Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
276 results about "Polysorbates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sorbitan mono-9-octadecanoate poly(oxy-1,2-ethanediyl) derivatives; complex mixtures of polyoxyethylene ethers used as emulsifiers or dispersing agents in pharmaceuticals.
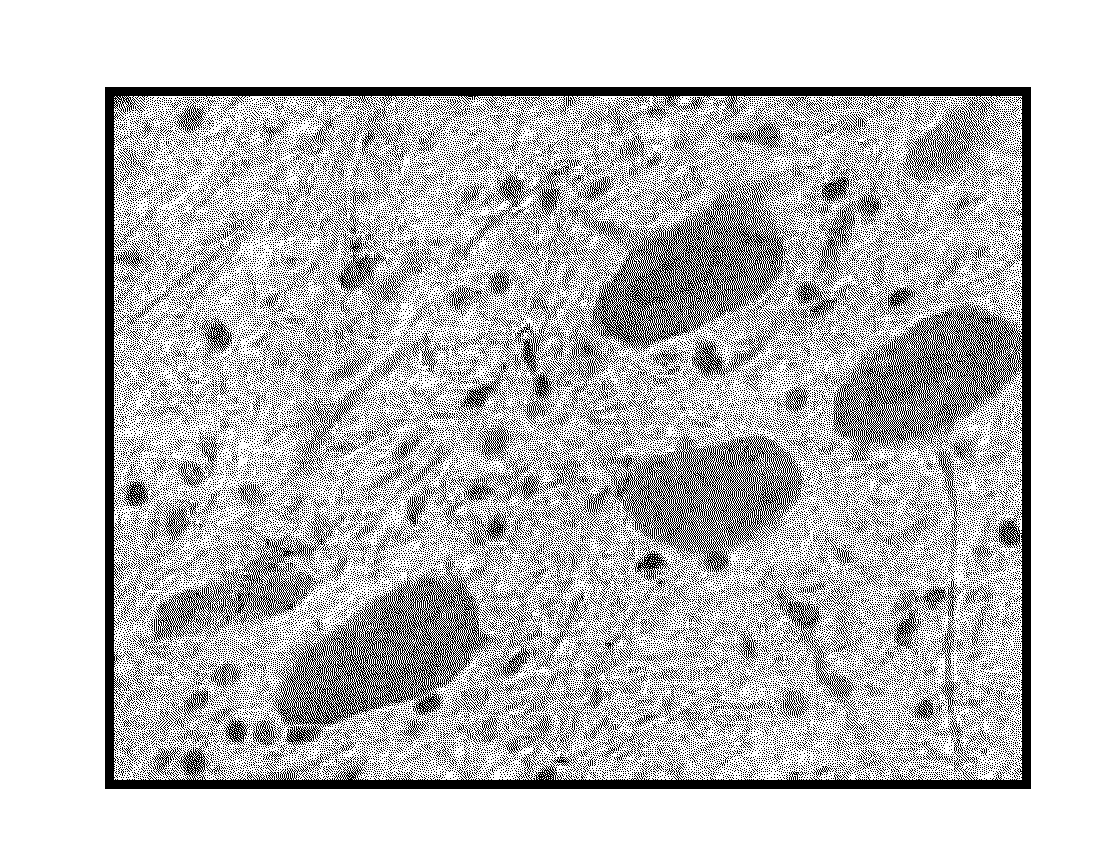
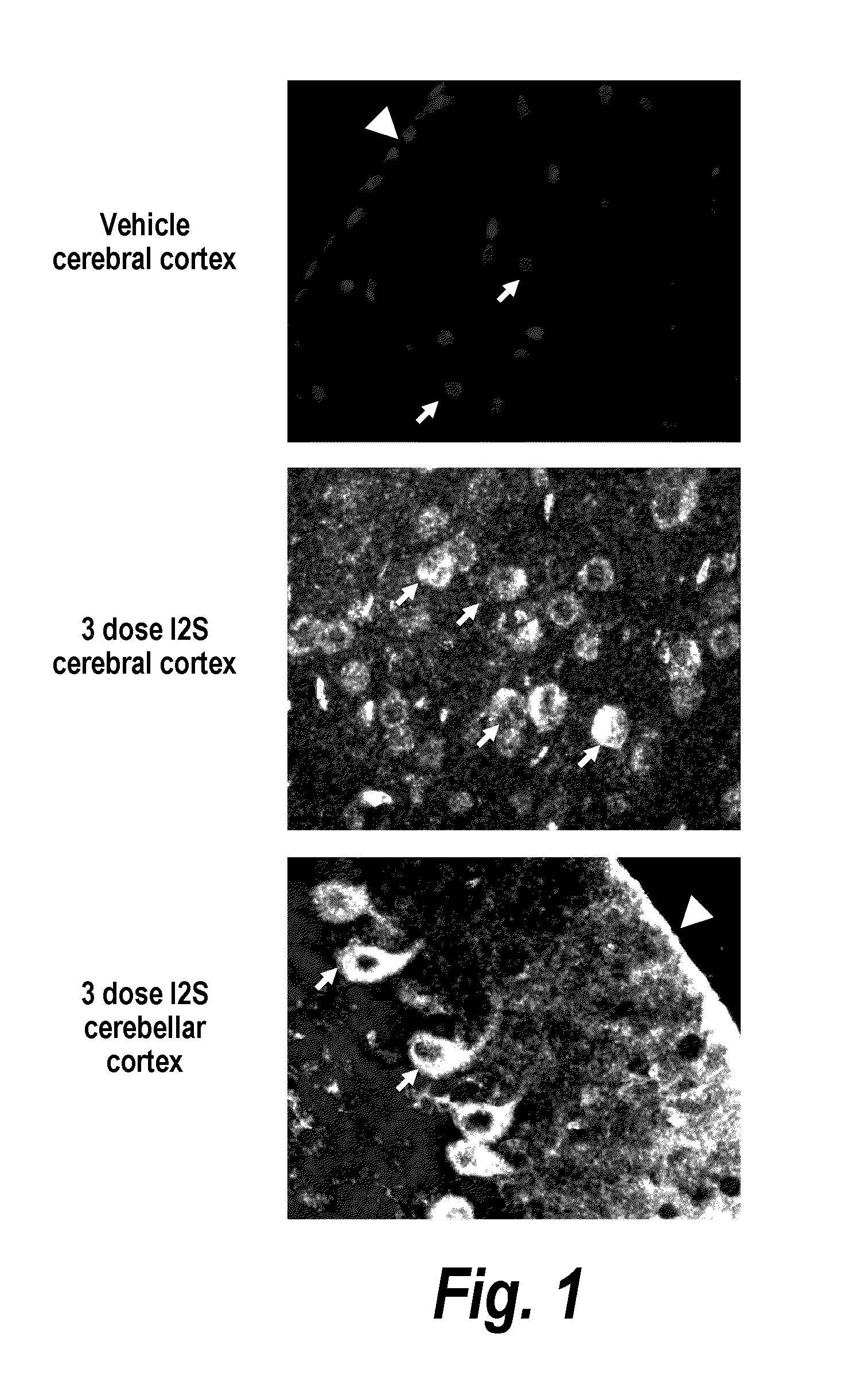
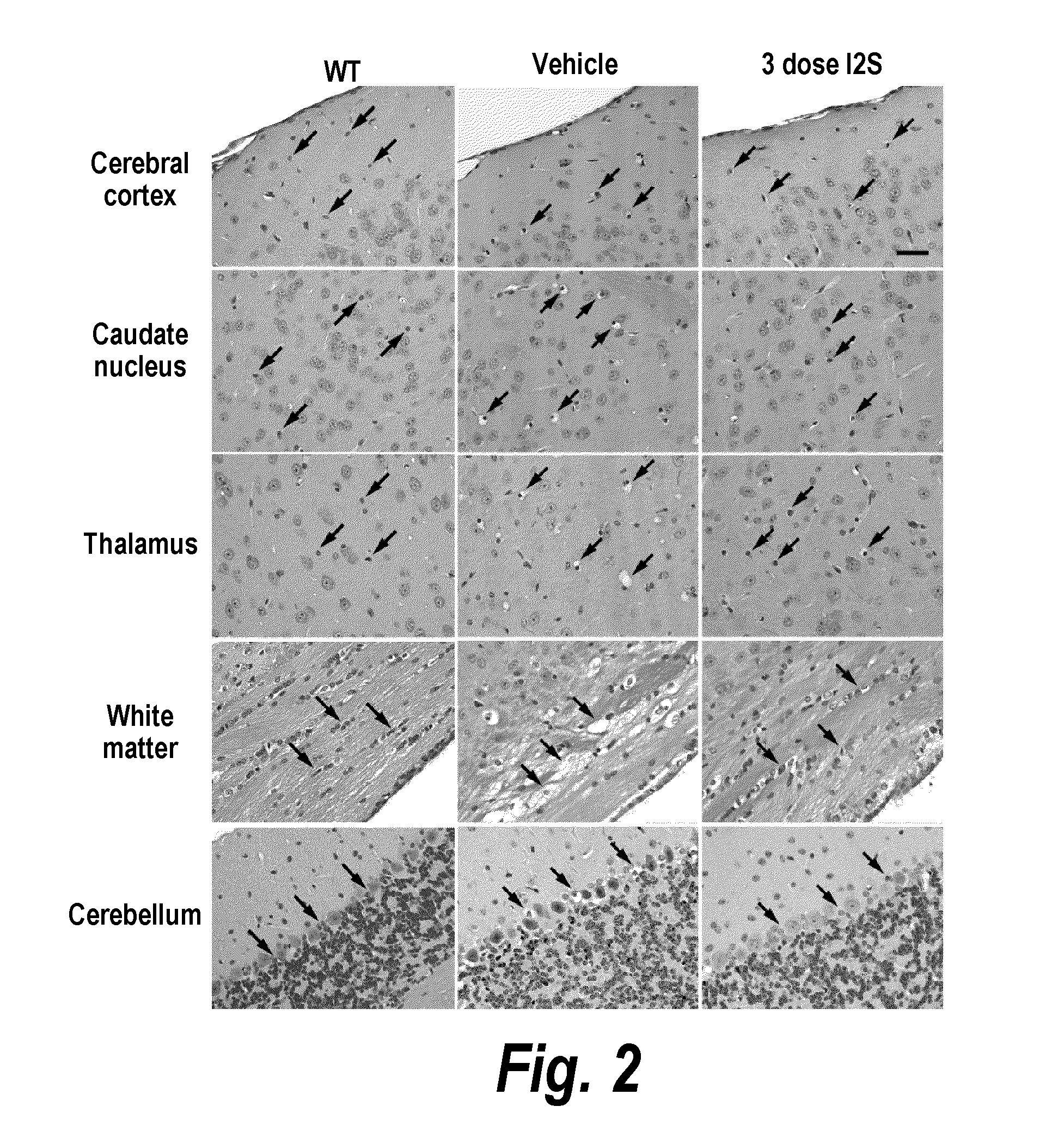
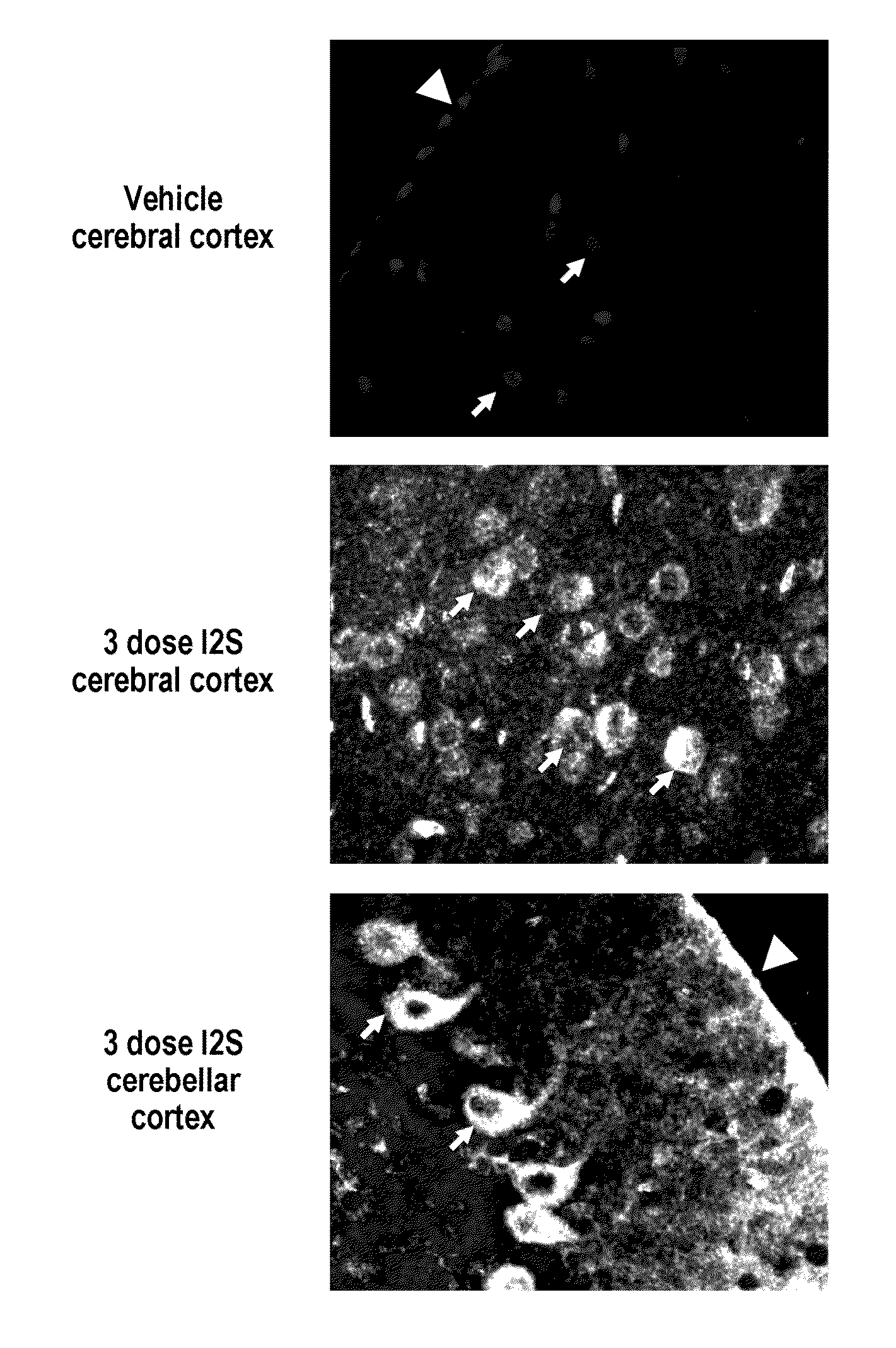
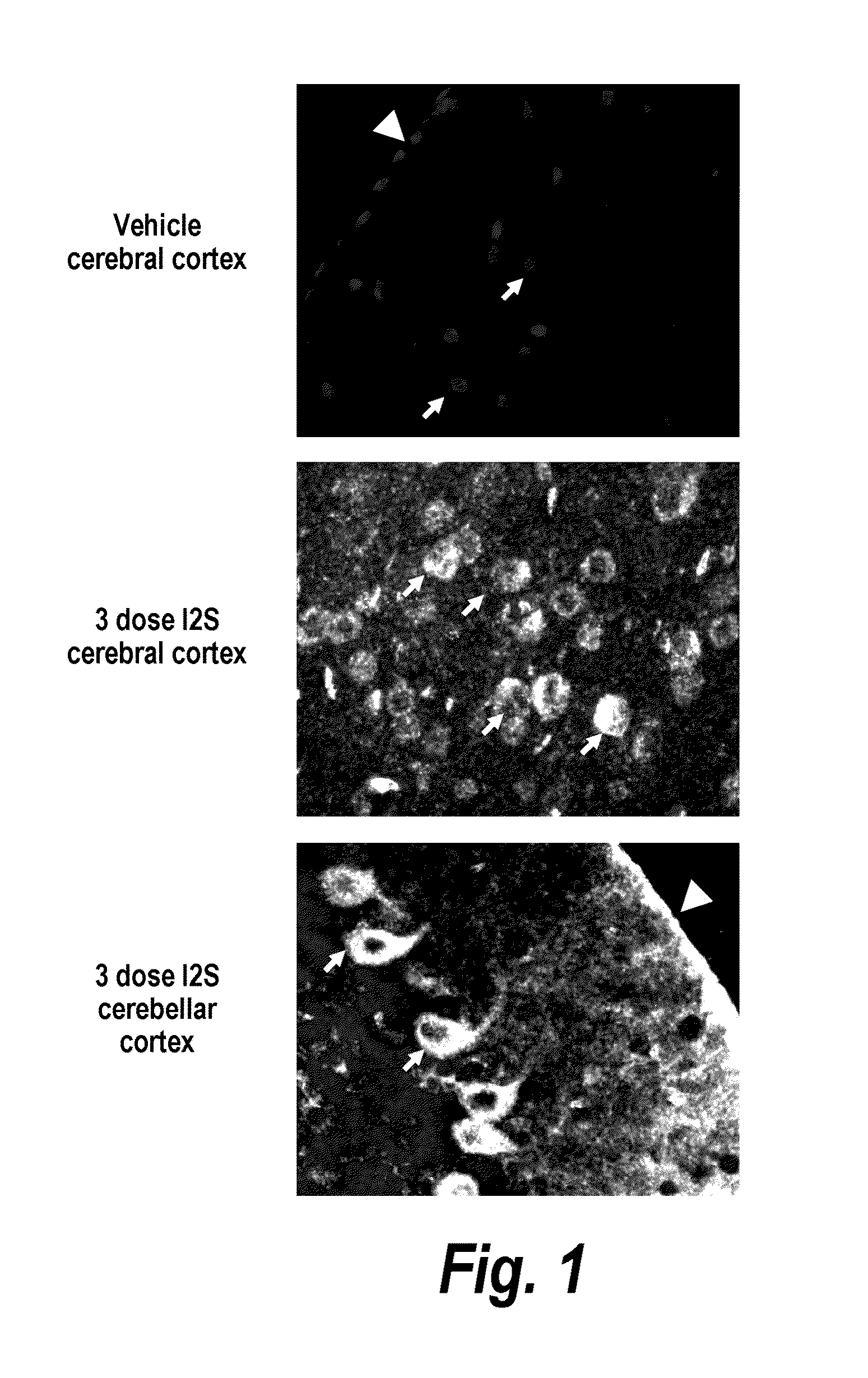
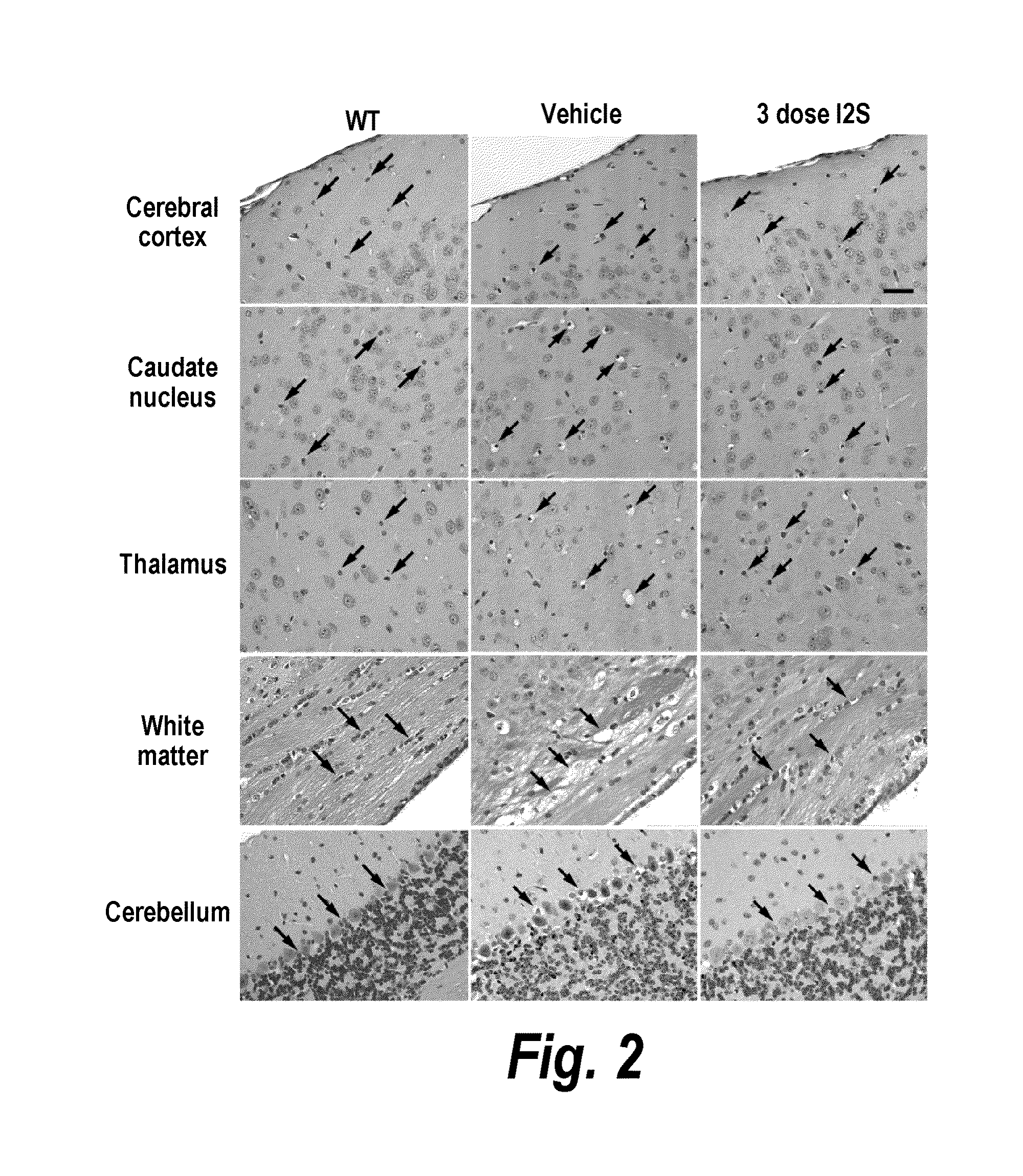
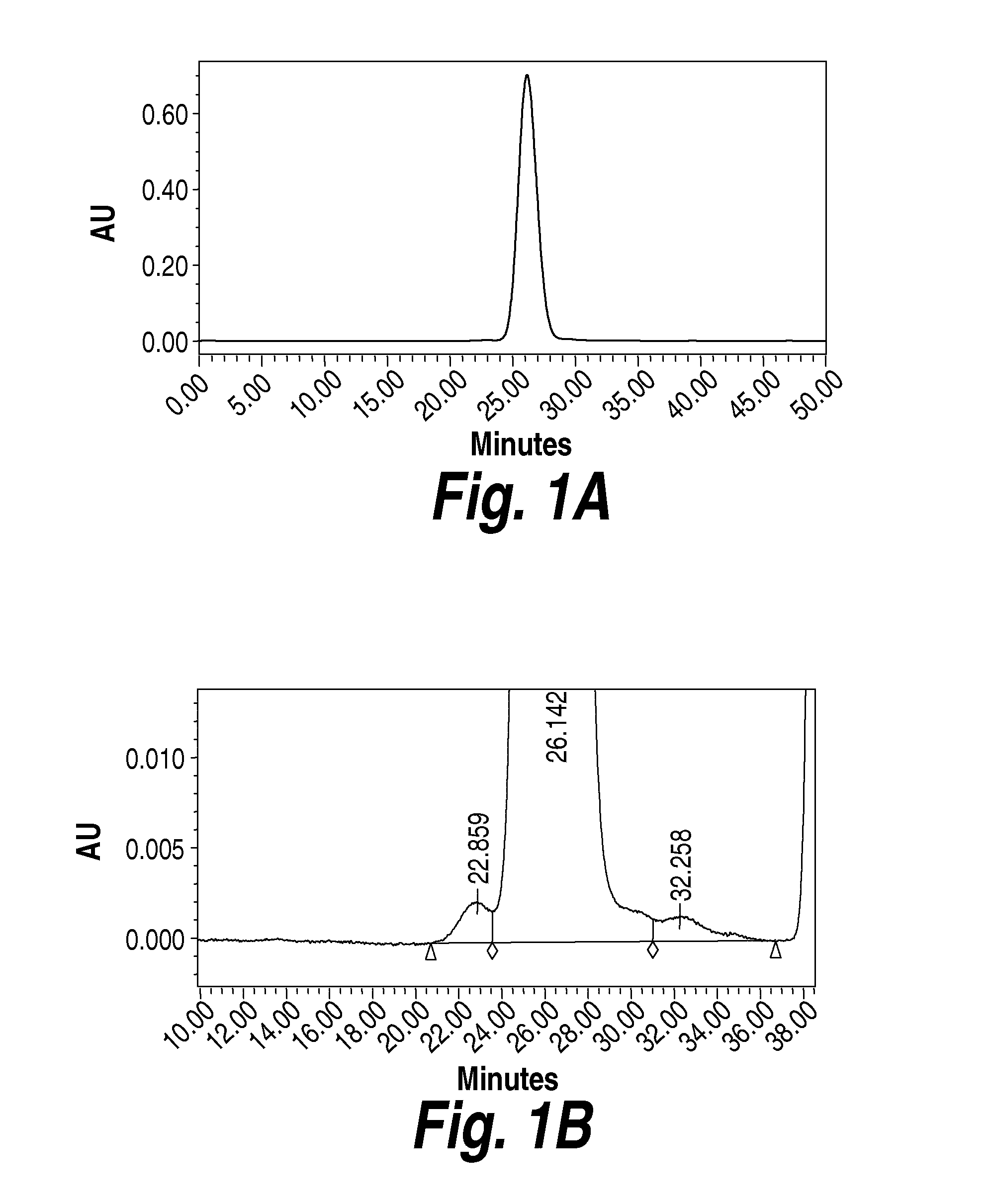
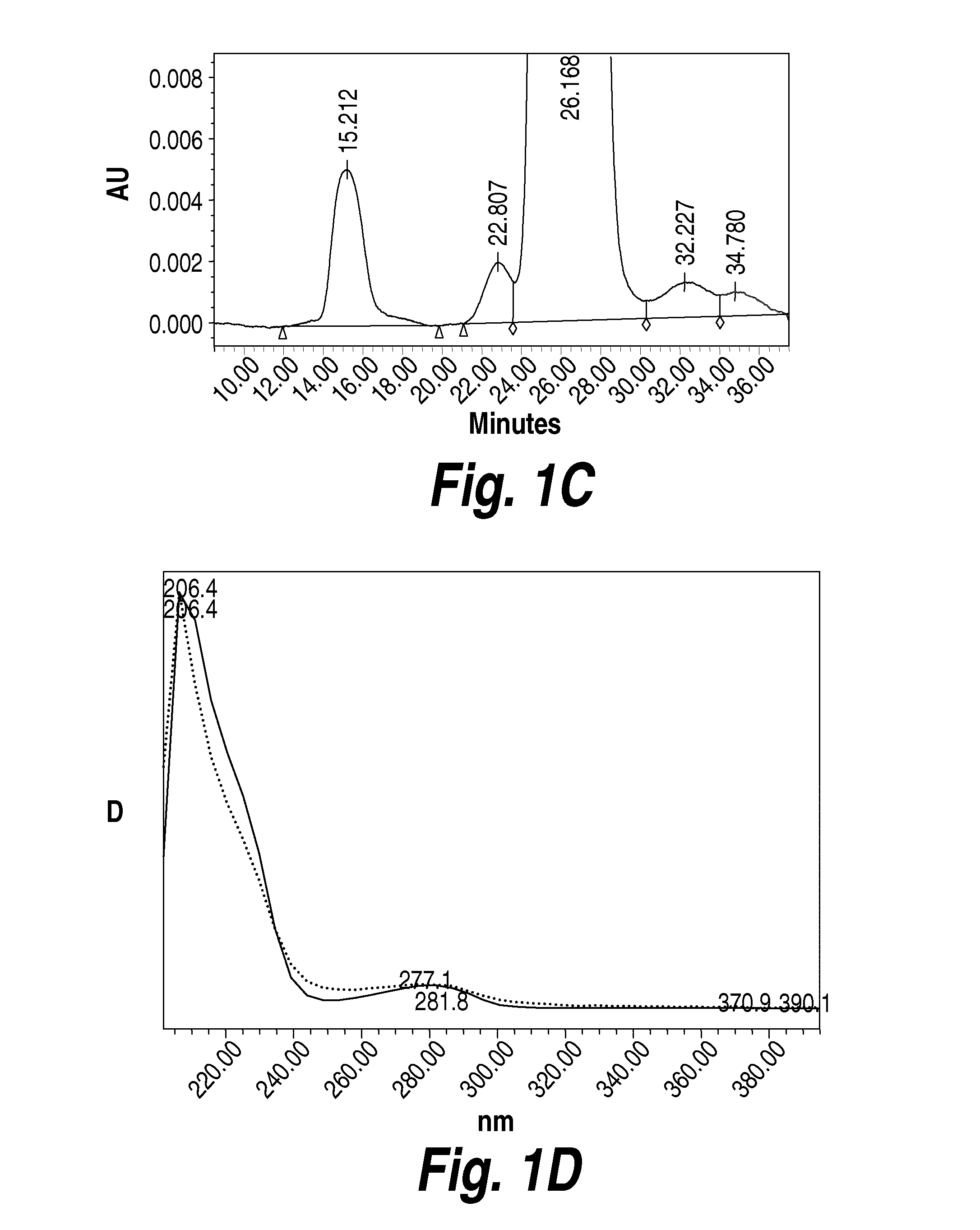
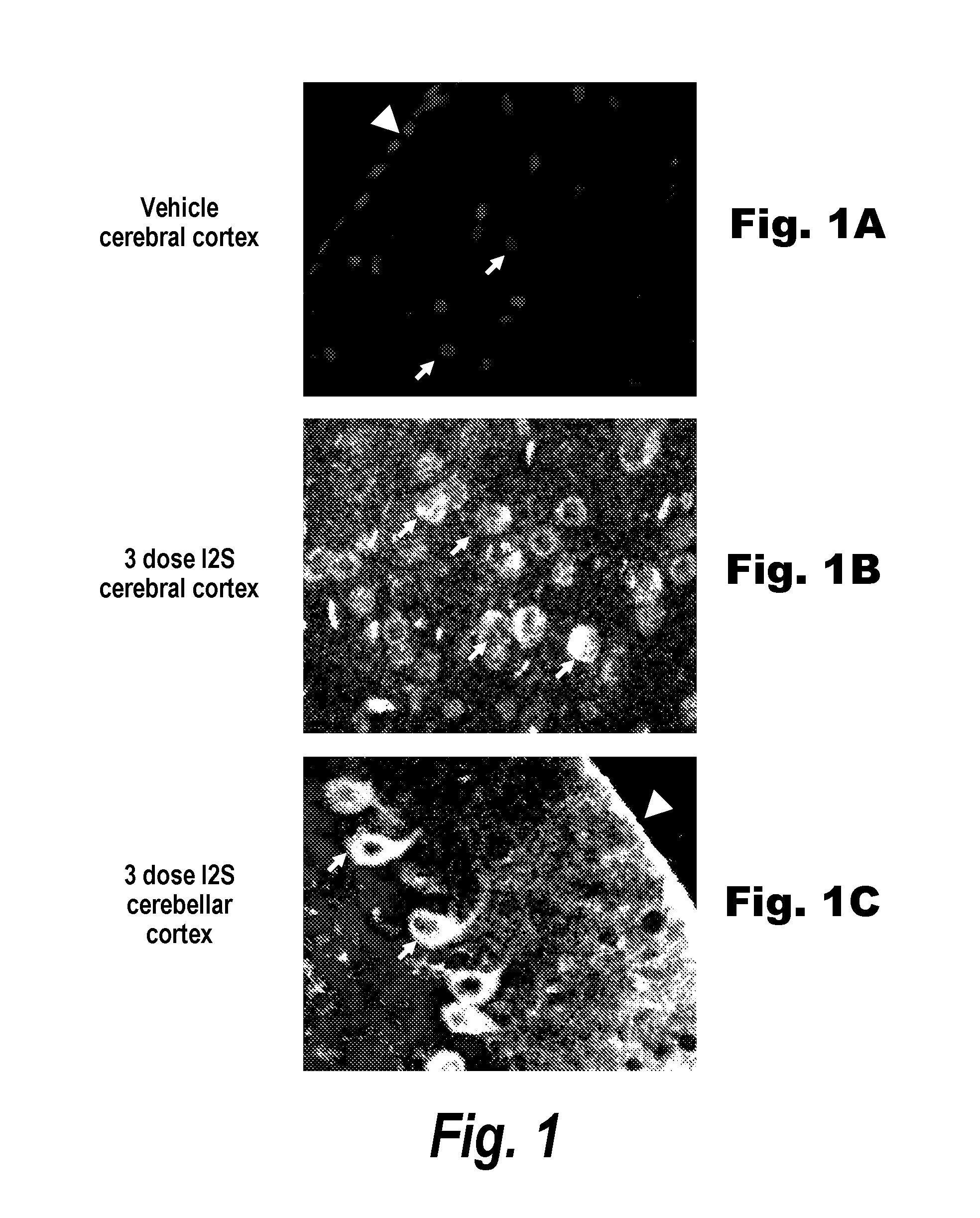
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS20110318323A1Effective and less approachEffectively and extensivelyNervous disorderHydrolasesIduronate-2-sulfataseHunter syndrome
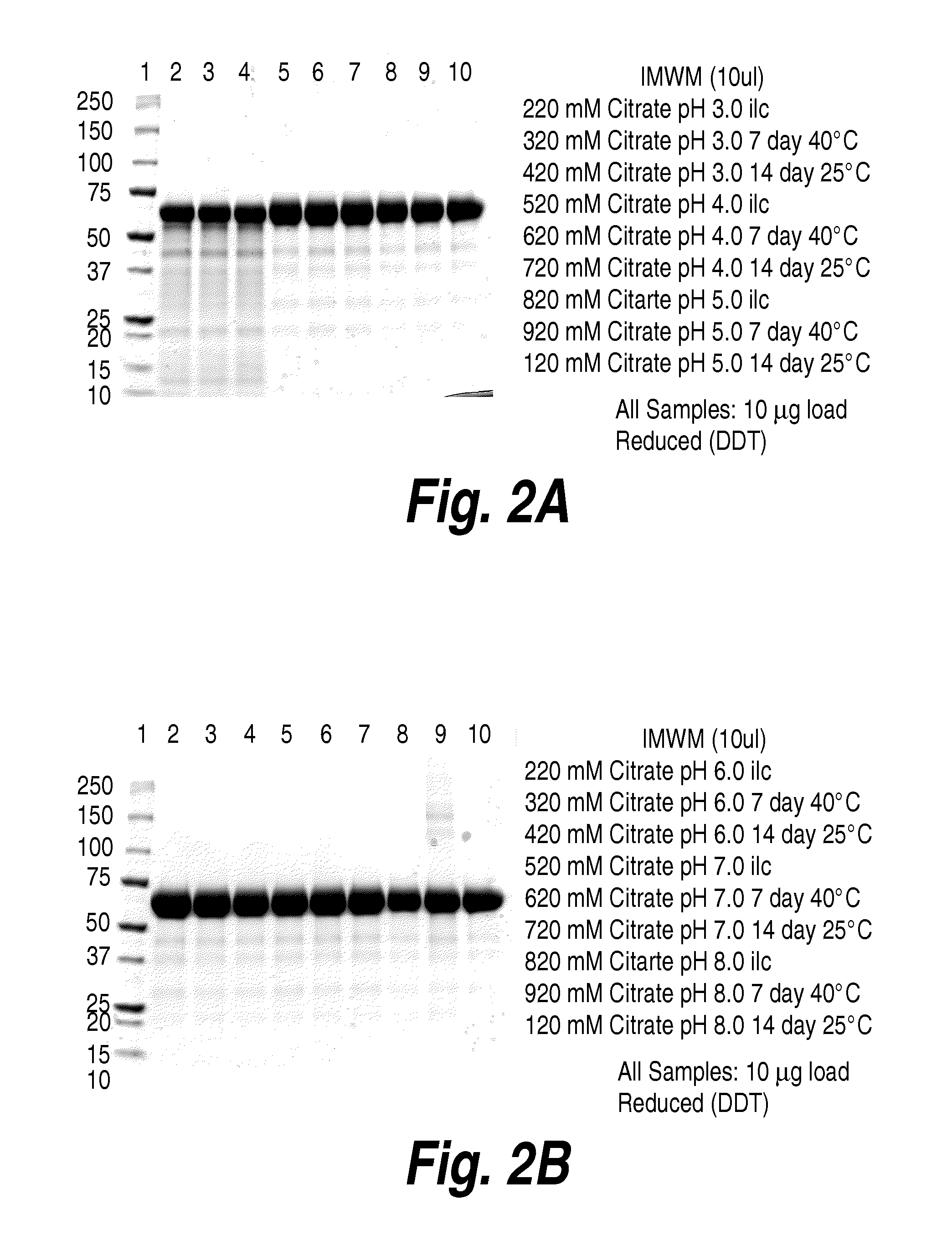
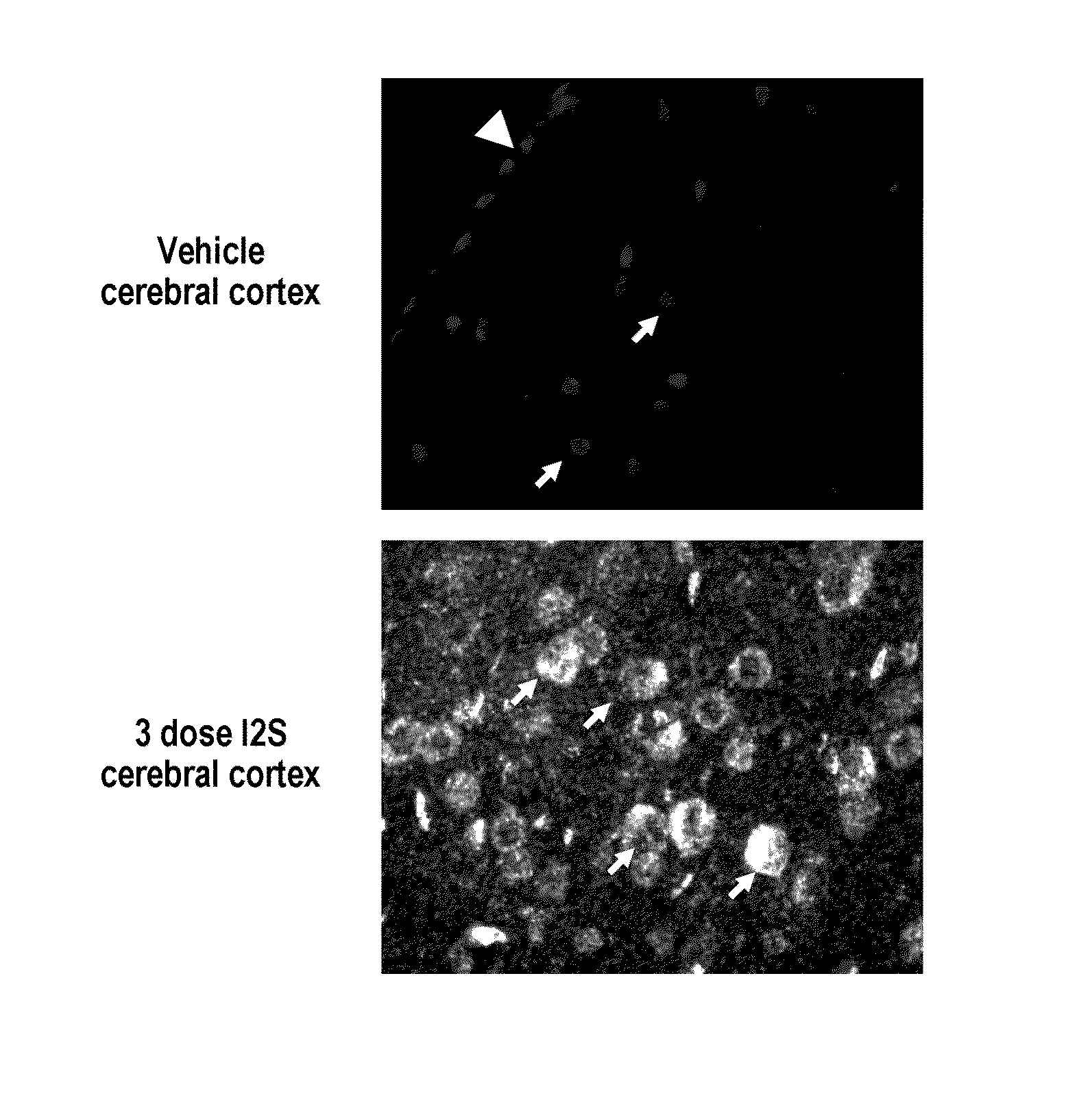
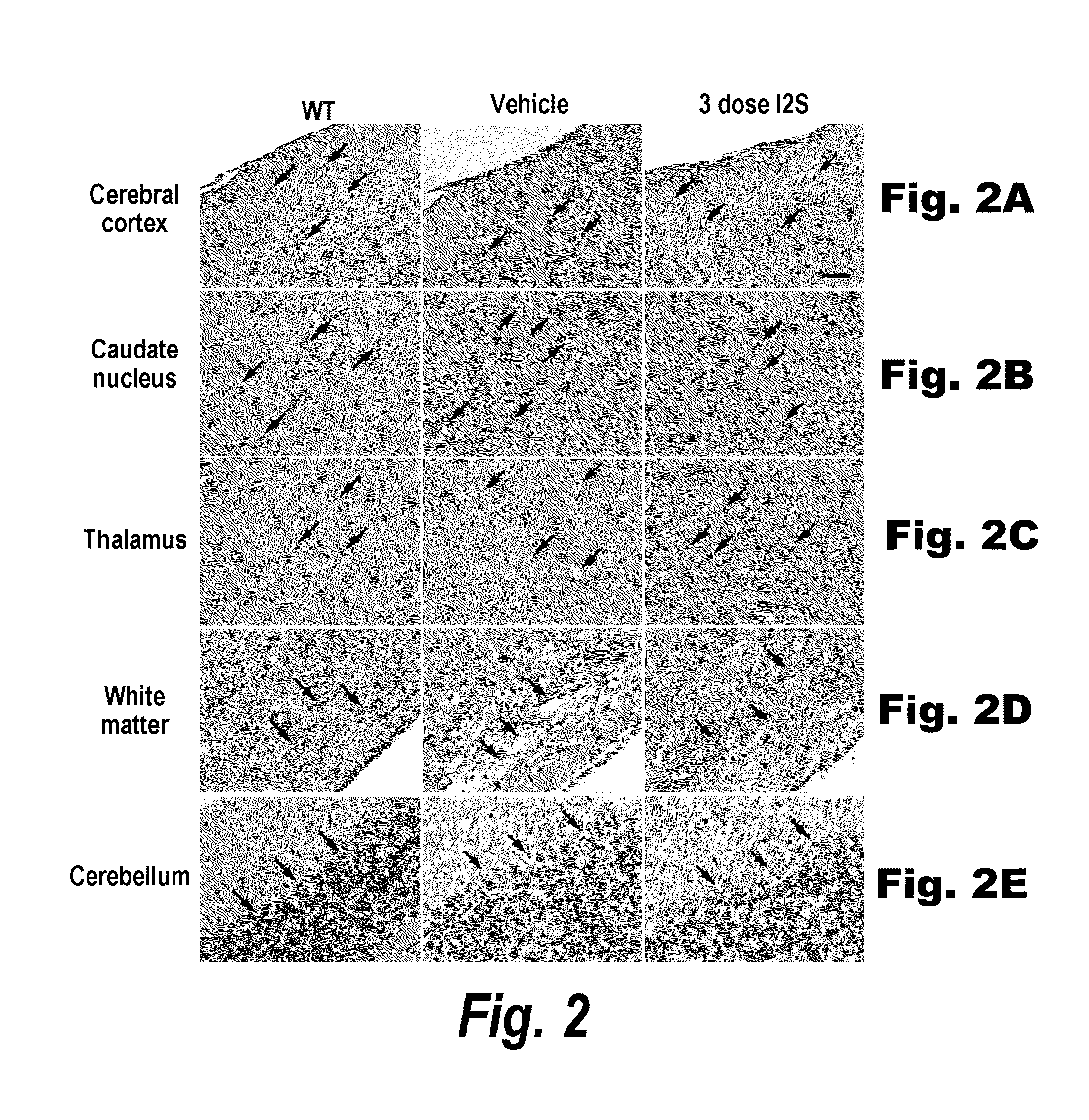
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising an iduronate-2-sulfatase (I2S) protein, salt, and a polysorbate surfactant for the treatment of Hunters Syndrome.
Owner:TAKEDA PHARMA CO LTD
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS8545837B2Effective and less invasiveEffectively and extensivelyNervous disorderPeptide/protein ingredientsIduronate-2-sulfataseHunter syndrome
Owner:TAKEDA PHARMA CO LTD
Methods and compositions for CNS delivery of heparan n-sulfatase
ActiveUS20120014936A1Effective and less approachReduce deliverySenses disorderNervous disorderSanfilippo syndrome type aLysosomal enzyme defect
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising a heparan N-sulfatase (HNS) protein, salt, and a polysorbate surfactant for the treatment of Sanfilippo Syndrome Type A.
Owner:TAKEDA PHARMA CO LTD
Moisturizing antimicrobial composition
