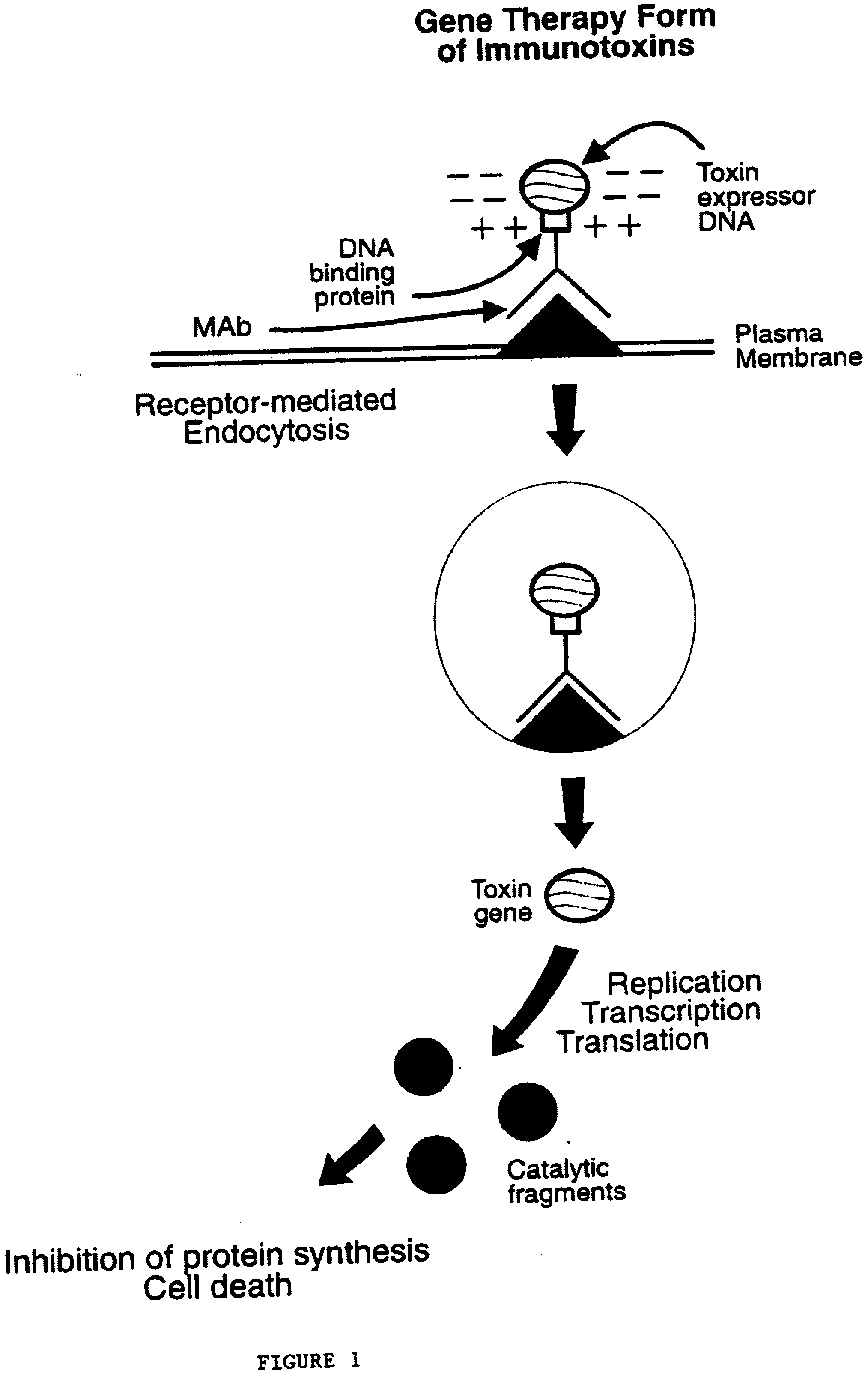
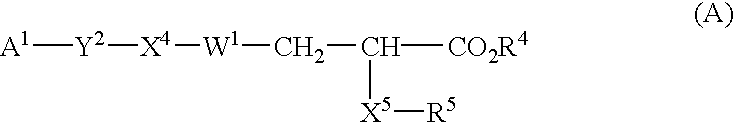Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
172results about How to "High binding activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antibodies and methods for generating genetically altered antibodies with enhanced effector function
InactiveUS20050054048A1Increase variabilityBetter pharmacokinetic profileAnimal cellsSugar derivativesGenetic diversityMonoclonal antibody
Dominant negative alleles of human mismatch repair genes can be used to generate hypermutable cells and organisms. By introducing these genes into cells and transgenic animals, new cell lines and animal varieties with novel and useful properties can be prepared more efficiently than by relying on the natural rate of mutation. These methods are useful for generating genetic diversity within immunoglobulin genes directed against an antigen of interest to produce altered antibodies with enhanced biochemical activity. Moreover, these methods are useful for generating antibody-producing cells with increased level of antibody production. The invention also provides methods for increasing the effector function of monoclonal antibodies and monoclonal antibodies with increased effector function.
Owner:EISAI INC
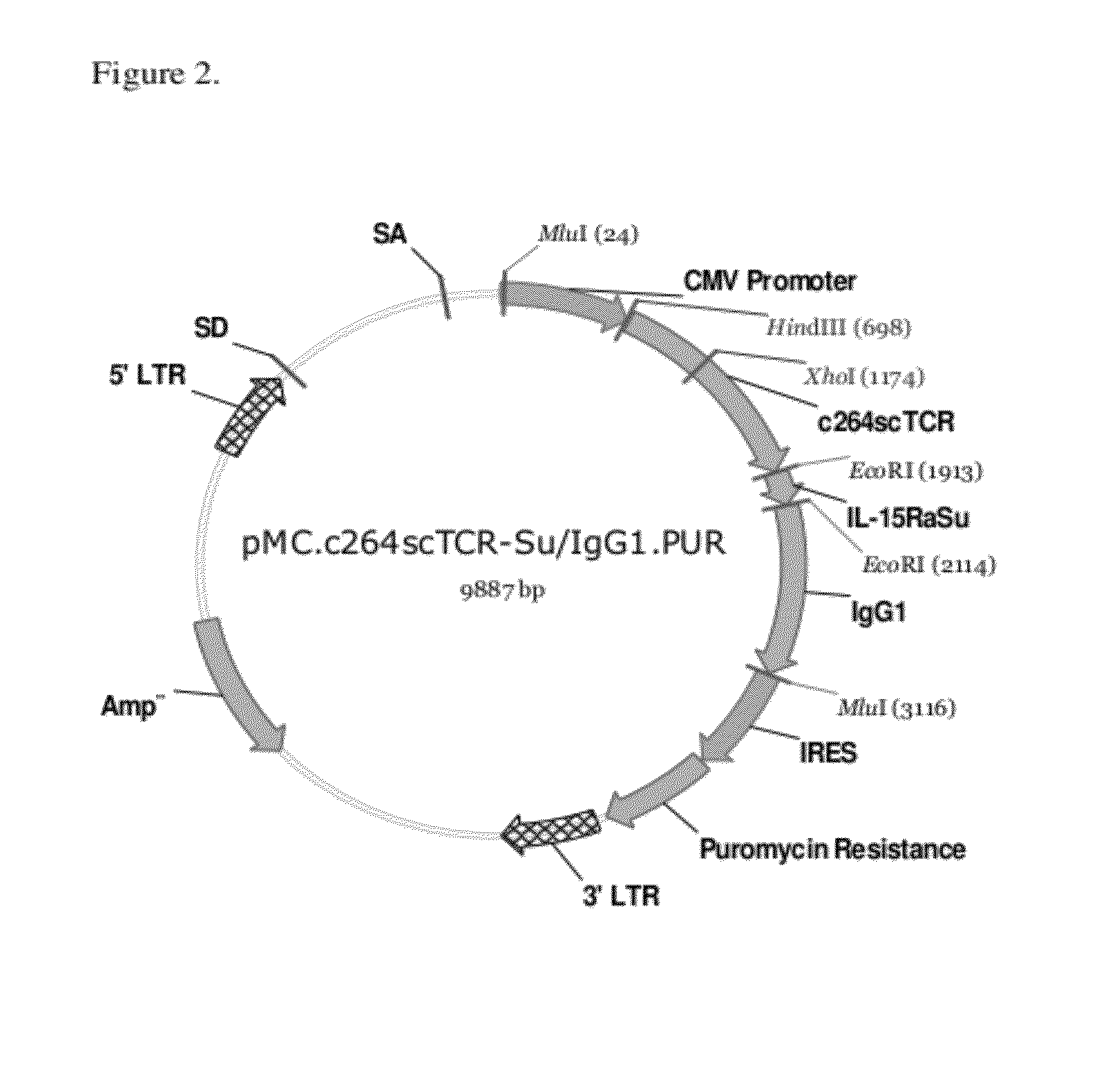
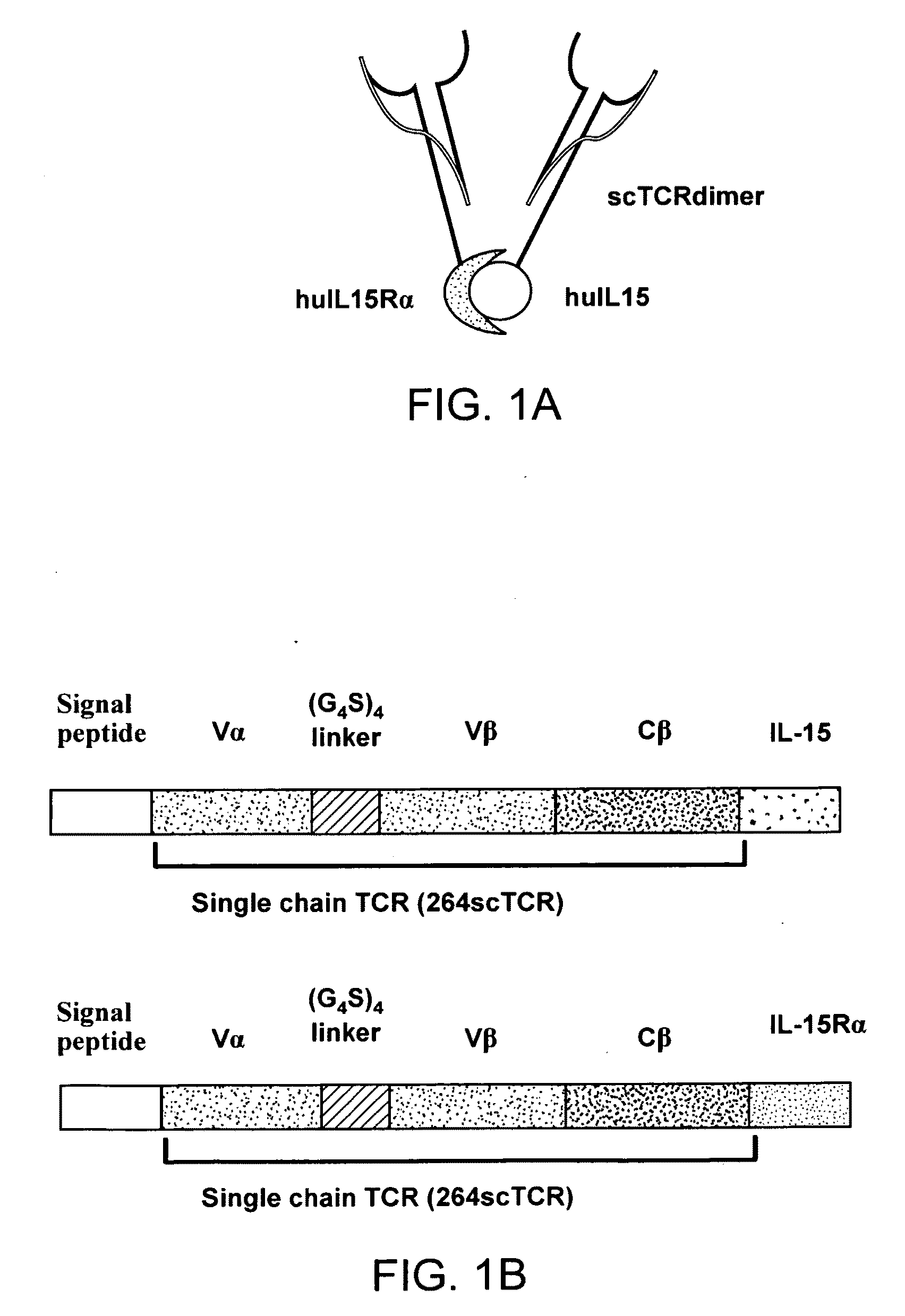
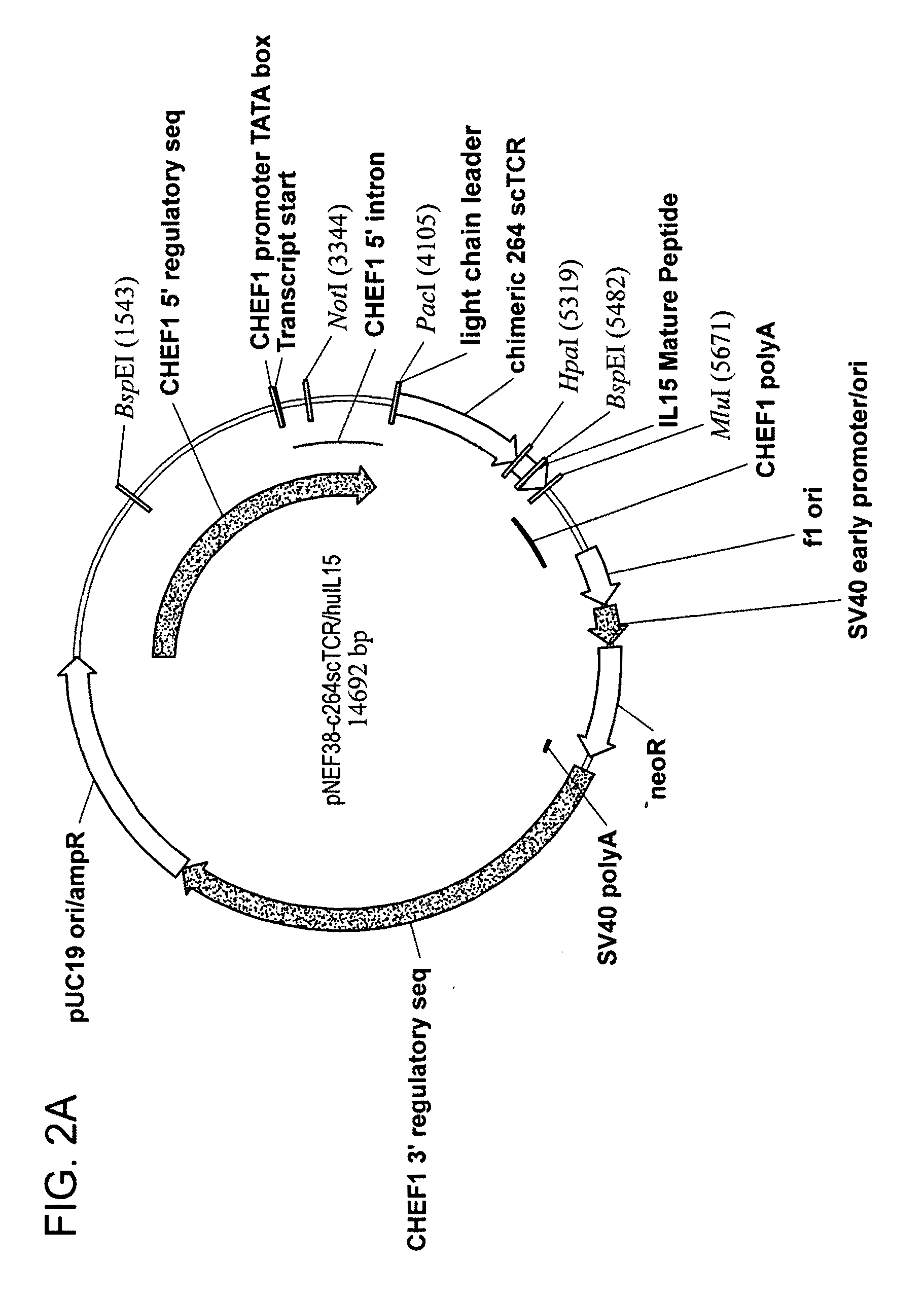
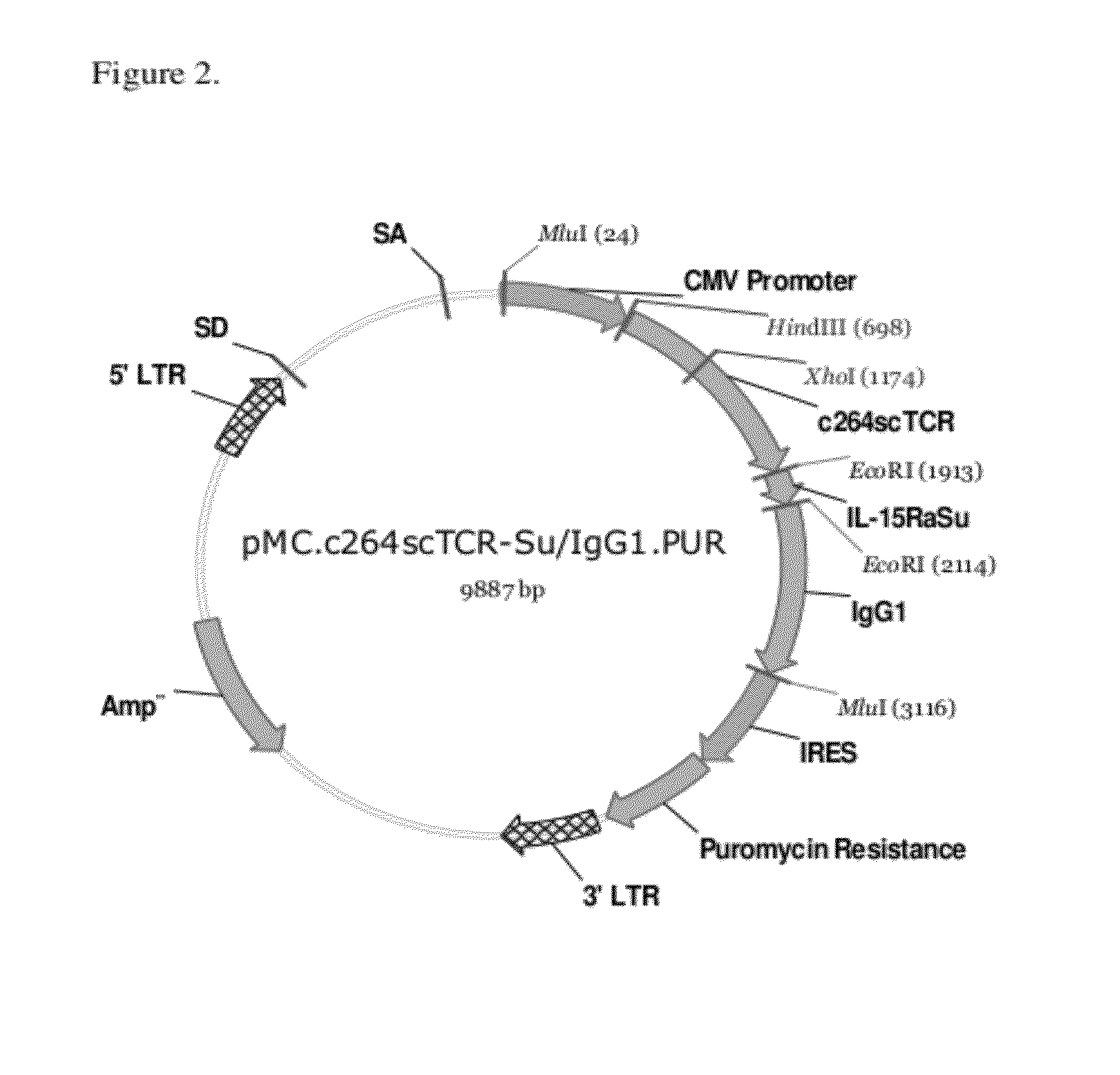
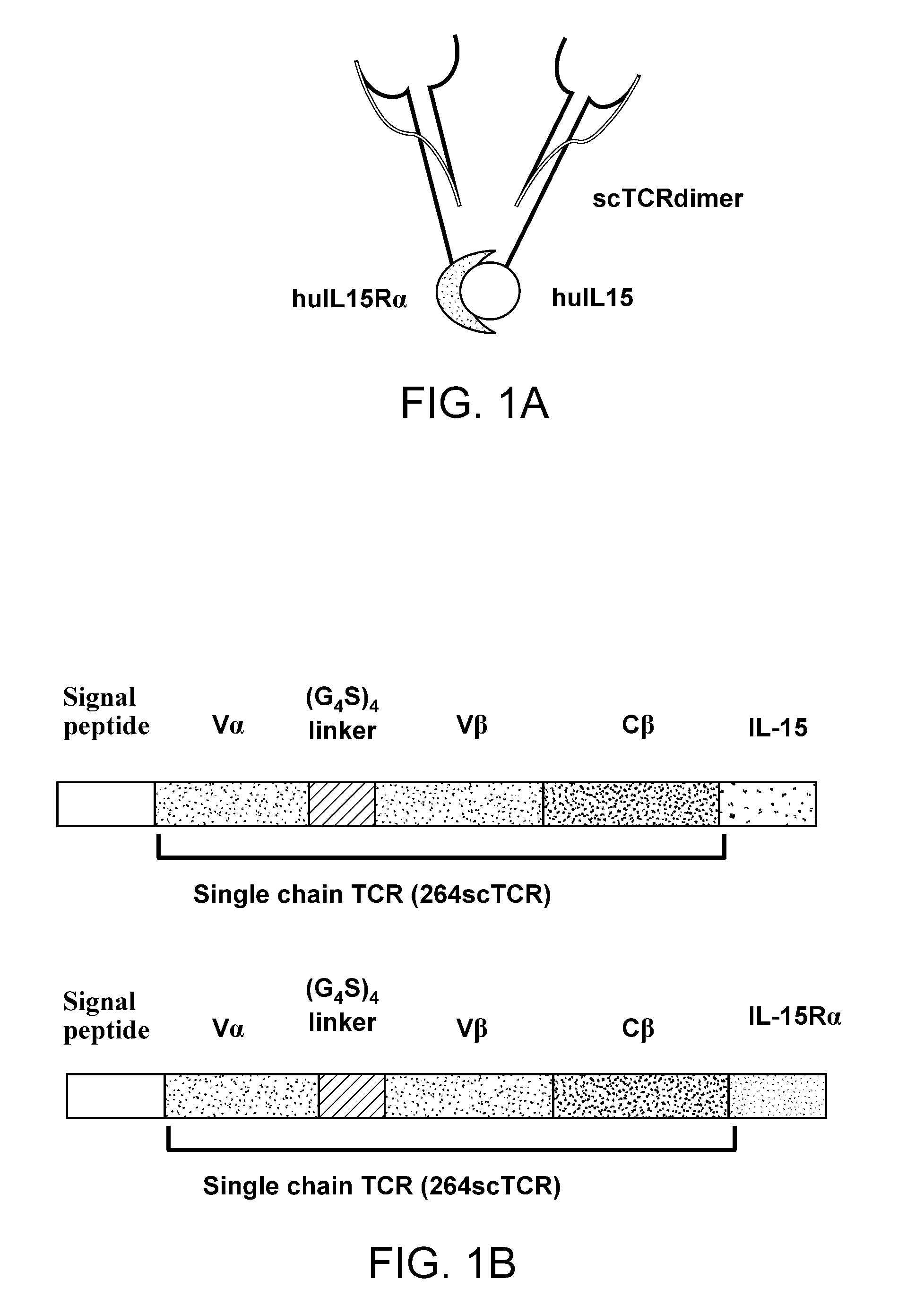
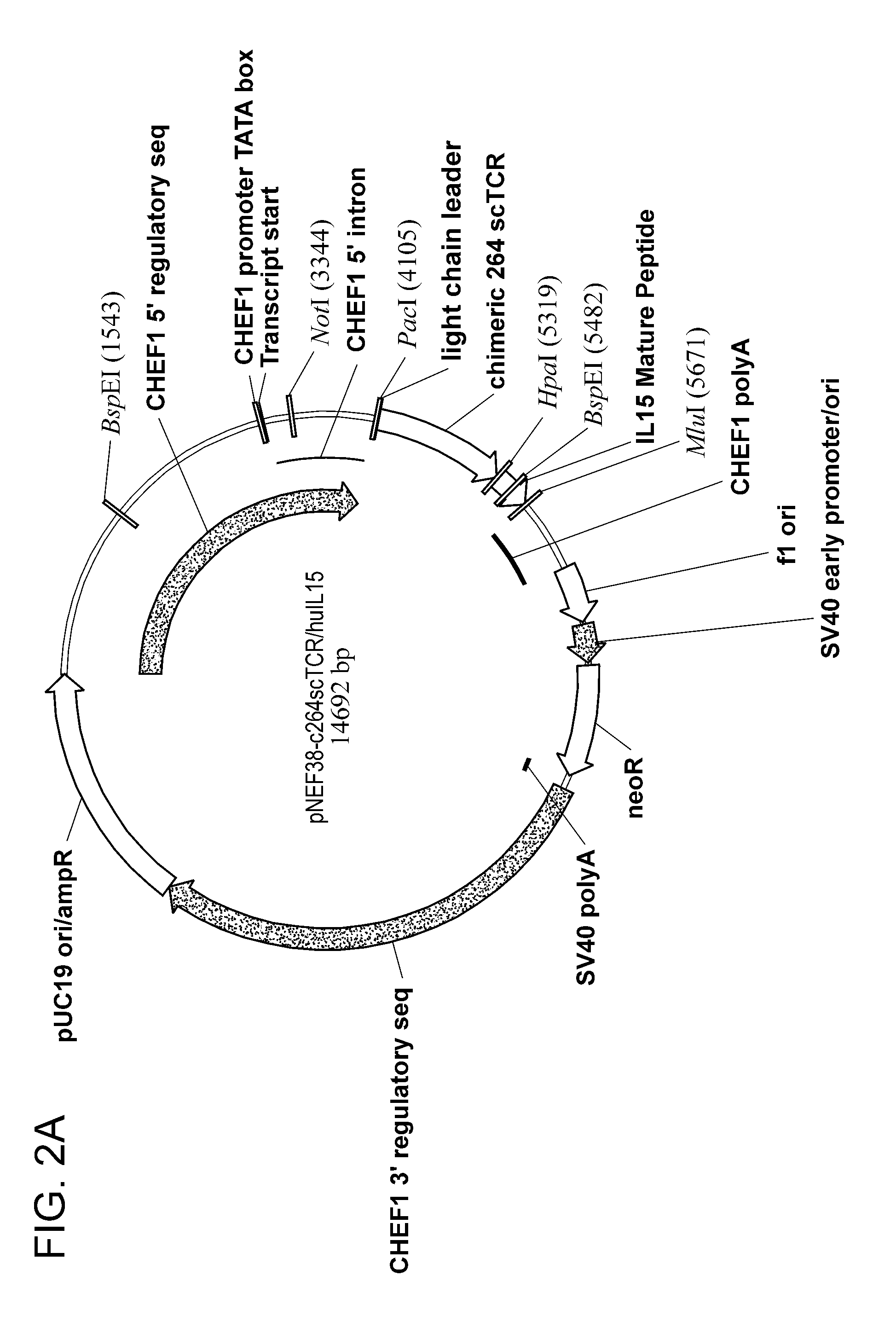
Multimeric IL-15 soluble fusion molecules and methods of making and using same
ActiveUS8507222B2High binding activityAvoid developmentOrganic active ingredientsBacteriaInterleukin-15 receptorCoordination complex
The invention provides soluble fusion protein complexes having at least two soluble fusion proteins. The first fusion protein is a biologically active polypeptide covalently linked to an interleukin-15 (IL-15) polypeptide or a functional fragment thereof. The second fusion protein is a second biologically active polypeptide covalently linked to a