Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Dihydropyrimidinuria" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A number sign (#) is used with this entry because of evidence that dihydropyrimidinase deficiency is caused by homozygous or compound heterozygous mutation in the DPYS gene ... DPYS deficiency is an autosomal recessive disease characterized by the presence of dihydropyrimidinuria. The clinical phenotype is highly variable, ranging from early ...
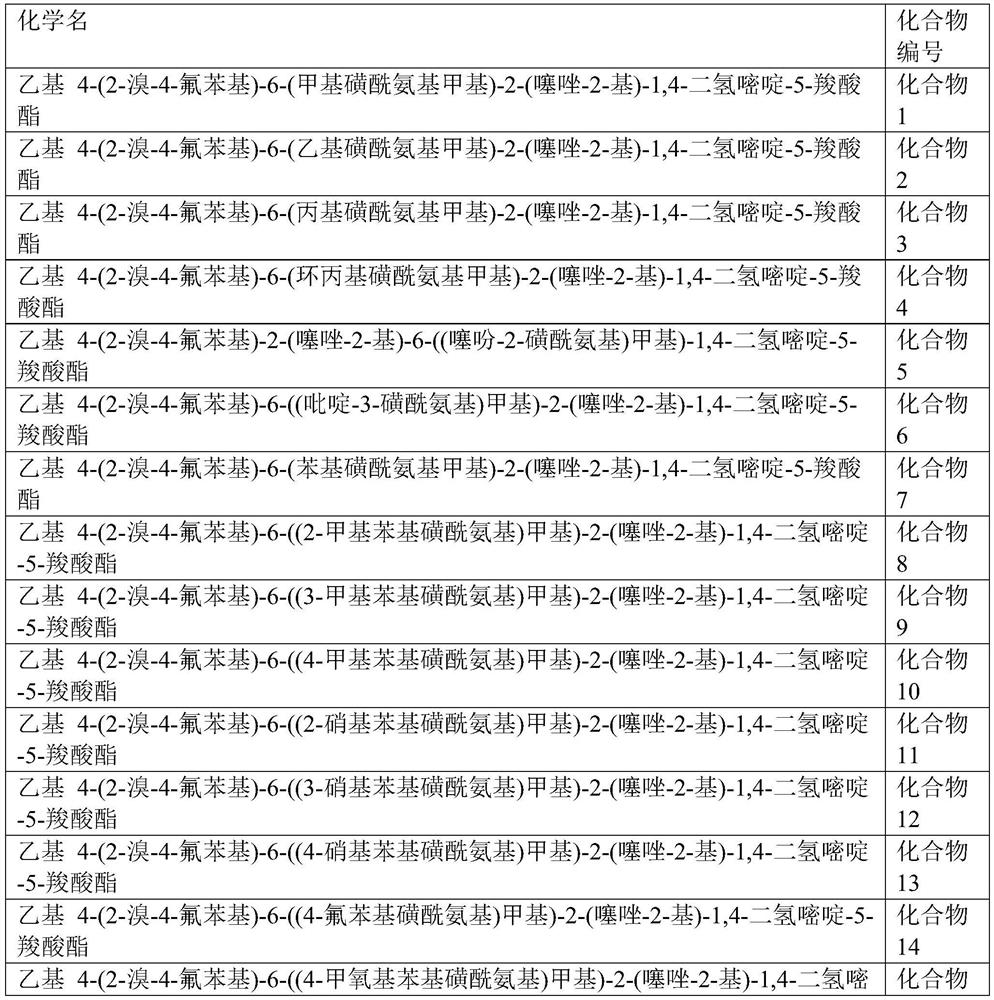
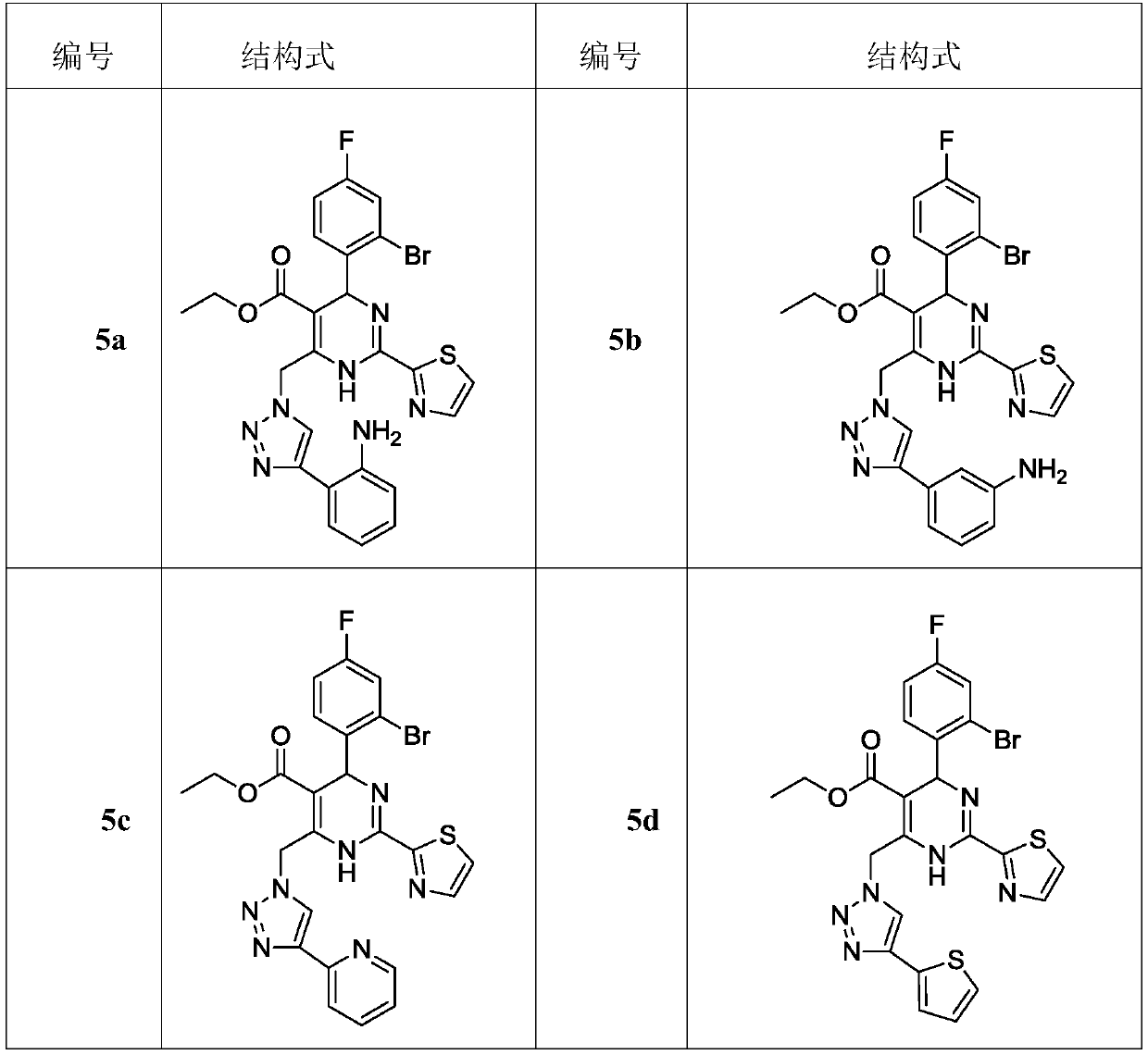
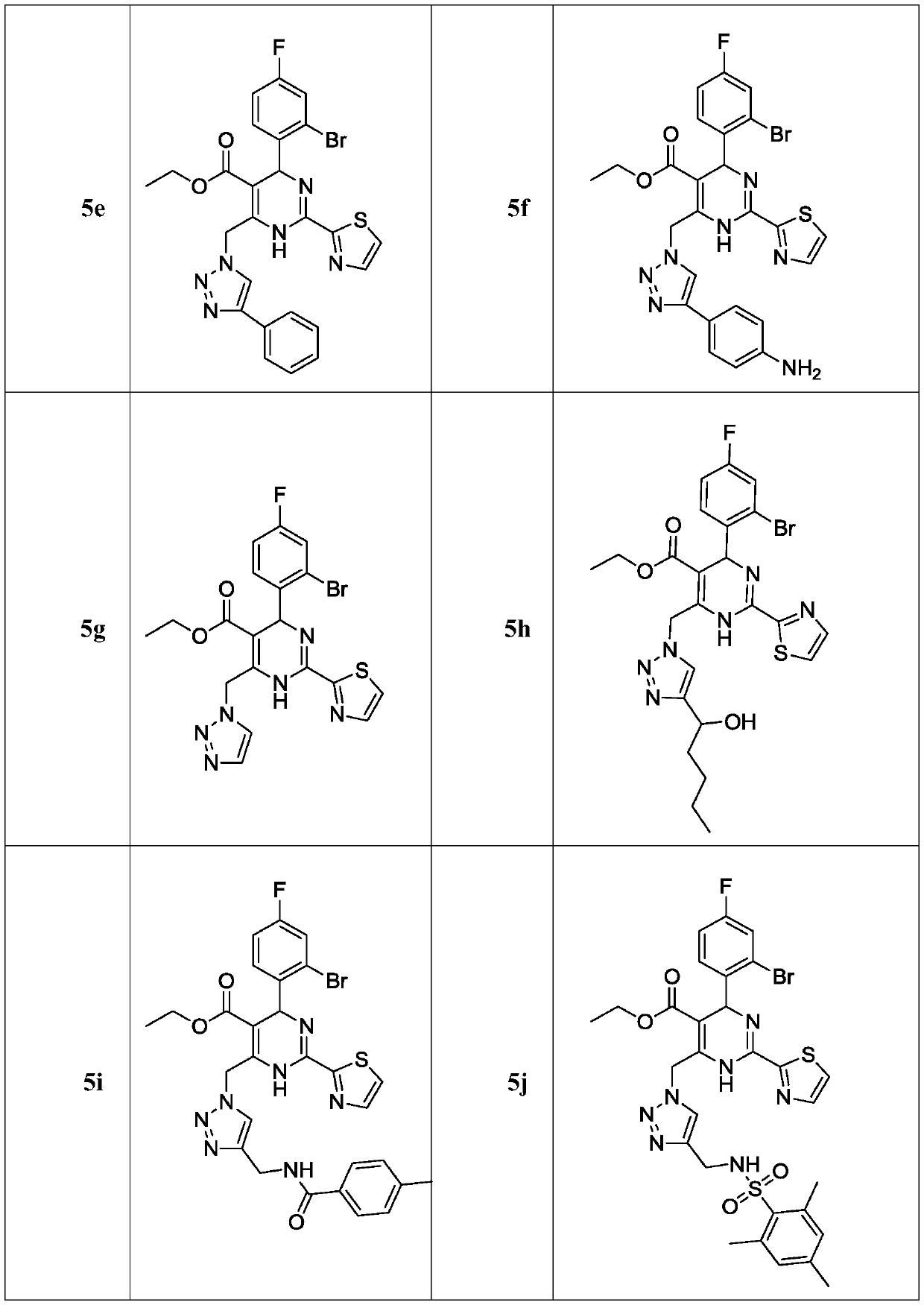
Dihydropyrimidine compounds and their application in pharmaceuticals
ActiveUS20150152096A1Avoid infectionReduce severityOrganic active ingredientsOrganic chemistryDiseaseDihydropyrimidinuria
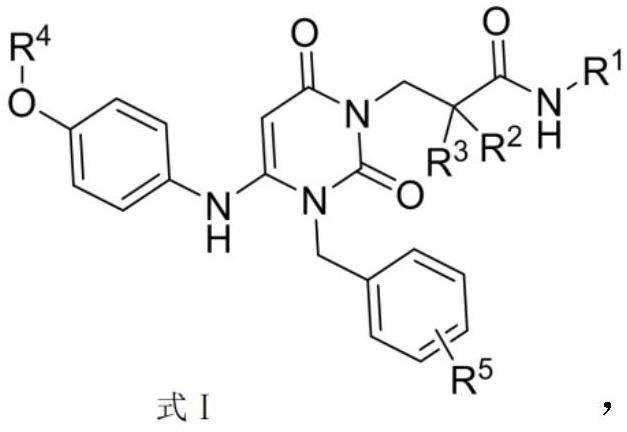
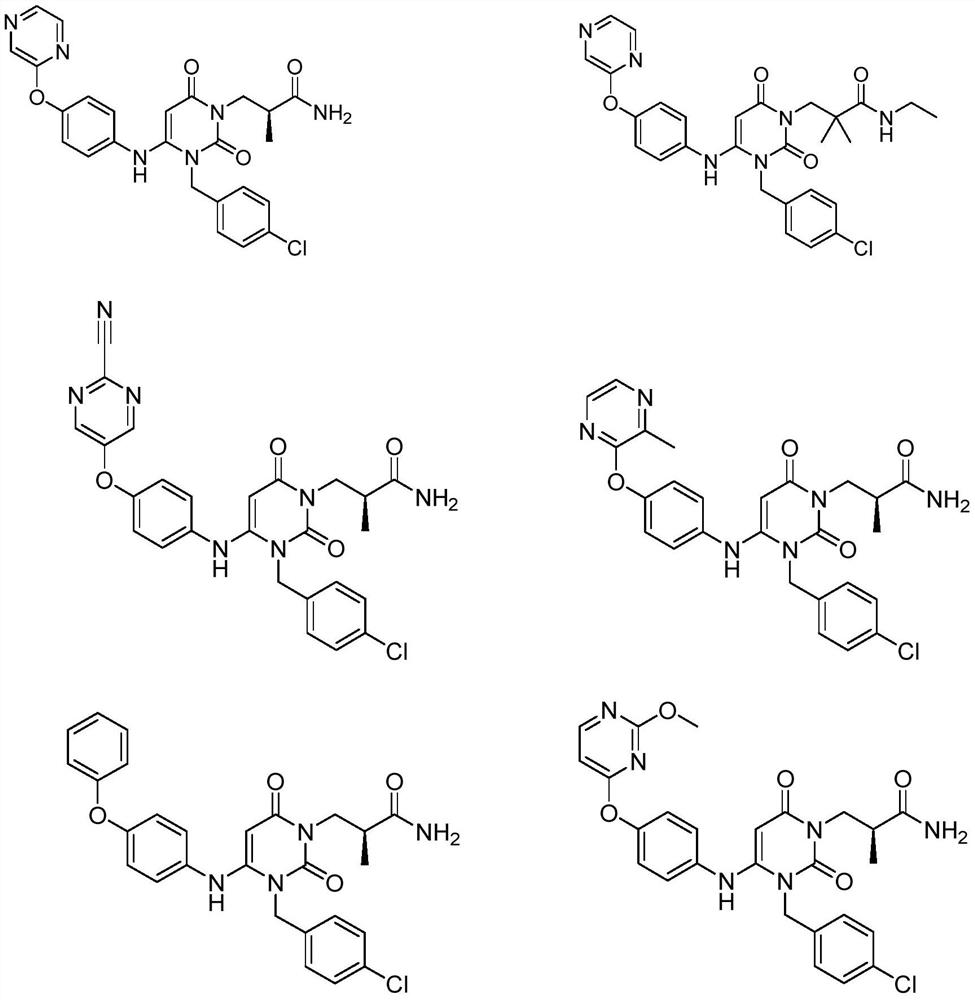
