Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
868results about How to "Improve oral bioavailability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Milled particles
InactiveUS6634576B2Increase incorporationOptimal for incorporationPowder deliveryInorganic non-active ingredientsParticulatesPolymer science
A process for milling a solid substrate in the milling chamber of a dispersion or media mill in the presence of a two or more compositions of milling media bodies is disclosed wherein all milling media bodies contribute to the grinding of the solid substrate and wherein at least one composition of media bodies provides fragments of milling media bodies that are retained with the milled solid substrate particles in the form of a synergetic commixture produced in the milling process. More specifically, a process is disclosed for preparing a synergetic commixture comprising small particles of a solid substrate and small particulates of a first material of a desired size comprising the steps of (a) providing to the milling chamber of a media mill a contents comprising a pre-mix of a solid substrate, a fluid carrier, a plurality of milling bodies of a first material having a fracture toughness Kc1, and a plurality of milling bodies of a second material having a fracture toughness Kc2; (b) operating the media mill to grind the solid substrate and degrade at least a portion of the milling bodies of first material to produce a dispersion in the fluid carrier comprising a synergetic commixture of small particulates of the first material and small particles of the solid substrate having a desired size equal to or less than a size Sp; (c) separating the dispersion from any milling bodies and solid substrate particles having a size larger than Sp; and (d) optionally removing the fluid carrier from the dispersion to form a synergetic commixture free of fluid and comprising the particles and the small particulates, wherein KC2 is greater than KC1.
Owner:RTP PHARMA +1
Integrase inhibitor compounds
InactiveUS20070072831A1Increasing cellular accumulationImprove bioavailabilityBiocideAntiviralsBiochemistryIntegrase inhibitor
