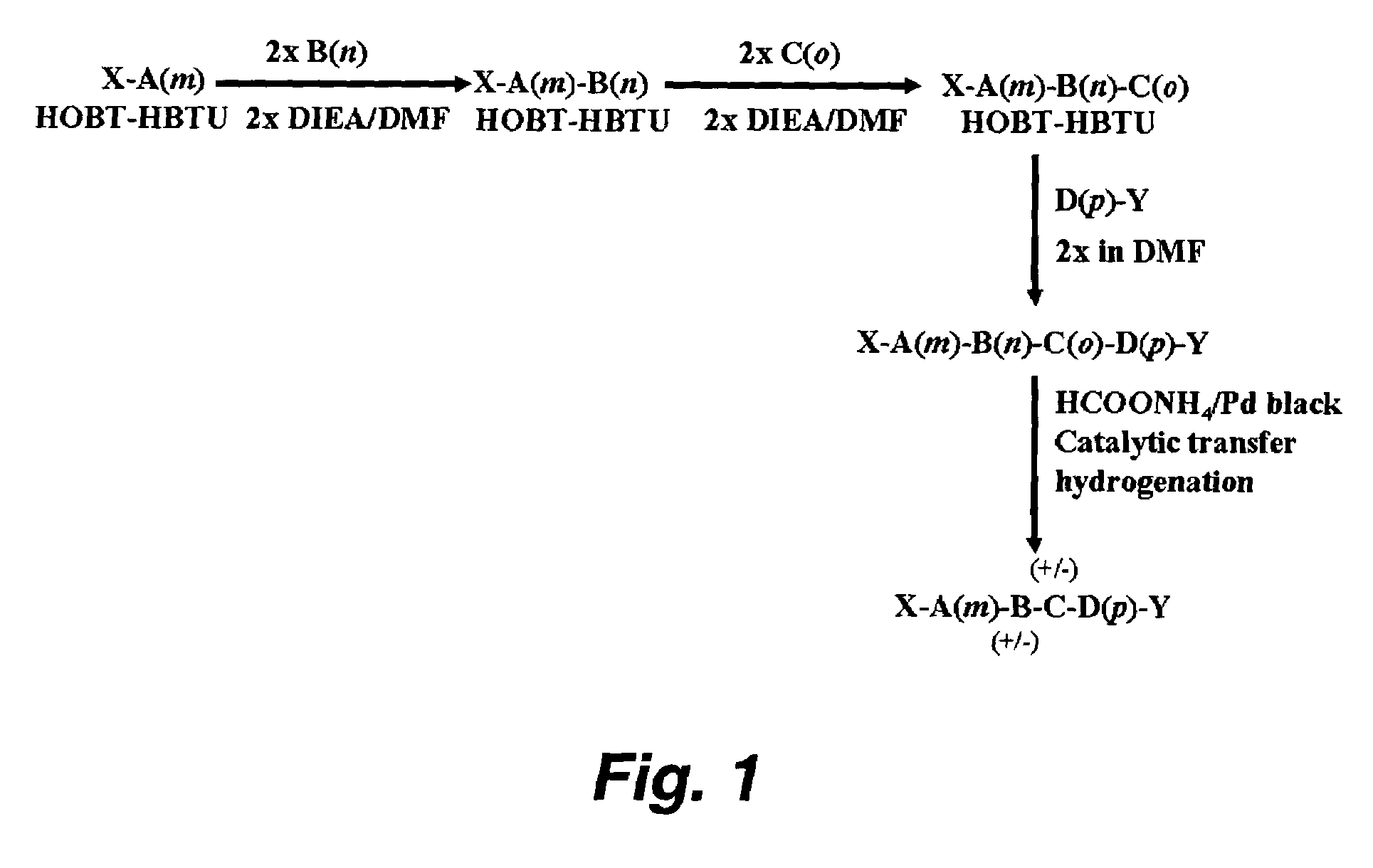
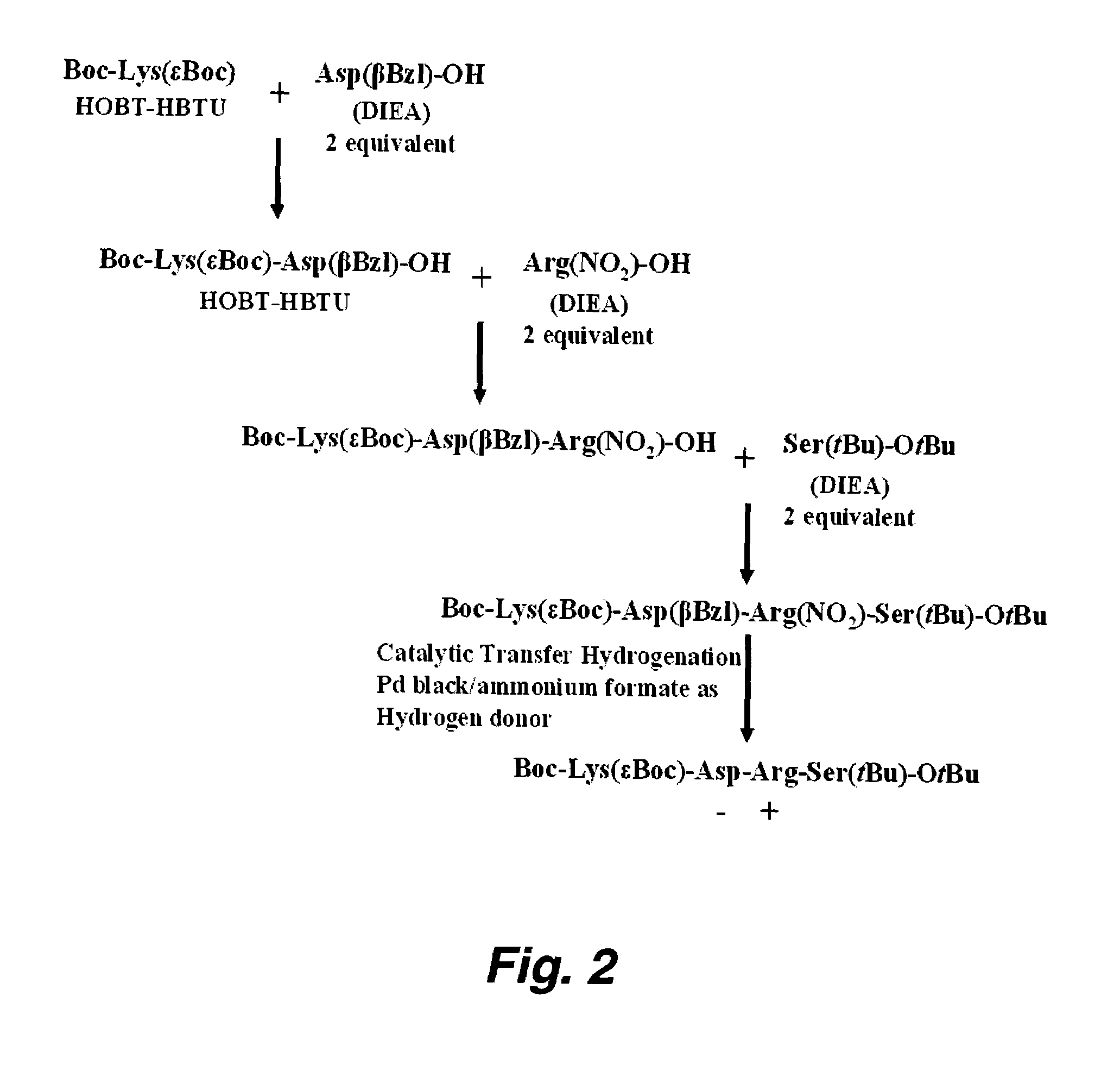
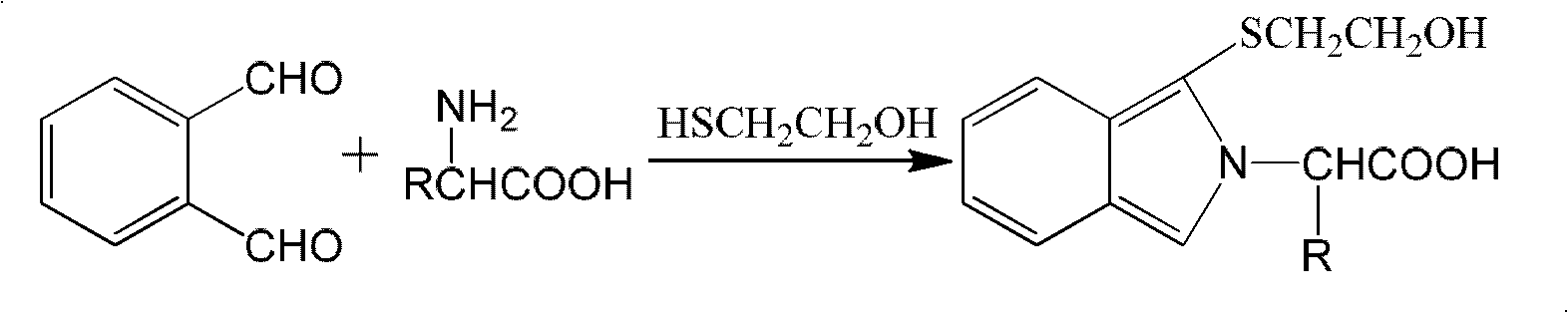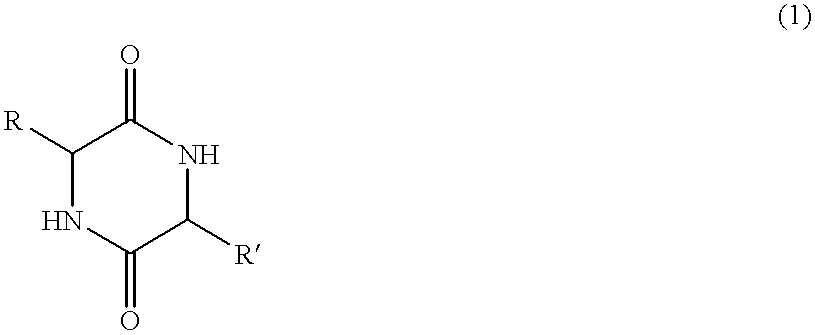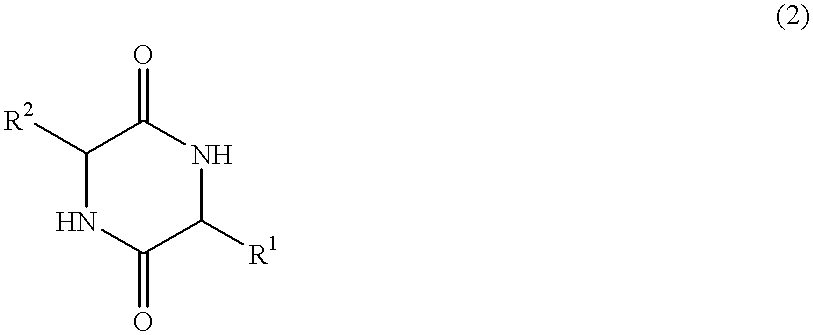Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1275 results about "Isoleucine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH⁺₃ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO⁻ form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms). It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes in other organisms such as bacteria. It is encoded by the codons AUU, AUC, and AUA.
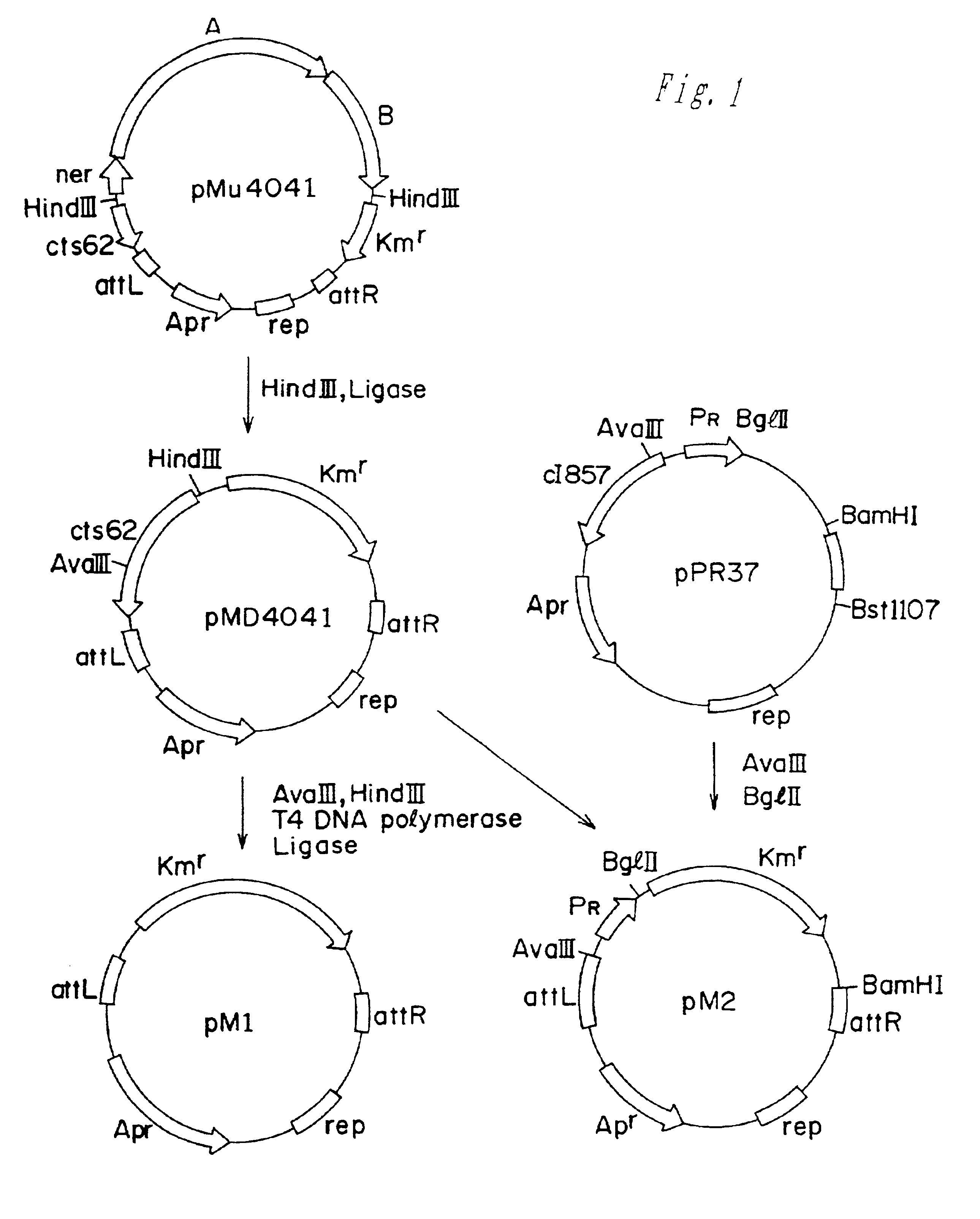
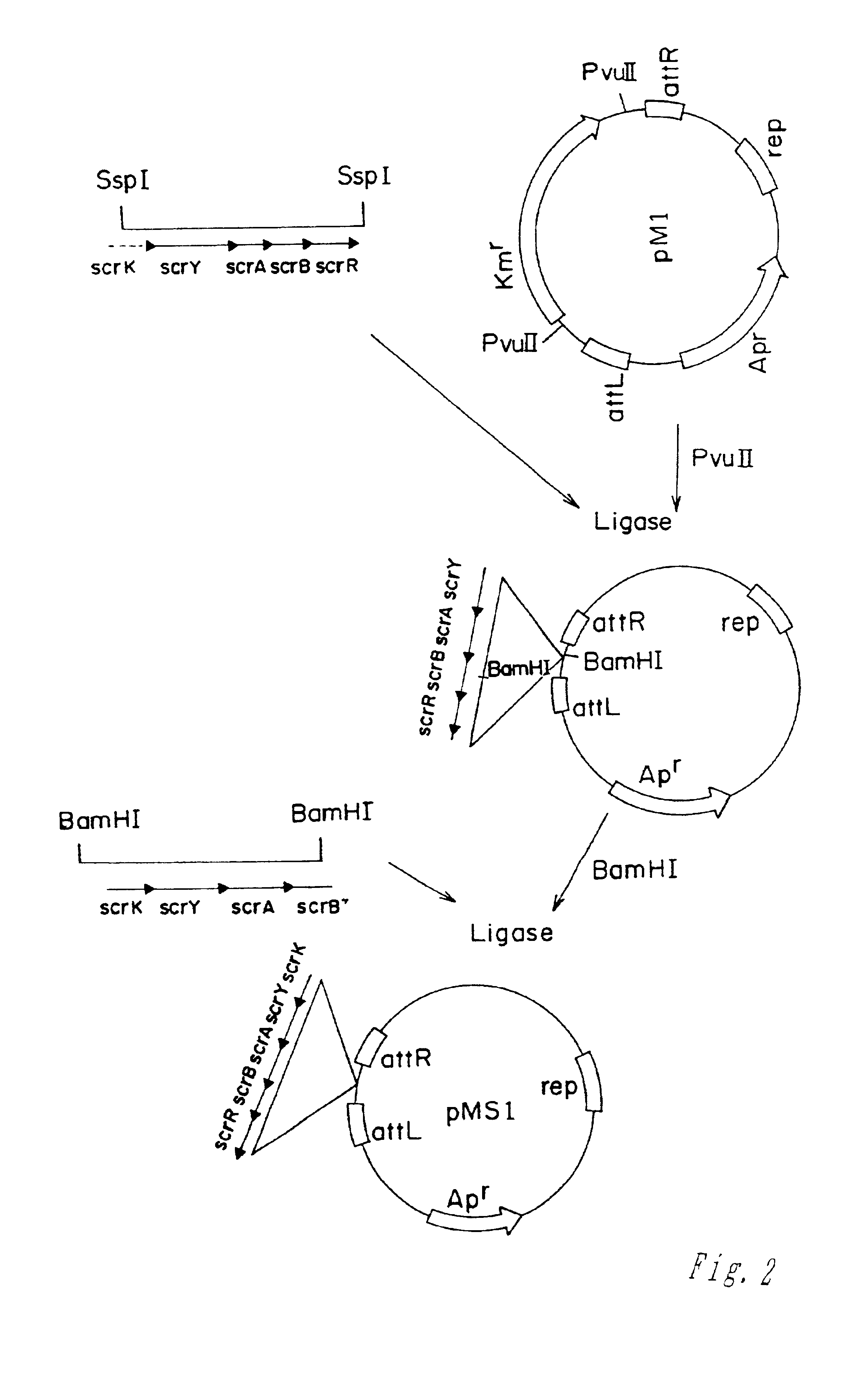
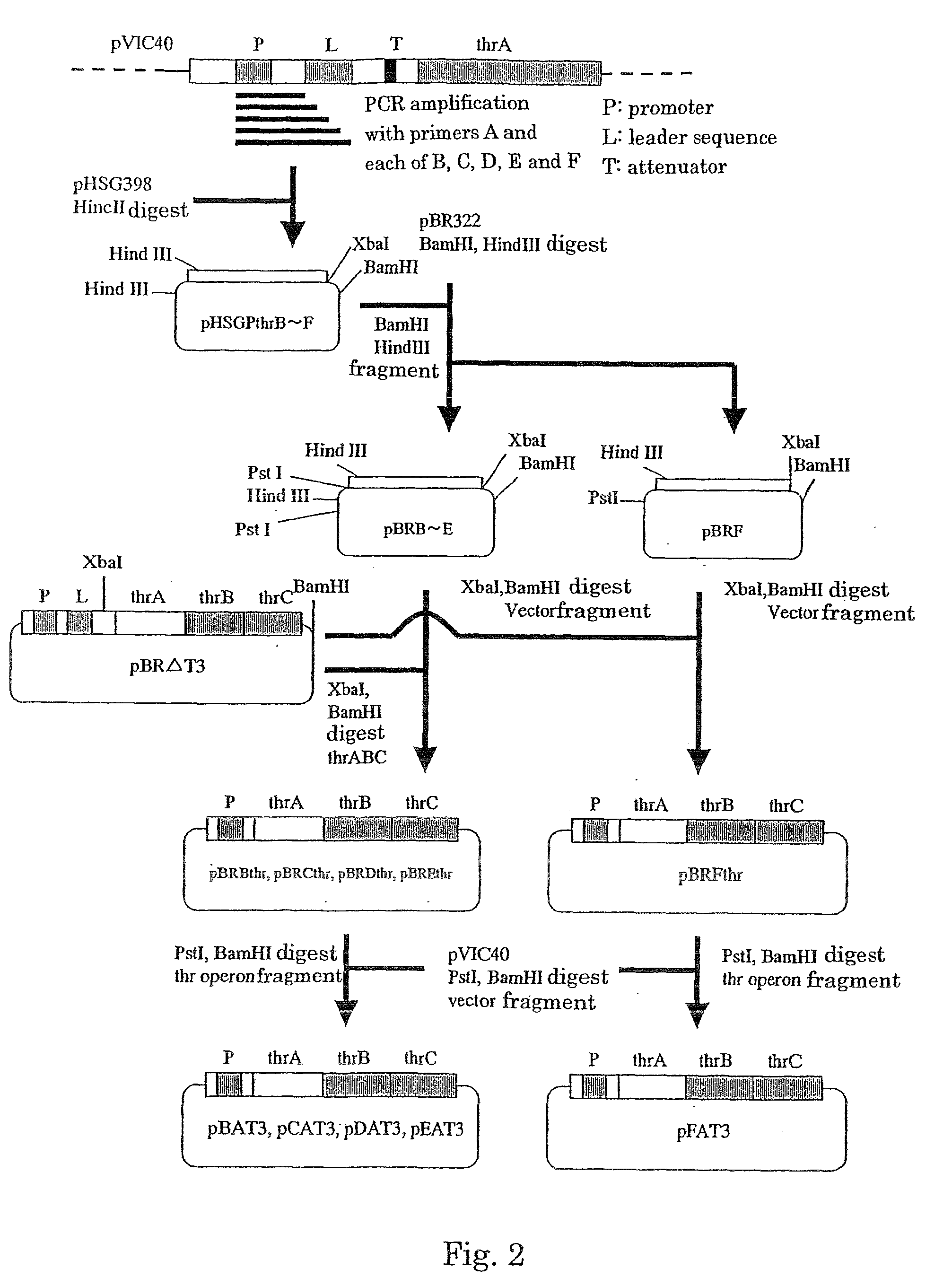
Methods of making amino acids using E. coli transformed with csc genes
An amino acid such as threonine, homoserine, isoleucine, lysine, valine and tryptophan is produced using a bacterium belonging to the genus Escherichia which has been constructed from sucorse non-assimilative strain belonging to the genus Escherichia and which harbors sucrose non-PTS (phosphoenol pyruvate-dependent sucrose-6-phosphotransferase system) genes and has an ability to produce the amino acid.
