Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58 results about "Vasopressin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
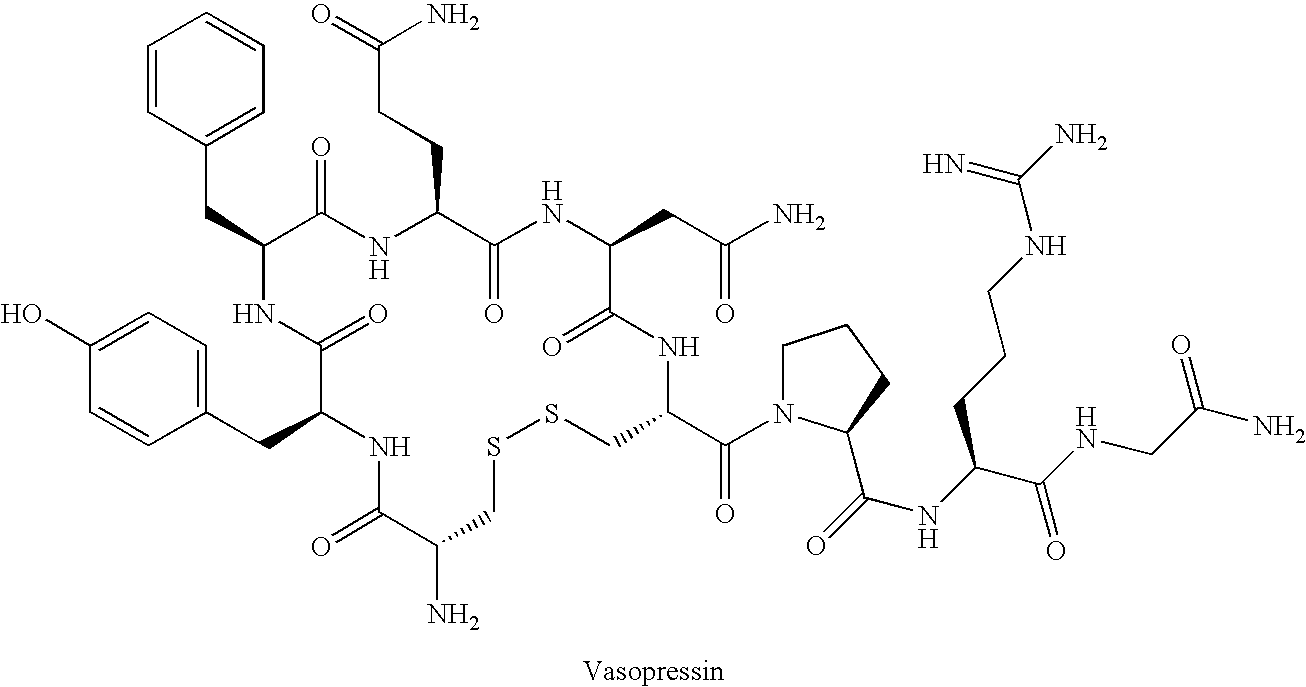
Vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon of that cell, which terminates in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure.
Nonpeptide agonists and antagonists of vasopressin receptors
The disclosed invention is a composition agonists and / or antagonists of V.sub.2, V.sub.1a or both receptors, in a host, including animals, and especially humans, using a small molecule or its pharmaceutically acceptable salt or prodrug.
Owner:EMORY UNIVERSITY
Fused azepine derivatives and their use as antidiuretic agents
Compounds according to general formulae (1 and 2), wherein G<1 >is an azepine derivative and G<2 >is a group according to general formulae (9-11) are new. Compounds according to the invention are vasopressin V2 receptor agonists. Pharmaceutical compositions of the compounds are useful as antidiuretic agents.
Owner:VANTIA
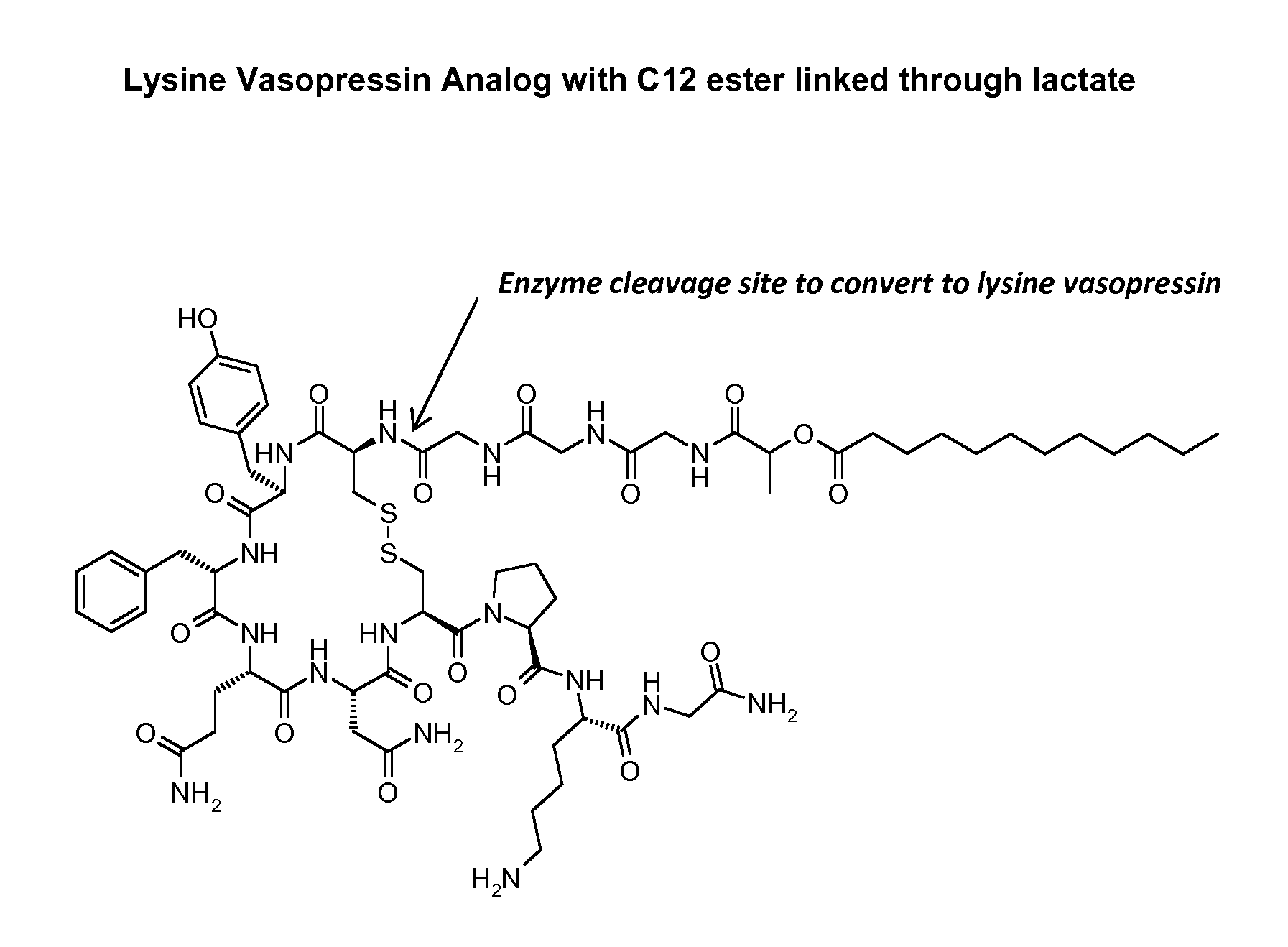
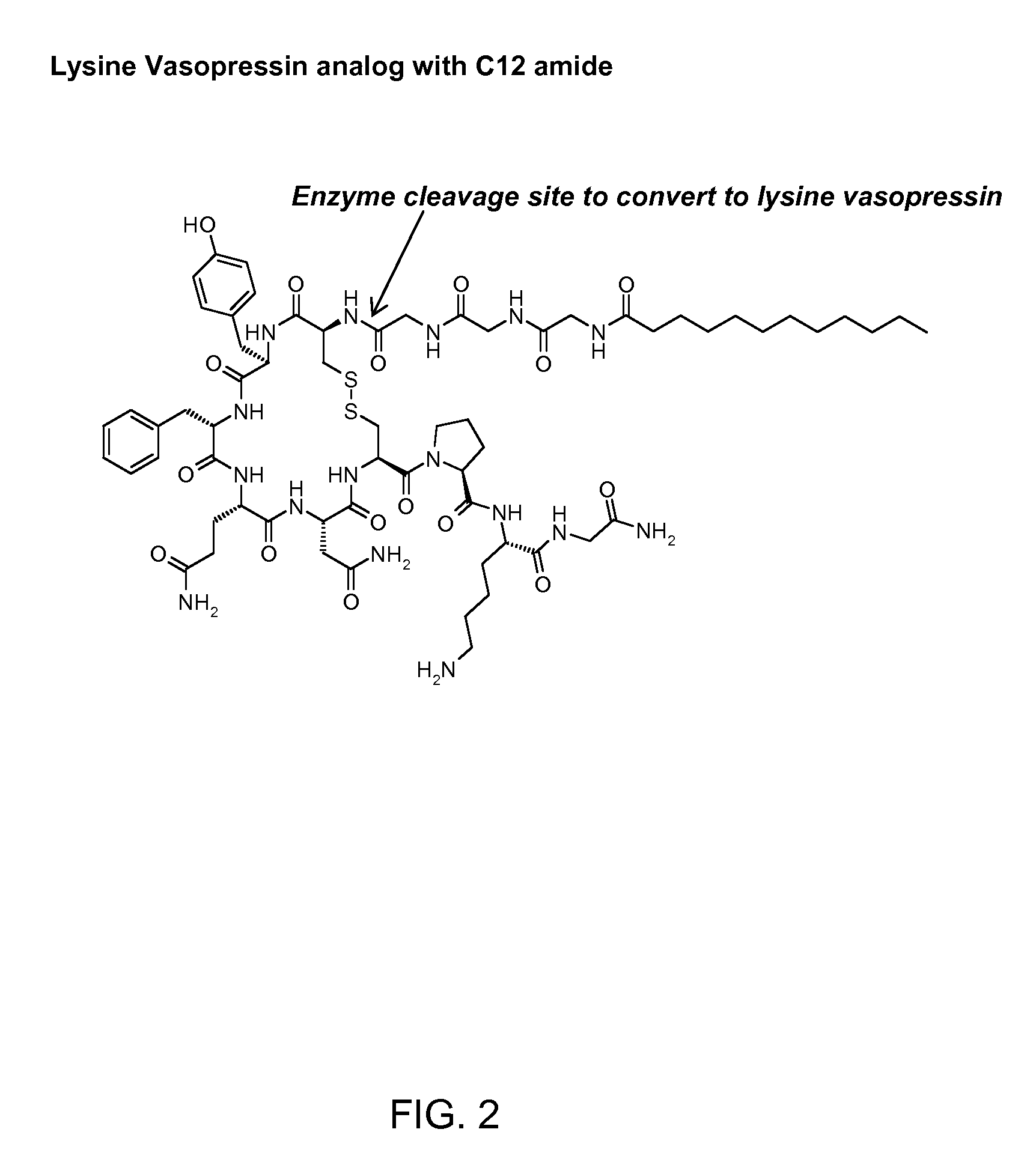
Composition for long-acting peptide analogs
ActiveUS7960336B2Increase perfusionImprove the level ofAntibacterial agentsPeptide/protein ingredientsHalf-lifeArginine
Owner:PHARMAIN CORP
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
InactiveUS7151084B2Nervous disorderPeptide/protein ingredientsMembrane TransportersPancreatic hormone
A complex is provided for the treatment of neurogenic conditions having the formula:where R1 isM is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium(II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium(II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium(II):transporter ligand.
