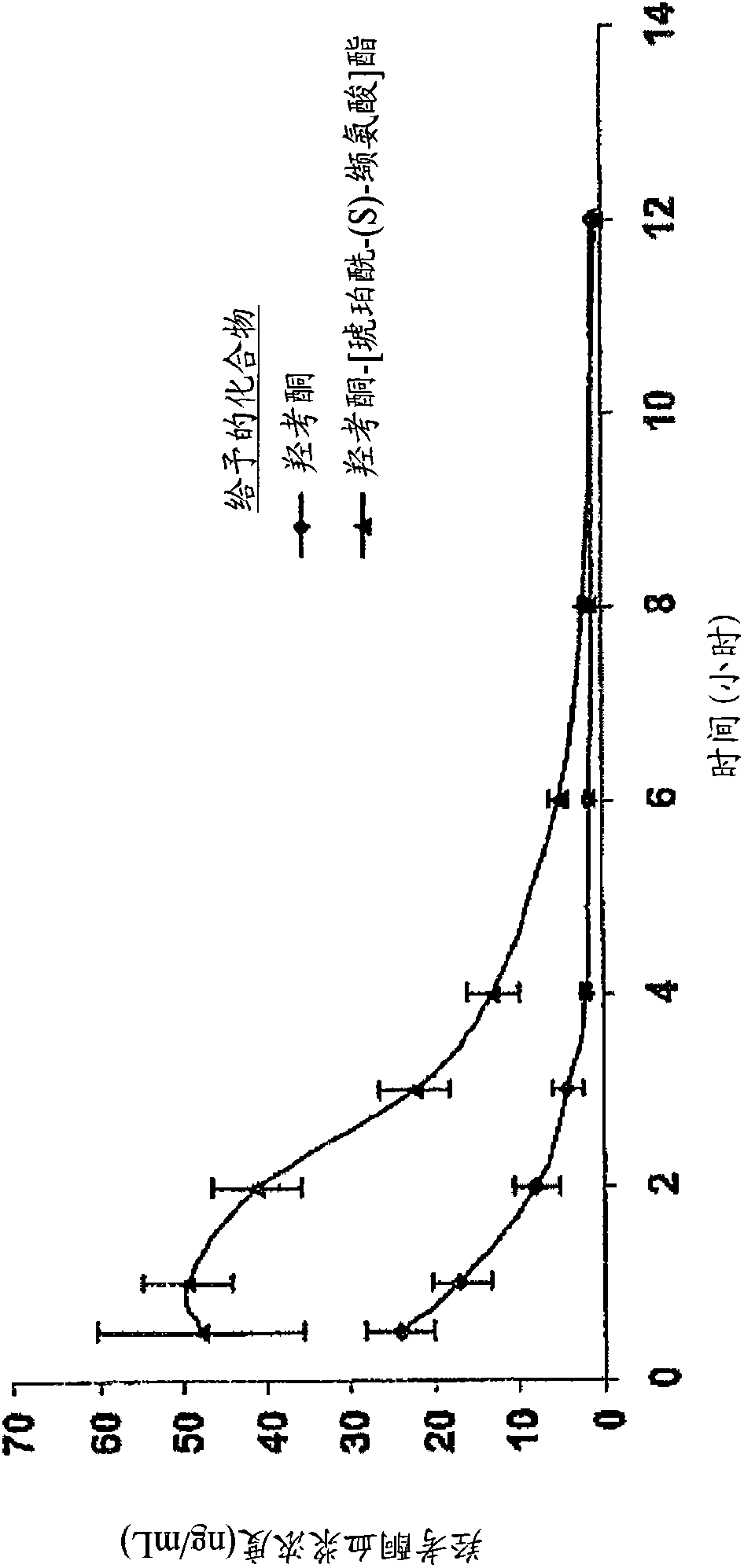
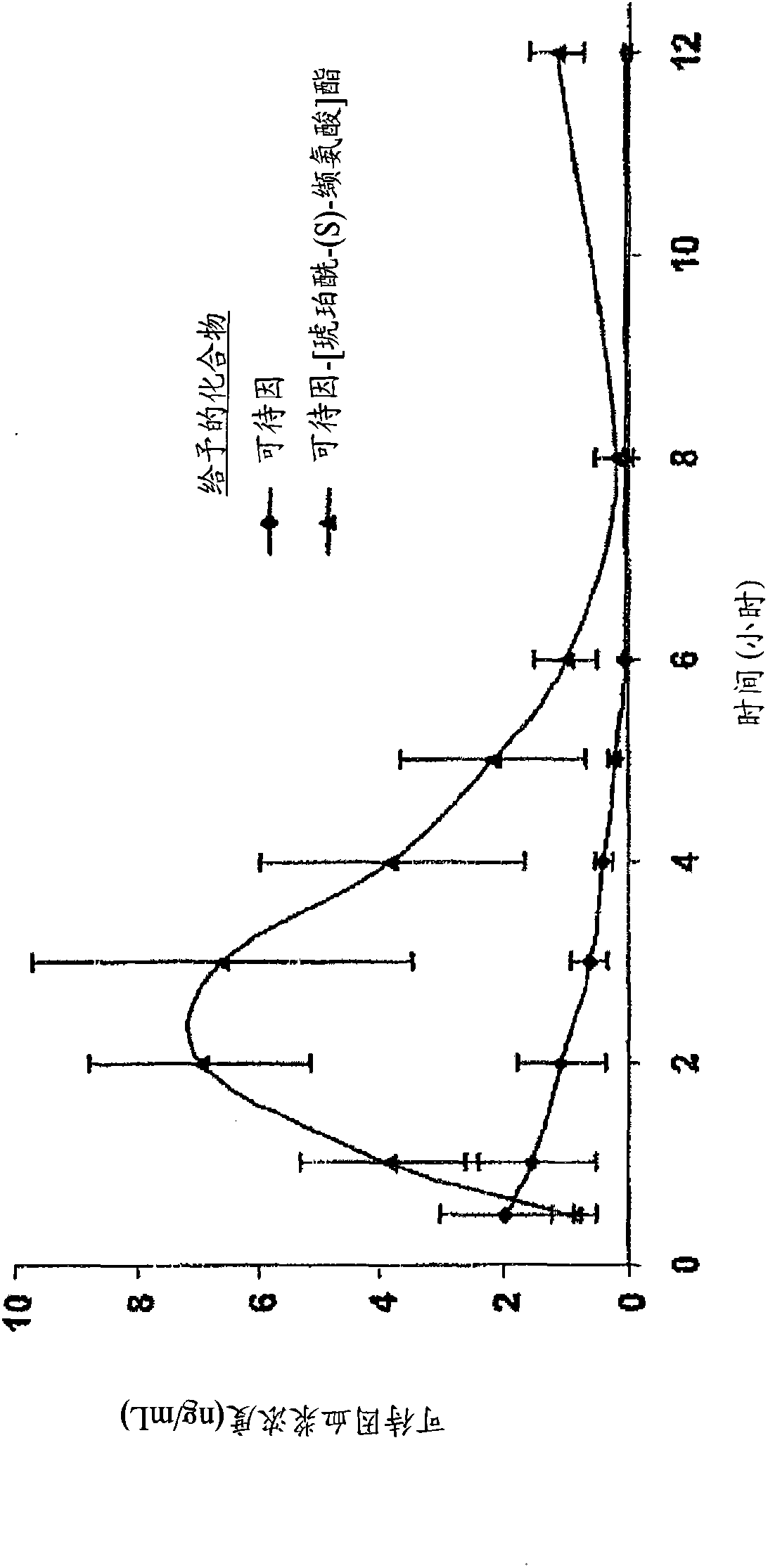
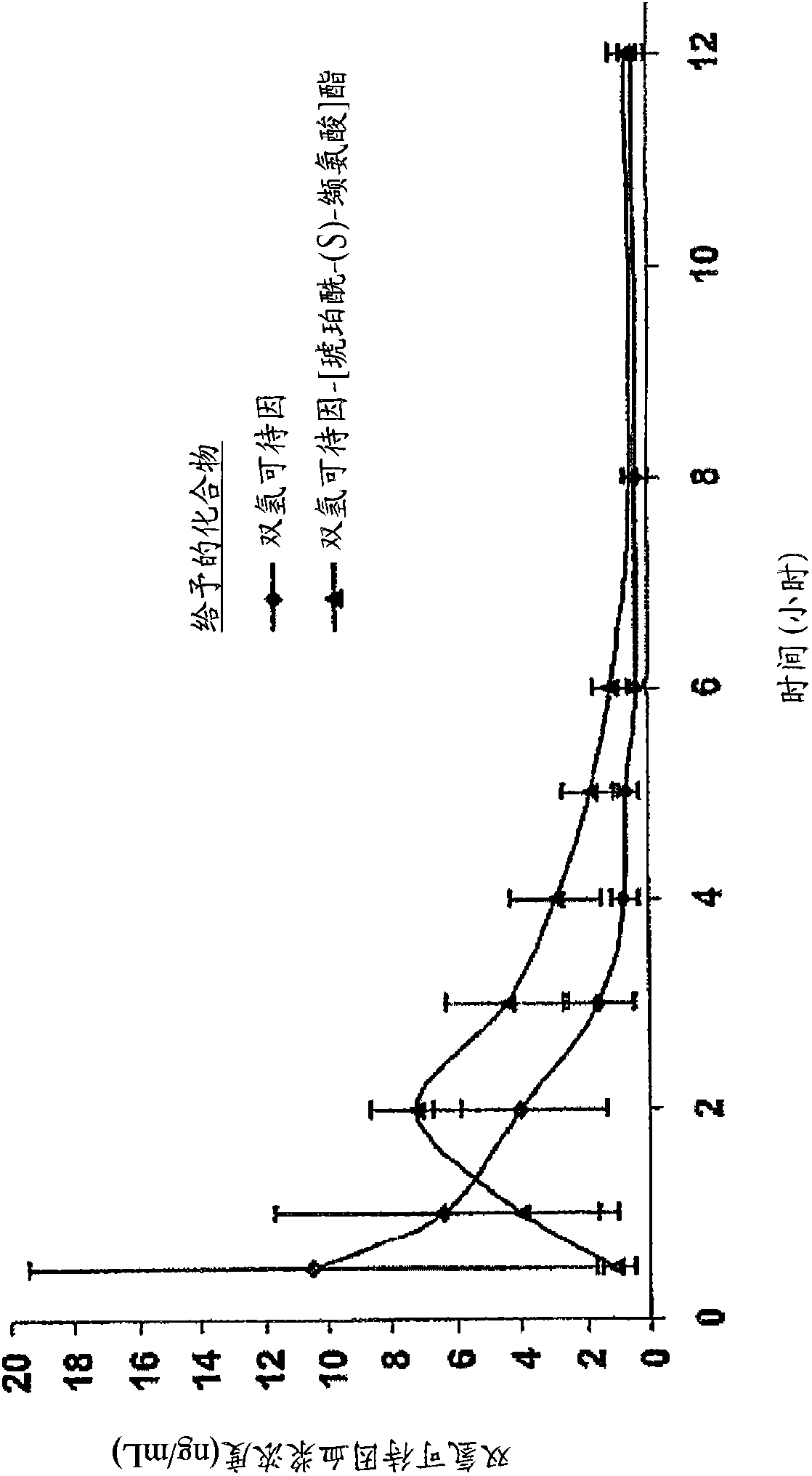
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
204 results about "Amino acid side chain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
There are three amino acids that have basic side chains at neutral pH. These are arginine (Arg), lysine (Lys), and histidine (His).
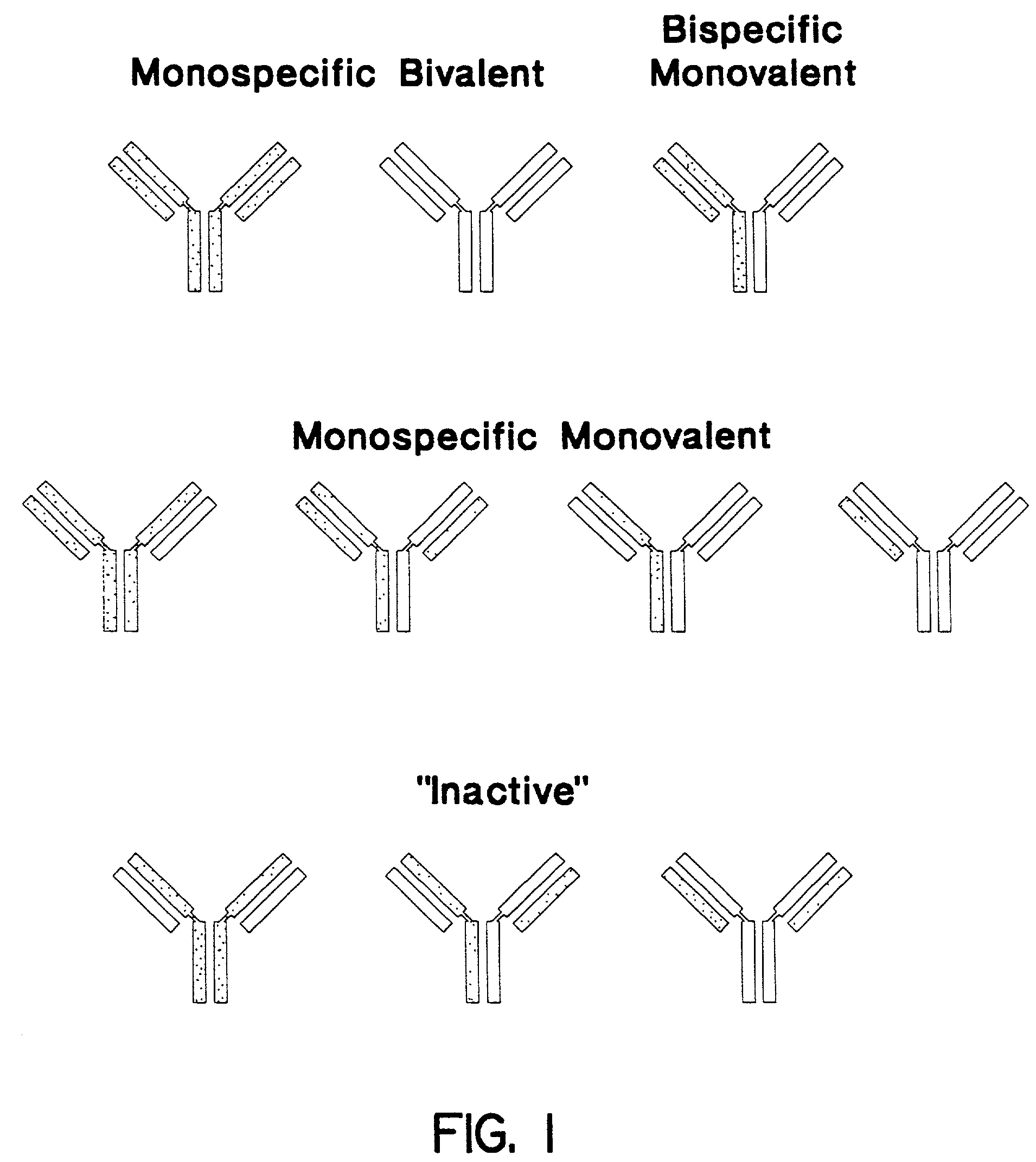
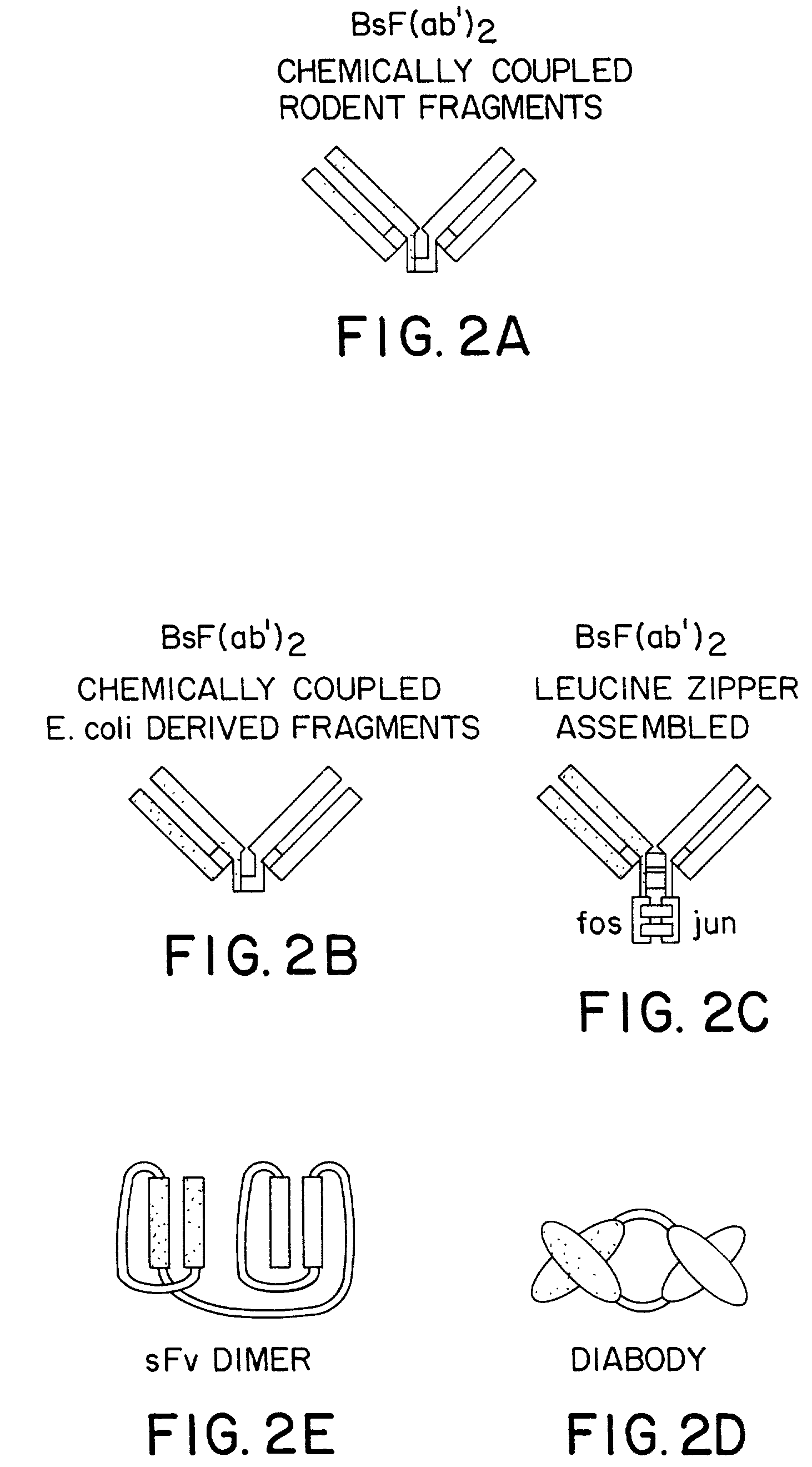
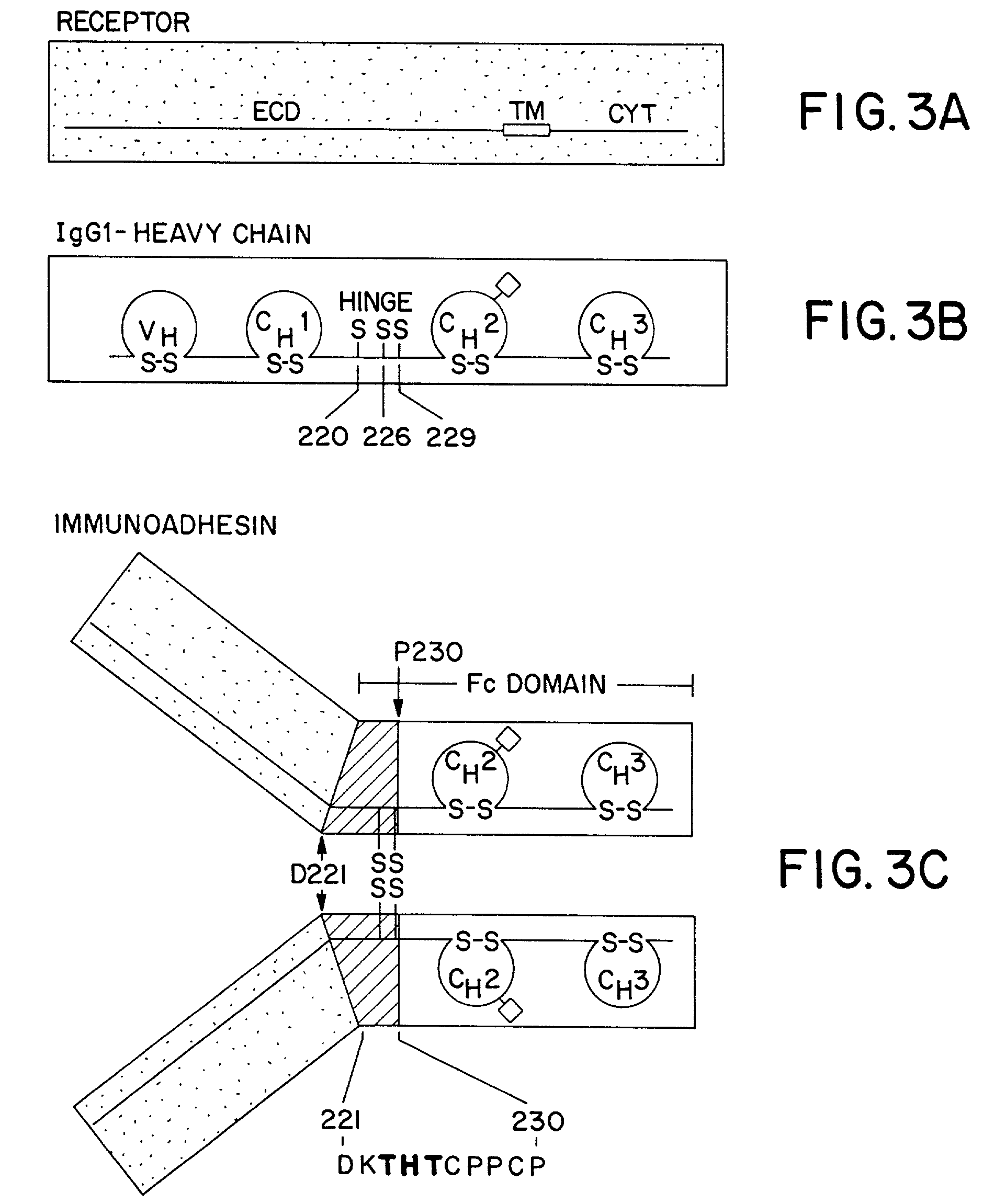
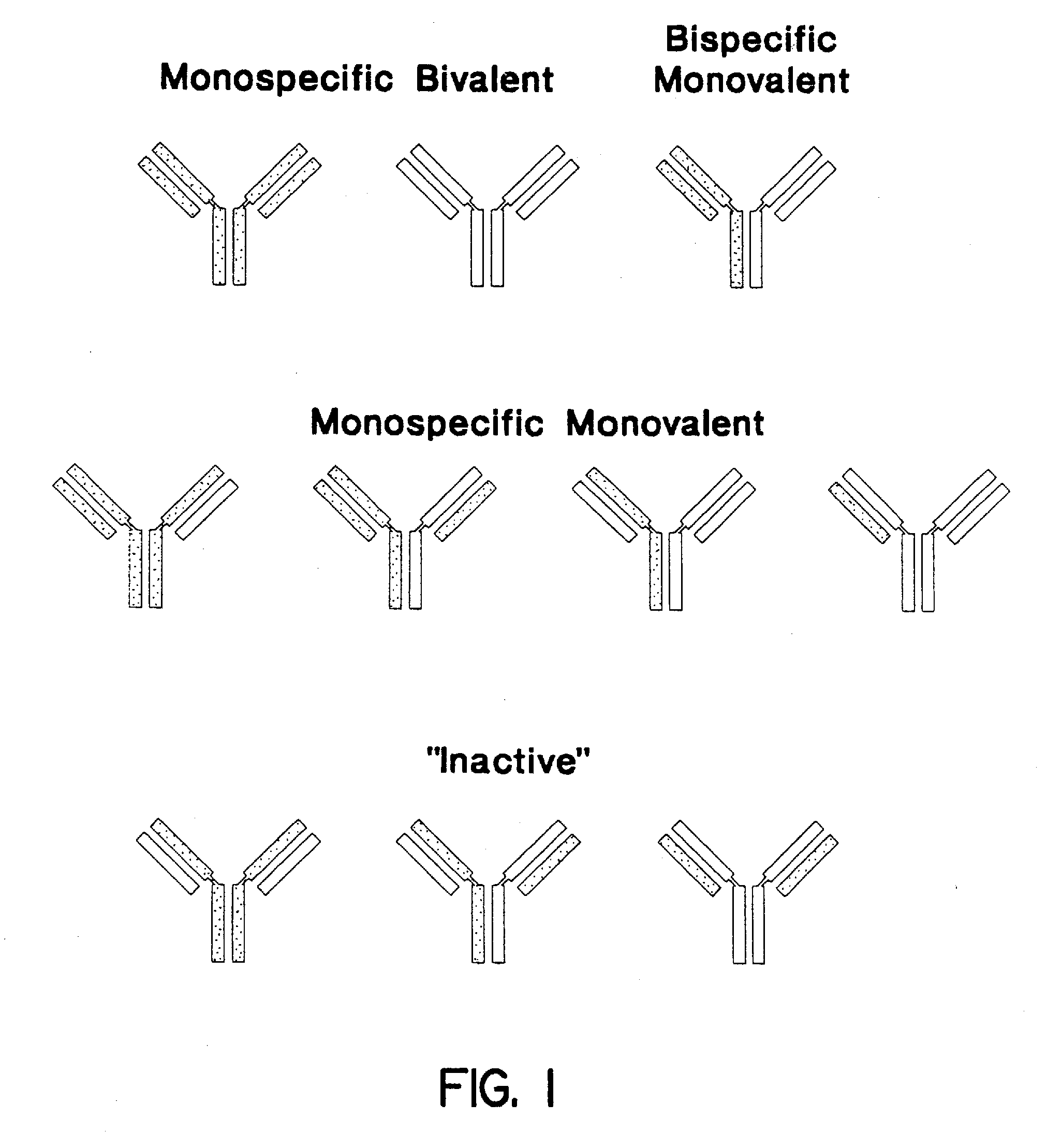
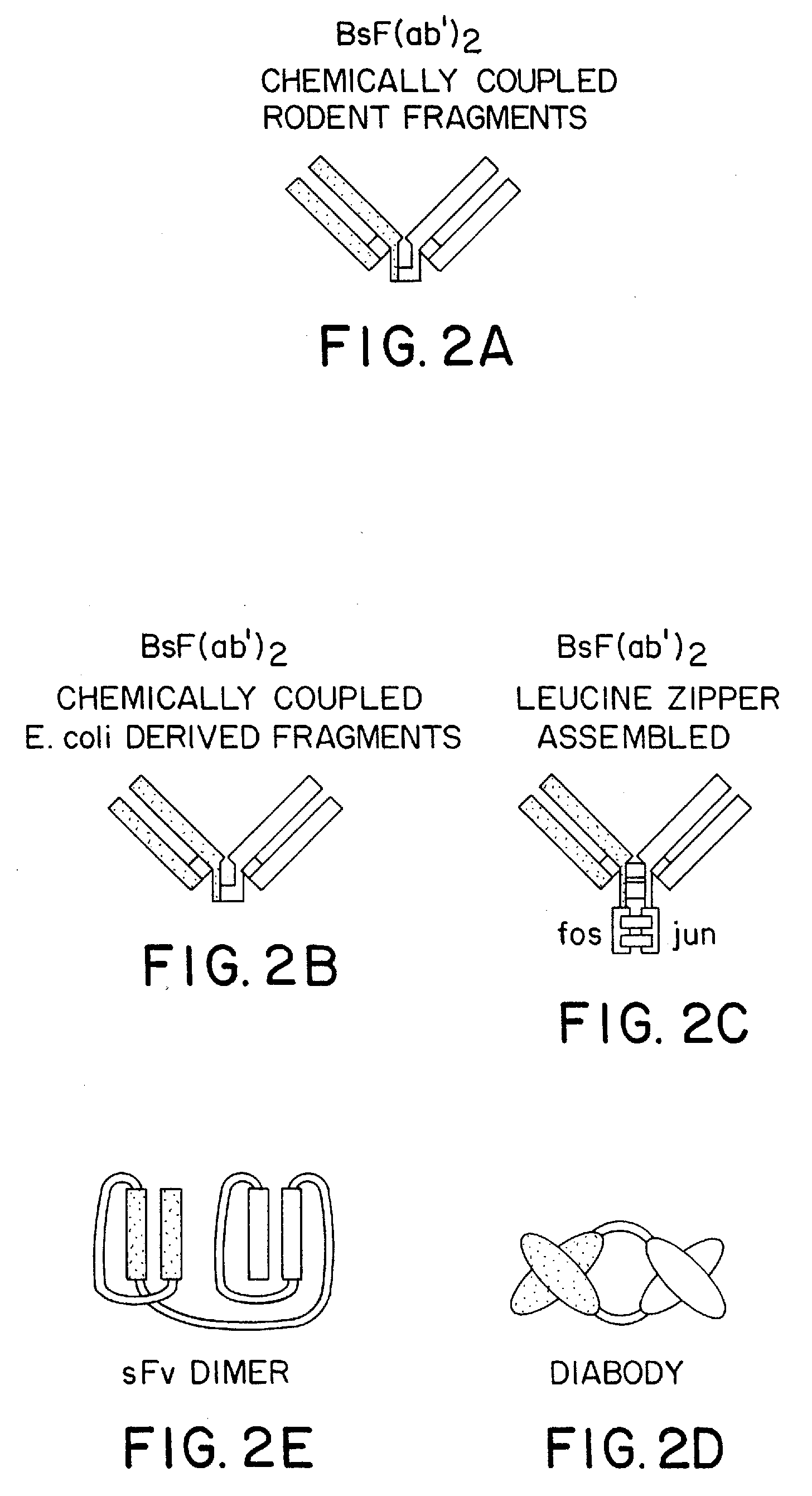
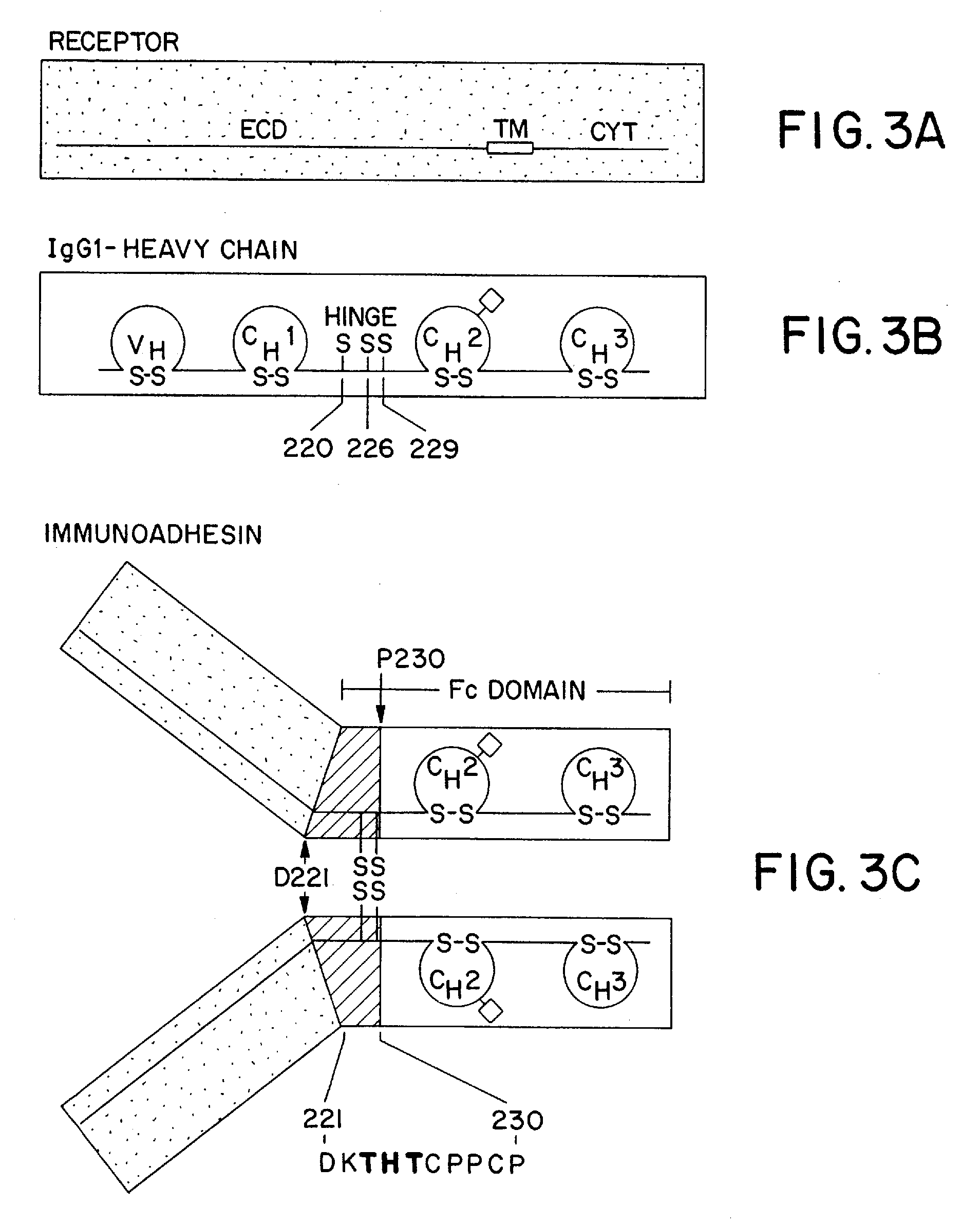
Method for making heteromultimeric polypeptides
InactiveUS7642228B2Increase productionAntibacterial agentsPeptide/protein ingredientsCrystallographyAmino acid side chain
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method involves introducing a protuberance at the interface of a first polypeptide and a corresponding cavity in the interface of a second polypeptide, such that the protuberance can be positioned in the cavity so as to promote heteromultimer formation and hinder homomultimer formation. “Protuberances” are constructed by replacing small amino acid side chains from the interface of the first polypeptide with larger side chains (e.g. tyrosine or tryptophan). Compensatory “cavities” of identical or similar size to the protuberances are created in the interface of the second polypeptide by replacing large amino acid side chains with smaller ones (e.g. alanine or threonine). The protuberance and cavity can be made by synthetic means such as altering the nucleic acid encoding the polypeptides or by peptide synthesis.
