Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
224results about How to "Good pharmacokinetic properties" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
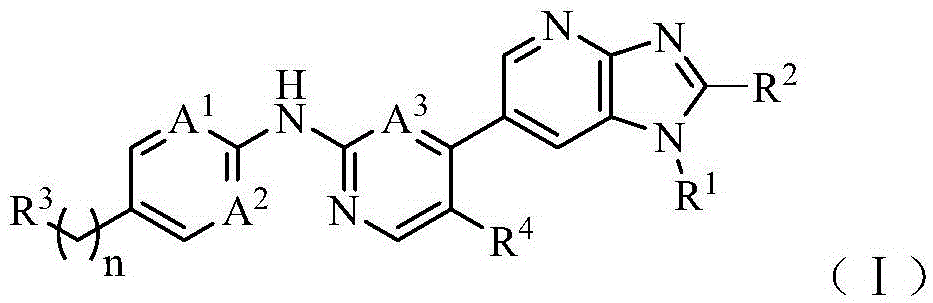
Pyrimidoheterocyclic compound, medicinal composition and application thereof
ActiveCN104418860ASelectiveGood pharmacokinetic propertiesOrganic active ingredientsOrganic chemistryErlotinibMedicine
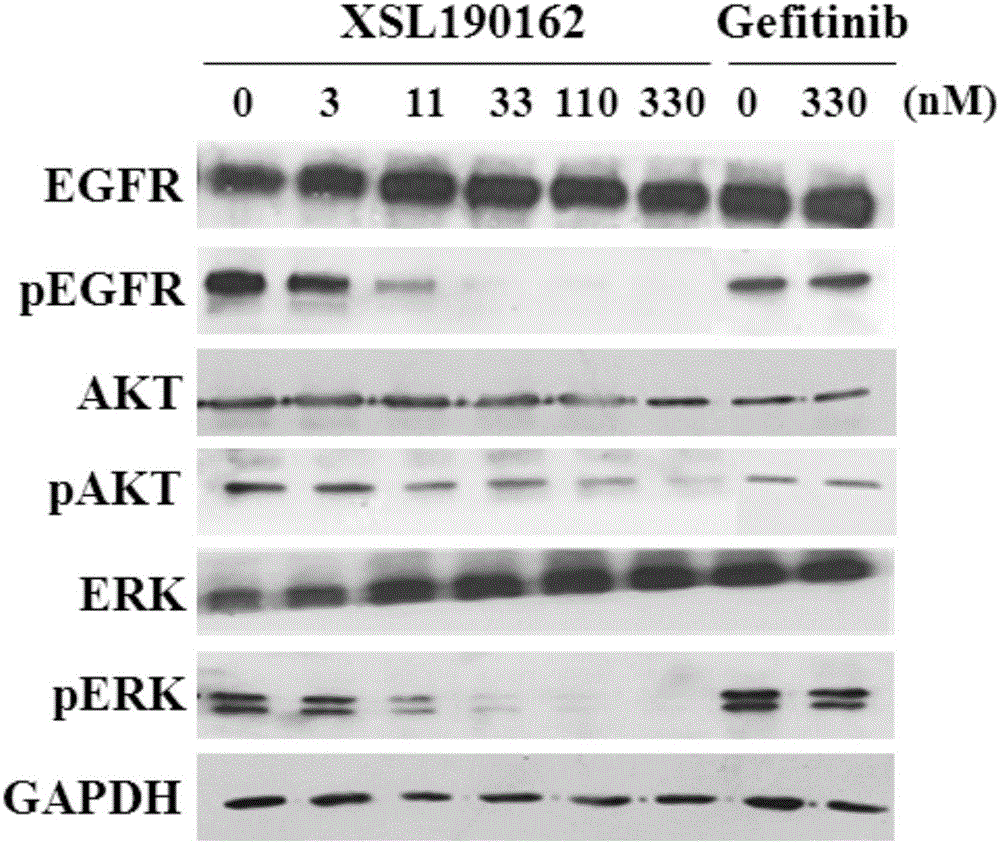
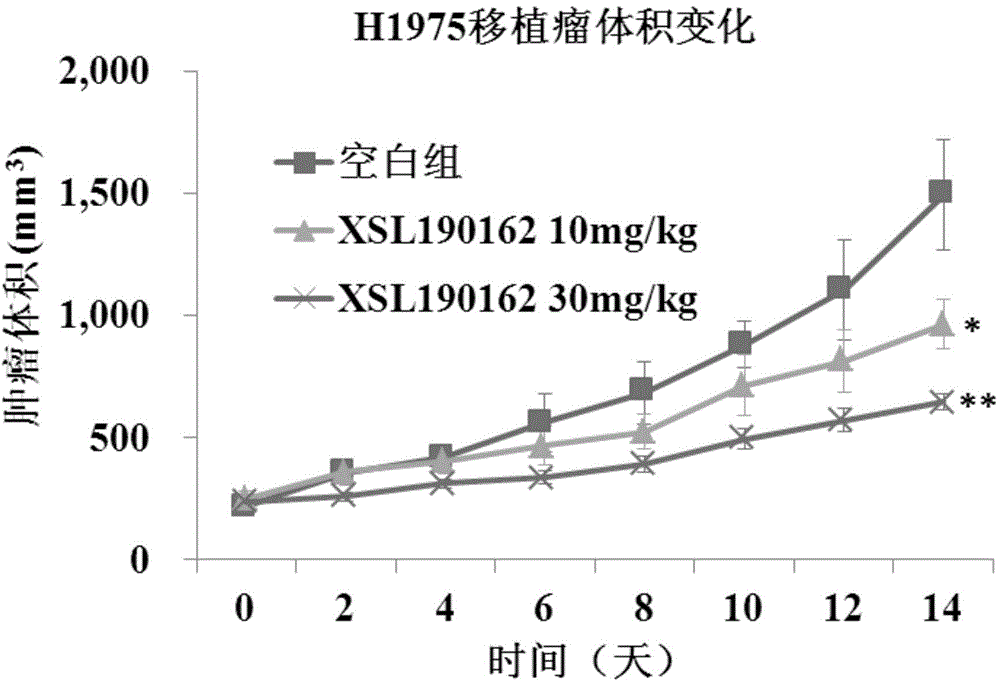
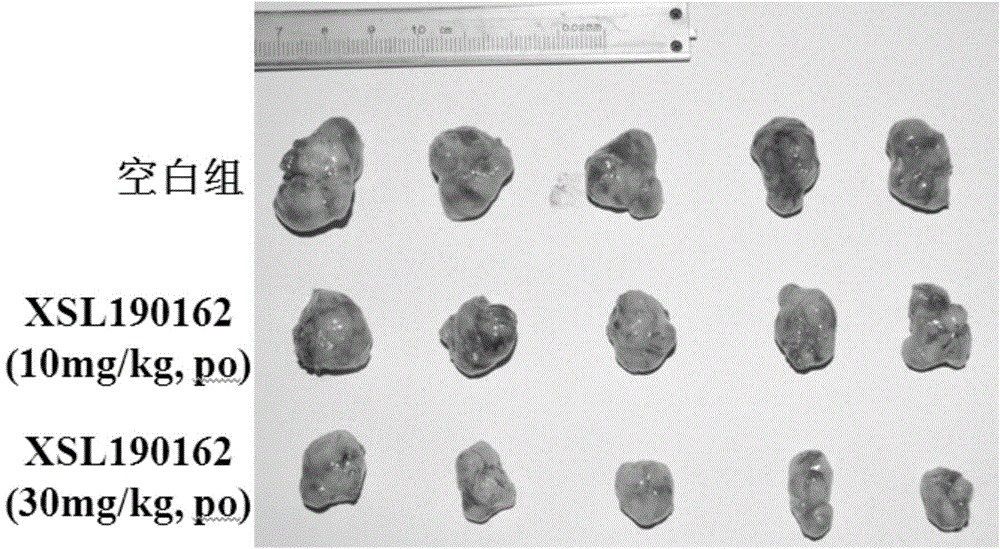
The invention discloses a pyrimidoheterocyclic compound represented as the formula (I), or a pharmaceutically acceptable salt or a stereisomer thereof, or a prodrug molecule thereof. The pyrimidoheterocyclic compound can effectively inhibit growth of various tumor cells and has an inhibiting effect on EGFR protease. The pyrimidoheterocyclic compound can be used for preparing an anti-tumor drug, and can overcome drug resistance caused by medicines in the prior art, such as gefitinib, erlotinib and the like, has a selectivity on wild non-small cell lung cancer and is excellent in pharmacokinetic property.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Caspase inhibitors and uses thereof
InactiveUS20020058630A1Enhanced inhibitory effectGood effectAntibacterial agentsOrganic active ingredientsCaspase inhibitorsAryl
Described herein are compounds that are useful as caspase inhibitors having the formula: wherein Ring A is an optionally substituted piperidine, tetrahydroquinoline or tetrahydroisoquinoline ring; R1 is hydrogen, CN, CHN2, R, or CH2Y; R is an optionally substituted group selected from an aliphatic group, an aryl group, or an aralkyl group; Y is an electronegative leaving group; R2 is CO2H, CH2CO2H, or esters, amides or isosteres thereof; and R3 is hydrogen, an optionally substituted aryl group, an optionally substituted aralkyl group, or an optionally substituted C1-6 aliphatic group, R4 is an optionally substituted group selected from an aryl group or a heterocyclyl group, or R3 and R4 taken together with the nitrogen to which they are attached optionally form a substituted or unsubstituted monocyclic, bicyclic or tricyclic ring.
Owner:VERTEX PHARMA INC
Pyrimidoheterocyclic compound and its pharmaceutical composition and application
ActiveCN104418860BGood pharmacokinetic propertiesSelectiveOrganic active ingredientsOrganic chemistryErlotinibTumor cells
The invention discloses a pyrimidoheterocyclic compound represented as the formula (I), or a pharmaceutically acceptable salt or a stereisomer thereof, or a prodrug molecule thereof. The pyrimidoheterocyclic compound can effectively inhibit growth of various tumor cells and has an inhibiting effect on EGFR protease. The pyrimidoheterocyclic compound can be used for preparing an anti-tumor drug, and can overcome drug resistance caused by medicines in the prior art, such as gefitinib, erlotinib and the like, has a selectivity on wild non-small cell lung cancer and is excellent in pharmacokinetic property.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Compstatin Analogs with Improved Pharmacokinetic Properties
ActiveUS20150158915A1High activityGood pharmacokinetic propertiesAntibacterial agentsSenses disorderChemical compoundPharmaceutical drug
Compounds comprising peptides capable of binding C3 protein and inhibiting complement activation are disclosed. The compounds comprise compstatin analogs in which the N-terminus contains an added or substituted component that improves (1) the peptide's binding affinity to C3 or its fragments, (2) the peptide's solubility in aqueous liquids, (3) the peptide's plasma stability, (4) the peptide's in vivo retention and / or (5) the peptide's bioavailability, as compared with an unmodified compstatin peptide under equivalent conditions. Pharmaceutical compositions and methods of using the compounds are also disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
2-aminopyrimidine compounds as well as pharmaceutical compositions and applications thereof
ActiveCN105601573ANovel structureGrowth inhibitionOrganic chemistryAntineoplastic agentsErlotinibWild type
The invention discloses 2-aminopyrimidine compounds as well as pharmaceutical compositions and applications thereof. The structure of the 2-aminopyrimidine compounds is shown in the formula I, definitions of R1, R2, R3, R4, R5, X, Y, Z and W in the formula are shown in the specification and the claim. The compounds can effectively inhibit growths of a plurality of tumor cells, generate inhibition effects for EGFR and IGF1R protease, and is used for preparing antitumor drugs; the compounds can overcome drug resistance which is induced by prior medicaments Gefitinib and Erlotinib and the like, has selectivity for tumor, especially wild type non-small cell lung cancers, and has good pharmacokinetics property.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +2
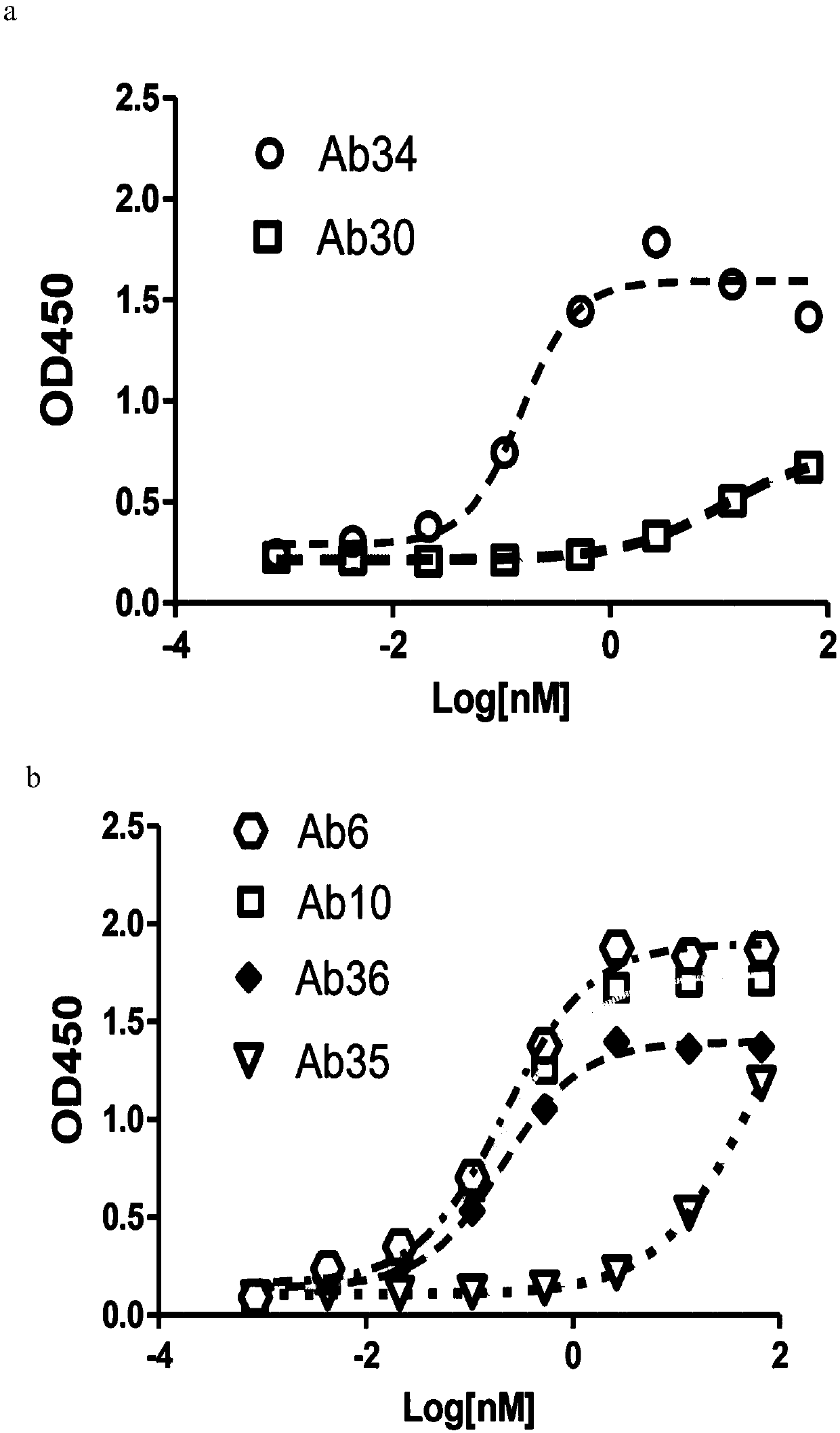
Antibody drug conjugate of CLDN 18.2-resistant antibody and preparation method and application of antibody drug conjugate
PendingCN111110862AGood pharmacokinetic propertiesPrevent proliferationOrganic active ingredientsImmunoglobulins against animals/humansDrug conjugationComplementarity determining region
The present invention discloses an antibody drug conjugate of a CLDN 18.2-resistant antibody. The structure of the antibody drug conjugate (ADC) is shown as Ab-[(L2)n-L1-D]y in a formula I, wherein Dis a small-molecule cytotoxic drug, L1 and L2 are connected to the drug and the antibody separately, and n is 0 or 1; y represents the average number of D which is coupled to Ab, 0 < y <= 10, and Ab is an antibody which can be specifically bound to human CLDN 18.2, and includes a light-chain variable region (VL) and / or a heavy-chain variable region (VH); and the CLDN 18.2-resistant antibody correspondingly contains at least one specific complementary determining region (CDR) sequence or a mutant sequence of the specific complementary determining region (CDR) sequence in the light-chain variable region (VL) and / or a heavy-chain variable region (VH), and binding of the antibody to CLDN 18.2 is maintained or improved through the mutation. The invention also discloses a ADC-containing pharmaceutical composition and a preparation method and application of ADC. The antibody drug conjugate of the antibody has a large security window and low toxic and side effects, and provides more specific,more effective and better treatment option for tumor patients.
