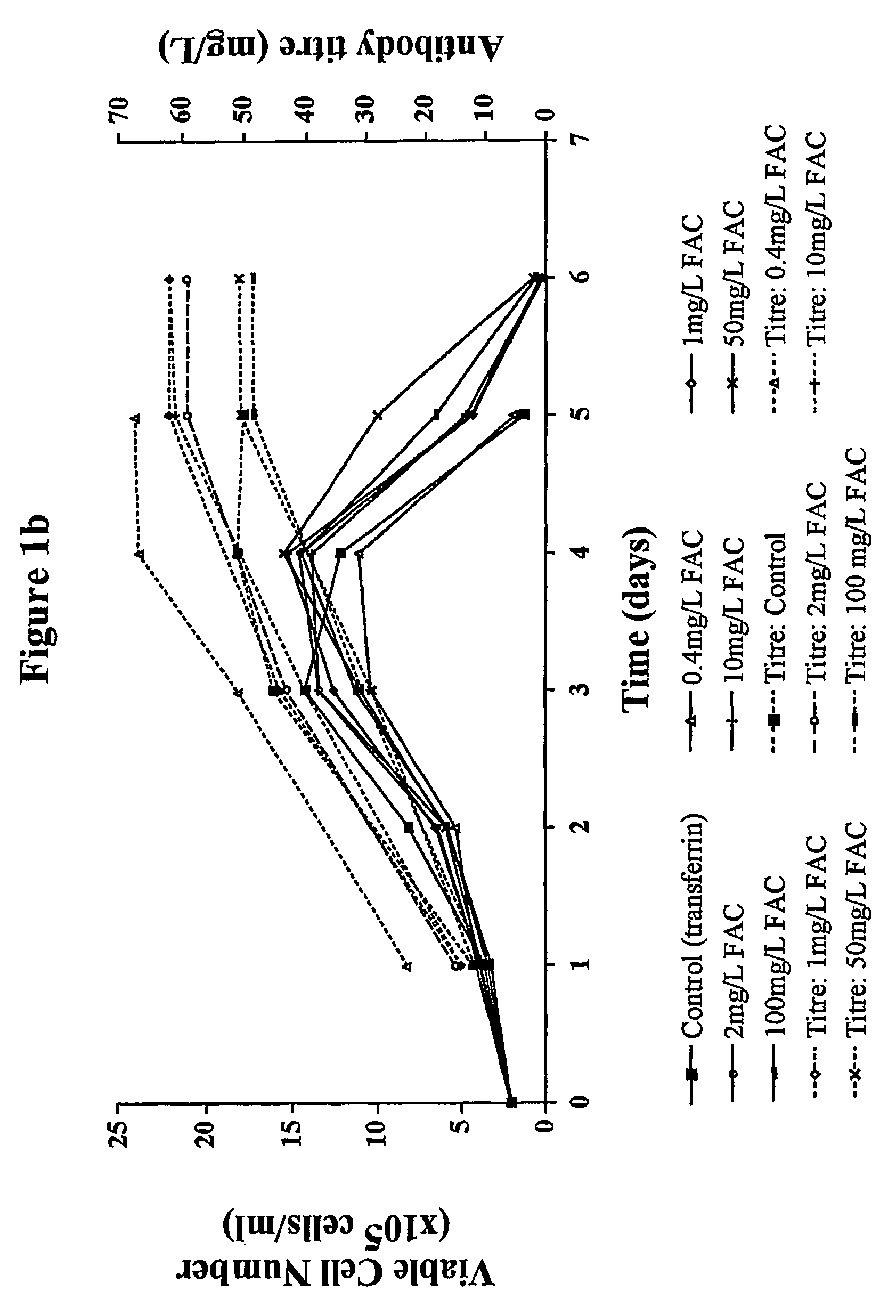
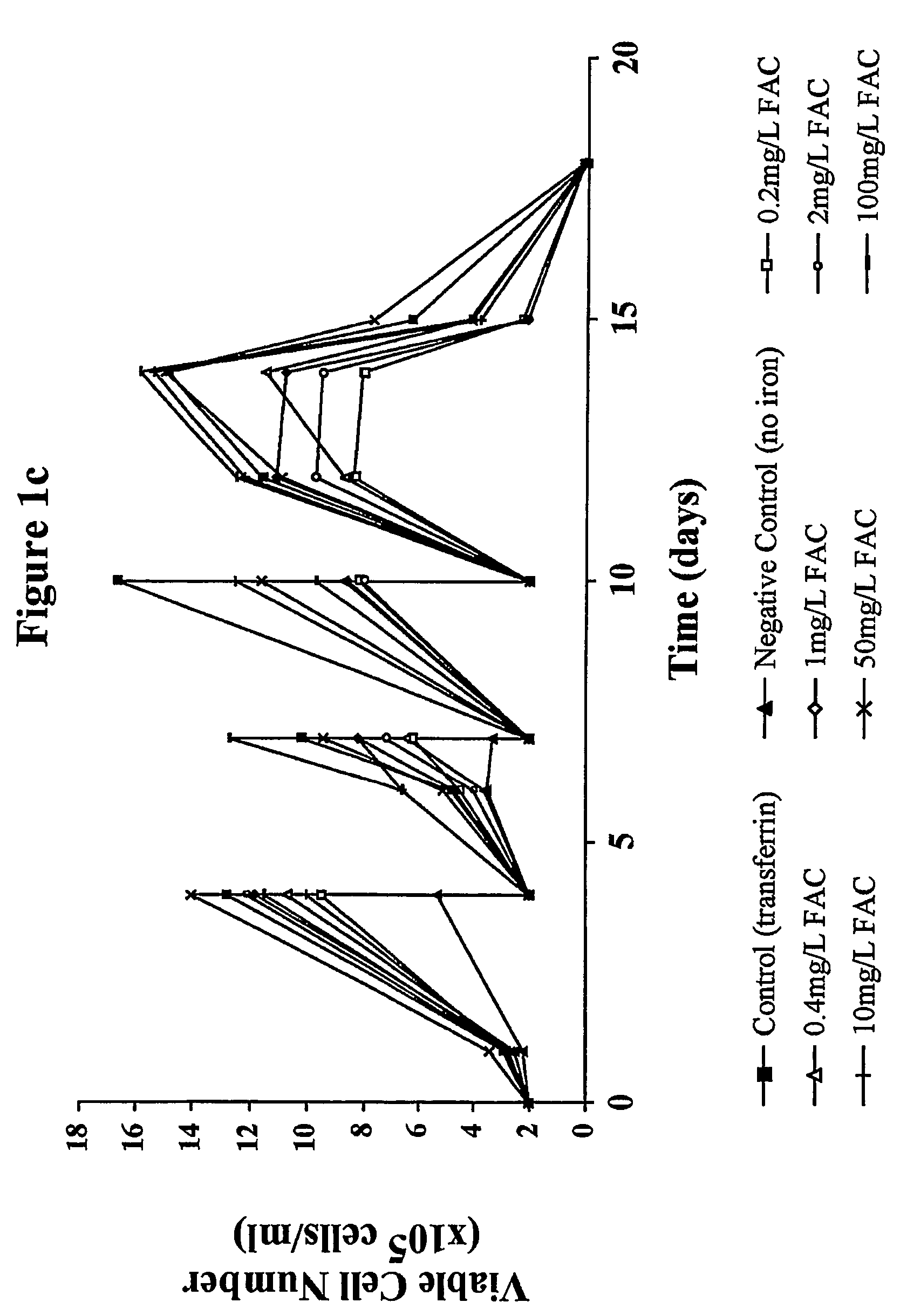
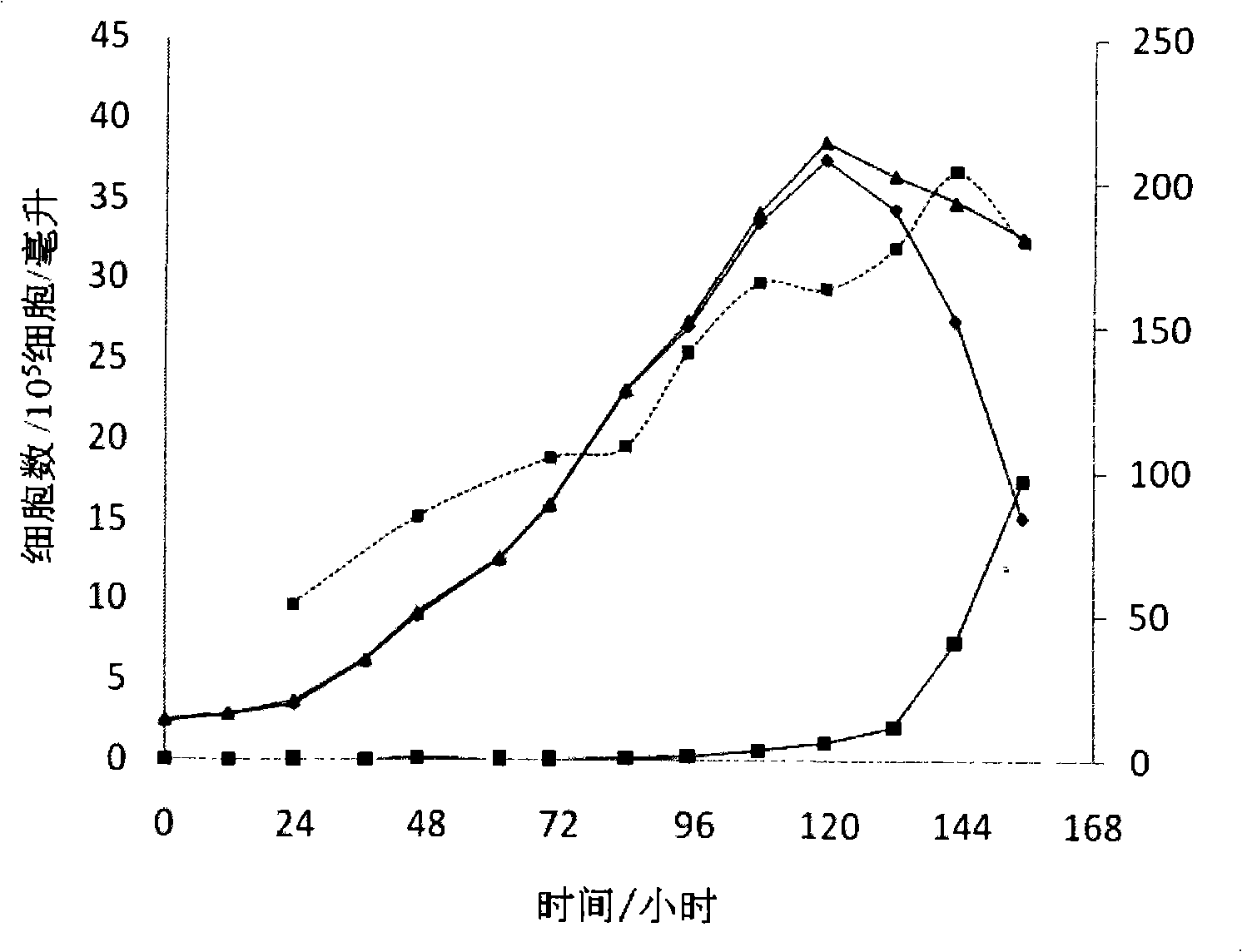
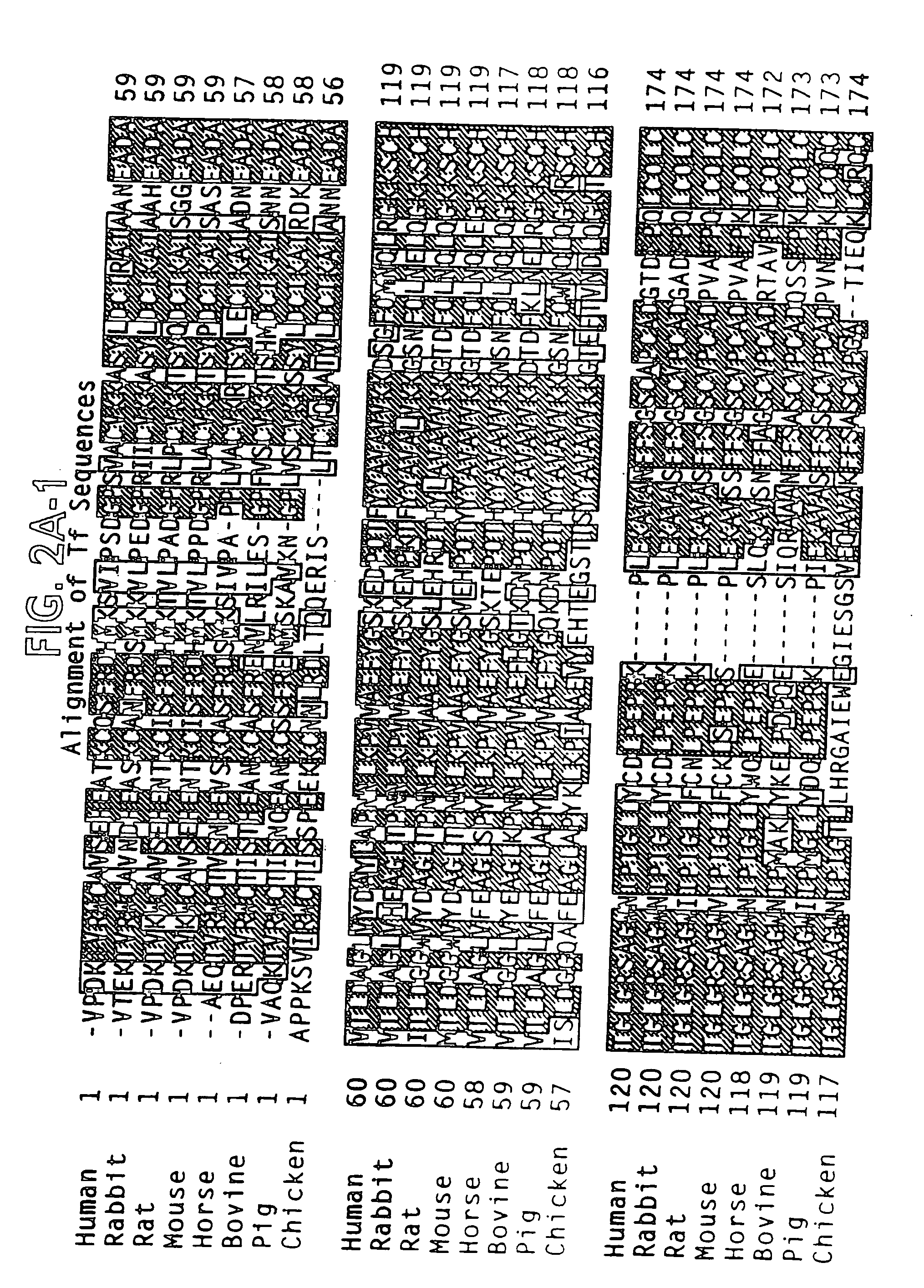
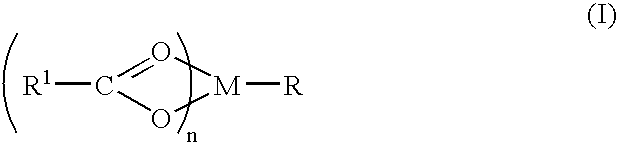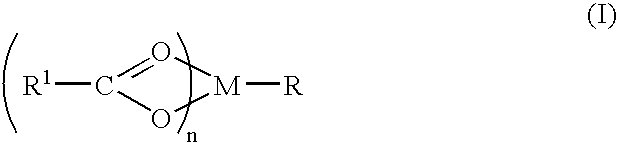Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
877 results about "Transferrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
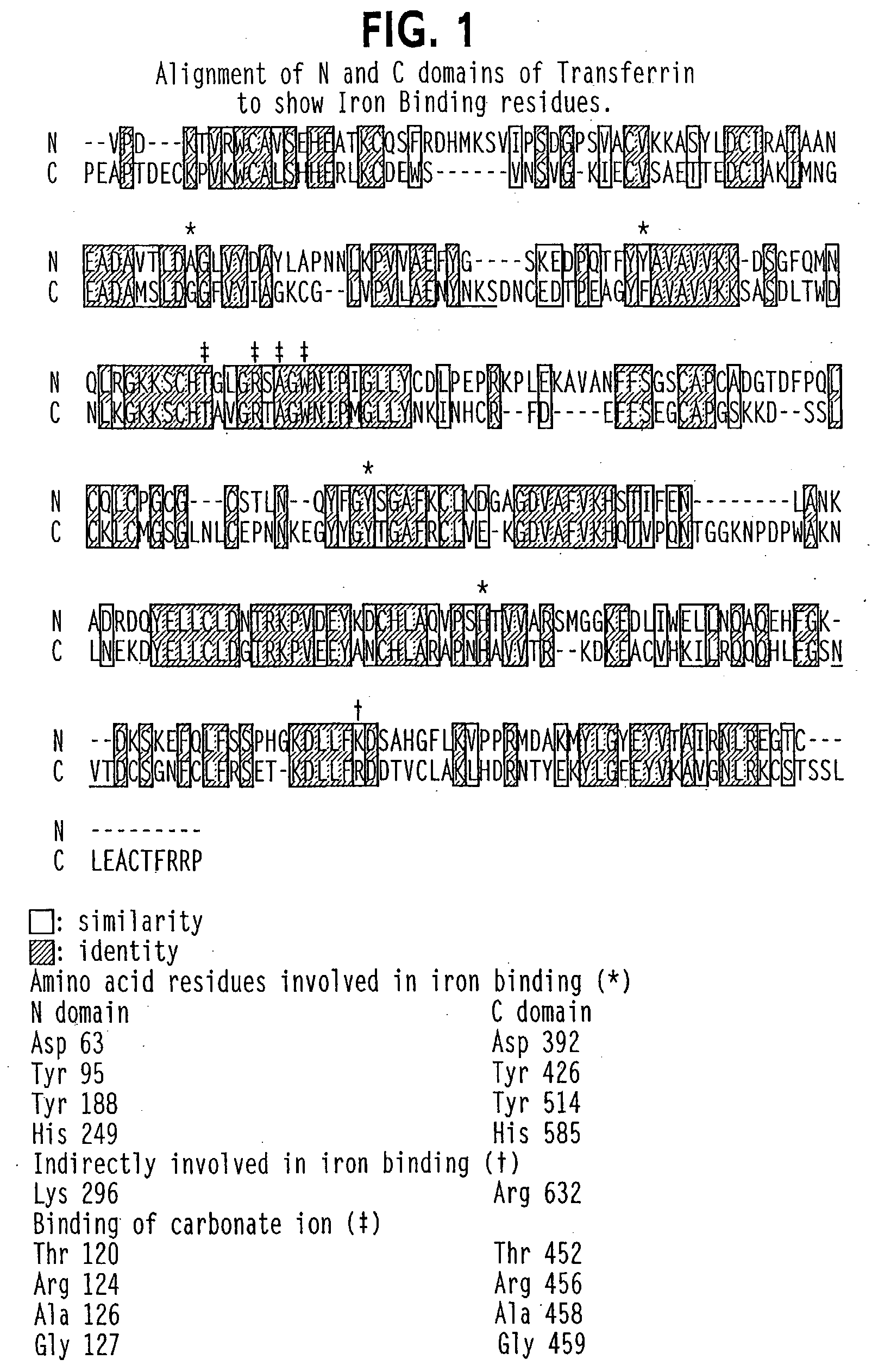
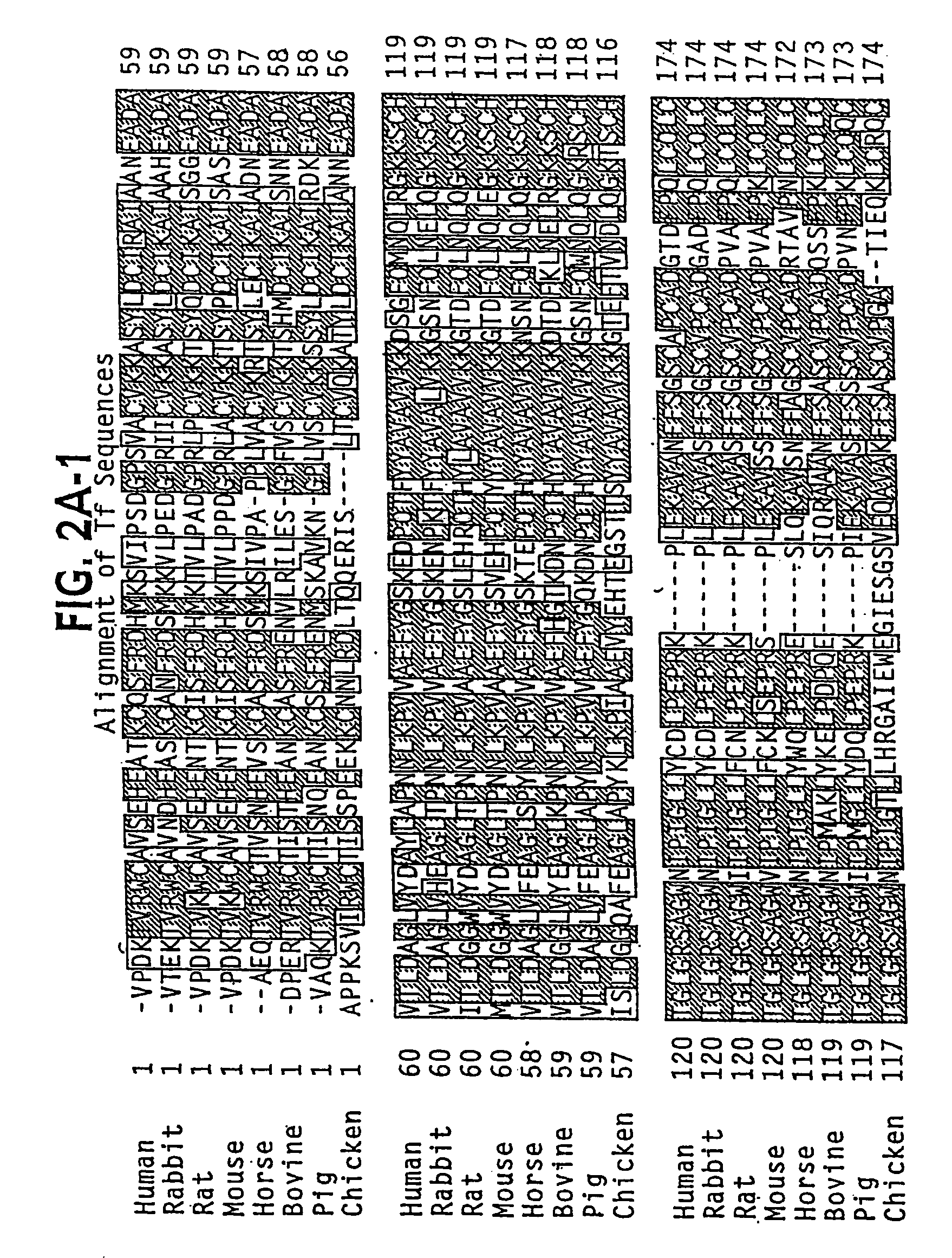
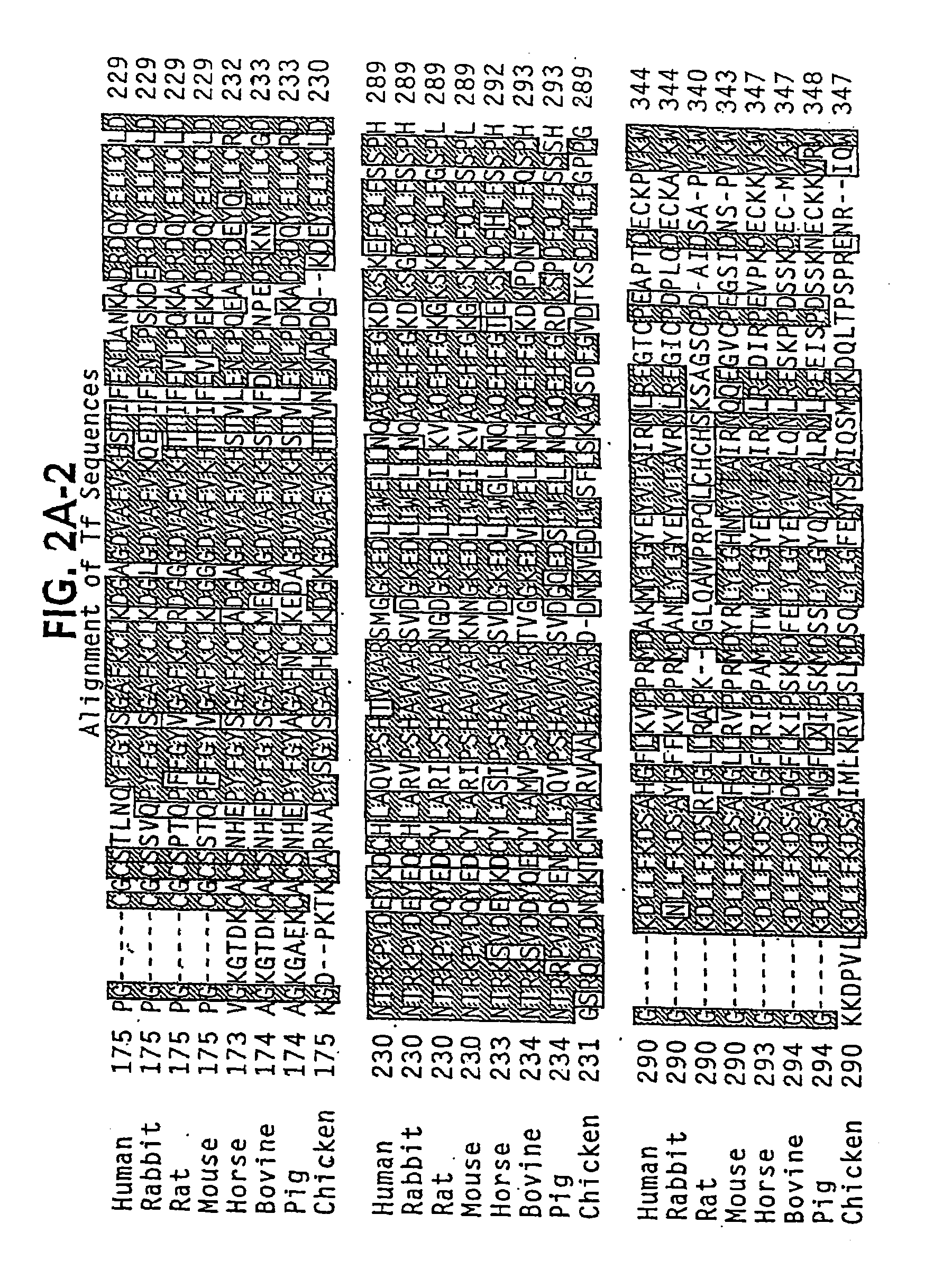
Transferrins are iron-binding blood plasma glycoproteins that control the level of free iron (Fe) in biological fluids. Human transferrin produced in the liver is encoded by the TF gene and as a 76-kDa glycoprotein.
Methods of combining metagenome and the metatranscriptome in multiplex profiles
The present invention describes changes in bacterial gastrointestinal, cutaneous and nasal microbiota associated various mammalian medical conditions. Described are diagnostic tests that arise from combining phylogenetic information about the families, genus, and species of the microbiome and their relative abundance with the metabolic information contained in the metatranscriptome to determine the presence and absence of a disease or medical condition. Provided are compositions of bacteria, co-cultures of bacteria and a carrier for use in treating the disclosed medical conditions. The described compositions restore or correct disease- or medical condition-related imbalances in the microbiome profile with culture-conditioned formulations in which the transcriptome activity of the administered organisms is optimized. Alternatively, formulations of metabolites that drive changes in the metatranscriptome native to the mammal that treat disease or a medical condition or restore health are taught.
Owner:ENSISHEIM PARTNERS
Modified transferrin fusion proteins
InactiveUS7176278B2Increased serum half-lifeImprove bioavailabilityAntibody mimetics/scaffoldsVirus peptidesDiseaseSerum ige
The present invention discloses fusion proteins comprising transferrin, lactoferrin or melanotransferrin fused to glucagon-like peptide 1 (GLP-1). In one embodiment of the invention, the fusion protein displays increased serum half-life as compared to a GLP-1 peptide in an unfused state. The invention includes a pharmaceutical composition comprising the GLP-1 fusion protein of the invention and a carrier. The fusion protein of the invention can be administered to a subject for treatment of diseases or conditions treatable by GLP-1, including, but not limited to, diabetes, obesity, congestive heart failure and inflammatory bowel syndrome.
