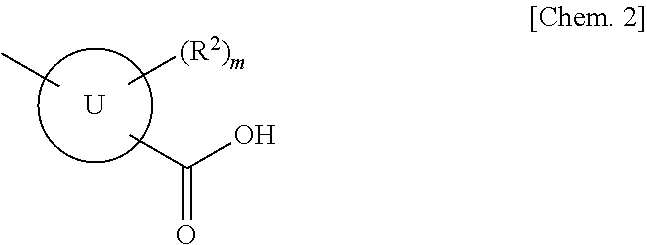Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
548 results about "Xanthine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
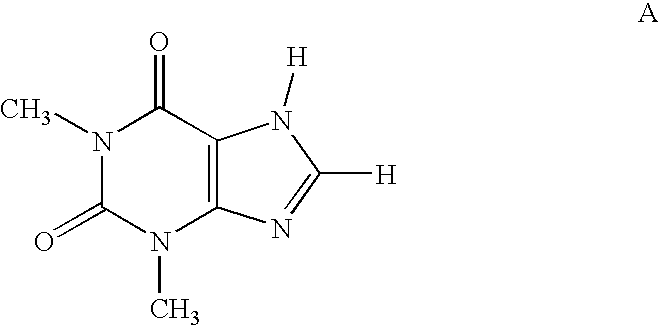
Xanthine (/ˈzænθiːn/ or /ˈzænθaɪn/; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base found in most human body tissues and fluids and in other organisms. Several stimulants are derived from xanthine, including caffeine and theobromine.
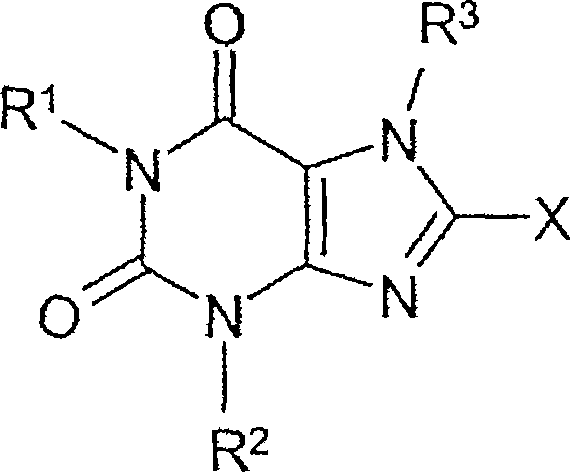
Derivatives of 8-substituted xanthines
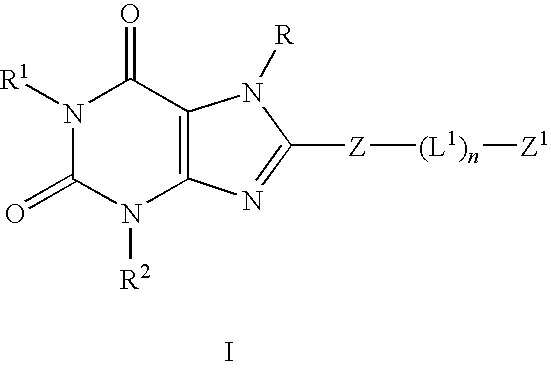
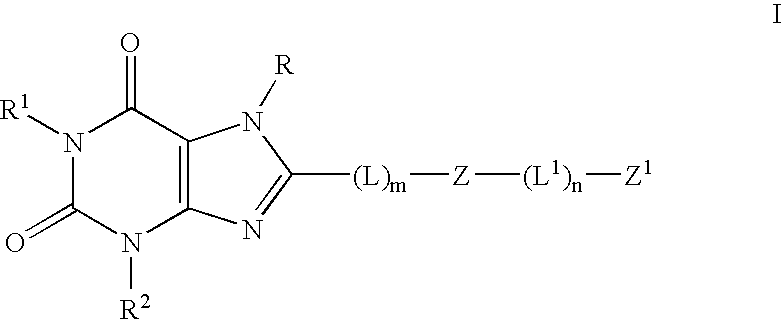
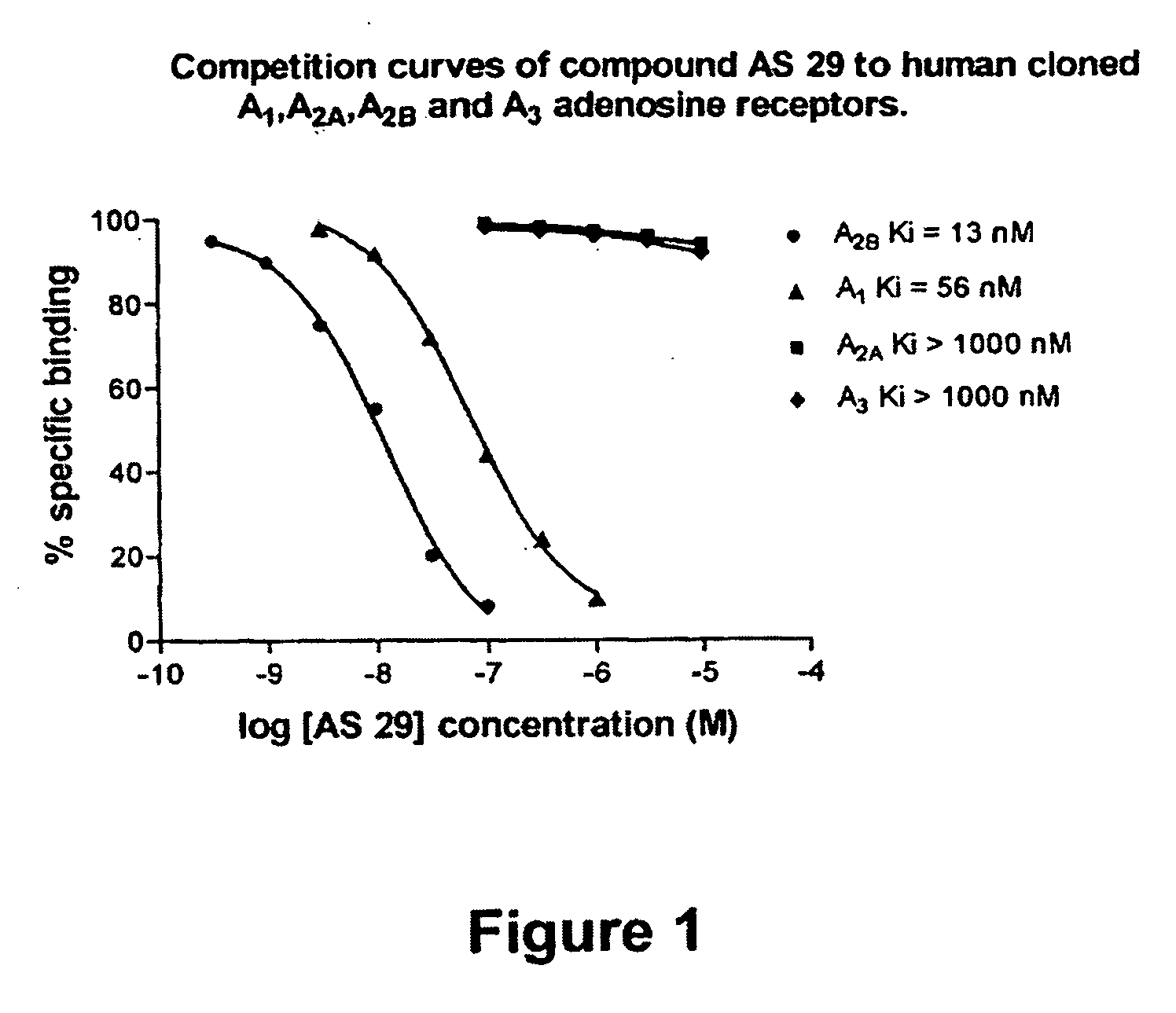
The present invention provides compounds and pharmaceutical compositions that are substituted pyridyl-linked-xantbines of formula Iwhich are selective antagonists of A2B adenosine receptors (ARs). These compounds and compositions are useful as pharmaceutical agents.
Owner:ADENOSINE THERAPEUTICS +1
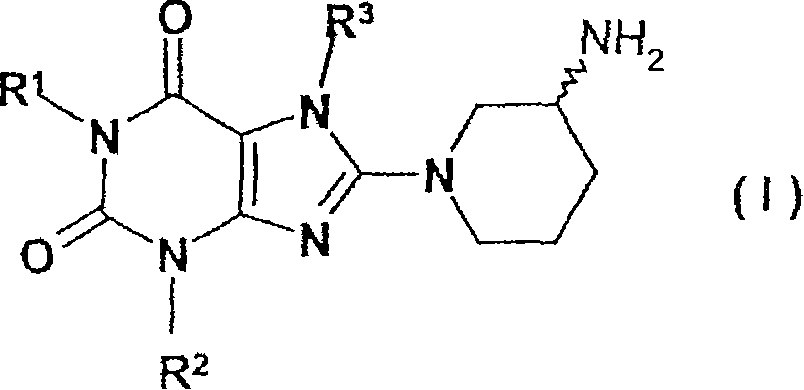
8-(3-amino-piperidin-1-yl)-xanthines, their preparation, and their use as pharmaceuticals
Owner:BOEHRINGER INGELHEIM INT GMBH
Combination products
Owner:SIGMOID PHARM LIMITED
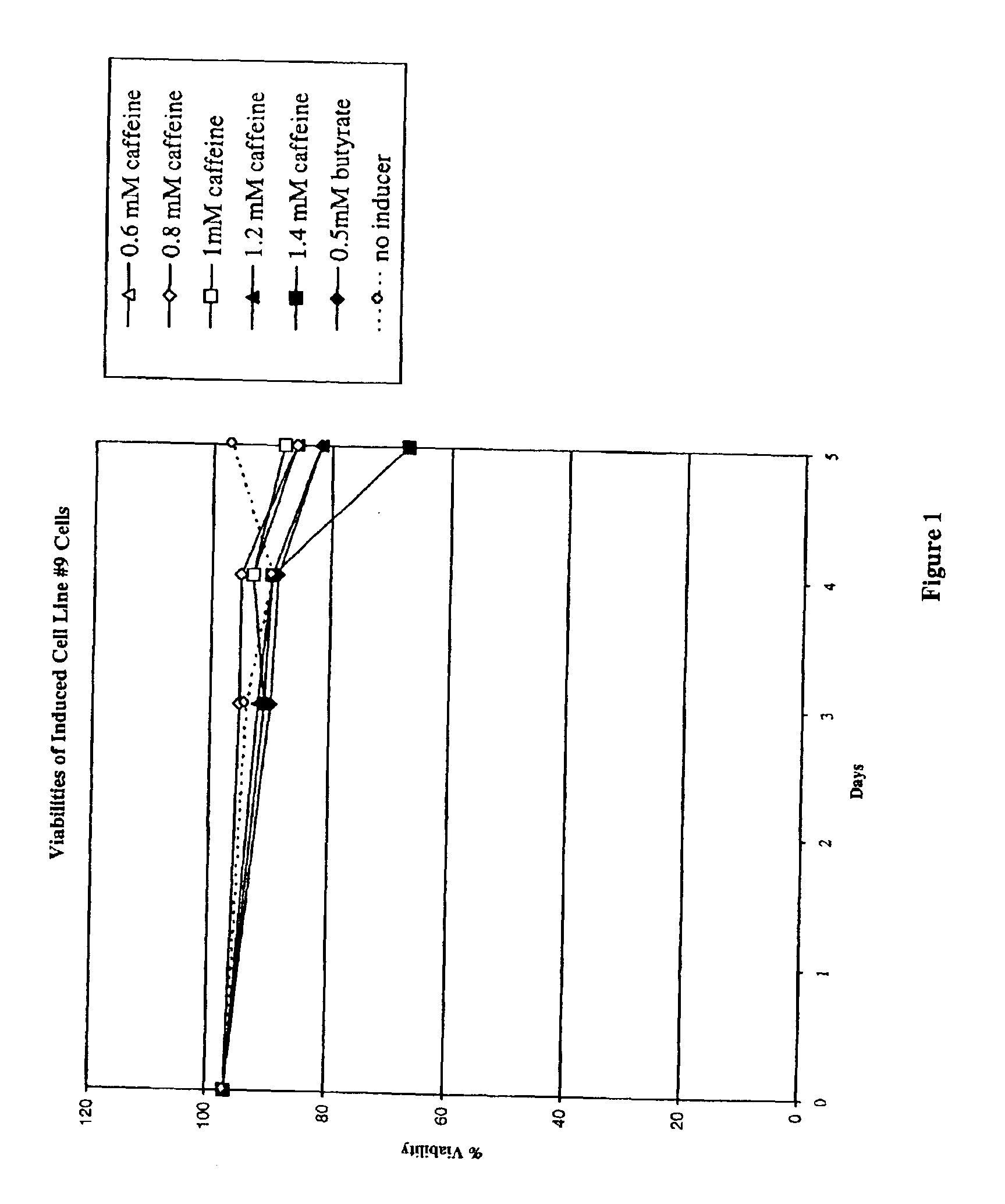
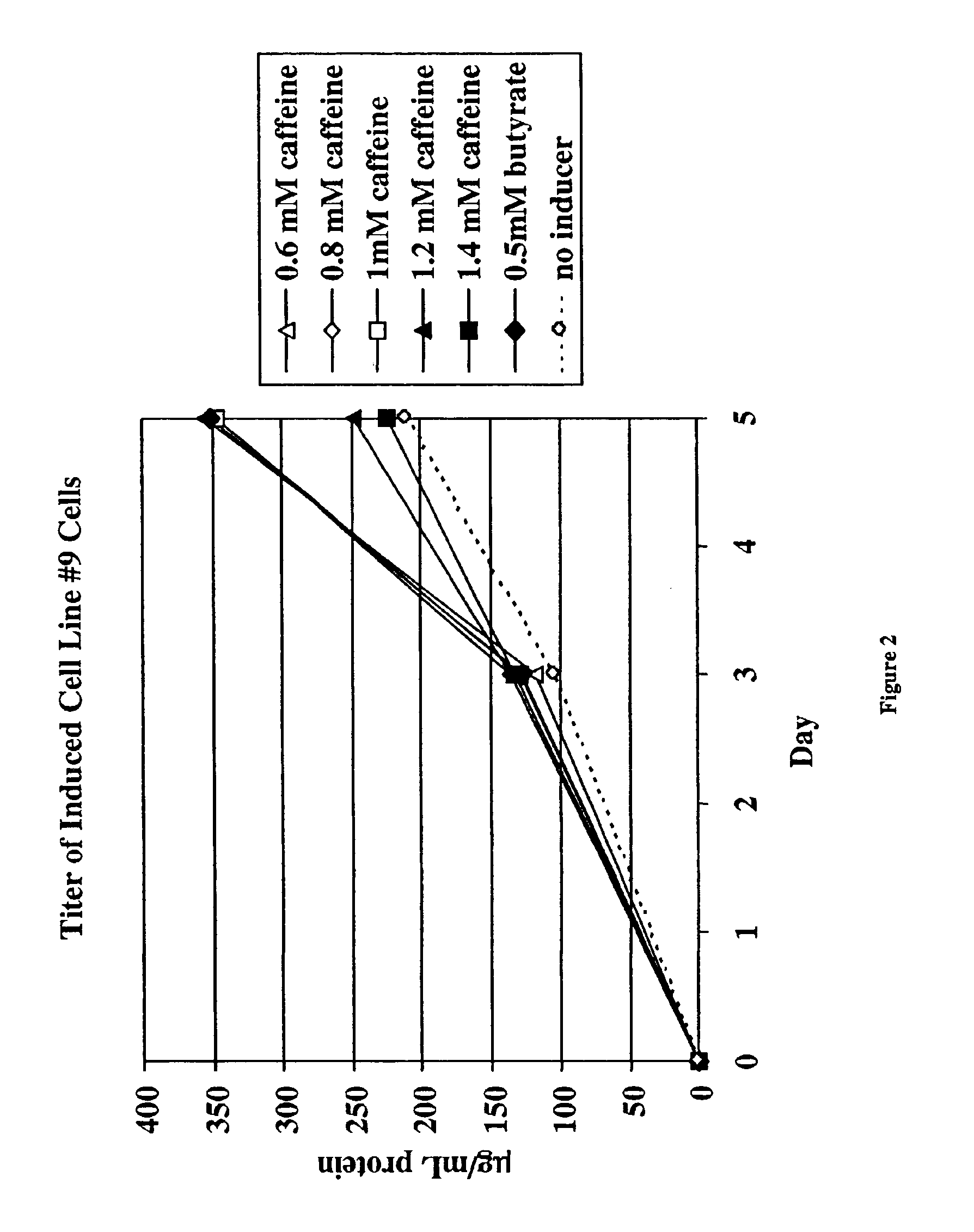
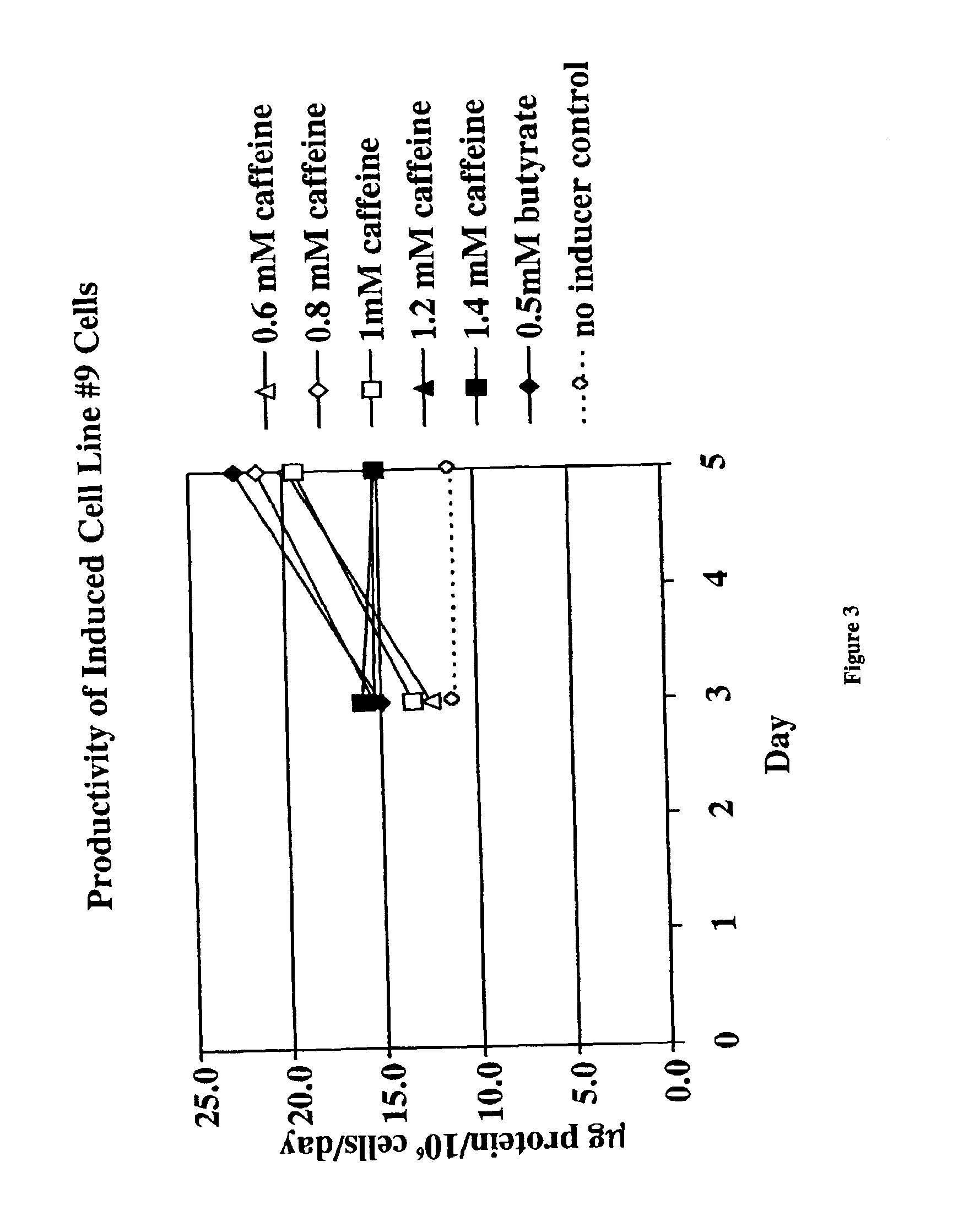
Methods for increasing polypeptide production
InactiveUS6872549B2Increase polypeptide expressionInduce productionAntibody mimetics/scaffoldsImmunoglobulinsXanthineInducer
The invention provides methods of increasing the production of polypeptides, optionally recombinant polypeptides, from mammalian cells using xanthine derivatives or hybrid polar compounds and cultures containing the same. Combinations of inducers including a hybrid polar compound and / or a xanthine derivative and / or an alkanoic acid can also be used, optionally at temperatures less than 37° C.
