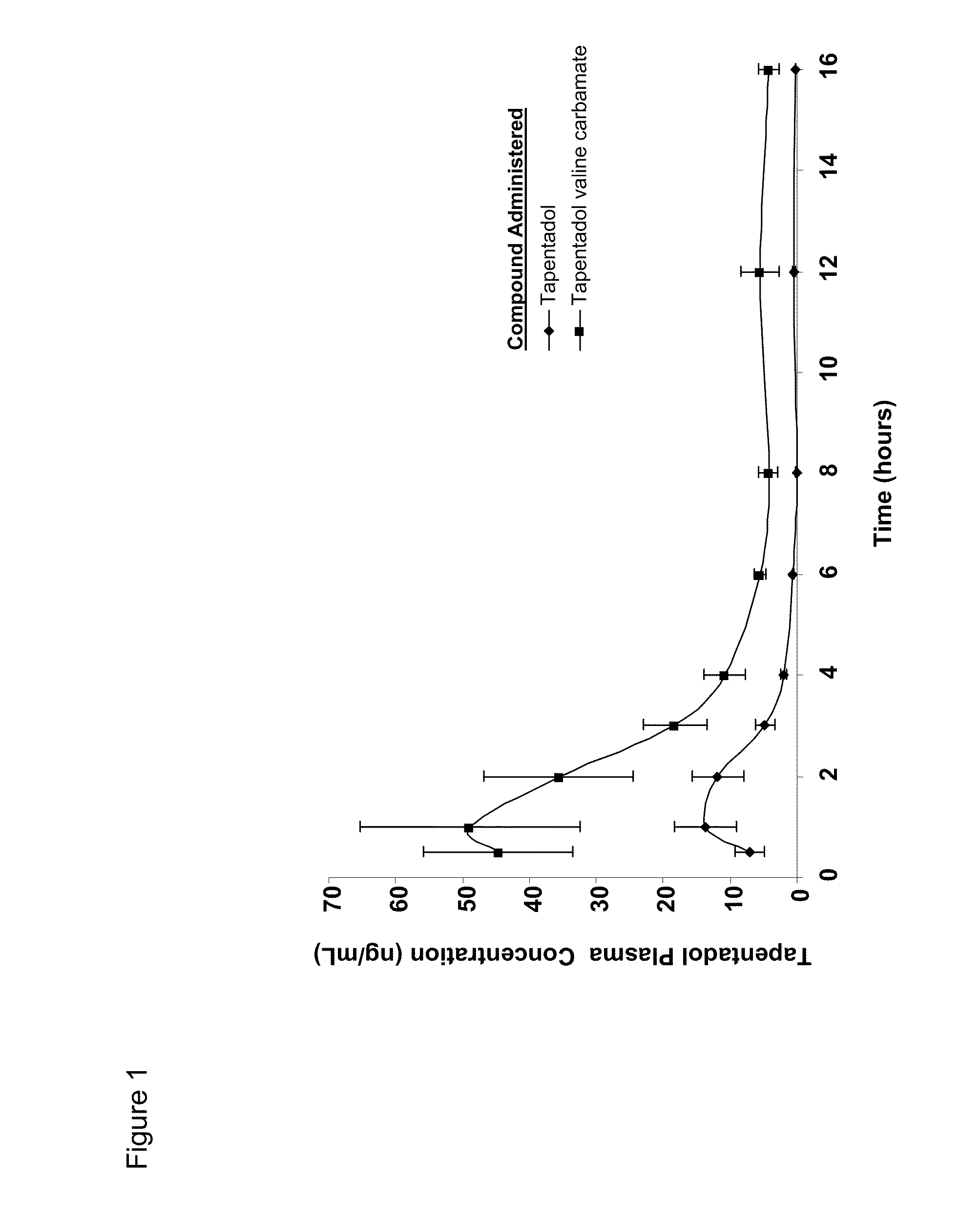
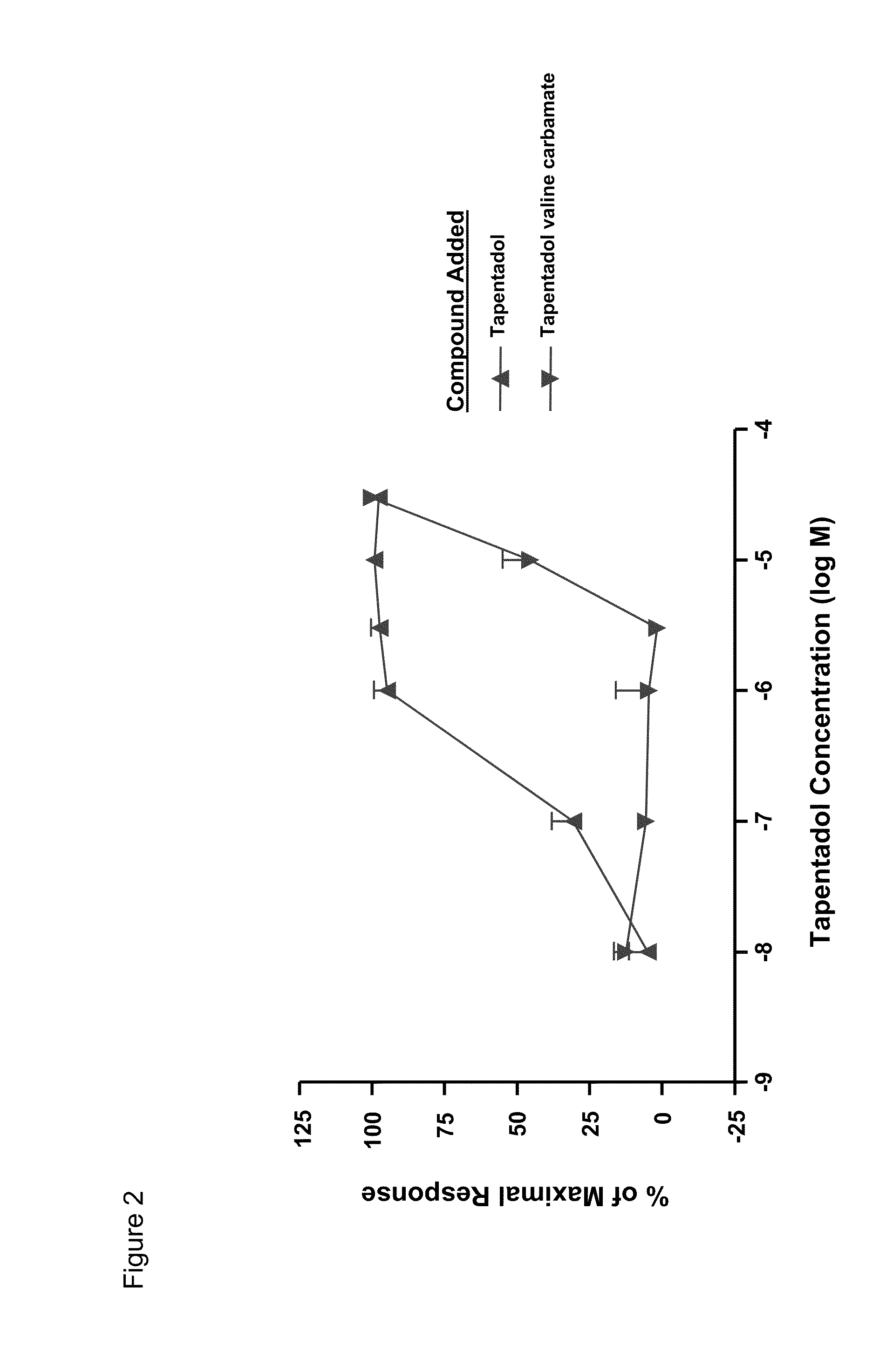
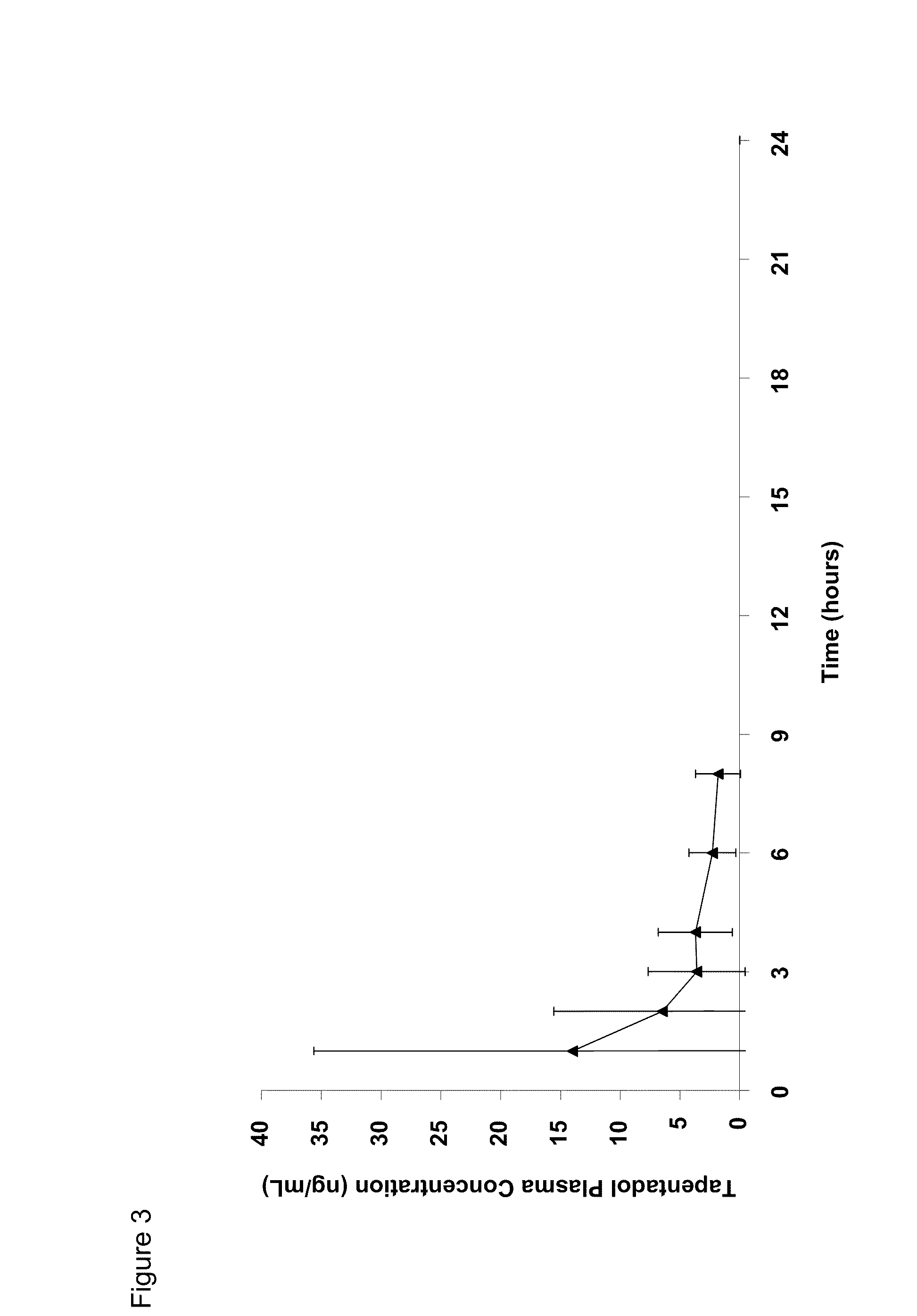
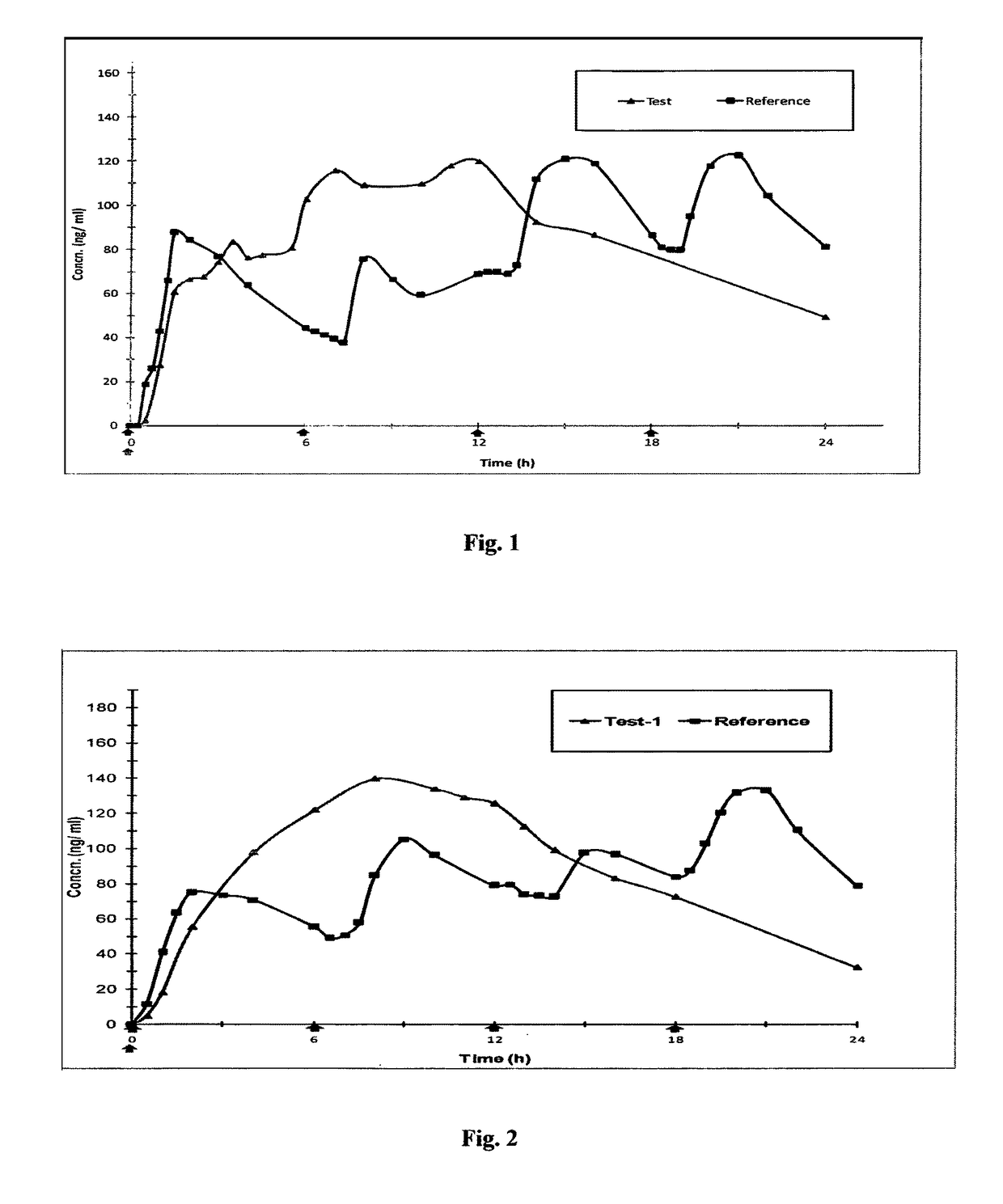
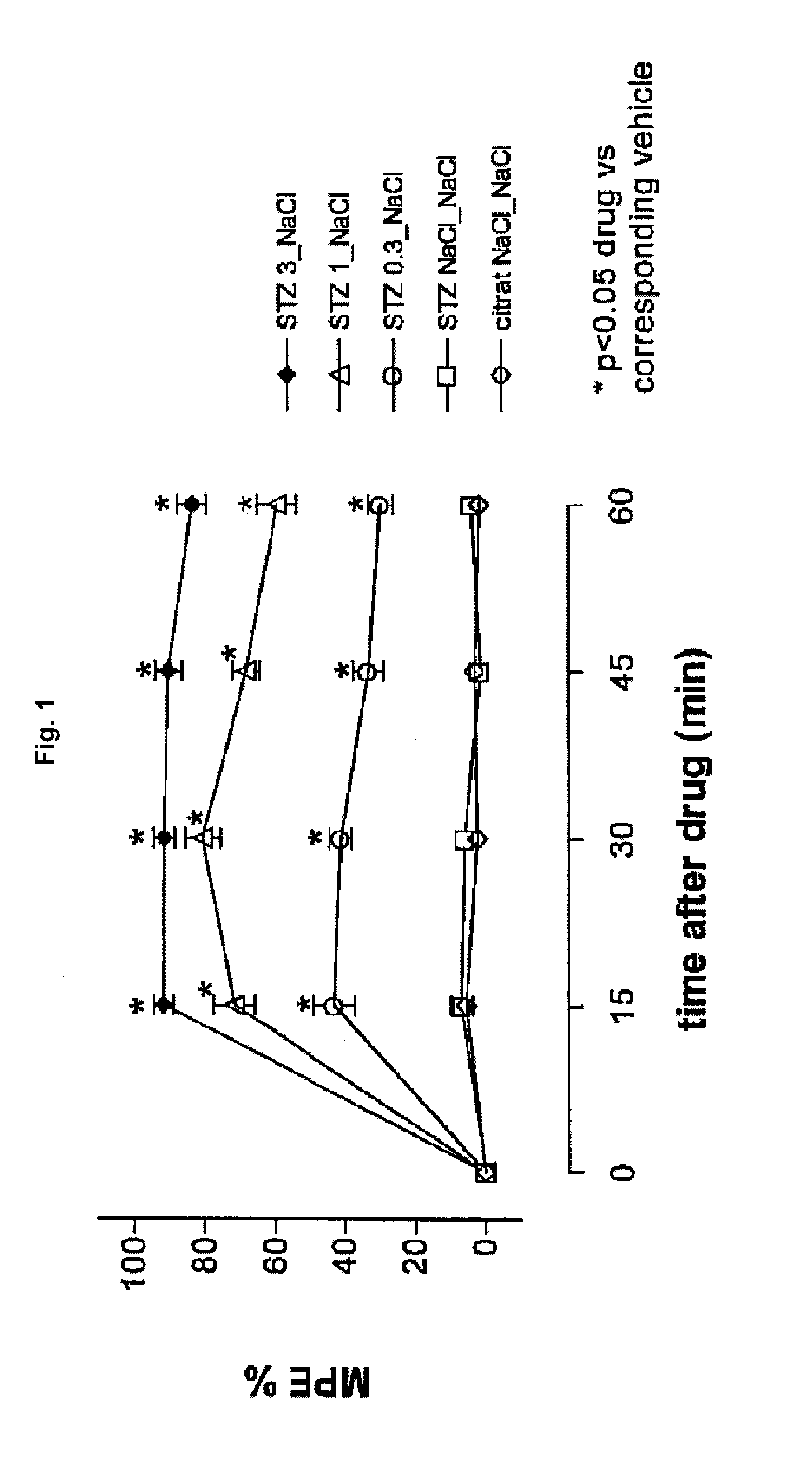
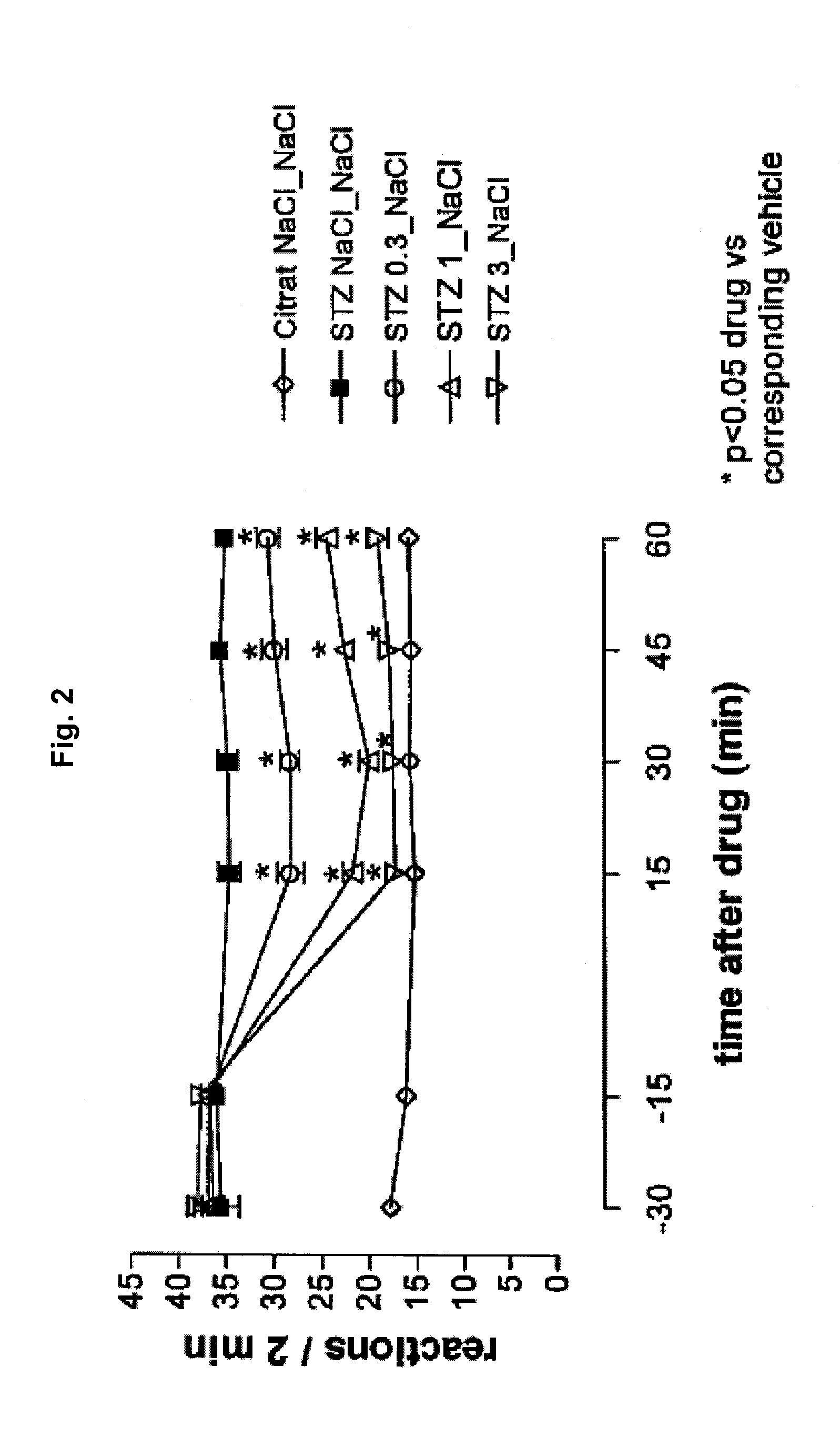
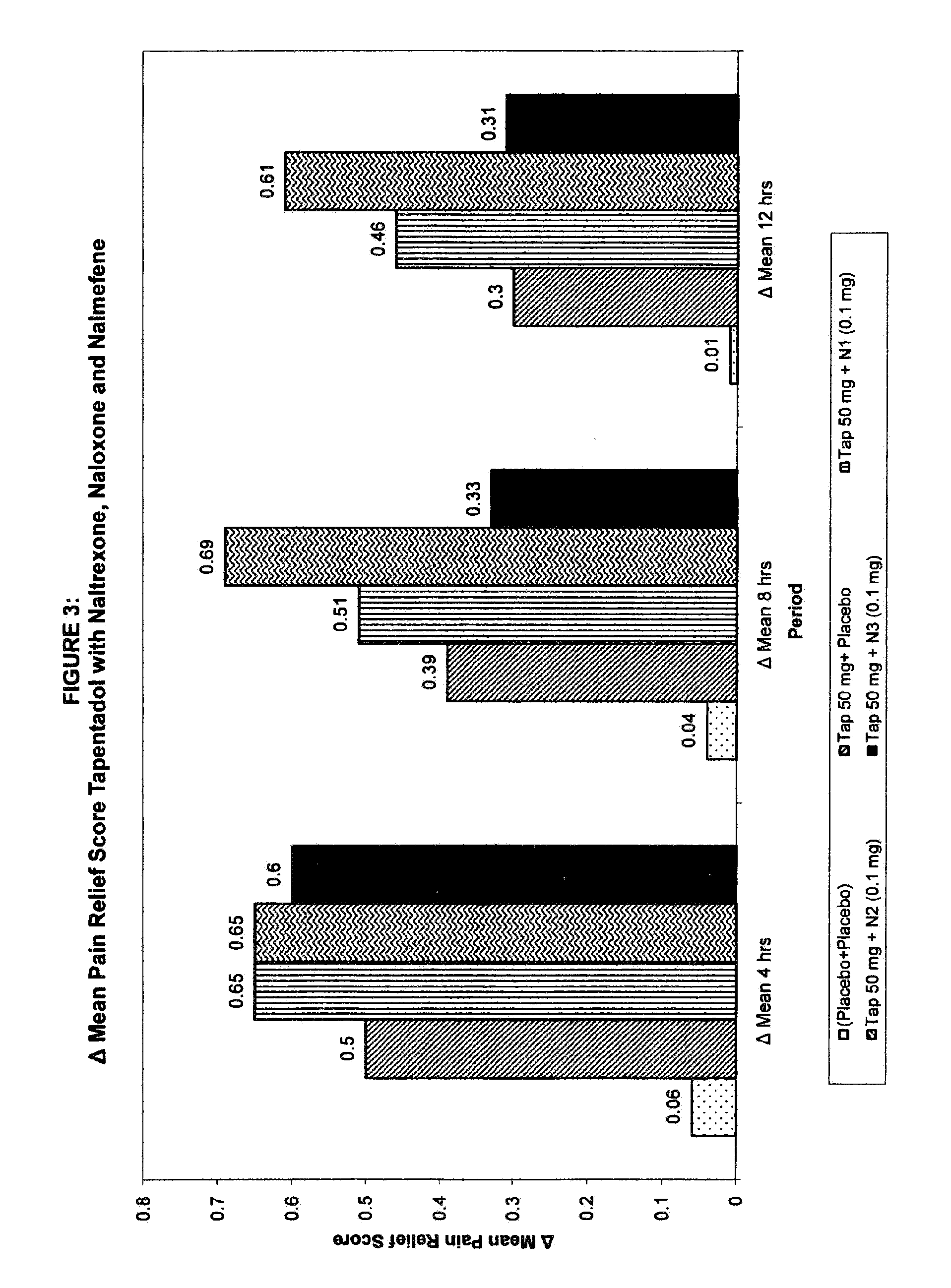
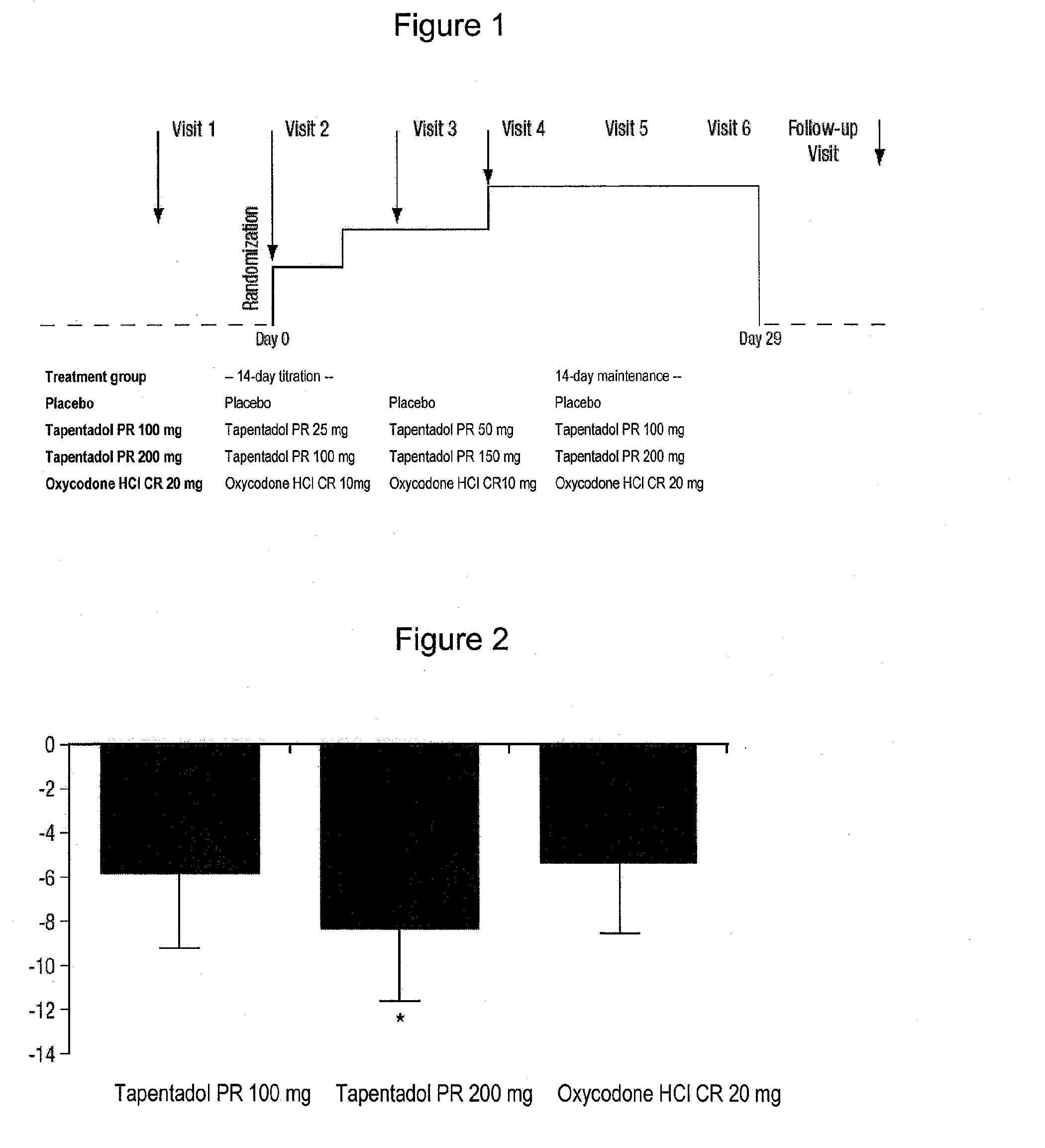
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "Tapentadol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