Owner:AJINOMOTO CO INC
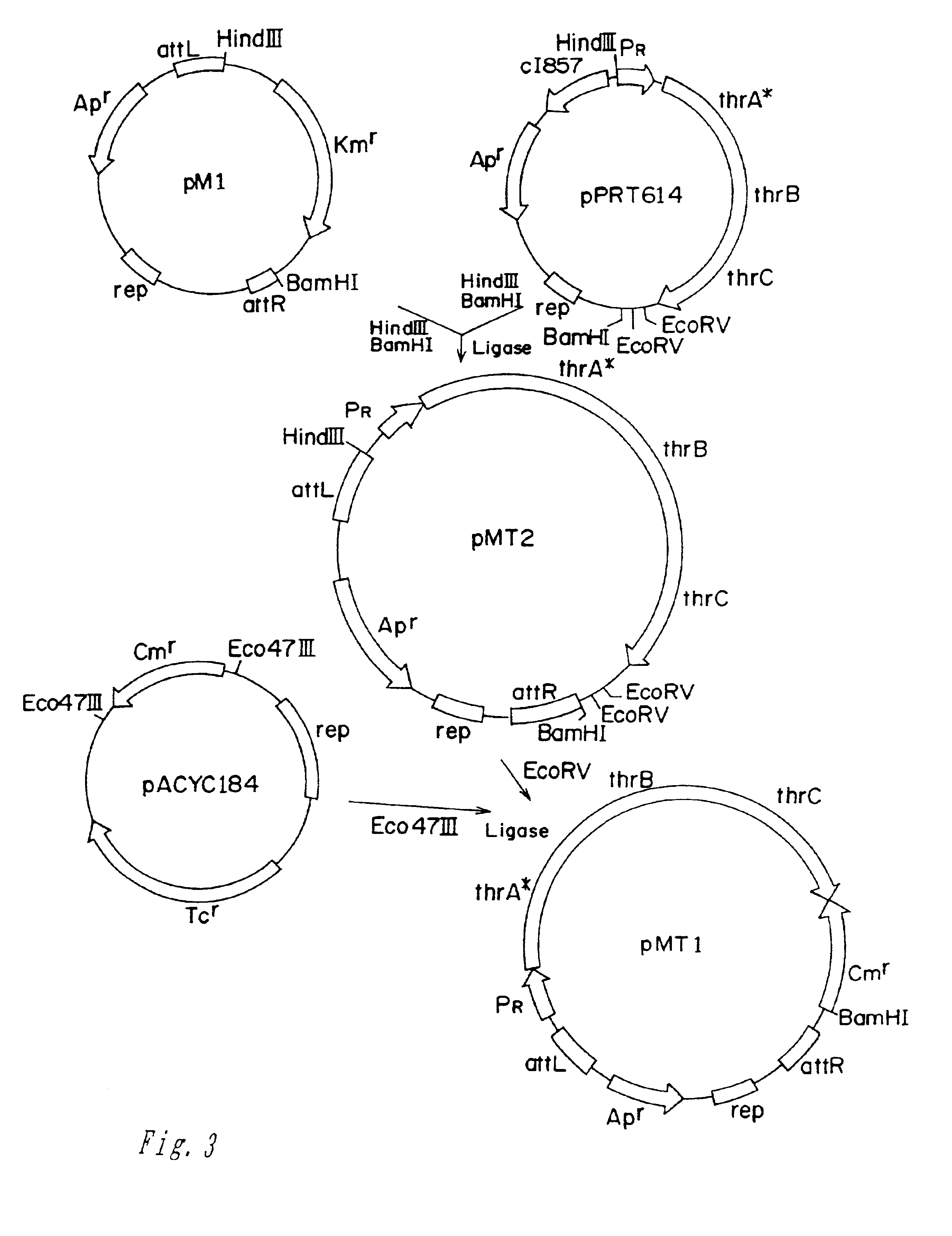
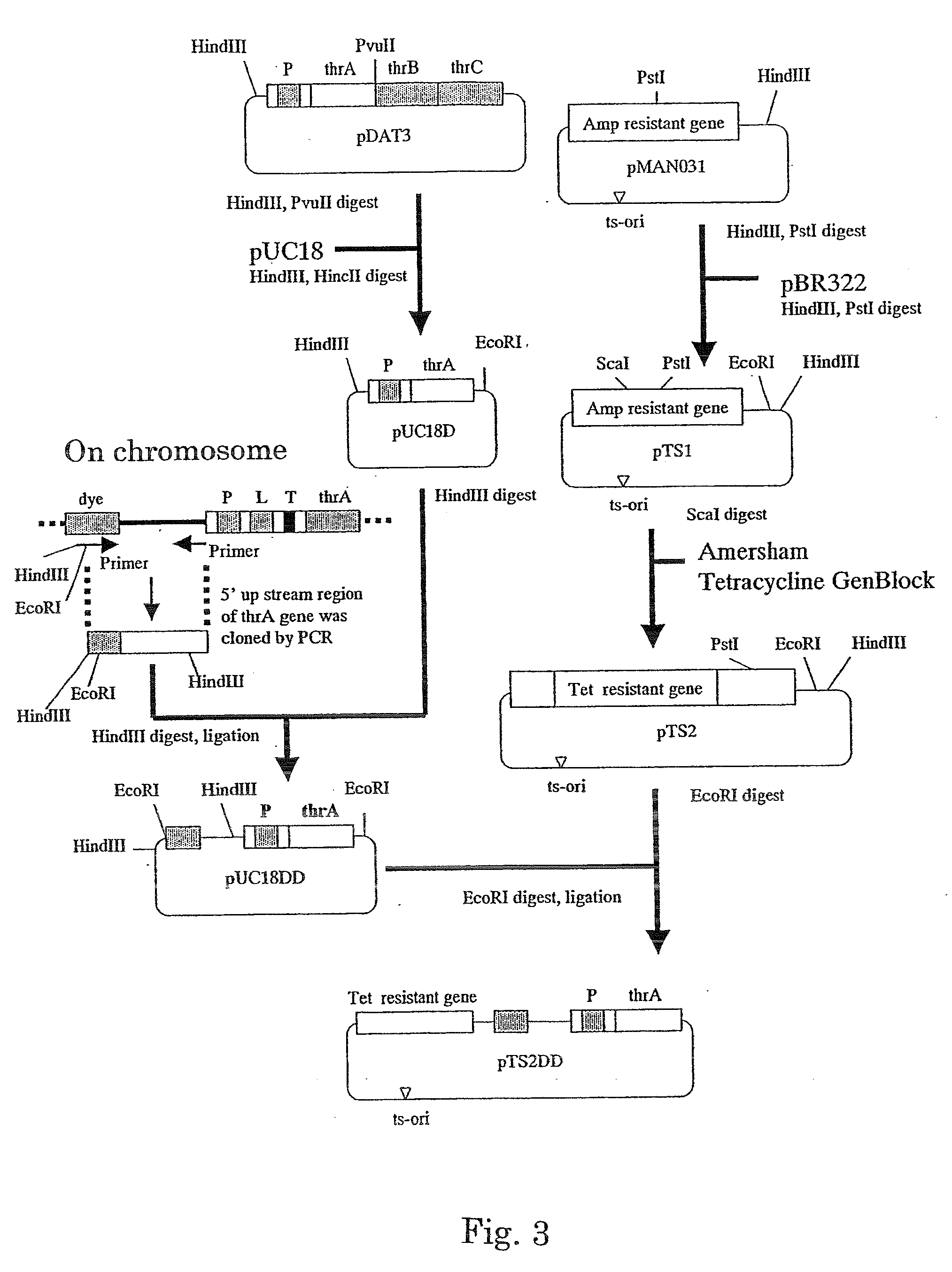
Method for producing l-amino acid by fermentation
InactiveUS20060216796A1Improve abilitiesEnhancing threonine biosynthetic pathwayBacteriaFermentationL-threonineMicrobiology
L-threonine or L-isoleucine is produced by culturing a bacterium which belongs to the genus Escherichia and has an ability to produce L-threonine or L-isoleucine, and wherein expression of a threonine operon is directed by its native promoter, and from which at least a leader sequence and an attenuator are deleted, in a medium and collecting the L-threonine or L-isoleucine from the medium.
Owner:AJINOMOTO CO INC
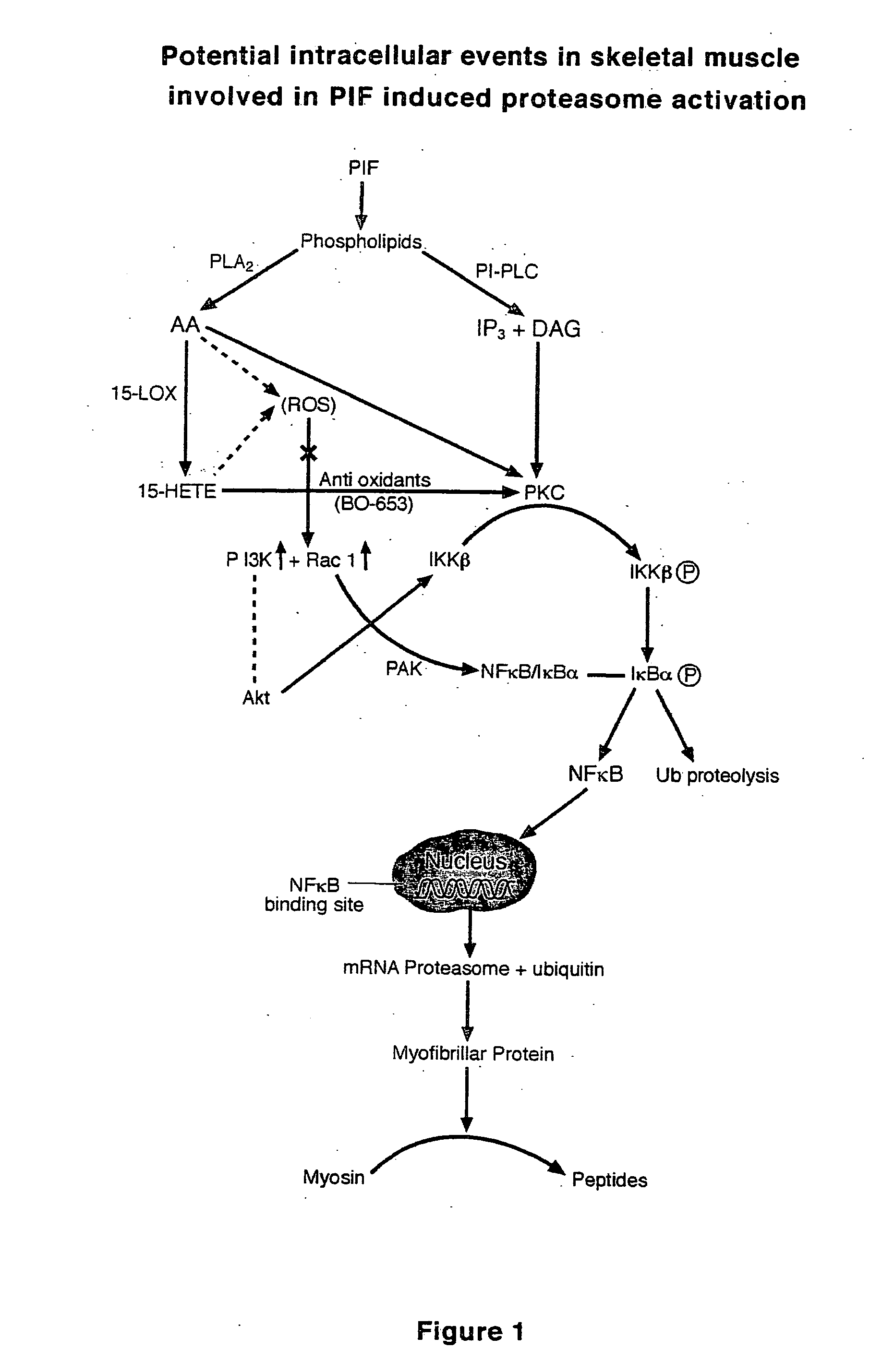
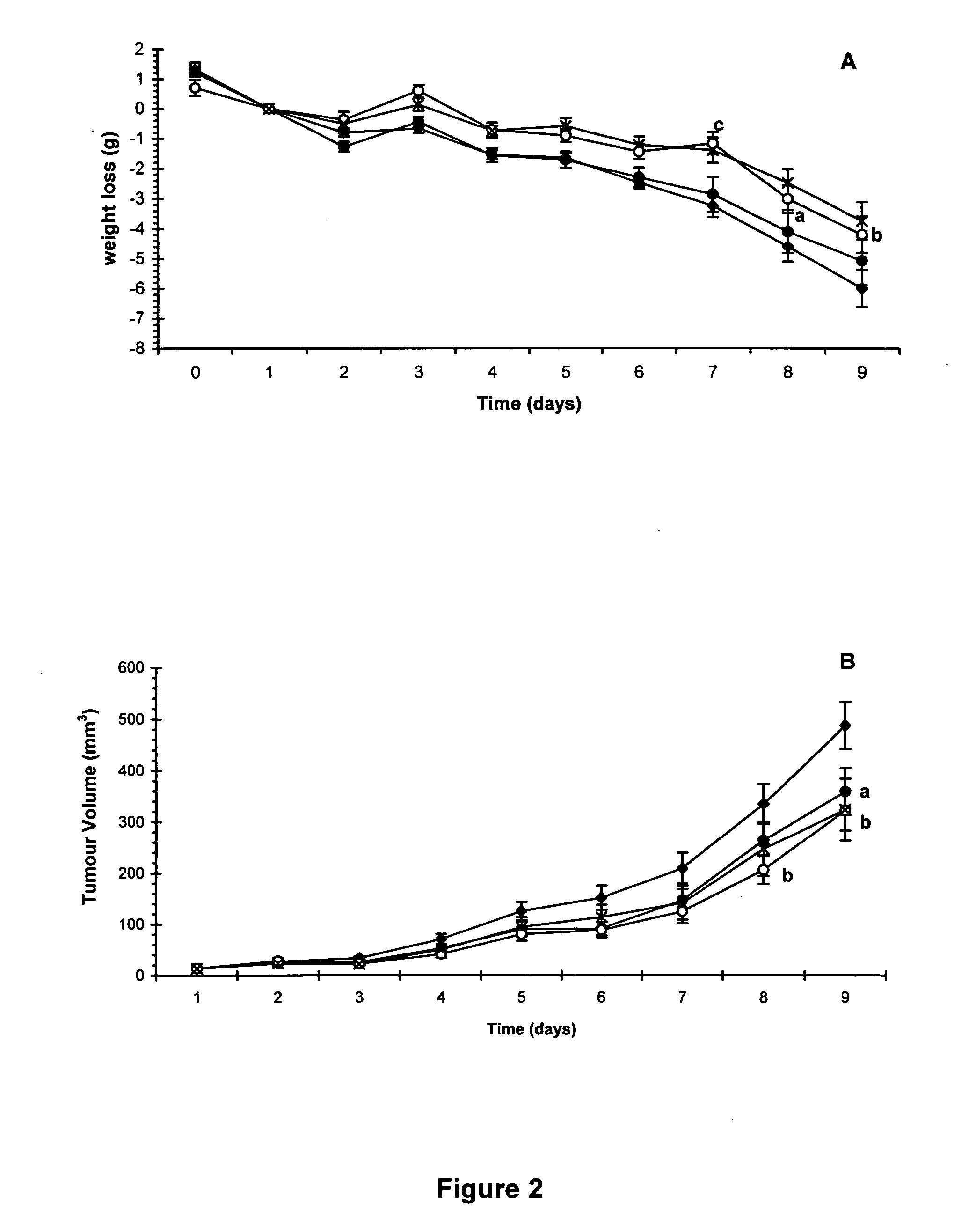
HMB compositions and uses thereof
InactiveUS20050215640A1Reduce tumor growth rateReduce rateBiocideNervous disorderInvoluntary weight lossNeutral Amino Acids
The present invention relates to methods for the prevention and treatment of chronic inflammatory diseases, cancer, and involuntary weight loss. In the practice of the present invention patients are enterally administered HMB alone or alternatively in combination with eicosapentaenoic (20:5 ω-3), FOS, carnitine and mixtures thereof. HMB may be added to food products comprising a source of amino-nitrogen enriched with large neutral amino acids such as leucine, isoleucine, valine, tyrosine, threonine and phenylalanine and subtantially lacking in free amino acids.
Owner:ABBOTT LAB INC
Antibodies immunoreactive with mutant 5-enolpyruvlshikimate-3-phosphate synthase
Antibodies immunoreactive to double mutant EPSPS are provided, and in an embodiment the double mutant EPSPS is one in which the wild-type EPSPS is substituted at residue 102 with isoleucine and at residue 106 with serine. Also provided are hybridomas producing the antibodies, as well as methods of making and using the antibodies.
Owner:M S TECH
Peptide for high performance inhibition of angiogenesis and method for preparing same and use thereof
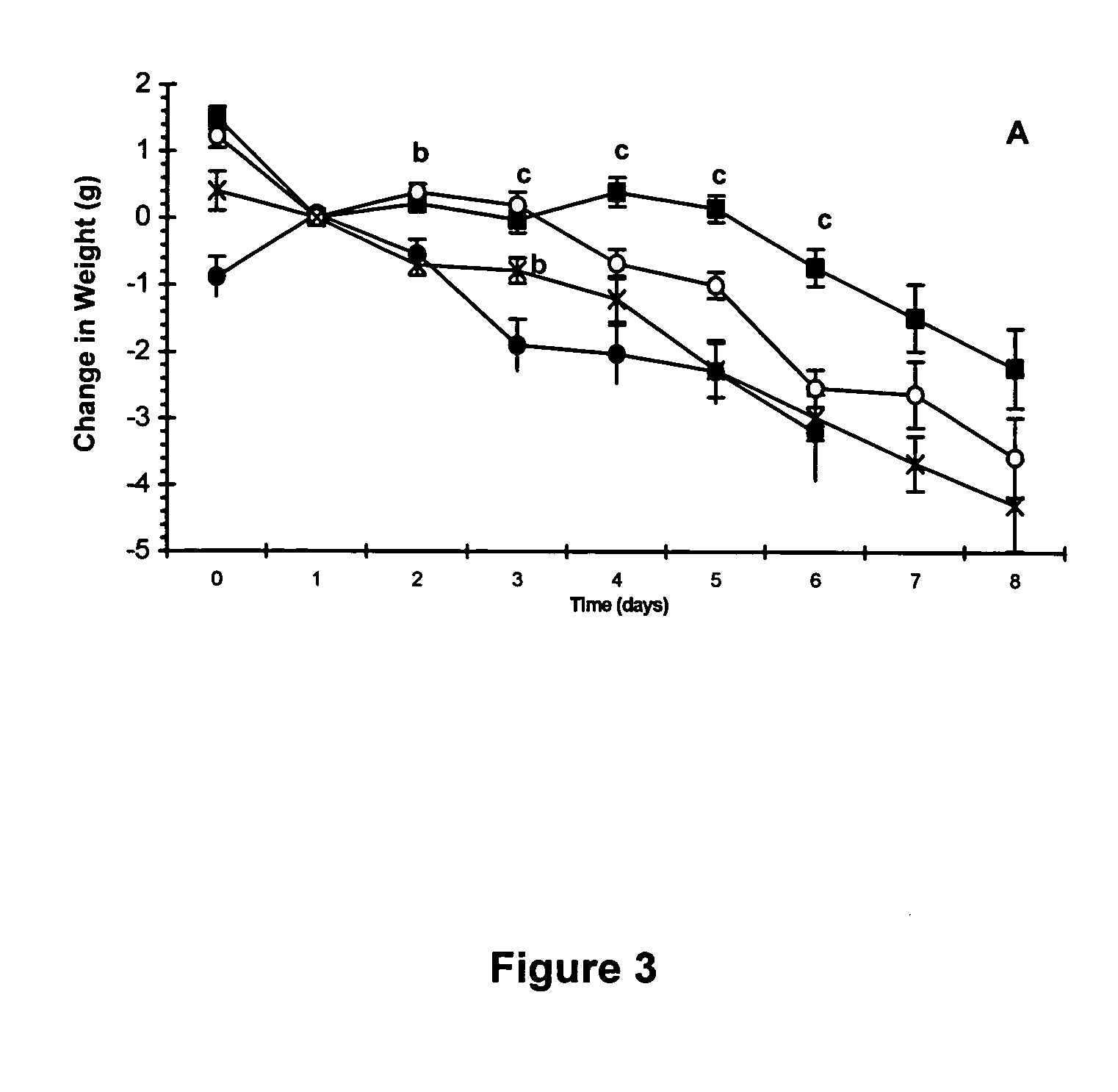
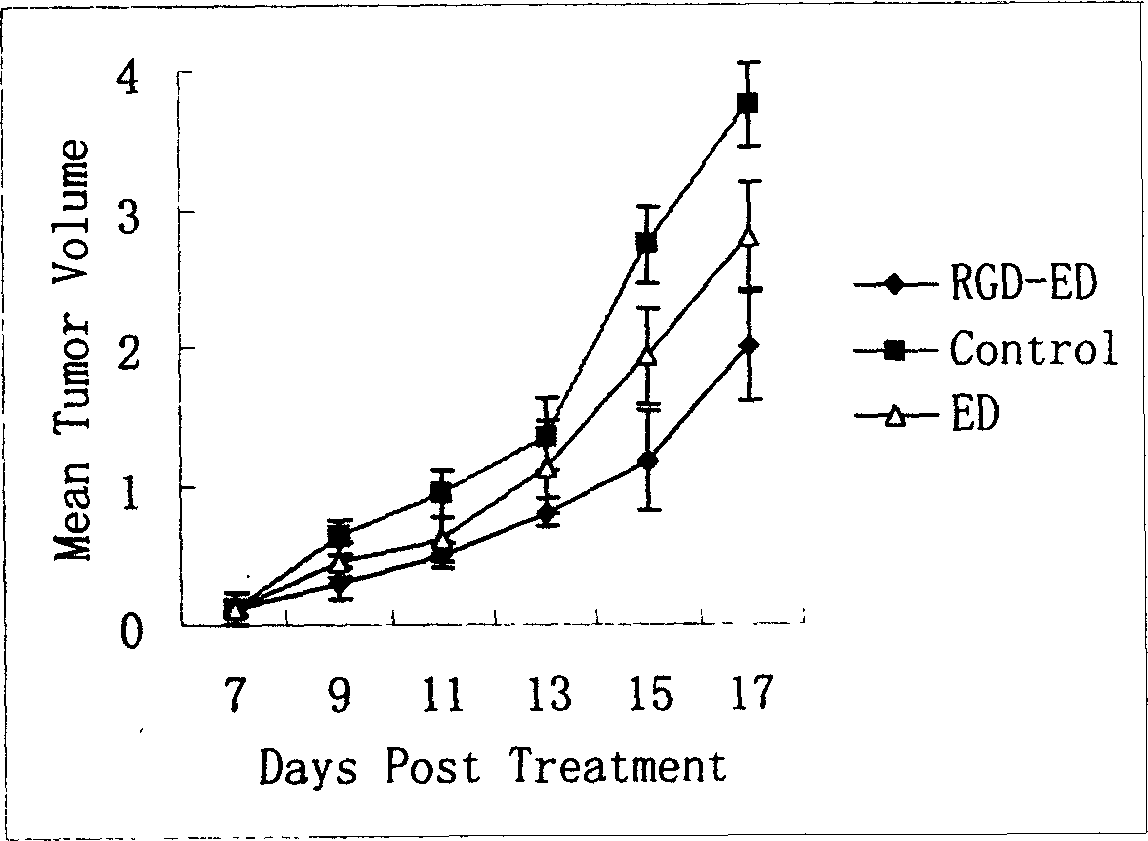
ActiveCN1699408AImprove and enhance growthImprove and enhance the anti-tumor effectPeptide/protein ingredientsSkeletal disorderEscherichia coliInclusion bodies
The invention relates to a peptide for high performance inhibition of angiogenesis and method for preparing same and use, wherein high performance blood vessel production inhibiting agent RGD-ED with integration compatibility is designed, the inhibiting agent comprises polypeptide polypeptide-valine-arginine-arginine-alanine-aspartate-arginine-alanine-alanine-valine-praline, its one or two ends are connected with polypeptides containing arginine-glycine-aspartic acid sequence. The RGD-ED provided by the invention can be synthesized. The invention also discloses the expression of one RGD-ED in bacillus coli through gene engineering method, wherein the RGD-ED is prepared through the steps of inclusion body protein segregation, dissolution and renaturation, and ion-exchange chromatography segregation and purification.
