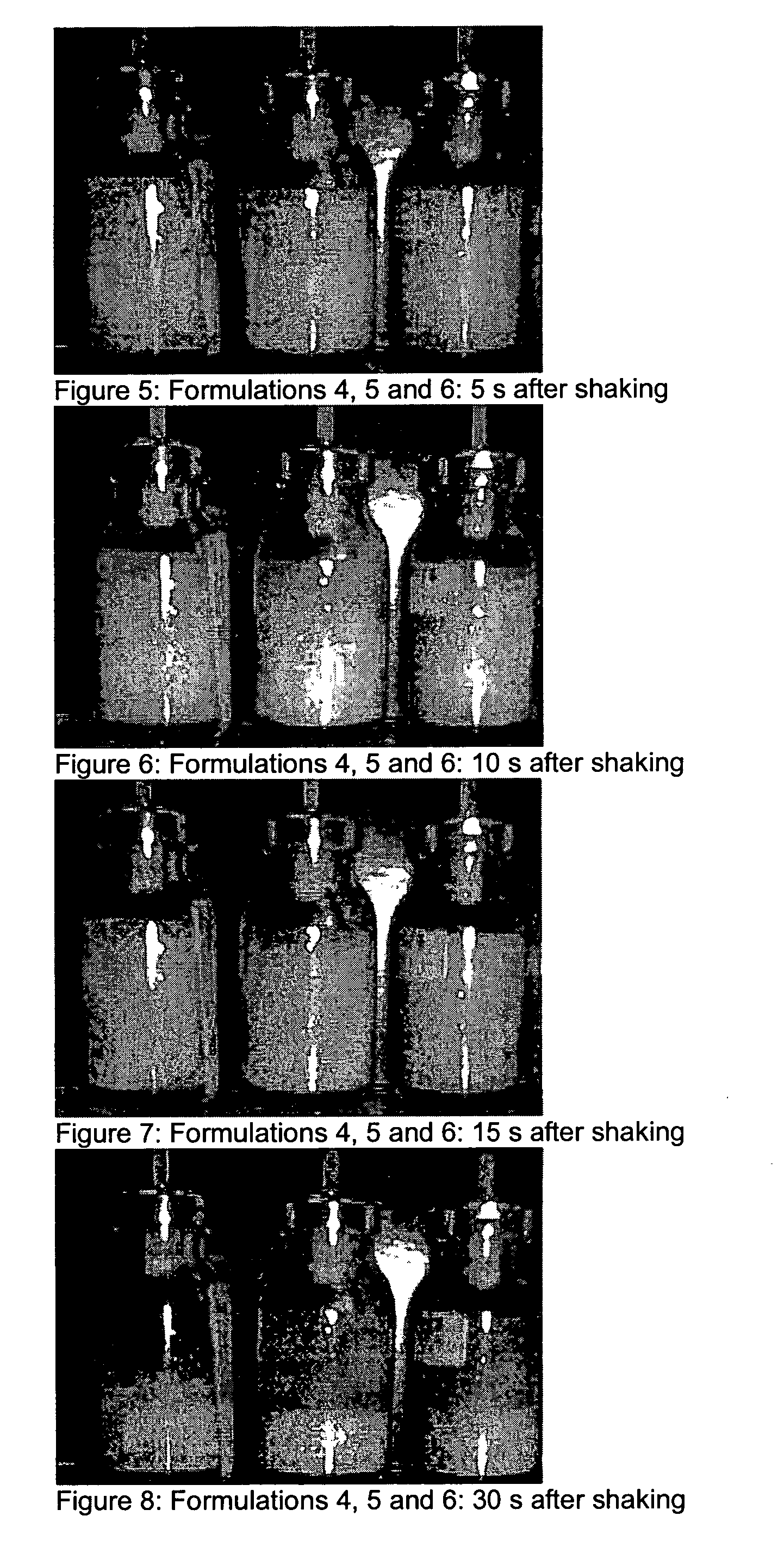
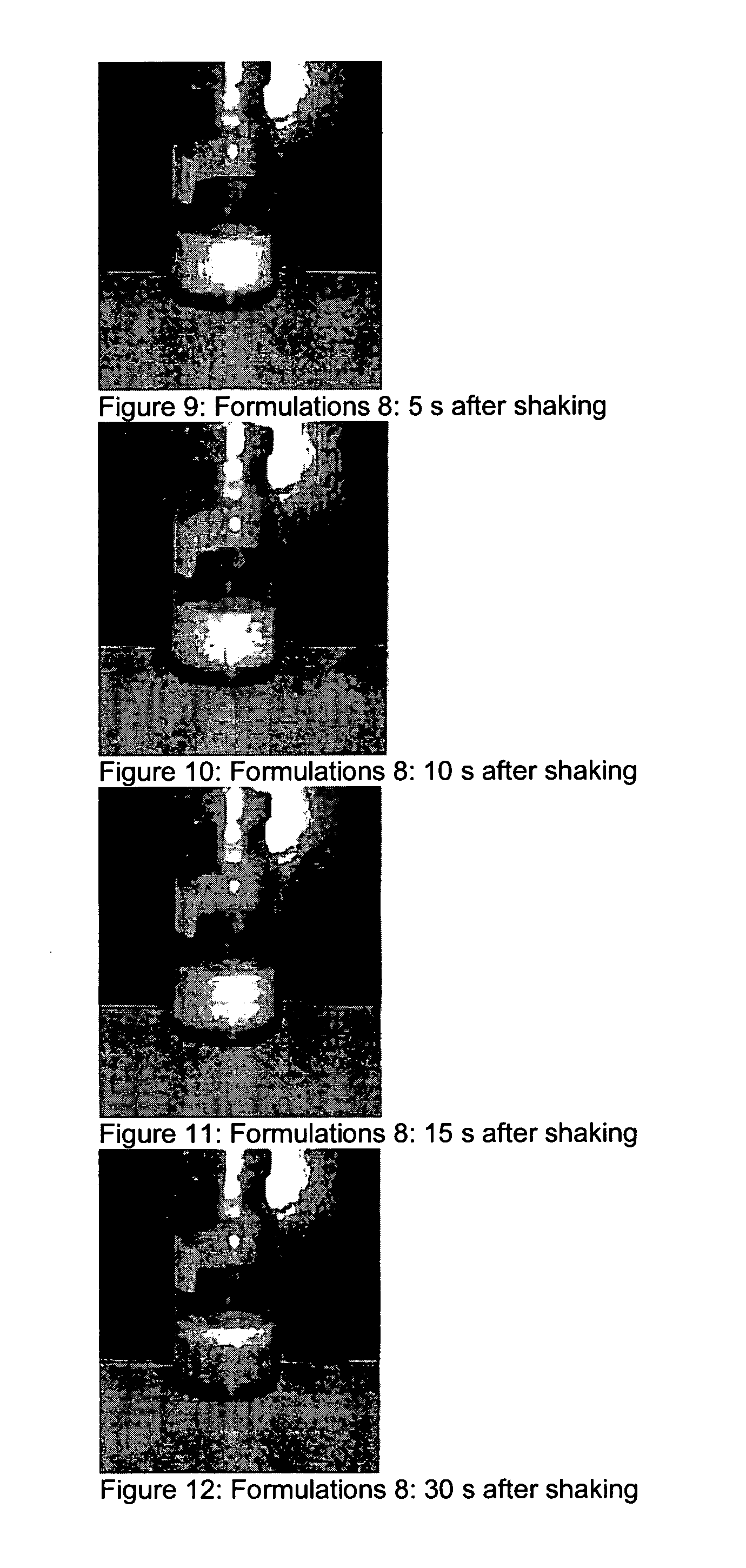
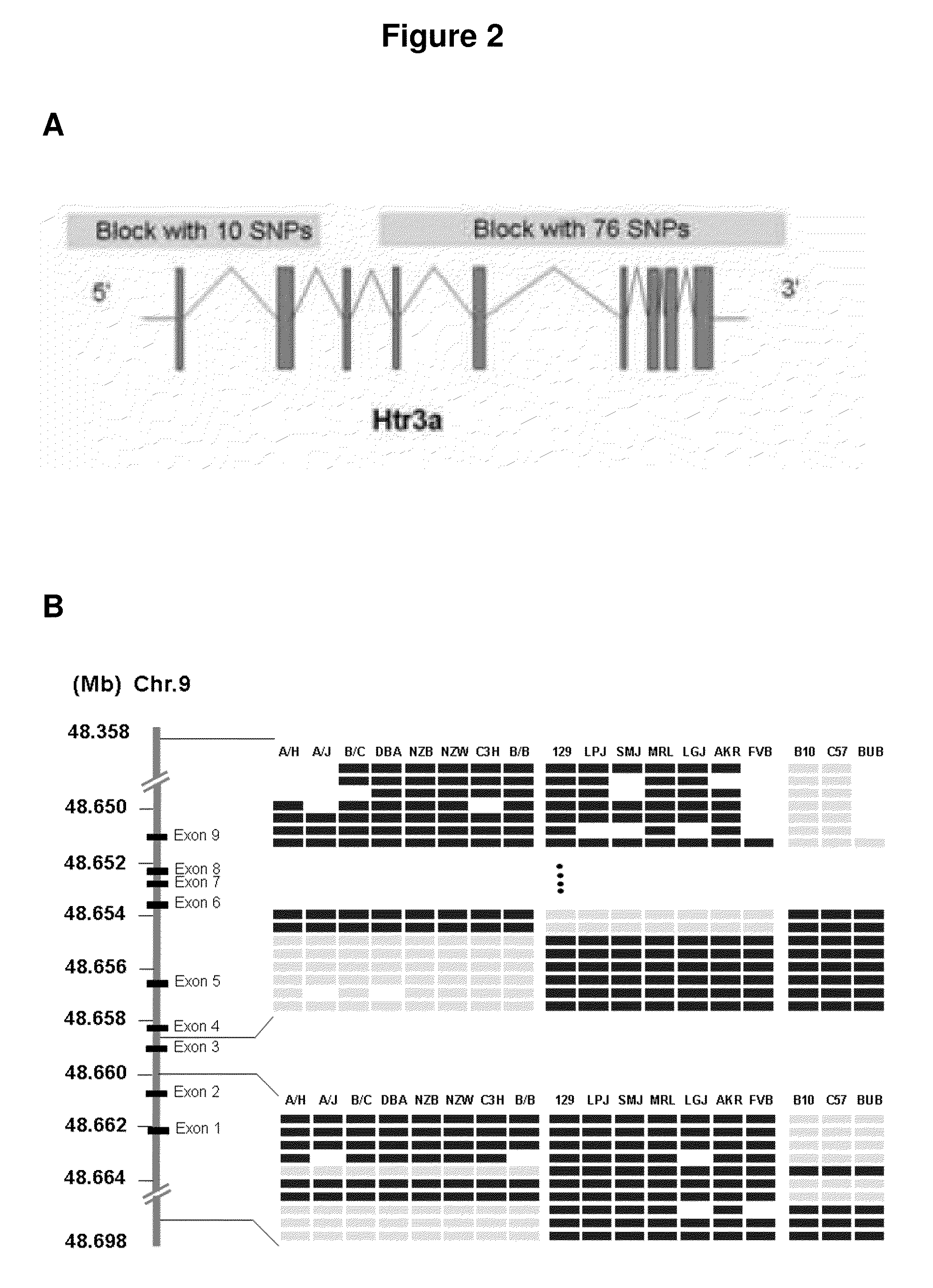
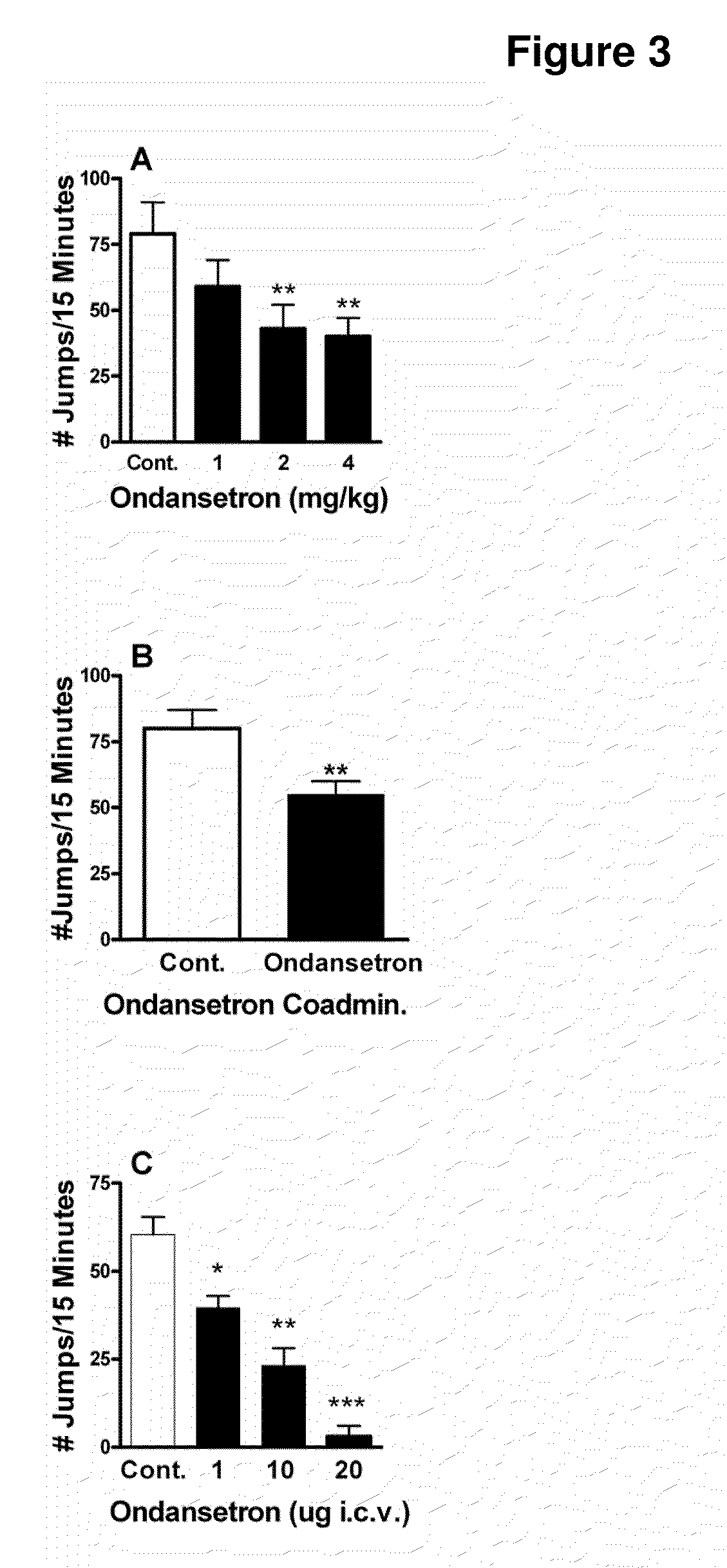
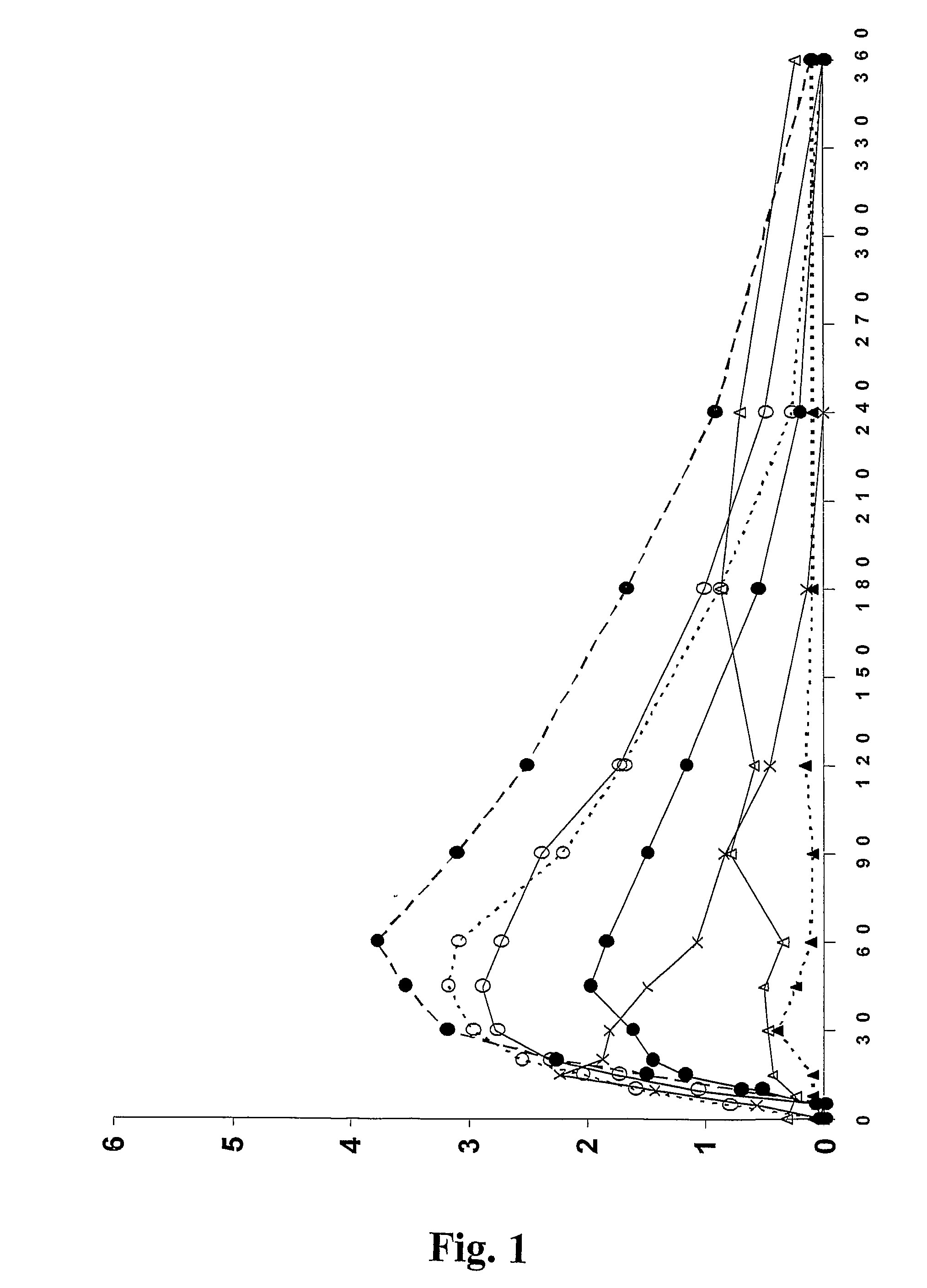
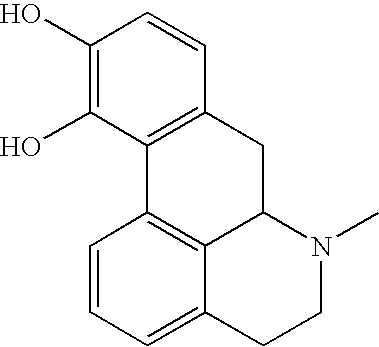
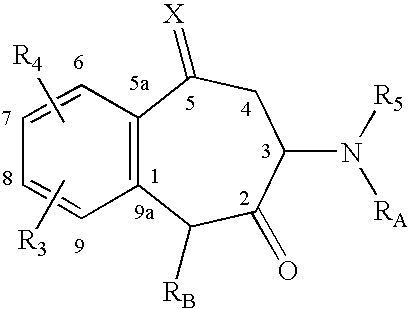
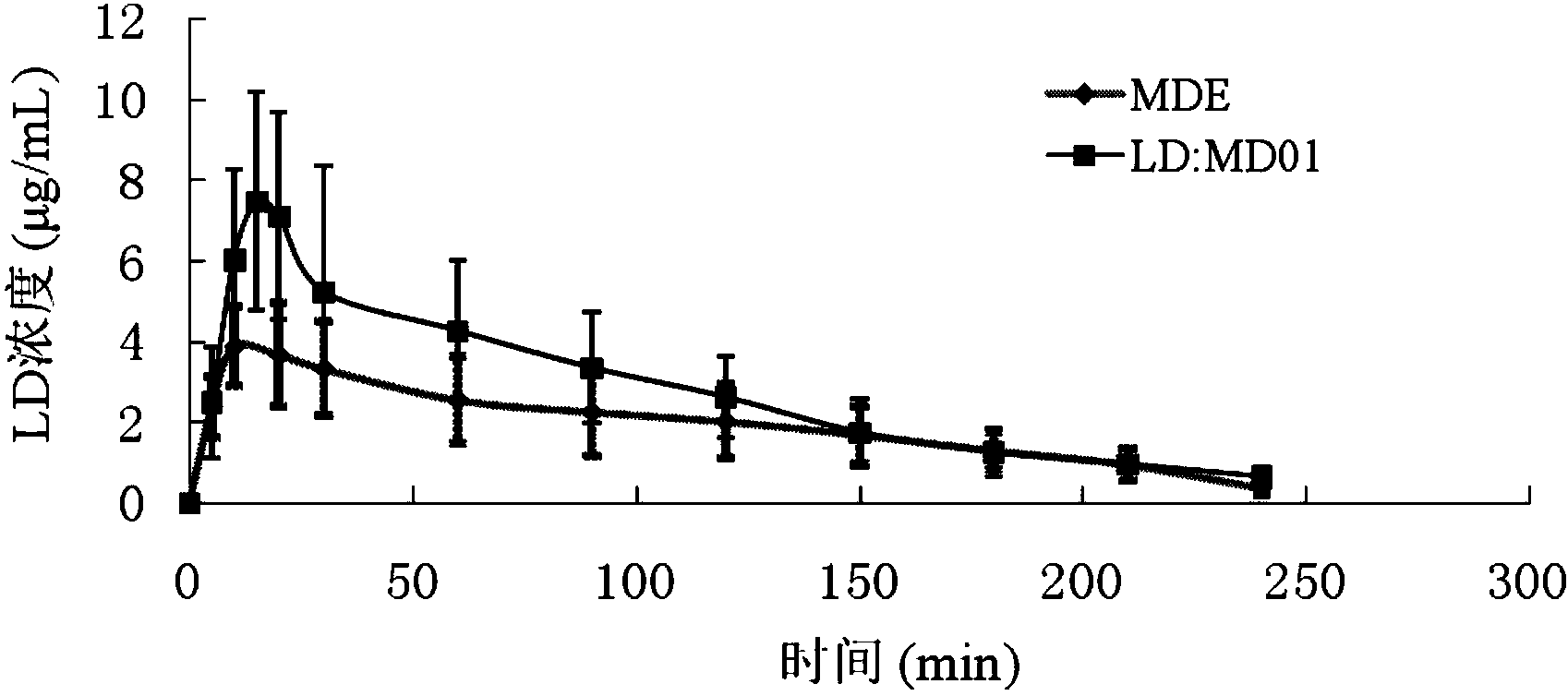
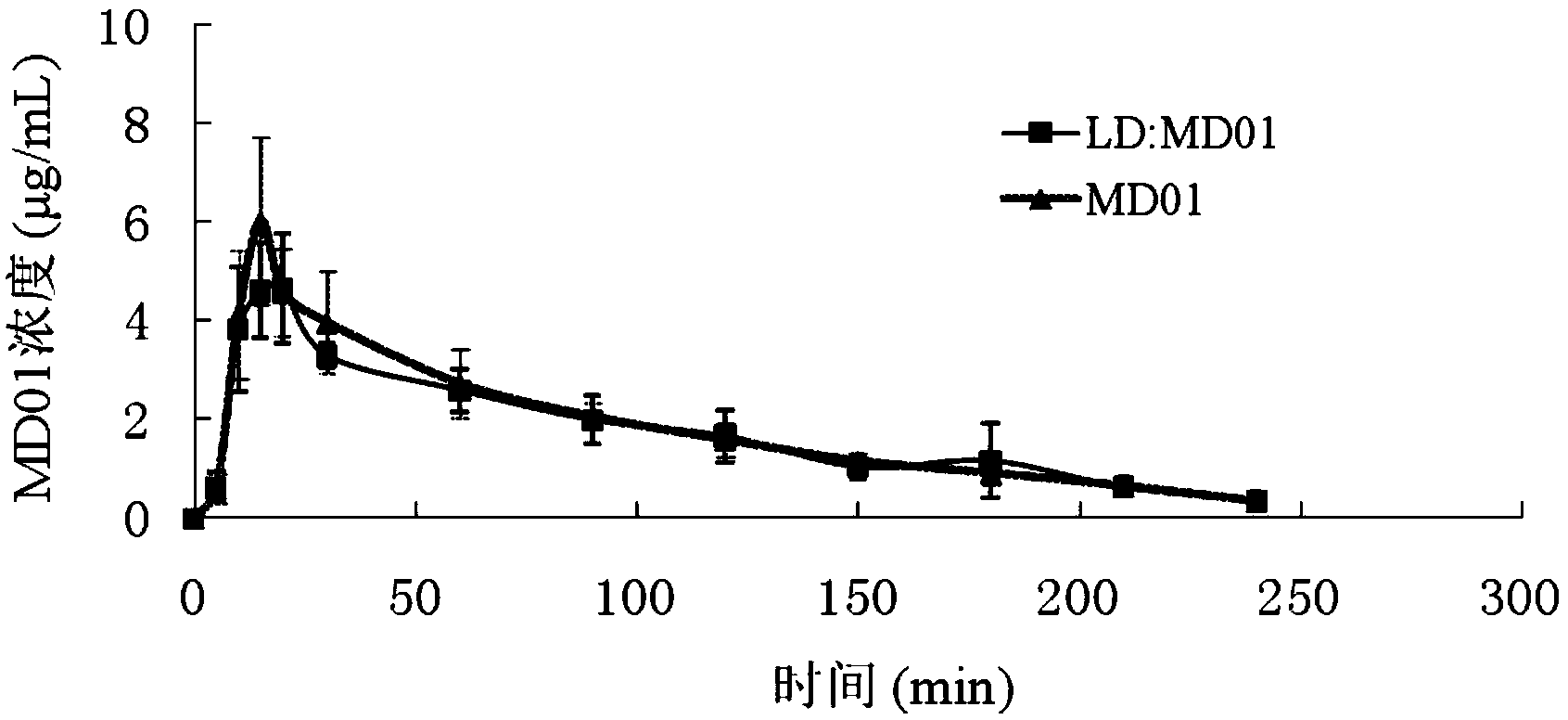
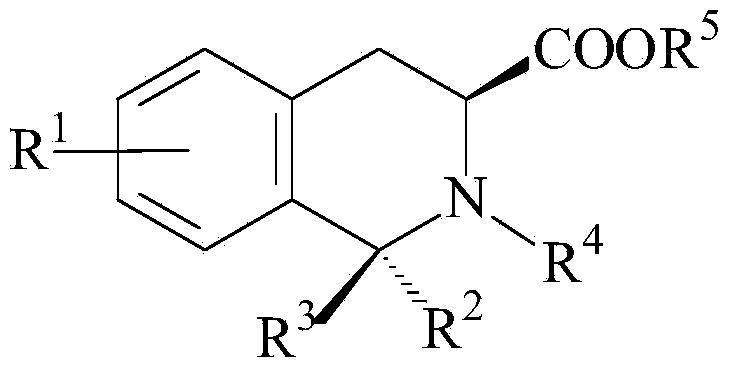
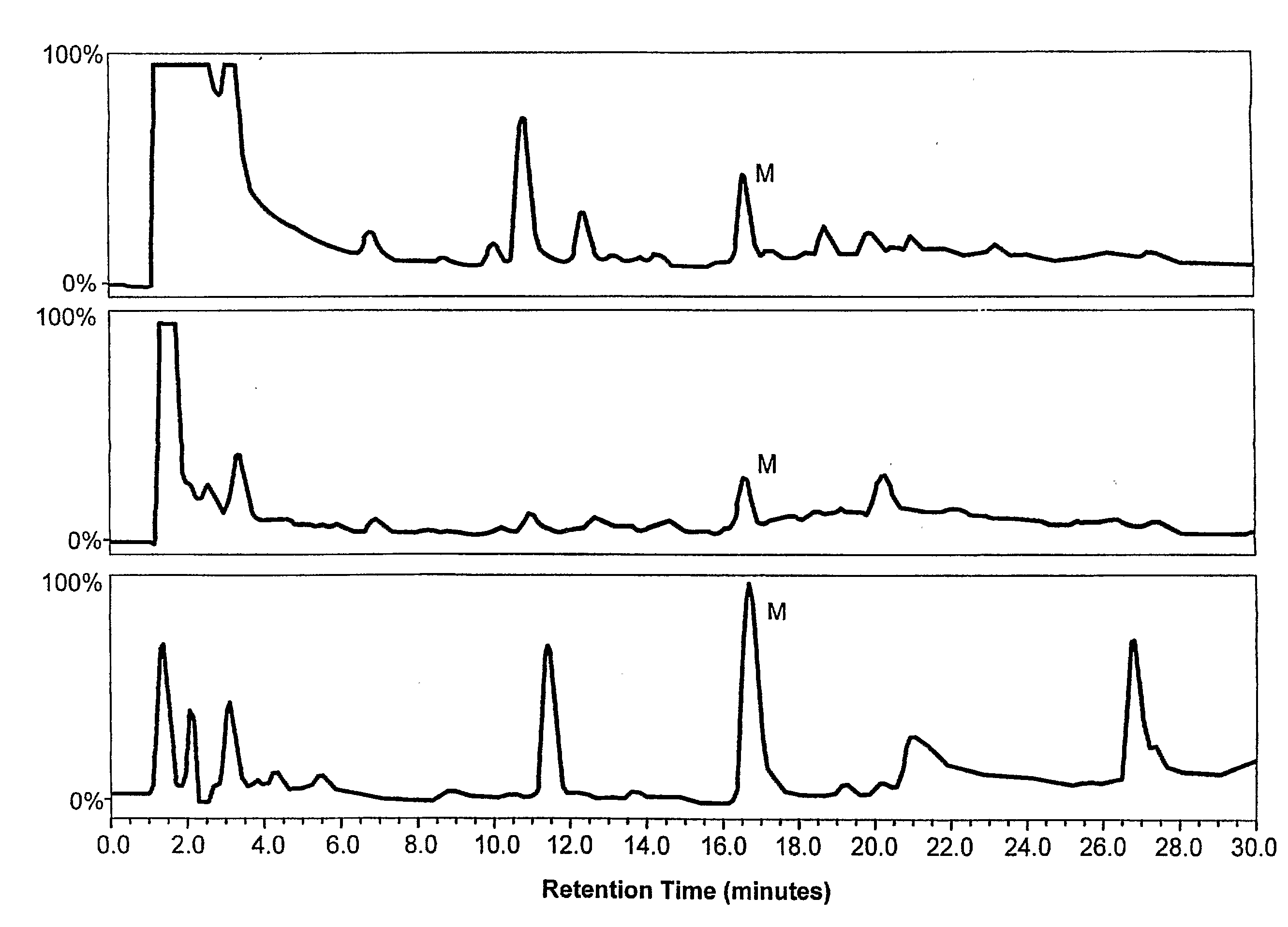
