum, desired 3D implant position data can be transferred from the preoperative image to the acetabulum during an operation. The custom made guide allows surgeons to place guide rods into either the acetabulum itself, or into the sidewall of the pelvis (ileum or ischium) according to a preoperative plan. This guiding system is also coupled with an offset stemmed acetabular component to allow precise implantation and secure fixation of an acetabular component in a predetermined 3-D position relative to the pelvis.
Owner:LIU FEI +1
Compositions and methods for treating insulin resistance and non-insulin dependent diabetes mellitus (type II diabetes)
ActiveUS9757346B2Increase muscle massReduce fatBiocidePeptide/protein ingredientsDiseaseInsulin dependent diabetes
The invention provides methods of treatment that induce satiety in a subject for a period of at least around twenty-four hours by once-daily administration to the subject of a controlled release dosage form, wherein the dosage form is administered while the subject is in the fasted state and at a time of around six to around nine hours prior to the subject's next intended meal, and wherein the dosage form comprises a controlled release composition, which comprises an enterically-coated, ileum hormone-stimulating amount of a nutritional substance and releases the majority of the nutritional substance in vivo upon reaching the subject's ileum. The invention also provides a diagnostic tool for probing the health and disease state of the ileal hormones, excess or deficiencies. The invention provides a safe vehicle for targeted deliveries of chemical, pharmaceuticals, natural substances and nutrition to the ileum. The present invention also provides a method for treating noninsulin dependent diabetes mellitus, pre-diabetic symptoms, and insulin resistance, as well as a number of disease states and conditions including gastrointestinal disorders as otherwise described herein.
Owner:SAPIENZA RES LLC +1
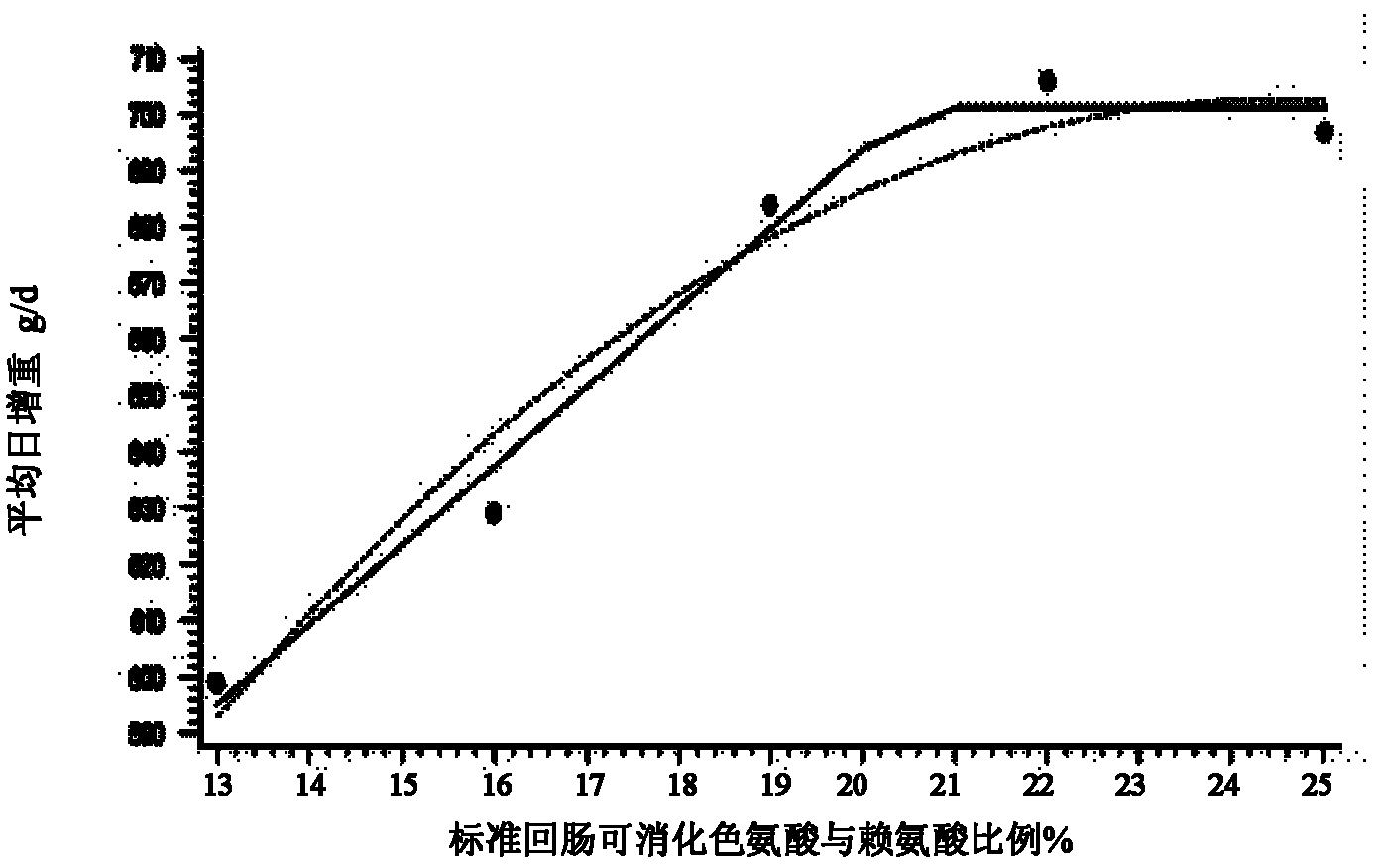
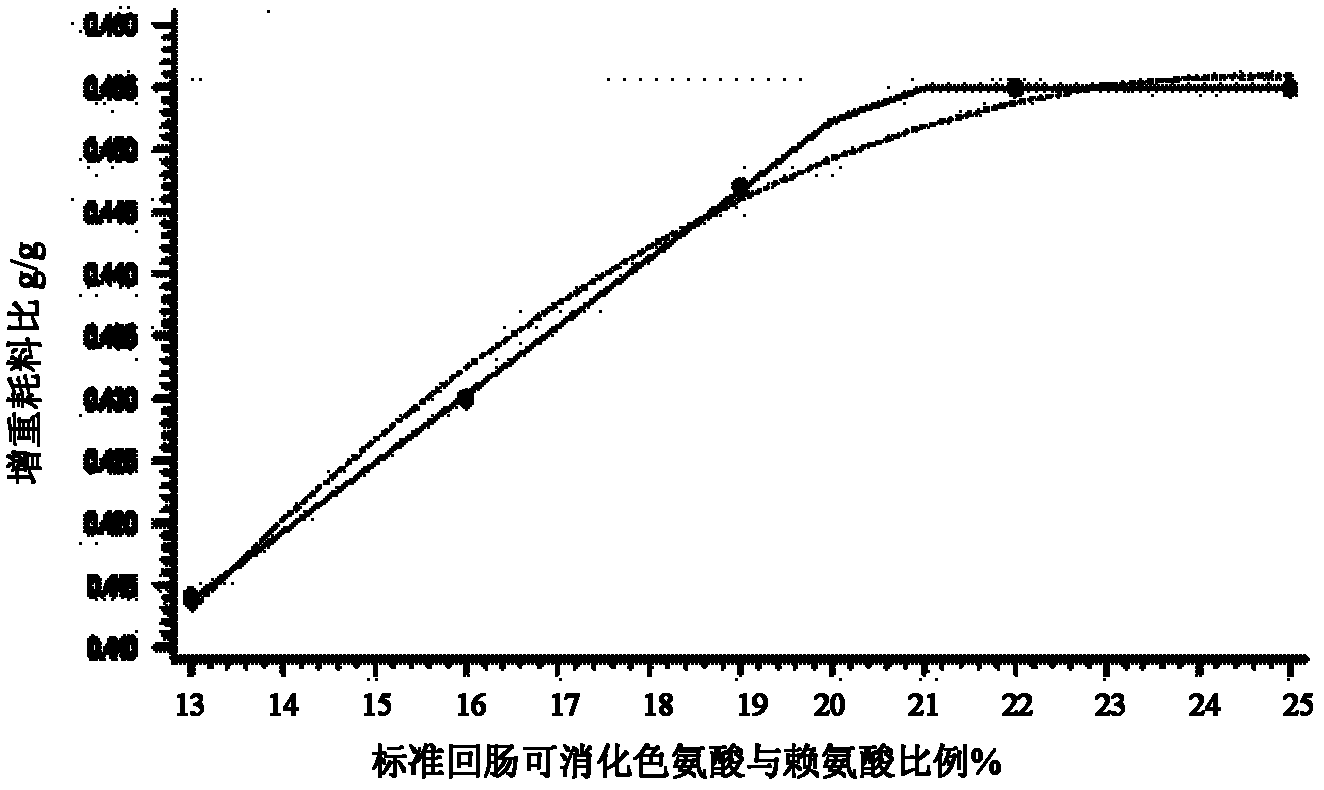
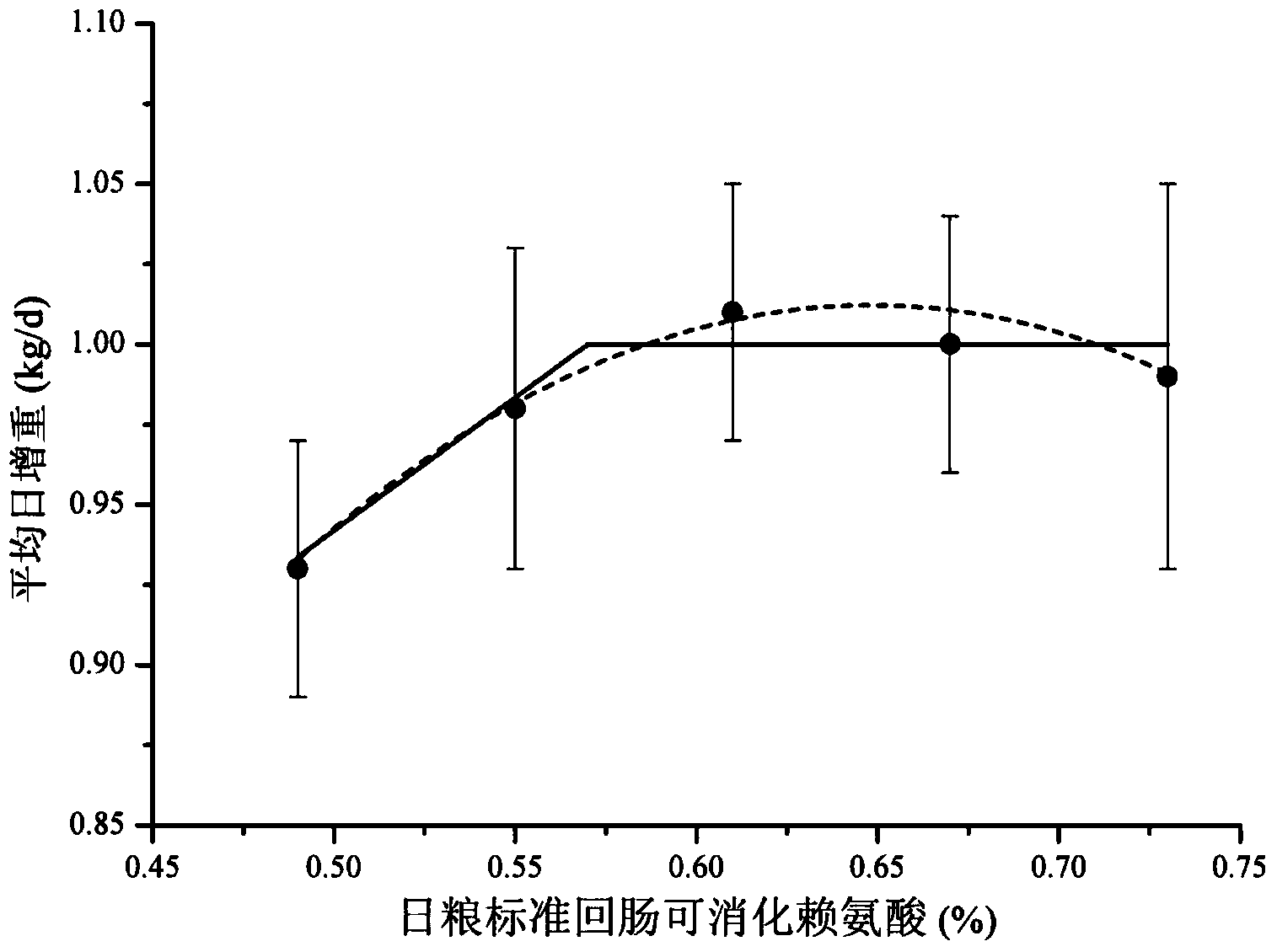
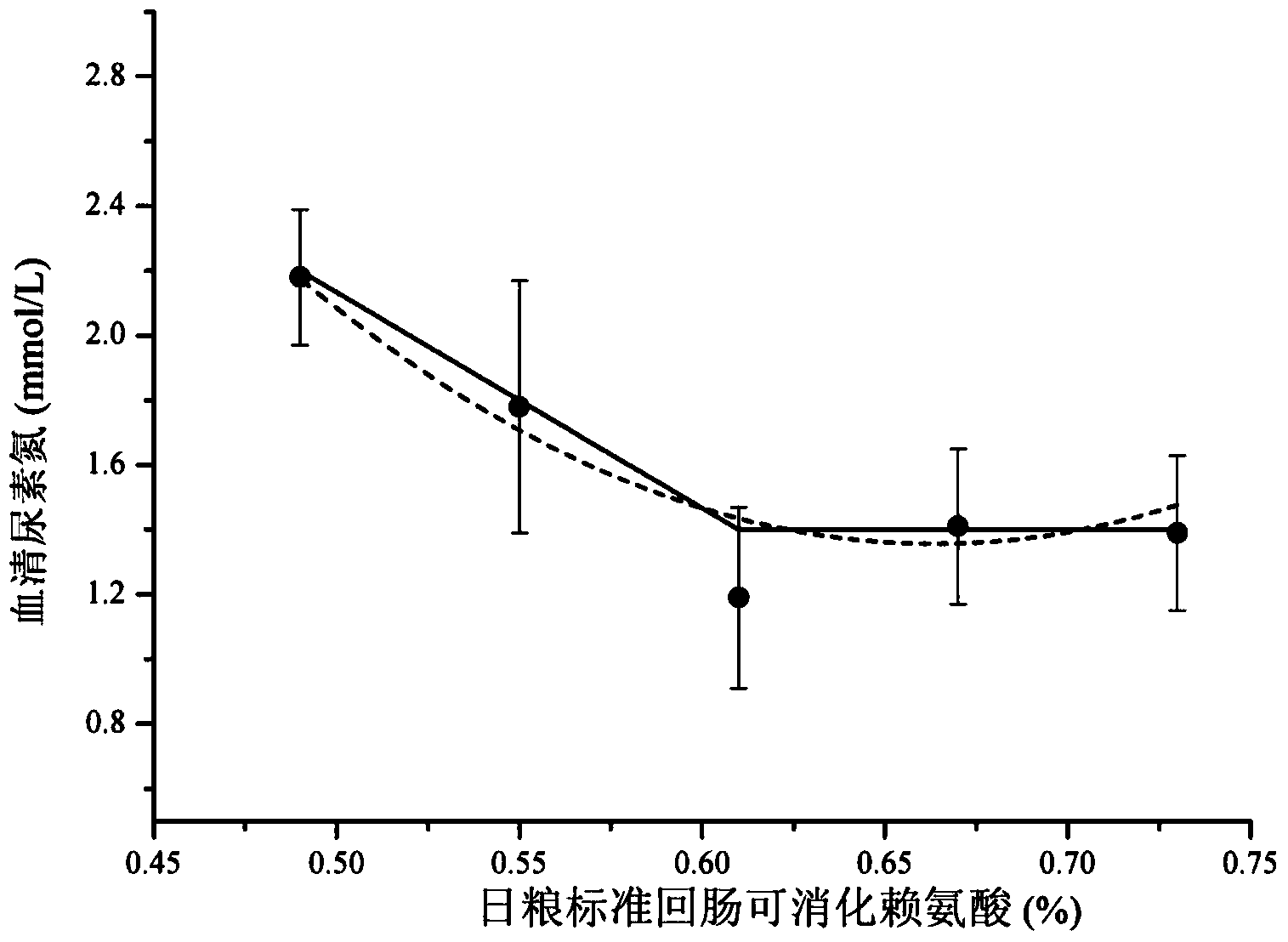
Low-nitrogen-emission daily ration for growing pigs
InactiveCN102217739ALow costEmission reductionAnimal feeding stuffAccessory food factorsFeed conversion ratioLow nitrogen
