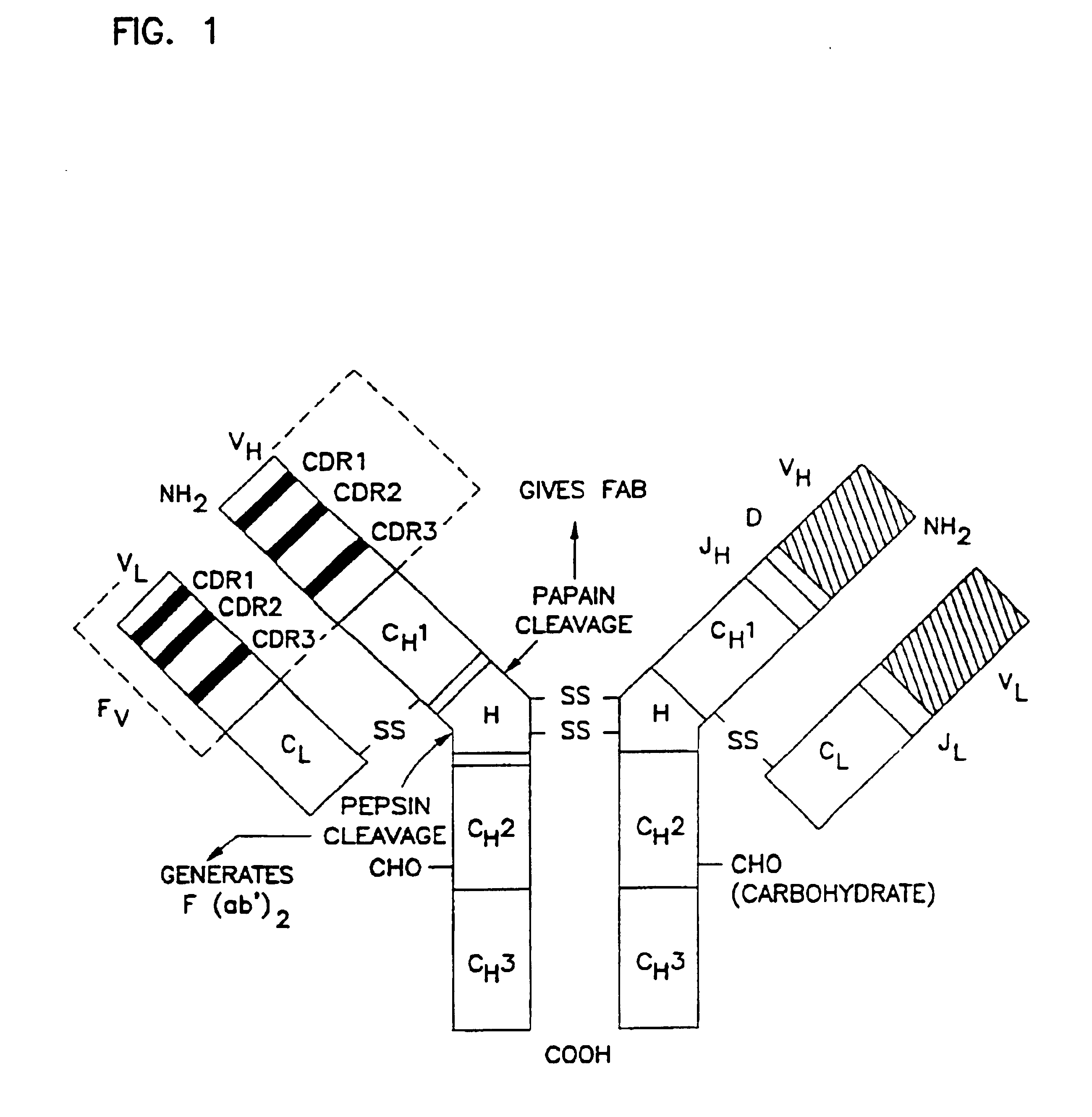
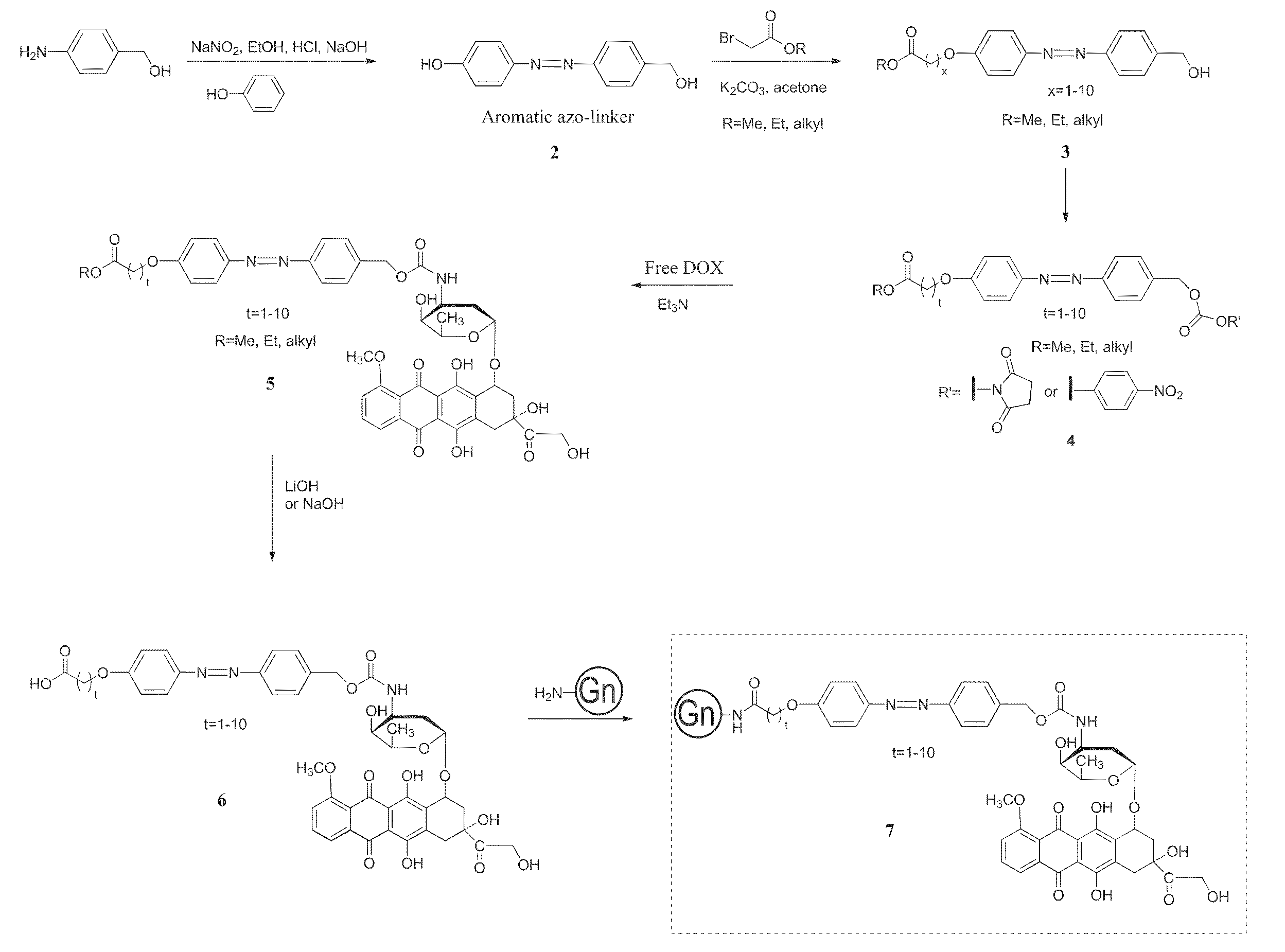
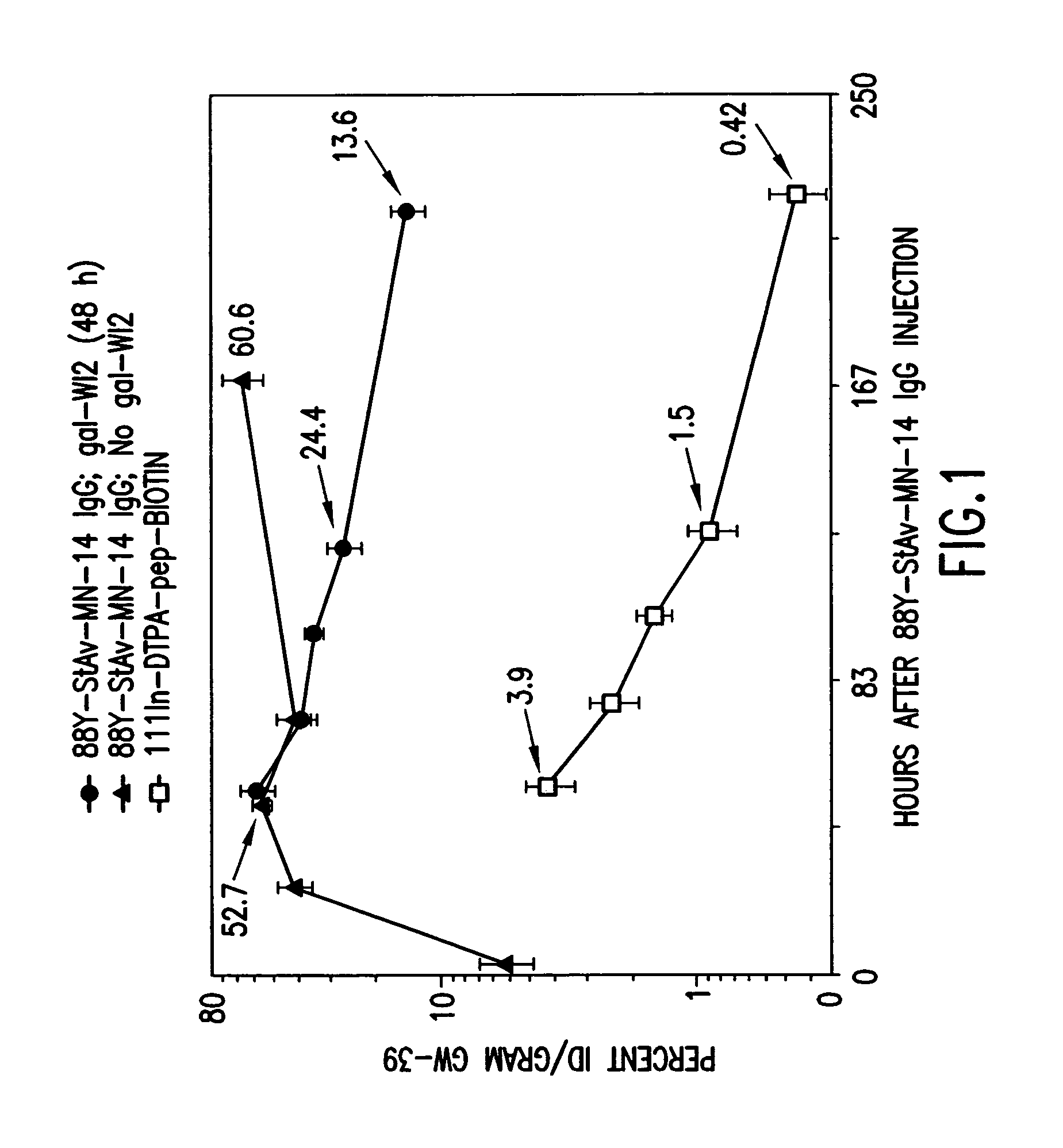
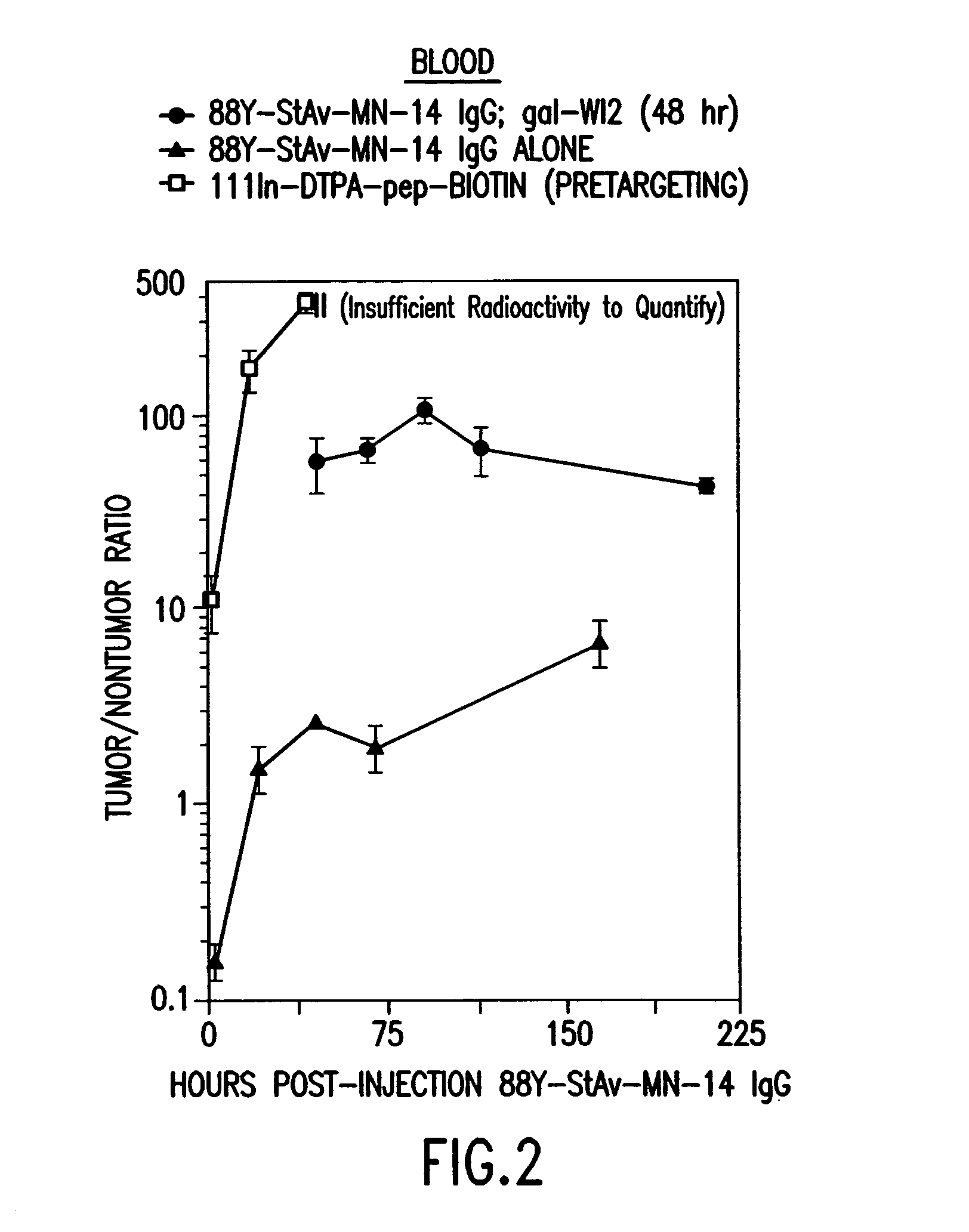
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4747results about "Radioactive preparation carriers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug administration method
InactiveUS7427607B2Reduce weightPrevention of the adhesion of an organBiocidePowder deliveryBiopolymerDrug administration
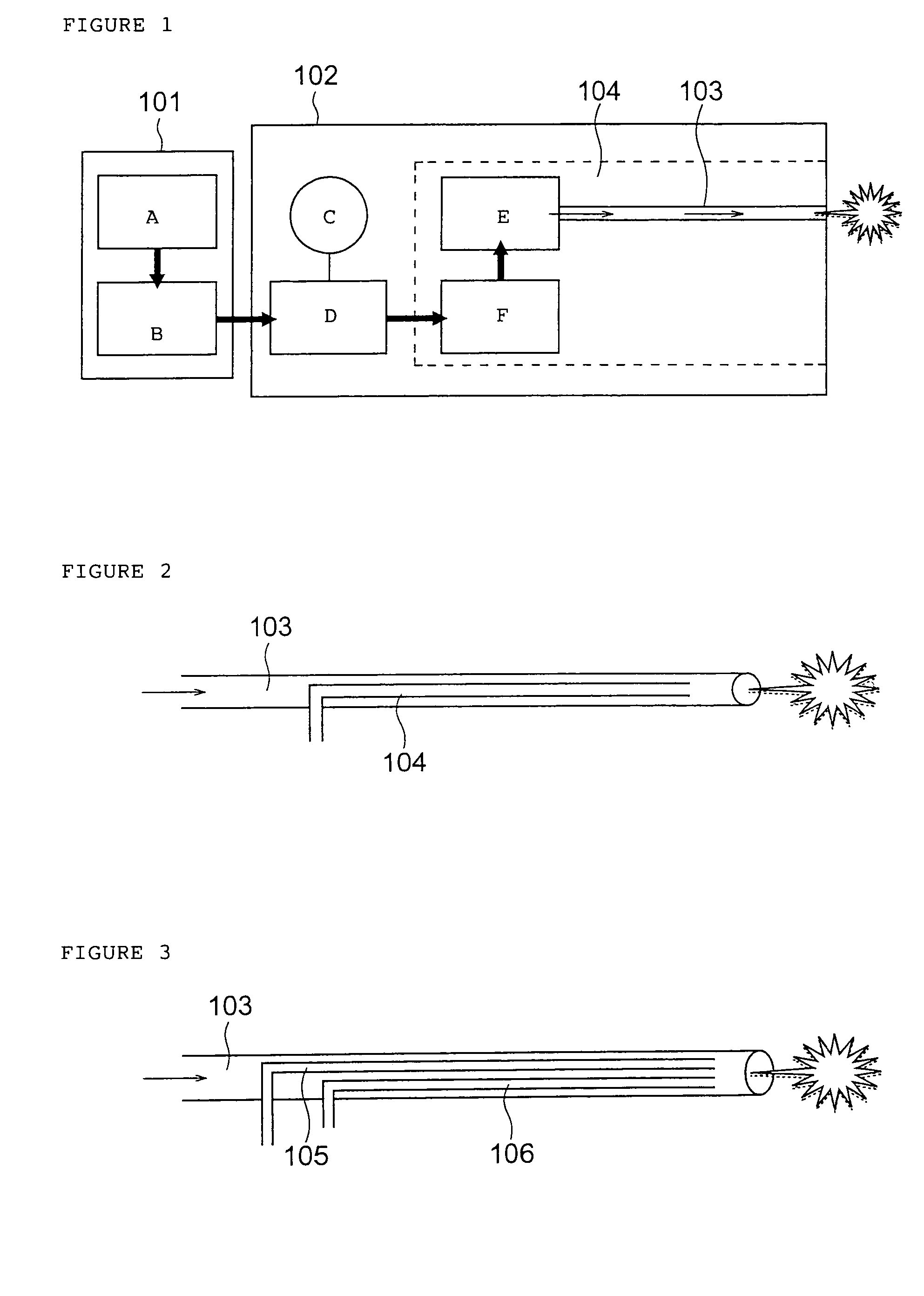
A method of administering a drug whereby a fine drug powder can be accurately administered to a target site (in particular, a target site in the body cavity) via fluidization and spraying with a gas by using a micro tube. Concerning the administration mode, in particular, the drug alone or a biopolymer is administered or the biopolymer is employed as a carrier in the above method. More specifically speaking, a method of administering a fine drug powder which comprises finely milling one or more types fine particles of the drug and / or the biopolymer, blending them each other, fluidizing the blend with a gas, then transporting the fluidized matter in a micro tube by the gas stream and spraying the fine drug powder from the tip of the micro tube toward the target site. Further, an administration method which comprises concentrically providing a capillary tube in the micro tube, supplying an aqueous solution of the drug and / or the biopolymer from the capillary tube into the gas stream and then mixing it with other fine particles of the drug and / or the biopolymer under transportation by the gas.
