Morphine and Morphine Precursors
a technology of morphine and precursors, applied in the field of morphine and morphine precursors, can solve the problems of few successful treatments available for these people, and achieve the effect of reducing the risk of overdosing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Reticuline Exposure to Invertebrate Ganglia Increases Endogenous Morphine Levels
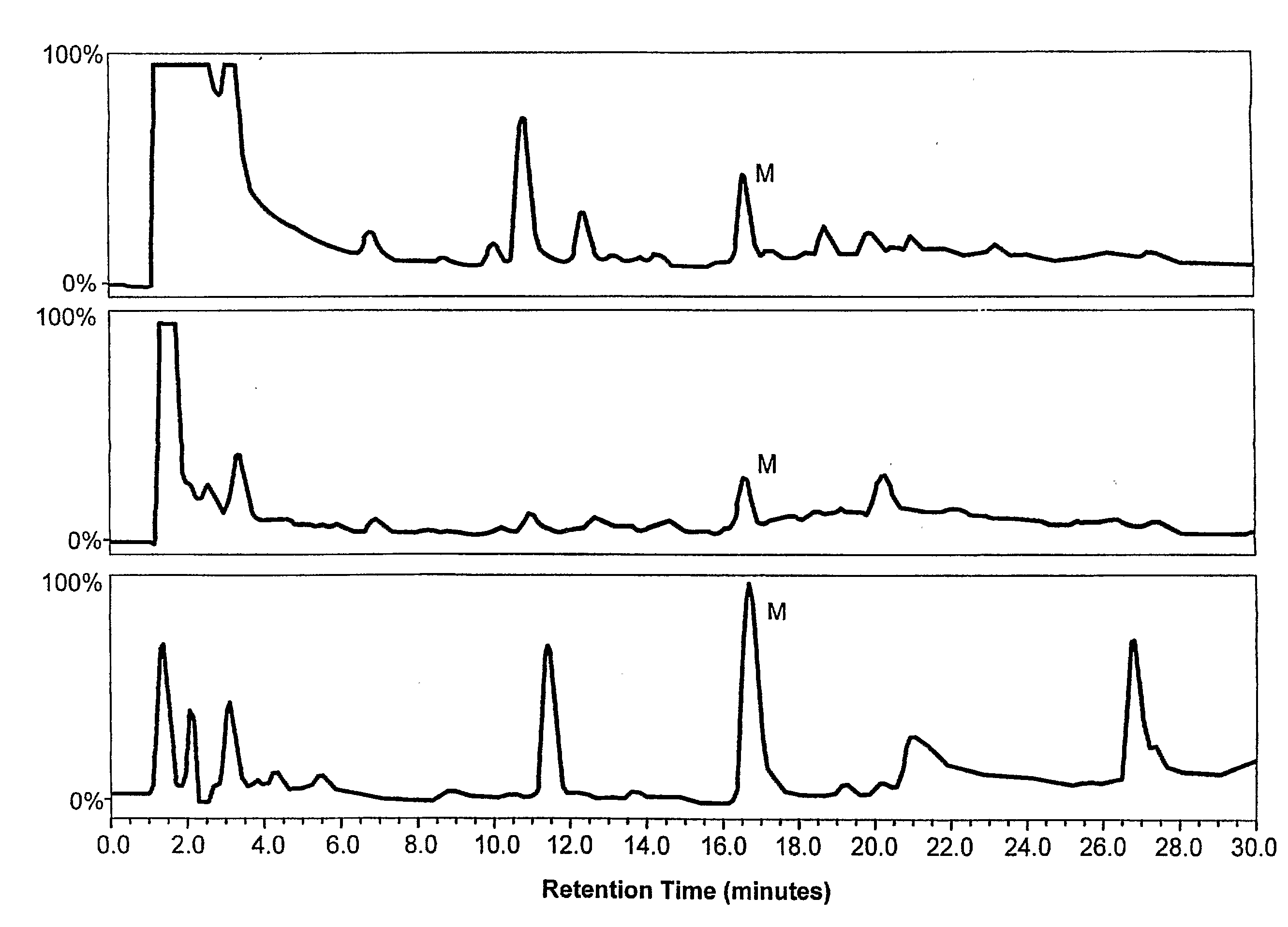
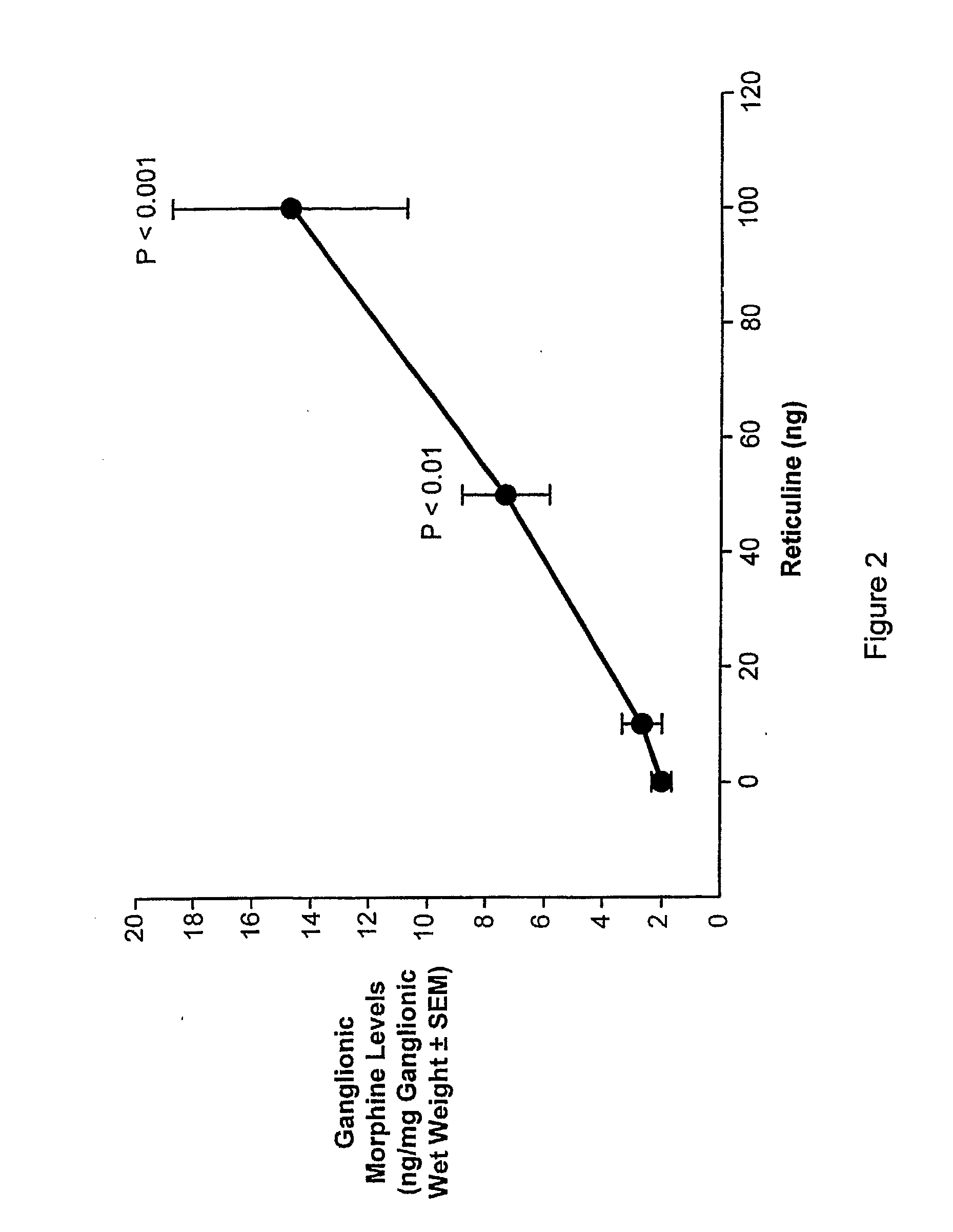
[0100]The following experiments were performed to determine if exposing tissues to an opiate alkaloid precursor, reticuline, would result in increasing endogenous morphine levels.
Material and Methods
[0101]Mytilus edulis collected from the local waters of Long Island Sound were maintained under laboratory conditions for at least 14 days prior using in experiments. Mussels were kept in artificial seawater (Instant Ocean, Aquarium Systems, Mentor, Ohio) at a salinity of 30 PSU and at a temperature of 18° C. as previously described (Stefano et al., Electro-Magnetobiol., 13:123-36 (1994)).
[0102]For reticuline exposure, 400 animals were placed and maintained in artificial seawater at 24° C., whereas control animals (100) were exposed to vehicle (PBS). For the biochemical analysis, groups of 20 animals had their pedal ganglia excised at different time periods after incubation with reticuline.
[0103]The extractio...