Owner:GENENTECH INC
Method for making heteromultimeric polypeptides
InactiveUS20070014794A1Increase productionAntibacterial agentsPeptide/protein ingredientsCrystallographyAmino acid side chain
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method involves introducing a protuberance at the interface of a first polypeptide and a corresponding cavity in the interface of a second polypeptide, such that the protuberance can be positioned in the cavity so as to promote heteromultimer formation and hinder homomultimer formation. “Protuberances” are constructed by replacing small amino acid side chains from the interface of the first polypeptide with larger side chains (e.g. tyrosine or tryptophan). Compensatory “cavities” of identical or similar size to the protuberances are created in the interface of the second polypeptide by replacing large amino acid side chains with smaller ones (e.g. alanine or threonine). The protuberance and cavity can be made by synthetic means such as altering the nucleic acid encoding the polypeptides or by peptide synthesis.
Owner:GENENTECH INC
Metallopeptide compositions for treatment of sexual dysfunction
Owner:PALATIN TECH INC
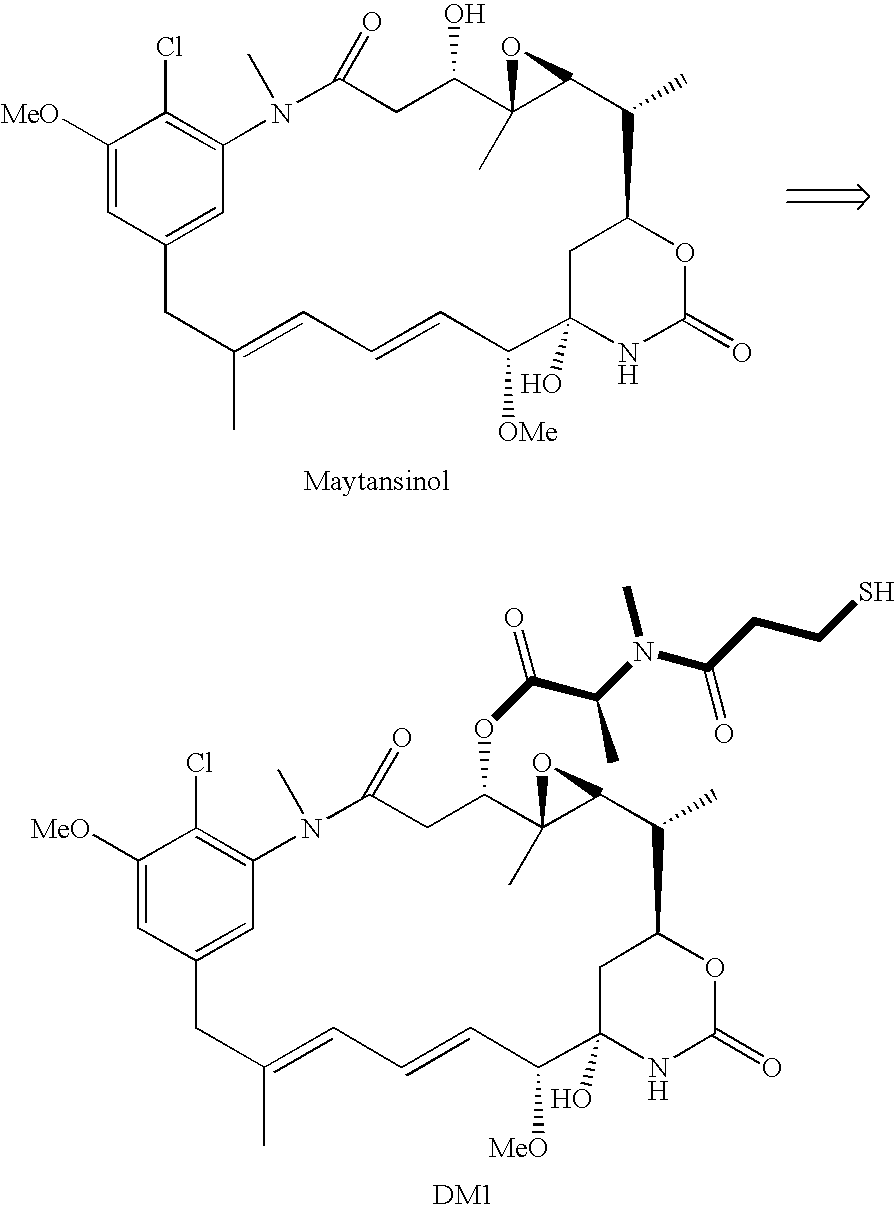
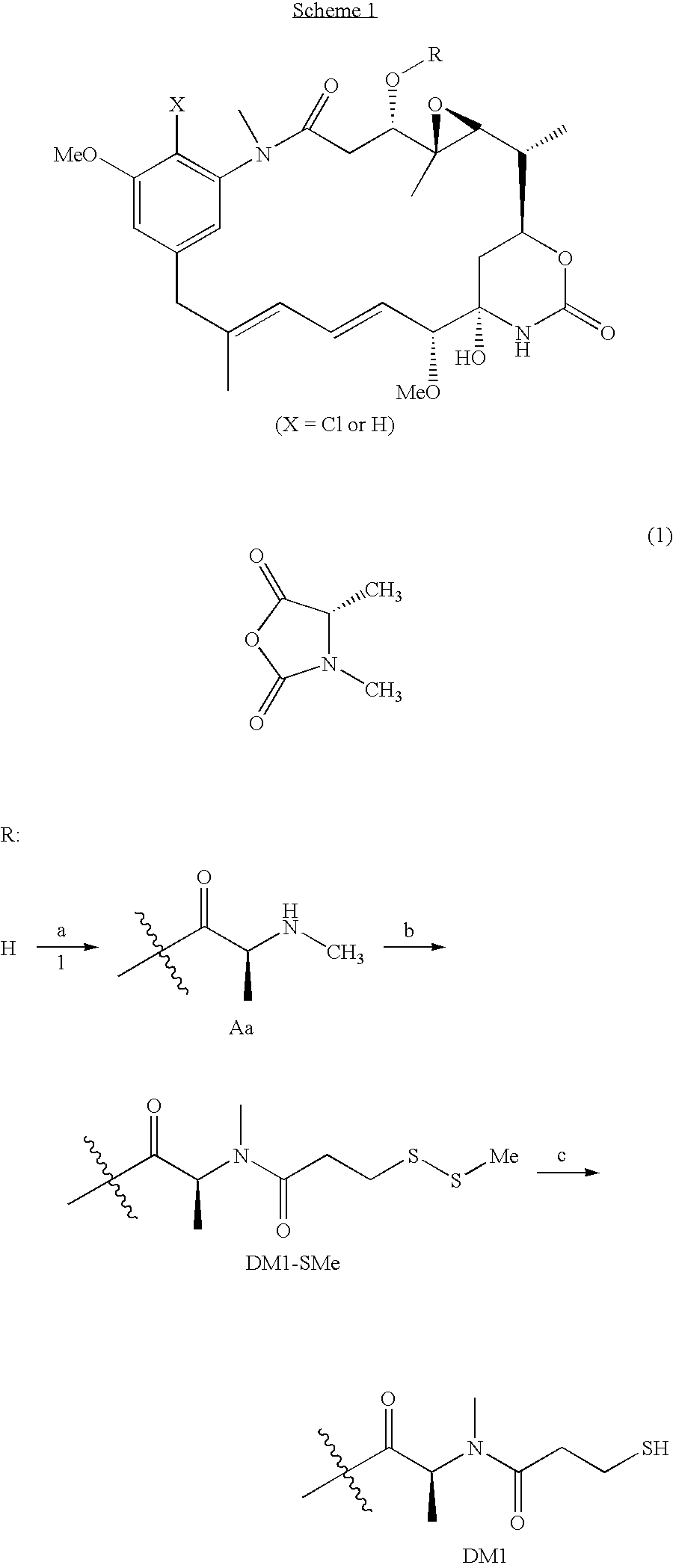
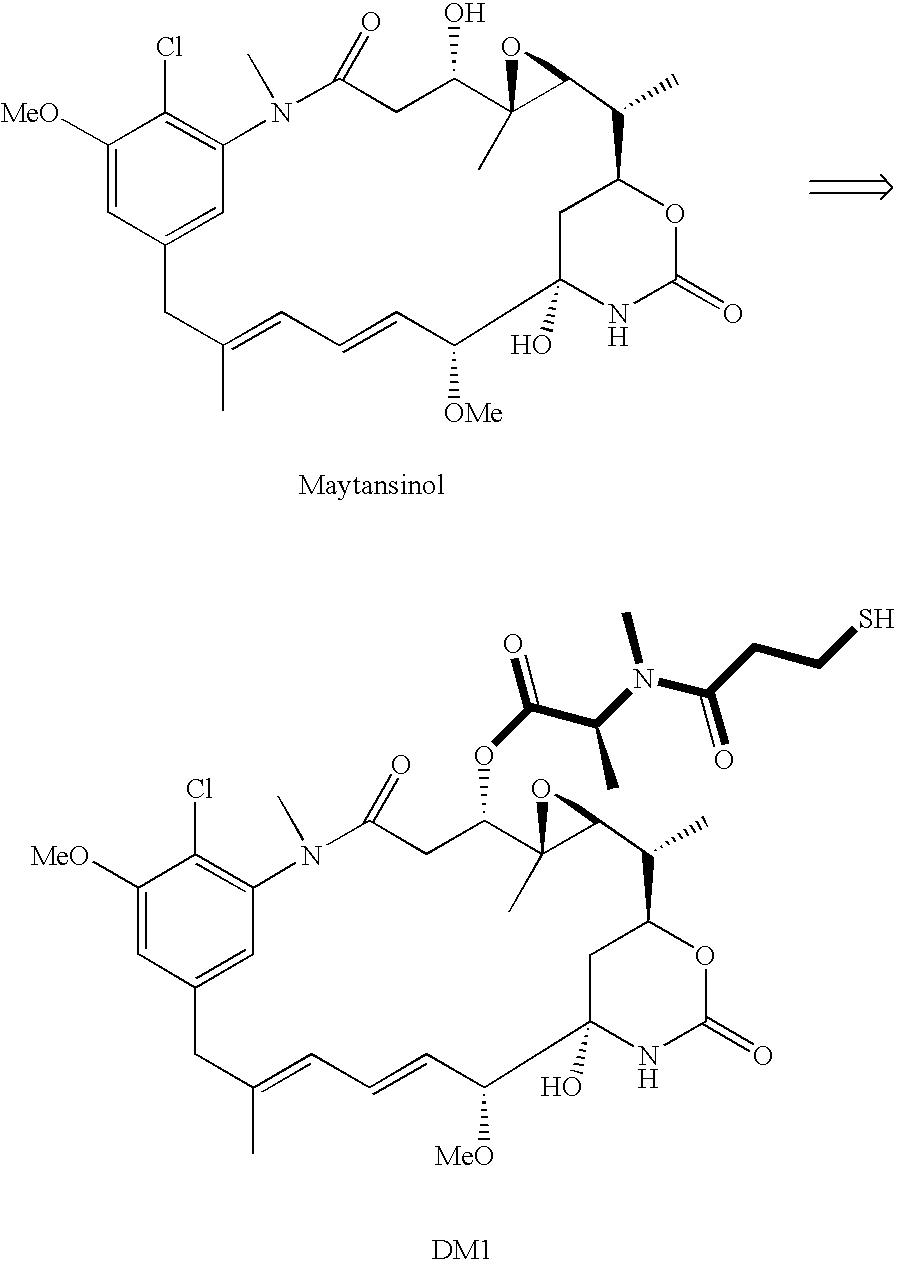
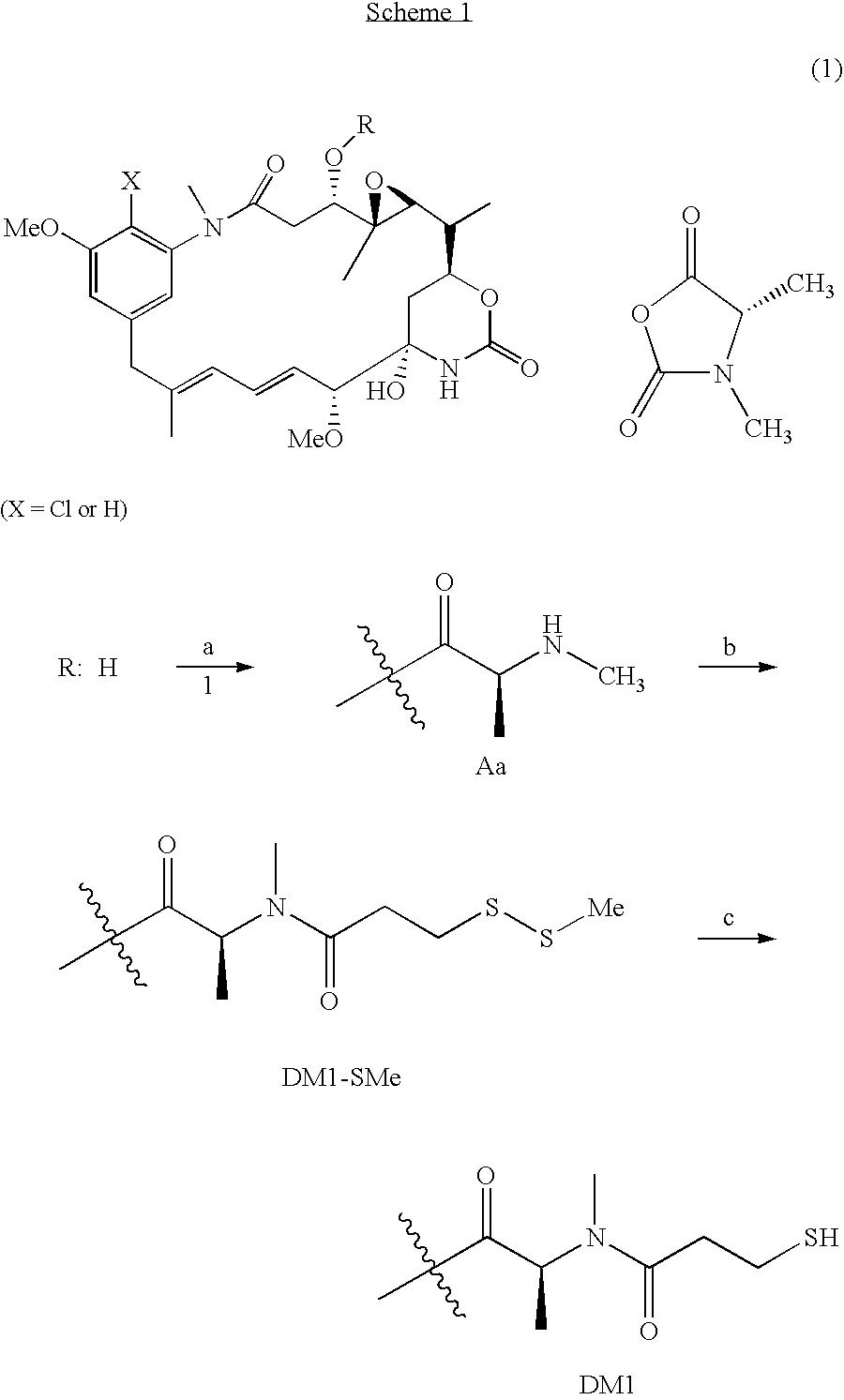
Method of acylating maytansinol with chiral amino acids
This invention provides a method for making maytansinoids having a chiral amino acid side chain, such as DM1 and DM4 that are used to treat cancer. According to this method, the side chain may be added with little epimerization of the amino acid chiral center.