Owner:GILEAD SCI INC
Novel nanoemulsion formulations
InactiveUS20070148194A1Reducing surfactant side-effectsImprove oral bioavailabilityDispersion deliveryEmulsion deliveryBuffering agentAntioxidant
An oil-in-water nanoemulsion delivery system that includes at least one oil having a concentration of greater than or equal to 2% (w / w) of at least one polyunsaturated fatty acid, preferably of the omega-3 or omega-6 family, is disclosed. The delivery system further includes at least one emulsifier and also an aqueous phase. Preferably, one or more hydrophobic therapeutic, monitoring and / or diagnostic agents are dispersed in the oil phase. The nanoemulsions may optionally contain other conventional pharmaceutical aids such as stabilizers, preservatives, buffering agents, antioxidants, polymers, proteins and charge inducing agents. The invention also relates to a process for preparing the nanoemulsions and to their use in the oral, parenteral, opthalmic, nasal, rectal or topical delivery of hydrophobic therapeutic, monitoring or diagnostic agents.
Owner:NORTHEASTERN UNIV +1
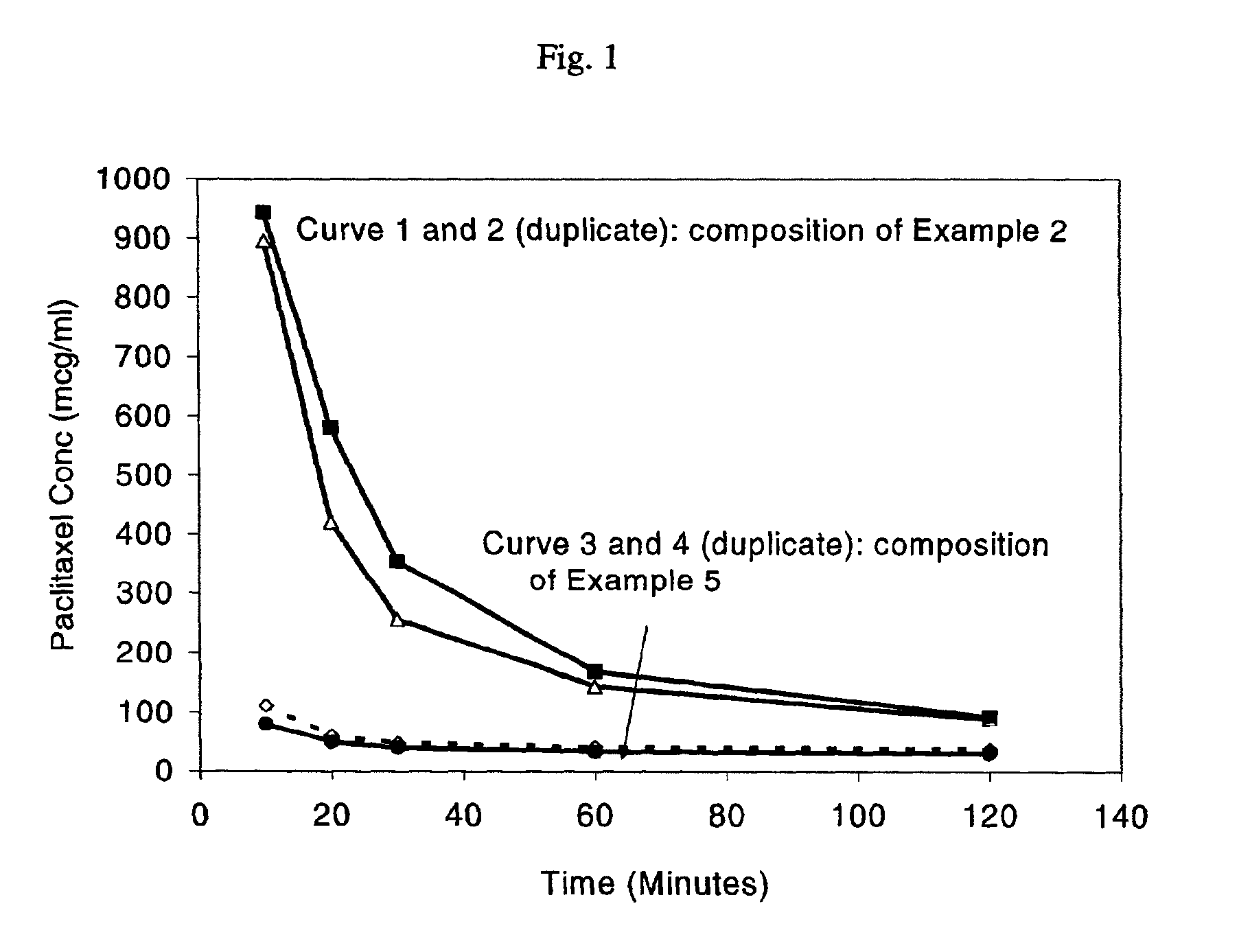
Oral pharmaceuticals formulation comprising paclitaxel, derivatives and methods of administration thereof
InactiveUS20040092428A1Improve bioavailabilityImprove oral bioavailabilityOrganic active ingredientsCyclic peptide ingredientsOral medicationBioavailability
The invention concerns excipients or combinations thereof suitable for preparing an oral formulation containing a pharmaceutical agent. More particularly, the invention is directed to stable, efficacious and bioavailable oral pharmaceutical formulations comprising paclitaxel, derivatives of paclitaxel and pharmaceutically acceptable salts thereof. The formulations of the invention increase bioavailability of paclitaxel when dissolved in the gastrointestinal system. The formulations of the invention are useful for administering paclitaxel, its derivatives, or pharmaceutically acceptable salts of such derivatives to patients in need thereof. The formulations of the invention are particularly suitable for oral administration to mammals including humans.