Owner:MILLER LANDON C G
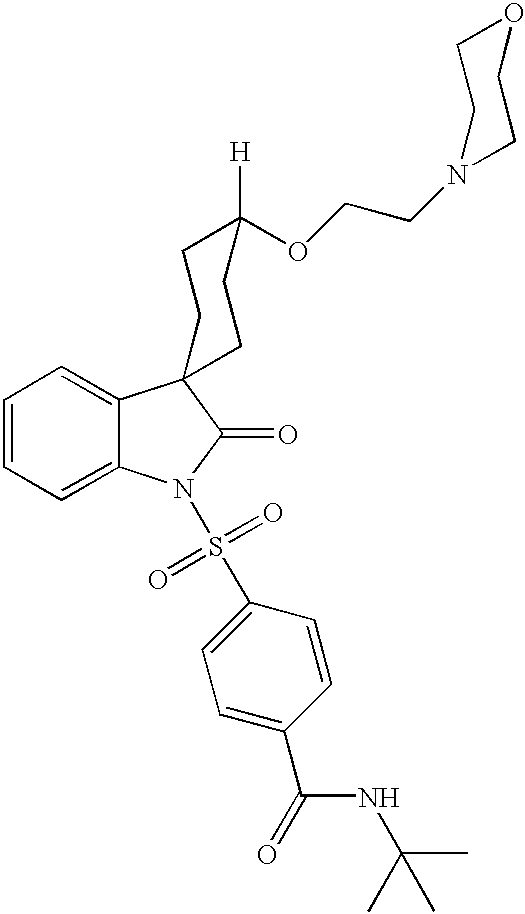
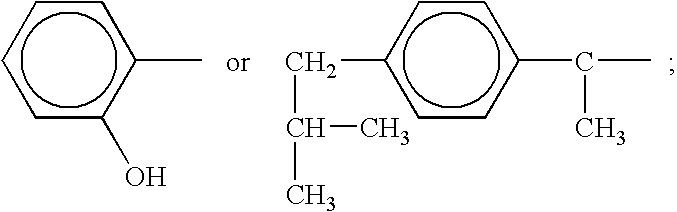
Heteroaryl-cyclohexyl-tetraazabenzo[e]azulenes
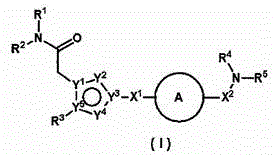
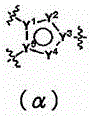
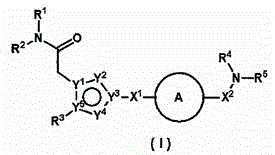
ActiveUS20110275801A1BiocideNervous disorderObsessive-compulsive disordersAutistic spectrum disorder
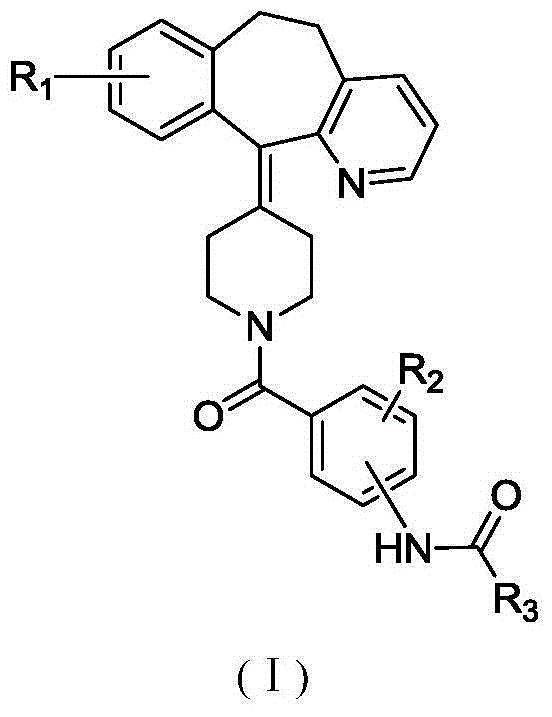
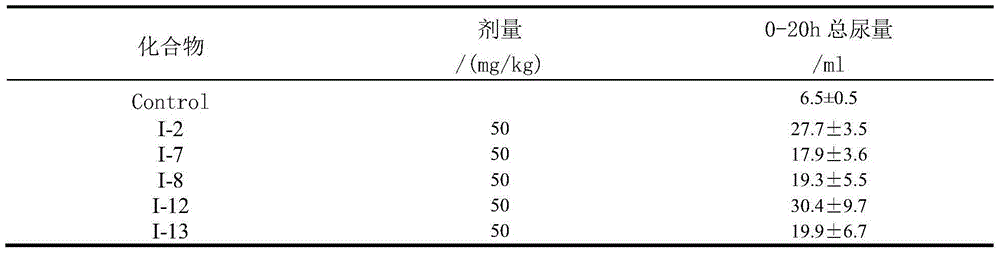
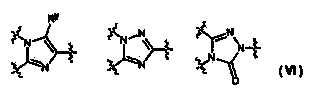
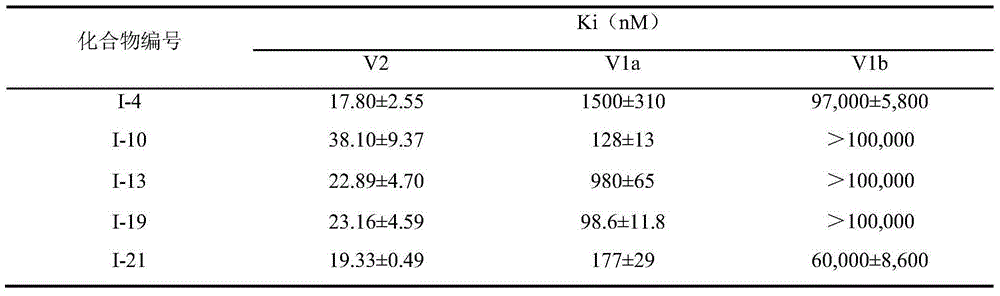
The present invention provides heteroaryl-cyclohexyl-tetraazabenzo[e]azulenes of formula Iwherein R1, R2 and R3 are as described herein.The compounds according to the invention act as V1a receptor modulators, and in particular as V1a receptor antagonists, their manufacture, pharmaceutical compositions containing them and their use as medicaments. The active compounds of the present invention are useful as therapeutics acting peripherally and centrally in the conditions of dysmenorrhea, male or female sexual dysfunction, hypertension, chronic heart failure, inappropriate secretion of vasopressin, liver cirrhosis, nephrotic syndrome, anxiety, depressive disorders, obsessive compulsive disorder, autistic spectrum disorders, schizophrenia, and aggressive behavior.
Owner:F HOFFMANN LA ROCHE & CO AG
Method for preparing vasopressin tannate
InactiveCN104672308AAvoid the problem of excessive volume and diluted concentrationSuitable for continuous productionOxytocins/vasopressinsPeptide preparation methodsDivinylbenzeneDesalination
The invention discloses a method for preparing vasopressin tannate. The preparation method of the vasopressin comprises the following step: sequentially carrying out reverse-phase purification and reverse-phase desalination on a vasopressin crude product solution by a high performance liquid chromatography, so as to obtain the vasopressin tannate, wherein packing of the high performance liquid chromatography, is a polystyrene-divinylbenzene (PS-DVB) copolymer. By combination of reverse-phase purification and reverse-phase desalination, the latest application of the polymer packing polystyrene-divinylbenzene is designed; and the vasopressin tannate can be prepared on a large scale.