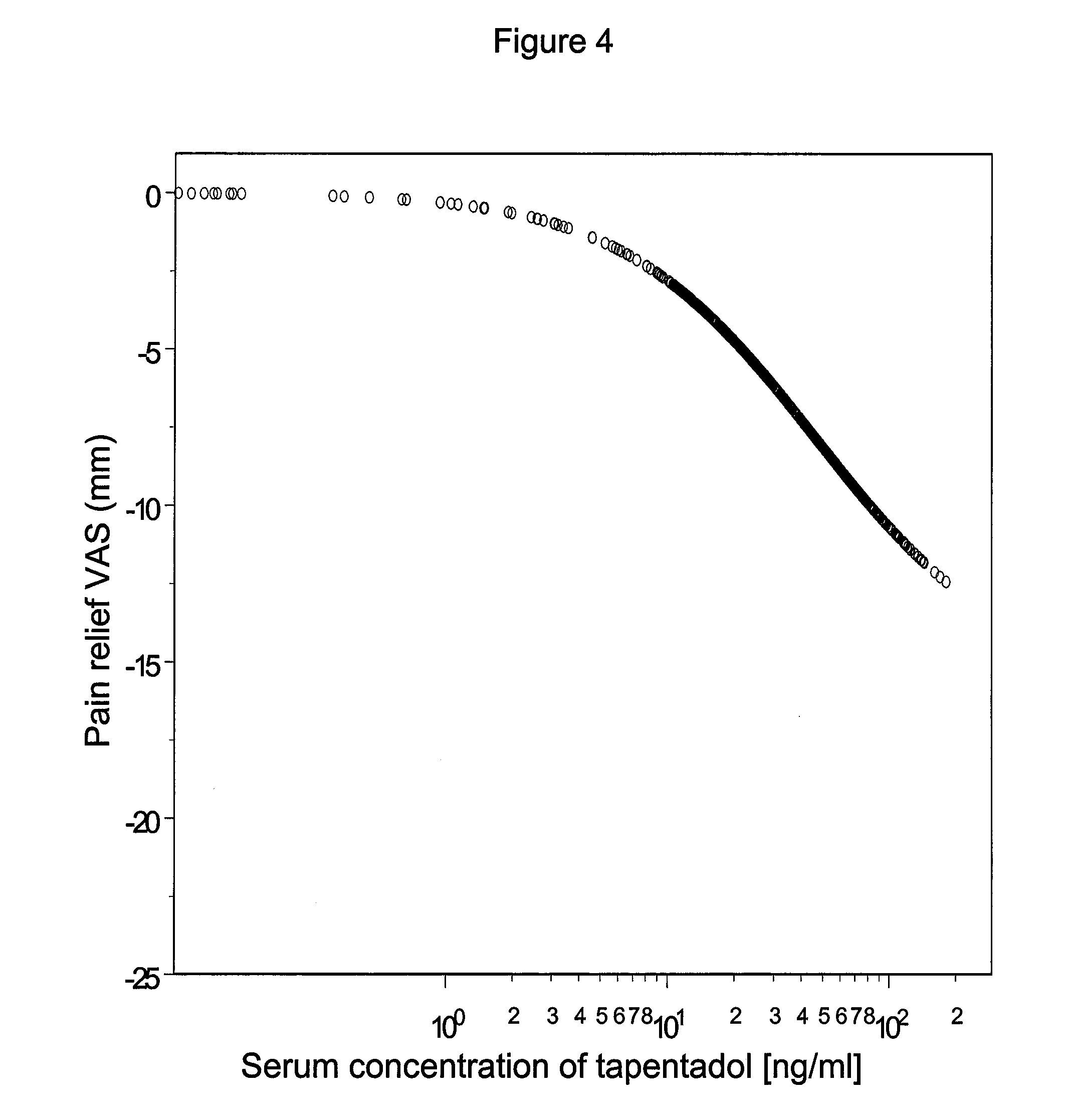
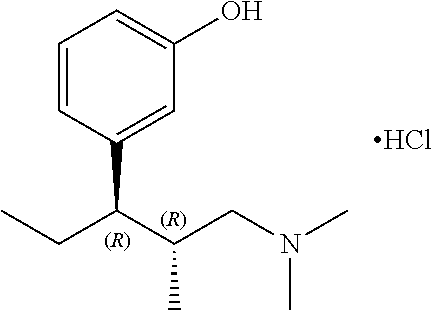
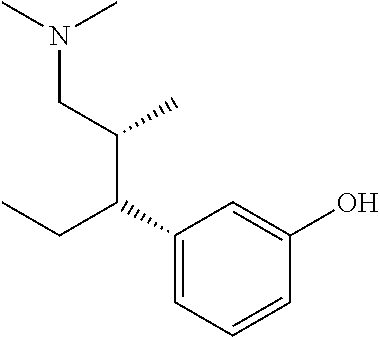
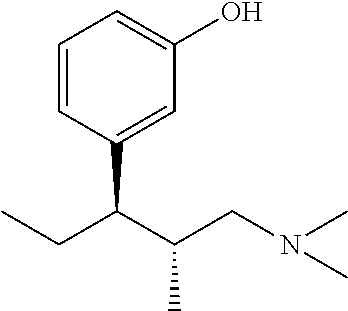
Tapentadol, brand names Nucynta among others, is a centrally acting opioid analgesic of the benzenoid class with a dual mode of action as an agonist of the μ-opioid receptor and as a norepinephrine reuptake inhibitor (NRI). Analgesia occurs within 32 minutes of oral administration, and lasts for 4–6 hours.