Owner:TRANSFORM PHARMACEUTICALS INC
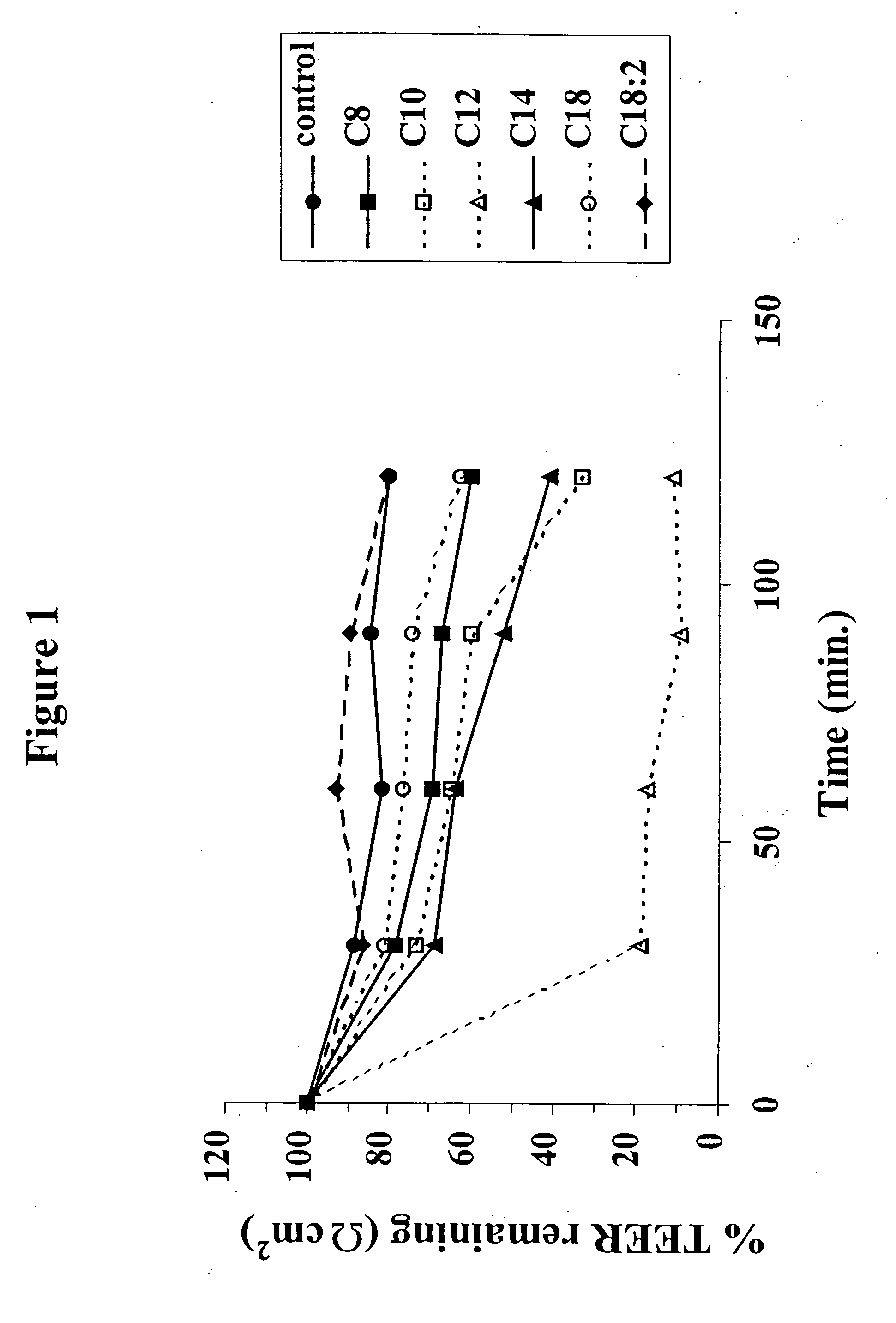
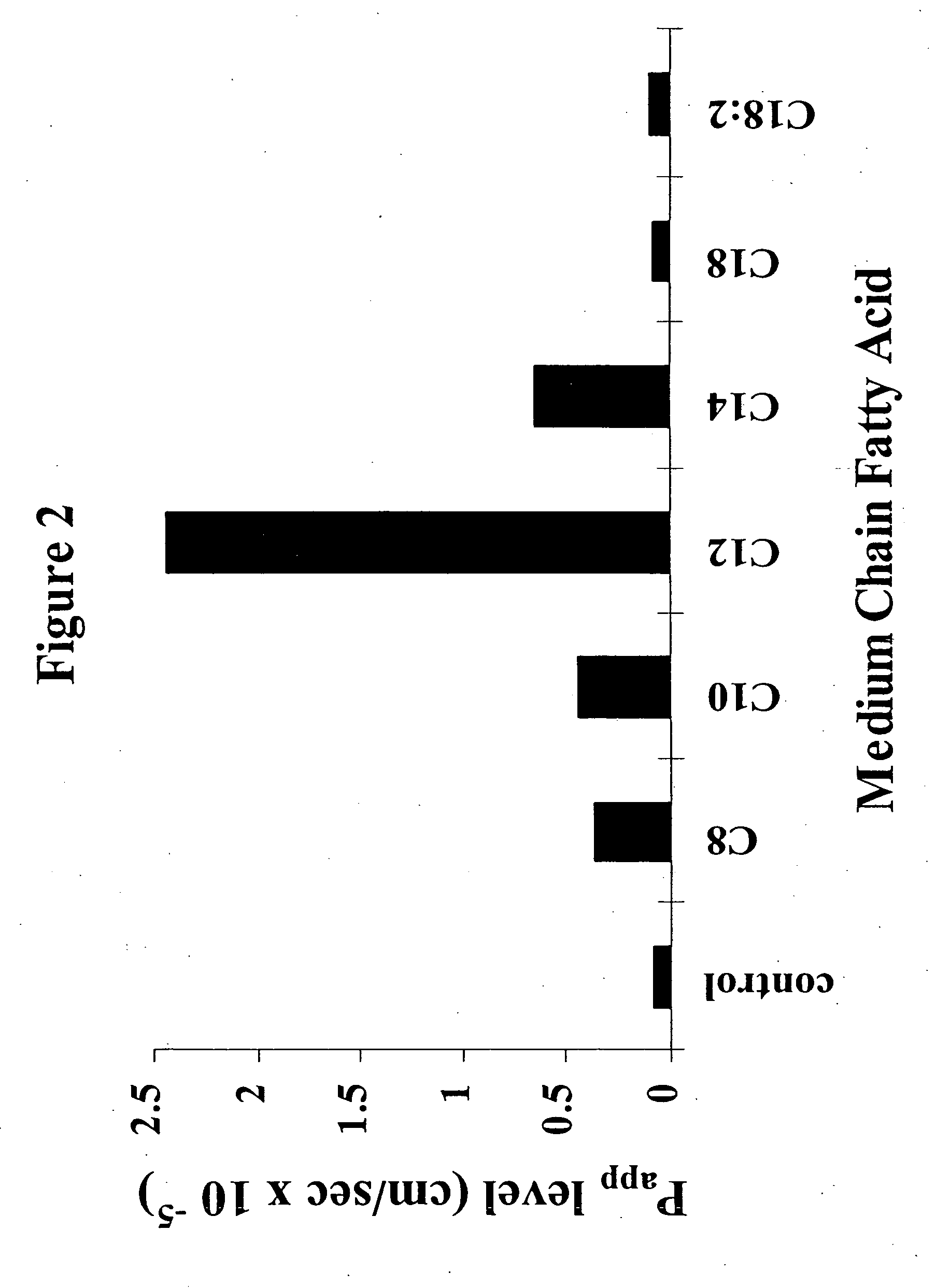
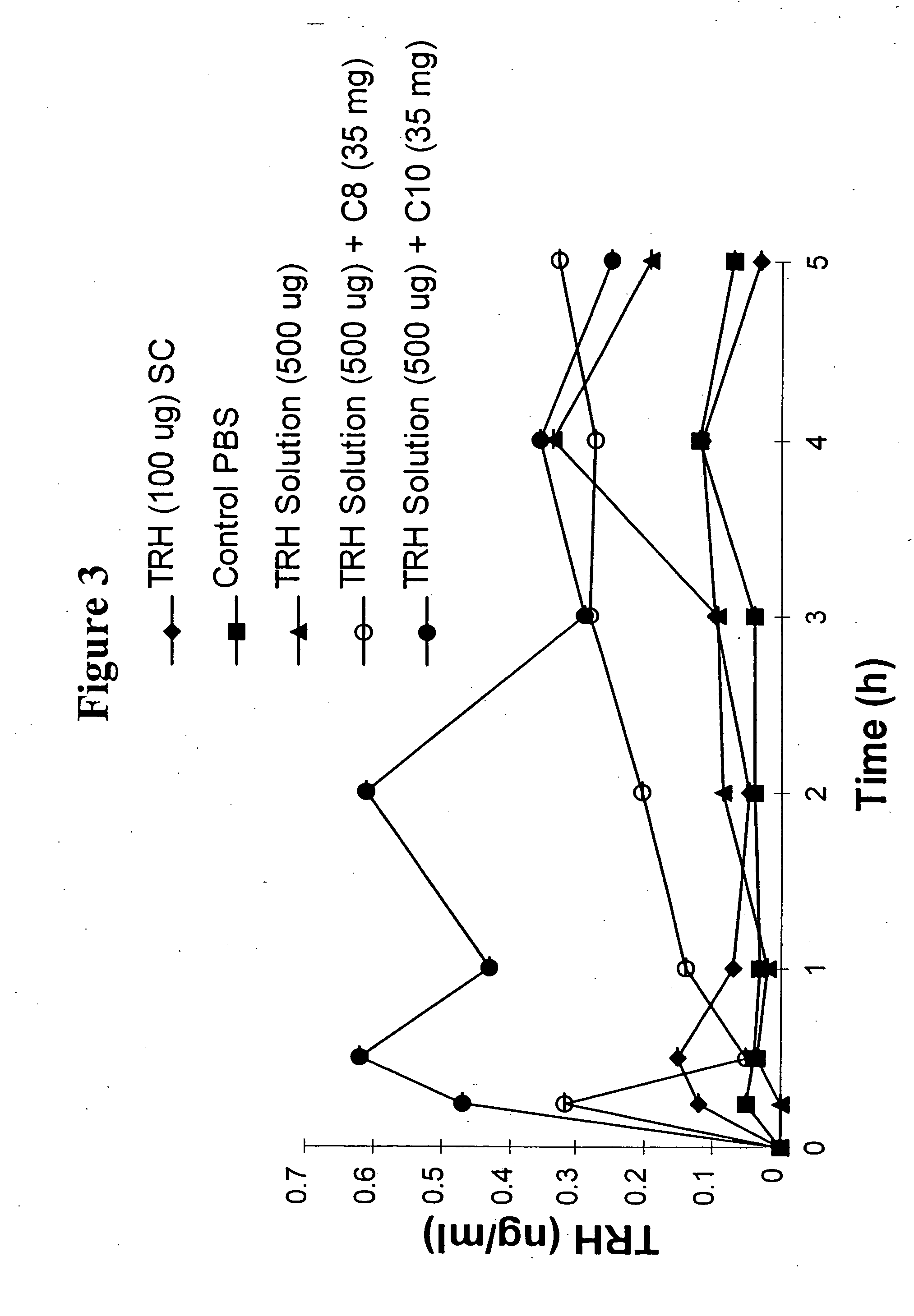
Solid oral dosage form containing an enhancer
InactiveUS8119159B2Minimizes risk of local irritationImprove oral bioavailabilityBiocidePhosphorous compound active ingredientsDelayed Release Dosage FormCarbon chain
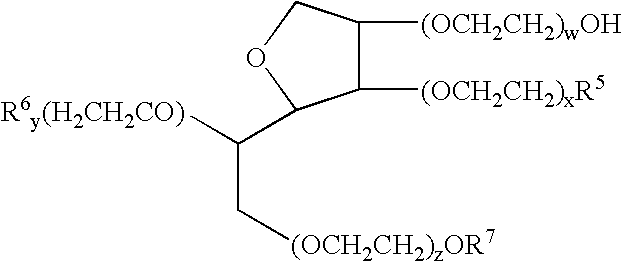
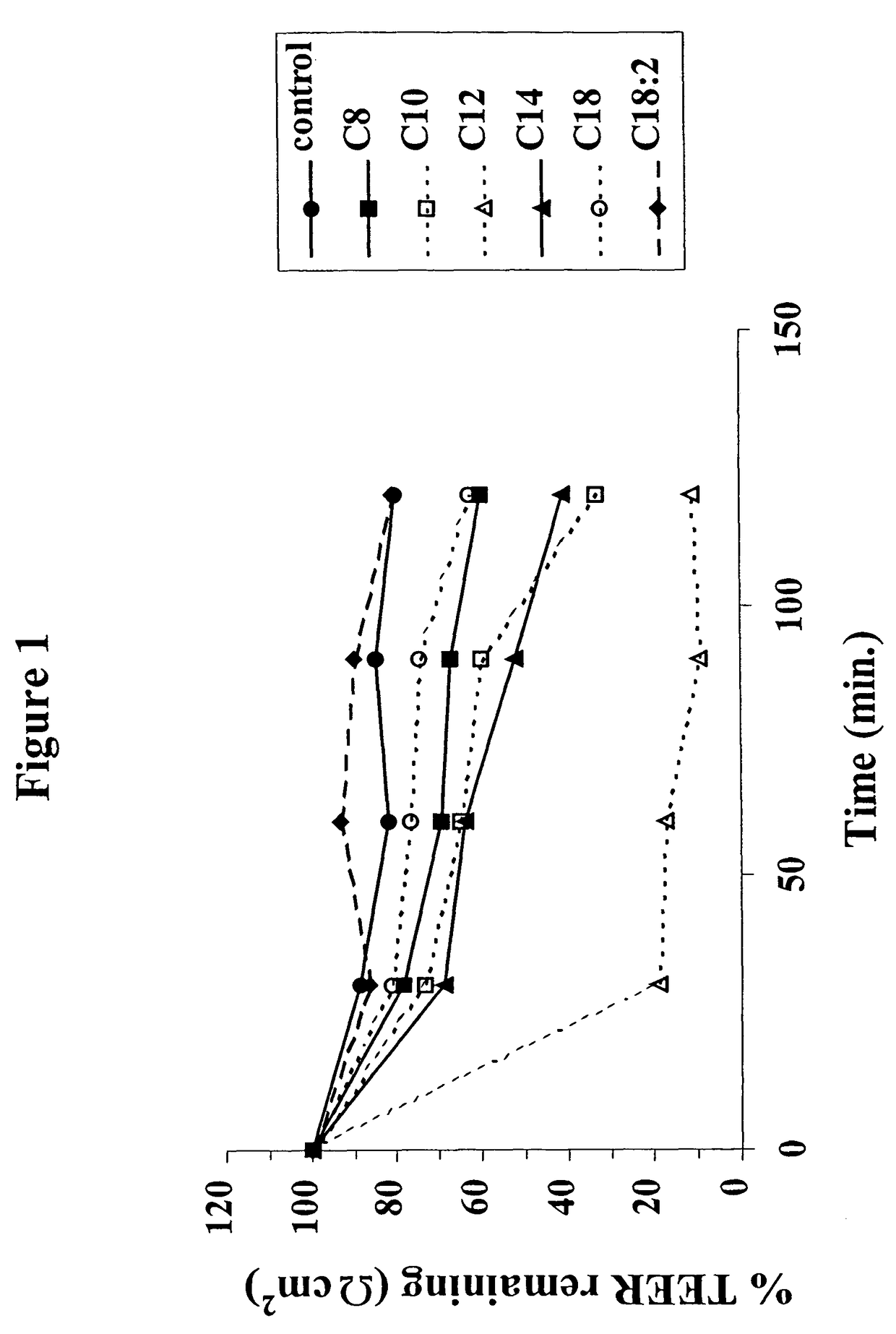
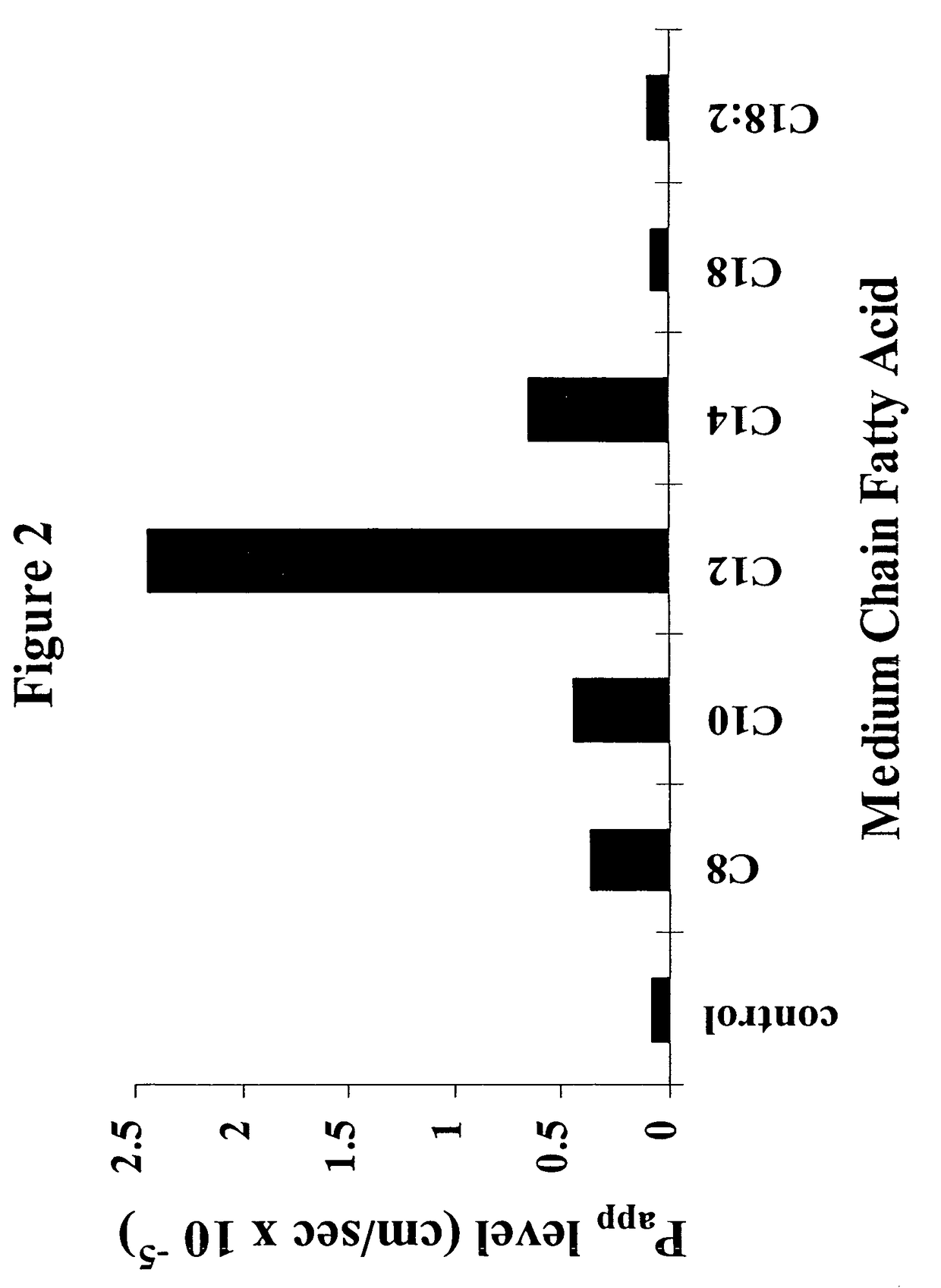
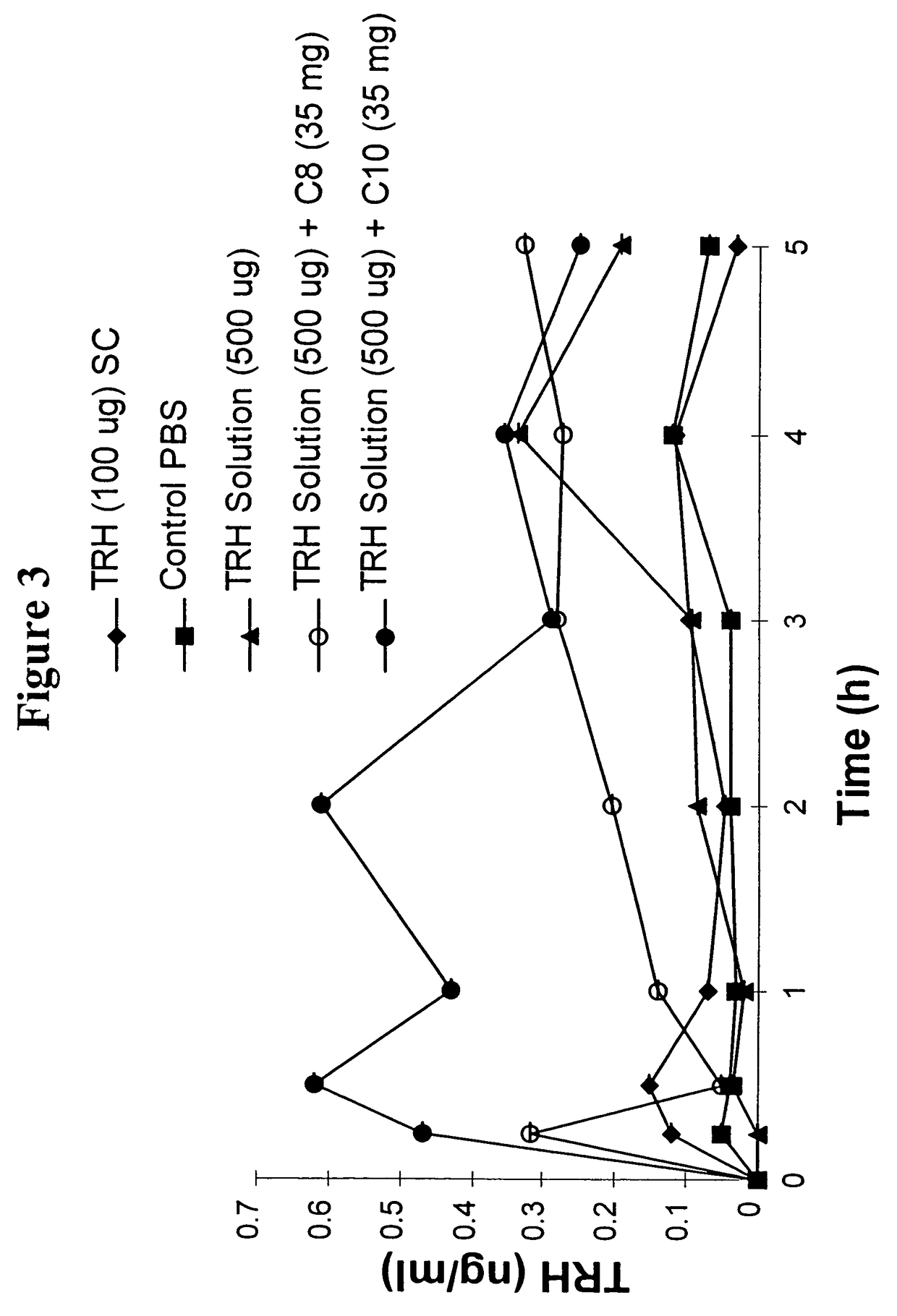
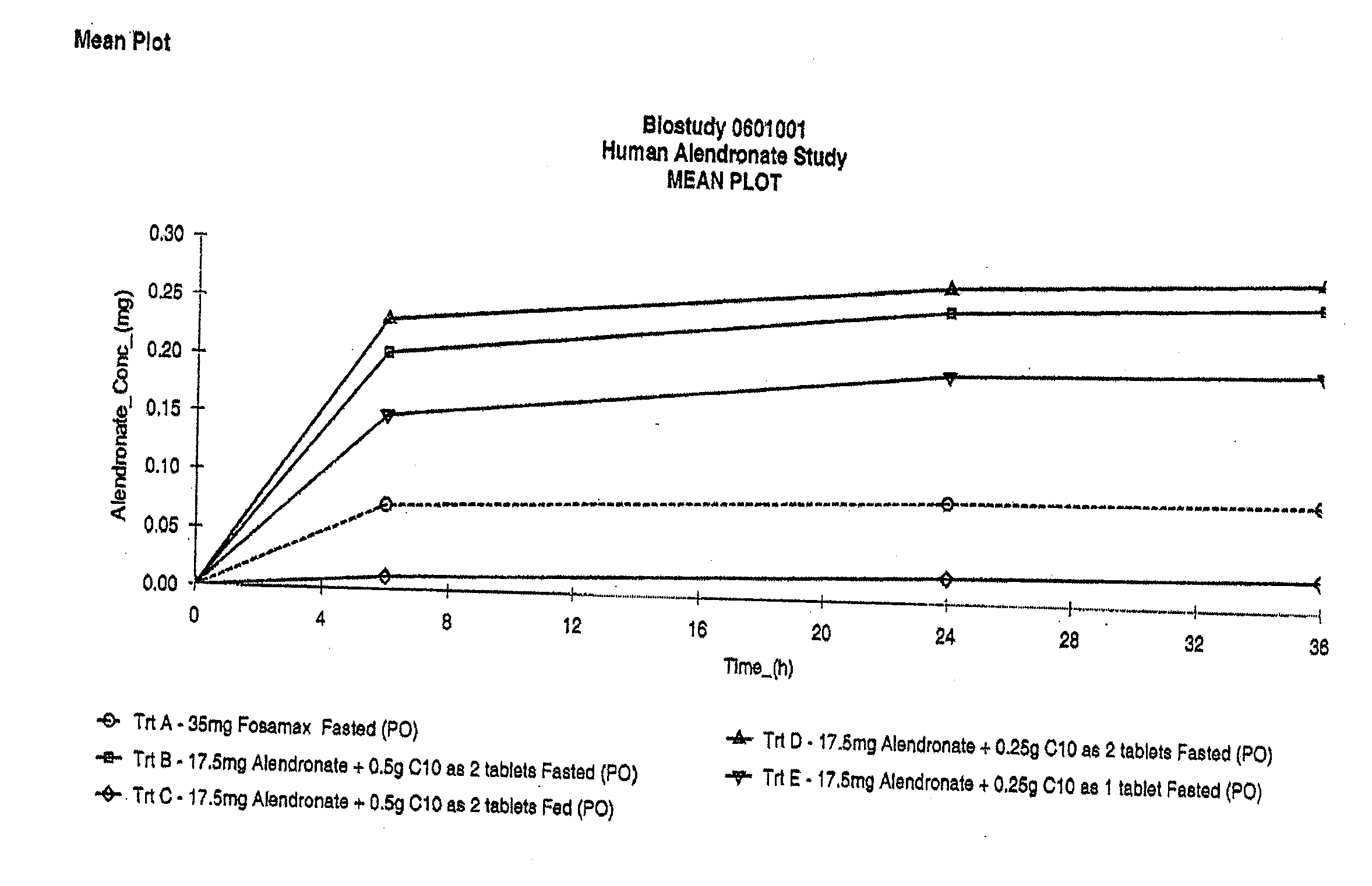
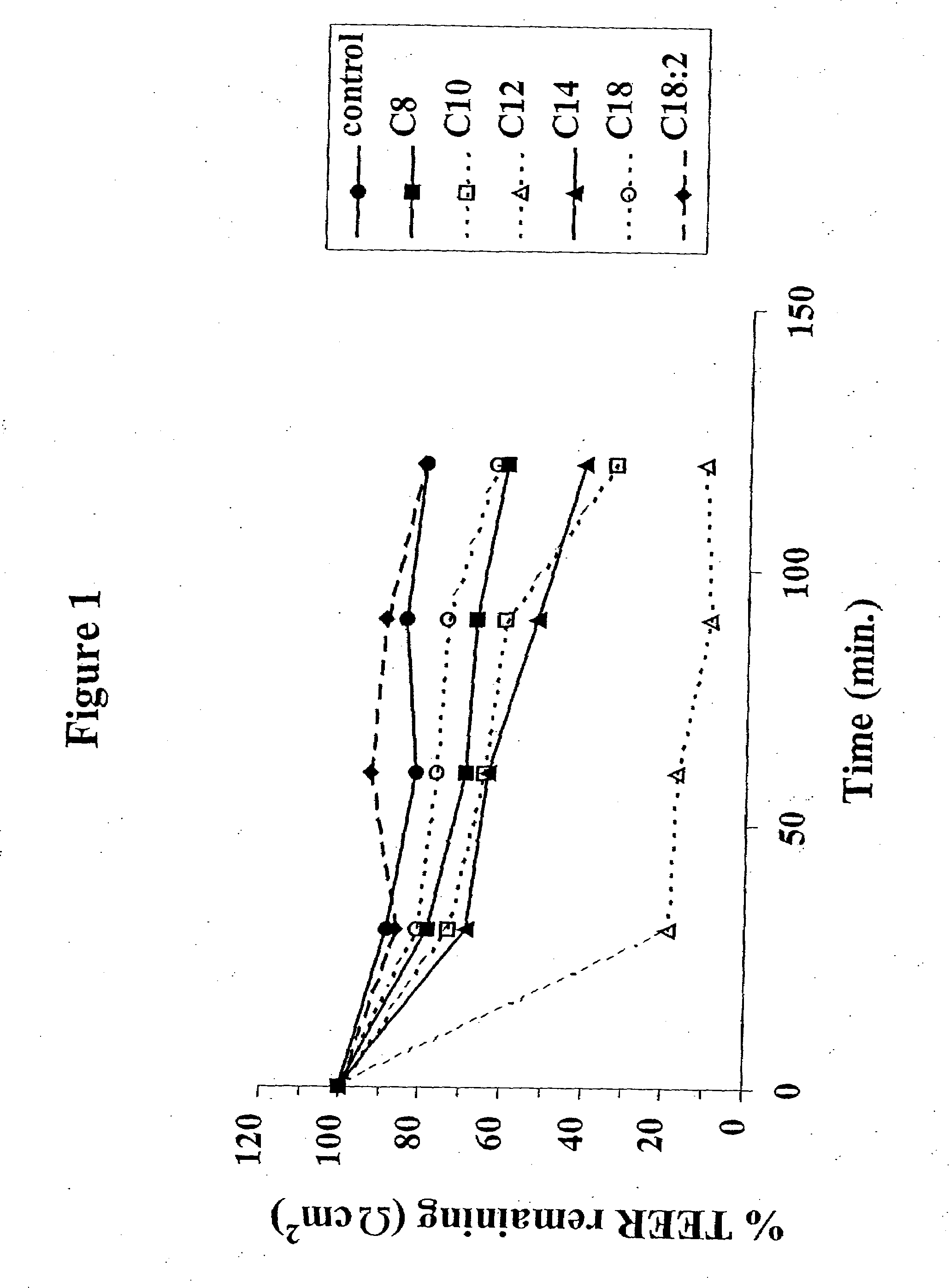
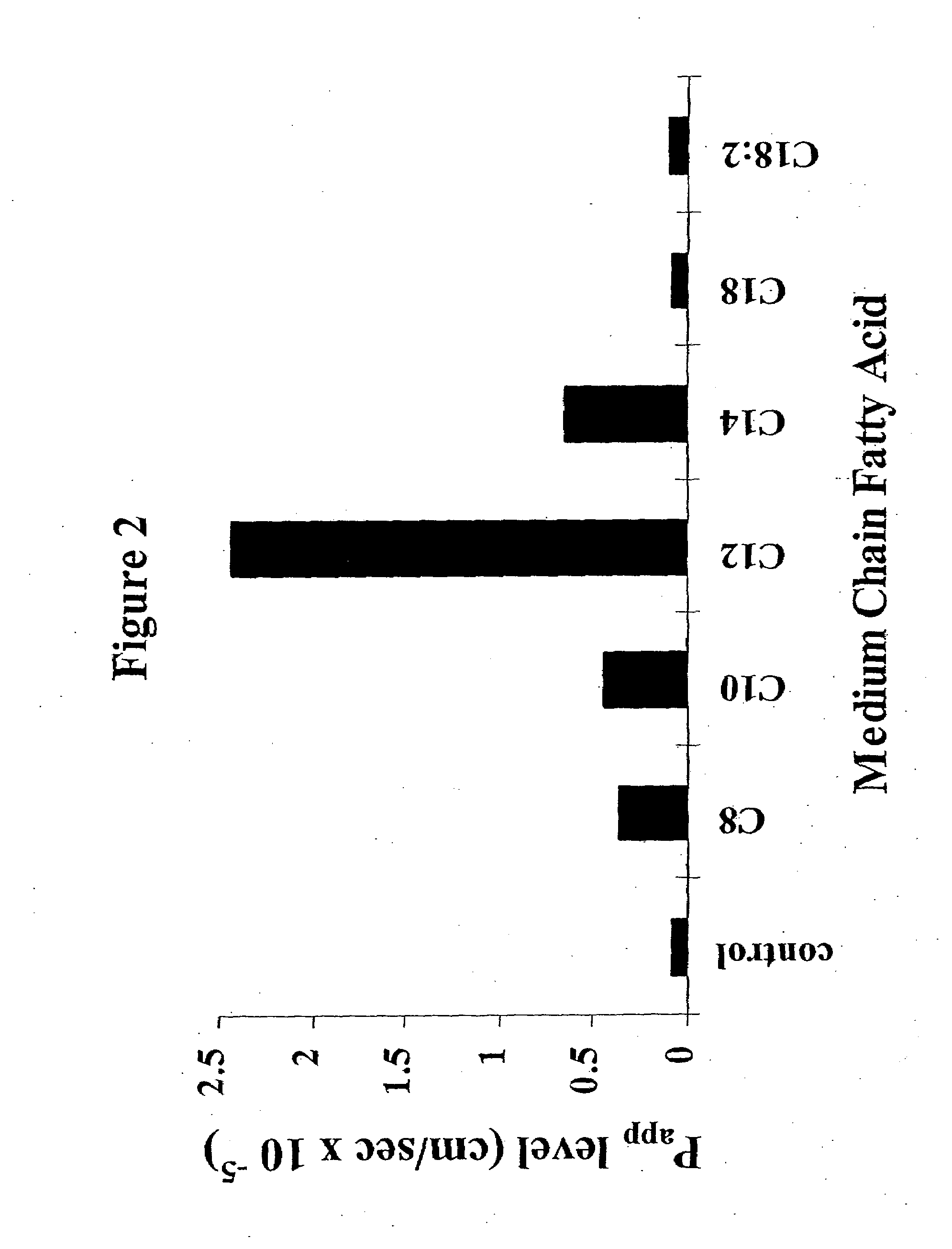
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to promote absorption of the bisphosphonate at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Solid Oral Dosage Form Containing an Enhancer
InactiveUS20070238707A1Improve oral bioavailabilityMinimizes risk of local irritationBiocideAntipyreticDelayed Release Dosage FormDiphosphonates
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to enhance intestinal delivery of the bisphosphonate to the underlying circulation. Preferably, the enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms, and the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Antiviral compounds
InactiveUS20070078081A1Improve bioavailabilityEffective treatmentBiocideDipeptide ingredientsStereochemistryMedicinal chemistry
Owner:GILEAD SCI INC
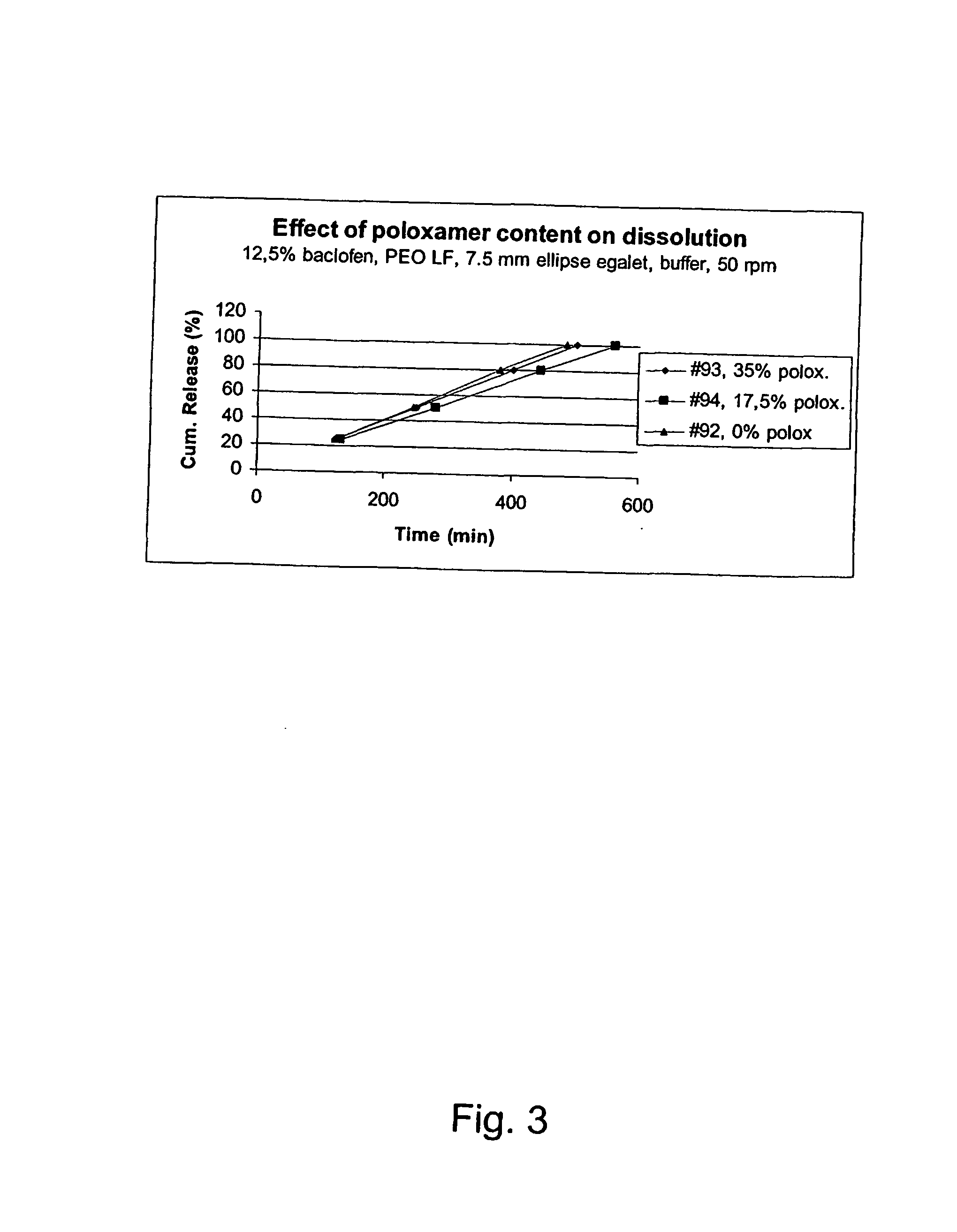
Matrix compositions for controlled delivery of drug substances
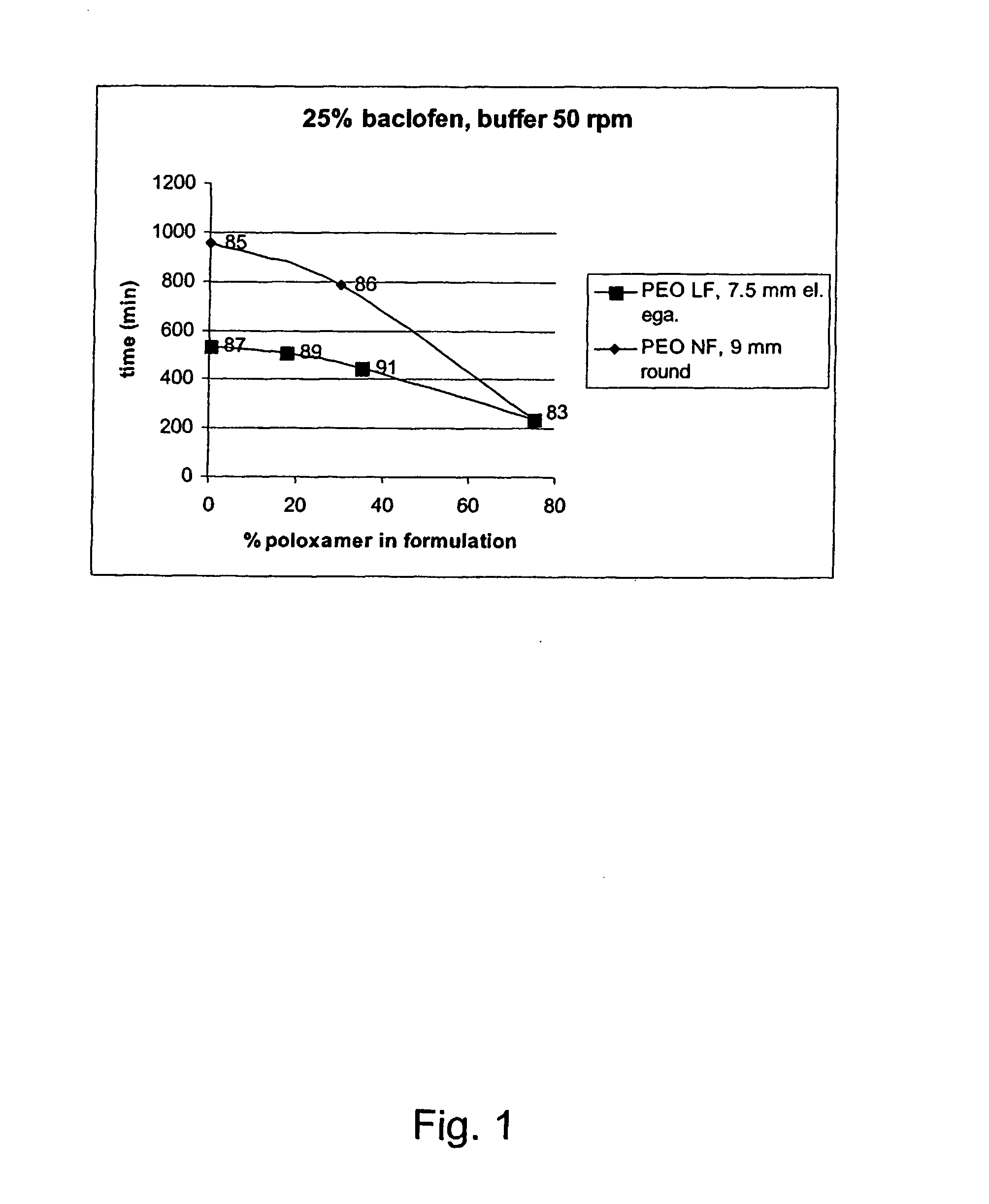
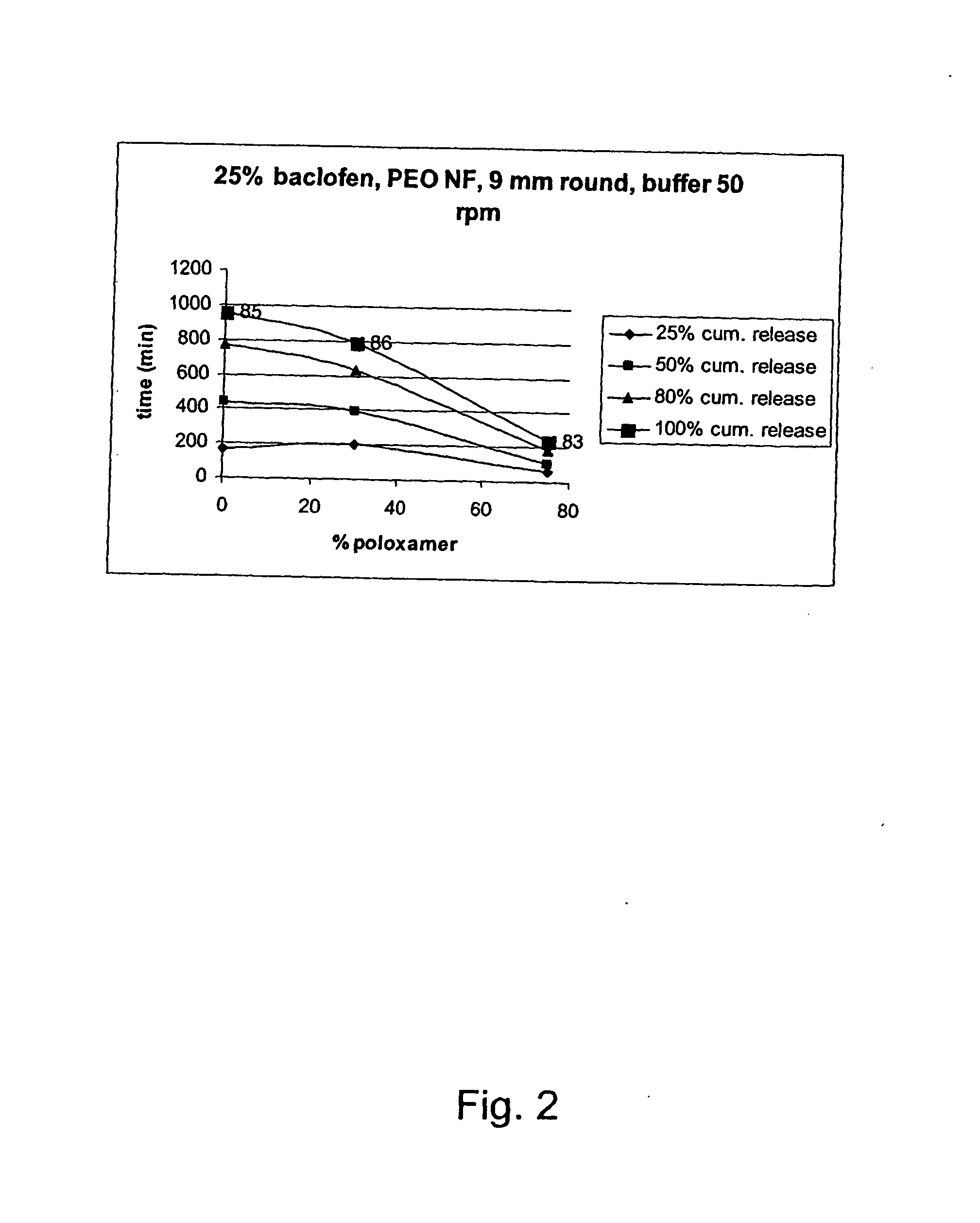
InactiveUS20070042044A1Improve solubilityImprove oral bioavailabilityBiocidePowder deliveryPolyethylene oxidePEG-PLGA-PEG
A novel matrix composition for pharmaceutical use. The matrix composition has been designed so that it is especially suitable in those situation where an improved bioavailability is desired and / or in those situation where a slightly or insoluble active substance is employed. Accordingly, a controlled release pharmaceutical composition for oral use is provided in the form of a coated