Owner:IMMUNEX CORP
Personal care compositions and methods for the beautification of mammalian skin and hair
Personal care composition comprising from about 0.05% to about 5% of at least one aquaporin-stimulating compound selected from the group consisting of xanthine, caffeine; 2-amino-6-methyl-mercaptopurine; 1-methyl xanthine; 2-aminopurine; theophylline; theobromine; adenine; adenosine; kinetin; p-chlorophenoxyacetic acid; 2,4-dichlorophenoxyacetic acid; indole-3-butyric acid; indole-3-acetic acid methyl ester; beta-naphthoxyacetic acid; 2,3,5-triiodobenzoic acid; adenine hemisulfate; n-benzyl-9-(2-tetrahydropyranyl)adenine; 1,3-diphenylurea; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)urea; zeatin; indole-3-acetic acid; 6-benzylaminopurine; alpha-napthaleneacetic acid; 6-2-furoylaminopurine; green tea extract; white tea extract; menthol; tea tree oil; ginsenoside-RB1; ginsenoside-RB3; ginsenoside-RC; ginsenoside-RD; ginsenoside-RE; ginsenoside-RG1; ginseng root extract; ginseng flower extract; pomegranate extract, extracts from Ajuga turkestanica; extracts from viola tricolor and combinations thereof; an additional ingredient selected from the group consisting of niacinamide, glycerin and mixtures thereof, and a dermatologically-acceptable carrier.
Owner:THE PROCTER & GAMBLE COMPANY
8-(3-amino-piperidin-1-yl)-xanthines, their preparation, and their use as pharmaceuticals
Owner:BOEHRINGER INGELHEIM INT GMBH
Nitrogenated heterocyclic compound and pharmaceutical composition comprising the same
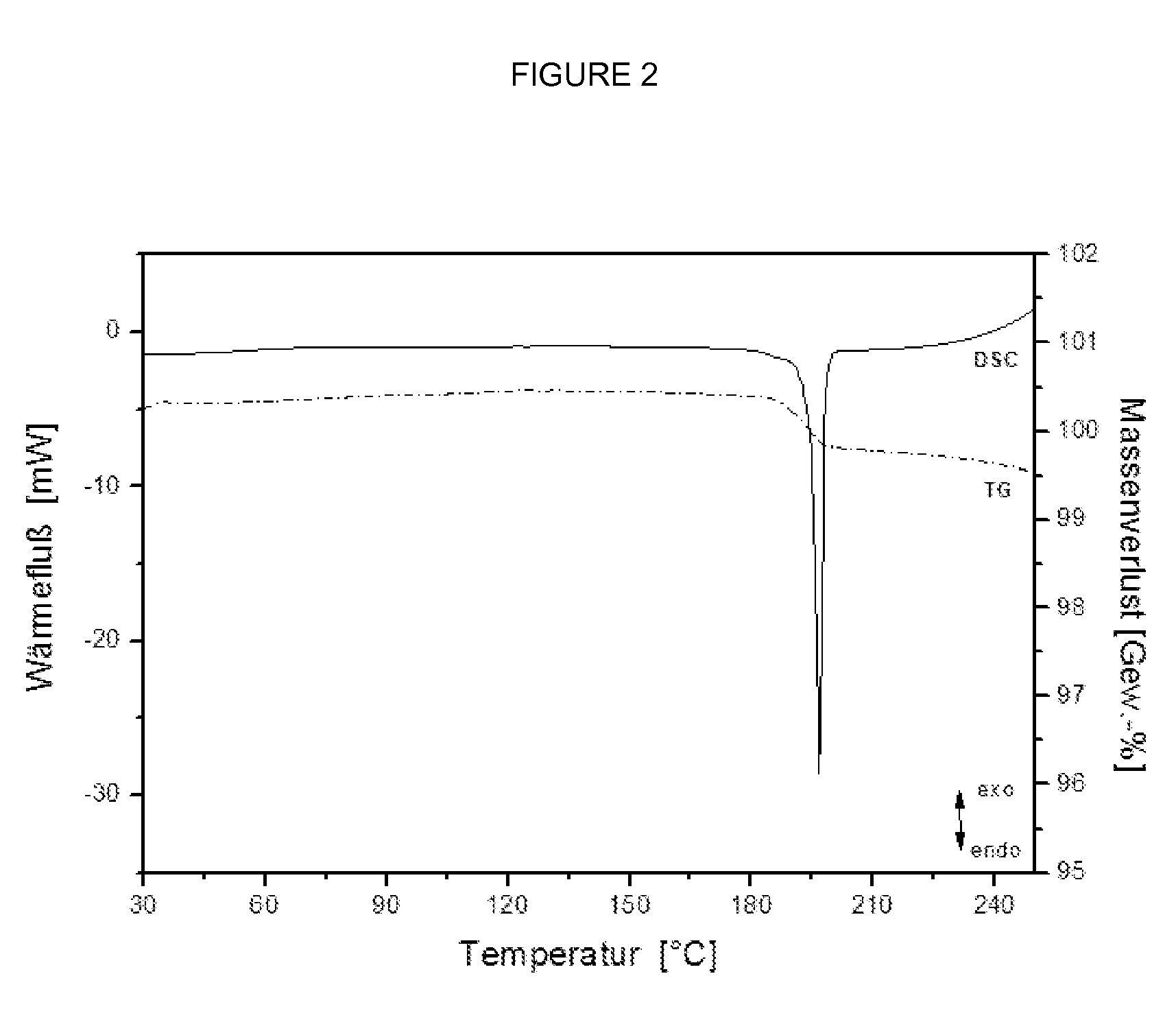
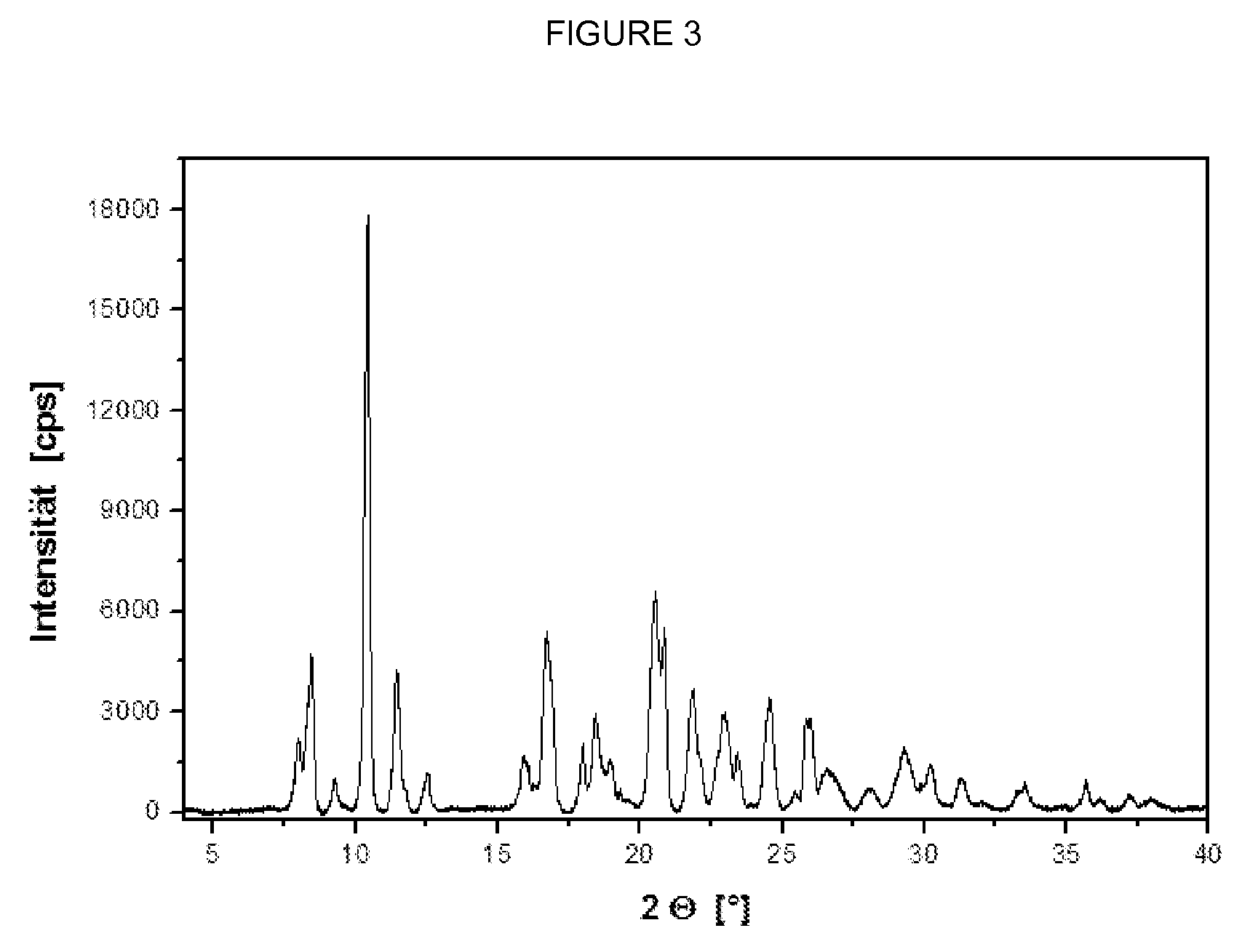
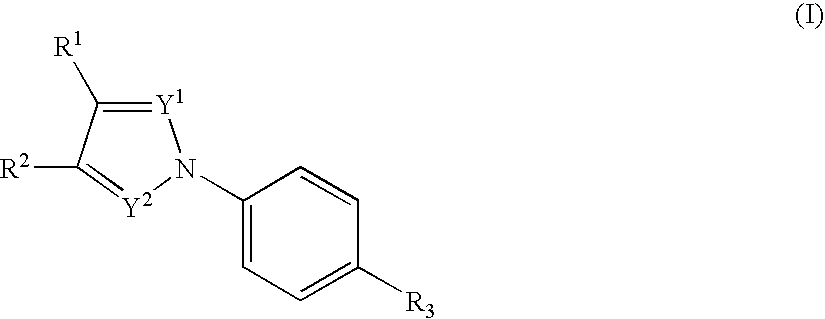
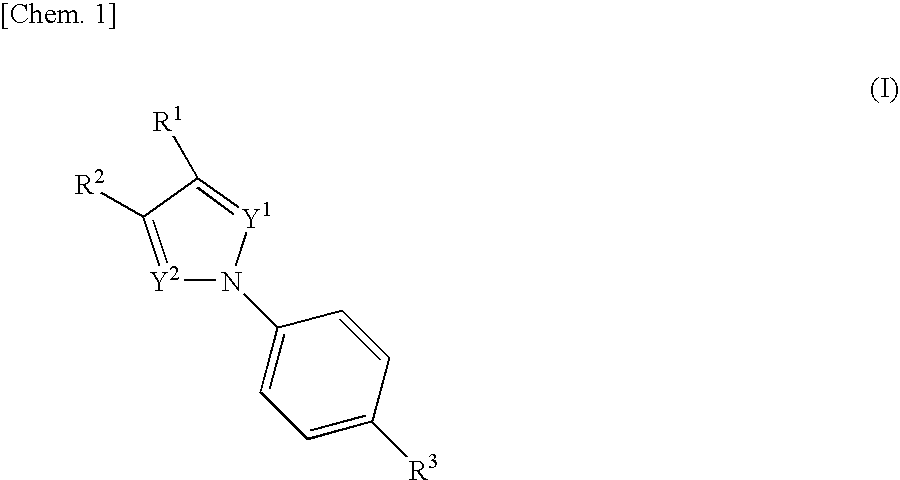
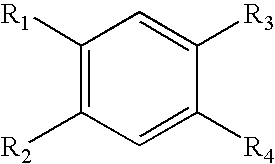
The present invention relates to novel compounds having a xanthine oxidase inhibitory effect and an uricosuric effect and pharmaceutical compositions comprising the same as an active ingredient. That is, the present invention relates nitrogen-containing heterocyclic compounds represented by the following general formula (I):wherein Y1 represents N or C(R4); Y2 represents N or C(R5); R4 and R5 independently represent an alkyl group, a hydrogen atom etc.; one of R1 and R2 represents an optionally substituted aryl group, an alkoxy group or an optionally substituted heterocyclic group; the other of R1 and R2 represents a haloalkyl group, a cyano group, a halogen atom etc.; and R3 represents a 5-tetrazolyl group or a carboxy group, and pharmaceutically acceptable salts thereof, and pharmaceutical compositions comprising the same as an active ingredient.
