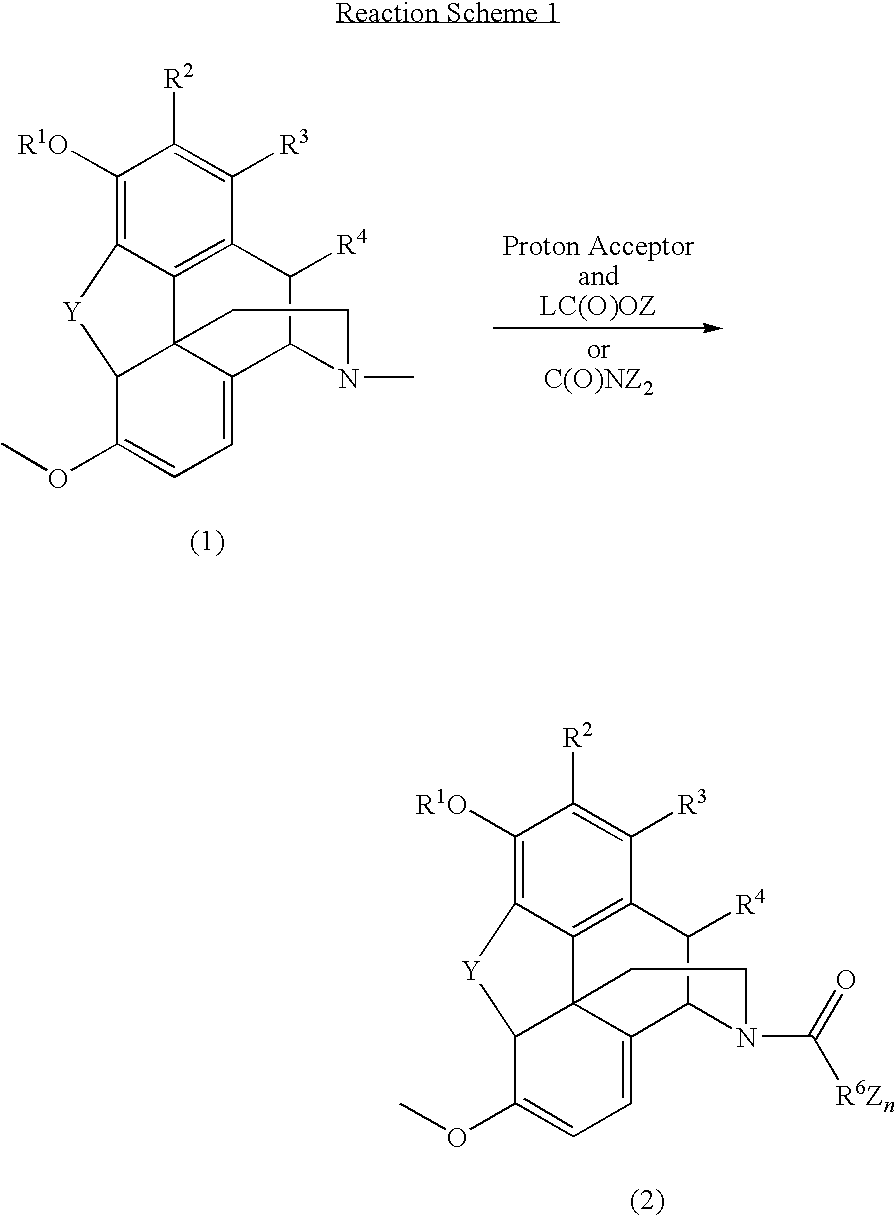
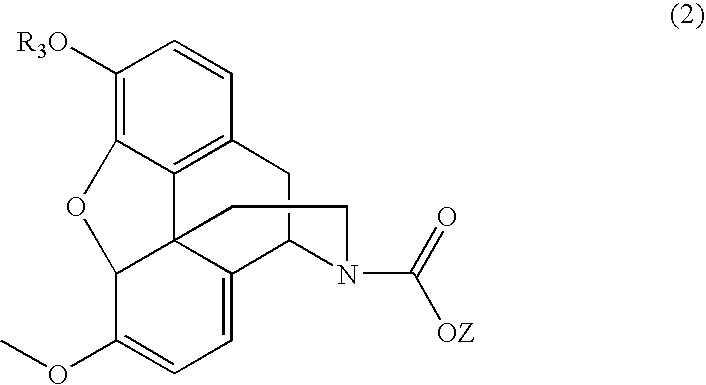
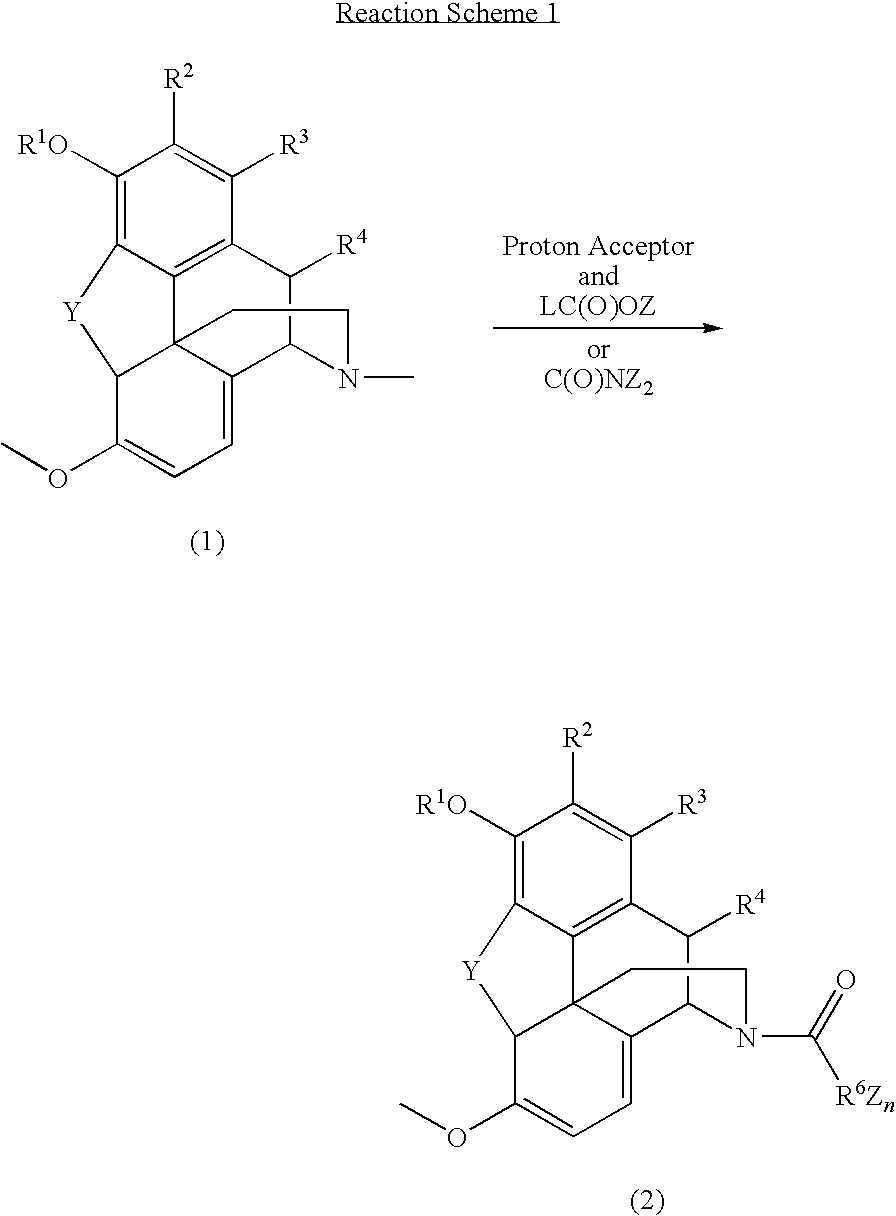
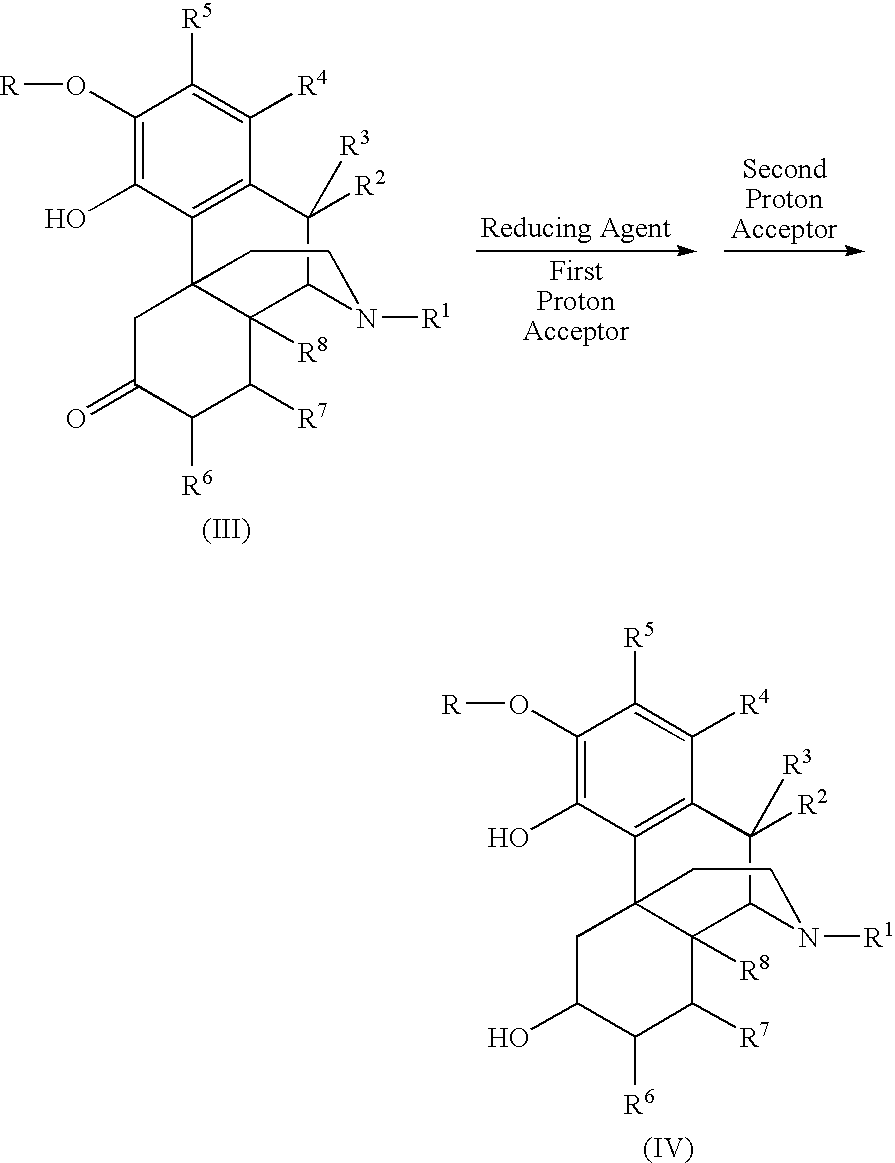
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
176 results about "Morphinan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
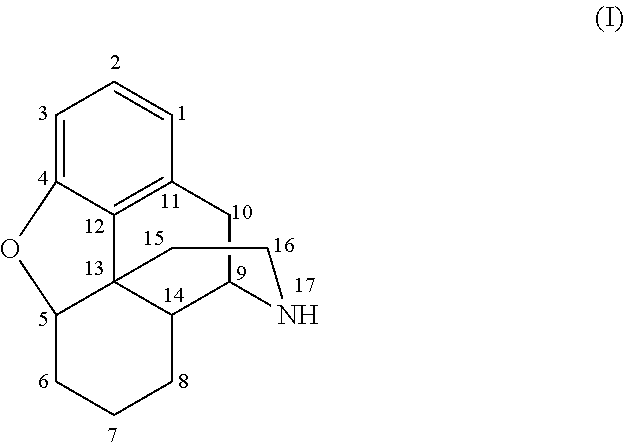
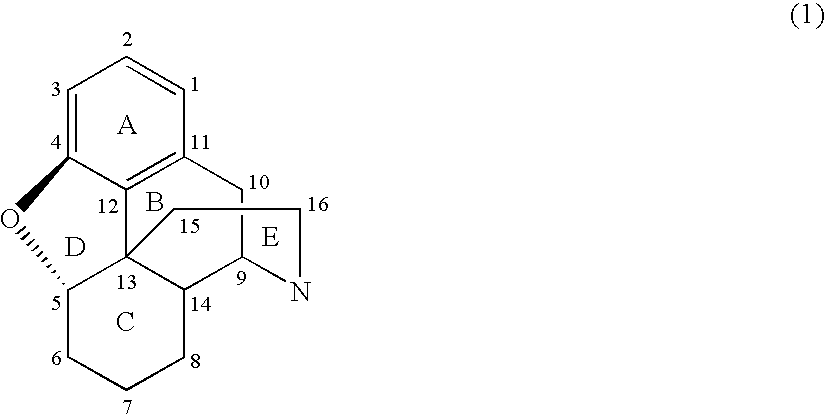
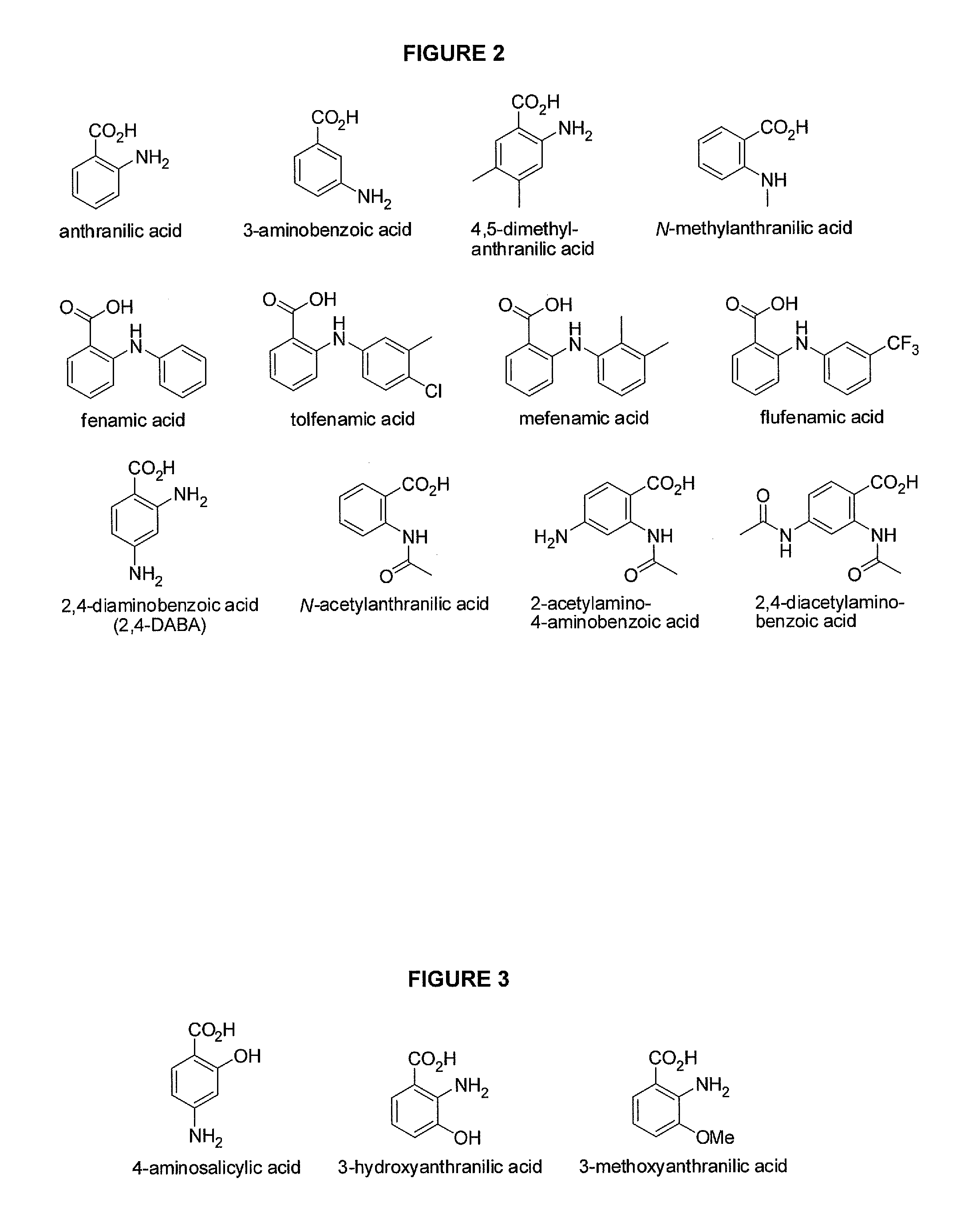
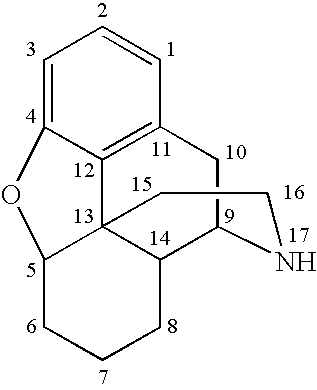
Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others.