matrix composition, the matrix composition comprising i) a mixture of a first and a second polymer that have plasticizing properties and which have melting points or melting intervals of a temperature of at the most 200° C., the first polymer being selected from the group consisting of polyethylene glycols and polyethylene oxides, and the second polymer being selected form block copolymer of ethylene oxide and propylene oxide including poly(ethylene-glycol-b-(DL-lactic acid-co-glycolic acid)-b-ethylene glycol (PEG-PLGA PEG), poly((DL-lactic acid-co-glycolic acid)-g-ethylene glycol) (PLGA-g-PEG), poloxamers and polyethylene oxide-polypropylene oxide (PEO-PPO), ii) a therapeutically, prophylactically and / or diagnostically active substance, the matrix composition being provided with a coating having at least one opening exposing at one surface of said matrix, wherein the active substance is released with a substantially zero order release.
Owner:EGALET LTD
Antiviral compounds
InactiveUS8178491B2Inhibitory and pharmacokinetic propertyImprove oral bioavailabilityBiocideDigestive systemCombinatorial chemistryAnti virals
Owner:GILEAD SCI INC
Compositions and dosage forms for gastric delivery of irinotecan and methods of treatment that use it to inhibit cancer cell proliferation
InactiveUS6881420B2Improve oral bioavailabilityReduced bioavailabilityBiocideCapsule deliveryWhole bodyCancer cell proliferation
The present invention provides oral dosage forms and compositions for administering antineoplastic agents, such as irinotecan, etoposide, paclitaxel, doxorubicin and vincristine, whose oral effectiveness is limited by pre-systemic and systemic deactivation in the GI tract. Gelling of the gastric retention vehicle composition, and in the case of solid forms concomitant expansion of the composition, retains the antineoplastic drug in the patient's stomach, minimizing pre-systemic and / or systemic deactivation of the drug.
Owner:TEVA PHARM USA INC
Formulation for oral administration of apoptosis promoter
InactiveUS20100297194A1Improve oral bioavailabilityConvenient route of administrationOrganic active ingredientsBiocideDiseaseAntioxidant