An antimicrobial moisturizing composition includes benzethonium chloride or benzalkonium chloride, a non-benzyl cationic surfactant, and an aqueous carrier. The composition of the present invention provides a significant and unexpected reduction of irritation, inflammation, dryness and / or redness, all issues associated with known alcohol-based skin disinfectants. In particular, the present invention provides a stable, aesthetically-pleasing, long-lasting, and moisturizing antimicrobial composition that is substantially free of ethanol, polysorbates, and anionic compounds that are known to inhibit the activity of benzethonium chloride or benzalkonium chloride.
Owner:CHATTEM
Cabazitaxel formulations and methods of preparing thereof
InactiveUS20120065255A1Organic active ingredientsBiocideHydrotropeTocopherol polyethylene glycol succinate
Pharmaceutical formulations comprising cabazitaxel, solubilizer, tocopherol polyethylene glycol succinate (TPGS), one or more hydrotropes, optionally one or more agents having a pKa of about 3 to about 6, and optionally one or more antioxidizing agents, wherein the formulations are substantially free of polysorbates and polyethoxylated castor oil. The solubilizer may comprise glycofurol or ethanol. Pharmaceutical formulations may alternatively comprise cabazitaxel, solubilizer, optionally one or more agents having a pKa of about 3 to about 6, and optionally one or more antioxidizing agents, wherein the formulations are substantially free of polysorbates and polyethoxylated castor oil. These formulations may be combined with a diluent, which comprises TPGS and one or more hydrotropes. Methods of administering the cabazitaxel formulations include combining the formulations with an infusion solution.
Owner:SCIDOSE
Atorvastatin calcium tablet and preparation method thereof
ActiveCN102920675AHigh dissolution rateImprove bioavailabilityMetabolism disorderPharmaceutical non-active ingredientsFiller ExcipientHardness