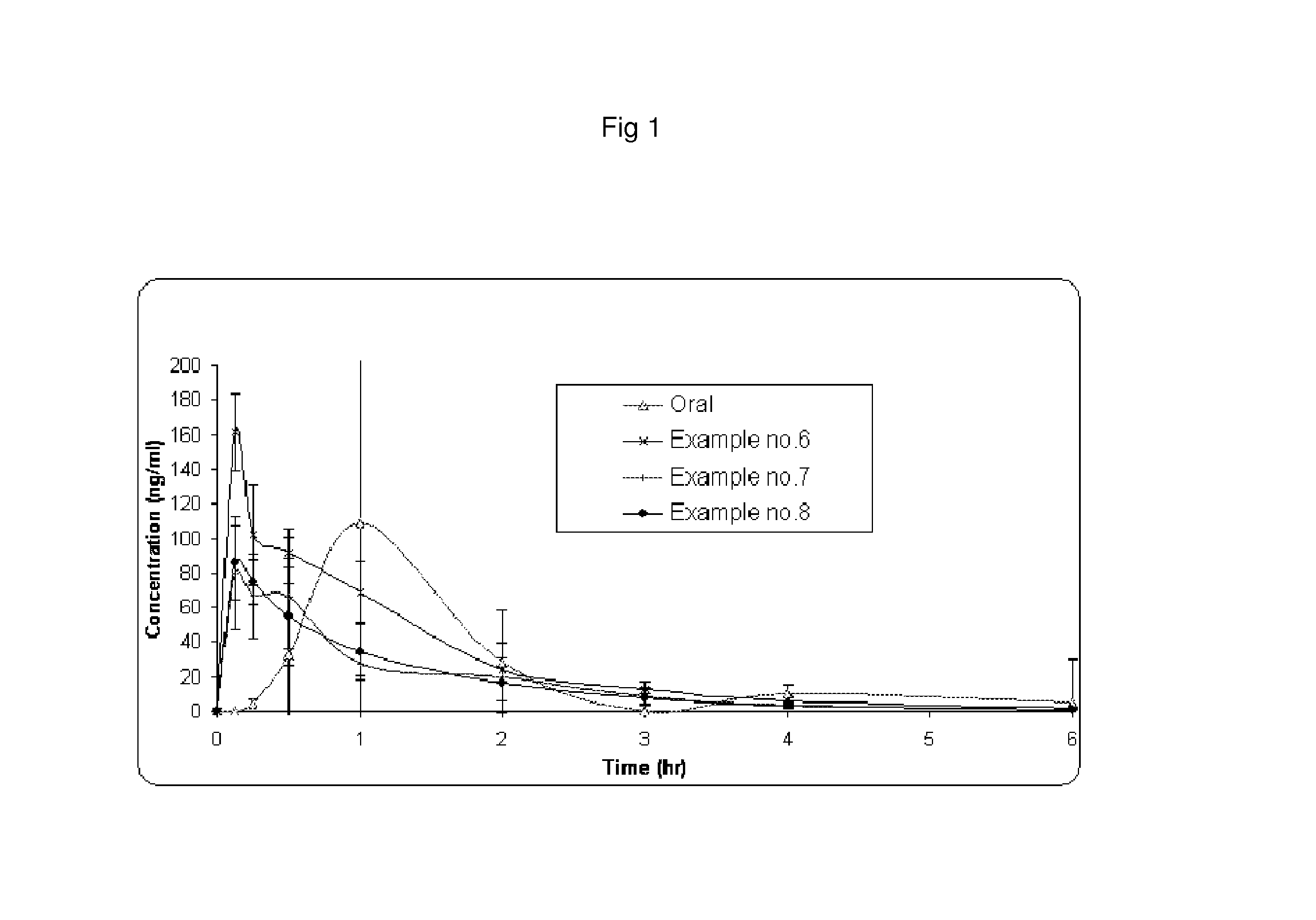
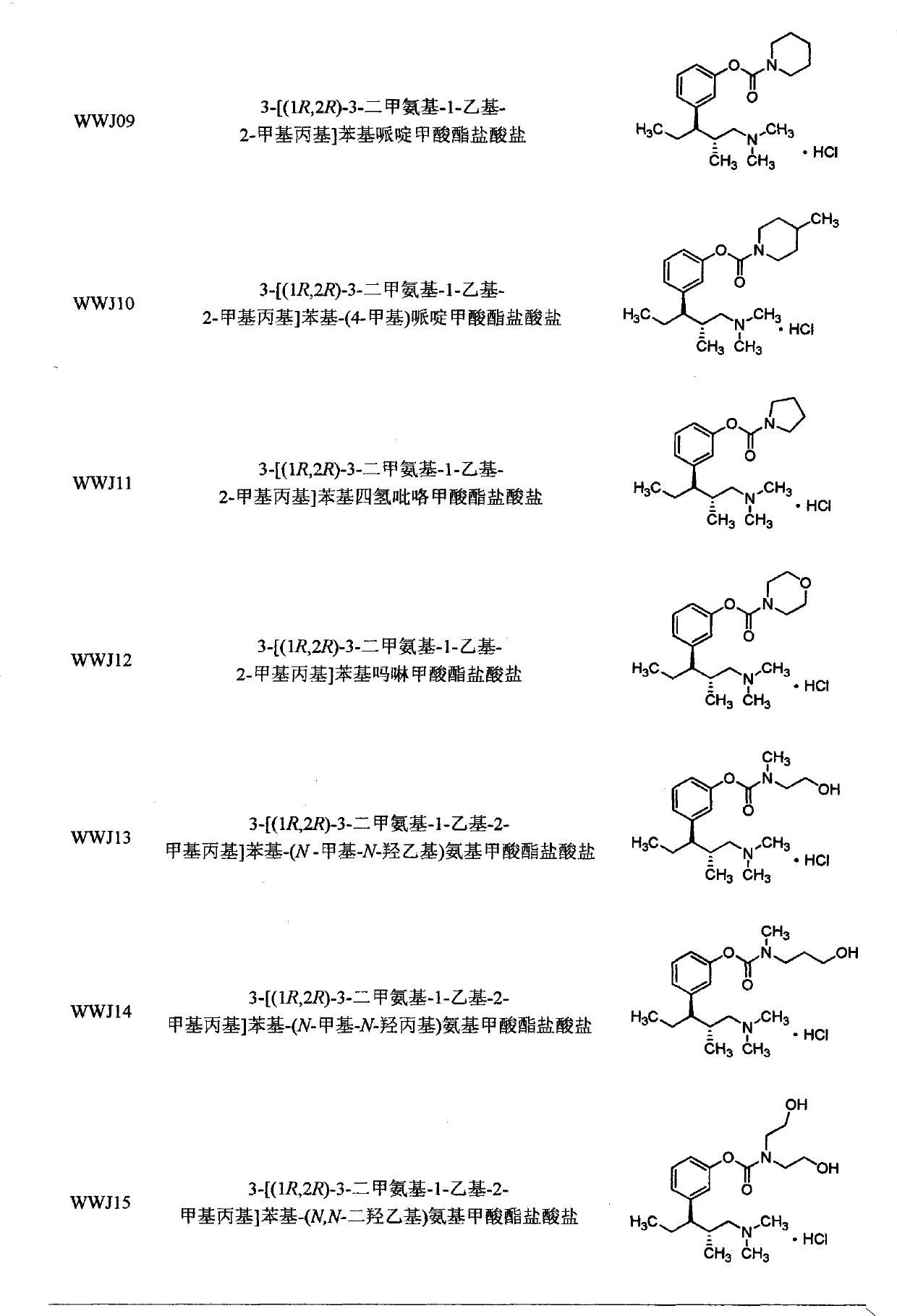
Amino acid and peptide carbamate prodrugs of tapentadol and uses thereof
InactiveUS20100227921A1Sufficient amountMinimizing the gastrointestinal (GI) side effectsBiocideNervous disorderCarbamateSide effect
Prodrugs of tapentadol with amino acids or short peptides, pharmaceutical compositions containing such prodrugs and a method for providing pain relief with the tapentadol prodrugs are provided herein. Prodrugs having side chains of valine, leucine, isoleucine and glycine amino acids and mono-, di- and tripeptides thereof are preferred. Additionally, methods for avoiding or minimizing the adverse gastrointestinal side effects associated with tapentadol administration, as well as increasing the oral bioavailability of tapentadol are provided herein.
