Synthetic method of tapentadol
A synthetic method, the technology of tapentadol, applied in the field of drug synthesis, can solve the problems of complex reaction route, no one-step ammoniation, complicated post-treatment, etc., and achieve the effect of simplifying the reaction route, reducing production cost and reducing post-processing steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
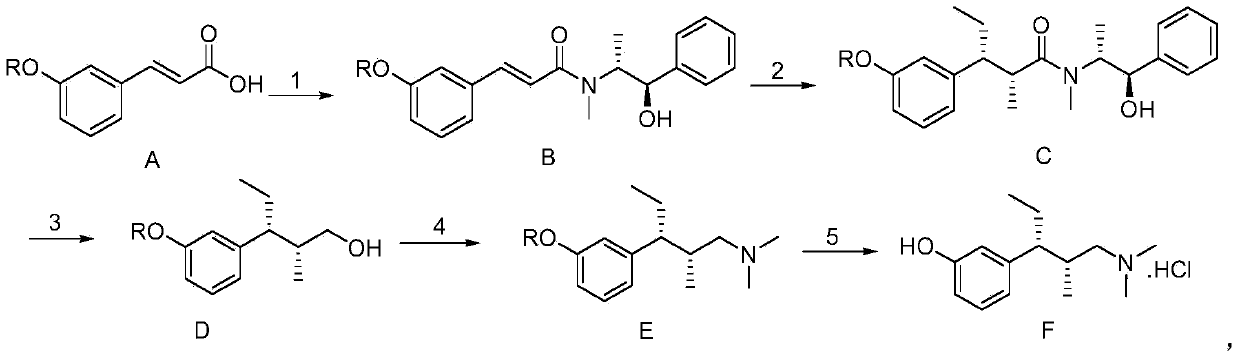
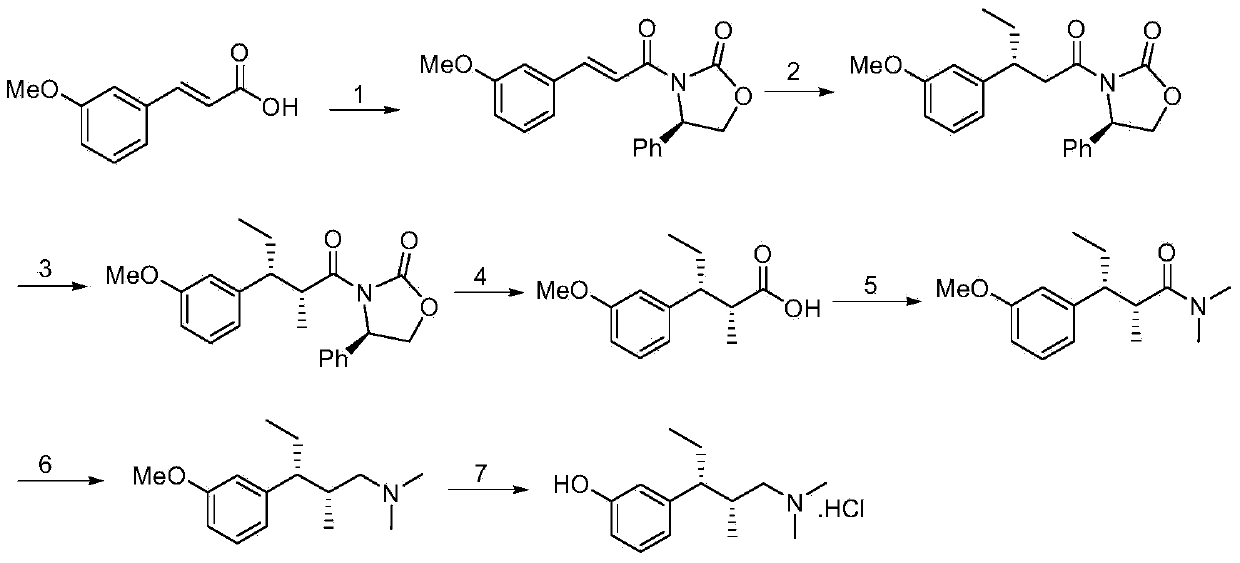
[0038] This embodiment provides a synthesis method of tapentadol, the method comprising the following steps: using meta-substituted cinnamic acid as a starting material, reacting with a chiral prosthetic group to obtain an amide; Reaction, two chiral centers are introduced to obtain a chiral product; the chiral product is reduced and deprotected to obtain a chiral alcohol; the chiral alcohol is subjected to amination reaction and substitution reaction to obtain tapentadol.
[0039] The method includes the following processes:
[0040]
[0041] Specifically, the method includes the following steps:
[0042] Step 1, using m-methoxycinnamic acid (compound A, R=Me, 1 times the amount) as the starting material, dissolved in dichloromethane (10 times the amount), cooled to 0 degrees, and added triethylamine (3 times the amount), slowly drop pivaloyl chloride (1 times the amount). After stirring for 3 hours, (1R,2R)-pseudoephedrine (1 times the amount) was added, and stirred ove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com