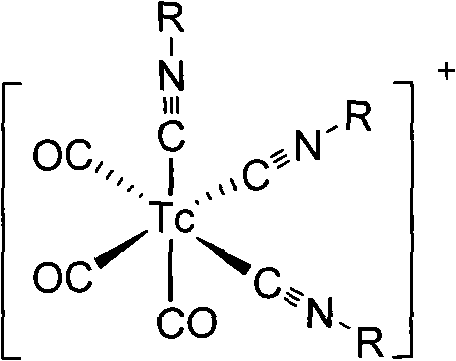
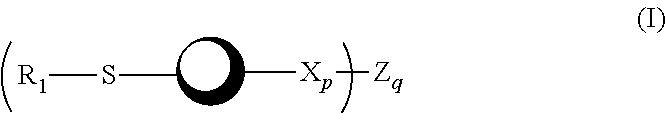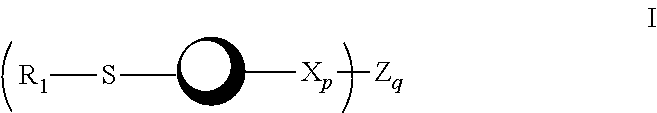Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
314 results about "Technetium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Technetium is a chemical element with the symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive; none are stable, excluding the fully ionized state of ⁹⁷Tc. Nearly all technetium is produced as a synthetic element, and only about 18,000 tons can be found at any given time in the Earth's crust. Naturally occurring technetium is a spontaneous fission product in uranium ore and thorium ore, the most common source, or the product of neutron capture in molybdenum ores. This silvery gray, crystalline transition metal lies between manganese and rhenium in group 7 of the periodic table, and its chemical properties are intermediate between those of these two adjacent elements. The most common naturally occurring isotope is ⁹⁹Tc.
Assembly for transmutation of a long-lived radioactive material
InactiveUS6233299B1Conversion outside reactor/acceleratorsNuclear energy generationRadioactive agentTechnetium-99
A new transmutation assembly permits an efficient transmutation of a long-lived radioactive material (long-lived FP nuclides such as technetium-99 or iodine-129) which was produced in the nuclear reactor. Wire-type members of a long-lived radioactive material comprised of metals, alloys or compounds including long-lived FP nuclides are surrounded by a moderator material and installed in cladding tubes to form FP pins. The FP pins, and nothing else, are housed in a wrapper tube to form a transmutation assembly. The wire-type members can be replaced by thin ring-type members. The transmutation assemblies can be selectively and at least partly loaded into a core region, a blanket region or a shield region of a reactor core in a fast reactor. From a viewpoint of reducing the influence on the reactor core characteristics, it is optimal to load the transmutation assemblies into the blanket region.
Owner:JAPAN ATOMIC ENERGY AGENCY INDEPENDANT ADMINISTRATIVE CORP
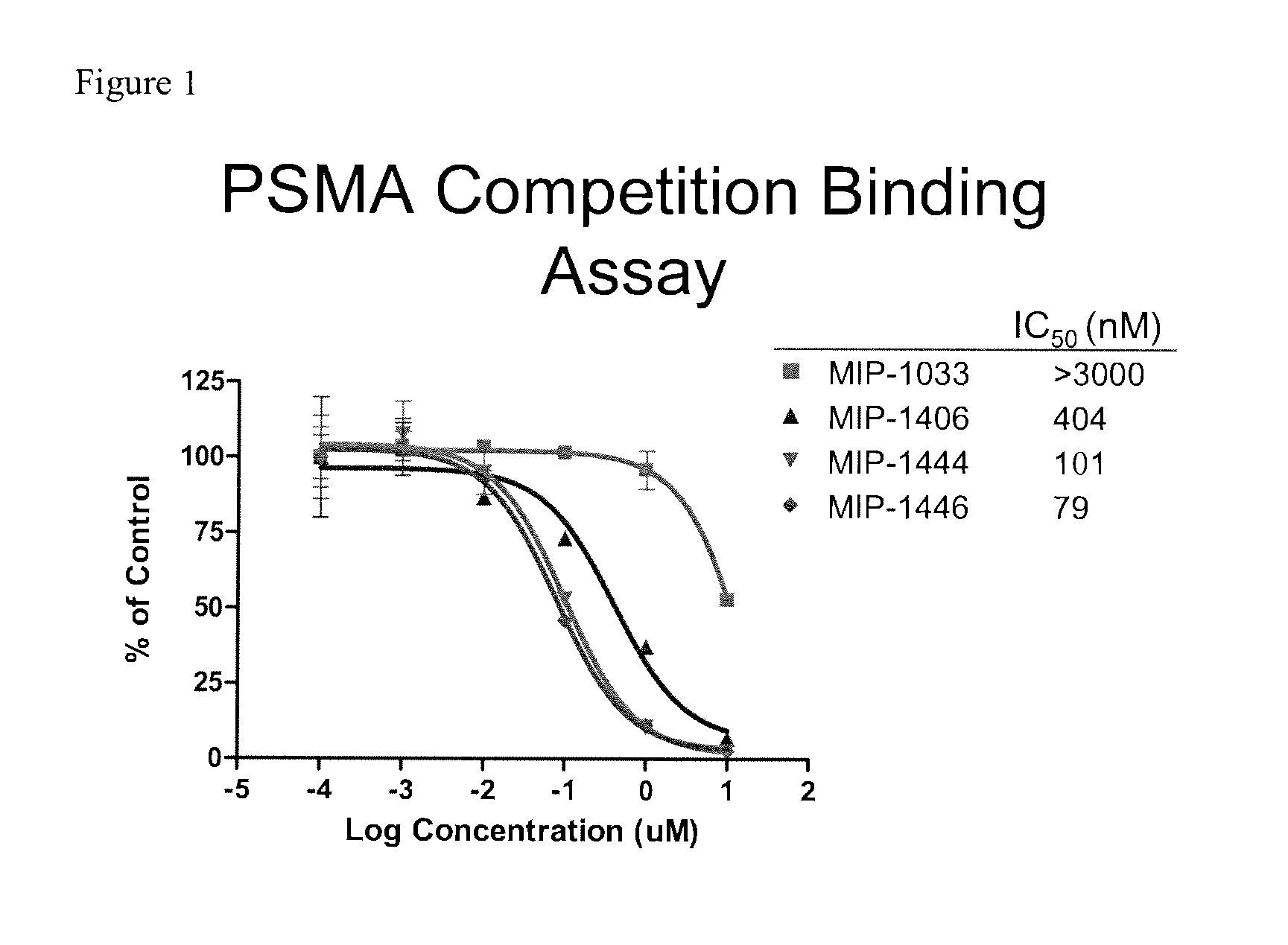
Technetium- and rhenium-bis(heteroaryl) complexes and methods of use thereof for inhibiting PSMA
Owner:MOLECULAR INSIGHT PHARMA
Imaging Agents
InactiveUS20070082879A1High yieldExcessive reactionBiocideIn-vivo radioactive preparationsRheniumAbnormal tissue growth
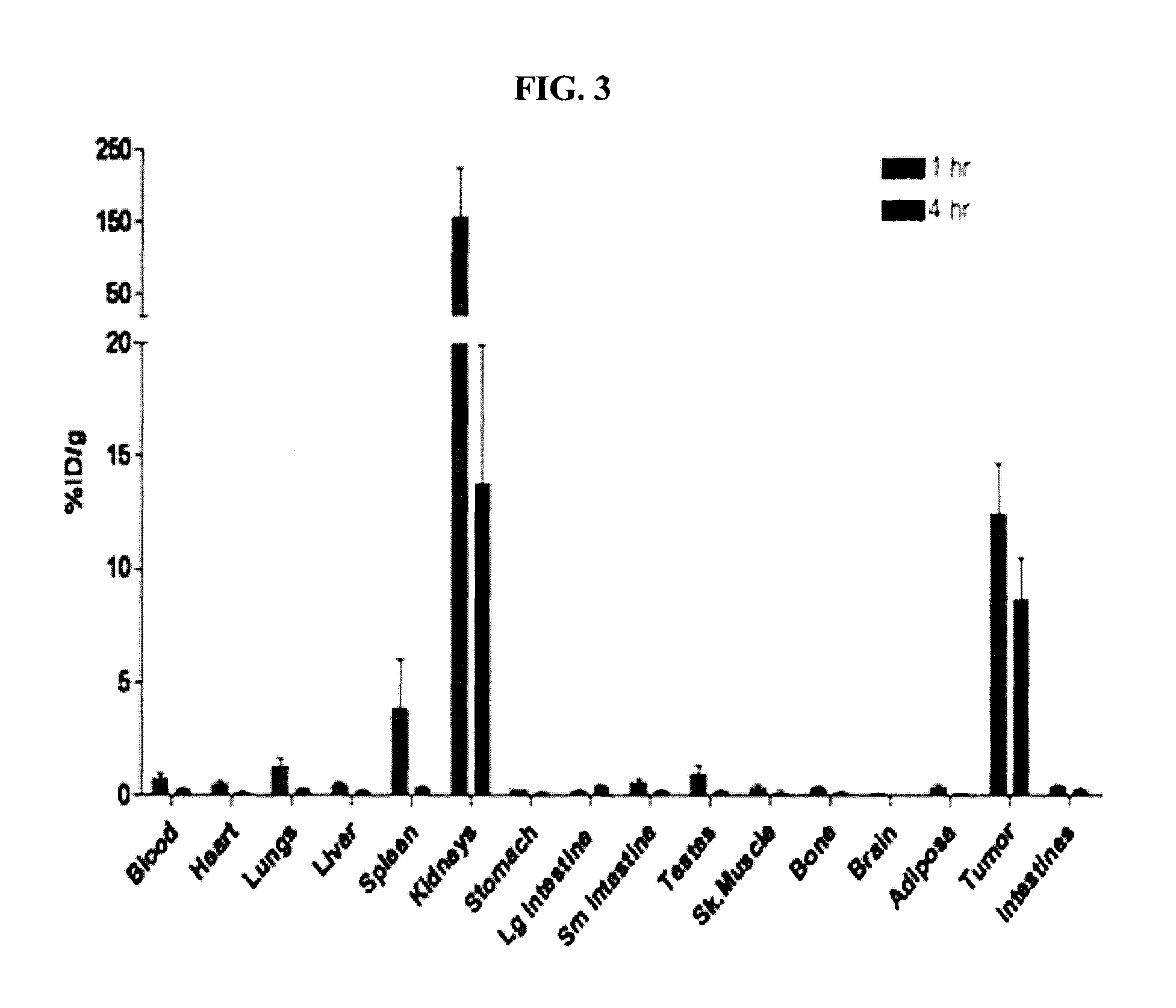
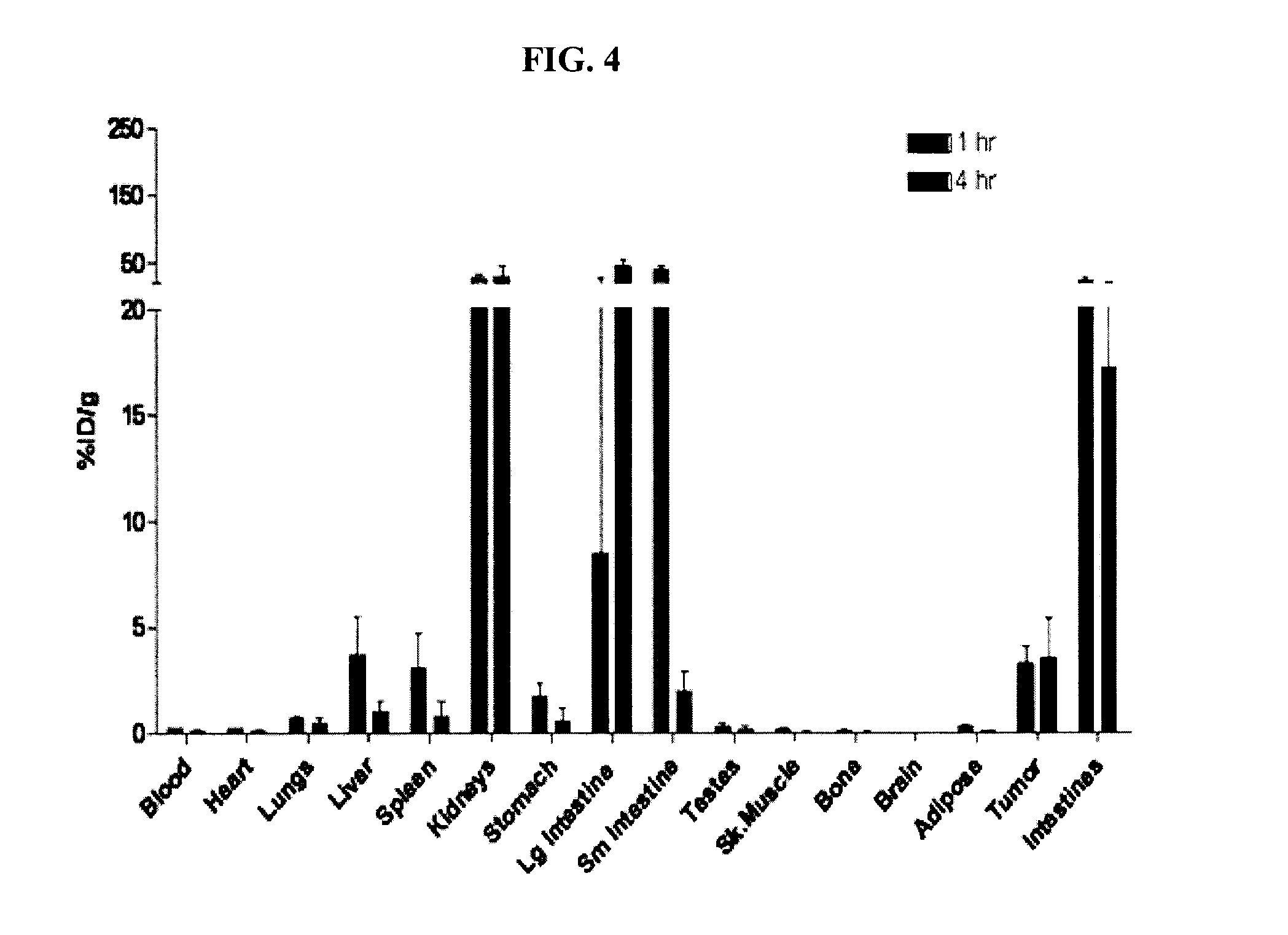
The present invention provides novel amino acid compounds useful in detecting and evaluating brain and body tumors. These compounds have the advantageous properties of rapid uptake and prolonged retention in tumors and can be labeled with halogen isotopes such as fluorine-18, iodine-123, iodine-124, iodine-125, iodine-131, bromine-75, bromine-76, bromine-77, bromine-82, astatine-210, astatine-211, and other astatine isotopes. These compounds can also be labeled with technetium and rhenium isotopes using known chelation complexes. The compounds disclosed herein bind tumor tissues in vivo with high specificity and selectivity when administered to a subject. Preferred compounds show a target to non-target ratio of at least 2:1, are stable in vivo and substantially localized to target within 1 hour after administration. Preferred compounds include 1-amino-2-[18F]fluorocyclobutyl-1-carboxylic acid (2-[18F]FACBC) and 1-amino-2-[18F]fluoromethylcyclobutyl-1-carboxylic acid (2-[18F]FMACBC). The labeled amino acid compounds of the invention are useful as imaging agents in detecting and / or monitoring tumors in a subject by PET or SPECT.
Owner:EMORY UNIVERSITY
Magnetoresistance element, magnetic memory, and magnetic head
There is provided a magnetoresistance element including a free layer that includes a first ferromagnetic layer and a second ferromagnetic layer whose magnetization directions are equal to each other and a nonmagnetic film intervening between the first and second ferromagnetic layers, a pinned layer including a third ferromagnetic layer that faces the free layer, and a nonmagnetic layer intervening between the free layer and the pinned layer, the nonmagnetic film containing a material selected from the group including titanium, vanadium, zirconium, niobium, molybdenum, technetium, hafnium, tungsten, rhenium, alloys thereof, semiconductors and insulators.