Owner:SHIRE PLC
Controlled release pharmaceutical compositions of tapentadol
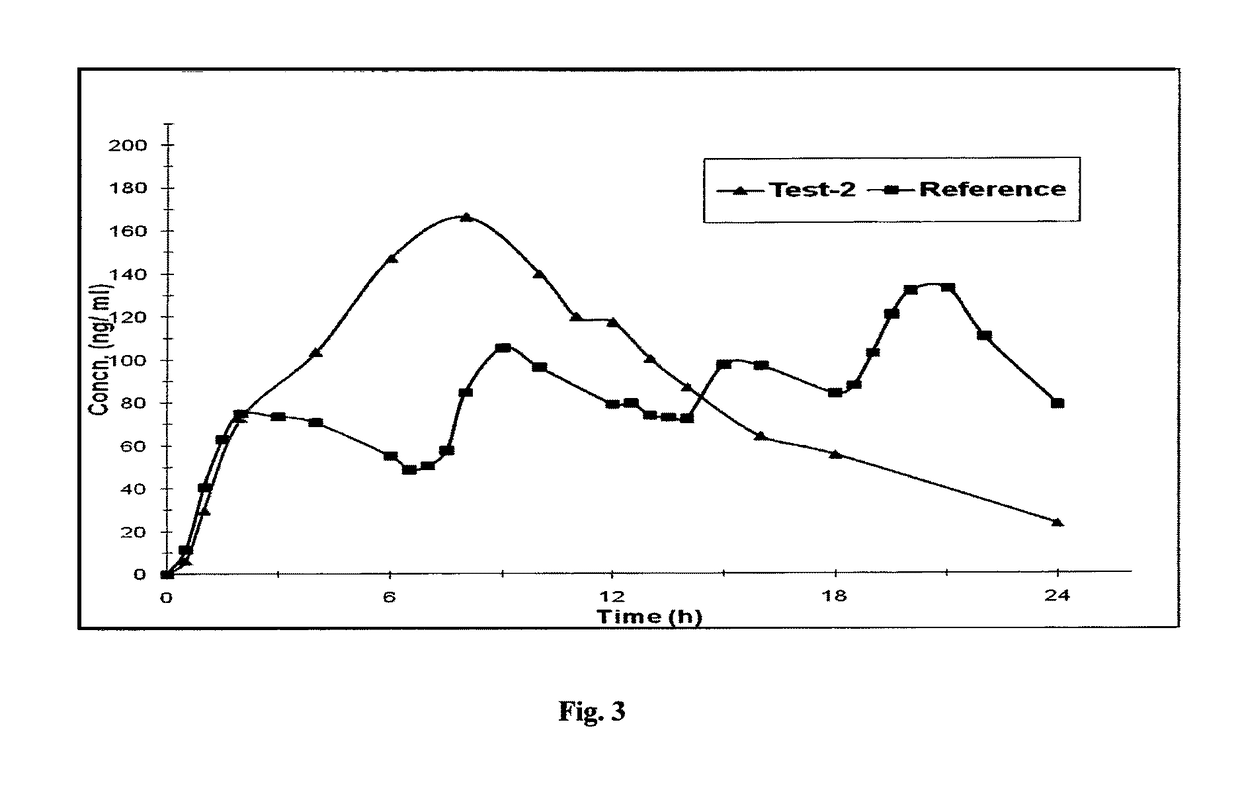
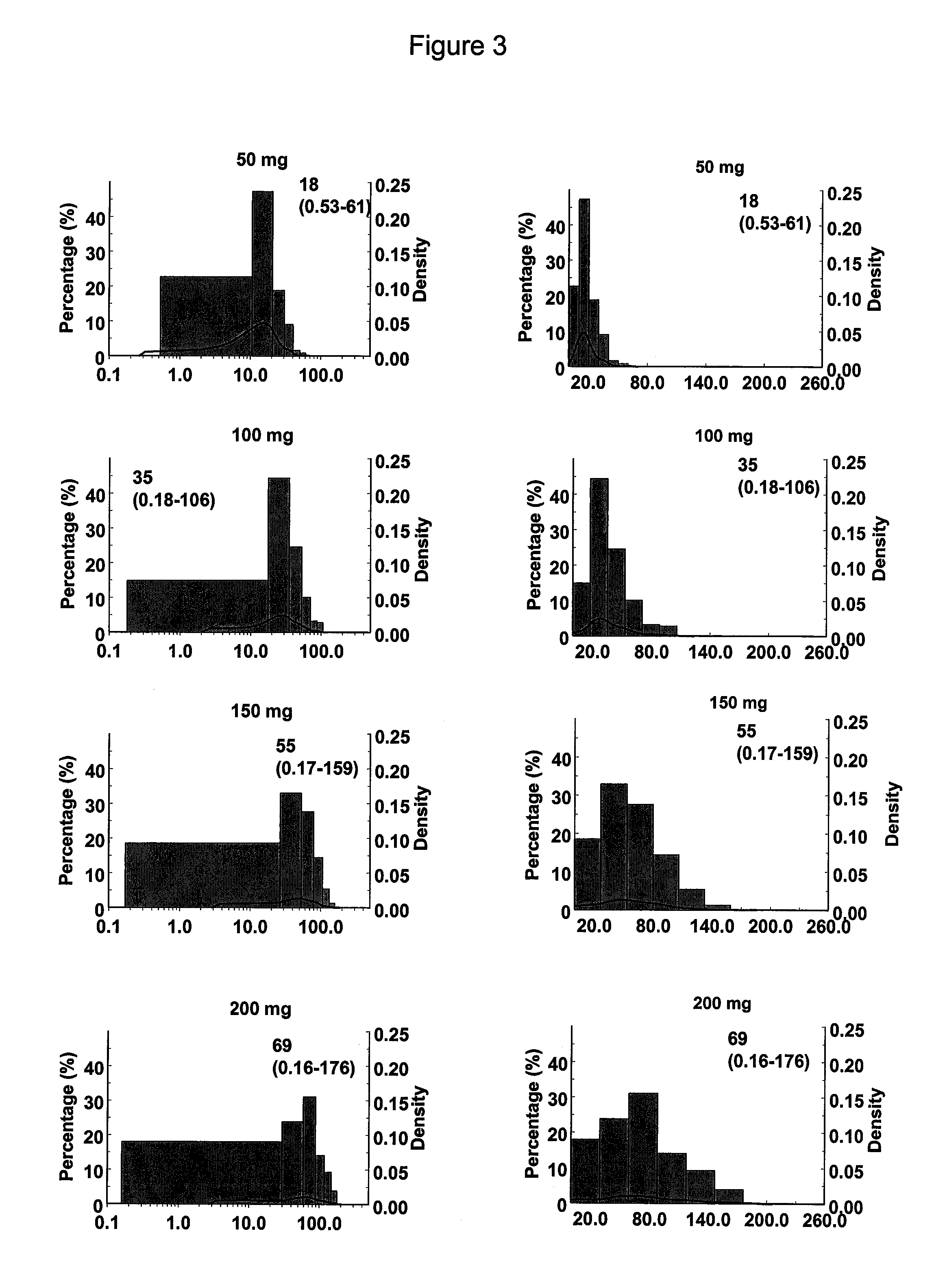
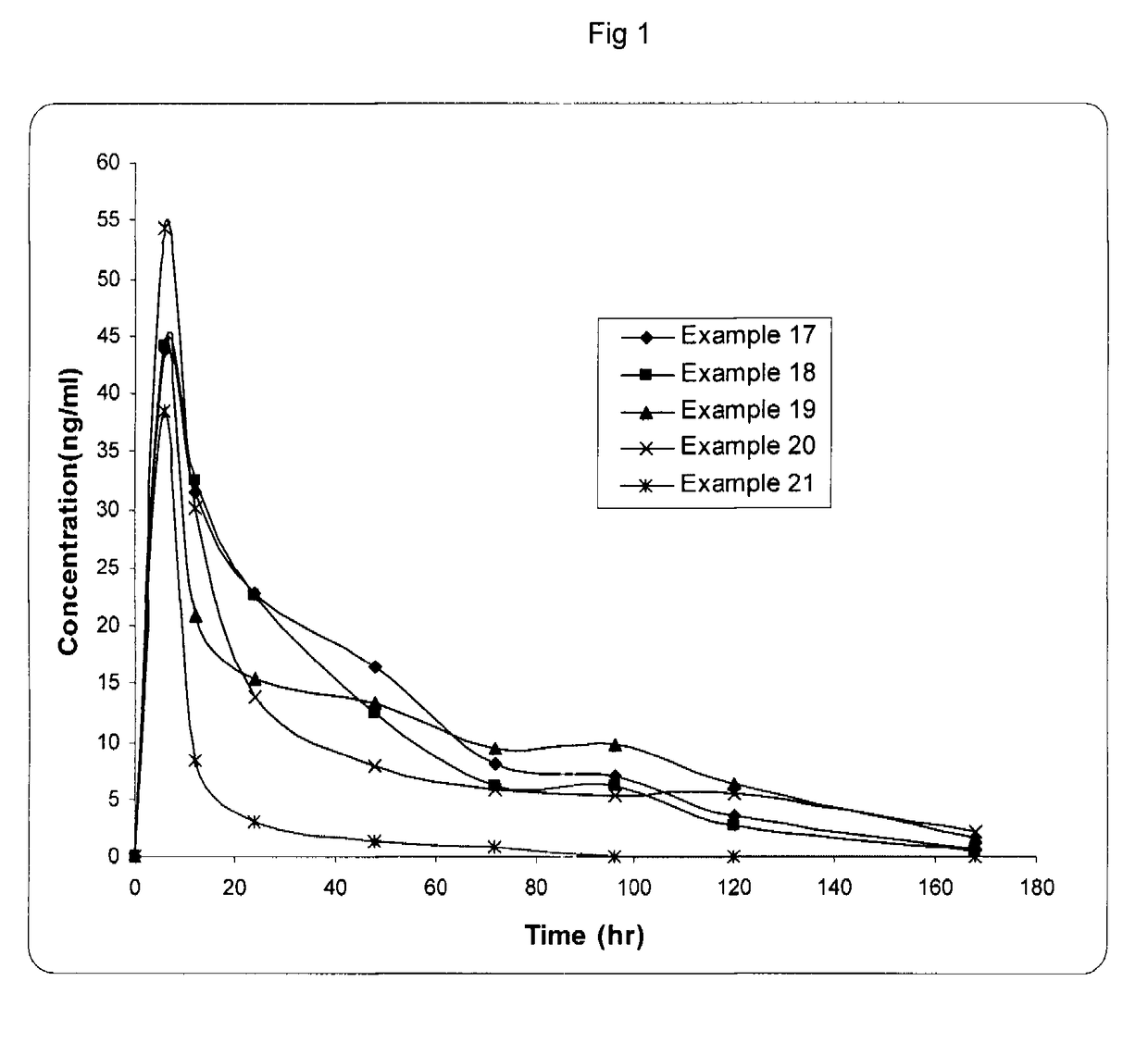
A once daily controlled release pharmaceutical compositions comprising tapentadol, wherein preferably the mean Tmax of tapentadol is reached after 10 hours of administration of the composition. The composition comprises tapentadol, such that it maintains serum concentration of tapentadol of at least about 20 ng / ml for at least about 17 hours after oral administration of the composition. According to one embodiment the controlled release pharmaceutical composition comprises tapentadol, which is gastroretentive.
Owner:LUPIN LTD
Controlled release pharmaceutical compositions of tapentadol
A once daily controlled release pharmaceutical compositions comprising tapentadol, wherein preferably the mean Tmax of tapentadol is reached after 10 hours of administration of the composition. The composition comprises tapentadol, such that it maintains serum concentration of tapentadol of at least about 20 ng / ml for at least about 17 hours after oral administration of the composition. According to one embodiment the controlled release pharmaceutical composition comprises tapentadol, which is gastroretentive.
Owner:LUPIN LTD
Titration of Tapentadol
InactiveUS20090012180A1Improve toleranceReduce frequencyOrganic active ingredientsBiocideTapentadolPharmacology
The use of tapentadol for the manufacture of a medicament comprising at least one administration unit A containing dose a of tapentadol and at least one administration unit B containing dose b of tapentadol, where dose a<dose b, for the treatment of pain.
Owner:GRUNENTHAL GMBH
Parenteral Administration of Tapentadol
InactiveUS20120225951A1Improve efficacyPain is efficientlyOrganic active ingredientsBiocideTapentadolPharmacology
An aqueous pharmaceutical composition adapted for parenteral administration of tapentadol or a physiologically acceptable salt thereof having a pH value of at least 5.4.
Owner:GRUNENTHAL GMBH
Novel and potent tapentadol dosage forms
InactiveUS20110281855A1Good curative effectIncreasing and decreasing cumulative daily doseBiocideNervous disorderOpioid antagonistSide effect
The present invention provides a dosage form comprising at least one form of tapentadol, with or without a second analgesic, and at least one opioid antagonist, wherein tapentadol is present in an optimal or suboptimal amount and the said antagonist is present in an amount effective to improve the efficacy and or reduce the side effects of tapentadol. The present invention further provides a method of treating pain and pain related conditions by administering to a patient in need thereof, a dosage form comprising at least one form of tapentadol, with or without a second analgesic, and at least one opioid antagonist, wherein tapentadol is present in an optimal or suboptimal amount and the said antagonist is present in an amount effective to improve the efficacy and or reduce the side effects of tapentadol.
Owner:GRUNENTHAL GMBH
Tapentadol for Treating Pain due to Osteoarthritis
InactiveUS20090005458A1Good effectEliminate side effectsBiocideOrganic active ingredientsAnesthesiaTapentadol
Owner:GRUNENTHAL GMBH
Semisolid aqueous pharmaceutical composition containing tapentadol
ActiveUS9446008B2Enhance and ensure stabilityComposition is stableBiocideOrganic active ingredientsTapentadolChemistry
A semisolid aqueous pharmaceutical composition containing tapentadol or a physiologically acceptable salt thereof.
Owner:GRUNENTHAL GMBH
Aqueous Pharmaceutical Formulation of Tapentadol for Oral Administration
InactiveUS20130022670A1Sufficient shelf lifeSufficient in use stabilityBiocideOrganic active ingredientsOral medicationPreservative
An aqueous pharmaceutical composition containing tapentadol or a physiologically acceptable salt thereof and being adapted for oral administration. The composition has excellent storage stability without relying on the presence of high amounts of preservatives.