Owner:BIOREXIS TECH INC
Process for chromatographic separation of peptides and nucleic acid, and new high affinity ion exchange matrix
InactiveUS6090288ACation exchanger materialsComponent separationChromatographic separationTransferrin
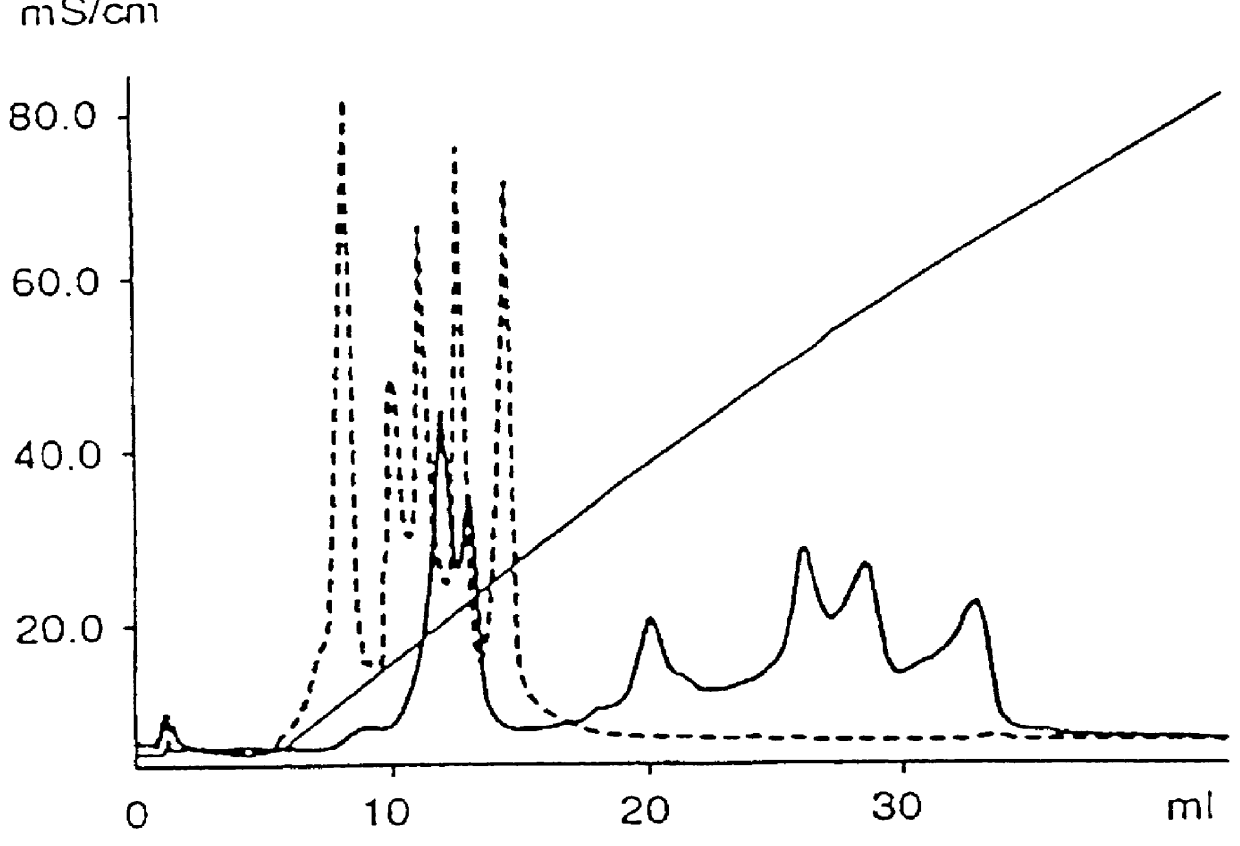
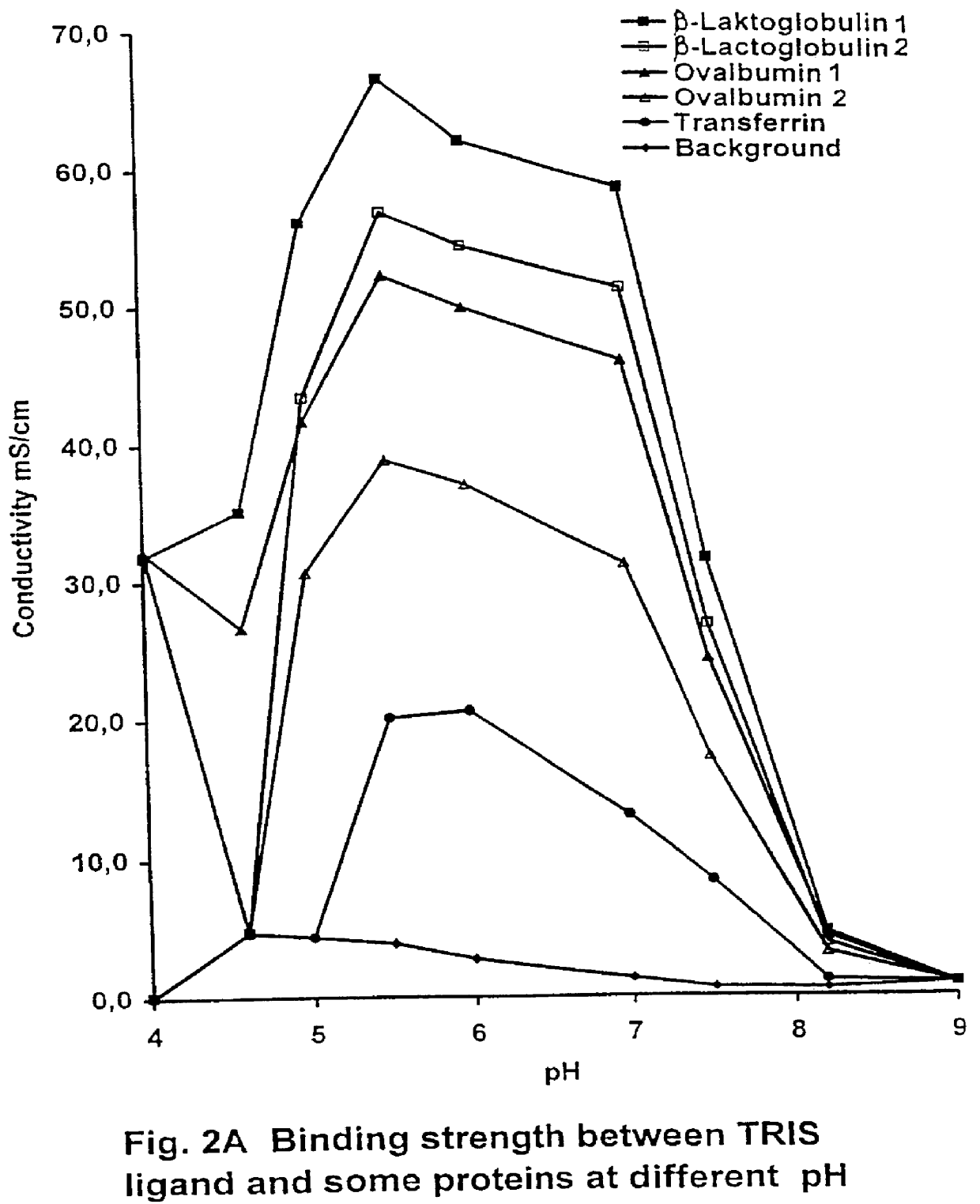
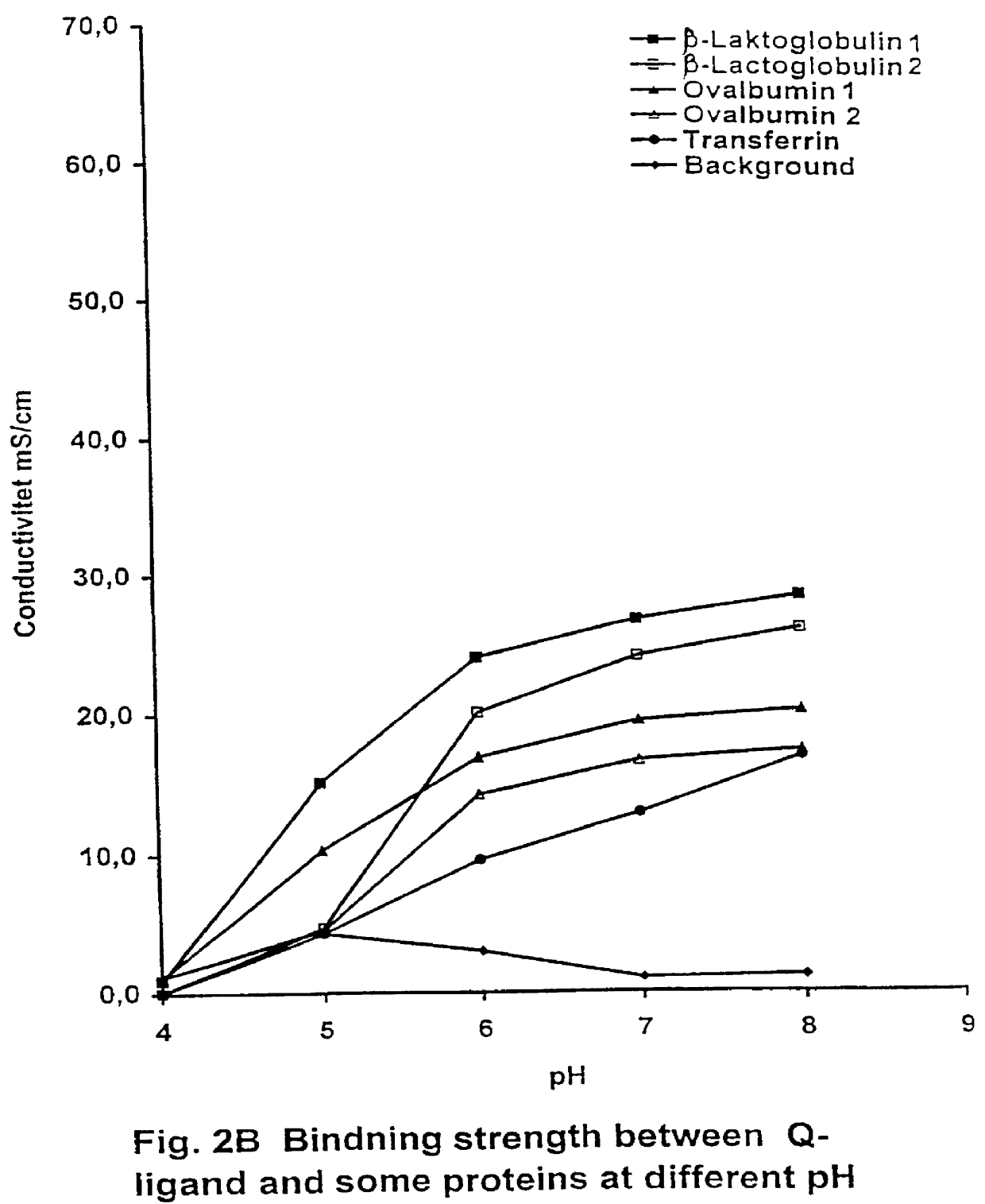
PCT No. PCT / SE97 / 00237 Sec. 371 Date Dec. 29, 1998 Sec. 102(e) Date Dec. 29, 1998 PCT Filed Feb. 14, 1997 PCT Pub. No. WO97 / 29825 PCT Pub. Date Aug. 21, 1997Process for separating off a peptide or a nucleic acid by an anion exchanger (I) characterized in that a) the anion exchanger (I) exhibits ligands, which (i) contain a primary, secondary or tertiary amino group and (ii) are covalently bound to an organic polymer (matrix), b) there on a carbon atom at a distance of 2 or 3 atoms away from an amino nitrogen in the ligands is a hydroxyl group or a primary, secondary or tertiary amino group, and c) the maximum elution ionic strength in the pH range 2-14 for at least one of the proteins transferrin, ovalbumin 1, ovalbumin 2, beta -lactoglobulin 1 and beta -lactoglobulin 2 on the anion exchanger is higher than the elution ionic strength required for a quaternary comparative ion exchanger.
Owner:GE HEALTHCARE BIOPROCESS R&D
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
A complex is provided for the treatment of neurogenic conditions having the formula: where R1 is M is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium (II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium (II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium (II):transporter ligand.
Owner:MILLER LANDON C G
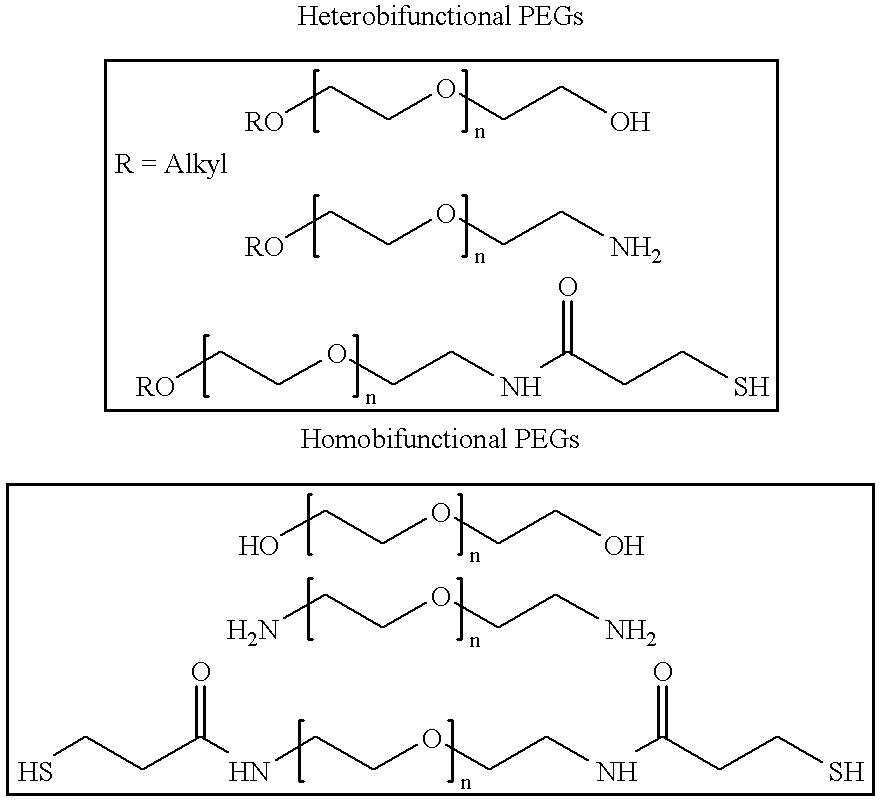
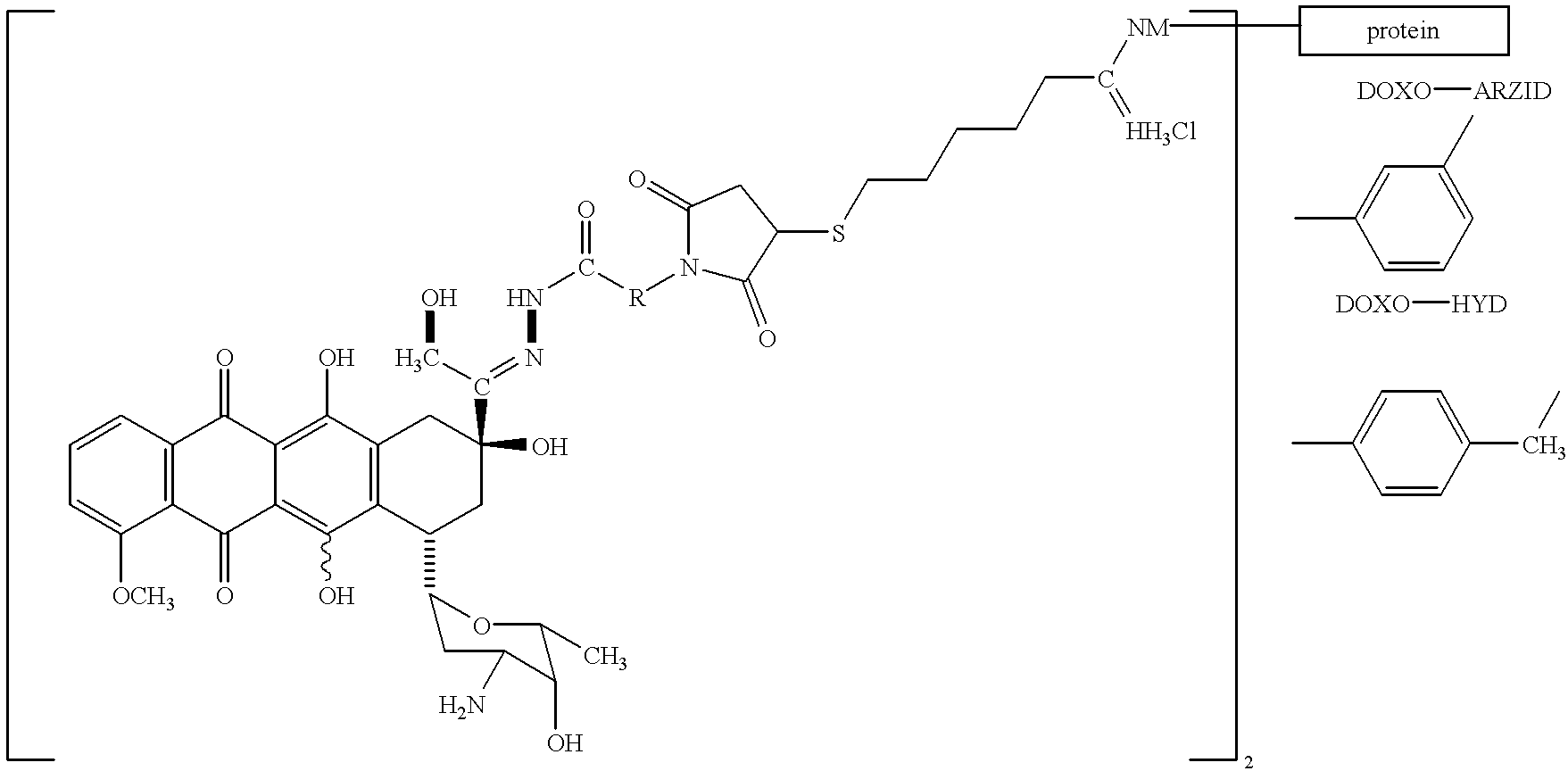
Antineoplastic conjugates of transferrin, albumin and polyethylene glycol
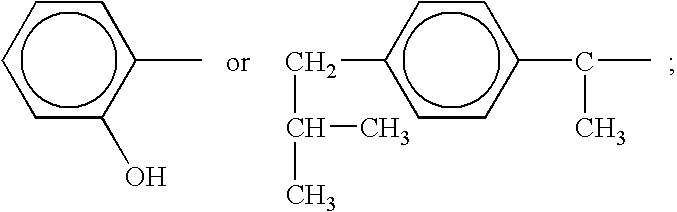
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R* H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
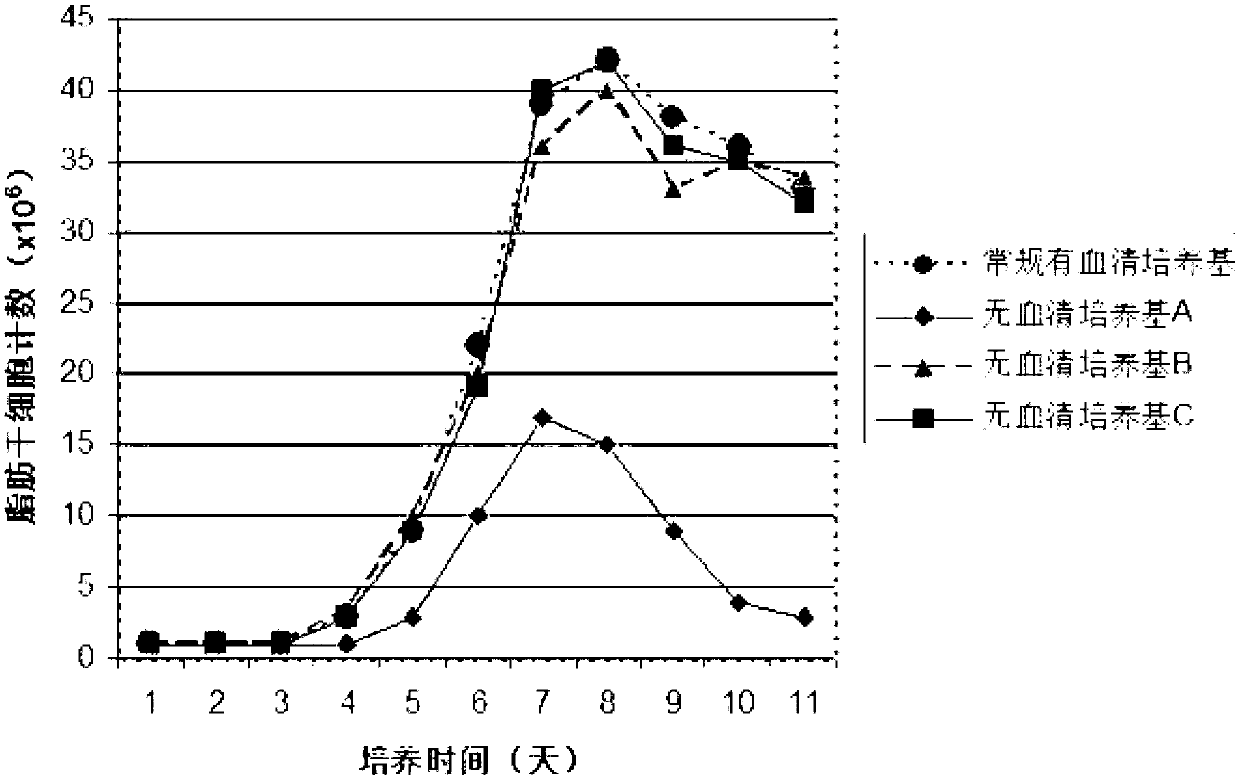
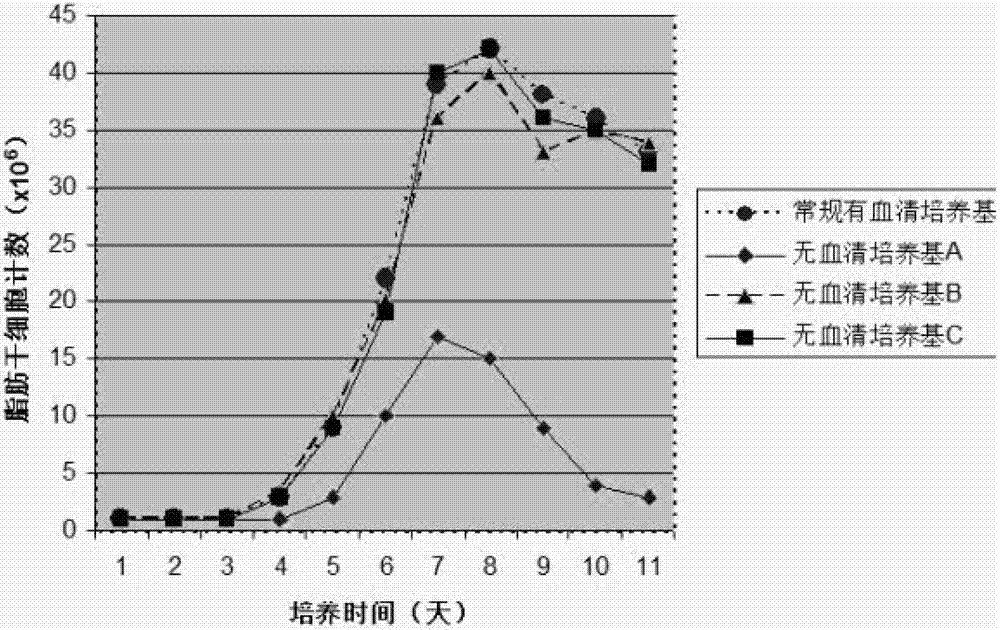
Serum-free medium for mesenchymal stem cells
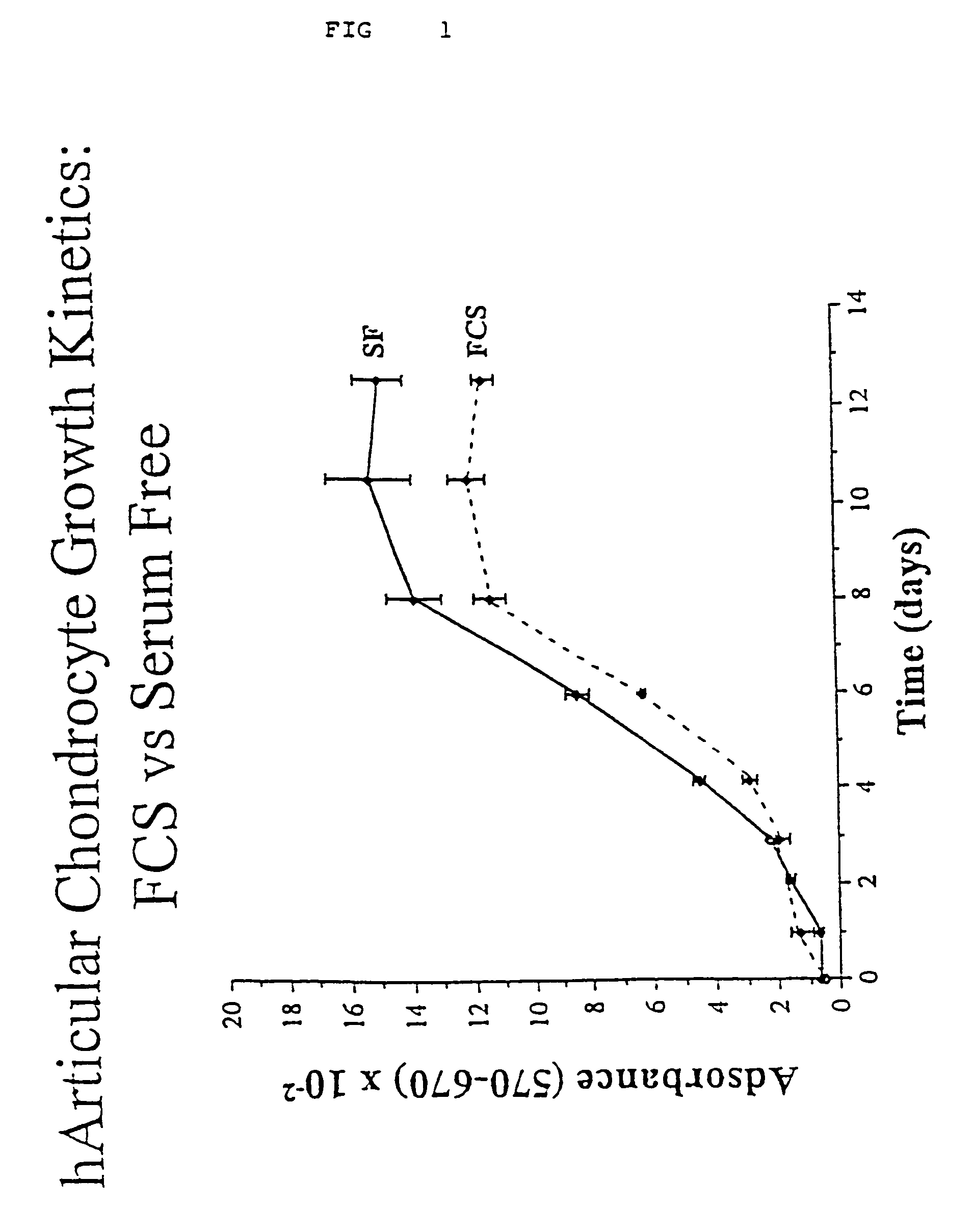