soluble interleukin-15 receptor alpha (IL-15Rα) polypeptide or a functional fragment thereof. In the complexes of the invention, one or both of the first and second fusion proteins further includes an immunoglobulin Fc domain or a functional fragment thereof; and the first fusion protein binds to the soluble IL-15Rα domain of the second fusion protein to form a soluble fusion protein complex. The invention further provides methods for making and using the complexes of the invention.
Owner:ALTOR BIOSCI LLC
Fusion molecules and IL-15 variants
ActiveUS20090324538A1Enhanced growthInhibit proliferationPeptide/protein ingredientsAntipyreticBiophysicsMammal
The instant invention provides soluble fusion protein complexes and IL-15 variants that have therapeutic and diagnostic use, and methods for making thesuch proteins. The instant invention additionally provides methods of stimulating or suppressing immune responses in a mammal using the fusion protein complexes and IL-15 variants of the invention.
Owner:ALTOR BIOSCI LLC
Multimeric il-15 soluble fusion molecules and methods of making and using same
ActiveUS20120177595A1Increasing per-molecule IL-1 activityHigh binding activityOrganic active ingredientsBacteriaChemistryInterleukin-15 receptor
The invention provides soluble fusion protein complexes having at least two soluble fusion proteins. The first fusion protein is a biologically active polypeptide covalently linked to an interleukin-15 (IL-15) polypeptide or a functional fragment thereof. The second fusion protein is a second biologically active polypeptide covalently linked to a soluble interleukin-15 receptor alpha (IL-15Rα) polypeptide or a functional fragment thereof. In the complexes of the invention, one or both of the first and second fusion proteins further includes an immunoglobulin Fc domain or a functional fragment thereof; and the first fusion protein binds to the soluble IL-15Rα domain of the second fusion protein to form a soluble fusion protein complex. The invention further provides methods for making and using the complexes of the invention.
