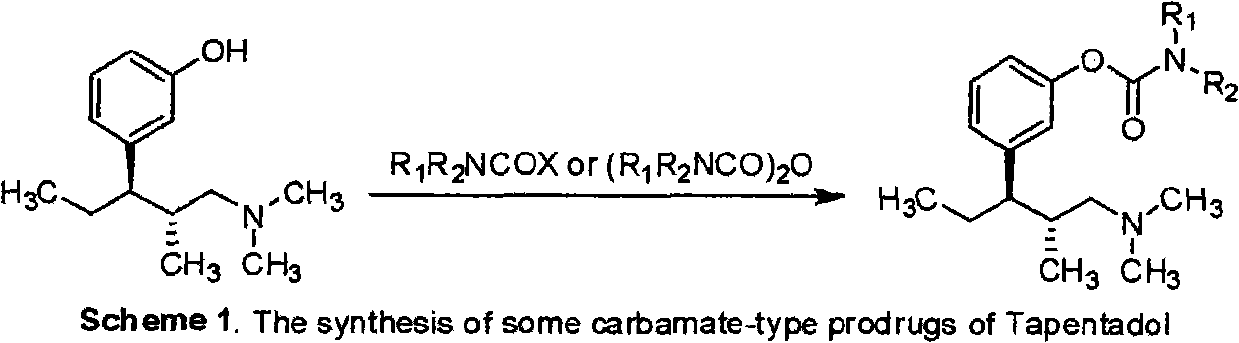
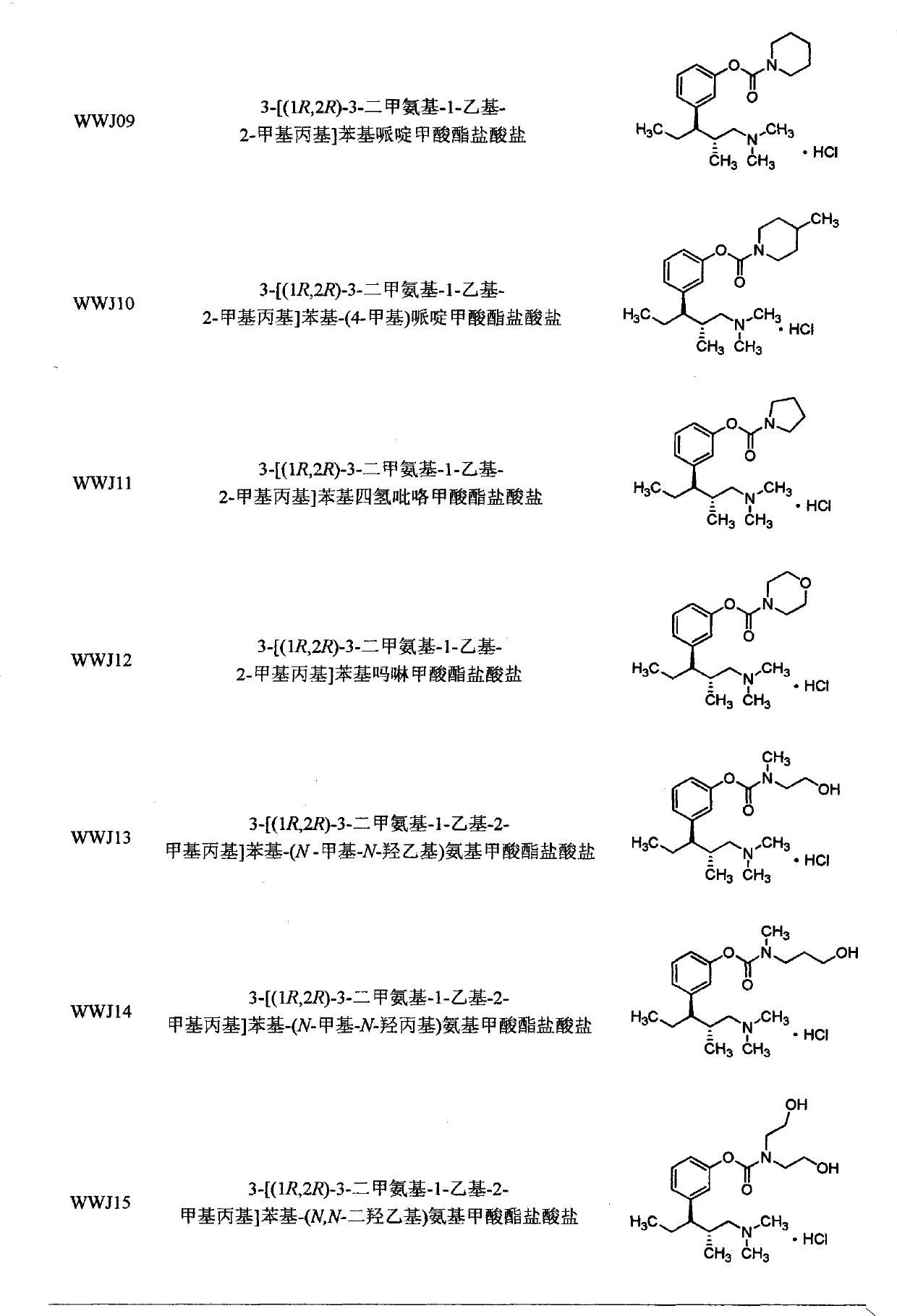
Tapentadol carbamate derivative and preparation method and application thereof
A carbamate, tapentadol technology, applied in the preparation of carbamate derivatives, the preparation of organic compounds, the active ingredients of esters, etc. Stability, effect on improving oral bioavailability
Inactive Publication Date: 2010-09-29
SHENYANG PHARMA UNIVERSITY +1
View PDF8 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
This kind of carrier fragment enters the body and is difficult to metabolize and release the prodrug, and the aromatic amine structure will have unpredictable toxicity, and the druggability is extremely low
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention belongs to the field of pharmacy, in particular to a tapentadol carbamate derivative with a general formula I, pharmaceutically acceptable salts, a preparation method and an application thereof. In the invention, the structure of phenolic hydroxyl groups in tapentadol molecules is improved by a chemical synthesis method, and proper carrier groups are introduced in the position to synthesize carbamate type prodrug. All amines adopted by the carbamate are fatty amines, wherein dimethylamine or methylamine fragments can generate demethylation metabolism easily in vivo, thereby being convenient for releasing raw tapentadol. Meanwhile, the existence of the carbamate structure increases the chemical stability