Owner:KISSEI PHARMA
(AZA)indolizine derivative and pharmaceutical use thereof
InactiveUS20130217878A1Inhibit productionExcellent xanthine oxidase inhibitory activityOrganic active ingredientsOrganic chemistryDiseaseXanthine
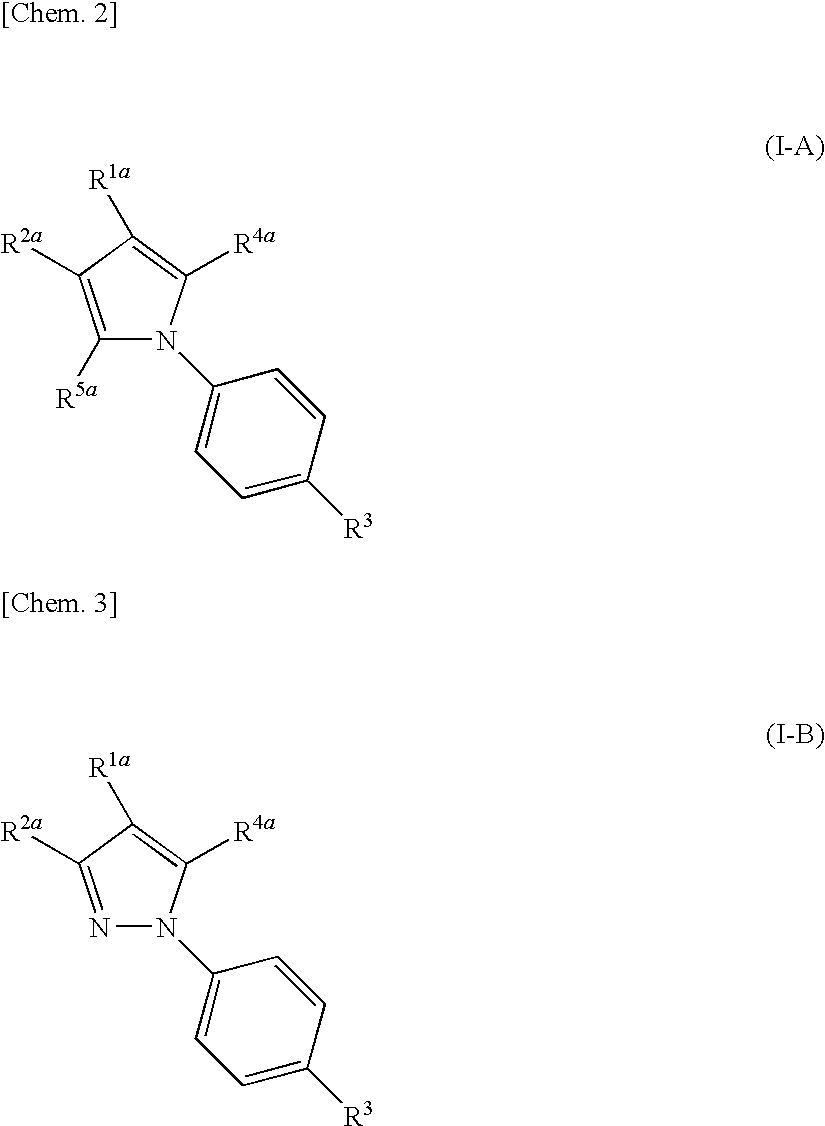
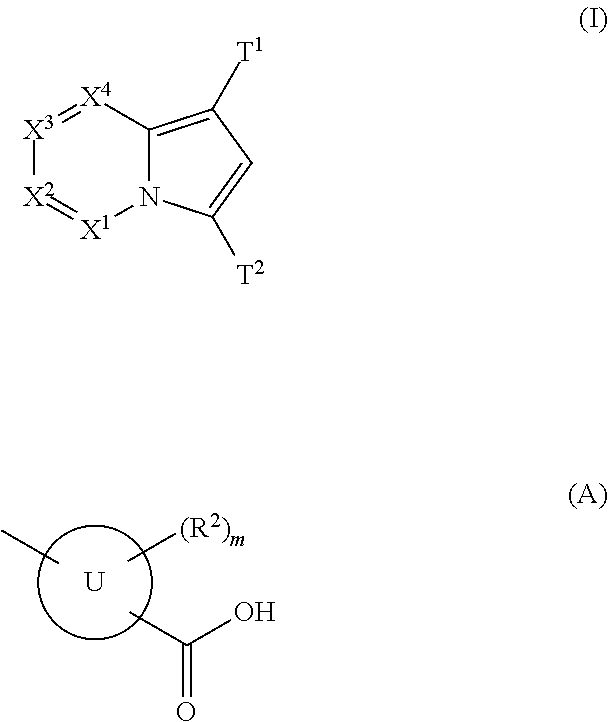
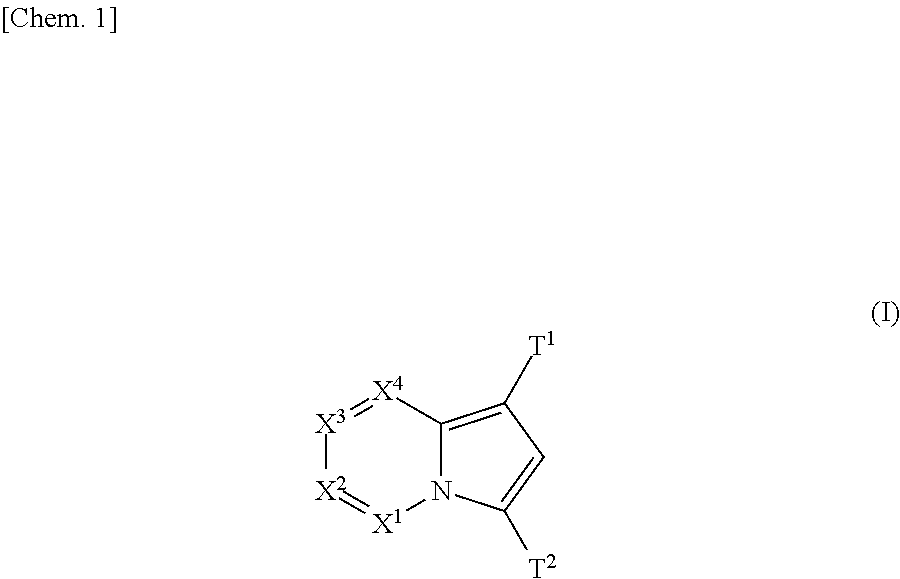
(Aza)indolizine derivatives represented by Formula (I) having xanthine oxidase inhibitory activities and useful as agents for the prevention or treatment of a disease associated with abnormality of serum uric acid level, prodrugs thereof, salts thereof or the like. In Formula (I), 0 to 2 of X1, X2, X3 and X4 are a nitrogen atom and the others are CR1; one of T1 and T2 represents cyano and the other represents a group represented by Formula (A), with the proviso that when T1 is cyano, at least one of X1 to X4 is a nitrogen atom; R1 independently represents a hydrogen atom, a halogen atom, a hydroxy group, C1-6 alkyl, C1-6 alkoxy or the like; ring U represents a benzene ring or the like; m represents integral number from 0 to 2; R2 independently represents a fluorine atom, a hydroxy group or the like.
Owner:KISSEI PHARMA
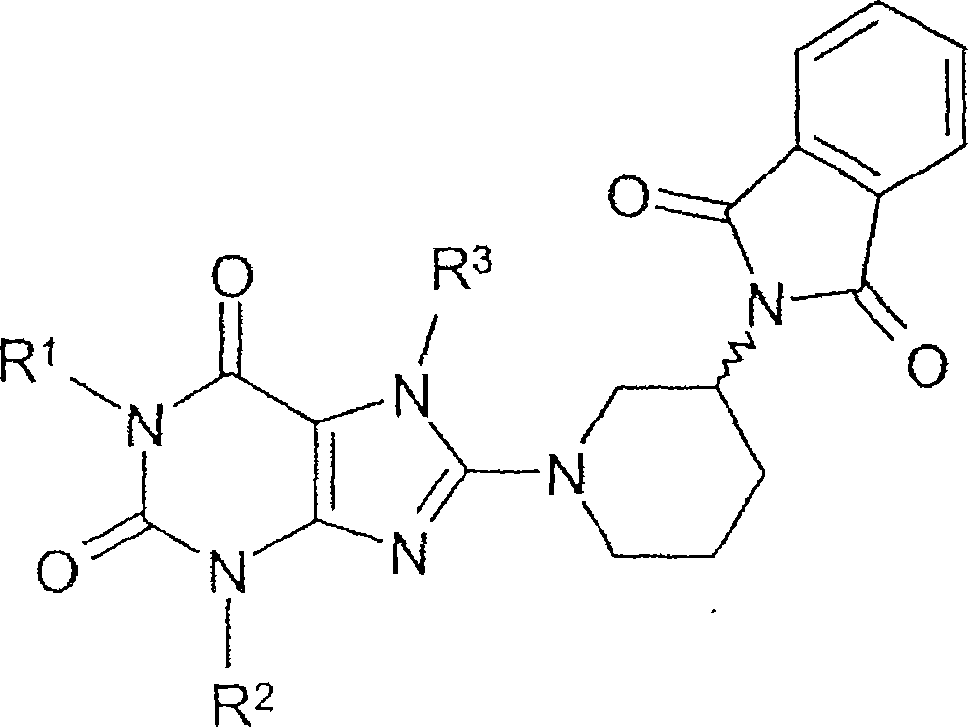
Method for producing chiral 8-(3-amino-piperidin-1-yl)-xanthines
Method for preparing 8-(3-aminopiperidin-1-yl)-xanthine derivatives (I) and their enantiomers and salts. Method for preparing 8-(3-aminopiperidin-1-yl)-xanthine derivatives of formula (I) and their enantiomers and salts by: (a) reacting an 8-X-xanthine (III) with 3-phthalimidopiperidine (A), or its enantiomer; (b) deprotecting the product (II); and (c) optionally conversion to salt. X : halo or sulfonate ester, especially bromo; R 1>phenylcarbonylmethyl, benzyl, naphthylmethyl, pyridinylmethyl, pyrimidinylmethyl, (iso)quinolinylmethyl, quinazolinylmethyl, quinoxalinylmethyl, naphthyridinylmethyl or phenanthridinylmethyl, all optionally substituted by one or two, same or different, Ra; R 2>1-3C alkyl, cyclopropyl or phenyl; R 3>2-buten-1-yl, 3-methyl-2-buten-1-yl, 2-butyn-1-yl, or 2-(fluoro, chloro, bromo, iodo, methyl, trifluoromethyl or cyano)-benzyl; Ra : hydrogen, fluoro, chloro, bromo, cyano, methyl, trifluoromethyl, ethyl, phenyl, methoxy, di- or tri-fluoromethoxy, or two Ra on adjacent C atoms complete OCH 2O or OCH 2CH 2O Independent claims are also included for the following: (1) (R) and (S)-3-phthalimidopiperidine as new compounds; and (2) methods for preparing the compounds of (1). [Image] [Image] - ACTIVITY : Antidiabetic; Antiarthritic; Anorectic; Osteopathic. No details of tests for these activities are given. - MECHANISM OF ACTION : Inhibition of dipeptidylpeptidase IV.