The invention discloses a low-nitrogen-emission daily ration for growing pigs. The low-nitrogen-emission daily ration contains protein, lysine, tryptophan, threonine and sulfur-containing amino acid, wherein the net energy content of the low-nitrogen-emission daily ration is 2.30 to 2.45 Mcal / kg, and the content of the protein is 11.5 to 15.5%; the content of the lysine capable of being digested by standard ileums in the low-nitrogen-emission daily ration is 0.9 to 1.02%, the ratio of the tryptophan capable of being digested by the standard ileums to the lysine capable of being digested by the standard ileums is not lower than 0.23, the ratio of the threonine capable of being digested by the standard ileums to the lysine capable of being digested by the standard ileums is not lower than 0.66, and the ratio of the sulfur-containing amino acid capable of being digested by the standard ileums to the lysine capable of being digested by the standard ileums is not lower than 0.61. According to the low-nitrogen-emission daily ration disclosed by the invention, by decreasing the crude protein level in a daily ration prescription in Feeding Standard of Swine (2004) and supplementing synthetic amino acids, the absorptivity of the amino acids in animals is increased, the feed cost is lowered, and the economic benefits of swine industry is enhanced; the decrease of nitrogen emission in environment is beneficial to alleviation of the problem of protein feed insufficiency to a certain extent; and the weight gain of growing pigs and the feed conversion rate are not influenced.
Owner:CHINA AGRI UNIV +1
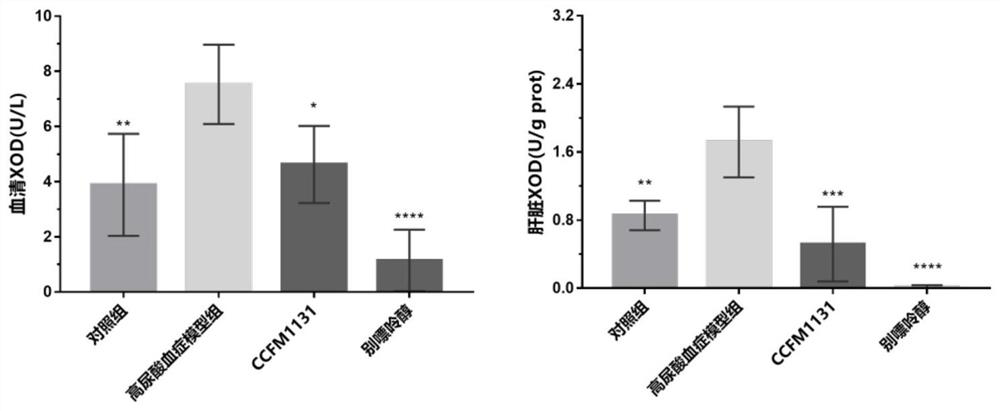
Lactobacillus rhamnosus CCFM1131 for relieving hyperuricemia
ActiveCN112574917ADecreased xanthine oxidase (XOD) activityLower serum uric acid levelsBacteriaMetabolism disorderBiotechnologyLactobacillus rhamnosus
The invention discloses lactobacillus rhamnosus CCFM1131 for relieving hyperuricemia, and belongs to the technical field of microorganisms. The lactobacillus rhamnosus CCFM1131 can reduce the serum uric acid level of a hyperuricemia mouse, inhibit the xanthine oxidase (XOD) activity of serum and the liver, and reduce the occurrence of hyperuricemia and gout; the lactobacillus rhamnosus CCFM1131 can be used for down-regulating serum blood sugar, down-regulating serum total triglyceride (TG), down-regulating serum total cholesterol (TC) level and inhibiting serum alkaline phosphatase (ALP) activity; in addition, the lactobacillus rhamnosus CCFM1131 can promote the expression of the ileum uric acid transporter ABCG2. Besides, the lactobacillus rhamnosus CCFM1131 can tolerate gastrointestinaltract environment, can be used for preparing functional microbial inoculants, foods and medicines for relieving hyperuricemia and gout, and has a wide application prospect.
Owner:无锡特殊食品与营养健康研究院有限公司
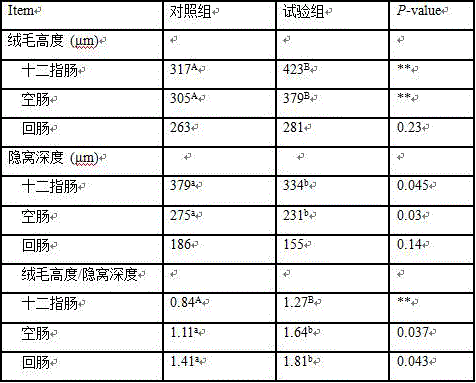
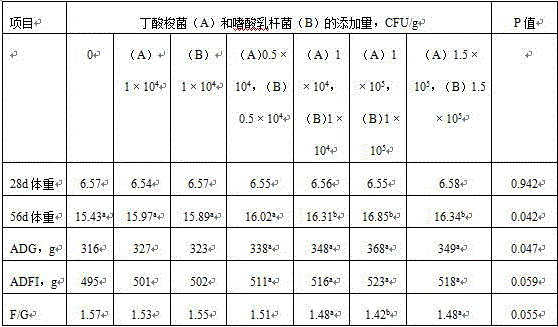
Feed formula for improving weaned piglet disease resistance and application
The invention discloses a feed formula for improving weaned piglet disease resistance and application. The feed formula comprises the following raw materials: 58 to 63 parts of corn, 16 to 24 parts of bean pulp, 2 to 5 parts of whole milk powder, 4 to 9 parts of fish meal, 1 to 5 parts of wheat bran, 1 to 3 parts of glucose, 0.1 to 0.5 part of acidifying agent, 0.5 to 1.5 parts of calcium hydrophosphate, 0.5 to 1.1 parts of stone powder, 0.1 to 0.5 part of table salt, 0.3 to 0.7 part of L-lysine hydrochloride, 0.2 to 0.7 part of DL-methionine, 0.2 to 0.6 part of threonine, 1 to 4 parts of piglet premix, 0.001 to 0.01 part of clostridium butyricum additive and 0.001 to 0.01 part of lactobacillus acidophilus additive, wherein the weight part ratio of clostridium butyricum to lactobacillus acidophilus is 1 to 1, and the viable content of the two types of freeze-dried powder is 1*10<9>CFU / g (the concentration in a feed is 1*10<4> to 1*10<5> CFU / g). As proved by a result, the mRNA (messenger Ribonucleic Acid) level relative expressions of NLRP3, NLRP6 and NLRP12 in jejunum in an experimental group are improved remarkably; the villus heights of the middle segment of the jejunum and each intestinal segment of an ileum are increased remarkably, and the crypt depths of a duodenum, the middle segment of the jejunum and an ileum are decreased remarkably; the IL-1 beta content of serum is decreased, and the IL-10 content of the serum is increased. In the feed for weaned piglets, the clostridium butyricum additive and the lactobacillus acidophilus additive are added according to a proper proportion, so that the weaned piglet disease resistance can be improved remarkably.