Owner:QINGDAO KANGYUAN PHARMA
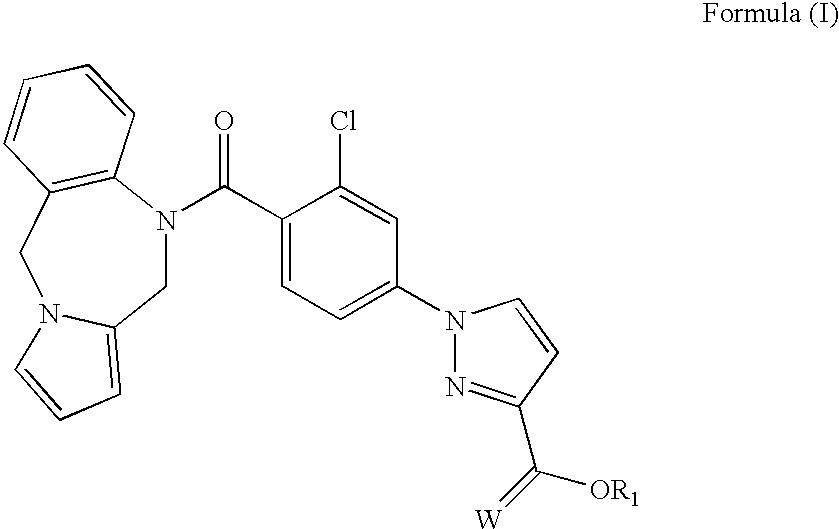
Biologically active vasopressin agonist metabolites
Novel compounds of Formula (I)and pharmaceutically acceptable salts thereof, and pharmaceutical formulations containing such compounds which are useful as anti-aquaretic agents in the treatment of nocturnal urinary enuresis, nocturnal urinary urgency, and / or similar conditions.
Owner:WYETH LLC
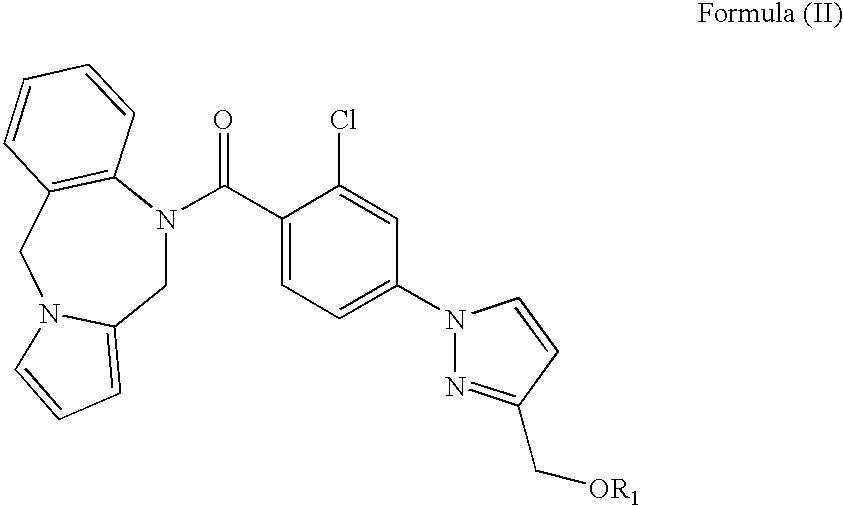
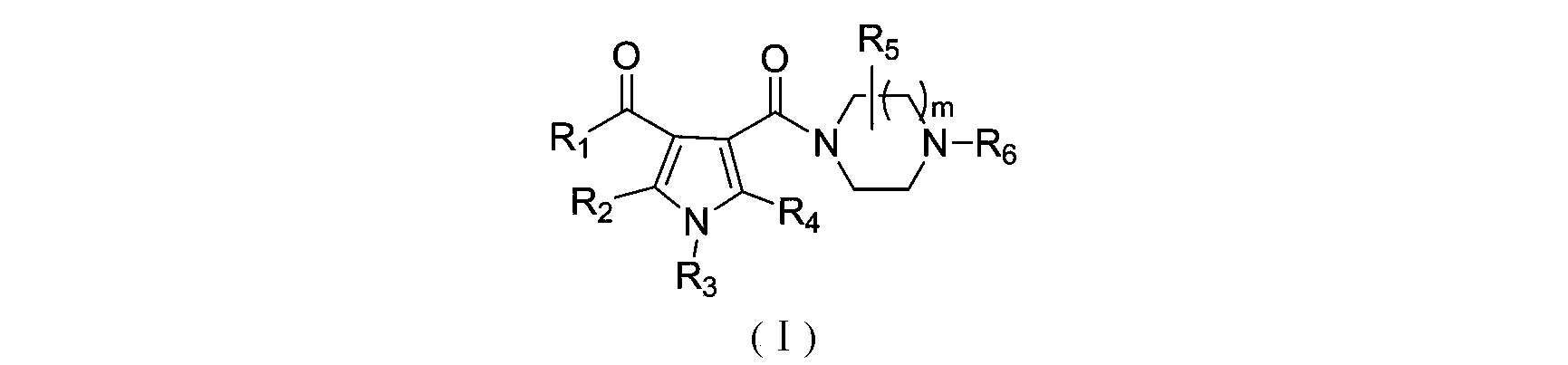
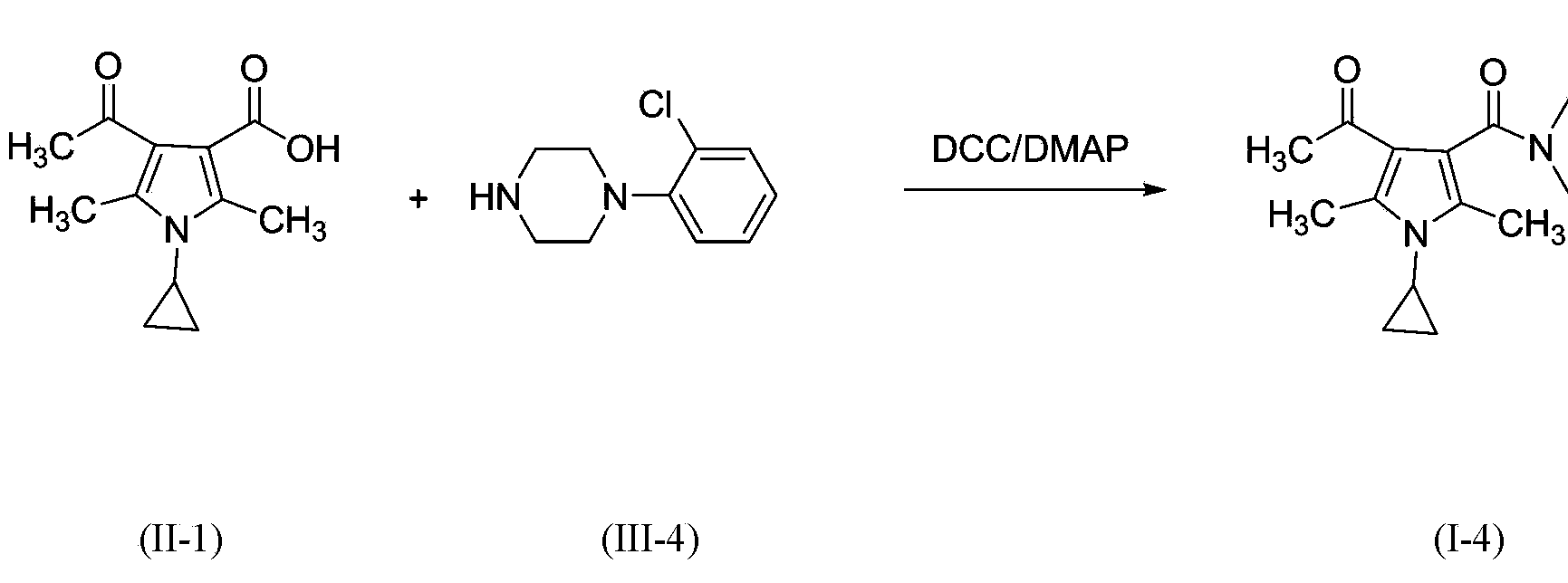
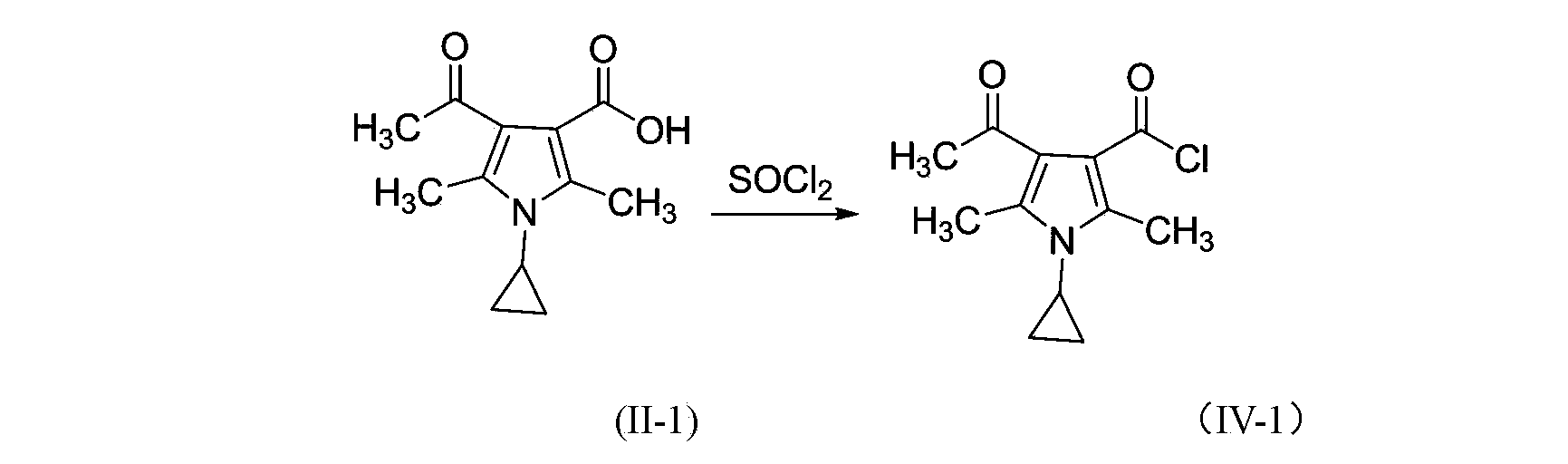
3-pyrrole carboxylic acid derivatives, and preparing method and application thereof
InactiveCN103420890AHas receptor antagonistic effectReceptor antagonismOrganic active ingredientsNervous disorderArginineAcid derivative
The invention relates to 3-pyrrole carboxylic acid derivatives, and a preparing method and an application thereof, and particularly relates to the 3-pyrrole carboxylic acid derivatives having a structure represented by formula I and pharmaceutically acceptable salts thereof and the preparing method of the derivatives, pharmaceutical compositions with the 3-pyrrole carboxylic acid derivatives having the structure represented by the formula I and the pharmaceutically acceptable salts of the 3-pyrrole carboxylic acid derivatives as active ingredients, and applications of the pharmaceutical compositions in prevention or treatment of diseases related with an arginine vasopressin V1a receptor, an arginine vasopressin V1b receptor, an arginine vasopressin V2 receptor, a sympathetic nervous system or a renin-angiotensin-aldosterone system.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Azole derivative
The present invention provides agents for treating or preventing diseases such as mood disorder, anxiety disorder, schizophrenia, Alzheimer's disease, Parkinson's disease, Huntington's chorea, eating disorder, hypertension, gastrointestinal disease, drug addiction, epilepsy, cerebral infarction, cerebral ischemia, cerebral edema, head injury, inflammation, immune-related disease, alopecia, and so forth. Specifically, the invention provides azole derivatives represented by general formula (I), or pharmaceutically acceptable salts thereof that have an antagonistic action against the arginine-vasopressin (AVP) V1b receptor:
Owner:TAISHO PHARMACEUTICAL CO LTD
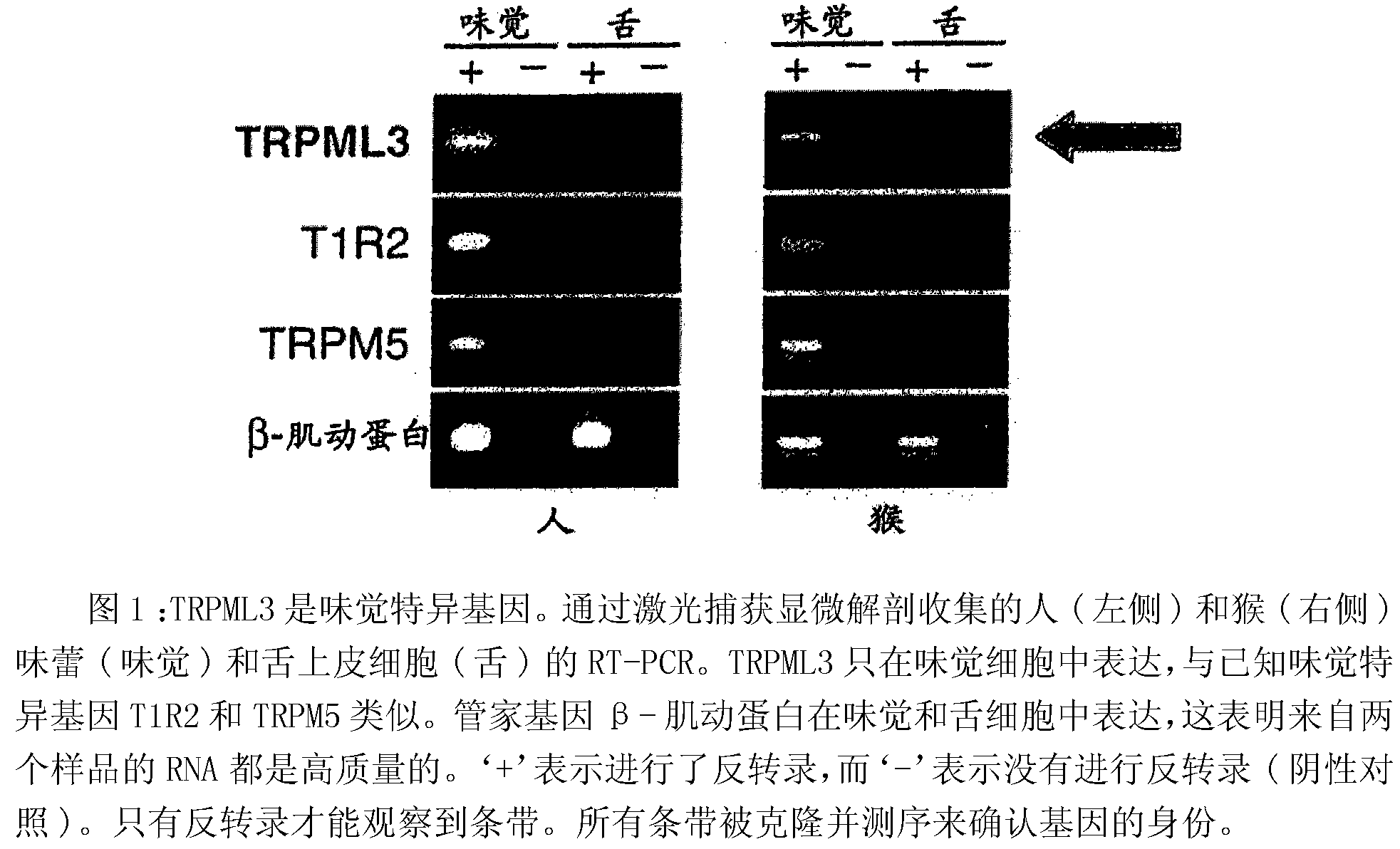
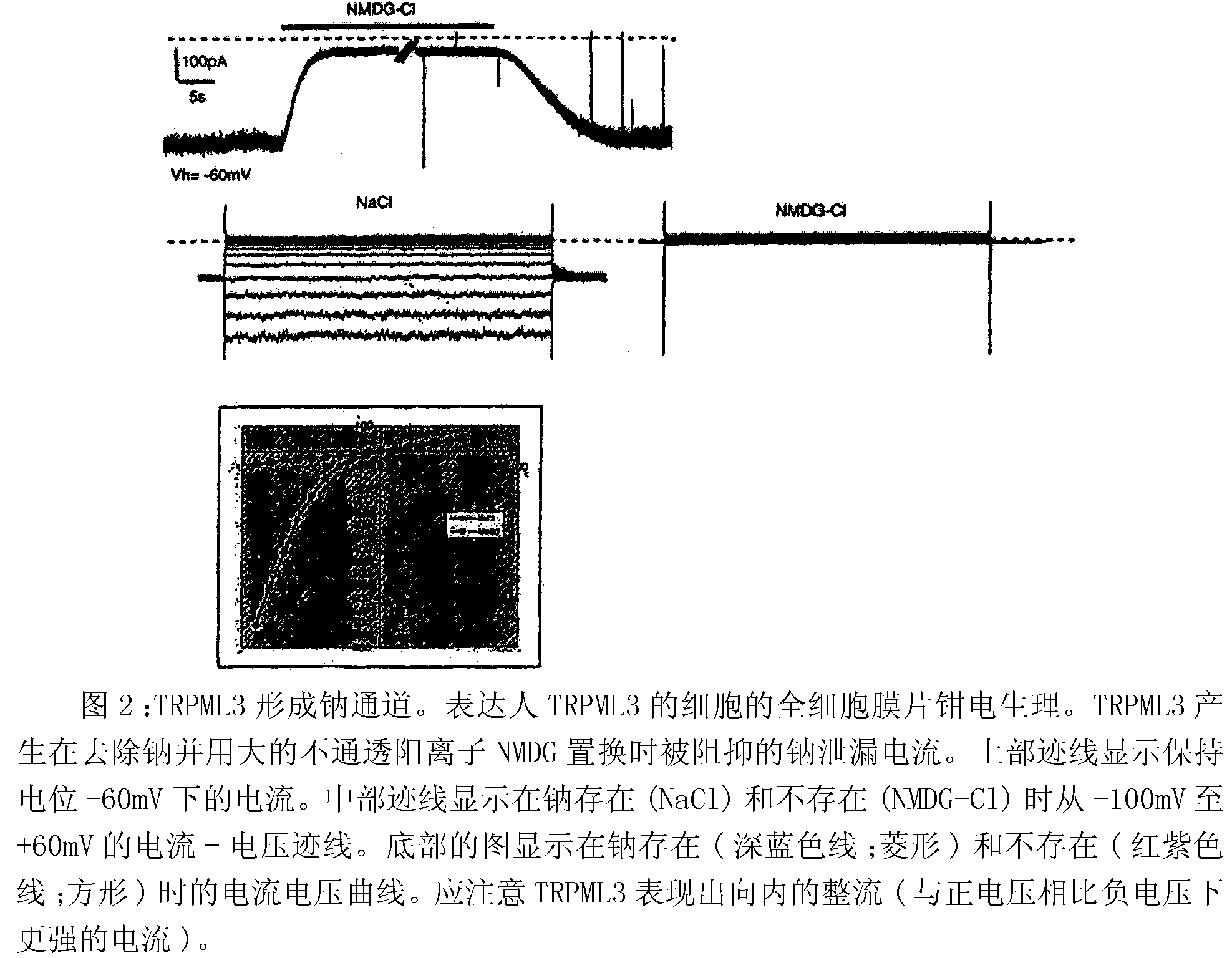
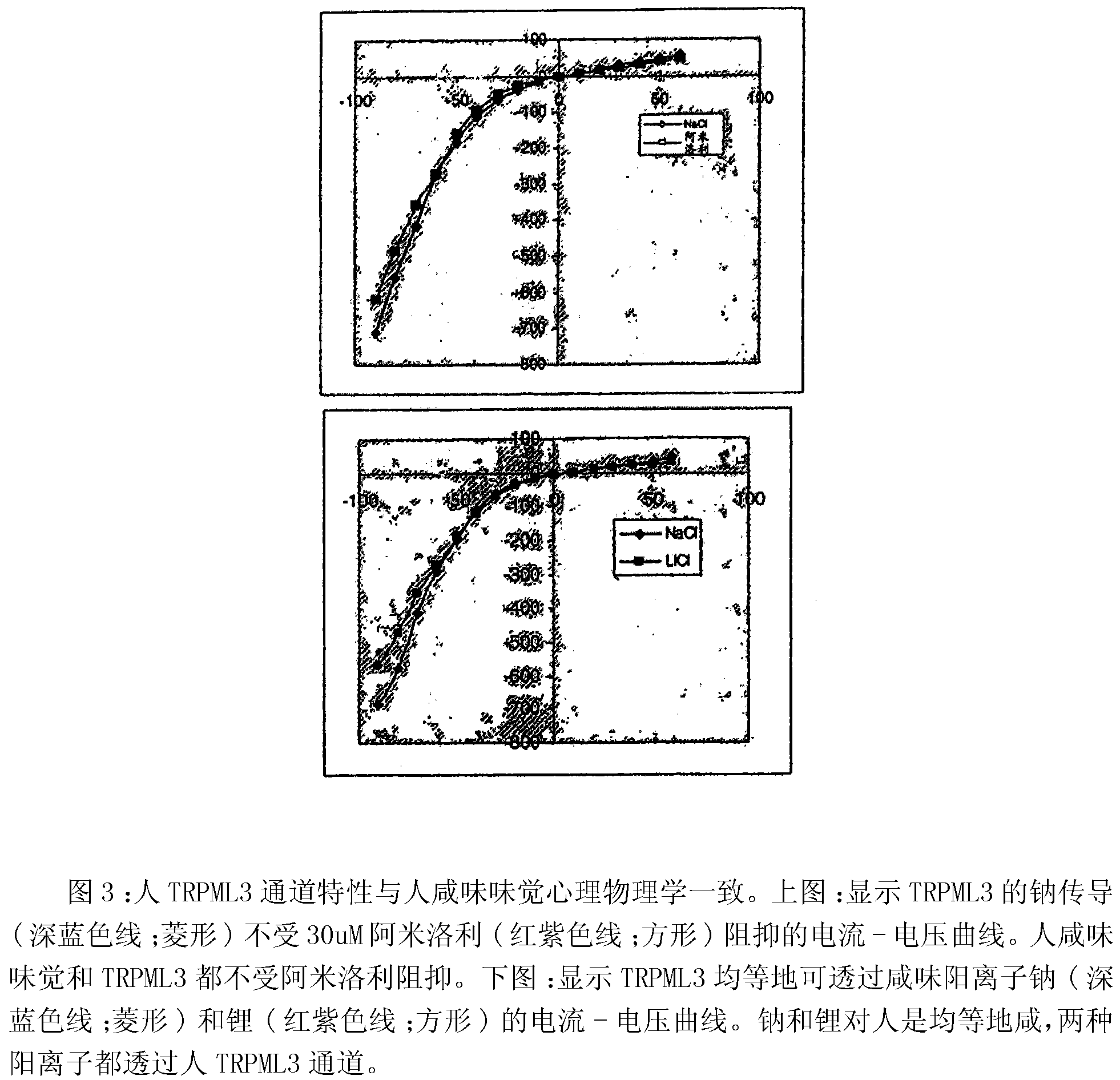