Owner:ALTOR BIOSCI LLC
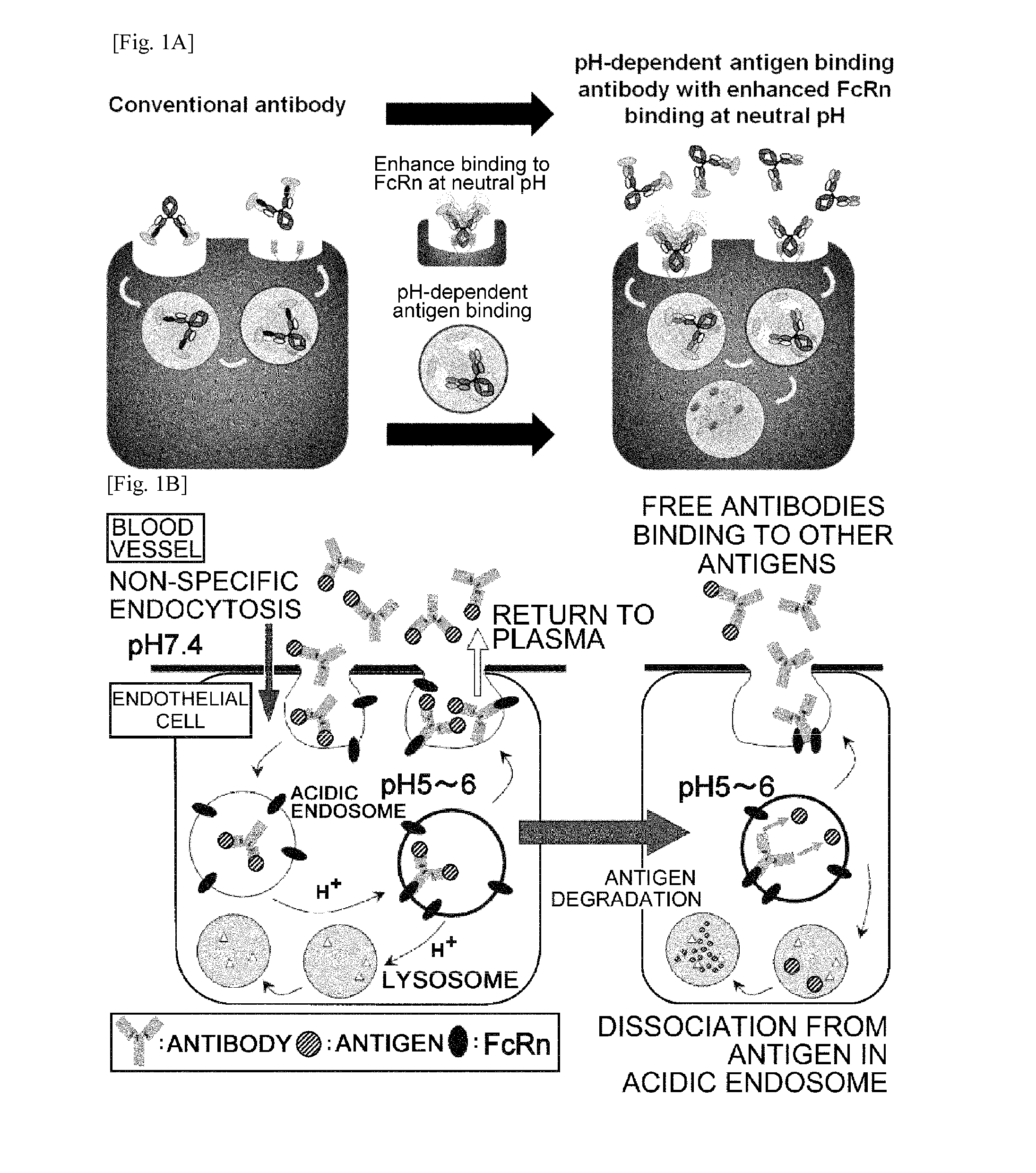
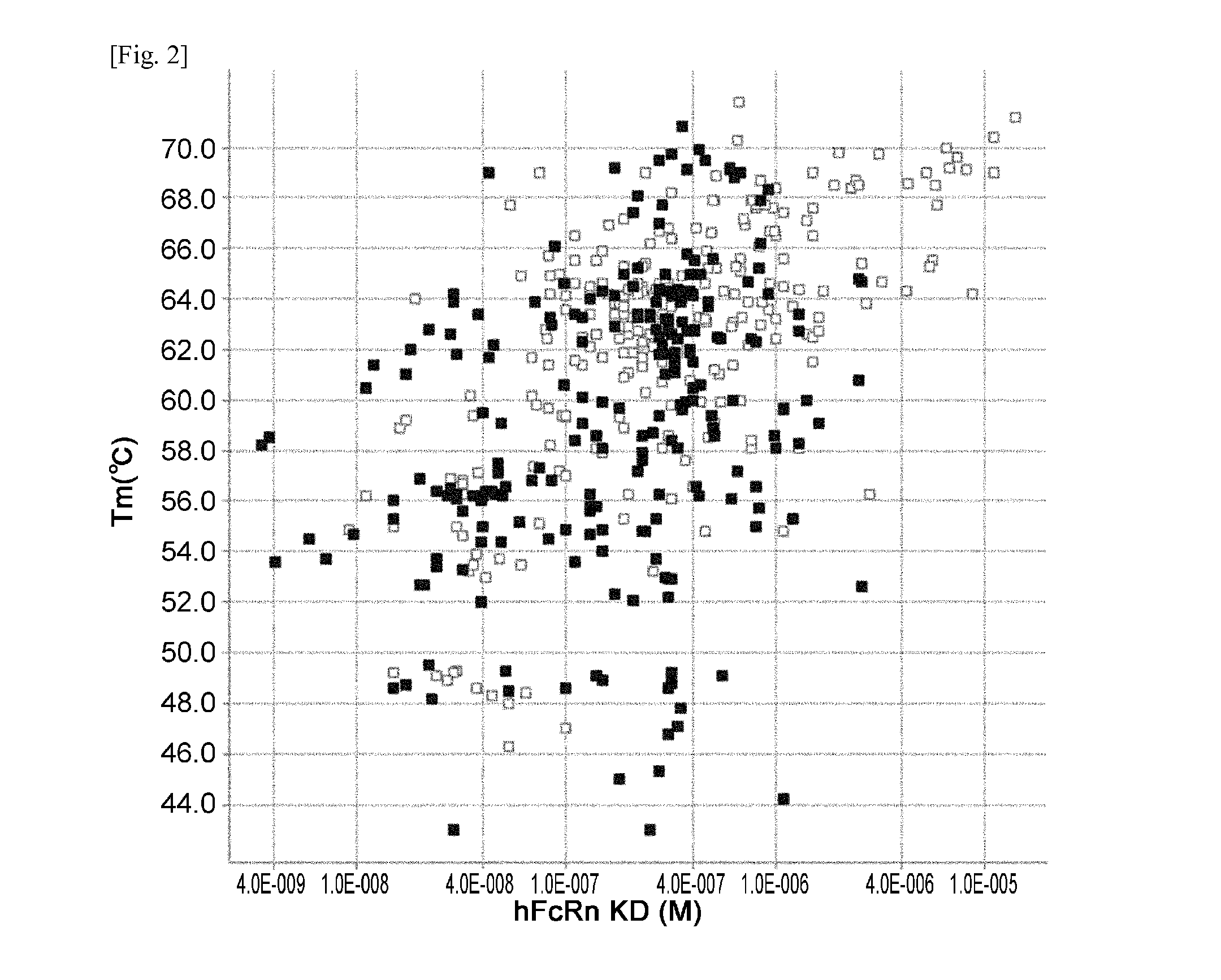
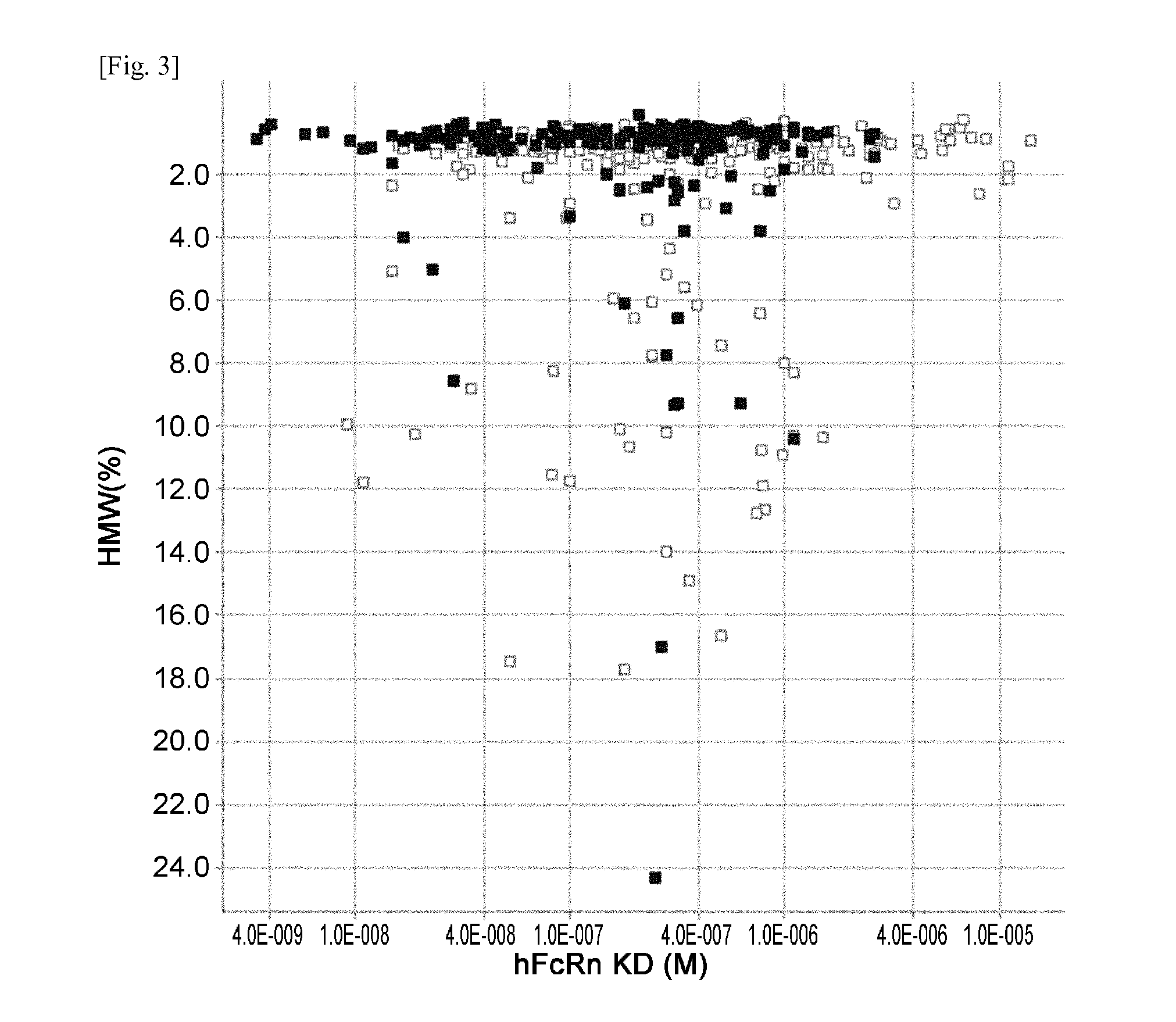
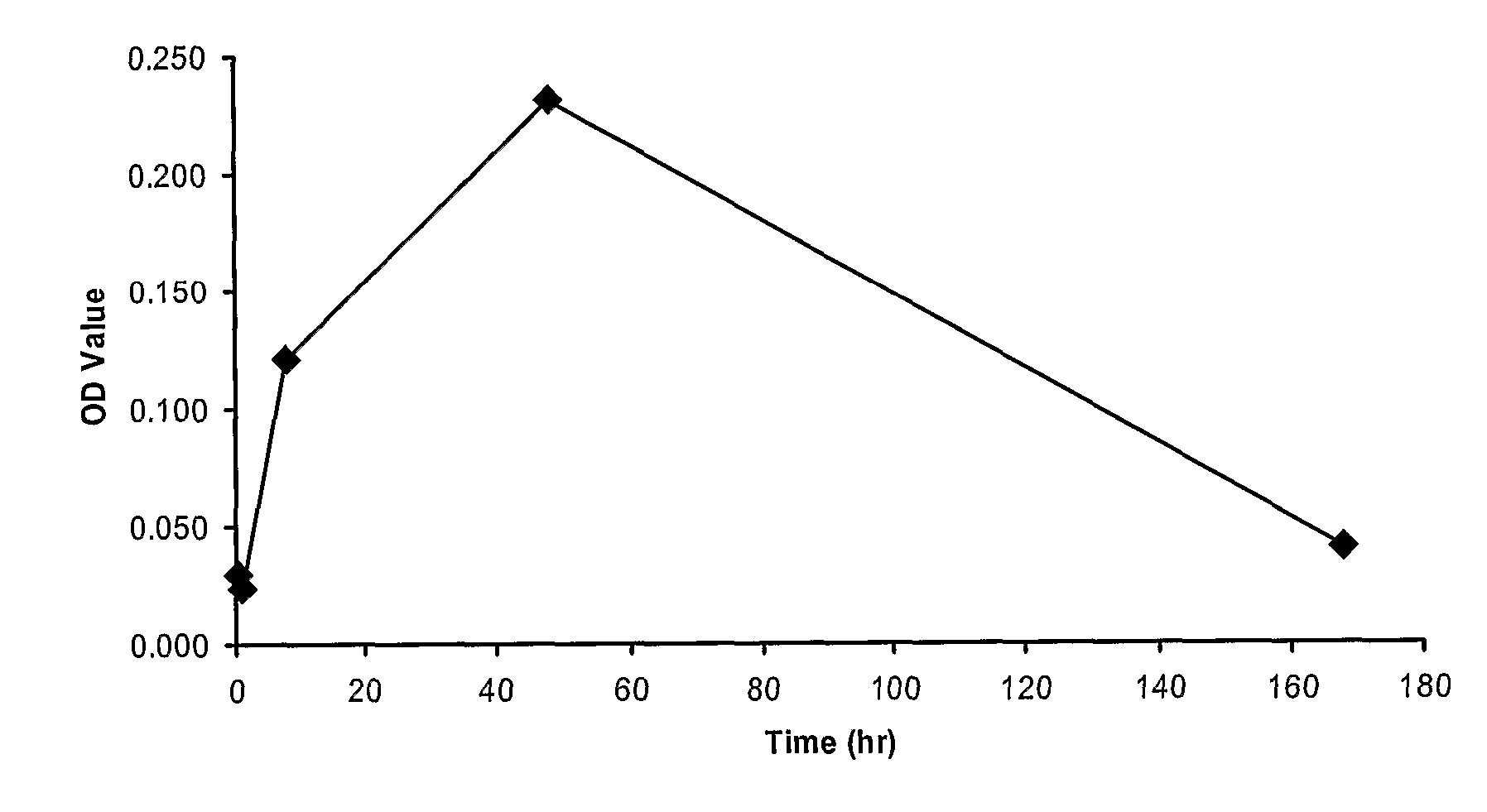
THERAPEUTIC ANTIGEN-BINDING MOLECULE WITH A FcRn-BINDING DOMAIN THAT PROMOTES ANTIGEN CLEARANCE
ActiveUS20140363428A1Low immunogenicityImprove stabilityAntipyreticAnalgesicsFc(alpha) receptorNeutral ph
The present invention provides: a modified FcRn-binding domain having an enhanced affinity for the Fc Receptor neonatal (FcRn) at neutral pH; an antigen-binding molecule comprising said FcRn-binding domain, which has low immunogenicity, high stability and form only a few aggregates; a modified antigen-binding molecule having an increased FcRn-binding activity at neutral or acidic pH without an increased binding activity at neutral pH for a pre-existing anti-drug antibody; use of the antigen-binding molecules for improving antigen-binding molecule-mediated antigen uptake into cells; use of the antigen-binding molecules for reducing the plasma concentration of a specific antigen; use of the modified FcRn-binding domain for increasing the total number of antigens to which a single antigen-binding molecule can bind before its degradation; use of the modified FcRn-binding domain for improving pharmacokinetics of an antigen-binding molecule; methods for decreasing the binding activity for a pre-existing anti-drug antibody; and methods for producing said antigen-binding molecules.
Owner:CHUGAI PHARMA CO LTD
Ligands That Enhance Endogenous Compounds
InactiveUS20070298041A1Increase the amount addedHigh activityVirusesImmunoglobulins against cytokines/lymphokines/interferonsHalf-lifeReactive site
The invention relates to ligands that comprise a moiety (e.g., a dAb) that has a binding site with binding specificity for an endogenous target compound but do not substantially inhibit the activity of said endogenous target compound. Preferably, the ligand does not bind to the active site of an endogenous target compound. The invention relates to the use of such a ligand for the manufacture of a medicament for increasing the half-life, bioavailability, activity or amount of an endogenous target compound to which the ligand binds.
Owner:DORMANTIS LTD
Novel antibody molecule aiming at human CLDN18.2, antigen binding fragment and medical application thereof
ActiveCN110606891AHigh binding activityInanimate material medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsComplement-dependent cytotoxicityAntigen Binding Fragment
The invention discloses an anti-human CLDN18.2 antibody as well as an antigen binding fragment and a medical application thereof. Specifically, the present invention relates to a murine antibody comprising a CDR region of the anti-human CLDN18.2 antibody, a chimeric antibody and a humanized antibody, a fully humanized antibody, an antibody-dependent cytotoxicity (ADCC) comprising the antibody andthe antigen binding fragment thereof, complement dependent cytotoxicity (CDC), the killing effect on CLDN18.2 positive cells, the effect of inhibiting the growth of CLDN18.2 positive tumors, the in-vivo drug effect, a medicine containing the anti-human CLDN18.2 antibody and the antigen binding fragment thereof, a composition of the medicine thereof, and an application of the medicine in tumor treatment, in particular to treatment of CLDN18.2 positive tumor patients..