Morphinan-derivatives for treating diabetes and related disorders
ActiveUS20150087669A1Risk of developingDelay progressBiocideMetabolism disorderDiseaseNR1 NMDA receptor
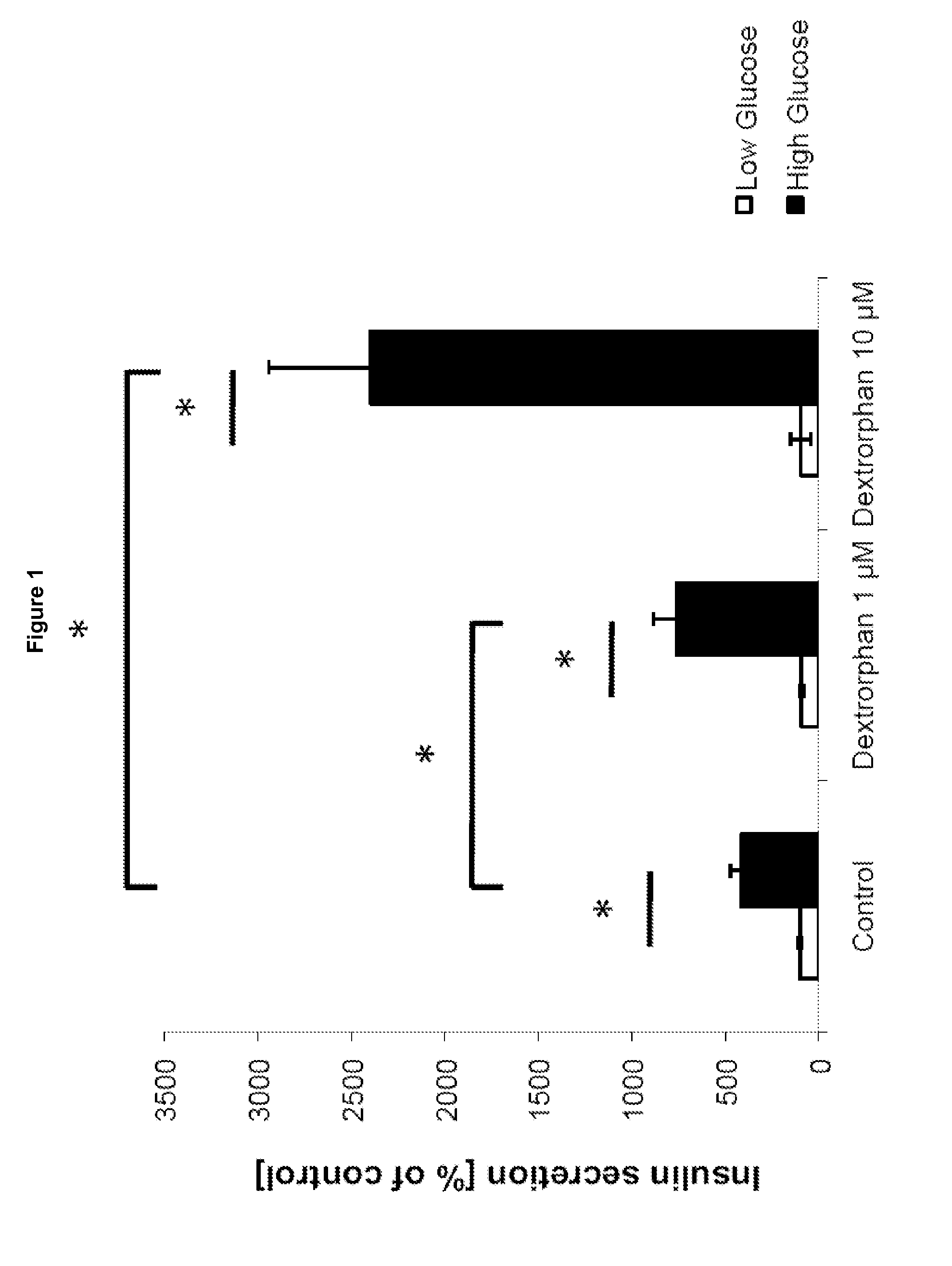
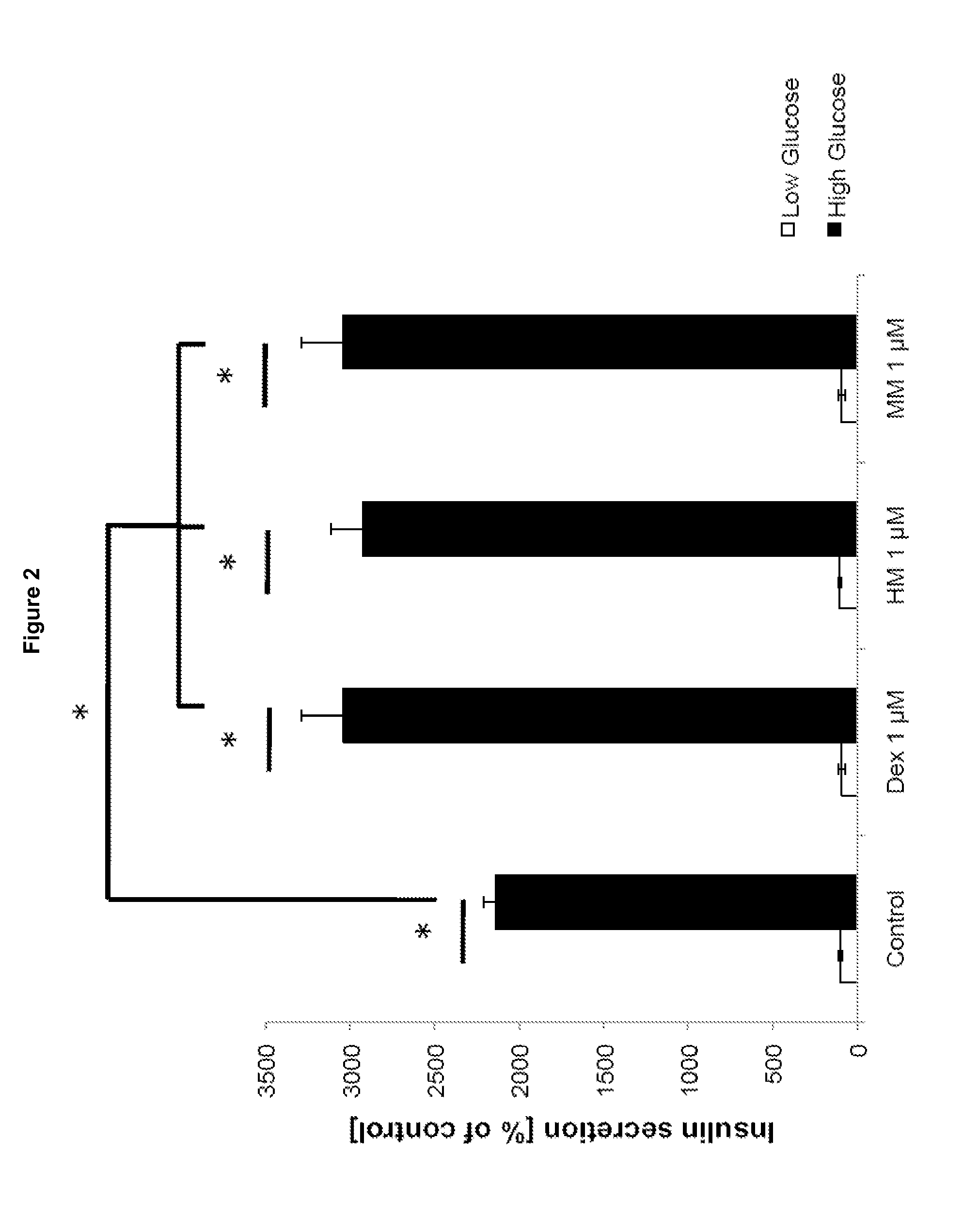
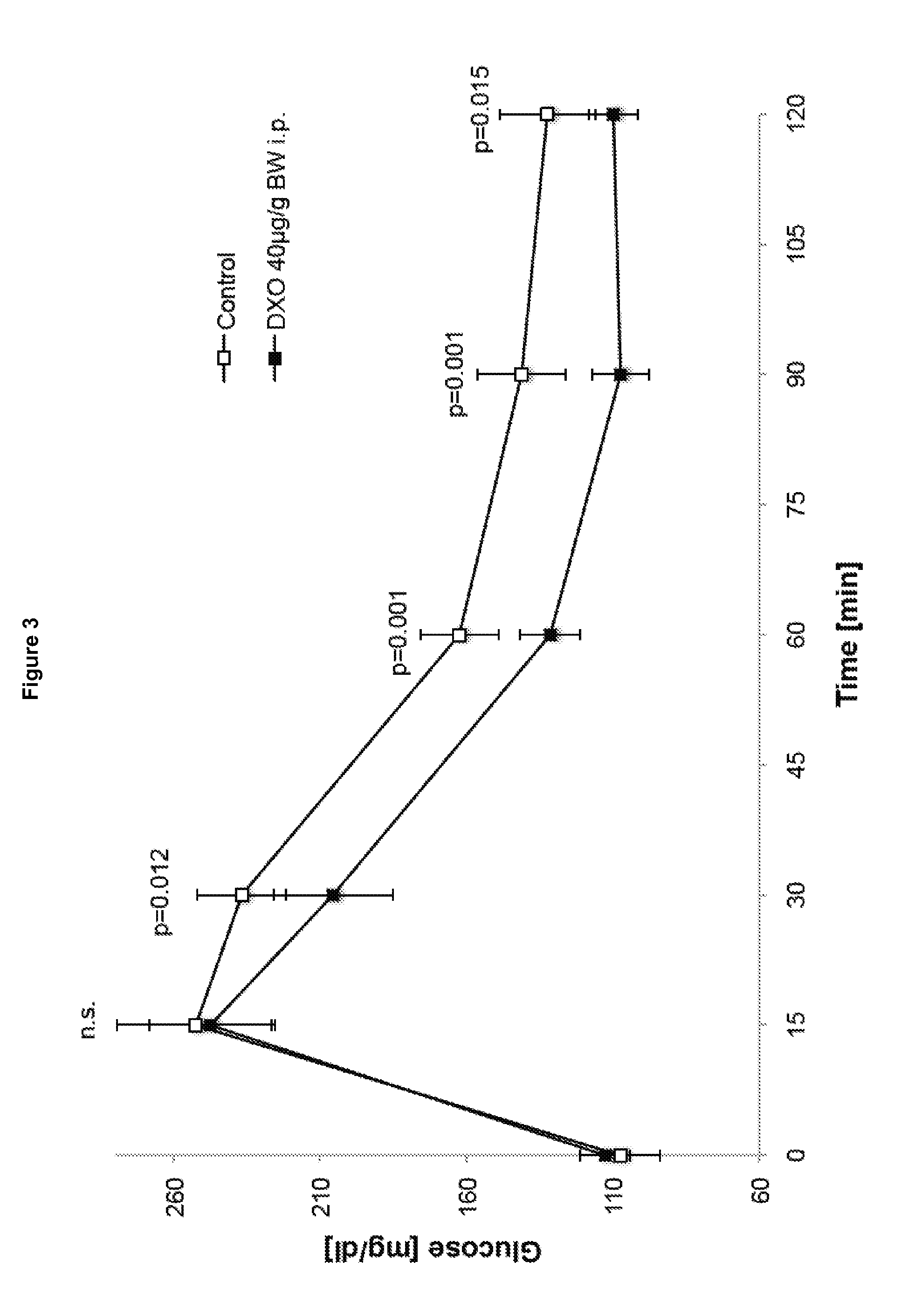
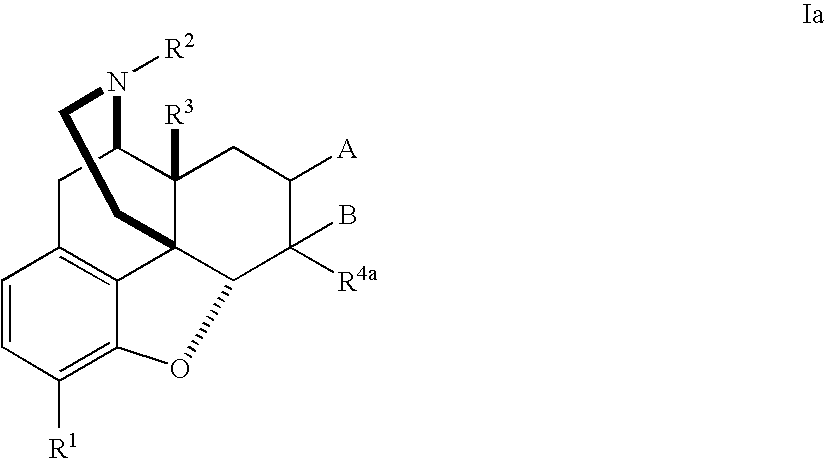
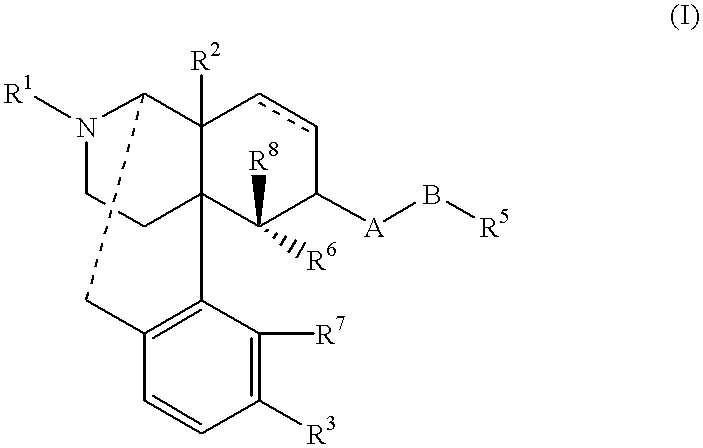
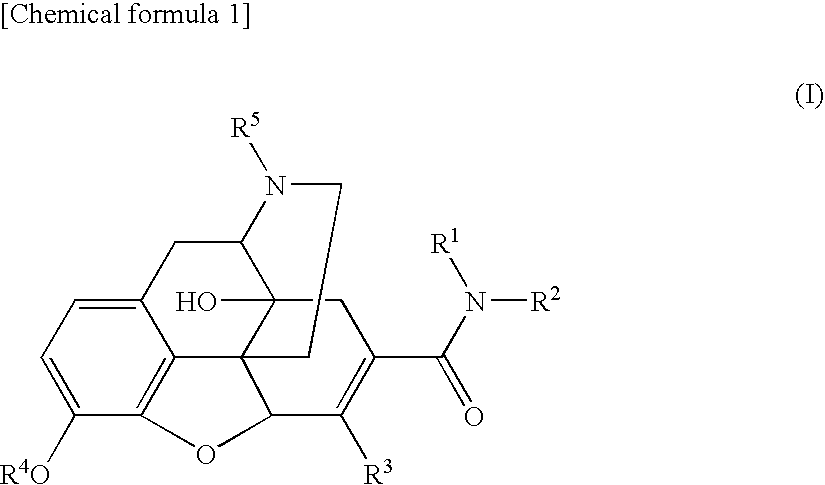
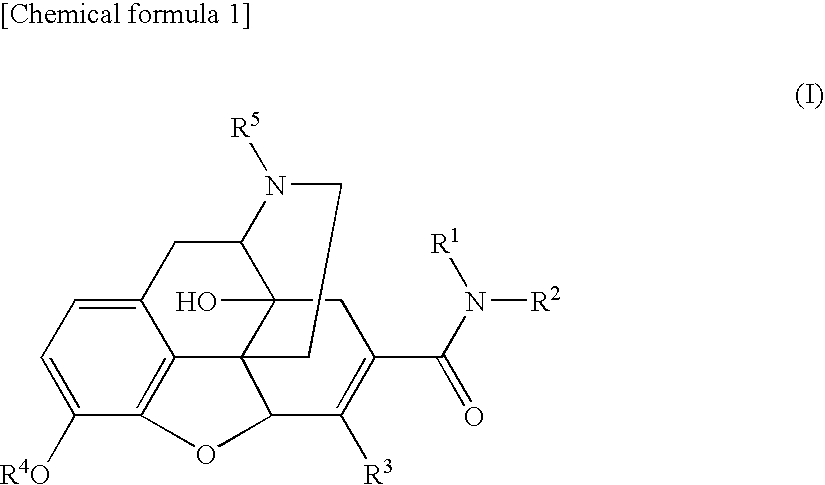
The invention relates to a morphinan-derivative that targets NMDA receptors on pancreatic islets and has the general formula (I)whereinR1 is selected from —OH, —CO2H, —R0, —OR0, —OC(═O)R0, —OC(═O)OR0 or —OC(═O)NHR0; and R2 is selected from —H, —R0, —C(═O)R0, —C(═O)OR0, —C(═O)NHR0 or —C(═NH)—NH—C(═NH)—NH2; wherein R0 is in each case independently selected from —C1-C6-alkyl, -aryl, -heteroaryl, —C1-C6-alkyl-aryl or —C1-C6-alkyl-heteroaryl, in each case independently unsubstituted or substituted;or its physiologically acceptable salt and / or stereoisomer, including mixtures thereof in all ratios, for use in the treatment of a disease or condition, where the disease or condition is insulin-dependent diabetes mellitus, non-insulin-dependent diabetes mellitus, obesity, and / or diabetic nephropathy.