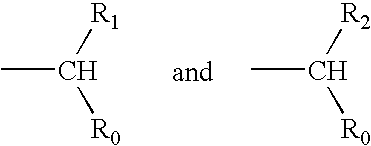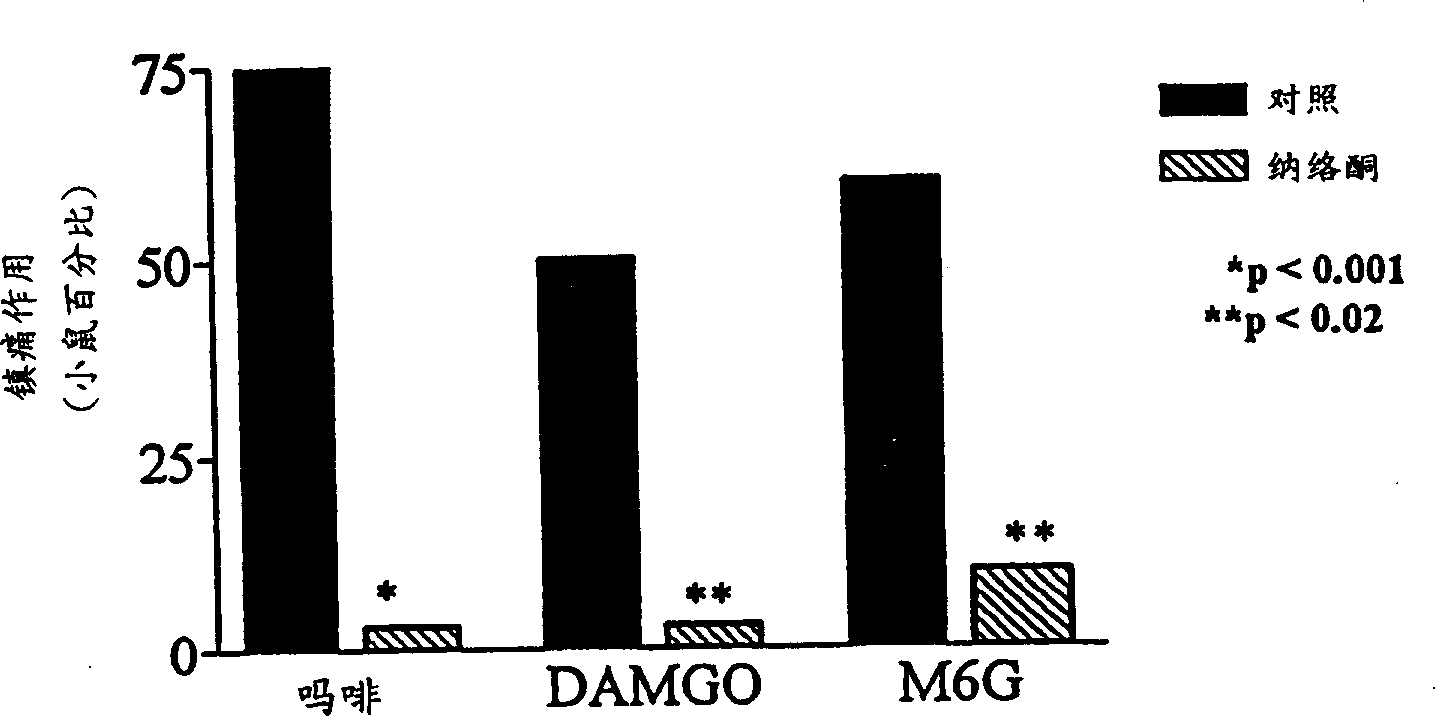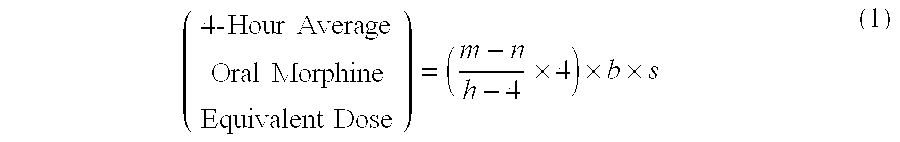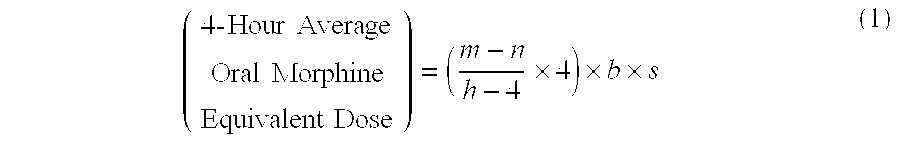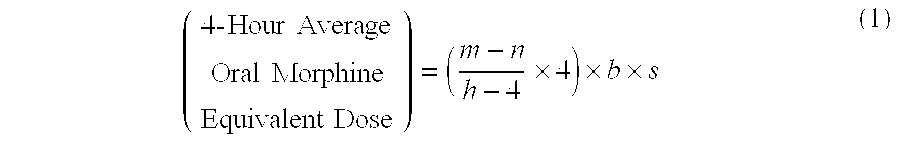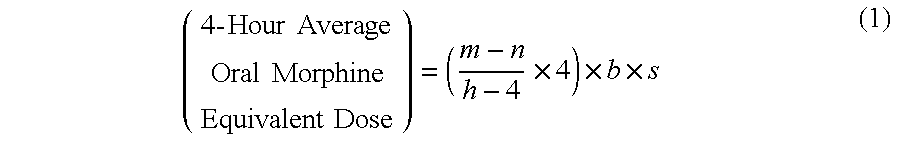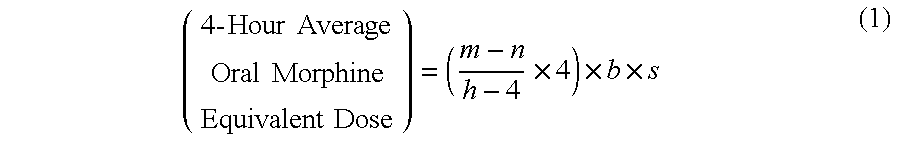Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
60 results about "Morphea" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Morphea (mor-FEE-uh) is a rare skin condition that causes painless, discolored patches on your skin.