Serum-free media for growth and proliferation of chondrocytes and mesenchymal stem cells in culture are provided. A serum-free medium for growth of chondrocytes includes a serum-free composition comprising FGF-2, linoleic acid, ascorbic acid, B-mercaptoethanol, transferrin and dexamethasone. Further, the composition comprises EGF, PDGFbb, insulin and albumin. A method for growing chondrocytes in a serum free medium comprising the compostion is also provided. Also provided for mesenchymal stem cell growth, is a serum-free medium which includes a composition comprising FGF-2, LIF, SCF, pantotenate, biotin and selenium and method, therefore.
Owner:CONSORZIO PER LA GESTIONE DEL CENT DI BIOTECNOLOGIA AVANZATA +1
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Culture process of human nerve stem cell
InactiveCN1389565APromote proliferation and divisionPromote growthNervous system cellsGlutethimideCortisone
The invention discloses a cultivated method of nervous stem cells, it includes following steps:1, Nervous stem cells substrate of human being is made up of base cultivate liquid which is compounded by mixing DMEM and F12 by rate of 1:1, and insulin, muriatic acid butyl amic, selenium natrium, ydrogen cortisone, L-glutamic acyl amic, man-turn iron albumen, flavone; 2, Noumenon serum of patient themselves is gathered immediately; 3. Noumenon serum of patient themselves is put into compounded Nervous stem cells substrate of human being, them, it is incubated by adding 3-7% CO2 warmer under conditino of 35C-38C. The method of the invention is simple, and invention has better repetition and its operation is simple and convenient; it plays an inestimable part in researching, teaching, clinic application, etc all of which relate to nervous stem cell and its application.
Owner:徐如祥 +1
Compositions comprising reproductive cell media and methods for using such compositions
InactiveUS6849394B2Mammal material medical ingredientsDead animal preservationInsulin-like growth factorCell culture media
Disclosed are compositions for mammalian, avian or piscian reproductive cells and methods for the collection, holding, processing, in vitro fertilization, sexing culturing, or storing (including long-term cryopreservation) of mammalian, avian, or piscian reproductive sperm cells. The compositions comprise a suitable reproductive cell media and a transforming growth factor, an insulin-like growth factor, or zinc, and, optionally, inositol, transferrin, or fructose, or combinations thereof.