Owner:TIANJIN INST OF ANIMAL HUSBANDRY & VETERINARY
Diagnostic tests for inflammatory bowel diseases
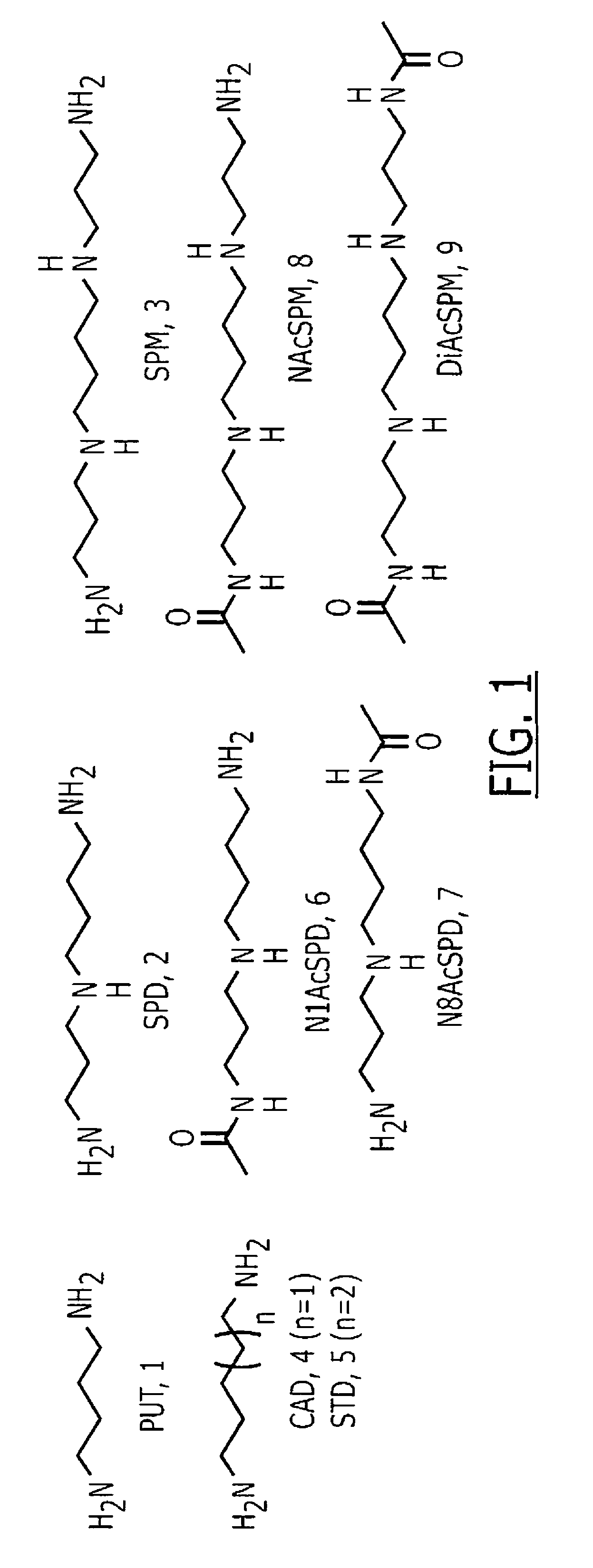
InactiveUS7972807B1Improved diagnostic testingMicrobiological testing/measurementDisease diagnosisWhite blood cellCadaverine
Disclosed are diagnostic tests helpful in indicating presence of an inflammatory bowel disease (IBD) in a human patient. In one embodiment, the test comprises obtaining a sample of mucosal tissue from the ileum or sigmoid colon of the patient; evaluating sample quality by testing for cadaverine and continuing the diagnostic test if the sample has no detectable cadaverine; testing the cadaverine negative sample for N-acetylated spermine; and correlating a detectable level of N-acetylated spermine in the sample with presence of IBD in the patient. Another, less invasive, method disclosed includes a diagnostic test comprising isolating mononuclear leukocytes from the patient's blood; testing the isolated mononuclear leukocytes for level of spermidine; and correlating a level of spermidine higher than that in mononuclear leukocytes of normal subjects as indicative of an inflammatory bowel disease in the patient.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Implantable and non-invasive stimulators for gastrointestinal therapeutics
ActiveUS20190358450A1Lower Level RequirementsReduce inflammationExternal electrodesDigestive electrodesGastroparesisNon invasive
Systems and methods for implementation of a disposable miniaturized implant for treatment of Post-Operative Ileums (POI),a miniaturized implant for treating chronic GI dysmotility (e.g., dysphagia, gastroesophageal reflux disease (GERD), nausea, functional dyspepsia, blockage of transit, and gastroparesis, inflammatory bowel disease) and obesity, by providing electrical stimulation to the part of bowel going through surgery to expedite the healing process while recording the smooth muscle activities simultaneously, or providing stimulation on a treatment location of the GI tract or the branch of the vagus nerve. Systems and methods are also provided for non-invasive, transcutaneous stimulation of anatomy within the abdomen of the patient.
Owner:RGT UNIV OF CALIFORNIA
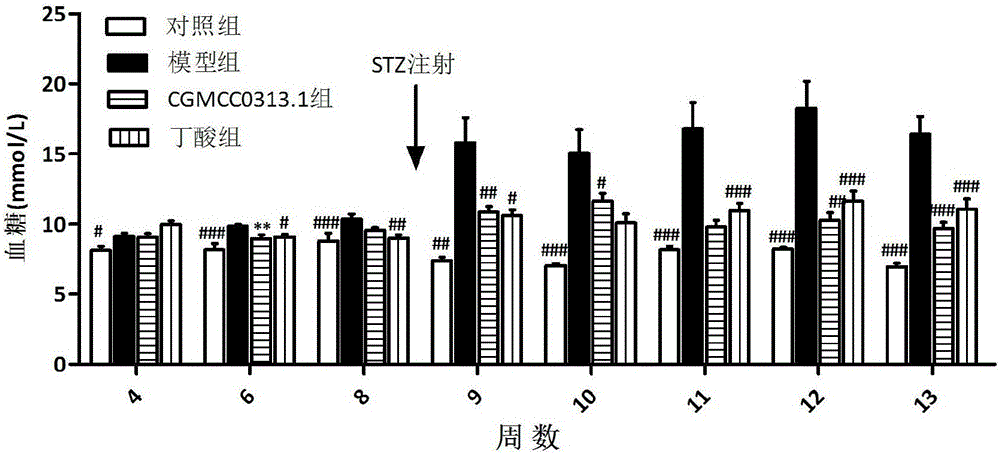
Application of clostridium butyrate in preparation for prevention and treatment or adjuvant therapy of hyperglycemia
ActiveCN105769928AChange compositionRecovery functionMetabolism disorderDigestive systemAcute hyperglycaemiaGlucagon-like peptide-1
The invention discloses an application of clostridium butyrate in a preparation for prevention and treatment or adjuvant therapy of hyperglycemia and belongs to the technical field of prevention and treatment of diabetes. According to the application, during the prevention and treatment or adjuvant therapy of the hyperglycemia, butyric acid is produced in an intestinal tract, meanwhile, florae of the intestinal tract are changed, signals are sent to an ileum, a liver, a pancreatic gland and a brain by a portal vein blood sugar sensor, the ileum is promoted to secrete (GLP-1) glucagon-like peptide-1, the gluconeogenesis of the liver is lowered, pancreatic beta cells are protected, the secretion of insulin of the pancreatic gland is promoted, and thus, the action of reducing blood sugar is exerted. The clostridium butyrate is a medicine which has been proven by clinical drug trials and can be directly taken orally.