Morphinan-derivatives for treating diabetes and related disorders
ActiveUS20150087669A1Risk of developingDelay progressBiocideMetabolism disorderDiseaseNR1 NMDA receptor
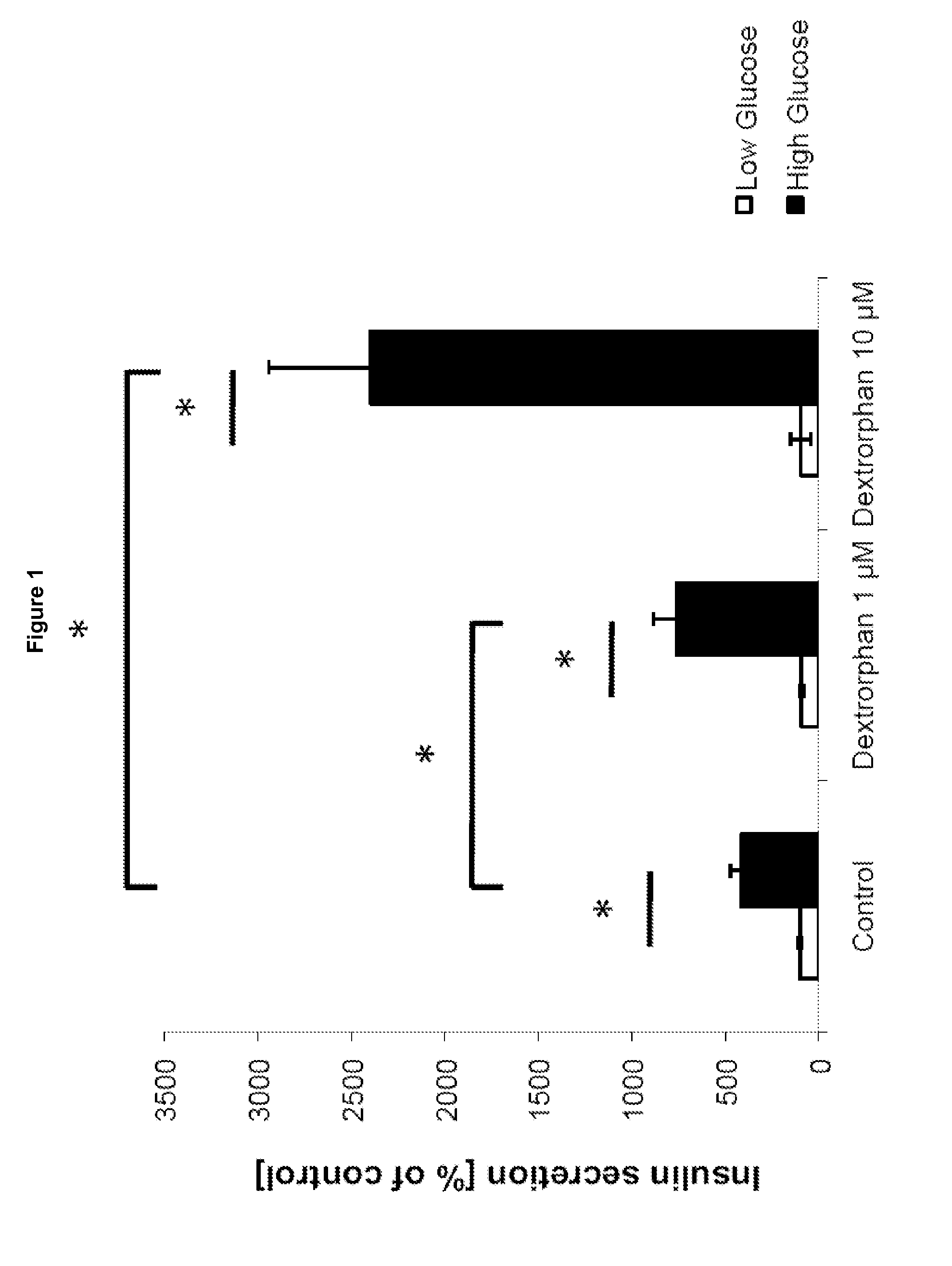
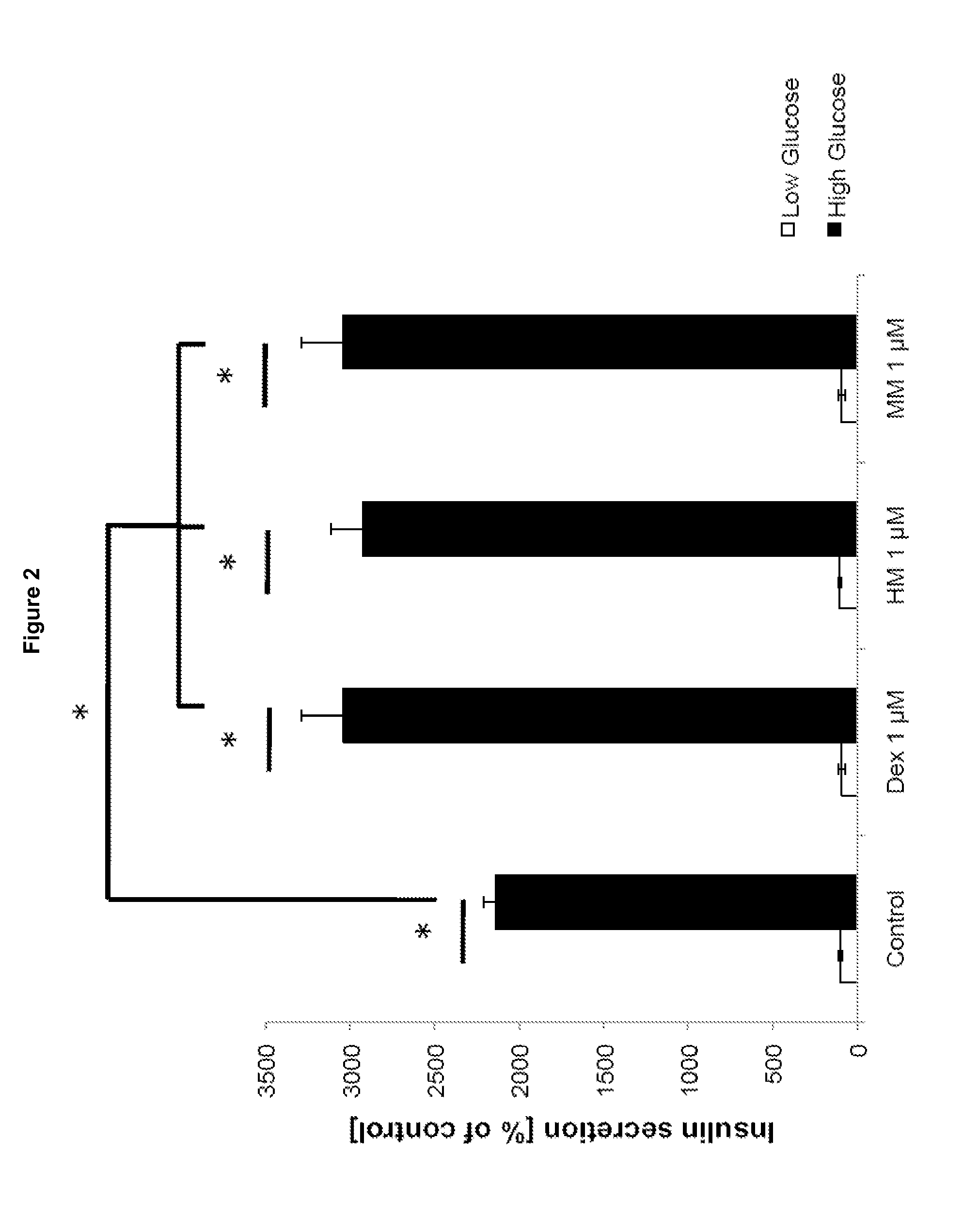
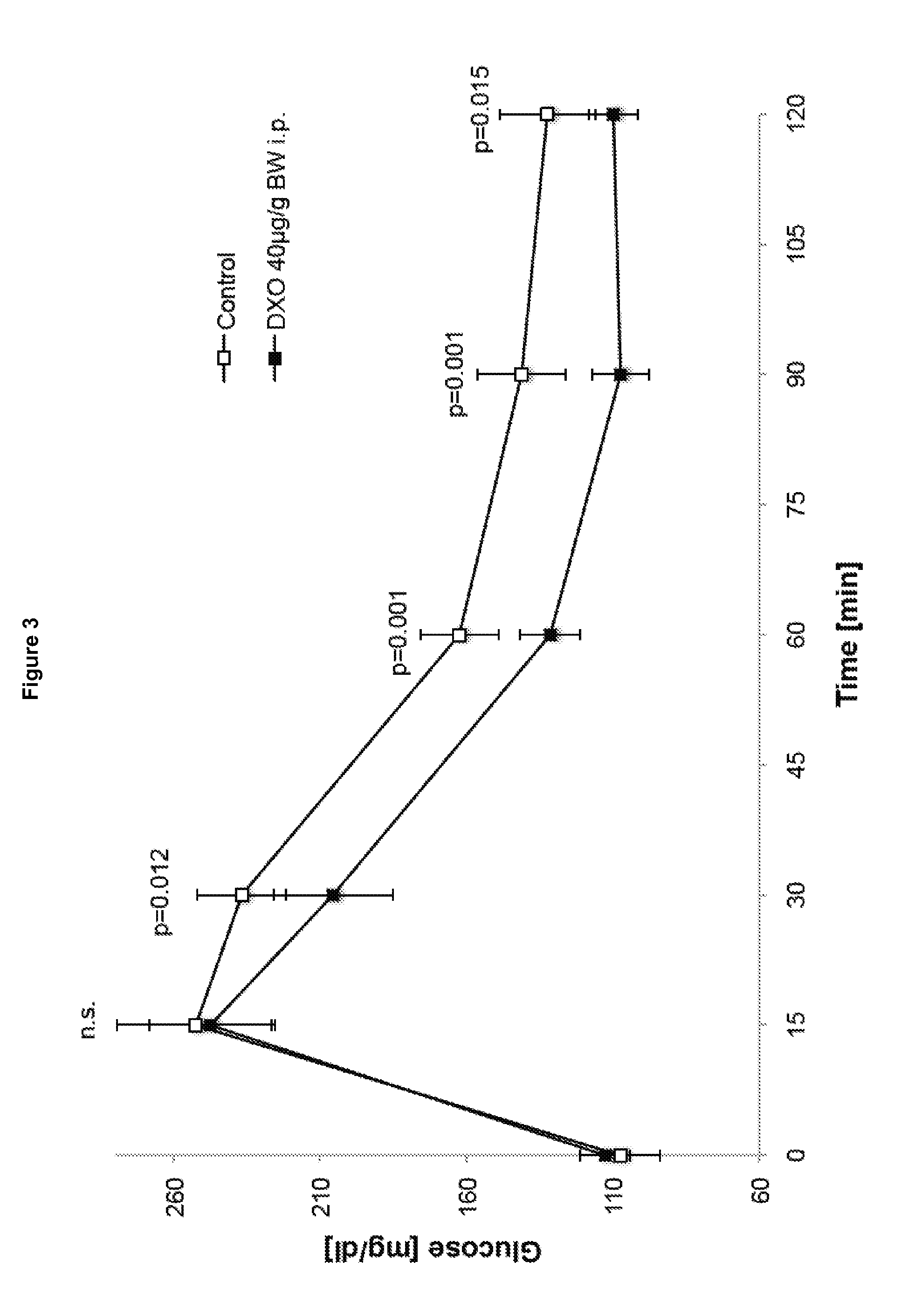
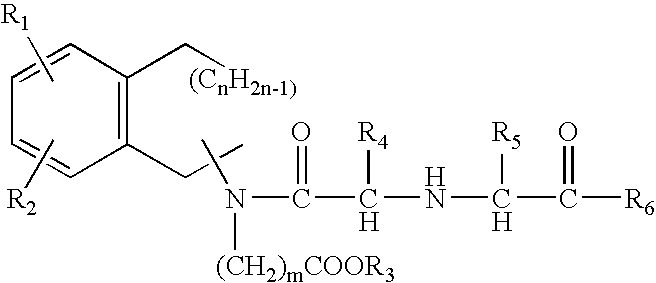
The invention relates to a morphinan-derivative that targets NMDA receptors on pancreatic islets and has the general formula (I)whereinR1 is selected from —OH, —CO2H, —R0, —OR0, —OC(═O)R0, —OC(═O)OR0 or —OC(═O)NHR0; and R2 is selected from —H, —R0, —C(═O)R0, —C(═O)OR0, —C(═O)NHR0 or —C(═NH)—NH—C(═NH)—NH2; wherein R0 is in each case independently selected from —C1-C6-alkyl, -aryl, -heteroaryl, —C1-C6-alkyl-aryl or —C1-C6-alkyl-heteroaryl, in each case independently unsubstituted or substituted;or its physiologically acceptable salt and / or stereoisomer, including mixtures thereof in all ratios, for use in the treatment of a disease or condition, where the disease or condition is insulin-dependent diabetes mellitus, non-insulin-dependent diabetes mellitus, obesity, and / or diabetic nephropathy.
Owner:DEUTE DIABETES FORSCHUNGSGES
Compositions for treating parkinson's disease
The present invention relates to improved treatment of diseases and disorders of the central nervous system by administration of apomorphine. In particular, the administration is via pulmonary inhalation. The invention provides the means for improving the treatment of a number of conditions, including Parkinson's Disease.
Owner:VECTURA LTD
Methods and Compositions for Treating or Preventing Narcotic Withdrawal Symptoms
ActiveUS20100144754A1Reduces physical dependenceAlleviate withdrawal symptomsBiocideNervous disorderDrug withdrawal symptomsPhysical dependence
This invention provides methods and pharmaceutical compositions for preventing or treating physical dependence and / or withdrawal associated with narcotic use, in particular by modulating a 5-HT3 receptor. Using a computational genetic approach in mice, a gene conserved between mice and humans was identified as candidate as a modulator of physical dependence to morphine. Administration of compounds that modulate 5-HT3 receptors was found to control withdrawal from morphine in mice and humans.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Pharmaceutical formulation of apomorphine for buccal administration
InactiveUS20090023766A1Improve sexual functionIncrease libidoBiocideNervous disorderSexual functionDisease
The present invention provides a kit comprising, in separate compartments of a container, the following components (a) and (b): (a) a combination of apomorphine or a pharmaceutically acceptable acid addition salt thereof and a pharmaceutically acceptable excipient or carrier; and (b) a solution which comprises a diluent and a pH modifying agent; the components being presented such that they can be combined at the point of use into a formulation which is adjusted to a pH ranging from mildly acidic to alkaline and which is suitable for buccal administration. The formulation is useful in treating Parkinson's disease and in promoting sexual function.
Owner:AMARIN PHARMA IRELAND
Conjoint administration of morphogens and ACE inhibitors in treatment of chronic renal failure
InactiveUS20050272649A1Preventing delaying needReducing necessary frequencyBiocidePeptide/protein ingredientsRenal disorderMorphine
The present invention provides reagents and methods for the treatment, and pharmaceuticals for use in the prevention and / or treatment, of chronic renal failure and other renal disorders in subjects (particularly mammalian subjects) renal replacement therapy. The methods involve the conjoint administration of ACE (Angiotensin-Converting Enzyme) inhibitors or Angiotensin II Receptor Antagonists (AIIRAs) with one or more OP / BMP family of proteins (morphogens, or inducers of morphogens, or agonists of the corresponding morphogen receptors, etc.). The invention also provides methods for implantation of renal cells induced with the conjoint administration of ACE inhibitors or AIIRAs with those morphogens.
Owner:BARNES JEWISH HOSPITAL +1
Apomorphine inhibitors of amyloid-beta (Abeta) fibril formation and their use in amyloidosis based disease
Described is a new class of small molecule inhibitors of amyloid beta protein (Abeta) aggregation, based on apomorphine. These molecules target the nucleation phase of Abeta self-assembly and interfere effectively with aggregation of Abeta 1-40 into amyloid fibrils in vitro as determined by transmission electron microscopy, Thioflavin T (ThT) fluorescence, and velocity sedimentation. Structure-activity studies using apomorphine analogues demonstrate that 10,11-dihydroxy substitutions of the D ring are preferred for the inhibitory effectiveness of these aporphines, and that methylation of these hydroxyl groups reduces their inhibitory potency. The ability of these small molecules to inhibit Abeta amyloid fibril formation appears to be linked to their ability to undergo auto-oxidation in solution, implicating an auto-oxidation product as the active Abeta inhibitor. Sedimentation velocity and electron microscopy studies demonstrate that apomorphine and analogues facilitate oligomerization of Abeta into short nonfibrillar soluble assemblies, but inhibit Abeta fibrillization.
Owner:CYTOKINE PHARMASCI
Dual opioid pain therapy
InactiveUS20090291975A1Eliminate side effectsGood analgesic effectBiocideDispersion deliveryPain therapySide effect
Provided are pharmaceutical compositions and methods for the alleviation of pain in a patient with optimal ratios of morphine and oxycodone that provide superior analgesic efficacy and lower incidence of adverse side effects compared to morphine and oxycodone alone. The pharmaceutical compositions comprise morphine and oxycodone, or pharmaceutically acceptable salts thereof, in ratios of about 3 to 2 to about 1 to 2, morphine to oxycodone by weight.