Owner:KAI TADASHI +3
Psma-targeted dendrimers
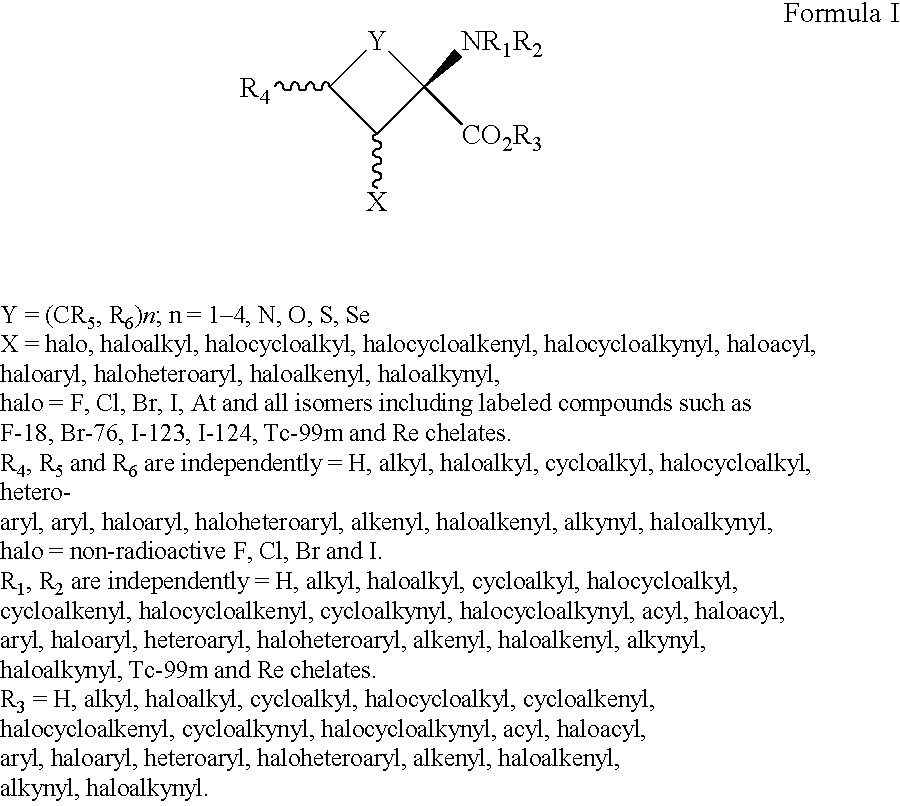
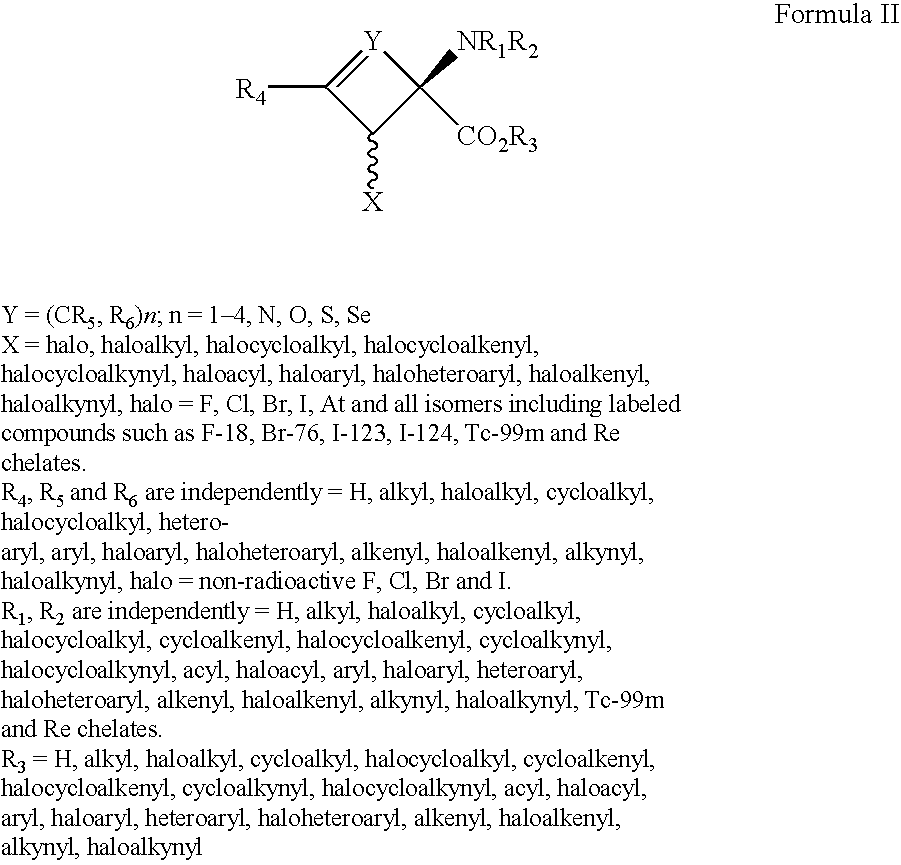
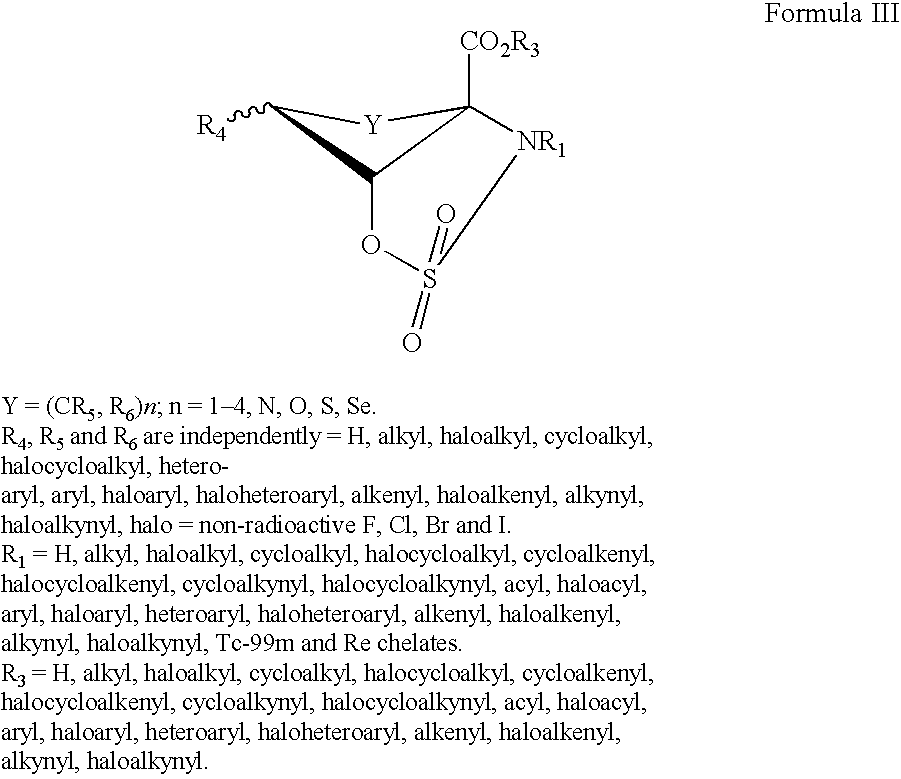
A dendrimer conjugate according to Formula (I), or its pharmaceutically acceptable salt, or solvate thereof: and complexes of Formula I conjugates with metals radionuclides of elements such as rhenium, technetium, yttrium, lutetium and others to provide a complex for imaging tissues or for the radiotherapeutic treatment of cancer tissue. Such complexes are specific to PSMA protein and can therefore be used in imaging or treating cancer of the prostate and other tissue where the protein is expressed.
Owner:MOLECULAR INSIGHT PHARMA
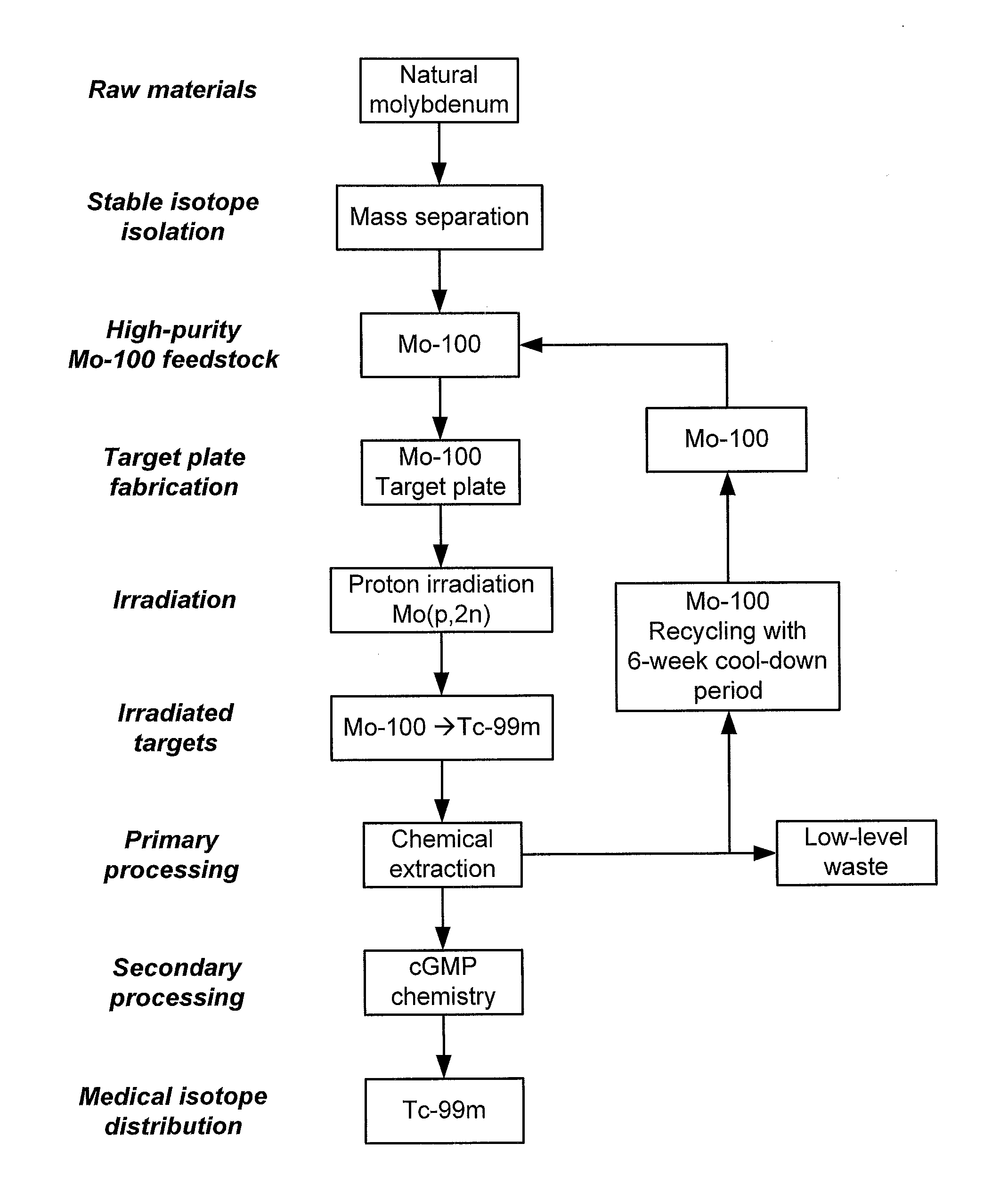
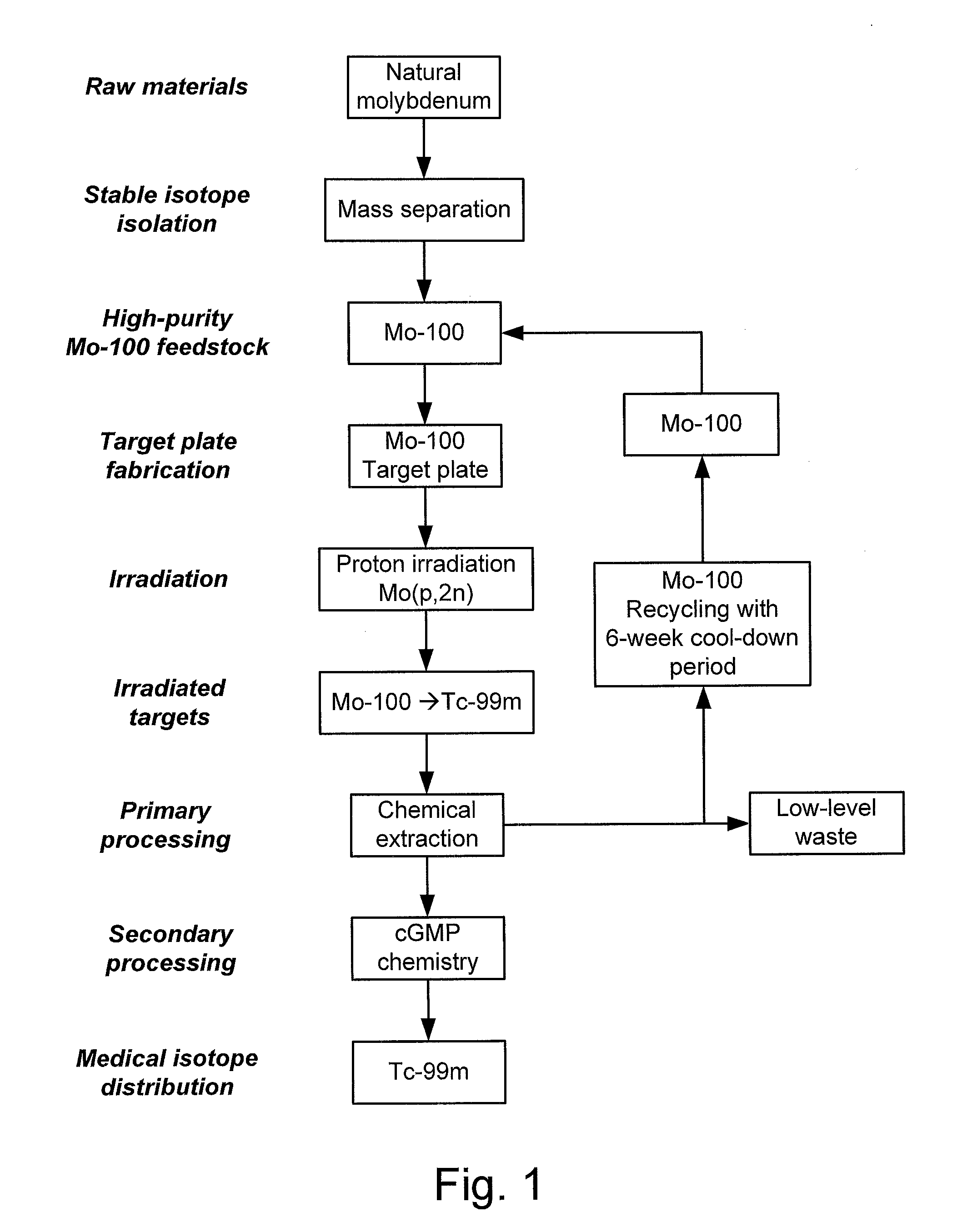
Processes, systems, and apparatus for cyclotron production of technetium-99m
A process for producing technetium-99m from a molybdenum-100 metal powder, comprising the steps of:(i) irradiating in a substantially oxygen-free environment, a hardened sintered target plate coated with a Mo-100 metal, with protons produced by a cyclotron;(ii) dissolving molybdenum ions and technetium ions from the irradiated target plate with an H2O2 solution to form an oxide solution;(iv) raising the pH of the oxide solution to about 14;(v) flowing the pH-adjusted oxide solution through a resin column to immobilize K[TcO4] ions thereon and to elute K2[MoO4] ions therefrom;(vi) eluting the bound K[TcO4] ions from the resin column;(vii) flowing the eluted K[TcO4] ions through an alumina column to immobilize K[TcO4] ions thereon;(viii) washing the immobilized K[TcO4] ions with water;(ix) eluting the immobilized K[TcO4] ions with a saline solution; and(x) recovering the eluted Na[TcO4] ions.