Owner:NEXT21 KK
Compositions for targeting the vasculature of solid tumors
InactiveUS6051230AEffectively compete for bindingReduce TEC-4 or TEC-11 bindingHybrid immunoglobulinsPeptide/protein ingredientsAntigenCell Surface Antigens
The present invention relates generally to methods and compositions for targeting the vasculature of solid tumors using immunological- and growth factor-based reagents. In particular aspects, antibodies carrying diagnostic or therapeutic agents are targeted to the vasculature of solid tumor masses through recognition of tumor vasculature-associated antigens, such as, for example, through endoglin binding, or through the specific induction of endothelial cell surface antigens on vascular endothelial cells in solid tumors.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Intraoperative, intravascular, and endoscopic tumor and lesion detection, biopsy and therapy
InactiveUS6096289ADiscriminationImprove discriminationUltrasonic/sonic/infrasonic diagnosticsSurgeryNon malignantAntibody fragments
Methods are provided for close-range intraoperative, endoscopic and intravascular deflection and treatment of lesions, including tumors and non-malignant lesions. The methods use antibody fragments or subfragments labeled with isotopic and non-isotopic agents. Also provided are methods for detection and treatment of lesions with photodynamic agents and methods of treating lesions with a protein conjugated to an agent capable of being activated to emit Auger electron or other ionizing radiation. Compositions and kits useful in the above methods are also provided.
Owner:IMMUNOMEDICS INC
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Owner:GLAXO SMITHKLINE LLC
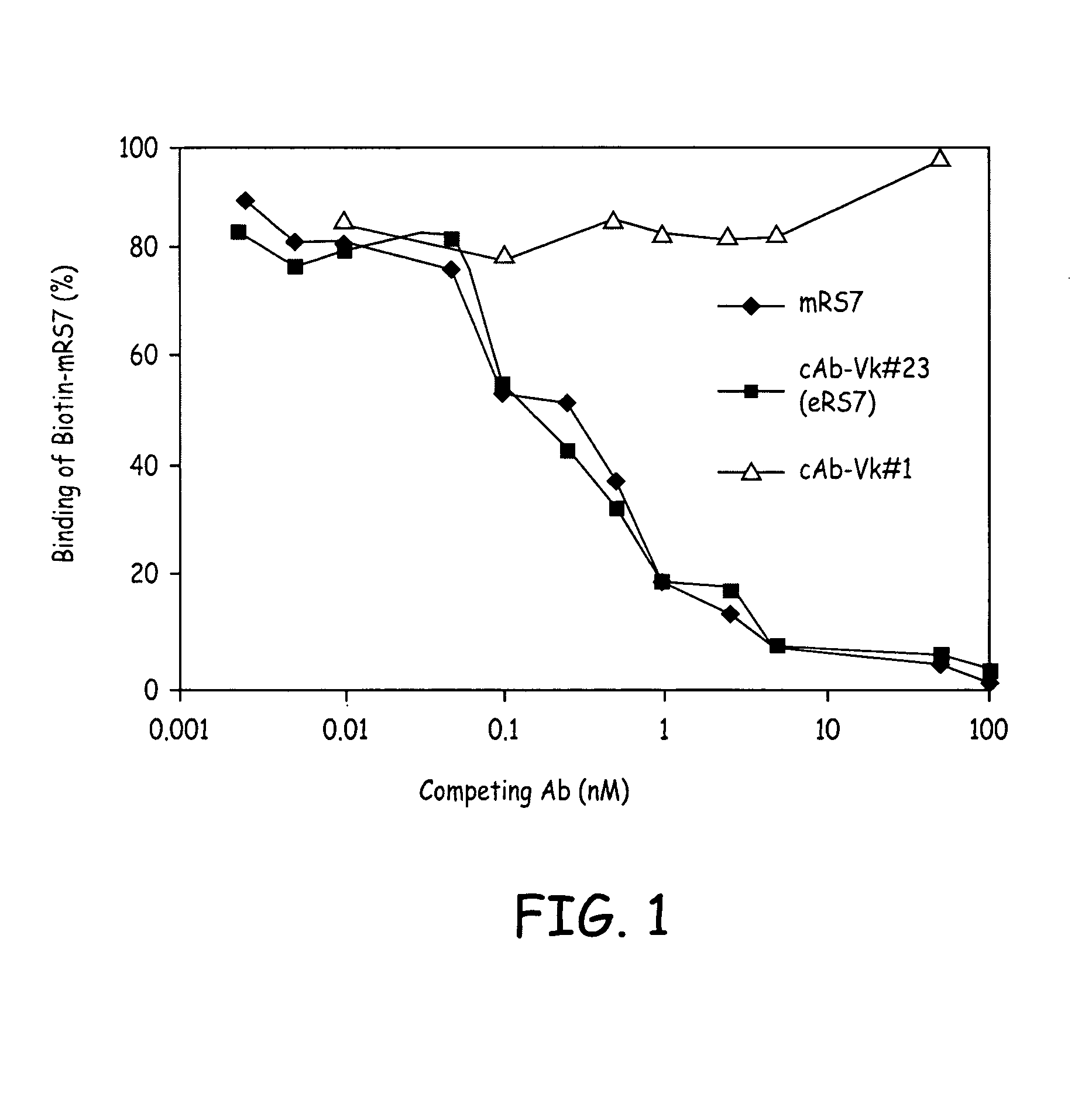
RS7 antibodies
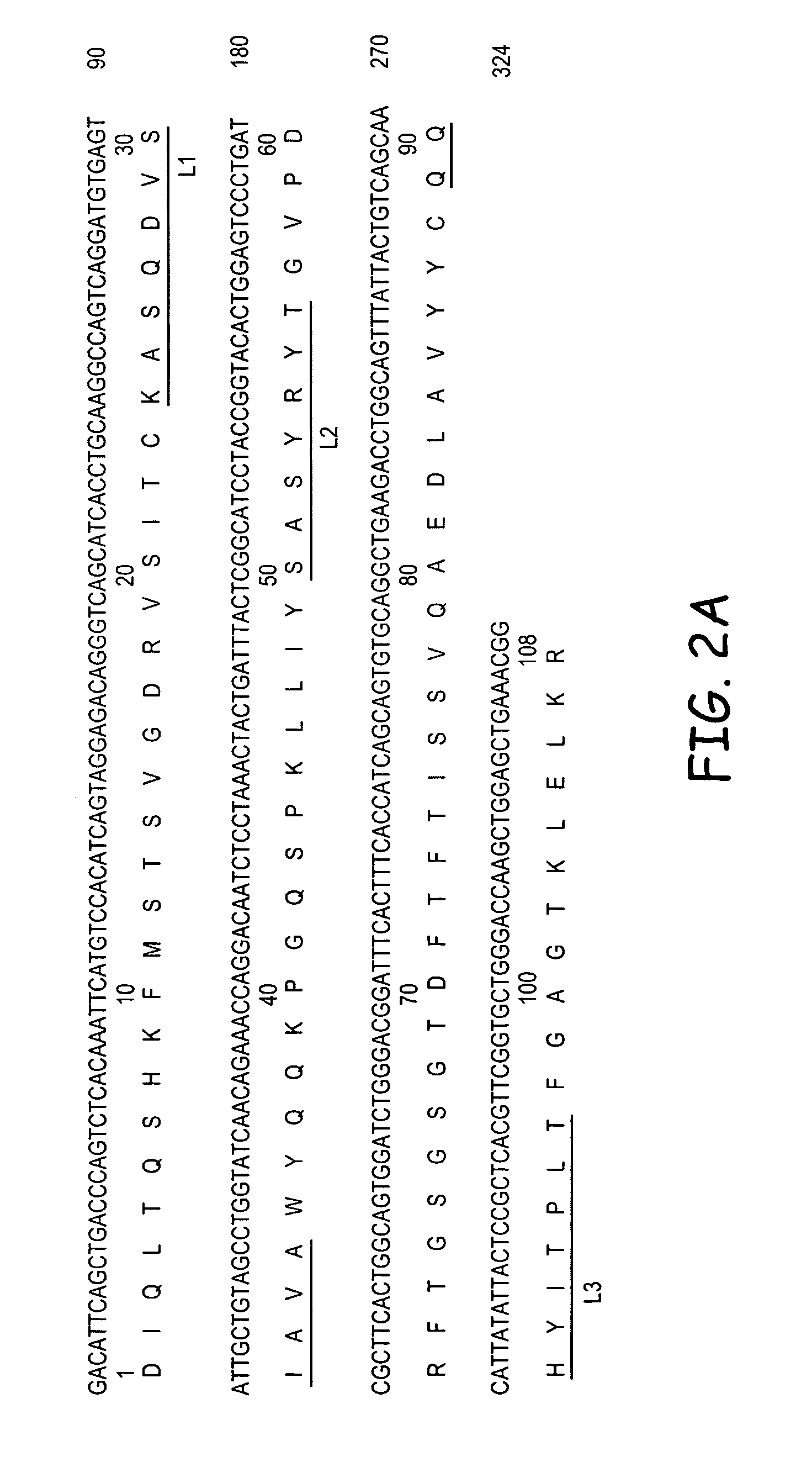
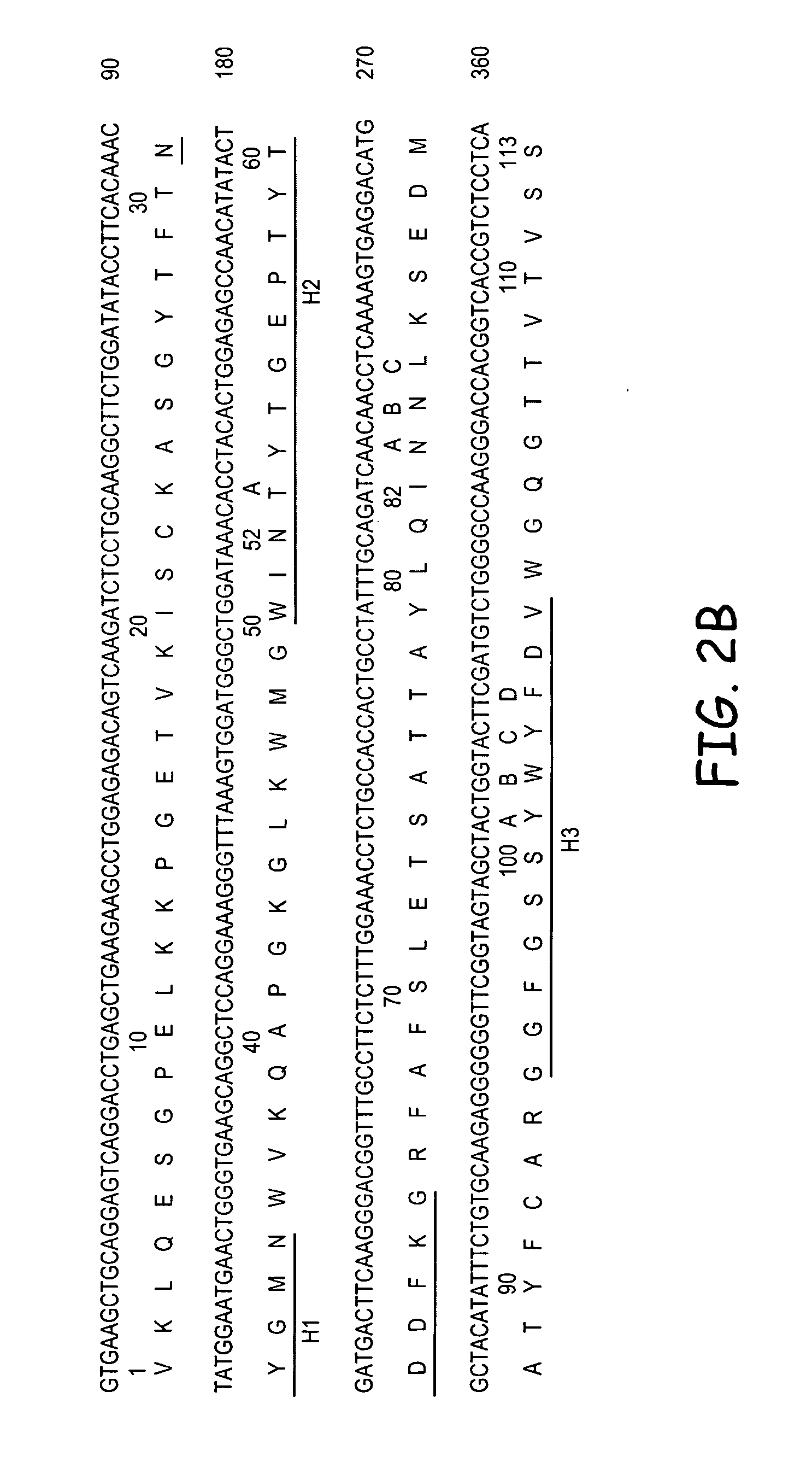
InactiveUS7238785B2Diagnosing and treating malignancyRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopeBinding site
Owner:IMMUNOMEDICS INC
Intrathecal administration of rituximab for treatment of central nervous system lymphomas
InactiveUS20020009444A1Prevent intermolecular disulfide formationPromote recoveryBiocidePeptide/protein ingredientsMeningesImmunocompromised patient
This invention describes methods of using anti-B cell antibodies, preferably anti-CD20 antibodies, and most preferably Rituximab, to treat B cell lymphomas of the brain, especially primary central nervous system lymphomas (PCNSLs), and to prevent meningeal relapse. The antibodies can be administered intrathecally alone, or in combination with other chemotherapeutics, such as methotrexate, or other anti-B cell antibodies to treat PCNSL in both immunocompromised and non-immunocompromised patients. These antibodies can also be used to diagnose patients with CNS lymphoma, especially in immunocompromised patients.
Owner:BIOGEN INC
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Methods for the treatment of lymphoma by administration of a B cell-specific antibody are described. The invention encompasses providing to a patient both unlabeled antibodies and antibodies labeled with a radioisotope. A principal advantage of the method is that tumor responses can be obtained in a radiometric dose range that does not require hematopoietic stem cell replacement as an adjunct therapy also described is a composition useful in the treatment of lymphoma.
Owner:RGT UNIV OF MICHIGAN +2
Medical device rapid drug releasing coatings comprising a therapeutic agent and a contrast agent
ActiveUS20080255510A1Improve the immunityReduction tendencyBiocideMedical devicesTherapeutic effectDrug release
The invention relates to a coated medical device for rapid delivery of a therapeutic agent to a tissue in seconds to minutes. The medical device has a layer overlying the exterior surface of the medical device. The layer contains a therapeutic agent, a contrast agent, and an additive.