Owner:BOEHRINGER INGELHEIM INT GMBH
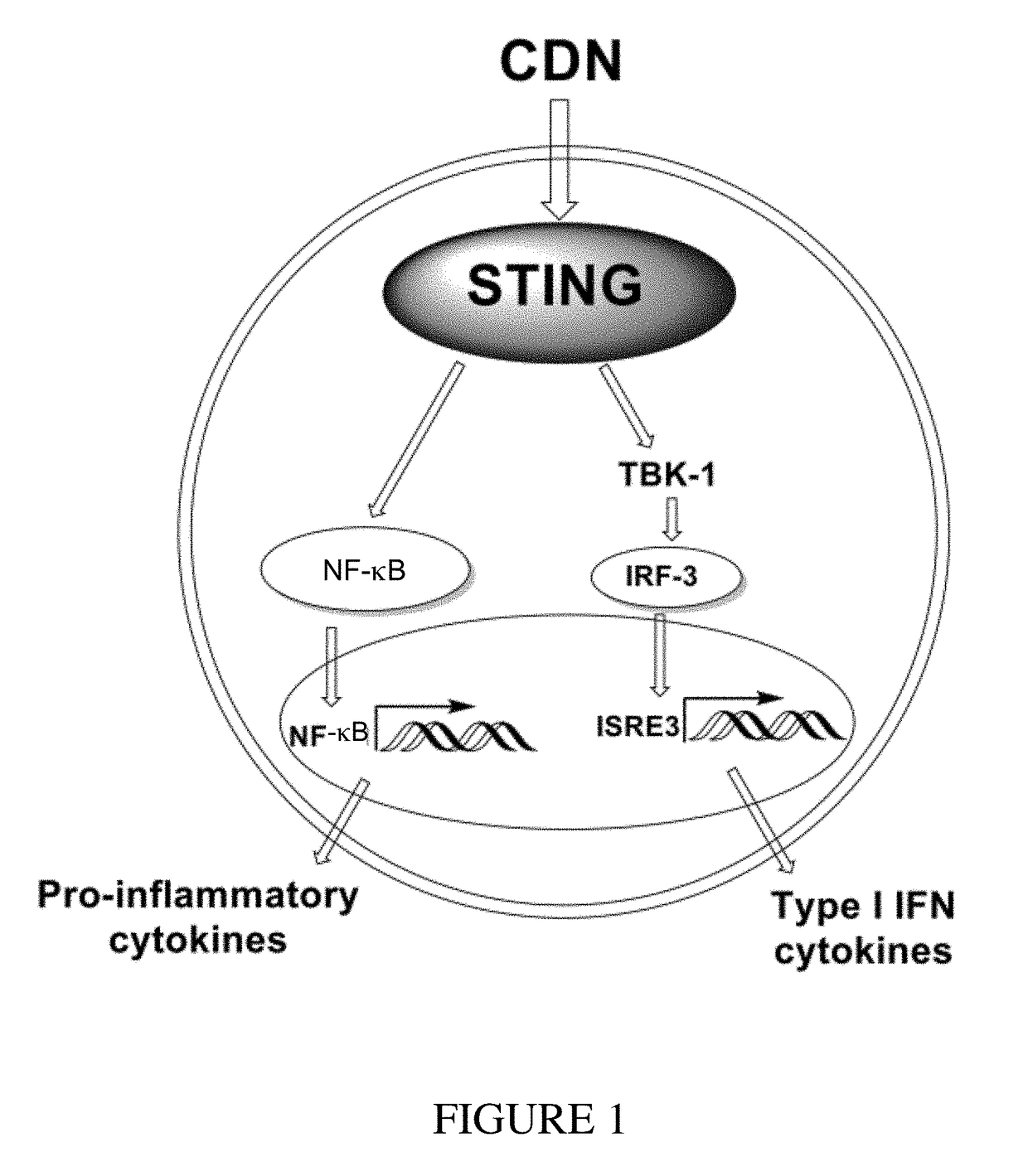
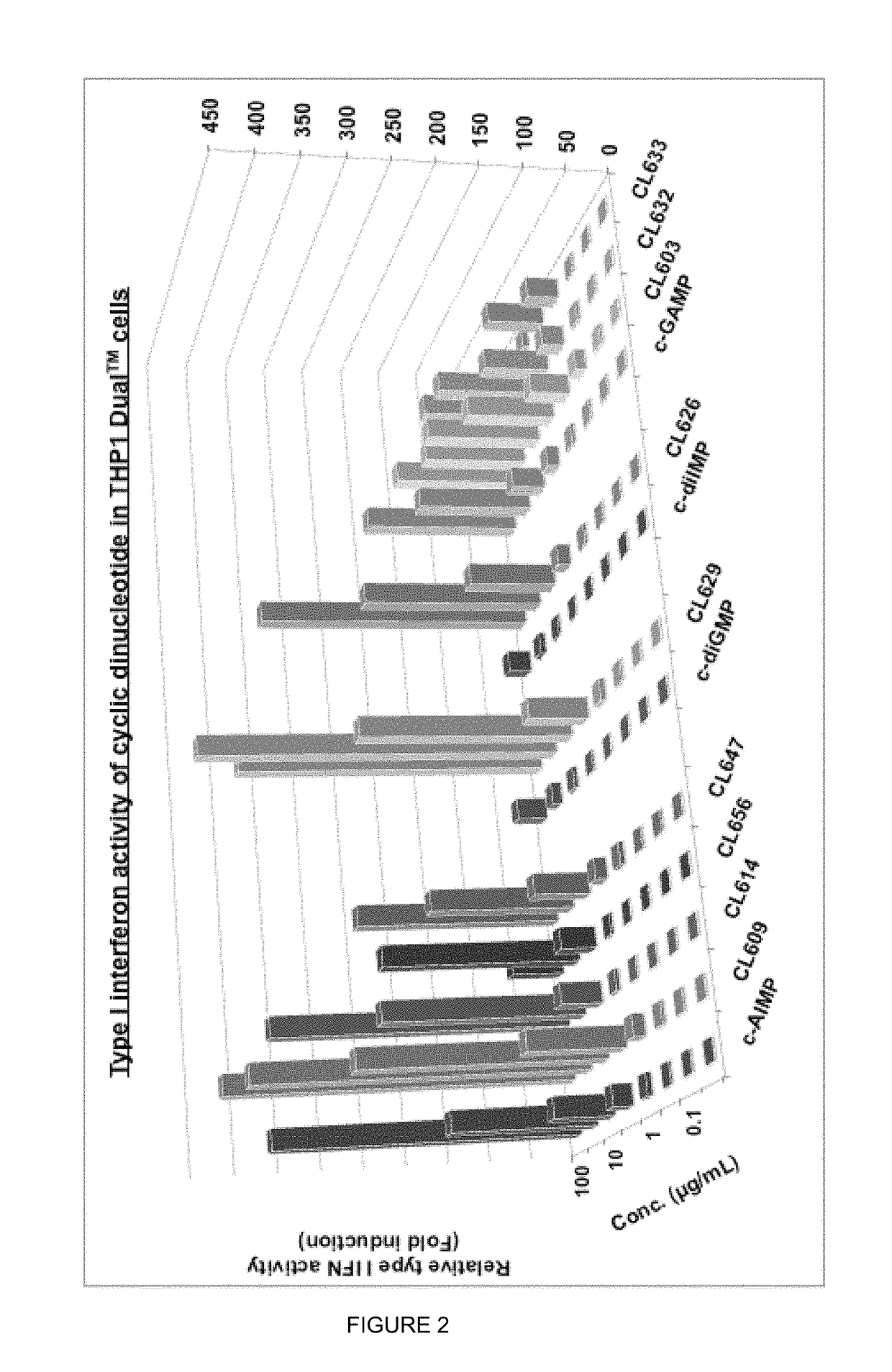
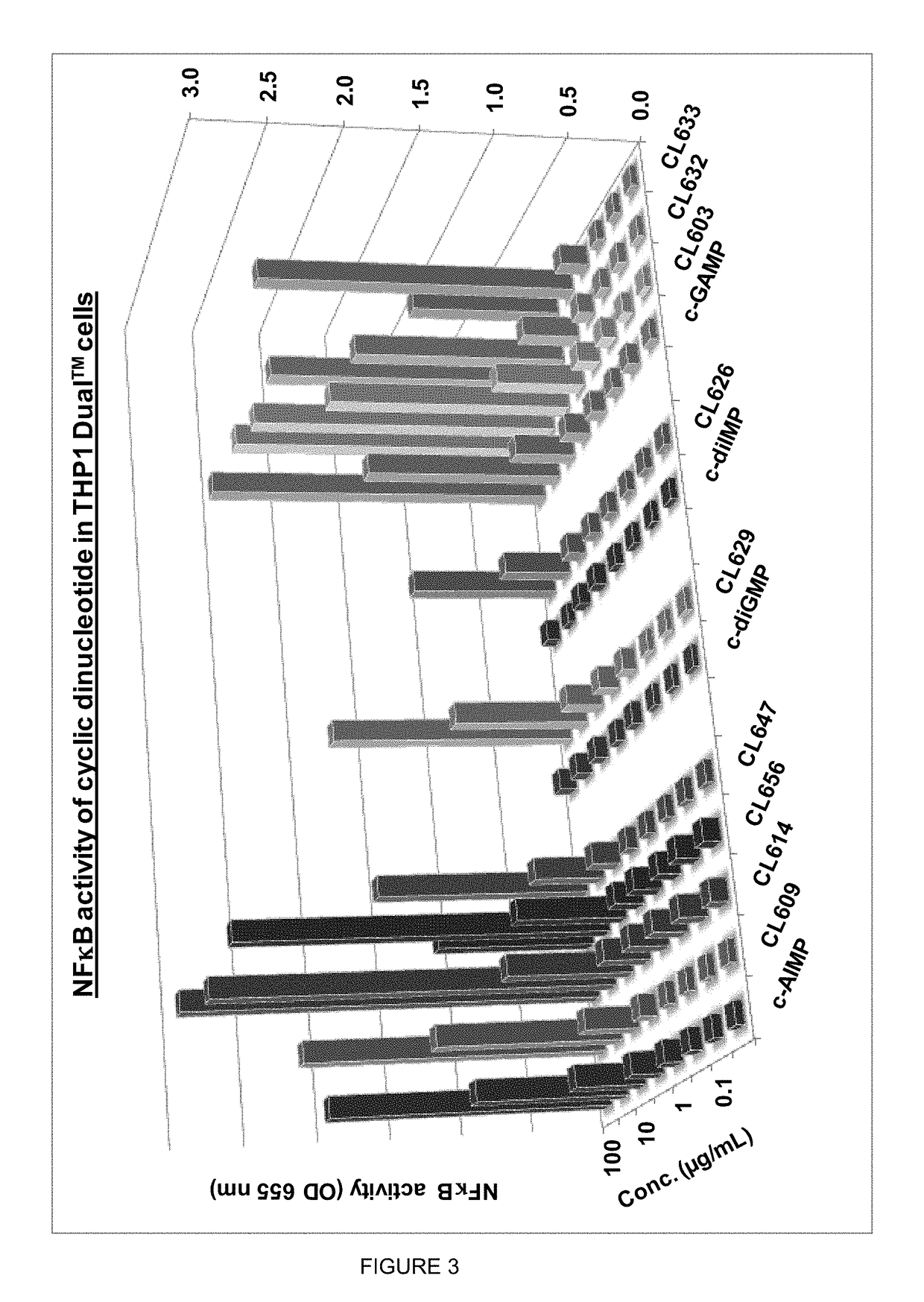
Cyclic dinucleotides for cytokine induction
ActiveUS10011630B2High activityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsPurineIn vivo
A cyclic dinucleotide compound of Formula (I):wherein X1 is H or F; X2 is H or F; at least one among X1 and X2 is a fluorine atom; Z is OH, OR1, SH or SR1, wherein: R1 is Na or NH4, or R1 is an enzyme-labile group which provides OH or SH in vivo such as pivaloyloxymethyl; B1 and B2 are bases chosen from Adenine, Hypoxanthine or Guanine, and B1 is a different base than B2 and a pharmaceutically acceptable salt thereof. Pharmaceutical compositions including the cyclic dinucleotide, as well as their use in the treatment of a bacterial infection, a viral infection or a cancer are also described.
Owner:KAYLA THERAPEUTICS
Preparation method and use of moringa oleifera leaf polysaccharides
InactiveCN104829743AHigh purityHigh deproteinization rateOrganic active ingredientsSkeletal disorderBiotechnologyXanthine
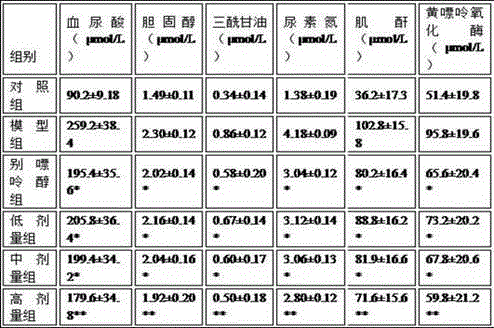
The invention belongs to the chemical field of natural products and particularly relates to a preparation method and use of moringa oleifera leaf polysaccharides. The preparation method comprises the steps of carrying out combined microwave-ultrasound extraction, hydrochloric acid method deproteinization, hydrogen peroxide method decoloration and AB-8 macroporous adsorption resin column separation, so as to prepare the moringa oleifera leaf polysaccharides. The preparation method of the moringa oleifera leaf polysaccharides has the advantages of short extraction time, low extraction temperature, low energy consumption and high extraction rate and purity. Besides, an animal experiment proves that the prepared moringa oleifera leaf polysaccharides have remarkable treatment effect to hyperuricosuria; by decreasing the content of cholesterol, triacylglycerol, urea nitrogen, creatinine and xanthine oxidase, the production of uric acid is achieved; meanwhile, by directly decomposing uric acid, renal functions are improved, the excretion of uric acid is promoted, and blood vessels are protected, thereby being favorable to the recovery of patients with the hyperuricosuria. The preparation method has a wide medical application prospect.
Owner:隽觅(广州)生物科技有限公司
Application of celery seed extract to preparation of medicine or health-care food for resisting to hyperuricemia and gout
InactiveCN105535048AEasy to solveGood treatment effectSkeletal disorderNatural extract food ingredientsSerum uric acidAdditive ingredient
The invention relates to application of celery seed extract to preparation of medicine or health-care food for resisting to hyperuricemia and gout and further provides medicine or health-care food for treating hyperuricemia and gout. Celery seed extract serves as the active constituent, and an appropriate number of medical carriers or auxiliary constituents are added, so that a preparation is obtained. The medicine for health-care food is simple in preparation process, the celery seed extract can obviously lower serum uric acid of model mice suffering from hyperuricemia caused by oteracil potassium salt, lower the activity of xanthine oxidase to different degrees, achieve an obvious inhibition effect on arthritis of the mice, prevent and treat hyperuricemia or gout and be applied to preparation of the medicine or health-care food for resisting to hyperuricemia and gout.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
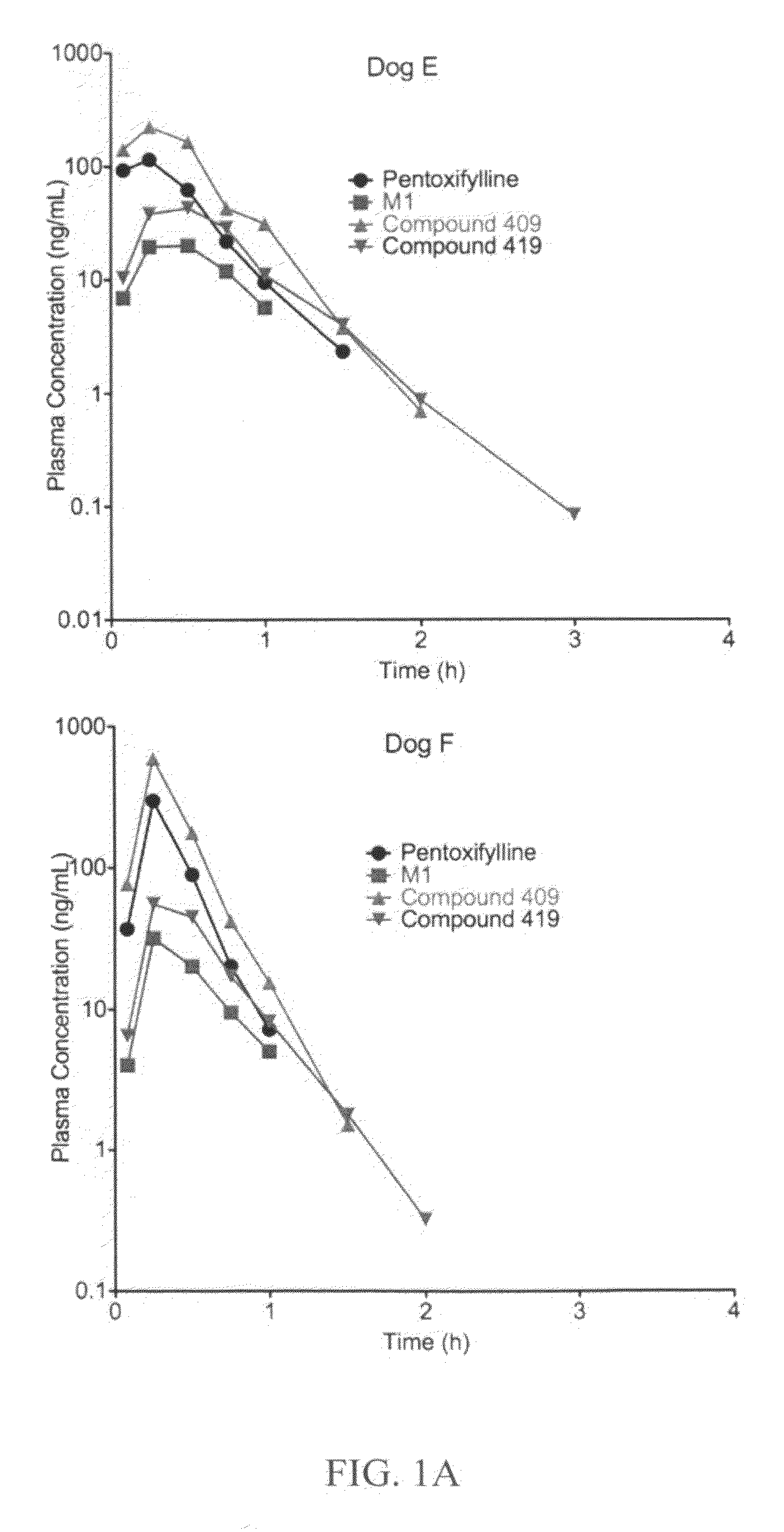
Substituted xanthine derivatives
This invention relates to novel compounds that are substituted xanthine derivatives and pharmaceutically acceptable salts thereof. For example, this invention relates to novel substituted xanthine derivatives that are derivatives of pentoxifylline. This invention also provides compositions comprising one or more compounds of this invention and a carrier and the use of the disclosed compounds and compositions in methods of treating diseases and conditions for which pentoxifylline and related compounds are beneficial.