Owner:DEUTE DIABETES FORSCHUNGSGES
Selective opioid compounds
ActiveUS20090209569A1Reducing lipid permeability of drugReduce penetrationAntibacterial agentsBiocideDiseaseInterstitial cystitis
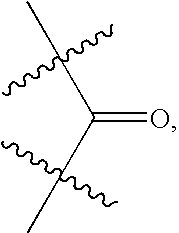
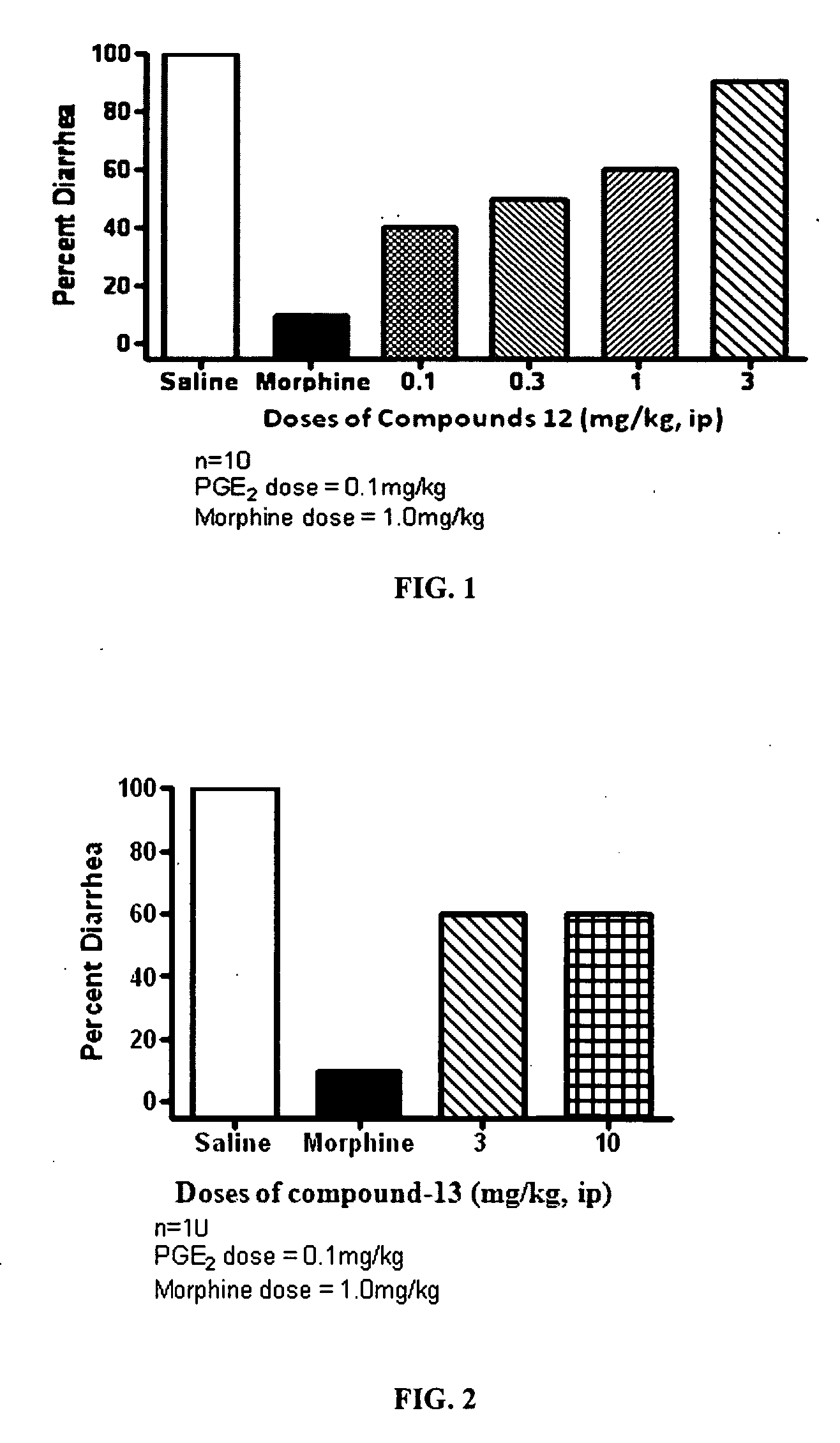
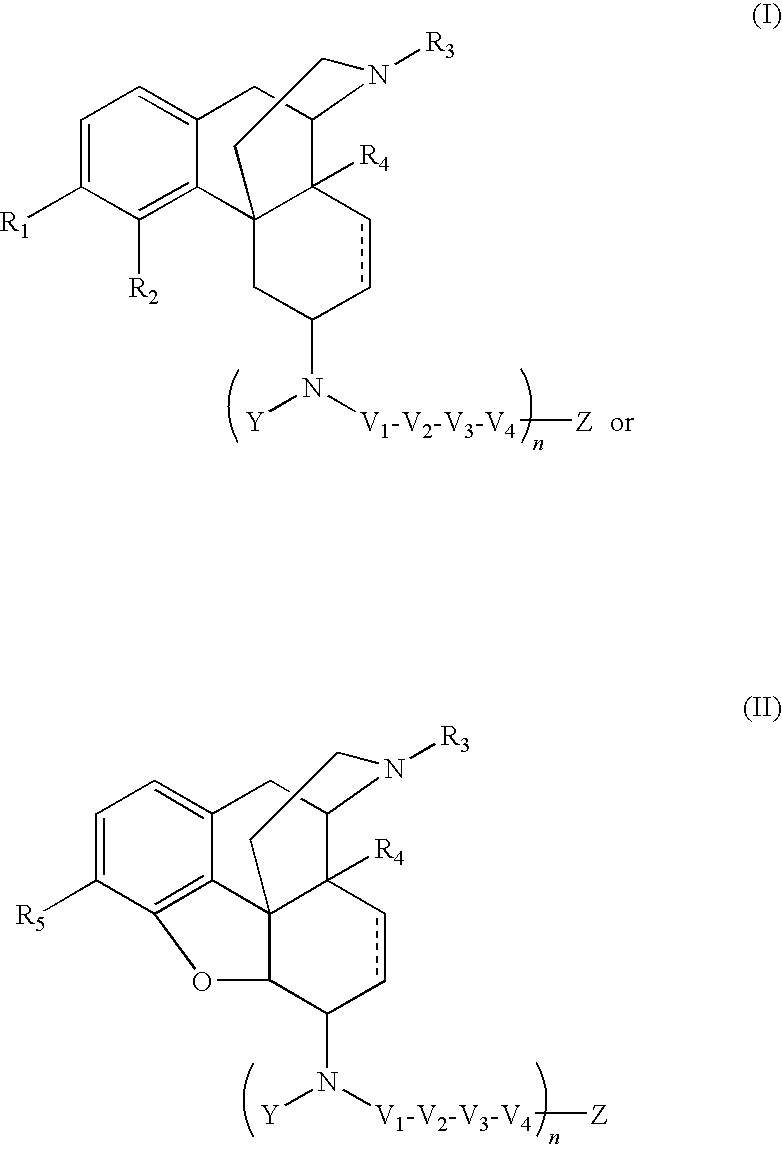
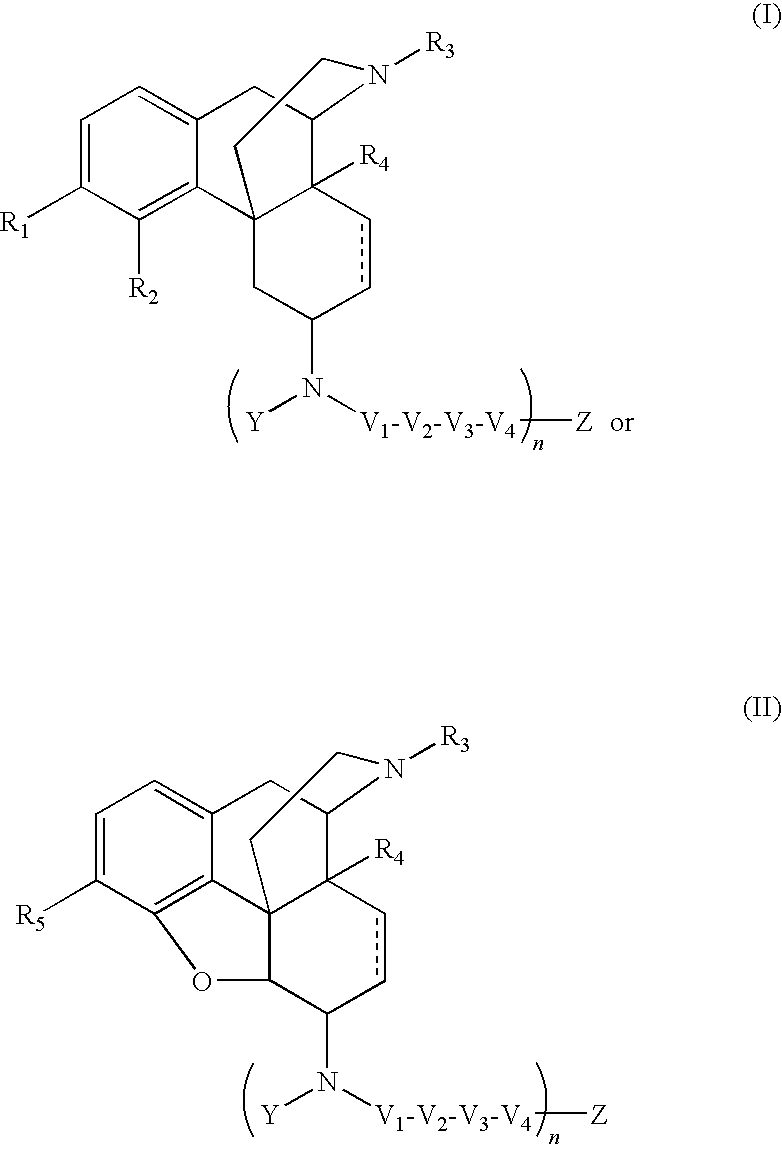
The present invention relates to compounds of Formula I or II, or pharmaceutically acceptable salts, esters, or prodrugs thereof:which relates to morphinan compounds useful as μ, δ, and / or κ receptor opioid compounds and pharmaceuticals containing same that may be useful for mediating analgesia, combating drug addiction, alcohol addiction, drug overdose, mental illness, bladder dysfunctions, neurogenic bladder, interstitial cystitis, urinary incontinence, premature ejaculation, inflammatory pain, peripherally mediated and neuropathic pain, cough, lung edema, diarrhea, cardiac disorders, cardioprotection, depression, and cognitive, respiratory, diarrhea, irritable bowel syndrome and gastro-intestinal disorders, immunomodulation, and anti-tumor agents.
Owner:ALKERMES INC
Antipruritic agent
Owner:TORAY IND INC
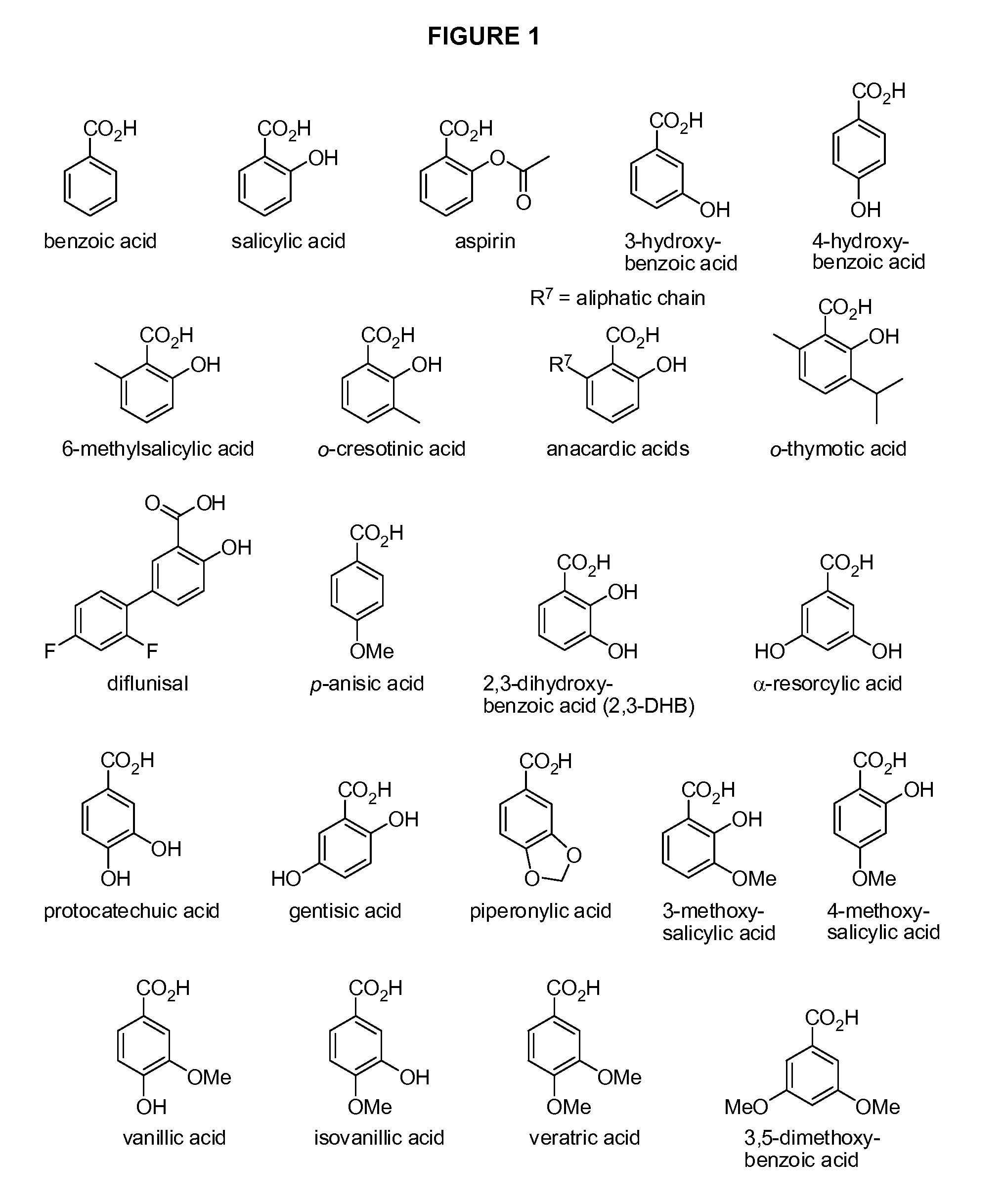
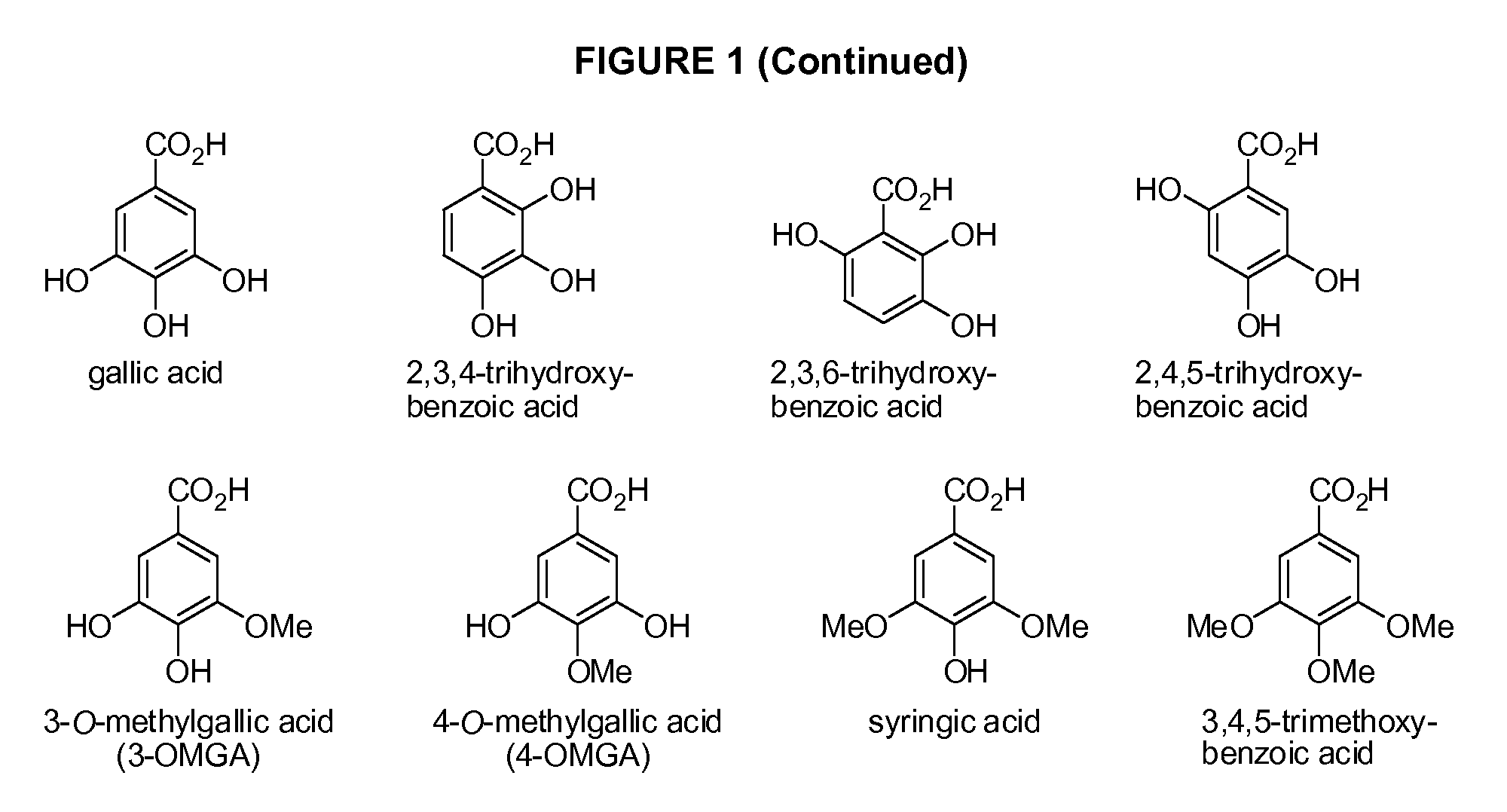
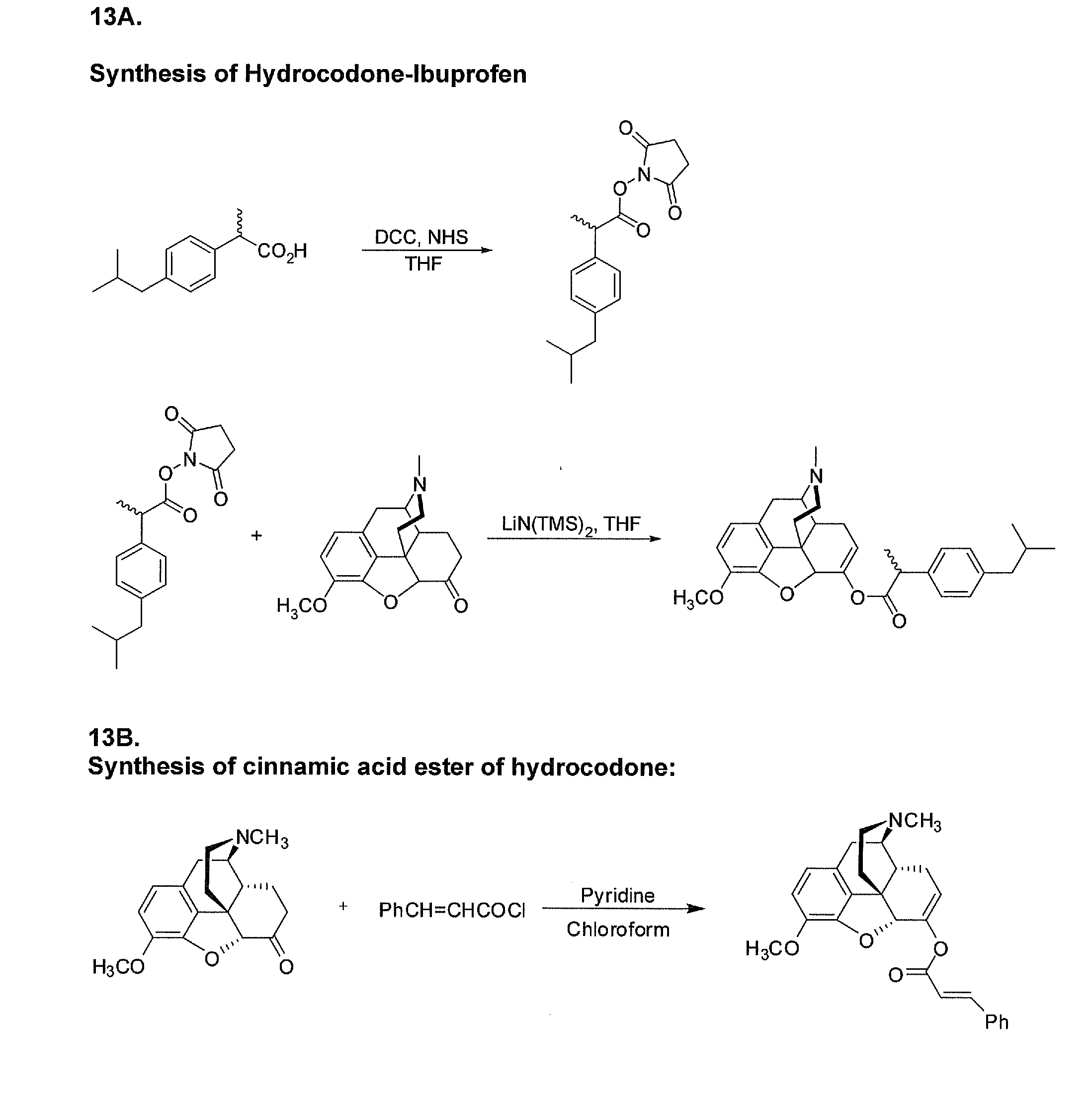
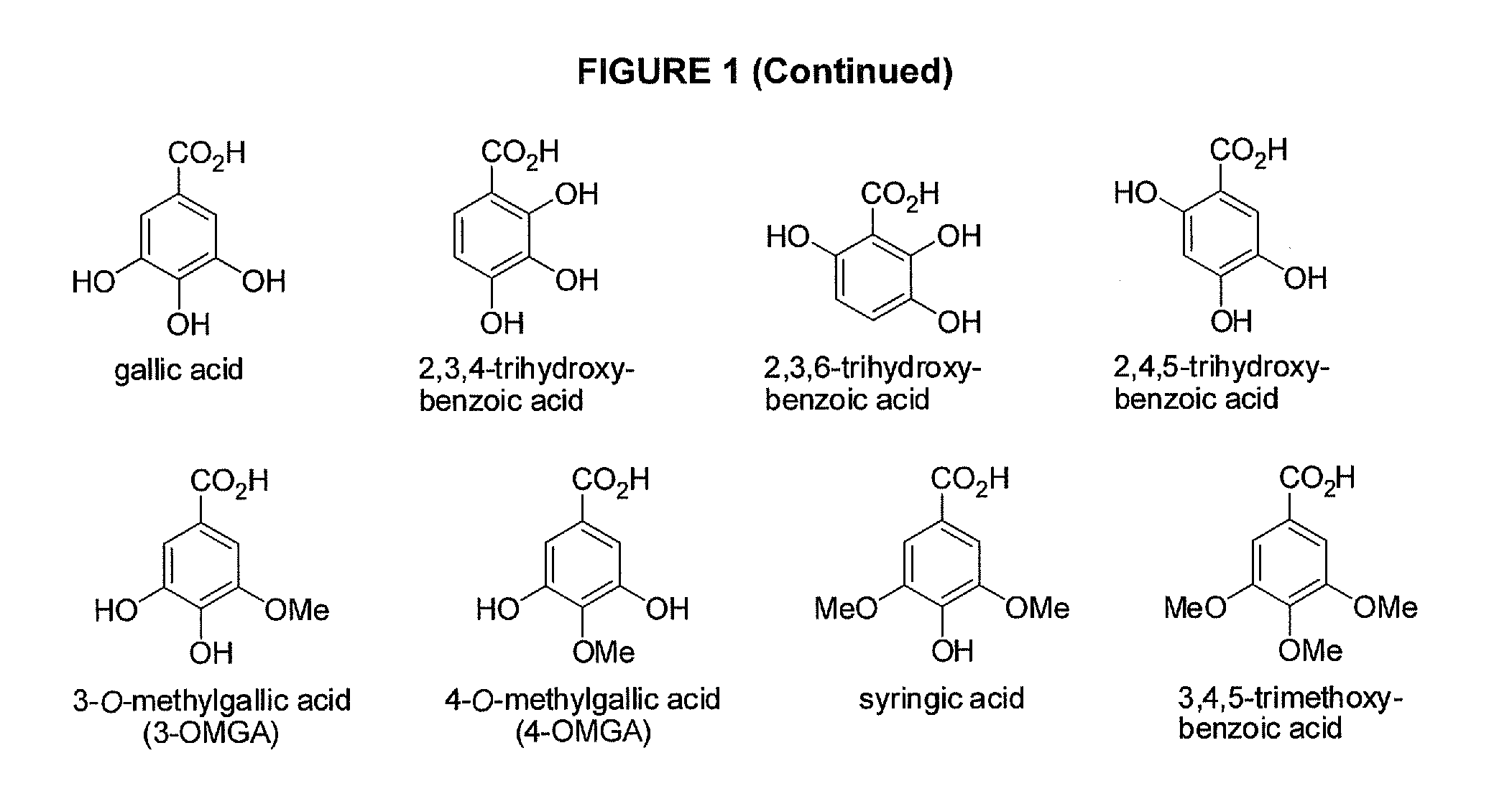
Benzoic acid, benzoic acid derivatives and heteroaryl carboxylic acid conjugates of hydrocodone, prodrugs, methods of making and use thereof
