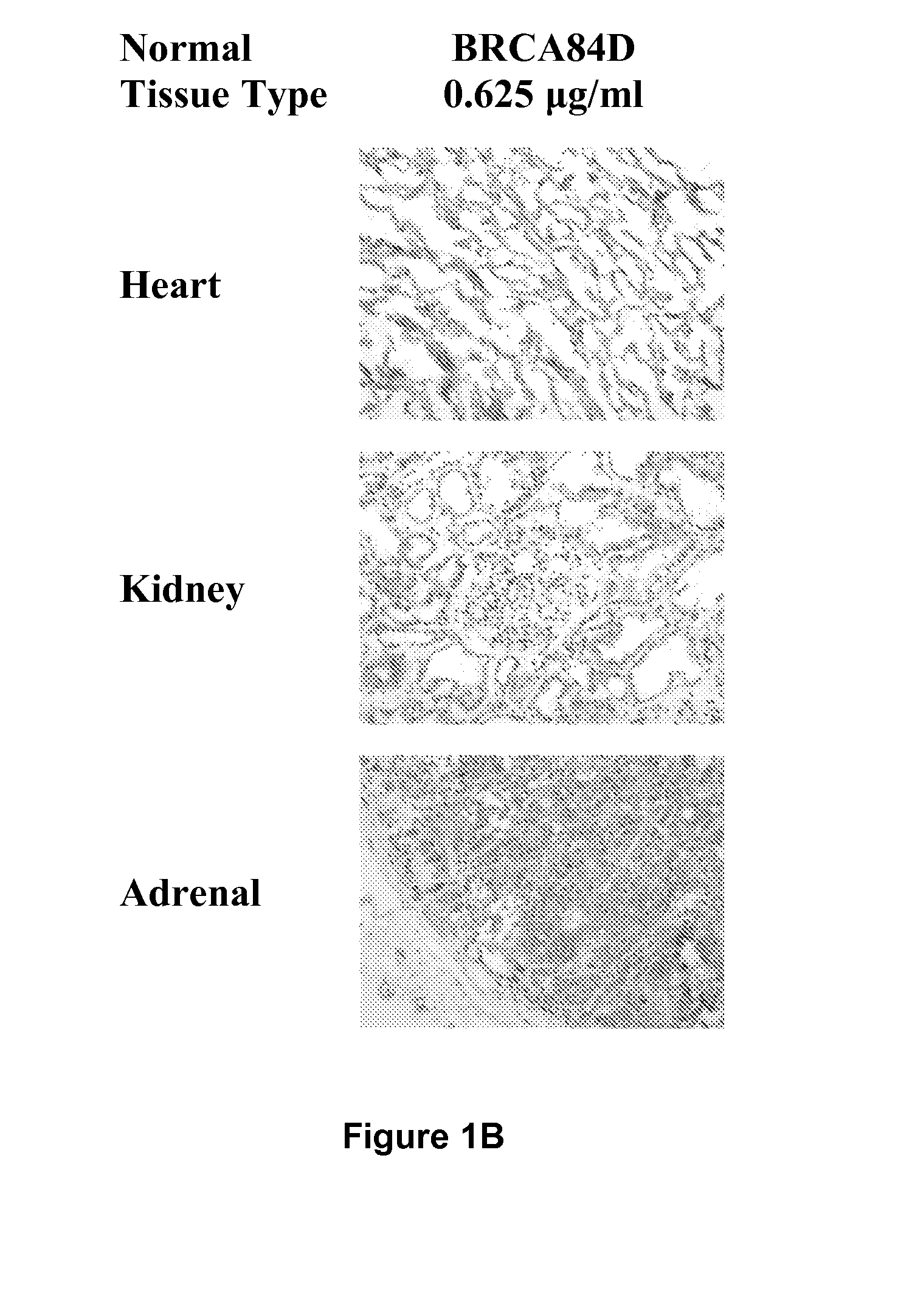
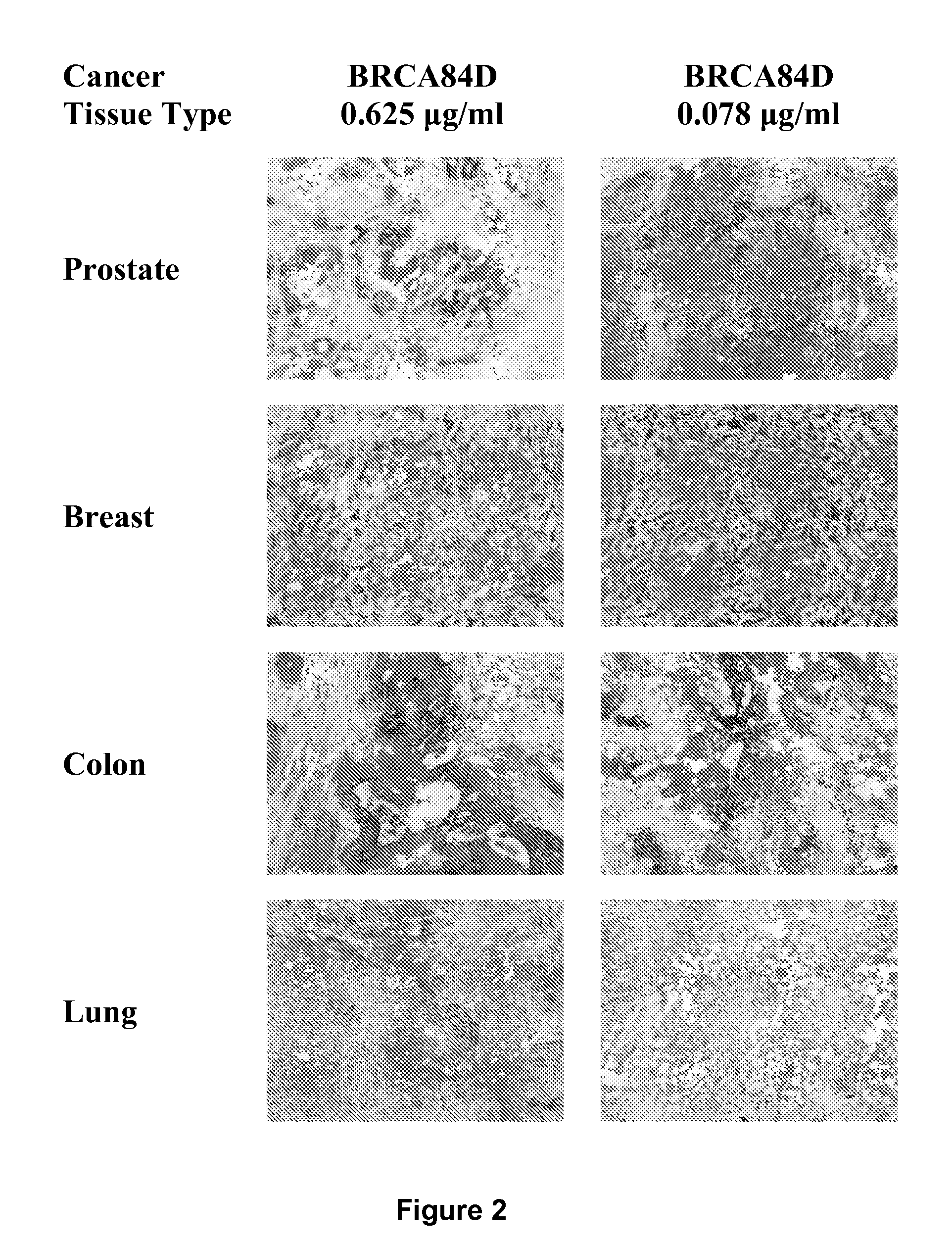
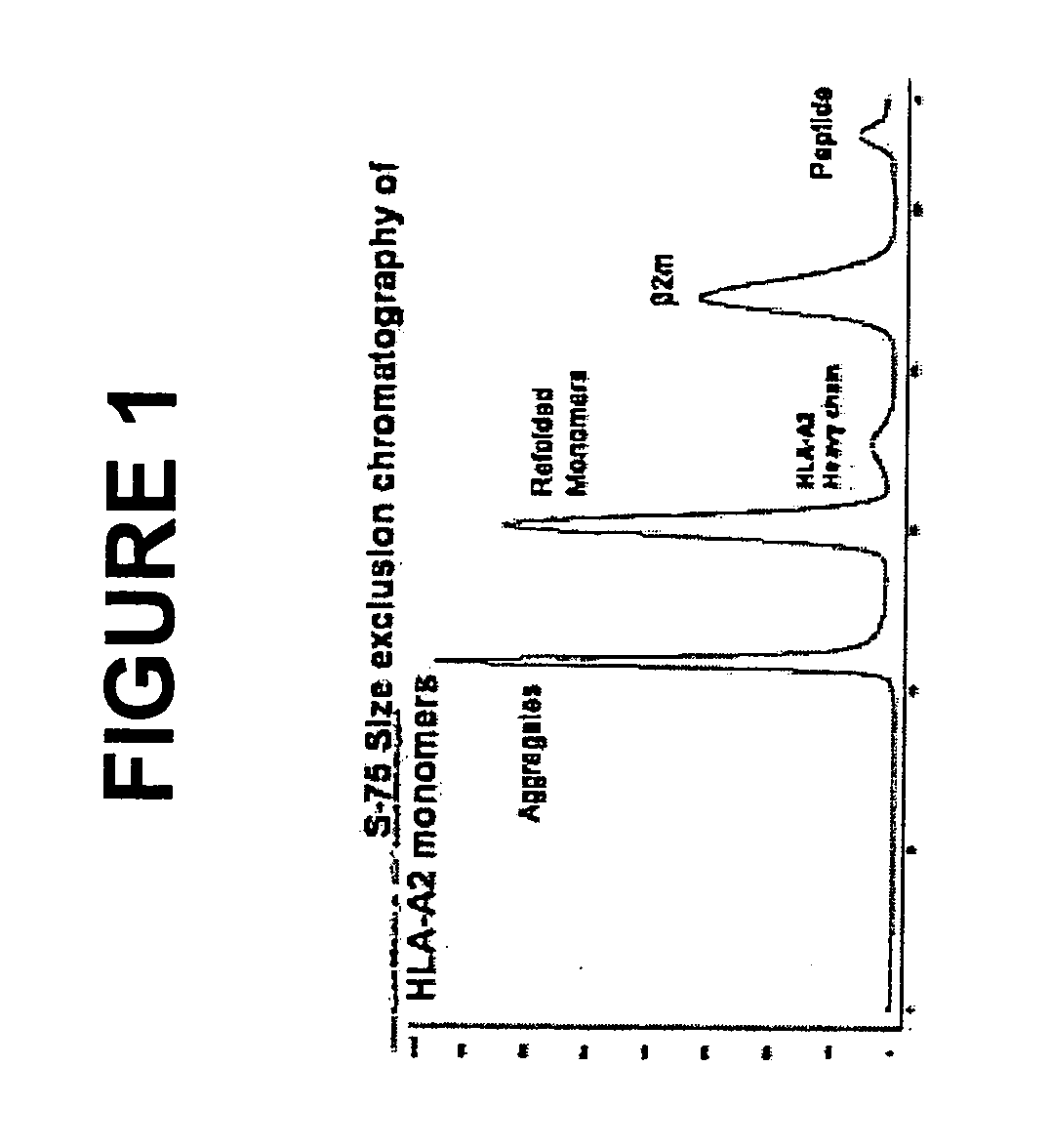
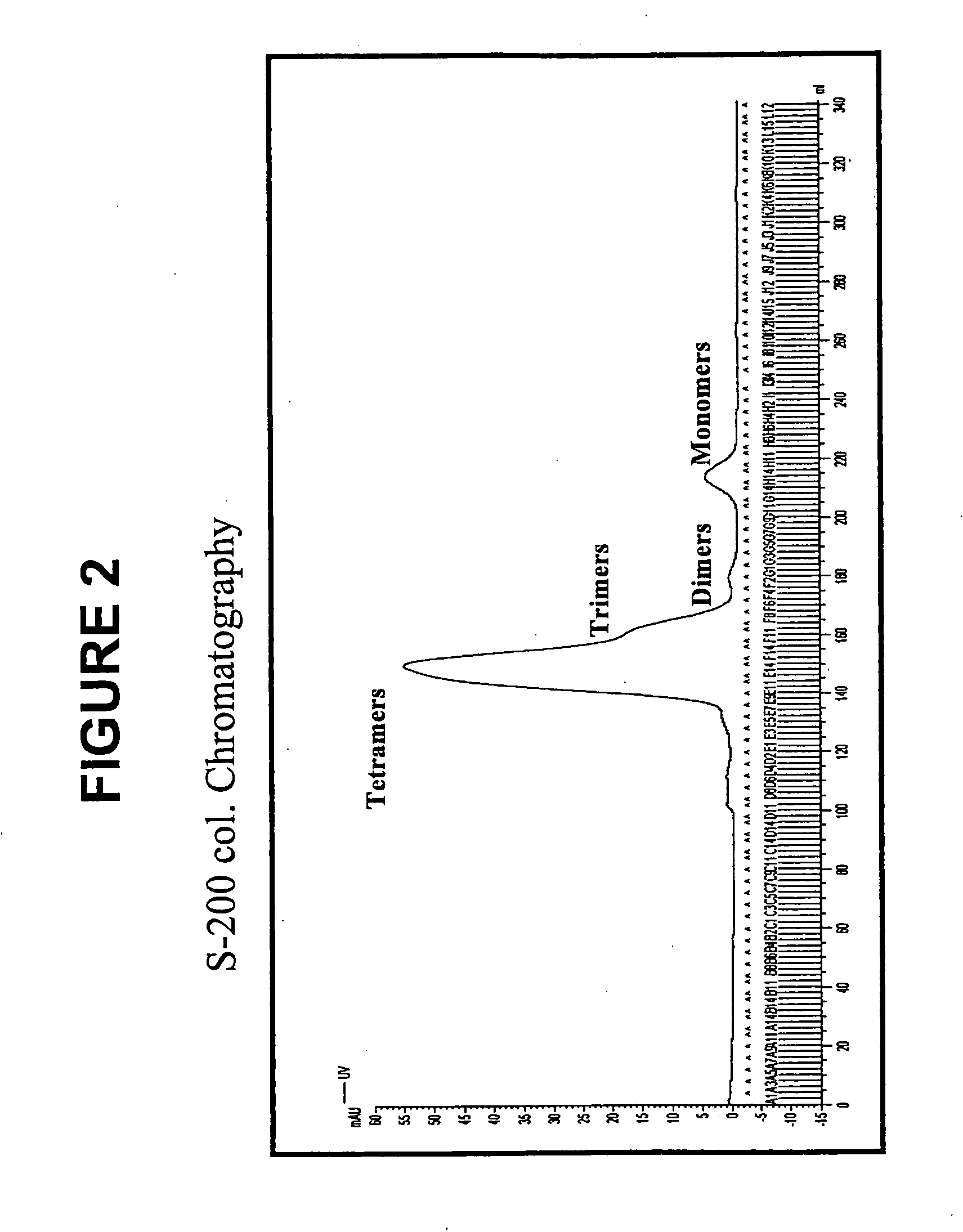
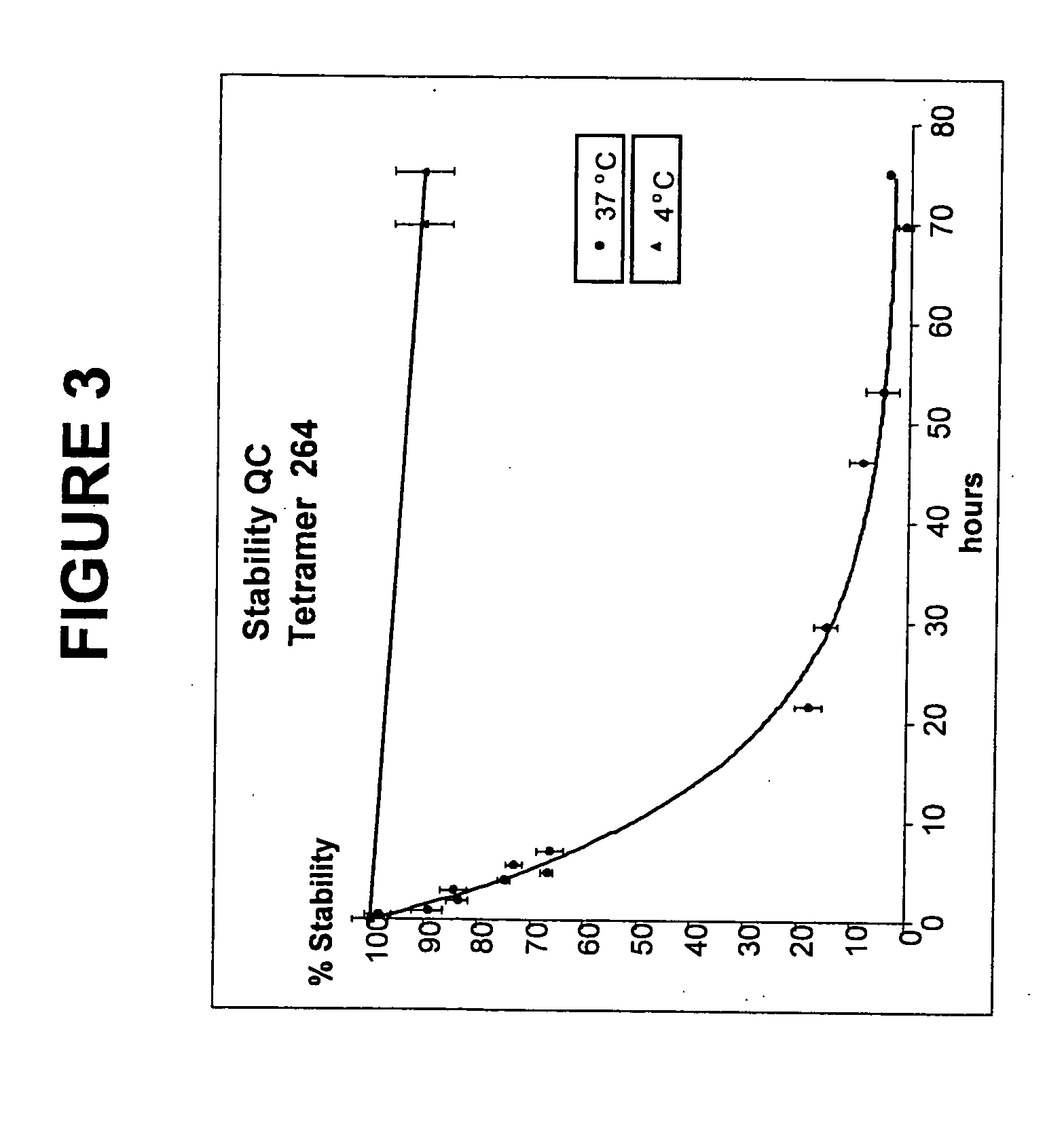
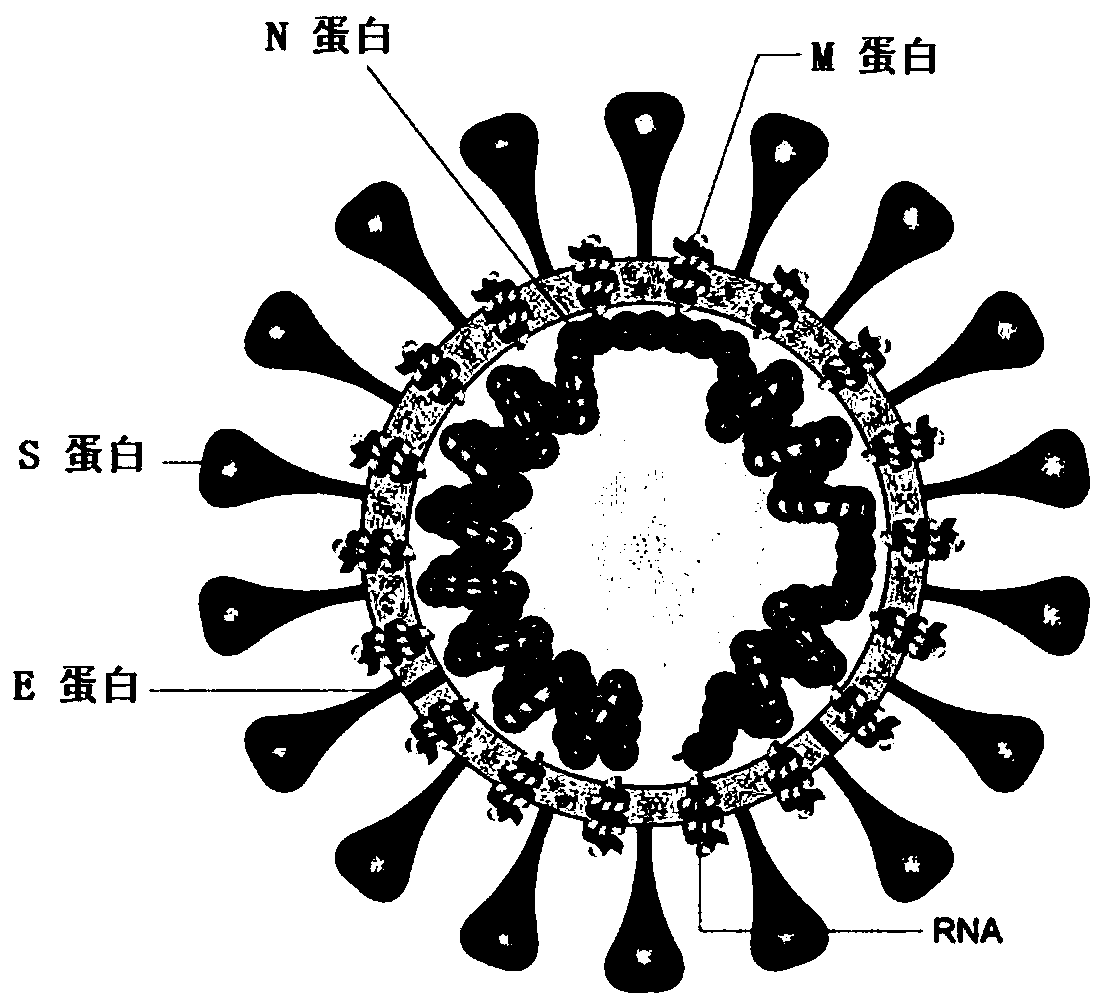
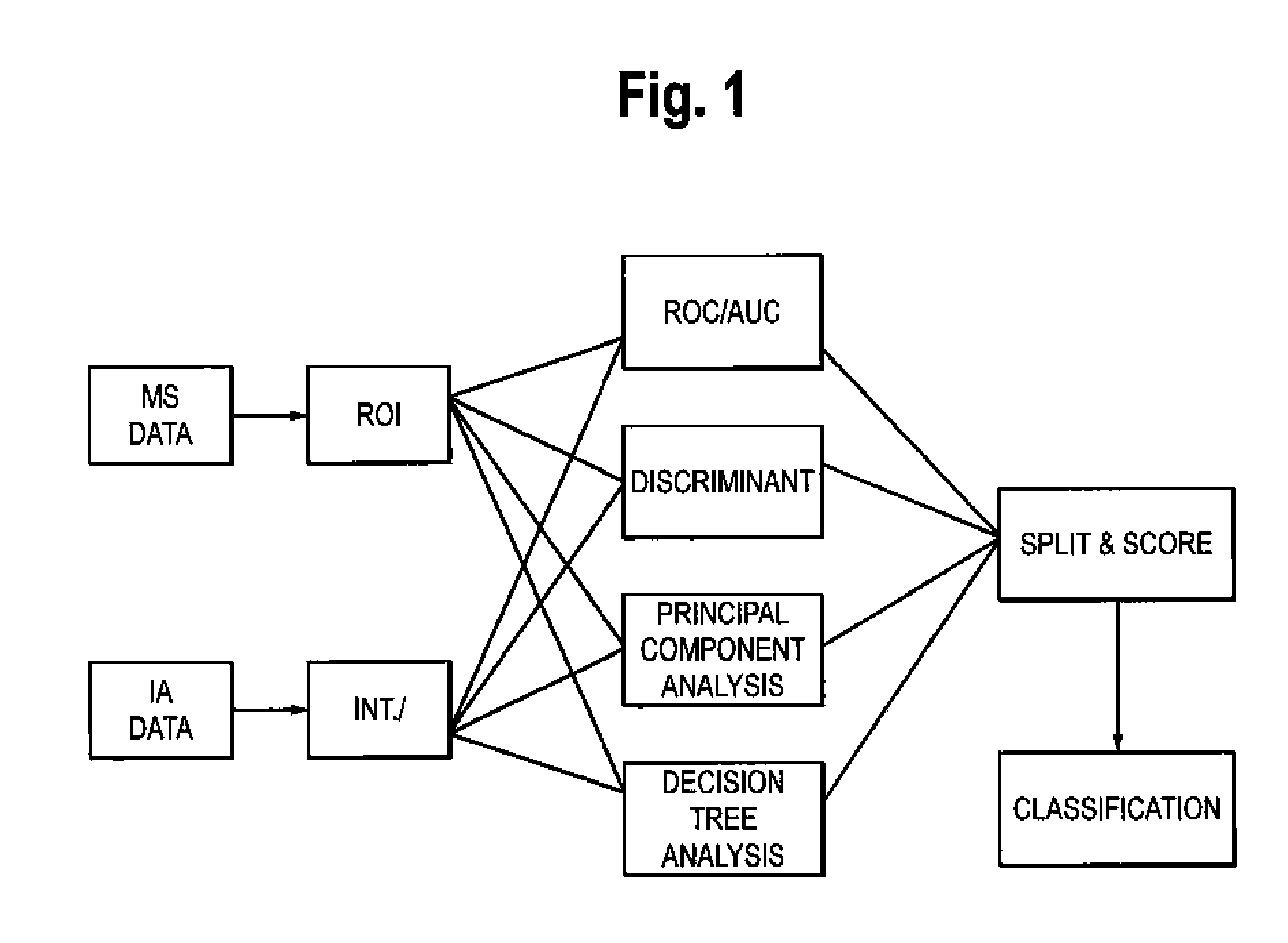
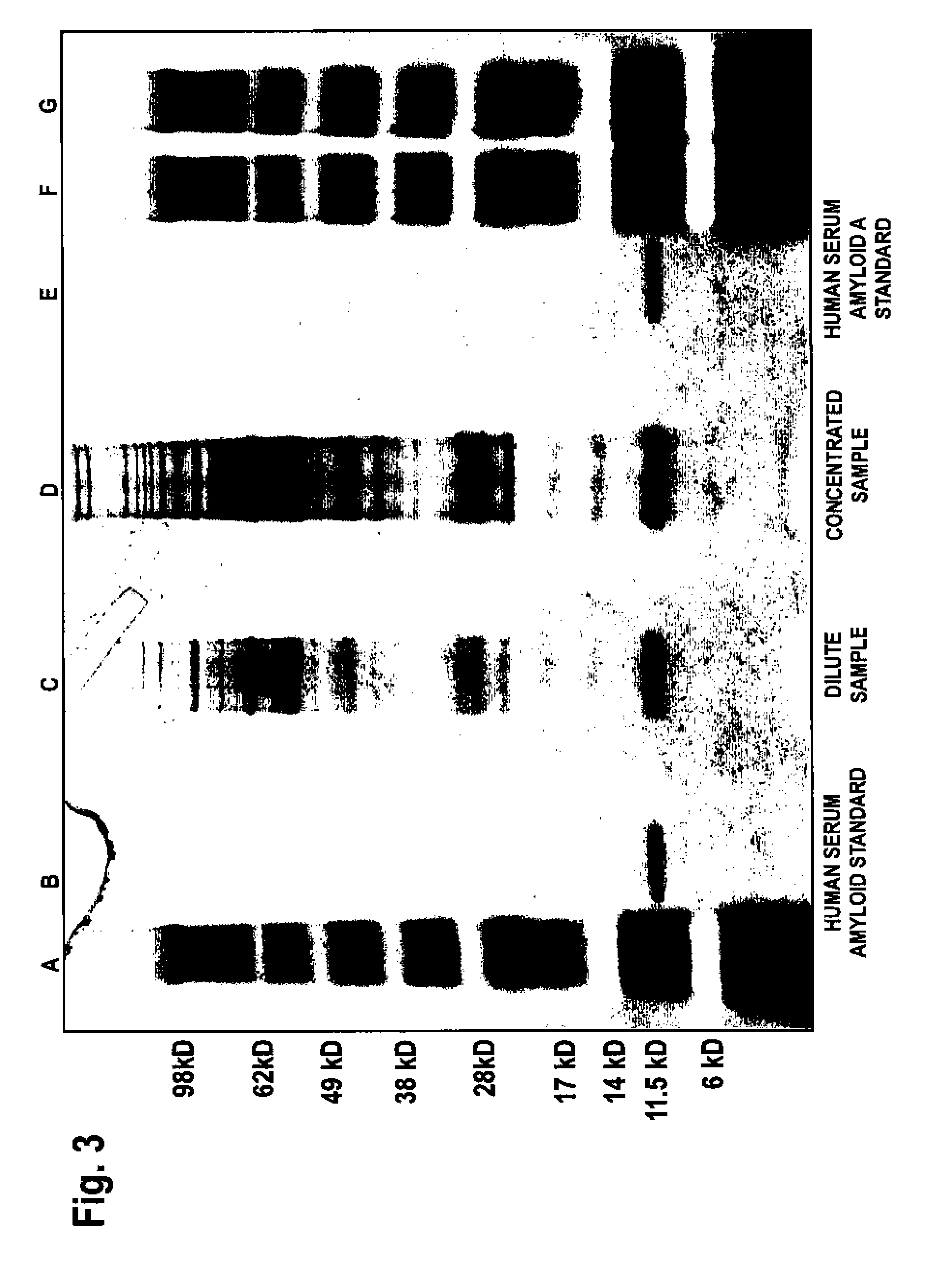
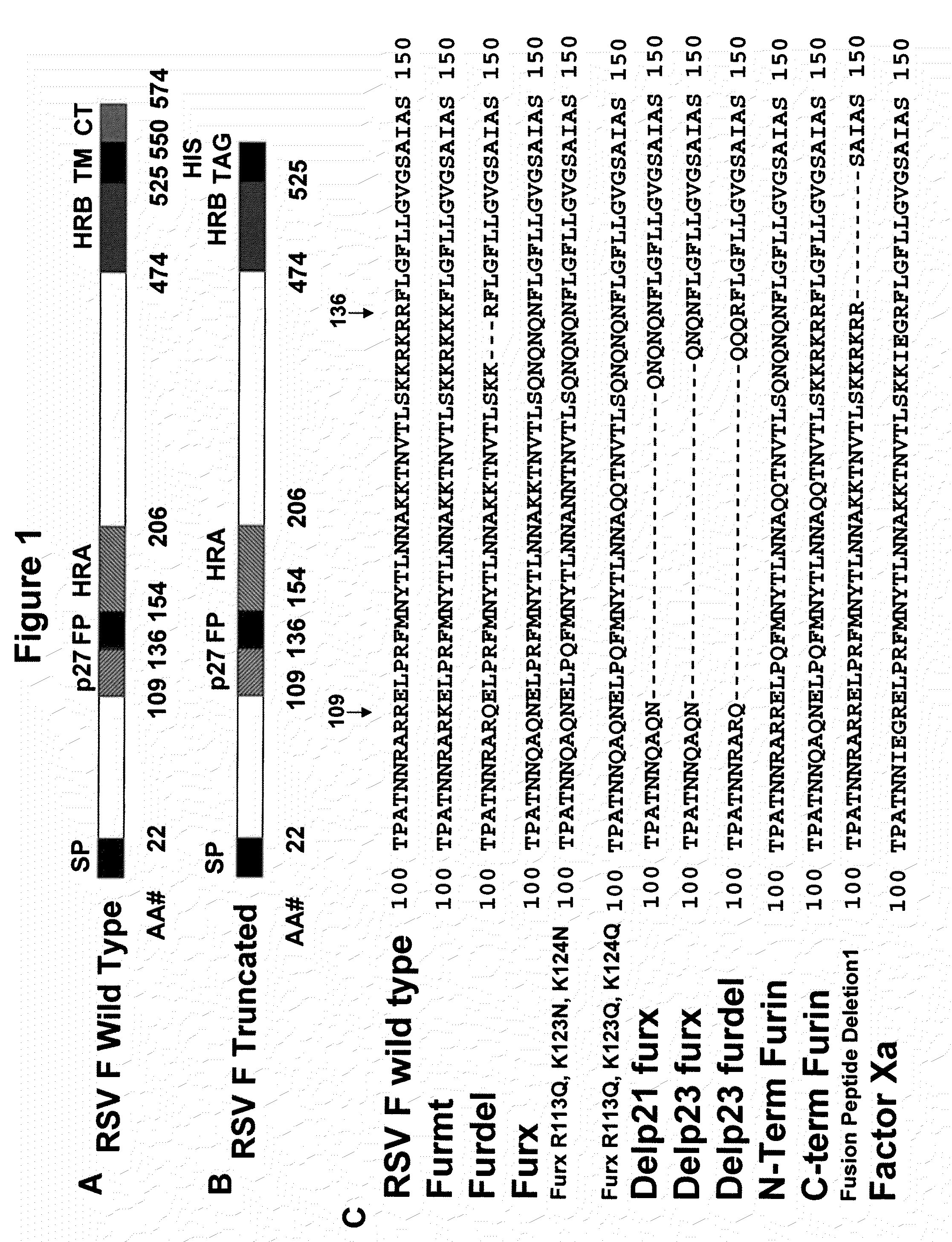
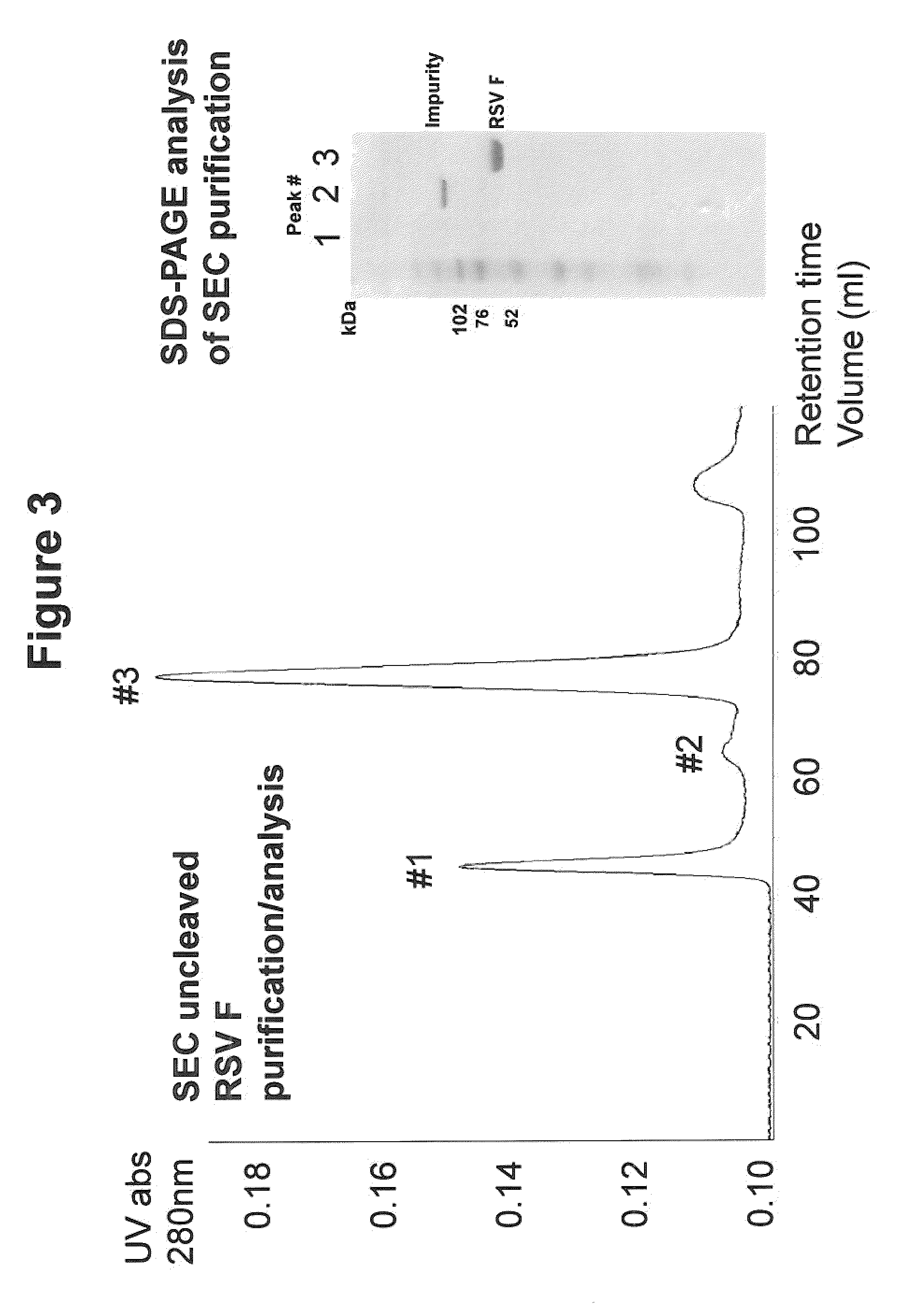
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3255 results about "Immune reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of immune response. : a bodily response to an antigen that occurs when lymphocytes identify the antigenic molecule as foreign and induce the formation of antibodies and lymphocytes capable of reacting with it and rendering it harmless. — called also immune reaction.