The invention discloses an atorvastatin calcium tablet and a preparation method thereof. The tablet consists of the following components in parts by mass: 7.22 parts of main medicine atorvastatin calcium, 84.55 parts of filler, 6 parts of disintegrating agent croscarmellose sodium, 1.33 parts of adhesive hydroxy propyl cellulose, 800.4 parts of cosolvent polysorbate and 0.5 part of lubricating agent magnesium stearate, wherein the filler comprises the following raw materials in parts by mass: 22.01 parts of calcium carbonate, 21.87 parts of milk sugar and 40.67 parts of microcrystalline cellulose. The atorvastatin calcium tablet has the characteristics of short disintegrating time, fast dissolving-out speed, high bioavailability and small particle diameter, and is convenient to take. Furthermore, the hardness of the tablet can reach 60-70N, so that the tablet is hardly broken, and therefore, the packing and transporting costs are reduced, and the industrialized popularization of the tablet is easily realized.
Owner:HENAN RUNHONG PHARMA
Micromolecular donkey-hide gelatin essence anti-aging facial mask
InactiveCN108785241AStable moisturizingFade fine linesCosmetic preparationsToilet preparationsWrinkle skinChondrus crispus extract
The invention relates to a micromolecular donkey-hide gelatin essence anti-aging facial mask, which contains the following components: glycerin, methylpropanediol, glyceryl polyether-26, 1,2-hexanediol, p-hydroxyacetophenone, butanediol, PEG-60 hydrogenated castor oil, panthenol, xanthan gum, allantoin, carbomer, glycerin polyacrylate, tromethamine, sodium hyaluronate, EDTA disodium, glycerin acrylate / acrylic copolymer, phenoxyethanol, propylene glycol, proplis extract, a PVM / MA copolymer, dipotassium glycyrrhizinate, essence, silk amino acids, beta-glucan, BAMBUSA VULGARIS WATER bambusa vulgaris water, hydrolyzed collagen, rice extract, glycine ussuriensis seed extract, sesame seed extract, chondrus crispus extract, ethylhexylglycerin, polysorbates-20, potassium chloride, locust