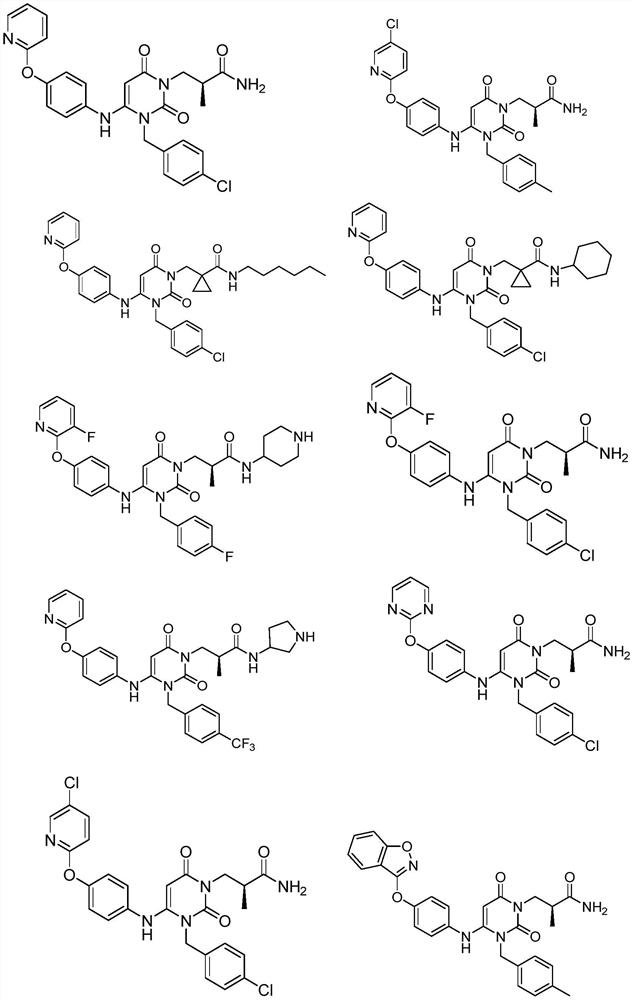
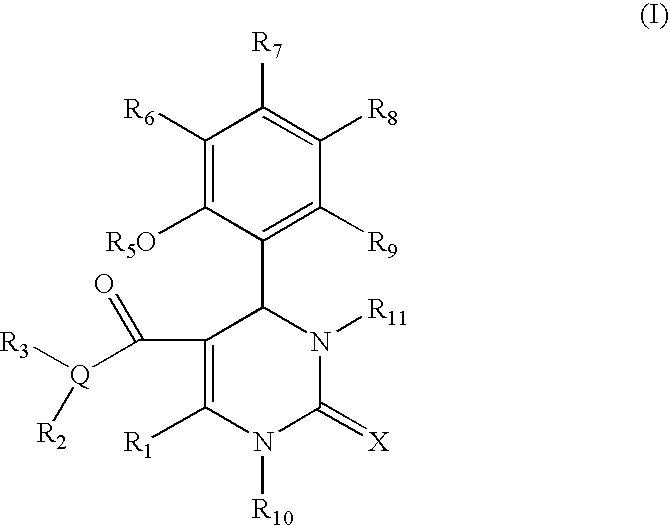
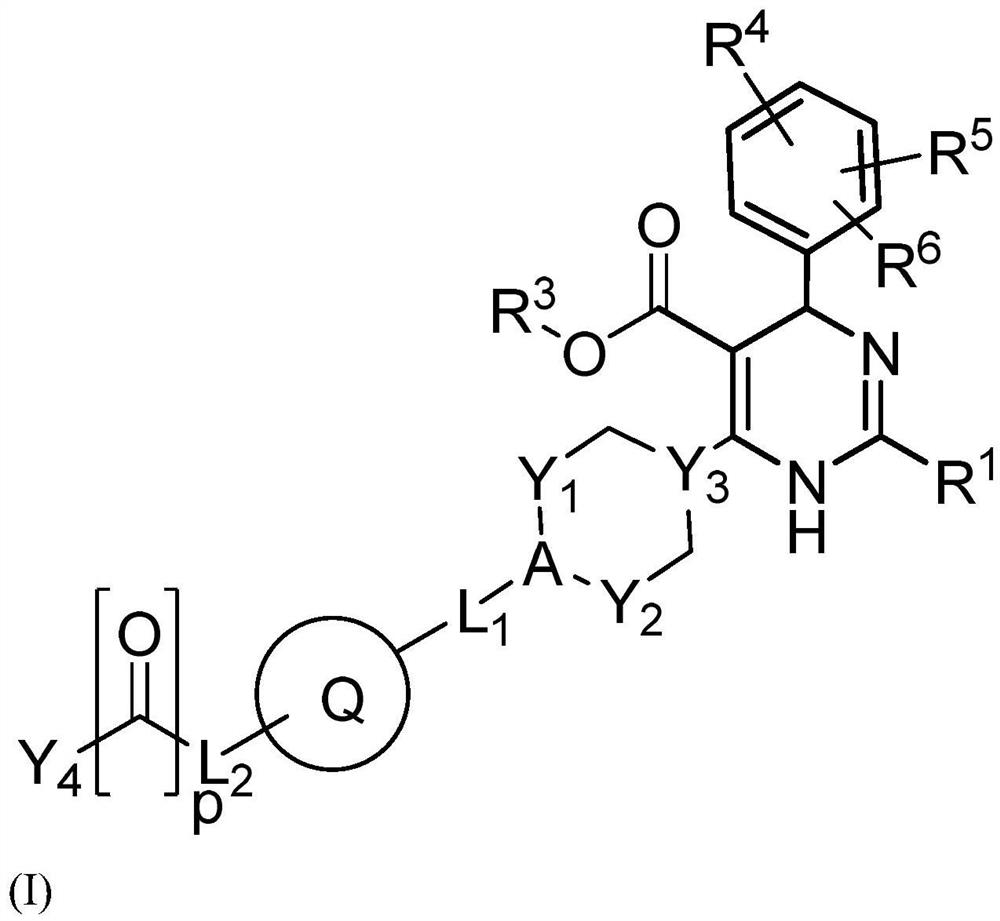
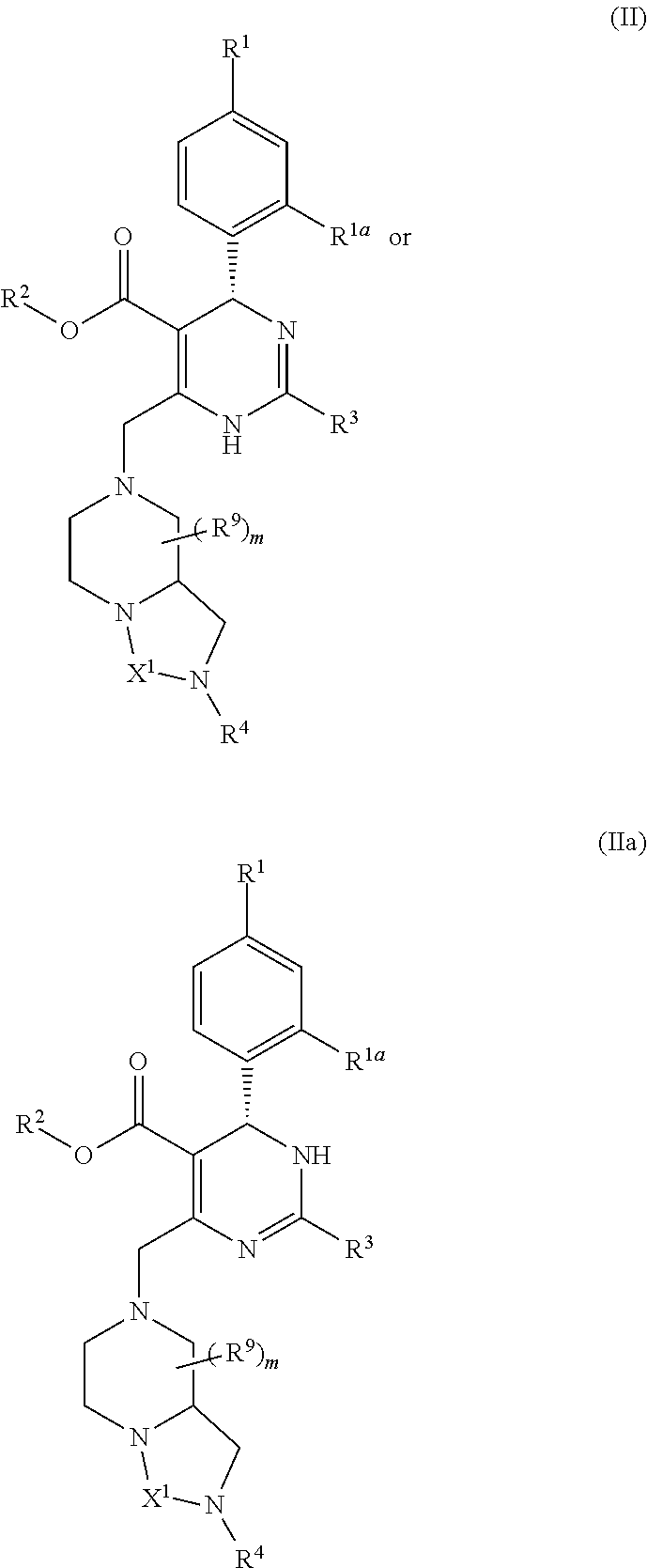
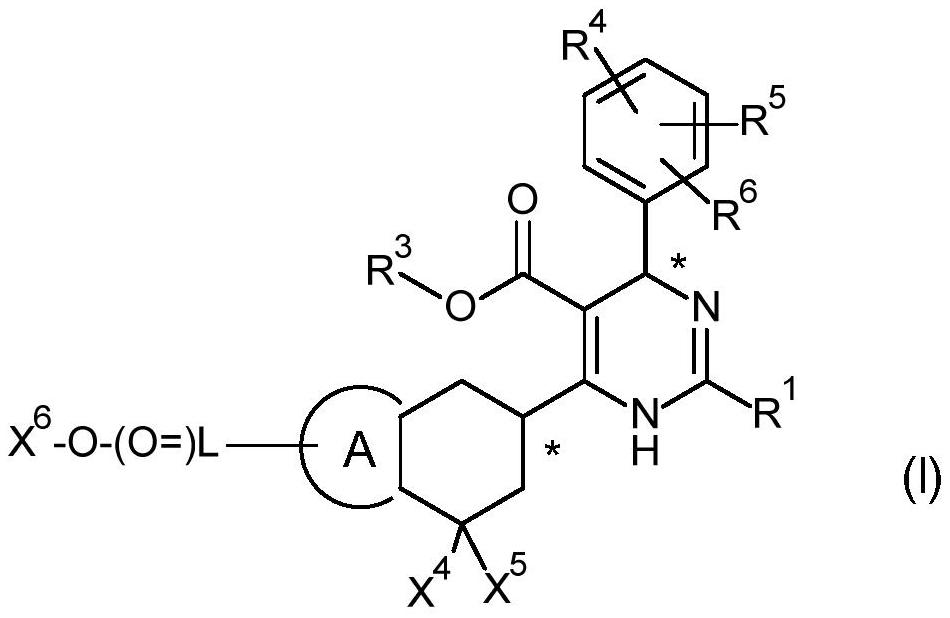
Provided herein are dihydropyrimidine compounds and their pharmaceutical applications, especially for use in treating and preventing HBV diseases. Specifically, provided herein are compounds having Formula (I) or (Ia), or an enantiomer, a diastereoisomer, a tautomer, a hydrate, a solvate, or a pharmaceutically acceptable salt thereof, wherein the variables of the formulas are as defined in the specification. Also provided herein is the use of the compounds having Formula (I) or (Ia), or an enantiomer, a diastereoisomer, a tautomer, a hydrate, a solvate, or a pharmaceutically acceptable salt thereof for treating and preventing HBV diseases.