Owner:L&L BIOPHARMA CO LTD
Carbamate caspase inhibitors and uses thereof
InactiveUS7074782B2Good effectGood cell penetrationBiocideSenses disorderCaspase inhibitorsCarbamate
This invention provides caspase inhibitors of formula I:wherein Z is oxygen or sulfur; R1is hydrogen, —CHN2, R, CH2OR, CH2SR, or —CH2Y; Y is an electronegative leaving group; R2 is CO2H, CH2CO2H, or esters, amides or isosteres thereof; R3 is a group capable of fitting into the S2 subsite of a caspase enzyme; R4 and R5 are taken together with the intervening nitrogen to form heterocyclic ring and R is as described in the specification. The compounds are effective inhibitors of apoptosis and IL-1β secretion.
Owner:VERTEX PHARMA INC
Radioactive label polypeptide coordination complex and preparation method and application thereof
ActiveCN102911256APromote commercial applicationSimple radiolabeling methodRadioactive preparation carriersPeptide preparation methodsCoordination geometryRadioactive Label
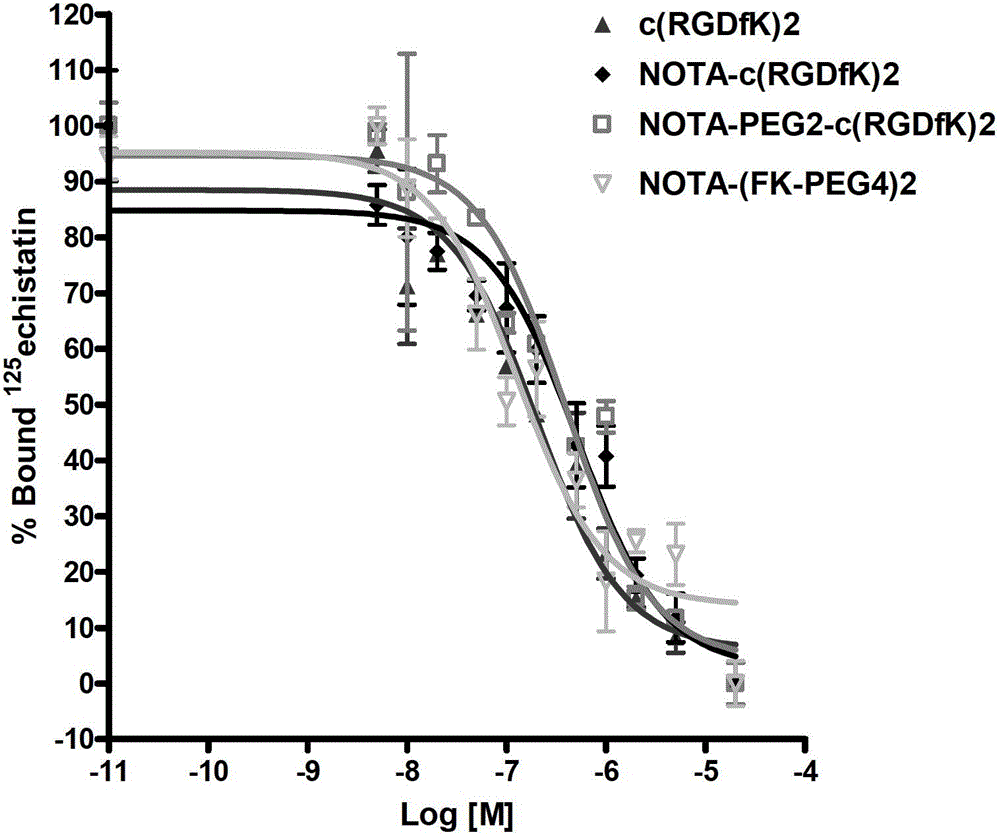
The invention provides an RGD polypeptide coordination complex of a radioactive nuclide label. According to the structure of the coordination complex, the radioactive nuclide is selected from 64Cu, 68Ga, 111In, 62Cu, 67Cu, 67Ga, 86Y, 89Zr or 18F; the ligand is a ligand compound containing an RGD structure, which is shown in a formula (I). The coordination complex has stronger ligand stability and high target / non-target ratio, and the appetency of the ligand with integrin AlphavBeta3 is stronger. The invention further provides a preparation method of the coordination complex, and an application of the coordination complex in preparing tumor developers.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
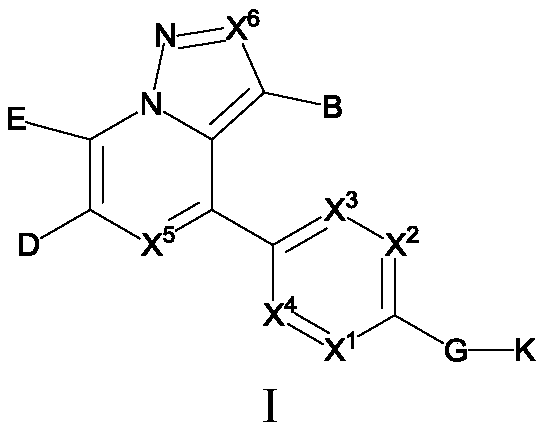
Fused cyanopyridine compound and preparation method and application thereof
ActiveCN112142735AGood selective inhibitionGood pharmacodynamicsOrganic active ingredientsOrganic chemistryCombinatorial chemistryDiastereomer
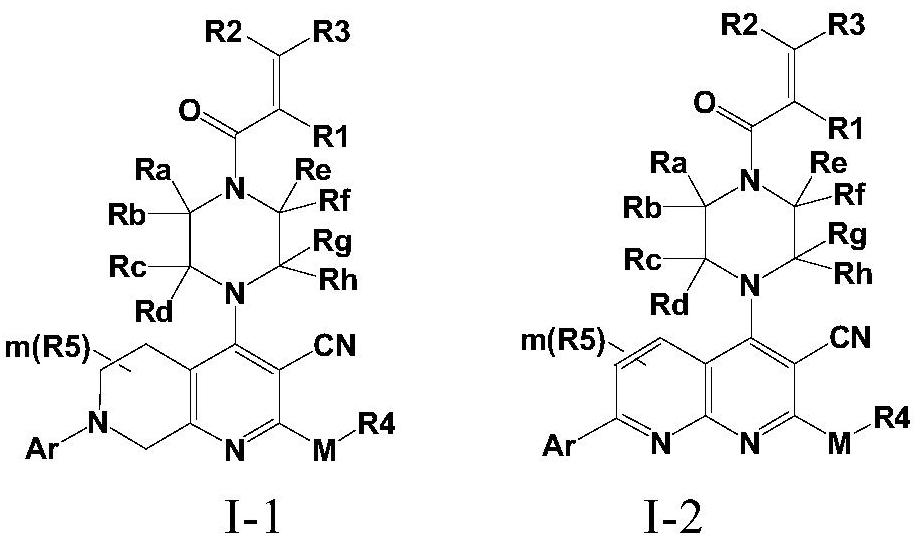
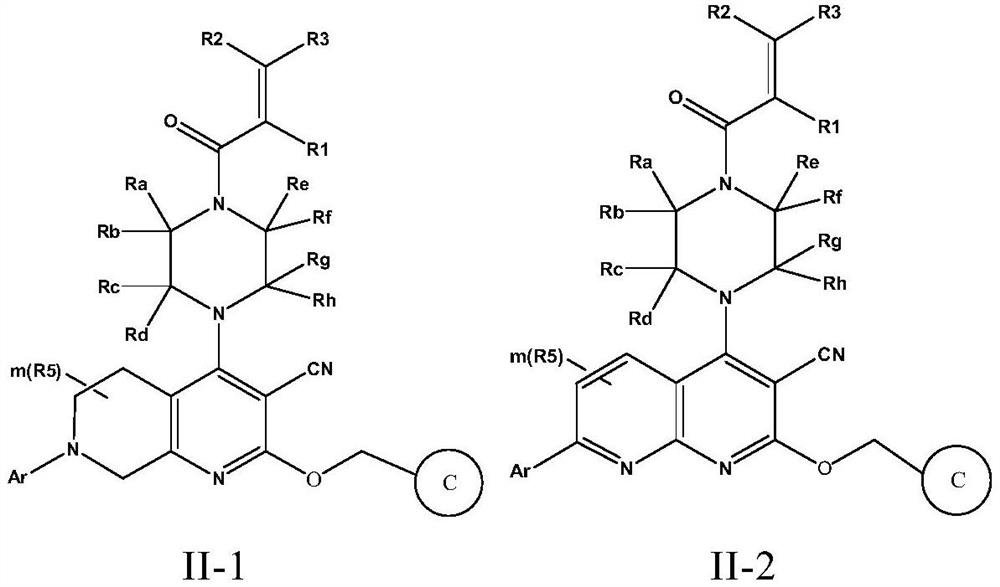
The invention discloses a fused cyanopyridine compound shown as a general formula I-1 or I-2, or a pharmaceutically acceptable salt thereof, or an enantiomer, a diastereoisomer, a tautomer, a torsional isomer, a solvate, a polymorphic substance or a prodrug thereof, and a preparation method and a pharmaceutical application of the fused cyanopyridine compound, wherein the definition of each group is shown in the specification.
Owner:RUDONG RINGENE PHARMA CO LTD +1
Novel Tepotinib derivative and preparation method thereof and application of derivative in antitumor drug
InactiveCN108752322AImprove bioavailabilityGood antitumor activityIsotope introduction to heterocyclic compoundsAntineoplastic agentsSulfurC-Met
The invention discloses a novel Tepotinib derivative and a preparation method thereof and an application of the derivative in an antitumor drug. According to the invention, a chiral structure is introduced in molecules, a hydrogen isotope deuterium is introduced in an easily metabolical part in the molecules, and atoms of sulfur, selenium and sulfoxide or groups are introduced in the molecules. The antineoplastic active experiments (having c-Met-expressed tumor cells) on a cell level proves that the compound has excellent antineoplastic activity, and the stability of the antineoplastic compound is obviously increased.