Owner:L&L BIOPHARMA CO LTD
Acetylcholine receptor-mediated targeting D-configuration polypeptide and application thereof
ActiveCN104558117AHigh binding activityImprove stabilityPowder deliveryNervous disorderDiseaseIn vivo
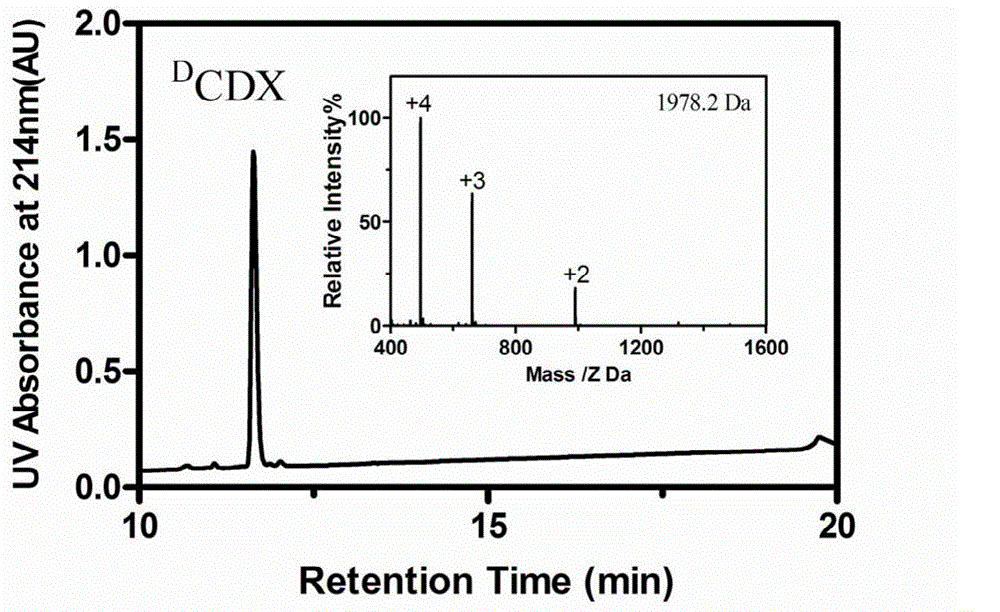
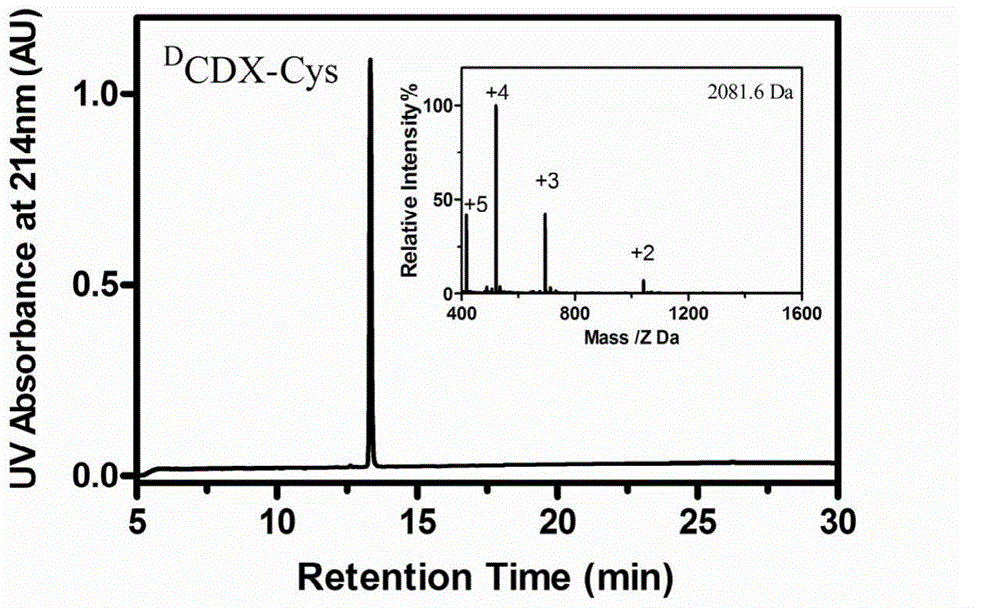
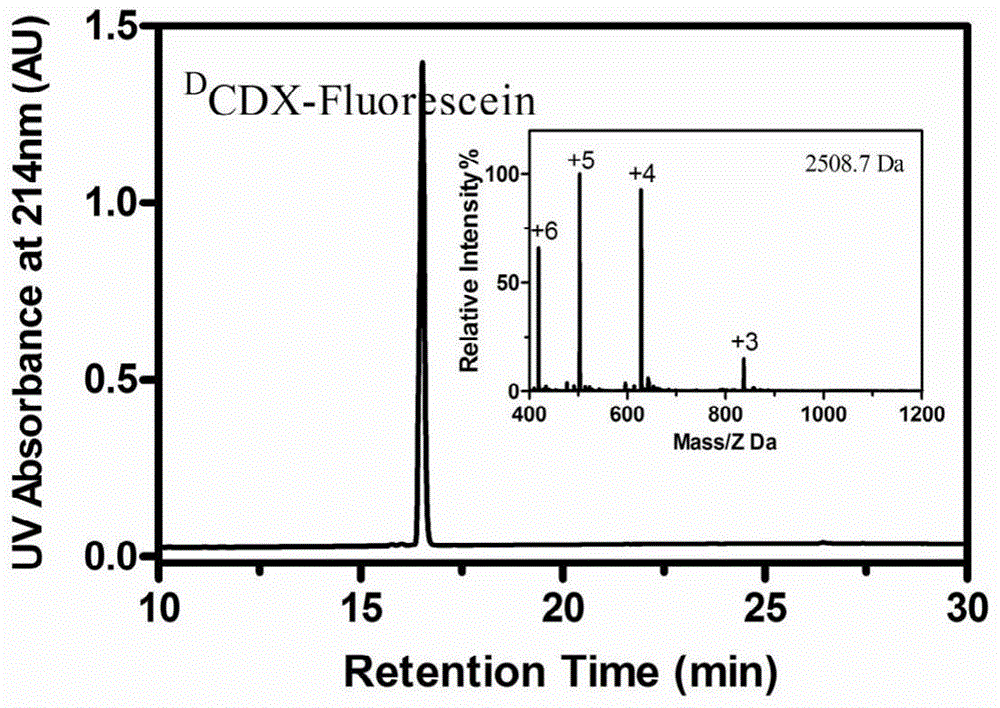
The invention belongs to the field of medicine, and relates to D-configuration polypeptide with high stability and capable of realizing mediated targeting of acetylcholine receptor high-expression cells and crossing corresponding barrier membranes and a nano drug delivery system thereof as well as an application in in-vivo and in-vitro brain targeting and in treatment of brain diseases and the like. Test results indicate that DCDX and the acetylcholine receptor are combined with IC50 to obtain 84.5nM which is stable in serum and tolerates hydrolysis of protease; the model drug carried by DCDX is specifically taken in by the positive cells expressing the acetylcholine receptor and has an ability of crossing the barrier formed by the kind of cells; and the nano drug delivery system made of a DCDX-modified polymer carrier material can deliver the entrapped model drug to the target tissue while the drug effect is remarkably improved. The D-configuration polypeptide DCDX provided by the invention can mediate active targeting of the drug or nano drug delivery system and has a good application prospect in the diagnosis and treatment of multiple diseases.
Owner:FUDAN UNIV
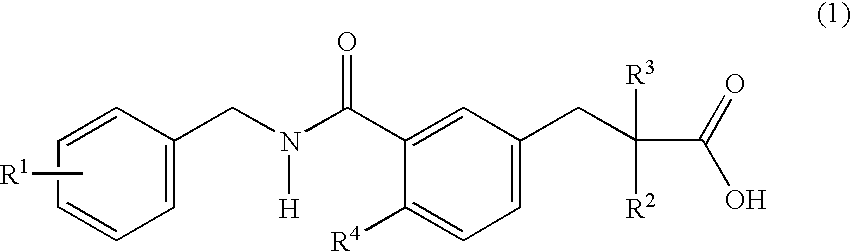
Substituted phenylpropionic acid derivatives as agonists to human peroxisome proliferator-activated receptor (PPAR) alpha
InactiveUS6506797B1Improve securityHigh binding activityBiocideOrganic chemistry3-phenylpropanoic acidAcid derivative
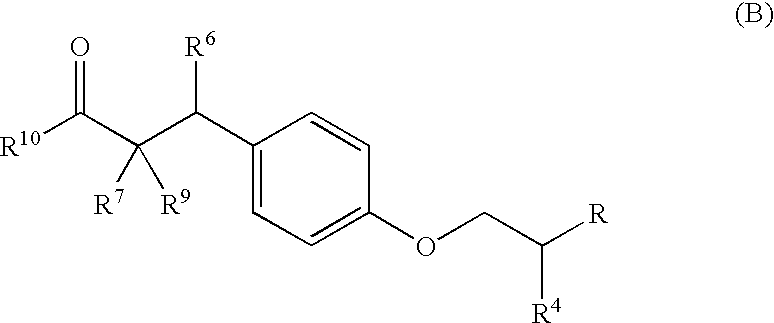
The invention provides novel substituted phenylpropanoic acid derivatives that activate by binding to receptor as ligands of human peroxisome preliferant-activated receptor alpha (PPARalpha), and exhibit potent decreasing action on lipids in blood (cholesterol and triglyceride).It relates to a substituted phenylpropanoic acid derivatives represented by a general formula (1),their pharmaceutically acceptable salts and their hydrates, and processes for preparing them.
Owner:KYORIN PHARMA CO LTD
Methods for increasing red blood cell levels and treating sickle-cell disease
InactiveUS20150361163A1Increase productionIncreased erythropoiesisSenses disorderPeptide/protein ingredientsPrimateSickle Cell Diseases
In certain aspects, the present disclosure provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans. In some embodiments, the compositions of the disclosure may be used to treat or prevent sickle-cell disease or one or more complications associated with sickle-cell disease.
Owner:ACCELERON PHARMA INC
D-configuration polypeptide with brain tumor targeting and tumor tissue penetrating capabilities and gene delivery system thereof
ActiveCN104072581AHigh transfection efficiencyProlong lifeGenetic material ingredientsPeptidesGene deliveryNeuropilins
The invention belongs to the field of pharmacy and relates to a D-configuration polypeptide and a gene delivery system of the D-configuration polypeptide and particularly relates to the D-configuration polypeptide which has high combining activity with neuropilin NRP-1 and has the brain tumor targeting and tumor tissue penetrating capabilities. The D-configuration polypeptide provided by the invention can mediate a nano delivery system to deliver drugs to tumors in a targeted manner for realizing targeted treatment of brain in-situ glioma. In vivo and in vitro experiments show that the genetic vector modified by the D-configuration polypeptide can remarkably improve the gene transfection efficiency. The genetic vector entrapping the therapeutic gene pORF-hTRAIL can remarkably prolong the lifetime of a nude mouse of brain glioma in-situ mode.
Owner:FUDAN UNIV
Antibody drug conjugate of CLDN 18.2-resistant antibody and preparation method and application of antibody drug conjugate
PendingCN111110862AGood pharmacokinetic propertiesPrevent proliferationOrganic active ingredientsImmunoglobulins against animals/humansDrug conjugationComplementarity determining region
The present invention discloses an antibody drug conjugate of a CLDN 18.2-resistant antibody. The structure of the antibody drug conjugate (ADC) is shown as Ab-[(L2)n-L1-D]y in a formula I, wherein Dis a small-molecule cytotoxic drug, L1 and L2 are connected to the drug and the antibody separately, and n is 0 or 1; y represents the average number of D which is coupled to Ab, 0 < y <= 10, and Ab is an antibody which can be specifically bound to human CLDN 18.2, and includes a light-chain variable region (VL) and / or a heavy-chain variable region (VH); and the CLDN 18.2-resistant antibody correspondingly contains at least one specific complementary determining region (CDR) sequence or a mutant sequence of the specific complementary determining region (CDR) sequence in the light-chain variable region (VL) and / or a heavy-chain variable region (VH), and binding of the antibody to CLDN 18.2 is maintained or improved through the mutation. The invention also discloses a ADC-containing pharmaceutical composition and a preparation method and application of ADC. The antibody drug conjugate of the antibody has a large security window and low toxic and side effects, and provides more specific,more effective and better treatment option for tumor patients.