bean gum,dipotassium phosphate, sodium citrate, hydrolyzed hyaluronic acid, glucose, calcium lactate, glucomannan, sodium acetylated hyaluronate and acetyl hexapeptide-8. The micromolecular donkey-hide gelatin essence anti-aging facial mask has the efficacies of supplementing skin nutrition, moisturizing, removing wrinkles and resisting senility.
Owner:SHAN DONG DONG E E JIAO
Formula and method for immobilizing tick sample for scanning electron microscopy
InactiveCN105486554ADry and completeShort timePreparing sample for investigationFreeze-dryingHigh energy
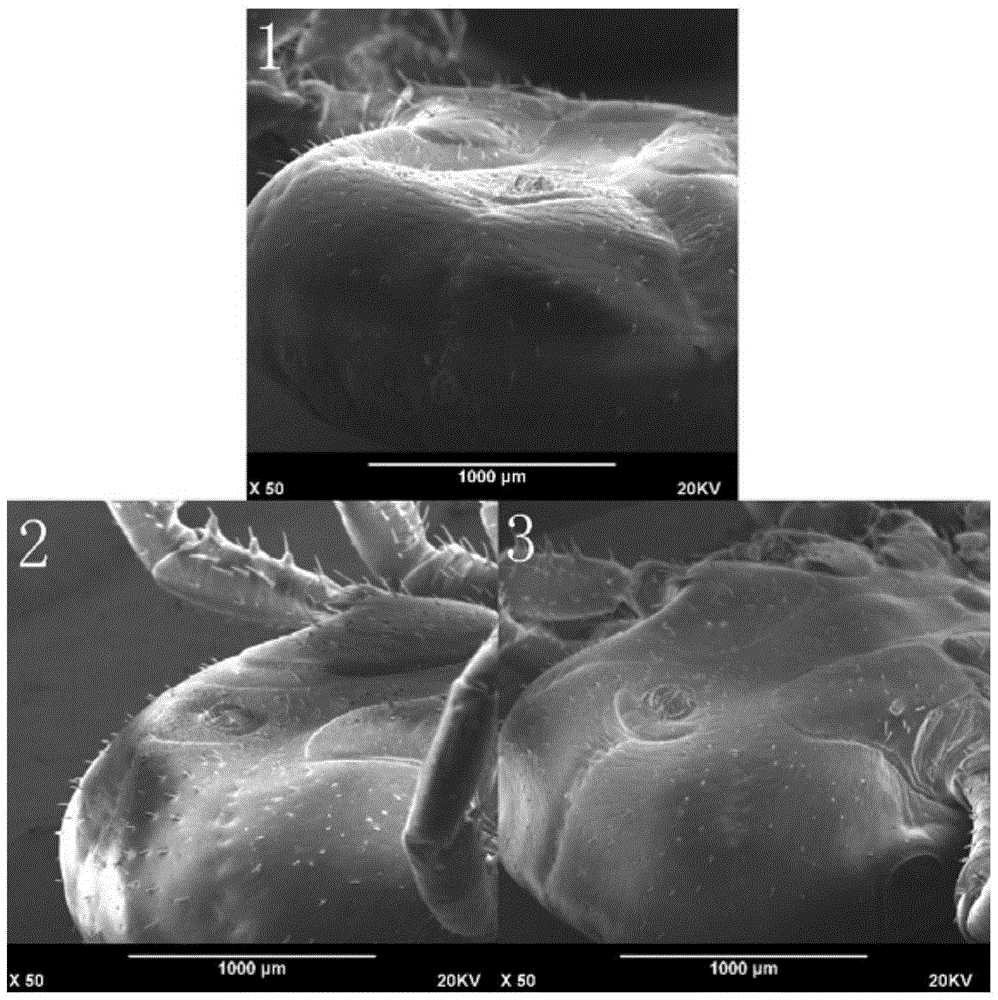
The invention relates to a formula and method for immobilizing a tick sample for scanning electron microscopy, which belongs to the technical field of methods for immobilizing samples for scanning electron microscopy. The immobilization formula comprises a PBS buffer solution, polysorbate and glutaraldehyde, and a glutaraldehyde immobilization liquid is prepared from the above-mentioned components. The method comprises the following steps: (1) observing whether host tissue is left in basis capituli of the tick sample and if so, removing the host tissue; (2) cleaning the surface of the tick sample; (3) immobilizing the tick sample at room temperature by using the glutaraldehyde immobilization liquid; (4) carrying out gradient dehydration with ethanol on the immobilized tick sample; (5) putting the tick sample into mixed liquor of absolute ethyl alcohol and acetone for displacement, and then putting the tick sample into acetone for displacement; and (6) drying the tick sample in a vacuum freeze drying instrument for drying and spraying gold on the tick sample. During scanning electron microscopic observation, the phenomenon of fuzzy and foggy images or incapable imaging due to ionization discharging of the sample as water vapor produced after electron beam bombardment of the sample encounters high-energy electron streams is prevented.