Potentiation of immune responses with liposomal adjuvants
InactiveUS6090406AGood water solubilityPractical and convenientBacterial antigen ingredientsViral antigen ingredientsLipid formationOrganic acid
A high integrity liposome comprising at least one stabile lipid and at least one peptide-like therapeutic agent associated with said liposome, adapted for parenteral administration to an animal, including a human, and method according to manufacture and use. Immunizing dosage forms comprising a liposome and an immunogen, wherein said liposome and immunogen are present in an immunization dose. Additionally, a dosage form, including such form particularly adapted to producing an immune response, comprising a salt according to an organic acid derivative of a sterol and an immunogen wherein said organic acid derivative of a sterol and immunogen are present in an immunization dose, and method according to use is disclosed. Further, a dosage form, including such form particularly adapted to producing an immune response, comprising dimyristoylphosphatidylcholine (DMPC) / cholesterol liposomes, optionally in an aluminum hydroxide gel, and an immunogen wherein said DMPC / cholesterol and immunogen are present in an immunization dose, and method according to use.
Owner:TRANSAVE
Device and method for attenuating an immune response
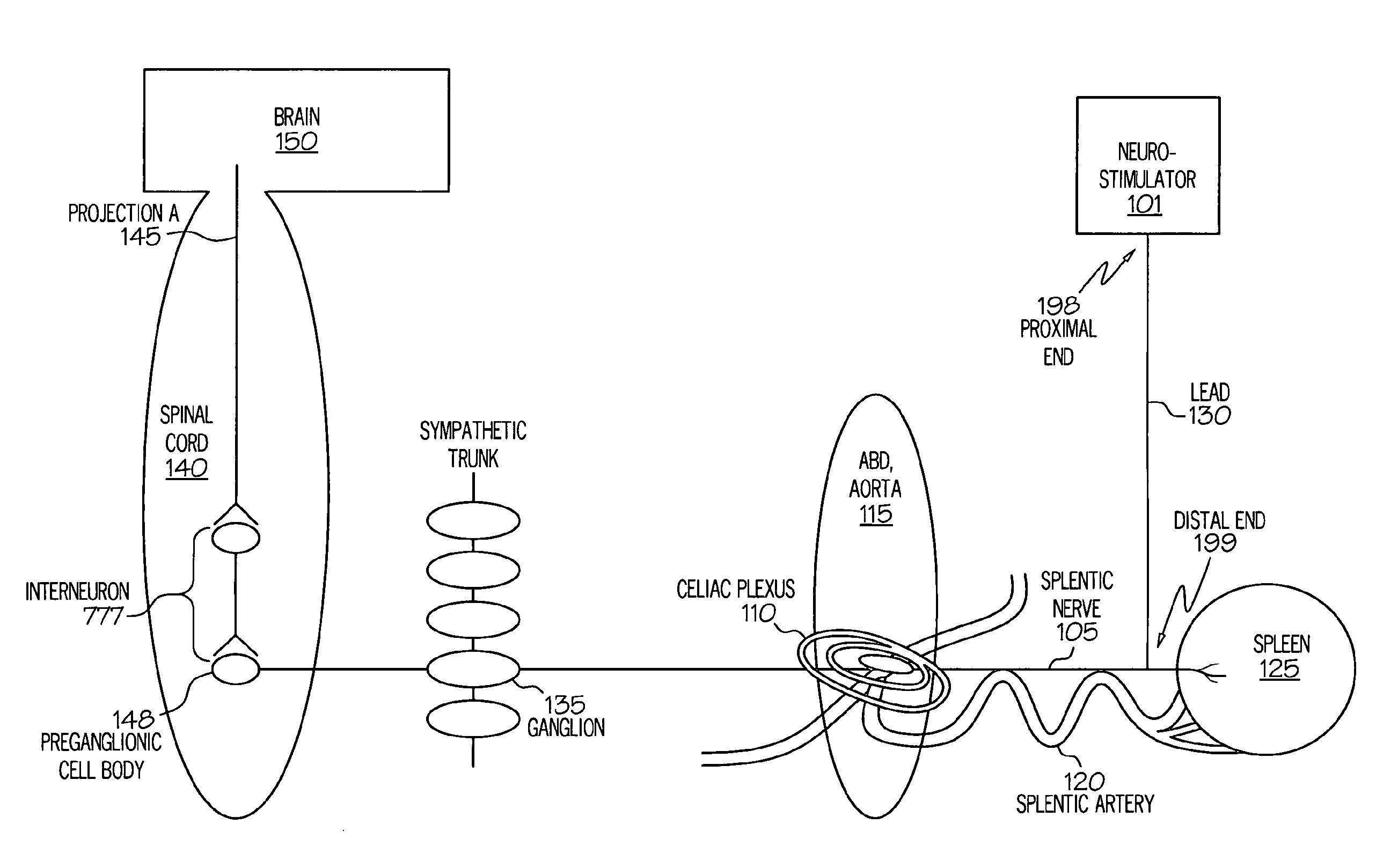
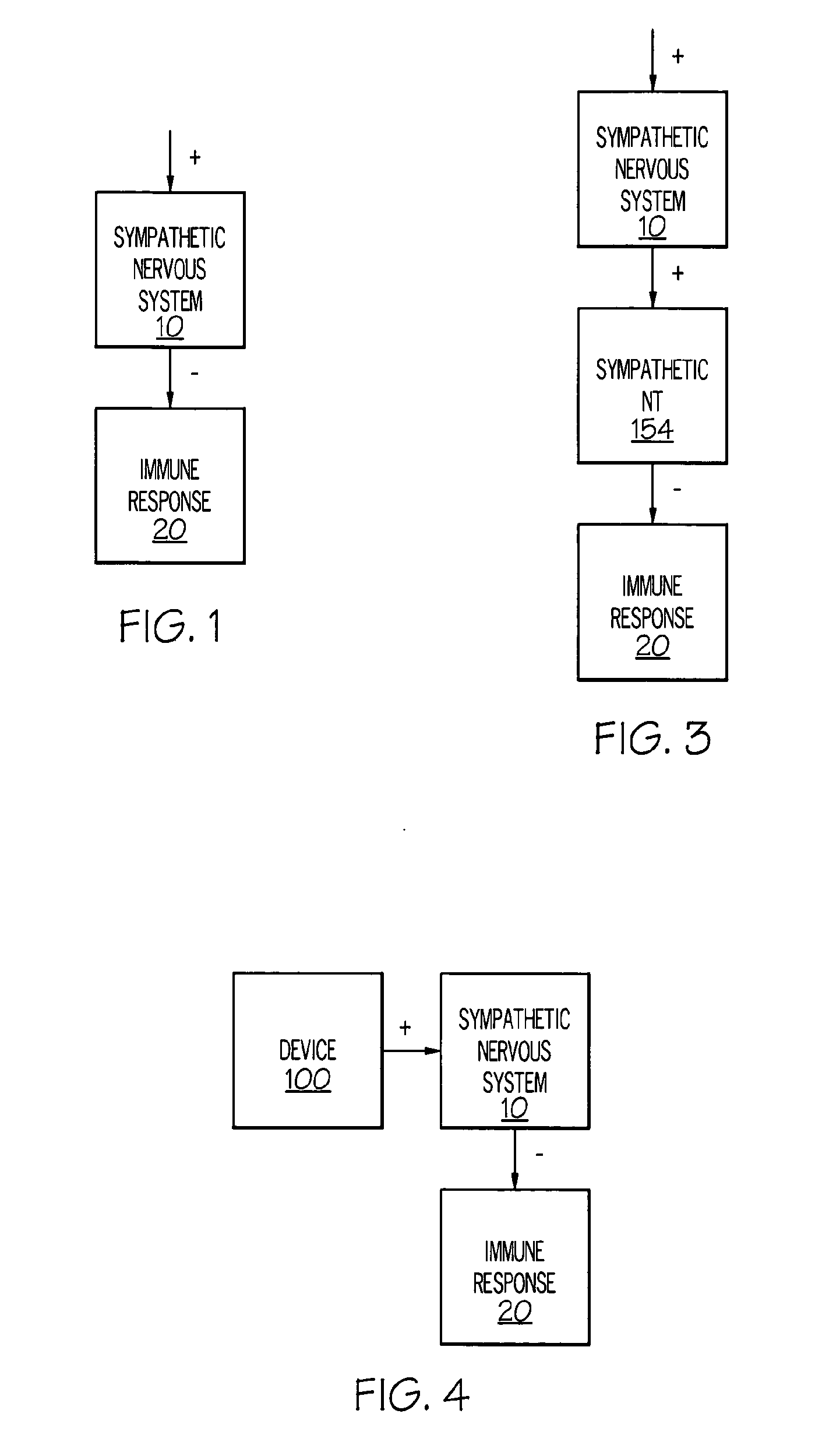
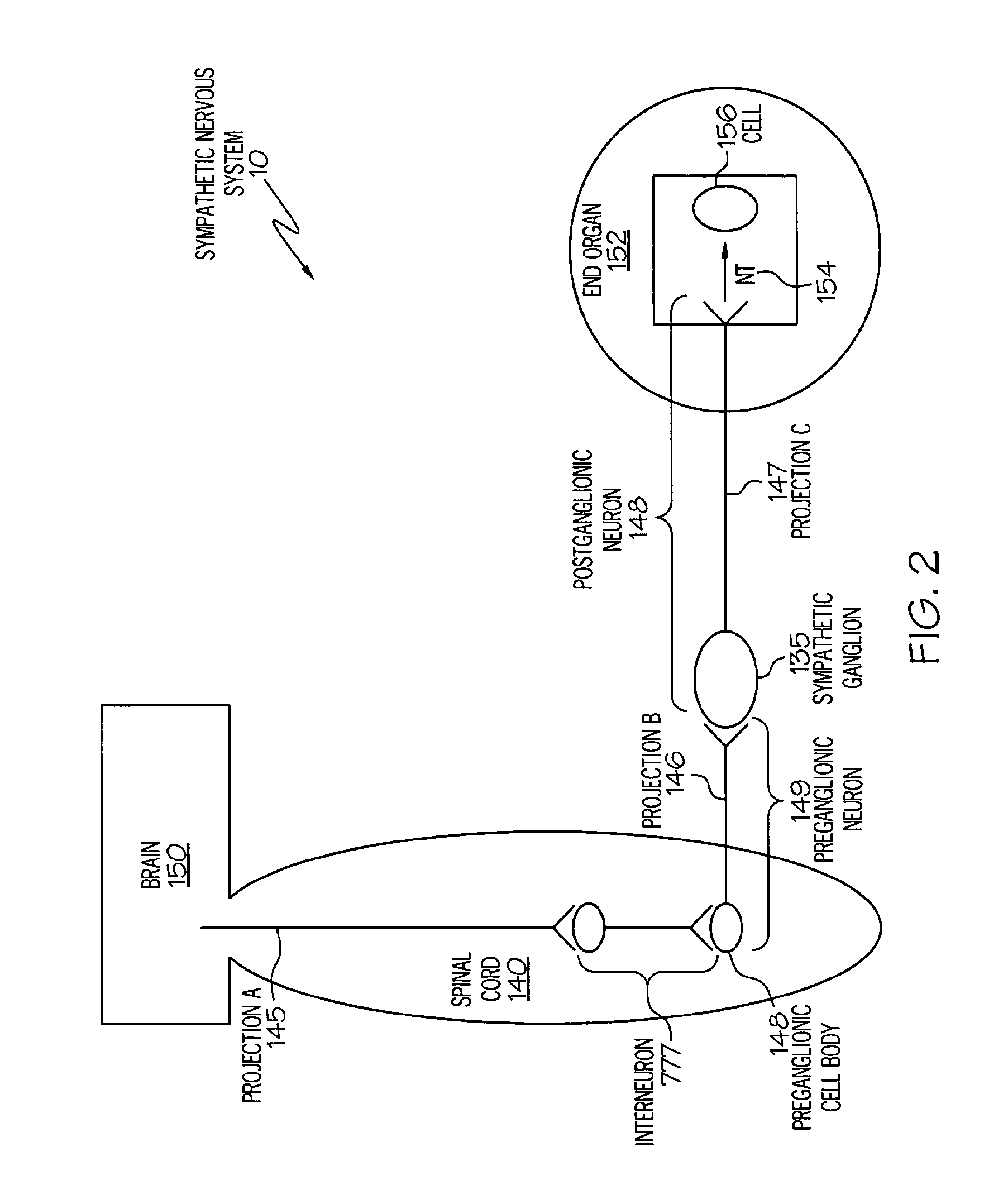
ActiveUS20050075701A1Good flexibilityMore levelsSpinal electrodesImplantable neurostimulatorsNervous systemNeuron
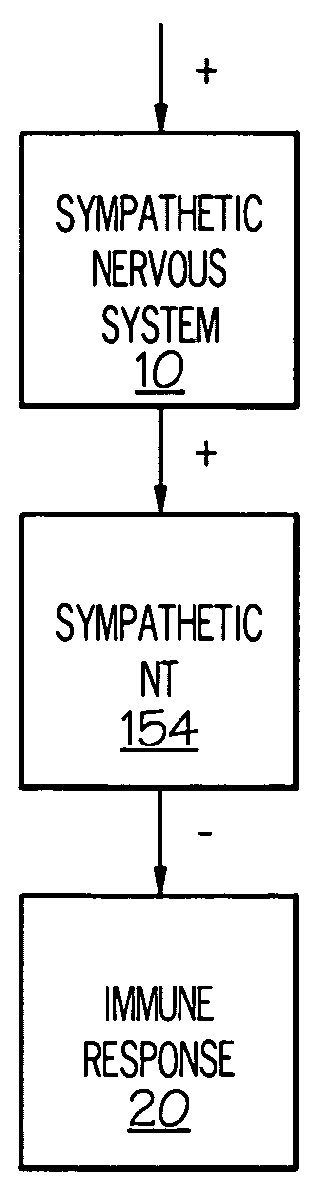
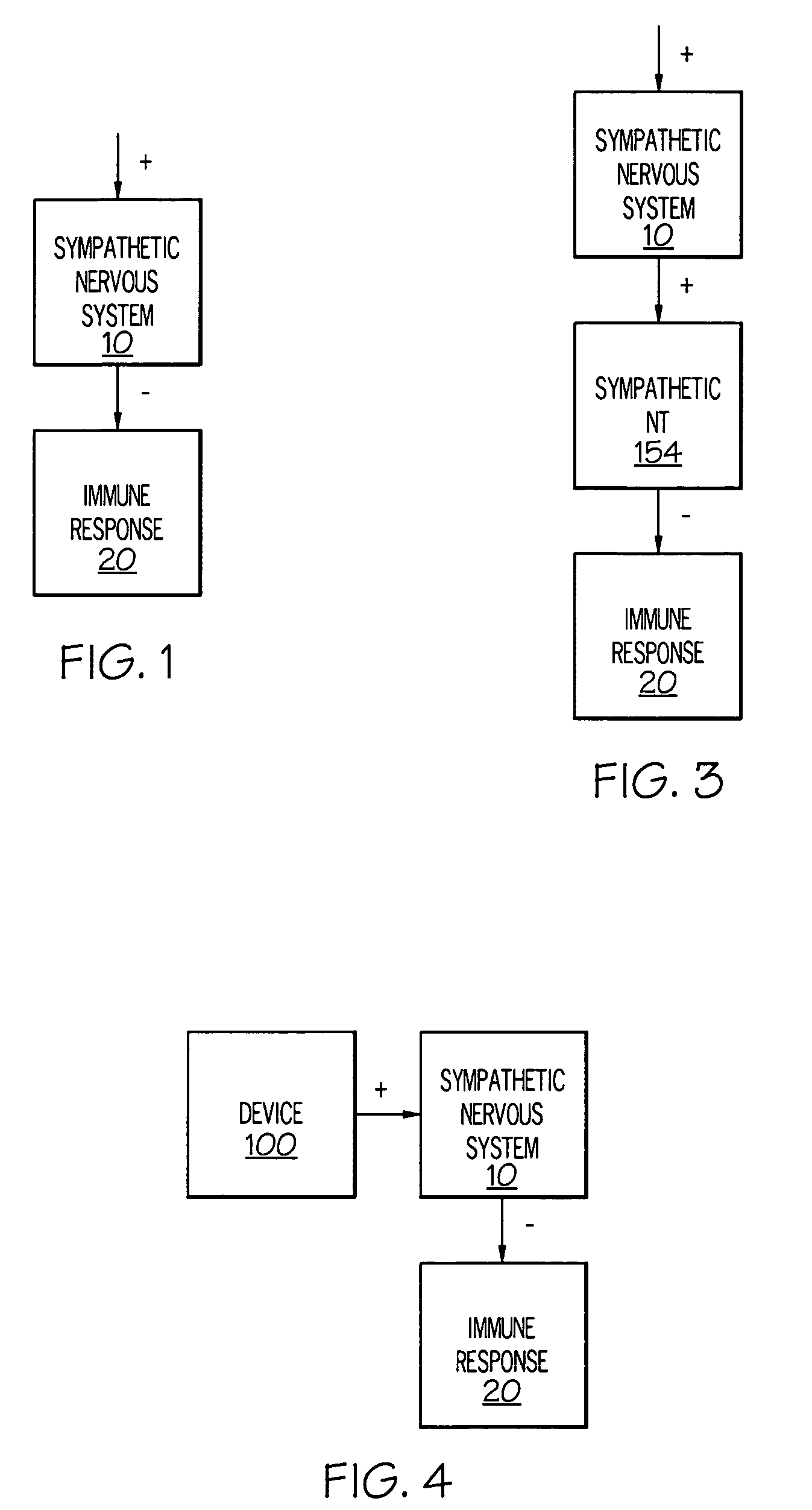
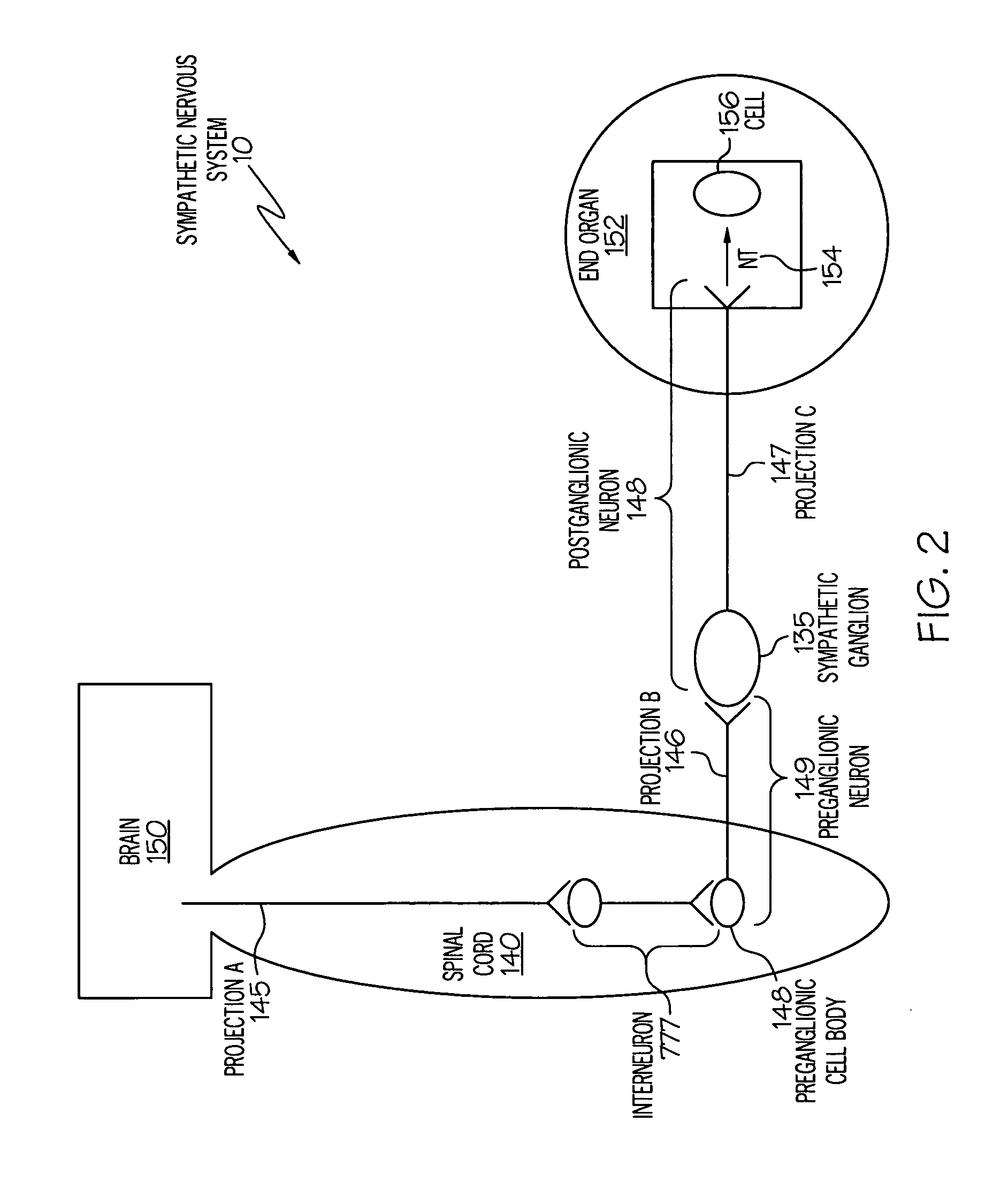
Stimulation of one or more neurons of the sympathetic nervous system, including the splenic nerve, to attenuate an immune response, including an inflammatory immune response, is discussed. Devices and systems to stimulate the sympathetic nervous system to attenuate an immune response are also discussed. Devices