Identification of TRPML3 (MCOLN3) as a salty taste receptor and use in assays for identifying taste (salty) modulators and/or therapeutics that modulate sodium transport, absorption or excretion and/or aldosterone and/or vasopressin production or release
The present invention relates to high-throughput mammalian and medium-throughput oocyte-based electrophysiological assays for identifying human TRPML3 modulators, preferably TRPML3 enhancers. Compounds that modulate TRPML3 function in the assay are expected to affect salty taste in humans. The inventive electrophysiological assays, such as the two-electrode voltage-clamp technique, facilitate the identification of compounds which specifically modulate human TRPML3. The assays of the invention provide a robust screen useful to detect compounds that facilitate (enhance) or inhibit TRPML3 function. Compounds that enhance or block TRPML3 channel activity should thereby modulate salty taste. In addition, these compounds may be used to regulate sodium excretion, urinary output and other biological functions relating to sodium levels. This invention relates to the elucidation that TRPML3 is involved in salty taste perception in primates including humans and likely other mammals (given the significance of sodium and other ions to physiological functions and conditions this phenotype is likely strongly conserved in different animals). The TRPML3 gene also modulates one or more of sodium metabolism, sodium excretion, blood pressure, fluid retention, cardiac function and urinary functions such as urine production and excretion. The inventors have identified TRPML3 as encoding a salty taste receptor in primates and humans (and likely other mammals) based on gene expression assays which have determined that TRPML3 is expressed specifically in taste bud cells and not in lingual epithelial cells, similar assays that have determined that TRPML3 is specifically expressed or enriched in the top half of taste bud cells in a subset of taste cells which do not express TRPM5 or PKD2L1, prior reports that document the expression of TRPML3 in other sensory organs such as the ear (therefore further substantiating the role of TRPML3 as a 'professional' sensory gene).
Owner:SENOMYX INC
Modulators of vasopressin receptors with therapeutic potential
Owner:THE SCRIPPS RES INST
1,2,4-Triazolone Derivative