An orally deliverable pharmaceutical composition comprises as a sole or first active ingredient a compound of Formula I defined herein or a pharmaceutically acceptable salt thereof, for example ABT-263 free base or ABT-263 bis-HCl salt, dispersed, in a free base equivalent amount of at least about 2.5% by weight of the composition, in a pharmaceutically acceptable carrier; wherein said active ingredient is in solid-state form and / or the composition further comprises, dispersed in the carrier, a pharmaceutically acceptable heavier-chalcogen antioxidant in an amount effective to inhibit oxidation of the active ingredient at a thioether linkage thereof. The composition is suitable for oral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBOTT LAB INC +1
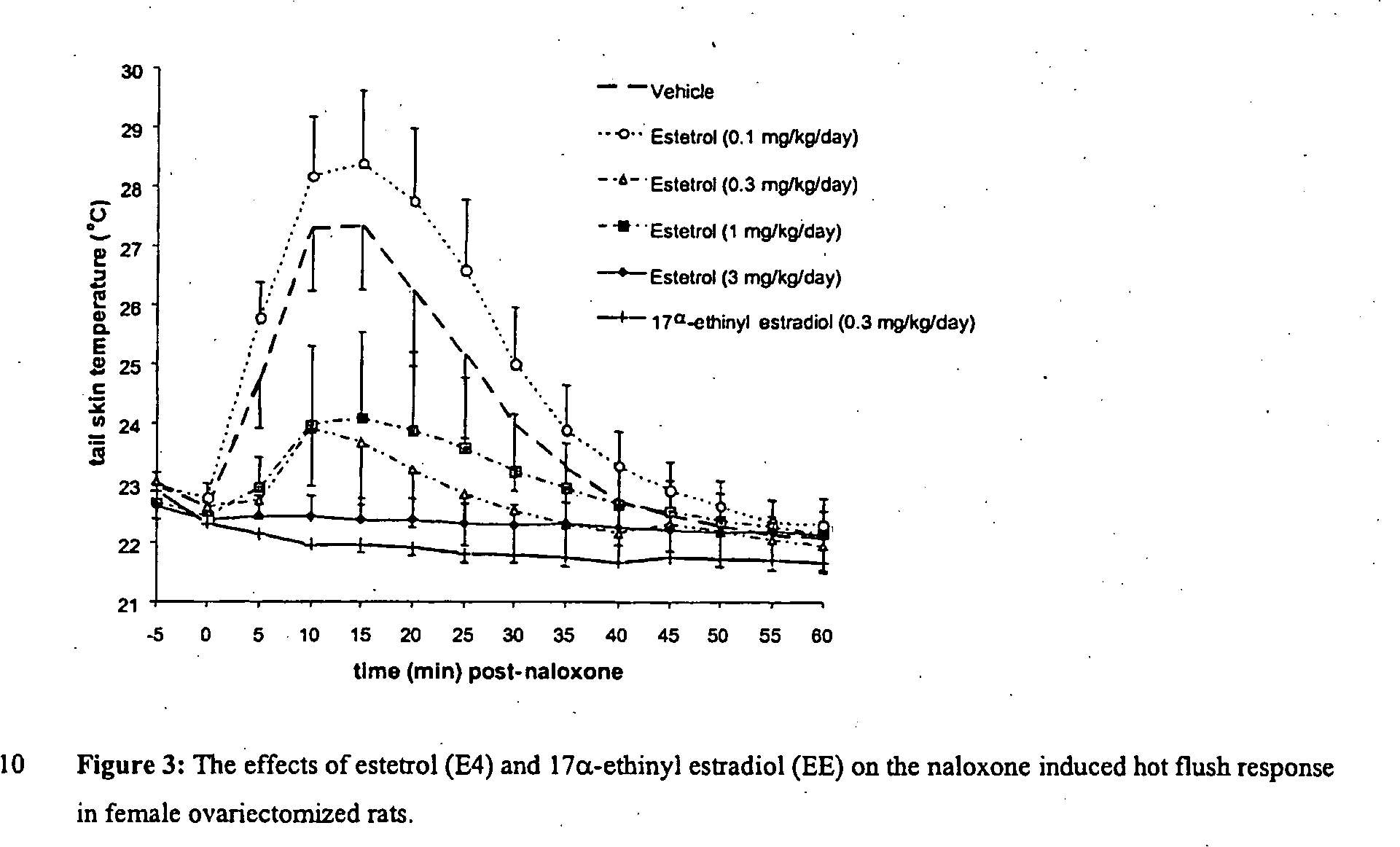
Estrogenic compounds in combination with progestogenic compounds in hormone-replacement therapy
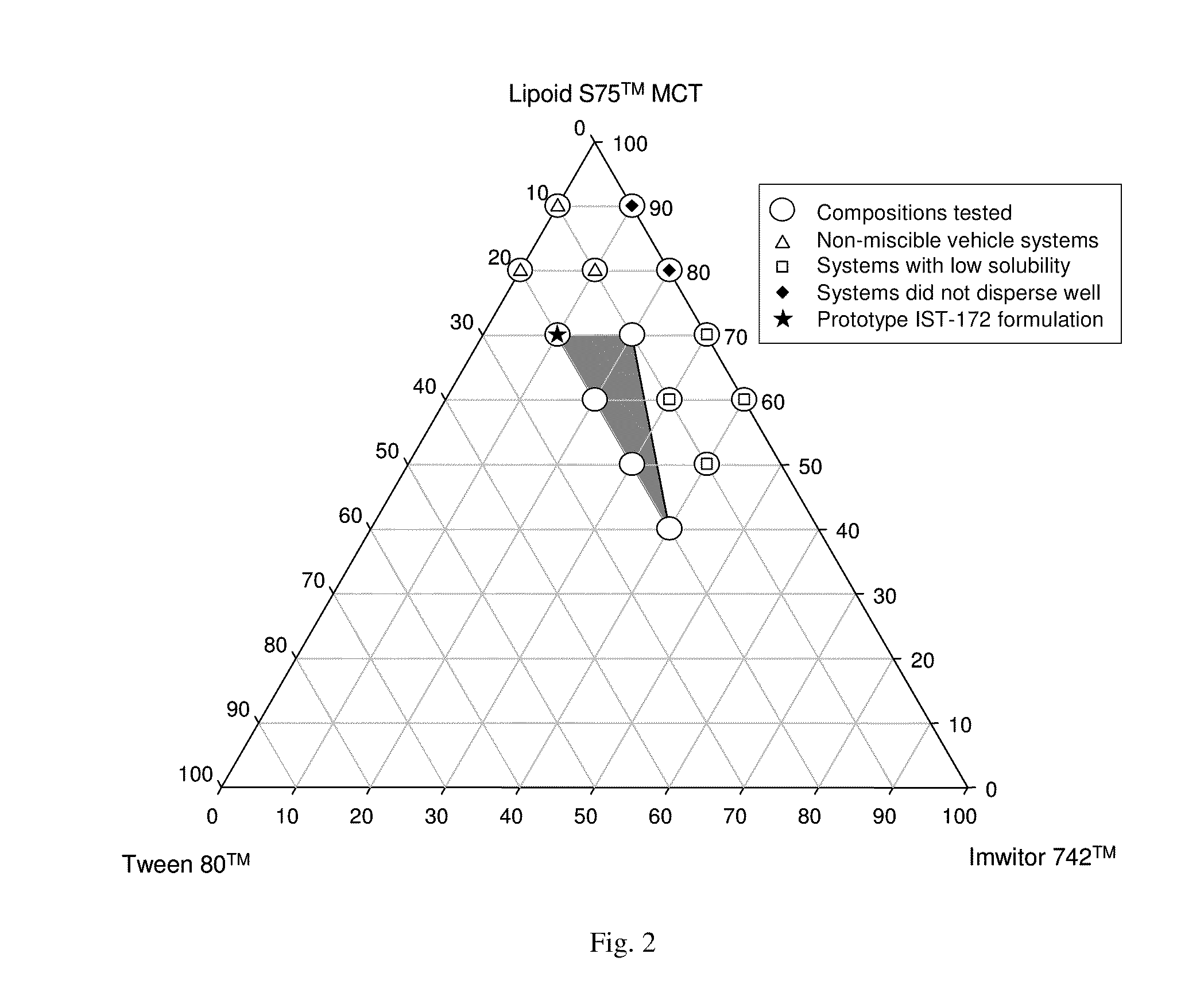
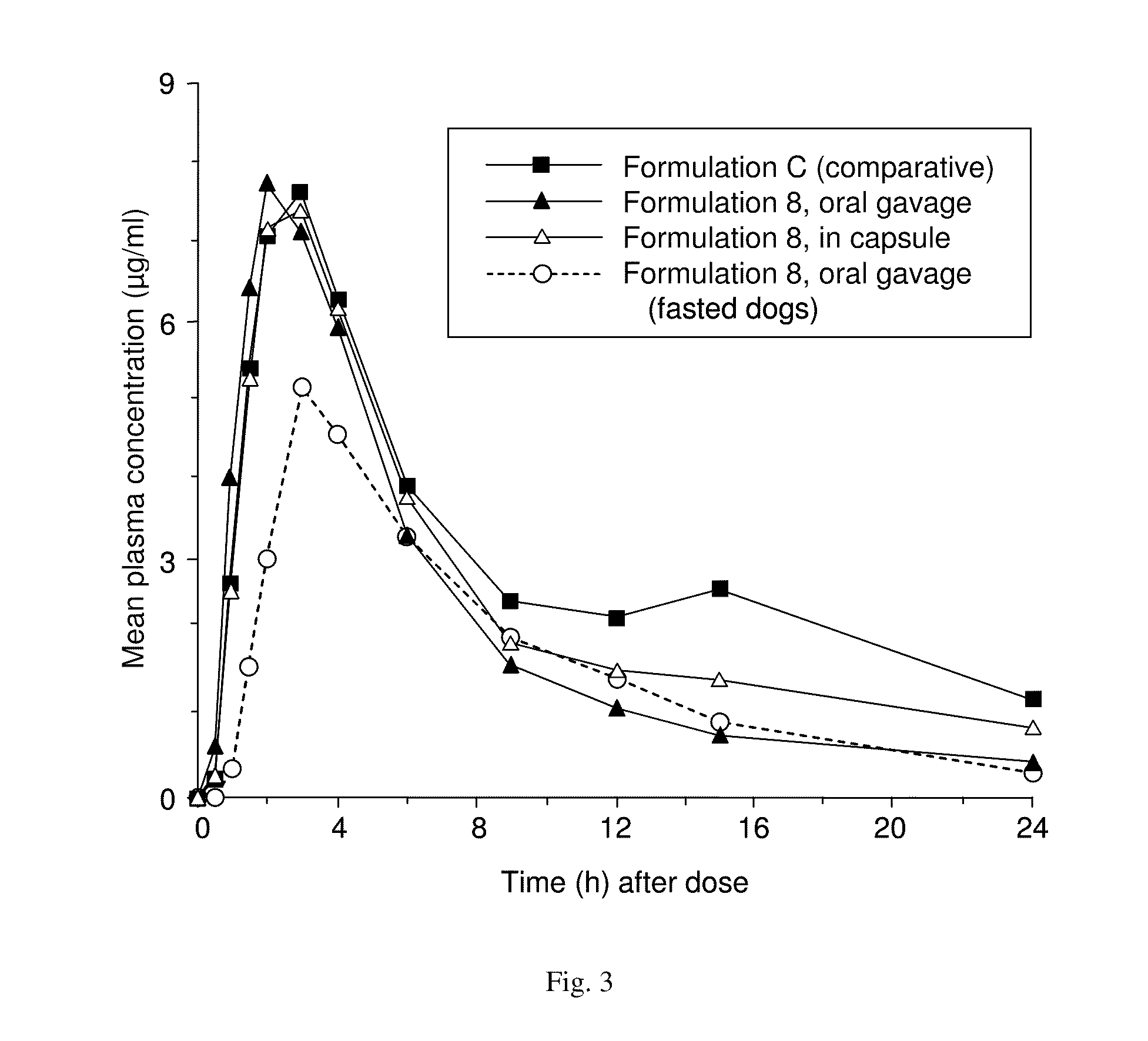
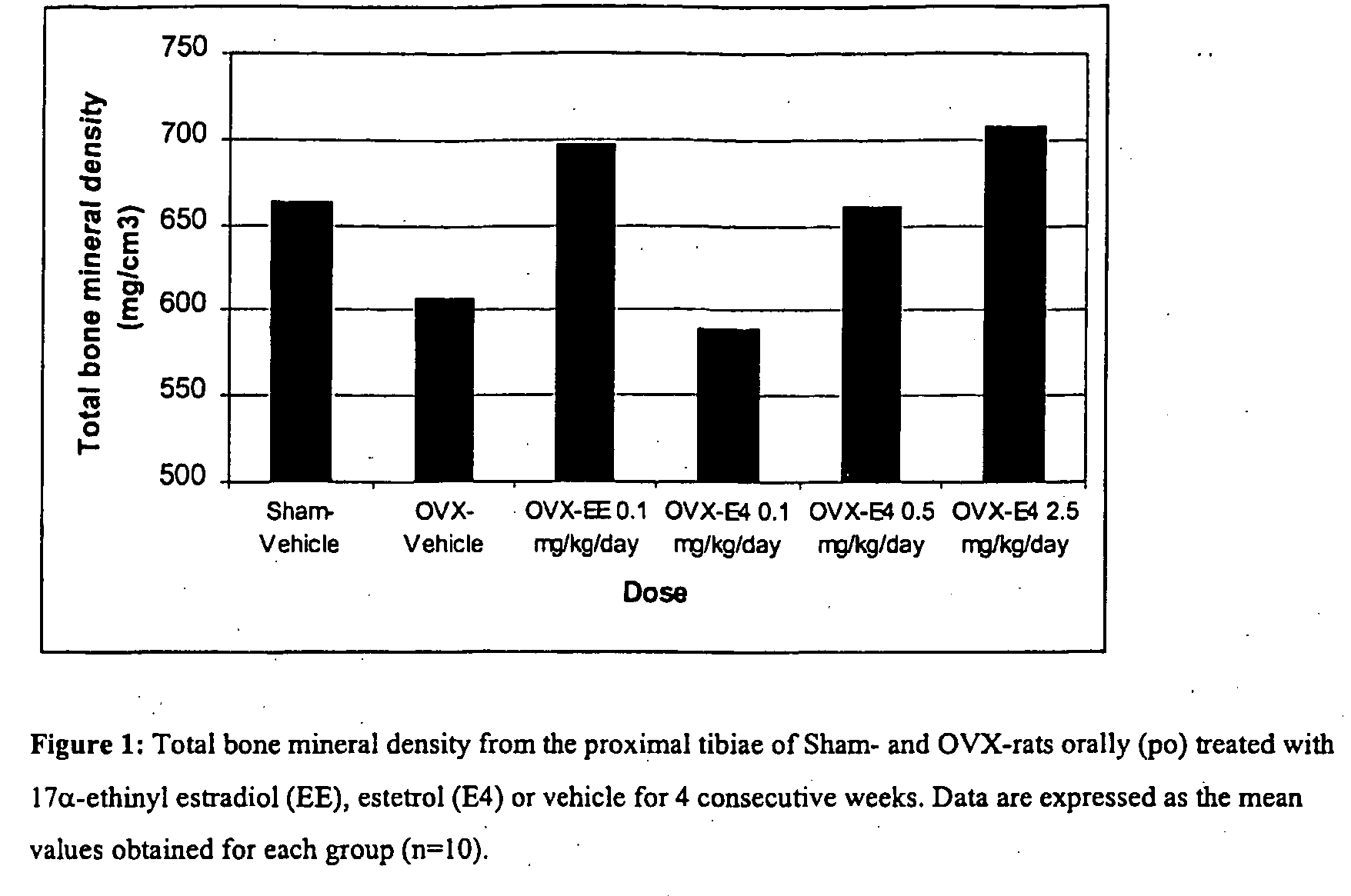
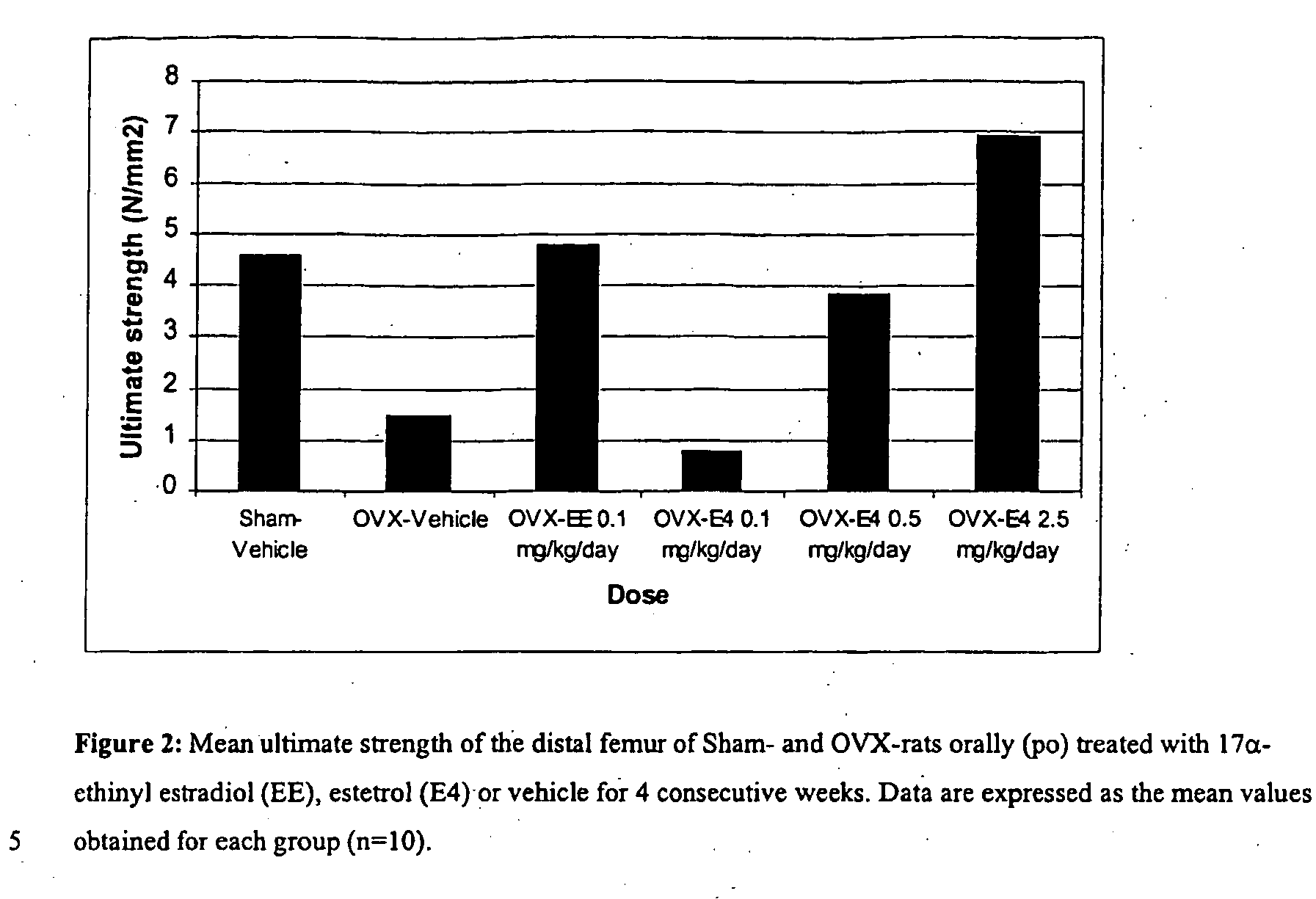
ActiveUS20050070488A1Reliable efficacyImprove oral bioavailabilityBiocideOrganic active ingredientsProgestinCompound (substance)
One aspect of the present invention relates to a method of hormone replacement in mammals, which method comprises the oral administration of an estrogenic component and a progestogenic component to a mammal in an effective amount to prevent or treat symptoms of hypoestrogenism, wherein the estrogenic component is selected from the group consisting of substances represented by the above formula in which formula R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1-5 carbon atoms; each of R5, R6, R7 is a hydroxyl group; and no more than 3 of R1, R2, R3, R4 are hydrogen atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method; and mixtures of one or more of the aforementioned substances and / or precursors. Another aspect of the invention concerns a pharmaceutical kit comprising oral dosage units that contain the aforementioned estrogenic component and a progestogenic component as well as an androgenic component.
