Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
120 results about "Intrathecal use" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intrathecal administration is a route of administration for drugs via an injection into the spinal canal, or into the subarachnoid space so that it reaches the cerebrospinal fluid (CSF) and is useful in spinal anaesthesia, chemotherapy, or pain management applications.
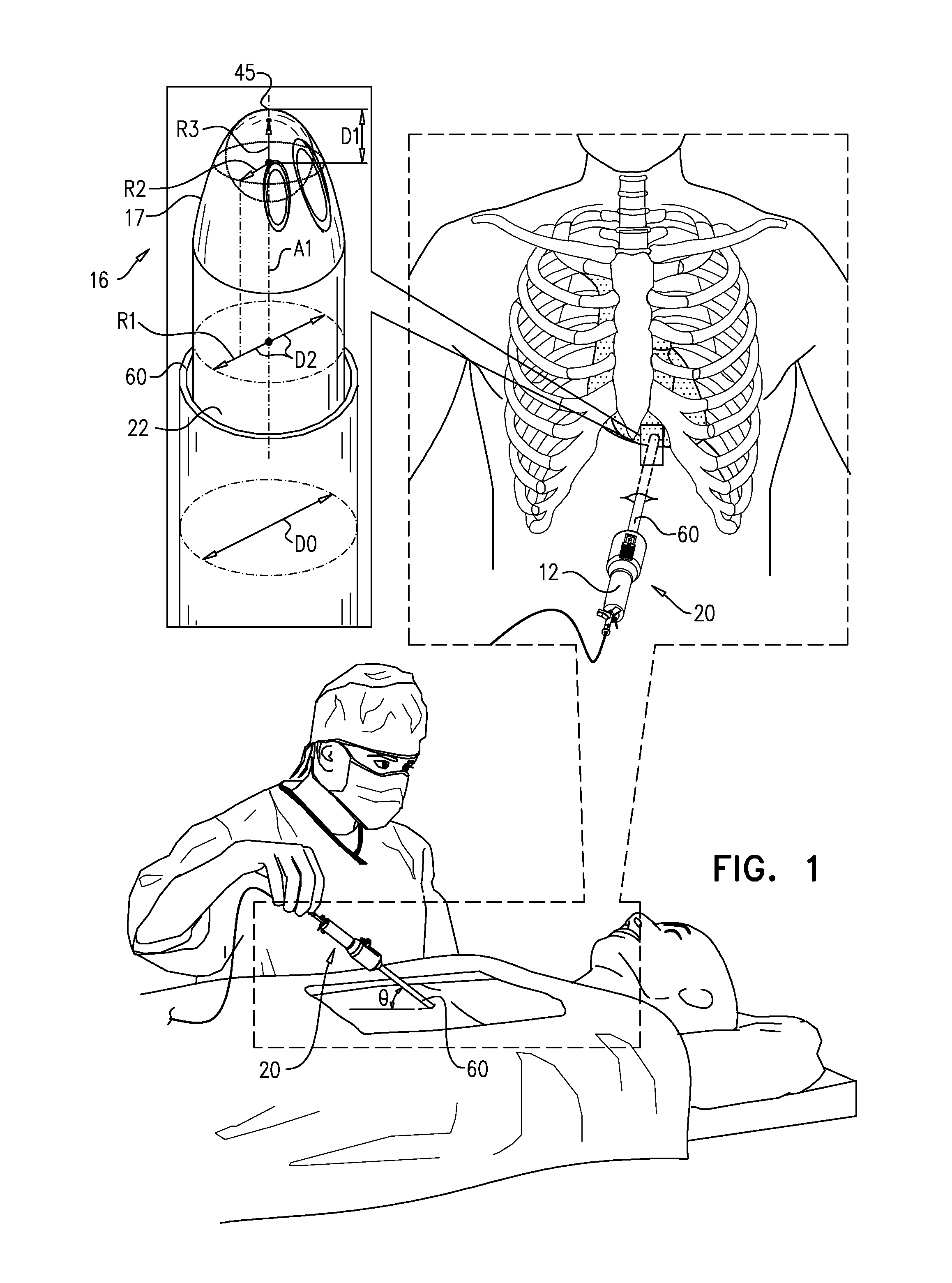
Intrathecal administration of rituximab for treatment of central nervous system lymphomas
InactiveUS20020009444A1Prevent intermolecular disulfide formationPromote recoveryBiocidePeptide/protein ingredientsMeningesImmunocompromised patient
This invention describes methods of using anti-B cell antibodies, preferably anti-CD20 antibodies, and most preferably Rituximab, to treat B cell lymphomas of the brain, especially primary central nervous system lymphomas (PCNSLs), and to prevent meningeal relapse. The antibodies can be administered intrathecally alone, or in combination with other chemotherapeutics, such as methotrexate, or other anti-B cell antibodies to treat PCNSL in both immunocompromised and non-immunocompromised patients. These antibodies can also be used to diagnose patients with CNS lymphoma, especially in immunocompromised patients.
Owner:BIOGEN INC
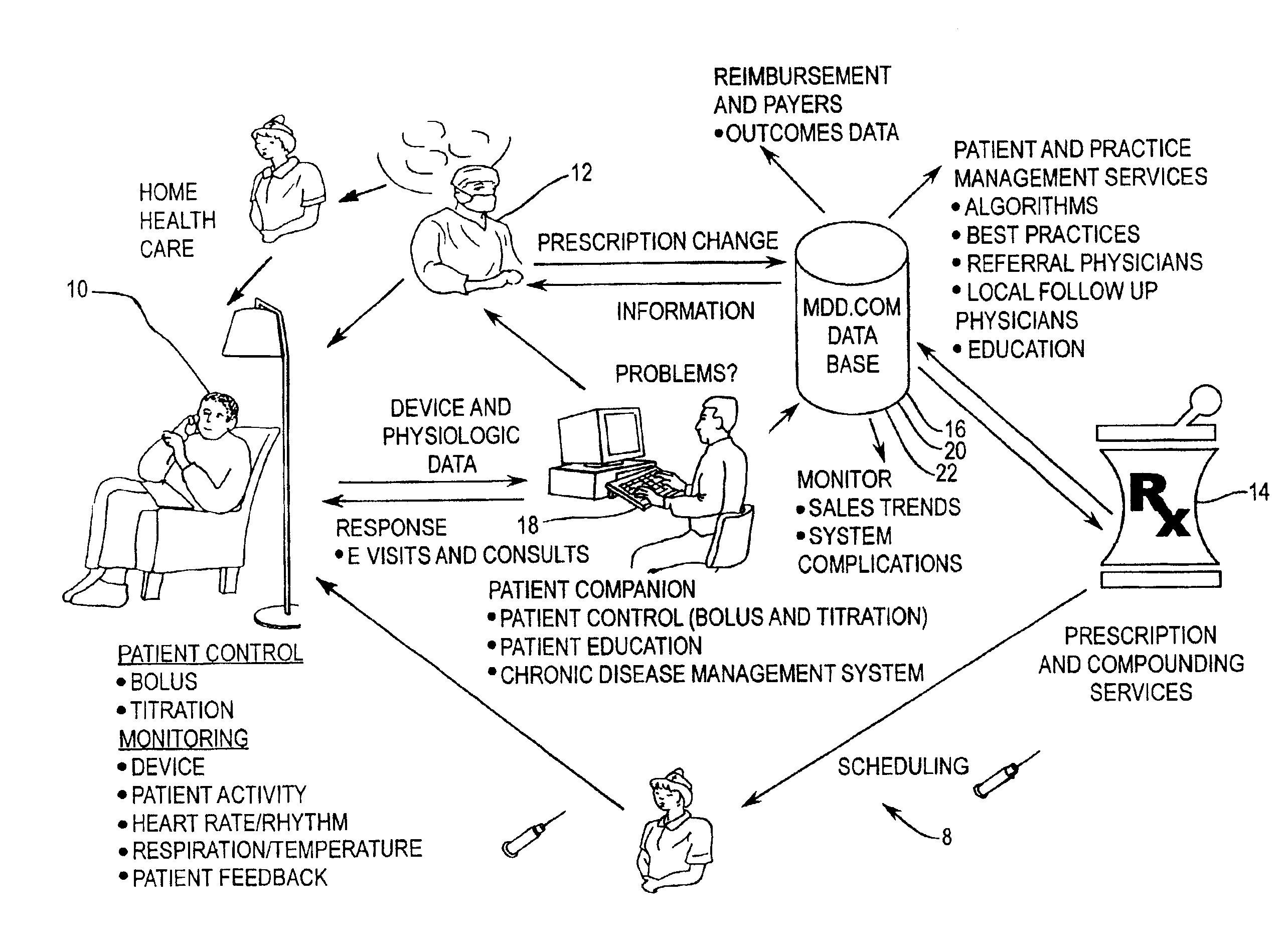
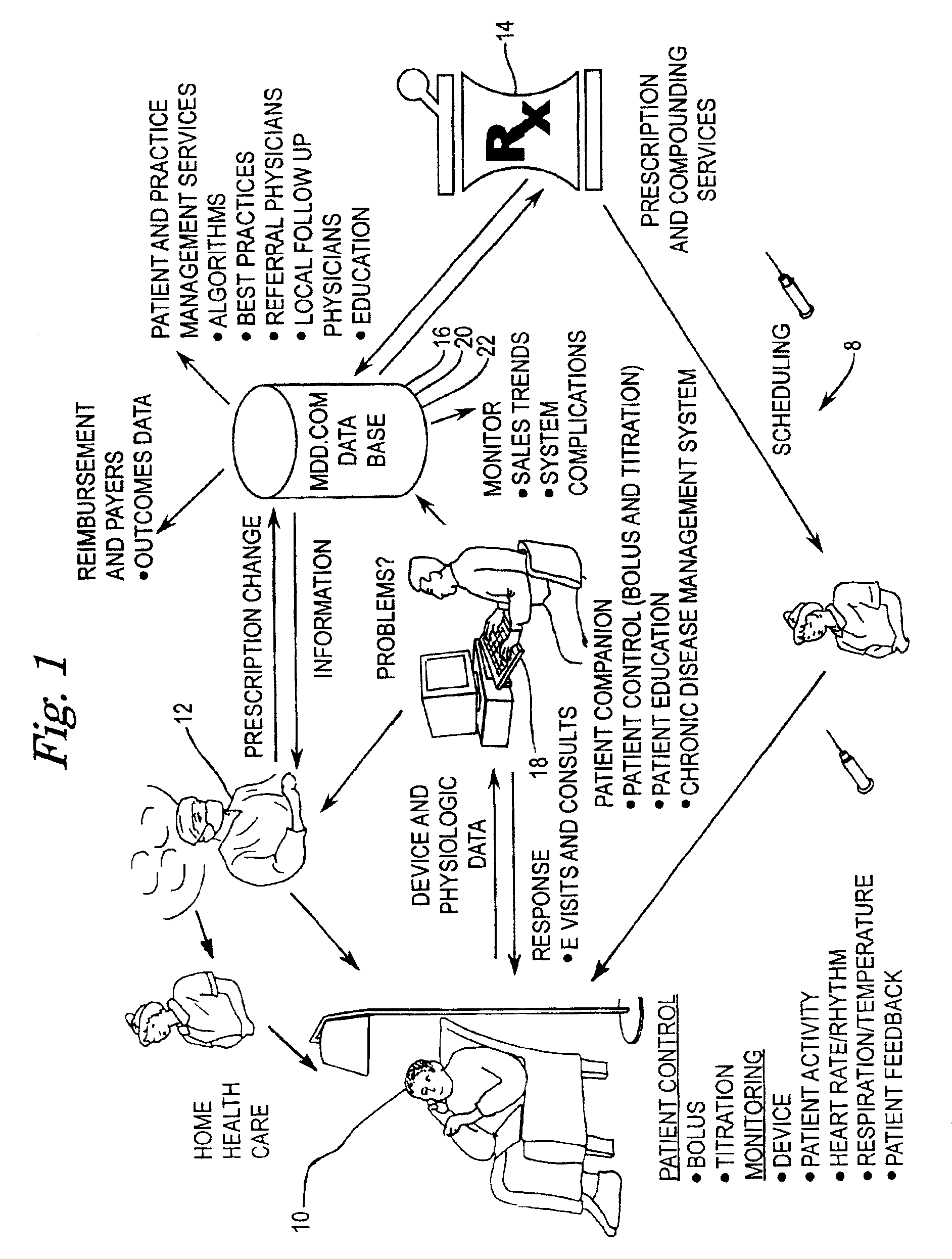
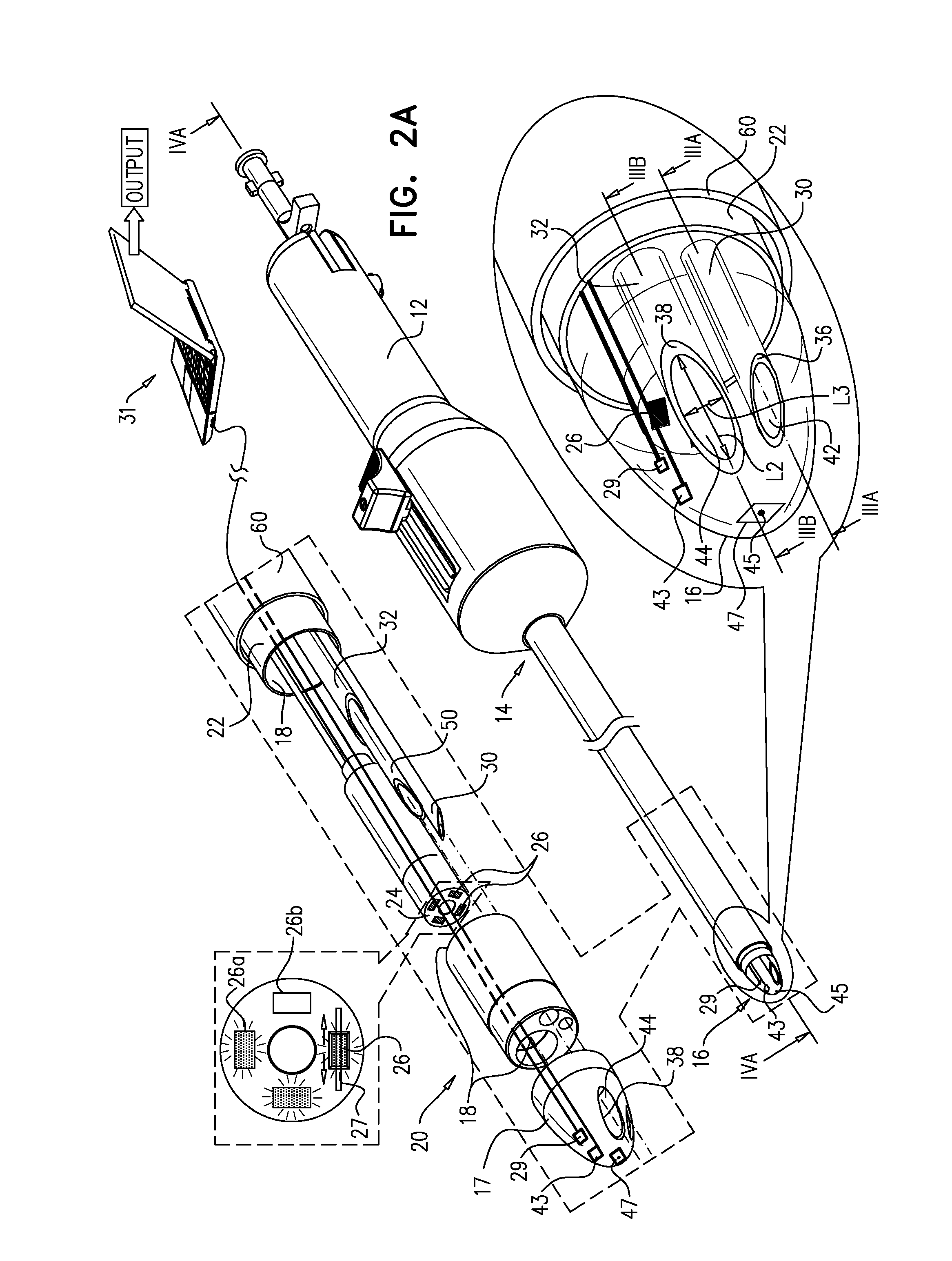
Implantable therapeutic substance infusion device configuration system
InactiveUS7072725B2Opportunities decreaseMinimize potential for overdoseLevel controlDrug and medicationsCommunications systemIntrathecal therapy
The present invention provides for a software-based computing environment that uses data communication systems or networks, including the Internet and World Wide Web technologies, to implement a dosage calculator, which assists the clinician in managing patients receiving intrathecal therapy. The invention provides for a communications environment for clinicians, pharmacists, drug pump manufactures, and patients to assess the information supplied by an implantable drug pump, and to integrate data from other data sources in order to provide optimization of the life of the drug pump and the therapeutic substance formulations for the patient.