discussed include pulse generators and drug pumps. Systems are described as optionally having one or more sensors and operator instructions. In specific examples, stimulation of the splenic nerve of pigs with a pulse generator is shown to be safe and effective in attenuating a lipopolysaccharide-induced immune response.
Owner:MEDTRONIC INC
Method for the generation of antigen-specific lymphocytes
InactiveUS20070116690A1Function increaseEnhancing function of T cellBiocideVirusesAutoimmune conditionAutoimmune disease
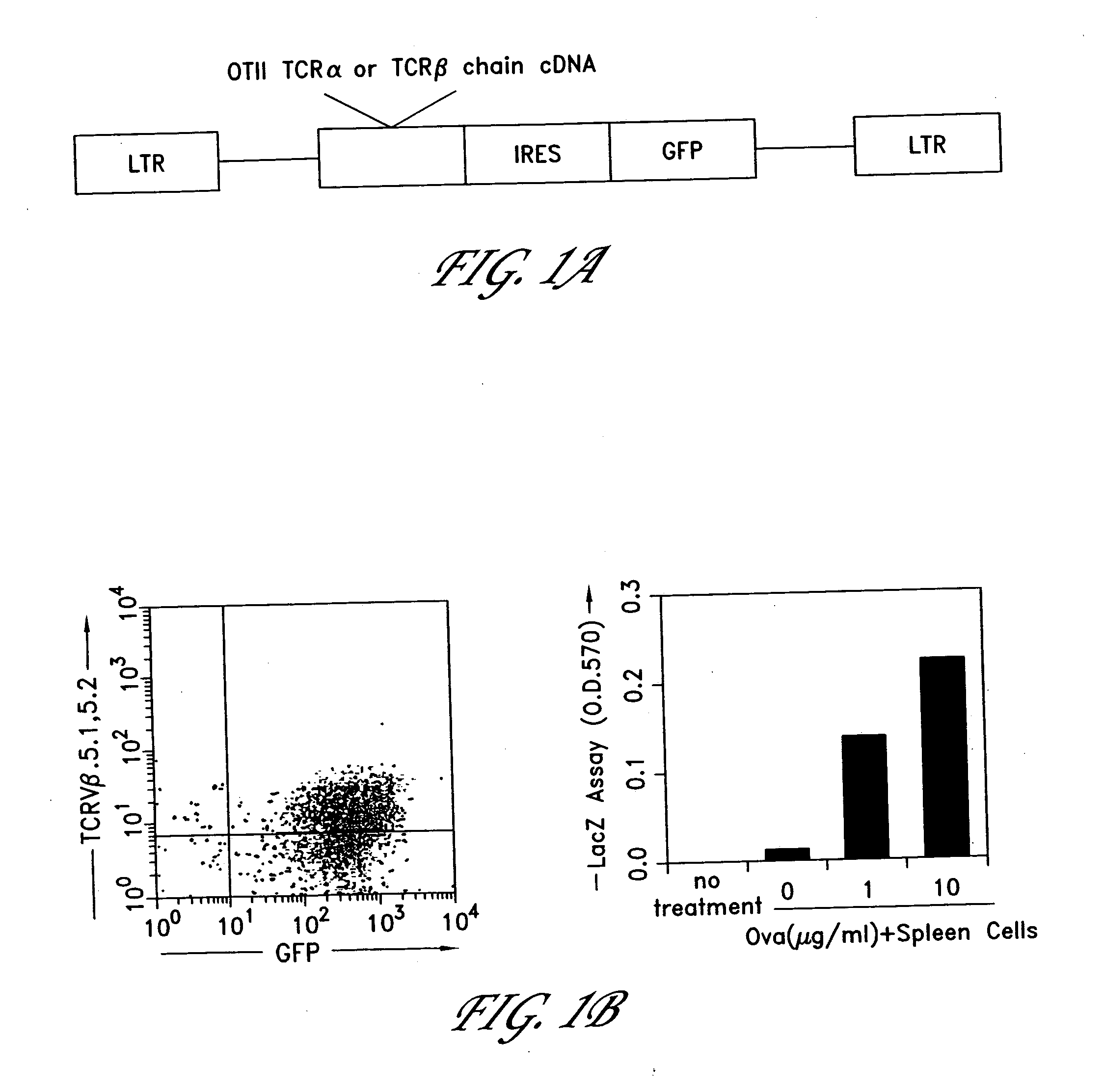
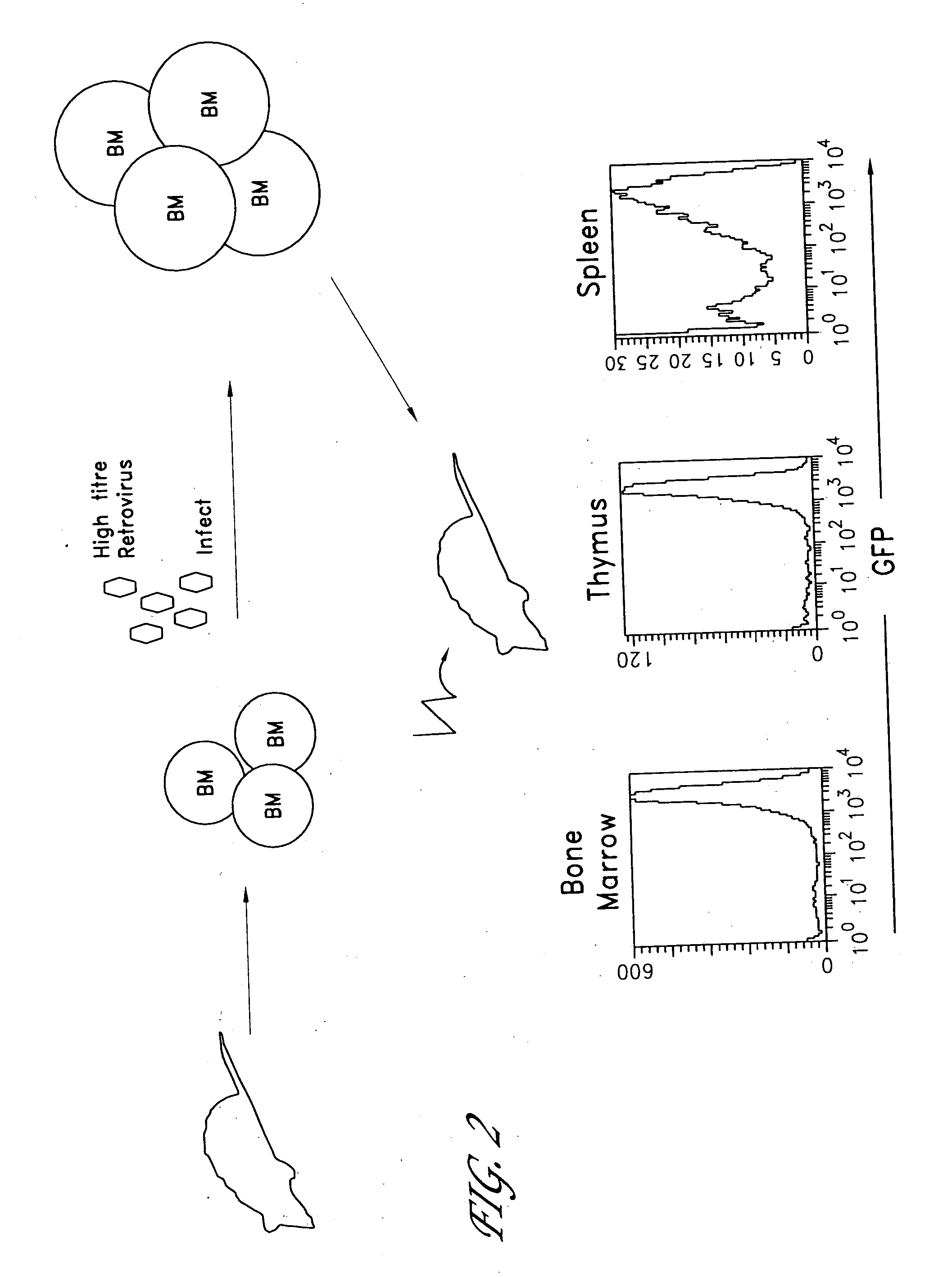
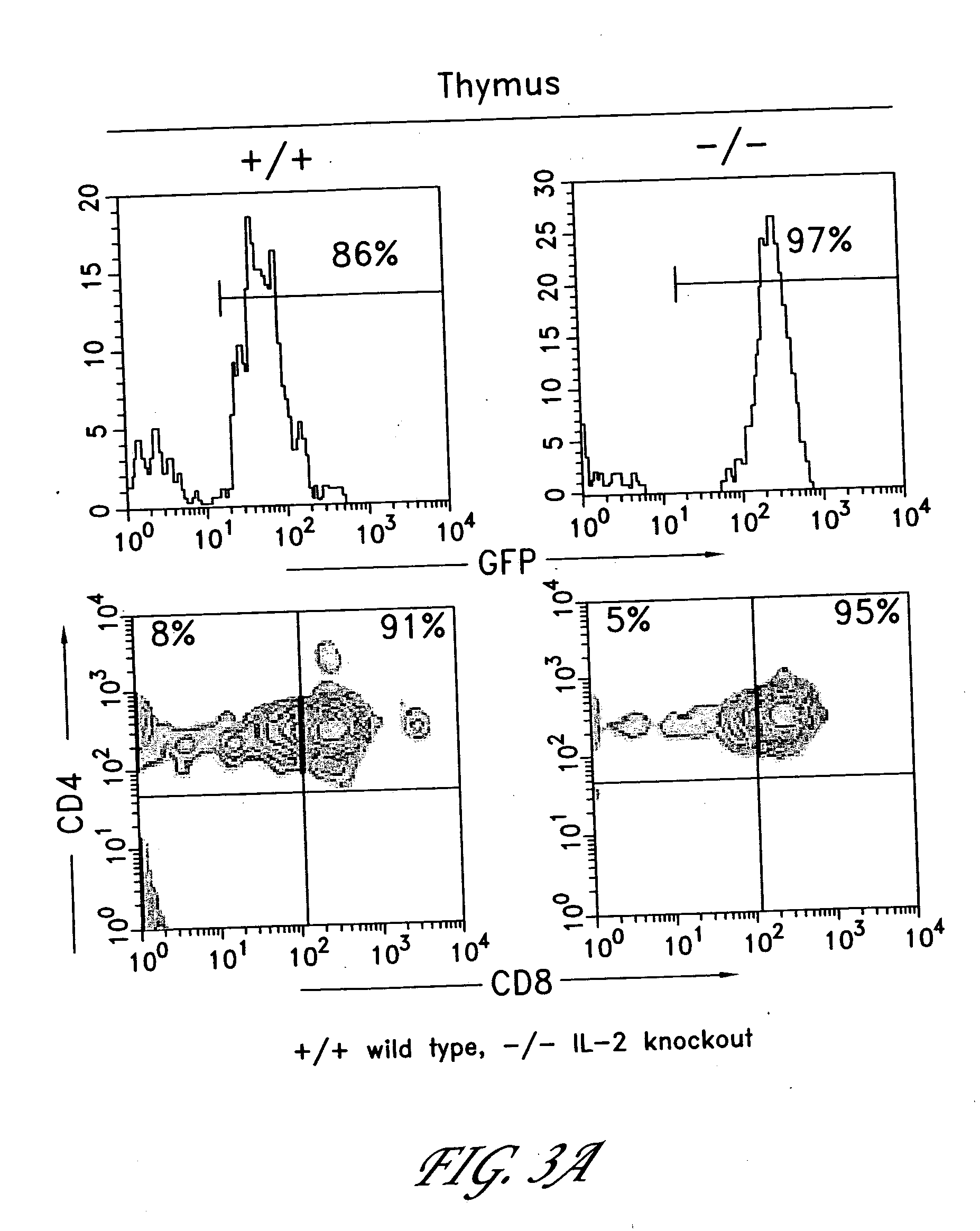
The invention provides systems and methods for the generation of lymphocytes having a unique antigen specificity. In a preferred embodiment, the invention provides methods of virally infecting cells from bone marrow with one or more viral vectors that encode antigen-specific antibodies for the production of, for example B cells and T cells. In some embodiments, the viral vectors include an IRES or 2A element to promote separation of, for example, the α subunit and β subunit of a T cell receptor (TCR) or heavy and light chains of a B-cell antibody. The resulting lymphocytes, express the particular antibody that was introduced in the case of B cells and TCR in the case of T cells. The lymphocytes generated can be used for a variety of therapeutic purposes including the treatment of various cancers and the generation of a desired immune response to viruses and other pathogens. The resulting cells develop normally and respond to antigen both in vitro and in vivo. We also show that it is possible to modify the function of lymphocytes by using stem cells from different genetic backgrounds. Thus our system constitutes a powerful tool to generate desired lymphocyte populations both for research and therapy. Future applications of this technology may include treatments for infectious diseases, such as HIV / AIDS, cancer therapy, allergy, and autoimmune disease.