The presently described technology provides compositions comprising aryl carboxylic acids chemically conjugated to hydrocodone (morphinan-6-one, 4,5-alpha-epoxy-3-methoxy-17-methyl) to form novel prodrugs / compositions of hydrocodone, including benzoates and heteroaryl carboxylic acids, which have a decreased potential for abuse of hydrocodone. The present technology also provides methods of treating patients, pharmaceutical kits and methods of synthesizing conjugates of the present technology.
Owner:KEMPHARM INC
Methods of Producing Stabilized Solid Dosage Pharmaceutical Compositions Containing Morphinans
Methods for producing stabilized solid dosage form pharmaceutical compositions are provided. In particular, methods for preparing protected granules containing morphinans, and solid dosage form pharmaceutical compositions produced using the morphinan-protected granules are provided.
Owner:MALLINCKRODT INC
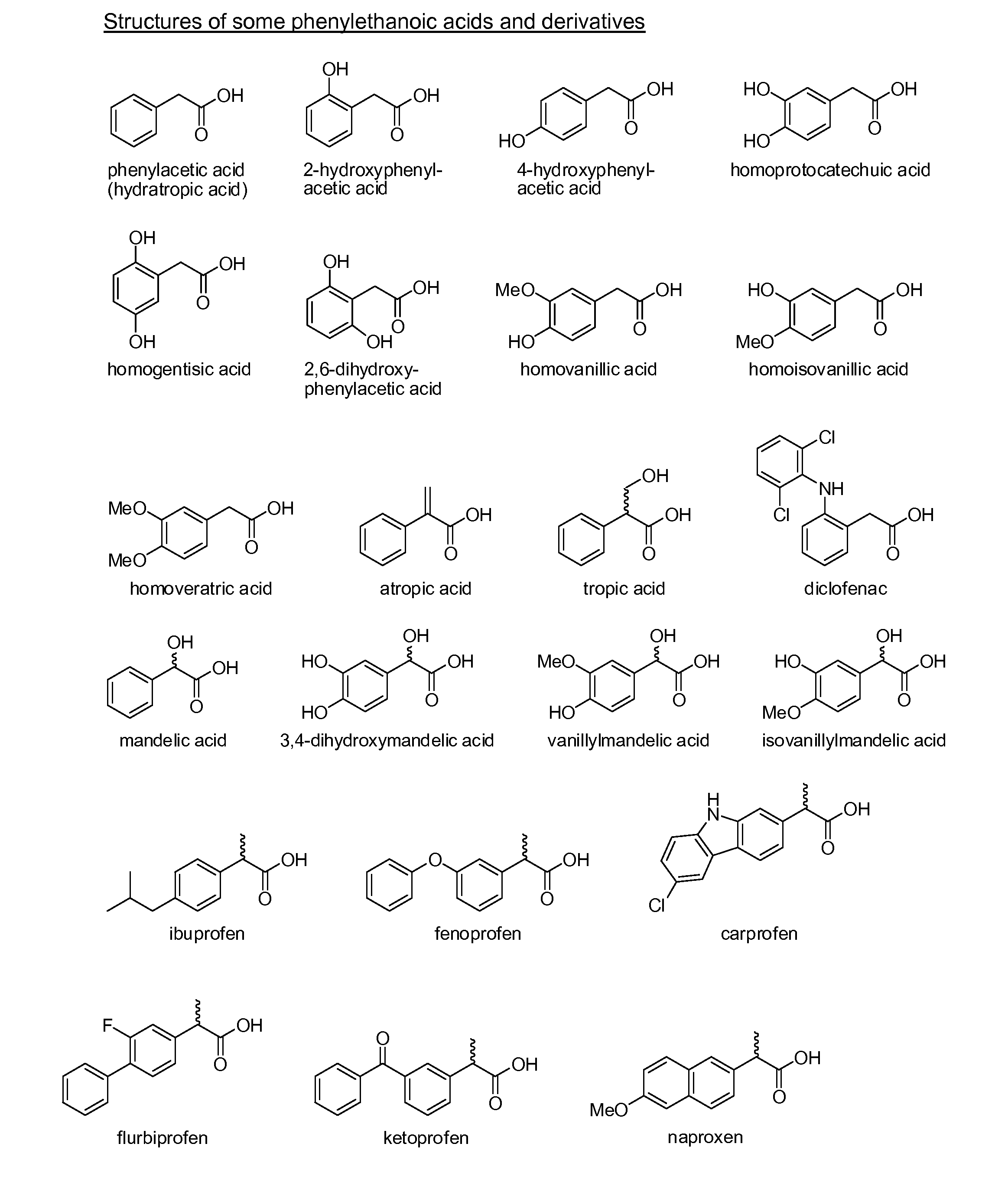
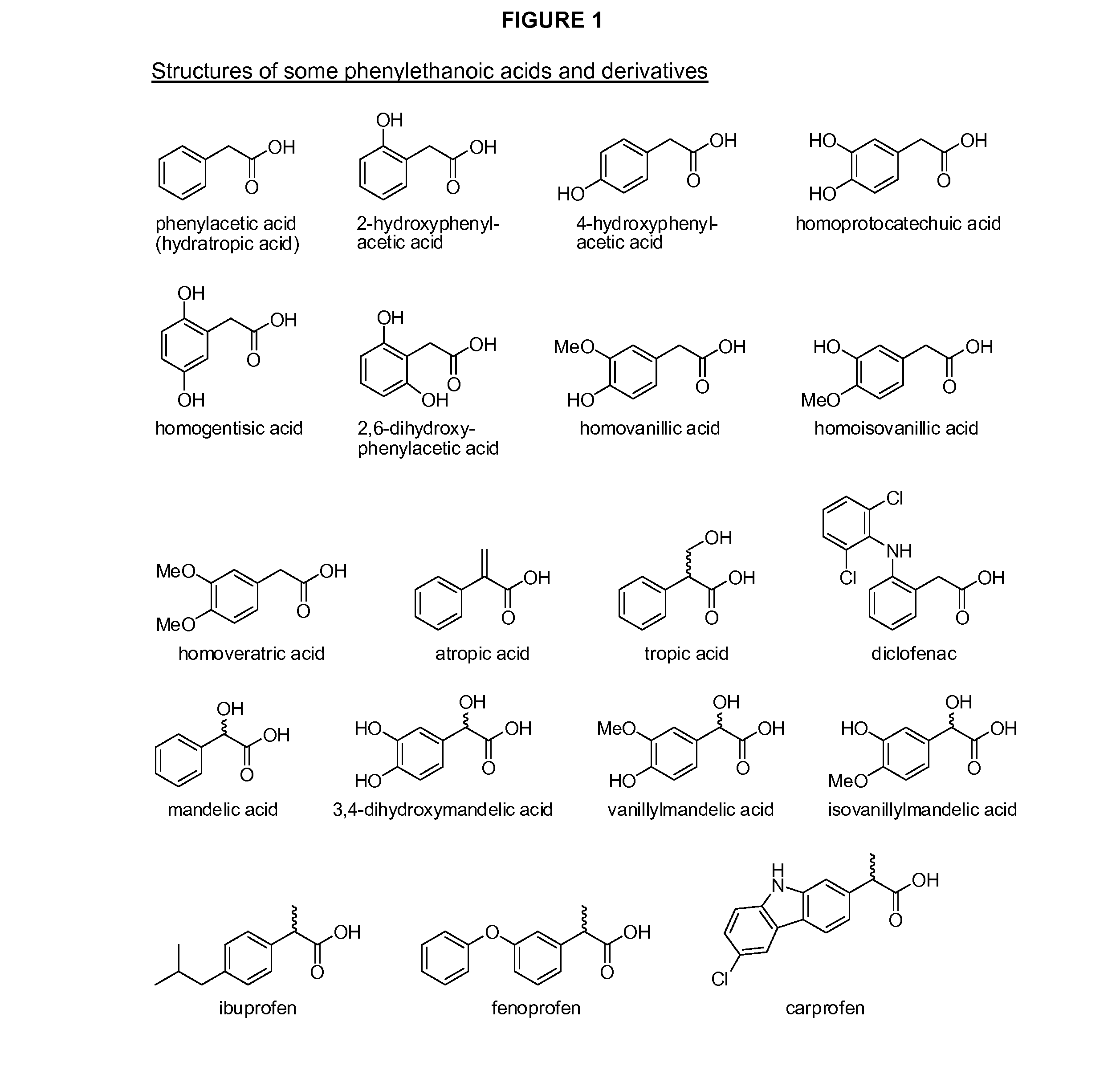
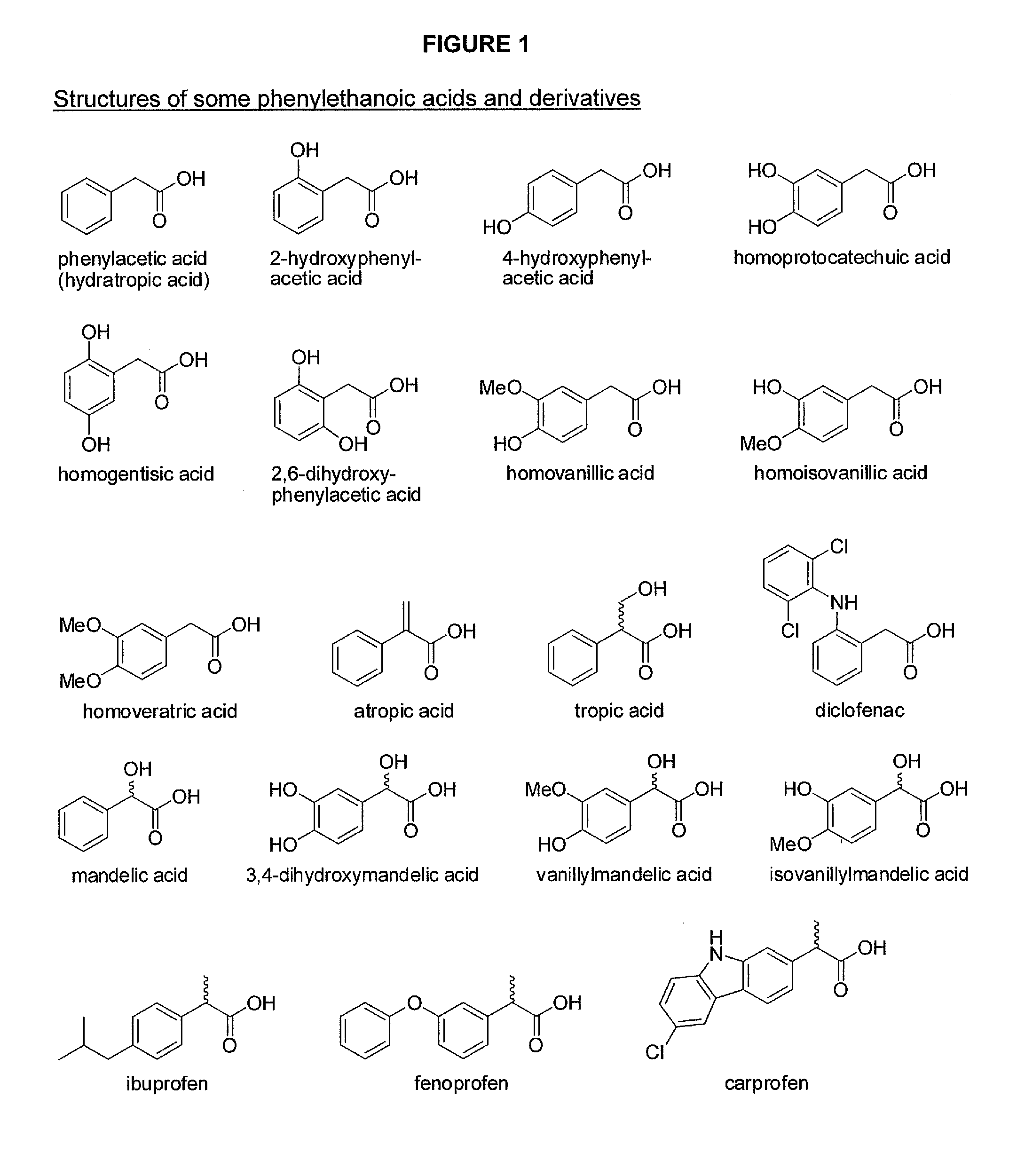
Phenylethanoic acid, phenylpropanoic acid and phenylpropenoic acid conjugates and prodrugs of hydrocodone, method of making and use thereof
ActiveUS20110002991A1Lower potentialReduce addictionBiocideNervous disorder3-phenylpropanoic acidEpoxy
The presently described technology provides phenylethanoic acid, phenylpropanoic acid, phenylpropenoic acid, a salt thereof, a derivative thereof or a combination thereof chemically conjugated to hydrocodone (morphinan-6-one, 4,5-alpha-epoxy-3-methoxy-17-methyl) to form novel prodrugs or compositions of hydrocodone which have a decreased potential for abuse of hydrocodone. The present technology also provides methods of treating patients, pharmaceutical kits and methods of synthesizing conjugates of the present technology.