Owner:MEDTRONIC INC
Delivery of therapeutic compounds to the brain and other tissues
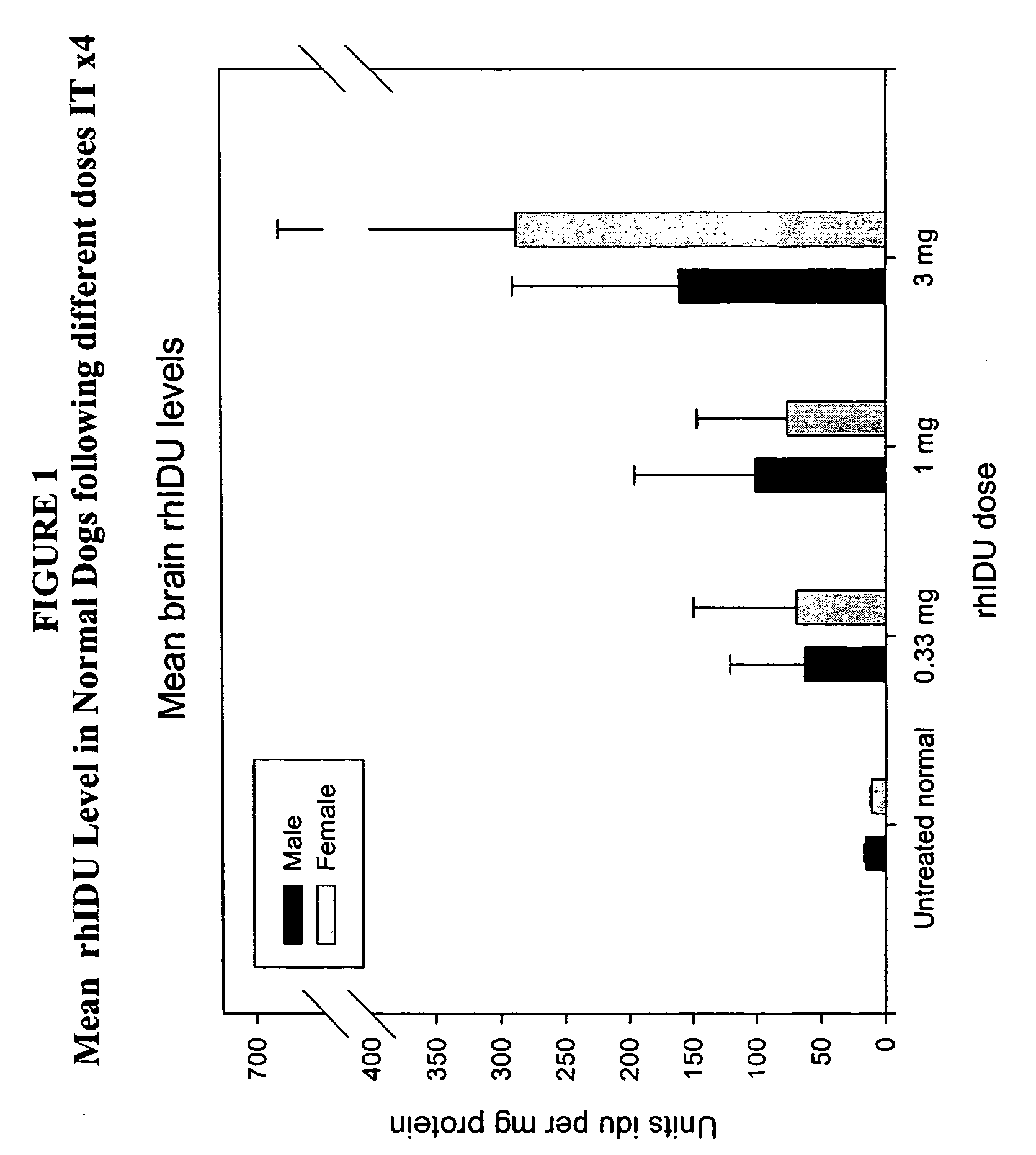
ActiveUS20050048047A1Reduces and eliminates glycogen storage granuleReduce eliminateNervous disorderPeptide/protein ingredientsMeningesIntrathecal use
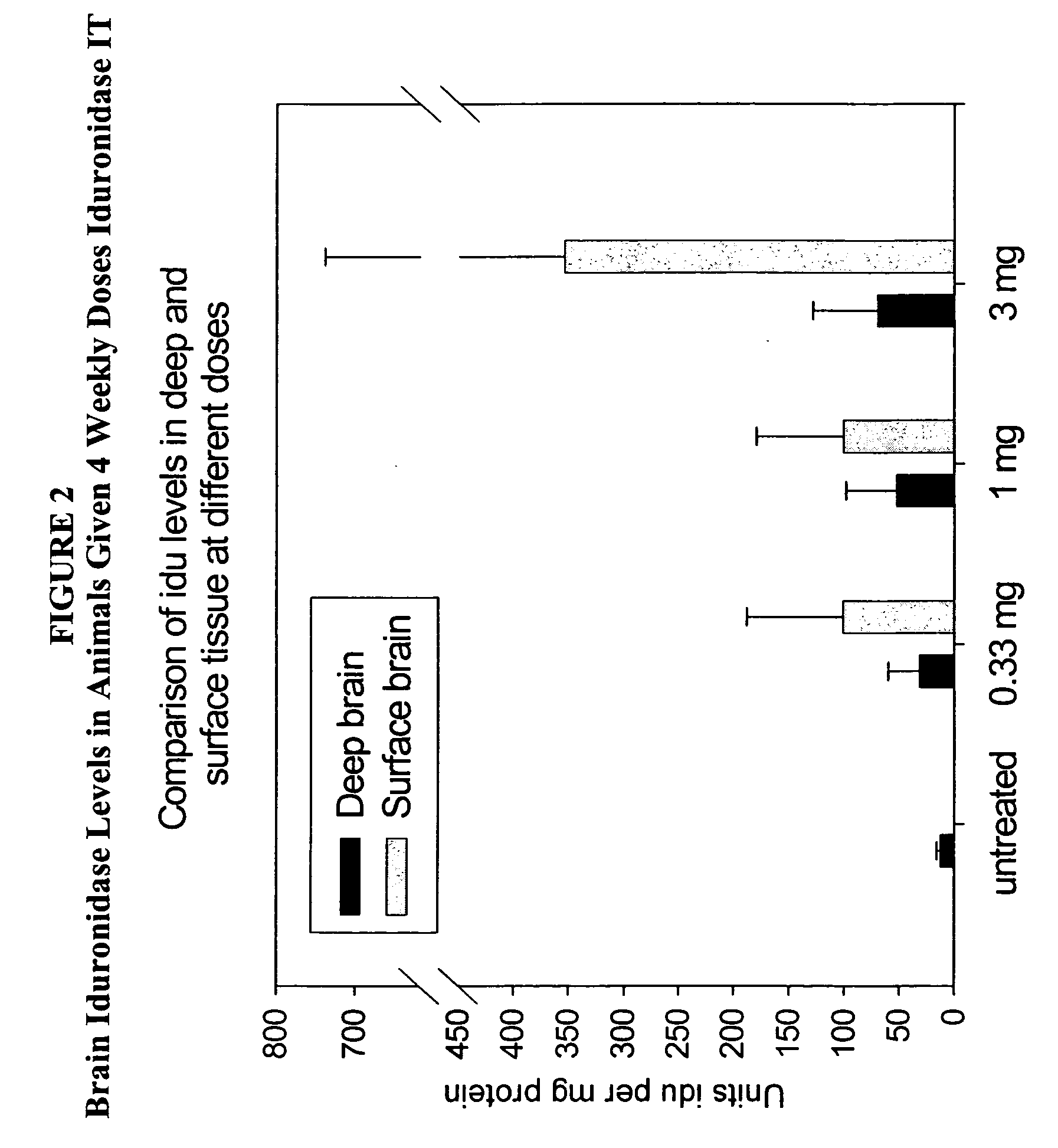
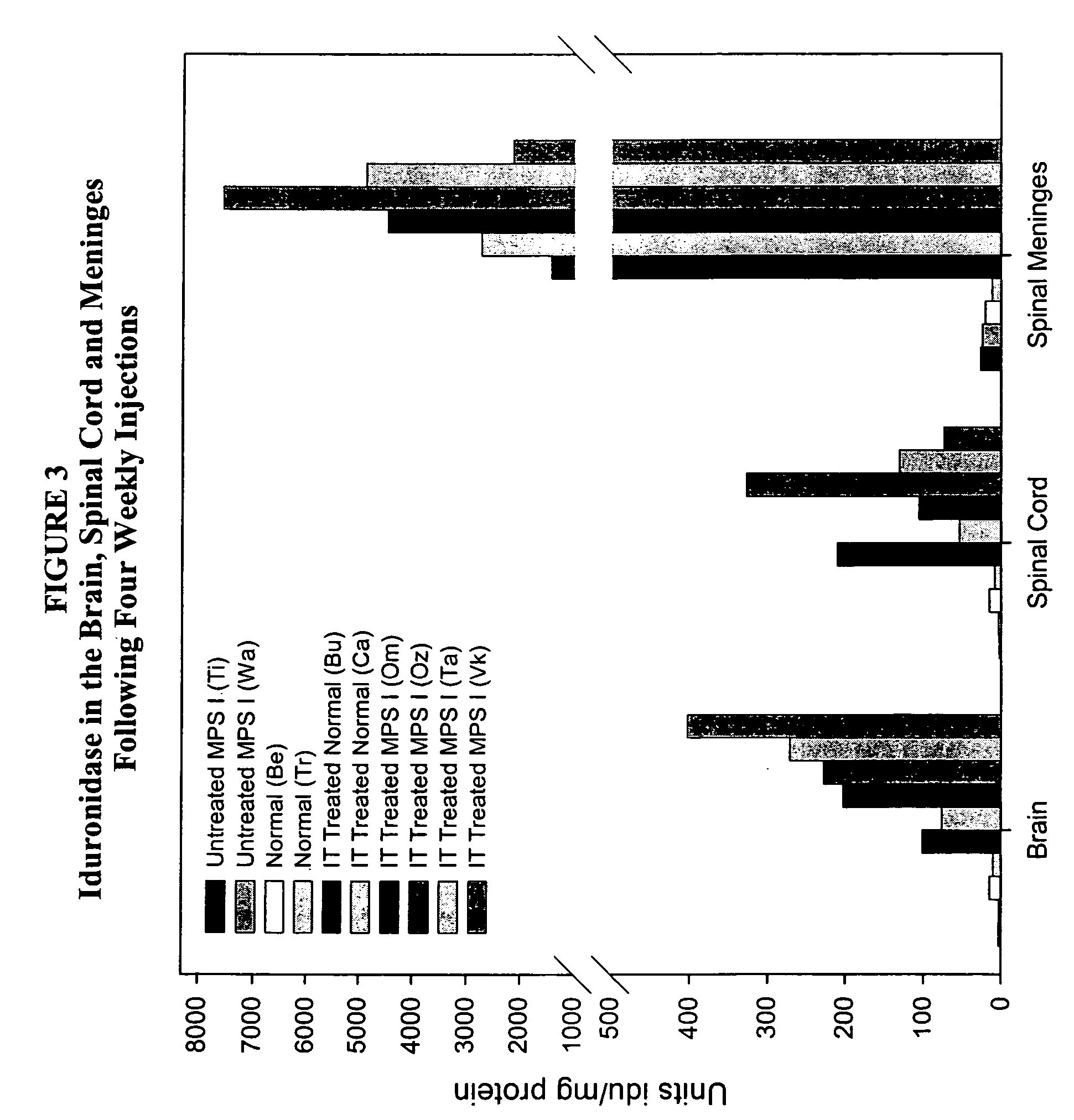
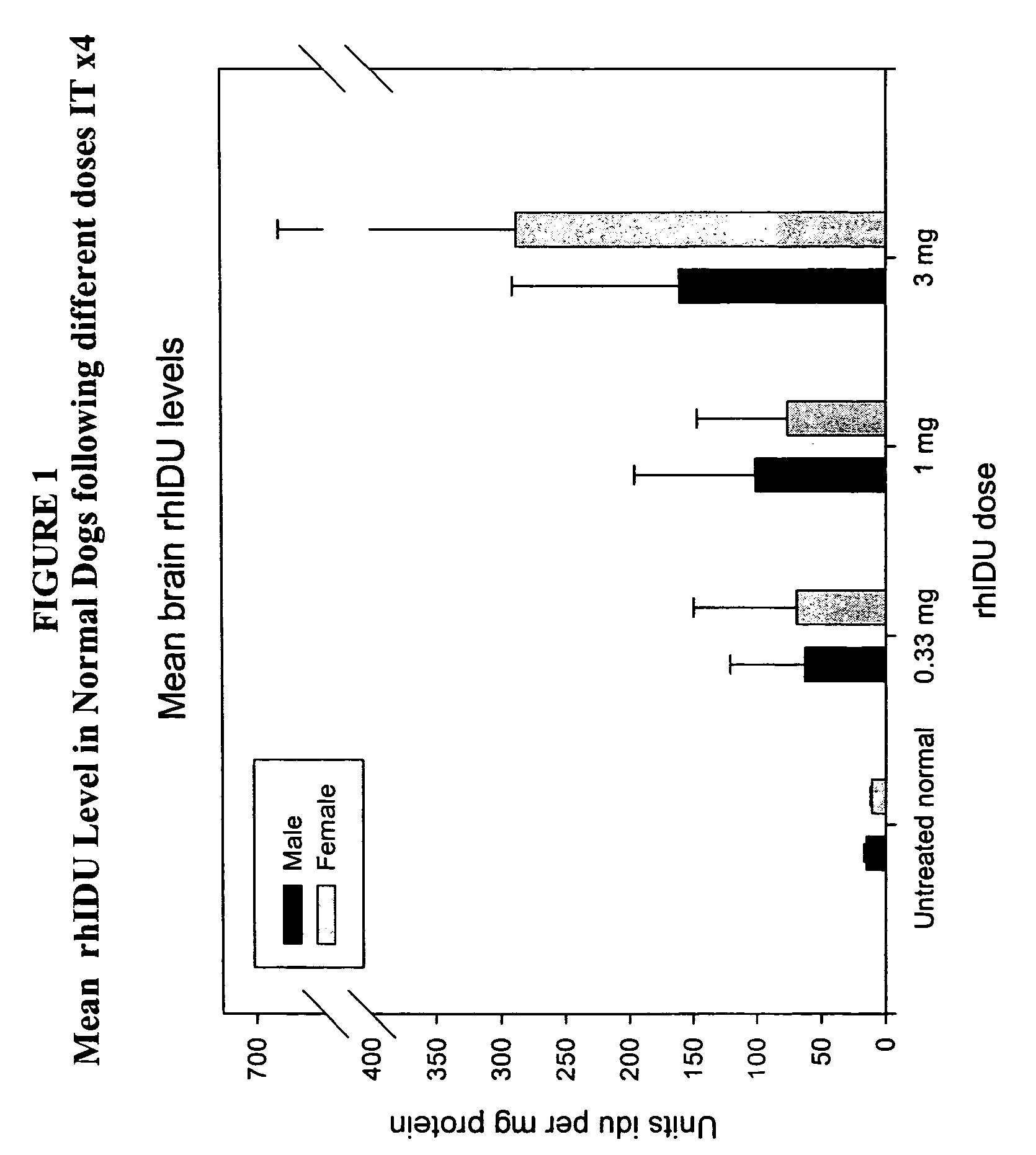
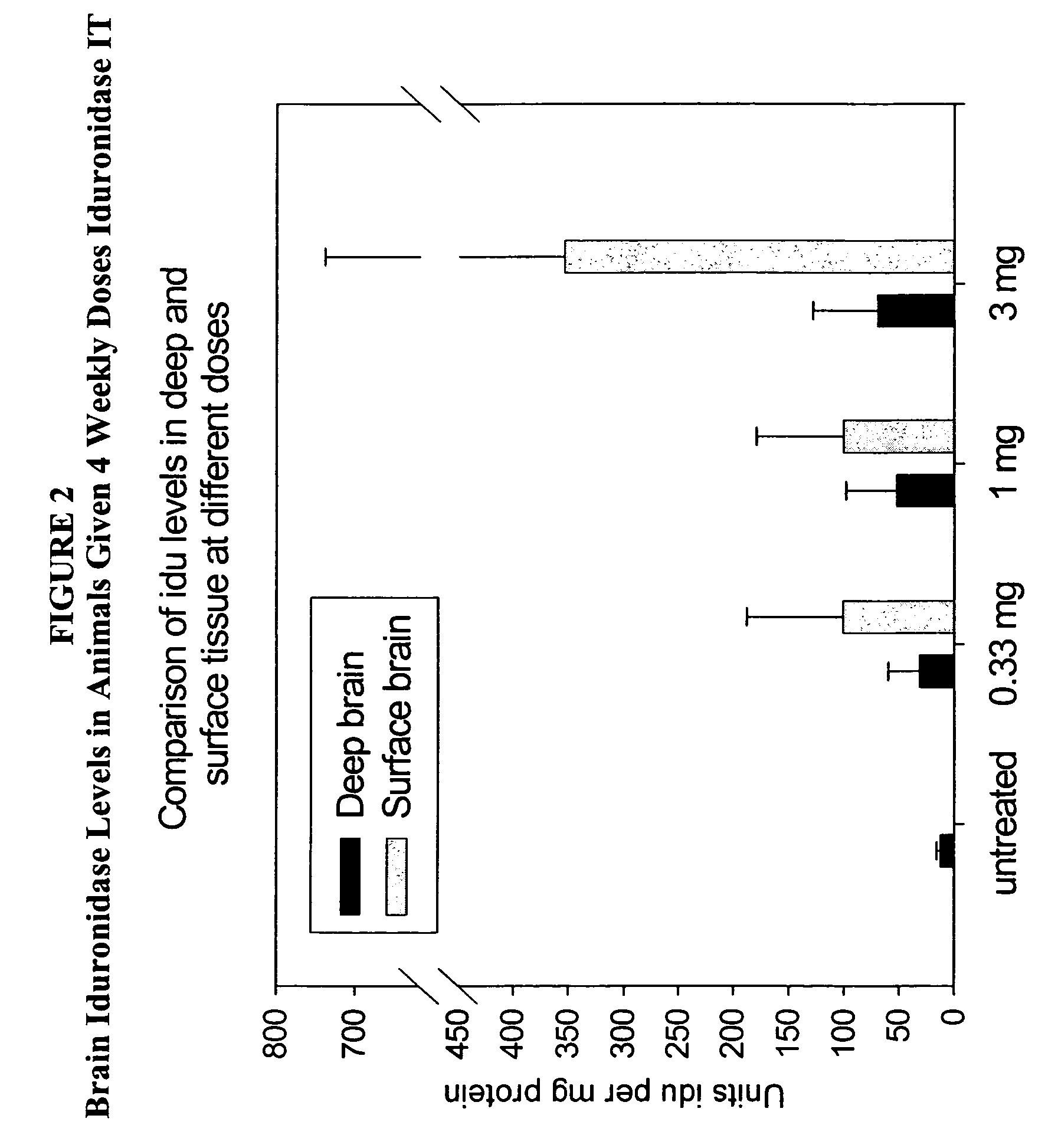
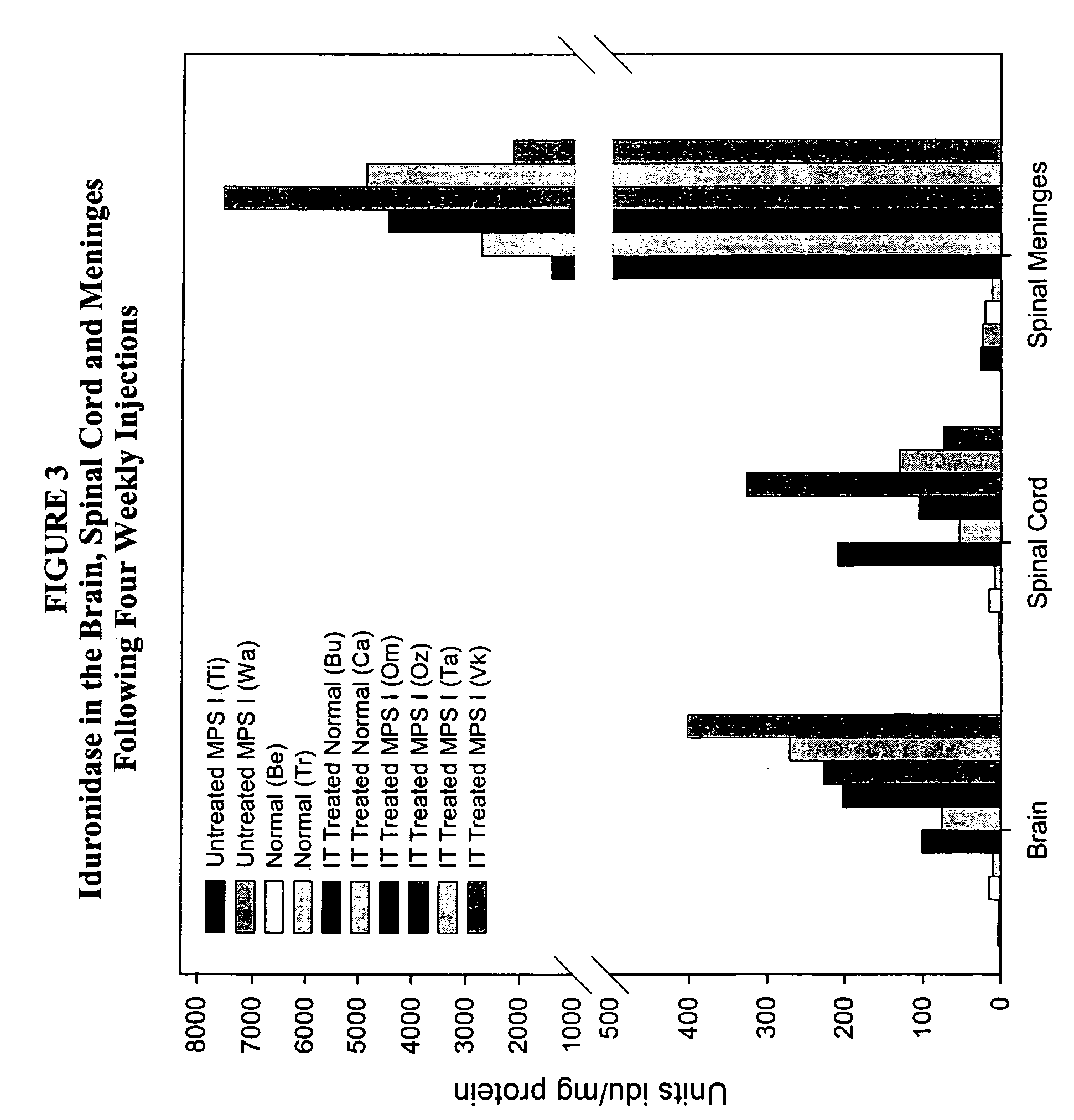
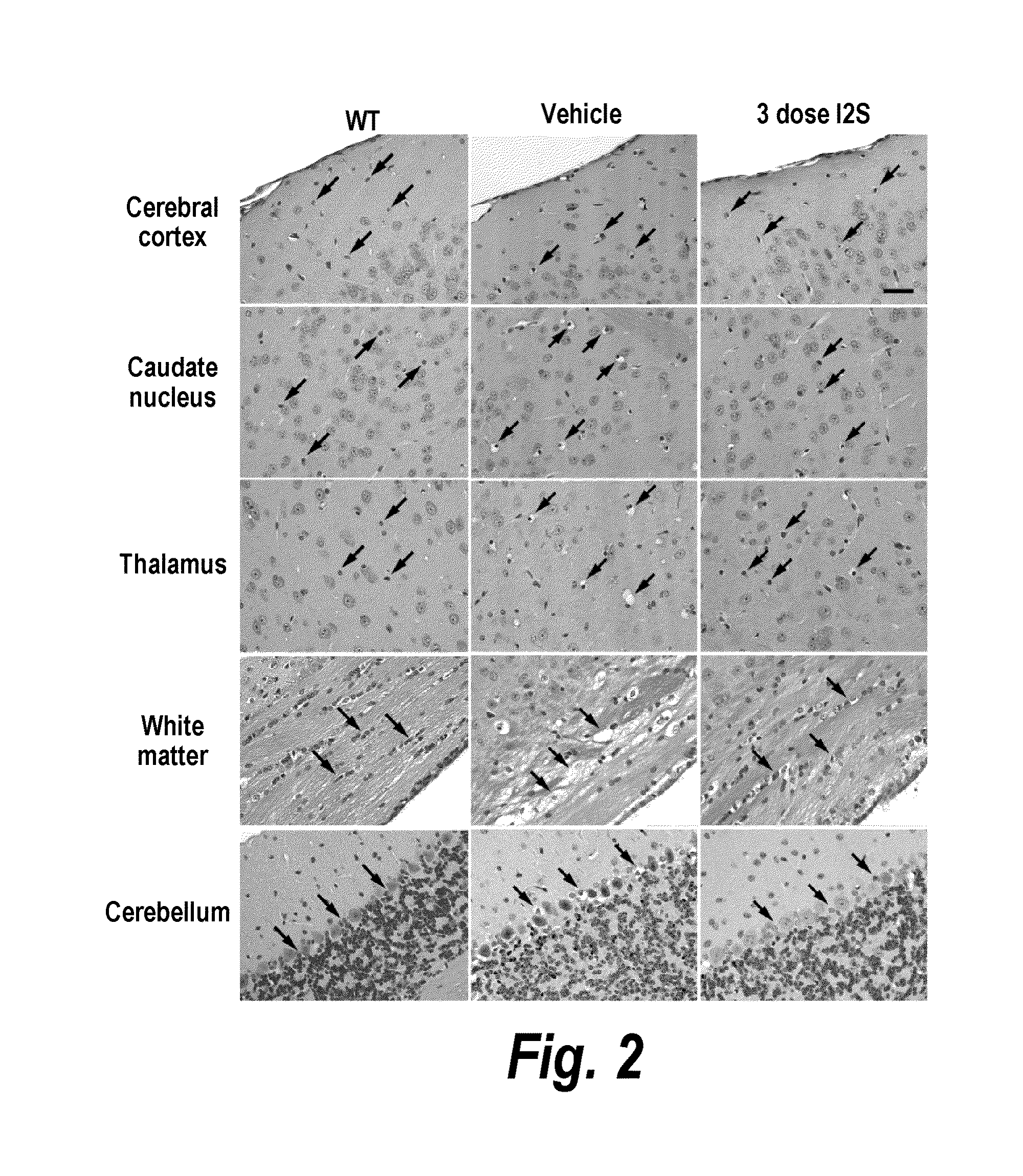
The present invention relates to the intrathecal (IT) administration of recombinant enzyme to treat lysosomal storage disorders. In an exemplary embodiment, intrathecal administration of human α-L-iduronidase (rhIDU) injections in MPS I affected animals resulted in significant enzyme uptake, significant rh-iduronidase activity in brain and meninges and a decrease of glycosaminoglycan (GAG) storage in cells of MPS I subjects to that of normal subjects. Intrathecal administration proved more effective than intravenous treatment at alleviating MPS I symptoms, indicating it is a useful method of treating lysosomal storage disorders.
Owner:BIOMARIN PHARMA INC
Intrathecal treatment of neuropathic pain with a2ar agonists
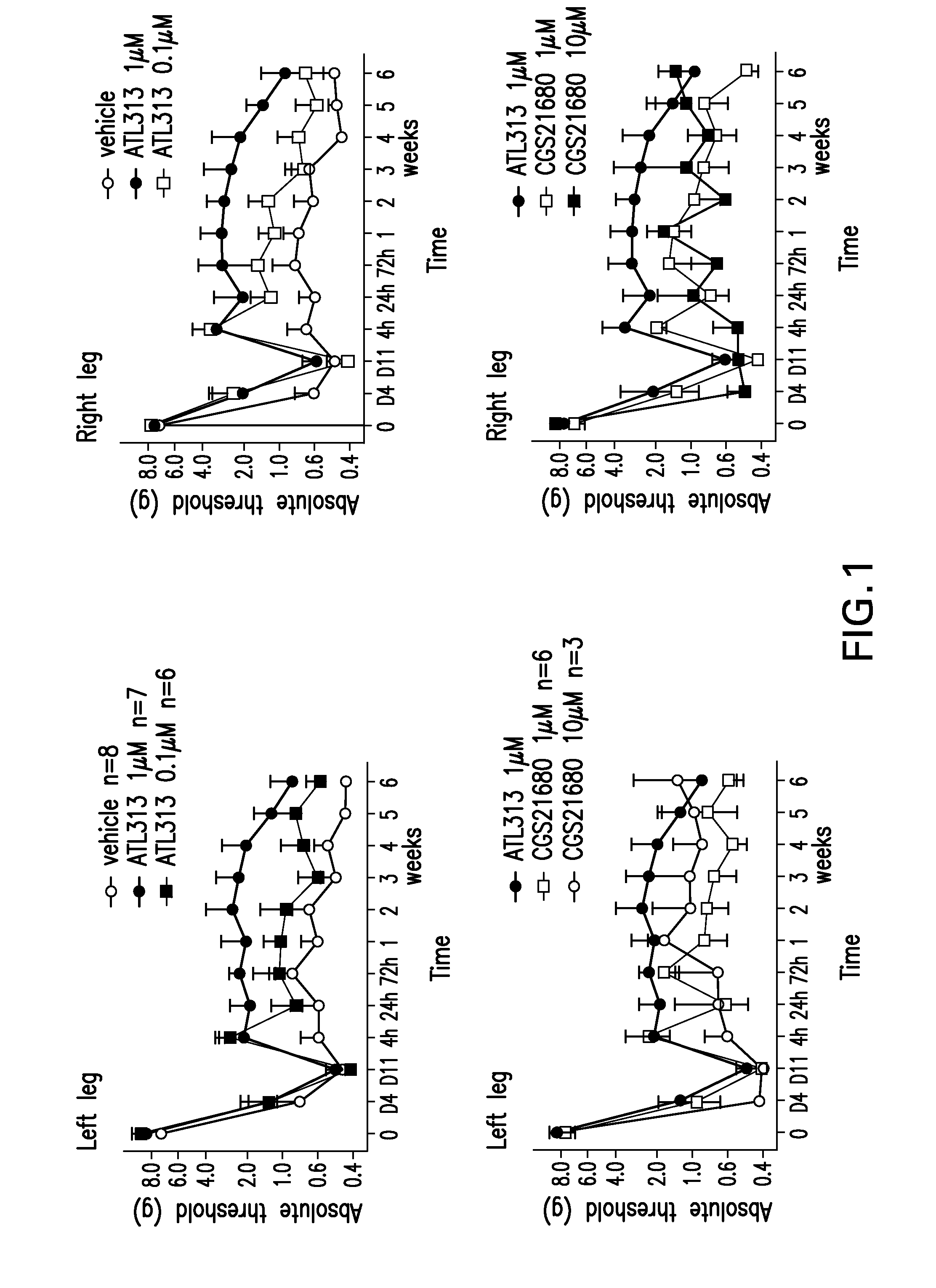
The present invention relates to a method of treating neuropathic pain via intrathecal administration of agonists of A2A adenosine receptors (ARs).
Owner:TROVIS PHARM LLC +1
Delivery of therapeutic compounds to the brain and other tissues
ActiveUS7442372B2Reduces and eliminates glycogen storage granuleReduce eliminateNervous disorderPeptide/protein ingredientsMeningesIntrathecal use
