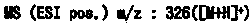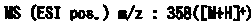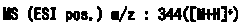Fused azole derivative
A technology of condensed ring azoles and derivatives, applied in the field of condensed ring azole derivatives, can solve problems such as unreported compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0313] Hereinafter, the present invention will be described in more detail with reference examples, examples, and test examples, but the present invention is not limited thereto, and changes can be made without departing from the scope of the present invention.
[0314] In Reference Examples and Examples, "Phase Separator" in the following treatments refers to Biotage's ISOLUTE (registered trademark) Phase Separator. For "SNAP Cartridge KP-NH" purified by column chromatography, use Biotage's SNAP Cartridge KP-NH; for "SNAP Cartridge KP-Sil", use Biotage's SNAP Cartridge KP-Sil; for "SNAP Cartridge HP For "-Sil", SNAP Cartridge HP-Sil from Biotage Co., Ltd. was used; for "Chromatorex NH", Cromatrex (registered trademark) NH manufactured by Fuji Silysia Chemical Co., Ltd. was used; TLC Platinum NH (manufactured by Fuji Silysia Corporation) was used for thin-layer chromatography (TLC); In addition, Silikagel 60 and Siricagel 60N refer to the use of silica gel sold by Kanto Chemic...
reference example P-A1
[0339] · Reference Example P-A1: Synthesis of 2-nitro-4-[3-(piperidin-1-yl)propoxy]aniline
[0340] [chem 36]
[0341]
[0342] 4-Amino-3-nitrophenol (2.15g) was dissolved in THF (40mL), and 1-piperidine propanol (2.00g), triphenylphosphine (5.49g), DEAD (2.2mol / L toluene solution, 9.52 mL), stirred at room temperature for 16 hours. The residue obtained by concentrating the reaction solution under reduced pressure was purified by silica gel column chromatography (Cromatrex NH, mobile phase: n-hexane / EtOAc=80 / 20-65 / 35; v / v) to obtain the title compound (3.41 g, reddish-brown oil). the
[0343] .
[0344] · Reference example P-A2: Synthesis of 3-chloro-N-{2-nitro-4-[3-(piperidin-1-yl)propoxy]phenyl}benzamide
[0345] [chem 37]
[0346]
[0347] CHCl of the compound (3.40 g), pyridine (4.92 mL) obtained in Reference Example P-A1 3 (40 mL) was added 3-chlorobenzoyl chloride (1.87 mL) and stirred at room temperature for 1.5 hours. The reaction solution was washed su...
reference example P-A15
[0412] · Reference example P-A15: 2-[5-bromo-2-(4-fluoro-3-methoxyphenyl)-1H-benzimidazol-1-yl]-N-isopropylacetamide and 2 Synthesis of a mixture of -[6-bromo-2-(4-fluoro-3-methoxyphenyl)-1H-benzimidazol-1-yl]-N-isopropylacetamide
[0413] [chemical 50]
[0414]
[0415] The title compound (0.33 g, colorless solid) was obtained from the compound (0.45 g) obtained in Reference Example P-A14 by the same method as in Reference Example P-A10. the
[0416] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com