Owner:LUTONIX INC
Combinations and modes of administration of therapeutic agents and combination therapy
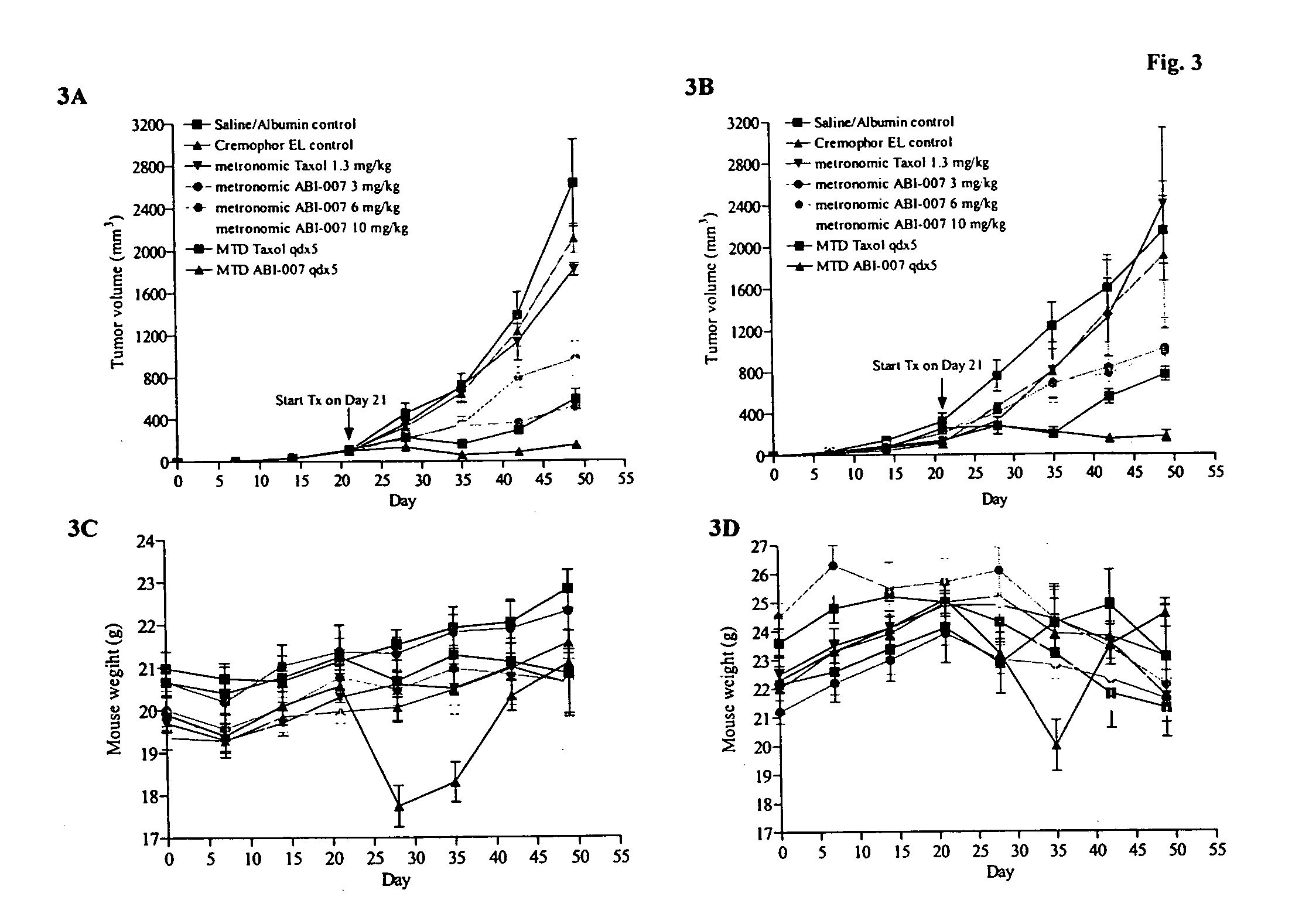
The present invention provides combination therapy methods of treating proliferative diseases (such as cancer) comprising a first therapy comprising administering to an individual an effective amount of a taxane in a nanoparticle composition, and a second therapy which may include, for example, radiation, surgery, administration of chemotherapeutic agents, or combinations thereof. Also provided are methods of administering to an individual a drug taxane in a nanoparticle composition based on a metronomic dosing regime.
Owner:ABRAXIS BIOSCI LLC
Pancreatic cancer associated antigen, antibody thereto, and diagnostic and treatment methods
The present invention is directed to an antigen found on the surface of rat and human pancreatic cancer cells and provides antibodies of high specificity and selectivity to this antigen as well as hybridomas secreting the subject antibodies. Methods for both the diagnosis and treatment of pancreatic cancer are also provided. This tissue marker of pancreatic adenocarcinoma, an approximately 43.5 kD surface membrane protein designated PaCa-Ag1, is completely unexpressed in normal pancreas but abundantly expressed in pancreatic carcinoma cells. Moreover, a soluble form of PaCa-Ag1 exists, having a molecular weight about 36 to about 38 kD, that is readily identified in sera and other body fluids of pancreatic cancer patients, using a subject antibody.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Novel nanoparticles and use thereof
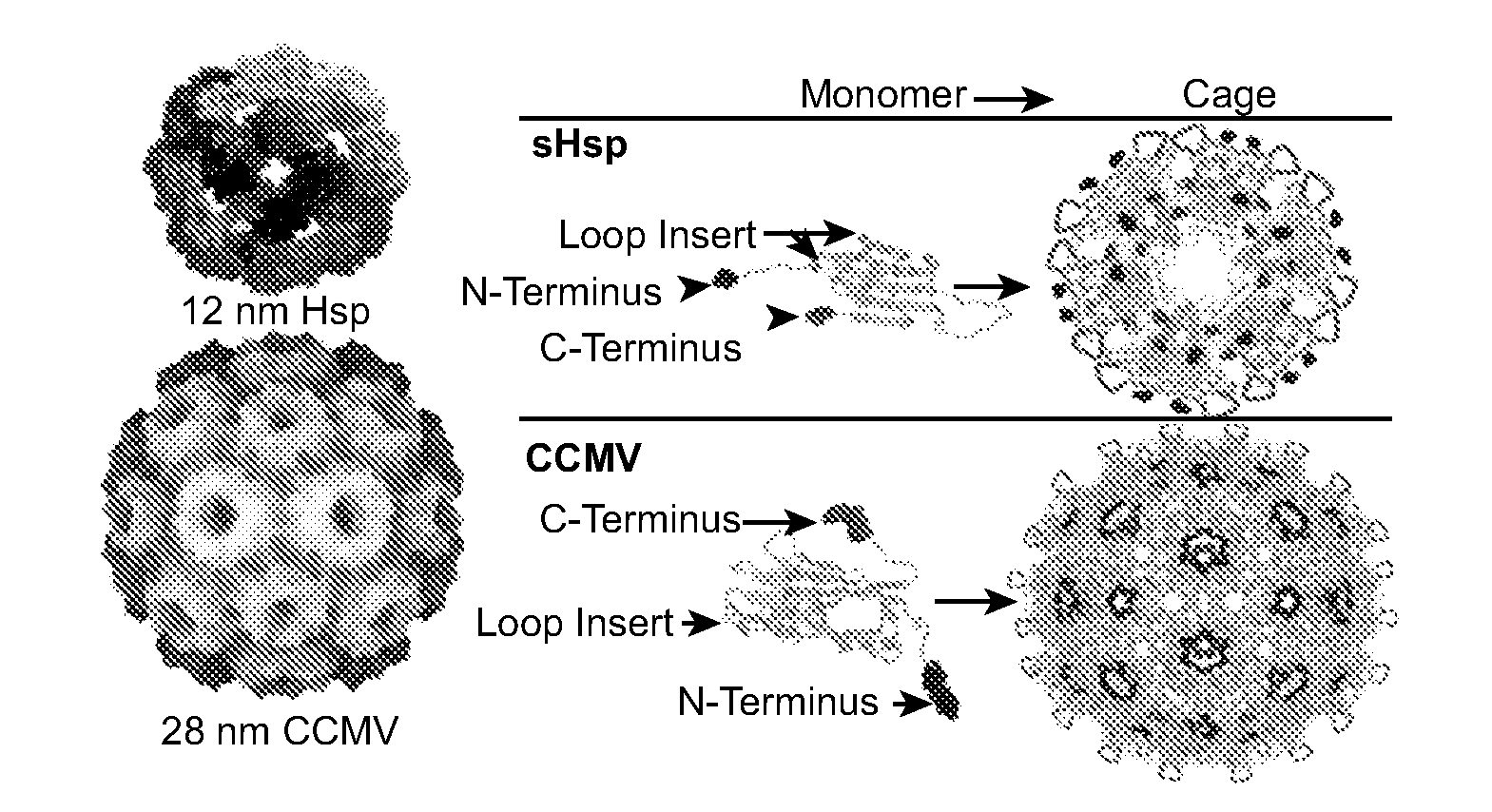
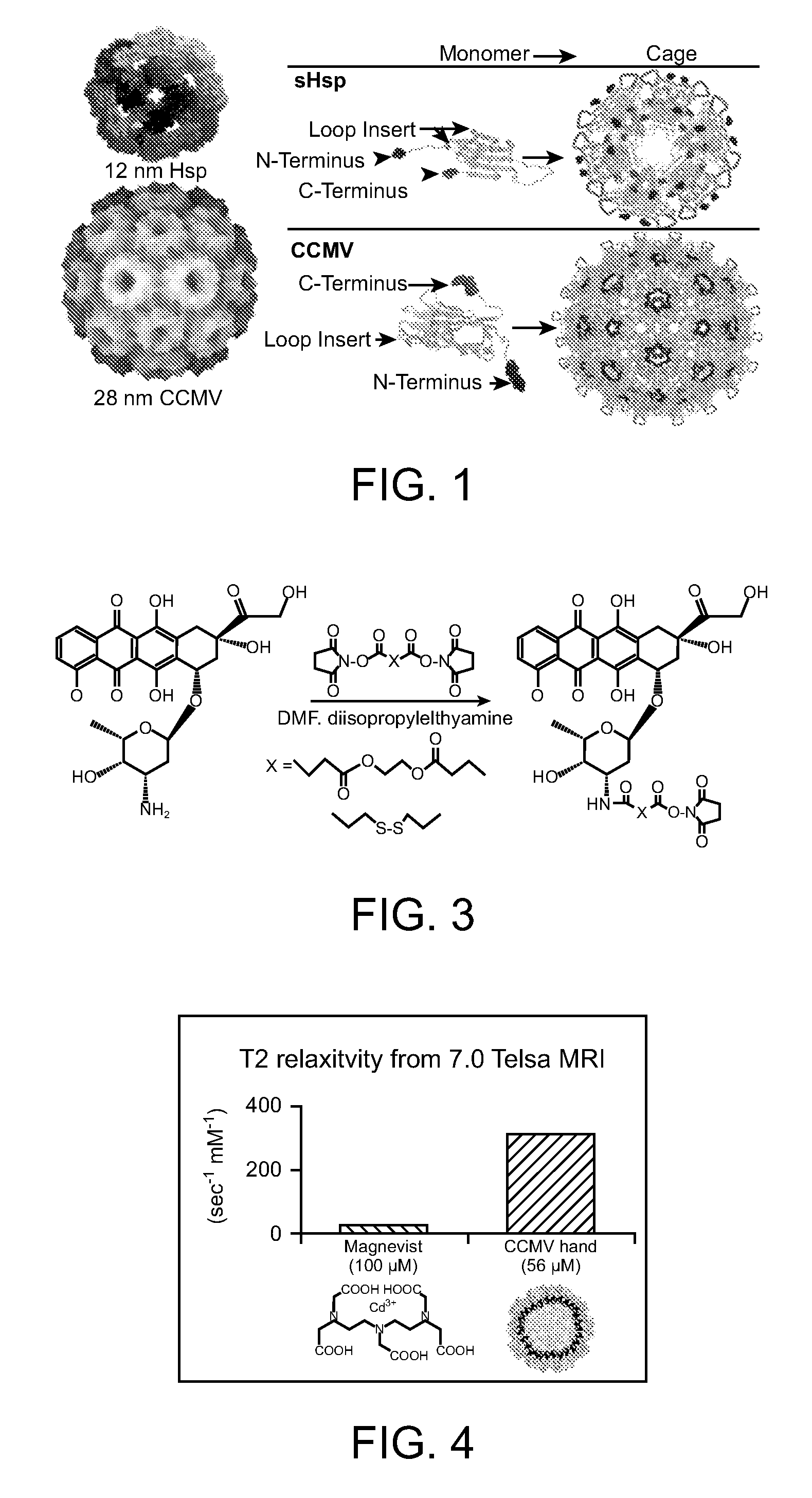
InactiveUS20070258889A1Increase the number ofFacilitate aggregation and crystallizationPowder deliveryLuminescence/biological staining preparationImaging agentProtein cage
The present invention is directed to novel compositions and methods utilizing delivery agents or nanoparticles that include protein cages with modified and / or unmodified subunits and various agents, such as therapeutic and / or imaging agents located on the interior and / or exterior surface of the protein cages.