The present invention relates to the intrathecal (IT) administration of recombinant enzyme to treat lysosomal storage disorders. In an exemplary embodiment, intrathecal administration of human α-L-iduronidase (rhIDU) injections in MPS I affected animals resulted in significant enzyme uptake, significant rh-iduronidase activity in brain and meninges and a decrease of glycosaminoglycan (GAG) storage in cells of MPS I subjects to that of normal subjects. Intrathecal administration proved more effective than intravenous treatment at alleviating MPS I symptoms, indicating it is a useful method of treating lysosomal storage disorders.
Owner:BIOMARIN PHARMA INC
Method of delivering a TNF antagonist to the brain of a human by perispinal administration without direct intrathecal injection
InactiveUS7214658B2Improve cognitive functionReduce deliveryAnimal cellsOrganic active ingredientsEtanerceptTnf antagonists
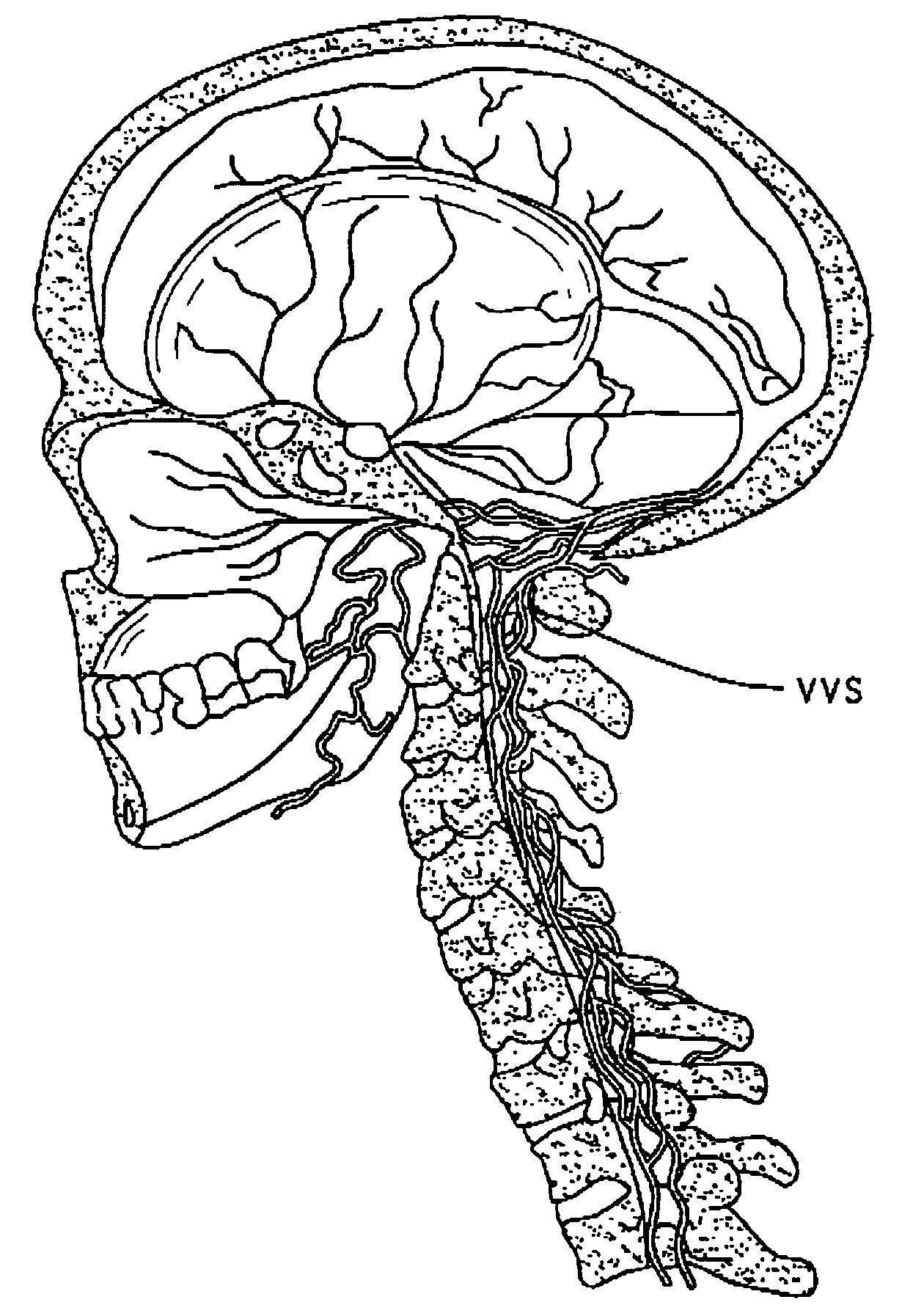
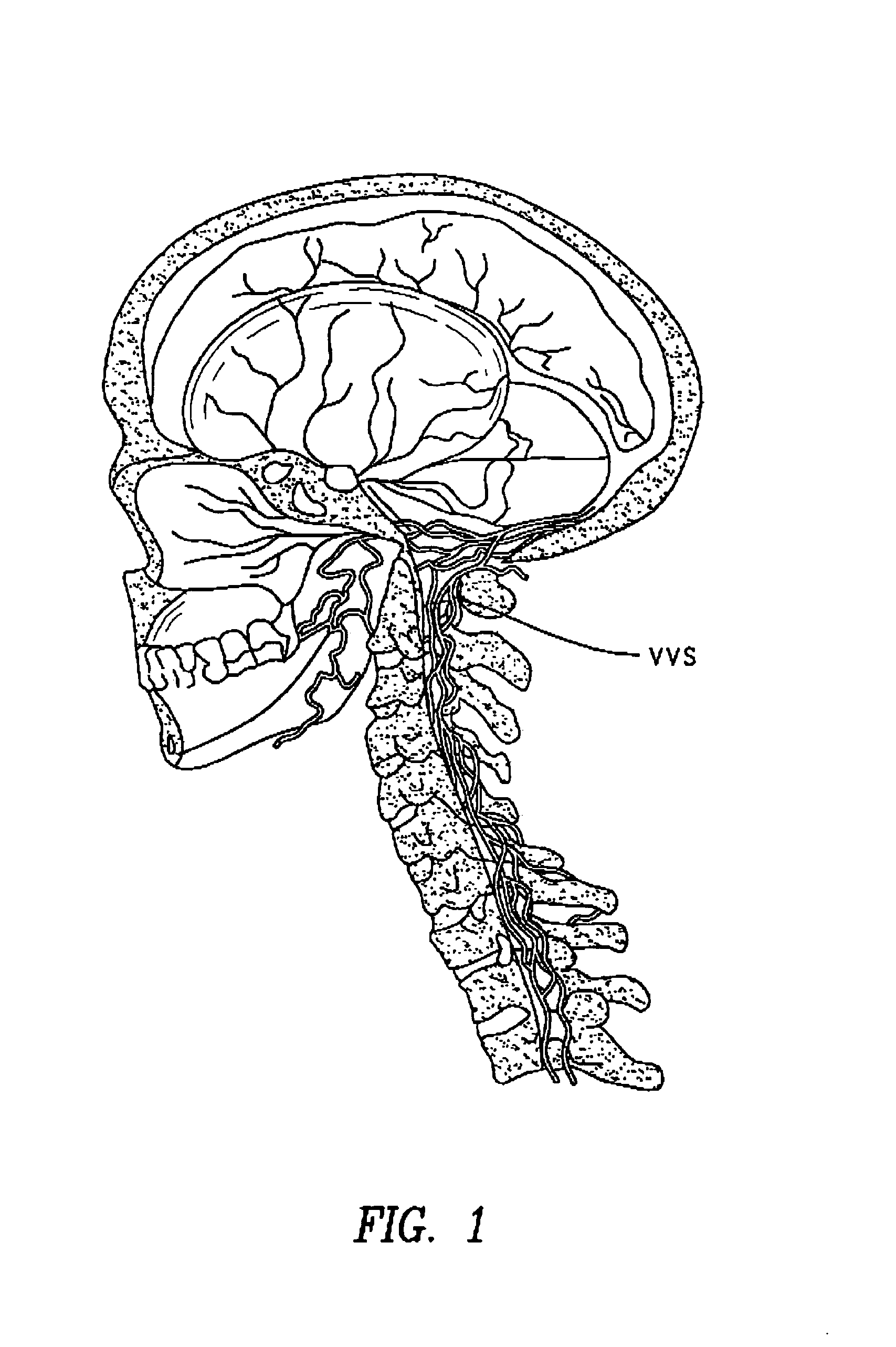
The present invention provides specific methods of using and administering etanercept to improve cognitive function in a human, for both the treatment and prevention of cognitive impairment, or, alternatively, to enhance cognitive function including Alzheimer's Disease, Idiopathic Dementia, and Traumatic Brain Injury. The methods of the present invention include the perispinal administration of etanercept. For the purposes of this patent “perispinal” is to be considered as referring to “perispinal extrathecal;” therefore direct intrathecal administration is excluded. Perispinal administration leads to enhanced delivery of etanercept to the brain in a therapeutically effective amount, via the vertebral venous system and / or the cerebrospinal fluid. Delivery of etanercept to the brain utilizing the methods of the present invention includes the use of the vertebral venous system to deliver etanercept to the brain via retrograde venous flow. Physical maneuvers are used to enhance delivery of etanercept to the brain via this route.