Owner:IMMUNOGEN INC
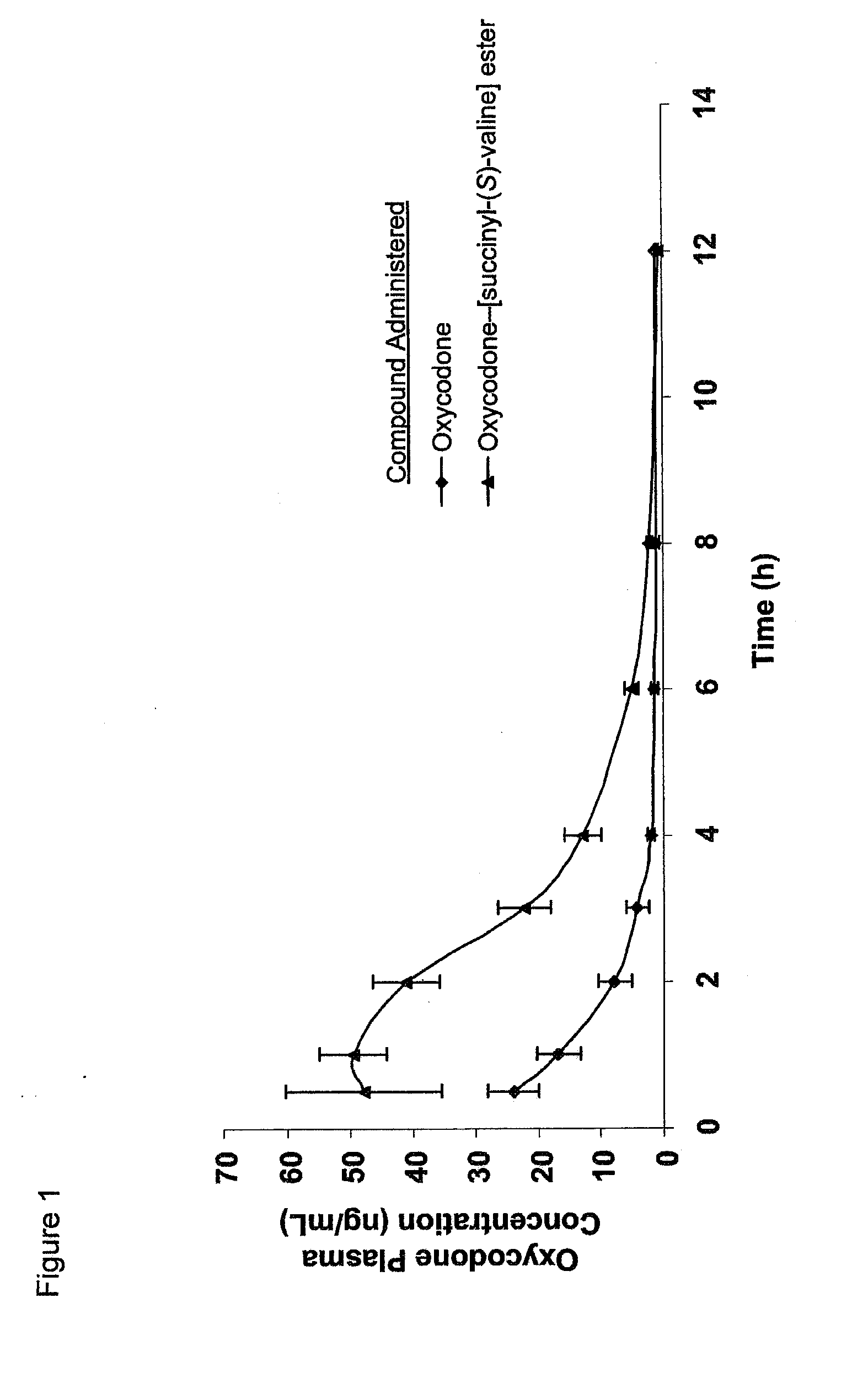
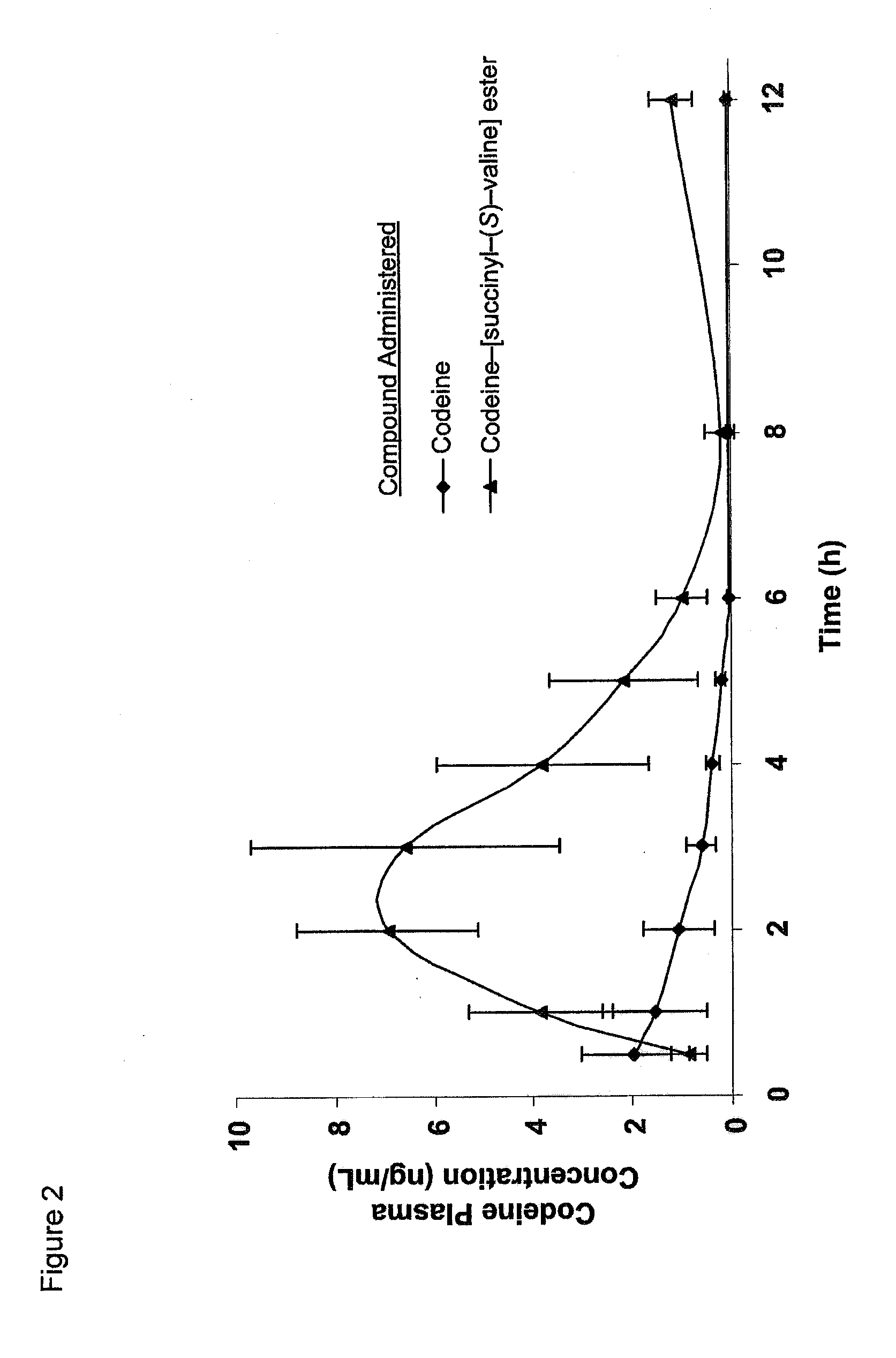
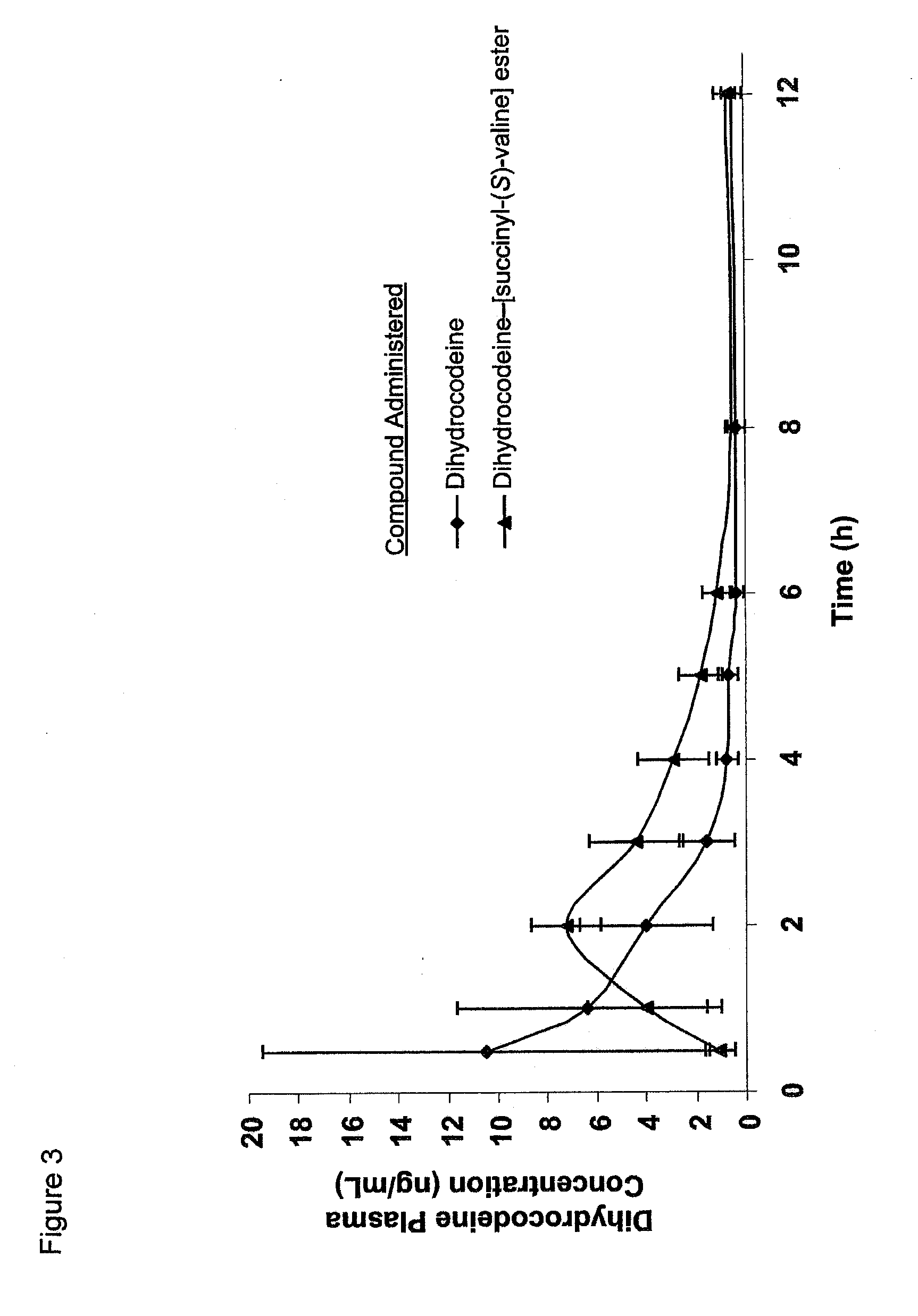
Novel dicarboxylic acid linked amino acid and peptide prodrugs of opioids and uses thereof
InactiveUS20100286186A1Low variabilityReduction and elimination of painBiocideNervous disorderSide effectAmino acid side chain
The present invention concerns dicarboxylic acid linked amino acid and peptide prodrugs of opioid analgesics and pharmaceutical compositions containing such prodrugs. Methods for providing pain relief, decreasing the adverse GI side effects of the opioid analgesic and increasing the bioavailability of the opioid analgesic with the aforementioned prodrugs are also provided. In one embodiment, prodrugs having the amino acid side chains of valine, leucine, isoleucine and glycine; and mono-, di- and tripeptides thereof are provided.