Owner:MOFA GRP
Serum-free medium for in vitro cultivation and amplification of mesenchymal stem cells
ActiveCN101412985AResidue reductionMaintain multilineage potentialSkeletal/connective tissue cellsSerum free mediaAntioxidant
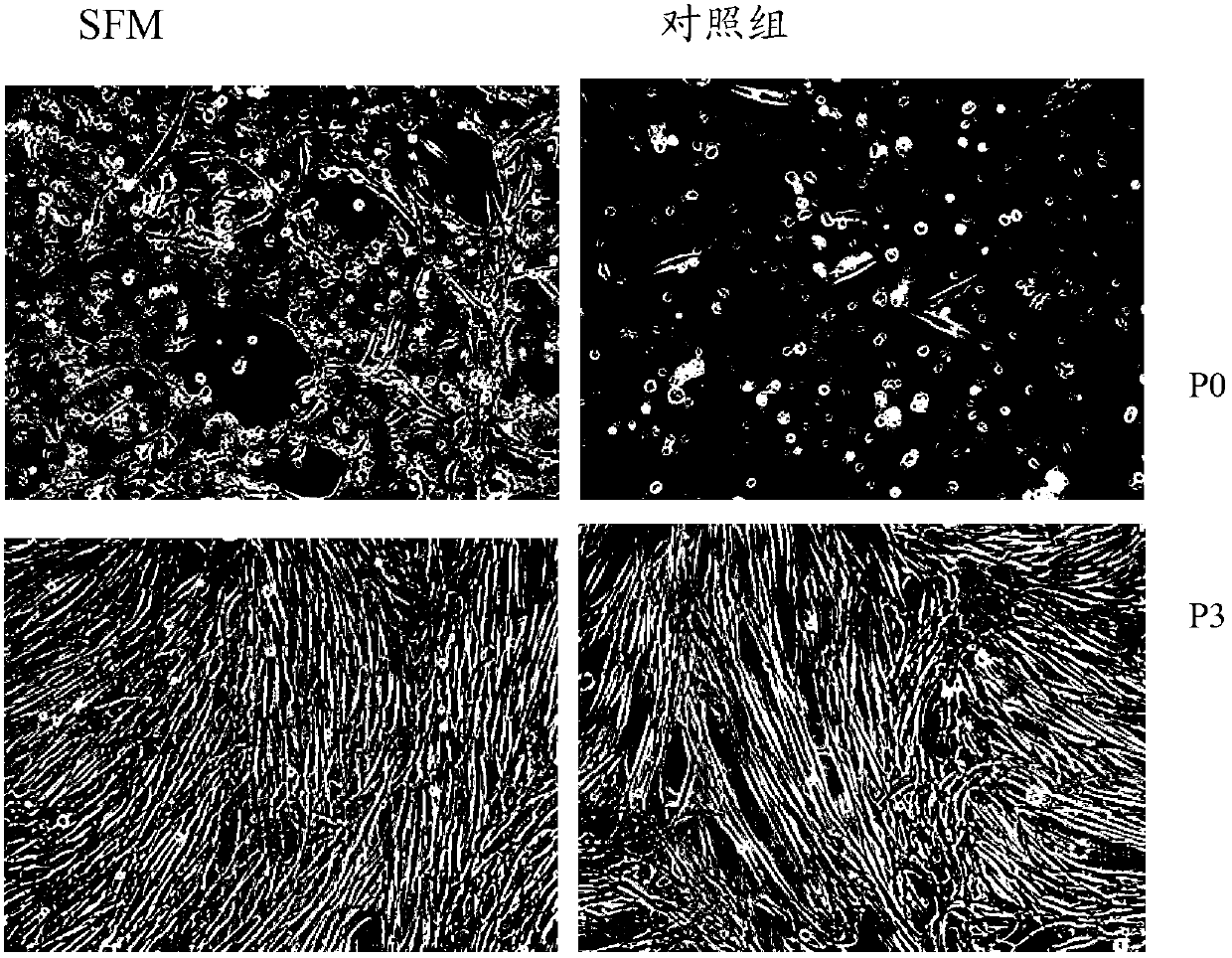
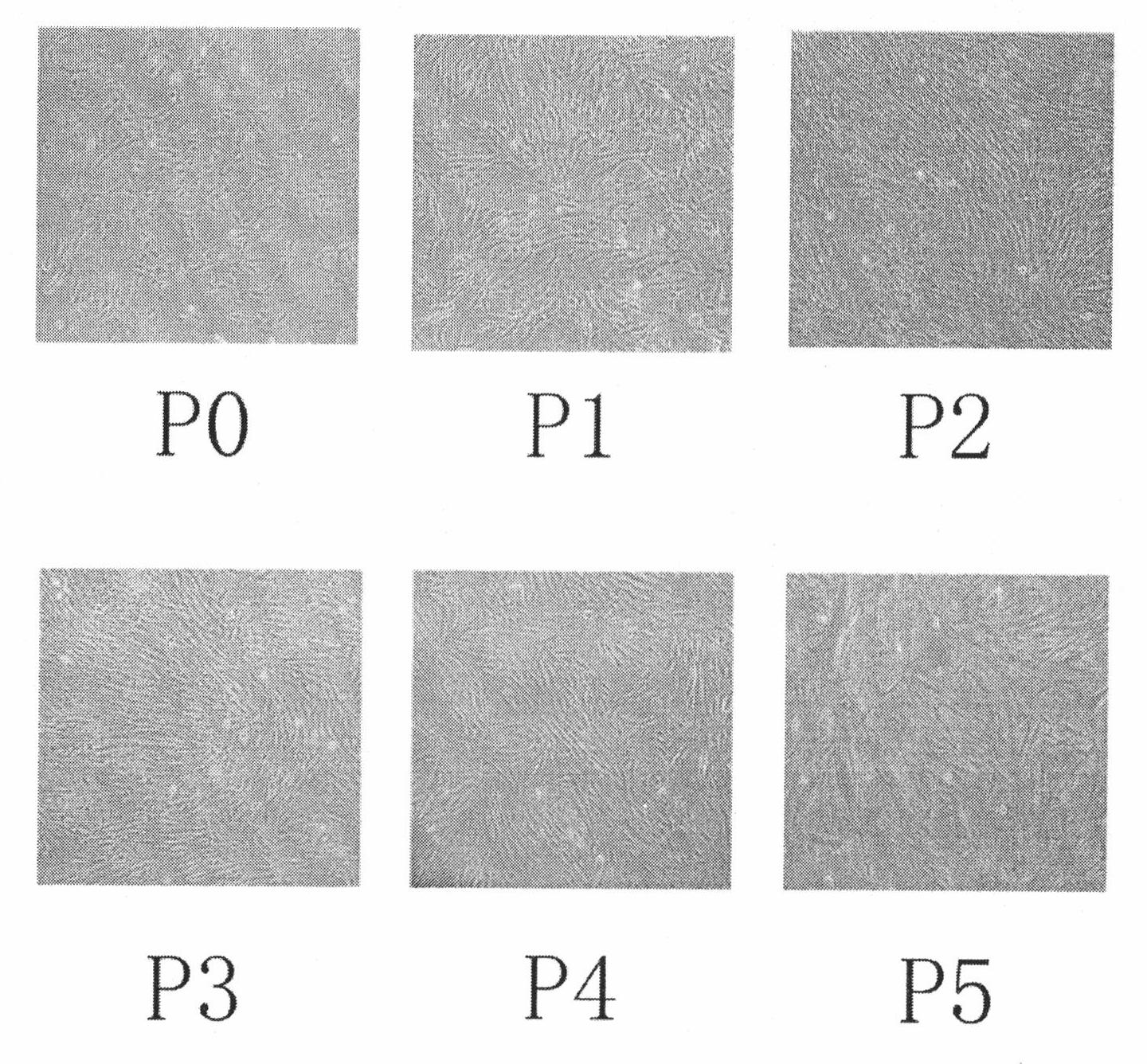
The present invention belongs to the field of biotechnology, and discloses a serum-free culture medium with specific chemical compositions for in vitro culture and amplification of bone marrow mesenchymal stem cells. By adding insulin, transferrin, ethanolamine, sodium selenite, growth factors, adherent factors, hormone, putrescine, inorganic salt, vitamin, albumin and antioxidant into a basic culture medium, the bone marrow mesenchymal stem cells can attach to the culture medium under a serum free condition, so the in vitro culture and amplification are realized, the potential of multi-directional differentiation is maintained, and the amplified cells can be induced to be osteoblast and lipocyte in vitro. The serum-free culture medium has the advantages that the clinic level cell products for human produced by the serum free culture medium can effectively avoid the potential risk of producing cell products by serum culture medium. The drawing appended is a photo of the confluence of the bone marrow mesenchymal stem cells cultured by the serum-free culture medium.
Owner:EAST CHINA UNIV OF SCI & TECH
Myeloma cell culture in transferrin-free low iron medium
ActiveUS8361797B2High titerSupport growthGenetically modified cellsCulture processCulture cellMicrobiology
The present invention relates to a method for culturing mammalian cells in a culture medium which is transferrin free and which contains no lipophilic or synthetic nitrogen-containing chelators. Also provided is the use of the medium and a process for providing a mammalian product by culturing cells capable of producing the product in the medium.
Owner:MEDIMMUNE LTD
Serum-free medium for culturing placenta mesenchymal stem cells
ActiveCN103805562AIncrease growth rateMaintain stem cell propertiesSkeletal/connective tissue cellsFibroblast growth factor receptor 2Cell culture media
The invention discloses a serum-free medium for culturing placenta mesenchymal stem cells. The serum-free medium takes a DMEM (Dulbecco Modified Eagle Medium) culture solution as a basis and also contains a fibroblast growth factor receptor 2, growth hormone, insulin, transferrin, glutathione, BMP-4, L-glutamine, sodium pyruvate, non-essential amino acids and beta-mercaptoethanol. According to various serum-free media provided by the invention, growth and proliferation of the placenta mesenchymal stem cells in a serum-free medium system can be effectively promoted, the placenta mesenchymal stem cells have higher growth and proliferation rate in the serum-free medium system compared with a serum cell culture medium, the characteristics of the stem cells are preserved, the serum-free medium has multiple differential potentials, and the stem cells can be directionally induced into fat cells and osteoblasts.
Owner:章毅 +10
Processes for clonal growth of hepatic progenitor cells
A method of propagating mammalian endodermally derived progenitors such as hepatic progenitors, their progeny, or mixtures thereof is developed which includes culturing mammalian progenitors, their progeny, or mixtures thereof on a layer of embryonic mammalian feeder cells in a culture medium. The culture medium can be supplemented with one or more hormones and other growth agents. These hormones and other growth agents can include insulin, dexamethasone, transferrin, nicotinamide, serum albumin, beta-mercaptoethanol, free fatty acid, glutamine, CUSO4, and H2SeO3. The culture medium can also include antibiotics. Importantly, the culture medium does not include serum. The invention includes means of inducing the differentiation of the progenitors to their adult fates such as the differentiation of hepatic progenitor cells to hepatocytes or biliary cells by adding, or excluding epidermal growth factor, respectively. The method of producing mammalian progenitors is useful in that the progenitors can be used subsequently in one or more of the following processes: identification of growth and differentiation factors, toxicological studies, drug development, antimicrobial studies, or the preparation of an extracorporeal organ such as a bioartificial liver.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Compositions and methods for targeting a polypeptide to the central nervous system
InactiveUS20050100986A1Nervous disorderPeptide/protein ingredientsPseudomonas aeruginosa exotoxin AInsulin-like growth factor
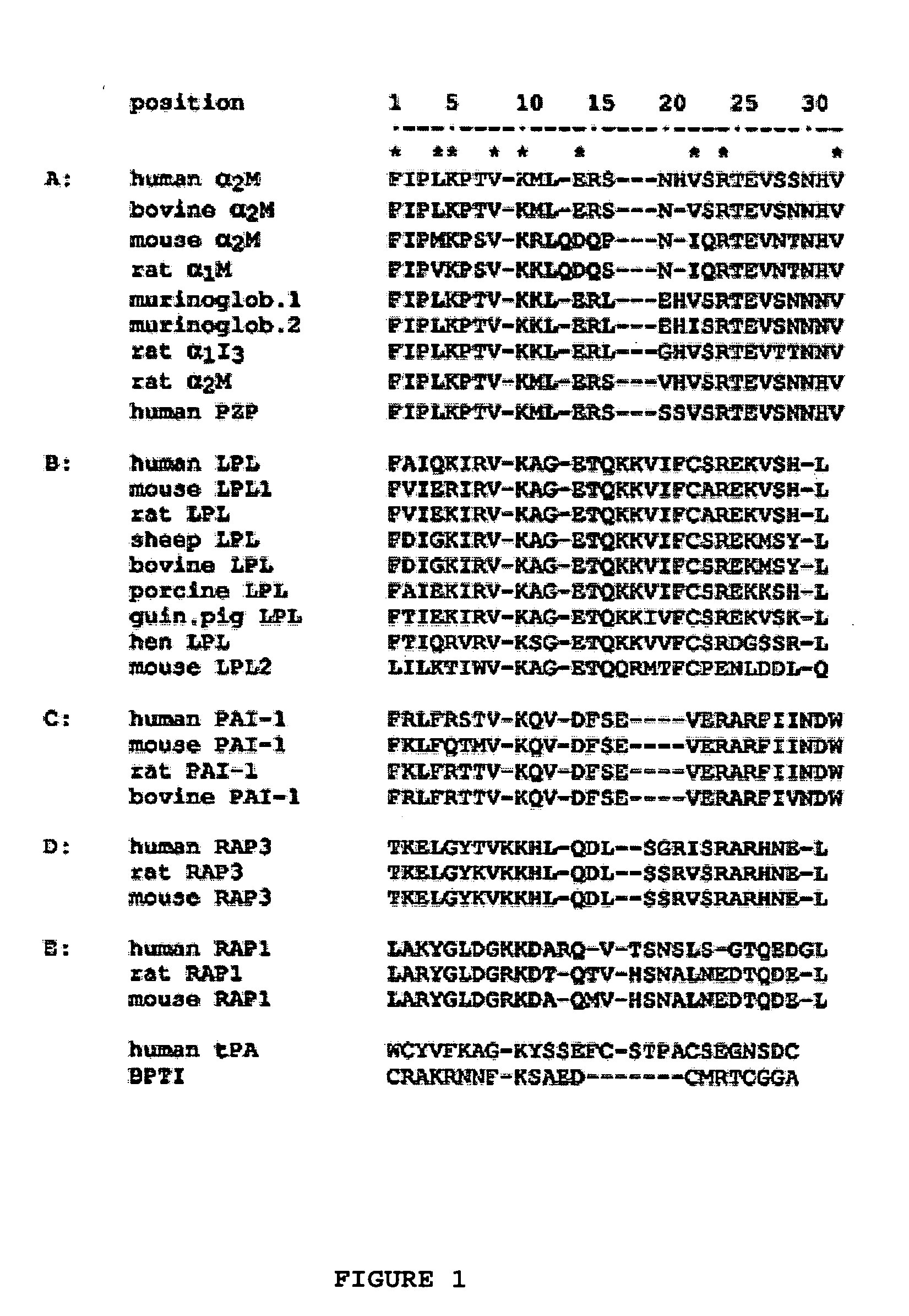
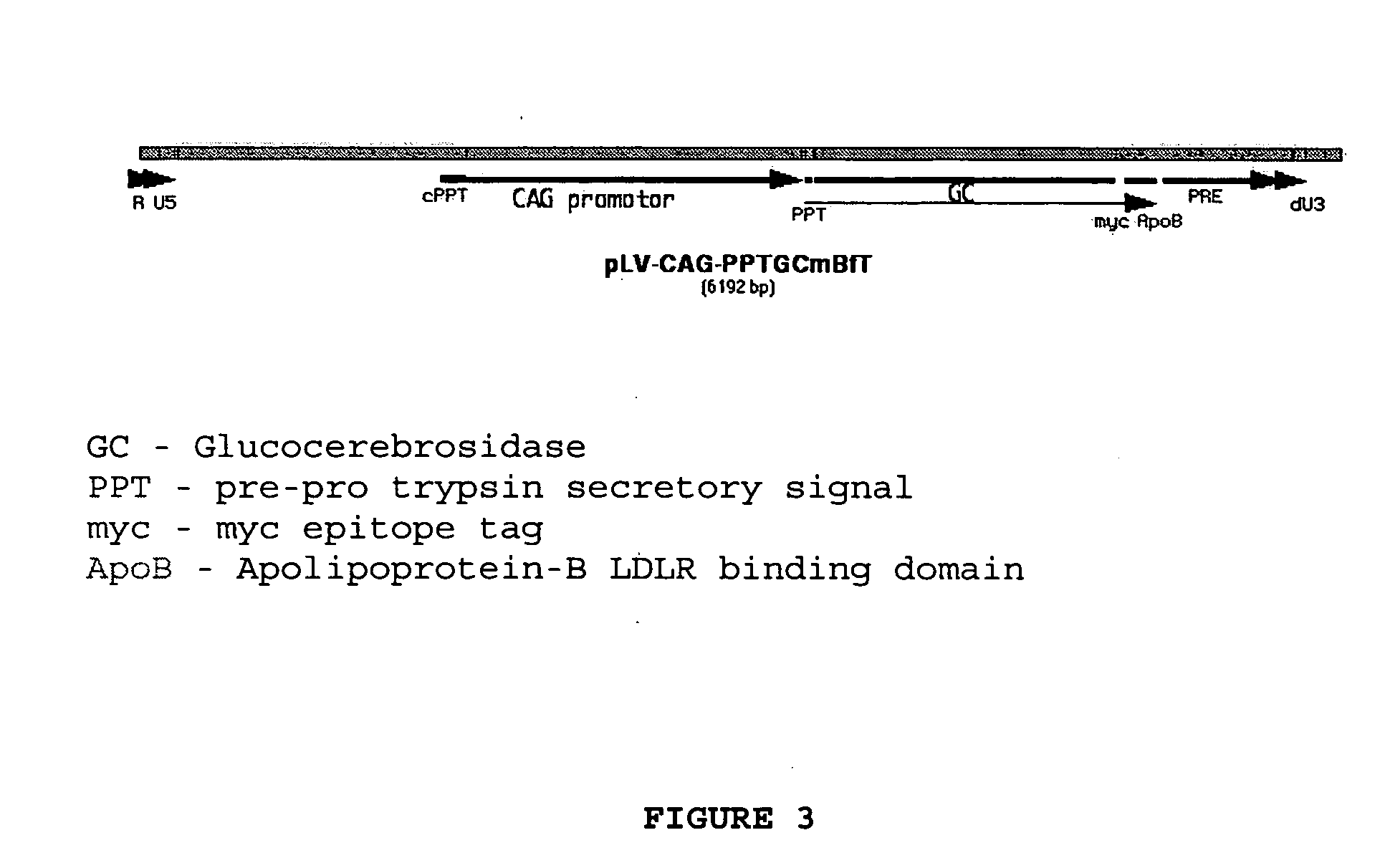
The invention provides a chimeric CNS targeting polypeptide having a BBB-receptor binding domain and a payload polypeptide domain. The chimeric CNS targeting polypeptide can have a BBB-receptor binding domain consisting of a receptor binding domain from ApoB, ApoE, aprotinin, lipoprotein lipase, PAI-1, pseudomonas exotoxin A, transferrin, α2-macroglobulin, insulin-like growth factor, insulin, or a functional fragment thereof. Nucleic acids encoding a chimeric CNS targeting polypeptide are also provided. Further provided is a method of delivering a polypeptide to the CNS of an individual. The method consists of administering to the individual an effective amount of a chimeric CNS targeting polypeptide, said chimeric CNS targeting polypeptide comprising a BBB-receptor binding domain and a payload polypeptide domain. The method also can deliver a polypeptide to the lysosomes of CNS cells.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Serum-free cell culture fluid suitable for enriching and culturing tumour stem cells
The invention provides a serum-free cell culture fluid suitable for enriching and culturing tumour stem cells. DMEM / f12 serves as the basic culture fluid of the serum-free cell culture fluid and transferrin, insulin and other substances are also added as the components of the serum-free cell culture fluid. The serum-free cell culture fluid provided by the invention can efficiently enrich tumour stem cells from malignant tumour cell strains and tumour tissues. In addition, the serum-free cell culture fluid can promote stable growth of the tumour stem cells and maintain fine cell activity and physiological properties of the tumour stem cells, thus being very suitable for research fields related to tumour cells and tumour stem cells.