Owner:TACT IP
Intrathecal and intratumoral superantigens to treat malignant disease
InactiveUS20060052295A1Improve effectivenessStrong specificityPeptide/protein ingredientsSnake antigen ingredientsDiseaseAbnormal tissue growth
The presence of tumor nodules in organs often results in serious clinical manifestations and the permeation by cancer cells of sheaths surrounding organs often produces clinical manifestations of pleural effusion, ascites or cerebral edema. The present invention addresses this problem by providing a method for treating tumors comprising (a) intratumoral administration of a superantigen and / or (b) intrathecal or intracavitary administration of a superantigen directly into the sheath. Intratumoral superantigen results in significant and sustained reduction of the tumor size. Intrathecal administration produces significant sustained reduction of the fluid accumulation associated with clinical improvement and prolonged survival. Useful superantigen compositions for intrathecal and intratumoral injection include tumoricidally effective homologues, fragments and fusion proteins of native superantigens. Also disclosed is combined therapy that includes intratumoral or intrathecal superantigen compositions in combination with (i) intratumoral low, non-toxic doses of one or more chemotherapeutic drugs or (ii) systemic chemotherapy at reduced and non-toxic doses of chemotherapeutic drugs.
Owner:JENQUEST
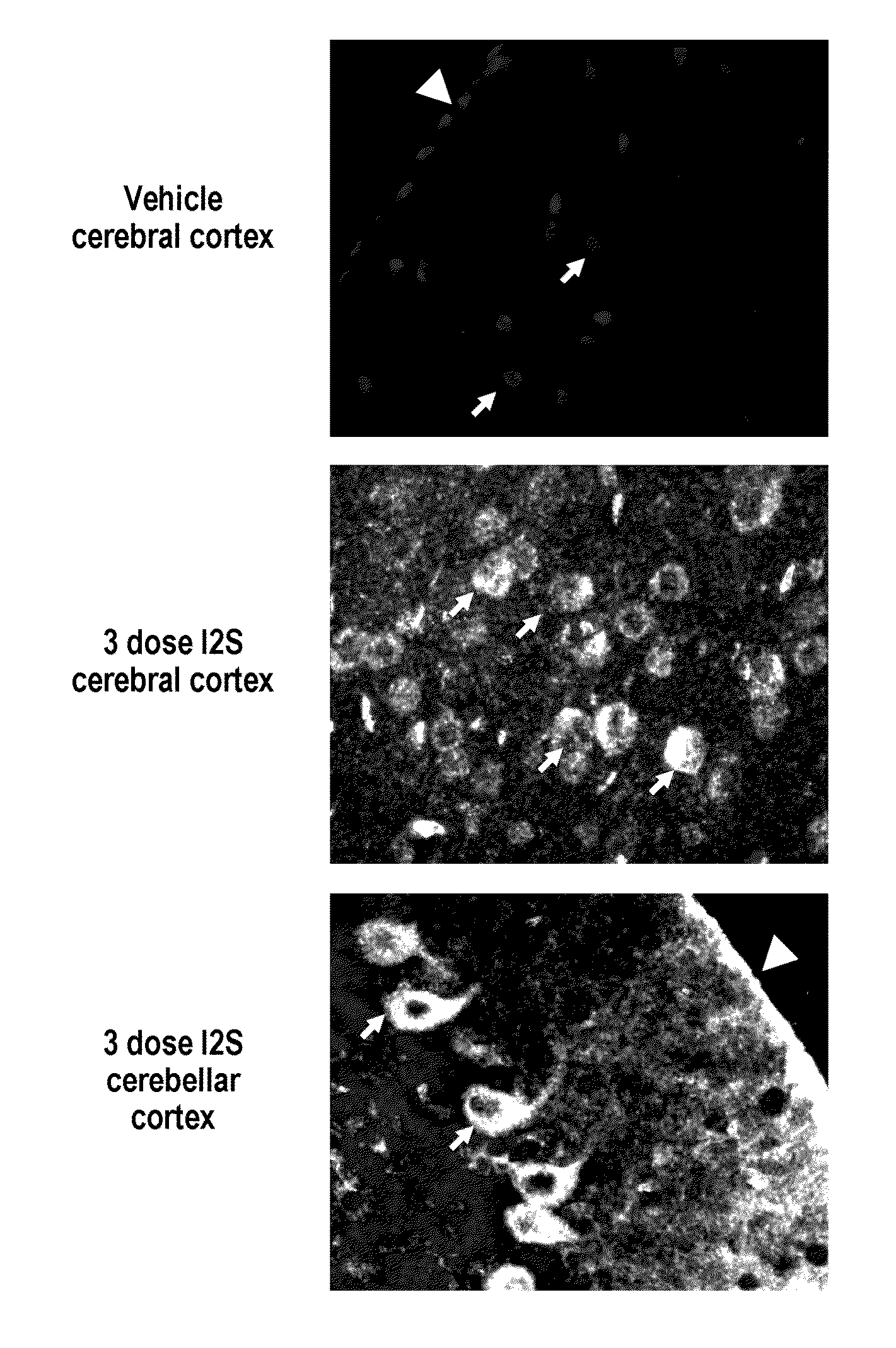
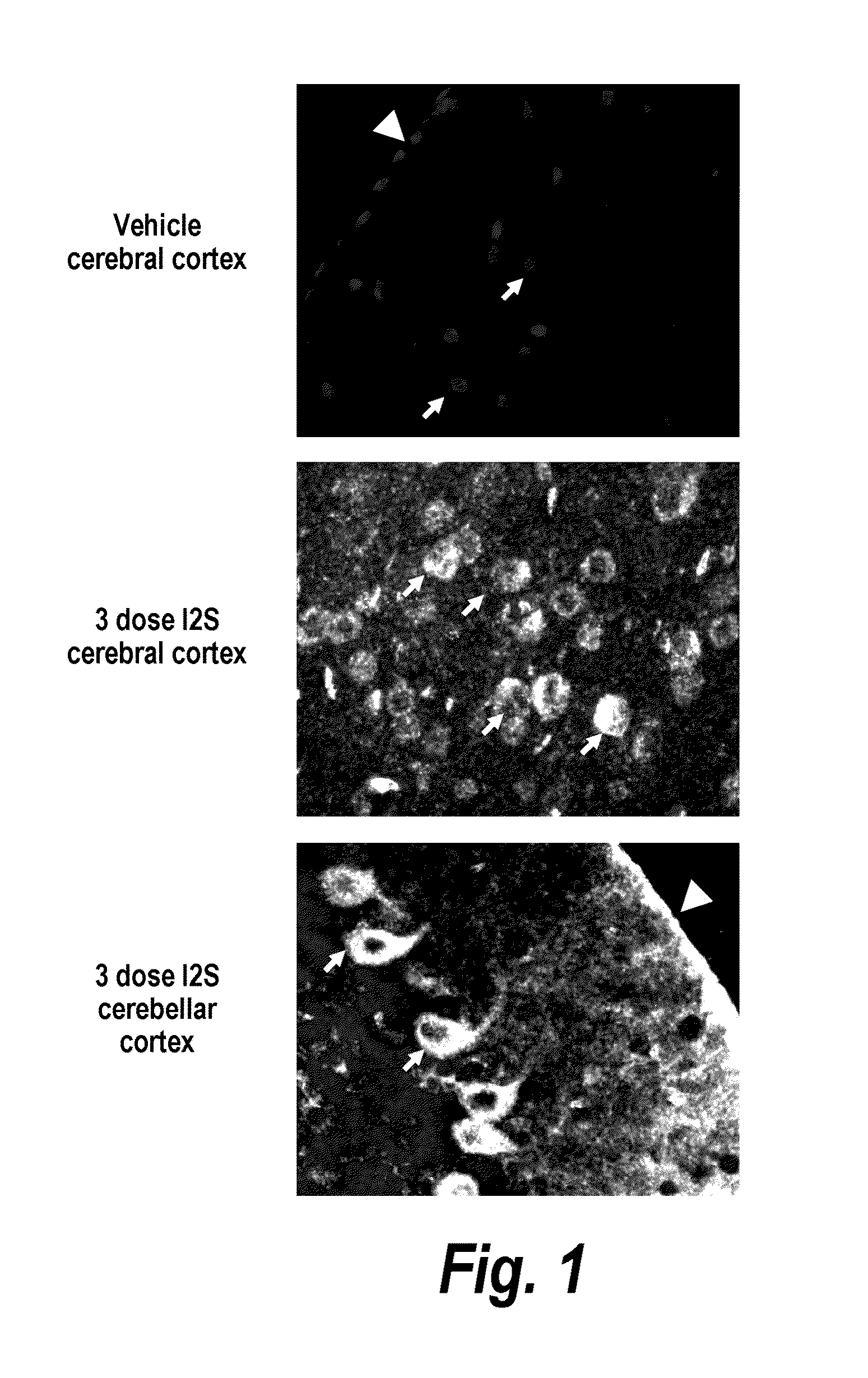
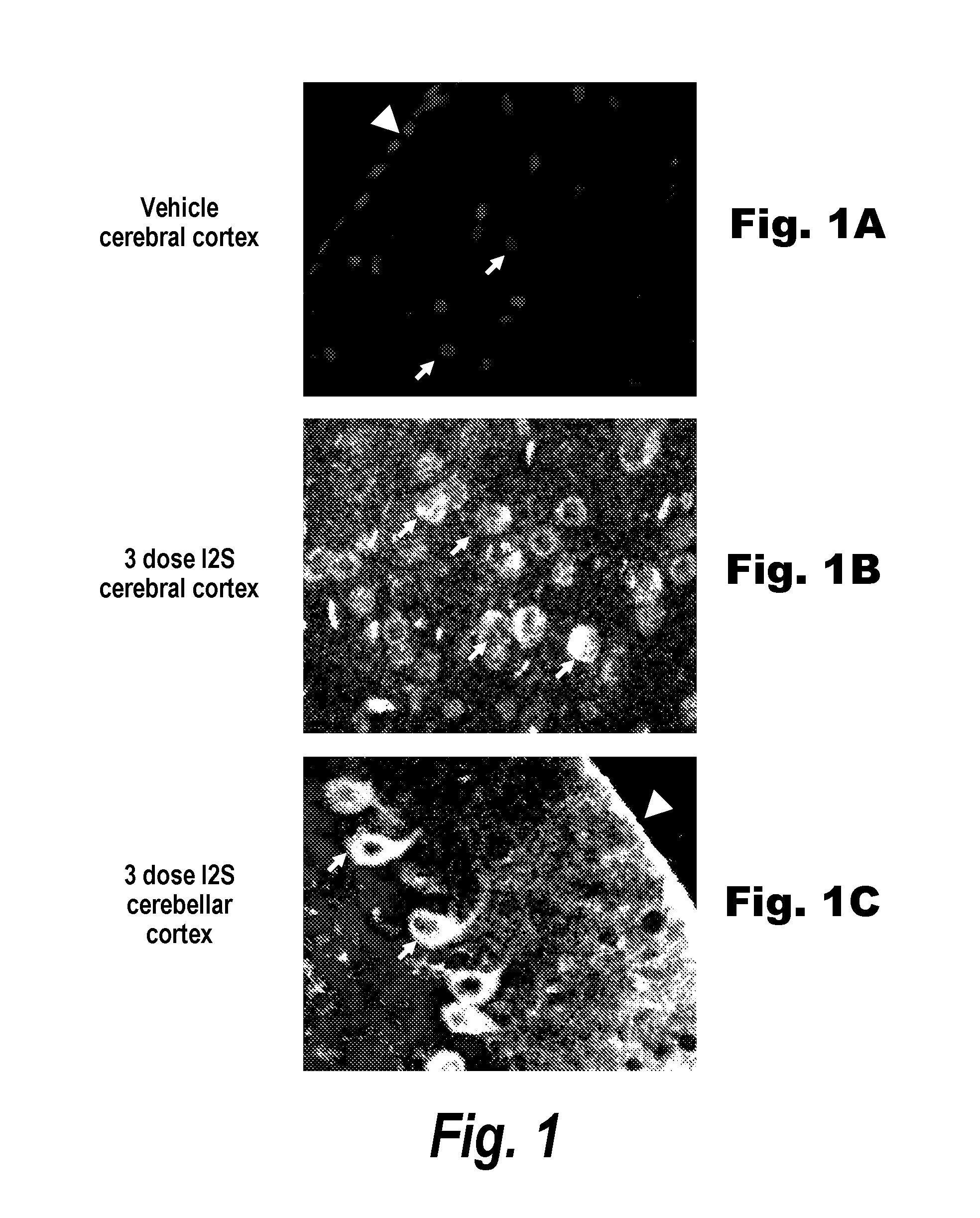
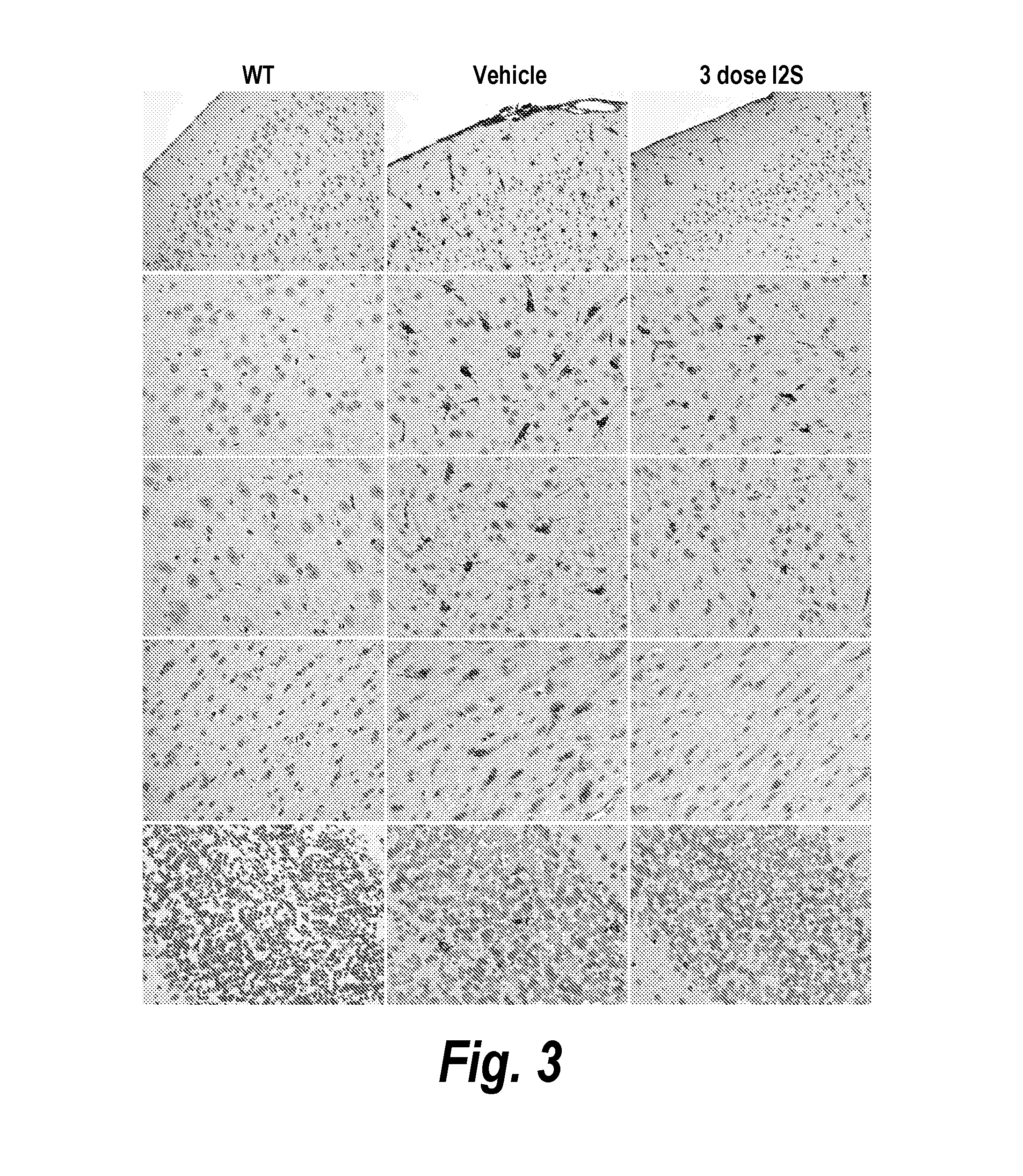
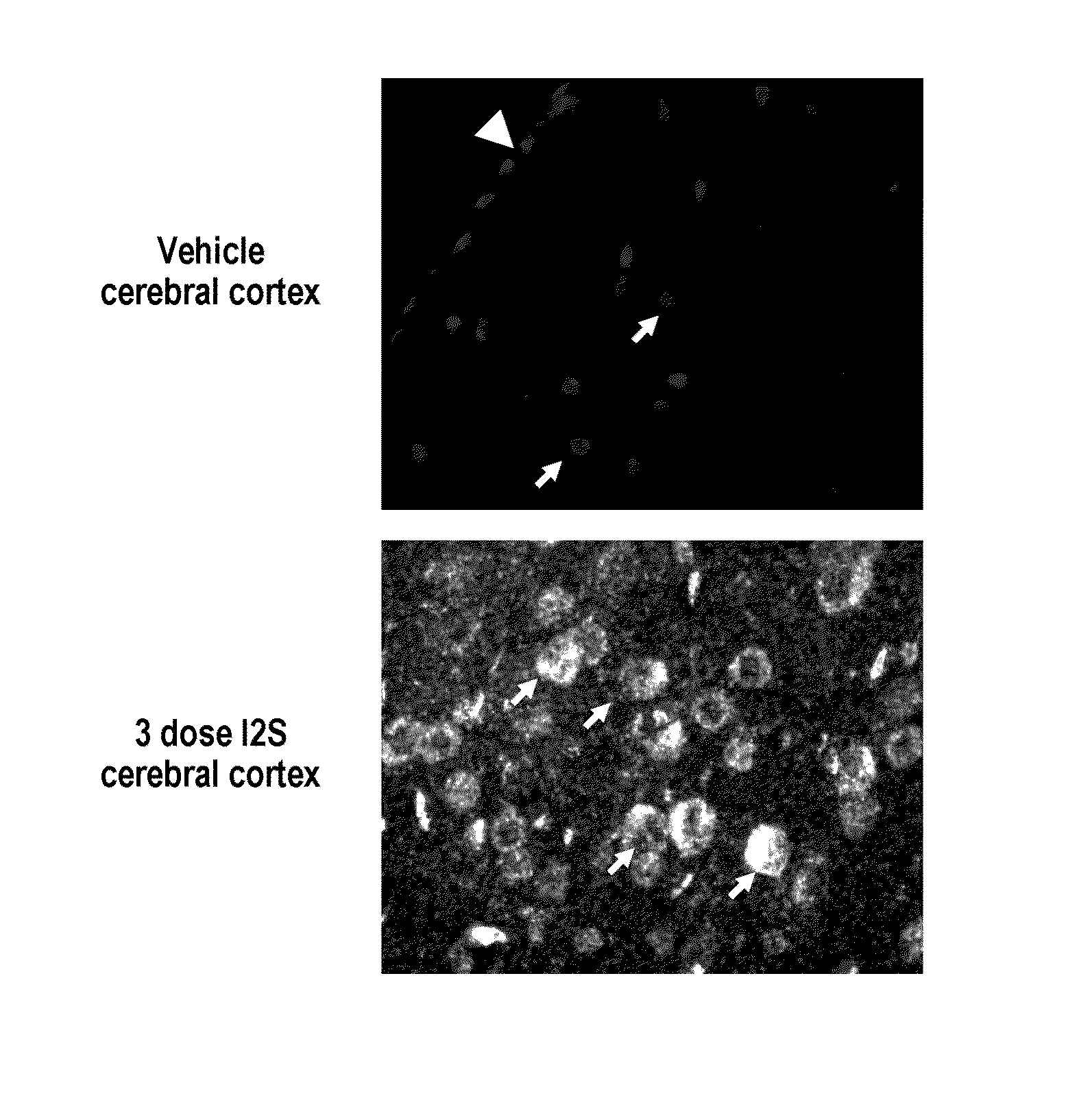
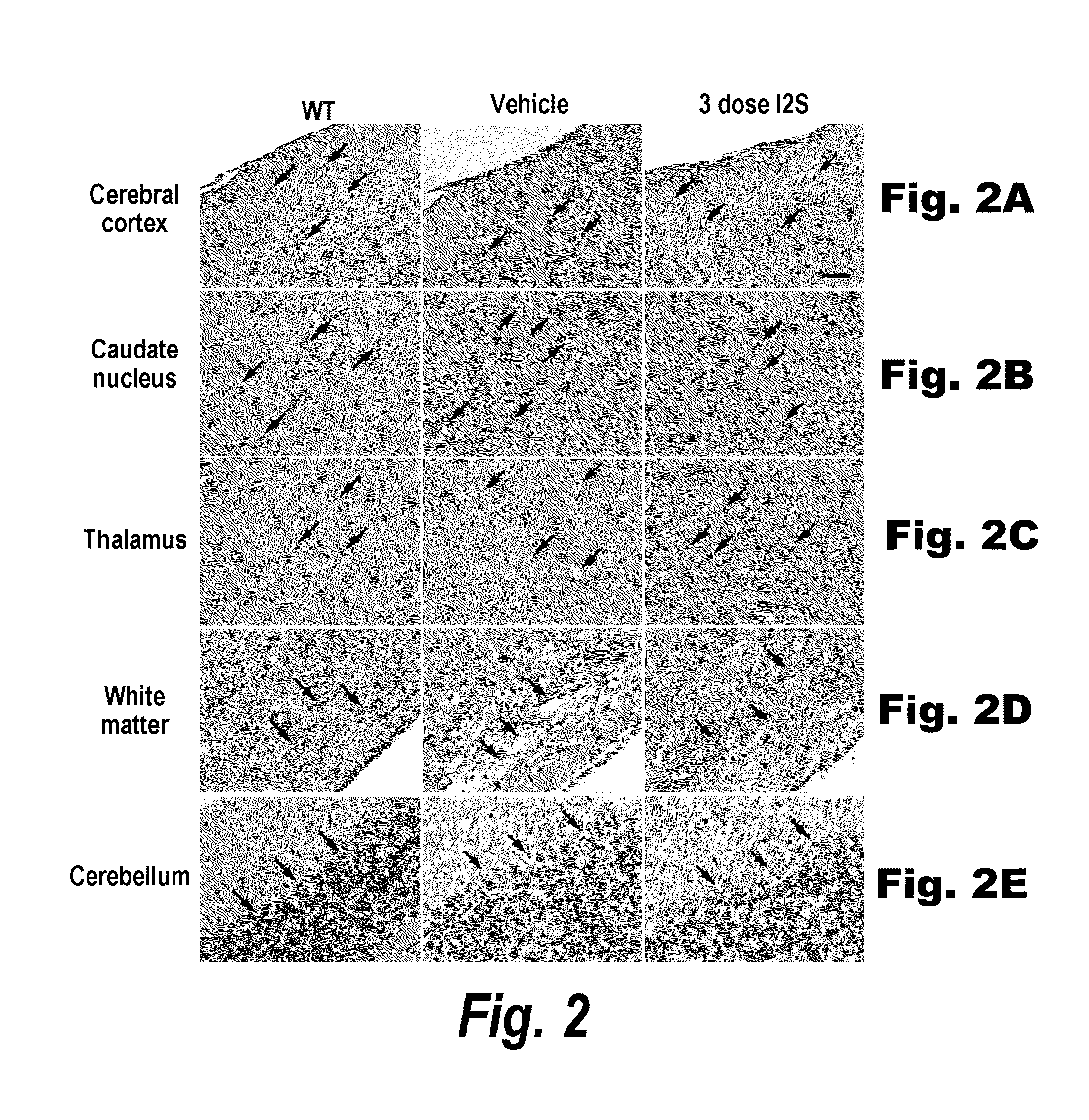
Methods and compositions for CNS delivery of iduronate-2-sulfatase
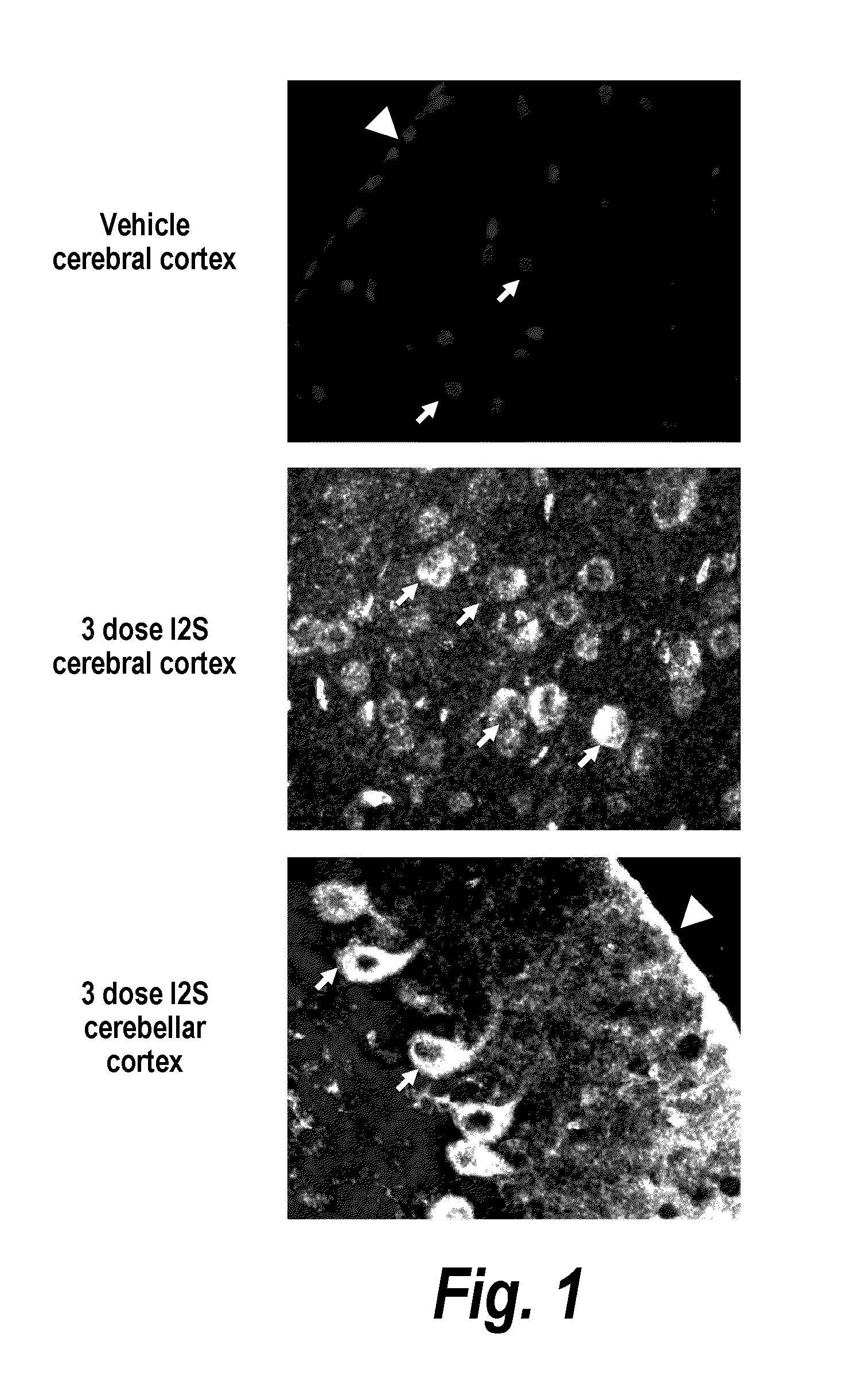
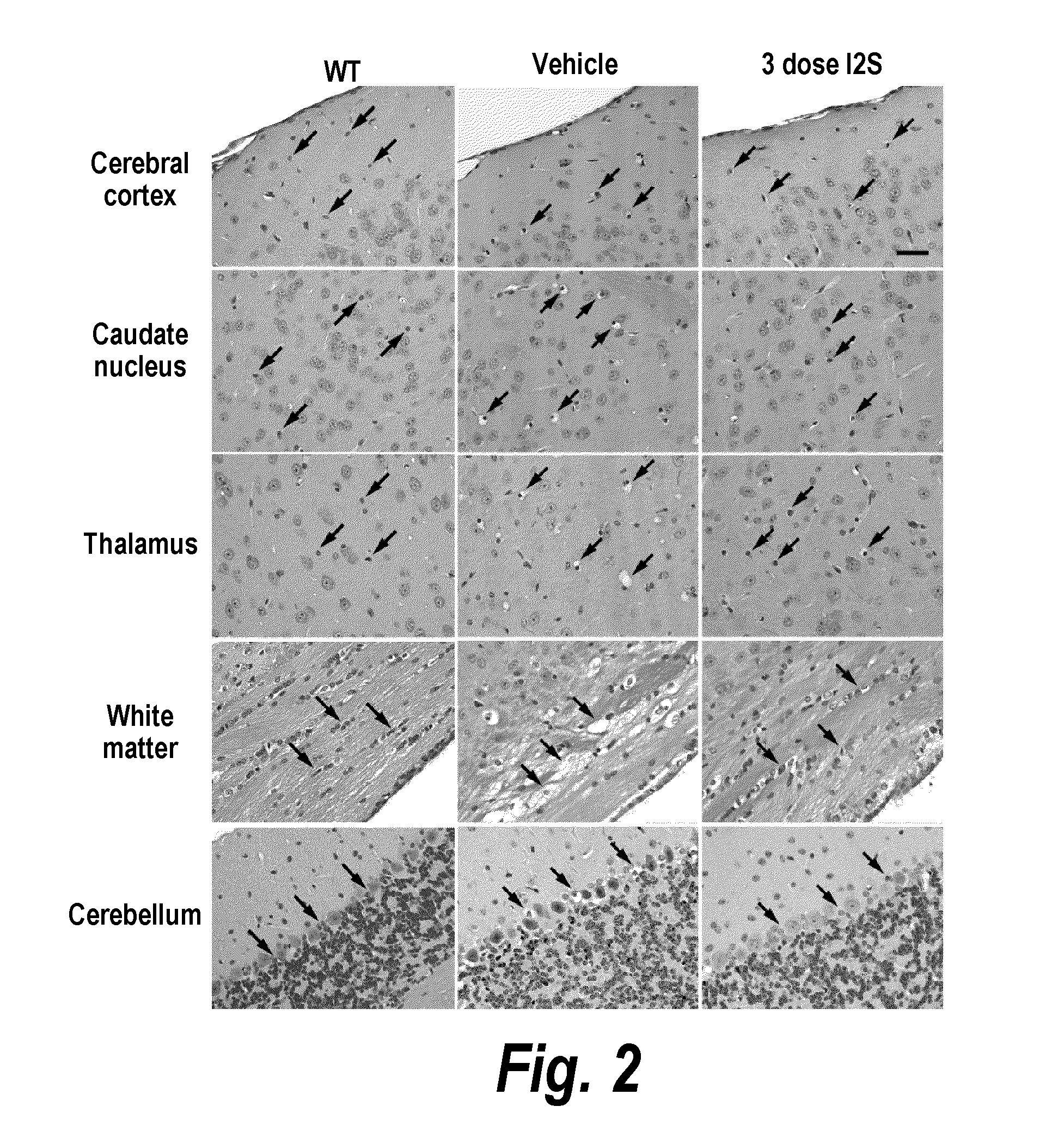
ActiveUS20110318323A1Effective and less approachEffectively and extensivelyNervous disorderHydrolasesIduronate-2-sulfataseHunter syndrome