Owner:SYMEPILIN PHARMA CO LTD
Phenylpiperazine derivatives for inhibiting tumor metastasis and tumor angiogenesis
ActiveCN102260225AGrowth inhibitionGood pharmacokinetic propertiesOrganic active ingredientsOrganic chemistryPhenylpiperazineTumor chemotherapy
The invention discloses phenylpiperazine derivatives and pharmaceutically acceptable salts thereof. The invention is characterized in that: the derivatives have the structure shown as a formula I; and in the formula, R1 refers to -H, -R, -OR, -COOR, halogen or -CN group, R refers to alkyl, the substitution position of R1 on a benzene ring is a para-position, ortho-position or meta-position of piperazine, m is a natural number ranging from 0 to 2, and R2 refers to a substituted or unsubstituted carboxyl group or sulfonic acid group. The phenylpiperazine derivatives and pharmaceutically acceptable salts thereof can be prepared into medicines for inhibiting tumor cell metastasis and tumor angiogenesis, medicines for treating liver cancer, breast cancer, ovarian cancer, gastric cancer, colon cancer, lung cancer or melanoma, tumor chemotherapy medicines and auxiliary medicines in surgical therapy.
Owner:蒋杰 +1
2-aminopyrimidine compound and application thereof
PendingCN110305161ANovel structureInhibitionOrganic active ingredientsGroup 5/15 element organic compoundsNon-small cell lung cancer (NSCLC)Mutant
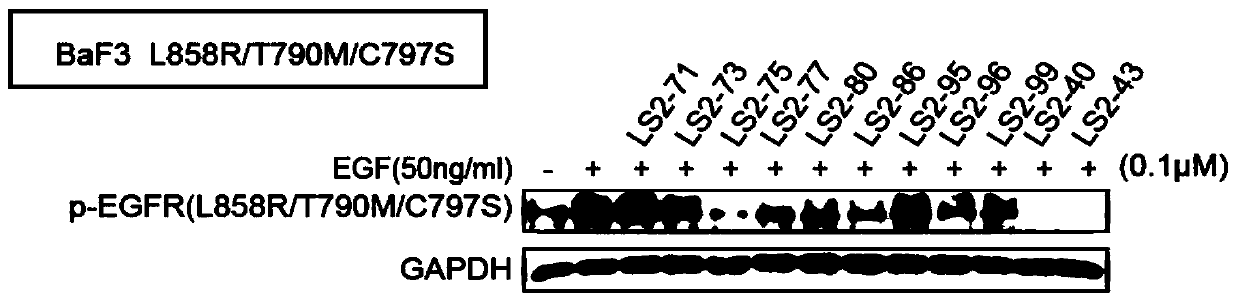
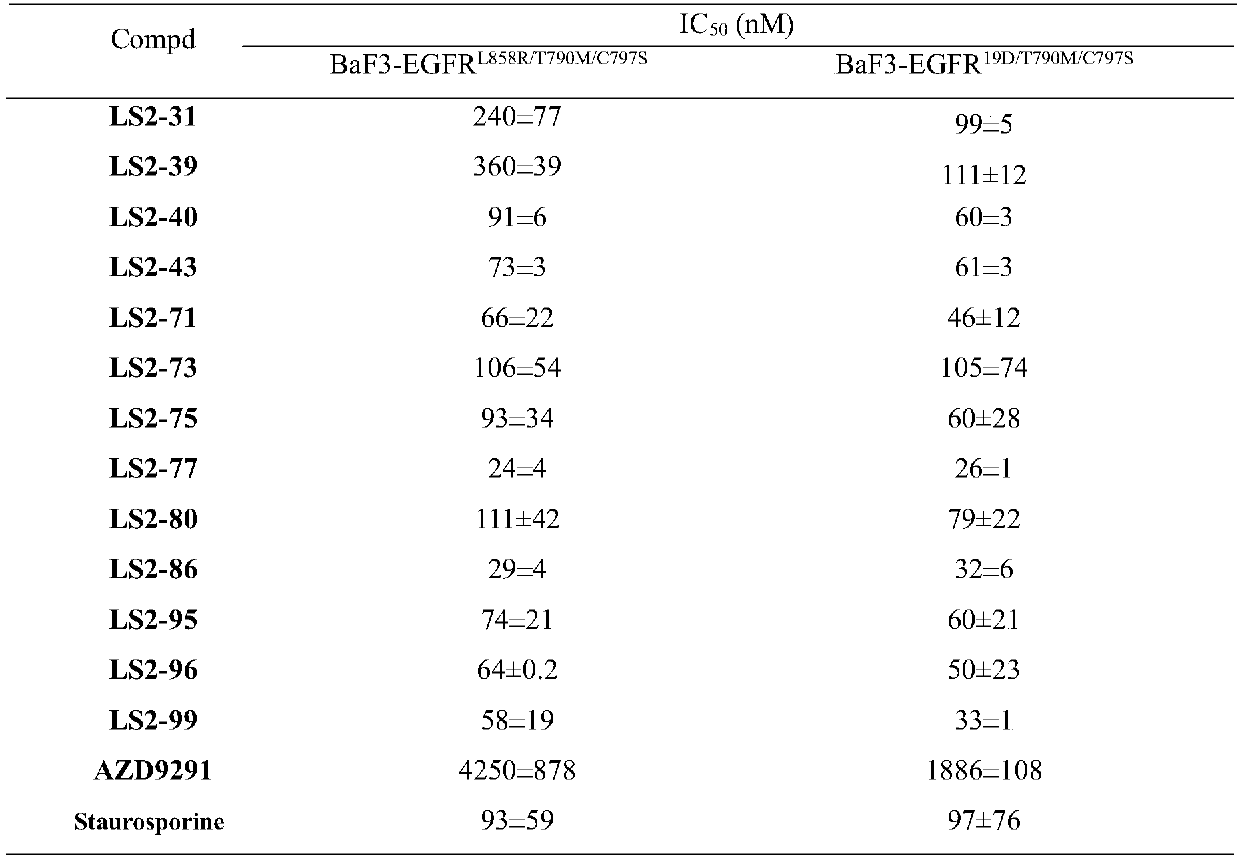
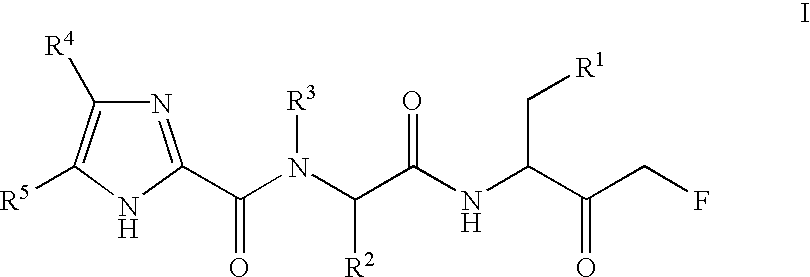
The invention relates to a 2-aminopyrimidine compound and application thereof. The structure of the 2-aminopyrimidine compound is shown as I. The compound can effectively inhibit the activity of EGFRprotein kinase resistance mutants (such as EGFRT790M and EGFRT790M / C797S), and can overcome clinical drug resistance of tumor patients such as patients suffering from non-small cell lung cancer induced by an existing third-generation selective EGFRT790M small molecule inhibitors Osimertinib (AZD9291), Olmutinib (HM6171), Rocketinib (CO-1686) and the like.
Owner:JINAN UNIVERSITY +1
Imidazole and benzimidazole caspase inhibitors and uses thereof
InactiveUS7205327B2Enhanced inhibitory effectGood effectBiocideSenses disorderCaspase inhibitorsMedicinal chemistry