Owner:RUYUAN WEI XIANG TECH CO LTD
Dihydropyrimidine compound as well as preparation method and application thereof
ActiveCN113801097AImprove securityGood inhibitory effectOrganic chemistryAntipyreticDiseaseMetabolite
The invention discloses a dihydropyrimidine compound as well as a preparation method and an application thereof, and belongs to the technical field of medicinal chemistry. The structure of the dihydropyrimidine compound provided by the invention is as shown in a formula I in the specification. The preparation method provided by the invention comprises the following steps: carrying out substitution reaction on a compound a and a compound k under the catalysis of alkali to generate a compound b; performing substitution reaction on the compound c and the compound d under an alkaline condition to obtain a compound e; carrying out Mitsunobu reaction on the compound e and f to obtain an intermediate g, and carrying out coupling reaction on the compound g and b to generate a compound h. The invention provides the application of the compound shown in the formula I or salt, solvate, allomer, metabolite, nitric oxide and prodrug thereof in preparation of drugs for treating or preventing P2X3 and / or / P2X2 / 3 receptor related diseases. The dihydropyrimidine compound disclosed by the invention has a good P2X3 receptor antagonism effect and better safety.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
Dihydropyrimidine compound as well as preparation method and application thereof
ActiveCN113620888AHigh affinityGood antitussive effectOrganic active ingredientsOrganic chemistryDiseaseDihydropyrimidinuria
The invention discloses a dihydropyrimidine compound as well as a preparation method and application thereof, belongs to the technical field of medicinal chemistry, and solves the problems of poor effect and large adverse reaction of a P2X3 receptor inhibitor in the prior art. The structure of the dihydropyrimidine compound is as shown in a formula I in the specification. The invention further provides a preparation method of the compound shown in the formula I and application of the compound in preparation of drugs for treating or preventing P2X3 and / or P2X2 / 3 receptor related diseases. The dihydropyrimidine compound provided by the invention has good affinity with P2X3, has a relatively strong antagonistic effect on a P2X3 receptor, and is safe and effective.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
Dihydropyrimidine compound and preparation method and application thereof
ActiveCN113666914AHigh affinityStrong antagonistic effectOrganic active ingredientsOrganic chemistryDiseaseMetabolite
The invention discloses a dihydropyrimidine compound and a preparation method and application thereof, and belongs to the technical field of medicinal chemistry. The structure of the dihydropyrimidine compound is as shown in a formula I which is described in the specification. The invention also discloses a preparation method of the dihydropyrimidine compound. The invention provides application of a compound as shown in a formula I or a salt, a solvate, an allomer, a metabolite, nitrogen oxide and a prodrug of the compound in preparation of drugs for treating or preventing P2X3 and / or / P2X2 / 3 receptor related diseases. The antitussive action time of the compound is obviously prolonged compared with that of a compound in a contrast 1; the inhibitory activity of the compound on P2X3 is superior to that of a compound in a contrast 1 and a positive control drug gefapirant, and the compound almost has no influence on the taste of mice under 10mg / kg intravenous administration, and has a significant statistical difference from that of the positive control drug gefapirant.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
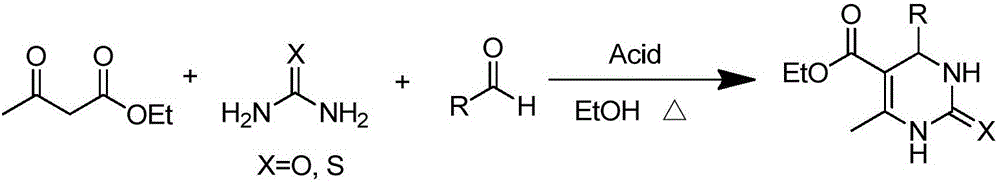
Method for synthesizing 3,4-dihydropyrimidine-2-one derivative