Owner:SHIRE PLC
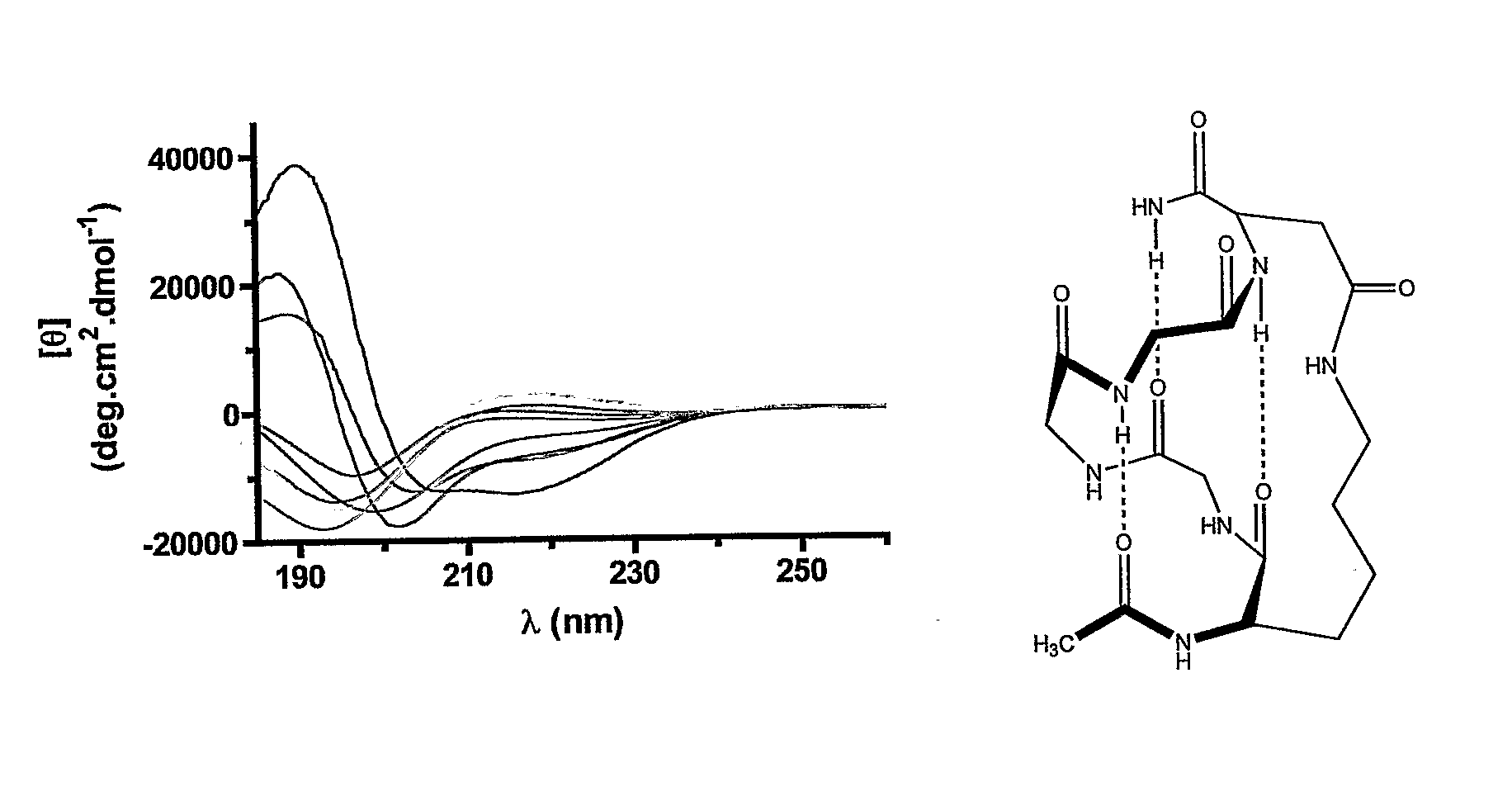
Alpha Helical Mimics, Their Uses and Methods For Their Production
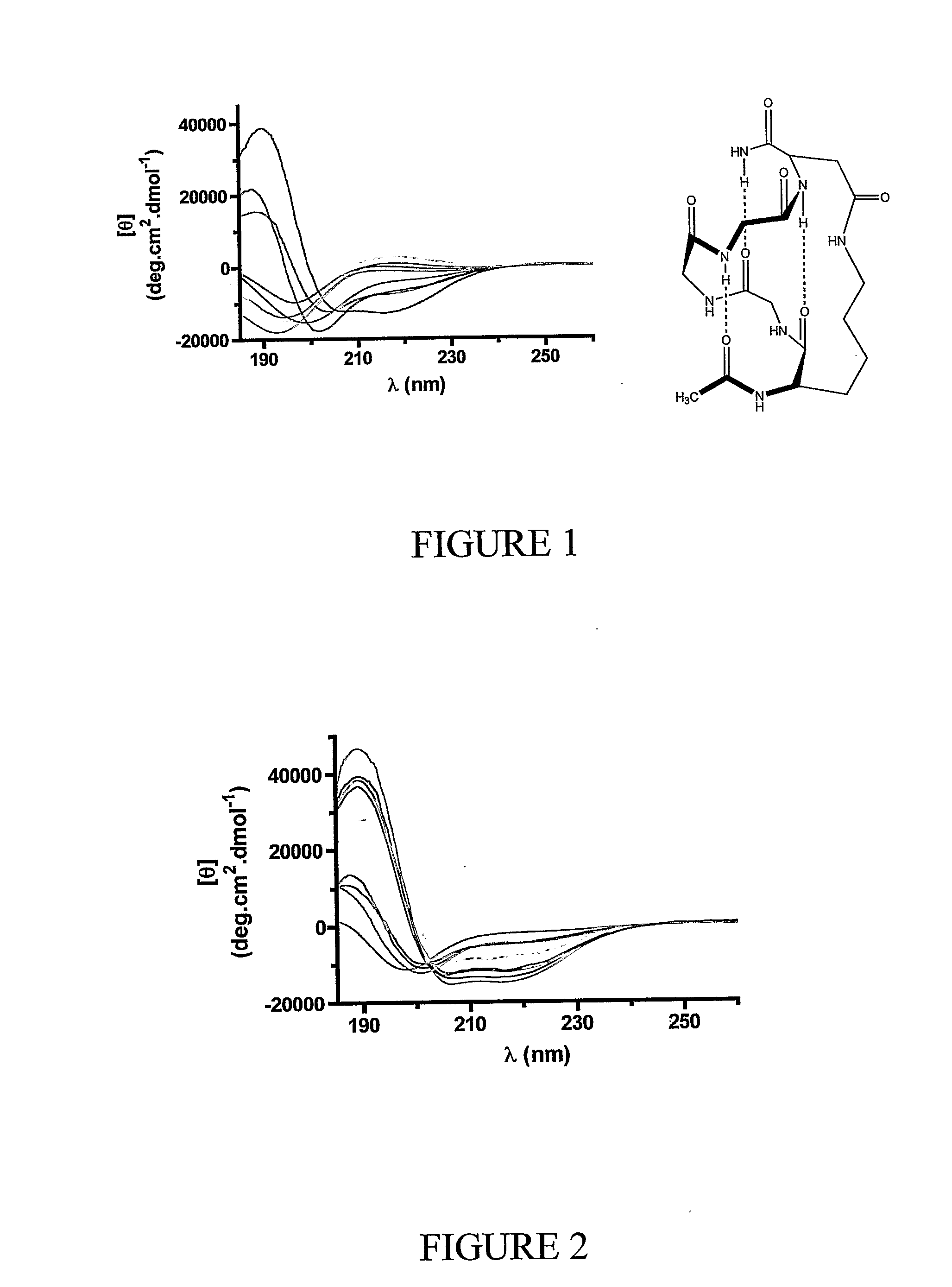
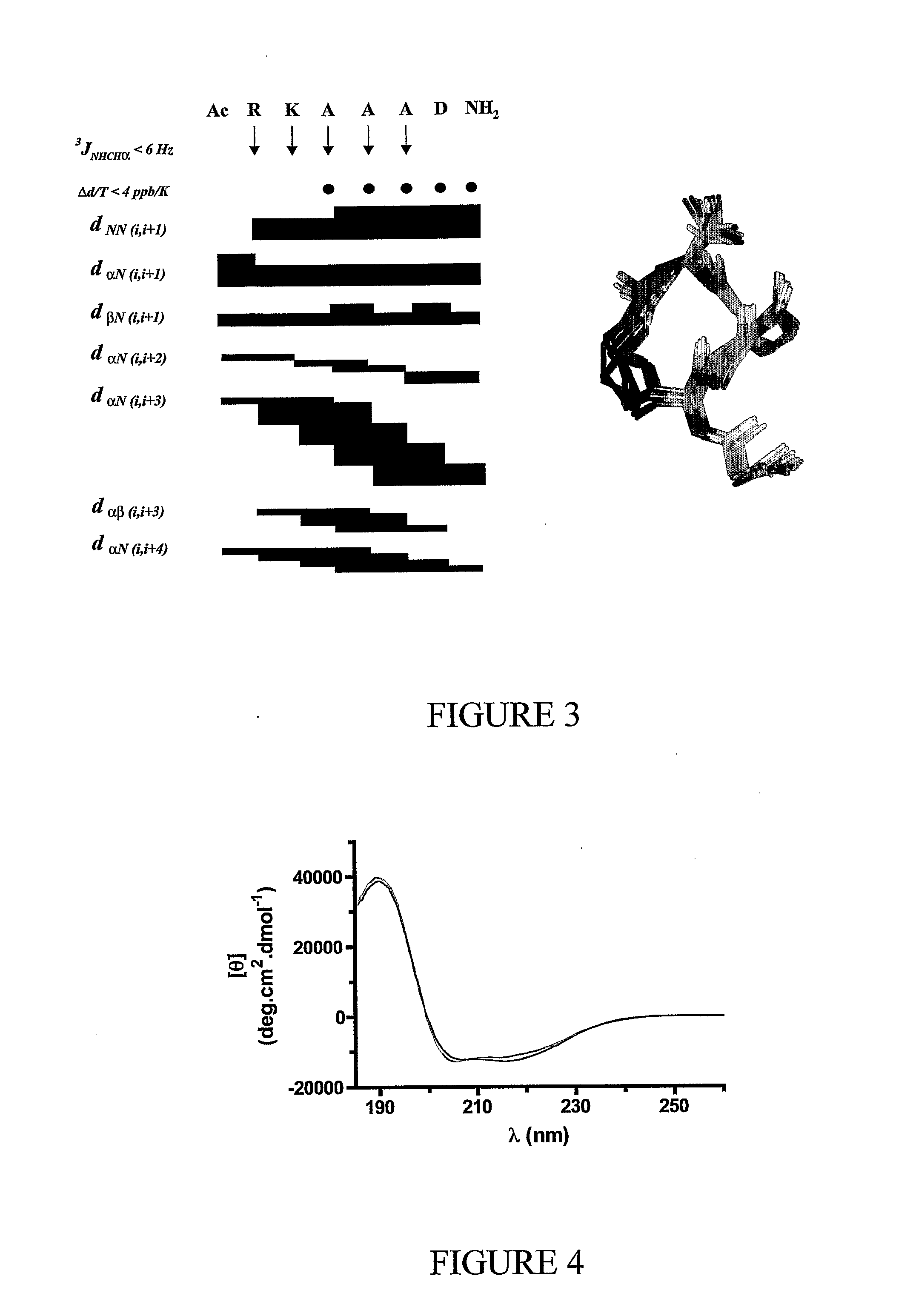
ActiveUS20080242598A1Enhance stability and bioavailability and activityImprove bioavailabilityNervous disorderDepsipeptidesMacrocyclic peptideAmino acid side chain
This invention discloses short chain peptides that have been constrained to adopt an alpha helical conformation and their use as alpha helical scaffolds for directing amino acid side chains into positions analogous to those found in longer chain alpha helical peptides and for attaching peptidic or non-peptidic appendages in order to mimic side chains of longer alpha helical peptides. More particularly the invention discloses alpha helical cyclic pentapeptides and their use as alpha helical scaffolds or macrocyclic alpha helical modules, either alone, or within longer chain peptides or attached to other macrocyclic peptides or attached to non-peptidic structures, for the purpose of mimicking naturally occurring peptides or proteins, and as agonists or antagonists of the biological activity of naturally-occurring peptides or proteins or for the preparation of new materials.
Owner:THE UNIV OF QUEENSLAND
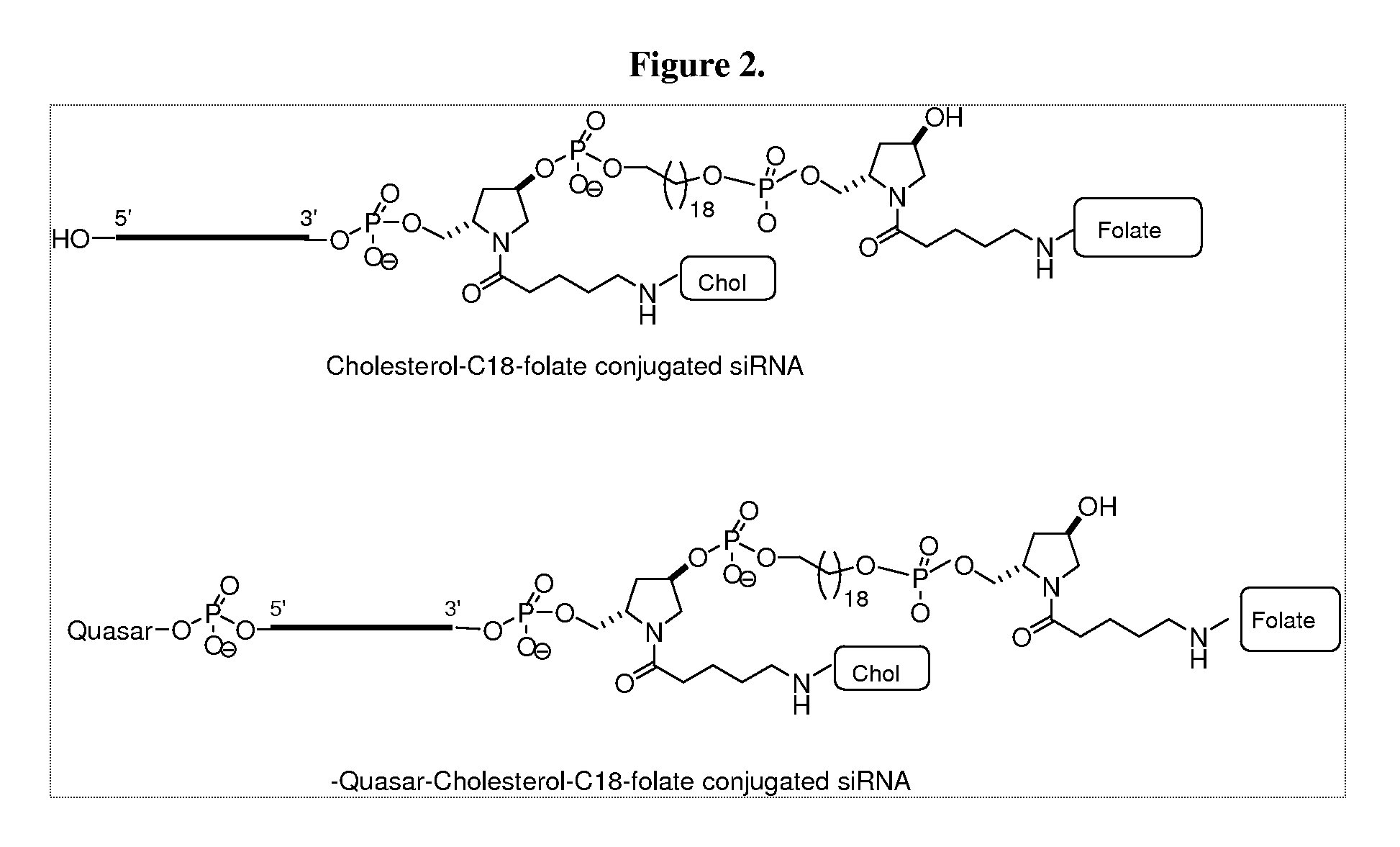
Folate conjugates
Owner:ALNYLAM PHARMA INC
Controlled modulation of amino acid side chain length of peptide antigens
ActiveUS20050169934A1Extend and shortenReduce decreaseBacterial antigen ingredientsPeptide/protein ingredientsPeptide antigenEpitope
The invention provides a method for the creation of peptide antigens comprising epitopes with at least a first modification comprising a shortened or lengthened amino acid side chain. By extension or shortening of the side chain with CH3 / CH2 groups, for example, made by computer assisted modeling of the tumor antigen (peptide) bound in the MHC-I-groove, immunogenicity can be improved with minimal modification of adjacent tertiary structure, thereby avoiding cross-reactivity. Provided by the invention are methods of creating such antigens, as well as methods for therapeutic or prophylactic treatment of various conditions comprising administration of the antigens.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Method of acylating maytansinol with chiral amino acids
This invention provides a method for making maytansinoids having a chiral amino acid side chain, such as DM1 and DM4 that are used to treat cancer. According to this method, the side chain may be added with little epimerization of the amino acid chiral center.
Owner:IMMUNOGEN INC
Thermo-responsive hydrogel compositions
InactiveUS20120231072A1Good biological propertiesDesirable release kineticsBiocideOrganic active ingredientsAmino acid side chainNanoparticle
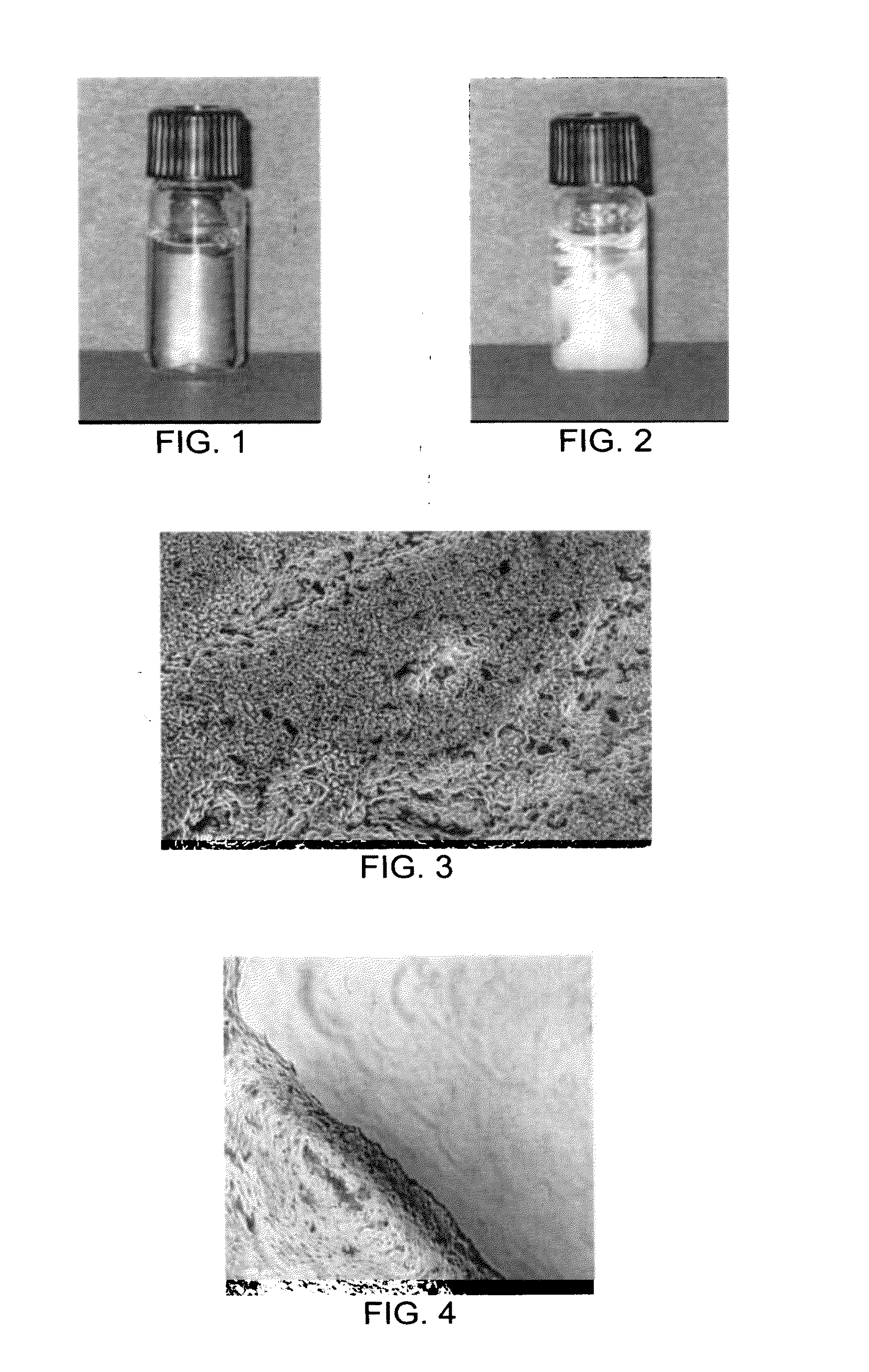
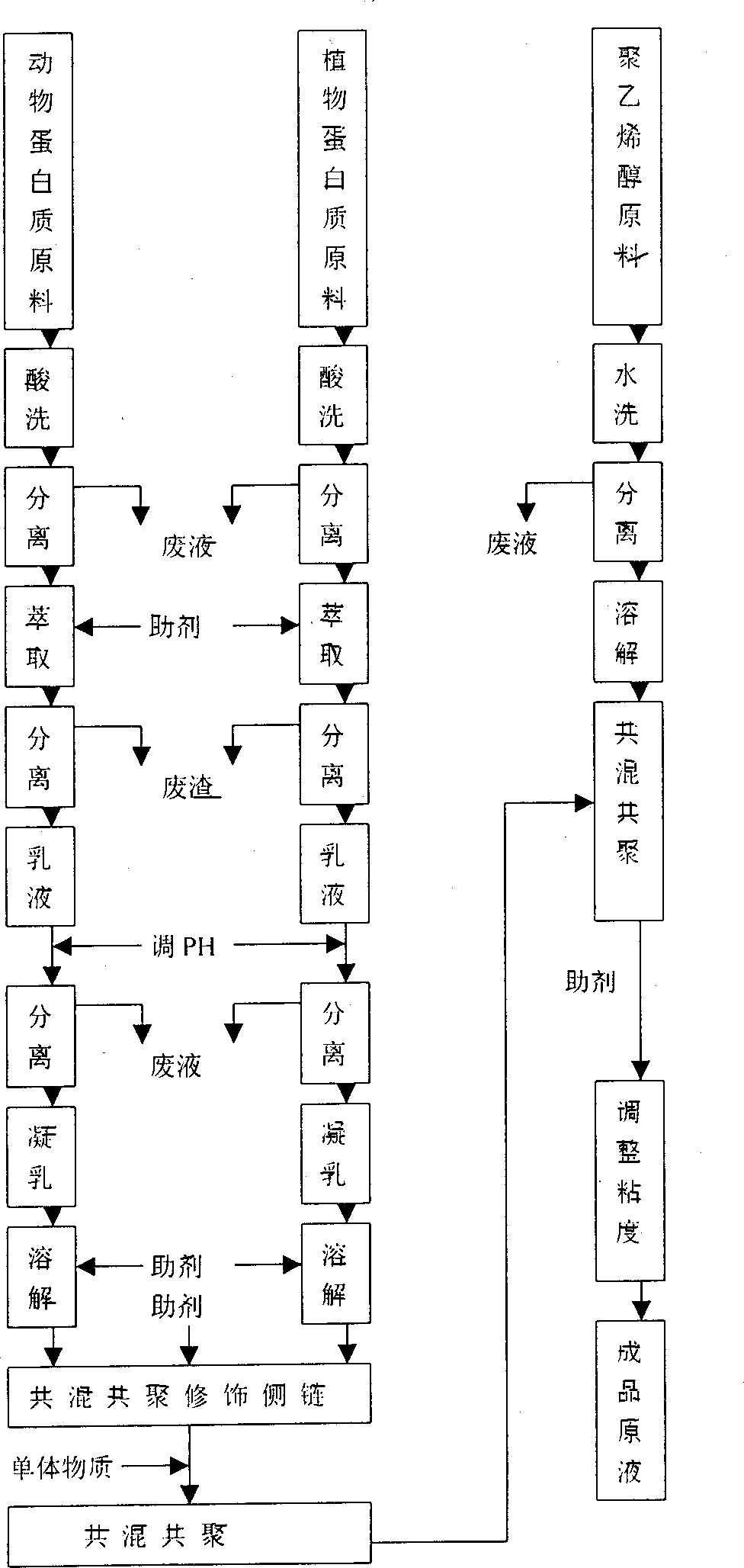
A thermo-responsive hydrogel, including a biocompatible monomer and / or polymer having an amino acid side chain. The hydrogel is thermo-responsive at a physiological temperature, and can include, incorporate, or encapsulate a treatment agent, such as a drug composition, a biomolecule, and / or a nanoparticle. The hydrogel is useful in delivering the treatment agent. The hydrogel is in a first physicochemical state for administration to a mammal. The hydrogel is thermo-responsive at a physiological temperature of the mammal, and changes to a second physicochemical state that is more solid than the first physicochemical state. In the second physicochemical state the thermo-responsive hydrogel releases the treatment agent.