This invention provides caspase inhibitors having the formula:wherein R1 is CO2H, CH2CO2H, or esters, amides or isosteres thereof; R2 and R3 are each independently selected from hydrogen or an optionally substituted C1–C6 aliphatic group; and R4 and R5 are each independently selected from hydrogen, an optionally substituted C1–C6 aliphatic group, or R4 and R5 taken together with the ring to which they are attached form an optionally substituted bicyclic ring. The caspase inhibitors are useful for treating a number of diseases such as cancer, acute inflammatory and autoimmune disorders, ischemic diseases and certain neurodegenerative disorders.
Owner:VERTEX PHARMA INC
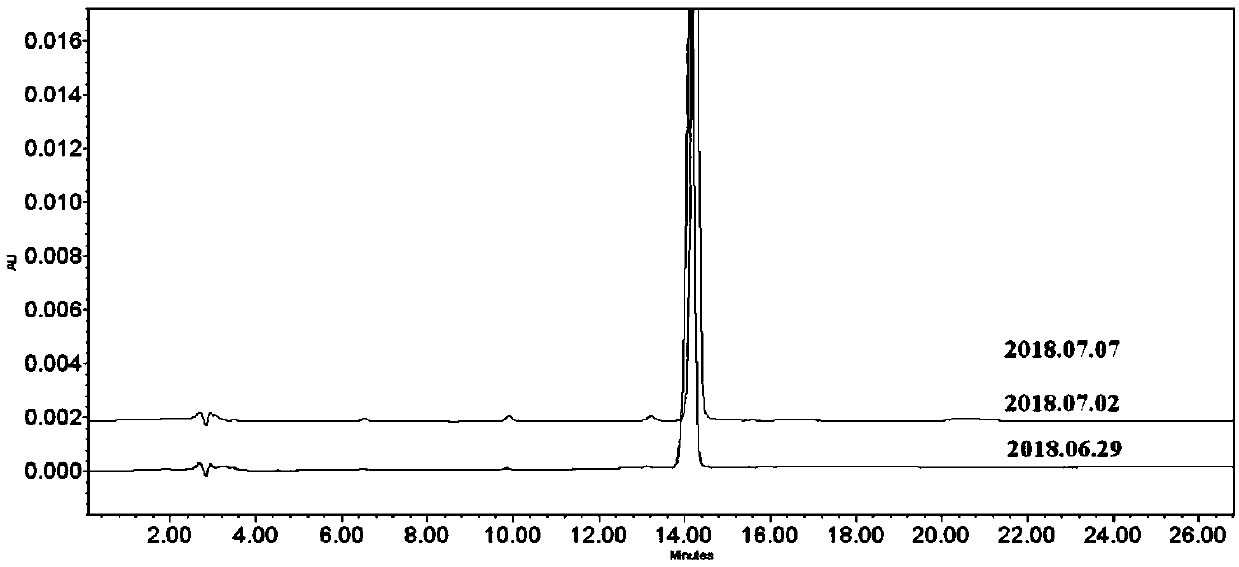
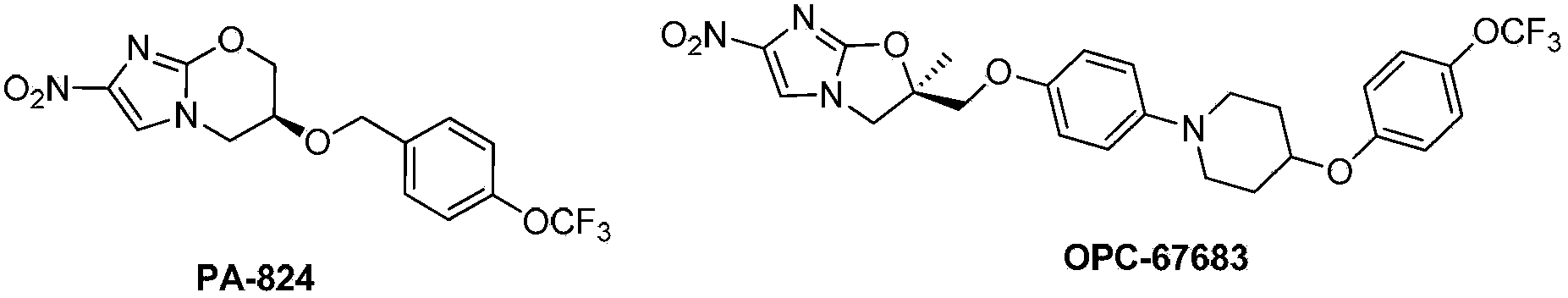
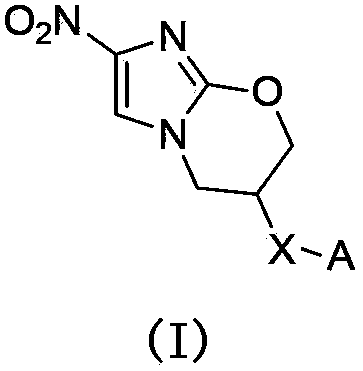
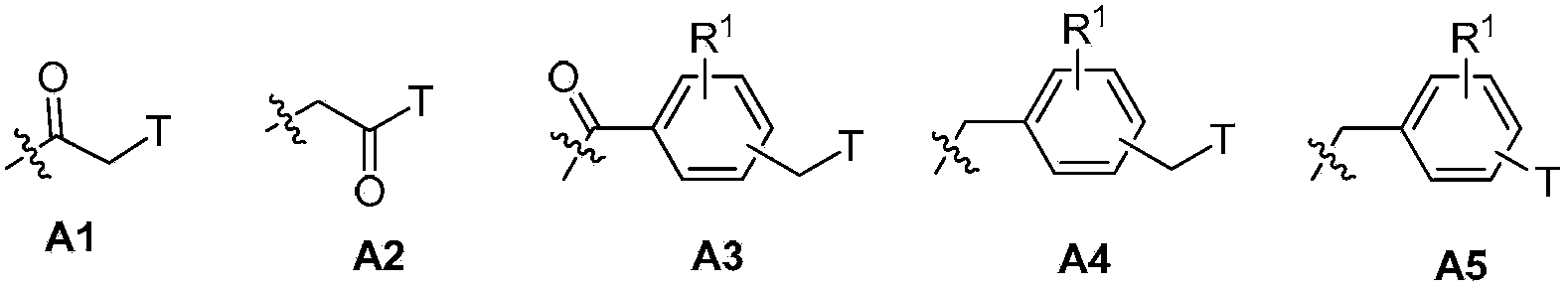
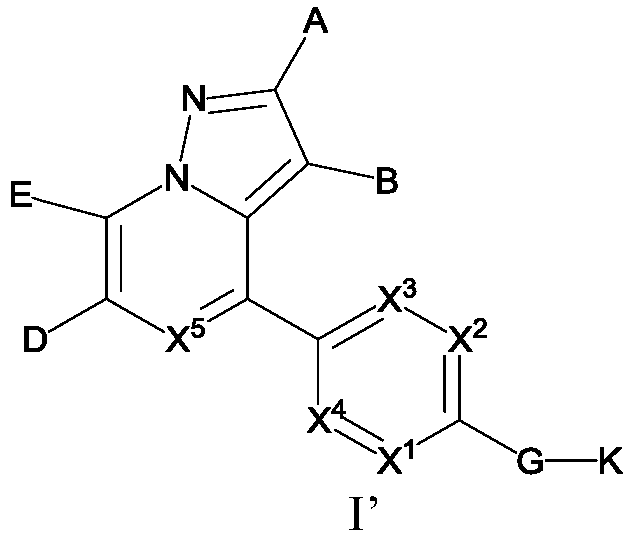
Pyrazolo[1, 5-a]pyridine compound and use thereof
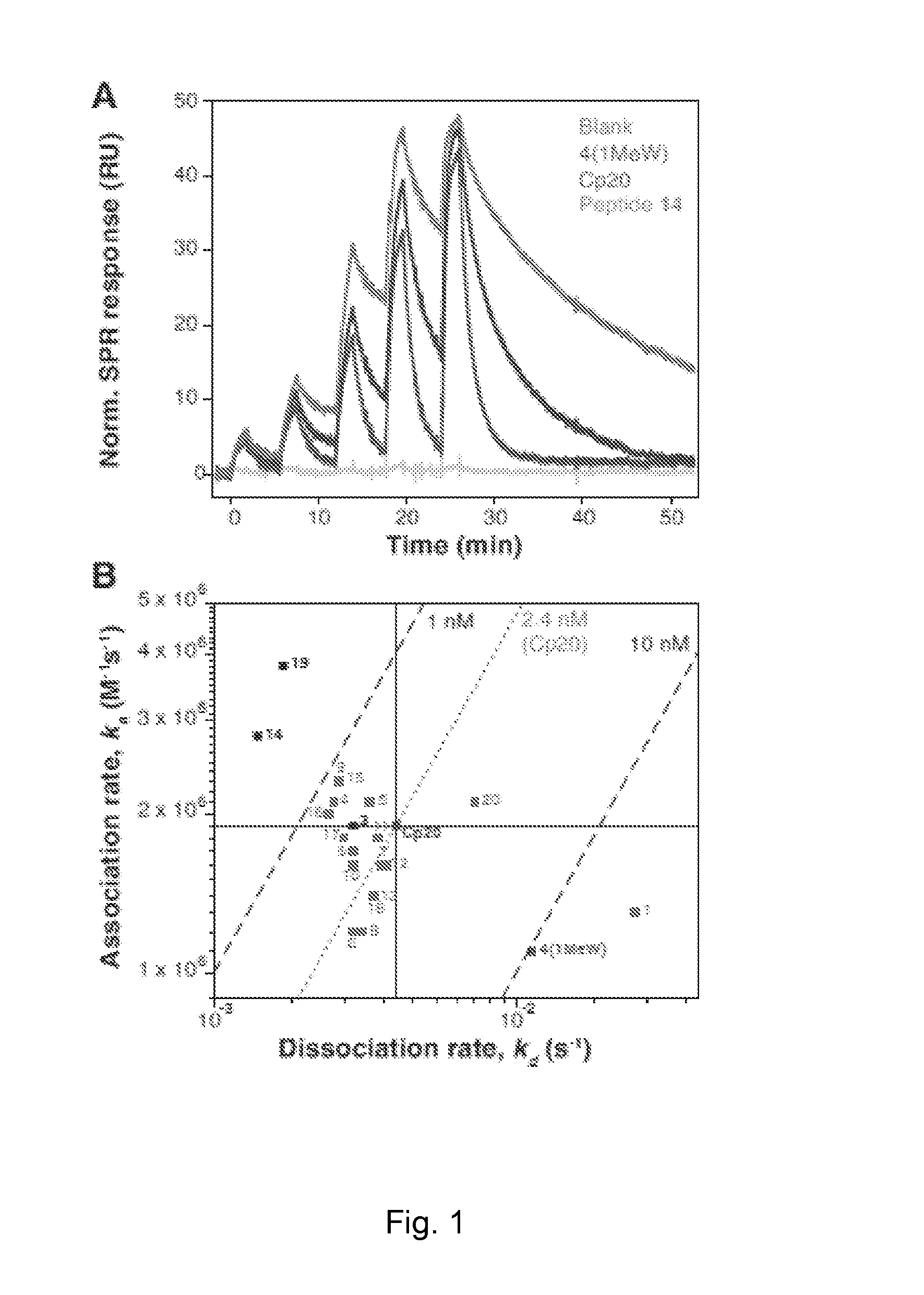
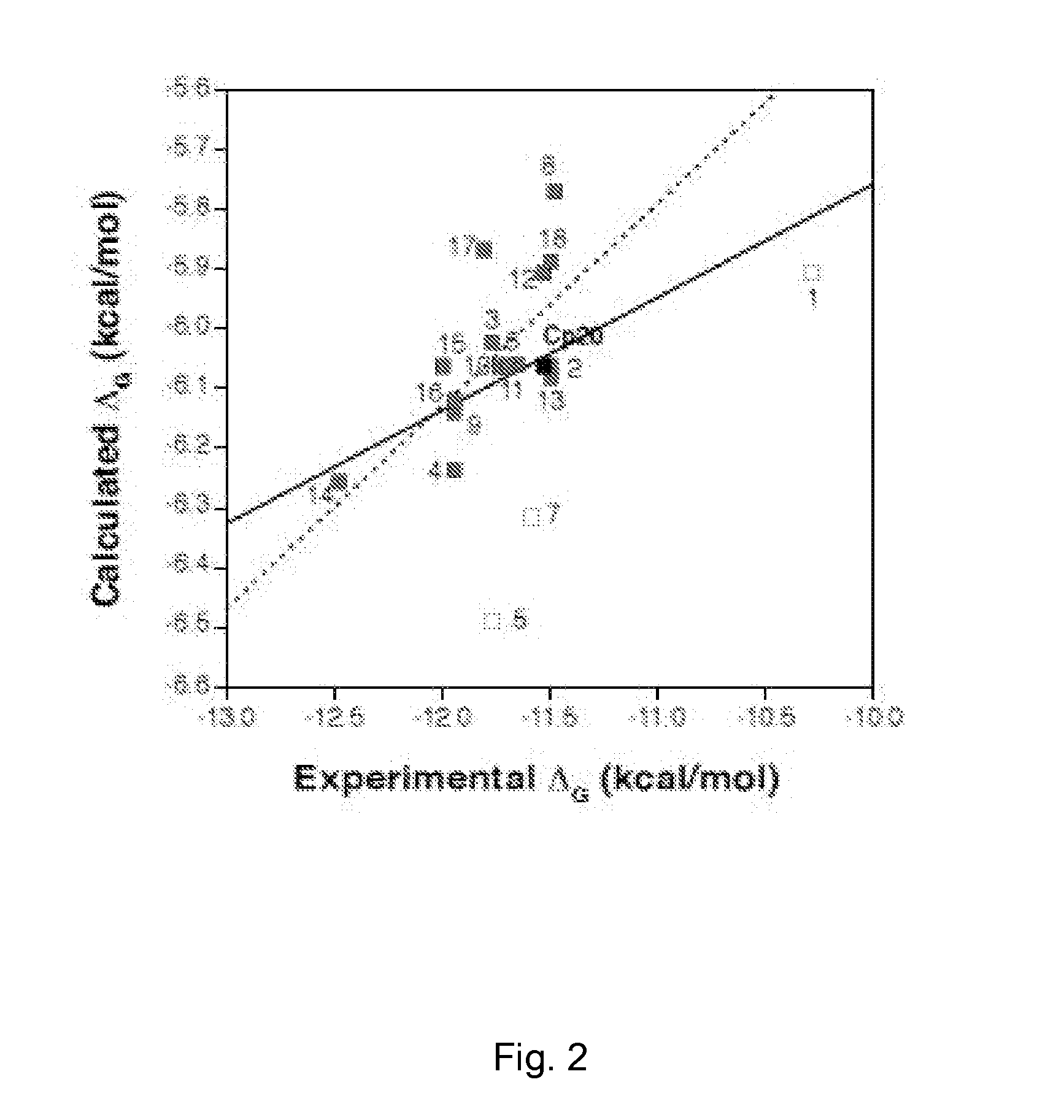
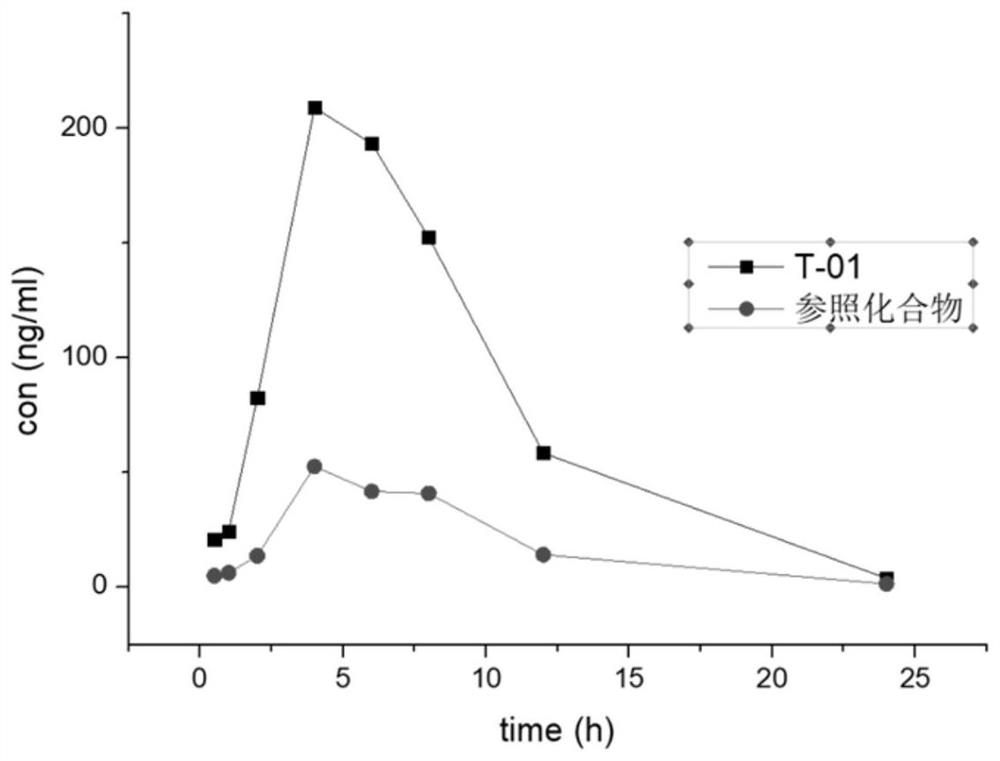
ActiveCN105524058AEnhanced inhibitory effectGood in vitro anti-tuberculosis activityAntibacterial agentsOrganic active ingredientsMulti-drug-resistant tuberculosisMinimum inhibitory concentration
The invention discloses a pyrazolo[1, 5-a]pyridine compound with the structure characteristic shown in the formula (I) or its pharmaceutically acceptable salt, stereoisomer or prodrug molecule and a use thereof. The compound has good in-vitro anti-tubercle bacillus activity, has the minimal inhibitory concentration (MIC) less than 0.1 micrograms per milliliter and partial MIC of 0.01 micrograms per milliliter and has strong inhibition effects on a clinically sorted multi-drug-resistant tuberculosis (MDR-TB) strain. In an in-vivo experiment, at a dosage of 20mg / kg / d, the pyrazolo[1, 5-a]pyridine compound can effectively eliminate H37Ra infection in a mouse and is a novel anti-tuberculosis compound.
Owner:GUANGDONG GOOD MEDICINE & HEALTH TECH CO LTD
CDK kinase inhibitor
ActiveCN105732615AExcellent CDK kinase inhibitory activityGood biological stabilityOrganic active ingredientsOrganic chemistryDiseaseDrugs preparations
The invention belongs to the technical field of medicines and particularly relates to a CDK kinase inhibitor represented as the general formula (I), pharmaceutically-accepatable salt, ester and solvate thereof, and stereisomers thereof. The R1, R2, R3, R4, A1, A2, A3 and n are defined as the specification. The invention also relates to a preparation method of the compounds, a medicine preparation and a medicine composition comprising the compounds, and an application of the compounds, the pharmaceutically accepatable salt, ester and solvate thereof, and the stereisomers thereof in preparation of a medicine for treating and / or preventing cancer-related diseases induced by the CDK kinase.