Owner:CHINA PHARM UNIV
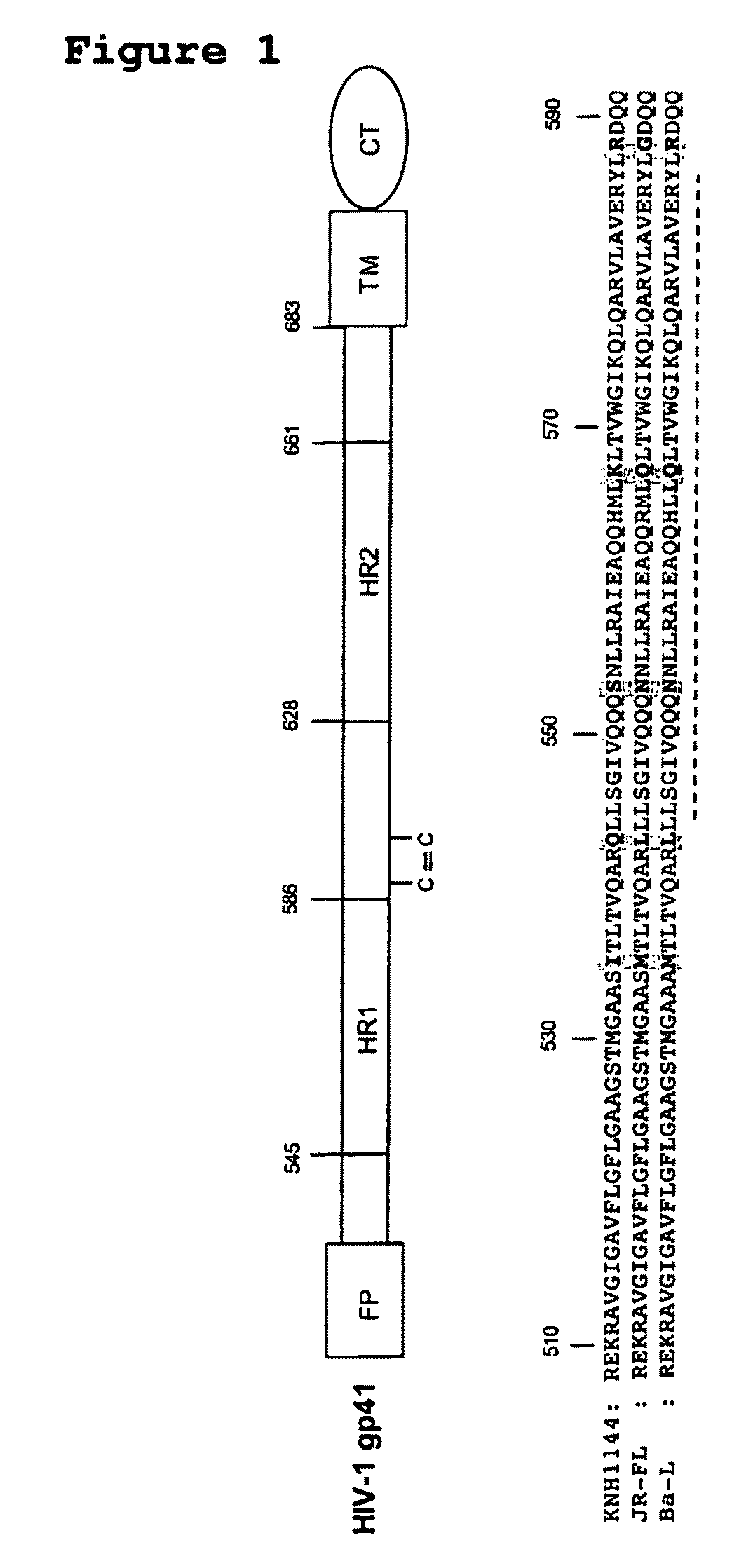
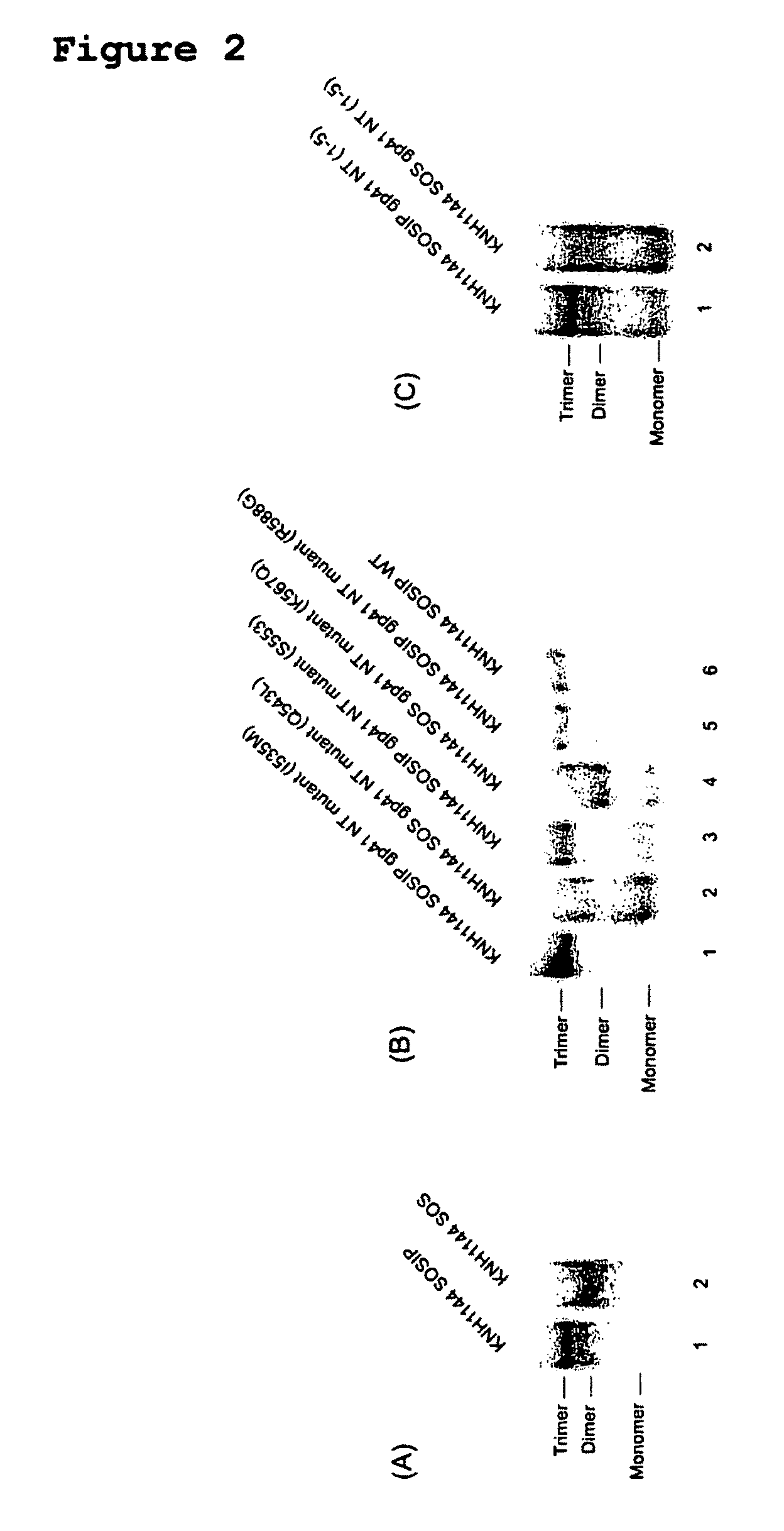
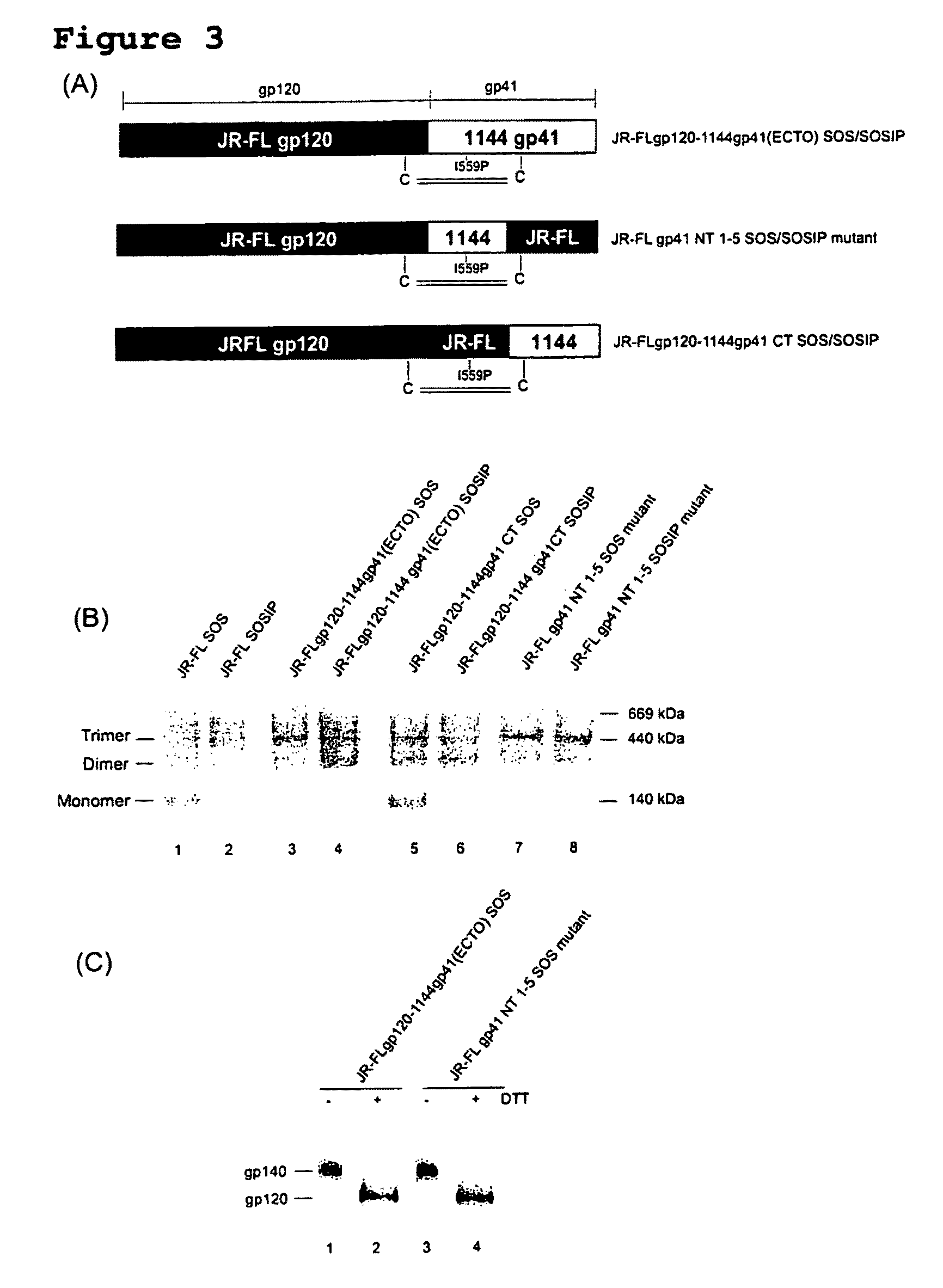
Soluble, stabilized, proteolytically cleaved, trimeric HIV-1 gp140 proteins comprising modifications in the N-terminus of the gp41 ectodomain
InactiveUS7939083B2Improve stabilityOverall antigenic structure of the trimer not adversely affectedViral antigen ingredientsVirus peptidesArginineGlutamine
Owner:CORNELL RES FOUNDATION INC +1
Black garlic, process and device for fermenting same
InactiveCN101518319AEasy to peel offNo irritating odorClimate change adaptationFruits/vegetable preservation using acidsS-Allyl cysteinePesticide residue
The invention discloses black garlic, a process and a device for fermenting the same, which overcomes technical defects of the prior product, processing method and processing device. The black garlic is integral in bulk, dry in outer peel and easy to peel. Garlic cloves are in preserved fruit paste shape, are free from pungent taste, have jelly taste, taste good and are easy to preserve. Every 100 grams of ripening black garlic contains 140 milligrams of isoleucine, 250 milligrams of leucine, 170 milligrams of lysine, 160 milligrams of cystine, S-allyl cysteine and polyphenol of which the content is 22 times that of raw garlic, 36 milligrams of sodium, 930 milligrams of potassium, 52 milligrams of magnesium and 13 milligrams of calcium. The black garlic has the advantages of low heavy-metal content, no pesticide residue and no additive. The fermenting process comprises the steps of taking integral garlic as raw material, initially washing the raw material with clear water, soaking the raw material in brine, placing the raw material in a tray, fermenting, steaming and baking the raw material, performing aging treatment, sterilizing and disinfecting the obtained product, and packing the obtained product in vacuum or with nitrogen. The fermenting process has the advantages of adopting mineral water vapor and discharging air in the whole process, along with few links and low cost. The fermenting device is heated by a steam boiler, has a humidifying vapor pipe, and uses all-wooden fermentation frame and a container.
Owner:DALIAN HUAGU GARLIC
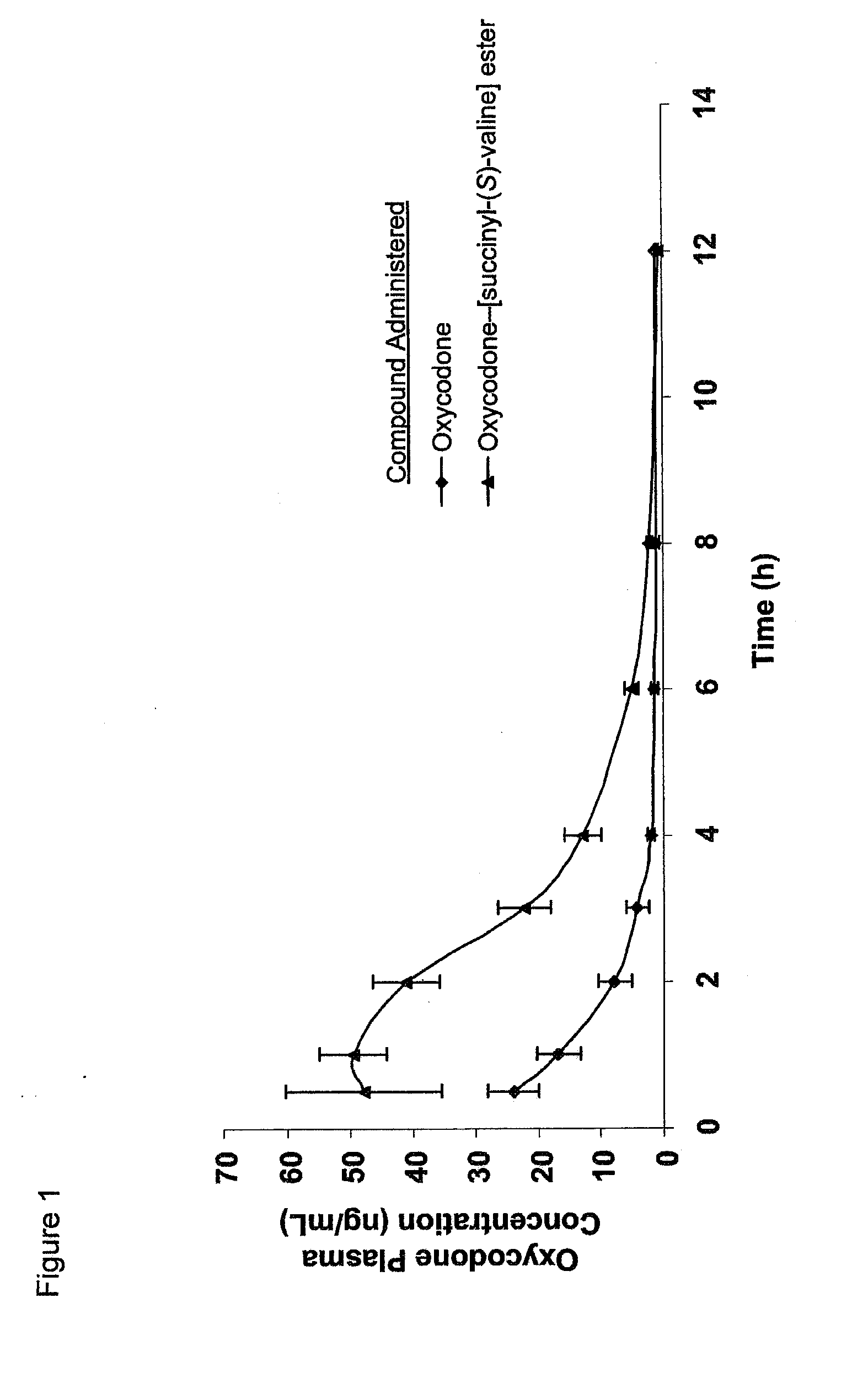
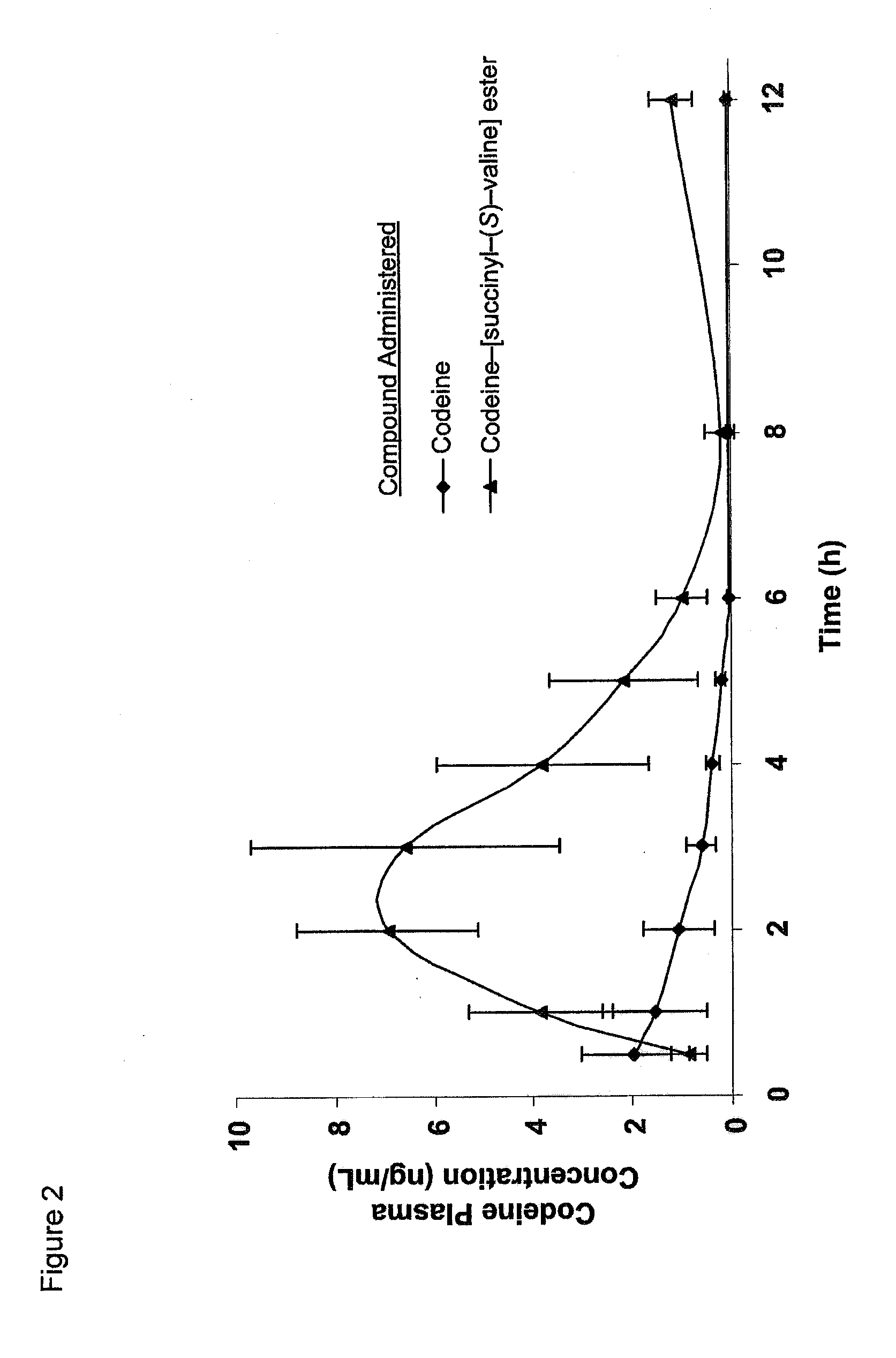
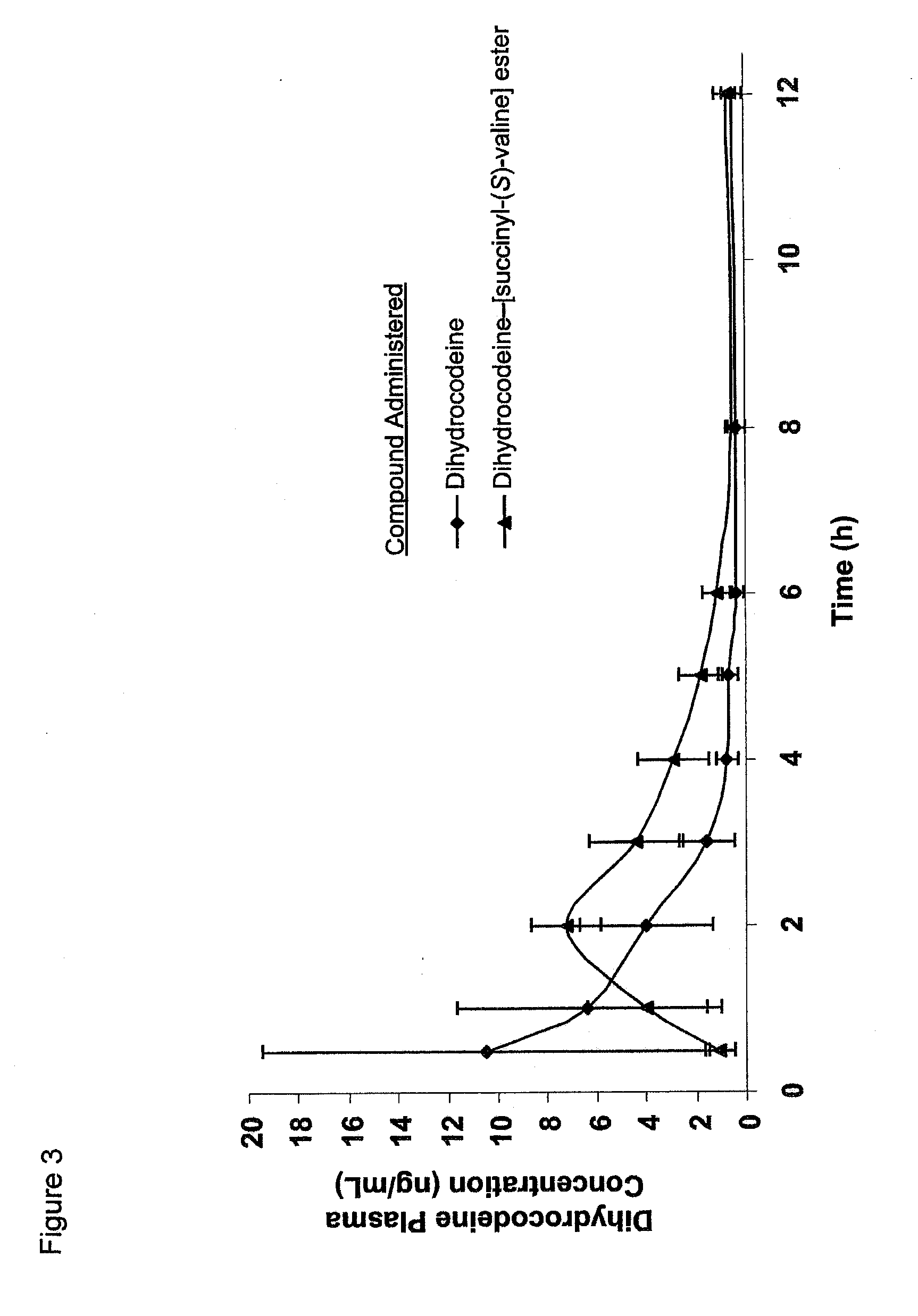
Novel dicarboxylic acid linked amino acid and peptide prodrugs of opioids and uses thereof
InactiveUS20100286186A1Low variabilityReduction and elimination of painBiocideNervous disorderSide effectAmino acid side chain
The present invention concerns dicarboxylic acid linked amino acid and peptide prodrugs of opioid analgesics and pharmaceutical compositions containing such prodrugs. Methods for providing pain relief, decreasing the adverse GI side effects of the opioid analgesic and increasing the bioavailability of the opioid analgesic with the aforementioned prodrugs are also provided. In one embodiment, prodrugs having the amino acid side chains of valine, leucine, isoleucine and glycine; and mono-, di- and tripeptides thereof are provided.