Owner:TRIUMF
Photosensitizing transition metal complex and its use for photovoltaic cell
InactiveUS20050081911A1Improve efficiencyIncreased durabilityRuthenium organic compoundsElectrolytic capacitorsRheniumO-Phosphoric Acid
A photosensitizing transition metal complex of the formula (Ia) MLY1, (Ib) MLX3 (Ic) MLY2X, (Id) MLY3X or (Ie) MLY4X in which M is a transition metal selected from ruthenium, osmium, iron, rhenium and technetium, preferably ruthenium or osmium. X is a co-ligand independently selected from NCS—, Cl—, Br—, I—, CN—, H2O; pyridine unsubstituted or substituted by at least one group selected from vinyl, primary, secondary or tertiary amine, OH and C1-30 alkyl, preferably NSC and CN—; L is a tridentate polypyridine ligand, carrying at least one carboxylic, phosphoric acid or a chelating group and one substituted or unsubstituted alkyl group having 1 to 50 carbon atoms, substituted or unsubstituted alkylamide group having 2 to 30 carbon atoms or substituted or unsubstituted aralkyl group having 7 to 50 carbon atoms. A dye-sensitized electrode includes a substrate having an electrically conductive surface, an oxide semiconductor film formed on the conductive surface, and the above sensitizer of formula (Ia), (Ib), (Ic), (Id) or (Ie) as specified above, supported on the film. A solar cell includes the above electrode, a counter electrode, and an electrolyte deposited there between.
Owner:SHARP KK
Photosensitizing transition metal complex containing quaterpyridine and photovoltaic cell with the metal complex
InactiveUS20050139257A1Improve efficiency and durability and stabilityRuthenium organic compoundsLight-sensitive devicesRheniumPhosphoric acid
A photosensitizer complex of formula (I) MLX2 in which M is a transition metal selected from ruthenium, osmium, iron, rhenium and technetium; each X is a co-ligand independently selected from NCS−, Cl−, Br−, I−, CN−, H2O; pyridine unsubstituted or substituted by at least one group selected from vinyl, primary, secondary or tertiary amine, OH and C1-30 alky, preferably NSC− and CN−. L is a tetradentate polypyridine ligand, carrying at least one carboxylic, phosphoric acid or a chelating group and one substituted or unsubstituted alkyl group having 1 to 50 carbon atoms, substituted or unsubstituted alkylamide group having 2 to 30 carbon atoms or substituted or unsubstituted aralkyl group having 7 to 50 carbon atoms. A dye-sensitized electrode includes a substrate having an electrically conductive surface, an oxide semiconductor film formed thereon, and the above sensitizer of formula (I) as specified above, supported on the film.
Owner:SHARP KK
PSMA-targeted dendrimers
A dendrimer conjugate according to Formula (I), or its pharmaceutically acceptable salt, or solvate thereof: and complexes of Formula I conjugates with metals radionuclides of elements such as rhenium, technetium, yttrium, lutetium and others to provide a complex for imaging tissues or for the radiotherapeutic treatment of cancer tissue. Such complexes are specific to PSMA protein and can therefore be used in imaging or treating cancer of the prostate and other tissue where the protein is expressed.
Owner:MOLECULAR INSIGHT PHARMA
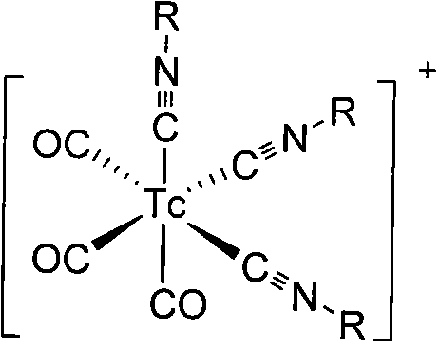
Radioactive transition metal nitride heterocomplex
InactiveUS6270745B1Impairing activityHigh yieldGroup 5/15 element organic compoundsRadioactive preparation carriersRheniumTechnetium
The present invention provides a single radioactive transition metal nitride heterocomplex which permits labeling of a physiologically active substance such as a peptide, hormone or the like without impairing the activity of the substance. The radioactive transition metal nitride heterocomplex of the present invention is represented by the following formula (I):wherein a radioactive transition metal M is radioactive technetium or radioactive rhenium, N is a nitrogen atom, X is a diphosphine compound or a diarsine compound, and Y is a bindentate ligand having a combination of electron-donating atoms.
Owner:NIHON MEDI PHYSICS CO LTD
Method and reactor for cracking hydrocarbon
ActiveUS20110295051A1Reduces and eliminates build-upThermal non-catalytic crackingHydrocarbon oil cracking processIndiumCerium
A method for cracking hydrocarbon, comprises: providing steam and hydrocarbon; and feeding steam and hydrocarbon into a reactor accessible to hydrocarbon and comprising a perovskite material of formula AaBbCcDdO3-δ, wherein 0<a<1.2, 0≦b≦1.2, 0.9<a+b≦1.2, 0<c<1.2, 0≦d≦1.2, 0.9<c+d≦1.2, −0.5<δ<0.5; A is selected from calcium, strontium, barium, and any combination thereof; B is selected from lithium, sodium, potassium, rubidium and any combination thereof; C is selected from cerium, zirconium, antimony, praseodymium, titanium, chromium, manganese, ferrum, cobalt, nickel, gallium, tin, terbium and any combination thereof; and D is selected from lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, ebium, thulium, ytterbium, lutetium, scandium, titanium, vanadium, chromium, manganese, ferrum, cobalt, nickel, copper, zinc, yttrium, zirconium, niobium, molybdenum, technetium, ruthenium, rhodium, palladium, silver, cadmium, hafnium, tantalum, tungsten, rhenium, osmium, iridium, platinum, gold, gallium, indium, tin, antimony and any combination thereof.
Owner:BL TECH INC
Catalyst for use in catalytic oxidation or ammoxidation of propane or isobutane in the gaseous phase
InactiveUS7087551B2High selectivityEasy to useOrganic compound preparationHeterogenous catalyst chemical elementsRheniumIridium