Owner:CONCERT PHARMA INC
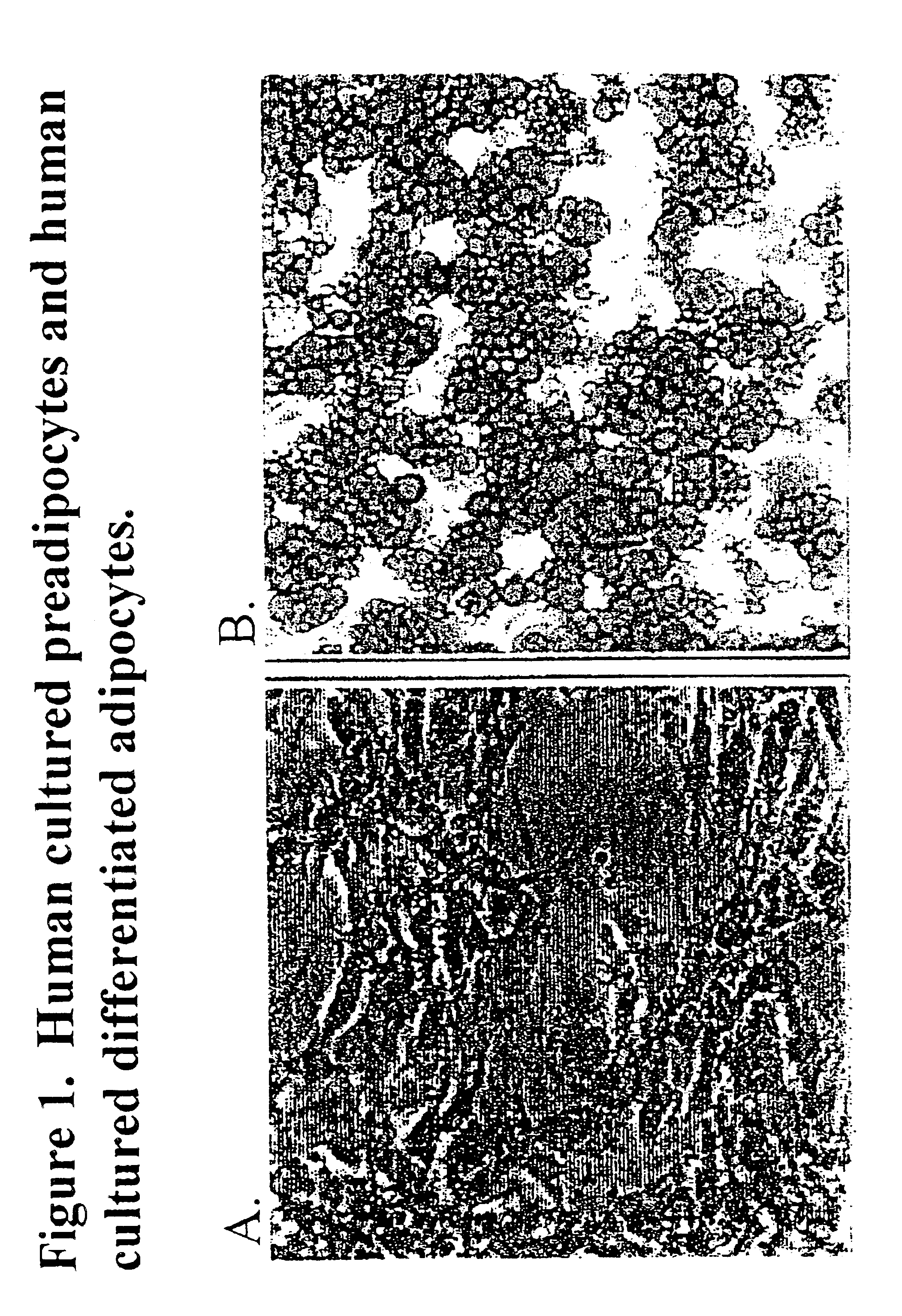
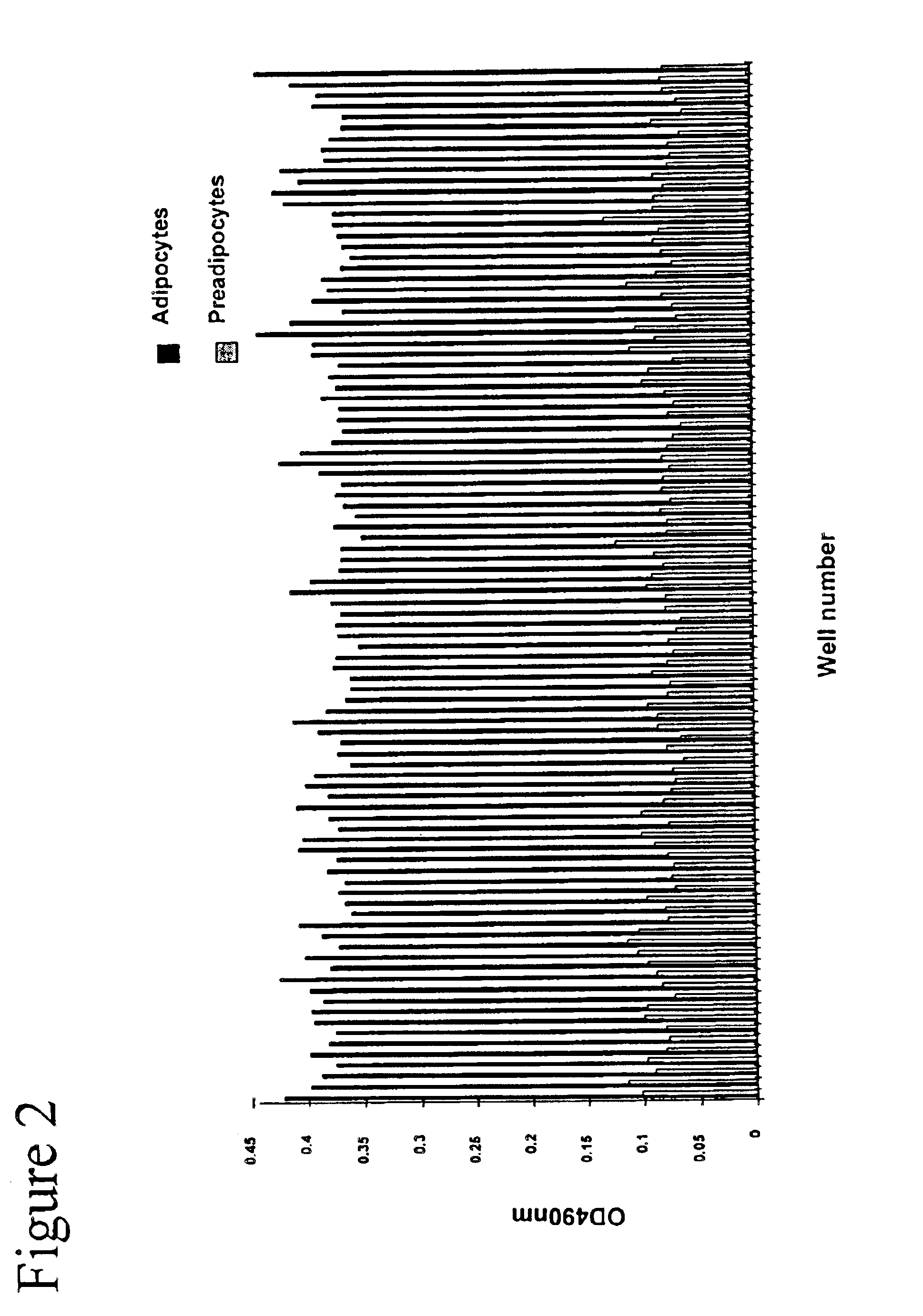
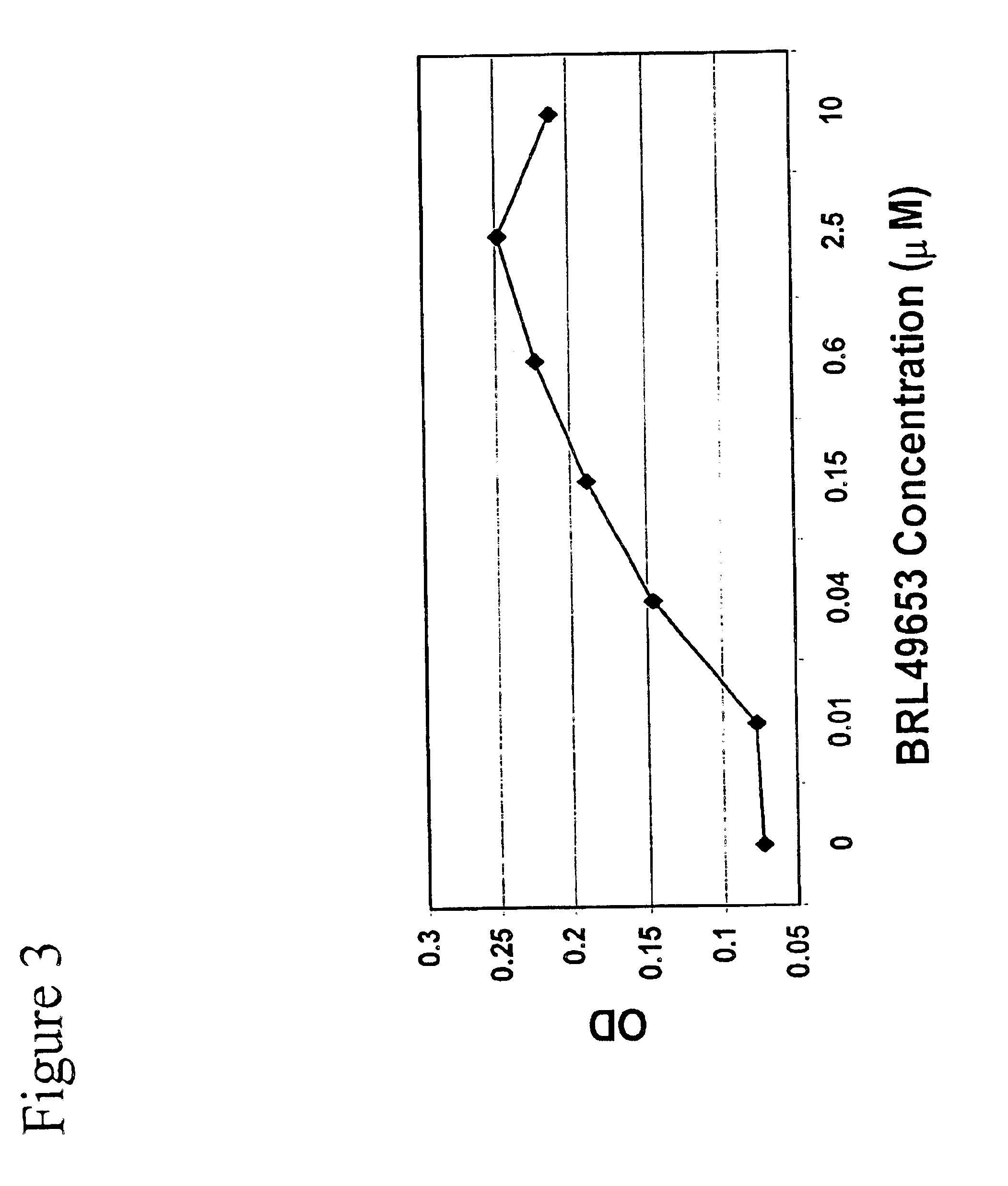
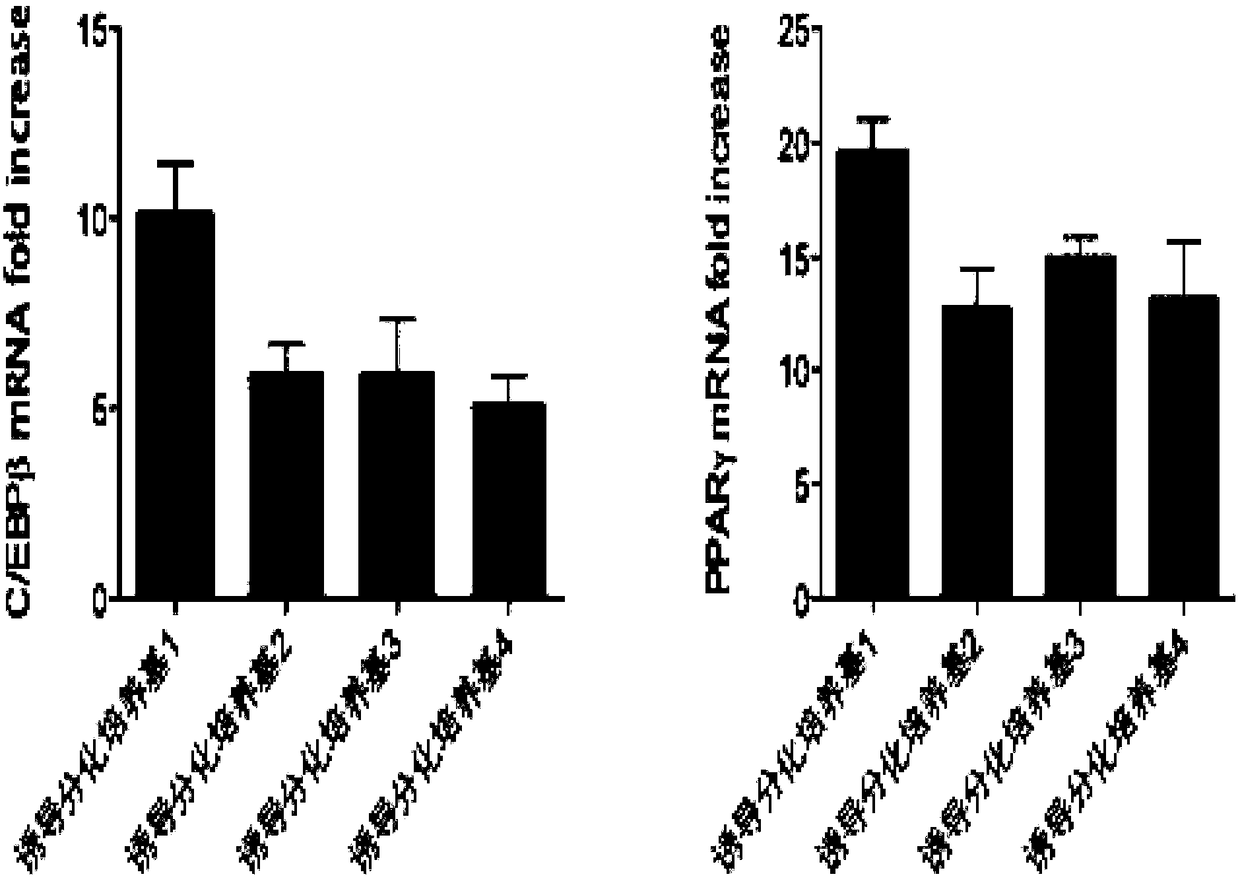
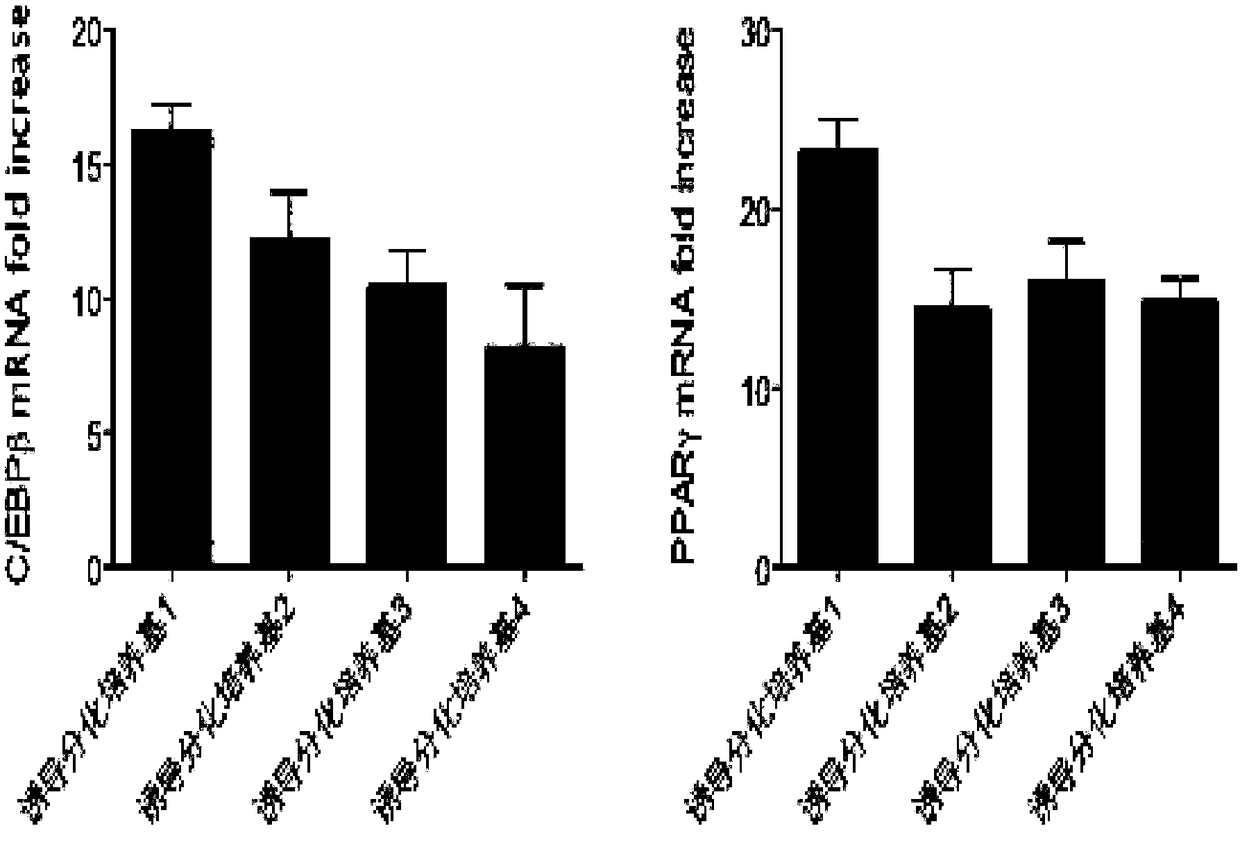
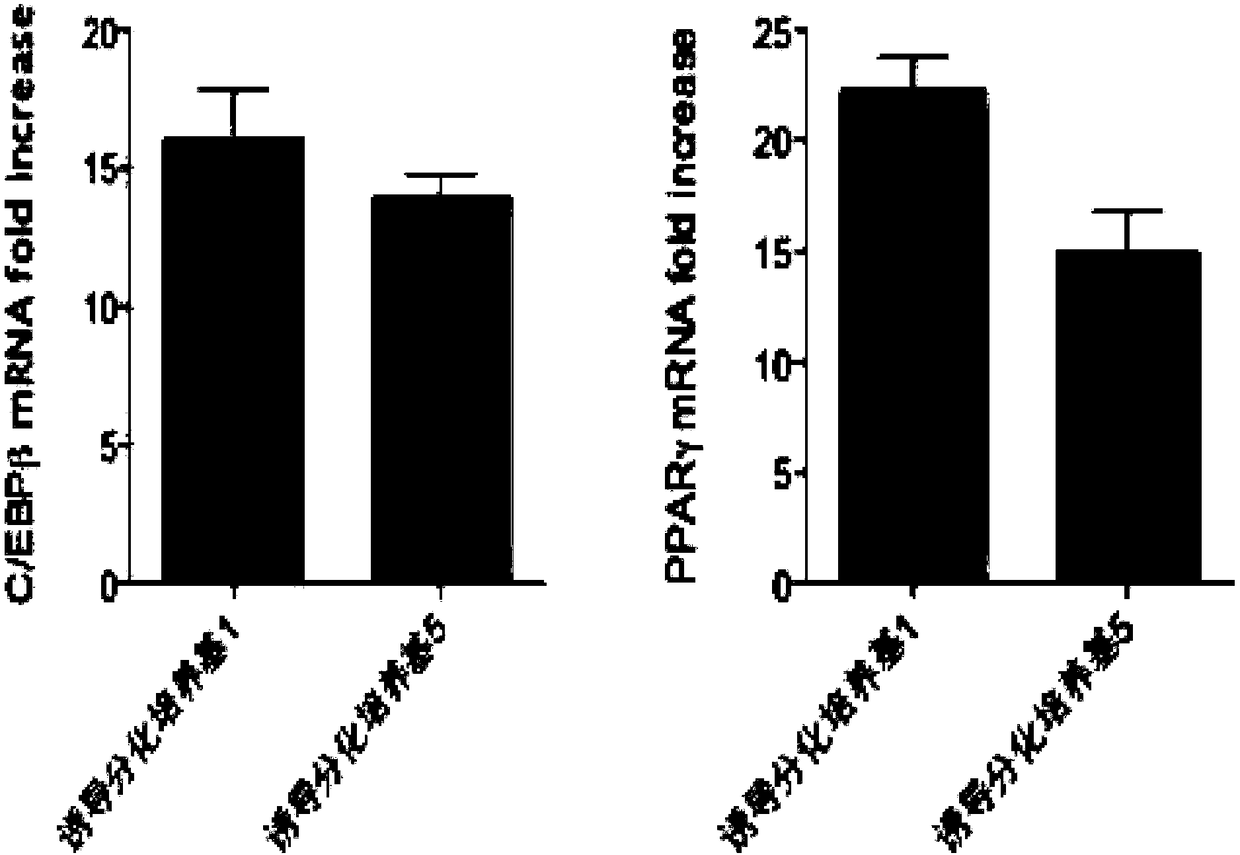
Methods and compositions for the differentiation of human preadipocytes into adipocytes
The present invention provides methods and compositions for the consistent and quantitative differentiation of human preadipocytes isolated from adipose tissue into adipocytes bearing biochemical, genetic, and physiological characteristics similar to that observed in isolated primary adipocytes. The methods of the invention comprise incubating isolated human preadipocytes, plated at least about 25,000 cells / cm2, in a medium containing, glucose, a cyclic AMP inducer such as isobutylmethylxanthine or forskolin, a glucocorticoid or glucocorticoid analogue, insulin or an insulin analogue and a PPARγ agonist or a RXR agonist. The compositions of the invention include media for the differentiation of human preadipocytes, human adipocytes differentiated by the methods of the invention and transfected adipocytes.The present invention also provides methods for determining the ability of a compound to affect the differentiation of human preadipocytes to adipocytes, for determining the ability of a compound to act as a PPARγ antagonist. a glucocorticoid, a glucocoticoid analogue, or an insulin analogue, for transfecting cultured human adipocytes, and as a means to identify novel polypeptides secreted from human adipocytes into the conditioned medium. The methods and compositions have use in the drug discovery of compounds having relevance to the disease states of diabetes, obesity, and cardiovascular disease and in the studies of these diseases.
Owner:SEED INTPROP LAW GRP
Agents and Methods for Osteogenic Oxysterols Inhibition of Oxidative Stress on Osteogenic Cellular Differentiation
InactiveUS20090202660A1Eliminating and minimizing effectInhibition of osteogenic differentiationBiocideOrganic active ingredientsPurine-Xanthine OxidaseBone Marrow Stromal Cell
The present invention discloses oxygenic oxygenic oxysterols. Also disclosed, agents and methods for protecting, blocking or rescuing marrow stromal cells from the inhibitory effects of oxidative stress on their osteoblastic cellular differentiation. Exemplary agents include oxysterols, rhBMP2, alone or in combination which are demonstrated to specifically combat oxidative stress caused by inflammatory oxidized lipids, such as xanthine / xanthine oxidase and minimally oxidized LDL. The synergistic effects of oxysterols and bone morphogenic proteins are disclosed.
Owner:RGT UNIV OF CALIFORNIA
Composition For External Use
InactiveUS20080207560A1High crystallinityLong orientationCosmetic preparationsBiocideVitamin CVitamin A Alcohol
An object of the present invention is to provide a composition for external use in which the percutaneous absorbability of vitamin A, vitamin A derivative(s), vitamin C, specific vitamin C derivative(s), xanthine derivative(s), ubiquinone(s) and / or hyaluronic acid is improved.The composition for external use is prepared by blending (i) a phospholipid and (ii) a mono- or oligo-glycol ether, together with (iv) at least one bioactive component selected from the group consisting of hyaluronic acid, hyaluronic acid derivatives, vitamin A, vitamin A derivatives, vitamin C, specific vitamin C derivatives, xanthine derivatives, ubiquinones, and salts thereof.
Owner:ROHTO PHARM CO LTD
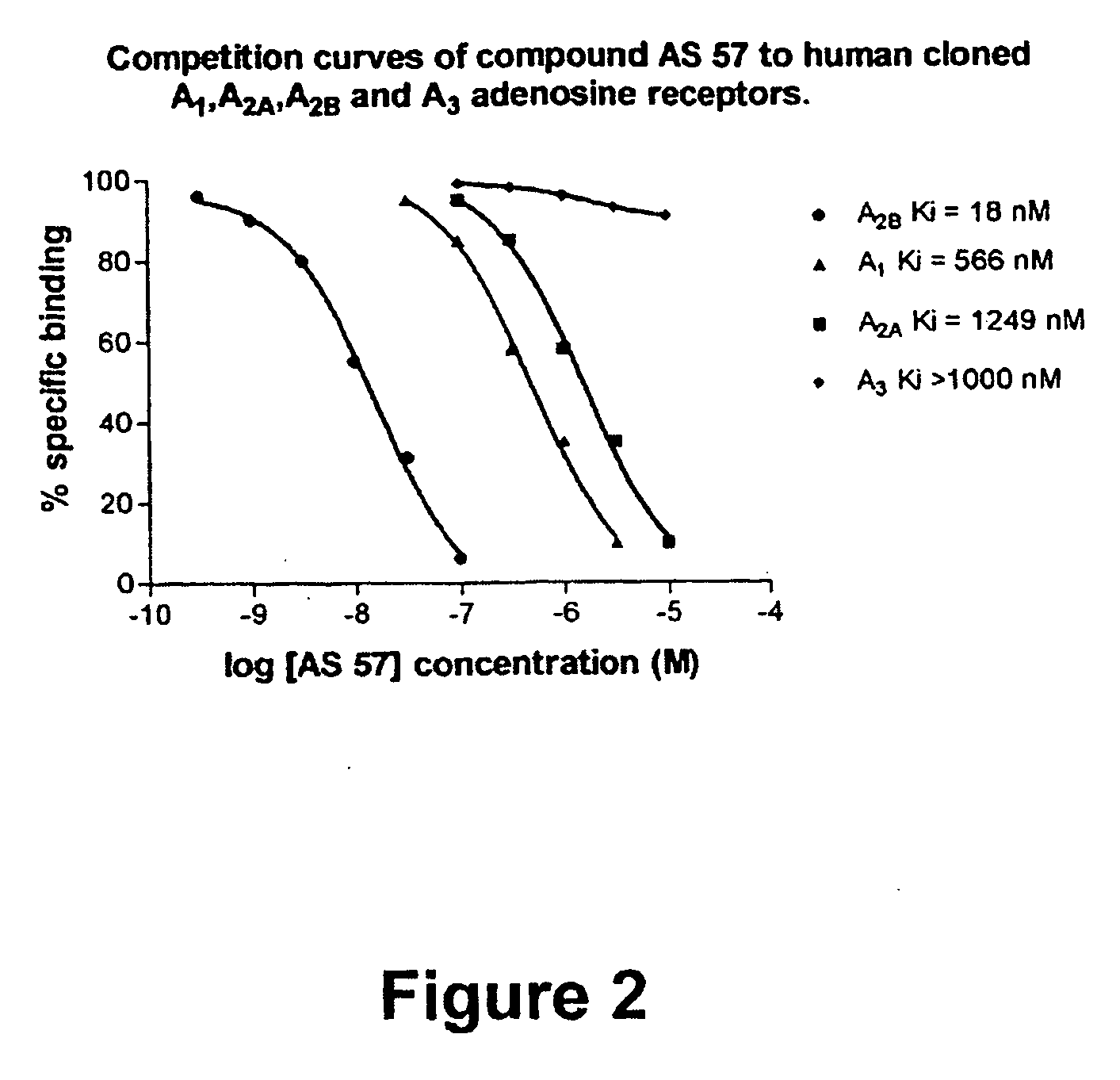
Substituted 8-heteroaryl xanthines