Owner:CALIFORNIA INST OF TECH
Enhanced purification of antibodies and antibody fragments by apatite chromatography
ActiveUS20090187005A1Peptide/protein ingredientsPeptide preparation methodsApatiteAntibody fragments
Methods are disclosed for use of apatite chromatography, particularly without reliance upon phosphate gradients, for purification or separation of at least one intact non-aggregated antibody, or at least one immunoreactive antibody fragment, from an impure preparation. Integration of such methods into multi-step procedures with other fractionation methods are additionally disclosed.
Owner:BIO RAD LAB INC
Compositions and methods for improved skin care
InactiveUS20070077292A1Safe and effective amount of collagenPrevent adverse side effectsOrganic active ingredientsCosmetic preparationsMedicineInstability
Compositions and methods for administering collagen to a human subject have been developed. The collagen-containing lipid vesicles of the invention provide a delivery system for human collagen which eliminates problems associated with chemical and physical instability of the collagen as well as immune responses to non-human collagen.
Owner:PINSKY MARK A
Electrochemcial immunoassay for tumor marker and small size immunoassay chip
InactiveCN1614405ARapid separation-free assayAvoid pollutionMaterial analysis by electric/magnetic meansBiological testingAntigenContact free
An electrochemical method for determining immunity of tumor marker includes forming microreaction cell, placing reference and counter electrode above it and fixing antigen molecular functioning film at pool bottom, connecting enzyme labelled antibody to pool bottom at time of only proper quantity of horseradish peroxidase to label tumor marker antibody, obtaining cataltyic current on working electrode by contacting free immune matter with electronic media and having positive ratio of the current to antigen. The microvolume immune determining chip is also disclosed.
Owner:NANJING UNIV +1
Compositions and methods for priming monocytic dendritic cells and t cells for th-1response
InactiveUS20050059151A1Mammal material medical ingredientsBlood/immune system cellsDendritic cellMonocyte
The present invention provides compositions and methods for inducing maturation of immature dendritic cells (DC) and for priming those cells for inducing a type 1 immune response. The present invention also provides dendritic cell populations useful for activating and for preparing T cells polarized towards production of type 1 cytokines and / or a type 1 response. Similarly, activated, polarized T cell populations, and methods of making the same are provided.
Owner:NORTHWEST BIOTHERAPEUTICS INC
Analysis of circulating tumor cells, fragments, and debris
InactiveUS20050181463A1Avoid further damageInhibit further damageBioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceApoptosis
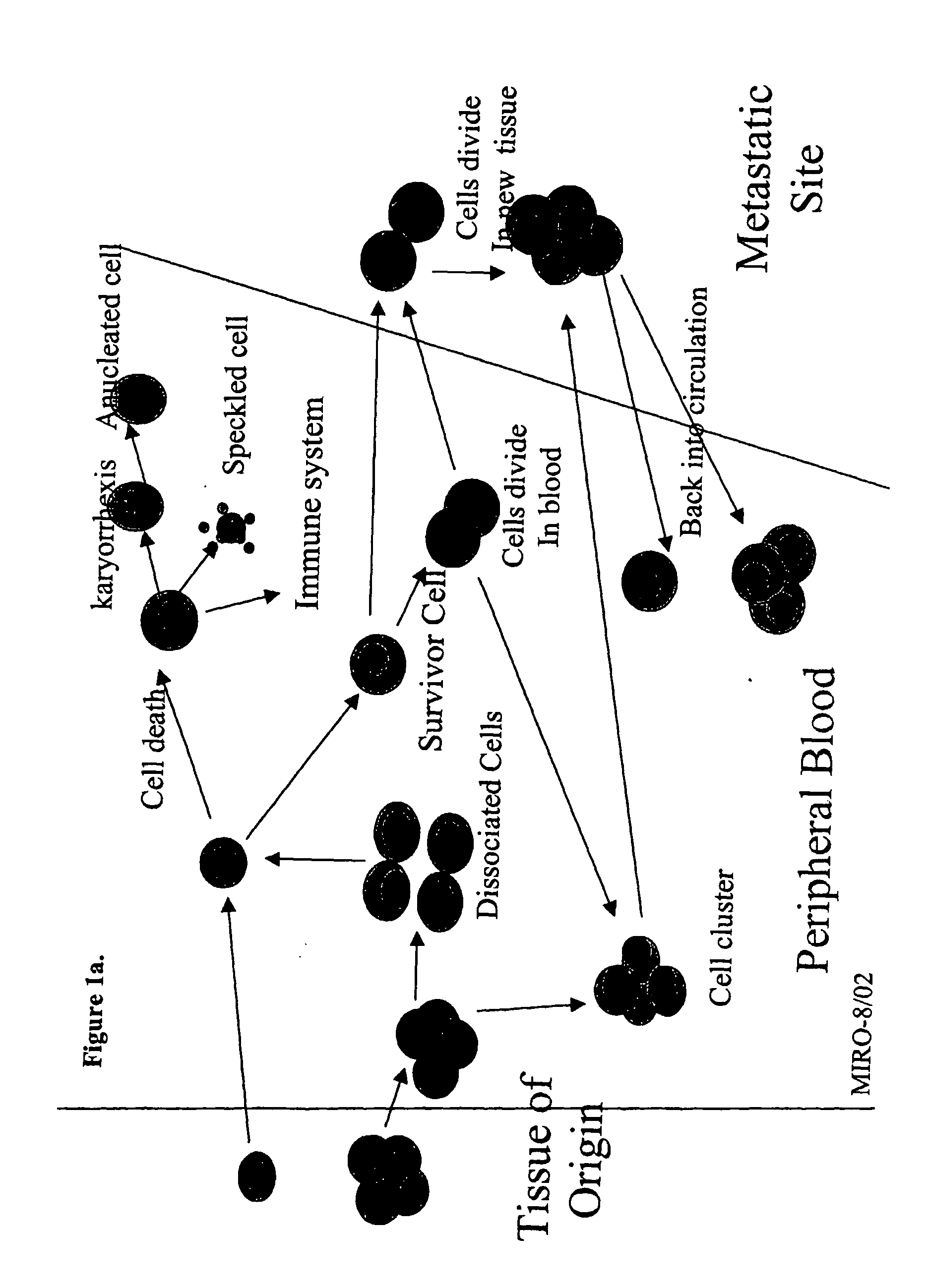
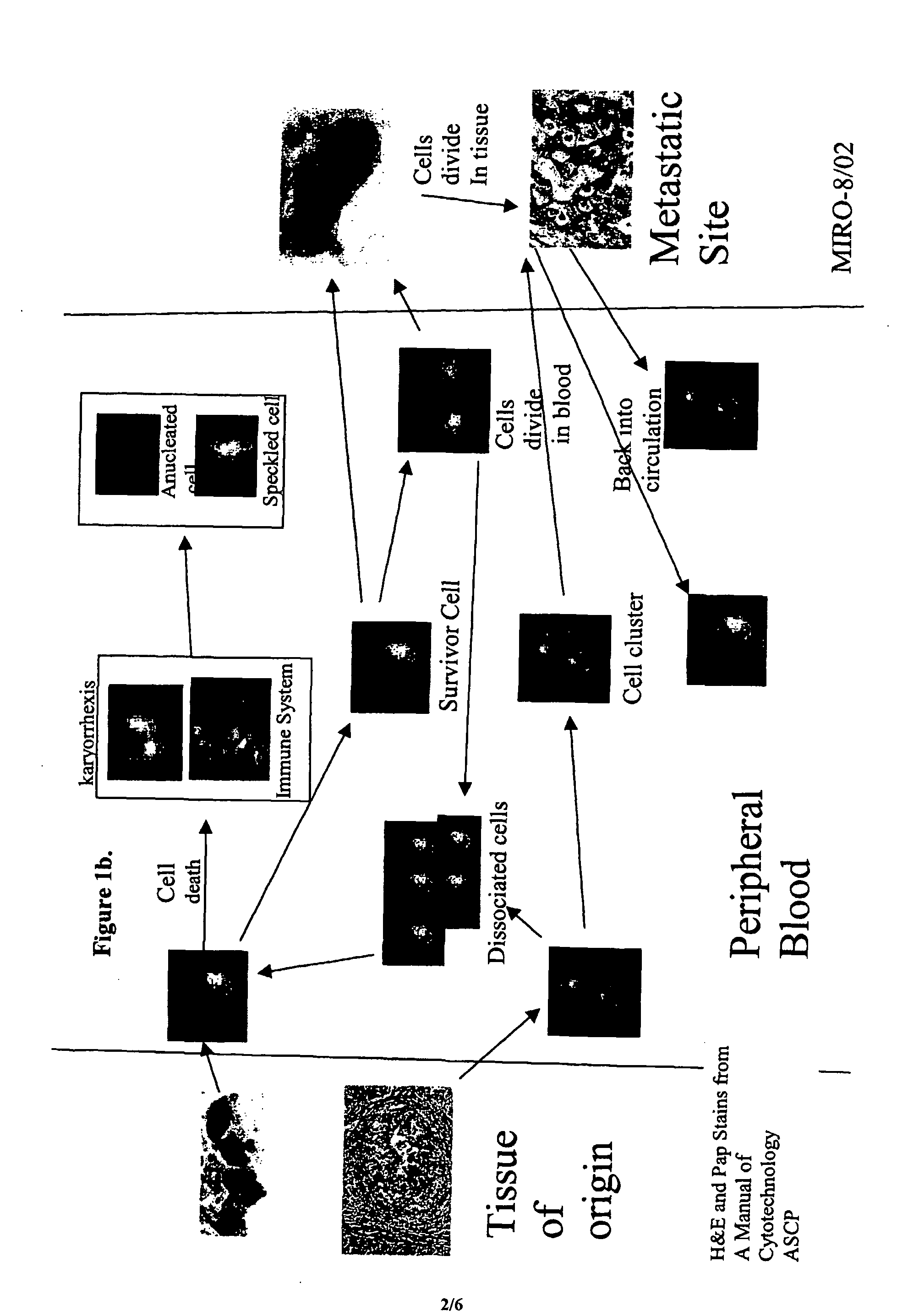
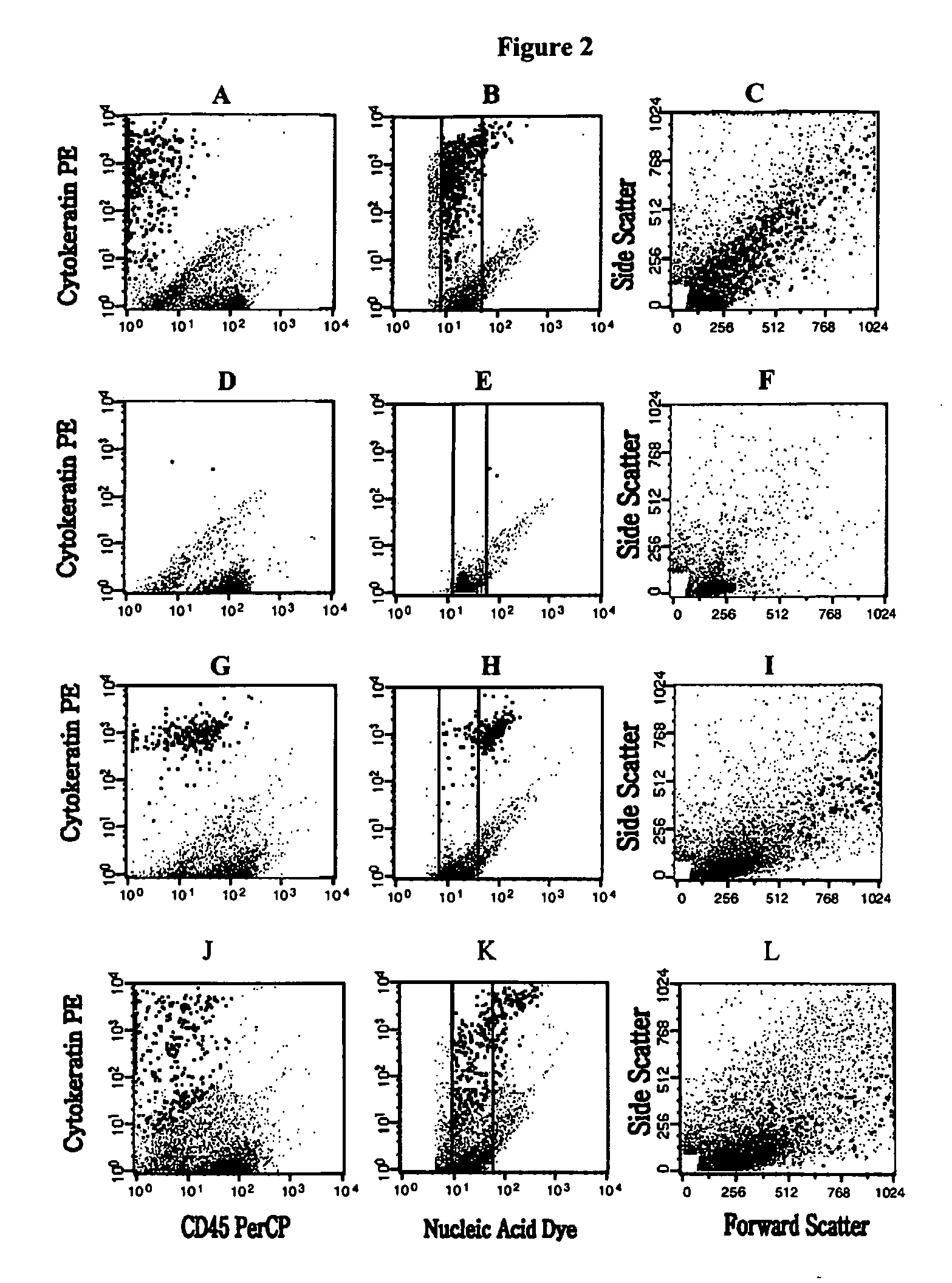
The methods and reagents described in this invention are used to analyze circulating tumor cells, clusters, fragments, and debris. Analysis is performed with a number of platforms, including flow cytometry and the CellSpotter® fluorescent microscopy imaging system. Analyzing damaged cells has shown to be important. However, there are two sources of damage: in vivo and in vitro. Damage in vivo occurs by apoptosis, necrosis, or immune response. Damage in vitro occurs during sample acquisition, handling, transport, processing, or analysis. It is therefore desirable to confine, reduce, eliminate, or at least qualify in vitro damage to prevent it from interfering in analysis. Described herein are methods to diagnose, monitor, and screen disease based on circulating rare cells, including malignancy as determined by CTC, clusters, fragments, and debris. Also provided are kits for assaying biological specimens using these methods.
Owner:MENARINI SILICON BIOSYSTEMS SPA
Antibodies Reactive with B7-H3 and Uses Thereof
ActiveUS20120294796A1Increased activationImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationHuman cancerCancer cell
The present invention relates to antibodies that are immunoreactive to the mammalian, and more particularly, the human B7-H3 receptor and to uses thereof, particularly in the treatment of cancer and inflammation. The invention thus particularly concerns humanized B7-H3-reactive antibodies that are capable of mediating, and more preferably enhancing the activation of the immune system against cancer cells that are associated with a variety of human cancers.