Disclosed is an oxide catalyst comprising an oxide represented by the formula Mo1VaNbbXcYdZeQfOn (wherein: X is at least one element selected from the group consisting of Te and Sb; Y is at least one element selected from the group consisting of Al and W; Z is at least one element selected from the group consisting of elements which individually form an oxide having a rutile structure and a Z oxide having a rutile structure is used as a source of Z for producing the catalyst; Q is at least one element selected from the group consisting of titanium, tin, germanium, lead, tantalum, ruthenium, rhenium, rhodium, iridium, platinum, chromium, manganese, technetium, osmium, iron, arsenic, cerium, cobalt, magnesium, nickel and zinc, and a Q compound not having a rutile structure is used as a source of Q for producing the catalyst; and a, b, c, d, e, f and n are, respectively, the atomic ratios of V, Nb, X, Y, Z, Q and O, relative to Mo). Also disclosed is a process for producing an unsaturated carboxylic acid or an unsaturated nitrile by using the above-mentioned oxide catalyst.
Owner:ASAHI KASEI KK
Tumor imaging compounds
ActiveUS20050192458A1Rapid uptakeHigh retention rateBiocidePeptide/protein ingredientsAbnormal tissue growthRhenium
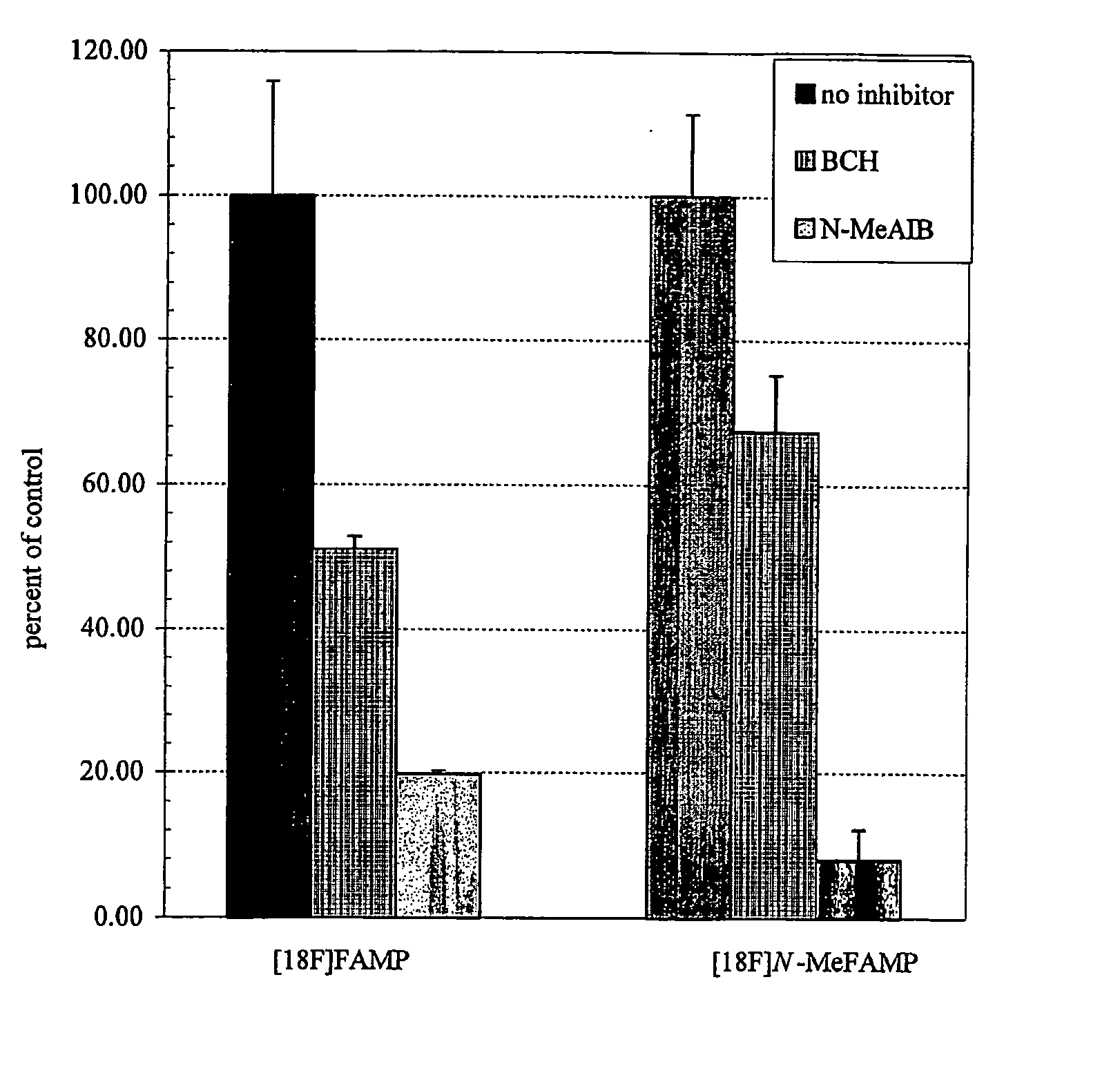
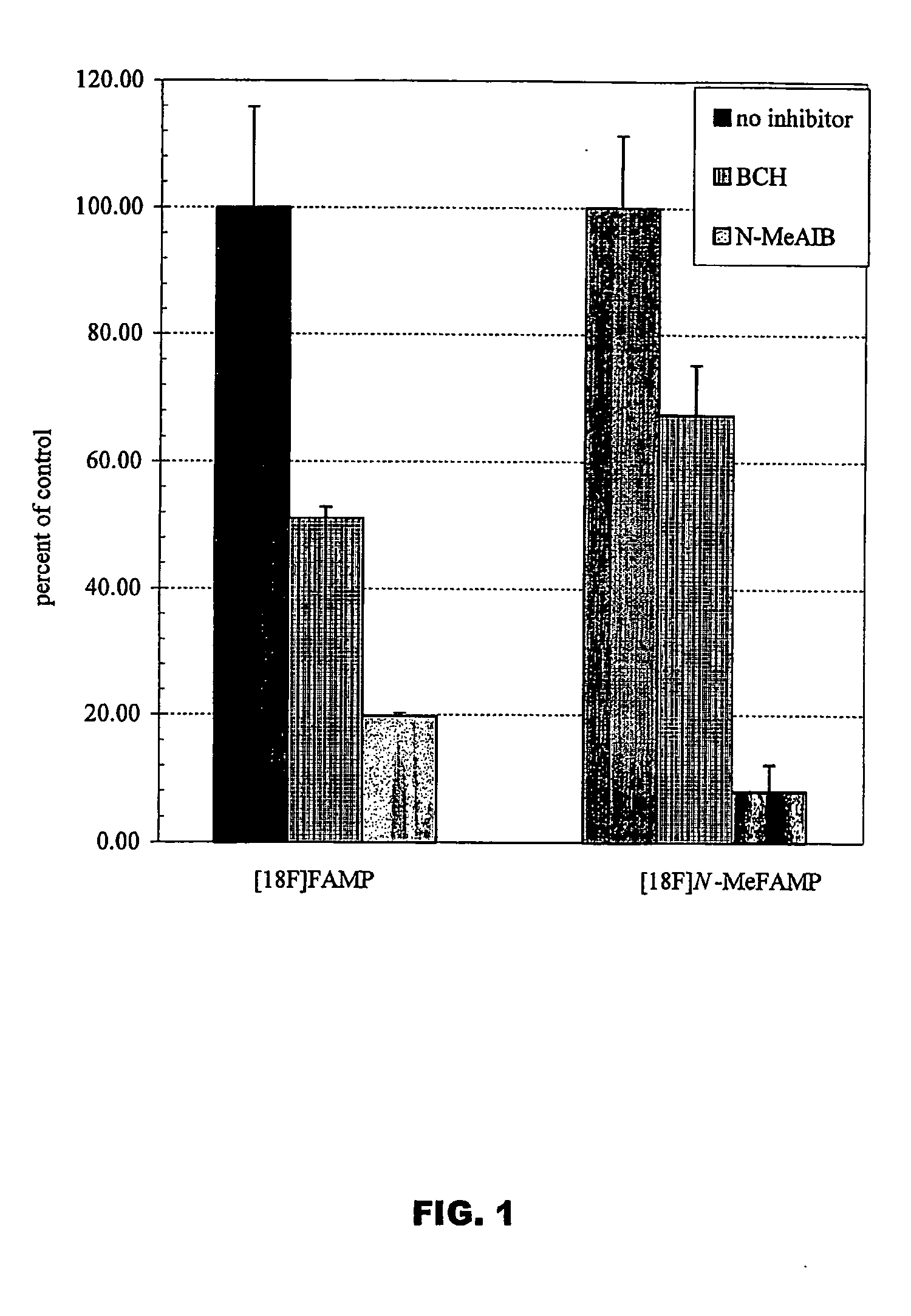
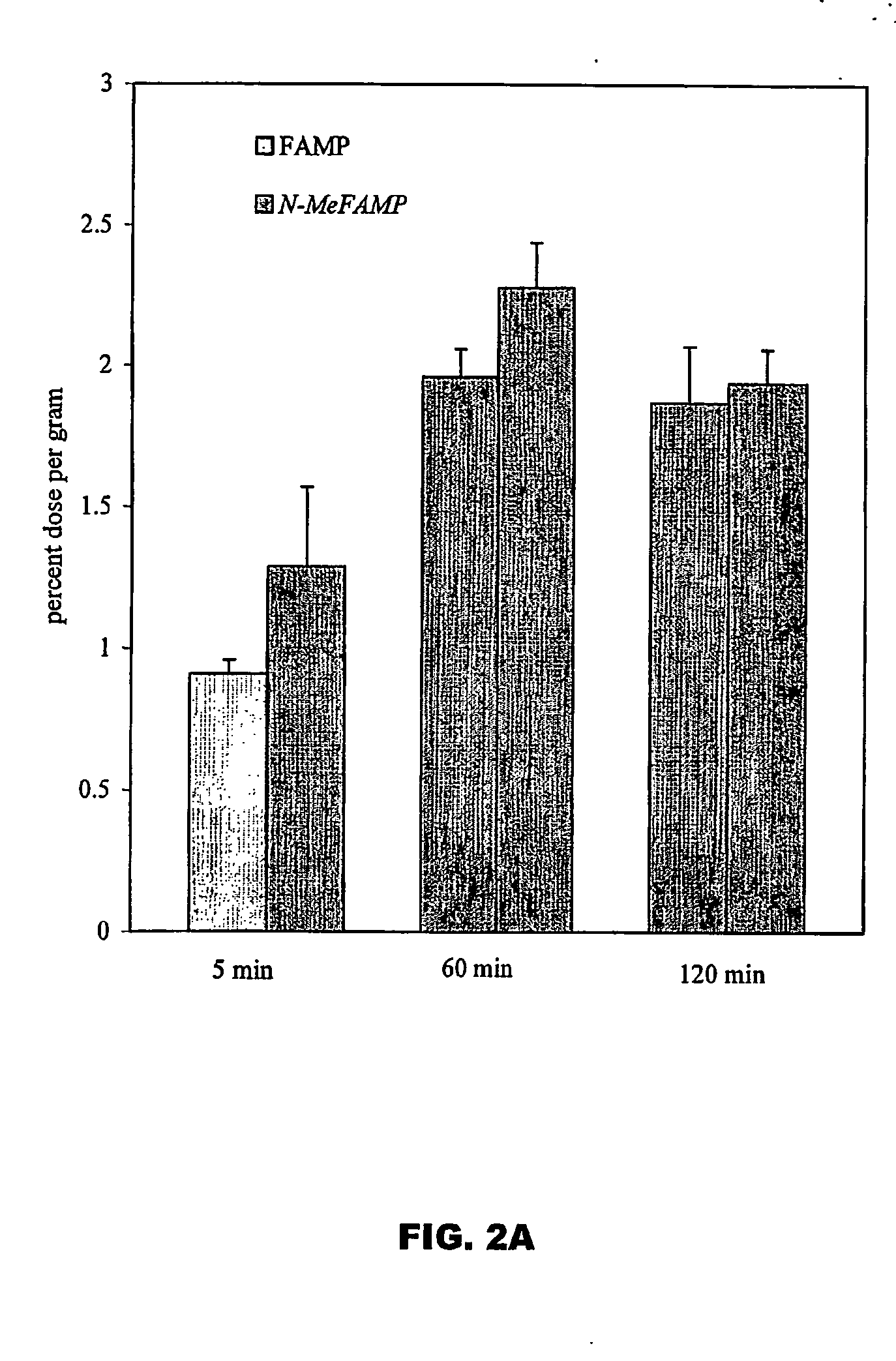
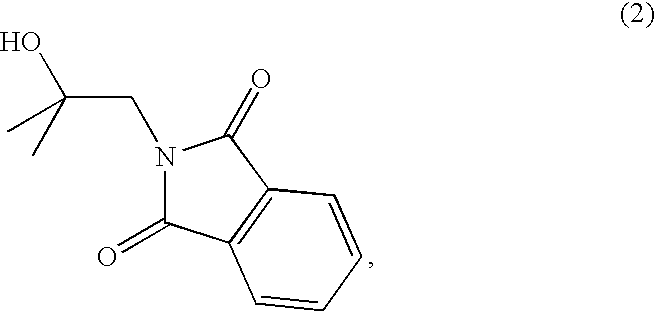
The invention provides novel amino acid compounds of use in detecting and evaluating brain and body tumors. These compounds combine the advantageous properties of α-aminoisobutyric acid (AIB) analogs namely, their rapid uptake and prolonged retention in tumors with the properties of halogen sustituents, including certain useful halogen isotopes such as fluorine-18, iodine-123, iodine-124, iodine-125, iodine-131, bromine-75, bromine-76, bromine-77, bromine-82, astatine-210, astatine-211, and other astatine isotopes. In addition the compounds can be labeled with technetium and rhenium isotopes using known chelation complexes. The amino acid compounds disclosed herein have a high specificity for target sites when administered to a subject in vivo. Preferred amino acid compounds include [18F] FAMP, ([18F]5a) and [18F]N-MeFAMP, ([18F]5b). The invention further features pharmaceutical compositions comprised of an α-amino acid moiety attached to eiher a four, five or a six member carbon-chain ring. The labeled amino acid compounds are useful as imaging agents in detecting and / or monitoring tumors in a subject by Positron Emission Tomography (PET) and Single Photon Emission Computer Tomography (SPECT).
Owner:EMORY UNIVERSITY
Technetium-and rhenium-bis(heteroaryl) complexes, and methods of use thereof
InactiveUS20060093552A1In-vivo radioactive preparationsGroup 3/13 element organic compoundsRheniumAryl
One aspect of the invention relates to complexes of technetium (Tc) and rhenium (Re) with various heteroaromatic ligands, e.g., quinolinyl and isoquinolinyl ligands, and their use in fluoresence and radioimaging for a variety of clinical diagnostic applications, as well as radiopharmaceuticals for therapeutic applications. Another aspect of the invention relates to quinolinyl and isoquinolinyl ligands that form a portion of the aforementioned complexes. Methods for the preparation of the technetium and rhenium complexes are also described. Another aspect of the invention relates to quinolinyl and isoquinolinyl ligands based on derivatized lysine, alanine and bis-amino acids for conjugation to small peptides by solid phase synthetic methods. Additionally, the invention relates to methods for imaging regions of a mammal using the complexes of the invention.
Owner:SYRACUSE UNIVERSITY +3
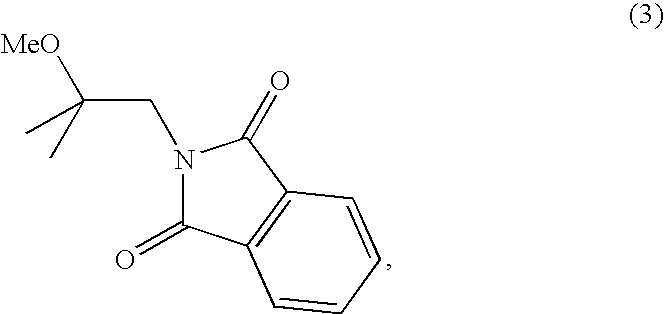
Methods for preparing 2-methoxyisobutylisonitrile and tetrakis(2-methoxyisobutylisonitrile)copper(i) tetrafluoroborate
ActiveUS20080102028A1Isocyanic acid derivatives preparationCarboxylic acid nitrile preparationTechnetiumTetrafluoroborate
The present invention relates to new synthetic methods for preparing 2-methoxyisobutylisonitrile and metal isonitrile complexes, such as tetrakis(2-methoxyisobutylisonitrile)copper(I) tetrafluroroborate, which are used in the preparation of technetium (99mTc) Sestamibi, and novel intermediate compounds useful in such methods.
Owner:JUBILANT DRAXIMAGE
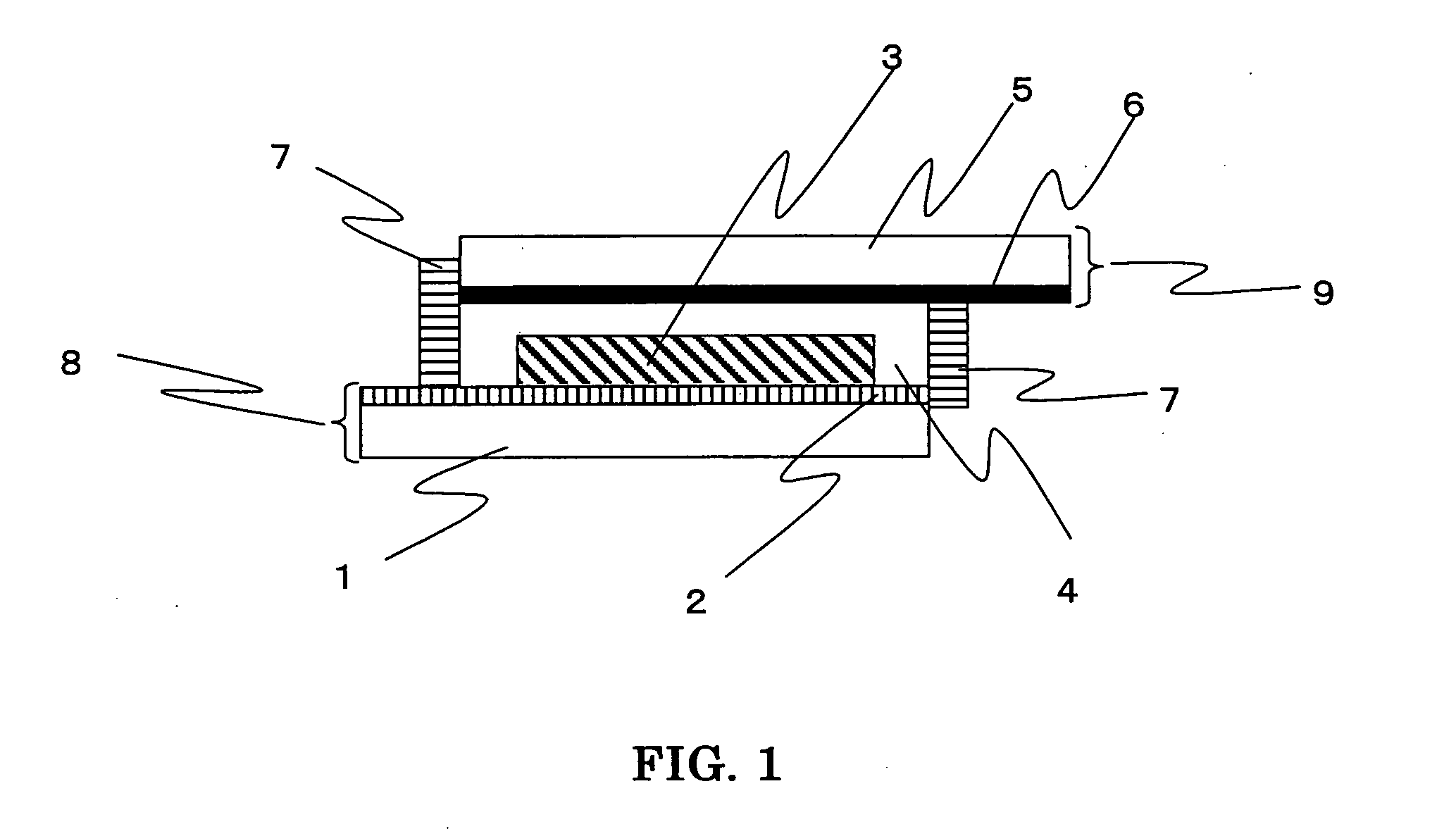
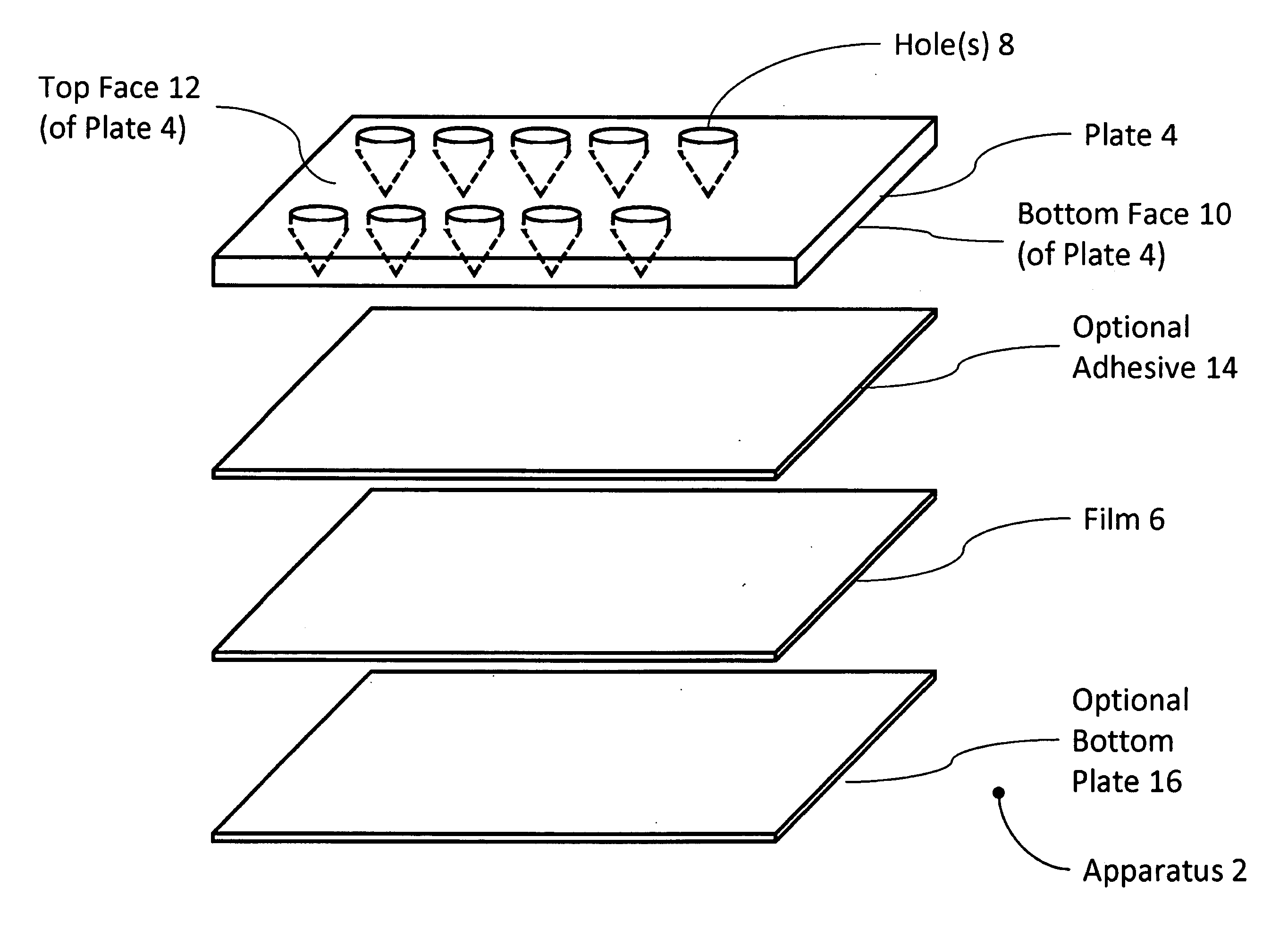
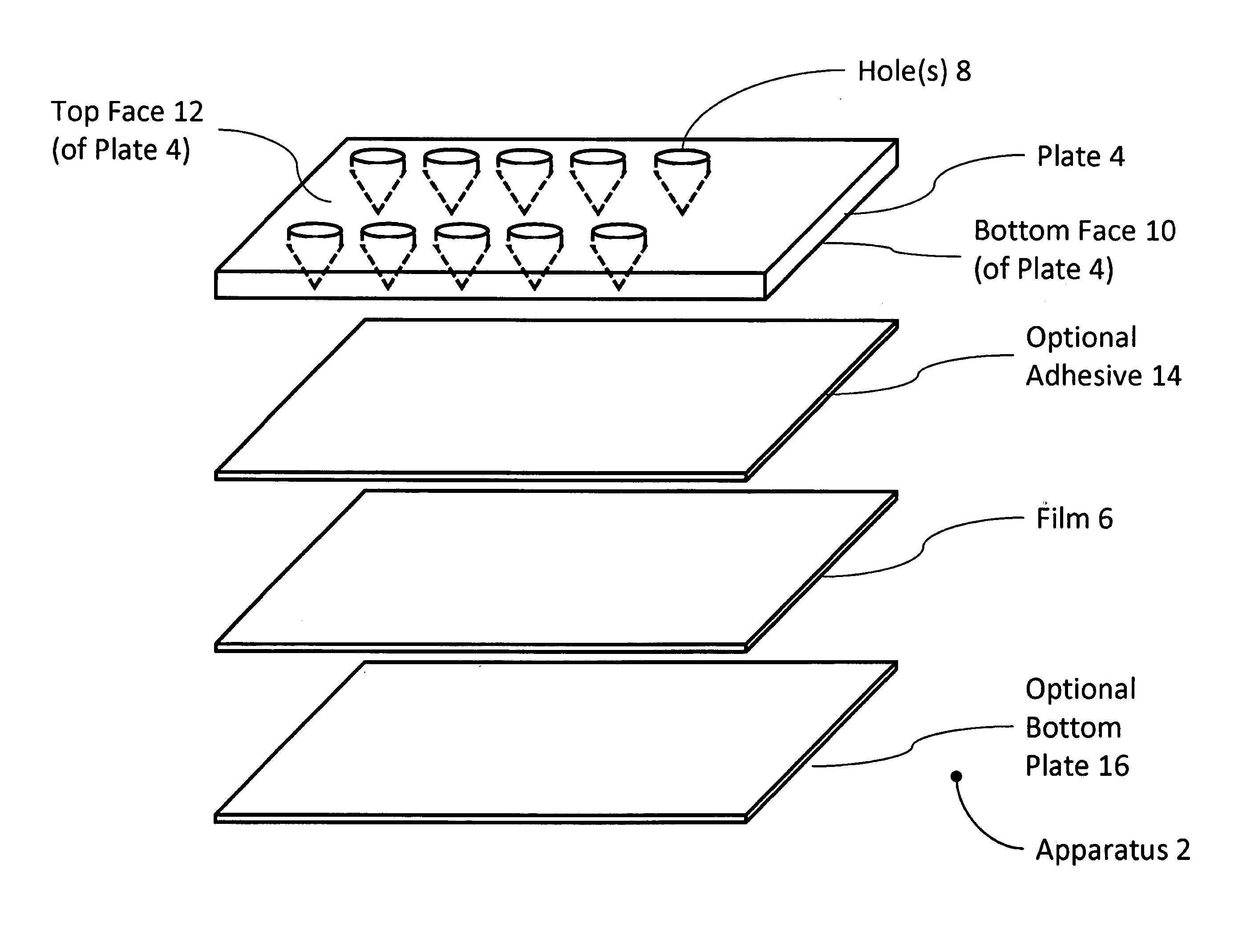
Well Plate
ActiveUS20090046832A1Material analysis using wave/particle radiationX-ray spectral distribution measurementRh elementPlatinum
The present invention includes an apparatus for preparing samples for measurement by x-ray fluorescence spectrometry. The apparatus comprises a plate having one or more holes passing through the plate. The holes are covered by a film on one side of the plate. The holes are less than 500 micrometers across in one dimension where the film covers the holes. The film is translucent to x-rays. The present invention also includes an apparatus for preparing samples for measurement by x-ray fluorescence spectrometry. The apparatus comprises a plate having one or more holes passing through the plate. The holes are covered on one side of the plate by a detachable cover forming a water-tight seal against the plate. The cover is substantially free of the elements osmium, yttrium, iridium, phosphorus, zirconium, platinum, gold, niobium, mercury, thallium, molybdenum, sulfur, lead, bismuth, technetium, ruthenium, chlorine, rhodium, palladium, argon, silver, and thorium. The holes are less than about 500 micrometers across in one dimension where the cover covers the holes. The present invention also includes a method for preparing samples for measurement by x-ray fluorescence spectrometry. The method comprises providing a solution of with less than 10 micromolar solute and a volume of between about 2 microliters and about 2 milliliters. The solution is concentrated and analyzed using x-ray fluorescence spectrometry.