The present invention provides a 1,2,4-triazolone derivative represented by Formula (1A) having an antagonistic activity on the arginine-vasopressin 1b receptor or a pharmaceutically acceptable salt thereof and provides a pharmaceutical composition comprising the compound or the salt as an active ingredient, in particular, a therapeutic or preventive agent exhibiting favorable pharmacokinetics in a disease such as mood disorder, anxiety disorder, schizophrenia, Alzheimer's disease, Parkinson's disease, Huntington's chorea, eating disorder, hypertension, gastrointestinal disease, drug addiction, epilepsy, cerebral infarction, cerebral ischemia, cerebral edema, head injury, inflammation, immune-related disease, or alopecia.
Owner:TAISHO PHARMACEUTICAL CO LTD
Bicycle vasporession agonists
The compounds of general formula (I) and their pharmaceutically acceptable salts are novel, wherein V is a covalent bond or NH, X is selected from CH2, O and N-alkyl, Z is S or -CH=CH-, R1 and R2 are independently selected from H, F, Cl, Br and alkyl, R3 is selected from OH, O-alkyl and NR4R5, R4 and R5 are each independently H or alkyl, or together they are -(CH2)q -, p is 0, 1, 2, 3 or 4, and q is 4 or 5. They are agonists of vasopressin V2 receptors and can be used as diuretics and procoagulants.
Owner:FERRING BV
Stable pharmaceutical composition of vasopressin
ActiveUS11318183B2Maintain stabilityIncrease pressurePeptide/protein ingredientsPharmaceutical delivery mechanismVascular dilationPharmaceutical medicine
Owner:MANKIND PHARMA LTD
Fused azole derivative
Provided is a therapeutic or prophylactic agent for mood disorders, anxiety, schizophrenia, Alzheimer's disease, Parkinson's disease, Huntington's chorea, eating disorders, hypertension, digestive diseases, drug dependence, epilepsy, cerebral infarction, cerebral ischemia, cerebral edema, head injury, inflammation, immune related diseases, depilation, and so on. More specifically, provided is a fused azole derivative represented by general formula (I), which has arginine vasopressin 1b receptor antagonism, or a pharmaceutically acceptable salt thereof.
Owner:TAISHO PHARMACEUTICAL CO LTD
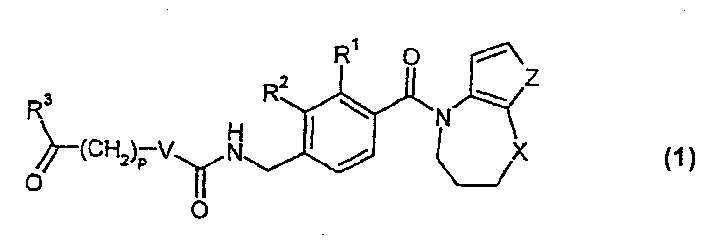
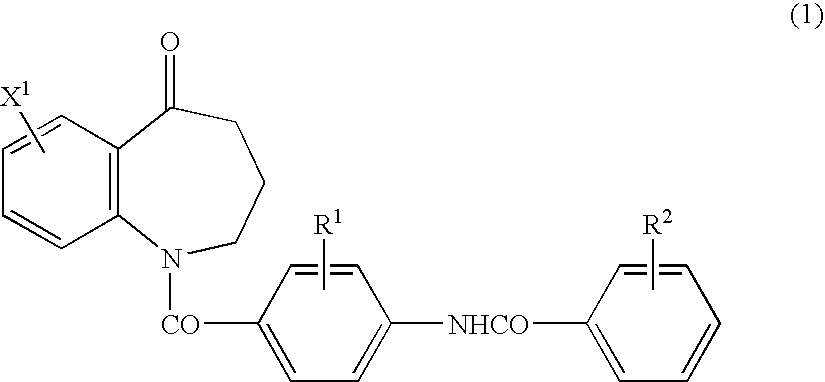
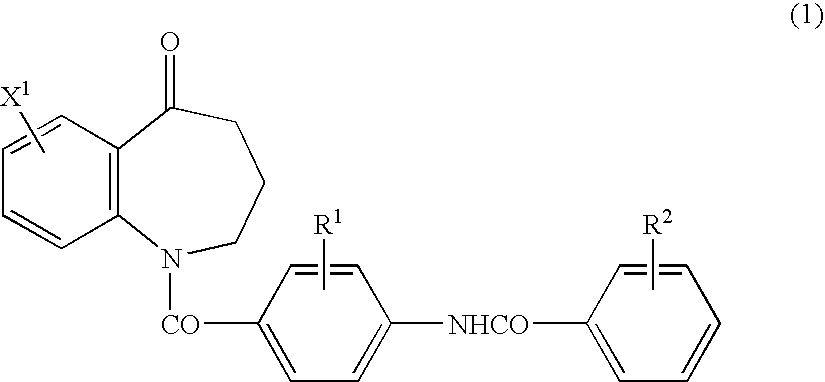
Novel benzoazepine compound, composition and applications of novel benzoazepine compound and composition
The invention relates to a novel benzoazepine compound, which comprises a pharmaceutically acceptable salt thereof. The invention also provides a pharmaceutical composition containing the compound and the pharmaceutically acceptable salt thereof. The present invention also relates to the use of the compounds and said compositions in the prevention or treatment of diseases associated with the arginine vasopressin V1a receptor, the arginine vasopressin V1b receptor, the arginine vasopressin V2 receptor, the sympathetic nervous system or the renin-angiotensin-aldosterone system.
