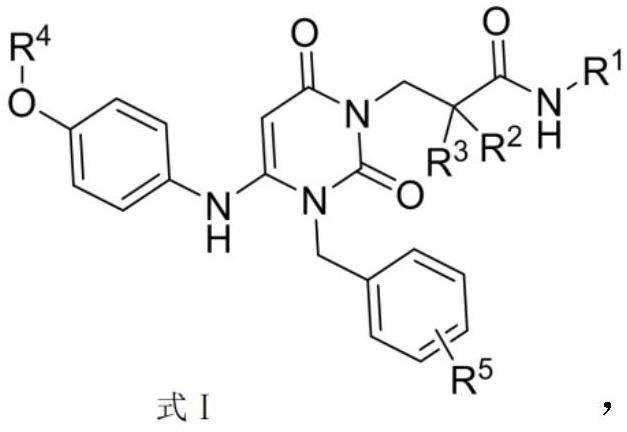
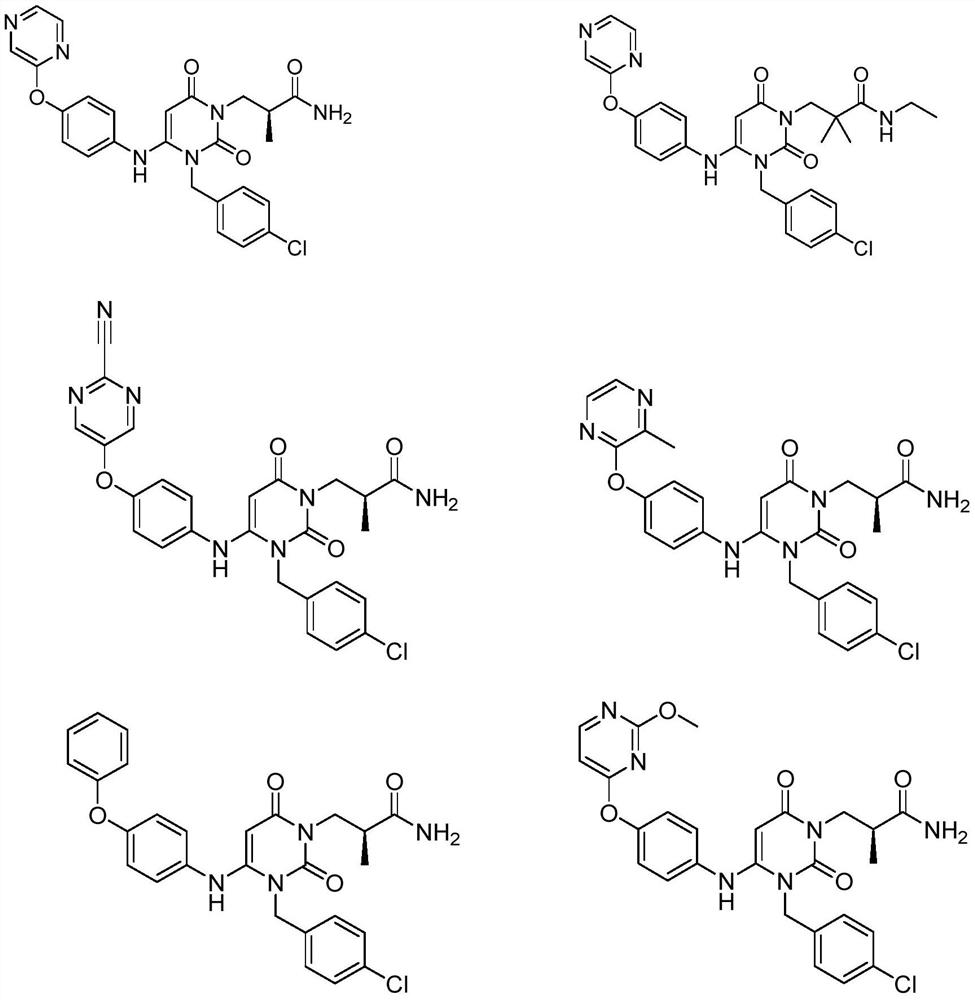
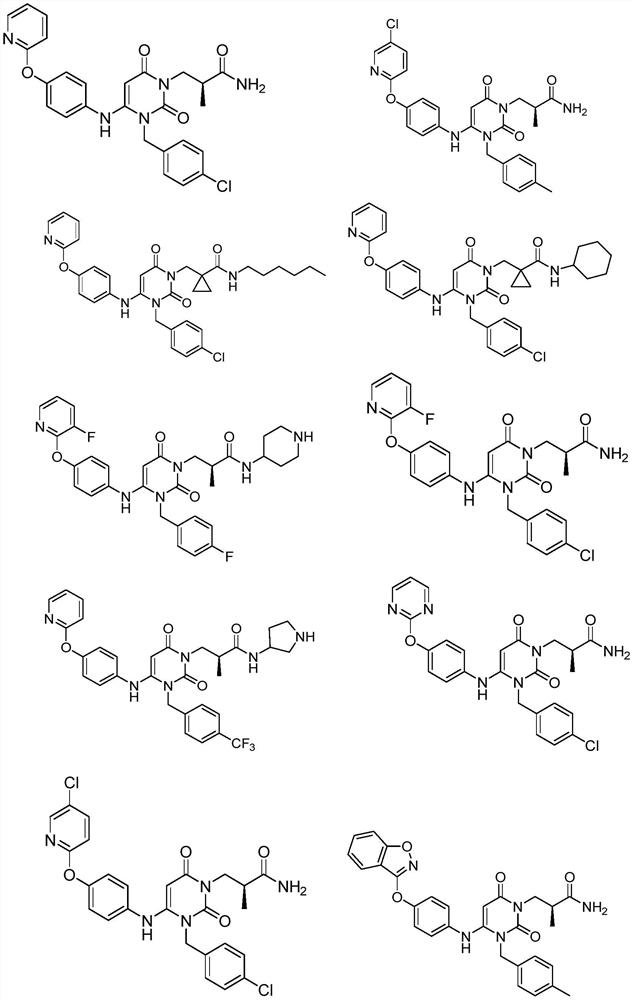
Dihydropyrimidine compound as well as preparation method and application thereof
A compound and solvate technology, applied in the field of medicinal chemistry, can solve the problems of high incidence of taste-related adverse events and failure to reach the main efficacy endpoint, and achieve good antitussive effect, easy operation, and strong antagonistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: (S)-3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyrazine-2-oxyl)phenyl)amino)-3, Preparation of 6-dihydropyrimidin-1(2H)-yl)-2-methylpropionamide (Compound 1)
[0054]
[0055] Step 1: Preparation of 4-(pyrazine-2-oxyl)aniline (compound b-1)
[0056] 2-Fluoropyrazine (50.0g, 0.52mol) and p-aminophenol (53.5g, 0.49mol) were dissolved in dimethylsulfoxide (360ml), cesium carbonate (320g, 0.98mol) was added to obtain a reaction mixture, and mechanically Stir the reaction mixture at constant speed. Then the internal temperature of the reaction system was raised to 80° C. for 2 h. The progress of the reaction was tracked by thin-layer chromatography. After the reaction was complete, the reaction mixture was added to three times the volume (about 1 L) of water and kept stirring. Then extract the product three times with ethyl acetate, combine and dry the ethyl acetate and concentrate to obtain the crude product. The crude product is slurried with 500ml of water for...
Embodiment 2