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising an iduronate-2-sulfatase (I2S) protein, salt, and a polysorbate surfactant for the treatment of Hunters Syndrome.
Owner:TAKEDA PHARMA CO LTD
Compositions comprising biomembrane sealing agent for treatment of pain or inflammation, and methods of use
The invention provides methods and kits for treatment of pain or inflammation. In one embodiment, the kit comprises a biomembrane sealing agent, such as PEG, and a bioactive agent, such as a magnesium compound. The biomembrane sealing agent and / or the bioactive agent an intravenous administration, an intramuscular administration, an intrathecal administration, a subcutaneous administration, an epidural administration, a parenteral administration, an intra-articular administration, a direct application onto or adjacent to a site of the pathological condition, and any combinations thereof. Alternatively, the biomembrane sealing agent and / or the bioactive agent may be delivered from a pump or an implant.
Owner:WARSAW ORTHOPEDIC INC
Pericardial access device
InactiveUS20150313633A1Reduce harmEnhance the imageCannulasSurgical needlesPericardiumIntrathecal use
Apparatus and methods are described, including apparatus comprising a longitudinal guide member comprising a blunt distal end having an outer surface at least part of which is transparent. The guide member is advanced distally toward a heart of a subject. A sheath is shaped and sized to surround the guide member and shaped to define an at least partially distally-facing suction port at a distal end of the sheath. A portion of a pericardium of the heart is drawn through the suction port and into the sheath, when the suction port is distal to the guide member. A puncturing element punctures the portion of the pericardium while the portion of the pericardium is in the sheath, and a puncturing-element-restraining element inhibits passage of a distal tip of the puncturing element out of the distal end of the sheath. Other applications are also described.