Owner:XUANZHU BIOPHARMACEUTICAL CO LTD
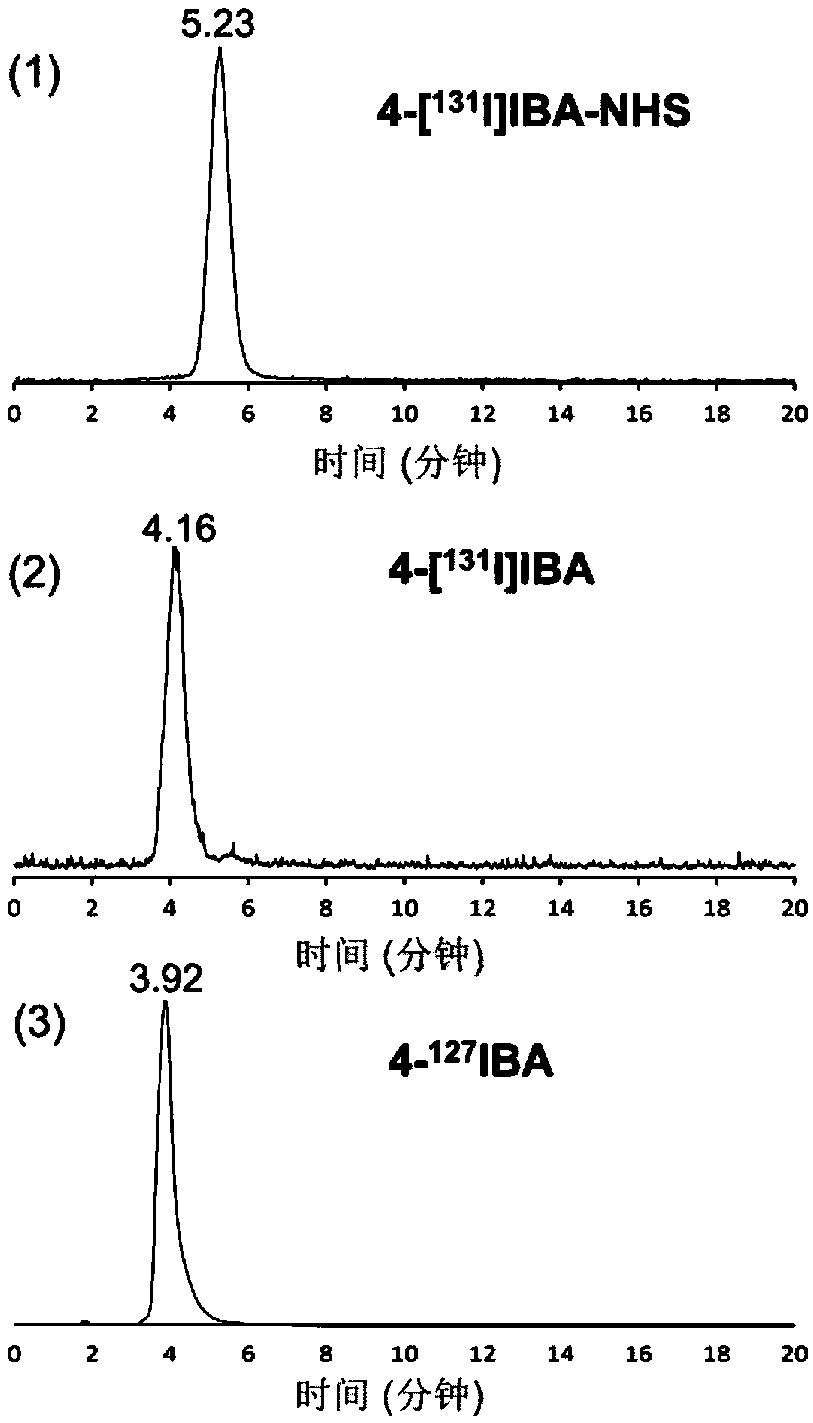
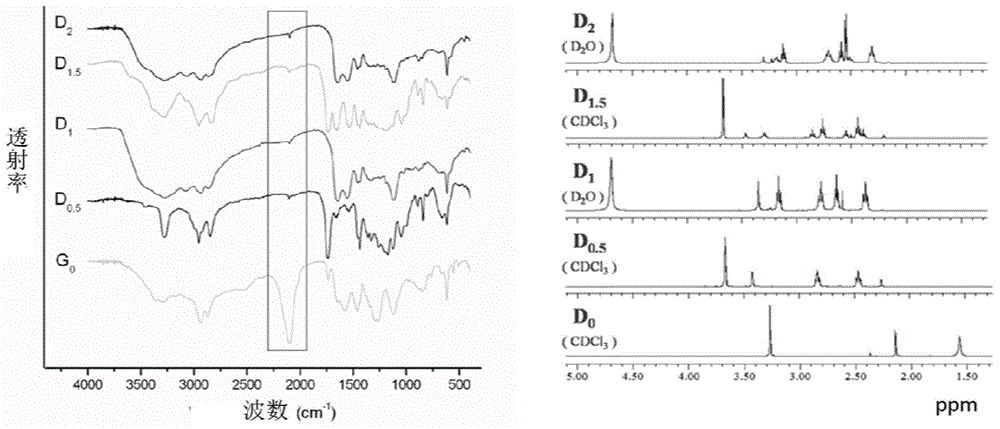
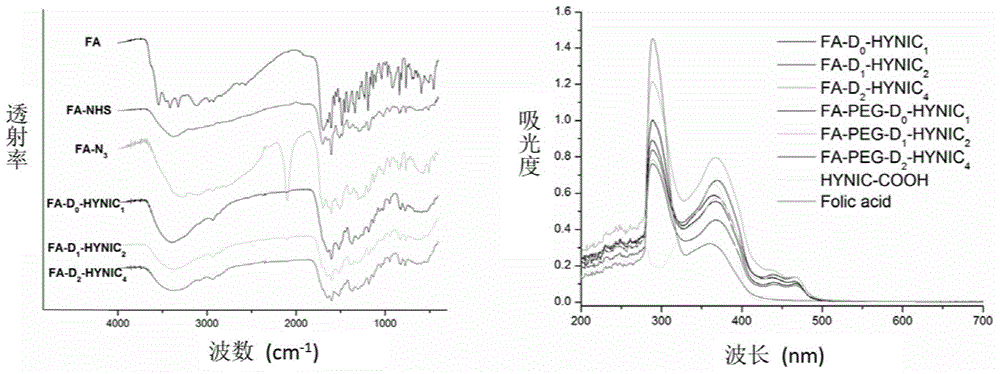
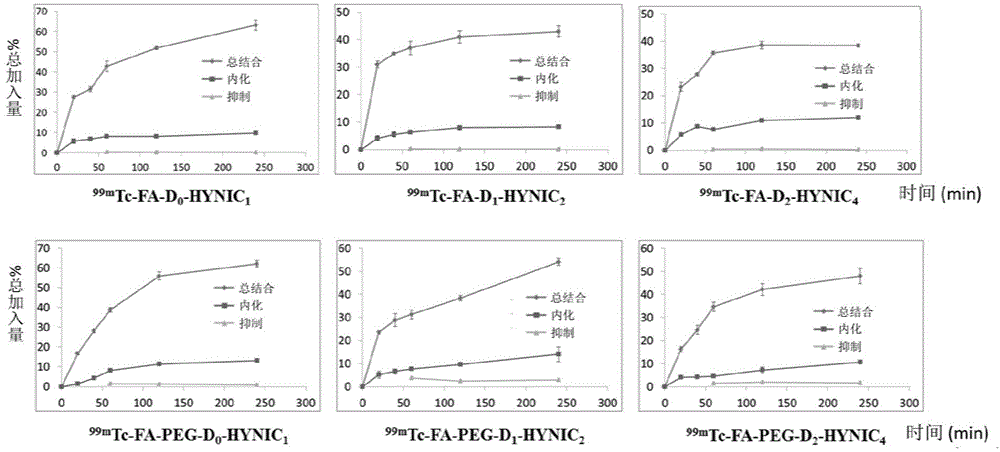
Radioactive iodine marked protein binding ligand and application thereof
ActiveCN108434468AImprove stabilityIncrease intakeOrganic compound preparationRadioactive preparation carriersStructural formulaRadioactive Label
The invention discloses a radioactive iodine marked protein binding ligand and application thereof. A structural formula of the radioactive iodine marked protein binding ligand is shown in a followingimage, wherein the I is radioactive iodine nuclide, the R is OH or derived from PEG, folic acid, RGD, octreotide, epidermal growth factor, protein, nucleic acid or polysaccharide. The radioactive iodine marked protein binding ligand disclosed by the invention has the advantages of simple marking method, low cost and good stability. Animal live test results show that the albumin binding ligand hashigher uptake and longer-time retention in blood and further has a higher target / non-target specific value. When the radioactive iodine marked protein binding ligand is modified to a target group orother functional groups, pharmacokinetic characters of compound can be obviously improved, a blood half-life period of the compound is prolonged, and the compound is suitable for being utilized as a blood pool, lymph and tumor diagnosing and treating reagent.
Owner:XIAMEN UNIV
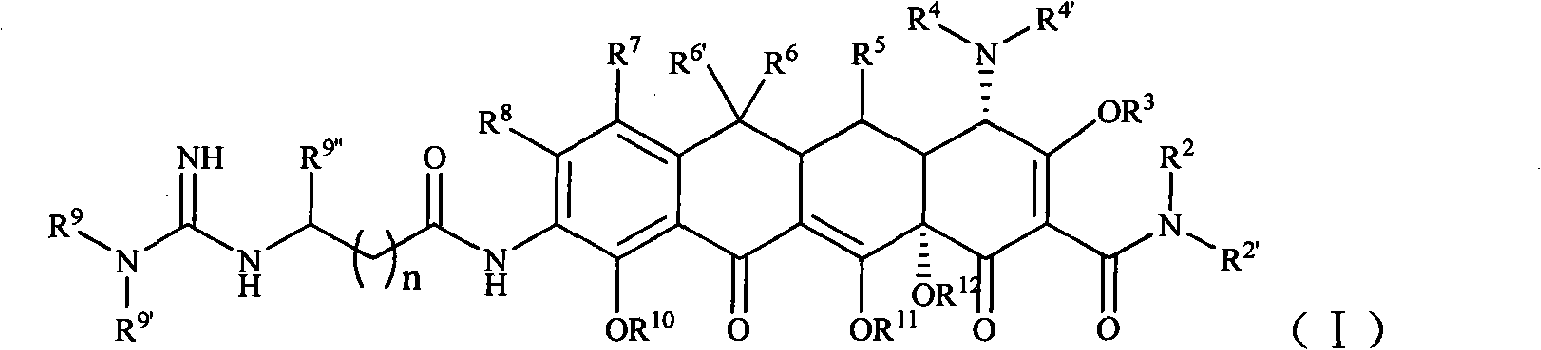
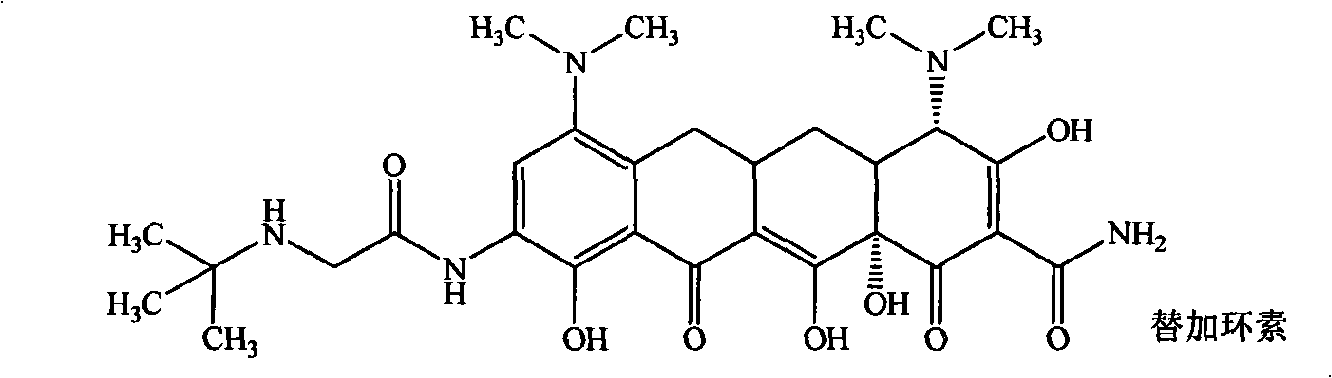
Guanidinoalkanoylamino substituted tetracycline derivatives
ActiveCN101684083ABroad spectrum antibacterialHigh antibacterial activityOrganic chemistryTetracycline active ingredientsInfective disorderStereochemistry
The invention belongs to the technical field of medicament, in particular to guanidinoalkanoylamino substituted tetracycline derivatives shown in a general formula (I), and pharmaceutically acceptablesalts or isomers thereof, wherein R<2>, R<2'>, R<3>, R<4>, R<4'>, R<5>, R<6>, R<6'>, R<7>, R<8>, R<9>, R<9'>, R<9'> and n are defined in the specification. The invention also relates to a method forpreparing the compounds, medicinal composition containing the compounds, as well as application of the compounds in the preparation of medicaments for treating and / or preventing tetracycline sensitivediseases, particularly in the preparation of medicaments for treating infectious diseases.