[0076] Example 2: 3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyrazine-2-oxyl)phenyl)amino)-3,6-dihydro Preparation of pyrimidin-1(2H)-yl)-N-ethyl-2,2-dimethylpropionamide (compound 2)
[0077] Compared with Example 1, the preparation method of this example replaces (S)-3-hydroxyl-2-methyl propionate methyl ester with equimolar 3-hydroxyl-2,2-dimethyl propionate in step 3 Acid methyl ester, and the ammonium chloride in step 6 was replaced by equimolar ethylamine, and the rest of the conditions were the same; Compound 2 was obtained as a white solid, with a yield of 66.5% and a purity of 99.78%.
[0078] ESI-MS:m / z=549.2(M+H) + .
[0079] 1HNMR (400MHz, DMSO-d6) δ9.52(s, 1H), δ8.66(s, 1H), δ8.16(s, 1H), 7.85–7.76(m, 1H), 7.48–7.38(m, 1H),7.35–7.26(m,2H),7.16(m,4H),7.14–7.11(m,1H),7.04(d,J=8.3Hz,1H),5.42–5.15(s,2H),4.62 (s, 1H), 3.92 (s, 2H), 3.22-3.16 (m, 2H), 1.09 (s, 6H), 1.02-0.96 (m, 3H).
Embodiment 3
[0080] Example 3: (S)-3-(3-(4-chlorobenzyl)-4-(4-(2-cyanopyrimidine-5-oxyl)phenyl)-amino)-2,6-di Preparation of oxo-3,6-dihydropyrimidin-1(2H)-yl)-2-methylpropionamide (compound 3)
[0081] Compared with Example 1, the preparation method of this example is that 2-fluoropyrazine in step 1 is replaced by equimolar 5-fluoro-2-cyanopyrimidine, and the rest of the conditions are the same. Compound 3 was obtained with a yield of 67.3% and a purity of 99.76%.
[0082] ESI-MS:m / z=532.2(M+H) + .
[0083] 1 HNMR (400MHz, DMSO-d6) δ8.63(s, 1H), δ8.11(s, 2H), 7.95(s, 2H), 7.86–7.76(m, 1H), 7.35–7.26(m, 2H) ,7.18-7.09(m,4H),7.14–7.11(m,1H),5.32(s,2H),4.62(s,1H),3.88-3.79(m,2H),2.72-2.68(m,1H) ,1.01-0.96(m,3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com