Owner:SUN YAT SEN UNIV
Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
The invention relates to a human amnion mesenchymal stem cell serum-free culture medium and a culture method thereof. The culture medium is formed by adding human serum albumin, human transferrin, human insulin and sodium selenite into a DMEM / F12 basic culture medium. The culture method for the culture medium comprises the following steps of: digesting human amnion by using trypsin, then digesting the human amnion by using collagenase IV and deoxyribonuclease I, and filtering the mixture to obtain single cell suspension; and adding the human serum albumin, the transferrin, the insulin and the sodium selenite into the DMEM / F12 basic culture medium in a ratio of VDMEM to VF12 of 1:1, and putting human amnion mesenchymal stem cells in a 37 DEG C CO2 incubator with saturated humidity and volume fraction of 5 percent under the serum-free condition, wherein culture in vitro and amplification are realized by solution change and transfer of culture, potentiality of multi-direction differentiation is maintained, and the amplified cells can be induced in vitro to form cartilage cells, osteoblasts and adipocytes. The culture medium and the culture method have the characteristics of no other animal sources, wide source and no limitation of ethics.
Owner:辽宁艾米奥干细胞与再生医学研究院有限公司
Animal origin-free low-protein culture medium suitable for animal cell product production
ActiveCN101603026AEasy to separate and purifySuitable for productionTissue cultureFermentationLipid formationAntioxidant
The invention relates to an animal origin-free low-protein culture medium suitable for animal cell product production, comprising 24 basic metabolism nutrients, 11 vitamins, 3 transferrin substitute compounds, 5 lipid compounds, 2 nucleic acid compounds, 4 hormones and growth factors, 3 antioxidants, 1 shear-resistant protective agent, 1 pH indicator, 2 pH buffers, 9 other inorganic salts, soy hydrolysates adopted to substitute animal origin component, and composition of ferrous sulfate, ferric nitrate and EDTA-2Na adopted to substitute transferrin. The culture medium can be made by dissolving the aforementioned components in triply distilled water. The positive effects of the culture medium are as follows: the culture medium contains no animal origin component, the total protein content is lower than 10mg / L, which helps separate and purify products and is suitable for production of recombinant protein medicaments; the culture medium supports normal growth and long-term subculturing of animal cells; the culture medium can be used without adaptation, is easily prepared and is suitable for massive production of animal cell products.
Owner:EAST CHINA UNIV OF SCI & TECH
Modified transferrin fusion proteins
InactiveUS20060205037A1Reduced glucose levelIncrease satietyPeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeTransferrin
Modified fusion proteins of a transferrini moiety, a GLP-1 moiety and a linker moiety, with increased productivity, bioactivity and serum half-life are disclosed. Preferred fusion proteins include those modified so that the transferrin moiety exhibits no or reduced glycosylation. The fusion proteins of the invention are useful for the treatment of Type 2 diabetes, Type 1 diabetes, obesity, congestive heart failure, and non-fatty liver disease.
Owner:BIOREXIS PHARMA CORP
Serum-free culture medium for mesenchymal stem cells
ActiveCN102433302AGood growthFast growthSkeletal/connective tissue cellsINSULIN HUMANPancreatic hormone
The invention discloses a serum-free culture medium for mesenchymal stem cells. The serum-free culture medium for the mesenchymal stem cells comprises the following ingredients: fibronectin at the final concentration of 25mu g / ml, basic fibroblast growth factors at the final concentration of 10ng / ml, human epidermal growth factors at the final concentration of 15ng / ml, recombinant human insulin at the final concentration of 1mg / ml, human transferrin at the final concentration of 0.55mg / ml, human blood albumin in a volume ratio of 5 percent, sodium selenite at the final concentration of 0.67mug / ml, L-carnitine at the final concentration of 5mM and resveratrol at the final concentration of 30mu M. When the mesenchymal stem cells are cultured by the serum-free culture medium for the mesenchymal stem cells, animal-derived serum is not contained, so infection risks can be controlled; the L-carnitine and the resveratrol which are added into the serum-free culture medium for the mesenchymalstem cells can effectively improve the growth state of the mesenchymal stem cells; and the growth speed of the mesenchymal stem cells is remarkably improved, and the biological characteristics of themesenchymal stem cells are kept unchanged.
Owner:CHENGDU QINGKE BIOTECH
Methods and Compositions for Isolating, Maintaining and Serially Expanding Human Mesenchymal Stem Cells
InactiveUS20100015710A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSodium bicarbonateLipid formation
Compositions and methods for isolating and expanding human mesenchymal stem / progenitor cells through multiple passages in defined serum-free environments are provided. The culture media compositions includes a basal medium supplemented with a nutrient mixture such as Ham's F12 nutrient mixture, glutamine, buffer solutions such as sodium bicarbonate and hepes, serum albumin, a lipid mixture, insulin, transferrin, putrescine, progesterone, fetuin, hydrocortisone, ascorbic acid or its analogues such as ascorbic acid-2-phosphate, fibroblast growth factor and transforming growth factor β, and are free of serum or other undefined serum substitutes such as platelet lysate. Methods employing these compositions and protein-coated surfaces for the isolation of mesenchymal stem / progenitor cells from human bone marrow and other tissues such as adipose tissue are also provided. Finally, methods are also provided for serially expanding these cells through multiple passages without losing mesenchymal stem cell-specific proliferative, phenotypical and differentiation characteristics.
Owner:UTI LLP
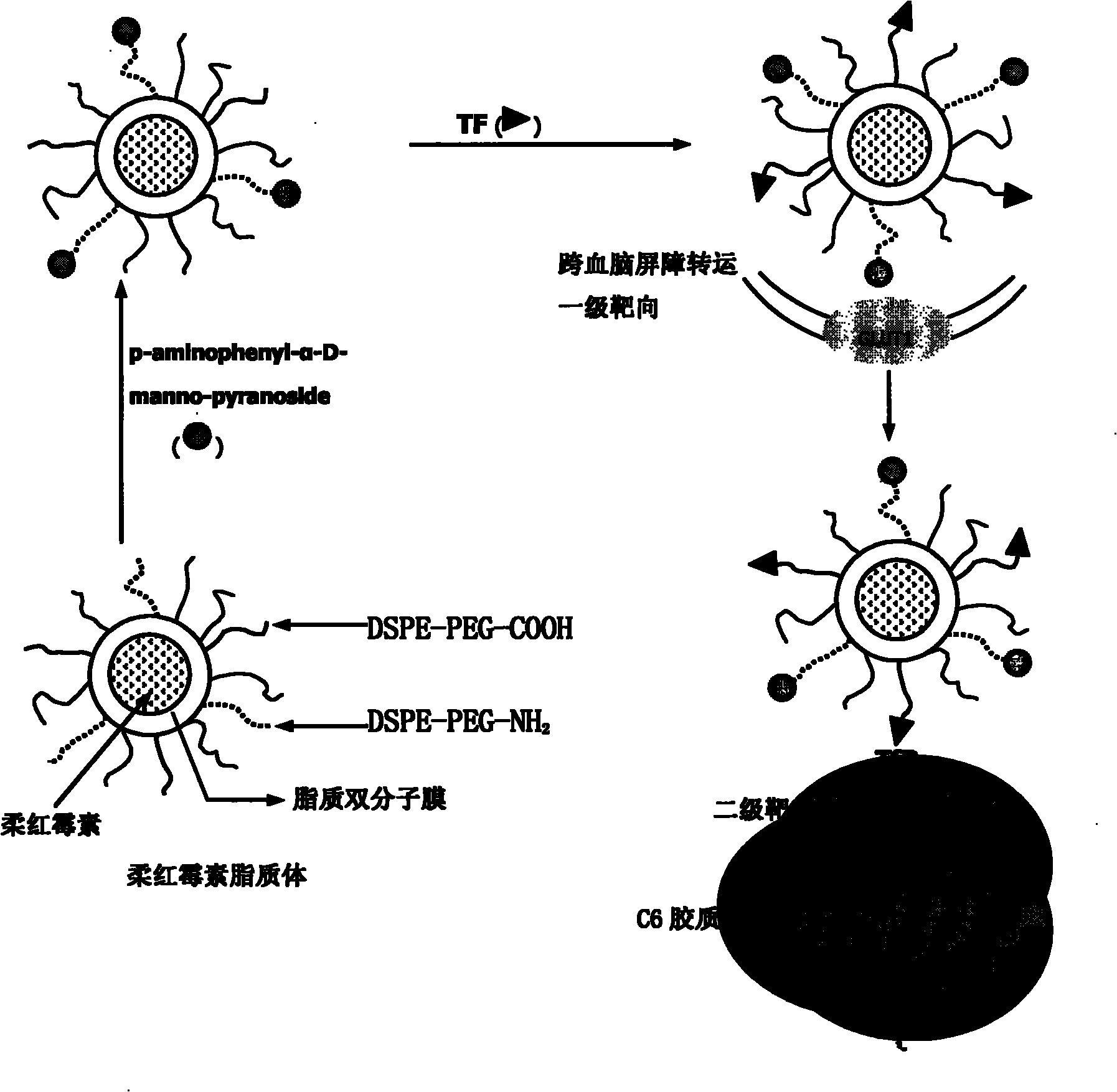
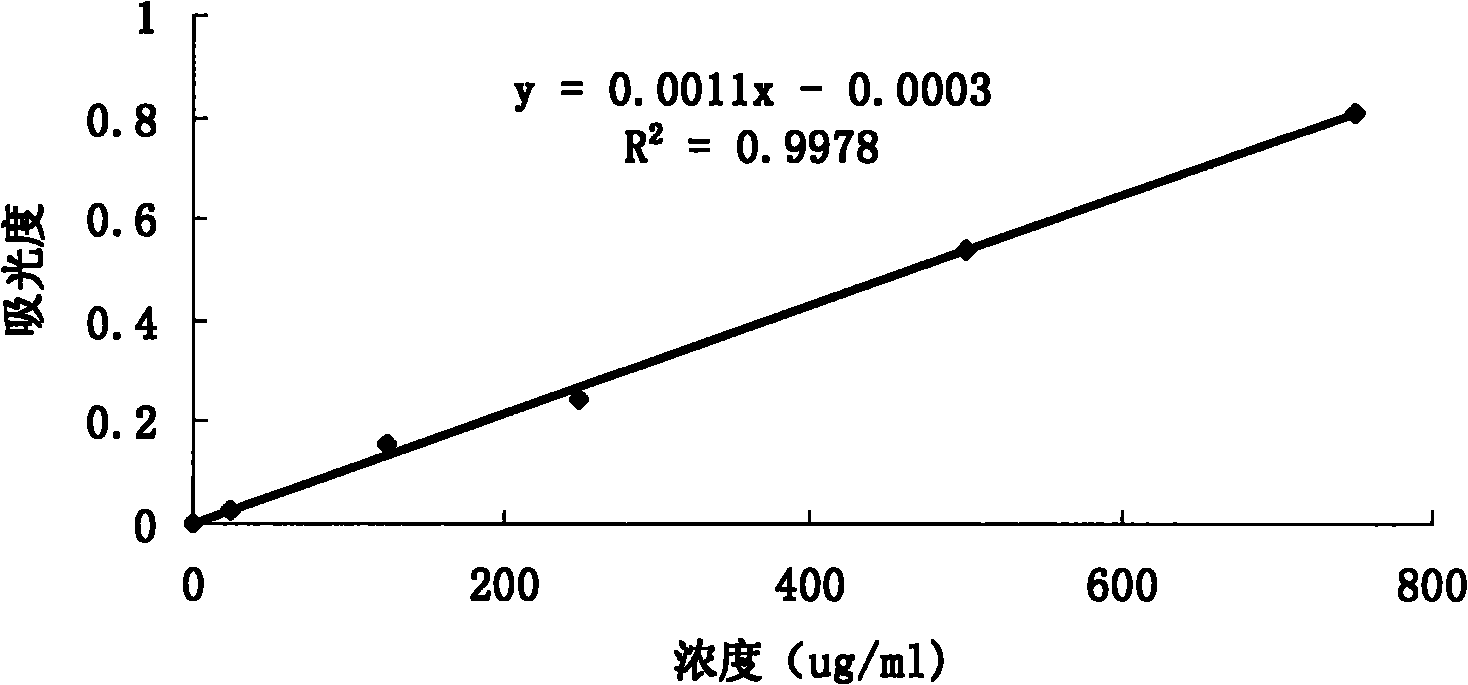
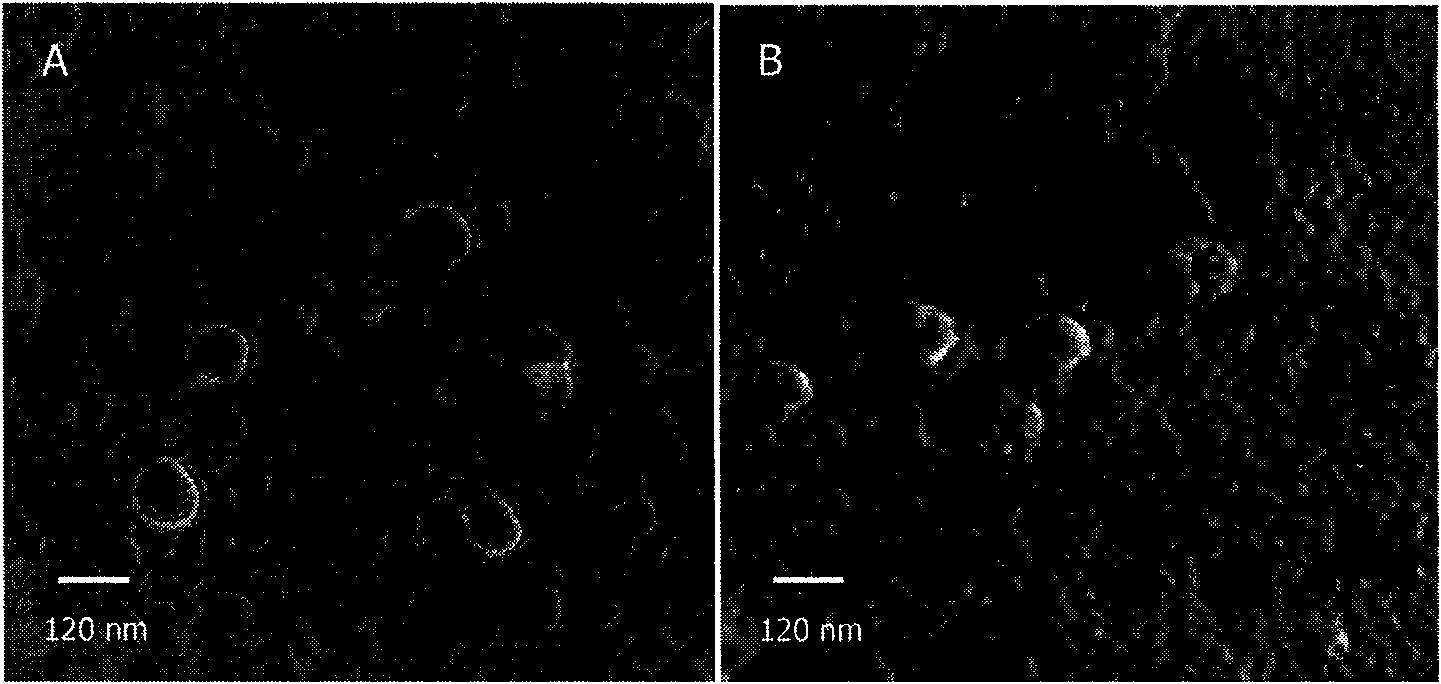
Dual target liposome and preparation method and application thereof
InactiveCN101816629AIncrease drug concentrationResearch to aid in non-invasive treatmentsOrganic active ingredientsMacromolecular non-active ingredientsDaunorubicinNon invasive
The invention discloses a dual target liposome and a preparation method and application thereof. The target liposome provided by the invention consists of liposome and modifiers on the surface of the liposome, wherein the modifiers on the surface of the liposome comprise p-aminophenyl-alpha-D-manno-pyranoside and transferrin. The invention also discloses a medicament-loaded liposome, which is obtained by wrapping daunorubicin by using the target liposome. The obtained target liposome has good capability of crossing the blood brain barrier, targets brain glioma, and can be used as a medicament carrier. The medicament-loaded liposome can target the medicament to a brain glioma site after crossing the blood brain barrier so as to greatly increase the concentration of the medicament at a tumor site and improve the effect of chemotherapy. The target liposome provides a new measure for brain glioma chemotherapy, contributes to the research of non-invasive therapy of the brain glioma, and has important theoretical meaning and clinical meaning.