Owner:KEMPHARM INC
Methods of producing stabilized solid dosage pharmaceutical compositions containing morphinans
Methods for producing stabilized solid dosage form pharmaceutical compositions are provided. In particular, methods for preparing protected granules containing morphinans, and solid dosage form pharmaceutical compositions produced using the morphinan-protected granules are provided.
Owner:MALLINCKRODT INC
Methods of Producing Stabilized Solid Dosage Pharmaceutical Compositions Containing Morphinans
Methods for producing stabilized solid dosage form pharmaceutical compositions are provided. In particular, methods for preparing protected granules containing morphinans, and solid dosage form pharmaceutical compositions produced using the morphinan-protected granules are provided.
Owner:MALLINCKRODT INC
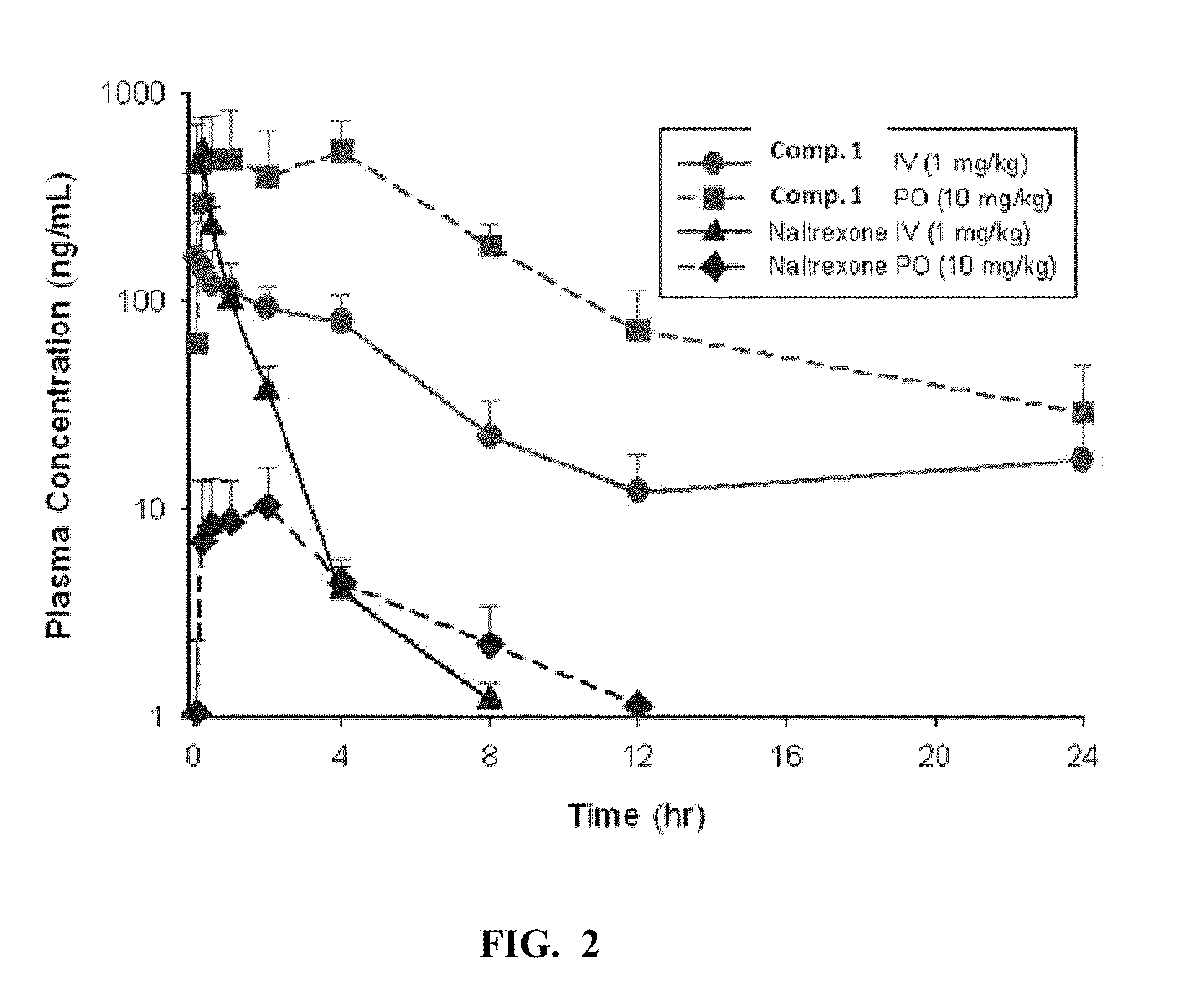
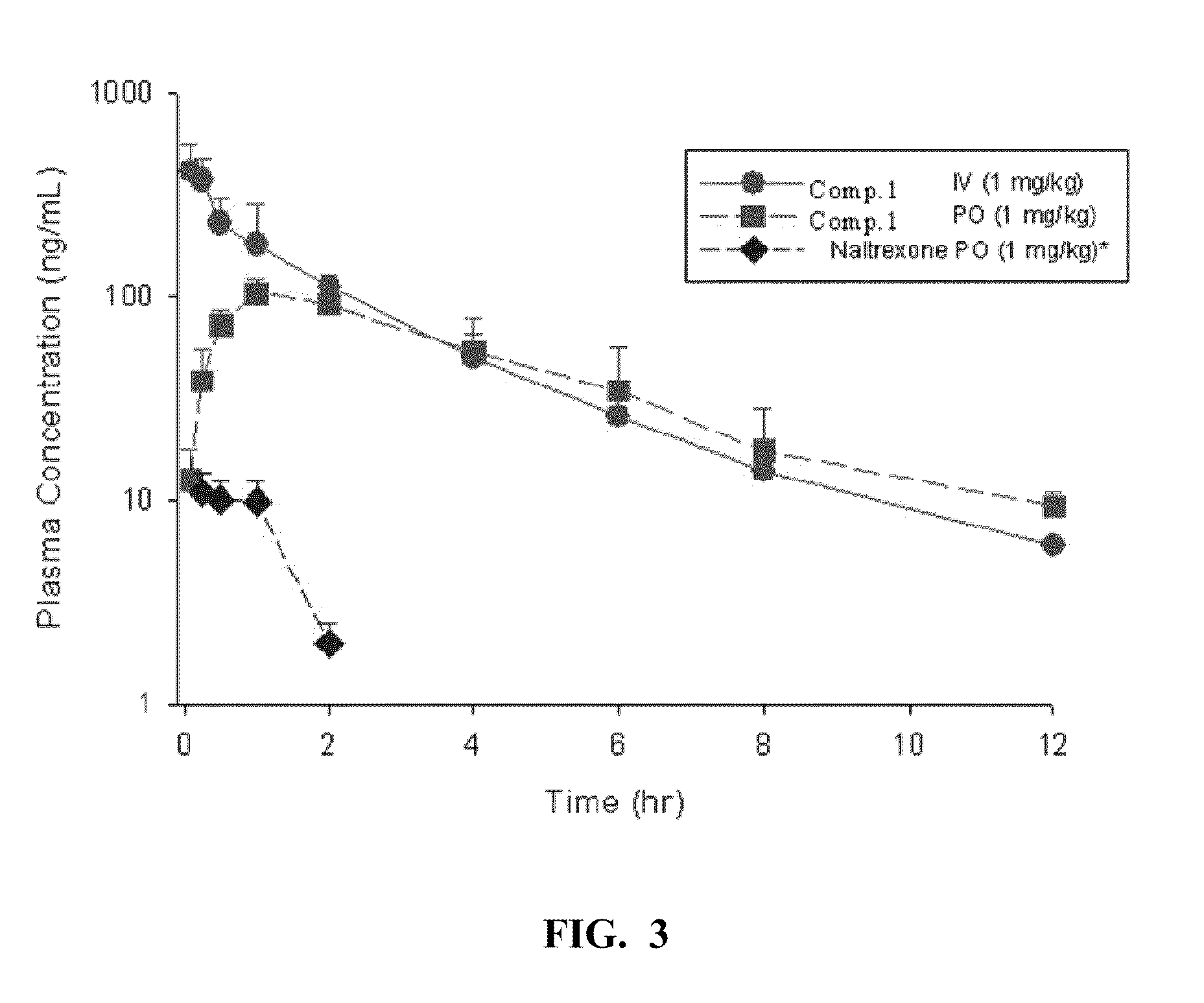
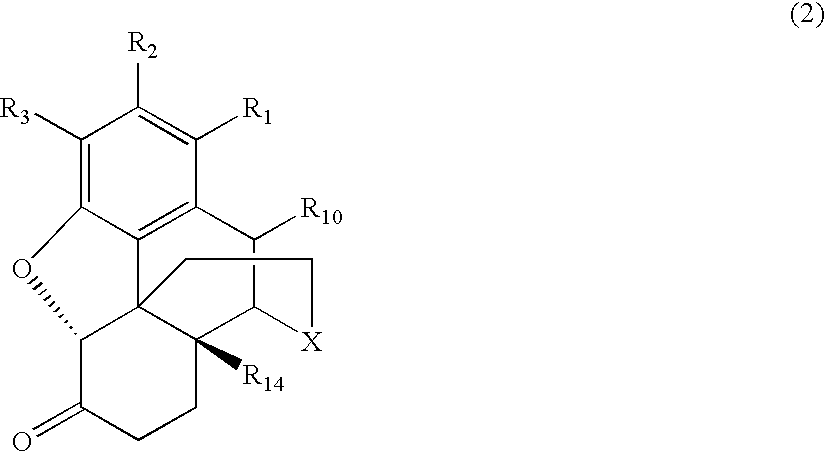
Morphinan derivatives with high oral bioavailability
InactiveUS20100240691A1Improve oral bioavailabilityImprove bioavailabilityBiocideNervous disorderDiseaseAlcohol
Owner:ALKERMES PHARMA IRELAND LTD
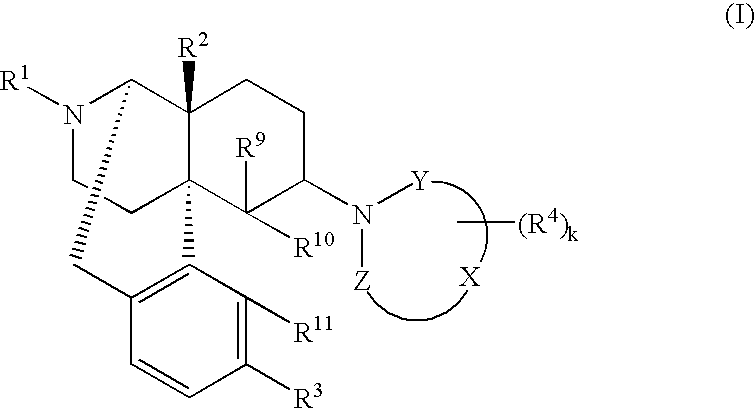
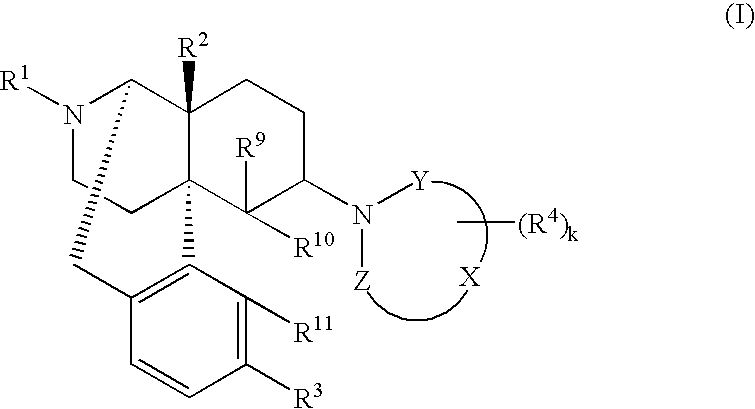
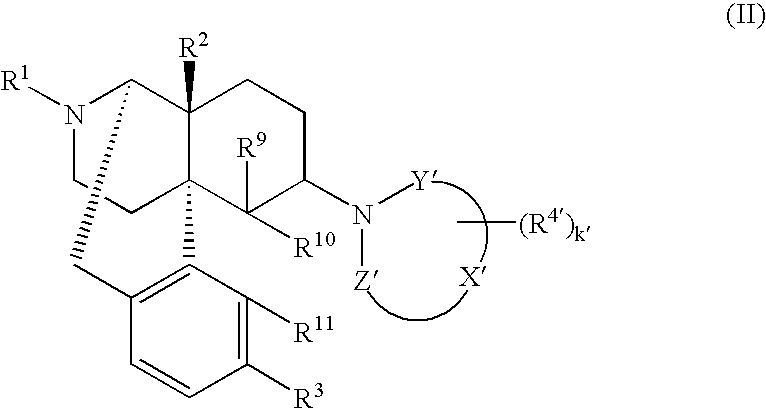
Remedies or preventives for urinary frequency or urinary incontinence and morphinan derivatives having nitrogen-containing heterocyclic group
InactiveUS20060040970A1Efficient processImprovement of side effectsBiocideOrganic chemistryAdditive ingredientMorphine
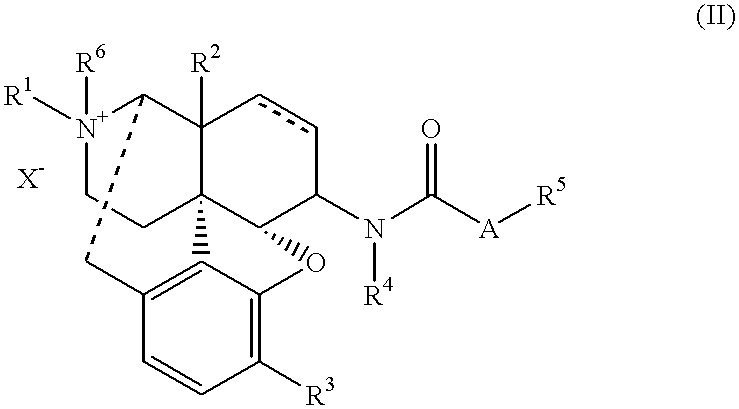
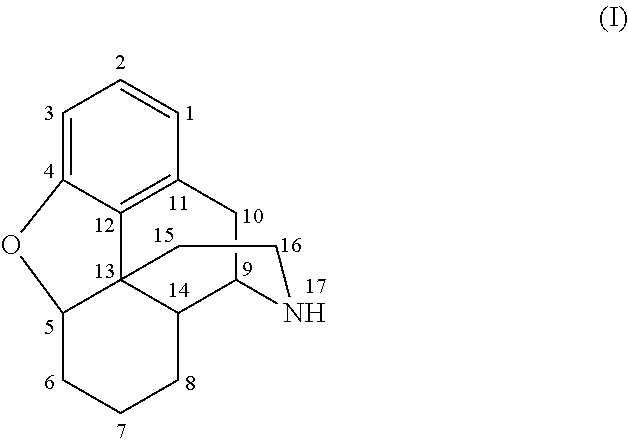
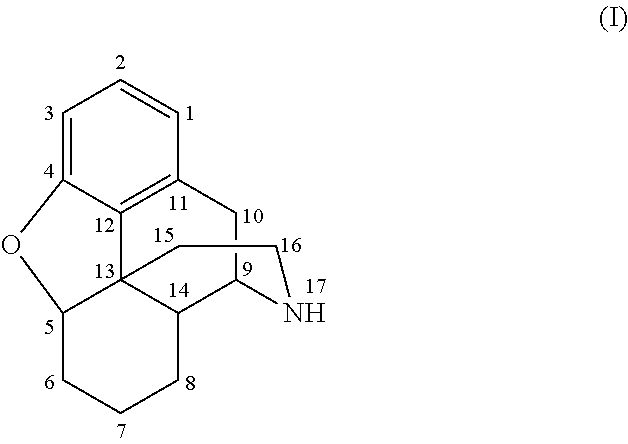