Owner:MONTANA STATE UNIVERSITY
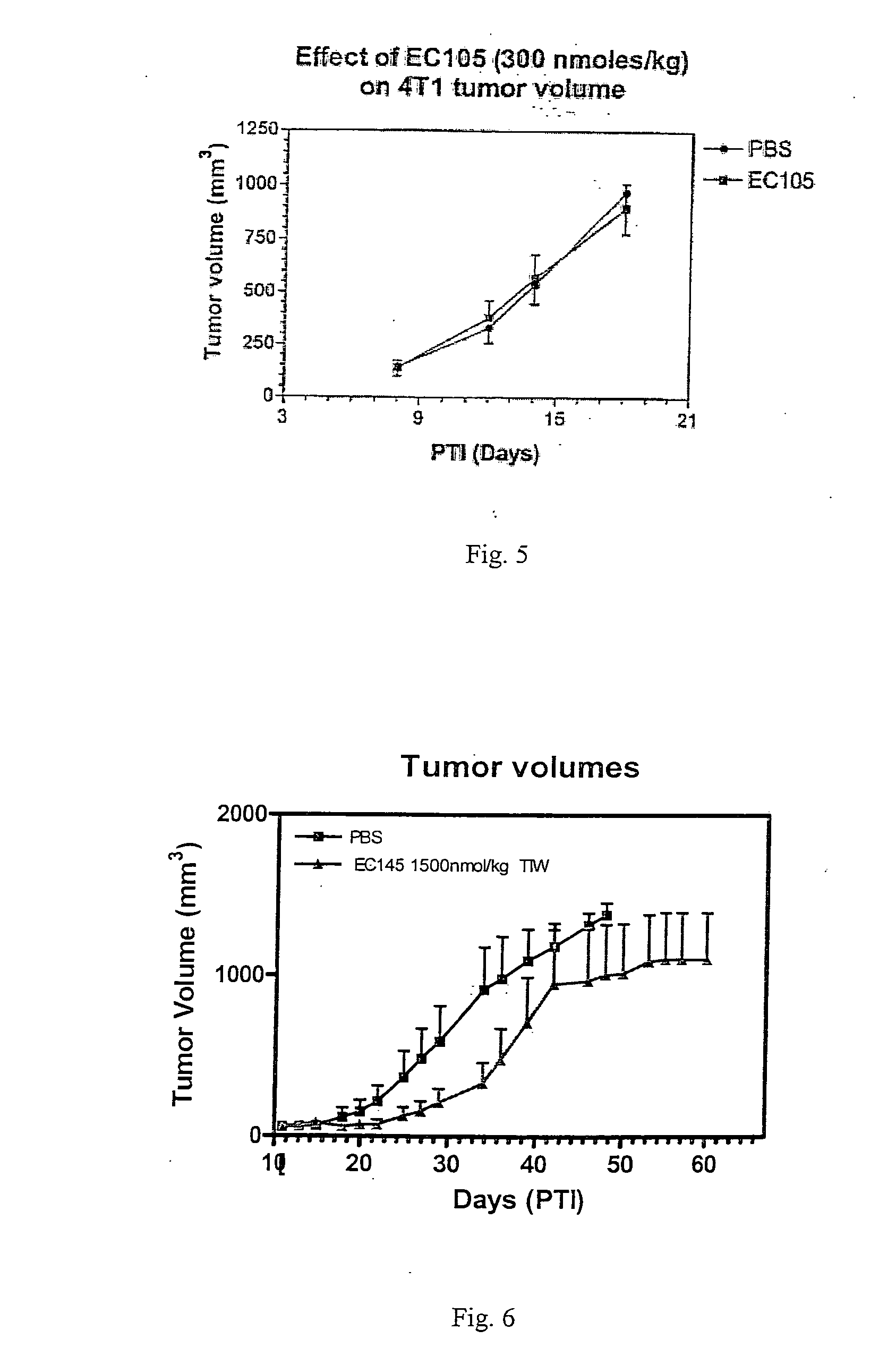
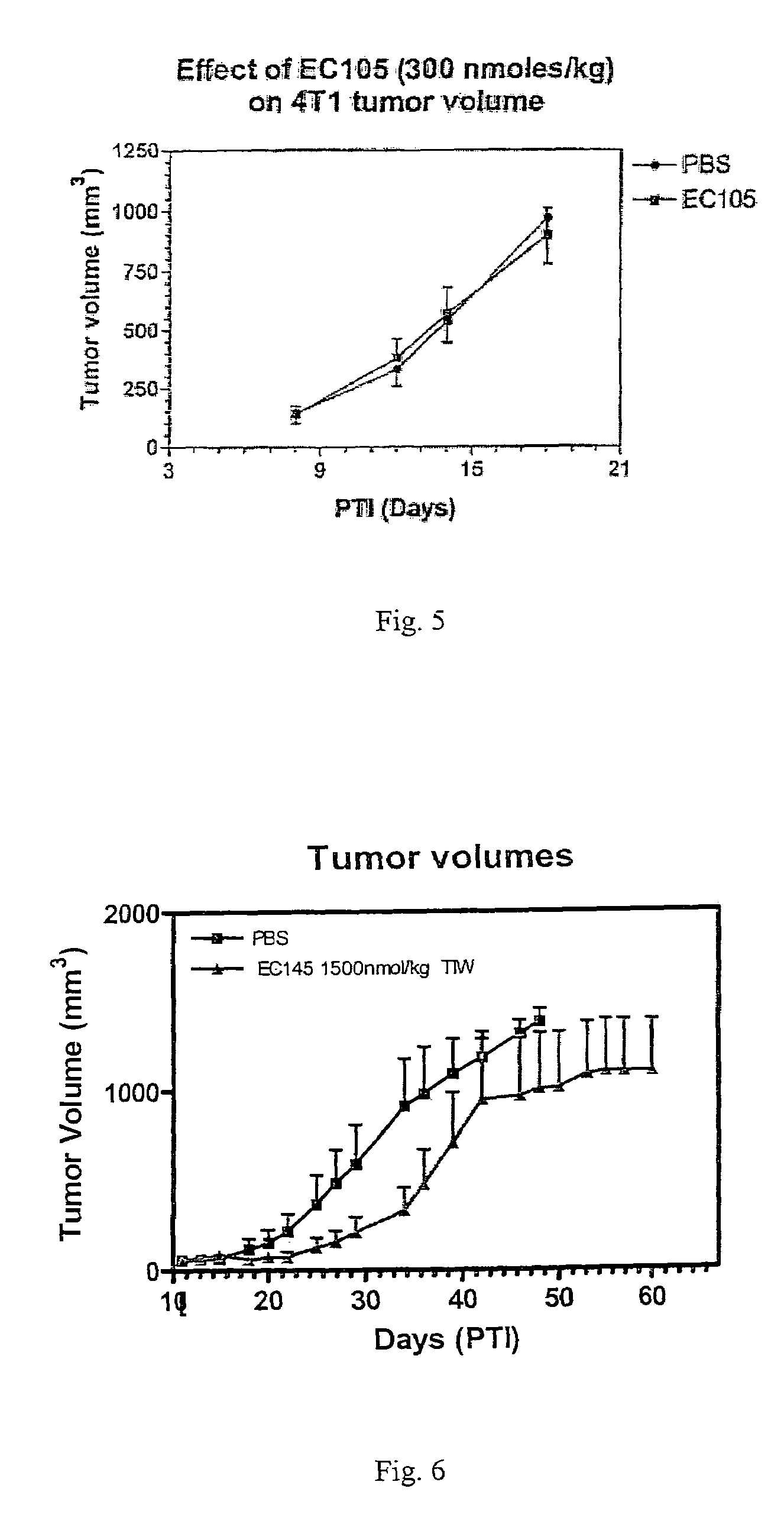
Vitamin-targeted imaging agents
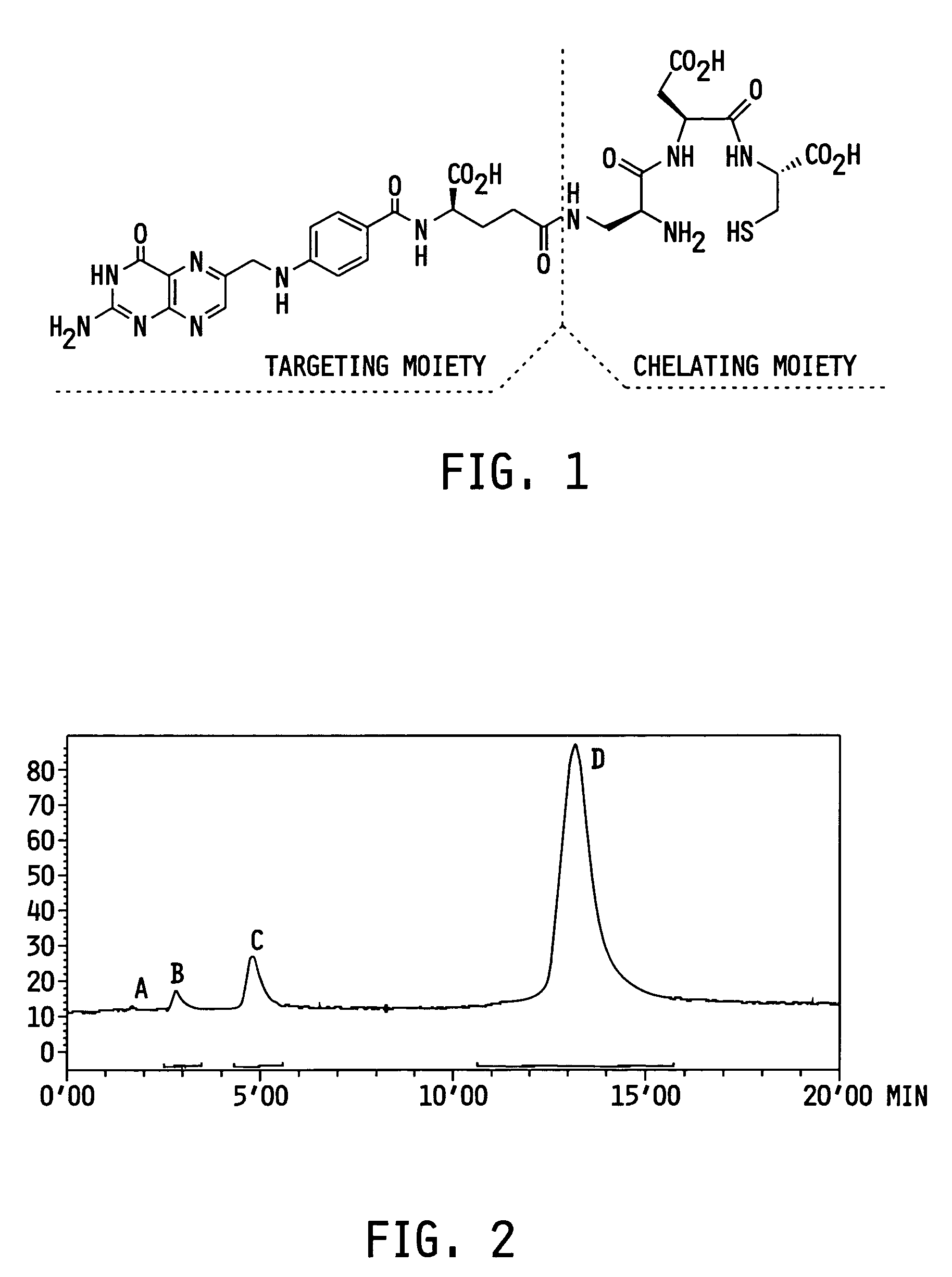
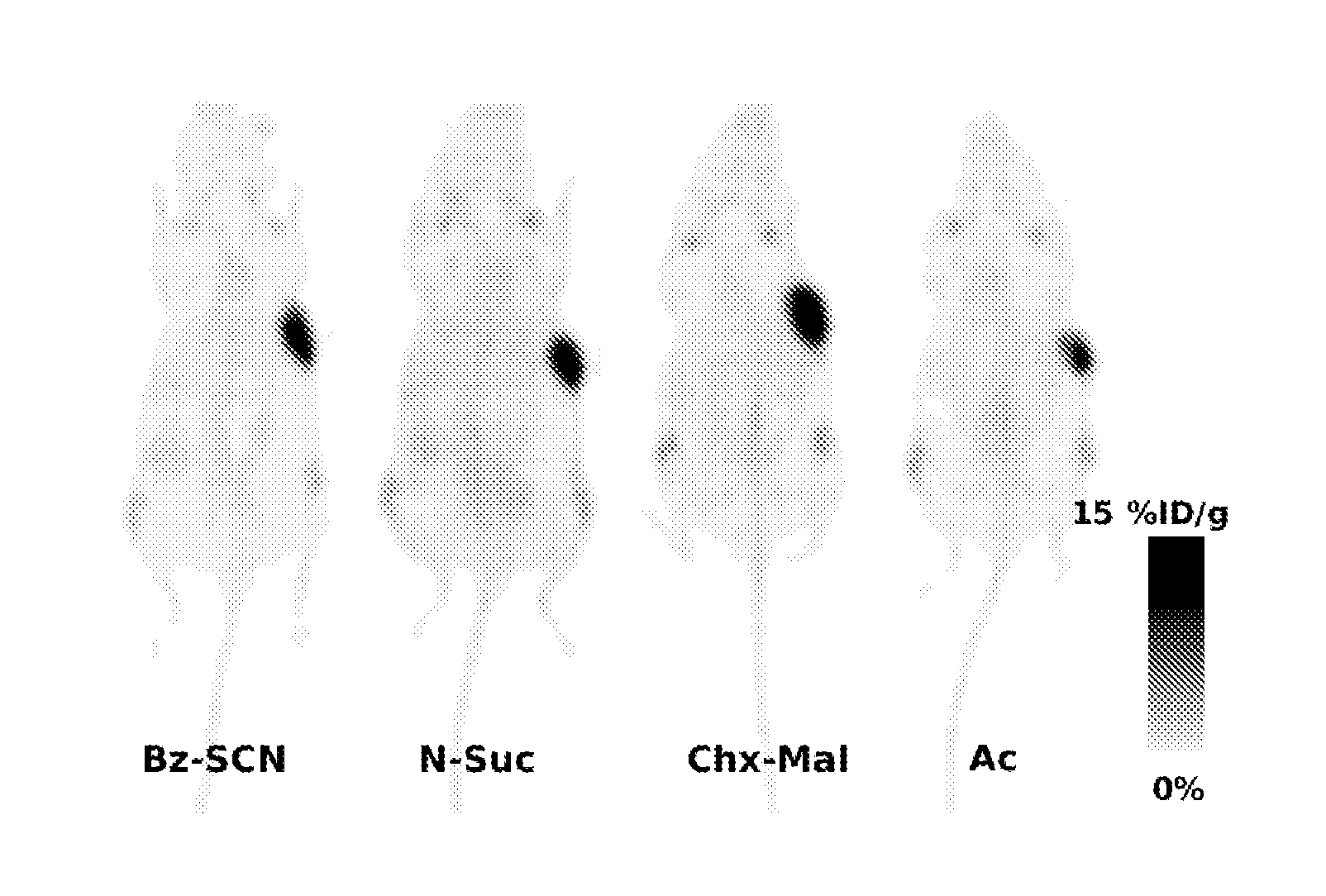
ActiveUS7128893B2Easy to getProducing cost is highPeptide/protein ingredientsRadioactive preparation carriersVitamin receptorSide chain
The invention relates to compounds and methods for targeting radionuclide-based imaging agents to cells having receptors for a vitamin, or vitamin receptor binding derivative or analog thereof, by using such a vitamin as the targeting ligand for the imaging agent. The invention provides a compound of the formulafor use in such methods. In the compound, V is a vitamin that is a substrate for receptor-mediated transmembrane transport in vivo, or a vitamin receptor binding derivative or analog thereof, L is a divalent linker, R is a side chain of an amino acid, M is a cation of a radionuclide, n is 1 or 0, K is 1 or 0, and the compound can be in a pharmaceutically acceptable carrier therefor. The vitamin-based compounds can be used to target radionuclides to cells, such as a variety of tumor cell types, for use in diagnostic imaging of the targeted cells.
Owner:ENDOCTYE INC
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:IDEC PHARM CORP
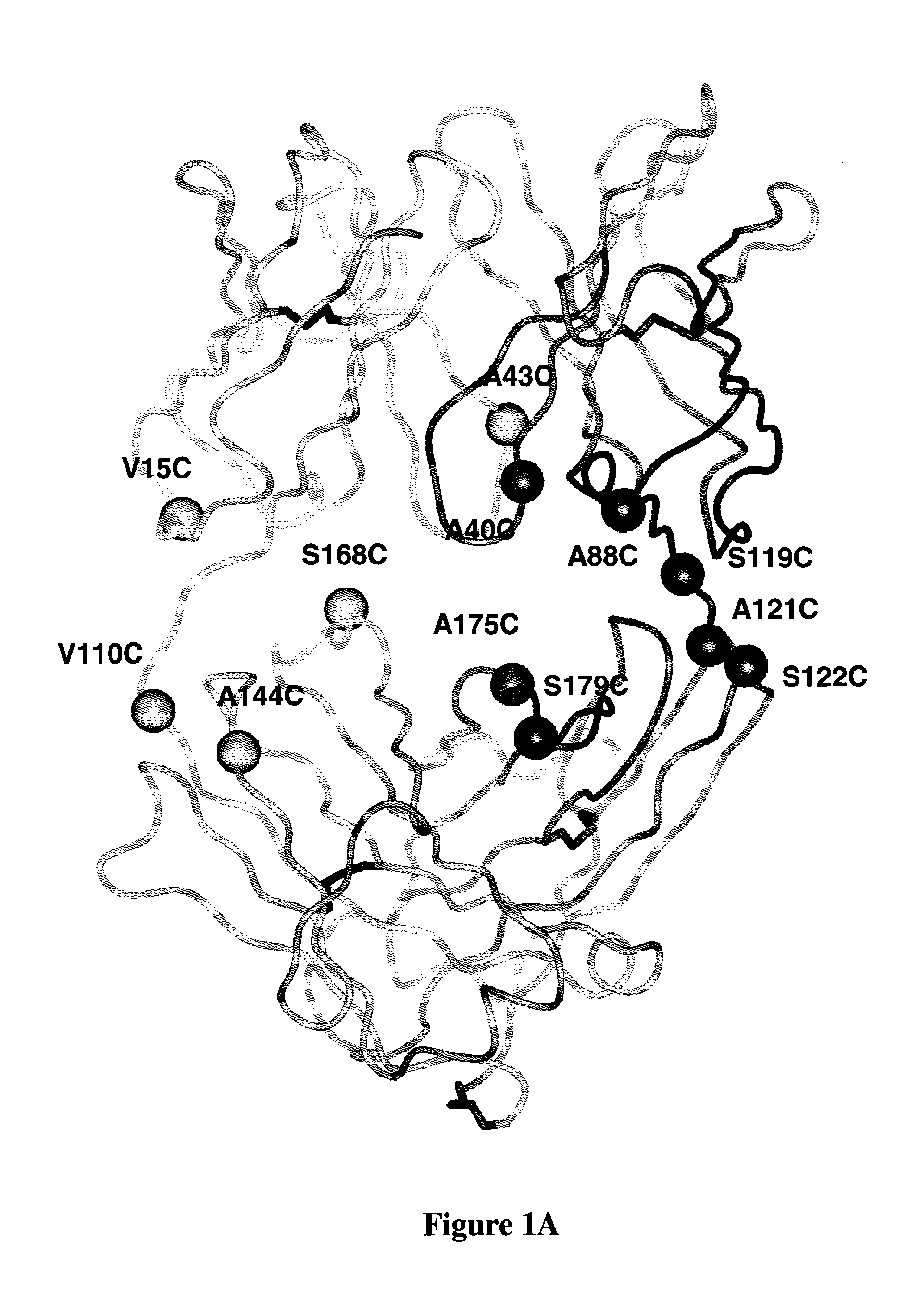
Zirconium-radiolabeled, cysteine engineered antibody conjugates
InactiveUS20100111856A1Peptide/protein ingredientsGenetic material ingredientsAntibody conjugateAntibody fragments
Antibodies are engineered by replacing one or more amino acids of a parent antibody with non cross-linked, highly reactive cysteine amino acids. Antibody fragments may also be engineered with one or more cysteine amino acids to form cysteine engineered antibody fragments (ThioFab). Methods of design, preparation, screening, and selection of the cysteine engineered antibodies are provided. Cysteine engineered antibodies (Ab) are conjugated with one or more zirconium complex (Z) labels through a linker (L) to form cysteine engineered zirconium-labeled antibody conjugates having Formula I:Ab-(L-Z)p Iwhere p is 1 to 4. Imaging methods and diagnostic uses for zirconium-radiolabeled, cysteine engineered antibody conjugate compositions are disclosed.