Owner:QRXPHARMA
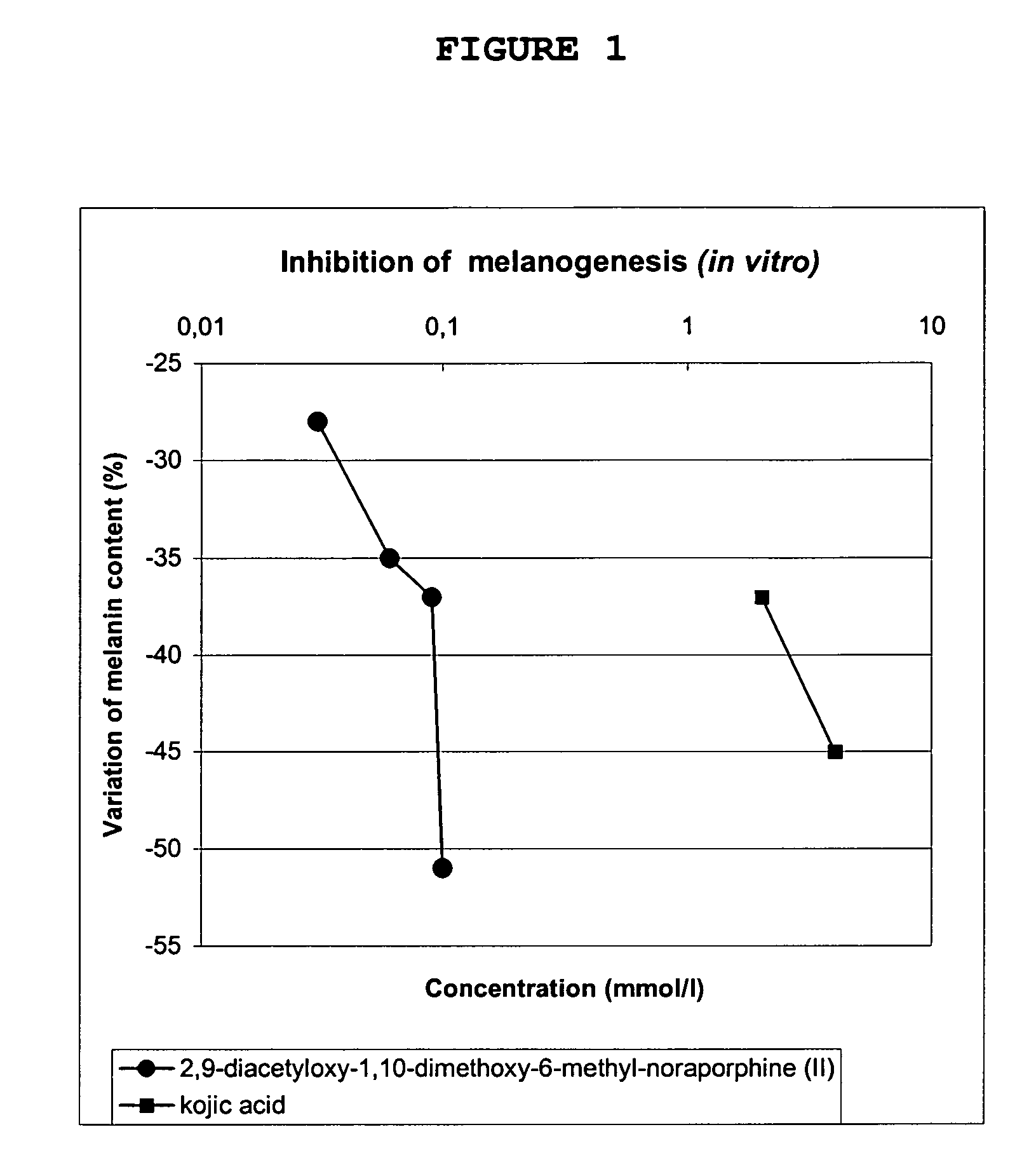
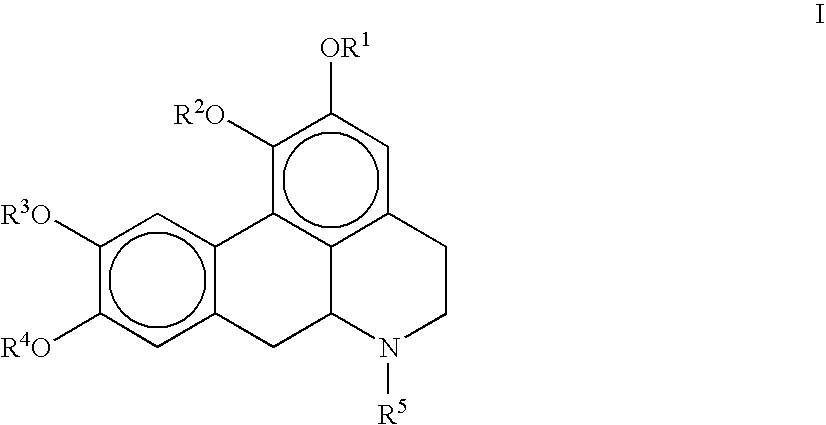
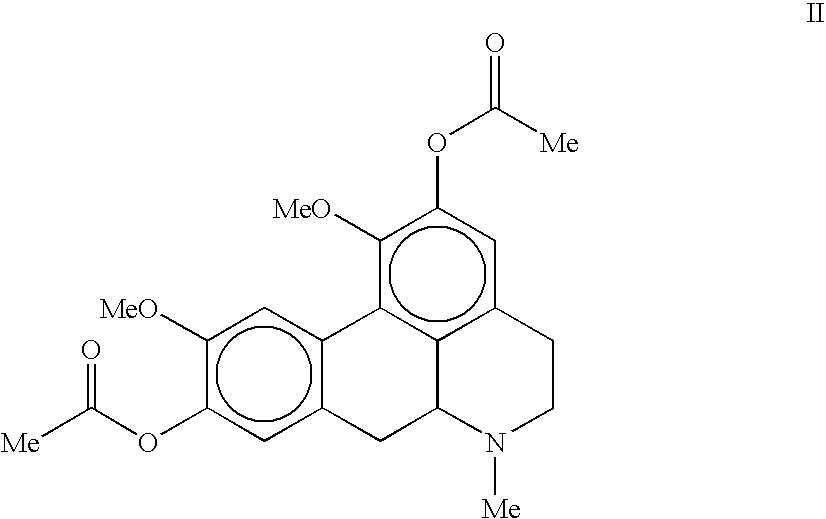
Molecules derived from noraporphine
The invention concerns novel molecules derived from noraporphine, as well as cosmetic and dermopharmaceutical compositions containing one or several of said derivatives, alone or combined with a plant extract, in particular glaucium flavum, and particularly preparations for reducing pigmentation, with anti-ageing effect or for slimming.
Owner:SEDERMA SA
Sublingual apomorphine
ActiveUS9044475B2Alleviating dyskinesiaEffectively alleviatedBiocidePowder deliveryMedicineSexual dysfunction
The invention features sublingual formulations of apomorphine that is a mucoadhesive polymer film or a strip having a first portion including an acid addition salt of apomorphine and a second portion including a pH neutralizing agent, and methods of treating Parkinson's disease, sexual dysfunction, and depressive disorders by administering sublingually the film or strip.
Owner:SUNOVION PHARMA INC
Morphogen compositions and use thereof to treat wounds
InactiveUS20060105950A1Preventing and treating and reducing severityPromote wound healingPeptide/protein ingredientsMetabolism disorderDiseaseMorphine
Disclosed are compositions and methods for promoting or accelerating wound healing and preventing, treating, or reducing symptoms associated with wounds or wounding disorders such as diabetic ulcers and burns. In one embodiment, the method includes administering a therapeutically effective amount of a nucleic acid encoding at least one morphogen, or an effective fragment thereof. Preferred morphogens include the human Sonic Hedghog (Shh), human Desert Hedgehog (Dhh), and human Indian Hedgehog (Ihh) proteins. The methods can be used alone or in combination with other methods involving administration of an angiogenic protein, a hematopoietic protein, or cells such as endothelial cells or endothelial precursor cells.
Owner:STEWARD RES & SPECIALTY PROJECTS
Transdermal patch and topical compositions comprising propylnorapomorphine
A pharmaceutical composition is characterized by: an active principle selected from one or more components of the group consisting of R(-)-propylnorapomorphine hydrochloride, S(+)-propylnorapomorphine hydrochloride, derivatives of R(-)-propylnorapomorphine hydrochloride and derivatives of S(+)-propylnorapomorphine hydrochloride, in pharmaceutically acceptable and effective doses, and further comprising components selected from the group consisting of stabilizers, solubilizers and permeation activators to facilitate the passage of the active principle through the skin. A transdermal patch that includes the pharmaceutical composition is also disclosed as well as a method of treatment for Parkinson's disease, hemicrania, sexual impotence, and psychotic disorders using the pharmaceutical composition or a transdermal patch that includes the pharmaceutical composition are described.
Owner:UNIHART CORP
Anaesthesia possessing lubricant for alimentary tract and respiratory tract endoscope
The invention relates to a lubricant with paresis used in enteron endoscope and respiratory tract endoscope. Components of said lubricant for realizing paresis comprises: phosphate methylmorphine and levogyration tetrahydropalmatine; the components of said lubricant for realizing lubricate effect comprises: disilyloxy, methyl ethylene glycol, chloromycetin in glycerin and ammonium bithiolicum glycerin; and other components of said lubricant comprises: adhesion promoter of Kapo 934, preservative agent of gluconic acid chlorhexidine and pH value adjuster of triethanolamine. And the weight proportion of said lubricant comprises: 1-10% phosphate methylmorphine, 1-10% levogyration tetrahydropalmatine, 20-50% disilyloxy, 5-35% methyl ethylene glycol, 3-30% chloromycetin in glycerin, 3-30% ammonium bithiolicum glycerin, 1-10% adhesion promoter of Kapo 934, 1-10% preservative agent of gluconic acid chlorhexidine, and 1-10% pH value adjuster of triethanolamine.
Owner:陈嘉农
Erectile dysfuction treatments comprising momentary vacuum therapy alone or with medications
InactiveUS7037257B1Prevents the breakdown of these chemical messagesHeart workload is reducedElcosanoid active ingredientsDiagnosticsVascular dilatationMorphine
The present invention relates to treatments for erectile dysfunction comprising momentary vacuum therapy alone or with medications. The treatments may be effective when other treatments (PDE-5 inhibitors (e.g., VIAGRA®); vasodilators (e.g., ALPROSTADIL®); combination pharmacotherapies (e.g., VIAGRA® and MUSE); central nervous system-acting agents (e.g., Apomorphine) fail. All treatments use a finding of the present invention that momentary vacuum therapy increases the elasticity of arteries to overcome, to a significant degree, the arterial inelasticity of atherosclerosis, a primary cause of erectile dysfunction. When momentary vacuum therapy is used with VIAGRA®, e.g., the atherosclerosis problem and the chemical message problem are treated simultaneously. Momentary vacuum therapy may be useful in the treatment of peripheral (e.g., arms and legs) artery disease and congestive heart failure.
Owner:KOENIG J FR +1
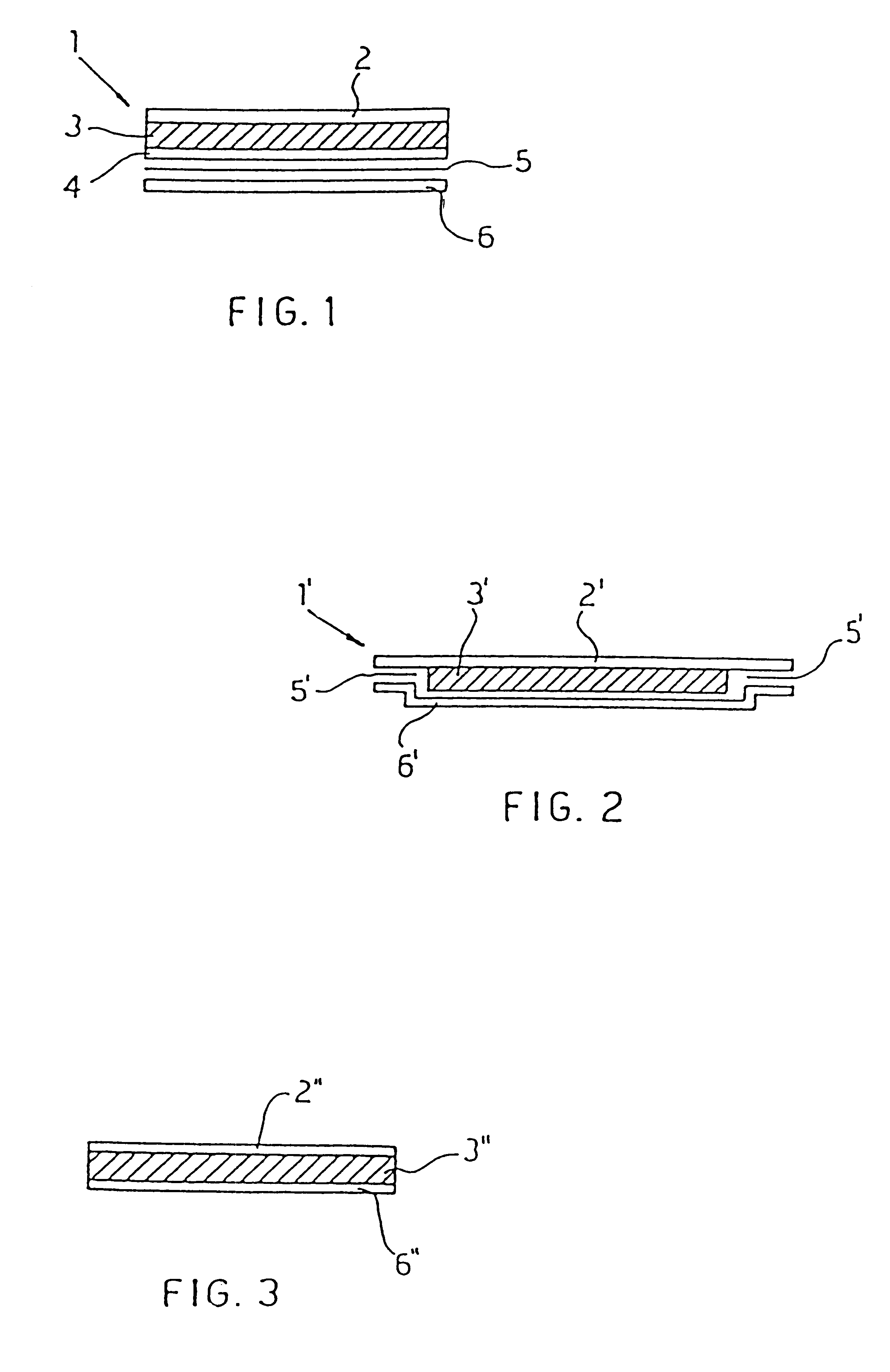
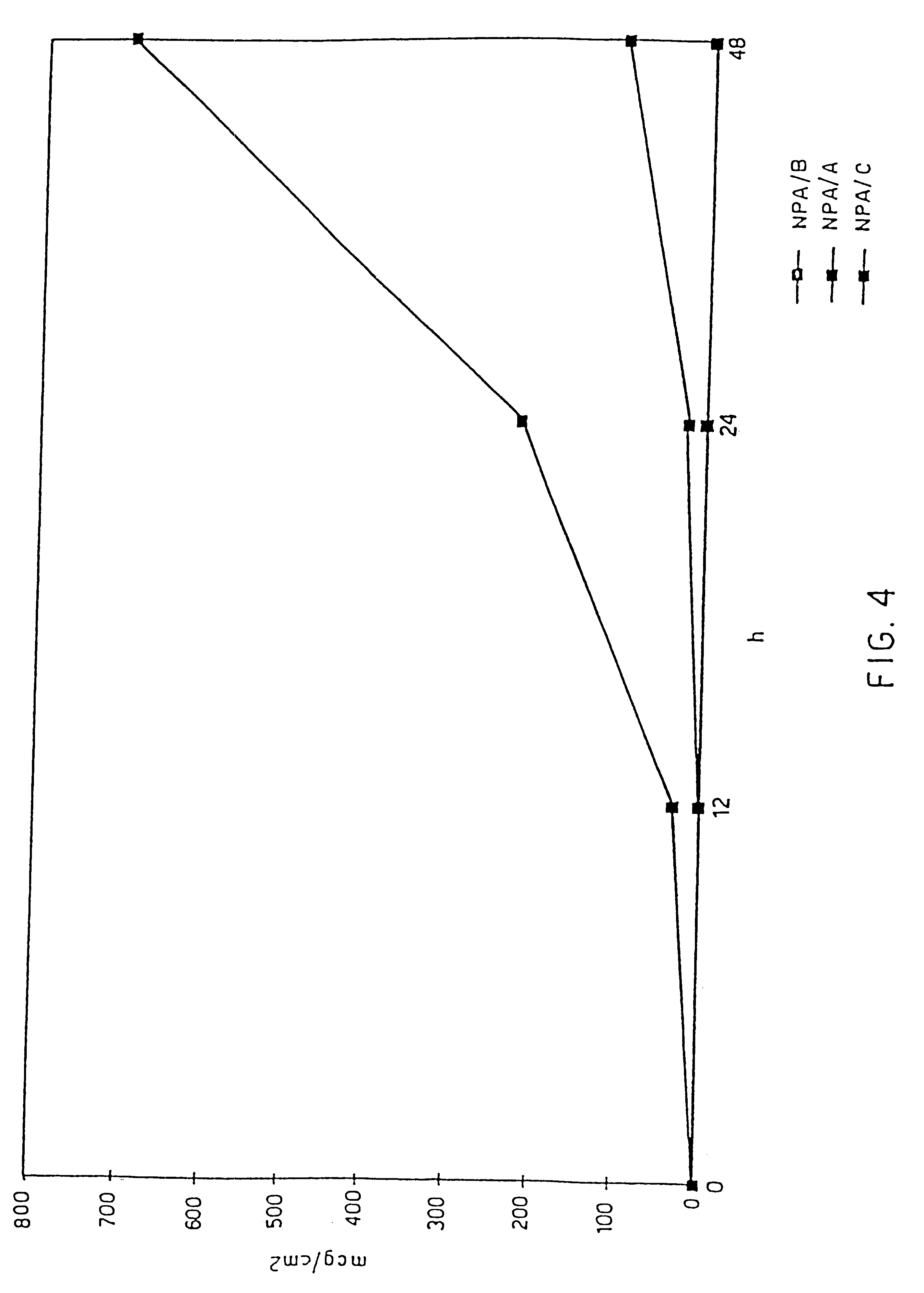
Medicinal composition for transdermal absorption, medicinal composition storing unit and transdermal absorption preparation using the same
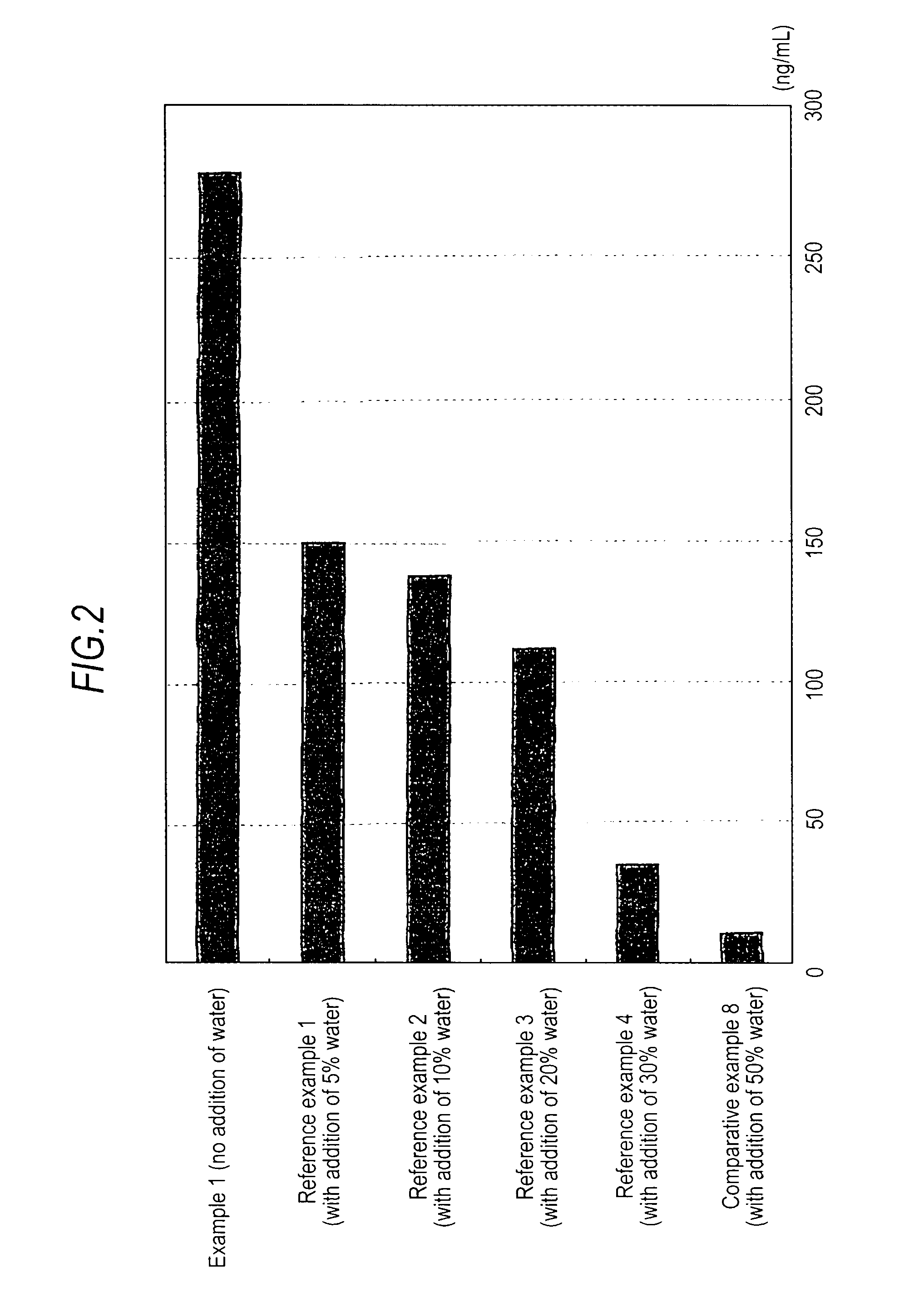
An object of the invention is to provide a transdermal absorption preparation capable of sustaining the blood morphine concentration at an effective level over at least 48 hours, etc. This transdermal absorption preparation comprises a medicinal composition for transdermal absorption in which an active ingredient selected from morphine and salts thereof is blended in such an amount as corresponding to the saturation solubility or more in an active ingredient-holding vehicle having fluidity at a temperature around the human skin surface temperature and at least a portion of the active ingredient is held in a crystalline form, characterized in that, in the case where a preparation obtained from the above-described medicinal composition for transdermal absorption is applied to the uninjured skin of the back of a white rabbit having been shaven with electrical clippers for 72 hours, the available amount of the active ingredient per single dose of the preparation is from 10 mg to 400 mg in terms of morphine base, and the plasma concentrations of the active ingredient 24 hours and 48 hours after the application of the preparation under the above-described conditions are each at least 40 ng / mL in terms of morphine base, and so on.