InactiveCN105712938AAchieve synthesisPhysical/chemical process catalystsOrganic chemistryAlcoholThiourea
The invention relates to an efficient and environment-friendly catalyst. On the mild reaction condition, a method for synthesizing 3,4-dihydropyrimidine-2-one derivative in a one-pot mode is achieved. The method comprises the steps that magnetic nano-particle load SnCl2 is adopted as a catalyst, ethyl alcohol is adopted as a reaction medium, and the three components of aldehyde, an alpha, beta-dicarbonyl compound and urea (thiourea) are condensed in a one-pot mode to obtain the 3,4-dihydropyrimidine-2-one derivative. The magnetic nano-particle load SnCl2 is repeatedly adopted seven times, and the catalytic reaction yield is not lowered obviously. The method is easy to implement, high in yield, short in reaction time, mild in reaction condition and wide in industrial application prospect, and the reusability of a catalytic reaction system is good.
Owner:TAIZHOU UNIV
Substituted dihydropyrimidines, dihydropyrimidones and dihydropyrimidinethiones as calcium channel blockers
The present invention is directed in part towards methods of modulating the function of calcium channels with pyrimidine-based compounds. In addition, the invention describes methods of preventing and treating calcium channel-related abnormal conditions in organisms with a compound identified by the invention. Furthermore, the invention pertains to pyrimidine-based compounds and pharmaceutical compositions comprising these compounds.
Owner:PULLELA PHANI KUMAR +3
Preparation method for dihydropyrimidine derivative
InactiveCN104402832ALow costConducive to industrial productionOrganic chemistrySolventDimethyl phosphite
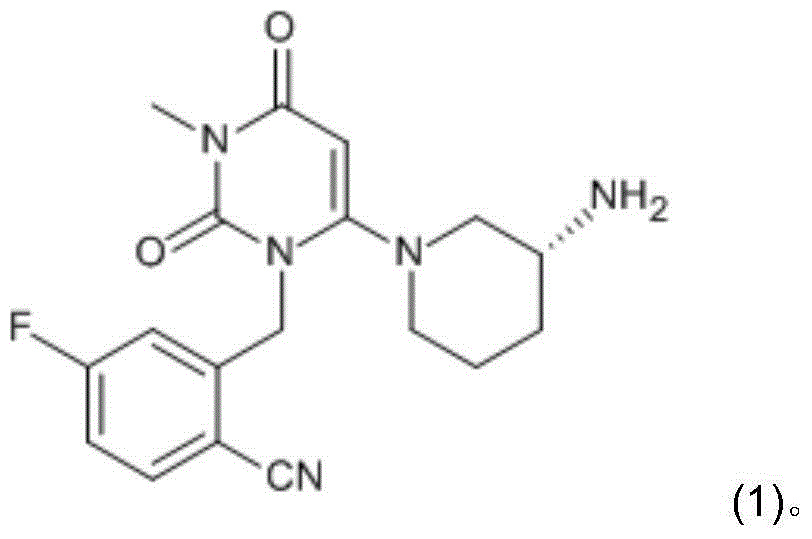
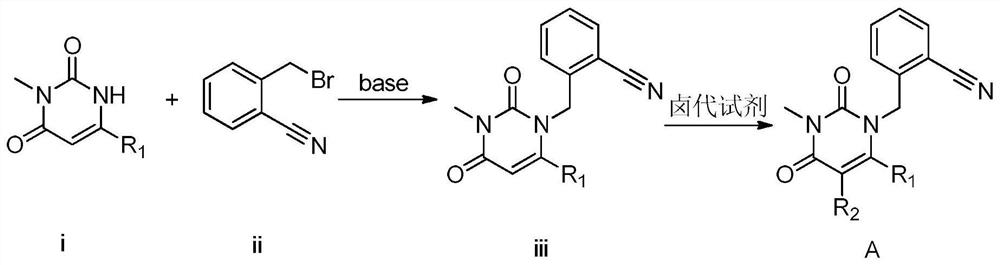
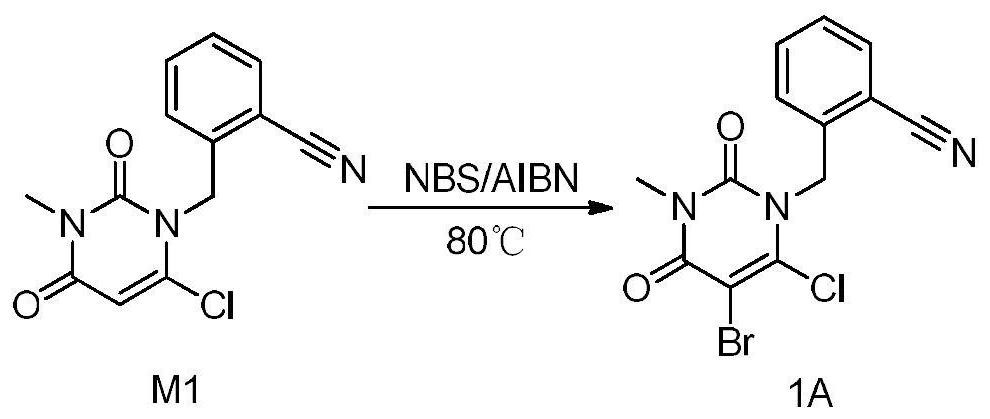
The invention provides a method for preparing 2-[(6-chloro-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl]-4-fluorobenzonitrile, and belongs to the technical field of pharmacy. The method comprises: performing bromination reaction on 4-fluoro-2-methylbenzonitrile and a bromination reagent to obtain a first product, then reacting the first product with 6-chloro-3-methyluracil in an alkali and a solvent for a period, then adding a catalyst, and performing reaction and post-processing, so as to obtain the target product. The catalyst is selected from one or more of diethyl phosphite, dimethyl phosphite and diphenyl phosphite. The provided method does not need to separate and purify 2-bromomethyl-4-fluorobenzonitrile, and is capable of obtaining the high-purity product at a high yield by adding the catalyst, and is low in cost and suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
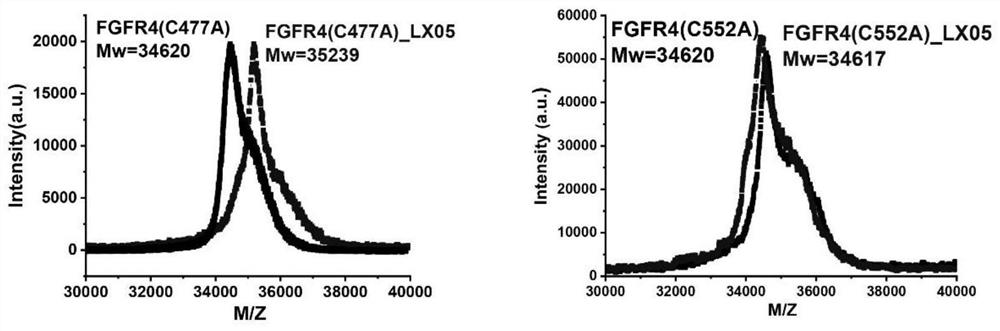
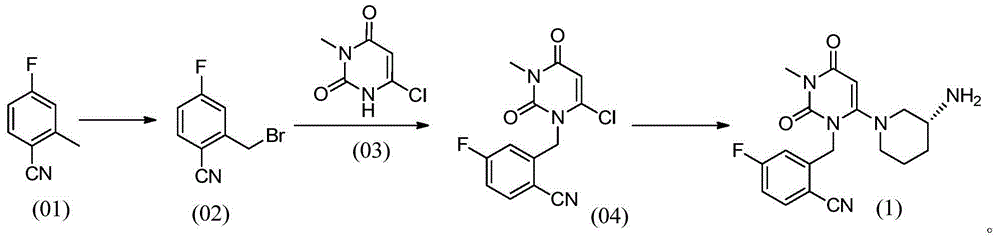
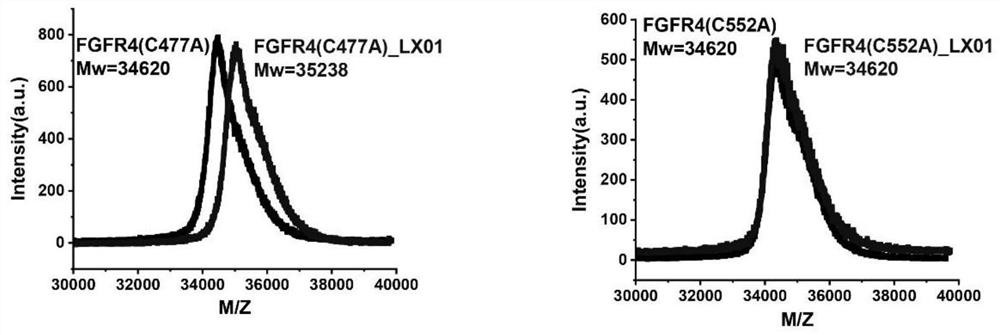
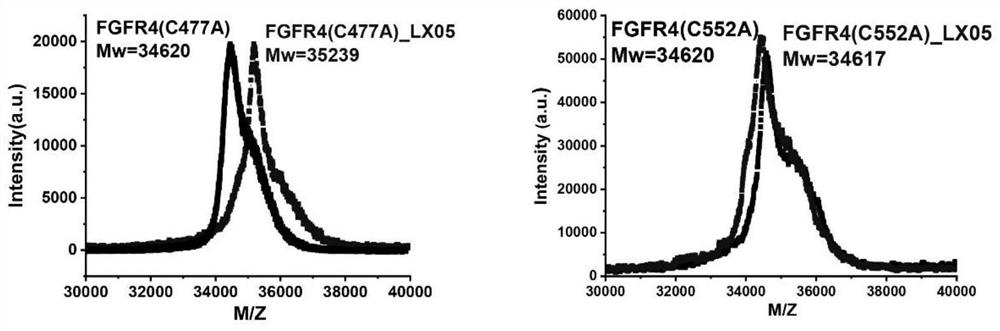
FGFR4 inhibitor, composition and use thereof in drug preparation
The invention provides an FGFR4 inhibitor which takes 3,4-dihydropyrimidine[4,5-d]pyrimidine-2(1H)-ketone as a mother nucleus and has a covalent structure. According to the embodiment, nine specific compounds are provided, kinase inhibitory activity tests are carried out on the nine compounds, the semi-inhibitory concentration of LX08 on FGFR4 kinase inhibitory activity is only 7 nM, the semi-inhibitory concentration is lower than that of active control FIN-2, and thus LX08 has potential application prospects. Besides, through MALDI-TOF mass spectrum combination experiments on the synthesized compounds, it is found that compounds such as LX01, LX05, LX06, LX07 and LX08 can only be covalently combined with Cys552 in the FGFR4 and cannot be covalently combined with Cys477 in the FGFR4, and LX09 is an FGFR4 inhibitor which can be covalently combined with two cysteine Cys552 and Cys477 in the FGFR4.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