Owner:ESTETRA SRL
Solid oral dosage form containing an enhancer
InactiveUS20070196464A1Improve oral bioavailabilityMinimizes risk of local irritationBiocidePhosphorous compound active ingredientsDelayed Release Dosage FormCarbon chain
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to promote absorption of the bisphosphonate at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
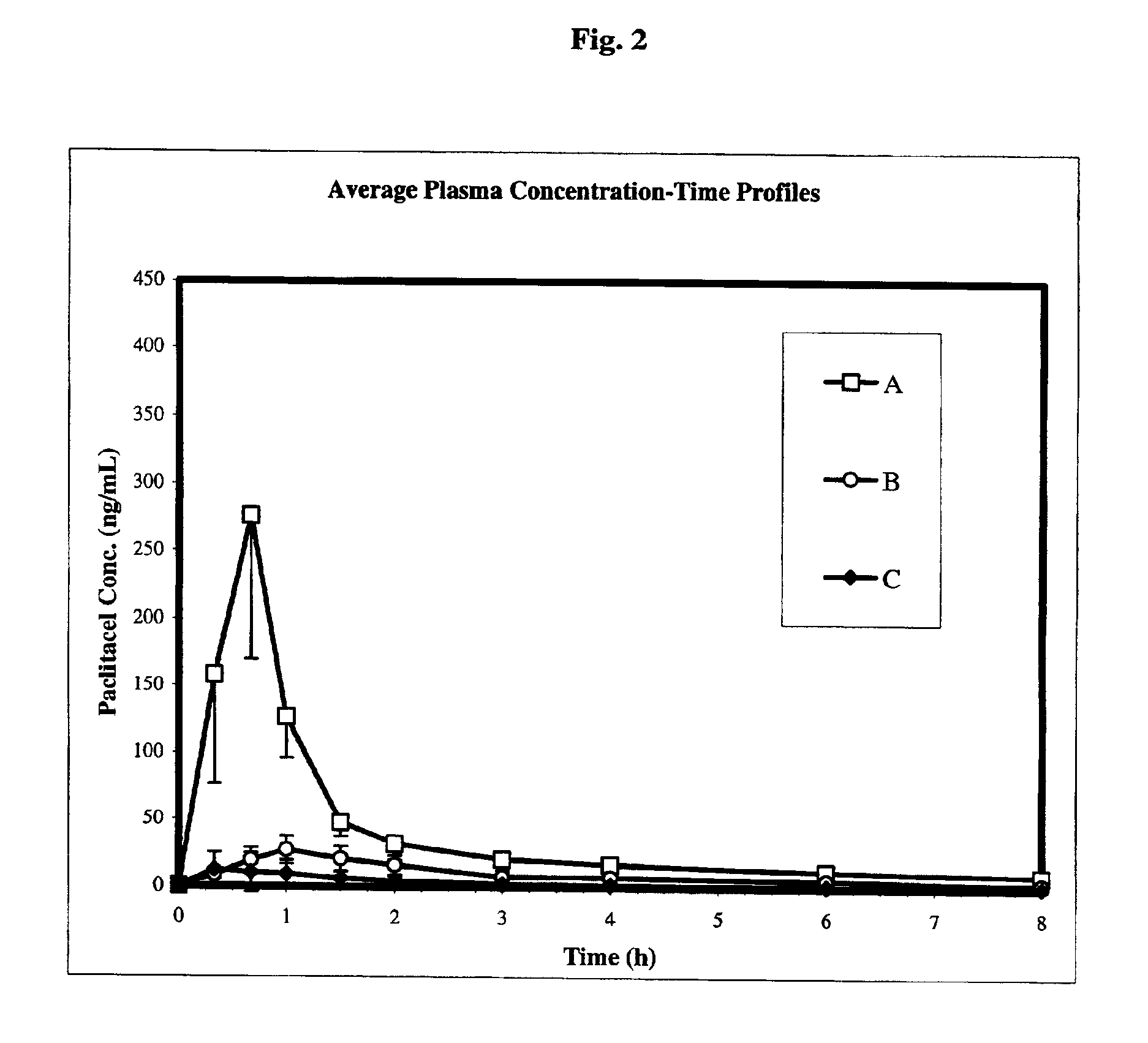
Chemotherapeutic microemulsion compositions of paclitaxel with improved oral bioavailability
InactiveUS7115565B2Rapid and efficient absorptionImprove bioavailabilityBiocideNervous disorderMonoglycerideSolvent
Pharmaceutical compositions suitable for oral administration comprising paclitaxel, a solvent, a surfactant, a substituted cellulosic polymer, and optionally but preferably a P-glycoprotein inhibitor. The composition may further comprise a diglyceride or mixture of diglyceride and monoglyceride. The composition generates a supersaturated paclitaxel microemulsion upon contact with water resulting in improved oral bioavailability of paclitaxel.
Owner:PHARMACIA & UPJOHN CO
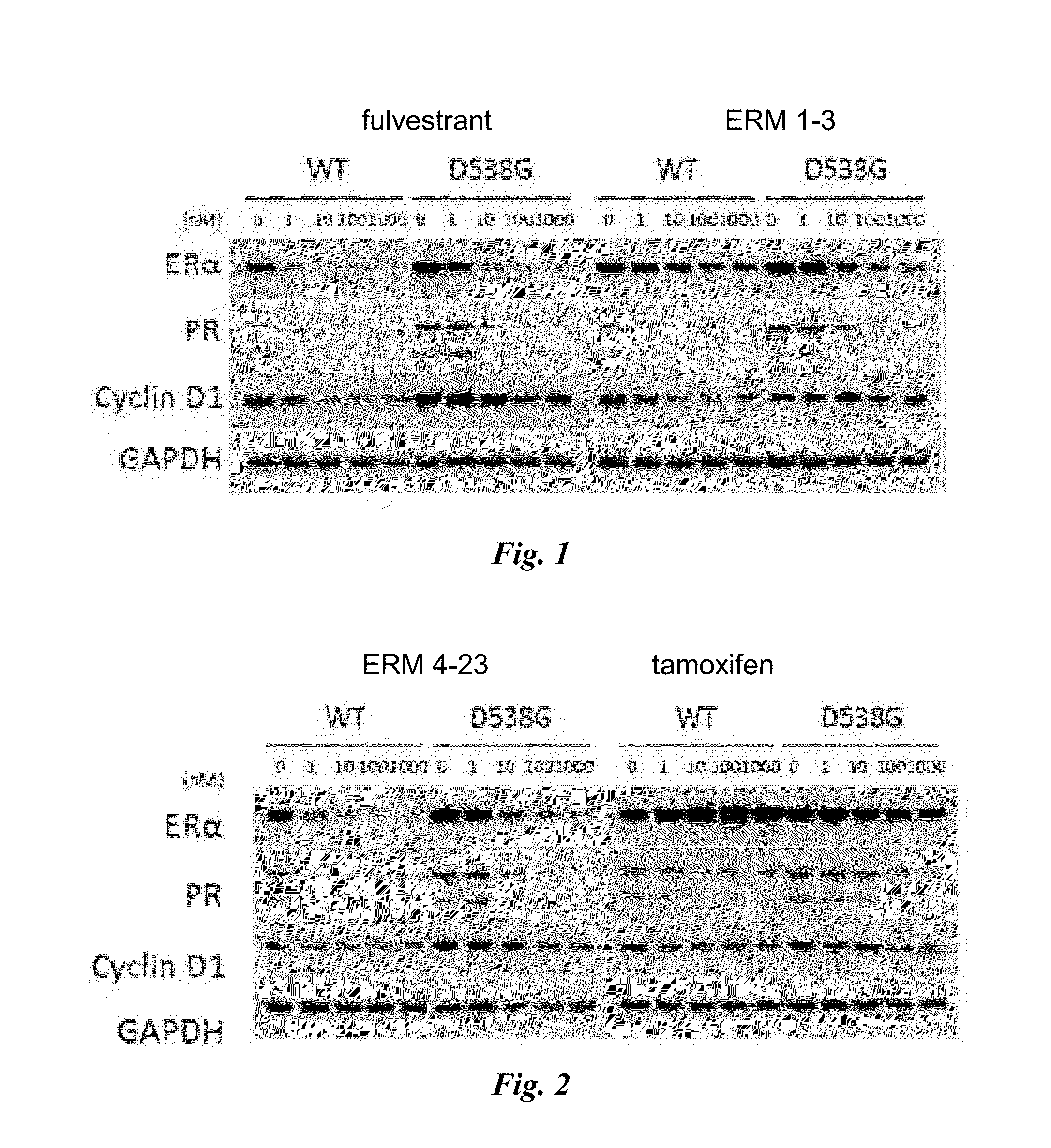
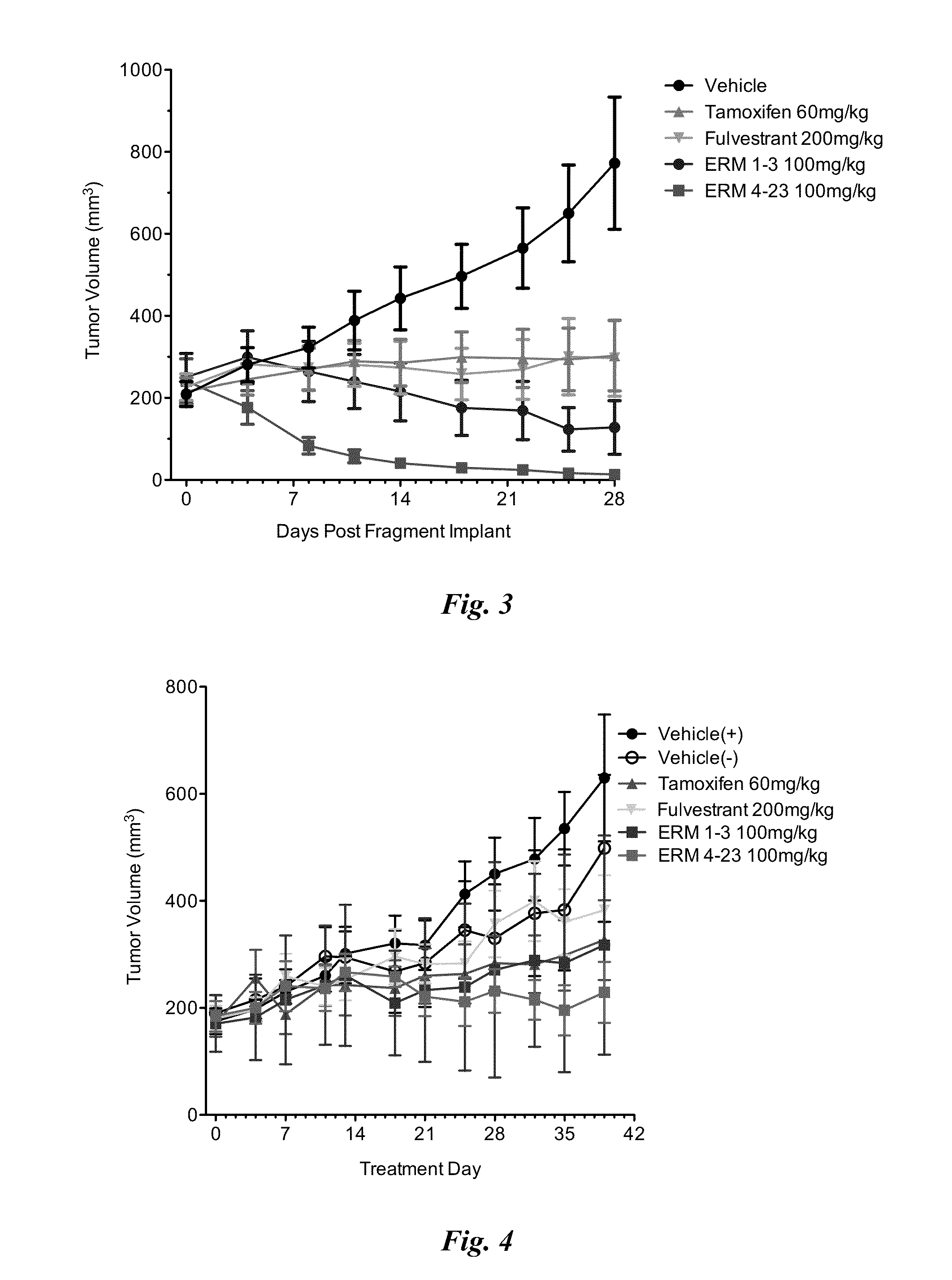
Methods and compositions for modulating estrogen receptor mutants
InactiveUS20150258099A1Altered interactionImprove treatment outcomesBiocideMicrobiological testing/measurementDiseaseEstrogen receptor modulator
Described herein are methods and compositions for treating an ER-related disease condition characterized by a mutation in the ESR1 gene by administering an estrogen receptor modulator. Also described herein are methods of treating hormone resistant-estrogen receptor (ER) positive breast cancers characterized by a mutation in the ESR1 gene by administering an estrogen receptor modulator.
Owner:F HOFFMANN LA ROCHE & CO AG +1
Dosage form comprising liquid formulation
InactiveUS20010001280A1Improve oral bioavailabilityImprove bioavailabilityPowder deliveryPill deliveryPharmaceutical formulationDosage form
Owner:ENCINAL PHARMA INVESTMENTS
2-Substituted and 4-substituted aryl nitrone compounds
InactiveUS20050182060A1High oral bioavailabilityLow toxicityBiocideOrganic chemistryNitroneMedicinal chemistry
Owner:RENOVIS
Polylactide nanoparticles
InactiveUS8003128B2Easy to produceHigh molecular weightOrganic active ingredientsNervous disorderNanoparticleLactide
A drug targeting system for administering a pharmacologically active substance to the central nervous system of a mammal across the animal's blood brain barrier. The drug targeting system comprises nanoparticles made of poly(DL-lactide) and / or poly(DL-lactide-co-glycolide), a pharmacologically active substance which is absorbed to, adsorbed to, and / or incorporated into the nanoparticles, and either contains TPGS or comprises a pluronic 188 surfactant coating deposited on the drug-loaded nanoparticles.
Owner:DAPHOT ENTERPRISES
Lewis acid mediated synthesis of cyclic esters
ActiveUS20050282782A1Increasing and improving propertyIncrease the number ofBiocideOrganic active ingredientsDiolMedicinal chemistry
Methods for the synthesis of cyclic phosphonic acid diesters from 1,3-diols are described, whereby cyclic phosphonic acid diesters are produced by reacting a chiral 1,3-diol and an activated phosphonic acid in the presence of a Lewis acid.