Owner:XINJIANG AGRI UNIV
Topical administration carrier composition and therapeutic formulations comprising same
Owner:CELMATRIX CORP
Medicament composition for water-soluble injection of paclitaxel, preparation method and uses thereof
ActiveCN101366696AOrganic active ingredientsPharmaceutical delivery mechanismFreeze-dryingHydroxystearic Acid
The invention discloses a water-soluble drug composition of taxol for injection. The drug composition comprises taxol as an active component, polyethyleneglycol 15- hydroxy stearic acid ester as a solubilizer, alcohol as a latent solvent and injection water as a solvent. Polysorbate, cyclodextrin derivatives, poloxamer, polyethylene glycol (PEG) and reducing sugar are also added and are favorable for improving the solubility and stability of the taxol in the composition. The composition is prepared to freeze-dried powder injection for injection, can not produce serious anaphylactic reaction, has small simulation, remarkably improves the clinical compliance of patients and has good water solubility and high convenience for medication by clinicians. The powder injection has good stability, can be stored at room temperature and is favorable for storage and transportation. The powder injection is simple in preparation process, easy to control quality, low in production cost and convenient for industrialized production and also greatly reduces the economic burden of the patients for medication. The invention also discloses a method for preparing the drug composition and clinical application thereof.
Owner:姚定全
Composition with azelaic acid
The invention relates to a pharmaceutical composition having the following constituents: azelaic acid, polyacrylic acid, triacylglyceride, propylene glycol, polysorbate, soya lecithin, water and salts. The composition is a hydrogel which is suited for the treatment of rosacea, presbyderma, melasma or skin irritations.
Owner:LEO PHARMA AS
Process for preparing aqueous dispersions containing high concentration of nano/submicron, hydrophobic, functional compounds
InactiveUS20120244134A1Good dispersionReduce in quantityBiocidePowder deliveryHigh concentrationSucrose
The present invention provides a process for preparing an aqueous dispersion containing a high concentration of nano / submicron, hydrophobic, functional compounds. The process is carried out by using a complex stabilizer having an HLB value of about 10 to about 17, comprising lecithin and at least one non-phospholipid selected from polysorbate, sucrose ester, and polyglycerol fatty acid ester; selecting a specific weight ratio of the hydrophobic functional compounds and the stabilizer; and using homogenization technique, media milling technique, and / or centrifugal technique. The aqueous dispersion containing a high concentration of nano / submicron, hydrophobic, functional compound produced by the process of the invention has stable dispersibility and improved bioavailability, and can be applied to the fields of foods and pharmaceuticals.
Owner:FOOD IND RES & DEV INST
Preparation method of high-purity 1,4-sorbitan
Owner:NANJING WELL BIOCHEM
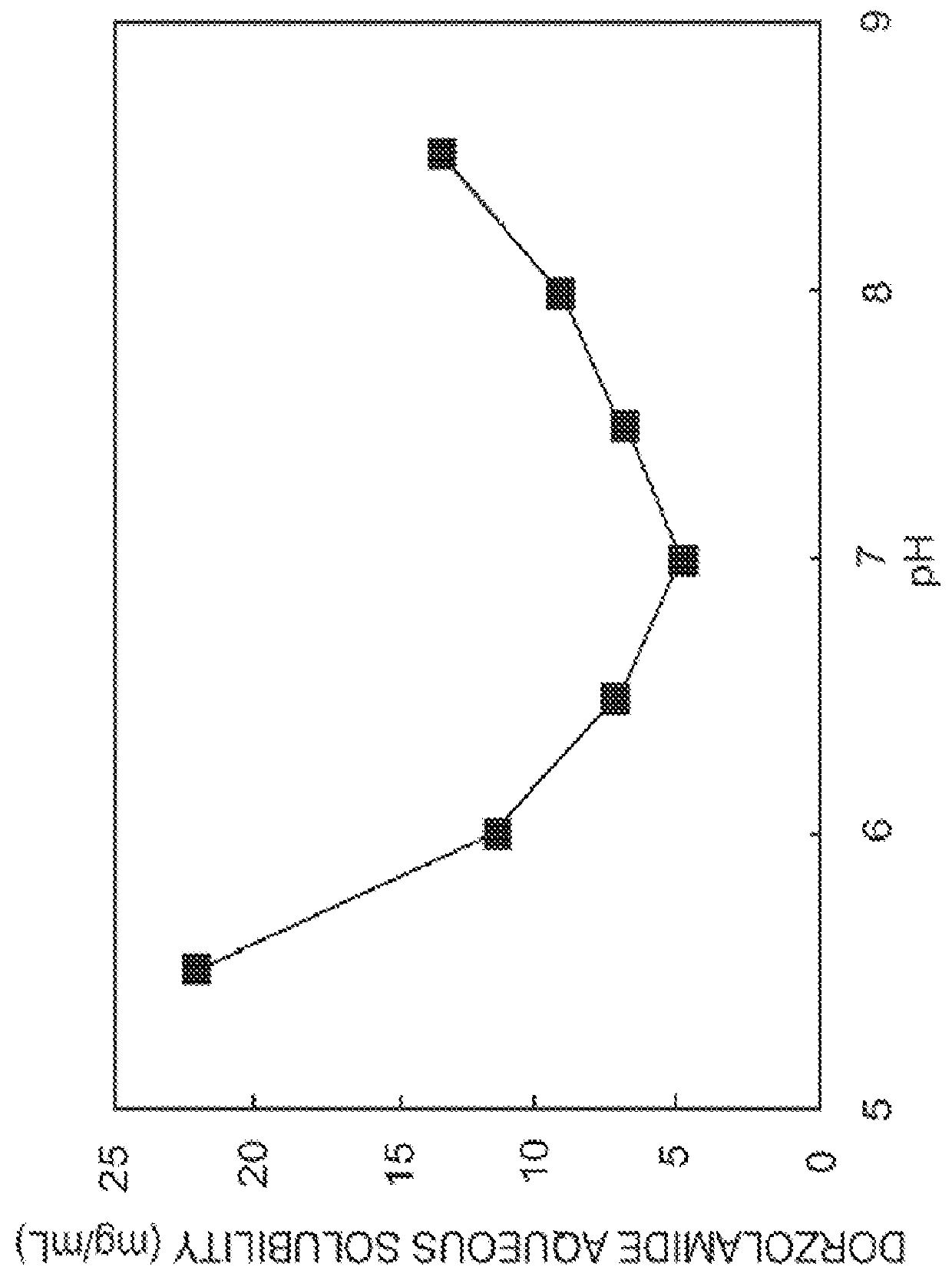
Ophthalmic composition
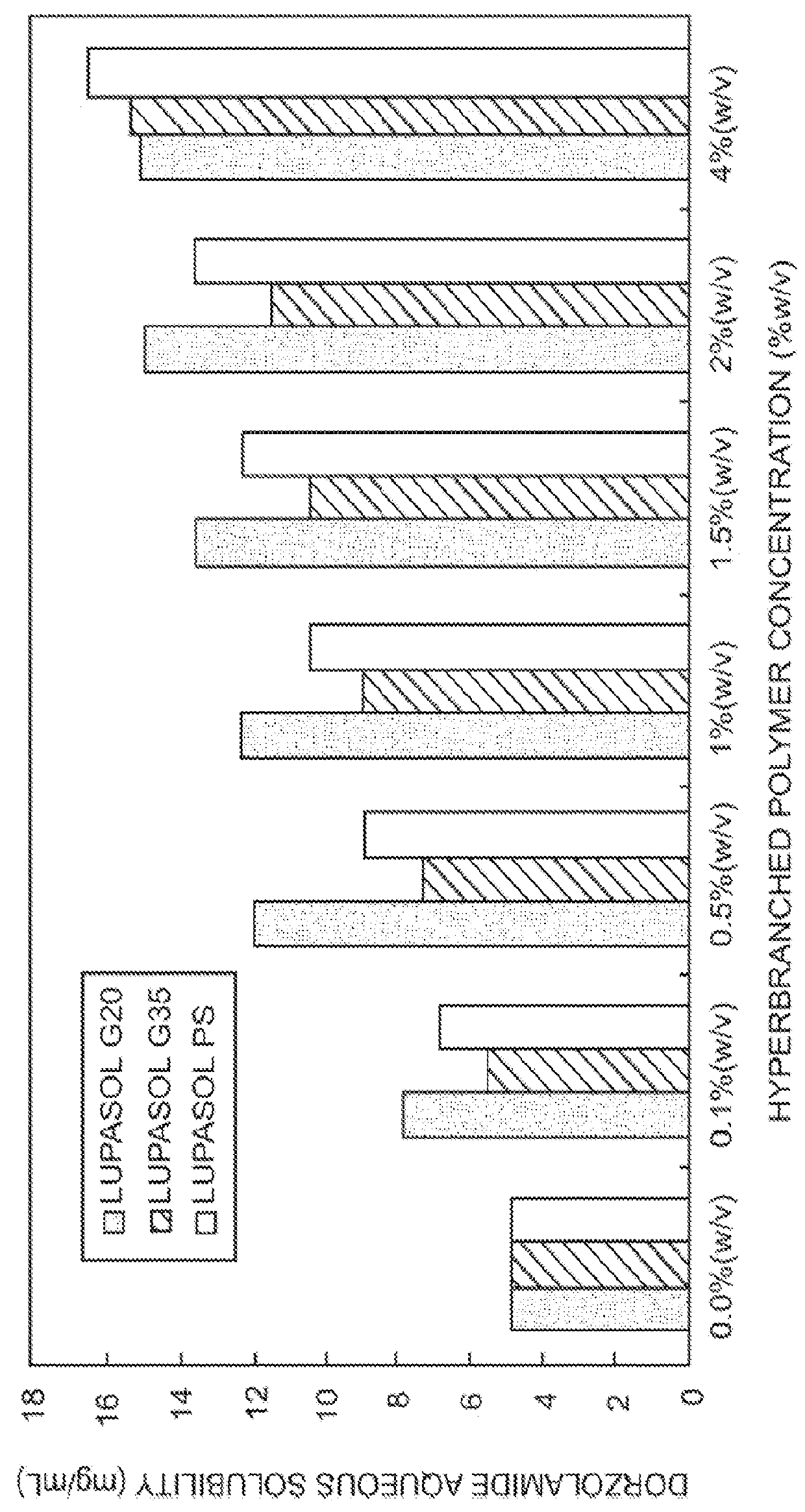
InactiveUS20130053374A1Good water solubilitySafely employedBiocideSenses disorderCarteololBeta blocker
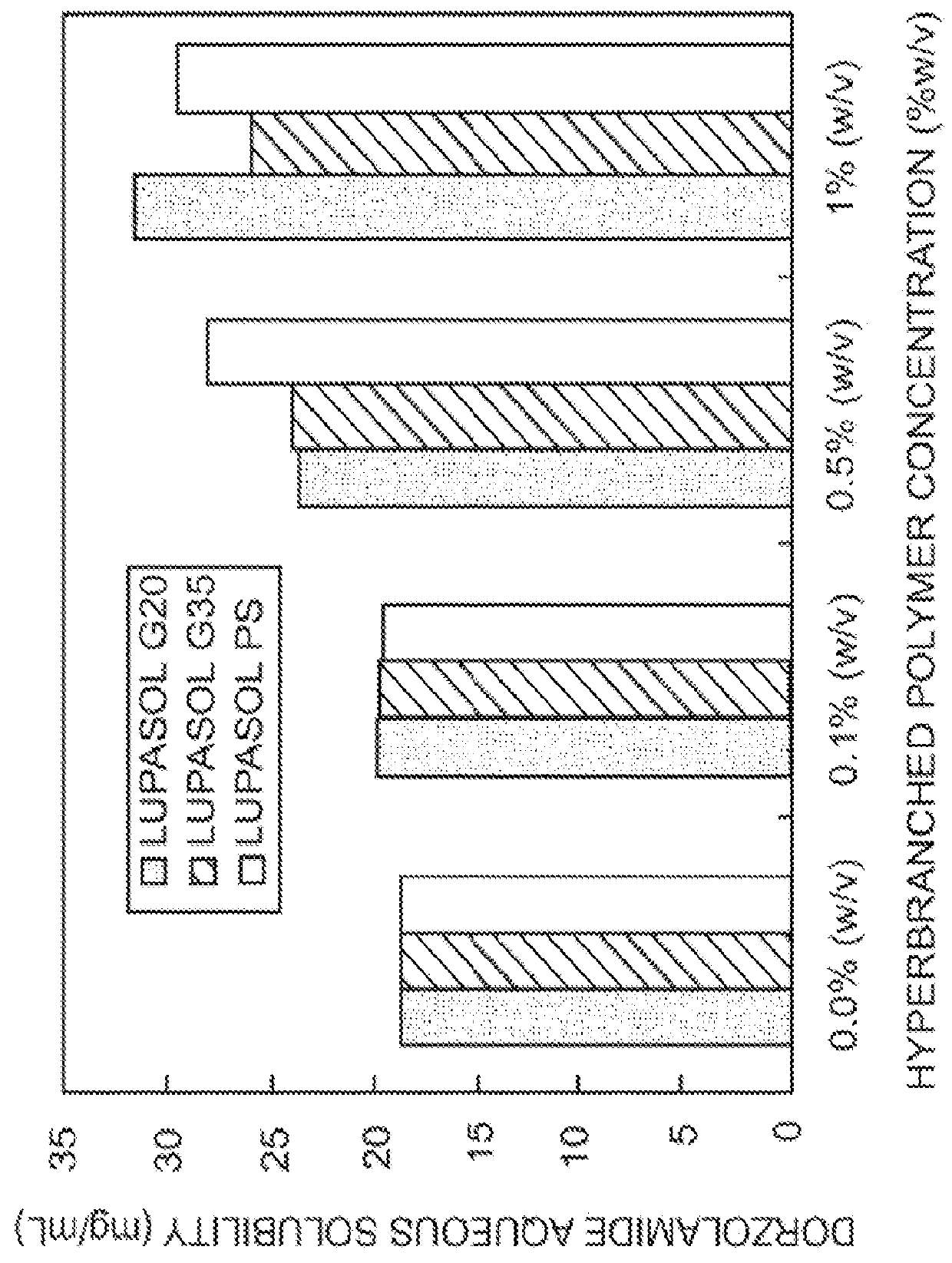
The present invention provides an ophthalmic composition comprising a hyperbranched polyester. The ophthalmic compositions may also comprise carbonic anhydrase inhibitors, wherein the hyperbranched polyester increases the aqueous solubility of the carbonic anhydrase inhibitor, and increases corneal permeation of the active agent. The ophthalmic compositions may also comprise non-ionic surfactants, such as PEG, Polysorbate, HPMC or HEC, and beta-blockers, such as Carteolol, Levobunolol, Betaxolol, Metipranolol, Timolol or Propranolol. The concentration of the hyperbranched polyester in the ophthalmic formulation should be less than or equal to 4% (w / v) in order to avoid any cytotoxic effects on human corneal cells and thus the eye irritation.