example 2
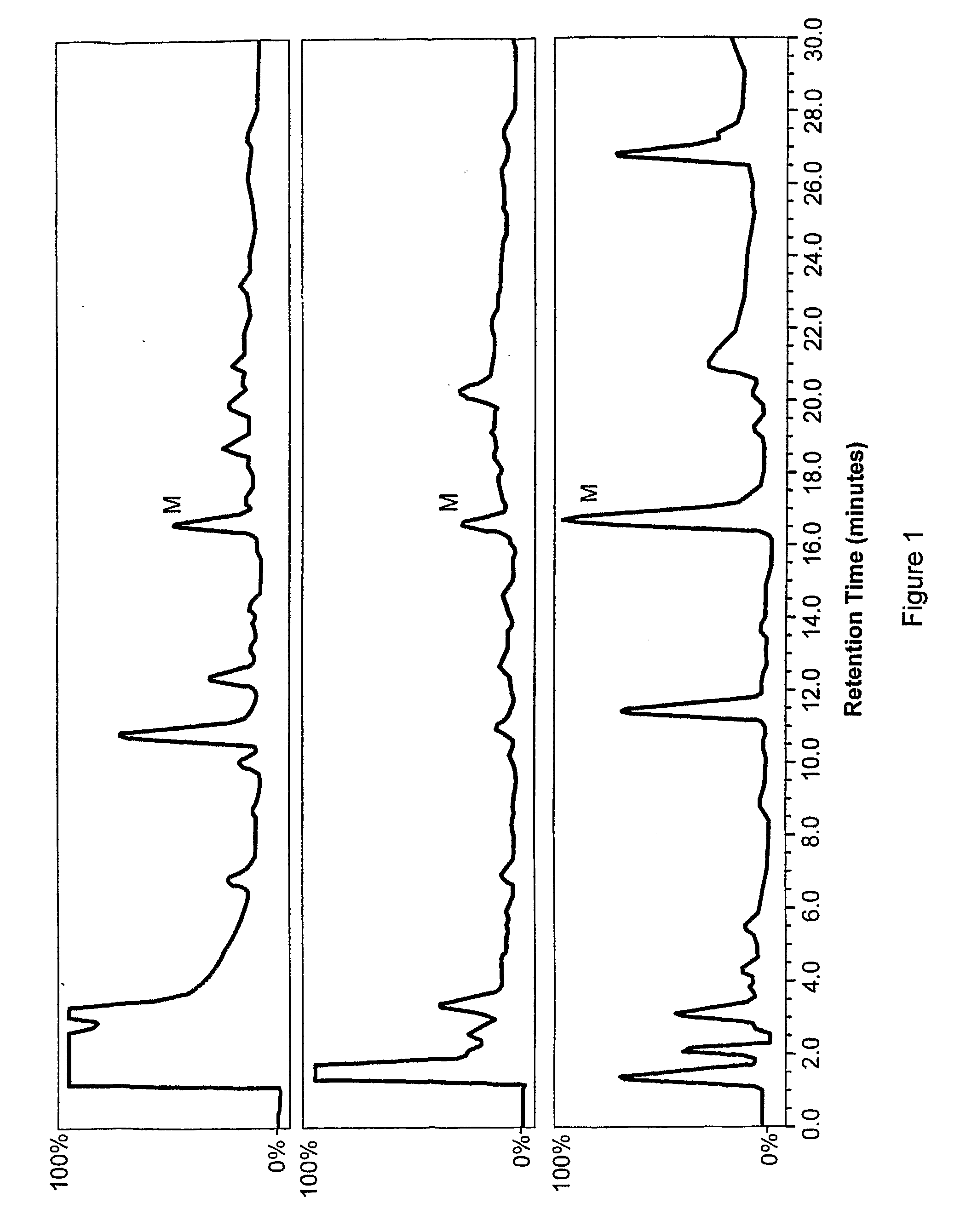
Mammalian Cells Produce Morphine from Reticuline
[0118]Human cells (NCI-H295R) were adapted from the NCI-H295 pluripotent adrenocortical carcinoma cell line (ATCC CRL-10296), which is from a carcinoma of the adrenal cortex. The original cells were adapted to a culture medium that decreased the population doubling time from 5 days to 2 days. While the original cells grew in suspension, the adapted cells were selected to grow in a monolayer. These cells retained the ability to produce adrenal androgens and were responsive to angiotensin II and potassium ions. To propagate these cells, the culture medium was a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium containing 15 mM HEPES, 0.00625 mg / mL insulin, 0.00625 mg / mL transferrin, 6.25 ng / mL selenium, 1.25 mg / mL bovine serum albumin, and 0.00535 mg / mL linoleic acid, 97.5%; Nu-Serum I, 2.5%. See, e.g., Rainey et al., Mol. Cell. Endocrinol., 100:45-50 (1994); Gazdar et al., Cancer Res., 50:5488-5496 (1990); and Bird ...
example 3
The Combination of L-DOPA and Dopamine Inhibits Endogenous Morphine Production
[0121]Mytilus pedal ganglia were obtained and incubated with 10 μg L-DOPA alone, 10 μg dopamine alone, or 10 μg L-DOPA plus 10 μg dopamine. The control ganglia were not incubated with L-DOPA or dopamine and exhibited 11.9 ng / mL morphine. The ganglia incubated with either L-DOPA alone or dopamine alone exhibited 9.31 and 8.82 ng / mL morphine, respectively. In contrast, ganglia incubated with both L-DOPA and dopamine exhibited 5.52 ng / mL of morphine. These results demonstrate that treatment with both L-DOPA and dopamine can reduce morphine production.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com