Owner:SHIRE PLC
TGF-B inhibitors and methods
A family of small peptides have been found to be inhibitory to TGF-beta activity, and preferably have the primary structure of Formula I:wherein AA1 is leucine, phenylalanine, alpha-aminoisobutric acid, N-methylalanine, N-methylisoleucine, or isoleucine; AA2 is the same or a different amino acid residue as in AA1; and AA3 is alanine or N-methylalanine.
Owner:RGT UNIV OF CALIFORNIA
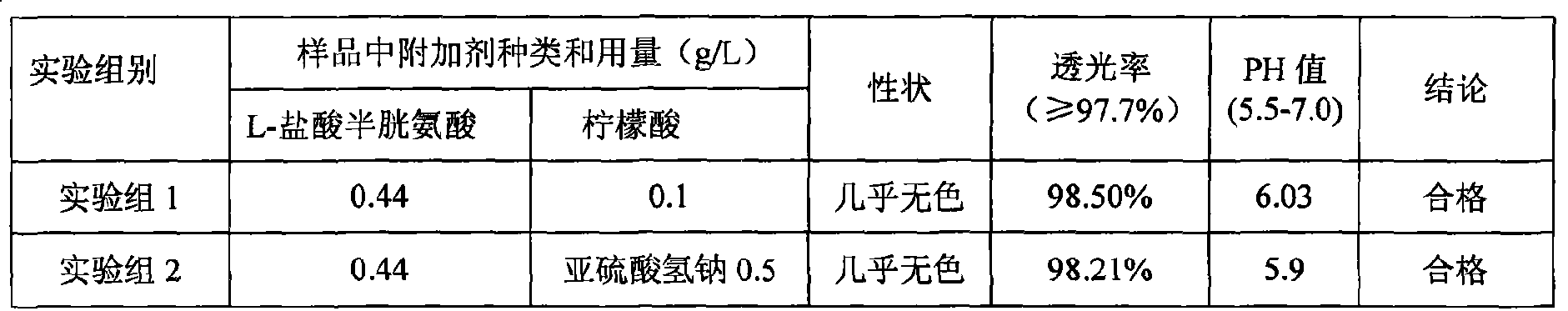
Compound amino acid injecta, and preparation method and detection method thereof
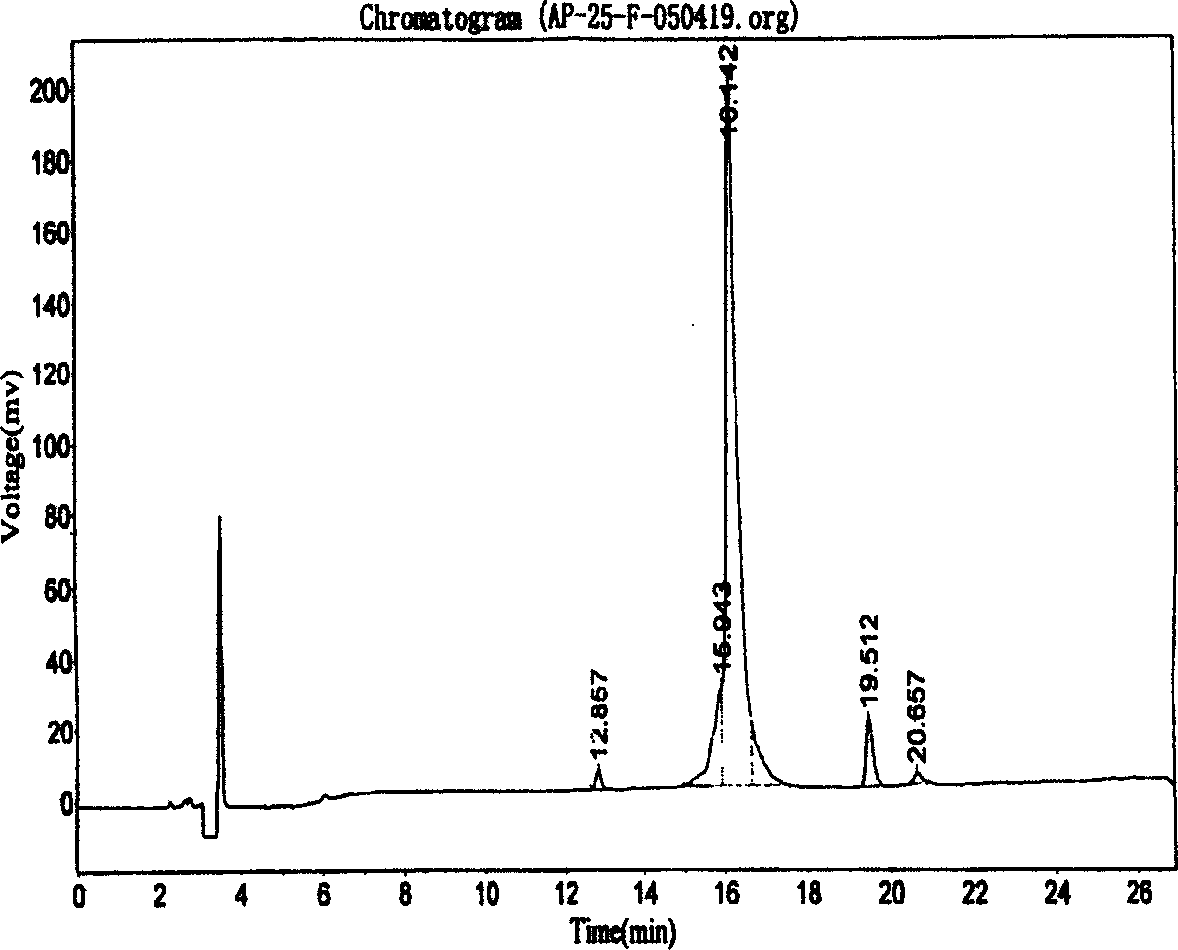
ActiveCN102440989AAccurate measurementExact reproductionOrganic active ingredientsMetabolism disorderIon chromatographySulfate radicals
The invention discloses compound amino acid injecta, which contains arginine hydrochloride, histidine monohydrochloride, leucine, isoleucine, lysine hydrochloride and the like; meanwhile, the invention further discloses a preparation method of the compound amino acid injecta (18AA); in the event of ensuring the product quality, the compound amino acid injecta has the characteristics of saving energy and enhancing efficacy; by means of repetitive verification on precision, detection limit, quantitation limit, linear range and the like, a simple, convenient, rapid and reliable method with cleaning verification is ensured; furthermore, the residual quantity of antioxygen, namely sodium hydrogensulfite, in the compound amino acid injecta and sulphate ion generated by degrading sodium hydrogensulfite are detected according to an ion chromatography principle, thus, the amount of the antioxygen charged in the raw material proportioning can be accurately measured, and the stability of the raw material proportioning process is judged according to the measurement result; and a detection method of the compound amino acid injecta provided by the invention is an online derivative amino acid content measuring method, the speed is rapid, and the method is applied to large-scale detection.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
HMB compositions and uses thereof
The present invention relates to methods for the prevention and treatment of chronic inflammatory diseases, cancer, and involuntary weight loss. In the practice of the present invention patients are enterally administered HMB alone or alternatively in combination with eicosapentaenoic (20:5 ω-3), FOS, carnitine and mixtures thereof. HMB may be added to food products comprising a source of amino-nitrogen enriched with large neutral amino acids such as leucine, isoleucine, valine, tyrosine, threonine and phenylalanine and substantially lacking in free amino acids.
Owner:ABBOTT LAB INC
Mutant-type lipases and applications thereof
The present invention provides mutant-type lipases which demonstrate superior lipolytic and esterific activities. The mutant-type lipases are characterized by an amino acid alteration at the residue immediately followed either the serine residue or the histidine residue or both residues of the Ser-His-Asp catalytic triad. The Ser-His-Asp catalytic triad is known to be the three residues, although occur far apart in the amino acid sequence of a lipase, that contribute to the hydrolytic activity in the active site of the lipase. The amino acid residue that follows the serine residue of the Ser-His-Asp catalytic triad is alanine. The amino acid residue that follows the histidine residue of the Ser-His-Asp catalytic triad is isoleucine. The wild-type lipase is preferably originated from Staphylococcus, particularly Staphylococcus epidermindis. The present invention also relates to a method for preparing the mutant-type lipases by site-directed mutagenesis using PCR and a method for utilizing the mutant-type lipase to catalyze synthesis of flavor esters to be used in food industry.
Owner:ACAD SINIC
Detergent composition containing amino acid component for washing fruits, vegetables and dishes
InactiveCN102492571APromote degradationMaintain stain releaseNon-ionic surface-active compoundsOrganic detergent compounding agentsAntioxidantArginine
The present invention relates to a detergent composition containing an amino acid component for washing fruits, vegetables and dishes, which comprises the following components of an anionic surfactant, a non-ionic surfactant, an amphoteric surfactant, amino acid, a viscosity modifier, a chelating agent, an antiseptic, a light stabilizer, an antioxidant, a flavor and deionized water. The amino acid composition is one or a mixture with more than two of alanine, arginine, asparagine, aspartic acid, cystine, glutamine, glutamic acid, leucine, lysine, methionine, phenylalanine, prolinol acid, serine, threonine, glycine, histidine, isoleucine, tryptophan, tyrosine and valine. The detergent of the invention can maintain good decontamination capability, improve the feeling when the detergent composition is used, reduce the hands skin irritation and enhance the hand skin moisturizing ability to improve dry feeling.
Owner:GUANGZHOU LIBY
Amino acid composition
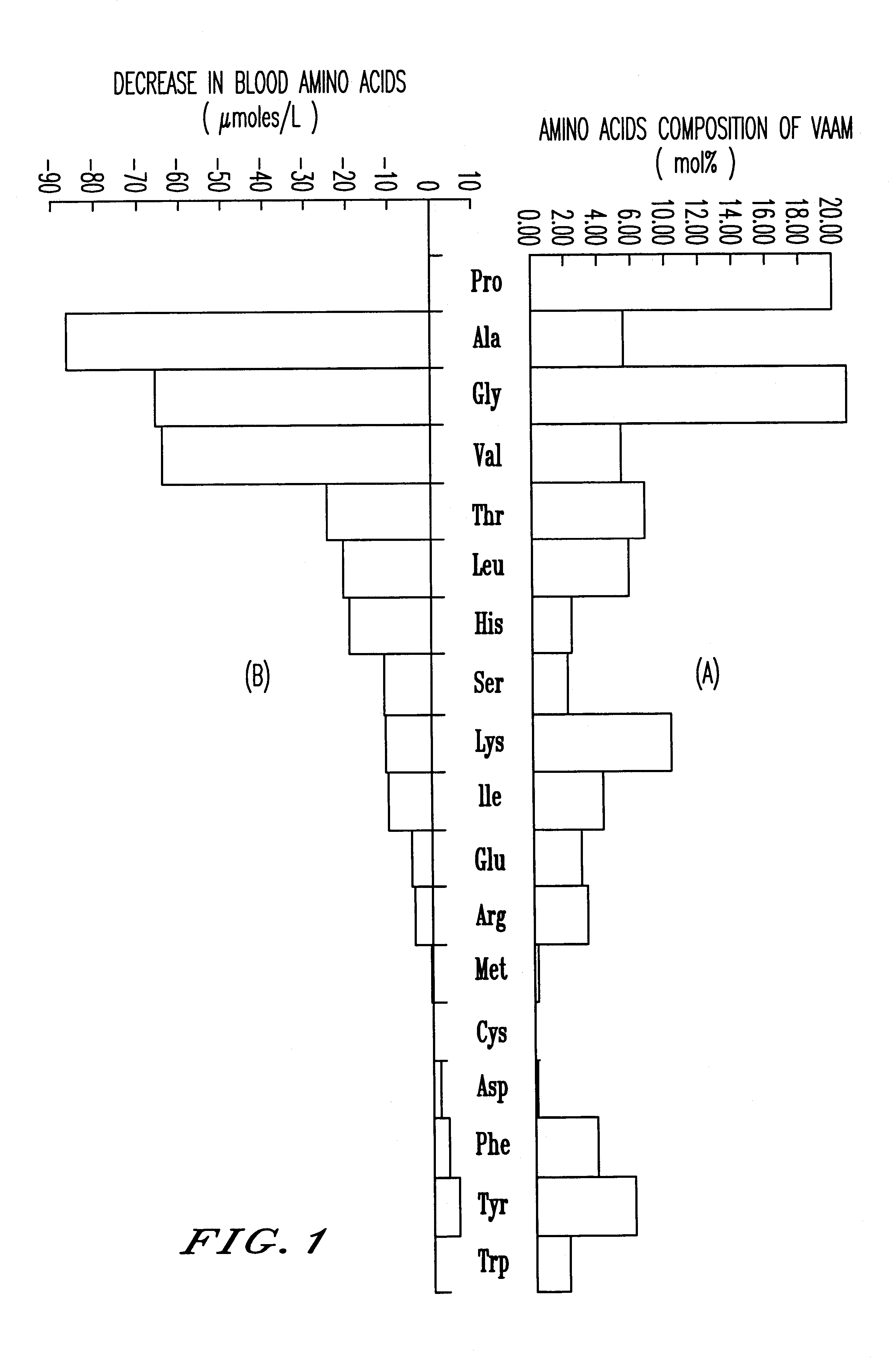
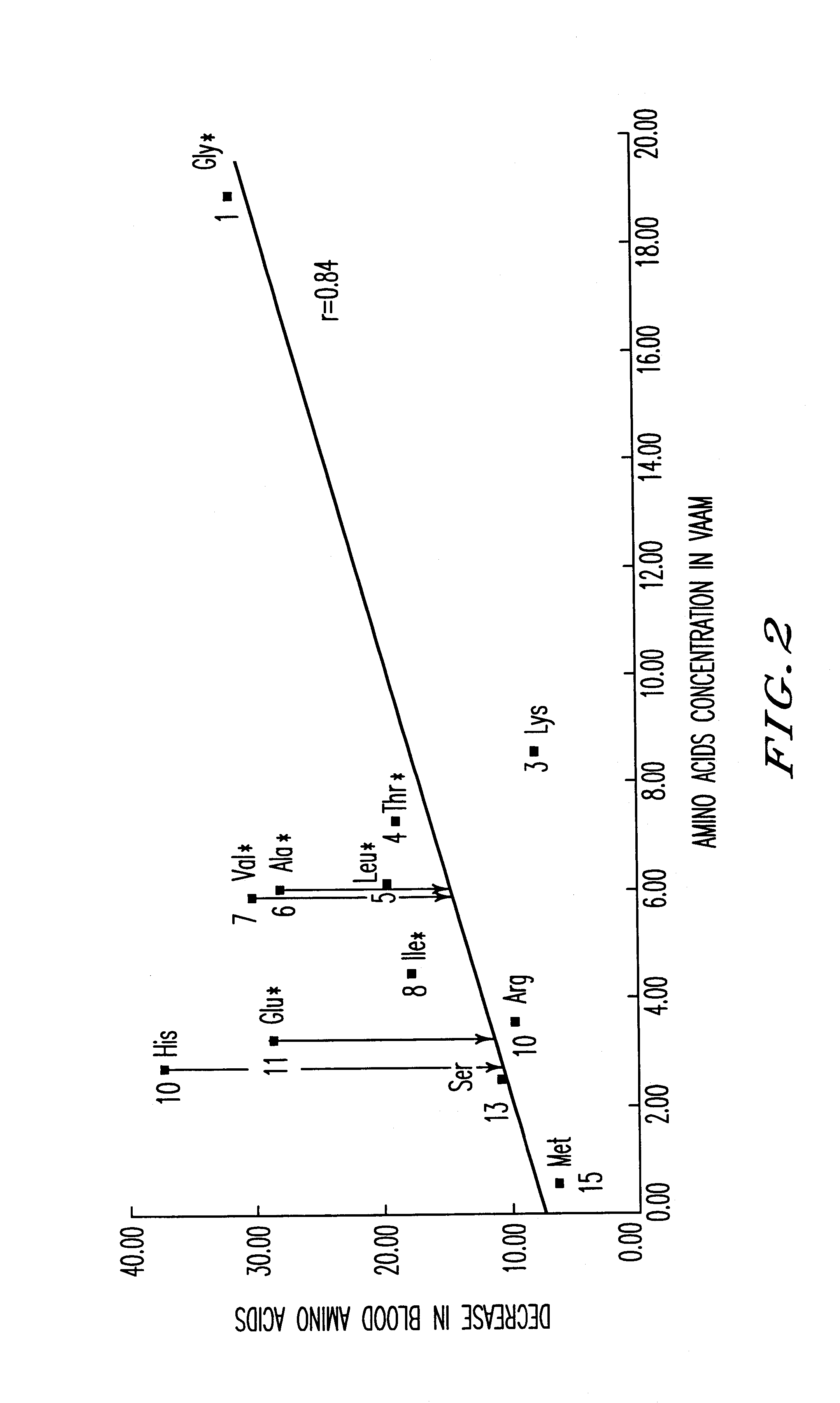
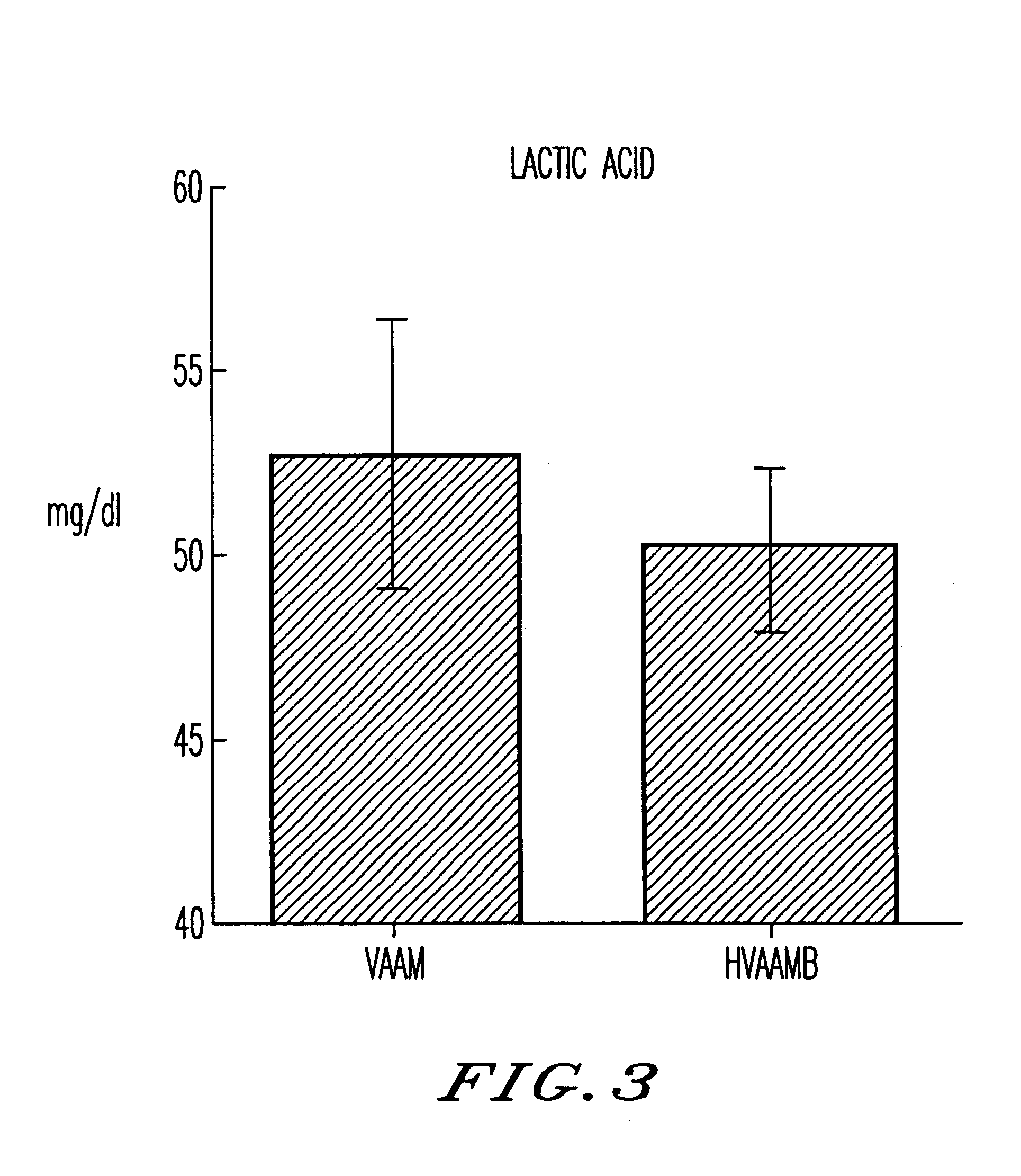
An amino acid composition comprising the following amino acids at the following molar ratio: proline (12.6 to 23.4), alanine (8.4 to 15.6), glycine (13.3 to 24.9), valine (8.2 to 15.4), threonine (5.0 to 9.4), eucine (4.3 to 8.1), histidine (1.8 to 11.9), serine (1.7 to 3.3), lysine (6.0 to 11.2), isoleucine (3.1 to 5.9), glutamic acid (2.2 to 10.4), arginine (2.4 to 4.6), phenylalanine (2.6 to 5.0), tyrosine (4.2 to 7.8) and trypsin (1.5 to 2.9). The composition supplements blood amino acids reduced during hard exercise and shows effects to improve motor function, to reduce fatigue after exercise and to help recovery from the fatigue.