Owner:L&L BIOPHARMA CO LTD
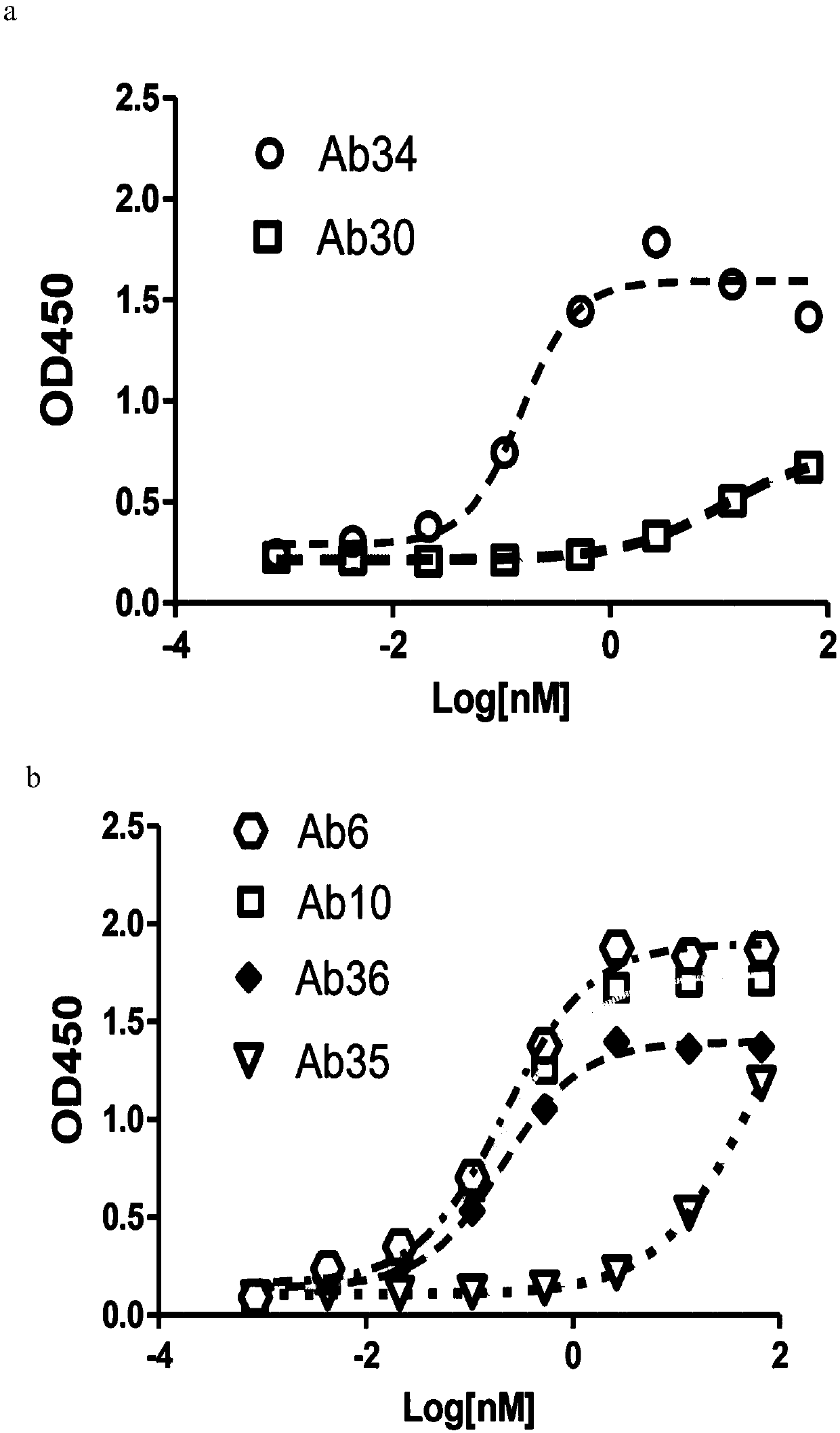
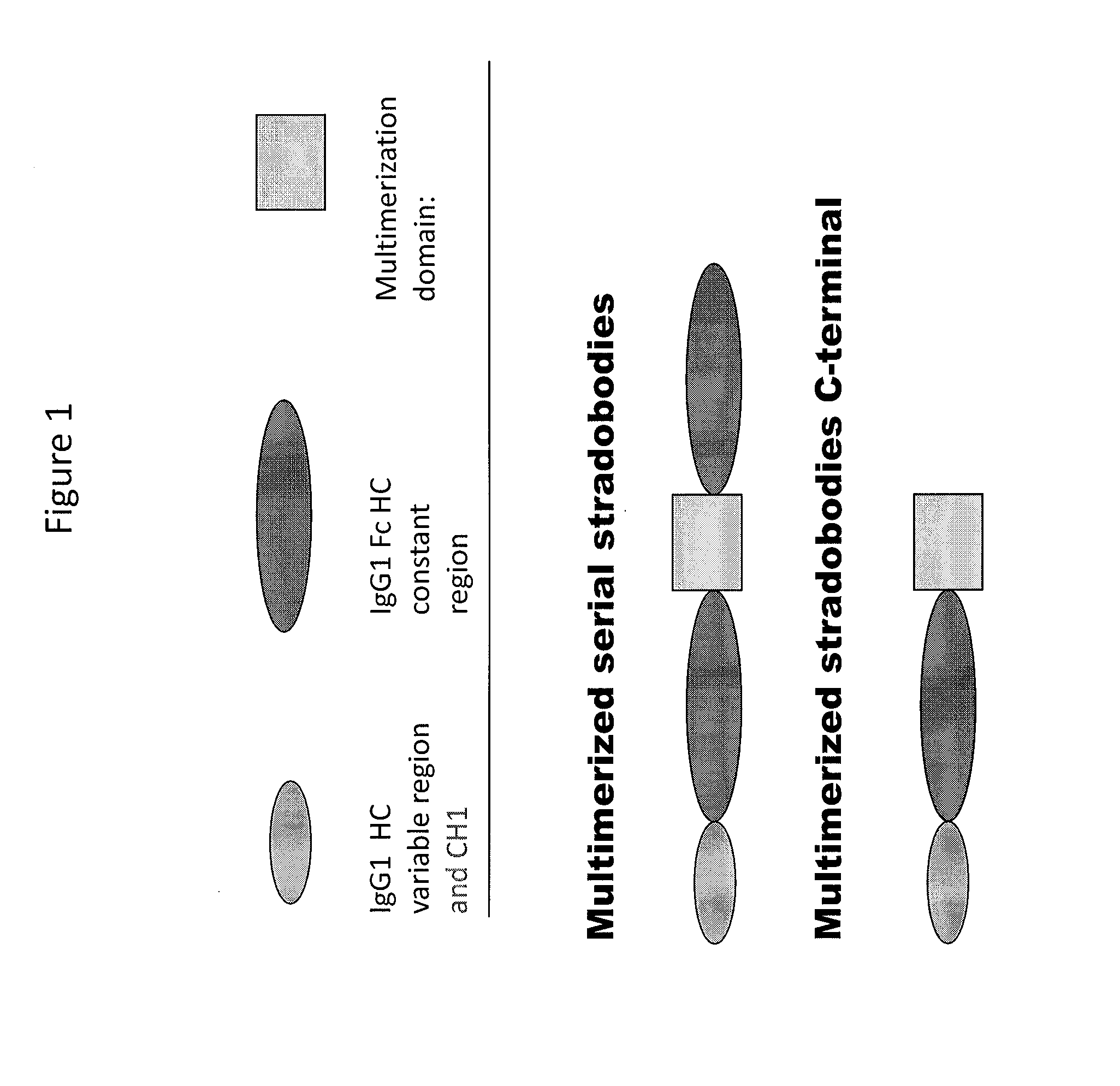
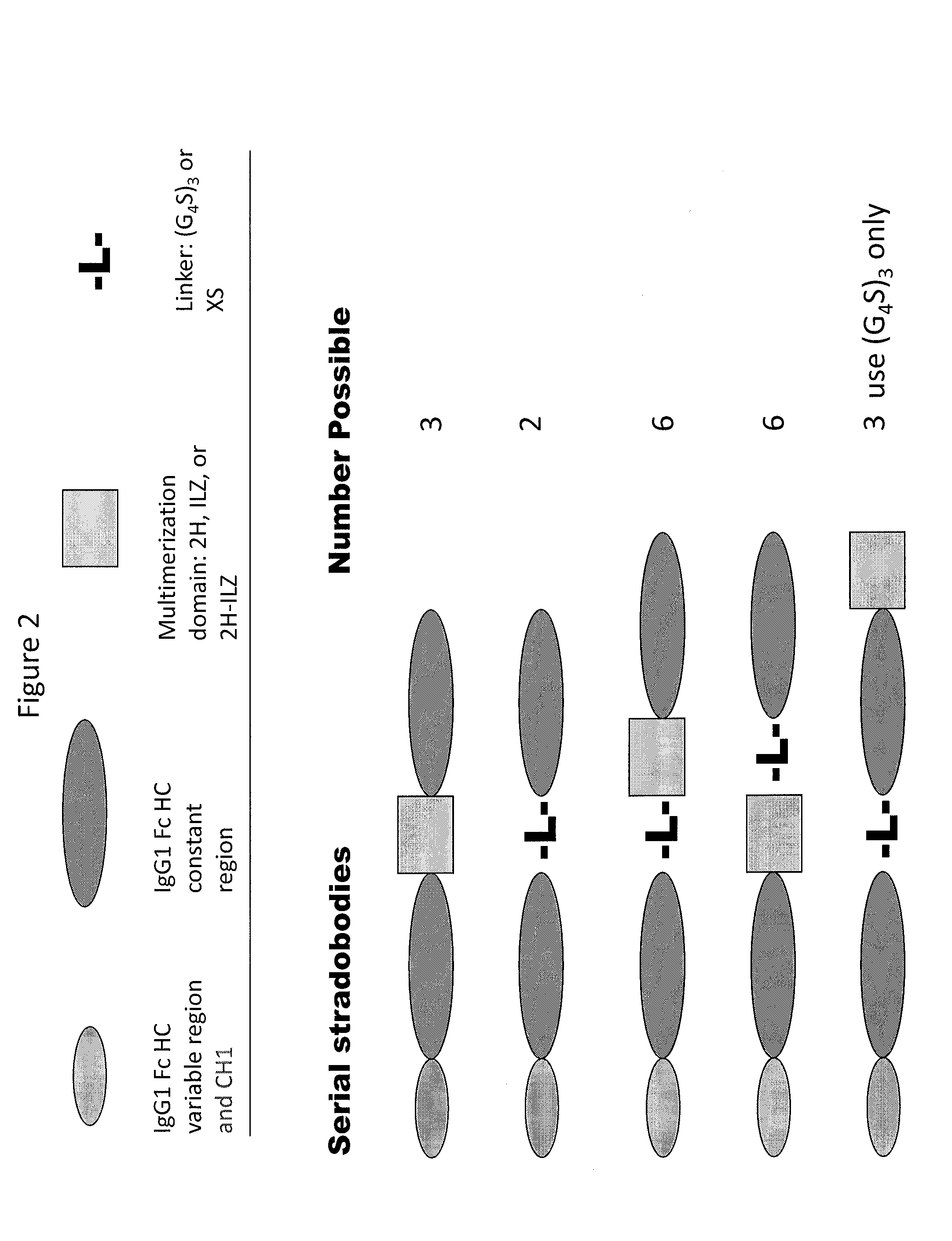
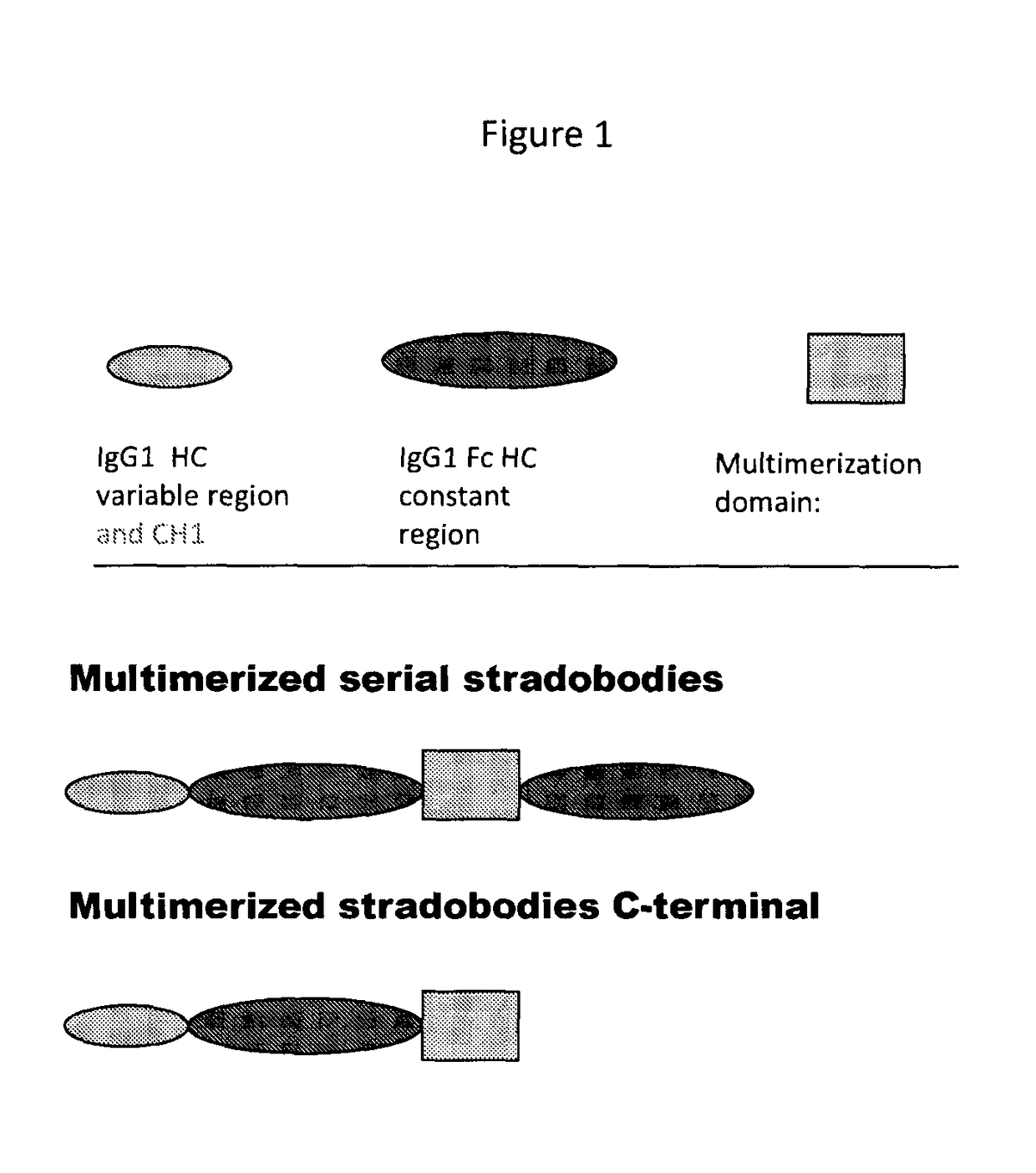
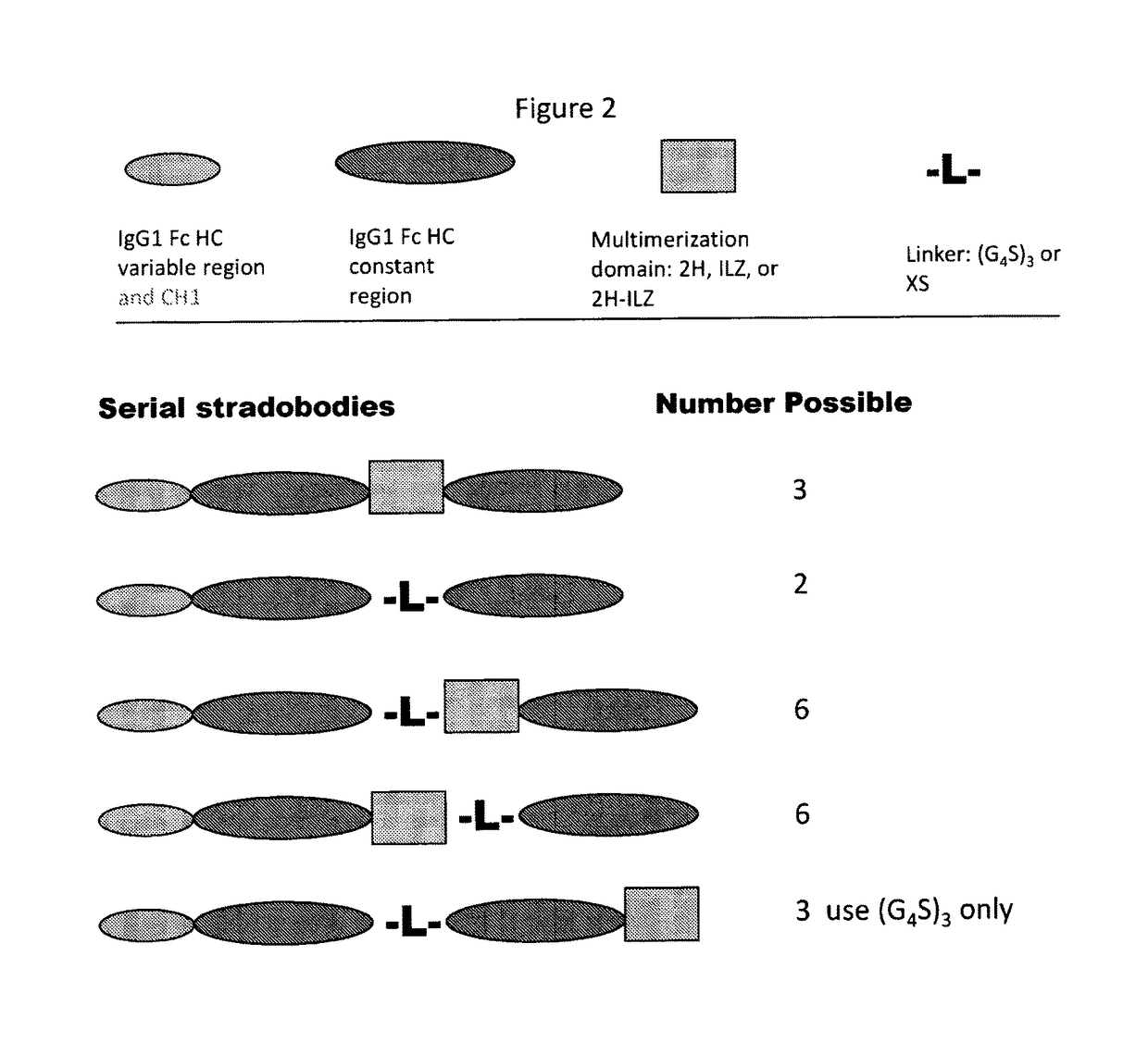
Molecules with antigen binding and polyvalent fc gamma receptor binding activity
ActiveUS20140072582A1Potent antibody-mediated cell cytoxicityPotent complement-dependent cell cytoxicity and direct cytotoxicityAntibacterial agentsSenses disorderFc receptorAntigen binding
The current invention involves biologically active proteins termed stradobodies. The stradobodies have two or more domains that create stradobody multimers. The stradobodies have both antigen-binding capacity and the ability to bind Fc receptors (FcR), and are useful in the treatment and prevention of disease.
