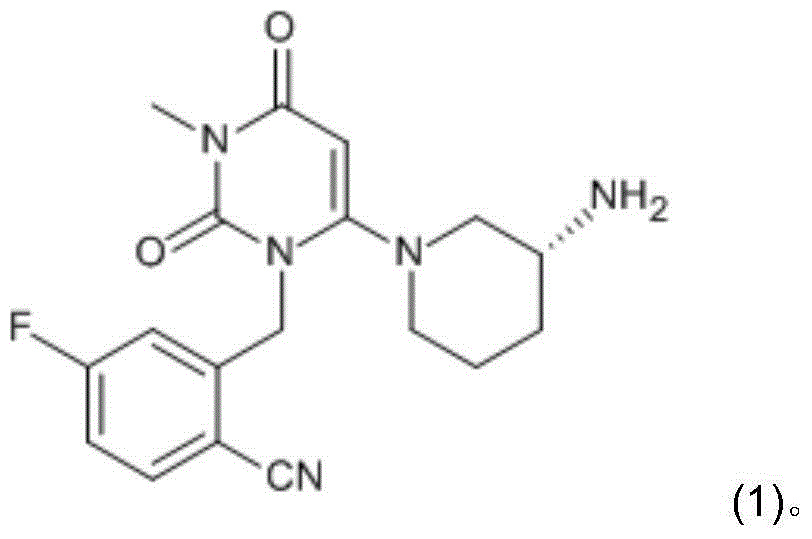
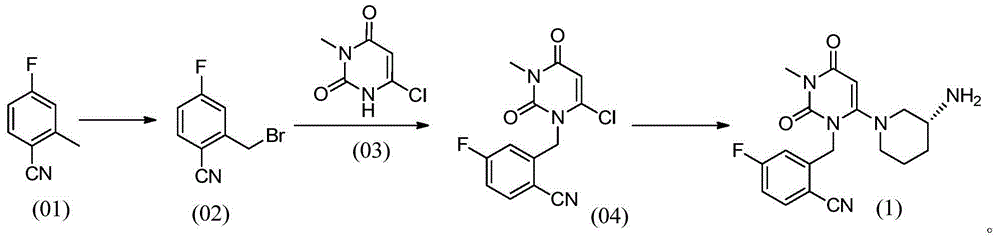
Preparation method for dihydropyrimidine derivative
A technology of dihydropyrimidine and methyluracil, applied in the direction of organic chemistry, can solve the problems of low yield, many impurities, affecting the purity and yield of the final product, etc., and achieve the effect of low cost and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0031] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0032] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0033] In the present invention, g means gram, and mL means milliliter.
Embodiment 1
[0035] Put 1.00kg of 4-fluoro-2-methylbenzonitrile, 1.98kg of N-bromosuccinimide, 0.025kg of azobisisobutyronitrile, and 7.40kg of chloroform into the reaction kettle, and control the temperature at 60°C- Stir and react at 70°C for 3 hours, cool down to 25°C, add 15% sodium sulfate solution (mass concentration) for washing, collect the organic phase and concentrate to dryness to obtain the first product.
[0036] Add 1.18kg of 6-chloro-3-methyluracil, 1.12kg of triethylamine, and 7.40kg of dimethylacetamide to the first product, and stir at a temperature of 65°C-70°C for 1 hour; then add Diethyl phosphite 0.31kg, stirred for 2.5 hours. Then the temperature was lowered to 25° C., 5.00 kg of water was added to the reaction system, and the resulting mixture was stirred for 0.5 hours. Then the mixture was cooled to 0°C-5°C and stirred for 0.5 hours. The mixture was centrifuged, and the resulting solid was dried at 40°C for 12 hours to obtain 1.97 kg of a light yellow solid, namely...
Embodiment 2
[0038] Put 1.00kg of 4-fluoro-2-methylbenzonitrile, 2.35kg of N-bromosuccinimide, 0.050kg of azobisisobutyronitrile, and 9.00kg of chloroform into the reaction kettle, and control the temperature at 65°C- Stir and react at 70°C for 3 hours, cool down to 25°C, add 20% sodium sulfate solution (mass concentration) for washing, collect the organic phase and concentrate to dryness to obtain the first product.
[0039] Add 1.19kg of 6-chloro-3-methyluracil, 1.34kg of triethylamine, and 8.00kg of dimethylacetamide to the first product, and stir at a temperature of 65°C-75°C for 1 hour; then add Dimethyl phosphite 0.45kg, stirred for 2 hours. Then the temperature was lowered to 25° C., 10.00 kg of water was added to the reaction system, and the resulting mixture was stirred for 0.5 hours. Then the mixture was cooled to 0°C-5°C and stirred for 0.5 hours. The mixture was centrifuged, and the resulting solid was dried at 40°C for 12 hours to obtain 1.96 kg of a light yellow solid, name...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com