Owner:RAINBOW MEDICAL LTD
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS8545837B2Effective and less invasiveEffectively and extensivelyNervous disorderPeptide/protein ingredientsIduronate-2-sulfataseHunter syndrome
Owner:TAKEDA PHARMA CO LTD
Method for reducing pain
ActiveUS20050192218A1Relieve painRetain potencyBiocideNervous disorderIntrathecal usePharmaceutical formulation
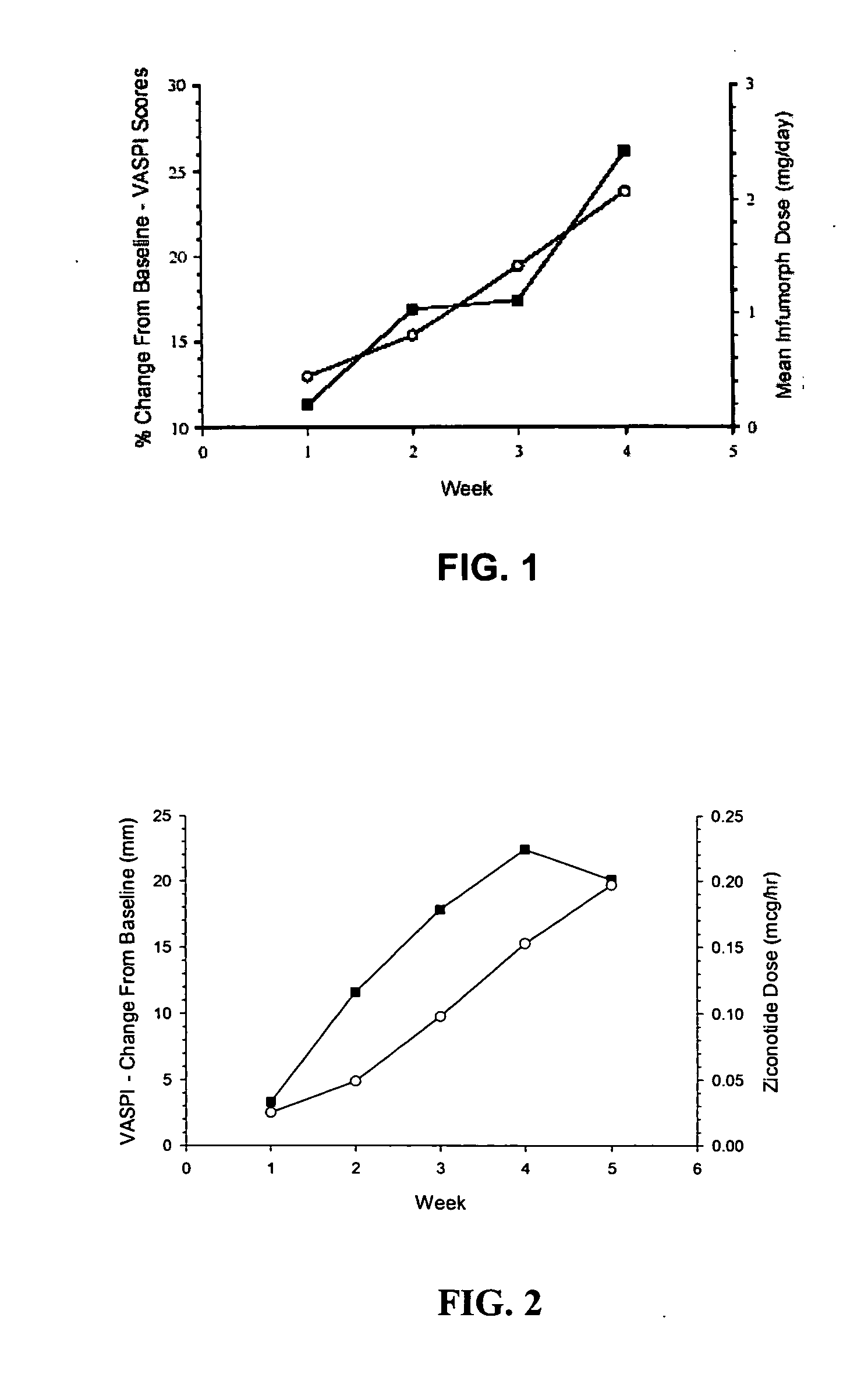
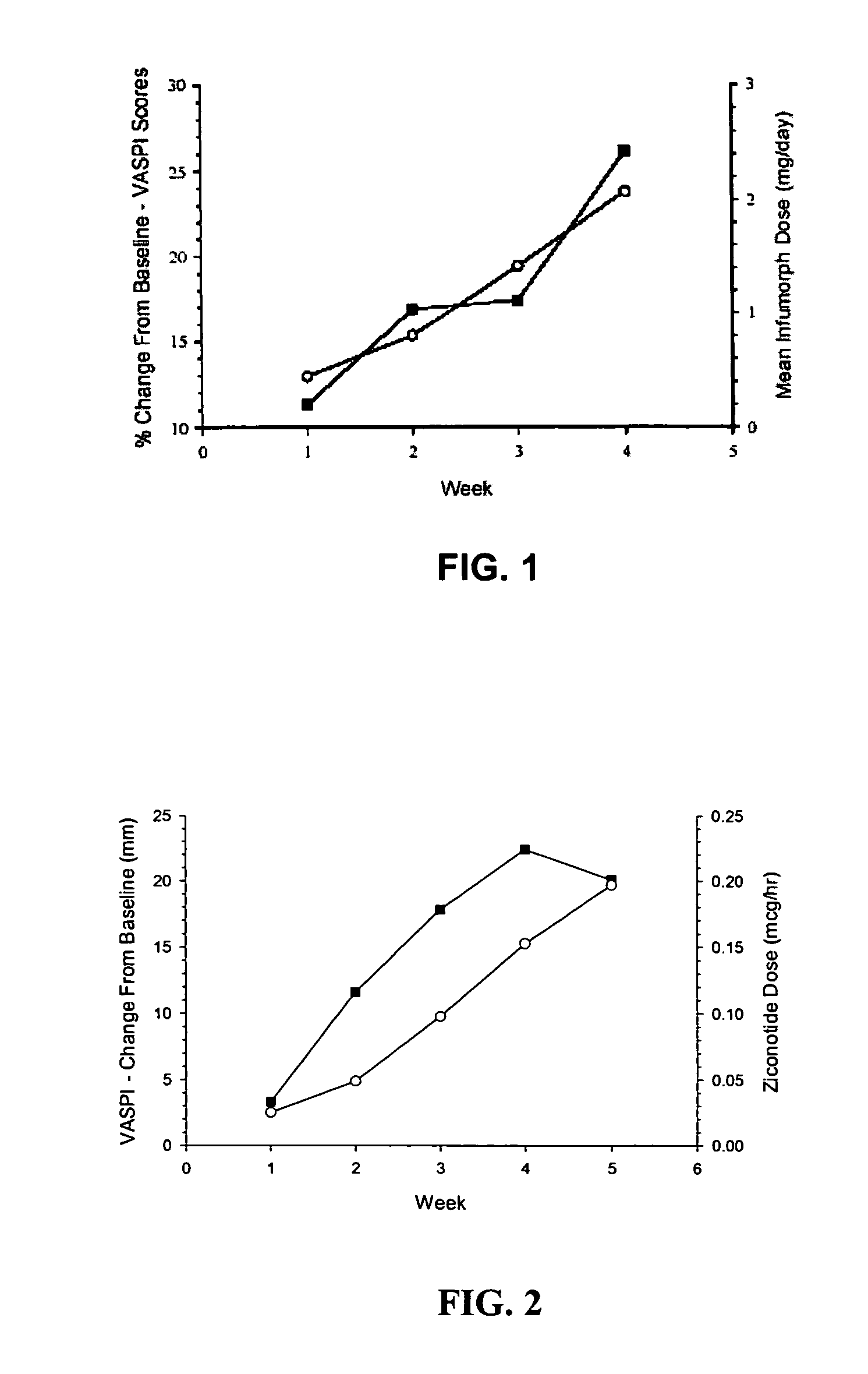
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
Methods and compositions for CNS delivery of heparan n-sulfatase
ActiveUS20120014936A1Effective and less approachReduce deliverySenses disorderNervous disorderSanfilippo syndrome type aLysosomal enzyme defect
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising a heparan N-sulfatase (HNS) protein, salt, and a polysorbate surfactant for the treatment of Sanfilippo Syndrome Type A.
Owner:TAKEDA PHARMA CO LTD
Compositions comprising biomembrane sealing agent for treatment of neuronal injury, and methods of use
The invention provides compositions, kits, and methods for treatment of neuronal injury. In one embodiment, the composition comprises a biomembrane sealing agent, such as PEG, and a bioactive agent, such as a magnesium compound. The biomembrane sealing agent and / or the bioactive agent an intravenous administration, an intramuscular administration, an intrathecal administration, a subcutaneous administration, an epidural administration, a parenteral administration, a direct application onto or adjacent to a site of the pathological condition, and any combinations thereof. Alternatively, the biomembrane sealing agent and / or the bioactive agent may be delivered from a pump or an implant.
Owner:WARSAW ORTHOPEDIC INC
Intrathecal administration of adeno-associated-viral vectors for gene therapy
ActiveUS20180339065A1Readily apparentAntibacterial agentsNervous disorderIntrathecal useViral vector
A composition comprising at least one AAV vector formulated for intrathecal delivery to the central nervous system is described. The composition comprises at least one expression cassette which contains sequences encoding an immunoglobulin construct linked to expression control sequences therefor and a pharmaceutically acceptable carrier. The immunoglobulin construct may be an immunoglobulin modified to have decreased or no measurable affinity for neonatal Fc receptor (FcRn).
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
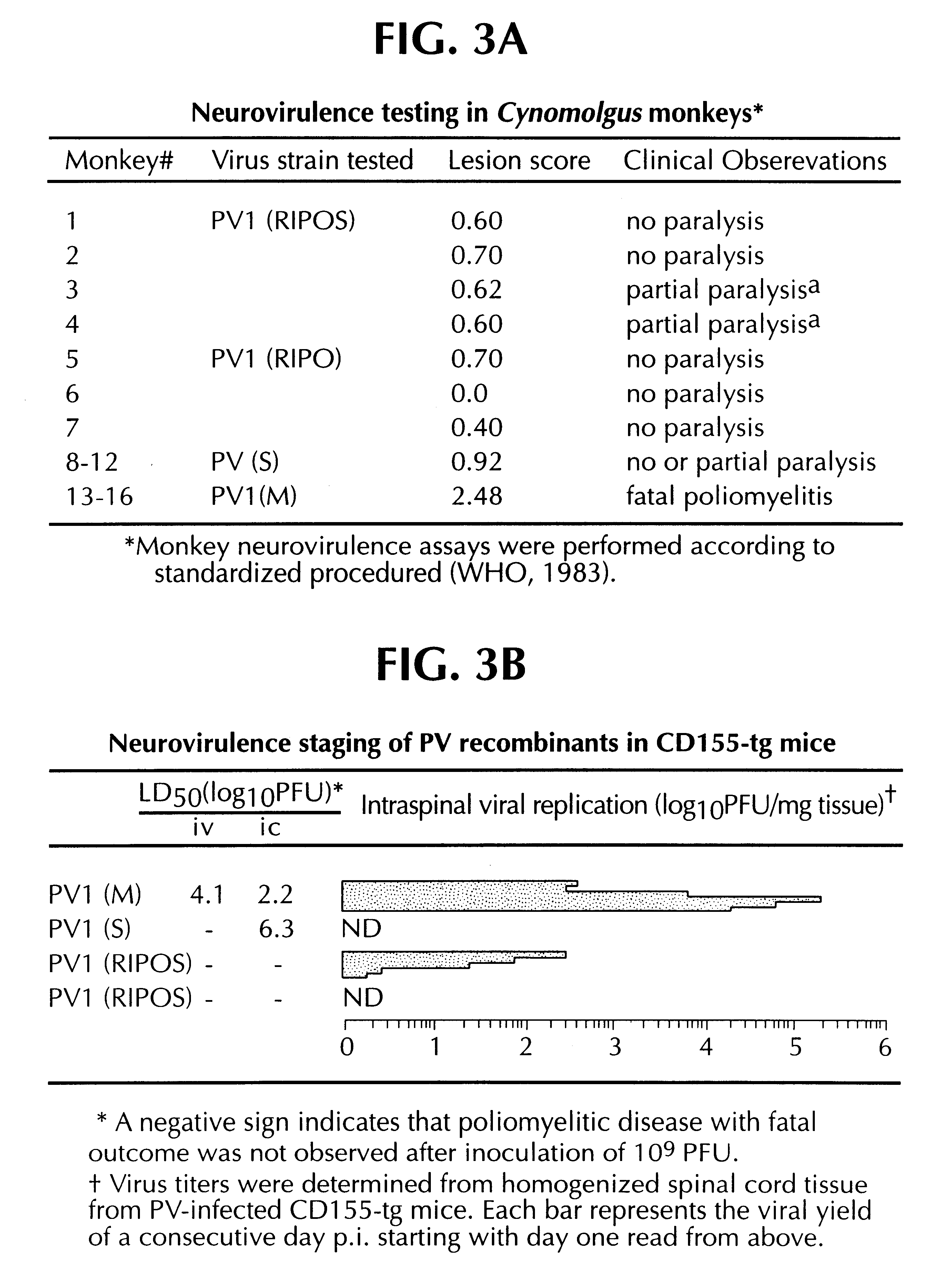
Recombinant poliovirus for the treatment of cancer
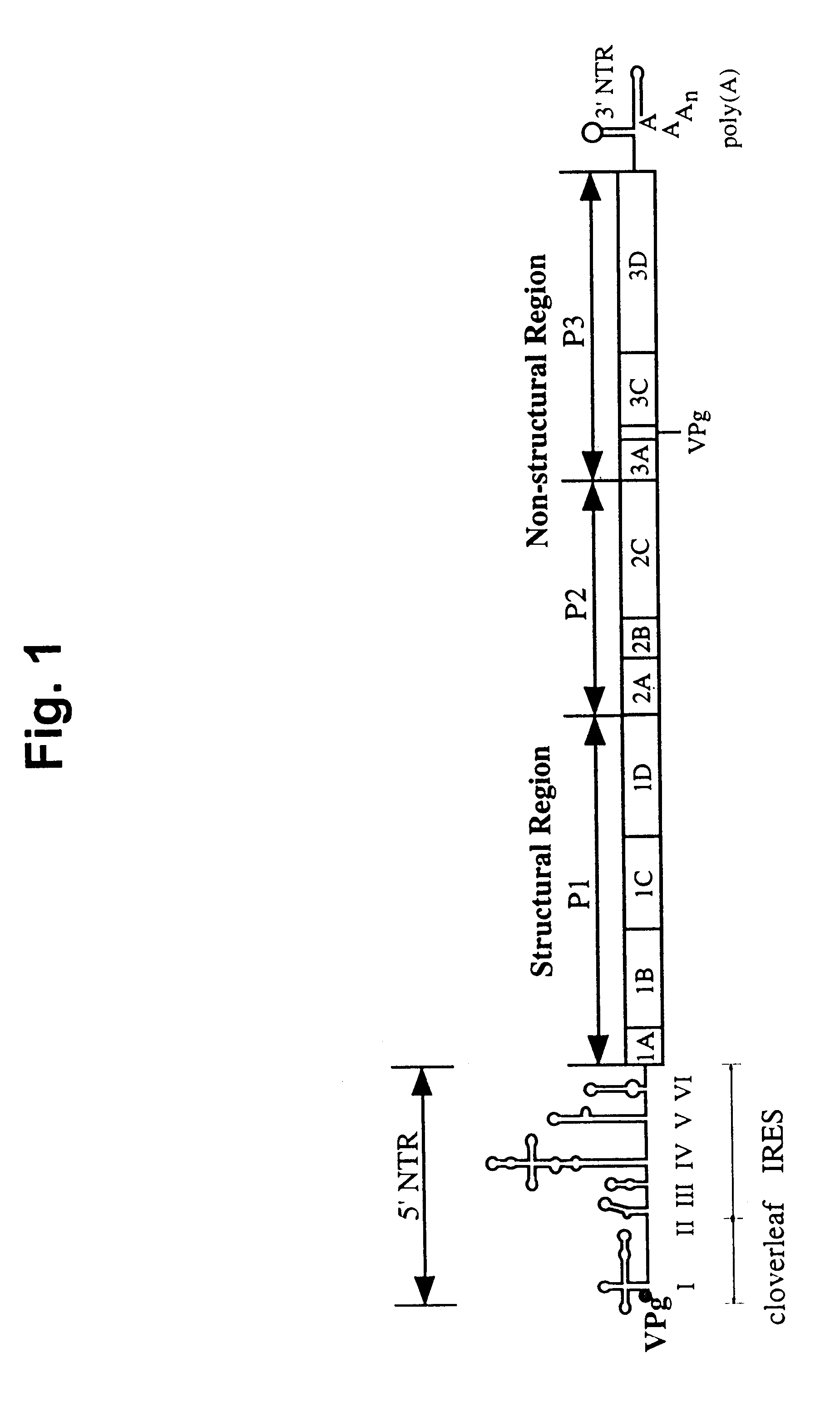
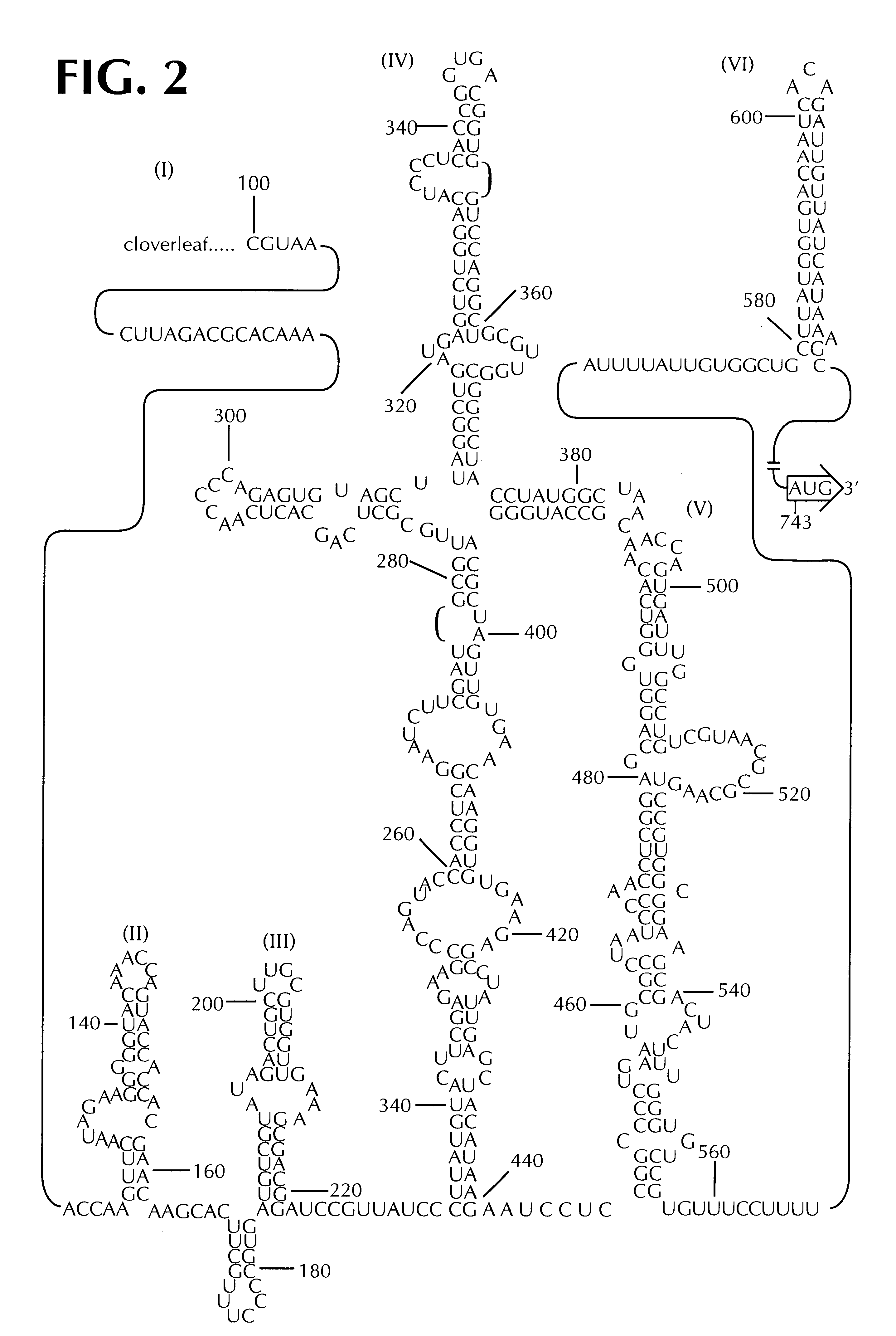