Owner:TEIKA PHARMA CO LTD
Anti-drug capsule prepared from pure traditional Chinese medicines
InactiveCN102114195ANo psychological desireAchieve the purpose of rehabilitationNervous disorderAnthropod material medical ingredientsDrug capsuleGastrodia
The invention provides an anti-drug capsule prepared from pure traditional Chinese medicines, which comprises the following traditional Chinese medicines in parts by weight: 10-30 parts of smallflower milkwort herb with root, 10-30 parts of maggot, 10-30 parts of ginseng, 10-30 parts of rhubarb, 10-30 parts of prepared scorpion, 10-30 parts of milk vetch, 10-30 parts of scorpion, 10-30 parts of corydalis tuber, 10-30 parts of angelica, 10-30 parts of prepared aconite root, 10-30 parts of cassia twig, 10-30 parts of bupleurum, 10-30 parts of gastrodia tuber, 10-30 parts of clematis root, 10-30 parts of angelica, 10-30 parts of white peony root, 10-30 parts of eucommia, 10-30 parts of pseudo-ginseng, 10-30 parts of safflower, 10-30 parts of american lopseed and 10-20 parts of licorice. The anti-drug capsule provided by the invention has the advantages of no toxic side effects, short treatment time and low cost; and the anti-drug capsule can also be used for comprehensively conditioning the body of a druggy and improving the immunity of the body, so that the druggy can realize drug rehabilitation and can recover simultaneously. After the anti-drug capsule is taken by 20 patients clinically, the patients have no adverse reactions; after the anti-drug capsule is taken by a patient for 7 days, the patient has ruddy complexion and pleasant mood and likes exercising, the mental state of the patient is the same as the mental state of a normal person, the weight of the patient obviously increases, and the patient has no craving for drugs; and after a course of treatment (10 days), morphine detection is carried out, and the detection result is negative. The effective rate of the anti-drug capsule is 100%.
Owner:陈都
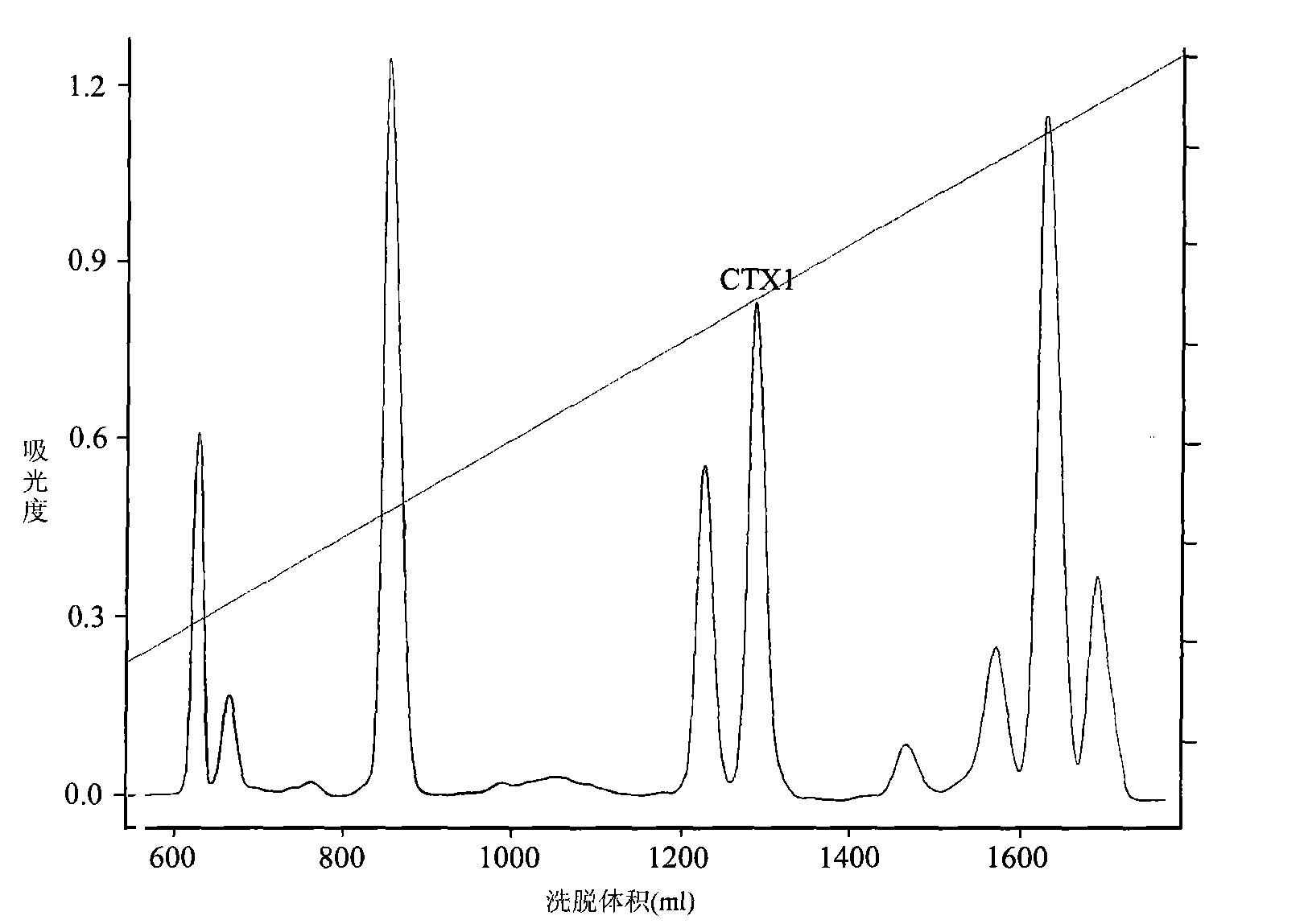
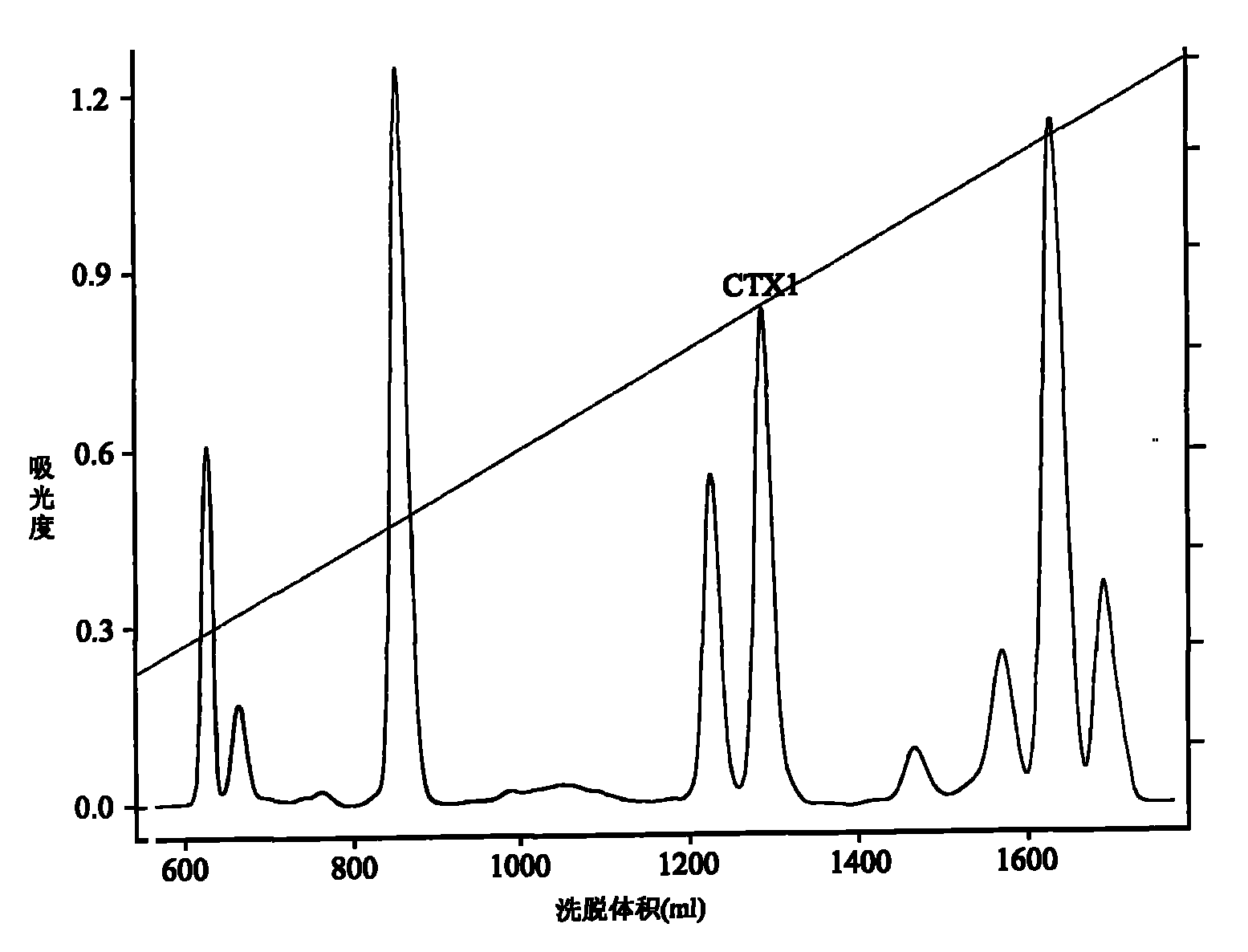
Application of cytotoxin (CTX1) from snake venom to preparation of medicament for rehabilitating
InactiveCN101926980ADesensitization of behaviorEliminate withdrawal symptomsNervous disorderPeptide/protein ingredientsDrug withdrawal symptomsThirst
The invention discloses application of cytotoxin (CTX1) from snake venom to preparation of a medicament for rehabilitating. Proved by the experiment, the CTX1 can better eliminate the behavioral sensitization of addict rats by morphine, eliminate the medicament thirst behavior and the abstinence syndrome of rats and has favorable rehabilitating effect. In addition, the invention can improve the function state, the diet and the activities of the addict rats and remarkably increase the weight, thereby recovering normal physiological activity.
Owner:SUN YAT SEN UNIV
Preparation method and application for Parkinson's disease rat model
InactiveCN104997761AHigh modeling success rateModeling success rate is lowOrganic active ingredientsIn-vivo testing preparationsMedicineMorphine
The invention discloses a preparation method for a Parkinson's disease rat model. The preparation method comprises the following steps: obtaining a locus of a rat MFB according to a brain three-dimensional positioning map or / and magnetic resonance imaging data of an experimental rat, wherein the locus is in the position which is 1.72 mm behind the bregma and 2.13 mm beside a middle line; respectively injecting 1-2 [um]l of 6-OHDA in positions 8.5 mm and 8.7 mm below the surface of the skull; after model making, injecting apomorphine hydrochloride to modeled animals for four weeks continuously for carrying out rotary experimental detection, and rotating towards the normal side, if the number of turns is larger than 7 r / min, regarding that model making is successful, and if the rotating direction is wrong or the number of revolutions is not enough, regarding that model making is unsuccessful. According to the invention, medicine is injected and fed to the relatively thick part of the MFB, and 6-OHDA damages nigral dopaminergic neurons through the dopaminergic neuron axon retrograde property, so that the success rate of model making is high, the success rate of 84% can be realized one week after model making and the success rate can reach 92% two weeks after model making; for model making reported at present, model making at other selected loca can be successful until four weeks mostly and the success rate of model making is usually lower than 80%.
Owner:SHANDONG NORMAL UNIV
Application of Lorcaserin hydrochloride in preparation of medicine for inhibiting opioid addiction and withdrawal syndrome
InactiveCN103877096AInhibitory reactivityNervous disorderHeterocyclic compound active ingredientsWithdrawal syndromeStimulant
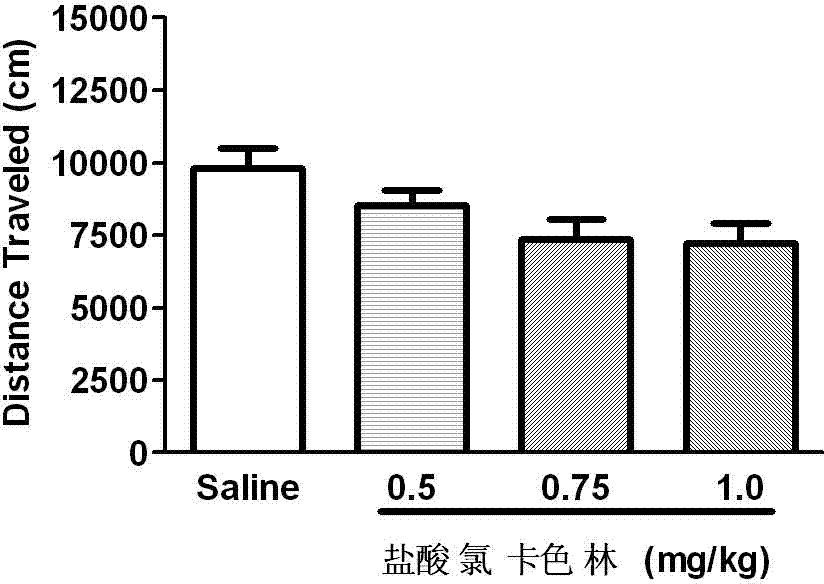
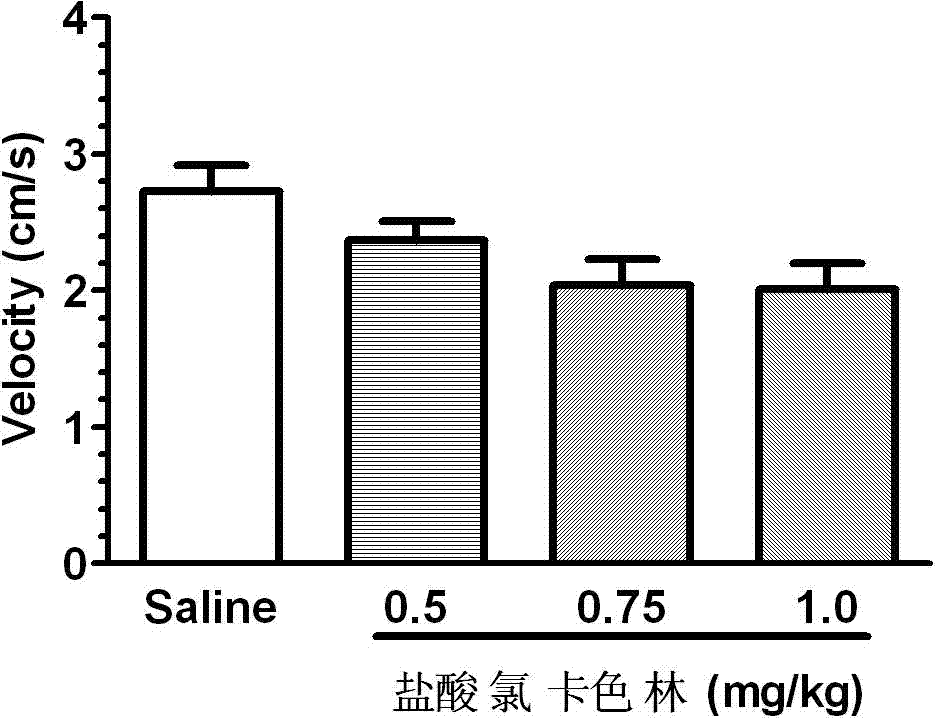
The invention relates to an application of Lorcaserin hydrochloride in preparation of a medicine for inhibiting opioid addiction and withdrawal syndrome. The Lorcaserin hydrochloride is approved to be a weight-reducing aid by the America Food and Drug Administration (FDA) in 2012. The Lorcaserin hydrochloride is a 5-hydroxytryptamine 5-HT2C receptor stimulant with high selectivity. The latest research of the invention finds that the Lorcaserin hydrochloride (0.5-1.0mg.kg<-1>) greatly reduces the naloxone-urged horizontal motion distance and speed of a morphine dependent mice and the withdrawal syndrome of the mice. It is found in the invention for the first time that the Lorcaserin hydrochloride can be applied clinically for rescuing and treating addiction and withdrawal syndrome generated by morphine and other opiates.