Owner:HAINAN SIHUAN PHARMA
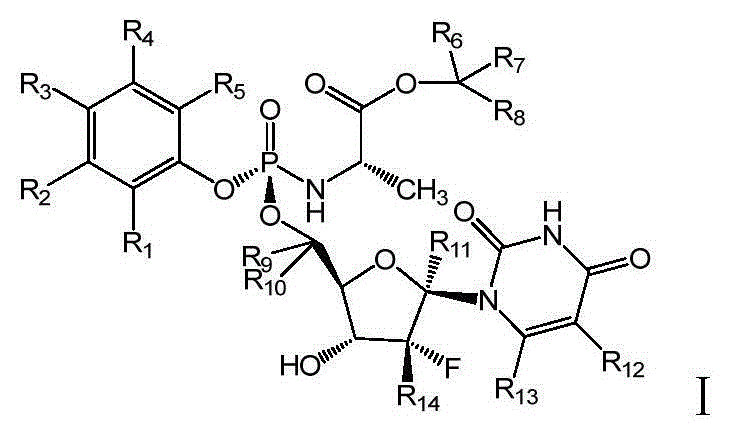
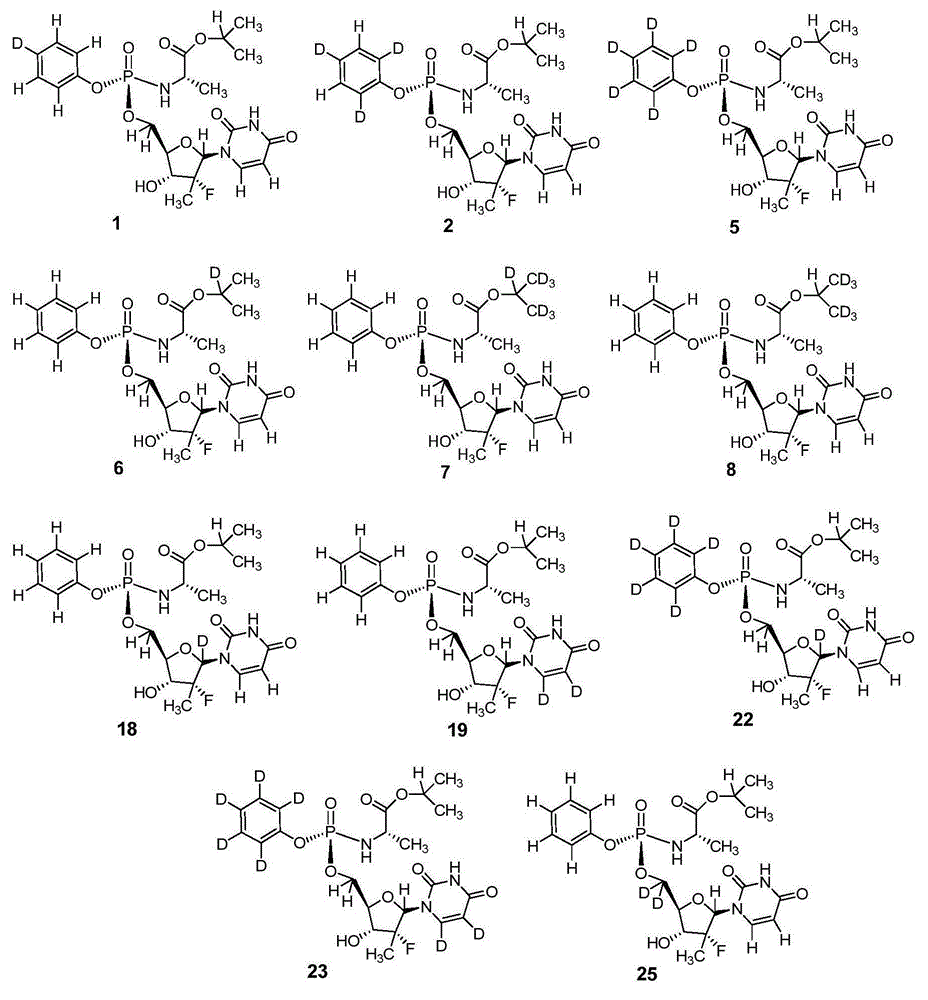
Deuterated nucleoside derivative
ActiveCN105254694AHigh degree of Sp enrichmentHigh degree of enrichmentOrganic active ingredientsSugar derivativesStereochemistry
The present invention relates to deuterated nucleoside derivative, particularly to a compound having a structure represented by a formula I or a pharmaceutically acceptable salt thereof. According to the present invention, the compound represented by the formula I has excellent pharmacokinetic properties and is expected to reduce the clinical dose so as to reduce the treatment and benefit more patients. The formula I is defined in the specification.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Tumor necrosis factor related apoptosis ligand fusion protein, and method of preparation and use thereof
ActiveCN104177500AExtended half-lifeGood pharmacokinetic propertiesBacteriaPeptide/protein ingredientsPeptideTreatment effect
The invention belongs to the biotechnical field, and concretely relates to a tumor necrosis factor family cell apoptosis protein fusion protein, and a preparation method and a use thereof. The fusion protein is composed of annexin, a connecting peptide and a tumor necrosis factor family cell apoptosis protein, and coding gene of the fusion protein is constructed through cloning. The tumor necrosis factor family cell apoptosis protein fusion protein has a substantial enhanced cell apoptosis induction effect, can induce the apoptosis of tumor cells insensitive to the cell apoptosis, and can reduce the protein administration dosage needed by the obtaining of the treatment effect.
Owner:JIANGSU TARGET BIOMEDICINE RES INST
Targeted probe for nuclide labeling and preparation method and application of targeted probe
ActiveCN104830316AStrong marking abilityShort marking timeOrganic chemistryRadioactive preparation carriersDiseaseTherapeutic effect
The invention discloses a targeted probe for nuclide labeling and a preparation method and an application of the targeted probe. A general structural formula of a complex is as shown in the specification, wherein R' is a nuclide chelating group; R is a targeted group; Dn is a molecular skeleton which is formed by repeated michael addition reaction and amidation reaction employing propargylamine as an initial reactant, the peripheral group is amino, n represents different algebras, and the numerical value of n is an integer greater than or equal to 0; and m is equal to 2n. A polymer in the targeted probe for nuclide labeling is beneficial to improvement of the specific activity; a high-quality developing result can be obtained by instrument scanning; the targeted probe can play a role in effectively monitoring tumors or inflammatory diseases; meanwhile, carrying of relatively many nuclides on a molecule is facilitated by the formed polymer; the nuclide concentration of focus location is improved; and the targeted probe plays a relatively good treatment role.
Owner:XIAMEN UNIV
Substituted pyrazole fused ring derivative as well as preparation method and application thereof
ActiveCN110964008AHigh activityGood pharmacokinetic propertiesOrganic active ingredientsOrganic chemistryDiseasePharmaceutical drug
The invention relates to the field of medicinal chemistry, and mainly relates to a compound represented by a formula I, a stereoisomer, a despinner, a tautomer, an isotope marker, NOx or a pharmaceutically acceptable salt thereof, a preparation method of the compound, and an application of the compound in preparation of a medicine for treating RET kinase mediated diseases.
Owner:APPLIED PHARMA SCI
A Peptide Modified Segment and Its Application in the Modification of Peptide Molecular Imaging Probes
InactiveCN102276688AGood pharmacokinetic propertiesIncrease contrastRadioactive preparation carriersPeptidesMolecular imagingImaging quality
The invention discloses a peptide modified segment, the structural formula is as follows: (L)m-(A)n-(S)o, wherein L is a connecting segment, m is 0-10; A is a PN or NP amino acid pair , n is 1-10; S is a spacer segment, and o is 0-10. The beneficial effects of the present invention are: the peptide modified segment provided by the present invention and its application in the modification of peptide molecular imaging probes can significantly improve the pharmacokinetic properties of targeting peptides, increase the tumor uptake value and tumor The contrast with the background optimizes the image quality. The target peptide product can be conveniently prepared by using an automatic peptide synthesizer through Fmoc protection chemistry, which is suitable for large-scale production.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Farnesoid X receptor (FXR) stimulant
ActiveCN109320517AExcellent FXR receptor agonistic activityPrevent non-alcoholic fatty liver diseaseOrganic active ingredientsSenses disorderDiseaseStimulant
The invention belongs to the technical field of medicines, and particularly relates to a compound as shown in the formula (I), and pharmaceutically acceptable salt and ester or a stereisomer thereof.R1, R2, R3, M1, M2, m, n, Q, L, ring A, ring B and ring C are as defined in the specification. The invention further relates to a preparation method of the compounds, and application in preparing medicines for treating and / or preventing related diseases such as non-alcoholic fatty liver disease, primary biliary cirrhosis, lipid metabolism disorders, diabetic complication and malignant tumors mediated by an FXR. The formula (I) is shown in the description.
Owner:XUANZHU BIOPHARMACEUTICAL CO LTD
Novel compounds and methods for their production
InactiveCN101578268APromote absorptionImprove solubilityOrganic active ingredientsOrganic chemistryAutoimmune diseaseMalaria
The present invention relates to ansamycin analogues that are useful, e.g. in the treatment of cancer, B-cell malignancies, malaria, fungal infection, diseases of the central nervous system and neurodegenerative diseases, diseases dependent on angiogenesis, autoimmune diseases or a prophylactic pretreatment for cancer. The present invention also provides methods for the production of these compounds and their use in medicine.
Owner:BRISTOL MYERS SQUIBB CO
Compound used as CDK7 kinase inhibitor and application thereof
InactiveCN112661745AEnhanced inhibitory effectSmall toxicityOrganic active ingredientsAntipyreticDiseaseCyclin
The invention relates to a compound used as a CDK7 kinase inhibitor and application thereof. Specifically, the compound disclosed by the invention has a structure shown as a formula I, and the definitions of all groups and substituents are as described in the specification. The compounds of the present invention are useful as inhibitors of cyclin-dependent kinase 7 (CDK7) for the treatment or prevention of proliferative diseases such as cancer, especially for modulating and treating diseases associated with abnormal activity of cyclin-dependent kinase 7 (CDK7).
Owner:TYK MEDICINES INC
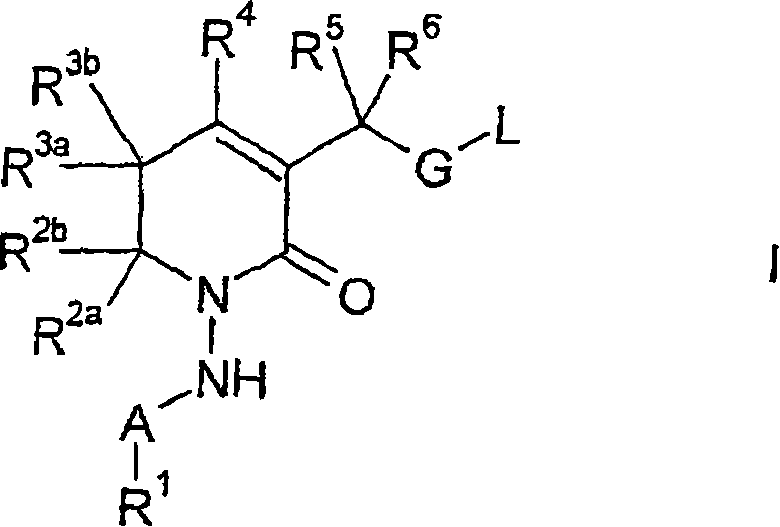
New 5,6-dihydropyrin-2-one compounds useful as inhibitors of thrombin
InactiveCN1894214AGood effectLow toxicityOrganic active ingredientsOrganic chemistryAnticoagulantThrombin activity
There is provided a compound of formula (I) wherein R<1>, R<2a>, R<2b>, R<3a>, R<3b>, R<4>, R<5>, R<6>, A, G and L have meanings given in the description, which compounds are useful as, or are useful as prodrugs of, competetive inhibitors of trypsin-like proteases, such as trombin, and thus, in particular, in the treatment of conditions where inhibition of thrombin is beneficial (e,g. conditions, such as thrombo-embolisms, where inhibition of trombin is required or desired, and / or conditions wherea anticoagulant thererapy is indicated).
Owner:ASTRAZENECA AB
Ketohexokinase (KHK) inhibitor and application thereof
InactiveCN111978296AExcellent KHK inhibitory activityGood pharmacokinetic propertiesOrganic active ingredientsNervous disorderDiseasePharmaceutical drug
The invention relates to the technical field of medicine, in particular to a ketohexokinase (KHK) inhibitor compound, pharmaceutically acceptable salts of the compound, esters of the compound or stereoisomers of the compound, a pharmaceutical composition and preparation containing the compound, the pharmaceutically acceptable salts of the compound, the esters of the compound or the stereoisomers of the compound, and application of the compound, the pharmaceutically acceptable salts of the compound, the esters of the compound or the stereoisomers of the compound to preparation of drugs for treating and / or preventing KHK-mediated diseases and related diseases.