Owner:GLIKNIK
Nucleic acid delivery system, methods of synthesis and use thereof
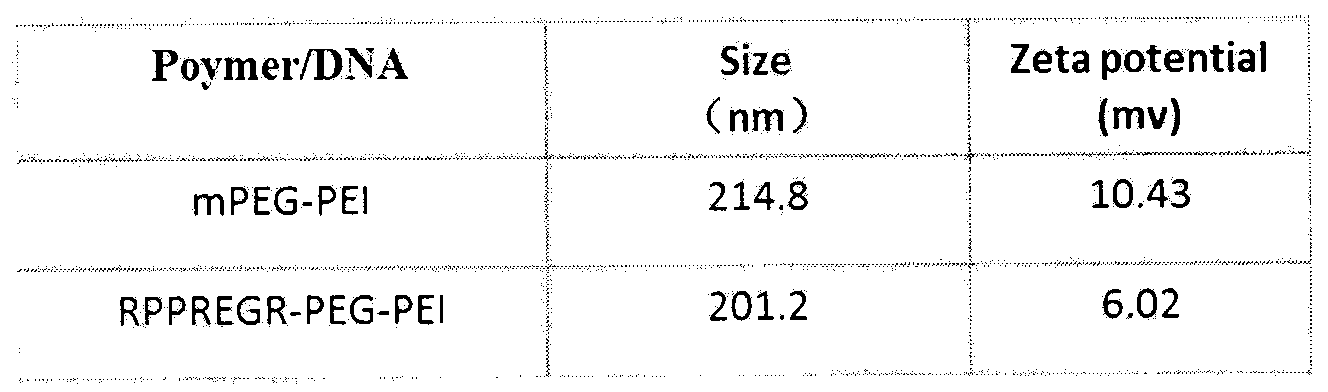
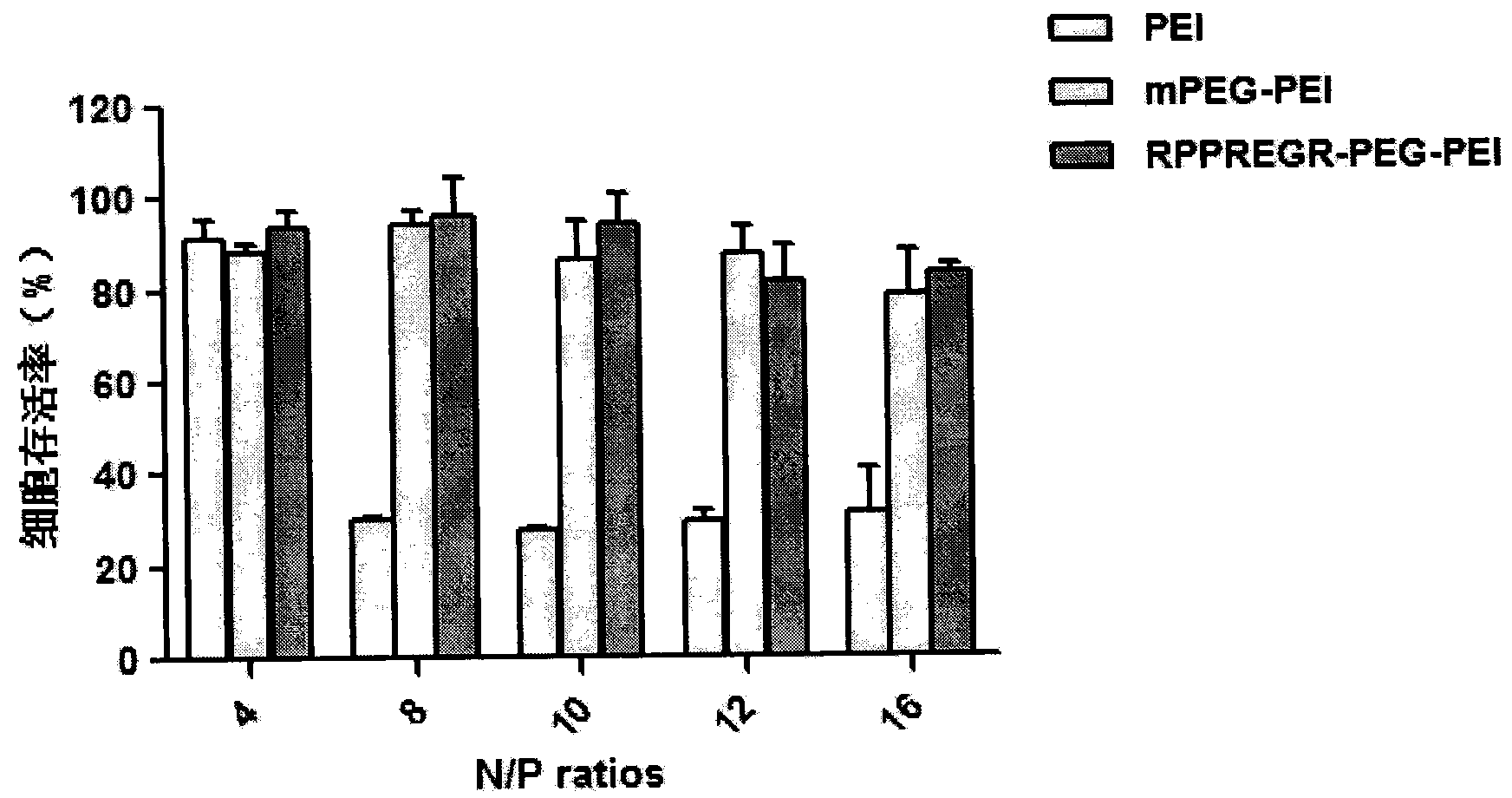
InactiveUS20040023902A1Efficiently produceHigh binding activityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntibodyMolecular biology
A nucleic acid delivery system is described. The delivery system contains a fusion protein having a target moiety and a nucleic acid binding moiety, and a nucleic acid sequence bound to the nucleic acid binding moiety of the fusion protein. The target moiety can be an antibody or a ligand. The use of this nucleic acid delivery system to transienntly or stably express a desired nucleic acid sequence in a cell is disclosed. Also disclosed is the use of this delivery system to target a cell and deliver a desired product.
Owner:DANA FARBER CANCER INST INC
Preparation method and applications of monoclonal antibody against human CD47
ActiveCN109438576AHigh binding activityAntigen epitope identification is clearImmunoglobulin superfamilyBiological material analysisEpitopeMonoclonal antibody
The invention belongs to the field of antibodies, and more particularly relates to a preparation method and applications of a monoclonal antibody against human CD47. The invention discloses the heightvariable region sequence of the monoclonal antibody. The monoclonal antibody against the human CD47 provided by the invention has good binding activity, can effectively recognize the expression of CD47 on the surfaces of tumor cells, and can effectively recognize the extracellular domain recombinant protein of the human CD47 at the protein level. In addition, the antigen epitope identification ofthe monoclonal antibody is clear, thereby being effectively applied to the diagnosis of CD47 target molecules and preparation and development of monoclonal antibody drugs against the human CD47.
Owner:SHANGHAI JIAO TONG UNIV
Recombinant Polypeptides and Methods for Detecting and/or Quantifying Autoantibodies Against Tsh Receptor
InactiveUS20080305098A1Facilitate detection and isolation and identificationHigh binding activitySugar derivativesBacteriaAutoantibodyAutoimmune condition
The present invention relates to unglycosylated isolated and purified recombinant polypeptides comprising a fusion protein able to bind to autoantibodies produced in response to an autoimmune disease associated with an immune reaction to a TSH-receptor (TSHR). Also disclosed are methods of detecting and / or quantifying such autoantibodies using the isolated and purified recombinant polypeptides and respective kits.
Owner:DR FENNING BIOMED +1
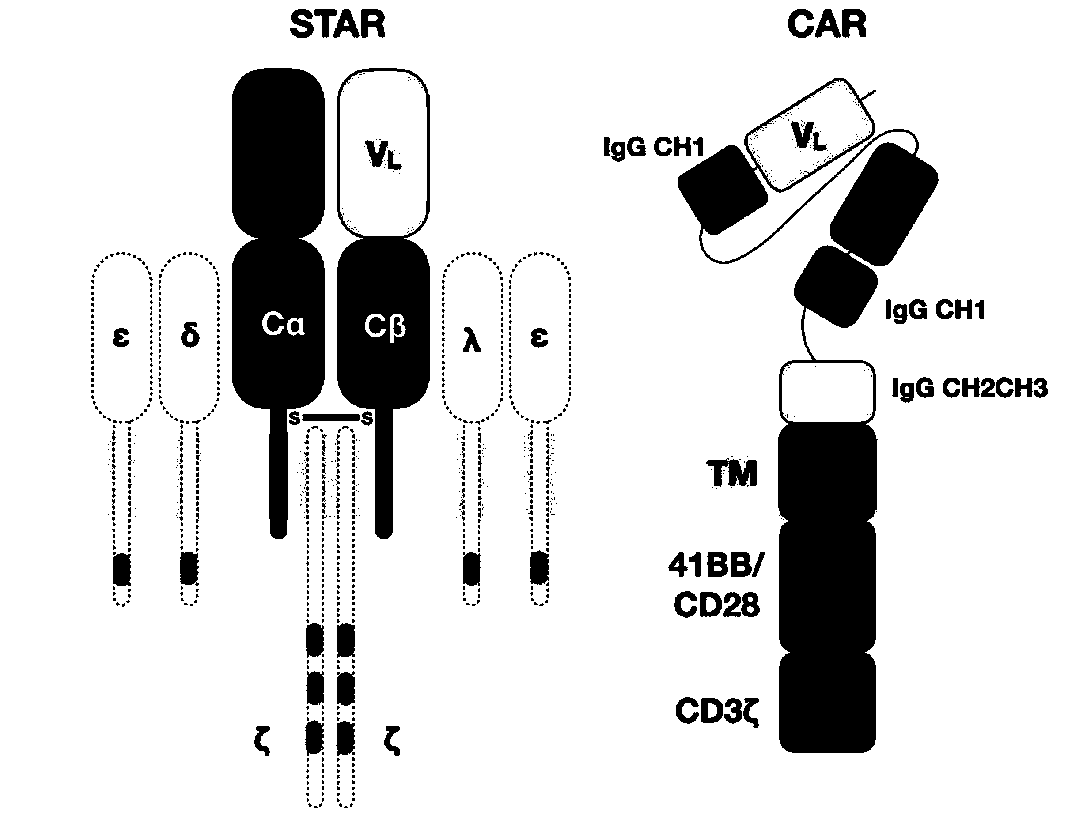
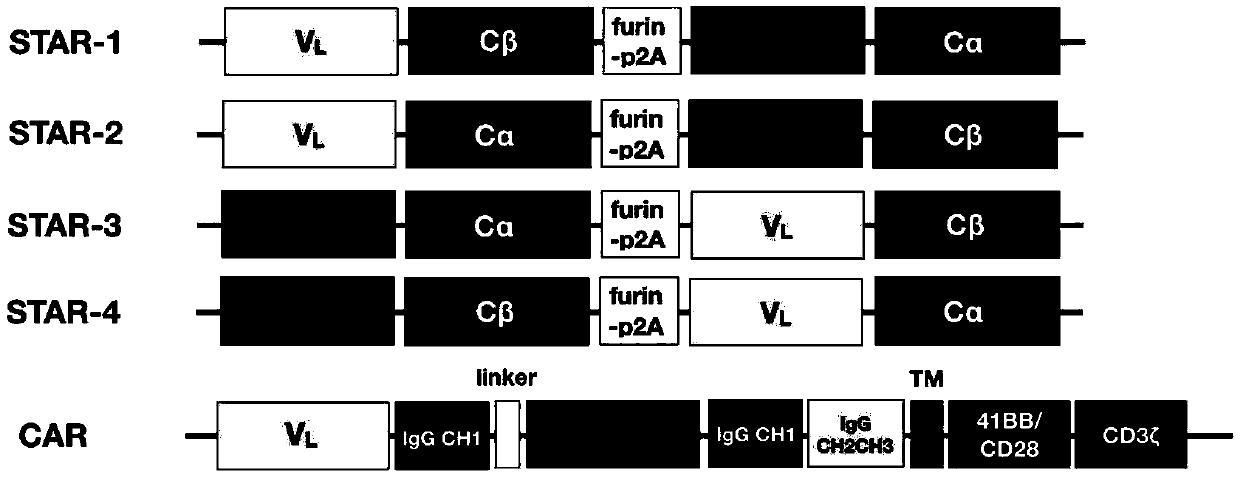
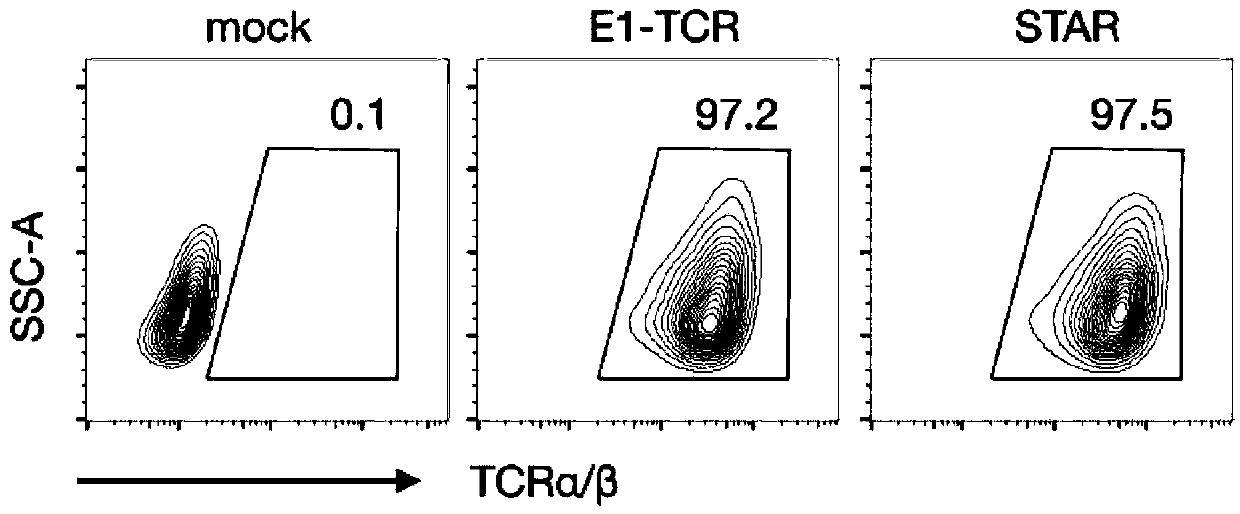
Chimeric T cell receptor STAR and application thereof
ActiveCN110818802AImprove matchReduce the introductionPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorsPharmaceutical drug
The invention discloses a chimeric T cell receptor STAR (Synthetic T Cell Receptor and Antigen Receptor), related preparations and drugs, and application of the chimeric T cell receptor STAR in preparation of cell drugs, and also relates to the preparation or an drug composition used for treatment of corresponding diseases such as tumors or infectious diseases.