Owner:ILLINOIS INSTITUTE OF TECHNOLOGY +1
Treatment of rhinitis
ActiveUS20140294738A1Extended stayOrganic active ingredientsAntipyreticAmino acid side chainDiketopiperazines
The invention provides a method of treating rhinitis. The method comprises administering an effective amount of a pharmaceutical composition comprising a diketopiperazine with amino acid side chains of aspartic acid and alanine (DA-DKP) formulated for nasal administration. The invention also provides a pharmaceutical product comprising a DA-DKP containing composition.
Owner:AMPIO PHARMA
Protein synthetic fibre spinning solution and its producing method
InactiveCN1364948ASkin-friendlyBreathableConjugated cellulose/protein artificial filamentsPolymer scienceAmino acid side chain
The protein fiber spinning solution is produced by using animal and plant material and through the processes of acid pickling, extraction, pH regulation to obtain protein curd, dissolving with cosolvent to form water solution, mixing, modifying side chain of amino acid with modifying agent, copolymerization with coloring monomer, mixing with PVA water solution and addition crosslinking agent to regualte viscosity. It may be used in spinning fiber with the same strengt has chemical fiber, the skin friendship, air penetrability and hygroscopicity the same as natural protein fiber, and improved hot water resistance, shrinkage and color.
Owner:卓宝松 +1
Ethoxy diphenyl ethane derivative and preparation method and application thereof
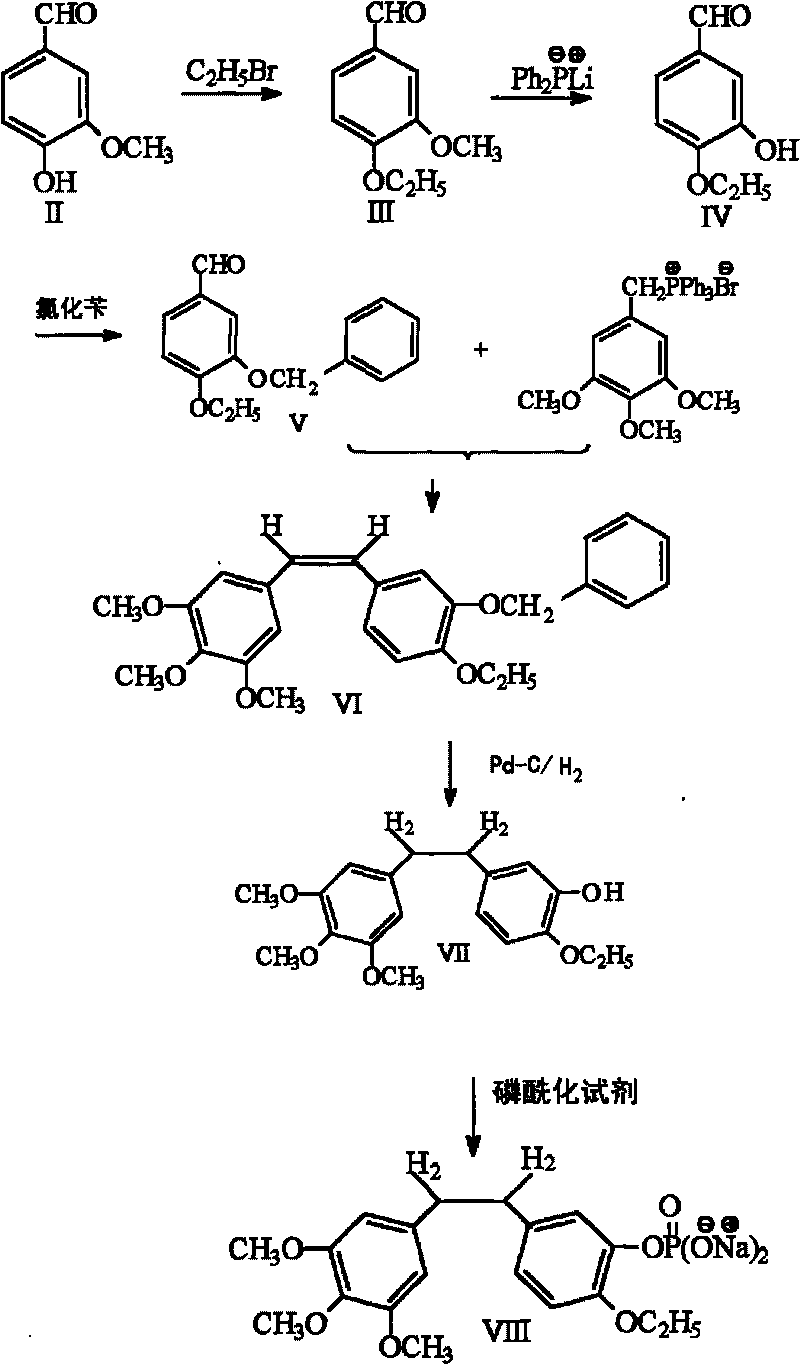
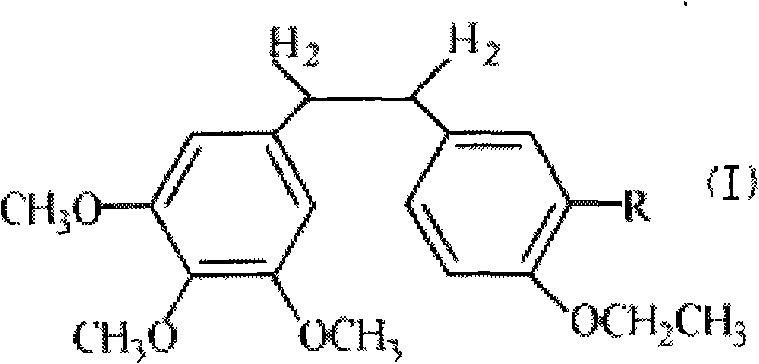
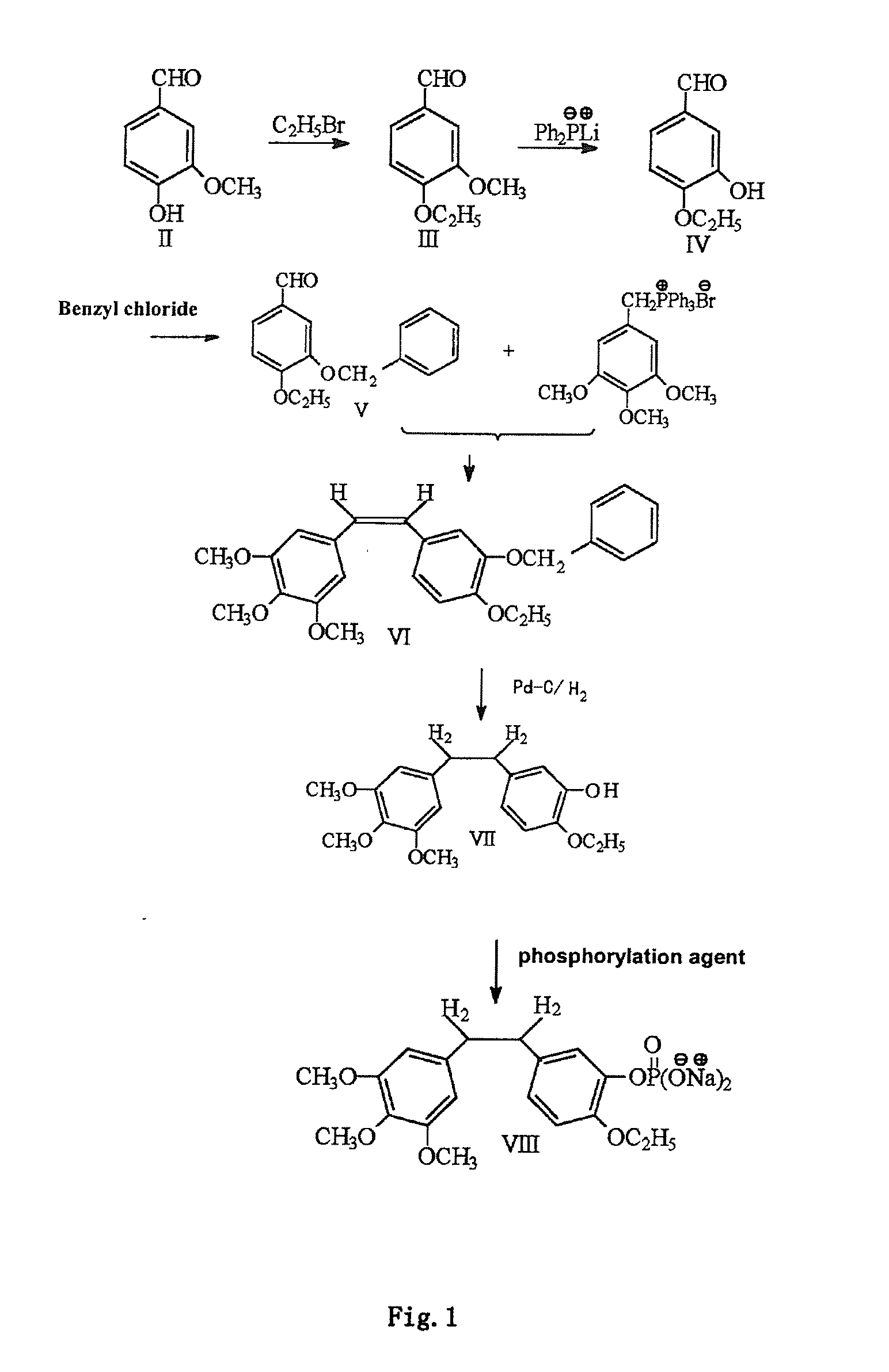
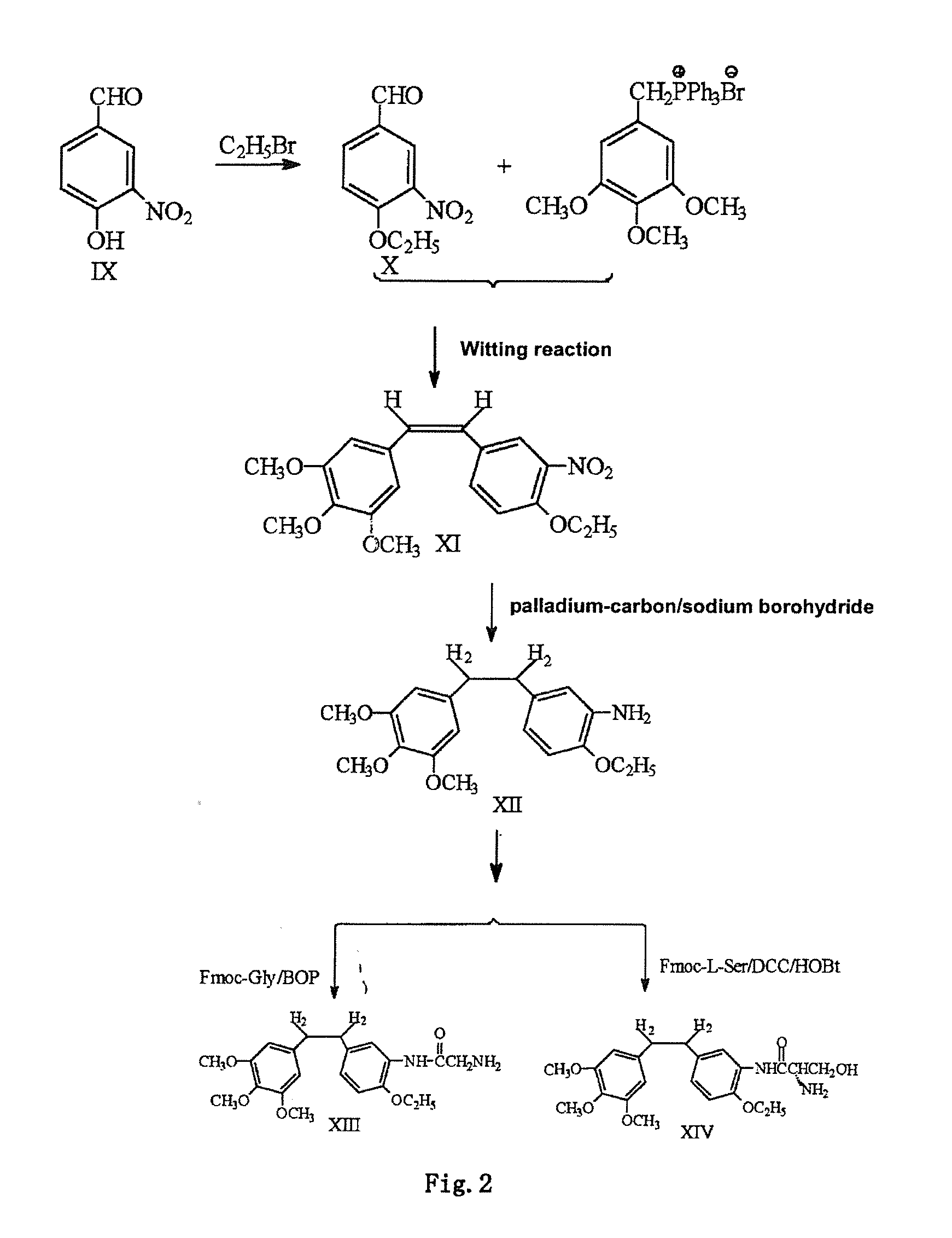
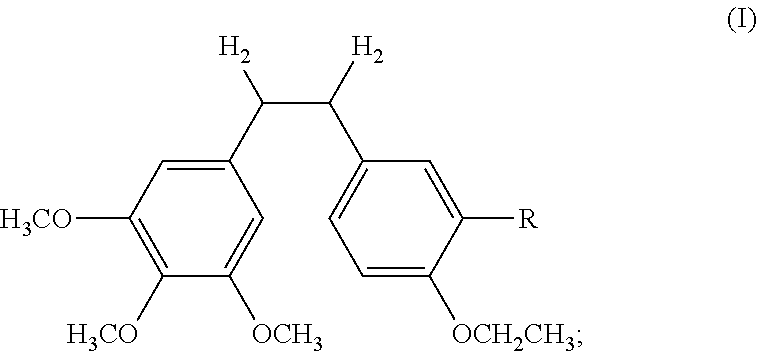
InactiveCN101723813AImprove stabilityReduce lossGroup 5/15 element organic compoundsEther/acetal active ingredientsAmino acid side chainLymphatic Spread
The invention discloses an ethoxy diphenylethane derivative and a preparation method and application thereof. The 4, position of a B aromatic ring of phenylethane is chemically modified by an ethoxyl group, and simultaneously, a hydroxyl group at the 3, position of the B aromatic ring of the phenylethane is modified into water-soluble pro-drugs such as phosphate and the like, and likewise, an amino acid side chain is introduced into an amino group at the 3, position to form an amino acid amide water-soluble pre-drug having a structure as shown by a structural formula (I). An ethoxy phenylethane derivative and a pre-drug thereof have strong capacity of inhibiting tubulin aggregation, have obvious targeted destructive functions to tumor vessels, selectively cause the tumor vessels to have dysfunctions and structural damages, induce the apoptosis of vascular endothelial cells so that tumor cells lose the support of nutrition and oxygen gas, and give play to the functions of killing and wounding the tumor cells or inhibiting the tumor metastasis.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD +1
Treatment of degenerative joint disease
ActiveUS20130090292A1Improve throughputLimit deliverySkeletal disorderPharmaceutical delivery mechanismAmino acid side chainDiketopiperazines
The invention provides a method of treating a degenerative joint disease. The method comprises administering an effective amount of a pharmaceutical composition comprising a diketopiperazine with amino acid side chains of aspartic acid and alanine (DA-DKP). The invention also provides a pharmaceutical product as well as a kit comprising DA-DKP.
Owner:AMPIO PHARMA
Treatment of degenerative joint disease
ActiveUS20140256642A1Improve throughputLimit deliveryPharmaceutical delivery mechanismSkeletal disorderAmino acid side chainDiketopiperazines
The invention provides a method of treating a degenerative joint disease. The method comprises administering an effective amount of a pharmaceutical composition comprising a diketopiperazine with amino acid side chains of aspartic acid and alanine (DA-DKP). The invention also provides a pharmaceutical product as well as a kit comprising DA-DKP.
Owner:AMPIO PHARMA INC
Capture compounds, collections thereof and methods for analyzing the proteome and complex compositions
InactiveUS8569481B2Effective treatmentEffective therapyPeptide librariesPeptide/protein ingredientsAmino acid side chainComplex protein
Capture compounds and collections thereof and methods using the compounds for the analysis of biomolecules are provided. In particular, collections, compounds and methods are provided for analyzing complex protein mixtures, such as the proteome. The compounds are multifunctional reagents that provide for the separation and isolation of complex protein mixtures. Each compound has the formula:wherein: Z is a trityl derivative; X, the reactivity function, covalently binds to amino acid side chains of proteins; Y, the selectivity function, modulates binding of X to the amino acid side chains in proteins such that X binds to fewer proteins when Y is present than in its absence; and Q permits separation or immobilization of the capture compound. Automated systems for performing the methods also are provided.
Owner:IONIS PHARMA INC
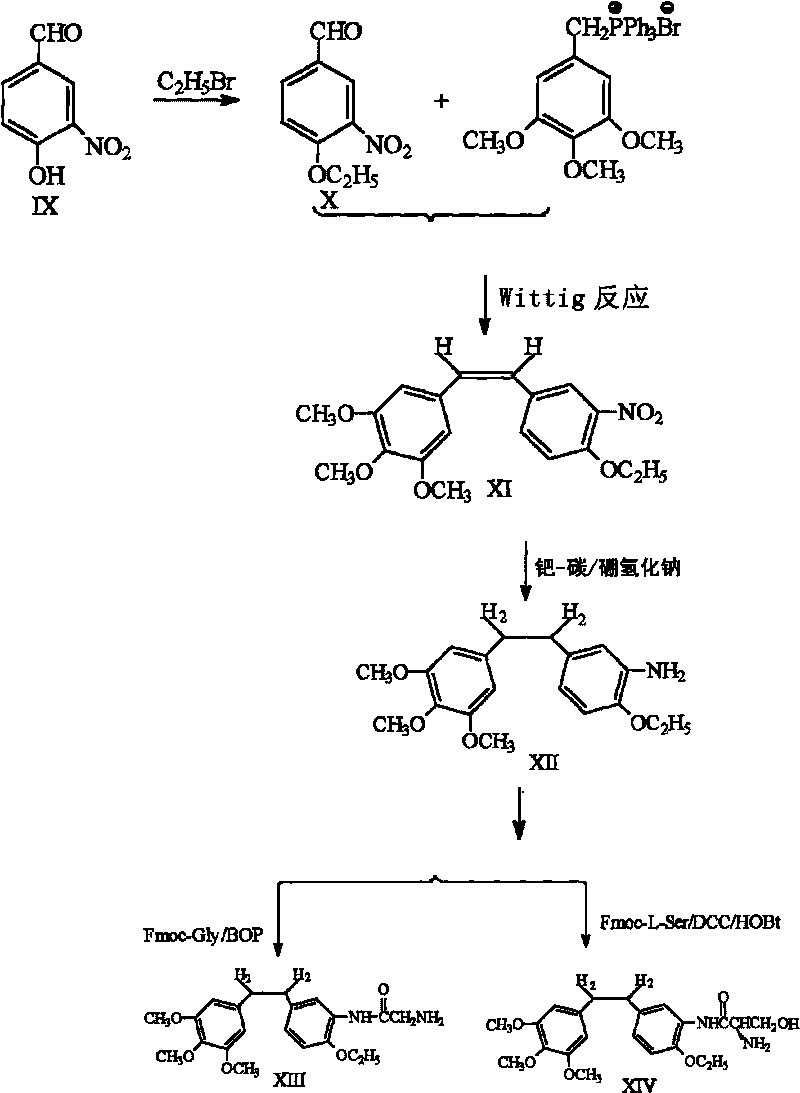
Ethoxy Diphenyl Ethane Derivatives, Preparation Processes and Uses Thereof
ActiveUS20120046492A1Improve drug stabilityLow toxicityOrganic compound preparationGroup 5/15 element organic compoundsAmino acid side chainVascular endothelium
The invention discloses an ethoxydiphenylethane derivative and a synthetic method and uses thereof 4′ position of phenylethane B aromatic ring is chemically modified by ethoxy and hydroxy at position 3′ thereof is simultaneously modified to water soluble prodrug such as phosphate, and similarly, amino acid side chain is introduced to amino at position 3′ to form amino acid amide water soluble prodrug having the structure shown as formula (I)the ethoxydiphenylethane derivative and the prodrug thereof include strong tubulin aggregation inhibiting ability and obvious target damage effect for tumor vessels, selectively cause dysfunction and structural damage of tumor vessels and induce apoptosis of vascular endothelial cells in order to play the role of killing tumor cells or inhibiting tumor metastasis in case that the tumor cells are free from the support of nutrition and oxygen.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD +1
Treatment of joint conditions
ActiveUS20160045493A1Improve throughputLimit deliveryOrganic active ingredientsPeptide/protein ingredientsDiseaseDosing regimen
The invention provides a method of treating a joint condition. The method comprises administering a multi-dose regimen of a pharmaceutical composition comprising a diketopiperazine with amino acid side chains of aspartic acid and alanine (DA-DKP). The invention also provides a method of treating osteoarthritis with multiple doses of a low-molecular weight fraction of human serum albumin.