Owner:THE INST OF PHYSICAL & CHEM RES WAKO +1
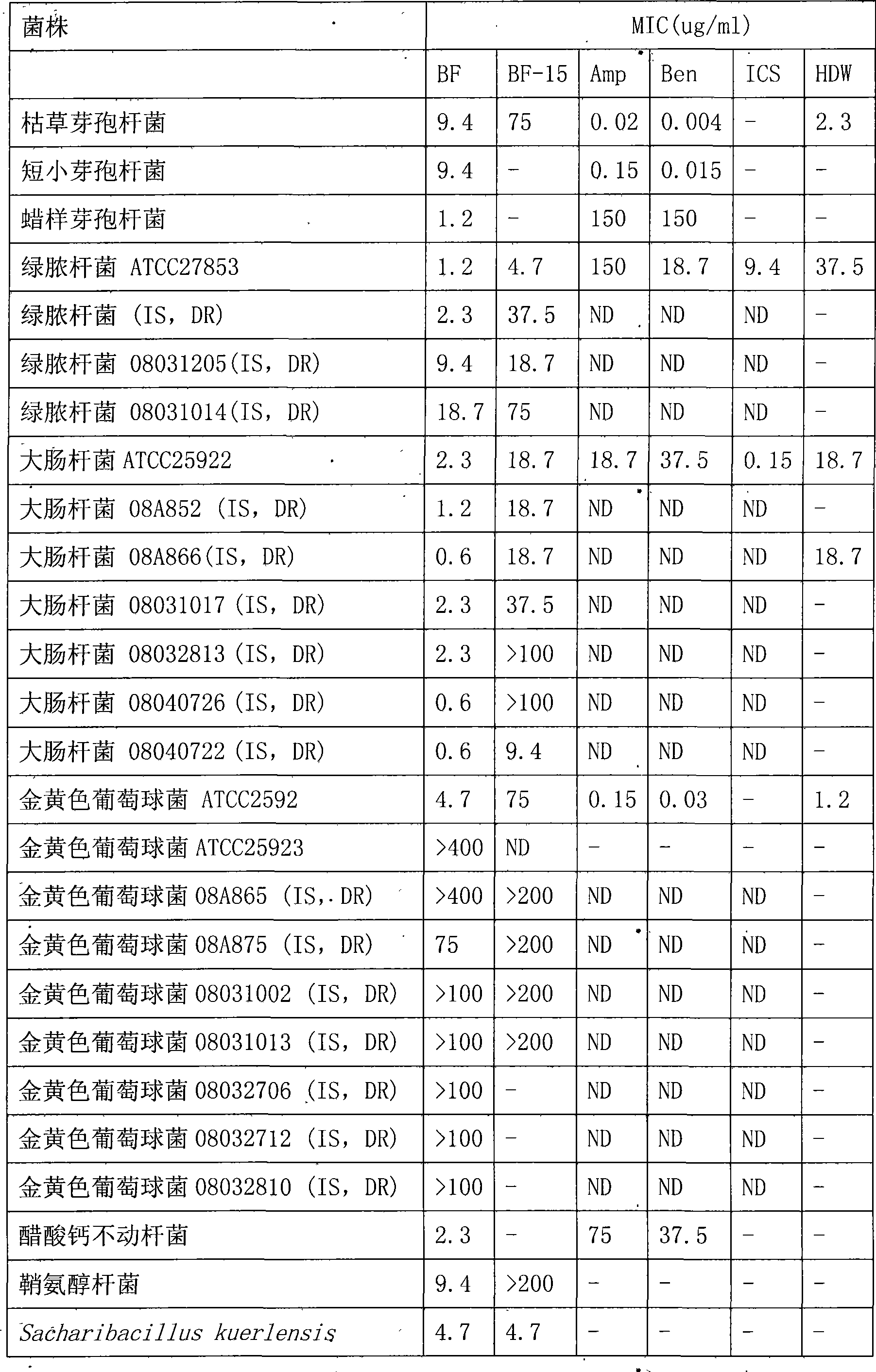
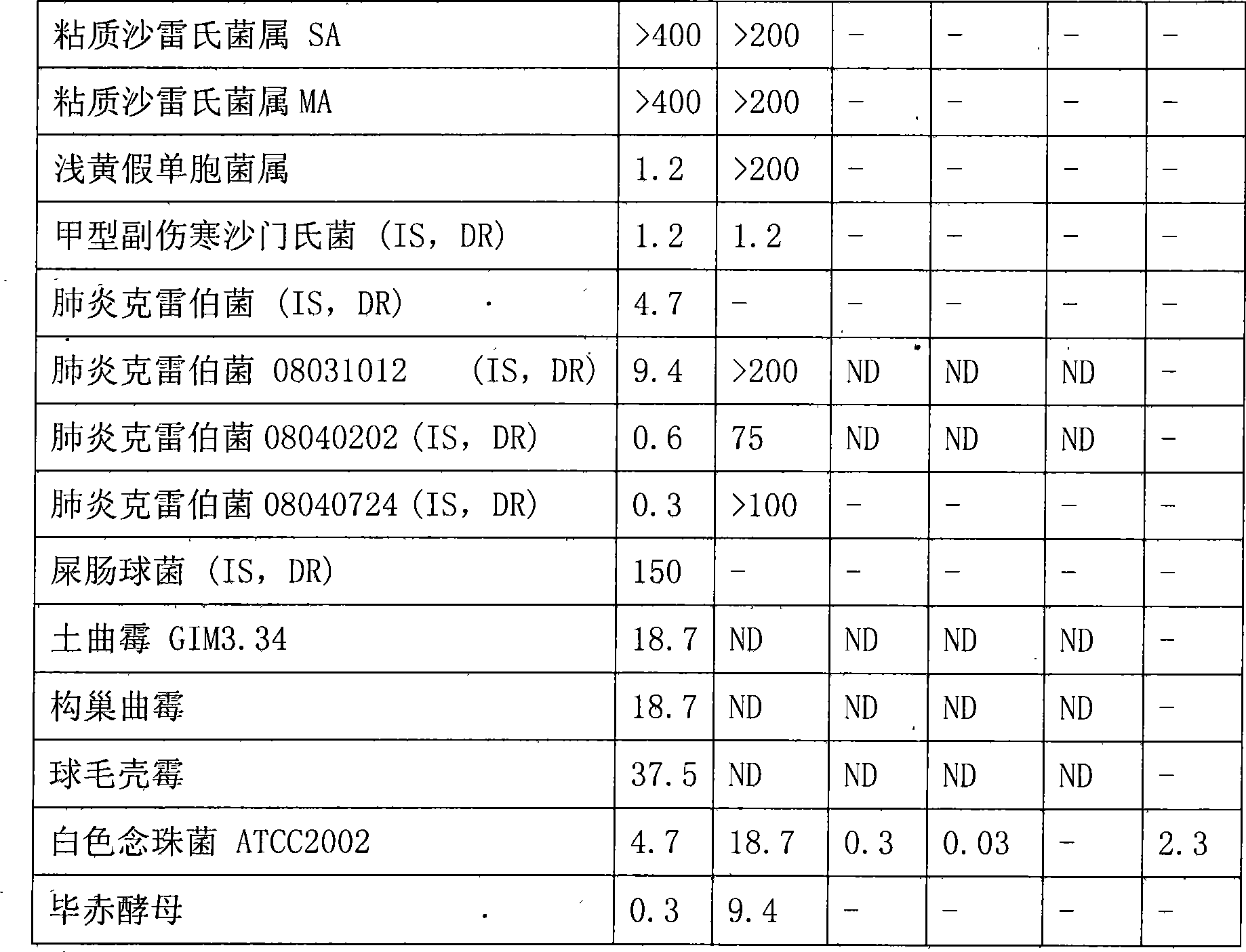
Bungarus fasciatus antibacterial peptide cathelicidin-BF, and genes and uses thereof
InactiveCN101412753ASmall molecular weightImprove the bactericidal effectFermentationAnimals/human peptidesArginineAntibiotic Y
The invention discloses cathelicidin-BF and a gene and application thereof, which belong to the field of biomedicine. The cathelicidin-BF is straight chain polypeptide and contains thirty amino acid residues, the molecular weight is 3,637.54Da, and the isoelectric point is 11.79. The complete sequence of the cathelicidin-BF is lysine-phenyl alanine-phenyl alanine-arginine-lysine-leucine- lysine-lysine-serine-valine-lysine-lysine-arginine-lactamine-lysine-glutamic acid-phenyl alanine- phenyl alanine-lysine-lysine-proline-arginine-valine-isoleucine-glycin-valine-serine-isoleucine- praline-phenyl alanine. The gene for encoding the cathelicidin-BF consists of 750 ribonucleotides, wherein 484th to 573rd ribonucleotides are used for encoding a mature peptide part. The cathelicidin-BF has small molecular weight, strong sterilization effect, and quick action time, and has quite strong killing function to a plurality of kinds of clinical drug-fast bacteria. In addition, the cathelicidin-BF also has the advantages of broad-spectrum antibiotics, salt independence and so on.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
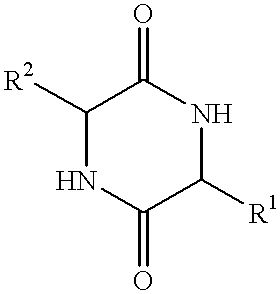
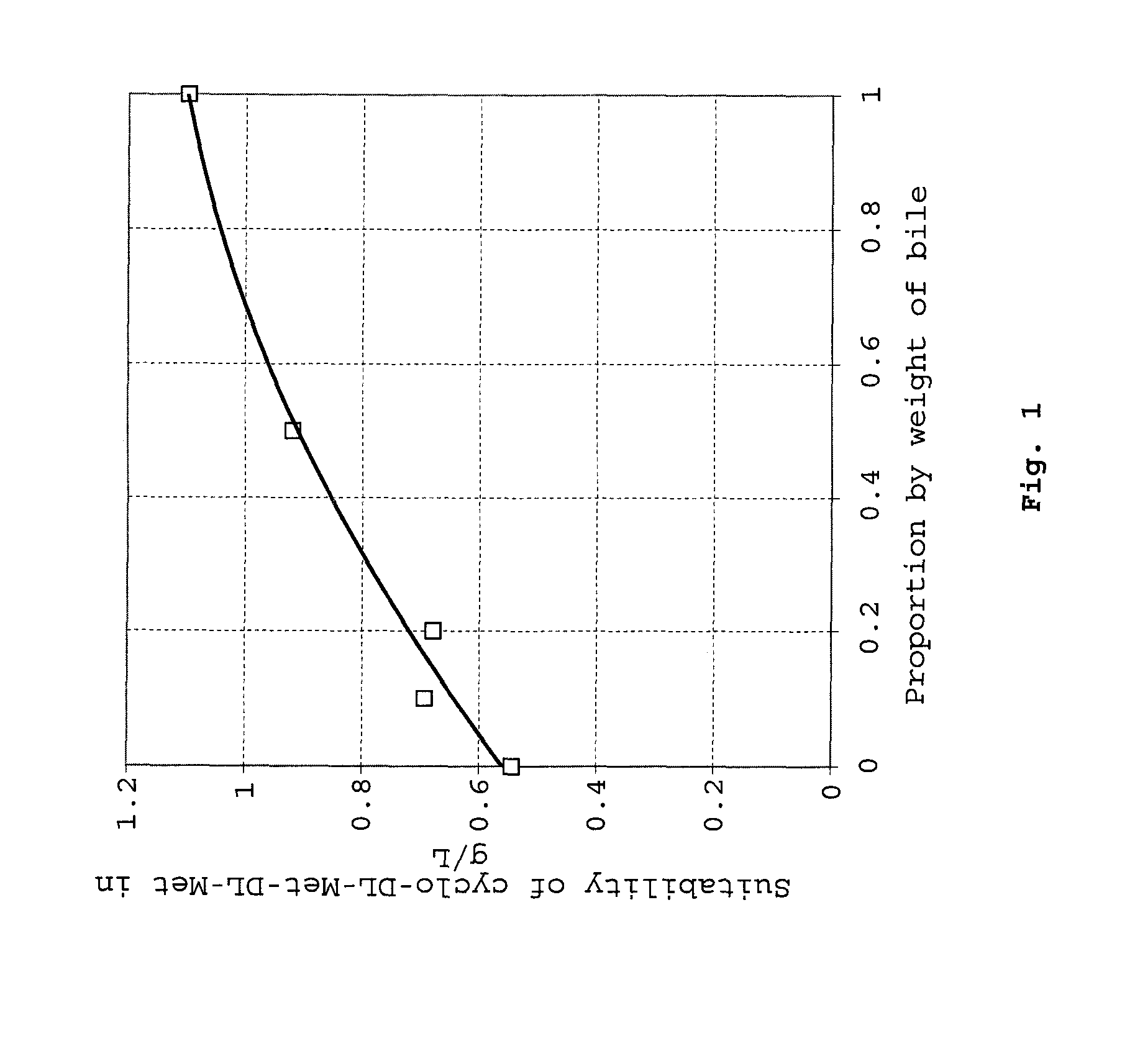
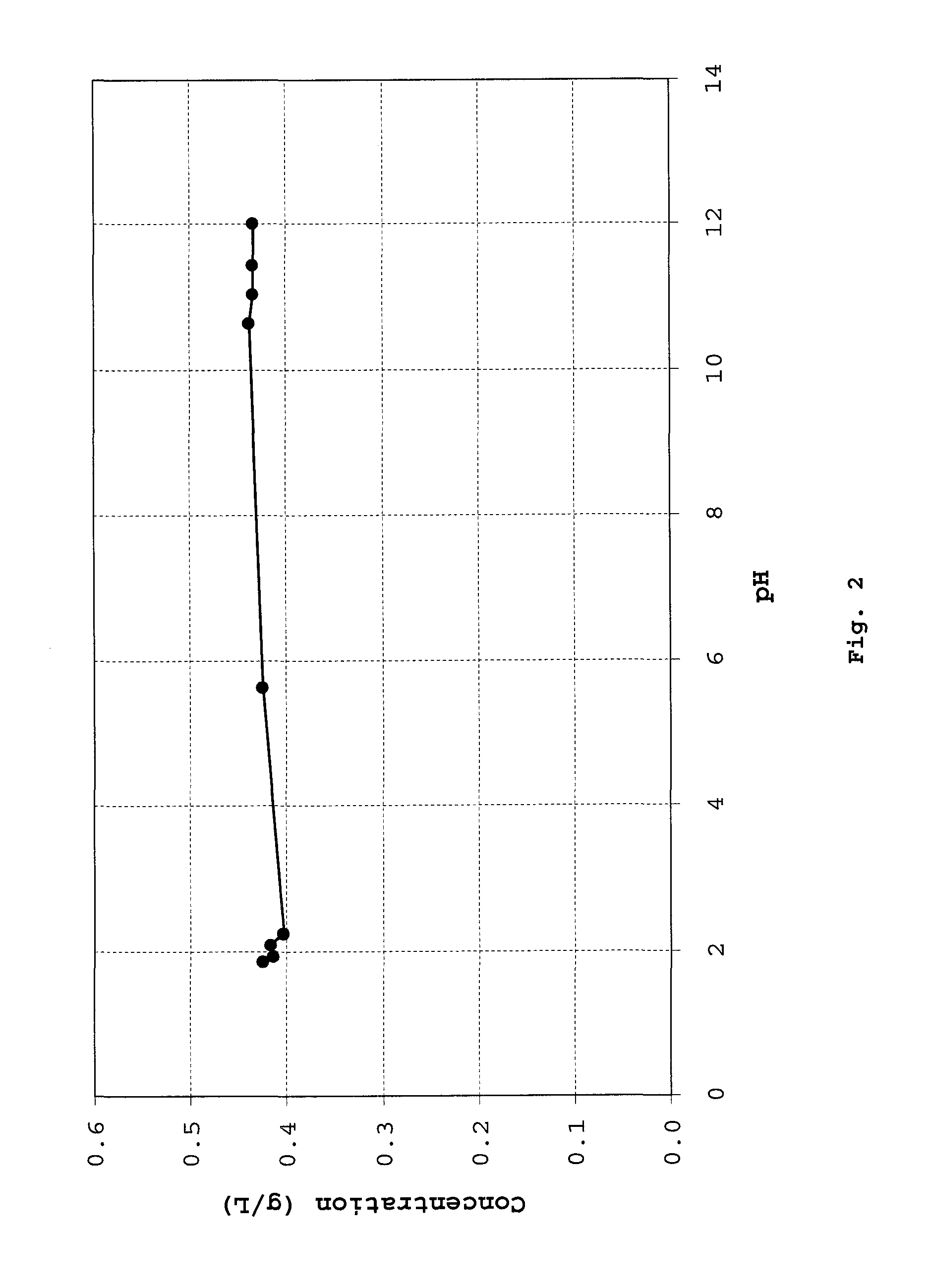
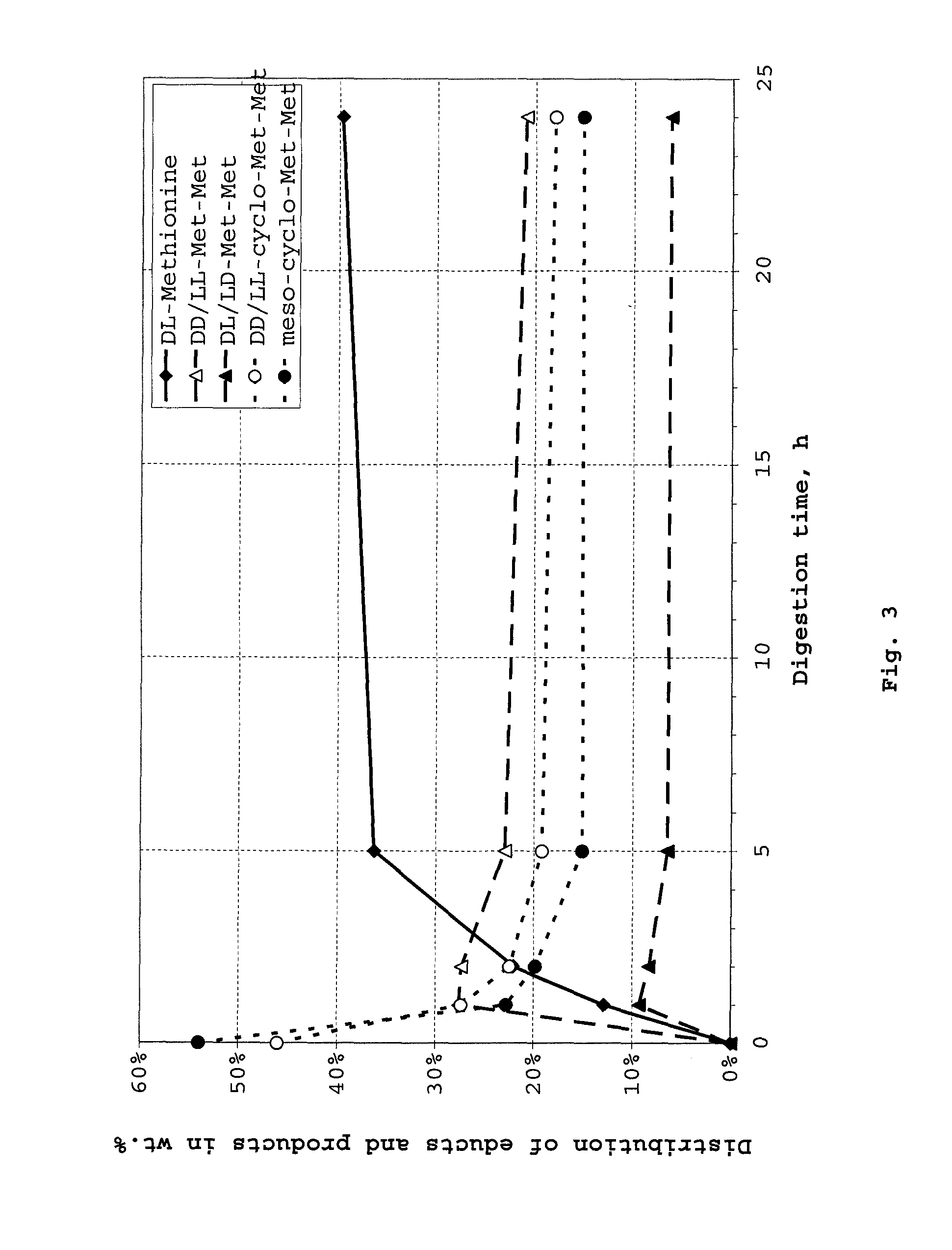
Method of synthesizing diketopiperazines
InactiveUS6967202B2Prevent unwanted side reactionInhibit side effectsOrganic active ingredientsDipeptide ingredientsSide chainTyrosine
The invention provides a method of synthesizing a diketopiperazine of the formula: wherein:R1 is —CH2COR3, or —CH2CH2COR3;R2 is the side chain of an amino acid selected from the group consisting of glycine, alanine, valine, leucine, isoleucine, serine, threonine, aspartic acid, asparagine, glutamic acid, glutamine, lysine, hydroxylysine, histidine, arginine, phenylalanine, tyrosine, tryptophan, thyroxine, cysteine, methionine, norvaline and ornithine;R3 is —OH, —NH2, —OR4, —NHR4, or —NR4R4; andeach R4 is independently an alkyl, aryl, alkylaryl, or arylalkyl.