Owner:MACROGENICS INC
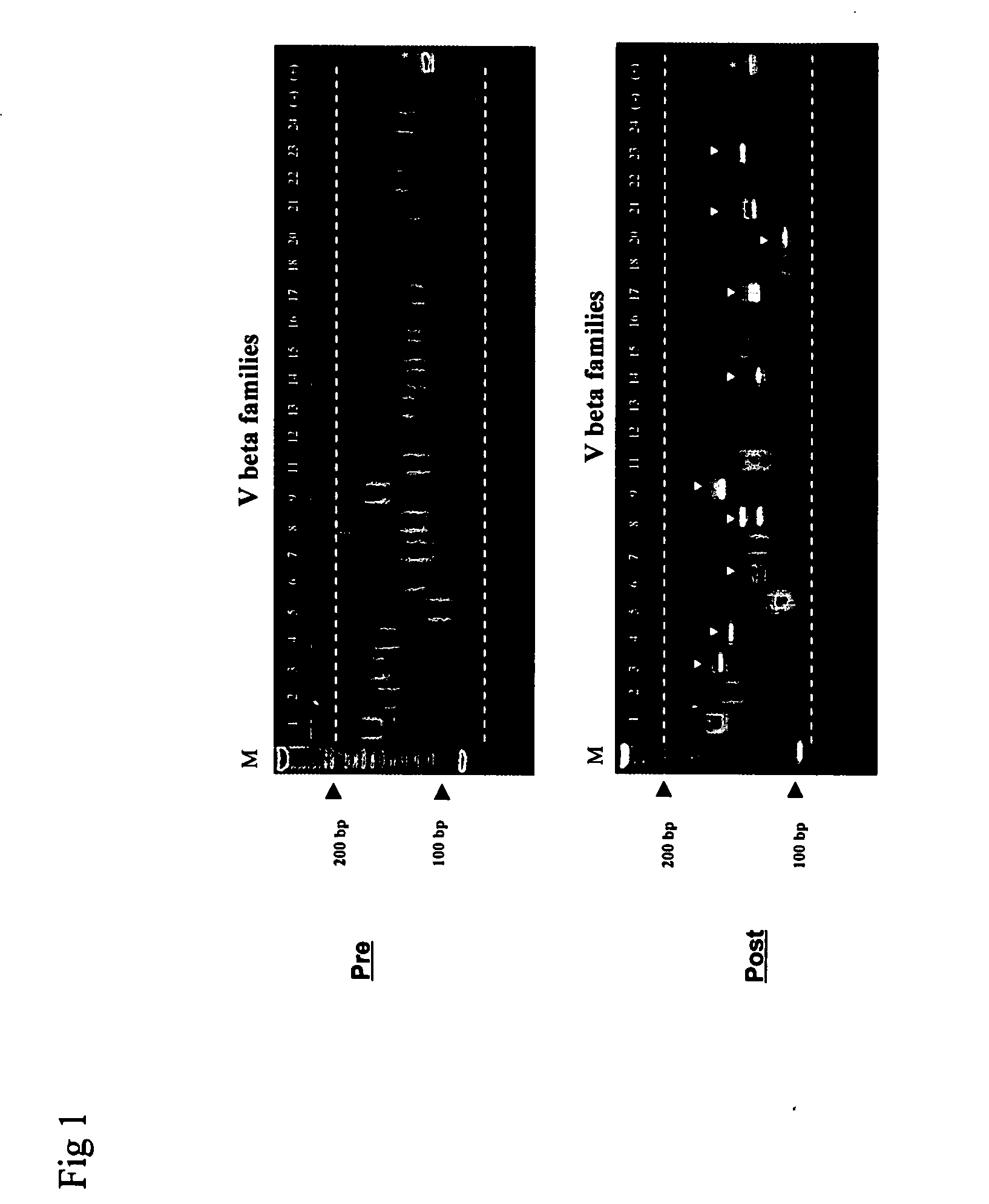
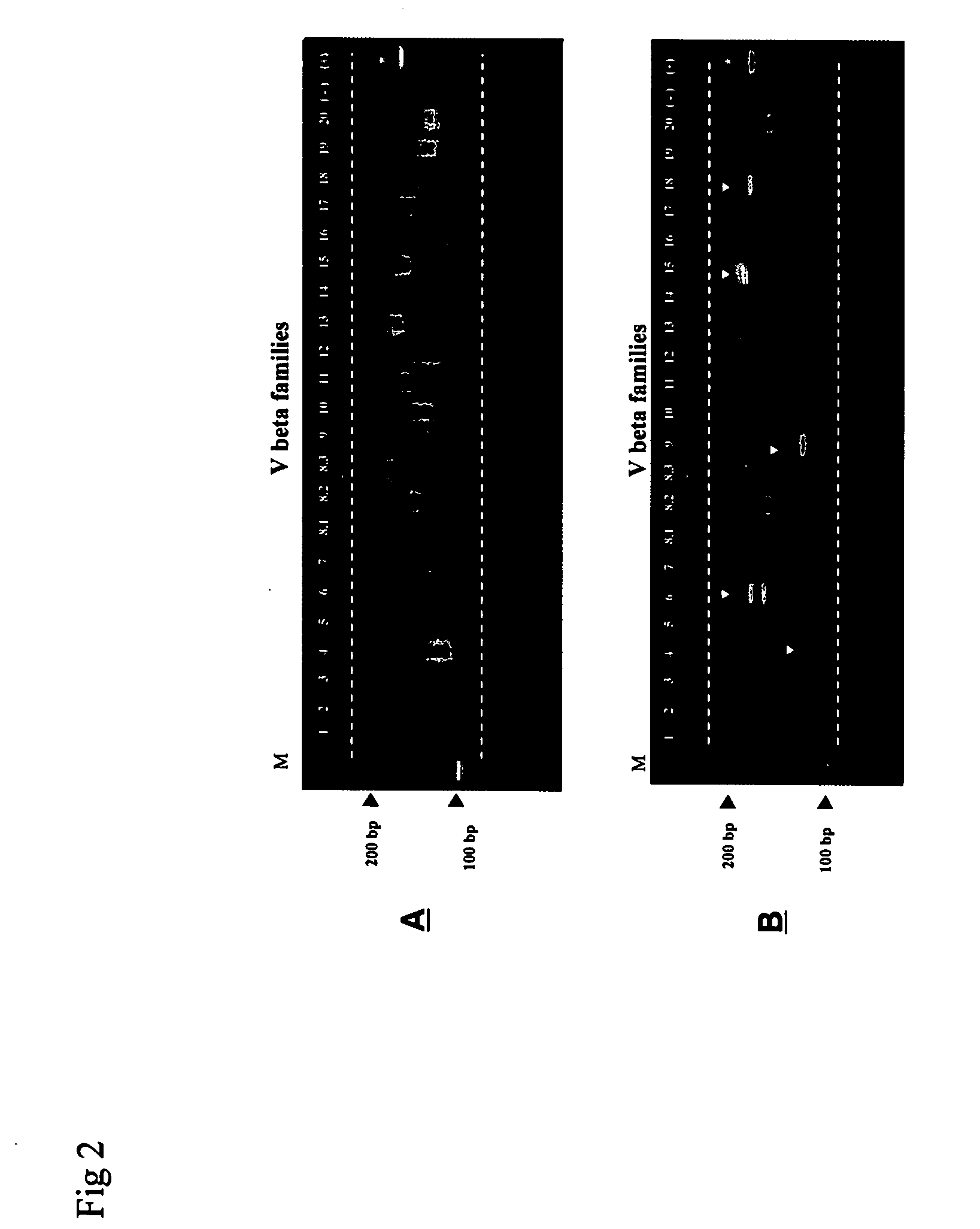
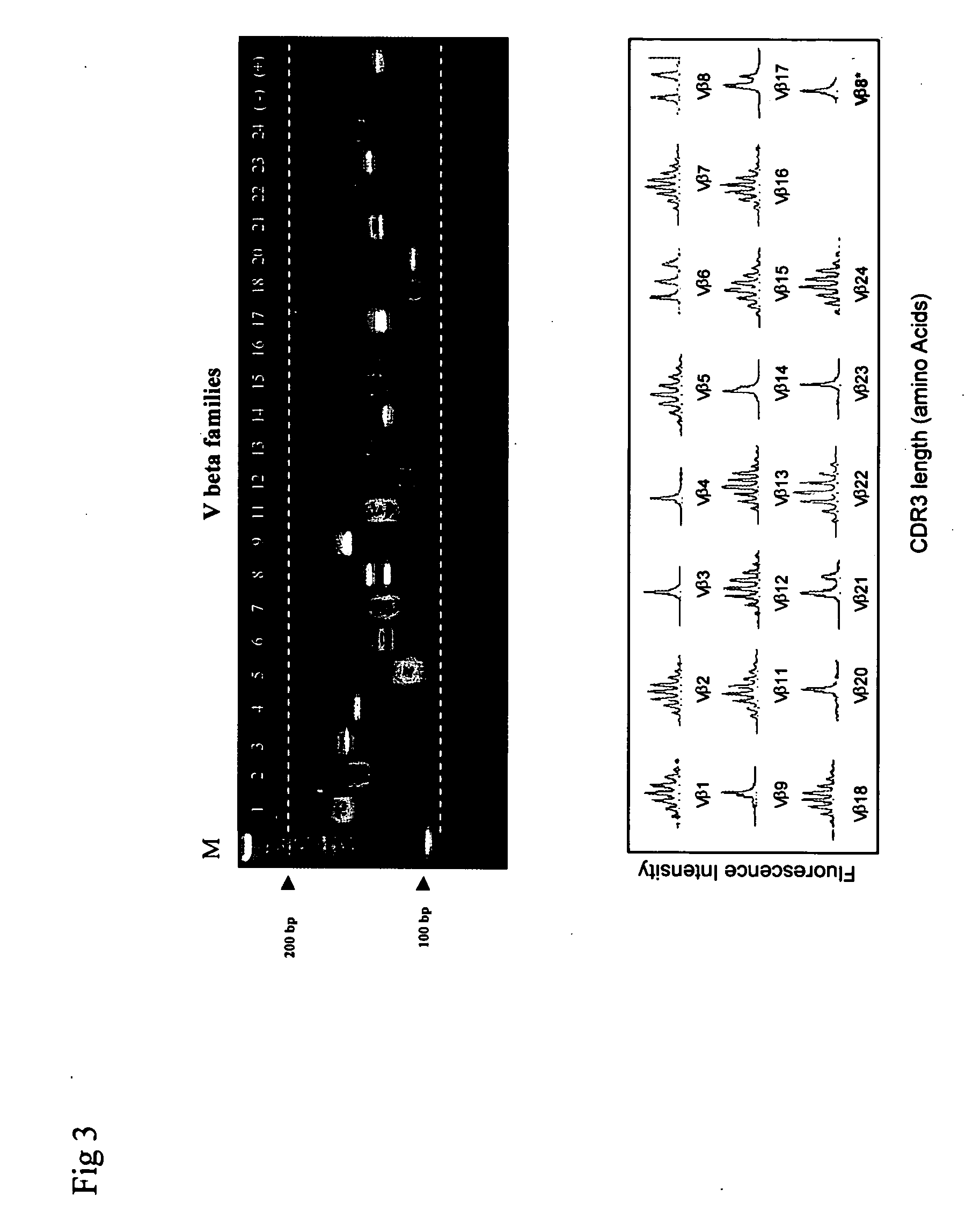
Method for detection and quantification of T-cell receptor Vbeta repertoire
ActiveUS20070117134A1Enhances PCR reaction sensitivityRapid determinationAnalysis using chemical indicatorsSugar derivativesVaccinationAntigen stimulation
The invention is a method for detecting and measuring T-cell receptor (TCR) repertoires from mammalian lymphocytes. The method is based on the use of the multiple sets of unique primers to amplify 22 regions of the TCR Vβ region and thereby detect clonal expansions related to antigen stimulation of the immune system. Kits containing sets of primers and specialized analytical statistical software for use in determining clonal expansion in humans and mice are disclosed. The reliability, efficiency and short assay time in using the method is well suited to monitoring immune response to vaccination and therapeutic treatments for immune disorders.
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
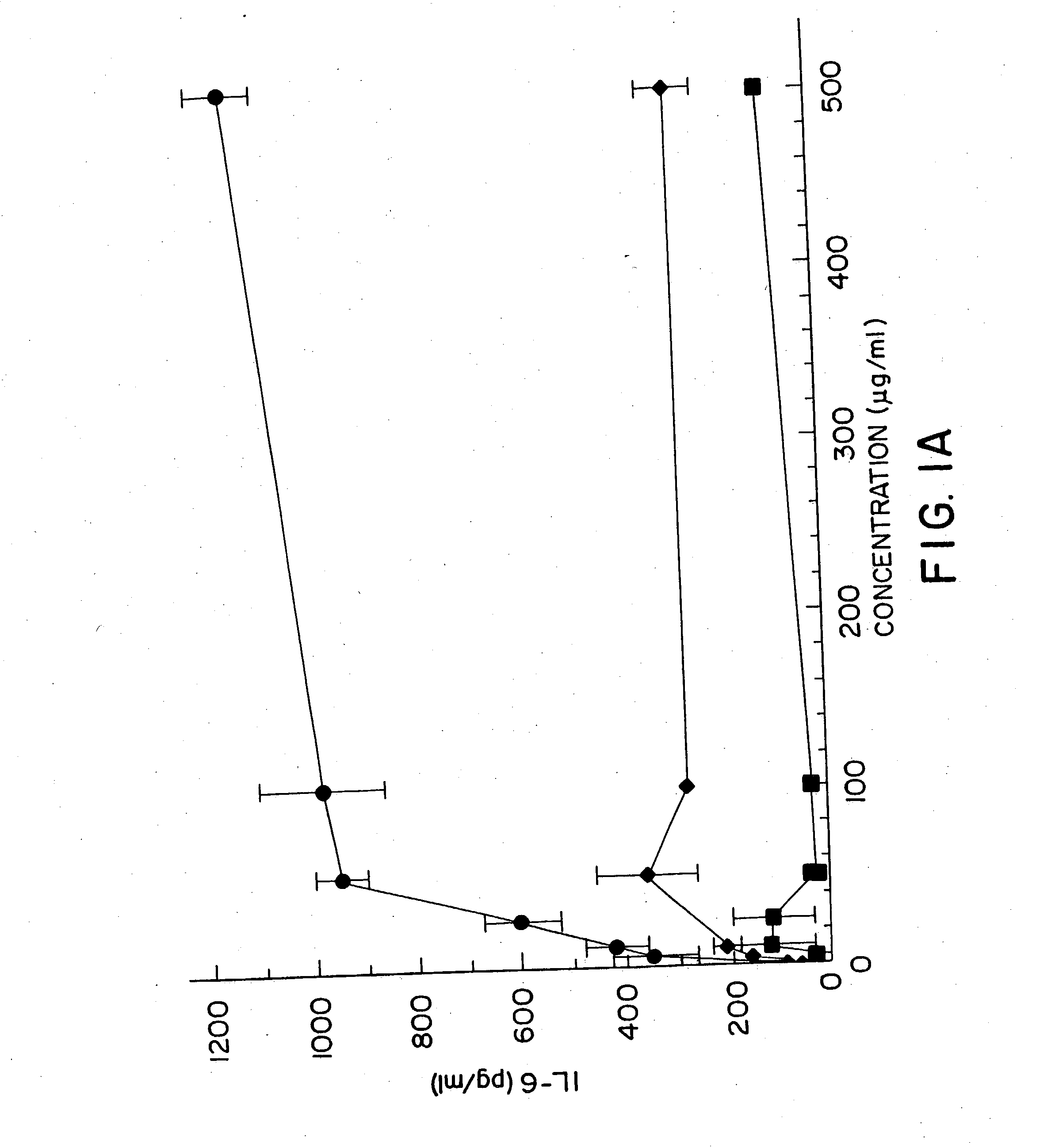
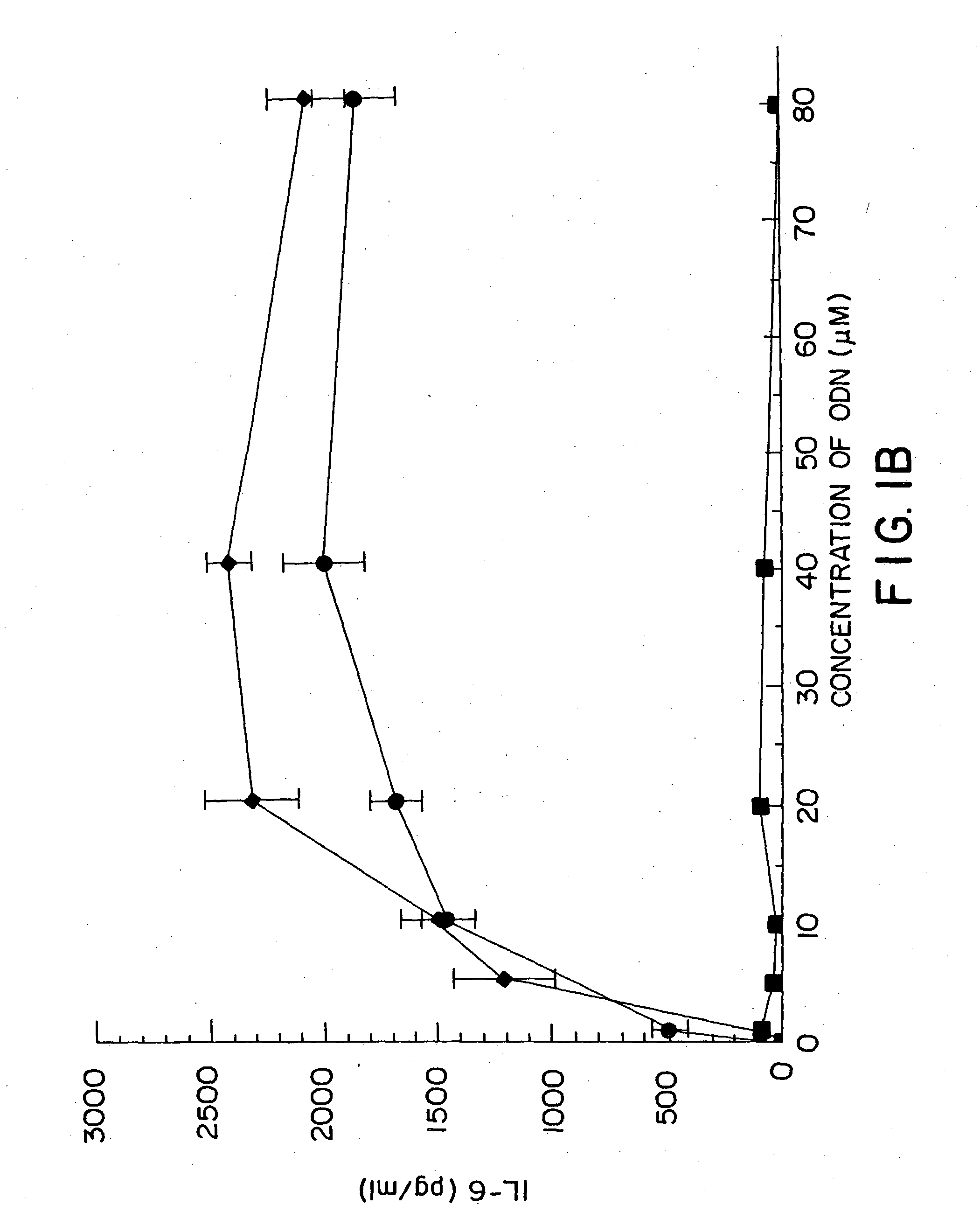
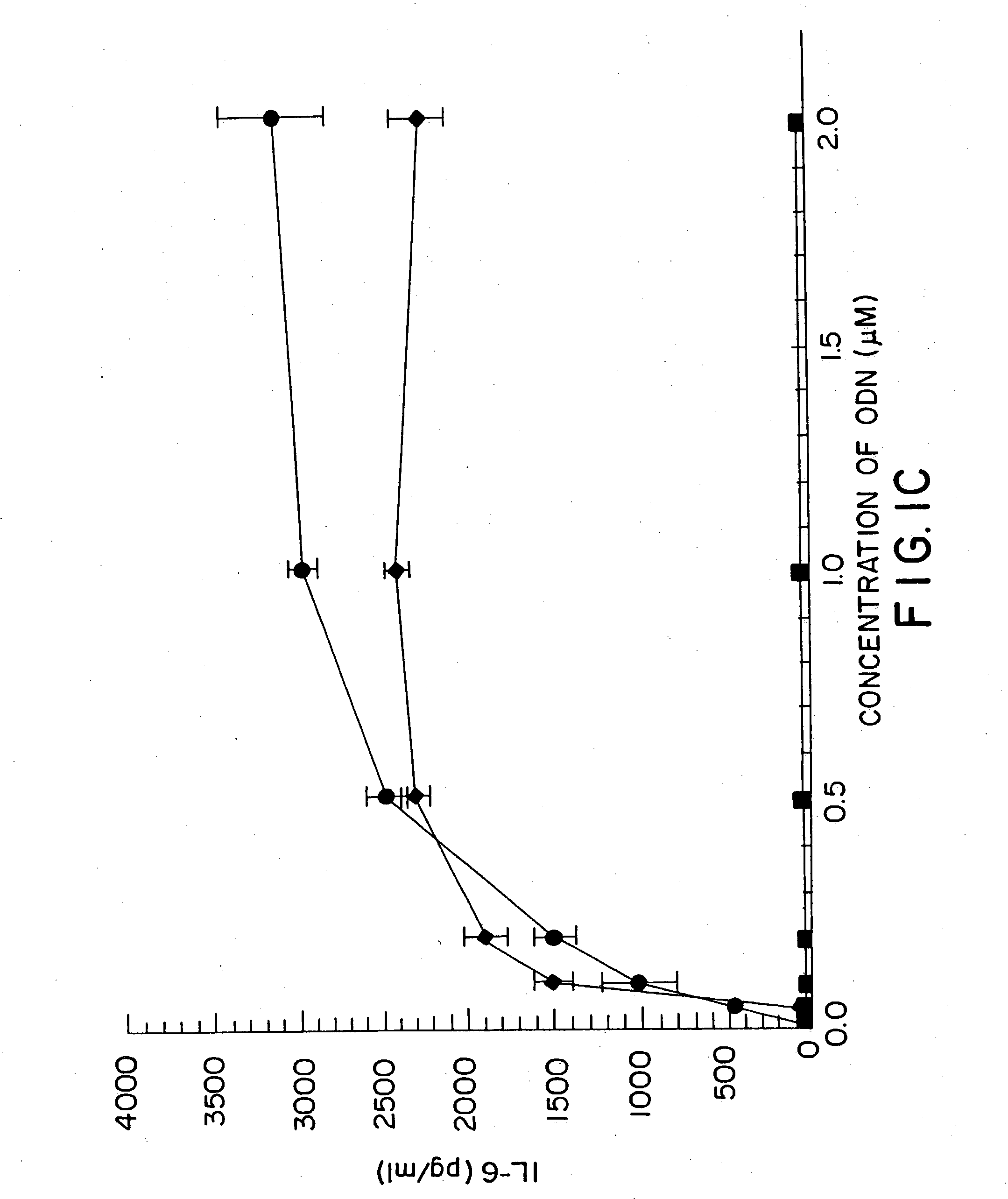
Methods for treating and preventing infectious disease
InactiveUS20050101554A1High sensitivityAntibacterial agentsGenetic material ingredientsAdjuvantNucleic acid sequencing
Nucleic acid sequences containing unmethylated CpG dinucleotides that modulate an immune response including stimulating a Th1 pattern of immune activation, cytokine production, NK lytic activity, and B cell proliferation are disclosed. The sequences are also useful as a synthetic adjuvant.