Owner:GRUNENTHAL GMBH
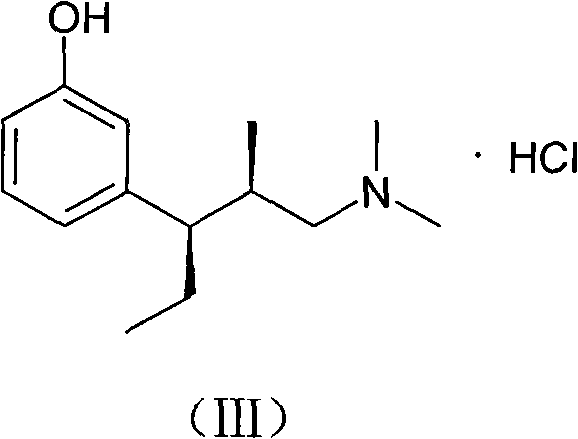
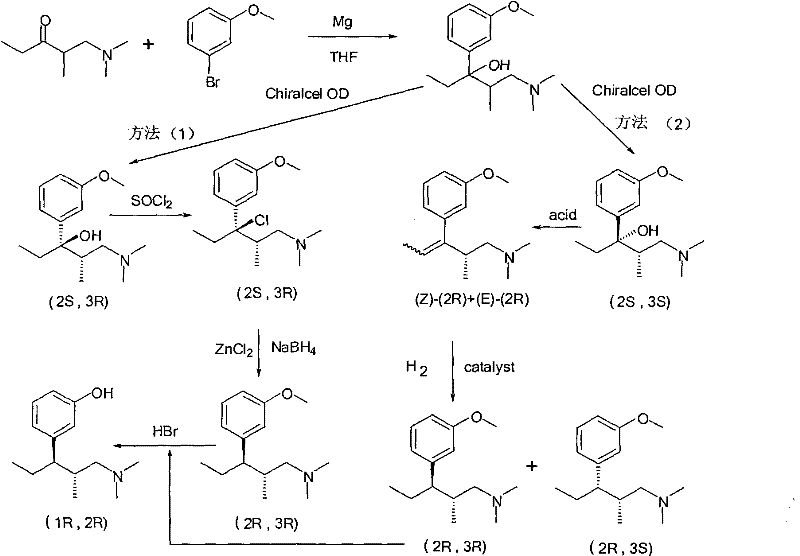
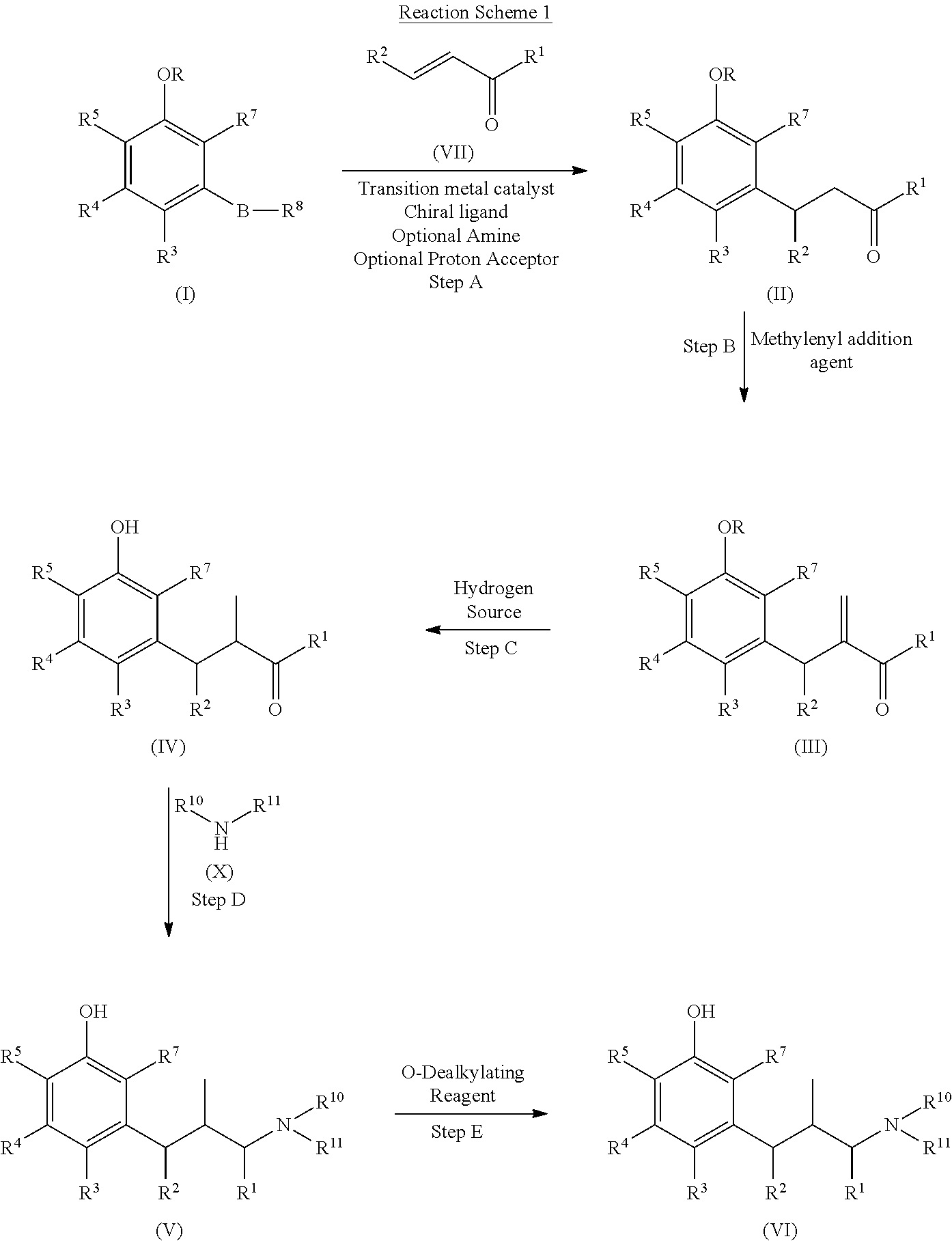
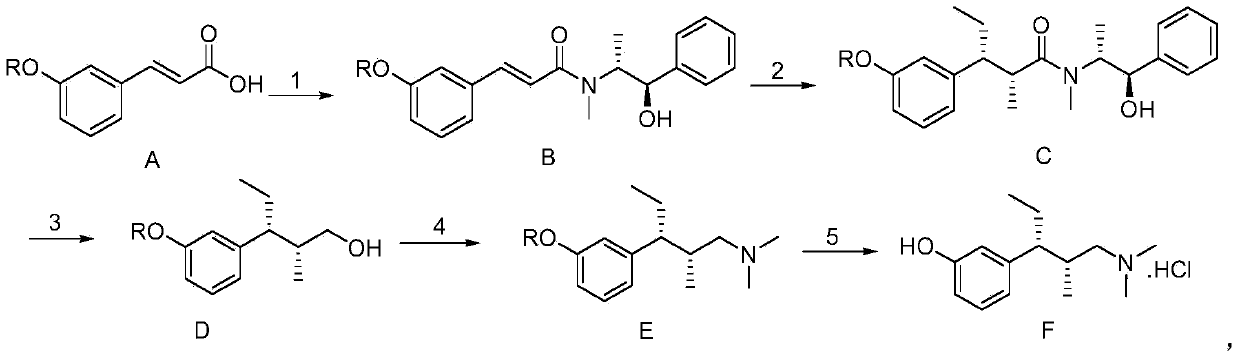
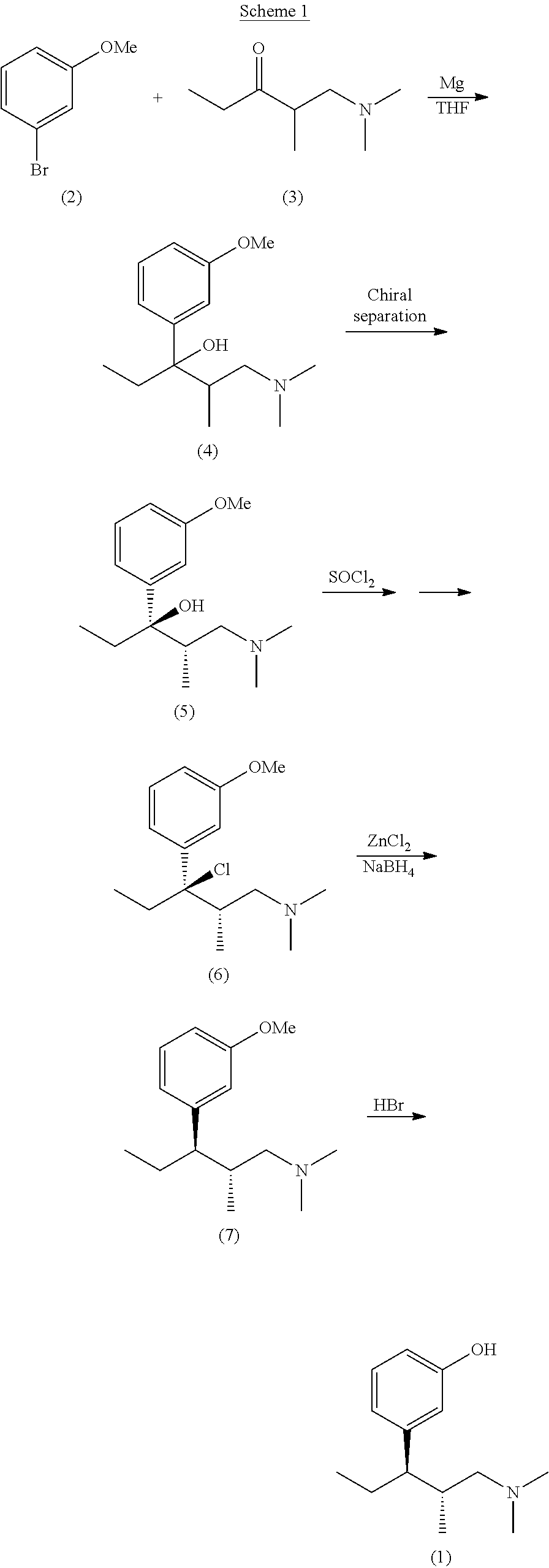
Method for preparing tapentadol intermediate
InactiveCN102206164AOrganic compound preparationAmino-hyroxy compound preparationAfter treatmentDrugs synthesis
The invention relates to the field of drug synthesis, in particular to a method for preparing an analgesic tapentadol intermediate (2R,3R)-3-(3-methoxyl phenyl)-N,N,2-trimethyl amylamine hydrochloride. The method is characterized by comprising the following steps of: chlorinating optically active (2S,3R)-1-dimethylamino-3-(3-methoxyl phenyl)-2-methyl amyl-3-ol and phosphorus pentachloride and salifying with hydrogen chloride to obtain hydrochloride, and then carrying out stereoselective hydrogenolysis in the presence of a catalyst, and salifying with the hydrogen chloride to obtain the analgesic tapentadol intermediate. The method provided by the invention has the advantages of cleanness, safety, convenience for after treatment and lower production cost. Compared with a method in a literature, the method has reaction time shortened by nearly 80 hours.
Owner:CHINA PHARM UNIV
Pharmaceutical invention of tapentadol
The present invention relates to a pharmaceutical composition of tapentadol for nasal administration. Present invention also relates to the process of preparation of pharmaceutical composition of tapentadol for nasal administration and its use in the treatment of pain.
Owner:TORRENT PHARMA LTD
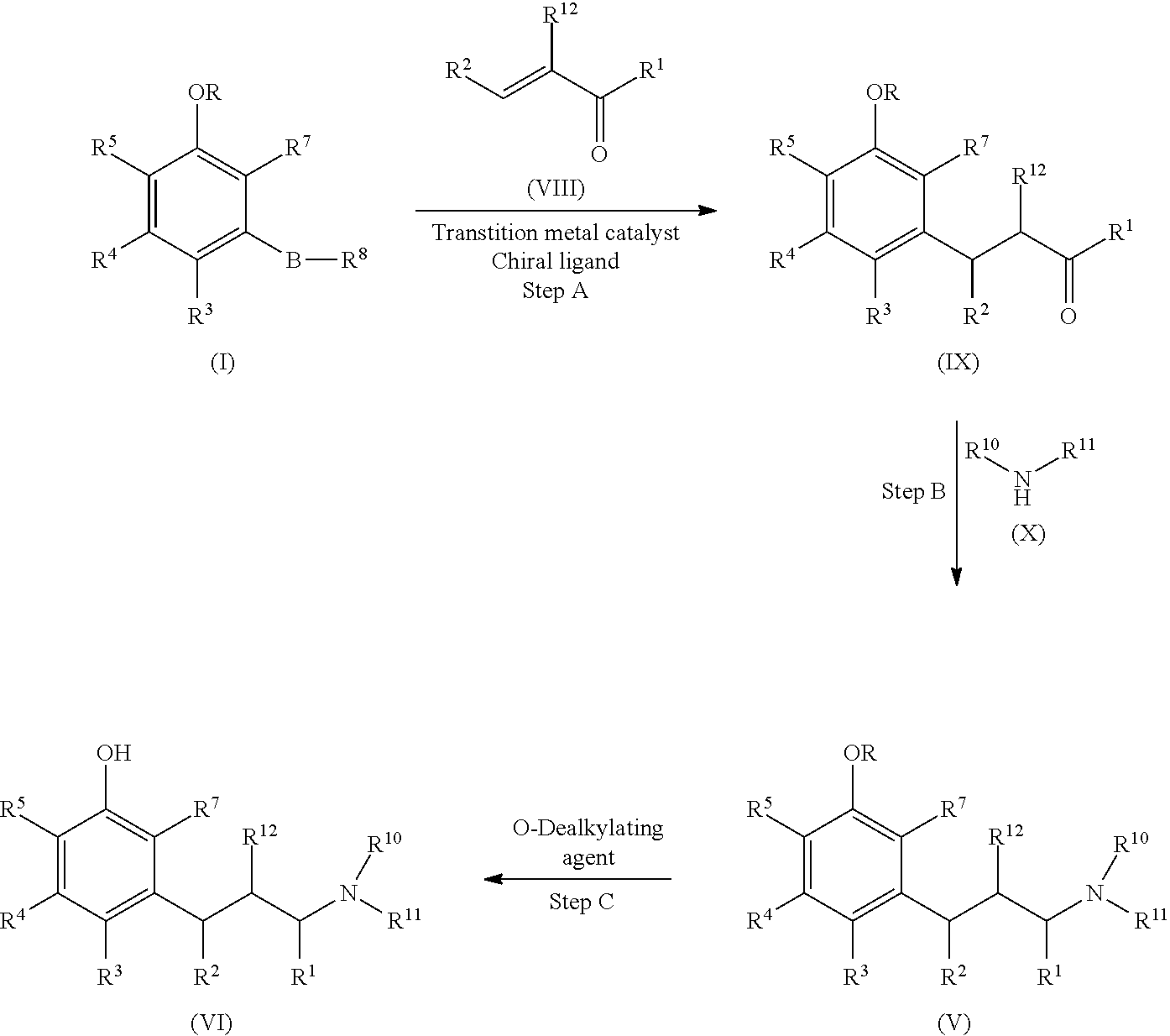
Process for preparing substituted phenylalkanes
The invention provides methods for preparing substituted phenylalkanes. In particular, the processes comprise reacting a phenyl boronic compound with an α-β unsaturated carbonyl-containing compound via an asymmetric 1,4-addition reaction. The processes may be useful in the synthesis of tapentadol.