Owner:AMPIO PHARMA
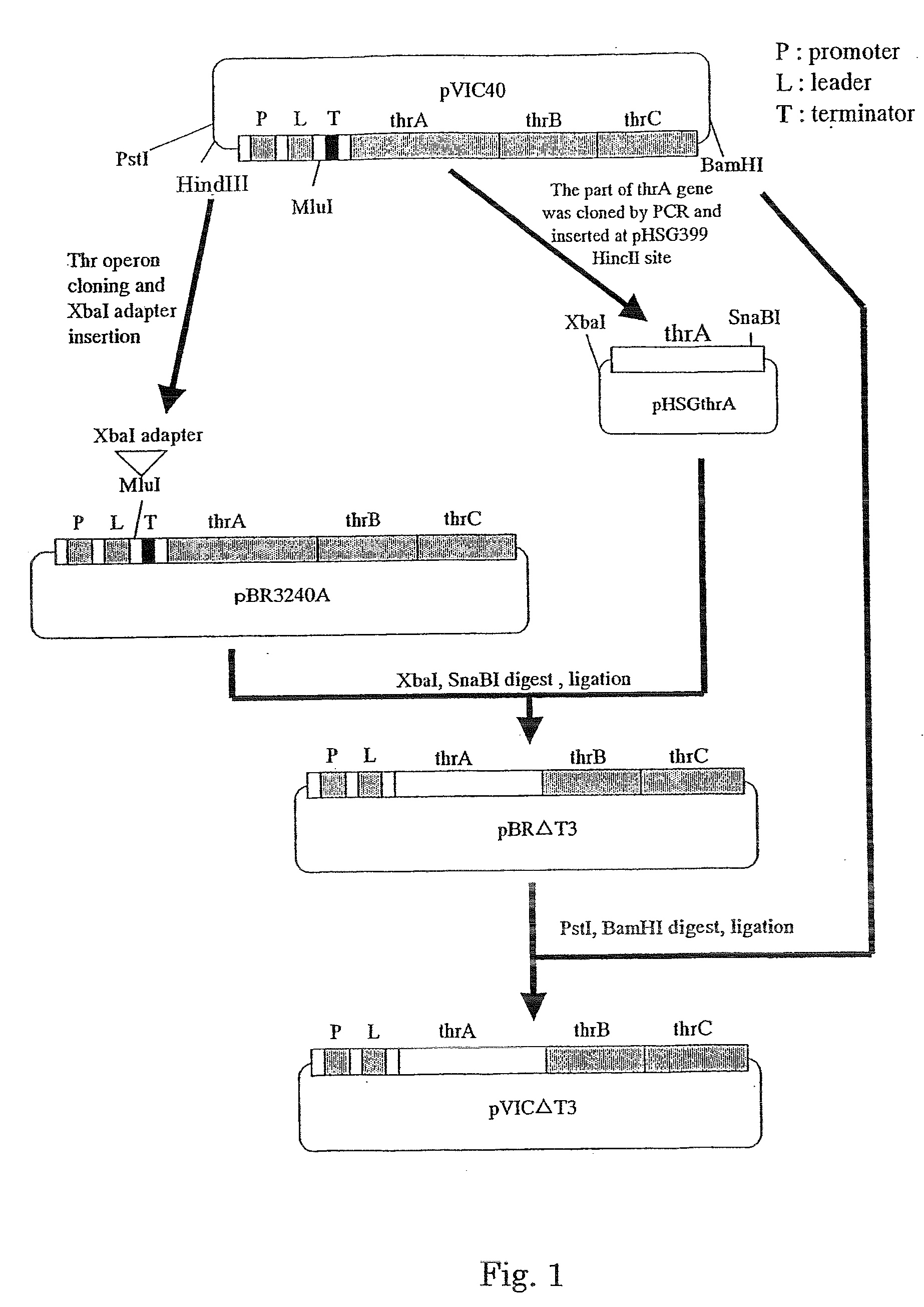
Corynebacterium glutamicum and method for producing high-yield isoleucine with same
ActiveCN105886431ASignificant progressBacteriaMicroorganism based processesGenes mutationMicroorganism
The invention relates to Corynebacterium glutamicum and a method for producing high-yield isoleucine with the same, and belongs to the field of microorganisms and genomics. The Corynebacterium glutamicum, specifically Corynebacterium glutamicum YI is preserved with a preservation number CGMCC NO. 12153. The Corynebacterium glutamicum is obtained by means of mutagenesis and is fermented in a 30L fermentation cylinder for 32-40 hours; the yield of isoleucine is up to 28g / L; accordingly, the method has the advantages of short time for fermentation and high yield and is superior to the prior art. The invention also discloses a gene mutation site of the strain relative to a standard strain, providing a new direction for correlational researches.
Owner:TIANJIN UNIV OF SCI & TECH
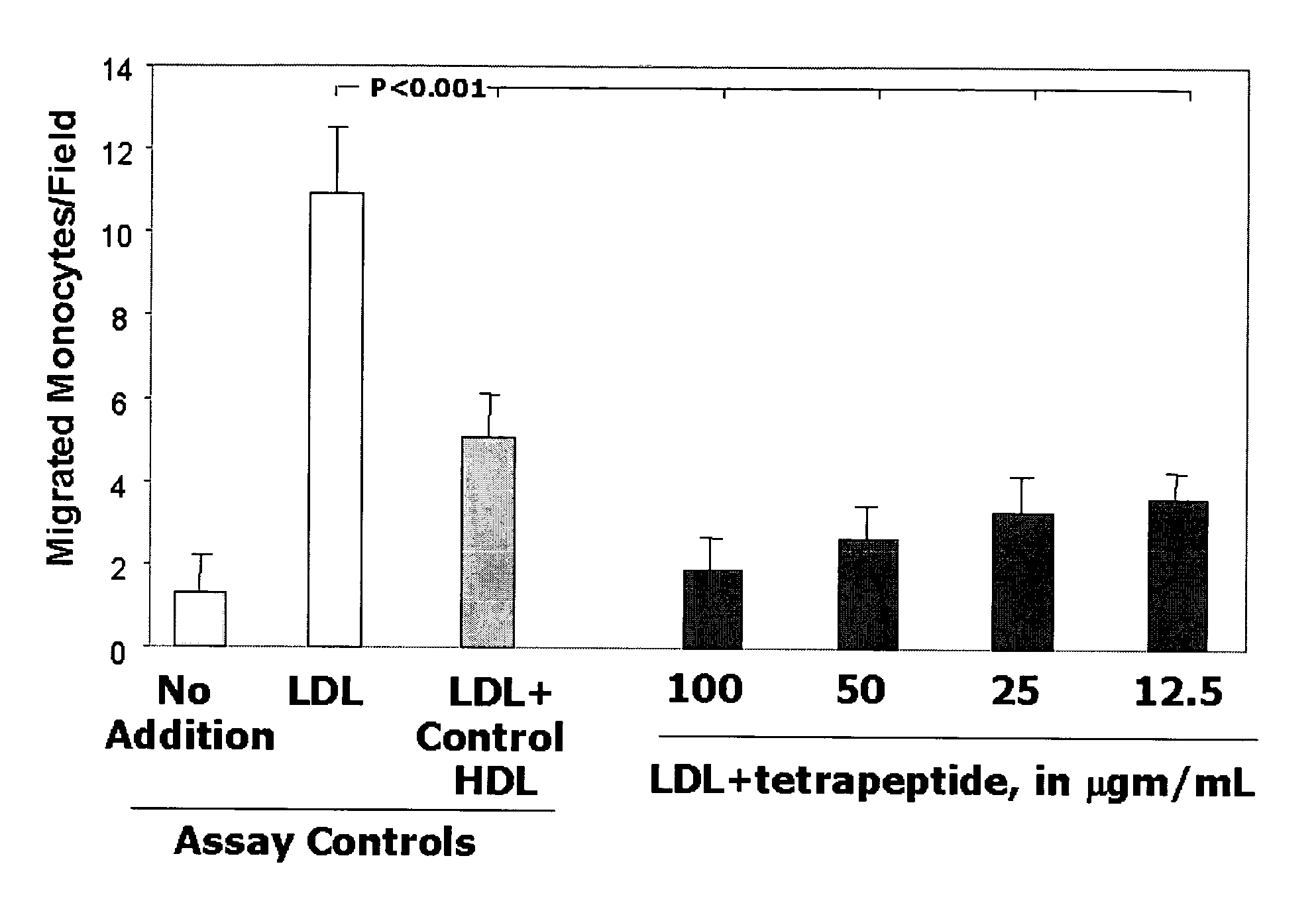
Orally administered small peptides synergize statin activity
InactiveUS7148197B2Readily taken up and deliveredMany symptomOrganic active ingredientsPeptide/protein ingredientsThreonineTyrosine
This invention provides novel peptides for the treatment of atherosclerosis. In certain embodiments the peptide is X1-X2-X3-X4 where X1 and X4 are independently selected from the group consisting of alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), tryptophan (Trp), methionine (Met), serine (Ser) bearing a hydrophobic protecting group, beta-naphthyl alanine, alpha-naphthyl alanine, norleucine, cyclohexylalanine, threonine (Thr) bearing a hydrophobic protecting group, tyrosine (Tyr) bearing a hydrophobic protecting group, lysine (Lys) bearing a hydrophobic protecting group, arginine (Arg) bearing a hydrophobic protecting group, ornithine (Orn) bearing a hydrophobic protecting group, aspartic acid (Asp) bearing a hydrophobic protecting group, cysteine (Cys) bearing a hydrophobic protecting group, and glutamic acid (Glu) bearing a hydrophobic protecting group; X2 and X3 are independently selected from the group consisting of Asp, Arg, and Glu; and the peptide converts pro-inflammatory HDL to anti-inflammatory HDL or makes anti-inflammatory HDL more anti-inflammatory.
Owner:RGT UNIV OF CALIFORNIA +1
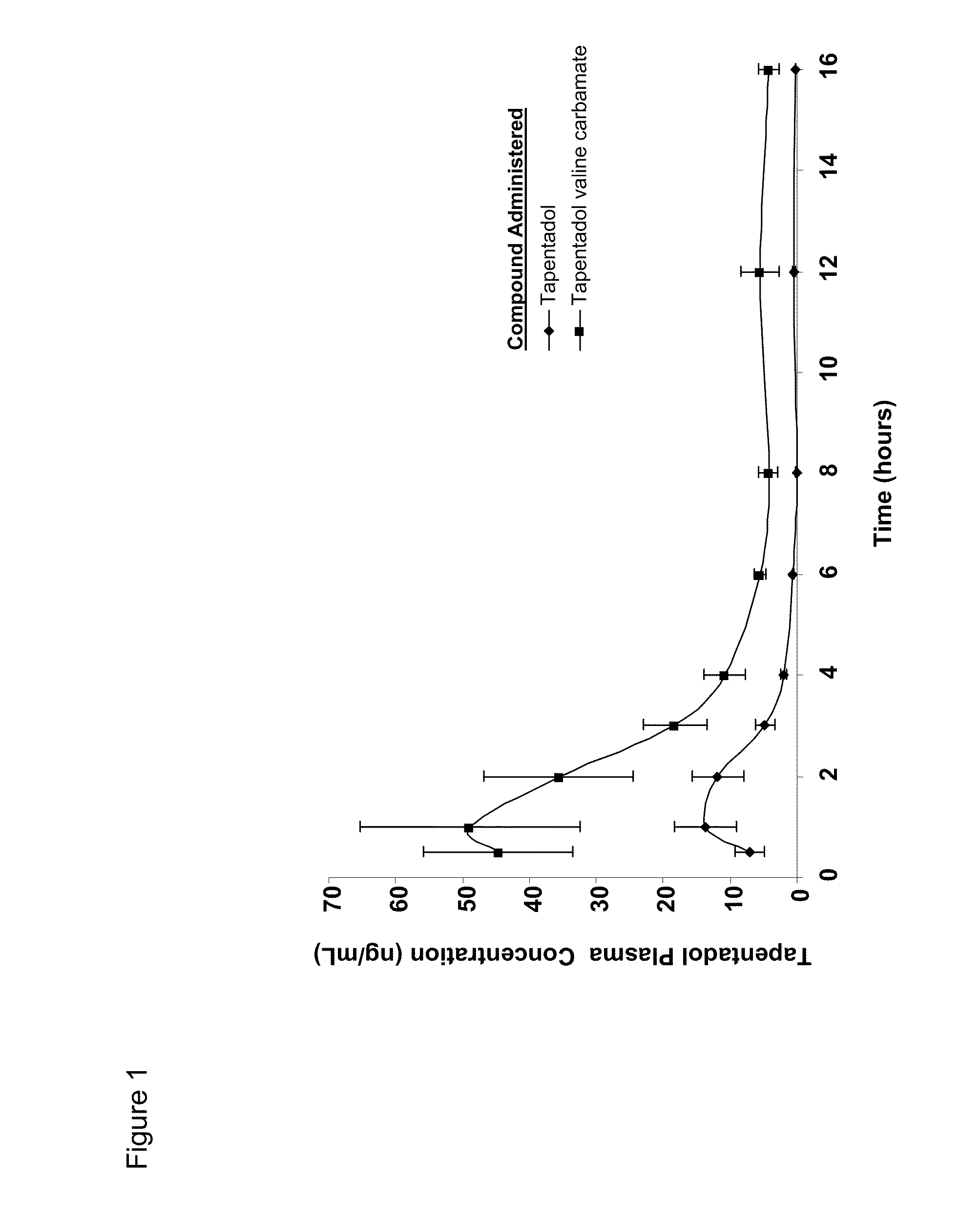
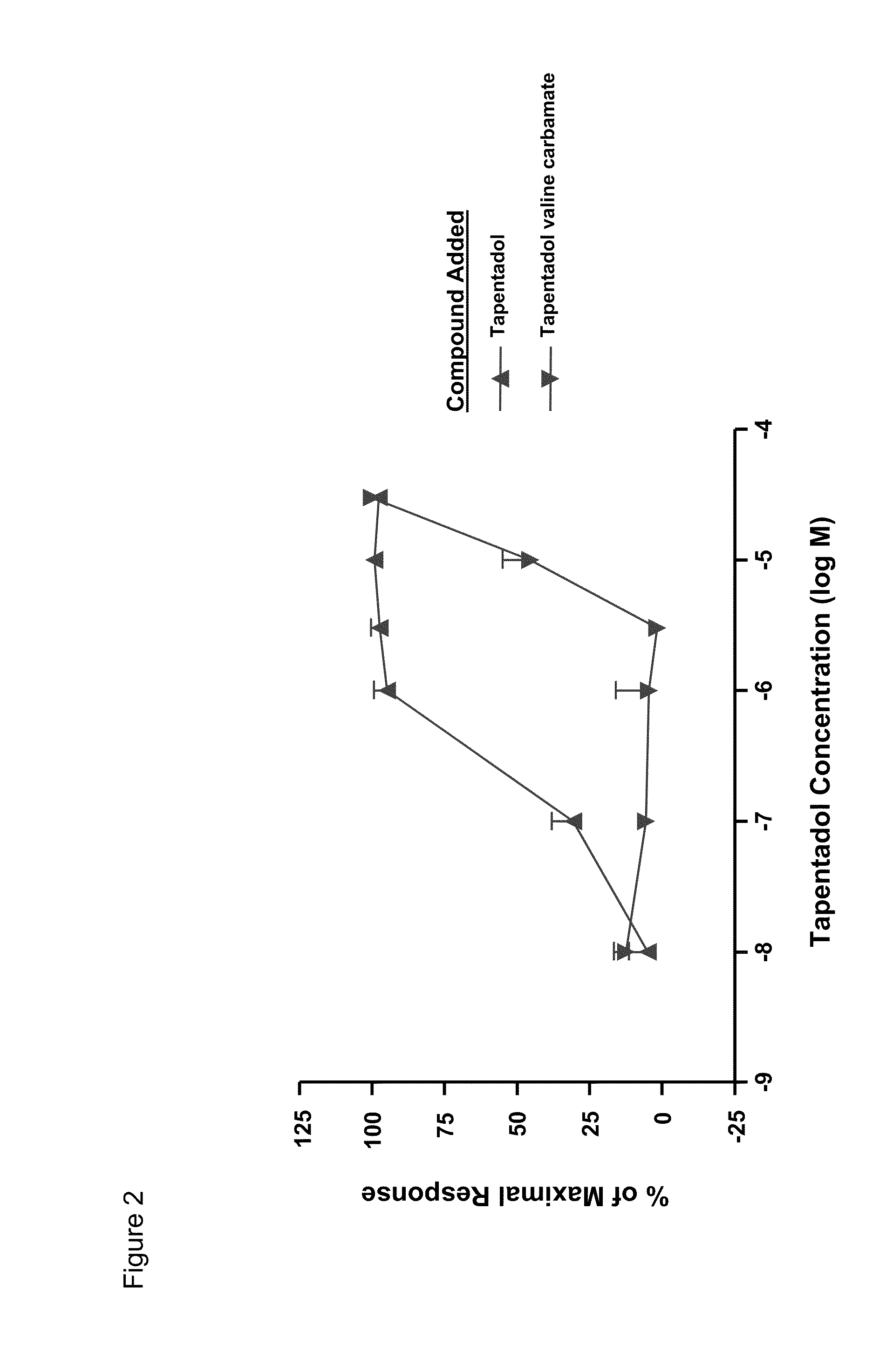
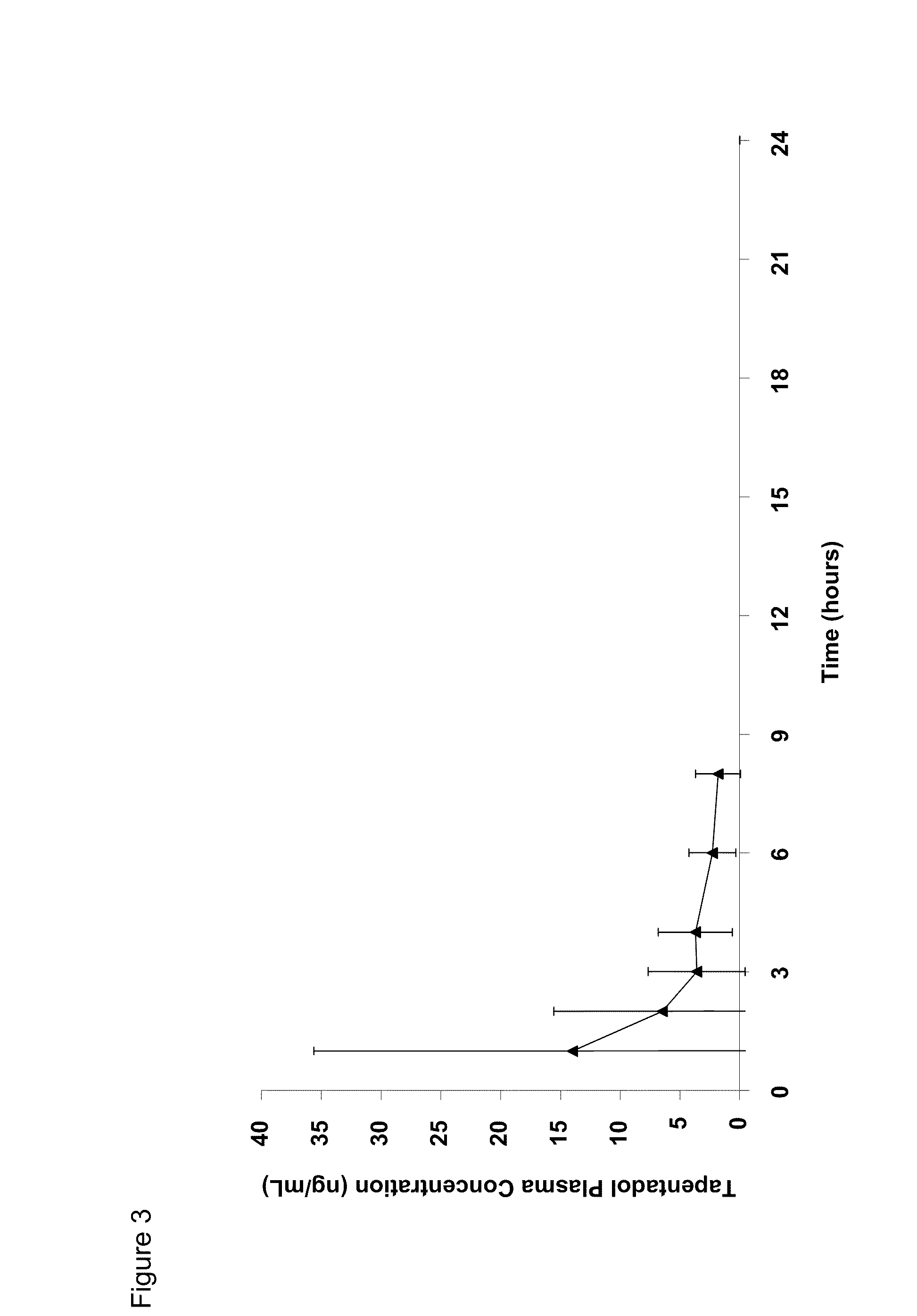
Amino acid and peptide carbamate prodrugs of tapentadol and uses thereof
InactiveUS20100227921A1Sufficient amountMinimizing the gastrointestinal (GI) side effectsBiocideNervous disorderCarbamateSide effect
Prodrugs of tapentadol with amino acids or short peptides, pharmaceutical compositions containing such prodrugs and a method for providing pain relief with the tapentadol prodrugs are provided herein. Prodrugs having side chains of valine, leucine, isoleucine and glycine amino acids and mono-, di- and tripeptides thereof are preferred. Additionally, methods for avoiding or minimizing the adverse gastrointestinal side effects associated with tapentadol administration, as well as increasing the oral bioavailability of tapentadol are provided herein.