Owner:SENJU USA
Effective skin care product having effects of whitening skin and removing freckles and preparation method of skin care product
InactiveCN105687044AImprove securityNo allergic reactionCosmetic preparationsToilet preparationsGlycyrrhiza glabra RootGlycerol
The invention discloses an effective skin care product having effects of whitening skin and removing freckles and a preparation method of the skin care product. The skin care product is prepared from raw materials in percentage by weight as follows: 5%-8% of Asian Betula alba juice, 2.5%-4.5% of the other effective skin care product additive, 10%-12% of glycerin, 1.5%-2.0% of ceramide 3, 0.8%-1.5% of sodium hyaluronate, 1.5%-2.5% of polysorbate -20 and 73%-78.7% of water, wherein the other effective skin care product additive is prepared from raw materials in percentage by weight as follows: 5%-8% of a glycyrrhiza glabra root extract, 3%-5% of a strawberry extract, 3%-7% of arbutin and 4.5%-7.5% of aloe vera leaf juice. Trials on the effective skin care product having effects of whitening skin and removing freckles by consumers find that the skin care product has effects of whitening skin, removing freckles and resisting oxidation; during the trials, feedback of trial allergic reactions is not received, and the skin care product is suitable for people with various skin types at ages prone to freckles (20-50 years old).
Owner:珀蒂珍(广东)健康产品有限公司
Pharmaceutical composition containing nocathiacin antibiotics
ActiveCN102018953AImprove solubilityLow efficiencyAntibacterial agentsAerosol deliveryPolyethylene glycolAntibiotic Y
The invention belongs to the field of pharmaceutical compositions and specially relates to a solution agent of a pharmaceutical composition containing nocathiacin antibiotics, in particular to the pharmaceutical composition containing the nocathiacin antibiotics, which comprises nocathiacin, a drug carrier and a hydrophilic substance, wherein the hydrophilic substance is selected from a latent solvent or a solubilizer; the drug carrier is a physiological dissolution medium with the pH of 4-9, the latent solvent is one or the mixture of more than two selected from ethanol, propylene glycol, glycerin or polyethylene glycol, and the solubilizer is one or the mixture of more than two selected from sorbitan fatty acid, polysorbate, polyvidone, poloxamer and lecithin. The pharmaceutical composition can significantly improve the solubility of the nocathiacin, the preparation process is safe, materials used by the pharmaceutical composition are economic, devices used for preparing the pharmaceutical composition are simple, and the process steps are few; and as the nocathiacin has significant anti-drug resistance activity, the pharmaceutical composition has important clinical application value.
Owner:NANJING BIOTICA PHARMA
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS20140271598A1Effective and less approachEffectively and extensivelySenses disorderNervous disorderIduronate-2-sulfataseHunter syndrome
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising an iduronate-2-sulfatase (I2S) protein, salt, and a polysorbate surfactant for the treatment of Hunters Syndrome.
Owner:TAKEDA PHARMA CO LTD
Cream for chap removal
InactiveCN104546562APrevent chappingCracked isolationCosmetic preparationsToilet preparationsGlycerolOil phase
The invention discloses a cream for chap removal, which is composed of a water phase, an oil phase and a functional phase, wherein in parts by weigh, the water phase is composed of 45-85 parts of water, 0.1-0.5 part of allantoin and 0.05-0.2 part of diazolidinyl urea, the oil phase is composed of 0.5-1.5 parts of cetostearyl alcohol, 1-3 parts of glyceryl stearate, 0.5-1.5 parts of PEG-100 stearate, 2.5-5 parts of glycerin, 1-5 parts of polydimethylsiloxane, 2.5-5 parts of propylene glycol, 1-3 parts of isopropyl palmitate, 1-3 parts of petrolatum, 0.5-1.5 parts of polysorbate-60, 5-15 parts of mineral oil, 0.1-0.3 part of methylparaben, and 0.05-0.15 part of propyl hydroxybenzoate; and the functional phase is composed of 1-4 parts of one or more of ammonium polyacrylate, C13-16 isoparaffin, and laureths-25, 0.15-0.45 part of essence and 0.25-0.75 part of vitamin E. Experiments show that the cream both has the functions of water locking and moisturizing and has the effects of chap prevention and chap removal, therefore, the cream has a good market prospect.
Owner:无锡樱花梦美容制品有限公司
Improved process for analyzing for separating, and for isolating polar protic monomers and/or oligomers
An improved process for separating and isolating individual polar protic monomer(s) and / or oligomer(s) on the basis of degree of polymerization. A liquid sample containing polar protic monomer(s) and / or oligomer(s) is introduced into a liquid chromatography (LC) column packed with a polar bonded stationary chromatographic phase. The individual polar protic monomer(s) and / or oligomer(s) are separated via a binary mobile phase elution. One or more individual fractions containing the monomer(s) and / or oligomer(s) are eluted. The polar protic monomer(s) and / or oligomer(s) may be proanthocyanidins, hydrolyzable tannins, oligosaccharides, oligonucleotides, peptides, acrylamides, polysorbates, polyketides, poloxamers, polyethylene glycols, polyoxyethylene alcohols or polyvinyl alcohols. The binary mobile phase comprises an A phase consisting essentially of a polar aprotic solvent and a B phase consisting essentially of a polar protic solvent. A process for separating and isolating xanthine(s) (e.g., caffeine and theobromine) from polar protic monomer(s) and / or oligomer(s). A liquid sample containing xanthine(s) and polar protic monomer(s) and / or oligomer(s) is introduced into an LC column packed with a polar bonded stationary chromatographic phase. The xanthines are separated via an isocratic mobile phase elution, and one or more individual fractions containing the xanthines are eluted.
Owner:MARS INC
Methods and compositions for CNS delivery of heparan N-sulfatase
ActiveUS9320711B2Effective and less invasiveReduce deliveryPowder deliverySenses disorderSanfilippo syndrome type aLysosomal enzyme defect
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising a heparan N-sulfatase (HNS) protein, salt, and a polysorbate surfactant for the treatment of Sanfilippo Syndrome Type A.
Owner:TAKEDA PHARMA CO LTD
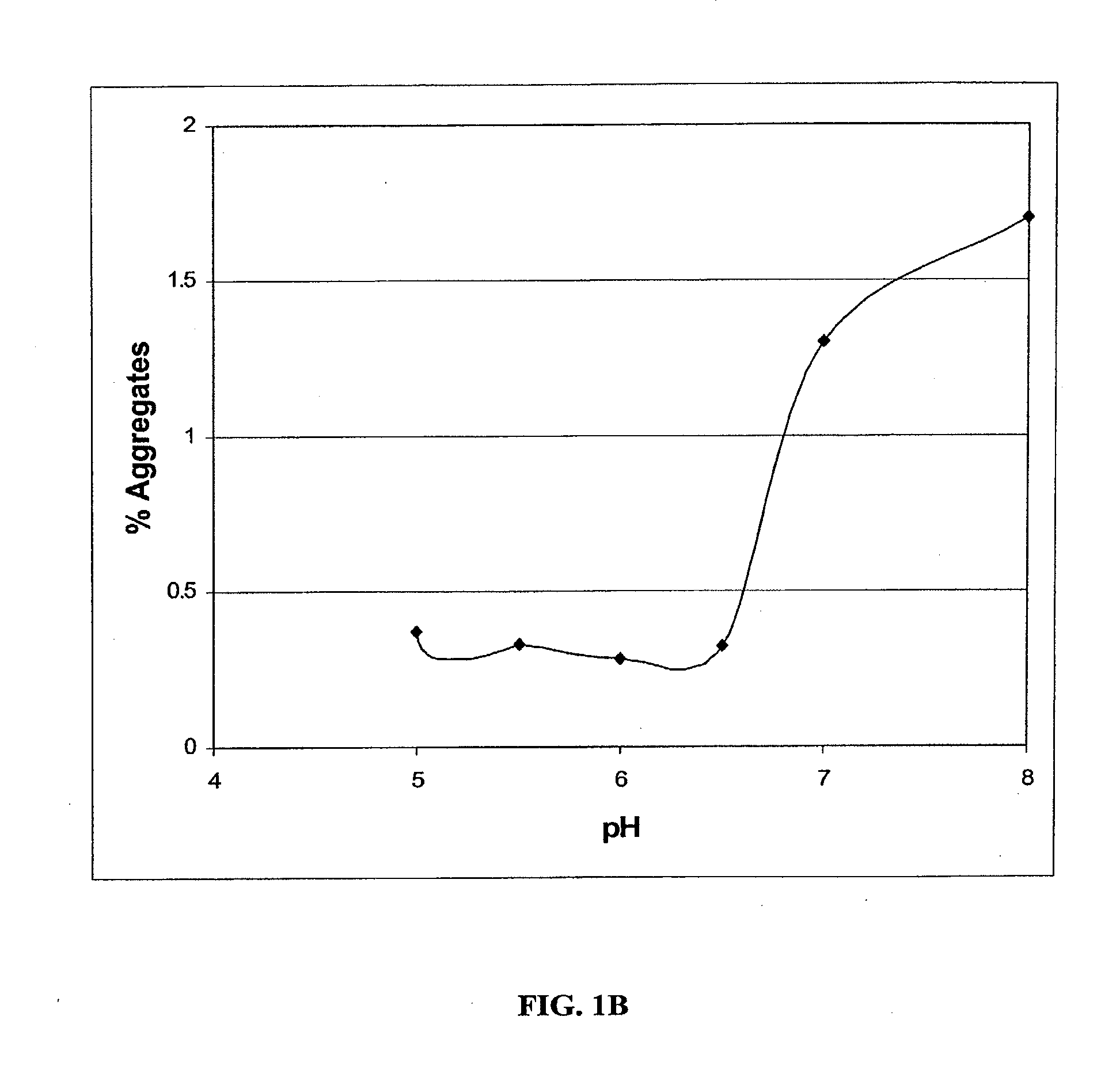
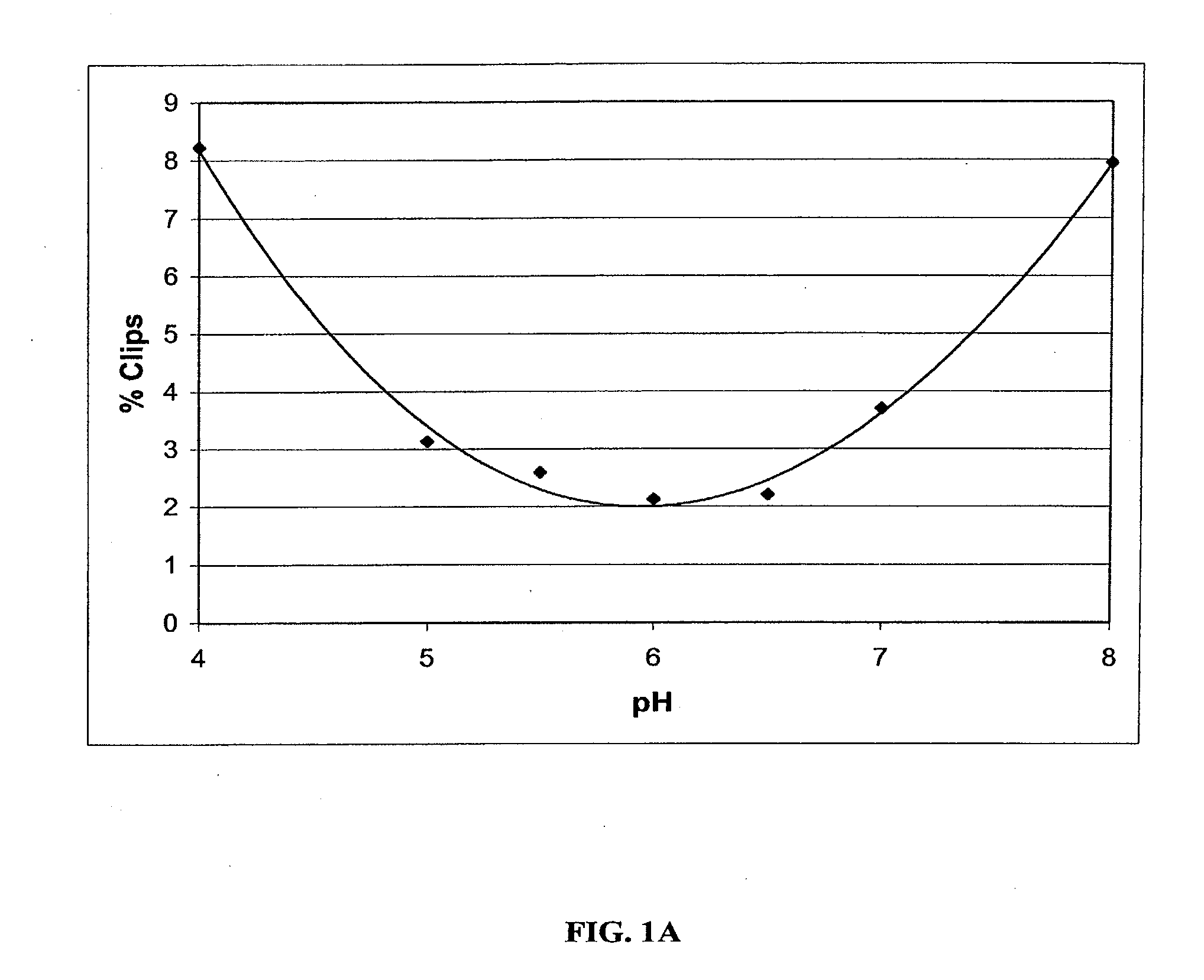
Stable Liquid Pharmaceutical Formulation Of IgG Antibodies
InactiveUS20110318343A1Avoid formingInorganic non-active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsHigh concentrationIL-2 receptor
This invention is directed to a stable liquid pharmaceutical formulation comprising a high concentration, e.g. 50 mg / ml or more, of antibody in about 20-60 mM succinate buffer or 30-70 mM histidine buffer, having pH from about pH 5.5 to about pH 6.5, about 0.01-0.1% polysorbate, and a tonicity modifier that contributes to the isotonicity of the formulation. This liquid formulation is stable at refrigerated temperature (2-8° C.) for at least 1 year, and preferably 2 years. This liquid formulation is suitable for subcutaneous injection. The preferred antibodies include Daclizumab, a humanized anti-IL-2 receptor monoclonal antibody; HAIL-12, a humanized anti-IL-12 monoclonal antibody; HuEP5C7, a humanized anti-L selectin monoclonal antibody; and Flintozumab, a humanized anti-gamma interferon monoclonal antibody.