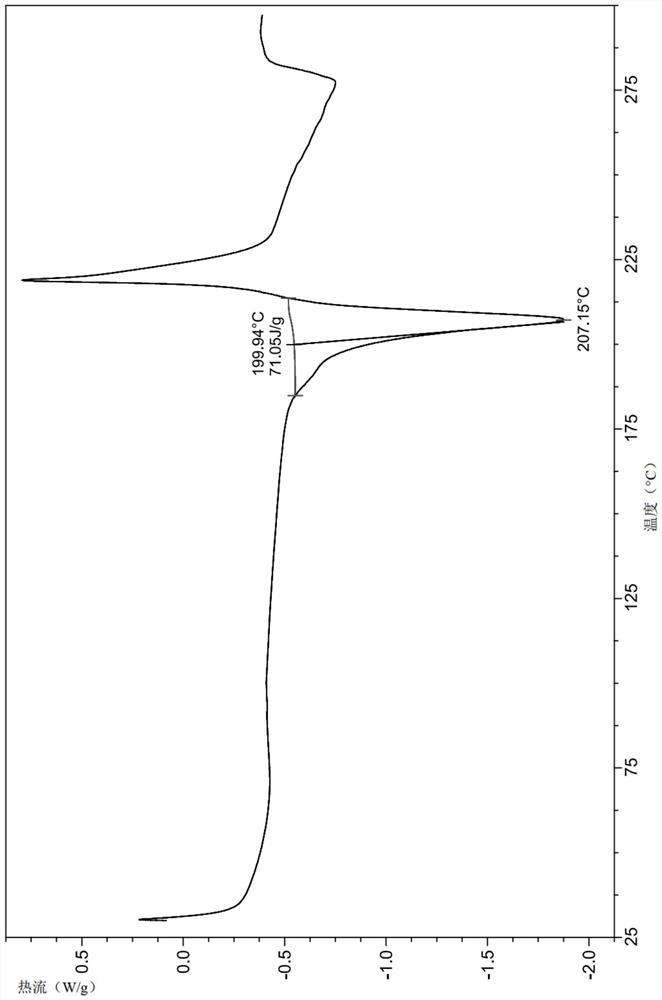
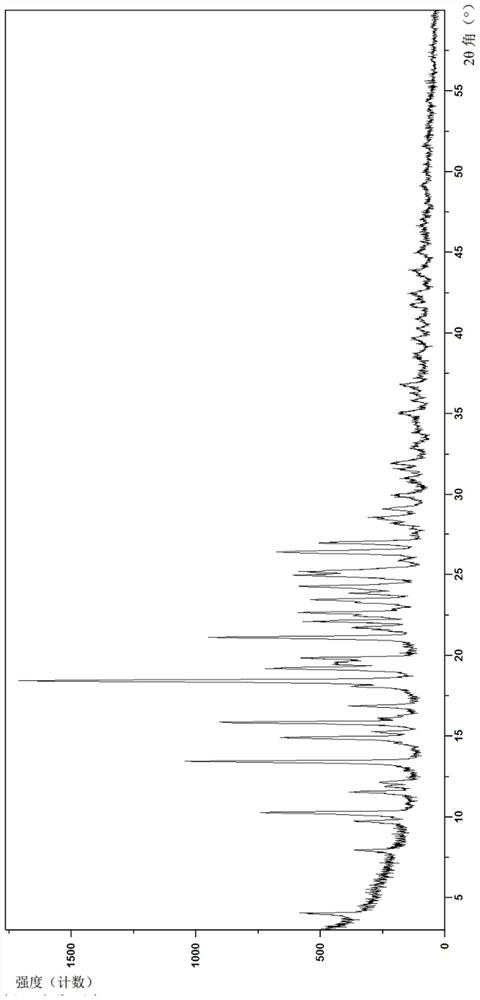
Crystal form of dihydropyrimidine derivative, preparation method of crystal form and application of crystal form in medicine
ActiveCN114702497AImprove stabilityGood pharmacokinetic propertiesOrganic active ingredientsOrganic chemistry methodsHigh humidityDihydropyrimidinuria
The invention discloses a crystal form of a dihydropyrimidine derivative, a preparation method of the crystal form and application of the crystal form in medicines. Specifically, the invention relates to a crystal form A-1 or a crystal form B-1 having a compound represented by a formula (I) or a formula (Ia), a preparation method thereof and an application of the crystal form A-1 or the crystal form B-1 in drugs, and the crystal form A-1 and the crystal form B-1 have high stability under high temperature, high humidity and illumination conditions, and have good pharmacokinetic properties in beagle bodies.
Owner:SOUTH CHINA UNIV OF TECH
Dihydropyrimidine compounds and their application in pharmaceuticals
ActiveUS9340538B2Avoid infectionReduce severityOrganic active ingredientsBiocideDiseaseDihydropyrimidinuria
Owner:RUYUAN WEI XIANG TECH CO LTD
Dihydropyrimidine derivatives and uses thereof in the treatment of hbv infection or of hbv-induced diseases
The application describes dihydropyrimidine derivatives which are useful in the treatment or prevention of HBV infection or of HBV-induced diseases, more particularly of HBV chronic infection or of diseases induced by HBV chronic infection, as well as pharmaceutical or medical applications thereof.
Owner:JANSSEN SCI IRELAND UC
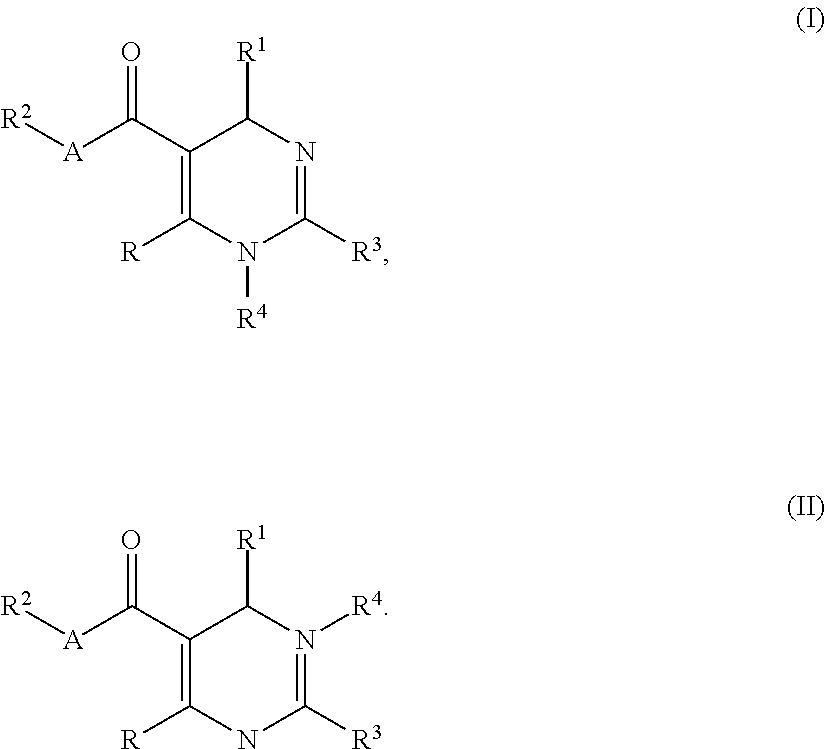
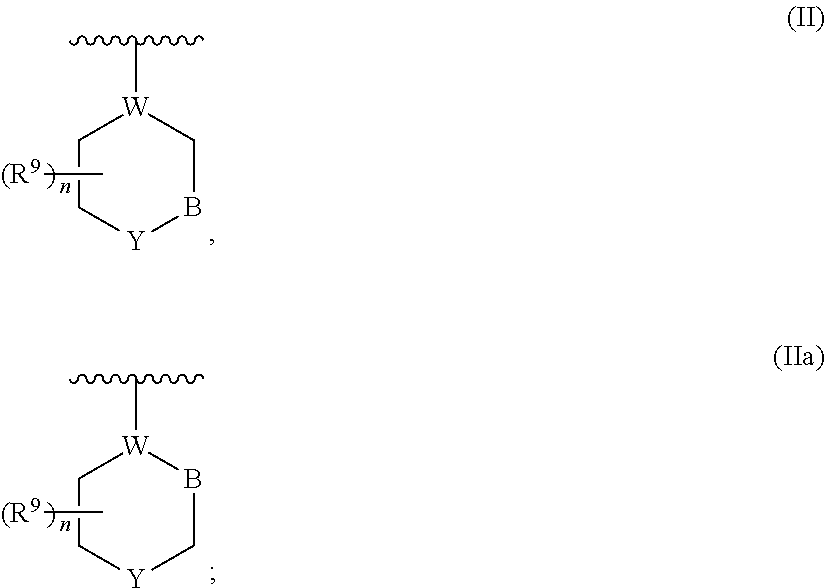
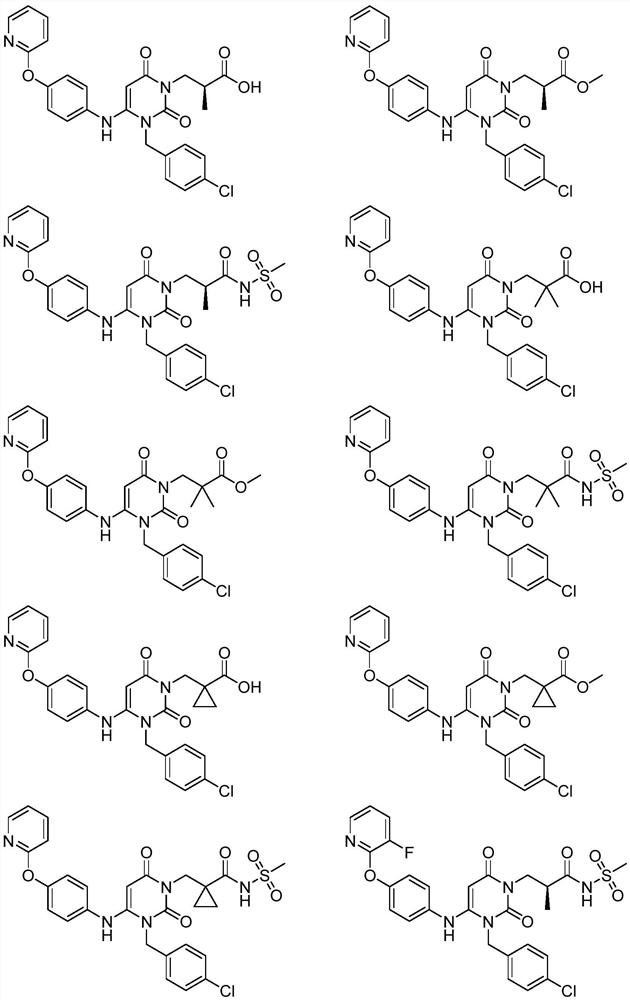
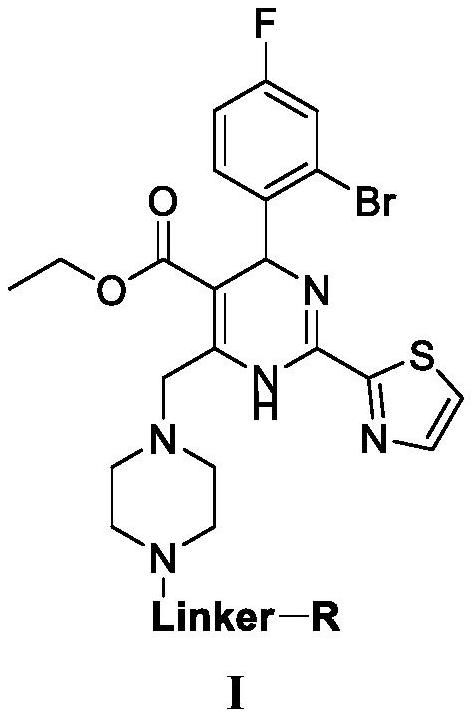
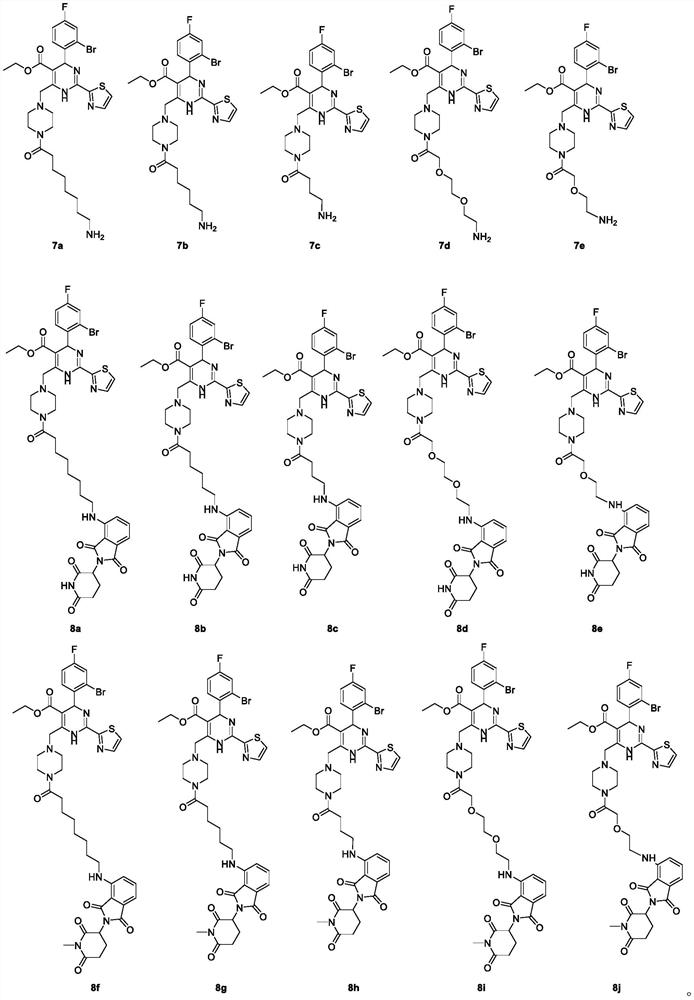
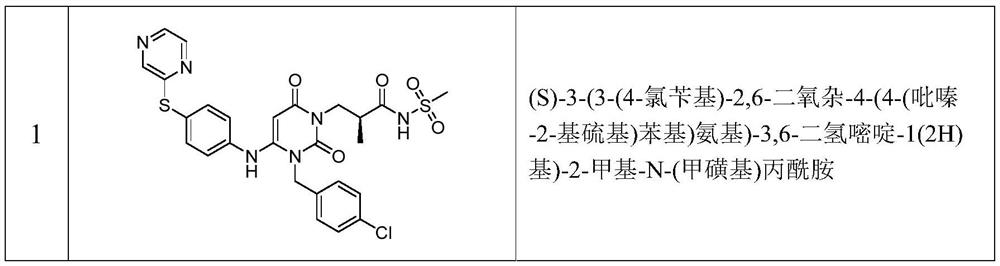
Urea compounds containing 3,4-dihydropyrimido[4,5-d]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors
ActiveUS20180065970A1Excellent ability to inhibit activityStrong inhibitory activityOrganic active ingredientsOrganic chemistryPTK InhibitorsStereochemistry
Owner:KOREA INST OF SCI & TECH
Acid addition salt of dihydropyrimidine derivative and application of acid addition salt in medicine
ActiveCN114702498AImprove stabilityGood pharmacokinetic propertiesOrganic active ingredientsOrganic chemistry methodsHydrobromidePhosphate crystals
The invention discloses an acid addition salt of a dihydropyrimidine derivative and application of the acid addition salt in medicines. The invention particularly relates to a hydrochloride crystal form A, a hydrochloride crystal form B, a sulfate crystal form A, a hydrobromide crystal form A, a phosphate crystal form A, a mesylate crystal form A or a mesylate crystal form B of a compound as shown in a formula (I) or a formula (Ia) and application of the hydrochloride crystal form A, the hydrochloride crystal form B, the sulfate crystal form A, the hydrobromide crystal form A, the phosphate crystal form A, the mesylate crystal form A or the mesylate crystal form B in medicines. The salts provided by the invention have better stability under the conditions of high temperature, high humidity and illumination, and have good pharmacokinetic properties in beagle bodies.
Owner:SOUTH CHINA UNIV OF TECH
3, 4-dihydropyrimidine benzonitrile derivative and preparation method and application thereof
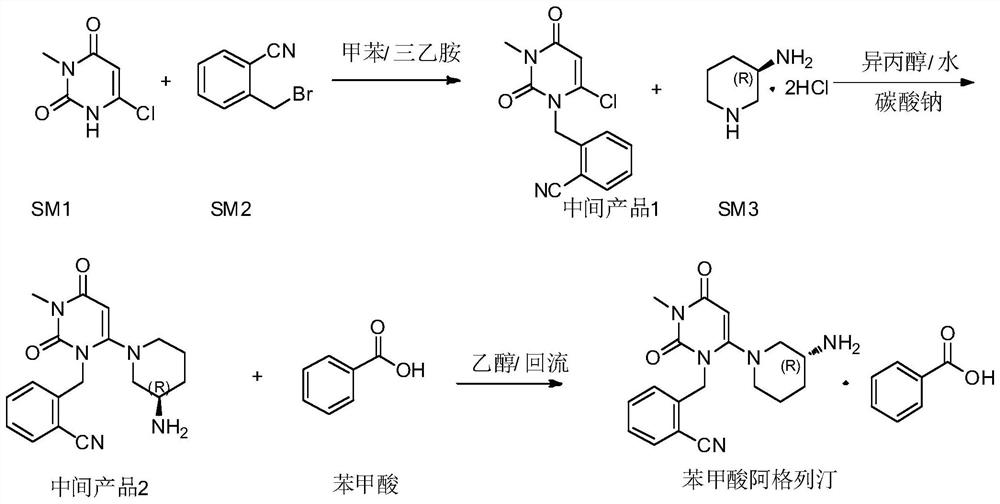
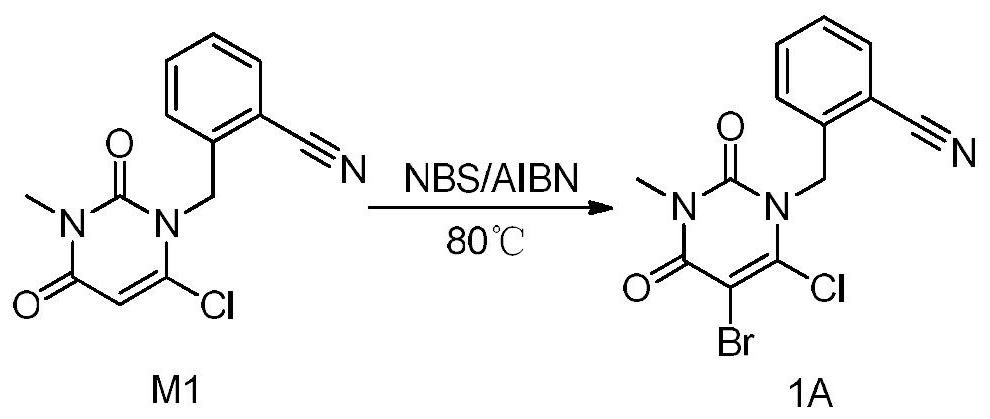
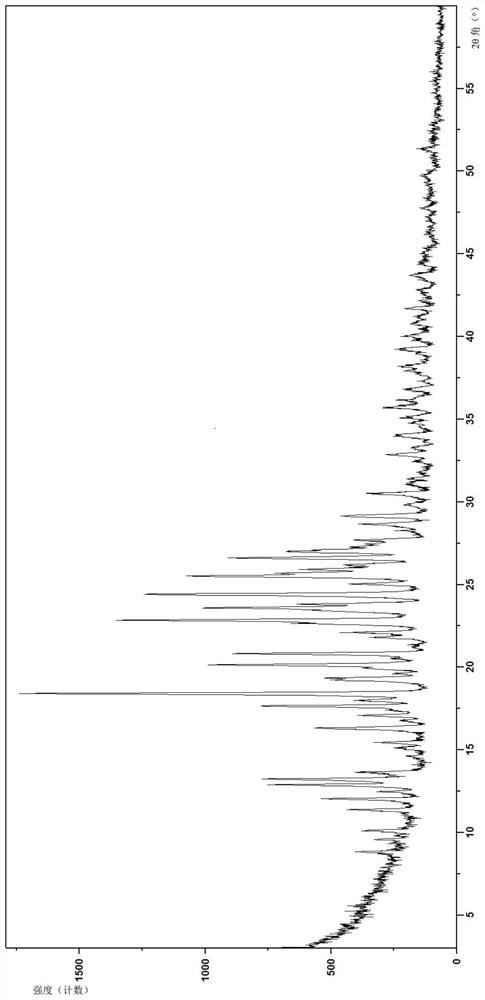
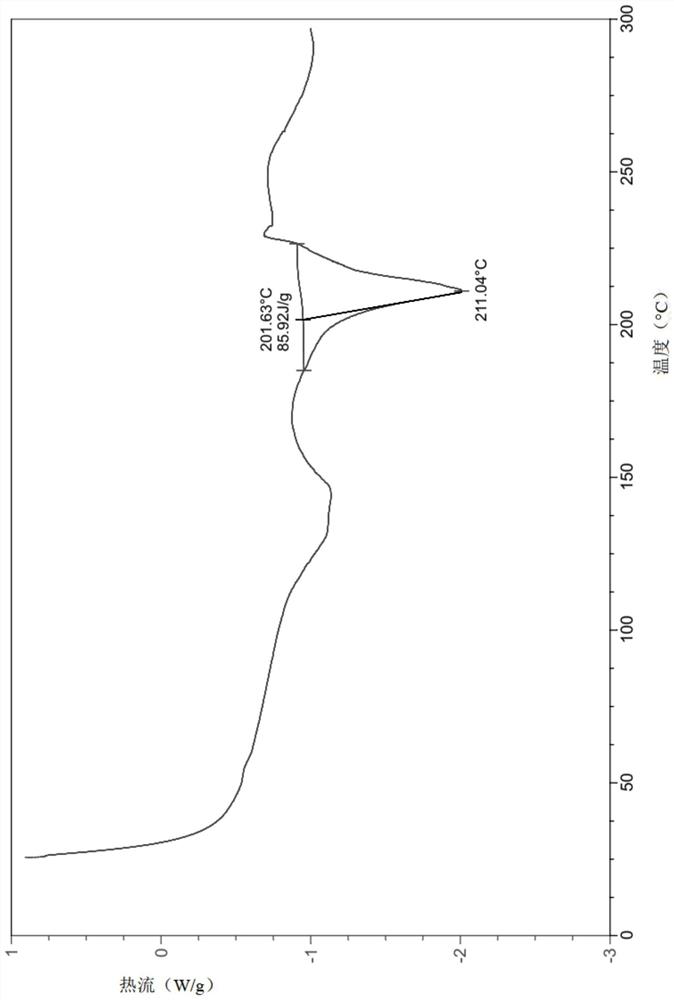
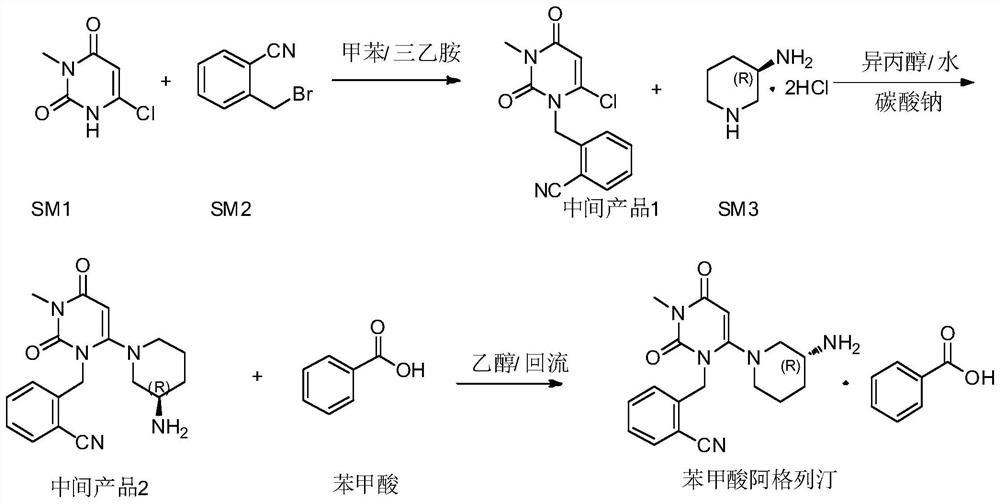
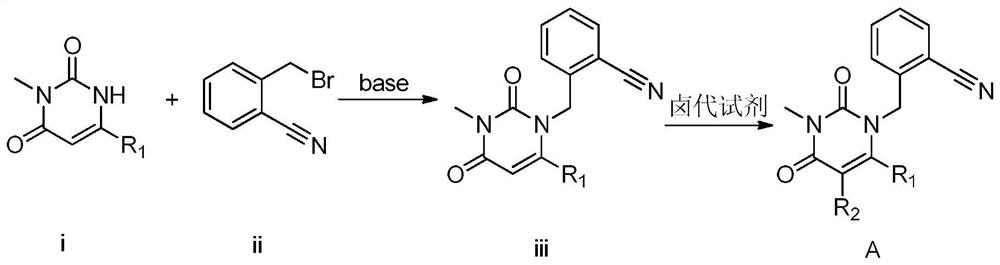
InactiveCN112480013AAchieve quality controlOrganic chemistryComponent separationBenzoic acidDihydropyrimidinuria
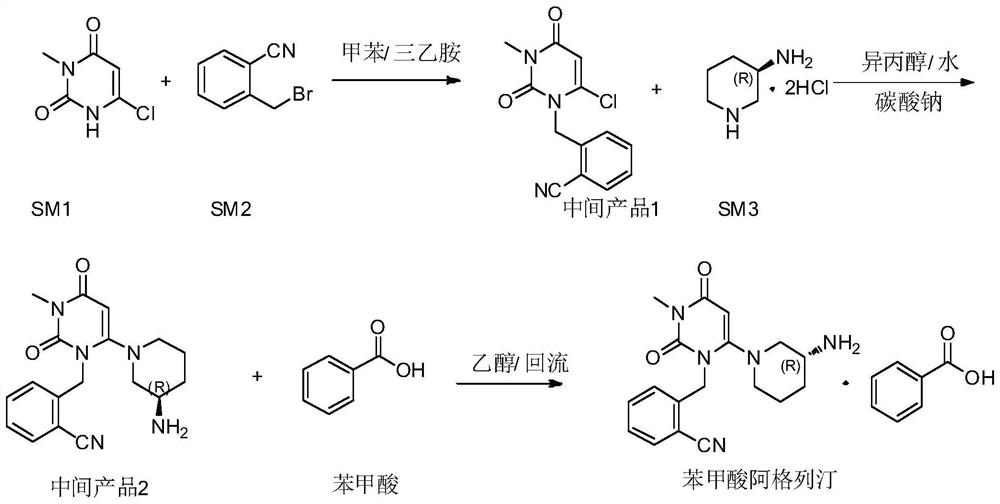