Owner:CHINA IMMUNOTECH BEIJING BIOTECH CO LTD +2
Fusion molecules and il-15 variants
ActiveUS20100278774A1High binding activityAvoid developmentBacteriaPeptide/protein ingredientsMammalProtein C
Owner:ALTOR BIOSCI LLC
Dental prosthetic material for promoting dentin remineralization and application of dental prosthetic material
ActiveCN106890095AHigh degree of bionicEasy to operateImpression capsDentistry preparationsIonType I collagen
The invention relates to a dental prosthetic material for promoting dentin remineralization. The dental prosthetic material for promoting dentin remineralization contains a polypeptide solution and a nanometer amorphous calcium phosphate suspension which are successively applied to the surface of a dentin surface, wherein the polypeptide solution and the nanometer amorphous calcium phosphate suspension are physically isolated before use; the polypeptide solution contains polypeptide and water, wherein the polypeptide has functions of adsorbing calcium ions and binding to type I collagen, and the polypeptide has a concentration of 0.5 to 0. 8 mg / ml; the nanometer amorphous calcium phosphate suspension contains nanometer amorphous calcium phosphate and water, wherein the concentration of nanometer amorphous calcium phosphate is 0.2 to 0.3 g / ml. The parental function polypeptide is combined with the nanometer amorphous calcium phosphate to be used, and the nanometer amorphous calcium phosphate can effectively realize the in situ remineralization on the dentin, with high degree of mineralization and bionic degree by setting the appropriate concentration ratio.
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
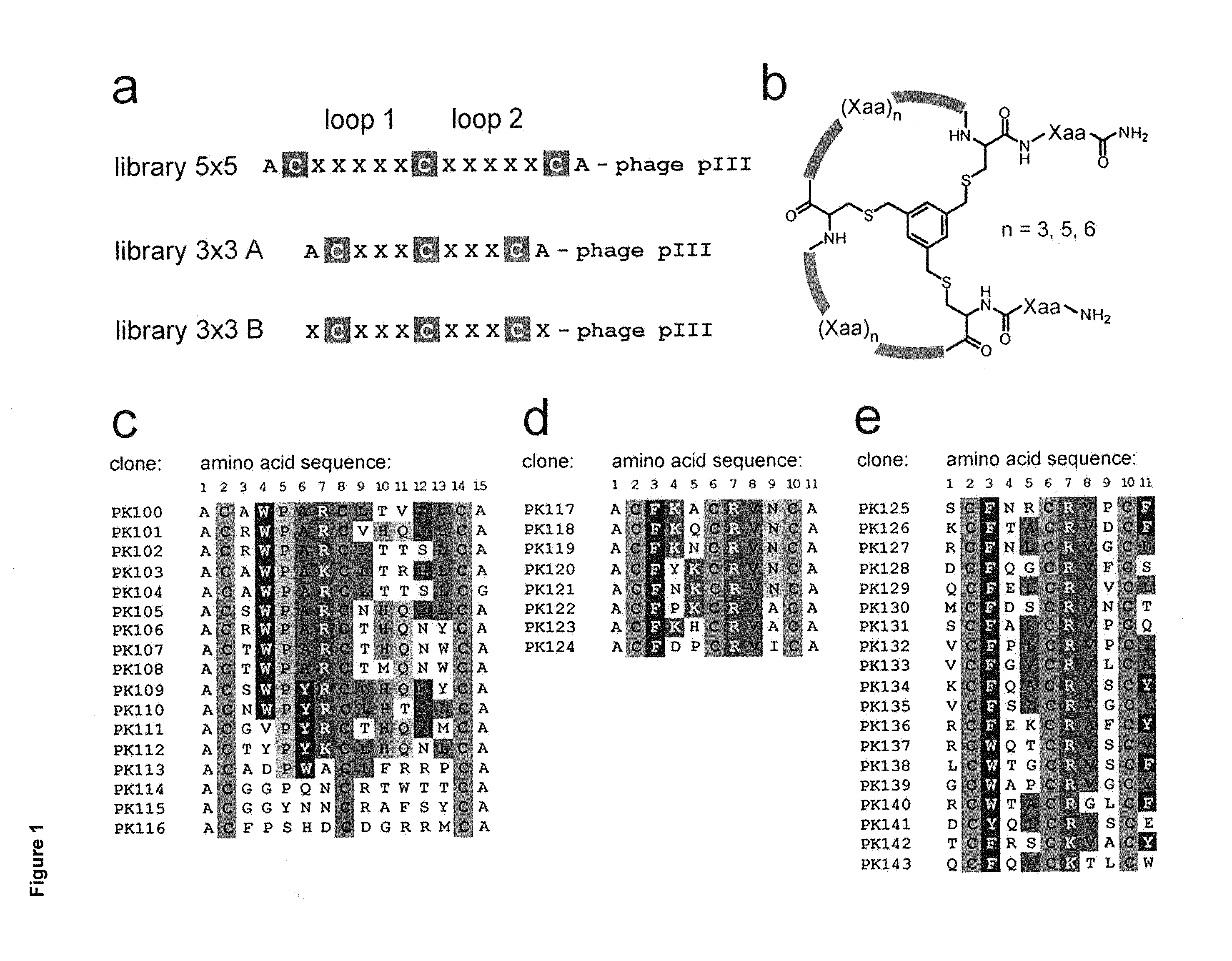
Modulation of structured polypeptide specificity
ActiveUS20140256596A1Small loss of entropyLess flexibleAntibacterial agentsSenses disorderCrystallographyPeptide ligand
Owner:BICYCLERD LTD
Methods for treating myelodysplastic syndromes and sideroblastic anemias
ActiveUS20160289286A1Increase productionIncreased erythropoiesisPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed blood cell
In certain aspects, the present disclosure provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans. In some embodiments, the compositions of the disclosure may be used to treat or prevent sideroblastic anemias and myelodysplastic syndromes or one or more complications associated sideroblastic anemias and myelodysplastic syndromes.
Owner:ACCELERON PHARMA INC
Bispecific antibody and application thereof
ActiveCN110669135AHigh binding activityStrong binding activityHybrid immunoglobulinsBiological material analysisNatural Killer Cell Inhibitory ReceptorsBispecific antibody
The invention discloses a bispecific antibody and an application thereof. Wherein the bispecific antibody comprises a first protein functional area and a second protein functional area; wherein the first protein functional area is the protein functional area targeting TIM-3; the TIM-3 full-length antibody of the targeting TIM-3 corresponding to the first protein functional area has weak binding activity with macaca TIM-3, has strong binding activity with marmoset and human TIM-3, and can activate the killing effect of human NK cells on tumor cells. The bispecific antibody not only can retain the activity of activating PBMC (NK) to kill tumor cells of the single TIM-3 antibody, but also can retain the original activity of the other protein functional area, and can achieve the activity equivalent to or better than the activity of activating T lymphocytes by combining two molecules (synergistic effect). The bispecific antibody (e.g., LB141) of the present invention has a more excellent effect in therapy than PD-1 antibody treatment alone or combined use of the TIM-3 antibody and the PD-1 antibody.
Owner:L&L BIOPHARMA CO LTD
Antibody, and coding gene and application thereof
The invention discloses an antibody, and a coding gene and application thereof. An amino acid sequence of a heavy chain variable region of the antibody is shown in the sequence 2 in a sequence table, while the amino acid sequence of a light chain is shown in the sequence 3 in the sequence table. Experimental results prove that the antibody of the invention has high binding activity (affinity is 2.7*10-8 mol / L) and high tumor cell growth and migration suppression capacity; and the affinity of an anti-epidermal growth factor receptor (EGFR) human-mouse chimeric antibody Cetuximab in foreign markets is 1.1*10-9 M. The humanized antibody of the invention can be better bound with an EGFR so as to ensure anti-tumor effect thereof. An anti-body preparation method of the invention has the advantage of simultaneously expressing the light chain and the heavy chain variable region. After all, the antibody and the preparation method thereof have vast application prospect in the field of the prevention and / or treatment of tumors.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Methods for treating myelodysplastic syndromes and sideroblastic anemias
ActiveUS10189882B2High selectivityRaise the ratioPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed blood cell
In certain aspects, the present disclosure provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans. In some embodiments, the compositions of the disclosure may be used to treat or prevent sideroblastic anemias and myelodysplastic syndromes or one or more complications associated sideroblastic anemias and myelodysplastic syndromes.
Owner:ACCELERON PHARMA INC
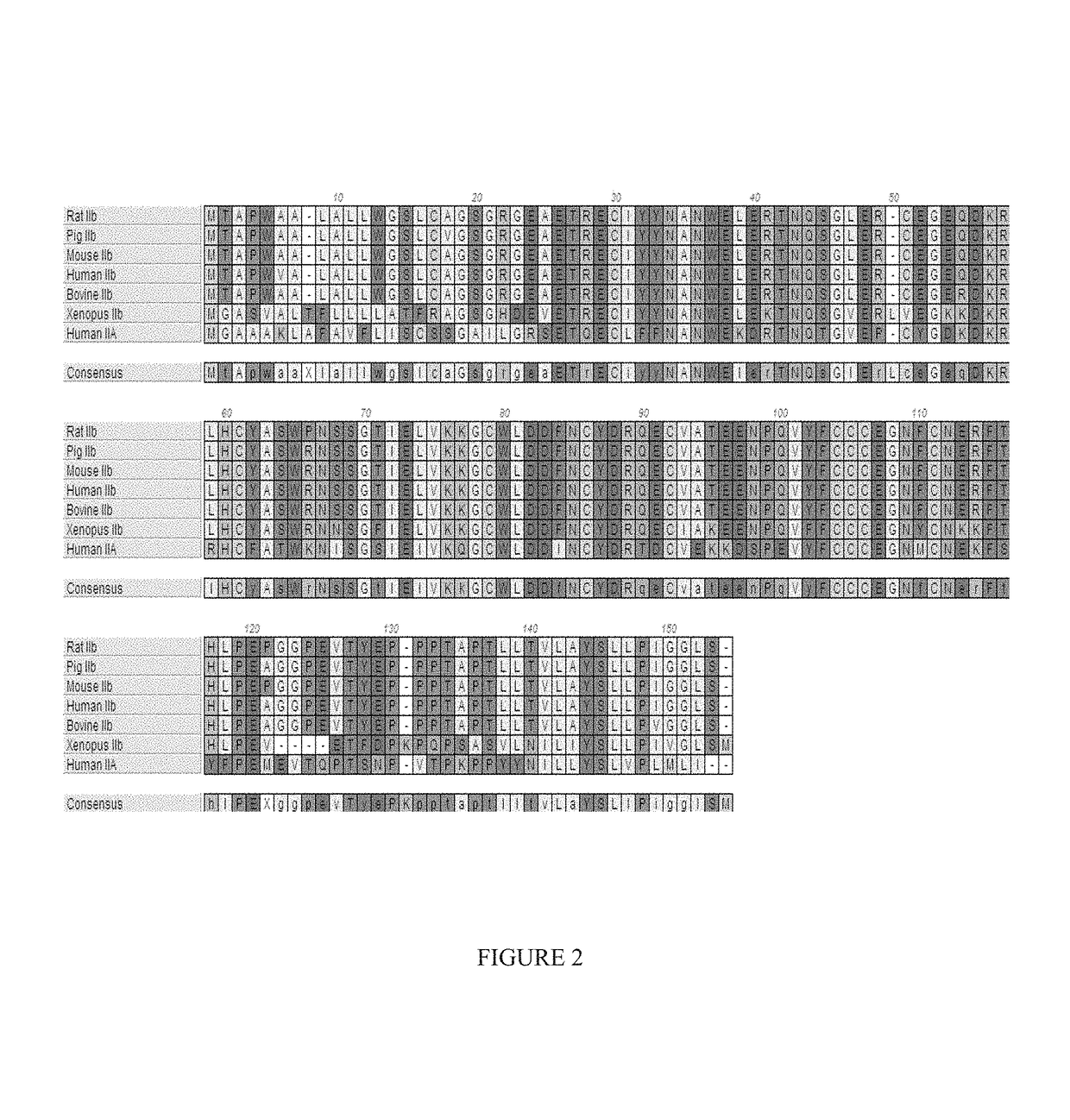
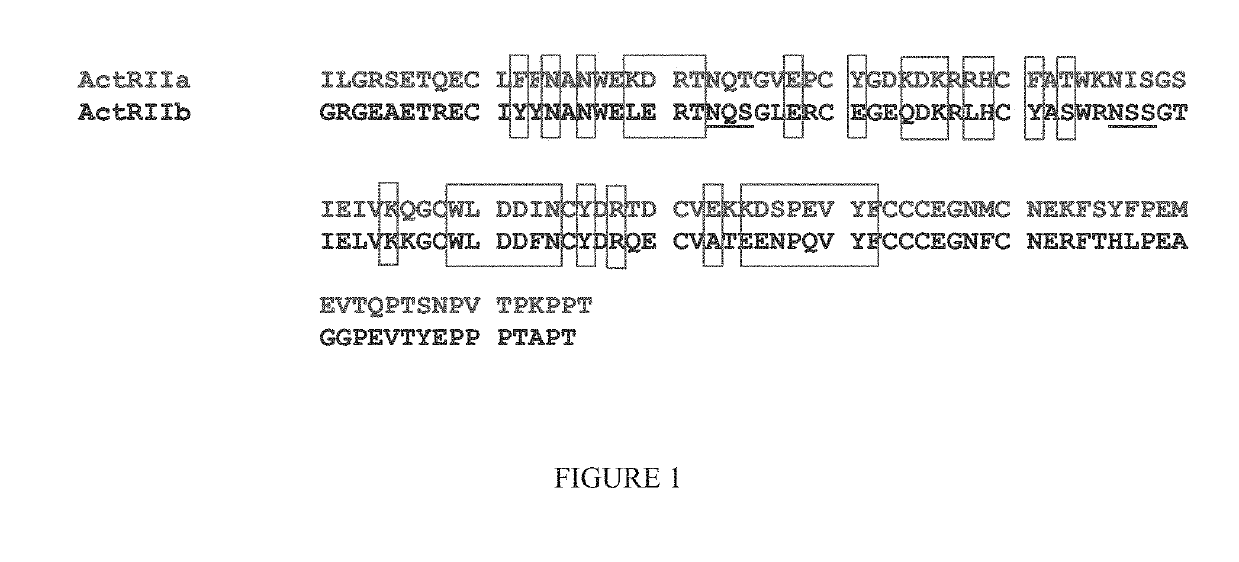
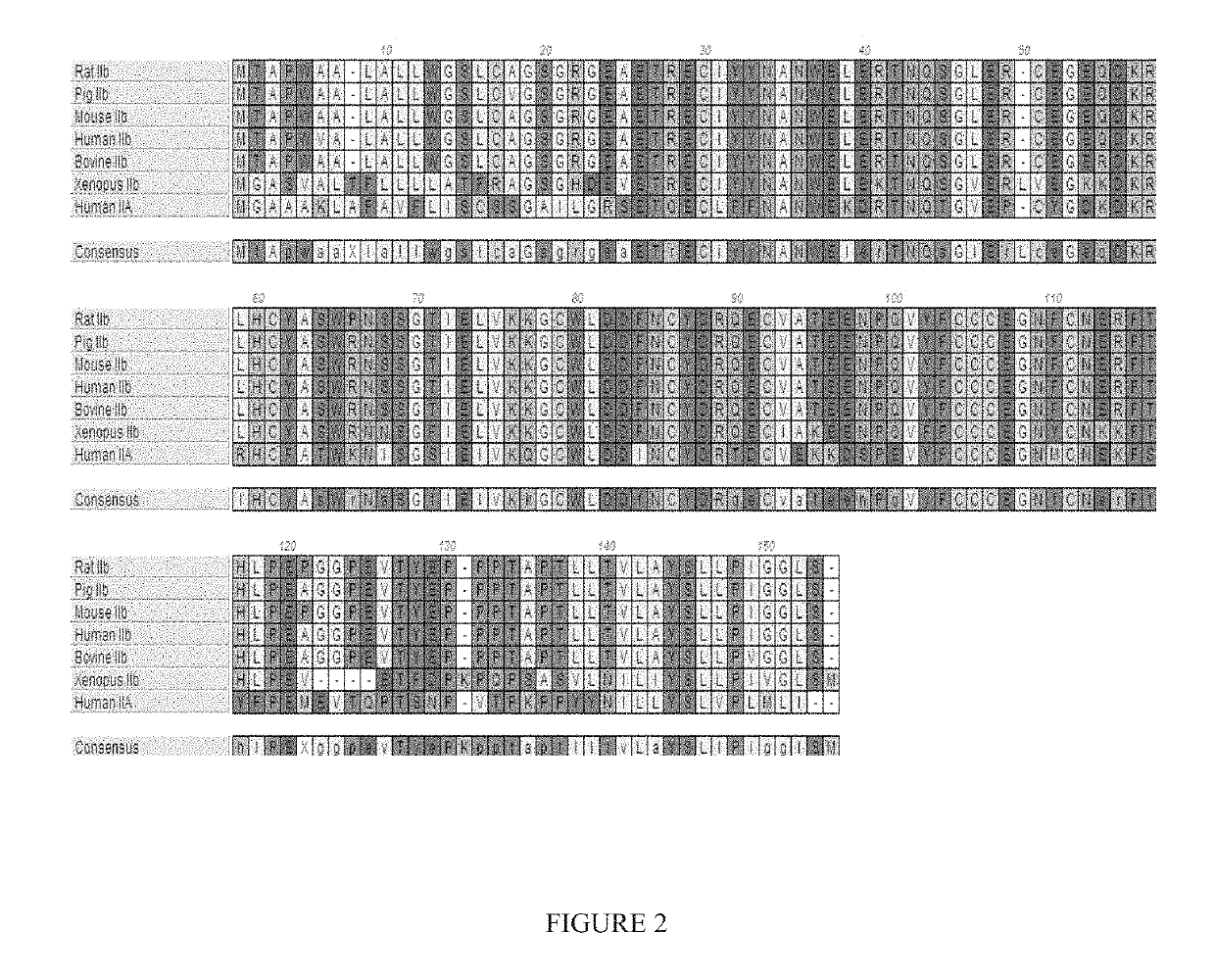
Methods for treating vascular eye disorders with actrii antagonists
ActiveUS10550170B2High selectivityRaise the ratioCompound screeningSenses disorderOcular diseasePharmacology
Disclosed herein are compositions and methods for increasing visual acuity in patients in need thereof and for treating vascular disorders of the eye.