Owner:ICAGEN LLC
Well plate
ActiveUS8238515B2Material analysis using wave/particle radiationX-ray spectral distribution measurementRh elementPlatinum
The present invention includes an apparatus for preparing samples for measurement by x-ray fluorescence spectrometry. The apparatus comprises a plate having one or more holes passing through the plate. The holes are covered by a film on one side of the plate. The holes are less than 500 micrometers across in one dimension where the film covers the holes. The film is translucent to x-rays. The present invention also includes an apparatus for preparing samples for measurement by x-ray fluorescence spectrometry. The apparatus comprises a plate having one or more holes passing through the plate. The holes are covered on one side of the plate by a detachable cover forming a water-tight seal against the plate. The cover is substantially free of the elements osmium, yttrium, iridium, phosphorus, zirconium, platinum, gold, niobium, mercury, thallium, molybdenum, sulfur, lead, bismuth, technetium, ruthenium, chlorine, rhodium, palladium, argon, silver, and thorium. The holes are less than about 500 micrometers across in one dimension where the cover covers the holes. The present invention also includes a method for preparing samples for measurement by x-ray fluorescence spectrometry. The method comprises providing a solution of with less than 10 micromolar solute and a volume of between about 2 microliters and about 2 milliliters. The solution is concentrated and analyzed using x-ray fluorescence spectrometry.
Owner:ICAGEN LLC
Method and reactor for cracking hydrocarbon
ActiveUS9499747B2Reduces and eliminates build-upThermal non-catalytic crackingHydrocarbon by dehydrogenationIndiumHafnium
A method for cracking hydrocarbon, comprises: providing steam and hydrocarbon; and feeding steam and hydrocarbon into a reactor accessible to hydrocarbon and comprising a perovskite material of formula AaBbCcDdO3-δ, wherein 0<a<1.2, 0≦b≦1.2, 0.9<a+b≦1.2, 0<c<1.2, 0≦d≦1.2, 0.9<c+d≦1.2, −0.5<δ<0.5; A is selected from calcium, strontium, barium, and any combination thereof; B is selected from lithium, sodium, potassium, rubidium and any combination thereof; C is selected from cerium, zirconium, antimony, praseodymium, titanium, chromium, manganese, ferrum, cobalt, nickel, gallium, tin, terbium and any combination thereof; and D is selected from lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, ebium, thulium, ytterbium, lutetium, scandium, titanium, vanadium, chromium, manganese, ferrum, cobalt, nickel, copper, zinc, yttrium, zirconium, niobium, molybdenum, technetium, ruthenium, rhodium, palladium, silver, cadmium, hafnium, tantalum, tungsten, rhenium, osmium, iridium, platinum, gold, gallium, indium, tin, antimony and any combination thereof.
Owner:BL TECH INC
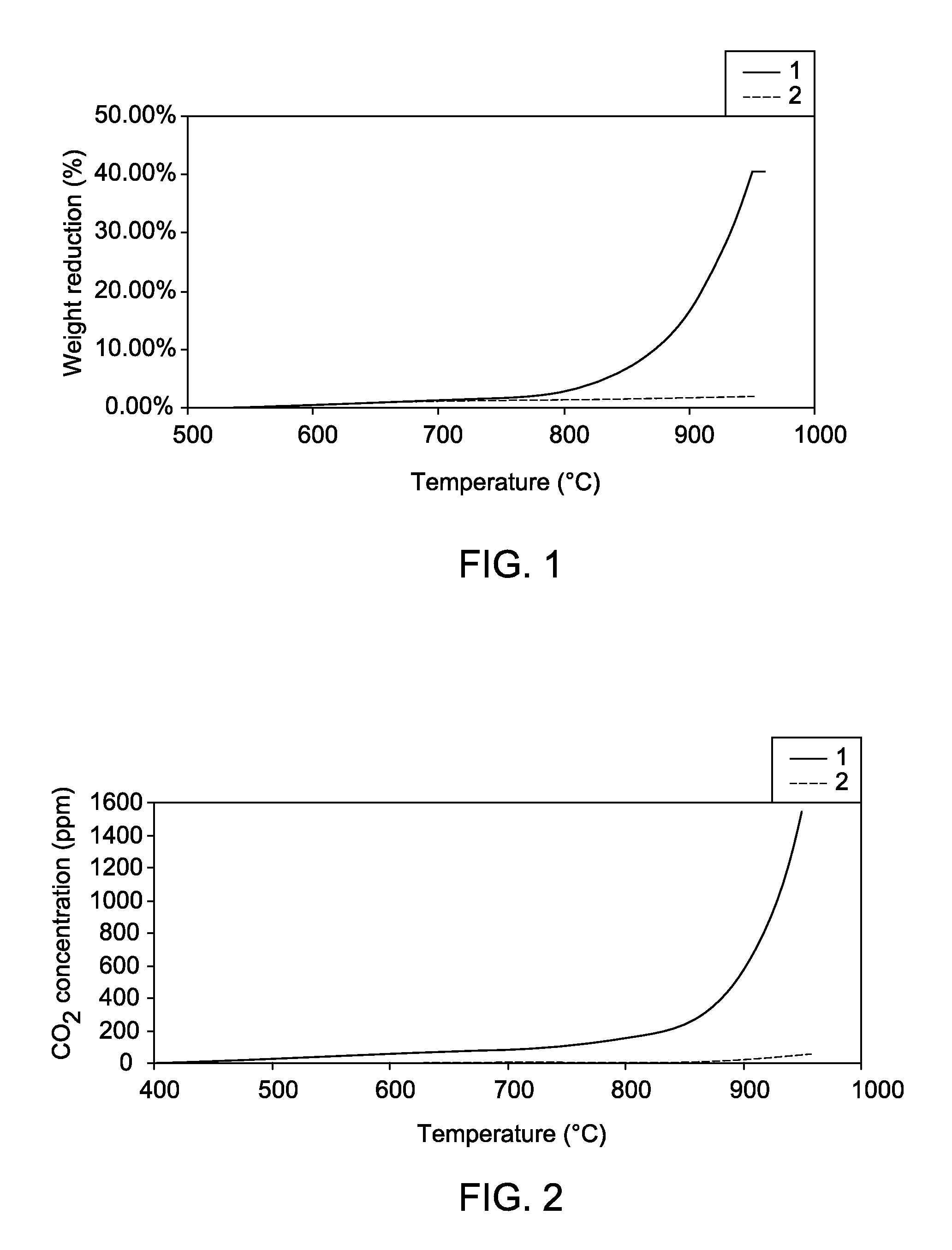
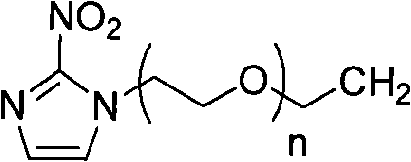
Caobonyl technetium labeled 2-azomycin composition, preparation method and application
InactiveCN101654465AHigh affinityIncrease intakeRadioactive preparation carriersGroup 7/17 element organic compoundsNitroimidazoleTechnetium
The invention discloses a radioactive nuclide labeled compound which is caobonyl technetium labeled 2-azomycin composition, a preparation method of the compound and a purpose of the compound. The preparation method of the caobonyl technetium labeled 2-azomycin composition comprises the following steps: firstly synthesizing a ligand NC-PEGn-NIM and heating the ligand and a newly prepared midbody <99m>Tc(CO)3(H2O)3<+>, the pH value of which is regulated to 9 to 10, to obtain the caobonyl technetium labeled 2-azomycin composition. The caobonyl technetium labeled 2-azomycin composition is concentrated in anoxybiotic tumor cells by the targeting function of azomycin and can be used for the SPECT raster display diagnosis of anoxybiotic tumors.
Owner:LANZHOU UNIVERSITY
Technetium-And Rhenium-Bis (Heteroaryl) Complexes, And Methods Of Use Thereof
ActiveUS20080025915A1Improve throughputLimit deliveryRadioactive preparation carriersGroup 3/13 element organic compoundsRheniumSmall peptide
One aspect of the invention relates to complexes of a radionuclide with various heteroaryl ligands, e.g., imidazolyl and pyridyl ligands, and their use in radiopharmaceuticals for a variety of clinical diagnostic and therapeutic applications. Another aspect of the invention relates to imidazolyl and pyridyl ligands that form a portion of the aforementioned complexes. Methods for the preparation of the radionuclide complexes are also described. Another aspect of the invention relates to imidazolyl and pyridyl ligands based on derivatized lysine, alanine and bis-amino acids for conjugation to small peptides by solid phase synthetic methods. Additionally, the invention relates to methods for imaging regions of a mammal using the complexes of the invention.
Owner:MOLECULAR INSIGHT PHARMA
Selenium-rich ceramic appliance and preparation technology thereof
The invention relates to a selenium-rich ceramic appliance. A ceramic appliance body is fired from raw materials in parts by mass as follows: 30-50 parts of selenium-rich soil, 35-45 parts of petalite, 5-15 parts of spodumene, 1-15 parts of quartz, 10-15 parts of kaolin, 5-8 parts of microcrystalline ceramic powder, 1-3 parts of halloysite nanotubes, 0.5-1 part of nano tungsten carbide powder, 0.5-1 part of nano technetium oxide powder and 0.3-0.5 parts of polyacrylamide. The inner wall of the ceramic appliance body is coated with a selenium-containing coating. The selenium-rich ceramic appliance is prepared from main materials including cheaper petalite and selenium-rich soil, is coated with the selenium-rich coating, has good thermal shock resistance, crack resistance, wear resistance and heat conductivity and can separate out trace selenium element to supplement the human body with selenium.
Owner:JIANGXI JINGSHANG IND CO LTD
Petalite heat-resistant ceramic wok and preparation process thereof
ActiveCN108101525AIncrease productionImprove control effectCooking-vessel materialsCrack resistanceTalc
The invention relates to a petalite heat-resistant ceramic wok. The petalite heat-resistant ceramic wok is prepared by firing the following raw materials in parts by weight: 45 to 65 parts of petalite, 15 to 40 parts of spodumene, 12 to 20 parts of quartz, 15 to 20 parts of kaolin, 6 to 9 parts of talc, 5 to 8 parts of microcrystalline ceramic powder, 1 to 3 parts of halloysite nanotubes, 0.5 to 1part of nanometer tungsten carbide powder, 0.5 to 1 part of nanometer technetium oxide powder, 0.3 to 0.5 part of polyacrylamide. The petalite heat-resistant ceramic wok utilizes the cheaper petaliteas a main raw material, and the prepared petalite heat-resistant ceramic wok has good thermal shock resistance, good crack resistance, good wear resistance and good thermal conductivity.