Owner:SHIRE PLC
Prevention of incorporation of non-standard amino acids into protein
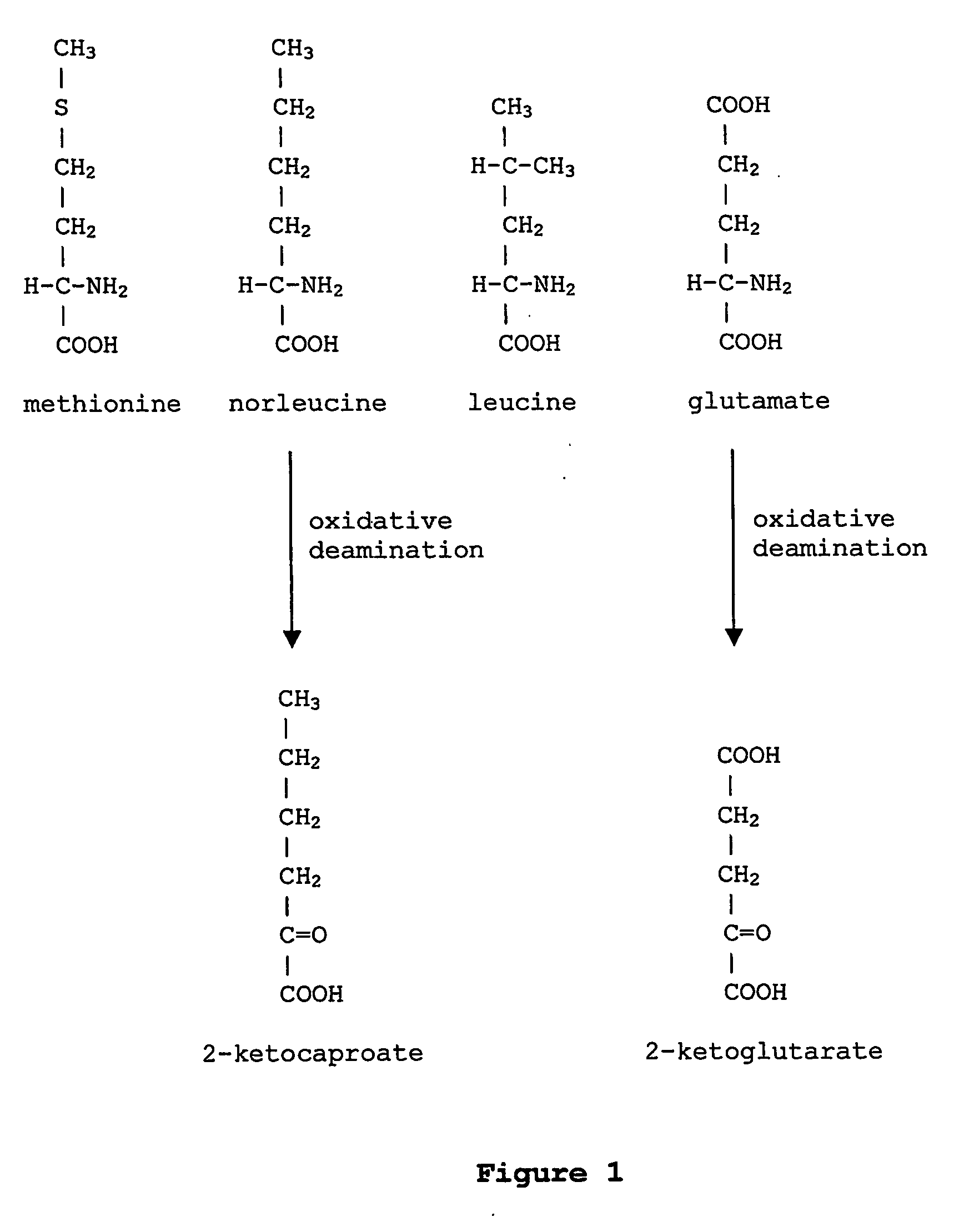
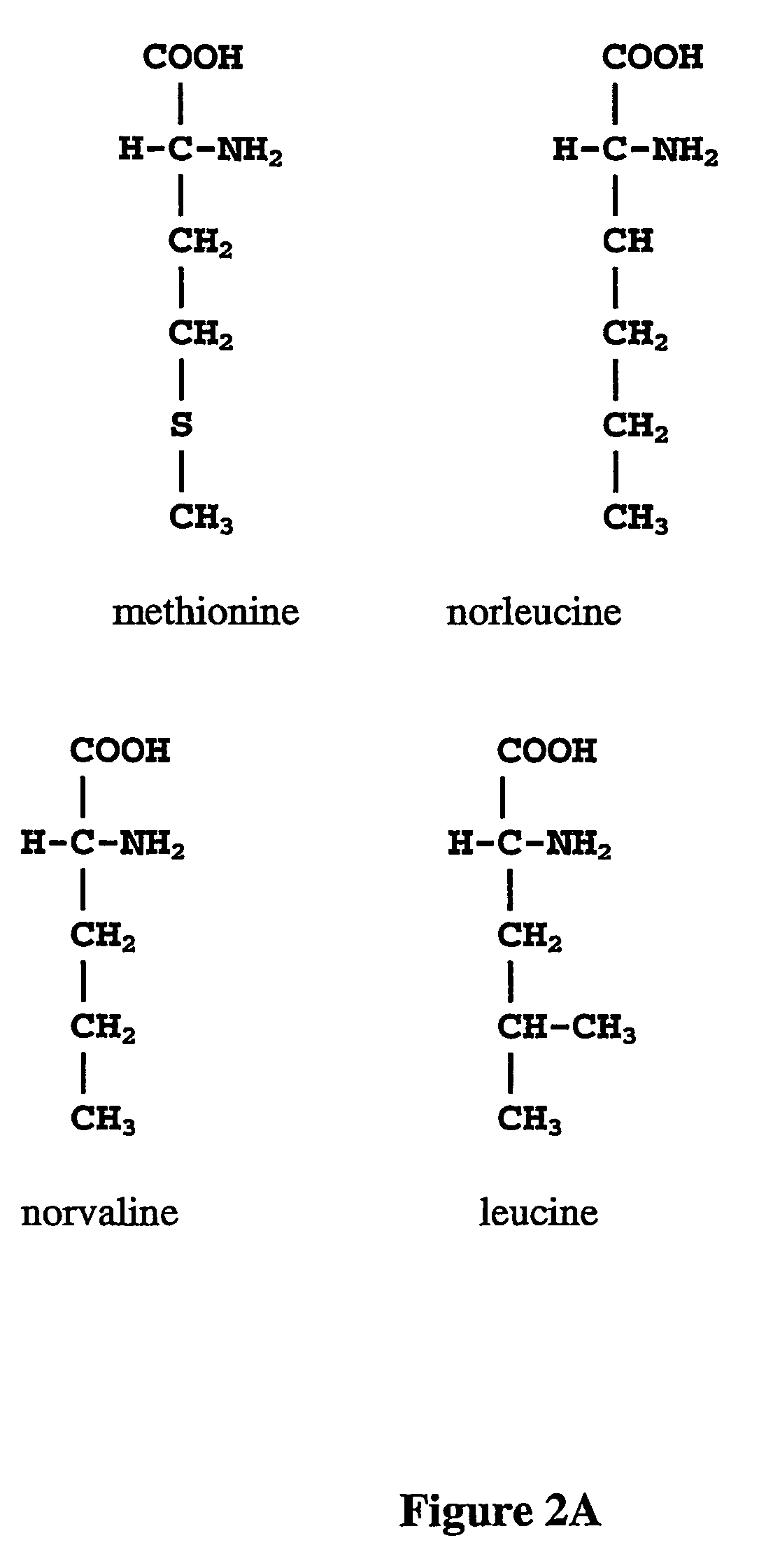
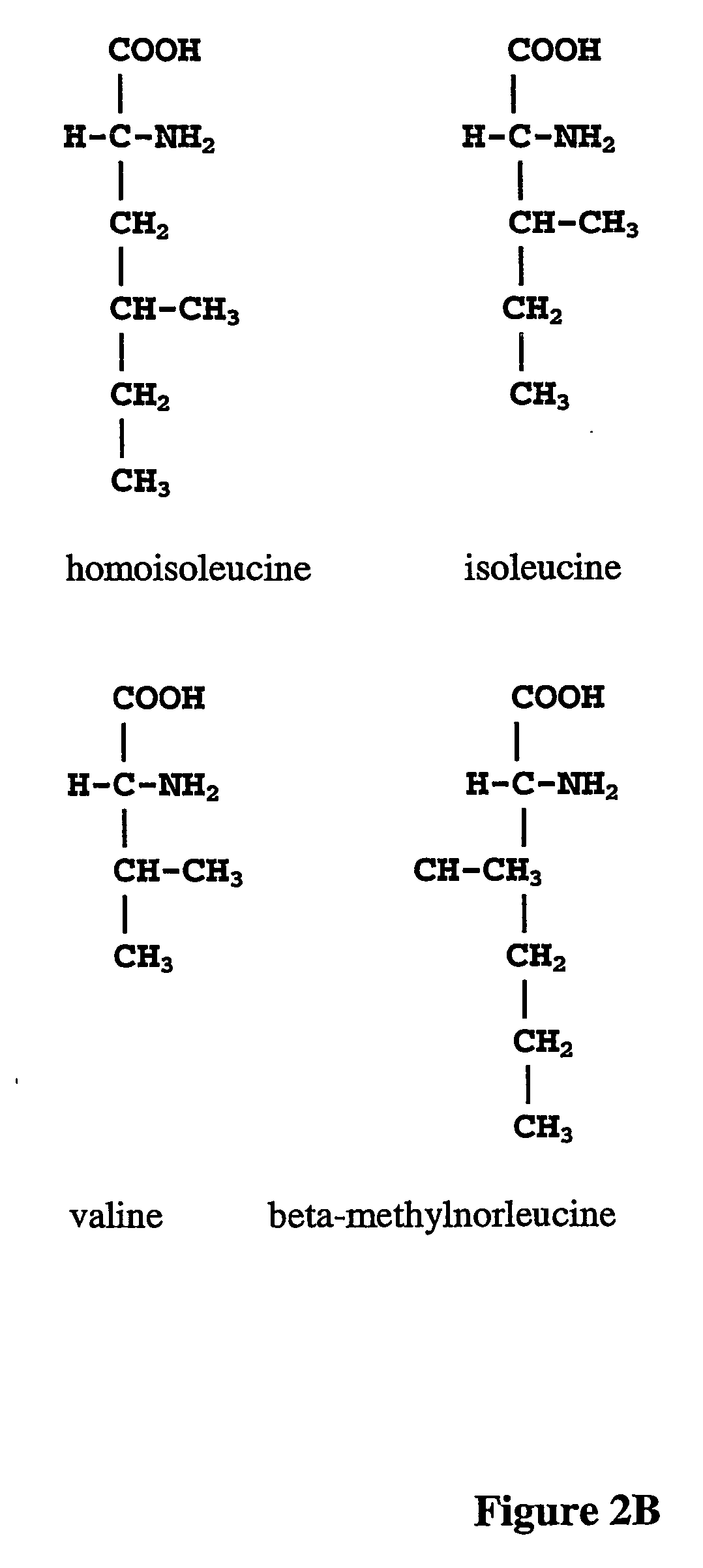
ActiveUS20070009995A1Cellular level is reducedReducing, or substantially eliminating, endogenous cellular levels of norleucineBacteriaPeptide/protein ingredientsBeta-methylnorleucinePhenylalanine dehydrogenase
The instant invention is drawn to the methods and compositions necessary to provide recombinant proteins with a substantially reduced or eliminated content of norleucine or other non-standard amino acids. Various embodiments of the invention provide for the substantial elimination of the incorporation of non-standard amino acids into recombinant proteins by the co-expression or enhanced expression of a protein (or the enzymatically active portion thereof) capable of degrading norleucine or other non-standard amino acids, including norvaline, beta-methylnorleucine, and homoisoleucine. In certain particular embodiments of the invention, the norleucine is degraded by a glutamate dehydrogenase, a leucine dehydrogenase, a valine dehydrogenase, a phenylalanine dehydrogenase, a glutamate / leucine / phenylalanine / valine dehydrogenase, or an opine dehydrogenase. Also provided are the cells and DNA constructs for carrying out these methods.
Owner:MONSANTO TECH LLC
Dietary Supplement Cognitive Support System
The present invention relates to a nutritional supplement composition, comprising a therapeutically effective amounts of Vitamin C, Vitamin D3, Thiamin, Riboflavin, Niacin, Vitamin B6, Folic acid, Vitamin B12, Pantothenic acid, Calcium, Magnesium, Zinc, Chromium, Sugar, Protein, Acetyl-L-Carnitine, Dimethylaminoethanol complex, Phosphatidylserine complex, L-Glutamine, N-Acetyl-L-Tyrosine, L-Phenylalanine, Taurine, Methionine, Valine, Isoleucine, 5 Hydroxytryptophan, L-Taurine, N-Acetyl-Tyrosine, N-Acetyl-L-Cysteine, Alpha Lipoic Acid, Alpha Glycerylphosphoricholine complex, Bacopa Monnieri extract, Gingko Biloba extract, Passion flower, Lemon Balm, Gotu Kola, Ashwagandha, Choline Bitartrate complex, Panax Ginseng extract, Turmeric, Organic freeze dried fruit juice blends (concord grape, red raspberry, pineapple, cranberry, acai, pomegranate, acerola cherry, bilberry, lingonberry, black currant, aronia, sour cherry, black raspberry), Organic freeze dried greens blends (barley grass, broccoli, beet, carrot, alfalfa, oat), and Protein digestive enzyme blends (Protease 4.5, peptidase, bromelain, protease 6.0, protease 3.0, L planatrum, B bifidum) in a mixture to provide optimal cognitive function.
Owner:FANTZ DAVID R
Mutated Immunoglobulin-Binding Polypeptides
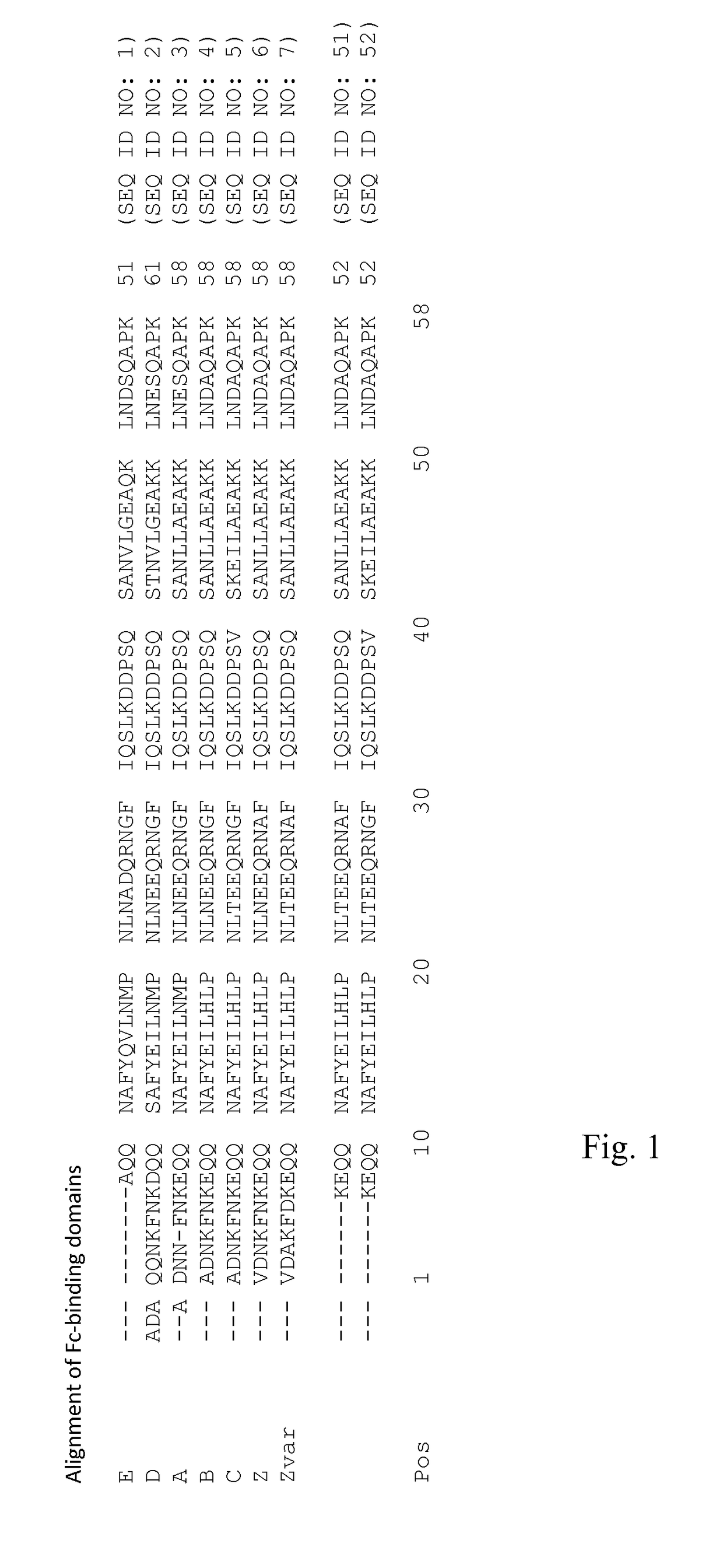
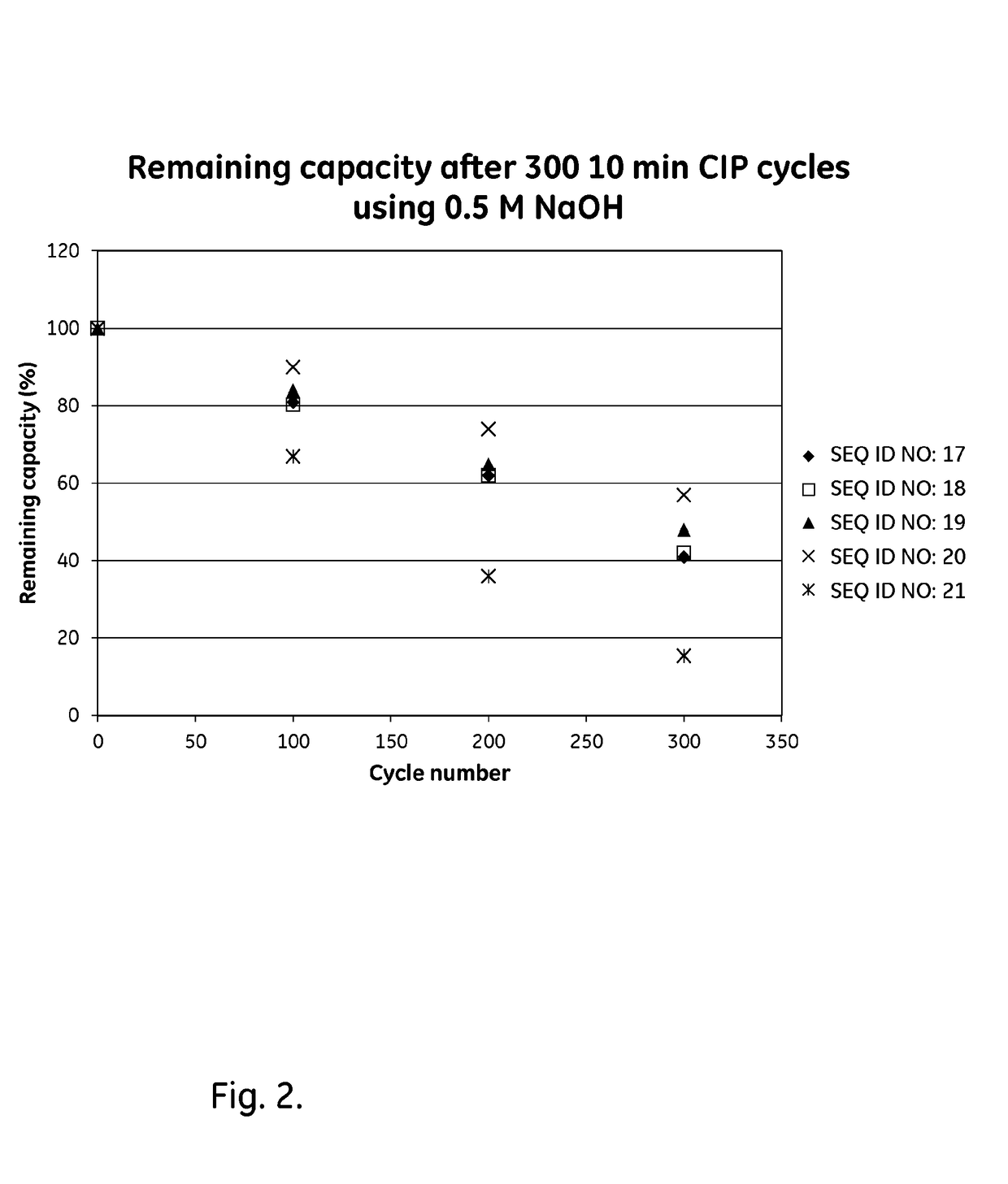
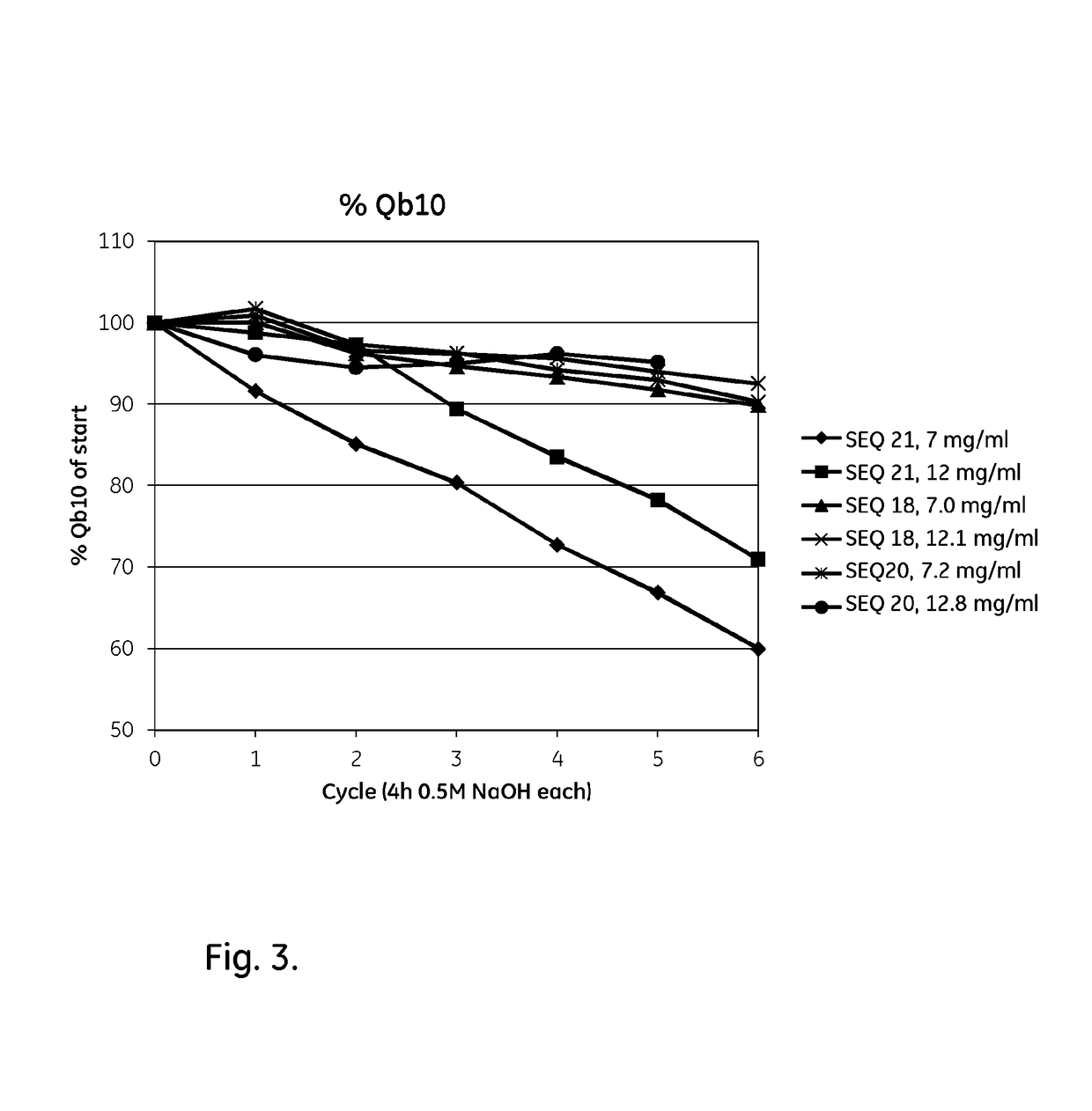
ActiveUS20170334954A1Improved alkaline stabilityImprove stabilityHybrid immunoglobulinsSolid sorbent liquid separationArginineFc binding
An Fc-binding polypeptide of improved alkali stability, comprising a mutant of an Fc-binding domain of Staphylococcus Protein A (SpA), as defined by SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO:3, SEQ ID NO: 4, SEQ ID NO: 5, SEQ ID NO:6, SEQ ID NO:7, SEQ ID NO:22, SEQ ID NO 51 or SEQ ID NO 52 wherein at least the asparagine or serine residue at the position corresponding to position 11 in SEQ ID NO:4-7 has been mutated to an amino acid selected from the group consisting of glutamic acid, lysine, tyrosine, threonine, phenylalanine, leucine, isoleucine, tryptophan, methionine, valine, alanine, histidine and arginine.
Owner:CYTIVA BIOPROCESS R&D AB
Hair and skin protecting compositions based on esters or ethers of betulin
Personal care compositions and formulations, which include conventional concentrations of known hair and skin altering components and a cosmetically acceptable amount of oil-soluble betulin and allo-betulin esters or ethers derived from betulin or allo-betulin in effective amounts are disclosed.
Owner:GLINSKI JAN
Peptide, a method for its preparation and a pharmaceutical composition containing the peptide
A peptide of the formula Iwherein X is hydrogen, glycine, alanine, leucine, isoleucine, valine, N-valine, proline, tyrosine, phenylalanine, tryptophan, D-alanine, D-leucine, D-isoleucine, D-valine, D-N-valine, D-proline, D-tyrosine, D-phenylalanine, D-tryptophan, gamma-aminobutyric acid or ζ-aminocaproic acid; A is D-gluptamic acid or D-y-glutamic acid; and Y is glycine, alanine, leucine, isoleucine, valine, N-valine, proline, tyrosine, phenylalanine, tryptophan, D-alanine, D-leucine, D-isoleucine, D-valine, D-N-valine, D-proline, D-tyrosine, D-phenylalanine, D-tryptophn, gamma-aminobutyric acid, ζ-aminocaproic acid, hydroxyl, or an amide group.
Owner:IMMUNOTECH DEV
Sport drink containing amino acids and carbohydrates
InactiveUS20070270355A1Quick changeSpeed recovery timeBiocidePeptide/protein ingredientsArginineTryptophan
A composition includes a plurality of amino acids. The plurality of amino acids includes at least one essential amino acid and at least one non-essential amino acid. The plurality of amino acids also includes at least one branch-chain amino acid. The composition also includes a source of carbohydrates. The compositions also includes purified water. The plurality of amino acids comprises about 1 wt % of the composition. A composition includes a plurality of amino acids, sodium citrate, sodium chloride, potassium phosphate, flavoring, a source of carbohydrates, and purified water. In some embodiments of the composition, the plurality of amino acids includes alanine, arginine, aspartate, cystine, glutamine, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonin, tryptophan, tyrosine, and valine.