Owner:F HOFFMANN LA ROCHE & CO AG
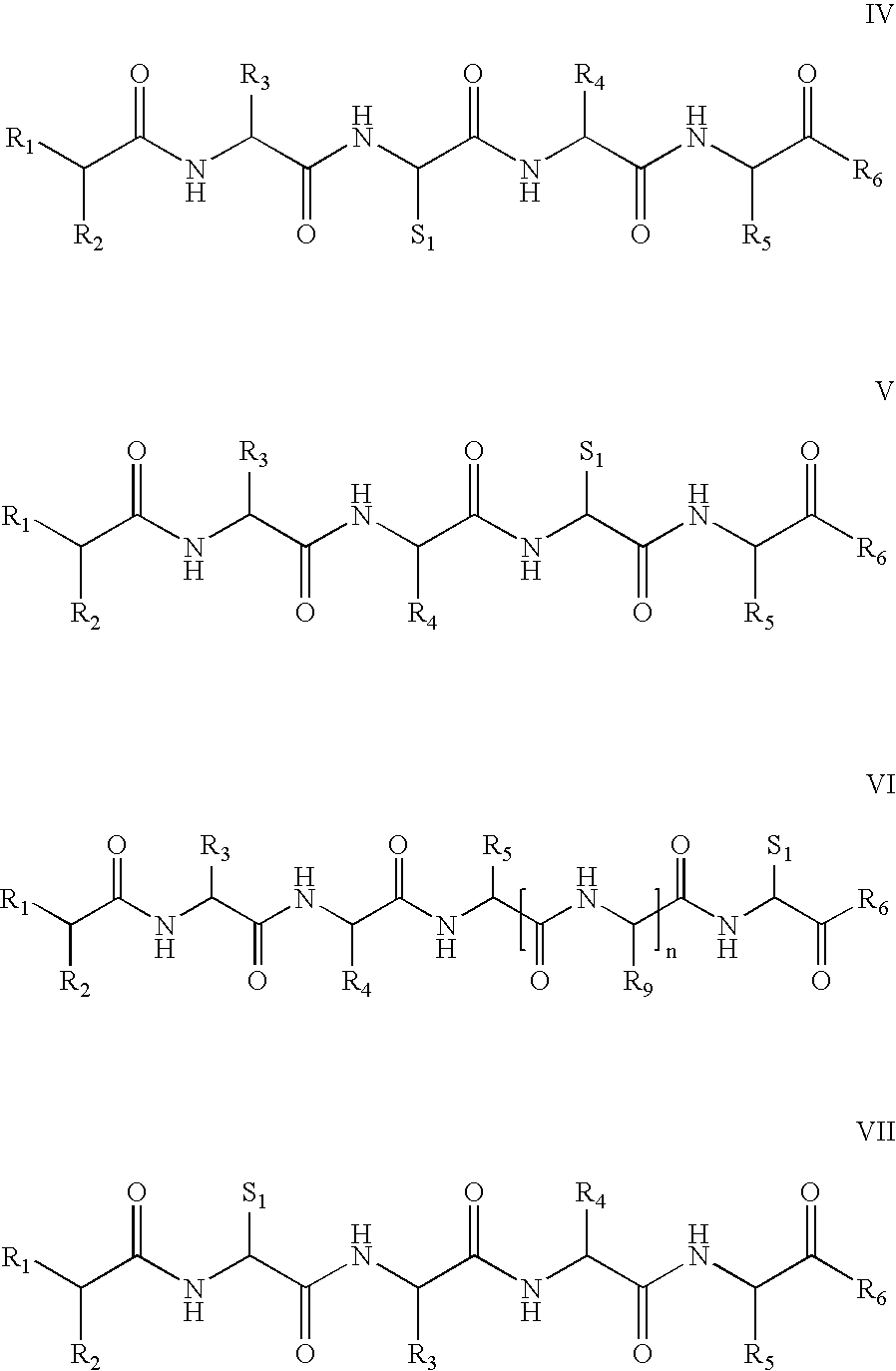
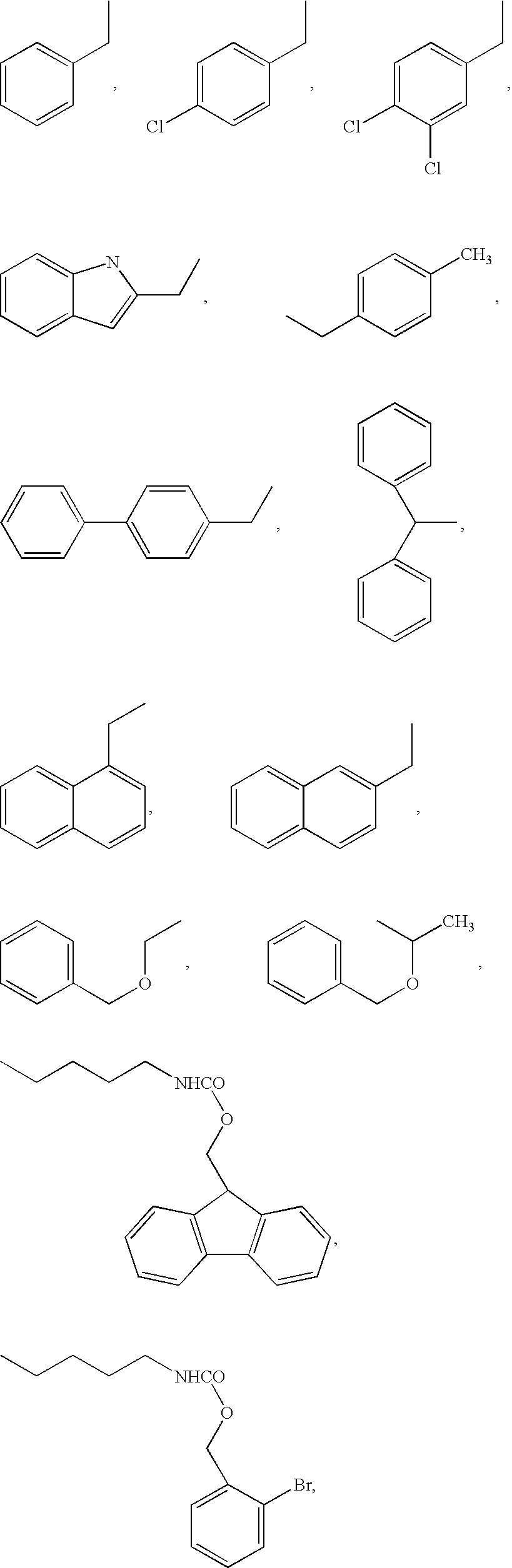
Vitamin receptor binding drug delivery conjugates
The invention describes a vitamin receptor binding drug delivery conjugate, and preparations therefor. The drug delivery conjugate consists of a vitamin receptor binding moiety, a bivalent linker (L), and a drug. The vitamin receptor binding moiety includes vitamins, and vitamin receptor binding analogs and derivatives thereof, and the drug includes analogs and derivatives thereof. The vitamin receptor binding moiety is covalently linked to the bivalent linker, and the drug, or the analog or the derivative thereof, is covalently linked to the bivalent linker, wherein the bivalent linker (L) includes components such as spacer linkers, releasable linkers, and heteroatom linkers, and combinations thereof. Methods and pharmaceutical compositions for eliminating pathogenic cell populations using the drug delivery conjugate are also described.
Owner:ENDOCTYE INC
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:BIOGEN INC
Metallopeptide compositions for treatment of sexual dysfunction
Owner:PALATIN TECH INC
Multivalent complexes, their production and method of use
Owner:PAUL SCHERRER INSTITUT
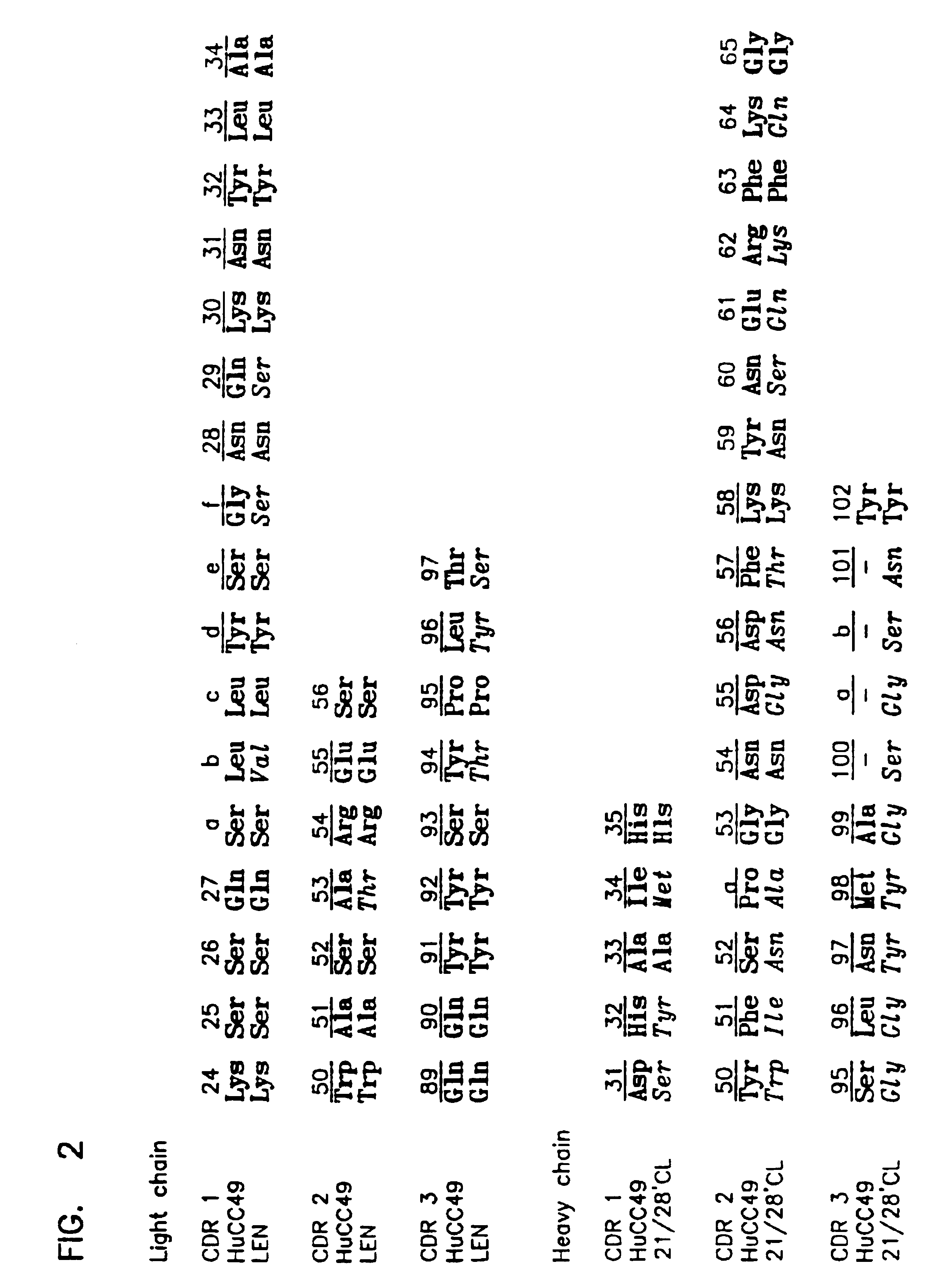
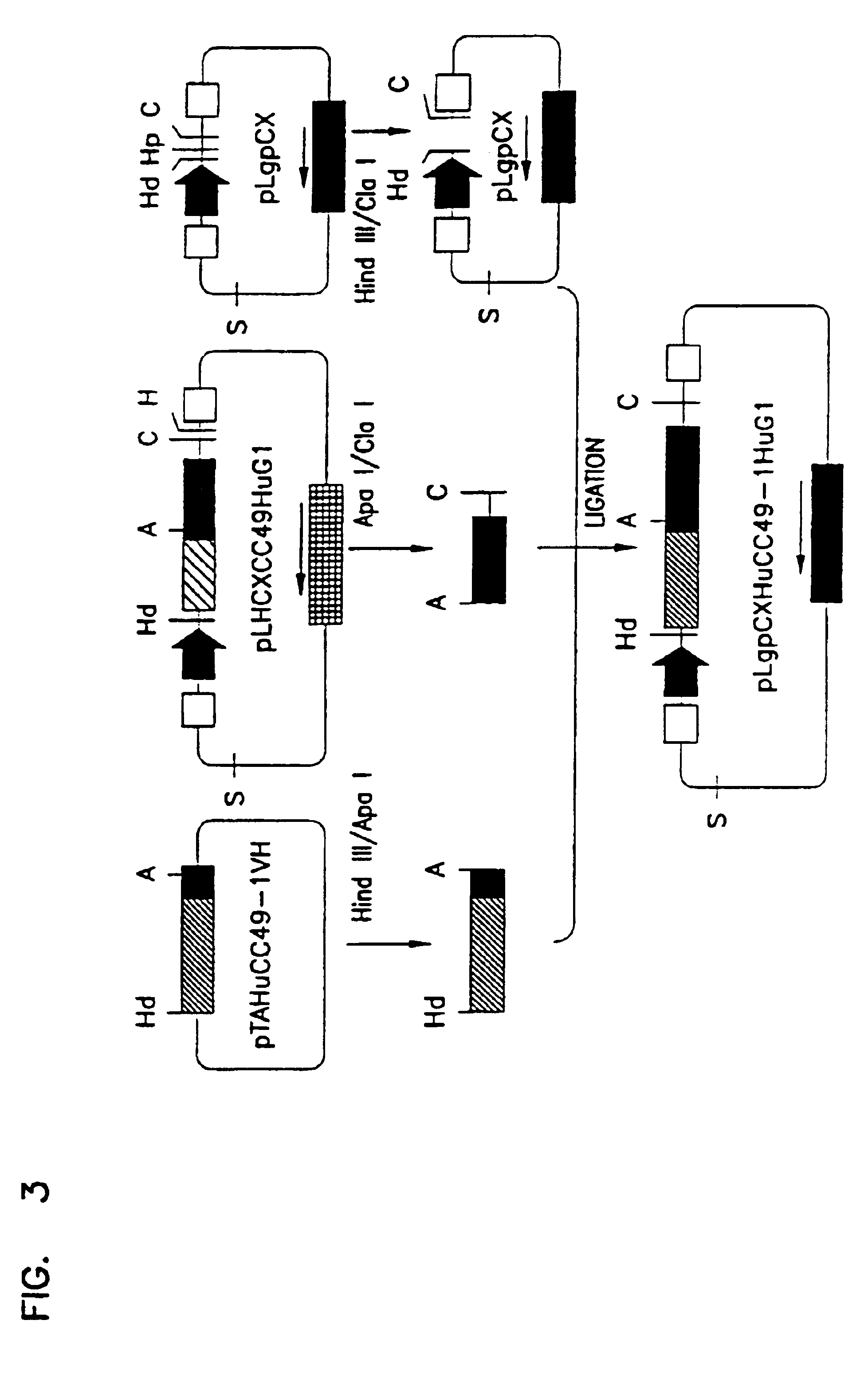
Variants of humanized anti carcinoma monoclonal antibody cc49
InactiveUS6818749B1Elicit adverse responseVirusesPeptide/protein ingredientsComplementarity determining regionHeavy chain
The invention is directed towards mouse-human chimeric variants of CC49 monoclonal antibodies with minimal murine content. A first aspect of the invention provides CDR variants of humanized monoclonal antibody (HuCC49) in which less than all six (three heavy chain and three light chain) Complementarity Determining Regions (CDRs) of CC49 are present. A second aspect of the invention provides SDR variants of humanized monoclonal antibody (HuCC49) in which only Specificity Determining Regions (SDRs) of at least one CDR from CC49 are present. The invention is also directed towards biotechnological methods of making the variants and therapeutic methods of using the variants.