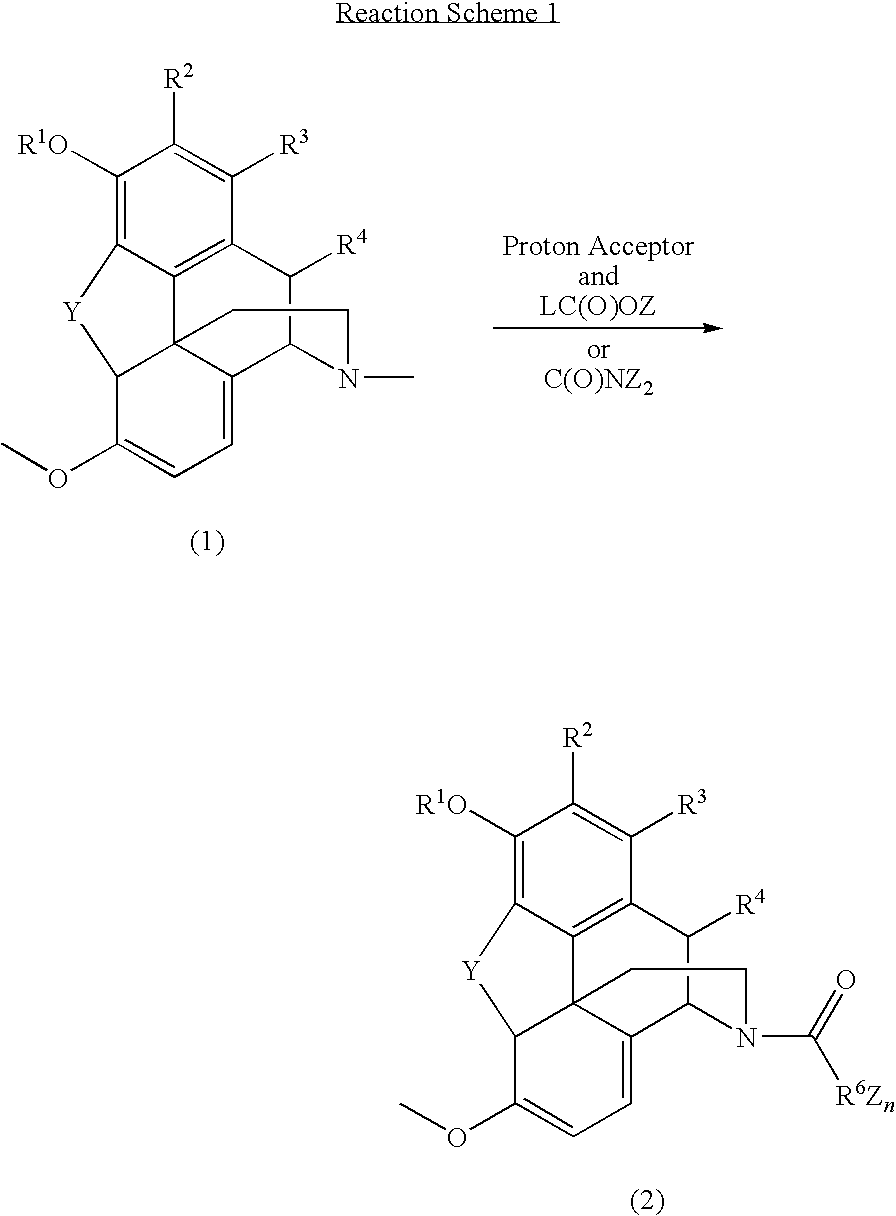
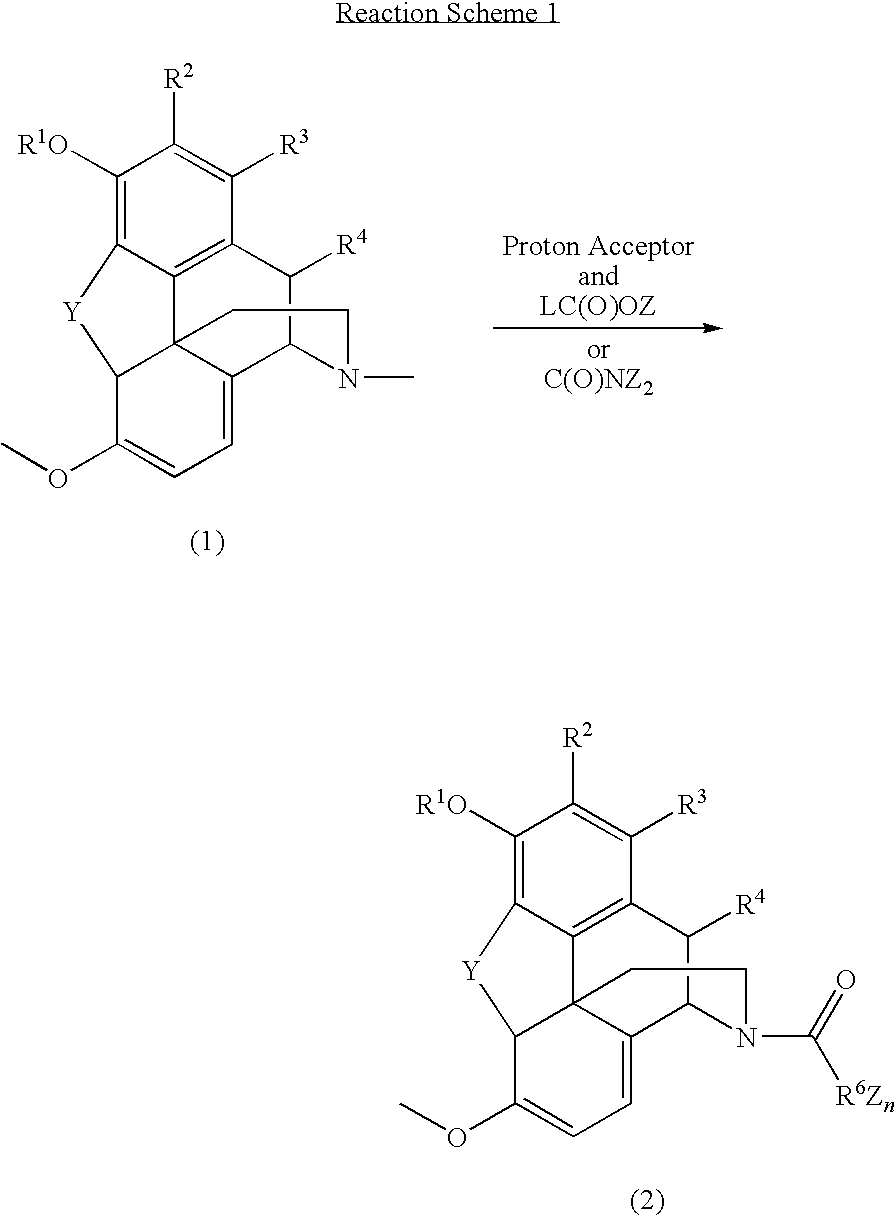
The invention provides a morphinan derivative of the Formula (I): (wherein R1 is methyl, cyclopropylmethyl or the like; R2 and R3 are hydroxy, methoxy, acetoxy or the like; both Y and Z are valence bonds, —C(═O)— or the like; X is C2-C5 carbon chain (one of the carbon atoms may be substituted by oxygen, sulfur or nitrogen) constituting a part of the ring structure, or the like; (R4)k is substituted or non-substituted benzene fused ring, carbonyl group or the like; R9 is hydrogen or the like; R10 and R11 are bound to represent —O—, or the like, and R6 is hydrogen or the like) or a pharmaceutically acceptable acid addition salt thereof. The invention also provides a therapeutic or prophylactic agent for urinary frequency or urinary incontinence, comprising as an effective ingredient the morphinan derivative or the pharmaceutically acceptable acid addition salt thereof; a method for therapy or prophylaxis of the diseases.
Owner:TORAY IND INC
PROCESS FOR MAKING MORPHINAN-6alpha-OLS
The present invention provides a process whereby morphinan-6-ones can be converted stereospecifically to the corresponding morphinan-6α-ols by catalytic hydrogenation under basic conditions.
Owner:TASMANIAN ALKALOIDS
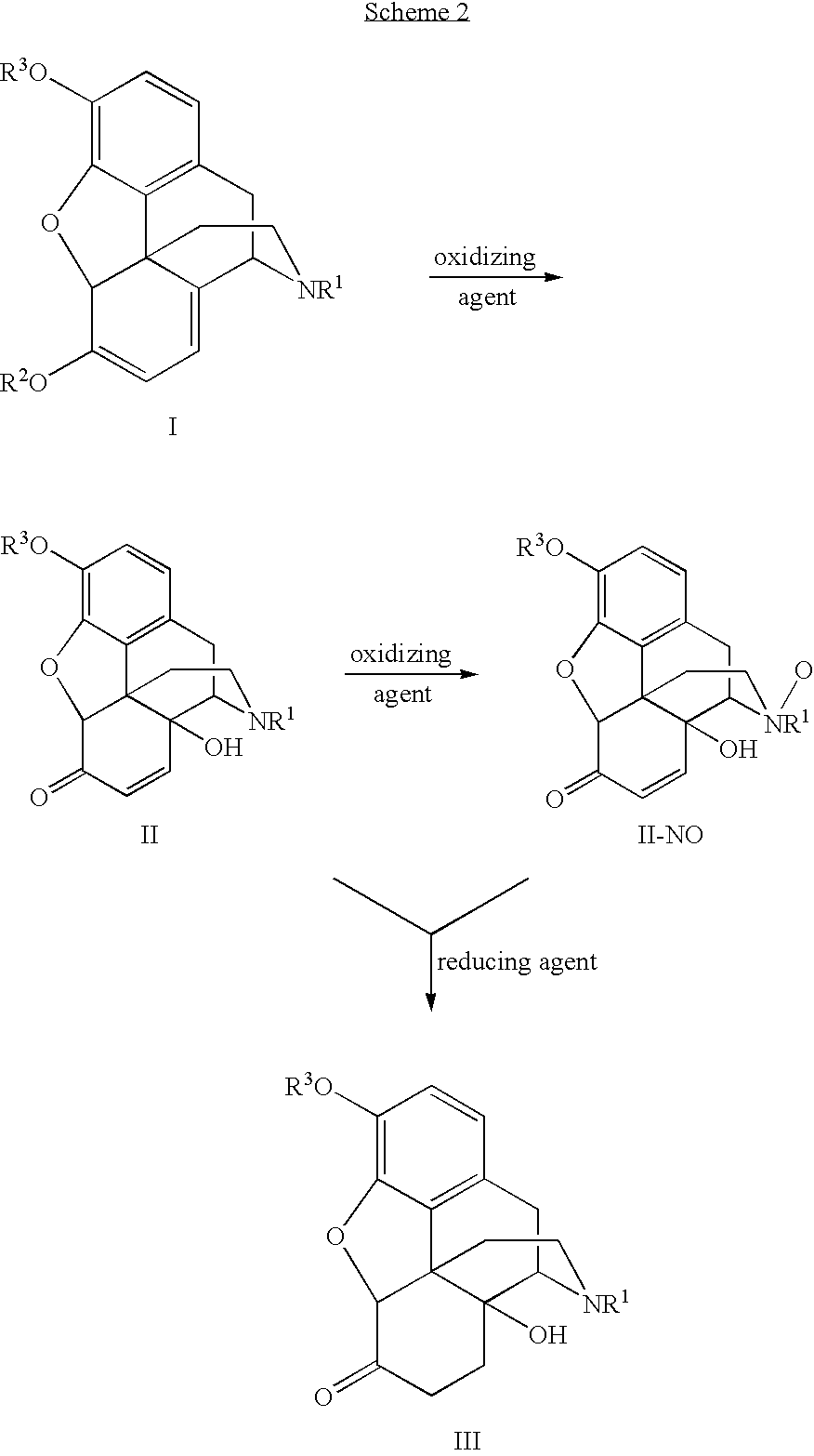
Stable medicinal compositions containing 4,5-epoxymorphinan derivatives
A stable pharmaceutical composition includes a 4,5-epoxy-morphinan derivative, and includes at least one of the group consisting of a water soluble antioxidant, a fat soluble antioxidant, a synergist, a sugar, and a surfactant.