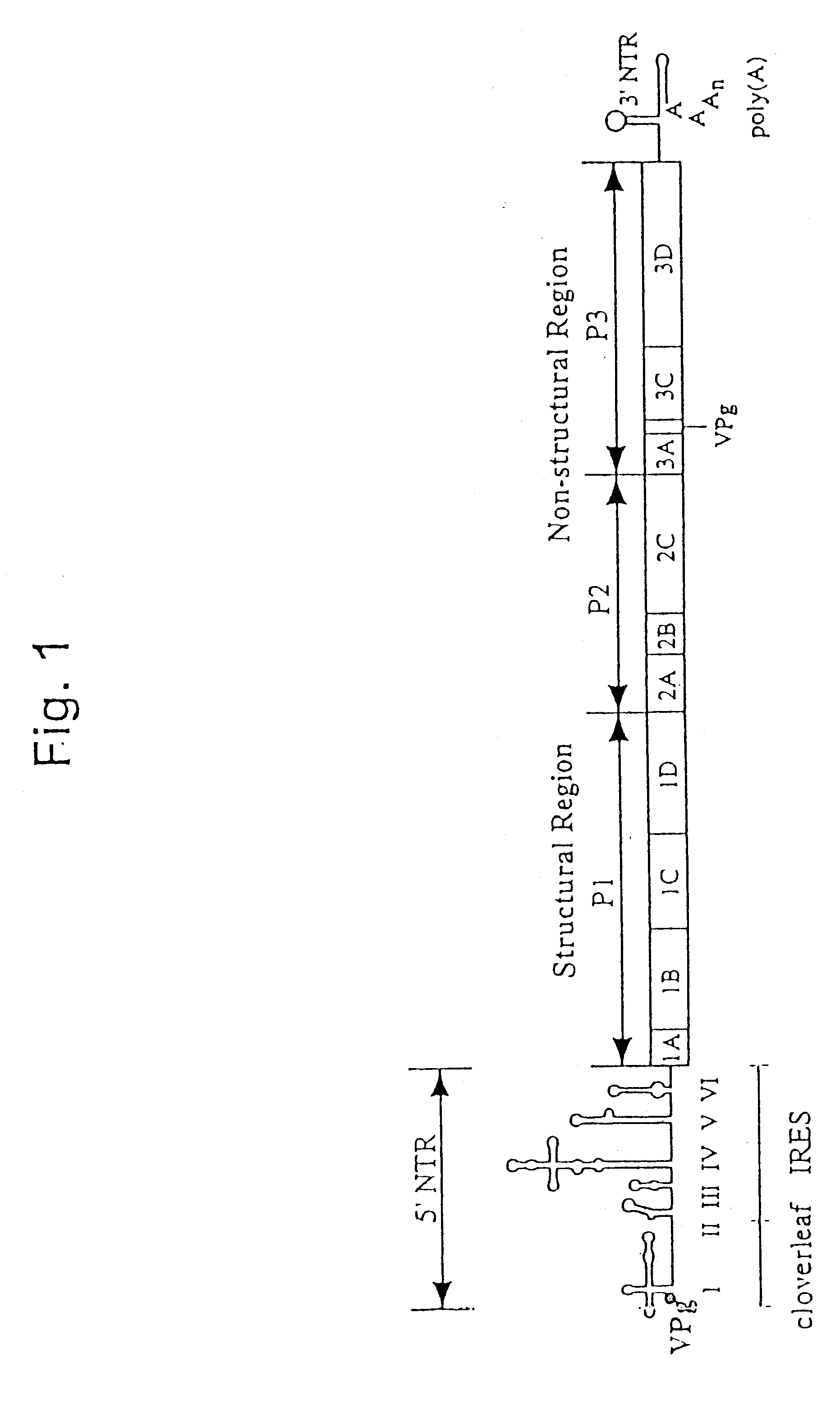
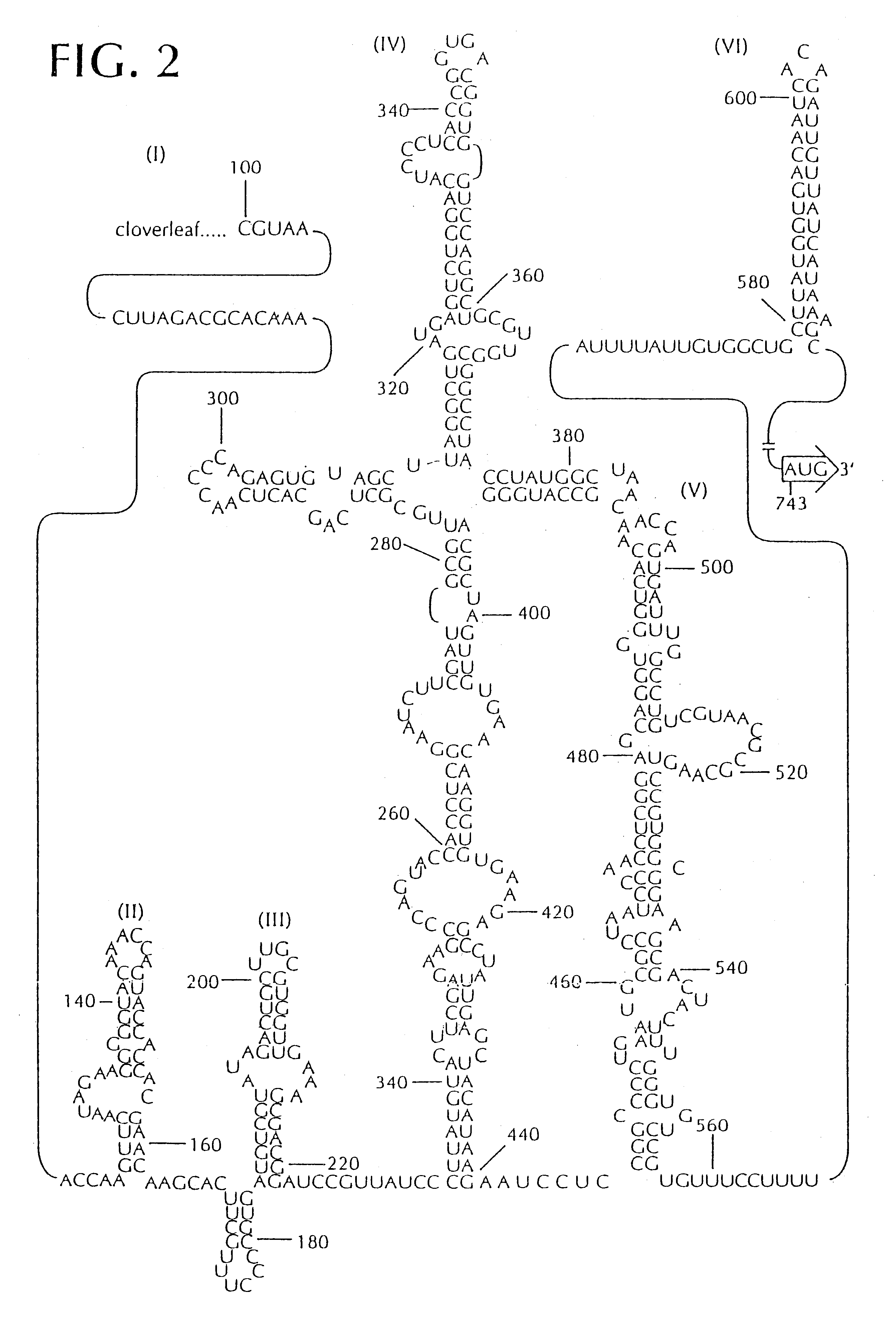
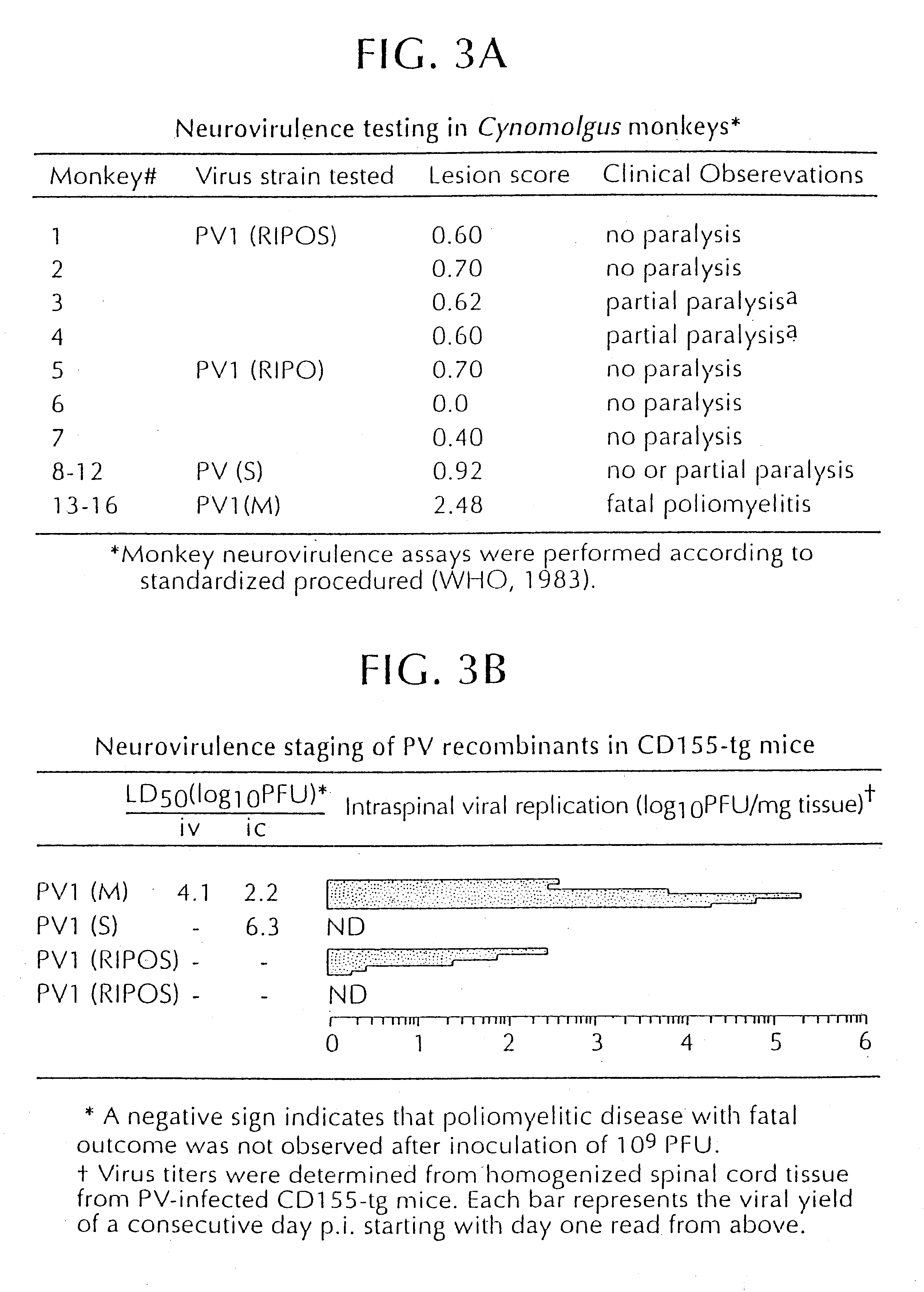
The present invention is directed to non-pathogenic, oncolytic, recombinant polioviruses for the treatment of various forms of malignant tumors. The recombinant polioviruses of the invention are those in which the internal ribosomal entry site (IRES) of the wild type poliovirus was exchanged with the IRES of other picornaviruses, and optionally P1, P3 or the 3'NTR thereof was exchanged with that of poliovirus Sabin type. More particularly, the present invention is directed to the administration of the non-pathogenic, oncolytic, recombinant poliovirus to the tumor directly, intrathecally or intravenously to cause tumor necrosis. The method of the present invention is particularly useful for the treatment of malignant tumors in various organs, such as: breast, colon, bronchial passage, epithelial lining of the gastrointestinal, upper respiratory and genito-urinary tracts, liver, prostate and the brain. Astounding remissions in experimental animals have been demonstrated for the treatment of malignant glioblastoma multiforme, an almost universally fatal neoplasm of the central nervous system.
Owner:NEW YORK UNIV OF RES FOUND OF THE
Method for reducing pain
ActiveUS7268109B2Nervous disorderPeptide/protein ingredientsPharmaceutical formulationAnalgesic agents
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS20140271598A1Effective and less approachEffectively and extensivelySenses disorderNervous disorderIduronate-2-sulfataseHunter syndrome
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising an iduronate-2-sulfatase (I2S) protein, salt, and a polysorbate surfactant for the treatment of Hunters Syndrome.
Owner:TAKEDA PHARMA CO LTD
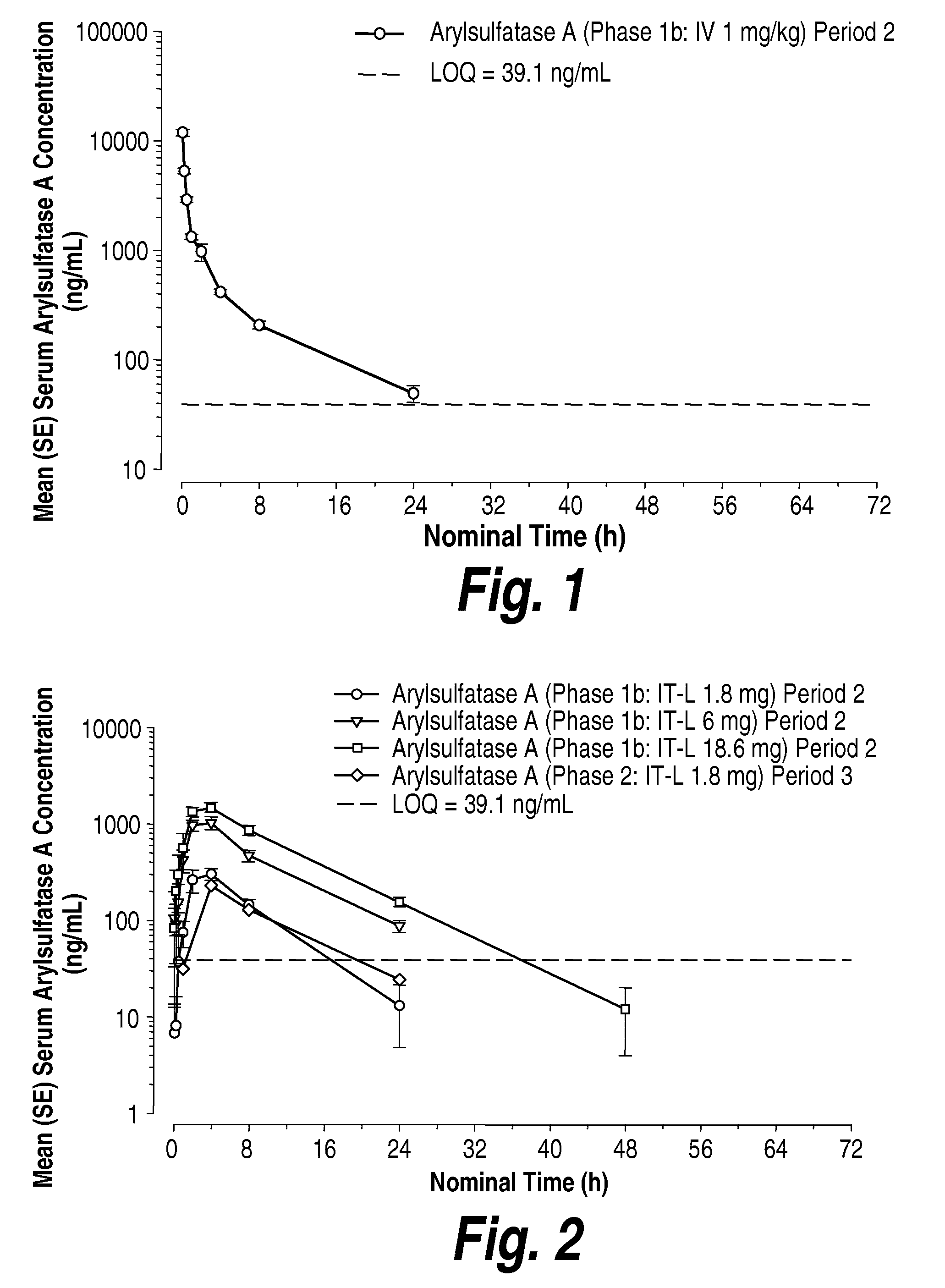
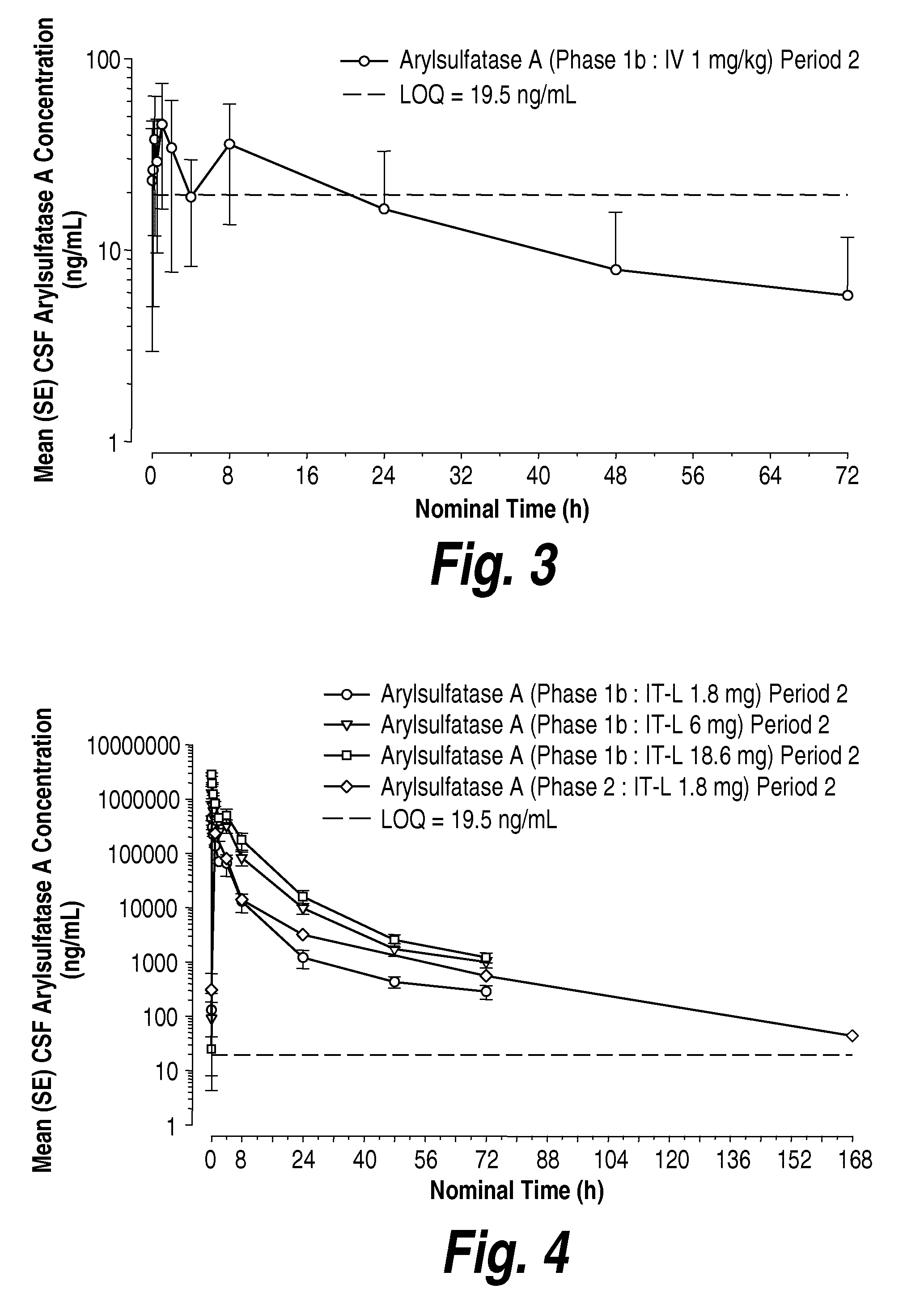
Methods and compositions for CNS delivery of arylsulfatase a
ActiveUS20120009171A1Effective and less approachReduce deliveryNervous disorderHydrolasesMetachromatic leucodystrophyLysosomal enzyme defect
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising an arylsulfatase A (ASA) protein, salt, and a polysorbate surfactant for the treatment of Metachromatic Leukodystrophy Disease.
Owner:TAKEDA PHARMA CO LTD
Methods and compositions for CNS delivery of heparan N-sulfatase
ActiveUS9320711B2Effective and less invasiveReduce deliveryPowder deliverySenses disorderSanfilippo syndrome type aLysosomal enzyme defect
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising a heparan N-sulfatase (HNS) protein, salt, and a polysorbate surfactant for the treatment of Sanfilippo Syndrome Type A.