Owner:XUZHOU MEDICAL UNIV +1
A kind of extraction process of vasopressin solution
ActiveCN106632615BRaw materials are cheap and easy to getEasy to operateOxytocins/vasopressinsPeptide preparation methodsBiotechnologyFluid phase
The invention discloses extraction process of a vasopressin solution. The extraction process includes the steps of raw material extraction, protein removal, resin exchange, ultrafiltration, and purification through preparative high performance liquid chromatography. The process combines various separation and purification methods to directly separate and purify the vasopressin from pituitary of animals. The raw material is low in cost and is easy to obtain. The product is high in yield and purity. The process has simple operations, is environment-friendly, is short in production period and is low in production cost, and is suitable for large-scale industrial production.
Owner:NANJING XINBAI PHARMA
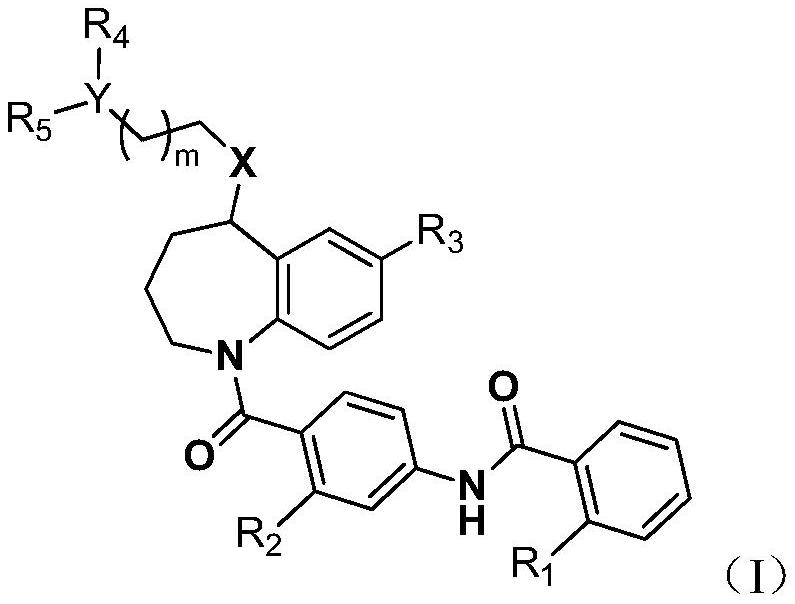
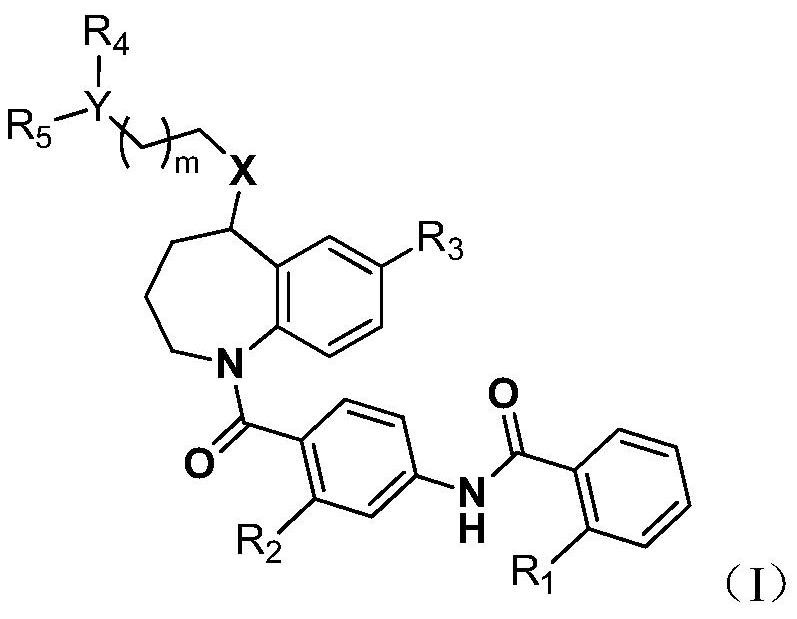
Benzazepine derivatives
PendingCN110891939AFavorable metabolic stabilityFavorable absorption propertiesOrganic chemistryUrinary disorderAntagonismPerylene derivatives
The present invention provides novel benzazepine compounds of Formula (1), or salts thereof, having vasopressin V1a and V2 antagonisms, and medical uses thereof. In the formula, R1 is optionally substituted C1-6 alkyl, etc.; L is -C(=O)-NH-, etc.; the ring A1 is a hydrocarbon ring, etc.; the ring A2 is a hydrocarbon ring, etc.; and each of the rings A1 and A2 may have at least one substituent.
Owner:OTSUKA PHARM CO LTD
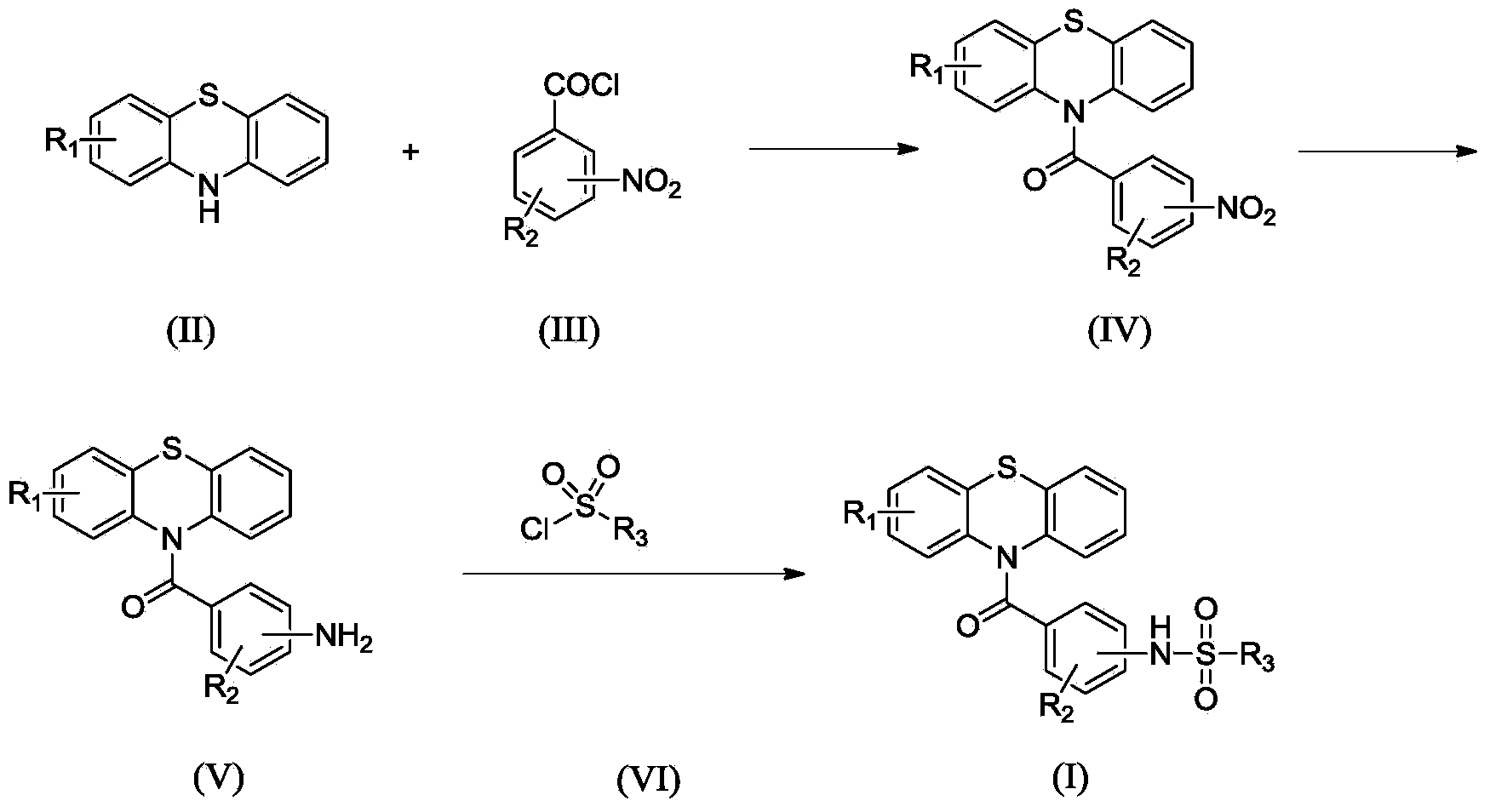
N-substituted benzoyl phenothiazine compounds as well as preparation method and application thereof
The invention relates to N-substituted benzoyl phenothiazine compounds as well as a preparation method and application thereof, and particularly relates to N-substituted benzoyl phenothiazine compounds having a structure as shown in a formula I described in the specification as well as a preparation method of the compounds, pharmaceutical compositions taking the N-substituted benzoyl phenothiazine compounds having a structure as shown in the formula I as active ingredients and use of the pharmaceutical compositions for preventing or treating diseases related to an arginine vasopressin V1a receptor, an arginine vasopressin V1b receptor, an arginine vasopressin V2 receptor, a sympathetic nervous system or a rennin-angiotensin-aldosterone system.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Phosphate ester-containing piperazine derivative and preparation method as well as application thereof
InactiveCN102627669AReceptor antagonismOrganic active ingredientsGroup 5/15 element organic compoundsDiseasePhosphate
The invention relates to a phosphate ester-containing piperazine derivative and a preparation method as well as application thereof, in particular to one type of phosphate ester-containing piperazine derivatives with a structure shown by a formula I and a preparation method thereof, a medicinal composition with the phosphate ester-containing piperazine derivative with the structure shown by the formula I as an active ingredient and application thereof to preventing or treating diseases related to arginine vasopressin V1a receptors, arginine vasopressin V1b receptors, arginine vasopressin V2 receptors, a sympathetic nervous system or a renin-angiotensin-aldosterone system.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Process for preparing benzazepine compounds or salts thereof
ActiveUS20090306369A1High yieldHigh purityOrganic compound preparationCarboxylic acid amides preparationBenzoic acidBenzene
This invention provides a process for preparing benzazepine compounds of the formula (1): wherein X1 is a halogen atom, R1 and R2 are a lower alkyl group, or salts thereof as well as intermediate benzoic acid compounds in high yield and high purity on industrial scale, which are useful as an intermediate for preparing a pharmaceutically active 2,3,4,5-tetrahydro-1H-1-benzazepine compound having vasopressin antagonistic activity.