Owner:TORAY IND INC
Phenylethanoic Acid, Phenylpropanoic Acid and Phenylpropenoic Acid Conjugates and Prodrugs of Hydrocodone, Methods of Making and Use Thereof
Owner:KEMPHARM INC
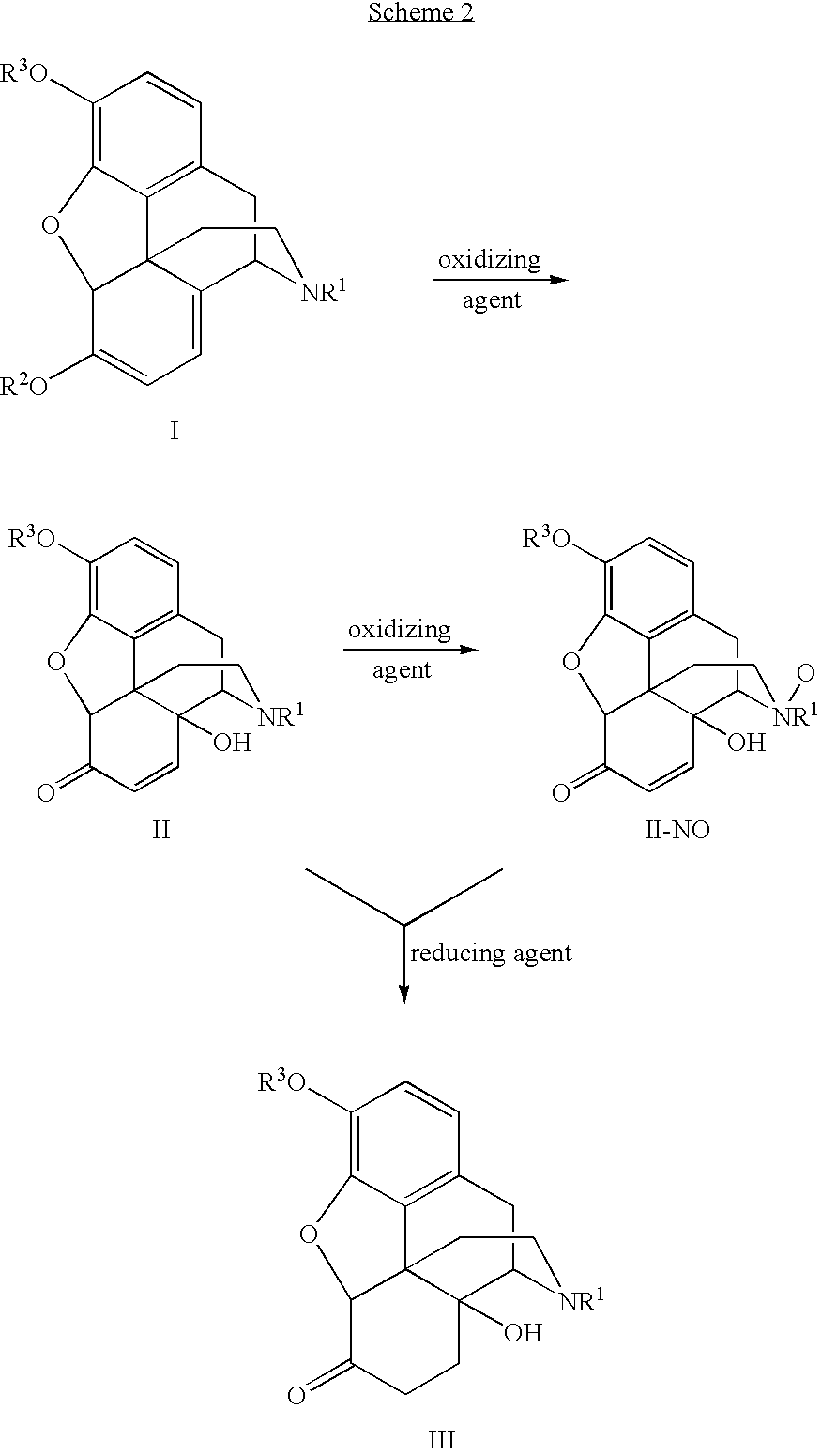
N-demethylation of N-methyl morphinans
The present invention provides a synthetic process for the N-demethylation of N-methyl morphinans. In particular, the invention provides improved synthetic methods for the preparation of N-demethylated morphinan compounds that may be employed as starting materials, for example, commonly available N-methyl opiates such as oripavine and thebaine, and C(3)-protected hydroxy derivatives of oripavine.
Owner:SPECGX LLC
Process for Preparing Morphinan-6-One Products with Low Levels of Alpha, Beta-Unsaturated Ketone Compounds
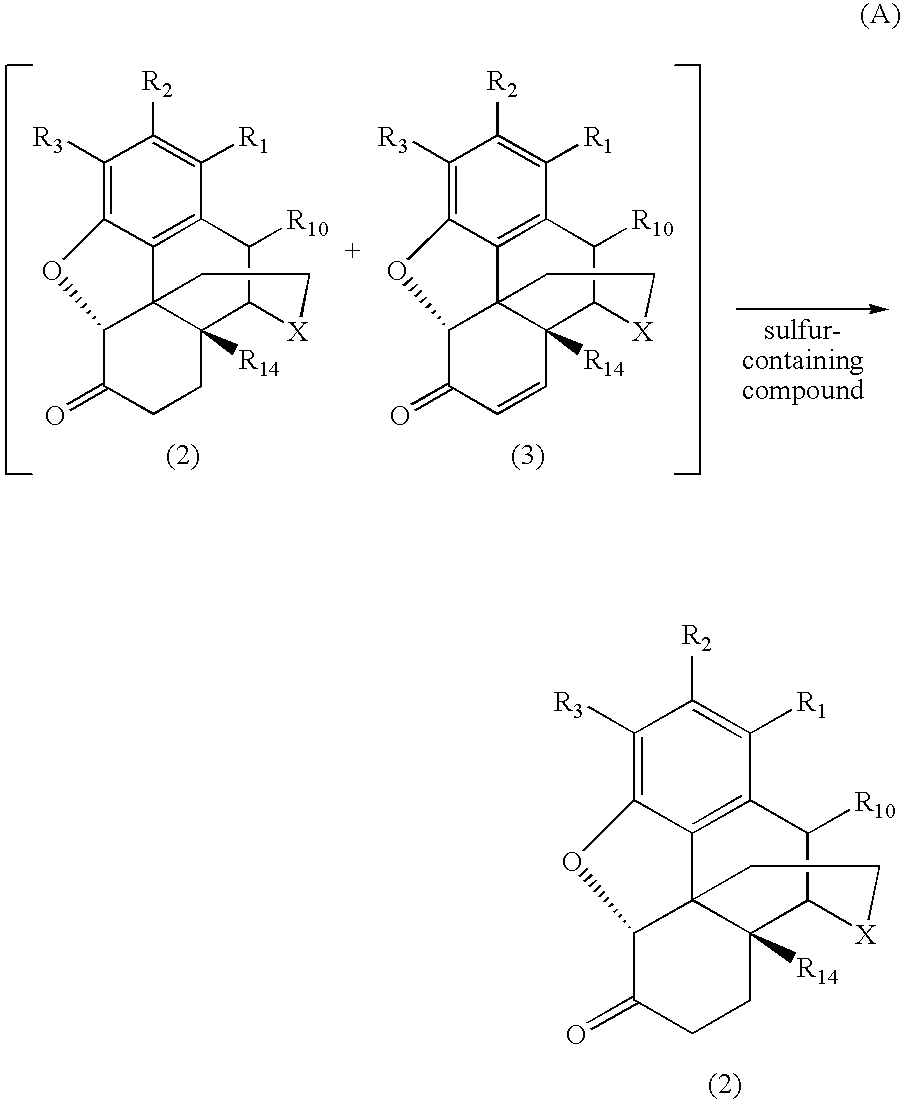
The present invention generally relates to processes for preparing highly pure morphinan-6-one products. The processes involve reducing the concentration of α,β-unsaturated ketone compounds present as impurities in morphinan 6 one products or reaction mixtures including morphinan 6 one compounds by treatment with a sulfur-containing compound. (A)
Owner:SPECGX LLC
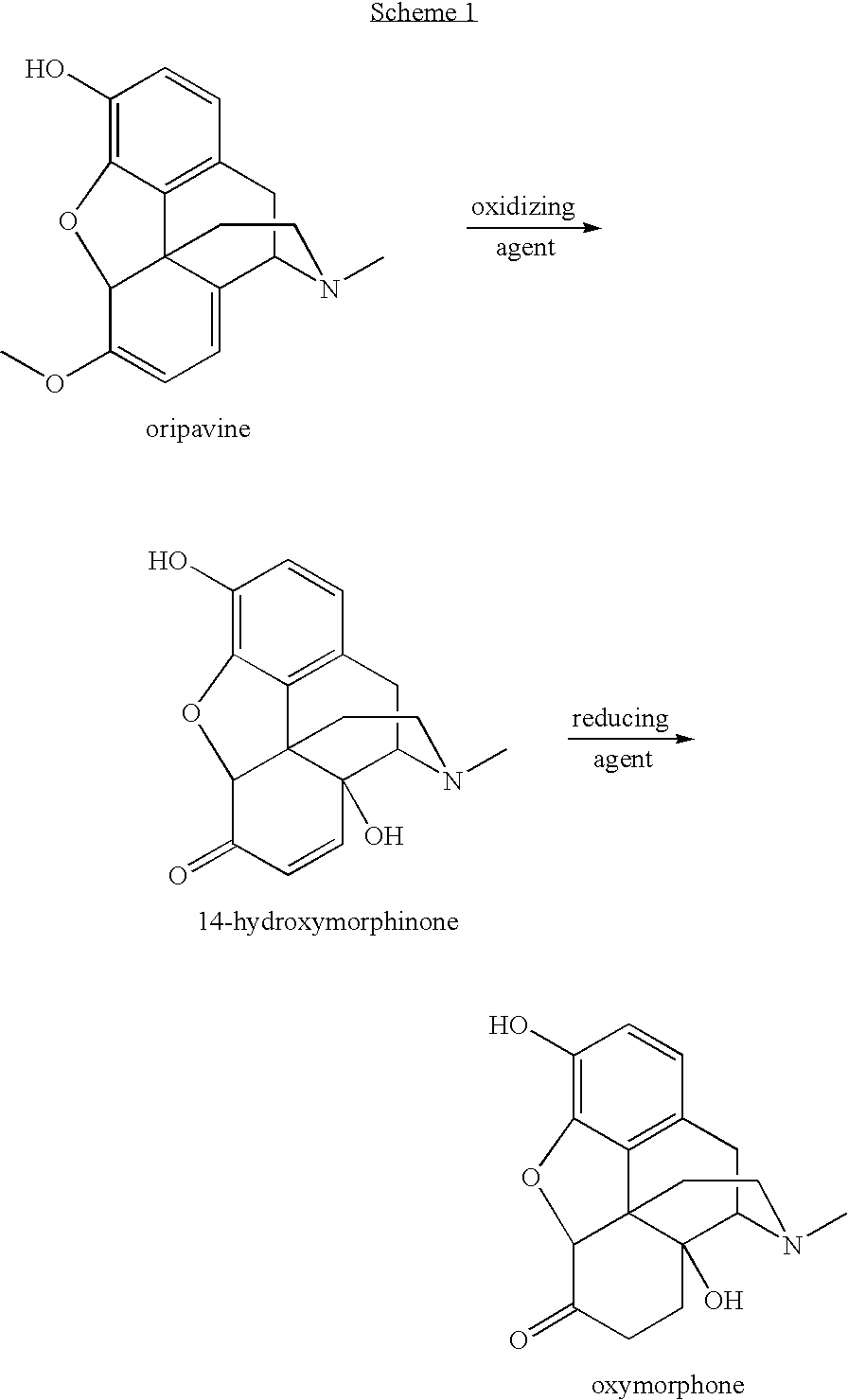
Preparation of oxymorphone from oripavine
An improved method for the preparation of oxymorphone from oripavine is provided. Oripavine is oxidized to form 14-hydroxymorphinone after which the oxidation reaction is quenched to prevent the formation of 1-1′-dimer side products. The 14-hydroxymorphinone is then reduced, typically by catalytic hydrogenation to form oxymorphone. The inventive method disclosed is further applicable to the production of morphinan derivatives.
Owner:SPECGX LLC
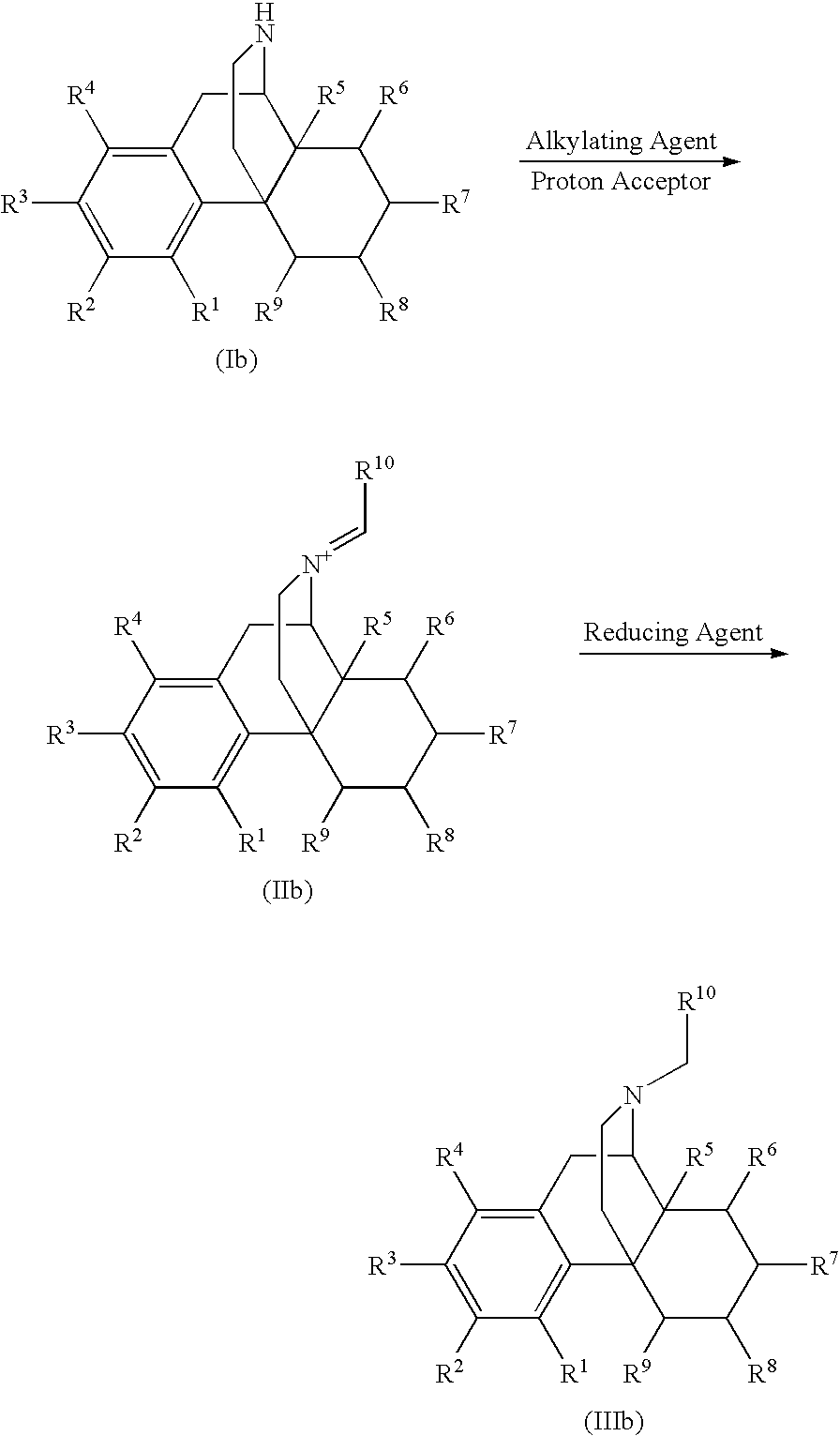
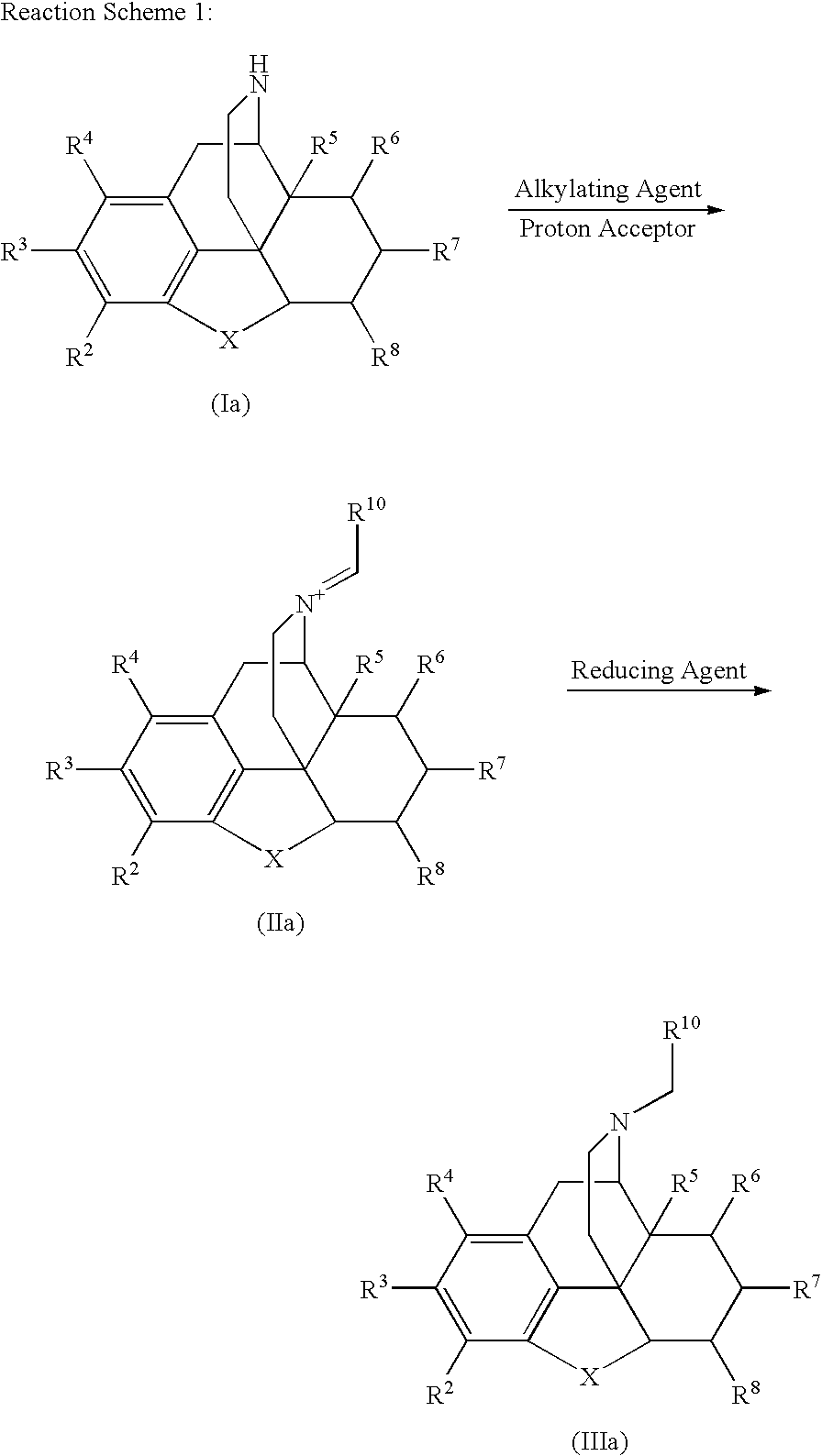
Process for the Reductive Alkylation of Normorphinans
The invention provides a process for the N-alkylation of normorphinan compounds to produce N-alkylated morphinan compounds. In particular, the process relates to the alkylation of a normorphinan compound by a carboxaldehyde in the presence of a reducing agent to form an N-alkylated morphinan.