Owner:UNIV OF IOWA RES FOUND +2
Method for treating an IgE-mediated disease in a patient using anti-CD40 monoclonal antibodies
InactiveUS6899879B2Inhibition of differentiationInhibit growthOrganic active ingredientsVirusesDiseaseEpitope
Methods for preventing or treating an IgE-mediated allergic disease in a patient are presented, the methods comprising administration of a monoclonal antibody capable of binding to a human CD40 antigen located on the surface of a human B cell, wherein binding of the antibody to the CD40 antigen prevents the growth or differentiation of the B cell. Monoclonal antibodies useful in these methods, and epitopes immunoreactive with such monoclonal antibodies are also presented.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Passive immunization of Alzheimer's disease
InactiveUS6913745B1Peptide/protein ingredientsAntibody mimetics/scaffoldsPassive ImmunizationsAmyloid disease
Disclosed are pharmaceutical compositions and methods for preventing or treating a number of amyloid diseases, including Alzheimer's disease, prion diseases, familial amyloid neuropathies and the like. The pharmaceutical compositions include immunologically reactive amounts of amyloid fibril components, particularly fibril-forming peptides or proteins. Also disclosed are therapeutic compositions and methods which use immune reagents that react with such fibril components.
Owner:JANSSEN SCI IRELAND UC
Cytokine antagonists for the treatment of sensorineural hearing loss
InactiveUS6423321B2Improve hearingReduce developmentPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsIntravenous routeSensorineural hearing loss
Specific Cytokine Antagonists, including TNF antagonists and / or Interleukin-1 antagonists, are used as novel therapeutic agents for the treatment of hearing loss, including presbycusis and other forms of sensorineural hearing loss. The present invention provides a method for inhibiting the action of TNF and / or IL-1 antagonists for treating hearing loss in a human by administering a TNF antagonist and / or an IL-1 antagonist for reducing the inflammation affecting the auditory apparatus of said human, or for modulating the immune response affecting the auditory apparatus of said human, by administering a therapeutically effective dosage level to said human of a TNF antagonist and / or an IL-1 antagonist. Administration may be systemic, through the subcutaneous, intramuscular, oral, or intravenous routes; or by delivering an anatomically localized application in the region of the head. The TNF antagonist is selected from the group consisting of etanercept, infliximab, D2E7, CDP 571, or thalidomide; and the IL-1 antagonist is either IL-1 RA or IL-1R type II receptor. Antiviral agents may be added for treating certain patients.
Owner:TACT IP
Methods of elicit, enhance and sustain immune responses against MHC class I-restricted epitopes, for prophylactic or therapeutic purposes
InactiveUS20050079152A1Improve responseEfficient amplificationAntibacterial agentsBiocideEpitopeMHC class I
Embodiments relate to methods and compositions for eliciting, enhancing, and sustaining immune responses, preferably against MHC class I-restricted epitopes. The methods and compositions can be used for prophylactic or therapeutic purposes.
Owner:MANNKIND CORP
Device and method for inhibiting release of pro-inflammatory mediator
ActiveUS20060287678A1Good flexibilityMore levelsSpinal electrodesArtificial respirationNervous systemNeuron
Stimulation of one or more neurons of the sympathetic nervous system, including the splenic nerve, to attenuate an immune response, including an inflammatory immune response, is discussed. Devices and systems to stimulate the sympathetic nervous system to attenuate an immune response are also discussed. Devices discussed include pulse generators and drug pumps. Systems are described as optionally having one or more sensors and operator instructions. In specific examples, stimulation of the splenic nerve of pigs with a pulse generator is shown to be safe and effective in attenuating a lipopolysaccharide-induced immune response.
Owner:MEDTRONIC INC
Pharmaceutical compositions and methods for treatment of amyloid diseases
Disclosed are pharmaceutical compositions and methods for preventing or treating a number of amyloid diseases, including Alzheimer's disesase, prion diseases, familial amyloid neuropathies and the like. The pharmaceutical compositions include immunologically reactive amounts of amyloid fibril components, particularly fibril-forming peptides or proteins. Also disclosed are therapeutic compositions and methods which use immune reagents that react with such fibril components.
Owner:JANSSEN ALZHEIMER IMMUNOTHERAPY
Vitamin D3 mimics
The present invention relates to non-secosteroidal compounds which activate and modulate the vitamin D receptor (VDR). Because compounds of the present invention display many of the beneficial properties of 1,25(OH)2D3, but with reduced calcium mobilization effects, they may be used advantageously to treat and prevent conditions that show vitamin D sensitivity. Such disease states typically show abnormal calcium regulatory, abnormal immune responsive, hyperproliferative, and / or neurodegenerative characteristics.
Owner:LIGAND PHARMA INC
Dock-and-lock (DNL) vaccines for cancer therapy
Owner:IBC PHARMACEUTICALS INC
Antibodies as T cell receptor mimics, methods of production and uses thereof
InactiveUS20060034850A1Aid in stabilizationAnimal cellsImmunoglobulin superfamilyHla moleculesDisease cause
The present invention relates to a methodology of producing antibodies that recognize peptides associated with a tumorigenic or disease state, wherein the peptides are displayed in the context of HLA molecules. These antibodies will mimic the specificity of a T cell receptor (TCR) but will have higher binding affinity such that the molecules may be used as therapeutic, diagnostic and research reagents. The method of producing a T-cell receptor mimic of the present invention includes identifying a peptide of interest, wherein the peptide of interest is capable of being presented by an MHC molecule. Then, an immunogen comprising at least one peptide / MHC complex is formed, wherein the peptide of the peptide / MHC complex is the peptide of interest. An effective amount of the immunogen is then administered to a host for eliciting an immune response, and serum collected from the host is assayed to determine if desired antibodies that recognize a three-dimensional presentation of the peptide in the binding groove of the MHC molecule are being produced. The desired antibodies can differentiate the peptide / MHC complex from the MHC molecule alone, the peptide alone, and a complex of MHC and irrelevant peptide. Finally, the desired antibodies are isolated.
Owner:TEXAS TECH UNIV SYST
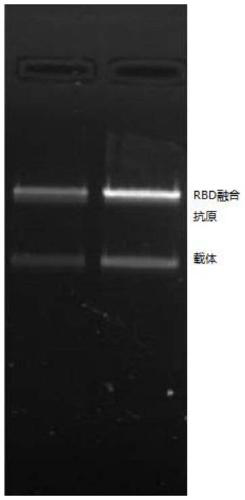
SARS-CoV-2 vaccine and preparation method thereof
ActiveCN111217917AEnter to helpImproving immunogenicityPolypeptide with localisation/targeting motifSsRNA viruses positive-senseAntigenDisease
The invention relates to a preparation method for a vaccine capable of treating and / or preventing SARS-CoV-2 infection or COVID-19 diseases. The core antigen of the vaccine comprises the RBD (receptor binding zone) fusion protein of the SARS-CoV-2, and a vaccine form comprises an RBD fusion protein subunit vaccine, an RBD fusion protein mRNA vaccine or an RBD fusion protein adenovirus vector vaccine. The above vaccine immunizes an organism, and immune reaction for treating and / or preventing the SARS-CoV-2 infection can be generated so as to be used for treating and / or preventing COVID-19. The invention also relates to an RBD fusion gene, the RBD fusion protein, a carrier, a cell, a preparation method, a treatment method or a pharmacy purpose of the SARS-CoV-2.
Owner:CANSINO BIOLOGICS INC
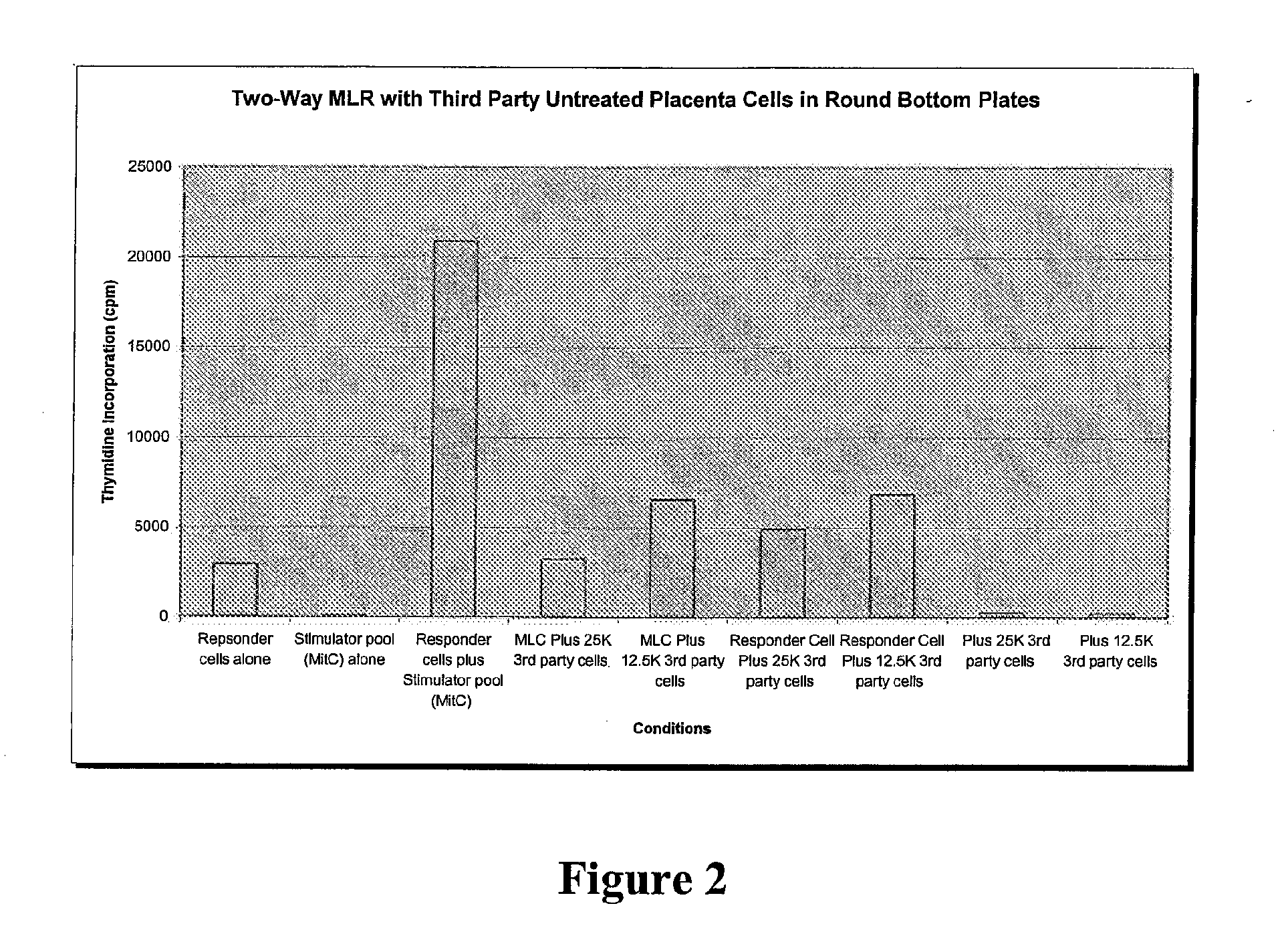
Compositions and methods for inhibiting adverse immune response in histocompatibility-mismatched transplantation
ActiveUS20070264269A1Effective for adverse immune responseAntipyreticAnalgesicsGraft versus host disease inductionCell based
Cell-based compositions and methods of their use to inhibit an adverse immune response such as graft versus host disease or rejection of transplanted tissue in a transplant recipient that is histocompatibility mismatched to the transplant donor are disclosed. The compositions and methods utilize postpartum-derived cells, such as cells derived from the placenta or umbilicus.