Owner:PEKING UNIV
Human adipose-derived stem cell serum-free basic medium
ActiveCN102732477BOvercome riskSkeletal/connective tissue cellsMineral ascorbatesAscorbic acid 2-sulfate
The invention discloses a human adipose-derived stem cell serum-free basic medium. The medium uses a serum substitute, and is composed of a high glucose type DMEM basic medium, human serum albumin, transferrin, taurine, reduced glutathione, ceruloplasmin, L-ascorbic acid-2-sulfate, alpha-tocopherol succinate, linoleic acid, alpha-ketoglutarate and selenium. The serum-free basic medium can exempt potential threats caused by animal serum in conventional serum-containing mediums to human health, and the adipose-derived stem cells cultured by the medium is more suitable for clinical application.
Owner:JIANGSU RE STEM BIOTECH
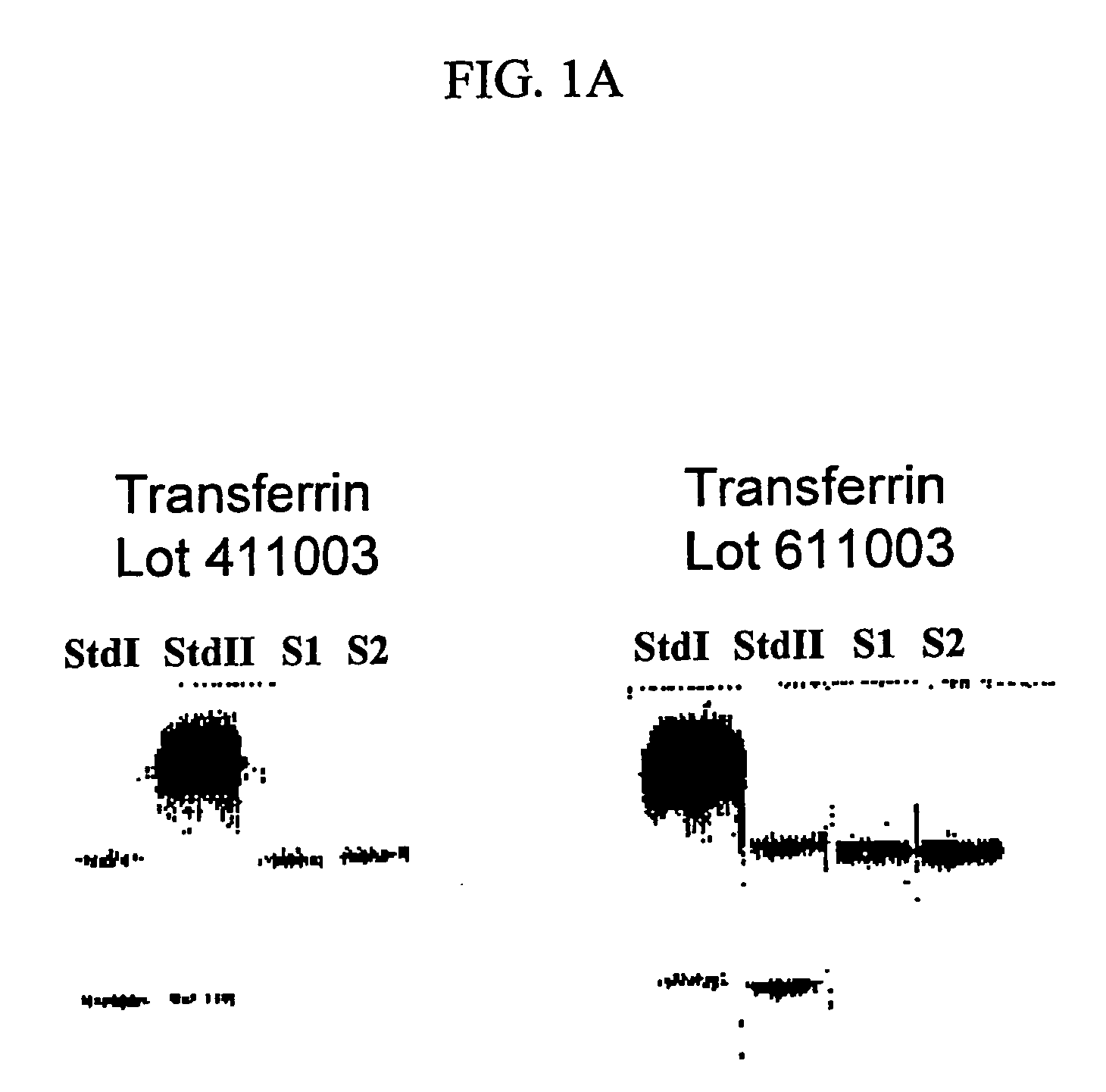
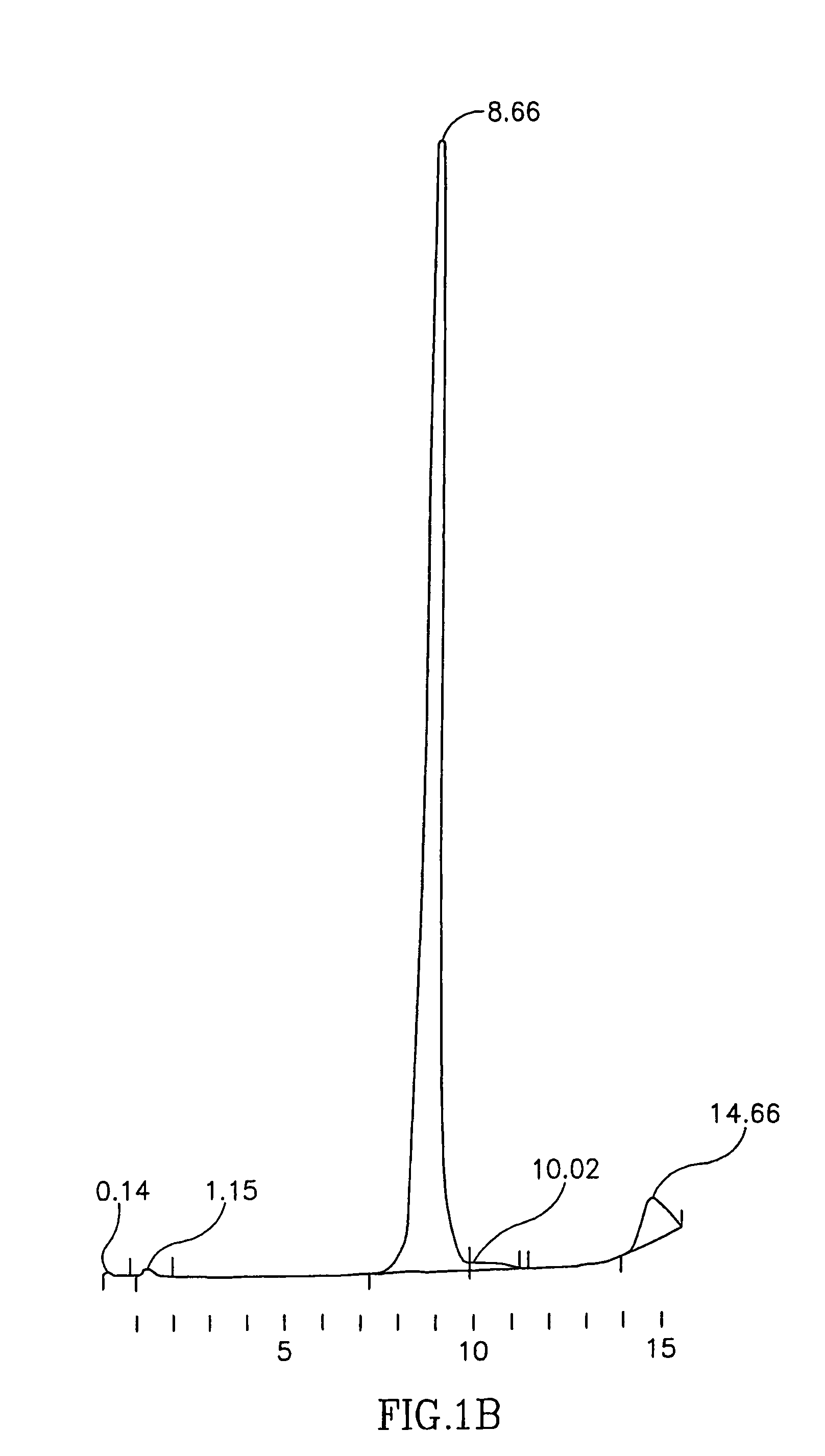
Ultrapure transferrin for pharmaceutical compositions
The present invention relates to an ultrapure human transferrin, and to methods for manufacturing the ultrapure transferrin. The transferrin may be holo-, apo- or at any desired degree of iron saturation. The invention further relates to the use of ultrapure transferrin as the protein moiety of conjugates, and to pharmaceutical compositions comprising ultra transferrin alone as well as in the form of a conjugate.
Owner:KAMADA
Human adipose-derived stem cell serum-free basic medium
ActiveCN102732477AIncrease viscosityFree from mechanical damageSkeletal/connective tissue cellsAscorbic acid 2-sulfateMineral ascorbates
The invention discloses a human adipose-derived stem cell serum-free basic medium. The medium uses a serum substitute, and is composed of a high glucose type DMEM basic medium, human serum albumin, transferrin, taurine, reduced glutathione, ceruloplasmin, L-ascorbic acid-2-sulfate, alpha-tocopherol succinate, linoleic acid, alpha-ketoglutarate and selenium. The serum-free basic medium can exempt potential threats caused by animal serum in conventional serum-containing mediums to human health, and the adipose-derived stem cells cultured by the medium is more suitable for clinical application.
Owner:JIANGSU RE STEM BIOTECH
Fragments of p97 and uses thereof
ActiveUS20140322132A1Reduce cardiotoxicityAntibacterial agentsHydrolasesTransferrinMelanotransferrin
Provided are fragments of human p97 (melanotransferrin) polypeptides having blood-brain barrier (BBB) transport activity, including variants and combinations thereof, conjugates comprising said p97 fragments, and related methods of use thereof, for instance, to facilitate delivery of therapeutic or diagnostic agents across the BBB.
Owner:BIOASIS TECH
SFM (serum-free medium) for culturing MSCs (mesenchymal stem cells)
ActiveCN103555665AClear ingredientsAvoid heterogeneous contaminationSkeletal/connective tissue cellsSodium bicarbonateSerum free media
The invention relates to an SFM (serum-free medium) for culturing MSCs (mesenchymal stem cells). Based on volume, the SFM comprises the following components: 10.2 grams per liter of alpha-MEM (alpha-minimum essential medium), 2.4 grams per liter of sodium bicarbonate, 1 to 5 millimoles of L-glutamine, 50 to 300 milligrams per liter of poloxamer 188, 2 to 8 grams per liter of recombinant human albumin, 10 to 20 milligrams per liter of recombinant human transferrin, 2 to 10 milligrams per liter of recombinant human insulin, 1 to 5 millimoles per liter of Hepes, 50 nanomoles of beta-mercaptoethanol, 0.1 to 1 milligram per liter of lipid, 1 to 5 milligrams per liter of trace element, 0.1 to 5 milligrams per liter of glutathione, 0.5 to 5 milligrams per liter of para-aminobenzoic acid, 1 to 50 nanograms per milliliter of hydrocortisone, 20 to 50 milligrams per liter of vitamin PP, 5 to 50 milligrams per liter of vitamin C, 2 to 10mu M of compound shown in a formula I, 5 to 20mu M of compound shown in a formula II, 10 to 20 nanograms per milliliter of progestin, 1 to 10 milligrams per liter of putrescine, 1 to 10 international units per liter of heparin, 1 to 10 nanograms per milliliter of EGF (epidermal growth factor), 1 to 10 nanograms per milliliter of b-FGF (b-fibroblast growth factor), 1 to 10 nanograms per milliliter of HGF (hepatocyte growth factor) and 1 to 10 nanograms per milliliter of VEGF (vascular endothelial growth factor). The SFM for culturing the MSCs is a BPS-SFM which has determinate chemical components and is free of animal-derived substances.