Owner:METABASIS THERAPEUTICS INC
Matrix compositions for controlled delivery of drug substances
InactiveUS20100166866A1Improve solubilityImprove oral bioavailabilityPowder deliveryBiocidePolyethylene oxidePEG-PLGA-PEG
Owner:EGALET LTD
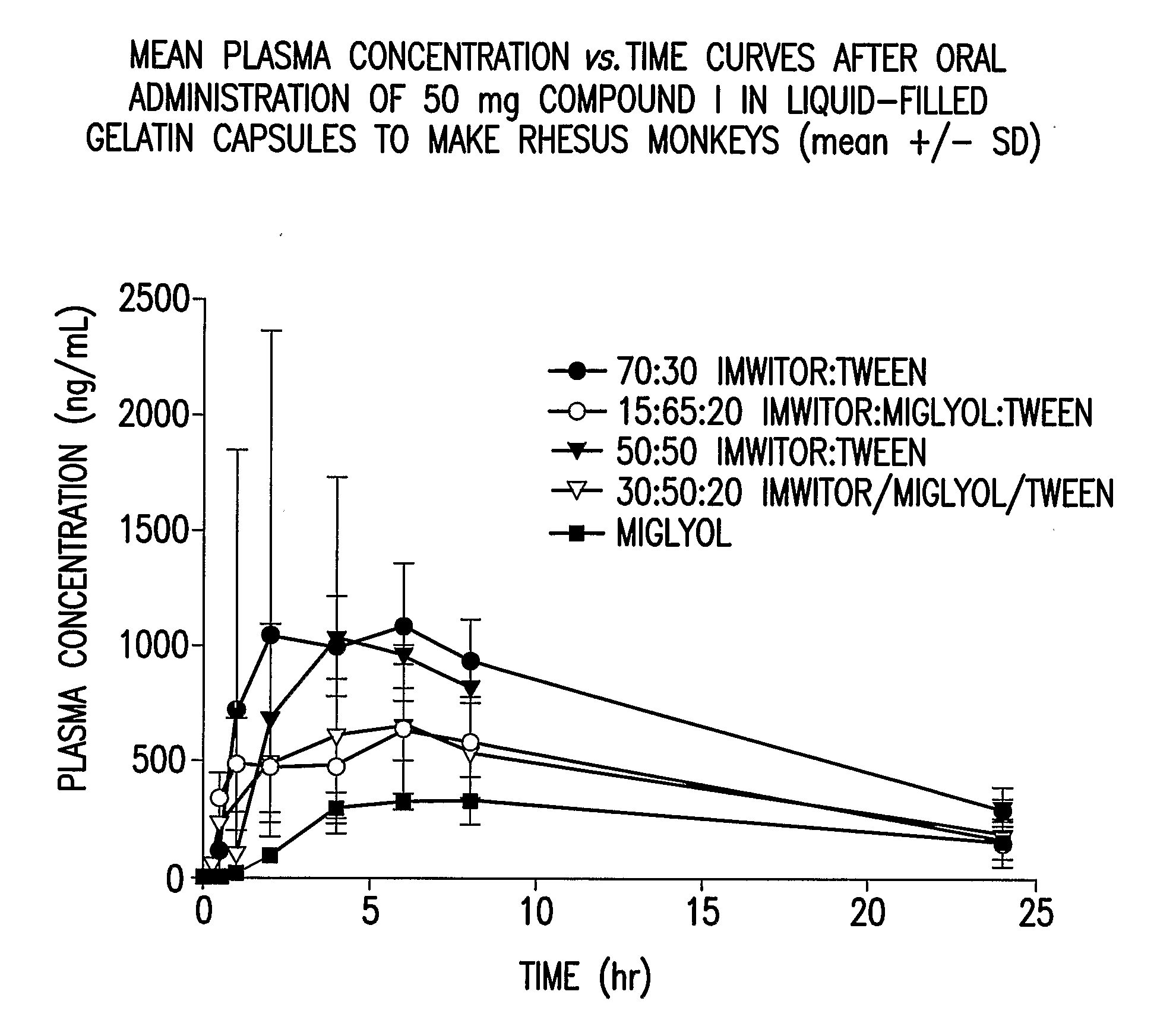
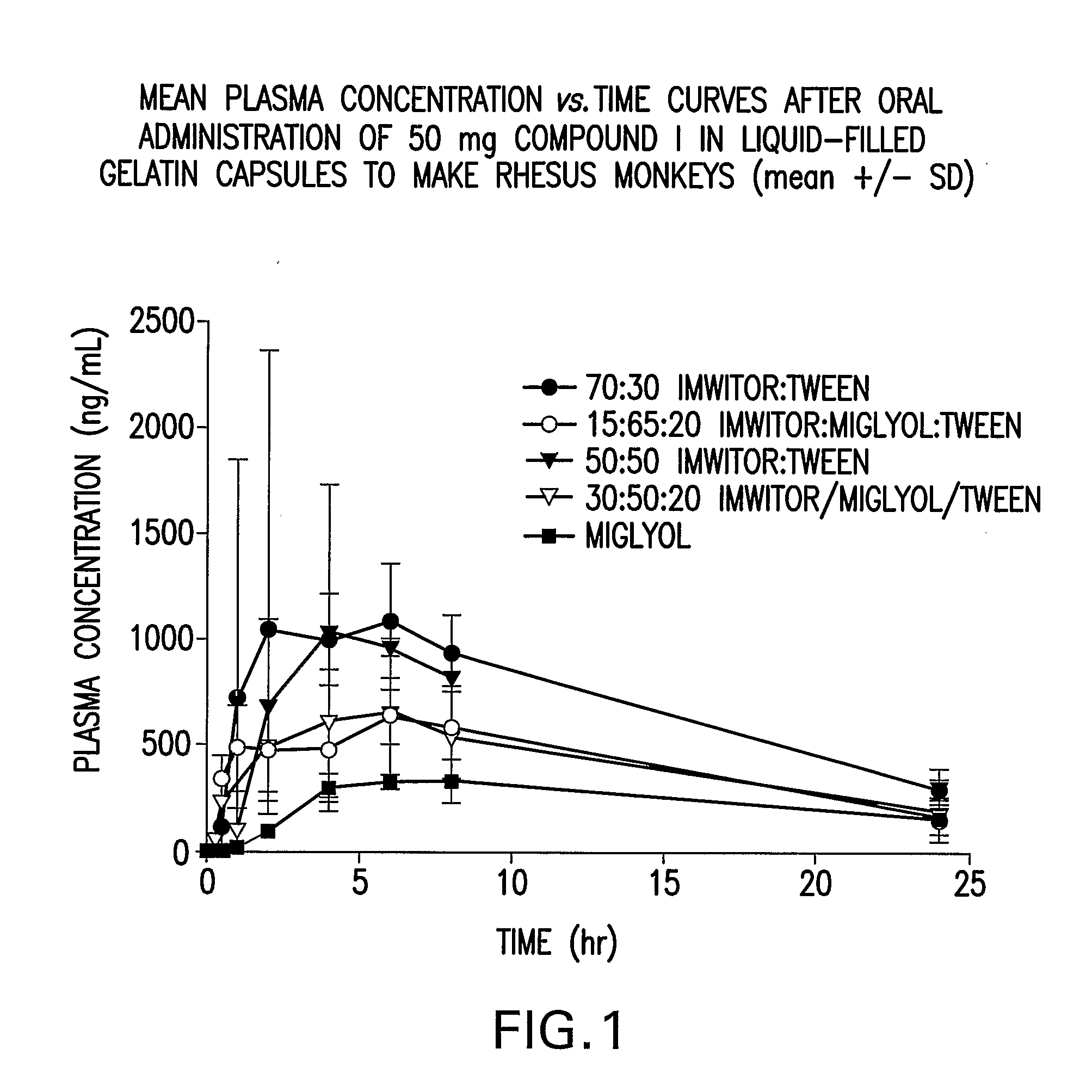
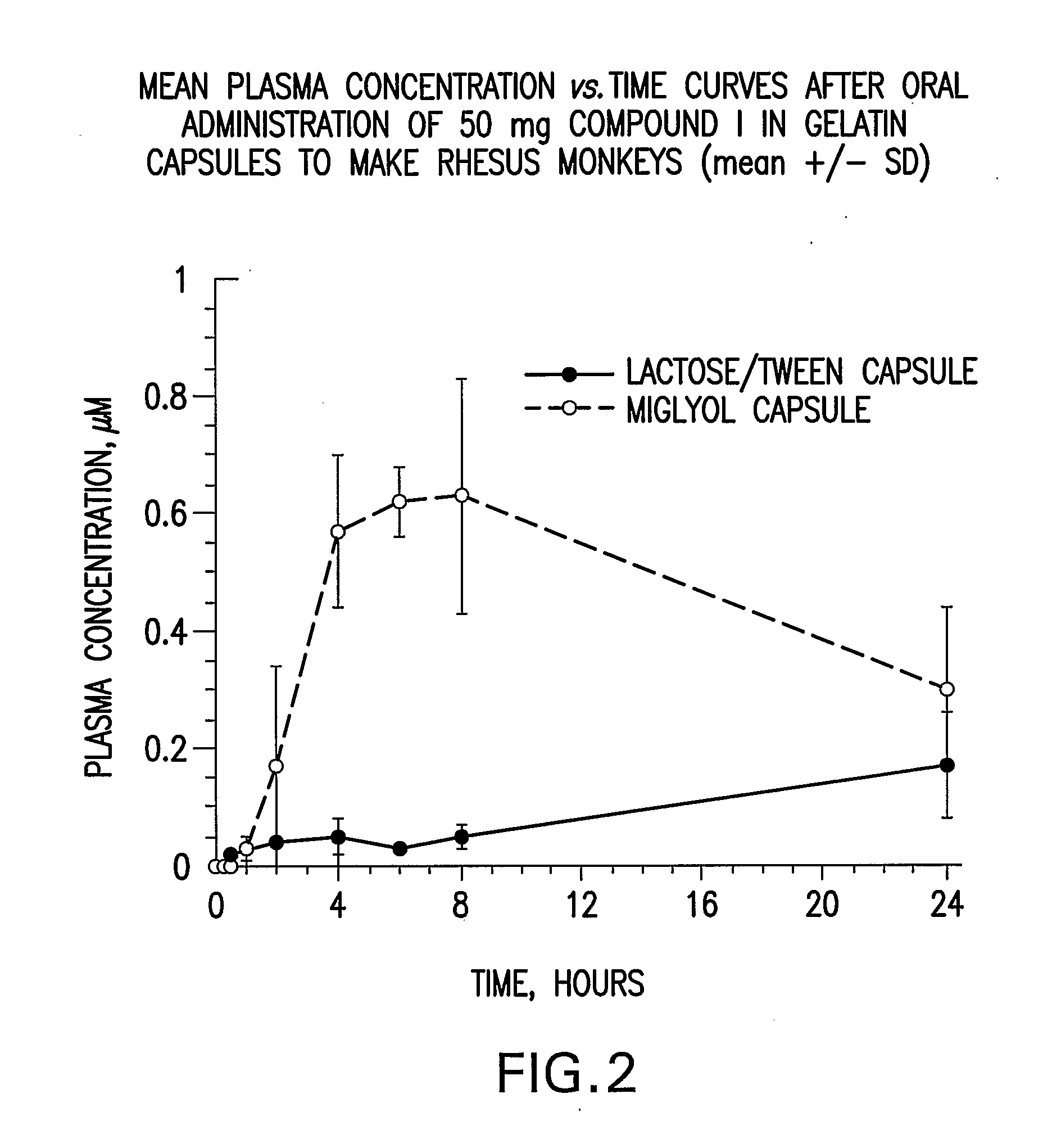
Liquid and Semi-Solid Pharmaceutical Formulations for Oral Administration of a Substituted Amide
InactiveUS20070298099A1Improve oral bioavailabilityImprove compoundBiocideNervous disorderAntioxidantSolvent
N-[1S,2S]-3-(4-chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-{[5-trifluoromethyl]pyridine-2-yl}oxy}propanamide (Compound I) has surprisingly improved solubility and bioavailability in a lipophilic vehicle comprising a pharmaceutically acceptable digestible oil, a surfactant, or a cosolvent, or a mixture of any two or more thereof. In one embodiment of the present invention are self-emulsifying or self-microemulsifying composition comprising 1) Compound I; 2) a surfactant having an HLB of 1 to 8; and 3) a surfactant having an HLB of over 8 to 20; and optionally, 4) a digestible oil and / or cosolvent and / or antioxidant or preservative.
Owner:MERCK SHARP & DOHME CORP
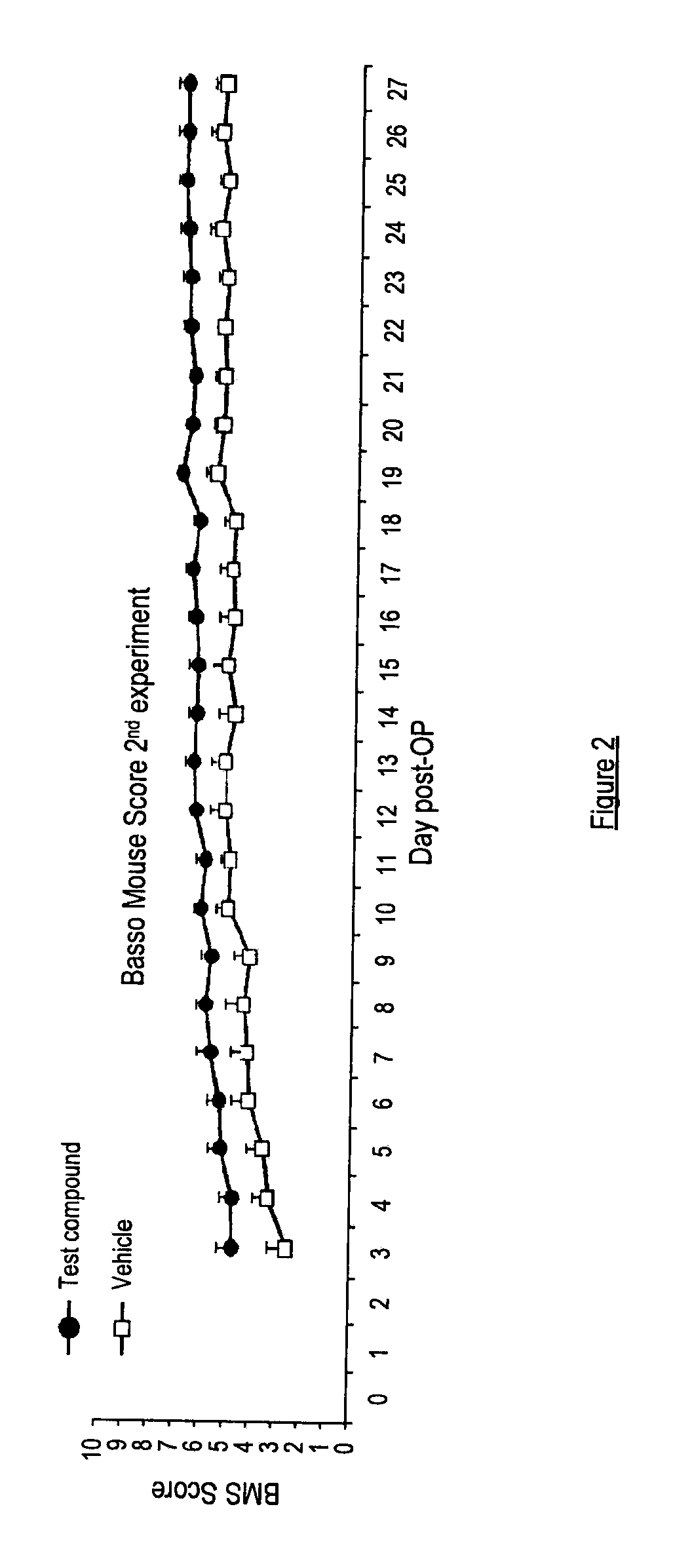
Use of angiotensin ii agonists
InactiveUS20120035232A1High expressionPromoting reinnervationOrganic active ingredientsBiocideMedicineAgonist
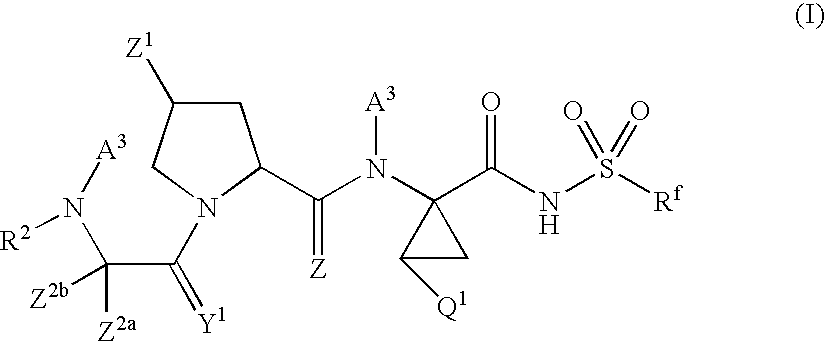
There is provided a compound of formula I,wherein Y1, Y2, Y3, Y4, Z1, Z2, R1, R2, R3, R4 and R5 have meanings given in the description, and pharmaceutically-acceptable salts thereof, for use in the treatment of spinal cord injury.