Owner:ABBVIE BIOTHERAPEUTICS
Non-protein clostridial toxin compositions
Pharmaceutical compositions that stabilize a Clostridial toxin active ingredient are described. The compositions can be liquid or solid compositions, and comprise a surfactant and an antioxidant. In some embodiments, the compositions comprise a surfactant selected from a poloxamer and a polysorbate; an antioxidant selected from methionine, N-acetyl cysteine, ethylenediaminetetraacetic acid and combinations thereof, and, optionally, a tonicity agent and / or a lyoprotector selected from, for example, trehalose, sucrose.
Owner:ALLERGAN INC
Topical administration carrier composition and therapeutic formulations comprising same
ActiveUS20070141004A1Reduce evaporative lossReduce the evaporative lossBiocideCosmetic preparationsWhole bodyBULK ACTIVE INGREDIENT
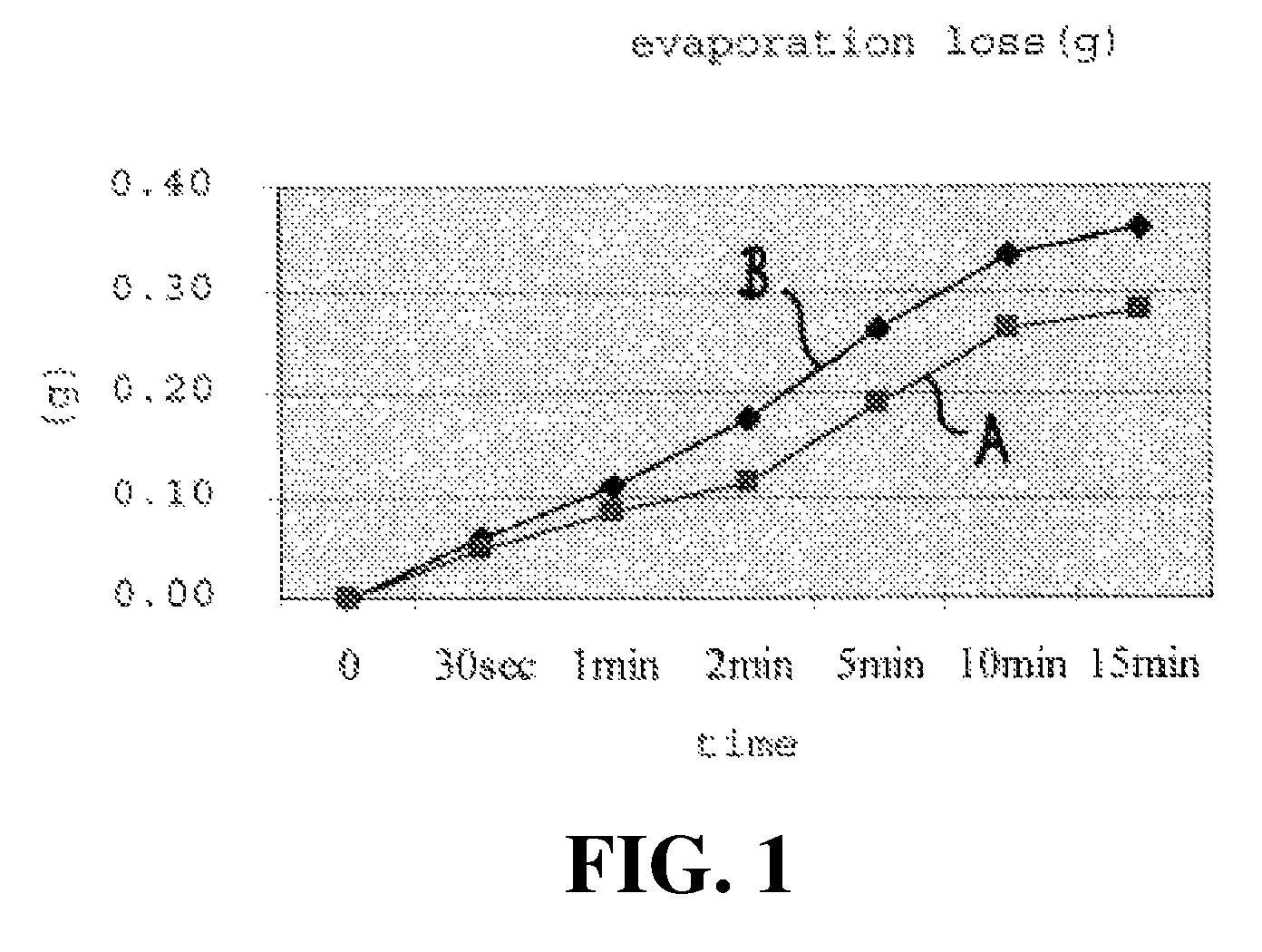
A topical administration carrier composition including water, glycerin and polysorbate, suitable for use with compositions containing active ingredients such as minoxidil that are susceptible to volatilization and transdermal penetration in application to the body. The carrier formulation retards the evaporative and systemic migration losses of the active ingredient composition, to provide sustained topical action, in relation to formulations lacking the components of the inventive composition.
Owner:CELMATRIX CORP
Concentrates of active agents, such as W-3 fatty acids, and polysorbate
InactiveUS8377494B2Effective and reliableLipidic food ingredientsFood preparationActive agentLanolin
The invention relates to the processing of substances that are not soluble, or soluble with difficulty, in water, in such a manner that, introduced into water or oil, these substances can yield a clear solution and can be easily integrated with finest homogeneous distribution into foodstuff, cosmetics, pharmaceuticals, and nutrient solutions. To achieve that, the invention provides a concentrate, consisting of an active substance from the group, which includes an algae oil, an essential oil, a terpene, phosphatidylserine, an ω 3 fatty acid, lanolin, a linoleic acid triglyceride, citral or tea tree oil, and a surplus amount of a polysorbate with at least two and half times the weight of the active substance.
Owner:AQUANOVA AG
Stable liquid pharmaceutical formulation of igg antibodies
InactiveUS20110070231A1Avoid formingInorganic non-active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsHigh concentrationSubcutaneous injection
This invention is directed to a stable liquid pharmaceutical formulation comprising a high concentration, e.g. 50 mg / ml or more, of antibody in about 20-60 mM succinate buffer or 30-70 mM histidine buffer, having pH from about pH 5.5 to about pH 6.5, about 0.01-0.1% polysorbate, and a tonicity modifier that contributes to the isotonicity of the formulation. This liquid formulation is stable at refrigerated temperature (2-8° C.) for at least 1 year, and preferably 2 years. This liquid formulation is suitable for subcutaneous injection. The preferred antibodies include Daclizumab, a humanized anti-IL-2 receptor monoclonal antibody; HAIL-12, a humanized anti-IL-12 monoclonal antibody; HuEP5C7, a humanized anti-L selectin monoclonal antibody; and Flintozumab, a humanized anti-gamma interferon monoclonal antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
High-fat powder grease and preparation method thereof
The invention discloses high-fat powder grease, which is characterized in that the high-fat powder grease comprises the following components in percent by weight: 81-91 percent of edible grease, 4-10 percent of edible emulsifier, 0.5-3 percent of silicon dioxide and dipotassium phosphate, 1-5 percent of animal and plant protein and 3-10 percent of starch sugar, wherein the edible emulsifier is one of the following matters or mixture of two or more than two of the following matters: monoglycerol / diglycerol fatty acid ester, diacetyl tartaric acid monoglyceride / diglyceride, sodium stearyl lactate, sucrose fatty acid ester, succinic acid glycerin fatty acid ester, polysorbate, polyglycerol fatty acid ester, fatty acid sorbitol anhydride ester, and lactic acid and fatty glyceride. The preparation method of the high-fat powder grease adopts spray drying. The fat content of the high-fat powder grease is high, the product stability is good and the grease needed by products can be provided when the high-fat powder grease is used for baked products; since the provided grease is in a powder state, the grease can be very easily mixed with flour and the like and the use is greatly facilitated; and at the same time, the high-fat powder grease simultaneously has a performance of shortening and the shortening capacity of the baked products can be remarkably improved.
Owner:BEIJING ALCHEMIST TECH
Hydrating and skin rejuvenating mask and preparation method thereof
ActiveCN108042388AAllergenicity reductionNo stimulationCosmetic preparationsToilet preparationsAbsorption of waterMoisture
The invention discloses a hydrating and skin rejuvenating mask and a preparation method thereof, and belongs to the technical field of masks. The hydrating and skin rejuvenating mask comprises the following raw materials in percentage by weight: 3 to 10 percent of glycerinum, 1.5 to 4.5 percent of butanediol, 1 to 4 percent of deep-sea fish collagen peptide, 3 to 8 percent of mycose, 0.05 to 2 percent of squalane, 0.15 to 2 percent of sodium hyaluronate, 1 to 5 percent of propylene glycol, 0.02 to 0.35 percent of xanthan gum, 0.02 to 0.45 percent of hydrogenated castor oil, 0.02 to 2 percent of polysorbate, 0.01 to 1 percent of phenoxyethanol, 0.01 to 0.5 percent of methylparaben, 0.1 to 2 percent of beta-glucan, 0.01 to 0.5 percent of a grape seed extract, 0.01 to 3 percent of an aloe extract, 0.01 to 1 percent of dipotassium glycyrrhizinate and the balance of deionized water. The mask provided by the invention promotes the absorption of water by the skin, is favorable for the skin topreserve moisture for a long time, is non-irritant and has a good effect of moisturizing skin.
Owner:MARINE BIOMEDICAL RES INST OF QINGDAO CO LTD
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS9220677B2Effective and less invasiveEffectively and extensivelyPowder deliverySenses disorderIduronate-2-sulfataseHunter syndrome
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising an iduronate-2-sulfatase (I2S) protein, salt, and a polysorbate surfactant for the treatment of Hunters Syndrome.