Owner:JIANGXI JINGSHANG IND CO LTD
Production of technetium from a molybdenum metal target
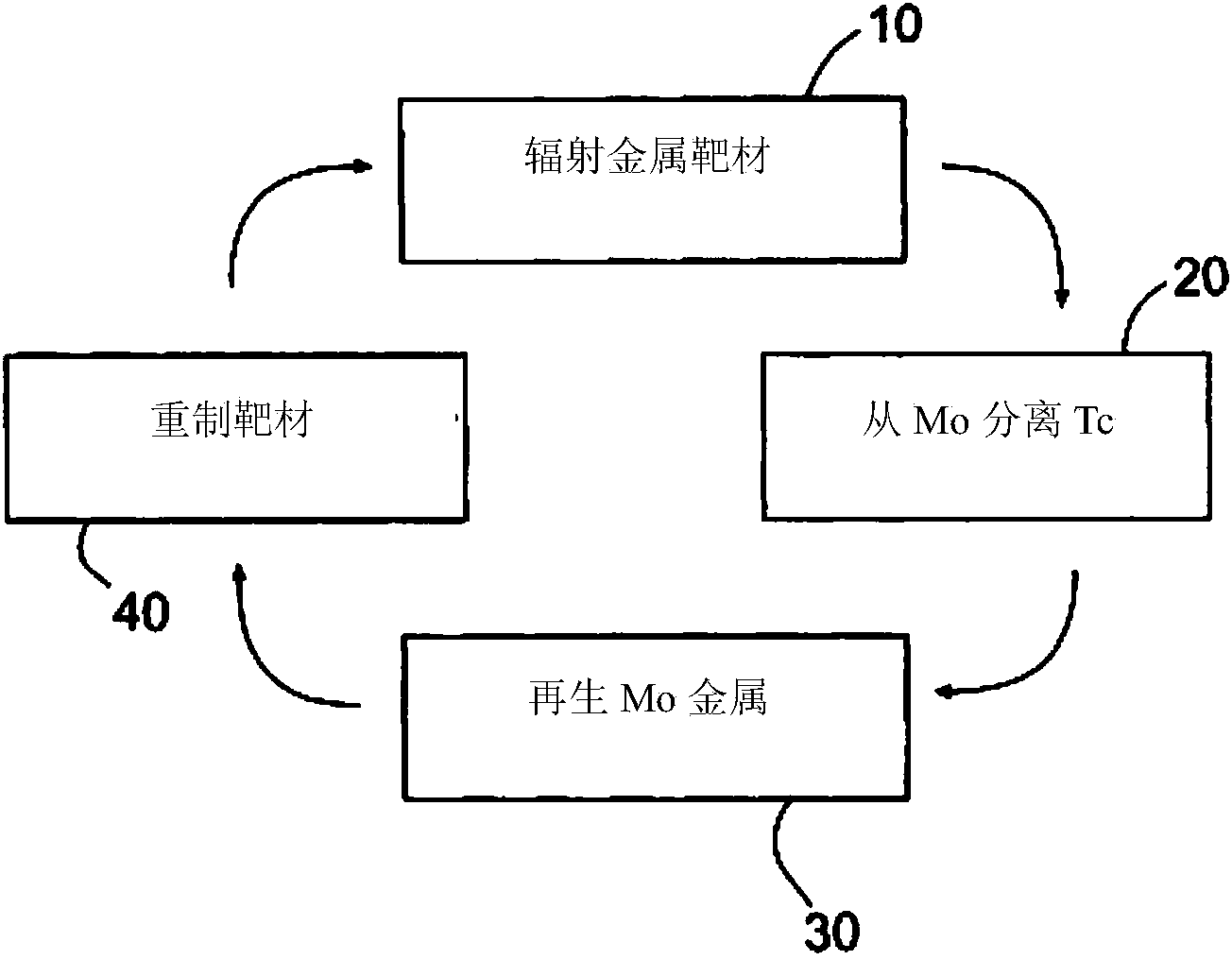
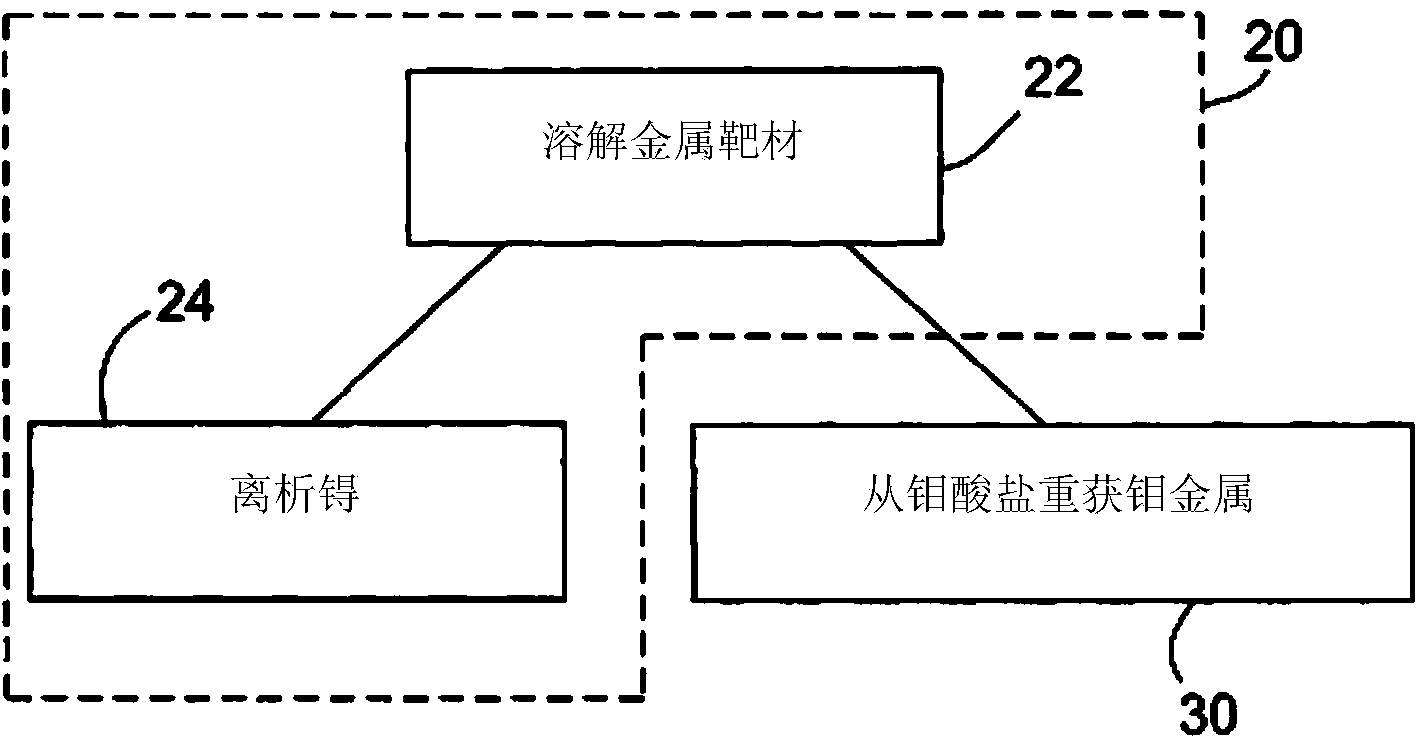
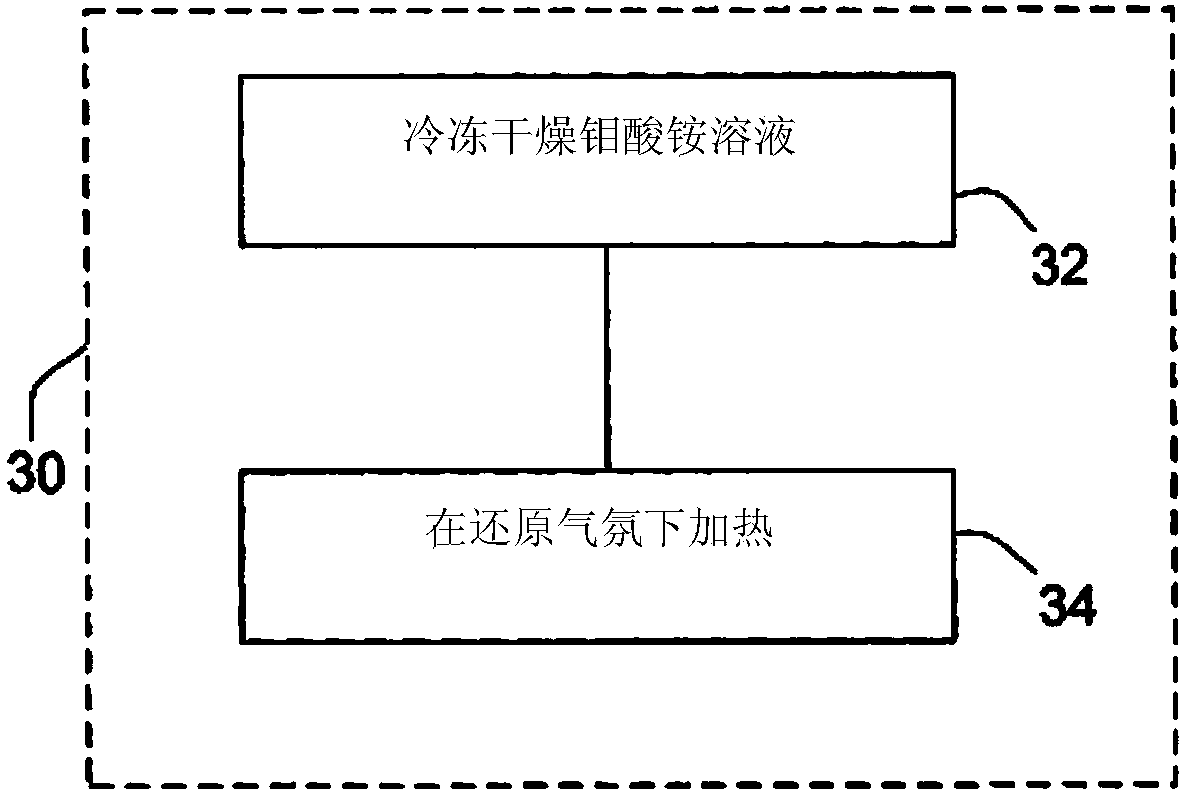
InactiveCN103733270ASpecific isotope recoveryConversion outside reactor/acceleratorsMolybdateTechnetium
Recycling of isotopically enriched molybdenum metal targets that are suitable for the large scale cyclotron production of 99mTc or 94mTc includes the charged particle irradiation of an enriched molybdenum metal target to produce a technetium isotope, separation of the technetium isotope following irradiation of the molybdenum, re-claiming the molybdenum metal and reformation of the molybdenum target for a further irradiation step. This process may then be repeated. Separation of the technetium isotope preferably is achieved by oxidative dissolution of the molybdenum thereby removing it from a target support plate, and forming molybdate and pertechnetate. The technetium isotope is isolated by various means, such as the ABEC process. To reuse the molybdenum, additional steps of isolating the molybdate and reducing it back to molybdenum metal are required. The recovered molybdenum metal may then be reformed as a target for example by pressing or pressing and sintering, followed by bonding to a target support plate.
Owner:阿尔伯塔大学董事会
High-technetium acid radical adsorbent as well as synthesis method and application thereof in radioactive wastewater treatment
ActiveCN106732481AImprove adsorption capacityStable structureOther chemical processesRadioactive contaminantsOrganic solventTechnetium
The invention discloses a high-technetium acid radical adsorbent as well as a synthesis method and application thereof in radioactive wastewater treatment. The synthesis method comprises the following steps: by taking silver nitrate and tert[4-(1-imidazolyl) phenyl] methane as a raw material and an organic solvent and water as mediums, performing hydrothermal reaction; after the reaction is completed, washing, filtering a reaction liquid, and drying obtained filter cakes, so as to obtain the high-technetium acid radical adsorbent. The high-technetium acid radical adsorbent disclosed by the invention is of a porous three-dimensional unlimited extension structure, and high-technetium acid radicals can be effectively exchanged with free nitrate inside pores, therefore, the radioactive wastewater can be efficiently treated.
Owner:SUZHOU UNIV
Low-temperature solidification of radioactive and hazardous wastes
Treatment of a radioactive waste stream is provided by adding sodium hydroxide (NaOH) and / or potassium hydroxide (KOH) together with a rapidly dissolving form of silica, e.g., fumed silica or fly ash. Alternatively, the fumed silica can be first dissolved in a NaOH / KOH solution, which is then combined with the waste solution. Adding a binder that can be a mixture of metakaolin (Al2O3.2SiO2), ground blast furnace slag, fly ash, or other additives. Adding an “enhancer” that can be composed of a group of additives that are used to further enhance the immobilization of heavy metals and key radionuclides such as 99Tc and 129I. An additional step can involve simple mixing of the binder with the activator and enhancer, which can occur in the final waste form container, or in a mixing vessel prior to pumping into the final waste form container, depending on the particular application.