Owner:SPECGX LLC
Compositions comprising enzyme-cleavable phenol-modified tapentadol prodrug
ActiveUS9238020B2Eliminate side effectsPatient compliance is goodBiocideCarbamic acid derivatives preparationControlled releaseBiological activation
Owner:SIGNATURE THERAPEUTICS
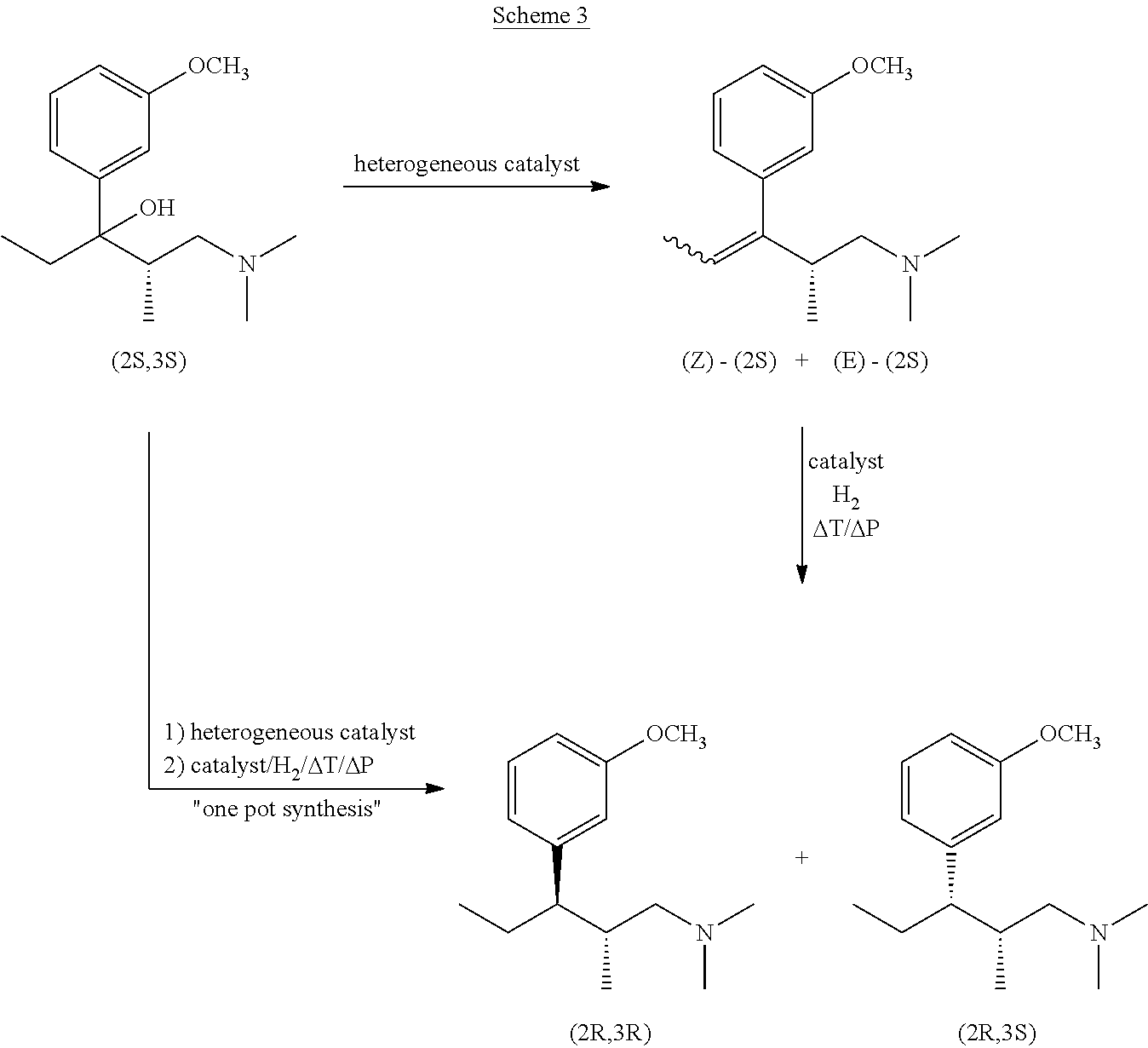
Stereoselective synthesis of tapentadol and its salts
InactiveUS20130150622A1Organic compound preparationCarbonyl compound preparationTapentadolMedicinal chemistry
Owner:BOEHRINGER INGELHEIM INT GMBH
Synthesis and application of intermediate of tapentadol
InactiveCN102477016AAmino preparation from aminesOrganic compound preparationCombinatorial chemistryTapentadol
The invention discloses a preparation method of a key intermediate (1R,2R)-1-ethyl-2-methylphenylethane compound of tapentadol and a method for preparing tapentadol and derivatives thereof from the key intermediate.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Parenteral Administration of Tapentadol
InactiveUS20160106688A1Efficacy of the composition containing tapentadolImprove efficacyOrganic active ingredientsBiocideTapentadolPharmacology
Owner:GRUNENTHAL GMBH
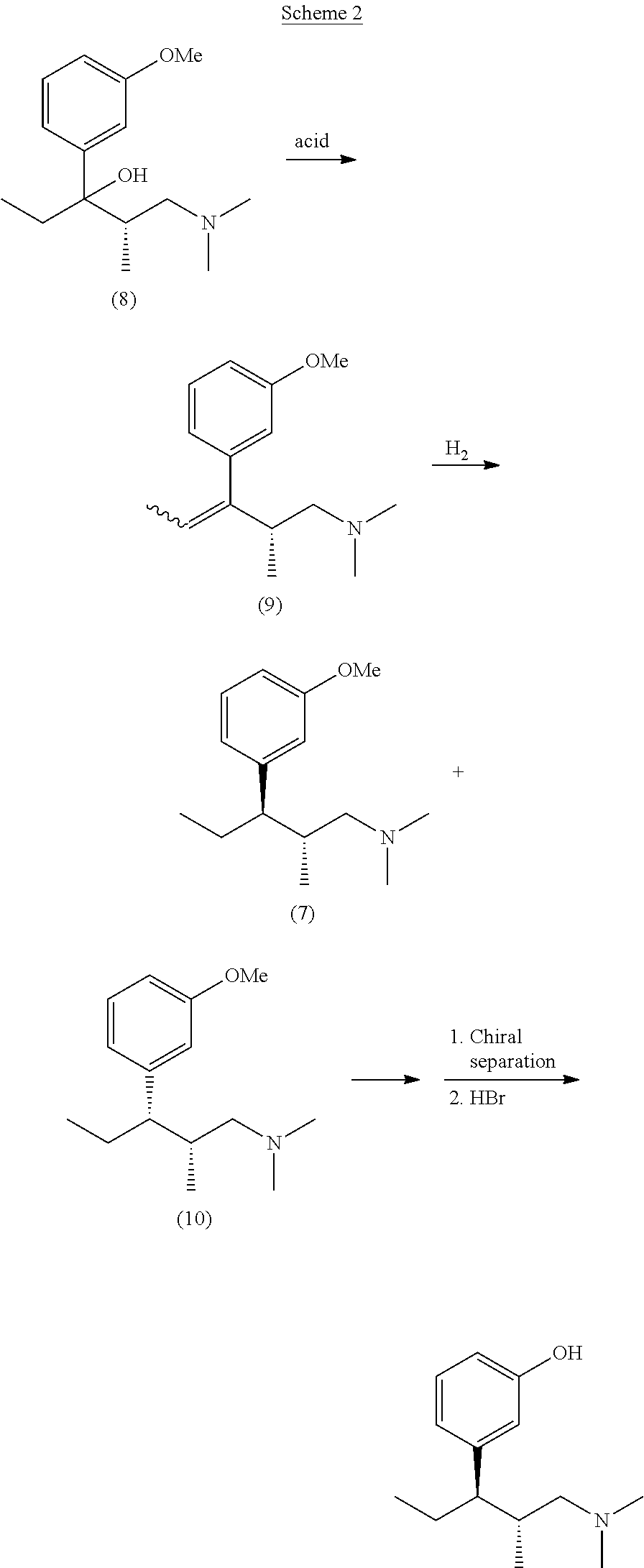
Intermediate compounds and processes for the preparation of tapentadol and related compounds
The present invention discloses processes for the preparation of 3-[(1R,2R)-3-(dimethyl-amino)-1-ethyl-2-methyl-propyl]phenol (Tapentadol), salts thereof and related compounds of formula (A), including stereoisomers and pharmaceutically acceptable salts thereof, and to certain intermediates used in such process.
Owner:MAPI PHARMA
Method of Inhibiting Chronification of Pain
InactiveUS20120309841A1Useful treatmentUseful inhibitionOrganic active ingredientsBiocideTreatment painMigraine
The use of tapentadol in the treatment of pain and / or pain chronification in a subject suffering from pain chronification and / or in the treatment of pain and inhibition of pain chronification in a subject suffering from pain and at risk of pain chronification, as well as the use of tapentadol for the treatment or inhibition of migraine.
Owner:GRUNENTHAL GMBH
Treatment of Irritable Bowel Syndrome
InactiveUS20130116334A1Avoid side effectsIncrease the amount of tapentadolOrganic active ingredientsBiocideIrritable bowel syndromeTapentadol
A method of treating irritable bowel syndrome in a patient in need thereof by administering to said patient a pharmaceutically effective amount of tapentadol.
Owner:GRUNENTHAL GMBH
Compositions for Use in Treating Parkinson's Disease and Related Disorders
ActiveUS20180147160A1Improvement of side effectsLow efficacyNervous disorderKetone active ingredientsSide effectActive agent
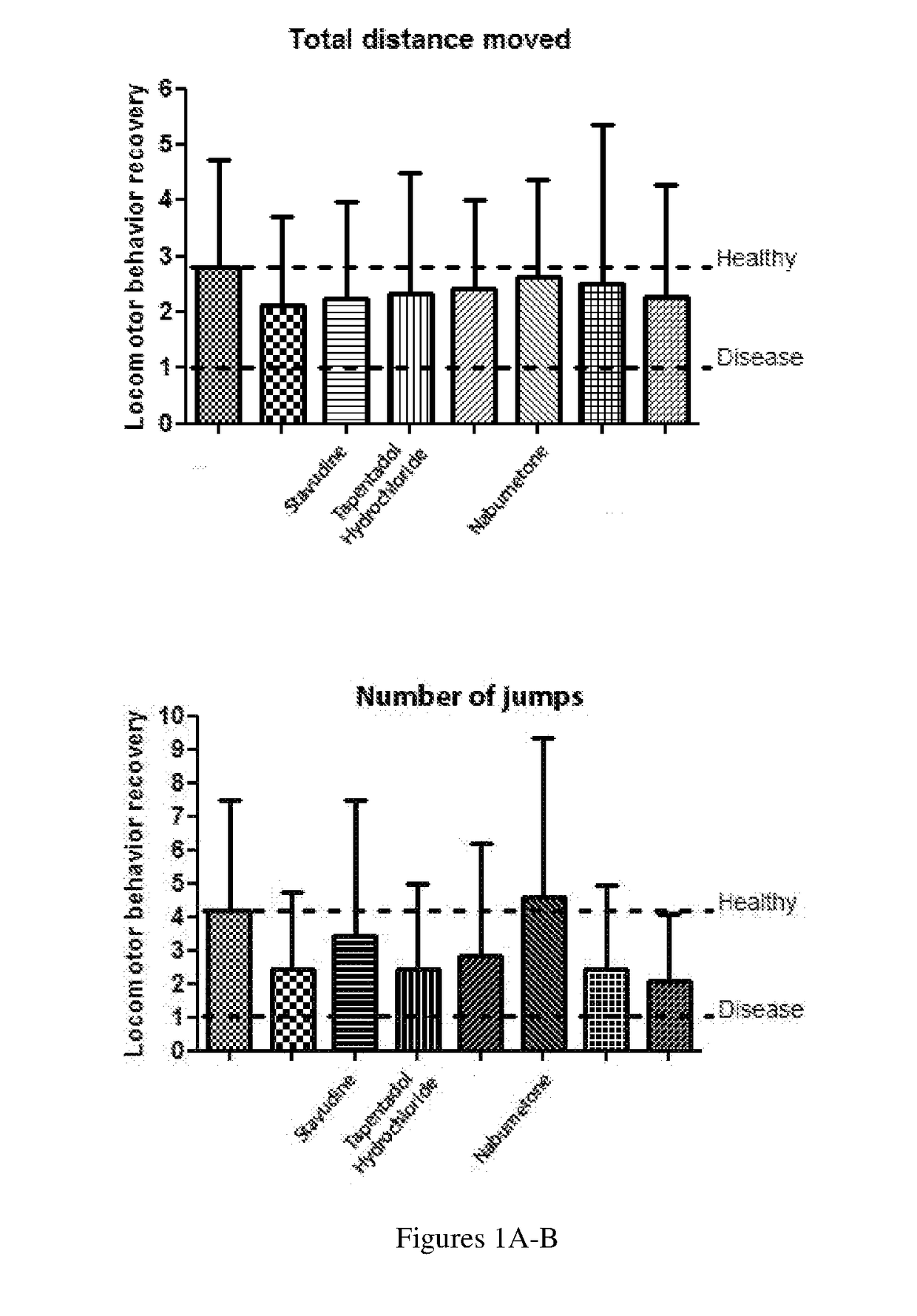
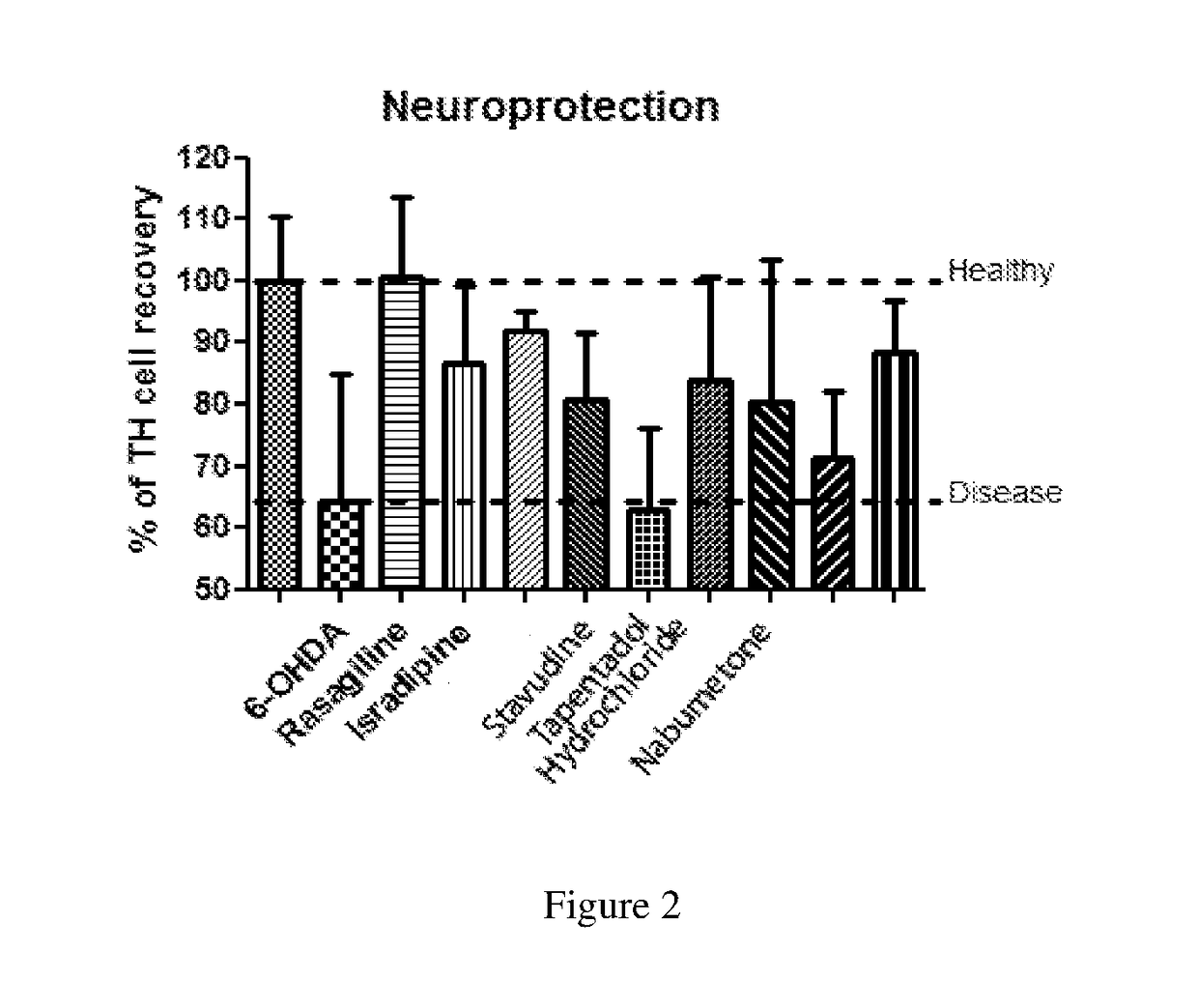
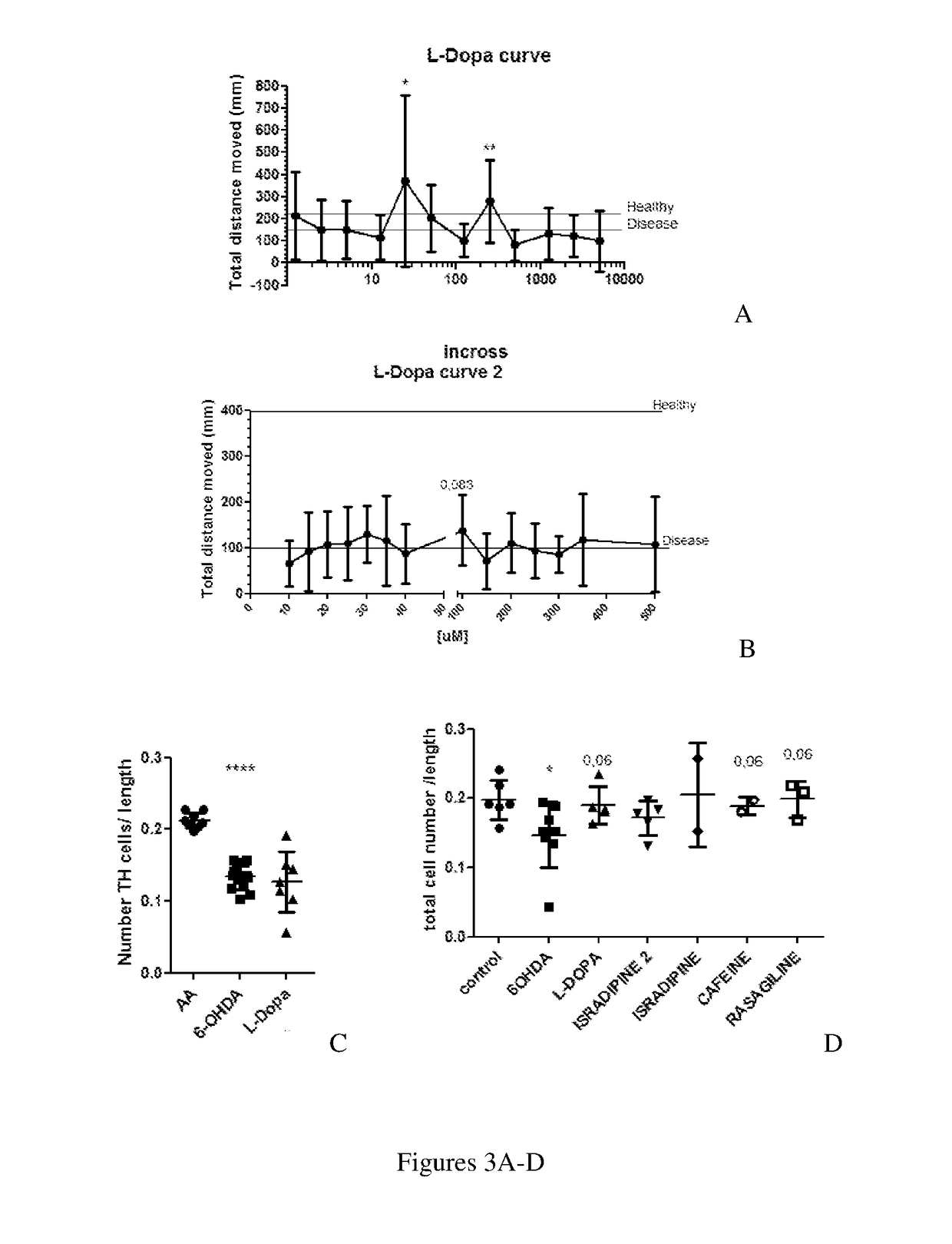
The present invention relates to methods of treating and managing Parkinson's disease and related disorders. The methods especially find use in managing motor symptoms, including gait problems, particularly during advanced stages when effectiveness of standard medications wear off or side effects become problematic, as seen in Parkinson's disease, other disorders treated with dopaminergic agents, and other conditions associated with motor problems, such as aging or stroke. The treatment also may include disease-modifying effects, neuroprotection of, or neurorescue effects on neuronal cells in patients with Parkinson's disease and other neurodegenerative disorders. In particular, the invention relates to method of administering pharmaceutical compositions comprising effective amounts of tapentadol or a pharmaceutically acceptable salt or derivative thereof or, in other embodiments, stavudine or nabumetone, or a derivative thereof, for treating symptoms associated with Parkinson's disease, either as individual active agents, in combination with each other, or in combination with agents known to treat Parkinson's disease, such as the dopaminergic agent levodopa. The invention also relates to methods of preparing pharmaceutical compositions comprising effective amounts of tapentadol, stavudine, or nabumetone, or a derivative thereof, or further in combination with a dopaminergic agent, or derivative thereof, as well as to methods of using the pharmaceutical compositions in treating Parkinson's disease, related disorders, other conditions treated with dopaminergic agents, and other conditions with gait problems, for example by oral administration of the compositions.