Owner:UNITED STATES OF AMERICA
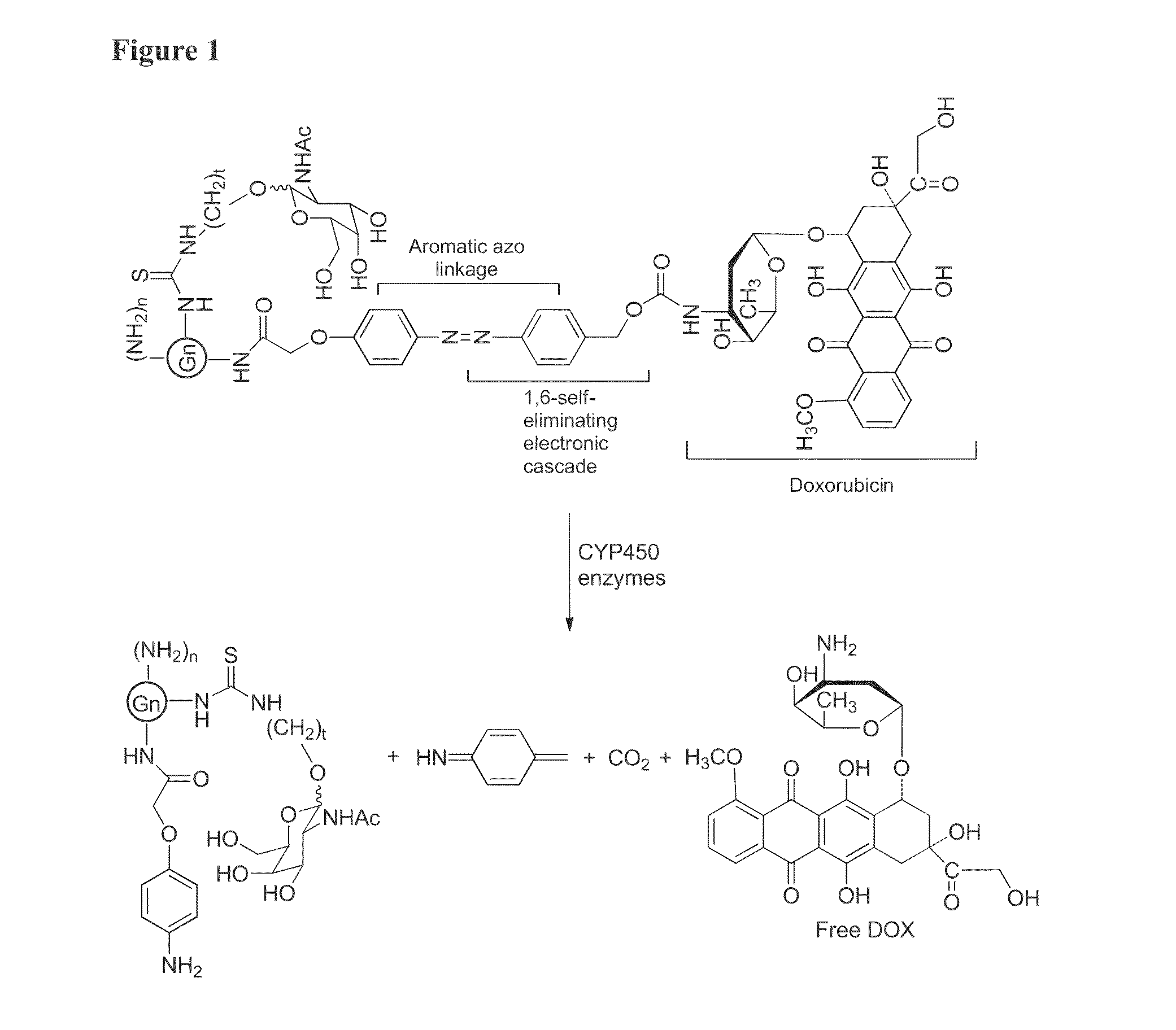
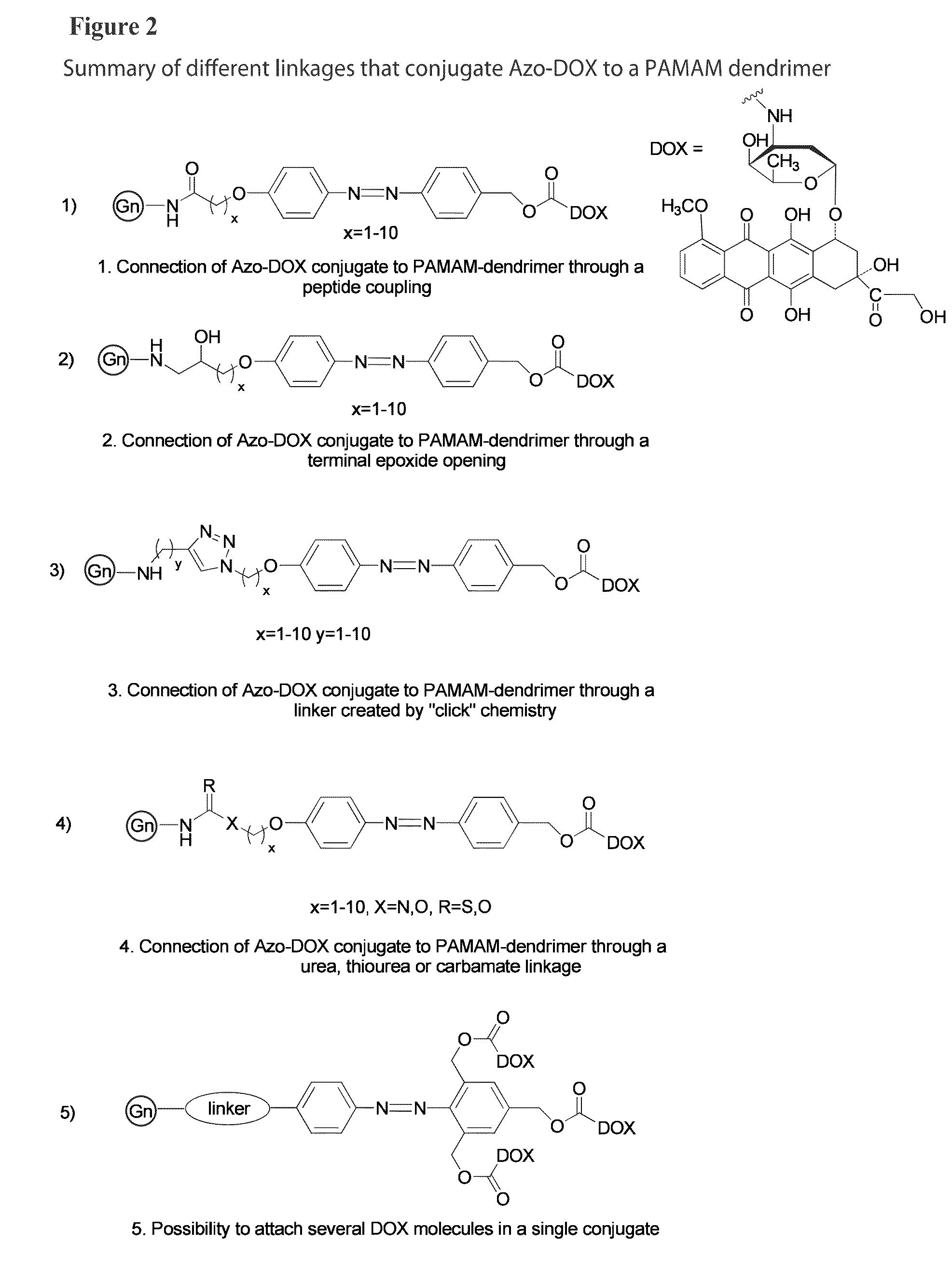
Targeted dendrimer-drug conjugates
InactiveUS20130004427A1Improve solubilityStrong therapeutic activityBiocideSugar derivativesDendrimerEnzyme
Owner:RGT UNIV OF MICHIGAN
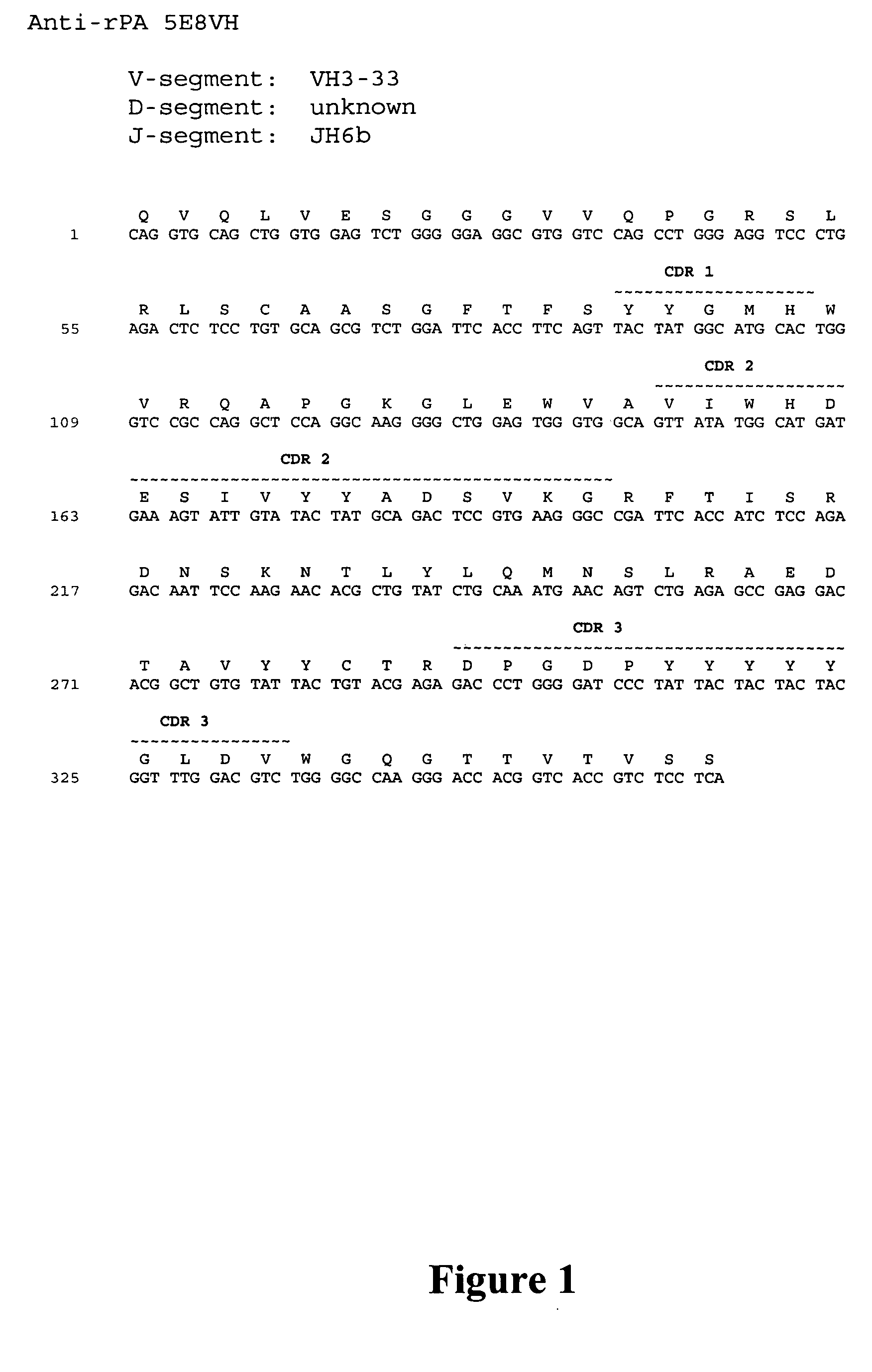
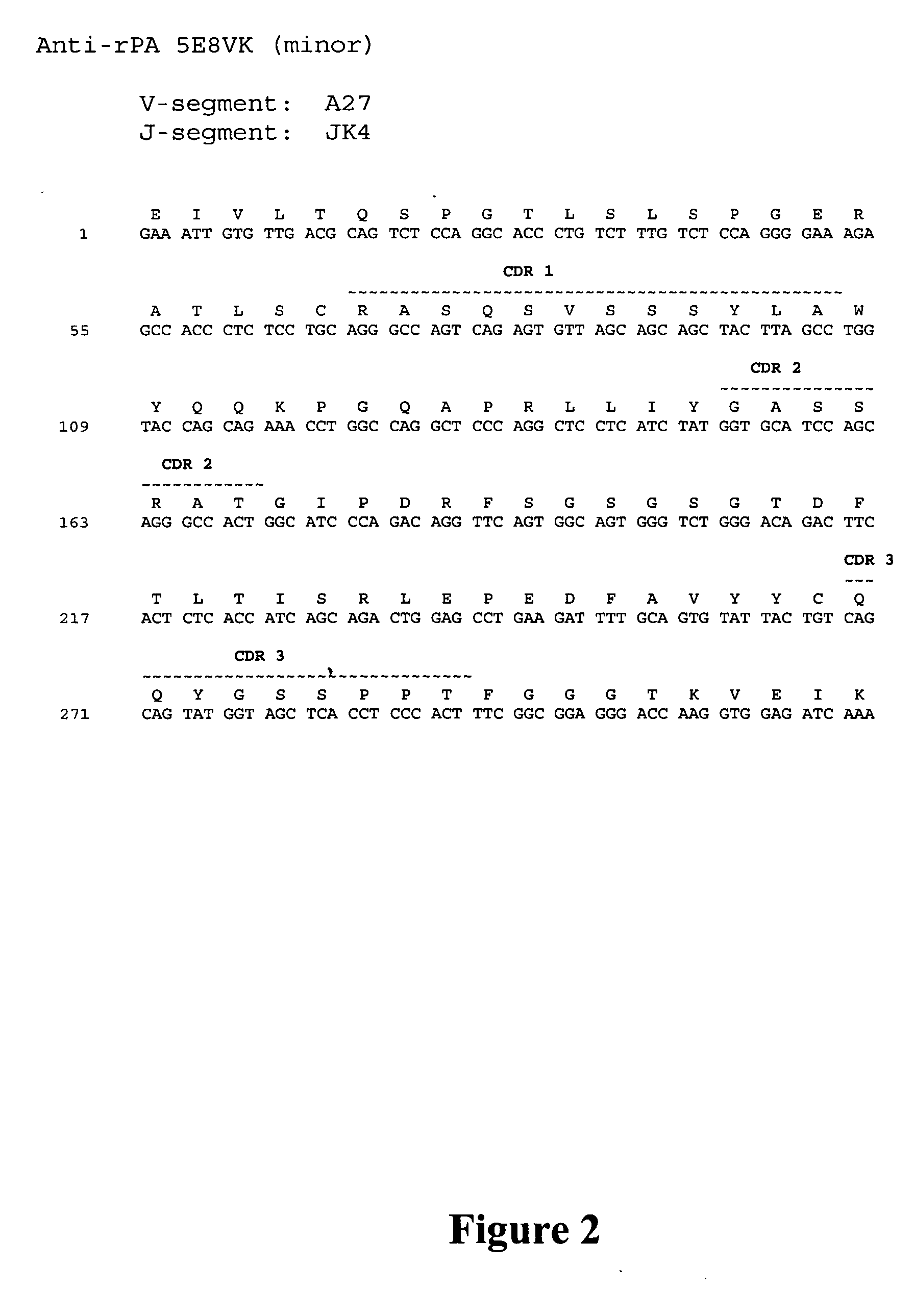
Human monoclonal antibodies against bacillus anthracis protective antigen
ActiveUS20050287149A1Neutralizing activityLess immunogenicAntibacterial agentsBacteriaV(D)J recombinationBispecific antibody
Isolated human monoclonal antibodies which bind to Anthrax protective antigen are disclosed. The human antibodies can be produced in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are derivatives of the human antibodies (e.g., bispecific antibodies and immunoconjugates), pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:MEDAREX LLC
Use of bi-specific antibodies for pre-targeting diagnosis and therapy
InactiveUS7074405B1Increased toxicityLow toxicityHybrid immunoglobulinsInorganic boron active ingredientsEpitopeDiagnostic agent
The present invention relates to a bi-specific antibody or antibody fragment having at least one arm that specifically binds a targeted tissue and at least one other arm that specifically binds a targetable conjugate. The targetable conjugate comprises a carrier portion which comprises or bears at least one epitope recognizable by at least one arm of said bi-specific antibody or antibody fragment. The targetable conjugate further comprises one or more therapeutic or diagnostic agents or enzymes. The invention provides constructs and methods for producing the bi-specific antibodies or antibody fragments, as well as methods for using them.
Owner:IMMUNOMEDICS INC
Vitamin receptor binding drug delivery conjugates
The invention describes a vitamin receptor binding drug delivery conjugate, and preparations therefor. The drug delivery conjugate consists of a vitamin receptor binding moiety, a bivalent linker (L), and a drug. The vitamin receptor binding moiety includes vitamins, and vitamin receptor binding analogs and derivatives thereof, and the drug includes analogs and derivatives thereof. The vitamin receptor binding moiety is covalently linked to the bivalent linker, and the drug, or the analog or the derivative thereof, is covalently linked to the bivalent linker, wherein the bivalent linker (L) includes components such as spacer linkers, releasable linkers, and heteroatom linkers, and combinations thereof. Methods and pharmaceutical compositions for eliminating pathogenic cell populations using the drug delivery conjugate are also described.
Owner:ENDOCTYE INC
Therapeutic using a bispecific antibody
Multivalent, multispecific molecules having at least one specificity for a pathogen and at least one specificity for the HLA class II invariant chain (Ii) are administered to induce clearance of the pathogen. In addition to pathogens, clearance of therapeutic or diagnostic agents, autoantibodies, anti-graft antibodies, and other undesirable compounds may be induced using the multivalent, multispecific molecules.