Owner:SHANDONG XUANZHU PHARMA TECH CO LTD
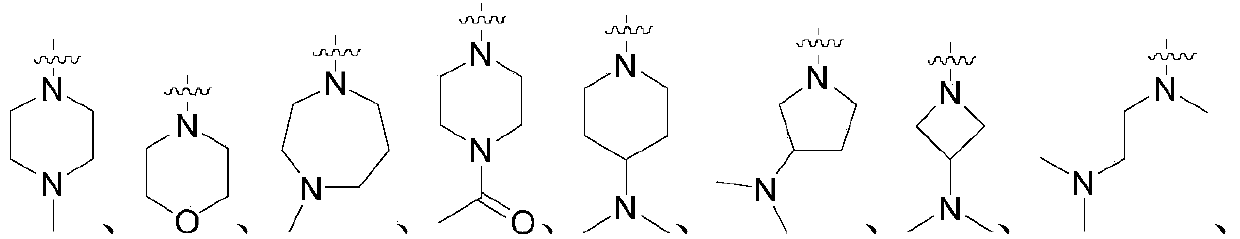
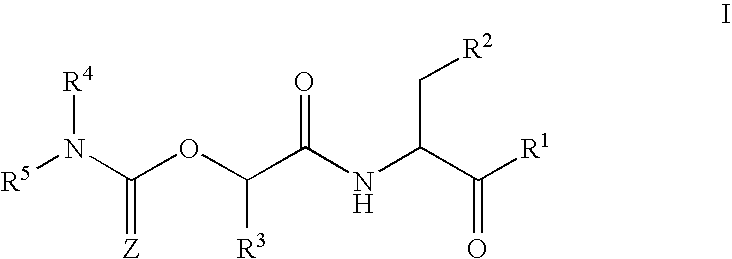
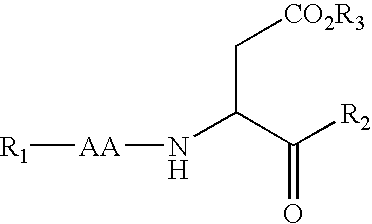
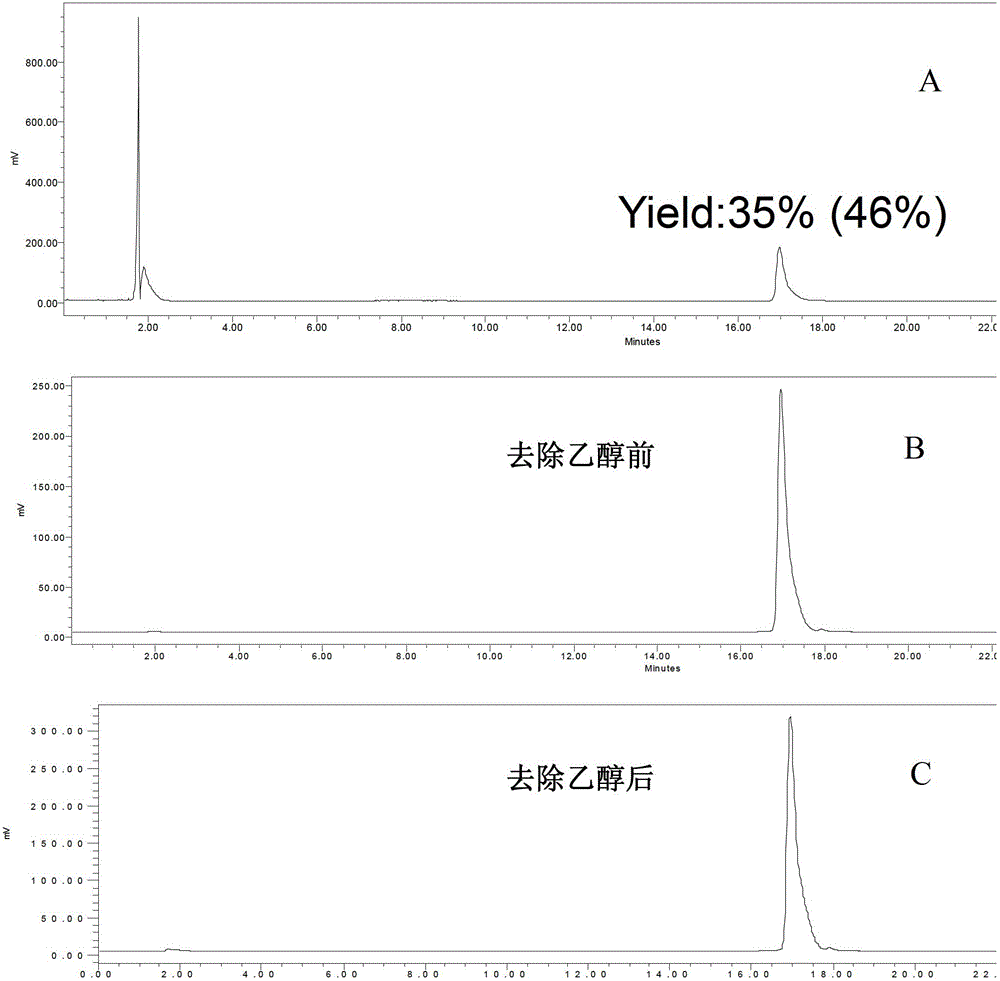
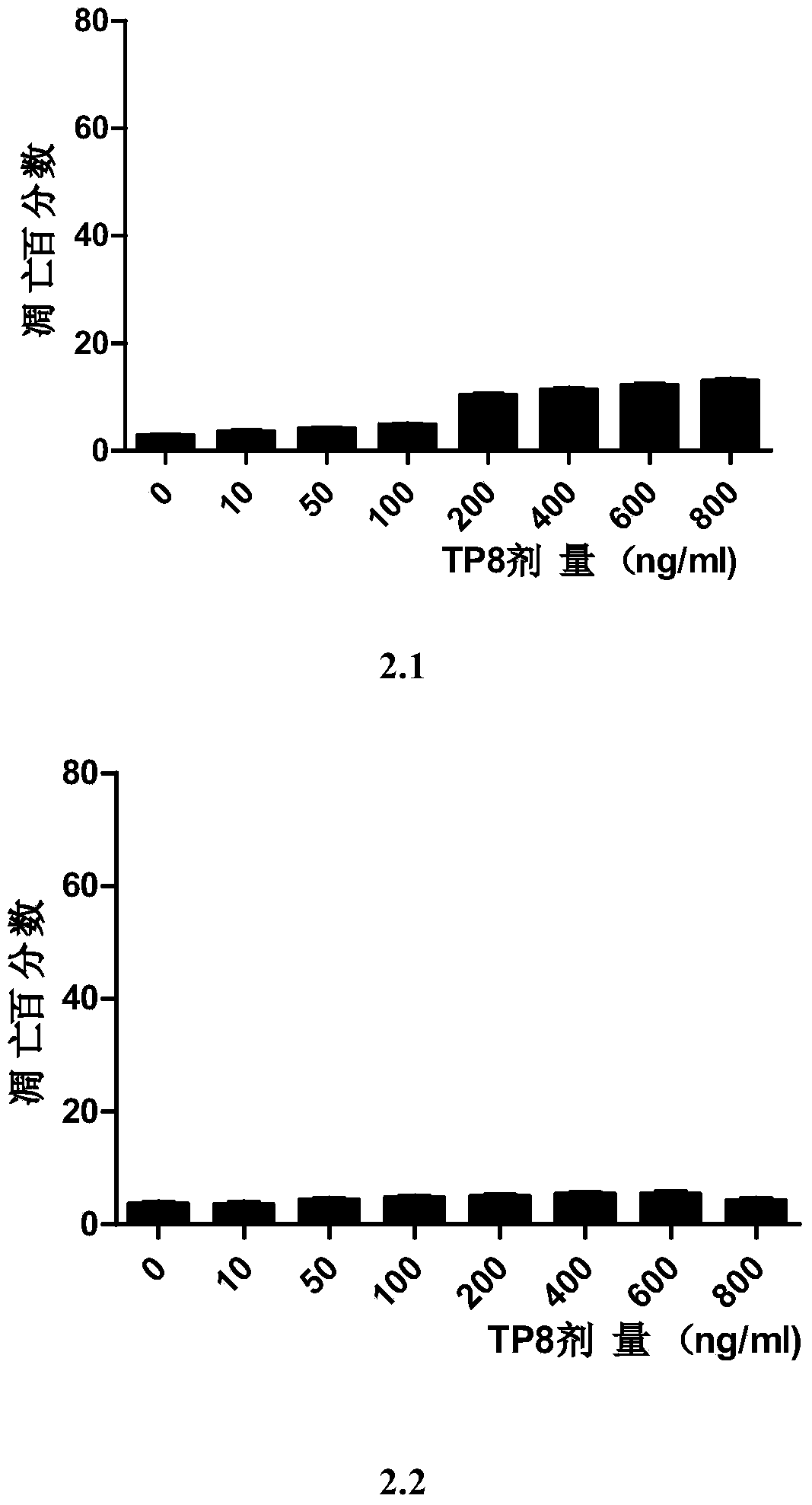
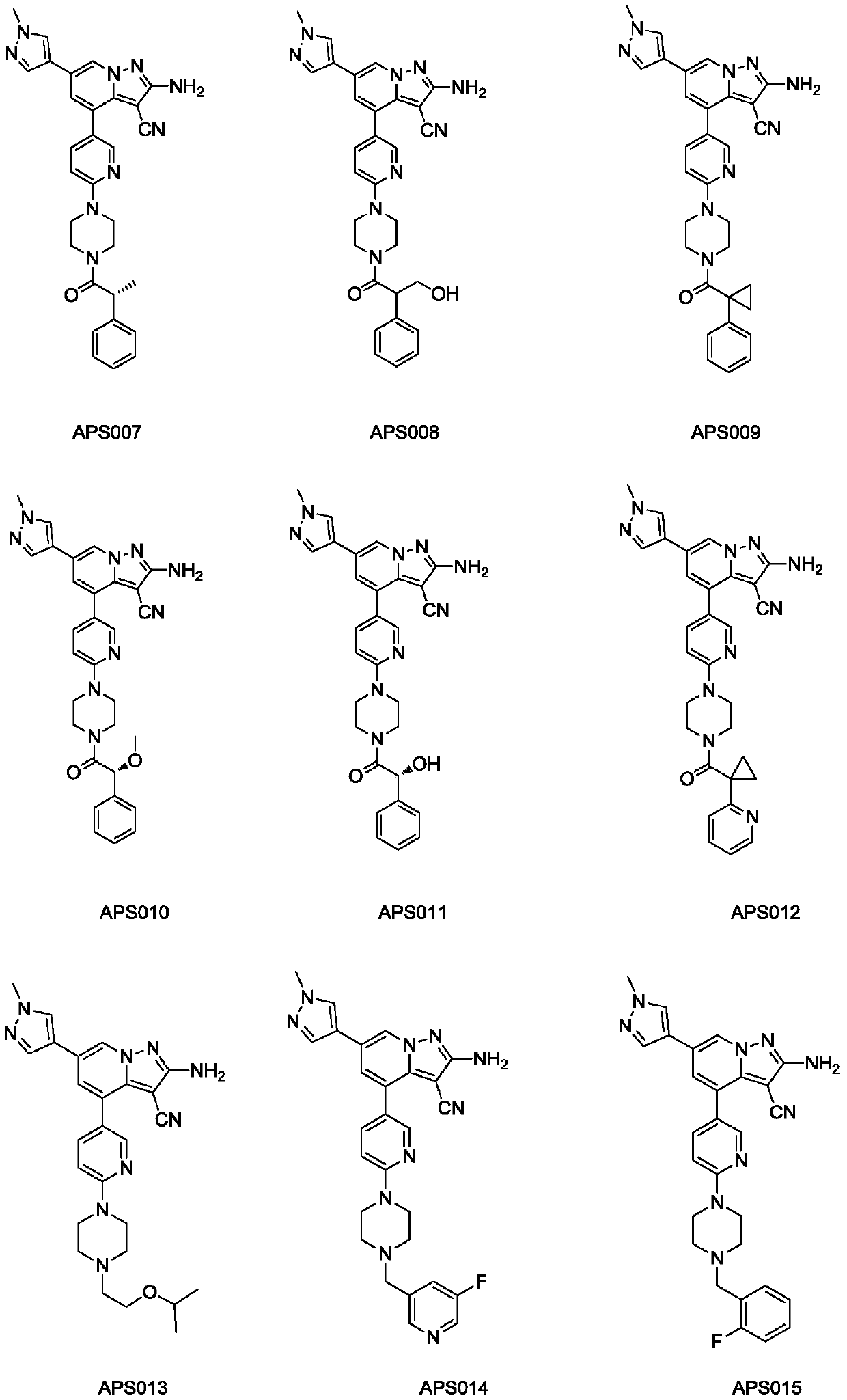
Casein kinase 1[epsilon] inhibitor, pharmaceutical composition and application thereof
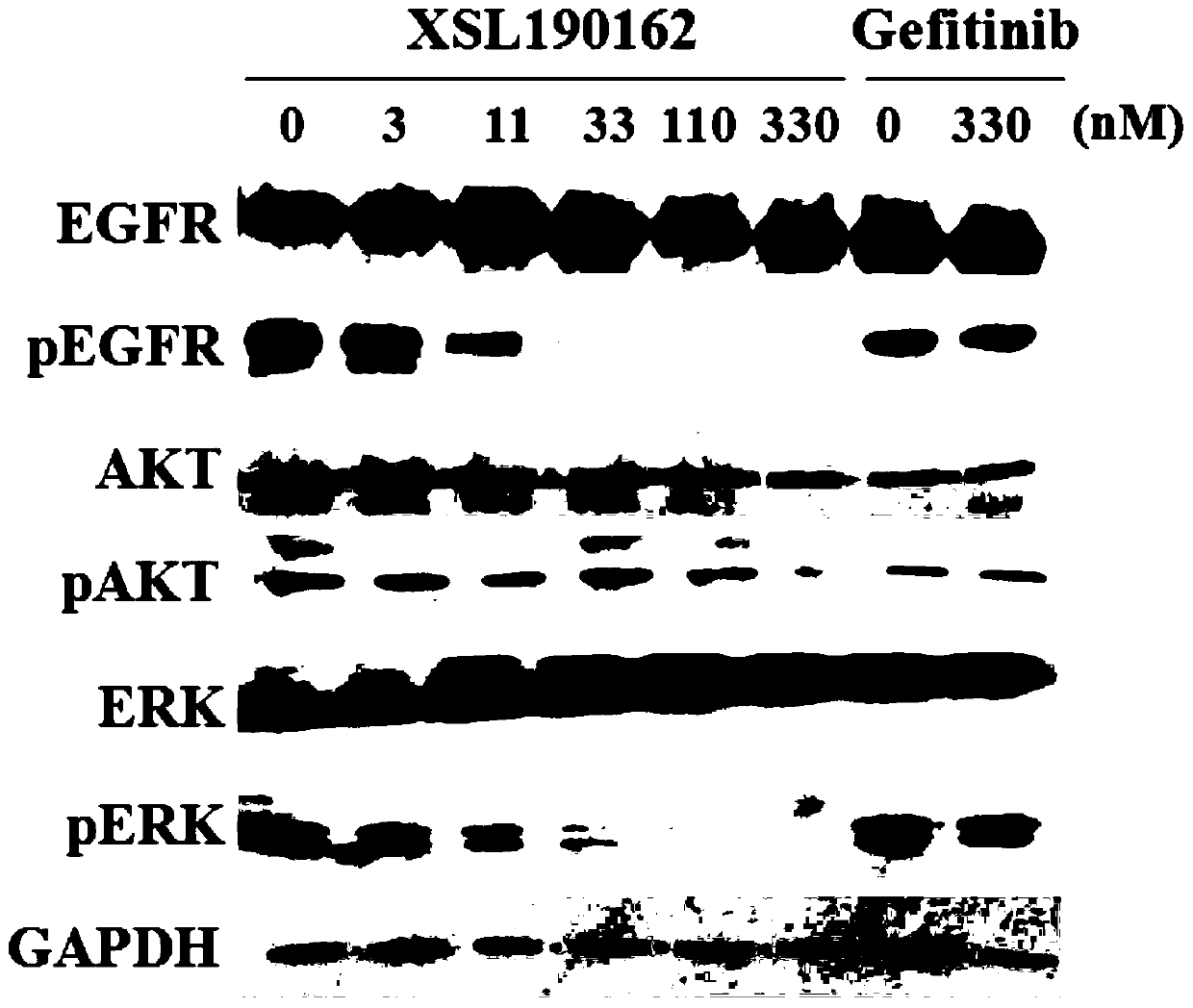
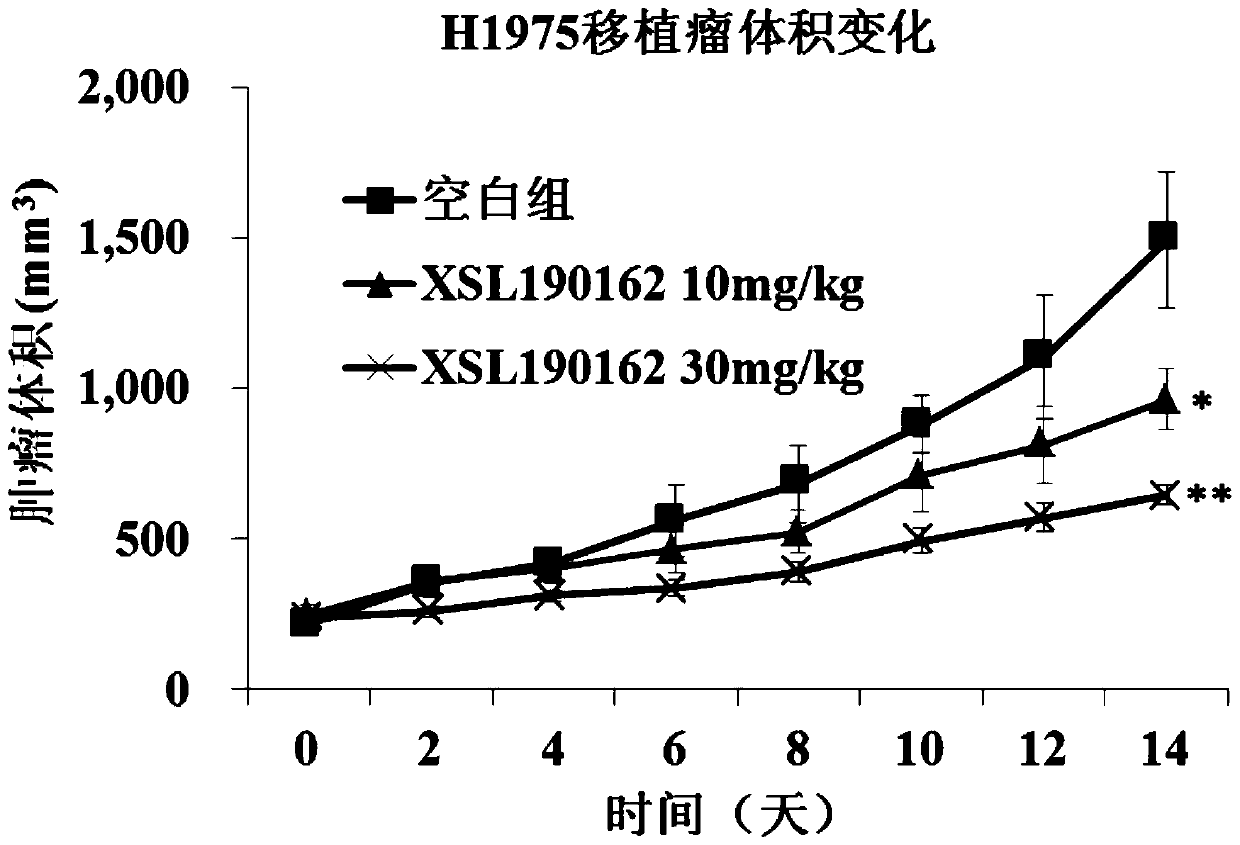
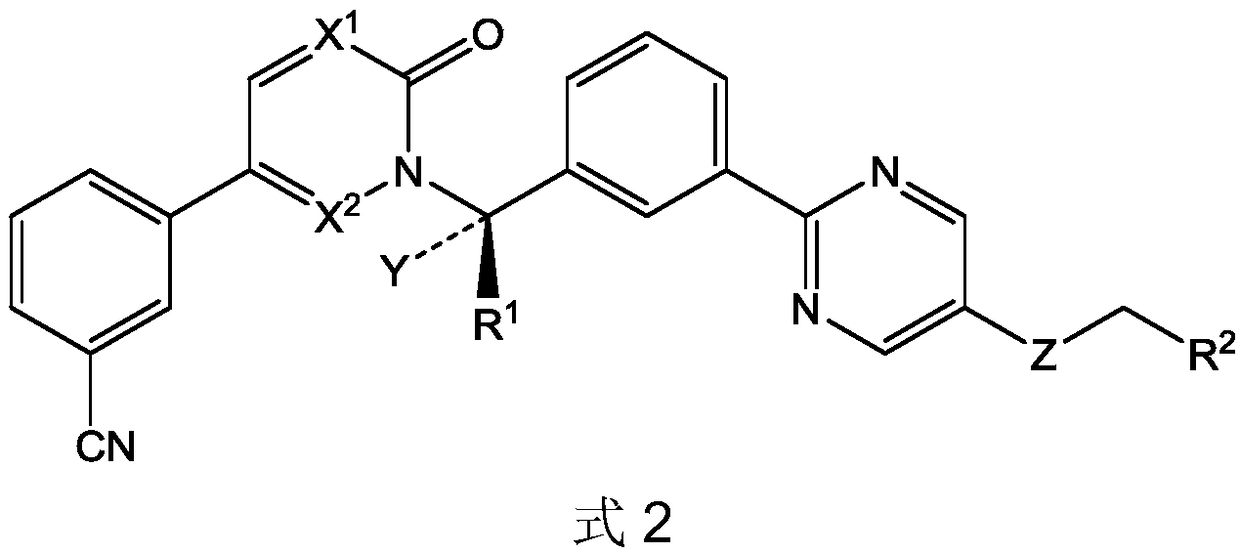
ActiveCN112142745AImprove anti-tumor effectGood pharmacokinetic propertiesOrganic active ingredientsOrganic chemistryImmunologic disordersAutoimmune condition
The invention discloses a novel substituted pyrazolopyrimidine compound for inhibiting the activity of casein kinase 1[epsilon] (CK1[epsilon]), a stereoisomer or a stereoisomer mixture of the novel substituted pyrazolopyrimidine compound, a pharmaceutically acceptable salt or solvate of the novel substituted pyrazolopyrimidine compound, and application of the compound to preparation of medicine for treating diseases, disorders or symptoms benefiting from the inhibition of the activity of casein kinase 1[epsilon] (CK1[epsilon]). The compound has inhibitory activity on CK1[epsilon] kinase, OCI-LY10 cells and Karpas299 cells, shows good anti-tumor activity in an OCI-LY10 subcutaneous xenogeneic model, shows excellent synergistic anti-tumor activity when being combined with a BTK inhibitor, has good pharmacokinetic properties, and can be applied to treatment of diseases, disorders or symptoms, including cancers, autoimmune diseases and the like, which benefit from inhibition of casein kinase 1[epsilon] activity, alone or in combination with other drugs.
Owner:HANGZHOU HERTZ PHARMA
Salts prepared from 2-(1-acyloxy-n-amyl)benzoic acid and basic amino acid or aminoguanidine, and preparation method and application thereof
ActiveCN109678715AImprove performanceGood water solubilityOrganic active ingredientsOrganic compound preparationSolubilityAcute toxicity testing
The invention discloses salts prepared from 2-(1-acyloxy-n-amyl)benzoic acid and basic amino acid or aminoguanidine, a preparation method thereof, a medicinal preparation containing the salts, and application of the salts to preparing of medicines used for preventing or treating ischemic cardiovascular and cerebral vascular diseases, resisting thrombus and relieving cardio-cerebral circulatory disturbance. A compound not only has good water solubility, aqueous solution stability and pharmacokinetics performance, but also has strong activity for resisting platelet aggregation, resisting thrombus, resisting cerebral ischemia and protecting nerves, the effect is