ActiveUS20050065341A1Avoid problemsSymptoms improvedBiocideSenses disorderSelective antagonistXanthine
The present invention provides compounds and pharmaceutical compositions that are selective antagonists of A2B adenosine receptors (ARs). These compounds and compositions are useful as pharmaceutical agents.
Owner:ALLERGAN SALES LLC
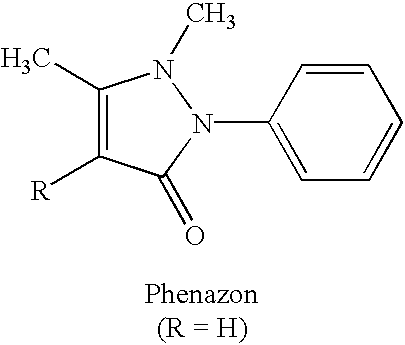
Xanthine-and phenazone-acesulfame-H complexes having improved taste, process for their preparation and their use
Complex compounds or adducts of xanthine derivatives, for example propentofylline or pentoxyfylline, or phenazone derivatives, for example phenazone, propylphenazone and aminophenazone, and acesulfame-H, in which the components are present in a molar ratio of 1:1 or 1:2, have a pleasantly sweet taste and are suitable for numerous applications, for example in pharmaceuticals. The compounds can be prepared from the dissolved components by simple reaction.
Owner:NUTRINOVA NUTRITION SPECIALTIES & FOOD ENGREDIENTS GMBH
Aryl thioxanthines
InactiveUS6066641AReduce smooth muscle cell proliferationIncrease pulmonary vasodilationBiocideNervous disorderArylMedicinal chemistry
Owner:EURO-CELTIQUE SA
Substituted xanthine derivatives
This invention relates to novel compounds that are substituted xanthine derivatives and pharmaceutically acceptable salts thereof. For example, this invention relates to novel substituted xanthine derivatives that are derivatives of pentoxifylline. This invention also provides compositions comprising one or more compounds of this invention and a carrier and the use of the disclosed compounds and compositions in methods of treating diseases and conditions for which pentoxifylline and related compounds are beneficial.
Owner:CONCERT PHARMA INC
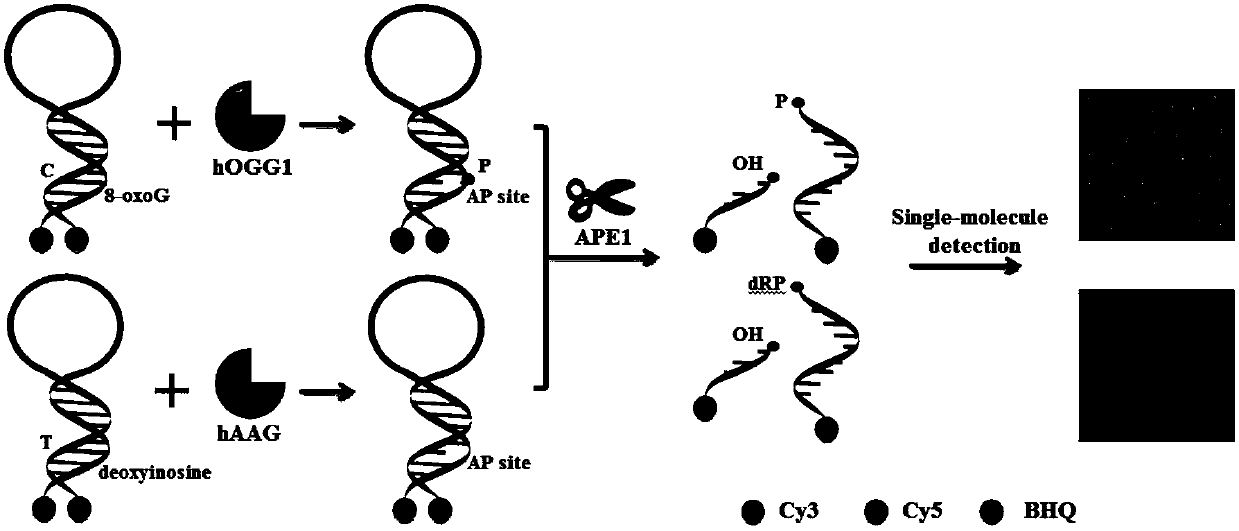
Fluorescence chemical sensor and method for simultaneously detecting diversified DNA (deoxyribonucleic acid) glycosylases on single-molecular levels and application of fluorescence chemical sensor
ActiveCN107723338AEasy to prepareThe detection method is simpleMicrobiological testing/measurementFluorescence/phosphorescenceMethylpurine DNA GlycosylaseMolecular level
The invention discloses a fluorescence chemical sensor and a method for simultaneously detecting diversified DNA (deoxyribonucleic acid) glycosylases on single-molecular levels and application of thefluorescence chemical sensor. The fluorescence chemical sensor, the method and the application have the advantages that the fluorescence chemical sensor is based on two different molecular beacons including a molecular beacon modified by 8-hydroxyl guanine and a molecular beacon modified by deoxygenated hypoxanthine, the tail end of the molecular beacon modified by the 8-hydroxyl guanine is labeled by cyanine 3 (Cy3) and quenching groups, the tail end of the molecular beacon modified by the deoxygenated hypoxanthine is labeled by cyanine 5 (Cy5) and quenching groups, the fluorescence chemicalsensor is used for detecting 8-hydroxyl guanine DNA glycosylases and N-methylpurine DNA glycosylases and is different from the traditional molecular beacons which can be severely affected by dynamicsand thermodynamics, signal restoration of the Cy3 and the Cy5 depends on molecular beacon splitting with the DNA glycosylases used as media, the DNA glycosylases can be simultaneously sensitively detected by the aid of the method without optional signal amplification, the activity of hOGG1 and hAAG can be detected by the aid of the method in an ultra-sensitive manner without optional signal amplification, the fluorescence chemical sensor can be easily, conveniently and quickly operated, and accurate and reliable test results can be obtained.