of the prodrug and can obviously improve the oral bioavailability, reduce the administration dosage and lower the toxic side effect, and the tapentadol carbamate derivative can be further developed into novel tapentadol analgesics.
Description
technical field The invention belongs to the field of pharmacy and relates to carbamate derivatives of tapentadol and its preparation and application. Background technique Tapentadol HCl is a new type of centrally acting analgesic drug developed by Johnson & Johnson Company of the United States with a dual mechanism of action. It is used as a single isomer of (1R, 2R), molecular formula C 14 h 24 ClNO, chemical name (1R, 2R)-3-(3-dimethylamino-1-ethyl-2-methylpropyl)-phenol Hydrochloride. Tapentadol belongs to 1-phenyl-3-dimethylaminopropane compounds, which have various pharmacological activities and can be used to relieve pain (EP693475), and can also be used to treat psychosis (DE102007012165), depression (DE10233048 ), urinary incontinence (WO2002043715), etc. Tapentadol was approved for marketing by the U.S. Food and Drug Administration (FDA) on November 21, 2008. It has been clinically shown that it has good efficacy between morphine and tramadol, and can achieve...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07C271/44C07C271/48C07C271/00C07D295/205C07D211/16C07C269/00A61K31/27A61K31/4453A61K31/40A61K31/445A61K31/5375A61P29/00
Inventor 许佑君王文娟傅鼎鼎常燕南何仲贵
Owner SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com