superior to that of (S)-butylphthalide and (R / S)-2-(1-hydroxy-n-amyl)potassium benzoate salt (PHPB), acute toxicity of the compoundto mouse intravenous injection dosing is remarkably lower than that of butylphthalide and PHPB, the inhibition ratio of the compound to an hERG potassium channel of a CHO-hERG cell is lower than thatof the (S)-butylphthalide, and a result of a bacterial reverse mutation test (Ames test) is negative.
Owner:JIANGSU KANION PHARMA CO LTD
Novel nitroimidazole compound and application thereof in pharmacy
ActiveCN103450220AStrong anti-tuberculosis effectGood effectAntibacterial agentsOrganic active ingredientsPharmacyNitroimidazole
The invention relates to a novel nitroimidazole compound as well as a preparation method and an application thereof. The invention in particular relates to nitroimidazole compounds shown in a formula (I) and a preparation method thereof as well as an application of the compounds shown in the formula (I) and pharmaceutically acceptable salts of the compounds shown in the formula (I) in preparation of medicines used for treating diseases related to infection caused by mycobacterium tuberculosis.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com












![Pyrazolo[1, 5-a]pyridine compound and use thereof Pyrazolo[1, 5-a]pyridine compound and use thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/909a5abe-a2d3-4ecd-9adb-643e3707a5fe/HDA0000770441560000011.PNG)
![Pyrazolo[1, 5-a]pyridine compound and use thereof Pyrazolo[1, 5-a]pyridine compound and use thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/909a5abe-a2d3-4ecd-9adb-643e3707a5fe/HDA0000770441560000012.PNG)
![Pyrazolo[1, 5-a]pyridine compound and use thereof Pyrazolo[1, 5-a]pyridine compound and use thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/909a5abe-a2d3-4ecd-9adb-643e3707a5fe/HDA0000770441560000021.PNG)














![Casein kinase 1[epsilon] inhibitor, pharmaceutical composition and application thereof Casein kinase 1[epsilon] inhibitor, pharmaceutical composition and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0e134e61-8d45-49ed-8aba-e0572ad009e2/FDA0002551856950000011.png)
![Casein kinase 1[epsilon] inhibitor, pharmaceutical composition and application thereof Casein kinase 1[epsilon] inhibitor, pharmaceutical composition and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0e134e61-8d45-49ed-8aba-e0572ad009e2/FDA0002551856950000021.png)
![Casein kinase 1[epsilon] inhibitor, pharmaceutical composition and application thereof Casein kinase 1[epsilon] inhibitor, pharmaceutical composition and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0e134e61-8d45-49ed-8aba-e0572ad009e2/FDA0002551856950000031.png)