The invention provides a 3, 4-dihydropyrimidine benzonitrile derivative and a preparation method and application thereof. The invention discloses a structure of a specific impurity (RRT=1.22 impurity)generated in the process of preparing alogliptin benzoate by adopting the route of the original research patent for the first time. The series of impurities have a warning structure, and the contentof the impurities in the alogliptin benzoate finished product needs to be detected according to related limits of the genetically toxic impurities. The invention further provides a preparation and purification method of the high-purity compound shown in the formula (A) and a detection method of the content of the impurity compound in the alogliptin benzoate medicine, and therefore quality controlover the alogliptin benzoate medicine is achieved.
Owner:湖南千金湘江药业股份有限公司 +1
Crystal forms, salts and complexes of dihydropyrimidine derivatives and their application in medicine
ActiveCN107400125BOrganic active ingredientsOrganic chemistry methodsPropanoic acidDihydropyrimidinuria
Disclosed are salts of dihydropyrimidine derivative and uses thereof in medicine. Specifically, it relates to citric acid complex, acid addition salt of 3-((R)-4-(((R)-6-(2-bromo-4-fluorophenyl)-5-(ethoxycarbonyl)-2-(thiazol-2-yl)-3,6-dihydropyrimidin-4-yl)methyl)morpholin-2-yl)propanoic acid or tautomer thereof, or crystalline form thereof, and pharmaceutically compositions thereof.
Owner:SUNSHINE LAKE PHARM CO LTD
3, 4-dihydropyrimidine benzonitrile derivative as well as preparation method and application thereof
InactiveCN112480074AAchieve quality controlOrganic chemistryComponent separationBenzoic acidDihydropyrimidinuria
The invention provides a 3, 4-dihydropyrimidine benzonitrile derivative as well as a preparation method and application thereof. The invention discloses a structure of a specific impurity (RRT is an impurity of 1.22) generated in the process of preparing alogliptin benzoate by adopting the route of the original research patent for the first time. The series of impurities have a warning structure,and the content of the impurities in the alogliptin benzoate finished product needs to be detected according to related limits of the genetically toxic impurities. The invention further provides a preparation and purification method of the high-purity compound shown in the formula (A) and a detection method of the content of the impurity compound in the alogliptin benzoate medicine, and thereforequality control over the alogliptin benzoate medicine is achieved.
Owner:湖南千金湘江药业股份有限公司 +1
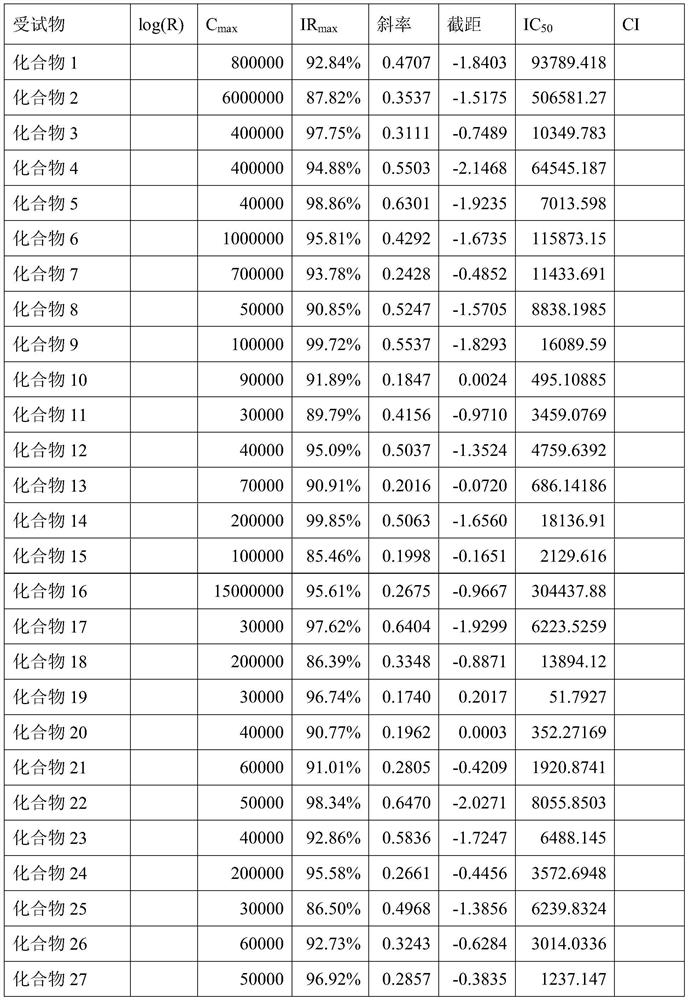
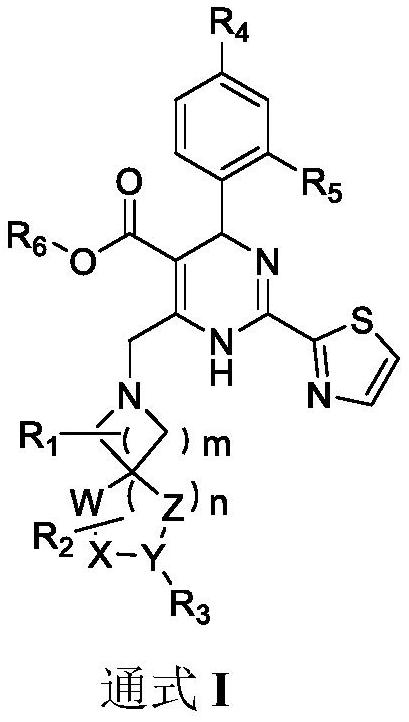
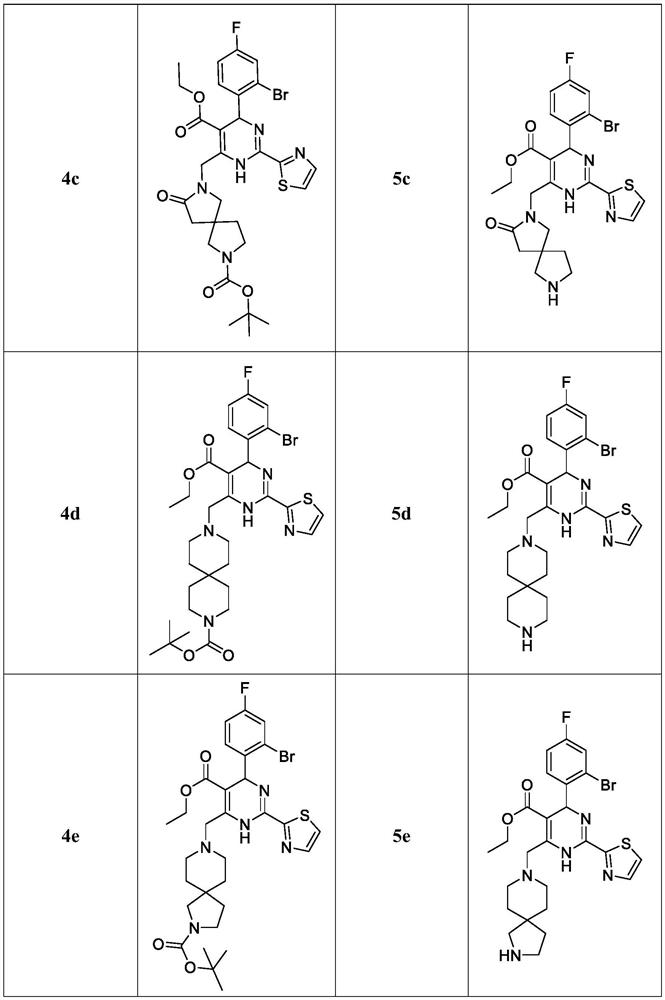
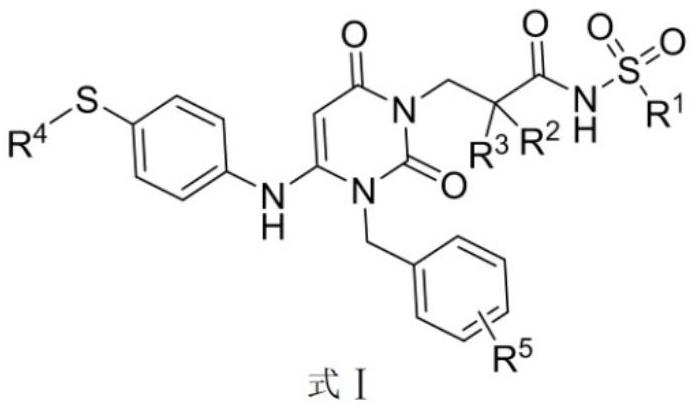
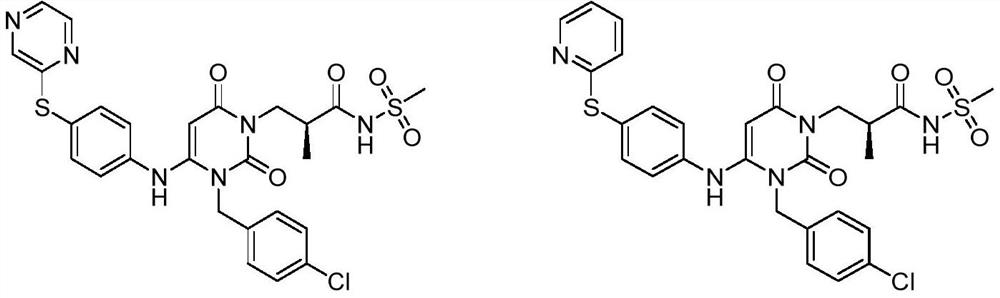
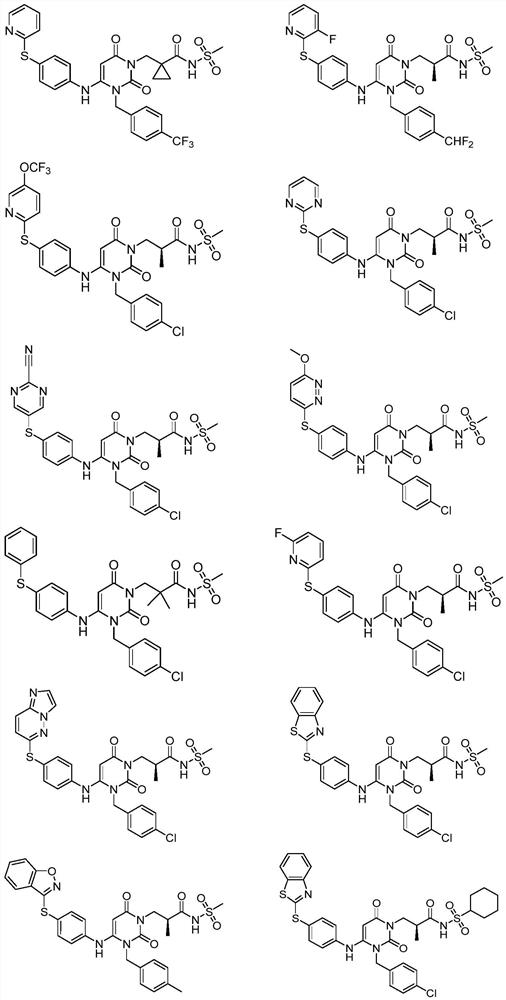
Dihydropyrimidine-sulfonamide derivatives, preparation method and application thereof
ActiveCN108947996BOrganic active ingredientsOrganic chemistryDihydropyrimidinuriaCombinatorial chemistry
Owner:SHANDONG UNIV
Dihydropyrimidine compounds and uses thereof in medicine
ActiveUS11142527B2Improved pharmacokinetic propertiesInducing effectOrganic active ingredientsGroup 5/15 element organic compoundsDiseaseDihydropyrimidinuria
A dihydropyrimidine compound and a pharmaceutical application thereof, especially the application used for treating and preventing HBV diseases. Specifically, a compound having Formula (I) or (Ia), or an enantiomer, a diastereoisomer, a tautomer, a hydrate, a solvate, or a pharmaceutically acceptable salt thereof, wherein the variables of the formulas are as defined in the specification. Also, use of the compound having Formula (I) or (Ia), or an enantiomer, a diastereoisomer, a tautomer, a hydrate, a solvate, or a pharmaceutically acceptable salt thereof as a medicine, especially for treating and preventing HBV diseases.