Owner:AMPIO PHARMA
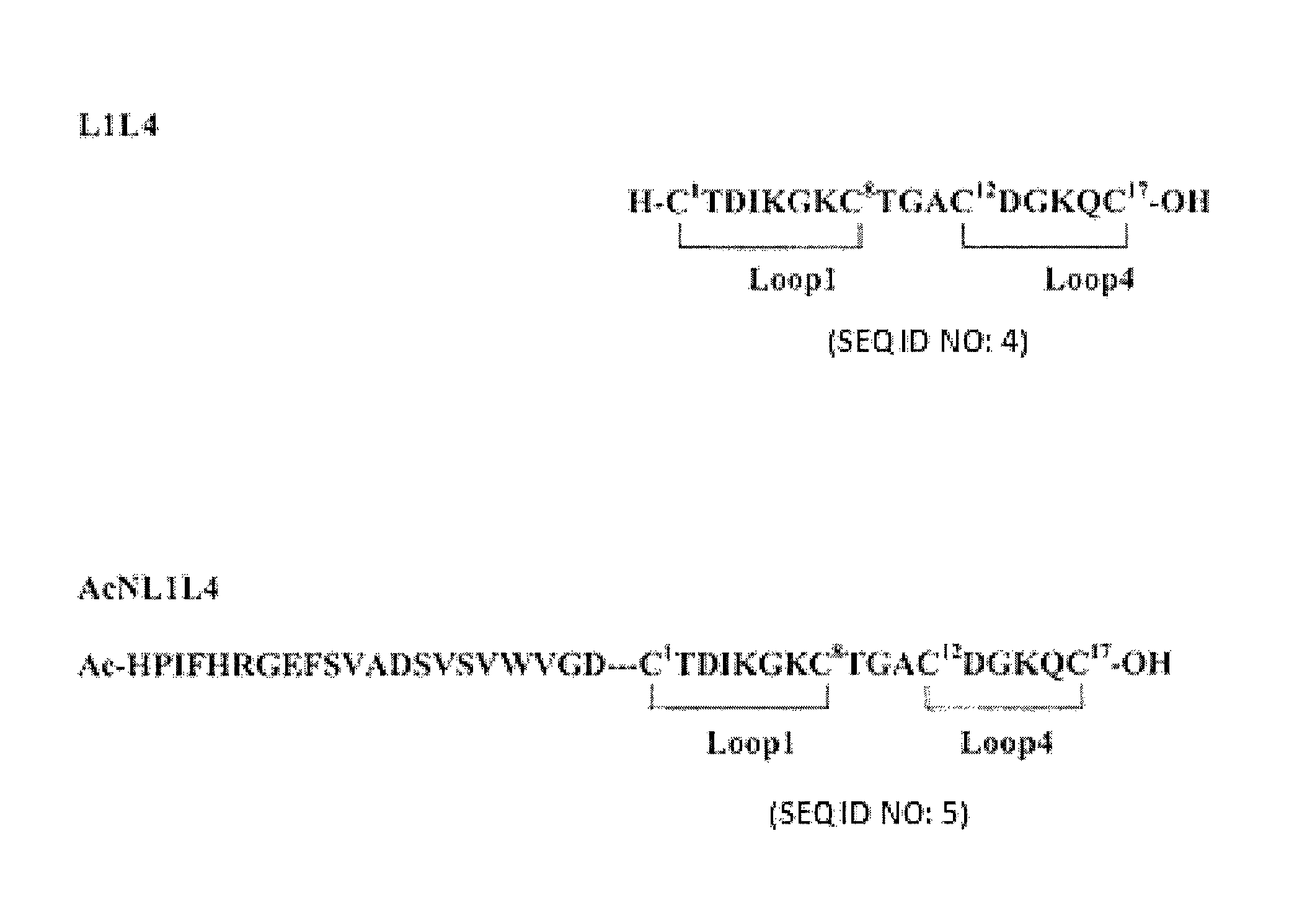
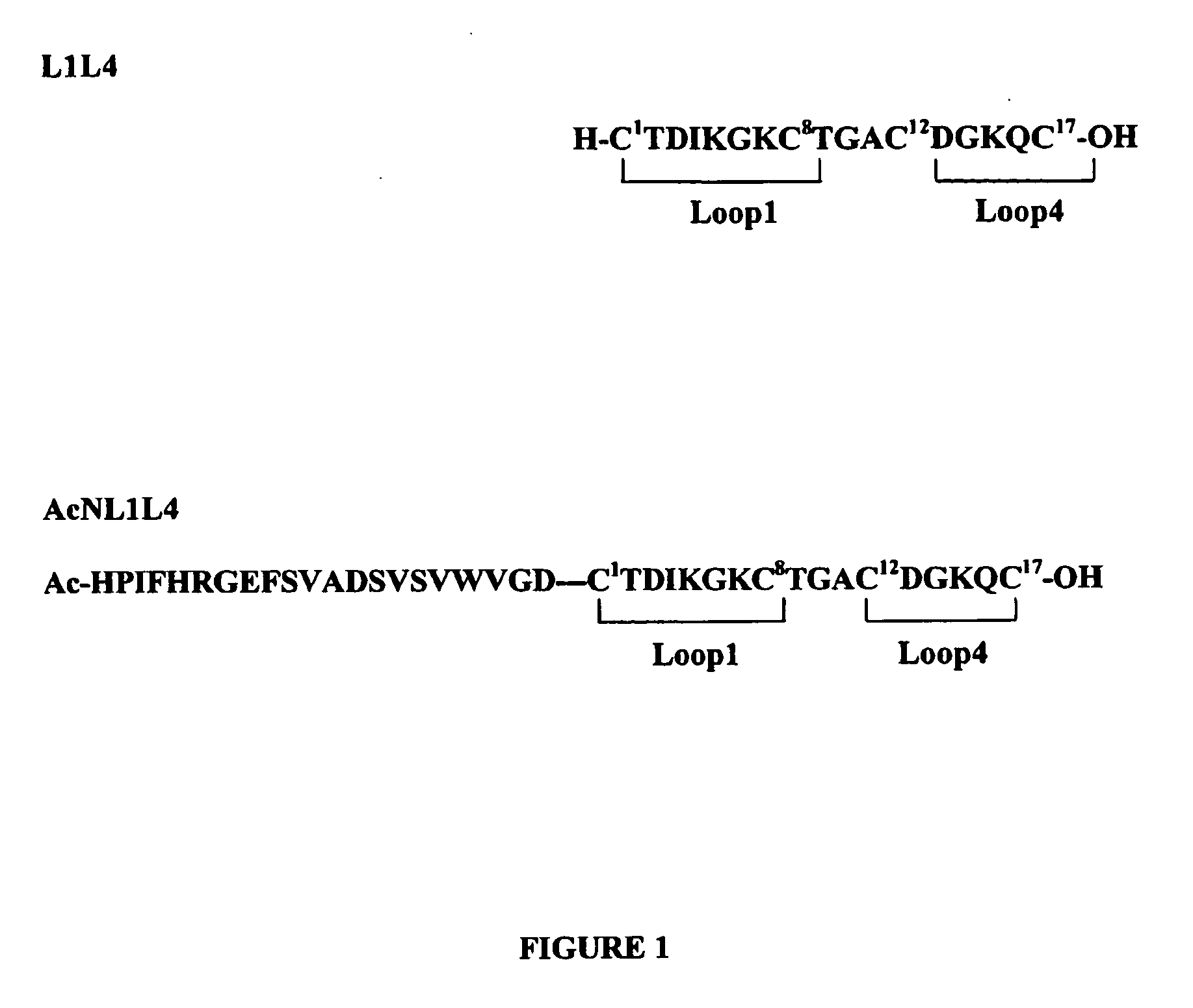
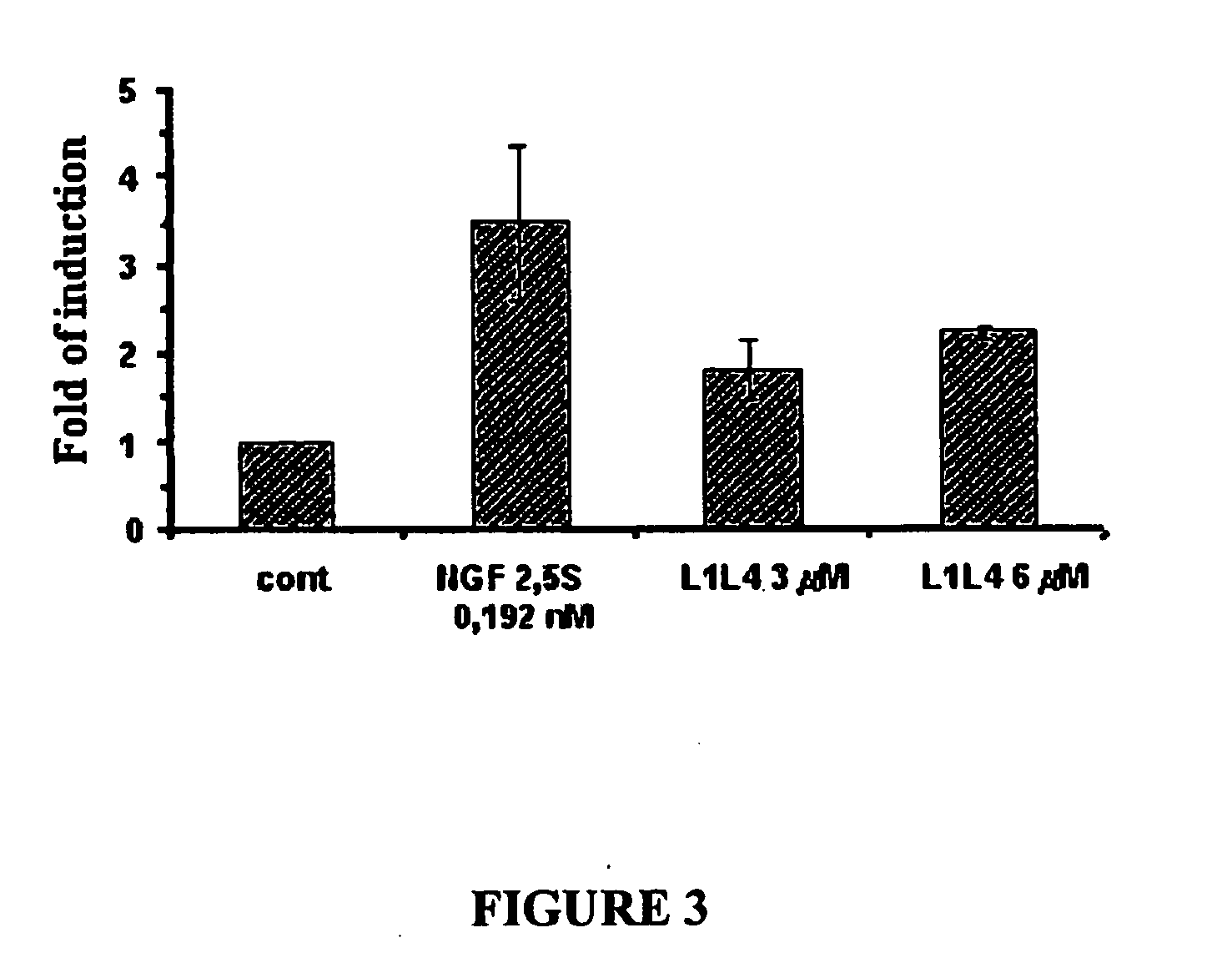
Heterodimeric peptide compounds displaying NGF activity and their use to treat neurodegenerative disorders
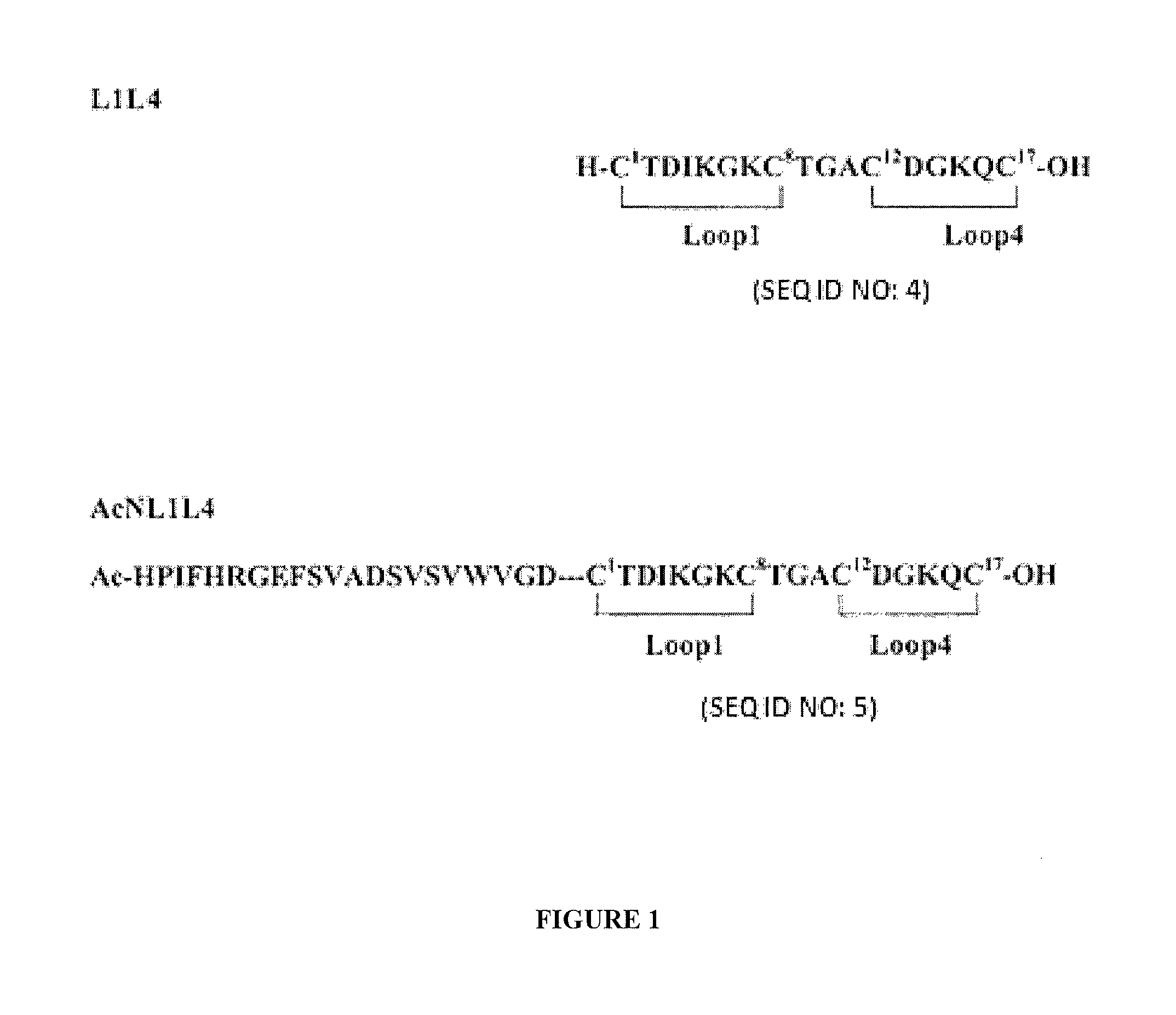
Peptides having a structure characterized by the presence of two loops constrained in cyclic structure by the presence of covalent bonds between amino acid side chains, the amino acid sequences of the first and the second loop being substantially homologues to that of loop 1 (residues 29-38) and of loop 4 (residues 92-97) of NGF, respectively, displaying nerve growth factor (NGF) agonist or partial agonist activity.
Owner:BLUEPRINT PHARMA
Peptide identification and sequencing by single-molecule detection of peptides undergoing degradation
InactiveUS20150087526A1Quick identificationMicrobiological testing/measurementLibrary member identificationAmino acid side chainFluorophore
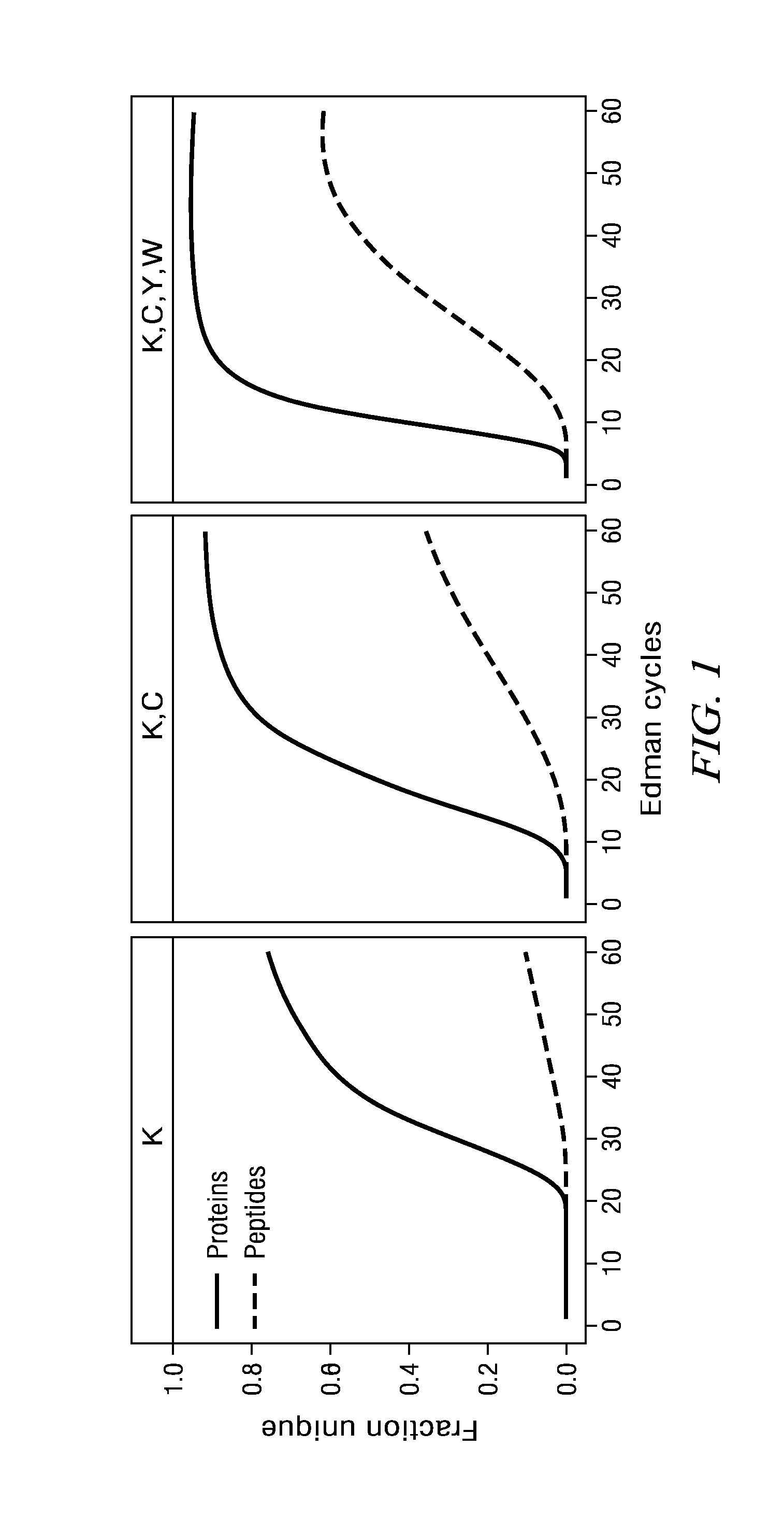
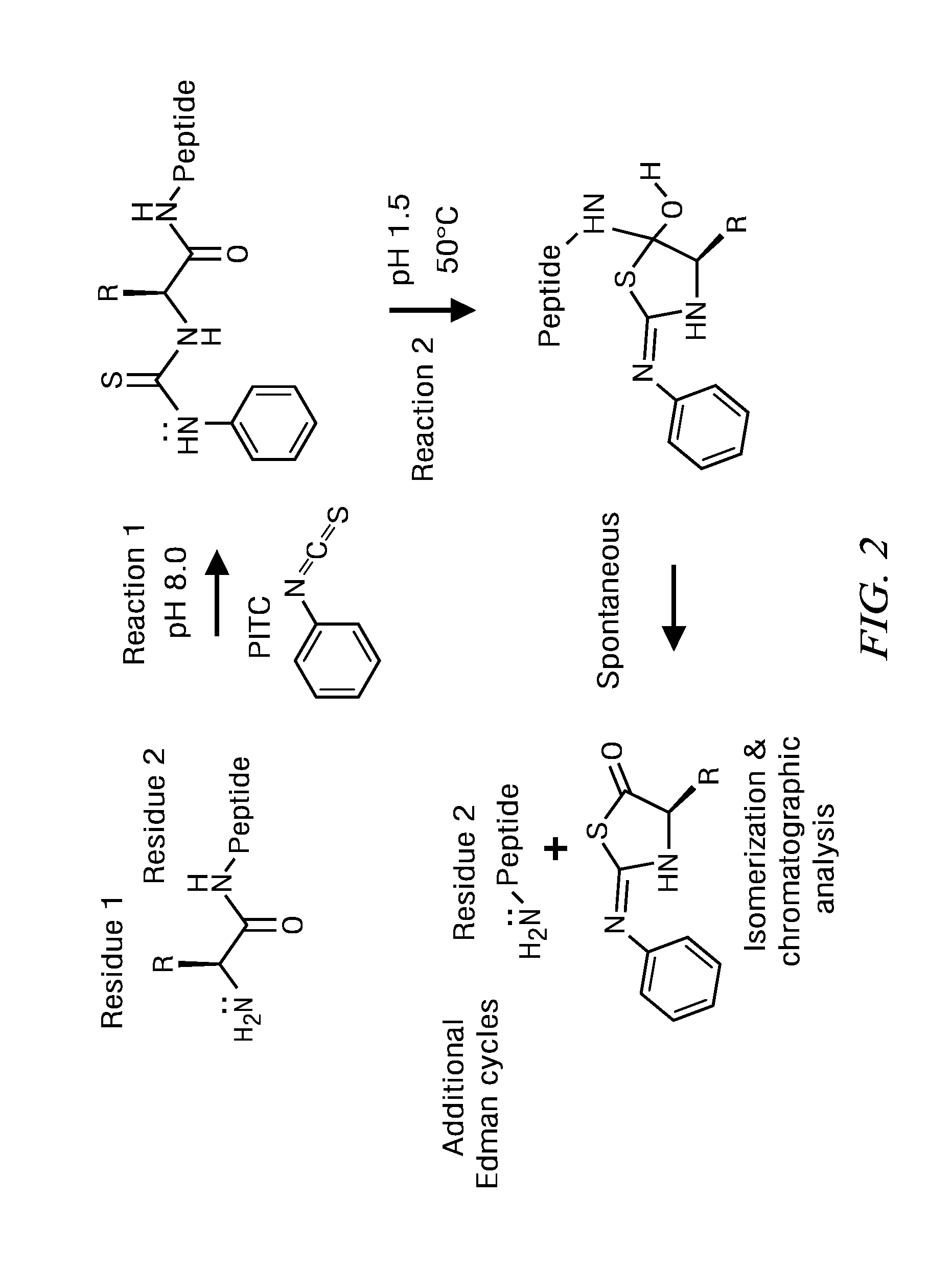
The present disclosure provides peptide amino acid sequencing and identification methods and kits for performing such methods. For example, single-molecule detection of fluorophore-labeled peptides is disclosed using multiple rounds of standard Edman degradation or using digestion by chemicals or enzymes. Different fluorophores covalently attached to each of a specific type of amino acid side chain of a peptide provide for the derivation of the peptide's encoded amino acid sequence following image alignments of multiple Edman cycles or following digestion by chemicals or enzymes. The amino acid sequence of a peptide and / or the identity of the peptide can be determined by bioinformatic analysis based on the encoded amino acid sequence. The present disclosure further provides peptide derivatization and immobilization strategies to enable the sequencing and identification of a single peptide or a plurality of peptides.