Owner:DEPUY SYNTHES PROD INC
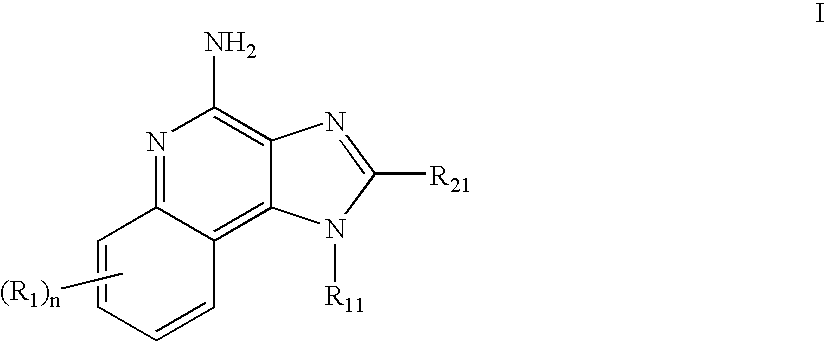
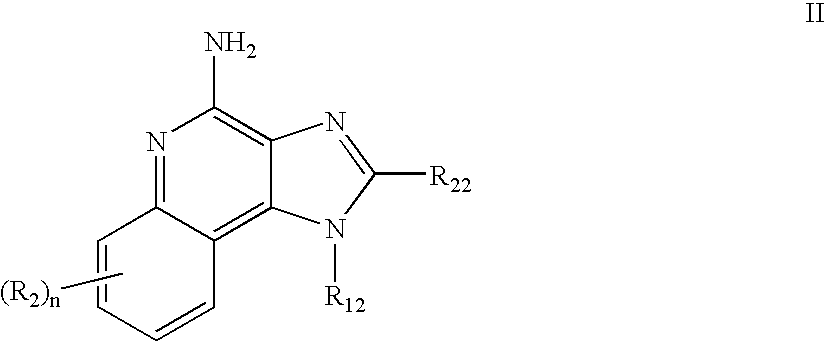
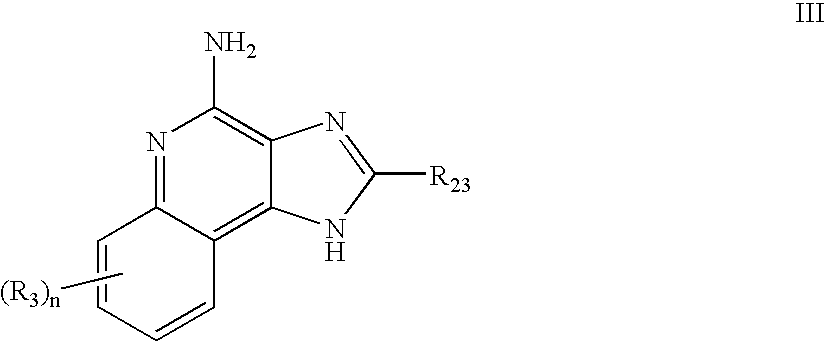
Immune response modifier formulations and methods
Pharmaceutical formulations including an immune response modifier (IRM) compound having a 2-aminopyridine moiety fused to a five-membered nitrogen-containing heterocyclic ring; a preservative system including a sorbic acid preservative selected from the group consisting of sorbic acid, esters thereof, salts thereof, and combinations thereof; an antioxidant; and an optional chelating agent.
Owner:MEDICIS PHARMA CORP
Adjuvanted influenza vaccines for pediatric use
ActiveUS8506966B2Enhance immune responseHigh seroprotection rateSsRNA viruses negative-senseViral antigen ingredientsAdjuvantSeroconversion
An influenza vaccine adjuvanted with a sub-micron oil-in-water emulsion elicits significantly higher immune responses in human pediatric populations. Compared to an existing unadjuvanted pediatric influenza vaccine, the adjuvanted vaccines provided herein can induce in children a longer persistence of high serum antibody titers and also longer seroconversion and seroprotection. The improvement in immune responses is seen for both influenza A virus and influenza B virus strains, but it is particularly marked for influenza B virus. Moreover, while the existing vaccine provides poor immunity in children after a single dose, the adjuvanted vaccine provides high seroprotection rates against the influenza A virus H3N2 subtype even after a single dose. Furthermore, the adjuvanted vaccine offers significantly better seroprotection against mismatched strains of influenza A virus.
Owner:SEQIRUS UK LTD
Methods and marker combinations for screening for predisposition to lung cancer
InactiveUS20070178504A1Microbiological testing/measurementBiological testingOncologyLung cancer susceptibility
Owner:ABBOTT LAB INC
Rsv f protein compositions and methods for making same
InactiveUS20110305727A1Consistent stabilitySsRNA viruses negative-senseSsRNA viruses positive-senseF proteinImmunogenicity
The present invention relates to immunogenic compositions comprising RSV F protein, methods for preparing compositions that contain RSV F protein ecto-domain polypeptides, and to certain engineered RSV F proteins and nucleic acids that encode the engineered RSV F proteins. Compositions prepared using the methods can contain RSV F protein ecto-domain polypeptides in a predominant or single desired form and conformation. The invention also relates to methods for inducing an immune response to RSV F.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Antibodies immunoreactive with mutant 5-enolpyruvlshikimate-3-phosphate synthase
Antibodies immunoreactive to double mutant EPSPS are provided, and in an embodiment the double mutant EPSPS is one in which the wild-type EPSPS is substituted at residue 102 with isoleucine and at residue 106 with serine. Also provided are hybridomas producing the antibodies, as well as methods of making and using the antibodies.
Owner:M S TECH
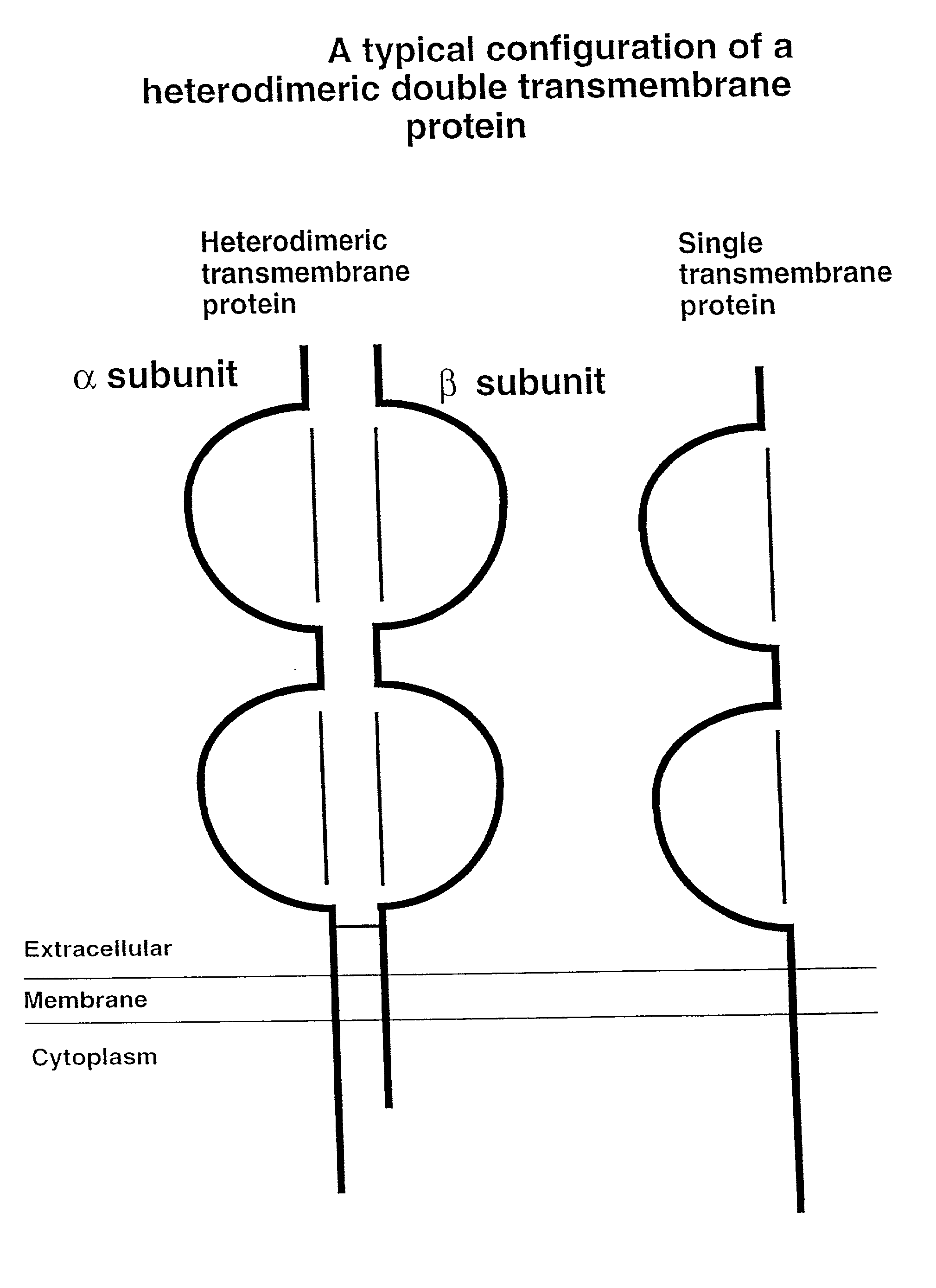
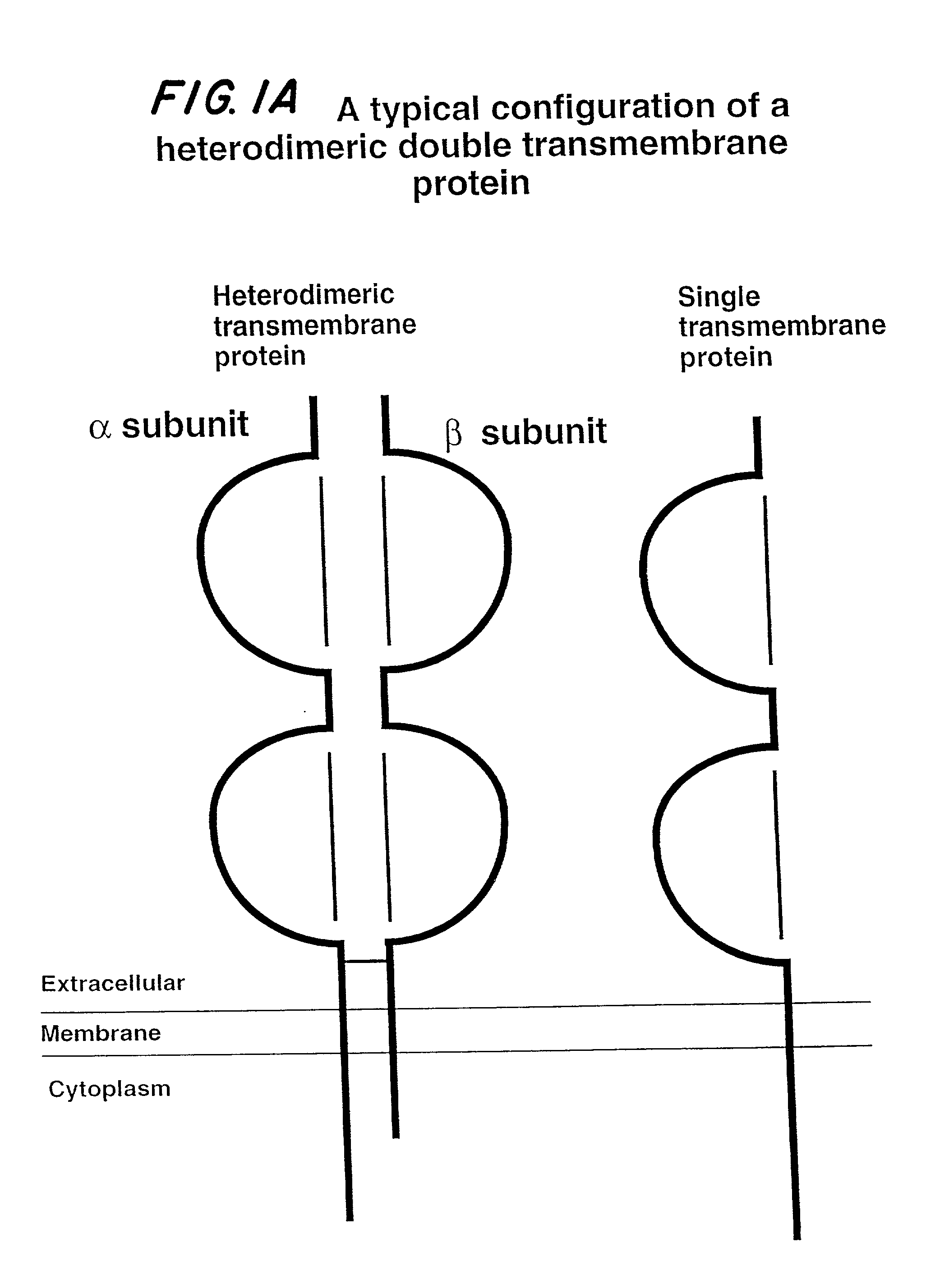
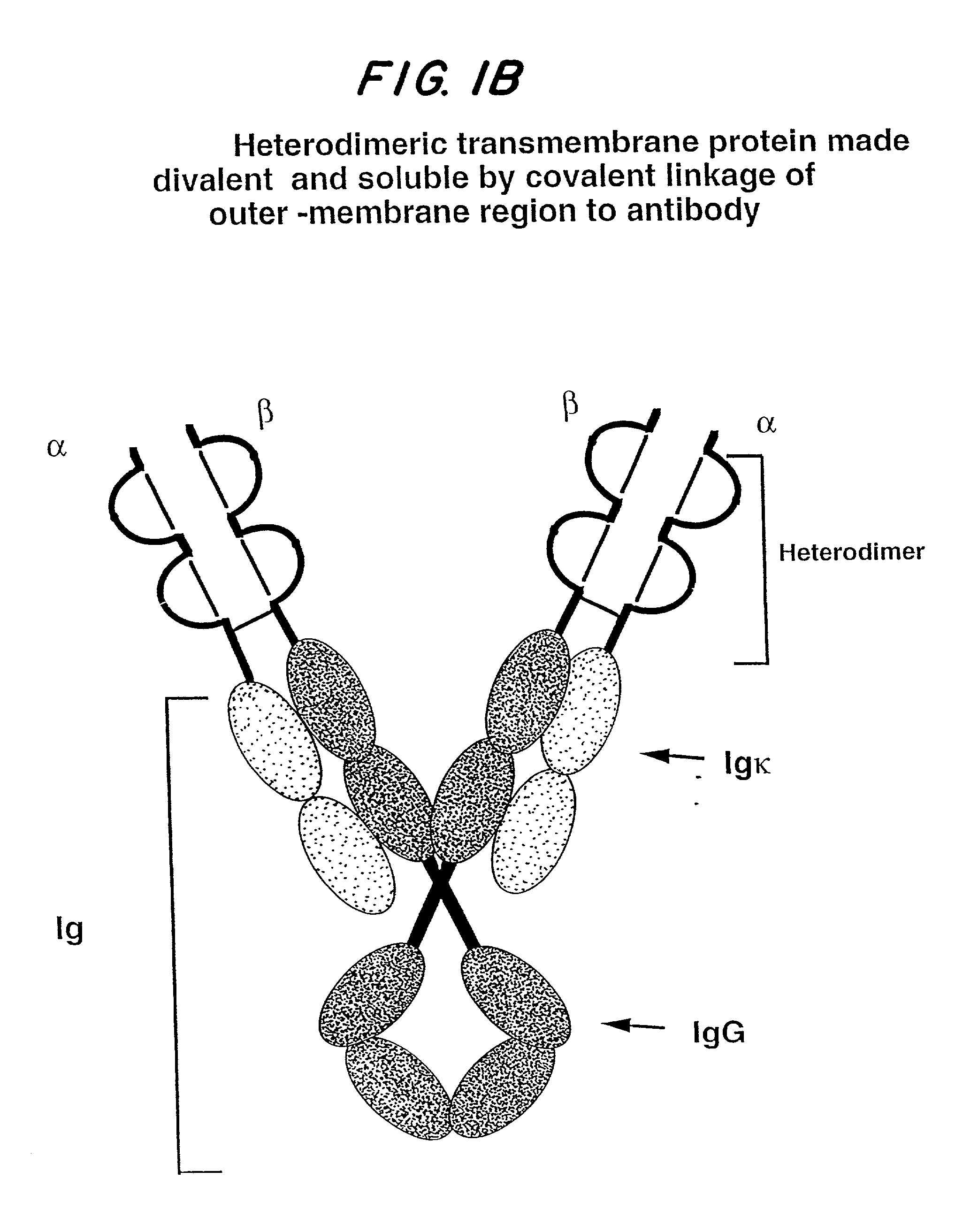
Soluble divalent and multivalent heterodimeric analogs of proteins
InactiveUS20020127231A1Well representedHigh affinityVirusesPeptide/protein ingredientsADAMTS ProteinsSpecific immunity
Specificity in immune responses is in part controlled by the selective interaction of T cell receptors with their cognate ligands, peptide / MHC molecules. The discriminating nature of this interaction makes these molecules, in soluble form, good candidates for selectively regulating immune responses. Attempts to exploit soluble analogs of these proteins has been hampered by the intrinsic low avidity of these molecules for their ligands. To increase the avidity of soluble analogs for their cognates to biologically relevant levels, divalent peptide / MHC complexes or T cell receptors (superdimers) were constructed. Using a recombinant DNA strategy, DNA encoding either the MHC class II / peptide or TCR heterodimers was ligated to DNA coding for murine Ig heavy and light chains. These constructs were subsequently expressed in a baculovirus expression system. Enzyme-linked immunosorbant assays (ELISA) specific for the Ig and polymorphic determinants of either the TCR or MHC fraction of the molecule indicated that infected insect cells secreted approximately 1 .mu.g / ml of soluble, conformnationally intact chimeric superdimers. SDS PAGE gel analysis of purified protein showed that expected molecular weight species. The results of flow cytometry demonstrated that the TCR and class II chimeras bound specifically with high avidity to cells bearing their cognate receptors. These superdimers will be useful for studying TCR / MHC interactions, lymphocyte tracking, identifying new antigens, and have possible uses as specific regulators of immune responses.
Owner:SCHNECK JONATHAN +1
Active immunization of AScr for prion disorders
Disclosed are pharmaceutical compositions and methods for preventing or treating a number of amyloid diseases, including Alzheimer's disease, prion diseases, familial amyloid neuropathies and the like. The pharmaceutical compositions include immunologically reactive amounts of amyloid fibril components, particularly fibril-forming peptides or proteins. Also disclosed are therapeutic compositions and methods which use immune reagents that react with such fibril components.
Owner:PROTHENA BIOSCI LTD
Immunogenic combination compositions and uses thereof
PendingUS20140227346A1Induce immune responseReduce in quantityPowder deliverySsRNA viruses positive-senseImmunogenicityVirology
This invention generally relates to immunogenic compositions that comprise an RNA component and a polypeptide component. Immunogenic compositions that deliver antigens in two different forms—a first antigen from a pathogen, in RNA-coded form; and a second antigen from a different pathogen, in polypeptide form—are effective in inducing immune response to both pathogens.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com