Owner:SUNSHINE LAKE PHARM CO LTD
Solid form of dihydropyrimidine compound and preparation method therefor and use thereof
ActiveUS11434235B2Good effectGood physical and chemical stabilityOrganic active ingredientsOrganic chemistry methodsDiseasePolymer science
Disclosed are a solid form of (E)-3-((R)-4-(((R)-6-(2-chloro-4-fluorophenyl)-5-(methoxycarbonyl)-2-(thiazol-2-yl)-3,6-dihydropyrimidin-4-yl)methyl)morpholin-2-yl)acrylic acid, a preparation method therefor, a pharmaceutical composition comprising same, and the use thereof in the preparation of drugs for preventing or treating viral diseases.
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
Dihydropyrimidine-triazole derivatives, preparation method and application thereof
ActiveCN107501257BOrganic active ingredientsOrganic chemistryDihydropyrimidinuriaTriazole derivatives
The invention discloses a dihydropyrimidine-triazole derivative, a preparation method therefor and an application of the dihydropyrimidine-triazole derivative. The compound has a structure represented by a formula I shown in the description. The invention further relates to the preparation method for the compound with the structure represented by the formula I and a pharmaceutical composition and provides the application of the compound in preparation of anti-HBV drugs.
Owner:SHANDONG UNIV
Dihydropyrimidine derivatives and uses thereof in treatment of HBV infection or of HBV-induced diseases
Owner:JANSSEN SCI IRELAND UC
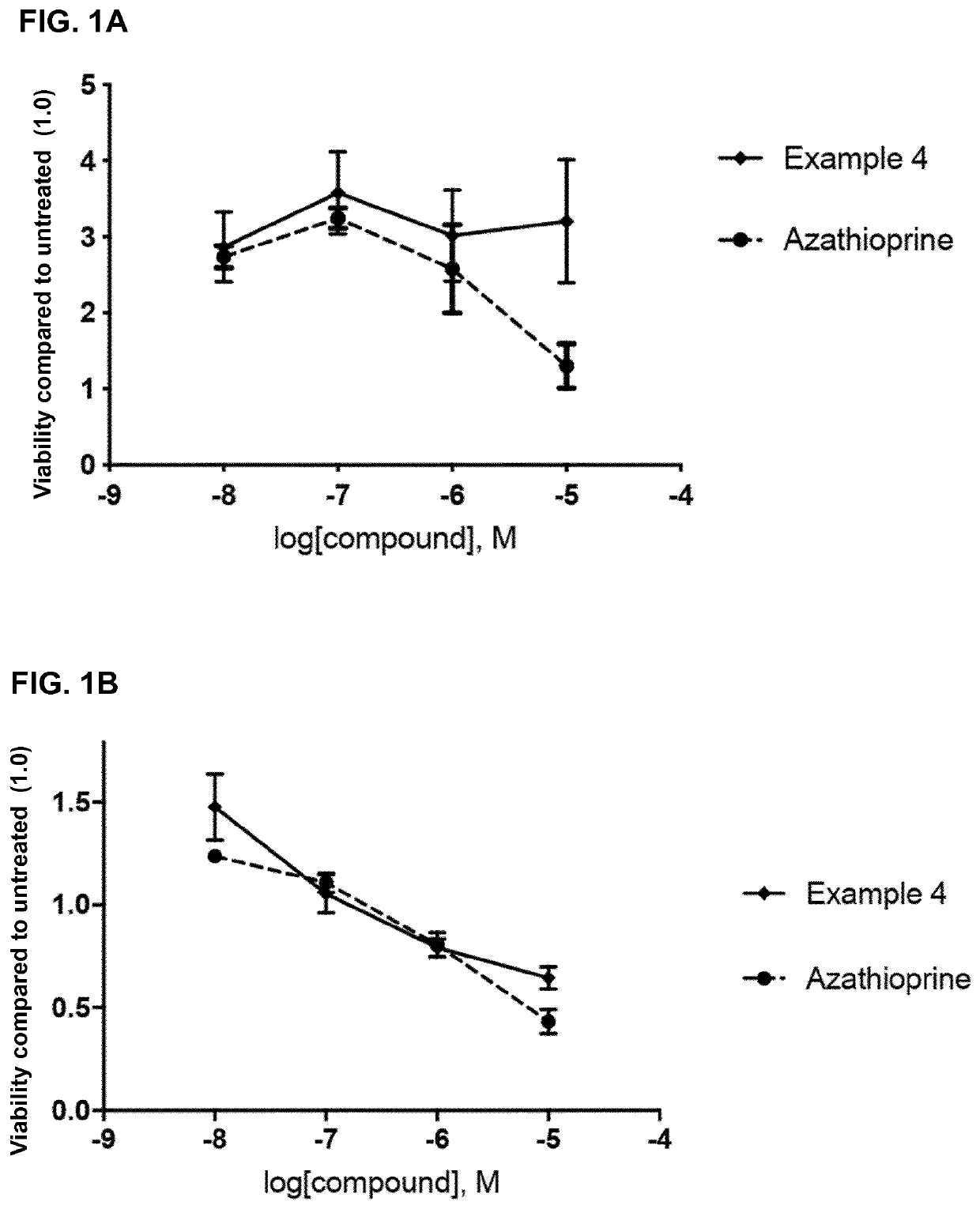
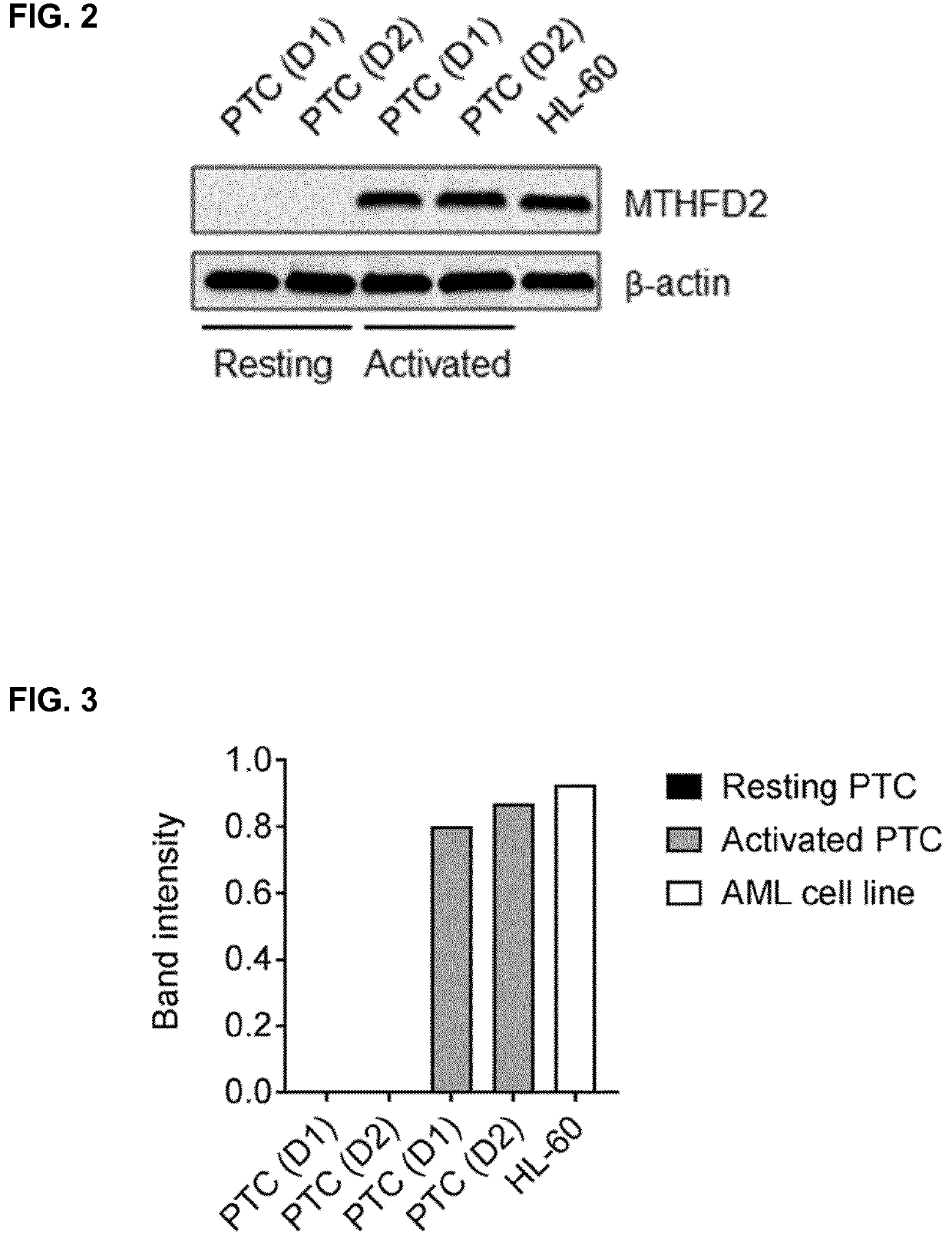
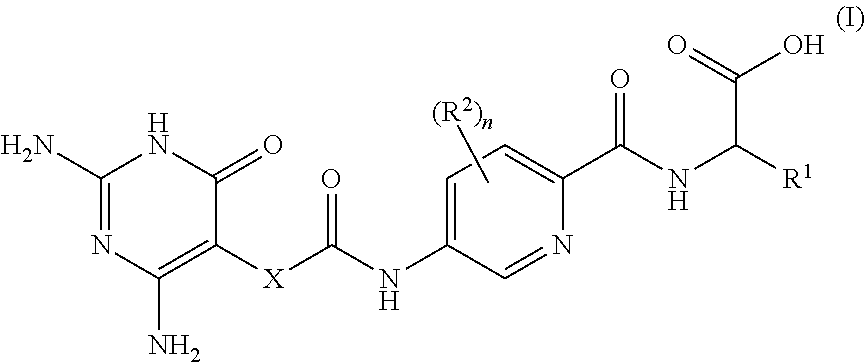
2,6-diamino-3,4-dihydropyrimidin-4-one derivatives and use thereof in therapy
ActiveUS20210052587A1Effective inhibitorOrganic active ingredientsOrganic chemistryImmunologic disordersAutoimmune condition
A compound of formula (I) or a pharmaceutically acceptable salt thereof. The compound is useful in therapy, e.g. for the treatment of cancers, inflammation, autoimmune diseases and graft-versus host diseases (e.g. in transplantation patients). A pharmaceutical composition comprising the compound or its salt and a method for preparing the compound.