Owner:ANHUI MEDICAL UNIV
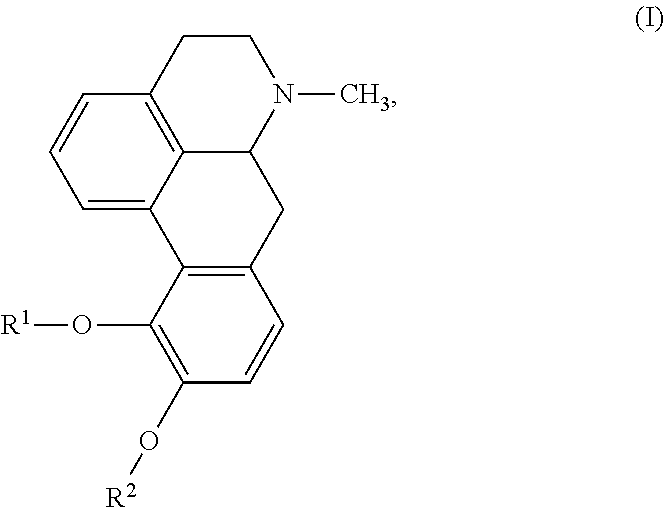
Aporphine esters and their use in therapy
InactiveUS7238705B2Improve bioavailabilityLong duration of actionBiocideNervous disorderDiseaseSexual dysfunction
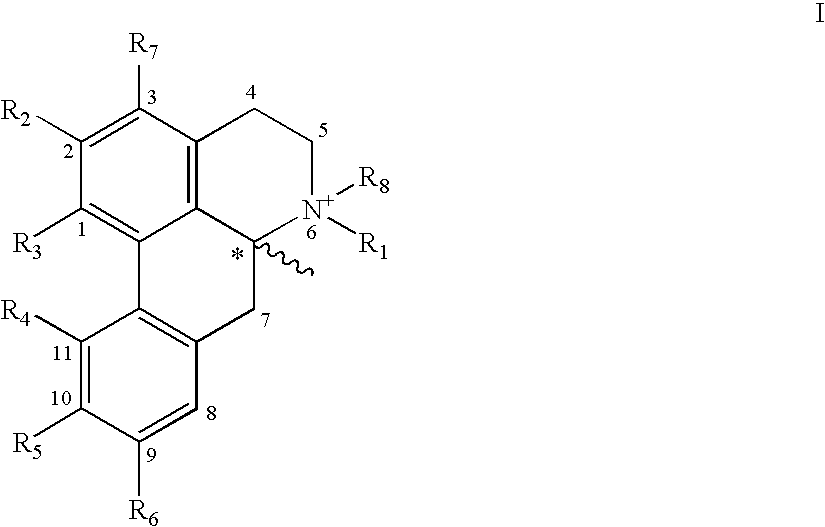
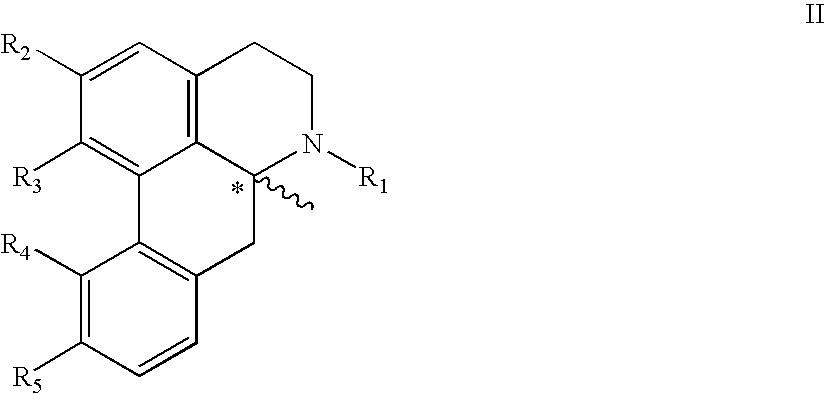
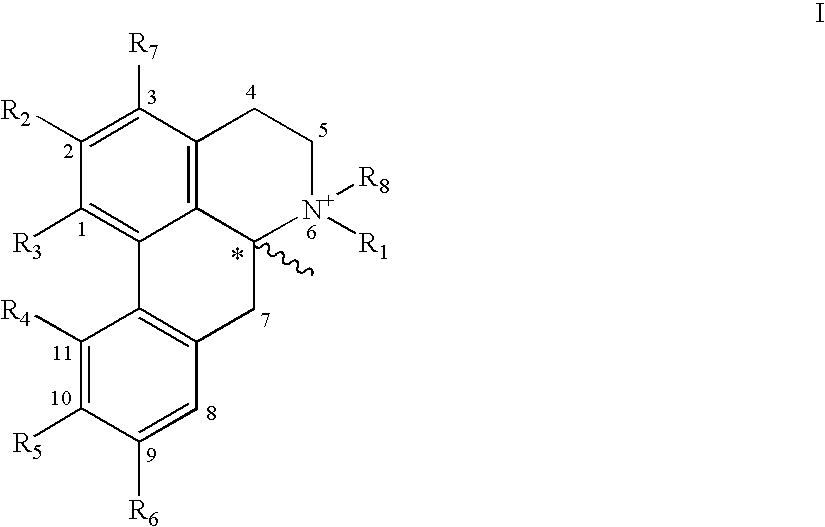
New aporphine derivatives are disclosed which have formula (I) and the physiologically acceptable salts thereof. Said derivatives may be used for the treatment of Parkinson's disease, hemicrania, restless legs syndrome (RLS), sexual dysfunction in men and women, hyperprolactemia and psychotic disorders, and / or evaluation of Parkinson's disease. Processes for the preparation of such derivatives are also disclosed.
Owner:AXON BIOCHEMICALS BV
Mixed snuff prepn. for reducing side effect of Apomorphinum
InactiveCN1375292AExtended stayEliminate or reduce nauseaOrganic active ingredientsNervous disorderNasal cavitySide effect
An apomorphine preparation compound of nasal cavity feeding used for curing Parkinson syndrom, male erection disturbance and female sexual disturbance can eliminate or decrease the side effects of bad feeling, dizziness, vomiting and abnormal taste sense occured in the treatment of apomarphine through using preparation compound adding with the vomiting stop medicine. The aromatic substance is added in order to let the nasal cavity have feelings of refreshing and comfort after the medicine is fed and the natural or synthetic high polymer compound is added to liquid preparation in order to delay the retaining period of medicine in nasal cavity. The preparation has the advantage of transmitting the medicine towards the brain and furthermore can be used with convenience, safety, effectivenessand good compliance.
Owner:ZHEJIANG ACAD OF MEDICAL SCI +1
Topical compositions comprising opioid analgesic and NMDA antagonist
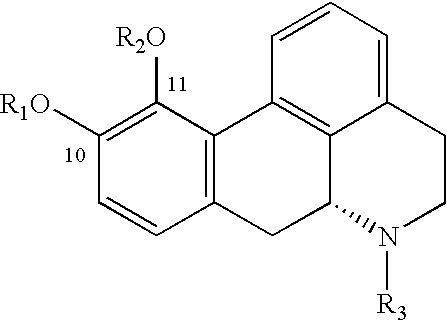
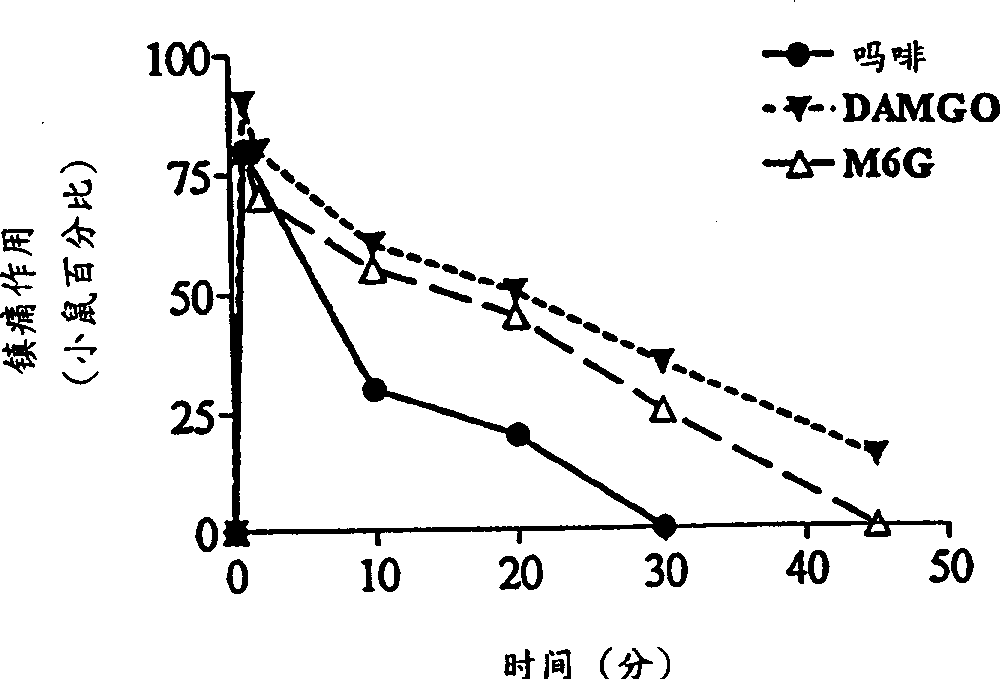
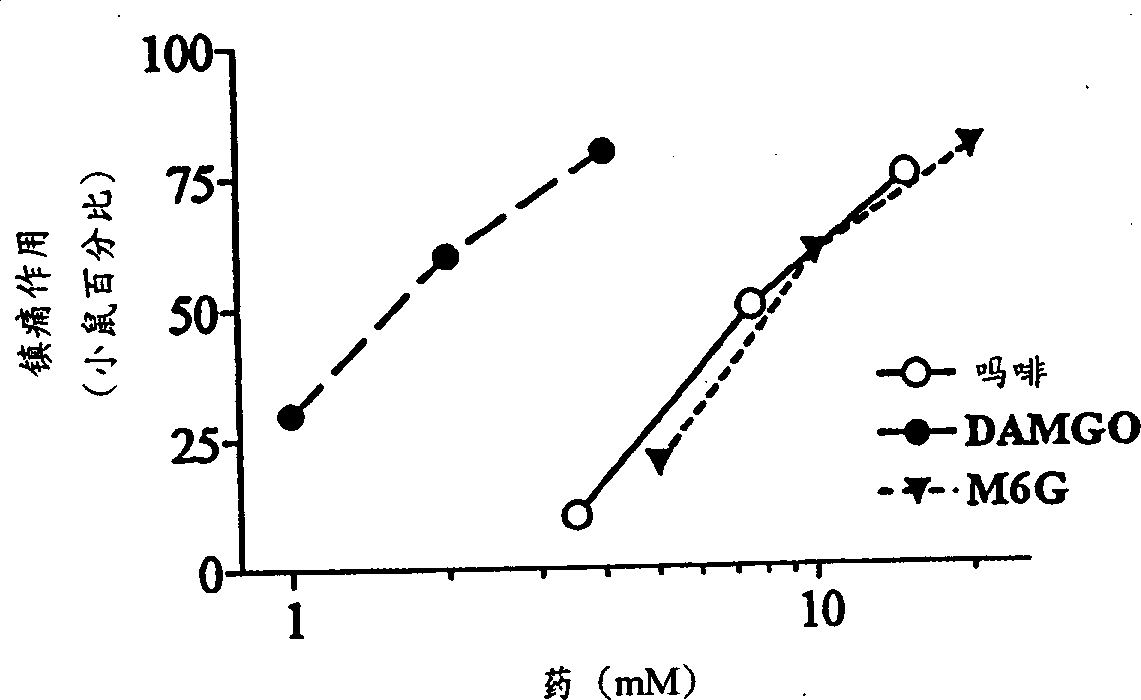
InactiveCN1309562AReduce inhibitionNervous disorderPeptide/protein ingredientsNR1 NMDA receptorWhole body
A topical opioid paradigm was developed to determine analgesic peripheral effects of morphine. Topical morphine as well as peptides such as [D-Ala2,MePhe4,Gly(ol)5]enkephalin (DAMGO) produced a potent, dose-dependent analgesia using the radiant heat tailflick assay. The topical drugs potentiated systemic agents, similar to the previously established synergy between peripheral and central sites of action. Local tolerance was rapidly produced by repeated daily topical exposure to morphine. Topical morphine tolerance was effectively blocked by the N-Methyl-D-Aspartate (NMDA) receptors antagonist MK801 and ketamine given either systemically or topically. NMDA receptor antagonists reversed pre-existing morphine tolerance. The activity of topical NMDA antagonists to block local morphine tolerance suggests that peripheral NMDA receptors mediate topical morphine tolerance. Morphine was cross tolerant to [D-Ala2,MePhe4,Gly(ol)5]enkephalin (DAMGO), but not to morphine-6 beta -glucuronide, implying different mechanisms of action. These observations have great importance in the design and use of opioids clinically. Topical pharmaceutical compositions comprising an analgesic that functions through an opiate receptor and an NMDA receptor antagonist for producing analgesia without inducing tolerance are described.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
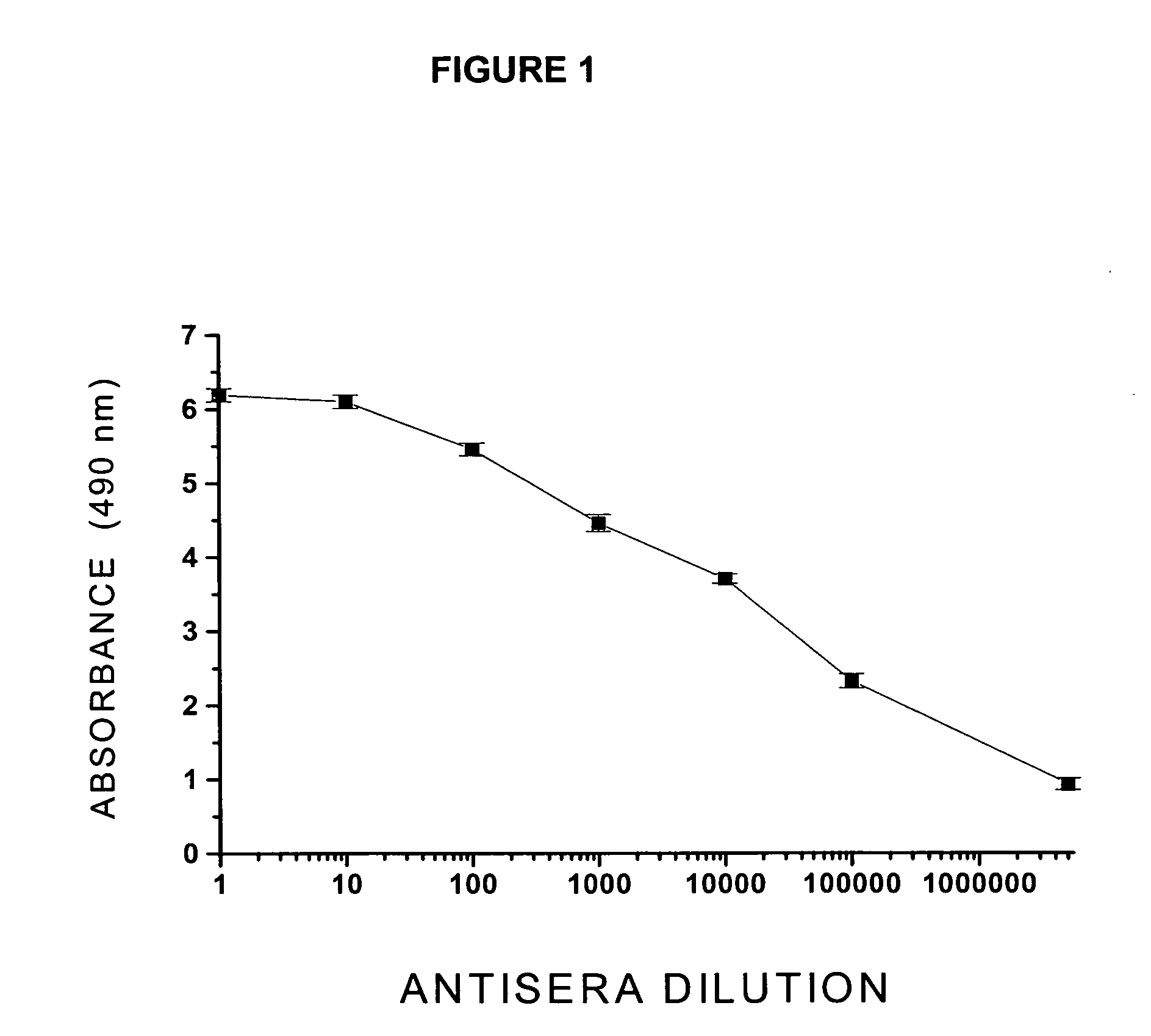
Process For The Preparation And Use Of A Bivalent Vaccine Against Morphine-Heroine Addiction
ActiveUS20080241183A1Easy to solveMaintain curative effectNervous disorderBacteria peptidesHuman useVaccination
The structural design, preparative methods and chemical composition of two structural formulations of bivalent vaccines against morphine-heroin addiction (morphine-6-hemisuccinyl-EDC-TFCS-tetanus toxoid and 3-O-carboxymethylmorphine-EDC-TFCS-tetanus toxoid), are disclosed. These vaccines are suitable for human use in which they are capable of triggering the synthesis of polyclonal antibodies against morphine opiate and its structural analogue, heroin, through the repeated in vivo administration of these formulations, in active vaccination protocols, in pre-clinical studies in rodents. The active vaccination paradigm through which these immunogens trigger a humoral immune response consolidated with a long-term immunological memory, characterized by the presence of high titers of specific antibodies against these two drugs of abuse, is also disclosed. Furthermore, the present invention reveals the efficacy of these conjugate formulations for triggering a sustained immunoprotection against morphine and heroin addiction using an intravenous self-administration paradigm of these two opiate substances in the rodent. Finally, a discussion is also made on the potential future use of these immunoconjugates in active vaccination protocols for treating both morphine and heroin addiction in the humans.