Owner:QINGDAO EASTSEA PHARMA
Precise visual jejunum and ileum tube
InactiveCN105996972APrecise positioningFast penetrationGastroscopesOesophagoscopesDiseaseStomach part
The invention discloses a precise visual jejunum and ileum tube which belongs to the technical field of medical apparatuses and instruments. The precise visual jejunum and ileum tube comprises an ileum tube body and a guide wire, and the tail end of the guide wire is provided with a tubular tip head which is made of a magnet; the portion, acting in vitro, of the visual jejunum and ileum tube is provided with an external magnet, and the external visual jejunum and ileum tube and the tubular tip head are mutually attracted; the base portion of the tubular tip head is provided with a camera, a wire of the camera is arranged in the direction of the guide wire, and the wire of the camera and the guide wire are connected into a whole. The precise visual jejunum and ileum tube can rapidly penetrate into the stomach under magnetic attraction and can precisely position to the duodenum, jejunum and ileum; a drug is delivered through the guide wire, nutritional foods are delivered to the intestines through attraction, the drug and the nutritional foods can be directly absorbed by the jejunum and ileum, and the parts with diseases and the position where the tube itself reaches can be observed at any time by means of the visual function.
Owner:南京医事达生物科技有限公司
Regimen for suppressing organ rejection
ActiveUS9168246B2Low fluctuation and swingSuppression of rejectionAntibody ingredientsPill deliveryOral treatmentRegimen
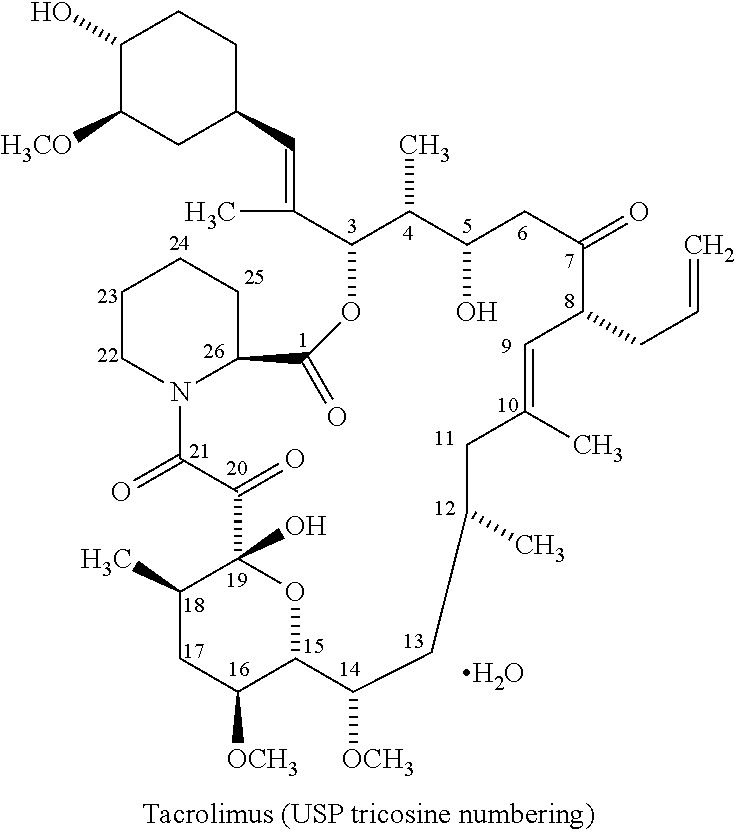
The present invention relates to a method of suppressing organ rejection in a patient receiving an organ transplant by initiating oral treatment with a once-daily extended release tacrolimus dosage form, for example, at an initial dose of from about 0.15 to about 0.20 mg / kg / day within 24 or 48 hours following transplantation. The once-daily extended release tacrolimus dosage form (i) provides low fluctuation and / or swing of tacrolimus, (ii) provides a significantly lower Cmax than an immediate release formulation of tacrolimus while providing the same or greater area under the curve (AUC), (iii) releases the tacrolimus substantially in the colon and / or the lower ileum, (iv) releases at most 63.5% of the tacrolimus in the dosage form at the 12 hour time point, or (v) any combination of any of the foregoing.
Owner:VELOXIS PHARM INC
Method to Increase the Growth Velocity of Human Infants
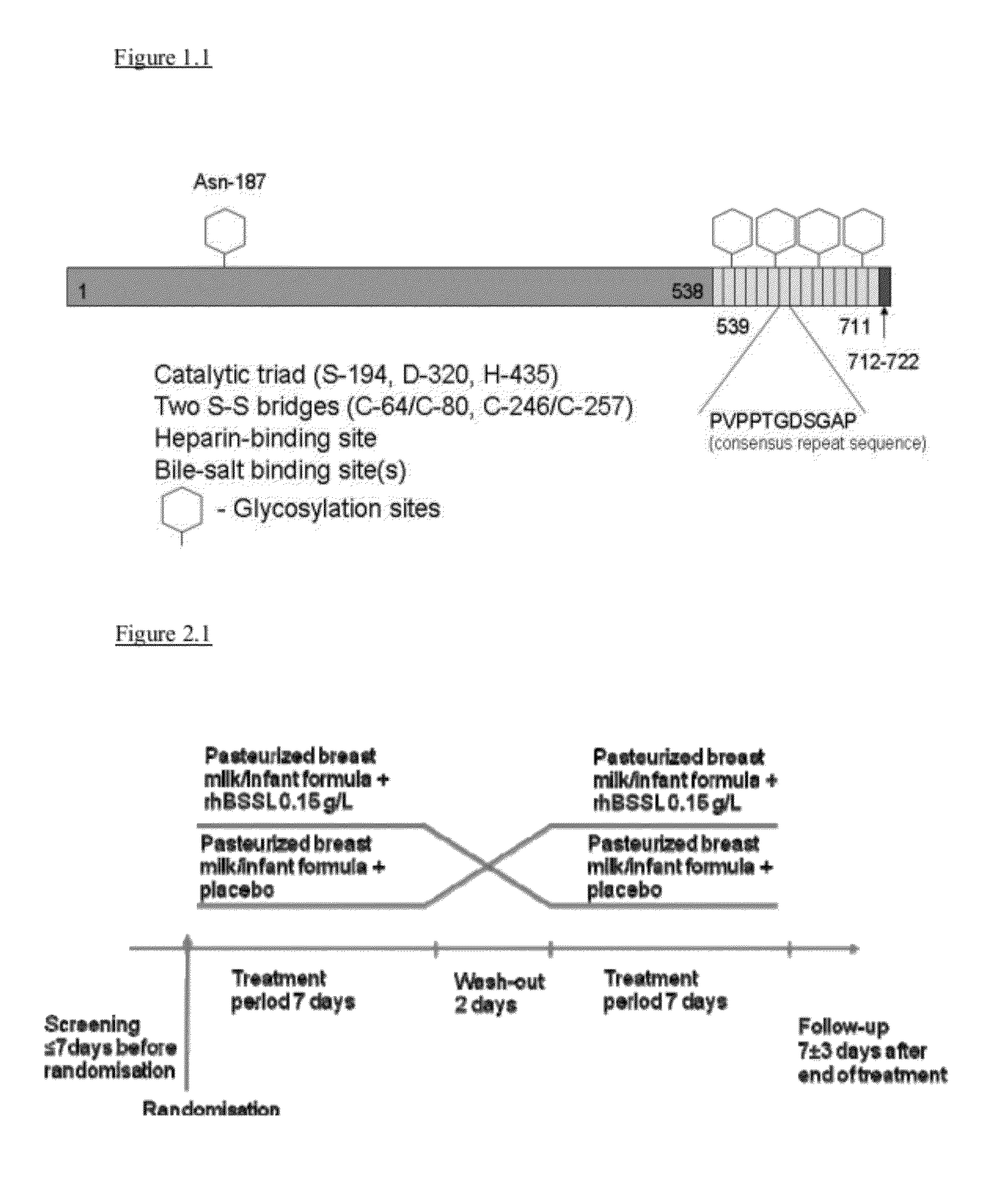
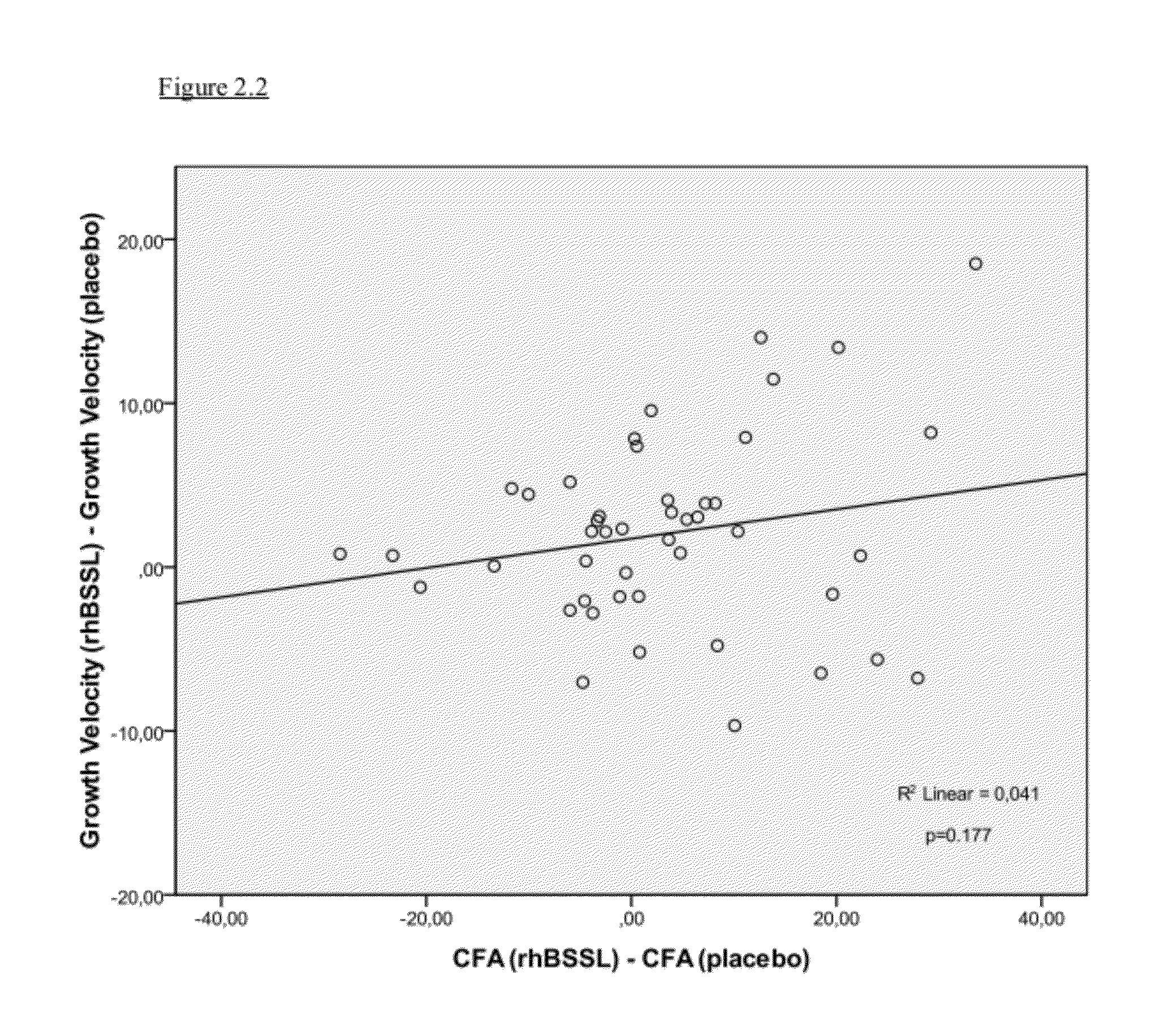