Owner:IMMUNOMEDICS INC
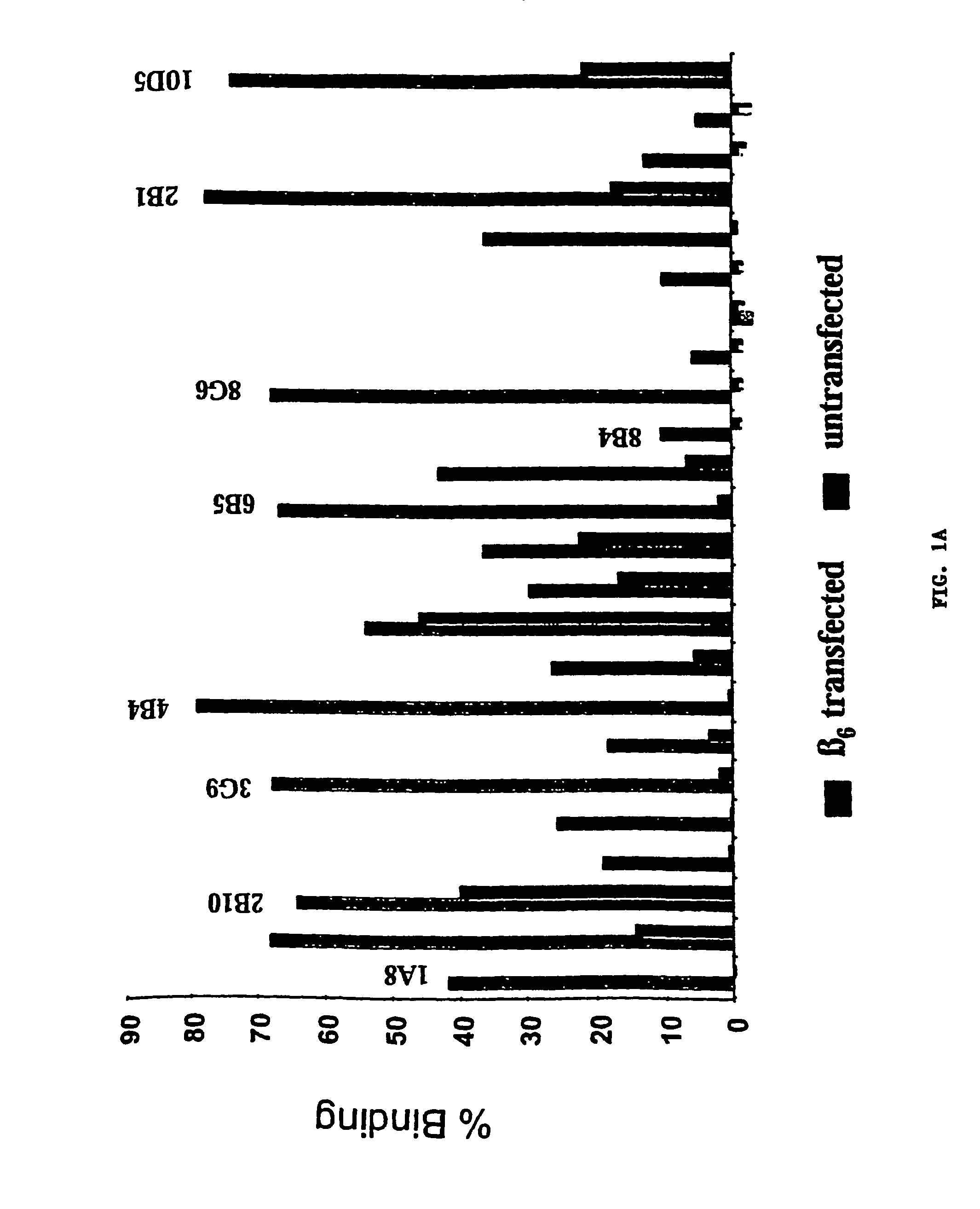
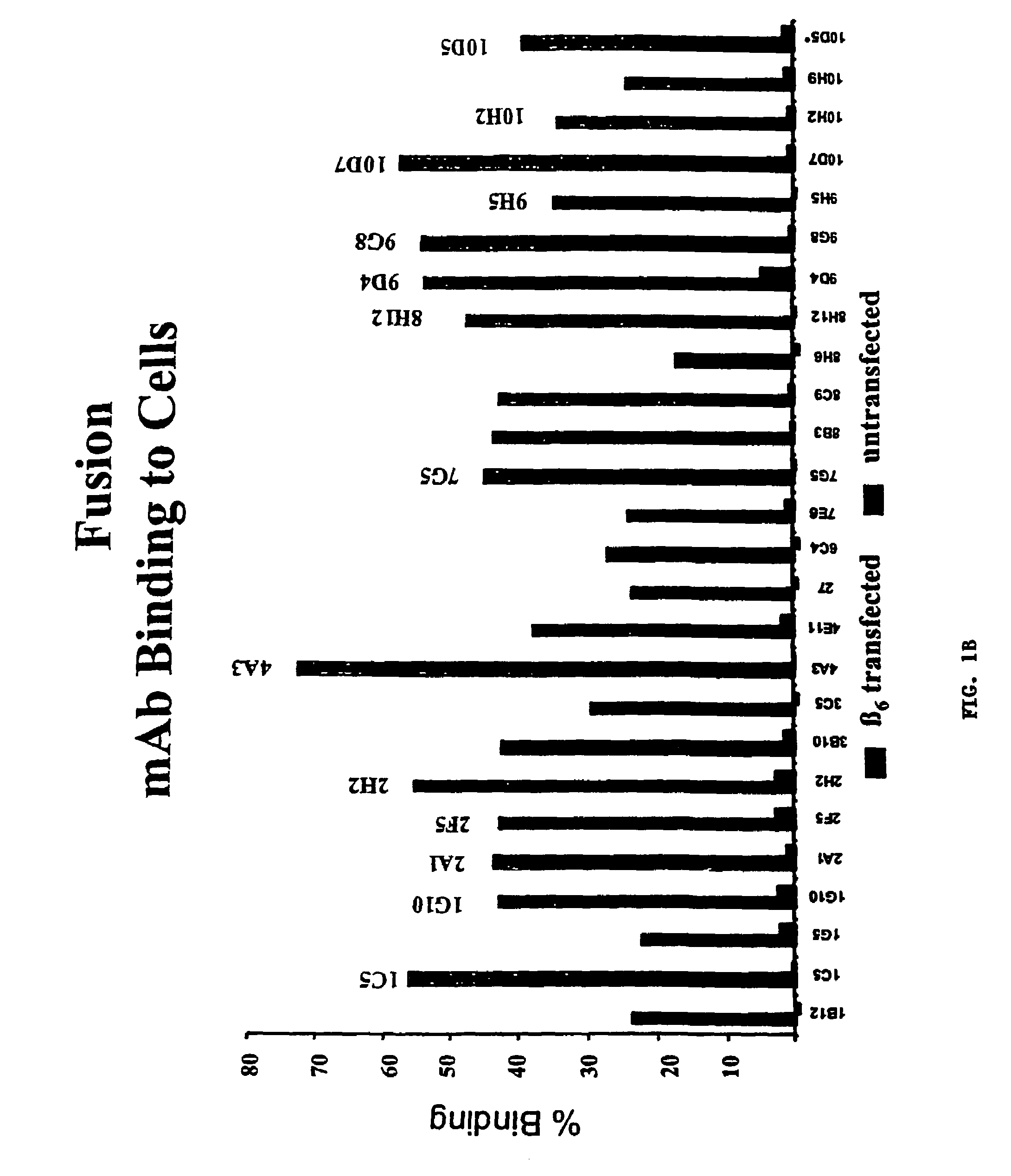
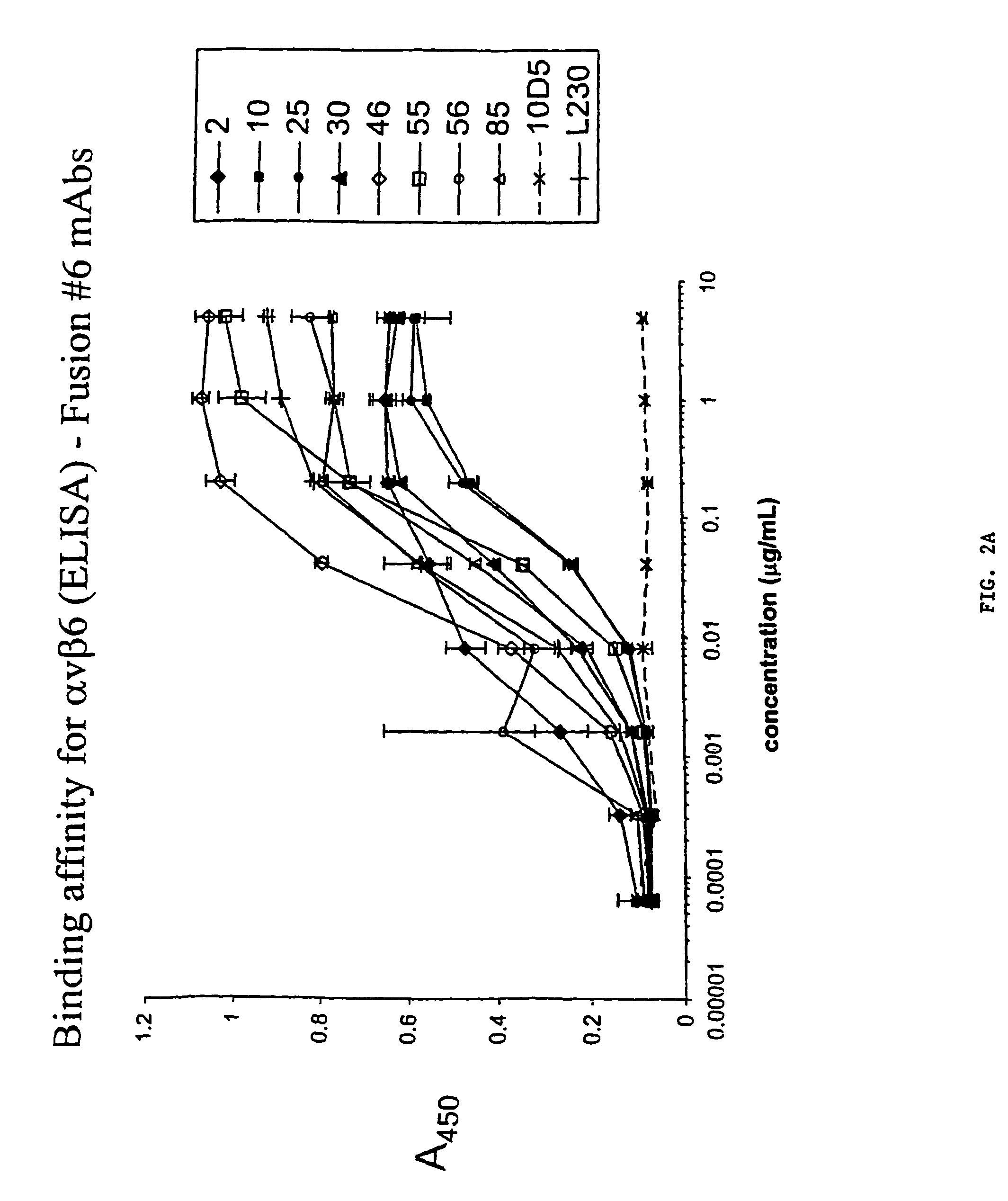
Anti-alphavbeta6 antibodies
InactiveUS7465449B2Reduce functionHigh affinitySkeletal disorderImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntiendomysial antibodies
Monoclonal antibodies that specifically bind to M.96. Also included are methods of using these antibodies to treat mammals having or at risk of having 006-mediated diseases, or to diagnose % Qmediated diseases.
Owner:BIOGEN MA INC +1
Treatment of B cell malignancies using combination of B cell depleting antibody and immune modulating antibody related applications
InactiveUS20020028178A1Prevent and reduce proliferation of cellReduce and prevent proliferationRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsCD20Antiendomysial antibodies
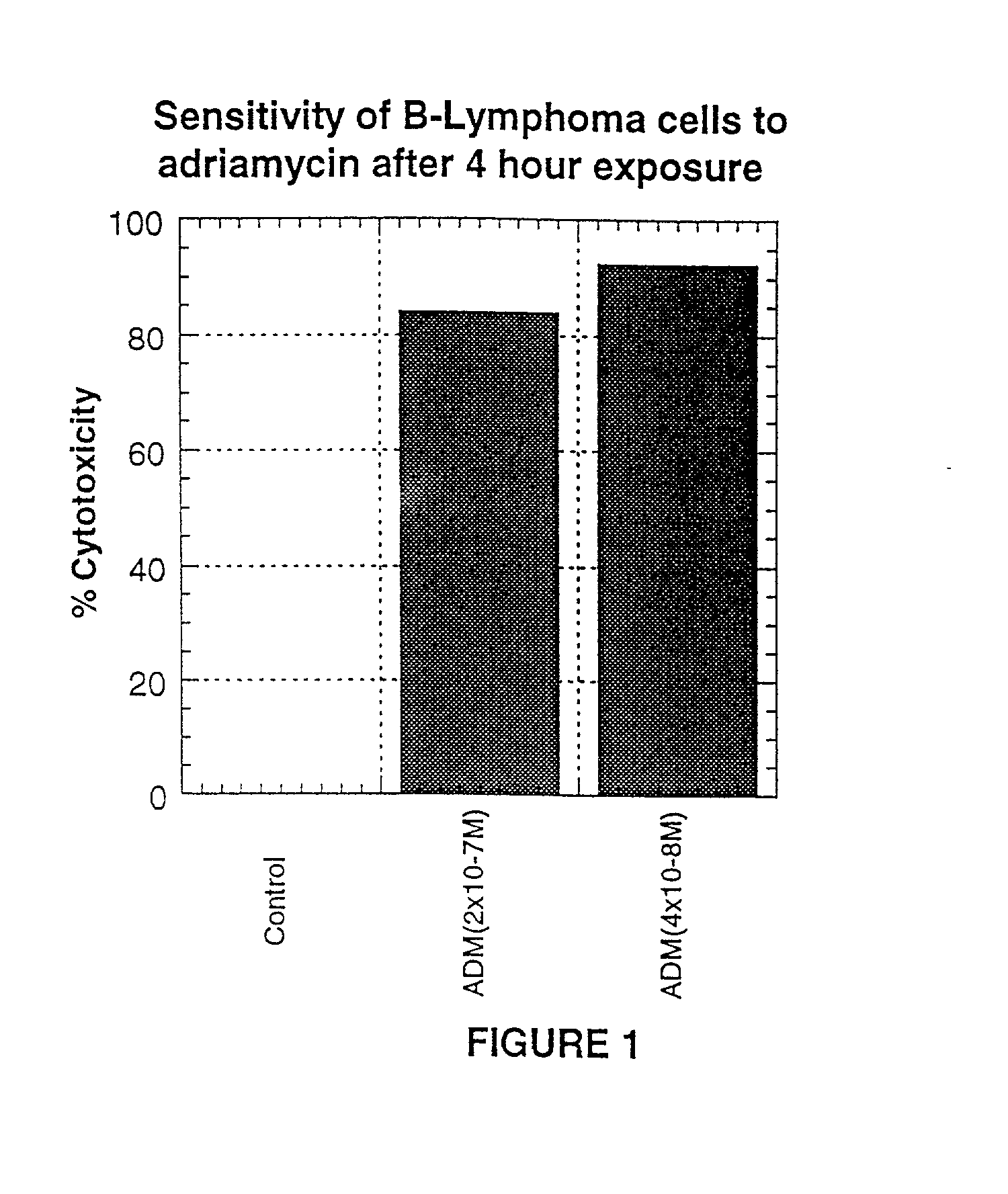
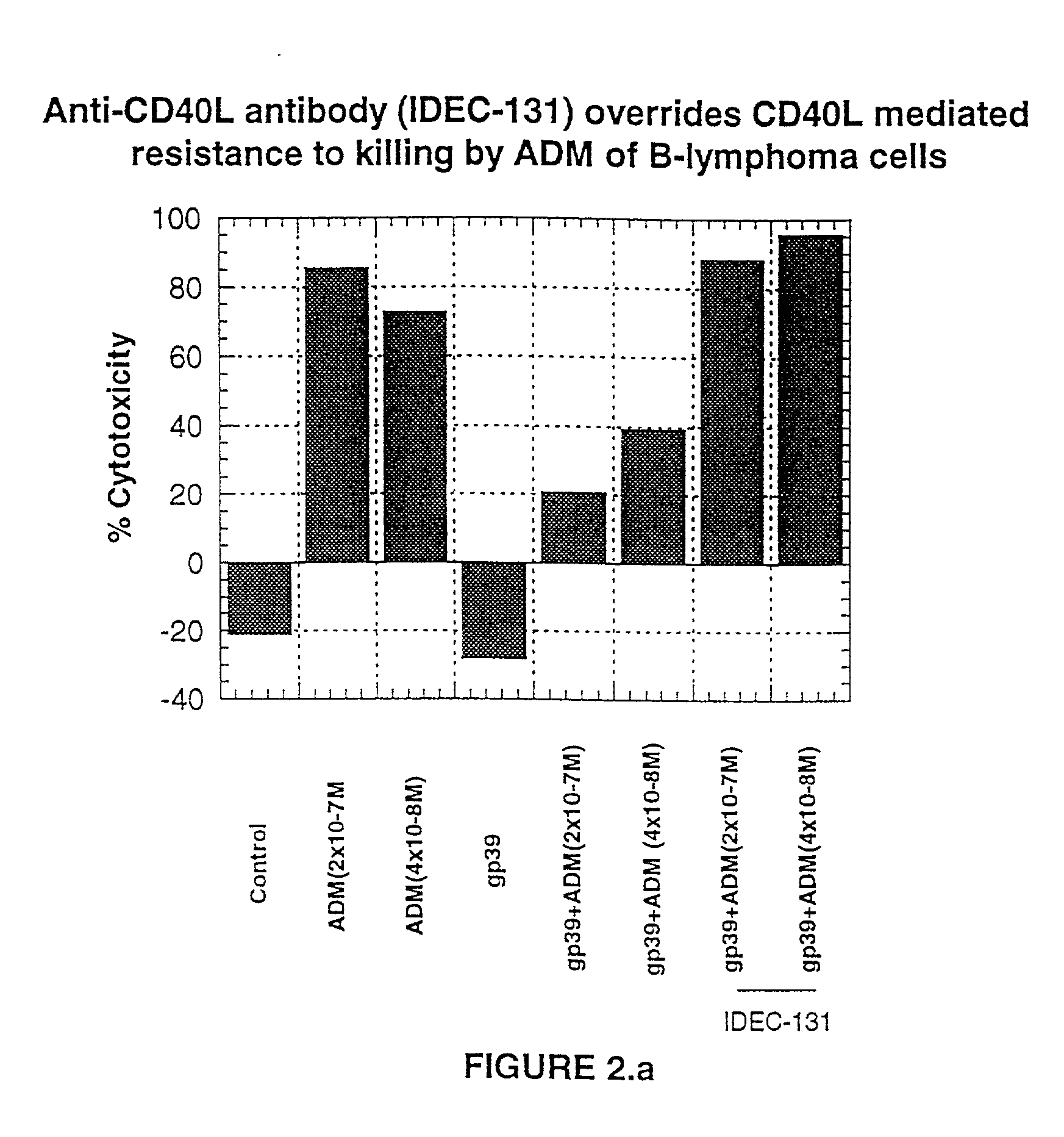
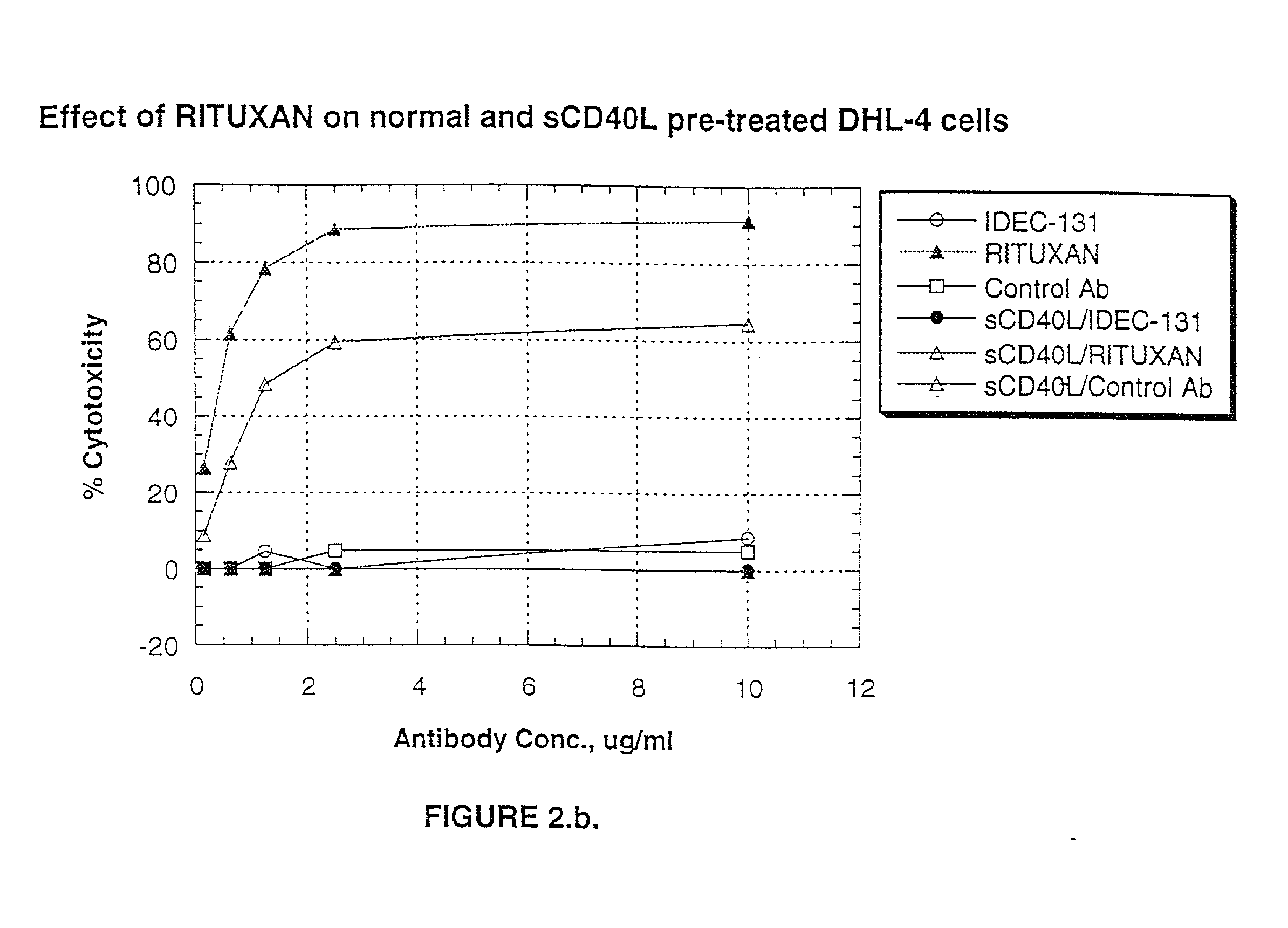
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN MA INC
Specific binding proteins including antibodies which bind to the necrotic center of tumors, and uses thereof
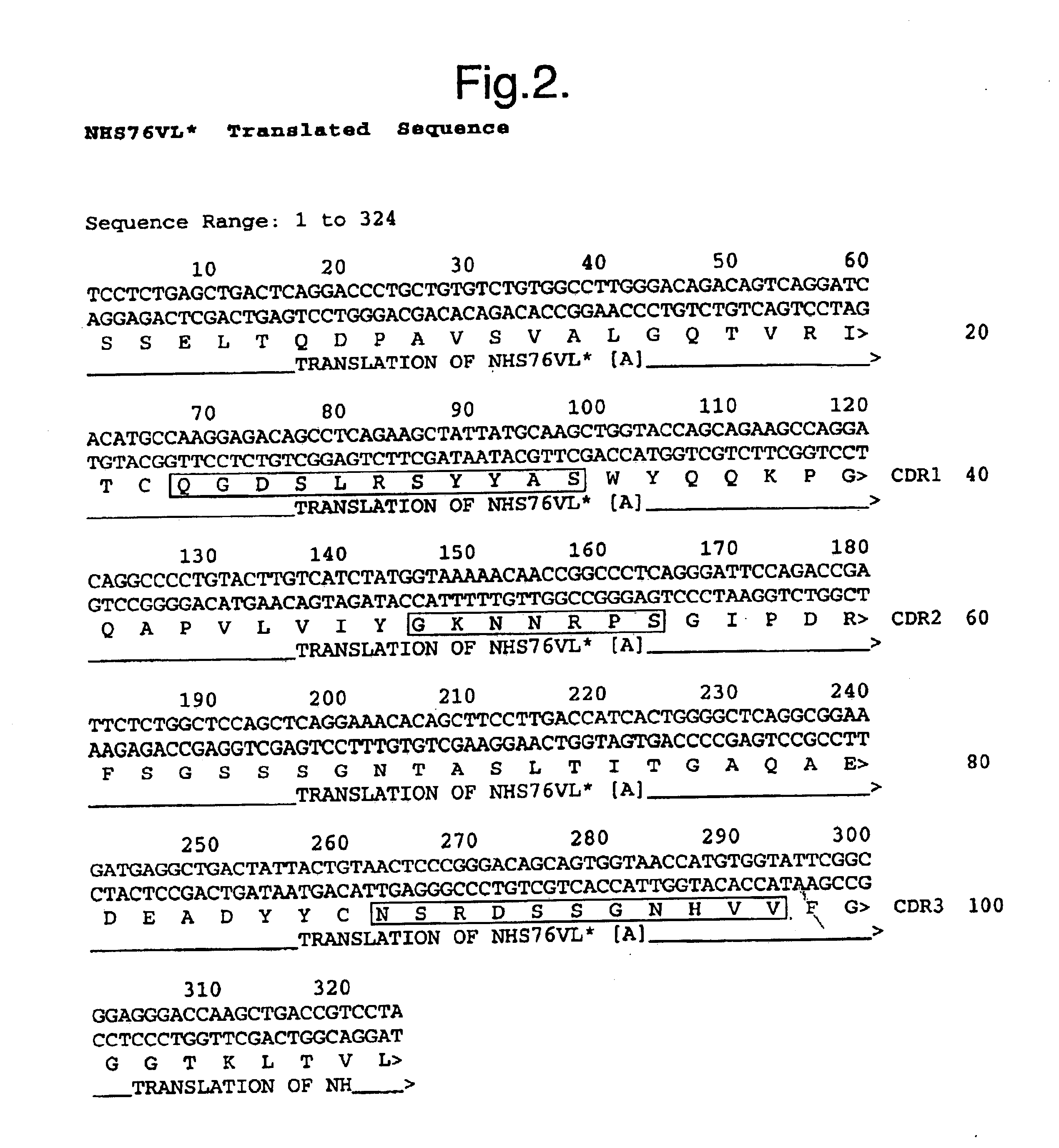
Specific binding members, based on the third CDR of the antibody NHS76 (SEQ ID NO: 2) are provided, together with their use in methods of treatment and diagnosis.
Owner:PEREGRINE PHARMA INC +1
Treatment of B-cell associated diseases
InactiveUS6846476B2Enhanced killing and depletionReduce the possibilityRadioactive preparation carriersAntibody ingredientsDiseaseAutoimmune responses
Treatment of B-cell associated diseases including autoimmune and B-cell malignancies such as leukemias, lymphomas, using the combination of an anti-CD20 antibody, preferably RITUXAN® and a radiolabeled anti-CD22 antibody, preferably an 90Y labeled humanized anti-CD22 antibody, is described. These therapeutic regimens provide for enhanced depletion of B cells, and therefore reduce the risk in B cell malignancy treatment of relapse associated with RITUXAN® and, moreover, provide for prolonged immunosuppression of B-cell immune responses, especially in the context of autoimmune diseases and transplant.