Owner:BEIJING DONGFANG HUAHUI BIOMEDICAL TECH
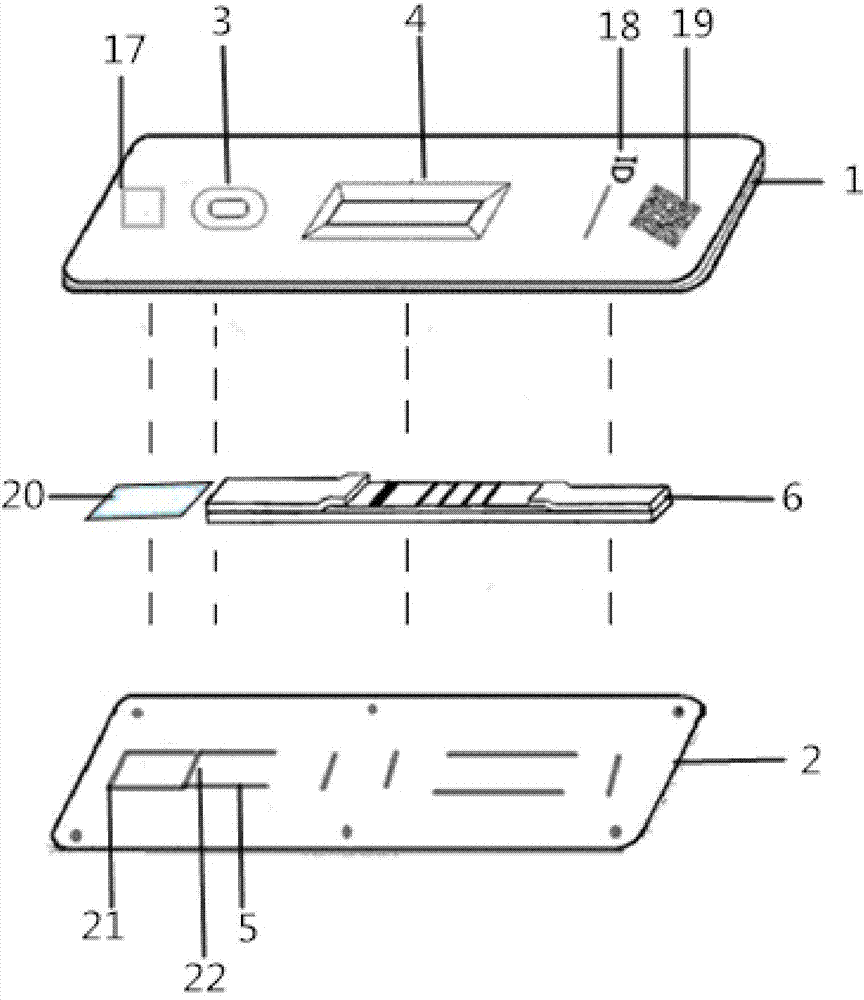
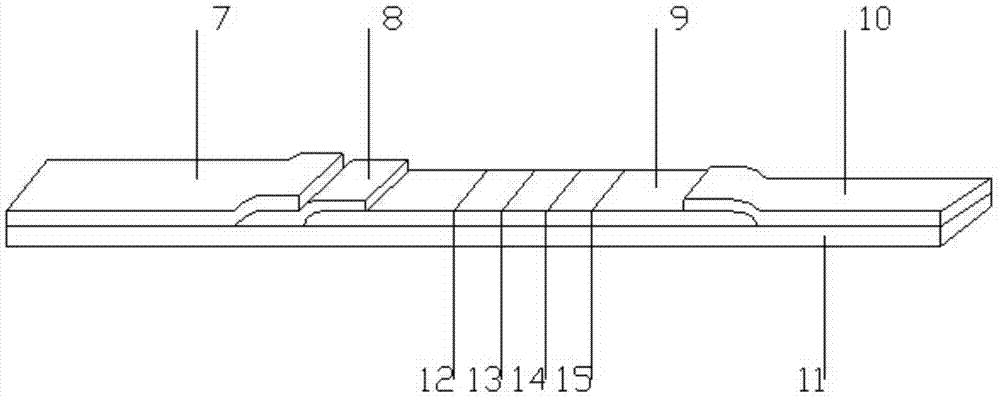
Hemoglobin, hemoglobin-haptoglobin composite and transferrin joint examination kit and preparation method and detection method thereof
The invention provides a hemoglobin, hemoglobin-haptoglobin composite and transferrin joint examination kit. The hemoglobin, hemoglobin-haptoglobin composite and transferrin joint examination kit comprises an upper shell, a lower shell, an immunostrip, test paper and the like; a gold-labeled composite containing a hemoglobin monoclonal antibody, a hemoglobin-haptoglobin composite monoclonal antibody and a transferrin monoclonal antibody is scribed on a nitrocellulose membrane; 3 test lines, a quality control line (15) and a gold-labeled composite membrane scribing line (8) are arranged on the nitrocellulose membrane in parallel. The invention further provides a preparation method and a detection method of the hemoglobin, hemoglobin-haptoglobin composite and transferrin joint examination kit. The hemoglobin, hemoglobin-haptoglobin composite and transferrin joint examination kit is convenient and simple in operation, stable in performance and accurate in result, has a relatively good reference value for early diagnosis and identification of colorectal cancer or colon cancer tumor or other tumors with lower gastrointestinal bleeding symptoms in clinic, significantly improves the positive detection rate of digestive hemorrhagic diseases, is suitable for clinical hospital examination and household self-examination, and provides measures for large-scale general examination of such diseases.
Owner:HANGZHOU HUIYUANTAI MEDICAL DEVICES
Devices and Methods for Detection of Occult Blood
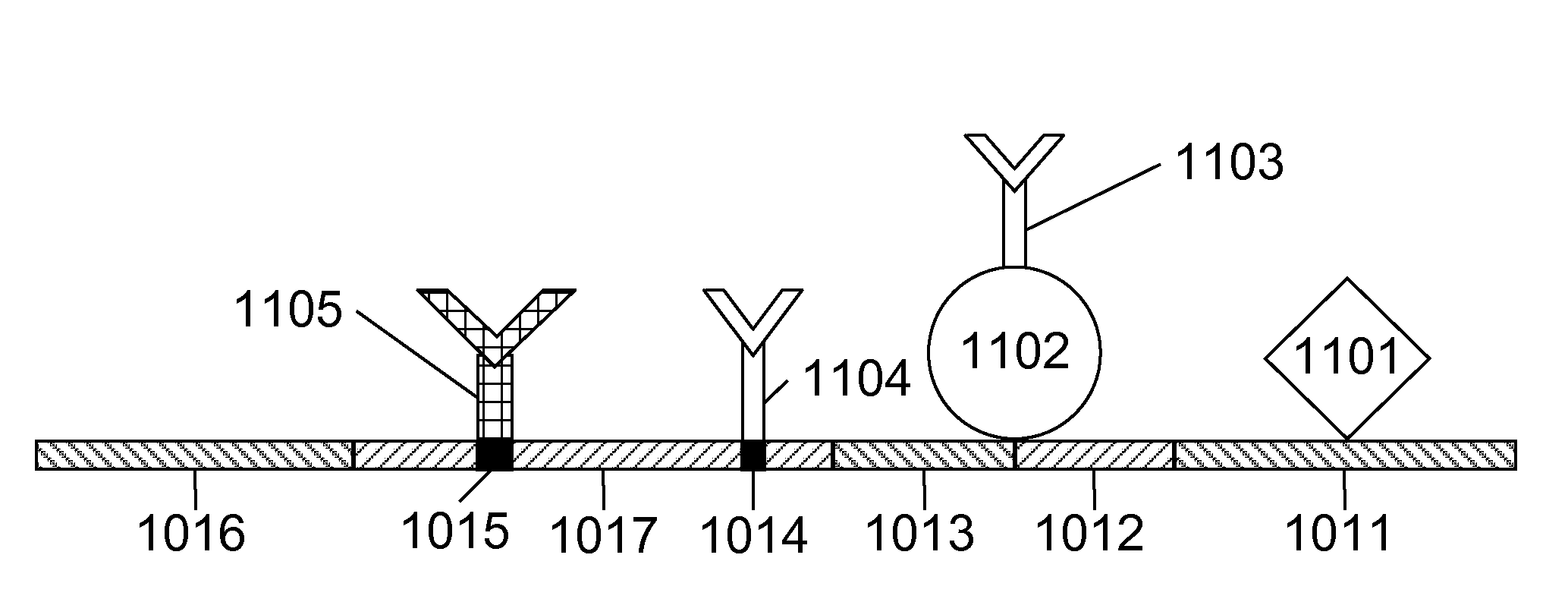
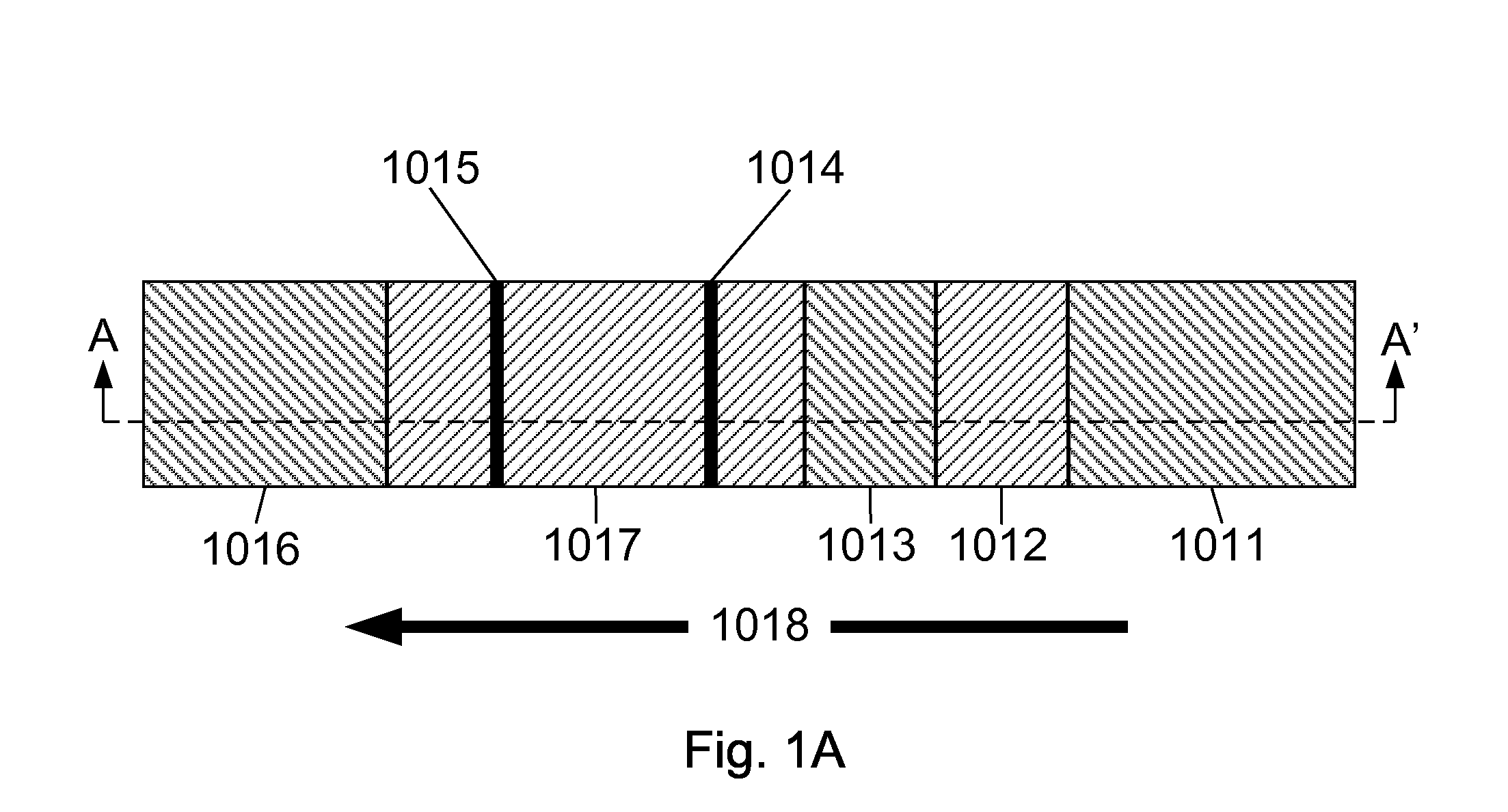
InactiveUS20080227208A1Simple methodHigh-precision detectionBiological testingParticle suspension analysisHemoglobin-haptoglobin complexTest sample
The present invention relates to devices and methods for the detection of occult blood in a test sample. These devices and methods can detect the presence of one or more of the following components of occult blood: hemoglobin, transferrin, hemoglobin-haptoglobin complex, and albumin. Methods for the detection of occult blood in a test sample include the following steps: exposing said test sample to two or more of the following antibodies: anti-hemoglobin antibodies, anti-hemoglobin-haptoglobin-complex antibodies, anti-transferrin antibodies, and anti-albumin antibodies or one or more of the following antibodies: anti-hemoglobin-haptoglobin-complex antibodies, anti-transferrin antibodies, and anti-albumin antibodies; determining the level of reactions between the antibodies and their corresponding components of occult blood that may be in the test sample; and deciding on the presence of occult blood. The devices of this invention can have two or more of test areas containing two or more of the following antibodies: anti-hemoglobin antibodies, anti-transferrin antibodies, anti-hemoglobin-haptoglobin complex antibodies, and anti-albumin antibodies or one or more of test areas containing one or more of the following antibodies: anti-transferrin antibodies, anti-hemoglobin-haptoglobin complex antibodies, and, anti-albumin antibodies. The devices and methods of this invention are simple and produce rapid responses.
Owner:YEE HSIAO CHING +2
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formula NH2CH2CH2CHR1C(O)N—R (I) where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
N2S2 chelate-targeting ligand conjugates
ActiveUS20050129619A1Sufficient amountHybrid immunoglobulinsRadioactive preparation carriersAngiostatinAbnormal tissue growth
The invention provides, in a general sense, a new labeling strategy employing compounds that are are N2S2 chelates conjugated to a targeting ligand, wherein the targeting ligand is a disease cell cycle targeting compound, a tumor angiogenesis targeting ligand, a tumor apoptosis targeting ligand, a disease receptor targeting ligand, amifostine, angiostatin, monoclonal antibody C225, monoclonal antibody CD31, monoclonal antibody CD40, capecitabine, a COX-2 inhibitor, deoxycytidine, fullerene, herceptin, human serum albumin, lactose, leuteinizing hormone, pyridoxal, quinazoline, thalidomide, transferrin, or trimethyl lysine. The present invention also pertains to kits employing the compounds of interest, and methods of assessing the pharmacology of an agent of interest using the present compounds.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com