Owner:P&T GLOBAL SOLUTIONS
PUREX process for separating technetium
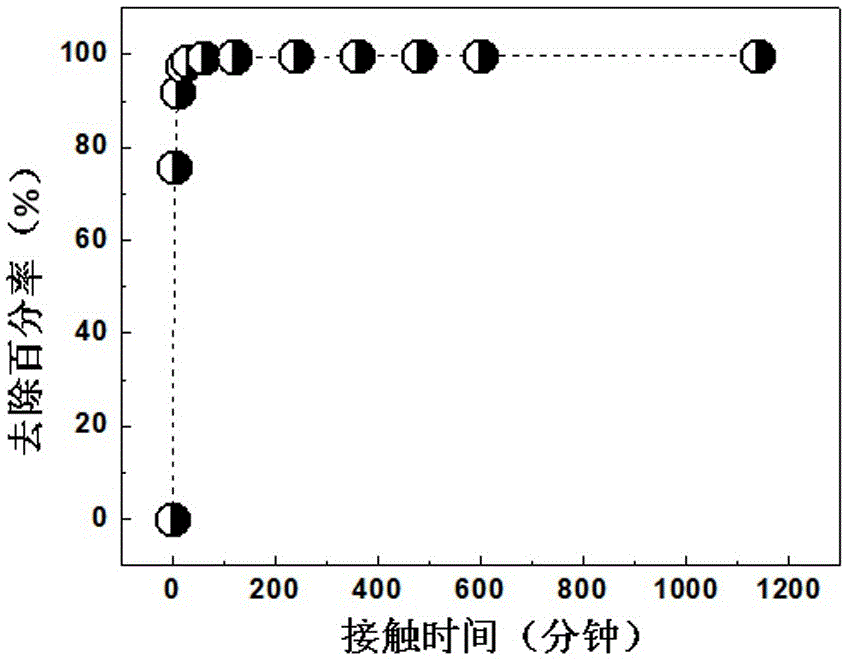
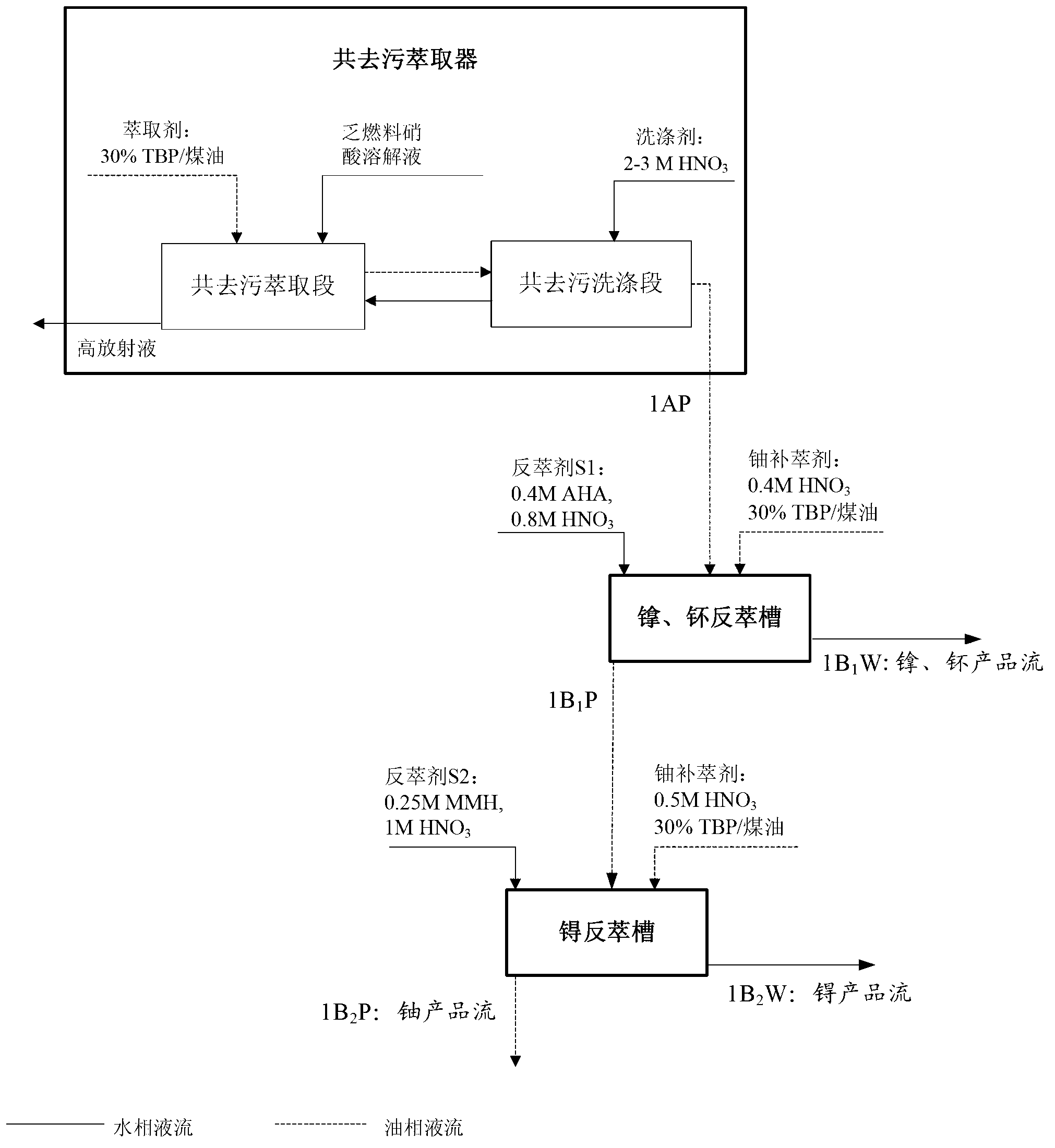
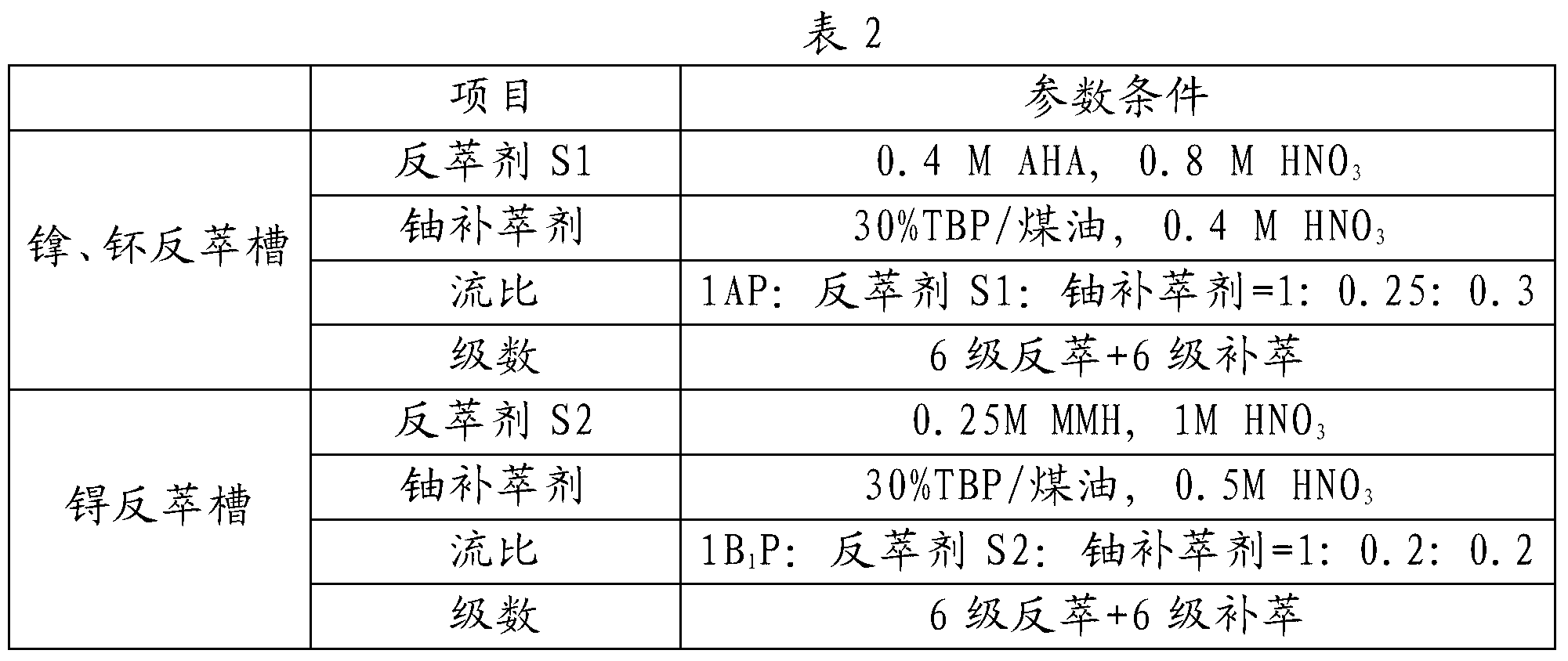
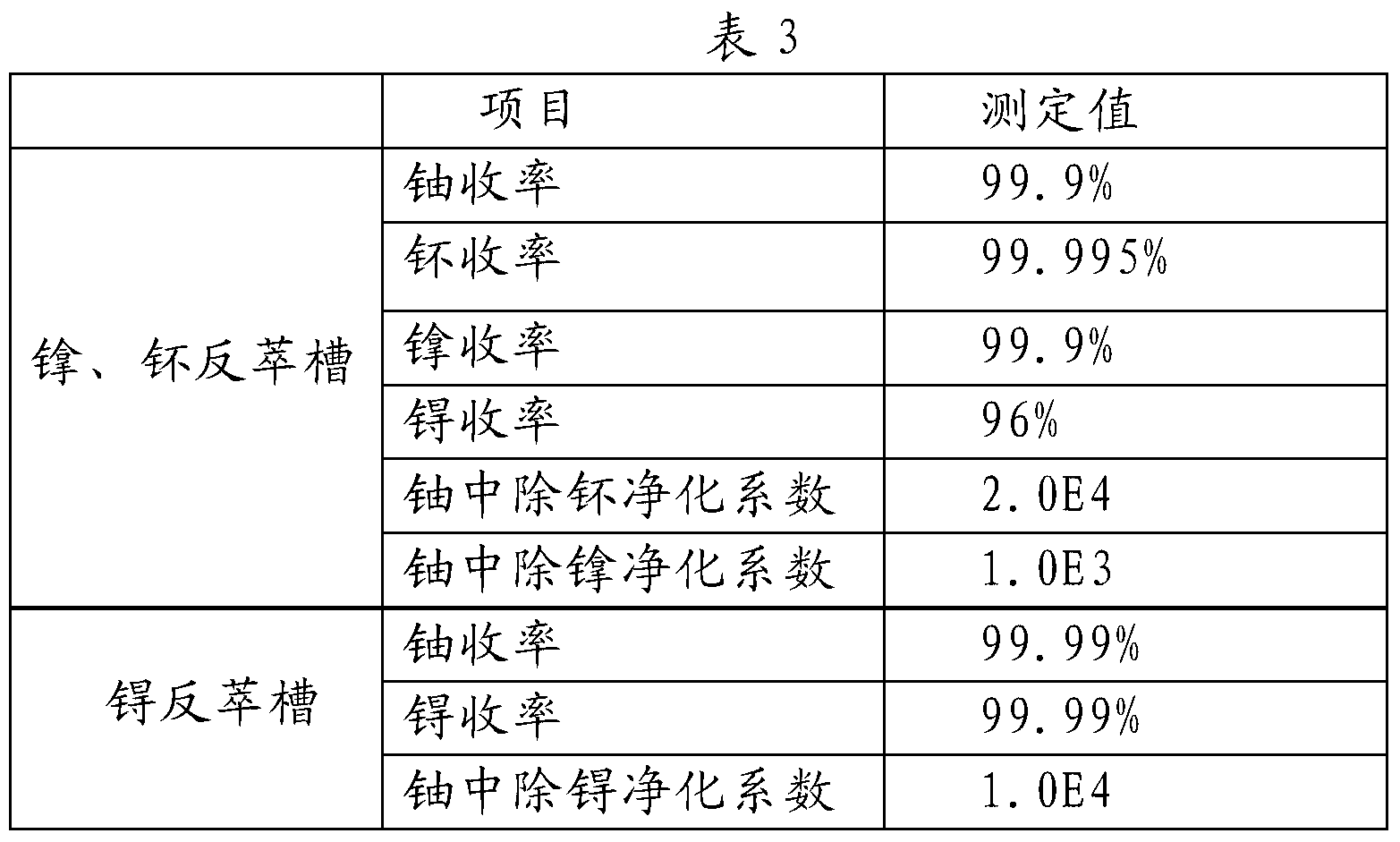
ActiveCN103325431AStep-by-step restore implementationGo simpleRadioactive decontaminationTechnetiumSalt free
The invention relates to a PUREX process for separating technetium. The PUREX process includes the steps: (1) co-decontamination and co-extraction: co-extracting uranium, plutonium, neptunium and the technetium in spent fuel nitric acid solution into an organic phase and washing co-extraction liquid; (2) plutonium and neptunium reverse extraction: reversely extracting the plutonium and the neptunium in the co-extraction liquid into a water phase by reverse extraction agents S1 containing AHA and then adding uranium supplement extraction agents for supplement extraction to obtain the water phase containing the plutonium and the neptunium and an oil phase containing the uranium and the technetium; (3) technetium reduction and reverse extraction: reducing and reversely extracting the technetium in the oil phase containing the uranium and the technetium into the water phase by reverse extraction agents S2 containing reducing agents and then adding uranium supplement extraction agents for supplement extraction to obtain a water phase containing the technetium and an oil phase containing the uranium, wherein the oil phase containing the uranium enters a subsequent uranium purification process. The neptunium, the plutonium and the technetium are reduced step by step through a step-by-step reduction method, the technetium can be separated out, the trend of elements is simpler and more uniform, all the reducing agents are salt-free reagents, remaining reagents are easily damaged, and the subsequent process is less affected.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
Technetium-dipyridine complexes, and methods of use thereof
One aspect of the invention relates to novel complexes of technetium (Tc) with various heteroaromatic ligands, e.g., pyridyl and imidazolyl ligands, and their use in radiopharmaceuticals for a variety of clinical diagnostic and therapeutic applications. Another aspect of the invention relates to novel pyridyl ligands that form a portion of the aforementioned complexes. Methods for the preparation of the technetium complexes are also described. Another aspect of the invention relates to novel pyridyl ligands based on derivatized lysine, alanine and bis-amino acids for conjugation to small peptides by solid phase synthetic methods. Additionally, the invention relates to methods for imaging regions of a mammal using the complexes of the invention.
Owner:BIOSTREAM
Electrode composition for removing nitrogen oxides, and apparatus and method thereof
The invention relates to an electrode composition for removing nitrogen oxides, and an apparatus and a method thereof. The electrode composition for removing nitrogen oxides comprises a catalysis material and an adsorption material, wherein the adsorption material is a perovskite material with the chemical formula of AaBbO3-delta, wherein a is greater than 0.9 and not greater than 1.2, b is greater than 0.9 and not greater than 1.2, and delta is greater than -0.5 and lower than 0.5; A comprises a first element and a selectable second element, the first element is selected from calcium, strontium, barium, lithium, sodium, potassium, rubidium and an arbitrary combination thereof, and the second element is selected from yttrium, bismuth, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium and an arbitrary combination thereof; and B is selected from silver, gold, cadmium, cerium, cobalt, chromium, copper, dysprosium, erbium, europium, iron, gallium, gadolinium, hafnium, holmium, indium, iridium, lanthanum, lutetium, manganese, molybdenum, niobium, neodymium, nickel, osmium, palladium, promethium, praseodymium, platinum, rhenium, rhodium, ruthenium, antimony, scandium, samarium, tin, tantalum, terbium, technetium, titanium, thulium, vanadium, tungsten, yttrium, ytterbium, zinc, zirconium, and an arbitrary combination thereof. The invention also relates to a corresponding apparatus and a corresponding method.