Owner:TAKEDA PHARMA CO LTD
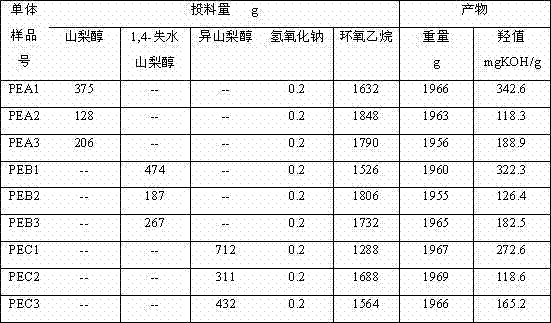
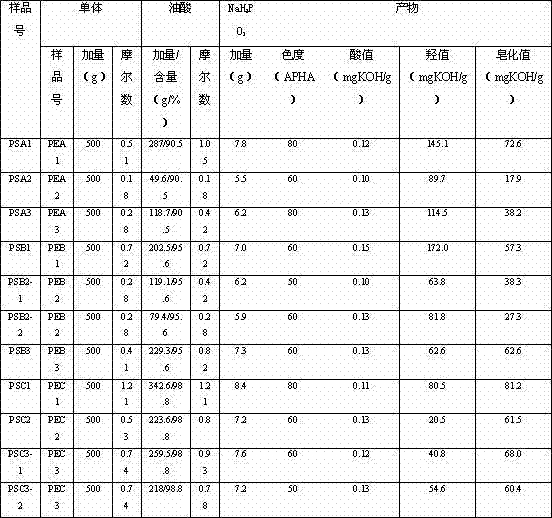
Method for preparing high purity polysorbate 80 through synthesizing three effective components and mixing
The invention discloses a method for preparing high purity polysorbate 80 through synthesizing three effective components and mixing, comprising the following steps: carrying out etherification of high purity sorbitol and ethylene oxide to prepare sorbitol polyoxyethylene ether (II), and carrying out esterification of the sorbitol polyoxyethylene ether (II) and high purity oleic acid to obtain sorbitol polyoxyethylene ether oleate (V); carrying out etherification of high purity sorbitan and oxirane to obtain sorbitan polyoxyethylene ether (III), and carrying out esterification of the sorbitanpolyoxyethylene ether (III) and high purity oleic acid to obtain sorbitan polyoxyethylene ether oleate (VI); carrying out etherification of high purity isosorbide and oxirane to obtain isosorbide polyoxyethylene ether (IV), and carrying out esterification of the isosorbide polyoxyethylene ether (IV) and high purity oleic acid to obtain isosorbide polyoxyethylene ether oleate (VII); and mixing V, VI and VII and refining to obtain the high purity polysorbate 80 (I). The prepared polysorbate 80 disclosed herein has high content of effective components, low content of nonactive components and impurities, and improvement of the applicability, can be applied for injection preparation.
Owner:NANJING WELL BIOCHEM
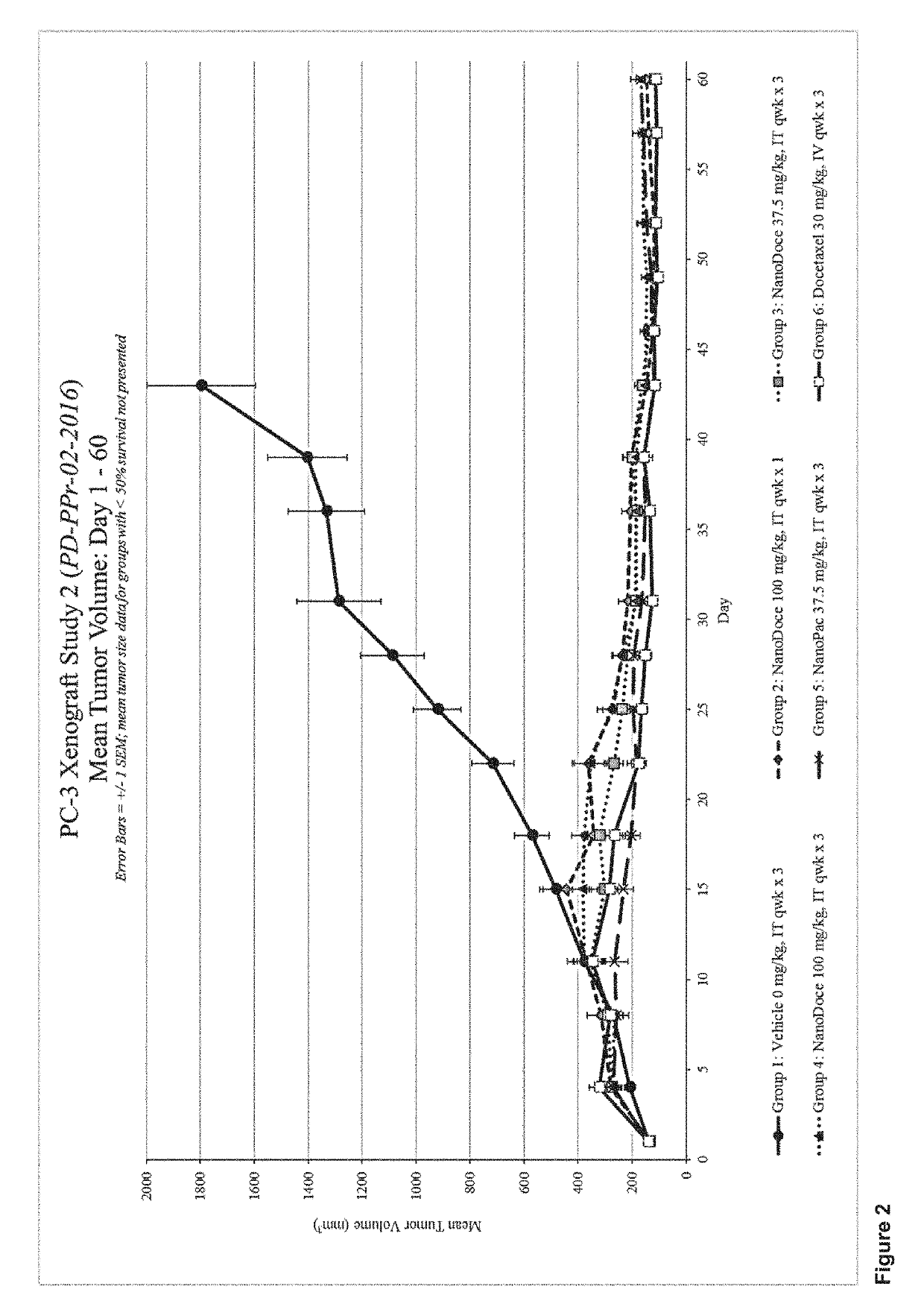
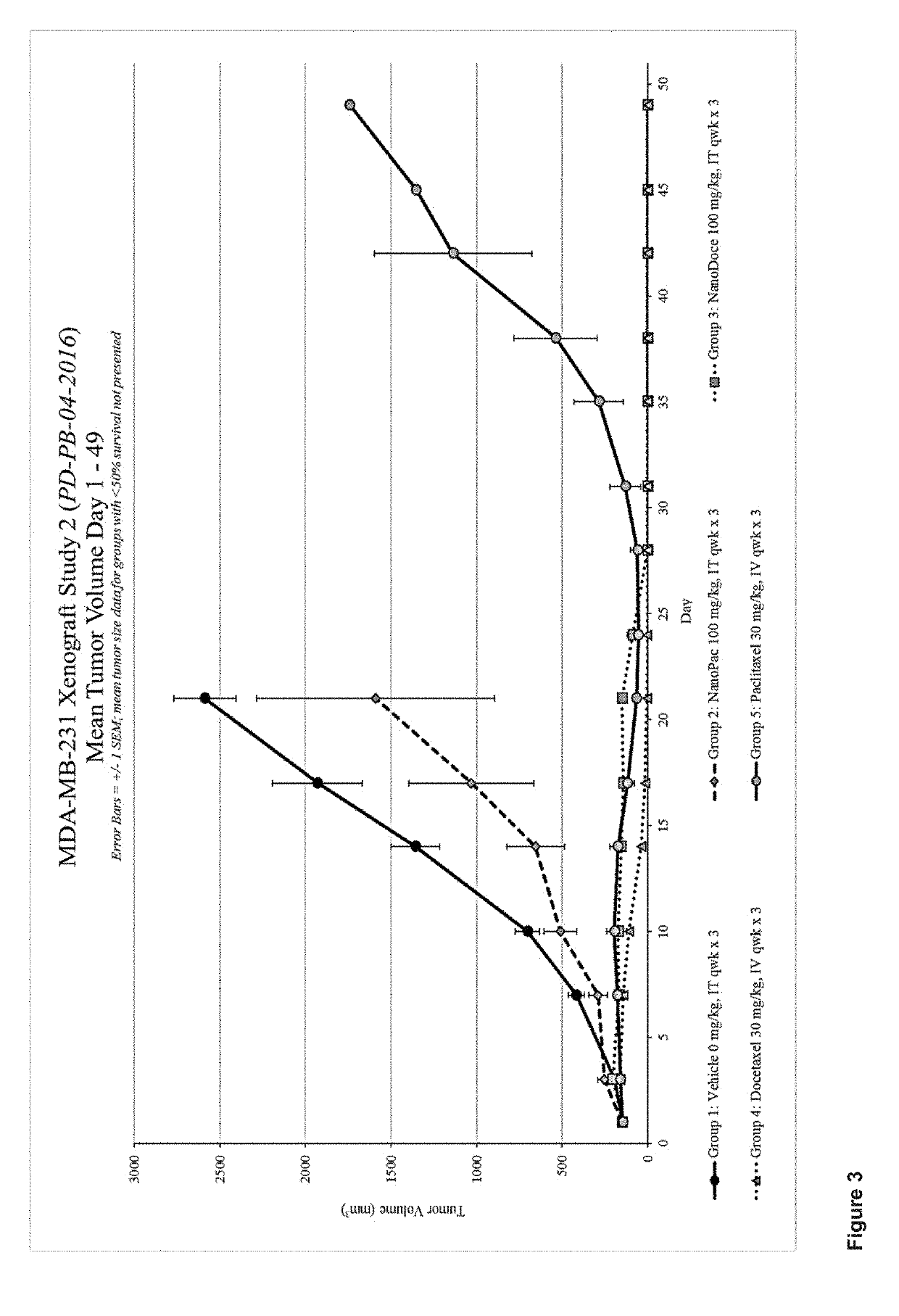
Methods for solid tumor treatment
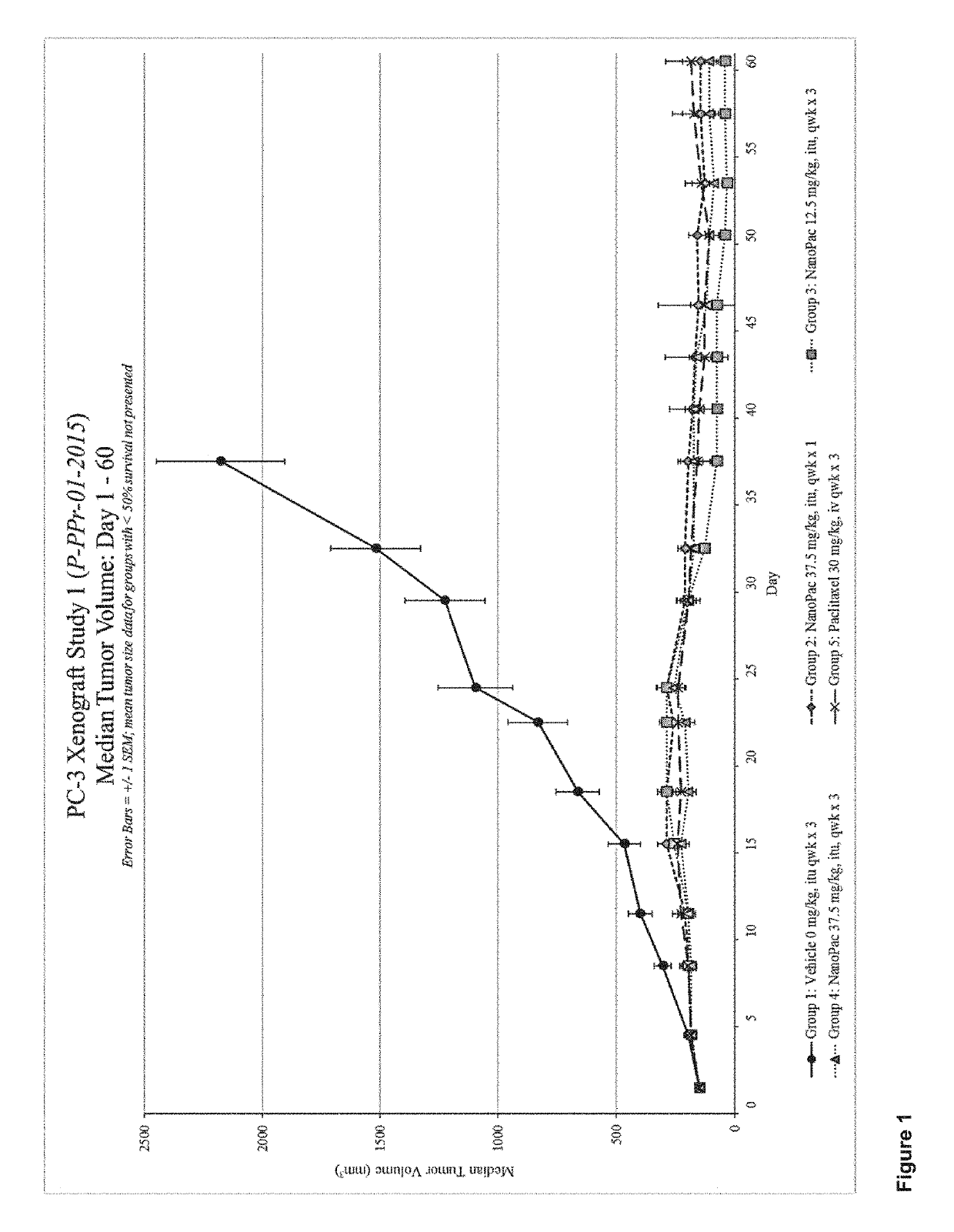
Disclosed herein are methods for treating solid tumors by direct injection into the tumors of chemotherapeutic particles, methods for inhibiting tumor metastasis by administering chemotherapeutic particles to a subject having a tumor, and compositions that include chemotherapeutic particles, small, amounts of a polysorbate, and a carrier.
Owner:CRITITECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com