Owner:SHANDONG NORMAL UNIV
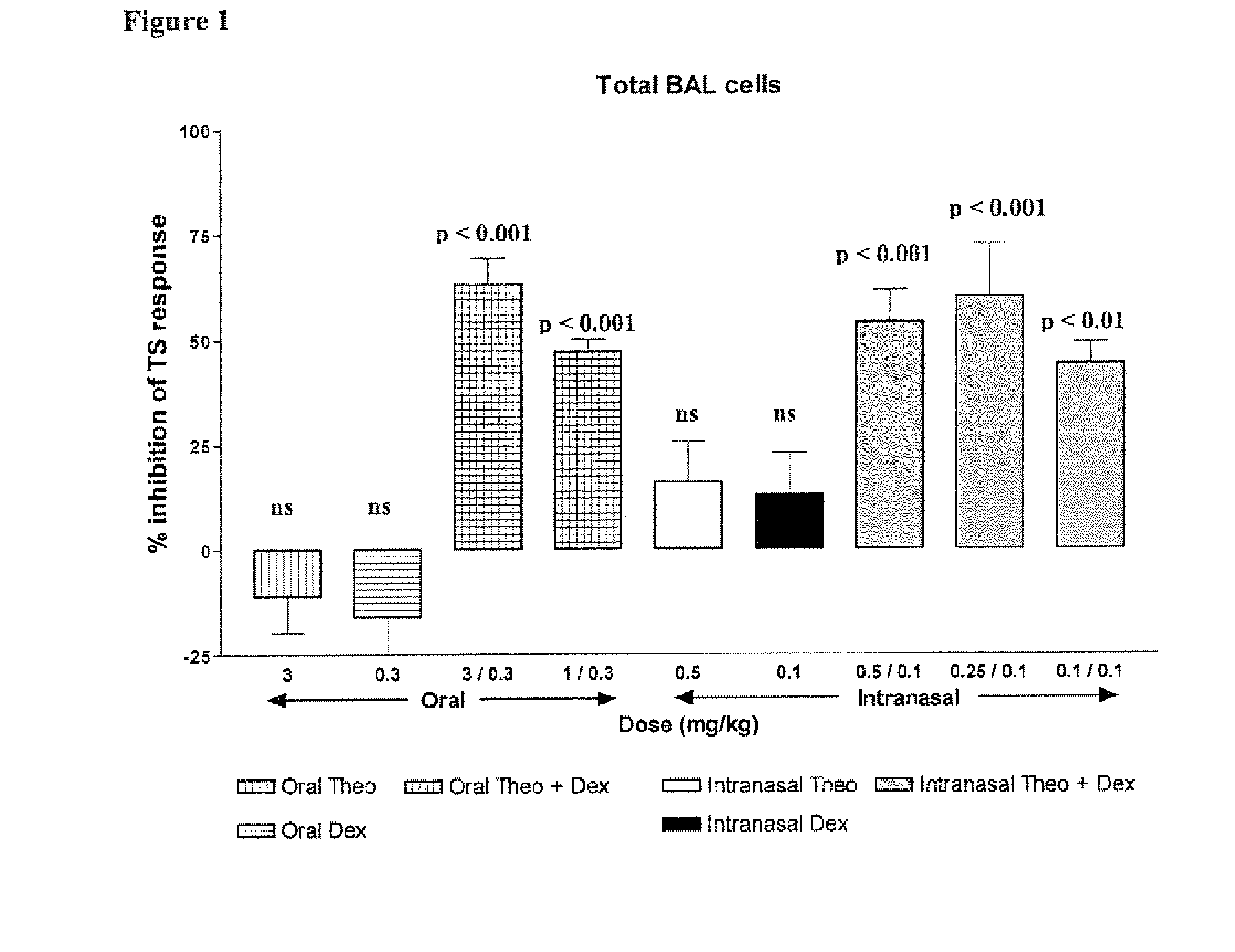
Inhaled combination therapy
InactiveUS20100324002A1Function increaseImprove inflammationBiocideRespiratory disorderMethyl xanthineObstructive Pulmonary Diseases
Owner:PULMAGEN THERAPEUTICS SYNERGY
Pyridyl substituted xanthines
InactiveUS20070072843A1Avoid problemsSymptoms improvedBiocideOrganic chemistryXanthineStereochemistry
The present invention provides compounds and pharmaceutical compositions that are selective antagonists of A2B adenosine receptors (ARs). These compounds and compositions are useful as pharmaceutical agents.
Owner:ADENOSINE THERAPEUTICS +1
8-Heteroaryl xanthine adenosine A2B receptor antagonists
The present invention relates generally to compounds of the formula (I):wherein:X is a five or six-membered heteroaromatic ring, containing one to four heteroatoms, selected from nitrogen, oxygen, or sulfur, provided that at least one heteroatom is nitrogen; andG1 and G2 are independently CH or N.The present invention also relates to the preparation of the compounds, pharmaceutical formulations thereof, and their use in medicine as potent or selective A2B adenosine receptor antagonists and their uses for treating asthma, autoimmune diseases and retinal vascular diseases.
Owner:KING PHARMA RES & DEV
Lactobacillus gasseri and application thereof in relieving and treating hyperuricemia
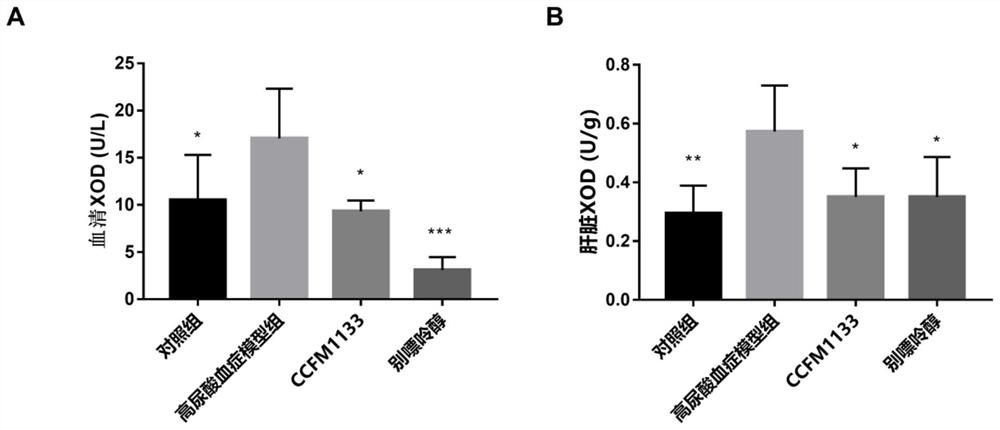
ActiveCN112458027ALower blood sugarGood for healthBacteriaMicroorganism based processesTG - TriglycerideXanthine
The invention discloses lactobacillus gasseri and application thereof in relieving and treating hyperuricemia, and belongs to the technical field of microorganisms. According to the lactobacillus gasseri CCFM1133 disclosed by the invention, the serum uric acid level of hyperuricemia mice and the xanthine oxidase (XOD) activity of serum and a liver can be reduced, and the occurrence of hyperuricemia and gout is reduced; the blood glucose and serum triglyceride (TG) level of a patient with hyperuricemia can be regulated, and the activity of liver catalase (CAT) and glutathione peroxidase (GSH-Px) is improved; expression of ileum ABCG2 is improved, and excretion of intestinal uric acid is promoted; and the intestinal short-chain fatty acid level is improved, and the health is promoted. The lactobacillus gasseri CCFM1133 disclosed by the invention can be used for preparing foods, functional foods or medicines, and has a wide application prospect.
Owner:JIANGNAN UNIV
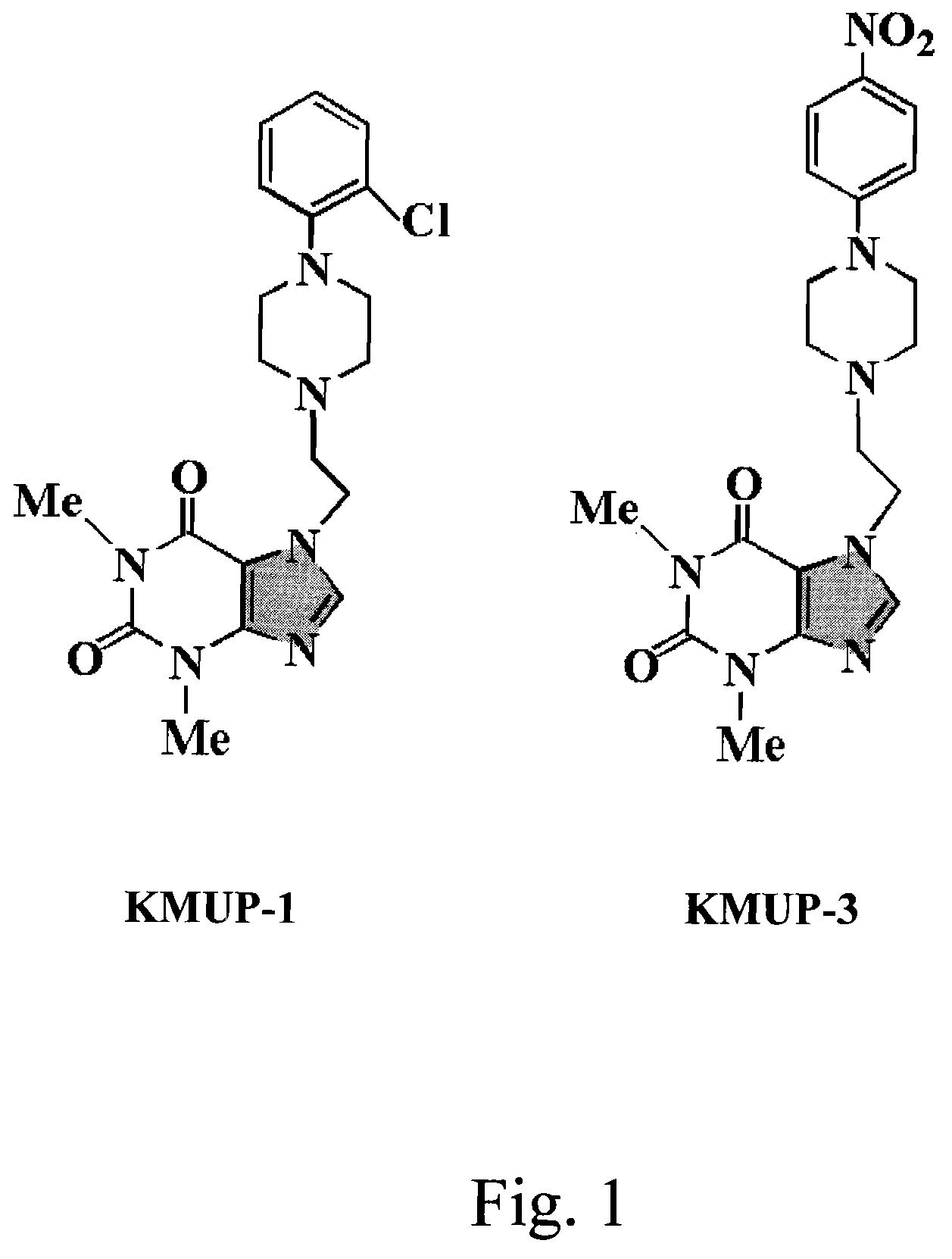
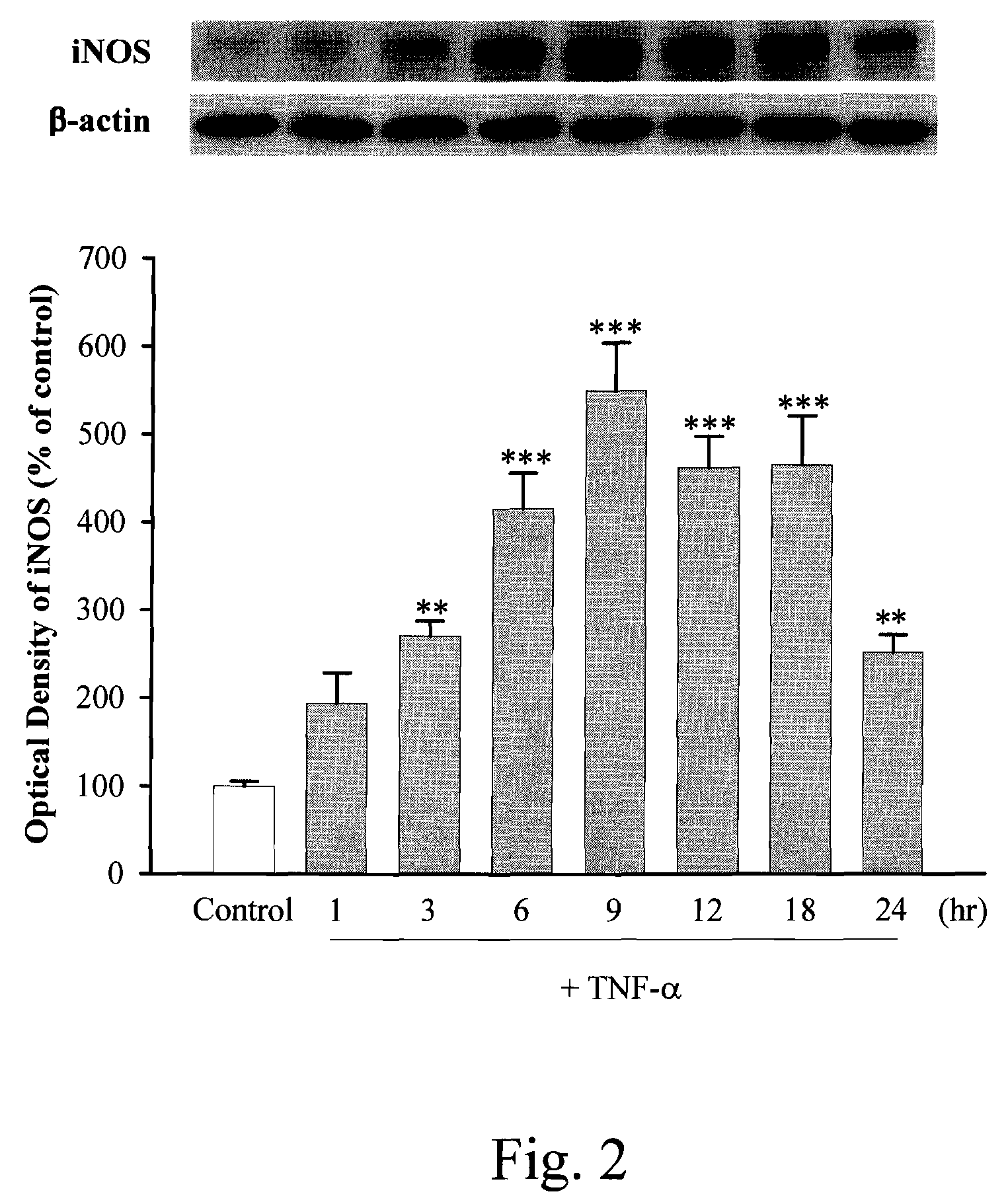
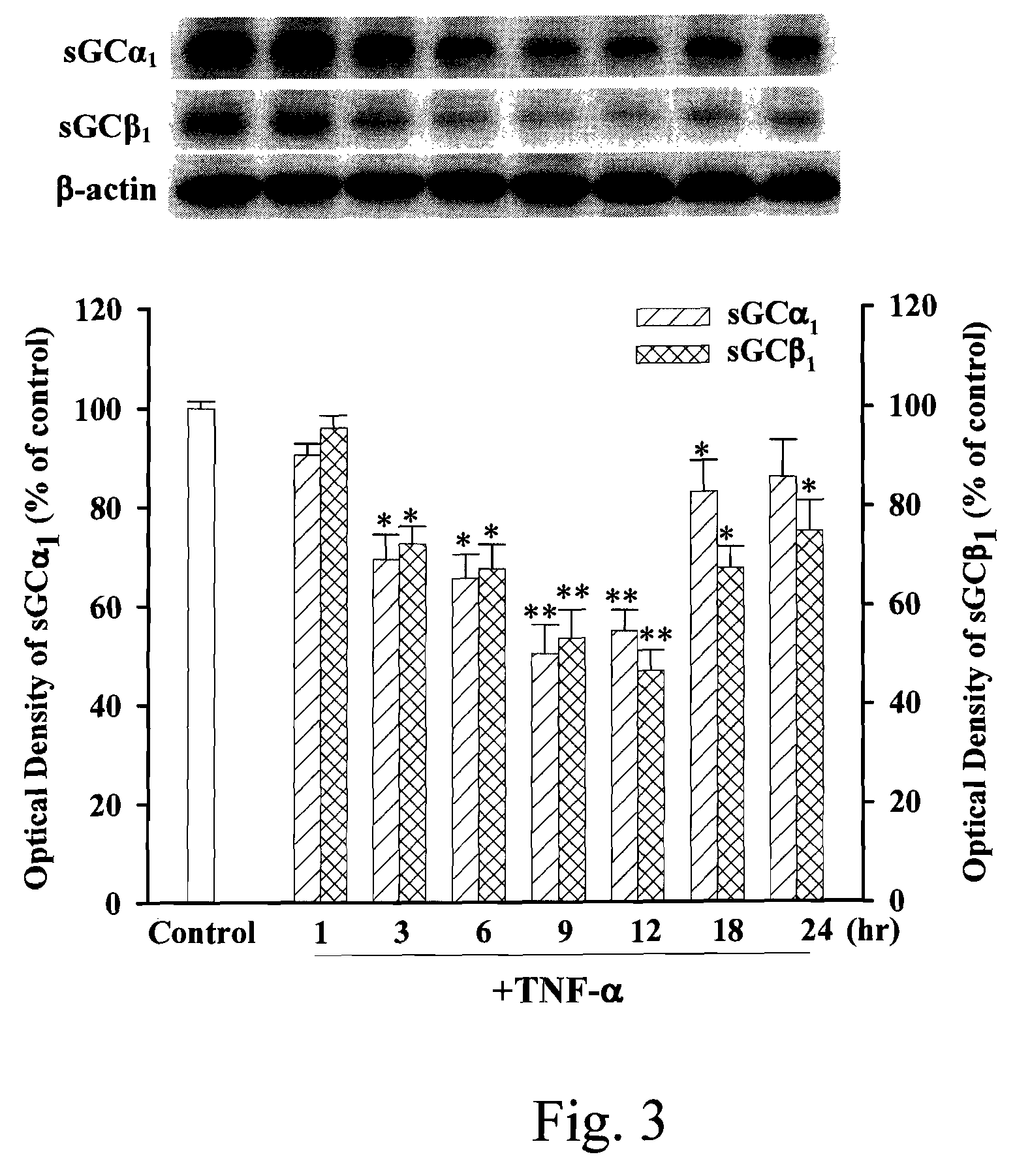
Anti-inflammation activity of newly synthesized xanthine derivatives kmup-1 and kmup-3
InactiveUS20080081816A1Inhibit expressionPreventing airway constrictionOrganic chemistryHeterocyclic compound active ingredientsChlorobenzeneNitrobenzene
An anti-inflammation substrate for decreasing the proinflammation induced by the cytokines and inhibiting the lung function degeneration is provided. The anti-inflammation substrate includes one selected from the group consisting of a 7-[2-[4-(2-chlorobenzene)piperazinyl]ethyl]-1,3-dimethylxanthine, a 7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1,3-dimethylxanthine, a respective pharmaceutical acceptable salt thereof, and a combination thereof.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
Human mesenchymal stem cell adipogenesis inducing and differentiating culture medium and preparation method thereof
InactiveCN108588015AEfficient adipogenic induction of differentiationShorten induction time of adipogenic differentiationCulture processSkeletal/connective tissue cellsDexamethasoneAdipogenesis