Owner:INST NACIONAL DE PSIQUIATRIA RAMON DE LA FUENTE MUNIZ
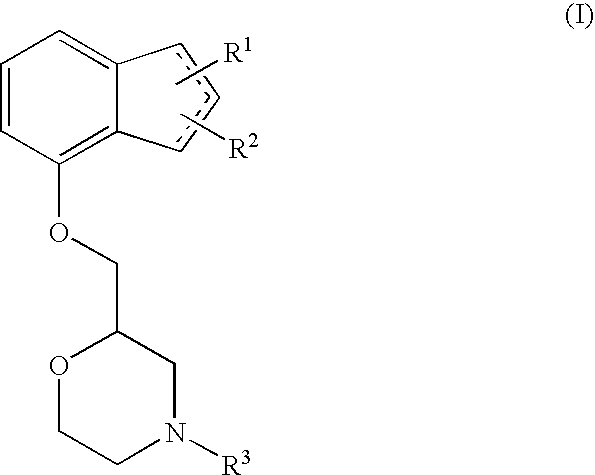
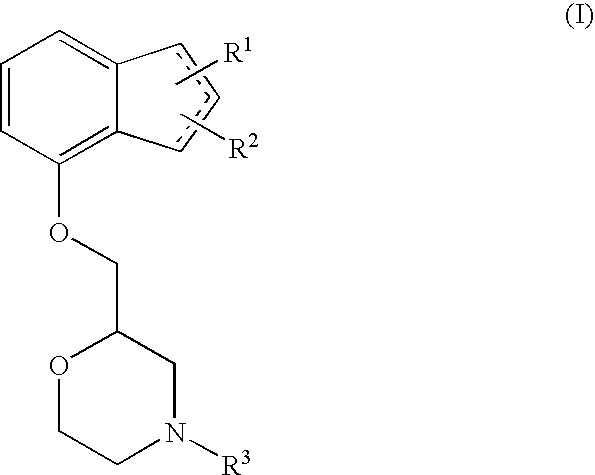
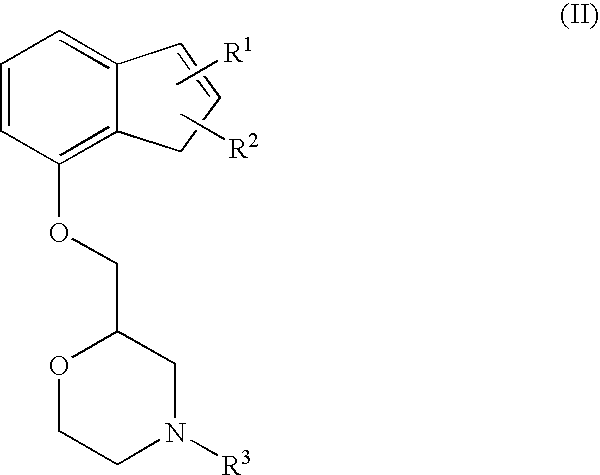
Novel preventive and/or therapeutic agent for neuropathic pain
InactiveUS20100087439A1Prevent goodExcellent therapeutic agentOrganic active ingredientsNervous disorderLeg painFibromyalgia
The present invention relates to an agent for preventing and / or treating neuropathic pain comprising a 2-[(substituted-inden-7-yloxy)methyl]morpholine of the formula (I) or a pharmaceutically acceptable salt thereof as an active ingredient. The invention is useful for providing an excellent agent for preventing and / or treating neuropathic pain, and particularly useful for providing an agent for preventing and / or treating allodynia, hyperalgesia, hyperesthesia, spontaneous pain, cancer pain, trigeminal neuralgia, phantom limb pain, postherpetic neuralgia, fibromyalgia, low back / leg pain, thalamic pain, entrapment (compressive) peripheral neuropathy, atypical facial pain, pain accompanying spinal cord injury, pain accompanying multiple sclerosis, pain accompanying chemotherapy-induced neuropathy, cancer pain on which an analgesic effect of a narcotic analgesic such as morphine is insufficient, pain accompanying diabetic neuropathy, and the like.
Owner:ASTELLAS PHARMA INC
Applications of tetrahydroisoquinoline-3-carboxylic acid derivatives in preparation of medicines treating dopaminergic nerve diseases
ActiveCN104161758AReduce the degradation rateImprove utilizationOrganic active ingredientsNervous disorderDiseaseChemical synthesis
Applications of tetrahydroisoquinoline-3-carboxylic acid derivatives in preparation of medicines treating dopaminergic nerve diseases are disclosed. The derivatives can be separated and obtained from the seed of stizolobium cochinchinensis, and can also be obtained by chemical synthesis by subjecting amino acid precursors phenylalanine and tyrosine, or levodopa with corresponding aldehydes or ketones to Pictet-Spengler condensation. Rat experiments show that: when the derivatives are administrated in combination with a levodopa component, the concentration of the levodopa in blood and brain tissues can be significantly increased, thus improving the bioavailability of the levodopa. In addition, the derivatives significantly inhibit highly-spontaneous rat movement induced by morphine, and penetrate the blood brain barrier, so that the derivatives can adjust the dopaminergic nerve functions. The derivatives can be used for treating the Parkinson's disease, psychosis, and other dopaminergic nerve diseases.
Owner:苏州必益必诚医药科技有限公司
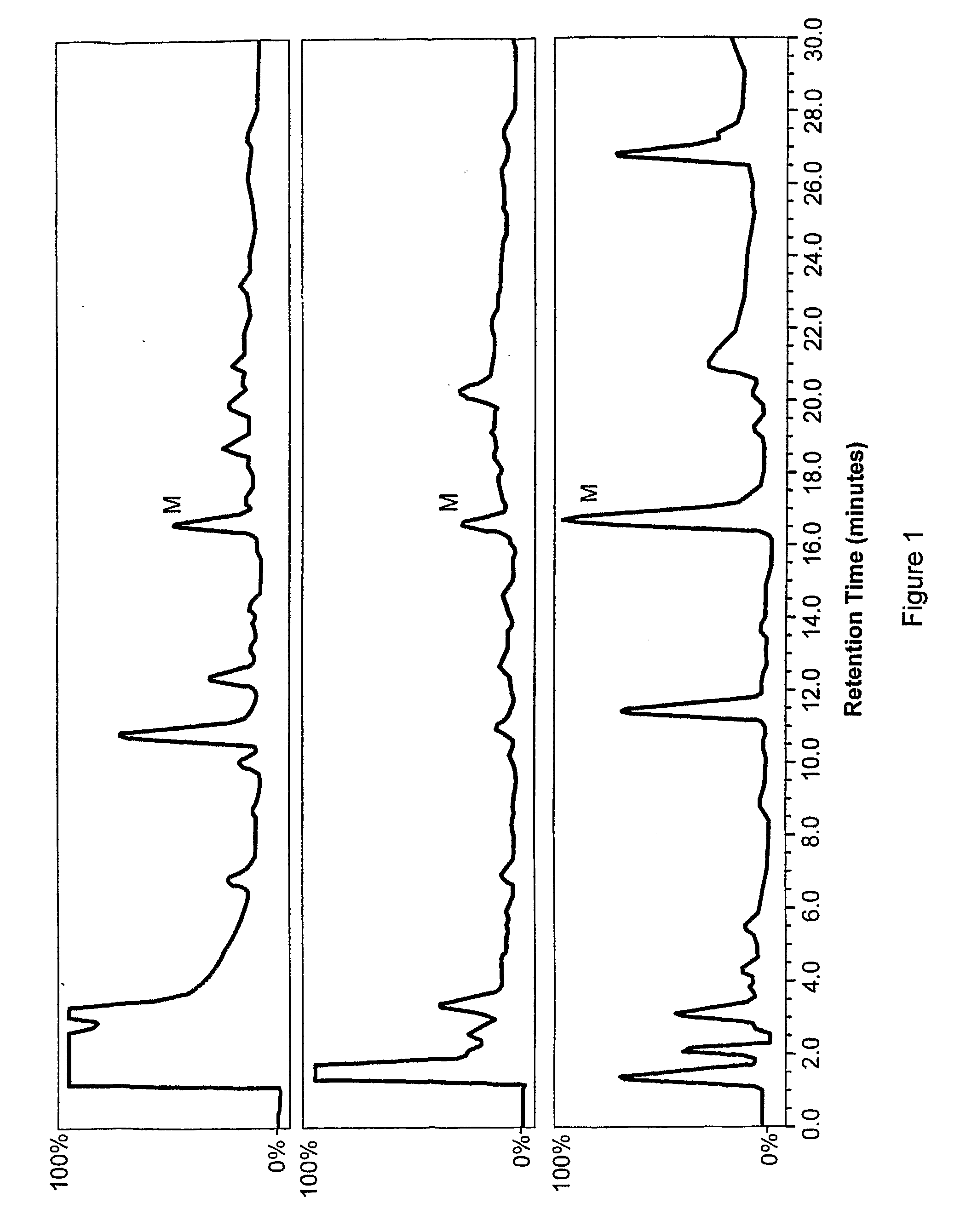
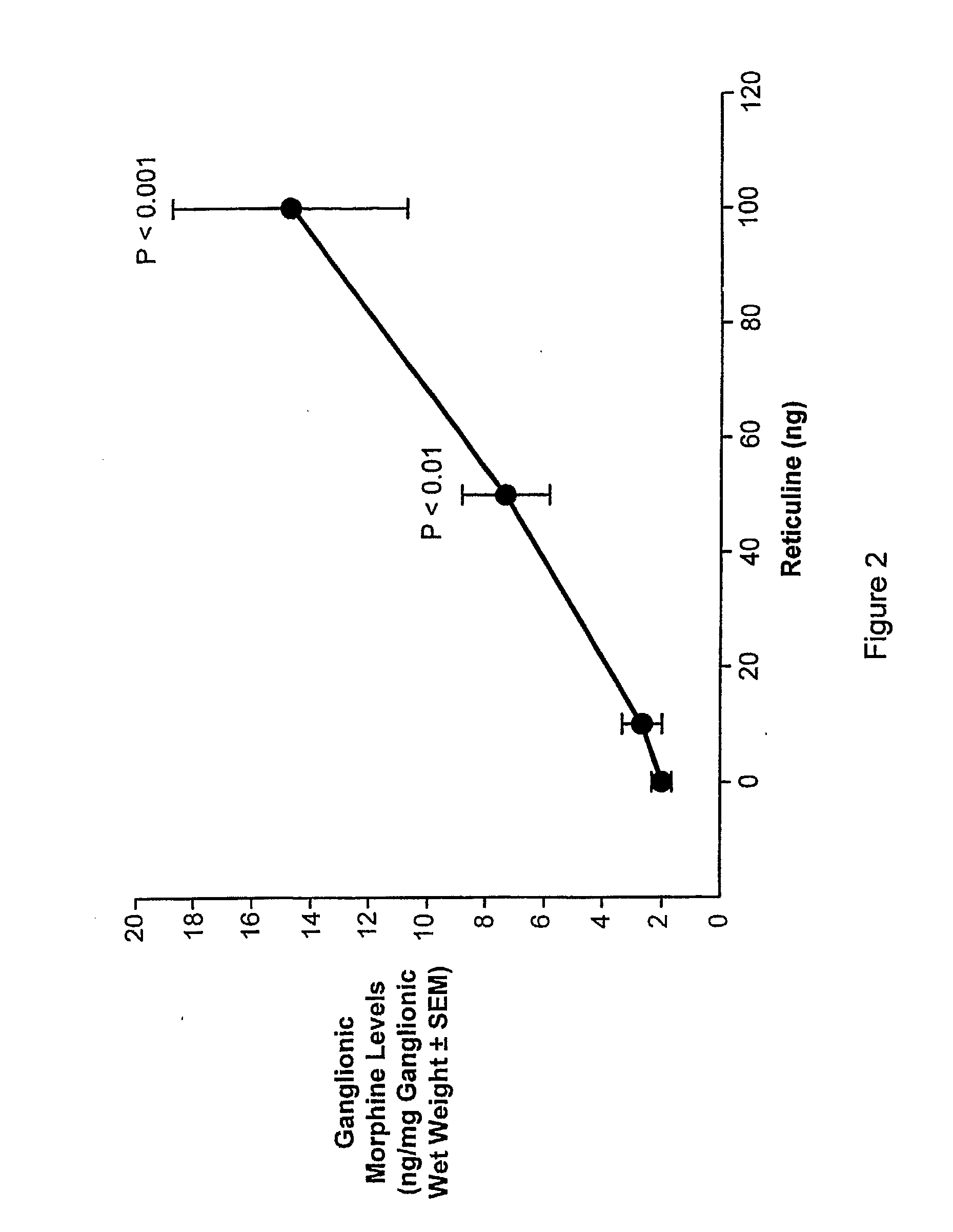
Morphine and Morphine Precursors
Methods and materials related to the use of morphine, morphine precursors (e.g., reticuline), and inhibitors of morphine synthesis or activity to treat diseases, to reduce inflammation, or to restore normal function are provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Methods of Converting a Patient's Treatment Regimen from Intravenous Administration of an Opioid to Oral Co-Administration of Morphine and Oxycodone Using a Dosing Algorithm to Provide Analgesia
A method of converting a treatment for pain comprising intravenous administration of opioids, to a treatment for pain comprising oral administration of a first dose of an immediate release morphine-oxycodone combination in patients in need of analgesia. The method may comprise (1) determining a four-hour average oral morphine equivalents or determining a net average hourly intravenous dose, and (2) orally administering to the patient a first dose of a morphine-oxycodone combination in a 3:2 ratio by weight every four to six hours. Also, a method of treating pain in patients who had been administered opioids intravenously, comprising using a dosing algorithm to determine the first dose of the immediate release morphine-oxycodone combination.
Owner:QRXPHARMA
Extract effective in treating drug addiction and preparation method therefor
ActiveUS20190151375A1Organic active ingredientsNervous disorderDrug withdrawal symptomsAcute toxicity testing
Disclosed are an extract that is effective in treating drug addiction and a preparation method therefor. An effective component of the extract has the following chemical structural characteristics: a cholestenol compound with hydroxyl (OH) at position 3 and a double bond between position 5 and position 6, or a cholestenol compound with hydroxyl (OH) at position 3, a double bond between position 5 and position 6 and a double bond between position 22 and position 23. The extract can be extracted from the traditional Chinese medicine Agriolima agrestis. The extract of the present invention is safe in acute toxicity, sedative and hypnotic without physical and psychic dependence, has an inhibitory effect on excitability in mouse caused by morphine and benzedrine and a detoxification treatment effect on the withdrawal symptoms in morphine-dependent rats, and is useful in the development of medications or food for treating drug addiction.
Owner:GUANGXI JIUFU BIOTECH CO LTD
Methods of Converting a Patient's Treatment Regimen from Intravenous Administration of an Opioid to Oral Co-Administration of Morphine and Oxycodone Using a Dosing Algorithm to Provide Analgesia
A method of converting a treatment for pain comprising intravenous administration of opioids, to a treatment for pain comprising oral administration of a first dose of an immediate release morphine-oxycodone combination in patients in need of analgesia. The method may comprise (1) determining a four-hour average oral morphine equivalents or determining a net average hourly intravenous dose, and (2) orally administering to the patient a first dose of a morphine-oxycodone combination in a 3:2 ratio by weight every four to six hours. Also, a method of treating pain in patients who had been administered opioids intravenously, comprising using a dosing algorithm to determine the first dose of the immediate release morphine-oxycodone combination.