The present invention relates to a method to increase the growth velocity of a human infant, said method comprising the enteral administration to said infant of recombinant human bile-salt-stimulated lipase (rhBSSL). Such method has particular utility for underweight or preterm human infants, particular those in medical need of increasing their growth velocity. The invention also relates to compositions, including infant feeds, kits, packaged-pharmaceutical-products and pharmaceutical compositions, and also to methods to prepare infant feeds. In another aspect, the present invention relates to methods to: (X) protect the small bowel mucosa of a human infant from damage; to (Y) protect an immature intestinal epithelium of a human infant from the deleterious effects of incompletely digested and / or excess fat and / or lipid; and / or to (Z) limit accumulation of incompletely digested and / or excess fat and / or lipid in the ileum of a human infant; said methods in each case comprising the step of enteral administration of rhBSSL.
Owner:SWEDISH ORPHAN BIOVITRUM AB
Daily ration with low nitrogen emission in later stage of finishing pigs with weight being 90-120kg
InactiveCN104041713AEmission reductionImprove utilization efficiencyAnimal feeding stuffAccessory food factorsLow nitrogenThreonine
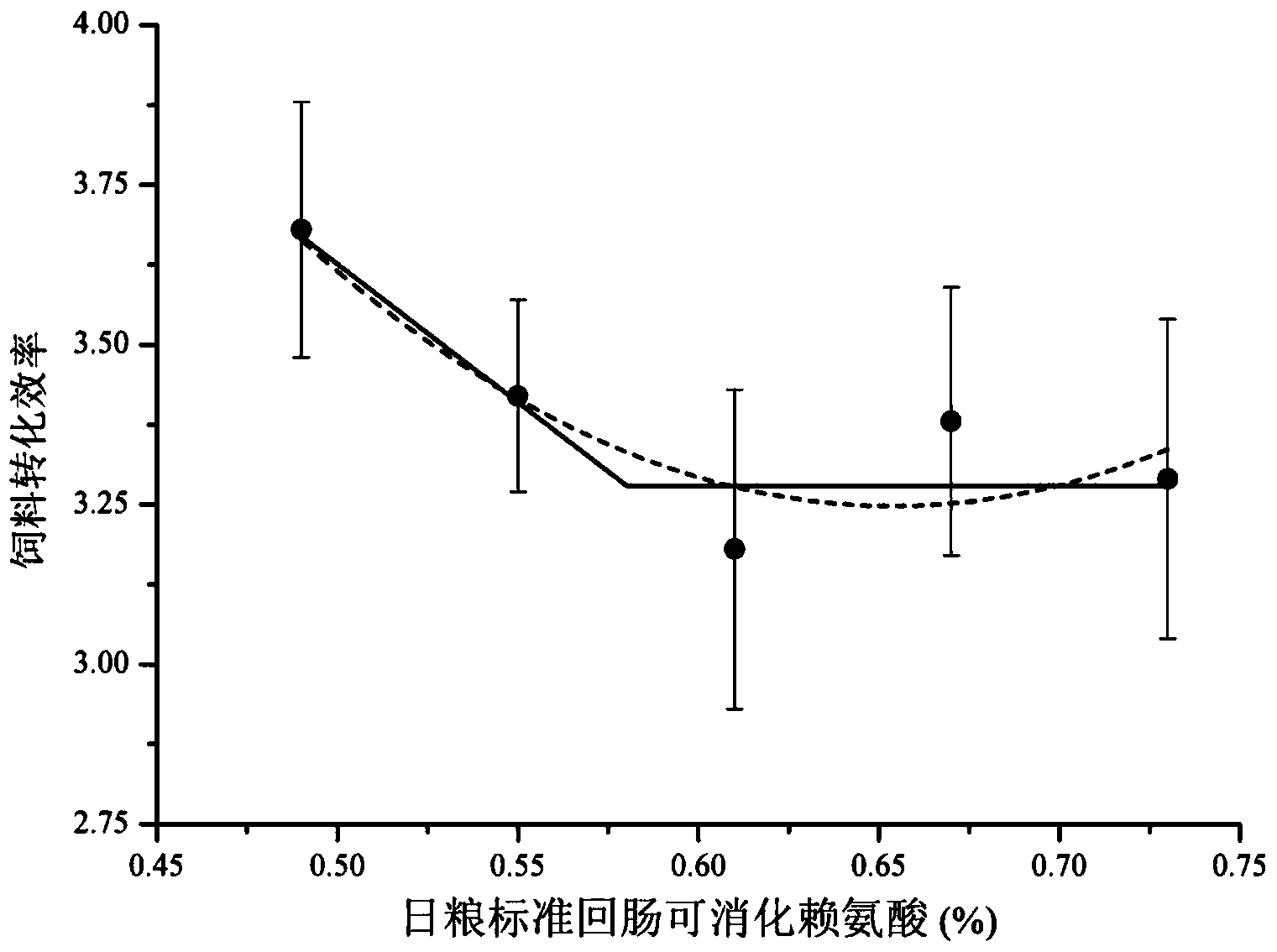
The invention discloses a daily ration with low nitrogen emission in later stage of finishing pigs with weight being 90-120kg. The daily ration comprises the following components by weight percent: 8.7-13.5% of protein and 0.49-0.73% of standard ileum digestible lysine, wherein the weight ratio of the standard ileum digestible tryptophan to the standard ileum digestible lysine is not less than 0.16%; the weight ratio of standard ileum digestible threonine to the standard ileum digestible lysine is not less than 0.61; the weight ratio of standard ileum digestible sulfur-containing amino acid to the standard ileum digestible lysine is not less than 0.57; the content of the net energy of daily ration with low nitrogen emission is 2.48Mcal / kg. Results show that the daily ration is capable of obtaining growth property and carcass quality similar to normal proteome, reducing the emission amount of animal nitrogen on the basis of cost consumption, thereby greatly improving the utilization efficiency of nitrogen in the animal.
Owner:CHINA AGRI UNIV
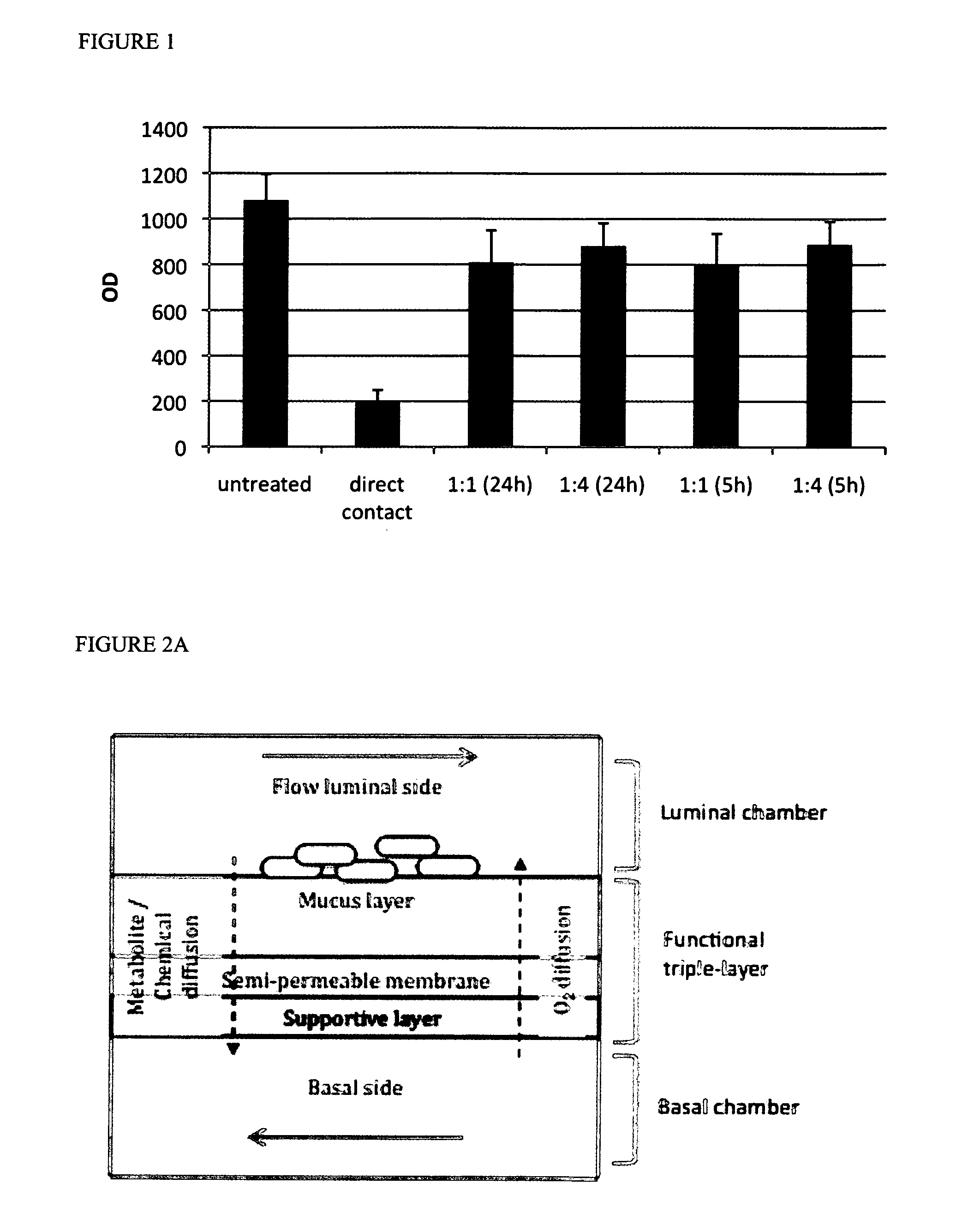
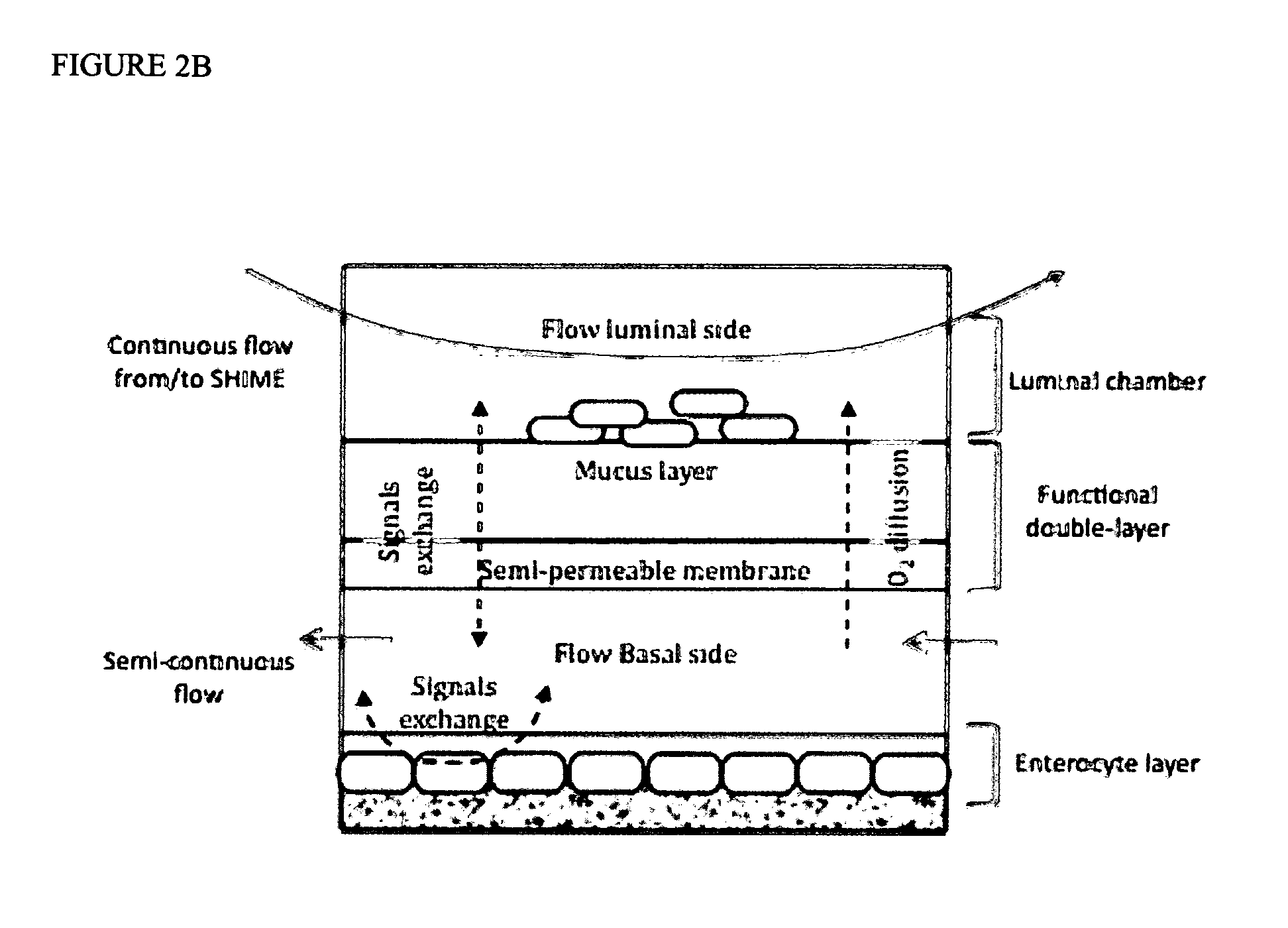
Technology and method to study microbial growth and adhesion to host-related surfaces and the host-microbiota interactions
InactiveUS20120058551A1Bioreactor/fermenter combinationsBiological substance pretreatmentsMicrobial ecosystemBiofilm