Owner:UNIV OF COLORADO THE REGENTS OF
Metallopeptide compositions for treatment of sexual dysfunction
Metallopeptide compositions are provided for treatment of sexual dysfunction in mammals, including male sexual dysfunction, such as erectile dysfunction, and female sexual dysfunction. The metallopeptides include at least one, and preferably two, aromatic amino acid side chain moieties, and are further characterized in that the metallopeptides preferably do not bind or significantly bind to a melanocortin receptor.
Owner:PALATIN TECH INC
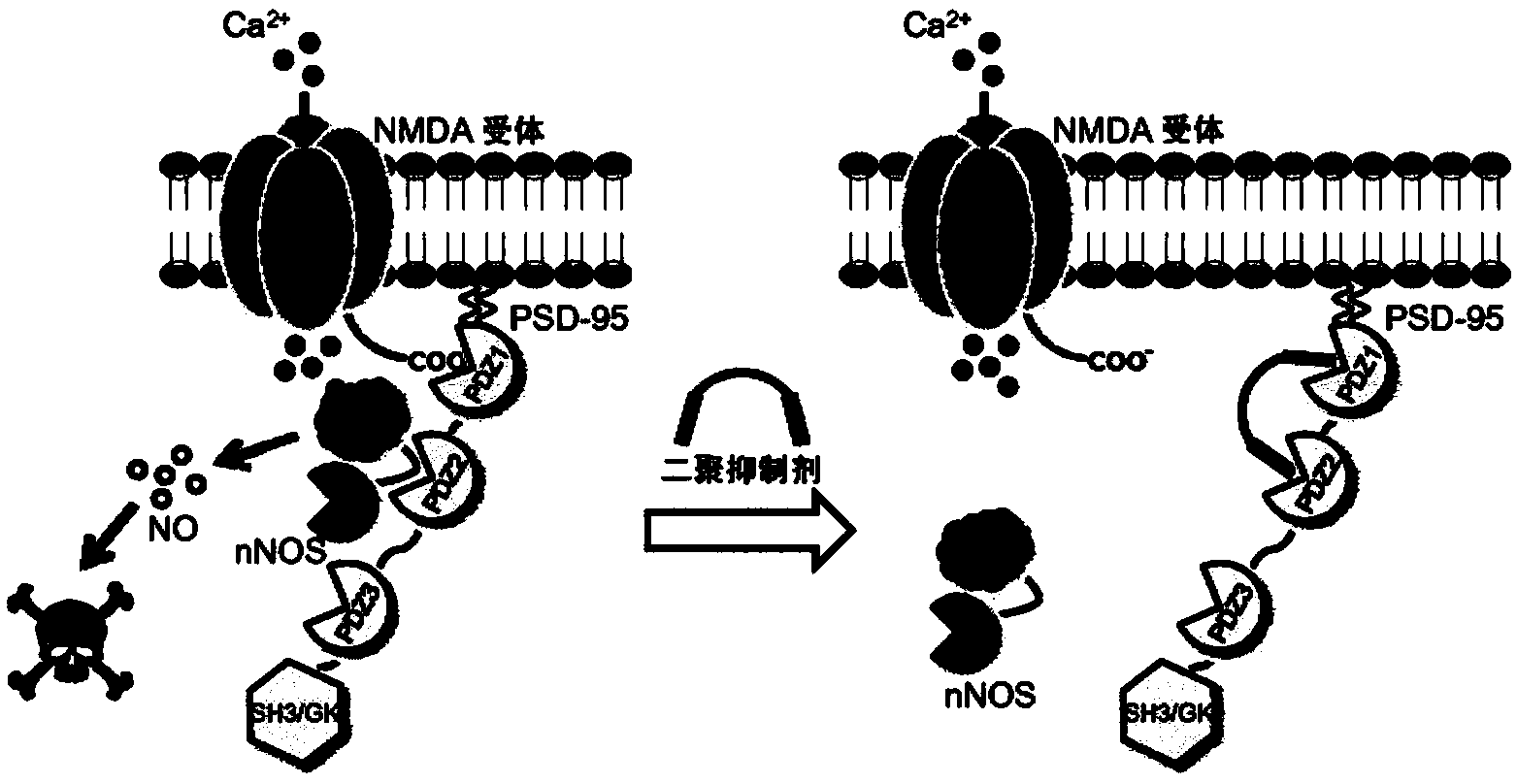
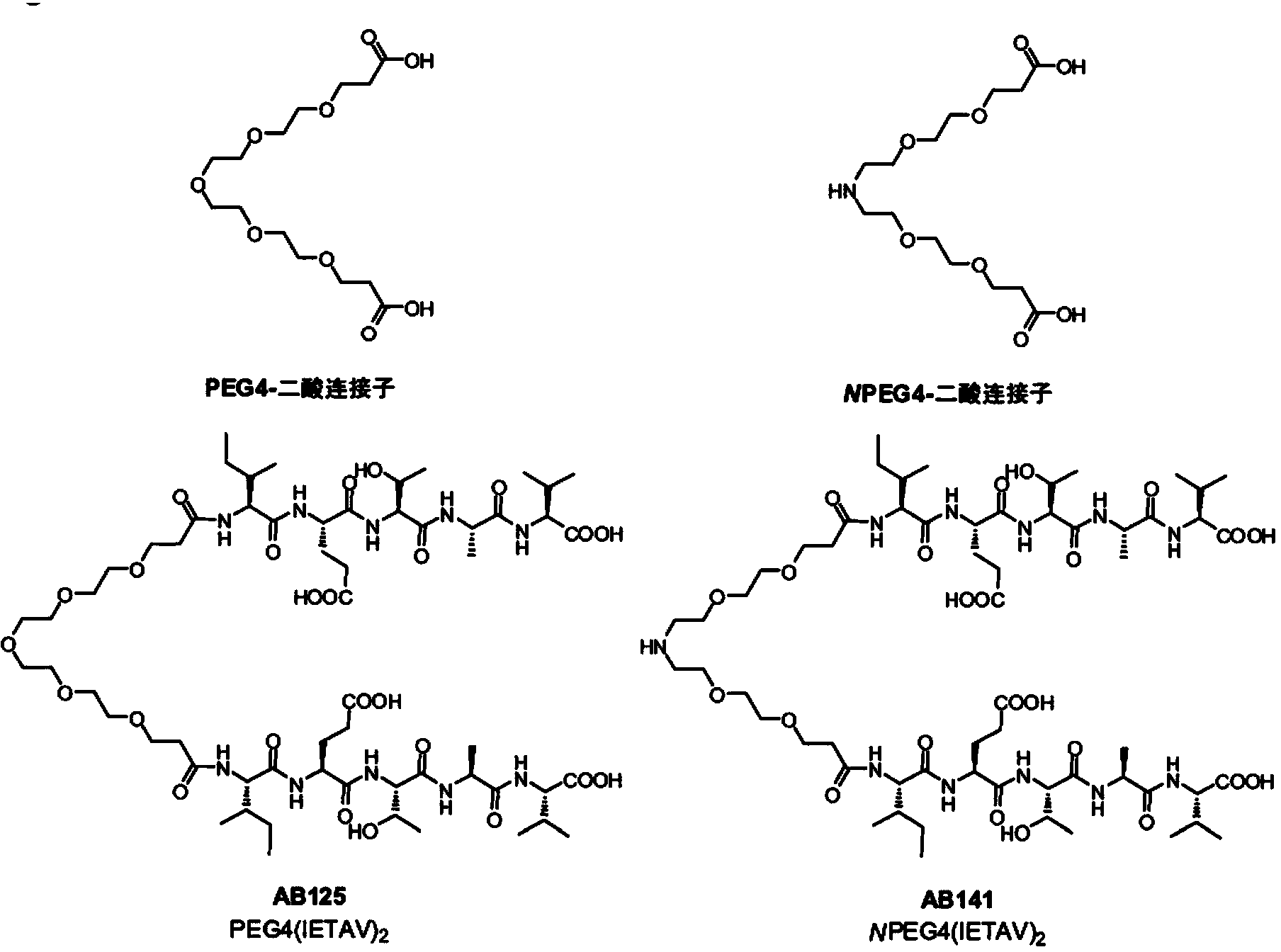
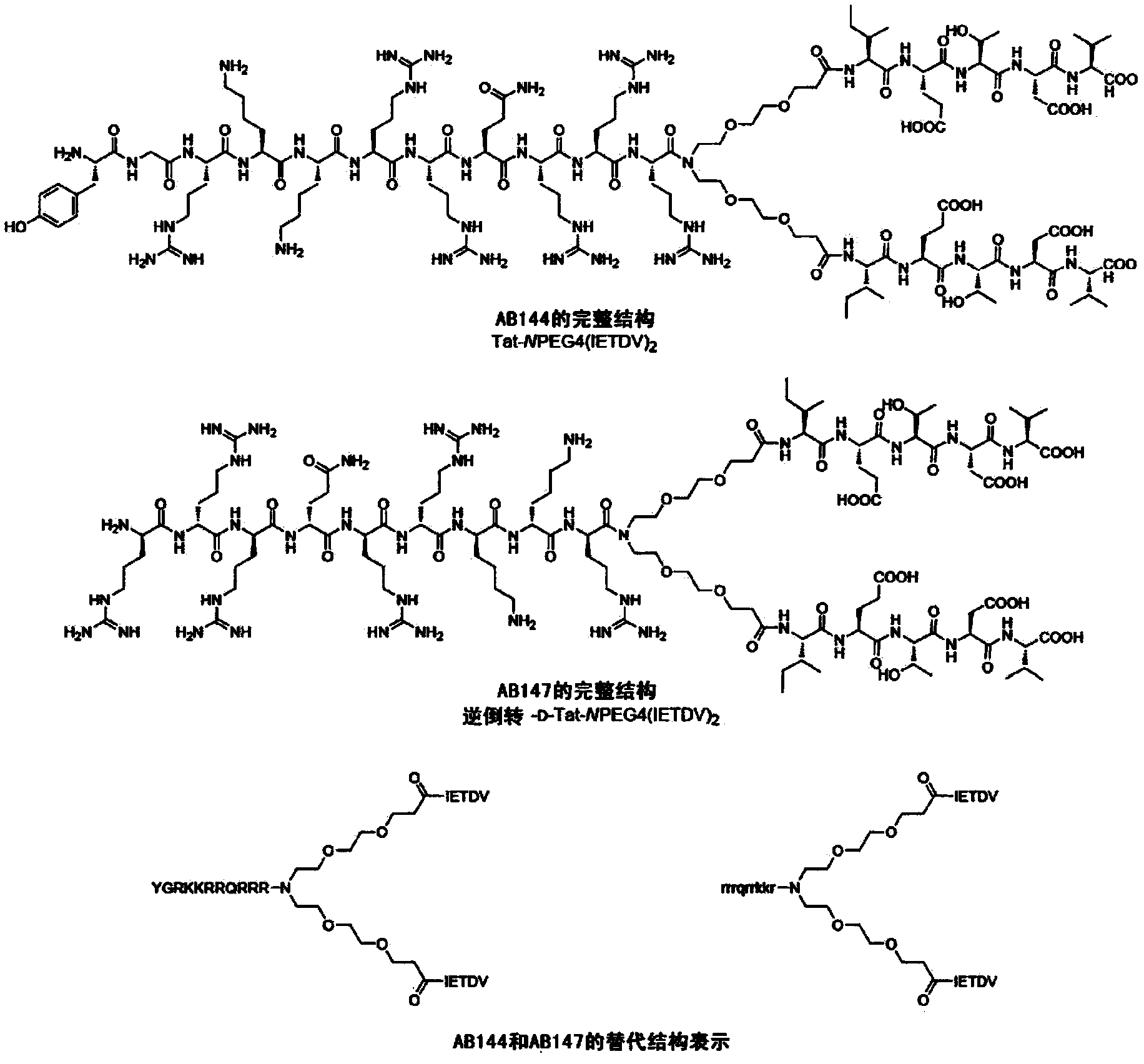
High-affinity, dimeric inhibitors of psd-95 as efficient neuroprotectants against ischemic brain damage and for treatment of pain
The invention provides novel potent inhibitors of the ternary protein complex of nNOS, PSD-95, and the NMDA receptor and pharmaceutical compositions comprising the inhibitors for prophylaxis and / or treatment of excitotoxic-related disease and chronic pain conditions in a subject. The inhibitors are dimeric PSD-95 inhibitors comprising a first peptide or peptide analogue linked to a second peptide or peptide analogue by a linker, wherein the first and the second peptide or peptide analogue comprise at least four amide-bonded residues having a sequence YTXV or YSXV, wherein a. Y is selected from among E, Q, and A, or an analogue thereof, and b. X is selected from among A, Q, D, N, N-Me-A, N-Me-Q, N-Me-D, and N-Me-N or an analogue thereof, and wherein a Cell Penetrating Peptide (CPP) is linked to the linker or to an amino acid side chain of the first and second peptide or peptide analogue. The linker can be a PEG or NPEG linker.
Owner:UNIVERSITY OF COPENHAGEN
Nogo-A receptor binding peptide as well as derivative and application thereof
ActiveCN105061560AInhibit bindingPromote regenerationNervous disorderPeptide/protein ingredientsProtein detectionAmino acid side chain
The invention belongs to the field of biological medicine, and particularly relates to a Nogo-A receptor binding peptide as well as a derivative and an application thereof. The amino acid residue sequence of the Nogo-A receptor binding peptide is HIYTALV or GFATITG. The derivative of the binding peptide is a product obtained through conventional modification of an amino terminal or a carboxyl terminal of a Nogo-A receptor binding peptide fragment on a Nogo-A receptor binding peptide amino acid side chain group or a product obtained by connecting a label used for peptide or protein detection or purification on the Nogo-A receptor binding peptide. The binding peptide and the derivative of the binding peptide can be bound with NgR in vitro, nerve regeneration is promoted by preventing binding of NgR and Nogo-A, nerve regeneration barriers caused by central nervous injury diseases can be treated, recovery of a central nervous function is promoted, and the binding peptide and the derivative can be widely applied in the medical and biological fields.
Owner:JINAN UNIVERSITY
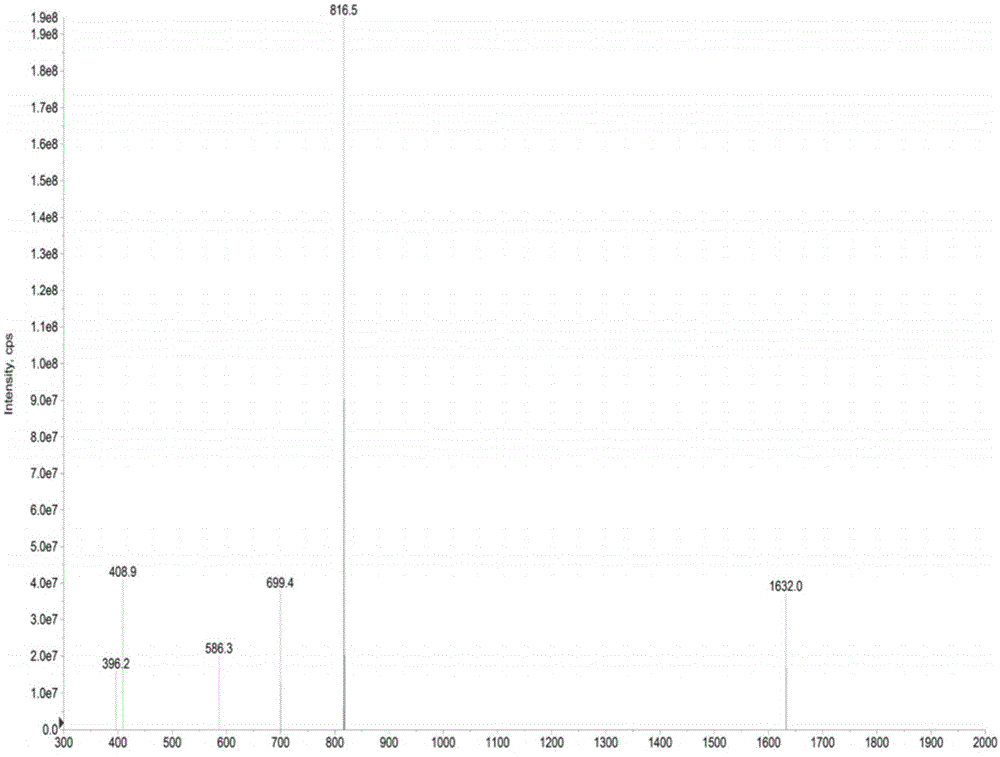
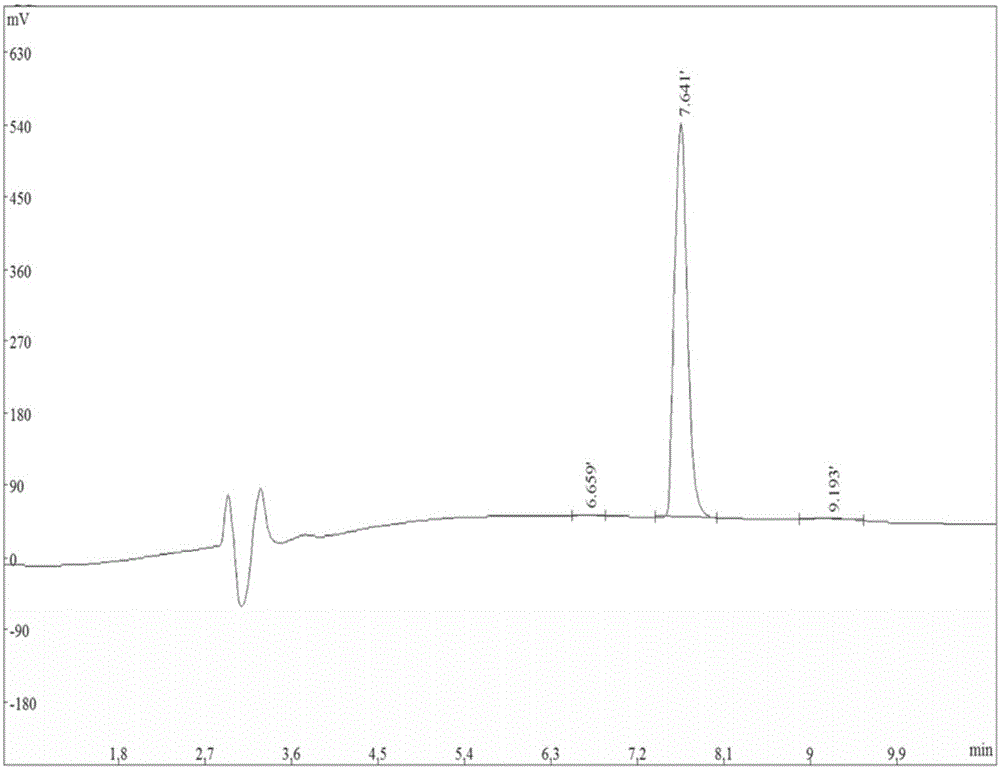
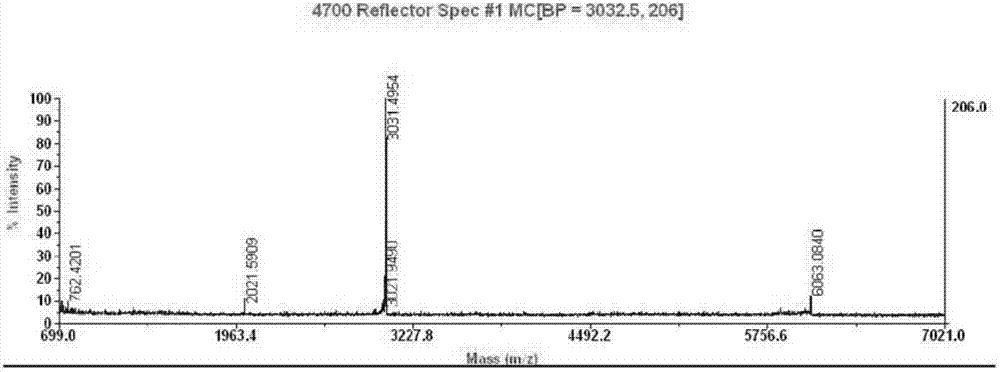
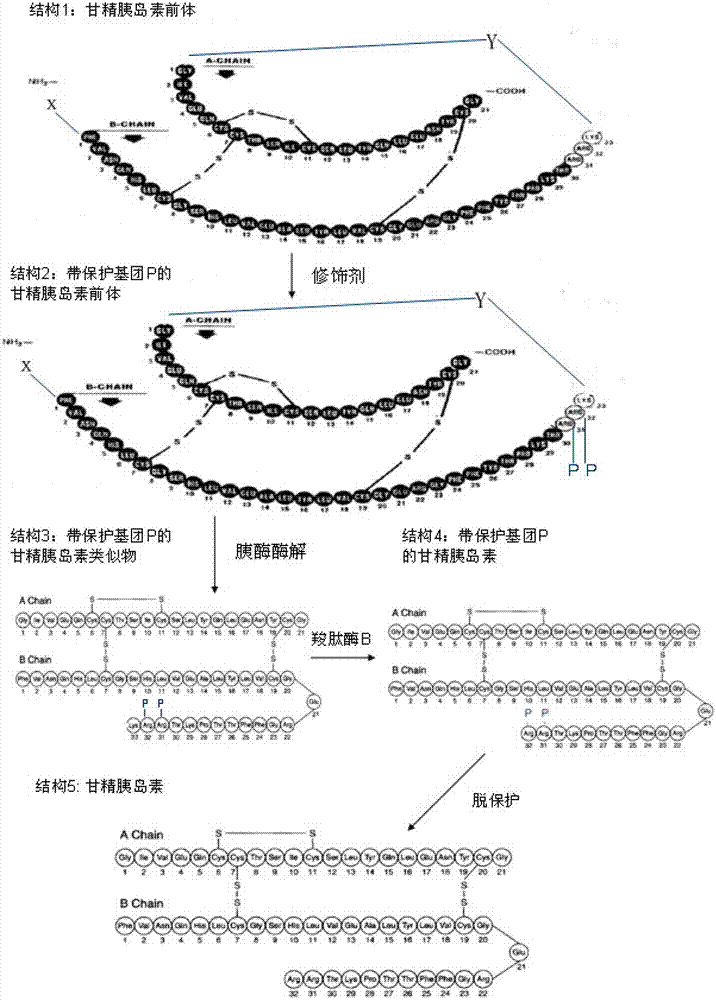
Preparation method of insulin glargine and analogue thereof
The invention discloses a preparation method of insulin glargine and an analogue thereof. The preparation method includes the following steps: (1) a gene engineering method is used for preparing a precursor of the insulin glargine and the analogue of the insulin glargine with a chain B and an end C containing a plurality of basic amino acids; (2) an amino acid side chain protective agent is used for distinguishing arginine or lysine through pancreatic enzyme specificity, and the insulin glargine and the analogue of the insulin glargine are provided with protecting groups and obtained under the effect of the protective agent and pancreatic enzyme; or the specificity is used for acting on clostripain of the arginine (Arg) or endoproteinase lysine (Lys) C of the Lys directly without protection; (3) carboxypeptidase is added optionally to remove unprotected basic amino acids at the tail end of the C; and (4) the glargine and the analogue of the insulin glargine are obtained through deprotection. The preparation method is simple and convenient, high in yield, wide in application range and suitable for introduction of more than two basic amino acids.