Owner:OTSUKA PHARM CO LTD
Bis-sulfonamide compound and its preparation method and use
The invention relates to bisulfamide compounds as well as a preparation method and use thereof, and specifically relates to bisulfamide compounds having the structure as shown in a formula I and pharmaceutically acceptable salts thereof as well as a preparation method of the bisulfamide compounds, pharmaceutical compositions with the bisulfamide compounds having the structure as shown in the formula I and the pharmaceutically acceptable salts thereof as active effective constituents and use of the pharmaceutical compositions in preventing or treating diseases associated with an arginine vasopressin V1a receptor, an arginine vasopressin V1b receptor, an arginine vasopressin V2 receptor, the sympathetic nervous system or the renin-angiotensin-aldosterone system; the formula I is as shown in the specification.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Benzazepine compound
InactiveCN104447555AExcellent vasopressin antagonismLittle side effectsOrganic chemistryMetabolism disorderMethyl benzeneCombinatorial chemistry
An object of the present invention is to provide a novel benzazepine compound or a salt thereof, which has excellent vasopressin antagonistic activity. The benzazepine compound or a salt thereof of the present invention is represented by Formula (1): wherein R 1 , R 2 and R 5 may be the same or different and each represents H or D; and R 3 and R 4 each represents a C 1-6 alkyl group, a C 1-6 deuteroalkyl group, or a C 1-6 perdeuteroalkyl group.
Owner:OTSUKA PHARM CO LTD
Recombinant human vasopressin and its application
ActiveCN104861057BExtended half-lifeHigh glycoform complexityPeptide/protein ingredientsPeptide preparation methodsElectrophoresesBinding site
The invention relates to high-molecular-weight recombinant human kininogenase, and a preparation method and application thereof. Specifically, the invention relates to the high-molecular-weight recombinant human kininogenase prepared by using a mammal cell culture production method and specific purifying technology for the first time. According to results of SDS-PAGE electrophoresis determination, the molecular weight of the high-molecular-weight recombinant human kininogenase is 43 to 49 KDa; the high-molecular-weight recombinant human kininogenase is glycoprotein and contains three N-Link glycosylation binding sites which are respectively located at Asn78, Asn84 and Asn141; and compared with kininogenase originated from human urine, the high-molecular-weight recombinant human kininogenase has a more complicated and more complete glycosylation level and obviously reduces a deamidation level. The high-molecular-weight recombinant human kininogenase obtained in the invention can substantially prolong in-vivo half-life and reduce side effects. The invention also relates to application of the high-molecular-weight recombinant human kininogenase in treatment of kidney failure.
Owner:UNICOHEALTH CO LTD
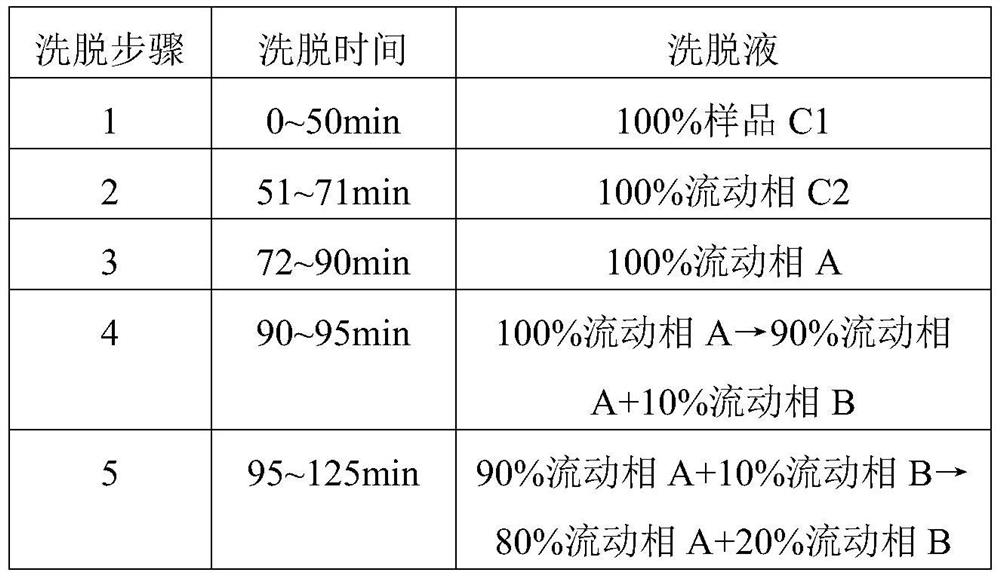
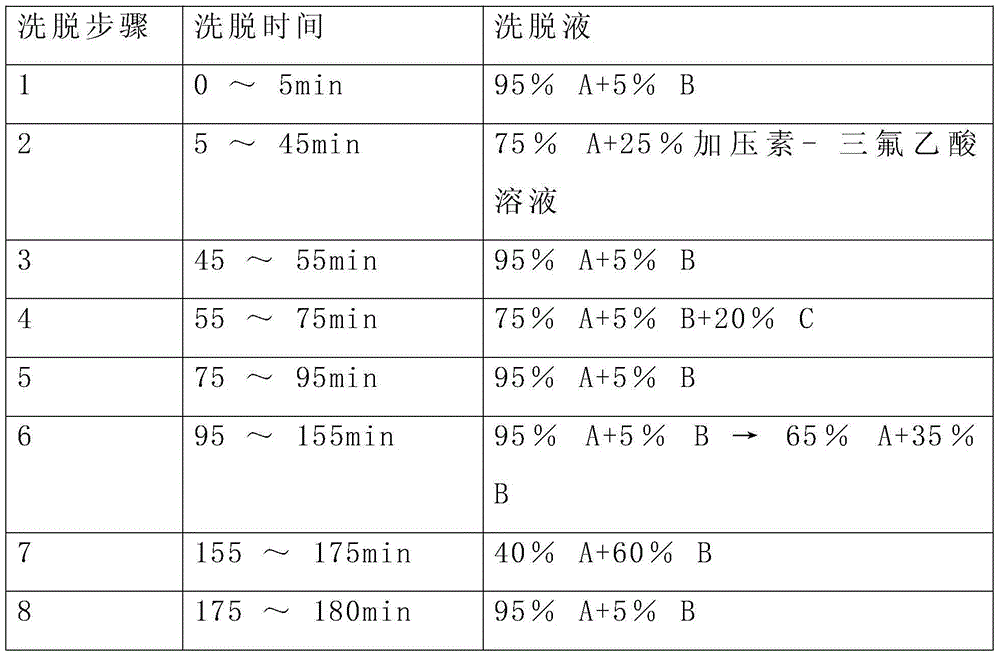
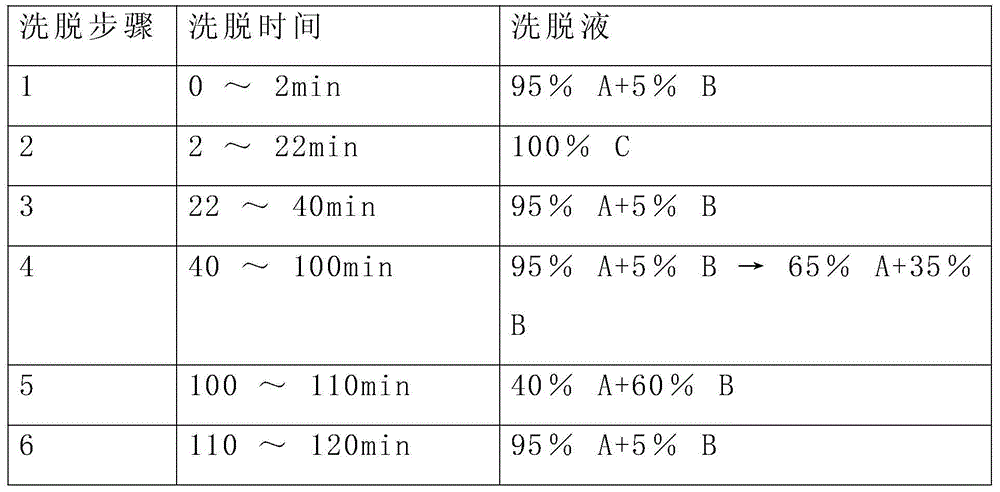
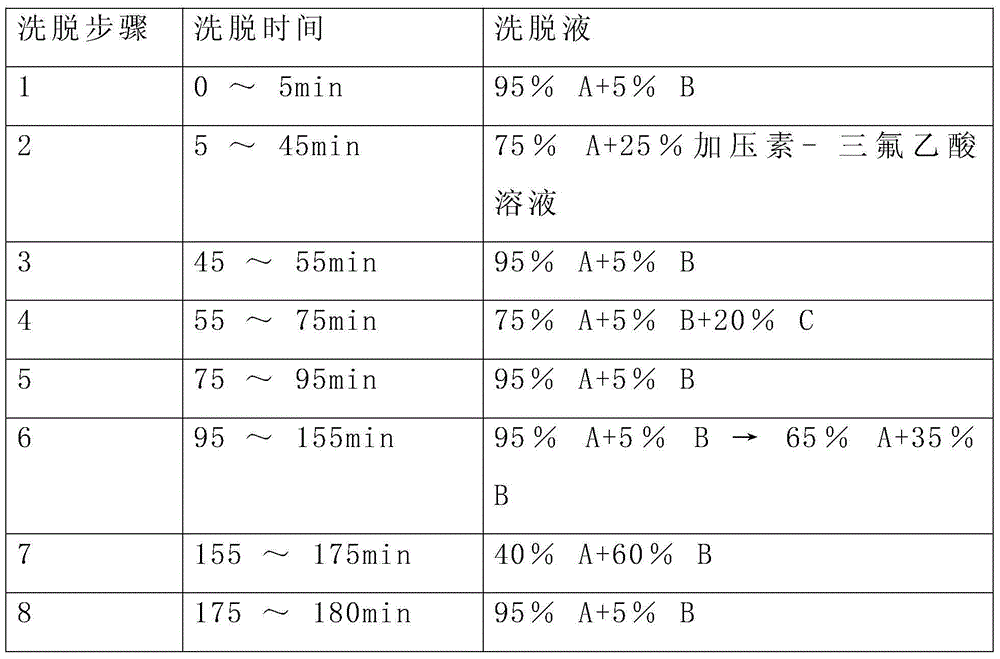
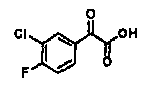
A kind of refining method of vasopressin [5-asp] impurity
ActiveCN109929011BSuitable for continuous productionSimple production processOxytocins/vasopressinsPeptide preparation methodsPurification methodsPhysical chemistry
The invention discloses a method for refining impurities of vasopressin [5‑Asp]. The refining method of vasopressin [5-Asp] impurity comprises the steps of: adopting high performance liquid phase chromatography to carry out reverse-phase enrichment, reverse-phase salt conversion, reverse phase Phase purification can be done; the packing of high performance liquid phase chromatography is super water-resistant packing; reverse-phase enrichment, reverse-phase salt conversion, and reverse-phase purification are all completed in a one-step reverse-phase elution process. The purification method of the vasopressin [5‑Asp] impurity of the present invention produces mostly waste water in the purification process, which can be reused after simple treatment at a sewage station, and is economical and environmentally friendly.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Bisamide compounds and preparation method and use thereof
InactiveCN104098543AHas receptor antagonistic effectReceptor antagonismOrganic active ingredientsNervous disorderDiseaseArginine
The invention relates to a compound with two amido bonds and a preparation method and use thereof, and particularly relates to the compound with the two amido bonds and a structure shown as the formula I, A pharmaceutically acceptable salt, a preparation method thereof, a pharmaceutical composition using the compound with the two amido bonds and the structure shown as the formula I and the pharmaceutically acceptable salt as active ingredients, and the use of the pharmaceutical composition in prevention or treatment of arginine vasopressin V1a receptor, arginine vasopressin V1b receptor, arginine vasopressin V2 receptor, sympathetic nervous system or renin-angiotensin-aldosterone system related diseases.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
A kind of preparation method of vasopressin [4-glu]
ActiveCN106749540BIncrease the difficultyRandom combinationOxytocins/vasopressinsPeptide preparation methodsCombinatorial chemistryOrganic chemistry