The present invention relates to in vitro adhesion modules that allow growth, stabilization and study of microbial communities that adhere to and colonize host-related surfaces, that mimic transport of chemical compounds across epithelial surfaces and simulate host-microorganism interactions and adaptation. It includes the provision of micromolar amounts of oxygen via the basal side of a mucus layer towards the adhered microorganisms thus establishing the microaerophilic conditions prevailing at the base of a biofilm. It can also include cells, simulating the host, in a chamber on the basal side of a functional layer comprising said mucus layer. The adhesion module of the present invention can be placed between the different compartments of the SHIME—the Simulator of the Human Intestinal Microbial Ecosystem. An extension of the SHIME is made where the duodenum, jejunum and ileum are separately mimicked.
Owner:UNIV GENT
Ileum fistulization tube
The invention discloses an ileum fistulization tube. One end of an outlet tube is communicated with intestine inner tube and the intestine inner tube is divided into a far-end tube body and a near-end tube body. The intestine inner tube is placed in the intestinal tract. Although strap-shaped adhesion or sheet-shaped adhesion is generated on the wound surface of an abdominal wall notch and the intestinal canal, due to the supporting of the intestine inner tube, small angles of the intestinal tract are not prone to generating, the angulation deformity of the intestinal canal is not prone to generating, and mechanical intestinal obstruction, volvulus, intestinal strangulation necrosis caused by internal hernia are prevented after an operation. According to the ileum fistulization tube, intestinal content can be smoothly discharged out of the human body only through the abdominal pressure without the dependence on a negative-pressure suction device, so that the burden of a patient is lessened and use is convenient. A designed double-layer air bag enables the intestinal tract to be completely obstructed, the intestinal content is prevented from polluting an anastomotic stoma or damaging a repairing portion; a contrast medium can be conveniently injected, the healing condition of the anastomotic stoma or the repairing portion can be observed, the number of times for which the fistulization tube is inserted in and pulled out of the patient is reduced, and diagnoses and treatment of a doctor are facilitated.
Owner:任东林 +1
Enteric sodium butyrate microcapsules as well as preparation method and application thereof
InactiveCN110897048ANot easy to absorb moistureImprove liquidityAccessory food factorsWorking-up animal fodderBiotechnologyEscherichia coli