Owner:VICORE PHARMA AB
Anticancer oral formulation
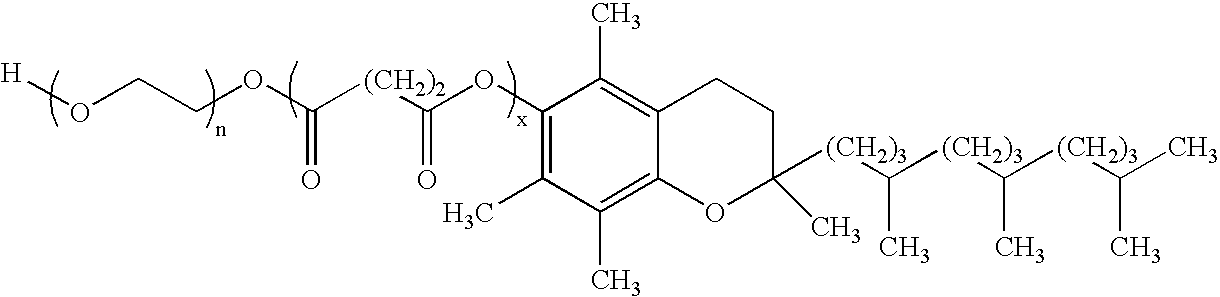
InactiveUS20100010059A1Improve oral bioavailabilityBiocideDispersion deliveryPolyethylene glycolSuccinates
This invention relates to an oral formulation containing an effective amount of the compound of the following formula I:d-alpha-tocopheryl polyethylene glycol 1000 succinate (“TPGS”); and 2-(2-ethoxyethoxy)ethanol (“Transcutol”). R1 through R4 and n are defined herein. Also disclosed is a method of treating cancer by administering this formula to a subject orally.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Cell-penetrating peptide modified nanoparticle and its preparation method
InactiveCN102988295AIncreased cellular uptakeImprove biostability in vivoPowder deliveryPeptide/protein ingredientsSide effectWhole body
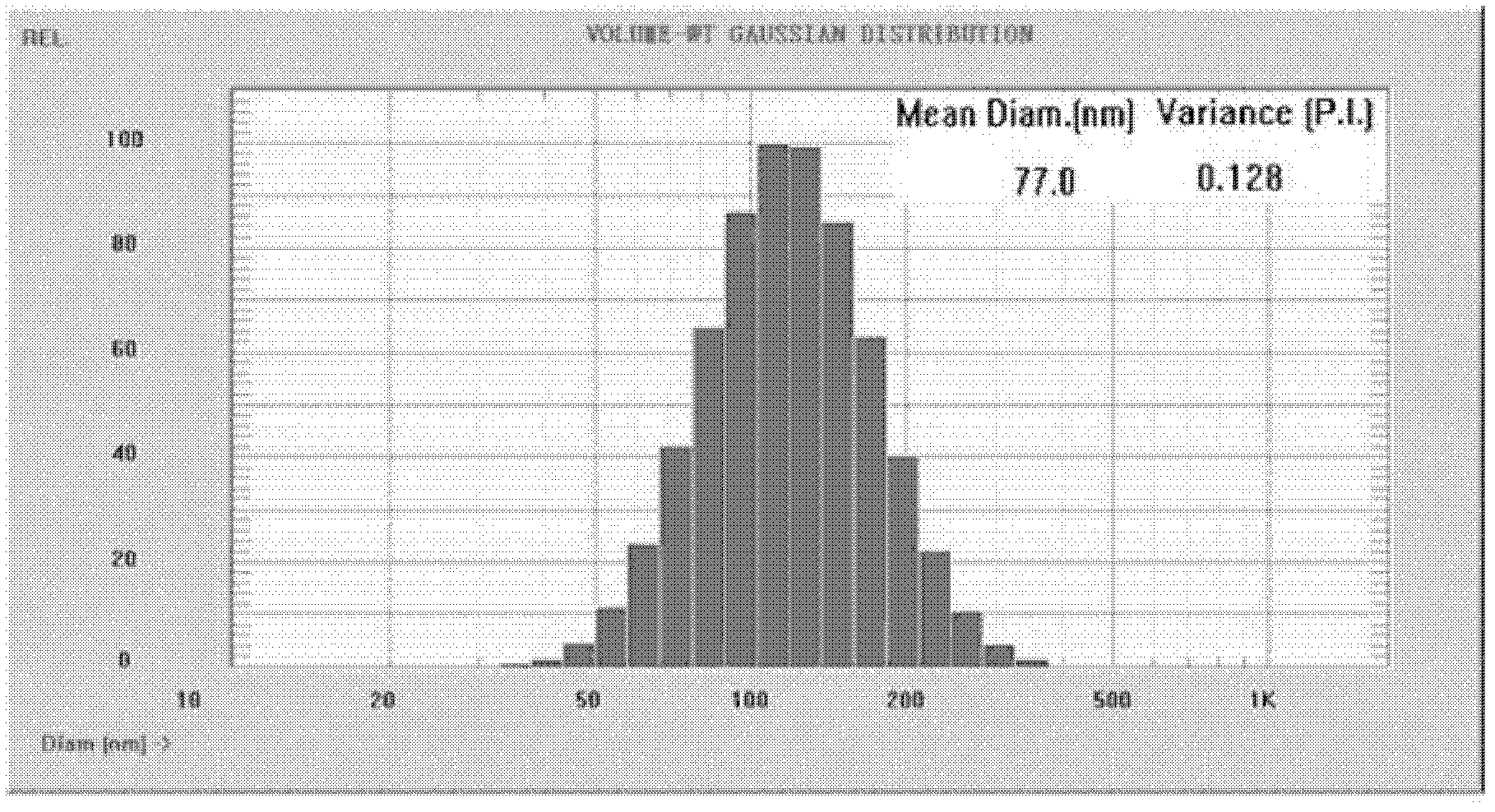
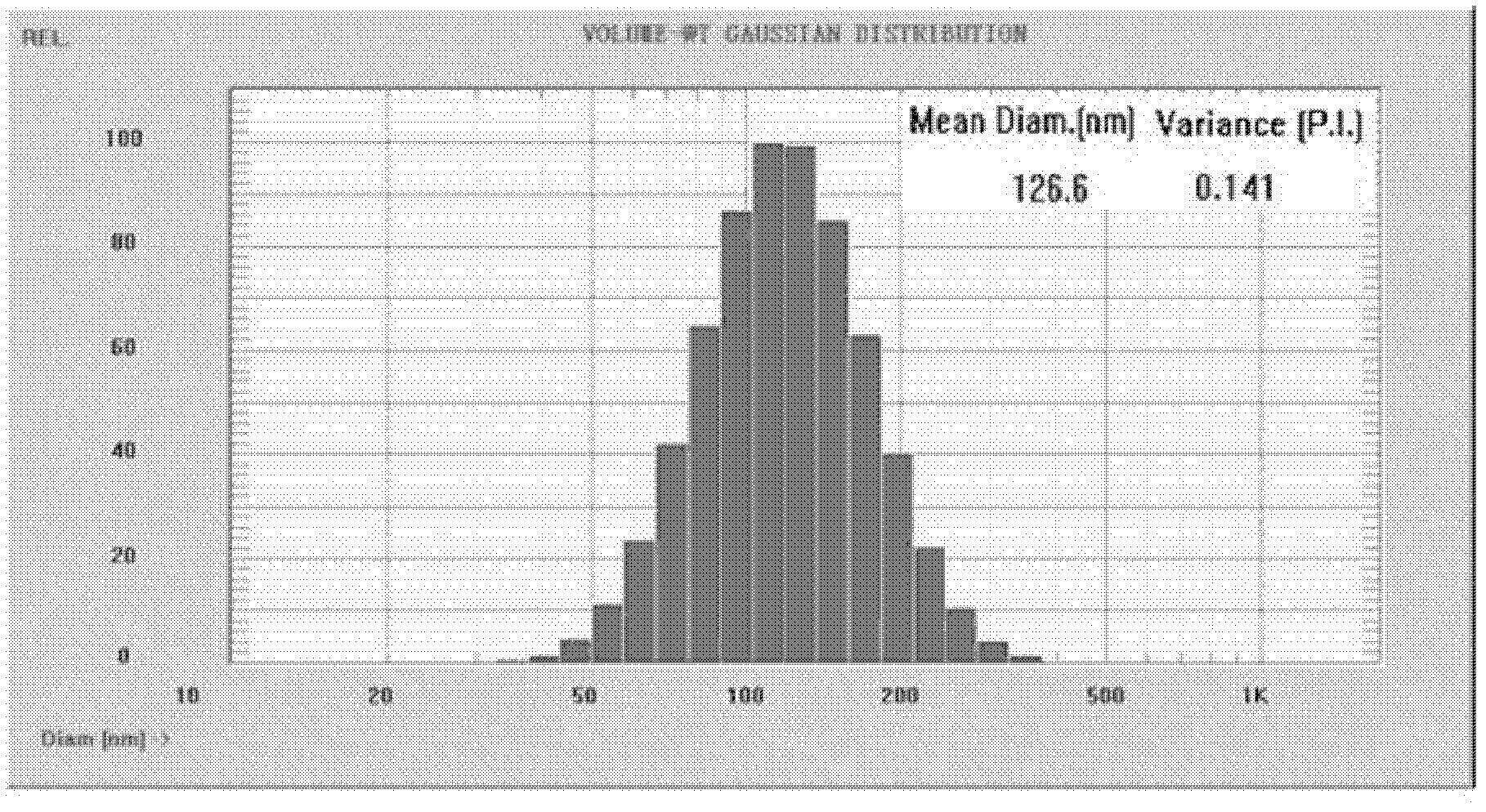
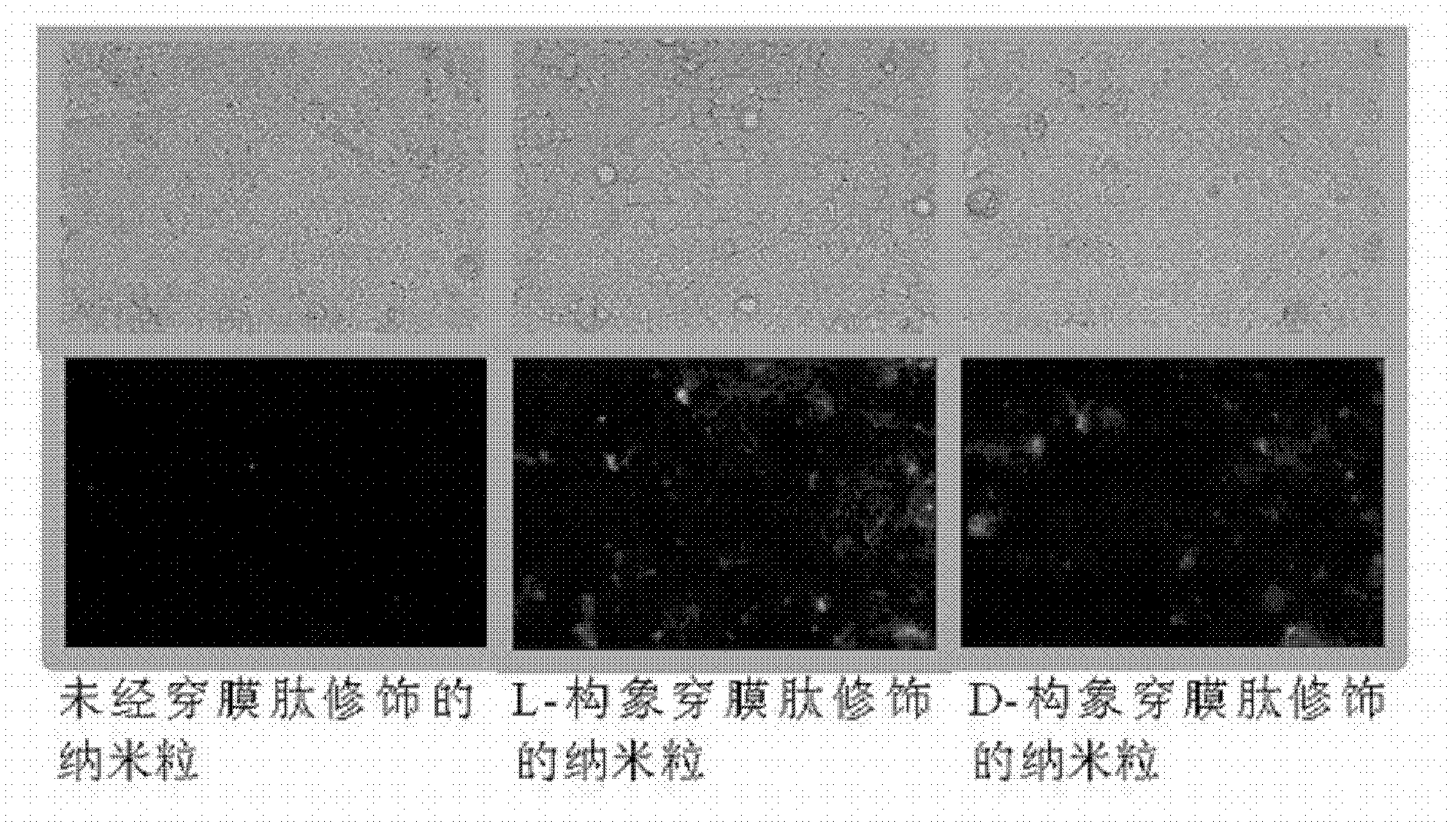
The invention belongs to the medicinal preparation field, relates to a cell-penetrating peptide modified nanoparticle, and concretely relates to a nanoparticle delivery system for oral polypeptide and protein medicines, and its preparation method. The medicine delivery system is composed of the nanoparticle, cell-penetrating peptides modified on the surface of the nanoparticle, and the polypeptide protein medicines sealed by the nanoparticle, wherein the average particle size range of the medicine delivery system is 10-500nm. The cell-penetrating peptide modified nanoparticle and glucosaminoglycan having electronegative cell surfaces undergo electric property attraction and then undergo endocytosis, so the cell-penetrating peptide modified nanoparticle and the glucosaminoglycan are integrally taken into cells; and the cell-penetrating peptide modified nanoparticle has an alimentary canal mucous membrane penetrating capability after the cell-penetrating peptide modified nanoparticle is orally taken, and can deliver the polypeptide protein medicines carried by the cell-penetrating peptide modified nanoparticle to the whole body for blood circulation, so the oral biological utilization degree of the medicines are improved. The cell-penetrating peptide modified nanoparticle has the advantages of improvement of the stability of polypeptide and protein medicines in the alimentary canal, reduction of the application amount of cell-penetrating peptides, and reduction of possible toxic side effects caused by the cell-penetrating peptides.
Owner:FUDAN UNIV
Antiviral compounds
InactiveUS20090047252A1Inhibitory and pharmacokinetic propertyImprove oral bioavailabilityBiocidePeptide/protein ingredientsCombinatorial chemistryAnti virals
Owner:GILEAD SCI INC
Morphinan derivatives with high oral bioavailability
InactiveUS20100240691A1Improve oral bioavailabilityImprove bioavailabilityBiocideNervous disorderDiseaseAlcohol
Owner:ALKERMES PHARMA IRELAND LTD
Ultra low dose doxepin and methods of using the same to treat sleep disorders
InactiveUS20100105614A1Improve oral bioavailabilityGood effectBiocideNervous disorderUltra low doseProdrug
Owner:SOMAXON PHARMA +1
Oral formulations of chemotherapeutic agents
InactiveUS20150231069A1Increase probabilityEffectively infiltrate across the inflamed leakyPowder deliveryOrganic active ingredientsCyclosporinsEnhanced absorption
A composition and method of using the composition for treating a patient in need thereof, the composition comprising an oral formulation for enhanced bioavailability of therapeutic agents such as the taxane chemotherapeutic agents. The composition comprises the therapeutic agent and an absorption enhancing agent either co-administered with the agent or administered separately, the therapeutic agent in a polymer matrix resulting in a microbead and including an edible oil resulting in an emulsion. The absorption enhancing agent is a cyclosporin in one embodiment. The absorption enhancing agent is a P glycoprotein inhibitor in another embodiment.
Owner:MODI PANKAJ
Novel 2'-c-methyl and 4'c-methyl nucleoside derivatives
InactiveUS20090118223A1Improve efficacyImprove securityBiocideSugar derivativesHepatitis c viralPhosphoric acid
Novel 2′-C-methyl nucleoside 5′-monophosphate and 4′-C-methyl nucleoside 5′-monophosphate derivatives, stereoisomers, and pharmaceutically acceptable salts or prodrugs thereof, their preparation, and their uses for the treatment of hepatitis C viral infection are described.
Owner:METABASIS THERAPEUTICS INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com