Owner:ACCELERON PHARMA INC
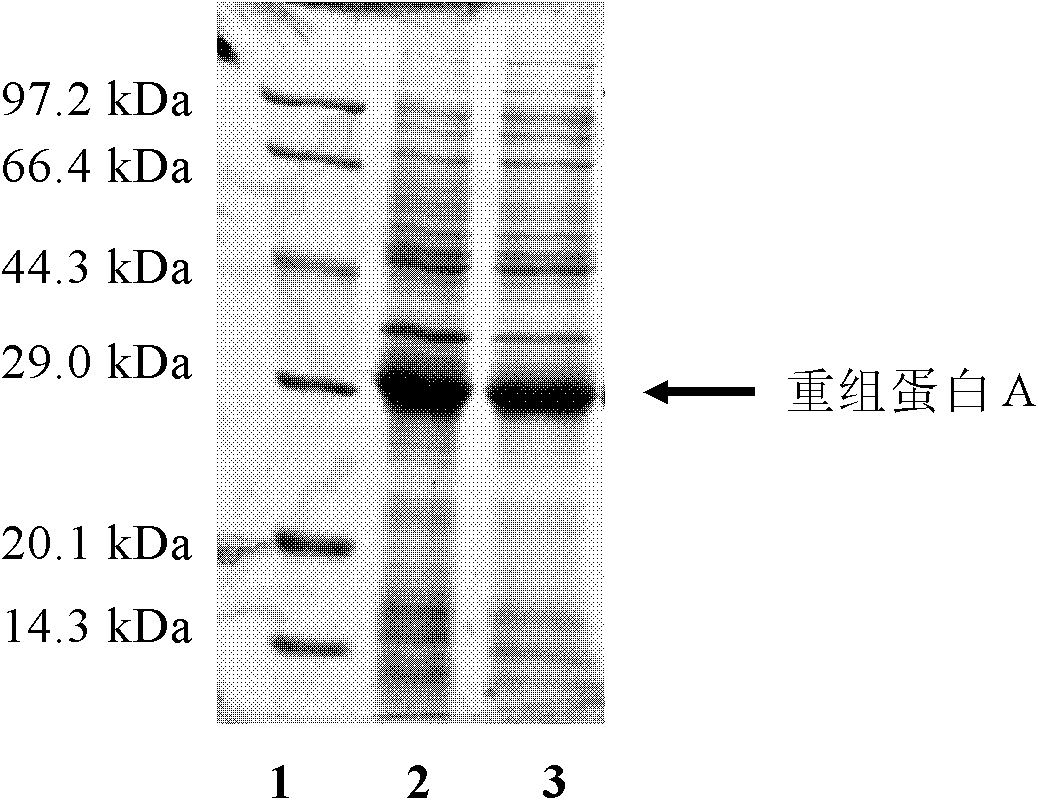
Method for producing recombinant protein A
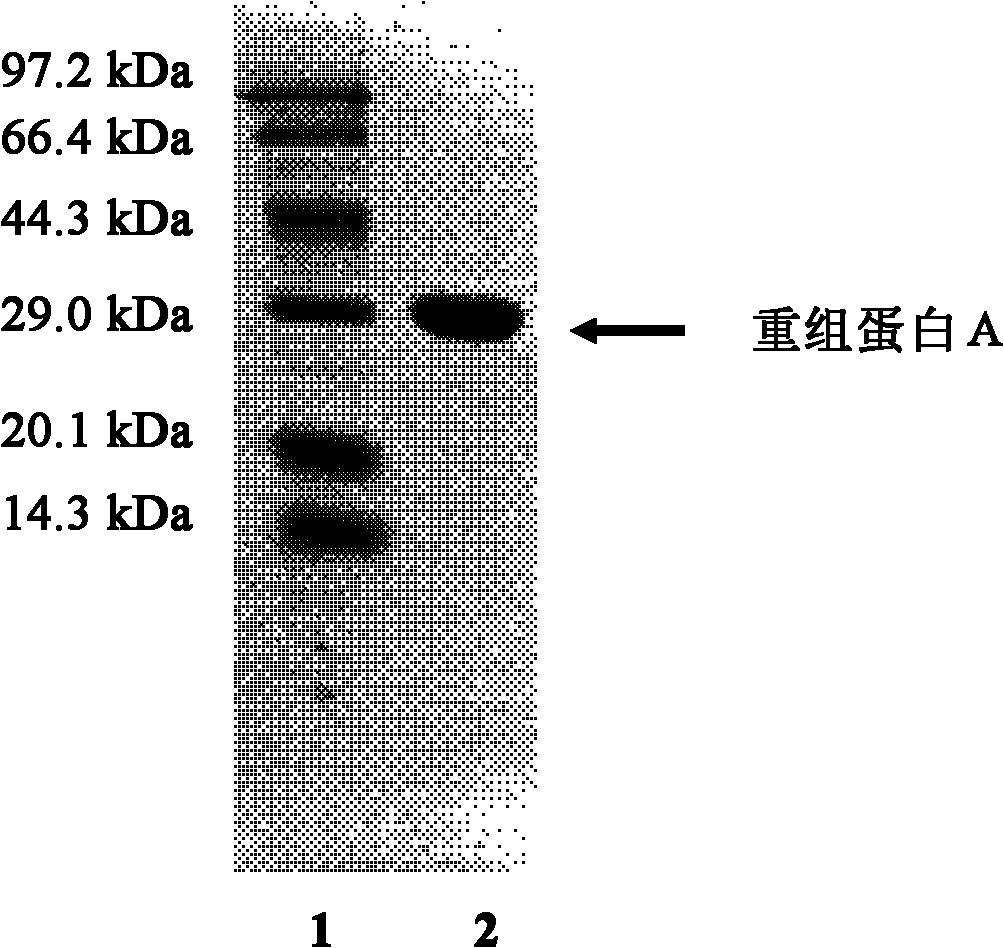
ActiveCN101921818AHigh purityHigh binding activityMicroorganism based processesPeptide preparation methodsDry weightTotal protein
The invention relates to a method for producing recombinant protein A, which belongs to the technical field of biological engineering and provides a technological condition for fermenting and expressing the recombinant protein A in high density by using genetic engineering bacteria, and a method for separating and purifying the recombinant protein A in high purity by using Fc segment of IgG as anaffinity ligand by a one-step affinity chromatography method. By applying the method for producing the recombinant protein A, the culture density of the recombinant bacteria fermented in high densityreaches OD600nm=80-100; the dry weight of the bacteria is 40-50g / L; the expression quantity of the protein A occupies 30% to 50% of total protein of the bacteria; the quantity of the protein A produced by each liter of bacterial liquid reaches 3g; and the purity of the protein A detected by SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis) and HPLC (High Performance Liquid Chromatography) is more than 95%. The method has the advantages of large yield, low cost, high product quality, and the like, and provides a practicable approach for preparing the recombinant protein A.
Owner:DALIAN UNIV OF TECH
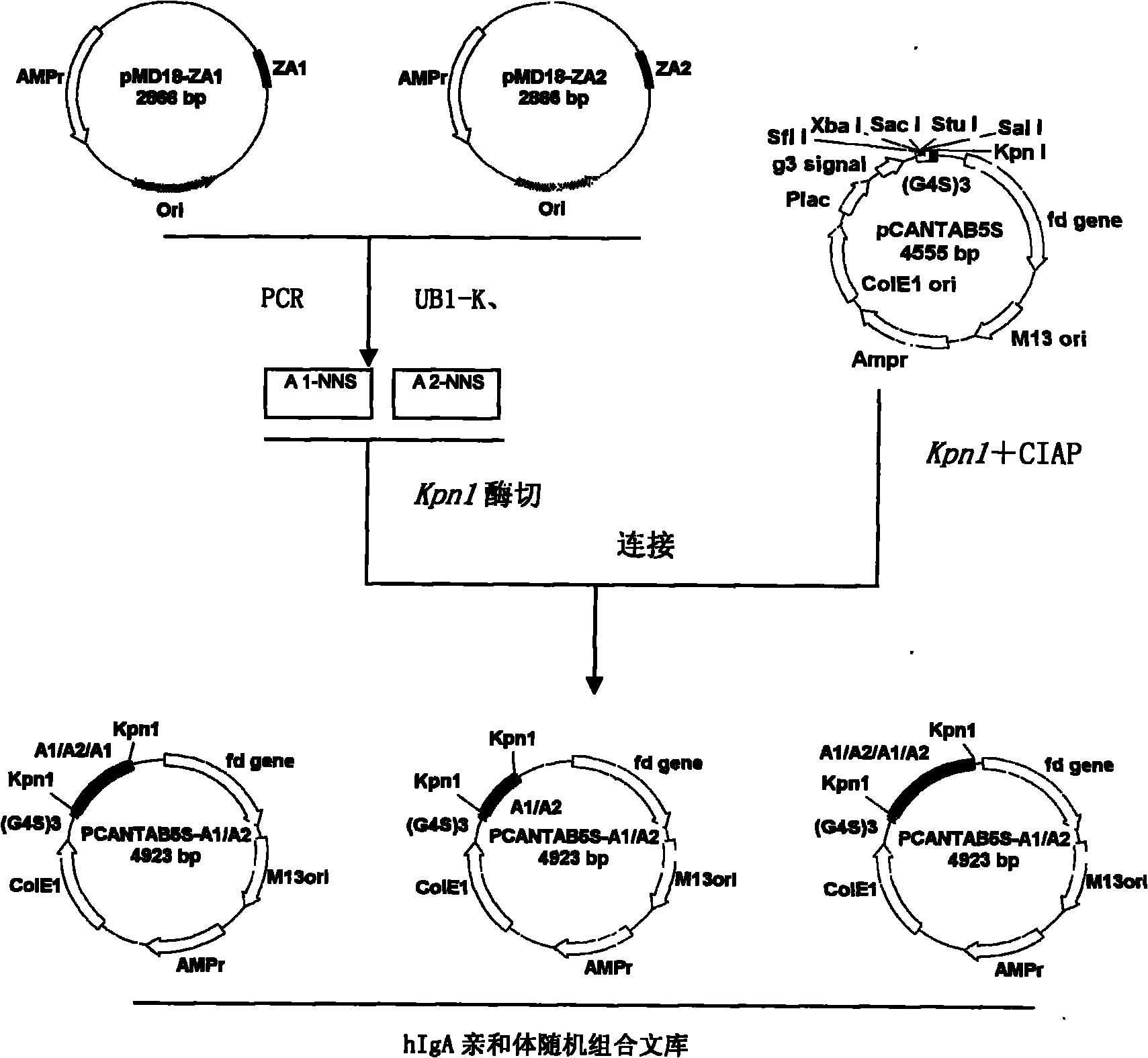
Human IgA immunoglobulin combination molecule having intramolecular affinity effect
InactiveCN102115497AHigh binding activityHigh activityBacteriaMicrobiological testing/measurementGlobin bindingBacteriophage
The invention discloses a human IgA immunoglobulin combination molecule having intramolecular affinity effect, a preparation method and an application thereof. The invention also discloses genes coding the human IgA immunoglobulin combination molecule, a preparation method and an application of the human IgA immunoglobulin combination molecule based on bacteriophage molecule evolution. The human IgA immunoglobulin combination molecule of the invention, especially repeated molecule of human IgA affibody, has intramolecular affinity effect when binding the human IgA, demonstrates very high human IgA binding activity, can be used for purification of high-specific IgA and research and development of detecting reagent, and purification for detection of human IgA antibody by ELISA adsorption method, immunity chromatography, immunohistochemical method and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Molecules with antigen binding and polyvalent FC gamma receptor binding activity
ActiveUS9683044B2Potent antibody-mediated cell cytoxicityPotent complement-dependent cell cytoxicity and direct cytotoxicityAntibacterial agentsSenses disorderFc receptorDisease
The current invention involves biologically active proteins termed stradobodies. The stradobodies have two or more domains that create stradobody multimers. The stradobodies have both antigen-binding capacity and the ability to bind Fc receptors (FcR), and are useful in the treatment and prevention of disease.
Owner:GLIKNIK
Methods and reagents for preparing and using immunological agents specific for P-glycoprotein
InactiveUS7144704B2Improve bindingRapid and reliable and cost-effective characterizationVirusesPeptide/protein ingredientsMammalWild type
This invention relates to immunological reagents and methods specific for a mammalian, transmembrane protein termed Pgp, having a non-specific efflux pump activity established in the art as being a component of clinically-important multidrug resistance in cancer patients undergoing chemotherapy. The invention provides methods for developing and using immunological reagents specific for certain mutant forms of Pgp and for wild-type Pgp in a conformation associated with substrate binding or in the presence of ATP depleting agents. The invention also provides improved methods for identifying and characterizing anticancer compounds.
Owner:ONCOTECH
IgG BINDING PEPTIDE
ActiveUS20100297606A1High binding activitySlow dissociation ratePeptide/protein ingredientsTissue cultureIgG bindingCysteine
The present invention provides a peptide capable of specifically binding to human IgG. In particular, the present invention relates to a human IgG binding peptide tag of 11 to 16 amino acids in length, comprising at least an amino acid sequence of the formula I:C-(X)n-W-X-X-X-W-(X)m-C(I)(SEQ ID NO: 17)wherein n and m are each an integer of 1 or more and the sum n+m is 4 or 5, wherein X-X-X in the formula I contains no cysteine residue, andwherein said amino acid sequence satisfies either or both of a) and b):a) (X)n—W in the formula I denotes Za-G-Y—W (SEQ ID NO: 18); andb) W—(X)m in the formula I denotes W-G-L-Zb (SEQ ID NO: 19)wherein Za and Zb are each 0, 1, or more amino acid residues.
Owner:KAGOSHIMA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com