Owner:MEDICINSK FORSKNING
Dihydropyrimidine-pomalidomide conjugate as well as preparation method and application thereof
PendingCN113512035AOrganic active ingredientsOrganic chemistryDihydropyrimidinuriaCombinatorial chemistry
The invention provides a dihydropyrimidine-pomalidomide conjugate as well as a preparation method and application thereof. The conjugate has a structure as shown in a formula I. The invention further relates to a preparation method of the compound with the structure as shown in the formula I, a pharmaceutical composition and application of the compound in preparation of anti-HBV drugs.
Owner:SHANDONG UNIV
Dihydropyrimidine compounds and their preparation and use
ActiveCN113666914BHigh affinityProlonged cough suppressant actionOrganic active ingredientsOrganic chemistryDiseaseMetabolite
The invention discloses a dihydropyrimidine compound, a preparation method and an application thereof, and belongs to the technical field of medicinal chemistry. The structure of the dihydropyrimidine compounds provided by the present invention is shown in Formula I. The invention also discloses a preparation method of the dihydropyrimidine compound. The present invention provides the application of the compound represented by formula I or its salt, solvate, isomer, metabolite, nitrogen oxide and prodrug in the preparation of medicines for treating or preventing P2X3 and / or / P2X2 / 3 receptor-related diseases . The antitussive action time of the compound of the present invention is significantly longer than that of the compound of Comparative Example 1; the inhibitory activity to P2X3 is better than that of the compound of Comparative Example 1 and the positive control drug gefapixant, and it has almost no effect on the taste of mice under 10mg / kg intravenous administration, which is comparable to that of the compound of Comparative Example 1. The positive control drug gefapixant had a statistically significant difference.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
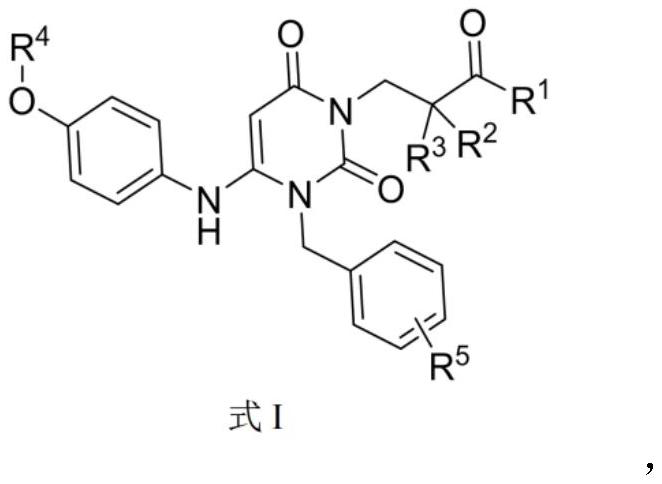
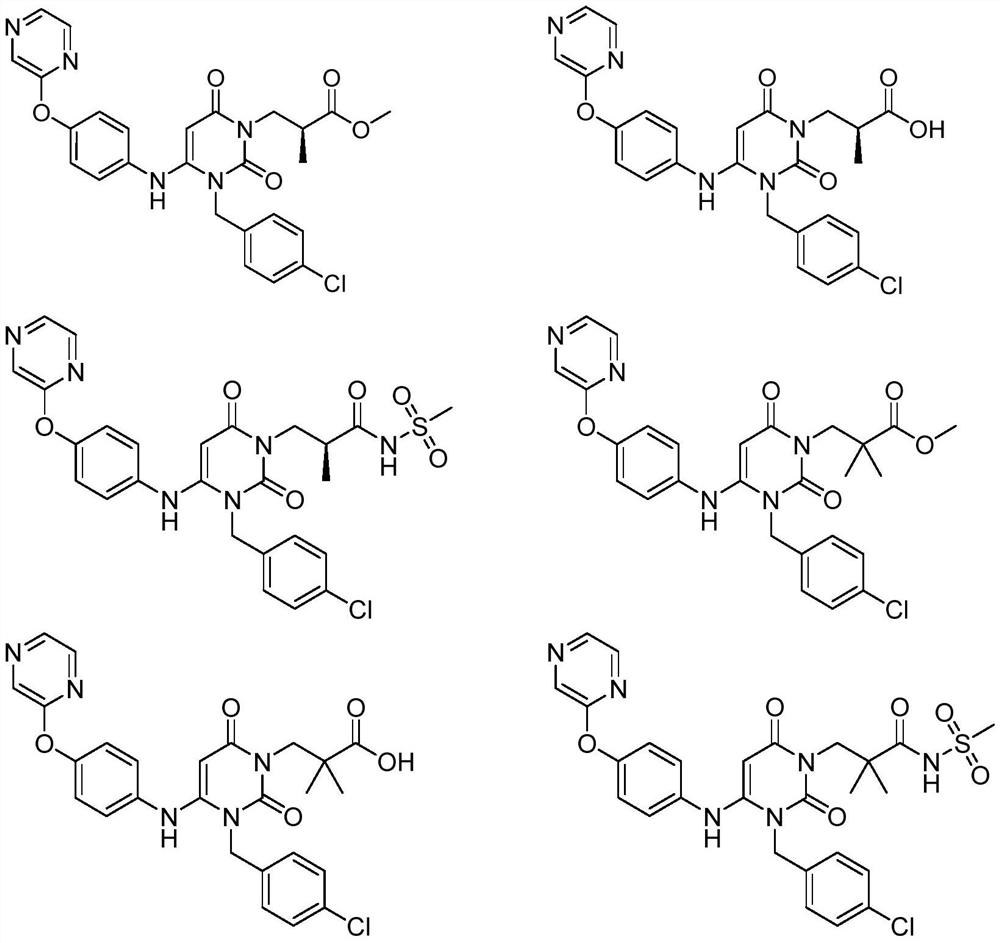
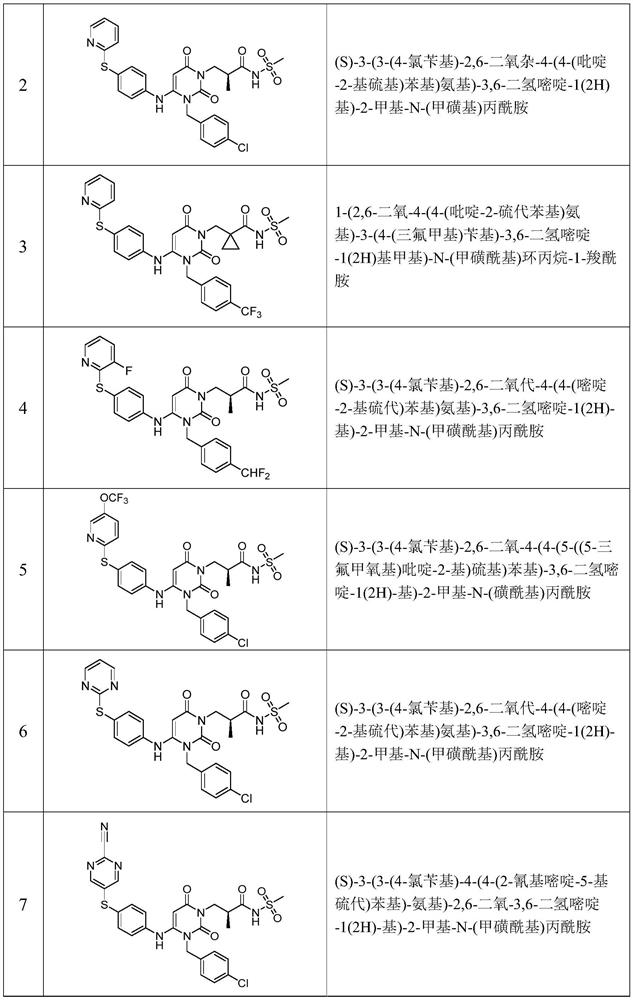
Urea compounds containing 3,4-dihydropyrimido[4,5-D]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors
ActiveUS10059717B2Excellent ability to inhibit activityStrong inhibitory activityOrganic active ingredientsOrganic chemistryPTK InhibitorsTyrosine-kinase inhibitor
Owner:KOREA INST OF SCI & TECH
Composition containing orlistat and dihydropyrimidine compounds and use thereof
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Dihydropyrimidine-spiro derivatives, preparation method and application thereof
ActiveCN113135921BOrganic active ingredientsOrganic chemistryDihydropyrimidinuriaCombinatorial chemistry
Owner:SHANDONG UNIV
2,6-diamino-3,4-dihydropyrimidin-4-one derivatives and use thereof in therapy
ActiveUS11504368B2Effective inhibitorOrganic active ingredientsOrganic chemistryImmunologic disordersAutoimmune condition
Owner:MEDICINSK FORSKNING
3, 4-dihydropyrimidine benzonitrile derivative as well as preparation method and application thereof
PendingCN112500356AAchieve quality controlOrganic chemistryComponent separationBenzoic acidDihydropyrimidinuria
The invention provides a 3,4-dihydropyrimidine benzonitrile derivative as well as a preparation method and application thereof. The invention discloses a structure of a specific impurity (RRT is an impurity of 1.22) generated in the process of preparing alogliptin benzoate by adopting the route of the original research patent for the first time. The series of impurities have a warning structure, and the content of the impurities in an alogliptin benzoate finished product needs to be detected according to related limits of the genetically toxic impurities. The invention further provides a preparation and purification method of the high-purity compound shown in the formula (A) and a detection method of the content of the impurity compound in the alogliptin benzoate medicine, and therefore quality control over the alogliptin benzoate medicine is achieved.
Owner:湖南千金湘江药业股份有限公司 +1
fgfr4 inhibitor, composition and use in pharmaceutical preparation
ActiveCN113527311BOrganic active ingredientsOrganic chemistryDihydropyrimidinuriaMass Spectrometry-Mass Spectrometry
The present invention proposes a FGFR4 inhibitor with 3,4-dihydropyrimidin[4,5-d]pyrimidin-2(1H)-one as the core and a covalent structure. The embodiment gives 9 specific compounds and tested the kinase inhibitory activity of these 9 compounds, wherein the half-inhibitory concentration of LX08 to the FGFR4 kinase inhibitory activity is only 7nM, which is lower than the FIIN-2 of the active control, and has potential application prospects . In addition, by performing MALDI-TOF mass spectrometry binding experiments on the synthesized compounds, we found that compounds such as LX01, LX05, LX06, LX07, and LX08 can only covalently bind to Cys552 in FGFR4, but cannot covalently bind to Cys477 in FGFR4, while LX09 is a FGFR4 inhibitor that can covalently bind to two cysteines Cys552 and Cys477 in FGFR4.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![Urea compounds containing 3,4-dihydropyrimido[4,5-d]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors Urea compounds containing 3,4-dihydropyrimido[4,5-d]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/67df311f-b49a-4075-b5fa-508165c18594/US20180065970A1-20180308-D00000.png)
![Urea compounds containing 3,4-dihydropyrimido[4,5-d]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors Urea compounds containing 3,4-dihydropyrimido[4,5-d]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/67df311f-b49a-4075-b5fa-508165c18594/US20180065970A1-20180308-D00001.png)
![Urea compounds containing 3,4-dihydropyrimido[4,5-d]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors Urea compounds containing 3,4-dihydropyrimido[4,5-d]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/67df311f-b49a-4075-b5fa-508165c18594/US20180065970A1-20180308-C00001.png)













![Urea compounds containing 3,4-dihydropyrimido[4,5-D]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors Urea compounds containing 3,4-dihydropyrimido[4,5-D]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7c06d6b5-520a-4b98-92b9-a6885ec43ad2/US10059717-D00001.png)
![Urea compounds containing 3,4-dihydropyrimido[4,5-D]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors Urea compounds containing 3,4-dihydropyrimido[4,5-D]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7c06d6b5-520a-4b98-92b9-a6885ec43ad2/US10059717-C00001.png)
![Urea compounds containing 3,4-dihydropyrimido[4,5-D]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors Urea compounds containing 3,4-dihydropyrimido[4,5-D]pyrimidin-2(1H)-one skeleton as protein kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7c06d6b5-520a-4b98-92b9-a6885ec43ad2/US10059717-C00002.png)