Owner:GENERAL ELECTRIC CO
Developing apparatus and electrostatic recording apparatus using the same
InactiveUS20050123312A1Low costIncrease printing speedElectrographic process apparatusTechnetiumEngineering
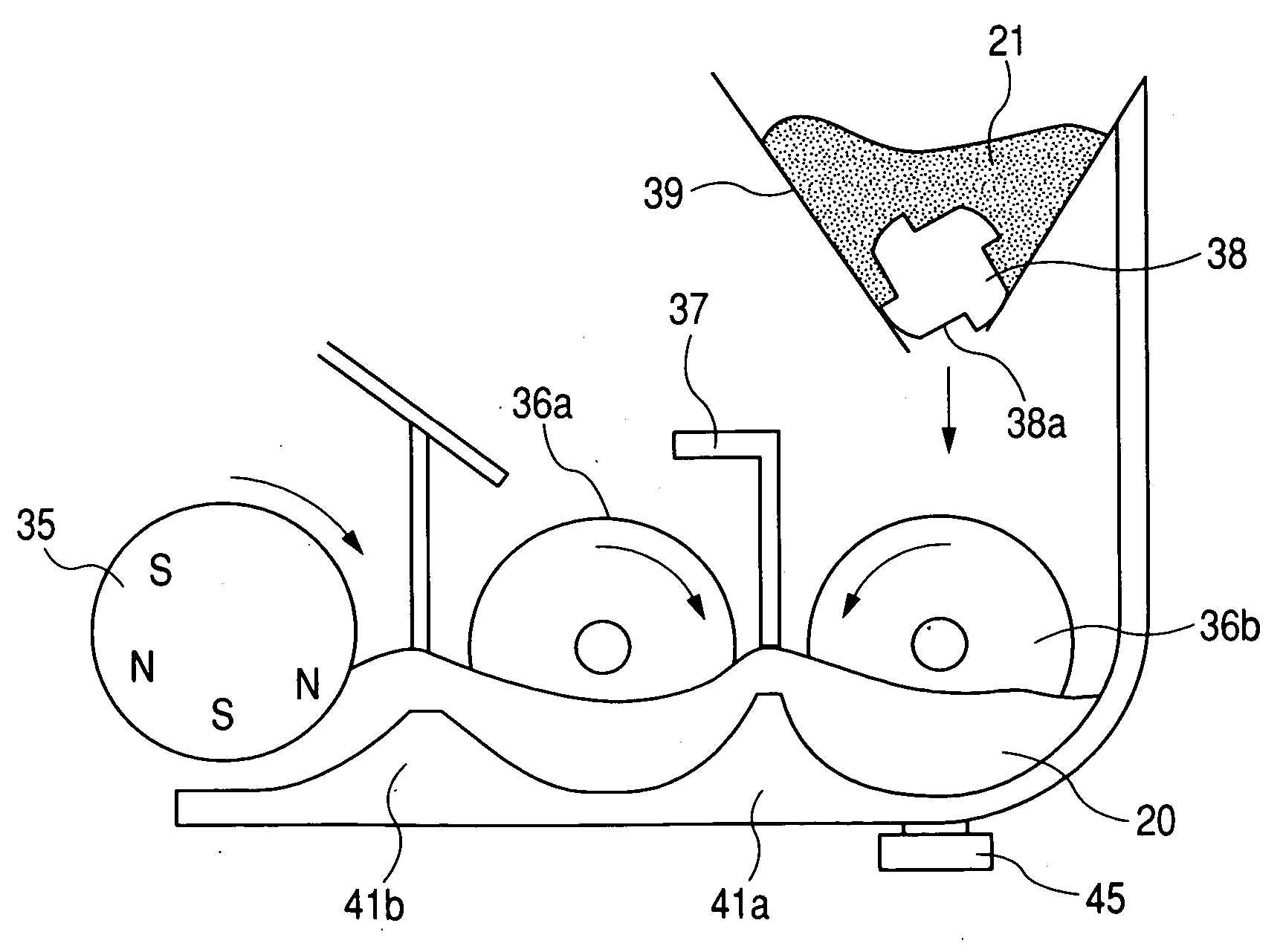
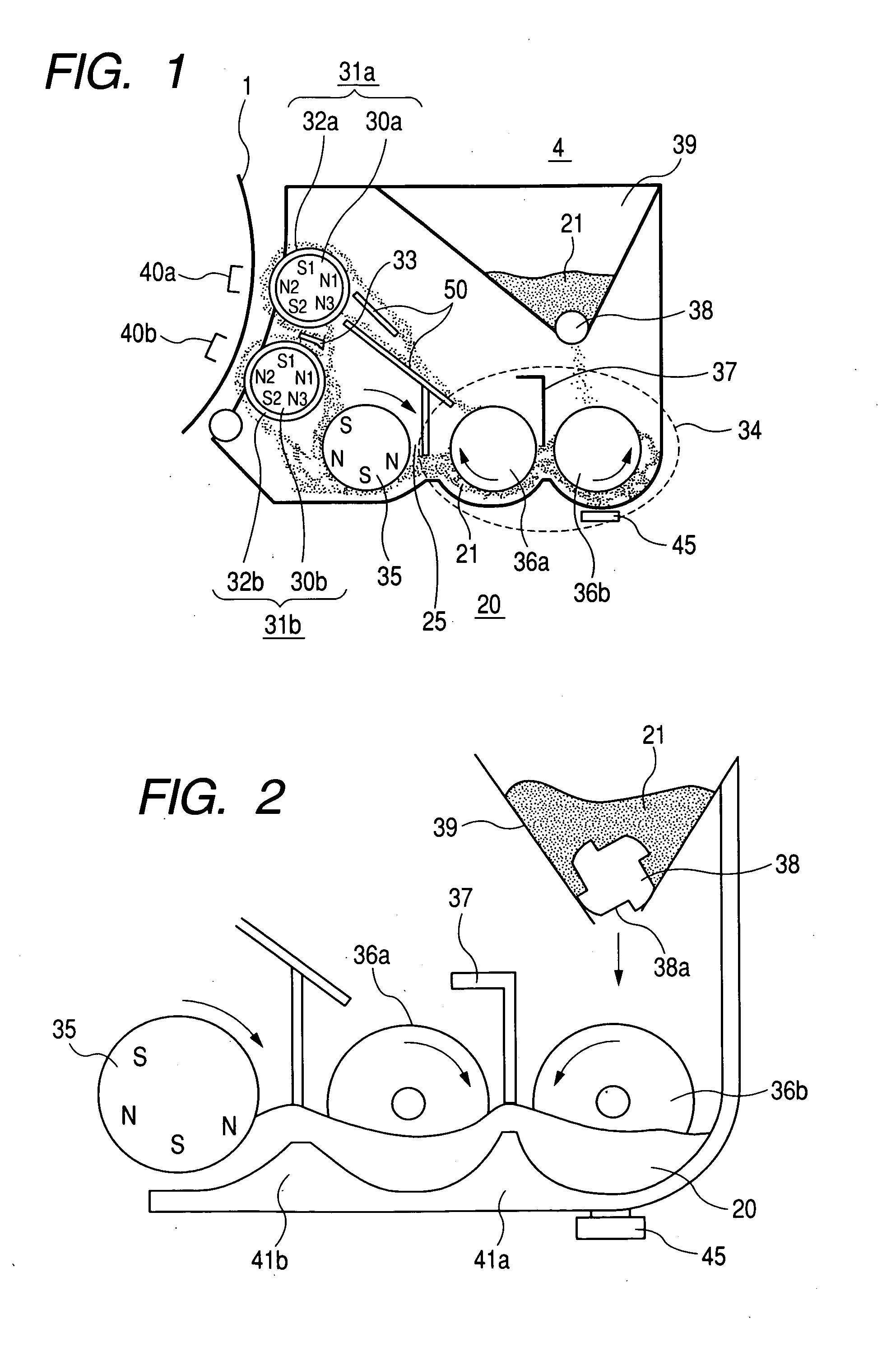
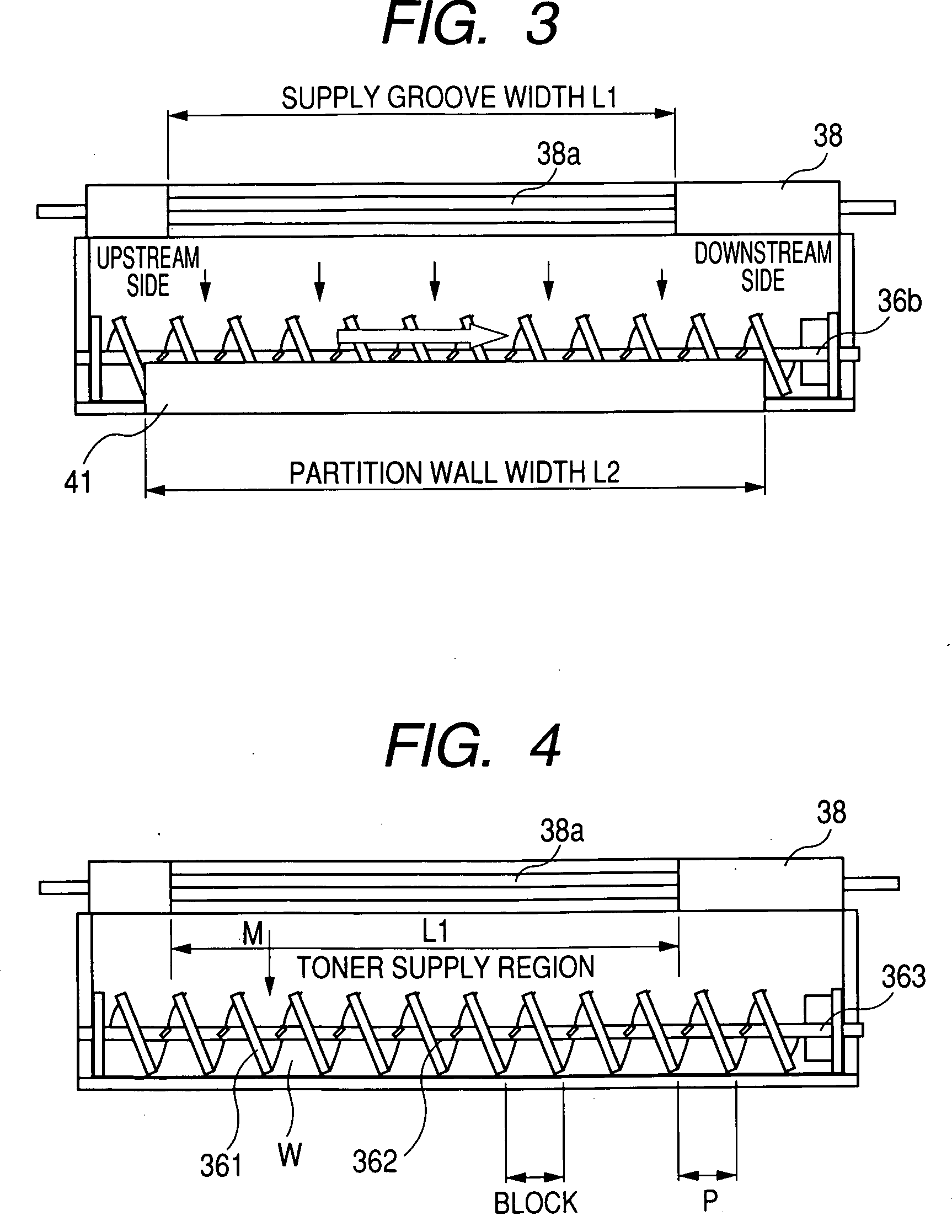
A developing apparatus characterized in being constituted to satisfy Equation (1) shown below when an amount of a developer carried by 1 pitch of a carrying member of an auger or the like is designated by notation W(g), a toner amount supplied by 1 pitch during a time period in which the developer carried by 1 pitch passes a supply region of a toner supply roller is designated by notation M(g), a toner concentration of the developer detected by a toner concentration sensor is designated by notation Tc (%), and a mixing limit toner concentration is designated by notation Tmax (%). (W×(Tc / 100)+M) / (W+M)≦Tmax / 100 (1)
Owner:RICOH PRINTING SYST
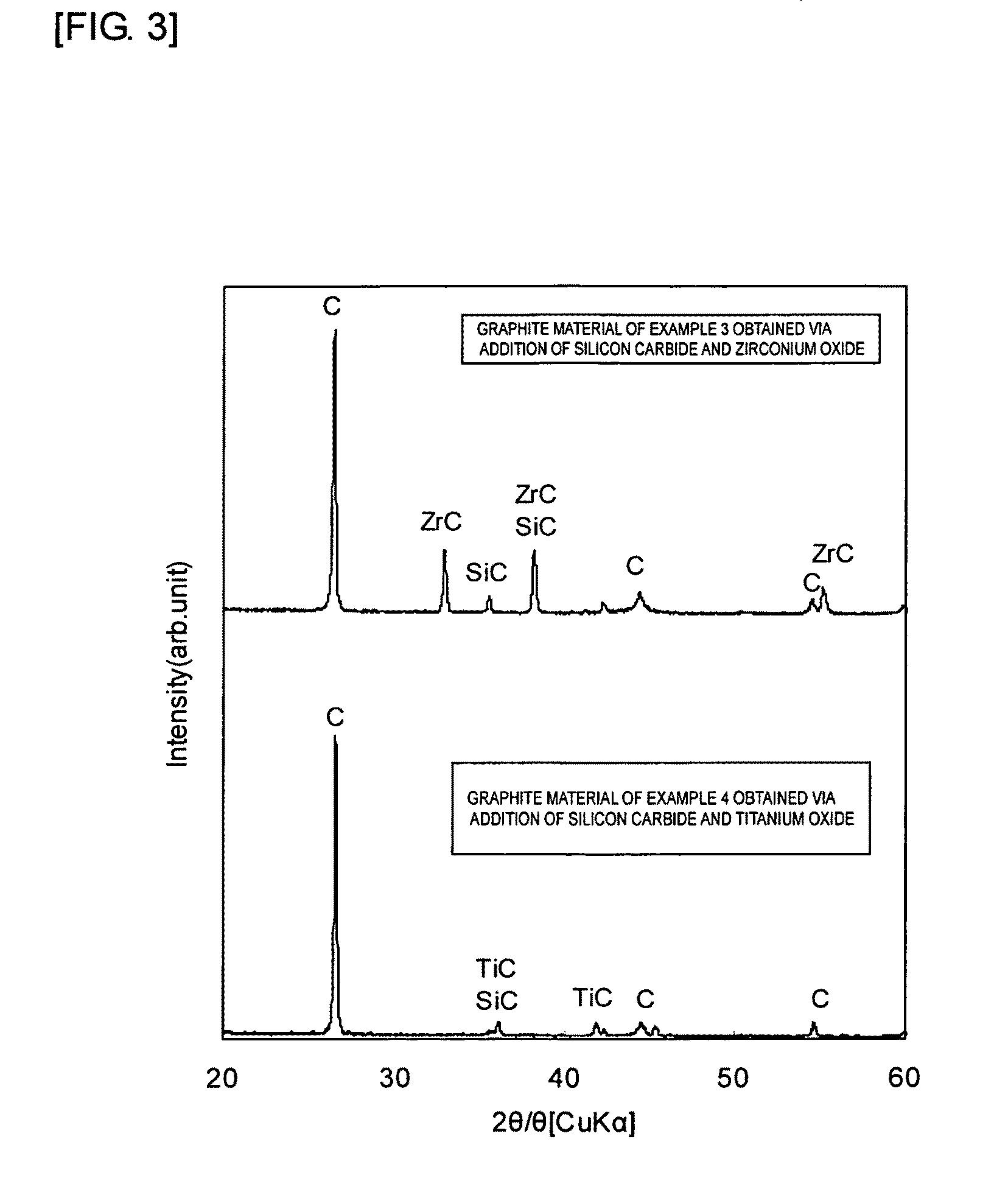
Graphite material method for manufacturing the same
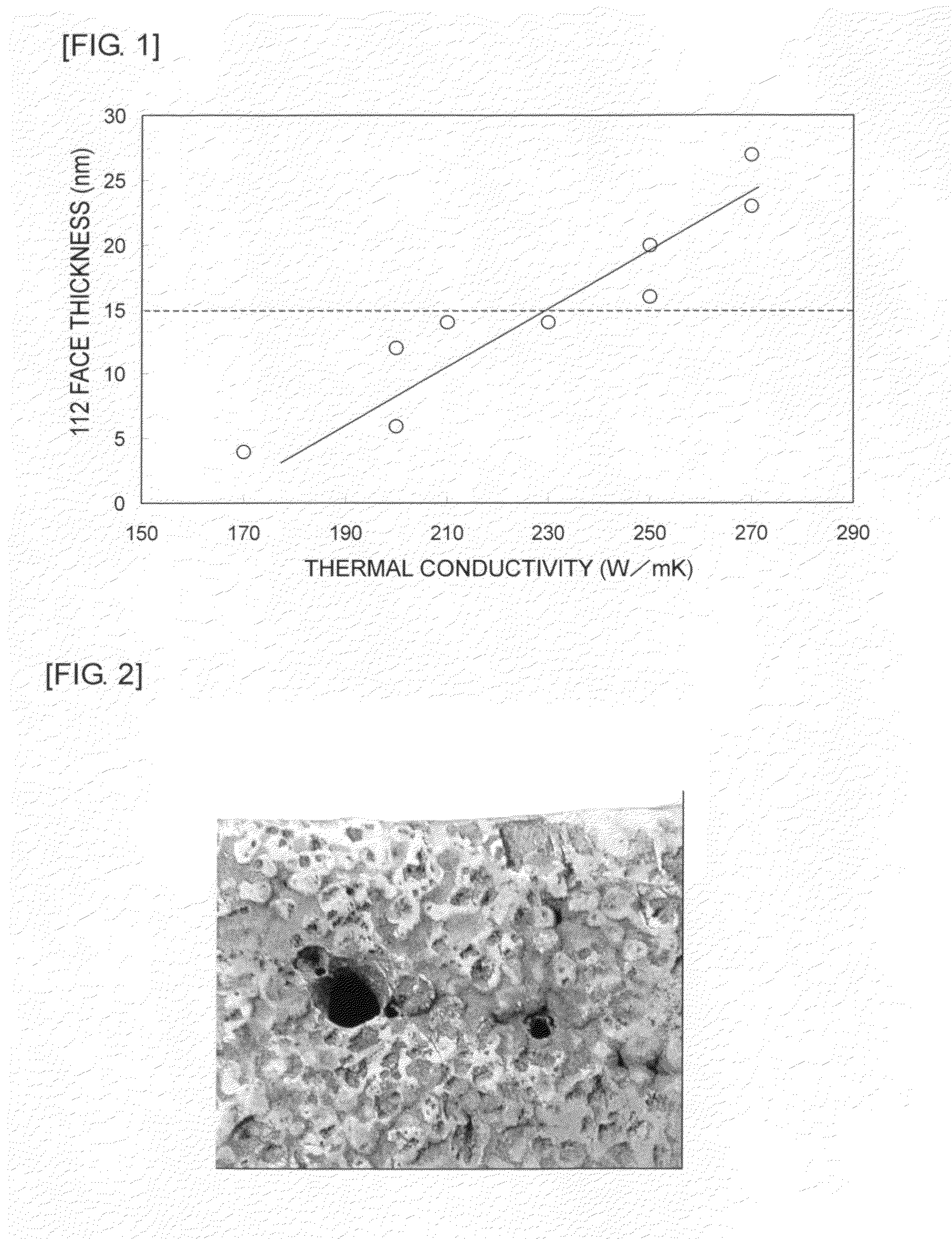
InactiveUS20100009193A1Improve thermal conductivityEffective coolingGraphiteSemiconductor/solid-state device detailsNiobiumX-ray
Owner:TOYO TANSO KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com