Owner:GARCIA RAMON D +3
Methods for affecting homeostasis and metabolism in a mammalian body
InactiveUS20050233014A1Effective quantityLow production costOrganic active ingredientsBiocideCysteine thiolateTryptophan
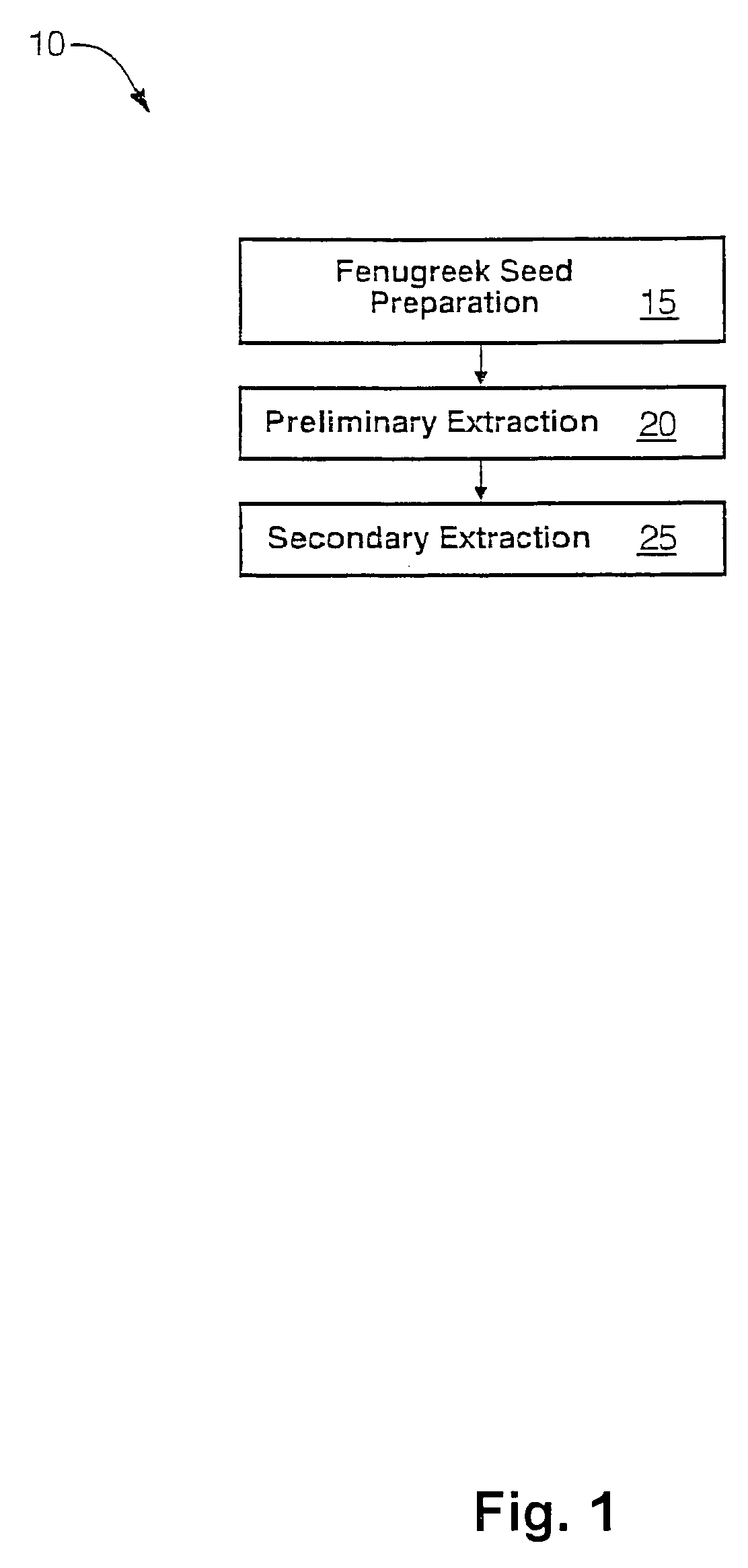
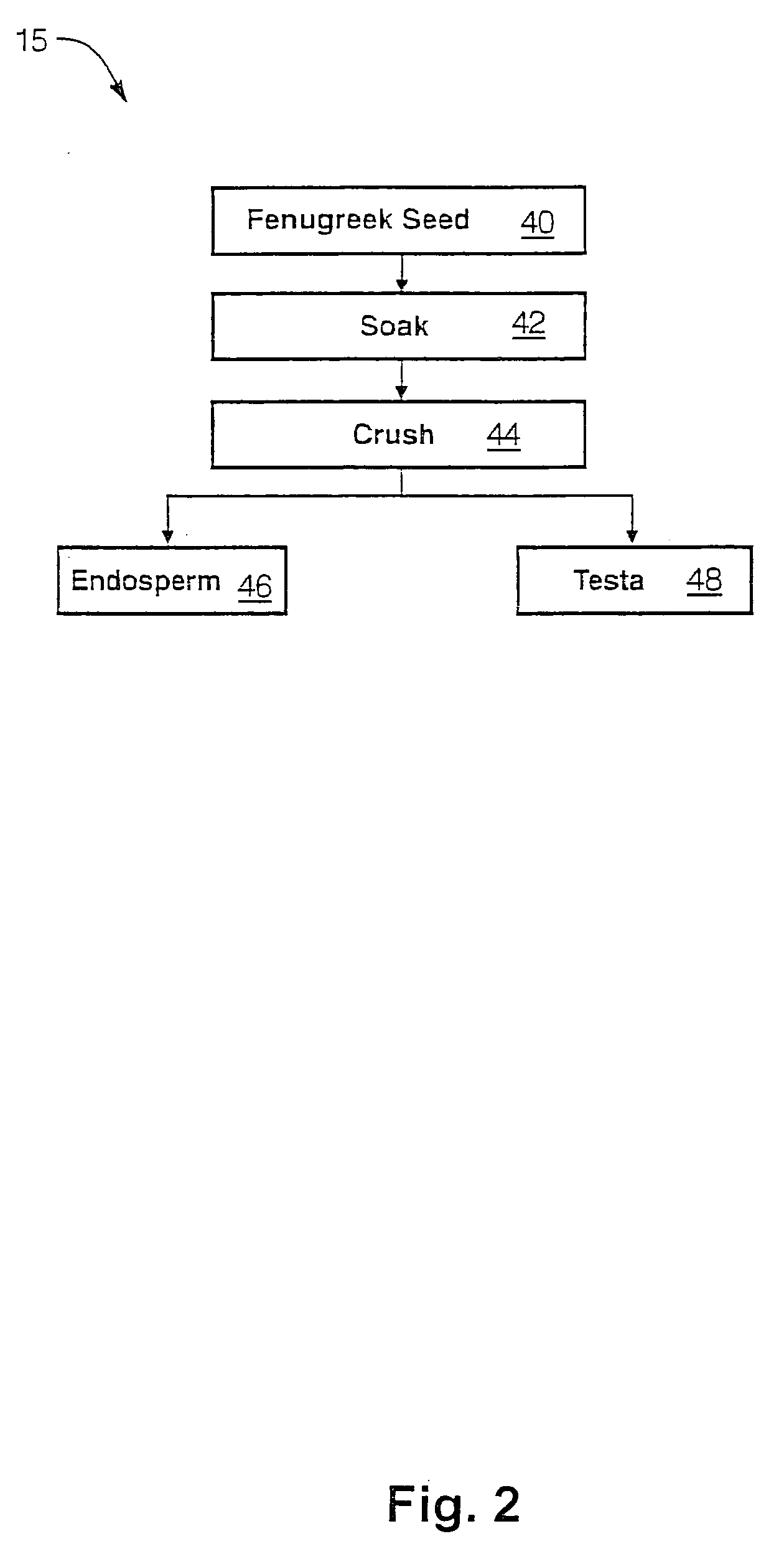
The present invention is directed to novel compositions of bio-active compounds comprising 4-hydroxyisoleucine and one or more compounds selected from the group of amino acids, alkaloids, glycosides, volatile oils, saponins, sapogenins, mannans, flavonoids, fatty acids, vitamins and provitamins, minerals, and carbohydrates. Preferably, the novel compositions of bio-active compounds include 4-hydroxyisoleucine and one or more amino acids selected from the group consisting of arginine, aspartate, threonine, serine, glutamate, proline, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tryptophan, phenylalanine, ornithine, proline, lysine, histidine, and gamma-aminobutyrate. The composition of bio-active compounds preferably include between about ten percent and about seventy percent of 4-hydroxyisoleucine and between about twenty percent and about forty percent of other amino acids. The bio-active compounds of the novel composition of the present invention may be derived, isolated, and / or extracted from Fenugreek seeds. A preferred method for extracting the bio-active compounds from Fenugreek seeds includes the steps of: (1) providing a plurality of Fenugreek seeds; (2) preparing the Fenugreek seeds; and (3) extracting a novel composition of bio-active compounds from the Fenugreek seeds, which include a preliminary extraction step and a secondary extraction step. The compositions of bio-active compounds have been found to be helpful in restoring healthy energy balance in humans and animals, aiding in weight management efforts, and for balancing blood sugar levels by way of assisting the body to make more efficient use of existing (i.e., endogenous) insulin.
Owner:TSI INC
Pharmaceutical composition containing 18 kinds of amino acid
ActiveCN101439036AInhibition of oxidative decomposition reactionsQuality assuranceOrganic active ingredientsMetabolism disorderAntioxidantTryptophan
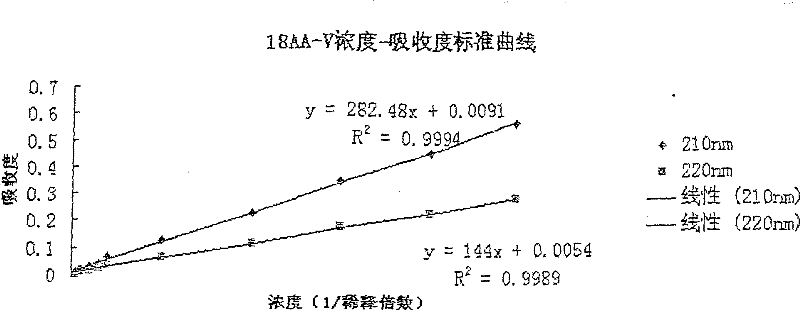
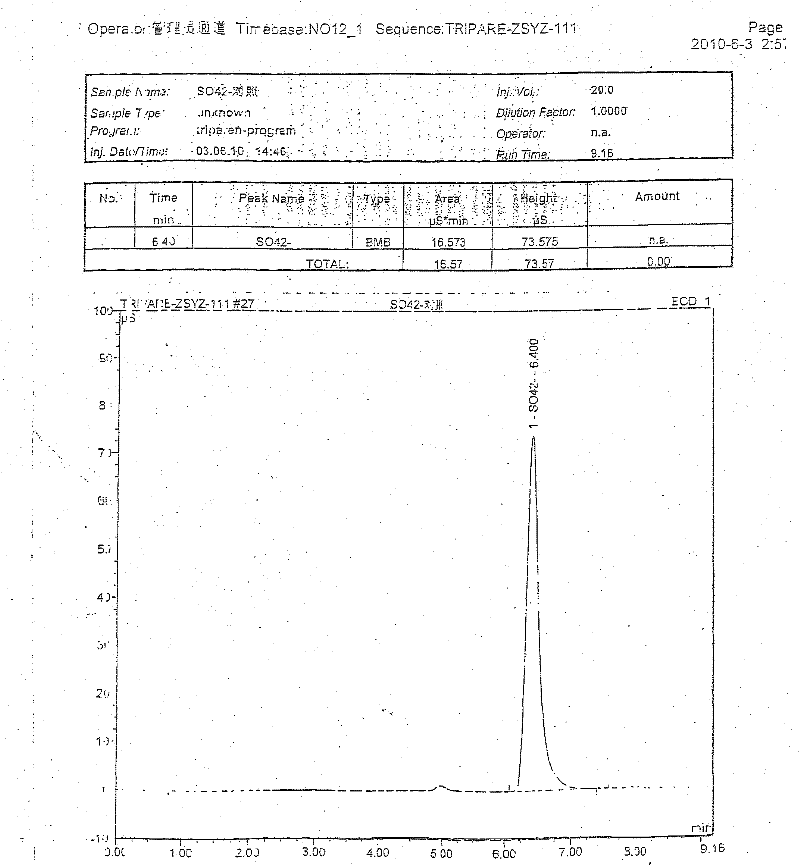
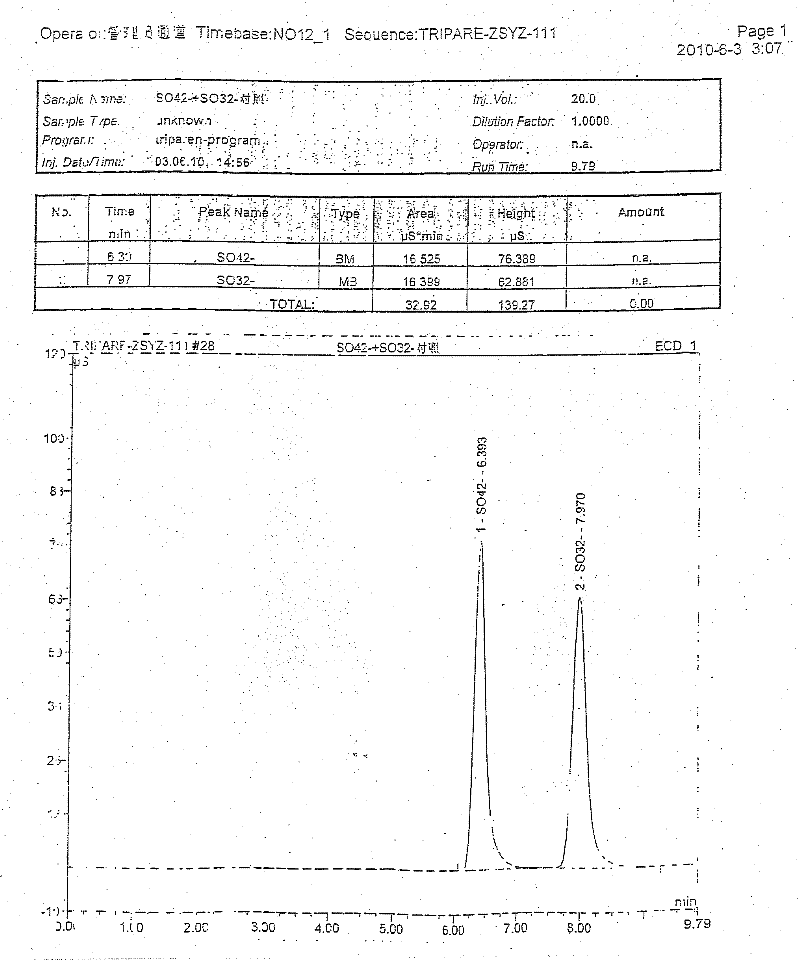
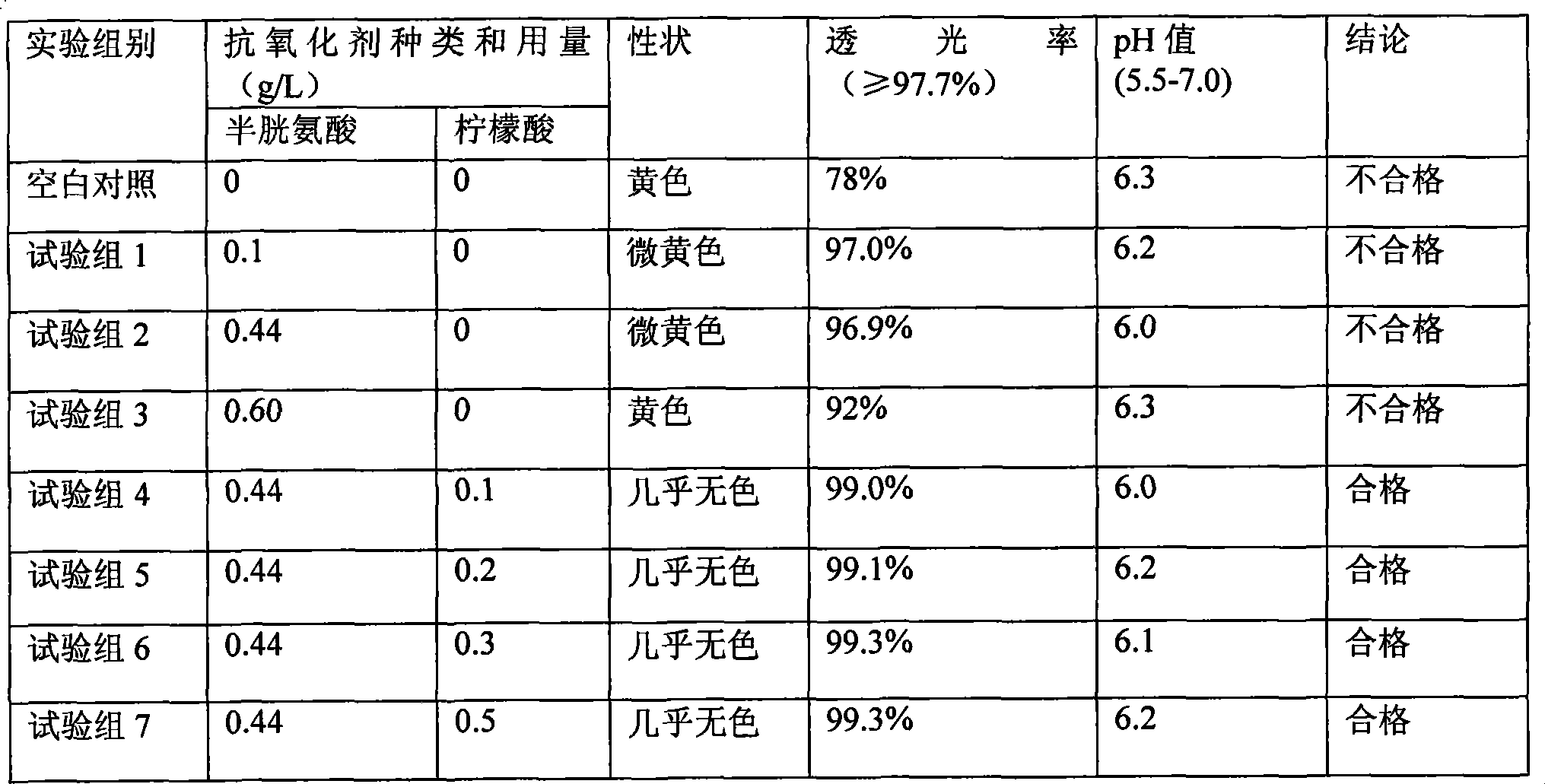
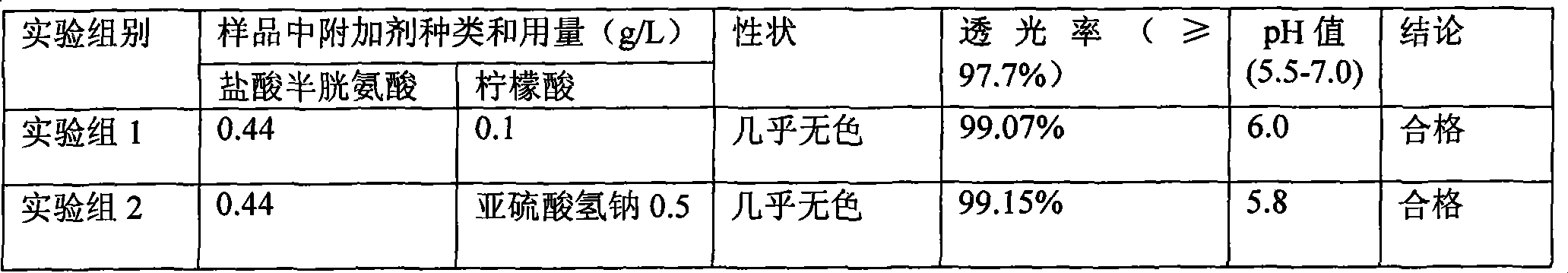
The invention discloses a pharmaceutical composition containing 18 amino acids. The pharmaceutical composition is characterized in that a compound amino acid injection (18AA-V) with varied concentration is prepared by using the following components according to the following ratios of parts by weight: 2.89 of arginine hydrochloride, 2.46 of histidine hydrochloride, 3.79 of leucine, 1.70 of isoleucine, 3.33 of lysine hydrochloride, 2.83 of phenylalanine, 1.97 of threonine, 1.36 of valine, 1.06 of methionine, 0.39 of tryptophan, 3.24 of glycine, 1.88 of alanine, 1.00 of proline, 0.11 of tyrosine, 0.67 of serine, 0.44 of cysteine hydrochloride, 1.15 of aspartic acid, 1.97 of glutamic acid, 50 of xylitol, 0.10 to 0.30 of citric acid and injection water with proper amount. The pharmaceutical composition does not contain a sulfite antioxidant so that the pharmaceutical composition is clinically used in a safer manner. After an accelerated test and a quality test, results show that the pharmaceutical composition is as stable as or more stable than like products (18AA-V) which are sold in the markets and contain sulfites.
Owner:福州凯瑞医药咨询有限公司
Cyclic dipeptides as feed additives
Feed additives containing essential amino acids which are diketopiperazines of formulas (IV) or (V) or salts thereof are provided:In formulas (IV) and (V), R1 and R2 may be an amino acid residue such as methionine, lysine, threonine, tryptophan, histidine, valine, leucine, isoleucine, phenylalanine, arginine, and cysteine, and may optionally be the same residue.Additionally provided are the diketopiperazines of formulas (IV) and (V) and a method to for their production.
Owner:EVONIK DEGUSSA GMBH
Skin external preparation
InactiveUS20080058400A1Good moisturizing effectMaintain abilityCosmetic preparationsBiocideHydroxyprolineTyrosine
A skin external preparation includes at least arginine, aspartic acid, isoleucine, leucine, lysine, threonine, glycine, histidine, serine, valine, tyrosine, cysteine, phenylalanine, hydroxyproline and acylglutamine among amino acids, or salts thereof, and a skin external preparation includes: at least arginine, aspartic acid, isoleucine, leucine, lysine and threonine among amino acids, or salts thereof, and a hydrolyzed silk.
Owner:FUJIFILM CORP
Food ingredient having milk taste-enhancing action, production method thereof, method of enhancing milk taste of food or seasoning and milk taste-enhanced milk-based hard candy
InactiveUS20110081473A1Great tasteLimited supplyMilk preparationConfectioneryAdditive ingredientArginine
An object of the invention is to provide a food ingredient having strong milk taste-enhancing action without providing coarse taste, a production method thereof, a method of improving milk taste, a milk-based hard candy, in particular a milk-based nonsugar hard candy, having a delicious milk taste that is produced with a food ingredient that can replace part or most of the dairy product at low cost.The present invention relates to a food ingredient having milk taste-enhancing action, prepared by heating an aqueous solution containing an amino acid mixture containing at least three kinds of amino acids selected from valine, proline, isoleucine, lysine, glutamic acid and arginine as the principal components and a carbohydrate. The total amount of the at least three kinds of amino acids selected from valine, proline, isoleucine, lysine, glutamic acid, and arginine is preferably 70 wt % or more with respect to the total amount of all amino acids.
Owner:UHA MIKAKUTO CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com