Owner:BIOGEN INC
Radiolabeling method using multivalent glycoligands as hepatic receptor imaging agent
InactiveUS20110097264A1Low toxicityImprove securityRadioactive preparation carriersSaccharide peptide ingredientsImaging agentClinical trial
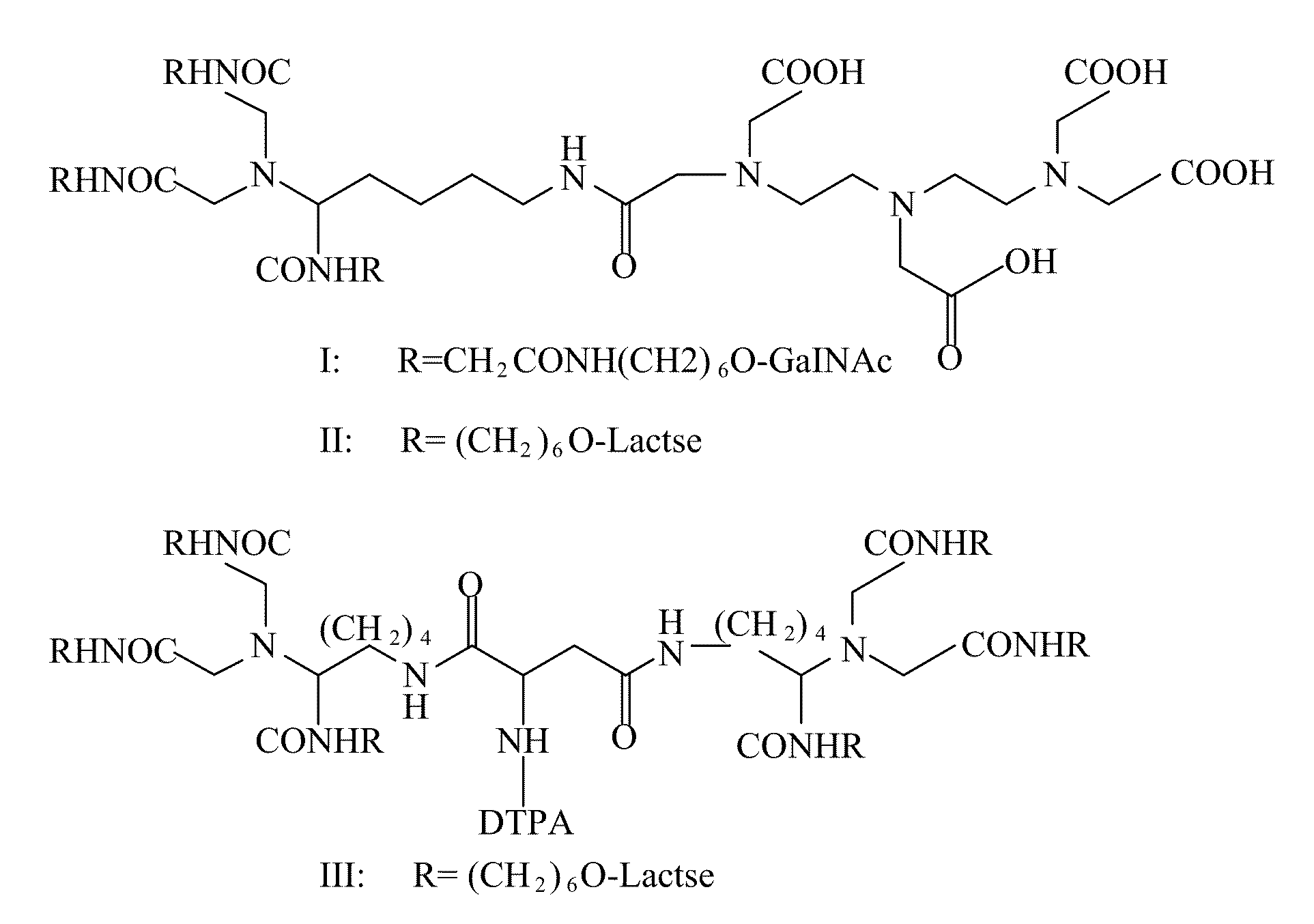
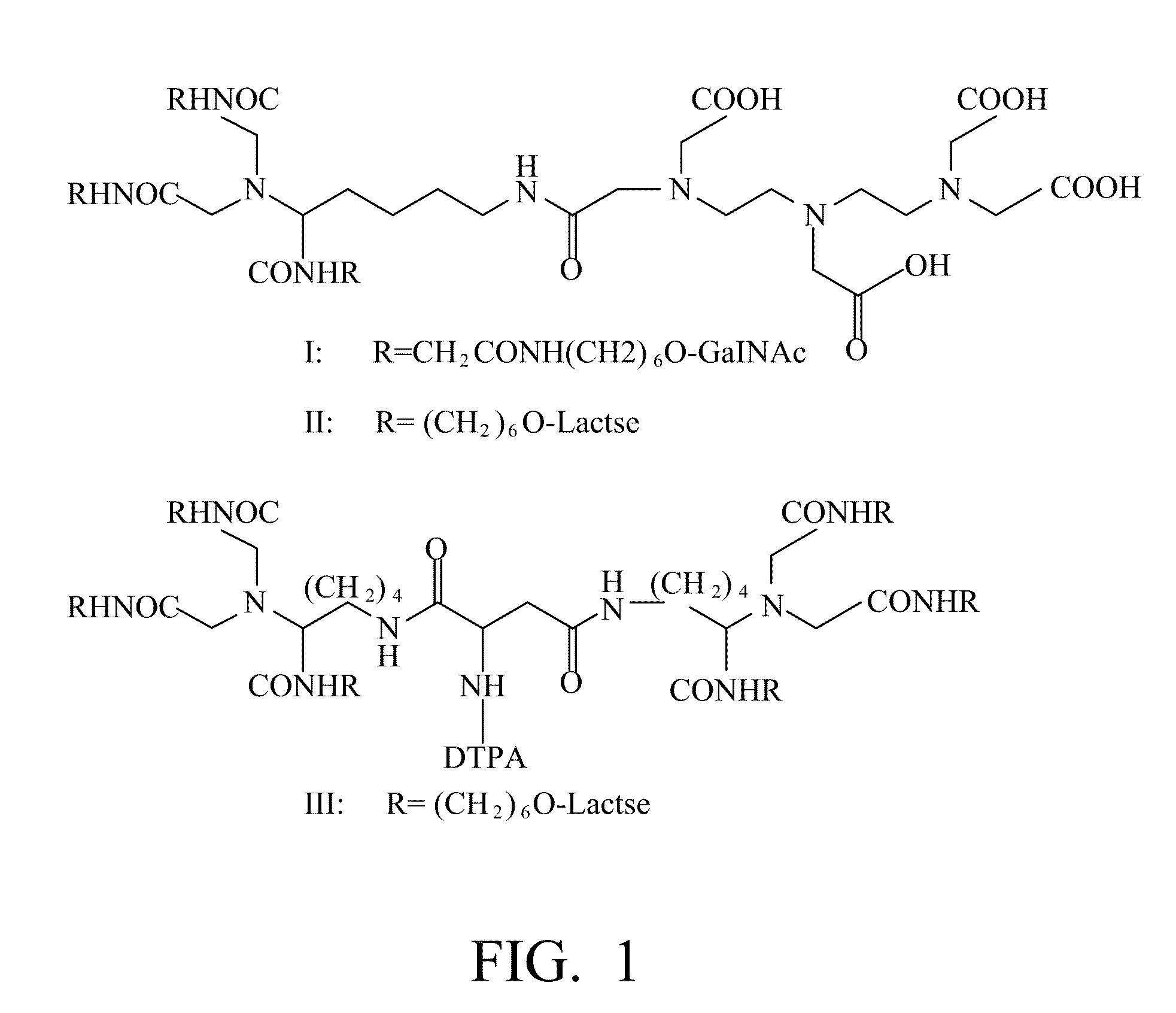
A radiolabeling method using a multivalent glycoligand as hepatic receptor imaging agent is provided. The multivalent glycoligand-DTPA derivatives (In-111-DTPA-hexa lactoside and In-111-DTPA-tri-galactosamine glycoside) labeled with In-111 are used as hepatic receptor imaging agent. The effects of imaging of a hepatic receptor in different species are evaluated, the lowest specific radioactivity values of hepatic receptor imaging required in different species are discovered. Since the specificity of the human ASGPR closely resembles that of the mouse. This kind of radiolabelling method, agent and related study about specific radioactivity could be used in clinical trial in the future.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Targeted combination immunotherapy of cancer and infectious diseases
InactiveUS7011812B1Efficient deliveryMinimize ToxicityHeavy metal active ingredientsOrganic active ingredientsCancer therapyWilms' tumor
The present invention is directed to methods for treating cancer wherein more than one therapeutic agent is used, with each of the therapeutic agents having different tumor-killing capabilities, and wherein the therapeutic agents are delivered to the tumor sites using combined targeting and pre-targeting methods. The methods of the present invention achieve good tumor to non-tumor ratios of the therapeutic agents, and are effective for cancer therapy.
Owner:IMMUNOMEDICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com