Owner:SHANGHAI HUAYI BIO LAB CO LTD
Novel dicarboxylic acid linked amino acid and peptide prodrugs of opioids and uses thereof
InactiveCN102573845AReduce frequencyImprove complianceNervous disorderOrganic chemistryAmino acid side chainSide effect
The present invention concerns dicarboxylic acid linked amino acid and peptide prodrugs of opioid analgesics and pharmaceutical compositions containing such prodrugs. Methods for providing pain relief, decreasing the adverse GI side effects of the opioid analgesic and increasing the bioavailability of the opioid analgesic with the aforementioned prodrugs are also provided. In one embodiment, prodrugs having the amino acid side chains of valine, leucine, isoleucine and glycine; and mono-, di-and tripeptides thereof are provided.
Owner:SHIRE PLC
Silk fibroin conductive hydrogel and preparation method thereof
The invention discloses a silk fibroin conductive hydrogel and a preparation method thereof. The silk fibroin conductive hydrogel is a silk fibroin conductive hydrogel constructed by using a silk fibroin molecule as a matrix, and introducing a host, a guest, a horseradish peroxidase and a polyphenol-modified graphene nanosheet in an amino acid side chain of the silk fibroin molecule, and has bothsuper-strong stretching and self-attaching functions. The prepared hydrogel has a conductivity of 10 s / m, and has good mechanical properties, the tensile deformation capacity can be up to 500%, and the hydrogel can be closely attached to the surface of human tissues or other articles, and can be repeatedly attached. More importantly, the hydrogel material can still retain excellent electrical conductivity, tensile resistance and self-attaching properties under high frequency use. The preparation of the silk fibroin conductive hydrogel provides a better material platform for production and application of a new generation of flexible bio-electronic products.
Owner:QINGDAO UNIV
Heterodimeric Peptide Compounds Displaying Ngf Activity and Their Use to Treat Neurodegenerative Disorders
Peptides having a structure characterized by the presence of two loops constrained in cyclic structure by the presence of covalent bonds between amino acid side chains, the amino acid sequences of the first and the second loop being substantially homologues to that of loop 1 (residues 29-38) and of loop 4 (residues 92-97) of NGF, respectively, displaying nerve growth factor (NGF) agonist or partial agonist activity.
Owner:BLUEPRINT PHARMA
Novel chiral chromatographic column fixed phase and preparation method thereof
InactiveCN102172518AImprove bindingChemically stableOther chemical processesChemical LinkageStationary phase
The invention belongs to the field of chiral fixed phases and relates to a chiral chromatographic column fixed phase in which an amino acid derivative is taken as a matrix of a chiral selective agent and the amino acid derivative is modified onto the surface of a carrier with a chemical bonding method. The chiral fixed phase consists of a chiral monomer and a carrier, wherein the general structure of the chiral monomer is shown in the specifications, wherein m is any integer from 1 to 17; n is any integer from 1 to 10; R is an amino acid side chain group; and the carrier is silica gel. The chiral selective agent is modified on the surface of the carrier by taking amino acid as the matrix with a bonding method, so that the bonding force between the chiral selective agent and the carrier isgood, and the obtained chiral chromatographic column fixed phase has stable chemical property, a good separation effect and high column efficiency, is convenient to use, and is prevented from tailing.
Owner:SUZHOU UNIV
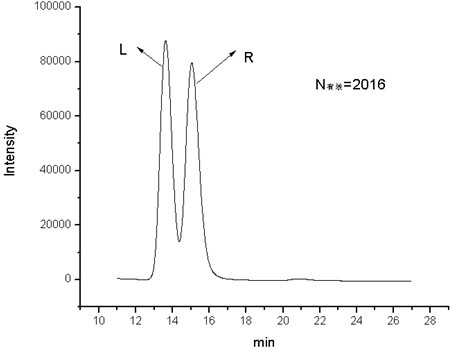
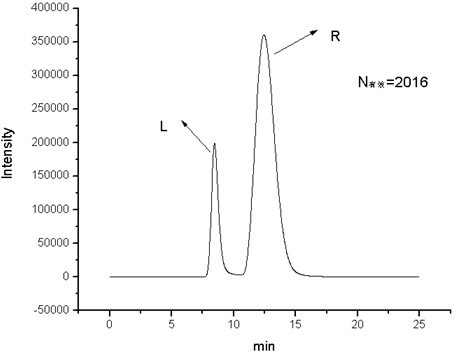
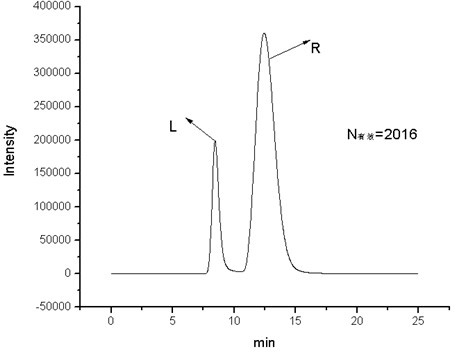
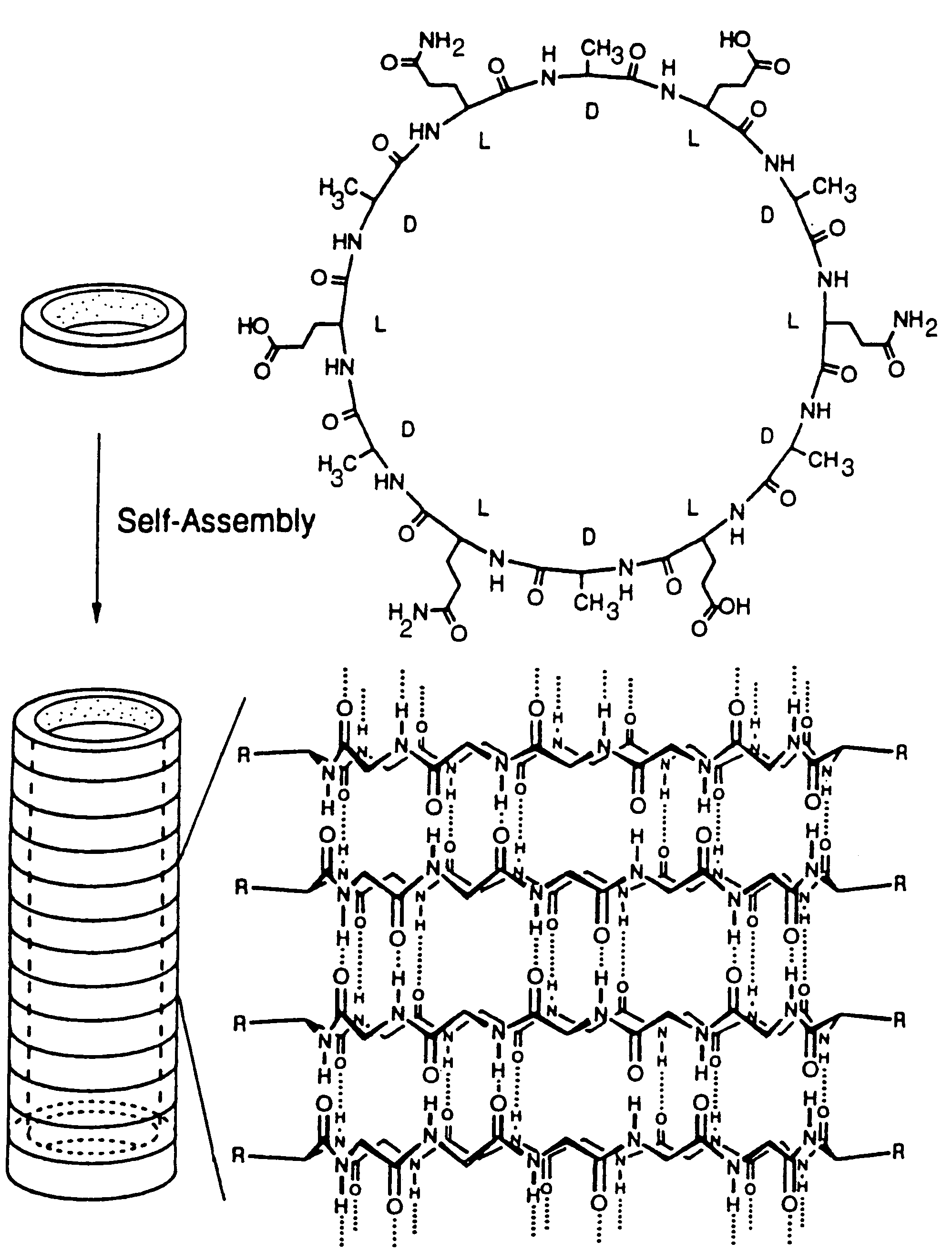
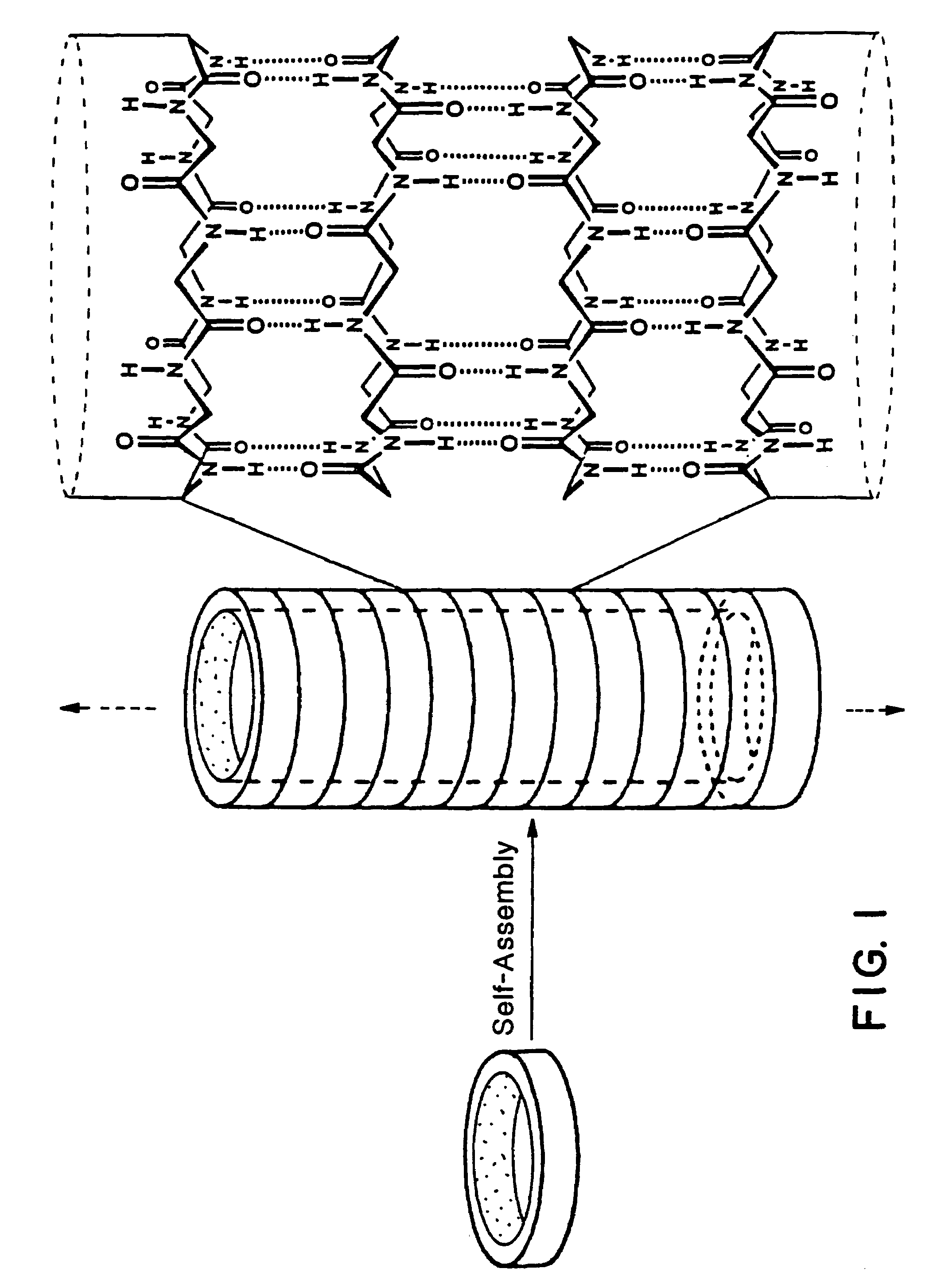
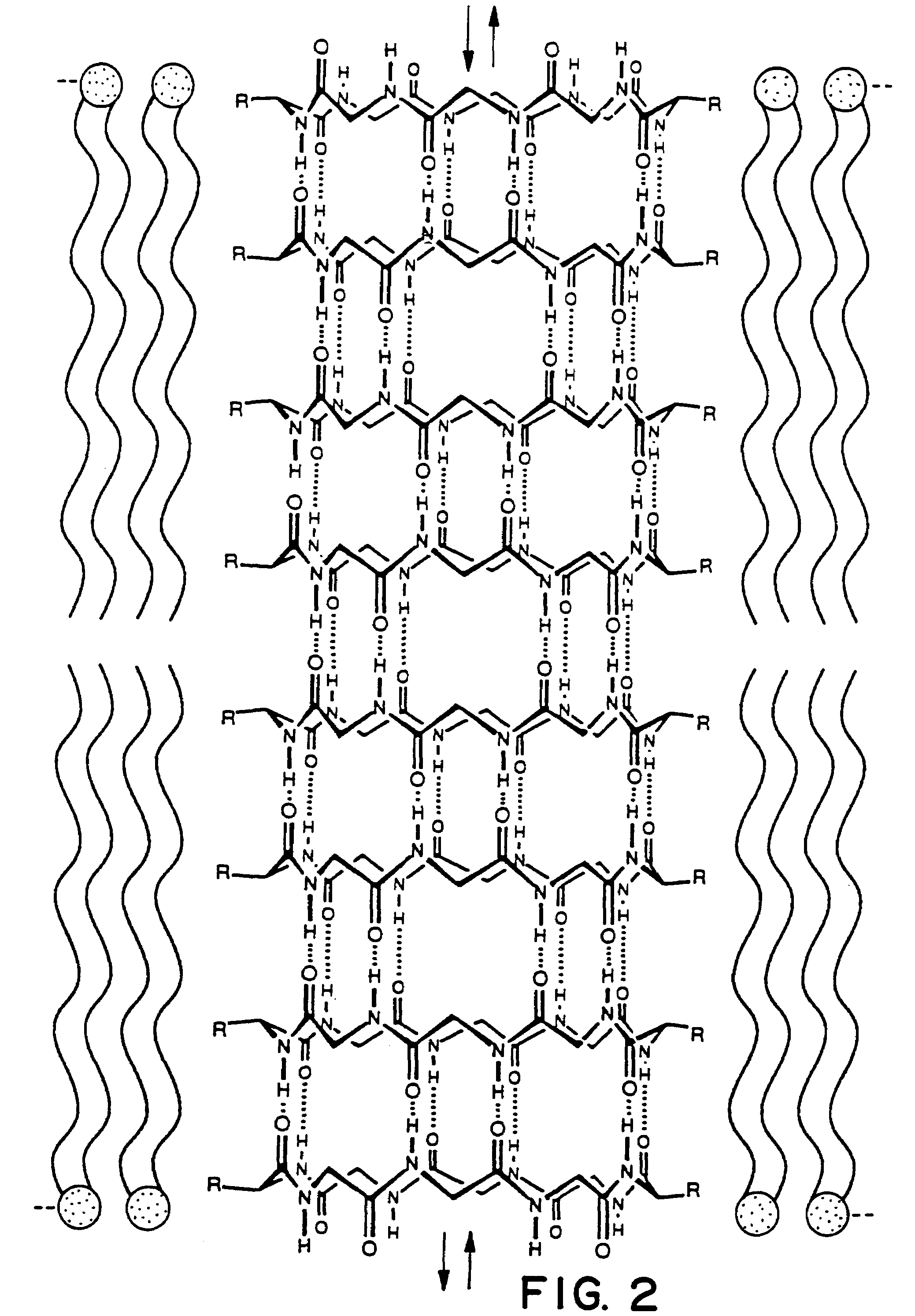
Cyclic peptide tube
InactiveUS7288623B2Easy to controlPromotes self-assemblyPeptide preparation methodsImmunoglobulinsLipid formationCyclic peptide
Cyclic homodetic peptides having a repeating D-L-chirality motif are shown to have a stable disk conformation with the amino acid side chains extending radially outward and the carbonyl and amino groups extending axially upward or downward. Such cyclic peptides can be employed as subunits in the assembly of molecular tubes. Cyclic peptides having a repeating D-L-chirality motif and lacking mutually repulsive side-chains are shown to stack atop one another in an anti-parallel fashion and are shown to be held together by the formation of β-sheet hydrogen bonding. The stacked cyclic peptides form a molecular tube having a central channel. The diameter of the channel is determined by the size cyclic peptide. If the cyclic peptide includes ionizable amino acid residues, e.g. glutamic acid or lysine, assembly and disassembly of the molecular tubes can be controlled by varying the pH. If the cyclic peptide includes hydrophobic amino acid residues, the molecular tube will insert into a lipid membrane. In such instances, the molecular tube provides a transmembrane channel. The channel can be gated or ungated. Molecular tubes can be terminated with a terminal cyclic peptide having methylated amino groups in one orientation. Molecular tubes may be employed as drug carriers, molecular sieves, reaction vessels, membrane channels, and other uses.
Owner:THE SCRIPPS RES INST
Specific antagonist peptide for advanced glycation end product receptor as well as derivatives and application of specific antagonist peptide
The invention belongs to the biomedical field and particularly relates to a specific antagonist peptide for an advanced glycation end product receptor as well as derivatives and an application of the specific antagonist peptide. An amino acid residue sequence of the specific antagonist peptide for the advanced glycation end product receptor is Ala-Pro-Asp-Thr-Lys-Thr-Gln. The derivatives of the specific antagonist peptide are products obtained by conventionally modifying an amino terminal or a carboxyl terminal of a specific antagonist peptide segment on a specific antagonist peptide amino acid side chain group, or products obtained by connecting a label for detecting or purifying polypeptide or protein to the specific antagonist peptide. The specific antagonist peptide of the advanced glycation end product receptor and the derivatives of the specific antagonist peptide can be specifically combined with RAGE in vivo and in vitro, so that neurodegenerative diseases can be treated through the effects of antagonist RAGE, and the relevant mechanism research also can be carried out. The results can be widely applied to the medical and biological fields, so that great social and economic benefits are produced.
Owner:JINAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com