Owner:SPECGX LLC
Benzoic acid, benzoic acid derivatives and heteroaryl carboxylic acid conjugates of hydrocodone, prodrugs, methods of making and use thereof
ActiveUS20120142719A1Lower potentialEliminate the effects ofBiocideNervous disorderBenzoic acidEpoxy
The presently described technology provides compositions comprising aryl carboxylic acids chemically conjugated to hydrocodone (morphinan-6-one, 4,5-alpha-epoxy-3-methoxy-17-methyl) to form novel prodrugs / compositions of hydrocodone, including benzoates and heteroaryl carboxylic acids, which have a decreased potential for abuse of hydrocodone. The present technology also provides methods of treating patients, pharmaceutical kits and methods of synthesizing conjugates of the present technology.
Owner:KEMPHARM INC
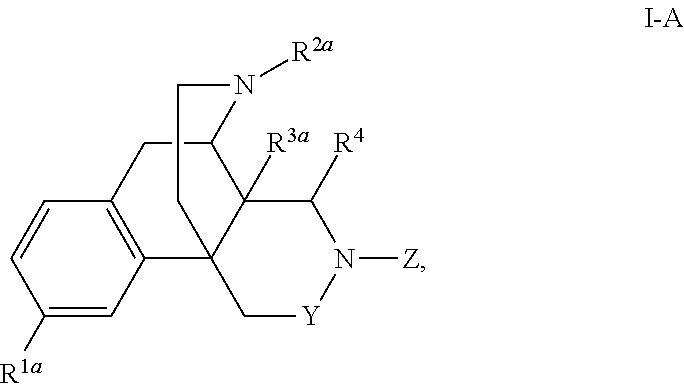
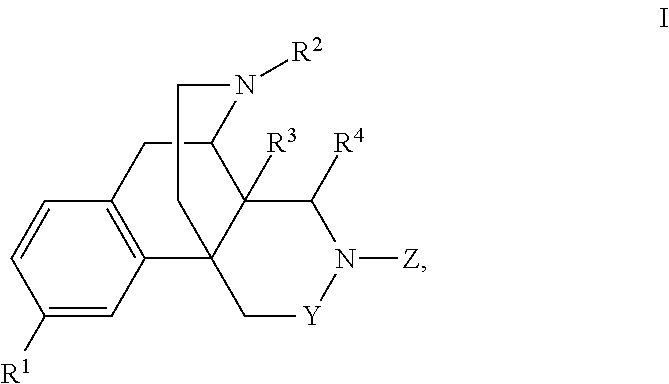
Nitrogen containing morphinan derivatives and the use thereof
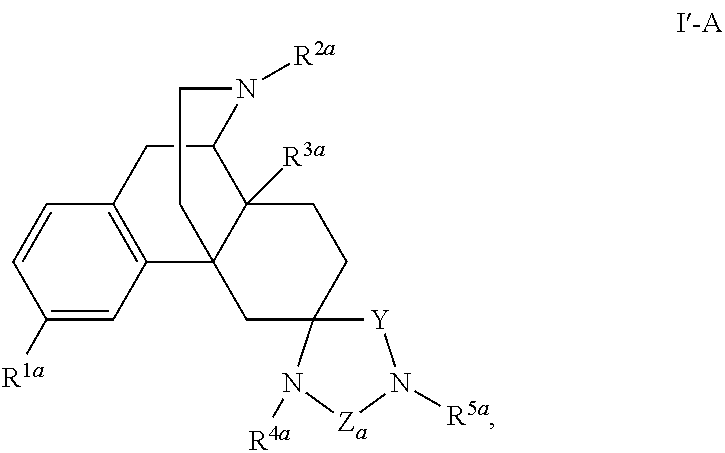
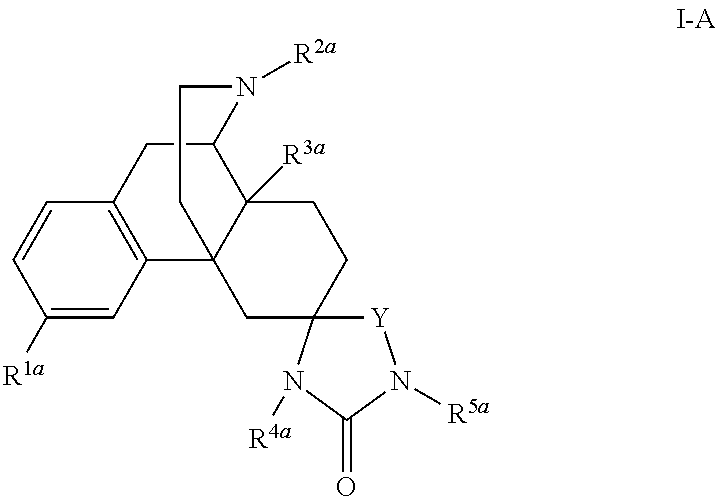
The application is directed to compounds of Formula I-Aand pharmaceutically acceptable salts and solvates thereof, wherein R1a-R3a, R4, Y, and Z are defined as set forth in the specification. The invention is also directed to use of compounds of Formula I-A to treat disorders responsive to the modulation of one or more opioid receptors, or as synthetic intermediates. Certain compounds of the present invention are especially useful for treating pain.
Owner:PURDUE PHARMA LP
Antipruritic
Owner:TORAY IND INC
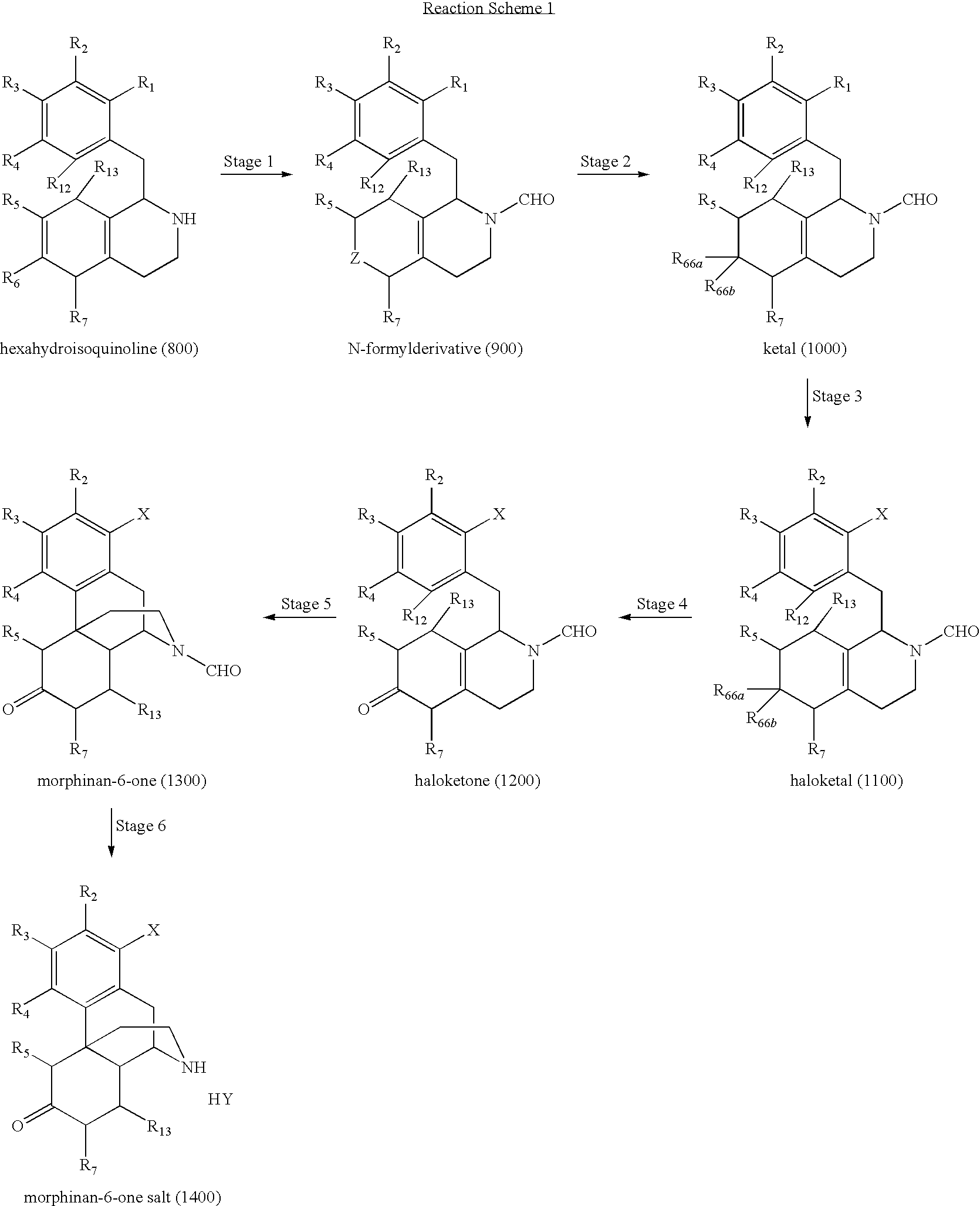
Preparation of Substituted Morphinan-6-Ones and Salts and Intermediates Thereof
The present invention is directed to processes for the synthesis of morphinan-6-ones and salts, intermediates, and analogs thereof.
Owner:SPECGX LLC
6,7-unsaturated-7-carbamoyl substituted morphinan derivative
ActiveUS20090203723A1Prevent constipationEliminate side effectsBiocideNervous disorderArylAcyl group
A novel compound which is useful as an agent for treating and / or preventing emesis, vomiting and / or constipation.A compound represented by the formula (I):wherein R1 and R2 are each independently hydrogen, optionally substituted lower alkyl, optionally substituted lower alkenyl, optionally substituted cycloalkyl, optionally substituted aryl etc., R3 is hydrogen, hydroxy, optionally substituted lower alkyl, optionally substituted lower alkenyl, optionally substituted lower alkynyl, optionally substituted lower alkoxy etc., R4 is hydrogen or lower alkyl, R5 is hydrogen, lower alkyl, cycloalkyl lower alkyl or lower alkenyl,or a pharmaceutically acceptably salt, or a solvate thereof is provided.
Owner:SHIONOGI & CO LTD
Compositions of (-)-17-(cyclobutylmethyl)morphinan-3,14-diol
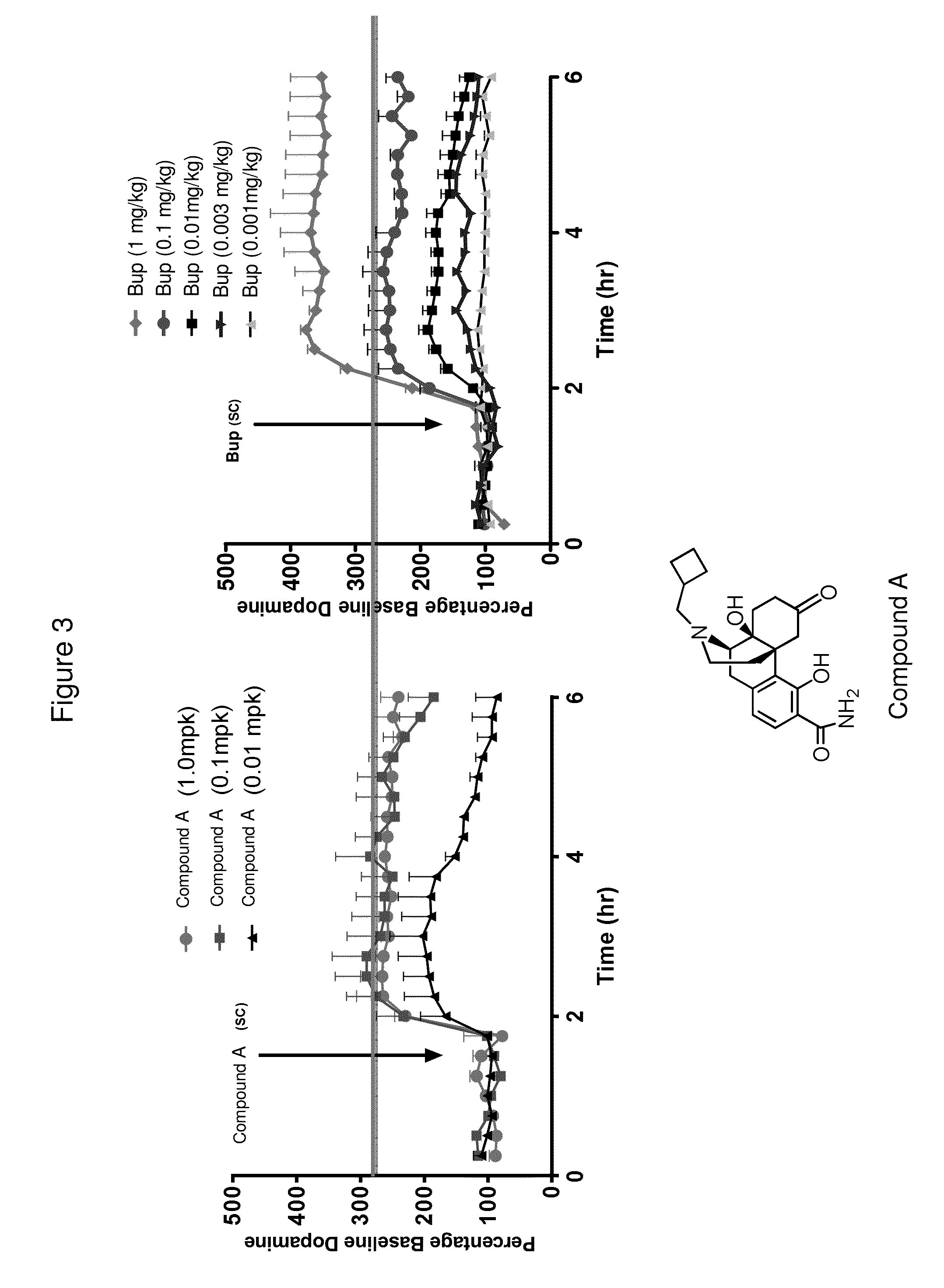
ActiveUS9364430B2Increase rangeSlow onsetOrganic active ingredientsGranular deliveryModified Release Dosage FormMorphinan
The present invention is directed to oral, therapeutically effective modified release pharmaceutical compositions of (−)-17-(cyclobutylmethyl)morphinan-3,14-diol and it pharmaceutically acceptable salts and the use thereof, including delayed onset and extended release dosage forms. The present invention is also directed at modified release dosage forms of oral (−)-17-(cyclobutylmethyl)morphinan-3,14-diol which provide robust efficacy and reduced potential for abuse and misuse.
Owner:RELMADA THERAPEUTICS
Spirocyclic morphinans and their use
Owner:PURDUE PHARMA LP
N-demethylation of N-methyl morphinans
The present invention provides a synthetic process for the N-demethylation of N-methyl morphinans. In particular, the invention provides improved synthetic methods for the preparation of N-demethylated morphinan compounds that may be employed as starting materials, for example, commonly available N-methyl opiates such as oripavine and thebaine, and C(3)-protected hydroxy derivatives of oripavine.
Owner:SPECGX LLC
Cytochrome P450 fusion protein
ActiveUS10006010B2Good flexibilityEfficient and rapid and easy assemblyAntibody mimetics/scaffoldsOxidoreductasesMorphinansCytochrome P450
This disclosure relates to the isolation of a nucleic acid molecule[s] that encode a novel cytochrome P450 and an oxidoreductase from a Papaver somniferum [P. somniferum] cultivar, transgenic cells transformed with said nucleic acid molecule and sequence variants thereof; and including methods for the production of intermediates in the production of morphinans.
Owner:THE UNIV OF YORK +1
Preparation of 6-Alpha-Amino N-Substituted Morphinans by Catalytic Hydrogen Transfer
The present invention provides processes for the stereoselective synthesis of 6-alpha-amino N-substituted morphinans. In particular, the invention provides processes for the reductive amination of 6-keto N-substituted morphinans by catalytic hydrogen transfer.
Owner:SPECGX LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com