The invention discloses a human mesenchymal stem cell adipogenesis inducing and differentiating culture medium and a preparation method thereof. The human mesenchymal stem cell adipogenesis inducing and differentiating culture medium is produced by the following components of an alpha-MEM / HG-DMEM culture medium, 5-50% of percent by volume of fetal calf serum, 0.5-10% of percent by volume of glutamine, 100-400 [mu]M volume of indomethacin, insulin with the concentration of 0.1-20 [mu]g / ml, 10-200 [mu]M volume of 1-methyl-3-isobutyl xanthine, 10-200 nM volume of dexamethasone and 0.1-20 [mu]M volume of spermine. The preparation method includes the following steps that a culture dish is cleaned; the culture medium is provided, and the alpha-MEM / HG-DMEM culture medium is prepared in the culture dish according to a formula; materials are mixed; and mixed liquor is filtered. Multiple histologic origin human mesenchymal stem cells including human mesenchymal stem cells, umbilical cord mesenchymal stem cells and adipose tissue-derived stromal cells are induced to the directional differentiation of adipogenesis cells; differentiating and inducing time of the human mesenchymal stem cells isshortened, the preparation method is convenient, and the differentiating and the inducing of human mesenchymal stem cell adipogenesis can be achieved stably and efficiently.
Owner:安徽瑞杰赛尔生物科技有限公司
8-Heteroaryl xanthine adenosine A2B receptor antagonists
The present invention relates generally to compounds of formula (I): wherein X is a five or six-membered heteroaromatic ring, containing one to four heteroatoms, selected from nitrogen, oxygen, or sulfur, provided that at least one heteroatom is nitrogen; and G1 and G2 are independently CH or N. The present invention also relates to the preparation of the compounds, pharmaceutical formulations thereof, and their use in medicine as potent or selective A2B adenosine receptor antagonists and their uses for treating asthma, autoimmune diseases and retinal vascular diseases.
Owner:KING PHARMA RES & DEV
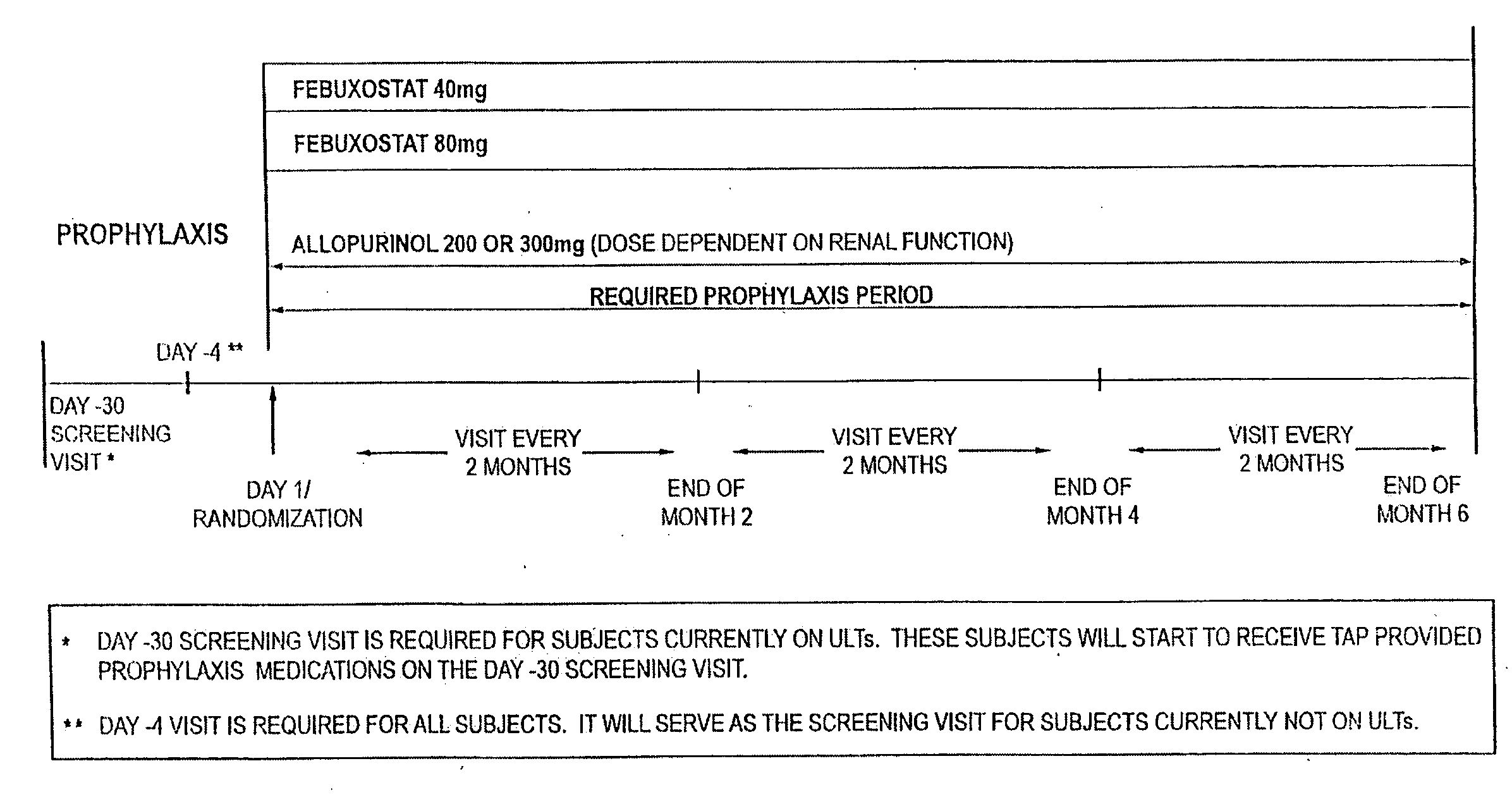
Methods for Preventing or Reducing the Number of Gout Flares Using Xanthine Oxidoreductase Inhibitors and Anti-Inflammatory Agents
The present invention relates to methods of preventing gout flares in a subject in need thereof by administering to the subject a therapeutically effective amount of at least one xanthine oxidoreductase inhibiting compound or salt thereof and at least one non-steroidal anti-inflammatory drug for a period of six months on a regular basis.
Owner:TAKEDA PHARMA U S A
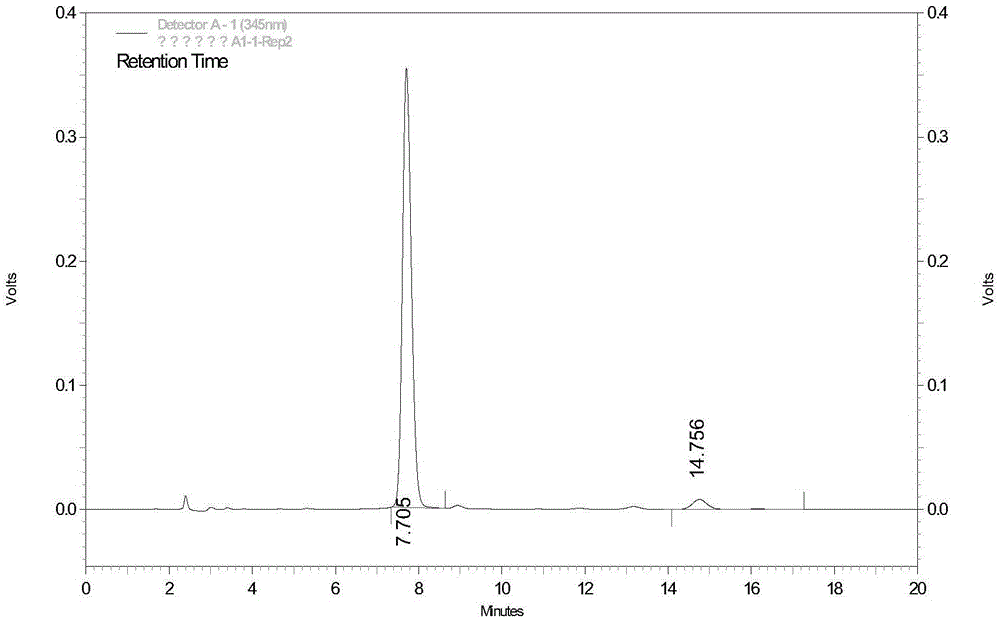
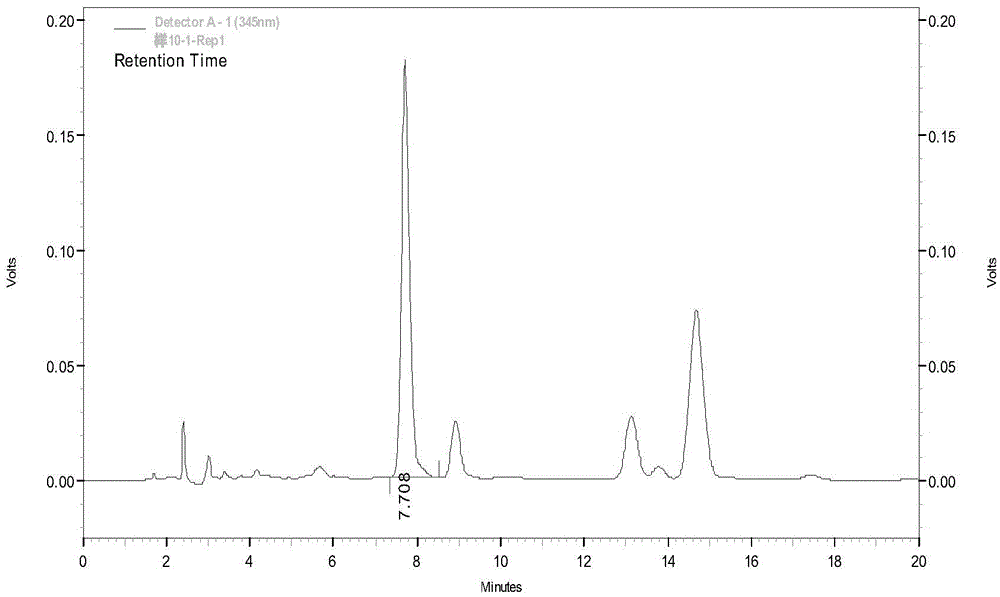
Improved process for analyzing for separating, and for isolating polar protic monomers and/or oligomers
An improved process for separating and isolating individual polar protic monomer(s) and / or oligomer(s) on the basis of degree of polymerization. A liquid sample containing polar protic monomer(s) and / or oligomer(s) is introduced into a liquid chromatography (LC) column packed with a polar bonded stationary chromatographic phase. The individual polar protic monomer(s) and / or oligomer(s) are separated via a binary mobile phase elution. One or more individual fractions containing the monomer(s) and / or oligomer(s) are eluted. The polar protic monomer(s) and / or oligomer(s) may be proanthocyanidins, hydrolyzable tannins, oligosaccharides, oligonucleotides, peptides, acrylamides, polysorbates, polyketides, poloxamers, polyethylene glycols, polyoxyethylene alcohols or polyvinyl alcohols. The binary mobile phase comprises an A phase consisting essentially of a polar aprotic solvent and a B phase consisting essentially of a polar protic solvent. A process for separating and isolating xanthine(s) (e.g., caffeine and theobromine) from polar protic monomer(s) and / or oligomer(s). A liquid sample containing xanthine(s) and polar protic monomer(s) and / or oligomer(s) is introduced into an LC column packed with a polar bonded stationary chromatographic phase. The xanthines are separated via an isocratic mobile phase elution, and one or more individual fractions containing the xanthines are eluted.
Owner:MARS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com