Owner:TAKEDA PHARMA CO LTD
Recombinant poliovirus for the treatment of cancer
The present invention is directed to non-pathogenic, oncolytic, recombinant polioviruses for the treatment of various forms of malignant tumors. The recombinant polioviruses of the invention are those in which the internal ribosomal entry site (IRES) of the wild type poliovirus was exchanged with the IRES of other picornaviruses, and optionally P1, P3 or the 3'NTR thereof was exchanged with that of poliovirus Sabin type. More particularly, the present invention is directed to the administration of the non-pathogenic, oncolytic, recombinant poliovirus to the tumor directly, intrathecally or intravenously to cause tumor necrosis. The method of the present invention is particularly useful for the treatment of malignant tumors in various organs, such as: breast, colon, bronchial passage, epithelial lining of the gastrointestinal, upper respiratory and genito-urinary tracts, liver, prostate and the brain. Astounding remissions in experimental animals have been demonstrated for the treatment of malignant glioblastoma multiforme, an almost universally fatal neoplasm of the central nervous system.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
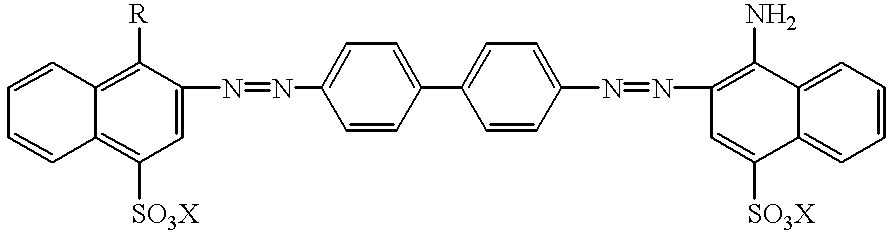
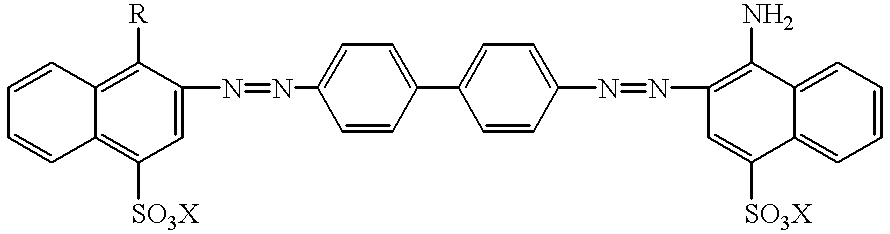
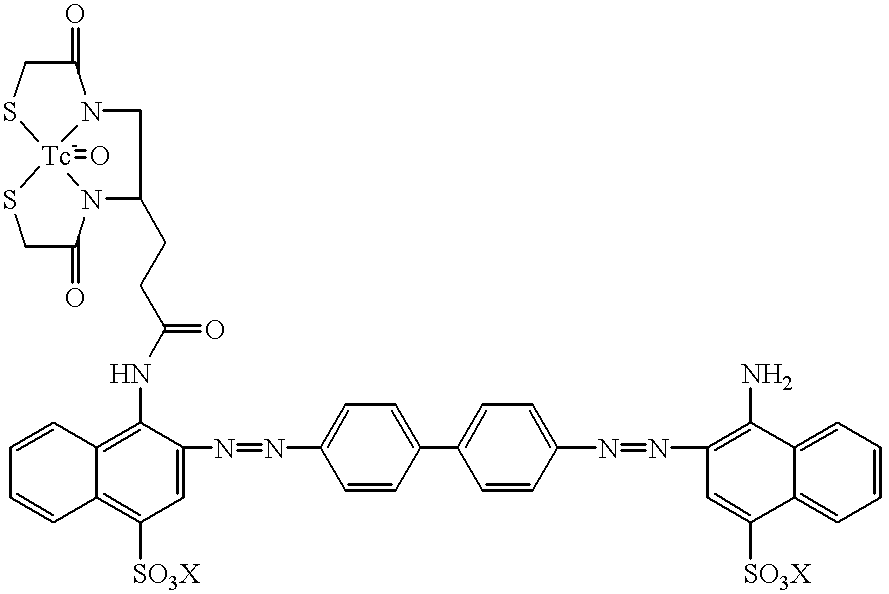
Technetium 99m-N2S2-congo red complexes utilizing diamide dithiolate ligand systems for radioimaging
The present invention discloses a group of technetium 99m-N2S2-congo red complexes that utilize diamide dithiolate ligand systems to synthesize compounds for radioimaging. The complexes have the general formula:wherein R represents a diamide dithiolate ligand system and X represents a cation. Specifically, disclosed are two moieties, wherein the first moeity utilizes a 99mTc-4,5-bis(thioacetamido)pentanoyl(N2S2) ligand system conjugated to the congo red molecule. In a preferred embodiment, the second moeity utilizes a 4-[2,5,5-Trimethyl-4-aza-2-mercaptohexyl]-6,6-dimethyl-2-thiomorpholinone ligand system conjugated to the congo red molecule. Both moeities can be used for radioimaging amyloid in animals and man by either intravenous or intrathecal administration.
Owner:ASHTON WESLEY SCOTT
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS9220677B2Effective and less invasiveEffectively and extensivelyPowder deliverySenses disorderIduronate-2-sulfataseHunter syndrome
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising an iduronate-2-sulfatase (I2S) protein, salt, and a polysorbate surfactant for the treatment of Hunters Syndrome.
Owner:TAKEDA PHARMA CO LTD
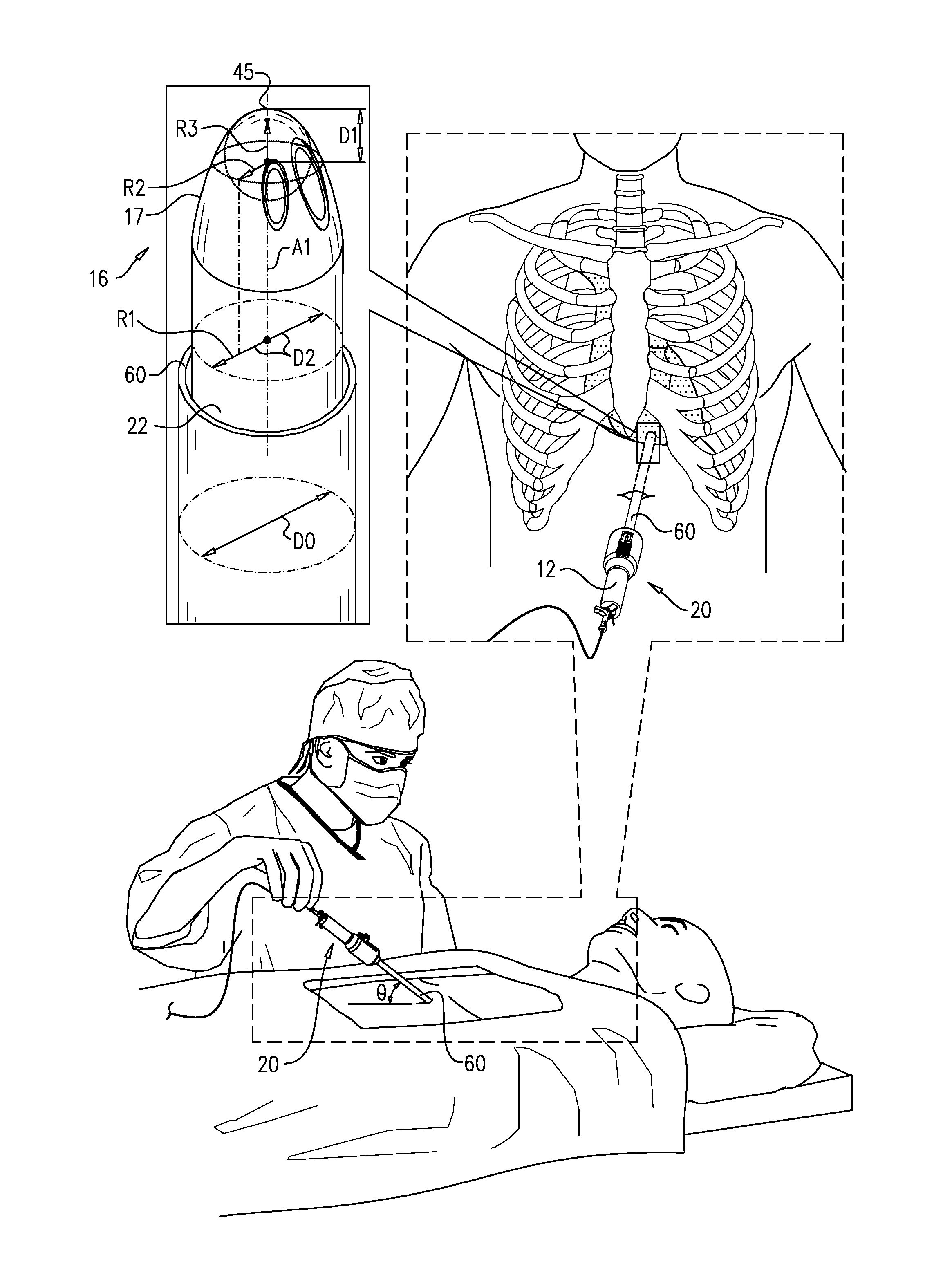
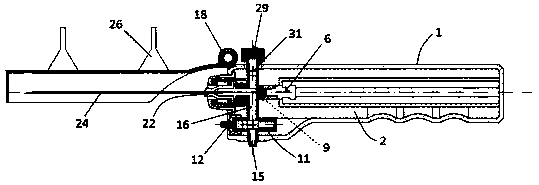
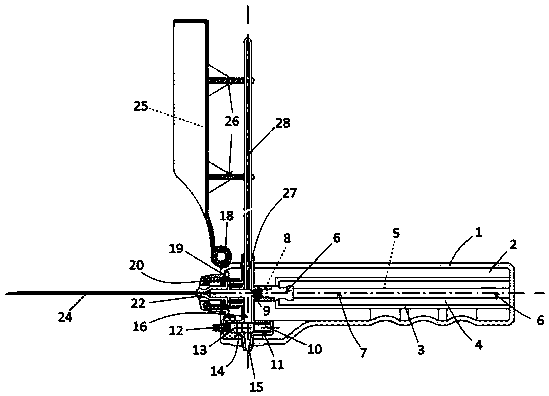
Integrated safe lumbar puncture device
ActiveCN108338825AGuarantee the safety of lifeQuick pull outSurgical needlesVaccination/ovulation diagnosticsCerebrospinal fluid pressureCerebral hernia
The invention discloses an integrated safe lumbar puncture device used clinically. The integrated safe lumbar puncture device is mainly applied to the conditions that cerebrospinal fluid puncture needs to be conducted in the clinical diagnosis and treatment process, for example, the cerebrospinal fluid pressure test is conducted during lumbar puncture as well as a cerebrospinal fluid sample is remained to perform laboratory examination. The integrated safe lumbar puncture device is characterized in that the traditional lumbar puncture needle can be replaced; the cerebrospinal fluid pressure test can be realized in the same device; the cerebrospinal fluid sample is remained according to the requirement and intrathecal injection is conducted; cerebral hernia caused by high cranial pressure excessive drainage in the lumbar puncture process can be avoided; the operation processes of sample retention, pressure test and intrathecal injection can be completed in the same device; the operationflow is simplified; puncture complications are avoided; and the safety of patients and operators is guaranteed.
Owner:迈德微创(天津)医疗器械有限责任公司
Intrathecal administration of mesenchymal stem cells and derivatives thereof for treatment of pain
InactiveUS20180036348A1Pharmaceutical delivery mechanismUnknown materialsWharton's jellyIntrathecal use
The invention discloses means of treating pain through administration of mesenchymal stem cells or derivatives thereof via an intrathecal manner at a concentration and frequency sufficient to reduce pain. In one embodiment of the invention, treatment of discogenic pain is provided by administration of mesenchymal stem cells intrathecally that are derived from the Wharton's Jelly. In another embodiment the invention teaches administration of supernatants from mesenchymal stem cells, in one embodiment said supernatants comprising of concentrated microvesicles.
Owner:AIDAN RES & CONSULTING
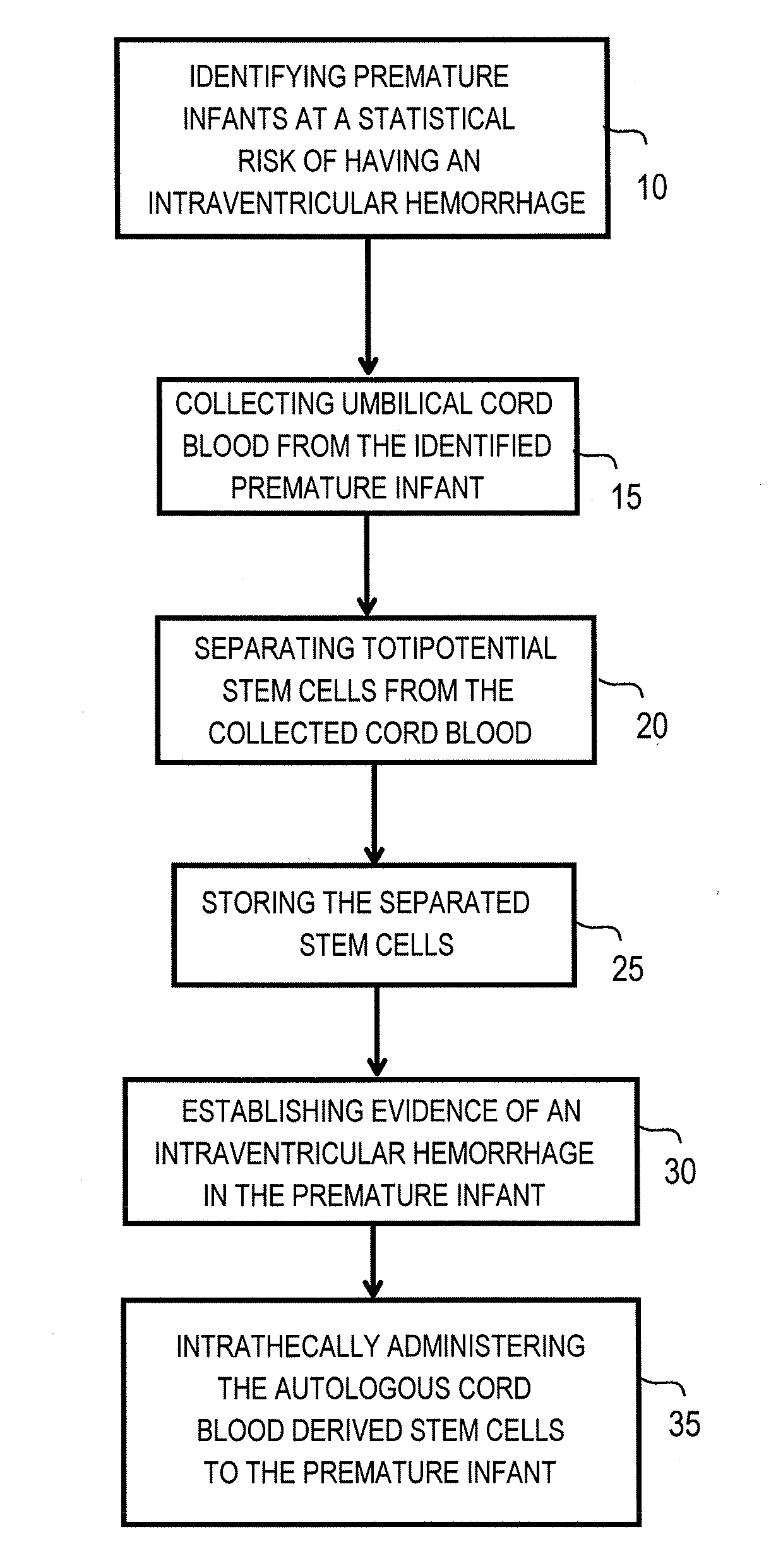
Method for intrathecal administration of autologous stem cells in premature infants
ActiveUS20100226896A1Improve pathophysiologyReduce severityBiocideMammal material medical ingredientsPremature thelarcheCord blood stem cell
The invention provides a method of treating a premature infant at a statistical risk of sustaining an intraventricular hemorrhage. The method, or therapeutic protocol, can include at least one or more of the following steps: identifying premature infants at a statistical risk of having an intraventricular hemorrhage, collecting umbilical cord blood from the identified premature infant, separating totipotential stem cells (e.g., having the ability to proliferate and differentiate as neural stem cells) from the collected cord blood, storing the separated stem cells, establishing evidence of an intraventricular hemorrhage (preferably prior to Grade III / IV if possible) in the premature infant, and intrathecally administering the autologous cord blood derived stem cells to the premature infant.
Owner:DRACKER ROBERT A
Treating neuropathic pain with seh inhibitors
Provided are methods for treating, reducing, alleviating, and / or inhibiting neuropathic pain by orally, intravenously or intrathecally administering an effective amount of an inhibitor of soluble epoxide hydrolase ("sEH"), to a patient in need thereof. The neuropathic pain treated is selected from the group consisting of post-herpetic neuralgia, trigeminal neuralgia, focal peripheral nerve injury, and anesthesia dolorosa, central pain due to stroke or mass lesion, spinal cord injury, or multiple sclerosis, and peripheral neuropathy due to diabetes, HIV, or chemotherapy.
Owner:RGT UNIV OF CALIFORNIA
Antimicrobial articles produced by additive manufacturing
InactiveCN106456835AAntibacterial agentsOrganic active ingredientsSelective laser meltingSelective laser sintering
An antibiotic-eluting article for implantation into a mammalian subject, produced by an additive manufacturing process wherein a polymeric material is concurrently deposited with a selected antibiotic. The additive manufacturing process may be a selective laser sintering process or a selective laser melting process or a selective heat sintering process or an electron beam melting process. The antibiotic-eluting article may be temporary or permanent orthopaedic skeletal component, an orthopaedic articulating joint replacement component, and / or an external hard-shell casing for an implantable device. One or more bone-growth-promoting compositions may be concurrently deposited with the polymeric material. The implantable device may be a cardiac pacemaker, a spinal cord stimulator, a neurostimulation system, an intrathecal drug pump for delivery of medicants into the spinal fluid, and infusion pump for delivery of chemotherapeutics and / or anti-spasmodics, an insulin pump, an osmotic pump, and a heparin pump.
Owner:ORTHOPAEDIC INNOVATION CENT
Rotational intrathecal analgesia
ActiveUS20030040486A1Reduce systemic side effectsMinimizing systemic side effectBiocideNervous disorderActive agentDrug administration
Owner:RGT UNIV OF CALIFORNIA +1
Compositions comprising biomembrane sealing agent for treatment of neuronal injury, and methods of use
The invention provides compositions, kits, and methods for treatment of neuronal injury. In one embodiment, the composition comprises a biomembrane sealing agent, such as PEG, and a bioactive agent, such as a magnesium compound. The biomembrane sealing agent and / or the bioactive agent an intravenous administration, an intramuscular administration, an intrathecal administration, a subcutaneous administration, an epidural administration, a parenteral administration, a direct application onto or adjacent to a site of the pathological condition, and any combinations thereof. Alternatively, the biomembrane sealing agent and / or the bioactive agent may be delivered from a pump or an implant.
Owner:WARSAW ORTHOPEDIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com