Owner:LUSOMEDICAMENTA +2
Semisolid Aqueous Pharmaceutical Composition Containing Tapentadol
ActiveUS20120225950A1Enhance and ensure stabilityComposition is stableOrganic active ingredientsBiocideTapentadolChemistry
A semisolid aqueous pharmaceutical composition containing tapentadol or a physiologically acceptable salt thereof.
Owner:GRUNENTHAL GMBH
Intermediate for preparing tapentadol or analogues thereof
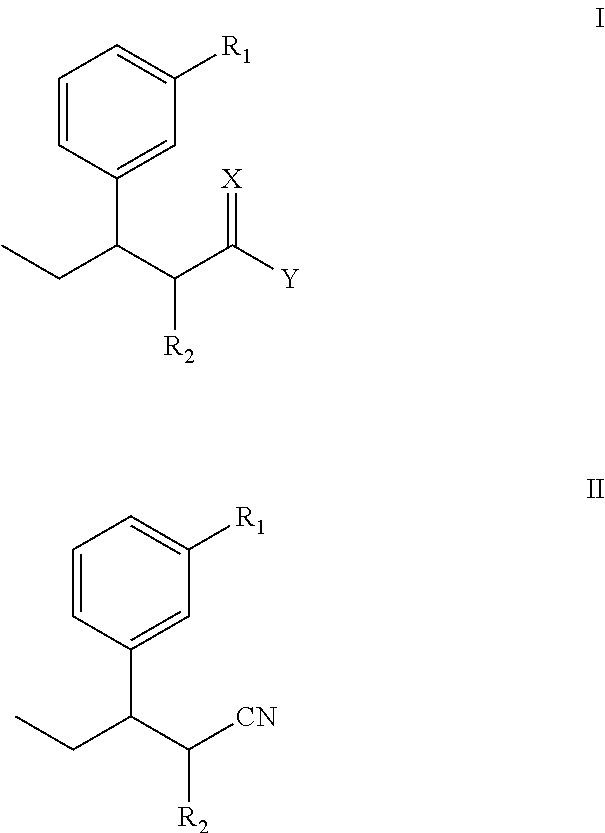
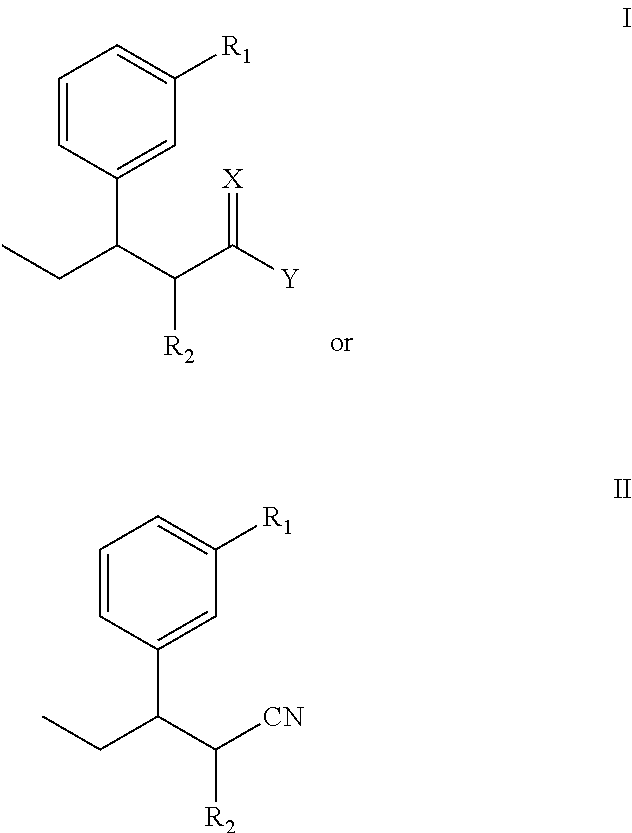
InactiveUS8853393B2Improve production yieldLow production costOrganic compound preparationCarboxylic acid esters preparationStructural formulaTapentadol
The invention discloses a novel intermediate for preparing tapentadol and analogues thereof, wherein the structural formula is shown as formula I or II, and the groups are defined as the specification. The invention further discloses a method for preparing the novel intermediate and use of the intermediate for preparing tapentadol and analogues thereof. The invention can remarkably improve the product yield and quality of tapentadol, reduce the production cost, and simplify the production procedure. The preparation process is environment friendly, thus more suitable for the requirements of industrial production.
Owner:ANHUI NEW STAR PHARMA DEV
Tapentadol carbamate derivative and preparation method and application thereof
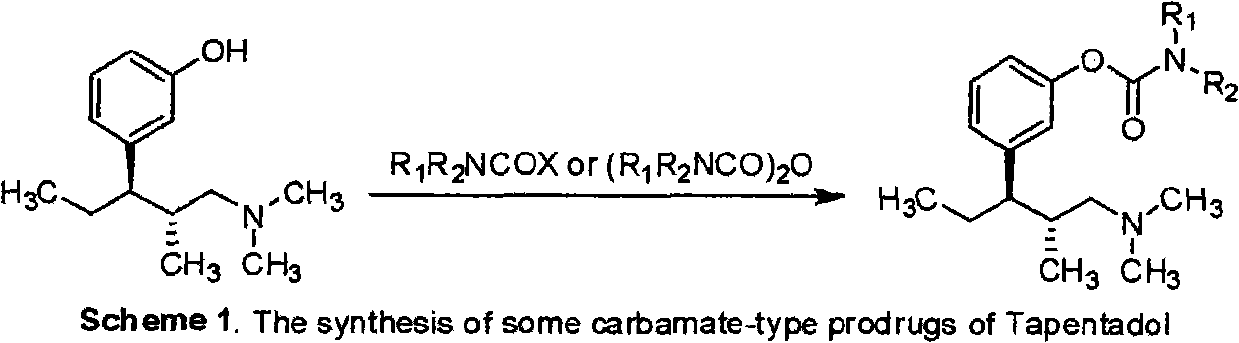
InactiveCN101845002AGood chemical stabilityEasy demethylationCarbamic acid derivatives preparationOrganic compound preparationChemical synthesisCarbamate
The invention belongs to the field of pharmacy, in particular to a tapentadol carbamate derivative with a general formula I, pharmaceutically acceptable salts, a preparation method and an application thereof. In the invention, the structure of phenolic hydroxyl groups in tapentadol molecules is improved by a chemical synthesis method, and proper carrier groups are introduced in the position to synthesize carbamate type prodrug. All amines adopted by the carbamate are fatty amines, wherein dimethylamine or methylamine fragments can generate demethylation metabolism easily in vivo, thereby being convenient for releasing raw tapentadol. Meanwhile, the existence of the carbamate structure increases the chemical stability of the prodrug and can obviously improve the oral bioavailability, reduce the administration dosage and lower the toxic side effect, and the tapentadol carbamate derivative can be further developed into novel tapentadol analgesics.
Owner:SHENYANG PHARMA UNIVERSITY +1
Process for preparing substituted phenylalkanes
The invention provides methods for preparing substituted phenylalkanes. In particular, the processes comprise reacting a phenyl boronic compound with an α-β unsaturated carbonyl-containing compound via an asymmetric 1,4-addition reaction. The processes may be useful in the synthesis of tapentadol.
Owner:SPECGX LLC
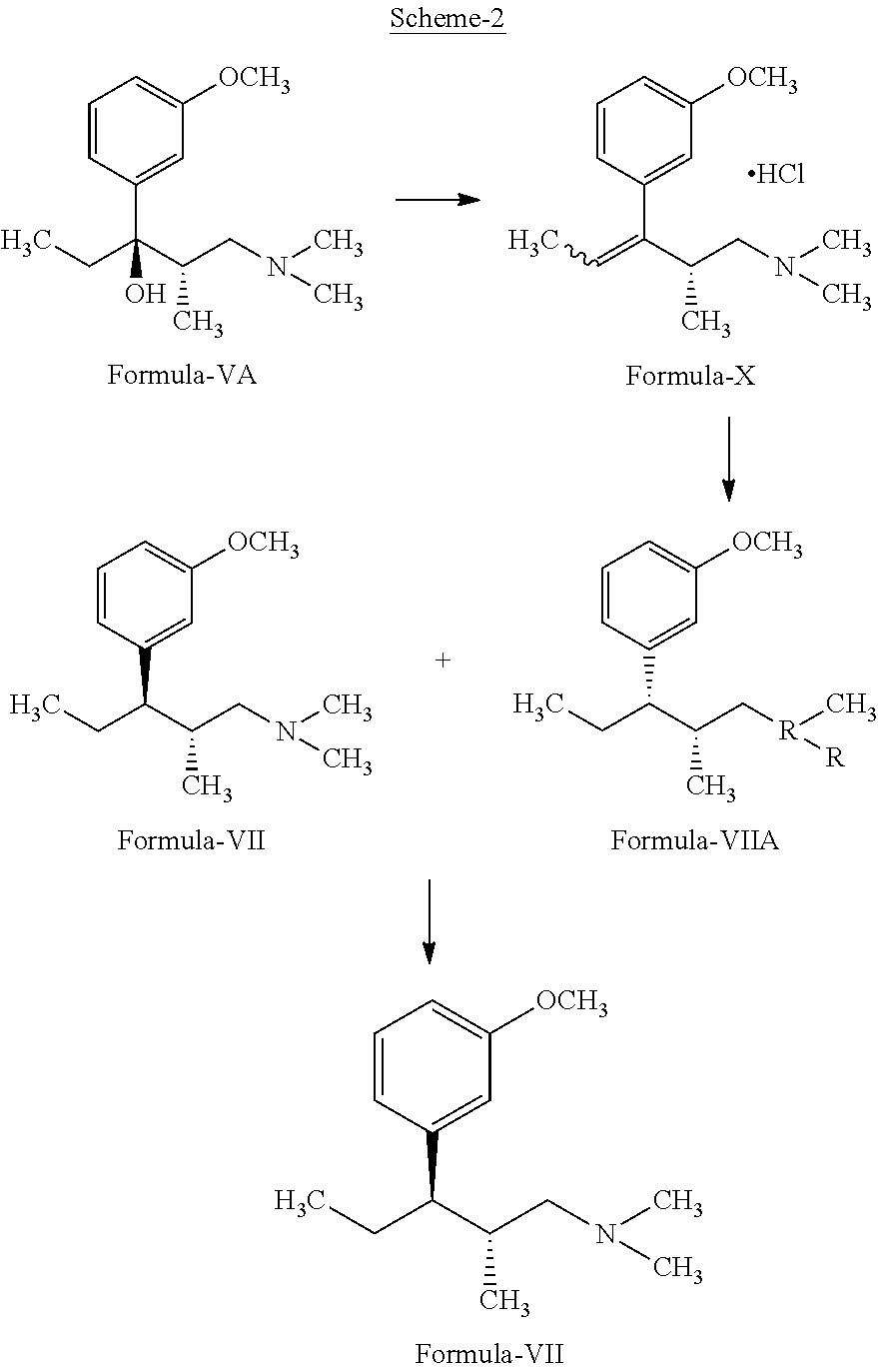
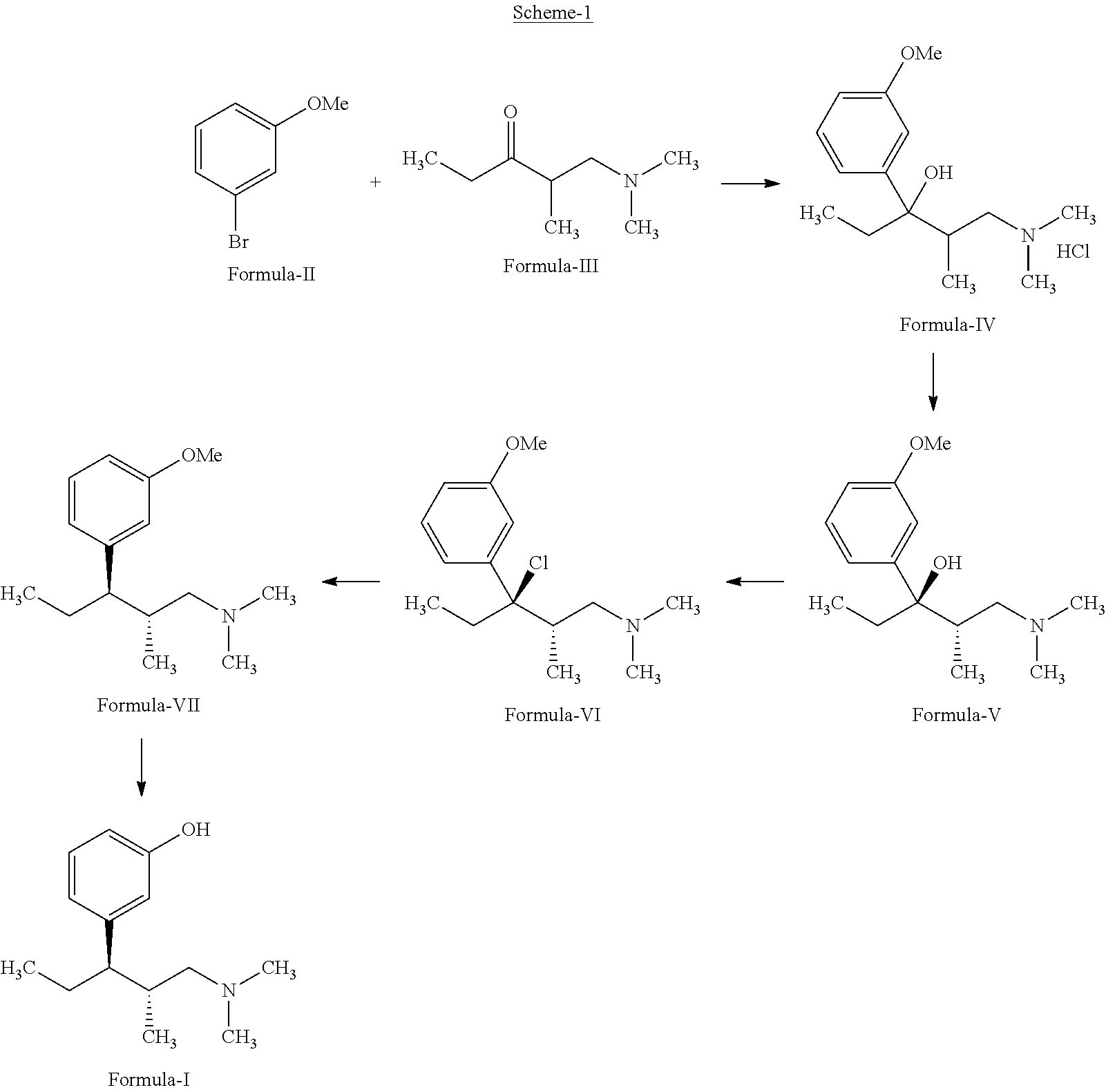
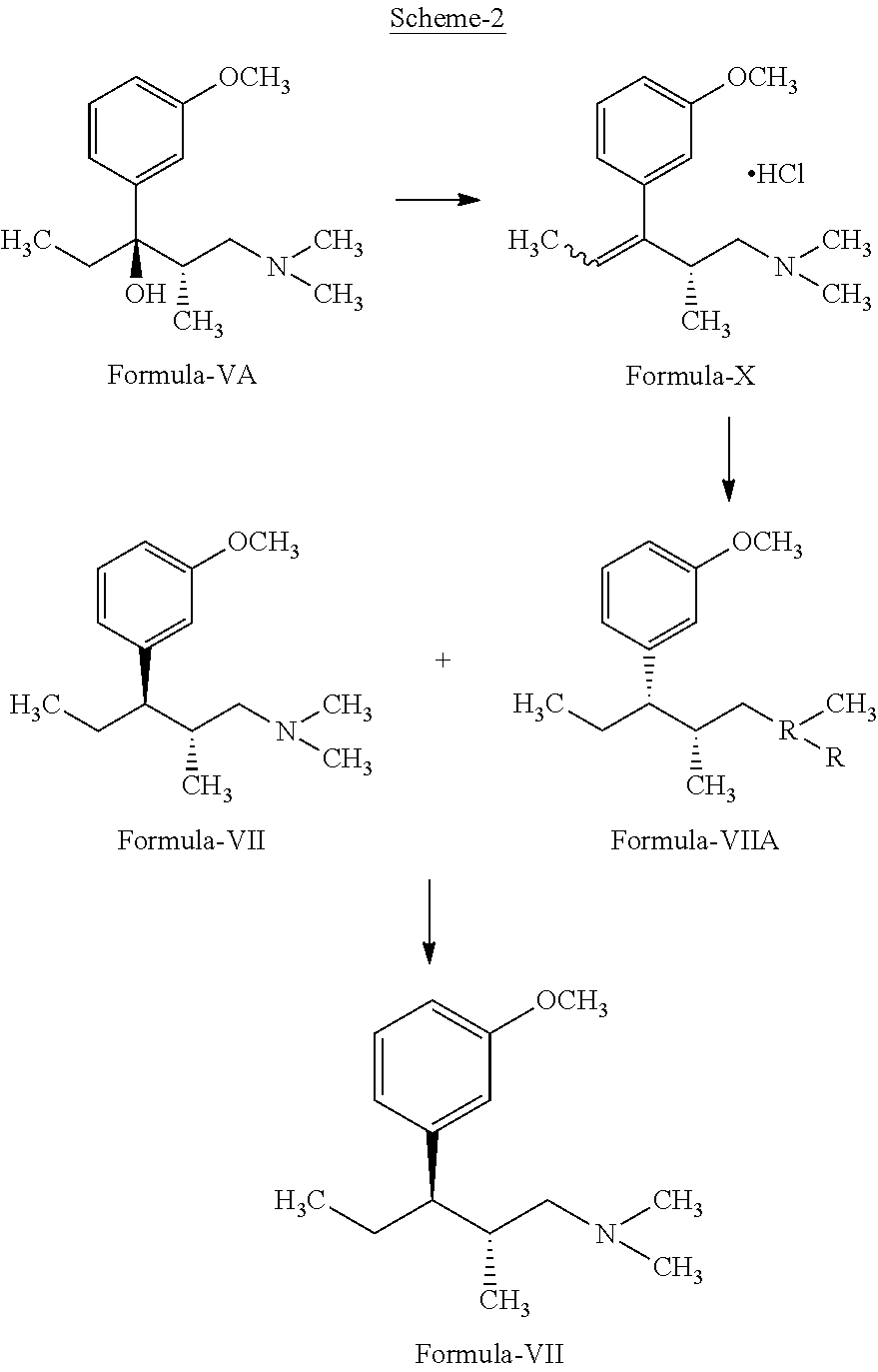
Process for the preparation of tapentadol
Disclosed herein is an improved process for the preparation of 3-[(2R,3R)-1-(dimethylamino)-2-methylpentan-3-yl]phenol of Formula-I and its pharmaceutically acceptable salt which comprises the reaction of (S)-1-(dimethylamino)-2-methylpentan-3-one of formula VIII with 3-bromo anisole of formula II under Grignard conditions to get the compound (2S,3R)-1-(dimethylamino)-3-(3-methoxyphenyl)-2-methyl pentan-3-ol of formula V followed by activation of the —OH group of the formula V to convert into sulfonate esters of formula IX, which are on reductive deoxygenation to yield (2R,3R)-3-(3-methoxyphenyl)-N,N,2-trimethylpentan-1-amine of formula VII and demethylation of formula VII to obtain the compound 3-[(2R,3R)-1-(dimethylamino)-2-methylpentan-3-yl]phenol of Formula-1.