The invention relates to an enteric sodium butyrate microcapsule as well as a preparation method and an application thereof. The enteric sodium butyrate microcapsule comprises an inner core layer anda coating material layer coating the surface of the inner core layer, the inner core layer comprises a sodium butyrate core and an auxiliary material layer wrapping the sodium butyrate core. The enteric sodium butyrate microcapsule preparation provided by the invention does not have rancidity-like smell, is not easy to absorb moisture, has good fluidity and dispersibility, can be slowly dissolvedin intestinal tracts, achieves targeted release of the intestinal tracts, and significantly improves the absorption effect. Compared with common sodium butyrate powder, when the sodium butyrate powderis used as a feed additive for feeding animals, the average daily feed intake and daily gain of the animals can be remarkably improved, the feed-to-weight ratio is reduced, and the diarrhea rate is reduced; the content of harmful bacteria such as escherichia coli in ileum and colons of animals can be remarkably reduced, and meanwhile beneficial bacteria such as lactic acid bacteria and bifidobacteria in the colons and cecum are remarkably increased.
Owner:GUANGZHOU GREAT BIOLOGICAL TECH
Anti-intussusception ileal stent and its use as an Anti-hyperglycemic method
InactiveUS20170128187A1Less complicatedLess invasiveStentsStomachIntestinal structureNutritional deficiency
Selected diabetic patients reveal significant glycemic control after bariatric surgical or endoscopic duodenal bypass. This controlling effect appears to occur partly because of an enhanced Glucagon-Like Peptide 1 (GLP1) release from intestinal L-cells. These procedures may also cause complications such as nutritional deficiencies. The present invention provides a stent. The method that places this stent in the ileum to activate the endogenous GLP1 release is a safer alternative to control the diabetes mellitus. However, the less mobile wall of the ileum next to the proximal stent rim can trigger an unwanted telescoping of the intestine, which is known as intussusception. An embodiment of this invention illustrates the characteristics of a stent that can prevent a likely intussusception and consequently be more confidently applied in the ileum to maintain or restore the ileal patency in general or for its anti-hyperglycemic effect in particular.
Owner:ZIAPOUR DR BEHRAD
Controlled release of vegetarian pore-sealing capsule in stomach, duodenum, jejunum, ileum and colon
InactiveCN105520917ACapsule deliveryMacromolecular non-active ingredientsControlled releaseJejunal lymph node
A vegetarian pore-sealing capsule is provided. The pore-sealing capsule may release active substances in a gastrointestinal tract, including stomach, duodenum, jejunum, ileum and colon, under control. The pore-sealing capsule includes a capsule shell having one or more pores and a pore-sealing material for sealing at least one of the pores. When the pore-sealing capsule is located at a destination inside the gastrointestinal tract, the pore-sealing material will leave the pores.
Owner:BIO PEPTIDE ENHANCER TECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com