Owner:QRXPHARMA
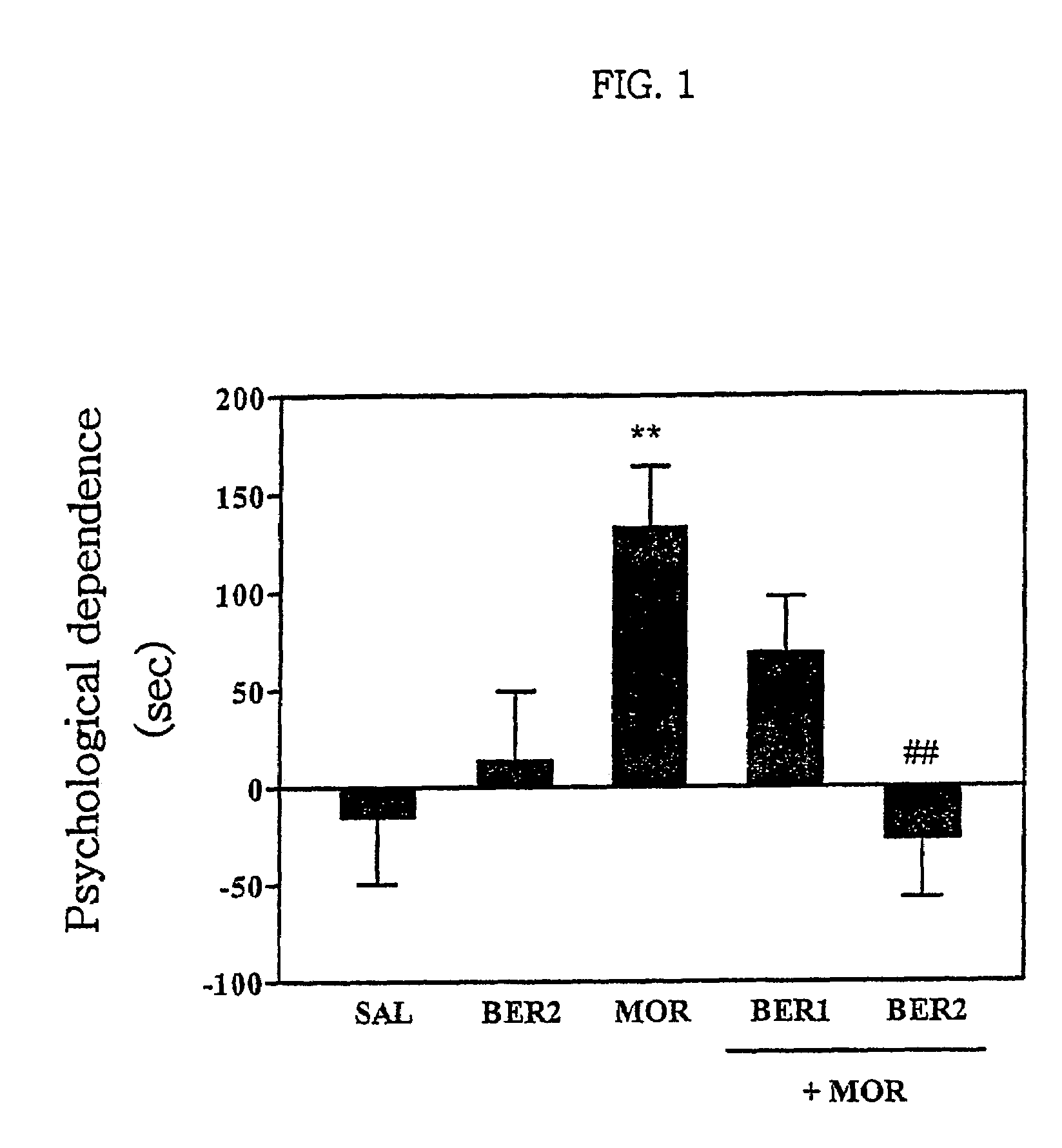
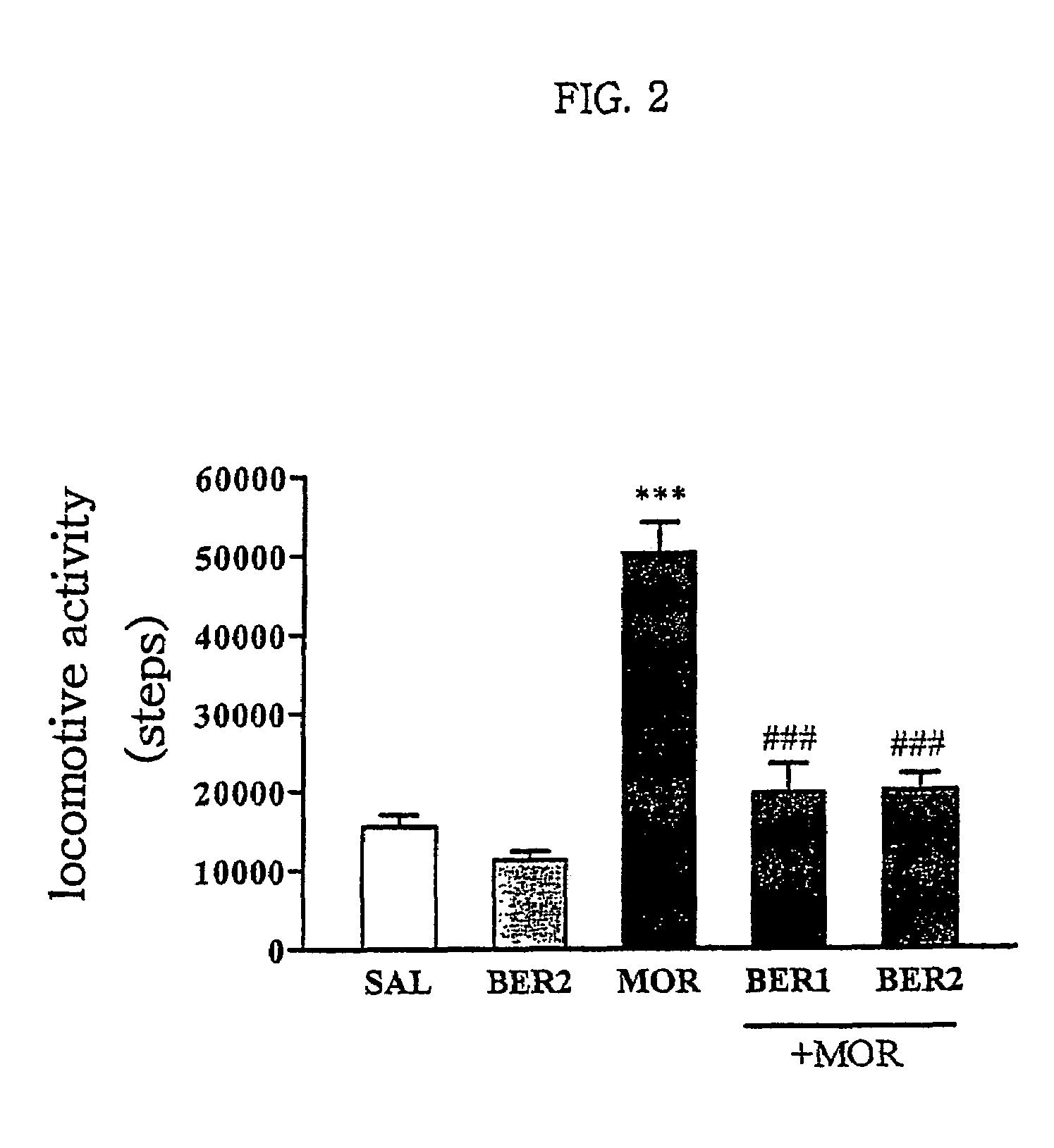
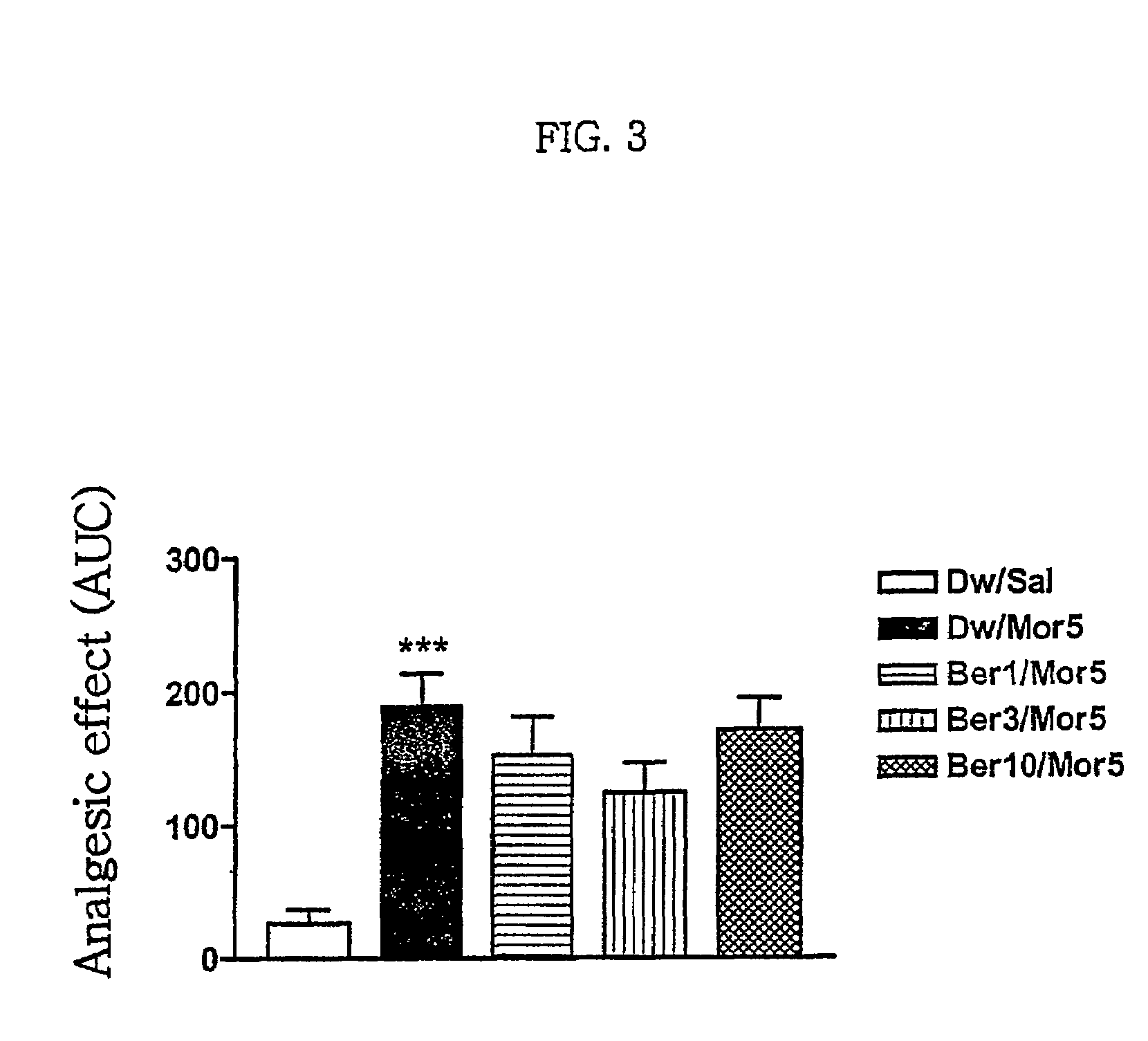
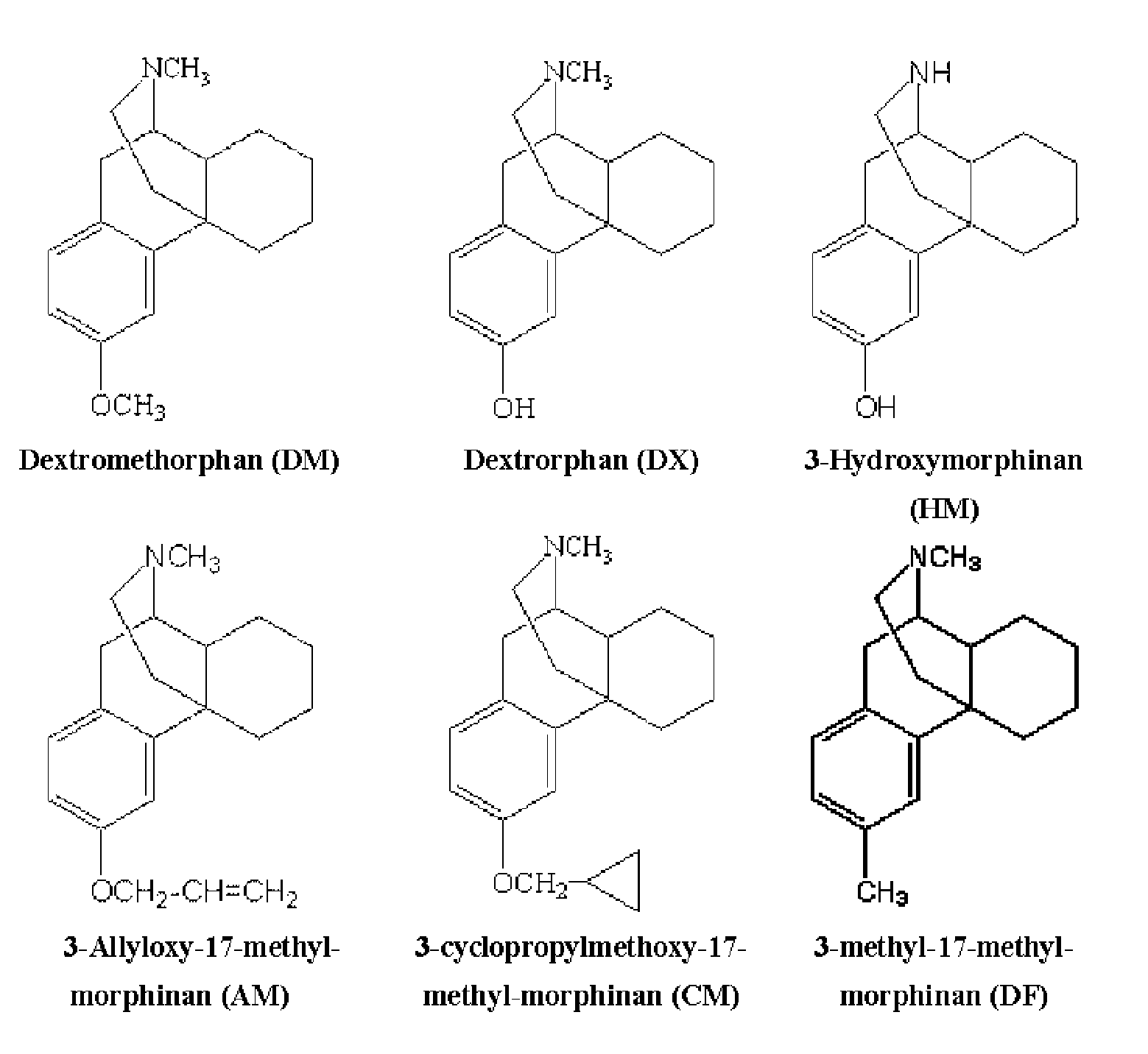
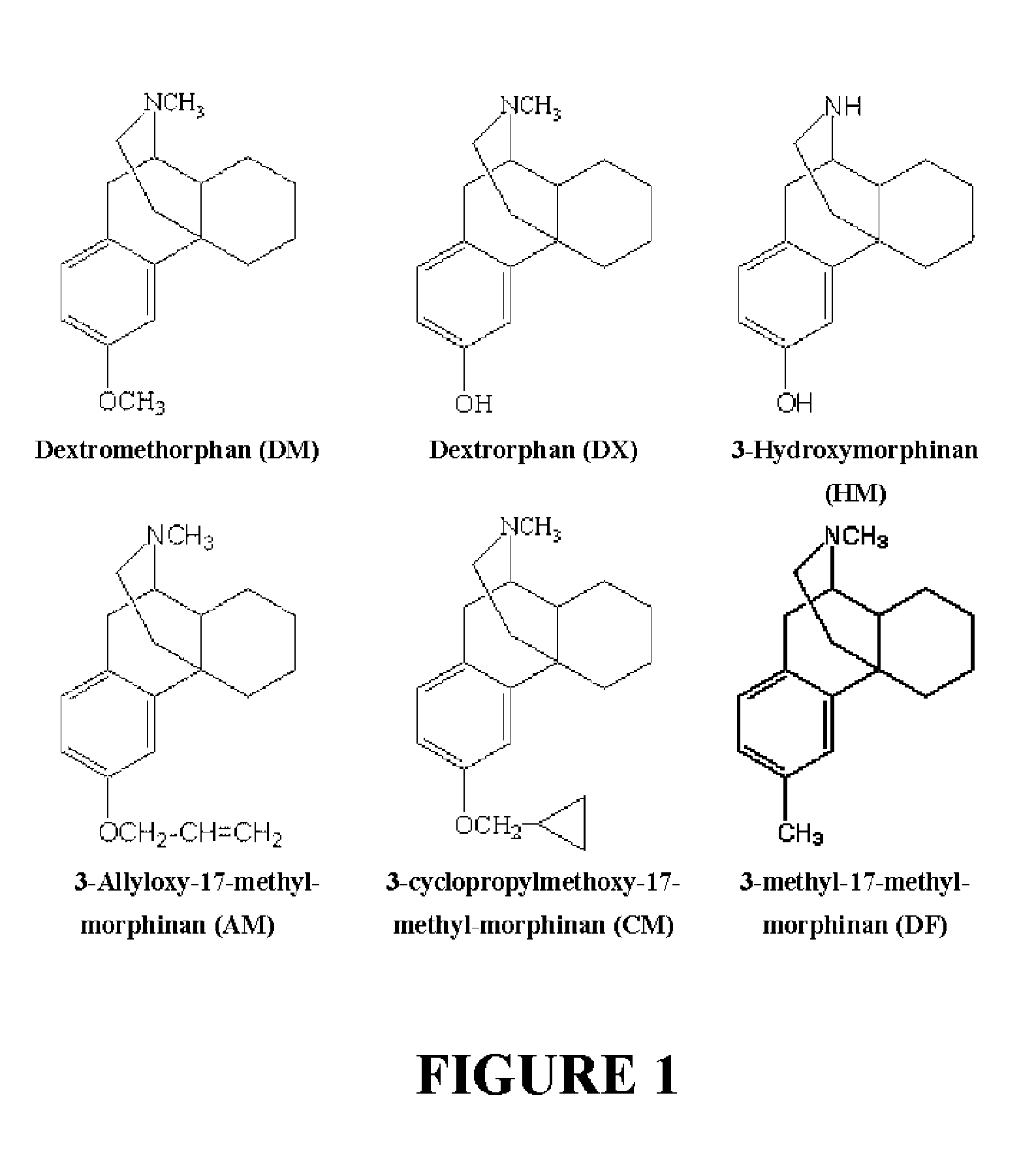
Pharmaceutical composition containing berberine as effective ingredient for preventing and treating addiction or tolerance to morphine
InactiveUS7250422B2Preventing and inhibiting developmentBiocideNervous disorderAbuse drugsAdditive ingredient
Disclosed is a pharmaceutical composition for preventing and treating addiction to morphine or preventing and inhibiting the development of tolerance to the analgesic effects of morphine, containing berberine as an effective ingredient, wherein the berberine has an inhibitory effect versus psychological dependence on abused drugs such as morphine and the increase of spontaneous locomotor activity upon administration of the drugs, The pharmaceutical composition and a Coptis japonica plant extract of the present invention, which contain berberine, are highly effective in inhibiting the aforementioned symptoms of morphine addiction, and are thus useful for prevention and treatment of addiction to abused drugs such as morphine. In addition, the pharmaceutical composition additionally containing a pharmaceutical acceptable carrier can be applied to prevent and inhibit morphine tolerance caused by repeated administration of morphine, while not affecting the analgesic effects of morphine upon a single administration.
Owner:SUNGKYUNKWAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com