Owner:INDOCO REMEDIES
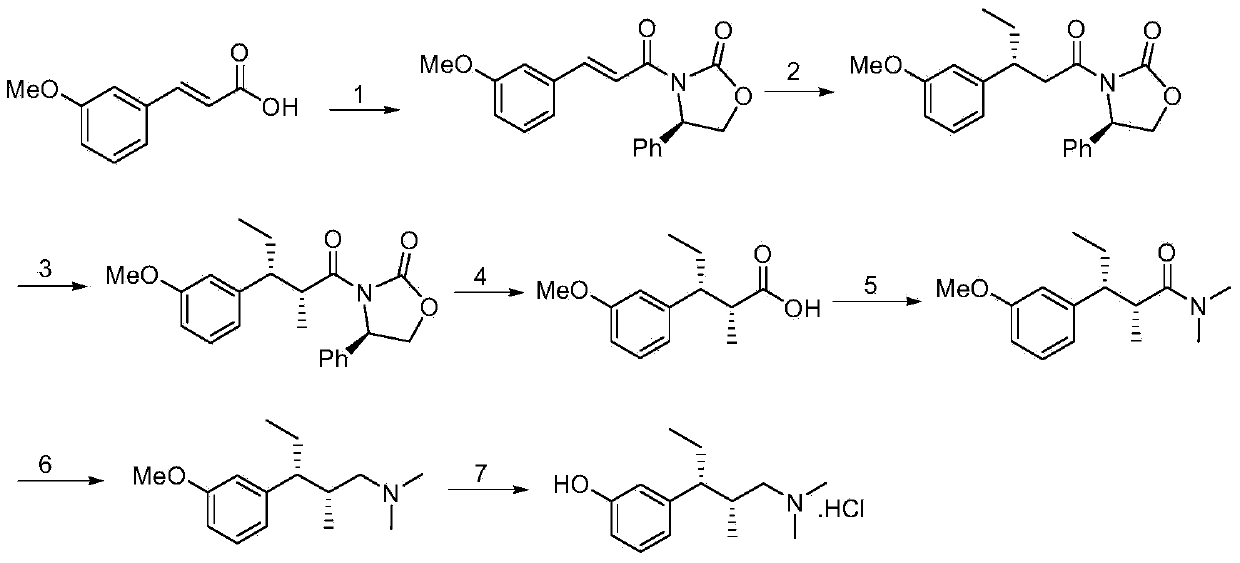
Synthetic method of tapentadol
ActiveCN103787898AThe reaction route is simpleFewer post-processing stepsOrganic compound preparationAmino-hyroxy compound preparationEphedrineAlcohol
The invention discloses a synthetic method of tapentadol. The method comprises the following steps: reacting meta-substitution cinnamic acid serving as a starting material with a chiral adjuvant to obtain amide; reacting the amide with a nucleophilic reagent and an electrophilic reagent, and introducing two chiral centers to obtain a chiral product; performing reduction and deprotection on the chiral product to obtain chiral alcohol; performing an amination reaction and a substitution reaction on the chiral alcohol to obtain the tapentadol. The tapentadol is synthesized by taking cheap and readily-available (1R,2R)-psedo-ephedrine as the chiral adjuvant, forming two chiral centers by using a one-pot method and performing simper deprotection and subsequent transformation. By adopting the synthetic method, the reaction route can be simplified, post-treatment steps can be reduced, and the production cost is reduced. The synthetic method is more suitable for industrial application.
Owner:暨明医药科技(苏州)有限公司
Pharmaceutical composition of tapentadol for parenteral administration
InactiveUS9629818B2Facilitated releaseOrganic active ingredientsPowder deliveryProlonged releaseTapentadol
Owner:TORRENT PHARMA LTD
Intermediate compounds and processes for the preparation of tapentadol and related compounds
The present invention discloses processes for the preparation of 3-[(1R,2R)-3-(dimethyl-amino)-1-ethyl-2-methyl-propyl]phenol (Tapentadol), salts thereof and related compounds of formula (A), including stereoisomers and pharmaceutically acceptable salts thereof, and to certain intermediates used in such process.
Owner:MAPI PHARMA
Process for the preparation of tapentadol
InactiveUS20130137890A1Clean reactionImprove securityAmino preparation from aminesOrganic compound preparationPhenolDeoxygenation
Disclosed herein is an improved process for the preparation of 3-[(2R,3R)-1-(dimethylamino)-2-methylpentan-3-yl]phenol of Formula-I and its pharmaceutically acceptable salt which comprises the reaction of (S)-1-(dimethylamino)-2-methylpentan-3-one of formula VIII with 3-bromo anisole of formula II under Grignard conditions to get the compound (2S, 3R)-1-(dimethylamino)-3-(3-methoxyphenyl)-2-methyl pentan-3-ol of formula V followed by activation of the —OH group of the formula V to convert into sulfonate esters of formula IX, which are on reductive deoxygenation to yield (2R,3R)-3-(3-methoxyphenyl)-N,N,2-trimethylpentan-1-amine of formula VII and demethylation of formula VII to obtain the compound 3-[(2R,3R)-1-(dimethylamino)-2-methylpentan-3-yl]phenol of Formula-1.
Owner:INDOCO REMEDIES
Process for the preparation of tapentadol and intermediates thereof
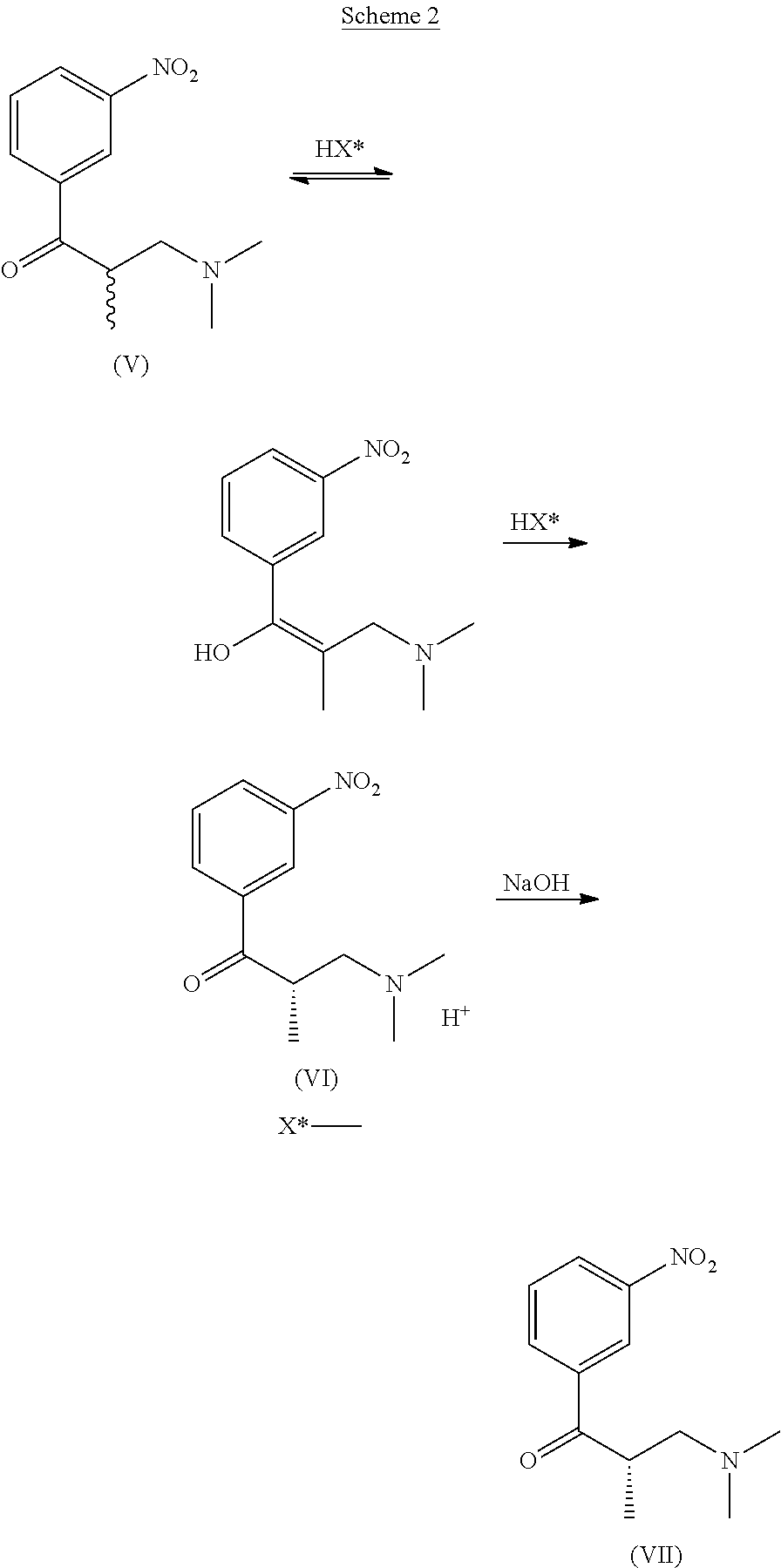
The present invention refers to a new process for the synthesis of tapentadol comprising the quantitative resolution of the racemic mixture (V) to obtain the stereoisomer of (S)-3-(dimethylamino)-2-methyl-1-(3-nitrophenyl)-propan-1-one (VII) according to the Scheme 2 belowusing the (2R,3R)—O,O′-dibenzoyltartaric chiral acid wherein said resolution is quantitative.The present invention also refers to some intermediate compounds of the new synthesis process of tapentadol.
Owner:AMRI ITAL SRL
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com