The invention discloses a preparation method for vasopressin[4-Glu]. The preparation method comprises the following step: successively subjecting a solution of a crude vasopressin[4-Glu] precursor product to reversed-phase cyclization, reversed-phase purification and reversed-phase desalination by adopting a highly-efficient liquid-phase reversed-phase chromatography process, wherein a filling material for the highly-efficient liquid-phase reversed-phase chromatography process is a styrene-divinyl benzene copolymer; and the crude vasopressin[4-Glu] precursor product contains two free sulfhydryl groups. The preparation method provided by the invention innovatively adopts a reversed-phase adsorption method for cyclization, purification and desalination, solves the problems of cyclization, purification and desalination once for all, optimizes production process, and is applicable to continuous industrial production.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Spiro-thiazolones
ActiveUS10092551B2Nervous disorderCarbonyl compound preparation by condensationPhase shiftedSleeping disorders
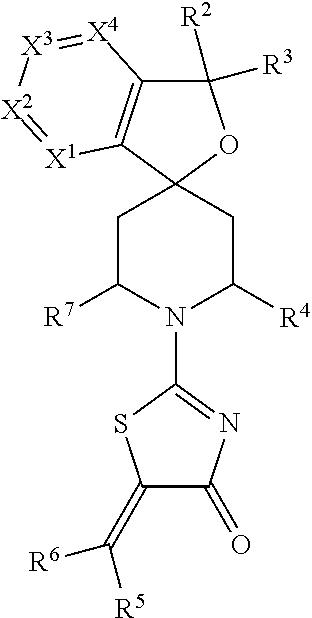
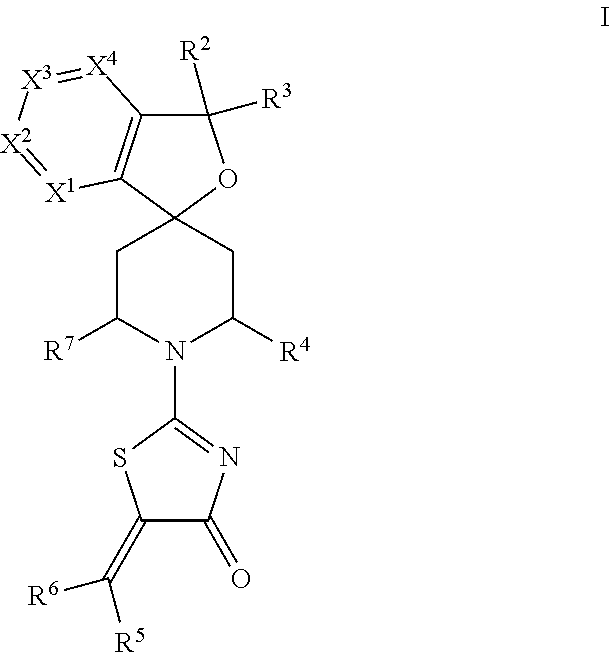
Spiro-thiazolones of formula Iwherein X1, X2, X3, X4, R2, R3, R4, R5, R6 and R7 are as defined herein, which act as V1a receptor modulators, and in particular as V1a receptor antagonists, their manufacture, pharmaceutical compositions containing them and their use as medicaments for treatment of inappropriate secretion of vasopressin, anxiety, depressive disorders, obsessive compulsive disorder, autistic spectrum disorders, schizophrenia, aggressive behavior and phase shift sleep disorders, in particular jetlag.
Owner:F HOFFMANN LA ROCHE & CO AG
Azole derivatives
The invention of the present application provides mood disorder, anxiety disorder, schizophrenia, Alzheimer's disease, Parkinson's disease, Huntington's disease, eating disorder, hypertension, digestive organ disease, drug dependence, epilepsy, cerebral infarction, Therapeutic or preventive agents for diseases such as cerebral ischemia, cerebral edema, head trauma, inflammation, immune-related diseases, and alopecia. Specifically, an azole derivative represented by the general formula (I) having an arginine vasopressin 1b receptor antagonistic activity, or a pharmaceutically acceptable salt thereof is provided. 【Chemical 1】
Owner:TAISHO PHARMACEUTICAL CO LTD
A kind of refining method of vasopressin
ActiveCN109929010BSuitable for productionHigh removal rateOxytocins/vasopressinsSolid sorbent liquid separationSewage treatmentAqueous solution
The invention discloses a refining method of vasopressin. The refining method comprises the following steps: using high-performance liquid phase chromatography to sequentially carry out reverse-phase enrichment, reverse-phase salt conversion, and reverse-phase purification of the crude vasopressin solution , that is, the crude product solution of vasopressin is obtained by oxidizing the crude product solution of reduced vasopressin synthesized in solid phase; the filler for high performance liquid phase chromatography is a super water-resistant filler. The present invention uses the one-step method of on-line reverse-phase enrichment, reverse-phase salt conversion, and reverse-phase purification to obtain pure polypeptide products. The removal rate of impurities in crude vasopressin products is high, and the purity of pure vasopressin products is higher. The production process, in which the mobile phase in the stages of column balance, sample enrichment and salt conversion is an aqueous solution, which is environmentally friendly and pollution-free, and the effluent waste liquid can be directly treated for sewage and recycled.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




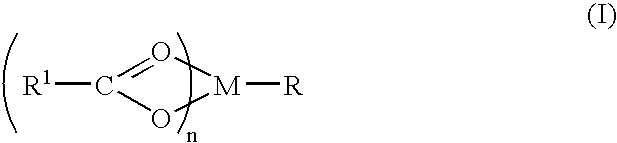
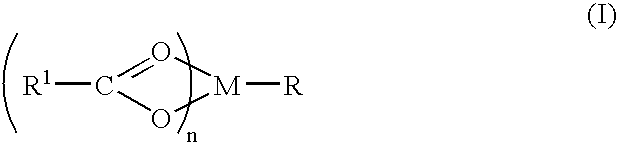
![Heteroaryl-cyclohexyl-tetraazabenzo[e]azulenes Heteroaryl-cyclohexyl-tetraazabenzo[e]azulenes](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e323e1ec-1df7-4ae2-9b60-16096f3a355e/US20110275801A1-20111110-C00001.png)
![Heteroaryl-cyclohexyl-tetraazabenzo[e]azulenes Heteroaryl-cyclohexyl-tetraazabenzo[e]azulenes](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e323e1ec-1df7-4ae2-9b60-16096f3a355e/US20110275801A1-20111110-C00002.png)
![Heteroaryl-cyclohexyl-tetraazabenzo[e]azulenes Heteroaryl-cyclohexyl-tetraazabenzo[e]azulenes](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e323e1ec-1df7-4ae2-9b60-16096f3a355e/US20110275801A1-20111110-C00003.png)






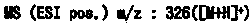
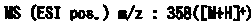
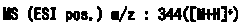













![A kind of refining method of vasopressin [5-asp] impurity A kind of refining method of vasopressin [5-asp] impurity](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/462553a6-0c93-462f-82f9-e97c846ee297/DEST_PATH_IMAGE004.png)
![A kind of refining method of vasopressin [5-asp] impurity A kind of refining method of vasopressin [5-asp] impurity](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/462553a6-0c93-462f-82f9-e97c846ee297/BDA0002050227930000031.png)
![A kind of refining method of vasopressin [5-asp] impurity A kind of refining method of vasopressin [5-asp] impurity](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/462553a6-0c93-462f-82f9-e97c846ee297/BDA0002050227930000085.png)